Note: Descriptions are shown in the official language in which they were submitted.
CA 02380954 2002-04-08
SUPERCAPACITOR DEVICE WITH EXTENDED CAPABILITY
FIELD OF THE INVENTION:
[0001 ] This invention relates to energy storage devices, and particularly the
present invention relates to such energy storage devices that provide high
power with
high voltage compliance, low impedance, small size, and enhanced discharge
capability.
Such energy storage devices are typically configured as a supercapacitor; and
as such,
supercapacitors provide peak power requirements of variable pulse loads,
particularly
when functioning otherwise in association with -- typically in parallel with --
a battery for
an electronic device.
BACKGROUND OF THE INVENTION:
[0002] There is an ever increasing requirement for batteries and other energy
storage devices to be used in conjunction with electrical devices of all
sorts. Some such
electrical devices may operate in such a manner that they have varying energy
requirements for electrical energy to be delivered to them from time to time.
Particularly, many electrical devices such as cellular telephones, personal
digital
assistants, laptop and portable computers, and the like, will function as a
pulsed load on
the battery which is providing energy to such electrical device, thus creating
a demand
for electrical energy storage devices that will satisfy the pulse load
requirements of such
electrical devices.
[0003] There is, therefore, a requirement for low impedance power sources that
will not suffer undue voltage losses particularly during millisecond current
pulses of the
electrical device. For example, it is not unusual for the voltage response of
a battery in
a present day cellular telephone to cause the telephone to prematurely shut
down during
a "talk" pulse energy requirement, even though the battery still retains quite
sufficient
electrical energy otherwise to provide power to the cellular telephone.
[0004] An approach that has been taken to provide a longer battery life under
variable pulse loads is to separate the power component and the energy
component of
the power source, by the parallel addition of a low impedance supercapacitor.
The
additional supercapacitor will support the pulse load during high current
pulses, and will
be recharged during the "off' or low current periods of operation of the
electrical device.
CA 02380954 2002-04-08
[0005] Unfortunately, however, prior art supercapacitors are expensive, and
they
do not have a suitable charge storage capacity. Moreover, such prior art
supercapacitors generally employ a non-aqueous electrolyte, resulting in low
conductivity within the supercapacitor. This, in turn, necessitates employment
of high
surface area electrode components, thereby resulting in expensive
construction.
[0006] Still further, in stand-alone applications, a prior art supercapacitor
may
have insufficient charge content to provide adequate runtime for the
electrical device
with which the supercapacitor is associated. For example, if a supercapacitor
were
providing auxiliary power to a portable or laptop computer, it would be
convenient to the
user of the computer if the supercapacitor were to provide full operational
capability for
the computer while the main batteries for the computer were being exchanged.
This,
however, would require an excessively large 12 Volt supercapacitor, one having
capacitance values in excess of 50 Farads.
(0007] Moreover, pulse load situations exist which would not benefit from the
addition of the supercapacitor. Those situations include pulse loads which do
not allow
sufficient time for recharge of the supercapacitor, and thereby which
continuously
deplete the energy storage of the supercapacitor. This renders the use of a
supercapacitor to be superfluous for high current management in many
circumstances.
[0008] Still further, mismatched impedances may result in a load sharing
circumstance which would render use of the supercapacitor to be ineffective.
[0009] Where pure carbon-carbon supercapacitors are employed, yet another
particular problem arises, one which is associated with such pure carbon-
carbon
supercapacitors. That is, there is a requirement for strict and close
maintenance of a
voltage window for discharge circumstances for the supercapacitor, otherwise
electrolyte decomposition may ensue. A normal precaution which ensures long
operating lifetimes for such supercapacitors is to operate them with a reduced
voltage
range. Leakage currents will generally then be lower, with correspondingly
less gas
generation, but this is also underutilization of expensive supercapacitors.
[0010] The purpose of the present invention is to provide for the construction
and implementation of a small, low cost, high current carrying device which
combines
the features, characteristics, and advantages, of both rechargeable batteries
and
supercapacitors. As will be noted hereafter, energy storage devices in keeping
with
2
CA 02380954 2002-04-08
present invention will provide for voltage compliance which will be greater
than 1 Volt.
Moreover, the present invention provides for low impedance devices which can
therefore
be used in a number of applications that place high current requirements on
the energy
storage devices which provide electrical energy for the respective electrode
devices.
[0011] Energy storage devices in keeping with present invention can be
customized as to their design so as to maximize their lifetime and yet, at the
same time,
minimize their size.
