Note: Descriptions are shown in the official language in which they were submitted.
CA 02385346 2001-12-20
WO 00/78849 PCT/CA00/00741
HETEROGENEOUS ION EXCHANGE MEMBRANE
AND METHOD OF MANUFACTURING THEREOF
Field of Invention
The present invention relates to novel heterogeneous ion exchange membranes,
methods
for producing such membranes, and apparatus employing such membranes.
Background of the Invention
Membranes that selectively allow diffusion and adsorption of ions while
excluding
certain other ions and non-ionized solutes and solvents, typically referred to
as ion exchange
membranes, have numerous important industrial applications. Such membranes are
used in
electrodialysis and electrodeionization equipment as well as in devices for
fractionation, transport
depletion and electro-regeneration, and purification or treatment of water,
food, beverages,
chemicals and waste streams. The membranes are also used in electrochemical
devices such as
caustic/chlorine electrolysis equipment, electropaint purification equipment,
and electro-organic
synthesis equipment. Additionally, ion exchange membranes are used in
electrophoresis devices
and analytical equipment as adsorbents, and as suppressor devices for ion
chromatography. They
are used in chemical treatment and concentration applications via the
processes of Donnan
dialysis and diffusion dialysis, and they are also used in batteries and fuel
cells for the production
of electricity.
In each of the applications described above, numerous membrane properties must
be
balanced against one another in order to achieve a membrane that satisfies the
desired objectives
of the particular application. Among these, it is an objective to employ ion
exchange membranes
that have high selectivity, low solvent and non-ionized solute transfer, low
diffusion resistance
of the ions selected, high physical strength, and good chemical resistance.
Additionally, it is
desirable that such membranes be easily manufactured at low cost without the
use of hazardous
substances. Furthermore, ideal membranes should be easy to handle and process
and should also
be amenable to low cost assembly techniques during the production of devices
containing such
membranes.
Current commercially available ion exchange membranes are primarily of two
general
types: homogeneous membranes and heterogeneous membranes. A homogeneous
membrane is
one in which the entire volume of the membrane (excluding any support material
that may be
CA 02385346 2001-12-20
WO 00/78849 PCT/CA00/00741
used to improve strength) is made from the reactive polymer. Examples include
membranes
made of sulfonated or aminated styrene-divinylbenzene polymers (SDVB
membranes),
polymerized perfluorosulfonic acids (PFSO membranes) or various thermoplastics
with active
groups grafted onto the base polymer.
Unfortunately, homogeneous membranes tend to be difficult to manufacture. They
also
tend to employ the use of hazardous materials during their manufacturing
process since, for the
most part, they must be made from base monomers. Additionally, they are
difficult to modify
chemically because each modification requires a change in the fundamental
chemistry of the
membrane.
Homogeneous membranes also tend to have limited physical strength (therefore
often
requiring a screen or cloth support) because the polymer produced cannot
readily combine both
the required physical and electrochemical properties to operate efficiently in
a fabricated device.
Homogeneous membranes may be either crosslinked (to provide the membrane with
dimensional
stability, but increased brittleness and sensitivity upon drying), or they may
be non-crosslinked
(to provide membranes which may be dried, but lack dimensional stability and
resistance to
swelling and various solvents).
In contrast, heterogeneous membranes are formed of 1) a composite containing
an ion
exchange resin to impart electrochemical properties and 2) a binder to impart
physical strength
and integrity. Heterogeneous membrane may also include inert support material
to impart extra
strength and stability. Typical heterogeneous membranes may be produced as
"micro-heterogeneous" membranes by the paste method (in which ion exchange
resin monomers
are reacted to form the ultimate ion exchange resin polymer in the presence of
a finely-ground
inert binder polymer), or in the alternative, as "macro-heterogeneous"
membranes by the physical
blending of pre-polymerized ion exchange resin and binder.
