Note: Descriptions are shown in the official language in which they were submitted.
CA 02383415 2002-03-13
WO 01/20042 PCT/US00/25423
METHOD FOR PROCESSING BIOLOGICAL SAMPLES
BACKGROUND OF THE INVENTION
The present invention relates to methods, compositions and systems for
isolating
materials from biological samples.
Multiplexed short tandem repeat ("STR") analysis of human DNA has been found
to be a very useful technique for the identification of persons for law
enforcement
purposes. In order for the data generated to be accepted in a court of law,
the STR
method requires the use of DNA from a single individual that has been
isolated, purified
and quantified using a highly reproducible protocol. When applied to the
analysis of
sexual assault evidence, difficulties are encountered because the samples
which are
~ 5 collected often contain cellular material from both the victim and the
suspect. Extraction
of the total DNA from the cellular mixture yields DNA from both persons in an
unknown
ratio, often with more of the victims DNA than the suspect's. Current
protocols call for
the separation of the male (sperm) and female (e.g. epithelial) components
before the
DNA isolation step by using a single differentiating feature of these cell
types: epithelial
2o cells tend to lysis more quickly with detergents than do sperm cell (under
non-reducing
conditions). There is no a priori reason to expect these protocols to yield a
complete
separation of the male and female fractions. If the sample contains far more
of the
victims DNA then the suspects, which is often the case, then STR analysis
often reveals
only the victim's genotype, leaving the suspect unidentified.
25 Much effort has been invested in the generation of highly selective
antibodies for
binding to specific biological targets, such as human sperm. Hybridoma cell
lines which
produce antibodies which are found to be useful for in vitro assays (i.e.
ELISA) and
pharmaceutical applications (e.g. birth control) are commercially available
(e.g. ATCC
HB-9762, HB-255 and HB-10039).
3o Photoaffinity labeling has become a popular technique for studying the
binding
interactions between biomolecules which accompany most biological events.
According
to this technique, one biomolecule expected to be involved in a binding event
is decorated
with a chemical group which will form covalent bonds to a second involved
biomolecule
during or after light activation.
CA 02383415 2002-03-13
WO 01/20042 PCT/US00/25423
One popular photoaffinity labeling technique uses the arylazide group for the
light
activated attachment step. When light activated, arylazides lose diatomic
nitrogen
producing the reactive nitrene intermediate. Nitrenes are known to form
covalent bonds
to neighboring molecules by addition to unsaturated linkages or insertion into
single
covalent bonds (C-H or C-C). Thus, attachment of the arylazide group to one
molecule
allows it to be covalently coupled to a second molecule. This property of the
arylazide
group has been applied in the area of bioconjugation (e.g. biotin labeling)
and protein
crosslinking, as described in B. Lacey & W.N. Grant, Anal. Biochem. (1987)
Vol. 163, p.
151 and U.C. Krieg, et al., Proc. Natl. Acad. Sci. USA (1986) Vol. 83, p.
8604.
Active esters of photoaffinity labels such as arylazides are commercially
available
and protocols for their use in decorating proteins are well developed, as
described in D.A.
Geselowitz & R.D. Neumann, Bioconjugate Chem. (1995) Vol. 6, p. 502 and A.C.
Forster, et al., Nucleic Acids Research (1985) Vol. 13, p. 745. NHS activated
esters, for
example, will react with primary amino groups of proteins (e.g. lysine
residues)
~ 5 producing stable amide bonds. Photoactivation of the decorated protein,
after binding to
a receptor, will produce a covalent adduct between the photoaffinity label and
the
receptor.
The automated isolation of DNA for PCR amplification is a topic of current
interest. The development of magnetic bead methods for DNA isolation is seen
as being a
2o generally useful activity, increasing the throughput and reproducibility of
the PCR
method as a whole. Many new technologies of this type have appeared recently
which
have not been tested for STR amplification. For example, Dynal Corporation has
introduced a magnetic bead protocol which has become quite popular in
molecular
biology research and is finding applications in the clinical laboratory.
Hardware for the
25 automation of the Dynal method is commercially available (Biomek 2000,
Beckman
Coulter, Inc., Fullerton, California). Automated assays employing magnetic
beads, such
as the Isolex 300i system for CD34 cell isolation available from Nexell
Therapeutics,
Inc., of Irvine, California, have reported and are commercially available.
