Note: Descriptions are shown in the official language in which they were submitted.
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
VASCULAR DEVICE FOR EMBOLI, THROMBUS AND
FOREIGN BODY REMOVAL AND METHODS OF USE
Field Of The Invention
The present invention relates to apparatus
and methods for filtering or removing matter from
within a vascular system. More particularly, the
present invention provides a low profile self-expanding
vascular device useful for capturing emboli or foreign
bodies generated during interventional procedures, and
for thrombectomy and embolectomy.
Background Of The Invention
Percutaneous interventional procedures to
treat occlusive vascular disease, such as angioplasty,
atherectomy and stenting, often dislodge material from
the vessel walls. This dislodged material, known as
emboli, enters the bloodstream, and may be large enough
to occlude smaller downstream vessels, potentially
blocking blood flow to tissue. The resulting ischemia
poses a serious threat to the health or life of a
patient if the blockage occurs in critical tissue, such
as the heart, lungs, or brain.
The deployment of stents and stent-grafts to
treat vascular disease, such as aneurysms, also
involves the introduction of foreign objects into the
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
2 -
bloodstream, and also may result in the formation of
clots or release of emboli. Such particulate matter,
if released into the bloodstream, also may cause
infarction or stroke.
Furthermore, interventional procedures may
generate foreign bodies that are left within a
patient's bloodstream, thereby endangering the life of
the patient. Foreign bodies may include, for example,
a broken guide wire, pieces of a stent, or pieces of a
catheter.
Numerous previously known methods and
apparatus have been proposed to reduce complications
associated with embolism, release of thrombus, or
foreign body material generation. U.S. Patent No.
5,833,644 to Zadno-Azizi et al., for example, describes
the use of a balloon-tipped catheter to temporarily
occlude flow through a vessel from which a stenosis is
to be removed. Stenotic material removed during a
treatment procedure is evacuated from the vessel before
the flow of blood is restored. A drawback of such
previously known systems, however, is that occlusion of
antegrade flow through the vessel may result in damage
to the tissue normally fed by the blocked vessel.
U.S. Patent No. 5,814,064 to Daniel et al.
describes an emboli filter system having a radially
expandable mesh filter disposed on the distal end of a
guide wire. The filter is deployed distal to a region
of stenosis, and any interventional devices, such as
angioplasty balloons or stent delivery systems, are
advanced along the guide wire. The filter is designed
to capture emboli generated during treatment of the
stenosis while permitting blood to flow through the
filter. Similar filter systems are described in U.S.
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 3 -
Patent No. 4,723,549 to Wholey et al. and U.S. Patent
No. 5,827,324 to Cassell et al.
One disadvantage of radially expandable
filter systems such as described in the foregoing
patents is the relative complexity of the devices,
which typically comprise numerous parts. Connecting
more than a minimal number of such parts to a guide
wire generally increases delivery complications. The
ability of the guide wire to negotiate tortuous anatomy
is reduced, and the profile of the device in its
delivery configuration increases. Consequently, it may
be difficult or impossible to use such devices in small
diameter vessels, such as are commonly found in the
carotid artery and cerebral vasculature. Moreover,
such filter devices are generally incapable of
preventing material from escaping from the filter
during the process of collapsing the filter for
removal.
International Publication No. WO 98/39053
describes a filter system comprising an elongated
member, a radially expandable hoop and a cone-shaped
basket. The hoop is affixed to the elongated member,
and the cone-shaped basket is attached to the hoop and
the elongated member, so that the hoop forms the mouth
of the basket. The filter system includes a specially
configured delivery catheter that retains the mouth of
the basket in a radially retracted position during
delivery.
While the filter system described in the
foregoing International Publication reduces the number
of components used to deploy the cone-shaped basket, as
compared to the radial strut-type filter elements
described hereinabove, it too has drawbacks. Chief
among these, it is expected that it will be difficult
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
4 -
to reduce the diameter of the radially expandable hoop
to its retracted position. In particular, as the hoop
is contracted through smaller radii of curvature, the
stiffness of the hoop is expected to increase
dramatically. This increased stiffness prevents the
hoop from being contracted more tightly, and is
expected to result in a delivery profile too large to
permit use of the device in critical regions of the
body, such as the smaller coronary arteries, carotid
arteries, and cerebral vasculature.
In view of the foregoing disadvantages of
previously known apparatus and methods, it would be
desirable to provide a vascular device, e.g., for use
as a vascular filter, that overcomes such disadvantages
and employs few components.
It would be desirable to provide a reliable
and multi-functional delivery system for use with the
vascular device.
It would be desirable to provide an
integrated vascular device with a thrombectomy element
and a vascular filter.
It also would be desirable to provide a
vascular device that is capable of being contracted to
a small delivery profile, thus permitting use of the
device in small vessels.
It further would be desirable to provide a
vascular device that is capable of being contracted to
a sufficiently small profile that it may be retrieved
using the guide wire lumen of previously known
treatment devices, and without the need for specialized
delivery catheters.
It still further would be desirable to
provide a vascular device that reduces the risk of
emboli or thrombus removed from the vessel wall
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 5 -
escaping from the device when the device is collapsed
and removed.
It also would be desirable to provide a
vascular device that permits a rapid exchange
deployment modality.
Summary Of The Invention
In view of the foregoing, it is an object of
the present invention to provide a vascular device that
overcomes disadvantages of previously known vascular
filters, thrombectomy/embolectomy and foreign body
removal devices, and employs few components.
It is an object of the present invention to
provide a reliable and multi-functional delivery system
for use with the vascular device.
It is an object to provide an integrated
vascular device with a thrombectomy element and a
vascular filter.
It also is an object of this invention to
provide a vascular device that is capable of being
contracted to a small delivery profile, thus permitting
use of the device in small vessels.
It is a further object to provide a vascular
device that is capable of being contracted to a
sufficiently small profile that it may be retrieved
using the guide wire lumen of previously known
treatment devices, and without the need for specialized
delivery catheters.
It is another object to provide a vascular
device that reduces the risk of emboli or thrombus
removed from the vessel wall escaping from the device
when the device is collapsed and removed.
CA 02378715 2002-01-09
WO 01/08743 PCT/USOO/20754
- 6 -
It also is an object to provide a vascular
device that permits a rapid exchange deployment
modality.
These and other objects of the present
invention are accomplished by providing a vascular
device, suitable for use as a vascular filter or
thrombectomy/embolectomy device that comprises a blood
permeable sac affixed at its perimeter to a support
hoop having an articulation region. The support hoop
is attached to a distal region of an elongated member,
such as a guide wire, and supports a proximally-
oriented mouth of the sac when the device is deployed
in a vessel. The device may also comprise a nose cone
to facilitate percutaneous introduction, and a delivery
sheath having one or more lumens. The lumens may
further be configured for a rapid exchange mode of
introduction along the guide wire.
In a first embodiment, the support hoop
includes one or more reduced-thickness articulation
regions that enable the support hoop to be contracted
to very small radii of curvature without the problems
of increased stiffness and kinking of previously known
devices. In an alternative embodiment, the
articulation region may comprise a gap in the support
hoop bridged by the perimeter of the blood permeable
sac.
The support hoop preferably also has a curved
profile that prevents the articulation region, when
folded, from damaging the wall of the vessel. The
curved profile permits the device to effectively
contact the walls of the vessel and reduce emboli or
thrombus removed from the vessel wall from bypassing
the sac. Moreover, the articulation region, when
combined with a support hoop having a curved profile,
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 7 -
causes the sides of the support hoop to fold inwards
towards one-another when the vascular device is
collapsed into a sheath for removal. This, in turn,
closes the mouth of the sac and reduces the potential
for emboli or thrombus to be released from the vascular
device during removal.
Advantageously, use of an articulation region
permits vascular devices of the present invention to be
contracted to very small diameters, thereby enabling
the use of delivery catheters having diameters as small
as 3 Fr. Moreover, the vascular devices may be
retracted within the guide wire lumens of conventional
treatment devices, such as angioplasty catheters and
stent delivery systems, thereby obviating the need to
re-insert a.specialized delivery catheter to remove the
vascular device. However, a retrieval sheath having a
distal region that flares or expands outwardly to
receive the emboli-filled sac upon completion of an
interventional procedure, and which reduces risk of
rupture to the sac, optionally may be provided in
accordance with the present invention.
In embodiments suitable for use as embolic
filters, the vascular device may include a separate
guide wire for introducing treatment devices proximal
of the deployed vascular device. Additionally, the
vascular device may have a second support hoop attached
to the distal end of the sac. During retrieval,
multiple hoops ensure that emboli are retained within
the sac and prevent the sac from bunching. Where
multiple hoops are rotated, they may be arranged such
that they rotate independently of the guide wire,
thereby reducing risk that the sac wall will become
twisted during advancement.
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
8 -
In alternative embodiments, sac bunching is
mitigated by tapering the sac and attaching it to one
or more support hoops, or to the guide wire. Sac
porosity may also be specified to ensure passage of
blood cells and capture of emboli, as well as to
control a pressure drop across the vascular device. In
other embodiments, a delivery sheath is provided that
permits a lesion to first be crossed with an
unencumbered guide wire prior to passing the vascular
device across the lesion. In still further
embodiments, several support hoops may be provided at
the mouth of a single sac to facilitate opening and
closing of the sac.
In thrombectomy applications, a separate
thrombectomy element may be provided in addition to the
vascular filter. The thrombectomy element may be
attached to the elongated member proximal of the
vascular filter or may comprise a separate catheter.
