Note: Descriptions are shown in the official language in which they were submitted.
CA 02384427 2002-03-08
O.Z. 5493-WO
Copolymers of acrvloylaminoalkyl compounds
The invention relates to antimicrobial polymers which are obtained by
copolymenzing acryloylaminoalkyl compounds with other monomers. The
invention further relates to a process for preparing these antimicrobial
polymers, and to their use.
The invention also relates to antimicrobial polymers which are obtained by
graft copolymerization of acryloylaminoalkyl compounds with other
1 o monomers on a substrate, and also to a process for their preparation, and
to their use.
For the purposes of the present invention, acryloylaminoalkyl compounds
are in particular dialkylaminoalkyl acrylates and acryloylaminoalkyl
ammonium salts.
It is highly undesirable for bacteria to become established or to spread on
the surfaces of piping, or of containers or packaging. Frequently, slime
layers form and permit sharp rises in microbial populations, and these can
2 0 lead to persistent impairment of the quality of water or of drinks or
foods,
and even to spoilage of the product and harm to the health of consumers.
Bacteria must be kept away from all fields of life where hygiene is
important. This affects textiles for direct body contact, especially in the
genital area, and those for the care of the sick or elderly. Bacteria must
also be kept away from surfaces of the furniture and instruments used in
patient-care areas, especially in areas for intensive care or for neonatal
care, and in hospitals, especially in areas for medical intervention, and also
in isolation wards for critical cases of infection, and in toilets.
3 o A current method of treating equipment, or the surfaces of furniture or of
textiles, to resist bacteria either when this becomes necessary or else as a
precautionary measure is to use chemicals or solutions of these, or else
mixtures which are disinfectant and therefore have fairly broad general
antimicrobial action. Chemical agents of this type act nonspecifically and
are themselves frequently toxic or irritant, or form degradation products
which are hazardous to health. In addition, people frequently exhibit
intolerance to these materials once they have become sensitized.
CA 02384427 2002-03-08
O.Z. 5493-WO - 2 -
Another procedure to counteract the surface spread of bacteria is the
incorporation of antimicrobial substances into a matrix.
In another technical sector, US 4 532 269 discloses a terpolymer of butyl
methacrylate, tributyltin methacrylate, and tert-butylaminoethyl
methacrylate. This polymer is used as an antimicrobial paint for ships: the
hydrophilic tert-butylaminoethyl methacrylate promotes gradual erosion of
the polymer, thus liberating the highly toxic tributyltin methacrylate as
1 o antimicrobial agent.
In these applications, the copolymer prepared using aminomethacrylates is
merely a matrix or carrier substance for added microbial agents which can
diffuse or migrate out of the carrier substance. Sooner or later, polymers of
this type lose their effectiveness once the necessary "minimum inhibitory
concentration" (MIC) at the surface has been lost.
European patent applications 0 862 858 and 0 8fi2 859 have disclosed
that homo- and copolymers of tert-butylaminoethyl methacrylate, a
2 o methacrylate having a secondary amino function, have inherent
microbicidal properties. For effective avoidance of undesirable resistance
phenomena in microbes, particularly bearing in mind that the development
of resistance by microbes is known from antibiotics research, systems
developed in the future will again have to be based on novel compositions
2 5 with improved effectiveness.
Tert-butylaminoethyl methacrylate is a commercially available monomer in
methacrylate chemistry and is used in particular as a hydrophilic
constituent in copolymerization reactions. For example, EP 0 290 676
3 o describes the use of various polyacrylates and polymethacrylates as a
matrix for immobilizing bactericidal quaternary ammonium compounds.
Dialkylaminoalkylmethacrylamides are widely used as a comonomer unit,
in particular as a constituent of materials which improve dispersion and
viscosity in lubricating oils. For example, EP 0 750 031 describes a
35 terpolymer from two alkyl acrylates, the alkyl chains present in each case
being of different lengths, and from a nitrogen-containing monomer,
including dimethylaminoacrylamides. US 5 821 313 describes analogous
' CA 02384427 2002-03-08
O.Z. 5493-WO 3 replacement sheet
systems with a proportion by weight of up to 45% of amino-containing
monomer.
As described in EP 0 416 762, dimethylaminopropylmethacrylamide is also
used as a terpolymer constituent in cationic electrodeposition paint
s compositions.
The preparation of antimicrobial copolymers using
dialkylaminoalkylacrylamides is not known.
The object on which the invention is based is therefore to develop novel
antimicrobial polymers which prevent bacteria from colonizing surfaces and
io spreading thereon.
