Note: Descriptions are shown in the official language in which they were submitted.
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
COMPOSITIONS AND THERAPEUTIC METHODS USING
MORPHOGENIC PROTEINS HORMONES AND HORMONE RECEPTORS
BACKGROUND OF THE INVENTION
The Transforming Growth Factor-Beta ("TGF-13") superfanuly represents a
large number of evolutionarily conserved morphogenic proteins with diverse
activities in
growth, differentiation, tissue morphogenesis and repair. This superfamily
includes
osteogenic proteins ("OPs") and bone morphogenic proteins ("BMPs"). OPs and
BMPs
share a highly conserved, bioactive cysteine-rich domain near their C-termini
and have a
propensity to form homo- and hetero-dimers.
Many morphogenic proteins belonging to the BMP family have been
described. Some were isolated using purification techniques on the basis of
osteogenic
activity. Others were identified and cloned by virtue of DNA sequence
homologies within
conserved regions that are common to the BMP family. These homologs are
referred to as
consecutively numbered BMPs whether or not they have demonstrable osteogenic
activity.
While several of the earliest members of the BMP family were identified by
virtue of their
ability to induce new cartilage and bone, a number of other BMPs have
different or
additional tissue-inductive capabilities. For example, BMP-12 and BMP-13
(identified by
DNA sequence homology) reportedly induce tendon/ligament-like tissue formation
in vivo
(WO 95/16035). Several BMPs, including some of those originally isolated on
the basis of
their osteogenic activity, can induce neuron proliferation and promote axon
regeneration
(WO 95/05846; Liem et al., Cell, 82, pp. 969-79 (1995)). Thus, it appears that
BMPs may
have a variety of potential tissue-inductive capabilities whose final
expression depends on a
complex set of developmental and environmental cues.
Many of the mammalian BMPs have been recombinantly expressed as active
homo- or heterodimers in a variety of host systems, making therapeutic
treatments using
morphogenic proteins feasible. Implantable osteogenic devices comprising
mammalian
osteogenic protein for promoting bone healing and regeneration have been
described (see,
e.g., Oppermann et al., U. S. Patent No. 5,354,557). Some osteogenic devices
contain
porous, biocompatible matrices that allow the diffusion of osteogenic proteins
into the
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
implantation site as well as the influx and efflux of progenitor cells.
Osteogenic protein-
coated prosthetic devices that enhance the bond strength between the
prosthesis and
existing bone have also been described (Rueger et al., U. S. Patent No.
5,344,654).
SUMMARY OF THE INVENTION
This invention is based on the discovery that the tissue-inductive activity of
a morphogenic protein can be enhanced by a hormone in the presence of a
soluble receptor
of the hormone.
Accordingly, this invention features a method for improving the tissue
inductive capability of a morphogenic protein at a target locus in a mammal.
In this
method, the morphogenic protein and a first effective amount of a hormone and
a second
effective amount of a soluble receptor of the hormone are administered to the
target locus,
wherein the morphogenic protein is capable of inducing tissue formation when
accessible to
a progenitor cell in the mammal, and the hormone and the receptor in
combination enhance
that capability. The morphogenic protein, hormone and hormone receptor can be
administered simultaneously to the target locus. Alternatively, the three
components are
administered separately, in any order: for instance, the morphogenic protein
can be
administered first, and then the hormone and hormone receptor are administered
together;
or the morphogenic protein and the hormone are administered together first,
and then the
hormone receptor is administered. In one embodiment, the morphogenic protein
is
administered via a nucleic acid (e.g., a plasmid, a viral vector, or naked
DNA) that
comprises a sequence encoding the morphogenic protein and is capable of
expressing the
morphogenic protein in the appropriate progenitor cells of a patient.
The morphogenic protein may comprise a pair of subunits disulfide-bonded
to produce a dimeric species, wherein at least one of the subunits comprises a
polypeptide
belonging to the BMP protein family. For instance, the morphogenic protein may
comprise
an amino acid sequence sufficiently duplicative of the amino acid sequence of
a reference
BMP such as BMP-2, BMP-3, BMP-4, BMP-5, BMP-6, BMP-7 (OP-1), BMP-8, BMP-9,
BMP-10, BMP-11, BMP-12, BMP-13, COP-5, or COP-7, such that it has morphogenic
activity similar to that of the reference BMP. In one preferred embodiment,
the
morphogenic protein is a homo- or heterodimer comprising a BMP-2 or BMP-7 (OP-
1)
subunit.
-2-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
The morphogenic protein is capable of inducing tissue formation. For
instance, it may be capable of inducing the progenitor cell to form tissue
tendon/ligament-
like or neural-like tissue; or it may be an osteogenic protein that is capable
of inducing the
progenitor cell to form endochondral or intramembranous bone, or cartilage.
The method
of this invention thus can be used to induce tissue regeneration or repair in
a variety of
tissue defects such as bone, cartilage, soft tissue and neural tissue defects.
Hormones useful in this invention include but are not limited to cytokines
(e.g., interleukins 1 through 18), growth factors (e.g., fibroblast growth
factor, vascular
endothelial growth factor, platelet-derived growth factor, TGF-(3, or
prostaglandin) or
morphogenic proteins.
The invention also features pharmaceutical compositions and kits
comprising a hormone and a soluble receptor thereof for improving the tissue
inductive
activity of a morphogenic protein. This invention also provides implantable
morphogenic
devices for inducing tissue formation in allogeneic and xenogeneic implants.
Such devices
comprise a morphogenic protein, a hormone and a soluble receptor thereof
disposed within
a carrier. Methods for inducing local tissue formation from a progenitor cell
in a mammal
using those compositions and devices are also provided. A method for
accelerating
allograft repair in a mammal using those morphogenic devices is provided. This
invention
also provides a prosthetic device comprising a prosthesis coated with a
morphogenic
protein, a hormone and a soluble receptor thereof, and a method for promoting
in vivo
integration of an implantable prosthetic device to enhance the bond strength
between the
prosthesis and the existing target tissue at the joining site. Methods for
treating tissue
degenerative conditions in a mammal using the pharmaceutical compositions are
also
provided.
Unless otherwise defined, all technical and scientific terms used herein have
the same meaning as commonly understood by one of ordinary skill in the art to
which this
invention belongs. Exemplary methods and materials are described below,
although
methods and materials similar or equivalent to those described herein can also
be used in
the practice or testing of the present invention. All publications and other
references
mentioned herein are incorporated by reference in their entirety. In case of
conflict, the
present specification, including definitions, will control. The materials,
methods, and
examples are illustrative only and not intended to be limiting.
-,
_,_
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
Other features and advantages of the invention will be apparent from the
following drawings, detailed description, and the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
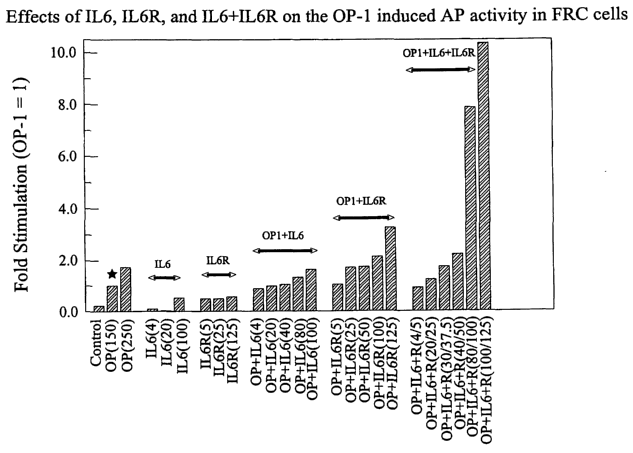
Fig. 1 is a bar graph showing that a combination of interleukin 6 ("IL-6")
and soluble IL-6 receptor ("sIL-6R") significantly increases the ability of OP-
1 to induce
alkaline phosphatase ("AP") activity in fetal rat calvaria ("FRC") cells. "OP"
stands for
"OP-1"; "IL-6R'' refers to "sIL-6R." The parenthesized numbers indicate the
protein
concentrations (ng/ml) used in the assay; in the case of IL-6/sIL-6R
combinations, the two
numbers separated by a backslash in the parenthesis indicate the protein
concentrations of
IL-6 and sIL-6R, respectively.
Fig. 2 is a photograph showing results of a mineralized bone nodule
formation assay using OP-1 and IL-6. Dark spots inside the wells represent
mineralized
bone nodules.
Fig. 3 is a photograph showing results of a mineralized bone nodule
formation assay using OP-l, IL-6 and sIL-6R. Dark spots inside the wells
represent
mineralized bone nodules.
Fig. 4 is a bar graph showing the mRIVA levels of BMPR-IA, BMPR-IB,
ActR-I, and BMPR-II in various test groups. "sR" stands for sIL-6R. Values in
the graph
represent the means~SE of twelve Northern blots with RNA isolated from two
different
FRC cell preparations.
Fig. 5 is a bar graph showing that the AP activity in FRC cells transfected
with the OP-1-encoding pW24 plasmid is enhanced by exogenous sIL-6R alone or a
combination of IL-6 and sIL-6R ("IL-6!R"). "IL6R" stands for sIL-6R. Values in
the
graph represent the mean~SE of five independent determinations with three
different FRC
cell preparations and two different DNA preparations. "IL-6/R (X/Y)" refers to
X ng/ml
IL-6 and Y ng/ml sIL-6R.
DETAILED DESCRIPTION OF THE INVENTION
In order that the invention herein described may be fully understood, the
following detailed description is set forth.
-4-
CA 02385887 2002-03-18
WO 01/23563 PCT/CTS00/26528
The term "biocompatible" refers to a material that does not elicit detrimental
effects associated with the body's various protective systems, such as cell
and humoral-
associated immune responses, e.g., inflammatory responses and foreign body
fibrotic
responses. This term also implies that no specific undesirable effects,
cytotoxic or
systemic, are caused by the material when it is implanted into the patient.
The term "BMP" refers to a protein belonging to the BMP family of the
TGF-f3 superfamily of proteins defined on the basis of DNA and amino acid
sequence
homology. According to this invention, a protein belongs to the BMP family
when it has at
least 50% (e.g., at least 70% or even 85%) amino acid sequence homology with a
known
BMP family member within the conserved C-terminal cysteine-rich domain that
characterizes the BMP family. Members of the BMP family may have less than 50%
DNA
or amino acid sequence homology overall.
The term "morphogenic protein" refers to a protein having morphogenic
activity. For instance, this protein is capable of inducing progenitor cells
to proliferate
and/or to initiate differentiation pathways that lead to the formation of
cartilage, bone,
tendon, ligament, neural or other types of tissue, depending on local
environmental cues.
Thus, morphogenic proteins useful in this invention may behave differently in
different
surroundings. A morphogenic protein of this invention may comprise at least
one
polypeptide belonging to the BMP family.
The term "osteogenic protein" refers to a morphogenic protein that is
capable of inducing a progenitor cell to form cartilage and/or bone. The bone
may be
intramembranous bone or endochondral bone. Most osteogenic proteins are
members of
the BMP family and are thus also BMPs. However, the converse may not be true.
According to this invention, a BMP identified by sequence homology must have
demonstrable osteogenic or chondrogenic activity in a functional bioassay to
be an
osteogenic protein.
The terms "morphogenic activity," "inducing activity" and "tissue inductive
activity" alternatively refer to the ability of an agent to stimulate a target
cell to undergo
one or more cell divisions (proliferation) that may optionally lead to cell
differentiation.
Such target cells are referred to generically herein as progenitor cells. Cell
proliferation is
typically characterized by changes in cell cycle regulation and may be
detected by a number
of means which include measuring DNA synthetic or cellular growth rates. Early
stages of
-5-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
cell differentiation are typically characterized by changes in gene expression
patterns
relative to those of the progenitor cell; such changes may be indicative of a
commitment
towards a particular cell fate or cell type. Later stages of cell
differentiation may be
characterized by changes in gene expression patterns, cell physiology and
morphology.
Any reproducible change in gene expression, cell physiology or morphology may
be used to
assess the initiation and extent of cell differentiation induced by a
morphogenic protein.
The term "synergistic interaction" refers to an interaction in which the
combined effect of two or more agents is greater than the algebraic sum of
their individual
effects.
The term "hormone/receptor pair" refers to a combination of a hormone and
a soluble receptor thereof. The hormone (e.g., a cytokine, a growth factor, or
a
morphogenic protein) can be of any mammalian origin (e.g., human, bovine, or
murine). A
soluble receptor of a hormone is a compound that binds specifically to the
hormone, and
can, for example, be a polypeptide containing only the hormone-binding domain
(e.g., an
extracellular domain) of a native cellular receptor of the hormone, an
antibody specific for
the hormone, or a chemical compound that specifically interacts with the
hormone. A
soluble receptor can also be a compound (e.g., a protein) containing a domain
that
specifically binds to the hormone and another domain that specifically binds
to the native
cellular receptor of the hormone such that the soluble receptor facilitates
the binding of the
hormone to its native cellular receptor; an example of such a soluble receptor
is the IGF-
binding protein.
