Note: Descriptions are shown in the official language in which they were submitted.
CA 02377118 2002-04-05
ANTIPSORIATIC COMPOSITIONS, = z
METHOD OF MAKING, AND METHOD OF USING
BACKGROUND OF INVENTION
1. Field of the Invention
The present invention relates to skin treating
compositions, to a method of making the compositions, and to a
method of using the compositions in the treatment of skin. In
another aspect, the present invention relates to antipsoriasis
compositions, to a method of making the compositions, and to a
method of using the compositions to treat psoriasis. In even
another aspect, the present invention relates to botanical-
derived skin treating compositions, to a method of making the
compositions, and to a method of using the compositions to treat
skin.
This application is a division of Canadian Patent
Application No. 2,249,458 filed March 19, 1997.
2. Description of the Related Art
Psoriasis is a chronic skin condition characterized by
itchy, flaky skin. It is estimated that two percent of the
United States population, more than four million people, will
suffer from psoriasis during their lives. Psoriasis conditions
can range from mild to severe.
In the United States, between about 150, 000 and 250, 000
new cases of psoriasis occur each year, with about 40,000 of
these cases classified as severe. Sufferers of psoriasis must
endure not only the irritating disease itself, but also the
embarrassment of skin disfigurement.
The total annual cost for treating psoriasis on an
outpatient basis is estimated at more than $1.5 billion. It is
estimated that psoriasis sufferers are spending an average of
$500 per year on psoriasis treatment to achieve only temporary
relief. Severe cases that require hospitalization may require
an expenditure of up to $10,000.
The compound 3-methylanthralin has long been utilized
in the treatment of psoriasis, and is listed in the Merck Index
as an antipsoriatic. Chrysarobin is a mixture of compounds
CA 02377118 2002-04-05
derived from Goa powder, and includes 3-methylanthralin. Goo
powder itself is derived from the wood and bark of Andria
Araroba Aguiar (Fam. Legum.inosae) . Literature references
describing the isolation of and structure of Chrysarobin date
back to the early 1.800s. A method of reducing Chrysarobin to
obtain 3-methylanthralin was known as early as 1931.
Known psoriasis treatments include: antimetabolites such
as methotrexate; corticosteroids such as triamcinolone creams
or injection, clobeasol propinate cream, and hydrocortisone;
keratolytic/destructive agents such as anthralin or salicylic
acid; lubricants such as hydrogenated vegetable oils and white
petroleum; oral retinoids such as etretinate or isotretinoin
tablets; photochemotherapy such as methoxsalen or trioxsalen
capsules, and coal tar; and topical cholecalciferol analogs
such as calcipotriene ointment, a topical vitamin D3; known
commercially as Dovonex ~, (Squib, Huffalo, NY). ,
Numerous botanical protocols for the treatment of
psoriasis are known, including the use of extracts of various
herbs, roots, seeds, flowers, berries, and twigs. See
Therapeutic Botanical Protocol for Psoriasis, Protocol
Journal of Botanical Medicines, August 1994, pp. 1-38.
A composition for the treatment of burns and erythema has
been described in CH-A-671336, the composition including
Aspbodelus verus Albus'root extract and four other plant
species.
However, the known psoriasis treatments suffer from one
or more deficiencies, including potential toxic side effects
and achieving only temporary relief. Thus-there is a need for
improved psoriasis treatment.
SUI~lARY OF INVENTION
It is an object of the present invention to provide a
composition and method of treating psoriasis.
It is another object of the present invention to provide
~S a method for making 3-methylanthralin.
It is even another object of the present invention to
CA 02377118 2002-04-05
2a
provide a method of making chrysophanol.
It is still another object of the present invention to
provide a method of making aloe-emodin.
CA 02377118 2002-04-05
-3-
It is a further object to provide a method of making
aloe-emodin monoacetate.
It is an additional object to provide a composition
extracted from a botanical specimen that has efficacy in treating
psoriasis.
It is another object to provide such a composition
having a plurality of polyphenols therein.
It is a further abject to provide a method of treating
psoriasis with such a composition.
It is yet another object to provide a method of
extracting such a composition from the botanical specimen.
These and other objects of the present invention are
achieved by the composition and methods of the present invention.
According to even still another embodiment of the
present invention there is provided a method of making a product
comprising at least one of 3-methylanthralin, chrysophanol, aloe-
emodin, and aloe-emodin monoacetate comprising contacting root
of the plant Asphodelus Microcarpus with acetic acid to form the
product. A further embodiment of the method includes recovering
3-methylanthralin, chrysophanol, aloe-emodin, or aloe-emodin
monoacetate from the product. Any of the products may be further
derivatized,
According to yet even another embodiment of the present
invention there is provided a method of obtaining either 3-
methylanthralin, chrysophanol, or aloe-emodin by recovery of the
desired compound from the root of the plant Asphodelus
Microcarpus. The polyphenols may be further derivatized.
BRIEF DESCRIPTION OF THE DRAWINGS
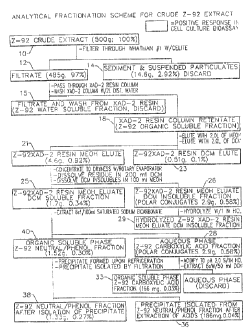
3o FIG. 1 is a schematic representation of the analytical
fractionation scheme utilized in Example 4.
FIG. 2 shown the peak assignments used to generate the
data of Table 3 for the XAD-2 resin DCM eluate fraction 23.
FIGS. 3 and 4, show, respectively, the direct probe
(DP) mass spectrometry data and liquid secondary ion mass
CA 02377118 2002-04-05
-4 -
spectrometry (LSIMS? data pertaining to the XAD-2 resin DCM
eluate fraction 23 of FIG. 1.
FIGS. 5, 6, and 7 show, respectively, a GC-MS
chromatogram, DP data, and LSIMS data for the chemical
composition of XAD-2 resin MeOH eluate DCM-insoluble fraction 26
of FIG. 1.
FIGS. 8, 9, and 10 show, respectively, a GC-MS
chromatogram, DP data, and LSIMS data for the hydrolyzed XAD-2
resin MeOH eluate DCM-insoluble fraction 29 of FIG. 1.
FIGS. 11, 12, and 13 show, respectively, a GC-MS
chromatogram, DP data, and LSIMS data for carboxylic acid
fraction 28.
FIG. ~ 14 shows GC-MS chromatogram data from the
methylated carboxylic acids of Example 5.
FIGS. 15, 16, and 17 show, respectively, a GC-MS
chromatogram, DP data, and LSIMS data for the neutral/phenol
fraction 36 of FIG. 1.
FIGS. 18-23 show, respectively, GC-MS chromatograms,
DP data, and LSIMS data for the neutral/phenol fraction DCM
silica gel eluate and the neutral/phenol fraction MeOH silica gel
eluate.
FIG. 24 is a plot showing the effects of the treatment
solution of Example 2 on HNF in fibroblasts.
DETAILED DESCRIPTION OF THE INVENTION
In the practice of the present invention, a composition
is made from the plant Asphodelus Microcarpus that comprises
compounds useful in the treatment of mammalian skin disorders,
especially psoriasis. Asphodelus Microcarpus is a plant that is
native to the Middle East; specifically, it can be easily found
in the Northern regions of Israel, as well as in regions from the
Canary Islands to Asia Minor.
Tn the practice of the present invention, the root of
Asphodelus Microcarpus is utilized. In a specific embodiment of
the present invention, the root or portions thereof may be
CA 02377118 2002-04-05
-5-
administered to treat mammalian skin disorders. Preferably, in
such a treatment, the outer skin of the root is first removed,
and then the inner portion of the root is applied to the
afflicted skin region. Additionally, raw extract from the roots
may also be applied~to the afflicted skin region.
It is to be understood in the practice of the present
invention that the roots of Asphodelus M.icrocarpus may be
harvested at any time. However, it is preferred that the roots
be harvested at a time when they are full of liquid which for
Asphode3us Microcarpus growing in Israel is generally from
February to May.