In a preferred embodiment, the thrombectomy element is
similar in construction to the vascular filter and may
be retracted independently. Alternatively, the
thrombectomy element may be any conventional
atherectomy device used in conjunction with the
vascular filter and may be advanced and retracted
either in conjunction or independently of the vascular
filter.
A delivery system in accordance with the
present invention, configured for use with the vascular
devices described herein, is also provided. The
delivery system integrates the functions of a Touhy
Borst, a torquer, and a pusher into a single device,
thereby facilitating introduction and retrieval of
embodiments of the present invention. The torqueing
function allows a vascular device to navigate tortuous
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
9 -
anatomy. For example, the distal end of a guide wire
may be rotated to selectively orient the vascular
device in a selected branch of a bifurcated vessel.
The Touhy-Borst adapter permits liquid to be introduced
or withdrawn through the lumen of the vascular device
delivery catheter. The pusher feature of the delivery
system allows deployment and retraction of the vascular
device from within the delivery catheter.
Methods of using embodiments of the present
invention are also provided, including use of novel
radiopaque features, and use of a previously known
balloon catheter to arrest antegrade flow through a
vessel until the vascular device of the present
invention is deployed.
Brief Description Of The Drawings
The above and other objects and advantages of
the present invention will be apparent upon
consideration of the following detailed description,
taken in conjunction with the accompanying drawings, in
which like reference characters refer to like parts
throughout, and in which:
FIGS. 1A and 1B are, respectively, a side-
sectional view of a previously known vascular device
contracted within a delivery sheath, and an end view of
that vascular device deployed in a vessel;
FIGS. 2A and 2B are, respectively, a
perspective view of a vascular device constructed in
accordance with the principles of the present invention
in a deployed state, and a detailed view of the
articulation region of the device of FIG. 2A;
FIG. 3 is a perspective view of the vascular
device of FIGS. 2 in a folded configuration, prior to
removal;
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 10 -
FIG. 4 is a plan view of the vascular device
of FIGS. 2;
FIGS. 5A-5D are side sectional views
depicting a method of deploying, using, and retrieving
the vascular device of FIGS. 2-4;
FIG. 6 is a perspective view of an
alternative embodiment of a vascular device of the
present invention in a deployed state;
FIGS. 7A and 7B are, respectively, a
perspective view and a plan view of a further
alternative embodiment of the present invention in a
deployed state;
FIGS. 8A-8E are sectional views of a vascular
device disposed within alternative embodiments of
delivery sheaths of the present invention;
FIG. 9 is a side view of a previously known
balloon catheter;
FIGS. 1OA-10D are views illustrating the
steps of using the balloon catheter of FIG. 9 with the
vascular device of FIGS. 2;
FIGS. 11A-11C are perspective views of
further alternative embodiments of vascular devices
constructed in accordance with the principles of the
present invention;
FIG. 12 is a perspective view of an
alternative embodiment of the vascular device of the
present invention with two support hoops, shown in a
deployed state;
FIG. 13 is a perspective view of an
alternative embodiment of the vascular device of FIG.
12 with a smaller distal support hoop;
FIG. 14 is a perspective view of a still
further alternative embodiment of the vascular device
CA 02378715 2002-01-09
WO 01/08743 PCT/USOO/20754
- 11 -
of FIG. 12 that allows the vascular device to
independently rotate with respect to the guide wire;
FIG. 15 is a perspective view of an
alternative embodiment of the present invention with a
tapered blood permeable sac, shown in a deployed state;
FIG. 16 is a perspective view of a radiopaque
support hoop constructed in accordance with one aspect
of the present invention;
FIGS. 17A-17C illustrate another alternative
embodiment of the vascular device of the present
invention in which the articulation region comprises a
gap in the support hoop bridged by the perimeter of the
blood permeable sac;
FIGS. 18A and 18B are side-sectional views
depicting an integrated vascular device of the present
invention suitable for thrombectomy, disposed,
respectively, within a delivery sheath and in a
deployed state;
FIGS. 19A-19E are side-sectional views
depicting a method of deploying, using, and retrieving
the integrated vascular device of FIGS. 18;
FIGS. 20A and 20B are side-sectional views
depicting an alternative embodiment of the integrated
vascular device of FIGS. 18, disposed, respectively,
within a delivery sheath and in a deployed state;
FIGS. 21A and 21B are side sectional views of
a delivery system constructed in accordance with the
present invention coupled to the vascular device of
FIG. 5A, shown, respectively, in a delivery
configuration and in a deployed configuration;
FIGS. 22A-22E are side sectional views
depicting a method of deploying, using, and retrieving
a vascular device of the present invention in
CA 02378715 2002-01-09
WO 01/08743 PCT/USOO/20754
- 12 -
conjunction with a specially configured retrieval
sheath; and
FIGS. 23A and 23B are side sectional views
depicting a method of using and retrieving the vascular
device in conjunction with an alternative embodiment of
the specially configured retrieval sheath.
Detailed Description Of The Invention
Referring to FIGS. 1A and 1B, some of the
disadvantages associated with previously known vascular
devices, such as the emboli filters described in the
above-mentioned International Publication WO 98/39053,
are described. In FIGS. 1, the vascular filter
comprises guide wire 10 having hoop 12 coupled to its
end. Filter sac 14 is affixed to hoop 12, so that when
delivery catheter 16 is retracted proximally and guide
wire 10 is held stationary, hoop 12 radially expands to
contact the walls of vessel V.
As described hereinabove, one difficulty with
such vascular filters is that the hoop used to support
the filter sac experiences increased stiffness when
contracted to small diameters, i.e., due to the sharp
directional change at the tip of the hoop, thereby
limiting the minimum delivery profile achievable for
such instruments. Although this effect may be reduced
by decreasing the thickness of the wire employed in
hoop 12, at the point at which the wire becomes
sufficiently thin to accommodate the bending stresses,
the wire is too thin to effectively radially expand and
urge the filter sac into engagement with the vessel
wall.
On the other hand, as shown in FIGS. 1A and
1B, the bending stresses imposed upon the hoop of such
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 13 -
previously known devices, if drawn within a delivery
catheter, may be sufficiently high to result in the
formation of kink 18 at the tip of the hoop. This
"kinking" effect becomes more severe in sheaths having
a small inner diameter. Thus, for example, applicant
has observed that when sheaths having inner diameters
of 0.035" or smaller are used, a hoop of nitinol or
multi-strand nitinol cable having a diameter of 0.0055"
will form kink 18. Kink 18 in turn may apply
relatively high localized pressure and friction against
wall 17 of sheath 16, thereby making the vascular
filter difficult to deploy. In particular, the kink
may impale wall 17 of delivery sheath 16 and may make
it difficult or impossible to deploy the vascular
filter, especially in tortuous anatomy.
In addition, when the filter is subsequently
deployed in vessel V, as shown in FIG. 1B, kink 18 may
deform the pre-formed shape of hoop 12, impairing the
ability of the filter to seal against the walls of
vessel V. This may in turn lead to the presence of
gaps G between the perimeter of the hoop and the vessel
wall, depending upon the severity of the kink.
Consequently, emboli may pass through the gaps with
antegrade flow and significantly reduce the efficacy of
the filter. Additionally, kink 18 may be sufficiently
sharp to damage or dissect the wall of vessel V when
the filter is deployed.
The vascular device of the present invention
solves the above-described disadvantages, providing a
vascular device, suitable for use as a vascular filter
or thrombectomy/embolectomy device, with a self-
expanding support hoop that is sufficiently thick to
radially expand and urge a blood permeable sac into
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 14 -
engagement with the vessel wall, but which includes an
articulation region that overcomes the problems
associated with kinking. In particular, the vascular
device of the present invention includes a reduced
thickness articulation region and a pre-formed curved
profile that avoids the difficulties of previously
known systems while providing a high degree of efficacy
in capturing emboli or thrombus, and ease of deployment
and retrieval.
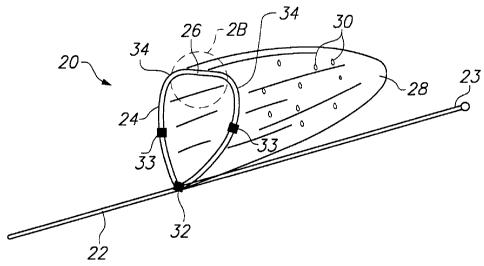
Referring now to FIGS. 2A and 2B, vascular
device 20 constructed in accordance with the principles
of the present invention, illustratively an embolic
filter, comprises guide wire 22, support hoop 24 having
articulation region 26, and blood permeable sac 28
affixed to support hoop 24. Sac 28 is coupled to
support hoop 24 so that the support hoop 24 forms an
opening for the sac. Support hoop 24 preferably is
connected to guide wire 22 near distal end 23 of the
guide wire.
Sac 28 preferably is constructed of a thin,
flexible biocompatible material, such as polyethylene,
polypropylene, polyurethane, polyester, polyethylene
tetraphlalate, nylon or polytetrafluoroethylene, or
combinations thereof. The material should be
sufficiently thin, such that the sac is non-
thrombogenic. Sac 28 includes openings or pores 30
that permit blood cells to pass through the sac
substantially unhindered, while capturing any larger
emboli, thrombus, or foreign bodies that may be
released during a procedure, such as angioplasty or
stent placement. In a preferred embodiment, sac 28 has
openings or pores 30 in a range of about 20 to 400
microns in diameter, and more preferably, about
approximately 80 microns. These pore sizes permit red
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 15 -
blood cells (which have a diameter of approximately 5
microns) to easily pass through the sac, while
capturing thrombus or emboli.
