Note: Descriptions are shown in the official language in which they were submitted.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
SMALL CYCLIC MIMICS OF BRAIN-DERIVED NEUROTROPHIC FACTOR (BDNF)
Field of the Invention
This invention relates to methods and
compositions for promoting nerve cell growth and in
particular to agonists of brain-derived neurotrophic
factor. The invention relates more particularly to
agonists which are derivatives of peptides based on the
structures of the solvent-exposed loops 2 and 4 of brain-
derived neurotrophic factor.
Background of the Invention
Brain-derived neurotrophic factor (BDNF) is a
member of the neurotrophin family of neurotrophic factors,
which includes nerve growth factor (NGF), neurotrophin-3
(NT-3), neurotrophin-4/5 (NT-4/5) and neurotrophin-6
(Thoenen, 1991; Gotz et al, 1994).
These factors promote the survival of neurons
during embryonic development, and thus play a vital role in
shaping the vertebrate nervous system. Between them, the
neurotrophins support the survival of a wide range of
peripheral and central neurons, although each individual
neurotrophin acts on specific neuronal populations (for
review sea Thoenen, 1991; Gotz et al, 1994).
In a variety of in vitro and in vivo models, BDNF
has been shown to promote neuronal survival during
embryonic development, and to prevent neuronal degeneration
resulting from disease or injury. Furthermore, several
BDNF-responsive neuronal populations have been implicated
in human neurodegenerative disease. For example, in the
central nervous system BDNF acts as a potent neurotrophic
factor for cranial and spinal motor neurons which
degenerate in amyotrophic lateral sclerosis (Thoenen et a1,
1993), as well as for dopaminergic neurons of the
substantia nigra which are lost a.n Parkinson's disease
(Spina et a1, 1992). In the periphery, BDNF has
neurotrophic actions on small fibre sensory neurons
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 2 -
involved in several types of sensory neuropathy (Lindsay,
1994).
The biological effects of BDNF and the other
neurotrophins are mediated by binding to two classes of
cellular receptor: members of the trk family of receptor
tyrosine kinases, and the low affinity neurotrophin
receptor, p75. Specific neurotrophins bind with high
of f inity (Kd approximately 10-11 M) to particular trk
members expressed by responsive neurons: thus NGF and NT-3.
bind to trkA; BDNF and NT-4/5 bind to trkB; NT-3 binds to
trkC. Binding of a neurotrophin to its specific-trk
receptor causes receptor homodimerisation, triggering the
intrinsic kinase domains of the receptors to
autophosphorylate intracellular tyrosine residues, and thus
initiating signal transduction cascades leading to neuronal
survival (Barbacid, 1994). In contrast, p75 acts as a
common low affinity receptor for the neurotrophins, and
binds each with comparable affinity (Kd approximately 10-9
M); p75 is expressed widely on central and peripheral
neurons as well as on other cell types, such as Schwann
cells (for review see Chao and Hempstead, 1995).
Vrhile the role of the trk members in signalling
the neurotrophic effects of the neurotrophins is well
established, the function of p75 remains controversial.
Although there is compelling evidence that p75 either
modulates responses mediated by trk members or itself plays
a part in survival signalling, the final effect of p75
appears to depend on the relative levels of expression of
p75 and trk (Kaplan and Miller, 1997). Of particular
interest are the observations that p75 may, under certain
circumstances, cause apoptosis either in the absence
(Rabizadeh et a1, 1993; Barrett and Bartlett, 1994) or
presence (Frade et a1, 1996) of bound neurotrophin. This
~~death signal's of p75 may be mediated by an intracellular
region homologous to the death-signalling domains of tumour
necrosis factor (TNF) receptor-1 and Fas (Chapman, 1995).
The neurotrophins are homodimers which consist of
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 3 -
two identical protomers of approximately 120 amino acids,
held together by hydrophobic interactions. The overall
amino acid homology between the different neurotrophins is
approximately 50%, and sequence alignment between the
members reveals a common pattern of sequence homology and
variability (Ibanez et al, 1993). X-ray crystal structures
have been determined for the NGF homodimer (McDonald et a1,
1991) and for a BDNF/NT-3 heterodimer (Robinson et a1,
1995), revealing a common fold for the neurotrophins. This
structure is depicted in Figure 1. In the neurotrophin
protomer, the regions of high sequence homology exist as
seven (3-strands, which contribute to three longitudinal
anti-parallel twisted (3-sheets. This structure is locked
by a "cystine knot" of three disulphide bridges. The six
cysteine residues which participate in the cystine knot
structure are fully conserved in all the neurotrophins.
The three pairs of ~3-strands are linked by three ~i-hairpin
loops (loop 1, loop 2 and loop 4) and a longer loop (loop
3), which correspond predominantly to the regions of
sequence variability.
It has been postulated that the ~3-hairpin loop
regions of the neurotrophins are responsible for the
specificity of different trk receptors, and thus are
important regions in receptor binding and activation. In
general, structure-activity data obtained from the
neurotrophins support this hypothesis. Site-directed
mutagenesis studies have identified amino acid residues of
in loop 2 of BDNF, which are important for binding to trkB
and for biological activity. Insertion of this region of
BDNF into NGF gave a chimeric protein which, unlike native
NGF bound to trkB and displayed BDNF-like biological
activity (Ibanez et a1, 1993). Additional residues in loop
3 ( G1n84 ) and loop 4 ( Lys96 and Arg9' ) have been shown to be
important in activation of trkB, but are thought not to be
involved in receptor banding. Tn~hen mapped on to the three-
dimensional structure of BDNF, these residues are solvent-
accessible, and together form a binding surface that almost
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 4 -
exclusively spans the top half of the molecule.
Other site-directed mutagenesis studies have
shown that three positively charged residues in each of the
neurotrophins are of paramount importance in binding to p75
(Ibanez et a1, 1992; Ryden et a1, 1995). These data are
consistent with the idea that p75 shares a common binding
interface with the neurotrophin family. There are,
however, differences in the position of these three
residues in different neurotrophins: in NGF, NT-3 and NT-
4/5, the three positively-charged residues are spread
across two adjacent loops, while in BDNF the three
positively-charged residues are contiguous amino acids
(Lys95-Lys96-Arg9~) , located on loop 4 (Figure 1) .
The ability of exogenously administered
neurotrophic factors such as BDNF to rescue neurons in a
variety of in vivo models of neurodegeneration has led to
the widespread belief that neurotrophins and other
neurotrophic factors offer exciting prospects for the
treatment of neurodegenerative diseases, such as motor
neuron disease and peripheral neuropathies (for review see
Hefti, 1994). Unfortunately, because they are proteins,
neurotrophic factors are orally inactive, are unable to
cross the blood-brain barrier, and typically have a short
half-life in plasma (Dittrich et a1, 1994). Thus the
recombinant human neurotrophic factors themselves are
unlikely to be optimal agents for the long-term treatment
of neurodegenerative disease. Indeed, the lack of success
thus far of neurotrophic factors a.n clinical trials for the
treatment of motor neuron disease has been attributed to
the inability of the proteins to reach their targets in the
central nervous system (CNS) following subcutaneous
administration (Penn et al, 1997). One means of
circumventing these problems would be to develop low
molecular weight, non-peptidic analogues of neurotrophic
factors with improved pharmacokinetic characteristics.
For example, Australian Patent Application No.
24264/97 by Regents of the University of California, and
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 5 -
Longo et al, 1997, disclose low molecular weight compounds,
8-9 amino acids long and incorporating a D-penicillamine
group, which bind to the p75 region of the NGF receptor,
and promote neuronal survival in primary cultures of chick
dorsal root ganglia. The compounds form undefined (either
parallel or anti-parallel) monocyclic dimers of 16-18 amino
acids. International Patent Application No. W095/15593 by
Queen's University at Kingston discloses bicyclic peptides
based on the cysteine knot region, which is distal from
loops 1-4 of BDNF; these bicyclic peptides act as
neurotrophin antagonists in the chick dorsal root ganglion
assay. Cyclic peptides based on various loop regions of
NGF have been shown to interfere with NGF-mediated
biological activity (LeSauter et al, 1995).
We have used a model of the three-dimensional
structure of BDNF to design small, conformationally-
constrained peptides that mimic the receptor-binding loops
of.BDNF. These peptides have been synthesised and
purified, and then assayed in cultures of embryonic chick
sensory neurons, a subpopulation of which require BDNF for
survival.
Monomeric cyclic peptides designed in this manner
from loop 2 - believed to be the major region of BDNF
contributing to trkB binding and activation - act as
specific antagonists of BDNF-mediated neuronal survival in
cell culture. We have investigated structure-activity
relationships in these peptides by conducting an alanine
scan, and have used pharmacodynamic simulations to model
the anticipated competitive mode of antagonism of these
peptides (O'Leary and Hughes, 1998).
We have now used the structure-activity data
obtained for these monomeric cyclic peptides to design
bicyclic dimeric peptides with BDNF agonist activity. The
peptides consist of two monocyclic peptides connected by a
linking moiety. It is surprising that the linking moiety
in some of the dimers found to be active is incorporated at
sites other than those predicted to be optimal on the basis
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 6 -
of the structure-activity relationships. In addition, the
linker distance found to give optimal activity was shorter
than that predicted from our design template.
By combining these data, we have been able to
create a novel class of tricyclic dimeric peptides with
markedly improved potency over the dimeric peptides.
In contrast, monomeric cyclic peptides based on
the p75-binding tripeptide sequence found in loop 4 had no
lnhlbltOry effects on the neuronal survival activity of
either BDNF or NGF (Zwar and Hughes, 1997). To our
surprise, however, when tested in the absence of
neurotrophin some of these monomeric peptides acted as
BDNF-like agonists, able to promote the survival of chick
sensory neurons in culture.
Summary of the Invention
According to a first aspect, the invention
provides a cyclic compound of one or more cyclic moieties,
which has a biological activity of brain-derived
neurotrophic factor (BDNF).
In one embodiment, the compound is a bicyclic
dimeric compound (that is, a compound composed of two
monocyclic compounds connected by a chemical linker) based
on loop 2 of BDNF, of general formula (I):
monomeric manameric
loop 2 sequence-linker-laop 2 sequel a
~constraint~ constraint
(I)
wherein:
monomeric loop 2 sequence means a sequence of
amino acid residues or functional equivalents thereof,
which is substantially homologous to the loop 2 region of
BDNF, and which comprises all or part of the following
sequence:
Glu°°-Lys°1-Va142-Pro43-Va144-Ser45-Lys46-Glya'-
G1n48-
Leu49-Lyss°-Glnsi
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
constraint means any chemically and biologically
compatible grouping of atoms serving to limit the
flexibility of the monomeric loop 2 sequence; and
linker means any chemically and biologically
compatible grouping of atoms serving to link two monomeric
loop 2 sequences and their associated constraints to give a
bicyclic, dimeric compound.
Generally, the preferred linking groups have 0 to
20 carbon atoms, and 0 to 10 heteroatoms (N, O, S, P etc.),
and may be straight chain or branched, may contain
saturated, unsaturated and/or aromatic rings, may contain
single and/or double bonds, and may contain chemical groups
such as amide, ester, disulphide, thioether, ether,
phosphate, amine and the like.
The "constraint" can be obtained by several
methods, including but not limited to:
(i) cyclising the N-terminal amine with the C-
terminal carboxyl acid function, either directly via an
amide bond between the N-terminal nitrogen and C-terminal
carbonyl, or indirectly via a spacer group, for example by
condensation with an w-amino carboxylic acid;
(ii) cyclising via the formation of a covalent
bond between the side chains of two residues, such as an
amide bond between a lysine residua and either an aspartic
acid or glutamic acid residue, or a disulphide bond between
two cysteine residues, or a thioether bond between a
cysteine residue and an w-halogenated amino acid residue,
either directly or via a spacer group as described in (i)
above. The residues contributing the side chains may be
derived from the monomeric loop 2 sequence itself, or may
be incorporated into or added on to the monomeric loop 2
sequence for this purpose; and,
(111) cyclising via the formation of an amide
bond between a side chain (for example of a lysine or
aspartate residue) and either the C-terminal carboxyl or N-
terminal amine, either directly or a spacer group as
described in (i) above. The residues contributing the side
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
_ g _
chains may be derived from the monomeric loop 2 sequence
itself, or may be incorporated into or added on to the
monomeric loop 2 sequence for this purpose.