Preparation of the Asphodelus Microcarpus roots is
generally as follows. Excess dirt and other foreign matter
should be removed from the roots, generally by shaking and water
washing. After dirt and other foreign matter have been removed
from the root, the next step is to remove the outer layer of the
root. This can be accomplished by using a scraper, knife, a
peeler such as a potato peeler, or the like.
The next step is to extract liquid from the roots.
Methods for obtaining liquid from a compressible liquid
containing solid are well known to those of skill in the art, and
any such method may be utilized. Simple methods include mashing,
squeezing, pulverizing, liquefying, or compressing the roots.
Preferably, the roots are liquefied in a commercially available
"juicer."
The liquid thus obtained is mixed with acetic acid.
Generally for this step, the volume ratio of Asphodelus
Microcarpus root juice to acetic acid is in the range of about
1:20 to about 20:1. Preferably, the volume ratio is in the range
of about 1:10 to about 10:1, more preferably in the range of
about 1:5 to about 5:1, even more preferably approximately 4:1.
The Asphodelus Microcarpus root liquid is next mixed
with acetic acid at any temperature suitable for creating a
mixture of 3-methylanthralin, chrysophanol, aloe-emodin, and
aloe-emodin monoacetate. It is preferable that the mixing occur
CA 02377118 2002-04-05
_""'~
-6-
with both the Asphodelus Microcarpus root liquid and acetic acid
in the liquid state. Thus the contacting temperature is above
the freezing point but below the boiling point for the mixture:
Optionally, in the practice of the present invention,
brimstone may be mixed in with the Asphodelus Microcarpus root
and acetic acid. The brimstone may be in any suitable form, but
is preferably ground, and more preferably ground to a flour-like
consistency.
The Asphodelus Microcarpus root/acetic acid mixture
comprises 3-methylanthralin, chrysophanol, aloe-emodin, and aloe
emodin monoacetate and has been shown to be useful in the
treatment of psoriasis. While this mixture is shown herein as
being derivable from the Asphodelus Microcarpus root, it should
be understood that the 3-methylanthralin, chrysophanol, and aloe
emodin can be obtained by the methods that are known in the art.
Aloe-emodin monoacetate can be made by contacting aloe-emodin
with acetic acid.
3-Methylanthralin, chrysophanol, aloe-emodin, and aloe
emodin monoacetate can be individually recovered from the mixture
using separation techniques as are well known in the art.
The treatment composition of the present invention may
include a wide range of the polyphenol components and/or their
derivatives. In the method of the present invention fox
treating psoriasis, the composition may be administered to a
mammalian organism by any route known in the art. Nonlimiting
examples of suitable routes of administration include oral,
parenteral, topical, and the like. Specific nonlimiting examples
of carrying out such routes of administration include injection,
IV administration, pills, tablets, capsules, liquids, gels,
creams, soaps, shampoos, dermal patches, inhaled aerosols,
sprays, suppositories, and the like. Topical administration is
preferred for human psoriasis.
The frequency of administration varies with the
strength of the composition, but an exemplary treatment schedule
comprises a topical application once per day until symptoms are
CA 02377118 2002-04-05
_ '7
eradicated. The course of treatment naturally is dependent upon
the severity of the affliction, and may last from 14 days up to
56 days, although this is not intended as limiting.
The present invention is described as being suitable
for the treatment of humans for psoriasis, which is to be
understood to include, but not be limited to, exfoliative
psoriatic dermatitis, pustular psoriasis, and guttatte variant
psoriasis.
It is also believed that the compositions of the
present invention axe useful in the treatment of other skin
disorders and conditions, including eczema.
In addition to being used in the treatment of skin
disorders, the compositions of the present invention are also
believed to be useful in the treatment of psoriatic arthritis.
EXAMPLES
The following examples are provided 'merely to
illustrate the invention and are not intended to limit the scope
of the invention in any manner.
Example 1 Obtaining Raw Extract
Raw extract was obtained from the Asphodelus
Microcarpus plant as follows. Approximately 1 1b of roots of the
plant were obtained from a location in Israel. Dirt and other
foreign matter was removed from the roots by shaking, and then
by washing with water, after which the outer skin of the roots
was removed. Liquid was extracted from the peeled roots
utilizing a commercially available "juicer". The 1 1b of roots
yielded approximately 300 cc of raw extract.
Example 2 Pretaaration of Treatment Solution
Approximately 650 cc (although 400-800 cc are usable,
depending upon the severity of the affliction) of the raw extract
obtained by the method of Example 1, approximately 350 cc
(although 200-600 cc are usable) of acetic acid, and
CA 02377118 2002-04-05
-~L
.8.
approximately 1 teaspoon of brimstone, ground to flour-like
texture, were mixed together. This mixture does not require
refrigeration, although the raw extract should be refrigerated
until the acetic acid is introduced if kept in an unmixed state.
Example 3 Analysis of the Treatment Solution
The treatment solution of Example 2 was subjected to
various types of chemical and physical analysis, the results of
which are presented in Tables 1 and 2.
to
Table 1
Proximate Analysis Data*
Physical description of sample Dark brown liquid containing sediment
and suspended particulate matter,
acetic acid like odor
Ph (as received) 1.95
Acetic acid content (by titration)5.05! wiw
Insoluble matter 2.14! wlw
Ash weight (mineral content) 0.746! wJw
Specific gravity (filtrate) 1.103 glml
Total dissolved solids (filtrate)12.94! wlw
Chloroform extractables (filtrate)0.84% wlw
(organic soluble components)
*Analysis performed by Stillwell & Cladding Testing Laboratories,
3 o Inc., New York, N.Y.
CA 02377118 2002-04-05
_: L
-9-
Table 2
Elemental/Heavy Metal Composition*
Element ~ . Conc. ppm: Element Canc: ppm:.
Sulfur 4260 Bismuth NO < 3 ppm
Potassium 1640 Lead ND < 3 ppm
to Calcium 1075 Antimony _ ND < 3 ppm
Sodium 216 Boron ND < 2 ppm
Phosphorous 142 Indium ND < 2 ppm
Magnesium 100 Molybdenum ND < 1 ppm
is Silicon 7 Arsenic ND < 1 ppm
Iron - 7 Selenium ND < 1 ppm
Aluminum 5 Tellurium ND < 1 ppm
Zinc 2 Thallium ND < 1 ppm
20
Tin 1 Lithium ND < 1 ppm
Strontium 0.9 Cobalt ND < 1 ppm
Cop~aer 0.9 Niobium ND < 1 ppm
Manganese 0.4 Gallium NO < 1 ppm
Titanium 0.4 Germanium ND < 1 ppm
Nickel 0.4 Silver ND < 1 ppm
l
Chromium 0.2 Cadmium ND < .5
ppm ~~
l
Barium 0.1 Mercury NO < .5
ppm
30
Vanadium 0.06 Be Ilium ND < .1
m l
3 s * Elemental/heavy metal analysis was conducted by Umpire and Control
Services; lnc., West Babylon, N.Y.
CA 02377118 2002-04-05
-10-
Example 4 Fractionation of Treatment Solution
The solution of Example 2 was also subjected to
analysis . As the solution of Example 2 is too complex for direct
analysis, it was fractionated according to the scheme in FiG. 1
using a combination of wet analytical chemistry, column
chromatography and solvent extraction procedures. Throughout the
analytical fractionation, isolates were subjected to cell culture
bioassay, as described in Example 6. Fractions testing positive
in the cell culture bioassay were then directed to additional
to separation and/or chemical characterization until nearly pure
isolates were obtained.
A 500 g sample 10 of the treatment solution of
Example 2 was filtered through a Buchner funnel containing
Whatman #1 filter paper and a 1 cm bed volume of Celite°
analytical filtering aid. The filtrate 12 (485 g, 97% of
original sample) tested posiaive in the cell culture bioassay and
was equivalent in potency to the unfiltered treatment solution
of Example 2. The filter retentate 14 (14.6 g, 2.92%) was a dark
reddish brown colored material with a clay-like consistency.
2o This substance tested negative in the cell culture bioassay.
Analysis of this sediment indicates it to be largely composed of
sucrose, complex carbohydrates, cellulosic debris, lignins,
inorganic minerals, and clay.
