Note: Descriptions are shown in the official language in which they were submitted.
20-09-2001 2001 17:21 J A KEMP & CO No.08b9 FUS0026111
CA 02381815 2002-02-12
CVO 01/Z1106 . PCTNSOOl2611X
-1-
xAnxo~c~ car oR cv~
' ~ 1_ FieldoftheInvention
1'he present invention xetates generally to the field of medical devices, and
more
particularly, to devices and, methods to avoid vascular restaaosis.
~ ' ' 2. I2~s~.~ '
M~my devices such as steats have been used by physicians to prevent restenosis
of
blood vessels following tr~Gats to oxpa~nd vessels nairoovsd by
arteriosclerosis:
' Following angioplasty, to correct arteriosclerosis reatmosis often. oconrs
because such '
try stimulate excess a prolifetatioa
IO Another solution to 'the problem of na:rowed vessels is to surgically
bypass them
with a prosthesis. Polytetsaftuoroethyleae (PTA has prvvetn adv. as a material
from which to fabricate blood vessel grabs ar prostheses. This is partially
because PTFE is
ex~mely biocampadble causing little or no immuaogenic xeaction when placed
within the
Zm~nan body: ?his is also because in its prafcrred form, expanded: P,3'FE
.(~''~E)~ ~
~g , material is light and porous and can be readily colonized by living cells-
so teat it becomes a
p~a~d part of the body. Unforttmately, the process of suturing such a
pmstsiesis of a
living vessel often stimulates cellular proliferation sim~ar to angioplasty.
The. fas'lure modes
of vascular grafts are fi~equently related -to a ~ lum~al hyper-pmliferative
cellular response
that eveamally affects the flow dy~ics resulting in thrombotic events and
occlasion 'vf , .
EmpfangsAMENDED SHEET
CA 02381815 2002-02-12
WO 01/21106 PCT/US00/26111
-2-
blood flow. Dialysis access grafts typically fail at the venous anastomotic
site due to flow
related intimal hyperplasia. Peripherally placed bypass grafts often fail due
to intimal
thickening at the suture sites.
Clinical research has indicated that ionizing radiation is capable of reducing
S restenosis and preventing cellular proliferation in vascular applications.
However, the
concept of Vascular Brachytherapy is relatively new. Although radiation has
been used for
years in Oncology, its use for the reduction of smooth muscle cell
proliferation and the
reduction of restenosis in vascular applications is recent. Ionizing radiation
has the ability to
damage cellular DNA and can either prevent the cells from dividing or can kill
them
outright. The utilization of a radiation source in vascular graft, especially
if incorporated into
ePTFE, may have the ability to maintain graft patency for longer periods of
time by
preventing the hyper-proliferative responses mentioned above.
A major problem with some current methods of treating restenosis through
radiation
therapy is that the radiation source is present as a fluid within the vascular
lumen, such that
the possibility of leakage is present, potentially causing major injury to the
patient. For
example, U.S. Patent No. 5,616,114 to Thorton et al. discloses an apparatus
and method to
deliver radiation to the walls of a blood vessel through the use of a catheter
with a balloon
tip which balloon can be inflated with radioactive liquid. It would be
desirable to provide
radioactive therapy to areas of the body without the risk of causing injury to
the patient in
the event of a leak caused by balloon breakage.
2001 17:22 J A KEMP & CO No.O$59 P °''"
20-09-2001 . , . . CA 02381815 2002-02-12 US0026111
-3-
Other methods of using radiation to treat restenosis extzploy radioactive
sources (often metallic) delivered by catheter. A d~fficutty with this
approach i9
leaving the catheter in the patient's circulatory system for a long enough
time to
adequately affect restmaosis. A radioactive source of adequate strength so as
to
minimize the indwelling time of ttxe catheter may be so strong as to have a
potential
for overexposure and prove dangerous to work with. A s ounce weak enough to
avoid
overexposure danger may result in problcxns caused by tb~e lengthy indwelling
of the
delivery catheter. Yet another alternative is to place the radioactive sources
oz~ a
metal stem. This approach may cause damage through direct eoutact between the
radioactive stmt and the vessel. Also, it may be difficult to achieve the
desired
pattern of radiation because the pattern is determined by the physical
canstrucdon of
the stmt.
US-A-5873811 discloses an admixture of polymcrie ac~esive witb~
radioactive particles which was cured aver application to a vessel wall.