MorpJ e~ nic proteins
The morphogenic proteins of this invention are capable of stimulating a
progenitor cell to undergo cell division and/or differentiation. They may
belong to the
TGF-13 protein superfamily, and include, but are not limited to, OP-l, OP-2,
OP-3, BMP-2,
BMP-3, BMP-3b, BMP-4, BMP-5, BMP-6, BMP-9, BMP-10, BMP-11, BMP-12,
BMP-13, BMP-14, BMP-15, GDF-1, GDF-2, GDF-3, GDF-5, GDF-6, GDF-7, GDRB,
GDF-9, GDF-10, GDF-11, GDF-12, DPP, Vg-l, Vgr-1, 60A protein, NODAL, UNIVIN,
SCREW, ADMP, NEURAL, and TGF-(3.
One of the preferred morphogenic proteins is OP-1. Nucleotide and amino
acid sequences for hOP-1 are provided in SEQ ID NOs: l and 2, respectively.
For ease of
description, hOP-1 is recited as a representative morphogenic protein. It will
be
-6-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
appreciated by the ordinarily skilled artisan that OP-1 is merely
representative of a family of
morphogens.
Other useful morphogenic proteins also include polypeptides having at least
50% (e.g., at least 70% or even 85%) sequence homology with a known
morphogenic
protein, particularly with a known BMP within the conserved C-terminal
cysteine-rich
domain that characterizes the BMP protein family. These morphogenic proteins
include
biologically active variants of any known morphogenic protein, including
variants
containing conservative amino acid changes. For instance, useful morphogenic
proteins
include those containing sequences that share at least 70% amino acid sequence
homology
with the C-terminal seven-cysteine domain of hOP-1, which domain corresponds
to the C-
terminal 102-106 amino acid residues of SEQ ID N0:2. The C-terminal 102 amino
acid
residues corresponds to residues 330-431 of SEQ ID N0:2. In one embodiment of
this
invention, the morphogenic protein consists of a pair of subunits disulfide-
bonded to
produce a dimer, wherein at least one of the subunits comprises a recombinant
polypeptide
belonging to the BMP family.
As used herein, "amino acid sequence homology" is understood to include
both amino acid sequence identity and similarity. Homologous sequences share
identical
and/or similar amino acid residues, where similar residues are conservative
substitutions
for, or "allowed point mutations" of, corresponding amino acid residues in an
aligned
reference sequence. Thus, a candidate polypeptide sequence that shares 70%
amino acid
homology with a reference sequence is one in which any 70% of the aligned
residues are
either identical to, or are conservative substitutions of, the corresponding
residues in a
reference sequence. Certain particularly preferred morphogenic polypeptides
share at least
60% (e.g., at least 65%) amino acid sequence identity with the C-terminal
seven-cysteine
domain of human OP-1.
As used herein, "conservative substitutions" are residues that are physically
or functionally similar to the corresponding reference residues. That is, a
conservative
substitution and its reference residue have similar size, shape, electric
charge, chemical
properties including the ability to form covalent or hydrogen bonds, or the
like. Preferred
conservative substitutions are those fulfilling the criteria defined for an
accepted point
mutation in Dayhoff et al., Atlas of Protein Seqz~ence arad StYUCtzcne 5:345-
352 ( 1978 &
Supp.). Examples of conservative substitutions are substitutions within the
following
-7_
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
groups: (a) valine, glycine; (b) glycine, alanine; (c) valine, isoleucine,
leucine; (d) aspartic
acid, glutamic acid; (e) asparagine, glutamine; (f) serine, threonine; (g)
lysine, arginine,
methionine; and (h) phenylalanine, tyrosine. The term "conservative variant"
or
"conservative variation" also includes the use of a substituting amino acid
residue in place
of an amino acid residue in a given parent amino acid sequence, where
antibodies specific
for the parent sequence are also specific for, i.e., "cross-react'' or "immuno-
react" with, the
resulting substituted polypeptide sequence.
Amino acid sequence homology can be determined by methods well known
in the art. For instance, to determine the percent homology of a candidate
amino acid
sequence to the sequence of the seven-cysteine domain, the two sequences are
first aligned.
The alignment can be made with, e.gT., the dynamic programming algorithm
described in
Needleman et al., J. Mol. Biol. -X8:443 (1970), and the Align Program, a
commercial
software package produced by DNAstar, Inc. The teachings by both sources are
incorporated by reference herein. An initial alignment can be refined by
comparison to a
mufti-sequence alignment of a family of related proteins. Once the alignment
is made and
refined, a percent homology score is calculated. The aligned amino acid
residues of the
two sequences are compared sequentially for their similarity to each other.
Similarity
factors include similar size, shape and electrical charge. One particularly
preferred method
of determining amino acid similarities is the PAM250 matrix described in
Dayhoff et al.,
supra. A similarity score is first calculated as the sum of the aligned
pairwise amino acid
similarity scores. Insertions and deletions are ignored for the purposes of
percent
homology and identity. Accordingly, gap penalties are not used in this
calculation. The
raw score is then normalized by dividing it by the geometric mean of the
scores of the
candidate sequence and the seven-cysteine domain. The geometric mean is the
square root
of the product of these scores. The normalized raw score is the percent
homology.
Morphogenic proteins useful herein include any known naturally occurring
native proteins, including allelic, phylogenetic counterparts and other
variants thereof.
These variants include forms having varying glycosylation patterns, varying N-
termini, and
active truncated or mutated forms of a native protein. Useful morphogenic
proteins also
include those that are biosynthetically produced (e.g., "muteins" or "mutant
proteins") and
those that are new, morphogenically active members of the general morphogenic
family of
proteins. Particularly useful sequences include those comprising the C-
terminal 96 to 102
_g_
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
amino acid residues o~ DPP (from DYOSOphila), Vg-1 (fromXenopus), Vgr-1 (from
mouse), the OP1 and OP2 proteins (U.S. Patent No. 5,011,691), as well as the
proteins
referred to as BMP-2, BMP-3, BMP-4 (WO 88/00205, U.S. Patent No. 5,013,649 and
WO
91/18098), BMP-5 and BMP-6 (WO 90/11366), BMP-8 and BMP-9. Other proteins
useful in the practice of the invention include active forms of OP1, OP2, OP3,
BMP-2,
BMP-3, BNLP-3b, BMP-4, BMP-5, BMP-6, BMP-9, BMP-10, BMP-11, BMP-12,
BMP-13, BMP-14, BMP-15, DPP, Vg-1, Vgr-1, 60A protein, GDF-l, GDF-2, GDF-3,
GDF-5, GDF-6, GDF-7, GDF-8, GDF-9, and GDF-10, GDF-11, GDF-12, GDF-13,
UNIVIN, NODAL, SCREW, ADMP, NEURAL, and TGF-(3.
Osteogenic proteins useful as morphogenic proteins of this invention include
those containing sequences that share greater than 60% identity with the seven-
cysteine
domain. In other embodiments, useful osteogenic proteins are defined as
osteogenically
active proteins having any one of the generic sequences defined herein,
including OPX
(SEQ ID N0:3) and Generic Sequences 7 (SEQ 117 N0:4), 8 (SEQ ID NO:S), 9 (SEQ
ID
N0:6) and 10 (SEQ ID N0:7).
Generic Sequence 7 (SEQ ID N0:4) and Generic Sequence 8 (SEQ ID
NO:S), disclosed below, accommodate the homologies shared among preferred
protein
family members identified to date, including OP-1, OP-2, OP-3, BMP-2, BMP-3,
BMP-4,
BN1P-5, BMP-6, 60A, DPP, Vg-1, Vgr-1, and GDF-1. The amino acid sequences for
these
proteins are described herein and/or in the art. The generic sequences include
the identical
amino acid residues shared by these sequences in the C-terminal six- or seven-
cysteine
skeletal domains (represented by Generic Sequences 7 and 8, respectively), as
well as
alternative residues for the variable positions within the sequences. The
generic sequences
provide an appropriate cysteine skeleton where inter- or intra-molecular
disulfide bonds can
form. Those sequences contain certain specified amino acids that may influence
the tertiary
structure of the folded proteins. In addition, the generic sequences allow for
an additional
cysteine at position 36 (Generic Sequence 7) or position 41 (Generic Sequence
8), thereby
encompassing the biologically active sequences of OP-2 and OP-3.
GENERIC SEQUENCE 7
3 0 Leu Xaa Xaa Xaa Phe Xaa Xaa Xaa Gly Trp Xaa Xaa Xaa Xaa Xaa Xaa
-9-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
1 5 10 15
Pro Xaa Xaa Xaa Xaa Ala Xaa Tyr Cys Xaa Gly Xaa Cys Xaa Xaa Pro Xaa Xaa
20 25 30
Xaa Xaa Xaa Xaa Xaa Xaa Asn His Ala Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
35 40 45 50
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Cys Cys Xaa Pro Xaa Xaa Xaa Xaa Xaa Xaa
55 60 65 70
Xaa Xaa Leu Xaa Xaa Xaa Xaa Xaa Xaa Xaa Val Xaa Leu Xaa Xaa Xaa Xaa Xaa
75 80 85
Met Xaa Val Xaa Xaa Cys Xaa Cys Xaa (SEQ ID N0:4)
90 95
wherein each Xaa is independently defined as follows ("Res." means "residue"):
Xaa at
res.2 = (Tyr or Lys); Xaa at res.3 = (Val or Ile); Xaa at res.4 = (Ser, Asp or
Glu); Xaa at
res.6 = (Arg, Gln, Ser, Lys or Ala); Xaa at res.7 = (Asp or Glu); Xaa at res.8
= (Leu, Val
or lle); Xaa at res. l l = (Gln, Leu, Asp, His, Asn or Ser); Xaa at res.l2 =
(Asp, Arg, Asn or
Glu); Xaa at res. 13 = (Trp or Ser); Xaa at res.14 = (Ile or Val); Xaa at
res.15 = (Ile or
Val); Xaa at res. l6 (Ala or Ser); Xaa at res.18 = (Glu, Gln, Leu, Lys, Pro or
Arg); Xaa at
res. l9 = (Gly or Ser); Xaa at res.20 = (Tyr or Phe); Xaa at res.21 = (Ala,
Ser, Asp, Met,
His, Gln, Leu or Gly); Xaa at res.23 = (Tyr, Asn or Phe); Xaa at res.26 =
(Glu, His, Tyr,
Asp, Gln, Ala or Ser); Xaa at res.28 = (Glu, Lys, Asp, Gln or Ala); Xaa at
res.30 = (Ala,
Ser, Pro, Gln, Ile or Asn); Xaa at res.31 = (Phe, Leu or Tyr); Xaa at res.33 =
(Leu, Val or
Met); Xaa at res.34 = (Asn, Asp, Ala, Thr or Pro); Xaa at res.35 = (Ser, Asp,
Glu, Leu,
Ala or Lys); Xaa at res.36 = (Tyr, Cys, His, Ser or Ile); Xaa at res.37 =
(Met, Phe, Gly or
Leu); Xaa at res.38 = (Asn, Ser or Lys); Xaa at res.39 = (Ala, Ser, Gly or
Pro); Xaa at
res.40 = (Thr, Leu or Ser); Xaa at res.44 = (Ile, Val or Thr); Xaa at res.45 =
(Val, Leu,
Met or Ile); Xaa at res.46 = (Gln or Arg); Xaa at res.47 = (Thr, Ala or Ser);
Xaa at res.48
_ (Leu or Ile); Xaa at res.49 = (Val or Met); Xaa at res.50 = (His, Asn or
Arg); Xaa at
res.51 = (Phe, Leu, Asn, Ser, Ala or Val); Xaa at res.52 = (Ile, Met, Asn,
Ala, Val, Gly or
Leu); Xaa at res.53 = (Asn, Lys, Ala, Glu, Gly or Phe); Xaa at res.54 = (Pro,
Ser or Val);
Xaa at res.55 = (Glu, Asp, Asn, Gly, Val, Pro or Lys); Xaa at res.56 = (Thr,
Ala, Val, Lys,
Asp, Tyr, Ser, Gly, lle or His); Xaa at res.57 = (Val, Ala or Ile); Xaa at
res.58 = (Pro or
Asp); Xaa at res.59 = (Lys, Leu or Glu); Xaa at res.60 = (Pro, Val or Ala);
Xaa at res.63 =
-10-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
(Ala or Val); Xaa at res.65 = (Thr, Ala or Glu); Xaa at res.66 = (Gln, Lys,
Arg or Glu);
Xaa at res.67 = (Leu, Met or Val); Xaa at res.68 = (Asn, Ser, Asp or Gly); Xaa
at res.69 =
(Ala, Pro or Ser); Xaa at res.70 = (Ile, Thr, Val or Leu); Xaa at res.71 =
(Ser, Ala or Pro);
Xaa at res.72 = (Val, Leu, Met or Ile); Xaa at res.74 = (Tyr or Phe); Xaa at
res.75 = (Phe,
Tyr, Leu or His); Xaa at res.76 = (Asp, Asn or Leu); Xaa at res.77 = (Asp,
Glu, Asn, Arg
or Ser); Xaa at res.78 = (Ser, Gln, Asn, Tyr or Asp); Xaa at res.79 = (Ser,
Asn, Asp, Glu
or Lys); Xaa at res.80 = (Asn, Thr or Lys); Xaa at res.82 = (Ile, Val or Asn);
Xaa at res.84
_ (Lys or Arg); Xaa at res.85 = (Lys, Asn, Gln, His, Arg or Val); Xaa at
res.86 = (Tyr, Glu
or His); Xaa at res.87 = (Arg, Gln, Glu or Pro); Xaa at res.88 = (Asn, Glu,
Trp or Asp);
Xaa at res.90 = (Val, Thr, Ala or Ile); Xaa at res.92 = (Arg, Lys, Val, Asp,
Gln or Glu);
Xaa at res.93 = (Ala, Gly, Glu or Ser); Xaa at res.95 = (Gly or Ala); and Xaa
at res.97 =
(His or Arg).