The filtrate was then passed through a preconditioned
chromatographic column packed with a 20 x 350 mm bed volume of
XAD-2 resin. (Supelcopak-2° absorbent) at a flow rate of 5
ml/minute. XAD-2 resin is a hydrophobic porous polymer absorbent
based on styrene-divinylbenzene copolymer. This resin has a high
affinity for absorbing nonpolar, organic-soluble components from
aqueous solutions. The column was preconditioned by washing it
with 1.0 L of dichloromethane (DCM), followed by 1.0 L of
methanol (MeOH) and finally 1.0 L of distilled/deionized water.
All solvents were ultra-high-purity ~~capillary-analyzed~~ grade
suitable for trace-level chemical analysis procedures. The
distilled water used throughout the analysis was obtained from
CA 02377118 2002-04-05
-11-
a Milli-Q~ purification system. It was double distilled in glass
and then further purified by passing it through ion-exchange and
activated carbon filters . After the filtrate was passed through
the XAD-2 resin, the column was washed with 2 L of distilled
water. The filtrate and wash 15 from the XAD-2 resin column
tested negative in the call culture bioassay and were discarded.
This dark brown colored fraction contains the water-soluble
compounds of the treatment solution, such as acetic acid, sugars,
low-molecular-weight polar organic acids, tannins, and other
l0 biologically inert components.
The XAD-2 resin column containing the treatment
solution retentate 16 was blown dry to remove as much of the
residual wash water as possible. The treatment solution organic-
soluble components were then eluted from the column with 2.0 L
of MeOH followed by 2.0 L of DCM. The MeOH and DCM XAD-2 resin
eluates were collected separately. The treatment solution XAD-2
resin MeOH eluate 21 yielded 4.6 g, which corresponds to 0.92%
of the original treatment solution on a weight basis. The eluate
was amber colored. The treatment solution XAD-2 resin DCM eluate
ltf 23 contained 0.51 g or O.lx of the original sample. This eluate
was bright yellow colored. Both of these fractions tested
positive in the cell culture bioassay. However, higher activity
was observed in the treatment solution XAD-2 resin MeOH eluate.
Therefore, this isolate was subjected to additional
fractionation.
The treatment solution XAD-2 resin MeOH eluate 21 was
concentrated to dryness in a round bottom flask using a rotary
evaporator at reduced temperature and pressure. The residue was
a dark red crystalline substance. The residue was dissolved in
200 ml of DCM, producing an amber-colored solution. However, not
all the residue was soluble in DCM. The DCM-insoluble materials
were then dissolved in 100 m1 of MEOH. The methanol-soluble
components had a dark red color. Thus two additional fractions
were prepared. The treatment solution XAD-2 resin MEOH eluate
s~.~ I)CM-soluble fraction 25 contained 1.7 g (0.34x) , and the
CA 02377118 2002-04-05
-~i
-12-
treatment solution XAD-2 resin MeOH eluate DCM-insoluble fraction
26 yielded 2.9 g (0.58%). Both of these fractions tested
positive in the cell culture bioassay.
The treatment solution XAD-2 resin MEOH eluate DCM
soluble fraction 40 contains organic-soluble neutral, phenolic,
and acidic components. It was extracted 6 times with 100 ml
portions of saturated sodium bicarbonate solution to remove the
carboxylic acid fraction. The saturated sodium bicarbonate
extract 28 was acidified to Ph 2.0 using 1 N HCL and then back
extracted 6 times with 50 ml portions of DCM to partition the
carboxylic acids into the organic phase. The treatment solution
carboxylic acid fraction 33 contained 166 mg (0.03%) and was
weakly positive in the cell culture bioassay. The fraction had
an amber-colored appearance.
The treatment solution XAD-2 resin MeOH eluate DCM-
soluble fraction, after extraction of the carboxylic acids,
contained 1.52 g or 0.30% of the original sample. This isolate
31 was named the treatment solution "neutral/phenol fraction"
based on its chemical composition. It yielded a strong positive
response in the cell culture bioassay. Upon refrigeration this
fraction was observed to form a yellow-orange-colored crystalline
precipitate. The precipitate was isolated by filtration through
a sintered glass type Gooch crucible. The precipitate 36
isolated from the neutral/phenol fraction after extraction of
acids yielded 186 mg (0.04%) of bright yellow-orange crystals.
This isolate was found to be the most highly active fraction in
the cell culture bioassay. The neutral/phenol fraction 38 after
harvesting of the crystalline precipitate was found to contain
1.33 g (0.27%) . This sample also tested strongly positive in the
cell culture bioassay.
Some additional subfractions were prepared from the
isolates described above. A portion of the treatment solution
carboxylic acid fraction was methylated with diazomethane reagent
to produce the corresponding methyl esters of the sample. This
was done to enhance the volatility of the acids to improve the
CA 02377118 2002-04-05
- L
-i3-
gas chromatographic separation. The treatment solution XAD-2
resin MeOH eluate DCM-insoluble fraction was hydrolyzed with 1
N Hcl for 4 hours at 100°C to break down glycosidically
conjugated species. The treatment solution neutral/phenol
fraction 29 after harvesting of precipitate was subjected to
additional minicolumn fractionation using a silica gel solid-
phase extraction column. These procedures are described in more
detail Example 5.
Example 5 - Chemical Characterization of the Fractions of Example 4
The nonvolatile components of the fractions were
analyzed by a combination of electron ionization direct probe
mass spectrometry (DP data) and by a high-mass technique, liquid
secondary ion mass spectrometry (LSIMS data).
The chemical composition of the XAD-2 resin DCM eluate
fraction 23 is shown in Table 3, with peak assignments
corresponding to the GC-MS chromatogram shown in FIG. 2. The DP
and T.~SIMS spectra pertaining to the XAD-2 resin DCM eluate
fraction 23 are shown in FIGS. 3 and 4, respectively. A
homologous series of nonvolatile compounds with molecular weights
474, 490, 506, and 508 were detected in the DP data. Accurate
mass measurements were performed on these peaks using high-
resolution (R = 10,000) mass spectrometry in order to determine
their elemental formulas. The empirical formulas for these
2 5 compounds were found to be C23H22~11 ( 4 74 MW ) , C23HZZOlx ( 4 90 MW ) ,
C13H22O,3 (506 MW) , and C23Hz401a (508 MW) . In other fractions
related homologues with molecular weights 478 and 492 were also
observed. Exact chemical structures for these compounds are
unknown. However, their elemental formulas and mass spectral
fragmentation patterns indicate that they are a class of
polyphenolic chemical compounds called bisflavanoids.
The chemical composition of XAD-2 resin MeOH eluate
DCM-insoluble fraction 26 is summarized in Table 4. The GC-MS
chromatogram, DP data, and LSIMS data pertaining to this fraction
are presented in FIGS. 5, 6 and 7. Most of this fraction was
CA 02377118 2002-04-05
-14-
nonvolatile, and so the GC-MS peaks described make up only a
small portion of this sample. The majority of the mass in this
fraction consists of high-molecular-weight nonvolatile compounds.
The DP data show relatively trace levels of several
S bisflavanoids. The LSIMS data show a complex mixture of high-
mass compounds in the range 300-1000 to be present in this
fraction. This fraction largely consists of ~~bound~~ compounds
such as glycosides (phenolic compounds bound to sugar molecules)
and other polar high-molecular-weight conjugates. This fraction
was digested with HC1 and heat in order to hydrolyze the
conjugates down to small molecules, which could then be
identified.
The data on the hydrolyzed XAD-2 resin MeOH eluate DCM-
insoluble fraction 29 are summarized in Table 5. The GC-MS
chromatogram, DP data, and LSIMS data for this fraction are shown
in FIGS. 8, 9, and 10, respectively. Please note that the
compounds in Table 3 that appear following hydrolysis are all
present as bound components in the original fraction.
CA 02377118 2002-04-05
-'..-i
-15-
Table 3
Chemical Composition XAD-2 Resin DCM Eluate
Fraction 23 of FIG. 1
M5 Compound (Synonyms, Common Names,CAS~# Peak
Spec Comments, Ete.) Area%..