In a second embodiment, the compound is a
tricyclic dimeric compound (that is, a compound composed of
two monocyclic compounds connected by two chemical linkers)
based on loop 2 of BDNF of general formula (II):
manomeric-linkerl-monameric
loop 2 sequence-linker2-loop 2 sequence
~constraint~ ~~constraint~
(II)
wherein:
monomeric loop 2 sequence means a sequence of
amino acid residues or functional equivalents thereof,
which is substantially homologous to the loop 2 region of
BDNF, and which comprises all or part of the following
sequence:
Glu4°-LyS41-Va142-pr043-va144-Ser45-ZayS46-G1y47 _ G1n48 -
Leu49-LySS~-G1n51;
constraint means any chemically and biologically
compatible grouping of atoms serving to limit the
flexibility of the monomeric loop 2 sequence; and
linker means any chemically and biologically
compatible grouping of atoms serving to link two monomeric
loop 2 sequences and their associated constraints to give a
tricyclic, dimeric compound. These linkers may be the same
or dif f erent .
Generally, the preferred linking groups have 0 to
20 carbon atoms, and 0 to 10 heteroatoms (N, O, S, P etc.),
and may be straight or branched, may contain saturated,
unsaturated and/or aromatic rings, may contain single
and/or double bonds, and may contain chemical groups such
as amide, ester, disulphide, thioether, ether, phosphate,
amine and the like.
The "constraint" can be obtained by several
methods, including but not limited to:
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
_ g _
(i) cyclising the N-terminal amine with the
C-terminal carboxyl acid function either directly via an
amide bond between the N-terminal nitrogen and C-terminal
carbonyl, or indirectly via a spacer group, for example by
condensation with an co-amino carboxylic acid;
(ii) cyclising via the formation of a
covalent bond between the side chains of two residues, such
as an amide bond between a lysine residue and either an
aspartic acid or glutamic acid residue, or a disulphide
bond between two cysteine residues, or a thioether bond
between a cysteine residue and an (~-halogenated amino acid
residue, either directly or via a spacer group as described
in (i) above. The residues contributing the side chains
may be derived from the ~~monomeric loop 2 sequence" itself,
or may be incorporated into or added onto the monomeric
loop 2 sequence for this purpose; and,
(iii) cyclising via the formation of an amide
bond between a side chain (for example of a lysine or
aspartate residue) and either the C-terminal carboxyl or N-
terminal amine, either directly or via the intermediacy of
a spacer group as described in (i) above. The residues
contributing the side chains may be derived from the
monomeric loop 2 sequence itself, or may be incorporated
into or added onto the monomeric loop 2 sequence for this
purpose.
In third embodiment, the compound is a monomeric,
monocyclic compound based on the p75-binding region of loop
4 of BDNF and incorporating a molecular spacer of the
general formula (III):
monomeric
loop 4 sequenJ
~constraint~
wherein:
monomeric loop 4 sequence means a sequence of
amino acid residues or functional equivalents thereof,
which is substantially homologous to the p75-binding region
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 10 -
of loop 4 of BDNF, and comprises all or part of the
following sequence:
Lysss-Lysss-~g3y and,
constraint' means any chemically and biologically
compatible grouping of atoms serving to limit the
flexibility of the monomeric loop 4 sequence. For example
by covalently linking all or part of the "monomeric loop 4
sequence" to form a cyclic structure (ring).
The "constraint "' can be derived by several
methods, including but not limited to:
(i) cyclising the N-terminal amine with the
C-terminal carboxyl acid function, either directly via an
amide bond between the N-terminal nitrogen and C-terminal
carbonyl, or indirectly via a spacer group, such as one or
more additional amino acid residues, including a.-and
amino carboxylic acid residues;
(ii) cyclising via the formation of a
covalent bond between the side chains of two residues, such
as an amide bond between a lysine residue and either an
aspartic acid or glutamic acid residue, or a disulphide
bond between two cysteine residues, or a thioether bond
between a cysteine residue and an cep-halogenated amino acid
residue, either directly or via a spacer group as described
in (i) above. The residues contributing the side chains
may be derived from the "monomeric loop 4 sequence" itself,
or may be incorporated into or added on to the "monomeric
loop 4 sequence" for this purpose; and
(iii) cyclising via the formation of an amide
bond between a side chain (for example of a lysine or
aspartate residue) and either the C-terminal carboxyl or N-
terzninal amine, either directly or via a spacer group as
described in (i) above. The residues contributing the
side chains may be derived from the monomeric loop 4
sequence itself, or may be incorporated into or added on to
the monomeric loop 4 sequence for this purpose.
Sequences encompassing conservative substitutions
of amino acids are within the scope of the invention,
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 11 -
provided that the biological activity is retained.
It is to be clearly understood that the compounds
of the invention include peptide analogues, including but
not limited to the following:
1. Compounds in which one or more amino acids
is replaced by its corresponding n-amino acid. The skilled
person will be aware that retro-inverso amino acid
sequences can be synthesised by standard methods. See for
example Chorev and Goodman, 1993;
2. Peptidomimetic compounds, in which the
peptide bond is replaced by a structure more resistant to
metabolic degradation. See for example Olson et a1, 1993.
3. Compounds a.n which individual amino acids
are replaced by analogous structures, for example gem-
diaminoalkyl groups or alkylmalonyl groups, with or without
modified termini or alkyl, acyl or amine substitutions to
modify their charge.
The use of such alternative structures can
provide significantly longer half-life a.n the body, since
they are more resistant to breakdown under physiological
conditions.
Methods for combinatorial synthesis of peptide
analogues and for screening of peptides and peptide
analogues are well known in the art (see for example Gallop
et al, 1994). It is particularly contemplated that the
compounds of the invention are useful as templates for
design and synthesis of compounds of improved activity,
stability and bioavailability.
Preferably where amino acid substitution is used,
the substitution is conservative, i.e., an amino acid is
replaced by one of similar size and with similar charge
properties.
In particularly preferred embodiments the
bicyclic dimers are of formula (IV) to (VI):
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 12 -
(L2-8P2C)2
I V C V S R G Q L I I V C V S R G Q L I
(IV)
(L2-8S4C)2
I-V-P-V-C-R-G-Q-L-I I-V-P-V-C-R-G-Q-L-)
(V)
where the dimeric bicyclic peptides (L2-8P2C)2
and (L2-8S4C)2 consist of monomeric loop 2 sequences
constrained by disulphide bonds formed between cysteine
residues added to the loop 2 sequence and joined by a
linker consisting of a disulphide bond formed between
cysteine residues substituted into the loop 2 sequence, or
(L2-8&E+K)2
Ac-L V-P-V-S-R-G-Q-L-i-E-NHZ Ac-i-V-P-V-S-R-G-Q-L-i-R-NfI2
(VI)
where the dimeric bicyclic peptide (L2-8&E+K)2
consists of monomeric loop 2 sequences constrained by
disulphide bonds formed between cysteine residues added to
the loop 2 sequence and joined by a linker consisting of an
amide bond formed between a glutamate and a lysine residue
added to the loop 2 sequence.
In a particularly preferred embodiment the
tricyclic dimer is of formula (VII):
WO 00/75176 CA 02376729 2001-12-10 PCT/AU00/00641
- 13 -
(L2-8S4C&E+K)2
Ac-C-V-P-V-C- G-Q-L-C-E-NH2Ac-C-V-P-V-C-K-G-Q-L-C-K-NHZ
I[ t
(vII)
wherein the dimeric tricyclic peptide (L2-
8S4C&E+K)2consists of monomeric loop 2 sequences
constrained by disulphide bonds formed between cysteine
residues added to the loop 2 sequence, and joined by one
linker consisting of a disulphide bond formed between
cysteine residues substituted into the loop 2 sequence and
a second substituted consisting of an amide bond formed
between a glutamate and a lysine residue added to the loop
2 sequence.
In a particularly preferred embodiment the
monomeric, monocyclic compound is of formula (VIII):
L4-3pA
rnPro-Ala-Lys-Lys-Argue
(VIII)
wherein the monomeric cyclic compound is cyclised
by condensing its amino- and carboxyl-termini directly via
as amide bond.
The invention further provides a pharmaceutical
composition, comprising a compound according to the
inveation together with a pharmaceutically acceptable
carrier.
The composition may be formulated so as to be
suitable for a variety of routes of administration, for
example intravenous, subcutaneous, intramuscular or
intrathecal or intraventricular injection, oral or for
topical administration.
The exact formulation will depend on the
individual route of administration. Methods and
pharmaceutical carriers for preparation of pharmaceutical
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 14 -
compositions are well known in the art; as set out in
textbooks such as Remington's Pharmaceutical Sciences, 17th
Edition, Mack Publishing Company, Easton, Pennsylvania,
USA. Pharmaceutically acceptable carriers include
conventional carriers which are suitable for use with
peptide-based drugs, including diluents, excipients, and
preservatives and the like. For example, carriers such as
dextrose, mannitol, sucrose, or lactose, buffer systems
such as acetate, citrate and phosphate, and bulking agents
such as serum albumin, preferably human serum albumin, may
be used.
The invention also provides a culture medium
additive for promotion of growth of neuronal cells in
vitro, comprising a compound according to the invention
together with a carrier or diluent which does not adversely
effect the growth of cells in culture. Suitable carriers
and diluents will be well known to the person skilled in
art, and include physiologically acceptable fluids such as
water, saline solution, or buffer solutions.
The optimal concentration of compound will vary
according to the cell type and the culture conditions, but
will generally be a.n the range 1-500~.1M, preferably 1-100~.1M.
The invention further provides a method of
treatment of a condition characterised by neuronal deficit
or neuronal death, comprising the step of administering an
effective amount of a compound of the invention to a
subject in need of such treatment.
It is contemplated that the method of the
invention is suitable for treatment of conditions including
but not limited to neurodegenerative diseases such as motor
neurone disease (amyotrophic lateral sclerosis),
progressive spinal muscular atrophy, infantile muscular
atrophy, Charcot-Marie-Tooth disease, Parkinson's Disease,
Parkinson-Plus syndrome, Guamanian Parkinsonian dementia
complex, progressive bulbar atrophy, Alzheimer's disease
and the like, other neurodegenerative conditions such as
those arising from ischaemia, hypoxia, neural injury,
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 15 -
surgery, exposure to neurotoxins such as N-methyl-4-phenyl-
1,2,3,6-tetrahydropyridine), and peripheral sensory
neuropathies, including those resulting from exposure to
drugs (such as cis-platin) and toxins and resulting from
diabetes, for example mononeuropathy multiplex.
The dose required will depend on the nature and
severity of the condition to be treated, and the route of
administration, and will be at the discretion of the
attending physician or surgeon. A suitable route,
frequency of administration, and dosage can readily be
established using conventional clinical trial methodology.
Throughout this specification, the amino acid
numbering is the same as in mature BDNF, and conventional
single-letter or three-letter amino acid code is used.
For the purposes of this specification it will be
clearly understood that the word "comprising" means
"including but not limited to", and that the word
"comprises" has a corresponding meaning.
The term ~functional equivalents thereof", when
used with reference to amino acid residues of the monomeric
loop 2 or loop 4 sequence, means amino acid sequence
variants of said sequence are encompassed. For example,
one or more of the amino acids Glua°-Lys41-Va142-Pro43-Val4a-
Ser45-Lys46-Gly4'-G1n48-Leua9-Lyss°-G1n51 may be deleted, and
optionally substituted by one or more amino acid residues;
or Wherein an amino acid residue has been covalently
modified so that the resulting product is a non-naturally
occurring amino acid. Amino acid sequence variants may be
made synthetically, for example, by peptide synthesis, or
may exist naturally.
An amino acid sequence variant of the monomeric
loop 2 or loop 4 sequence of BDNF is included within the
scope of the invention provided that it is functionally
active. As used herein, "functionally active" and
"functional activity" in reference to the monomeric loop 2
or loop 4 sequence of BDNF means that the compound
generated therefrom is able to promote or enhance the
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 16 -
growth, survival, function, and/or differentiation of
neurons and glia, especially axon fasciculation and process
outgrowth, whether the neurons be central, peripheral,
motorneurons, or sensory neurons, e.g. photoreceptors,
vestibular ganglia, spinal ganglia and auditory hair cells.
Therefore, monomeric loop 2 or loop 4 amino acid sequence
variants generally will share at least about 75%
(preferably greater than 80% and more preferably greater
than 90%) sequence identity with the amino acid sequence
Glu4°-Lys41-Va142-Pro43-Va144-Ser45-Lys46-Glya~-Gln4$-Leu49-LysS~-
Glnsl, after aligning the sequences to provide for maximum
homology, as determined, for example, by the Fitch, et al.,
Proc. Nat. Acad. Sci. USA 80:1382-1386 (1983), version of
the algorithm described by Needleman, et al., J. Mol. Biol.
48:443-453 (1970).
