Note: Descriptions are shown in the official language in which they were submitted.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
TITLE OF THE INVENTION
REDUCED ANTIGENICITY TISSUE (RAT) IMPLANTS
FIELD OF THE INVENTION
The present invention relates to bone, cartilage and other tissue implant
materials
comprising reduced antigenicity bone (RAB), reduced antigenicity cartilage
(RAC) or
reduced antigenicity tissue (RAT) by virtue of having been treated to remove
substantially all non-collagenous proteins from the bone, cartilage- or other
tissue implant
to matrix. In specific applications of the invention the RAB, RAC and RAT
implants are
treated with osteogenic (osteoinductive or osteoconductive) compositions,
including but
not limited to bone morphogenic proteins, cartilage derived morphogenic
proteins,
growth factors, cells, natural or recombinant, expressing such proteins or
growth factors,
and expressible nucleic acids encoding such proteins and growth factors.
BACKGROUND OF THE INVENTION
In the art of orthopedic medicine, there is frequently the need for
implantation of
materials in order to provide support or to replace damaged or diseased bone
tissue.
2o Classically, such implants have comprised titanium or other relatively
inert metals,
synthetic polymeric substances, and the like. Autologous bone, harvested from
a first
anatomical location, and reimplanted into a second anatomical location, has
also been
relied upon by surgeons. However, such methods, while effective at the second
anatomical location, are less than ideal, due to morbidity at the first
anatomical location.
Use of allograft (from another individual of the same species) or xenograft
(from another
species) bone, cartilage or other material has gained increasing acceptance,
as techniques
for removal of potentially pathogenic organisms have become increasingly
sophisticated
and reliable. However, residual problems exist with induction of immune
responses to
antigenic proteins present in allograft and xenograft implants. Accordingly,
there has
3o been a long felt need for materials which may reliably be obtained in
substantially
unlimited quantities, such as xenograft bone (e.g. bovine, ovine, equine,
porcine, canine,
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
2
etc.), suitable for implantation in various orthopedic applications, such as
spinal fusion,
and the like.
Spinal fusion is indicated to provide stabilization of the spinal column for
painful spinal
motion and disorders such as structural deformity, traumatic instability,
degenerative
instability, and post-resection iatrogenic instability. Fusion, or
arthrodesis, is achieved by
the formation of an osseous bridge between adjacent motion segments. This can
be
accomplished within the disc space, anteriorly between contiguous vertebral
bodies or
posteriorly between consecutive transverse processes, laminae or other
posterior aspects
to of the vertebrae.
An osseous bridge, or fusion mass, is biologically produced by the body upon
skeletal
injury. This normal bone healing response is used by surgeons to induce fusion
across
abnormal spinal segments by recreating spinal injury conditions along the
fusion site and
then allowing the bone to heal. A successful fusion requires the presence of
osteogenic
or osteopotential cells, adequate blood supply, sufficient inflammatory
response, and
appropriate preparation of local bone. This biological environment is
typically provided
in a surgical setting by decortication, or removal of the outer, cortical bone
to expose the
vascular, cancellous bone, and the deposition of an adequate quantity of high
quality graft
2o material.
A fusion or arthrodesis procedure is often performed to treat an anomaly
involving an
intervertebral disc. Intervertebral discs, located between the endplates of
adjacent
vertebrae, stabilize the spine, distribute forces between vertebrae, and
cushion vertebral
bodies. A normal intervertebral disc includes a semi-gelatinous component, the
nucleus
pulposus, which is surrounded and confined by an outer, fibrous ring called
the annulus
fibrosis. In a healthy, undamaged spine, the annulus fibrosis prevents the
nucleus
pulposus from protruding outside the disc space. Spinal discs may be displaced
or
damaged due to trauma, disease or aging. Disruption of the annulus fibrosis
allows the
3o nucleus pulposus to protrude into the vertebral canal, a condition commonly
referred to as
a herniated or ruptured disc. The extruded nucleus pulposus may press on the
spinal
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
3
nerve, which may result in nerve damage, pain, numbness, muscle weakness and
paralysis. Intervertebral discs may also deteriorate due to the normal aging
process or
disease. As a disc dehydrates and hardens, the disc space height will be
reduced leading
to instability of the spine, decreased mobility and pain.
Sometimes the only relief from the symptoms of these conditions is a
discectomy, or
surgical removal of a portion or all of an intervertebral disc, followed by
fusion of the
adjacent vertebrae. The removal of the damaged or unhealthy disc will allow
the disc
space to collapse. Collapse of the disc space can cause instability of the
spine, abnormal
1o joint mechanics, premature development of arthritis or nerve damage, in
addition to
severe pain. Pain relief via discectomy and arthrodesis requires preservation
of the disc
space and eventual fusion of the affected motion segments. Bone grafts are
often used to
fill the intervertebral space to prevent disc space collapse and promote
fusion of the
adjacent vertebrae across the disc space. In early techniques, bone material
was simply
disposed between the adjacent vertebrae, typically at the posterior aspect of
the vertebrae,
and the spinal column was stabilized by way of a plate or rod spanning the
affected
vertebrae. Once fusion occurred the hardware used to maintain the stability of
the
segment became superfluous and was a permanent foreign body. Moreover, the
surgical
procedures necessary to implant a rod or plate to stabilize the level during
fusion were
frequently lengthy and involved. It was therefore determined that a more
optimal solution
to the stabilization of an excised disc space is to fuse the vertebrae between
their
respective end plates, preferably without the need for anterior or posterior
plating.
There have been a number of attempts to develop an acceptable intra-discal
implant that
could be used to replace a damaged disc and maintain the stability of the disc
interspace
between the adjacent vertebrae, at least until complete arthrodesis is
achieved. To be
successful the implant must provide temporary support and allow bone ingrowth.
Success of the discectomy and fusion procedure requires the development of a
contiguous
growth of bone to create a solid mass, because the implant may not withstand
the cyclic
compressive spinal loads for the life of the patient. Many attempts to restore
the
intervertebral disc space after removal of the disc have relied on metal
devices. U.S.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
4
Patent No. 4,878,915 to Brantigan teaches a solid metal plug. U.S. Patent Nos.
5,044,104; 5,026,373 and 4,961,740 to Ray; 5,015,247 to Michelson and U.S.
Patent No.
4,820,305 to Harms et al., U.S. Patent No. 5,147,402 to Bohler et al. and
5,192,327 to
Brantigan teach hollow metal cage structures. Unfortunately, due to the
stiffness of the
material, some metal implants may stress shield the bone graft, increasing the
time
required for fusion or causing the bone graft to resorb inside the cage.
Subsidence, or
sinking of the device into bone, may also occur when metal implants are
implanted
between vertebrae if fusion is delayed. Metal devices are also foreign bodies
which can
never be fully incorporated into the fusion mass.
Various bone grafts and bone graft substitutes have also been used to promote
osteogenesis and to avoid the disadvantages of metal implants. Autograft is
often
preferred because it is osteogenic. Both allograft and autograft are
biological materials
which are replaced over time with the patient's own bone, via the process of
creeping
substitution. Unlike a metal implant, over time a bone graft may virtually
disappear,
while a metal implant persists long after its useful life. Stress shielding is
avoided
because bone grafts have a similar modulus of elasticity as compared with the
surrounding bone. Commonly used implant materials have stiffness values far in
excess
of both cortical and cancellous bone. Titanium alloy has a stiffness value of
114 Gpa and
316L stainless steel has a stiffness of 193 Gpa. Cortical bone, on the other
hand, has a
stiffness value of about 17 Gpa. Moreover, bone as an implant also allows
excellent
postoperative imaging because it does not cause scattering like metallic
implants on CT
or MRI imaging.
Various implants have been constructed from bone or graft substitute materials
to fill the
intervertebral space after the removal of the disc. For example, the Cloward
dowel is a
circular graft made by drilling an allogeneic or autogeneic plug from the
illium. Cloward
dowels are bicortical, having porous cancellous bone between two cortical
surfaces.
Such dowels have relatively poor biomechanical properties, in particular a low
3o compressive strength. Therefore, the Cloward dowel is not suitable as an
intervertebral
spacer without internal fixation due to the risk of collapsing prior to fusion
under the
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
intense cyclic loads of the spine. Bone dowels having greater biomechanical
properties
have been produced and marketed by the Regeneration Technologies, Inc., (RTI),
1
Innovation Drive, Alachua, Florida 32615, and have been patented, see U.S.
Patent No.
5,814,084. Unicortical dowels from allogeneic femoral or tibial condyles are
available.
5 RTI has also developed a diaphysial cortical dowel having superior
mechanical
properties, which forms the basis of the 5,814,084 patent (the '814 patent).
