Note: Descriptions are shown in the official language in which they were submitted.
CA 02384265 2002-03-06
1
BENZODIAZEPIN DERIVATIVES, THE PRODUCTION AND USE THEREOF
The present invention relates to novel benzodiazepine
derivatives, their preparation and the use as inhibitors of the
enzyme poly(ADP-ribose) polymerise or PARP (EC 2.4.2.30) for
producing drugs.
Poly(ADP-ribose) polymerise (PARP) or, as it is also called,
poly(ADP-ribose) synthase (PARS) is a regulatory enzyme which is
found in cell nuclei (K. Ikai et al., J. Histochem. Cytochem.
1983, 31, 1261-1264). It is assumed that PARP is involved in the
repair of DNA breaks (M. S. Satoh et al., Nature 1992, 356,
356-358). Damage or breaks in DNA strands activate the enzyme
pARP which, when it is activated, catalyzes the transfer of
ADP-ribose from NAD (S. Shaw, Adv. Radiat. Biol., 1984, 11,
1-69). During this, nicotinamide is released from NAD.
Nicotinamide is converted back into NAD by other enzymes with
consumption of the energy carrier ATP. Overactivation of PARP
would accordingly result in nonphysiologically large consumption
of ATP, and this leads in the extreme case to cell damage and
cell death.
It is known that free radicals such as superoxide anion, NO and
hydrogen peroxide may lead to DNA damage in cells and thus
activate PARP. The formation of large amounts of free radicals is
observed in a number of pathophysiological states, and it is
assumed that this accumulation of free radicals leads or
contributes to the observed cell or organ damage. This includes,
for example, ischemic states of organs as in stroke, myocardial
infarct (C. Thiemermann et al., Proc. Natl. Acid. Sci. USA, 1997,
94, 679-683) or ischemia of the kidneys, but also reperfusion
damage as occurs, for example, after lysis of myocardial infarct
(see above: C. Thiemermann et al.). Inhibition of the enzyme PARP
might accordingly be a means of at least partly preventing or
moderating this damage. PARP inhibitors might thus represent a
novel therapeutic principle for treating a number of diseases.
The enzyme PARP influences the repair of DNA damage and might
thus also play a part in the therapy of cancers, since a greater
action potential on tumor tissue was observed (G. Chen et al.
Cancer Chemo. Pharmacol. 1988, 22, 303) in combination with
substances with cytostatic activity.
Nonlimiting examples of tumors are leukemia, glioblastomas,
lymphomas, melanomas, and carcinomas of the breast and cervix.
~
CA 02384265 2002-03-06
. t
'' 2
It has additionally been found that PARP inhibitors may show an
immunosuppressant effect (D. Weltin et al. Int. J.
Immunopharmacol. 1995, 17, 265-271).
It has likewise been discovered that PARP is involved in
immunological disorders or diseases in which the immune system
plays an important part, such as, for example, rheumatoid
arthritis and septic shock, and that PARP inhibitors may show a
beneficial effect on the course of the disease (H. Rroger et al.
Inflammation 1996, 20, 203-215; W. Ehrlich et al. Rheumatol. Int.
1995, 15, 171-172; C. Szabo et al., Proc. Natl. Acad. Sci. USA
1998, 95, 3867-3872; S. Cuzzocrea et al. Eur. J. Pharmacol. 1998,
342, 67-76 ) .
PARP means for the purpose of this invention also isoenzymes of
the PARP enzyme described above.
In addition, the PARP inhibitor 3-aminobenzamide showed
protective effects in a model of circulatory failure (S.
Cuzzocrea et al., 9r. J. Pharmacol. 1997, 121, 1065-1074).
There is likewise experimental evidence that inhibitors of the
enzyme PARP might be of benefit as agents for treating diabetes
mellitus (V. Burkart et al. Nature Med. 1999, 5, 314-319).
Benzodiazepines and benzodiazepinones and their derivatives
represent a class of chemicals which have been widely used in
organic synthesis. Derivatives of these compounds additionally
having a fused-on imidazo ring, that is to say
imidazobenzodiazepinones, have scarcely been described, however.
Aminodibenzodiazepinones were prepared in P.V. Khadikar et al. J.
J Heterocycl. Chem. 1998, 35, 675. Thus, simple derivatives having
radicals such as chlorine or nitro on the benzo ring and a methyl
group on the imidazo ring were prepared in Geneste et al.,
Eur. J. Chem. Chim. Ther. 1978, 13, 53. In M.J. Kukla et al., J.
Med. Chem. 1991, 34, 3187, a dihydroimidazobenzodiazepinone was
prepared as intermediate for active substances said to have an
anti-HIV effect.
The compounds of the general formula I according to this
invention have not previously been described and are accordingly
novel.
It has additionally been found, surprisingly, that benzodiazepine
derivatives having a fused-on ring are very effective inhibitors
of the enzyme PARP.
v~.,~~ f. NUSU/507(71._ CA 02384265 2002-03-06
. v
3
The present invention describes novel benzodiazepine derivatives
of the general formula I which are potent PARP inhibitors.
The present invention relates to substituted benzodiazepine
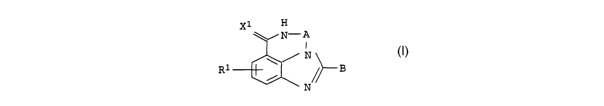
derivatives of the general formula I
X1 H
N-A
I
N
R1 \ ~~ B I
N
in which
A can be a C1-C3 chain where each carbon atom may also carry one
or two of the following substituents: C1-C4-alkyl, OH,
O-Cl-C4-alkyl, COON, COO-C1-C4-alkyl and phenyl or one C atom
may also carry an =O group, and
X1 can be S, O and NH, and
R1 is hydrogen, chlorine, fluorine, bromine, iodine, branched
and unbranched Cl-C6-alkyl, OH, nitro, CF3, CN, NR11R12,
NH-CO-R13, O-C1-C4-alkyl, where Rll and R12 are, independently
of one another, hydrogen or C1-C4-alkyl, and R13 is hydrogen,
C1-C4-alkyl, C1-C4-alkyl-phenyl or phenyl, and
B can be an unsaturated, saturated or partially unsaturated
mono-, bi- or tricyclic ring with a maximum of 15 carbon
atoms, an unsaturated, saturated or partially unsaturated
mono-, bi- or tricyclic ring with a maximum of 14 carbon
atoms and 0 to 5 nitrogen atoms, 0 to 2 oxygen atoms or 0 to
2 sulfur atoms, each of which may also be substituted by one
R4 and a maximum of 3 different or identical RS radicals, and
one or two carbon or sulfur atoms may also carry one or two
=O groups, such as, for example, keto groups, sulfones or
sulfoxides, or is a radical~L~,-Y-MW in which
L can be a straight-chain or branched, saturated or
unsaturated carbon chain of 1 to 8 C atoms, it being
possible for each carbon atom to be substituted by one or
two R4 radicals and a maximum of two different or
identical R5 radicals, and
M has, independently of L, the same meaning as L, and
..~~~i ~u i of
CA 02384265 2002-03-06
.' 4
Y is a bond, or can be S, O or NR3, where R3 can be
hydrogen, branched and unbranched C1-C6-alkyl,
C1-Ca-alkyl-phenyl, phenyl, and
v can be 0 and 1, and
w can be 0 and 1, and
when Y is a bond, R4 and R5 are not both hydrogen, and
when 8 is L"-Y-Mw, R1 is not chlorine or N02, and
R4 is hydrogen and -(D)p-(E)a-(F1)q -G1-(F2)r-(G2).G3, where
D can be S, NR43 and O
C ._
E can be phenyl,
~C=0, -S02-, -SOyNH-, -NHCO-, -CONH-, NHSOZ-,
-NHCOCH2X4,
and
X4 can be S, 0 or NH, and
F1 can be a straight-chain or branched saturated or
unsaturated carbon chain of 1 to 8 C atoms, and
FZ has, independently of F1, the same meaning as F1,
G1 is a bond or can be an unsaturated, saturated or
partially unsaturated mono-, bi- or tricyclic ring with a
maximum of 15 carbon atoms, an unsaturated, saturated or
partially unsaturated mono-, bi- or tricyclic ring with a
maximum of 14 carbon atoms and 0 to 5 nitrogen atoms, 0
to 2 oxygen atoms or 0 to 2 sulfur atoms, each of which
may also be substituted by a maximum of 3 different or
identical R5 radicals, and one or two carbon or sulfur
atoms may also carry one or two =O groups, and
GZ is NR41R42 and
vv.iv/ .7V / bZ
CA 02384265 2002-03-06
R7 R7 R7 R7
N N
5 N ~ N N- R9
U
R7 R7 R7
N~N~R9 N J N O
U
or a bond, and
G3 can be an unsaturated, saturated or partially unsaturated
mono-, bi- or tricyclic ring with a maximum of. l5 carbon
atoms, an unsaturated, saturated or partially unsaturated
mono-, bi- or tricyclic ring with a maximum of 14 carbon
atoms and 0 to 5 nitrogen atoms, 0 to 2 oxygen atoms or 0
to 2 sulfur atoms, each of which may also be substituted
by a maximum of 3 different or identical radicals R5, and
one or two carbon or sulfur atoms may also carry one or
two =O groups, or is hydrogen, and
p can be 0 and 1 and
s can be 0 and 1 and
q can be 0 and 1 and
r can be 0 and l and