Present macro-heterogeneous membranes tend to have inferior electrochemical
properties
as compared to micro-heterogeneous membranes, but they do offer a number of
advantages as
compared to membranes of the micro-heterogeneous variety. In particular, macro-
heterogeneous
membranes are easy to manufacture and can be readily chemically modified
since, within limits,
the binder and resin types and content can be varied without significantly
modifying the
manufacturing process. Notably, with micro-heterogeneous membranes, the binder
must be
compatible with the pre-cursor ion exchange monomers such that the binder does
not interfere
-2-
CA 02385346 2001-12-20
WO 00/78849 PCT/CA00/00741
with the polymerization of the ion exchange monomer or, as a consequence of
such
polymerization, becomes chemically altered with undesirable properties.
In some plate-and-frame type unit operations, ion exchange membranes are
typically
interposed between adj acent frame members to assist in defining individual
chambers or
compartments. For example, in filter-press type electrodeionization units, ion
exchange
membranes are interposed between adjacent frame members or spacers to form
separate diluting
and concentrating chambers. In assembling such units, a plurality of frame
members are
provided in a parallel manner with ion exchange membranes interposed between
the frame
members. The resulting structure is then forced together by clamping means
with a view to
providing a closed, tightly sealed unit. Tears and pinholes also contribute to
poor deionization
performance.
Unfortunately, present ion exchange membrane materials do not possess entirely
adequate
sealing characteristics. During prolonged operation of the afore-mentioned
unit operations, ion
exchange membrane materials have a tendency to creep, thereby receding from
contact with
adjacent frame members and potentially compromising positive sealing of the
unit. Present ion
exchange membranes also tend to be brittle and prone to tearing or pinhole
formation, thereby
further potentially compromising the sealing of the unit.
In addition, present ion exchange materials are not particularly suitable for
high
temperature applications. As a result, unit operations having ion exchange
membranes are
unlikely candidates for pharmaceutical applications, where the constituent
membranes would be
exposed to high temperatures during cleaning for purposes of disinfection.
With respect to membrane manufacturing, the prior methods used to make
heterogeneous
ion exchange membranes typically involved standard equipment for sheet
extrusion. This
equipment is very common. However, extruding filled materials like
heterogeneous ion
exchange membranes involves special difficulties. Gauge control, gear pump
pressure limits and
uniformity of dispersion of the phases axe all special difficulties
encountered when extruding the
materials in question. Yield rates as low as 30% are common.
Summary of Invention
A heterogeneous ion exchange material is provided comprising an ion exchange
resin
incorporated within a binder, the binder comprising a material selected from
the group consisting
-3-
CA 02385346 2001-12-20
WO 00/78849 PCT/CA00/00741
of (i) a Metallocene catalyzed linear low density polyethylene, (ii) a very
low density
polyethylene or ultra low density polyethylene processed using either Ziegler-
Natta catalysts or
Metallocene catalysts, (iii) a thermoplastic elastomeric olefin comprising a
polypropylene
continuous phase with an ethylene-propylene-dime monomer or ethylene-propylene
rubber
rubbery phase dispersed through the polypropylene continuous phase, and (iv) a
thermoplastic
vulcanizate comprising a polypropylene continuous phase with an ethylene-
propylene-dime
monomer, ethylene-propylene rubber, nitrile-butadiene rubber, natural rubber,
ethylene vinyl
acetate rubbery phase dispersed through the polypropylene continuous phase, a
co-polymer of
vinylidene fluoride and hexafluoropropylene, or a co-polymer of vinylidene
fluoride and
hexafluoropropylene and tetrafluoroethylene.
In one aspect, the binder is a Metallocene catalyzed linear low density
polyethylene.
In another aspect, the binder is a very low density polyethylene or ultra low
density
polyethylene processed using either Ziegler-Natta catalysts or Metallocene
catalysts.
In a further aspect, the binder is a thermoplastic elastomeric olefin
comprising a
1 S polypropylene continuous phase with an ethylene-propylene-dime monomer or
ethylene
propylene rubber rubbery phase dispersed through the polypropylene continuous
phase.
In yet a further aspect, the binder is a thermoplastic vulcanizate comprising
a
polypropylene continuous phase with an ethylene-propylene-dime monomer,
ethylene-propylene
rubber, nitrite-butadiene rubber, natural rubber or ethylene vinyl acetate
rubbery phase dispersed
through the polypropylene continuous phase.