This dowel
also provides the further advantage of having a naturally preformed cavity
formed by the
existing meduallary canal of the donor long bone. The cavity can be packed
with
osteogenic materials such as bone or bioceramic.
Unfortunately, the use of bone grafts can present several disadvantages.
Autograft is
available in only limited quantities. The additional surgery also increases
the risk of
infection and blood loss and may reduce structural integrity at the donor
site.
Furthermore, some patients complain that the graft harvesting surgery causes
more short-
term and long-term pain than the fusion surgery. Allograft material, which is
obtained
from donors of the same species, is more readily obtained. However, allogeneic
bone
does not have the osteoinductive potential of autogenous bone and therefore
may provide
only temporary support. The slow rate of fusion using allografted bone can
lead to
collapse of the disc space before fusion is accomplished. Both allograft and
autograft
2o present additional difficulties. Graft alone may not provide the stability
required to
withstand spinal loads. Internal fixation can address this problem but
presents its own
disadvantages such as the need for more complex surgery as well as the
disadvantages of
metal fixation devices. Also, the surgeon is often required to repeatedly trim
the graft
material to obtain the correct size to fill and stabilize the disc space. This
trial and error
approach increases the length of time required for surgery. Furthermore, the
graft
material usually has a smooth surface which does not provide a good friction
fit between
the adjacent vertebrae. Slippage of the graft may cause neural and vascular
injury, as well
as collapse of the disc space. Even where slippage does not occur, micromotion
at the
graft/fusion-site interface may disrupt the healing process that is required
for fusion. In
3o addition, even allograft material may be available in limited supply.
Accordingly, a
method for use of xenograft material has long been needed.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
6
Several attempts have been made to develop a bone graft substitute which
avoids the
disadvantages of metal implants and bone grafts while capturing advantages of
both. For
example Unilab, Inc. markets various spinal implants composed of
hydroxyapatite and
bovine collagen. In each case, developing an implant having the biomechanical
properties of metal and the biological properties of bone without the
disadvantages of
either, has been extremely difficult or impossible.
These disadvantages have led to the investigation of bioactive substances that
regulate the
complex cascade of cellular events of bone repair. Such substances include
bone
morphogenetic proteins, for use as alternative or adjunctive graft materials.
Bone
morphogenetic proteins (BMPs), a class of osteoinductive factors from bone
matrix, are
capable of inducing bone formation when implanted in a fracture or surgical
bone site.
Recombinantly produced human bone morphogenetic protein-2 (rhBMP-2) has been
demonstrated in several animal models to be effective in regenerating bone in
skeletal
defects. The use of such proteins has led to a need for appropriate Garners
and fusion
spacer designs.
Due to the need for safer bone graft materials, bone graft substitutes, such
as bioceramics,
have recently received considerable attention. The challenge has been to
develop a bone
graft substitute which avoids the disadvantages of metal implants and bone
grafts while
capturing the advantages of both. Calcium phosphate ceramics are biocompatible
and do
not present the infectious or immunological concerns of allograft materials. .
Ceramics
may be prepared in any quantity, which is a great advantage over autograft and
even
allograft bone graft material. Furthermore, bioceramics are osteoconductive,
stimulating
osteogenesis in bony sites. Bioceramics provide a porous matrix which further
encourages new bone growth. Unfortunately, ceramic implants typically lack the
strength
to support high spinal loads and therefore require separate fixation before
the fusion.
Of the calcium phosphate ceramics, hydroxyapatite(HA) and tricalcium phosphate
(TCP)
3o ceramics have been most commonly used for bone grafting. Hydroxyapatite is
chemically similar to inorganic bone substance and biocompatible with bone.
However,
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
7
it is slowly degraded. 13-tricalcium phosphate is rapidly degraded in vivo and
is too weak
to provide support under the cyclic loads of the spine until fusion occurs.
Thus,
developing an implant having the biomechanical properties of metal and the
biological
properties of bone without the disadvantages of either has been extremely
difficult or
impossible.
It recently became apparent that natural bone mineral is not actually as close
to the
chemistry and structure of hydroxyapatite as was previously believed.
(Spector, 21
Clinics in Plastic Surgery 437-444, 1994, the complete text of which is herein
to incorporated by reference.) Natural bone mineral contains carbonate ions,
magnesium,
sodium, hydrogenophosphate ions and trace elements. Bone mineral also has a
different
crystalline structure than HA. Other details of bone chemistry are disclosed
in U.S.
Patent No. 4,882,149 to Spector. Mimicking the chemistry and microstructure of
bone is
important to obtain a beneficial modulus of elasticity and resorbption rate.
Several attempts have been made to make materials which are closer to the
microstructure of bone. Some disclose removing organic material from bone to
yield
bone mineral. Some of the materials are used as drug carriers as disclosed in,
for
example, U.S. Patent No. 5,417,975. U.S. Patent No. 4,882,149 to Spector
describes a
2o bone mineral material which is free from fat and bone proteins. The result
is a powdery,
brittle radiopaque material which can be used to deliver bone growth proteins.
The
Spector mineral is thought to be closer to natural bone mineral than synthetic
calcium
phosphate ceramics, but it does not have characteristics which allow it to be
shaped into
formed objects. U.S. Patent Nos. 4,314,380 to Miyata et al. And 5,573,771
disclose
adding collagen or gelatin to bone mineral. However, it is unclear how close
these
materials are to the natural structure of bone, because the crystalline
structure is disrupted
when all of the proteins are removed from the treated bone. Urist et al. (110
Arch Surg.
416, 1975) discloses a chemosterilized, antigen-extracted, autodigested,
alloimp 1 ant
which is thought to preserve the morphogenetic potential of the material.
McKay,
3o W098/56433, published 17 December 1998, purported to disclose a bone graft
composite
and spacers comprising bone stated to have "been processed to remove
associated non-
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
8
collagenous bone proteins", followed by combination through soaking with a
bone
growth factor. However, in reviewing the methods disclosed therein for removal
of non-
collagenous proteins from bone, it is apparent that removal of non-collagenous
protein,
and preservation of the collagenous structure of bone, would not be
effectively
accomplished. The "deactivation" process consisted of: chemical and enzymatic
treatment to dissolve and remove all cellular and non-collagenous
proteinaceous material.
How the collagenous material is preserved is not disclosed. The thus treated
material was
then, purportedly, treated by soaking in isopronanol, peroxide, and SDS,
followed by a
rinse in water and gamma irradiation. None of these materials are thought to
yield a non-
lo collagenous-protein-free bone implant material comprising natural bone
collagen and
mineral which is identical to natural bone. Thus, a need has remained for
fusion spacers
which stimulate bone ingrowth and avoid the disadvantages of metal implants
and known
bone implants, yet provide sufficient strength to support the vertebral column
until the
adjacent vertebrae are fused. A need has also remained for bone graft
materials which
provide the osteogenic potential and low risk of infectious or immunogenic
complications
of autograft without the disadvantages of autograft.
SUMMARY OF THE INVENTION
2o In accordance with one aspect of the invention, bone graft compositions,
vertebral
spacers, and various other bone implants composed of bone graft compositions
are
provided. In one aspect, the invention provides reduced antigenicity bone
(RAB)
compositions, alone or in combination with a bone growth factor, bone
morphogenic
protein, cartilage derived growth factors, cells expressing such factors, or
nucleic acids
actively encoding such factors. In another aspect of this invention, reduced
antigenicity
cartilage (RAC) compositions, or reduced antigenicity tissue (RAT)
compositions are
prepared and used to replace damaged, diseased or otherwise compromised
tissues, either
in the spine or in any number of other biological locations.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
9
One object of the invention is to provide a bone graft implant having
substantially natural
mineral structure, reduced antigenicity (reduced immunogenicity), safety and
osteoinductive potential of autograft.
Another object of the invention is to provide a cartilage graft implant having
substantially
natural mineral structure, reduced antigenicity (reduced immunogenicity),
safety and
osteoinductive potential of autograft.
Another object of the invention is to provide a tissue graft implant having
substantially
l0 natural structure, reduced antigenicity (reduced immunogenicity), safety
and renewed
tissue growth and induction potential.
Another object of the invention is to provide spacers for engagement between
vertebrae
which restore the intervertebral disc space and which support the vertebral
column while
encouraging bone ingrowth and avoiding stress shielding.
Another object of the invention is to provide pins, suture anchors,
interference screws,
demineralized bone implants, including but not limited to ligaments, oral
maxilofacial
plates, dowels, posterior lumbar interbody fusion implants, trauma screws and
plates,
pericardium (for dura, plum, shoulder patch and perioligaments), wedges, chips
and
pastes comprising reduced antigenicity bone, cartilage or other tissues, alone
or in
combination with growth factors, or nucleic acids encoding growth factors,
including but
not limited to bone morphogenetic proteins, cartilage derived morphogenetic
proteins,
tissue growth factor (betal and the like).