SUMMARY OF THE INVENTION
In general, in one aspect, the invention features a method of processing a
biological sample. The method includes providing a separation reagent,
reacting the
2
CA 02383415 2002-03-13
WO 01/20042 PCT/US00/25423
biological sample with the separation reagent to capture a target species in
the biological
sample, creating an adduct of the target species and the separation reagent
and separating
the adduct from the biological sample. The separation reagent includes a
microparticle
and a receptor for a ligand on the target species
Preferred embodiments of the invention include one or more of the following
advantageous features. The covalent adduct is formed by activating a
photoaffinity label
coupled to the separation reagent. Separating the adduct includes capturing
the
microparticle magnetically, by filtration, or by centrifugation. The receptor
includes at
least one binding protein, which can be an antibody. The biological sample is
a forensic
sample and the target species is a sperm cell. The method includes releasing a
DNA from
the sperm cell, magnetically removing the adduct, and analyzing the DNA .
In general, in another aspect, the invention features a separation reagent for
a
biological sample. The separation reagent includes a microparticle, a receptor
coupled to
the microparticle, and a photoaffinity label coupled to the receptor.
Preferred
~5 embodiments of the invention include one or more of the following
advantageous
features. The microparticle is a magnetic bead. The receptor includes at least
one
binding protein, which can be an antibody. The photoaffinity label includes an
arylazide.
The arylazide includes a nitroarylazide.
In general, in another aspect, the invention features an automated system for
2o processing a biological sample. The automated system includes means for
providing a
separation reagent, means for reacting the biological sample with the
separation reagent
to capture a target species in the biological sample, means for creating an
adduct of the
target species and the separation reagent and means for separating the adduct
from the
biological sample. The separation reagent includes a microparticle and a
receptor for a
25 ligand on the target species
In general, in still another aspect, the invention features an apparatus for
processing a biological sample. The apparatus includes a first chamber for
receiving the
sample, a first capture means proximate to the first chamber for capturing a
separation
reagent, a second chamber in fluidic communication with the first chamber, and
a second
3o capture means proximate to the second chamber for capturing the separation
reagent. In
preferred embodiments, the first and second capture means can include
electromagnets.
Advantages that can be seen in implementations of the invention include one or
more of the following. Capturing the target species with a selective receptor
provides a
CA 02383415 2002-03-13
WO 01/20042 PCT/US00/25423
high degree of selectivity for the target species in the biological sample.
The permanent
attachment of the target species and separation reagent using a photoaffinity
label allows
for the complete separation of the target species from the biological sample.
The use of
microparticles provides a large surface area for the permanent attachment of
receptors,
enabling the efficient capture of a large percentage, or all, of the target
species molecules
from the biological sample. The use of magnetic beads for sample separation
and DNA
isolation avoids the need for centrifugation steps, thereby enabling the full
automation of
sample processing and yielding highly reproducible results.
The details of one or more embodiments of the invention are set forth in the
accompanying drawings and the description below. Other features and advantages
of the
invention will become apparent from the description, the drawings, and the
claims.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. I is a flowchart illustrating a method of isolating a target species from
a
~ 5 biological sample.
FIG. 2 illustrates the synthesis of a photoactivatable separation reagent
according
to the invention.
FIG. 3 illustrates the use of a photoactivatable separation reagent to isolate
DNA
from a target species in a biological sample.
20 FIG. 4 is a schematic diagram of an apparatus for isolating a target
species from a
biological sample according to the invention.
Like reference numbers and designations in the various drawings indicate
like elements.
4
CA 02383415 2002-03-13
WO 01/20042 PCT/US00/25423
DETAILED DESCRIPTION
The invention provides methods, compositions and systems for processing
biological samples using microparticulate separation reagents to capture a
target species
from a biological sample. As shown in FIG. 1, the methods begin with the
preparation of
a separation reagent (step 100). The separation reagent is formed by binding a
receptor -
for example, a binding protein having affinity for a target species in the
biological sample
- to a microparticle, such as a coated magnetic bead, as will be described in
more detail in
connection with FIG. 2 below. Microparticles for use in the invention
generally have
dimensions of from about 1 millimeter to about 1 nanometer and may be
fabricated from
a wide variety of materials, including latex polystyrene, colloidal metals or
other
appropriate substances using known techniques. The prepared separation reagent
is
reacted with a biological sample known or suspected to contain the target
species (step
110), and an adduct of the separation reagent and the target species is formed
(step 120),
as will be described in more detail in connection with FIG. 3 below. This
adduct is
separated from the biological sample (step 130), and the target species is
released for
further analysis free of the biological sample (step 140).