Pores 30 are preferably formed by a laser
drilling process. For example, a thin sheet of the
flexible biocompatible material may be thermoformed to
create sac 28, for example, by stretching the sheet
over a mandrel, by dip forming, or by blow molding.
Sac 28 may alternatively be fabricated from an extruded
tube of the biocompatible material. A flat metal mask,
with tiny holes approximately the size of pores 30, may
then be placed in front of the sac. A laser having a
beam diameter equal to or greater than the diameter of
the material illuminates the mask. The laser beam
passes through the holes in the mask and strikes the
material, thereby forming pores 30 in sac 28.
Laser drilling may also be accomplished with
a laser having a beam diameter approximately the size
of pores 30, in which case pores 30 may drilled
individually. Sac 28 may alternatively comprise a
woven material, for example, formed from the above-
mentioned polymers, having a pore diameter determined
as a function of the pattern and tightness of the
weave.
Support hoop 24 comprises a hoop having a
circular or rectangular cross-section that is formed of
a super-elastic material, such as a nickel-titanium
alloy ("nitinol"). During deployment and retrieval of
vascular device 20, described hereinafter, support hoop
24 folds in half and collapses to fit within a small
diameter delivery sheath. When vascular device 20 is
in a deployed state, as depicted in FIG. 2A, support
hoop 24 resumes its pre-formed shape. Support hoop 24
preferably comprises nitinol wire, although it may also
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 16 -
be formed from a multi-strand nitinol cable, a spring
tempered stainless steel, or other super-elastic
material.
In accordance with the principles of the
present invention, support hoop 24 includes one or more
reduced-thickness articulation regions 26, and pre-
formed curved regions 34. As depicted in FIG. 2B,
articulation region 26 includes a region having reduced
thickness t1 compared to thickness t of the remainder
of support hoop 24. Articulation region 26 and curved
regions 34 enable support hoop 24 to fold with a pre-
determined shape when vascular device 20 is collapsed
to a contracted state for delivery or retrieval.
In FIG. 2B, articulation region 26 is
depicted as a localized reduction in the thickness of
support hoop 24, as may be achieved, for example, using
conventional grinding, chemical etching, or electroless
polishing processes. Alternatively, support hoop 24
may be continuously tapered along its circumference, so
that articulation region 26 results from a more gradual
reduction in the wall thickness of the support hoop.
Tapering support hoop 24 may permit greater flexibility
in the vicinity of articulation region 26, thus
enabling support hoop 24 to fold more easily at the
articulation region. Such tapering of the thickness of
the support hoop along a portion of its circumference
also may reduce the potential for stress-induced
fracture typically associated with abrupt changes in
diameter.
In a preferred embodiment of vascular device
20 of the present invention, vascular device 20 easily
fits within a delivery sheath having an inner diameter
of 0.03311, and, more preferably, may be used with a
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 17 -
delivery sheath having an inner diameter as small as
0.026". The deployed diameter of support hoop 24
preferably is approximately 7 mm, while guide wire 22
preferably has a diameter of 0.014". The distal end of
guide wire 22 also may be tipped with a spring section
or coil tip, as is per se known.
Support hoop 24 preferably is constructed of
0.0055" nitinol wire tapered (by a grinding, chemical
etching, or electroless polishing process) to 0.0025"
at articulation region 26. Specifically, articulation
region 26 preferably consists of a length about 0.05"
long and having a diameter of 0.0025", coupled on
either side to curved regions 34. Each of curved
regions 34 includes a length of wire that is tapered
from a diameter of 0.055" to a diameter of 0.0025" over
a length of about 0.025". Support hoop 24 also may
include radiopaque features, such as gold or platinum
bands 33, spaced at intervals around the circumference
of support hoop 24, or a coil of radiopaque material
wrapped around the support hoop, as described
hereinafter with respect to FIG. 16, or a gold plated
coating.
Referring to FIGS. 3 and 4, additional
features of vascular device 20 are described. FIG. 3
depicts vascular device 20 of FIG. 2A in a contracted
state, while FIG. 4 illustrates a directional change in
support hoop 24 preferably caused by the presence of
curved regions 34. Advantageously, use of articulation
region 26 and the curved profile of support hoop 24
introduced by curved regions 34 also cause support hoop
24 to fold in half during retrieval. As shown in FIG.
3, support hoop 24 folds in half, effectively closing
the mouth of blood permeable sac 28 and preventing the
escape of collected emboli or thrombus. This feature
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 18 -
also may permit the use of a smaller or shallower sac
than would otherwise be possible, without increasing
the risk of material escaping from the device when the
sac is collapsed for retrieval. Use of a smaller or
shallower sac also enables vascular device 20 to be
delivered in a smaller delivery sheath, having an inner
diameter as small as 0.026" for the preferred
embodiment.
Referring now to FIGS. 5A-5D, methods of
using the vascular device of the present invention as a
vascular filter are described. In FIG. 5A, guide wire
22 and delivery sheath 40 are manipulated into position
within vessel V using well-known percutaneous
techniques. Vascular device 20 of FIG. 2A is disposed
in its contracted delivery state within distal end 42
of delivery sheath 40, and delivery sheath 40 is
advanced through the vessel using distal end 23 of
guide wire 22. Articulation region 26 and curved
regions 34 of support hoop 24 enable the sides of the
support hoop to fold together and become elongated when
drawn within delivery sheath 40. The size of delivery
sheath 40 and guide wire 22 have been exaggerated to
illustrate structure. In reality, the diameter of
delivery sheath 40 is approximately an order of
magnitude smaller than the internal diameter of vessel
V.
With respect to FIG. 5B, once delivery sheath
40 is disposed at a desired location within a patient's
vessel V, such as a coronary artery or carotid artery,
as determined, for example, by the position of
radiopaque band 43 under a fluoroscope, guide wire 22
is held stationary while delivery sheath 40 is
retracted proximally. Alternatively, delivery sheath
CA 02378715 2002-01-09
WO 01/08743 PCT/USOO/20754
- 19 -
40 may be held stationary while guide wire 22 is
advanced. In either case, when vascular device 20 is
no longer confined within delivery sheath 40, support
hoop 24 expands to seal against the walls of vessel V.
When in its deployed state, curved regions 34 of
support hoop 24 orient articulation region 26
concentrically against the inside wall of the vessel,
thus reducing the risk of impaling the vessel wall, as
might be expected of the kinked support hoop of FIG.
1B. Blood continues to flow unimpeded through vessel V
in direction D.
In FIG. 5C, once vascular device 20 is
deployed in vessel V, other interventional instruments,
such as angioplasty catheters, atherectomy devices, or
stent delivery systems may be advanced along guide wire
22 to position such devices at treatment zones located
proximally of vascular device 20. For example, in FIG.
5C, angioplasty balloon catheter 44 has been advanced
along guide wire 22 to a position proximal of vascular
device 20 to trap emboli E, i.e., pieces of plaque
dislodged from the walls of vessel V by balloon 46.
With respect to FIG. 5D, upon completion of
the angioplasty procedure using angioplasty balloon
catheter 44, guide wire 22 is pulled proximally to
cause the sides of support hoop 24 to collapse together
to close the mouth of sac 28 (see FIG. 3). Additional
proximal retraction of guide wire 22 causes support
hoop 24 and sac 28 to enter at least partially within
the guide wire lumen of angioplasty catheter 44. As
depicted in FIG. 5D, only a portion of support hoop 24,
near articulation region 26, and a distal portion of
sac 28 extend out of the guide wire lumen of
angioplasty catheter 44. Alternatively, vascular
CA 02378715 2002-01-09
WO 01/08743 PCT/USOO/20754
- 20 -
device 20 may be fully retracted within the guide wire
lumen. Angioplasty catheter 44 then is withdrawn with
vascular device 20 and any trapped emboli E.
Advantageously, the compliant design of
vascular device 20 permits the device to be contracted
to its delivery state within the guide wire lumen of
conventional previously known interventional devices.
Accordingly, unlike previously known vascular devices,
which require removal of the interventional device
followed by re-insertion of a specially designed
catheter to retrieve the vascular device, the system of
the present invention reduces the time, effort and
trauma of this additional step. Instead, the vascular
device may be readily closed and retrieved upon
completion of the interventional procedure.
Vascular device 20 alternatively may be used
in performing thrombectomy/embolectomy. In this case,
the vascular device is deployed in a vessel at a
location distal to a lesion, in the manner depicted in
FIGS. 5A and 5B. Once support hoop 24 is deployed into
contact with the vessel wall, vascular device 20 may be
retracted proximally to scrape along the wall of the
vessel, and excise thrombus so that it is captured in
sac 28. Delivery sheath 44 may then be re-inserted
into the vessel along guide wire 22, and vascular
device 20 is retracted and removed from the vessel.
Additional thrombectomy embodiments are described
hereinbelow with respect to FIGS. 18-20.
As discussed hereinabove, sac 28 is porous so
that blood cells may pass through while emboli E are
captured. As seen in FIG. 5B, if the sum of the area
of all these pores Al is less than the internal cross-
sectional area A2 of vessel V, a pressure drop is
CA 02378715 2002-01-09
WO 01/08743 PCT/USOO/20754
- 21 -
expected across the vascular device. This may lead to
hemolysis and insufficient downstream flow. If Al is
greater than or equal to A2, the pressure drop is
expected to decrease. Proper selection of pore
diameter (in the range of 20-400 microns) and pore
density ensures that Al is greater than or equal to A2.
