Note: Descriptions are shown in the official language in which they were submitted.
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
TITLE
HYDROXYALKANOYLAMINOLACTAMS AND RELATED STRUCTURES AS
INHIBITORS OF A~ PROTEIN PRODUCTION
FIELD OF THE INVENTION
This invention relates to novel lactams having drug
and bio-affecting properties, their pharmaceutical
compositions and methods of use. These novel compounds
inhibit the processing of amyloid precursor protein and,
more specifically, inhibit the production of A(3-peptide,
thereby acting to prevent the formation of neurological
deposits of amyloid protein. More particularly, the
present invention relates to the treatment of neurological
disorders related to (3-amyloid production such as
Alzheimer's disease and Down's Syndrome.
BACKGROUND OF THE INVENTION
Alzheimer's disease (AD) is a degenerative brain
disorder characterized clinically by progressive loss of
memory, temporal and local orientation, cognition,
reasoning, judgment and emotionally stability. AD is a
common cause of progressive dementia in humans and is one
of the major causes of death in the United States. AD has
been observed in all races and ethnic groups worldwide, and
is a major present and future health problem. No treatment
that effectively prevents AD or reverses the clinical
symptoms and underlying pathophysiology is currently
available (for review, Dennis J. Selkoe; Cell Biology of
the amyloid (beta)-protein precursor and the mechanism of
Alzheimer's disease, Annu Rev Cell Biol, 1994, 10: 373-
403).
Histopathological examination of brain tissue derived
upon autopsy or from neurosurgical specimens in effected
individuals revealed the occurrence of amyloid plaques and
neurofibrillar tangles in the cerebral cortex of such
patients. Similar alterations were observed in patients
with Trisomy 21 (Down's syndrome), and hereditary cerebral
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
hemorrhage with amyloidosis of the Dutch-type.
Neurofibrillar tangles are nonmembrane-bound bundles of
abnormal proteinaceous filaments and biochemical and
immunochemical studies led to the conclusion that their
principle protein subunit is an altered phosphorylated form
of the tau protein (reviewed in Selkoe, 1994).
Biochemical and immunological studies revealed that
the dominant proteinaceous component of the amyloid plaque
is an approximately 4.2 kilodalton (kD) protein of about 39
to 43 amino acids. This protein was designated A~i, (3-
amyloid peptide, and sometimes (3/A4; referred to herein as
A~3. In addition to its deposition in amyloid plaques, A(3
is also found in the walls of meningeal and parenchymal
arterioles, small arteries, capillaries, and sometimes,
venules. A(3 was first purified and a partial amino acid
reported in 1984 (Glenner and Wong, Biochem. Biophys. Res.
Commun. 120: 885-890). The isolation and sequence data for
the first 28 amino acids are described in U.S. Pat. No
4,666,829.
Compelling evidence accumulated during the last decade
revealed that A(3 is an internal polypeptide derived from a
type 1 integral membrane protein, termed (3 amyloid
precursor protein (APP). a APP is normally produced by
many cells both in vivo and in cultured cells, derived from
various animals and humans. A~3 is derived from cleavage of
(3 APP by as yet unknown enzyme (protease) system(s),
collectively termed secretases.
The existence of at least four proteolytic activities
has been postulated. They include (3 secretase(s),
generating the N-terminus of A~3, a secretase(s) cleaving
around the 16/17 peptide bond in A(3, and y secretases,
generating C-terminal A(3 fragments ending at position 38,
39, 40, 42, and 43 or generating C-terminal extended
precursors which are subsequently truncated to the above
polypeptides.
-2 -
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Several lines of evidence suggest that abnormal
accumulation of A(3 plays a key role in the pathogenesis of
AD. Firstly, A~i is the major protein found in amyloid
plaques. Secondly, A(3 is neurotoxic and may be causally
related to neuronal death observed in AD patients.
Thirdly, missense DNA mutations at position 717 in the 770
isoform of ~3 APP can be found in effected members but not
unaffected members of several families with a genetically
determined (familiar) form of AD. In addition, several
other (3 APP mutations have been described in familiar forms
of AD. Fourthly, similar neuropathological changes have
been observed in transgenic animals overexpressing mutant
forms of human ~3 APP. Fifthly, individuals with Down's
syndrome have an increased gene dosage of ~3 APP and develop
early-onset AD. Taken together, these observations
strongly suggest that A~3 depositions may be causally
related to the AD.
It is hypothesized that inhibiting the production of
A~3 will prevent and reduce neurological degeneration, by
controlling the formation of amyloid plaques, reducing
neurotoxicity and, generally, mediating the pathology
associated with A(3 production. One method of treatment
methods would therefore be based on drugs that inhibit the
formation of A(3 in vivo.
Methods of treatment could target the formation of A(3
through the enzymes involved in the proteolytic processing
of (3 amyloid precursor protein. Compounds that inhibit (3
or ysecretase activity, either directly or indirectly,
could control the production of A~i. Advantageously,
compounds that specifically target y secretases, could
control the production of A~3. Such inhibition of (3 or
ysecretases could thereby reduce production of A(3, which,
thereby, could reduce or prevent the neurological disorders
associated with A~3protein.
-3-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
SUMMARY OF THE INVENTION
One object of the present invention is to provide
novel compounds which are useful as inhibitors of the
production of A(3 protein or pharmaceutically acceptable
salts or prodrugs thereof.
It is another object of the present invention to
provide pharmaceutical compositions comprising a
pharmaceutically acceptable carrier and a therapeutically
effective amount of at least one of the compounds of the
present invention or a pharmaceutically acceptable salt
form or prodrug form thereof.
It is another object of the present invention to
provide a method for treating degenerative neurological
disorders comprising administering to a host in need of
such treatment a therapeutically effective amount of at
least one of the compounds of the present invention or a
pharmaceutically acceptable salt form or prodrug form
thereof.
These and other objects, which will become apparent
during the following detailed description, have been
achieved by the inventors' discovery that compounds of
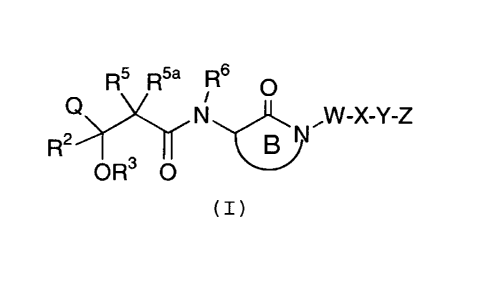
Formula (I):
R5 R5a Rs
O
N ,W-X-Y-Z
R2 B ~ N
OR3 O
(I)
or pharmaceutically acceptable salt form or prodrug forms
thereof, wherein Q, R2, R3, R5, RSa, R6, B, W, X, Y, and Z
are defined below, are effective inhibitors of the
production of A(3.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
Thus, in a first embodiment, the present invention
provides a novel compound of Formula (I):
-4-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R5 Rsa Rs
O
N ,W-X-Y-Z
R2 ~ B_N
OR3 O
(I)
or a pharmaceutically acceptable salt form or prodrug
thereof, wherein:
Q is Q1,
(C1-C3 alkyl)-O-Q1,
(C1-C3 alkyl)-S-Q1,
(C1-C3 alkyl)-S(=O)-Q1,
(C1-C3 alkyl)-S(=O)2-Q1, or
(C1-C3 alkyl)-N(R20)_Q1;
Q1 is C1-Cg alkyl substituted with 0-3 Rla;
C2-Cg alkenyl substituted with 0-3 Rla;
C2-Cg alkynyl substituted with 0-3 Rla;
C3-C1p cycloalkyl substituted with 0-3 Rlb;
C3-C1p carbocycle substituted with 0-3 Rlb;
C6-C1p aryl substituted with 0-3 Rlb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
Rla, at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, C1, F, Br, I, NR15R16~ CF3
C3-C1p carbocycle substituted with 0-3 Rlb;
C6-Clp aryl substituted with 0-3 Rlb; and
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
_5-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
Rlb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, C1-C4 haloalkyl-S-, and
(C1-C6 alkyl)-O-C(=O)-;
R2 is H, methyl, ethyl, propyl, or butyl;
R3 is H, C1-C6 alkyl, -C(=0)(C1-C6 alkyl), -C(=S)(C1-C6
alkyl), or -C(=0)NR21R22;
alternatively, R2 and OR3 are combined to form C=O or
C=N-OH;
R5 is H, OR14;
C1-C6 alkyl substituted with 0-3 RSb;
C1-C6 alkoxy substituted with 0-3 R5b;
C2-C6 alkenyl substituted with 0-3 RSb;
C2-C6 alkynyl substituted with 0-3 RSb;
C3-C1p cycloalkyl substituted with 0-3 R5c;
C3-C1p carbocycle substituted with 0-3 R5c;
C6-C1p aryl substituted with 0-3 R5c; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3R5c;
R5a is H, C1-C4 alkyl, or C2-Cg alkenyl;
alternatively, R5 and R5a may be combined to form a 3-7
membered cycloalkyl ring substituted with 0-3 RSc;
optionally the cycloalkyl ring formed by combining R5
-6-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
and R5a may be benzo fused, wherein the benzo fused
ring may be substituted with 0-3 Rsc;
RSb, at each occurrence, is independently selected from:
H, C1-C6 alkyl, CF3, OR14, C1, F, Br, I, =O, CN, NO2,
NR15R16, acetyl, SCH3, S (=O) CH3, S (=0) 2CH3, C2-C6
alkenyl, C2-C6 alkynyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, C1-Cg haloalkyl-S-,
C3-Clp cycloalkyl substituted with 0-3 RSc;
C3-Clp carbocycle substituted with 0-3 R5c;
C6-C1p aryl substituted with 0-3 R5c; and
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 RSc;
RSc, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-Cg alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
R6 is H or C1-C6 alkyl;
W is - (CR8R8a) p-;
p is 0, 1, 2, 3, or 4;
R8 and R8a, at each occurrence, are independently selected
from H, F, C1-C4 alkyl, C2-C4 alkenyl, C2-C4 alkynyl
and C3-Cg cycloalkyl;
X is a bond;
C6-C1p aryl substituted with 0-3 RXb;
C3-C1p cycloalkyl substituted with 0-3 RXb;
C3-C1p carbocycle substituted with 0-3 RXb; or
5 to 10 membered heterocycle substituted with 0-2 RXb;
-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
RXb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-Cg alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
Y is a bond or -(CR9R9a)t-V-(CR9R9a)u-;
t is 0, 1, 2, or 3;
a is 0, 1, 2, or 3;
R9 and R9a, at each occurrence, are independently selected
from H, F, C1-C6 alkyl or C3-Cg cycloalkyl;
V is a bond, -C(=O)-, -O-, -S-, -S(=0)-, -S(=O)2-,
-N (R19 ) -, -C (=O) NRl9b_ ~ -NRl9bC (=0) -. -NRl9bS (=0) 2-.
-S (=O) 2NR19b-, -C (=O) O-, or -OC (=O) -;
Z is H;
C1-Cg alkyl substituted with 0-2 R12;
Cz-C4 alkenyl substituted with 0-2 R12;
CZ-C4 alkynyl substituted with 0-2 R12;
C6-Clp aryl substituted with 0-4 Rl2b;
C3-C1p carbocycle substituted with 0-4 Rl2b; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rl2b;
Ring B is a 6, 7, or 8 membered lactam,
wherein the lactam is saturated, partially saturated
or unsaturated;
wherein each additional lactam carbon is substituted
with 0-2 R11; and,
-g-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
optionally, the lactam contains a heteroatom selected
from -N=, -NH-, -N(R10)-, -O-, -S-, -S(=O)-, and
-S(=O)2-;
additionally, two R11 substituents on adjacent atoms may be
combined to form C3-C6 carbocycle fused radical, a
benzo fused radical, or a 5 to 6 membered heteroaryl
fused radical,
wherein said 5 to 6 membered heteroaryl fused radical
comprises 1-2 heteroatoms selected from N, O, and S;
wherein said benzo fused radical or 5 to 6 membered
heteroaryl fused radical is substituted with 0-3 R13;
R10 is H, C (=O) R17, C (=O) OR17, C (=O) NR18R19, S (=O) 2NR18R19
S(=O)zRl7;
C1-C6 alkyl substituted with 0-2 RlOa;
C6-C1p aryl substituted with 0-4 Rlob;
C3-C1p carbocycle substituted with 0-3 RlOb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is optionally substituted with 0-3
RlOb;
RlOa, at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, C1, F, Br, I, =O, CN, N02, NR15R16~
CF3 ;
aryl substituted with 0-4 Rlob;
C3-C1p carbocycle substituted with 0-3 Rlob; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is optionally substituted with 0-3
RlOb;
-9-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
RlOb~ at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, C1-Cg alkoxy, Cl, F, Br, I, CN, N02,
NR15R16, CF3, acetyl, SCH3, S(=O)CH3, S(=O)2CH3, C1-C6
alkyl, C1-C4 alkoxy, C1-C4 haloalkyl, C1-C4 haloalkoxy,
and C1-C4 haloalkyl-S-;
R11, at each occurrence, is independently selected from H,
C1-C4 alkoxy, C1, F, Br, I, =O, CN, N02, NR18R19,
C ( =0 ) R17 ~ C ( =O ) OR17 , C ( =0 ) NR18R19 ~ S ( =O ) 2NR18R19
CF3;
C1-C6 alkyl substituted with 0-1 Rlla;
C6-C1p aryl substituted with 0-3 Rllb;
C3-C1p carbocycle substituted with 0-3 Rllb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rllb;
Rlla~ at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, Cl, F, Br, I, =O, CN, N02, NR15R16~
CF3;
phenyl substituted with 0-3 Rllb;
C3-C1p carbocycle substituted with 0-3 Rllb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rllb;
Rllb~ at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-Cg
haloalkyl, C1-Cg haloalkoxy, and C1-C4 haloalkyl-S-;
R12 at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16~ -C(=O)NR15R16~ CF3
-10-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
acetyl, SCH3, S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-Cq
alkoxy, C1-C4 haloalkyl, C1-C4 haloalkoxy, and C1-C4
haloalkyl-S-;
C6-C1p aryl substituted with 0-4 Rl2b;
C3-C1p carbocycle substituted with 0-4 Rl2b; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rl2b;
Rl2b~ at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=0)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
R13, at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, C1-C4 alkoxy, Cl, F, Br, I, CN, N02,
NR15R16 , and CF3 ;
R14, at each occurrence, is independently selected from H,
phenyl, benzyl, C1-C6 alkyl, and C2-C6 alkoxyalkyl;
Rl4a is H, phenyl, benzyl, or C1-C6 alkyl;
R15, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6 alkyl)-
C(=O)-, (C1-C6 alkyl)-O-C(=O)- and (C1-C6 alkyl)-
S(=O)2-;
R16, at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6
alkyl)-C(=O)-, (C1-C6 alkyl)-O-C(=O)- and (C1-C6
alkyl ) -S (=O) 2-;
-11-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
alternatively, -NR15R16 may be a heterocyclic ring selected
from the group piperidinyl, morpholinyl,
thiomorpholinyl, pyrrolidinyl, homopiperidinyl,
piperazinyl, and N-methylpiperizinyl;
R17 is H, C1-C6 alkyl, or C2-C6 alkoxyalkyl,
aryl substituted by 0-4 Rl7a, or
aryl-CH2- wherein said aryl is substituted by by 0-4
Rl7a;
Rl7a is H, methyl, ethyl, propyl, butyl, methoxy, ethoxy,
propoxy, butoxy, -OH, F, C1, Br, I, CF3, OCF3, SCH3,
S(=O)CH3, S(=O)2CH3, -NH2, -N(CH3)2, or C1-C4
haloalkyl;
R18, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6 alkyl)-
C(=O)- and (C1-C6 alkyl)-S(=O)2-;
alternatively, -NR17R18 may be a heterocyclic ring selected
from the group piperidinyl, morpholinyl,
thiomorpholinyl, pyrrolidinyl, homopiperidinyl,
piperazinyl, and N-methylpiperizinyl;
R19, at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6
alkyl)-C(=O)- and (C1-C6 alkyl)-S(=O)2-;
Rl9b, at each occurrence, is independently selected from H
and C1-C6 alkyl;
R2~ is H, OH, C1-C4 alkyl, phenyl, benzyl, or phenethyl;
R21, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, and phenethyl; and
-12-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R22, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, and phenethyl.
[2] In a preferred embodiment the present invention
provides a compound of Formula (I) wherein:
Q is Q1,
(C1-C3 alkyl)-0-Q1,
(C1-C3 alkyl)-S-Q1,
(C1-C3 alkyl)-S(=O)-Q1,
(C1-C3 alkyl)-S(=0)2-Q1, or
(C1-C3 alkyl)-N(R2o)-Q1;
Q1 is C1-C6 alkyl substituted with 0-3 Rla;
C2-C6 alkenyl substituted with 0-3 Rla;
C2-C6 alkynyl substituted with 0-3 Rla;
C3-C1p cycloalkyl substituted with 0-3 Rlb;
C3-Clp carbocycle substituted with 0-3 Rlb;
C6-C1p aryl substituted with 0-3 Rlb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
Rla, at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, Cl, F, Br, I, NR15R16~ CF3;
C3-Clp carbocycle substituted with 0-3 Rlb;
C6-C1p aryl substituted with 0-3 Rlb; and
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
-13-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Rlb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-Cg alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, C1-C4 haloalkyl-S-, and
(C1-C6 alkyl)-O-C(=O)-;
R2 is H, methyl, ethyl, propyl, or butyl;
R3 is H, C1-C4 alkyl, -C(=O)(C1-C4 alkyl), -C(=S)(C1-Cg
alkyl ) , or -C ( =O ) NR21R22 ;
R5 is H, OR14;
C1-C6 alkyl substituted with 0-3 R5b;
C1-C6 alkoxy substituted with 0-3 R5b;
C2-C6 alkenyl substituted with 0-3 RSb;
C2-C6 alkynyl substituted with 0-3 R5b;
C3-Clp cycloalkyl substituted with 0-3 RSc;
C3-C1p carbocycle substituted with 0-3 RSc;
C6-C1p aryl substituted with 0-3 R5c; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3R5c;
R5a is H, C1-C4 alkyl, or C2-C4 alkenyl;
alternatively, R5 and R5a may be combined to form a 3-7
membered cycloalkyl ring substituted with 0-3 R5c;
R5b, at each occurrence, is independently selected from:
H, C1-C6 alkyl, CF3, OR14, C1, F, Br, I, =O, CN, N02,
NR15R16, acetyl, SCH3, S(=O)CH3, S(=O)2CH3, C2-C6
alkenyl, C2-C6 alkynyl, C1-C4 alkoxy, Cl-C4
haloalkyl, C1-C4 haloalkoxy, and
C1-C4 haloalkyl-S-,
C3-Clp cycloalkyl substituted with 0-3 R5c;
C3-Clp carbocycle substituted with 0-3 RSc;
-14-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
C6-Cip aryl substituted with 0-3 RSc; and
to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
5 heterocycle is substituted with 0-3 RSc;
RSc, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)ZCH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-Cg haloalkoxy, and C1-C4 haloalkyl-S-;
R6 is H, methyl, or ethyl;
W is -(CR8R8a)p-;
p is 0, 1, or 2;
Rg and Rga, at each occurrence, are independently selected
from H, F, methyl, and ethyl;
X is a bond;
phenyl substituted with 0-3 RXb;
C3-C6 cycloalkyl substituted with 0-3 RXb; or
5 to 6 membered heterocycle substituted with 0-2 RXb;
RXb, at each occurrence, is independently selected from H,
OH, Cl, F, Br, I, CN, NO2, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-Cg
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
Y is a bond or -(CR9R9a)t-V-(CR9R9a)u-;
t is 0, 1, or 2;
a is 0, 1, or 2;
-15-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R9 and R9a, at each occurrence, are independently selected
from H, F, methyl, and ethyl;
V is a bond, -C(=0)-, -O-, -S-, -S(=O)-, -S(=O)2-,
-N(R19)-, -C(=O)NH-, -NHC(=O)-, -NHS(=O)2-, or
-S(=O)2NH-;
Z is H, halo;
C1-C4 alkyl substituted with 0-2 R12;
C2-C4 alkenyl substituted with 0-2 R12;
C2-C4 alkynyl substituted with 0-2 R12;
C6-C1p aryl substituted with 0-4 Rl2b;
C3-C6 carbocycle substituted with 0-4 Rl2b; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rl2b;
Ring B is a 7 membered lactam,
wherein the lactam is saturated, partially saturated
or unsaturated;
wherein each additional lactam carbon is substituted
with 0-2 R11; and,
optionally, the lactam contains a heteroatom selected
from -N=, -NH-, -N(R1~)-, -O-, -S-, -S(=O)-, and
-S(=0)2-;
additionally, two R11 substituents on adjacent atoms may be
combined to form C3-C6 carbocycle fused radical, a
benzo fused radical, or a 5 to 6 membered heteroaryl
fused radical,
wherein said 5 to 6 membered heteroaryl fused radical
comprises 1-2 heteroatoms selected from N, O, and S;
wherein said benzo fused radical or 5 to 6 membered
heteroaryl fused radical is substituted with 0-3 R13;
-16-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R1o is H, C (=O) R17, C (=O) OR17, C (=O) NR18R19, S (=O) 2NR18R19
S (=O) 2817;
C1-C6 alkyl substituted with 0-2 Rloa;
C6-C1o aryl substituted with 0-4 Rlob;
C3-C1p carbocycle substituted with 0-3 Rlob; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is optionally substituted with 0-3
RlOb;
Rloa~ at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, C1, F, Br, I, =O, CN, N02, NR15R16~
CF3, or aryl substituted with 0-4 Rlob;
RlOb~ at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, C1-C4 alkoxy, Cl, F, Br, I, CN, N02,
NR15R16, CF3, acetyl, SCH3, S(=O)CH3, S(=O)2CH3, C1-C6
alkyl, C1-C4 alkoxy, C1-C4 haloalkyl, C1-C4 haloalkoxy,
and C1-C4 haloalkyl-S-;
R11, at each occurrence, is independently selected from H,
C1-C4 alkoxy, C1, F, Br, I, =O, CN, N02, NR18R19,
C (=0) R17, C (=0) OR17, C (=0) NR18R19, S (=0) 2NR18R19
CF3;
C1-C6 alkyl substituted with 0-1 Rlla
C6-C1o aryl substituted with 0-3 Rllb;
C3-C1o carbocycle substituted with 0-3 Rllb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rllb;
-17-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Rlla~ at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, C1, F, Br, I, =O, CN, N02, NR15R16~
CF3;
phenyl substituted with 0-3 Rilb;
C3-Cip carbocycle substituted with 0-3 Rllb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rllb;
Rllb~ at each occurrence, is independently selected from H,
OH, Cl, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
R12 at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16~ -C(=O)NR15R16~ CF3,
acetyl, SCH3, S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4
alkoxy, C1-Cg haloalkyl, C1-C4 haloalkoxy, and C1-C4
haloalkyl-S-;
C6-Cip aryl substituted with 0-4 Rl2b;
C3-Cip carbocycle substituted with 0-4 Rl2b; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rl2b;
Rl2b~ at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
R13, at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, C1-C4 alkoxy, C1, F, Br, I, CN, N02,
3 5 NR15R16 , and CF3 ;
-18-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R14, at each occurrence, is independently selected from H,
phenyl, benzyl, C1-C6 alkyl, and C2-C6 alkoxyalkyl;
R15, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6 alkyl)-
C(=0)-, (C1-C6 alkyl)-O-C(=O)- and (C1-C6 alkyl)-
S(=0)2-;
R16, at each occurrence, is independently selected from H,
OH, C1-Cg alkyl, phenyl, benzyl, phenethyl, (C1-C6
alkyl)-C(=O)-, (C1-C6 alkyl)-O-C(=O)- and (C1-C6
alkyl)-S(=O)2-;
alternatively, -NR15R16 may be a heterocyclic ring selected
from the group piperidinyl, morpholinyl,
thiomorpholinyl, pyrrolidinyl, homopiperidinyl,
piperazinyl, and N-methylpiperizinyl;
R17 is H, aryl, aryl-CH2-, C1-C6 alkyl, or CZ-C6
alkoxyalkyl;
Rlg, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6 alkyl)-
C(=O)- and (C1-C6 alkyl)-S(=O)2-;
R19, at each occurrence, is independently selected from H,
OH, C1-Cg alkyl, phenyl, benzyl, phenethyl, (C1-C6
alkyl)-C(=O)- and (C1-C6 alkyl)-S(=O)2-;
alternatively, -NR17R18 may be a heterocyclic ring selected
from the group piperidinyl, morpholinyl,
thiomorpholinyl, pyrrolidinyl, homopiperidinyl,
piperazinyl, and N-methylpiperizinyl;
R2~ is H, OH, C1-C4 alkyl, phenyl, benzyl, or phenethyl;
-19-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R21, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, and phenethyl; and
R22, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, and phenethyl.
[2] In a preferred embodiment the present invention
provides a compound of Formula (I) wherein Ring B is
selected from:
O O O
R11' ~N~ R11 O R11
R1o
O ~ O
S~~R11 Rii~ ~ ~ 11~~
R
O O O
fV / ~ f IV / ~ ~ IV
~ ~
I R11 ~R11 ~R11
O O
f N/~ f
or
O
R10 R10
-20-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
wherein each benzo fused radical is substituted with 0-3
R13 .