Refernng to FIG. 2, in one embodiment photoactivatable separation reagents for
specific target species are prepared by decorating microparticles 200 with
target-specific
receptors 210 and attaching chemical species 220 to these receptors that will
form
2o covalent bonds between the receptor and the target species after
photoactivation. In the
described embodiment, the target species is human sperm and the receptor is a
binding
protein having an affinity for human sperm - for example, an anti-sperm
antibody or
mixture of anti-sperm antibodies such as those produced by cell lines ATCC HB-
9762.
HB-25~ and HB-10039. Ideally, the receptor should (1) not be crossreactive
with other
cell types found in the biological sample (or be minimally crossreactive); (2)
bind to the
target species with high affinity; and (3) and in this embodiment, bind
selectively to the
head of the sperm cell. The selective binding to the sperm head is important
because the
head contains the DNA targeted for isolation and the tails of the sperms are
often found
missing in case samples.
CA 02383415 2002-03-13
WO 01/20042 PCT/US00/25423
Appropriate receptors can be identified by screening for affinity and the
orientation of binding using known techniques -- for example, microtiter plate
based
fluorescence assays and optical microscopy. While the invention is described
in this
embodiment, those skilled in the art will recognize that the principles of the
invention can
be applied advantageously to other targets and receptors, such as detection of
pathogens
in food, profiling organisms present in environmental samples, separating
fetal blood
cells from maternal blood cells for cytogenetic analysis, isolation of
lymphocytes or other
cells from whole blood, etc.
As shown in FIG. 2, selective reduction of the disulfides which connect the
heavy
chains of an antibody 210 (e.g. with 2-aminoethanethiol) produces antibody
fragments
230 which carry reactive sulfhydryl groups 240 on a portion of the antibody
fragment
which is a distance from the Fab region 250 of the antibody binding site.
Fragments 230
are attached to microparticles such as coated magnetic beads 200, such as
those available
from Dynal Corporation of Oslo, Norway, or Bangs Laboratories, Inc., of
Fishers,
~ 5 Indiana, by the reaction of sulfhydryl groups 240 with haloacetyl or
maleimide groups on
the microparticle surface, as described in K. Kato, et al., J. Immunology
(1976) Vol. 116
(6), p. 1554. Optionally, fragments 230 are attached to microparticles 200
through an
additional linker such as a secondary antibody. Because of the location of
sulfhydryl
groups 240, the chemical bonding of sulfhydryl groups 240 to the surface of
the
2o microparticles will thus place Fab region 250 a distance from the surface
of the
microparticle 200 such that it can bind to the target antigen with out
interference.
After purification of adduct 260 by magnetic capture, filtration,
centrifugation or
other separation techniques. photoaffinity label 220 is introduced by
acylation with the
NHS ester of a nitroarylazide, such as 5-azido-2-nitrobenzoyloxysuccinimide
270 (ANB-
25 NOS, available from Pierce Chemicals) under known conditions. See U.C.
Krieg, et al.,
Proc. Natl. Acad. Sci. USA ( 1986) Vol. 83, p. 8604. The nitroarylazide group
has a red
shifted absorbance spectrum relative to other known arylazides, making its
photoactivation possible with visible light, which is advantageous for many
biological
samples of interest, such as those collected in sexual assault cases, which
may contain UV
30 absorbing impurities. Separation reagent 280 is then isolated by magnetic
capture,
filtration, centrifugation or other separation techniques, and washed to
remove impurities.
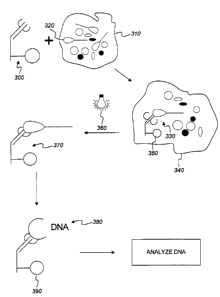
Referring to FIG. 3, the binding of an intact sperm cell to the modified
antibody,
after light activation, produces a permanent (covalent) antibody-sperm cell
adduct 370.