One benefit of the present invention is that it solves many of the problems
associated with
the use of bone and other graft materials, either from allograft or xenograft
materials. The
antigen removal process disclosed herein removes immunogenic and potentially
disease
causing agents while retaining the natural microstructure of bone and other
tissues
described herein. This feature allows the use of allograft or xenograft, which
is available
in virtually unlimited supply. Fortifying the graft with a bone growth factor,
cells
CA 02379665 2002-O1-21
WO 01/08715 PCT/iJS00/20629
expressing bone growth factors, or nucleic acids which actively encode bone
growth
factors or osteogenic proteins, makes the graft osteoinductive, thereby making
the pain
and risk of harvesting autograft unnecessary. An additional benefit is that
the invention
provides a stable scaffold for bone, cartilage or other tissue ingrowth as the
process of
5 fusion or new cartilage or tissue generation occurs.
A further object and another benefit of this invention is that it allows the
use of bone
grafts without the need for metal cages or internal fixation, due to the
increased speed of
fusion.
to
Other objects and further benefits of the present invention will become
apparent to
persons of ordinary skill in the art from the following written description
and
accompanying Figures.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a top perspective view of a bone dowel implant according to US
Patent No.
5,814,084, treated according to the method of the present invention to remove
non-
collagen protein, and optionally treated with BMP, cells expressing growth
factors, or
2o nucleic acids encoding growth factors.
FIG. 2 shows bilateral dowel placement between LS and the sacrum, using a RAB
dowel
such as that shown in figure 1.
FIG. 3 is a perspective view of a cortical RAB dowel such as that shown in
figure 1,
having a chamber and a threaded external feature.
FIG. 4 is a side perspective view of a RAB dowel according to this invention.
FIG. 5 is a cross-section of a RAB dowel of this invention.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
11
FIG. 6 is a side elevational view of the RAB dowel shown in FIG. 5.
FIG. 7 is a RAB cortical ring packed with an osteogenic material.
FIG. 8 is a representation of a RAB cortical ring embodiment provided by this
invention.
FIG. 9 is another embodiment of a RAB cortical ring provided by this
invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
to
For the purposes of promoting an understanding of the principles of the
invention,
reference will now be made to the embodiments illustrated in the drawings and
specific
language will be used to describe the same. It will nevertheless be understood
that no
limitation of the scope of the invention is thereby intended, such alterations
and further
modifications in the illustrated spacers, and such further applications of the
principles of
the invention as illustrated therein being contemplated as would normally
occur to one
skilled in the art to which the invention relates.
The present invention provides bone graft compositions, spacers and surgical
procedures.
2o The bone graft compositions include reduced antigenicity bone (RAB) grafts,
optionally
in combination with an osteogenic material, such as a bone morphogenic protein
(BMP),
cartilage derived morphogenic protein, growth factors, cells expressing such
proteins,
peptides (e.g. p15) or nucleic acids actively encoding such growth factors,
peptides or
proteins. As is now known in the art, delivery of nucleic acids encoding
desirable gene
products results in uptake of such nucleic acids and expression of the encoded
proteins.
The nucleic acids may be so-called naked DNA or RNA, comprising appropriate
transcription and translation start and stop signals, as are known in the art.
The nucleic
acid may also comprise viral replication signals.
3o This invention also provides pins, suture anchors, interference screws,
demineralized
bone implants, including but not limited to ligaments, oral maxilofacial
plates, dowels,
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
12
posterior lumbar interbody fusion implants, trauma screws and plates,
pericardium (for
dura, plum, shoulder patch and perioligaments), wedges, chips and pastes
comprising
reduced antigenicity bone, cartilage or other tissues, alone or in combination
with growth
factors, or nucleic acids encoding growth factors, including but not limited
to bone
morphogenetic proteins, cartilage derived morphogenetic proteins, tissue
growth factor
(betal and the like). Thus, while emphasis may be placed herein on RAB
implants for
spinal fusions, those skilled in the art will appreciate, based on the instant
disclosure, that
RAC and RAT implants for a wide variety of orthopedic and non-orthopedic
applications
may benefit by treating such tissues to reduce antigenicity, and optionally
treating such
tissues with appropriate growth factors, cells, nucleic acids and the like.
The bone grafts according to this invention are treated according to the
method disclosed
herein to remove all of the cellular material, fat and non-collagenous protein
that is
otherwise associated with bone graft compositions. In preferred embodiments,
free
collagen is also removed, leaving structural or bound collagen which is
associated with
bone mineral to form the trabecular struts of bone.
Although the RAB graft of this invention is depleted of non-collagenous
proteins and
non-structural collagens and is defatted, it still contains the natural
crystalline structure of
2o bone. Therefore, the RAB bone graft compositions of this invention have the
natural
microstructure of bone without the risk of disease transmission or significant
immunogenicity or antigenicity.
The natural crystalline structure of bone is maintained by the presence of
structural
collagen in association with the natural bone minerals. This yields a bone
graft material
with preferred physical and biological characteristics, including the ability
to deliver bone
growth factors and osteogenic proteins, cells, and nucleic acids, without
attendant
immunogenicity or antigenicity.
3o The presence of structural collagen and the natural mineral structure of
bone results in an
elasticity and radioopacity which is identical or nearly identical to bone.
The material
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
13
has sufficient resilience and elasticity to retain a formed body and yet
remains rigid
enough to maintain an open space between bone portions to result in a fusion
mass.
Other allograft materials such as demineralized bone matrix do not have the
optimal
physical properties to accomplish this without the assistance of a support.
When the RAB graft materials of this invention are combined with an osteogenic
factor
such as bone morphogenetic protein, growth factor, cells expressing BMPs or
growth
factors, TGF-beta, TGF-beta superfamily members, FGF, PDGF, P15, or nucleic
acids
encoding BMPs, CDMPs (cartilage derived morphogenic proteins), TGF-beta, TGF-
beta
1o superfamily members, FGF, PDGF, P15, other growth factors, or nucleic acids
encoding
such factors, the composite is an ideal bone graft substitute. The composite
has the
natural calcium phosphate structure of bone. This facilitates incorporation
and
substitution of the graft material, giving the composites a desirable
resorbption rate of a
few months. This compares favorably to the resorbption rates of known
materials which
are typically either too fast, slow or unpredictable. For example, allograft
typically is
resorbed within 12-60 months but may, on the other hand, resorb too quickly
before
fusion can occur due to an immunogenic response by the patient.
The combination of BMP and other osteogenic factors with the RAB graft
according to
2o this invention provides the osteoinductive potential of autograft without
the need for a
harvesting surgical procedure at a secondary location, where morbidity may
occur. The
osteoinductive composites of this invention enhance bone growth into and
incorporation
of the graft, resulting in fusion more quickly than would occur using RAB
graft material
alone. Allograft alone typically requires many months to incorporate and
sometimes is
never fully incorporated, but is merely encased within the patient's bone. The
quicker
fusion, occurring within about five months, provided by this invention
compensates for
the less desirable biomechanical properties of graft and makes the use of
internal fixation
and metal interbody fusion devices unnecessary. The spacers of this invention
are not
required to support the cyclic loads of the spine for very long because of the
quick fusion
3o rates which reduce the biomechanical demands on the spacer. However, when
required,
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
14
the compositions of this invention may be used with internal fixation devices
or may be
reinforced as needed, see W098/56319, hereby incorporated by reference.
A further advantage provided by this invention is that, because the bone graft
material of
this invention has been treated to remove essentially all non-collagenous
proteins and
non-structural collagens, the graft may be autogeneic, allogeneic or
xenogeneic. The
components of bone which could cause disease or prompt the patient's body to
reject the
graft are removed by the treatment process disclosed herein. Xenogenic bone,
such as
bovine, ovine, porcine, canine, equine or other bone, is available in
virtually unlimited
supply. Several osteogenic factors are also available in unlimited supply
thanks to
recombinant DNA technology. Therefore, the present invention solves all of the
problems associated with autograft, allograft and xenograft, including supply,
immunogenicity, disease transmission and the need for surgical procedures at
secondary
sites.
