Note: Descriptions are shown in the official language in which they were submitted.
CA 02380957 2002-04-08
DUAL CHEMISTRY HYBRID BATTERY SYSTEMS
FIELD OF THE INVENTION:
[0001 ] This invention relates to hybrid battery systems, and particularly to
hybrid battery
systems having a high power, low impedance battery in parallel with a high
energy battery, where
the battery chemistries of the two component batteries are different.
BACKGROUND OF THE INVENTION:
[0002] Hybrid battery systems are becoming known, and are or will be finding
more
acceptance in various configurations and situations where battery power is
required, either as a prime
energy source - such as in many kinds of portable electrical equipment of all
sorts, power tools,
telephones, computers, and the like - as well as in traction vehicles of all
sorts, and in stand-by
situations. In all configurations of hybrid battery systems, there is a
parallel arrangement of two
disparate batteries, one of which is typically a high power battery, or a
supercapacitor, and the other
of which is a high energy battery. Each has its own charge and discharge
characteristics, its own
impedance characteristics, and its own contribution to the provision of power
to the load. In many
instances, the high energy battery may be called upon to recharge the high
power battery, even
during ongoing load requirements being placed on the hybrid battery system.
[0003 ] Typically, the kind of load requirements that are being contemplated
are those which
~~re pulsed loads, as opposed to steady state loads; moreover, the kind of
Load is such that it may have
periods of high energy requirements and longer periods of low energy
requirements, all of which are
expected to be satisfied by the hybrid battery system.
[0004] The combination of two rechargeable batteries which deliver an enhanced
performance as a "tuned" system is quite simple in concept. As noted, one unit
is designed to have
a relatively low ampere hour capacity, 'but it will have the capability of
high power delivery. The
other unit will have greater ampere hour capacity - sometimes, much greater -
and it is designed to
rnaximize energy density.
1
CA 02380957 2002-04-08
[0005] Put together, such a hybrid battery system can offer a number of
specific advantages
as power sources for devices that have variable or "pulse" load profiles.
Those advantages include
the following:
~ Higher overall energy density.
~ High pulse capability without truncation of life times of the constituent
battery units.
~ Greater service life.
~ Higher safety levels for more volatile high energy density batteries.
[0006] Also, as noted, hybrid battery systems contemplate the use of
supercapacitors as well
.as the use of high power batteries. However, there are certain advantages to
be gained by using low
ampere hour capacity rechargeable batteries instead of supercapacitors. One of
those advantages is,
of course, the higher available ampere hour capacity for high current delivery
from a battery as
opposed to a supercapacitor. Moreover, sustained pulses are possible from a
rechargeable, high
power battery as apposed to a supercapacitor, due to the prospect of the
depletion of the capacitive
charge storage of the battery, and Faradaic energy arising from the
electrochemical reaction in the
battery.
[0007] This is particularly important when it is considered that the demand
for pulsed energy
does not necessarily allow sufficient time for immediate recharge of the high
power pulse device -
t:he supercapacitor or high power battery. Moreover, in such instances as when
a portable computer
is being started at the same time as the onset of wireless communication
through an associated
modem andlor mobile telephone, for example, there may be a sustained high
current drain as the
hard drive of the computer is spun up and wireless communication is initiated.
The concern is, of
course, that the energy requirements that are placed on such as a
supercapacitor may be greater than
the ampere hour capacity of the supercapacitor to deliver.
[0008] Still further, it is now becoming known that there are a number of
combinations of
rechargeable chemistries that may be possible in the configuration of a hybrid
battery system. Thus,
by pairing or combining the highest power density batteries and the highest
energy density batteries,
the greatest performance benefits may be achieved. For example, a high energy
nickel zinc battery
rnay be combined with a high power density lead acid battery; or a high energy
density lithium
2
CA 02380957 2002-04-08
polymer battery may compliment a high power nickel zinc battery or a nickel
metal hydride battery,
or a lead acid battery.
[0009] However, the chose of a particular system must contemplate the
"electrochemical"
compatibility of the constituent batteries, as well as the complexity of the
electronic management
system for the hybrid battery configuration. By referencing the
electrochemical compatibility, it is
meant that care must be taken in interfacing batteries that have different
chemistries which have
distinct thermodynamic signatures. Each battery has unique voltage ranges
associated with its
conditions of charge, discharge, and very little electrochemical activity.
Also, t he voltage ranges
for those various conditions may vary quite considerably from battery to
battery.
[0010] However, those voltage ranges for various conditions of batteries can
be modified
either chemically, or by battery design, so as to fine tune battery
compatibility.