Generic Sequence 8 (SEQ ID NO:S) includes all of Generic Sequence 7 and
in addition includes the following five amino acid at its N-terminus: Cys Xaa
Xaa Xaa Xaa
(SEQ ID N0:8), wherein Xaa at res.2 = (Lys, Arg, Ala or Gln); Xaa at res.3 =
(Lys, Arg or
Met); Xaa at res.4 = (His, Arg or Gln); and Xaa at res.5 = (Glu, Ser, His,
Gly, Arg, Pro,
Thr, or Tyr). Accordingly, beginning with residue 7, each "Xaa" in Generic
Sequence 8 is
a specified amino acid as defined as for Generic Sequence 7, with the
distinction that each
residue number described for Generic Sequence 7 is shifted by five in Generic
Sequence 8.
For example, "Xaa at res.2 = (Tyr or Lys)" in Generic Sequence 7 corresponds
to Xaa at
res.7 in Generic Sequence 8.
Generic Sequences 9 (SEQ ID N0:6) and 10 (SEQ ID N0:7) are composite
amino acid sequences of the following proteins: human OP-1 ("hOP-1"), hOP-2,
hOP-3,
hBMP-2, hBMP-3, hBMP-4, hBMP-5, hBMP-6, hBMP-9, hBMPlO, hBMP-11,
Drosophila 60A, Xenopus Vg-1, sea urchin LTNIVIN, hCDMP-1 (mouse GDF-5 or
''mGDF-5"), hCDMP-2 (mGDF-6, hBMP-13), hCDMP-3 (mGDF-7, hBMP-12), mGDF-3,
hGDF-1, mGDF-1, chicken DORSALIN, DPP, Drosophila SCREW, mouse NODAL,
mGDF-8, hGDF-8, mGDF-9, mGDF-10, hGDF-11, mGDF-11, hBMP-15, and rat BMP3b.
Like Generic Sequence 7, Generic Sequence 9 accommodates the C-terminal six-
cysteine
skeleton and, like Generic Sequence 8, Generic Sequence 10 accommodates the C-
terminal
seven-cysteine skeleton.
-11-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
GENERIC SEQUENCE 9
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
1 5 10 15
Pro Xaa Xaa Xaa Xaa Xaa Xaa Xaa Cys Xaa Gly Xaa Cys Xaa Xaa Xaa Xaa
20 25 30
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
35 40 45 50
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Cys Xaa Pro Xaa Xaa Xaa
55 60 65
Xaa Xaa Xaa Xaa Xaa Leu Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
70 75 80
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Cys Xaa Cys Xaa (SEQ ID N0:6)
85 90 95
wherein each Xaa is independently defined as follows: Xaa at res. l = (Phe,
Leu or Glu);
Xaa at res.2 = (Tyr, Phe, His, Arg, Thr, Lys, Gln, Val or Glu); Xaa at res.3 =
(Val, Ile, Leu
or Asp); Xaa at res.4 = (Ser, Asp, Glu, Asn or Phe); Xaa at res.5 = (Phe or
Glu); Xaa at
res.6 = (Arg, Gln, I,ys, Ser, Glu, Ala or Asn); Xaa at res.7 = (Asp, Glu, Leu,
Ala or Gln);
Xaa at res.8 = (Leu, Val, Met, Ile or Phe); Xaa at res.9 = (Gly, His or Lys);
Xaa at res.10 =
(Trp or Met); Xaa at res. l l = (Gln, Leu, His, Glu, Asn, Asp, Ser or Gly);
Xaa at res.12 =
(Asp, Asn, Ser, Lys, Arg, Glu or His); Xaa at res.l3 = (Trp or Ser); Xaa at
res. l4 = (Ile or
Val); Xaa at res.15 = (Ile or Val); Xaa at res.16 = (Ala, Ser, Tyr or Trp);
Xaa at res.18 =
(Glu, Lys, Gln, Met, Pro, Leu, Arg, His or Lys); Xaa at res.19 = (Gly, Glu,
Asp, Lys, Ser,
Gln, Arg or Phe); Xaa at res.20 = (Tyr or Phe); Xaa at res.21 = (Ala, Ser,
Gly, Met, Gln,
His, Glu, Asp, Leu, Asn, Lys or Thr); Xaa at res.22 = ( Ala or Pro); Xaa at
res.23 = (Tyr,
Phe, Asn, Ala or Arg); Xaa at res.24 = (Tyr, His, Glu, Phe or Arg); Xaa at
res.26 = (Glu,
Asp, Ala, Ser, Tyr, His, Lys, Arg, Gln or Gly); Xaa at res.28 = (Glu, Asp,
Leu, Val, Lys,
Gly, Thr, Ala or Gln); Xaa at res.30 = (Ala, Ser, Ile, Asn, Pro, Glu, Asp,
Phe, Gln or Leu);
Xaa at res.31 = (Phe, Tyr, Leu, Asn, Gly or Arg); Xaa at res.32 = (Pro, Ser,
Ala or Val);
Xaa at res.33 = (Leu, Met, Glu, Phe or Val); Xaa at res.34 = (Asn, Asp, Thr,
Gly, Ala,
Arg, Leu or Pro); Xaa at res.35 = (Ser, Ala, Glu, Asp, Thr, Leu, Lys, Gln or
His); Xaa at
res.36 = (Tyr, His, Cys, Ile, Arg, Asp, Asn, Lys, Ser, Glu or Gly); Xaa at
res.37 = (Met,
Leu, Phe, Val, Gly or Tyr); Xaa at res.38 = (Asn, Glu, Thr, Pro, Lys, His,
Gly, Met, Val or
-12-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
Arg); Xaa at res.39 = (Ala, Ser, Gly, Pro or Phe); Xaa at res.40 = (Thr, Ser,
Leu, Pro, His
or Met); Xaa at res.41 = (Asn, Lys, Val, Thr or Gln); Xaa at res.42 = (His,
Tyr or Lys);
Xaa at res.43 = (Ala, Thr, Leu or Tyr); Xaa at res.44 = (Ile, Thr, Val, Phe,
Tyr, Met or
Pro); Xaa at res.45 = (Val, Leu, Met, Ile or His); Xaa at res.46 = (Gln, Arg
or Thr); Xaa at
S res.47 = (Thr, Ser, Ala, Asn or His); Xaa at res.48 = (Leu, Asn or Ile); Xaa
at res.49 =
(Val, Met, Leu, Pro or Ile); Xaa at res.50 = (His, Asn, Arg, Lys, Tyr or Gln);
Xaa at res.51
_ (Phe, Leu, Ser, Asn, Met, Ala, Arg, Glu, Gly or Gln); Xaa at res.52 = (Ile,
Met, Leu,
Val, Lys, Gln, Ala or Tyr); Xaa at res.53 = (Asn, Phe, Lys, Glu, Asp, Ala,
Gln, Gly, Leu or
Val); Xaa at res.54 = (Pro, Asn, Ser, Val or Asp); Xaa at res.55 = (Glu, Asp,
Asn, Lys,
Arg, Ser, Gly, Thr, Gln, Pro or His); Xaa at res.56 = (Thr, His, Tyr, Ala,
Ile, Lys, Asp, Ser,
Gly or Arg); Xaa at res.57 = (Val, Ile, Thr, Ala, Leu or Ser); Xaa at res.58 =
(Pro, Gly,
Ser, Asp or Ala); Xaa at res.59 = (Lys, Leu, Pro, Ala, Ser, Glu, Arg or Gly);
Xaa at res.60
_ (Pro, Ala, Val, Thr or Ser); Xaa at res.61 = (Cys, Val or Ser); Xaa at
res.63 = (Ala, Val
or Thr); Xaa at res.65 = (Thr, Ala, Glu, Val, Gly, Asp or Tyr); Xaa at res.66
= (Gln, Lys,
Glu, Arg or Val); Xaa at res.67 = (Leu, Met, Thr or Tyr); Xaa at res.68 =
(Asn, Ser, Gly,
Thr, Asp, Glu, Lys or Val); Xaa at res.69 = (Ala, Pro, Gly or Ser); Xaa at
res.70 = (Ile,
Thr, Leu or Val); Xaa at res.71 = (Ser, Pro, Ala, Thr, Asn or Gly); Xaa at
res.72 = (Val,
Ile, Leu or Met); Xaa at res.74 = (Tyr, Phe, Arg, Thr, Tyr or Met); Xaa at
res.75 = (Phe,
Tyr, His, Leu, Ile, Lys, Gln or Val); Xaa at res.76 = (Asp, Leu, Asn or Glu);
Xaa at res.77
= (Asp, Ser, Arg, Asn, Glu, Ala, Lys, Gly or Pro); Xaa at res.78 = (Ser, Asn,
Asp, Tyr,
Ala, Gly, Gln, Met, Glu, Asn or Lys); Xaa at res.79 = (Ser, Asn, Glu, Asp,
Val, Lys, Gly,
Gln or Arg); Xaa at res.80 = (Asn, Lys, Thr, Pro, Val, Ile, Arg, Ser or Gln);
Xaa at res.81
_ (Val, Ile, Thr or Ala); Xaa at res.82 = (Ile, Asn, Val, Leu, Tyr, Asp or
Ala); Xaa at res.83
_ (Leu, Tyr, Lys or Ile); Xaa at res.84 = (Lys, Arg, Asn, Tyr, Phe, Thr, Glu
or Gly); Xaa at
res.85 = (Lys, Arg, His, Gln, Asn, Glu or Val); Xaa at res.86 = (Tyr, His, Glu
or Ile); Xaa
at res.87 = (Arg, Glu, Gln, Pro or Lys); Xaa at res.88 = (Asn, Asp, Ala, Glu,
Gly or Lys);
Xaa at res.89 = (Met or Ala); Xaa at res.90 = (Val, Ile, Ala, Thr, Ser or
Lys); Xaa at res.91
_ (Val or Ala); Xaa at res.92 = (Arg, Lys, Gln, Asp, Glu, Val, Ala, Ser or
Thr); Xaa at
res.93 = (Ala, Ser, Glu, Gly, Arg or Thr); Xaa at res.95 = (Gly, Ala or Thr);
and Xaa at
res.97 = (His, Arg, Gly, Leu or Ser). Further, after res.53 in rat BMP3b and
mGDF-10
there is an Ile; after res.54 in GDF-1 there is a Thr; after res.54 in BMP3
there is a Val;
-13-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
after res.78 in BMP-8 and DORSALIN there is a Gly; after res.37 in hGDF-1
there are Pro,
Gly, Gly, and Pro.
Generic Sequence 10 (SEQ ID N0:7) includes all of Generic Sequence 9
and in addition includes the following five amino acid residues at its N-
terminus: Cys Xaa
Xaa Xaa Xaa (SEQ ID N0:9), wherein Xaa at res.2 = (Lys, Arg, Gln, Ser, His,
Glu, Ala,
or Cys); Xaa at res.3 = (Lys, Arg, Met, Lys, Thr, Leu, Tyr, or Ala); Xaa at
res.4 = (His,
Gln, Arg, Lys, Thr, Leu, Val, Pro, or Tyr); and Xaa at res.5 = (Gln, Thr, His,
Arg, Pro,
Ser, Ala, Gln, Asn, Tyr, Lys, Asp, or Leu). Accordingly, beginning at res.6,
each "Xaa" in
Generic Sequence 10 is a specified amino acid defined as for Generic Sequence
9, with the
distinction that each residue number described for Generic Sequence 9 is
shifted by five in
Generic Sequence 10. For example, "Xaa at res. l = (Phe, Leu or Glu)" in
Generic
Sequence 9 corresponds to Xaa at res.6 in Generic Sequence 10.
As noted above, certain preferred bone morphogenic proteins useful in this
invention have greater than 60%, preferably greater than 65%, identity with
the C-terminal
seven-cysteine domain of hOP-1. These particularly preferred sequences include
allelic and
phylogenetic variants of the OP-1 and OP-2 proteins, including the Drosophila
60A
protein. Accordingly, in certain particularly preferred embodiments, useful
proteins include
active proteins comprising dimers having the generic amino acid sequence "OPX"
(SEQ ID
N0:3), which defines the seven-cysteine skeleton and accommodates the
homologies
between several identified variants of OP-1 and OP-2. Each Xaa in OPX is
independently
selected from the residues occurring at the corresponding position in the C-
terminal
sequence of mouse or human OP-1 or OP-2.