[0012] The present inventors have quite unexpectedly discovered that these
goals can be achieved in an energy storage device which employs a nickel-based
positive electrode, a carbon-based negative electrode, and an aqueous
electrolyte;
where redox couples are associated with the negative electrode.
PRIOR ART:
[0013] Stepanov et al United States patents 5,986,876 issued November 16,
1999 and 6,181,546 issued Jan. 31, 2001 each teach double layer capacitors.
The' 876
patents teaches a double layer capacitor where one of the electrodes is a
fibrous
carbonic material, and the other electrode is a nickel hydroxide positive
electrode,
together with an aqueous alkali-metal carbonate electrolyte. The fibrous
carbonic
material may be metallized by nickel or copper, in the range of 9% to 60% by
weight of
the electrode.
[0014] The' 546 patents teaches a double layer capacitor where one of the
electrodes is polarizable, and the other of the electrodes is non-polarizable.
SUMMARY OF THE INVENTION:
[0015] The present invention provides an energy storage device having a nickel-
based positive electrode, a carbon-based negative electrode, a separator, and
an
aqueous electrolyte.
[0016] The positive electrode comprises from 3% to 95% by weight of nickel
hydroxide, with the balance being chosen from the group consisting of: nickel
powder,
cobalt powder, carbon powder, and mixtures thereof.
[0017] The negative electrode comprises from 10% to 95% by weight of carbon,
with the balance being at least one redox couple.
3
CA 02380954 2002-04-08
[0018] An electrolyte is employed which is an aqueous alkaline solution.
[0019] Typically, the separator is chosen from the group consisting of: non-
woven nylon separator material, microporous polypropylene, microporous
polyethylene,
and combinations and mixtures thereof.
[0020] Also, typically, an energy storage device in accordance with the
present
invention will be configured as a supercapacitor.
[0021] In keeping with the provisions of the present invention, the positive
electrode is a thin film electrode chosen from the group consisting of: a
vacuum
deposited layer of positive electrode formulation material on a conductive
substrate, a
pasted positive electrode formulation in a conductive nickel foam substrate,
an electro-
precipitated or chemically precipitated positive electrode formulation in a
thin substrate
of a conductive sintered nickel matrix, and combinations thereof.
[0022] Also, the negative electrode is a thin film electrode comprising a
conductive substrate chosen from the group consisting of: porous nickel foam,
porous
copper foam, porous silver foam, and mixtures and combinations thereof,
together with
said negative electrode formulation being loaded thereinto.
[0023] In general, the negative electrode formulation comprises powdered
carbon in the range of 10% to 95% by weight, together with at least one metal-
based
redox couple.
[0024] Typically, the at least one redox couple is chosen from the group of
active
redox couples which include, as their active material, bismuth oxide, indium
oxide, cobalt
oxide, iron oxide, iron hydroxide, hydrides of metals from the Groups IIIA,
IIIB, IVA, IVB,
VB, VIB, VIIB, and VIII, of the Periodic Table, hydrides of mischmetals,
oxides of
mischmetals, and combinations thereof.
[0025] The present invention provides that an energy storage device in keeping
with present invention may have a group of redox couples that is chosen so as
to have
overlapping oxidation voltages, and thereby so as to extend the range of
discharge
voltage of the energy storage device during a discharge operation.
[0026] In the formulation and construction of a negative electrode in keeping
with the present invention, the basis weight of the conductive substrate is in
the range of
200 g/mz to 500 g/m2.
4
CA 02380954 2002-04-08
[0027] Either electrode in keeping with the present invention may have a
thickness of the thin film electrode which is in the range of 0.003 inch to
0.012 inch.
(0028] Still further, the formulation of the positive electrode may further
comprise
at least one of silver (I) oxide, silver (II) oxide, and mixtures thereof.
[0029] Also, the charge and discharge voltage ranges of the positive electrode
may be extended by the addition of selected amounts of cobalt oxide to various
portions
of the positive electrode formulation.
[0030] Another aspect of the present invention is the provision of hybrid
batteries
which will comprise an energy storage device in keeping with the present
invention, in
parallel with a high energy capacity battery, which may be a nickel zinc
battery or a
lithium ion battery.