[0011 ] It must be noted, however, that m even easier, and more readily
available approach,
may be arrived at by varying the number of cells in each battery. Careful
matching of the numbers
of cells will ensure appropriate load sharing and efficient transfer of charge
between two parallel
battery strings.
[0012] Of course, the relative impedances of two batteries, and their relative
capacities, must
also be considered.
[0013] Several arrangements may be contemplated, where the operation and
responsibility
of one or the other of the constituent battery units in a hybrid battery
system may vary, depending
on the purposes to which the hybrid battery system is to be put. They include
the following sets of
circumstances, which are discussed in greater detail hereafter;
~ A configuration where the energy battery maintains the power battery fully
charged
over its entire operational range.
~ A configuration where the energy battery maintains the power battery fully
charged
over most of its operational range.
~ A configuration where the energy battery maintains the power battery fully
charged
only when the energy battery itself is fully charged.
~ A configuration where the energy battery is not capable of recharging the
power
battery over the entire voltage range of operation of the hybrid battery
system.
3
CA 02380957 2002-04-08
[0014] A number of various hybrid battery system configurations are
contemplated by the
present invention, having differing electrical couples with respect to the
high power battery and the
high energy battery. Examples of such hybrid battery systems conftgurations,
having different
chemistries - that is having different electrical couples - of the high power
battery and the high
energy battery, are described in greater detail hereafter.
SUMMARY OF THE INVENTION:
[0015] In accordance with one aspect of the present invention, there is
provided a plurality
~of hybrid battery system configurations. However, it will be noted that the
present invention is
particularly directed to dual chemistry hybrid battery systems; and to that
end, the present invention
'teaches a hybrid battery system which comprises a high power, low impedance
battery in parallel
with a high energy battery.
[0016] The high power, low impedance battery, and the high energy battery,
have
substantially equal terminal voltages when they are each fully charged, and at
rest.
[0017] However, the ampere hour capacity of the high energy battery to a
predetermined
cutoff voltage is at least twenty times the ampere hour capacity of the high
power battery to the same
cutoff voltage.
[0018] The electrical couples of the high power battery and the high energy
battery are
different one from the other.
[0019] Indeed, the electrical couples of the high power battery and the high
energy battery
may be chosen from the group of pairs of electrical couples consisting of lead
acid and nickel zinc
batteries, lead acid and lithium ion batteries, lead acid and lithium polymer
batteries, nickel zinc and
lithium polymer batteries, nickel metal hydride and lithium polymer batteries,
and carbon nickel
oxide and nickel zinc batteries.
[;0020] In the case where the pair of electrical couples is carbon nickel
oxide and nickel zinc,
the carbon nickel oxide battery may comprise carbon impregnated into a nickel
foam substrate, and
the nickel electrode is a compressed pasted nickel oxide electrode having a
thickness in the range
of 0.007 to 0.012 inches.
4
CA 02380957 2002-04-08
[0021 ] In that case, the carbon electrode of the carbon nickel oxide battery
may be doped
with a doping material chosen from the group consisting of bismuth oxide, iron
hydroxide, and
combinations thereof.
[0022] Typically, the number of cells in the high power battery differs from
the number of
cells in the high energy battery.
BRIEF DESCRIPTION OF THE DRAWINGS:
[0023] The novel features which are believed to be characteristic of the
present invention,
.as to its structure, organization, use and method of operation, together with
further objectives and
.advantages thereof, will be better understood from the following drawings in
which a presently
preferred embodiment of the invention will now be illustrated by way of
example. It is expressly
understood, however, that the drawings axe for the purpose of illustration and
description only and
~~re not intended as a definition of the limits of the invention. Embodiments
of this invention will
now be described by way of example in association with the accompanying
drawings in which:
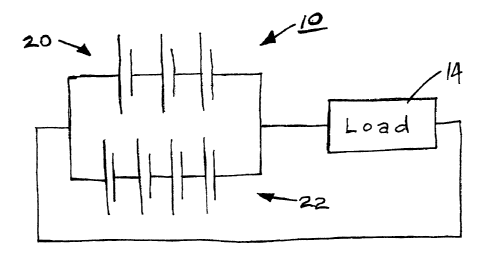
[0024] Figure 1 is a simple circuit showing a hybrid battery system in series
with a load;
[0025] Figure 2 is a simple circuit showing several additions to the circuit
of Figure 1;
[0026] Figure 3 is a composite set of curves showing the effect of coupling a
lead acid
power battery with a nickel zinc energy battery, compared with the performance
of the nickel zinc
battery alone; and
[0027] Figure 4 is a further composite curve showing the effect of coupling a
carbon nickel
oxide battery with a nickel zinc battery, compared with the nickel zinc
battery alone;
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS:
[0028] The novel features which are believed to be characteristic of the
present invention,
as to its structure, organization, use and method of operation, together with
further objectives and
advantages thereof, will be better understood from the following discussion.