Amino acid sequence variants of the monomeric
loop 2 or loop 4 sequence of BDNF are prepared by
introducing appropriate amino changes into amino acid
sequence, or by in vitro synthesis. Such variants include,
for example, deletions from, or insertions or substitutions
of, amino acid residues within Glu4°-Lys41-Vald2-Pro43-Ya144-
Ser°5-Lys46-Gly4~-Glna$-Leu49-LysS°-G1n51. Any combination
of
deletion, insertion, and substitution may be made to arrive
at an amino acid sequence variant of the monomeric loop 2
or loop 4 sequence of BDNF, provided that such variant
possesses the desired characteristics described herein.
There are two principal variables in the
construction of amino acid sequence variants of the
monomeric loop 2 or loop 4 sequence of BDNF: the location
of the mutation site and the nature of the mutation. In
general, the location and nature of the mutation chosen
will depend upon the monomeric loop 2 or loop 4 sequence of
BDNF characteristic to be modified.
For example, functionally active amino acid
sequence variants of the monomeric loop 2 or loop 4
sequence of BDNF may be selected, for example, by
substituting one or more amino acid residues in the amino
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 17 -
acid sequence Glu4°-Lysal-Val4~-Pro43-Va144-Ser45-Lys46-Gly°'
G1n48-Leu49-Lyss°-Glnsl with other amino acid residues of a
similar or different polarity or charge.
One useful approach is called "alanine scanning
mutagenesis." Here, an amino acid residue or group of
target residues are identified (e. g., charged residues such
as arg, asp, his, lys, and glu) and, by means of
recombinant DNA technology, replaced by a neutral or
negatively charged amino acid (most preferably alanine or
polyalanine) to affect the interaction of the amino acids
with the surrounding aqueous environment in or outside the
cell. Cunningham, et al., Science 244: 1081-1085 (1989).
Those domains demonstrating functional sensitivity to the
substitutions then are refined by introducing further or
other variants at or for the sites of substitution.
Amino acid sequence deletions generally range
from about 1 to 6 residues, more preferably about 1 to 3
residues, and typically are contiguous. Generally, the
number of consecutive deletions will be selected so as to
preserve the tertiary structure of the monomeric loop 2 or
loop 4 sequence of BDNF.
Amino acid sequence insertions include amino-
and/or carboxyl-terminal fusions, or intrasequence
insertions (i.e., insertions made within the amino acid
sequence Glu4°-Lys41-Vala2-Pro43-Vale-Ser45-Lys°6-Gly°'-
G1n48-
Leu49-Lys5°-Glnsl) may range generally from about 1 to 10
residues, more preferably 1 to 5, most preferably 1 to 3.
The third group of variants are those in which at
least one amino acid residue in the amino acid sequence
3 0 Glu4°-Lysal-Va142-Pro43-Va144-Ser45-Lys46-Glya'-Gln4$-Leu49-
Lyss°-
G1n51, preferably one to four, more preferably one to
three, even more preferably one to two, and most preferably
only one, has been removed and a different residue inserted
in its place. The sites of greatest interest for making
such substitutions are those sites most likely to be
important to the functional activity of the monomeric loop
2 or loop 4 sequence of BDNF. Accordingly, to retain
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 18 -
functional activity, those sites, especially those falling
within a sequence of at least three other identically
conserved sites, are substituted in a relatively
conservative manner. Such conservative substitutions are
shown in Table A under the heading of preferred
substitutions. If such substitutions do not result in a
change in functional activity, then more substantial
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 19 -
Table A
Original Exemplary Preferred
Resi due Substitutions Substitutions
Ala (A) val; leu; ile val
Arg (R) lys; gln; asn lys
Asn (N) gln; his; lys; arg gln
Asp (D) glu glu
Cys (C) ser ser
Gln (Q) asn asn
Glu (E) asp asp
Gly (G) pro pro
His (H) asn; gln; lys; arg arg
Ile (I) leu; val; mat; ala; phe;
norleucine leu
Leu (L) norleucine;
ile;
val;
met; ala; phe ile
Lys (K) arg; gln; asn arg
Mat (M) leu; phe; ile leu
Phe (F) leu; val; ile; ala leu
Pro (P) gly gly
Ser (S) thr thr
Thr (T) ser ser
Trp (W) tyr tyr
Tyr (Y) trp; phe; thr; ser phe
Val (V) ile; leu; met; phe;
ala; norleucine leu
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 20 -
changes, denominated exemplary substitutions in Table A, or
as further described below in reference to amino acid
classes, may be introduced and the resulting variant the
monomeric loop 2 or loop 4 sequence of BDNF analyzed for
functional activity.
Insertional, deletional, and substitutional
changes in the amino acid sequence Glu4°-Lys41-Va142-Pro4a-
Va144-Ser45-Lys46-Gly4~-G1n48-Leu49-LysS°-Glnsl may be made to
improve the stability of the monomeric loop 2 or loop 4
sequence of BDNF.
Covalent modifications of the monomeric loop 2 or
loop 4 sequence of BDNF are also included within the scope
of this invention. For example, covalent modifications are
introduced into the monomeric loop 2 or loop 4 sequence of
BDNF by reacting targeted amino acid residues of the
monomeric loop 2 or loop 4 sequence of BDNF with an organic
derivatizing agent that is capable of reacting with
selected amino acid side chains or the N- or C-terminal
residues.
Cysteinyl residues most commonly are reacted with
oc-haloacetates (and corresponding amines), such as
chloroacetic acid or chloroacetamide, to give carboxymethyl
or carboxyamidomethyl derivatives. Cysteinyl residues also
are derivatized by reaction with bromotrifluoroacetone, a.-
bromo-(3-(5-imidozoyl)propionic acid, chloroacetyl
phosphate, N-alkylmaleimides, 3-nitro-2-pyridyl disulfide,
methyl 2-pyridyl disulfide, p-chloromercuribenzoate, 2-
chloromercuri-4-nitrophenol, or chloro-7-nitrobenzo-2-oxa-
1,3-diazole.
Histidyl residues are derivatized by reaction
with diethyl-pyro-carbonate at pH 5.5-7.0 because this
agent is relatively specific for the histidyl side chain.
Para-bromophenacyl bromide also is useful; the reaction is
preferably performed in O.iM sodium cacodylate at pH 6Ø
Lysinyl and amino terminal residues are reacted with
succinic or other carboxylic acid anhydrides.
Derivatization with these agents has the effect of
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 21 -
reversing the charge of the lysinyl residues. Other
suitable reagents for derivatizing oc-amino-containing
residues include imidoesters such as methyl picolinimidate;
pyridoxal phosphate; pyridoxal; chloroborohydride;
trinitrobenzenesulfonic acid; O-methylisourea; 2,4-
pentanedione; and transaminase-catalyzed reaction with
glyoxylate.
Arginyl residues are modified by reaction with
one or several conventional reagents, among them
phenylglyoxal, 2,3-butanedione, 1,2-cyclohexanedione, and
ninhydrin. Derivatisation of arginine residues requires
that the reaction be performed in alkaline conditions
because of the high pKa of the guanidine functional group.
Furthermore, these reagents may react with the groups of
lysine as well as the arginine epsilon-amino group.
The specific modification of tyrosyl residues may
be made, with particular interest in introducing spectral
labels into tyrosyl residues by reaction with aromatic
diazonium compounds or tetranitromethane. Most commonly,
N-acetylimidizole and tetranitromethane are used to form O-
acetyl tyrosyl species and 3-vitro derivatives,
respectively.
Carboxyl side groups (aspartyl or glutamyl) are
selectively modified by reaction with carbodiimides (R'-
N=C=N-R'), where R and R' are different alkyl groups, such
as 1-cyclohexyl-3-(2-morpholinyl-4-ethyl) carbodiimide or
1-ethyl-3-(4-azonia-4,4-dimethylpentyl) carbodiimide.
Furthermore, aspartyl and glutamyl residues are converted
to asparaginyl and glutaminyl residues by reaction with
ammonium ions.
Glutaminyl and asparaginyl residues are
frequently deamidated to the corresponding glutamyl and
aspartyl residues, respectively. Alternatively, these
residues are deamidated under mildly acidic conditions.
Either form of these residues falls within the scope of
this invention.
Other modifications include hydroxylation of
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 22 -
proline and lysine, phosphorylation of hydroxyl groups of
seryl or threonyl residues, methylation of theoc-amino
groups of lysine, arginine, and histidina side chains,
acetylation of the N-terminal amine, and amidation of any
C-terminal carboxyl group. Creighton, Proteins: Structure
and Molecular Properties, pp.79-86 (W. H. Freeman & Co.,
1983) .
The term "substantially homologous" means that an
amino acid sequence is quite similar to that of the
monomeric loop 2 or loop 4 sequence of BDNF, and have at
least about 85°0 (preferably at least about 90%, and most
preferably at least about 95%) of the amino acids matching
with at least 7 of the amino acids found in the sequence
Glu4°-Lys41-Va142-Pro43-Va144-Ser45-Lys46-Glya'-Gln4$-Leu49-LySS~-
Glnsl.
The term "more resistant to metabolic
degradation" means that the compound of the invention has
been modified such that the resulting compound is more
stable under acidic conditions than the "native" sequence
of the monocyclic loop 2 or loop 4 sequence of BDNF. For
example, amino acid substitutions as discussed previously
may be undertaken which produce compounds more resistant to
metabolic degradation. It is well known in the art that D-
amino acids, and amino acids analogues are more resistant
to acidic environments. Conjugates of small peptides and
cholic acid have reduced metabolic degradation problems.
The term biological activity with reference to
BDNF means a biological activity which is normally
promoted, either directly or indirectly, by the presence of
BDNF, and includes, but is not limited to, BDNF binding to
the p75 receptor or the trkB receptor, neuron survival,
neuron differentiation, including neuron process formation
and neurite outgrowth, and biochemical effects such as
induction of enzymes which are stimulated by BDNF. Such
biological activities can be measured by conventional in
vitro and in vivo assays, such as the chick dorsal root
ganglion assay described herein by Barde et a1 (1980) and
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 23 -
the neurite outgrowth, and in vivo kindling assays
described a.n W097/15593 and by Riopelle et a1 (1982).
It will be clearly understood that the compounds
of the invention may be synthesised by any suitable method.
Solid phase methods such as those developed for synthesis
of peptides and peptidomimetic compounds are preferred,
including but aot limited to the Fmoc solid phase peptide
synthesis method described herein, the Boc solid phase
peptide synthesis method, and PIN synthesis methods (for
review, see Maeji et al., 1995). Those skilled in the art
will readily be able to select the most suitable method for
any given compound of the invention.
Brief Description of the Figures
Figure 1 shows the backbone trace of the three-
dimensional structure of BDNF dimer (one monomer in black,
the other grey), showing the positions of the loop 2 (trkB
binding) and loop 4 (predominantly p75 binding) regions.
Side chains of the p75 binding tripeptide in loop 4 (Lys-
Lys-Arg) are shown.
Figure 2 illustrates the molecular modelling of
monomeric cyclic loop 2 analogues. An a-carbon to a-
carbon trace of the native loop 2 of BDNF is shown,
superimposed with low-energy conformations of loop 2
analogues L2-12, L2-10, L2-8 and L2-6, each of which is
constrained by a disulphide bridge (indicated by arrows).
Figure 3 shows the concentration-response curves
of monomeric cyclic loop 2 analogues in competition with
BDNF. The monomeric cyclic loop 2 analogues L2-12 (closed
triangles), L2-12a (open triangles), L2-10 (open squares),
L2-8 (closed diamonds) and L2-6 (open diamonds) and the
monomeric linear peptide L2-12b (closed squares) were
assayed in competition with BDNF (4 x 10-11 M) in cultures
of E8 to E10 chick sensory neurons. Surviving neurons were
counted after 48 hrs in culture, and these counts were then
expressed as a percentage of originally-plated viable
neurons and normalised such that survival in cultures
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 24 -
containing BDNF alone (P; positive control; closed circle)
was set to 100% and survival in cultures with neither BDNF
nor loop 2 analogue (N; negative control; open circle) to
0%. Data are expressed as the mean ~ SEM from at least 8
observations (n=8) from 4 independent experiments.
Figure 4 shows the concentration-response curves
of monomeric cyclic peptide L2-12, alone and in competition
with NGF. Surviving neurons were counted after 48 hrs in
culture, and these counts expressed as a percentage of
originally-plated viable neurons and normalised such that
survival in cultures containing NGF alone (P; positive
control; closed circle) was set to 100°o and survival in
cultures with neither NGF nor L2-12 (N; negative control;
open circle) to 0%. When assayed in the absence of NGF,
L2-12 (closed triangles) produced no significant effect on
neuronal survival when compared to survival in negative
control cultures (p> 0.05, ANOVA; n=6). L2-12 (open
triangles) assayed in competition with NGF (4 x 10-11 M)
produced no significant effect on NGF-mediated survival (p>
0.05, ANOVA; n=6). Data are expressed as the mean ~ SEM.