R41 can be hydrogen, C1-C6-alkyl, it being possible for each
carbon atom also to carry up to two R6 radicals, phenyl which
may also carry a maximum of two R6 radicals, and (CH2)t-K and
R42 can be hydrogen, C1-C6-alkyl, -CO-R8, C02-R8, SOZNH2, SOZ-Re,
-(C=NH)-Re and -(C=NH)-NHR8 and
R43 can be hydrogen and Cl-C4-alkyl and
t can be 1, 2, 3, 4 and
x can be NR11R12, NRli-C1-C4_alkyl-phenyl, pyrrolidine,
piperidine, 1,2,5,6-tetrahydropyridine, morpholine,
homopiperidine, piperazine, which may also be substituted by
~1 ~..rm.~V/0Z
CA 02384265 2002-03-06
6
an alkyl radical Ci-C6-alkyl, and homopiperazine which may
also be substituted by an alkyl radical Ci-C6-alkyl, and
R5 can be hydrogen, chlorine, fluorine, bromine, iodine, pH,
vitro, CF3, CN, NRiiRi2, NH-CO-R13, Ci-C4-alkyl-CO-NH-R13
r COR ,
Co-C4-alkyl-0-CO-Ri3, Ci-Ca-alkyl-phenyl, phenyl,
C02-Ci-C4-alkyl, and branched and unbranched Ci-C6-alkyl,
O-Ci-C4-alkyl, S-Ci-C4-alkyl, it being possible for each
C atom of the alkyl chains to carry up to two R6 radicals,
and for the alkyl chains also to be unsaturated, and
R6 can be hydrogen, chlorine, fluorine, bromine, iodine,
branched and unbranched Ci-C6-alkyl, OH, vitro, CF3, CN,
NRiiRi2~ NH-CO-R13, 0-Ci-C4-alkyl,
,_. ,
R~ can be hydrogen, Ci-C6-alkyl, phenyl, it being possible
for
the ring also to be substituted by up to two R~i radicals, and
an amine NRiiRi2 or a cyclic saturated amine which has 3 to 7
members, and may also be substituted by an alkyl radical
Ci-C6-alkyl, and homopiperazine which may also be substituted
by an alkyl radical Ci-Cs-alkyl,
and where the radicals Rii, R12 and R13 in K, R5, R6 and R~.may,
independently of one another, assume the same meaning as for Ri,
and
R~i can be OH, Ci-C6-alkyl, O-Ci-CQ-alkyl, chlorine, bromine,
iodine, fluorine, CF3, vitro, NH2, and
RB can be Ci-C6-alkyl, CF3, phenyl, Ci-C4-alk 1- hen
P y~.. it being
possible for the ring also to be substituted by up to two R81
radicals, and
R81 can be OH, Ci-C6-alkyl, O-Ci-C4-alkyl, chlorine, bromine,
iodine, fluorine, CF3, vitro, NH2, and
R9 can be hydrogen, Ci-C6-alkyl, Ci-C4-alkyl-phenyl,
C02-Ci-C4-alkyl-phenyl, C02-Ci-C4-alkyl, So2-phenyl, CORE and
phenyl, it being possible for the phenyl rings also to be
substituted by up to two R91 radicals, and
R91 can be OH, Ci-C6-alkyl, O-Ci-C4-alkyl, chlorine, bromine,
iodine, fluorine, CF3, vitro, NH2,
and their tautomeric forms, possible enantiomeric and
diastereomeric forms, and prodrugs thereof.
CA 02384265 2002-03-06
0050/50761
n
7
Preferred compounds of the formula I are those where
A is a C2 chain, which may be substituted, and
X1 is 0, and
R1 is hydrogen.
Preferred compounds of the formula I are those as indicated
above, in which
B can be an unsaturated, saturated or partially unsaturated
mono-, bi- or tricyclic ring with a maximum of 15 carbon
atoms, an unsaturated, saturated or partially unsaturated
mono-, bi- or tricyclic ring with a maximum of 14 carbon
atoms and 0 to 5 nitrogen atoms, 0 to 2 oxygen atoms or 0 to
2 sulfur atoms, each of which may also be substituted by one
R4 and a maximum of 3 different or identical R5 radicals, and
one or two carbon or sulfur atoms may also carry one or two
=O groups.
Particularly preferred radicals for B are:
B phenyl, cyclohexyl, piperidine, pyridine, pyrimidine,
pyrrole, pyrazole, thiophene, furan, oxazole, naphthalene,
piperazine, quinoline, pyrazine, which may also be
substituted by one R4 or a maximum of 2 R5,
Very particularly preferred compounds of the formula I are those
where
R4 is h dro en or~ D 1 2 3 3
y g o, i-F o, i-G -G with G equal to hydrogen and
D is O and NR43, where R43 is hydrogen and C1-C3-alkyl and
F1 is CZ-C4-alkyl.
Additional particularly preferred compounds of the formula I are
those where B is L"-Y-Mw where
v is 0, and
w is 1, and
Y is a bond, and
vv~tr/~p751
' J 1 ~: Y
CA 02384265 2002-03-06
8
can be a straight-chain or branched carbon chain of 2 to 8
C atoms, which contains at least one double bond, it being
possible for each carbon atom to be substituted by one or two
R4 radicals and a maximum of two different or identical R5
radicals, and
R1 is hydrogen, and
R4 is Do,l-Flo~l-G1-G2-G3 with G3 equal to hydrogen, and
D is O and NR43, where R43 is hydrogen and Cl-C3-alkyl and
Fl is C2-C4-alkyl.
The use of compounds of the general formula I for producing
medicines With a PARP-inhibiting effect is likewise claimed, with
R1. X1 and A having the same meaning as above, and it being
possible for B to be hydrogen and a C1-C6 alkyl chain.
The compounds of the formula I can be employed as racemates, as
enantiomerically pure compounds or as diastereomers. If
enantiomerically pure compounds are required, they can be
obtained, for example, by carrying out a conventional racemate
resolution with the compounds of the formula I or their
intermediates using a suitable optically active base or acid.
Alkyl chains may in each case be branched or unbranched.
Unbranched alkyl chains are preferred.
The invention thus also relates to compounds Which are mesomers
or tautomers of compounds of the formula I.
The invention further relates to the physiologically tolerated
salts of the compounds I, which can be obtained by reacting
compounds I with a suitable acid or base. Suitable acids and
bases are .listed, for example, in Fortschritte der
Arzneimittelforschung, 1966, Hirkhauser Verlag, volume 10, pages
224-285. These include, for example, hydrochloric acid, citric
acid, tartaric acid, lactic acid, phosphoric acid,
methanesulfonic acid, acetic acid, formic acid, malefic acid,
fumaric acid etc., and sodium hydroxide, lithium hydroxide,
potassium hydroxide and tris.
Prodrugs mean compounds which are metabolized in vivo to
compounds of the general formula I. Typical prodrugs are
phosphates, carbamates of amino acids, esters and others.
vvw~5v761
CA 02384265 2002-03-06
9
The benzodiazepine derivatives I according to the invention can
be prepared in various ways, as outlined in synthesis schemes
1-3.
The possible methods of synthesis are essentially already known
or are based on analogous routes which are known.
Synthesis scheme 1
CHO R1 O
NH
II ~ III
NHy H 1' A
R1 O
NH
N~ N-A
I
B
Condensation of the aldehyde II with diamines III results in the
benzimidazole I, this preferably being done in polar solvents
such as ethanol or dimethylformamide with addition of acids such
,as acetic acid at elevated temperature, ordinarily 80-120~C. It is
beneficial for the reaction to add weak oxidizing.agents such as,
for example, copper(II) salts which are added, for example, as
aqueous solutions.
40
~ , . , 0050/50761 CA 02384265 2002-03-06
Scheme 2
R1
5 COO-Alkyl COON Xi
I --~ I + H
B
IV V H2N N- A
10 H III
Ri
Xi
H
I ~--
l HN N-A
.. ~ H
O
As an alternative to the aldehydes II shown in scheme I, it is
also possible to employ acids such as V (see scheme 2) or
nitriles such as VII (see scheme 3) in place of the aldehyde.
Reaction of these derivatives takes place in analogy to the
preparation from the substituted aldehydes II. Starting from V
the condensation to II takes place in two stages. Firstly, the
acid V is reacted with the aniline III in a peptide-like coupling
to give the amide VI. This is .carried out under conventional
conditions which are listed, for example, in Houben-Weyl,
Methoden der Organischen Chemie, 4th Edition, E5, chapter V, and
R.C. Larock, Comprehensive Organic Transformations, VCH
Publisher, 1989, pages 972 et seq. The ring closure to the
benzimidazole then takes place at elevated temperature, for
example 60 to 180~C, with or without solvents such as .
dimethylformamide, with the addition of acids such as acetic acid
or directly in acetic acid itself.
Scheme 3
R1
R1 X1
~ N X1 / \ H
+ ~ H
g ~ /
~ N N- A
H2N N A
VII H III I
., ....~v~ qtr ~ et
CA 02384265 2002-03-06
11
Reaction of the diamine III With a nitrile VII likewise takes
place under conventional conditions: This may entail the use of
solvents such as dimethylformamide with the addition of acids or
else the use of polyphosphoric acid at elevated temperature such
as 60 to 200~C. It is, however, also possible to use the
conventional methods for preparing amidines from benzonitriles as
described in Houben-Weyl, Methoden der organischen Chemie, E5,
pages 1304 et seq., J. Amer. Chem. Soc. 1957, 427 and J. Org.