Selection of a larger pore diameter within
the provided range may also reduce the pressure drop by
decreasing drag as blood passes through sac 28. Drag
may further be decreased by providing elliptical pores
through the sac that project round relative to
bloodflow when sac 28 is deployed. Furthermore, the
porosity of sac 28 may be specified such that, if
distal pores become occluded with thrombus, emboli,
etc., proximal pores remain open to ensure continuous
blood flow. It should also be noted that flow through
vessel V is substantially unaffected by placement of
sac 28 and hoop 24 in the flow path.
Referring now to FIG. 6, an alternative
embodiment of the vascular device of the present
invention, again illustratively a vascular filter, is
described. Vascular device 50 comprises guide wire 51
and support hoops 52 and 53 connected to blood
permeable sac 54. As discussed hereinabove, vascular
device 50 includes articulation regions 55 and 56
formed at the intersection of opposing curved regions
57 and 58 of support hoops 52 and 53. Sac 54
preferably also is connected to guide wire 51 along its
entire length, thereby providing more controlled
deployment and removal of vascular device 50. Support
hoop 53 serves to stabilize and deploy the distal
portion of sac 54. In addition, affixing sac 54 to
guide wire 51 may provide a more compact arrangement
CA 02378715 2007-06-11
22 -
within a delivery sheath, and prevent bunching of the
sac material.
In FIGS. 7A and 7B, a further alternative
embodiment of the vascular device of the present
invention is described. Vascular device 60, shown in
the deployed state, comprises guide wire'61 having
multi-turn helical support hoop 63 connected at weld
point 62. Blood permeable sac 64 is affixed to the
distal-most portion of support hoop 63. Support hoop
63 includes one or more side turns 65 that terminate in
curved regions 66, as described hereinabove. Curved
regions 66 in turn are joined together by articulation
region 67. Preferably, side turns 65 are coupled to
one another and to the distal region of guide wire 61,
e.g., by a weld bead, at point 68.
In accordance with this aspect of the present
invention, vascular device 60 may be contracted to
small profile delivery state. When deployed.from a
delivery catheter, such as delivery sheath 40 of FIG.
5A, side turns 65 expand into contact with the walls of
the vessel proximal to the location at which curved
regions 66 contact the vessel wall. Side turns 65
serve to stabilize the support hoop 63 and sac 64 when
vascular device 60 is deployed within a blood vessel.
In addition, side turns 65 are expected to assist in
orienting the axis of support hoop 63 and sac 64 in
alignment with the longitudinal axis of vessel V.
Accordingly, support hoop 63 is expected to reduce the
risk of tilting of the vascular device within the
vessel, and thus enhance the safety and reliability of
the device.
Referring now to FIGS. 8A-8E, several
embodiments of a delivery sheath suitable for use with
CA 02378715 2002-01-09
WO 01/08743 PCT/USOO/20754
- 23 -
the vascular device of the present invention are
described. Each of these embodiments are designed to
permit the physician to first pass a guide wire across
a lesion before passing the vascular device of the
present invention across the lesion. Thus, the risk of
generating emboli, during the step of positioning the
vascular device of the present invention distal to a
lesion, is expected to be reduced.
In particular, in FIG. 8A, vascular device 70
of the present invention comprises guide wire 71,
support hoop 72 and blood permeable sac 73 folded in a
contracted delivery state within lumen 74 of delivery
sheath 75. Vascular device 70 is similar in design to
vascular device 20 of FIG. 2A, except that device 70
includes nose cone 76 affixed to distal region 77 of
guide wire 71. Delivery sheath 75 includes hemostatic
fitting 78 at its proximal end and guide wire lumen 79.
In accordance with the methods of the present
invention, vascular device 70 and guide wire 80 are
used as follows. First, unencumbered guide wire 80 is
advanced through a vessel until distal region 81 of the
guide wire crosses a lesion. The proximal end of guide
wire 80 then is inserted into the distal end of guide
wire lumen 79 of delivery sheath 75 using previously
known "over the wire" techniques.
Delivery sheath 75 then is advanced over
guide wire 80, which is held stationary, until nose
cone 76 and a distal portion of the delivery sheath
cross the lesion. Once support hoop 72 and sac 73 of
vascular device 70 are positioned distal to the lesion,
guide wire 80 is withdrawn from the vessel and delivery
sheath 75 is retracted proximally, thereby deploying
vascular device 70 to its deployed state. As will of
course be understood, nose cone 76 remains in the
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 24 -
vessel, distal to sac 73, during deployment of the
vascular device. Upon completion of use of vascular
device 70, delivery sheath 75 may once again be
advanced along guide wire 71 and the support hoop and
sac retracted within lumen 74 of delivery sheath 75.
Alternatively, an interventional device may be advanced
over guide wire 71 to perform a medical procedure, and
the vascular device may be retrieved within a guide
wire lumen of the interventional device, as discussed
hereinabove with respect to FIGS. 5.
Vascular device 90 of FIG. 8B is similar in
construction to that of FIG. 8A, and includes guide
wire 91, support hoop 92, blood permeable sac 93 and
nose cone 94. Delivery sheath 95 includes lumen 96
housing device 90, guide wire lumen 97, and hemostatic
fitting 98. Guide wire lumen 97 opens through skive 99
in lateral wall 100 of delivery sheath 95. Guide wire
101 therefore may be used in accordance with well-known
"rapid exchange" techniques, wherein the length of
unencumbered guide wire 101 may be significantly
shorter than in the case of the "over the wire"
arrangement depicted in FIG. 8B. Operation of delivery
sheath 95 and vascular device 90 is similar to that
described hereinabove with respect to FIG. 8A, except
that the proximal end of unencumbered guide wire 101 is
passed through the distal end of lumen 97 and passes
out through skive 99.
In FIG. 8C, delivery sheath 105 includes
lumen 106 that opens through the lateral wall via skive
107, and guide wire lumen 108 that opens through the
lateral wall via skive 109. Accordingly, as will be
apparent to one of ordinary skill, both vascular device
110 and guide wire 112 may be used as described
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 25 -
hereinabove with respect to FIG. 8A and further in
accordance with "rapid exchange" techniques.
Vascular device 113 of FIG. 8D is similar in
construction to those described hereinabove. Delivery
sheath 114 includes lumen 115, guide tube 116, and
hemostatic fitting 117. Lumen 115 houses device 113
during delivery and retrieval. Guide tube 116
comprises guide wire lumen 118, which is configured to
receive unencumbered guide wire 119. In operation, the
proximal end of guide wire 119 is passed through guide
wire lumen 118 of guide tube 116. Thus, guide wire 119
may be used in accordance with "rapid exchange"
techniques described with respect to FIG. 8B and with
"over the wire" techniques described with respect to
FIG. 8A.
Vascular device 120 of FIG. 8E is also
similar to those described hereinabove. Delivery
sheath 121 includes lumen 122 and hemostatic fitting
123. Lumen 122 houses device 120. Guide wire 124 is
coupled to and terminates at the proximal end of
delivery sheath 121. Thus, distal end 126 of guide
wire 125 of vascular device 120 is first to cross the
lesion. Then, nose cone 127, attached to guide wire
125, and a distal portion of delivery sheath 121 cross
the lesion. Guide wire 124 and attached delivery
sheath 121 are retracted proximally, thereby deploying
vascular device 120 to its deployed state. Device 120
may then be retrieved within sheath 121 or within an
interventional device, as discussed hereinabove.
Referring now to FIG. 9, a previously known
balloon catheter is described. Catheter 130 is
constructed of materials typically used in catheters,
such as polyethylene or polyurethane, and includes
compliant balloon 131 disposed in distal region 132.
CA 02378715 2007-06-11
2.6 -
Compliant balloon, which may be formed of nylon or
latex, is inflated using inflation port 133'at proximal
end 134 of the catheter. Catheter 130 also includes
hemostatic port 136 and an interior lumen through which
a delivery sheath may be advanced to pass out of an
opening in distal end 137.
With respect to FIGS. 10A-10C, a method of
using catheter 130 of FIG. 9 in conjunction with the
vascular device of the present invention is described.
In accordance with this aspect of the present
invention, antegrade blood flow through a vessel is
occluded while a vascular device constructed in
accordance with the present invention is advanced
across a lesion. Once the vascular device,
illustratively a vascular filter, is deployed, the
balloon is deflated, thereby permitting antegrade flow
to be established. Importantly, because flow through
the vessel is stopped prior to deployment of the
vascular-device, few or no emboli are expected to
bypass the filter.
More particularly, with respect to FIG. 10A,
catheter 130 is disposed in vessel V at a location
proximal to lesion L, with the vascular device of the
present invention disposed in its contracted delivery
state in delivery sheath 138. In FIG. 10B, balloon 131
is inflated via inflation port 133 (see FIG. 9) to
engage the interior wall of vessel V, thereby
arresting antegrade flow in the vessel.
As shown in FIG. 10C, delivery sheath 130
then is advanced across lesion L so that the support
hoop and sac of the vascular device will be disposed
distal to lesion L when deployed. During this step,
delivery sheath 138 may generate emboli E as it passes
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 27 -
across the lesion. However, because antegrade flow in
the vessel is stopped, the emboli will not travel
distally in the vessel.