Surprisingly, it has now been found that copolymerization of
acryloylaminoalkylamines with aliphatically unsaturated monomers or graft
copolymerization of these components on a substrate gives polymers with a
surface which is lastingly microbicidal, is not damaged by solvent or physical
is action, and exhibits no migration. There is no need here to use any other
biocidal active ingredient.
The use of 2-methacryloyloxyethyl derivatives as a cationic constituent in
copolymerization reactions is known from other technical sectors. In this
connection, EP 0 322 234 describes the synthesis of terpolymers which are
2o dewatering auxiliaries auxiliaries for dye systems, as described in US 4
168
976. There is no discussion in this context of acryloylaminoalkyl derivatives,
which are in chemical terms a different class of substance.
FR-A-2757866 discloses polymers or copolymers having quaternary amino
groups, the quaternary amino group containing an alkyl or aryl radical having
2s from 5 to 20 carbon atoms.
EP 331 528 describes copolymers composed of from 40 to 95% by weight of
ethylene and from 5 to 60% by weight of at least one
dialkylaminoalkylacrylamide.
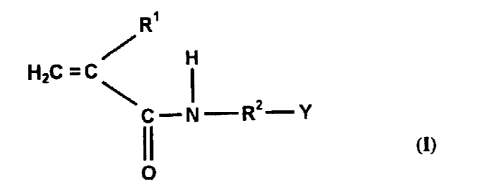
3o The present invention therefore provides antimicrobial copolymers which are
obtained by copolymerizing a monomer of the formula I
CA 02384427 2002-03-08
O.Z. 5493-WO 4 replacement sheet
R'
H
HzC =C'
C--N-Rz-Y
(1)
O
where
R' - -H or -CH3,
R2 - a branched or unbranched aliphatic hydrocarbon
s radical having from 1 to 5 carbon atoms,
Y - NR3R4
R3, R4 - H or a branched or unbranched aliphatic hydrocarbon
radical having from 1 to 5 carbon atoms, where R3
and R4 may be identical or different,
io with at least one other aliphatically unsaturated monomer excluding
ethylene.
The invention further provides a process for preparing antimicrobial
copolymers, where monomers of the formula I
R'
HZC =C~ H
'C- ~ -RZ--Y
(I)
O
is where
R' - -H or -CH3,
R2 - a branched or unbranched aliphatic hydrocarbon
radical having from 1 to 5 carbon atoms,
Y - NR3R4
Zo R3, R4 - H or a branched or unbranched aliphatic hydrocarbon
radical having from 1 to 5 carbon
CA 02384427 2002-03-08
O.Z. 5493-WO 5 replacement sheet
atoms, where R3 and R4 may be identical or different,
are copolymerized with at least one other aliphatically unsaturated monomer
excluding ethylene.
s The monomers of the formula I which may be used to prepare the inventive
copolymers may therefore also be described by the formulae II
(dialkylaminoacrylamides) and III (acryloylaminoalkylammonium salts):
R'
Ra
z , Ra
C-N-R -N
(II)
O
R~
Rs
C-N-R2-Nr R4 X'
R5 (III}
O
To obtain adequate antimicrobial action from the copolymer or graft polymer,
io the proportion of monomers of formula I or, respectively, 3-
methacrylaminopropyltrimethylammonium chloride or 3-acryl-
amidopropyltrimethylammonium chloride in the reaction mixture during the
preparation of the antimicrobial copolymers or in the process according to
the invention should be from 5 to 98 mol%, preferably from 30 to 98 mol%,
is particularly preferably from 40 to 98 mol%, based on the entirety of the
monomers.
The aliphatically unsaturated monomers used may be any of the monomers
which copolymerize with the monomers of formula I. Examples of those
suitable are acrylates and methacrylates, such as acrylic acid, tert-butyl
2o methacrylate, or methyl methacrylate, styrene, vinyl chloride, vinyl
ethers,
acrylamides, acrylonitriles, olefins (propylene, butylene, isobutylene), allyl
compounds, vinyl ketones, vinylacetic acid,
CA 02384427 2002-03-08
O.Z. 5493-WO 6 replacement sheet
vinyl acetate, and vinyl esters, e.g. in particular methyl methacrylate, ethyl
methacrylate, butyl methacrylate, tert-butyl methacrylate, methyl acrylate,
ethyl acrylate, butyl acrylate, tert-butyl acrylate, tert-butylaminoethyl
esters,
2-diethylaminoethyl methacrylate, 2-diethylaminoethyl vinyl ether, 2-meth-
s acryloyloxyethyltrimethylammonium methosulfate, and 2-meth-
acryloyloxyethyltrimethylammonium chloride.