Chem. 1987, 1017.
Compounds III are synthesized as shown in scheme 4 by reacting a
substituted nitrobenzoic ester IX with a suitable diamine in a
polar solvent such as dimethylformamide in the presence of a base
such as potassium carbonate at 100~C to 150~C, preferably at 110~C
(.~--
to 130~C, in particular at about 120~C, followed by hydrogenation
in the presence of a suitable catalyst such as 10% palladium on
carbon.
Scheme 4
C02R2 H
X
,A~ uN-A
Rl \ 1) H2N NHy NH
~ R1
N02 2) H2 - Pd/C
NH2
IX
Y = Halogen
III
The invention additionally relates to the intermediates of the
formula III
3 5 p,
I
NH
R1 ' III
NH2
in which
A is a C1-C3 chain it being possible for each carbon atom also
to carry one or two of the following substituents:
Ci-Ca-alkyl, OH, 0-C1-C4-alkyl, COZH, C02-C1-C4-alkyl and
phenyl or one C atom may also carry an =O group, and
X1 H
~/ N-
- vu~u~~W of
CA 02384265 2002-03-06
12
X1 and R1 have the meanings stated previously,
excluding the compounds
9-amino-3-methyl-1,2,3,4-tetrahydro-SH-1,4-benzodiazepin-5-one,
9-amino-3-methyl-3,4-dihydro-iH-1,4-benzodiazepine-2,5-dione,
6,8-diamino-2,4-(1H,3H)-quinazolinedione,
8-amino-2,4-(1H,3H)-quinazolinedione
and the salts thereof.
Additionally a process for preparing compounds of the formula III
and their salts, where 2-halo-3-nitrobenzoic esters are reacted
with a suitable diamine in a polar solvent in the presence of a
base, and then the nitro group is hydrogenated with hydrogen in
the presence of a suitable catalyst,
and the use of compounds of the formula III in the synthesis of
PARp inhibitors.
The substituted benzodiazepine derivatives I contained in the
present invention are inhibitors of the enzyme poly(ADP-ribose)
polymerise or PARP (EC 2.4.2.30).
The inhibitory effect of the substituted benzodiazepine
derivatives I can be determined using an enzyme assay which has
already been disclosed in the literature, with a Ki being
determined as a gauge of the effect. The benzodiazepine
derivatives I were measured in this way for an inhibitory effect.
on the enzyme poly(ADP-ribose) polymerise or PARP (EC 2.4.2.30).
The substituted benzodiazepine derivatives of the general formula
I are inhibitors of poly(ADP-ribose) polymerise (PARP) or, as it
is also called, poly(ADP-ribose) synthase (PARS) and can thus be
used for the treatment and prophylaxis of diseases associated
with an increased activity of.these enzymes.
The compounds of the formula I can be employed to produce drugs
for treating damage following ischemias and for the prophylaxis
of expected ischemias in various organs.
The present benzodiazepine derivatives of the general formula I
can accordingly be used for the treatment and prophylaxis of
neurodegenerative disorders occurring after ischemia, trauma
(craniocerebral trauma), massive bleeding, subarachnoid
hemorrages and stroke, and of neurodegenerative disorders such as
multi-infarct dementia, Alzheimer~s disease, Huntington~s disease
and of epilepsies, in particular of generalized epileptic
seizures such as, for example, petit mil and tonoclonic seizures
. .
0050/50761 CA 02384265 2002-03-06
13
and partial epileptic seizures such as temporal lobe, and complex
partial seizures, and further for the treatment and prophylaxis
of damage to the heart after cardiac ischemias and damage to
the
kidneys after renal ischemias, for example of acute renal
insufficiency caused by drug therapies such as, for example,
associated with cyclosporin treatment, of acute kidney failure
o
r
of damage occurring during and after a kidney~transplant. The
compounds of the general formula I can further be used for
treatment of acute myocardial infarct and damage occurring
during
and after medical lysis thereof (for example with,TPA, reteplase,
streptokinase or mechanically with a laser or Rotablator) and
of
microinfarcts during and after heart~valve replacement, aneurysm
resections and heart transplants. The present benzodiazepine
derivatives I can likewise be used for treatment in cases of
revascularization of critically narrowed coronary arteries
for
,
example in PTCA and bypass operations, and critically narrowed
peripheral arteries, for example leg arteries. In addition,
the
benzodiazepine derivatives I can be beneficial in the treatment
of tumors arid metastasis thereof, and be used for treating
inflammations and rheumatic disorders, such as, for example,
rheumatoid arthritis, and for the treatment of diabetes mellitus,
for the treatment of multiorgan failure, for example associated
with septic shock, and for the treatment of ARDS ("acute
respiratory distress syndrome", shock lung).
The pharmaceutical preparations according to the invention
contain a therapeutically effective amount of the compounds I in
addition to conventional pharmaceutical excipients.
For local external use, for example, in dusting powders,
ointments or sprays, the active substances can be present in the
usual concentrations. The active substances are ordinarily
present in an amount of 0.001 to 1% by weight, preferably 0.001
to 0.1% by weight.
For internal use, the preparations.are administered in single
doses. From 0.1 to 100 mg are given per kg of body weight in a
single dose. The preparation may be administered in one or more
doses each day, depending on the nature and severity of the
disorders.
Appropriate for the required mode of administration, the
pharmaceutical preparations according to the invention comprise
conventional carriers and diluents, in addition to the active
substance. For local external use, it is possible to use
pharmaceutical excipients such as ethanol, isopropanol,
ethoxylated castor oil, ethoxylated hydrogenated castor oil,
v
0050/50761 CA 02384265 2002-03-06
14
polyacrylic acid, polyethylene glycol, polyethylene glyco7i.
stearate, ethoxylated fatty alcohols, liquid paraffin, petrolatum
and wool fat. Examples suitable for internal use are lactose,
propylene glycol, ethanol, starch, talc and polyvinylpyrrolidone.
It is also possible for antioxidants such as tocopherol and
butylated hydroxyanisole, and butylated hydroxytoluene,
flavor-improving additives, stabilizers, emulsifiers and
lubricants to be present.
The substances present in the preparation in addition to the
active substance, and the substances used in the production of
the pharmaceutical preparations are toxicologically acceptable
and compatible with the particular active substance. The
pharmaceutical preparations are produced in a conventional way,
for example by mixing the active substance with conventional
carriers and diluents.
The pharmaceutical preparations can be administered in various
ways, for example orally, parenterally such as intravenously by
infusion, subcutaneously, intraperitoneally and topically. Thus,
possible presentations are tablets, emulsians, infusion and
injection solutions, pastes, ointments, gels, cremes, lotions,
dusting powders and sprays.
Pharmacological example:
Inhibition of the enzyme poly(ADp-ribose) polymerase or PARP
(EC 2.4.2.30)
A 96-well microtiter plate (Falcon) is coated with histories (type
II-AS; SIGMA H7755). For this purpose, histories are dissolved in
.. a concentration of 50 ~g/ml in carbonate buffer (0.05 M NaHC03; pH
9.4). The individual wells of the microtiter plates are each
incubated with 100 ~1 of this histone solution overnight. The
histone solution is then removed, and the individual wells are
incubated with 200 ~1 of a 1% strength HSA (bovine serum albumin)
solution in carbonate buffer at room temperature for 2 hours.'
This is followed by washing three times with washing buffer
(0.05% Tweenl0 in PBS). For the enzyme reaction, 50 ~.1 of the
enzyme reaction solution (5 ~1 of reaction buffer (1 M tris-HC1
pH 8.0, 100 mM MgCl2, 10 mM DTT), 0.5 ~,1 of PARP,(c = 0.22 ~,g/~1),
4 ~1 of activated DNA (SIGMA D-4522, 1 mg/ml in Water), 40.5 ~1
of H20) are preincubated in each well with 10 ~1 of an ~.nhibitor
solution for 10 minutes. The enzyme reaction is started by adding
40 ~,1 of a substrate solution (4 ~,l of reaction buffer (see
above), 8 ~1 of NAD solution (100 E,iM in HZO), 28 ~1 of HZO). The
reaction time is 20 minutes at room temperature. The reaction is
_ 0050/50761
CA 02384265 2002-03-06
stopped by washing three times with washing buffer (see above).
This is followed by incubation at room temperature with a
specific anti-poly(ADP-ribose) antibody for one hour. The
antibodies used were ~~lOH~~ monoclonal anti-poly(ADP-ribose)
5 antibodies (Kawamaitsu H et al. (1984) Monoclonal antibodies to
poly(adenosine diphosphate ribose) recognize different
structures. Biochemistry 23, 3771-3777). It is likewise possible
to use polyclonal antibodies.