[4] In a preferred embodiment the present invention
provides a compound of Formula (Ia):
R5 R5a O
Q N ,W-X-Y-Z
R2 ~ B . N
OH O
(Ia)
or a pharmaceutically acceptable salt form or prodrug
thereof, wherein:
Q is Q1,
(C1-C3 alkyl)-0-Q1,
(C1-C3 alkyl)-S-Q1,
(C1-C3 alkyl)-S(=O)-Q1,
(C1-C3 alkyl)-S(=0)2-Q1, or
(C1-C3 alkyl)-N(R2o)-Q1;
Q1 is C1-C6 alkyl substituted with 0-3 Rla;
C2-C6 alkenyl substituted with 0-3 Rla;
~C2-C6 alkynyl substituted with 0-3 Rla;
C3-C1p cycloalkyl substituted with 0-3 Rlb;
C3-C1p carbocycle substituted with 0-3 Rlb;
C6-C1p aryl substituted with 0-3 Rlb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
Rla, at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, C1, F, Br, I, NR15R16~ CF3;
C3-C1p carbocycle substituted with 0-3 Rlb;
-21-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
C6-Cip aryl substituted with 0-3 Rib; and
to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
5 heterocycle is substituted with 0-3 Rlb;
Rib, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=0)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, C1-C4 haloalkyl-S-, and
(C1-C6 alkyl)-O-C(=O)-;
R2 is H, methyl, or ethyl;
RS is H, OR14;
C1-C6 alkyl substituted with 0-3 RSb;
C1-C6 alkoxy substituted with 0-3 R5b;
C2-C6 alkenyl substituted with 0-3 R5b;
C2-C6 alkynyl substituted with 0-3 R5b;
C3-Cip cycloalkyl substituted with 0-3 RSc;
C3-Cip carbocycle substituted with 0-3 RSc;
C6-Cip aryl substituted with 0-3 RSc; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3R5c;
R5a is H, C1-C4 alkyl, or C2-C4 alkenyl;
alternatively, R5 and R5a may be combined to form a 3-7
membered cycloalkyl ring substituted with 0-3 RSc;
RSb, at each occurrence, is independently selected from:
H, C1-C6 alkyl, CF3, OR14, Cl, F, Br, I, =O, CN, N02,
NR15R16, acetyl, SCH3, S (=O) CH3, S (=O) 2CH3, C2-C6
-22-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
alkenyl, C2-C6 alkynyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and
C1-C4 haloalkyl-S-,
C3-Clp cycloalkyl substituted with 0-3 R5c;
C3-Clp carbocycle substituted with 0-3 R5c;
C6-Clp aryl substituted with 0-3 R5c; and
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 RSc;
RSc, at each occurrence, is independently selected from H,
OH, Cl, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-Cg alkoxy, C1-Cg
haloalkyl, C1-Cg haloalkoxy, and C1-C4 haloalkyl-S-;
W is - (CRgRBa) p-;
p is 0, 1, or 2;
Rg and R8a, at each occurrence, are independently selected
from H, F, methyl, and ethyl;
X is a bond;
phenyl substituted with 0-3 RXb;
C3-C6 cycloalkyl substituted with 0-3 RXb; or
5 to 6 membered heterocycle containing 1 to 3
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-2 RXb;
RXb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-Cg
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
Y is a bond or -(CR9R9a)t-V-(CR9R9a)u-;
-23-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
t is 0, 1, or 2;
a is 0, 1, or 2;
R9 and R9a, at each occurrence, are independently selected
from H, F, methyl, and ethyl;
V is a bond, -C(=O)-, -0-, -S-, -S(=O)-, -S(=O)2-,
-N(R19)-, -C(=O)NH-, or -NHC(=O)-;
Z is H, halo;
C1-C4 alkyl substituted with 0-2 R12;
C2-C4 alkenyl substituted with 0-2 R12;
C2-C4 alkynyl substituted with 0-2 R12;
C6-Clp aryl substituted with 0-4 Rl2b;
C3-C6 carbocycle substituted with 0-4 Rl2b; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rl2b;
Ring B is selected from:
O
O ~~N/~ { O /
13 N
N/ N~ ,/R
11 \~ > R1 ' ~ ~ yRl3
R ~I~/~R13
R11 ; R13 ; and R13/-J ~-r~Rl3 .
R11, at each occurrence, is independently selected from H,
C1-C4 alkoxy, C1, F, Br, I, =O, CN, N02, NR18R19,
C ( =O ) R17 ~ C ( =0 ) OR17 , C ( =O ) NR18R19 ~ S ( =O ) 2NR18R19
CF3;
C1-C6 alkyl substituted with 0-1 Rlla;
-24-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
C6-Clp aryl substituted with 0-3 Rllb;
C3-C1p carbocycle substituted with 0-3 Rllb; or
to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
5 sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rllb;
Rlla~ at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, C1, F, Br, I, =O, CN, N02, NR15R16~
CF3;
phenyl substituted with 0-3 Rllb;
C3-C1p carbocycle substituted with 0-3 Rllb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rllb;
Rllb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and Cl-C4 haloalkyl-S-;
R12 at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16~ -C(=O)NR15R16~ CF3
acetyl, SCH3, S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4
alkoxy, C1-C4 haloalkyl, C1-C4 haloalkoxy, and C1-C4
haloalkyl-S-;
C6-Clp aryl substituted with 0-4 Rl2b
C3-C1p carbocycle substituted with 0-4 Rl2b; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rl2b;
-25-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Rl2b~ at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
R13, at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, C1-C4 alkoxy, C1, F, Br, I, CN, N02,
NR15R16 , and CF3 ;
R14, at each occurrence, is independently selected from H,
phenyl, benzyl, C1-C6 alkyl, and C2-C6 alkoxyalkyl;
R15, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6 alkyl)-
C(=O)-, (C1-C6 alkyl)-O-C(=O)- and (C1-C6 alkyl)-
S (=O) 2-;
R16, at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6
alkyl)-C(=O)-, (C1-C6 alkyl)-O-C(=O)- and (C1-C6
alkyl)-S(=O)2-;
alternatively, -NR15R16 may be a heterocyclic ring selected
from the group piperidinyl, morpholinyl,
thiomorpholinyl, pyrrolidinyl, homopiperidinyl,
piperazinyl, and N-methylpiperizinyl;
R17 is H, aryl, aryl-CH2-, C1-C6 alkyl, or C2-C6
alkoxyalkyl;
R18, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6 alkyl)-
C(=O)- and (C1-C6 alkyl)-S(=O)2-;
-26-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R19, at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6
alkyl)-C(=O)- and (C1-C6 alkyl)-S(=O)2-;
alternatively, -NR18R19 may be a heterocyclic ring selected
from the group piperidinyl, morpholinyl,
thiomorpholinyl, pyrrolidinyl, homopiperidinyl,
piperazinyl, and N-methylpiperizinyl; and
R2~ is H, OH, C1-C4 alkyl, phenyl, benzyl, or phenethyl.
[5] In a preferred embodiment the present invention
provides a compound of Formula (Ia):
R5 R5a ~..~ O
Q~~ N ,W-X-Y-Z
R
OH O
(Ia)
wherein:
Ring B is selected from:
O ~ O N ~ O
/ ~ ~ N
13
N ~ N~ / yR 13 , R13
y R ~ \ / i
R11
2 0 R11 ; R13 ; and - ;
Q is Q1 or (C1-C3 alkyl)-O-Q1;
Q1 is C1-Cg alkyl substituted with 0-3 Rla;
C2-C6 alkenyl substituted with 0-3 Rla;
C2-C6 alkynyl substituted with 0-3 Rla;
C3-Clp cycloalkyl substituted with 0-3 Rlb;
C3-Clp carbocycle substituted with 0-3 Rlb;
C6-C1p aryl substituted with 0-3 Rlb; or
-27-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
5
Rla, at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, C1, F, Br, I, NR15R16~ CF3;
C3-Clp carbocycle substituted with 0-3 Rlb;
C6-C1p aryl substituted with 0-3 Rlb; and
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
Rlb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-Cg alkoxy, C1-C4
haloalkyl, Cl-C4 haloalkoxy, C1-C4 haloalkyl-S-, and
(C1-C6 alkyl)-O-C(=O)-;
R2 is H, methyl, or ethyl;
RS is H, OR14;
C1-C6 alkyl substituted with 0-3 R5b;
C2-C6 alkenyl substituted with 0-3 R5b;
C2-C6 alkynyl substituted with 0-3 R5b;
C3-C6 cycloalkyl substituted with 0-3 RSc;
C3-C6 carbocycle substituted with 0-3 RSc;
phenyl substituted with 0-3 RSc; or
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3R5c;
R5a is H, C1-C4 alkyl, or C2-C4 alkenyl;
-28-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
alternatively, R5 and R5a may be combined to form a C4-C7
cycloalkyl ring;
RSb, at each occurrence, is independently selected from:
H, C1-C6 alkyl, CF3, OR14, C1, F, Br, I, =O, CN, N02,
NR15R16, acetyl, SCH3, S(=O)CH3, S(=O)2CH3, C2-C6
alkenyl, CZ-C6 alkynyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and
C1-Cg haloalkyl-S-,
C3-Clp cycloalkyl substituted with 0-3 RSc;
C3-C1o carbocycle substituted with 0-3 RSc;
C6-C1p aryl substituted with 0-3 RSc; and
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 RSc;
R5c, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-Cg alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
W is - (CRgR8a) p-;
p is 0, 1, or 2;
R8 and R8a, at each occurrence, are independently selected
from H, methyl, and ethyl;
X is a bond;
phenyl substituted with 0-3 RXb;
C3-C6 cyclolakyl substituted with 0-3 RXb; or
5 to 6 membered heterocycle containing 1 to 3
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-2 RXb;
-29-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
RXb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I , CN, N02 , NR15R16 , CF3 , acetyl , SCH3 ,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
Y 1s a bond or -(CR9R9a)t-V-(CR9R9a)u-%
t is 0, 1, or 2;
a is 0, 1, or 2;
R9 and R9a, at each occurrence, are independently selected
from H, F, methyl, and ethyl;
V is a bond, -C(=O)-, -0-, -S-, -S(=O)-, -S(=O)2-,
-N(R19)-, -NHC(=O)-, or -C(=O)NH-;
Z is H, F, C1, Br;
C1-C4 alkyl substituted with 0-2 R12;
C2-C4 alkenyl substituted with 0-2 R12;
C2-C4 alkynyl substituted with 0-2 R12;
Cg-Clp aryl substituted with 0-4 Rl2b%
C3-C6 carbocycle substituted with 0-4 Rl2b; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rl2b%
R11, at each occurrence, is independently selected from
H, =O, NR18R19, C (=O) R17, C (=O) OR17, C (=O) NR1gR19,
S(=O)2NR1gR19, CF3;
C1-C6 alkyl substituted with 0-1 Rlla;
phenyl substituted with 0-3 Rllb;
C3-C6 carbocycle substituted with 0-3 Rllb; or
-30-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 Rllb;
5
Rlla~ at each occurrence, is independently selected from H,
C1-C4 alkyl, OR14, Cl, F, Br, =O, CN, N02, NR15R16~
CF3;
phenyl substituted with 0-3 Rllb;
C3-C6 carbocycle substituted with 0-3 Rllb; or
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 Rllb;
Rllb~ at each occurrence, is independently selected from H,
OH, C1, F, Br, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
R12 at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16~ -C(=O)NR15R16~ CF3
acetyl, SCH3, S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4
alkoxy, C1-C4 haloalkyl, C1-C4 haloalkoxy, and C1-C4
haloalkyl-S-;
C6-Clo aryl substituted with 0-4 Rl2b;
C3-C1p carbocycle substituted with 0-4 Rl2b; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rl2b;
Rl2b~ at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
-31-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-Cg
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
R13, at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, C1-C4 alkoxy, C1, F, Br, I, CN, N02,
NR15R16, and CF3;
R14, at each occurrence, is independently selected from H,
phenyl, benzyl, C1-C6 alkyl, and C2-C6 alkoxyalkyl;
R15, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6 alkyl)-
C(=O)-, (C1-C6 alkyl)-O-C(=O)- and (C1-C6 alkyl)-
S (=O) 2-;
R16, at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6
alkyl)-C(=O)-, (C1-C6 alkyl)-O-C(=O)- and (C1-C6
alkyl)-S(=O)2-;
alternatively, -NR15R16 may be a heterocyclic ring selected
from the group piperidinyl, morpholinyl,
thiomorpholinyl, pyrrolidinyl, homopiperidinyl,
piperazinyl, and N-methylpiperizinyl; and
R17 is H, aryl, aryl-CH2-, C1-C6 alkyl, or Cz-C6
alkoxyalkyl;
R18, at each occurrence, is independently selected from H,
C1-C6 alkyl, phenyl, benzyl, phenethyl, (C1-C6 alkyl)-
C(=O)- and (C1-C6 alkyl)-S(=O)2-;
alternatively, -NR18R19 may be a heterocyclic ring selected
from the group piperidinyl, morpholinyl,
-32-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
thiomorpholinyl, pyrrolidinyl, homopiperidinyl,
piperazinyl, and N-methylpiperizinyl; and
R19, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, butyl, phenyl, benzyl, and
phenethyl.
[5] In a preferred embodiment the present invention
provides a compound of Formula (Ia) wherein:
Q is Q1;
Q1 is C1-C6 alkyl substituted with 0-3 Rla;
C2-C6 alkenyl substituted with 0-3 Rla;
CZ-C6 alkynyl substituted with 0-3 Rla;
C3-C1o cycloalkyl substituted with 0-3 Rlb;
C3-Clp carbocycle substituted with 0-3 Rlb;
C6-C1p aryl substituted with 0-3 Rlb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
Rla, at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, C1, F, Br, I, NR15R16~ CF3;
C3-Clp carbocycle substituted with 0-3 Rlb;
C6-C1p aryl substituted with 0-3 Rlb; and
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
Rlb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=0)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-Cg
-33-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
haloalkyl, C1-C4 haloalkoxy, C1-C4 haloalkyl-S-, and
(C1-C6 alkyl)-O-C(=O)-;
R2 is H, methyl, or ethyl;
R5 is H, OR14;
C1-C6 alkyl substituted with 0-3 R5b;
C2-C6 alkenyl substituted with 0-3 Rsb; or
C2-C6 alkynyl substituted with 0-3 R5b;
R5a is H, methyl, ethyl, propyl, butyl, or C2-C4 alkenyl;
alternatively, R5 and R5a may be combined to form a C4-C7
cycloalkyl ring;
RSb, at each occurrence, is independently selected from:
H, methyl, ethyl, propyl, butyl, CF3, OR14, C1, F, Br,
I, =O, NR15R16~
C3-C7 cycloalkyl substituted with 0-3 R5c;
C3-C7 carbocycle substituted with 0-3 Rsc;
phenyl substituted with 0-3 RSc; and
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 R5c;
R5c, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C4 alkyl, C1-C3 alkoxy, C1-C2
haloalkyl, and C1-C2 haloalkoxy;
W is -(CHR8)p-;
p is 0 or 1;
R8 is H, methyl, or ethyl;
-34-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
X is a bond;
phenyl substituted with 0-2 RXb;
C5-C6 cycloalkyl substituted with 0-3 RXb; or
to 6 membered heterocycle containing 1 to 3
5 heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-2 RXb;
RXb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=0)2CH3, C1-C6 alkyl, C1-Cg alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
Y is a bond, -V-, -CH2-V-, -V-CH2-, or -CH2-V-CH2-;
V is a bond, -C(=O)-, -O-, -S-, -S(=O)-, -S(=O)2-, or
-N(R19) -
Z is H, F, C1, Br,
C1-C4 alkyl substituted with 0-2 R12;
C2-C4 alkenyl substituted with 0-2 R12;
C2-C4 alkynyl substituted with 0-2 R12;
C6-C1p aryl substituted with 0-4 Rl2b
C3-C6 carbocycle substituted with 0-4 Rl2b; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rl2b
R11, at each occurrence, is independently selected from
H~ =O~ NR18R19~ C(=O)R17~ C(=0)OR17, C(=O)NR18R19~
S(=O)ZNR18R19~ CF3:
C1-C6 alkyl substituted with 0-1 Rlla
phenyl substituted with 0-3 Rllb
C3-C6 carbocycle substituted with 0-3 Rllb; or
-35-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 Rllb; wherein said 5 to 7
5 membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, oxazolyl,
isoxazolyl, and tetrazolyl;
Rlla~ at each occurrence, is independently selected from H,
methyl, ethyl, propyl, butyl, methoxy, ethoxy,
propoxy, Cl, F, =O, NR15R16, CF3;
phenyl substituted with 0-3 Rllb;
C3-C6 carbocycle substituted with 0-3 Rllb; or
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 Rllb; wherein said 5 to 7
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, oxazolyl,
isoxazolyl, and tetrazolyl;
Rllb~ at each occurrence, is independently selected from H,
OH, C1, F, NR15R16, CF3, acetyl, SCH3, S(=O)CH3,
S(=O)2CH3, methyl, ethyl, propyl, butyl, methoxy,
ethoxy, propoxy, C1-C2 haloalkyl, and C1-C2
haloalkoxy;
R12 at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16~ -C(=O)NR15R16~ CF3
acetyl, SCH3, S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4
-36-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
alkoxy, C1-C4 haloalkyl, C1-C4 haloalkoxy, and C1-C4
haloalkyl-S-;
C6-C1p aryl substituted with 0-4 Rl2b;
C3-Clp carbocycle substituted with 0-4 Rl2b; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rl2b;
Rl2b, at each occurrence, is independently selected from H,
OH, Cl, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
R13, at each occurrence, is independently selected from H,
OH, Cl-C6 alkyl, Cl-C4 alkoxy, C1, F, Br, I, CN, N02,
NR15R16 , and CF3 ;
R14, at each occurrence, is independently selected from H,
phenyl, benzyl, C1-C4 alkyl, and C2-C4 alkoxyalkyl;
R15, at each occurrence, is independently selected from H,
methyl, ethyl, propyl, butyl, phenyl, benzyl, and
phenethyl;
R16, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, butyl, phenyl, benzyl,
phenethyl, CH3CHZC(=O)-, CH3C(=O)-, CH3CH20C(=O)-,
CH30C(=O)-, CH3CH2S(=O)2- and CH3S(=O)2-;
R17 is H, phenyl, benzyl, C1-C4 alkyl, or C2-C4 alkoxyalkyl;
R18, at each occurrence, is independently selected from H,
methyl, ethyl, propyl, butyl, phenyl, benzyl, and
phenethyl; and
-37-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R19, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, and butyl.
[7] In a preferred embodiment the present invention
provides a compound of Formula (Ia) wherein:
Q is Q1,
Q1 is C1-C6 alkyl substituted with 0-3 Rla;
C2-C6 alkenyl substituted with 0-3 Rla;
C2-C6 alkynyl substituted with 0-3 Rla;
C3-C6 cycloalkyl substituted with 0-3 Rlb;
phenyl substituted with 0-3 Rlb; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rlb;
Rla, at each occurrence, is independently selected from
H, methyl, ethyl, propyl, butyl, OR14, C1, F, Br, I,
NR15R16~ CF3;
C3-C6 carbocycle substituted with 0-3 Rlb;
phenyl substituted with 0-3 Rlb; and
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rlb;
Rlb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, methyl, ethyl, propyl, butyl,
methoxy, ethoxy, propoxy, C1-C2 haloalkyl, C1-C2
haloalkoxy, (methyl)OC(=O)-, (ethyl)OC(=O)-,
(propyl)OC(=O)-, and (butyl)OC(=O)-;
-38-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R2 is H or methyl ;
R5 is H, OR14;
C1-C4 alkyl substituted with 0-1 R5b;
C2-C4 alkenyl substituted with 0-1 RSb; or
C2-C4 alkynyl substituted with 0-1 R5b;
R5a is H, methyl, ethyl, propyl, or butyl;
alternatively, R5 and R5a may be combined to form a
cyclobutyl, cyclopentyl, cyclohexyl, or cycloheptyl
ring;
RSb, at each occurrence, is independently selected from:
H, methyl, ethyl, propyl, butyl, CF3, OR14, C1, F,
=O, NR15R16~
C3-C7 cycloalkyl substituted with 0-3 R5c;
C3-C7 carbocycle substituted with 0-3 RSc;
phenyl substituted with 0-3 RSc; and
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 RSc; wherein said 5 to 7
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, oxazolyl,
isoxazolyl, and tetrazolyl;
R5c, at each occurrence, is independently selected from H,
OH, C1, F, NR15R16 , CF3 , acetyl , SCH3 , S ( =O ) CH3 ,
S(=0)2CH3, methyl, ethyl, propyl, butyl, methoxy,
ethoxy, propoxy, C1-C2 haloalkyl, and C1-C2
haloalkoxy;
W is a bond, -CH2-, or -CH(CH3)-;
-39-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
X is a bond;
phenyl substituted with 0-1 RXb;
C5-C6 cycloalkyl substituted with 0-1 RXb; or
5 to 6 membered heterocycle containing 1 to 3
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-1 RXb; wherein said 5 to 6
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
pyrazolyl, imidazolyl, oxazolyl, and isoxazolyl;
RXb, at each occurrence, is independently selected from
H, OH, C1, F, Br, NR15R16, CF3, acetyl, SCH3, S (=O) CH3,
S(=O)2CH3, methyl, ethyl, propyl, butyl, methoxy,
ethoxy, propoxy, C1-C2 haloalkyl, and C1-C2
haloalkoxy;
Y is a bond, -V-, -V-CH2-, or -CH2V-;
V is a bond, -C(=O)-, -O-, -S-, -S(=O)-, -S(=O)2-, or
-N(R19)-;
Z is H, F, C1, Br,
C1-C4 alkyl substituted with 0-2 R12;
C2-C4 alkenyl substituted with 0-2 R12;
C2-C4 alkynyl substituted with 0-2 R12;
C6-Clp aryl substituted with 0-4 Rl2b;
C3-C6 carbocycle substituted with 0-4 Rl2b; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rl2b
-40-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Rll, at each occurrence, is independently selected from
H, NR18R19, CF3;
C1-C4 alkyl substituted with 0-1 Rlla;
phenyl substituted with 0-3 Rllb;
C3-C6 carbocycle substituted with 0-3 Rllb; or
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 Rllb; wherein said 5 to 7
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, oxazolyl,
isoxazolyl, and tetrazolyl;
Rlla~ at each occurrence, is independently selected from H,
methyl, ethyl, propyl, butyl, methoxy, ethoxy,
propoxy, C1, F, =O, NR15R16, CF3;
phenyl substituted with 0-3 Rllb;
C3-C6 carbocycle substituted with 0-3 Rllb; or
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 Rllb; wherein said 5 to 7
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, oxazolyl,
isoxazolyl, and tetrazolyl;
Rllb~ at each occurrence, is independently selected from H,
OH, C1, F, NR15R16, CF3, methyl, ethyl, propyl, butyl,
methoxy, ethoxy, propoxy, C1-C2 haloalkyl, and C1-C2
haloalkoxy;
-41-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R12 at each occurrence, is independently selected from H,
OH, Cl, F, Br, NR15R16, -C(=O)NR15R16, CF3, acetyl,
SCH3, S(=O)CH3, S(=O)2CH3, methyl, ethyl, propyl,
butyl, methoxy, ethoxy, propoxy, C1-C2 haloalkyl, and
C1-C2 haloalkoxy;
phenyl substituted with 0-4 Rl2b
C3-C6 carbocycle substituted with 0-4 Rl2b; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rl2b;
Rl2b~ at each occurrence, is independently selected from
H, OH, C1, F, Br, NR15R16, CF3, acetyl, SCH3, S (=O) CH3,
S(=O)2CH3, methyl, ethyl, propyl, butyl, methoxy,
ethoxy, propoxy, C1-C2 haloalkyl, and C1-C2
haloalkoxy;
R13, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, butyl, methoxy, ethoxy,
propoxy, C1, F, Br, CN, NR15R16, and CF3;
R14, at each occurrence, is independently selected from H,
phenyl, benzyl, methyl, ethyl, propyl, and butyl;
R15, at each occurrence, is independently selected from H,
methyl, ethyl, propyl, or butyl;
R16, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, butyl, phenyl, benzyl, and
phenethyl;
Rlg, at each occurrence, is independently selected from H,
methyl, ethyl, propyl, butyl, phenyl, benzyl and,
phenethyl; and
-42-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R19, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, butyl, phenyl, benzyl and,
phenethyl.
[8] In a preferred embodiment the present invention
provides a compound of Formula (Ia) wherein:
Q is -CH3, -CH2CH3, -CH2CH2CH3, -CH2CH2CH2CH3,
-CH2CH2CH2CH2CH3, -CH2CH2CH2CH2CH2CH3, -CH(CH3)2.
-CH(CH3)CH2CH3, -CHZCH(CH3)2, -CH2C(CH3)3.
-CF3, -CHZCF3, -CH2CH2CF3, -CHZCH2CH2CF3,
-CH=CH2, -CHZCH=CH2, -CH2C(CH3)=CH2, -CH2CH=C(CH3)z.