[0029] Each of Figures 1 and 2 shows a typical configuration of a hybrid
battery system 10
amd 12, respectively. Each of these hybrid battery configurations comprises a
low impedance, high
5
CA 02380957 2002-04-08
power battery 20 connected in parallel with a high energy battery 22. In each
case, the hybrid battery
configuration 10 or 12 is shown in series with a load 14.
[0030] The hybrid battery configuration 12 also includes a DC to DC converter
16, in series
with a switch 18 which may function to put the DC to DC converter into the
circuit between the high
energy battery 22 and the high power battery 20, in the manner described
hereafter. A pair of diodes
24, which may also be Field Effect Transistors, are shown; one or either of
which may function at
.any time, in the manner described hereafter.
[0031 ] The ampere hour capacity of the high energy battery 22, to a
predetermined cutoff
voltage, is typically at least twenty times the ampere hour capacity of the
high power battery 20, to
ahe same cutoff voltage.
[0032] However, the electrical couples of the high power battery 20 and of the
high energy
battery 22, are different one from the other.
x!:0033] Various examples of disparate pairs of electrical couples of the high
power battery
and of the high energy battery, respectively, are as follows, each of which is
described in greater
detail hereafter;
~ Lead acid high power battery and nickel zinc high energy battery.
~ Lead acid high power battery and lithium ion high energy battery.
~ Lead acid high power battery and lithium polymer high energy battery.
~ Nickel zinc high power battery and lithium polymer high energy battery .
~ Nickel metal hydride high power battery and lithium polymer high energy
battery.
~ Carbon nickel oxide high power battery and nickel zinc high energy battery.
[0034] It will also be noted, as examples only, that the number of cells in
the high power
battery 20 may differ from the number of cells in the high energy battery 22.
Examples of such
configurations are given below.
[0035] Several types of conditions for varying configurations of hybrid
battery systems, in
beeping with the present invention, have been noted above. Specific examples
are now given.
[0036] The first type of configuration is that in which the high energy
battery will maintain
the high power battery fully charged over its entire operational range.
Reference is made to
Examples 1, 2, 3, and 4, as follows;
6
CA 02380957 2002-04-08
Example 1 Lead Acid/Lithium Ion
Cell Lead Acid 3 Cell LiIon Battery Voltage
2.52 Chg 4.2 Chg 12.6
2.28 Chg 3.8 11.4
5 2.16 3.6 10.8
2.04 3.4 10.2
1.80 3.0 9.0
1.68 2.8 8.4
Example 2 Lead Acid/Lithium Polymer
5 Cell Lead Acid :3 Cell Li Polymer Battery Voltage
2.52 Chg 4.2 Chg 12.6
2.40 Chg 4.0 12.0
2.28 Chg 3.8 11.4
2.22 Slo Chg 3. ~ 11.1
1.80 3.0 9.0
Example 3 Nickel Zinc/Lithium Polymer
8 Cell NiZn 4 Cell Li Polymer Battery Voltage
2.10 Hi Chg 4.2 Chg 16.8
2.00 Hi Chg 4.0 16.0
1.90 Chg 3.8 15.2
1.85 Chg 3.7 14.8
1.50 3.0 12.0
Example 4 Lithium Polymer/Nickel Metal Hydride
10 Cell NiMH 4 Cell LiPolymer Battery Voltage
7
CA 02380957 2002-04-08
1.68 Hi Chg 4.2 Chg 16.8
1.60 Hi Chg 4.0 Dischg 16.0
1.52 Chg 3.8 Dischg 15.2
1.48 Chg 3.7 Dischg 14.8
1.20 3.0 12.0
[0037] Each of these examples show the effect, in various dual chemistry
hybrid battery
configurations, of the changes in battery voltage over the entire operational
range of the hybrid
battery. Referring to Example 4, over the normal discharge and charge range of
the lithium polymer
lbattery - that is, from 3.7 volts to 4.2 volts - the high power battery
operates in such a voltage range
that it is always fully charged. In the case of Example 4, in particular, the
available pulse energy is
derived from the double layer capacity of the high power battery. The Faradaic
current of the high
power battery is accessed only in cases of long high current pulses, or in the
event of extremely high
current drains.