BRIEF DESCRIPTION OF THE DRAWINGS:
(0031] The novel features which are believed to be characteristic of the
present
invention, as to its structure, organization, use and method of operation,
together with
further objectives and advantages thereof, will be better understood from the
following
drawings in which a presently preferred embodiment of the invention will now
be
illustrated by way of example. It is expressly understood, however, that the
drawings
are for the purpose of illustratian and description only and are not intended
as a
definition of the limits of the invention. Embodiments of this invention will
now be
described by way of example in association with the accompanying drawings in
which:
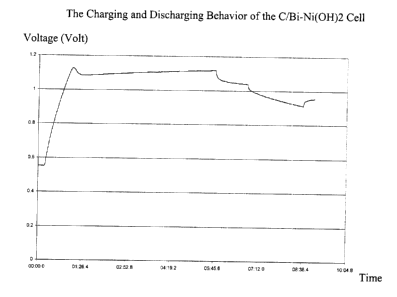
[0032] Figure 1 shows the charge behavior of a fully discharged energy storage
device manufactured in keeping with present invention;
[0033] Figure 2 illustrates the manner in which an energy storage device in
keeping with present invention extends the run time of a high energy battery
by damping
the voltage response thereof to a cut off voltage; and
[0034] Figure 3 demonstrates the extended pulse capability of an energy
storage device in keeping with the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS:
[0035] The novel features which are believed to be characteristic of the
present
invention, as to its structure, organization, use and method of operation,
together with
5
CA 02380954 2002-04-08
further objectives and advantages thereof, will be better understood from the
following
discussion.
[0036] As noted above, the purpose of the present invention is to provide a
carbon-based energy storage device that permits for high current delivery,
which allows
wider voltage compliance and other aqueous based supercapacitors, and which
provides for extended discharge times of the device and any high energy
battery with
which the energy storage device of the present invention is connected in
parallel. All of
this is achieved, as will be discussed hereafter, by the employment of
electrochemical
redox couples at the negative electrode.
[0037] Accordingly, energy storage devices in keeping with present invention
may function alone, or in conjunction with high energy batteries --
particularly, such high
energy batteries which are incapable of supporting high current drains either
as a
consequence of safety concerns or as a consequence of their own high
impedance.
The present invention contemplates that energy storage devices in keeping
herewith
may be used directly in parallel with a high energy battery, but that more
often they will
be used in a circuit with a high energy battery together with a DC/DC
converter and a
switch arrangement which will ensure more controlled and complete charging of
the
energy storage device in keeping with present invention from the high energy
battery
and/or from an external power source.
[0038] A preferred configuration for an energy storage device in keeping with
present invention, so as to provide for low impedance and high current
capability, is
achieved by the provision of highly conductive positive and negative
electrodes which
are also designed to have maximum electrolyte accessibility. A typical
electrolyte which
may be employed with energy storage devices in keeping with present invention,
and
which will promote a good electrolyte conductivity, is 30% potassium hydroxide
solution.
[0039] Each of the positive and negative electrodes is a thin film electrode,
and
each is designed to provide for a high cycle life with high energy density and
high
conductivity.
[0040] Typically, a positive electrode for an energy storage device in keeping
with present invention is nickel-based, comprising nickel hydroxide as the
active
component. Various forms for positive electrodes in keeping with present
invention are
contemplated, including a thin film of positive electrode formulation which
may be a
6
CA 02380954 2002-04-08
vacuum deposited onto a suitable conductive metal substrate, a thin pasted
layer within
a three-dimensional nickel foam, or an electro-precipitated or chemically
precipitated
positive electrode formulation in a thin sintered nickel matrix.
[0041 ] Indeed, the formulation of the positive electrode in keeping with the
present invention may comprise from 3% up to 95% of nickel hydroxide, with the
balance of the active material of the positive electrode formulation being
nickel powder,
cobalt powder, carbon powder, and mixtures thereof. Typically, the addition of
carbon
powder, nickel powder, and cobalt powder, to the positive electrode
formulation is in
excess of 10% by weight of the positive electrode, so as to provide additional
conductivity and capacitive charge for the positive electrode.
[0042] Likewise, a typical negative electrode in keeping with present
invention is
a thin layer of highly conductive carbon which is embedded in a nickel foam,
or silver
foam substrate. The metallic foam substrate, of course, provides particularly
for
electron conductivity within the negative electrode.
[0043] However, in keeping with present invention, the formulation for the
negative electrode may comprise from 10% up to 95% of carbon, with the balance
being
at least one redox couple.
[0044] The employment of redox couples in the negative electrode will extend
the discharge capability thereof beyond that which is normally associated with
the
capacitance and the pseudo-capacitance which is associated with carbon per se.