SLmrIIVIARY OF ~~N~',LON
The present invention is directed to radioactive grafts or cuffs, wherein
radioactive therapy is localized to an afflicted area This can be accomplished
in
several different ways by incorporating radioactive elements into vascular
gza~es ox
similar irnplantable medical devices.
It is an object of this invc~auon to provide an i~ao~pla~atable m~sdical~
device this-... .
utilizes radiation therapy to prevent excess tissue proliferation especially
proliferation resulting in restenosis of blood vessels. '
It is also an object of tbui5 invention to provido a device for radiation
therapy
that does not involve the transport of leak-prone radioactive ~,uid ~kbxough
the body.
EmDfang:AMENDED SHEET
..._.. .w.r:.~,t"" .
~n co~ 2001 11:22 J A KEMP & CO ~o,OgSg n
20-09-2001 ' - ' ' ~ CA 02381815 2002-02-12 US0026111
..
These and additional objects are accomplished by a first aspect of the
invention as defned in claim 1 and by a second aspect of the invention as
defined in
claim 4. The radioactivity may be imparted through tbie incozpoxataon of
radioactive
"seeds", coals, wixes, fluids, etc. eathex epcapsulated, impregnated, wrapped
around or
otbexwise attached to a vascular graft, patch, drape or other implantable
nnedzcal
device. The material of constxuctian could be cy'TFE, polyester, silicon,
polyurethane ox any athet bxo~xedical material. The design of the device and
choice
of the radioisotope material dictates the duration and strength of the
ra,dioaetivxty.
The biomedical material envelops and encapsulates tbo radioactive souxce
preventing
accidental release and providing a "spacer" between the source and the
cellular tissue
to be treated.
The present uavenlaon contemplates five pximary ezzzbadizztents, although one
slailled in the art can appreciate a greaser numbs' of possibilities based on
the
inventive concepts hereiua_ ,A, fast eznbodirn~ent includes incorporating
radioactive
"seeds" (grains, granules, encapsulated radioactive fluid or other radioactive
particles) into a graft eithex alozag ats length ox at proximal and distal
ends. Tb~e seeds
can be placed into the ePTI?'E or other biomedical material prior to extrusion
(e.g.,
coextruded) or fabrication so that they will be embedded into the graft and ~l
havo
au even distribution withita the gz~a~'t. ,A. second embodirttcut uses
radioactive secd$
implanted in the biomedical matcnial as in the first embodiment, but the end
product
is in the form of a "bandage" that is wrapped around a synthetic or natural
vessel, -
axxadiaxing the vessel to inhibit the tissue proliferation. A third
embodime~;t
incorporates radioactive ware into a graft by coiling~the wane along its
length or at
isolated positions (e.g, near the point of aoastamosis with the living
vasculatuare). Zn
the case ofwoven biomedical zaatexials (e.g., polyester) the wire can be
cowoven
with the
Empfanss:AMENDED SHEET
CA 02381815 2002-02-12
WO 01/21106 PCT/US00/26111
=5-
biomedical material. The radioactive "wire" can actually be a beading of solid
plastic (e.g.,
PTFE) which contains radioactive powder or seeds. Such beading can be readily
laminated
to biomedical graft material. A fourth embodiment includes impregnating
radioactive agents
into the wall of a graft. Finally, a fifth embodiment utilizes an encapsulated
stmt graft with .
pockets that are filled with radioactive material. This embodiment can be used
intraluminally or as an interposition graft.
A more complete understanding of the radioactive grafts or cuffs will be
afforded to
those skilled in the art, as well'as a realization of additional advantages
and objects thereof,
by a consideration of the following detailed description of the preferred
embodiment.
Reference will be made to the appended sheets of drawings which will first be
described
briefly.
