Note: Descriptions are shown in the official language in which they were submitted.
. _ . . . ~ _
CA 02383226 2002-02-26 L J w ~~~f4:
. .. .s " "s, .. .
. ~ .. . . . . . , , ,
Subs ~.i tote ~he~t"; ,
. . . . . . . .
-_ 1. _ .. .. .. . .. ...
._2104.10 PCT
10
~ FLOW CONTROL AND
FILTRATATION METHOD AND APPARATUS
This application claims benefit and incorporates by
reference the entire disclosure of U.S. Provisional
Application No. 60/153,647, filed September 13, 1999.
FIELD OF THE INVENTION
The present invention relates to flow control and
filtration methods and apparatuses applicable to
medical devices, drug delivery devices, food dispensing
devices, aerosol generation devices and the like.
BACKGROUND OF THE INVENTION
Fluid dispensing devices are used for a variety of
applications, including, for example, the delivery of
medicaments, the dispensing of food stuffs, the
AMENDED SHEET
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
- 2 -
dispensing of cleansers or hair sprays and the like.
Such devices may typically include an aerosol
generation surface and a channel for conveying fluid,
with the channel having an inlet in fluid communication
with a reservoir and an outlet positioned above the
aerosol generation surface. The fluid to be delivered
is contained in the reservoir.
Conventional fluid dispensing apparatuses, however,
lack cost effective and adequate methods and devices
for controlling the flow of fluid. Moreover,
conventional fluid dispensing devices, especially drug
delivery devices, suffer from lack of effective
mechanisms for controlling evaporation and
contamination of the fluid reservoir. Mechanical
devices are not considered a viable option due to their
high cost and unreliability (high rate of failure).
Thus, there exists a need to develop a cost effective
and reliable shut-off valve for controlling fluid flow
and preventing evaporation and contamination of the
fluid reservoir.
SUMMARY AND DESCRIPTION OF THE INVENTION
The present invention presents new and unique methods
and apparatuses for closing off and controlling the
fluid flow in a fluid dispensing system used in medical
devices, drug delivery systems, food dispensing,
aerosol generation and the like.
A porous material is used in the present invention for
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
- 3 -
both a flow control regulator and/or a pressure
dependent shut-off valve for fluid dispensing. The
porous material may also be used to prevent seepage of
fluid from a pressurized fluid source, minimizes
evaporation of fluid contained therein, and/or to
prevent contamination.
The present invention presents a closure and/or a flow
control valve for fluid dispensing systems and the
like. The fluid metering and dispensing system
according to the present invention delivers a fluid
from a source reservoir to a dispensing location by
application of a pressure drop.
To protect the source reservoir and minimize
evaporation, a shut-off valve in the fluid conveyance
path is established. Mechanical shut off valves such
as check valves, poppet valves, flapper valves,
duckbill valves and others are too costly and too
unreliable.
Thus, the present invention provides novel methods and
apparatuses wherein a porous media inserted in the
fluid conveyance path of fluid dispensing systems acts
as a shut-off valve, fluid flow control mechanism, and
contamination preventative device.
The porous media requires a predetermined activation
pressure drop or threshold pressure to establish a flow
of fluid. At any pressure below the activation
pressure, fluid will not flow. Thus, the pressure drop
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
- 4 -
of the porous media acts as a shut-off valve when the
pressure drops below a certain level. Such a pressure
drop occurs in an inhalable drug delivery device upon
the inspiratory effort of a user.
The size of pores, overall pore volume, and to a
certain extent the hydrophillic/hydrophobic balance of
the porous material, determines the amount of pressure
necessary to initiate flow (i.e., the threshold
pressure). These variables also determine the rate of
flow in the material. For example, some porous media
promote free-flow after the threshold pressure has been
reached. The flow promoted in other porous media, once
the threshold pressure has been met, increases as the
pressure increases, reaching a maximum predetermined
value.