With respect to FIG. 10D, once vascular
device 140 is deployed, so that support hoop 141 and
sac 142 span vessel V, balloon 131 is deflated. This,
in turn, causes antegrade flow to become re-established
in vessel V, urging emboli E into sac 142. Catheter
130 then may be withdrawn, and additional treatment
devices advanced along guide wire 143 of vascular
device 140. Removal of vascular device 140 may be by
any of the methods described hereinabove with respect
to FIG. 5D.
Referring now to FIGS. 11A-11C, still further
alternative embodiments of vascular devices constructed
in accordance with the present invention are described.
Each of the devices of FIGS. 11A-11C, which are shown
in the deployed state, includes two or more support
hoops to support the blood permeable sac. Each of
those support hoops in turn includes an articulation
region that permits the sides of the support hoops to
collapse inwards to each other as described hereinabove
with respect to FIGS. 3 and 4.
Specifically, in FIG. 11A vascular device
150, illustratively an embolic filter, comprises guide
wire 151, support hoops 152 and 153 having articulation
regions 154 and 155, respectively, and blood permeable
sac 156 affixed to support hoops 152 and 153. Sac 156
is coupled to support hoops 152 and 153 so that the
support hoops form an opening for the sac. Support
hoops 152 and 153 preferably are connected to guide
wire 151 near its distal end.
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 28 -
Sac 156 is also attached to the distal end of
guide wire 151 at point 157. Sac 156 preferably is
constructed of a thin, flexible biocompatible material,
as for the embodiments described hereinabove, and
includes openings or pores 158 that permit blood cells
to pass through the sac substantially unhindered, while
capturing any larger material that may be released
during a procedure such as angioplasty or stent
placement. Pore sizes are selected as described
hereinabove with respect to FIG. 2A.
Support hoops 152 and 153 comprise hoops
having circular or rectangular cross-sections that are
formed of a super-elastic material, such as a
nickel-titanium alloy ("nitinol"). During deployment
and retrieval of vascular device 150, support hoops 152
and 153 fold in half and collapse to fit within a small
diameter delivery sheath. When the delivery sheath is
retracted, support hoops 152 and 153 resume their
pre-formed shape and deploy the perimeter of sac 156
into contact with the vessel walls. Support hoops 152
and 153 preferably comprise a nitinol wire, but also
may be formed from a multistrand nitinol cable, or
other super-elastic material.
In accordance with the principles of the
present invention, support hoops 152 and 153 are
affixed to guide wire 151 at ring 159 and include
reduced-thickness articulation regions 154 and 155,
constructed as described hereinabove. More
particularly, support hoops 152 and 153 are pre-formed
to form structures having curved regions 160 and 161,
respectively, so that articulation regions 154 and 155
are disposed in a portion of the support hoop that is
approximately concentric with a vessel wall when
vascular device 150 is deployed. Articulation regions
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 29 -
154 and 155 and curved regions 160 and 161 thus enable
support hoops 152 and 153 to fold with a pre-determined
shape when vascular device 150 is collapsed to a
contracted state for delivery or retrieval.
In a preferred embodiment of vascular device
150 of the present invention, vascular device 150
easily fits within a delivery sheath having an inner
diameter of 0.033", and more preferably, may be used
with a delivery sheath having an inner diameter as
small as 0.026". The deployed diameter of vascular
device 150 preferably is approximately 7 mm.
Compared to vascular device 20 of FIGS. 2-4,
vascular device 150 of FIG. 11A employs two support
hoops instead of one and provides central location of
guide wire 151 and attachment of blood permeable sac
156 to the distal end of the guide wire. These
differences may provide more controlled deployment and
removal of vascular device 150. In addition, affixing
sac 156 to guide wire 151 may provide a more compact
arrangement within a delivery sheath, and prevent
bunching of the sac material.
Referring now to FIG. 11B, another
alternative embodiment of the vascular device of the
present invention, again illustratively a vascular
filter, is described. Vascular device 170 is similar
in construction to vascular device 150, except that
vascular device 170 employs three support hoops instead
of two. Device 170 comprises guide wire 151 and
support hoops 171, 172 and 173 connected to blood
permeable sac 156.
As discussed hereinabove, vascular device 170
includes articulation regions 174, 175 and 176 formed
at the intersection of opposing curved regions 178, 179
and 180 of support hoops 171, 172 and 173. Support
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 30 -
hoops 171, 172 and 173 preferably are connected to the
distal end of guide wire 151 at ring 177. Sac 156
preferably also is connected to guide wire 151 at point
157. Vascular device 170 is expected to provide
similar advantages to those contemplated for vascular
device 150.
With reference to FIG. 11C, yet another
alternative embodiment of the vascular device of the
present invention, again illustratively a vascular
filter, is described. Vascular device 190 is similar
in construction to vascular devices 150 and 170, except
that vascular device 190 employs four articulated
support hoops. Device 190 comprises guide wire 151 and
support hoops 191, 192, 193 and 194 connected to blood
permeable sac 156, with articulation regions 195, 196,
197 and 198 formed at the intersection of opposing
curved regions 200, 201, 202 and 203 of the respective
support hoops 191-194. Support hoops 191-194 are
preferably connected to the distal end of guide wire
151 at ring 199.
Alternative embodiments of vascular devices
of the present invention have been described with one
to four support hoops. As will be apparent to one of
ordinary skill in the art of interventional device
design, any number of support hoops may be used with
minor modifications to the designs described
hereinabove.
Referring now to FIGS. 12-15, further
alternative embodiments of the vascular device of the
present invention are described. In FIG. 12, vascular
device 250, illustratively an embolic filter, comprises
guide wire 252, support hoops 253 and 254 having
articulation regions 255 and 256, respectively, and
blood permeable sac 258 affixed to support hoops 253
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 31 -
and 254. Sac 258 is coupled to support hoop 253 at its
proximal end so that the support hoop forms an opening
for the sac. Sac 258 is coupled to support hoop 254 at
its distal end to prevent emboli from spilling from sac
258 during retrieval. Support hoops 253 and 254
preferably are connected to guide wire 252 near distal
end 259 of the guide wire. Sac 258 has openings or
pores 260 that permit red blood cells to easily pass
through the sac.
During deployment and retrieval of vascular
device 250, support hoops 253 and 254 expand and
collapse as discussed hereinabove with respect to
support hoop 24 of FIGS. 2. Support hoops 253 and 254
are attached to guide wire 252 at attachment points 261
and 262, respectively, and further comprise curved
regions 263 and 264, respectively. Support hoops 253
and 254 may include radiopaque features, such as gold
or platinum bands 265, spaced at intervals around the
circumference of the hoops.
Applicant expects that vascular device 250
may further reduce the risk that captured emboli could
spill during retrieval, and also may provide a better
seal against the artery.
With reference to FIG. 13, an alternative
embodiment of vascular device 250 that prevents
bunching is disclosed that may provide even further
benefits. Vascular device 270 comprises guide wire 272
on which proximal support hoop 273 and distal support
hoop 274 are disposed. The proximal and distal
portions of blood permeable sac 275 are affixed to
support hoops 273 and 274, respectively. Proximal
support hoop 273 is attached to distal end 271 of guide
wire 272 at attachment point 276 and includes
articulation region 277 and curved regions 278.
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 32 -
Likewise, distal support hoop 274 is attached to guide
wire 272 at attachment point 279 and includes
articulation region 280 and curved regions 281. Sac
275 includes blood permeable pores 282. Hoops 273 and
274 may include radiopaque features, such as gold or
platinum bands 283, spaced at intervals around the
circumference of the hoops.
Proximal support hoop 273 is significantly
larger in circumference than distal hoop 274. Proximal
hoop 273 seals against the artery walls and defines the
diameter of the mouth of sac 275. Smaller distal hoop
274 prevents emboli from spilling from sac 275 when
retrieving device 270. It also allows the diameter of
sac 275 to decrease along its length. This taper in
sac 275 is expected to reduce the risk that sac 275
will bunch when the sac is retrieved. Sac 275 may
further by attached to guide wire 272.
Applicant has determined that where multiple
support hoops are employed, as in the embodiments of
FIGS. 12 and 13, twisting of the guide wire during
deployment may prevent the sac of the vascular device
from properly sealing against the vessel wall. For
example, if guide wire 252 in the embodiment of FIG. 12
is rotated after distal hoop 254 has been deployed, but
before proximal hoop 253 has been deployed, proximal
hoop 253 may deploy at an angle with respect to distal
hoop 254. This, in turn, may constrict, or all
together close, the opening of sac 258, thereby
rendering the vascular device ineffective.
FIG. 14 discloses a vascular device in
accordance with the present invention that overcomes
problems associated with twisting of the guide wire
during deployment. Vascular device 290 comprises guide
wire 292 with distal end 293, and support hoops 294 and
CA 02378715 2002-01-09
WO 01/08743 PCT/USOO/20754
- 33 -
295. Support hoops 294 and 295 further comprise
articulation regions 296 and 297, respectively, and
curved regions 298 and 299, respectively. The proximal
and distal portions of blood permeable sac 300 are
attached to support hoops 294 and 295, respectively.
Sac 300 includes pores 301. Support hoops 294 and 295
are attached to sheath 302 at attachment points 303 and
304, respectively. Sheath 302 preferably comprises a
flexible, 0.001" thick tube made of a biocompatible
material, such as polyamide or polytetraethylene.