This invention provides the further advantage of exploiting the discovery that
bone
mineral is an excellent Garner for osteogenic factors, such as bone
morphogenic proteins,
CDMP, nucleic acids encoding such factors, peptides (e.g. p15) and cells
expressing such
factors. Hydroxyapatite, which is similar in chemical composition to the
mineral in
2o cortical bone, is an osteogenic factor-binding agent which controls the
rate of delivery of
certain proteins to the fusion site. Calcium phosphate compositions such as
hydroxyapatite are thought to bind bone morphogenic proteins and prevent BMP
from
prematurely dissipating from the spacer before fusion can occur. It is further
believed
that retention of the BMP by the agent permits the protein to initiate the
transformation of
mesenchymal stem cells into bone producing cells or osteoblasts within the
device at a
rate that is conducive to complete and rapid bone formation and ultimately,
fusion across
the disc space. The RAB spacers of this invention have the advantage of
including a load
bearing member composed of bone which naturally binds and provides controlled
delivery of osteogenic factors such as bone morphogenic proteins, without at
the same
3o time inducing undesirable immune responses in the recipient thereof. We
have also
unexpectedly noted an approximately twelve-fold greater response to BMP when
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
incorporated into a bone Garner matrix and implanted, as compared to a
collagen sponge
impregnated with BMP.
This invention also capitalizes on the discovery that cortical bone, like
metal, can be
5 conveniently machined into the various shapes disclosed herein. In some
embodiments,
the load bearing members define threads on an outer surface. Machined
surfaces, such as
threads, provide several advantages that were previously only available with
metal
implants. Threads allow better control of spacer insertion than can be
obtained with a
smooth surface. This allows the surgeon to more accurately position the
spacer, which is
10 extremely important around the critical neurological and vascular
structures of the spinal
column.
Threads and the like also provide increased surface area which facilitates the
process of
bone healing and creeping substitution for replacement of the donor bone
material and
15 fusion. These features also increase post-operative stability of the spacer
by engaging the
adjacent vertebral endplates and anchoring the spacer to prevent expulsion.
This is a
major advantage over smooth grafts. Surface features also stabilize the bone-
spacer
interface and reduce micromotion to facilitate incorporation and fusion.
2o The RAB graft compositions of this invention can be prepared according to
methods
disclosed herein. Bone of human or animal source is obtained according to
known
procedures. The bone is cleaned to remove tissue and blood and is then treated
v~rith
agents to remove cellular material, fats, noncollagenous proteins, and non-
structural
collagens. Typical agents include alcohols and peroxides. In preferred
embodiments, the
bone material is also treated to remove free coilagen, leaving only bound or
structural
collagen in association with bone minerals. This reduces
immunogenicity/antigenicity,
without compromising the structural integrity of the bone material. One
preferred agent
for removing free collagen and associated non-structural antigenic proteins
and any
remaining fat is a chaotropic agent, such as urea, guanidinium hydrochloride,
Triton X-
100, Tween, TNBP, or the like, in combination with alcohol and peroxide
treatment. The
RAB bone material is then preferably washed with sterile, deionized water and
terminally
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
16
sterilized by suitable methods, including but not limited to gamma
irradiation, vapor-
phase peroxide treatment, and the like.
RAB allograft or xenograft bone dowels or other appropriately shaped implants
can be
packaged fresh frozen or freeze-dried, preferably freeze dried. Sterilization
can be
provided via aseptic processing or terminally sterilized by ETO, E-beam, or
gamma
irradiation preferably gamma irradiation. Gamma irradiation allows the
procurement and
processing of the allograft under less rigorous environmentally controlled
conditions
since terminal sterilization offers a significantly higher degree of
sterility.
to
The RAB graft according to this invention is treated to remove all of the non-
collagenous
bone proteins leaving a non-immunogenic, disease-free, bone graft implant
material
having the natural mineral, microcrystalline structure of bone, with a
consistency which
retains desired forms. The composition of this invention is preferred because
it has a
microstructure which is the closest to natural bone of all of the known
treated bone
products. This bone product also has the radioopacity of natural bone and does
not show
the dense white image of the bone products of Spector and Geistlich. The
product of this
invention also provides superior resorbability, particularly when combined
with an
osteogenic protein, cell, nucleic acid or other osteogenic factor. Resorbption
has been
found to advantageously occur within several months as opposed to several
years
required for the Spector and Geistlich materials or the few weeks of the Urist
product.
When the material is combined with a bone growth factor, the resorbption time
is ample
for forming the bony bridge required for fusion and bone healing. The RAB
material of
this invention also has an elasticity similar to normal bone while the Spector
and
Geistlich materials have been found to be brittle and weak.
The RAB materials of this invention are preferably combined with an osteogenic
composition or material containing a bone growth factor, proteins, peptides,
or cells
expressing such factors, or nucleic acids which actively encode such factors.
An
3o osteogenic material can be applied to the bone material by impregnating the
graft with a
solution including an osteogenic composition. The allograft or xenograft is
allowed to
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
17
soak for sufficient time to allow the allograft or xenograft to absorb the
protein, nucleic
acid, or is cultured with appropriate cells which encode growth factors.
Additional
protein could be used with the allograft or xenograft produced according to
the method of
this invention by the incorporation of the protein in a delivery vehicle
placed around or in
the allograft. In some embodiments, an osteogenic composition can be packed
into a
chamber defined within a body of the material. The various osteogenic factors,
growth
factors, proteins, peptides or nucleic acids may be forced into the
interstices of the bone
or other reduced antigenicity implants under vacuum or pressure, or by
oscillation
between high and low pressure in an appropriate chamber or vessel. The
composition
to may be applied by the surgeon during surgery or the spacer may be supplied
with the
composition preapplied. In such cases, the osteogenic composition may be
stabilized for
transport and storage such as by freeze-drying. The stabilized composition can
be
rehydrated and/or reactivated with a sterile fluid such as saline or water or
with body
fluids applied before or after implantation.
The term "osteogenic composition" as used herein means virtually any material
that
promotes bone growth or healing, including natural, synthetic and recombinant
proteins,
hormones and the like, cells expressing such factors, and nucleic acids
actively encoding
such factors. By "actively encoding" is meant the inclusion in a nucleic acid
construct of
2o all required signals, including transcriptional promoters and terminators,
enhancers, and
the like, as known in the art, in order to achieve efficient expression of
encoded factors.
The osteogenic compositions used in this invention preferably comprise an
amount of
such composition sufficient to stimulate or induce bone growth or healing of a
substantially pure bone inductive factor such as a bone morphogenetic protein
in a
pharmaceutically acceptable carrier. The preferred osteoinductive factors
include, but are
not limited to, the recombinant human bone morphogenic proteins {rhBMPs),
CDMPs,
and nucleic acids encoding such factors, because they are available in
unlimited supply
and do not transmit infectious diseases. Most preferably, the bone
morphogenetic protein
is a rhBMP-2, rhBMP-4 or heterodimers thereof. The concentration of rhBMP-2 or
other
3o growth factors (e.g. TGF-(31, TGF-(32, p15, and the like, available from
many
commercial sources, including but not limited to R&D Systems, Minneapolis, MN;
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
18
Peptide Innovations, Texas) is generally between about 0.4 mg/ml to about 1.5
mg/ml,
preferably near 1.5 mg/ml. However, any bone morphogenetic protein is
contemplated
including bone morphogenetic proteins designated as BMP-1 through BMP-13,
CDMP1
and CDMP2, and various growth factors know in the art to be beneficial for the
induction
of bone growth and tissue regeneration. BMPs are available from Genetics
Institute, Inc.,
Cambridge, Massachusetts and may also be prepared by one skilled in the art as
described
in U.S. Patent Nos. 5,187,076 to Wozney et al.; 5,366,875 to Wozney et al.;
4,877,864 to
Wang et al.; 5,108,922 to Wang et al.; 5,116,738 to Wang et al.; 5,013,649 to
Wang et al.;
5,106,748 to Wozney et al.; and PCT Patent Nos. W093/00432 to Wozney et al.;
1o W094/26893 to Celeste et al.; and W094/26892 to Celeste et al. All
osteoinductive
factors are contemplated whether obtained as above or isolated from bone or
other
sources. Methods for isolating bone morphogenic protein from bone are
described in
U.S. Patent No. 4,294,753 to Urist and Urist et al., 81 PNAS 371, 1984.