Pore size also limits the size of the particles
entering and leaving the porous media. Thus, the size
of the particles leaving the fluid dispensing apparatus
are limited to a particular size, as well as
substantially eliminating fluid loss due to vapor
pressure (evaporation of the fluid) in the fluid
reservoir. Accordingly, the porous valve may be
considered a filtration device as well, filtering the
fluid to dispense only fluid particles of a certain
size.
The particle size limitation also results in
substantially eliminating contamination of the fluid in
the fluid reservoir. Biological contamination is
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
- 5 -
effectively prevented from traversing the porous
material since the biological contaminants will not fit
through the pores of the porous valve. The pore size
can be manufactured to a particular pore size to
prevent a particular type of biological cell from
contaminating the fluid reservoir.
For example, pores can be sized to prevent the
following biological bodies from entering the fluid
path or reservoir:
- viruses: from 0.05 to 0.1 microns;
- bacteria: from 0.5 to 1.5 microns;
- red blood cells: 5 microns; and
- lymphocytes: from 5 to 8 microns.
Additionally, sizing of the particles leaving the fluid
reservoir is important in many fluid dispensing
systems. For example, in drug delivery systems,
especially inhaler devices, the medication dose for
dispensing into a patient is more readily absorbable at
a particular particle size.
The hydrophillic/hydrophobic balance of the porous
material and porosity can be adjusted to minimize
evaporation of fluid from the source reservoir through
the porous media valve. Porous valves according to the
present invention have reduced evaporative losses by
60$ over mechanical valves.
Thus, by changing the parameters such as the thickness
of the porous material, the size of the pores, the pore
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
- 6 -
volume, and the hydrophillic/hydrophobic balance of the
material, an infinite number of activation/closure
pressures and/or flow rates can be achieved.
Accordingly, in one aspect of the present invention, a
pressure valve for an outlet of a fluid reservoir of a
fluid dispensing device includes a porous media.
In another aspect of the present invention, a pressure
valve for a fluid dispensing device includes a fluid
conveyance channel having an internal diameter, an
outlet and an inlet in fluid communication with a fluid
reservoir. The valve also includes a porous media
positioned adjacent to the outlet.
In yet another aspect of the present invention, a
dispensing tube for a fluid dispensing device includes
a fluid conveyance channel having an internal diameter
where the channel includes an outlet positioned above
an aerosol generation surface and an inlet in fluid
communication with a fluid reservoir. The dispensing
tube also includes a pressure valve adjacent to the
outlet where the valve includes a porous media.
In yet another aspect of the present invention, a
pressure regulator for an outlet of a fluid reservoir
of a fluid dispensing device includes a porous media.
In yet another aspect of the present invention, a
regulator for a fluid dispensing device includes a
fluid conveyance channel having an internal diameter
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
with an outlet positioned above an aerosol generation
surface and an inlet in fluid communication with a
fluid reservoir. The regulator also includes a porous
media positioned adjacent the outlet.
In yet another aspect of the present invention, a
porous media for a fluid dispensing device includes a
porous plastic having a pore size between approximately
about 0.02 microns to 0.80 microns.
In yet another aspect of the present invention, a
pressure valve system for a fluid dispensing device
includes means for conveying a fluid from a fluid
reservoir to an aerosol generation surface and porous
means in fluid communication with the conveying means.
The porous means allows fluid to flow onto the aerosol
generation surface upon the application of a pressure
drop.
In yet another aspect of the present invention, a
method of dispensing a fluid in a fluid dispensing
device is presented wherein the fluid dispensing device
includes a fluid reservoir having an outlet positioned
adjacent an aerosol generation surface, an airflow
flowing over the surface, and a valve comprising a
porous media positioned adjacent the outlet. The
porous media includes a predetermined activation
pressure. The method includes the steps of applying
the predetermined activation pressure across the porous
media, dispensing the medicament onto the aerosol
generation surface and entraining aerosolized
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
_ g _
particles generated by the surface into the airflow.
