Note: Descriptions are shown in the official language in which they were submitted.
CA 02378913 2002-01-10
WO 01/05459 PCT/US00/19034
SYSTEM FOR EFFECTING SMOKE CESSATION
FIELD OF THE INVENTION
This invention relates generally to a method for treating conditions
responsive to nicotine
therapy. More specifically, the invention relates to pulmonary administration
of nicotine to effect
smoking cessation.
BACKGROUND OF THE INVENTION
Diseases related to cigarette smoking, such as lung disease, heart disease and
cancer, claim
an estimated 400,0001ives each year. The combustion of tobacco produces
poisons and carcinogens
that present a significant health hazard for smokers and non-smokers alike.
Nicotine is a principal
component of tobacco, and the most pharmacologically active. It is physically
addictive, making it
extremely difficult for a smoker to quit.
Smoking a cigarette delivers nicotine vapors to the lungs, where nicotine is
rapidly absorbed
through the arteries and delivered to the brain. Nicotine interacts with
nicotinic cholinergic
receptors in the brain to induce the release of neurotransmitters and produce
an immediate reward--
the "rush" that smokers experience--that is associated with a rapid rise in
blood level. A persistent
stimulus is also produced, and is associated with a high blood level of
nicotine. Complex behavioral
and social aspects of smoking, e.g., the hand-to-mouth ritual, etc., are also
habit-forming.
A therapeutic approach to aid in smoking cessation is to provide the smoker
with nicotine
from sources other than cigarettes. A number of nicotine replacement therapies
have been
developed to accomplish this result. Commercially available therapies deliver
nicotine to the
systemic circulation via absorption through mucosal membranes or the skin.
These include
nicotine-containing chewing gum, sachets, transdermal patches, capsules,
tablets, lozenges, nasal
sprays and oral inhalation devices.
Nicotine delivery via inhalation offers the benefit of addressing the
psychological
component of cigarette smoking in addition to the physiological dependence on
nicotine. Nicotine
inhalation systems release nicotine as a vapor (see U.S. Patent Nos.
5,167,242; 5,400,808;
5,501,236; 4,800,903; 4,284,089; 4,917,120; 4,793,366), aerosol (see U.S.
Patent Nos. 5,894,841;
5,834,011) or dry powder (see U.S. Patent No. 5,746,227) when air is inhaled
through the inhaler. A
droplet ejection device (U.S. Patent No. 5,894,841) has also been described
that delivers a controlled
dose of nicotine via inhalation. These systems deliver low doses of nicotine
to the mouth and throat,
where nicotine is absorbed through the mucosal membranes into the circulation.
Some inhalation
therapies feature devices that simulate or approximate the look, feel and
taste of cigarettes.
-1-
CA 02378913 2002-01-10
WO 01/05459 PCTIUSOO/19034
Currently available nicotine replacement therapies, such as transdermal and
buccal systems,
provide a low, steady-state blood level of nicotine to the patient. The need
remains for an smoking
cessation therapy that delivers a precise dose of nicotine to the lungs in a
profile that mimics the
blood levels achieved by cigarette smoking--providing an initial sharp rise in
blood level followed
by a slow release of nicotine--making it possible for the user to be weaned
off of nicotine and to quit
smoking.
SUMMARY OF THE INVENTION
A system for aiding a patient in quitting smoking is disclosed. The system is
comprised of a
means for the delivery of aerosolized nicotine which makes it possible to
gradually decrease the
amount of nicotine that the patient receives. The system comprises a means for
aerosolizing a
formulation comprised of nicotine and a means for decreasing the amount of
nicotine formulation
which is aerosolized and/or the amount which actually reaches the patient's
circulatory system. The
amount of nicotine aerosolized or effectively delivered to the patient can be
changed in several
different ways using either the device aerosolization mechanism, the
formulation or forinulation
containers loaded into the device.
A preferred system of the invention aerosolizes the liquid formulation by
applying force to a
container of nicotine formulation and causing the nicotine formulation to be
moved through a porous
membrane which results in creating particles of nicotine formulation which are
inhaled by the
patient. This system modifies the amount of nicotine aerosolized by providing
a plurality of
different containers or different groups of containers wherein the different
containers or groups of
containers contain different concentrations of nicotine. A patient using the
system can utilize
packets of nicotine formulation containing a high concentration initially and
then gradually switch
towards lower and lower concentrations so that the patient receives
essentially the same amount of
aerosolized formulation but receives gradually reduced amounts of nicotine due
to the reduced
concentration of the nicotine in the formulation.
The same procedure described above can also be carried with a dry powder
inhaler (DPI).
Using the dry powder inhaler technology the packets of dry powder nicotine
formulation loaded into
the device can initially contain a relatively high concentration of nicotine.
Thereafter, the
concentration of nicotine in the dry powder formulation added into the device
is gradually
decreased. Thus, using this system the same amount of dry powder is
aerosolized, but the amount of
nicotine is gradually decreased by decreasing the concentration or simply the
total amount of
nicotine in the dry powder package loaded into the device. The same procedure
can be utilized with
a conventional metered dose inhaler (MDI) device. It is somewhat more
difficult to utilize the
invention with an MDI device. However, small pressurized canisters
conventionally used with
-2-
CA 02378913 2002-01-10
WO 01/05459 PCTIUSOO/19034
MDIs can contain different concentrations of nicotine along with the
propellant. By using a first
container which includes the highest concentration of nicotine and gradually
changing to lower and
lower concentrations of nicotine in the pressurized canister the desired
result of reducing the amount
of nicotine delivered to the patient can be obtained. The same results could
be obtained by gradually
decreasing the amount of formulation released when the value of a container is
opened.
When using a dry power inhaler or a system which aerosolizes a liquid
formulation by
moving the formulation through a porous membrane it is possible to decrease
the amount of nicotine
gradually by making changes in the device, or more specifically the operation
of the device. For
example, a dry powder inhaler often utilizes a burst of air in order to
aerosolize the dry powder. The
burst of air could be decreased so that not all of the powder is fully
aerosolized or so that the powder
is not aerosolized in a completely efficient manner. In a more preferred
embodiment the system for
aerosolizing liquid forinulation is adjusted at different points so that
different amount of pressure are
applied to the formulation making it possible to aerosolize decreasing amounts
of forrnulation and
allowing the patient to be gradually weaned off of nicotine.
The most preferred embodiment of the invention involves the use of a system
which
aerosolizes liquid formulations of nicotine contained within individual
packets which packets
include a porous membrane. As indicated above the amount of nicotine that can
be changed by
changing the amount of or concentration of nicotine in the packets. However,
it is also possible to
decrease the amount of nicotine actually delivered to the patient's
circulatory system by changing the
size of the pores in the membrane. When the pore size is in a preferred range
then a relatively high
concentration of the formulation aerosolized will reach the patient's lungs
and move from the lungs
into the patient's circulatory system. However, by making the pores larger the
aerosolized particles
created also become larger. The larger particles will not move into the lungs
as efficiently as the
smaller particles. Further, the larger particles may be deposited in areas
where they are not readily
absorbed into the patient's circulatory system. Thus, in accordance with a
preferred embodiment of
the invention a plurality of different containers are produced. The containers
are different from each
other in that they contain different amounts or concentrations of nicotine.
Alternatively, the
containers are different from each other in that they have different porous
membranes on them
which make it possible to aerosolize the forinulation in a somewhat less
efficient manner over time.
It is possible to combine both or all three features together. More
specifically, it is possible to
produce containers which contain (1) smaller concentrations of nicotine; (2)
smaller amounts of
nicotine; or (3) have porous membranes which have different size or amounts of
pores so as to less
efficiently aerosolize the formulation present in the container.
A method for aiding in smoking cessation and for treating conditions
responsive to nicotine
therapy by the administration of nicotine is disclosed. A formulation
comprised of nicotine is
-3-
CA 02378913 2002-01-10
WO 01/05459 PCTIUSOO/19034
aerosolized. The aerosol is inhaled into the lungs of the patient. Once
inhaled, particles of nicotine
deposit on lung tissue and from there enter the patient's circulatory system.
Because delivery is to
the lungs, the patient's seruxn nicotine level is quickly raised to a desired
level--as quickly as if the
user were smoking. The methods of the invention produce arterial
concentrations of nicotine similar
to cigarette smoking.
Subsequently, the patient's dependence on nicotine is reduced by gradually
reducing the
dose of nicotine. The dose of nicotine is reduced by progressively increasing
the size distribution of
the aerosolized nicotine particles delivered to the patient. This decreases
the amount of nicotine
delivered to the patient's lungs, with the result that nicotine absorption is
less immediate and the
blood plasma level is lower.
A method of treatment is disclosed, comprising:
(a) aerosolizing a formulation comprised of nicotine creating aerosolized
particles which
are sufficiently small as to enter the alveolar ducts;
(b) allowing a patient to inhale the aerosolized particles of (a) thereby
causing nicotine to
enter the patient's blood at air/blood diffusion membranes;
(c) repeating (a) and (b) a plurality of times;
(d) aerosolizing a formulation comprised of nicotine creating aerosolized
particles which
are too large to enter alveolar ducts but sufficiently small to enter primary
and secondary
bronchioles;
(e) allowing the patient to inhale the aerosolized particles of (d) into
primary and secondary
bronchioles; and
(f) repeating (d) and (e) a plurality of times.
The method is preferably further comprised of:
(g) aerosolizing a formulation comprised of nicotine creating aerosolized
particles which
are too large to enter primary and secondary bronchioles but sufficiently
small to enter the small
bronchi;
(h) allowing the patient to inhale the aerosolized particles of (g) into small
bronchi; and
(i) repeating (g) and (h) a plurality of times.
An aspect of the invention is a method of treatment whereby nicotine or a
nicotine substitute
is aerosolized, inhaled into areas of the respiratory tract including the
lungs and provided to the
circulatory system of the patient at levels sufficient to simulate cigarette
smoking.
An advantage of the invention is that the nicotine levels are raised almost
inunediately on
administration.
-4-
CA 02378913 2009-02-05
Another advantage of the invention is that the patient can gradually be weaned
off
of the immediate effect of nicotine obtained via smoking and gradually weaned
off of the
need of nicotine by, respectively, increasing particle size and decreasing
dose size or
concentration.
A feature of the invention is that aerosolized particles of nicotine having a
diameter
of about 0.5 to 8 microns ( ) are created and inhaled deeply into the lungs,
thereby
enhancing the speed and efficiency of administration.
In one aspect of the present invention, there is provided delivering nicotine
by
inhalation as a means of treating conditions responsive to nicotine therapy,
and
particularly for smoking cessation therapy.
In another aspect of the present invention, there is provided varying the
distribution
of aerosolized particles of nicotine inhaled as a means of treating smokers
wishing to quit.
In another aspect of the present invention, there is provided liquid
formulations
(which includes suspensions) of nicotine and derivatives thereof appropriate
for
pulmonary delivery.
In another aspect of the present invention, there is provided how nicotine
delivered
via the lung can quickly increase blood plasma levels.
An aspect of the invention is a method whereby larger and larger particles of
aerosolized nicotine are administered to a patient over time in order to first
wean a
smoking patient off of the addiction to immediate nicotine and thereafter
reduce the
amount of nicotine in order to wean the patient completely off of the
addiction to nicotine,
thereby allowing the patient to quit smoking.
A feature of this invention is that it allows for the formation of nicotine
particles in
different sizes designed for delivery to different areas of a patient's lungs.
An advantage of the invention is that it allows the patient to be weaned off
of (1)
the need for immediate nicotine delivery as obtained when smoking, and (2) the
need for
nicotine at all.
In another aspect of the present invention, there is provided a kit for aiding
a
patient in quitting smoking, comprising: a first plurality of containers
having therein a
formulation of nicotine and a pharmaceutically acceptable carrier, wherein the
nicotine is
present in a first concentration; a second plurality of containers having
therein a
formulation of nicotine and a pharmaceutically acceptable carrier wherein the
nicotine is
-5-
CA 02378913 2009-02-05
present in a second concentration which is less than the first concentration;
and, wherein
the formulation in the first plurality of containers and the formulation in
the second
plurality of containers are liquid flowable formulations wherein the nicotine
is present in
each liquid flowable formulation in a solution or suspension. Each plurality
may comprise
two or more canisters, the canisters containing a formulation of nicotine
which may
comprise a low boiling point propellant. In various embodiments, the
containers may
comprise a porous membrane.
In another aspect of the present invention, there is provided a kit described
herein
for aiding a patient in quitting smoking further comprising: a device for
aerosolizing each
formulation, wherein each container of each plurality of containers can be
loaded into the
device for aerosolizing. The device may be a hand-held self-contained device.
In another aspect of the present invention, there is provided a kit wherein
the
formulation in the first plurality of containers and the formulation in the
second plurality
of containers is a dry powder formulation.
In another aspect of the present invention, there is provided a kit wherein
the first
plurality of containers comprises two or more canisters which canisters
contain the
formulation in the form of nicotine and low boiling point propellant and
wherein the
second plurality of containers comprises two or more canisters wherein the
formulation in
the canisters comprises nicotine and a low boiling point propellant.
In another aspect of the present invention, there is provided a kit wherein
the first
plurality of containers each comprise a porous membrane wherein the pores have
a first
average size, and wherein the second plurality of containers each comprise a
porous
membrane wherein the pores have a second average size which is larger than the
first
average size of the pores in the membrane of the first plurality of
containers.
In another aspect of the present invention, there is provided a kit wherein
the first
plurality of containers and second plurality of containers hold the nicotine
and
pharmaceutically acceptable carrier in a liquid flowable form.
In another aspect of the present invention, there is provided a use for
smoking
cessation.
In another aspect of the present invention, there is provided a use for an
aerosol
formulation suitable for inhalation comprising nicotine, the formulation
further comprising
aerosolized particles which are sufficiently small as to target alveoli of a
respiratory tract
and particles which are sized to target bronchi of a respiratory tract, for
smoking cessation.
-5a-
CA 02378913 2007-05-16
In another aspect of the present invention, there is provided a kit wherein
the device for
aerosolizing formulation is a hand-held, self-contained device.
In another aspect of the present invention, there is provided a kit further
comprising: a
third plurality of containers which are for being loaded into the device for
aerosolizing
formulation wherein the containers are comprised of nicotine and a
pharmaceutically
acceptable carrier and wherein the nicotine is present in a third
concentration which is less than
the second concentration.
In another aspect of the present invention, there is provided a kit wherein
the
formulation in the first plurality of containers and the formulation in the
second plurality of
containers is a liquid flowable formulation wherein the nicotine is present in
the formulation in
a solution or suspension.
In another aspect of the present invention, there is provided a kit wherein
the first
plurality of containers comprises a porous membrane for each container and
further wherein
the second plurality of containers comprises a porous membrane for each
container.
In another aspect of the present invention, there is provided a kit wherein
the
formulation in the first plurality of containers and the formulation in the
second plurality of
containers is a dry powder formulation.
In another aspect of the present invention, there is provided a kit wherein
the first
plurality of containers comprises two or more canisters which canisters
contain the formulation
in the form of nicotine and low boiling point propellant and wherein the
second plurality of
containers comprises two or more canisters wherein the formulation in the
canisters comprises
nicotine and a low boiling point propellant.
In another aspect of the present invention, there is provided a kit wherein
the first
plurality of containers each comprise a porous membrane wherein the pores have
a first
average size, and wherein the second plurality of containers each comprise a
porous membrane
wherein the pores have a second average size which is larger than the first
average size of the
pores in the membrane of the first plurality of containers.
In another aspect of the present invention, there is provided a kit wherein
the first
plurality of containers and second plurality of containers hold the nicotine
and
pharmaceutically acceptable carrier in a liquid flowable form.
In another aspect of the present invention, there is provided a use for
smoking
cessation.
In another aspect of the present invention, there is provided a use for an
aerosol
formulation suitable for inhalation comprising nicotine, the formulation
further comprising
aerosolized particles which are sufficiently small as to target alveoli of a
respiratory tract and
particles which are sized to target bronchi of a respiratory tract, for
smoking cessation.
5a
CA 02378913 2007-05-16
In another aspect of the present invention, there is provided a use for an
aerosol
fonnulation suitable for inhalation comprising nicotine, the formulation
comprising aerosolized
particles which are sufficiently small as to target alveoli of a respiratory
tract and particles
which are sized to target bronchi of a respiratory tract, for preparation of a
medicament for
smoking cessation.
In another aspect of the present invention, there is provided a use wherein
the particles
which are sized to target bronchi of a respiratory tract have a diameter of 2
m to about 4 m.
In another aspect of the present invention, there is provided a use wherein
the
aerosolized particles which are sufficiently small as to target alveoli of a
respiratory tract have
a diameter of 0.5 m to about 29m.
In another aspect of the present invention, there is provided a use wherein
the
formulation comprises 0.25mg or more of nicotine.
These and other aspects, objects, advantages, and features of the invention
will become
apparent to those skilled in the art upon reading this disclosure.
BRIEF DESCRIPTION OF THE DRAWINGS
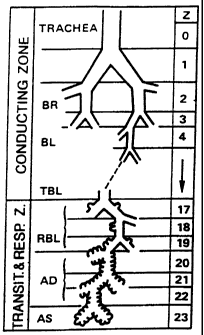
FIG. 1 is a schematic view of a human lung branching pattern.
FIG. 2 is a schematic view of a human respiratory tract.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Before the devices, formulations, and methodology of the present invention are
described, it is to be understood that this invention is not limited to the
particular device,
components, formulations and methodology described, as such may, of course,
vary. It is also
to be understood that the terminology used herein is with the purpose of
describing particular
embodiments only, and
5b
CA 02378913 2007-05-16
is not intended to limit the scope of the present invention which will be
limited only by the appended
claims.
It must be noted that as used herein and in the appended claims, the singular
forms "a,"
"aad," and "the" include plural referents unless the context clearly dictates
otherwise. Thus, for
example, reference to "a formulation" includes mbttures of different
formulations and reference to
"the method of treatment" includes reference to equivalent steps and methods
known to those skilled
in the art, and so forth.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meanmg as cominonly understood by one of ordinary sldll m the art to which
this invention belongs.
Although any methods and materials sumlar or equivalent to those descnbed
herein can be used in
the practice or testing of the invention, the preferred methods and inatmals
are now descnbed_
DEFINITIONS
The term "nicotine" is intended to mean the naturally occurring allcaloid
known as nicotine,
having the chemical name S-3-(1-methyl-2-pyrrolidinyl)pyridine, which may be
isolated and
purified from nature or synthetically produced in any manner. This term is
also intended to
encompass the commonly occurring salts containing pharinacologically
acceptable anions, such as
hydrochloride, hydrobromide, hydroiodide, nitrate, sulfate or bisulfate,
phosphate or acid phosphate,
acetate, lactate, citrate or acid citrate, tartrate or bitartrate, succinate,
maleate, funnarate, gluconate,
saccharate, benzoate, methanesulfonate, ethanesnlfonate, beazenesulfanate, p
tohiene sulfonate,
aamphorate and pamoate satts. Nicotine is a colorless to pale yellow, strongly
alkaline, oily,
volatile, hygroscopic liquid having a molecular weight of 162.23 and the
formula:
I-
GH3
N
CH3
N
=-
-6-
CA 02378913 2007-05-16
Structure and ionisatian of nicotine. Nicotine is approximately 10% of the
particulate weight in
cigarette smoke. Brand differences change this percentage. It is
monoprotonated at most
physiological pH values. The diprotonated ion would exist at pH values found
in the stomach.
Metabolism is largely due to oxidation. Cotinine is a major matabolite;
howover, there are at least 4
primary metabolites of nicotine and aU are encompassed by the use of this term
herein.
The term "nicaRine" furthet includes any pharmacologieally acceptable
derivative,
metabolite or analog of nicotine which exhibits pharmacotherapeutic properties
similar to nicotine.
Such derivatives and metabolites are known in the art, and iaclude cortinmc,
norcotinuie, nomicotine,
nicotine N-oxide, cotinine N-oxide, 3-hydroxycoRinine and 5-hydroxycotinine or
phannaceutically
aeoeptable salta thereoà A number of usefid derivatives of nicatine are
disclosed within the
Physician's Desk Reference (53d edition) as well as Harrison's Principles of
Internal Medicine (14`h
edition). In addition, applicants refer to U.S. Patent Nos. 5,776,957;
4,965,074; 5,278,176; 5,276,043;
5,227,391; 5,214,060; 5,242,934; 5,223,497; 5,278,045; 5,232,933; 5,138,062;
4,966,916; 4,442,292;
4,321,387; 5,069,094; 5,721,257; which describe nicotine derivatives and
formulations.
The physiologically active form of nicotine is the S-(-)-isomer. Certain
e.onpounds of the
pnseat invention may exist in particular geometric or stereoisomeric forms.
The present invention
contemplates all such compounds, including cis and trans isomers, R and S
enantiomers,
diastereomers, the racemic nuxtures thereof, and other mixtures thereof, as
falling within the scope
of the invention. Additional asynunetric carbon atoms may be present in a
substituent such as an
allcyl group. All such isomers, as well as mixtures thereot are intended to be
included in this
invention.
The term "upper airways" and the like are used interchangeably herein to
define an area of
the respiratorysystem which includes the oropharyngeal region and trachea.
This area is the first
area which air eeders upon inhalation (see FIG. 1).
The terms "central airways," "bronchial airways" and the like are used
interchangeably
hereia to refer to a region of the respiratory system that includes
generations 1 through 16 of the
airways (see FIG. 1) which removes particles larger than 3 in diameter. They
are the conductive
ai=ways that also clean particles finaa t6e lung using a niucosal clearance
mechanism. Upon
inhalatiian, air passes through the upper airways into the ccntral airways.
The tenns "puhnonary region," "peripheral region" and the like are used
iatercbangeably
hereia to defina a region of the respiratory system where gas exchange occurs
between the lungs and
the circulatory systern, i.e., where oxygea enters the blood and carbon
dioxide leaves the blood.
The peripheral region includes generations 17 through 23 of the airways (see
FIG. 1). Drugs
delivered to this area generally have a systemic effect.
-7-
CA 02378913 2002-01-10
WO 01/05459 PCTIUSOO/19034
The terms "alveolar ducts," "alveoli" and the like refer to components in the
pulmonary
region of the lung which are approximately 3 in diameter where gas exchange
occurs between the
air in the lungs and the circulatory system.
The term "diameter" is used herein to refer to particle size as given in the
"aerodynamic"
size of the particle. The aerodynamic diameter is a measurement of a particle
of unit density that
has the same terminal sedimentation velocity in air under normal atmospheric
conditions as the
particle in question. This is pointed out in that it is difficult to
accurately measure the diameter of
small particles using current technology and the shape of such small particles
may be continually
changing. Thus, the diameter of one particle of material of a given density
will be said to have the
same diameter as another particle of the same material if the two particles
have the same terminal
sedimentation velocity in air under the same conditions. In connection with
the present invention, it
is important that particles, on average, have the desired diameter so that the
particles can be inhaled
and targeted to a specific area of the lungs. It is also important not to have
particles which are too
small in that such particles would be inhaled into the lungs and then exhaled
without depositing on
the lung tissue in the same manner that particles of smoke can be inhaled and
exhaled with only a
small amount of the particles being deposited on the lung tissue. An
acceptable range for particle
diameter varies depending on the area of the respiratory tract being targeted.
To target the alveolar
ducts and alveoli the particles should have a diameter in a range of about 0.5
to about 2 . To
target the area above the alveolar ducts and below the small bronchi the
diameter should be in the
range of from about 2 to about 4 , and to target the small bronchi and
above the particles should
have a diameter of from about 4 to about 8 .
The term "porous membrane" shall be interpreted to mean a membrane of material
in the
shape of a sheet having any given outer perimeter shape, but preferably
covering a package opening
which is in the form of an elongated rectangle, wherein the sheet has a
plurality of openings therein,
which openings may be placed in a regular or irregular pattern, and which
openings have a diameter
in the range of 0.25 to 4 and a pore density in the range of I x 1 04 to
about 1 x 108 pores per
square centimeter. Alternatively, the porous membrane may be merely an area of
the package which
has pores therein wherein the pores have a size and a density as described
above. The configuration
and arrangement of the pore density may be changed so as to provide pores
which are capable of
creating the desired amount of aerosol. For example, the porous membrane or
area of the container
may have some 10 to 10,000 pores therein which pores are positioned in an area
of from about 1
mm2 to about 1 cm2. The membrane is preferably comprised of a material having
a density in the
range of 0.25 to 3.0 mg/cmz, more preferably 1.7 mg/cm2, and a thickness of
about 2 to 20 , more
preferably about 8 to 12 . The membrane material is preferably hydrophobic
and includes
materials such as polycarbonates and polyesters which may have the pores
formed therein by any
-8-
CA 02378913 2009-02-05
suitable method including anisotropic etching or by etching through a thin
film of metal or other
suitable material. Pores can be created in the membrane which may be an area
of the container by
use of techniques such as etching, plating or laser drilling. The membrane
materials may have pores
with a conical configuration and have sufficient structural integritv so that
it is maintained intact
(will not rupture) when subjected to force in the amount of about 20 to 200
psi while the formulation
is forced through the pores. The membrane functions to form an aerosofized
mist when the
formulation is forced through it. Those skilled in the art may contemplate
other materials which
achieve this function as such materials are intended to be encompassed by this
invention.
The terms "treatment;" "treating," and the like are used interchangeably
herein to generally
mean obtaining a desired pharmacological and/or physiological effect. The
terms are used in a
manner somewhat differently than the terms are typically used in that what is
intended by the
method of treatment of the invention is to allow a patient to overcome an
addiction to_nicotine and
thereby allow the patient to quit smoking. The treating effect of the
invention provides a
psychological effect in that the invention originally delivers high doses of
nicotine in a manner that
simulates the nicotine delivery obtained from a cigarette. The patient then
becomes accustomed to
relying on the methodology of the invention to provide an immediate "rush" of
nicotine. Thereafter,
the particles of the aerosol are made larger. This prevents the particles from
penetrating deeply into
the lung and, therefore, to some extent, dirninishes the "rush" of nicotine.
However, the same
amount of nicotine is still given to the patient in order to satisfy the
overall nicotine craving.
Eventually, the treatment of the invention reduces the amount of nicotine so
as to allow the patient
to completely "wean" off of nicotine and to quit smoking.
The publications
discussed herein are provided solely for their stated disclosure prior to the
filing date of the present
application. Nothing herein is to be construed as an admission that the
invention is not entitled to
antedate such publications by virtue of prior invention. Further, the actual
publication date may be
different from that stated on the publication and as such may require
independent verification of the
actual publication dates.
GENERAL METHODOLOGY
The steady state delivery of nicotine as therapy for smokers wishing to quit
is characterized
by slow absorption and low blood levels of nicotine, which limits its utility.
The present invention
replaces the nicotine that a smoker receives from smoking a cigarette in a
therapeutically effective
manner by providing a rapid pulse of bioavailable nicotine to the smoker on
demand.
-9-
CA 02378913 2002-01-10
WO 01/05459 PCT/US00/19034
One means currently available for a true pulsatile, rapid onset replacement
therapy is
intravenous administration. Although preparations of nicotine appropriate for
intravenous
administration have been available for some time, intravenous cannulation as a
means for gaining
access to the circulation for the administration of nicotine on demand is not
a socially acceptable
alternative to cigarette smoking.
The treatment methodology of the present invention creates an aerosol of
nicotine particles.
The nicotine particles may be formed from any liquid containing nicotine
including a solution or
suspension of nicotine and aerosolized in any known manner including (1)
moving the formulation
through a porous membrane in order to create particles or (2) a dry powder
where the particles of
powder have been designed to have a desired diameter. The rate of particle
absorption is directly
proportional to the surface area of the tissue on which the particles are
deposited. Accordingly,
nicotine is absorbed more slowly through the mucosal membranes of the upper
respiratory tract
which have a smaller surface area than through the airways in the lower
respiratory tract which have
a larger surface area. Thus, the overall effect of increasing the size of the
nicotine particles is to
reduce the rate at which nicotine is absorbed into the circulation, thereby
reducing the smoker's
physiological dependence on the quick rush of nicotine experienced when
smoking.
METHOD OF TREATMENT
The penetration of aerosolized nicotine particles into the respiratory tract
is determined
largely by the size distribution of the particles formed. Larger particles, i.
e., particles with a
diameter _ 5 , deposit on the upper airways of the lungs (see FIG. 1).
Particles having a diameter in
a range of about >2 y to <5 /c penetrate to the central airways. Smaller
particles having a diameter
s2 /.c penetrate to the peripheral region of the lungs.
An important feature of the invention is that the treatment methodology begins
with
particles of a given size, carries out treatment for a given period of time
after which the particles are
increased in size. The particles initially administered to the patient
penetrate deeply into the lung,
i.e., the smallest particles (e.g. 0.5 to 2 ) target the alveolar ducts and
the alveoli. When the
deepest part of the lung is targeted with the smallest particles the patient
receives an immediate
"rush" from the nicotine delivered which closely matches that received when
smoking a cigarette.
These small particles can be obtained by milling powder into the desired size
and inhaling the
powder or by creating a solution or suspension and moving the solution or
suspension through the
pores of a membrane. In either case, the desired result is to obtain particles
which have a diameter
in the range of 0.5 to about 2 R. Those skilled in the art will understand
that some of the particles
will fall above and below the desired range. However, if the majority of the
particles (50% or more)
fall within the desired range then the desired area of the lung will be
correctly targeted.
-10-
CA 02378913 2002-01-10
WO 01/05459 PCTIUSOO/19034
The patient is allowed to continually, from time to time, target the outermost
area of the
lung with the smallest particles. For example, the patient would be instructed
to repeatedly
administer the smallest size particles when the patient would normally smoke a
cigarette. In this
manner, the patient will become accustomed to finding that the device
administers nicotine into the
patient in the same manner that a cigarette does. In one embodiment of the
invention the
concentration of the nicotine in the liquid formulation could be reduced
gradually over time. This
could be done over a sufficiently long period of time so as to allow the
patient to "wean" off of
nicotine. However, in a more preferred embodiment of the invention the amount
of nicotine is kept
substantially constant but the size of the aerosolized particles created are
increased.
The second phase of the treatment methodology is to increase the size of the
particles so as
to target the respiratory tract above the alveolar ducts and below the small
bronchi. This can
generally be accomplished by creating aerosolized particles of nicotine which
have a size and range
of about 2 to about 4 R. Administration is carried out in the same manner as
described above.
Specifically, the patient administers the aerosolized nicotine at the same
time when the patient
would be smoking a cigarette. Since the patient has become adjusted to
receiving the nicotine
"rush" from the smaller sized particles, the patient will expect and is
therefore likely to experience
the same "rush" when administering the slightly larger particles. However, the
effect will be less
immediate. This procedure is camed out over a period of time, e.g., days or
weeks. In one
embodiment of the invention it is possible to reduce the dose of aerosolized
nicotine delivered to the
patient during this second phase. However, the dose may remain constant.
The treatment can be completed after any phase, e.g. after the second phase.
However, in
accordance with a more preferred embodiment of the invention a third phase of
treatment is carried
out. Within the third phase the particle size of the aerosolized nicotine is
increased again. The
particles are increased to a size in a range from about 4 to about 8 or,
alternatively, perhaps as
large as 12 . These larger particles will target the upper airways. The
larger particles will give a
very small immediate "rush" but will still be absorbed through the mucous
membranes of the
patient's respiratory tract. Accordingly, the patient will be administering
nicotine doses which may
be the same as those doses administered at the beginning of treatment. At this
point the treatment
can take a number of different directions. The patient can attempt to stop
administration by
immediate and complete cessation of nicotine delivery. Alternatively, the
patient can try to wean off
of nicotine by delivering fewer doses during a given time period. In another
alternative, the same
size dose (volume of aerosol formulation) is administered and delivered,
creating the same amount
of aerosol, but wherein the aerosolized particles contain progressively less
nicotine (i.e., more dilute
concentration). The amount of nicotine can be decreased until the patient is
receiving little or no
-11-
CA 02378913 2002-01-10
WO 01/05459 PCTIUSOO/19034
nicotine. Those skilled in the art reading this disclosure will recognize
variations on the overall
method and methods for stopping treatment.
There are a number of aspects of the invention which will result in the
ability of the smoker
to use the invention and, eventually, quit smoking. Firstly, the invention is
particularly suited for
smokers in that smokers are accustomed to inhaling their source of nicotine.
Other treatments such
as those involving the transdermal delivery of nicotine via a nicotine "patch"
or buckle delivery via a
nicotine "gum" do not match the means which a smoker usually obtains nicotine.
Further, the present invention provides a method wherein the patient obtains
an influx of
nicotine into the circulatory system at a rate which substantially matches the
rate which nicotine
would enter the circulatory system when smoking. This is obtained because, at
least at first, the
invention provides sufficiently small particles such that they are inhaled
deeply into the lung, i.e.
50% or more of the particles are inhaled deeply into the lung and thereby
quickly enter the patient's
circulatory system.
Thirdly, the present invention is advantageous in that the rate at which the
delivered nicotine
enters the circulatory system can be gradually decreased by gradually
increasing the size of the
aerosolized particles delivered to the patient. This can be done over any
desired period of time and
in any desired number of phases.
Lastly, the invention provides a means whereby the amount of nicotine
delivered to the
patient can be gradually decreased in a number of different ways. Firstly, it
can be decreased by
decreasing the concentration of nicotine in the aerosolized formulation.
Secondly, it can be
decreased by merely decreasing the number of administrations of aerosolized
doses. Thirdly, it can
be decreased by decreasing the size of the dose aerosolized and inhaled by the
patient.
One aspect of the invention is a method of treatment, comprising:
(a) aerosolizing a formulation comprised of nicotine creating aerosolized
particles
which are sufficiently small to target a particular lower area of the
respiratory tract such as the
alveoli. The particles targeting this area will have a relatively small size,
e.g. 0.5 micron to about 2
microns in diameter.
(b) in the next step the patient inhales the aerosolized particles of (a) into
the respiratory
tract, preferably targeted to a specific area of the lower respiratory tract
where the deposited
particles cross into the patient's circulatory system.
In step (c), steps (a) and (b) are repeated a plurality of times.
Specifically, the patient may
repeat these steps any number of times such as every time the patient would
normally smoke a
cigarette. At this point the patient could continue the treatment protocol in
this manner and
gradually decrease the number of times the patient administers aerosolized
nicotine until the patient
is no longer addicted to nicotine. Decreasing the amount of aerosolized
nicotine could also be done
-12-
CA 02378913 2002-01-10
WO 01/05459 PCT/US00/19034
by decreasing the concentration of nicotine within the aerosolized particles
decreasing the
concentration of nicotine in the formulation and/or decreasing the size of the
aerosolized dose.
Preferably the method of the invention continues with a step (d) which
involves aerosolizing
formulation comprised of nicotine in order to create aerosolized particles
which are larger in size
than the aerosolized particles produced in step (a). These larger particles
are directed towards a
particular area of the patient's respiratory tract, e.g. the mid-region of the
patient's respiratory tract.
(See Figures 1 and 2) These particles could have a size in the range of about
2 microns to about 4
microns.
In the following step (d) the patient inhales the aerosolized particles of (d)
thereby targeting
the particular desired area of the patient's respiratory tract such as the mid
region. Thereafter, steps
(d) and (e) are repeated a plurality of times. At this point the patient can
decrease the amount of
nicotine being delivered as indicated in the same manner as indicated above
step (c). Alternatively,
the method of the invention can be continued so that a third phase of
treatment can be carried out
which phase is similar to the two phases described above. In accordance with
the above invention it
is possible to carry out the treatment in any number of phases. For example,
the treatment could
involve as many as 24 phases which target specific defined regions of a
patients respiratory tract
using particles which are continually larger in size in each of the 24 phases
(see Figure 1 and Table 1
below). Because it may not be practical to specifically design the particles
so that they are all larger
in each of the phases the formulations may be designed so that a certain
percentage of the particles
within each phase of delivery is larger than the particles in the preceding
phase.
The method of the invention can be carried out using 1 to 24 different phases
with each
phase targeting a higher level of the respiratory tract (See Table 1). The
higher levels of the
respiratory tract can be targeted using larger and larger particles.
-13-
CA 02378913 2007-05-16
Table 1:
Subdivision of the Respiratory Tree
Generation Name
0 Trachea
1 Prinlary bronchi
2 Lobar bronchi
3 Segmental bronchi
4 subsegmental bronclu
5 Small bronchi
t
11 Bronchioles, pritnary and secondary
13
14 Terminal bronchioles
16 Respiratory bronchioles
!
18
19 Alveolar ducts
23
24 Alveoli
NICOTINE DELIVERY DEVICES
Precision delivery of sma11 molecule drugs via the lung for systemic effect is
possible. An
electronic inhaler capable of delivering a liquid formulated drug stored in a
unit dose packages has
been described and disclosed in U.S. Patent No. 5,718,222 entitled "Disposable
Package for Use in
Aerosolized Delivery of Drugs ` A formulation of nicotine
can be prepared for delivery with this system. Quantitative delivery of
nicotine on demand provides
a mechanism for nicaRine replacement therapy which is unlikely to be
associated with recidivism
precipitated by the symptoms of physical withdrawal.
In the present invention, a nicotine formulation is forced through the
openings or pores of a
porous membrane to create an aerosol. In the prefeired embodiment, the
openings are all uniform in
size and are positioned at uniform distances from each other. However, the
opeaings can be varied
in size and randomly placed on the membrane. If the size of the openings is
varied, the size of the
particles formed will also vary. In general, it is preferable to maintain
uniform opening sizes in
order to create uniform particle sizes, and it is particularly preferable to
have the opening sizes
within the rangc of about 0.25 p to abrnrt 6 which will create particle
sizes of about 0.5 p to 12 p
which are preferred with respect to inhalation applications. When the openings
have a pore size in
the range of 0.25 to I they will produce an aerosol having particle sizes
in the range of 0.5 to 2
-14-
CA 02378913 2002-01-10
WO 01/05459 PCT/USOO/19034
, which is particularly useful for delivering nicotine to the alveolar ducts
and alveoli. Pore sizes
having a diameter of about 1 to 2 will produce particles having a diameter
of about 2 to 4 ,
which are particularly useful for delivering nicotine to the area above the
alveolar ducts and below
the small bronchi. A pore size of 2 to 4 will create particles having a
diameter of of 4 to 8 ,
which will target the area of the respiratory tract from the small bronchi
upward.
Increasing the size of the openings of the porous membranes produces nicotine
particles of
increasing size. A strategy in which the blood level of nicotine is reduced
gradually will be the most
effective in treating the symptoms of withdrawal, and thereby increase the
chances of successful
smoking cessation. In one embodiment of the invention, the size of the
aerosolized nicotine
particles is increased in a stepwise manner by using porous membranes that
create "monodisperse"
aerosols, wherein all the particles within the aerosol created have
essentially the same particle size.
Nicotine particles of increasing size are produced by using membranes of
increasing pore sizes.
In another embodiment, the size of the aerosolized nicotine particles is
increased in gradient
fashion by using porous membranes that create "multi-disperse" aerosols,
wherein the particles
within the aerosol created have different particle sizes. Membranes which have
an increasing range
of pore sizes are used to produce nicotine particles of increasing size.
Nicotine can be administered orally. However, after oral administration it is
absorbed from
the gut into the portal blood and degraded promptly by the liver. Thus,
insignificant amounts reach
the patient's systemic circulation. Nicotine can also be administered
parenterally. However, when
so administered it is rapidly absorbed and metabolized making it difficult to
sustain therapeutic
levels in plasma over time. In view of such, effective therapy has been
carried out using other
means of delivery (e.g., transdermal patches, gum). The present invention uses
intrapuhnonary
delivery to avoid first pass liver metabolism and to obtain quick infusion
into the patient's systemic
circulatory system. The present invention administers sufficient nicotine by
inhalation to
temporarily produce a rapid increase in the patient's blood level, and
thereafter allow the patient's
nicotine level to return to a therapeutically effective level.
Because intrapulmonary administration is not 100% efficient, the amount of
drug
aerosolized will be greater than the amount that actually reaches the
patient's circulation. For
example, if the inhalation system used is only 50% efficient then the patient
will aerosolize a dose
which is twice that needed to raise the patient's nicotine level to the extent
needed to obtain the
desired results. More specifically, when attempting to administer 1 mg of
nicotine with a delivery
system known to be 50% efficient, the patient will aerosolize an amount of
formulation containing
about 2 mg of nicotine.
A device comprised of a container that includes an opening covered by a porous
membrane,
such as the device disclosed in U.S. Patent No. 5,906,202, may be used to
deliver nicotine. The
-15-
CA 02378913 2007-05-16
device may be designed to have the shape and/or bear the markings of a pack of
cigarettes, and may
include the scent of tobacco. These features and others that address the
behavioral component of
cigarette smoking may enhance the effectiveness of the method described
herein.
DOSING
Cigarettes contain 6 to 11 nig of nicotine, of which the smoker typically
absorbs 1-3 mg; see
Henningfield NEngi JMed 333:1196-1203 (1995). Factors influencing nicotine
absorption include
subject-dependent factors, such as smoking behavior, lung clearance rate,
etc., morphological
factors, and physiological factors, such as tidal volume, inspiratory and
expiratory flow rate, particle
size and density. See Darby et al., Clin Pharmacokinet 9:435-439 (1984). The
systemic dose of
nicotine per puff is extremely variable, however, peak plasma concentrations
of 2540 ng/mL of
nicotine, achieved within 5-7 minutes by cigarette smoking, are believed
typical. In accordance with
the present invention, 0.1 mg to 10 mg, preferably I to 3 mg, and more
preferably about 2 mg of
nicotine are delivered to the lungs of the patient in a single dose to achieve
peak blood plasma
concentrations of 15-40 ng/mL.
= The amount of a nicotine administered will vary based on factors such as the
age, weight
and frequency of smoking or nicotine tolerance of the smoker. Other factors,
such as daily stress
patterns, demographic factors may also determine, in part, the amount of
nicotine sufficient to
satisfy the smoker's craving for nicotine. Administering nicotine using the
methods of the present
invention can involve the daily administration of anywhere from 5 mg to 200 mg
of nicotine, but
more preferably involves the administration of approximately 10 to 100 mg per
day.
It is noted that nicotine can be administered in toxic amounts. Care should be
taken not to
overdose the patient. The amount of nicotine which an individual can tolerate
will vary on a number
of factors including size, sex, weight and ainount of cigarette smoking the
patient is accustomed to.
In order to avoid overdosing it is possible to program a lock-out system into
the delivery device
wbich prtweats administration of aerosolized doses beyond a given point. Such
a system is
described within U.S. Patent 5,735,263 issued Apri17, 1998.,
The nicotine is in a liquid form or is dissolved or dispersed within a
pharmaceutically
acceptable, liquid excipient material to provide a liquid, flowable
formulation which can be readily
aerosolized. The container will include the formulation having nicotine
therein in an amount of
about 10 mL to 300 mL, more preferably about 200 mL. The large variation in
the amounts which
might be delivered is due to different delivery efficiencies for different
devices. Administration may
involve several inhalations by the patient, with each inhalation providing
nicotine from the device.
For example, the device can be programmed so as to release the contents of a
single container or to
-16-
CA 02378913 2002-01-10
WO 01/05459 PCT/US00/19034
move from one container to the next on a package of interconnected containers.
Delivering smaller
amounts from several containers can have advantages. Since only small amounts
are delivered from
each container and with each inhalation, even a complete failure to deliver
nicotine with a given
inhalation is not of great significance and will not seriously disturb the
reproducibility of the dosing
event. Further, since relatively small amounts are delivered with each
inhalation, the patient can
safely administer a few additional micrograms (or milligrams) of nicotine
without fear of
overdosing.
In one embodiment of the invention the patient is treated in the three
different phases. In the
first phase the aerosolized liquid particles or dry powder particles have a
size and a range of 0.5 to
about 2 . The particles of nicotine having this size are administered in a
dosage amount which is
substantially equivalent to the doses or amount which the patient would
received from a single
cigarette or, alternatively, the dosage amount which the patient would
received from a single puff on
a single cigarette. Assuming that the patient receives the dosage amount of a
single cigarette then
the patient will be administered approximately 1 to 3 mg of nicotine each time
the formulation is
aerosolized. The particles having a size of 0.5 to about 2 will be
administered to the patient over
a plurality of days (e.g., 2 to 7 days) or perhaps a plurality of weeks (e.g.,
2 to 4 weeks). If the
device and/or dosage containers are designed to deliver a dosage equivalent to
a puff on a cigarette
then substantially smaller doses are delivered. If each dose corresponds to a
puff on a cigarette then
a patient may be directed to continually take aerosolized doses equivalent to
a cigarette puff over a
period of one to ten minutes or any period of time equivalent to what that
patient normally takes to
smoke one cigarette. This constitutes the first phase of treatment.
After completing the first phase of the treatment the method of the invention
may be
completed. However, as indicated above the method may be continued by
repeating phases such as
the first phase using continually larger particles and/or continuing more
dilute solutions of nicotine
and/or smaller doses of nicotine.
Within the second phase of treatment the patient is preferably administered
the same dosage
amount of nicotine with each inhalation, e.g., the patient is administered 1
to 3 mg of nicotine each
time formulation is aerosolized. However, during the second phase the size of
the particles is
increased to a size and range from 2 to about 4 R. The particle size is
increased in order to target
an area of the lungs where the nicotine will be absorbed into the circulatory
system more slowly.
Specifically, the larger particles target an area of the lungs above the
alveolar ducts and below the
small bronchi. Administration is carried out over a plurality of days or a
plurality of weeks in the
same manner as indicated above. Within all phases the patient preferably
administers nicotine from
a device of the invention when the patient would normally smoke a cigarette.
The treatment can be
-17-
CA 02378913 2002-01-10
WO 01/05459 PCT/USOO/19034
completed pursuant to the present invention by using only the two phases.
However, it is preferable
to include three or more phases.
In accordance with the third phase, the same dose is administered each time
nicotine
formulation is aerosolized. Accordingly, 1 to 3 mg of nicotine is delivered to
the patient at each
dose. However, the dose is delivered by using aerosolized particles which have
a diameter of 4 or
more, e.g., in the range of from 4 to about 8 R. These larger particles are
designed to target the
area of the respiratory tract at the small bronchi or higher. When the
nicotine targets the upper
airways it will not immediately enter the patient's circulatory system.
However, the nicotine will,
eventually, cross the mucous membranes of the upper respiratory tract and
enter the circulatory
system. Thus the patient will be administered nicotine but will become less
accustomed to having
the immediate "rush" obtained from smoking. Thus, within the third phase the
patient has been
weaned away from the need for the "rush" of nicotine. The third phase is then
used to continually
reduce the number of administrations needed and thereby reduce the amount of
nicotine
administered. By this process the patient's dependency on nicotine is slowly
reduced and then
eliminated thereby allowing the patient to quit smoking.
When nicotine enters the circulatory system of a human patient it is oxidized
to cotinine
within four to six hours. The present invention includes the administration of
cotinine and other
nicotine derivatives provided such derivatives do not result in unacceptable
adverse effects.
INDICATIONS
The method of the invention has applicability to smokers wishing to quit or
trying to quit
who have experienced all or any of the nicotine withdrawal symptoms associated
with smoking
cessation, such as craving for nicotine, irritability, mood ability,
frustration or anger, anxiety,
drowsiness, sleep disturbances, impaired concentration, nervousness,
restlessness, decreased heart
rate, increased appetite and weight gain.
While particularly applicable to smoking cessation, pulmonary administration
of nicotine
could be of value for the treatment of other diseases, such as for patients
suffering from
neurodegenerative diseases, psychiatric disorders and other central nervous
system disorders
responsive to nicotinic receptor modulation (see U.S. Patent Nos. 5,187,169;
5,227,391; 5,272,155;
5,276,043; 5,278,176; 5,691,365; 5,885,998; 5,889,029; 5,914,328). Such
diseases include, but are
not limited to, senile dementia of the Alzheimer's type, Parkinson's disease,
schizophrenia,
obsessive-compulsive behavior, Tourette's Syndrome, depression, attention
deficit disorder,
myasthenia gravis and drug addiction.
-18-
CA 02378913 2007-05-16
FORMULATIONS
Pharmaceutical grade nicotine can be produced as a colorless to pale vellow
liquid. The
pure liquid could be aerosolized and inhaled by itself. Alternatively, a
formulation may include a
buffer to enhance absorption. Any absorption enhancers including arnmonia
could be used with the
formulation. However, a typical formulation is only nicotine dissolved in
water or dry powder
nicotine. Methods of formulating liquids and liquid inhalers are disclosed in
U.S. Patent Nos.
5,364,838; 5,709,202; 5,497,763; 5,544,646; 5,718,222; 5,660,166; 5,823,178;
and 5,910,301.
Formulations of nicotine include
aqueous formulations, aqueous saline formulations, and ethanol formulations.
All of these
formulations may be included with additional components such as peimeation
enhancers, buffers,
preservatives and excipient and carrier components and additives nonnally
included within
fornwlaiions for aerosolized drug delivery.
Nicotine is freely soluble in water. An aqueous nicotine solution may be
readily aerosolized
and inhaled. The nicotine solution can be placed in a low boiling point
propellant in a pressurized
canister and released using a conventional metered dose inhaler (MDI) dcvice.
Preferably, the MDI
device is modified so that the aerosolized dose is released each time at the
same inspiratory flow rate
and inspiratory volume. When tbis is done the patient is more likely to
receive the same dose each
time. A device for obtaining repeating dosing with an MDI canister is taught
in U.S. Patent No.
5,404,871.
In accordance with the present invention it is preferable to load the nicotine
solution inRo a
container which opens to a porous membrane. When the formulation is forced
through the
mecnbrane it is aarosolized. Such a container is taught in U.S. Patent No.
5,497,763 and is loaded
into a device and delivered via a nwthod as taught in U.S. Patent No.
5,823,178.
A dry powder formulation caanprising a pharinacologically acceptable salt of
nicotine alone
or with additives such as components to prevent the particles froin stiaking
together may be used
SUPPLEMENTAL TREATMENT METHODOLOGY
Smokers wishing to quit may be treated solely with respiratory nicotine as
indicated above,
i.e. by intrapulmonary delivery. However, it is possible to treat such
patients with a combination of
pularonary administration and other mean.s of administration, such as
traasderinal adminisbration.
Transdermal nicotine is preferably administered to maintain a steady state
level of nicotine within
the circulatory system Nasal or buccal formulation could be used for nasal or
buccal delivery which
could supplement aerosolized delivery.
-19-
CA 02378913 2002-01-10
WO 01/05459 PCT/US00/19034
Based on the above, it will be understood by those skilled in the art that a
plurality of
different treatments and means of administration can be used to treat a single
patient. For example,
a patient can be simultaneously treated with nicotine by transdermal
administration, nicotine via
pulmonary administration, in accordance with the present invention, and
nicotine which is
administered to the mucosa.
The instant invention is shown and described herein in a manner which is
considered to be
the most practical and preferred embodiments. It is recognized, however, that
departures may be
made therefrom which are within the scope of the invention and that obvious
modifications will
occur to one skilled in the art upon reading this disclosure.
-20-