Note: Descriptions are shown in the official language in which they were submitted.
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
METHOD FOR TREATING CHRONIC PAIN
USING MEK INHIBITORS
BACKGROUND
The invention features a method for treating chronic pain using MEK
inhibitors. Chronic pain includes neuropathic pain, and chronic inflammatory
pain.
Abnormality anywhere in a nerve pathway disrupts nerve signals, which
in turn are abnormally interpreted in the brain, causing neuropathic pain.
Neuropathic pain may be, for example, a deep ache, a burning sensation, or
hypersensitivity to touch. Diseases or conditions associated with neuropathic
pain include, without limitation, diabetic neuropathy, causalgia, plexus
avulsion, neuroma, vasculitis, crush injury, viral infections (e.g., herpes
virus
infection or HIV), constriction injury, tissue injury, nerve injury from the
periphery to the central nervous system, limb amputation, hypothyroidism,
uremia, chronic alcoholism, post-operative pain, arthritis, back pain, and
vitamin deficiencies.
Infections such as herpes zoster (shingles) can cause nerve
inflammation and produce postherpetic neuralgia, a chronic burning localized
to the area of viral infection. Hyperalgesia is when an already noxious
stimulus becomes more painful, and allodynia, when a previously non-noxious
stimulus becomes painful (such as contact of clothing or a breeze). Reflex
sympathetic dystrophy is accompanied by swelling and sweating or changes
in local blood flow, tissue atrophy, or osteoporosis. Causalgia, including
severe burning pain and swelling, sweating, and changes in blood flow, may
follow an injury or disease of a major nerve such as the sciatic nerve. Some
~ types of chronic low back pain can have a neuropathic component (e.g.,
sciatica, postpoliomyelitis and CPRM). Neuropathic pain may also be induced
by cancer or chemotherapy.
1
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Neuropathic pain is currently treated with anticonvulsants such as
carbamazepine and antidepressants such as amitryptaline. NSAIDS and
opioids generally have little effect (Fields et al 1994 Textbook of Pain p 991-
996 (pub: Churchill Livingstone), James & Page 1994
J.Am.Pediatr.Med.Assoc, 8: 439-447, Galer, 1995 Neurology 45 S17-S25.
Neuropathic conditions that have been treated with gabapentin include:
postherpetic neuralgia, postpoliomyelitis, CPRM, HIV-related neuropathy,
trigeminal neuralgia, and reflex sympathetic dystrophy (RSD).
The generally weak efficacy of antiinflammatory agents suggests that the
mechanism for chronic pain is separate from hyperalgesia.
SUMMARY OF THE INVENTION
The invention features a method for treating chronic pain, which
method includes the step of administering a composition including a MEK
inhibitor to a patient in need of such treatment. Chronic pain includes
neuropathic pain, idiopathic pain, and pain associated with vitamin
deficiencies, uremia, hypothyroidism post-operative pain, arthritis, back
pain,
and chronic alcoholism. The invention also features compositions as
disclosed, formulated for the treatment of chronic pain. Such a composition
may include one or more MEK inhibitor compounds having a structure
disclosed in patent application PCT/US99/30416, international filing date
December 21, 1999.
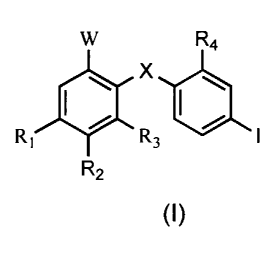
Examples of MEK inhibitors include a compound having the formula (I)
below:
W R4
\ X I \
R~ ~ R3 / I
R2
2
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
W is one of the following formulae (i) - (xiii):
X2 H O O
N_ XZ RSN N NRs ~ O N O p-S
N XI N~ ~ ~ / X2 Nv X~ O / ~ N-H N~ NRs
(i) (ii) (iii) (i~ (V) (Vi) (vii)
N NRs ~N ~ X2 N-N ~N-NRs Xl N X2 N
N. ~ N.~ RSN / N / \ \ Xi
N O N ,X2 ,X2 ~X2
I I
(viii) (ix) (x) (xi) (xii) (xiii)
HO O O
-N ~O O ~N- \
X2 \ X~ X~ \ N X~ ~ N N~N
(XIV) (XV) (XVI) (XVII)
1~
X~ is O, S, or NRF. X2 is OH, SH, or NHRE. Each of RE and RF is H or
C ~_4 alkyl; each of R~ and R2 is independently selected from H, F, N02, Br
and CI; R~ can also be S02NR~RH, or R~ and R2 together with the benzene
ring to which they are attached constitute an indole, isoindole, benzofuran,
benzothiophene, indazole, benzimidazole, or benzthioazole. R3 is selected
from H and F; each of R~, RH, and R4 is independently selected from H, CI
and CH3. R5 is H or C ~~ alkyl. Each hydrocarbon radical above is optionally
substituted with between 1 and 3 substituents independently selected from
halo, hydroxyl, amino, (amino)sulfonyl, and N02. Each heterocyclic radical
3
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
above is optionally substituted with between 1 and 3 substituents
independently selected from halo, C ~~ alkyl, C 3_6 cycloalkyl, C 3~ alkenyl,
C 3_
4 alkynyl, phenyl, hydroxyl, amino, (amino)sulfonyl, and N02, wherein each
substituent alkyl, cycloalkyl, alkenyl, alkynyl or phenyl is in turn
optionally
substituted with between 1 and 2 substituents independently selected from
halo, C ~_2 alkyl, hydroxyl, amino, and N02. The invention also features a
pharmaceutically acceptable salt or C ~_$ ester of a disclosed compound. For
example, the disclosed alcohol compounds may form esters having the
structure obtained by replacing the H of a hydroxyl group with a -C(=O)C ~_~
acyl group.
Preferred embodiments of the invention include methods of using one
or more of the following compounds:
(a) said MEK inhibitor has a structure selected from: 2,4-bis-(2-chloro-4-iodo-
phenylamino)-3-fluoro-5-nitro-benzoic acid; 5-[3,4difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-[1,3,4]oxadiazol-2-ylamine; 5-[4-fluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-[1,3,4]oxadiazol-2-0l; (2,3-difluoro-6-
[1,3,4]oxadiazol-2-yl-phenyl)(-(4-iodo-2-methyl-phenyl)-amine; 5-[3,4-difluoro-
2-(4-iodo-2-methyl-phenylamino)-phenyl]-[1,3,4]oxadiazole-2-thiol; 5-
[3,4difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-[1,2,4]triazole-3-
ylamine; and 5-[3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-
[1,2,4]triazole-3-thiol; and
(b) said MEK inhibitor has the structure selected from: 2,4-bis-(2-chloro-4-
iodo-phenylamino)-3-fluoro-5-nitro-benzoic acid.
Other aspects of the invention are provided in the description,
examples and claims below.
BRIEF DESCRIPTION OF THE FIGURES
FIG. 1 is a bar graph representing the paw withdrawal threshold (PWT)
in grams as a function of time in days. The empty, cross-hatched, and single-
4
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
hatched bars are vehicle, PD 198306, and pregabalin, respectively. The
arrows indicate time of drug administration (30 mg/kg, p.o.).
FIG 2. is a bar graph representing the force required in grams to elicit
paw withdrawal using von Frey hair filaments as a function of time in days.
Baseline (BL) measurements were taken before treatment. Animals received
a single p.o. administration of PD 198306 (3-30mg/kg), or pregabalin
(30mg/kg) and withdrawal thresholds were re-assessed 1 h after treatment.
Treatments were repeated twice a day for two days. Results are expressed
median ~ 1St and 3~d quartiles. *P<0.05, **P<0.01, ***P<0.001 significantly
different from vehicle treated animals (Mann-Whitney t test; n=7-8).
FIG. 3. is a bar graph representing the force required in grams to elicit
paw withdrawal using von Frey hair filaments as a function of time in days.
Baseline (BL) measurements were taken before treatment. Animals received
a single p.o. administration of PD 198306 (3-30mg/kg), or pregabalin
(30mg/kg) and withdrawal thresholds were re-assessed 1 h after treatment.
Treatments were repeated twice a day for two days. Results are expressed
median ~ 1St and 3~d quartiles. **P<0.01 significantly different from vehicle
treated animals (Mann-Whitney t test; n=6).
FIG. 4. is a bar graph representing the force required in grams to elicit
paw withdrawal using von Frey hair filaments as a function of time in days.
Baseline (BL) measurements were taken before treatment. Animals received
a single i.t. administration of PD 198306 (1-30~g/10~1), or pregabalin
(100~g/10~,1) and withdrawal thresholds were re-assessed at 30min, 1 h and
2h after treatment. Results are expressed median ~ 1St and 3'd quartiles.
*P<0.05, ***P<0.001 significantly different from vehicle treated animals (Mann-
Whitney t test; n=7-9).
FIG. 5. is a bar graph representing the force required in grams to elicit
paw withdrawal using von Frey hair filaments as a function of time in days.
5
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Baseline (BL) measurements were taken before treatment. Animals received
a single i.t. administration of PD 198306 (1-30~g/10p1), or pregabalin
(100pg/10w1) and withdrawal thresholds were re-assessed at 30min, 1 h and
2h after treatment. Results are expressed median ~ 1 St and 3'd quartiles.
*P<0.05, **P<0.01, ***P<0.001 significantly different from vehicle treated
animals (Mann-Whitney t test; n=6-8).
FIG. 6 is a bar graph representing the force required in grams to elicit
paw withdrawal using von Frey hair filaments as a function of time in days .
Animals received a single intraplantar (i.pl.) administration of PD 198306
(3mg/100~.1), or an intrathecal injection of PD 198306 (30p,g/10p1) and
withdrawal thresholds were re-assessed 1 h after treatment. Results are
expressed median ~ 1St and 3'd quartiles. **P<0.01 significantly different
from
vehicle treated animals (Mann-Whitney t test; n=6-9).
FIG. 7. is a bar graph representing the force required in grams to elicit
paw withdrawal using von Frey hair filaments as a function of time in days.
Animals received a single intraplantar (i.pl.) administration of PD 198306
(3mg/100~.1), or an intrathecal injection of PD 198306 (30wg/10p.1) and
withdrawal thresholds were re-assessed 1 h after treatment. Results are
expressed median ~ 1St and 3'd quartiles. **P<0.01 significantly different
from
vehicle treated animals (Mann-Whitney t test; n=6).
FIG. 8 is a bar graph representing the force required in grams to elicit
paw withdrawal using von Frey hair filaments. Baseline (BL) measurements
were taken before treatment. Animals received a single i.t. administration of
PD219622, PD297447, PD 184352, or PD 254552 (30pg/10~1), or pregabalin
(100pg/10p1) and withdrawal thresholds were re-assessed at 30min, 1h and
2h after treatment. Results are expressed median ~ 1St and 3'd quartiles.
*P<0.05, **P<0.01, ***P<0.001 significantly different from vehicle treated
animals (Mann-Whitney t test; n=7-8).
6
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
DETAILED DESCRIPTION
The compounds disclosed herein are pharmaceutically active, for
example, they inhibit MEK. MEK enzymes are dual specificity kinases
involved in, for example, immunomodulation, inflammation, and proliferative
diseases such as cancer and restenosis.
Proliferative diseases are caused by a defect in the intracellular
signaling system, or the signal transduction mechanism of certain proteins.
Defects include a change either in the intrinsic activity or in the cellular
concentration of one or more signaling proteins in the signaling cascade . The
cell may produce a growth factor that binds to its own receptors, resulting in
an autocrine loop, which continually stimulates proliferation. Mutations or
overexpression of intracellular signaling proteins can lead to spurious
mitogenic signals within the cell. Some of the most common mutations occur
in genes encoding the protein known as Ras, a G-protein that is activated
when bound to GTP, and inactivated when bound to GDP. The above-
mentioned growth factor receptors, and many other mitogenic receptors, when
activated, lead to Ras being converted from the GDP-bound state to the GTP-
bound state. This signal is an absolute prerequisite for proliferation in most
cell types. Defects in this signaling system, especially in the deactivation
of
the Ras-GTP complex, are common in cancers, and lead to the signaling
cascade below Ras being chronically activated.
Activated Ras leads in turn to the activation of a cascade of
serine/threonine kinases. One of the groups of kinases known to require an
active Ras-GTP for its own activation is the Raf family. These in turn
activate
MEK (e.g., MEK1 and MEK2) which then activates MAP kinase, ERK (ERK~
and ERK2). Activation of MAP kinase by mitogens appears to be essential for
proliferation; constitutive activation of this kinase is sufficient to induce
cellular
transformation. Blockade of downstream Ras signaling, for example by use of
a dominant negative Raf-1 protein, can completely inhibit mitogenesis,
whether induced from cell surface receptors or from oncogenic Ras mutants.
Although Ras is not itself a protein kinase, it participates in the activation
of
7
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Raf and other kinases, most likely through a phosphorylation mechanism.
Once activated, Raf and other kinases phosphorylate MEK on two closely
adjacent serine residues, S218 and S222 in the case of MEK-1, which are the
prerequisite for activation of MEK as a kinase. MEK in turn phosphorylates
MAP kinase on both a tyrosine, Y185, and a threonine residue, T183,
separated by a single amino acid.
This double phosphorylation activates MAP kinase at least 100-fold.
Activated MAP kinase can then catalyze the phosphorylation of a large
number of proteins, including several transcription factors and other kinases.
Many of these MAP kinase phosphorylations are mitogenically activating for
the target protein, such as a kinase, a transcription factor, or another
cellular
protein. In addition to Raf-1 and MEKK, other kinases activate MEK, and
MEK itself appears to be a signal integrating kinase. Current understanding
is that MEK is highly specific for the phosphorylation of MAP kinase. In fact,
no substrate for MEK other than the MAP kinase , ERK, has been
demonstrated to date and MEK does not phosphorylate peptides based on
the MAP kinase phosphorylation sequence, or even phosphorylate denatured
MAP kinase. MEK also appears to associate strongly with MAP kinase prior
to phosphorylating it, suggesting that phosphorylation of MAP kinase by MEK
may require a prior strong interaction between the two proteins. Both this
requirement and the unusual specificity of MEK are suggestive that it
may have enough difference in its mechanism of action to other protein
kinases that selective inhibitors of MEK, possibly operating through
allosteric
mechanisms rather than through the usual blockade of the ATP binding site,
may be found.
The effect of the MEK inhibitor PD 198306 has been investigated in two
animal models of neuropathic pain by assessing static allodynia with von Frey
hairs.
Oral administration of PD 198306 (3-30mg/kg) had no effect in the model
of chronic constriction injury of the sciatic nerve (CCI). However, after
repeated
administration (3 doses over two days) it had a transient effect in the
diabetic
neuropathy model (streptozocin). This may be due to disorders of the blood
8
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
brain barrier induced by the diabetic condition in these animals, thus
allowing
central action of the compound. Intrathecal administration of PD 198306 (1-
30~.g) dose-dependently blocked static allodynia in both the streptozocin and
the
CCI models of neuropathic pain, with minimum effective doses (MED) of 3 and
10~g respectively. The highest dose used (30~g) totally blocked the
maintenance of static allodynia, for up to 1 h. Intraplantar administration of
PD
198306 (3mg/100p1) at a dose 100-fold higher than the dose shown to be
effective intrathecally (30~g/10~1) had no effect on static allodynia in
either of the
neuropathic pain models. This finding confirms the lack of effect seen after
systemic administration and suggests a central site of action for the
compound.
From this study we can suggest the use of MEK inhibitors as potential
new therapeutic tools for chronic pain. The study of potential side-effects,
especially related to memory, of future brain-penetrant MEK inhibitors will
indicate the therapeutic window for this novel class of compounds in the
treatment of pain.
9
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
A. Terms
Certain terms are defined below and by their usage throughout this
disclosure.
Alkyl groups include aliphatic (i.e., hydrocarbyl or hydrocarbon radical
structures containing hydrogen and carbon atoms) with a free valence. Alkyl
groups are understood to include straight chain and branched structures.