OPX
Cys Xaa Xaa His Glu Leu Tyr Val Ser Phe Xaa Asp Leu Gly Trp Xaa Asp Trp
1 5 10 15
Xaa Ile Ala Pro Xaa Gly Tyr Xaa Ala Tyr Tyr Cys Glu Gly Glu Cys Xaa Phe Pro
20 25 30 35
Leu Xaa Ser Xaa Met Asn Ala Thr Asn His Ala Ile Xaa Gln Xaa Leu Val His Xaa
40 45 SO 55
Xaa Xaa Pro Xaa Xaa Val Pro Lys Xaa Cys Cys Ala Pro Thr Xaa Leu Xaa Ala
-14-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
60 65 70
Xaa Ser Val Leu Tyr Xaa Asp Xaa Ser Xaa Asn Val Ile Leu Xaa Lys Xaa Arg
75 80 85 90
Asn Met Val Val Xaa Ala Cys Gly Cys His (SEQ ID N0:3)
95 100
wherein Xaa at res.2 = (Lys or Arg); Xaa at res.3 = (Lys or Arg); Xaa at res.
l l = (Arg or
Gln); Xaa at res.16 = (Gln or Leu); Xaa at res.19 = (Ile or Val); Xaa at
res.23 = (Glu or
Gln); Xaa-at res.26 = (Ala or Ser); Xaa at res.35 = (Ala or Ser); Xaa at
res.39 = (Asn or
Asp); Xaa at res.41 = (Tyr or Cys); Xaa at res.50 = (Val or Leu); Xaa at
res.52 = (Ser or
Thr); Xaa at res.56 = (Phe or Leu); Xaa at res.57 = (Ile or Met); Xaa at
res.58 = (Asn or
Lys); Xaa at res.60 = (Glu, Asp or Asn); Xaa at res.61 = (Thr, Ala or Val);
Xaa at res.65 =
(Pro or Ala); Xaa at res.71 = (Gln or Lys); Xaa at res.73 = (Asn or Ser); Xaa
at res.75 =
(Ile or Thr); Xaa at res.80 = (Phe or Tyr); Xaa at res.82 = (Asp or Ser); Xaa
at res.84 =
(Ser or Asn); Xaa at res.89 = (Lys or Arg); Xaa at res.91 = (Tyr or His); and
Xaa at res.97
= (Arg or Lys).
In another embodiment, the morphogenic proteins comprise species of the
generic amino acid sequence
1 10 20 30 40 50
CXXXXLXVXFXDXGWXXWXXXPXGXXAXYCXGXCXXPXXXXXXXXNHAXX
60 70 80 90 100
QXXVXXXNXXXXPXXCCXPXXXXXXXXLXXXXXXXVXLXXYXXMXVXXCXCX
(SEQ ID NO:10)
or residues 6-102 of SEQ ID NO:10, where the letters indicate the amino acid
residues of
standard single letter code, and the Xs represent any amino acid residues.
Cysteine
residues are highlighted.
Preferred amino acid sequences within the foregoing generic sequence (SEQ
ID NO:10) are:
1 10 20 30 40 50
LYVDFRDVGWNDWIVAPPGYHAFYCHGECPFPLADHLNSTNHAIV
K S S L QE 'JIS E FD Y E A AY MPESMKAS VI
F E K I DN L N S Q ITK F P TL
-15-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
A S K
60 70 80 90 100
QTLVNSVNPGKIPKACCVPTELSAISMLYLDENENVVLKNYQDMWEGCGCR
SI HAI SEQV EP EQMNSLAI FFNDQDK I RK EE T DA H H
RF T S K DPV V Y N S H RN RS
N S K P E
and
1 10 20 30 40 50
CKRHPLYVDFRDVGWNDWIVAPPGYHAFYCHGECPFPLADHLNSTNHAIV
RRRS K S S L QE VIS E FD Y E A AY MPESMKAS VI
KE F E K I DN L N S Q ITK F P TL
Q A S K
60 70 80 90 100
QTLVNSVNPGKIPKACCVPTELSAISMLYLDENENVVLKNYQDMWEGCGCR
SI HAI SEQV EP EQMNSLAI FFNDQDK I RK EE T DA H H
RF T S K DPV V Y N S H RN RS
N S K P E
wherein each of the amino acids arranged vertically at each position in the
sequence may be
used alternatively in various combinations (SEQ ID NO:10). These generic
sequences have
6 or 7 cysteine residues where inter- or intra-molecular disulfide bonds can
form. These
sequences also contain other critical amino acids that influence the tertiary
structure of the
proteins.
In still another embodiment, useful morphogenic proteins comprise an amino
acid sequence encoded by a nucleic acid that hybridizes, under low, medium or
high
stringency hybridization conditions, to DNA or RNA encoding reference
morphogenic
protein coding sequences. Exemplary reference sequences include the C-terminal
sequences defining the conserved seven-cysteine domains of OP-1, OP-2, BMP-2,
BMP-4,
BMP-5, BMP-6, 60A, GDF-3, GDF-5, GDF-6, GDF-7, and the like. High stringent
hybridization conditions are herein defined as hybridization in 40% formamide,
SX SSPE,
SX Denhardt's Solution, and 0.1% SDS at 37°C overnight, and washing in
0.1X SSPE,
0.1% SDS at 50°C. Standard stringency conditions are well characterized
in commercially
available, standard molecular cloning texts. See, for example, Molecular
Cloning, A
Labor°ato~y Mamtal, 2nd Ed., ed. by Sambrook et al. (Cold Spring Harbor
Laboratory
Press 1989); DNA Cloning, Volumes I and II (D. N. Glover ed., 1985);
Oligonucleotide
-16-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
Synthesis (M. J. Gait ed., 1984); Nucleic Acid Hybridization (B. D. Hames & S.
J. Higgins
eds. 1984); and B. Perbal, A Practical Guide To Molecular Cloning (1984).
Suitable in vitro, ex vivo and in vivo bioassays known in the art, including
those described herein, may be used to ascertain whether a new BMP-related
gene product
has a morphogenic activity. Expression and localization studies defining where
and when
the gene is expressed may also be used to identify potential morphogenic
activities. Nucleic
acid and protein localization procedures are well known to those of skill in
the art (see,
e.g., Ausubel et al., eds. Current Protocols in Molecurlar Cloning, Greene
Publishing and
Wiley Interscience, New York, 1989).
Many of the identified BMPs are osteogenic and can induce bone and
cartilage formation when implanted into mammals. Some BMPs identified based on
sequence homology to known osteogenic proteins possess other morphogenic
activities and
a combination of a hormone and a soluble receptor thereof may be used to
enhance those
activities. For example, BMP-12 and BMP-13 reportedly induce ectopic formation
of
tendon/ligament-like tissue when implanted into mammals (Celeste et al., WO
95/16035).
Using this bioassay, a skilled practitioner can readily identify one or more
combinations of
hormones and soluble receptors thereof that can stimulate the ability of the
BMP to induce
tendon/ligament-like tissue formation.
Certain BMPs which are known to be osteogenic can also induce neuronal
cell differentiation. Embryonic mouse cells treated with BMP-2 or OP-1
differentiate into
astrocyte-like (filial) cells, and peripheral nerve regeneration using BMP-2
has been
reported (Wang et al., WO 95/05846). In addition, BMP-4, BMP-5 and OP-1 are
expressed in epidermal ectoderm flanking the neural plate. Ectopic recombinant
BMP-4
and OP-1 proteins can induce neural plate cells to initiate dorsal neural cell
fate
differentiation (Liem et al., Cell, 82, pp. 969-79 (1995)). At the spinal cord
level, OP-1
and other BMPs can induce neural crest cell differentiation. It is suggested
that OP-1 and
these BMPs can induce many or all dorsal neural cell types, including roof
plate cells,
neural crest cells, and commissural neurons, depending on localized positional
cues.
That several osteogenic proteins originally derived from bone matrix are
involved in neural development suggests that these and other members of the
BMP family
have additional tissue inductive properties that are not yet disclosed. It is
envisioned that
the hormone/receptor combinations set forth in this invention can be used to
enhance new
-17-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
or known tissue inductive properties of various known morphogenic proteins. It
is also
envisioned that the invention described herein will be useful for stimulating
tissue inductive
activities of new morphogenic proteins as they are identified in the future.
Production of Morphogenic Proteins
The morphogenic proteins of this invention can be derived from a variety of
sources. For instance, they may be isolated from natural sources,
recombinantly produced,
or chemically synthesized.
A. Naturally Derived Morphogenic Proteins
The morphogenic proteins of the invention can be purified from tissue
sources, e.g., mammalian tissue sources, using well known techniques. See,
e.g.,
Oppermann et al., U.S. Patent Nos. 5,324,819 and 5,354,557. If a purification
protocol is
unpublished, as for a newly identified morphogenic protein, conventional
protein
purification techniques (e.g., immunoaffinity) may be performed in combination
with
morphogenic activity assays. Such assays allow the trace of the morphogenic
activity
through a series of purification steps.
B. Recombinantly Expressed Morphogenic Proteins
In another embodiment of this invention, the morphogenic protein is
produced by expressing an appropriate recombinant DNA molecule in a host cell.
The
DNA and amino acid sequences of many BMPs and OPs have been reported, and
methods
for their recombinant production are published and otherwise known to those of
skill in the
art. For a general discussion of cloning and recombinant DNA technology, see
Ausubel et
al., supra; see also Watson et al., Recombir2ant DNA, 2d ed. 1992 (W.H.
Freeman and Co.,
New York).
The DNA sequences encoding bovine and human BMP-2 (formerly BMP-
2A) and BMP-4 (formerly BMP-2B), and processes for recombinantly producing the
corresponding proteins are described in U.S. Patent Nos. 5,011,691, 5,013,649,
5,166,058
and 5,168,050. The DNA and amino acid sequences of human and bovine BMP-5 and
BMP-6, and methods for their recombinant production, are disclosed in U.S.
Patent Nos.
5,106,748, and 5,187,076, respectively; see also U.S. Patent Nos. 5,011,691
and
5,344,654. Methods for OP-1 recombinant expression are disclosed in Oppermann
et al.,
U.S. Patent Nos. 5,011,691 and 5,258,494. For an alignment ofBMP-2, BMP-4, BMP-
5,
BMP-6 and OP-1 (BMP-7) amino acid sequences, see WO 95/16034. DNA sequences
-18-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
encoding BMP-8 are disclosed in WO 91/18098, and DNA sequences encoding BMP-9
in
WO 93/00432. DNA and deduced amino acid sequences encoding BMP-10 and BMP-11
are disclosed in WO 94/26893, and WO 94/26892, respectively. DNA and deduced
amino
acid sequences for BMP-12 and BMP-13 are disclosed in WO 95/16035. The above
patent
disclosures, which describe DNA and amino acid sequences, and methods for
producing the
BMPs and OPs encoded by those sequences, are incorporated herein by reference.
To clone genes that encode new BMPs, OPs and other morphogenic
proteins identified in extracts by bioassay, methods entailing "reverse
genetics" may be
employed. Such methods start with a protein of known or unknown function to
obtain the
gene that encodes that protein. Standard protein purification techniques may
be used as an
initial step. If enough protein can be purified to obtain a partial amino acid
sequence, a
degenerate DNA probe capable of hybridizing to the DNA sequence that encodes
that
partial amino acid sequence may be designed, synthesized and used as a probe
to isolate
full-length clones that encode that or a related morphogenic protein.
Alternatively, a partially-purified extract containing the morphogenic protein
may be used to raise antibodies directed against that protein. Morphogenic
protein-specific
antibodies may then be used as a probe to screen expression libraries made
from cDNAs
(see, e.g., Broome and Gilbert, Proc. Natl. Acad. Sci. U.S.A., 75, pp. 2746-49
(1978);
Young and Davis, Pnoc. Natl. Acad. Sci. U.S.A., 80, pp. 31-35 (1983)).
For cloning and expressing new BMPs, OPs and other morphogenic
proteins identified based on DNA sequence homology, the homologous sequences
may be
cloned and sequenced using standard recombinant DNA techniques. With the DNA
sequence available, a DNA fragment encoding the morphogenic protein may be
inserted
into an expression vector selected to work in conjunction with a desired host
expression
system. The DNA fragment is cloned into the vector such that its transcription
is
controlled by a heterologous promoter in the vector, preferably a promoter
which may be
optionally regulated.
Some host-vector systems appropriate for the recombinant expression of
BMPs and OPs are disclosed in the references cited above. Useful host cells
include but
are not limited to bacteria such as E. coli, yeasts such as Sacclraromyces and
Picia, insects
cells and other primary, transformed or immortalized eukaryotic cultured
cells. Preferred
eukaryotic host cells include CHO, COS and BSC cells (see below).
-19-
CA 02385887 2002-03-18
WO 01/23563 PCT/CTS00/26528
An appropriate vector is selected according to the host system selected.
Useful vectors include but are not limited to plasmids, cosmids,
bacteriophage, insect and
animal viral vectors, including those derived from retroviruses and other
single and double-
stranded DNA viruses.
In one embodiment of this invention, the morphogenic protein may be
derived from a recombinant DNA molecule expressed in a prokaryotic host. Using
recombinant DNA techniques, various fusion genes have been constructed to
induce
recombinant expression of naturally sourced osteogenic sequences in E. co7i
(see, e.g.,
Oppermann et al., U. S. Patent No. 5,354,557, incorporated herein by
reference). Using
analogous procedures, DNAs comprising truncated forms of naturally sourced
morphogenic sequences may be prepared as fusion constructs linked by a
sequence coding
for the acid labile cleavage site (Asp-Pro) to a leader sequence (such as the
"MLE leader")
suitable for promoting expression in E. coli.