10 The antibodies were employed in a 1:5000 dilution in antibody
buffer (1% BSA in PBS; 0..05% Tween20). Washing three times with
washing buffer is followed by incubation at room temperature with
the secondary antibody for one hour. In this case the monoclonal
antibody used was an anti-mouse IgG coupled to peroxidase
15 (Boehringer Mannheim), and the rabbit antibody was an anti-rabbit
IgG coupled to peroxidase (SIGMA A-6154), each in a 1:10,000
dilution in antibody buffer. After washing three times with
washing buffer, the color reaction is carried out using
100 ~1/well color reagent (SIGMA, TMB mixture, T8540) at room
temperature for about 15 min. The color reaction is stopped by
adding 100 ~1 of 2 M H2S04. Measurement is carried out immediately
thereafter (450 nm versus 620 nm; "Easy Reader~~ EAR340AT ELISA
plate reader, SLT-Labinstruments, Austria). The IC50 of an
inhibitor to be measured is the concentration of inhibitor at
which a half-maximum change in color concentration occurs.
Examples
Example 1
2-(4-(4-Methylpiperazin-1-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
a) 9-Nitro-1,2,3,4-tetrahydro-5H-1,4-benzodiazepin-5-one
24 g (0.11 mol) of methyl 2-chloro-3-nitrobenzoate were
dissolved in 250 ml of dimethylformamide. 15.4 g (0.11 mol)
of potassium carbonate and 22.3 ml (0.33 mol) of
ethylenediamine were successively added, and the mixture was
heated at 120~C for 3 hours. The mixture was then
concentrated to half the volume in vacuo, and the residue was
poured into water, whereupon the product precipitated. 19.7 g
of the product were obtained.
b) 9-Amino-1,2,3,4-tetrahydro-5H-1,4-benzodiazepin-5-one
1.7 g of 10% palladium/carbon were added to 19 g (91.7 mmol)
of the intermediate la in 500 ml of ethanol, and it was then
hydrogenated with hydrogen. The mixture was then filtered.
0~5~~5~761 CA 02384265 2002-03-06
I6
The filtrate was concentrated in vacuo, and the residue was
recrystallized from isopropanol/ether. The crystals which
separated out were filtered off with suction. 14.4 g of the
product were obtained.
c) 2-(4-(4-Methylpiperazin-1-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
2Øg (11.3 mmol) of the intermediate 1b and 2.8 ml
(45.15 mmol) of concentrated acetic acid were dissolved in
200 ml of methanol and, at room temperature, a solution of
3.0 g (14.7 mmol) of 4-(4-methylpiperazin-1-yl)benzaldehyde
in 50 ml of methanol was added dropwise. The mixture was
stirred at room temperature for 1 hour. Then 2.9 g
(14.7 mmol) of copper(II) acetate dissolved in 100 ml of
water were added dropwise, and the mixture was refluxed for.
30 minutes. During this time, in parallel a solution of 4.1 g
(17 mmol) of sodium sulfide x 9 H20 in 70 ml of water and a
solution of 17 ml of f M hydrochloric acid in 50 ml of water
were added. After cooling, the resulting precipitate was
filtered off with suction, and the filtrate was concentrated
in vacuo. The resulting residue was partitioned between
aqueous sodium bicarbonate solution and ethyl acetate. The
organic phase was separated off, dried and concentrated in
vacuo. The residue was crystallized from ethyl acetate/ether.
2.4 g of the product were obtained.
1H-NMR (D6-DMSO): 8 = 2.2 (3H.), 2.5.(4H), 3.3 (4H), 3.5 (2H),
4.4 (2H), 7.1 (2H), 7.3 (1H), 7.7-7.9 (4H) and 8.4 (1H) ppm.
[M+ = 361]
Example 2
2-(4-Nitrophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-
7(4H)-one
The product was obtained in analogy to the method in lc from
9-amino-1,2,3,4-tetrahydro-5H-1,4-benzodiazepin-5-one and
4-nitrobenzaldehyde.
1H-NMR (D6-DMSO): 8 = 3.6 (2H), 4.5 (2H), 7.4 (1H) and
7.9-8.6 (7H) ppm.
[M+ = 308]
0050/50761
CA 02384265 2002-03-06
w
17
Example 3
2-(4-(2-N,N-Diethylaminoeth-1-yloxy)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
The product was obtained in analogy to the method in lc from
9-amino-1,2,3,4-tetrahydro-5H-1,4-benzodiazepin-5-one and
4-(2-N,N-diethylaminoeth-1-yloxy)benzaldehyde.
1H-NMR (D6-DMSO): b = 1.0 (6H), 2.6 (4H), 2.8 (1H), 3.5 (2H),
4.1 (2H), 4.5 (2H), 7.1 (2H), 7.4 (1H), 7.7-7.9 (4H) and
8.4 (1H) ppm.
[M+ = 378]
The following further examples were prepared in analogy to the
above methods:
Example 4
2-(4-(2-Piperidin-1-yleth-1-yloxy)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk]-[1,4]benzodiazepin-7(4H)-one
The product was obtained in analogy to the method in lc from
9-amino-1,2,3,4-tetrahydro-5H-1,4-benzodiazepin-5-one and
4-(2-piperidin-1-yleth-1-yloxy)benzaldehyde.
1H-NMR (D6-DMSO): 8 = 1.3-1.6 (6H), 2.5 (4H), 2.7 (2H), 3.6 (2H),
4.2 (2H), 4.5 (2H), 7.1 (2H), 7.4 (1H), ?.7-7.9 (4H) and
8.4 (1H) ppm.
[M+ = 390]
Example 5
2-(4-(N-(2-N,N-Diethylaminoeth-1-yl)-N-methylamino)phenyl)-5,6-
dihydroimidazo[4,5,1-jk]-[1,4]benzodiazepin-7(4H)-one
The product was obtained in analogy to the method in lc from
9-amino-1,2,3,4-tetrahydro-5H-1,4-benzodiazepin-5-one and
4(N-(2-N,N-diethylaminoeth-1-yl)-N-methylamino)benzaldehyde.
1H-NMR (D6-DMSO): b = 0.9 (6H), 2.5 (6H), 3.0 (3H), 3.4-3.6 (4H),
4.45 (2H), 6..8 (2H), 7.3 (1H), 7.6-7.9 (4H) and 8.45 (1H) ppm.
[M+ = 391]
~~50~5~76Z CA 02384265 2002-03-06
Example 6
18
2-(4-(4-(tert-Butyloxycarbonyl)piperazin-1-yl)phenyl)-5,6-
dihydroimidazo[4,5,1-jk]-[1,4]benzodiazepin-7(4H)-one
The product was obtained in analogy to the method in lc from
9-amino-1,2,3,4-tetrahydro-5H-1,4-benzodiazepin-5-one and
4-(4-(tert-butyloxycarbonyl)piperazin-1-yl)benzaldehyde.
1H-NMR (D6-DMSO): 8 = 1.4 (9H), 3.3 (4H), 3.4-3.6 (6H), 4.45 (2H),
7.1 (2H), 7.3 (1H), 7.7-7.9 (4H) and 8.4 (1H) ppm.
[M+ = 447 j
Example 7
2-(4-(4(tert-Butyloxycarbonyl)homopiperazin-1-yl)phenyl)-5,6-
dihydroimidazo[4,5,1-jk]-[1,4]benzodiazepin-7(4H)-one
The product was obtained in analogy to the method in lc from
9-amino-1,2,3,4-tetrahydro-5H-1,4-benzodiazepin-5-one and
4-(4-(tert-butyloxycarbonyl)homopiperazin-1-yl)benzaldehyde.
1H-NMR (D6-DMSO): b = 1.2-1.3 (9H), 1.8-1.9 (2H), 3.2-3.8 (10H),
4.45 (2H), 6.9 (2H), 7.3 (1H), 7.7 (2H), 7.8 (2H) and
8.4 (1H) ppm.
[M+ = 461]
Example 8
2-(4-(Homopiperazin-1-yl)phenyl)-5,6-dihydroimidazo~[4,5,1-jk]-
[l,4jbenzodiazepin-7(4H)-one
The product was prepared from the product from Example 7 in
analogy to Example 9.
[M* = 361)
Example 9
2-(4-(Piperazin-1-yl)phenyl-5,6- dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one trihydrochloride
0.5 g of Example 6 was added to 30 ml of isopropanolic hydrogen
chloride solution at room temperature and stirred for several
hours. The mixture was then concentrated in vacuo, and the
resulting residue was recrystallized from ethanol. The product
was obtained as trihydrochloride.
w~ui5o~6~
' CA 02384265 2002-03-06
19
iH-NMR (D6-DMSO): 8 = 3.2-3.8 (10H), 4..5 (2H), 7.2 (2H),
7.5-8.0 (5H), 8.6 (1H) and 9.6 (broad) ppm.