#
66 phenol 108-95-2 1.43
282 sulfur (elemental sulfur six memberedNA 0.90
ring)
372 dibutylphthaiate (plasticizer) 84-74-2 0.29
441 hexadecanoic acid (palmitic acid)57-10-3 0.11
445 sulfur (S-8, cyclic sulfur, orthothrombic10544-50-09.02
sulfur,
molecular sulfur)
452 1-hexadecanol (hexadecyl alcohol)36653-82-40.81
485 linoleic acid 60-33-3 0.91
519 3-methyl-1,8,9-anthra~netriol 491-59-8 5.86
(3-methylanthralin, Chrysarobin)
527 1,8-dihydroxy-3-methyl-9,10-anthracenedione481-74-3 11.76
(Chrysvphanol, Chrysophanic acid,
2 0 1.8-dihydroxy-3-methyl-1,8-anthraquinone)
549 1,8-dihydroxy-3-(hydroxymethyl)-9,10-NA 0.14
anthracenedione .
(Aloe-Emodin)
574 2,4-bis(dimethylbenzyl)-6-t-butylphenolNA 0.27
583 di-2-ethylhexylphthalate (plasticizer)117-81-7 65.44
619- monoacetate derivative of 1,8-dihydroxy-3-NA 2.23
622 (hydroxymethyl)-9,10-anthracenedione
(Aloe-Emodin monoa~tate)
~DP bis-flavanoids with m.w.'s 474 NA 1.73
(C2,HZZO"), 490 (est.
Data (C~,Hz=O,Z), 506 (C=,HziO") and total
508 (Cz,Hz~O") for
exact chemicals structures unknown DP 8~
3 0 LSIMS)
LSIMS unknown high mass compounds m.w. NA 1.73
608 8 (est.
Data 696 total
for
OP 8~ S
t_SI
data
DP = Data is from Electron Ionization Direct Insertion Probe
LSIMS = Liquid Secondary Ion Mass Spectrometry
CA 02377118 2002-04-05
-16-
Table 4
Chemical Composition XAD-2 Resin MeOH Eluate
DCM-Insoluble Fraction !Fraction 26 of FIG. 1)
MS Compound {Synonyms, Common Names, CAS # Peak
Spec Comments;; Etc.) Area I
#
423 palmitic acid 57-10-3 17.05
447 unk. 216 m.w. aromatic NA 2.41
468 linoleic acid 60-33-3 28.08
519 3-methyl-1,8,9-anthracenetriol 491-59-8 27.82
(3-rnethylanthralin, Chrysarobin)
527 1,8-dihydroxy-3-methyl-9,10-anthracenedione481-74-3 12.78
(Chrysophanol, Chrysophanic acid,
1,8-dihydroxy-3-methyl-1,8-anthraquinone)
583 di-2-ethylhexylphthalate (plasticizes) 117-81-7 11.87
DP bis-flavanoids with m.w.'s 492 (CZ,H24O,z)NA trace
and
Data 506 (C2,Hu0,~) exact chemicals structures
unknown
LSIMS complex mixture of unknown compounds NA major
m.w.
Data range 300-1000 (bound components,
glycosides, higfi m.w. polar conjugates
etc.)
OP = Data is from Electron Ionization Direct Insertion Probe raw data file
FM10647
LSIMS = liquid Secondary Ion Mass Spectrometry Data file VG2268
CA 02377118 2002-04-05
-17-
Table 5
Chemical Composition of Hydro~zed XAD-2 Resin MeOH
Eluate DCM-Insoluble (Fraction 29 of FIG. 1)
MS Compound (Synonyms, CommonCAS Peak
Spec Names, # Area
# Comments, Etc.) /;
57 tumaric acid 110-17-84.14
97 2-ethyl-t-hexanol (ptaslicizer104-7871.B
degradate)
123 2-furancarboxylic acid 88-14-22.64
151 4-oxo-pentanoic acid (fevutinic123-76-25.36
acid)
183 a-hydroxyhexanoic acid NA 0.62
271 branched dodecanol isomerNA 1.56
278 1-chlorododecane (probably112-52-70.91
a hydrolysis
art'rfact)
282 1-dodecatlol (lauryl alcohol)112-53-815.7
318 dodecanoic acid (lauric 143-07-72.54
acid)
345 tributylphosphate (plasticizer)126-7381.29
348 1-tetradecanol (myristyl 112-72-15.89
alcohol)
36t 2-ethoxy-1dodecanot 297184-37.09
365 2.8-dihydroxy-3-methyl-1,4-napthoquinoneNA 3.29
(Droserone)
1 S 378 tetradecanoic acid (myristic544-63-82.31
acid)
420 2-ethoxy-1-tetradecanol NA 3.01
422 methyl palmilate 112-39-00.48
428 7-hydroxy5-melhoxy-2-methyl-4-oxo-4H-1-7338-5t-41.15
benzopyran-6tarboxaldehyde
4a hexadecanoic acid (palmitic57-l0-37.54
t acid)
440 a-hydroxytauric acid NA 3.4
461 heptadecanoic acid (margaric508-12-70.32
acid)
449 oleic acid 112-80-10.69
?
0
. 480 linotoic acid Gt1.33-34.ti3
511 a-hydroxymyrislic acid 2507-5530.42
488 odadecanoic acid (stearic57-1 1.35
acid) i-4
518 3-methyl-1,8.9-anthracenetriol49159-80.42
-
(3-methytanthralin, Chrysarobin)
520 1,8-dihydroxy-3-methyl-9,10-anthracenedione48174-34.71
(Chrysophano4 Chrysophanic
acid.
1,8-dihydroxy-3-methyl-1,8-anthraquinone)
574 di-2-ethy~exytphthalate 11781-74.48
25 (plasticizer)
OP bis-llavanoids with m.w.'sNA 11.98
Data 478 (Ci=HyO,=). 492
(C"Hr,O,,) and 306 (C=,H1,0")
exact
chemicals structures unknown
OP = Data is from Electron Ionization 0'uect Insertion Probe raw data file
FM10827
CA 02377118 2002-04-05
The carboxylic acid fraction 28 data are summarized in
Table 6. The GC-MS chromatogram, DP data, and LSIMS data
pertaining to this fraction 28 are presented in FIGS. 11, 12, and
13. Carboxylic acid fractions often contain nonvolatile species
that do not readily pass through gas chromatography. These
compounds can be chemically derivatized into more volatile forms
using methylation or silylation reagents. Therefore, the
carboxylic acid fraction 28 was methylated using freshly prepared
diazomethane reagent. This procedure converts the carboxylic
acids into their corresponding methyl esters. Phenols are
converted into methyl ethers. The methyl esters and methyl
ethers are more volatile and chromatograph better than the free
acids.
The GC-MS chromatogram from the methylated carboxylic
acids is shown in FIG. 14. No additional compounds were detected
in the methylated sample. All the same compounds observed in the
underivatized carboxylic acid fraction were found as their
methylated counterparts.
Table 7 summarizes the chemical composition of the
2.O yellow precipitate which was isolated from the neutral/phenol
fraction 36 after extraction of the carboxylic acids. This
fraction, which contains relatively few components, possesses the
highest degree of activity in the cell culture bioassay. The GC-
MS chromatogram, DP data, and LSIMS data pertaining to this
fraction are shown in FIGS. 15-17.