The choice of carrier material for the osteogenic composition is based on the
application
desired, biocompatibility, biodegradability, and interface properties. The
bone growth
inducing composition can be introduced into the pores of the bone material in
any
suitable manner. For example, the composition may be injected into the pores
of the
graft. In other embodiments, the composition is dripped onto the graft or the
graft is
soaked in or sprayed with a solution containing an effective amount of the
composition to
stimulate osteoinduction. Alternatively, the osteogenic composition is infused
into the
bone under elevated or reduced pressure, or both. In any event, the pores of
the RAB
matrix are exposed to the composition for a period of time sufficient to allow
the
osteogenic composition to thoroughly soak, coat and infuse into the graft. The
osteogenic
factor, preferably a BMP, may be provided in freeze-dried form and
reconstituted in a
pharmaceutically acceptable liquid or gel Garner such as sterile water,
physiological
saline or any other suitable Garner. The carrier may be any suitable medium
capable of
delivering the proteins, cells or nucleic acids to the RAB graft. Preferably
the medium is
supplemented with a buffer solution as is known in the art. In one specific
embodiment
of the invention, rhBMP-2 is suspended or admixed in a carrier, such as MFR
buffer,
water, saline, liquid collagen or injectable bicalcium phosphate. In a
preferred
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
19
embodiment, BMP is applied to the pores of the graft and then lypholized or
freeze-dried.
The graft-BMP composition can then be stored in a sterile container, at room
temperature, or at decreased temperatures for storage and transport.
Alternatively, the
osteoinductive protein, cells or nucleic acids can be added at the time of
surgery.
Other osteoinductive protein carriers are available to deliver proteins to a
chamber
defined within the bone material or to locations around the implantation site
of the bone
material. Potential Garners include calcium sulphates, polylactic acids,
polyanhydrides,
collagen, calcium phosphates, polyesters, polyphoazines, polyamines,
polycarbonates,
l0 and the like, and demineralized bone. The Garner may be any suitable
carrier capable of
delivering the proteins. Most preferably, the carrier is capable of being
eventually
resorbed into the body. One preferred carrier is an absorbable collagen sponge
marketed
by Integra LifeSciences Corporation under the trade name Helistat~ Absorbable
Collagen Hemostatic Agent, (a biologically derived bovine achilles tendon
collagen).
Another preferred carrier is an open cell polylactic acid polymer (OPLA).
Other
potential matrices for the compositions may be biodegradable and chemically
defined
calcium sulfates, calcium phosphates such as tricalcium phosphate (TCP) and
hydroxyapatite (HA) and including injectable bicalcium phosphates (BCP), and
polyanhydrides. Other potential materials are biodegradable and are
biologically derived,
such as bone or dermal collagen. Further matrices are comprised of pure
proteins or
extracellular matrix components. The osteoinductive material may also be an
admixture
of BMP and a polymeric acrylic ester carrier, such as polymethylmethacrylate,
polyvinylacetate, polyhydroxyethyl methacrylate, and the like. For packing the
chambers
of the spacers of the present invention, the Garners are preferably provided
as a sponge
which can be compressed into the chamber or as strips or sheets which may be
folded to
conform to the chamber. Preferably, the Garner has a width and length which
are each
slightly greater than the width and length of the chamber. In the most
preferred
embodiments, the Garner is soaked with a rhBMP-2 solution and then compressed
into
the chamber. The sponge is held within the chamber by the compressive forces
provided
3o by the sponge against the wall of the dowel. It may be preferable for the
Garner to extend
out of the openings of the chamber to facilitate contact of the osteogenic
composition
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
with the highly vascularized tissue surrounding the fusion site. The Garner
can also be
provided in several strips sized to fit within the chamber. The strips can be
placed one
against another to fill the interior. As with the folded sheet, the strips can
be arranged
within the spacer in several orientations. Preferably, the osteogenic
material, whether
5 provided in a sponge, a single folded sheet or in several overlapping
strips, has a length
corresponding to the length and width of the chamber.
Another preferred carrier is a biphasic calcium phosphate ceramic.
Hydroxyapatite/tricalcium phosphate ceramics are useful as carriers because of
their
1o desirable bioactive properties and degradation rates in vivo. A preferred
ratio of
hydroxyapatite to tricalcium phosphate is between about 0:100 and about 65:35.
Any
size or shape ceramic carrier which will fit into the chambers defined in the
load-bearing
member are contemplated. Ceramic blocks are commercially available from
Sofamor
Danek Group, B. P. 4-62180 Rang-du-Fliers, France and Bioland, 132 Route
d:Espagne,
15 31100 Toulouse, France. Of course, rectangular and other suitable shapes
are
contemplated. The osteoinductive factor is introduced into the carrier in any
suitable
manner. For example, the Garner may be soaked in a solution containing the
factor.
The present invention also provides spacers for maintaining a space between
adjacent
2o bones. The spacers include a body composed of RAB graft in combination with
a bone
growth factor. The bone source is any suitable bone material preferably of
vertebrate
origin, including tibial, fibial, humeral, iliac, etc. The RAB graft bodies of
this invention
include flat spacers, bone dowels, cortical rings, bone chips and any other
suitably shaped
bone piece. A preferred body is obtained from the diaphysis of a long bone
having a
medullary canal which forms a natural chamber in the graft.
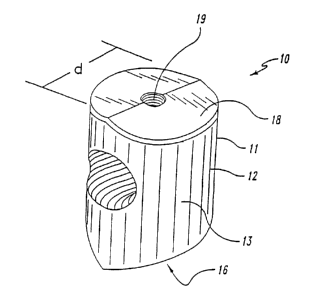
In one specific embodiment depicted in Figure l, the invention provides a
spacer 10 for
maintaining a space between adjacent bones in a patient. The spacer 10
includes a load-
bearing member or body 11 sized and shaped to fit within the space. The body
11 is
3o preferably composed of a natural RAB material which has been processed to
remove
associated non-collagenous bone proteins. The bone material contains native
collagen
CA 02379665 2002-O1-21
WO 01108715 PCT/US00/20629
21
materials and naturally associated bone minerals but is substantially free
from native non-
collagenous protein. The chemical composition of the bone material allows it
to
resiliently retain a shaped body. The shape of the body is preferably formed,
and the
body machined to have desired surface features, before the bone material is
processed
according to the methods of this invention. However, in some embodiments a
mass of
bone is treated as disclosed herein, and then is shaped or machined to form a
particular
body.
Refernng now to Figures 1 and 2, in some embodiments, the body 11 is shaped as
a
1o dowel. Dowel shaped bodies are sometimes preferred when the bones are
vertebrae to be
fused. The dowel 10 includes a wall 12 sized for engagement within the
intervertebral
space (IVS) to maintain the IVS in proper physiologic orientation. The wall 12
defines
an outer engaging surface 13 for contacting the adjacent vertebrae. The wall
12 is
preferably cylindrical, so that the bone dowel 10 has a diameter d which is
larger than the
height h of the IVS between adjacent vertebrae V or the height of the space
between the
lowest lumbar vertebrae LS and the sacrum S as depicted in Figure 2.
In another embodiment depicted in Figure 3, the body is a bone dowel 20 which
includes
a wall 22 having an engagement surface 23. The wall 22 defines a chamber 25
2o therethrough. Preferably, the load-bearing member is a bone graft obtained
from the
diaphysis of a long bone having a medullary canal which forms the chamber 25.
Such
dowels are available from Regeneration Technologies, Inc., 1 Innovation Drive,
Alachua,
Florida 32615. The chamber 25 can be packed with an osteogenic composition to
stimulate osteoinduction. The chamber 25 is preferably defined through a pair
of outer
engaging surfaces 23 so that the composition has maximum contact with the
endplates of
the adjacent vertebrae. Referring now to FIG. 4, the spacer 20 preferably
includes a solid
protective wall 26 which is positionable to protect the spinal cord from
escape or leakage
of material packed within the chamber 25. In anterior approaches, the
protective wall 26
is posterior. Preferably, the osteogenic composition has a length which is
greater than the
length of the chamber (Figures 5 and 6) and the composition is disposed within
the
chamber 25 to contact the end plates of adjacent vertebrae when the spacer 20
is
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
22
implanted between the vertebrae. This provides better contact of the
composition with
the end plates to stimulate osteoinduction.
Various features can be machined on the outer surfaces of the dowels of this
invention.
In one embodiment shown in Figure 3, the dowel 20 includes an outer engaging
surface
23 defining threads 24. Referring again to Figure 1, in some embodiments, the
dowel 10
is provided with a tool-engaging hole 19 in a wall 18 opposite the solid
protective wall
16. The tool engaging hole 19 is provided in a surface of the dowel which is
adj acent the
surgeon and opposite the initial thread 17. For an anterior procedure, the
tool engaging
l0 tool hole 19 would be provided in the anterior surface of the dowel 10.
Other machined
features are contemplated in the outer or bone engaging surfaces 23. Such
machine
features include surface roughenings such as knurlings and ratchetings.