BRIEF DESCRIPTION OF THE DRAWINGS
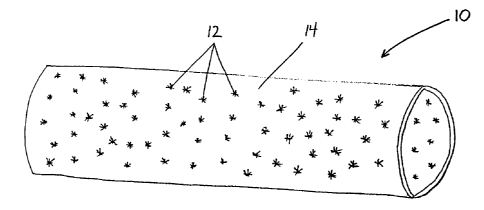
Fig. 1 is a perspective view of a first embodiment of the present invention
with
radioactive seeds dispersed throughout a graft;
Fig. 2 is a cut-away view of a second embodiment of the present invention with
the
radioactive seeds of Fig. 1 dispersed throughout a cuff like device;
Fig. 3 is a perspective view of a third embodiment of the present invention
vaith a
radioactive coil (wire or beading) wrapped around a graft;
Fig. 4 is a perspective view of a fourth embodiment of the present invention
with
pockets of radioactive fluid dispersed throughout a graft;
CA 02381815 2002-02-12
WO 01/21106 PCT/US00/26111
-6-
Fig. 5 is a diagrammatic cross-sectional view of a mandrel and die assembly
which is
used to extrude a graft (especially PTFE) containing radioactive agents.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
The present invention satisfies the need for a medical device to prevent
restenosis
through radioactive elements incorporated therein. This can be accomplished by
mixing the
radioactive seeds or powders into PTFE or other biomedical material before
extrusion of the
device so that the radioactive elements are evenly distributed throughout the
resulting graft
or cuff. Another method of incorporation is to wrap a solid radioactive
element (wire or
beading) around the device during manufacture or just prior to implanting into
the body. It
should be appreciated that the various devices can be fabricated with a non-
radioactive
component that is then rendered radioactive by neutron bombardment prior to
use. This
allows device manufacture without need to worry about radioactive
contamination of the site
or workers. Also, neutron bombardment can be used to generate radioactive
isotopes with
such short half lives that normal manufacture and delivery would be
impractical (that is, the
radioactivity would be significantly decayed by the time the device was
delivered).
Referring now to the drawings, in which like reference numbers represent
similar or
identical structures throughout, Fig. 1 illustrates a first embodiment of a
radioactive graft 10.
The graft 10 is composed of a biomedical material (e.g., ePTFE) 14 with
embedded
radioactive seeds 12. The seeds 12 (either solid particles or radioactive
fluid droplets) are
either co-extruded with the material already in a radioactive state, or are
treated after
CA 02381815 2002-02-12
WO 01/21106 PCT/US00/26111
_7_
extrusion by neutron bombardment or the like. The latter method of forming the
radioactive
seeds 12 would present various advantages with respect to manufacturability,
safety and
shelf life. In the case where the material is PTFE or a similar substance
extrusion will be
explained with reference to Fig. 5 below.
By implanting the radioactive seeds 12 into the ePTFE covering 14, the use of
the
radioactive substance becomes wide ranging. The flexibility of the radioactive
graft 10
enables it to be used in many applications such as on the inside or outside of
a stmt. With
the radioactive element in the form of seeds, the radioactive properties can
be manipulated
based on need. Thus, if a longer duration of treatment is necessary, a long
life, low energy
isotope can be utilized; conversely, if a shorter more intense treatment is
desired, a different
isotope can be employed.
Fig. 2 illustrates a second embodiment of the present invention which is akin
to the
first embodiment. Radioactive cuff 20 includes a strip 24 made of any
biomedical material,
such as ePTFE that is nonabsorbable, with imbedded radioactive seeds 12. As
shown in Fig.
2, the radioactive cuff 20 is wrapped around a body vessel 70 like a bandage,
so that the
radioactive seeds 12 irradiate the proliferating cells 72 within the vessel.
This cuff 20 is
extremely versatile and can be used in many different applications, including
treatment of
non-vascular malignancies such as those found in the synthetic replacement of
a bile duct. In
fact, radioactive cuff 20 can be utilized for nearly any tube or lumen in the
body that is being
occluded by a growth. As with the first embodiment, the radioactive properties
of the seeds
12 can be chosen based on the specific application of the device, although an
isotope with a
CA 02381815 2002-02-12
WO 01/21106 PCT/US00/26111
_g_
very long half life emitting relatively low energy radiation will normally be
preferred. As in
all cases of the present invention, the ePTFE prevents direct contact between
the vascular
tissue and the radioactive source. Thickness of the ePTFE can be selected to
provide the
ideal spacing between the source and the tissue.
Fig. 3 shows a third embodiment of the present invention. The graft structure
30
includes a graft or tubular member 34 with a radioactive wire 32 coiled
radially around the
outside surface, extending along its length. Beading as of PTFE containing
radioactive
material can be used in place of wire. Beading can be readily laminated to the
graft as can
wire clad in an appropriate plastic material. If the biomedical material is
woven or knitted,
the wire or beading can also be woven or knitted into the structure. The graft
structure 30
can be used in conjunction with a stmt in which the graft structure 30 is used
to cover either
a luminal or abluminal stent surface for insertion into a body lumen. The
radioactive wire 32
would then act to reduce the possibility of restenosis after the stmt was
deployed by
eliminating the proliferating cells. Most likely the radioactive wire or
beading would be
limited to the end regions of the graft 34 where it is sutured to the
patient's vasculature. The
radioactive wire 32 can be attached to the graft with adhesives.