-CH2CH2CH=CHz, -CH2CH2C(CH3)=CH2, -CH2CH2CH=C(CH3)2,
cis-CH2CH=CH(CH3), cis-CH2CH2CH=CH(CH3),
trans-CH2CH=CH(CH3), trans-CH2CH2CH=CH(CH3);
-C=CH, -CH2C=CH, -CH2C=C(CH3),
cyclopropyl-, cyclobutyl-, cyclopentyl-, cyclohexyl-,
cyclopropyl-CH2-, cyclobutyl-CHz-, cyclopentyl-CH2-,
cyclohexyl-CH2-, cyclopropyl-CH2CH2-, cyclobutyl-CHZCH2-,
cyclopentyl-CH2CH2-, cyclohexyl-CHZCH2-,
phenyl-, 2-F-phenyl-, 3-F-phenyl-, 4-F-phenyl-,
4-methoxyphenyl-,4-ethoxyphenyl-, 4-propoxyphenyl-,
phenyl-CH2-, (2-F-phenyl)CH2-, (3-F-phenyl)CH2-,
(4-F-phenyl)CH2-, (2-C1-phenyl)CHZ-, (3-C1-phenyl)CH2-,
(4-C1-phenyl)CH2-,
(2,3-diF-phenyl)CH2-, (2,4-diF-phenyl)CH2-,
(2,5-diF-phenyl)CH2-, (2,6-diF-phenyl)CH2-,
(3,4-diF-phenyl)CH2-, (3,5-diF-phenyl)CH2-,
(2,3-diCl-phenyl)CH2-, (2,4-diCl-phenyl)CHZ-,
(2,5-diCl-phenyl)CH2-, (2,6-diCl-phenyl)CHZ-,
(3,4-diCl-phenyl)CH2-, (3,5-diCl-phenyl)CH2-,
-43-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
(3-F-4-Cl-phenyl)CH2-, (3-F-5-C1-phenyl)CH2-,
(3-C1-4-F-phenyl)CH2-,
2-furanyl-CH2-, 3-furanyl-CH2-, 2-thienyl-CH2-,
3-thienyl-CH2-, 2-pyridyl-CH2-, 3-pyridyl-CH2-,
4-pyridyl-CH2-, 1-imidazolyl-CH2-, 2-oxazolyl-CH2-,
4-oxazolyl-CH2-, 5-oxazolyl-CH2-, 3-isoxazolyl-CH2-,
4-isoxazolyl-CH2-, 5-isoxazolyl-CHZ-,
phenyl-CHZCH2-, (2-F-phenyl)CH2CH2-,
(3-F-phenyl)CH2CH2-, (4-F-phenyl)CH2CH2-,
(2-Cl-phenyl)CHZCH2-, (3-C1-phenyl)CH2CH2-,
(4-C1-phenyl)CHZCH2-,
(2,3-diF-phenyl)CH2CHZ-, (2,4-diF-phenyl)CH2CH2-,
(2,5-diF-phenyl)CH2CH2-, (2,6-diF-phenyl)CH2CH2-,
(3,4-diF-phenyl)CH2CH2-, (3,5-diF-phenyl)CH2CH2-,
(2,3-diCl-phenyl)CH2CH2-, (2,4-diCl-phenyl)CH2CH2-,
(2,5-diCl-phenyl)CH2CH2-, (2,6-diCl-phenyl)CH2CH2-,
(3,4-diCl-phenyl)CH2CH2-, (3,5-diCl-phenyl)CH2CH2-,
(3-F-4-C1-phenyl)CH2CH2-, (3-F-5-C1-phenyl)CH2CH2-;
furanyl-CH2CH2-, thienyl-CH2CH2-, pyridyl-CHZCH2-,
1-imidazolyl-CH2CH2-, oxazolyl-CH2CH2-,
isoxazolyl-CH2CHZ-, 3,5-dimethylisoxazol-4-yl-CH2CH2-,
phenyl-propyl-;
benzyl-CH(NH2)-, benzyl-CH(NHC(=O)-O-tBu)-,
benzyloxy-CH2-, pyrrolidin-2-yl-, or
3-t-butoxycarbonylpyrrolidin-2-yl-;
R2 is H or methyl;
R5 is -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CH2CH2CHZCH3,
-CH(CH3)CHZCH3, -CH2CH(CH3)2, -CH2C(CH3)3,
-CH2CH2CH2CH2CH3, -CH(CH3)CH2CH2CH3, -CHZCH(CH3)CHZCH3,
-CH2CH2CH(CH3)2, -CH(CH2CH3)2.
-CF3, -CH2CF3, -CH2CH2CF3, -CHZCH2CHZCF3, -CH2CH2CH2CH2CF3,
-44-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
-CH=CH2, -CH2CH=CH2, -CH2CH2CH=CH2, -CH=CHCH3,
cis-CH2CH=CH(CH3), traps-CH2CH=CH(CH3),
traps-CH2CH=CH(C6H5), -CH2CH=C(CH3)2, cis-CH2CH=CHCH2CH3,
traps-CH2CH=CHCH2CH3, cis-CH2CH2CH=CH(CH3), trans-
CH2CH2CH=CH(CH3), traps-CH2CH=CHCHZ(C6H5),
-C=CH, -CH2C=CH, -CH2C=C(CH3), -CH2C=C(C6H5),
-CHZCH2C=CH, -CH2CH2C=C(CH3), -CH2CHZC=C(C6H5),
-CH2CH2CH2C=CH, -CH2CH2CH2C=C(CH3), -CH2CHZCH2C=C(C6H5),
cyclopropyl-CH2-, cyclobutyl-CH2-, cyclopentyl-CH2-,
cyclohexyl-CH2-, (2-CH3-cyclopropyl)CH2-,
(3-CH3-cyclobutyl)CHZ-, cyclopropyl-CHZCH2-,
cyclobutyl-CHZCH2-, cyclopentyl-CH2CH2-,
cyclohexyl-CH2CH2-, (2-CH3-cyclopropyl)CH2CH2-,
(3-CH3-cyclobutyl)CH2CH2-,
phenyl-CH2-, (2-F-phenyl)CH2-, (3-F-phenyl)CH2-,
(4-F-phenyl)CHZ-, (3,5-diF-phenyl)CH2-, 2-furanyl-CH2-,
3-furanyl-CH2-, 2-thienyl-CH2-, 3-thienyl-CH2-,
2-pyridyl-CH2-, 3-pyridyl-CH2-,
4-pyridyl-CH2-, 1-imidazolyl-CH2-, 2-oxazolyl-CH2-,
4-oxazolyl-CH2-, 5-oxazolyl-CH2-, 3-isoxazolyl-CH2-,
4-isoxazolyl-CH2-, 5-isoxazolyl-CH2-,
phenyl-CH2CH2-, (2-F-phenyl)CH2CH2-, (3-F-phenyl)CH2CH2-,
(4-F-phenyl)CH2CH2-, furanyl-CH2CH2-, thienyl-CHZCH2-,
pyridyl-CH2CH2-, 1-imidazolyl-CH2CH2-, oxazolyl-CHZCH2-,
isoxazolyl-CH2CH2-;
methoxy, ethoxy, propoxy, or butoxy;
R5a is H;
alternatively, R5 and R5a may be combined to form
cyclopentyl, cyclohexyl, or cycloheptyl;
-45-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
W is a bond, -CHZ-, or -CH(CH3)-;
X is a bond;
W ~ I N
/ / N '~ N /
, , , N , ,
N~' ~~ '~ ~ N
or ~ ;
Y is a bond, -CH2-V-, -V-, or -V-CH2-;
V is a bond, -C(=O)-, -O-, -S-, -S(=O)-, -S(=O)2-, -NH-, or
-N (CH3 ) -;
Z is H, F, C1, Br, methyl, ethyl, n-propyl, i-propyl, n-
butyl, i-butyl, s-butyl, t-butyl,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
phenyl, 2-F-phenyl, 3-F-phenyl, 4-F-phenyl,
2-C1-phenyl, 3-C1-phenyl, 4-C1-phenyl,
2,3-diF-phenyl, 2,4-diF-phenyl, 2,5-diF-phenyl,
2,6-diF-phenyl, 3,4-diF-phenyl, 3,5-diF-phenyl,
2,3-diCl-phenyl, 2,4-diCl-phenyl, 2,5-diCl-phenyl,
2,6-diCl-phenyl, 3,4-diCl-phenyl, 3,5-diCl-phenyl,
3-F-4-C1-phenyl, 3-F-5-C1-phenyl, 3-C1-4-F-phenyl,
2-Me0-phenyl, 3-Me0-phenyl, 4-Me0-phenyl, 2-Me-phenyl,
3-Me-phenyl, 4-Me-phenyl, 2-MeS-phenyl, 3-MeS-phenyl,
4-MeS-phenyl, 2-CF30-phenyl, 3-CF30-phenyl,
4-CF30-phenyl,
furanyl, thienyl, pyridyl, 2-Me-pyridyl, 3-Me-pyridyl,
4-Me-pyridyl, 1-imidazolyl, oxazolyl, isoxazolyl,
1-benzimidazolyl, morpholino, N-piperinyl,
phenyl-CH2-, (2-F-phenyl)CH2-, (3-F-phenyl)CH2-,
-46-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
(4-F-phenyl)CH2-, (2-C1-phenyl)CH2-, (3-C1-phenyl)CH2-,
(4-C1-phenyl)CH2-, (2,3-diF-phenyl)CH2-,
(2,4-diF-phenyl)CH2-, (2,5-diF-phenyl)CH2-,
(2,6-diF-phenyl)CH2-, (3,4-diF-phenyl)CH2-,
(3,5-diF-phenyl)CH2-, (2,3-diCl-phenyl)CH2-,
(2,4-diCl-phenyl)CH2-, (2,5-diCl-phenyl)CH2-,
(2,6-diCl-phenyl)CH2-, (3,4-diCl-phenyl)CH2-,
(3,5-diCl-phenyl)CHZ-, (3-F-4-C1-phenyl)CH2-,
(3-F-5-C1-phenyl)CH2-, (3-C1-4-F-phenyl)CH2-,
(2-Me0-phenyl)CH2-, (3-Me0-phenyl)CHZ-,
(4-Me0-phenyl)CHZ-, (2-Ph0-phenyl)CH2-,
(3-Ph0-phenyl)CH2-, (4-Ph0-phenyl)CH2-,
(2-Me-phenyl)CHZ-, (3-Me-phenyl)CH2-,
(4-Me-phenyl)CH2-, (2-MeS-phenyl)CH2-,
(3-MeS-phenyl)CH2-, 4-MeS-phenyl)CH2-,
(2-CF30-phenyl)CH2-, (3-CF30-phenyl)CH2-,
(4-CF30-phenyl)CH2-, (furanyl)CH2-, (thienyl)CH2-,
(pyridyl)CH2-, (2-Me-pyridyl)CH2-, (3-Me-pyridyl)CH2-,
(4-Me-pyridyl)CHZ-, (1-imidazolyl)CH2-, (oxazolyl)CH2-,
(isoxazolyl)CH2-, (1-benzimidazolyl)CH2-,
(cyclopropyl)CH2-, (cyclobutyl)CH2-, (cyclopentyl)CH2-,
(cyclohexyl)CH2-, (morpholino)CH2-, (N-pipridinyl)CH2-,
phenyl-CH2CH2-, (phenyl)2CHCH2-, (2-F-phenyl)CH2CH2-,
(3-F-phenyl)CH2CH2-, (4-F-phenyl)CH2CH2-,
(2-C1-phenyl)CH2CHZ-, (3-C1-phenyl)CH2CH2-,
(4-C1-phenyl)CH2CH2-, (2,3-diF-phenyl)CH2CH2-,
(2,4-diF-phenyl)CH2CH2-, (2,5-diF-phenyl)CHZCH2-,
(2,6-diF-phenyl)CH2CH2-, (3,4-diF-phenyl)CH2CH2-,
(3,5-diF-phenyl)CH2CH2-, (2,3-diCl-phenyl)CH2CH2-,
(2,4-diCl-phenyl)CH2CH2-, (2,5-diCl-phenyl)CH2CH2-,
(2,6-diCl-phenyl)CH2CH2-, (3,4-diCl-phenyl)CH2CH2-,
(3,5-diCl-phenyl)CH2CH2-, (3-F-4-C1-phenyl)CH2CH2-,
(3-F-5-Cl-phenyl)CH2CH2-, (3-C1-4-F-phenyl)CH2CH2-,
(2-Me0-phenyl)CH2CH2-, (3-Me0-phenyl)CH2CH2-,
(4-Me0-phenyl)CH2CH2-, (2-Me-phenyl)CH2CH2-,
(3-Me-phenyl)CH2CH2-, (4-Me-phenyl)CH2CH2-,
(2-MeS-phenyl)CH2CH2-, (3-MeS-phenyl)CH2CH2-,
-47-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
(4-MeS-phenyl)CHZCH2-, (2-CF30-phenyl)CH2CH2-,
(3-CF30-phenyl)CH2CH2-, (4-CF30-phenyl)CH2CH2-,
(furanyl)CH2CH2-,(thienyl)CH2CH2-, (pyridyl)CH2CH2-,
(2-Me-pyridyl)CH2CH2-, (3-Me-pyridyl)CH2CH2-,
(4-Me-pyridyl)CH2CH2-, (imidazolyl)CH2CH2-,
(oxazolyl)CH2CH2-, (isoxazolyl)CH2CH2-,
(benzimidazolyl)CH2CH2-,(cyclopropyl)CHZCH2-,
(cyclobutyl)CH2CH2-,(cyclopentyl)CH2CH2-,
(cyclohexyl)CH2CH2-,(morpholino)CH2CH2-, or
(N-pipridinyl)CH2CH2-;
R11, at each occurrence, is independently selected from H,
methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, s
butyl, t-butyl, phenyl, benzyl, phenethyl, cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl,
cyclopropylmethyl, cyclobutylmethyl, cyclopentylmethyl,
cyclohexylmethyl, cycloheptylmethyl, cyclopropylethyl,
cyclobutylethyl, cyclopentylethyl, cyclohexylethyl,
2-F-phenyl-, 3-F-phenyl, 4-F-phenyl, 4-C1-phenyl, 4-CH3-
phenyl, 4-Me0-phenyl-, 4-CF3-phenyl, (4-F-phenyl)CH2-,
(4-C1-phenyl)CH2-, (4-CH3-phenyl)CH2-,
(4-CF3-phenyl)CH2-, (4-F-phenyl)CHZCH2-,
(4-C1-phenyl)CH2CH2-, (4-CH3-phenyl)CH2CH2-,
(4-CF3-phenyl)CH2CH2-, pyridin-2-yl-, pyridin-3-yl-,
4-CF3-pyridin-2-yl-, 4-CH3-pyridin-2-yl-, thiazol-2-yl-,
azapan-1-yl, N,N-dimethylamino, N,N-diethylamino, N,N-
dipropylamino, and N,N-dibutylamino; and
R13, at each occurrence, is independently selected from H,
MeO, F, and C1.
[9] In a preferred embodiment the present invention
provides a compound of Formula of Formula (Ic);
R5 Rsa H O
Q~~~ N ,W-X-Y-Z
R2 _N
OH O ~Rli
-48-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
(Ic)
or a pharmaceutically acceptable salt form or prodrug
thereof.
[10] In a preferred embodiment the present invention
provides a compound of Formula (Id);
R5 R5a H O
RQ'~ N ~ N.W _X_Y_Z
OH O N~ ~ ''!R13
R11 ~\R13
(Id)
or a pharmaceutically acceptable salt form or prodrug
thereof.
[11] In a preferred embodiment the present invention
provides a compound of Formula (Ie):
O -Y-Z
R2
R13
R
R5 R5a ~..~ O
~~N W_X
'N
OH O /
'i
13i
(Ie)
or a pharmaceutically acceptable salt form or prodrug
thereof.
[12] In a preferred embodiment the present invention
provides a compound selected from:
3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-5-
phenylpentyl)amino-1-methyl- 5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-5-
phenylpentyl)amino-1-methyl-5-(4-fluoro-phenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
-49-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2(R)-Benzyl-3(S)-hydroxyl-1-oxo-5-phenylpentyl)amino-1-
methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Isopropyl-3(S)-hydroxyl-1-oxo-5-phenylpentyl)amino
1-methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-4-(3,5-
difluorophenoxy)butyl)amino-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one;
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-4-(3,5-
difluorophenoxy)butyl)amino-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one;
3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-4-
phenoxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one;
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-4-phenoxybutyl)amino-
7-chloro-1-methyl-5-(4-fluorophenyl)-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(2(R)- Isobutyl -3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-4-phenoxybutyl)amino-7-
chloro-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-
2-one;
-50-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-4-phenoxybutyl)amino-
7-chloro-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2(R)-Benzyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-ISObutyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Isopropyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Methoxy-3(S)-hydroxyl-1-oxo-4-(4-
trifluoromethylbenzyloxy)butyl)amino-7-chloro-1-methyl-5-
phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-4-(2,4-
difluorobenzyloxy)butyl)amino-7-chloro-1-methyl-5-phenyl-
2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Vinyl-3(S)-hydroxyl-1-oxo-4-benzyloxybutyl)amino-7-
chloro-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-
2-one;
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
-51-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-5- phenylpentyl)amino-
1-methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-3-
cyclopropylpropyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-heptyl)amino-1-methyl-
5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(R)-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-heptyl)amino-1-
methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(S)-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-heptyl)amino-1-
methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-nonyl)amino-1-methyl-
5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-hexyl)amino-1-methyl-
5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-4- phenylbutyl)amino
1-methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-5- phenylpentyl)amino-1-
methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-6- phenylhexyl)amino-1-
methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-butyl)amino-1-methyl-
5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-octyl)amino-1-methyl-
5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
-52-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-heptyl)amino-1-methyl-5-
phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-3- phenylpropyl)amino-1-
methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-5,5-dimethyl-
hexyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-hexyl)amino-1-methyl-5-
phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-3-(4-
propoxyphenyl)propyl)amino-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one;
2-(R)-cyclopropylmethyl-3-(S)-hydroxylheptanoic acid (2-
oxo-1-(3-phenoxybenzyl)azapan-3-(S)-yl) amide;
2(R)-cyclopropylmethyl-5-(3,5-difluorophenyl)-3-(S)-
hydroxypentanoic acid (2-oxo-1-(3-phenoxybenzyl)azapan-3-
(S)-yl)amide;
4-cyclopentyl-2-(R)-cyclopropylmethyl-3-(S)-hydroxybutanoic
acid (2-oxo-1-(3-phenoxybenzyl)azapan-3-(S)-yl) amide;
2-(R)-cyclopropylmethyl-3-(S)-hydroxyheptanioc acid (1-(5-
bromo-3-pyridinyl)methyl-2-oxo-azapan-3-(S)-yl) amide;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(2-fluorophenyl)-1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(azapan-1-yl)-1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
-53-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2-(R)-cyclopropylmethyl-5-(3,5-difluorophenyl)-3(S)-
hydroxyl-1-oxopentyl)amino-1-methyl-5-(pyridn-2-yl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxopentyl)amino-1-methyl-5-(4-chlorophenyl)-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(4-methoxyphenyl)1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(4-methoxyphenyl)-1-methyl-2,3-dihydro-
1H-1,4-benxodiazepin-2-one;
3-(S)-(4-cyclopentyl-2-(R)-cyclopropylmethyl-3-(S)-
hydroxyl-1-oxobutyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(S)-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-oxohept-6-
enyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-oxohept-6-
enyl)amino-1-methyl-5-(4-trifluoromethyl-phenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(S)-(2-(R)-cyclopropylmethyl-5-(3,5-dimethylisoxazol-4-
yl)-3-(S)-hydroxyl-1-oxopentyl)amino-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-7-chloro-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
-54-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-(pyridin-2-yl)-2,3-dihydro-1H-1,4
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(4-fluorophenyl)-1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifouoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(5-cyclopentyl-2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxopentyl)amino-1-methyl-5-(pyridin-2-yl)-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(2-(R)-iso-butyl-3-(S)-hydroxyl-1-oxoheptyl)amino-1-
methyl-5-(4-trifluoromethylphenyl)-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(S)-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-oxo-5-
(thiophen-2-yl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(S)-(2-(R)-cyclopropylmethyl-5-(furan-2-yl)3-(S)-
hydroxyl-1-oxopentyl)amino-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one;
3-(5-cyclopentyl-2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxopentyl)amino-5-(4-fluorophenyl)-1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(S)-(2-(R)-cyclopropylmethyl-5-(3,5-difluorophenyl)-3-
(S)-hydroxy-1-oxopentyl)amino-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
-55-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(S)-(3-(S)-hydroxyl-2-(R)-(thiophen-2-yl)methyl-1-
oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(S)-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-7-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-7-methoxy-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(S)-(2-(R)-cyclobutylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(S)-(2-(R)-(3,5-difluorobenzyl)-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydor-1H-1,4-
benzodiaxepin-2-one;
3-(S)-(2-(R)-(furan-2-yl)methyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxl-1-oxo-5-
pheylpentyl)amino-1-methyl-5-(pyridin-2-yl)-2,3-dihydro-1H-
benzodiazepin-2-one;
3-(2-(R)-iso-butyl-3-(S)-hydroxyl-1-oxoheptyl)amino-5-(4-
fluorophenyl)-1-methyl-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
3-(2-(R)-iso-butyl-3-(S)-hydroxyl-1-oxoheptyl)amino-5-(4-
fluorophenyl)-1-methyl-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
-56-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-oxo-5-
phenylpentyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-5-(furan-2-yl)-3-(S)-hydroxyl-1-
oxopentyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(5-cyclopentyl-2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxopentyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-oxooctyl)amino-
1-methyl-5-(4-trifluoromethylphenyl)-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-oxononyl)amino-
1-methyl-5-(4-trifluoromethylphenyl)-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethyl(pyridin-2-
yl))-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(R)-cyclobutylmethyl-3-(S)-hydroxyl-1-oxoheptyl)amino-
1-methyl-5-(40trifluoromethylphenyl)-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopentylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-methyl-2-pyridiyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
-57-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-methyl-2-pyridyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-oxobutyl)amino-
1-methyl-5-(4-trifluoromethylphenyl)-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(S)-(2-(R)-(3-butenyl)-3-(S)-hydroxyl-1-oxoheptyl)amino-
1-methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(S)-(2-(R)-(3-methylbutyl)3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(S)-(2-(R)-ethyl-3-(S)-hydroxyl-1-oxoheptyl)amino-1-
methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(S)-(2-(R)-propyl-3-(S)-hydroxyl-1-oxoheptyl)amino-1
methyl-5-phenyl-2,3-dihydro-1,4-benzodiazepin-2-one;
3-(S)-(2-(R)-butyl-3-(S)-hydroxyl-1-oxoheptyl)amino-1-
methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(4-(S)-amino-3-(R)-hydroxyl-2-(R)-methyl-1-oxo-5-
phenylpentyl)amino-7-chloro-5-(2-fluorophenyl)-1-methyl-
2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(4-(S)-(tert-butoxycarbonylamino-3-(R)-hydroxyl-2-(R)-
methyl-1-oxo-5-phenylpentyl)amino-7-chloro-5-(2-
fluorophenyl)-1-methyl-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
3-(3-(tert-butoxycarbonylpyrrolidin-2-(R)-yl)-3-(R)-
hydroxyl-2-(R)-methyl-1-oxopropyl)amino-7-chloro-5-(2-
-58-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
fluorophenyl)-1-methyl-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
3-(3-(R)-hydroxyl-2-(R)-methyl-1-oxo-3-(pyrrolidin-2-(R)-
yl)propyl)-amino-7-chloro-5-(2-fluorophenyl)-1-methyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(4-benzyloxy-3-(R)-hydroxyl-2-(R)-iso-propyl-1-oxobutyl-
amino-7-chloro-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
2-(4-(S)-amino-3-(S)-hydroxyl-2-(S)-methyl-1-oxo-5-
phenylpentyl)amino-7-chloro-5-(2-flourophenyl)-1-methyl-
2,3-dihydro-1H-1,4-benzodiazepin-2-one;
2-(4-(S)-(tert-butoxycarbonylamino-3-(S)hydroxyl-2-(S)-
methyl-1-oxo-5-phenylpentyl)amino-7-chloro-5-(2-
fluorophenyl)-1-methyl-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(thiazol-2-yl)-2,3-dihydro-1H-
1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-cyclopropylmethyl-5-(thiazol-2-yl)-2,3-
dihydro-1H-l,4benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-cyclopropylmethyl-5-(4-
trifluoromethylphenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-benzyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
-59-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(3-phenoxybenzyl)-5-(4-trifluoromethyl-
phenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(3-pyridinylmethyl)-5-(4-trifluoromethyl-
phenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(S)-cyclopropylmethyl-3-(R)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethyl-phenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(S)-cyclopropylmethyl-3-(R)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(R)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(S)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(S)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-3-(S)-methyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethyl-phenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
-60-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(3-phenoxybenzyl)-5-methyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one;
3-(2-(R)-cyclopropylmehtyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-benzyl-5-methyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(3-(S)-acetoxy-2-(R)-iso-butyl-1-oxoheptyl)amino-5-(4-
fluorophenyl)-1-methyl-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
3-(S)-(5-cyclopentyl-2-(R)-cyclopropylmethyl-3-(S)-methoxy-
1-oxopentyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
1-(1-hydroxypentyl)cyclohexanecarboxylic acid(5-(4-
fluorophenyl)-1-methyl-2-oxo-2,3-dihydro-1H-1,4-
benzodiazepin-3-yl)amide;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-methyl-5H,7H-dibenzo[b,d]azepin-6-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-oxooctyl)amino-
5-methyl-5H,7H-dibenzo[b,d]azepin-6-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-oxononyl)amino-
5-methyl-5H,7H-dibenzo[b,d]azepin-6-one;
3-(2-(R)-cyclopropylmethyl-5-(furan-2-yl)-3-(S)-hydroxyl-1
oxopentyl)amino-5-methyl-5H,7H-dibenzo[b,d]azepin-6-one;
2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-heptanoic acid (2-
oxo-1-(3-phenylamino-benzyl)azapan-3-(S)-yl) amide;
-61-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-cyclopentyl-1-methyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-benzyl-1-methyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-benzyl-1-butyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-cycloheptyl-1-methyl -2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cycloropylmethyl-3-(S)-hydroxyl-1-oxoheptyl)amino-
1-benzyl-5-cycloheptyl-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-butyl-5-cycloheptyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(2-pyridinylmethyl)-5-(4-
trifluoromethylphenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(3-pyridinylmethyl)-5-(2-fluorophenyl)-
2,3-dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(S)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxopentyl)amino-1-(3-pyridynylmethyl)-5-(4-
trifluoromethylphenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
-62-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3-(2-1(R)-Cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(N,N-dibutylamino)-2,3-dihydro-
1H-1,4-benzodiazepin-2-one;
3-(2-(R)-Cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-n-butyl-5-t-butyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-Cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(2-oxo-3,3-dimethylbutyl)-5-n-butyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one;
3-(2-(R)-Cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-benzyl-5-t-butyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-Cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(2-picolyl)-5-n-butyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one;
3-(2-(R)-Isobutyl-3-(S)-hydroxyl-1-oxoheptyl)amino-1-
methyl-5-homopiperidino-2,3-dihydro-1H-1,4-benzodiazepin-2-
one;
3-(2-(R)-cyclopropylmethyl-1, 3-dioxoheptyl)amino-1-methyl-
5-(4-trifluoromethylphenyl)-2,3-dihydro-1H-1,4-
benzodiazepin-2-one; and
1-pentyrylcyclohexanecarboxylic acid (5-(4-fluorophenyl)-1-
methyl-2-oxo-2,3-dihydro-1H-1,4-benzodiazepin-3-yl) amide.