In another embodiment of this invention, the morphogenic protein is
expressed using a mammalian host-vector system (e.g., transgenic production or
tissue
culture production). A morphogenic protein so expressed may resemble more
closely the
naturally occurring protein. While the glycosylation pattern of the
recombinant protein may
sometimes differ from that of the natural protein, such differences are often
not essential for
biological activity of the recombinant protein. Techniques for transfection,
expression and
purification of recombinant proteins are well known in the art. See, e.g.,
Ausubel et al.,
supra, and Bendig, Genetic Engineering, 7, pp. 91-127 (1988).
Mammalian DNA vectors should include appropriate sequences to promote
expression of the gene of interest. Such sequences include transcription
initiation,
termination and enhancer sequences; efficient RNA processing signals such as
splicing and
polyadenylation signals; mRNA-stabilizing sequences; translation-enhancing
sequences
(e.g., Kozak consensus sequence); protein-stabilizing sequences; and when
desired,
sequences that enhance protein secretion.
Restriction maps and sources of various exemplary expression vectors
designed for OP-1 expression in mammalian cells have been described in U.S.
Patent No.
5,354,557. Each of these vectors employs a full-length hOP-1 cDNA sequence
inserted
into the pUC-18 vector. It will be appreciated by those of skill in the art
that DNA
sequences encoding truncated forms of morphogenic proteins may also be used,
provided
-20-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
that the expression vector or host cell provides the sequences necessary to
direct
processing and secretion of the expressed protein.
Useful promoters include, but are not limited to, the SV40 early and late
promoters, the adenovirus major late promoter, the mouse metallothionein-I
("mMT")
promoter, the Rous sarcoma virus ("RSV") long terminal repeat ("LTR"), the
mouse
mammary tumor virus ("MMTV") LTR, and the human cytomegalovirus ("CMV") major
intermediate-early promoter. For instance, a combination of the CMV or MMTV
promoter
with an enhancer sequence from the RSV LTR has been found to be particularly
useful in
expressing human osteogenic proteins.
Preferred DNA vectors also include a marker gene (e.g., neomycin or
DHFR) and means for amplifying the copy number of the gene of interest. DNA
vectors
may also comprise stabilizing sequences (e.g., ori- or ARS-like sequences and
telomere-like
sequences), or may alternatively be designed to favor directed or non-directed
integration
into the host cell genome.
One method of gene amplification in mammalian cell systems is the use of
the selectable dihydrofolate reductase (DHFR) gene in a dhfr cell line.
Generally, the
DHFR gene is provided on the vector carrying the gene of interest, and
addition of
increasing concentrations of the cytotoxic drug methotrexate (MTX) leads to
amplification
of the DHFR gene copy number, as well as that of the gene physically
associated with it.
DHFR as a selectable, amplifiable marker gene in transfected Chinese hamster
ovary (CHO)
cell lines is particularly well characterized in the art. Other useful
amplifiable marker genes
include the adenosine deaminase (ADA) and glutamine synthetase (GS) genes.
Gene amplification can be further enhanced by modifying marker gene
expression regulatory sequences (e.g., enhancer, promoter, and transcription
or translation
initiation sequences) to reduce the levels of marker protein produced.
Lowering the level
of DHFR transcription increases the DHFR gene copy number (and the physically-
associated gene) to enable the transfected cell to adapt to growth in even low
levels of
methotrexate (e.g., 0.1 ~M MTX). Preferred expression vectors such as pH754
and
pH752 (Oppermann et al., U. S. Patent No. 5,354,557, Figs. 19C and D) have
been
manipulated, using standard recombinant DNA technology, to create a weak DHFR
promoter. As will be appreciated by those skilled in the art, other useful
weak promoters,
-21-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
different from those disclosed herein, can be constructed using standard
methods. Other
regulatory sequences also can be modified to achieve the same effect.
Another gene amplification scheme relies on the temperature sensitivity (ts)
of BSC40-tsA58 cells transfected with an SV40 vector. Temperature reduction to
33°C
stabilizes the temperature-sensitive SV40 T antigen, which leads to the
excision and
amplification of the integrated transfected vector DNA, thereby amplifying the
physically-
associated gene of interest.
The choice of cells/cell lines depends on the needs of the skilled
practitioner.
Monkey kidney cells (COS) provide high levels of transient gene expression and
are thus
useful for rapidly testing vector construction and the expression of cloned
genes. COS
cells expressing the gene of interest can be established by transfecting the
cells with, e.g., an
SV40 vector carrying the gene. Stably transfected cell lines, on the other
hand, can be used
for long term production of morphogenic proteins. By way of example, both CHO
cells
and BSC40-tsA58 cells can be used as host cells. Recombinant OP-1 has been
expressed in
three different cell expression systems: COS cells for rapidly screening the
functionality of
the various expression constructs, CHO cells for the establishment of stable
cell lines, and
BSC40-tsA58 cells as an alternative means of producing recombinant OP-1
protein.
Several bone-derived osteogenic proteins (OPs) and BMPs are found as
homo- and heterodimers comprising interchain disulfide bonds in their active
forms. For
instance, BMP-2, BMP-4, BMP-6 and BMP-7 (OP-1) -- originally isolated from
bone --
are bioactive as either homodimers or heterodimers. The ability of OPs and
BMPs to form
heterodimers may confer additional or altered morphogenic activities on
morphogenic
proteins. Heterodimers may exhibit qualitatively or quantitatively different
binding affinities
than homodimers for OP and BMP receptors. Altered binding affinities may in
turn result
in differential activation of receptors that mediate different signalling
pathways, ultimately
leading to different biological activities. Altered binding affinities can
also be manifested in
a tissue or cell type-specific manner, thereby inducing only particular
progenitor cell types
to undergo proliferation and/or differentiation.
The dimeric proteins can be isolated from the culture media and/or refolded
and dimerized irz vitro to form biologically active compositions. Heterodimers
can be
formed in vita°o by combining separate, distinct polypeptide chains.
Alternatively,
heterodimers can be formed in a single cell by co-expressing nucleic acids
encoding
-22-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
separate, distinct polypeptide chains. See, e.g., WO 93/09229 and U.S. Patent
No.
5,411,941, for exemplary protocols for heterodimer protein production.
C. In Vivo Expression of Morpho~enic Proteins
The morphogenic protein of the invention can also be produced ire vivo in a
patient. To achieve this, an expression vector comprising a promoter
operatively linked to
a coding sequence of the morphogenic protein may be introduced into progenitor
cells in
the patient. Alternatively, one can isolate the appropriate progenitor cells
from the patient,
transfect or transduce the cells with the expression vector, and re-introduce
the treated cells
to the patient at a desired locus.
(1) Vectors
A nucleic acid construct according to this invention is derived from a non-
replicating linear or circular DNA or RI~1A vector, or from an autonomously
replicating
plasmid or viral vector. Alternatively, the construct is integrated into the
host genome.
Any vector that can transfect or transduce the desired progenitor cell may be
used.
Preferred vectors are viral vectors, including those derived from replication-
defective
retroviruses (see, e.g., W089/07136; Rosenberg et al., N. Eng. J. Med. 323(9):
570-578
(1990)), adenovirus (see, e.g., Morsey et al., .l. Cell. Biochem., Supp. 17E
(1993)), adeno-
associated virus (Kotin et al., Proc. Natl. Acad. Sci. USA 87:2211-2215
(1990)),
replication-defective herpes simplex viruses (HSV; Lu et al., Abstract, page
66, Abstracts
of the Meeting on Gene Therapy, Sept. 22-26, 1992, Cold Spring Harbor
Laboratory, Cold
Spring Harbor, New York), vaccinia virus (Mukherjee et al., C.'ancer Gene
They°. 7:663-70
(2000)), and any modified versions of these vectors. Methods for constructing
expression
vectors are well known in the art. See, e.g., Sambrook et al., Molecular
Cloning.' A
Laboratory Manual, Cold Spring Harbor Laboratory, 2nd Edition, Cold Spring
Harbor,
New York, 1989).
(2) Expression Control Sequences
In these vectors, expression control sequences are operably linked to the
nucleic acid sequence encoding the morphogenic protein useful in this
invention. For
eukaryotic cells, expression control sequences may include a promoter, an
enhancer, such
as one derived from an immunoglobulin gene, SV40, cytomegalovirus, etc., and a
polyadenylation sequence. A nucleic acid construct of this invention may also
contain an
internal ribosome entry site ("IRES"), and an intron that may be desirably
located between
-23-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
the promoter/enhancer sequence and the morphogenic protein-coding sequence.
Selection
of these and other common vector elements are conventional. See, e.g.,
Sambrook et al,
supra; Ausubel et al., Current Protocols in Molecular Biology, John Wiley &
Sons, New
York, (1989); and references cited therein.
In one embodiment of the present invention, high-level constitutive
expression is desired. Exemplary promoters for this purpose include, without
limitation,
the retroviral Rous sarcoma virus (RSV) LTR promoter/enhancer, the
cytomegalovirus
(CMV) immediate early promoter/enhancer (see, e.g., Boshart et al, Cell 41:521-
530
(1985)), the SV40 promoter, the dihydrofolate reductase promoter, the
cytoplasmic (3-actin
promoter, the phosphoglycerol kinase (PGK) promoter. Useful promoters for BMP
expression in osteoblasts also include the Type I collagen gene promoter and
the CBFA
gene promoter. Useful promoters for BMP expression in chondrocytes and
chondroblasts
include the Type II collagen gene promoter and the Type X collagen gene
promoter. In
another embodiment, the native transcription-regulatory elements of the
desired
morphogenic protein can be used.
Using the guidance provided by this application, one of skill in the art may
make a selection among the above expression control sequences and modified
versions
thereof without departing from the scope of this invention.
(3) Administration of Nucleic Acid Constructs
The nucleic acid constructs of this invention may be formulated as a
pharmaceutical composition for use in any form of transient and/or stable gene
transfer irr
vivo and ire vitro. The composition comprises at least the nucleic acid
construct and a
pharmaceutically acceptable carrier such as saline. Other aqueous and non-
aqueous sterile
suspensions known to be pharmaceutically acceptable carriers and well known to
those of
skill in the art may be employed also. The construct may be used for in vivo
and ex vivo
gene therapy, in vit~~o protein production and diagnostic assays.
The nucleic acid construct can be introduced into target cells as naked
DNA, or by, e.g., liposome fusion (see, e.g., Nabel et al., Scier7ce 249:1285-
8 (1990);
Ledley, JPediat~~ics 110:1-8 and 167-74 (1987); Nicolau et al., Proc Natl Acad
Sci USA
80:1068-72 (1983)), erythrocyte ghosts, or microsphere methods
(microparticles; see. e.g.,
United States patent 4,789,734; United States patent 4,925,673; United States
patent
-24-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
3,625,214; Gregoriadis, D~~ug Carf~iens in Biology and Medicine, pp. 287-341,
Academic
Press, 1979).
If the nucleic acid construct is viral-based, it can also be packaged as a
virion which then is used to transduce a cell (e.g., an autologous T cell
isolated from a
patient) in vitro. The infected cell is then introduced into the patient.
Alternatively, the
recombinant virus may be administered to a patient directly, e.g., locally at
the tissue defect
site; or intravenously, intraperitoneally, intranasally, intramuscularly,
subcutaneously,
and/or intradermally, as determined by one skilled in the gene therapy art. A
slow-release
device, such as an implantable pump, may be used to facilitate delivery of the
recombinant
virus to a cell. Where the virus is administered to a subject, the specific
cells to be infected
may be targeted by controlling the method of delivery. The treatments of the
invention may
be repeated as needed, as determined by one skilled in the art.
Dosages of the nucleic acid construct of this invention in gene therapy will
depend primarily on factors such as the condition being treated. The dosage
may also vary
depending upon the age, weight and health of the patient. For example, an
effective human
dosage of a BMP-coding virus is generally in the range of from about 0.5 ml to
50 ml of
saline solution containing the virus at concentrations of about 1 x 10', 1 x l
OR, 1 x 109, 1 x
10'°, 1 x 10", 1 x 1012, 1 x 10'', 1 x 10'x, 1 x 10'', or 1 x 10'~
viral particles per dose
administered. The dosage will be adjusted to balance the corrective benefits
against any
adverse side effects. The levels of expression of BMP may be monitored to
determine the
type and frequency of dosage administration.
D. Synthetic Non-native Morpho~enic Proteins
In another embodiment of this invention, a morphogenic protein may be
prepared synthetically. Morphogenic proteins prepared synthetically may be
native, or may
be non-native proteins, i.e., those not otherwise found in nature.
Non-native morphogenic proteins can be made by mutating nature
morphogenic proteins. Methods for making mutations that favor refolding and/or
assembling subunits into forms that exhibit greater morphogenic activity have
been
described. See, e.g., U.S. Patent No. 5,399,677.
Non-native morphogenic proteins can also be synthesized using a series of
consensus sequences (U. S. Patent No. 5,324,819). These consensus sequences
were
designed based on partial amino acid sequence data obtained from native
osteogenic
-25-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
products and on their homologies with other proteins reportedly having a
presumed or
demonstrated developmental function. Several biosynthetic consensus sequences
(called
consensus osteogenic proteins or "COPS") have been expressed as fusion
proteins in
prokaryotes. Purified fusion proteins may be cleaved, refolded, combined with
a hormone
and a soluble receptor thereof, implanted in an established animal model and
examined for
their bone- and/or cartilage-inducing activity. Certain preferred synthetic
osteogenic
proteins comprise one or both of two synthetic amino acid sequences designated
COPS
(SEQ ID NO:11) and COP7 (SEQ ID N0:12).