Guide wire 292 passes through the lumen of sheath 302.
Sheath 302 is able to rotate with respect to guide wire
292 but is translationally restrained by stops 305 and
306, for example, solder beads.
By attaching support hoops 294 and 295 to
sheath 302, rotational problems are mitigated. Sheath
302 only transmits translational motion of guide wire
292 to support hoops 294 and 295. Thus, twisting
moments applied to wire 292 will not affect the
performance of vascular device 290. Sac 300 may also
be attached to sheath 302.
With reference to FIG. 15, a further
alternative embodiment of the vascular device of the
present invention is disclosed that also prevents
bunching. Vascular device 310 comprises guide wire 312
on which support hoop 313 is disposed. Tapered blood
permeable sac 314 is affixed to support hoop 313. Hoop
313 is attached to distal end 311 of guide wire 312 at
attachment point 315 and includes articulation region
316 and curved regions 317. Tapered sac 314 includes
blood permeable pores 318. Hoop 313 may include
radiopaque features, such as gold or platinum bands
319, spaced at intervals around the circumference of
the hoop.
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 34 -
As with vascular device 270 of FIG. 13, the
diameter of tapered sac 314 decreases along its length
to reduce the risk of bunching when the sac is
retrieved. Tapering also reduces the amount of
material that must fit within the lumen of a delivery
sheath, and thereby allows a delivery sheath of smaller
profile to be used. Furthermore, tapering the blood
permeable sac reduces the risk that the sac will snag
on a stent during retrieval.
Because vascular device 310 lacks the distal
support hoop of the embodiments of FIGS. 12 and 13,
there is a reduced risk of problems associated with
twisting. In a preferred embodiment, the diameter at
the distal end of tapered sac 314 is less than the
internal diameter of the retrieval sheath with which
the apparatus is used. Tapered sac 314 may optionally
be attached to guide wire 312, for example, to further
mitigate bunching.
Referring now to FIG. 16, a support hoop
including a radiopaque feature is disclosed. Support
hoop 320, illustratively shown in the deployed state,
comprises articulation region 321, curved regions 322,
attachment point 323, and wound radiopaque wire 324.
In the preferred embodiment, wire 324 is platinum and
is either round or a strip approximately 0.001" in
diameter. Wire 324 is wrapped around hoop 320 all
along its circumference.
One method of making a vascular device
radiopaque is to electroplate platinum or gold onto the
device. However, electroplating can be complex and
expensive, and may cause manufacturing difficulties.
Because the hoop must change shape during deployment
and retrieval, increased thickness or flaking of plated
gold are undesirable characteristics and may promote
CA 02378715 2007-06-11
35 -
failure of the support hoop. By wrapping wire 324,
hoop 320 maintains its strength and flexibility.
Radiopaque wire 324 may be used in conjunction with any
of the vascular devices discussed herein. Radiopaque
wire 324 may further be used with a wide variety of
other vascular filter devices, as are known in the art.
Referring now to FIGS. 17A-17C, another
alternative embodiment of the vascular device of the
present invention is described. As illustrated in FIG.
17A, vascular device 330 comprises guide wire 332 with
distal region 333, wishbone support hoop 335, and blood
permeable sac 336. Wishbone hoop 335 comprises spines
337 and 338 separated by a gap that serves as
articulation region 339. Articulation region 339 is
shown in greater detail in FIG. 17B, which corresponds
to the area circled in FIG. 17A taken along section
line B--B. Blood permeable sac 336 is wrapped around
and attached to itself all along its perimeter,
creating hem bond 340 and lumen 341. Sac 336 includes
pores 342. Lumen 341 is configured to receive spines
337 and 338 and bridge the gap between them. FIG. 17C
is a sectional view taken along line C--C of FIG. 17A,
showing hem bond 340 and lumen 341 with spine 338
passing there through.
Referring again to FIG. 17A, wishbone support
hoop 335 is attached to sheath 343 at attachment point
344. Sheath 343 is similar to sheath 302 of the
embodiment of FIG. 14, and preferably comprises a
flexible, 0.001" thick tube made of a biocompatible
material, such as polyamide or polytetraethylene.
Distal end 333 of guide wire 332 passes through the
lumen of sheath 343. Sheath 343 may rotate with
respect to guide wire 332 but is translationally
restrained by stops 345 and 346, for example, solder
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 36 -
beads. Sheath 343 mitigates rotational problems by
only transmitting translational motion of guide wire
332 to wishbone hoop 335. Twisting moments applied to
wire 332 do not affect the performance of vascular
device 330.
The wishbone design of support hoop 335
advantageously enables a wider variety of materials to
be used to fabricate the support hoop. Articulation
region 339 allows vascular device 330 to deploy and
contract in a manner similar to that described above
for alternative embodiments. Deployment and retraction
of wishbone hoop 335 induces minimal deformation of
spines 337 and 338, thereby permitting use of materials
such as spring steel. As will of course be apparent,
the support hoop of the embodiment of FIGS. 17A-17C may
advantageously be incorporated in any of the foregoing
embodiments.
Referring now to FIGS. 18A and 18B, an
integrated vascular device suitable for thrombectomy is
described. The integrated device comprises a
thrombectomy element and a vascular filter. In a
preferred embodiment, the thrombectomy element is
similar in construction to vascular filter 20 described
above and is connected to the guide wire proximal of
the vascular filter. Alternatively, the thrombectomy
element may be disposed on a separate catheter. The
thrombectomy element may be retracted independently of
the vascular filter.
In FIGS. 18, integrated vascular device 350
comprises guide wire 351, thrombectomy element 352
including support hoop 353 and blood permeable sac 354,
and vascular filter element 355 including support hoop
356 and blood permeable sac 357. Filter hoop 356 is
attached to guide wire 351 while thrombectomy hoop 353
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 37 -
is attached to ring 358. Ring 358 is attached to pull
wire 359 and has a bore through which guide wire 351
passes. Ring 358 therefore acts as a linear bearing
and allows thrombectomy hoop 353 to be moved by pull
wire 359 independently of guide wire 351.
Alternatively, thrombectomy element 352 may omit sac
354 and simply comprise a wire hoop; in this case
severed thrombus is captured by vascular filter 355.
In FIG. 18A, support hoops 353 and 356 and
blood permeable sacs 354 and 356 are contracted to a
delivery state within lumen 360 of delivery sheath 361.
Delivery sheath 361 includes nose cone 362 affixed to
distal region 363 of guide wire 351. In FIG. 18B,
integrated vascular device 350 is shown deployed in a
vessel. As illustrated in FIG. 18B, vascular filter
355 expands to engage the perimeter of the vessel and
prevent thrombus from bypassing the blood permeable
sac, while thrombectomy element 352 engages the vessel
wall proximal of vascular filter 355. As described
hereinbelow, proximal movement of thrombectomy device
352 scrapes thrombus from the wall of the vessel when
pull wire 359 pulls ring 358 and support hoop 353
proximally.
Referring now to FIGS. 19A-19E, an
illustrative method of using the integrated vascular
device of the present invention for thrombectomy is
described. In FIG. 19A, guide wire 351 is manipulated
into position proximal to thrombus T within vessel V
using well-known percutaneous techniques. Vascular
device 350 of FIGS. 18A and 18B is disposed in its
contracted delivery state within the distal end of
delivery sheath 361 and the delivery sheath is advanced
through the vessel using distal end 363 of guide wire
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 38 -
351. The sides of support hoops 353 and 356 are folded
together and become elongated when drawn within
delivery sheath 361, as described with respect to
vascular device 20 of FIGS. 2-4.
With respect to FIG. 19B, once delivery
sheath 361 is disposed at the desired location proximal
to thrombus T within a patient's vessel V, such as a
coronary artery or carotid artery, based on the
position of, for example, radiopaque bands under a
fluoroscope, integrated vascular device 350 is advanced
through thrombus T. Distal end 363 of guide wire 351
is advanced through the lesion, then nose cone 362
gradually increases the diameter of the void within
thrombus T so that the remainder of delivery sheath 361
can be advanced far enough that thrombectomy element
352 (still within delivery sheath 361) is located
distal to thrombus T.
With integrated vascular device 350 in
position, guide wire 351 is held stationary while
delivery sheath 361 is retracted proximally, as seen in
FIG. 19C. Alternatively, delivery sheath 361 may be
held stationary while guide wire 351 is advanced. In
either case, when vascular device 350 is no longer
confined within delivery sheath 361, support hoops 353
and 356 expand to seal against the walls of the vessel
V and deploy blood permeable sacs 354 and 357,
respectively. Blood continues to flow through vessel V
in direction A, impeded only by thrombus T.
In FIG. 19D, once vascular device 350 is
deployed in vessel V, thrombus T is removed in the
following manner. Vascular filter support hoop 353 is
rigidly attached to guide wire 351, while thrombectomy
support hoop 353 is attached to pull wire 359 via ring
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 39 -
358. Thrombectomy element 352 then is retracted
proximally to scrape along the wall of the vessel V by
motion at the proximal end of pull wire 359. Thrombus
T, located proximal to thrombectomy element 352, is
excised so that it is captured in blood permeable sac
354 during the retraction.