In yet another aspect of the present invention, a
system for dispensing a fluid in a fluid dispensing
device is presented. The fluid dispensing device
includes a fluid reservoir having an outlet positioned
adjacent an aerosol generation surface, an airflow
flowing over the surface, and a valve comprising a
porous media positioned adjacent the outlet. The
porous media includes a predetermined activation
pressure. The system comprises means for applying the
predetermined activation pressure across the porous
media, means for dispensing said medicament onto the
aerosol generation surface, and means for entraining
aerosolized particles generated by the surface into the
airflow.
In yet another aspect of the present invention, a
method of dispensing a medicament in a drug delivery
device is presented. The drug delivery device includes
a medicament reservoir having an outlet positioned
adjacent an aerosol generation surface, an airflow
flowing over the surface, and a valve comprising a
porous media positioned adjacent the outlet. The
porous media includes a predetermined activation
pressure. The method includes the steps of applying
the predetermined activation pressure across the porous
media, dispensing the medicament onto the aerosol
generation surface, and entraining aerosolized
particles generated by the surface into the airflow.
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
_ g _
The above aspects of the present invention will become
more clear with reference to the following figures and
written description.
BRIEF DESCRIPTION OF THE DRAWINGS
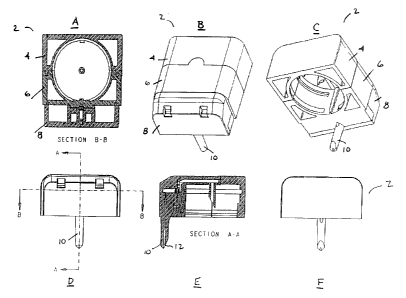
Figs. lA-1F illustrate views of an assembled three-
section fluid dispensing system.
Figs. 2A-2F illustrate views the end section of the
fluid dispensing system shown in Figs. lA-1F.
Figs. 3A-3G illustrate views of the center section of
the fluid dispensing system illustrated in Figs. lA-1F.
Figs. 4A-4F illustrate views of the rear section of the
fluid dispensing system illustrated in Figs. lA-1F.
Fig. 5 is a side-sectional view of a dispensing tube
having a porous material valve according to the present
invention.
Fig. 6 illustrates a view of the porous valve according
to the present invention.
Fig. 7 shows a chart illustrating efficiency of a fluid
dispensing system using a porous valve according to the
present invention.
Fig. 8 shows a chart illustrating the performance of a
porous valve according to the present invention.
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
- 10 -
Fig. 9 shows a chart illustrating the performance
summary of a drug delivery device using the porous
valve according to the present invention.
Fig. 10 shows a chart illustrating water flowrate
versus tube dimension.
Fig. 11 shows a chart illustrating the tube pressure
drop for the inner tube diameters listed in Fig. 10.
Fig. 12 illustrates the pressure calculation for an
inhaler device.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Figs. lA-1F illustrate various views of an assembled
three-section fluid dispensing system 2 having a porous
valve according to the present invention. The system
includes rear section 4, center section 6, and spout
section 8, containing spout 10 and spout tip 12.
Figs. 2A-2F illustrate various views the end section of
the fluid dispensing system shown in Figs. lA-1F
containing a spout which includes the porous material
valve according to the present invention.
Figs. 3A-3G illustrate various views of the center
section of the fluid dispensing system illustrated in
Figs. lA-1F.
Figs. 4A-4F illustrate various views of the rear
section of the fluid dispensing system illustrated in
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
- 11 -
Figs. lA-1F.
Fig. 5 is a side-sectional view of a dispensing tube 14
for a fluid dispensing system having a porous material
valve 18 at the end 16 of the dispensing tube 14 which
is in fluid communication with a fluid reservoir.
Fig. 6 illustrates various views of the porous valve
according to the present invention.
Fig. 7 shows a chart illustrating the efficiency of a
fluid dispensing system using a porous valve according
to the present invention versus particle size. The x-
axis represents the size of particles allowed to pass
through the porous filter and the y-axis represents the
device efficiency in percentage. The graph illustrates
that as the particle size increases, so does the
efficiency of the device. In regard to the present
invention, efficiency increases with the porous valve
according to the present invention over simply using a
long tube average. Average efficiency increase is over
7~.