A
typical redox couple which may be employed in keeping with the present
invention may
include bismuth/bismuth oxide, iron (II) hydroxide/iron (III) hydroxide,
indium oxide,
cobalt oxide, metal hydrides, ather metallmetal oxide materials, and oxides
and
hydroxides of mischmetals.
[0045] It is been discovered that the following are particularly useful for
the at
least one redox couple when it is chosen from the group of active redox
couples which
include, as their active material, bismuth oxide, indium oxide, cobalt oxide,
iron oxide,
iron hydroxide, hydrides of metals from the Groups IIIA, IIIB, IVA, IVB, VB,
VIB, VIIB,
and VIII, of the Periodic Table, hydrides of mischmetals, oxides of
mischmetals, and
combinations thereof.
[0046] The ratio of the carbon to redox couple can be varied, and will
typically be
in the range of 95: 5 to 10: 90, as to the carbon:redox couple ratio. The
chosen ratio will
7
CA 02380954 2002-04-08
be dependent on the size requirement for the energy storage device, as well as
the
required duty cycle lifetime for the energy storage device.
[0047] Moreover, the chemical energy component of the negative electrode may
support the current requirements placed on the negative electrode in a number
of
different ways. For example, the chemical energy component may independently
support the load current; or for an intermittent or pulsed load, it may
provide both load
support and recharge of the double layer capacity of the negative electrode.
However,
because the stored chemical energy component is energy dense, a much smaller
device
than prior art supercapacitors is achieved.
[0048] It should be noted that typical separator materials that may be
employed
in the construction of energy storage devices in keeping with present
invention, to
electrically isolate the negative and positive electrodes, may include
microporous
polypropylene and microporous polyethylene. However, typically conventional
non-
woven nylon separator materials are preferred, so as to enhance any oxygen
recombination which may occur within the energy storage device.
[0049] Several examples now follow which illustrate various embodiments and
utilizations of energy storage devices in keeping with the present invention.
Each of the
examples is described below, and each has resulted in specific test results
that are
illustrated in the accompanying Figures.
Examlale 1:
[0050] A 4 Volt test unit was assembled using four cells which were connected
in series. Each cell contained a high porosity nickel foam, which was such as
to have a
basis weight of less then 500 gm/m2. This conductive substrate was employed
for both
the positive and negative electrodes. The foam was pre-compressed to thickness
of
0.015 inch, and was loaded with active material for each of the respective
positive and
negative electrodes before being compressed to a final thickness of 0.011
inch. Each
cell comprised one positive electrode and two negative electrodes; and the
size of the
electrodes was approximately 1.2 inches by 0.9 inches.
[0051 ] Each of the negative electrodes of each of the cells was loaded with
0.32
g of a mixture of carbon (58%), bismuth oxide (40%), and PTFE binder (2%
8
CA 02380954 2002-04-08
[0052] Each of the positive electrodes of each of the cells was loaded with a
mixture of nickel hydroxide (82%), cobalt oxide (3%), zinc oxide (3%), and a
mixture of
nickel and cobalt powders (10%). The remaining 2% of the formulation comprised
PTFE emulsion binder.
[0053] Each of the individual cells was given a forming charge over 14 hours
at
6 mA, the 4 Volt unit was assembled, and was then tested. The testing
comprised fully
discharging the 4 Volt unit, and then charging it at 312 rriA for
approximately six minutes
before allowing for a rest period, followed by a 312 mA discharge. The result
of the test
is shown in Figure 1.
[0054] It was noted that initially the voltage rose quickly, which is
attributed to
the double layer capacity of the unit. According to the following equation,
that double
layer capacity is calculated as 27 Farads.
Q = CV (Equation 1 )
Where Q is the coulombs passed;
Where C is the capacitance in Farads; and
Where V is the voltage in Volts
[0055] After the initial sharp rise in voltage, is noted that the voltage
profile
flattens out as the bismuth oxide is transformed to bismuth. During the
discharge
period, both the bismuth and the double layer capacitance are discharged.
Example 2:
[0056] Then, the device as described above was placed in parallel with a 1 Ah
lithium ion battery. The lithium ion battery, and the combination of the
lithium ion
battery, were each subjected to a 1.2 mS discharge pulse of 750 mA every 5.2
mS.