CA 02377118 2002-04-05
-19-
Table 6
Chemical Composition of Carboxylic Fraction
(Fraction 28 FIG. 1)
MS Compound (Synonyms.CAS Peek
SpeeCommon Hamea, a Area
al Commrnts, Ete.) X
37-102anetnylacrytitacid79-41-414.4
19 2-memylbulyrie acd 1185302.04
57 pentanore aed 109-52J2.2
1t8hexanas acid tt2-82-t3.52
t202.furancarboxyficaud118-t4-29.t8
t unknown 138 m.w, NA 2.7
s3 audw containing
compound
1672atnymexanoicaud t49-575t
t8tbenzoic add 658502.91
t97ttrtanoie acid t24-07.28.29
2t7Denzerleacetieaad 10382-2t.2
227rlonanoicacid 1t2-03-04.74
242decanoicaeid 734-4855.2
2772Jyaroxy:l.,~~~ 87-4090.77
~p (uesotic sad.
homosaicylic add)
277yneenylproperwie 621-82-90.69
sad (unnamic sad)
3053.4dactrbrtt0ertzoicaeidStJ4-50.88
318dodersnoic sad (lauric147-07-70.82
add)
323tOwmdecenoic acid 112-18-90.72
328tuuunerfioic acid 123-38-90.54
- 329pemacnbrophend (wood87-86-SO.
preservative) t
t
342I~meUaxy~-hydroxybentaldehyde(van4tin)12133-50.75
772p-coumaric acid. 39s3-97-31.23
methyl ester
378telradenrioic add 544-63-80.72
(mynsttc acid)
385methylfmuWla. 23080710.72
408methyl4ister o! 596-6450.71
3-4.dimethoxydnnamic
acd
41ata-0enzene dicarboxylic10021-00.6T
acid IterePhthalic
xd)
423Lt-dihydf0-4.648tydtouy-3.methyi-i:ocoumann30951172U,42
- i8-hYdroxymeeein)
a279.Nexadecenaic acid209 0.55
(paknilokie acid) 1.29.1
434hexaaecaivoic sad 57.10-7t.9
Ipa4nitic acd)
4389.octadecerwrc add 1 0.49
(oleic acdl t2-80-1
4789.12octmlacadienoicadd(Nnokicacd)80-33-31.03
464oaaaec>noic scd s7-t 0.69
(atearic add) 1.4
4913-memyl-1.8.9snttuacenetrrol491-59-80.55
-
(3-methylanttuatin.
Ctuysarobin)
518t,84ihydroxy-3-methyl9.t0-anthracened'rone481-74.31.3
(Chryaoptunol, Chrysophanie
acid.
t.8 J4rydroxy.3.n;ethyl
L.B~anthrequmonel
572dl2edryltiexytphlhatatelplaatrdur)ItT.Bt.70.8
833saualene 768384-90.74
OP 0is-Ilavanoids wrilhNA 27.22
Datam.w's 492 IC"H"O,~ eat.
and total
508 (C~H~,O,rj a:act
chemicals atrutttuea for
unknown OP
d
ISIMS
LSIMSunknown hr~b mass 14A 27
Drleconytotmrts rn 22
w. 530. 854 eW
rrH174U total
laDPB
LSIMS
OP ~ Data a Iron Eleeuon lonitatron Direct Insenwn Prone taw oats file FM
IOB48
LSIMS ~ LMrmf SeconJary Ion MaaaSpectrane~ry Data tile Vti2267
CA 02377118 2002-04-05
-20-
Table 7
S
Chemical Composition Precipitate Neutral/Phenol
Fraction after Extraction of Acids (Fraction 36 of FIG. 1)
MS Compound (Synonyms, Common Names, CAS # Peak
Spec Comments, Etc.) Area
#
419 methyl palmitate 112-39-0 0.53
464 methyl linoleate 1 i 2-63-0 1.09
510 3-methyl-1.8,9-anthracenetriol 491-59-8 19.69
l o (3-methylanthralin, Chrysarobin) ,
517 1,8-dihydroxy-3-methyl-9,10-anthracenedione481-74-3 57.41
(Chrysophanol, Chrysophanic acid,
1,8-dihydroxy-3-methyl-1,8-anthraquinone)
583 di-2-ethylhexylphthalate (plasticizes)117-81-7 18.35
DP bis-flavanoids with m.w.'s 474 (C23H220"),NA 2.93 (est.
492
Data (Cz,H2,0,2). 506 (C2~Hu0") and 508 total
(C23H2,O") for
1 s exact chemicals structures unknown DP
LSIMS)
LSIMS unknown high mass compounds m.w. 618, NA 2.93 (est.
662,
Data 696, 718, 736 and 758 total
for
DP &
LSIMS
data
DP'= Data is from Electron Ionization Direct Insertion Probe raw data file
FM10645
LSIMS = Liquid Secondary fon Mass Spectrometry Data file VG2270
The neutral/phenol fraction following extraction of
carboxylic acids and harvesting of the yellow precipitate was
still too complex for direct analysis. Therefore, it was
subjected to additional fractionation using a silica gel solid
CA 02377118 2002-04-05
-21-
phase extraction minicolumn procedure. The fraction was passed
through the silica gel column and was washed with DCM. A second
more polar fraction was then eluted from the column using
methanol. Out of the original 1.32 g of neutral/phenol fraction
S were recovered 0.73 g (0.15% of original crude extract? in the
DCM eluate and 0.59 g (0.12%) in the MeOH eluate. Therefore, the
neutral/phenol fraction following extraction of carboxylic acids
and harvesting of yellow precipitate was split into two
additional subfractions for analysis. These were named the
neutral/phenol fraction DCM silica gel eluate and the
neutral/phenol fraction MEOH silica gel eluate. The chemical
compositions of these two fractions are summarized in Tables 8
and 9. The GC-MS chromatograms, DP data, and LSIMS data
pertaining to these two fractions are shown in FIGS. 17
through 22.
CA 02377118 2002-04-05
-22-
Table 8
Chemical Comeosition Neutral/Phenol Fraction
Silica GeI MEOH Eluate
MS Compound (5ynonyma. CAS Peak
Sp~cCommon Nama. s Area
a Commrrrtts, Ete.) Y.
134 3.5,5-trimed~yl-2ctrdchexenet-oneT859t 0.83
((soptuxone)
205-5-hydroxymothyUurturyl1055 6.24
225 acetate (HMf acetate)t-58-3
231 gykxrytmonoaeetate 26sa6-35-51.03
1- ~ 244 glyCeryWiacetate 2539531-70.6
246 gtycerydriacetate 102-7811
(triaeetin)
254 deesrwic aad 334.48-S0.18
295 t-4.butanadiold(acetate628-6T.t0.57
311 unknovm aromatic NA 0.53
acetate
318 unknown arana8c acetateNA 0-65
328 dodecanok acid (tawlc113-07.72.
acid) t
1
334 t-benzettediol monoaeetateNA 2.03
(hydroquinone
monos
353 unkraowm aromatic NA 0.29
acetate
1 S 359 unknown aromatic NA 0.13
acetate
3n myri:tir:rda ss4.s3-a2.ss
399 unknown aromatic NA 0.5
acetate
405 pentadecanoicaeid 100284-2t.6
421 t.d-0eruenedfearbo:yie100-2100.58
acid perephlha6e
acid)
a28 hexadecanoie add 57.10-3t
(palmitic acid) l.se
436 hepfadecanoicadd 508-1271.67
474 dnokicacid 8033-32.,88
500 oraddecaraic add 57-t 39-02
(stearic acid) t-4
520 3-mettryl-t:8:9anthraeenetriot~9t-5982.57
2 0 1n: CttrysarobYr)
529 t.8-dfiydroxy:3metby49,t0anthraeenedionea81-7435.7
(Clttysolthtatd.
Chysophanic acid.
1.S:dfiydroay3methyt.t,8-anlhraquinorte)
539-rntxitne of brp chainNA 4
575 allphatle sutates
583 dl-2-athylhexytphthalate(PIasIkIzer)1178170.55
818 t.8-A9rydroay-3-(hyd<oxymedtyp-9.10-NA 3.82
-
anthraeened-rone
(Aioe.Enrodkt)
622 monoacelate derivativeNA 1.34
of l,8dihydroxyJ-
(hydroxymethyl)-9.10-anMracenedione
(Aloe-Emadinmonoaeetate)
2 5 6~ tmlawwn 378 m.w. NA 0.77
aranalie aceute
644 aQualene T683-8d-90.2T
OP bb-Ilavandds with NA 4.3
Oatsm.w.'s 474 (Cr,HnO"), (cal
478 total
(CafipO,~. 490 (CrrH~On). lot
492 (C~hla,Op). OP
506 d
(CHrrO") snd 5011 ~SIMS)
(C~H"O,~ exact chemicals
stnretures unknown
lSIMSunknown high masa NA 4
Oalacompounds m.w. 608, 3
880 (est.
and 898 total
br
OP
b
~SIMS
data
DP . Oats is from Eacarwr lonizadon Okeet lnseribn Probe raw dau Gk FM 10850
t.SIMS ~ liquid Secondary Ion Mass Spsdromelry Oata (1e vG2265
:()
CA 02377118 2002-04-05
-23-
Table 9
Chemical Composition Neutral/Phenol
Fraction Silica Gel DCM Eluate
MS Compound (Synonyms, CAS Psak
Spo Common Namss; a Ar~x
At t
Comments. Etc.)