Examples include methyl, ethyl, propyl, isopropyl, butyl, n-butyl, isobutyl, t-
butyl, pentyl, isopentyl, 2,3-dimethylpropyl, hexyl, 2,3-dimethylhexyl, 1,1-
dimethylpentyl, heptyl, and octyl. Cycloalkyl groups include cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
Alkyl groups can be substituted with 1, 2, 3 or more substituents which
are independently selected from halo (fluoro, chloro, bromo, or iodo),
hydroxy,
amino, alkoxy, alkylamino, dialkylamino, cycloalkyl, aryl, aryloxy,
arylalkyloxy,
heterocyclic radical, and (heterocyclic radical)oxy. Specific examples include
fluoromethyl, hydroxyethyl, 2,3-dihydroxyethyl, (2- or 3-furanyl)methyl,
cyclopropylmethyl, benzyloxyethyl, (3-pyridinyl)methyl, (2- or 3-
furanyl)methyl,
(2-thienyl)ethyl, hydroxypropyl, aminocyclohexyl, 2-dimethylaminobutyl,
methoxymethyl, N-pyridinylethyl, diethylaminoethyl, and cyclobutylmethyl.
Alkenyl groups are analogous to alkyl groups, but have at least one
double bond (two adjacent sp2 carbon atoms). Depending on the placement
of a double bond and substituents, if any, the geometry of the double bond
may be entgegen (E), or zusammen (Z), cis, or trans. Similarly, alkynyl
groups have at least one triple bond (two adjacent sp carbon atoms).
Unsaturated alkenyl or alkynyl groups may have one or more double or triple
bonds, respectively, or a mixture thereof; like alkyl groups, unsaturated
groups
may be straight chain or branched, and they may be substituted as described
both above for alkyl groups and throughout the disclosure by example.
Examples of alkenyls, alkynyls, and substituted forms include cis-2-butenyl,
trans-2-butenyl, 3-butynyl, 3-phenyl-2-propynyl, 3-(2'-fluorophenyl)-2-
propynyl,
3-methyl(5-phenyl)-4-pentynyl, 2-hydroxy-2-propynyl, 2-methyl-2-propynyl, 2-
propenyl, 4-hydroxy-3-butynyl, 3-(3-fluorophenyl)-2-propynyl, and 2-methyl-2-
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
propenyl. In formula (I), alkenyls and alkynyls can be C 2~ or C 2_8, for
example, and are preferably C 3_4 or C 3_8.
More general forms of substituted hydrocarbon radicals include
hydroxyalkyl, hydroxyalkenyl, hydroxyalkynyl, hydroxycycloalkyl, hydroxyaryl,
and corresponding forms for the prefixes amino-, halo- (e.g., fluoro-, chloro-
,
or bromo-), nitro-, alkyl-, phenyl-, cycloalkyl- and so on, or combinations of
substituents. According to formula (I), therefore, substituted alkyls include
hydroxyalkyl, aminoalkyl, nitroalkyl, haloalkyl, alkylalkyl (branched alkyls,
such
as methylpentyl), (cycloalkyl)alkyl, phenylalkyl, alkoxy, alkylaminoalkyl,
dialkylaminoalkyl, arylalkyl, aryloxyalkyl, arylalkyloxyalkyl, (heterocyclic
radical)alkyl, and (heterocyclic radical)oxyalkyl. R~ thus includes
hydroxyalkyl, hydroxyalkenyl, hydroxyalkynyl, hydroxycycloalkyl, hydroxyaryl,
aminoalkyl, aminoalkenyl, aminoalkynyl, aminocycloalkyl, aminoaryl,
alkylalkenyl, (alkylaryl)alkyl, (haloaryl)alkyl, (hydroxyaryl)alkynyl, and so
forth.
Similarly, RA includes hydroxyalkyl and aminoaryl, and RB includes
hydroxyalkyl, aminoalkyl, and hydroxyalkyl(heterocyclic radical)alkyl.
Heterocyclic radicals, which include but are not limited to heteroaryls,
include: furyl, oxazolyl, isoxazolyl, thiophenyl, thiazolyl, pyrrolyl,
imidazolyl,
1,3,4-triazolyl, tetrazolyl, pyridinyl, pyrimidinyl, pyridazinyl, indolyl, and
their
nonaromatic counterparts. Further examples of heterocyclic radicals include
piperidyl, quinolyl, isothiazolyl, piperidinyl, morpholinyl, piperazinyl,
tetrahydrofuryl, tetrahydropyrrolyl, pyrrolidinyl, octahydroindolyl,
octahydrobenzothiofuranyl, and octahydrobenzofuranyl.
Selective MEK 1 or MEK 2 inhibitors are those compounds which
inhibit the MEK 1 or MEK 2 enzymes, respectively, without substantially
inhibiting other enzymes such as MKK3, PKC, Cdk2A, phosphorylase kinase,
EGF, and PDGF receptor kinases, and C-src. In general, a selective MEK 1
or MEK 2 inhibitor has an IC5o for MEK 1 or MEK 2 that is at least one-
fiftieth
(1/50) that of its IC5o for one of the above-named other enzymes. Preferably,
a selective inhibitor has an ICSO that is at least 1/100, more preferably
1/500,
and even more preferably 1/1000, 1/5000, or less than that of its ICSO or one
or
more of the above-named enzymes.
11
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
B. Compounds
One aspect of the invention features the disclosed compounds shown
in formula (I) in the Summary section.
Embodiments of the invention include compounds wherein: (a) R~ is
bromo or chloro; (b) R2 is fluoro; (c) R3 is H; (d) each of R2 and R3 is H;
(e) each of R2 and R3 is fluoro; (f) R~ is bromo; (g) each of R~, R2 and R3 is
fluoro; (h) R2 is nitro; (i) R3 is H; (j) R4 is chloro; (k) R4 is methyl; (I)
R5 is H;
(m) R5 is CH3; (n) X~ is O or S; (o) X~ is NH or NCH3; (p) X2 is OH, SH, or
NH2; (q) X2 is OH; (r) X2 is NHRE; (s) R4 is H; (t) R4 is chloro or methyl; or
combinations thereof.
Preferably, where one of the substituents on a heterocyclic radical is an
alkenyl or alkynyl group, the double or triple bond, respectively, is not
adjacent
the point of attachment when it is a heteroatom. For example, in such a
situation, the substituent is preferably prop-2-ynyl, or but-2 or 3-enyl, and
less
preferably prop-1-ynyl or but-1-enyl.
Examples of compounds include: [5-fluoro-2-(1 H-tetrazol-5-yl)-
phenyl]-(4-iodo-2-methyl-phenyl)-amine; [2,3-difluoro-6-(1 H-tetrazol-5-yl)-
phenyl]-(4-iodo-2-methyl-phenyl)- amine; (4-iodo-2-methyl-phenyl)-(2,3,4-
trifluoro-6-(1 H-tetrazol-5-yl)-phenyl]-amine; [4-bromo-2,3-difluoro-6-(1 H-
tetrazol-5-yl)-phenyl]-(4-iodo-2-methyl-phenyl)-amine; [5-fluoro-4-nitro-2-(1
H-
tetrazol-5-yl)-phenyl]-(4-iodo-2-methyl-phenyl)-amine; [2-(4,4-dimethyl-4,5-
dihydro-oxazol-2-yl)-5-fluoro-phenyl]-(4-iodo-2-methyl-phenyl)-amine; [6-(4,4-
dimethyl-4,5-dihydro-oxazol-2-yl)-2,3-difluoro-phenyl]-(4-iodo-2-methyl-
phenyl)-amine; [6-(4,4-dimethyl-4,5-dihydro-oxazol-2-yl)-2,3,4-trifluoro-
phenyl]-(4-iodo-2-methyl-phenyl)-amine; [4-bromo-6-(4,4-dimethyl-4,5-
dihydro-oxazol-2-yl)-2,3-difluoro-phenyl]-(4-iodo-2-methyl-phenyl)-amine; [2-
(4,4-dimethyl-4,5-dihydro-oxazol-2-yl)-5-fluoro-4-nitro-phenyl]-(4-iodo-2-
methyl-phenyl)-amine; 5-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-
[1,3,4]thiadiazol-2-0l; 5-[3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-
phenyl]-
[1,3,4]thiadiazol-2-0l; 5-[3,4,5-trifluoro-2-(4-iodo-2-methyl-phenylamino)-
phenyl]-[1,3,4]thiadiazol-2-0l; 5-[5-bromo-3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-[1,3,4]thiadiazol-2-0l; 5-[4-fluoro-2-(4-iodo-2-methyl-
12
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
phenylamino)-5-nitro-phenyl]-[1,3,4]thiadiazol-2-0l; 5-[4-fluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-[1,3,4]oxadiazol-2-0l; 5-[3,4-difluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-[1,3,4]oxadiazol-2-0l; 5-[3,4,5-trifluoro-2-(4-
iodo-
2-methyl-phenylamino)-phenyl]-[1,3,4]oxadiazol-2-0l; 5-[5-bromo-3,4-difluoro-
2-(4-iodo-2-methyl-phenylamino)-phenyl]-[1,3,4]oxadiazol-2-0l; 5-[4-fluoro-2-
(4-iodo-2-methyl-phenylamino)-5-nitro-phenyl]-[1,3,4]oxadiazol-2-0l; 5-[4-
fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-[1,2,4]triazol-3-0l; 5-[3,4-
difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-[1,2,4]triazol-3-0l; 5-
[3,4,5-trifluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-[1,2,4]triazol-3-
0l;
5-[5-bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-
[1,2,4]triazol-3-0l; and 5-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-5-nitro-
phenyl]-4H-[1,2,4]triazol-3-0l.
Further examples include: 5-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-
phenyl]-[1,3,4]thiadiazol-2-ylamine; 5-[3,4-Difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-[1,3,4]thiadiazol-2-ylamine; 5-[3,4,5-Trifluoro-2-(4-iodo-
2-methyl-phenylamino)-phenyl]-[1,3,4]thiadiazol-2-ylamine; 5-[5-bromo-3,4-
difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-[1,3,4]thiadiazol-2-ylamine;
5-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-5-nitro-phenyl]-[1,3,4]thiadiazol-
2-ylamine; 5-[4-Fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-
[1,3,4]oxadiazol-2-ylamine; 5-[3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-
phenyl]-[1,3,4]oxadiazol-2-ylamine; 5-[3,4,5-trifluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-[1,3,4]oxadiazol-2-ylamine; 5-[5-bromo-3,4-difluoro-2-
(4-iodo-2-methyl-phenylamino)-phenyl]-[1,3,4]oxadiazol-2-ylamine; 5-(4-fluoro-
2-(4-iodo-2-methyl-phenylamino)-5-nitro-phenyl]-[1,3,4]oxadiazol-2-ylamine;
5-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-[1,2,4]triazol-3-
ylamine; 5-[3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-
[1,2,4]triazol-3-ylamine; 5-[3,4,5-trifluoro-2-(4-iodo-2-methyl-phenylamino)-
phenyl]-4H-[1,2,4]triazol-3-ylamine; 5-[5-bromo-3,4-difluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-4H-[1,2,4]triazol-3-ylamine; 5-(4-fluoro-2-(4-
iodo-2-methyl-phenylamino)-5-nitro-phenyl]-4H-[1,2,4]triazol-3-ylamine; 5-[4-
fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-[1,3,4]thiadiazole-2-thiol; 5-
[3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-[1,3,4]thiadiazole-2-
13
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
thiol; 5-[3,4,5-trifluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-
[1,3,4]thiadiazole-2-thiol; 5-[5-bromo-3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-[1,3,4]thiadiazole-2-thiol; 5-[4-fluoro-2-(4-iodo-2-
methyl-
phenylamino)-5-nitro-phenyl]-[1,3,4]thiadiazole-2-thiol; 5-[4-fluoro-2-(4-iodo-
2-
methyl-phenylamino)-phenyl]-[1,3,4]oxadiazole-2-thiol; 5-[3,4-difluoro-2-(4-
iodo-2-methyl-phenylamino)-phenyl]-[1,3,4]oxadiazole-2-thiol; 5-[3,4,5-
trifluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-[1,3,4]oxadiazole-2-thiol; 5-
[5-bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-
[1,3,4]oxadiazole-2-thiol; 5-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-5-nitro-
phenyl]-[1,3,4]oxadiazole-2-thiol; 5-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-
phenyl]-4H-[1,2,4]triazole-3-thiol; 5-[3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-4H-[1,2,4]triazole-3-thiol; 5-[3,4,5-trifluoro-2-(4-iodo-
2-
methyl-phenylamino)-phenyl]-4H-[1,2,4]triazole-3-thiol; 5-[5-bromo-3,4-
difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-[1,2,4]triazole-3-thiol;
and 5-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-5-nitro-phenyl]-4H-
[1,2,4]triazole-3-thiol.
Additional examples are: 5-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-
phenyl]-isothiazol-3-0l; 5-[3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-
phenyl]-isothiazo1-3-01; 5-[3,4,5-trifluoro-2-(4-iodo-2-methyl-phenylamino)-
phenyl]-isothiazol-3-0l; 5-[5-bromo-3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-isothiazol-3-0l; 5-[4-fluoro-2-(4-iodo-2-methyl-
phenylamino)-5-nitro-phenyl]-isothiazol-3-0l; 5-[4-fluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-isoxazol-3-0l; 5-[3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-isoxazol-3-0l; 5-[3,4,5-trifluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-isoxazol-3-0l; 5-[5-bromo-3,4-difluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-isoxazol-3-0l; 5-[4-fluoro-2-(4-iodo-2-methyl-
phenylamino)-5-nitro-phenyl]-isoxazol-3-0l; 5-[4-fluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-1 H-pyrazol-3-0l; 5-[3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-1 H-pyrazol-3-0l; 5-[3,4,5-trifluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-1 H-pyrazol-3-0l; 5-[5-bromo-3,4-difluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-1 H-pyrazol-3-0l; 5-[4-fluoro-2-(4-iodo-2-methyl-
phenylamino)-5-nitro-phenyl]-1 H-pyrazol-3-0l; 4-[4-fluoro-2-(4-iodo-2-methyl-
14
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
phenylamino)-phenyl]-isothiazol-3-0l; 4-[3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-isothiazo1-3-01; 4-[3,4,5-trifluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-isothiazol-3-0l; 4-[5-bromo-3,4-difluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-isothiazol-3-0l; 4-[4-fluoro-2-(4-iodo- 2-methyl-
phenylamino)-5-nitro-phenyl]-isothiazol-3-0l; 4-[4-fluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-isoxazol-3-0l; 4-[3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-isoxazol-3-0l; 4-[3,4,5-trifluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-isoxazol-3-0l; 4-[5-bromo-3,4-difluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-isoxazol-3-0l; 4-[4-fluoro-2-(4-iodo-2-methyl-
phenylamino)-5-nitro-phenyl]-isoxazol-3-0l; 4-[4-fluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-1-methyl-1 H-pyrazol-3-0l; 4-[3,4-difluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-1-methyl-1 H-pyrazol-3-0l; 1-methyl-4-[3,4,5-
trifluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-1 H-pyrazol-3-0l; 4-[5-
bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-1-methyl-1 H-
pyrazol-3-0l; and 4-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-5-nitro-phenyl]-
1-methyl-1 H-pyrazol-3-0l.
The invention also features compounds such as: 5-[2-(2-amino-4-iodo-
phenylamino)-4-fluoro-phenyl]-1-methyl-1H-[1,2,3]triazol-4-0l; 5-[2-(2-amino-4-
iodo-phenylamino)-3,4-difluoro-phenyl]-1-methyl-1H-[1,2,3]triazol-4-0l; 5-[2-
(2-
amino-4-iodo-phenylamino)-3,4,5-trifluoro-phenyl]-1-methyl-1H-[1,2,3]triazol-
4-0l; 5-[2-(2-amino-4-iodo-phenylamino)-5-bromo-3,4-difluoro-phenyl]-1-
methyl-1 H-[1,2,3]triazol-4-0l; 5-[2-(2-amino-4-iodo-phenylamino)-4-fluoro-5-
nitro-phenyl]-1-methyl-1H-[1,2,3]triazol-4-0l; 5-[4-fluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-3-methyl-3H-[1,2,3]triazol-4-0l; 5-[3,4-difluoro-2-(4-
iodo-
2-methyl-phenylamino)-phenyl]-3-methyl-3H-[1,2,3]triazol-4-0l; 3-methyl-5-
[3,4,5-trifluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-3H-[1,2,3]triazol-4-
0l;
5-[5-bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-3-methyl-
3H-[1,2,3]triazol-4-0l; 5-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-5-nitro-
phenyl]-3-methyl-3H-[1,2,3]triazol-4-0l; 4-[4-fluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-2-methyl-2H-pyrazol-3-0l; 4-[3,4-difluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-2-methyl-2H-pyrazol-3-0l; 2-methyl-4-[3,4,5-
trifluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-2H-pyrazol-3-0l; 4-[5-
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-2-methyl-2H-
pyrazol-3-0l; 4-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-5-nitro-phenyl]-2-
methyl-2H-pyrazol-3-0l; 1-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-
4-methyl-1,4-dihydro-tetrazol-5-one; 1-[3,4-difluoro-2-(4-iodo-2-methyl-
phenylamino)-phenyl]-4-methyl-1,4-dihydro-tetrazol-5-one; 1-methyl-4-[3,4,5-
trifluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-1,4-dihydro-tetrazol-5-one;
1-[5-bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4-methyl-
1,4-dihydro-tetrazol-5-one; 1-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-5-
nitro-phenyl]-4-methyl-1,4-dihydro-tetrazol-5-one; 1-[4-fluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-1H-[1,2,3]triazol-4-0l; 1-[3,4-difluoro-2-(4-iodo-
2-
methyl-phenylamino)-phenyl]-1H-[1,2,3]triazol-4-0l; 1-[3,4,5-trifluoro-2-(4-
iodo-
2-methyl-phenylamino)-phenyl]-1H-[1,2,3]triazol-4-0l; 1-[5-bromo-3,4-difluoro-
2-(4-iodo-2-methyl-phenylamino)-phenyl]-1H-[1,2,3]triazol-4-0l; and 1-[4-
fluoro-2-(4-iodo-2-methyl-phenylamino)-5-nitro-phenyl]-1 H-[1,2,3]triazol-4-
0l.