The spacers of this invention can be inserted using conventional techniques
and known
tools. In accordance with additional aspects of the present invention, methods
for
implanting an interbody fusion spacer, such as the spacer 20, are
contemplated. The
spacers of this invention can also be inserted using laporoscopic technology
as described
in Sofamor Danek USA's Laproscooic Bone Dowel Surgical Technique, 1995, 1800
Pyramid Place, Memphis, Tennessee 38132,1-800-933-2635. Devices of this
invention
can be conveniently incorporated into Sofamor Danek's laproscopic bone dowel
system
that facilitates anterior interbody fusions with an approach that is much less
surgically
morbid than the standard open anterior retroperitoneal approaches. This system
includes
templates, trephines, dilators, reamers, ports and other devices required for
laproscopic
dowel insertion.
The body may also include other shapes such as cortical rings as shown in
Figure 7.
Such cortical rings 50 are obtained by a cross-sectional slice of the
diaphysis of a long
bone and include a superior surface 51 and an inferior surface 52. The graft
shown in
Figure 7 includes an outer surface 53 which is adjacent and between the
superior 51 and
3o inferior 52 surfaces. In one embodiment bone growth through-holes 53a are
defined
through the outer surface 53 to facilitate fusion. The holes 53a allow
mesenchymal stem
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
23
cells to creep in and bone growth protein to diffuse out of the graft. This
facilitates bone
graft incorporation and possibly accelerates fusion by forming anterior and
lateral bone
bridging outside and through the device. In another embodiment the outer
surface 53
defines a tool-engaging hole 54 for receiving an implanting tool. In a
preferred
embodiment, at least one of the superior and/or inferior surfaces 51, 52 are
roughened for
gripping the end plates of the adjacent vertebrae. The surface roughenings may
include
teeth 56 on ring 50' as shown in Figure 8 or waffle pattern 57 as shown on
ring 50" in
Figure 9. When cortical rings are used as the graft material the ring 50 may
be trimmed
for a more uniform geometry as shown in Figure 7 or left in place as shown in
Figure 9.
to
The graft can also be formed into a square shape to be conveniently
incorporated into
current surgical procedures such as, the Smith-Robinson technique for cervical
fusion
(Smith, M.D., G.W. and R.A. Robinson, M.D., "The Treatment of Certain Cervical-
Spine
Disorders By Anterior Removal Of The Intervertebral Disc And Interbody
Fusion", J.
Bone And Joint Surgery, 40-A:607-624 (1958) and Cloward, M.D., R.B., "The
Anterior
Approach For Removal Of Ruptured Cervical Disks", in meeting of the Harvey
Gushing
Society, Washington, D.C., April 22, 1958). In such procedures, the surgeon
prepares the
endplates of the adjacent vertebral bodies to accept a graft after the disc
has been
removed. The endplates are generally prepared to be parallel surfaces with a
high-speed
2o burr. The surgeon then typically sculpts the graft to fit tightly between
the bone surfaces
so that the graft is held by compression bet~~een the vertebral bodies. The
bone graft is
intended to provide structural support and promote bone ingrowth to achieve a
solid
fusion of the affected joint. The spacers of this invention avoid the need for
this graft
sculpting as spacers of known size and dimensions are provided. This invention
also
avoids the need for a donor surgery because the osteoinductive properties of
autograft are
provided by the allograft or xenograft RAB implants prepared according to the
present
invention. The spacers can be combined with osteoinductive materials that make
allograft or xenograft RAB implants osteoinductive. Therefore, the spacers of
this
invention speed the patient's recovery by reducing surgical time, avoiding a
painful donor
surgery and inducing quicker fusion. The following specific examples are
provided for
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
24
purposes of illustrating the invention, and no limitations on the invention
are intended
thereby.
EXAMPLE 1
REMOVAL OF ANTIGENS FROM BONE GRAFT MATERIAL
This procedure, and variations on the specifics thereof, is employed to remove
non-
collagenous protein from bone graft materials. The bone graft material may be
allograft
or xenograft, selected from bovine, porcine, equine, ovine, canine or the
like. This
to procedure removes proteins, fats, polysaccharides, glycosaminoglycans and
other non-
collagenous antigens from bone matrix, and may be conducted in any order of
steps,
although carrying the process out in the order provided herein has provided
consistently
excellent results:
1. Peroxide Treatment:
This procedure is followed to de-fat the bone tissue, to remove blood and
other proteins,
and to inactivate microorganisms that might be present in or on the bone.
Prior to
initiating this treatment, the bone was cleaned of any attached adventitious
tissue.
a. The bone tissue was placed into a container, covered with peroxide
solution, and
permitted to soak with agitation, sonication or both for about 15 minutes.
b. The peroxide solution and removed debris was decanted, and the bone tissue
was
rinsed with warm sterile water.
Treatment at this stage with a TNBP/Triton X-100 or like solutions, such as
hydrogen
peroxide/SDS helps to remove additional non-structural proteins and residual
fat.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
2. Acetone Treatment:
This procedure was followed to remove residual fatty tissue:
5 a. The bone tissue was placed into a container, covered with acetone, and
heated to
between about 35 to 40 degrees centigrade, and permitted to soak with
agitation
for about 15 minutes. This step was repeated until no fat was visible in the
solution after being allowed to cool (three to five cycles is usually
adequate).
b. The bone tissue was then rinsed with sterile water and permitted to dry.
Variations on this treatment may include use of 99% isopropanol, hexane, and
combinations of these solvents. Treatment of the graft at this or a different
stage of the
process with acetic or other acid (acetic acid, hydrochloric acid,
hydrofluoric acid,
phosphoric acid, citric acid, formic acid, butyric acid, or mixtures thereof),
is useful to
produce a slightly demineralized bone graft of reduced antigenicity, with
concomitant
effects on the graft strength, growth factor binding capacity, resorbability,
removal of
acid soluble proteins and loosely associated collagens, and further reductions
in
antigenicity. We have discovered that reduction in the mineral content of
between about
0 to about 25%, or between about 1 to about 10% or even as little as 1% to 5%
as
2o compared to the normal bone mineral content confers significant advantages
on the
reduced antigenicity bone composition. The guiding principle in the level of
demineralization that should be conducted is to remove as much mineral as
possible,
without at the same time reducing the compressive strength of the bone. In
order to
achieve uniform, limited demineralization, the RAB is preferably contacted for
about
thirty minutes with acid, e.g. 1 % acetic acid, with the acid being introduced
into an
evacuated chamber containing the R.AB, such that uniform acid penetration
occurs. If
inorganic acids are used, e.g. HCI, the acid strength or period of acid
contact should be
reduced, to avoid complete demineralization of the RAB. We have found that
limited
removal of even as little as 1-2% of the normal bone mineral content results
in greater
3o predictability (reduced scatter in shear stress measurments) in the
strength of bone grafts
thus treated. Additional benefits of this treatment include dissolution of
acid soluble
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
26
proteins, efficient removal of SDS or other ionic solvents or contaminants,
enhanced
binding of growth factors, reduced time to remodel implanted bone, and further
reduction
in antigenicity.
3. Urea Treatment:
This procedure was followed in order to remove associated non-collagenous
proteins
from the bone tissue.
to a. The tissue was transferred to a container sufficient to contain the
tissue and a
large excess volume (approximately five-fold) of urea solution (6 M).
b. Non-collagenous proteins were extracted from the bone tissue for
approximately
48 hours, with agitation.
c. The urea/protein solution was then decanted, and the bone tissue was rinsed
with
sterile water, several times(about three) using at least a two-fold volume of
water.
Each rinse was permitted to continue with agitation for about 20 minutes.
d. A final water wash was conducted for 24 hours with agitation, followed by
decantation of the water and freeze-drying of the tissue.
The foregoing procedure was conducted with bovine bone blocks and cancellous
chips.
Bone cubes of 1 cm were cut from bovine condyles. As a final sterilization
step, the thus
treated bone was subjected to lyophilization and then gamma irradiation. Bone
implant
material treated according to this procedure was implanted into a primate
model. Little or
no adverse immune response (swelling, inflammation) was detected. Furthermore,
bone
implant treated in this manner was soaked with bone morphogenic protein and
implanted
in a primate model. Excellent induction of new bone growth into and around the
implant
bovine bone was detected, without adverse immune response. Based on the
success
achieved in using bone implant prepared as described herein for delivery of
growth
factors in the form of active protein, success in delivering cells expressing
growth factors,
3o or nucleic acids actively encoding growth factors is expected. Allograft or
xenograft
bone infused or coated with cells, recombinant or natural, which produce
growth factors,
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
27
or nucleic acid constructs, such as those disclosed in US Patent No.
5,763,416, hereby
incorporated by reference, or WO 99/06563, also hereby incorporated, are
anticipated to
actively induced bone growth without induction of adverse immune responses.
Alternatives to the above-described treatment includes the use of guanidinium
hydrochloride, TritonX-100, Tween, TNBP and the like, optionally including
combinations of chaotropic agents and surfactants such as SDS (sodium dodecyl
sulfate).