[M* = 347 ]
Example 10
2-(4-Aminophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one x 2 HCl
[M+ = 280 ]
Example 11
2-(Piperidin-4-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one x HC1
[M+ = 271
Example 12
(,~ 2-(1-n-Propylpiperidin-4-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4X)-one x HC1
[M+ = 313 ]
Example 13
2-(1-Benzylpiperidin-4-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one x HC1
[M+ = 361
Example 14
2-(Pyridin-4-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-
7(4X)-one x HC1
[M+ = 265 ]
Example 15
2-(Thien-3-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-
7(4X)-one x HC1
[M+ = 270)
Example 16
2-(Quinolin-3-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-
7(4X)-one x HC1
[M+ = 315 ]
Example 17
2-(Naphth-2-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-
7(4X)-one
[M+ = 313 ]
$xample 18
2-(1X-Imidazol-1-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one x HC1
vv~u~ 5uv o1
CA 02384265 2002-03-06
[M+ = 330]
Example 19
2-(4-(3-Formylpyrrol-1-yl)phenyl)-5,6-dihydroimidazo[4,5,1-j k]_
5 [1,4]benzodiazepin-7(4H)-one
[M+ = 356 ]
Example 20
2-(4-(3-Trifluoroacetamidomethylpyrrol-1-yl)phenyl)-5,6-di-
10 hydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one x HC1
[M+ = 453]
Example 21
2-(4-(4-(Piperidin-1-yl)piperidin-1-yl)phenyl)-5,6-dihydro-
15 imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one x 2 HC1
(... [M+ = 432 ]
Example 22
2-(4-(3-(Piperidin-1-ylmethyl)pyrrol-1-yl)phenyl)-5,6-dihydro-
20 imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one x HCl
(M+ = 427]
Example 23
2-(4-(3-Aminomethylpyrrol-1-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4X)-one x HC1
[M+ = 358]
Example.24
2-(3-(2-(N,N-Dimethylamino)eth-1-yl)-4-nitrophenyl)-5,6-di-
hydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4X)-one x HC1
[M+ = 380]
Example 25
5,6-Dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4X)-one
[M+ = 187]
Example 26
2-(Pyrazin-2-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-
7(4H)-one x HC1
[M+ = 266]
Example 27
2-(2-(tert-Butyloxycarbonylaminomethyl)thiazol-4-yl)-5,6-di-
hydroimidazo[4,5,1-jk][l,4jbenzodiazepin-7(4H)-one
IM+ = 399]
ooSO/5~761 CA 02384265 2002-03-06
21
Example 28
2-(2-(Aminomethyl)thiazol-4-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one x HC1
IM+ = 300]
Example 29
2-(2-fluoro-4-(pyridin-4-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+ = 358]
Example 30
2-(1-(1-Methylpiperidin-4-yl)piperidin-4-yl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4X)-one x 2 HC1
[M+ = 369]
-- Example 31
2-[(Z)-1-(4-Fluorophenyl)-2-(pyridin-3-yl)ethenyl]-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+ = 384]
Example 32
2-(1-Henzylpiperidin-3-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M+ = 360]
Example 33
2-(1-phenylcyclopent-1-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
IM+ = 331]
Example 34
2-(1-Phenylcyclohex-1-yl)-5,6-dihydroimidazo[4,5,1-jk}[1,4]-
benzodiazepin-7(4X)-one
[M+ = 345] .
Example 35
6-(4-(Aminomethyl)cyclohex-1-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M* = 298]
Example 36
2-IIE)-2-(Pyridin-4-yl)ethenyl]-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4N)-one
[M+ = 290]
' ;' uuSO/50761
" CA 02384265 2002-03-06
22
Example 37
2-[3-Cyanophenyl]-5,6-dihydroimidazo[4,5,1-jk][I,4]benzodiazepin-
7(4H)-one
[M+-I = 288 ]
Example 38
2-(2-Phenyl-1H-imidazol-4-yl)-5,6-dihydroimidazo(4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
(M+-1 = 329]
Example 39
2-[2-(4-Methylphenyl)-1,3-oxazol-4-yl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
IM+-1 = 344]
.~ 15
Example 40
2-[1-(4-Fluorophenyl)-5-methyl-1H-pyrazol-4-yl]-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
IM+-I = 361]
Example 4.1
2-[1-(4-Chlorophenyl)-1H-pyrazol-5-yl]-5,6-dihydroimidazo-
(4,5,1-jk][1,4]benzodiazepin-7(4H)-one
(M+-1 = 363]
Example 42
2-(3-Propyl-5-isoxazolyl)-5,6-dihydroi.midazo.[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
(M+-1 = 296]
Example 43
2-(1-(4-Methoxyphenyl)-iH-pyrrol-3-yl]-5,6-dihydroimidazo-
(4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 358]
Example 44
2-(1,2,5-Trimethyl-1H-pyrrol-3-yl)-5,6-dihydroimidazo-
(4,5,1-jk](1,4]benzodiazepin-?(4H)-one
[M+-1 = 294]
Example 45
2-(4-Benzoyl-1-methyl-1H-pyrrol-2-yl)-5,6-dihydroimidazo-
[4,5,1-jk](1,4]benzodiazepin-7(4H)-one
(M*-1 = 370]
CA 02384265 2002-03-06
Z3
Example 46
2-~4-Methyl-5-[4-(trifluoromethyl)phenyl]-3-isoxazolyl}-5,6-di-
hydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 412]
Example 47
2-(5-Methyl-2-furyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M+-1 = 267]
Example 48
2-[1-(2-Chlorophenyl)-5-(trifluoromethyl)-1H-pyrazol-4-yl]-
5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 431]
Example 49
2-(5-Methyl-1H-i.midazol-4-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M+-1 = 267]
Example 50
2-(1-Methyl-1H-pyrazol-4-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
[M+-1 = 267]
Example 51
2-(1-Methyl-1H-indol-3-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M+-1 = 316]
Example 52
2-{6-[(4-Chlorophenyl)thio]imidazo[2,1-b][1,3]thiazol-5-yl}-
5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 451]
Example 53
2-[1-(4-Chlorophenyl)-1H-pyrrol-3-yl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 363]
Example 54
2-[2-(4-Fluorobenzoyl)-1-benzofuran-5-yl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 425]
_ _ _ _ __
CA 02384265 2002-03-06
24
Example 55
2-(2,5-Dibromo-3-thienyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M+-1 = 427 ]
Example 56
2-(2-Phenyl-1,3-oxazol-4-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[Mf-1 = 330]
Example 57
2-(6-Methyl-2-pyridinyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M+-1 = 278]
f_,._
Example 58
2-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1X-pyrazol-4-yl)-
5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 373]
Example 59
2-[1-(Benzylaminocarbonylmethyl)pyrrol-2-yl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]-benzodiazepin-7(4H)-one
[M+-1 = 399]
Example 60
2-(1-Phenyl-1H-pyrazol-4-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
[M+-1 = 329]
Example 61
2-[1-(3-Cyano-4-methoxypyridin-2-yl)pyrrol-2-yl]-5,6-dihydro-
imidazo[4,5,1-jk][1,4]-benzodiazepin-7(4H)-one '
[M+-1 = 384]
Example 62
2-{1-[(4-Methylphenyl)sulfonyl]-1H-indol-3-yl}-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 456]
Example 63
2-(5-Methoxy-1X-indol-3-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
[M+-1 = 332]
CA 02384265 2002-03-06
Example 64
2-[4-Bromo-1-(4-chlorobenzyl)-1H-pyrazol-5-yl]-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4X)-one
[M+-1 = 456]
5
Example 65
2-[1-(4-Methylphenyl)-1H-pyrrol-2-yl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
IM+-1 = 342]
Example 66
2-(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 377]
Example 67
2-[4-(4-Chlorobenzoyl)-1-methyl-1H-pyrrol-2-yl]=5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
IM+-I = 404]
Example 68
2-[4-(Diethylamino)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
IM+-I = 334]
Example 69
2-(4-Methoxy-1-naphthyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M+-1 = 343]
Example 70
.'