Further examples of the invention include 3-[4-fluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-2H-isoxazol-5-one; 3[3,4-difluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-2H-isoxazol-5-one; 3-[3,4,5-trifluoro-2-(4-iodo-2-
methyl-phenylamino)-phenyl]-2H-isoxazol-5-one; 3-[5-bromo-3,4-difluoro-2-
(4-iodo-2-methyl-phenylamino)-phenyl]-2H-isoxazol-5-one; 3-[4-fluoro-2-(4-
iodo-2-methyl-phenylamino)-5-nitro-phenyl]-2H-isoxazol-5-one; [5-fluoro-2-(2-
oxo-2,3-dihydro-21>4_-[1,2,3,5]oxathiadiazol-4-yl)-phenyl]-(4-iodo-2-methyl-
phenyl)-amine; [2,3-difluoro-6-(2-oxo-2,3-dihydro-21>4_-[1,2,3,5]oxathiadiazol-
4-yl)-phenyl]-(4-iodo-2-methyl-phenyl)-amine; (4-lodo-2-methyl-phenyl)-[2,3,4-
trifluoro-6-(2-oxo-2,3-dihydro-21>4_-[1,2,3,5]oxathiadiazol-4-yl)-phenyl]-
amine;
[4-bromo-2,3-difluoro-6-(2-oxo-2,3-dihydro-21>4 -[1,2,3,5]oxathiadiazol-4-yl)-
phenyl]-(4-iodo-2-methyl-phenyl)-amine; [5-fluoro-4-nitro-2-(2-oxo-2,3-dihydro-
21>4- [1,2,3,5]oxathiadiazol-4-yl)-phenyl]-(4-iodo-2-methyl-phenyl)-amine; 4-
[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-isoxazol-5-one; 4-[3,4-
difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-isoxazol-5-one; 4-[3,4,5-
trifluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-isoxazol-5-one; 4-[5-
bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-4H-isoxazol-5-
16
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
one; and 4-[4-fluoro-2-(4-iodo-2-methyl-phenylamino)-5-nitro-phenyl]-4H-
isoxazol-5-one.
Further compounds, where R~ can also be S02NR~RH, or R~ and R2
together with the benzene ring to which they are attached constitute an
indole,
isoindole, benzofuran, benzothiophene, indazole, benzimidazole, or
benzthioazole, include the following groups:
Group 1
(1) 2-Fluoro-5-(5-hydroxy-1,3,4-oxadiazol-2-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(2) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(5-hydroxy-1,3,4-oxadiazol-2-
yl)-N-methyl-benzenesulfonamide
(3) 2,3-Difluoro-5-(5-hydroxy-1,3,4-oxadiazol-2-yl)-4-(4-iodo-2-methy1-
phenylamino)-N, N-dimethyl-benzenesulfonamide
(4) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(5-hydroxy-1,3,4-
oxadiazol-2-yl)-N-methyl-N-(3-morpholin-4-yl-propyl)-
benzenesulfonamide
(5) 2-Fluoro-5-(5-hydroxy-1,3,4-oxadiazol-2-yl)-4-(4-iodo-phenylamino)-N-[2-
(2-methoxy-ethoxy)-ethyl]-benzenesulfonamide
(6) N-(2-Dimethylamino-ethyl)-2-fluoro-5-(5-hydroxy-1,3,4-oxadiazol-2-yl)-4-
(4-iodo-phenylamino)-N-methyl-benzenesulfonamide
Group 2
(1 ) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-2-fluoro-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(2) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-4-(2-chloro-4-iodo-phenylamino)-2-
fluoro- benzenesulfonamide
(3) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-2,3-difluoro-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
17
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
(4) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-4-(2-chloro-4-iodo-phenylamino)-2,3-
difluoro-benzenesulfonamide
(5) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-2-fluoro-4-(4-iodo-phenylamino)-
benzenesulfonamide
(6) 4-(5-Amino-1,3,4-oxadiazol-2-yl)-2-fluoro-5-(4-iodo-phenylamino)-
benzenesulfonamide
Group 2b
(1) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-2-fluoro-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(2) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-2-fluoro-4-(4-iodo-phenylamino)-N-
methyl-N-(3-morpholin-4-yl-propyl)-benzenesulfonamide
(3) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-2,3-difluoro-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(4) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-2-fluoro-4-(4-iodo-phenylamino)-
benzenesulfonamide
(5) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-4-(2-chloro-4-iodo-phenylamino)-2,3-
difluoro-N-[3-(4-methyl-piperazin-1-yl)-propyl]-benzenesulfonamide
(6) 5-(5-Amino-1,3,4-oxadiazol-2-yl)-4-(2-chloro-4-iodo-phenylamino)-2-
fluoro-N-(3-piperidin-1-yl-propyl)-benzenesulfonamide
Group 3
(1) 2-Fluoro-4-(4-iodo-2-methyl-phenylamino)-5-(5-mercapto-1,3,4-oxadiazol-
2-yl)-benzenesulfonamide
(2) 2-Fluoro-5-(4-iodo-phenylamino)-4-(5-mercapto-1,3,4-oxadiazol-2-y1)-
benzenesulfonamide
(3) 2,3-Difluoro-4-(4-iodo-2-methyl-phenylamino)-5-(5-mercapto-1,3,4-
oxadiazol-2-yl)-benzenesulfonamide
(4) 2-Fluoro-4-(4-iodo-phenylamino)-5-(5-mercapto-1,3,4-oxadiazol-2-y1)-
benzenesulfonamide
18
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
(5) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(5-mercapto-1,3,4-
oxadiazol-2-yl)-benzenesulfonamide
(6) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(5-mercapto-1,3,4-oxadiazol-
2-yl)-benzenesulfonamide
Group 4
(1) 2-Fluoro-5-(5-hydroxy-1,3,4-thiadiazol-2-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(2) 2-Fluoro-4-(5-hydroxy-1,3,4-thiadiazol-2-yl)-5-(4-iodo-phenylamino)-
benzenesulfonamide
(3) 2,3-Difluoro-5-(5-hydroxy-1,3,4-thiadiazol-2-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(4) 2-Fluoro-5-(5-hydroxy-1,3,4-thiadiazol-2-yl)-4-(4-iodo-phenylamino)-
benzenesulfonamide
(5) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(5-hydroxy-1,3,4-
thiadiazol-2-yl)-benzenesulfonamide
(6) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(5-hydroxy-1,3,4-thiadiazol-2-
yl)-benzenesulfonamide
Group 5
(1) 5-(5-Amino-1,3,4-thiadiazol-2-yl)-2-fluoro-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(2) 4-(5-Amino-1,3,4-thiadiazol-2-yl)-5-(4-iodo-phenylamino)-2-mercapto-
benzenesulfonamide
(3) 5-(5-Amino-1,3,4-thiadiazol-2-yl)-2,3-difluoro-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(4) 5-(5-Amino-1,3,4-thiadiazol-2-yl)-2-fluoro-4-(4-iodo-phenylamino)-
benzenesulfonamide
(5) 5-(5-Amino-1,3,4-thiadiazol-2-yl)-4-(2-chloro-4-iodo-phenylamino)-2,3-
difluoro-benzenesulfonamide
(6) 5-(5-Amino-1,3,4-thiadiazol-2-yl)-4-(2-chloro-4-iodo-phenylamino)-2-
fluoro-benzenesulfonamide
19
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Group 6
(1) 2-Fluoro-5-(5-hydroxy-4H-1,2,4-triazol-3-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(2) 2-Fluoro-4-(5-hydroxy-4H-1,2,4-triazol-3-yl)-5-(4-iodo-phenylamino)-
benzenesulfonamide
(3) 2,3-Difluoro-5-(5-hydroxy-4H-1,2,4-triazol-3-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(4) 5-[4-(2-Dimethylamino-ethyl)-5-hydroxy-4H-1,2,4-triazol-3-yl]-2-fluoro-4-
(4-iodo-phenylamino)-benzenesulfonamide
(5) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(5-hydroxy-4H-1,2,4-
triazol-3-yl)-benzenesulfonamide
(6) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(5-hydroxy-4-methyl-4H-1,2,4-
triazol-3-yl)-benzenesulfonamide
Group 7
(1 ) 5-(5-Amino-4H-1,2,4-triazol-3-yl)-2-fluoro-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(2) 4-(5-Amino-4H-1,2,4-triazol-3-yl)-2-fluoro-5-(4-iodo-phenylamino)-
benzenesulfonamide
(3) 5-(5-Amino-4-methyl-4H-1,2,4-triazol-3-yl)-2,3-difluoro-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(4) 5-[5-Amino-4-(3-dimethylamino-propyl)-4H-1,2,4-triazol-3-yl]-2-fluoro-4-(4-
iodo-phenylamino)-benzenesulfonamide
(5) 5-(5-Amino-4H-1,2,4-triazol-3-yl)-4-(2-chloro-4-iodo-phenylamino)-2,3-
difluoro-benzenesulfonamide
(6) 5-(5-Amino-4-methyl-4H-1,2,4-triazol-3-yl)-4-(2-chloro-4-iodo-
phenylamino)-2-fluoro-benzenesulfonamide
Group 8
(1) 2-Fluoro-5-(3-hydroxy-isoxazol-5-yl)-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
(2) 2-Fluoro-4-(3-hydroxy-isoxazol-5-yl)-5-(4-iodo-phenylamino)-
benzenesulfonamide
(3) 2,3-Difluoro-5-(3-hydroxy-isoxazol-5-yl)-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(4) 2-Fluoro-5-(3-hydroxy-isoxazol-5-yl)-4-(4-iodo-phenylamino)-
benzenesulfonamide
(5) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(3-hydroxy-isoxazol-5-yl)-
benzenesulfonamide
(6) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(3-hydroxy-isoxazol-5-yl)-
benzenesulfonamide
Group 9
(1 ) 5-(3-Amino-isoxazol-5-yl)-2-fluoro-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(2) 4-(3-Amino-isoxazol-5-yl)-2-bromo-5-(4-iodo-phenylamino)-
benzenesulfonamide
(3) 5-(3-Amino-isoxazol-5-yl)-2,3-difluoro-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(4) 5-(3-Amino-isoxazol-5-yl)-2-fluoro-4-(4-iodo-phenylamino)-
benzenesulfonamide
(5) 5-(3-Amino-isoxazol-5-yl)-4-(2-chloro-4-iodo-phenylamino)-2,3-difluoro-
benzenesulfonamide
(6) 5-(3-Amino-isoxazol-5-yl)-4-(2-chloro-4-iodo-phenylamino)-2-fluoro-
benzenesulfonamide
Group 10
(1) 2-Fluoro-4-(4-iodo-2-methyl-phenylamino)-5-(3-mercapto-isoxazol-5-yl)-
benzenesulfonamide
(2) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(3-mercapto-isoxazol-5-yl)-
benzenesulfonamide
(3) 2,3-Difluoro-4-(4-iodo-2-methyl-phenylamino)-5-(3-mercapto-isoxazol-5-
yl)-benzenesulfonamide
21
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
(4) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(3-mercapto-isoxazol-5-
yl)-benzenesulfonamide
(5) 2-Fluoro-4-(4-iodo-phenylamino)-5-(3-mercapto-isoxazol-5-yl)-
benzenesulfonamide
(6) 2-Bromo-5-(4-iodo-phenylamino)-4-(3-mercapto-isoxazol-5-yl)-
benzenesulfonamide
Group 11
(1 ) 2-Fluoro-5-(3-hydroxy-isoxazol-4-yl)-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(2) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(3-hydroxy-isoxazol-4-yl)-
benzenesulfonamide
(3) 2,3-Difluoro-5-(3-hydroxy-isoxazol-4-yl)-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(4) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(3-hydroxy-isoxazol-4-yl)-
benzenesulfonamide
(5) 2-Fluoro-5-(3-hydroxy-isoxazol-4-yl)-4-(4-iodo-phenylamino)-
benzenesulfonamide
(6) 2-Bromo-4-(3-hydroxy-isoxazol-4-yl)-5-(4-iodo-phenylamino)-
benzenesulfonamide
Group 12
(1 ) 5-(3-Amino-isoxazol-4-yl)-2-fluoro-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(2) 5-(3-Amino-isoxazol-4-yl)-4-(2-chloro-4-iodo-phenylamino)-2-fluoro-
benzenesulfonamide
(3) 5-(3-Amino-isoxazol-4-yl)-2,3-difluoro-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(4) 5-(3-Amino-isoxazol-4-yl)-4-(2-chloro-4-iodo-phenylamino)-2,3-difluoro-
benzenesulfonamide
(5) 5-(3-Amino-isoxazol-4-yl)-2-fluoro-4-(4-iodo-phenylamino)-
benzenesulfonamide
22
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
(6) 4-(3-Amino-isoxazol-4-yl)-2-chloro-5-(4-iodo-phenylamino)-
benzenesulfonamide
Group 13
(1) 5-(3-Amino-isoxazol-4-yl)-2-fluoro-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(2) 5-(3-Amino-isoxazol-4-yl)-4-(2-chloro-4-iodo-phenylamino)-2-fluoro-
benzenesulfonamide
(3) 5-(3-Amino-isoxazol-4-yl)-2,3-difluoro-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(4) 5-(3-Amino-isoxazol-4-yl)-4-(2-chloro-4-iodo-phenylamino)-2,3-difluoro-
benzenesulfonamide"
(5) 5-(3-Amino-isoxazol-4-yl)-2-fluoro-4-(4-iodo-phenylamino)-
benzenesulfonamide
(6) 4-(3-Amino-isoxazol-4-yl)-2-chloro-5-(4-iodo-phenylamino)-
benzenesulfonamide
Group 14
(1) 5-(2-Amino-5H-pyrrol-3-yl)-2-fluoro-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(2) 5-(2-Amino-5H-pyrrol-3-yl)-4-(2-chloro-4-iodo-phenylamino)-2-fluoro-
benzenesulfonamide
(3) 5-(2-Amino-5H-pyrrol-3-yl)-2,3-difluoro-4-(4-iodo-2-methyl-phenylamino)-
benzenesulfonamide
(4) 5-(2-Amino-5H-pyrrol-3-yl)-4-(2-chloro-4-iodo-phenylamino)-2,3-difluoro-
benzenesulfonamide
(5) 5-(2-Amino-5H-pyrrol-3-yl)-2-fluoro-4-(4-iodo-phenylamino)-
benzenesulfonamide
(6) 4-(2-Amino-5H-pyrrol-3-yl)-2-chloro-5-(4-iodo-phenylamino)-
benzenesulfonamide
23
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Group 15
(1 ) 2-Fluoro-5-(5-hydroxy-1-methyl-1 H-pyrazol-4-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(2) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(5-hydroxy-1 H-pyrazol-4-yl)-