[13] In a preferred embodiment the present invention
provides a compound of Formula (I) wherein the
stereochemistry of carbon 3 in lactam ring B is of the S
configuration.
-63-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
[14] In a preferred embodiment the present invention
provides a compound of Formula (I) wherein the
stereochemistry of carbon 3 in lactam ring B is of the R
configuration.
[15] In a preferred embodiment the present invention
provides a compound of Formula (Ib) .
R5
R5a H O
01 N ,W-X-Y-Z
OH O
(Ib)
wherein:
Ring B is selected from:
O ~ O N/~ O
/ ~ ~ N
13
N ~ N~ ~ /R 13 ~ /R13
R11 \ R ~ ~
R11 . R13 .
and ~ ~ ,
Q1 is C1-C6 alkyl substituted with 0-3 Rla;
C2-C6 alkenyl substituted with 0-3 Rla.;
C2-C6 alkynyl substituted with 0-3 Rla;
C3-C1p cycloalkyl substituted with 0-3 Rlb;
C3-Clp carbocycle substituted with 0-3 Rlb;
C6-C1p aryl substituted with 0-3 Rlb; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
Rla, at each occurrence, is independently selected from H,
C1-C6 alkyl, OR14, C1, F, Br, I, NR15R16~ CF3;
C3-C1o carbocycle substituted with 0-3 Rlb;
C6-C1p aryl substituted with 0-3 Rlb; and
-64-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rlb;
5
Rlb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-Cg
haloalkyl, C1-C4 haloalkoxy, C1-C4 haloalkyl-S-, and
(C1-C6 alkyl)-O-C(=0)-;
R5 is OR14;
C1-C6 alkyl substituted with 0-3 R5b;
C2-C6 alkenyl substituted with 0-3 R5b; or
C2-C6 alkynyl substituted with 0-3 RSb;
R5a is H, methyl, ethyl, propyl, butyl, or C2-C4 alkenyl;
alternatively, R5 and R5a may be combined to form a C4-C7
cycloalkyl ring;
R5b, at each occurrence, is independently selected from:
H, methyl, ethyl, propyl, butyl, CF3, OR14, C1, F, Br,
I, =O, NR15R16~
C3-C7 cycloalkyl substituted with 0-3 R5c;
C3-C7 carbocycle substituted with 0-3 R5c;
phenyl substituted with 0-3 RSc; and
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 R5c;
R5c, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C4 alkyl, C1-C3 alkoxy, C1-C2
haloalkyl, and C1-Cz haloalkoxy;
-65-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
W is -(CHRB)p-;
p is 0 or 1;
R8 is H, methyl, or ethyl;
X is a bond;
phenyl substituted with 0-2 RXb;
C5-C6 cycloalkyl substituted with 0-3 RXb; or
5 to 6 membered heterocycle containing 1 to 3
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-2 RXb;
RXb, at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)ZCH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-C4 haloalkoxy, and C1-C4 haloalkyl-S-;
Y is a bond, -V-, -CH2-V-, -V-CH2-, or -CH2-V-CH2-;
V is a bond, -C (=O) -, -O-, -S-, -S (=O) -, -S (=0) 2-, or
-N(R19) _;
Z is H, F, C1, Br,
C1-C4 alkyl substituted with 0-2 R12;
C2-C4 alkenyl substituted with 0-2 R12;
C2-C4 alkynyl substituted with 0-2 R12;
C6-C1p aryl substituted with 0-4 Rl2b;
C3-C6 carbocycle substituted with 0-4 Rl2b; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rl2b
-66-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R11, at each occurrence, is independently selected from
H, =O, NR18R19, C (=O) R17, C (=O) ORl7, C (=O) NR18R19
S(=O)2NR18R19~ CF3;
C1-C6 alkyl substituted with 0-1 Rlla;
phenyl substituted with 0-3 Rllb;
C3-C6 carbocycle substituted with 0-3 Rllb; or
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 Rllb; wherein said 5 to 7
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, oxazolyl,
isoxazolyl, and tetrazolyl;
Rlla~ at each occurrence, is independently selected from H,
methyl, ethyl, propyl, butyl, methoxy, ethoxy,
propoxy, Cl, F, =O, NR15R16, CF3;
phenyl substituted with 0-3 Rllb;
C3-C6 carbocycle substituted with 0-3 Rllb; or
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 Rllb; wherein said 5 to 7
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, oxazolyl,
isoxazolyl, and tetrazolyl;
Rllb~ at each occurrence, is independently selected from H,
OH, C1, F, NR15R16, CF3, acetyl, SCH3, S(=O)CH3,
S(=O)2CH3, methyl, ethyl, propyl, butyl, methoxy,
ethoxy, propoxy, C1-CZ haloalkyl, and C1-CZ
haloalkoxy;
-67-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R12 at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16~ -C(=O)NR15R16~ CF3,
acetyl, SCH3, S(=O)CH3, S(=0)2CH3, C1-C6 alkyl, Cl-C4
alkoxy, C1-C4 haloalkyl, C1-C4 haloalkoxy, and C1-C4
haloalkyl-S-;
C6-C1p aryl substituted with 0-4 Rl2b
C3-Clp carbocycle substituted with 0-4 Rl2b; or
5 to 10 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 10 membered
heterocycle is substituted with 0-3 Rl2b
Rl2b~ at each occurrence, is independently selected from H,
OH, C1, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)2CH3, C1-C6 alkyl, C1-C4 alkoxy, C1-C4
haloalkyl, C1-Cg haloalkoxy, and C1-C4 haloalkyl-S-;
R13, at each occurrence, is independently selected from H,
OH, C1-C6 alkyl, C1-C4 alkoxy, C1, F, Br, I, CN, N02,
NR15R16 , and CF3 ;
R14, at each occurrence, is independently selected from H,
phenyl, benzyl, C1-C4 alkyl, and C2-C4 alkoxyalkyl;
R15, at each occurrence, is independently selected from H,
methyl, ethyl, propyl, butyl, phenyl, benzyl, and
phenethyl;
R16, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, butyl, phenyl, benzyl,
phenethyl, CH3CH2C(=O)-, CH3C(=0)-, CH3CH20C(=O)-,
CH30C(=O)-, CH3CH2S(=O)2- and CH3S(=0)2-;
R17 is H, phenyl, benzyl, C1-C4 alkyl, or C2-C4 alkoxyalkyl;
-68-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R18, at each occurrence, is independently selected from H,
methyl, ethyl, propyl, butyl, phenyl, benzyl, and
phenethyl; and
R19, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, and butyl.
[16] In a preferred embodiment the present invention
provides a compound of Formula (Ib) .
Q1 is C1-C6 alkyl substituted with 0-3 Rla;
C2-C6 alkenyl substituted with 0-3 Rla;
CZ-C6 alkynyl substituted with 0-3 Rla;.
C3-C6 cycloalkyl substituted with 0-3 Rlb;
phenyl substituted with 0-3 Rlb; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rlb;
Rla, at each occurrence, is independently selected from
H, methyl, ethyl, propyl, butyl, OR14, C1, F, Br, I,
NR15R16, CF3;
C3-C6 carbocycle substituted with 0-3 Rlb;
phenyl substituted with 0-3 Rlb; and
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rlb;
Rlb, at each occurrence, is independently selected from H,
OH, Cl, F, Br, I, CN, N02, NR15R16, CF3, acetyl, SCH3,
S(=O)CH3, S(=O)ZCH3, methyl, ethyl, propyl, butyl,
methoxy, ethoxy, propoxy, C1-C2 haloalkyl, C1-C2
-69-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
haloalkoxy, (methyl)OC(=O)-, (ethyl)OC(=O)-,
(propyl)OC(=O)-, and (butyl)OC(=O)-;
R5 1s OR14;
C1-C4 alkyl substituted with 0-1 RSb;
CZ-C4 alkenyl substituted with 0-1 RSb; or
CZ-Cg alkynyl substituted with 0-1 RSb;
R5a is H, methyl, ethyl, propyl, or butyl;
alternatively, R5 and R5a may be combined to form a
cyclobutyl, cyclopentyl, cyclohexyl, or cycloheptyl
ring;
RSb, at each occurrence, is independently selected from:
H, methyl, ethyl, propyl, butyl, CF3, OR14, C1, F,
=O, NR15R16
C3-C7 cycloalkyl substituted with 0-3 R5c;
C3-C7 carbocycle substituted with 0-3 RSc;
phenyl substituted with 0-3 R5c; and
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 RSc; wherein said 5 to 7
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, oxazolyl,
isoxazolyl, and tetrazolyl;
R5c, at each occurrence, is independently selected from H,
OH, C1, F, NR15R16, CF3, acetyl, SCH3, S(=O)CH3,
S(=O)ZCH3, methyl, ethyl, propyl, butyl, methoxy,
ethoxy, propoxy, C1-C2 haloalkyl, and C1-C2
haloalkoxy;
W is a bond, -CH2-, or -CH(CH3)-;
-70-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
X is a bond;
phenyl substituted with 0-1 RXb;
C5-C6 cycloalkyl substituted with 0-1 RXb; or
to 6 membered heterocycle containing 1 to 3
5 heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-1 RXb; wherein said 5 to 6
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
pyrazolyl, imidazolyl, oxazolyl, and isoxazolyl;
RXb, at each occurrence, is independently selected from
H, OH, C1, F, Br, NR15R16, CF3, acetyl, SCH3, S(=O)CH3,
S(=O)ZCH3, methyl, ethyl, propyl, butyl, methoxy,
ethoxy, propoxy, C1-C2 haloalkyl, and C1-Cz
haloalkoxy;
Y is a bond, -V-, -V-CHZ-, or -CH2V-;
V is a bond, -C(=O)-, -O-, -S-, -S(=O)-, -S(=O)2-, or
-N(R19)-;
Z is H, F, C1, Br,
C1-C4 alkyl substituted with 0-2 R12;
C2-C4 alkenyl substituted with 0-2 R12;
C2-C4 alkynyl substituted with 0-2 R12;
C6-C1p aryl substituted with 0-4 Rl2b;
C3-C6 carbocycle substituted with 0-4 Rl2b; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rl2b;
R11, at each occurrence, is independently selected from
-71-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
H, NR18R19, CF3;
C1-C4 alkyl substituted with 0-1 Rlla;
phenyl substituted with 0-3 Rllb;
C3-C6 carbocycle substituted with 0-3 Rllb; or
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 Rllb; wherein said 5 to 7
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, oxazolyl,
isoxazolyl, and tetrazolyl;
Rlla, at each occurrence, is independently selected from H,
methyl, ethyl, propyl, butyl, methoxy, ethoxy,
propoxy, C1, F, =O, NR15R16 ~ CF3 ;
phenyl substituted with 0-3 Rllb;
C3-C6 carbocycle substituted with 0-3 Rllb; or
5 to 7 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 7 membered heterocycle
is substituted with 0-3 Rlib; wherein said 5 to 7
membered heterocycle is selected from pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl,
thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, oxazolyl,
isoxazolyl, and tetrazolyl;
Rllb, at each occurrence, is independently selected from H,
OH, C1, F, NR15R16, CF3, methyl, ethyl, propyl, butyl,
methoxy, ethoxy, propoxy, C1-C2 haloalkyl, and C1-C2
haloalkoxy;
R12 at each occurrence, is independently selected from H,
OH, C1, F, Br, NR15R16~ _C(-O)NR15R16, CF3, acetyl,
-72-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
SCH3, S(=O)CH3, S(=O)2CH3, methyl, ethyl, propyl,
butyl, methoxy, ethoxy, propoxy, C1-C2 haloalkyl, and
C1-CZ haloalkoxy;
phenyl substituted with 0-4 Rl2b;
C3-C6 carbocycle substituted with 0-4 Rl2b; or
5 to 6 membered heterocycle containing 1 to 4
heteroatoms selected from nitrogen, oxygen, and
sulphur, wherein said 5 to 6 membered heterocycle
is substituted with 0-3 Rl2b;
Rl2b~ at each occurrence, is independently selected from
H, OH, C1, F, Br, NR15R16, CF3, acetyl, SCH3, S(=O)CH3,
S(=O)ZCH3, methyl, ethyl, propyl, butyl, methoxy,
ethoxy, propoxy, C1-C2 haloalkyl, and C1-CZ
haloalkoxy;
R13, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, butyl, methoxy, ethoxy,
propoxy, C1, F, Br, CN, NR15R16, and CF3;
R14, at each occurrence, is independently selected from H,
phenyl, benzyl, methyl, ethyl, propyl, and butyl;
R15, at each occurrence, is independently selected from H,
methyl, ethyl, propyl, or butyl;
R16, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, butyl, phenyl, benzyl, and
phenethyl;
R18, at each occurrence, is independently selected from H,
methyl, ethyl, propyl, butyl, phenyl, benzyl and,
phenethyl; and
-73-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
R19, at each occurrence, is independently selected from H,
OH, methyl, ethyl, propyl, butyl, phenyl, benzyl and,
phenethyl.
[17] In a preferred embodiment the present invention
provides a compound of Formula (Ib)wherein:
Q1 is -CH3, -CHZCH3, -CH2CH2CH3, -CH2CH2CH2CH3,
-CH2CH2CH2CH2CH3, -CH2CH2CH2CHZCH2CH3, -CH(CH3)2,
-CH(CH3)CH2CH3, -CH2CH(CH3)2, -CH2C(CH3)3,
-CF3, -CH2CF3, -CH2CH2CF3, -CH2CH2CH2CF3,
-CH=CH2, -CH2CH=CH2, -CH2C(CH3)=CH2, -CH2CH=C(CH3)2.
-CHZCH2CH=CH2, -CH2CH2C(CH3)=CH2, -CH2CH2CH=C(CH3)z.
cis-CH2CH=CH(CH3), cis-CH2CH2CH=CH(CH3),
trans-CH2CH=CH(CH3), trans-CHZCH2CH=CH(CH3);
-C=CH, -CH2C=CH, -CH2C=C (CH3 ) ,
cyclopropyl-, cyclobutyl-, cyclopentyl-, cyclohexyl-,
cyclopropyl-CH2-, cyclobutyl-CH2-, cyclopentyl-CH2-,
cyclohexyl-CHZ-, cyclopropyl-CH2CH2-, cyclobutyl-CH2CH2-,
cyclopentyl-CH2CH2-, cyclohexyl-CH2CH2-,
phenyl-, 2-F-phenyl-, 3-F-phenyl-, 4-F-phenyl-,
4-methoxyphenyl-,4-ethoxyphenyl-, 4-propoxyphenyl-,
phenyl-CH2-, (2-F-phenyl)CHZ-, (3-F-phenyl)CH2-,
(4-F-phenyl)CH2-, (2-C1-phenyl)CH2-, (3-C1-phenyl)CH2-,
(4-Cl-phenyl)CH2-,
(2,3-diF-phenyl)CH2-, (2,4-diF-phenyl)CH2-,
(2,5-diF-phenyl)CHZ-, (2,6-diF-phenyl)CH2-,
(3,4-diF-phenyl)CH2-, (3,5-diF-phenyl)CH2-,
(2,3-diCl-phenyl)CHZ-, (2,4-diCl-phenyl)CH2-,
(2,5-diCl-phenyl)CH2-, (2,6-diCl-phenyl)CH2-,
(3,4-diCl-phenyl)CHZ-, (3,5-diCl-phenyl)CH2-,
-74-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
(3-F-4-C1-phenyl)CH2-, (3-F-5-C1-phenyl)CH2-,
(3-C1-4-F-phenyl)CH2-,
2-furanyl-CH2-, 3-furanyl-CH2-, 2-thienyl-CH2-,
3-thienyl-CH2-, 2-pyridyl-CH2-, 3-pyridyl-CH2-,
4-pyridyl-CH2-, 1-imidazolyl-CH2-, 2-oxazolyl-CH2-,
4-oxazolyl-CH2-, 5-oxazolyl-CH2-, 3-isoxazolyl-CH2-,
4-isoxazolyl-CH2-, 5-isoxazolyl-CH2-,
phenyl-CH2CH2-, (2-F-phenyl)CH2CH2-,
(3-F-phenyl)CH2CH2-, (4-F-phenyl)CHZCH2-,
(2-C1-phenyl)CH2CH2-, (3-C1-phenyl)CH2CH2-,
(4-Cl-phenyl)CH2CH2-,
(2,3-diF-phenyl)CHZCHZ-, (2,4-diF-phenyl)CH2CH2-,
(2,5-diF-phenyl)CH2CHz-, (2,6-diF-phenyl)CH2CH2-,
(3,4-diF-phenyl)CH2CH2-, (3,5-diF-phenyl)CH2CH2-,
(2,3-diCl-phenyl)CH2CH2-, (2,4-diCl-phenyl)CH2CH2-,
(2,5-diCl-phenyl)CH2CH2-, (2,6-diCl-phenyl)CH2CH2-,
(3,4-diCl-phenyl)CH2CH2-, (3,5-diCl-phenyl)CHZCH2-,
(3-F-4-C1-phenyl)CH2CH2-, (3-F-5-C1-phenyl)CH2CH2-;
furanyl-CH2CH2-, thienyl-CH2CH2-, pyridyl-CH2CH2-,
1-imidazolyl-CH2CHZ-, oxazolyl-CH2CH2-,
isoxazolyl-CH2CH2-, 3,5-dimethylisoxazol-4-yl-CH2CH2-,
phenyl-propyl-;
benzyl-CH(NH2)-, benzyl-CH(NHC(=O)-O-tBu)-,
benzyloxy-CH2-, pyrrolidin-2-yl-, or
3-t-butoxycarbonylpyrrolidin-2-yl-;
R5 is -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CH2CH2CH2CH3,
-CH(CH3)CH2CH3, -CH2CH(CH3)2, -CH2C(CH3)3,
-CH2CH2CH2CH2CH3, -CH(CH3)CH2CH2CH3, -CH2CH(CH3)CH2CH3,
-CH2CH2CH(CH3)2, -CH(CH2CH3)2,
-CF3, -CH2CF3, -CH2CH2CF3, -CH2CH2CH2CF3, -CH2CH2CH2CH2CF3,
-CH=CH2, -CH2CH=CH2, -CH2CH2CH=CH2, -CH=CHCH3,
-75-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
cis-CH2CH=CH(CH3), trans-CH2CH=CH(CH3),
trans-CH2CH=CH(C6H5), -CH2CH=C(CH3)2, cis-CHZCH=CHCHZCH3,
trans-CH2CH=CHCH2CH3, cis-CH2CH2CH=CH(CH3), trans-
CH2CH2CH=CH(CH3), trans-CH2CH=CHCH2(C6H5),
-C=CH, -CH2C=CH, -CH2C=C(CH3), -CHIC=C(C6H5),
-CH2CH2C=CH, -CH2CH2C=C(CH3), -CH2CH2C=C(C6H5),
-CH2CHZCH2C=CH, -CH2CHZCH2C=C(CH3), -CH2CH2CH2C=C(C6H5),
cyclopropyl-CH2-, cyclobutyl-CH2-, cyclopentyl-CHZ-,
cyclohexyl-CH2-, (2-CH3-cyclopropyl)CH2-,
(3-CH3-cyclobutyl)CH2-, cyclopropyl-CH2CH2-,
cyclobutyl-CH2CH2-, cyclopentyl-CH2CH2-,
cyclohexyl-CH2CH2-, (2-CH3-cyclopropyl)CH2CH2-,
(3-CH3-cyclobutyl)CH2CH2-,
phenyl-CH2-, (2-F-phenyl)CHZ-, (3-F-phenyl)CH2-,
(4-F-phenyl)CH2-, (3,5-diF-phenyl)CH2-, 2-furanyl-CH2-,
3-furanyl-CH2-, 2-thienyl-CH2-, 3-thienyl-CH2-,
2-pyridyl-CH2-,.3-pyridyl-CH2-,
4-pyridyl-CH2-, 1-imidazolyl-CH2-, 2-oxazolyl-CH2-,
4-oxazolyl-CH2-, 5-oxazolyl-CH2-, 3-isoxazolyl-CHZ-,
4-isoxazolyl-CH2-, 5-isoxazolyl-CH2-,
phenyl-CH2CH2-, (2-F-phenyl)CH2CH2-, (3-F-phenyl)CH2CH2-,
(4-F-phenyl)CHZCH2-, furanyl-CH2CH2-, thienyl-CH2CH2-,
pyridyl-CH2CH2-, 1-imidazolyl-CH2CH2-, oxazolyl-CH2CH2-,
isoxazolyl-CH2CH2-;
methoxy, ethoxy, propoxy, or butoxy;
R5a is H;
alternatively, R5 and R5a may be combined to form
cyclopentyl, cyclohexyl, or cycloheptyl;
W is a bond, -CH2-, or -CH(CH3)-;
-76-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
X is a bond;
\~ ~ ~ N ~ ~ ~ \~ ~ ~ \~ ~ ~ \~i
/ N ~ N /
N
N~ ~ '~ ~~ N
, ; ~ ; or ~ ;
Y is a bond, -CH2-V-, -V-, or -V-CH2-;
V is a bond, -C(=O)-, -O-, -S-, -S(=O)-, -S(=O)2-, -NH-, or
-N ( CH3 ) - ;
Z is H, F, C1, Br, methyl, ethyl, n-propyl, i-propyl, n-
butyl, i-butyl, s-butyl, t-butyl,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
phenyl, 2-F-phenyl, 3-F-phenyl, 4-F-phenyl,
2-Cl-phenyl, 3-C1-phenyl, 4-C1-phenyl,
2,3-diF-phenyl, 2,4-diF-phenyl, 2,5-diF-phenyl,
2,6-diF-phenyl, 3,4-diF-phenyl, 3,5-diF-phenyl,
2,3-diCl-phenyl, 2,4-diCl-phenyl, 2,5-diCl-phenyl,
2,6-diCl-phenyl, 3,4-diCl-phenyl, 3,5-diCl-phenyl,
3-F-4-C1-phenyl, 3-F-5-Cl-phenyl, 3-C1-4-F-phenyl,
2-Me0-phenyl, 3-Me0-phenyl, 4-Me0-phenyl, 2-Me-phenyl,
3-Me-phenyl, 4-Me-phenyl, 2-MeS-phenyl, 3-MeS-phenyl,
4-MeS-phenyl, 2-CF30-phenyl, 3-CF30-phenyl,
4-CF30-phenyl,
furanyl, thienyl, pyridyl, 2-Me-pyridyl, 3-Me-pyridyl,
4-Me-pyridyl, 1-imidazolyl, oxazolyl, isoxazolyl,
1-benzimidazolyl, morpholino, N-piperinyl,
phenyl-CH2-, (2-F-phenyl)CH2-, (3-F-phenyl)CH2-,
(4-F-phenyl)CH2-, (2-C1-phenyl)CH2-, (3-C1-phenyl)CH2-,
(4-C1-phenyl)CH2-, (2,3-diF-phenyl)CH2-,
-77-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
(2,4-diF-phenyl)CHZ-, (2,5-diF-phenyl)CHz-,
(2,6-diF-phenyl)CH2-, (3,4-diF-phenyl)CH2-,
(3,5-diF-phenyl)CH2-, (2,3-diCl-phenyl)CH2-,
(2,4-diCl-phenyl)CH2-, (2,5-diCl-phenyl)CH2-,
(2,6-diCl-phenyl)CH2-, (3,4-diCl-phenyl)CH2-,
(3,5-diCl-phenyl)CH2-, (3-F-4-C1-phenyl)CH2-,
(3-F-5-Cl-phenyl)CH2-, (3-C1-4-F-phenyl)CH2-,
(2-Me0-phenyl)CH2-, (3-Me0-phenyl)CH2-,
(4-Me0-phenyl)CH2-, (2-Ph0-phenyl)CH2-,
(3-Ph0-phenyl)CH2-, (4-Ph0-phenyl)CH2-,
(2-Me-phenyl)CH2-, (3-Me-phenyl)CH2-,
(4-Me-phenyl)CH2-, (2-MeS-phenyl)CH2-,
(3-MeS-phenyl)CHZ-, 4-MeS-phenyl)CHZ-,
(2-CF30-phenyl)CHZ-, (3-CF30-phenyl)CHZ-,
(4-CF30-phenyl)CH2-, (furanyl)CH2-, (thienyl)CHZ-,
(pyridyl)CH2-, (2-Me-pyridyl)CH2-, (3-Me-pyridyl)CH2-,
(4-Me-pyridyl)CH2-, (1-imidazolyl)CH2-, (oxazolyl)CH2-,
(isoxazolyl)CH2-, (1-benzimidazolyl)CH2-,
(cyclopropyl)CHZ-, (cyclobutyl)CH2-, (cyclopentyl)CHZ-,
(cyclohexyl)CH2-, (morpholino)CH2-, (N-pipridinyl)CH2-,
phenyl-CH2CH2-, (phenyl)2CHCH2-, (2-F-phenyl)CH2CH2-,
(3-F-phenyl)CH2CH2-, (4-F-phenyl)CH2CH2-,
(2-Cl-phenyl)CHZCH2-, (3-C1-phenyl)CH2CH2-,
(4-C1-phenyl)CH2CH2-, (2,3-diF-phenyl)CH2CH2-,
(2,4-diF-phenyl)CH2CH2-, (2,5-diF-phenyl)CH2CH2-,
(2,6-diF-phenyl)CH2CH2-, (3,4-diF-phenyl)CH2CH2-,
(3,5-diF-phenyl)CH2CH2-, (2,3-diCl-phenyl)CH2CH2-,
(2,4-diCl-phenyl)CH2CH2-, (2,5-diCl-phenyl)CH2CH2-,
(2,6-diCl-phenyl)CH2CH2-, (3,4-diCl-phenyl)CH2CH2-,
(3,5-diCl-phenyl)CH2CH2-, (3-F-4-C1-phenyl)CH2CH2-,
(3-F-5-C1-phenyl)CH2CH2-, (3-C1-4-F-phenyl)CH2CH2-,
(2-Me0-phenyl)CH2CH2-, (3-Me0-phenyl)CH2CH2-,
(4-Me0-phenyl)CH2CH2-, (2-Me-phenyl)CH2CH2-,
(3-Me-phenyl)CH2CH2-, (4-Me-phenyl)CH2CH2-,
(2-MeS-phenyl)CH2CH2-, (3-MeS-phenyl)CH2CH2-,
(4-MeS-phenyl)CH2CH2-, (2-CF30-phenyl)CH2CH2-,
-78-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
(3-CF30-phenyl)CHZCH2-, (4-CF30-phenyl)CH2CH2-,
(furanyl)CH2CH2-,(thienyl)CH2CH2-, (pyridyl)CHZCH2-,
(2-Me-pyridyl)CHZCH2-, (3-Me-pyridyl)CHZCH2-,
(4-Me-pyridyl)CH2CH2-, (imidazolyl)CH2CH2-,
(oxazolyl)CH2CH2-, (isoxazolyl)CH2CH2-,
(benzimidazolyl)CHZCH2-,(cyclopropyl)CHZCH2-,
(cyclobutyl)CH2CH2-,(cyclopentyl)CH2CH2-,
(cyclohexyl)CH2CH2-,(morpholino)CHZCH2-, or
(N-pipridinyl)CH2CH2-;
R11, at each occurrence, is independently selected from H,
methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, s-
butyl, t-butyl, phenyl, benzyl, phenethyl, cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl,
cyclopropylmethyl, cyclobutylmethyl, cyclopentylmethyl,
cyclohexylmethyl, cycloheptylmethyl, cyclopropylethyl,
cyclobutylethyl, cyclopentylethyl, cyclohexylethyl,
2-F-phenyl-, 3-F-phenyl, 4-F-phenyl, 4-C1-phenyl, 4-CH3-
phenyl, 4-Me0-phenyl-, 4-CF3-phenyl, (4-F-phenyl)CH2-,
(4-Cl-phenyl)CHZ-, (4-CH3-phenyl)CH2-,
(4-CF3-phenyl)CH2-, (4-F-phenyl)CH2CH2-,
(4-C1-phenyl)CH2CH2-, (4-CH3-phenyl)CH2CH2-,
(4-CF3-phenyl)CH2CH2-, pyridin-2-yl-, pyridin-3-yl-,
4-CF3-pyridin-2-yl-, 4-CH3-pyridin-2-yl-, thiazol-2-yl-,
azapan-1-yl, N,N-dimethylamino, N,N-diethylamino, N,N-
dipropylamino, and N,N-dibutylamino; and
R13, at each occurrence, is independently selected from H,
MeO, F, and C1.