The aliphatically unsaturated monomers are preferably the compounds of
acrylic or methacrylic acid, particularly preferably esters of acrylic or
to methacrylic acid.
The monomer used in the formula II is preferably
dimethylaminopropylmethacrylamide, diethylaminopropylmethacrylamide, or
N-3-dimethylaminopropylacrylamide.
The monomer used in the formula III is 3-
methacrylaminopropyltrimethylammonium chloride or 3-
acrylamidopropyltrimethylammonium chloride.
2o The antimicrobial copolymers of the invention may be obtained by
copolymerizing monomers of the formula I or II or III with one or more
aliphatically unsaturated monomers. The polymerization advantageously
takes place by a free-radical route using a free-radical initiator, or with
initiation by radiation. The examples describe typical procedures.
The antimicrobial copolymers of the invention may also be obtained by
copolymerizing monomers of the formula I or II or III with at least one
aliphatically unsaturated monomer on a substrate. This gives a physisorbed
coating made from the antimicrobial copolymer on the substrate.
CA 02384427 2002-03-08
O.Z. 5493-WO - 7 -
Particularly suitable substrate materials are any of the synthetic polymers,
e.g. polyurethanes, polyamides, polyesters, polyethers, polyether block
amides, polystyrene, polyvinyl chloride, polycarbonates,
polyorganosiloxanes, polyolefins, polysulfones, polyisoprene,
polychloroprene, polytetrafluoroethylene (PTFE), and corresponding
copolymers and blends, and also natural or synthetic rubbers, with or
without radiation-sensitive groups. The process of the invention may also
be employed on surfaces of products which have been made from metal,
from glass, or from wood and which have been painted or otherwise
1 o plastic-coated.
In another embodiment of the present invention, the copolymers may be
obtained by graft polymerization of a substrate using monomers of the
formula I or II or III and using at least one aliphatically unsaturated
monomer. The grafting of the substrate permits covalent linking of the
antimicrobial copolymer to the substrate. The substrates used may be any
polymeric material, such as the abovementioned plastics.
Prior to the graft copolymerization, the surfaces of the substrates may be
2 o activated by various methods. Any standard method for activating polymer
surfaces may be used here. For example, the substrate may be activated
prior to the graft polymerization by UV radiation, plasma treatment, corona
treatment, flame treatment, ozonization, electrical discharge, or y-radiation.
The surfaces are advantageously freed in advance in a known manner
2 5 from oils, fats, or other contamination, using a solvent.
The substrates may be activated using UV radiation in the wavelength
range from 170-400 nm, preferably from 170-250 nm. An example of a
suitable radiation source is a Noblelight UV excimer apparatus from
3 o HERAEUS, Hanau, Germany. However, mercury vapor lamps are also
suitable for substrate activation as long as they emit substantial
proportions of radiation in the abovementioned ranges. The exposure time
is generally from 0.1 second to 20 minutes, preferably from 1 second to 10
minutes.
The activation of the substrate using UV radiation prior to the graft
polymerization may also be effected using an additional photosensitizer.
For this, the photosensitizer, such as benzophenone, is applied to the
CA 02384427 2002-03-08
O.Z. 5493-WO - 8 -
substrate surface and irradiated. A mercury vapor lamp may again be used
here, with exposure times of from 0.1 second to 20 minutes, preferably
from 1 second to 10 minutes.
According to the invention, the activation may also be achieved by plasma
treatment using an RF or microwave plasma (Hexagon, Technics Plasma,
85551 Kirchheim, Germany) in air, nitrogen, or argon atmospheres. The
exposure times are generally from 2 seconds to 30 minutes, preferably
from 5 seconds to 10 minutes. The energy supplied in the case of
to laboratory devices is from 100 to 500 W, preferably from 200 to 300 W.
Corona devices (SOFTAL, Hamburg, Germany) may also be used for
activation. The exposure times in this case are generally from 1 to 10
minutes, preferably from 1 to 60 seconds.
Activation by electrical discharge, electron beam, or y-radiation (e.g. from a
cobalt 60 source), and also ozonization, permit short exposure times,
generally from 0.1 to 60 seconds.