Figure 5 shows the effect of a monomeric cyclic
loop 2 analogue (L2-12a) on the concentration-response
curve of BDNF. L2-12a (1 x 10-~ M) was assayed i.n
competition with BDNF (1.8 x 10-13 to 1.8 x 10-1° M, 0.51 og
increments) in cultures of E8-E10 sensory neurons.
Surviving neurons were counted after 48 hrs in culture;
these counts were expressed as a percentage of the number
of originally plated viable neurons, and logistic sigmoidal
curves fitted to the data. Compared to the BDNF control
curve (closed circles), the BDNF curve in the presence of
L2-12a (open circles) shows a significant depression in
maximum (40%; ** p< 0.005, Student's unpaired two-tailed t-
test) and a 1.6 fold shift in pECs°, although the latter is
insignificant (p> 0.05, Student's unpaired two-tailed t-
test). N (closed square) refers to negative control
cultures in the absence of both BDNF and L2-12a. Data are
expressed as the mean ~ SEM of 8 observations (n=8) from 4
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 25 -
independent experiments.
Figure 6 illustrate the maximal inhibition of
BDNF-mediated survival of cultured sensory neurons by
monomeric cyclic loop 2 analogues systematically
substituted with alanine. Loop 2 analogues were assayed in
competition with BDNF (4 x 10-11 M) in cultures of E8-E10
sensory neurons. Surviving neurons were counted after 48
hrs in culture, and these counts were expressed as a
percentage of originally-plated viable neurons then
normalised such that survival of cultures containing BDNF
was set as 100%, while that for cultures with neither BDNF
nor loop 2 analogue was set to 0%. Maximal inhibition of
BDNF-mediated survival was calculated by subtracting the
lowest value for BDNF-mediated survival from that of BDNF
alone (100%). Note that alanine substitution a.n the L2-12
sequence can affect the ability of these peptides to
modulate BDNF-mediated survival. Significant reduction in
inhibitory activity, compared to L2-12 (closed bar), was
observed when Ala was substituted for val3, Vals, Sers
(***p< 0.001; ANOVA Bonferroni multiple comparisons test
n=12), Lysll (*p < 0.05; n=12) and G1n12 (**p< 0.01; n=10).
No data (ND) were obtained for L2-12P4~A. Data are
expressed as the mean ~ SEM.
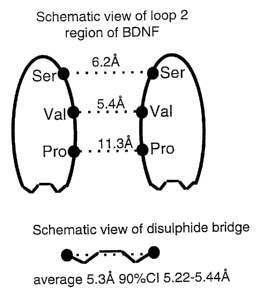
Figure 7A shows a schematic view of the two loop
two regions in the model of the three-dimensional structure
of the BDNF dimer, showing the interatomic distances (A)
between a-carbon atoms of selected residues.
Figure 7B shows a schematic view of the
disulphide bridge of the cysteine residue, showing the
average interatomic distance and 90% confidence interval
(90% CI) of oc-carbon atoms, determined by conformational
analysis.
Figure 8 shows a graph of the survival of sensory
neurons in the presence of the bicyclic dimeric peptides
(L2-8P2C)2, (L2-8V3C)2 and (L2-8S4C)2. Neurons were
prepared from chick dorsal root ganglia from embryonic
chicks (E8-E10), and surviving neurons counted after 48 hrs
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 26 -
in culture. Data are presented as a percentage of the
number of cells supported by BDNF (lng/ml; 100%) after the
same period in culture. Survival in negative control
cultures was set to 0%. Highly significant differences in
neuronal survival in the presence of (L2-8P2C)z, and (L2-
8S4C)2 were observed compared to survival in negative
controls (ANOVA, *** p<0.001, Bonferroni multiple
comparisons test).
Figure 9 shows a graph of the survival of sensory
neurons in the presence of the monomeric cyclic peptides
L2-8P2C(Acm) and L2-8S4C(Acm). Neurons were prepared from
dorsal root ganglia from embryonic chicks (E8-E10), and
surviving neurons counted after 48 hours in culture. Data
are presented as a percentage of the number of cells
supported by BDNF (lng/ml; 100%) after the same period in
culture. Survival in negative control cultures was set to
0%. Data were obtained from at least two independent
experiments.
Figure 10 shows a graph of the survival of
sensory neurons in the presence of the amide-linked dimeric
bicyclic peptide (L2-8&E+K)2. Neurons were prepared from
chick dorsal root ganglia obtained from embryonic chicks
(E8-E10), and surviving neurons counted after 48 hours in
culture. Data are expressed as a percentage of the number
of cells supported by BDNF (lng/ml; 100%) after the same
period in culture. Survival in negative control cultures
was set to 0%. A highly significant difference in neuronal
survival in the presence of (L2-8&E+K)2 was observed
compared to survival in negative controls (ANOVA, p<0.001,
Bonferroni multiple comparisons test).
Figure 11 shows a graph of the survival of
sensory neurons in the presence of the dimeric tricyclic
loop 2 analogue (L2-8S4C&E+K)Z. Neurons were prepared from
chick dorsal root ganglia obtained from embryonic chicks
3S (E8-E10), and surviving neurons counted after 48 hrs in
culture. Data are expressed as a percentage of the number
of cells supported by BDNF (lng/ml; 100%) after the same
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 27 -
period in culture. Survival in negative control cultures
was set to 0%. A highly significant difference in neuronal
survival in the presence of (L2-8S4C~E+K)z was observed
compared to survival in negative controls (ANOVA, p<0.001,
Bonferroni multiple comparisons test).
Figure 12 shows a graph of the survival of
sensory neurons in the presence of the monomeric cyclic
loop 4-derived L4-3pA(II) (closed circles). Neurons were
prepared from dorsal root ganglia from embryonic chicks
(E8-E10) and surviving neurons counted after 48 hrs in
culture. B: positive control (BDNF 1 ng/ml); N: negative
control (no peptide). *** Significantly different to
negative control (ANOVA, p< 0.001, Bonferroni multiple
comparisons test, n = 12).
Figure 13 shows a graph of the survival of
sensory neurons in the presence of the monomeric cyclic
loop 4-derived peptides L4-3pA(I), L4-3pA(II) and L4-3Hx,
and their linear homologues L4-3pAa and L4-3Hxa. All
peptides were added at a concentration of 10-6 M. BDNF was
added at 1 ng/ml. Neg: shows the survival in negative
control cultures containing neither BDNF nor peptide. ***
Significantly different to negative control (ANOVA, p<
0.001, Bonferroni multiple comparisons test, n = 12).
Figure 14 shows a graph of the affect of the
monomeric cyclic loop 4-derived peptides L4-3pA(I) (open
diamonds), L4-3pA(II) (open squares) and L4-3Hx (open
triangles), and their linear homologues L4-3pAa (crosses)
and L4-3Hxa (asterisks) on the neuronal survival effect
mediated by BDNF (1 ng/ml). Over the concentration range
3 0 tested ( 10-11 to 10-5 ) , none of the peptides exhibited a
significant effect on BDNF mediated neuronal survival.
Figure 15 shows a graph of the effect of the
monomeric cyclic loop 4-derived peptides L4-3pA(I) (open
diamonds), L4-3pA(II) (open squares) and L4-3Hx (open
triangles), and their linear homologues L4-3pAa (crosses)
and L4-3Hxa (asterisks) on the neuronal survival effect
mediated by NGF (1 ng/ml). Over the concentration range
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 28 -
tested (10-11 to 10-5), none of the peptides exhibited a
significant effect on NGF mediated neuronal survival.
Figure 16 shows a graph of the survival of
sensory neurons in the presence of the monomeric cyclic
loop 4 peptides, L4-3Ap(I), L4-3Ap(II), L4-3AP(I) and L4-
3AP(II). All peptides were added at a concentration of 10-
M. BDNF was added at a concentration of 1 ng/ml. Neg
shows the survival of control cultures containing neither
BDNF nor peptide. None of the peptides exhibited an effect
on neuronal survival that was significantly different to
that seen in negative control cultures.
Figure 17 shows a graph of the survival of
sensory neurons in the presence of the monomeric cyclic
loop 4 peptides L4-3K3ApA and L4-3K4ApA. The peptides were
added at a concentration of 10-6 M. BDNF was added at a
concentration of 1 ng/ml. Neg shows the survival of
control cultures containing neither BDNF nor peptide.
Neither of the peptides exhibited an effect on neuronal
survival that was significantly different to that seen in
negative control cultures.
Figure 18 shows the solution structure of peptide
L4-3pA(II) derived by nuclear magnetic resonance (NMR)
spectroscopy. Depicted is an overlay of the twenty
conformations of peptide L4-3pA(II) with the lowest target
function in the software package DYANA, following 10,000
steps of simulated annealing followed by 2,000 steps on
minimisation using the NMR-derived distance and dihedral
angle constraints. Residues are labelled and numbered.
Figure 19 shows the effects of peptide L4-3pA(II)
of the invention on neuronal loss following peripheral
nerve lesion. This was accomplished by comparing the
number of sensory neurones in the C8 dorsal root ganglia
and motor neurons a.n the central region of the spinal cord
in the lesioned side versus that in the intact
contralateral side five days following nerve lesion, and
administration of the peptide to the distal nerve stump.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 29
Detailed Description of the Invention
The invention will now be described by way of
reference only to the non-limiting examples, and to the
Figures.
Abbreviations
BDNF brain-derived neurotrophic factor
E embryonic day
NGF nerve growth factor
NT-3 neurotrophin-3
NT-4 neurotrophin-4
TFA trifluoroacetic acid
NMR nuclear magnetic resonance spectroscopy.
Materials
Mouse recombinant BDNF was a kind gift from Dr R
Kolbeck and Professor Y-A Harde (Max-Planck-Institute for
Psychiatry, Martinsried, Federal Republic of Germany).
NGF, purified from male mouse submaxillary gland was
purchased from Boehringer-Mannheim (Mannheim, Federal
Republic of Germany). Fertilised chicken eggs ware
obtained from Research Poultry Farms (Research, Victoria,
Australia), trypsin from Worthington (Freehold, NJ,
U.S.A.), L-15 from GIBCO BRL (Grand Island, NY, U.S.A.),
horse serum from CSL (Parkville, Victoria, Australia),
Nunclon 10 cm tissue culture dishes from Nalge Nunc
International (Roskilde, Denmark), Falcon Multiwell 48-well
tissue culture plates from Becton Dickinson (Franklin
Lakes, NJ, U.S.A.) and mouse laminin, isolated from
Englebreth-Holm-Swarm tumour cells, from Collaborative
Biomedical Products (Bedford, MA, U.S.A.). FSmoc-amino
acids and Wang resin were purchased from Auspep (Parkville,
Victoria, Australia), PR-500 resin from Calbiochem-
NovaBiochem (Alexandria, New South Wales, Australia) and
Econosil irregular packed HPLC columns from Alltech
Associates (Baulkham Hills, New South Wales, Australia).
Other reagents were purchased from Sigma (Castle Hill, New
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 30 -
South Wales, Australia).
Example 1 Homology Modelling of BDNF
A model of the three-dimensional structure of
murine BDNF was obtained by protein homology modelling
techniques from murine NGF. This was performed by the
Swiss-Model automated protein homology server running at
the Glaxo Institute for Molecular Biology in Geneva,
Switzerland, accessed via the Internet
(http://expasy.hcuge.ch/swissmod/SWISS-MODEL.html, Peitsch,
1995). Briefly, a three-dimensional model of the target
sequence is produced in the following manner: Swiss-Model
searches the Brookhaven Protein Data Bank for the sequences
of homologous proteins of known three-dimensional
structure. Once a template sequence is found, Swiss-Model
produces a structural framework for the target sequence,
using a combination of sequence alignment tools and three-
dimensional superimposition. Homologous regions of the
template protein form the structural backbone of the target
sequence, while non-conserved regions are built using the
three-dimensional structures of related sequences in the
Brookhaven Protein Data Bank. Side chains not present on
the template protein are inserted, and all side chains are
corrected using a library of allowed rotamers. The model
is then optimised by energy minimisation using the CHP~R~m
force-field.
The co-ordinates of the BDNF monomer were
downloaded and the BDNF dimer constructed by superimposing
two monomers over the co-ordinates of selected conserved oc-
carbon atoms in the NGF dimer, using the PC-based molecular
modelling software Hyperchem version 4.0 (Hypercube,
Ontario, Canada).
As expected, the model of the three-dimensional
structure of BDNF obtained by homology modelling possessed
an overall fold very similar to that of NGF, as shown in
Figure 2. The validity of this structure was further
confirmed by its similarity to the crystal structure of a
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 31 -
BDNF/NT-3 heterodimer (Robinson et a1, 1995), whose co-
ordinates were released some time after this work
commenced.