benzenesulfonamide
(3) 2,3-Difluoro-5-(5-hydroxy-1 H-pyrazol-4-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(4) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(5-hydroxy-1 H-pyrazol-4-
yl)-benzenesulfonamide
(5) 2-Fluoro-5-{5-hydroxy-1-[3-(4-methyl-piperazin-1-yl)-propyl]-1 H-pyrazol-4-
yl}-4-(4-iodo-phenylamino)-benzenesulfonamide
(6) 2-Fluoro-4-(5-hydroxy-1 H-pyrazol-4-yl)-5-(4-iodo-phenylamino)-
benzenesulfonamide
Group 16
(1) 2-Fluoro-5-(5-hydroxy-3-methyl-3H-1,2,3-triazol-4-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(2) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(5-hydroxy-3H-1,2,3-triazol-4-
yl)-benzenesulfonamide
(3) 2,3-Difluoro-5-(5-hydroxy-3H-1,2,3-triazol-4-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(4) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(5-hydroxy-3H-1,2,3-
triazol-4-yl)-benzenesulfonamide
(5) 2-Fluoro-5-(5-hydroxy-3H-1,2,3-triazol-4-yl)-4-(4-iodo-phenylamino)-
benzenesulfonamide
(6) 2-Fluoro-5-{5-hydroxy-3-[2-(2-methoxy-ethoxy)-ethyl]-3H-1,2,3-triazol-4-
yl}-4-(4-iodo-phenylamino)-benzenesulfonamide
Group 17
(1) 2-Fluoro-5-(5-hydroxy-3-methyl-3H-1,2,3-triazol-4-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
24
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
(2) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(5-hydroxy-3H-1,2,3-triazol-4-
y1)- benzenesulfonamide
(3) 2,3-Difluoro-5-(5-hydroxy-3H-1,2,3-triazol-4-yl)-4-(4-iodo-2-methyl-
phenylamino)-benzenesulfonamide
(4) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(5-hydroxy-3H-1,2,3-
triazol-4-yl)-benzenesulfonamide
(5) 2-Fluoro-5-(5-hydroxy-3H-1,2,3-triazol-4-yl)-4-(4-iodo-phenylamino)-
benzenesulfonamide
(6) 2-Fluoro-5-{5-hydroxy-3-[2-(2-methoxy-ethoxy)-ethyl]-3H-1,2,3-triazol-4-
yl}-4-(4-iodo-phenylamino)-benzenesulfonamide
Group 18
(1) 2-Fluoro-4-(4-iodo-2-methyl-phenylamino)-5-(5-oxo-4,5-dihydro-tetrazol-1-
yl)-benzenesulfonamide
~15 (2) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(5-oxo-4,5-dihydro-tetrazol-
1-
yl)-benzenesulfonamide
(3) 2,3-Difluoro-4-(4-iodo-2-methyl-phenylamino)-5-(5-oxo-4,5-dihydro-
tetrazol-1-yl)-benzenesulfonamide
(4) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(5-oxo-4,5-dihydro-
tetrazol-1-yl)-benzenesulfonamide
(5) 2-Fluoro-4-(4-iodo-phenylamino)-5-(5-oxo-4,5-dihydro-tetrazol-1-y1)-
benzenesulfonamide
(6) 2,6-Difluoro-3-(4-iodo-phenylamino)-4-(5-oxo-4,5-dihydro-tetrazo1-1-y1)-
benzenesulfonamide
Group 19
(1) 2-Fluoro-4-(4-iodo-2-methyl-phenylamino)-5-(4-methyl-5-oxo-4,5-dihydro-
tetrazol-1-yl)- benzenesulfonamide
(2) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(4-methyl-5-oxo-4,5-dihydro-
tetrazol-1-yl)-benzenesulfonamide
(3) 2,3-Difluoro-4-(4-iodo-2-methyl-phenylamino)-5-(5-oxo-4,5-dihydro-
tetrazol-1-yl)-benzenesulfonamide
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
(4) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(5-oxo-4,5-dihydro-
tetrazol-1-yl)-benzenesulfonamide
(5) 5-[4-(2-Dimethylamino-ethyl)-5-oxo-4,5-dihydro-tetrazol-1-yl]-2-fluoro-4-
(4-
iodo-phenylamino)-benzenesulfonamide
(6) 2-Fluoro-5-(4-iodo-phenylamino)-4-(4-methyl-5-oxo-4,5-dihydro-tetrazol-1-
yl)-benzenesulfonamide
Group 20
(1) 2-Fluoro-4-(4-iodo-2-methyl-phenylamino)-5-(2-oxo-2,3-dihydro-1,2,3,5-
oxathiadiazol-4-yl)-benzenesulfonamide
(2) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(2-oxo-2,3-dihydro-1,2,3,5-
oxathiadiazol-4-yl)-benzenesulfonamide
(3) 2,3-Difluoro-4-(4-iodo-2-methyl-phenylamino)-5-(2-oxo-2,3-dihydro-
1,2,3,5-oxathiadiazol-4-yl)-benzenesulfonamide
(4) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(2-oxo-2,3-dihydro-
1,2,3,5-oxathiadiazol-4-yl)-benzenesulfonamide
(5) 2-Fluoro-4-(4-iodo-phenylamino)-5-(2-oxo-2,3-dihydro-1,2,3,5-
oxathiadiazol-4-yl)-benzenesulfonamide
(6) 2-Fluoro-5-(4-iodo-phenylamino)-4-(2-oxo-2,3-dihydro-1,2,3,5-
oxathiadiazol-4-yl)-benzenesulfonamide
Group 21
(1 ) 2-Fluoro-4-(4-iodo-2-methyl-phenylamino)-5-(3-methyl-2-oxo-2,3-dihydro-
1,2,3,5-oxathiadiazol-4-yl)-benzenesulfonamide
(2) 2,6-Difluoro-3-(4-iodo-phenylamino)-4-(3-methyl-2-oxo-2,3-dihydro-
1,2,3,5-oxathiadiazol-4-yl)-benzenesulfonamide
(3) 2,3-Difluoro-4-(4-iodo-2-methyl-phenylamino)-5-(3-methyl-2-oxo-2,3-
dihydro-1,2,3,5-oxathiadiazol-4-yl)-benzenesulfonamide
(4) 2-Fluoro-4-(4-iodo-phenylamino)-5-(3-methyl-2-oxo-2,3-dihydro-1,2,3,5
oxathiadiazol-4-yl)-benzenesulfonamide
26
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
(5) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(3-methyl-2-oxo-2,3-
dihydro-1,2,3,5-oxathiadiazol-4-yl)-benzenesulfonamide
(6) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-N-methyl-5-(3-methyl-2-oxo-2,3-
dihydro-1,2,3,5-oxathiadiazol-4-yl)-benzenesulfonamide
Group 22
(1 ) 2-Fluoro-4-(4-iodo-2-methyl-phenylamino)-5-(5-oxo-2,5-dihydro-isoxazol-
3-yl)-benzenesulfonamide
(2) 2,6-Difluoro-3-(4-iodo-phenylamino)-4-(5-oxo-2,5-dihydro-isoxazo1-3-y1)-
benzenesulfonamide
(3) 2,3-Difluoro-4-(4-iodo-2-methyl-phenylamino)-5-(5-oxo-2,5-dihydro-
isoxazol-3-yl)-benzenesulfonamide
(4) 2-Fluoro-4-(4-iodo-phenylamino)-5-(5-oxo-2,5-dihydro-isoxazol-3-y1)-
benzenesulfonamide
(5) 4-(2-Chloro-4-iodo-phenylamino)-2,3-difluoro-5-(5-oxo-2,5-dihydro-
isoxazol-3-yl)-benzenesulfonamide
(6) 4-(2-Chloro-4-iodo-phenylamino)-2-fluoro-5-(5-oxo-2,5-dihydro-isoxazol-3-
yl)-benzenesulfonamide
Group 23
(1 ) 5-[6-(4-lodo-2-methyl-phenylamino)-1 H-benzimidazol-5-yl]-1,3,4-
oxadiazol-2-0l
(2) 5-[6-(4-lodo-phenylamino)-benzofuran-5-yl]-1,3,4-oxadiazol-2-0l
(3) 5-[7-Fluoro-6-(4-iodo-2-methyl-phenylamino)-benzoxazol-5-yl]-1,3,4-
oxadiazol-2-0l
(4) 5-[5-(4-lodo-phenylamino)-benzofuran-6-yl]-1,3,4-oxadiazol-2-0l
(5) 5-[6-(2-Chloro-4-iodo-phenylamino)-7-fluoro-1,3-dihydro-isobenzofuran-5-
yl]-1,3,4-oxadiazol-2-0l
(6) 5-[6-(2-Chloro-4-iodo-phenylamino)-1-methyl-1H-benzimidazol-5-yl]-1,3,4-
oxadiazol-2-0l
27
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Group 24
(1 ) 5-[2-Amino-6-(4-iodo-2-methyl-phenylamino)-1 H-benzimidazol-5-yl]-1,3,4-
oxadiazol-2-0l
(2) 5-[6-(4-lodo-phenylamino)-benzo[b]thiophen-5-yl]-1,3,4-oxadiazol-2-0l
(3) 5-[7-Fluoro-6-(4-iodo-2-methyl-phenylamino)-benzothiazol-5-yl]-1,3,4-
oxadiazol-2-0l
(4) 5-[5-(4-lodo-phenylamino)-benzo[b]thiophen-6-yl]-1,3,4-oxadiazol-2-0l
(5) 5-[6-(2-Chloro-4-iodo-phenylamino)-7-fluoro-1,3-dihydro-
benzo[c]thiophen-5-yl]-1,3,4-oxadiazol-2-0l
(6) 5-[6-(2-Chloro-4-iodo-phenylamino)-2-oxo-2,3-dihydro-1H-2$I>4- 2,1,3-
benzothiadiazol-5-yl]-1, 3,4-oxadiazol-2-0l
Group 25
(1) 5-[2-Amino-6-(4-iodo-2-methyl-phenylamino)-benzothiazol-5-yl]-1,3,4-
oxadiazol-2-0l
(2) 5-[6-(4-lodo-phenylamino)-1 H-indol-5-yl]-1,3,4-oxadiazol-2-0l
(3) 5-[7-Fluoro-6-(4-iodo-2-methyl-phenylamino)-benzothiazol-5-yl]-1,3,4-
oxadiazol-2-0l
(4) 5-[5-(4-lodo-phenylamino)-1H-indol-6-yl]-1,3,4-oxadiazol-2-0l
(5) 5-[6-(2-Chloro-4-iodo-phenylamino)-7-fluoro-2,3-dihydro-1 H-isoindol-5-yl]-
1,3,4-oxadiazol-2-0l
(6) 5-[5-(2-Chloro-4-iodo-phenylamino)-1H-indazol-6-yl]-1,3,4-oxadiazol-2-0l
Group 26
(1) 5-[2-Amino-6-(4-iodo-2-methyl-phenylamino)-benzothiazol-5-yl]-1,3,4-
oxadiazol-2-0l
(2) 5-[6-(4-lodo-phenylamino)-1 H-indol-5-yl]-1,3,4-oxadiazol-2-0l
(3) 5-[7-Fluoro-6-(4-iodo-2-methyl-phenylamino)-benzoxazol-5-yl]-1,3,4-
oxadiazol-2-0l
(4) 5-[5-(4-lodo-phenylamino)-benzoxazol-6-yl]-1,3,4-oxadiazol-2-0l
(5) 5-[6-(2-Chloro-4-iodo-phenylamino)-7-fluoro-2,3-dihydro-1 H-isoindol-5-yl]-
1, 3,4-oxad iazol-2-0l
28
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
(6) 5-(5-(2-Chloro-4-iodo-phenylamino)-1H-indazol-6-yl]-1,3,4-oxadiazol-2-0l
29
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
C. Synthesis
The disclosed compounds can be synthesized according to Schemes
1-25 or analogous variants thereof. These synthetic strategies are further
exemplified in Examples 1-8 below. The solvent between compounds 4 and 5
in Scheme 1 is toluene (PhMe).
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 1
OH O
H N
IizN' N O H Ra p N \ O H Ra ~ ~N-N O H R
~ H a
I \ N I \ Cl- -CI I \ N \ DMF I \ N I \
I ~--
R~ / R.~ / 1 PhCI R~ / R3 / I 0 Ri / R3 / I
Rz Rz Rz
1 2 3
O'~'
~S~CH3 '~~ HO
N ~ v0 ~ N
N\ O N\ O
H Ra H Ra
\ N \ \ N \
Ri I / R3 I / 1 R~ I / R3 I / I
Rz c Rz c
HO~ NHz
Ph/~
4
O~NHz
N
N\ O N3~N O
H Ra // H Ra
O
\ N I \ I \ N I \
R~ / R3 / 1 R~ / R3 / I
Rz Rz
OH
Ph
HN N_N S H R N\ S H Ra
H a
\ N \ conc.HCl \ N \
R I / R I / 1 EtOH R~ I / R I / I
1 3
Rz Rz
31
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 2
OH
N
Ph~O NH O N ~ NH
H Ra ~ 'NHz H Ra
\ N \ HzN ~ ~. \ N \
R~ I / R3 I ~ I Pyridine R~ I ~ R3 I / I
Rz Rz
11
OH
OH
i
CI iN N~ NH
H Ra H Ra
\ N \ K-N=S=O \ N \
Et3N/Ph
R~ ~ R3 I Ri ~ R3 I
Rz Rz
12 13
Ph
p HO
N -N
NH
O~S \ H Ra H Ra
\ N \ H2~ Hz \ N \
R ~ ~ R I / I EtOH R ~ / R3 I / 1
1 3 1
Rz Rz
14 15
HO O
~ O ~ O R
H a
OCH3 SOC12 / I OCH3 LDA / I N I \
R~ \ R3 HzN~OH R~ \ R3 H2N Ra R~ \ R3 / I
Rz Rz I ~ Rz
16 17 ~ I 18
32
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 3
OEt
O N
. N N ~ ,NH O OH
HzN~H \ F 1. AcONa/Brz F 1.SOCI2 F
I I \ /AcOH _ I \ 2. KSCN I \
R~ ~ R, R~ ~ R3 2. KOH, EtOH R~ ~ R3 3.~ EtOH R~ ~ R3
Rz Rz Rz 4. NHZNHZ Rz
19 20 21 22
'I HCl '
HzN~ H. NHz
O H N 'OH 4 N~OH
R
HO o ~ ,N p N~ NH Nv NH
HzN H HzN I w H Ra
\ \ F Nay \ F _ \ N \
R, I ~ R3 Ri I ~ R3 H20 Ri I ~ R3 LDA R~ ( ~ R3 I
Rz Rz Rz Rz
22 23 24 25
1. HCI/ HZO
NHZNHZ ~ 2. NaOH/H20
PyBOP H
H N~N O
2
\ F HOCN
I i + NHs
R~ R3
Rz
26
33
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 4
N- OH N- OH
H2N.N O O N H2N R N R
H a
\ F CI' -CI \ F 1 \ N \
R~ ~ / R3 R~ ~ / R3 LDA R~ ~ / R3 ~ / I
R2 R2 Rz
26 24 25
SII
CI~CI PCIS/POC13 1. PCIS/POC13
~SH ~CI
N -1 N -1
N O N O 2. HO~NHz
0
\ F \ F
Ri ~ / R3 Ri ~ / R3
R2 R2
27 28
HO~NH2
1. CH31
2. KMn04
O
''
N~SO HO NH N~O~~z
N y O W/\i 2 N \ O
Ra
F D H
\ N \
R~ R3 R~ ~ / R3 ~ / I
Rz Rz
29 30
34
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 5
N~OH N~OH
Ra
CH3 H ~~ N \ S H2N ~I w N \ S H Ra
\ F Ph'N~N OEt \ F ~I \ N \
FI
Lawesson's reagent I ~ LDA I / I
R, R3 R, R3 R, R3 I
R2 R2 R2
31 32 33
o OII
HS S O HO- v S S OII Ph~N~N.N S
F ~ -Br F Ph~N~N,NH2 H H F
I \ HO' v _ \
H H I \
Ri ~ R3 piperidine/acetone
R~ R3 Ri / Rs
Rz R2 R2
34 35 36
N~OH N~OH
Ra
N\ S F H2N I ~ N\ S N R
a
conc. HCl \ ~ 1 \ \
EtOH R~ I ~ R3 LDA RI I , I ,
R2 R2
37 9
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 6
R4
COOH HZN COOH R4
\F ~, \a \
I
LHMDS
R1 ~ _R3 R1 ~ ~R3 I
R2 R2
N~ O
(~ )(cocl)2 R4
(2) \ a \
HzN~~OH ~ ~ ~ ~ I
R1 ~ -R3
(3) SOCIz
R2
36
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 7
R4
COOH HZN COOH R4
\ F ~ i I \ ~ \
R1 I ~ R3 LHMDS R1 I ~ R3 I ~ I
R2 R2
O
O CzH ~ ~ ~ O R4
HZN R4
NHZNHZ,PY-BOP ~ CICOZCZHs
\ \~ \ \
R1 ~ ~ R3 I ~ I R1 ~ R3 / I
R2 R2
OH
N
Vila N ~ O
R4
R1 ~ / R3 ~ / I
R2
37
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 8
R4
COOH HZN COOH R4
i~ . ~ a
R1 I ~ R3 LHMDS R1 I ~ R3 I / I
R2 R2
O
~ ,a O
H N'a O R4 CZHS ' R4
a CICOZC2H6
NHZNHZ,PY-BOP \ \ ~ ~ a
R1 I ~ R3 I ~ I R1 ~ R3 ~ I
R2 R2
Lawesson's Reagent Lawesson's Reagent
Xylene Toluene
SH OH
N~ N
N~ S N~ S
R4 R4
a ~ ~ a
R1 ~ / R3 ~ / 1 R1 ~ ~ R3 ~ / I
R2
38
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 9
R4
COOH H2N
F I i
I
R1 ~ R3 ~HMDS R1
R2
NHZ
~ N~ S
HZN' \ ~ ~ O R4 [H~~ R4
(1)(COCI)Z
(2) NHZNHC(S)NHZ ~ ~ ~ \ ~ \
R1 I ~ R3 I ~ I R1 ~ R3 ~ I
R2 R2
NaOCH3/CH30H
Reflux
SH
N
N ~ NH
R4
,a
R1 I ~ R3 I / I
R2
39
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 10
R4
COOH HZN COOH R4
\ F I ~ I \ ~ \
R1 I ~ R3 LHMDS R1 I ~ R3 I / I
R2 R2
NHZ
N-
H N~a O R4 N ~ O
NHZNH2 2 BrCNINaHC03(aq.) ~ R4
PY-~ \ ~ \ Dioxane, r.t.