2 o Substrate surfaces may also be activated by flame treatment. Suitable
devices, in particular those with a barrier flame front, can readily be
constructed or, for example, purchased from ARCOTEC, 71297
Monsheim, Germany. They may be operated using hydrocarbons or
hydrogen as combustion gas. In all cases it is necessary to avoid damage
to the substrate by overheating, and this can readily be ensured if that
surface of the substrate facing away from the flame treatment side is in
intimate contact with a cooled metal surface. Activation by flame treatment
is therefore restricted to relatively thin, sheet-like substrates. The
exposure
times are generally from 0.1 second to 1 minute, preferably from 0.5 to 2
3 o seconds. The flames are exclusively nonluminous, and the distances
between the substrate surfaces and the outer side of the flame front are
from 0.2 to 5 cm, preferably from 0.5 to 2 cm.
The substrate surfaces activated in this way are coated by known
methods, such as dipping, spraying, or spreading, using monomers of the
formula I or II or III (component I) and using one or more aliphatically
unsaturated monomers (component II), where appropriate in solution.
Solvents which have proven useful are water and waterlethanol mixtures,
CA 02384427 2002-03-08
O.Z. 5493-WO - 9 -
but other solvents may also be used as long as they are sufficiently
capable of dissolving the monomers and give good wetting of the substrate
surfaces. Solutions with monomer contents of from 1 to 10% by weight, for
example about 5% by weight, have proven successful in practice and
generally give, in a single pass, coherent coatings which cover the
substrate surface and have thicknesses which can be more than 0.1 Nm.
The graft copolymerization of the monomers applied to the activated
surfaces may usefully be initiated by radiation in the short-wave segment
l o of the visible range, or in the long-wave segment of the UV range of
electromagnetic radiation. For example, the radiation from a UV excimer to
wavelengths of from 250 to 500 nm, preferably from 290 to 320 nm, is very
suitable. Mercury vapor lamps are also suitable here as long as they emit
substantial proportions of radiation in the abovementioned ranges. The
exposure times are generally from 10 seconds to 30 minutes, preferably
from 2 to 15 minutes.
Graft copolymerization of the comonomer compositions of the invention
may also be achieved by a process described in European patent
2 o application 0 872 512 and based on graft polymerization of molecules of
monomer and of initiator incorporated by swelling. The monomer used for
the swelling process may be component II.
The antimicrobial copolymers of the invention made from monomers of
formula I or II or III (component I) and from at least one other aliphatically
unsaturated monomer (component II) exhibit microbicidal or antimicrobial
behavior, even without grafting onto a substrate surface. In another
embodiment of the present invention, components I and II are
copolymerized on a substrate.
The components may be in solution when applied to the substrate.
Examples of suitable solvents are water, ethanol, methanol, methyl ethyl
ketone, diethyl ether, dioxane, hexane, heptane, benzene, toluene,
chloroform, dichloromethane, tetrahydrofuran, and acetonitrile. It is also
possible to use component II as solvent for component I.
The novel antimicrobial copolymers may also be used directly, i.e. not by
polymerizing the components on a substrate but as an antimicrobial
CA 02384427 2002-03-08
O.Z. 5493-WO - 10 -
coating. Suitable coating methods are application of the copolymers in
solution or as a melt.
The solution of the polymers of the invention may be applied to substrates
by dipping, spraying, or painting, for example.
If the copolymers of the invention are produced directly on the substrate
surface without grafting, conventional free-radical initiators may be added.
Examples of initiators which may be used in preparing the copolymers of
1 o the invention are azonitriles, alkyl peroxides, hydroperoxides, acyl
peroxides, peroxoketones, peresters, peroxocarbonates, peroxodisulfate,
persulfate, and any of the usual photoinitiators, such as acetophenones, a
hydroxyketones, dimethylketals, and benzophenone. The polymerization
may also be initiated thermally or, as previously stated, by electromagnetic
radiation, such as UV light or y-radiation.
Use of the modified polymer substrates
The present invention also provides the use of the antimicrobial
2 o copolymers of the invention for producing antimicrobial products, and
provides the resultant products per se. The products may comprise
polymer substrates modified according to the invention, or consist of these.
Products of this type are preferably based on polyamides, on
polyurethanes, on polyether block amides, on polyesteramides, or on
polyesterimides, on PVC, on polyolefins, on silicones, on polysiloxanes, on
polymethacrylate, or on polyterephthalates, which have surfaces modified
using polymers of the invention.
Examples of antimicrobial products of this type are machine parts for the
3 o processing of food or drink, components of air conditioning systems,
roofing, bathroom or toilet items, kitchen items, components of sanitary
equipment, components of animal cages or of animal houses, recreational
products for children, components of water systems, packaging for food or
drink, operating units (touch panels) of devices, and contact lenses.