Example 2 Molecular Modelling of Monomeric
Cyclic Loop 2 Analogues
The molecular modelling of peptide analogues was
carried out using Hyperchem, as follows: After visual
inspection of the model obtained by homology modelling,
loop 2 was defined and excised from the three-dimensional
structure of BDNF, and various means of constraining
peptides to the native loop 2 conformation were
investigated. Each constraint was built between the
terminal residues of the peptide, and these residues
geometrically optimised using the Polak-Ribiere algorithm
and MM+ force-field to a local low-energy conformation.
These modelled peptides were assessed for their ability to
mimic the native conformation by measuring the root mean
square deviation of the peptide backbone to that of the
native loop following least squares superimposition.
From this model of BDNF the second ~3-hairpin loop
( loop 2 ) was def fined as Glu°~-Lys41-Val4z-Pro43-Va144-Ser4s-
Lysds-Gly4'-G1n48-Leu49-Lyss°-Glnsl, where the amino acid
numbering is the same as in mature BDNF. Peptide analogues
of this loop were modelled to investigate
(i) what type of constraint would be most
appropriate to allow the peptides to mimic loop 2 in its
native loop conformation, and
(ii) where in the sequence this constraint would
be best positioned.
As a result of these studies four peptides, L2-
12, L2-10, L2-8 and L2-6, each constrained by a disulphide
bridge between terminal cysteine residues, were chosen for
synthesis and biological examination. The sites from which
these peptides are derived are illustrated in Figure 2.
Example 3 Synthesis of Monomeric Cyclic
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 32 -
Loop 2 Peptides
Linear peptides in the free acid form were
assembled manually from Fmoc-amino acids on Wang resin
using batch-type solid phase methods (Fields and Noble,
1990). Both coupling and deprotection reactions were
assessed with the trinitrobenzenesulphonic acid test
(Thompson et al, 1995). The linear peptide amide L2-12a
was synthesised using continuous flow methods on PR-500
resin; coupling and deprotection steps were monitored
spectrophotometrically. Cleavage of peptides from the
resin and side chain removal was accomplished with
trifluoroacetic acid (TFA)/ethanedithiol/H20 (18:1:1).
Crude peptide products were analysed and purified
by reversed phase HPLC over Econosil C-18 irregular packed
columns. Gradients were tailored to individual runs, using
combinations of solution A (0.1% TFA in HZO) and solution B
(0.1% TFA in 70 % acetonitrile, 30 % Hz0) .
Purified peptide products were cyclised by
oxidising terminal cysteine residues to the disulphide in
the presence of 10% dimethylsulphoxide in 0.1 M NH4HC03
solution at pH 8.0 (Tam et a1, 1991). Reactions were
monitored and the cyclised products purified by high
performance liquid chromatography. The purity of the
peptides was further assessed by capillary zone
electrophoresis (Applied Biosystems 270A). The identity of
each peptide was confirmed by mass spectrometry using
either electrospray (Micromass platform II with
electrospray source), or fast atom bombardment (Jeol JMS-Dx
300) techniques.
All the peptide analogues were synthesised with
free amino and carboxyl termini, except L2-12a, which had
acetylated amino and amidated carboxyl termini. The linear
peptide L2-12b was synthesised without terminal Cys
residues, to ensure that it remained in a linear form
during biological assays. The peptides L2-12E10A to Z2-
12Q120A contain alanine substitution at the indicated
positions in the L2-12 sequence.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 33 -
The peptide analogues synthesised are listed in
Table 1.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 34 -
Table 1
Monomeric Cyclic Loop 2 Analogue Sequences
Code Peptide Analogue Sequence SEQ ID NO.
L2-12 C-E-R-V-P-V-S-R-G-Q-L-R-Q-C SEQ ID N0.1
L2-12a Ac-C-E-R-V-P-V-S-R-G-Q-L-R-Q-C-NH2 SEQ
ID N0.2
L2-12b E-R-V-P-V-S-R-G-Q-L-R-Q SEQ ID N0.3
L2-10 C-R-V-P-V-S-R-G-Q-L-R-C SEQ ID N0.4
L2-8 C-V-P-V-S-R-G-Q-L-C SEQ ID N0.5
L2-6 C-P-V-S-R-G-Q-C SEQ ID N0.6
L2-12E1~A C-A-R-V-P-V-S-R-G-Q-L-R-Q-C SEQ ID N0.7
L2-12R2dA C-E-A-V-P-V-S-R-G-Q-L-R-Q-C SEQ ID N0.8
L2-12V30A C-E-R-A-P-V-S-R-G-Q-L-R-Q-C SEQ ID N0.9
L2-12P4aAa C E-R-V-A-V-S-R-G-Q-L-R-Q-C SEQ ID N0.10
L2-12V5~A C E-R-V-P-A-S-R-G-Q-L-R-Q-C SEQ ID N0.11
L2-12S6aA C-E-R-V-P-V-A-R-G-Q-L-R-Q-C SEQ ID N0.12
L2-12R7AA C-E-R-V-P-V-S-A-G-Q-L-R-Q-C SEQ ID N0.13
L2-12G8~A C E-R-V-P-V-S-R-A-Q-L-R-Q-C SEQ ID N0.14
L2-12Q9aA C E-R-V-P-V-S-R-G-A-L-R-Q-C SEQ ID N0.15
L2-12L10~A C-E-R-V-P-V-S-R-G-Q-A-R-Q-C SEQ ID N0.16
L2-12R11AA C-E-R-V-P-V-S-R-G-Q-L-A-Q-C SEQ ID N0.17
12Q12aA C-E-R-V-P-V-S-R-G-Q-L-R-A-C SEQ ID N0.18
L2- .
Amino acids are represented by their one letter coda,
reading left to right from amino to carboxyl termini. The
analogue coda, for example L2-12K9~A, refers to 12 residues
from the native loop 2 sequence of BDNF with lysine at
position 9 substituted with alanine. Cysteine residues not
found in the native BDNF sequence were incorporated to form
disulphide bridges, which are represented by lines between
side chains.
apeptide L2-12P4~A not synthesised.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 35 -
Example 4 Inhibition of BDNF-Mediated Sensory Neuron
Survival by Monomeric Cyclic Loop 2 Peptides
The biological activity of the monomeric cyclic
loop 2 peptide analogues was assayed in primary cultures of
sensory neurons prepared from embryonic chick dorsal root
ganglia, essentially as described by Barde et al (1980).
The survival of specific sub-populations of these neurons
in culture is supported by neurotrophins acting through the
appropriate member of the trk receptor family (Lindsay,
1996). Briefly, 80 dorsal root ganglia were dissected from
four embryonic day 8-10 chicks (E8-E10), treated with 0.1%
trypsin for 20 min at 37°C, washed twice with 2 ml medium
(L-15 (C02) , 5 % horse serum, 60 ~tg/ml streptomycin and 100
~g/ml penicillin) and gently triturated. Non-neuronal
cells were removed by pre-plating the neuronal suspension
on a 10 cm tissue culture dish for 3 h at 37°C, 5% COZ.
Prior to plating of neurons, 48-well tissue culture plates
were coated with poly-DL-ornithine (150 ail of 1 mg/ml in
0.15 M sodium borate buffer pH 8.3) overnight at 4°C and
then with laminin (125 ~.~.1 of 7.5 ~,1/ml in L-15 (C02) ) for 4
hrs at 37°C, 5% C02. Immediately after removal of laminin
solution, 200 ~.l of suspension, and where appropriate,
samples of test compounds (2 ~L1) were added to each well.
After 1 h neurotrophic factors were added (either 2 or 6
~.1), and viable neuron numbers were determined manually by
counting 40 standard fields at 200x magnification.
After 48 hrs incubation, phase-bright healthy
neurons with neurites at least twice the length of the cell
soma were counted in 20-30 fields at 200x magnification,
and counts expressed as a percentage of the original number
of viable neurons plated (°'° neuronal survival). Percentage
neuronal survival data was normalised, such that neuronal
survival in the presence of neurotrophin (4 x 10-11 M;
positive control) was set to 100°a, while survival in the
absence of both neurotrophin and monomeric cyclic loop 2
peptide analogue (negative control) was set to 0%. Values
were expressed as mean ~ SEM. Data from different
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 36 -
experiments were analysed for lack of significant variation
using a parametric one-way analysis of variance (ANOVA)
before being grouped. Statistics were performed using
Instat version 2.04a (GraphPad, San Diego, CA, U.S.A.).
Prism software (GraphPad, San Diego, CA, U.S.A.) was used
to fit sigmoidal curves to the data.
To investigate the ability of the loop 2
analogues L2-12, L2-12a, L2-10, L2-8 and L2-6 to modulate
BDNF-mediated survival, the peptides were assayed from 1 x
10-11 to 1 x 10-4 M in competition with BDNF at 4 x 10-11 M,
a concentration which produces near maximal survival. The
results are summarised in Figure 3. All five peptides
showed a similar pattern of concentration-dependent
inhibition of BDNF-mediated survival, causing an increase
in inhibition from 1 x 10-11 to a maximum at approximately 1
x 10-6 M; above this concentration inhibition either
reached a plateau (L2-10), or diminished (L2-12, L2-12a,
L2-8 and L2-6), giving the concentration-response curve an
inverted bell-shape. However, the maximal level of
inhibition produced by these peptides varied: L2-8 showed
the greatest maximum (50°~ ~5), followed by L2-12a (44% ~4),
L2-10 (41% ~2), L2-12 (40% ~3) and L2-6 (27% ~6). The
maximal inhibition and pICso values, the latter obtained
from logistic sigmoidal curves fit to the data, are
summarised in Table 2.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 37 -
Table 2
Summary of Data for L2-12, L2-12a, L2-10, L2-8
and L2-6 in Competition with BDNF in Cultures
of E8-E10 Sensory Neurons
Loop 2 Maximal pICso
analogue inhibition
L2-12 40% 3 9.93 0.15
L2-12a 440 4 9.38 0.30
L2-10 41% 2 8.16 t 0.26
L2-8 50% 5 9.54 0.16
L2-6 27~ 6 10.53 0.16
Maximal inhibition refers to the greatest % reduction in
normalised % BDNF-mediated neuronal survival. pICSO values
were calculated from logistic sigmoidal curves fitted to
the data given in Figure 3.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 38 -
In contrast to the results obtained with the
monomeric cyclic peptides, the monomeric linear peptide L2-
12b did not show significant inhibition of BDNF-mediated
survival over the concentration range tested (1 x 10-11 to
1 x 10-4 M; Figure 3 ) .
Example 5 Specificity of Inhibition of Neuronal
Survival Activity by Monomeric Cyclic
Loop 2 Analogues
To determine the specificity of the peptides in
inhibiting BDNF-mediated survival, monomeric cyclic peptide
L2-12 (1 x 10-11 to 1 x 10-4 M) was assayed in competition
with NGF (4 x 10-11 M), using the assay described in Example
4. As shown in Figure 4, at the concentrations tested
peptide L2-12 did not significantly inhibit NGF-mediated
survival. These data suggest that the inhibition of
neuronal survival seen in Example 4 is specific for BDNF.
Example 6 Lack of Intrinsic Neuronal Survival
Activity or Toxic Effects of Monomeric
Cyclic Loop 2 Analogues
then added to cultures alone, i.e. in the absence
of neurotrophin, the monomeric cyclic peptide L2-12 neither
intrinsically promoted neuronal survival nor exhibited non
specific toxic effects on neurons at the concentrations
tested ( 1 x 10-11 to 1 x 10-4 M) , giving neuronal survival
of around 5%, i.e. similar to that of negative controls, as
shown in Figure 4.
This lack of intrinsic neuronal survival
promoting activity of the monomeric cyclic loop 2 peptide
L2-12 was expected. L2-12 and the other monomeric cyclic
loop 2 peptides are monomeric, they are unlikely to
dimerise trkB, and the dimerization is crucial for trk-
mediated signalling (Ding et a1, 1992).
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 39 -
Example 7 Effect of Monomeric Cyclic Peptides on the
Concentration-Response Curve for BDNF
To examine the effect of inhibitory peptides on
the concentration-response curve of BDNF, monomeric cyclic
L2-12a (1 x 10-~ M) was added in competition with BDNF (1.8
x 10-13 to 1: 8 x 10-1° M in 0 . 5 log increments ) . The results
are shown in Figure 5. At this concentration, peptide L2-
12a caused a significant depression (40%) of the maximal
neuronal survival response of BDNF from 41°o to 24%, which
is consistent with the maximal inhibition of BDNF-mediated
survival exhibited by L2-12a. The peptide also caused a
small rightward shift of the BDNF concentration-response
curve (pECs° of BDNF alone: 11.2 ~0.2; BDNF + L2-12a: 10.9
~0.3), although this was not statistically significant.