\ \
R1 I ~ R3 I / I I ~ I ~ I
R1 ~ ~R3
R2 R2
1)CS2, KOH
2)HCI
SH
N
N~ O
R4
\ ~ \
R1 ~ / R3 ~ / I
R2
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 11
R4
COOH H N COOH R4
z
I
R1 I ~ R3 ~H~R1 I ~ R3 I / I
R2 R2
NHz
N
COO ~ 3 R4 N ~ NH
CH30H/H2S04 aminoguanidine nitrate H R4
\ ~ \ NaOCH3/CH30H ~ \ N
R1 / R3 / I
R1 ~ R3 ~ I
R2
S
MeNH~ NHZ
MeNCS
R4
\a \
R1 I ~ R3 I / I
R2
41
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 12
O COzCzHs
(1 )PPh3,CBr4 ~ F
R1 I / R3 (2)n-BuLi,
then CICOZCZH5R1 R3
R2 R2
COzC2Hs HO
-N
R4 ~ NHCH3
NHZNHCH3 R4
LHMDS _ ~ ~ ~ ~_
R4
/ ~ / ~ a
HZN ~ R1 R3 I
R2 R1 / R3 / I
R2
42
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 13
R4
COOH HZN COOH R4
\ F ~ , \ ~ \
_I
R1 I ~ R3 LHMDS R1 ~ R3 / I
RZ
R2
CH OH R4 CHZBr R4 CHZCN R4
2
[H] \ ~ \ Bromination \ ~ \ ~ \ ~ \
R1 ~ ~ R3 I ~ I R1 ~ / R3 I ~ I R1 ~ R3 / I
R2
N-S
HO ~ S03N R4
NaH,CS2 cess HZOZ \ ~ \
HCONMe2 R1 R1 ~ ~ R3 I / I
R2
N-S
~H+~ HO / ~ R4
\a \
R1 I ~ R3 I / I
R2
43
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 14
O COOH
COOH R4
COOH
HZN ~ / R4
R4
\ / I \ ~ \ ~O~ \ \
R1 ~ R3 LH S ~ / ~ / R1 ~ R3 ~ I
R2 R1 ~ ~R3 I R2
R2
O COSCZHS O~H.-O
R4 BFa H \ SC2H5R4
etherat [nee
\ ~\ ~ \ \
R1 ~ R3 / I R1 I ~ R3 I ~ I
R2
R
~N-N
NHZNHZ \ " R4 Alkalation \ off R4
\ ~ \ ~ ~ \ ~ \
R1 ~ R3 / I R1 ~ R3 ~ I
R2 R2
44
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 15
R4
COOH H N COOH R4
z \
\ F I ~ \ b \
_I
R1 I / R3 LHMDS R1 I / R3 I / I
R2 R2
OH
N-
CZH50 ~ i
N
R4 NHZNH2 EtOH \ R4
\ ~ \
/ I R1 I / R3 I / I
R2
NHZNHCH3,EtOH
OH
N
i
N~
R4
\ ~ \
R1 I / R3 I / I
R2
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 16
R4
COOH H N COOH R4
\ H
\ F I / \ N \
I
R1 I / R3 LH~R1 I / R3 I / I
R2 R2
EtOOC Br
CHZCOOEt R4 R4
\ N \ 1)LDA \ a \
2 ~1 ~ / R3 ~ / I
R1 ~ ~R3 I
R2 R2
NHZ
EtOOC N3 EtOOC N=N~ R4
R4
NaN3/DMF Hz \ a \
\ \
R1 I / R3 I / I R1 / R3 / I
R2 R2
R
~N-N
a N alk lation ~ N
y HO
R4 ~ R4
\a \ \a \
R1 I / R3 I / I R1 I / R3 I / I
R2 R2
alkylation
N=N
HO ~ N-R
R4
\a \
R1 I / R3 I / I
R2
46
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 17
R4
CN HZN CN R4
\ F I i \ ~ \
I
R1 ~ ~ R3 ~HMDS R1 / R3 ~ / I
R2 R2
O~
OH ~S-O
NHZOH.HCI HZN ~ N R4 HN / N R4
NaOCH3/CH30H H SOCh/Et3N
\ N \ \ ~ \
R1 I ~ R3 I ~ I R1 I ~ R3 I ~ I
R2 R2
O S-O
R5~N i N
alkylation ~ R4
\ \
R1 I / R3 I / I
R2
47
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 18
R4
COOH HZN COOH R4
\ F I ~ ~ \ ~ \
R1 I ~ R3 LHMDS R1 I ~ R3 I / I
R2 R2
OH
N
H N ~ NH
HZN~~ O R4 R4
NHZNHz,PY-BOP ~t"aa/KOH/EtOH
\ a \ \ ~ \
R1 I ~ R3 I ~ I
R1 ~ ~R3 I
R2 R2
R5NC0/NaOH/H20
OH
N
N ~ N~R5
R4
H
\ N \
R1 I / R3 ~ / I
R2
48
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 19
R4
CN H2N CN R4
\ F I ~ I \ ~ \
R1 I ~ R3 ~HMDS R1 ~ ~ R3 I / I
R2 R2
O
OH \L0
J
H2N , N N , N
R4 CICOOCZH6 R4
NHZOH.HCI
NaOCHsICH30H pyridine
\ a \ ~ \ ~ \
R1 I ~ R3 I ~ I
R1 ~ ~R3 I
R2 R2
49
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 20
O ~'v2~'2~5
LHMDS ~ R4
\ F (1)PPh3,CBr4
\ ~ \
R1 ~ R3 (2)n-BuLi,
then CICOZCZH5R1 ~N \ R1 ~ R3 ~ I
R2 I / R2
HO Me0
-N -N
O
NHzOH.HCI \ a R4 CHzN~~ \ ~ R4
a
\ \ ~\ ~\
R1 I ~ R3 I / I R1 ~ R3 ~ I
R2 R2
Me0
1 )DPPA/Et3N
1)n-BuLiITHF 2)TMS(CHz)zOH -N
3)H O R4 3)n-Bu4NFITHF HzN \ O R4
~\ ~ ~\ a ~\
I R1 ~ R3 ~ I
R2
HO
-N
O
HBrIHOAc HZN \ R4
,a
R1 I ~ R3 I ~ I
R2
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 21
R4
COOH H N COOH R4
z \
\ F ~ , \ ~ \
_I
R1 I / R3 LHMDS R1 I ~ R3 I / I
RZ R2
N~ N
EtO2C ~ O R4 HZNHNOC ~ O R4
NHZNHZ,EtOH
(1 )(COCI)z
(2)CNCH2COZCZHS ~ \ ~ \ I \ I \
Et3NITHF R1 ~ R3 / I R1 ~ R3 ~ I
RZ R2
HO
-N
NH
HCllreflux H2N R4
\ ~ \
R1 ~ / R3 ~ / I
R2
51
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 22
R4
NOz HzN \ NO R4 NH R4
\ F ~ ~ I \z a \ IHl \z a \
R1 I ~ R3 LH~R1 I ~ R3 I ~ 1 R1 I ~ R3 I ~ I
R2 Ry R2
O
_ ' 'H
N3 a R4 - N~N N R4
I \ ~ \ CHz CHO
-~ -~ \ a I \
R1 ~ R3 ~ I
R1 ~ R3 ~ I
R2 R2
~ N
~~~OH R4 ~N OH R4
\ a \ I\ a I\
R1 I ~ R3 I ~ I R1 ~ R3 ~ I
R2 R2
Alternate synthesis: OH(TMS)
N R4 OH TMS N.~OH(TMS) N
HC=J ( i N R4 N R4
aI~ ~ +
\a \ \aI\
I I
R1 R3 I R1 ~ R3 ~ I R1 ~ R3 / I
R2 R2 R2
52
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 23
R4
NOz I"IzN \ NOz R4 NHz R4
\ F I / I \ b \ LHl I \ b I \
R1 I ~ R3 LHMDS~R1 I ~ R3 I ~ I R1 ~ R3 / I
R2 RZ R2
N-N
N ii \
N~ 3 R4 N~N~ R4
H
HC(OEt)3, NaN~ I \ N I \ -----~ I \
R1 ~ R3 ~ I R1 ~ R3 ~ I
R2 R2
N _~ N _\~
\ ~ / 'OAr
Iz, KMnO4,H2S04 ~N I R4 ArOH,Ky03 N R4
H
\ N \
\ \ I I
R1 I ~ R3 I ~ I R1 ~ R3 / I
R2
R2
R5
N
N ~ N ~O
NaH2PO~IPd-C ~N~O R4 Alkylation ~N R4
\ I I\
R1 I ~ R3 I ~ I R1 ~ R3 / I
R2 R2
53
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 24
R4
CHO H N CHO R4
2
\ F I i \ ~ \
I
I / LHMDS I / I /
R1 ~ ~R3 R1 ~ ~R3 I
R2 R2
NHZ
O
HO ~ N
R4
KCN/(NH4)zC03/NH4Cl
EtOH/Hz0 \ a \
I / I /
R1 ~ ~ R3 I
R2
Alternate Sythesis: NH
2
O
CHO HO ~ ~N
KCN/(NH4)2C03/NH4CI
I \ EtOHIH20
R1 / R3 I
R2 R1 ~ ~R3
R2
NH2
R4
HZN
HO ~ N R4
I
\ ~ \
LHMDS
I / I /
R1 ~ ~R3 I
R2
54
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Scheme 25
R4
COOH H2N \ COOHH R4
\ F I i I \ N \
LHMDS I
R1 ~ ~R3 R1 ~ ~R3 I
R2 R2
O
_ COOH
N, O
O N H R4
H N~COOH R4 DMF/SOCIZ
\ a \
I \ I \ I ~ I ~
R1 ~ ~R3 I
R1 ~ R3 ~ I
R2
R2
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
D. Uses
The disclosed compositions are useful as both prophylactic and
therapeutic treatments for diseases or conditions relating to chronic pain,
including neuropathic pain, as provided in the Summary section, as well as
diseases or conditions modulated by the MEK cascade. For example, in one
embodiment, the disclosed method relates to postoperative pain, phantom
limb pain, burn pain, gout, trigeminal neuralgia, acute herpetic and
postherpetic pain, causalgia, diabetic neuropathy, plexus avulsion, neuroma,
vasculitis, crush injury, constriction injury, tissue injury, post-surgical
pain,
arthritis pain, or limb amputation
For example, local injuries can be treated with local or topical
administration. Chronic pain affecting the entire body, such as diabetic
neuropathy can be treated with systemic administration (injection or orally)
of
a disclosed composition. Treatment for chronic pain (e.g., post-operative
pain) confined to the lower body can be administered centrally, e.g.,
epidurally. Formulations and methods of administration can include the use of
more than one MEK inhibitor, or a combination of a MEK inhibitor and another
pharmaceutical agent, such as an anti-inflammatory, analgesic, muscle
relaxing, or anti-infective agent. Preferred routes of administration are
oral,
intrathecal or epidural, subcutaneous, intravenous, intramuscular, and, for
non-human mammals, intraplantar, and are preferably epidural.
1. Dosages
Those skilled in the art will be able to determine, according to known
methods, the appropriate dosage for a patient, taking into account factors
such as age, weight, general health, the type of pain requiring treatment, and
the presence of other medications. In general, an effective amount will be
between 0.1 and 1000 mg/kg per day, preferably between 1 and 300 mg/kg
body weight, and daily dosages will be between 10 and 5000 mg for an adult
subject of normal weight. Commercially available capsules or other
56
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
formulations (such as liquids and film-coated tablets) of 100 mg, 200 mg, 300
mg, or 400 mg can be administered according to the disclosed methods.
2. Formulations
Dosage unit forms include tablets, capsules, pills, powders, granules,
aqueous and nonaqueous oral solutions and suspensions, and parenteral
solutions packaged in containers adapted for subdivision into individual
doses.
Dosage unit forms can also be adapted for various methods of administration,
including controlled release formulations, such as subcutaneous implants.
Administration methods include oral, rectal, parenteral (intravenous,
intramuscular, subcutaneous), intracisternal, intravaginal, intraperitoneal,
intravesical, local (drops, powders, ointments, gels, or cream), and by
inhalation (a buccal or nasal spray).
Parenteral formulations include pharmaceutically acceptable aqueous
or nonaqueous solutions, dispersion, suspensions, emulsions, and sterile
powders for the preparation thereof. Examples of carriers include water,
ethanol, polyols (propylene glycol, polyethylene glycol), vegetable oils, and
injectable organic esters such as ethyl oleate. Fluidity can be maintained by
the use of a coating such as lecithin, a surfactant, or maintaining
appropriate
particle size. Carriers for solid dosage forms include (a) fillers or
extenders,
(b) binders, (c) humectants, (d) disintegrating agents, (e) solution
retarders, (f)
absorption acccelerators, (g) adsorbants, (h) lubricants, (i) buffering
agents,
and (j) propellants.
Compositions may also contain adjuvants such as preserving, wetting,
emulsifying, and dispensing agents; antimicrobial agents such as parabens,
chlorobutanol, phenol, and sorbic acid; isotonic agents such as a sugar or
sodium chloride; absorption-prolonging agents such as aluminum
monostearate and gelatin; and absorption-enhancing agents.
57
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
3. Related compounds
The invention provides the disclosed compounds and closely related,
pharmaceutically acceptable forms of the disclosed compounds, such as
salts, esters, amides, hydrates or solvated forms thereof; masked or protected
forms; and racemic mixtures, or enantiomerically or optically pure forms.
Pharmaceutically acceptable salts, esters, and amides include
carboxylate salts (e.g., C ,_$ alkyl, cycloalkyl, aryl, heteroaryl, or non-
aromatic
heterocyclic), amino acid addition salts, esters, and amides which are within
a
reasonable benefit/risk ratio, pharmacologically effective, and suitable for
contact with the tissues of patients without undue toxicity, irritation, or
allergic
response. Representative salts include hydrobromide, hydrochloride, sulfate,
bisulfate, nitrate, acetate, oxalate, valerate, oleate, palmitate, stearate,
laurate, borate, benzoate, lactate, phosphate, tosylate, citrate, maleate,
fumarate, succinate, tartrate, naphthylate, mesylate, glucoheptonate,
lactiobionate, and laurylsulfonate. These may include alkali metal and alkali
earth cations such as sodium, potassium, calcium, and magnesium, as well as
non-toxic ammonium, quaternary ammonium, and amine cations such as
tetramethyl ammonium, methylamine, trimethylamine, and ethylamine. See,
for example, S.M. Berge, et al., "Pharmaceutical Salts," J. Pharm. Sci., 1977,
66:1-19 which is incorporated herein by reference. Representative
pharmaceutically acceptable amides of the invention include those derived
from ammonia, primary C ~_6 alkyl amines and secondary di (C ,_6 alkyl)
amines. Secondary amines include 5- or 6-membered heterocyclic or
heteroaromatic ring moieties containing at least one nitrogen atom and
optionally between 1 and 2 additional heteroatoms. Preferred amides are
derived from ammonia, C ~_3 alkyl primary amines, and di (C ~_2 alkyl)amines.