The amino acid sequences of COPS and COP7 are shown below, as set forth
in Oppermann et al., U. S. Patent Nos. 5,011,691 and 5,324,819, which are
incorporated
herein by reference:
COPS LYVDFS-DVGWDDWIVAPPGYQAFYCHGECPFPLAD
COP7 LYVDFS-DVGWNDWIVAPPGYHAFYCHGECPFPLAD
COPS HFNSTN--H-AVVQTLVNSVNSKI--PKACCVPTELSA
COP7 HLNSTN--H-AVVQTLVNSVNSKI--PKACCVPTELSA
COPS ISMLYLDENEKVVLKYNQEMVVEGCGCR (SEQ ID NO:11)
COP7 ISMLYLDENEKVVLKYNQEMVVEGCGCR (SEQ ID N0:12)
In these amino acid sequences, the dashes (-) are used as fillers only to line
up comparable sequences in related proteins. Differences between the aligned
amino acid
sequences are highlighted.
In one embodiment of this invention, the morphogenic protein is a synthetic
osteogenic protein comprising a partial or complete sequence of a generic
sequence
described above (SEQ ID N0:4, 5, 6, 7, or 10) such that it is capable of
inducing tissue
formation when properly folded and implanted in a mammal. For instance, the
synthetic
protein can induce bone formation from osteoblasts when implanted in a
favorable
environment; or it can promote cartilage formation when implanted in an
avascular locus or
when co-administered with an inhibitor of full bone development.
-26-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
In another embodiment, the synthetic morphogenic protein of this invention
comprises a sequence suWciently duplicative of a partial or complete sequence
of a COP,
e.g., COPS (SEQ ID NO:11) or COP7 (SEQ ID N0:12). Biosynthetic COP sequences
are
believed to dimerize during refolding and appear not to be active when
reduced. Both
homodimeric and heterodimeric COPS are contemplated in this invention. In
certain
embodiments, this synthetic protein is less than about 200 amino acids long.
These and other synthetic non-native osteogenic proteins may be used in
concert with a hormone/receptor pair and tested using izmitro, ex vivo or in
vivo bioassays
for progenitor cell induction and tissue regeneration. The proteins in
conjunction with the
hormone/receptor pairs of this invention are envisioned to be useful for the
repair and
regeneration of bone, cartilage, tendon, ligament, neural and potentially
other types of
tissue.
Homol~ous Proteins Having~Morphogenic Activity
The morphogenic proteins useful in this invention may be produced by
recombinant expression of DNA sequences isolated based on homology with the
osteogenic
COP consensus sequences described above. Synthetic COP DNA sequences may be
used
as probes to retrieve related DNA sequences from a variety of species (see,
e.g.,
Oppermann et al., U.S. Patent Nos. 5,011,591 and 5,258,494, which are
incorporated
herein by reference).
Morphogenic proteins encoded by a gene that hybridizes with a COP
sequence probe are assembled into two subunits disulfide-bonded to produce a
heterodimer
or homodimer capable of inducing tissue formation when implanted into a
mammal.
Recombinant BMP-2 and BMP-4 have been shown to have cross-species osteogemc
activity as homodimers and as heterodimers assembled with OP-1 subunits.
Morphogenic protein-encoding genes that hybridize to synthetic COP sequence
probes
include genes encoding Vgl, inhibin, DPP, OP-1, BMP-2 and BMP-4. Vgl is a
known
Xezzopus laevis morphogenic protein involved in early embryonic patterning.
Inhibin is
another developmental gene that is a member of the BMP family of proteins from
Xezzopzzs
laevis. DPP is an amino acid sequence encoded by a Dnosophila gene responsible
for
development of the dorso-ventral pattern. OP-l, BMP-2 and BMP-4 are osteogemc
proteins that can induce cartilage, bone and neural tissue formation.
-27-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
In another embodiment of this invention, a morphogenic protein may
comprise a polypeptide encoded by a nucleic acid that hybridizes under
stringent conditions
to an "OPS" nucleic acid probe (Oppermann et al., U.S. Patent No. 5,354,557).
"OPS" --
standing for OP-1 "short" -- refers to the portion of the human OP-1 protein
defining the
conserved 6 cysteine skeleton in the C-terminal active region (97 amino acids;
SEQ ID
N0:2, residues 335-431).
One example of a stringent hybridization condition is hybridization in 4X
SSC at 65°C (or 10°C higher than the calculated melting
temperature for a hybrid between
the probe and a nucleic acid sequence containing no mis-matched base pairs),
followed by
washing in O.1X SSC at the hybridization temperature. Another stringent
hybridization
condition is hybridization in 50% formamide, 4X SSC at 42°C.
Thus, in view of this disclosure, the skilled practitioner can readily design
and synthesize genes, or isolate genes from cDNA or genomic libraries that
encode amino
acid sequences having morphogenic activity. These genes can be expressed in
prokaryotic
or eukaryotic host cells to produce large quantities of active osteogenic or
otherwise
morphogenic proteins. The recombinant proteins may be in native, truncated,
mutant,
fusion, or other active forms capable of inducing formation of bone,
cartilage, or other
types of tissue, as demonstrated by ire vit~~o and ex vivo bioassays and in
vivo implantation
in mammals, including humans.
Hormones and Receptors Thereof
A hormone/receptor pair of this invention is capable of stimulating the
ability of a morphogenic protein to induce tissue formation from a progenitor
cell. In a
method of this invention, the tissue inductive activity of a morphogenic
protein in a
mammal is improved by co-administering effective amounts of a hormone and a
soluble
receptor thereof Alternatively, the morphogenic protein and the
hormone/receptor pair are
administered sequentially. It has been found that the synergism between a
morphogenic
protein and a hormone/receptor pair is preserved even if the morphogenic
protein is
administered 4 to 8 hours before the hormone/receptor pair. The morphogenic
protein, the
hormone, and the hormone receptor can also be administered separately.
One or more hormone/receptor pairs can be selected for use in concert with
one or more morphogenic proteins according to the desired tissue type to be
induced and
the site at which the treatment will be administered. The particular choice of
a
-28-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
morphogenic protein(s)/hormone(s)/receptor(s) combination and the relative
concentrations
at which they are combined may be varied systematically to optimize the tissue
type
induced at a selected treatment site using the procedures described herein.
Hormones useful in this invention include, but are not limited to,
interleukins
1 throughl8, fibroblast growth factor, vascular endothelial growth factor,
platelet-derived
growth factor, TGF-(3, and prostaglandins (e.g., E1 and E2). It may be
preferred that the
target cell has a cell-surface receptor for the hormone. The hormones can also
be
morphogenic proteins such as GDFs; as a result, the composition of this
invention will
contain two morphogenic proteins and a soluble receptor of one of these
proteins.
One preferred hormone/receptor pair of this invention is IL-6/sIL-6R. IL-6
is a member of a subfamily of multifunctional hormones. It appears to play a
role in both
bone formation and bone resorption by affecting mitogenesis of target cells
and regulating
the synthesis of other local factors. Clinical studies show that IL-6 is
involved in a variety
of diseases, such as fibrous dysplasia, osteopenia, osteoporosis and Paget's
disease.
Recombinant full length human IL-6 (26 kD) expressed from E. coli can be
obtained from
Sigma (St. Louis, MI) and Promega (Madison, WI). Recombinant sIL-6R produced
from
baculovirus and containing the entire extracellular domain (residues 1-339; 38
kD) of
human IL-6R can be obtained from Sigma and R&D Systems (Minneapolis, MN). See
also
Examples 1 and 2, infi°a. Active allelic, species or other variants of
these IL-6 and sIL-6
products can also be used.
The hormone or the hormone receptor of this invention can be associated
with an agent that is capable of increasing the hormone's or receptor's bio-
activity, e.g.,
synthesis, half life, bio-availability, and reactivity with other bio-
molecules such as binding
proteins and receptors. These agents may contain carrier molecules such as
proteins and
lipids.
The hormone and hormone receptor are present in amounts capable of
synergistically stimulating the tissue inductive activity of the morphogenic
proteW W a
mammal. The relative concentrations of morphogenic protein, hormone and
hormone
receptor that optimally induce tissue formation may be determined empirically
by the skilled
practitioner using the procedures described herein.
Progenitor Cells
-29-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
Tile progenitor cell that is induced to proliferate and/or differentiate by
the
morphogenic protein of this invention is preferably a mammalian cell. Examples
of useful
progenitor cells are mammalian chondroblasts, osteoblasts and neuroblasts, all
earlier
developmental precursors thereof. and all cells that develop therefrom (e.g.,
pre-
chondroblasts and chondrocytes). The progenitor cell may be induced to form
one or more
tissue types such as endochondral or intramembranous bone, cartilage,
tendon/ligament-like
tissue, neural tissue and kidney tissue. The specific morphogenic activity
exhibited by a
morphogenic protein will depend in part on the type of the progenitor cell as
well as the
treatment site. These variables may be tested empirically.
I O Morphogenic proteins are highly conserved throughout evolution, and non-
mammalian progenitor cells are likely to be stimulated by same- or cross-
species
morphogenic proteins and hormone/receptor combinations. It is thus envisioned
that where
schemes are available for implanting xenogeneic cells into humans without
adverse
immunological reactions, non-mammalian progenitor cells stimulated by
morphogenic
15 protein and a hormone/receptor pair according to the procedures set forth
herein will be
useful for tissue regeneration and repair in humans.
Testing Mor~hogenic Activity
To identify a hormone/receptor pair capable of stimulating the tissue
inductive activity of a chosen morphogenic protein, an appropriate assay is
selected.
20 Initially, ire vitro assays can be performed. A useful in vitro assay may
monitor a nucleic
acid or protein marker whose expression is known to correlate with the
associated cell
differentiation pathway. See, e.g., Examples 3 and 4 of United States Patent
No.
5,854,207, Lee et al.; and Examples 1 and 2, irTfra.
Examples 1 and 2; infra, describe experiments using OP-1 to identify and to
25 optimize an effective concentration of IL-6 and sIL-6R. OP-1 is known to
have osteogemc
and neurogenic activity. Thus, to identify a hormone/receptor pair having
synergistic
effects with OP-l, one can conduct an ire vitro assay that examines the
expression of a
molecular marker, e.g., an osteogenic- or a neurogenic-associated marker, in
appropriate
progenitor cells.
30 One useful assay for testing potential hormone/receptor pairs with OP-1 for
osteogenic activity is the alkaline phosphatase ("AP") enzymatic assay. AP is
an osteoblast
differentiation marker in primary osteoblastic fetal rat calvarial ("FRC")
cells. The OP-1-
-3 0-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
stimulated AP activity results from increased steady-state AP mRNA levels.
Other useful
protein markers for monitoring osteogenic activity of a composition include,
but are not
limited to, type I collagen, osteocalcin, osteopontin, bone sialoprotein and
PTH-dependent
cAMP levels.
An AP assay is performed generally as follows. First, a hormone/receptor
pair is identified by picking various concentrations and ratios of the hormone
and hormone
receptor and testing them in the absence and presence of a morphogenic
protein. Second,
the amounts of hormone and hormone receptor required to achieve optimal,
preferably
synergistic, tissue induction in concert with the morphogenic protein is
determined by
generating dose response curves.
Optionally, additional hormone/receptor pairs that further stimulate or
otherwise alter the morphogenic activity induced by a morphogenic protein and
a first
hormone/receptor pair may be identified and a new mufti-factor dose response
curve
generated. See, e.g., Example 5 of United States Patent 5,854,207.
Bone Induction Assays
The various morphogenic compositions and devices of this invention can
also be evaluated with ex vivo or irr vivo bioassays. A rat bioassay for bone
induction may
be used to monitor osteogenic activity of osteogenic proteins in concert with
one or more
hormone/receptor pairs. See, e.g., Sampath et al., Proc. Natl. Acad. Sci. USA,
80, pp.
6591-95 (1983), and United States Patent No. 5,854,207, Example 7. Rat
bioassays are
useful as the first step in moving from irz vitro studies to ire vivo studies.
Large animal efficacy models for osteogenic device testing are known in the
art. Exemplary models are the feline femoral model, the rabbit ulnar model,
the dog ulnar
model and the monkey model. See, e.g., U. S. Patent Nos. 5,354,557, and
5,854,207
(Examples 10 and 11 therein)
In general, about 500-1000ng of active morphogenic protein and about 10-
200ng of active hormone and active hormone receptor are combined with 25mg of
a carrier
matrix for rat bioassays. In larger animals, typically about 0.8-lmg of active
morphogenic
protein per gram of carrier is combined with about 100ng or more of an active
hormone
and hormone receptor. The optimal ratios of morphogenic protein to hormone and
of
hormone to hormone receptor for a specific tissue type may be determined
empirically by
-3 1-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
those of skill in the art according to the procedures set forth herein.
Greater amounts may
be used for large implants.
Tendon/ligament-like tissue formation bioassay
Assays for monitoring tendon and ligament-like tissue formation induced by
morphogenic proteins are known in the art. See, e.g., Celeste et al., WO
95/16035, hereby
incorporated by reference. Such assays can be used to identify
hormone/receptor pairs that
stimulate tendon/ligament-like tissue formation by BMP-12, BMP-13 or other
morphogenic
proteins in a particular treatment site. The assays may also be used to
optimize
concentrations and treatment schedules for therapeutic tissue repair
regiments.