Example 8 Identification of Amino Acids Important for
the Inhibitory Effect of Monomeric Cyclic
Loop 2 Peptides
The contribution of individual residues within
the monomeric cyclic L2-12 sequence towards BDNF-inhibitory
activity was examined by conducting an alanine scan (Ala
scan), and testing the resulting monomeric cyclic peptides
from 1 x 10-11 to 1 x 10-4 M in log increments for their
ability to modulate BDNF-mediated survival at 4 x 10-11 M.
The sequences of the alanine substituted peptides are shown
in Table 1 above, and the results are shown in Figure 6.
A significant difference in maximal inhibition
compared to that produced by L2-12 (40% ~3) was seen when
Ala was substituted for Val3 (Va142 in native BDNF sequence;
0%~9) , Sers~as> (2°0 +7) ~ Lysllcso> (9 0 ~4) and Glnlacsl> (5/
~5), suggesting that these residues are important for BDNF-
inhibitory activity. Substitution of Ala for ValS~aa>
yielded a peptide which gave a slight, though
insignificant, potentiation of BDNF-mediated survival (-7°0
~9). However, the peptide did not show intrinsic survival
promoting activity in the absence of BDNF (data not shown).
No significant change in maximal inhibition was observed
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 40 -
when Ala was substituted for Glul~4o~ (39°0 ~13), Lysz~al> (26%
*-'1). Lys'~as> (33% ~8), Glyaca~> (46°0 ~13), Gln9cas~ (32% ~6)
and Leulocas~ (33 0 ~6) .
Example 9 Molecular Design of Disulphide-linked
Dimeric Bicyclic Loop 2 Analogues
The two loop 2 regions of BDNF are juxtaposed in
the three-dimensional model of the dimer (Figure 1), which
allows design of small dimeric peptides that mimic this
spatial arrangement. On the basis of observations made in
the highly analogous NGF-trkA receptor system, we predicted
that small dimeric loop 2 analogues could act as agonists
if they could facilitate dimerization of trkB. It has been
shown that divalent antibodies to trkA can cause the
homodimerisation of this receptor, leading to signal
transduction and NGF-like biological activity in vitro
(Clary et a1, 1994). Moreover, a small peptide mimetic of
erythropoietin, produced by a recombinant library
technique, possesses full erythropoietin-like biological
activity as a result of self-association to form a dimer
which dimerises the erythropoietin receptor (Wrighton et
al, 1996). Examination of the X-ray crystal structure of
the peptide-erythropoietin receptor complex (Livnah et al,
1996) reveals that the structure of the bound dimeric
peptide bears a striking resemblance to the loop 2 regions
of BDNF in our three-dimensional model.
The most effective of the monomeric, cyclic,
disulphide-linked loop 2 peptides which were shown in
Examples 4, 5, 7 and 8 were able to inhibit BDNF neuronal
survival activity, peptide L2-8, was chosen as the basis
for the design of dimeric peptides. This peptide consists
of 8 amino acid residues of BDNF plus the two terminal
cysteine residues oxidised to cyclic disulphide, i.e. a
total of 10 residues.
Examination of the model of the three-dimensional
structure of BDNF revealed three amino acid positions in
which the two loop 2 regions in BDNF are in close
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 41 -
proximity, thus presenting an opportunity to create dimeric
analogues of peptide L2-8. The positions, corresponding to
Pro2, Val3 and Ser4 of peptide L2-8, are characterised by
Ca-to-ca distances of 11.3A, 5.4A and 6.2A, respectively,
as shown in Figure 7A. Conformational analysis of a
cysteine residue (i.e. two disulphide-linked cysteine
residues) by computational chemical methods revealed that
the mean ca-to-ca distance of this residue was 5.4A (90%
CI: 5.22-5.44A), as shown a.n Figure 7B. These data
suggested to us that a cysteine residue could comfortably
be incorporated into peptide L2-8 at positions Val3 and
Ser4, to give dimeric, disulphide-linked peptides that
might mimic the spatial arrangement of the loop 2 regions
in native BDNF, but fitted considerably less well in place
of Pro2.
However, as shown in Example 8, examination of a
series of peptides based on a 14 amino acid monomeric loop
2 peptide inhibitor of BDNF action, in which amino acids
were systematically replaced with Ala, showed that residues
equivalent to Val3 and Ser4 in peptide L2-8 were required
for BDNF inhibitory activity, and therefore were presumably
involved in binding to BDNF receptors. No data were
available concerning an Ala replacement at a position
equivalent to Pro2.
On the basis of the structural data alone, we
chose to synthesise the, disulphide-linked dimeric bicyclic
peptides (L2-8V3C)2 and (L2-8S4C)2, incorporating a
cysteine bridge in place of Val3 and Ser4, respectively.
The disulphide-linked dimeric bicyclic peptide (L2-8P2C)2,
in which the cysteine linkage was incorporated in place of
Pro2, was also synthesised, as definitive information on
the role of Proz was not available, even though our
structural data suggested that cysteine would not provide
the optimal means of dimerization at this point.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 42 -
Example 10 Synthesis of Disulphide-Linked Dimeric
Bicyclic Loop 2 Analogues
The disulphide-linked dimeric bicyclic peptides
(L2-8P2C)2, (L2-8V3C)2 and (L2-8S4C)z were synthesised by
standard solid phase synthesis techniques using F~noc amino
acids, as described in Example 3, and using a mixed Cys
protection strategy (Cys(Trt) and Cys(Acm)). The general
method is illustrated in Scheme 1.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 43 -
a..t, e..,°
Synthesis of disulphide-linked dimeric bicyclic peptide
analogues of loop 2 of BDNr
H-Cys(Trt~------Cys(Acm) Cys(Trt}--Hesin
,i. 90% TFA + scavengers
H-Cys---Cys(Acm) Cys-OH
,i 10% DMSO pH 8.4
H-Cys Cys(Acm) Cys-OH
II
12 50mM in Acetic acid
quench
H-Cys C '- ~ys-OH
H-CyLs Cy~ ~~s-OH
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 44 -
Peptides and intermediates were purified by reverse-phase
high performance liquid chromatography, and characterised
by electrospray mass spectrometry. The structures of the
compounds synthesised are shown in Table 3.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 45 -
Table 3
Structures of Disulphide-linked Dimeric Bicyclic
Loop 2 Analogues and Their Monomeric Cyclic Precursors
S
L2-8 C-V-P-V-S-R-G-Q-L-C
(L2-8P2C)2 C-V-C-V-S-R-G-Q-L-C C-V-C-V-S-R-G-Q-L-C
SEQ ID N0.19
(L2-8V3C)2 C-V-P-C-S-R-G-Q-L-C C-V-P-C-S-R-G-Q-L-C
SEQ ID N0.20
(L2-8S4C)2 C-V-P-V-C-R-G-Q-L-C C-V-P-V-C-R-G-Q-L-C
SEQ ID N0.21
L28P2C(Acm) C-V-C(Acm)-V-S-R-G-Q-L-C
SEQ ID N0.22
L28V3C(Acm) C-V-P-C(Acm)-S-R-G-Q-L-C
SEQ ID N0.23
L28S4C(Acm) C-V-P-V-C(Acm)-R-G-Q-L-C
SEQ ID N0.24
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 46 -
Example 11 Intrinsic Neuronal Survival Activity of
Disulphide-Linked Dimeric Bicyclic Loop
2 Analogues
The disulphide-linked dimeric bicyclic loop 2
peptides were analysed for their ability to promote the
survival of sensory neurons in cultures prepared from
dorsal root ganglia obtained from embryonic day 8-10
chicks, as described in Example 4. Peptides (L2-8P2C)z and
(L2-8S4C)2 each displayed concentration-dependent neuronal
survival activity, maximally promoting the survival of 28%
and 30% of the neurons that would be supported by BDNF
itself. These results are shown in Figure 8.
The activity of these two disulphide-linked
dimeric bicyclic loop 2 peptides was surprising in view of
the number of the modifications. In peptide (L2-8S4C)2,
the serine residue (Sera) shown to be important for the
inhibitory action on BDNF-mediated neuronal survival of the
monomeric cyclic loop 2 peptides (see Example 8) was
replaced by the disulphide-linked cysteine residue. In
peptide (L2-8P2C)2, the COC-to-Ca distance of the cysteine
residue is likely to be much shorter than the corresponding
distance in our model of BDNF of the two proline residues
which it replaces.
In contrast, peptide (L2-8V3C)2 was inactive, as
shown in Figure 8, despite the likelihood that it could
best accommodate the cysteine residue, at least in terms of
interatomic distance.
Example 12 Lack of Intrinsic Neuronal Survival
Activity of Monomeric Precursors of
Dimeric Bicyclic Loop 2 Analogues
To determine whether the dimeric nature of the
disulphide-linked dimeric bicyclic loop 2 peptides was
required for intrinsic neuronal survival activity, we
assayed peptides L2-8P2C(Acm) and L2-8S4C(Acm), the
monomeric cyclic precursors of peptides (L2-8P2C)2 and (L2-
8S4C)2 a.n which the Acm groups on the internal Cys were
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 47 -
intact, for their ability to promote sensory neuronal
survival in culture. Unlike their dimeric counterparts,
both monomeric cyclic peptides were inactive, as shown in
Figure 9.
Example 13 Molecular Design of an Amide-Linked
Dimeric Bicyclic Loop 2 Analogue
Because of the position of the cysteine residue
in the disulphide-linked dimeric bicyclic loop 2 analogues
described in Examples 9 to 11 a.n the region required for
the inhibitory activity of the monomeric cyclic peptides,
we further examined the BDNF model to see if other dimeric
bicyclic peptides could be designed that did not involve
the replacement of these possible receptor-binding
residues. On the basis of Ca-to-Ccc distance measurements.
we reasoned that an amide-linked dimeric bicyclic peptide,
(L2-8&E+K)2, could be created by joining together two
analogues of the monomeric cyclic peptide L2-8 via an amide
bond between an additional lysine and glutamate residue
added to the C-terminus. An illustration of this is shown
in Table 4.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 48 -
Table 4
Structure of Amide-Linked Bicyclic Analogue
of Loop 2 of BDNF
S
(L2-8&E+K)2
Ac-C-V-P-V-S-K-G-Q-L-C-E-NH2Ac-C-V-P-V-S-K-G-Q-L-C-K-NH2
SEQ ID NO. 25 SEQ ID NO. 26
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 49 -
Example 14 Synthesis of an Amide-Linked Dimeric
Bicyclic Loop 2 Analogue
The amide-linked dimeric bicyclic peptide (L2-
8&E+K)z was prepared as shown in Scheme 2 by condensing two
cyclic N-acetylated, C-amidated, partially-protected
monomers synthesised by standard solid phase techniques on
Rink amide MBHA resin as described in Example 3 and shown
in Scheme 2.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- SO -
Scheme 2
Syr_thesis of an amide-linked dimeric bicyclic loop 2
analogue
Fmoc solid phase assembly
assembly
Ac-C(Trt)------S(8zl)-----K(Z)----C(Trt)-K(Boc)-~ Ac-C(Trt)----S(Bzl)-----K(Z)-
---C(Trt)-E(Trt)-
c~eavaa~ TFA f scavengers
oc.C....___~B~)____.K(~_..__C..K.NH Z
jQp DMSO in NH aC03solution
~;yclisationl
qc.' ....__..g(gZl)__..__.__~~__'C..K.NH2 p,c_ L
_.._.S(g~).._____K(Z..~..._C._E.NHZ
I
sjjmerisaton
HATU& DIEA in DMF '
ion
qc_~______.g(g~)__.....~Z)_.____~..K.NHZ
qc.C_.___..S(gzl)_______K(Z)......C_.E.NHZ
I
cleava~ae HF+ m-cresol
oc.~____.._S._...K._._______~..K.NHZ Ac.C...___.g___._K________._C__E_NH2
l i i I
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 51 -
The remaining Ser and Lys protecting groups were
removed by treatment of the partially protected bicyclic
dimer with hydrogen fluoride/m-cresol (10:1) for one hour
at 5°C. HF was removed by evaporation at room temperature.
The desired peptide (L2-8&E+K)z and intermediates were
purified by HPLC and characterised by mass spectrometry.
Example 15 Intrinsic Neuronal Survival Activity of
an Amide-Linked Dimeric Bicyclic Loop 2
Analogue
The amide-linked dimeric bicyclic loop 2 peptide
(L2-8&E+K)2 was assayed in cultures of sensory neurons
prepared from dorsal root ganglia obtained from embryonic
chicks, as described in Example 4. Peptide displayed
concentration dependent neuronal survival activity,
supporting the survival of 28% of those neurons supported
by BDNF (lng/ml) with an ECso in the order of 10-a M. The
results are shown in Figure 10.