With respect to FIG. 19E, once thrombus T has
been captured within sac 354, pull wire 359 is pulled
proximally to cause the sides of thrombectomy support
hoop 353 to collapse together to close the mouth of sac
354 (see FIG. 3). Additional proximal retraction of
pull wire 359 causes support hoop 353 and sac 354 to
enter within lumen 360 of delivery sheath 361,
restoring normal blood flow to vessel V. Meanwhile,
vascular filter 355 is in a position distal to
thrombectomy element 352 to trap emboli E, i.e., pieces
of plaque dislodged from either thrombus T or the walls
of vessel V by thrombectomy element 352. Once any
emboli E have been collected, filter hoop 356 and sac
357 are retracted into delivery sheath 361 by motion at
the proximal end of guide wire 351, in a manner similar
to the retraction of hoop 353 and sac 354. Once guide
wire 351 has been fully retracted, and nose cone 362 at
the distal end 363 of guide wire 351 is again in
contact with delivery sheath 361, the delivery sheath
is withdrawn with integrated vascular device 350, the
trapped thrombus T, and any trapped emboli E.
As with previous embodiments, the compliant
design of integrated vascular device 350 permits the
device to be contracted to its delivery state within
the guide wire lumen of conventional previously known
interventional devices, thereby reducing time, effort,
and trauma. The vascular device may be readily closed
CA 02378715 2007-06-11
40 -
and retrieved upon completion of the interventional
procedure.
Referring now to FIGS. 20A and 20B, an
alternative embodiment of the integrated vascular
device is described. Integrated vascular device 370
comprises guide wire 371, thrombectomy element 372, and
vascular filter 373 having support hoop 374 and blood
permeable sac 375. Filter hoop 374 is attached to
guide wire 371, while thrombectomy element 372 is
disposed to slide along guide wire 371. Alternatively,
thrombectomy element 372 may be disposed on a separate
catheter element that extends either through lumen 377
of delivery sheath 378 or is separately disposed
proximal to vascular filter 373. FIG. 20A shows
thrombectomy element 372 and vascular filter 373
contracted in a delivery state within lumen 377 of
delivery sheath 378. Delivery sheath 378 includes nose
cone 379 affixed to distal region 380 of guide wire
371. In FIG. 20B, integrated vascular device 370 is
shown in the deployed state.
Thrombectomy element 372 may comprise any of
a family of known thrombectomy, atherectomy, or,
alternatively, drug delivery devices suitable for use
in conjunction with vascular filter 373. Thrombectomy
element 372 may, for example, comprise any of: a rotary
ablation device, such as described in U.S. Patent Nos.
4,867,156 to Stack et al., 4,990,134 to Auth, and
5,314,407 to Auth et al.; an atherectomy technology,
such as described in U.S. Patent Nos. 5,181,920 to
Mueller et al., and 5,074,841 to Ademovic et al.; or a
balloon embolectomy technology, such as described in
U.S. Patent Nos. 3,923,065 to Nozick et al., 5,769,871
to Mers Kelly et al., 5,192,290 to Hilal, 5,112,347 to
Taheri, and 4,030,503 to Clark III.
CA 02378715 2007-06-11
41 -
Thrombectomy element 372 may alternatively comprise a
wire loop or ring, such as described for the embodiment
of FIGS. 1.8A and 18B, a laser ablation device, a
chemical flushing system, etc.
Referring now to FIGS. 21A and 21B, a
delivery system configured for use with embodiments of
the present invention is described. The delivery
system facilitates-deployment and retrieval of .the
embodiments by integrating the functions of a torquer,
a Touhy Borst adapter, and a pusher into a single
device. In FIGS. 21, the delivery system is
illustratively used in conjunction with vascular device
of FIGS. 2-5. In FIG. 21A, vascular device 20 is in
15 the retracted delivery configuration, while in FIG. 21B
vascular device 20 is in the expanded deployed
configuration. Delivery system 450 comprises proximal
screw cap 452, collet 456, handle 460, rod 464, central
screw cap 468, lumen flushing section 472, distal hub
20 479, and nose piece 486.
Proximal screw cap 452 includes bore 453 with
female screw thread 454 and guide wire lumen 455. Bore
453 extends proximally from the distal face of cap 452.
Guide wire lumen 455 extends from the proximal end of
bore 453 to the proximal end of cap 452.
Handle 460 comprises proximal male screw
thread 461 configured to engage female screw thread 454
of cap 452, and lumen 462 configured to receive collet
456 in its proximal end and rod 464 in its distal end.
Lumen 462 has a reduced diameter at the distal end of
handle 460 that captures a step on the proximal end of
rod 464. Thus, while collet 456 is removable received
within lumen 462, rod 464 may translate and rotate
within, but may not be removed from, lumen 462. Guide
CA 02378715 2007-06-11
42 -
wire 22.freely passes through collet 456 when screw
cap 452-is not securely fastened to handle 460. When
cap 452 is securely fastened to handle 460, it causes
collet 456 to elastically deform, decreasing the
diameter of the lumen extending through the collet, and
frictionally locking guide wire 22 into'rigid
attachment with collet 456. Guide wire 22 is thereby
rigidly connected to handle 460.
Rod 464 further comprises guide wire lumen
465 extending therethrough. Rod 464 has its distal end
rigidly and permanently affixed to central screw cap
468. Cap 468 comprises female screw thread 469 and
lumen 470. Lumen 470 includes a proximal reduced-
diameter step that captures rod 464 within the proximal
end of cap 468, and a distal portion that receives
lumen flushing or fluid port section 472.
Section 472 comprises. male screw thread-473,
side port 474, bore 475, guide wire lumen 476, and
fluid lumen 477. Male screw thread 473 is configured
to engage female thread 469 of cap 468. Section 472
includes a flange disposed just distal of thread 473
that is captured within lumen 470 of cap 468. Thus,
cap 468 may be tightened onto and loosened from, but
not removed from, section 472.
Rod 464 is received within bore 475 of
section 472. Guide wire 22 passes between bore 475 and
fluid lumen 477 within guide wire lumen 476. Fluid
lumen 477 connects side port 474 to the guide wire
lumen of delivery sheath 40. O-rings 478 provide a
fluid seal at the distal end of lumen 477.
Distal hub. 479 connects section 472 to nose
piece 486. Hub 479 comprises bore 483, female screw
thread 484, and annulus 485 containing tapered
projection 481. Bore 483 includes flange 482 that
CA 02378715 2002-01-09
WO 01/08743 PCT[USOO/20754
- 43 -
rotatably receives section 472 in its proximal end.
Nose piece 486 comprises male screw thread 487, tapered
bore 488, and delivery sheath lumen 489. Male screw
thread 487 is configured to engage female thread 484 in
annulus 485 of hub 479.. Tapered bore 488 allows
tapered projection 481 of hub 479 to extend within nose
piece 486 and permit delivery sheath 40 from delivery
sheath lumen 489 to extend therethrough. 0-rings 478
are disposed between the hub 479 and nose piece 486 and
between hub 479 and section 472.
Delivery system 450 advantageously may be
implemented in a variety of ways. For example, the
delivery system may be offered with a delivery catheter
or sheath pre-attached. In this embodiment, proximal
screw cap 452 is loosened, and the proximal end of
guide wire 22 may be passed through the delivery
catheter or sheath, and delivery system 450, until
vascular device 20 is in its retracted state within the
delivery catheter or sheath. Insertion of the vascular
device into the patient may then proceed.
Alternatively, delivery system 450 may be commercially
supplied in the configuration shown in FIG. 5A, i.e.,
pre-loaded with a delivery catheter or sheath, such as
sheath 40, already attached and a vascular device, such
as vascular device 20, retracted therein. As another
alternative, delivery system 450 may be offered without
either a delivery sheath or vascular device attached,
or the delivery catheter or sheath may be an
interventional instrument, such as an angioplasty,
atherectomy, or stent delivery catheter.
Referring again to FIGS. 5A-5D in conjunction
with FIGS. 21A and 21B, a method of using the delivery
system of the present invention in conjunction with a
vascular filter is described. With vascular device 20
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 44 -
contracted within distal end 42 of delivery sheath 40
(FIGS. 5A and 21A), delivery sheath 40 is attached to
delivery system 450 by loosening proximal screw cap 452
and extending the proximal end of guide wire 22 through
delivery system 450, with handle 460 in its proximal-
most position (FIG. 21A). Screw cap 452 is then
tightened to cause collet 456 to engage guide wire 22
to handle 460.
Delivery sheath 40 then is advanced through a
patient's vasculature using well-known percutaneous
techniques using distal end 23 of guide wire 22. If a
vessel bifurcation is to be crossed during advancement,
handle 460 may be rotated to divert the distal end of
sheath 40 into the desired branch of the bifurcation.
The rotational moment or torque applied to handle 460
is transmitted to guide wire 22 (when screw cap 452 is
tightened), which causes distal end 23 to rotate and
facilitates positioning of vascular device 20 in the
proper side of the bifurcation. As shown in FIG. 5A,
advancement continues until delivery sheath 40 is
disposed at a desired location within a patient's
vessel V, such as a coronary or carotid artery, as
determined, for example, by the position of radiopaque
band 43 under a fluoroscope.
With the vascular device in position, handle
460, and thus guide wire 22, is held stationary while
section 472 and attached delivery sheath 40 are
retracted proximally. Alternatively, handle 460 may be
advanced while section 472 and sheath 40 are held
stationary. In either case, when vascular device 20 is
no longer confined within delivery sheath 40, support
hoop 24 expands to seal against the walls of the vessel
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 45 -
V, as depicted in FIGS. 5B and 21B. Blood continues to
flow unimpeded through vessel V in direction A.