2-(4-Methoxy-2,5-dimethylphenyl)-5,6-dihydroimidazo[4,5,1-jk]-
w [1,4]benzodiazepin-7(4X)-one
IM+-1 = 321]
Example 71
2-[3-(4-Chlorophenoxy)phenyl]-5,6-dihydroimidazo[4,5,1-jk][I,4]-
benzodiazepin-7(4H)-one
IM+-1 = 389]
Example 72
2-[4-(Methylthio)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
[M+-1 = 309]
CA 02384265 2002-03-06
26
Example 73
2-[4-(Acetyloxy)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benza--
diazepin-7(4H)-one
[M+-1 = 321]
Example 74
2-[2,5-Bis(trifluoromethyl)phenyl]-5,6-dihydroimidazo[4,5,1-jk]_
[1,4]benzodiazepin-7(4H)-one
[M*-1 = 399]
Example 75
2-(2,3-Dimethoxyphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M*-1 = 323]
Example 76
2-(2-Methylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one,
[M*-1 = 277j
Example 77
2-[4-(Benzyloxy)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M*-1 = 369]
Example 78
2-(2-Chloro-6-fluorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
[M*-1 = 315]
Example 79
2-(2-Ethoxyphenyl)-5,6-dihydroimidazo[4,5,1-j k][1,4]benzo-
diazepin-7(4X)-one
[M*-1 = 307]
Example 80
2-(4-Isopropylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4)benzo-
diazepin-7(4H)-one
[M*-1 = 305]
Example 81
2-(6-Nitro-1,3-benzodioxol-5-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M*-1 = 352]
CA 02384265 2002-03-06
27
Example 82
2-(2,3-Dihydro-1,4-benzodioxin-6-yl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 321]
Example 83
2-[4-(Dimethylamino)-1-naphthyl]-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4X)-one
[M'*-1 = 355 ]
Example 84
2-[4-(Difluoromethoxy)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4~]-
benzodiazepin-7(4X)-one
[M+-1 = 329]
Example 85
2-(3,7-Dichloro-8-quinolinyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benz~odiazepin-7(4X)-one
[M+-1 ~ 383]
Example 86
2-[4-Chloro-3-(trifluoromethyl)phenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 365 ]
Example 87
2-(1-tert-Butyl-1X-pyrazol-4-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4X)-one
[M*-1 = 309]
Example 88
2-(4-Chloro-5-nitro-1-benzothien-2-yl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4X)-one
[M+-1 = 398]
Example 89 ,
2-[1-(4-Phthalimidobutan-1-yl)indol-3-yl]-5,6-dihydroimidazo-
[4,5,1-j k][1,4]benzodiazepin-7(4H)-one
[M+-1 = 503 ]
Example 90
2-(3-Isobutyl-5-isoxazolyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
[M+-1 = 310]
,. ......v, :avi vt
CA 02384265 2002-03-06
28
Example 91
2-[1-(4-Methoxyphenyl)-5-(trifluoromethyl)-1H-pyrazol-4-yl]-
5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 427]
Example 92
2-[2-(Dimethylamino)-1,3-thiazol-5-yl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 313]
Example 93
2-[3-(4-tert-Butylphenyl)-5-isoxazolyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 386]
_ 15
Example 94
2-[1-(4-Chlorophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]-5,6-dihydro-
imidazo[4,5,1-jk][l,4jbenzodiazepin-7(4X)-one
[M+-1 = 391]
Example 95
2-(3-Chlorophenyl)-5,6-dihydroimidazo.[4,5,1-jk][1,4]benzo-
diazepin-7(4x)-one
[M+-1 = 297]
Example 9.6
2-(3-Fluorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin
-7(4H)-one
[M+-1 = 281]
Example 97
2-(3-Phthalimidophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M'*-1 = 408 ]
Example 98
2-{4-[3-Chloro-5-(trifluoromethyl)-2-pyridinyl]phenyl}-5,6-di-
hydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 442]
Example 99
2-[5-(6-Methylnicotinamido)-2-chlorophenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]-benzodiazepin-7(4H)-one
[M+-1 = 431]
CA 02384265 2002-03-06
2g
Example 100
2-(4-tert-Butoxyphenyl)-5,6-dihydroimidaxo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M+-1 = 335]
Example I01
4-(7-Oxo-4,5,6,7-tetrahydroimidazo[4,5,1-jk][1,4]benzodiazepin-
2-yl)benzonitrile
[M*-1 = 288]
Example 102
2-[3-(Trifluoromethoxy)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M*-1 = 347]
i Example 103
2-[3-(3,5-Dichlorophenoxy)phenyl]-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4X)-one
[M+-1 = 423]
Example 104
2-(3-Bromo-4,5-dimethoxyphenyl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M*-1 = 402]
Example 105
2-[5-(Allyloxy)-1,3-dimethyl-1X-pyrazol-4-yl]-5,6-dihydroimidazo-
[4.,5,1-jk][1,4]benzodiazepin-7(4X)-one
[M+-1 = 337]
Example 106
2-~2-[3-(Trifluoromethyl)anilino]phenyl}-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4X)-one
[M+-1 = 422]
Example 107
2-[2-(2-Phenylethyl)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
[M+-1 = 367]
Example 108
2-(3-Benzoylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M+-1 = 367]
CA 02384265 2002-03-06
Example 109
2-(4-Acetamidophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M*-1 = 320]
5
Example 110
2-(I,3-Benzodioxol-5-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
f M+-1 = 307 ]
Example 111
2-(5-Aminosulfonyl-2,4-dichlorophenyl)-5,6-dihydroimidazo-
[4,5,1-jk]-[1,4]benzodiazepin-7(4H)-one
[ M*-1 = 411 ]
Example 112
2-(2-Benzoyloxymethylphenyl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M*-1 = 397]
Example 113
2-(2-N,N-Diethylaminocarbonyl-3,6-difluor-phenyl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 398]
Example 114
2-(2-(N-2,2,2-Trifluoracetamido)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk]-[1,4]benzodiazepin-7(4H)-one
[M*-1 = 374]
Example 115
2-[4-(Trifluoromethyl)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
fM+-1 = 331]
Example 116
2-[2-Fluoro-4-(trifluoromethyl)phenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4X)-one
[M*-1 = 349 ]
Example 117
2-(3-Chloro-4-methoxyphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
[M*-1 = 327
CA 02384265 2002-03-06
31
Example 118
2-(3-Bromo-4-fluorophenyl)-5,6-dihydroimidazo[4,5,1-jk)[1,4]_
benzodiazepin-7(4H)-one
[M+-1 = 360]
Example 119
2-(2,5-Dimethyl-1-phenyl-1H-pyrrol-3-yl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 356]
Example 120
2-[4-(2,4-Dichlorobenzoyl)-1-methyl-1H-pyrrol-2-yl]-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 439]
--..
Example 121
2-[1-(2-Fluorophenyl)-1X-pyrrol-2-yl]-5,6-dihydroimidazo-
[4,5,1-jk)[1,4]benzodiazepin-7(4H)-one
[M+-1 = 346]
Example 122
2-(3,5-Dimethoxyphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M+-1 = 323]
Example 123
2-(4-Bromo-2-fluorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M'"-1 = 360]
Example 124
2-(2-Chloro-4-fluorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M+-1 = 315]
Example 125
2-[2-(Benzyloxy)-3-methoxyphenyl]-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4X)-one
[M*-1 = 399]
Example 126
2-(2,4-Diethoxy-3-methylphenyl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M+-1 = 365]
uv~u~50761
CA 02384265 2002-03-06
32
Example 127
2-(5-Bromo-2,4-dimethoxyphenyl)-5,6-dihydroimidazo[4,5,1-jkj-
[1,4]benzodiazepin-7(4X)-one
[M+-1 = 402j
Example 128
2-[4-(Dimethylamino)-2-methoxyphenyl]-5,6-dihydroimidazo-
[4,5,1-jk][l,4jbenzodiazepin-7(4X)-one
IM+-1 = 336]
Example 129
2-[2-Chloro-5-(trif-luoromethyl)phenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4X)-one
IM+-1 = 366]
Example 130
2-(3,5-Dimethylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M+-1 = 291]
Example 131
2-[4-Fluoro-2-(trifluoromethyl)phenyl]-5,6-dihydroimidazo-
[4,5,1-jkj[1,4]benzodiazepin-7(4X)-one
[M'*-1 = 349]
Example 132
2-(5-Bromo-2-fluorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M+-1 = 360]
( Example 133
2-[4-(1-Pyrrolidinyl)phenyl]-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4X)-one
[M+-1 = 332j
Example 134
2-(4-Isopropoxyphenyl)-5,6-dihydroimidazo[4,5,1-jk][l,4jbenzo-
diazepin-7(4X)-one
[M+-1 = 321j
Example 135
2-(3,5-Dibromophenyl)-5,6-dihydroimidazo[4,5,1-jkj[1,4]benzo-
diazepin-7(4H)-one
[M*-1 = 421j
~ . vvw/~V/bl
' CA 02384265 2002-03-06
33
Example 136
2-[4-(Benzyloxy)-2-methoxyphenyl]-5,6-dihydroimidazo[4,5,1-jk]-
[1,4)benzodiazepin-7(4H)-one
[M*-1 = 399] .
Example 137
2-[3-Fluoro-4-(trifluoromethyl)phenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[ M+-1 = ~3 4 9 ]
Example 138
2-[5-(4-Nitrophenyl)-2-furyl]-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M+-1 = 374]
' Example 139
2-(3-Acetyloxyphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M+-1 = 321]
Example 140
2-[2-(tent-Butylthio)phenyl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M+-1 = 351]
Example 141
2-[2-Fluoro-5-(trifluoromethyl)phenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[ M+-1 = 3 4 ~9 ]
Example 142
2-(3,4-Dimethylphenyl)-5,6-dihydroimidazo[4,5,1-j k][1,4)benzo-
diazepin-7(4H)-one
[M+-1 = 291]
Example 143
2-[4-(Ethylthio)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M+-1 = 323)
Example 144
2-~4-[(Trifluoromethyl)thio]phenyl}-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 363]
" uv~u~50761
CA 02384265 2002-03-06
34
Example 145
2-~2-[(4-Chlorophenyl)thin]phenyl}-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 406]
Example 146
2-(4-Chloro-3-fluorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M+-1 = 316]
Example 147
2-(2-(4-Ethoxycarbonyl-piperidin-1-yl)-thiazol-5-yl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 425]
_ 15
Example 148
2-~1,3-Dimethyl-5-[4-(trifluoromethyl)phenoxy]-1H-pyrazol-4-yl}-
5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 441]
Example 149
2-~1-Methyl-3-(trifluoromethyl)-5-[3-(trifluoromethyl)phenoxy.]-
1H-pyrazol-4-yl}-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-
7(4X)-one
[M+-1 = 495]
Example 150
2-[2-(4-Benzyl-1-piperazinyl)-1,3-thiazol-5-yl]-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4X)-one
[M+-1 = 444]
Example 151
2-(5-Isopropyl-2-methylcyclohexyl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M+-1 = 325]
Example 152
2-(6,6-Dimethylbicyclo[3.1.1]kept-2-yl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one .
[M+-1 = 309]
Example 153
2-[5-(3-Nitrophenyl)-2-furyl]-5,6-dihydroimidazo[4,5,1-jk][1,4)-
benzodiazepin-7(4H)-one
[M+-1 = 374]
- vv~V/~p761
".