[18] In a preferred embodiment the present invention
provides a compound of Formula (If);
R5
Rsa ~..~ O
N N~W-X-Y-Z
OH O
R
(If)
-79-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
or a pharmaceutically acceptable salt form or prodrug
thereof .
[19] In a preferred embodiment the present invention
provides a compound of Formula (Ig);
R5
Rsa ~..~ O
N~N~W-X-Y-Z
OH O N~ ~ \'rR~3
R1~ ~~R13
(Ig)
or a pharmaceutically acceptable salt form or prodrug
thereof.
[20] In a preferred embodiment the present invention
provides a compound of Formula (Ih);
R5
R5a ~..~ O
W-X-Y-Z
Q1 N N
OH O / ~ ~ ~R13
Rise
(Ih)
or a pharmaceutically acceptable salt form or prodrug
thereof.
In another preferred embodiment the present invention
provides all herein disclosed embodiments with the proviso
that when R5 and R5a are not simultaneaously H.
In another preferred embodiment the present invention
provides all herein disclosed embodiments with the proviso
that when Q is a 9 membered benzofused heterocyclic group
substituted by 0, 1, or 2 Rla, then R3 is H.
-80-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
In another preferred embodiment the present invention
provides all herein disclosed embodiments with the proviso
that when -WXYZ is a tertiary butyl group and R5 is either
C1-C4 alkyl or Cz alkenyl, then Q is not phenyl substituted
by 0 , 1 or 2 R1a .
In another preferred embodiment the present invention
provides all herein disclosed embodiments with the proviso
that when R5 is C1-C3 alkyl, then Q is not phenyl
substituted by 0, 1 or 2 Rla.
In another preferred embodiment the present invention
provides all herein disclosed embodiments with the proviso
that the moiety:
Rs Rsa
Q
OH O
of Formula (I), et seq., is not a C1-Cg alkyl, C2-Cg
alkenyl, C2-Cg alkynyl, C3-C1o cycloalkyl-C1-Cg alkyl, C3-Clo
cycloalkyl, C1-C4 alkyl-0-C1-C4 alkyl, C1-C4 alkyl-S-C1-C4
alkyl, C2-C4 alkyl-NR2o-C2-C4 alkyl, C2-C4 alkyl-C6-C1o
aryl, C2-C4 alkyl-C6-C1o cycloalkyl, C2-Cg alkenyl, C6-Clo
aryl-C1-C4 alkyl, C6-C1o aryl-C2-C4-alkynyl, indol-3-yl-C1-
C3 alkyl, and imidazol-4-y1-C1-C3 alkyl; where any alkyl
group is substituted with OH.
In a second embodiment, the present invention provides
a pharmaceutical composition comprising a compound of
Formula (I) and a pharmaceutically acceptable carrier.
In a third embodiment, the present invention provides
a method for the treatment of a neurological disorder
associated with ~i-amyloid production comprising
administering to a host in need of such treatment a
-81-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
therapeutically effective amount of a compound of Formula
(I) .
In a preferred embodiment the neurological disorder
associated with (3-amyloid production is Alzheimer's
Disease.
In a fourth embodiment, the present invention provides
a method for inhibiting 'y-secretase activity for the
treatment of a physiological disorder associated with
inhibiting y-secretase activity comprising administering to
a host in need of such inhibition a therapeutically
effective amount of a compound of Formula (I) that inhibits
y-secretase activity.
In a preferred embodiment the physiological disorder
associated with inhibiting y-secretase activity is
Alzheimer's Disease.
In a fifth embodiment, the present invention provides
a compound of Formula (I) for use in therapy.
In a preferred embodiment the present invention
provides a compound of Formula (I) for use in therapy of
Alzheimer's Disease.
In a sixth embodiment, the present invention provides
for the use of a compound of Formula (I) for the
manufacture of a medicament for the treatment of
Alzheimer's Disease.
DEFINITIONS
As used herein, the term "A~3" denotes the protein
designated A(3, (3-amyloid peptide, and sometimes (3/A4, in
the art. A(3 is an approximately 4.2 kilodalton (kD)
protein of about 39 to 43 amino acids found in amyloid
plaques, the walls of meningeal and parenchymal arterioles,
-82-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
small capillaries, and sometimes, The
arteries, venules.
isolation and quencedata for the first 28
se amino
acids
are described U.S. Pat. 829. The amino
in No 43 acid
4,666,
sequence is:
1
Asp Ala Glu Phe Arg His Asp Ser Gly Tyr
11
Glu Val His His Gln Lys Leu Val Phe Phe
21
Ala Glu Asp Val Gly Ser Asn Lys Gly Ala
31
Ile Ile Gly Leu Met Val Gly Gly Val Val
41
Ile Ala Thr
The term "APP", as used herein, refers to the protein
known in the art as ~3 amyloid precursor protein. This
protein is the precursor for A~i and through the activity of
"secretase" enzymes, as used herein, it is processed into
A~3. Differing secretase enzymes, known in the art, have
been designated (3 secretase, generating the N-terminus of
A(3, ac secretase cleaving around the 16/17 peptide bond in
A(3, and "'y secretases", as used herein, generating C-
terminal A(3 fragments ending at position 38, 39, 40, 42,
and 43 or generating C-terminal extended precursors which
are subsequently truncated to the above polypeptides
The compounds herein described may have asymmetric
centers. Compounds of the present invention containing an
asymmetrically substituted atom may be isolated in
optically active or racemic forms. It is well known in the
art how to prepare optically active forms, such as by
resolution of racemic forms or by synthesis from optically
active starting materials. Many geometric isomers of
olefins, C=N double bonds, and the like can also be present
in the compounds described herein, and all such stable
isomers are contemplated in the present invention. Cis and
-83-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
trans geometric isomers of the compounds of the present
invention are described and may be isolated as a mixture of
isomers or as separated isomeric forms. All chiral,
diastereomeric, racemic forms and all geometric isomeric
forms of a structure are intended, unless the specific
stereochemistry or isomeric form is specifically indicated.
The term "substituted," as used herein, means that any
one or more hydrogens on the designated atom is replaced
with a selection from the indicated group, provided that
the designated atom's normal valency is not exceeded, and
that the substitution results in a stable compound. When a
substituent is keto (i.e., =O), then 2 hydrogens on the
atom are replaced.
When any variable (e. g., Rla, R4a, RZj etc.) occurs
more than one time in any constituent or formula for a
compound, its definition at each occurrence is independent
of its definition at every other occurrence. Thus, for
example, if a group is shown to be substituted with 0-3
Rla, then said group may optionally be substituted with up
to three Rla groups and R1a at each occurrence is selected
independently from the definition of Rla. Also,
combinations of substituents and/or variables are
permissible only if such combinations result in stable
compounds.
When a bond to a substituent is shown to cross a bond
connecting two atoms in a ring, then such substituent may
be bonded to any atom on the ring. When a substituent is
listed without indicating the atom via which such
substituent is bonded to the rest of the compound of a
given formula, then such substituent may be bonded via any
atom in such substituent. Combinations of substituents
and/or variables are permissible only if such combinations
result in stable compounds.
As used herein, "alkyl" or "alkylene" is intended to
include both branched and straight-chain saturated
aliphatic hydrocarbon groups having the specified number of
carbon atoms; for example, "C1-C6 alkyl" denotes alkyl
-84-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
having 1 to 6 carbon atoms. Examples of alkyl include, but
are not limited to, methyl, ethyl, n-propyl, i-propyl,
n-butyl, i-butyl, sec-butyl, t-butyl, pentyl, and hexyl.
Preferred "alkyl" group is "C1-C4 alkyl" wherein methyl,
ethyl, n-propyl, i-propyl, n-butyl, and i-butyl, are
specifically preferred. As used herein, "C1-C3 alkyl",
whether a terminal substituent or a alkylene group linking
two substituents, is understood to specifically include
both branched and straight-chain methyl, ethyl, and propyl.
As used herein, "alkenyl" or "alkenylene" is intended
to include hydrocarbon chains of either a straight or
branched configuration and one or more unsaturated
carbon-carbon bonds which may occur in any stable point
along the chain. Examples of "Cz-C6 alkenyl" include, but
are not limited to, ethenyl, 1-propenyl, 2-propenyl, 1-
butenyl, 2-butenyl, 3-butenyl, 3-methyl-2-butenyl, 2-
pentenyl, 3-pentenyl, hexenyl, and the like.
As used herein, "alkynyl" or "alkynylene" is intended
to include hydrocarbon chains of either a straight or
branched configuration and one or more carbon-carbon triple
bonds which may occur in any stable point along the chain,
such as ethynyl, 1-propynyl, 2-propynyl, 1-butynyl, 2-
butynyl, 3-butynyl, and the like.
"Alkoxy" or "alkyloxy" represents an alkyl group as
defined above with the indicated number of carbon atoms
attached through an oxygen bridge. Examples of alkoxy
include, but are not limited to, methoxy, ethoxy,
n-propoxy, i-propoxy, n-butoxy, s-butoxy, t-butoxy,
n-pentoxy, and s-pentoxy. Preferred alkoxy groups are
methoxy, ethoxy, n-propoxy, i-propoxy, n-butoxy, s-butoxy,
t-butoxy. Similarly, "alkylthio" or "thioalkoxy" is
represents an alkyl group as defined above with the
indicated number of carbon atoms attached through a sulphur
bridge.
"Halo" or "halogen" as used herein refers to fluoro,
chloro, bromo, and iodo. Unless otherwise specified,
preferred halo is fluoro and chloro. "Counterion" is used
-85-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
to represent a small, negatively charged species such as
chloride, bromide, hydroxide, acetate, sulfate, and the
like.
"Haloalkyl" is intended to include both branched and
straight-chain saturated aliphatic hydrocarbon groups
having the specified number of carbon atoms, substituted
with 1 or more halogen (for example -C~Fw where v = 1 to 3
and w = 1 to (2v+1)). Examples of haloalkyl include, but
are not limited to, trifluoromethyl, trichloromethyl,
pentafluoroethyl, pentachloroethyl, 2,2,2-trifluoroethyl,
2,2-difluoroethyl, heptafluoropropyl, and
heptachloropropyl. "Haloalkoxy" is intended to mean a
haloalkyl group as defined above with the indicated number
of carbon atoms attached through an oxygen bridge; for
example trifluoromethoxy, pentafluoroethoxy, 2,2,2-
trifluoroethoxy, and the like. "Halothioalkoxy" is
intended to mean a haloalkyl group as defined above with
the indicated number of carbon atoms attached through a
sulphur bridge.
"Cycloalkyl" is intended to include saturated ring
groups, having the specified number of carbon atoms. For
example, "C3-C6 cycloalkyl" denotes such as cyclopropyl,
cyclobutyl, cyclopentyl, or cyclohexyl.
As used herein, "carbocycle" is intended to mean any
stable 3- to 7-membered monocyclic or bicyclic or 7- to
13-membered bicyclic or tricyclic, any of which may be
saturated, partially unsaturated, or aromatic. Examples of
such carbocycles include, but are not limited to,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl, adamantyl, cyclooctyl, [3.3.0]bicyclooctane,
[4.3.0]bicyclononane, [4.4.0]bicyclodecane (decalin),
[2.2.2]bicyclooctane, fluorenyl, phenyl, naphthyl, indanyl,
adamantyl, or tetrahydronaphthyl (tetralin). Preferred
example of "C3-C1p carbocycle" or "C3-C6 carbocycle" is C3-
C6 cycloalkyl, specifically cyclopropyl, cyclobutyl,
cyclopentyl, and cyclohexyl.
-86-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
As used herein, the term "heterocycle" or
"heterocyclic ring" is intended to mean a stable 5- to 7-
membered monocyclic or bicyclic or 7- to 14-membered
bicyclic heterocyclic ring which is saturated partially
unsaturated or unsaturated (aromatic), and which consists
of carbon atoms and 1, 2, 3 or 4 heteroatoms, preferably 1,
2, or 3 heteroatoms, independently selected from the group
consisting of N, 0 and S and including any bicyclic group
in which any of the above-defined heterocyclic rings is
fused to a benzene ring. The nitrogen and sulfur
heteroatoms may optionally be oxidized. The heterocyclic
ring may be attached to its pendant group at any heteroatom
or carbon atom which results in a stable structure. The
heterocyclic rings described herein may be substituted on
carbon or on a nitrogen atom if the resulting compound is
stable. If specifically noted, a nitrogen in the
heterocycle may optionally be quaternized. It is preferred
that when the total number of S and O atoms in the
heterocycle exceeds 1, then these heteroatoms are not
adjacent to one another. It is preferred that the total
number of S and O atoms in the heterocycle is not more than
1.
Examples of heterocycles include, but are not limited
to, 1H-indazole, 2-pyrrolidonyl, 2H,6H-1,5,2-dithiazinyl,
2H-pyrrolyl, 3H-indolyl, 4-piperidonyl, 4aH-carbazole,
4H-quinolizinyl, 6H-1,2,5-thiadiazinyl, acridinyl,
azocinyl, benzimidazolyl, benzofuranyl, benzothiofuranyl,
benzothiophenyl, benzoxazolyl, benzthiazolyl,
benztriazolyl, benztetrazolyl, benzisoxazolyl,
benzisothiazolyl, benzimidazalonyl, carbazolyl,
4aH-carbazolyl, b-carbolinyl, chromanyl, chromenyl,
cinnolinyl, decahydroquinolinyl, 2H,6H-1,5,2-dithiazinyl,
dihydrofuro[2,3-b]tetrahydrofuran, furanyl, furazanyl,
homopiperidinyl, imidazolidinyl, imidazolinyl, imidazolyl,
1H-indazolyl, indolenyl, indolinyl, indolizinyl, indolyl,
isobenzofuranyl, isochromanyl, isoindazolyl, isoindolinyl,
isoindolyl, isoquinolinyl, isothiazolyl, isoxazolyl,
-87_
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
morpholinyl, naphthyridinyl, octahydroisoquinolinyl,
oxadiazolyl, 1,2,3-oxadiazolyl, 1,2,4-oxadiazolyl,
1,2,5-oxadiazolyl, 1,3,4-oxadiazolyl, oxazolidinyl,
oxazolyl, oxazolidinylperimidinyl, phenanthridinyl,
phenanthrolinyl, phenarsazinyl, phenazinyl, phenothiazinyl,
phenoxathiinyl, phenoxazinyl, phthalazinyl, piperazinyl,
piperidinyl, pteridinyl, piperidonyl, 4-piperidonyl,
pteridinyl, purinyl, pyranyl, pyrazinyl, pyrazolidinyl,
pyrazolinyl, pyrazolyl, pyridazinyl, pyridooxazole,
pyridoimidazole, pyridothiazole, pyridinyl, pyridyl,
pyrimidinyl, pyrrolidinyl, pyrrolinyl, pyrrolyl,
quinazolinyl, quinolinyl, 4H-quinolizinyl, quinoxalinyl,
quinuclidinyl, carbolinyl, tetrahydrofuranyl,
tetrahydroisoquinolinyl, tetrahydroquinolinyl,
6H-1,2,5-thiadiazinyl, 1,2,3-thiadiazolyl,
1,2,4-thiadiazolyl, 1,2,5-thiadiazolyl, 1,3,4-thiadiazolyl,
thianthrenyl, thiazolyl, thienyl, thienothiazolyl,
thienooxazolyl, thienoimidazolyl, thiophenyl, triazinyl,
1,2,3-triazolyl, 1,2,4-triazolyl, 1,2,5-triazolyl,
1,3,4-triazolyl, xanthenyl. Preferred 5 to 10 membered
heterocycles include, but are not limited to, pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl, thiazolyl,
pyrrolyl, pyrazolyl, imidazolyl, oxazolyl, isoxazolyl,
tetrazolyl, benzofuranyl, benzothiofuranyl, indolyl,
benzimidazolyl, 1H-indazolyl, oxazolidinyl, isoxazolidinyl,
benzotriazolyl, benzisoxazolyl, oxindolyl, benzoxazolinyl,
quinolinyl, and isoquinolinyl. Preferred 5 to 7 membered
heterocycles include, but are not limited to, pyridinyl,
pyrimidinyl, triazinyl, furanyl, thienyl, thiazolyl,
pyrrolyl, piperazinyl, piperidinyl, homopiperidinyl,
pyrazolyl, imidazolyl, oxazolyl, isoxazolyl, tetrazolyl;
more preferred 5 to 7 membered heterocycles include, but
are not limited to, pyridinyl, pyrimidinyl, triazinyl,
furanyl, thienyl, thiazolyl, piperazinyl, piperidinyl,
homopiperidinyl, pyrazolyl, imidazolyl, and tetrazolyl.
Preferred 5 to 6 membered heterocycles include, but are not
limited to, pyridinyl, pyrimidinyl, triazinyl, furanyl,
-88-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
thienyl, thiazolyl, pyrrolyl, piperazinyl, piperidinyl,
pyrazolyl, imidazolyl, oxazolyl, isoxazolyl, tetrazolyl;
Also included are fused ring and containing, for example,
the above heterocycles.
As used herein, the term "aryl", "C6-Clp aryl" or
aromatic residue, is intended to mean an aromatic moiety
containing the specified number of carbon atoms; for
example phenyl, pyridinyl or naphthyl. Unless otherwise
specified, "aryl" may be unsubstituted or substituted with
0 to 3 groups selected from H, OH, OCH3, C1, F, Br, I, CN,
N02 , NH2 , N ( CH3 ) H, N ( CH3 ) 2 , CF3 , OCF3 , C ( =O ) CH3 , SCH3 ,
S(=O)CH3, S(=O)2CH3, CH3, CH2CH3, C02H, and C02CH3.
The phrase "additional lactam carbons", as used
herein, is intended to denote the number of optional carbon
atoms in the lactam ring B of Formula (I). Formula (Ia):
O
2
3 ;N
B
(Ia)
represents the lactam ring B of Formula (I). Additional
lactam carbons are carbons in lactam ring B other than the
carbons numbered 2 and 3 in the backbone of the formula.
The additional lactam carbons may be optionally replaced by
a heteroatom selected from oxygen, nitrogen and sulfur.
Lactam ring B contains 1, 2, 3, 4, 5, 6 or 7 optional
carbons, wherein one optional carbon may optionally be
replaced by a heteroatom, such that the total number of
members of lactam ring B, including atoms numbered 1, 2 and
3 in the backbone, does not exceed 10. It is preferred
that the total number of atoms of lactam ring B is 6, 7 or
8; it is more preferred that the total number of atoms of
lactam ring B is seven. Examples of lactam ring B include:
_89_
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
O O O
N/~ f N,~ ~ Nr~
11
f3 11
N R11 O
i
R1o
B1 B2 B3
O O O
N/~ ~ N/~ ~ N/
\ N
S~~ R11 R11
Rii
B4 B5 B6
O U
O ~ N/~ ~ N~
N/~ O
\ / \
R11 R1o
B7 B8 B9
O O O
/ ~ ~ N,~ f N/
\J
R11 ~ R11 R11
B10 B11 B12
O O O
f N~~ ~ N/~ ~ N~
N \ R11 R11
R1o
-90-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
B13 B14 B15
O
N~~
R1°-N
O
B16
but are not intended to limit the invention. Preferred
examples of lactam ring B are B1, B2, B5, B6, B8, B9, B13,
and B16; more preferred examples of lactam ring B are B1,
B6, B8, B9, and B13. Preferred examples of substituent R1°
or R11 on lactam B are methyl, ethyl, phenyl, 4-
fluorophenyl, 4-chlorophenyl, 4-trifluorophenyl, (4-
fluorophenyl)methyl, (4-chlorophenyl)methyl, and (4-
trifluorophenyl)methyl. Preferred examples of substituent
R13 on fused rings of lactam B are methyl, fluoro, and
chloro.
The compounds herein described may have asymmetric
centers. One enantiomer of a compound of Formula (I) may
display superior biological activity over the opposite
enantiomer. For example carbon 3 of lactam ring B Formula
(I") may exist in either an S or R configuration. Thus, an
R or S configuration at carbon 3 in Formula (I") is
considered part of the invention. An example of such
configuration includes,
R5 R5
Q N~ Q H O
N~CH3 N N~CH3
HO p N ~ ~ / R13 HO O N ~ ~ / R1 s
11 ~ 11
R and R
but is not intended to be limited to this example of ring
B. When required, separation of the racemic material can
be achieved by methods known in the art. Additionally, the
-91-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
carbon atoms to which the OH and R5 are attached may
describe chiral carbons which may display superior
biiological activity over the opposite enantiomer. For
example, where Q and R5 are not H, then the configuration
of the two centers may be described as (2R,3R), (2R,3S),
(2S,3R), or (2S,3S). All configurations are considered
part of the invention; however, the (2R,3S) and the (2S,3R)
are preferred and the (2R,3S) is more preferred.
The phrase "pharmaceutically acceptable" is employed
herein to refer to those compounds, materials,
compositions, and/or dosage forms which are, within the
scope of sound medical judgment, suitable for use in
contact with the tissues of human beings and animals
without excessive toxicity, irritation, allergic response,
or other problem or complication, commensurate with a
reasonable benefit/risk ratio.
As used herein, "pharmaceutically acceptable salts"
refer to derivatives of the disclosed compounds wherein the
parent compound is modified by making acid or base salts
thereof. Examples of pharmaceutically acceptable salts
include, but are not limited to, mineral or organic acid
salts of basic residues such as amines; alkali or organic
salts of acidic residues such as carboxylic acids; and the
like. The pharmaceutically acceptable salts include the
conventional non-toxic salts or the quaternary ammonium
salts of the parent compound formed, for example, from
non-toxic inorganic or organic acids. For example, such
conventional non-toxic salts include those derived from
inorganic acids such as hydrochloric, hydrobromic,
sulfuric, sulfamic, phosphoric, nitric and the like; and
the salts prepared from organic acids such as acetic,
propionic, succinic, glycolic, stearic, lactic, malic,
tartaric, citric, ascorbic, pamoic, malefic, hydroxymaleic,
phenylacetic, glutamic, benzoic, salicylic, sulfanilic,
2-acetoxybenzoic, fumaric, toluenesulfonic,
methanesulfonic, ethane disulfonic, oxalic, isethionic, and
the like.
-92-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
The pharmaceutically acceptable salts of the present
invention can be synthesized from the parent compound which
contains a basic or acidic moiety by conventional chemical
methods. Generally, such salts can be prepared by reacting
the free acid or base forms of these compounds with a
stoichiometric amount of the appropriate base or acid in
water or in an organic solvent, or in a mixture of the two;
generally, nonaqueous media like ether, ethyl acetate,
ethanol, isopropanol, or acetonitrile are preferred. Lists
of suitable salts are found in Remington's Pharmaceutical
Sciences, 17th ed., Mack Publishing Company, Easton, PA,
1985, p. 1418, the disclosure of which is hereby
incorporated by reference.