A method for manufacturing an ion exchange membrane is also provided using
advanced
extrusion techniques, including computer-controlled material feed, computer-
controlled
automatic die thickness adjustment with independently adjustable lip segments
and nuclear gauge
detection with feed-back control. In one aspect, the method comprises the
steps of: (i) extruding
polymeric material through an auto-die, having a first lip block with a
plurality of segments and
a second lip block, at least one of the first lip block segments spaced from
said second lip block,
the at least one of the first lip block segments disposed at a first position,
(ii) measuring a first
thickness of the extruded polymeric material with a sensor, (iii) providing an
input signal
corresponding to the first thickness to a central processing unit (CPU),
processing the input
signal in said CPU by comparing said input signal to a setpoint corresponding
to a desired
thickness, (iv) providing an output signal, and (v) moving the at least one
first lip block segment
-4-
CA 02385346 2001-12-20
WO 00/78849 PCT/CA00/00741
to a second position in response to said output signal to change the spacing
between the at least
one first lip block segment and the second lip block.
A method for manufacturing an ion exchange membrane is also provided by
injection
molding.
Brief Description of Drawings
The present invention will be better understood with reference to the appended
drawings
in which:
Figure 1 is an illustration of an auto-die;
Figure 2 is a schematic of a method of manufacturing an ion exchange membrane.
Detailed Description of the Invention
The composite membrane of the present invention may be employed in various
applications, including but not limited to, polarity-based chemical
separations, such as
electrodeionization and electrodialysis, electrolysis, fuel cells and
batteries, pervaporation, gas
separation, dialysis separation and industrial electrochemistry, such as
chloralkali production and
other electrochemical applications.
Heterogeneous ion exchange membranes are provided comprising typical ground
ion
exchange resin such as Rohm and Haas AMBERLITET"~ IR120 and AMBERLITET"~ IRA
402
bound by a polymeric binder selected from: (i) a Metallocene-catalyzed linear
low density
polyethylene (M-LLDDE), (ii) a very low density polyethylene (VLDPE) or ultra
low density
polyethylene (ULDPE) processed using either Ziegler-Natta catalysts or
Metallocene catalysts,
(iii) a thermoplastic elastomeric olefin comprising a polypropylene continuous
phase with an
ethylene-propylene-dime monomer (EPDM) or ethylene-propylene rubber (EPR)
rubbery phase
dispersed through the polypropylene continuous phase, and (iv) a thermoplastic
vulcanizate
comprising a polypropylene continuous phase with an EPDM, EBR, nitrite-
butadiene rubber
(NBR), natural rubber (NR), ethylene vinyl acetate (EVA) rubbery phase
dispersed through the
polypropylene continuous phase, a co-polymer of vinylidene fluoride and
hexafluoropropylene,
or a co-polymer of vinylidene fluoride and hexafluoropropylene and
tetrafluoroethylene. The
M-LLDPE can be an ethylene alpha olefin copolymerized using Metallocene
catalysts or
-5-
' ~ 8 882 788 ...
, , ~___.....
ICA 02385346 2001-12-20
r
constrained geometry catalysts such as YNST1'E'~'u. The thermoplastic
vulcanizate can be AES
r
SANTOPRBNEz'M or DSM S.ARLIN1~TM, ox AES TREFSIrT~. '
in one embodiment, the thcrmop fastic based elastvmer is sn alloy comprising M-
LLDPE
and any of polypropylene (PP), low density polygthylene (LDPE), high density
polyethylene
(1-1DPE), EDPM (cross-linked, partially icmss-linked, or non-cross-linked),
EPR (cross-linked,
partially cross-linked, or non-cross-linked), EVA or other synthetic robbers
such as a co-polymer
of vinylidene fluoride and hexafluoropropyle'ne or a co polynner of vinylidene
fluoride and
! .
htxafluvropmpylcnc and tetrafluvrvethylenc. .
In another embodiment, the thermoplastic based elastomer is an alloy of VLDPE
or . .. _.