[0057] Two curves are shown in Figure 2, the first being identified by the
numeral 20, and the second being identified by the numeral 30. The first curve
20
shows the terminal voltage of the lithium ion battery itself, to a cut off
voltage of 3.6
Volts. It will be seen that the cut off voltage was reached at about 4:20
hours. The
second curve 30 shows the terminal voltage of the parallel combination of the
4 Volt unit
of the present invention together with the lithium ion battery; and it will be
seen that the
cut off voltage was reached at about 5:55 hours. Approximately 95 minutes was
added
to the discharge life of the combination 4 Volt unit/lithium ion battery over
the discharge
9
CA 02380954 2002-04-08
life of the lithium ion battery per se. In other words, the discharge life was
extended by
95/355 min., or approximately 26.7%.
Exam~~le 3:
S [0058] In order to achieve uniform current density in the negative
electrode, it is
advantageous to use a highly conductive powdered carbon having a high surface
area.
Two commercially available products which satisfied that criterion are Black
Pearls
2000T"" and Conductex 975T""
[0059] Moreover, metallic oxide additions to the carbon are favored as redox
couples because of their conversion to conductive metal during formation of
the
negative electrode. However, as noted above, the proportion of carbon to redox
couple
may be dictated in keeping with the specific application to which the energy
storage
device of the present invention is to be applied.
[0060] In the present example, a small prismatic cell was constructed, and
then
assembled into a 6 Volt battery which had dimensions of 4.87 inches by 2.0
inches by
2.25 inches. The 6 Volt battery weighed 800 g; and had a capacitance of 20
Farads.
This capacitance was primarily associated with the surface area of the carbon
(250
mz/gm).
[0061 ] A bismuth oxide/bismuth (Bi203)/Bi redox couple added more than 1
Ampere hour of stored energy to the battery. The device was then subjected to
a series
of 50 Ampere current pulses, each of which lasted for 0.1 seconds. Figure 3
illustrates
the results of that test; and it will be noted that there was minimal fade in
the terminal
voltage.
[0062] However, it should be noted that the theoretical calculation of the
capacitance of the carbon in the battery suggests that it should not have been
able to
support more than four pulses before reaching a cut off voltage of 4.6 Volts
[0063] In many cases, it may be beneficial simply to add a single redox couple
to
the negative electrode, so as to pravide voltage stability above a specific
cut off voltage.
However, there are some occasions when multiple redox couples will provide
certain
advantages. For example, if the energy storage device of the present invention
is to be
charged by the continuously fading voltage of a directly connected parallel
high energy
battery, it may be advantageous to provide several redox couples in order that
the
CA 02380954 2002-04-08
energy storage device of the present invention may be charged over a wider
range. It
will be understood that, typically, such a configuration is that of a
supercapacitor being
connected in parallel with a high energy battery.
[0064] Moreover, it should also be noted that various portions of the nickel
hydroxide active material of the positive electrode may be doped with varying
amounts
of cobalt oxide so as to vary the charge/discharge voltages over a range of
values.
Similarly, silver (I) oxide or silver (II) oxide may be employed by being
admixed to the
active material of the positive electrode. Such techniques as are discussed
immediately
above will obviate the necessity for DC/DC conversion which is otherwise aimed
at
boosting the charging voltages sufficiently high so as to provide for
effective charging.
[0065] Finally, it should be noted that energy storage devices in keeping with
the
present invention, particularly when configured as supercapacitors, may
function not
only as a high current partner to lithium ion batteries, but also as a voltage
limiting
device which is capable of shunting overcharge current around the lithium ion
battery.
In such a configuration, the nickel electrode is appropriately sized to
approach the full
charge before the redox couple in the negative electrode.
[0066] Thus, with the appropriate choice of a negative redox couple, and then
appropriate selection of the number of unit cells in the energy storage
device, the
oxygen recombination cycle for the negative redox couple can be used as a
voltage
limiter for the system.
[0067] There has been described an energy storage device which
advantageously may be configured as a high power battery, or more particularly
as a
supercapacitor. The effectiveness of the energy storage device in keeping with
the
present invention arises as a consequence particularly of the employment of
oxide redox
couples, particularly metal-based oxide redox couples, on the negative
electrode. The
methods of construction of energy storage devices in keeping with the present
invention
are simple and economic; the other components of the energy storage devices,
particularly the alkaline electrolyte and the separator are well known to
those skilled in
the art. Thus, energy storage devices in keeping with the present invention
may be
provided in an economic manner and in large quantities of varying
configurations, sizes,
capacities, and current capabilities.
It
CA 02380954 2002-04-08
[0068] Throughout this specification and the claims which follow, unless the
context requires otherwise, the word "comprise", and variations such as
"comprises" or
"comprising", will be understood to imply the inclusion of a stated integer or
step or
group of integers or steps but not to the exclusion of any other integer or
step or group
of integers or steps.
12