"Prodrugs" are intended to include any covalently
bonded carriers which release the active parent drug
according to formula (I) in vivo when such prodrug is
administered to a mammalian subject. Prodrugs of a
compound of formula (I) are prepared by modifying
functional groups present in the compound in such a way
that the modifications are cleaved, either in routine
manipulation or in vivo, to the parent compound. Prodrugs
include compounds of formula (I) wherein a hydroxy, amino,
or sulfhydryl group is bonded to any group that, when the
prodrug or compound of formula (I) is administered to a
mammalian subject, cleaves to form a free hydroxyl, free
amino, or free sulfhydryl group, respectively. Examples of
prodrugs include, but are not limited to, acetate, formate
and benzoate derivatives of alcohol and amine functional
groups in the compounds of formula (I), and the like.
"Stable compound" and "stable structure" are meant to
indicate a compound that is sufficiently robust to survive
isolation to a useful degree of purity from a reaction
mixture, and formulation into an efficacious therapeutic
agent.
SYNTHESIS
The compounds of the present invention can be prepared
in a number of ways well known to one skilled in the art of
-93-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
organic synthesis. The compounds of the present invention
can be synthesized using the methods described below,
together with synthetic methods known in the art of
synthetic organic chemistry, or variations thereon as
appreciated by those skilled in the art. Preferred methods
include, but are not limited to, those described below.
All references cited herein are hereby incorporated in
their entirety by reference.
The novel compounds of this invention may be prepared
using the reactions and techniques described in this
section. The reactions are performed in solvents
appropriate to the reagents and materials employed and are
suitable for the transformations being effected. Also, in
the description of the synthetic methods described below,
it is to be understood that all proposed reaction
conditions, including choice of solvent, reaction
atmosphere, reaction temperature, duration of the
experiment and workup procedures, are chosen to be the
conditions standard for that reaction, which should be
readily recognized by one skilled in the art. It is
understood by one skilled in the art of organic synthesis
that the functionality present on various portions of the
molecule must be compatible with the reagents and reactions
proposed. Such restrictions to the substituents which are
compatible with the reaction conditions will be readily
apparent to one skilled in the art and alternate methods
must then be used.
Scheme 1
-94-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
~( 5
HN' \ 1 ~ BuLi R ~N~ 1. Bu2BOTf, TEA
O 2.R5CH2COCI ~O 2.Q-CHO
Bn' ' B ' ~'n
2
LiOH, H2O2 O OH
Q O~N
- O
R5 ~ THF, H20 R5
Bn
3 4
Aldol derivatives can be prepared by the procedure of
Evans (D. A. Evans et al, Org. Synth. 1990, 68, 83-90),
which is outlined in Scheme 1 where acylation of an
oxazolidinone with an acid chloride provides structure 2.
Asymmetric aldol reaction to form 3 followed by cleavage of
the chiral auxiliary yielding (3-hydroxycarboxylic acid 4.
Additional examples are found in D. A. Evans Aldrichimica
Acta 1982, 25, 23-32. Alternative syntheses of structures
4 can be accomplished by the methods of Crimmins ( M. T.
Crimmins et al, J. Am. Chem. Soc. 1997, 119, 7883-7884),
Paterson (I. Paterson et al, Org. React. 1997, 51, 1-200)
and Mukaiyama (T. Mukaiyama et al, Org. React. 1994, 1-
104). Anti-aldols may be synthesized according to: (a) A.
K. Ghosh, J. Am. Chem. Soc. 1996, 118, 2527-2528, or (b) S.
Masamune et al., J. Am. Chem. Soc. 1997, 119, 2586.
Scheme 2
ZYXW R13 ZYXW R~3
O -i -i
N \ / EDC, HOBt OH O O N
Q OH + ~ ,
R5 H2N N R~i TEA, CH2CI2 O R5 H N R11
4
5 6
Carboxylic acids of formula 4 can be coupled to
anappropriate lactam intermediate using methods commonly
used in peptide syntheses, such as DCC, EDC, CDI, BOP,
PyBOP, HATU, HBTU and phenyl ester mediated coupling, as
-95-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
described in A. R. Chamberlin, Chem. Rev. 1997, 97, 2243-
2266. Compound 6 is synthesized, as illustrated in Scheme
2, by coupling acid 4 with 3-amino-1,4-benzodiazepin-2-one
under the catalysis of EDC and HOBt providing the final
5 compound 6 (S. Nozaki et al, Bull. Chem. Soc. Jpn. 1982,
55, 2165-2168).
Similarly, Schemes 2a and 2b illustrate synthesis of
bisbenzodiazepine and lactam compounds of the present
invention:
Scheme 2a
ZYXW R13
WXYZ 13 -I'
OH O O N R OH 00 N
~ ~ %,
Q~OH + H2N I / EDC, HOBt II
TEA, CH2CI ~N l
R5 _ 2 f35 H ~ 'R13
~R 13
4a 4b 4c
Scheme 2b
OH O WXYZ N XYZ
- O ' O
Q~OH + N EDC, HOBt
- H2N
R5 TEA, CH2C12 O . 5 H
R
4b 5b 5c
Methods for the synthesis of lactam intermediates as
contemplated by the present invention useful in the
synthesis of compounds of Formula (I), including amino
benzodiazepinones, dibenzo azepinones and other related
heterocycles, are known in the art and are disclosed in a
number of references including PCT publication number WO
98/28268, WO 99/66934, WO 00/07995, and WO 00/38618, which
are hereby incorporated by reference: Additional
references include Bock, et al, J. Org. Chem., 1987, 52,
3232-3239; Sherrill et al, J. Org. Chem., 1995, 60, 730-
-96-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
734; and Walsh, D. A., Synthesis, September 1980, p.677;
and Brown, et al., Tetrahedron Letters, 1971, 8, 667-670.
Synthetic approaches to aminobenzodiazepines are
widely described in the literature and well known to one
skilled in the art. The typical methods are illustrated,
but are not limited to, the following references. See (a)
M. G. Bock et al., J. Org. Chem., 1987, 52, 3232; (b) R. G.
Sherrill et al., J. Org. Chem., 1995, 60, 734; (c) M. G.
Bock et al., J. Med. Chem., 1989, 32, 13-16; (d) J. L.
Castro et al., J. Med. Chem., 1997, 40, 2491-2501; (e) M.
S. Chambers et al., Bioorg. & Med. Chem. Lett., 1993, 3
(10), 1919-1924; (f) J. H. Gogerty et al., J. Med. Chem.,
1977, 20 (7), 952; (g) G. Semple et al., Bioorg. & Med.
Chem. Lett., 1996, 6(1), 51-54; (h) G. Semple et al., J.
Med. Chem., 1997, 40, 331-341; (i) G. Semple et al.,
Bioorg. & Med. Chem. Lett., 1996, 6 (1), 55-58; (j) G.
Semple et al., Synth. Commun., 1996, 26 (4), 721-727; and
(k) G. A. Showell et al., J. Med. Chem., 1994, 37, 719-
721. For general synthetic descriptions of 2-
aminobenzophenone with various substitutions used in the
preparation of benzodiazepine, see D. A. Walsh, Synthesis
1980, 677.
Scheme 3
1. NaH, DMF ~ Os04, Na104 O
Q OH 2. allyl bromide, KI Q1'O Et20-H20 Q1~O~H
7 8 9
The preparation of aldehyde Q-CHO with general
structure of 9 is shown in Scheme 3 (H. C. Arndt, Synthesis
1979, 202-204). Allyl ether 8 can be made from the action
of an alkoxide generated in DMF with allyl bromide, which
is converted to a-alkoxy- or aryloxyaldehyde 9 using a two-
phase osmium tetraoxide oxidation.
Scheme 4
_97_
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
1. NaH, THF
2. allyl alcohol, KI
R R
11
/H
Os04, Na104 ~ \~ ~O
Et20-H20 ~ ~ O
R
12
As shown in Scheme 4, aldehyde Q-CHO of general
structure 12 can be prepared in the same fashion from the
5 corresponding allyl benzyl ether, which is readily
available according to the procedure described by P.
Kocienski (P. Kocienski Tetrahedron 1990, 46, 1767-1782).
The aldehydes used in Scheme 1 are either commercially
10 available, prepared from commercially available or readily
accessible alcohols, or prepared from commercially
available or readily accessible carboxylic acids. For
preparation of other non-commercially available aldehydes
from commercially available or readily accessible alcohols
by oxidation of the corresponding alcohols, see(a) S. V.
Ley et al Synthesis 1994, 639; (b) D. Swern, Synthesis
1981, 165-185; and (c) R. C. Larock, Comprehensive Organic
Transformations, Wiley-VCH: 1989; pp604-614. For
preparation of other non-commercially available aldehydes
from commercially available or readily accessible
carboxylic acids by reducing the corresponding Weinreb
amides or reduction of carboxylic acid derivatives, see (a)
S. M. Weinreb et al. Tetrahedron Lett. 1981, 22, 3815-3818;
(b) M. Braun, Synthesis 1989, 856; and (c) D. A. Evans, J.
Org. Chem. 1993, 58, 2446-2453.
Aminoaldehydes used in the synthesis of the compounds
of the invention may be prepared by oxidation of
corresponding amino alcohols or reduction of corresponding
amino acids; see(a) J. Jurczak et al., Synlett 1993, 241;
and (b) S. G. Davis et al., Synlett 1995, 700.
_98_
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Sulfur containing aldehydes used in the synthesis of
compounds of the invention may be made by conjugate
addition of a thiol to oc,(3-unsaturated aldehydes or
reaction of a thiol with a halosubstituted aldehyde. See
T. Cohen et al., J. Org. Chem. 1995, 60, 2022; Tetrahdron
1994, 50, 12793-12810; J. Org. Chem. 1992, 57, 6;
Phosphorus, Sulfur, and Silicon 1993, 74, 1; and
Tetrahdron 1994, 50, 11569-11584.
Sulfoxides and sulfones are prepared from the
corresponding sulfide by oxidation. See M. Hudlicky,
Oxidations in Organic Chemistry, ACS, 1990; pp 250-264.
The acid chlorides used in Scheme 1 are either
commercially available or prepared from commercially
available or readily accessible carboxylic acids by the
action of oxalyl chloride or thionyl chloride. See R. C.
Larock, Comprehensive Organic Transformations, Wiley-VCH:
1989; pp963-964.
Examples
Chemical abbreviations used in the Examples are
defined as follows: "DMPU" for 1,3-dimethyl-3,4,5,6-
tetrahydro-2(1H)-pyrimidone; "TBTU" for O-(1H-benzotriazol-
1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate;
"BOP" for benzotriazol-1-yloxytris-dimethylamino)-
phosphonium hexafluorophosphate; "Bu2BOTf" for dibutylboron
triflate; "EDC" for 1-[3-(dimethylamine)propyl]-3-
ethylcarbodiimide hydrochloride; "HOBt" for 1-
hydroxybenzotriazole; and "TEA" for triethylamine.
"HPLC" is an abbreviation used herein for high
pressure liquid chromatography. Compounds of the present
invention are generally purified by HPLC using conditions
known to one skilled in the art. However, unless otherwise
indicated, the following conditions are generally
applicable. Reverse-phase HPLC can be carried out using a
Vydac C-18 column with gradient elution from 10~ to 100
buffer B in buffer A (buffer A: water containing 0.1~
trifluoroacetic acid, buffer B: 10~ water, 900
_99-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
acetonitrile containing 0.1% trifluoroacetic acid).
Alternatively, reverse-phase HPLC can be carried out using
a Vydac C-18 column with gradient elution from 10% to 90
acetonitrile in water.
Example 1.
3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-5-
phenylpentyl)amino-1-methyl- 5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one.
(R)-3-(3-cyclopentyl-1-oxopropyl)-4-(phenylmethyl)-2-
oxazolidinone (2).
O O O
HN~ 1 ~ BuLi N
/O 2. 3-cyclopentylpropionyl chloride ~' \/O
Bn
1 2
A dry round bottom flask was charged with of (R)-4-
(phenylmethyl)-2-oxazilidinone (1, 17.7 g, 0.100 mol).
Anhydrous tetrahydrofuran (300 mL) was then added, and the
solution was cooled to -78°C. A solution of butyllithium
(42.0 mL, 0.105 mol, 2.50 M in hexane) was added to the
reaction flask over a 10-min period. After a few minutes,
3-Cyclopentylpropionyl chloride (16.8 mL, 0.110 mol) was
added. The resulting solution was stirred for 30 min at -
78°C, then allowed to warm to ambient temperature over a
30-min period. Excess 3-cyclopentylpropionyl chloride was
quenched by the addition of 60 mL of saturated aqueous
ammonium chloride. The bulk of the tetrahydrofuran and
hexane was removed on a rotary evaporator, and the slurry
was extricated with two 80 mL portion of dichloromethane.
The combined organic layers were washed with 75 mL of 1 M
sodium hydroxide and 75 mL of brine, dried over anhydrous
magnesium sulfate, and filtered. The solvent was removed
under reduced preddure. The residue was trituated with
hexane to provide 16.5 g of desired product 2 as a white
solid. 1H NMR (300 MHz, CDC13) 8 7.18 - 7.40 (5 H, m),
-100-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
4.67 (1 H, m), 4.12 - 4.22 (2 H, m), 3.30 (1 H, dd, J =
13.4, 3.1 Hz), 2.84 - 3.06 (2 H, m), 2.76 (1 H, dd, J =
13.4, 9.6 Hz), 1.42 - 1.96 (9 H, m), 1.15 (2 H, br, m).
3-(2(R)-cyclopentylmethyl-3(S)-hydroxyl-5-phenyl-1
oxopentyl)-4(R)-(phenylmethyl)-2-oxazolidinone (3).
O O OH O O
JJ 1. Bu2BOTf, TEA Ph N~O
~~O
2. PhCH2CH2CH0 B
2 3
To a solution of acyloxazolidinone 2 (1.57 g, 5.00
mmol) in 20 mL of dichloromethane, cooled to -78°C under
nitrogen atmosphere, dibutylboron triflate (1.40 mL, 5.50
mmol) was added dropwise, followed by the addition of
triethylamine. The mixture was warmed slowly to 0°C and
was stirred at 0°C for an additional hour. The resultant
boryl enolate solution was then cooled to -78°C, and 3-
phenylpropanal (0.80 mL, 5.5 mmol) was added over a 5-min
period time. The solution was stirred for 1 h at -78°C,
then for 1 h at 0°C. The reaction mixture was quenched by
the addition of 4 mL of a pH 7 aqueous phosphate buffer
and 12 mL of methanol. To this cloudy solution was added 8
mL of methanol and 10 mL of 30~ aqueous hydrogen peroxide
at such a rate as to keep the internal temperature below
10°C. After the solution was stirred for one additional
hour, the volatile material was removed in a rotary
evaporator. The resulting slurry was extracted with three
20 mL portions of diethyl ether. The combined organic
layers was washed with 20 mL of 5~ aqueous sodium
bicarbonate and 20 mL of brine, dried over anhydrous
magnesium sulfate, filtered and concentrated under reduced
pressure. Purification by flash column chromatography (25~
ethyl acetate - hexane) provided 1.11 g (56~) of aldol 3 as
a colorless oil. 1H NMR (300 MHz, CDC13) 8 7.15 - 7.38 (m,
10 H), 4.72 (m, 1 H), 4.12 - 4.28 (m, 3 H), 3.85 (m, 1 H),
-101-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
3.34 (1 H, dd, J = 13.4, 3.1 Hz); 2.80 - 2.95 (1 H, m),
2.60 - 2.78 (2 H, m), 1.95 - 2.05 (1 H, m), 1.40 - 1.90
(10 H, m) , 1.10 (2 H, m) .
2(R)-cyclopentylmethyl-3(S)-hydroxyl-5-phenylpentanoic acid
(4) .
OH O O OH O
LiOH, H202 Fh OH
~ ~O
THF, H20
3 4
Acyloxazolidinone 3 (226 mg, 0.500 mmol) was dissolved
in 3 mL of THF and 1 mL of distilled water. The resulting
solution was cooled to 0°C. To this solution was added 30g
aqueous hydrogen peroxide (0.40 mL, 4.0 mmol), followed by
a solution of lithium hydroxide (19 mg, 0.80 mmol) in 0.5
mL of distilled water. After the solution was stirred for
16 h, sodium sulfite (567 mg, 4.50 mmol) in 3 mL of
distilled water was added. The bulk of tetrahydrofuran was
removed under reduced pressure, and the resulting mixture
(pH 12 ~ 13) was extracted with three 20 mL portion of
methylene chloride to remove the oxazolidinone auxiliary.
The aqueous layer was cooled in an ice bath and acidified
to pH 1 with 6 M aqueous hydrochloric acid. The resulting
cloudy solution was extracted with five portion of 30 mL
ethyl acetate. The combined organic layers are dried over
anhydrous magnesium sulfate, filtered and concentrated
under reduced pressure to yield 230 mg (810) of the desired
acid 4 as a white solid. 1H NMR (300 MHz, CDC13) b 7.18 -
7.35 (5 H, m), 3.87 (1 H, m), 2.81 - 2.87 (1 H, m), 2.60 -
2.76 (1 H, m); 2.54 - 2.60 (1 H, m), 1.00 - 1.95 (m, 13 H).
3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-5-
phenylpentyl)amino-1-methyl- 5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one (6).
-102-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
EDC; HOBt OH O
4 +
TsOH~ H; TEA, CH2C12 Ph
~ 6
A mixture of acid 4 (250 mg, 0.900 mmol) and 3-amino-
1-methyl- 5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one
5 p-toluenesulfonate salt (364 mg, 0.820 mmol) in 4 mL of
methylene chloride was stirred at 0°C. 1-Hydroxy-
benzotriazole hydrate (133 mg, 0.980 mmol), 1-[3-
(dimethylamine)propyl]-3-ethylcarbodiimide hydrochloride
(314 mg, 1.64 mmol) and triethylamine (0.51 mL, 3.7 mmol)
were added sequentially. After the mixture was stirred for
16 h, 30 mL of ethyl acetate was added. The organic layer
was washed with 1 M HC1 (15 mL), 5% NaHC03 (30 mL) and
brine (30 mL), dried over anhydrous magnesium sulfate and
concentrated under reduced pressure. Purification by
chromatotron (30% ethyl acetate - hexane) afforded two
diastereomers A and B. A: 120 mg (25%); 1H NMR (300 MHz,
CDC13) 8 7.20 - 7.70 (15 H, m), 5.54 (1 H, d, J = 8.0 Hz),
4.02 (1 H, m), 3.48 (3 H, s), 2.83 - 3.00 (1 H, m), 2.62 -
2.74 (1 H, m), 2.40 - 2.48 (1 H, m), 1.00 - 2.00 (13 H, m);
MS (ESI): 524 (M+H), 546 (M+Na), 522 (M-H), 558 (M+C1). B:
120 mg (25%); 1H NMR (300 MHz, CDC13) $ 7.60 (1 H, d, J =
6.9 Hz), 7.20 - 7.45(14 H, m), 5.56 (1 H, d, J = 8.4 Hz),
3.84 (1 H, m), 3.48 (3 H, s), 2.83 3.00 (1 H, m), 2.62
- -
2.74 (1 H, m), 2.50 - 2.60 (1 H, 1.00 - 1.95 (13 H,
m), m);
MS (ESI): 524 (M+H),546 (M+Na), 522 (M-H).
Examples 2-135
The general procedure for Example 1 was followed using
the corresponding acid chloride, aldehyde, and substituted
benzodiazepine, azepane or bisbenzodiazepine. Starting
materials were either commercially available or prepared by
methods known to one skilled in the art.
-103-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 2. 3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-5-
phenylpentyl)amino-1-methyl-5-(4-fluoro-phenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 542 (M+H).
Example 3. 3-(2(R)-Benzyl-3(S)-hydroxyl-1-oxo-5-
phenylpentyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 532 (M+H).
Example 4. 3-(2(R)-Isopropyl-3(S)-hydroxyl-1-oxo-5-
phenylpentyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 484 (M+H).
Example 5. 3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-4-
(3,5-difluorophenoxy)butyl)amino-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 562 (M+H).
Example 6. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-4-(3,5-
difluorophenoxy)butyl)amino-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one. MS (ESI): 536 (M+H).
Example 7. 3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-4-
phenoxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one. MS (ESI): 560 (M+H).
Example 8. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-4-
phenoxybutyl)amino-7-chloro-1-methyl-5-(4-fluorophenyl)-
2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 552
(M+H).
Example 9. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 464 (M+H).
Example 10. 3-(2(R)- Isobutyl -3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 506 (M+H).
-104-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 11. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-4-
phenoxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one. MS (ESI): 492 (M+H).
Example 12. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-4-
phenoxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one. MS (ESI): 534 (M+H).
Example 13. 3-(2(R)-Benzyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 574 (M+H).
Example 14. 3-(2(R)-Cyclopentylmethyl-3(S)-hydroxyl-1-oxo-
4-cyclohexyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 566 (M+H).
Example 15. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 540 (M+H).
Example 16. 3-(2(R)-Isopropyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 526 (M+H).
Example 17. 3-(2(R)-Methoxy-3(S)-hydroxyl-1-oxo-4-(4-
trifluoromethylbenzyloxy)butyl)amino-7-chloro-1-methyl-5-
phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI):
590 (M+H).
Example 18. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-4-(2,4-
difluorobenzyloxy)butyl)amino-7-chloro-1-methyl-5-phenyl-
2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 542
(M+H).
-105-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 20. 3-(2(R)-Vinyl-3(S)-hydroxyl-1-oxo-4-
benzyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 536 (M+H).
Example 21. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-4-
cyclohexyloxybutyl)amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 498 (M+H).
Example 23. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-5-
phenylpentyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 498 (M+H).
Example 24. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-3-
cyclopropylpropyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 434 (M+H).
Example 25a. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-
heptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 450 (M+H).
Example 25b. 3-(R)-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-
heptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 450 (M+H).
Example 25c. 3-(S)-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo
heptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4
benzodiazepin-2-one. MS (ESI): 450 (M+H).
Example 26. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-
nonyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 478 (M+H).
Example 27. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-
hexyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 436 (M+H).
-106-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 28. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-4-
phenylbutyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 484 (M+H).
Example 29. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-5-
phenylpentyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 442 (M+H).
Example 30. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-6-
phenylhexyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 470 (M+H).
Example 31. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-
butyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 408 (M+H).
Example 32. 3-(2(R)-Isobutyl-3(S)-hydroxyl-1-oxo-
octyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 464 (M+H).
Example 33. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-
heptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 408 (M+H).
Example 34. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-3-
phenylpropyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 428 (M+H).
Example 35. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-5,5-
dimethyl-hexyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 422 (M+H).
Example 36. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-hexyl)amino-
1-methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one.
MS (ESI): 394 (M+H).
-107-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 37. 3-(2(R)-Methyl-3(S)-hydroxyl-1-oxo-3-(4-
propoxyphenyl)propyl)amino-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one. MS (ESI): 486 (M+H).
Example 38. 2-(R)-cyclopropylmethyl-3-(S)-
hydroxylheptanoic acid (2-oxo-1-(3-phenoxybenzyl)azapan-3-
(S)-yl) amide. MS (ESI): 493 (M+H), 491 (M-H), 527 (M+C1).
Example 39. 2(R)-cyclopropylmethyl-5-(3,5-difluorophenyl)-
3-(S)-hydroxypentanoic acid (2-oxo-1-(3-
phenoxybenzyl)azapan-3-(S)-yl)amide.
MS (ESI): 577 (M+H), 575 (M-H), 599 (M+Na).
Example 40. 4-cyclopentyl-2-(R)-cyclopropylmethyl-3-(S)-
hydroxybutanoic acid (2-oxo-1-(3-phenoxybenzyl)azapan-3-
(S)-yl) amide. MS (ESI): 519 (M+H), 541 (M+Na), 517 (M-H).
Example 41. 2-(R)-cyclopropylmethyl-3-(S)-hydroxyheptanioc
acid (1-(5-bromo-3-pyridinyl)methyl-2-oxo-azapan-3-(S)-yl)
amide. MS (ESI): 480 (M(79Br)+H), 482 (M(8lBr+H), 478
(M(79Br)+H), 480 (M(8lBr)-H).
Example 42. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(2-fluorophenyl)-1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 466 (M+H), 488 (M+Na),
464 (M-H). Chromatography Note b and Note c.
Example 43. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(azapan-1-yl)-1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 469 (M+H), 491 (M+Na),
467 (M-H). Chromatography Note b and Note c.
Example 44. 3-(2-(R)-cyclopropylmethyl-5-(3,5-
difluorophenyl)-3(S)-hydroxyl-1-oxopentyl)amino-1-methyl-5-
(pyridn-2-yl)-2,3-dihydro-1H-1,4-benzodiazepin-2-one.
MS (ESI): 533 (M+H), 555 (M+Na), 531 (M-H). Chromatography
Note b and Note c.
-108-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
The 3-(3,5-difluorophenyl)propanal used in the aldol
reaction was prepared from trans-3,5-difluorocinnamic acid
by: (1) hydrogenation to 3-(3,5-difluorophenyl)propionic
acid (L. Kruse et al J. Med. Chem. 1987, 30, 486-494); (2)
formation of Weinreb amide (M. Braun Synthesis 1989, 856);
and (3) reduction to aldehyde (D. A. Evans J. Org. Chem.
1993, 58, 2446-2453).
Example 45. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxopentyl)amino-1-methyl-5-(4-chlorophenyl)-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 482 (M+H), 504 (M+Na),
480 (M-H). Chromatography Note i and Note k.
Example 46. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(4-methoxyphenyl)1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 466 (M+H), 488 (M+Na),
464 (M-H). Chromatography Note b and Note c.
Example 47. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1
oxoheptyl)amino-5-(4-methoxyphenyl)-1-methyl-2,3-dihydro
1H-1,4-benxodiazepin-2-one. MS (ESI): 479 (M+H), 500
(M+Na), 476 (M-H). Chromatography Note m.