These assays may be used to test various combinations of morphogenic
protein and hormone/receptor combinations, and to produce an irmioo dose
response curve
for determining the effective relative concentrations of morphogenic proteins
and
hormones/receptors.
Neural Assays
The osteogenic proteins BMP-4 and BMP-7 (OP-1) can induce ventral
neural plate explants to undergo differentiation into dorsal neural cell fates
(Liem et al.,
Cell, 82, pp. 969-79 (1995)). Molecular markers of dorsal cell differentiation
are described
in Liem et al. These markers include PAX3 and MS~i, whose expression
delineates an
early stage of neural plate cell differentiation; DSL-l, a BMP-like molecule
delineating
differentiation of dorsal neural plate cells at a stage after neural tube
closure; and SLUG
protein, whose expression after neural tube closure defines pre-migratory
neural crest cells.
Expression of these dorsal markers can be induced in ventral neural plate
explants by
ectopic BMP4 and OP-1.
A peripheral nerve regeneration assay using BMP-2 has been described
(Wang et al., WO 95/05846, hereby incorporated by reference). The assay
involves the
implantation of neurogenic devices in the vicinity of severed sciatic nerves
in rats. This
procedure may be used to assess the ability of a putative hormone/receptor
pair to enhance
the neuronal inductive activity of homo- and heterodimers of morphogenic
proteins having
neurogenic activity, such as BMP-2, BMP-4, BMP-6 and OP-1, or of any selected
neurogenic protein/hormone/hormone receptor combinations.
Pharmaceutical Compositions
-3 2-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
The pharmaceutical compositions of this invention contain at least one (e.g.,
at least 2, 3 or 5) morphogenic protein, and at least one (e.g., at least 2 or
3)
hormone/receptor pair. These compositions are capable of inducing tissue
formation when
administered, e.g., implanted, into a patient. The compositions will be
administered at an
effective dose to induce the particular type of tissue at the desired
treatment site.
Determination of a preferred pharmaceutical formulation and a therapeutically
efficient
dose regiment for a given application is well within the skill of the art.
Factors that need to
be considered include, for example, the administration mode, the condition and
weight of
the patient, the extent of desired treatment and the tolerance of the patient
for the
treatment.
Doses expected to be suitable starting points for optimizing treatment
regiments are based on the results of in vitro assays, and ex vivo or in vivo
assays. Based
on the results of such assays, a range of suitable morphogenic protein and
hormone/receptor concentration ratios can be selected to test at a treatment
site in animals
and then in humans.
The pharmaceutical compositions of this invention may be in a variety of
forms. These include, for example, solid, semi-solid and liquid forms such as
tablets, pills,
powders, liquids, suspensions, suppositories, gels, pastes, and other
injectable and infusible
solutions. The preferred form depends on the intended mode of administration
and
therapeutic application. Modes of administration may include oral, parenteral,
subcutaneous, intravenous, intralesional or topical administration. In most
cases, the
pharmaceutical compositions of this invention will be administered in the
vicinity of or at
the treatment site in need of tissue regeneration or repair.
The pharmaceutical compositions of this invention may, for example, be
placed into sterile, isotonic formulations with or without co-factors which
stimulate uptake
or stability. For example, the compositions may contain a formulation buffer
comprising
5.0 mg/ml citric acid monohydrate, 2.7 mg/ml trisodium citrate, 41 mg/ml
mannitol, 1
mg/ml glycine and 1 mg/ml polysorbate 20. This solution can be lyophilized,
stored under
refrigeration and reconstituted prior to administration with sterile Water-For-
Injection
(USP).
The compositions may also include pharmaceutically acceptable carriers well
known in the art. See, for example, Remington's Pharmaceutical Sciences, 16th
Edition,
-33-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
1980, Mac Publishing Company. Such pharmaceutically acceptable carriers may
include
other medicinal agents, carriers, genetic carriers, adjuvants, and excipients
such as human
serum albumin or plasma preparations. The compositions may be in the form of a
unit dose
and will usually be administered as a dose regiment that depends on the
particular tissue
treatment.
The pharmaceutical compositions of this invention may also be administered
in form of a morphogenic device using, for example, microspheres, liposomes,
other micro-
particulate delivery systems or sustained release formulations placed in,
near, or otherwise
in communication with affected tissues or the bloodstream bathing those
tissues.
Liposomes containing the polypeptide mixtures of this invention can be
prepared by well-known methods. See, e.g. DE 3,218,121; Epstein et al., Proc.
Natl.
Acad. Sci. U.S.A., 82, pp. 3688-92 (1985); Hwang et al., Proc. Natl. Acad.
Sci. U.S.A., 77,
pp. 4030-34 (1980); U.S. Patent Nos. 4,485,045 and 4,544,545. Ordinarily the
liposomes
are of the small (about 200-800 Angstroms) unilamellar type in which the lipid
content is
greater than about 30 mol.% cholesterol. The proportion of cholesterol is
selected to
control the optimal release rate of the polypeptides of interest.
The polypeptide mixtures of this invention may also be attached to
liposomes containing other biologically active molecules to modulate the rate
and
characteristics of tissue induction. Such attachment may be accomplished by
cross-linking
agents such as heterobifunctional cross-linking agents that have been widely
used to couple
toxins or chemotherapeutic agents to antibodies for targeted delivery.
Conjugation to
liposomes can also be accomplished using the carbohydrate-directed cross-
linking reagent
4-(4-maleimidophenyl) butyric acid hydrazide. See, e.g., Duzgunes et al., J.
Cell. Biochem.
Abst., Suppl. 16E 77 (1992).
Mor_phogenic Devices
The pharmaceutical compositions of this invention can additionally contain
an implantable, biocompatible carrier. Such compositions are also called
morphogenic
devices. The carrier functions as a sustained release delivery system for the
therapeutic
proteins and protects the proteins from non-specific proteolysis. The carrier
may be
biodegradable in vivo. A sustained release carrier may contain semipermeable
polymer
matrices in the form of shaped articles, e.g., suppositories or capsules. Such
a carrier can
be made of polylactides (U.S. Patent No. 3,773,319; EP 58,481), copolymers of
L-glutamic
-34-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
acid and ethyl-L-glutamate (Sidman et al., Biopolymers, 22, pp. 547-56
(1985)); poly(2-
hydroxyethyl-methacrylate) or ethylene vinyl acetate (Langer et al., J.
Biomed. Mater. Res.,
15, pp. 167-277 (1981); Langer, Chem. Tech., 12, pp. 98-105 (1982)).
The carrier may also serve as a temporary scaffold and substratum for
recruitment of migratory progenitor cells and their subsequent anchoring and
proliferation,
until replaced by new bone or other appropriate tissue. For example, the
carrier may
contain a biocompatible matrix made up of particles or otherwise having the
desired
porosity or microtexture. The pores will permit migration, anchoring,
differentiation and
proliferation of the relevant progenitor cells. The particle size may be
within the range of
70qm-850qm, e.g., 70~m-420~m or 150qm-420pm. A particulate matrix may be
fabricated by close packing particulate materials into a shape spanning the
tissue defect to
be treated. Various matrices known in the art can be employed. See, e.g., U.
S. Patent
Nos. 4,975,526, 5,162,114 and 5,171,574, and WO 91/18558, all of which are
herein
incorporated by reference.
Useful matrix materials include but are not limited to collagen; celluloses,
including carboxymethyl cellulose; homo- or co-polymers of glycolic acid,
lactic acid, and
butyric acid, including derivatives thereof; and ceramics, such as
hydroxyapatite, tricalcium
phosphate and other calcium phosphates. See, e.g., U.S. No. 5,854,207, col.
25, line 59,
through col. 27, line 6. Various combinations of these or other suitable
matrix materials
may be useful as determined by the assays set forth herein. The choice of
material depends
in part on its irz vivo dissolution rate. In bones, the dissolution rates can
vary according to
whether the implant is placed in cortical or trabecular bone.
Other useful matrices include particulate, demineralized, guanidine-
extracted, allogenic bone; and specially treated, particulate, protein-
extracted,
demineralized xenogenic bone. See, e.g., Example 6 of U.S. Patent No.
5,854,207. Such
xenogenic bone powder matrices may be treated with proteases such as trypsin.
Preferably,
the xenogenic matrices are treated with one or more fibril-modifying agents to
increase the
intraparticle intrusion volume (porosity) and surface area. Useful modifying
agents include
solvents such as dichloromethane, trichloroacetic acid, acetonitrile and acids
such as
trifluoroacetic acid and hydrogen fluoride. A preferred fibril-modifying agent
is in the form
of a heated aqueous medium, preferably an acidic aqueous medium having a pH
less than
about 4.5, most preferably having a pH between about 2 and 4, inclusive. The
acidic
-3 5-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
aqueous medium can, for instance, be 0.1% acetic acid with a pH of about 3.
Heating
demineralized, delipidated, guanidine-extracted bone collagen in an aqueous
medium at
elevated temperatures (e.g., at about 37°C-65°C, preferably at
about 45°C-60°C) for
approximately one hour is generally sufficient to achieve the desired surface
morphology.
It is hypothesized that the heat treatment alters the collagen fibrils,
resulting in an increase
in the particle surface area.
Xenogenic bone matrices can be used in a variety of clinical settings. In
addition to its use as a matrix for bone formation in various orthopedic,
periodontal, and
reconstructive procedures, the matrix also may be used as a sustained release
carrier, or as
a collagenous coating for orthopedic or general prosthetic implants.
Demineralized guanidine-extracted xenogenic bovine bone contains a
mixture of additional materials that may be fractionated further using
standard biomolecular
purification techniques. For example, bone extracts can be fractionated by
chromatography, and the various extract fractions corresponding to the
chromatogram
peaks can be added back together to an active matrix. Doing so may remove
inhibitors of
bone or tissue-inductive activity, thereby improving matrix properties.
Besides morphogenic proteins and hormone/receptor pairs, the morphogenic
devices of this invention may additionally contain other hormones and trophic
agents. The
devices may also contain antibiotics, chemotherapeutic agents, enzymes, enzyme
inhibitors
and other bioactive agents. These ingredients may be adsorbed onto or
dispersed within
the carrier, and will be released over time at the implantation site as the
carrier material is
slowly absorbed.
General Consideration of Matrix Properties
Factors influencing the performance of a matrix include matrix geometry,
particle size (if the matrix is made up of particles), the methodology for
combining the
matrix and morphogenic proteins, the degree of both intra- and inter-particle
porosity, the
presence of mineral, and the presence of surface charge. For example, studies
have shown
that, in bone induction using OP-1 and a morphogenic protein stimulating
factor,
perturbation of the matrix charge by chemical modifications can abolish bone
inductive
responses. Particle size also influences the quantitative response of new
bone, with sizes
between 70~m and 420qm capable of eliciting the maximum response. Further,
contamination of the matrix with bone mineral may inhibit bone formation.
Individual
-3 6-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
heavy metal concentrations in a bone matrix can be reduced to less than about
1 ppm by the
methods described herein.
The sequential cellular reactions at the interface of the bone matrix and an
osteogenic protein implant are complex. The mufti-step cascade includes:
binding of fibrin
and fibronectin to the implanted matrix, migration and proliferation of
mesenchymal cells,
differentiation of the progenitor cells into chondroblasts, cartilage
formation, cartilage
calcification, vascular invasion, bone formation, remodeling, and bone marrow
differentiation. A successful matrix is capable of accommodating each of these
steps.
The matrix may be shaped as desired in anticipation of surgery or shaped by
the physician or technician during surgery. It has been shown that new bone is
formed
essentially with the dimensions of the implanted device. In the case where the
matrix
material is biodegradable in vivo, the matrix material is slowly absorbed by
the body and is
replaced by new bone in the shape of, or very nearly the shape of, the
implant. Thus, the
matrix is preferably shaped to span a tissue defect and to take the desired
form of the new
tissue. For example, in the case of bone repair of a non-union defect, it is
desirable to use
dimensions that span the non-union, and the new bone will eventually fill the
defect.
The matrix may be a shape-retaining solid made of loosely-adhered
particulate material, e.g., collagen. Alternatively, the matrix may be a
molded, porous
solid, or an aggregation of close-packed particles held in place by
surrounding tissue.
Masticated muscle or other tissue may also be used. Large allogenic bone
implants can act
as a carrier for the matrix if their marrow cavities are cleaned and packed
with particles
containing dispersed osteogenic protein and hormone/receptor pair.
The matrix may also take the form of a paste or a hydrogel. "Hydrogel"
refers to a three dimensional network of cross-linked hydrophilic polymers in
the form of a
gel. The gel is substantially composed of water, for instance, greater than
90% water.
Hydrogel matrices can carry a net positive or net negative charge, or may be
neutral. A
typical net negative charged matrix is alginate. Hydrogels carrying a net
positive charge
are, for example, extracellular matrix components such as collagen and
laminin. Examples
of commercially available extracellular matrix components include MATRIGELTM
and
VITROGENTM. Example of a net neutral hydrogel are highly cross-linked
polyethylene
oxide and polyvinyl alcohol.