The copolymers or graft copolymers of the invention may be used
wherever importance is placed on surfaces which are as free as possible
from bacteria, i.e. microbicidal surfaces or surfaces with release properties.
CA 02384427 2002-03-08
O.Z. 5493-WO - 11 -
Examples of the use of the copolymers or graft polymers of the invention
are in particular surface coatings, protective paints, and other coatings in
the following sectors:
- marine: ships' hulls, docks, buoys, drilling platforms, ballast water tanks
- construction: roofing, basements, walls, facades, greenhouses, sun
protection, garden fences, wood protection
- sanitary: public conveniences, bathrooms, shower curtains, toilet items,
swimming pools, saunas, jointing, sealing compounds
- food and drink: machines, kitchens, kitchen items, sponges, recreational
products for children, packaging for food or drink, milk processing,
drinking water systems, cosmetics
- machine parts: air conditioning systems, ion exchangers, process water,
solar-powered units, heat exchangers, bioreactors, membranes
- medical technology: contact lenses, diapers, membranes, implants
- consumer articles: automobile seats, clothing (socks, sports clothing),
hospital equipment, door handles, telephone handsets, public
conveyances, animal cages, cash registers, carpeting, wall coverings.
2 o The copolymers of the invention or coatings made from these copolymers
are also used as components for formulating inks or paints, e.g. as an
additive or as a coating for an additive or for a pigment.
The present invention also provides the use of the inventive polymer
substrates surface-modified using polymers of the invention or using the
process of the invention for producing hygiene products or items for
medical technology. The same descriptions of preferred materials are
applicable. Examples of hygiene products of this type are toothbrushes,
toilet seats, combs, and packaging material. The term hygiene item also
3 o includes other objects which may come into contact with a large number of
people, for example telephone handsets, stair rails, door handles, window
catches, and grab straps and grab handles in public conveyances.
Examples of items in medical technology are catheters, tubing, protective
or backing films, and surgical instruments.
The following examples are given to describe the present invention in
greater detail, but are not intended to limit its scope as set out in the
patent
claims.
CA 02384427 2002-03-08
O.Z. 5493-WO - 12 -
Example 1:
16 g of 3-methacryloylaminopropyltrimethylammonium chloride (50% by
weight solution in water) (Aldrich), 9 g of tert-butyl methacrylate (Aldrich),
and 60 ml of ethanol are charged to a three-necked flask and heated to
65°C under a stream of argon. 0.15 g of azobisisobutyronitrile
dissolved in
4 ml of ethyl methyl ketone is then slowly added dropwise, with stirring.
The mixture is heated to 70°C and stirred for 72 h at this
temperature.
1 o After expiry of this time, the reaction mixture is stirred into 0.5 I of
deionized water, whereupon the polymeric product precipitates. After the
product has been isolated by filtration, the filter residue is washed with
100 ml of deionized water in order to remove any residual monomers still
present. The product is then dried in vacuo at 52°C for 24 hours.
is
Examale 1 a:
0.05 g of the product from example 1 is shaken in 20 ml of a test microbial
suspension of Staphylococcus aureus. After a contact time of 15 minutes,
1 ml of the test microbial suspension is removed, and the number of
2 o microbes in the test mixture is determined. After expiry of this period,
the
number of microbes has fallen from 10' to 104.
Example 1 b:
0.05 g of the product from example 1 is shaken in 20 ml of a test microbial
2 5 suspension of Pseudomonas aeruginosa. After a contact time of 60
minutes, 1 ml of the test microbial suspension is removed, and the number
of microbes in the test mixture is determined. After expiry of this period,
the
number of microbes has fallen from 10' to 104.
3 o Example 2:
16 g of 3-methacryloylaminopropyltrimethylammonium chloride (50% by
weight solution in water) (Aldrich), 9 g of butyl methacrylate (Aldrich), and
60 ml of ethanol are charged to a three-necked flask and heated to 65°C
under a stream of argon. 0.15 g of azobisisobutyronitrile dissolved in 4 ml
35 Of ethyl methyl ketone is then slowly added dropwise, with stirring. The
mixture is heated to 70°C and stirred for 72 h at this temperature.
After
expiry of this time, the reaction mixture is stirred into 0.5 I of deionized
water, whereupon the polymeric product precipitates. After the product has
CA 02384427 2002-03-08
O.Z. 5493-WO - 13 -
been isolated by filtration, the filter residue is washed with 100 ml of
deionized water in order to remove any residual monomers still present.