[0038] In all of these examples, pulse energy is available over the discharge
life of the energy
battery. However, in cases where overcharging of the power battery 20 is
likely, a diode or FET 24
may be inserted into one or both arms of the hybrid battery configuration, as
shown in Figure 2.
[:0039] Another condition is the situation where the high energy battery 22
will maintain the
high power battery 20 fully charged over most of its operational range. In
this case, reference is
made to Examples 5 and 6.
Example 5 Lead Acid/Nickel Zinc
6 Cell Lead Acid 8 Cell NiZn Battery Voltage
2.45 Chg 1.84 Chg 14.72
2.20 Slo Chg 1.65 13.20
2.15 1.61 12.88
2.00 1.50 12.00
1.87 1.40 11.20
8
CA 02380957 2002-04-08
1.60 ~ 1.20 ~ 9.60
Example 6 bead Acid/Lithium Polymer
Cell Lead Acid 3 Cell Li Polymer Battery Voltage
S 2.52 Chg 4.2 Chg 12.6
2.40 Chg 4.0 12.0
2.28 Chg 3.8 11.4
2.22 Slo Chg 3.7 11.1
2.16 3.6 10.8
(0040] Here, it is seen that the high energy battery will operate with minimal
external
iinfluence so as to maintain the power battery fully charged over most of its
discharge range.
However, in the latter stages of discharge, the voltage of the high energy
battery may be insufficient
to charge or to rapidly replenish the power battery.
X0041 ] In that case, the power battery must be actively charge from the high
energy battery
through a DC to DC converter during the nonpulse periods.
(0042] Again, reference is made to Figure 2, where it can be seen that switch
18 can be
operated during the non pulse periods of the load 14, so as to supply energy
from the high energy
battery 22 through the DC to DC converter 16 to the high power battery 20.
x:0043 ] Alternatively, the power battery 20 may be sized with an appropriate
reserve capacity
that it is sufficient to deliver the remaining pulse energy without recharge.
This, of course, would
normally result in a significantly higher cost for the hybrid battery
configuration.
(0044] Yet another situation where a hybrid battery may be employed is that
when the high
energy battery maintains the high power battery fully charged only when the
high energy battery,
itself, is fully charged.
(0045] In this case, reference is made to Example 7. Here, the high energy
battery does not
have the capability of recharging the high power battery over much of its
discharge range. Such a
hybrid battery may be functional, for example, in automotive starting
situations where charge flow
9
CA 02380957 2002-04-08
is directed to the power battery preferentially. Otherwise, unless the high
power battery 20 is sized
to deliver the pulse loads without recharge, there is a need for DC to DC
conversion as shown in
Figure 2.
Example 7 Lithium PolymerlNickel Metal Hydride
12 Cell NiMH 4 Cell Li Polymer Battery Voltage
1.40 Chg 4.2 Chg 16.8
1.33 4.0 16.0
1.27 3.8 15.2
1.23 3.7 14.8
1.00 3.0 12.0
j0046] Yet another situation for dual chemistry hybrid battery systems, in
keeping with the
present invention, is the case when the high energy battery is not capable of
recharging the high
power battery over the entire voltage range of operation. Here, reference is
made to Example 8.
lExample 8 Nickel Zinc/Lithium Polymer
10 Cell NiZn 4 Cell Li Polymer Battery Voltage
1.68 4.2 Chg 16.8
1.60 4.0 16.0
1.52 3.8 15.2
1.48 3.7 14.8
1.44 3.6 14.4
(0047] It will be seen that both batteries, in this case, must be charged
independently, or the
high energy battery will be utilized to charge the high power battery using DC
to DC conversion.
Such a configuration may be particularly applicable in a situation where the
high energy battery 22
will replenish the high rate battery 20 through the DC to DC converter 16, and
where the high power
battery 22 is the conduit for all current which is supplied to the load 14.
CA 02380957 2002-04-08
[0048] Other tests of dual chemistry batteries in keeping with the present
invention are
discussed below, with reference to Figures 3 and 4.
[0049] First, turning to Figure 3, two discharge voltage characteristic curves
are illustrated
over a period of time. It is seen that the battery voltage axis ranges up to 8
volts, and the time axis
in Figure 3 is over 801 seconds.