6
CA 02383415 2002-03-13
WO 01/20042 PCT/US00/25423
The capture of the microparticles, followed by rigorous washing will allow for
the
permanent separation of the sperm cells, and most importantly the DNA that
they carry,
from any other cells or cellular debris present in the sample.
Separation reagent 300 is added to a solution of the biological sample to be
analyzed 310, which is prepared by suspending the sample cells, collected, for
example,
from a sexual assault victim, in a suitable buffer (for example, containing
surfactants for
disruption of epithelial cells and the like). Optionally, low energy
sonication is used to
ensure that all target cells are extracted into solution 310. Separation
reagent 300
captures target cell 320, forming receptor-ligand complex 330. In one
embodiment,
complex 330 is separated from mixture 340 by capture of microparticles 350, by
magnetic
capture, filtration, centrifugation or other separation techniques, arid is
resuspended in
fresh buffer. In another embodiment, mixture 340 is carried directly to the
irradiation
stage, described next.
The suspension containing complex 330 is irradiated with light from light
source
15 360, producing covalent adduct 370 and permanently attaching target cell
320 to bead
350. Adduct 370 is isolated by magnetic capture, filtration, centrifugation or
other
separation techniques, and purified by repeated washing, optionally including
low energy
sonication, until all traces of foreign cellular material (e.g., cellular
material from a
victim) are removed.
2o Because adduct 370 (or optionally complex 330) can be separated from
mixture
340 by magnetic capture, no centrifugation step is necessary to isolate the
target cells
from the biological sample. As a result, the isolation of target cells, and
analysis of target
DNA, can be completely automated, for example using known techniques and
hardware
such as the Biomek 2000 available from Beckman Coulter, Inc. This automation
results
25 in increased efficiency and reproducibility as compared to previously known
methods.
Target cell DNA 380 is released from adduct 370, for example by chemical
reduction (e.g. buffer containing 2-mercaptoethanol or dithiothreitol) and/or
proteinase K
digestion or other means, and the microparticles 390 are removed from the
sample by
magnetic capture, filtration, centrifugation or other separation techniques,
if desired.
3o Purified DNA 380 is then analyzed using known techniques, such as the prior
art
magnetic bead/PCR techniques described above.
One embodiment of an apparatus 400 for applying these techniques to the
processing of the forensics samples is illustrated in FIG. 4. A forensics
sample suspected
7
CA 02383415 2002-03-13
WO 01/20042 PCT/US00/25423
to contain a biological target species (e.g., sperm cells) is introduced
through inlet 410
into a chamber 420 loaded with a separation reagent or reagents as described
above. In
this embodiment, the sample can be of varying volume (e.g., from 1 to 100
milliliters or
more) and composition (e.g., clothing, upholstery, etc.), and inlet 410 and
chamber 420
are configured accordingly. A buffer solution is introduced into chamber 420
and the
target species is suspended in the solution, for example by mixing using
stirrer 430. After
the target species is captured by and attached to the separation reagent (to
form an adduct
as described above), the remaining solution is drained through outlet 440.
Optionally,
depending on the relative size of the separation reagent and outlet 440, the
adduct can be
captured prior to draining to prevent any loss of adduct (e.g., by magnetic
interaction
between electromagnet 450 and coated magnetic beads included in the separation
reagent,
by gravity, or other means as discussed above). The adduct can also be washed
to remove
all traces of contaminants by adding and draining additional solution through
inlet 410
and outlet 430 respectively.
~ 5 Optionally, the adduct is resuspended in clean buffer and valve 460 is
opened to
allow the mixture to flow to chamber 470 through tubing 480. As the mixture
flows
through chamber 470, the adduct is captured (e.g., by electromagnet 490).
Chamber 470
is then sealed, and the target species is released from the captured adduct as
described
above. This results in a concentrated sample of the target species suitable
for further
2o analysis - for example, PCR amplification and analysis. In other
embodiments, the
second chamber 470 can be omitted, in which case the target species is
released for
further analysis after processing in the first chamber 420. The invention has
been
described in terms of particular embodiments. Those skilled in the art will
recognize that
other embodiments are within the scope of the following claims.