This activity was similar both in maximal effect
and potency to that observed with the disulphide-linked
dimeric bicyclic loop 2 analogues (L2-8P2C)2 and (L2-8S4C)2
described in Example 11. Thus it appears that different
chemical linkers incorporated in different positions within
the dimeric bicyclic loop 2 analogues imparts neuronal
survival promoting activity to these peptides.
Example 16 Molecular Design of a Dimeric Tricyclic
Loop 2 Analogue
Although the dimeric bicyclic peptides described
in Examples 9 through 11 and 13 through 15 represent a
significant step in the discovery of small molecules which
mimic the action of BDNF, they are nonetheless considerably
less potent efficacious than the parent peptide. One
reason for the reduced activity of the dimeric bicyclic
peptides compared to BDNF could be their ability to rotate
relatively freely about their dimerising constraint, be it
a Cys-to-Cys disulphide or a Lys-to-Glu amide. To try and
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 52 -
create a molecule that might show either improved efficacy
(as evidenced by an increase in the maximal percent
neuronal survival), or increased potency, we reasoned that
we would need to restrict the freedom of rotation about the
S dimerising constraint. To do~this, we chose to combine, in
one molecule, the two different dimerising constraints used
in the disulphide-linked and the amide-linked dimeric
bicyclic loop 2 analogues. We anticipated that the
resultant dimeric tricyclic loop 2 analogue (L2-8S4C&E+K)2,
by restricting the rotation of the two loop 2 moieties
relative to one another would much better mimic the loop 2
orientation seen a.n the native protein, and therefore would
show improved efficacy and potency. This is shown in Table
5.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 53 -
Table 5
Structure of Dimeric Tricyclic Loop 2 Analogue
(L2-8S4C&E+K)2
Ac-C V-P-V-C-K-G-Q-L-C-E-NHZAc-C-~7-P-V-C-K-G-Q-L-C-K-NHZ
SEQ ID NO. 27 SEQ ID NO. 28
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 54 -
Example 17 Synthesis of a Dimeric Tricyclic Loop 2
Analogue
The dimeric tricyclic peptide (L2-8S4C&E+K)2 was
prepared as shown in Scheme 3 from two cyclic N-acetylated,
C-amidated, partially-protected monomers synthesised by
standard solid phase techniques on Rink amide MBHA resin as
described in Example 14.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 55 -
Scheme 3
Synthesis of a dimeric tricyclic loop 2 analogue
assembly Fmocsolid phase assembly
Ac_C(Trt)-------C(Acm)--.---K(Z)---C(Trt)- ~ Ac-C(Trt)------C(Acm)------K(Z)---
-C(Trt)-
cleav age TFA + scavengers
Ac:C--_----C(,acm)___---K(Z).___-_-C_.K_ 2 Ac:C-__..__C(qcm)__.-_-
K(~______.C__E_ 2
oxidation DMSO in NH<COJ solution
(cv clisation)
Ac_C..___..C(qcm).____.K~Z)._..iC..K. 2 qc.C..___..C(qcm)_.__._K(~-___.C__E. z
...
HATU & DIEA in DMF
a,c:~._._.._C(A,cm)_-._.K~Z).._-iC_.K_ Z qc.C..__...C(qcm).-____~Z~_...-C_.E_
II
2nd dimerisation i IZ+AcOH
reaction ii ascorbic acid
Ac:~-___.__C_..__.K(Z)._._~ C ~ K. Z ,ec:C._..__.C__..._K(Z)__._ C._E.
2
I
cleav a4e HF+ m-cresol
Ac-~--..__ ~C_....K_..__._~~ Ci_K_ Z p,c.C______.C_..__K.._.._.._.C..E_
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 56 -
The monomers were initially condensed in the first
dimerisation reaction via the free lysine and glutamate
side chains. A second dimerisation reaction was carried by
oxidising the internal cysteine residues, completing the
tricycle. The remaining Lys protecting groups were removed
by treating the partially protected tricyclic dimer with
hydrogen fluoride/m-cresol (10:1) for one hour at 5°C. HF
was removed by evaporation at room temperature. The
desired peptide (L2-8S4C&E+K)2 and intermediates were
purified by HPLC and characterised by mass spectrometry.
Example 18 Intrinsic Neuronal Survival Activity of
a Dimeric Tricyclic Loop 2 Analogue
The dimeric tricyclic loop 2 peptide (L2-
8S4C&E+K)2 was assayed in cultures of sensory neurons
prepared from dorsal root ganglia obtained from embryonic
chicks, as described in Example 4. Peptide displayed
concentration dependent neuronal survival activity,
supporting the survival of 35% of those neurons supported
2 0 by BDNF ( lng/ml ) with an ECS° in the order of 10-1° M. The
results are shown in Figure 11.
The maximal neuronal survival promoting effect of
the dimeric tricyclic loop 2 peptide (L2-8S4C&E+K)2 is
similar to that of the dimeric bicyclic loop 2 analogues.
However peptide (L2-8S4C&E+K)2 is approximately two orders
of magnitude more potent than the dimeric bicyclic
analogues. This activity is consistent with the hypothesis
that the presence of two dimerising constraints (Cys-to-Cys
disulphide and Lys-to-Glu amide) would create a molecule
which much better mimics the spatial arrangement of the two
loop 2 moieties than any of the dimeric bicyclic compounds,
which contain only a single dimerising constraint.
Example 19 Molecular Design of Monomeric Cyclic
Analogues of the P75 Binding Region of
Loop 4 of BDNF
The three positively-charged residues thought to
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 57 -
be important for the binding of BDNF to the low affinity
neurotrophin receptor p75 are contiguous (Lys95-Lys96-Arg9')
and are located on loop 4, as shown in Figure 1. This gave
us the opportunity to propose small monomeric cyclic
peptides that might mimic the conformation of this
tripeptide sequence, using the computer-aided molecular
design approach described in Example 2. On the basis of
these studies we chose to synthesise two cyclic monomeric
peptides: L4-3pA, a pentapeptide incorporating a relatively
conformationally restricted DPro residue; and L4-3Hx, a
tetrapeptide incorporating a conformationally flexible 6-
aminohexanoyl residue. Both peptides were cyclised by
condensing their amino-terminus with their carboxy-terminus
(head-to-tail cyclisation).
Example 20 Synthesis of Monomeric Cyclic Analogues of
the p75 Binding Region of Loop 4 of BDNF
The monomeric cyclic loop 4 peptides were
synthesised from 9-fluorenylmethoxycarbonyl (Fznoc) amino
acids, using standard solid phase synthesis protocols as
described in Example 3. The linear side chain-protected
peptides L4-3pAa and L4-3Hxa, suitable for head-to-tail
cyclisation to give the monomeric cyclic peptides L4-3pA
and L4-3Hx, respectively, were obtained by treating
peptides synthesised on acid-labile 2-chlorotrityl
derivatised resin (NovaBiochem, Australia) with acetic
acid/trifluoroethanol/dichloromethane (1:1:8) for 30
minutes (Barlos et al., 1991). The cyclic peptides were
obtained by stirring the appropriate linear side chain-
protected peptide (0.1 to 0.5 mg/ml) in dichloromethane in
the presence of the standard peptide bond-formation
reagents 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyl
uronium hexafluorphosphate (HBTU), 1-hydroxybenzotriazole
(HOBt) and diisopropylamine (DIEA) (HBTU:HOBt:DIEA 1:1:1.5
equivalents relative to peptide). Treatment of the product
of this reaction with TFA/scavengers yielded the desired
fully-deprotected product. The corresponding linear
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 58 -
homologues were prepared by treating the appropriate side
chain-protected linear peptide with TFA/scavengers, without
prior cyclisation.
Cyclisation reactions were monitored and peptides
purified by reverse phase HPLC on either analytical (4.6 mm
internal diameter) or semi-preparative (22.5 ~) C18
columns, using linear acetonitrile gradients i.n 0.1% TFA
solution at appropriate flow rates. Desired fractions were
collected and lyophilised for characterisation by mass
spectrometry.
Synthesis of peptide L4-3pA yielded two
stereoisomers, L4-3pA(I) and L4-3pA(II), each with the
desired molecular weight of 581 daltons. These isomers
were purified by HPLC and were assayed separately for
biological activity.
A list of the compounds synthesised is given in
Table 6.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 59 -
Table 6
Structures of Monomeric Cyclic Loop 4 Analogues and Their
Linear homologues
L4-3pA(I)
and ~-nPro-Ala-Lys-Lys-Argue SEQ ID N0.29
L4-3pA(II)
L4-3Hz ~-Ahg-Lys-Lys-Arg-~ SEQ ID N0.30
L4-3pAa H-DPro-Ala-Lys-Lys-Arg-OH SEQ ID N0.31
L4-3Hza H- Ahz-Lys-Lys-Arg-OH SEQ ID N0.32
All amino acid residues are given by their standard three
letter codes, except Ahx: 6-amino hexanoyl.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 60 -
Example 21 Intrinsic Neuronal Survival Activity of
a Monomeric Cyclic Analogue of the p75
Binding Region of Loop 4 of BDNF
The monomeric cyclic analogues of the p75 binding
region of loop 4, L4-3pA(I), L4-3pA(II), L4-3Hx and their
linear homologues L4-3pAa and L4-3Hxa, were assayed in
cultures of sensory neurons prepared from embryonic chicks
as described in Example 4. As shown in Figure 10, the
monomeric cyclic loop 4 peptide, L4-3pA(II) displayed
concentration-dependent neuronal survival activity. This
intrinsic neuronal survival activity of L4-3pA(II) was
surprising; unlike the loop 2 peptides described in Example
11, it is neither dimeric nor bicyclic. Moreover, the
activity was confined to L4-3pA(II). Neither its
stereoisomer L4-3pA(I), the other monomeric cyclic loop 4
peptide L4-3Hx constrained by the more conformationally
flexible aminohexanoyl residue, nor their linear
counterparts displayed neuronal survival activity in this
assay system, as shown in Figure 11.
Example 22 Lack of Inhibition of BDNF- and NGF-
Mediated Sensory Neuron Survival by
Monomeric Cyclic Loop 4 Analogues of
the p75 Binding Region of Loop 4 of
BDNF and Their Linear Homologues
The monomeric cyclic analogues of the p75 binding
region of loop 4 L4-3pA(I), L4-3pA(II), L4-3Hx and their
linear homologues L4-3pAa and L4-3Hxa, were assayed for
their ability to modulate the neuronal survival effects of
BDNF and NGF in cultures of sensory neurons prepared from
embryonic chicks as described in Example 4. Unlike the
monomeric cyclic loop 2 peptides, none of the monomeric
cyclic loop 4 peptides or their linear homologues showed
any significant inhibition of either BDNF- or NGF- mediated
neuronal survival, as shown in Figures 12 and 13,
respectively.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 61 -
Example 23 Role of DPro in the Neuronal Survival
Promoting Activity of Monomeric Cyclic
Loop 4 Analogues of the p75 Binding
Region of BDNF
Given the biological data obtained with L4-
3pA(II) described in Example 21, we decided to investigate
the role of the DPro residue in the neuronal survival
activity of the monomeric cyclic loop 4 peptides. We chose
to synthesise two compounds using the methods described in
Example 20: L4-3Ap, in which the position of the nPro
residue is swapped with the Ala residue; and L4-3AP, in
which the configuration of the Pro residue is L rather than
D. In a manner presumably analogous to that seen with L4-
3pA, both peptides yielded two isomers of identical
molecular weight: L4-3Ap(I), L4-3Ap(II), L4-3AP(I) and L4-
3AP(II). The sequences of these four peptides is shown in
Table 7.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 62 -
Table 7
Structure of Further Monomeric Cyclic Loop 4 Analogues
L 4-3Ap ( I )
and rAla-nPro-Lys-Lys-Arg-~ SEQ ID N0.33
L4-3Ap(II)
L 4-3AP( I )
and rAla-Pro-Lys-Lys-Arg-~ SEQ ID N0.34
L4-3AP(II )
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 63 -
The monomeric cyclic peptides were assayed in
cultures of sensory neurons prepared from embryonic chicks
as described in Example 4. Unlike peptide L4-3pA(II),
neither L4-3Ap(I), L4-3Ap(II), L4-3AP(I) nor L4-3AP(II)
displayed neuronal survival activity. These data, shown in
Figure 16, suggest that both the position in the cyclic
sequence and stereochemistry of the Pro residue are
important for the neuronal survival activity displayed by
peptide L4-3pA(II).