CA 02384265 2002-03-06
Example 154
2-(2,5-Dimethoxytetrahydro-3-furanyl)-5,6-dihydroimidazo-
(4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 317]
5
Example 155
2-(2-Thienyl)-5,6-dihydroimidazo[4,5,1-jk](1,4]benzodiazepin-
7(4H)-one
[M+-1 = 269]
Example 156
2-(1,3-Thiazol-2-yl)-5,6-dihydroimidazo(4,5,1-jk](1,4]benzo-
diazepin-7(4X)-one
[M+-1 = 270]'
__ 15
Example 157
2-(4-Methoxycyclohexyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M*-1 = 299]
Example 158.
2-(3,5-Dimethoxy-2-methoxycarbonylphenyl)-5,6-dihydroimidazo-
[4,5,1-jk](1,4]benzodiazepin-7-(4H)-one
(M+-1 = '381 ]
Example 159
2-~5-(1-Methyl-3-(trifluoromethyl)-lx-pyrazol-5-yl]-2-thienyl}-
5,6-dihydroimidazo(4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 417] .
Example 160
2-(2-Fluoro-5-methoxyphenyl)-5,6-dihydroimidazo(4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M+-1 = 311]
Example 161
2-(4-Butylphenyl)-5,6-dihydroimidazo[4,5,1-jk][I,4]benzodiazepin-
7(4H)-one
[M*-1 = 319]
Example 162
2-[2-(Trifluoromethoxy)phenyl]-5,6-dihydroimidazo(4,5,1-jk](1,4]-
benzodiazepin-7(4H)-one
[M+-1 = 347]
v. . _~-v~.rv~Vi
CA 02384265 2002-03-06
'' 3 6
Example 163
2-(4-Quinolinyl)-5,6-dihydroimidazo[4,5,I-jk][I,4]benzodiazepin-
7 ( 4H) -one
[M*-1 = 314]
Example I64
2-(2-Quinolinyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-
7(4x)-one
[ M*-1 ~ = 314 ]
Example 165 '
2-(2-Chloro-3-quinolinyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M*-1 = 348]
_ 15 '
Example 166
2-[4-(IH-Pyrrol-1-yl)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M*-1 = 328]
Example 167
2-(lH-Indol-6-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-
7(4H)-one
[M*-1 = 302]
Example 168
2-[4-(1,1-Dioxo-1,2-thiazinan-2-yl.)-phenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 396]
Example 169
2-(1,3-Benzothiazol-6-yl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M*-1 = 320]
Example 170
2-(2,3-Dihydro-1-benzofuran-5-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4X)-one
[M*-1 = 305]
Example 171
2-(4-(2-(2-Furylmethylthio)acetamido)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 432]
CA 02384265 2002-03-06
37
Example 172
2-~[5-(2-Fluorobenzoyl)-2-thienyl]methyl}-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
IM+-1 = 405]
Example 173
2-(2-(2-Acetamidopyridin-5-ylthio)pyridin-5-yl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 430]
Example 174
2-(4-(N-(3,4-Dioxo-2-ethoxy-1-cyclobuten-1-yl)amino)phenyl-
5,6-dihydroimidazo[4,5,1-jk][1,4]-benzodiazepin-7(4H)-one
[M+-1 = 402]
Example 175
2-[(2-Quinoxalinylthio)methyl]-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4X)-one
[M+-1 = 361]
Example I76
2-[4-(Methylamino)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M+-1 = 292]
Example 177
2-(5-(4-Aminosulfonylphenyl)furan-2-yl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 408]
Example 178
2-{2,5-Dimethyl-1-[4-(trifluoromethyl)phenyl]-1H-pyrrol-3-yl}-
5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 424]
Example 179
2-~I-[(2,4-Difluorophenyl)sulfonyl]-1H-pyrrol-2-yl}-5,6-dihydro-
imidazo[9,5,1-jk][1,4]benzodiazepin-7(4X)-one
[M+-I = 428]
Example I80
2-i1-[2,6-Dichloro-4-(trifluoromethyl)phenyl]-2,5-dimethyl-
1H-pyrrol-3-yl}-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-
7(4H)-one
[M+-1 = 493]
.. .. . _ ~~ "VI Vi
CA 02384265 2002-03-06
' 38
Example 181
2-[5-(Phenylethynyl)-2-thienyl]-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
IM+-1 = 369j
Example 182
2-~5-[2-(Trifluoromethoxy)phenyl]-2-furyl}r5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
IM+-1 = 413]
Example 183
2-(5-(2-Methoxycarbonylthiophen-3-yl)furan-2-yl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 393
Example 184
2-(2,5-Dimethylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M+-1 = 291]
Example 185
2-(4-Methoxycarbonylphenyl)-5,6-dihydroimidazo[4,5,1-jkj-
[1,4]benzodiazepin-7(4H)-one
IM+-1 = 321]
Example 186
2-(4-Methylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M+-1 = 277 ]
Example 187
2-(3,4-Difluorophenyl)-5,6-dihydroimidazo[4,5,1-jk][lf4jbenzo-
diazepin-7(4H)-one
IM'"-1 = 299
Example 188
2 -(4-Fluorophenyl)-5,6-dihydroimidazo[4,5,1-jkj[1,4)benzo-
diazepin-7(4H)-one
[ M+-1 = 2 81
Example 189
2-(3-Chloro-4-fluorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4j-
benzodiazepin-7(4H)-one
[M+-1 = 315]
, ~~wi5p761
CA 02384265 2002-03-06
39
Example 190
2-(3-Bromo-4-methoxyphenyl)-S,6-dihydroimidazo[4,5,1-jk][1,4j-
benzodiazepin-7(4X)-one
. [M+-1 = 372]
Example 191
2-[4-(Trifluoromethoxy)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4j-
benzodiazepin-7(4X)-one
[M+-1 = 374]
Example 192
2-(2,S-Difluorophenyl)-5,6-dihydroimidazo[4,5,1-jkj[1,4]benzo--
diazepin-7(4X)-one
[M+-1 = 299]
Example 193
2-[4-(1,1,2,2-Tetrafluoroethoxy)phenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4X)-one
[M*-1 = 379]
Example 194
2-[4-Fluoro-3-(trifluoromethyl)phenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4X)-one
[M'"-1 = 349 j
Example 195
2-(4-Cyanophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M+-1 = 288]
Example 196
2-(3-Bromo-4-fluorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4X)-one
[M+-1 = 360]
Example 197
2-(4-tent-Butyl-2-methylphenyl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4X)-one
[M+-1 = 333]
Example 198
2-[4-(1-Methoxy-1-methylethyl)phenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 335]
CA 02384265 2002-03-06
Example 199
2-(4-Bromophenyl)-5,6-dihydroimidazo[4,5,1-j k][1,4]benzodiazepin-
7(4H)-one
[M+-1 = 342]
5
Example 200
2-[4-(3,4-Dichlorophenoxy)phenyl]-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M*-1 = 424]
Example 201
2-[4-(2-Propynyloxy)phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M*-1 = 317]
Example 202
2-{4-[Chloro(difluoro)methyl]phenyl}-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 347]
Example 203
2-(4-Benzoylphenyl)-5,6-dihydroimidazo[4,5,1-j k][1,4]benzo-
diazepin-7(4H)-one
[M*-1 = 367]
Example 204
2-(4-Ethylphenyl)-5,6-dihydroimidazo[4,5,1-j k][1,4)benzodiazepin-
7(4H)-one
[M*-1 = 291]
Example 205
2-(2-Hydroxy-5-methylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M*-1 = 293]
Example 206
2-[4-(2,6-Difluorobenzoyl)-1-methyl-1H-pyrrol-2-yl]-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 406]
Example 207
2-[4-(3-Chlorobenzoyl)-1-methyl-1H-pyrrol-2-yl]-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 404]
.. . ~ ~ .y r v ~ ~7 1
CA 02384265 2002-03-06.