283 unknown 154 m.w. NA 0.29
rnmpound
268 2.3Eihydro-2.54imethyl~H-tbenzopyran-4-696878720.88
one
278 S.8 hexasulfide (sixNA t.35
membered ring structure)
283 2,6~it-butyl-p-hydroxyanisoleNA 2,12
(antioxidant)
295 butylated hydroxy 128-3703.65
toluene (8H1- antioxdant)
301 methyl lsurate t 1 1.47
t-82.0
323 diethytphthalate 846-2 1.08
(plasticizer)
329 dodecyi acetate t 12-66-31.82
1 5 358 unknown bauoata NA 0.65
785 methylleidecanoate 173188-01.35
369 oaxrs)Adruramic aldehyde101-8600.65
373 bePtylbenzaate 120-51-40.71
388 meewnoate 713254.10.82
390 telradecyl acetate 638-59-50
59
40a txnryl sa&cylate 11858-11.35
417 acetylterulic acid 2596-47.82.12
425 methyl patmitate 112J9~04.53
433 dibutylphthalate Sd74.22,00
141 sulfur (S-8, eyUie 10544-50-09.59
suNur; orthothrbmbie
sullur,
molecular sulfw)
447 hexadecylacetate 829-70-91.88
151 rtathylheptadecanoate17319260.94
471 methyl finobaee 112-63-015:A2
478 methylslesrate 112-61-80.76
25
515 3-meMy1-1,8.9anthracenettiol49159-85.06
(3erKdrylaMfualin,
Chrysarobin)
319 1.8-dihydroxy3-melhyl.g,l0.anthracenedione481-74.315.00
533 (Ctwysophar>al, CMysophanic
acid.
t.8-dihydroxy-3-methyl-1.8-anthraquinane)
543 di-2-ethylhexylpMhalate117-81-712.53
(plasticizer)
817 monoacetate derivativeNA BZ4
of l,8dihydroxy.3-
(hydroxyrtwthyp:9,10-anthracenedione
(aloe-Emodin monoacetate)
3 ~ 839 methyl tamesate 10485-JO-81-08
OP bb-8avanoida with NA 9.71
Oatsm.w.'s 474 (CnH,rO, (eat.
) 490 total
(Cr,11,r0"). 492 1w
(C~fl,.0"). 508 OP
(C"ItrrO.,) b
and 508 (CnHNO,~ lSIM$)
exaC chemicals sbudures
unknown; also unknown
peaks at m.w. 570
and
602
ISIMSunknawm h'gh mass NA 9.71
Oatseorr>pounda m.w. t (esl
540. 818. otal
648 and 682 for
OP
6
IStMS
data
OP ~ Oata is Iron Electron Ionization Oked Insertion Probe raw data file
FM10649
ISIMS = liquid Secondary ton Masa Spewomeby Oata tits VG2266
CA 02377118 2002-04-05
-24-
The data obtained from all of the fractions of
Example 4 were pooled together and normalized to produce a
comprehensive summary. These data are summarized in Tables l0A-
F, which describe the chemical composition of the organic soluble
S fraction, which constitutes approximately 1% of the treatment
solution on a w/w basis but contains 100% of the 'biological
activity. The compounds in this table are grouped together by
chemical class. It should be noted that the quantitative date
(% w/w) provided in this and the other tables are not exact but
l0 rather are semiquantitative. The data were derived from a
combination of GC area % integrations and from gravimetric
determinations made throughout the fractionation. Highly
accurate quantitation data would only be possible if analytical
reference standards were available for all compounds detected so
15 that detector response factors could be determined and the data
adjusted.
CA 02377118 2002-04-05
-25-
Data Summary: Composition of OrQani.c Soluble
Fraction Grouped by Chemical Class
Table 10A
Aiphatic Carboxylic Acids CAS # ! wlw
2-methylacryiic acid 79-41-4 0.27
2-methylbutyric acid 116-53-0 0.04
fumaric acid 110-17-8 1.50
I
pentanoic acid 109-52-4 0.04
hexanoic acid 142-&2-1 0.07
2-furancarboxyiic acid 88-14-2 1.13
2-ethylhexanoic acid 149-57-5 0.02
4-oxo-pentanoic acid ttevulinic acid)123-76-2 '1.94
a-hydroxyhexanoic acid NA 0.23
octanoic acid 124-07-2 0.118
nonanoic acid 112-05-0 0.09
decanoic acid 334-48-5 0.11
2 0 dodecanoic acid (iauric acid) 143-07-7 1.09
10-undecenoic acid 112-38-9 0.01
nonanedioic acid 123-3B-9 0.01
tetradecanoic acid (myristic acidj 544-63-8 1.05
pentadecanoic acid 1002084-20.12
2 5 hexadecanoic acid (palmitic acid) 57-10-3 9.82
a-hydroxyfauric acid NA 1.232
a-hydroxymyristic acid 2507-55-30.15
heptadecanoic acid 506-12-7 0.24
oleic acid 112-80-1 0.259
30
tinoteic acid 60-33-3 12.26
stearic acid 57-11-4 3:43
paimitoleic acid 2091-29-40:01
CA 02377118 2002-04-05
-26-
Table 10B
Aromatic Carboxylic Acids
...__
benzoic acid 65-85-0 0.06
benzeneacetic acid 103-82-2 0.02
3,4-dichlorobenzoic acid 51-44-5 0.02
1,4-benzenedicarboxylic acid ~terephthaiic acid)100-21-0 0.06
cinnamic acid 621-82-9 0.01
acetyl ferulic acid , 2596-47-6 0.20
20
30
CA 02377118 2002-04-05
-27-
Table lOC
Esters
glyceryl monoacetate 26446-35-50.08
glyceryl diacetate 25395-3i-70.04
gtyceryl triacetate (triacetin) 102-76-1 0.08
1,4-butanediol diacetate 628-67-1 0.04
2-hydroxy-5-methytfurfuryt acetate (HMF 10551-58-30.47
acetate)
diethytphthalate (ptasticizer) 84-66-2 0.10
dibutylphthalate (plasticizer) 84-74-2 - 0.21
di-2-ethylhexylphthalate (ptasticizer) 117-81-7 11.70
tributylphosphate (ptasticizer) 126-73-8 0.47
methyl taurate 111-82-0 0.14
methyl tridecanoate 1731-88-0 0.13
methyl pentadecanoate 7132-64-1 0.08
methyl palmitate 112-39-0 0.60
2 0 methyl heptadecanoate 1731-92-6 0.09
methyl linoleate 112-63-0 1.51
methyl stearate 112-61-8 0.07
methyl farnesate 10485-70-80.10
2 5 dodecyl acetate 112-66-3 0.17
tetradecyt acetate 638-59-5 0.06
hexadecyt acetate 629-70-9 0.18
benzyl benzoate 120-51-4 0.54
benzyl salicytate 118-58-1 0.13
30
methyl ester of 3,4-dimethoxy cinnamic acid5396-64-5 0.01
unknown aromatic acetates NA 0.16
mixture of long chain aliphatic acetates NA 0.30
unknown benzoate NA 0.06
3 5 unknown 378 m.w. aromatic acetate NA 0.06
-
CA 02377118 2002-04-05
-28-
Table lOD
Phenolic Compounds
phenol 108-95-2 0.09
I
2-hydroxy-3-methylbenZOic acid (cresotic 83-40-9 0.01
acid, .