Representative pharmaceutically acceptable esters of the invention include
C ~_~ alkyl, C 5_7 cycloalkyl, phenyl, and phenyl(C ~_6)alkyl esters.
Preferred
esters include methyl esters.
The invention also includes disclosed compounds having one or more
functional groups (e.g., hydroxyl, amino, or carboxyl) masked by a protecting
group. Some of these masked or protected compounds are pharmaceutically
58
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
acceptable; others will be useful as intermediates. Synthetic intermediates
and processes disclosed herein, and minor modifications thereof, are also
within the scope of the invention.
HYDROXYL PROTECTING GROUPS
Hydroxyl protecting groups include: ethers, esters, and protection for 1,2-
and
1,3-diols. The ether protecting groups include: methyl, substituted methyl
ethers, substituted ethyl ethers, substituted benzyl ethers, silyl ethers and
conversion of silyl ethers to other functional groups.
Substituted Methyl Ethers
Substituted methyl ethers include: methoxymethyl, methylthiomethyl, t-
utylthiomethyl, (phenyldimethylsilyl) methoxymethyl, benzyloxymethyl, p-
ethoxybenzyloxymethyl, (4-methoxyphenoxy) methyl, guaiacolmethyl, t-
butoxymethyl, 4-pentenyloxymethyl, siloxymethyl, 2-methoxyethoxymethyl,
2,2,2-trichloroethoxymethyl, bis(2-chloro- ethoxy)methyl, 2-
(trimethylsilyl)ethoxymethyl, tetrahydropyranyl, 3-bromotetrahydro-pyranyl,
tetrahydrothiopyranyl, 1-methoxycyclohexyl, 4-methoxytetrahydropyranyl, 4-
methoxytetrahydrothio-pyranyl, 4-methoxytetrahydrothiopyranyl S,S-dioxido,
1-[(2-chloro-4-methyl)phenyl]-4-methoxypiperidin-4-yl, 1,4-dioxan-2-yl,
tetrahydrofuranyl, tetrahydrothiofuranyl, and 2,3,3a,4,5,6,7,7a-octahydro-
7,8,8-trimethyl-4,7-ethanobenzofuran-2-yl.
Substituted Ethyl Ethers
Substituted ethyl ethers include: 1-ethoxyethyl, 1-(2,chloroethoxy)ethyl,
1-methyl-1-methoxyethyl, 1-methyl-1-benzyloxyethyl, 1-methyl-1-benzyloxy-2-
fluoroethyl, 2,2,2-trichloroethyl, 2-trimethylsilyethyl, 2-
(phenylselenyl)ethyl, t
butyl, allyl, p-chlorophenyl, p-methoxyphenyl, 2,4-dinitrophenyl, and benzyl.
Substituted Benzyl Ethers
Substituted benzyl ethers include: p-methoxybenzyl, 3,4-dimethoxybenzyl,
o-nitrobenzyl, p-nitrobenzyl, p-halobenzyl, 2,6-dichlorobenzyl, p-cyanobenzyl,
p-phenylbenzyl, 2- and 4-picolyl, 3-methyl-2-picolyl N-oxido, diphenylmethyl,
p, p'-dinitrobenzhydryl, 5-dibenzosuberyl, triphenylmethyl, a-naphthyldiphenyl-
methyl, p-methoxyphenyldiphenylmethyl, di(p-methoxyphenyl)phenylmethyl,
59
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
tri-(p-methoxyphenyl)methyl, 4-(4'-bromophenacyloxy)phenyldiphenylmethyl,
4,4',4"-tris(4,5-dichlorophthalimidophenyl)methyl, 4,4',4"-
tris(levulinoyloxyphenyl) methyl, 4,4',4"tris(benzoyloxyphenyl)methyl, 3-
(imidazol-1-ylmethyl)bis(4',4"-dimethoxyphenyl)-methyl, 1,1-bis(4-
methoxyphenyl)-1'-pyrenylmethyl, 9-anthryl, 9-(9-phenyl) xanthenyl, 9-(9-
phenyl-10-oxo) anthryl, 1,3-benzodithiolan-2-yl, and benzisothiazolyl S,S-
dioxido.
Silyl Ethers
Silyl ethers include: trimethylsilyl, triethylsilyl, triisopropylsilyl,
dimethylisopropylsilyl, diethylisopropylsilyl, dimethylthexylsilyl, t-
butyldimethylsilyl, t-butyldiphenylsilyl, tribenzylsilyl, tri-p-xylylsilyl,
triphenylsilyl,
diphenylmethylsilyl, and t butylmethoxyphenylsilyl.
ESTERS
Esters protecting groups include: esters, carbonates, assisted cleavage,
miscellaneous esters, and sulfonates.
Esters
Examples of protective esters include: formate, benzoylformate, acetate,
chloroacetate, dichloroacetate, trichloroacetate, trifluoroacetate,
methoxyacetate, triphenylmethoxyacetate, phenoxyacetate, p-
chlorophenoxyacetate, p-P-phenylacetate, 3-phenylpropionate, 4-
oxopentanoate (levulinate), 4,4-(ethylenedithio) pentanoate, pivaloate,
adamantoate,crotonate,4-methoxycrotonate, benzoate, p-phenylbenzoate,
and 2,4,6-trimethylbenzoate (mesitoate).
Carbonates
Carbonates include: methyl, 9-fluorenylmethyl, ethyl, 2,2,2-trichloroethyl,
2-(trimethylsilyl) ethyl, 2-(phenylsulfonyl) ethyl, 2-(triphenylphosphonio)
ethyl,
isobutyl, vinyl, allyl, p-nitrophenyl, benzyl, p-methoxybenzyl, 3,4-
dimethoxybenzyl, o-nitrobenzyl, p-nitrobenzyl, S-benzyl thiocarbonate, 4-
ethoxy-1-naphthyl, and methyl dithiocarbonate.
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Assisted Cleavage
Examples of assisted cleavage protecting groups include: 2-iodobenzoate, 4-
azido-butyrate, 4-nitro-4-methylpentanoate, o-(dibromomethyl) benzoate, 2-
formylbenzene-sulfonate, 2-(methylthiomethoxy) ethyl carbonate, 4-
(methylthiomethoxymethyl) benzoate, and 2-(methylthiomethoxymethyl)
benzoate.
Miscellaneous Esters
In addition to the above classes, miscellaneous esters include: 2,6-dichloro-4-
methylphenoxyacetate, 2,6-dichloro-4-(1,1,3,3-tetramethylbutyl)
phenoxyacetate,
2,4-bis(1,1-dimethylpropyl) phenoxyacetate, chlorodiphenylacetate,
isobutyrate, monosuccinoate, (E]-2-methyl-2-butenoate (tigloate), o-
(methoxycarbonyl) benzoate, p-P-benzoate, a-naphthoate, nitrate, alkyl N,N,N
' N'-tetramethylphosphorodiamidate, N-phenylcarbamate, borate,
dimethylphosphinothioyl, and 2,4-dinitrophenylsulfenate.
W ilfnnatac
Protective sulfates includes: sulfate, methanesulfonate(mesylate),
benzylsulfonate, and tosylate.
PROTECTION FOR 1,2- AND 1,3-DIOLS
The protection for 1,2 and 1,3-diols group includes: cyclic acetals and
ketals,
cyclic ortho esters, and silyl derivatives.
Cyclic Acetals and Ketals
Cyclic acetals and ketals include: methylene, ethylidene, 1-t-butylethylidene,
1-phenylethylidene, (4-methoxyphenyl) ethylidene, 2,2,2-trichloroethylidene,
acetonide (isopropylidene), cyclopentylidene, cyclohexylidene,
cycloheptylidene, benzylidene, p-methoxybenzylidene, 2,4-
dimethoxybenzylidene, 3,4-dimethoxybenzylidene, and 2-nitrobenzylidene.
Cyclic Ortho Esters
Cyclic ortho esters include: methoxymethylene, ethoxymethylene, dimethoxy-
methylene, 1-methoxyethylidene, 1-ethoxyethylidine, 1,2-
dimethoxyethylidene,
61
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
a-methoxybenzylidene, 1-(N,N-dimethylamino)ethylidene derivative, a-(N,N-
dimethylamino) benzylidene derivative, and 2-oxacyclopentylidene.
PROTECTION FOR THE CARBOXYL GROUP
ESTERS
Ester protecting groups include: esters, substituted methyl esters, 2-
substituted ethyl esters, substituted benzyl esters, silyl esters, activated
esters, miscellaneous derivatives, and stannyl esters.
Substituted Methyl Esters
Substituted methyl esters include: 9-fluorenylmethyl, methoxymethyl,
methylthiomethyl, tetrahydropyranyl, tetrahydrofuranyl, methoxyethoxymethyl,
2-(trimethylsilyl)ethoxy-methyl, benzyloxymethyl, phenacyl, p-bromophenacyl,
a-methylphenacyl, p-methoxyphenacyl, carboxamidomethyl, and N-
phthalimidomethyl.
2-Substituted Ethyl Esters
2-Substituted ethyl esters include: 2,2,2-trichloroethyl, 2-haloethyl, ~-
chloroalkyl, 2-(trimethylsily)ethyl, 2-methylthioethyl, 1,3-dithianyl-2-
methyl,
2(p-nitrophenylsulfenyl)-ethyl, 2-(p-toluenesulfonyl)ethyl, 2-(2'-
pyridyl)ethyl, 2-
(diphenylphosphino)ethyl, 1-methyl-1-phenylethyl, t butyl, cyclopentyl,
cyclohexyl, allyl, 3-buten-1-yl, 4-(trimethylsily)-2-buten-1-yl, cinnamyl, a-
methylcinnamyl, phenyl, p-(methylmercapto)-phenyl, and benzyl.
Substituted Benzyl Esters
Substituted benzyl esters include: triphenylmethyl, diphenylmethyl,
bis(o-nitrophenyl)methyl, 9-anthrylmethyl, 2-(9,10-dioxo)anthrylmethyl, 5-
dibenzo-suberyl, 1-pyrenylmethyl,2-(trifluoromethyl)-6-chromylmethyl, 2,4,6-
trimethylbenzyl, p-bromobenzyl, o-nitrobenzyl, p-nitrobenzyl, p-
methoxybenzyl, 2,6-dimethoxybenzyl, 4-(methylsulfinyl)benzyl, 4-sulfobenzyl,
piperonyl, and 4-P-benzyl.
Silyl Esters
Silyl esters include: trimethylsilyl, triethylsilyl, t butyldimethylsilyl, i-
propyldimethylsilyl, phenyldimethylsilyl, and di- t-butylmethylsilyl.
62
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Miscellaneous Derivatives
Miscellaneous derivatives includes: oxazoles, 2-alkyl-1,3-oxazolines, 4-alkyl-
5-oxo-1,3-oxazolidines, 5-alkyl-4-oxo-1,3-dioxolanes, ortho esters, phenyl
group, and pentaaminocobalt(III) complex.
Stannyl Esters
Examples of stannyl esters include: triethylstannyl and tri-n-butylstannyl.
AMIDES AND HYDRAZIDES
Amides include: N,N-dimethyl, pyrrolidinyl, piperidinyl, 5,6-
dihydrophenanthridinyl, o-nitroanilides, N-7-nitroindolyl, N-8-nitro-1,2,3,4-
tetrahydroquinolyl, and p-P-benzenesulfonamides. Hydrazides include: N-
phenyl, N,N'-diisopropyl and other dialkyl hydrazides.
PROTECTION FOR THE AMINO GROUP
CARBAMATES
Carbamates include: carbamates, substituted ethyl, assisted cleavage,
photolytic cleavage, urea-type derivatives, and miscellaneous carbamates.
Carbamates
Carbamates include: methyl and ethyl, 9-fluorenylmethyl, 9-(2-
sulfo)fluorenylmethyl, 9-(2,7-dibromo)fluorenylmethyl, 2,7-di-t-butyl-[9-
(10,10-
dioxo-10,10,10,10-tetrahydro- thioxanthyl)]methyl, and 4-methoxyphenacyl.
Substituted Ethyl
Substituted ethyl protective groups include: 2,2,2-trichloroethyl, 2-
trimethylsilylethyl, 2-phenylethyl, 1-(1-adamantyl)-1-methylethyl, 1,1-
dimethyl-
2-haloethyl, 1,1dimethyl-2,2-dibromoethyl, 1,1-dimethyl-2,2,2-trichloroethyl,
1-
methyl-1-(4-biphenylyl)ethyl, 1-(3,5-di-t butylphenyl)-1-methylethyl, 2-(2'-
and
4'-pyridyl)ethyl, 2-(N,N-icyclohexylcarboxamido)- ethyl, t-butyl, 1-adamantyl,
vinyl, allyl, 1-isopropylallyl, connamyl, 4-nitrocinnamyl, quinolyl, N-
hydroxypiperidinyl, alkyldithio, benzyl, p-methoxybenzyl, p-nitrobenzyl, p-
bromobenzyl, p-chlorobenzyl, 2,4dichlorobenzyl, 4-methylsulfinylbenzyl,
9-anthrylmethyl, and diphenylmethyl.
63
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Assisted Cleavage
Protection via assisted cleavage includes: 2-methylthioethyl,
2-methylsulfonylethyl, 2-(p-toluenesulfonyl)ethyl, [2-(1,3-dithianyl)]methyl,
4-methylthiophenyl, 2,4-dimethyl-thiophenyl, 2-phosphonioethyl,
2-triphenylphosphonioisopropyl, 1,1-dimethyl-2cyanoethyl, m-chloro-p-
acyloxybenzyl, p-(dihydroxyboryl)benzyl, 5-benzisoxazolyl-methyl, and
2-(trifluoromethyl)-6-chromonylmethyl.
Photolytic Cleavage
Photolytic cleavage methods use groups such as: m-nitrophenyl, 3,5-
dimethoxybenzyl, o-nitrobenzyl, 3,4-dimethoxy-6-nitrobenzyl, and phenyl(o-
nitrophenyl)methyl.
Urea-Type Derivatives
Examples of of urea-type derivatives include: phenothiazinyl-(10)-carbonyl
derivative, N'-p-toluenesulfonylaminocarbonyl, and N'-
phenylaminothiocarbonyl.
Miscellaneous Carbamates
In addition to the above, miscellaneous carbamates include: t amyl, S-benzyl
thiocarbamate, p-cyanobenzyl, cyclobutyl, cyclohexyl, cyclopentyl,
cyclopropylmethyl, p-decyloxy-benzyl, diisopropylmethyl, 2,2-
dimethoxycarbonylvinyl, o-(N,N-dimethyl-carboxamido)-benzyl, 1,1-dimethyl-
3(N,N-dimethylcarboxamido)propyl, 1,1-dimethyl-propynyl, di(2-pyridyl)methyl,
2-furanylmethyl, 2-iodoethyl, isobornyl, isobutyl, isonicotinyl, p(p'-
methoxyphenyl- azo)benzyl, 1-methylcyclobutyl, 1-methylcyclohexyl, 1-
methyl-1-cyclopropyl- methyl, 1-methyl-(3,5-dimethoxyphenyl)ethyl, 1-methyl-
1 (p-henylazophenyl)- ethyl, 1-methyl-1-phenylethyl, 1-methyl-1-(4-
pyridyl)ethyl, phenyl, p-(phenylazo)benzyl, 2,4,6-tri-t-butylphenyl, 4-
(trimethylammonium) benzyl, and 2,4,6-trimethylbenzyl.
64
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
AMIDES
Amides
Amides includes: N-formyl, N-acetyl, N-chloroacetyl, N-trichloroacetyl,
N-trifluoroacetyl, N-phenylacetyl, N-3-phenylpropionyl, N-picolinoyl, N-3-
pyridyl-carboxamide, N-benzoylphenylalanyl derivative, N-benzoyl, and N-p-
phenylbenzoyl.