Example 48. 3-(S)-(4-cyclopentyl-2-(R)-cyclopropylmethyl-
3-(S)-hydroxyl-1-oxobutyl)amino-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 474 (M+H),
496 (M+Na), 472 (M-H).
Example 49. 3-(S)-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-
1-oxohept-6-enyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 446 (M+H), 468 (M+Na),
444 (M-H).
Example 50. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxohept-6-enyl)amino-1-methyl-5-(4-trifluoromethyl-phenyl)-
2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 514
(M+H). Chromatography Note i.
-109-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 51. 3-(S)-(2-(R)-cyclopropylmethyl-5-(3,5-
dimethylisoxazol-4-yl)-3-(S)-hydroxyl-1-oxopentyl)amino-1-
methyl-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one.
MS (ESI): 515 (M+H), 537 (M+Na), 513 (M-H).
The 3-(3,5-dimethyl-4-isoxazole)propanal used in the
aldol reaction was prepared from: (1) methyl 3-(3,5-
dimethyl-4-isoxazole)propionate (M. C. Marcotullio J. Org.
Chem 1994, 59, 2884); (2) DIBAL-H reduction to alcohol ( N.
M. Yoon et al J. Org. Chem. 1985, 50, 2443-2450); and (3)
TPAP/NMO oxidation to aldehyde (S. V. Ley et al Synthesis
1994, 639).
Example 52. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-7-chloro-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 482 (M+H), 504 (M+Na),
480 (M-H). Chromatography Note n and Note o.
Example 53. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-(pyridin-2-yl)-2,3-dihydro-1H-1,4
benzodiazepin-2-one. MS (ESI): 449 (M+H), 471 (M+Na}, 447
(M-H). Chromatography Note b and Note c.
Example 54. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(4-fluorophenyl)-1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 466 (M+H), 500 (M+C1).
Chromatography Note h.
Example 55. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifouoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 516 (M+H),
514 (M-H), 550 (M+C1). Chromatography Note i.
The 3-cycloproyl propionic acid, which was converted
to 3-cycloproyl propionyl chloride used in the aldol
reaction, was prepared according to: A. Donetti J. Med.
Chem. 1972, 15, (6), 590-592.
-110-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 56. 3-(5-cyclopentyl-2-(R)-cyclopropylmethyl-3-
(S)-hydroxyl-1-oxopentyl)amino-1-methyl-5-(pyridin-2-yl)-
2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 489
(M+H), 511 (M+Na), 487 (M-H). Chromatography Note b and
Note c.
Example 57. 3-(2-(R)-iso-butyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 518 (M+H),
540 (M+Na), 516 (M-H). Chromatography Note b and Note c.
Example 58. 3-(S)-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-
1-oxo-5-(thiophen-2-yl)amino-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one. MS (ESI): 502 (M+H), 524
(M+Na), 500 (M-H).
Example 59. 3-(S)-(2-(R)-cyclopropylmethyl-5-(furan-2-
yl)3-(S)-hydroxyl-1-oxopentyl)amino-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 486 (M+H),
508 (M+Na), 484 (M-H).
Example 60. 3-(5-cyclopentyl-2-(R)-cyclopropylmethyl-3-
(S)-hydroxyl-1-oxopentyl)amino-5-(4-fluorophenyl)-1-methyl-
2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 506
(M+H), 528 (M+Na), 504 (M-H). Chromatography Note h.
Example 61. 3-(S)-(2-(R)-cyclopropylmethyl-5-(3,5-
difluorophenyl)-3-(S)-hydroxy-1-oxopentyl)amino-1-methyl-5-
phenyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one.
MS (ESI): 532 (M+H), 554 (M+Na), 530 (M-H).
Example 62. 3-(S)-(3-(S)-hydroxyl-2-(R)-(thiophen-2-
yl)methyl-1-oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one. MS (ESI): 448 (M+H), 470
(M+Na), 446 (M-H).
-111-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 63. 3-(S)-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-
1-oxoheptyl)amino-7-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 490 (M+H), 512 (M+Na), 488
(M-H) .
Example 64. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-7-methoxy-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 478 (M+H), 500 (M+Na),
476 (M-H). Chromatography Note 1.
Example 65. 3-(S)-(2-(R)-cyclobutylmethyl-3-(S)-hydroxyl-
1-oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 462 (M+H), 484 (M+Na).
The 3-cyclobutyl propionic acid, which was converted
to 3-cyclobutyl propionyl chloride used in the aldol
reaction, was prepared according to: A. Donetti J. Med.
Chem. 1972, 15, (6), 590-592.
Example 66. 3-(S)-(2-(R)-(3,5-difluorobenzyl)-3-(S)-
hydroxyl-1-oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydor-
1H-1,4-benzodiaxepin-2-one. MS (ESI): 520 (M+H), 518 (M-
H) .
Example 67. 3-(S)-(2-(R)-(furan-2-yl)methyl-3-(S)-
hydroxyl-1-oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one. MS (ESI): 474 (M+H), 472 (M-H).
Example 68. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxl-1-
oxo-5-pheylpentyl)amino-1-methyl-5-(pyridin-2-yl)-2,3-
dihydro-1H-benzodiazepin-2-one. MS (ESI): 514 (M+H), 536
(M+Na), 512 (M-H). Chromatography Note h.
Example 69. 3-(2-(R)-iso-butyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(4-fluorophenyl)-1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 497 (M+H), 519 (M+Na),
495 (M-H). Chromatography Note b and Note c.
-112-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 70. 3-(2-(R)-iso-butyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-(4-fluorophenyl)-1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 468 (M+H), 502 (M+C1).
Chromatography Note h.
10
Example 71. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxo-5-phenylpentyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 421 (M+H), 443 (M+Na),
419 (M-H). Chromatography Note b and Note c.
Example 72. 3-(2-(R)-cyclopropylmethyl-5-(furan-2-yl)-3-
(S)-hydroxyl-1-oxopentyl)amino-1-methyl-5-(4-
trifluoromethylphenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-
one. MS (ESI): 554 (M+H), 576 (M+Na), 552 (M-H).
Chromatography Note i.
Example 73. 3-(5-cyclopentyl-2-(R)-cyclopropylmethyl-3-
(S)-hydroxyl-1-oxopentyl)amino-1-methyl-5-(4-
trifluoromethylphenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-
one. MS (ESI): 542 (M+H), 564 (M+Na), 540 (M-H).
Chromatography Note i.
Example 74. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxooctyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 530 (M+H),
552 (M+Na), 528 (M-H). Chromatography Note i.
Example 75. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxononyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 544 (M+H),
566 (M+Na), 542 (M-H). Chromatography Note i.
Example 76. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethyl(pyridin-2-
yl))-2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 517
(M+H), 539 (M+Na), 515 (M-H). Chromatography Note i.
-113-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 77. 3-(2-(R)-cyclobutylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(40trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 530 (M+H),
552 (M+Na), 528 (M-H). Chromatography Note i.
Example 78. 3-(2-(R)-cyclopentylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 544 (M+H),
542 (M-H), 578 (M+C1). Chromatography Note i.
Example 79. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-methyl-2-pyridiyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 463 (M+H).
Chromatography Note cc.
Example 80. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-methyl-2-pyridyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 463 (M+H).
Chromatography Note dd.
Example 81. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxobutyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 474 (M+H).
Chromatography Note i.
Example 82. 3-(S)-(2-(R)-(3-butenyl)-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 448 (M+H).
Example 83. 3-(S)-(2-(R)-(3-methylbutyl)3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 464 (M+H).
Example 84. 3-(S)-(2-(R)-ethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 422 (M+H).
-114-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 85. 3-(S)-(2-(R)-propyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1,4-
benzodiazepin-2-one. MS (ESI): 436 (M+H).
Example 86. 3-(S)-(2-(R)-butyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-phenyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 450 (M+H).
Example 87. 3-(4-(S)-amino-3-(R)-hydroxyl-2-(R)-methyl-1-
oxo-5-phenylpentyl)amino-7-chloro-5-(2-fluorophenyl)-1-
methyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one.
MS (ESI): 523 (M+H), 521 (M-H). Chromatography Note x.
Example 88. 3-(4-(S)-(tert-butoxycarbonylamino-3-(R)-
hydroxyl-2-(R)-methyl-1-oxo-5-phenylpentyl)amino-7-chloro-
5-(2-fluorophenyl)-1-methyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 645 (M+H), 621 (M-H).
Chromatography Note a and Note u.
Example 89. 3-(3-(tert-butoxycarbonylpyrrolidin-2-(R)-yl)-
3-(R)-hydroxyl-2-(R)-methyl-1-oxopropyl)amino-7-chloro-5-
(2-fluorophenyl)-1-methyl-2,3-dihydro-1H-1,4-benzodiazepin-
2-one. MS (ESI): 523 (M+H), 521 (M-H). Chromatography Note
a and Note v.
Example 90. 3-(3-(R)-hydroxyl-2-(R)-methyl-1-oxo-3-
(pyrrolidin-2-(R)-yl)propyl)-amino-7-chloro-5-(2-
fluorophenyl)-1-methyl-2,3-dihydro-1H-1,4-benzodiazepin-2-
one. MS (ESI): 473 (M+H), 471 (M-H). Chromatography Note y
and Note z.
Example 91. 3-(4-benzyloxy-3-(R)-hydroxyl-2-(R)-iso-
propyl-1-oxobutyl-amino-7-chloro-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 534.(M+H),
556 (M+Na), 532 (M-H). Chromatography Note a and Note v.
-115-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 92. 2-(4-(S)-amino-3-(S)-hydroxyl-2-(S)-methyl-1-
oxo-5-phenylpentyl)amino-7-chloro-5-(2-flourophenyl)-1-
methyl-2,3-dihydro-1H-1,4-benzodiazepin-2-one.
MS (ESI): 523 (M+H), 521 (M-H). Chromatography Note w.
Example 93. 2-(4-(S)-(tert-butoxycarbonylamino-3-
(S)hydroxyl-2-(S)-methyl-1-oxo-5-phenylpentyl)amino-7-
chloro-5-(2-fluorophenyl)-1-methyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 645 (M+Na), 621 (M-H).
Chromatography Note a and Note v.
Example 94. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1
oxoheptyl)amino-1-methyl-5-(thiazol-2-yl)-2,3-dihydro-1H
1,4-benzodiazepin-2-one. MS (ESI): 455 (M+H), 477 (M+Na),
453 (M-H). Chromatography Note b and Note c.
The benzodiazepine was made from 2-aminophenyl-
2'thiazolylketone (see A. Furstner et al., Tetrahedron
1995, 51 (3), 773-786) following the synthetic sequence
from: R. G. Sherrill et al., J. Org. Chem. 1995, 60, 734.
Example 95. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-cyclopropylmethyl-5-(thiazol-2-yl)-2,3-
dihydro-1H-l,4benzodiazepin-2-one. MS (ESI): 495 (M+H),
517 (M+Na), 493 (M-H). Chromatography Note c.
The benzodiazepine was made from 2-aminophenyl-
2'thiazolylketone (see A. Furstner et al., Tetrahedron
1995, 51 (3), 773-786) following the synthetic sequence
from: R. G. Sherrill et al., J. Org. Chem. 1995, 60, 734.
Example 96. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-cyclopropylmethyl-5-(4-
trifluoromethylphenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-
one. MS (ESI): 556 (M+H), 578 (M+Na), 554 (M-H).
Chromatography Note j and Note p.
Example 97. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-benzyl-5-(4-trifluoromethylphenyl)-2,3-
-116-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 592 (M+H),
614 (M+Na), 590 (M-H). Chromatography Note b and Note c.
Example 98. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(3-phenoxybenzyl)-5-(4-trifluoromethyl-
phenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI):
684 (M+H), 706 (M+Na), 682 (M-H). Chromatography Note q and
Note r.
Example 99. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(3-pyridinylmethyl)-5-(4-trifluoromethyl-
phenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI):
593 (M+H), 615 (M+Na), 519 (M-H). Chromatography Note q and
Note r.
Example 100. 3-(2-(S)-cyclopropylmethyl-3-(R)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethyl-phenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 516 (M+H),
538 (M+Na), 514 (M-H). Chromatography Note i.
The syn-aldol was made according to Scheme 1, except
that (S)-4-(phenylmethyl)-2-oxazolidinone was used instead
of the (R)-isomer shown in Scheme 1.
Example 101. 3-(2-(S)-cyclopropylmethyl-3-(R)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 516 (M+H),
538 (M+Na), 514 (M-H). Chromatography Note k.
The syn-aldol was made according to Scheme 1, except
that (S)-4-(phenylmethyl)-2-oxazolidinone was used instead
of the (R)-isomer shown in Scheme 1.
Example 102. 3-(2-(R)-cyclopropylmethyl-3-(R)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 516 (M+H),
538 (M+Na), 514 (M-H). Chromatography Note i.
-117-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
NHTs O NHTs O pH
1. TiCl4; DIPEA
O 2. C4H9CH0, TiCl4 I / O
O OH
LiOH, THF-H20 HO
The anti-aldols were made by the method described in:
A. K. Ghosh, J. Am. Chem. Soc. 1996, 118, 2527-2528. The
carboxylic acid shown was coupled with the corresponding
benzodiazepine following a procedure analogous to the
procedure of the last step in Example 1.
Example 103. 3-(2-(S)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 516 (M+H),
538 (M+Na), 514 (M-H). Chromatography Note i.
Followed the synthetic sequence of Example 102, except
the opposite enantiomer of the chiral auxiliary was used.
Example 104. 3-(2-(S)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 516 (M+H),
514 (M-H). Chromatography Note k.
Followed the synthetic sequence of Example 102, except
the opposite enantiomer of the chiral auxiliary was used.
Example 105. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-3-
(S)-methyl-1-oxoheptyl)amino-1-methyl-5-(4-trifluoromethyl-
phenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI):
530 (M+H), 552 (M+Na), 528 (M-H). Chromatography Note i.
Addition of a methyl group to Example 135 was
performed with an organocerium reagent generated from
methylmagnesium bromide and cerium trichloride according
to: T. Imamoto et al (a) J. Org. Chem. 1984, 49, 3904-3912,
and (b) J. Am. Chem. Soc. 1989, 111, 4392-4398.
-118-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 106. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(3-phenoxybenzyl)-5-methyl-2,3-dihydro-
1H-1,4-benzodiazepin-2-one. MS (ESI): 554 (M+H).
Chromatography Note as and Note bb.
Example 107. 3-(2-(R)-cyclopropylmehtyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-benzyl-5-methyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 462 (M+H). Chromatography
Note b and Note c.
Example 108. 3-(3-(S)-acetoxy-2-(R)-iso-butyl-1-
oxoheptyl)amino-5-(4-fluorophenyl)-1-methyl-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 510 (M+H), 532 (M+Na),
508 (M-H). Chromatography Note h.
Example 109. 3-(S)-(5-cyclopentyl-2-(R)-cyclopropylmethyl-
3-(S)-methoxy-1-oxopentyl)amino-1-methyl-5-phenyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 502 (M+H),
524 (M+Na).
OH O ~ ~O p O
N O Me30BF4 N'
~/ proton sponge ~/
Bn' = Bn'
~O O
LiOH, THF-H20 OH
Methylation of the corresponding aldol was carried out
according to: (a) D. A. Evans et al., Tetrahedron Lett.
1994, 35 (39), 7171-7172; (b) G. R. Pettit, Synthesis 1996,
719-725. The carboxylic acid shown was then coupled with
the corresponding benzodiazepine following a procedure
analogous to the procedure of the last step in Example 1.
Example 113. 1-(1-hydroxypentyl)cyclohexanecarboxylic
acid(5-(4-fluorophenyl)-1-methyl-2-oxo-2,3-dihydro-1H-1,4-
-119-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
benzodiazepin-3-yl)amide. MS (ESI): 480 (M+H), 502 (M+Na),
478 (M-H). Chromatography Note t and Note h.
O IO' OH O O
N~N- 1. Bu2BOTf, TEA N~N'
Ph~ 2~ CaHsCHO
Ph
OH O
'OH
LiOH, THF-H20
The corresponding aldol was made according to (a) A.
S. Kende et al., Tetrahedron Lett. 1989, 30 (43), 5821-
5824; (b) H. Mulzer et al., Tetrahedron Lett. 1995, 36
(42), 7643-7646. The carboxylic acid shown was coupled
with benzodiazepine following a procedure analogous to the
procedure of the last step in Example 1.
Example 114. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-methyl-5H,7H-dibenzo[b,d]azepin-6-one.
MS (ESI): 421 (M+H), 443 (M+Na), 419 (M-H). Chromatography
Note s.
Example 115. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxooctyl)amino-5-methyl-5H,7H-dibenzo[b,d]azepin-6-one.
MS (ESI): 435 (M+H), 457 (M+Na), 433 (M-H). Chromatography
Note s.
Example 116. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxononyl)amino-5-methyl-5H,7H-dibenzo[b,d]azepin-6-one.
MS (ESI): 449 (M+H), 471 (M+Na), 447 (M-H). Chromatography
Note s.
Example 117. 3-(2-(R)-cyclopropylmethyl-5-(furan-2-yl)-3-
(S)-hydroxyl-1-oxopentyl)amino-5-methyl-5H,7H-
dibenzo[b,d]azepin-6-one. MS (ESI): 459 (M+H), 481 (M+Na),
457 (M-H). Chromatography Note s.
-120-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 118. 2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-
heptanoic acid (2-oxo-1-(3-phenylamino-benzyl)azapan-3-(S)-
yl) amide. MS (ESI): 492 (M+H), 514 (M+Na), 490 (M-H).
Step 1: [2-Oxo-1-(3-phenylamino-benzyl)-azepan-3-yl]-
carbamic acid tert-butyl ester: In a 100 ml round bottomed
flask Binap, (S)-(-)2,2'-Bis(diphenylphosphino)-1,1'-
binaphthyl, ( 0.210 g , 0.3375 mmol) dissolved in l5mL
toluene was stirred at 80°C for 1 minute . To the flask
cooled to room temperature under inert atmosphere Pd(OAC)2
(0.050 g , 0.225 mmol) was added and the solution was
stirred at room temperature for 2 minutes. To the reaction
mixture [1-(3-Iodo-benzyl)-2-oxo-azepan-3-yl]-carbamic acid
tert-butyl ester ( 1.0 g , 2.25 mmol) dissolved in l5mL
toluene , aniline (1.047 g , 11.25 mmol) and Sodium tert-
butoxide (0.259 g , 2.70 mmol ) were added and the solution
was stirred at 80°C for 18 h. The reaction was cooled to
room temperature, diluted with 200mL of water, and
extracted twice with 100 mL of ethyl acetate. The organic
layer was dried with anhydrous sodium sulfate, filtered and
concentrated to an oil. The oil was purified on flash
silica gel column using 10-30~ ethyl acetate in hexanes as
eluent to yield 0.562 g (610). MS (ESI) M+H = 432.5
Step 2: 3-Amino-1-(3-phenylamino-benzyl)-azepan-2-one,
trifluoroacetic acid salt: In a 25mL round bottomed flask
the ester from Step 1 above ( 0.025 g , 0.06 mmol ) was
dissolved in lOmL of 50~TFA / CH2C12 and was stirred at
room temperature for 1 h. The solvent was concentrated to
an oil and dried under high vacuum to yield 0.025 g (1000 .
MS (ESI) M+H = 310.4
Step 3: 2-Cyclopropylmethyl-3-hydroxy-heptanoic acid
[2-oxo-1-(3-phenylamino-benzyl)-azepan-3-yl]-amide: In a
25mL round bottomed flask 2-Cyclopropylmethyl-3-hydroxy-
heptanoic acid ( 0.01258 , 0.061mmo1 ) was dissolved in 1mL
DMF. To the reaction mixture HATU, O-(7-Azabenzotriazol-1-
yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate,
(0.029 g , 0.0734 mmol ) and N-Methyl morpholine (0.018 g,
0.018 mmol) were added and the reaction solution was
-121-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
stirred at room tenperature for 10 minutes. To the
reaction mixture the compound from Step 2 above (0.025 g,
0.06 mmol) dissolved in 1mL of DMF was added and the
reaction solution was stirred at room tenperature for 18 h.
The solution was diluted with 50 mL of water and extracted
twice with 20 mL of ethyl acetate. The combined organic
layers were dried with anhydrous Sodium sulfate, filtered,
and concentrated to an oil. The oil was purified on a
flash silica gel column using 20-40% ethyl acetate in
hexanes as eluent to yield 6.0 mg (200). MS (ESI) M+H =
492.6
Example 119. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-cyclopentyl-1-methyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 440 (M+H). Chromatography
Note a.
Synthesis of 2-aminophenyl cyclopentylmethanone: To a
solution of anthranilonitrile (15.0 g) in diethyl ether
(600 mL) was added a solution of 2.0 M cyclopentylmagnesium
bromide in diethyl ether (159 mL) at 0°C under nitrogen.
The mixture was stirred at room temperature overnight (20
hours). 500 ml of 5 N HC1 in H20 was added very slowly at
0°C. The mixture was stirred at room temperature for 1
hour. The aqueous layer was neutralized with 50~ NaOH/H20
to pH = 12. 2 X 500 mL of ethyl acetate was used to extract
the aqueous layer. The combined organic layers were dried
over Na2S04. The solvent was removed to give the crude
product 22.5 g (93.6 yield). H1NMR(CDC13): 86.62-7.82 (m,
4H), 3.64-3.78 (m, 1H), 1.50-1.96 (m, 8H).
The 2-aminophenyl cyclopentylmethanone was converted
to benzodiazepine following: R. G. Sherrill et al J. Org.
Chem. 1995, 60, 734.
Example 120. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-benzyl-1-methyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 462 (M+H), 460 (M-H).
Chromatography Note b and Note c.
-122-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
The benzodiazepine was made from 1-(2-aminophenyl)-2-
phenylethanone (see M. W. Partridge et al., J. Chem. Soc.
1964, 3673) following the synthetic sequence from: R. G.
Sherrill, J. Org. Chem. 1995, 60, 734.
Example 121. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-benzyl-1-butyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 504 (M+H), 502 (M-H).
Chromatography Note b and Note c.
Example 122. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-5-cycloheptyl-1-methyl -2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 468 (M+H), 466 (M-H).
Chromatography Note b and Note c.
Example 123. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-benzyl-5-cycloheptyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 544 (M+H), 542 (M-H).
Chromatography Note a.
Example 124. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-butyl-5-cycloheptyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 510 (M+H), 508 (M-H).
Chromatography Note b and Note c.
Example 125. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(2-pyridinylmethyl)-5-(4-
trifluoromethylphenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-
one. MS (ESI): 593 (M+H), 615 (M+Na), 591 (M-H).
Chromatography Note a.
Example 126. 3-(2-(R)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(3-pyridinylmethyl)-5-(2-fluorophenyl)-
2,3-dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 543
(M+H), 541 (M-H). Chromatography Note d and Note e.
-123-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 127. 3-(2-(S)-cyclopropylmethyl-3-(S)-hydroxyl-1-
oxopentyl)amino-1-(3-pyridinylmethyl)-5-(4-
trifluoromethylphenyl)-2,3-dihydro-1H-1,4-benzodiazepin-2-
one. MS (ESI): 565 (M+H), 563 (M-H). Chromatography Note f
and Note g.
Example 128. 3-(2-1(R)-Cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-(N,N-dibutylamino)-2,3-dihydro-
1H-1,4-benzodiazepin-2-one. MS (ESI): 499 (M+H).
Chromatography Note a.
Example 129. 3-(2-(R)-Cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-n-butyl-5-t-butyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 470 (M+H). Chromatography
Note b and Note c.
Example 130. 3-(2-(R)-Cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(2-oxo-3,3-dimethylbutyl)-5-n-butyl-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 512 (M+H).
Chromatography Note b.
Example 131. 3-(2-(R)-Cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-benzyl-5-t-butyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 504 (M+H). Chromatography
Note a.
Example 132. 3-(2-(R)-Cyclopropylmethyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-(2-picolyl)-5-n-butyl-2,3-dihydro-1H-1,4-
benzodiazepin-2-one. MS (ESI): 505 (M+H). Chromatography
Note b and Note c.
Example 133. 3-(2-(R)-Isobutyl-3-(S)-hydroxyl-1-
oxoheptyl)amino-1-methyl-5-homopiperidino-2,3-dihydro-1H-
1,4-benzodiazepin-2-one. MS (ESI): 471 (M+H).
Chromatography Note b and Note c.
-124-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Example 135. 3-(2-(R)-cyclopropylmethyl-1,3-
dioxoheptyl)amino-1-methyl-5-(4-trifluoromethylphenyl)-2,3-
dihydro-1H-1,4-benzodiazepin-2-one. MS (ESI): 514 (M+H),
536 (M+Na), 512 (M-H). Chromatography Note i.
Example 55 was oxidized to the dicarbonyl compound by
TPAP/NMO, see S. V. Ley et al., Synthesis 1994, 639.
Example 136. 1-pentyrylcyclohexanecarboxylic acid (5-(4-
fluorophenyl)-1-methyl-2-oxo-2,3-dihydro-1H-1,4-
benzodiazepin-3-yl) amide. MS (ESI): 478 (M+H), 500
(M+Na), 476 (M-H). Chromatography Note h.
Chromatography Notes:
Note a: epimeric mixture at BZD.
Note b: 1St eluting peak on CHIRALPAK AD chiral column with
10 - 35o i-PrOH/hexane.
Note c: 2nd eluting peak on CHIRALPAK AD chiral column
with 10 - 35~ i-PrOH/hexane.
Note d: 1St eluting peak on CHIRALCEL OD chiral column with
2/200/800 ratio of MeOH/i-PrOH/Hexane.
Note e: 2nd eluting peak on CHIRALCEL OD chiral column
with 2/200/800 ratio of MeOH/i-PrOH/Hexane.
Note f: 1St eluting peak on silica gel column with 2~
MeOH/CHZC12.