The product is then dried in vacuo at 50°C for 24 hours.
Example 2a:
0.05 g of the product from example 2 is shaken in 20 ml of a test microbial
suspension of Staphylococcus aureus. After a contact time of 15 minutes,
1 ml of the test microbial suspension is removed, and the number of
microbes in the test mixture is determined. After expiry of this period, the
1 o number of microbes has fallen from 10' to 103.
Example 2b:
0.05 g of the product from example 2 is shaken in 20 ml of a test microbial
suspension of Pseudomonas aeruginosa. After a contact time of 60
minutes, 1 ml of the test microbial suspension is removed, and the number
of microbes in the test mixture is determined. After expiry of this period,
the
number of microbes has fallen from 10' to 104.
Example 3:
2 0 12 g of 3-acrylamidopropyltrimethylammonium chloride (75% strength by
weight solution in water) (Aldrich), 9 g of tert-butyl methacrylate (Aldrich),
and 60 ml of ethanol are charged to a three-necked flask and heated to
65°C under a stream of argon. 0.15 g of azobisisobutyronitrile
dissolved in
4 ml of ethyl methyl ketone is then slowly added dropwise, with stirring.
The mixture is heated to 70°C and stirred for 72 h at this
temperature.
After expiry of this time, the reaction mixture is stirred into 0.51 of
deionized water, whereupon the polymeric product precipitates. After the
product has been isolated by filtration, the filter residue is washed with
100 ml of deionized water in order to remove any residual monomers still
3 o present. The product is then dried in vacuo at 50°C for 24 hours.
Example 3a:
0.05 g of the product from example 3 is shaken in 20 ml of a test microbial
suspension of Staphylococcus aureus. After a contact time of 15 minutes,
1 ml of the test microbial suspension is removed, and the number of
microbes in the test mixture is determined. After expiry of this period, the
number of microbes has fallen from 10' to 103.
CA 02384427 2002-03-08
O.Z. 5493-WO - 14 -
Example 3b:
0.05 g of the product from example 3 is shaken in 20 ml of a test microbial
suspension of Pseudomonas aeruginosa. After a contact time of 60
minutes, 1 ml of the test microbial suspension is removed, and the number
of microbes in the test mixture is determined. After expiry of this period,
the
number of microbes has fallen from 10'to 104.
Example 4:
12 g of 3-acrylamidopropyltrimethylammonium chloride (75% strength by
to weight solution in water) (Aldrich), 9 g of butyl methacrylate (Aldrich),
and
60 ml of ethanol are charged to a three-necked flask and heated to 65°C
under a stream of argon. 0.15 g of azobisisobutyronitrile dissolved in 4 ml
of ethyl methyl ketone is then slowly added dropwise, with stirring. The
mixture is heated to 70°C and stirred for 72 h at this temperature.
After
expiry of this time, the reaction mixture is stirred into 0.51 of deionized
water, whereupon the polymeric product precipitates. After the product has
been isolated by filtration, the filter residue is washed with 100 ml of
deionized water in order to remove any residual monomers still present.
The product is then dried in vacuo at 50°C for 24 hours.
Examale 4a:
0.05 g of the product from example 4 is shaken in 20 ml of a test microbial
suspension of Staphylococcus aureus. After a contact time of 15 minutes,
1 ml of the test microbial suspension is removed, and the number of
microbes in the test mixture is determined. After expiry of this period, the
number of microbes has fallen from 10' to 103.
Example 4b:
0.05 g of the product from example 4 is shaken in 20 ml of a test microbial
3 o suspension of Pseudomonas aeruginosa. After a contact time of 60
minutes, 1 ml of the test microbial suspension is removed, and the number
of microbes in the test mixture is determined. After expiry of this period,
the
number of microbes has fallen from 10'to 104.
Examale 5:
A nylon-12 film is exposed for 2 minutes at a pressure of 1 mbar to 172 nm
radiation from a Heraeus excimer source. The film activated in this way is
placed into an irradiator under an inert gas, and secured. Under a
CA 02384427 2002-03-08
O.Z. 5493-WO - 15 -
counterstream of inert gas, the film is then covered with 20 ml of a mixture
of 16 g of 3-methacryloylaminopropyltrimethylammonium chloride (50%
strength by weight solution in water) (Aldrich), 9 g of tert-butyl
methacrylate
(Aldrich), and 60 g of ethanol. The irradiation chamber is sealed and
placed at a distance of 10 cm from a Heraeus excimer source emitting at
wavelength 308 nm. Irradiation is begun and continues for 15 minutes. The
film is then removed and rinsed with 30 ml of ethanol, then dried in vacuo
at 50°C for 12 hours, then extracted 5 times with water for 6 hours at
30°C,
and then dried for 12 hours at 50°C.