Assisted Cleavage
Assisted cleavage groups include: N-o-nitrophenylacetyl, N-o-
nitrophenoxyacetyl, N-acetoacetyl, (N'-dithiobenzyloxycarbonylamino)acetyl,
N-3-(p-hydroxphenyl) propionyl, N-3-(o-nitrophenyl)propionyl, N-2-methyl-2-
(o-nitrophenoxy)propionyl, N-2-methyl-2-(o-phenylazophenoxy)propionyl, N-4-
chlorobutyryl, N-3-methyl-3-nitrobutyryl, N-o-nitrocinnamoyl, N-
acetylmethionine derivative, N-o-nitrobenzoyl, N-o-
(benzoyloxymethyl)benzoyl, and 4,5-diphenyl-3-oxazolin-2-one.
Cyclic Imide Derivatives
Cyclic imide derivatives include: N-phthalimide, N-dithiasuccinoyl,
N-2,3-diphenyl-maleoyl, N-2,5-dimethylpyrrolyl,
N-1,1,4,4-tetramethyldisilylazacyclopentane adduct, 5-substituted
1,3-dimethyl-1,3,5-triazacyclohexan-2-one, 5-substituted 1,3-dibenzyl-
1,3,5-triazacyclohexan-2-one, and 1-substituted 3,5-dinitro-4-pyridonyl.
SPECIAL -NH PROTECTIVE GROUPS
Protective groups for - NH include: N-alkyl and N-aryl amines, imine
derivatives, enamine derivatives, and N-hetero atom derivatives (such as N-
metal, N-N, N-P, N-Si, and N-S), N-sulfenyl, and N-sulfonyl.
N-Alkyl and N-Ar~Amines
N-alkyl and N-aryl amines include: N-methyl, N-allyl,
N-[2-(trimethylsilyl)ethoxyl]-methyl, N-3-acetoxypropyl,
N-(1-isopropyl-4-nitro-2-oxo-3-pyrrolin-3-yl), quaternary ammonium salts,
N-benzyl, N-di(4-methoxyphenyl)methyl, N-5-dibenzosuberyl,
N-triphenylmethyl, N-(4-methoxyphenyl)diphenylmethyl, N-9-phenylfluorenyl,
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
N-2,7-dichloro-9-fluorenylmethylene, N-ferrocenylmethyl, and
N-2-picolylamine N'-oxide.
Imine Derivatives
Imine derivatives include: N-1,1-dimethylthiomethylene, N-benzylidene,
N-p-methoxybenzylidene, N-diphenylmethylene,
N-[(2-pyridyl)mesityl]methylene, N-(N',N'-dimethylaminomethylene),
N,N'-isopropylidene, N-p-nitrobenzylidene, N-salicylidene,
N-5-chlorosalicylidene, N-(5-chloro-2-hydroxyphenyl)phenyl-methylene, and
N-cyclohexylidene.
Enamine Derivative
An example of an enamine derivative is
N-(5,5-dimethyl-3-oxo-1-cyclohexenyl).
N-Hetero Atom Derivatives
N-metal derivatives include: N-borane derivatives, N-diphenylborinic acid
derivative, N-[phenyl(pentacarbonylchromium- or -tungsten)]carbenyl, and
N-copper or N-zinc chelate. Examples of N-N derivatives include: N-nitro,
N-nitroso, and N-oxide. Examples of N-P derivatives include:
N-diphenylphosphinyl, N-dimethylthiophosphinyl, N-diphenylthiophosphinyl,
N-dialkyl phosphoryl, N-dibenzyl phosphoryl, and N-diphenyl phosphoryl.
Examples of N-sulfenyl derivatives include: N-benzenesulfenyl,
N-o-nitrobenzenesulfenyl, N-2,4-dinitrobenzenesulfenyl,
N-pentachlorobenzenesulfenyl, N-2-nitro-4-methoxy-benzenesulfenyl, N-
triphenylmethylsulfenyl, and N-3-nitropyridinesulfenyl. N-sulfonyl derivatives
include: N-p-toluenesulfonyl, N-benzenesulfonyl, N-2,3,6-trimethyl-
4-methoxybenzenesulfonyl, N-2,4,6-trimethoxybenzenesulfonyl, N-
2,6-dimethyl-4-methoxy-benzenesulfonyl, N-pentamethylbenzenesulfonyl, N-
2,3,5,6-tetramethyl-4-methoxybenzene- sulfonyl, N-
4-methoxybenzenesulfonyl, N-2,4,6-trimethylbenzenesulfonyl, N-
2,6-dimethoxy- 4-methylbenzenesulfonyl, N-
2,2,5,7,8-pentamethylchroman-6-sulfonyl, N-methanesulfonyl,
66
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
N-~trimethylsilylethanesulfonyl, N-9-anthracenesulfonyl, N-
4-(4',8'-dimethoxynaphthylmethyl)-benzenesulfonyl, N-benzylsulfonyl, N-
trifluoromethylsulfonyl, and N-phenacylsulfonyl.
S Disclosed compounds which are masked or protected may be prodrugs,
compounds metabolized or otherwise transformed in vivo to yield a disclosed
compound, e.g., transiently during metabolism. This transformation may be a
hydrolysis or oxidation which results from contact with a bodily fluid such as
blood, or
the action of acids, or liver, gastrointestinal, or other enzymes.
Features of the invention are further described in the examples below.
67
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
E. Examples
BIOLOGICAL EXAMPLES
Example 1
Effect of PD 198306 on streptozocin-induced static allodynia
Animals
Male Sprague Dawley rats (250-300g), obtained from Bantin and
Kingman, (Hull, U.K.) were housed in groups of 3. All animals were kept under
a 12h light/dark cycle (lights on at 07h OOmin) with food and water ad
libitum.
All experiments were carried out by an observer blind to drug treatments.
Development of diabetes in the rat
Diabetes was induced in rats by a single i.p. injection of streptozocin
(50 mg/kg) as described previously (Courteix et al., 1993).
Evaluation of static allodynia
Mechanical hypersensitivity was measured using Semmes-Weinstein
von Frey hairs (Stoelting, Illinois, U.S.A.). Animals were placed into wire
mesh
bottom cages allowing access to the underside of their paws. Animals were
habituated to this environment prior to the start of the experiment.
Mechanical
hypersensitivity was tested by touching the plantar surface of the animals
right
hind paw with von Frey hairs in ascending order of force ( 0.7, 1.2, 1.5, 2,
3.6,
5.5, 8.5, 11.8, 15.1 and 29g) for up to 6 sec. Once a withdrawal response was
established, the paw was re-tested, starting with the next descending von
Frey hair until no response occurred. The highest force of 29 g lifted the paw
as well as eliciting a response, thus represented the cut off point. The
lowest
amount of force required to elicit a response was recorded as the paw
withdrawal threshold (PWT) in grams.
68
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Drugs
PD 198306 [N-Cyclopropylmethoxy-3,4,5-trifluoro-2-(4-iodo-2-methyl-
phenylamino)-benzamideJ and CI-1008 (pregabalin) were synthesized at
Parke-Davis (Ann Arbor, MI, USA). PD 198306 was suspended in
cremophor:ethanol:water (1:1:8) vehicle. Pregabalin was dissolved in water.
Both compounds were administered orally. Streptozocin (Aldrich, UK) was
dissolved in 0.9% w/v NaCI and administered intraperitoneally. Drug
administrations were made in a volume of 1 ml/kg.
Statistics
The static allodynia data were analysed using a Kruskall-Wallis ANOVA
for non-parametric results, followed when significant by Mann-Whitney's t
test.
Experimental protocol
Static allodynia was assessed with von Frey hairs, before (baseline,
BL) and 1 h after oral administration of PD 198306 (30mg/kg, p.o.), vehicle
(cremophor:ethanol:water, 1:1:8) or pregabalin (30mg/kg, p.o.) (test).
Animals were administered again the same compounds on the following day,
both in the morning and the afternoon. Static allodynia was assessed only
before and 1 h after the afternoon administration, in order to minimise the
habituation of the animals to the testing conditions. Animals treated with
pregabalin received water in the morning administration, in order to avoid the
potential development of tolerance to the compound with repeated
administration.
Day 1: D
a.m.: PD 198306
Water
Vehicle
p.m.: BL p.m.: BL
69
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
PD 198306 PD 198306
Pregabalin Pregabalin
Vehicle Vehicle
Test Test
RESULTS
A single administration of pregabalin (30mg/kg, p.o.) significantly
blocked streptozocin-induced static allodynia 1 h after administration. In
contrast, a single administration of PD 198306 (30mg/kg, p.o) had no effect on
streptozocin-induced static allodynia 1 h after administration (see below).
However, after the compound had been administered twice more on the
following day, it significantly blocked streptozocin-induced static allodynia
1 h
after the third administration. The effects had disappeared by the following
day (see FIG. 1 ).
Example 2
MATERIALS AND METHODS
Animals
Male Sprague Dawley rats (250-300g), obtained from Charles River,
Margate, U.K.) were housed in groups of 3-6. All animals were kept under a
12h light/dark cycle (lights on at 07h OOmin) with food and water ad libitum.
All
experiments were carried out by an observer blind to drug treatments.
Diabetes was induced in rats by a single i.p. injection of streptozocin
(50mg/kg) as described previously (Courteix et al., 1993).
Development of Chronic Constriction Injury in the rat
Animals were anaesthetised with 2% isoflurane 1:4 02/N20 mixture
maintained during surgery via a nose cone. The sciatic nerve was ligated as
previously described by Bennett and Xie, 1988. Animals were placed on a
homeothermic blanket for the duration of the procedure. After surgical
preparation the common sciatic nerve was exposed at the middle of the thigh
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
by blunt dissection through biceps femoris. Proximal to the sciatic
trifurcation,
about 7mm of nerve was freed of adhering tissue and 4 ligatures (4-0 silk)
were tied loosely around it with about 1 mm spacing. The incision was closed
in layers and the wound treated with topical antibiotics.
Intrathecal infections
PD 198306 and pregabalin were administered intrathecally in a volume
of 10 p1 using a 100 p1 Hamilton syringe by exposing the spine of the rats
under
brief isoflurane anaesthesia. Injections were made into the intrathecal space
between lumbar region 5-6 with a 10 mm long 27 gauge needle. Penetrations
were judged successful if there was a tail flick response. The wound was
sealed
with an autoclip and rats appeared fully awake within 2-3 min following
injection.
Evaluation of static allod
Mechanical hypersensitivity was measured using Semmes-Weinstein
von Frey hairs (Stoelting, Illinois, U.S.A.). Animals were placed into wire
mesh
bottom cages allowing access to the underside of their paws. Animals were
habituated to this environment prior to the start of the experiment.
Mechanical
hypersensitivity was tested by touching the plantar surface of the animals
right
hind paw with von Frey hairs in ascending order of force ( 0.7, 1.2, 1.5, 2,
3.6,
5.5, 8.5, 11.8, 15.1 and 29g) for up to 6sec. Once a withdrawal response was
established, the paw was re-tested, starting with the next descending von
Frey hair until no response occurred. The highest force of 29g lifted the paw
as well as eliciting a response, thus represented the cut off point. The
lowest
amount of force required to elicit a response was recorded as the paw
withdrawal threshold (PWT) in grams.
Experimental protocol
Static allodynia was assessed with von Frey hairs, before (baseline,
BL) and 0.5h, 1 h and 2h after intrathecal or intraplantar administration of
PD
198306 (1-30~g, i.t.), vehicle (cremophor:ethanol:water, 1:1:8) or pregabalin
(10~g, i.t). For oral administration experiments, static allodynia was
assessed
71
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
with von Frey hairs, before (baseline, BL) and 1 h after oral administration
of
PD 198306 (3-30mg/kg, p.o.), vehicle (cremophor:ethanol:water, 1:1:8) or
pregabalin (30mg/kg, p.o.). Animals were administered again the same
compounds on the following day, both in the morning and the afternoon. Static
allodynia was assessed before and 1 h after the morning administration. In the
afternoon static allodynia was assessed before, 1 h, 2h and 3h after
administration for streptozocin treated animals. CCI animals were assessed
before, 1 h and 2h after administration
Druas used
PD 198306 and pregabalin were synthesised at Parke-Davis (Ann
Arbor, MI, USA). PD 198306 was suspended in cremophor:ethanol:water
(1:1:8) vehicle. Pregabalin was dissolved in water. Both compounds were
administered orally, intrathecally or intraplantar in volumes of 1 ml/kg, 101
and
100w1 respectively. Streptozocin (Aldrich, UK) was dissolved in 0.9% w/v NaCI
and administered intraperitoneally in a volume of 1 ml/kg.
Statistics
Data were analysed using a Kruskall-Wallis ANOVA for non-parametric
results, followed when significant by Mann-Whitney's t test vs vehicle group.
RESU LTS
1. Effects of PD 198306 on static allodynia, following systemic
administration
1.1. Effect of PD198306 on streptozocin-induced static allodynia
A single administration of pregabalin (30mg/kg, p.o.) significantly blocked
streptozocin-induced static allodynia 1h after administration. In contrast, a
single administration of PD 198306 (3-30mg/kg, p.o) had no effect on
streptozocin-induced static allodynia 1 h after administration (FIG. 2).
However, after the compound had been administered twice more on the
following day, PD 198306 (30mg/kg) significantly blocked streptozocin-
induced static allodynia for 2h after the third administration (FIG. 2).
72
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
1.2. Effect of PD198306 on CCI-induced static allod~nia
A single administration of pregabalin (30mg/kg, p.o.) significantly blocked
CCI-
induced static allodynia 1 h after administration. In contrast, neither a
single or
multiple administration of PD 198306 (3-30mg/kg, p.o) had any effect on CCI-
induced static allodynia (FIG. 3).
2. Effects of PD 198306 on static allodynia, following intrathecal
administration
Intrathecally administered PD198306 (1-30p.g) dose-dependently blocked the
maintenance of static allodynia in both streptozocin (FIG. 4) and CCI animals
(FIG. 5) with respective MEDs of 3 and 10 fig. This antiallodynic effect
lasted
for 1 h.
3. Effects of PD 198306 on static allodynia, following intraplantar
administration
An intrathecal administration of PD 198306 (30~g) significantly blocked static
allodynia in both neuropathic pain models (FIGS. 6,7). In contrast, a single
administration of PD 198306 at a dose 100-fold higher (3mg/100p,1) directly
into the paw had no effect on streptozocin (FIG. 6) or CCI-induced static
allodynia (FIG. 7).
REFERENCES
Bennett GJ, Xie Y-K. A peripheral mononeuropathy in rat that produces
disorders of pain sensation like those seen in man. Pain 1988;33:87-107.
Courteix C, Eschalier A and Lavarenne J. Streptozocin -induced rats:
behavioural evidence for a model of chronic pain. Pain 1993;53:81-8
73
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
Example 3
Effect of other MEK inhibitors in a neuropathic pain model in the rat
SUMMARY
The effect of several MEK inhibitors, with different binding affinities, has
been investigated in the CCI model of neuropathic pain in the rat, by
assessing
static allodynia with von Frey hairs. Intrathecal administration of PD219622
or
PD297447 (30~g) had no significant effect on allodynia. This lack of effect
may reflect the low affinity or solubility of the compounds. However,
intrathecal administration of PD 254552 or PD 184352 (30~g), which posses
higher binding affinities, blocked the maintenance of static allodynia in CCI
animals. The antiallodynic effect was only evident for 30min post-injection
and
thus, shorter than the one observed for pregabalin (100pg). The magnitude of
the effect was similar for 30~g of PD 184352 and 100~,g of pregabalin. From
this study it is concluded that MEK inhibitors exert an antiallodynic effect
in
CCI-induced neuropathic rats when administered intrathecally, and that the
antiallodynic effect correlates with the affinity of the compounds.
The animals and methods for developing chronic constriction injury in
the rat, injecting test compounds, and evaluation of static allodynia were
according to Example 2 above. PD219622, PD297447, PD 184352, PD
254552 and pregabalin were administered intrathecally at doses of 30~,g for
all
PD compounds and 100~g for pregabalin. Static allodynia was assessed with
von Frey hairs, before (baseline, BL) and 0.5h, 1 h and 2h after intrathecal
administration of the compounds
Drubs used
PD297447, PD219622, PD 254552, PD 184352 (CI-1040), and pregabalin
were synthesised at Parke-Davis (Ann Arbor, MI, USA). PD297447,
PD219622, PD 254552 and PD 184352 were suspended in
74
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
cremophor:ethanol:water (1:1:8) vehicle. Pregabalin was dissolved in water.