41
Example 208
2-(2-Ethoxy-1-naphthyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M+-1 = 357 ]
Example 209
2-[2-(Benzyloxy)-4,5-dimethoxyphenyl]-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 429 ]
Example 210
2-{4-[(2-Chloroethyl)(ethyl)amino]-2-methylphenyl}-5,6-dihydro-
imidazo[4,5,1-jkj[1,4]benzodiazepin-7(4H)-one
[M*-1 = 382]
_ 15
Example 211
2-(4,5-Dimethoxy-2-methylphenyl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M+-1 = 337
Example 212
2-(7-Methyl-2-naphthyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
[M+-1 = 327]
Example 213
2-(2,4-Dimethoxy-5-methylphenyl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M+-1 = 337 ]
Example 214
2-(3-Benzoyl-2,4-dichlorophenyl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
[M+-1 = 436]
Example 215
2-(6-Chloro-1,3-benzodioxol-5-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
IM+-1 = 341]
Example 216
2-[4-(Benzyloxy)-3,5-dimethoxyphenyl]-5,6-dihydroimidazo-
[4,5,1-j k][1,4]benzodiazepin-7(4H)-one
[M+-1 = 429
vu~u/Sp761
CA 02384265 2002-03-06
42
Example 217
2-(3,4-Diethoxyphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M*-1 = 351]
Example 218
2-(2-((Pyridin-2-yl)aminocarbonyl)eth-1-yl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M+-1 = 335]
Example 219
2-(3-((Pyridin-2-yl)aminocarbonyl)prop-1-yl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 349]
Example 220
2-((1,3-Dimethyl-3,7-dihydro-2,6-dioxo-1H-purin-8-yl)methyl-
5,6-dihydroimidazo[4,5,1-jk]-[1,4]benzodiazepin-7(4H)-one
[M+-1 = 379]
Example 221
2-(2-((Thiazol-2-yl)aminocarbonyl)eth-1-yl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-2-yl)-7(4H)-one
[M*-1 =.341]
Example 222
2-~2-[(1,3-Dimethyl-1X-pyrazol-5-yl)amino]phenyl}-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 372]
Example 223
.. 2-(2-(4-Chlorophenyl)methylthio-3-cyanopyridin-6-yl)-5,6-dihydro-
imidazo-(4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 445]
Example 224
2-(4-tent-Butylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
.[M*-1 = 319]
Example 225
2-{2,5-Dimethyl-1-[3-(trifluoromethyl)phenyl]-lH-pyrrol-3-yl}-
5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
[M*-1 = 424]
.. - - . -v. vi
CA 02384265 2002-03-06
' 43
Example 226
2-(5-Chloro-3-methyl-1-phenyl-1X-pyrazol-4-yl)-5,6-dihydro-
'midazo[4,5,1-jk][1,4]benzodiazepin-7(4X)-one
IM+-1 = 377]
Example 227
2-[2,5-Bis(trifluoromethyl)phenyl]-5,6-dihydroimidazo[4,5,1-jk]_
[1,4]benzodiazepin-7(4X)-one
[M+-1 = 399]
Example 228
2-[4-(4-tert-Butyl-1,3-thiazol-2-yl)phenyl]-5,6-dihydroimidazo-
[4,5,I-jk][1,4]benzodiazepin-7(4X)-one
[M+-1 = 402]
Example 229
2-(3-Cyano-4-N,N-dimethylamino-2-fluorophenyl)-5,6-dihydro-
imidazo-[4,5,1-jk][1,.4]benzodiazepin-7(4H)-one
[M*-1 = 349]
Example 230
2-(6-Methoxy-2-naphthyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M+-1 = 343]
Z5
Example 231
2-(4-Isobutylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4X)-one
[M*-1 = 319]
Example 232
2-(3-Bromo-4-methoxyphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
[M*-1 = 372]
The following compounds according to the invention can be
prepared in analogy to the methods described above:
1. 2-(4-(4-n-propylpiperazin-1-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jkj[1,4]benzodiazepin-7(4H)-one
2. 2-(4-(4-isopropylpiperazin-1-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
3. 2-(4-(4-benzylpiperazin-1-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
vu50/50761
CA 02384265 2002-03-06
44
4. 2-(4-(4-n-butylpiperazin-1-yl)phenyl)-5,6-dihydroimidazo
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
5. 2-(4-(4-ethylpiperazin-1-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
6. 2-(4-(2-N,N-dimethylaminoeth-1-yloxy)phenyl)-5,6-dihydro-
imidazo[4,5,1-k][1,4]benzodiazepin-7(4H)-one
7. 2-(4-(2-pyrrolidin-1-yleth-1-yloxy)phenyl)-5,6-dihydroimid-
azo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
8. 2-(4-(2-piperazin-1-yleth-1-yloxy)phenyl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
9. 2-(4-(2-(4-methylpiperazin-1-yl)eth-1-yloxy)phenyl)-5,6
dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
10. 2-(4-(2-(4-propylpiperazin-1-yl)eth-1-yloxy)phenyl)-5,6-
dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
11. 2-(4-(2-(4-ethylpiperazin-1-yl)eth-1-yloxy)phenyl)-5,6
dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
12. 2-(4-(2-(4-benzylpiperazin-1-yl)eth-1-yloxy)phenyl)-5,6-
dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
13. 2-(4-(2-(4-acetamidopiperazin-1-yl)eth-1-yloxy)phenyl)-
5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
14. 2-(4-(2-(4-benzamidopiperazin-1-yl)eth-1-yloxy)phenyl)-
5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
15. 2-(4-(4-methylhomopiperazin-1-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
16. 2-(4-(4-benzylhomopiperazin-1-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
17. 2-(4-(4-n-butylhomopiperazin-1-yl)phenyl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
18. 2-(4-(4-ethylhomopiperazin-1-yl)phenyl)-5,6-dihydroimidazo-
[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
,.......~ ~v, vt
CA 02384265 2002-03-06
19. 2-(4-methoxyphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
20. 2-(4-chlorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benza-
5 diazepin-7(4H)-one
21. 2-(4-aminophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
ZO 22. 2-(4-isopropylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
23. 2-(3-chlorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
_ 15
24. 2-(3-methylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
25. 2-(3-phenylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
20 diazepin-7(4H)-one
26. 2-(3-isopropylphenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]-
benzodiazepin-7(4H)-one
25 27. 2-(3-fluorophenyl)-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
28. 2-piperidin-4-yl-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
29. 2-(1-ethylpiperidin-4-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
30. 2-(1-n-propylpiperidin-4-yI)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
31. 2-(1-isopropylpiperidin-4-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
32. 2-pyridin-4-yl-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodi-
azepin-7(4H)-one
33. 2-pyridin-2-yl-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodi-
azepin-7(4H)-one
CA 02384265 2002-03-06
Y
46
34. 2-thien-2-yl-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
35. 2-indol-5-yl-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodi-
azepin-7(4H)-one
36. 2-indol-2-yl-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodi-
azepin-7(4H)-one
37. 2-quinolin-3-yl-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
38. 2-isoquinolin-1-yl-5,6-dihydroimidazo[4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
39. 2-quinoxalin-2-yl-5,6-dihydroimidazo(4,5,1-jk][1,4]benzo-
diazepin-7(4H)-one
40. 2-naphth-2-yl-5,6-dihydroimidazo(4,5,1-jk][1,4]benzodi-
azepin-7(4H)-one
41. 2-(2-(N,N-dimethylamino)eth-1-ylamino)phenyl)-5,6-dihydro-
imidazo[4,5,1-k][1,4]benzodiazepin-7(4H)-one
42. 2-(2-(N,N-diethylamino)eth-1-ylamino)phenyl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
43. 2-(2-piperidin-1-yleth-1-ylamino,)phenyl)-5,6-dihydroimid-
azo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
44. 2-(2-pyrrolidin-1-yleth-1-ylamino)phenyl)-5,6-dihydroimid-
azo[4,5,1-jk](1,4]benzodiazepin-7(4H)-one
45. 2-(3-(N,N-dimethylamino)prop-1-ylamino)phenyl)-5,6-dihydro-
imidazo[4,5,1-jk](1,4]benzodiazepin-7(4H)-one
96. 2-(3-(N,N-diethylamino)prop-1-ylamino)phenyl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
47. 2-(3-piperidin-1-ylprop-1-ylamino)phenyl)-5,6-dihydroimid-
azo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
48. 2-(3-pyrrolidin-1-ylprop-1-ylamino)phenyl)-5,6-dihydro-
imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one
CA 02384265 2002-03-06
47
49. 2-cyclohexyl-5,6-dihydroimidazo[4,5,1-jkj[1,4]benzodi-
azepin-7(4H)-one
50. 2-(cis-4-aminocyclohex-1-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
51. 2-(4-methoxycyclohex-1-yl)-5,6-dihydroimidazo[4,5,1-jk]-
[1,4]benzodiazepin-7(4H)-one
52. 2-phenyl-5,6-dihydroimidazo[5,4,1-jk][1,4]benzodiazepin-
7(4H)-one
53. 2-(3-aminophenyl)-5,6-dihydroimidazo[5,4,1-jk][1,4]benzo-
diazepin-7(4H)-one
54. 2-(4-N,N-dimethylaminomethylphenyl)-5,6-dihydroimidazo-
[5,4,1-jk][1,4]benzodiazepin-7(4H)-one
55. 2-(4-(2-N,N-dimethylaminoeth-1-yl)phenyl)-5,6-dihydro-
imidazo[5,4,1-jk][1,4]benzodiazepin-7(4H)-one
56. 2-(4-hydroxyphenyl)-5,6-dihydroimidazo[5,4,1-j k][1,4]benzo-
diazepin-7(4H)-one
57. 2-(4-pyrrolidinemethylphenyl)-5,6-dihydroimidazo[5,4,1-jk]-
[1,4]benzodiazepin-7(4H)-one
58. 2-(2-methylthiophenyl)-5,6-dihydroimidazo[5,4,1-jk][1,4]-
benzodiazepin-7(4H)-one
59. 2-(4-carboxyphenyl)-5,6-dihydroimidazo[5,4,1-jk][1,4]benzo-
diazepin-7(4H)-one
60. 2-(3,5-bis(trifluoromethyl)phenyl)-5,6-dihydroimidazo-
[5,4,1-jk][1,4]benzodiazepin-7(4H)-one
61. 2-(4-tert-butylphenyl)-5,6-dihydroimidazo[5,4,1-jk][1,4]-
benzodiazepin-7(4H)-one
62. 2-(3-(morpholin-4-ylmethyl)phenyl)-5,6-dihydroimidazo-
[5,4,1-jk][1,4]benzodiazepin-7(4X)-one