Note g: 2nd eluting peak on silica gel column with 2~
MeOH/CH2C12.
Note k: made from BZD-amine which in Cbz protected form was
the 2nd eluting peak on CHIRALPAK AD column with
acetonitrile.
Note m: made from BZD-amine which in Cbz protected form was
the 1St eluting peak on CHIRALPAK AS with
methanol.
Note n: made from BZD-amine which was the 1St eluting peak
on CHIRALPAK AS with O.lo diethylamine/methanol.
Note o: made from BZD-amine which was the 2nd eluting peak
on CHIRALPAK AS with 0.1~ diethylamine/methanol.
Note h: made from BZD-amine which was the 1St eluting peak
on CHIRALPAK AD column with 0.1~
diethylamine/MeOH.
Note i: made from BZD-amine which in Cbz protected form was
the 1St eluting peak on CHIRALPAK AD column with
acetonitrile.
Note 1: made from BZD-amine which in Cbz protected was the
1St eluting peak on CHIRALCEL OJ with 1:4 of
hexane/ethanol.
Note j: 1St eluting peak on CHIRALPAK AD column with
acetonitrile/water.
-125-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Note p: 2nd eluting peak on CHIRALPAK AD column with
acetonitrile/water.
Note q: 1St eluting peak on CHIRALCEL OD with 10$ i-
propanol/hexane.
Note r: 2nd eluting peak on CHIRALCEL OD with 10~ i-
propanol/hexane.
Note s: made from bisbenazapine amine which was the 1St
peak on CHIRALCEL OD with 20~ i-PrOH/hexane with
diethylamine.
Note t: made from BZD-amine which was the 2nd eluting peak
on CHIRALPAK AD column with 0.1~
diethylamine/MeOH.
Note w: derived from Example 93 by treatment with TFA.
Note x: derived from Example 88 by treatment with TFA.
Note u: 2nd eluting peak on silica gel column with 30-80%
EtOAc/hexane.
Note v: 1St eluting peak on silica gel column with 30-800
EtOAc/hexane.
Note y: derived from Example 89 by treatment with TFA.
Note z: derived from Example 89 by treatment with TFA.
Note aa: 1St eluting peak on CHIRALPAK AD with 20:80 of
water/MeCN.
Note bb: 2nd eluting peak on CHIRALPAK AD with 20:80 of
water/MeCN.
Note cc: made from BZD-amine which in Cbz protected form
was the 2nd eluting peak on CHIRALCEL OD column
with 1/300/700 ratio of diethtlamine/EtOH/C02.
Note dd: made from BZD-amine which in Cbz protected form
was the 1St eluting peak on CHIRALCEL OD column
with 1/300/700 ratio of diethtlamine/EtOH/C02.
Tables 1-8 below provide representative Examples of
the compounds of Formula (I) of the present invention.
-126-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Table 1
i Hs
OH O O N ~ / Rya
D HN N R11
Rs
Ex.# Q R5 R11 R13 Mass
(M+H)
1 phenethyl cyclopentylmethyl phenyl H 524
2 phenethyl cyclopentylmethyl 4-F-phenyl H 542
3 phenethyl benzyl phenyl H 532
4 phenethyl i-propyl phenyl H 484
5 3,5-diF- cyclopentylmethyl phenyl H 562
phenoxymethyl
6 3,5-diF- i-butyl phenyl H 536
phenoxymethyl
7 phenoxymethyl cyclopentylmethyl phenyl C1 560
8 phenoxymethyl i-butyl 2-F-phenyl C1 552
9 cyclohexyl- methyl phenyl H 464
oxymethyl
cyclohexyl- i-butyl phenyl H 506
oxymethyl
11 phenoxymethyl methyl phenyl C1 492
12 phenoxymethyl i-butyl phenyl C1 534
13 cyclohexyl- benzyl phenyl C1 574
oxymethyl
14 cyclohexyl- cyclopentylmethylphenyl C1 566
oxymethyl
cyclohexyl- i-butyl phenyl C1 540
oxymethyl
16 cyclohexyl- i-propyl phenyl C1 526
oxymethyl
17 4-CF3-benzyl- methoxy phenyl C1 590
oxymethyl
18 2,4-diF-benzyl-methyl phenyl C1 542
oxymethyl
benzyloxymethylvinyl phenyl C1 536
21 cyclohexyl- methyl phenyl C1 498
oxymethyl
22
23 phenethyl i-butyl phenyl H 498
24 cyclopropyl i-butyl phenyl H 434
25a n-butyl i-butyl phenyl H 450
25b n-butyl i-butyl phenyl H 450
25c n-butyl i-butyl phenyl H 450
26 n-hexyl i-butyl phenyl H 478
-127-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
27 n-propyl i-butyl phenyl H 436
28 benzyl i-butyl phenyl H 484
29 phenethyl methyl phenyl H 442
30 phenpropyl methyl phenyl H 470
31 methyl i-butyl phenyl H 408
32 n-pentyl i-butyl phenyl H 464
33 n-butyl methyl phenyl H 408
34 phenyl methyl phenyl H 428
35 2,2-dimethyl- methyl phenyl H 422
propyl
36 n-propyl methyl phenyl H 394
37 4-propoxyphenyl methyl phenyl H 486
25a: the chiral carbon of the benzodiazepine ring is racemic.
25b: the chiral carbon of the benzodiazepine ring is (R).
25c: the chiral carbon of the benzodiazepine ring is (S).
Table 2
W-X-Y-Z
i
OH O O N
Q N
H
Ex.# Q z-Y-x-w-
38 n-butyl 3-phenoxybenzyl
39 3,5-diF-phenethyl 3-phenoxybenzyl
40 cyclopentylmethyl 3-phenoxybenzyl
41 n-butyl 5-bromo-3-pyridinyl
118 n-butyl 3-(phenyl)amino-benzyl
-128-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Table 3
i Hs
OH O O N ~ ~ Ria
Q HN N R"
R5
Ex.# Q R5 R11 R13
42 n-butyl cyclopropylmethyl2-F-phenyl H
43 n-butyl cyclopropylmethylazapan-1-yl H
44 3,5-diF- cyclopropylmethylpyridin-2-yl H
phenethyl
45 n-butyl cyclopropylmethyl4-C1-phenyl H
46 n-butyl cyclopropylmethyl3-F-phenyl H
47 n-butyl cyclopropylmethyl4-Me0-phenyl H
48 cyclopentyl cyclopropylmethylphenyl H
methyl
49 but-3-enyl cyclopropylmethylphenyl H
50 but-3-enyl cyclopropylmethyl4-CF3-phenyl H
51 2-(3,5-dimethylcyclopropylmethylphenyl H
isoxazol-4-yl)-
ethyl
52 n-butyl cyclopropylmethylphenyl C1
53 n-butyl cyclopropylmethylpyridin-2-yl H
54 n-butyl cyclopropylmethyl4-F-phenyl H
55 n-butyl cyclopropylmethyl4-CF3-phenyl H
56 2-cyclopentyl- cyclopropylmethylpyridin-2-yl H
ethyl
57 n-butyl i-butyl 4-CF3-phenyl H
58 2-(thiophen-2- cyclopropylmethylphenyl H
yl)-ethyl
59 2-(furan-2-yl)-cyclopropylmethylphenyl H
ethyl
60 2-cyclopentyl- cyclopropylmethyl4-F-phenyl H
ethyl
61 3,5-diF- cyclopropylmethylphenyl H
phenethyl
62 n-butyl cyclopropylmethylphenyl H
-129-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
63 n-butyl thiophen-2-yl- phenyl H
methyl
64 n-butyl cyclopropylmethyl phenyl Me0
65 n-butyl cyclobutylmethyl phenyl H
66 n-butyl 3,5-diF-phenyl- phenyl H
methyl
67 n-butyl furan-2-yl-methyl phenyl H
68 phenethyl cyclopropylmethyl4-F-phenyl H
69 phenethyl cyclopropylmethylpyridin-2-yl H
70 n-butyl i-butyl 4-F-phenyl H
71 phenethyl cyclopropylmethylphenyl H
72 2-furan-2-yl- cyclopropylmethyl4-CF3-phenyl H
ethyl
73 2-cyclopentyl- cyclopropylmethyl4-CF3-phenyl H
ethyl
74 n-pentyl cyclopropylmethyl4-CF3-phenyl H
75 n-hexyl cyclopropylmethyl4-CF3-phenyl H
76 n-butyl cyclopropylmethyl4-CF3-pyridin- H
2-yl
77 n-butyl cyclobutylmethyl 4-CF3-phenyl H
78 n-butyl cyclopentylmethyl4-CF3-phenyl H
79 n-butyl cyclopropylmethyl4-methyl- H
pyridin-2-yl
80 n-butyl cyclopropylmethyl4-methyl- H
pyridin-2-yl
81 methyl cyclopentylmethyl4-CF3-phenyl H
82 n-butyl but-3-enyl phenyl H
83 n-butyl 3-methyl-butyl phenyl H
84 n-butyl ethyl phenyl H
85 n-butyl propyl phenyl H
86 n-butyl n-butyl phenyl H
87 1-(S)-amino- methyl 2-F-phenyl C1
phenethyl
88 1-(S)-(BOC-NH)-methyl 2-F-phenyl C1
phenethyl
89 N-BOC- methyl 2-F-phenyl C1
pyrrolidin-2-
(R)-yl
90 pyrrolidin-2- methyl 2-F-phenyl C1
(R)-yl
-130-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
91 benzyloxy-methyli-propyl phenyl C1
119 n-butyl cyclopropylmethylcyclopentyl H
120 n-butyl cyclopropylmethylbenzyl H
122 n-butyl cyclopropylmethylcycloheptyl H
128 n-butyl cyclopropylmethylN,N-dibutyl- H
amino
133 n-butyl i-butyl homopiperidinoH
134 n-butyl i-butyl spiro-cyclo- H
pentyl
Table 4
i Hs
OH O ~ N ~ ~ Ris
O~HN N'
R"
Rs
Ex.# Q R5 R11 R13
92 1-(S)-amino- Methyl 2-F-phenyl C1
phenethyl
93 1-(S)-(BOC-NH)-Methyl 2-F-phenyl C1
phenethyl
100 n-butyl cyclopropylmethyl4-CF3-phenyl H
101 n-butyl cyclopropylmethyl4-CF3-phenyl H
-131-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Table 5A
W -X-Y-Z
I
HO R2 O O N
N Ro
Ex.# Q R2 Z-Y-X-W- R11
94 n-butyl H methyl thiazol-2-yl
95 n-butyl H cyclopropylmethyl thiazol-2-yl
96 n-butyl H cyclopropylmethyl 4-CF3-phenyl
97 n-butyl H benzyl 4-CF3-phenyl
98 n-butyl H 3-phenoxy-benzyl 4-CF3-phenyl
99 n-butyl H 3-pyridinyl-methyl 4-CF3-phenyl
105 n-butyl Me methyl 4-CF3-phenyl
106 n-butyl H 3-phenoxy-benzyl methyl
107 n-butyl H benzyl methyl
121 n-butyl H n-butyl benzyl
123 n-butyl H benzyl cycloheptyl
124 n-butyl H n-butyl cycloheptyl
125 n-butyl H 2-pyridinyl-methyl 4-CF3-phenyl
126 n-butyl H 3-pyridinyl-methyl 2-F-phenyl
129 n-butyl H n-butyl t-butyl
130 n-butyl H 2-oxo-3,3- n-butyl
dimethylbutyl
131 n-butyl H benzyl t-butyl
132 n-butyl H 2-pyridinyl-methyl n-butyl
-132-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Table 5B
W -X-Y-Z
I
HO O O N
O~N~ ,
H N R11
Ex.# Q R2 Z-Y-X-W- R11
102 n-butyl H methyl 4-CF3-phenyl
Table 5C
W-X-Y-Z
I
HO O O IV
Q ~N N
H Rii
U
Ex.# Q R2 Z-Y-X-W- R11
103 n-butyl H methyl 4-CF3-phenyl
104 n-butyl H methyl 4-CF3-phenyl
127 ethyl H 3-pyridinyl-methyl 4-CF3-phenyl
-133-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Table 6
Hs
RIO O O N \
Q~HN
~o N
R5 R5a
Ex.# R3 Q R5~R5a R11
5 __
108 acetyl n-butyl butyl g-F-phenyl
l
H
R5a -
2-cyclo R5 - cyclopropyl
109 methyl pentyl methyl phenyl
ethyl R5a - H
CR5R5a
113 H n-butyl 4-F-phenyl
- 1,1-cyclohexyl
-134-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
Table 8
CH3
OH 0 0
Q'
H
Ex.#
Q
114 n-butyl
n-pentyl
115
n-hexyl
116
2-(furan-2-yl)-ethyl
117
UTILITY
A~i production has been implicated in the pathology of
Alzheimer's Disease (AD). The compounds of the present
invention have utility for the prevention and treatment of
AD by inhibiting A(3 production. Methods of treatment
target formation of A~3 production through the enzymes
involved in the proteolytic processing of (3 amyloid
precursor protein. Compounds that inhibit ~3 or ysecretase
activity, either directly or indirectly, control the
production of A(3. Such inhibition of ~3 or y secretases
reduces production of A~i, and is expected to reduce or
prevent the neurological disorders associated with
A~iprotein, such as Alzheimer's Disease.
Cellular screening methods for inhibitors of A(3
production, testing methods for the in vivo suppression of
A(3 production, and assays for the detection of secretase
activity are known in the art and have been disclosed in
-135-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
numerous publications, including PCT publication number V40
98/22493, EPO publication number 0652009, US patent 5703129
and US patent 5593846; all hereby incorporated by
reference.
The compounds of the present invention have utility
for the prevention and treatment of disorders involving A(3
production, such as cerebrovascular disorders.
Compounds of the present invention have been shown to
inhibit A~3 production, as determined by the secretase
inhibition assay described below.
Compounds of the present invention have been shown to
inhibit A(3 production, utilizing the C-terminus ~3 amyloid
precursor protein accumulation assay described below.
Compounds of Formula (I) are expected to possess y-
secretase inhibitory activity. The y-secretase inhibitory
activity of the compounds of the present invention is
demonstrated using assays for such activity, for example,
using the assay described below. Compounds of the present
invention have been shown to inhibit the activity of ~y-
secretase, as determined by the A~3 immunoprecipitation
assay.
Compounds provided by this invention should also be
useful as standards and reagents in determining the ability
of a potential pharmaceutical to inhibit A(3 production.
These would be provided in commercial kits comprising a
compound of this invention.
As used herein "ug" denotes microgram, "mg" denotes
milligram, "g" denotes gram, "~ZL" denotes microliter, "mL"
denotes milliliter, "L" denotes liter, "nM" denotes
nanomolar, "uM" denotes micromolar, "mM" denotes
millimolar, "M" denotes molar, "nm" denotes nanometer,
"SDS" denotes sodium dodecyl sulfate, and "DMSO" denotes
dimethyl sulfoxide, and "EDTA" denotes
ethylenediaminetetraacetato.
-136-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
A compound is considered to be active if it has an
ICSp or Ki value of less than about 100 uM for the
inhibition of A~3 production.
(3 amyloid precursor protein accumulation assay
A novel assay to evaluate the accumulation of A(3
protein was developed to detect potential inhibitors of
secretase. The assay uses the N 9 cell line, characterized
for expression of exogenous APP by immunoblotting and
immunoprecipitation.
The effect of test compounds on the accumulation of A(3
in the conditioned medium is tested by immunoprecipitation.
Briefly, N 9 cells are grown to confluency in 6-well plates
and washed twice with 1 x Hank's buffered salt solution.
The cells are starved in methionine/cysteine deficient
media for 30 min, followed by replacement with fresh
deficient media containing 150uCi S35 Translabel
(Amersham). Test compounds dissolved in DMSO (final
concentration 1~) are added together with the addition of
radiolabel. The cells are incubated for 4 h at 37°C in a
tissue culture incubator.
At the end of the incubation period, the conditioned
medium is harvested and pre-cleared by the addition of 5 u1
normal mouse serum and 50u1 of protein A Sepharose
(Pharmacia), mixed by end-over-end rotation for 30 minutes
at 4°C, followed by a brief centrifugation in a microfuge.
The supernatant is then harvested and transferred to fresh
tubes containing 5ug of a monoclonal antibody (clone
1101.1; directed against an internal peptide sequence in
A(3) and 50 u1 protein A Sepharose. After incubation
overnight at 4°C, the samples are washed three times with
high salt washing buffer (50mM Tris, pH 7.5, 500mM NaCl,
5mM EDTA, 0.5~ Nonidet P-40), three times with low salt
wash buffer (50mM Tris, pH 7.5, 150mM NaCl, 5mM EDTA, 0.5~
Nonidet P-40), and three times with lOmM Tris, pH 7.5. The
pellet after the last wash is resuspended in SDS sample
-137-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
buffer (Laemmli, 1970) and boiled for 3 minutes. The
supernatant is then fractionated on either 10-20%
Tris/Tricine SDS gels or on 16.5% Tris/Tricine SDS gels.
The gels are dried and exposed to X-ray film or analyzed by
phosphorimaging. The resulting image is analyzed for the
presence of A(3 polypeptides. The steady-state level of A(3
in the presence of a test compound is compared to wells
treated with DMSO (1%) alone. A typical test compound
blocks A(3 accumulation in the conditioned medium, and is
therefore considered active, with an ICSp less than 100 uM.
C-Terminus (~ Amyloid Precursor Protein Accumulation Assay
The effect of test compounds on the accumulation of C-
terminal fragments is determined by immunoprecipitation of
APP and fragments thereof from cell lysates. N 9 cells are
metabolically labeled as above in the presence or absence
of test compounds. At the end of the incubation period,
the conditioned medium are harvested and cells lysed in
RIPA buffer (10 mM Tris, pH 8.0 containing 1% Triton X-100,
1% deoxycholate, 0.1% SDS, 150mM NaCl, 0.125% NaN3).
Again, lysates are precleared with 5u1 normal rabbit serum
/ 50u1 protein A Sepharose, followed by the addition of BC-
1 antiserum (15~z1;) and 50u1 protein A Sepharose for 16
hours at 4°C. The immunoprecipitates are washed as above,
bound proteins eluted by boiling in SDS sample buffer and
fractionated by Tris/Tricine SDS-PAGE. After exposure to
X-ray film or phosphorimager, the resulting images are
analyzed for the presence of C-terminal APP fragments. The
steady-state level of C-terminal APP fragments is compared
to wells treated with DMSO (1%) alone. A typical test
compound stimulates C-terminal fragment accumulation in the
cell lysates, and is therefore considered active, with an
ICSp less than 100 uM.
A~3 Immunoprecipitation Assay
-138-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
This immunoprecipitation assay is specific for y
secretase (i.e., proteolytic activity required to generate
the C-terminal end of A(3 either by direct cleavage or
generating a C-terminal extended species which is
subsequently further proteolyzed). N 9 cells are pulse
labeled in the presence of a reported y secretase inhibitor
(MDL 28170) for 1 h, followed by washing to remove
radiolabel and MDL 28170. The media is replaced and test
compounds are added. The cells are chased for increasing
periods of times and A (3 is isolated from the conditioned
medium and C-terminal fragments from cell lysates (see
above). The test compounds are characterized whether a
stabilization of C-terminal fragments is observed and
whether A(3 is generated from these accumulated precursor.
A typical test compound prevents the generation of A(3 out
of accumulated C-terminal fragments and is considered
active with an ICSp less than 100 uM.
Dosaae and Formulation
The compounds of the present invention can be
administered orally using any pharmaceutically acceptable
dosage form known in the art for such administration. The
active ingredient can be supplied in solid dosage forms
such as dry powders, granules, tablets or capsules, or in
liquid dosage forms, such as syrups or aqueous suspensions.
The active ingredient can be administered alone, but is
generally administered with a pharmaceutical carrier. A
valuable treatise with respect to pharmaceutical dosage
forms is Remington's Pharmaceutical Sciences, Mack
Publishing.
The compounds of the present invention can be
administered in such oral dosage forms as tablets, capsules
(each of which includes sustained release or timed release
formulations), pills, powders, granules, elixirs,
tinctures, suspensions, syrups, and emulsions. Likewise,
they may also be administered in intravenous (bolus or
-139-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
infusion), intraperitoneal, subcutaneous, or intramuscular
form, all using dosage forms well known to those of
ordinary skill in the pharmaceutical arts. An effective
but non-toxic amount of the compound desired can be
employed to prevent or treat neurological disorders related
to (3-amyloid production or accumulation, such as
Alzheimer's disease and Down's Syndrome.
The compounds of this invention can be administered by
any means that produces contact of the active agent with
the agent's site of action in the body of a host, such as a
human or a mammal. They can be administered by any
conventional means available for use in conjunction with
pharmaceuticals, either as individual therapeutic agents or
in a combination of therapeutic agents. They can be
administered alone, but generally administered with a
pharmaceutical carrier selected on the basis of the chosen
route of administration and standard pharmaceutical
practice.
The dosage regimen for the compounds of the present
invention will, of course, vary depending upon known
factors, such as the pharmacodynamic characteristics of the
particular agent and its mode and route of administration;
the species, age, sex, health, medical condition, and
weight of the recipient; the nature and extent of the
symptoms; the kind of concurrent treatment; the frequency
of treatment; the route of administration, the renal and
hepatic function of the patient,and the effect desired. An
ordinarily skilled physician or veterinarian can readily
determine and prescribe the effective amount of the drug
required to prevent, counter, or arrest the progress of the
condition.
Advantageously, compounds of the present invention may
be administered in a single daily dose, or the total daily
dosage may be administered in divided doses of two, three,
or four times daily.
The compounds for the present invention can be
administered in intranasal form via topical use of suitable
-140-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
intranasal vehicles, or via transdermal routes, using those
forms of transdermal skin patches wall known to those of
ordinary skill in that art. To be administered in the form
of a transdermal delivery system, the dosage administration
will, of course, be continuous rather than intermittant
throughout the dosage regimen.
In the methods of the present invention, the compounds
herein described in detail can form the active ingredient,
and are typically administered in admixture with suitable
pharmaceutical diluents, excipients, or carriers
(collectively referred to herein as carrier materials)
suitably selected with respect to the intended form of
administration, that is, oral tablets, capsules, elixirs,
syrups and the like, and consistent with conventional
pharmaceutical practices.
For instance, for oral administration in the form of a
tablet or capsule, the active drug component can be
combined with an oral, non-toxic, pharmaceutically
acceptable, inert carrier such as lactose, starch, sucrose,
glucose, methyl callulose, magnesium stearate, dicalcium
phosphate, calcium sulfate, mannitol, sorbitol and the
like; for oral administration in liquid form, the oral drug
components can be combined with any oral, non-toxic,
pharmaceutically acceptable inert carrier such as ethanol,
glycerol, water, and the like. Moreover, when desired or
necessary, suitable binders, lubricants, disintegrating
agents, and coloring agents can also be incorporated into
the mixture. Suitable binders include starch, gelatin,
natural sugars such as glucose or ~3-lactose, corn
sweeteners, natural and synthetic gums such as acacia,
tragacanth, or sodium alginate, carboxymethylcellulose,
polyethylene glycol, waxes, and the like. Lubricants used
in these dosage forms include sodium oleate, sodium
stearate, magnesium stearate, sodium benzoate, sodium
acetate, sodium chloride, and the like. Disintegrators
include, without limitation, starch, methyl cellulose,
agar, bentonite, xanthan gum, and the like.
-141-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
The compounds of the present invention can also be
administered in the form of liposome delivery systems, such
as small unilamellar vesicles, large unilamallar vesicles,
and multilamellar vesicles. Liposomes can be formed from a
variety of phospholipids, such as cholesterol,
stearylamine, or phosphatidylcholines.
Compounds of the present invention may also be coupled
with soluble polymers as targetable drug carriers. Such
polymers can include polyvinylpyrrolidone, pyran copolymer,
polyhydroxypropylmethacrylamide-phenol,
polyhydroxyethylaspartamidephenol, or polyethyleneoxide-
polylysine substituted with palmitoyl residues.
Furthermore, the compounds of the present invention may be
coupled to a class of biodegradable polymers useful in
achieving controlled release of a drug, for example,
polylactic acid, polyglycolic acid, copolymers of
polylactic and polyglycolic acid, polyepsilon caprolactone,
polyhydroxy butyric acid, polyorthoesters, polyacetals,
polydihydropyrans, polycyanoacylates, and crosslinked or
amphipathic block copolymers of hydrogels.
Gelatin capsules may contain the active ingredient and
powdered carriers, such as lactose, starch, cellulose
derivatives, magnesium stearate, stearic acid, and the
like. Similar diluents can be used to make compressed
tablets. Both tablets and capsules can be manufactured as
sustained release products to provide for continuous
release of medication over a period of hours. Compressed
tablets can be sugar coated or film coated to mask any
unpleasant taste and protect the tablet from the
atmosphere, or enteric coated for selective disintegration
in the gastrointestinal tract.
Liquid dosage forms for oral administration can contain
coloring and flavoring to increase patient acceptance.
In general, water, a suitable oil, saline, aqueous dextrose
(glucose), and related sugar solutions and glycols such as
propylene glycol or polyethylene glycols are suitable
carriers for parenteral solutions. Solutions for
-142-
CA 02377221 2002-O1-22
WO 01/19797 PCT/US00/24967
parenteral administration preferably contain a water
soluble salt of the active ingredient, suitable stabilizing
agents, and if necessary, buffer substances. Antioxidizing
agents such as sodium bisulfite, sodium sulfite, or
ascorbic acid, either alone or combined, are suitable
stabilizing agents. Also used are citric acid and its
salts and sodium EDTA. In addition, parenteral solutions
can contain preservatives, such as benzalkonium chloride,
methyl- or propyl-paraben, and chlorobutanol.
Suitable pharmaceutical carriers are described in
Remington's Pharmaceutical Sciences, Mack Publishing
Company, a standard reference text in this field.
-143-