[0050] A first voltage discharge characteristic is shown at 32, and it is
representative of the
terminal voltage of a nickel zinc battery when operated on its own, in a
situation to be described
hereafter. The curve 34 is the discharge voltage characteristic of a hybrid
battery system, which
comprised the same nickel zinc battery together with a lead acid battery.
[0051 ] The configuration in this case was a dual chemistry hybrid battery
system which
comprised a three cell high power lead acid battery in parallel with a four
cell high energy density
nickel zinc battery. However, as noted the nickel zinc battery was first
tested on its own.
[0052] The pulse load was a 300 mA current for 1.2 millisecond, with a 4
millisecond rest
between pulses. The nominal voltage of the two batteries was 6 volts.
[0053] It will be seen that up to about 500 seconds, the voltage excursions of
the nickel zinc
battery alone, as shown in curve 32, were quite profound. Also, at about 400
seconds, a cutoff
voltage of 5 volts was being reached.
[0054] On the other hand, it is seen in curve 34 that because of the lower
impedance of the
lead acid battery, the voltage excursions of the hybrid battery were
relatively small up to about 500
seconds. This is because the low impedance of the lead acid battery permitted
such relatively small
voltage excursions during pulse applications. However, below a battery voltage
of about 5.8 volts,
the lead acid battery was unable to be recharged, and the pulse response
returned to normal - at
about 500 seconds. On the other hand, up to a 5 volt cutoff voltage - a quite
normal cutoff voltage
for a nominal 6 volt battery - there was between 50% and 100% increase in run
time.
[0055] Turning now to Figure 4, another set of curves is shown at 42 and 44.
In this case,
curve 42 is representative of the terminal voltage characteristic of a nickel
zinc battery alone; curve
44 is representative of the terminal voltage excursions of a hybrid battery
system. It is seen that the
terminal voltage in this case ranged from about 7 volts up to above 9 volts,
over a time period of
about 27 minutes.
11
CA 02380957 2002-04-08
[0056] The dual chemistries involved in this hybrid battery system are a high
power, thin
film, carbon nickel oxide battery, together with a nickel zinc high energy
battery.
[0057] The manufacture ofthe high power, thin film, carbon nickel oxide
battery comprised
impregnating carbon into a highly conducting nickel foam substrate. The nickel
electrode was
formed in a conventional pasted technology, except that it was compressed so
as to have a film
thickness in the range of 0.007 inches to 0.012 inches - typically, 0.010
inches.
[0058] The utilization of such a high power, carbon nickel oxide battery,
avoids certain
difficulties with respect to lead acid batteries, such as the interface
stability, sulphation of the battery
under discharge conditions, and the like.
[0059] Various carbon nickel oxide batteries were manufactured, including some
in which
the carbon electrode had doping material added to it. The doping material was
an electroactive
material such as bismuth oxide, iron hydroxide, or combinations thereof. The
use of this additional
electroactive material permitted delivery of more than double layer capacity
charge.
[0060] The tests shown in Figure 4 were with respect to a seven cell carbon
nickel oxide
battery having bismuth oxide doping material in the carbon electrode. The
nickel zinc battery was
<r five cell battery, having a capacity of approximately 80 milliampere hours.
The useful capacity
of the carbon nickel oxide battery, in the voltage range of 7 to 9 volts, was
about 3 milliampere
hours.
[0061 ] The nickel zinc battery, and the hybrid battery configuration, were
each subjected to
a 400 milliampere pulse having a pulse duration of 1.2 milliseconds and a 4
millisecond rest between
pulses. It is seen from Figure 4 that the pulse response is much greater for
the nickel zinc battery
alone, as seen by the height of the voltage excursions shown in curve 42.
[0062] More significantly, the pulse response of the hybrid battery system did
not fade as
the discharge of the battery proceeded.
[0063] Thus, to a 6.3 volt cutoff voltage, there was a 100% increase in run
time, as is clearly
seen in Figure 4.
[0064] Dual chemistry hybrid battery systems have been described in some
detail above. It
will, of course, be understood that other disparit chemistries can be
employed, with appropriate
snatching of the electrochemical compatibility, and with appropriate voltage
matching by the choice
~.2
CA 02380957 2002-04-08
~of the number of cells in each of the constituent batteries for the hybrid
battery system. Such choices
may be made, however, without departing from the spirit and scope of the
appended claims.
[0065] Throughout this specification and the claims which follow, unless the
context requires
otherwise, the word "comprise", and variations such as "comprises" or
"comprising", will be
understood to imply the inclusion of a stated integer or step or group of
integers or steps but not to
the exclusion of any other integer or step or group of integers or steps.
13