' !
ULDPE and any ofPP, LDPE, I;IDPE, M LLDPE, EPDM, (cross-linked, partially
cross-naked,
or non~ross-linked), EPR (cross-linked, partially cross-linked, or non-cross-
Iink~) or EVA. '
In another embodiment, the thermoplastic based elastomer is an alloy of (r) a
~ .
thcrmopiastic elastomeric olefin comprising a polypropylene continuous phase
with an EPDM
r
or EPR rubbery phase dispersed through the polypropylene continuous phase, and
('u) any of
LDPE, I~PE, M-LLDPE, or linear low(density polyethylene (LLDPE)_ .
In another embodiment, the . thermoplastic based elagtor~aer is an alloy of
(r) a '
thermoplastic vulcanizate comprising a poiypmpylene continuous phase with an
EPDM EPR,
NBlt, NR or EVA robbery phase dispersed through the polypropylene continuous
phase, and ('u)
anyof LDPE,.HDPE, M-LLDPE, or lin ~ar low density polyethyleuo (LLDPE). '
The heterogen~us ion exchange membrane ofthe present invention can be
manufacttued
with advanced extrusion technology including computer controlled material
~eed, computer
cornrolled automatic die thiclmcss adjustment with independently adjustable
lip segments. and
nuclear gauge detection with feed back control. Alternatively, the
heterogeneous ion exchange
. . E
membrane of the present invention can be mauerfactured using iqjection
molding.
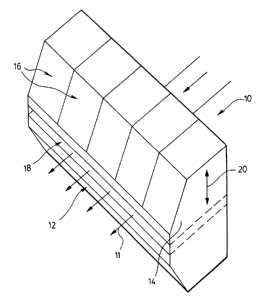
' Referring to Figures l arid 2, ~in done embodiment, the ion exchange
membrane of the
present invention is manufsctuted by advanced sheet extrusion technology to
manufacture
. . exchange membranes. The inventive process involves the use of 'very
accurate nuclear gauge
measuring instruments feeding back to a;control computer that automatically
adjusts an "auta
dic" 10 (see Figure 1). This auto-die has a first lip block 12 and a second
lip block 14. The
second lip block 14 is split into many individually adjustable segments or
zones I6 for precise
-6-
AMENDED ~SHE~T
C..n~...,s.....~i ~9 A.~s ~0~~,A
CA 02385346 2001-12-20
WO 00/78849 PCT/CA00/00741
gauge control. Other extruder parameters and gear pump parameters can also be
automatically
adjusted.
Membrane ingredients, including the polymeric binder and the ion exchange
resin, are
fed by an extruder 8 into the auto-die 10 through gate slot 18 in the
direction indicated by arrow
11. After exiting the autodie 10, the extruded material is fed through
calendaring rolls 26a, 26b
for flattening and solidifying the extruded sheet and smoothing its surface.
Thickness of the
extruded and calendared material is measured by a nuclear gauge sensor 24. At
this time, the
first lip block 12 is at a first position. The sensor provides an electrical
input signal
corresponding to the thickness of the extruded and calendared material to a
central processing
unit (CPU) 22. The CPU 22 compares the input signal with a setpoint
corresponding to a desired
thickness of the extruded and calendared material. The CPU 22 then provides an
output signal
to one or more of the zones 16 of the second lip block 14 of the auto-die 10.
In response to this
output signal, the zones 16 are actuated and move relative to the first lip
block 12 from a first
position to a second position in the direction indicated by arrows 20, thereby
adjusting the
spacing between the zone or zones 16 and the first lip block 12 and achieving
the desired spacing
A second embodiment of the invention involves the injection molding of ion
exchange
membranes. This reduces the production cost and further ensures dimensional
consistency and
adequate phase dispersion. Injection molding eases the processing of
beneficial binder materials
that may not be ideally suited to extrusion with a filler material such as ion
exchange resin
particles.
It will be understood, of course, that modification can be made in the
embodiments of the
invention described herein without departing from the scope and purview of the
invention as
defined by the appended claims.