Example 24 Role of Lys Residues in the Neuronal
Survival Promoting Activity of
Monomeric Cyclic Loop 4 Analogues of
the p75 Binding Region of BDNF
To investigate the importance of the two Lys
residues to the neuronal survival activity of the monomeric
cyclic loop 4 peptides, we chose to synthesise two
analogues of peptide L4-3pA using the methods described i.n
Example 20 in which a Lys residua is replaced by Ala: L4-
3K3ApA and L4-3K4ApA. Unlike L4-3pA, both L4-3K3ApA and
L4-3K4ApA yielded only single major products following
synthesis and cyclisation. The sequences of these two
peptides a.s shown in Table 8.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 64 -
Table 8
Structure of Further Monomeric Cyclic Loop 4 Analogues
Incorporating Ala for Lys
L4-3K3ApA rnPro-Ala-Ala-Lys-Arg-~ SEQ ID N0.35
L4-3K4ApA rnPro-Ala-Lys-Ala-Argue SEQ ID N0.36
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 65 -
The monomeric cyclic peptides L4-3K3ApA and L4-
3K4ApA were assayed in cultures of sensory neurons prepared
from embryonic chicks as described in Example 4. Compared
to L4-3pA(II), peptides L4-3K3ApA and L4-3K4ApA displayed
only marginal neuronal survival activity. These data,
shown in Figure 17, suggest that the two Lys residues of
the cyclic monomeric peptide L4-3pA are required for
neuronal survival activity.
Example 25 NMFt Analysis of Monomeric Cyclic
Analogue of the p75 Binding Region of
Loop 4 of BDNF
The neuronal survival activity of the monomeric
cyclic analogues of the p75 binding region of loop 4 of
BDNF is confined almost exclusively to peptide L4-3pA(II).
To examine a structural basis for this neuronal survival
activity, we chose to determine the structure of peptide
L4-3pA(II) i.n solution using NMIt techniques. A HPLC pure
sample of peptide L4-3pA(II) was lyophilised then taken up
in 550 ml of 10%2H20/90%H20 and the pH adjusted to 5.3. The
solution was then transferred into a 5 mm NMit tube. NNBt
spectra ware acquired at 400 MHz on a Varian Inova 400 MHz
NMft spectrometer. One-dimensional 1H spectra were acquired
with a sweep-width of 4000 Hz over 8K points.
Solvent suppression was achieved with selective
low-power presaturation. Spectra were acquired at a series
of sample temperatures (30°C, 15°C and 5°C) to check for
temperature dependence of the peptide spectrum. The
peptide did not show significant temperature dependence.
All subsequent spectra were recorded at 30°C.
A series of 2D 1H spectra were then recorded for
L4-3pA. Typically, each spectrum was acquired with a sweep
width of 4000 Hz over 1024 points, with 800 t1, increments.
TOCSY and DQF-COSY spectra were acquired for use in spin
system assignments, while ROESY spectra were acquired to
generate distance constraints. Spectra were initially
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 66 -
transformed using the ~Tarian VNNlR software package to check
for the quality of the data. Subsequently, spectra were
transformed using NNlltpipe, and analysed using NNlltview.
Complete assignment of all non-exchangeable proton
resonances was made. Dihedral constraints for the backbone
f angles were derived from the J3~_~~ coupling constants
measured from 1D spectra. A total of 61 structurally
important distance constraints and 3 backbone f angle
constraints were determined from the NNgt data for L4-
3pA(II).
Structure calculation was carried out using the
software package DYANA. Cyclisation of the peptide was
achieved by introducing a set of special distance
constraints to both bring the ends of the peptide together,
and restrain the peptide bond angle to 180°. A modified
version of the residue library containing a set of
parameters defining a DPro residue was produced to allow
calculation to include the nPro residue. A total of 100
structures were calculated on the basis of the NNgt-derived
constraint list by 10,000 steps of simulated annealing
followed by 2,000 steps of minimisation of the DYANA target
function. The 20 structures with the lowest target
function were then selected as the final family of
structures for the peptide. An overlay of these structures
of peptide L4-3pA(II) can be found in Figure 18.
As can be seen in Figure 18, the conformation of
the backbone of peptide L4-3pA(II) is uniquely defined in
solution. In addition, side chain of Lys4 adopts a single
conformation up to its gamma-carbon atom, while the
conformation of the side chain of ArgS is uniquely defined
to the delta-nitrogen. The presence of a single backbone
conformation and well-defined side chains for peptide L4-
3pA is consistent with the biological data showing that
compounds of closely related sequence to L4-3pA show either
markedly reduced, or no neuronal survival activity in cell
culture experiments. This exceptionally well-defined
conformation of L4-3pA will be used as a template for the
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 67 -
design of non-peptidic molecules with neuronal survival
promoting activity.
Example 26 Effect of Peptide L4-3pA(II) on Lesion-
Induced Neurodegeneration In Vivo
The ability of peptide L4-3pA(II) to prevent or
slow neurodegeneration in vivo was tested a.n a model of
peripheral nerve lesion. To do this, newborn (24-48 hrs)
Wistar rat pups (4 per treatment group) were rendered
unconscious by ice-induced hypothermia. The median and
ulnar nerve in the right forelimb has exposed, transected
and wrapped with a piece of gel foam containing 10 u1 a
solution a.n PBS of L4-3pA(II) at one of two doses (10 ug/ul
or 1 ug/ul) or PBS alone. Pups were re-united with their
mothers and after 5 days were killed with a lethal
injection of sodium pentobarbital (150 mg/kg) and perfused
with a buffered 4% solution of paraformaldehyde. Spinal
cords and DRGs were dissected out and embedded in paraffin,
and serial transverse sections were cut, mounted on glass
slides and.stained with 0.5% cresyl violet. Neurons
displaying prominent nucleoli ware counted in every fifth
section to include the entire rostrocaudal length of the
DRG. Effects of peptides on neuronal loss were determined
by comparing the number of neurons in the experimental side
versus that in the intact contralateral side. Statistical
comparisons between treatments was determined by one way
ANOVA followed by post hoc Tukey's test.
As can be seen in Figure 19, both doses of
peptide L4-3pA(II) significantly reduce the loss of of
sensory (panel A: 100ug, 31 ~5% loss; l0ug, 23 ~3% loss)
and motor (panel B: 100ug, 16 ~2°'° loss; l0ug, 11 ~6% loss)
neurons that would otherwise die (panel A: sensory neurons
45 ~2°~ loss; panel B: motor neurons 35 ~2% loss) as a
result of the lesion. The degree of rescue is similar to
that seen with other neurotrophic factors, such as LIF
(Cheema et a1 1994a; 1994b). It is worthy of note that the
best rescue of both sensory and motor neurons was obtained
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 68 -
with the smaller dose (10 ug) of L4-3pA(II). This may
reflect the nature of the apparently bell-shaped
concentration response curve we have observed for L4-
3pA(II).
It will be apparent to the person skilled in the
art that while the invention has been described in some
detail for the purposes of clarity and understanding,
various modifications and alterations to the embodiments
and methods described herein may be made without departing
specification.
References cited herein are listed on the
following pages, and are incorporated herein by this
ref erence .
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 69 -
REFERENCES
Barbacid, M. (1994). J. Neurobiol., 25, p1386-
1403.
Barde, Y.-A., Edgar, D. and Thoenen H. (1980).
Proc. Natl. Acad. Sci. USA, 77, p1199-1203.
Barrett, G.L. and Bartlett, P.F. (1994). Proc.
Natl. Acad. Sci. USA, 91, p6501-6505.
Chao, M.V. and Hempstead, B.L. (1995). Trends
Neurol Sci., 18, p321-326.
Chapman, B.S. (1995). FEBS Lett., 374, p216-220.
Cheema, S.S., Richards, L.J., Murphy, M. and
Bartlett, P.F. (1994a). J. Neurosci. Res., 37, p213-218.
Cheema, S.S., Richards, L.J., Murphy, M. and
Bartlett, P.F. (1994b). NeuroReport, 5, p989-992.
Chorev, M. and Goodman, M. (1993). Acc. Chem.
Res., 26, p266-273.
Clary, D.O., Weskamp, G., Austin, L.R. and
Reichardt L.T. (1994). Mol. Biol. Cell, 5, p549-563.
Dittrich , F., Thoenen, H. and Sendtner, M.
(1994). Ann Neurol., 35, p151-163.
Fields, G.B. and Noble, R.L. (1990). Int. J.
Peptide Protein Res., 35, p161-214.
Frade, J.F., Rodriguez-Tebar, A. and Barde, Y.-A.
(1996). Nature, 383, p166-168.
Gallop, M.A., Barrett, R.W., Dower, W.J., Fodor,
S.P.A. and Gordon, E.M. (1994). J. Mad. Chem., 37, p1233-
1251.
Gotz, R., Koster, R., Winkler, C., Raulf, F.,
Lottspeich, F., Schartl, M. and Thoenen, H. (1994).
Nature, 372, p266-269.
Hefti, F. (1994). J. Neurobiol., 25, p1418-1435.
Ibanez, C.F., Ebendal, T., Barbany, G., Murray-
Rust, J., Blundell, T.L. and Persson, H. (1992). Cell, 69,
p329-341.
Ibanez C.F., Ilag L.L., Murray-Rust J. and
Persson H. (1993). EMBO J., 12, p2281-2293.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 70 -
Jing, S., Tapley, P. and Barbacid, M. (1992).
Neuron, 9, p1067-1079.
Kaplan, D.R. and Miller, F.D. (1997). Curr
Opinion Cell Biol., 9, p213-221.
LeSauteur, L., Wei, L., Gibbs, B.F. and Saragovi,
H.U. (1995). J. Biol. Chem., 270, p6564-6569.
Lindsay, R.M. (1994). Neurobiol. Aging, 15, 249-
251
Lindsay, R.M. (1996). Phil. Trans. R. Soc. Lond.
B, 351, p365-373.
Longo, F.M., Manthorpe, M., Xie, Y.M. and Varon,
S. (1997). J. Neurosci. Res., 48, p1-17.
Livnah, O., Stura, E.A., Johnson, D.L.,
Middleton, S.A., Mulcahy, L.S., Wrighton, N.C., Dower,
W.J., Jolliffe, L.K. and Wilson, I.A. (1996). Science, 273,
p464-471.
Maeji, N.J., Bray, A.M., Valerio, R.M. and Wang,
W.,(1995). Peptide Res., 8, p33-38.
McDonald N.Q., Lapatto R., Murray-Rust J.,
Gunning J., Wlodawer A. and Blundell T.L. (1991). Nature,
354, p411-414.
O'Leary, P.D. and Hughes, R.A. (1998). J.
Neurochem., 70, p1712-1721.
Olson, G.L., Bolin, D.R., Bonner, M.P., Bos, M.,
Cook, C.M., Fry, D.C., Graves,B.J., Hatada, M., Hill, D.E.,
Kahn, M., Madison, V.S., Rusiecki, V.K., Sarabu, R.,
Sepiwall, J., Vincent, G.P. and Voss, M.E. (1993). J. Med.
Chem., 36, p3039-3049.
Peitsch, M.C. (1995). Biotechnology, 13, 658-660.
Penn, R.D. (1997). Neurosurgery, 40, p94-100
Rabizadeh S., Oh J., Zhong L.T., Yang J., Bitler
C.M., Butcher, L.L. and Bredesen, D.E. (1993). Science,
261, p345-348
Riopelle, R.J. and Kennedy, J.C. (1982). Can. J.
Physiol. Pharmacol., 66, p707.
Robinson, R.C., Radziejewski, C., Stuart, D.I.
and Jones, E.Y. (1995). Biochemistry, 34, p4139-4146.
CA 02376729 2001-12-10
WO 00/75176 PCT/AU00/00641
- 71 -
Ryden, M. Murray-Rust, J., Glass, D., Ilag, L.L., Trupp,
M., Yancopoulos, G.D., McDonald, N.Q. and Ibanez, C.F.
(1995). EMBO J., 14, p1979-1990.
Spina, M.B., Squinto, S.P., Miller, J., Lindsay,
R.M. and Hyman, C. (1992). J. Neurochem., 59, p99-106.
Tam, J.P., Wu, C.-R., Liu, W. and Zhang, J.-W.
(1991). J. Am. Chem. Soc., 113, p6657-6662.
Thoenen, H. (1991). Trends Neurosci., 14, p165-
170.
Thoenen, H., Hughes, R.A. and Sendtner, M.
(1993). Exp. Neurol., 124, p47-55
Thompson, P.E., Keah H.H., Gomme P.T., Stanton
P.G. and Hearn M.T.W. (1995). Int. J. Peptide Protein Res.,
46, p174-180.
Wrighton, N.C., Farrell, F.X., Chang, R.,
Kashyap, A.K., Barbone, F.P., Mulcahy, L.S., Johnson, D.L.,
Barrett, R.W., Jolliffe, L.K. and Dower, W.J. (1996).
Science, 273, p458-463.
Zwar, R.A. and R.A. Hughes. (1997). Proc. Aust.
Soc. Clin. Exp. Pharm. Toxicol., 4, p90.