Depending on the medical procedure prescribed
in conjunction with the use of vascular device 20,
delivery sheath 40 may retrieve vascular device 20 at
the conclusion of the procedure, or sheath 40 may be
detached from delivery system 450 and removed from the
patient. If sheath 40 is detached, guide wire 22 may
be removed from delivery system 450 so that other
interventional instruments, such as angioplasty
catheters, atherectomy devices, or stent delivery
systems may be advanced along guide wire 22 to position
such devices at treatment zones located proximally of
vascular device 20. Guide wire 22 and the
interventional catheter then may be passed through and
fastened to delivery system 450. For example, as shown
in FIG. 5C, angioplasty balloon catheter 44 may be
advanced along guide wire 22 to a position proximal of
vascular device 20 so that device 20 may trap emboli E,
i.e., pieces of plaque dislodged from the walls of
vessel V by balloon 46.
Upon completion of the angioplasty procedure
using angioplasty balloon catheter 44, handle 460 with
attached guide wire 22 is pulled proximally to cause
the sides of support hoop 24 to collapse together to
close the mouth of sac 28 (FIG. 3). Additional
proximal retraction of guide wire 22 causes support
hoop 24 and sac 28 to enter at least partially within
the guide wire lumen of angioplasty catheter 44. As
depicted in FIG. 4D, only a portion of support hoop 24,
near articulation region 26, and a distal portion of
sac 28 extend out of the guide wire lumen of
angioplasty catheter 44. Angioplasty catheter 44 then
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 46 -
is withdrawn with vascular device 20 and any trapped
emboli E.
It also may be beneficial during a medical
procedure to introduce or withdraw fluids from the
operative site. For example, it may be beneficial to
deliver medicaments, or draw suction to remove blood.
The delivery sheath lumen also may require flushing
with saline to prevent clotting within the lumen.
These and other procedures are made possible by side
port 474 of section 472, which, as described
hereinabove, is in fluid communication with the lumen
of delivery sheath 40.
In addition to applications with vascular
filters, delivery system 450 may be used as part of the
thrombectomy/embolectomy procedure described herein
above, as well as in a variety of other procedures.
Embodiments of the present invention may
optionally be used in conjunction with a specially
configured retrieval sheath. Applicant has determined
that bunching of sac 28 in FIG. 5D may occur during
retraction into catheter 44, resulting in a retrieval
profile that may be difficult to navigate through a
patient's vasculature. However, additional proximal
retraction of guide wire 22 in an attempt to decrease
the profile of sac 28 may generate stress loads
sufficient to tear sac 28 and release captured emboli.
With reference to FIGS. 22A-22E, a specially
configured retrieval sheath and methods of use with the
vascular device of the present invention are described.
As with FIGS. 5, sizes have been exaggerated to
illustrate structure. In FIG. 22A, guide wire 556 is
positioned within vessel V using well-known
percutaneous techniques. Vascular device 550 is
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 47 -
disposed in its contracted delivery state within distal
end 554 of delivery sheath 552. Retrieval sheath 560
and guide catheter 562 are advanced over delivery
sheath 552 to a position located just proximal of
distal end 554.
Retrieval sheath 560 includes collapsible
flared end region 564, which is shown in a contracted
delivery state within catheter 562 in FIG. 22A. Flared
end region 564 has a deployed state, wherein the wall
flares outward to form a frustrum of a cone, and a
contracted state, wherein the wall is substantially
cylindrical. Flared end region 564 preferably includes
radiopaque band 566.
With respect to FIG. 22B, once delivery
sheath 552 is disposed at a desired location within a
patient's vessel V, guide wire 556 is held stationary
while delivery sheath 552 is retracted proximally.
Alternatively, delivery sheath 552 may be held
stationary while guide wire 556 is advanced. In either
case, when vascular device 550 is no longer confined
within delivery sheath 552, support hoop 568 and
attached blood permeable sac 570, expands to seal
against the walls of the vessel V. Sac 570 further
comprises radiopaque band 572. When in the deployed
state, the curved regions of support hoop orient its
articulation region concentrically against the inside
wall of the vessel. Blood continues to flow unimpeded
through vessel V in direction A.
With vascular device 550 deployed, an
interventional procedure is performed proximal of the
device. For example, guide catheter 562 may be an
angioplasty balloon catheter similar to catheter 44 of
FIGS. 5C and 5D. The interventional procedure
CA 02378715 2002-01-09
WO 01/08743 PCT/US00/20754
- 48 -
generates emboli E proximal of device 550, which travel
downstream and are captured in sac 570.
With respect to FIG. 22C, upon completion of
the interventional procedure, guide wire 556 is pulled
proximally to cause the sides of support hoop 568 to
collapse together to close the mouth of sac 570 (see
FIG. 3). Additional proximal retraction of guide wire
556 causes support hoop 568 and sac 570 to partially
enter within distal end 554 of delivery sheath 552. If
bunching of the sac is anticipated or suspected, flared
sheath 560 may be advanced distally to expand end
region 564, which comprises a suitable elastomeric
material, such as latex, rubber, or a synthetic variant
thereof.
As depicted in FIG. 22D, delivery sheath 552
is retracted proximally while retrieval sheath 560 is
held stationary, until radiopaque bands 572 and 566 are
concentrically aligned, as determined, for example,
with a fluoroscope. Then, as illustrated in FIG. 22E,
sheaths 552 and 560 are simultaneously withdrawn
proximally while guide catheter 562 is held stationary.
This motion causes flared end region 564 to collapse
sac 570 to its contracted state. In so doing, flared
end region 564 applies a distributed load over the
surface of sac 570, thereby decreasing the retrieval
profile of sac 570 with reduced risk of rupture of sac
570.
Vascular device 550 also may be used in
performing thrombectomy/embolectomy. In this case,
vascular device 550 is advanced in its retracted state
within delivery sheath 552 to a location distal of a
lesion. Delivery sheath 552 is withdrawn proximally,
and vascular device 550 is deployed. With support hoop
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 49 -
568 in contact with the vessel wall, vascular device
550 may be retracted proximally to scrape along the
wall of the vessel and excise thrombus so that it is
captured in sac 570. Delivery sheath 552, as well as
flared sheath 560 and guide catheter 562, then may be
reinserted into the vessel along guide wire 556, and
vascular device 550 may be retracted and removed from
the vessel in the manner described hereinabove.
With reference to FIGS. 23A and 23B, an
alternative embodiment of the specially configured
retrieval sheath, and methods of use with the vascular
device of the present invention, are described. Again,
sizes have been exaggerated to illustrate structure.
In FIG. 23A, guide wire 582 has been positioned within
vessel V using well-known percutaneous techniques.
Vascular device 580 has been expanded to its deployed
state after delivery within delivery sheath 584, in the
manner discussed hereinabove. Support hoop 586 seals
against the walls of vessel V, and blood permeable sac
588 is positioned to capture emboli E generated by, for
example, an upstream interventional procedure. Blood
continues to flow unimpeded through vessel V in
direction A.
Delivery sheath 584 further comprises
atraumatic expander 590 disposed on a distal end.
Retrieval sheath 592 is advanced over delivery sheath
584 to a position located just proximal of expander
590. Retrieval sheath 592 includes expandable end
region 594, which is shown in a contracted delivery
state in FIG. 23A. Expandable end region 594 has a
deployed state, wherein the wall flares outward to form
a frustrum of a cone, and a contracted state, wherein
the wall is substantially cylindrical. Expander 590
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 50 -
has a larger maximum diameter than end region 594.
Expandable end region 594 preferably includes
radiopaque band 596, while expander 590 preferably
includes radiopaque band 598 so that their positions
relative to one another may be accurately determined.
With respect to FIG. 23B, upon completion of
the interventional procedure, guide wire 582 is pulled
proximally to cause the sides of support hoop 586 to
collapse together to close the mouth of sac 588 (see
FIG. 3). Additional proximal retraction of guide wire
582 causes support hoop 586 and sac 588 to partially
enter within the distal end of delivery sheath 584.
If bunching of the sac is anticipated or
suspected, delivery sheath 584 may be retracted
proximally while retrieval sheath 592 is held
stationery to expand end region 594 of retrieval sheath
592 with expander 590. Delivery sheath 584 is
retracted a sufficient distance to protect sac 588 and
its embolic contents within end region 594. The
distance may be determined by means of radiopaque bands
596 and 598. End region 594 comprises a suitable
elastomeric material, such as latex, rubber or a
synthetic variant thereof.
The profile of end region 594 in the expanded
state allows for retraction of retrieval sheath 592, as
well as delivery sheath 584 and vascular device 580
disposed therein, in a manner that mitigates dangerous
interaction with the vascular wall. It also allows
vascular device 580 to be retrieved in a partially
collapsed state that reduces the risk of sac 588
tearing. As with vascular device 550, vascular device
580 may be used in performing thrombectomy/embolectomy.
CA 02378715 2002-01-09
WO 01/08743 PCTIUSOO/20754
- 51 -
The support hoops depicted herein
illustratively are shown as oval or heart-shaped in the
deployed state, where the shape is exaggerated for the
sake of clarity. In preferred embodiments, the support
hoops are substantially round when deployed, to ensure
contact around the circumference of the support hoop
and provide a positive seal against the arterial wall.
Although preferred illustrative embodiments
of the present invention are described above, it will
be evident to one skilled in the art that various
changes and modifications may be made without departing
from the invention. It is intended in the appended
claims to cover all such changes and modifications that
fall within the true spirit and scope of the invention.