Alternatively, the wire. can
be coated with PTFE (e.g., by inserting the wire 32 into an elongate PTFE tube
of a slightly
greater diameter than the wire 32) or other plastic. This coated wire can then
be adhered to
the graft 34 through heat and pressure or through the use of an adhesive.
Fig. 4 illustrates a fourth embodiment of the present invention. In this
embodiment,
the radioactive agent is again incorporated into an ePTFE or other biomedical
material
20-09-2001 US0026111
.~rN. 1UU1 II:'tt ~ A KEMP & CO No.0869 P~ U/IV
CA 02381815 2002-02-12
wo oxnx~o6 ~ ~ rcr~soa~zsm
.9..
member. Flerc, !he radioactive substance is in the form of a liquid wharein a
radioaetrve
solutiQ~a is prepared sad small droplets of the solution aro encased by. tu~~
plastic shells. The
.rcsulnng radioactive balls 4Z arc co-a~t~ded with the biomedical material,
44; creat~ug a
radioactive grad 40 contaiaiag small pockets 46 filled with radioactive balls
42. Beside co-
. ~truding techniques, the graft 40 can also be created by implanting or
enezpsulating tire
t ''
radioactive balls 42 after graft fabrication.
In a Bdlh aabodiment; much Iike the first and third cmbodituents, radiosdive
or
radiophatmaceutieat aarc izupregaated directly into the wall of as ePTFE graft
without
boing aggregated as "sends". in this case, the radioactive agent can come iri
the foes of a
IO gnouad up solid or powder which is coextruded with the PT~1~. Whit
refexence to the first,
third and fifth embodiments, Fig. 5 illustrates a ram extruder assembly 50 for
co-acrtuding a
billet of material, whirls is this case consists of PTFE mixed wilt: any of
the radioactive
agents described. The ram extzuder assembly 50 includes as extrusion bxael
52,. an
extrnsiau die 54, a mandrel 55, and g ram 58. The billet of material S9 is
placed within tho .
I S cxtnuion barrel 5Z. Force is applied to i~a s8 which in tern cxpols
pressiu~e on Errs YtiIIet of ,
. ~aI 59. The gr~ causes the bola of tnataial 59 to be extruded around the
mandrel . .. _
56, through t~ttc ion die 54 so that it issue as a tubular a~ctrud~e 60. An
arrow 62
shows tbc direction of the ~ctrarion. The tubular adtudate b0 is then eapaaded
and s
is acdordaace wig , the atpsas:.on and sinteaag Procedures tmdcrtakea with
puss PTFE
ZO vascular grafts which errs well known in the art. 'Where plastic aarased
radioactive HqQid
ball 4,i are used, earapsulation materials arc selected so that the~exttusion
process does not
Emvfane; ~, ~~ ~ _ ,~..~
AMENDED SHEET
CA 02381815 2002-02-12
WO 01/21106 PCT/US00/26111
-10-
result in rupture of the balls 42 and release of radioactive liquid.
Having thus described a preferred embodiment of the radioactive graft, it will
be
apparent to those skilled in the art how certain advantages of the present
invention have been
achieved. It should also be appreciated that various modifications,
adaptations, and
alternative embodiments thereof may be made within the scope and spirit of~the
present
invention. Some examples have been illustrated with ePTFE as a biomedical
material, but it
should be apparent that the inventive concepts described above would be
equally applicable
to polyester, organosilicon, polyurethane or any other biomedical material
that can be
extruded or woven. Moreover, the words used in this specification to describe
the invention
and its various embodiments are to be understood not only in the sense of
their commonly
defined meanings, but to include by special definition in this specification.
Thus, if an
element can be understood in the context of this specification as including
more than one
meaning, then its use in a claim must be understood as being generic to all
possible
meanings supported by the specification and by the word itself. The
definitions of the words
or elements of the following claims are, therefore, defined in this
specification to include not
only the combination of elements which are literally set forth, but all
equivalent structure,
material or acts for performing substantially the same fimction in
substantially the same way
to obtain substantially the same result. The described embodiments are to be
considered
illustrative rather than restrictive. The invention is further defined by the
following claims.