All
compounds were administered intrathecally in a 10p1 volume.
Statistics
Data were analysed using a Kruskall-Wallis ANOVA for non-parametric
results, followed when significant by Mann-Whitney's t test vs vehicle group.
RESULTS
Intrathecally administered PD297447 or PD219622 (30~,g) had no
significant effect on allodynia. This lack of effect may reflect the low
affinity of
the compounds (965nM and 100nM respectively). However, intrathecal
administration of PD 184352 or PD 254552 (30~g) blocked the maintenance
of static allodynia in CCI animals (see FIG. 8). These compounds possess
higher affinity (2 and 5 nM respectively). The antiallodynic effect was only
evident for 30min post-injection and thus, shorter than the one observed for
pregabalin (100p,g). The magnitude of the effect was similar for 30~g of PD
184352 and 100~.g of pregabalin.
The results indicate that MEK inhibitors exert an antiallodynic effect in
CCI-induced neuropathic rats when administered intrathecally, and that the
antiallodynic effect correlates with the affinity of the compounds.
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
CHEMICAL EXAMPLES
EXAMPLE 1
S 14-Chloro-2-(4,4-Dimethyl-4,5-dih~dro-oxazol-2-yl)-phenyll-(4-iodo-2-methyl-
ehenyl)-amine (18). (Scheme 2, R~=CI, R2=R3=H, R4=CH3)
a). A mixture of 5-chloro-2-methoxybenzoic acid 16 (14.8 g, 0.0793 mole)
and SOC12 (28.31 g, 14.97 ml, 0.1584 mole) was refluxed for 2 hours and
excess SOC12 removed leaving a white residue. The solid was dissolved in
CH2C12 and added to a solution of 2-amino-2-methyl-1-propanol (13.98 g,
14.97 ml, 0.1584 mole) in CH2C12 cooled with ice-bath. The ice-bath was
removed, and after stirring at room temperature for 3 hours a white solid
precipitated. The precipitate was separated by filtration and discarded. The
filtrate was concentrated leaving a thick colorless oil. SOC12 (17.4 ml) was
added to the oil dropwise. An exothermic reaction took place resulting in to a
freely flowing solution. After stirring for 30 minutes, the reaction mixture
was
poured in to Et20 (200 ml). An oil separated out. The Et20 layer was
removed by decanting and discarded. The oily residue was dissolved in a
minimum amount of water, basified with aqueous 20% NaOH, and extracted
with Et20. The Et20 layer was dried (K2C03) and concentrated to give 17 as
tan oil. Yield 14.63 g (77%).
b). LDA (5 ml of 2.0 M solution in THF) was added to a solution of 4-iodo-
2-methylaniline (2.33 g, 0.010 mole) in THF (15 ml) at-78 °C. The
mixture
was stirred at -78 °C for 30 minutes. To this, a solution of 17 (1.199
g, 0.005
mole) in THF (15 ml) was added. The mixture stirred for 16 hours as it
warmed up to room temperature. The reaction mixture was quenched with
aqueous NH4C1 and extracted with Et20. The Et20 layer was dried (MgS04)
and concentrated to give crude 18 as brown oil. The oil was purified on silica
column chromatography. Eluting with CH2C12 gave pure 1.7 g (77%) of 18 as
brown oil. Four hundred and nine milligrams of the oil were dissolved in Et20
and treated with Et20-HCI giving the HCI salt as a light yellow solid
76
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
precipitate. Yield 356.4 mg (81 %); mp 324-330 °C; Anal. Calcd/found
for
C~BH~$N20CILHC1Ø5H20: C, 44.47/44.32; H, 4.15/3.94; N, 5.76/5.66.
EXAMPLE 2
[2,3-Difluoro-6-(1 H-tetrazol-5-yl)-phenyll-(4-iodo-2-methyl-phenyl)-amine
N=N
CN N ~ NH
H H
\ N I \ NaN3, (C2Hs)3N.HC1 N
\ \
toluene/reflux
~F I ~ F ~ I
F F
[2,3-Difluoro-6-cyano-phenyl]-(4-iodo-2-methyl-phenyl)-amine (1.11g,
3mmol) and Sodium azide(0.255g, 3.9mmol) and triethylamine hydrochloride
(0.537g, 3.9mmol) were all suspended in 10m1 toluene and stirred at
100°C
for 12 hours. The mixture was concentrated and the residue purified by
column chromatography with ethyl acetate/methanol (10/1) to give the product
as a foam-like solid. The yield: ~50%
m.p: 83.4-88.7°C
H NMR(CDC13, 400Hz): 8/ppm 7.69(1 H, m, Phenyl-H); 7.42(1 H, s, Phenyl-H);
7.27(1 H, m, Phenyl-H); 6.91 (1 H, dd, J=16.2Hz, 8.3Hz, Phenyl-H); 6.40(1 H,
dd, Phenyl-H); 2.28(3H, s, CH3)
77
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
EXAMPLE 3
[6-(4,4-DimethKl-4,5-dihydro-oxazol-2-yl)-2,3-difluoro a~henyll-(4-iodo-2-
methyl-phenyl)-amine
COOHH N ~ O
N ~ (1) (cocl)z H
(2)2-amino-2-methyl-1-propanol ~ N
F / I
F 'F I
F
A solution of 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid
(1.17g, 3mmol), oxalyl chloride (0.457g, 3.6mmol) in 30m1 dichloromethane
was treated with 2 drops of dimethylformamide, stirred at room temperature
for 3 hours then concentrated. The residue was dissolved in 25 ml
dichloromethane then the solution was added dropwise to a solution of 2
amino-2-methyl-1-propanol (0.623g, 7mmol) in 25 ml dichloromethane at
0°C,
then stirred at room temperature for 12 hours, filtered off the precipitate,
the
filtration was washed with water, 5% aqueous sodium bicarbonate, 1 N HCI,
brine, dried with sodium sulfate. Concentration gave the crude product, then
resuspended in 25 ml chloroform, then thionyl chloride was added at 0°C
and
stirred at room temperature for 15 hours, then concentrated and the residue
was dissolved in 30 ml dichloromethane , 1 N HCI was added to adjusted the
pH value to 11, the separated and extracted with chloroform, dried with
sodium sulfate. Concentrated and then run column with
hexanes/dichloromethane (20/1 ) to give the compound as a white crystal.
The yield: 65%
m.p.: 103.7-104.4°C
H NMR(CDC13, 400Hz): 8/ppm 10.2(1 H, s, NH), 7.48-7.58(1 H, m, Phenyl-H);
7.48(1 H, s, Phenyl-H); 7.38(1 H, d, J=8.5Hz, Phenyl-H), 6.66-6.72(1 H, m,
Phenyl-H); 6.58(1 H, t, J=8.OHz, Phenyl-H); 4.01 (2H, s, -CH2-); 2.31 (3H, s,
Phenyl-CH3); 1.32(6H, s, -C(CH3)2-)
78
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
EXAMPLE 4
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid methyl ester
COOH O O
H H
N ~ ~ HzS04/CH30H
reflux
/ F / I / F / I
F F
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid (5g) was dissolved
in 100m1 methanol and 5 drops of concentrated sulfuric acid was added, reflux
for 4 days. Run column with hexanes/dichloromethane to give the product as
a white solid, yield: 50%.
m.p.:90.1-90.4°C
' H NMR(CDC13, 400Hz): 8/ppm 8.92(1 H, s, NH), 7.75-7.78(1 H, m, Phenyl-H);
7.49(1 H, s, Phenyl-H); 7.38(1 H, dd, J=8.5Hz, 2.OHz, Phenyl-H), 6.66-6.73(1
H,
m, Phenyl-H); 6.56-6.60(1 H, m, Phenyl-H); 3.88(3H, s, -OCH3); 2.30(3H, s,
Phenyl-CH3)
79
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
EXAMPLE 5
5-[3.4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-phen~-4H-f 1,2,41triazol-3-
lay mine
HZN
\ ~- N
O O N ~ NH
H H
N ~ ~ aminohuanidine nitrate N
NaOCH3/CH30H
\F I ~ F / I
F F
Aminoguanidine nitrate (1.65g, 12mmol) was added to a solution of sodium
methoxide (0.648g, 12mmol) in methanol (12m1) at 0°C, then 3,4-Difluoro-
2-
(4-iodo-2-methyl-phenylamino)-benzoic acid methyl ester was added as a
solution of methanol and refulx for 20 hours, concentration and run column
with hexanes/ethyl acetate to give the product as a white crystal. The yield:
60%
m.p.: 191.7-192.0°C
' H NMR(DMSO, 400Hz): 8/ppm 9.45(1 H, s, -NH-); 7.79(1 H, t, J=7.3Hz,
Phenyl-H); 7.51 (1 H, s, Phenyl-H); 7.35(1 H, d, J=10.1 Hz, Phenyl-H); 7.05
7.11 (1 H, m, Phenyl-H); 6.44-6.48(1 H, m, Phenyl-H); 6.32(2H, s, -NH2),
2.32(3H, s, CH3)
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
EXAMPLE 6
5-f3,4-Difluoro-2-(4-iodo-2-methyl-phen lay mino)-phenyll-f 1,3,41oxadiazol-2-
lay mine
H2N
N
O NHNHz O , N
H H
N ~ ~ BrCN/NaHC03(aq) N
dioxane, r.t.
~F I ~ F ~ I
F F
To a solution of 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid
hydrazide (0.806g, 2mmol) in 5m1 of dioxane was added cyanogen bromide
(0.212g, 2mmol) followed by a solution of sodium bicarbonate (0.17g, 2mmol)
in 5m1 of water. The resulting mixture was stirred 18 hours at room
temperature the solution was concentrated and the residure was run column
with hexanes/ethyl acetate (3/1 ) to give the product which was recrystallized
from ethyl acetate / hexanes to provide a pale-yellow crystal. The yield: 58%
m. p.: 183.7-184.0°C
' H NMR(CDC13, 400Hz): 8/ppm 8.87(1 H, s, -NH-); 7.52(1 H, s, Phenyl-H);
7.45-7.49(1 H, m, Phenyl-H); 7.40(1 H, d, J=8.3Hz, Phenyl-H); 6.77-6.83(1 H,
m, Phenyl-H); 6.60-6.63(1 H, m, Phenyl-H); 5.02(2H, s, -NH2), 2.36(3H, s,
CH3)
81
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
EXAMPLE 7
2-f 3,4-Difluoro-2-(4-iodo-2-meth I-~ylamino)-
benzoyllhydrazinecarbothioamide
S
COOHH H N~N~N O
N \ (1) (COCI)z 2 H N
(2) thiosemicarbazide
F I ~ ~ ~ i i
~F 'I
F F
A solution of 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid (3.9g,
0.01 mol), oxalyl chloride (1.90g, 0.015mo1) in 40m1 dichloromethane was
treated with 2 drops of dimethylformamide, stirred at room temperature for 3
hours before concentration. The residue was dissolved in 10 ml
tetrahydrofuran and added to a solution of thiosemicarbazide (2.0g, 0.022mo1)
in 50m1 tetrahydrofuran at 0°C, stirred at room temperature for 14
hours.
Concentrated and run column chromatography with hexanes/ethyl acetate
(1/1) to give the product as a yellow solid. 2.91g. The yield: 63%
m.p.:159.5-160.0°C
H NMR(DMSO, 400Hz): 8/ppm 10.58(1 H, s, -NH-); 9.28(1 H, s, -NH-);
8.83(1 H, s, -NH-); 7.95(1 H, s, Phenyl-H); 7.12-7.75(2H, m, NH2); 7.51 (1 H,
s,
Phenyl-H); 7.37(1 H, dd, J=8.6Hz, 1.7Hz, Phenyl-H); 7.16(1 H,dd, J=17Hz,
9.OHz, Phenyl-H); 6.40-6.50(1 H, m, Phenyl-H); 5.02(2H, s, -NH2), 2.00(3H, s,
CH3)
82
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
EXAMPLE 8
5-(3,4-Difuoro-2-(4-iodo-2-methyl ~~henylamino)-phenyll-4H-[1,2,4~triazole-3-
thiol .
H S
S H N-
H N~N~N O N ~ NH
2
H \ N \ NaOCH3/CH30H H
N
refl ux
F / I ~ F ~ I
F F
2-[3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-
benzoyl]hydrazinecarbothioamide (1.386g, 3mmol) was dissolved in 15 ml
anhydrous methanol, sodium methoxide (25% wt% in methanol) 2.5m1 was
added at 0°C in one portion. The resulting mixture was heated at reflux
for 17
hours before concentration. Run column with hexanes/ethyl acetate to give
the product as a needle white crystal. The yield: 40%
m.p.: 196.5(dec.)
~ H NMR(DMSO, 400Hz): 8/ppm 13.87(1 H, s, -NH-); 13.80(1 H, s, -NH-);
8.16(1 H, s, -NH-); 7.61-7.65(1 H, m, Phenyl-H); 7.48(1 H, s, Phenyl-H);
7.32(1 H, dd, J=8.6Hz, 2.2Hz, Phenyl-H); 7.24(1 H,dd, J=16.4Hz, 9.5Hz,
Phenyl-H); 6.42-6.46(1 H, m, Phenyl-H); 5.02(2H, s, -NH2), 2.20(3H, s, CH3).
83
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
EXAMPLE 9
X2,3-Difluoro-6-f 1,3,41oxadiazol-2ylphenyl)-(4-iodo-2-methyl-phenyl)-amine
N
N O N~ O
HC(OEt)3 I \ N I \
\ N
pTsOH, EtOH
F I F I
F F
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid hydrazide (146
mg, 0.36 mmol) was suspended in 7 mL of absolute EtOH and 2 mL of
HC(OEt)3 was added along with approximately 3 mg of pTsOH> The reaction
was heated to reflux for 3h, cooled and concentration on a rotary evaporator.
The reaction was purified (Si02, 4:1 Hexane/EtOAc) to afford 117 mg (79%)
of (2,3-difluoro-6-[1,3,4]oxadiazol-2-yl-phenyl)-(4-iodo-2-methyl-phenyl)-
amine
as a yellow powder. M.p. = 144.4 - 145.5 °C. 'H NMR (400MHz, CDC13) 8
8.89 (s, 1 H), 8.44 (s, 1 H), 7.66 (m, 1 H), 7.52 (d, J = 1.7 Hz, 1 H), 7.38
(dd, J =
8.5, 1.9 Hz, 1 H), 6.83 (m, 1 H), 6.14 (dd, J = 8.5, 5.9 Hz, 1 H), 2.37 (s, 3
H).
EXAMPLE 10
5-f 3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-f 1,3,4]oxadiazole-2-
thiol
S
N
N O N~ O
CS2, KOH N
N \ ~ I \ I \
Et0 H
~F I ~F I
F F
3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid hydrazide
(170 mg, 0.42 mmol) was suspended in 7 mL of absolute EtOH and cooled to
84
CA 02377100 2001-12-11
WO 01/05391 PCT/US00/18346
0 °C. Carbon disulfide (74 mg, 0.97 mmol) was added followed by 24 mg
(0.42 mmol) of powdered KOH. The reaction was stirred for 1 h at 0 °C,
1 h at
rt, and refluxed for 3 h to afford a homogeneous reaction. The reaction was
cooled to rt, at which point a ppt formed. Water was added and the reaction
diluted with 5 mL of EtOAc. 1 N HCI was added to acidify the aqueous layer
(pH = 2). The aqueous layer was extracted with EtOAc (3x). The combined
organic layers were dried over Na2S04 and concentrated to obtain 96 mg
(51 %) of 5-[3,4-Difluoro-2-(4-iodo-2-methyl-phenylamino)-phenyl]-
[1,3,4]oxadiazole-2-thiol as a yellow powder. M.p. = 231.8 - 232.8 °C.
' H
NMR (400MHz, CDC13) 8 7.62 (m, 2H), 7.47 (s, 1 H), 7.30 (complex m, 2H),
6.44 (dd, J = 8.0, 4.5 Hz, 1 H), 2.19 (s, 3H).
F. OTHER EMBODIMENTS
From the above disclosure and examples, and from the claims below,
the essential features of the invention are readily apparent. The scope of the
invention also encompasses various modifications and adaptations within the
knowledge of a person of ordinary skill. Examples include a disclosed
compound modified by addition or removal of a protecting group, or an ester,
pharmaceutical salt, hydrate, acid, or amide of a disclosed compound.
Publications cited herein are hereby incorporated by reference in their
entirety.
What is claimed is: