Note: Descriptions are shown in the official language in which they were submitted.
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
LOW VISCOSITY ALKYL DIPHENYL OXIDE SULFONIC ACID BLENDS
This invention is directed to surfactant materials and compositions and to
methods for
making concentrated intermediates with good handling properties.
Rheological behavior is an important consideration in a liquid. An appropriate
viscosity in a
liquid product enables it to either be (a) usefully consumed as received or
(b) conveniently
received into a conditioning system for further adjustment of the viscosity to
a useful value
for the application. The utility of components used in a liquid blend is also
affected by
to viscosity; and, in this regard, highly concentrated alkyl diphenyl oxide
sulfonic acid as
manufactured has a relatively high liquid viscosity. DOWFAXT"~ surfactants
(DOWFAX is a
trademark of The Dow Chemical Company) are good examples of products from
alkyl
diphenyl oxide sulfonic acids. Highly concentrated alkyl diphenyl oxide
sulfonic acids have
solids concentrations from 60 percent to 95 percent and are denoted as High
Actives Acid,
15 or HAA, herein. While the high viscosity can be moderated to acceptable
levels with dilution
in some HAAs, other HAAs (for example DOWFAX Detergent Acid) demonstrate an
apparent liquid crystal region in the 40 percent to 80 percent solids range.
The liquid crystal
region is characterized by very high viscosity (greater than 1,000,000
centipoise) and the
material is accordingly too viscous at temperatures below 40 degrees C for
convenient
2o handling. When the material is heated to render the viscosity acceptably
convenient, the
material is unfortunately too hot for safe handling outside of relatively
expensive blending
environments optimized for safe operations at such temperatures. As noted
previously,
DOWFAX surfactants are good examples of products from alkyl diphenyl oxide
sulfonic
acids. DOWFAX surfactants have two ionic charges per molecule. Each molecule
consists
25 of a pair of sulfonate groups on a diphenyl oxide backbone. This double
charge density is
largely responsible for excellent solvating and coupling action in this
molecular family.
DOWFAX surfactants have excellent solubility and stability in concentrated
electrolytes and
are resistant to oxidative and thermal degradation. DOWFAX surfactants have
hydrophobes
of a linear or branched alkyl group comprised of from six to sixteen carbons,
depending upon
3o the particular surfactant. Example utility of DOWFAX surfactants is in
textile dyeing, polymer
emulsion processing, agricultural chemical manufacturing, and (as an additive)
cleaning fluid
formulating.
It has been desired for some time to be able to sell High Active Acid as a
concentrated
35 product for use in formulations prior to neutralization in order to
minimize shipping and
handling costs respective to the surfactant product water component; however,
(a) the
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
addition of water to HAA at room temperature has traditionally not been
convenient because
of the high viscosity of the HAA at room temperatures and (b) most customers
for the
surfactant product are not conveniently availed of a blending environment for
safe handling
of hot HAA. Speculated benefits, therefore, of efficiency in shipping and
handling and the
benefits in safety from an HAA which could be blended into water at room
temperature have
not been realized. What is needed is an HAA having a useful viscosity at room
temperature
which can be added to water. The present invention solves this problem by
providing HAA
formulation embodiments and methods for their formulation so that an HAA
having a
relatively low viscosity at room temperature is provided.
to
The room temperature viscosity of an alkyl Biphenyl oxide sulfonic acid blend
is beneficially
controlled according to the invention by admixing a fatty acid having a
carboxylic chain
length between 1 and 12 into the alkyl Biphenyl oxide sulfonic acid blend to
provide between
weight percentage and 50 weight percentage of fatty acid in the admixture. In
further
detail, the preferred embodiments are described as:
1 - A method for viscosity control in an alkyl Biphenyl oxide sulfonic acid,
characterized by
the step of:
admixing a fatty acid having a carboxylic chain length between 1 and 12 into
the alkyl
2o Biphenyl oxide sulfonic acid blend to provide between 5 weight percentage
and 50 weight
percentage of fatty acid in the admixture.
2 - A method for preparation of an alkyl Biphenyl oxide sulfonic acid blend
characterized by
the steps of:
admixing formic acid, acetic acid, propionic acid, butanoic acid, pentanoic
acid, valeric acid,
hexanoic acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid,
undecanoic acid,
or dodecanoic acid to provide between 5 weight percentage and 50 weight
percentage of
fatty acid in an admixture with an alkyl Biphenyl oxide characterized by
/ \
and
2
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
/ ~ o /
R _R
where R is an alkyl radical having between 6 and 16 carbon atoms; and
sulfonating said admixture with a sulfonating agent.
3 - An alkyl diphenyl oxide sulfonic acid blend having between 5 weight
percentage and 50
weight percentage of a fatty acid with a carboxylic chain length between 1 and
12.
l0 4 - An admixture composition of:
formic acid, acetic acid, propionic acid, butanoic acid, pentanoic acid,
valeric acid, hexanoic
acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid, undecanoic
acid, or
dodecanoic acid to provide between 5 weight percentage and 50 weight
percentage of fatty
acid in the admixture composition; and
alkyl diphenyl oxide characterized by
/ ~ ~ /
2o R - and
/ ~ .
R R
where R is an alkyl radical having between 6 and 16 carbon atoms.
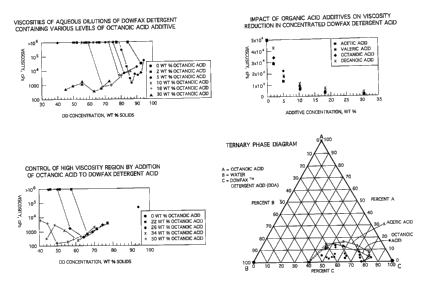
Turning now to an overview of the Figures, Figure 1 shows the impact of
various levels of
octanoic acid upon the viscosity of a DOWFAX alkyl diphenyl oxide sulfonic
acid surfactant
blend.
Figure 2 shows the impact of various levels of octanoic acid upon the
viscosity of a
DOWFAX alkyl diphenyl oxide sulfonic acid surfactant blend in the high
viscosity range.
Figure 3 shows the comparative impact of acetic, valeric, octanoic, and
decanoic fatty acids
on the viscosity of a DOWFAX alkyl diphenyl oxide sulfonic acid surfactant
blend.
3
CA 02379347 2002-O1-15
WO 01/08793 PCTNS00/18287
Figure 4 shows a ternary phase diagram showing significant liquid crystal
phase regions for
water, DOWFAX Detergent Acid, and fatty acid (acetic acid and octanoic acid).
In further discussion of details in the preferred embodiments, alkyl diphenyl
oxide sulfonate
surfactants are a Friedel-Crafts reaction product of an olefin and diphenyl
oxide using AICI3
as a catalyst as indicated in Formula I.
Forrmila I
O
~ ~ ~ ~ A1C13 R
+ Olefin ~ ~ O
R R
biphenyl oxide is present in excess and is recycled. The reaction yields a
mixture of
i5 monoalkyl Biphenyl oxide and dialkyl Biphenyl oxide. The ratio of
monoalkylation to
dialkylation can be optimized depending on the end use of the products.
The next step in the process is the reaction of the alkylate with a
sulfonating agent. This
reaction (Formula II) is conducted in a solvent to dilute the reactant and to
act as a diluent
2o for the S03 used in the reaction.
4
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
Formula II S03H
R
O
S03 R SO H S03H
3
Solvent SO H
3
R R / ~ O
to R R
S03H
O
15 R ~S03H ~R
The reaction generally yields a mixture of monosulfonates and disulfonates
according to
Formulas III - VI. The level of disulfonation is determined by the end use of
the product.
Generally, the disulfonation level is above 80 percent. The predominant
component in the
2o commercial reaction mixture is the monoalkyl diphenyl oxide disulfonate
(MADS) of Formula
IV, with monoalkyl Biphenyl oxide monosulfonate IMAMS) of Formula III, dialkyl
Biphenyl
oxide monosulfonate (DAMS) of Formula V, and dialkyl Biphenyl oxide
disulfonate (DADS) of
Formula VI essentially providing the remainder.
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
Formula III
S03H
/ ~ O /
R
Formula N
/ ~ /
t0 R S03H S03H
Formula V S03H
/ ~ O /
15 R ~R
Formula VI S03H
ao / ~ /
R ~S03H ~R
Alkyl diphenyloxide sulfonates and their traditional methods of preparation
are well-known
and reference is made thereto for purposes of describing this invention.
Representative
25 methods of preparation and handling are disclosed in U.S. Patents
2,990,375; 3,264,242,
3,634,272; 3,945,437; and 5,015,367. The commercially available species are
predominantly (greater than 85 percent) disulfonates (the DADS and MADS
described
above) and are a mixture of mono- and di- alkyl with the percentage of
dialkylation (the
DADS and DAMS described above) being 5 to 25 and the percentage of
monoalkylation (the
3o MAMS and MADS described above) being 75 to 95 percent. Most typically, the
commercially available species are 85 percent monoalkyl and 15 percent
dialkyl.
The traditional method taught by Steinhauer et al. (U.S. Pat. No. 2,990,375)
outlines a series
of steps, the first step comprising preparing an alkyldiphenyl ether by
reacting an olefin or an
6
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
olefin halide, such as tripropylenes, tetrapropylenes, pentapropylenes or
dodecyl bromide,
with diphenyl ether at a temperature between 50° C and 100° C in
the presence of the
Friedel-Crafts catalyst. The reaction mixture is washed with water to remove
the catalyst,
the phases separated, and the organic-rich phase subjected to distillation to
obtain a fraction
consisting of a mixture of monoalkylated Biphenyl ether and dialkylated
Biphenyl ether. The
number of alkyl substituents per Biphenyl ether molecule can be controlled by
adjusting the
relative proportions of the reactants. Alternatively, the distillation can be
performed so as to
separate the monoalkylated and dialkylated Biphenyl ethers from one another
and from lower
or higher boiling ingredients after which the monoalkylated and dialkylated
Biphenyl ether
to fractions can be combined at a desirable ratio.
The mixture of monoalkylated and dialkylated Biphenyl ethers is subsequently
reacted with a
sulfonating agent, such as chlorosulfonic acid, sulfuric acid, or sulfur
trioxide, in an inert
solvent.
The general process of today uses reaction of an unsaturated hydrocarbon such
as an
alpha-olefin in the range of 6 to 16 carbons with Biphenyl oxide in the
presence of AIC13.
Reaction of alpha-olefins in the higher range of 18-30 carbons with Biphenyl
oxide in the
presence of AIC13 holds some promise for fulfilling future surfactant needs.
The ratio of
2o mono- to dialkylation is controlled by the ratio of olefin to Biphenyl
oxide. Recycled excess
Biphenyl oxide is purified and reused. The rate of the reaction and the yield
are controlled by
the amount of catalyst and temperature of the alkylation. Excessively high
temperatures as
well as excessive amounts of catalyst yield higher levels of dialkylation and
trialkylation. Low
temperatures result in a low conversion of olefin. The ratios of
concentration, catalyst and
temperature are critical in keeping the reaction products consistent
throughout the
production cycle. The catalyst is removed from the process stream and the
crude reaction
mixture is then stripped of excess Biphenyl oxide. Additional purification is
optionally
effected prior to the sulfonation reaction.
3o Sulfonation is generally carried out in a solvent. The solvent provides
value in distributing
the sulfonating agent, preventing localized burning and yield loss of the
reaction product, and
acting as a heat removal medium in control of the reaction process
temperature. Current
commercial process routes use sulfur dioxide, methylene chloride, or air as
reaction
solvents. The air sulfonation process eliminates the need for the removal and
recycle of the
liquid reaction solvent and is amenable to onsite generation of S03. Liquid
solvents require
7
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
the use of liquid S03 that is diluted into the solvent prior to addition to
the sulfonation
reactors. Sulfur trioxide and chlorosulfonic acid are the two most common
sulfonating
agents.
After sulfonation, (1 ) the sulfonic acid is separated from its diluent, (2)
the anhydrous acid
(HAA) is diluted with water, and (3) neutralization of the diluted acid is
optionally executed
with an alkaline base such as sodium hydroxide. The material is packaged and
sold in
drums or bulk shipments as the customer requires.
to The high viscosity of concentrated HAA derives from properties related to
liquid crystal
presence. This effect initiates at hydrophobe chain lengths above 6, is
increasingly
pronounced in observed samples to chain lengths of 16, and is expected to
extend with
greater significance to cases such as those which are contemplated via
reaction of alpha-
olefins in the higher range of 18-30 carbons with diphenyl oxide. Accordingly,
a liquid crystal
15 disrupter, or crystal structure breaker, is highly desirable as an additive
for enabling useful
viscosity in a useful HAA solids region (that is in an 60-95 percent solids
range). In this
regard, an additional component in the blend is most desirable which disrupts
High Actives
Acid (HAA) liquid crystal structure without imparting undesirable attributes
to the resulting
blend. In this regard, dimethylformamide (DMF) and methyl formamide (MF)
effectively
2o disrupt the liquid crystal structure in alkyl diphenyl oxide sulfonic acid
blends used in deriving
DOWFAX surfactants; but DMF and MF are not favored for use because of asserted
health
concerns.
It has been discovered that addition of fatty acids, for instance, caprylic
(octanoic) or lauric
25 acid, to highly concentrated surfactant sulfonic acid can greatly reduce
the surfactant
viscosity and improve handling characteristics of HAA. The use of such an
additive to form
particular blends enables the manufacture and use of concentrated acid forms
of these
surfactants.
3o In an alternative embodiment, admixing the fatty acid with the alkyl
Biphenyl oxide prior to
sulfonation also provides reduction of surfactant viscosity and improved
handling
characteristics in the HAA material.
Formic acid, acetic acid, propionic acid, butanoic acid, pentanoic acid,
valeric acid, hexanoic
35 acid, heptanoic acid, octanoic acid, nonanoic acid, decanoic acid,
undecanoic acid, and
8
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
dodecanoic (lauric) acid all provide benefit in low viscosity HAA formulations
as further
described with reference to the sample data in the Examples and Figures.
EXAMPLE 1:
Samples containing straight-chain carboxylic acids from formic to lauric acid
were blended
with a representative alkyl diphenyl oxide sulfonic acid surfactant with a 16-
carbon
hydrophobe side chain (DOWFAX Detergent Acid, 94 wt percent concentration) at
levels of
wt percent carboxylic acid based upon DOWFAX amount. The viscosities of these
to samples were measured at 40 °C. The results are listed in Table 1.
A Brookfield programmable rheometer, Model HDAV-III, was used to measure the
viscosity
of DOWFAX acid samples. The spindle size used was SC4-21. The viscosities of
the
samples were measured at 40 °C, a temperature at which the Thermosel
temperature control
t5 stage was stable.
Approximately 8 mLs of sample were placed into the rheometer chamber. The
spindle was
inserted into the chamber so that the sample covered to 1/8 inch of the
spindle shaft. The
chamber was placed into the temperature control stage and the spindle
connected to the
rheometer. The rheometer was auto-zeroed. Stirring was started at 1 RPM and
the sample
was allowed to temperature equilibrate for ten minutes. After the ten minutes,
the motor was
stopped, the sample was allowed to sit for five minutes, then the motor was
started again. A
reading was taken after the spindle made 5 revolutions. The stirring was
increased and the
torque recorded until the allowable torque range on the instrument was
exceeded. The
equation below was used to convert torque to viscosity in units of cP:
Viscosity = 100/RPM * TK * SMC * Torque
Torque constant (TK) = 2
Spindle Multiply Constant (SMC) = 5
35
9
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
TABLE 1
Structure - Viscosity Modification Attributes
of Carboxylic Acid Additives in DOWFAX Detergent Surfactant
[9.1 wt percent carboxylic acid, 85.5 wt percent DOWFAX Detergent, 5.4 wt
percent water]
Carboxylic Acid Viscosity,
cP
Common (Systematic) (C~ 40.8 C)
Formic (methanoic) 7030
Acetic (ethanoic) 5847
Propanoic (propanoic) 4965
Butyric (butanoic) 5227
2o Valeric (pentanoic) 4970
Caproic (hexanoic) 6333
Enanthic (heptanoic) 6290
Caprylic (octanoic) 9360
Pelargonic (nonanoic) 9120
3o Capric (decanoic) 15820
Lauric (dodecanoic) 18040
EXAMPLE 2:
Samples containing a variety of concentrations (from 2 to 50 wt percent based
upon
DOWFAX acid amount) of a representative carboxylic acid, octanoic acid, were
blended with
a representative alkyl diphenyl oxide sulfonic acid surfactant with a 16-
carbon hydrophobe
side chain (DOWFAX Detergent Acid, or DD-HAA in Figures 1 and 2) at a variety
of aqueous
4o dilution levels (from 44 to 94 wt percent DOWFAX acid). Each sample was
blended until
homogeneous. The viscosities of these samples were measured at 40 °C by
the method
indicated in Example 1. The results of these measurements are shown in Figures
1 and 2.
Some of the samples (a) exhibited liquid crystal behavior with very high
viscosities and (b)
~5 turned solid-like in consistency. These samples typically exhibited
viscosities exceeding the
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
upper measuring limit of the rheometer (1,000,000 cP), and these samples are
shown as
having viscosities of 1,000,000 cP in the Figures. The behavior of DOWFAX
Detergent Acid
containing no carboxylic acid ("0 wt percent OA") is shown for comparison
purposes in both
Figures 1 and 2.
The onset of the liquid crystal phase in Figure 1 is apparent at the rapid
rise of viscosity with
decrease of solids in the 69 percent to 90 percent solids range (depending on
the particular
concentration of octanoic acid). Only at 30 percent octanoic acid is the
liquid crystal phase
evidently suppressed.
EXAMPLE 3:
Samples containing a variety of concentrations (from 2 to 30 wt percent) of
four
representative carboxylic acids (acetic, valeric, octanoic, and decanoic
acids) each were
blended with a representative alkyl Biphenyl oxide sulfonic acid surfactant
with a 16-carbon
hydrophobe side chain (DOWFAX Detergent Acid, 94 wt percent concentration).
Each
sample was blended until homogeneous. The viscosities of these samples were
measured
at 40 °C by the method indicated in Example 1. The results of these
measurements are
shown in Figure 3. The behavior of DOWFAX Detergent Acid containing no
carboxylic acid
(at "0 wt percent additive concentration" on the graph) is shown for
comparison.
Comparison of the data for all acids at concentrations above 0 percent in
Figure 3 with the 0
percent case help to further illustrate the general viscosity reducing
influence of fatty acids
on an HAA such as the tested DOWFAX Detergent Acid.
The data of Figure 3 indicate a higher significance of fatty acid chain length
toward viscosity
reduction at the 5 weight percent fatty acid concentration.
EXAMPLE 4:
3o Samples containing various ratios of either acetic or octanoic acid, as
representative
carboxylic acids, of a representative alkyl Biphenyl oxide sulfonic acid
surfactant with a 16-
carbon hydrophobe side chain (DOWFAX Detergent Acid), and water were prepared.
Each
sample was blended until homogeneous. Gross visual examination of each sample
was
made to identify the presence of a solid-like, liquid crystal phase. Data
defining the
composition of samples exhibiting such a highly viscous phase were plotted on
a ternary
11
CA 02379347 2002-O1-15
WO 01/08793 PCT/US00/18287
phase diagram to ascertain the phase boundary. Boundary regions for blends
with either
acetic acid or octanoic acid are shown in Figure 4.
The ternary phase diagram of Figure 4 shows significant liquid crystal phase
regions for
water, DOWFAX surfactant acid, and two fatty acids (acetic acid and octanoic
acid). The
phase boundary is indicated where the viscosity measures 1 million centipoise
or greater at
room temperature and pressure. The high viscosity area underscores the
importance of the
method of addition in admixing the alkyl Biphenyl oxide sulfonic acid
surfactant and fatty acid
blend of the described embodiments with water. It should be noted successful
combination
of HAA with water requires attentiveness to the issue of progression in
component
concentration with respect to phase control according to the depiction of
Figure 4. In this
regard, an alkyl Biphenyl oxide sulfonic acid surfactant acid / fatty acid
admixture should be
added to water in use of the highly concentrated HAA in creating a surfactant
for use and
sale; water should not be added to the alkyl Biphenyl oxide sulfonic acid
surfactant acid /
fatty acid admixture in use of the highly concentrated HAA in creating a
surfactant for use
and sale. In this regard, with reference to Figure 4, the addition of water to
the alkyl Biphenyl
oxide sulfonic acid surfactant acid / fatty acid admixture can function to
induce substantive
liquid crystal formation in the admixture and render the admixture too viscous
for use since
the dilution of HAA with water effects entry into the liquid crystal region.
EXAMPLE 5:
Octanoic acid at a 10 weight percent concentration based upon expected levels
of DOWFAX
Detergent Acid was added to alkylate during a sulfonation reaction. A control
reaction
containing no octanoic acid under identical conditions yielded DOWFAX
Detergent Acid
exhibited a viscosity of 40,200 cP. The product of the sulfonation reaction
containing the 10
weight percent octanoic acid had viscosity of 3,100 cP.
The beneficial results from use of fatty acids in the described embodiments
indicate that fatty
3o alcohols, fatty amines, or even linear alkanes in the C6 - C,e range
warrant consideration and
empirical study in contemplated embodiment blends.
12