The reverse side of the film is then treated in the same way, so that the
polyamide film finally obtained has been coated on both sides with grafted
polymer.
Example 5a:
A piece of coated film from example 5 (5 x 4 cm) is shaken in 30 ml of a
test microbial suspension of Staphylococcus aureus. After a contact time
of 15 minutes, 1 ml of the test microbial suspension is removed, and the
number of microbes in the test mixture is determined. After expiry of this
2 o time, the number of microbes has fallen from 10' to 104.
Examale 5b:
A piece of coated film from example 5 (5 x 4 cm) is shaken in 30 ml of a
test microbial suspension of Pseudomonas aeruginosa. After a contact
2 s time of 60 minutes, 1 ml of the test microbial suspension is removed, and
the number of microbes in the test mixture is determined. After expiry of
this time, the number of microbes has fallen from 10' to 104.
Example 6:
3 o A nylon-12 film is exposed for 2 minutes at a pressure of 1 mbar to 172 nm
radiation from a Heraeus excimer source. The film activated in this way is
placed into an irradiator under an inert gas, and secured. Under a
counterstream of inert gas, the film is then covered with 20 ml of a mixture
of 12 g of 3-acrylamidopropyltrimethylammonium chloride (75% strength by
35 weight solution in water) (Aldrich), 9 g of tert-butyl methacrylate
(Aldrich),
and 60 g of ethanol. The irradiation chamber is sealed and placed at a
distance of 10 cm from a Heraeus excimer source emitting at wavelength
308 nm. Irradiation is begun and continues for 15 minutes. The film is then
CA 02384427 2002-03-08
O.Z. 5493-WO - 16 -
removed and rinsed with 30 ml of ethanol, then dried in vacuo at 50°C
for
12 hours, then extracted 5 times with water for 6 hours at 30°C, and
then
dried for 12 hours at 50°C.
The reverse side of the film is then treated in the same way, so that the
polyamide film finally obtained has been coated on both sides with grafted
polymer.
Example 6a:
to A piece of coated film from example 6 (5 x 4 cm) is shaken in 30 ml of a
test microbial suspension of Staphylococcus aureus. After a contact time
of 15 minutes, 1 ml of the test microbial suspension is removed, and the
number of microbes in the test mixture is determined. After expiry of this
time, the number of microbes has fallen from 10'to 104.
Example 6b:
A piece of coated film from example 6 (5 x 4 cm) is shaken in 30 ml of a
test microbial suspension of Pseudomonas aeruginosa. After a contact
time of 60 minutes, 1 ml of the test microbial suspension is removed, and
2 o the number of microbes in the test mixture is determined. After expiry of
this time, the number of microbes has fallen from 10' to 104.
Example 7:
17 g of dimethylaminopropylmethacrylamide (Aldrich), 7 g of butyl
methacrylate (Aldrich) and 120 ml of ethanol are charged to a three
necked flask and heated to 65°C under a stream of argon. 0.3 g of
azobisisobutyronitrile dissolved in 8 ml of ethyl methyl ketone is then slowly
added dropwise, with stirring. The mixture is heated to 70°C and
stirred at
this temperature for 72 h. After expiry of this time, the reaction mixture is
3 o stirred into 0.6 I of cyclohexane, whereupon the polymeric product
precipitates. After the product has been isolated by filtration, the filter
residue is washed with 100 ml of n-hexane in order to remove any residual
monomers still present. The product is then dried in vacuo at 50°C for
24
hours.
Example 7a:
0.05 g of the product from example 7 is shaken in 20 ml of a test microbial
suspension of Staphylococcus aureus. After a contact time of 15 minutes,
CA 02384427 2002-03-08
O.Z. 5493-WO - 17 -
1 ml of the test microbial suspension is removed, and the number of
microbes in the test mixture is determined. After expiry of this time, no
remaining Staphylococcus aureus microbes are detectable.
Example 7b:
0.05 g of the product from example 7 is shaken in 20 ml of a test microbial
suspension of Pseudomonas aeruginosa. After a contact time of 60
minutes, 1 ml of the test microbial suspension is removed, and the number
of microbes in the test mixture is determined. After expiry of this time, the
1 o number of microbes has fallen from 10' to 103.