Examples of conditions for use of these agents include use of 4 M guanidinium
hydrochloride, and 1 % TNBP/TritonX-100.
to
EXAMPLE 2
PREPARATION OF DIAPHYSIAL CORTICAL BONE DOWEL
A consenting donor (i.e., donor card or other form of acceptance to serve as a
donor) was
screened for a wide variety of communicable diseases and pathogens, including
human
immunodeficiency virus, cytomegalovirus, hepatitis B, hepatitis c and several
other
pathogens. These tests may be conducted by any of a number of means
conventional in
the art, including but not limited to ELISA assays, PCR assays, or
hemagglutination.
Such testing follows the requirements of: (i) American Association of Tissue
Banks,
2o Technical Manual for Tissue Banking, Technical Manual - Musculoskeletal
Tissues,
pages M19-M20; (ii) The Food and Drug Administration, Interim Rule, Federal
Register/Vol. 50, No. 238/Tuesday, December 14, 1993/Rules and
Regulations/65517, D.
Infectious Disease Testing and Donor Screening; (iii) MMWR/Vol. 43/No. RR-8,
Guidelines for Preventing Transmission of Human Immunodeficiency Virus Through
Transplantation of Human Tissue and Organs, pages 4-7; (iv) Florida
Administrative
Weekly, Vol. 10, No. 34, August 21, 1992, 59A-1.001-014 59A-1.005(12)(c),
F.A.C.,
(12)(a)-(h), 59A-1.005(15), F.A.C., (4)(a)-(8). In addition to a battery of
standard
biochemical assays, the donor, or their next of kin, was interviewed to
ascertain whether
the donor engaged in any of a number of high risk behaviors such as having
multiple
3o sexual partners, suffering from hemophilia, engaging in intravenous drug
use etc. After
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
28
the donor was ascertained to be acceptable, the bones useful for obtention of
the dowels
were recovered and cleaned.
A dowel was obtained as a transverse plug from the diaphysis of a long bone
using a
diamond tipped cutting bit which was water cleaned and cooled. The bit was
commercially available (Starlite, Inc) and had a generally circular nature and
an internal
vacant diameter between about 10 mm to about 20 mm. The machine for obtention
of
endo- and cortical dowels consisted of a pneumatic driven miniature lathe
which is
fabricated from stainless steel and anodized aluminum. It has a spring-loaded
carriage
1o which travels parallel to the cutter. The carnage rides on two runners
which are 1.0 inch
stainless rods and has a travel distance of approximately 8.0 inches. One
runner has set
pin holes on the running rod which will stop the carnage from moving when the
set pin is
placed into the desired hole. The carnage is moveable from side to side with a
knob
which has graduations in metric and in English. This allows the graft to be
positioned.
On this carriage is a vice which clamps the graft and holds it in place while
the dowel is
being cut. The vice has a cut out area in the jaws to allow clearance for the
cutter. The
lathe has a drive system which is a pneumatic motor with a valve controller
which allows
a desired RPM to be set.
2o First, the carnage is manually pulled back and locked in place with a set
pin. Second, the
graft is loaded into the vice and is aligned with the cutter. Third, the
machine is started
and the RPM is set, by using a knob on the valve control. Fourth, the set pin,
which
allows the graft to be loaded onto the cutter to cut the dowel. Once the
cutter has cut all
the way through the graft the carriage will stop on a set pin. Fifth, sterile
water is used to
eject dowel out of the cutter. It is fully autoclavable and has a stainless
steel vice and/or
clamping fixture to hold grafts for cutting dowels. The graft can be
positioned to within
0.001" of an inch which creates dowel uniformity during the cutting process.
The cutter used in conjunction with the above machine can produce dowels
ranging from
5 mm to 30 mm diameters and the sizes of the cutters are 10.6 mm; 11.0 mm;
12.0 mm;
13.0 mm; 14.0 mm; 16.0 mm; and 18.0 mm. The composition of the cutters is
stainless
CA 02379665 2002-O1-21
WO 01/08715 PCT/IJS00/20629
29
steel with a diamond powder-cutting surface which produces a very smooth
surface on
the wall of the dowels. In addition, sterile water is used to cool and remove
debris from
graft and/or dowel as the dowel is being cut (hydro infusion). The water
travels down
through the center of the cutter to irngate as well as clean the dowel under
pressure. In
addition, the water aids in ejecting the dowel from the cutter.
The marrow was then removed from the medullary canal of the dowel and the
cavity
cleaned to create a chamber. The chamber interior may be scraped or machined
as
desired and may be filled with desired osteogenic materials, including
allograft, autograft,
1o ceramic, growth factors and the like. The final machined product may be
stored, frozen or
freeze-dried and vacuum-sealed for later use.
EXAMPLE 3
THREADING OF DOWELS
A diaphysial cortical bone dowel is prepared as described above. The plug is
then
machined, preferably in a class 10 clean room, to the dimensions desired. The
machining
is preferably conducted on a lathe such as a jeweler's lathe or machining
tools may be
specifically designed and adapted for this purpose. A hole is then drilled
through the
2o anterior wall of the dowel. The hole is then tapped to receive a threaded
insertion tool.
EXAMPLE 4
PREPARATION OF RAB DOWEL-rhBMP-2 COMPOSITE BY DRIPPING
A threaded R.AB dowel is obtained through the methods described above. A vial
containing 4.0 mg of lypholized rhBMP-2 (Genetics Institute) is constituted
with 1 mL
sterile water (Abbott Laboratories) for injection to obtain a 4.0 mg/mL
solution as
follows:
1. Using a 3-cc syringe and 22G needle, slowly inject 1.0 mL sterile water for
injection
into the vial containing lypholized rhBMP-2.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
2. Gently swirl the vial until a clear solution is obtained. Do not shake.
The dilution scheme below is followed to obtain the appropriate rhBMP-2
concentration.
This dilution provides sufficient volume for two dowels. The dilutions are
performed as
5 follows:
1. Using a 5-cc syringe, transfer 4.0 mL of MFR 906 buffer (Genetics
Institute) into a
sterile vial.
2. Using a 1-cc syringe, transfer 0.70 mL reconstituted rhBMP-2 into the vial
containing
10 the buffer.
3. Gently swirl to mix.
DILUTION SCHEME
INITIAL rhBMP-2 rhBMP-2 MFR-842 FINAL rhBMP-2
15 CONCENTRATION VOLUME VOLUME CONCENTRATION
~~mL) (mL) (mL) (mg/mL)
4.0 0.7 4.0 0.6
1. Using a 3-cc syringe and 22G needle, slowly drip 2.0 mL of 0.60 mg/mL rhBMP-
20 2 solution onto the Bone Dowel.
2. Implant immediately.
As an alternative to the above, a RAB implant is lyophilized, and then brought
into
contact with a solution containing osteogenic proteins, growth factors,
nucleic acids and
25 the like, in order to reconstitute the lyophilized RAB. In the process of
being
reconstituted, the RAB draws the growth factors or other osteogenic
compositions,
natural or recombinant, into the interstices of the RAB matrix.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
31
EXAMPLE 5
PREPARAION OF RAB ALLOGRAFT OR XENOGRAFT BONE
BMP COMPOSITE BY SOAKING
1. Freeze dried rhBMP-2 is reconstituted with sterile water for injection as
in Example
4.
2. A sterile RAB allograft or xenograft bone dowel is transferred to a sterile
"soaking"
container.
3. Reconstituted rhBMP-2 is added to the soaking container so that the
allograft is
1o completely submersed in a BMP solution.
4. The RAB allograft or xenograft bone dowel is allowed to soak in the rhBMP-2
solution for 30-60 minutes so that the graft absorbs the protein.
To enhance the efficiency of loading of BMPs or other grov~~th factors, the
RAB allograft
or xenograft is contacted with such factors under vacuum with swirling for
about 15
minutes.
EXAMPLE 6
BONE DOWEL PACKED WITH BMP-2/COLLAGEN COMPOSITION
A threaded RAB dowel is obtained through the methods of Examples 1-5. A vial
containing 4 .0 mg of lypholized rhBMP-2 (Genetics Institute) is constituted
with 1 mL
sterile water (Abbott Laboratories) for injection to obtain a 4.0 mg/mL
solution as
follows:
1. Using a 3-cc syringe and 22G needle, slowly inject 1.0 mL sterile water for
injection
into the vial containing lypholized rhBMP-2.
2. Gently swirl the vial until a clear solution is obtained. Do not shake. The
dilution
scheme below is followed to obtain the appropriate rhBMP-2 concentration. The
dilutions are performed as follows:
1. Using a 3-cc syringe, transfer 2.5 mL of MFR-842 buffer (Genetics
Institute) into a
sterile vial.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
32
2. Using a 1-cc syringe, transfer 0.30 mL of 4.0 mg/mL reconstituted rhBMP-2
into the
vial containing the buffer.