homosalicylic acid)
ferulic acid methyl ester 2309-07-1 0.01
benzyl salicylate 118-58-1 0.13
3,4-dihydro-4,8-dihydroxy-3-methylisocoumarin309-5i-1120.01
(6-hydroxymellein)
7-hydroxy-5-methoxy-2-me#hyt-4-axo-4H-1-benzopyran-6-7338-51-4 0.42
carboxaldehyde
2,6-di-t-butyl-4~-methylphenoi {BHT, antioxidant)128-37-0 0.34
2,6-di-t-butyl-p-methyla~isole {antioxidant)NA 0.20
,
2,4-bis-(dimethylbenzyt)-6-t-bvtylphenol NA 0.02
(antioxidant)
hydroquinone monoacetate NA 0.15
4-hydroxy-3-methoxybenzaldehyde (vanillin)121-33-5 0.01
p-coumaric acid methyl ester 3943-97-3 0.02
pentachlorophenol (PCP, wood preservative)87-86-5 0.002
3-methyl-1,8,9-anthracenetriol 491-59-8 11.77
(3-methylanthralin; Chrysarobin)
2 5 1,8-dihydroxy-3-methyl-9,10-anthracenedione481-74-3 10.37
(Chrysophanol, Chrysophanic acid,
1,8-dihydroxy-3-methyl-1,8-anthraquinone)
1,t3-dihydroxy-3-(hydroxymethyl)-9,10-anthracenedione481-72-1 0.30
(Aloe-Emodin)
monoacelate derivative of 1.,8-dit~ydroxy-3-NA 0.83
3 0 (hydroxymethyl)-9,10-anthracenedione I
(Aloe-Emodin monoacetate)
2,8-dihydroxy-3-methyl-1,4-naphihoquinone NA 1.7
(Droserone) 9
bis-flavanoids with m.w.'s 474 (Cz,HzzO"),NA 6.27
478 (CzzHzzO,z), ' est.
5 490 (Cz,HzzO,z), 492 (Cz,Hz,O,Z), 506 (CZ,HzzO,z) total
and 508 far
(Cz,Hz,O") exact chemical structures unknown DP &
LSIIvIS
CA 02377118 2002-04-05
-29-
Table 10E
Atcohols
2-ethyl-1-hexanol 104-76-7 0.65
branched dodecanol isomer NA 0.57
1-dodecanol (dodecyi alcohol) 112-53-8 5.69
_-
1-tetradecano! (tetradecyl alcohol] 112-72-1 2.14
i
2-ethoxy-1-dodecanol 29718-44-3 2.a,7
1-hexadecanoi (hexadecyi alcohol) 36653-82-4 0.05
2-ethoxy-1-tetradecanol NA 1.09
Table lOF
Misceltaneo~ts Carnpounds
3,5,5-trimethyl-2-cyclohexene-1-one (isophorone)78-59-1 0.06
2,3-dihydro-2,5-dimethyl-4H-1-ber~zopyran-4-one69687-87-2 0.08
2 o a-hexylcinnamic aldehyde 101-86-0 0.06
squalene 7683-64-9 0.03
1-chloro-dodecane (dodecyl chloride) 112-52-7 0.33
'
sulfur (elemental sulfur six membered ring) NA 0.18
2 5 sulfur (S-8, cyclic sulfur, ortho#hrombic sulfur,10544-50-0 0.90
molecular
sulfur)
unknown 138 m.w. sulfur-containing compound NA 0.05
unknown 216 m.w. aromatic compound NA 0.87
3 o unknown 154 m.w. aromatic compound NA D.03
complex mixture of high molecular weight unknownNA 6.27
compounds in the range 400-1000, many of these est.
compounds are conjugates of the compounds identified total
in for
this study such as glycosides, polar conjugates, DP &
high m.w. LSIMS
esters etc. I
35
CA 02377118 2002-04-05
-30-
Exam",ple 6 - In vivo Study. Application of the Treatment Solution
to a Flaky Skin Mouse
The flaky skin mouse (fsn) is a genetically engineered
mouse with an autosomal recessive mutation causing the skin to
resemble that exhibited in human psoriasis (see Sundberg et al.,
J. Vet. D.iagn. Invest. 4:312-17, 1992)
In the homozygous affected flaky skin mouse (fsn/fsn),
where the mutation is on both chromosomes, histological features
such as marked acanthosis, hyperkeratosis with focal
LU parakeratosis, subcorneal pustules, dermal capillary dilation,
and dermal infiltration of inflammatory cells are seen.
Ten affected mice (fsn/fsn) and 10 normal littermate
controls (fsn/-) were obtained from The Jackson Laboratory in Bar
Harbor, ME. The animals were maintained.using standard diet and
housing:
The dorsal surface of each animal was shaved. One-half
of the dorsal surface received weekly topical treatments of the
solution of Example 2, while the other half served as an
untreated control. The solution of Example 2 was applied to the
skin using a sterile cotton swab. Treatments continued for up
to seven weeks. Weekly biopsies of treated and untreated areas
were also removed for histological examination. Following the
seventh week of treatment, the animals were necropsied for gross
pathological changes.
The solution of Example 2 was found to induce dramatic
gross and microscopic changes only for the hyperproliferative
skin of the affected (fsn/fsn) mice with minimal to no effects
noted for the (fsn/-) control mice. Observations by a veterinary
dermatopathologist described the skin response of the affected
animals as being similar to a "chemical burn." Histologic
findings indicated that the keratinocyte growth of the affected
mouse skin was markedly reduced, and that the treated skin began
to slough off a bit but remained fully attached as a biological
bandage allowing healing of the underlying skin. This effect was
not observed in the control animals or untreated skin in the
affected animals. Results are presented in Table 11.
CA 02377118 2002-04-05
-31-
Table 11
Summary of Flaky Skin Mouse Study
I.D. NUMBERPhenotype Histological Gross PathologicComments
Assessment Changes _
1 (fsnlfsn)Flaky skin Reduction None observed
in
proliferation
of
keratinocytes
2 (fsnlfsn)Fiaky skin Reduction None observed
in
proliferation
of
0 keratinocytes
3 - (fsnlfsn)Flaky skin Reduction None observed
in
proliferation
of
keratinocytes
(fsnlfsn) Flaky skin Reduction None observed
in
proliferation
of
keratinocytes
(fsn/fsnjFlaky skin Reduction None observed
, in
proliferation
of
keratinocytes
6 (fsnlfsn)Flaky skin Reduction None observed
in
proliferation
of
keratinocytes
7 (fsnlfsn)Flaky skin Reduction None observed
in
proliferation
of
' 0 keratinocytes
8 (fsnlfsn)Flaky skin Reduction None observed
in
proliferation
of
keratinocytes
9 (fsnlfsn)Flaky skin Reduction None observed
in
proliferation
of
keratinocytes
>_ S 10 (fsn/fsn)Confusing Marginal changeNone observedJackson tabs
phenotype suggest mistyping
of animal
11 (+!-) Normal skin No change None observed
12 (+!-) Normal skin No change None observed
13 (+I-) Normal skin No change None observed
1 4 (+I-) Normal skin No change None observed
3 0 15 (+/-) Normal skin No change None observed
16 (+I-) Normal skin No change tVone observed
17 (+I-) Normal skin No change None observed
18 (+p) Normal skin No change None observed
19 (+I-) Normal skin No change None observed
20 (+/-) Normal skin No change None observed
CA 02377118 2002-04-05
-32-
Example 7 - Bioassay - In vivo Study Bioassay
The in vivo study of Example 6 using the flaky skin
mouse model was indicative of keratinocyte cell specifically with
respect to the activity of the treatment solution compound.
S In vitro cell testing is necessary to detect activity
with respect to cell type for purposes of developing a bioassay
for treatment solution activity, and second to further study
results obtained using the flaky skin mice of Example 6. Because
human skin contains both keratinocytes and fibroblasts, either
of which may be involved in the psoriasis disease process, pure
cultures of human fibroblasts were grown from normal adult human
skin, keloid scars (hyperproliferative fibroblasts), and
commercially available certified pure cultures of normal human
adult epidermal keratinocytes. These cell types were tested
:S separately for their growth responses to treatment with the
treatment solution of Example 2.