Example 8:
13 g of dimethylaminopropylmethacrylamide (Aldrich), 11 g of butyl
methacrylate (Aldrich) and 120 ml of ethanol are charged to a three
necked flask and heated to 65°C under a stream of argon. 0.3 g of
azobisisobutyronitrile dissolved in 8 ml of ethyl methyl ketone is then slowly
added dropwise, with stirring. The mixture is heated to 70°C and
stirred at
this temperature for 72 h. After expiry of this time, the reaction mixture is
stirred into 0.6 I of demineralized water, whereupon the polymeric product
2 o precipitates. After isolation of the product by filtration, the filter
residue is
washed with 100 ml of n-hexane in order to remove any residual
monomers still present. The product is then dried in vacuo at 50°C for
24
hours.
2 5 Example 8a:
0.05 g of the product from example 8 is shaken in 20 ml of a test microbial
suspension of Staphylococcus aureus. After a contact time of 15 minutes,
1 ml of the test microbial suspension is removed, and the number of
microbes in the test mixture is determined. After expiry of this time, the
3 o number of microbes has fallen from 10' to 103.
Example 8b:
0.05 g of the product from example 8 is shaken in 20 ml of a test microbial
suspension of Pseudomonas aeruginosa. After a contact time of 60
35 minutes, 1 ml of the test microbial suspension is removed, and the number
of microbes in the test mixture is determined. After expiry of this time, the
number of microbes has fallen from 10' to 104.
CA 02384427 2002-03-08
O.Z. 5493-WO - 18 -
Example 9:
14 g of dimethylaminopropylmethacrylamide (Aldrich), 10 g of tert-butyl
methacrylate (Aldrich) and 120 ml of ethanol are charged to a three-
necked flask and heated to 65°C under a stream of argon. 0.3 g of
azobisisobutyronitrile dissolved in 8 ml of ethyl methyl ketone is then slowly
added dropwise, with stirring. The mixture is heated to 70°C and
stirred at
this temperature for 72 h. After expiry of this time, the reaction mixture is
stirred into 0.6 I of demineralized water, whereupon the polymeric product
precipitates. After isolation of the product by filtration, the filter residue
is
to washed with 100 ml of n-hexane in order to remove any residual
monomers still present. The product is then dried in vacuo at 50°C for
24
hours.
Example 9a:
0.05 g of the product from example 9 is shaken in 20 ml of a test microbial
suspension of Staphylococcus aureus. After a contact time of 15 minutes,
1 ml of the test microbial suspension is removed, and the number of
microbes in the test mixture is determined. After expiry of this time, the
number of microbes has fallen from 10'to 103.
Example 9b:
0.05 g of the product from example 9 is shaken in 20 ml of a test microbial
suspension of Pseudomonas aeruginosa. After a contact time of 60
minutes, 1 ml of the test microbial suspension is removed, and the number
of microbes in the test mixture is determined. After expiry of this time, the
number of microbes has fallen from 10' to 103.
Example 10:
14 g of dimethylaminopropylmethacrylamide (Aldrich), 10 g of ethyl
3 o methacrylate (Aldrich) and 120 ml of ethanol are charged to a three
necked flask and heated to 65°C under a stream of argon. 0.3 g of
azobisisobutyronitrile dissolved in 8 ml of ethyl methyl ketone is then slowly
added dropwise, with stirring. The mixture is heated to 70°C and
stirred at
this temperature for 72 h. After expiry of this time, the reaction mixture is
3 5 stirred into 0.6 I of cyclohexane, whereupon the polymeric product
precipitates. After isolation of the product by filtration, the filter residue
is
washed with 100 ml of n-hexane in order to remove any residual
monomers still present. The product is then dried in vacuo at 50°C for
24
CA 02384427 2002-03-08
O.Z. 5493-WO - 19 -
hours.
Example 10a:
0.05 g of the product from example 10 is shaken in 20 ml of a test
microbial suspension of Staphylococcus aureus. After a contact time of 15
minutes, 1 ml of the test microbial suspension is removed, and the number
of microbes in the test mixture is determined. After expiry of this time, the
number of microbes has fallen from 10' to 103.
Example 10b:
0.05 g of the product from example 10 is shaken in 20 ml of a test
microbial suspension of Pseudomonas aeruginosa. After a contact time of
60 minutes, 1 ml of the test microbial suspension is removed, and the
number of microbes in the test mixture is determined. After expiry of this
time, the number of microbes has fallen from 10' to 104.