3. Gently swirl to mix.
DILUTION SCHEME
INITIAL rhBMP-2 rhBMP-2 MFR-842 FINAL rhBMP-2
CONCENTRATION VOLUME VOLUME CONCENTRATION
(mg/mL) (mL) (mL) (mg/mL)
l0 4.0 0 .3 2.5 0 .43
The rhBMP-2 solution is applied to a Helistat sponge (Genetics Institute) as
follows:
1. Using sterile forceps and scissors, cut a 7.5 cm x 2.0 cm strip of Helistat
off of a 7.5 x
10 cm (3" x 4") sponge.
2. Using a 1-cc syringe with a 22-G needle, slowly drip approximately 0.8 mL
of 0.43
mg/mL rhBMP-2 solution uniformly onto the Helistat sheet.
3. Using sterile forceps, loosely pack the sponge into the chamber of the RAB
dowel.
4. Using a 1-cc syringe with a 22-G needle, inj ect the remaining 0.8 mL of
0.43 mg/mL
rhBMP-2 into the sponge in the RAB dowel through the openings of the chamber.
5. Implant immediately.
EXAMPLE 7
RAB DOWEL PACKED rhBMP-2/HA/TCP COMPOSITION
A threaded RAB dowel is obtained through the methods of Examples 1-5. A vial
containing 4.0 mg of lypholized rhBMP-2 (Genetics Institute) is constituted
with 1 mL
sterile water (Abbott Laboratories) for injection to obtain a 4.0 mg/mL
solution as
3o follows:
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
33
1. Using a 3-cc syringe and 22G needle, slowly inject 1.0 mL sterile water for
injection
into the vial containing lypholized rhBMP-2.
2. Gently swirl the vial until a clear solution is obtained. Do not shake. A
cylindrical
block of biphasic hydroxyapatite/tricalcium phosphate (Bioland) is wetted with
a 0.4
mg/mL rhBMP-2 solution. The BMP-ceramic block is packed into the chamber of
the
RAB dowel and the thus packed RAB dowel is then implanted.
EXAMPLE 8
RAB ALLOGRAFT OR XENOGRAFT BONE CHIP-COMPOSITE PREPARATION
to
1. Allograft or xenograft bone chips are harvested, processed and prepared
according to
Example 1 to produce RAB allograft or xenograft bone chips.
2. Freeze dried rhBMP-2 is reconstituted with sterile water for injection as
described in
Example 4.
3. The sterile RAB allograft or xenograft bone chips are transferred to the
sterile
"soaking" container. Preferably, the RAB bone chips are first lyophilized, so
that upon
contact with the BMP solution, the bone chips reconstitute, thereby soaking up
the BMP
solution into the interstices of the chips.
4. Reconstituted rhBMP-2 is placed into the soaking container so that the RAB
allograft
or xenograft is completely submersed.
5. The RAB allograft or xenograft bone chips are soaked in the rhBMP-2
solution for 30-
60 minutes.
6. Using sterile forceps, the RAB allograft or xenograft bone chips are
removed from
the soaking container and placed into the posterolateral gutters of the level
of the
spine to be fused, or into any other bony location where bone fusion or repair
is
desired.
This procedure may be employed to make a gelatin sponge by injecting gelatin
into bone
chips prepared as described above and then lyophilizing the composition. As
with
3o Example 5, the efficiency of BMP or growth factor loading is enhanced when
contact is
made with the RAB under vacuum.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
34
EXAMPLE 9
PREPARATION OF RAB CORTICAL RING-COMPOSITES
A cortical ring is obtained as a cross-sectional slice of the diaphysis of a
human long
bone and then prepared using the methods described in Example 1 to produce a
RAB
cortical ring. The RAB ring is fashioned into a square hollow ring. The ring
is packed
with an osteogenic composition as described in the foregoing EXAMPLES.
to EXAMPLE 10
RAB SPACERS
A RAB D-shaped cervical spacer is obtained as a cross-sectional slice of a
diaphysis of a
long bone and treated according to the method of Example 1. The exterior
surfaces of the
walls are formed by machining the slice to a D-shape. The engaging surfaces of
the
spacer are provided with knurlings by a standard milling machine. A hole is
then drilled
through the anterior wall of the spacer. The hole is then tapped to engage a
threaded
insertion tool. The chamber of the spacer is then packed with an osteogenic
composition
as described in the foregoing EXAMPLES.
EXAMPLE 11
ANTEROR INTERBODY CERVICAL FUSION
The cervical spine is approached anteriorly according to known surgical
techniques. The
RAB composite material is placed within the interdiscal space.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
EXAMPLE 12
POSTEROLATERAL FUSION
The spine is approached posterolaterially according to known surgical
techniques. The
5 RAB composite material of this invention is placed between portions of
adjacent
vertebrae.
EXAMPLE 13
USE OF RAB COMPOSITE WITH BINDII~'G MATRIX
to
Processed RAB allograft or xenograft is added to a binding matrix to hold the
allograft
chips together improving their handling characteristics. RAB chips prepared
according to
Example 5 are lyophilized and then mixed with gelatin and water to form a
paste or
slurry, then, optionally, freeze dried into a sheet or any other desired form.
At the time of
15 surgery the surgeon hydrates the gelatin RAB allograft/xenograft composite
with an
osteoinductive protein solution. Alternative binding matrix materials include
glycosaminoglycans, hyaluronic acid, polymers, proteins and other suitable
materials.
With or without added demineralized bone matrix, the compositions described
herein
may have applications in diverse areas of the orthopedic arts. For example,
pre-formed
20 shapes may be prepared using appropriately proportioned quantities of RAB,
RAB that
has been demineralized (DRAB), gelatin, growth factors and the like. Similar
compositions may be envisioned for RACs and RATs. Compositions wherein gelatin
is
present at a sufficiently high concentration that the composition is in a semi-
liquid,
malleable solid or viscous liquid above normal body temperature of a
recipient, but
25 becomes a gel or solid at normal body temperature, upon implantation into
the recipient
are highly desirable. For such applications, gelatin concentrations of between
about one
to twenty-five percent are typically sufficient, depending on the average
molecular
weight of the gelatin employed in such compositions. In addition, compositions
wherein
gelatin is present at a sufficiently high concentration that the composition
is a solid at a
3o temperature above normal body temperature of a recipient but is a malleable
solid at a
slightly higher temperature, such that a solid of substantially any desired
form may be
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
36
made at the higher temperature, and upon implantation into a recipient, the
composition
maintains the formed shape, are also highly desirable. Typically, gelatin
concentrations
of between about ten and forty percent are sufficient for this purpose,
depending on the
molecular weight of the gelatin employed for such compositions.
EXAMPLE 14
CHARACTERISTICS AND APPLICATIONS FOR RAB
The combination of a bone growth factor with a RAB graft provides superior
results as
1o compared with other known implant materials. Quicker fusion rates provide
enhanced
mechanical strength sooner. The RAB of this invention is an excellent protein
carrier
which provides controlled release of BMP or other osteogenic compositions,
including
growth factors, cartilage derived morphogenic proteins, nucleic acids encoding
BMPs or
other growth factors, to the fusion site. The presence of structural collagen
and the
natural mineral structure of bone results in an elasticity and radioopacity
which is
identical or nearly identical to bone. The material has sufficient resilience
and elasticity
to retain a formed body and yet remains rigid enough to maintain an open space
between
bone portions to result in a fusion mass.
2o EXAMPLE 15
REDUCED ANTIGENICITY CARTILAGE AND OTHER TISSUES
In a manner similar to that used for preparation of RAB implants, reduced
antigenicity
cartilage (RAC) and reduced antigenicity tissues (RAT) may be produced by
treating
such tissues with chaotropic agents, as described above for bone. The thus-
treated
cartilage and other tissues may then optionally be contacted with various
growth factors,
nucleic acids and cells, as described above for the RAB implants of this
invention.
As discussed above, the goal of RAB/RAC/R.AT production is to remove all
unbound
3o substances. This goal is achieved according to the methods disclosed herein
for such
varied tissues as bone, cartilage, skin, fascia, dura and the like.
CA 02379665 2002-O1-21
WO 01/08715 PCT/US00/20629
37
While the invention has been illustrated and described in detail in the
drawings and
foregoing description, the same is to be considered as illustrative and not
restrictive in
character, it being understood that only the preferred embodiments and best
mode have
been shown and described and that all changes and modifications that come
within the
spirit of the invention are desired to be protected.