Fig. 8 shows a chart illustrating the performance of a
porous valve according to the present invention when
used with an open mouthpiece, inhaler-type drug
delivery device versus using the same device with no
valve at all. The graph illustrates respirable
fraction in percentage, respirable dose in ug and
device efficiency in percentage. When the porous valve
is use, each category shows improvement.
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
- 12 -
Fig. 9 shows a chart illustrating the performance
summary of a drug delivery device using the porous
valve according to the present invention. The chart
lists a particular specification, the target value, the
original value which was obtained by using a long metal
tube with a fluid dispensing system, the measured value
when using the porous filter according to the present
invention, and a benchmark which was obtained from a
best in class - nearest commercial product.
It is clear from the chart of Fig. 9, that placement of
the porous valve in the fluid conveyance path yields
the following:
- increased efficiency of a fluid dispensing
system;
- delivers the fluid more consistent and
uniform to aerosolizer mechanism of an
inhaler device (piezoelectric horn);
- lowers evaporative losses by approximately
60$;
- enhances metering accuracy by approximately
4~; and
- is relatively low in cost.
Fig. 10 shows a chart illustrating water flowrate
versus the dimension of the tube dispensing the fluid
from the fluid reservoir. The chart includes columns
for tube inner diameter (in inches and cm), tube
flowrate (in cm3/s and ,uL/s), droplet diameter (in cm),
the mass of the droplet (in grams), and finally, the
force (in grams) required to pass the droplet through
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
- 13 -
the porous material.
Fig. 11 shows a chart illustrating the tube pressure
drop for the inner tube diameters listed in Fig. 10.
For a given inner tube diameter, the pressure drop, or
pressure required to drive the fluid through the porous
material is listed (in millibar and psi).
Fig. 12 illustrates the maximum pressure calculation
for flow through the porous media. As shown, the
maximum force generated is 20N, and the maximum
pressure generated at the tip of the cannula is 2,571
1b. f/in2.
Examples of porous materials include polysulfone,
polyether sulfone, cellulose acetate, nylon,
polyvinylidene fluoride, polytetraflouoroethylene and
polyolefin. Specific examples of porous material that
may be used are Sartorious 0.2 or 0.45 micron cellulose
acetate CA 12587, Millipore 0.20 micron GVPH 2935,
Millipore 0.22 micron GSW P096 or Pall 0.22 micron
nylon media.
Pore sizes may be between approximately about 0.05 to
1.0 microns, and more preferably between approximately
about 0.15 to 0.90 microns, and most preferably between
approximately about 0.20 - 0.80 microns.
A preferred porous valve configuration for a drug
delivery device according to the present invention
includes a fluid conveyance channel having an internal
CA 02383226 2002-02-26
WO 01/19438 PCT/US00/25145
- 14 -
diameter of 0.5 mm, a 0.5 mm gap, with 2.0 mm above a
piezoelectric aerosolizer device (device for producing
aerosolized particles from a fluid).
Medicament is dispensed in a drug delivery device
according to the present invention by applying a
pressure drop across the porous media of the medicament
dispensing tube. Such a pressure drop may be applied
as a result of the inspiratory effort of a user of the
drug delivery device, which also causes an airflow
within the device. Concurrently, or shortly thereafter
the application of the pressure drop, medicament is
dispensed onto an aerosol generation surface of the
drug delivery device. The medicament is aerosolized
and then entrained in the airflow which was created by
the inspiratory effort.
The following example is illustrative of the present
invention:
A commercial, porous filtration material was inserted
into a small diameter cannula in a drug delivery
device. The material included hydrodynamic pores
having diameters of 0.45 microns (preferably no less
than 0.2 microns), and a total thickness less than
0.010". This material included an appropriate surface
area and pressure drop characteristics to enable the
drug delivery apparatus to dispense 300 micro liters
per second fluid flow at a 10 psi pressure drop. The
porous material allows particles and fluid with mean
diameters less than 200 nanometers to pass.