Protocol
Cell growth is measured by examining the amounts of DNA
synthesis, for as cells grow and divide, more DNA is produced.
>o Radioactive thymidine is added to the cell culture media. The
assay is conducted as follows: Cultured fibroblasts are grown
in
complete minimal essential medium (CMEM) containing 10% fetal
bovine serum as a growth factor source. Epidermal keratinocytes
were cultured in the presence of complete keratinocyte growth
medium (CKGM) supplemented with bovine pituitary extract,
hydrocortisone, and epidermal growth factor (EGF) as a growth
factor source.
Healthy growing cells were seeded into Corning 24 well
tissue culture plates at a density of 1.0 X 10' cells per well in
1.0 ml of either CMEM or CKGM depending on cell type. The cells
were incubated for 24-36 hours or until they reached 60-70%
confluency at 37°C in the presence of 5% CO2. The medium was
then changed to either MEM without the 10% serum or KGM without
35 hydrocortisone or ECF, but leaving the BPE in the media. This
allows starvation of the cells, or their regression to a
nongrowth phase where although they are not dividing, they remain
CA 02377118 2002-04-05
-33-
biochemically active. The cells were allowed to incubate under
these conditions for 24 hours. The starving media were then
removed and replaced with 1.0 ml/well of complete growth media
CMEM or CKGM containing no treatment, treatment solution of
v Example 2, or a subfraction from Example 4 at a dilution of
1:5000, or solvent alone such as acetic acid or DMSO.
Statistically significant numbers of repetitions were performed
for each treatment. Additionally 1 ~Ci tritiated thymidine (3H)
was also added per well. Following these treatments, the cells
o were allowed to incubate for 24 hours using identical conditions
as above. Following the end of the incubation period, the wells
were washed 3 times with phosphate buffered saline (PBS) and
fixed with 12.50 trichloroacetic acid (TCA) for 10 minutes
followed by methanol for 10 minutes. The plates were air dried
S and the cells solubilized in 1.0 ml of 0.2 N NaOH at 37°C for 1
hour. Growth was determined by measuring the level of
radioactivity present. This was accomplished by counting 0.9 ml
of the solubilized cells in a scintillation counter.
0 Results
Fibroblasts
Fibroblasts from normal adult skin and keloid scar were
cultured and assayed for effects of the treatment solution on
growth as described above. Neither normal nor keloid fibroblasts
were inhibited by the treatment solution. A typical graph for
these experiments is shown in FIG. 24.
Keratinocytes
The treatment solution of Example 2 was fractioned as
in Example 4, with the chemical compositions of those fractions
30 analyzed in Example 5 and results shown in Tables 3-9 above.
Tables 12-14 summarize the percentages of the 4 main
active ingredients in each of those fractions, and mean
percentages of inhibition in the keratinocyte bioassay for each
fraction. Finally, mean percentages of inhibition are
35 normalized to the percentages of each active ingredient in the
fractions mentioned above.
CA 02377118 2002-04-05
-34-
Table 12
1?ercentaaes of 4 Main Active Ingredients
in the Treatment Solution of Example 2,
Compiled from Tables 3-10
Fraction FIG.1 3-methyl chrysuobialoe. aloe-emodin
l~F. N0. .ttuhralin cmodin morxucetate
XAD-2 Resin DCM 23 ~ 5.8dSd I 1.76;E0.1496 2.2396
Eluate
XAD-2 26 27:8256 12.7896 09E Os6
1. 0 Rcsin MeOH Eiuate~DCht-
lnsol.
Hydrolyzed Z-92 29 0.4296 4 7t 091, 096
XAD 96
Resin-MeOH Eluate
DCM
huol.
Z-92 Carboxylic 28 ' O. S5 ~6 1. 3 0 c 05>:
'~~
Acid Fx
15 Precipitate 36 19.(9 57.41 05F; 096
~
From Neutral!
Phenol Fx
Ncucral/ 31 2.5T56 5.79E 3-8y'.Sb1.3496
Phenol Silica Gel
MeOH
8luatt
Neutral! 31 5.06 15.0096 096 6.2496
~~I Sihca Gel DCM
Eluete
Ctude Z-92 10 11. 77 10. 37 0. 30960. 83 96
9<: St
Table 13
Fraction Analysis Summary Results -
r5 % Growth Inhibition of Keratinoc~tes by Fraction
Fraction Ra! Meant 7/12 '.1217128 8!3 8!28 9/1;9115 10/6 10!13
No. Inhibit.
From FIG. 1
23 6~~ X96
26 50 9E ~ 44 56
_ j 'b 96
1
~
29 - 7 sr; 7
~
3 0 28 4096 5176 3596 359
.3b 635 5095 7
31 305'6 30~ 3996
31 9; 9:; 9a 99 98 80 94 89
56 ~ 9E 56 56 ~
?7 zip 1756 2a~
?? 17'~ 1596 18'b
7? 1b5:; 1656
775b 7756 7956 84~ 6996
3 5 ?? 94 ~ 94
5~
CA 02377118 2002-04-05
-35-
Table 14
Percent Inhibition per Percentage of Ingredient
in Fraction for 4 Main Active Ingredients in the Treatment
Solution of Examble 2 (Calculated From Mean %
Inhibition from Table 13?
Fraecion DescriptionFraction Ref. 3-cnethy(chrysarobiBloc- aloe-emodin
From No.
FIG. 1 From FIG. 1 aiuhralin emodinmonoaceute
XAD-Z Resin DCM 23 10.58'x5.27~'x 4439E 2896
~
Eluate ..
~D-2 26 1.?9~;!-3.9156 096 0~
Resin MeOH Eluatc-DCM-
Insol.
Hydrolyzed Z-92 29 16.66 1.48 09b 096
XAD
Resin-MeOH Eluate
DCM
lnsoi.
Z-92 Carboxylic 23 72.725'E30.769b 0x 096
~
Acid Fx
Precipitate 36 3.19 1.1596 05E 096
From Neutral!
Phenol Fx
Neutral! 31 11.67 S. ?696 7. 22. 38
~ 85 96
~
Phenol Silica Gcl
McOli
Eluate
Neutral! 3I ! 8.18 6. I 09'0 14. 74
~. 3 96 56
Pherbl Silica Gcl
DCM
Eluate
Ctude Z-92 10 6.545 7.4296 256.6592.7796
Example 8 - Neutralizata.on Study
'S As the pH of the treatment solution of Example 2 is
very acidic, studies were undertaken to neutralize the Ph to 7.0
in order to examine inhibitory properties in the neutral state.
Crude treatment solution was adjusted to pH 7.O with 1.0 N NaOH.
Eferatinocytes were assayed for growth in the presence of the
3o neutralized extract using bioassay methodology as described
above, except that neutralized treatment solution was added as
the test compound at a dilution of 1:5000.
Results of this analysis indicated that neutralization
removes activity of the treatment solution. While not wishing
3s to be limited by theory, it is hypothesized that the addition of
acetic acid during the preparation sequence for the treatment
solution may acetylate reactive molecules, conferring additional
biological activity: This may confer increased abilities to
CA 02377118 2002-04-05
-36-
enter cells, etc. Neutralization of such entities by raising the
pH to 7.0 may render the active moieties inactive, as reflected
by the dramatically decreased activity observed with these
studies.
S
Example 9'- Protein Study
This Example examines whether or not any proteins were
present in the treatment solution of Example 2. Toward this end,
treatment solution was denatured at 65°C in the presence of
sodium dodecyl sulfate (SDS) and electrophoresed on 12%
polyacrylamide gels (PAGE) in the presence of SDS.
Following electrophoretic analysis and staining with
Coomassie blue, no proteins were evident on visual inspection of
the gel.
t5 While the illustrative embodiments of the invention
have been described with particularity, it will be understood
that various other modifications will be apparent to and can be
readily made by those skilled in the art without departing from
the spirit and scope of the invention. Accordingly, it is not
intended that the scope of the claims appended hereto be limited
to the examples and descriptions set forth herein but rather that
the claims be construed as encompassing all the features of
patentable novelty that reside in the present invention,
including all features that would be treated as equivalents
25 thereof by those skilled in the art to which this invention
pertains.