Prosthetic Devices
-3 7-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
This invention also features an implantable prosthetic device comprising at
least one morphogenic protein and at least one hormone/receptor pair at
therapeutic
amounts and ratios. The device can be used in conjunction with a composition
containing
the same or other morphogenic protein or hormone/receptor pair. The prosthetic
device
may be made from a material containing metal or ceramic. Exemplary prosthetic
devices
are hip devices, screws, rods and titanium cages for spine fusion. The device
is implanted
in a mammal (e.g., a human) at a locus where the target tissue and the surface
of the
prosthetic device are maintained at least partially in contact for a time
sufficient to permit
enhanced tissue growth between the target tissue and the device.
The osteogenic composition may be disposed on the prosthetic implant on a
surface region that is to be positioned next to a target tissue in the mammal.
Preferably, the
mammal is a human patient. The composition is disposed in an amount sufficient
to
promote enhanced tissue growth into the implant or onto its surface. The
amount of the
composition to be used may be determined empirically by using bioassays such
as those
described herein and in Rueger et al., U.S. Patent No. 5,344,654, which is
incorporated
herein by reference. Preferably, animal studies are performed to optimize the
concentration
of the ingredients in the device before a similar prosthetic device is used in
a human patient.
The prosthetic devices will be useful for repairing orthopedic defects,
injuries or anomalies
in the treated mammal.
Utility of Morphog-enic Compositions and Devices
The compositions, devices and methods of this invention will permit a
physician to treat a variety of tissue injuries, tissue degenerations, and
other diseased tissue
conditions. The compositions and devices can ameliorate or remedy these
conditions by
stimulating local tissue formation or regeneration.
The devices of this invention may be implanted at the desired locus in a
mammal such that the implant is accessible to the appropriate progenitor cells
of this
mammal. The devices may be used alone or in combination with other therapies
for tissue
repair and regeneration.
The morphogenic devices of this invention may also be implanted in or
surrounding a joint for use in cartilage and soft tissue repair, or in or
surrounding nervous
system-associated tissue for use in neural regeneration and repair. The tissue
specificity of
the particular morphogenic protein -- or combination of morphogenic proteins
with other
-3 8-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
biological factors -- will determine the cell types or tissues that will be
amenable to such
treatments and can be selected by one skilled in the art. The ability to
enhance
morphogenic protein-induced tissue regeneration by co-administering a
hormone/receptor
pair according to the present invention is thus not believed to be limited to
any particular
cell-type or tissue.
The osteogenic compositions and devices of this invention will permit the
physician to obtain predictable bone, ligament and/or cartilage formation
using less
osteogenic protein to achieve at least about the same extent of bone or
cartilage formation.
The osteogenic compositions and devices of this invention may be used to treat
more
effectively the injuries, anomalies and disorders that have been described in
the prior art of
osteogenic devices. These include, for example, forming local bone in
fractures, non-union
fractures, fusions and bony voids such as those created in tumor resections or
those
resulting from cysts; treating acquired and congenital craniofacial and other
skeletal or
dental anomalies (see e.g., Glowacki et al., Lancet, 1, pp. 959-63 (1981));
performing
dental and periodontal reconstructions where lost bone replacement or bone
augmentation
is required such as in a jaw bone; and supplementing alveolar bone loss
resulting from
periodontal disease to delay or prevent tooth loss (see e.g., Sigurdsson et
al., J.
Periodontol., 66, pp. 511-21 (1995)).
An osteogenic device of this invention that comprises a matrix comprising
allogenic bone may also be implanted at a site in need of bone replacement to
accelerate
allograft repair and incorporation in a mammal. Another potential clinical
application of the
improved osteogenic devices of this invention is in cartilage repair, for
example, following
joint injury or in the treatment of osteoarthritis. The ability to enhance the
cartilage-
inducing activity of morphogenic proteins by co-administering a
hormone/receptor pair may
permit faster or more extensive tissue repair and replacement using the same
or lower levels
of morphogenic proteins.
The morphogenic compositions and devices of this invention will be useful
in treating certain congenital diseases and developmental abnormalities of
cartilage, bone
and other tissues. For example, homozygous OP-1-deficient mice die within 24
hours after
birth due to kidney failure (Luo et al., J. Bore Mirz Res., 10 (Supp. 1), pp.
S163 (1995)).
Kidney failure in these mice is associated with the failure to form renal
glomeruli due to
lack of mesenchymal tissue condensation. OP-1-deficient mice also have various
skeletal
_39_
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
abnormalities associated with their hindlimbs, rib cage and skull, are
polydactyl, and exhibit
aberrant retinal development. These results, in combination with those
discussed above
concerning the ability of OP-1 to induce differentiation into dorsal neural
cell fates, indicate
that OP-1 plays an important role in epithelial-mesenchymal interactions
during
development. It is anticipated that the compositions, devices and methods of
this invention
will be useful for ameliorating these and other developmental abnormalities.
Develapmental abnormalities of the bone may affect isolated or multiple
regions of the skeleton or of a particular supportive or connective tissue
type. These
abnormalities often require complicated bone transplantation procedures and
orthopedic
devices. The tissue repair and regeneration required after such procedures may
occur more
quickly and completely with the use of morphogenic compositions, devices and
methods of
this invention.
Examples of heritable conditions, including congenital bone diseases, for
which use of the morphogenic compositions and devices of this invention will
be useful
include osteogenesis imperfecta, the Hurler and Marfan syndromes, and several
disorders of
epiphyseal and metaphyseal growth centers such as is presented in
hypophosphatasia, a
deficiency in alkaline phosphatase enzymatic activity.
Inflammatory joint diseases may also benefit from the improved methods,
compositions and devices of this invention. These diseases include but are not
limited to
rheumatoid and psoriatic arthritis, bursitis, ulcerative colitis, regional
enteritis, Whipple's
disease, ankylosing spondylitis (also called Marie Strumpell or Bechterew's
disease), and
the so-called "collagen diseases" such as systemic lupus erythematosus (SLE),
progressive
systemic sclerosis (scleroderma), polymyositis (dermatomyositis), necrotizing
vasculitides,
Sjogren's syndrome (sicca syndrome), rheumatic fever, amyloidosis, thrombotic
thrombocytopenic purpura and relapsing polychondritis. Heritable disorders of
connective
tissue include Marfan's syndrome, homocystinuria, Ehlers-Danlos syndrome,
osteogenesis
imperfecta; alkaptonuria, pseudoxanthoma elasticum, cubs laxa, Hurler's
syndrome, and
myositis ossificans progressma.
Examples
The following are examples which illustrate the morphogenic compositions
and devices of this invention, and methods used to characterize them. These
examples
-40-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
should not be construed as limiting. They are included for purposes of
illustration and the
present invention is limited only by the claims.
Example 1
Fig. 1 shows the effects of Il-6, sIL-6R, and mixtures of recombinant human
IL-6 and recombinant human sIL-6R ("IL-6/R"), respectively, on the OP-1-
induced AP
activity in FRC cells. Confluent FRC cells were treated with the indicated
agents) for 24
hrs. The concentrations of agents) used (ng/ml) are indicated in parentheses.
For IL-6/R,
the molar ratio of the two was maintained at about 1.2, and their respective
amounts are
indicated also in parentheses. Total AP activity was determined
spectrophotometrically.
Total cellular protein was determined by the Bradford Assay. Specific AP
activity was
calculated as AP/protein unit. AP activity values were normalized to that of
150ng/ml OP-
1 (= 1) and represent the means of 8-12 independent determinations using 3
different FRC
cell preparations.
As shown in Fig. 1, IL-6 alone in the concentration range tested did not
stimulate the basal AP activity. SIL-6R alone stimulated the basal AP activity
slightly;
however, the stimulation did not seem to be sIL-6R dose-dependent.
Fig. 1 further demonstrates that IL-6 potentiates the OP-1-induced AP
activity in a dose-dependent manner. A maximum of about 2-fold stimulation was
observed
(p<0.05). SIL-6R also potentiated the OP-1-induced AP activity in a dose-
dependent
manner. A higher fold (about 3.5-fold; p<0.02) of stimulation than observed
with IL-6 was
achieved.
The effect of IL-6/R on the OP-1-induced AP activity was also examined.
At the highest tested dose of IL-6 plus its soluble receptor (100ng/ml IL-6
and 125ng/ml
sIL,-6R), the OP-1-induced AP activity was synergistically enhanced by about
10-fold. This
enhancement was reproducible. However, at the lower dose range, the IL-6/R
combination
did not appear to stimulate beyond what was achieved by either IL-6 or its
receptor alone;
on the contrary, the combination appeared to suppress AP activity.
Example 2
Fig. 2 shows that IL-6 alone enhanced OP-1 action in a mineralized bone
nodule formation assay. FRC cells were grown in aMEN (supplemented with 5%
FBS, 30
p,g/ml gentamycin, 100 ~g/ml ascorbic acid and 5 mM (3-glycerolphosphate), and
treated
-41-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
for various durations of time with (1) solvent vehicle, (2) 200ng/ml OP-1, or
(3) 200ng/ml
OP-1 plus IL-6 at various concentrations. The culture media were replenished
with the
same treatment agents) every three days. Progress of nodule formation was
monitored
every three days. After a total of 15 days, cells were fixed with formalin and
photographed.
As seen in Fig. 3, IL-6/R also enhanced OP-1's ability to induce the formation
of
mineralized bone nodules.
Example 3
To determine whether IL-6/R effects its synergy with OP-1 by directly
stimulating OP-1 responsive cells or by increasing the number of OP-1
responsive cells,
primary cultures of FRC were used as a model system, in which AP activity
levels were
used as a biochemical marker of OP-1 responsiveness. Histochemical data showed
that the
number of AP positive cells in cultures treated with IL-6/R (40 ng/ml IL-6 and
50 ng/ml
sIL-6R) and OP-1 (200 ng/ml) was similar to that in cultures treated with OP-1
alone (200
ng/ml). However, the AP activity level was higher in the former cultures than
the latter
cultures. IL-6 alone (40 ng/ml) did not stimulate AP positive cells; and sIL-
6R alone (50
ng/ml) or IL-6R (40 ng/ml IL-6 and 50 ng/ml sIL-6R) stimulated AP positive
cells to a
smaller extent, as compared to the combination of IL-6R and OP-1. These data
suggest
that IL-6/R's synergistic effect on OP-1 results from IL-6/R's direct
stimulation of OP-1
responsive cells.
Example 4
To investigate whether IL-6/R stimulates the expression of OP-1 receptors
on FRC cells, the mRNA levels of three BMP type I receptors (BMPR-IA, BMPR-IB,
and
ActR-I) and one BMP type II receptor (BMPR-II) were measured by Northern blot
analysis.
Briefly, confluent FRC cells were treated for 48 hours with (1) a vehicle; (2)
200 ng/ml OP-l; (3) 40 ng/ml IL-6 and 50 ng/ml sIL-6R; or (4) 200 ng/ml OP-1,
40 ng/ml
IL-6, and 50 ng/ml sIL-6R. Total RNA was isolated using the TRI reagent
(Sigma) and
loaded onto an AGAROSE GTG (FMC) gel containing formaldehyde. Northern blots
were prepared and probed with 32P-labeled cDNA encoding for the various BMPRs.
These
probes hybridized only to mRNA. The radioactive bands were detected and
quantified
using a PHOSPHORIMAGER (Molecular Dynamics, Sunnyvale, CA). To normalize the
-42-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
band intensity of the BMPR bands, the blots were also probed with an
oligonucleotide for
the 18 S rRNA.
The mRNA levels in control FRC cells, OP-1-treated cells, IL-6!R-treated
cells, and (OP-1 + IL-6/R)-treated cells were compared (Fig. 4). The data
showed that
OP-1 did not affect the mRNA level of the type I receptors, but stimulated the
BNIPR-II
mRNA level by about 2.2 fold. Likewise, IL-6/R did not alter the mRNA
expression level
of the type I receptors, but increased the BMPR-II mRNA level by about 1.5-
fold. In the
presence of OP-1 and IL-6/R, the mRNA level of the type I receptors was not
significantly
changed; however, the BMPR-II mRNA level was almost 3-fold higher than the
control.
These results suggest that IL-6/R can stimulate the osteogenic activity of OP-
1 by elevating
BMPR-II mRNA expression.
Example 5
The OP-1 protein used in Examples 1-5 was provided exogenously to the
test cells. To investigate whether the same IL-6/R synergistic effect would be
observed
when the OP-1 protein was expressed intracellularly in test cells, FRC cells
were
transfected with pW24, a plasmid carrying an OP-1 coding sequence under the
control of
the CMV promoter.
Briefly, confluent FRC cells were transfected with pW24 (2 ~g/ml). After
recovery, the transfected cells were treated with exogenous sIL-6R alone or IL-
6/R for 24
hours. Then the total AP activity levels were determined (Fig. 5).
The data showed that the levels of OP-1-induced AP activity in pW24-
transfected cells were enhanced by sIL-6R in a dose-dependent manner. At a
concentration
of 75 ng/ml, sIL-6R stimulated the OP-1-induced AP activity by as much as 4
fold.
The data also showed that the levels of OP-1-induced AP activity in pW24-
transfected cells were also enhanced by IL-6/R in a dose-dependent manner. A
2.5-fold
stimulation of the OP-1-induced AP activity was observed when IL-6/R was
applied to the
test cells at concentrations of 60 ng/ml for IL-6 and 75 ng/ml for sIL-6R.
-43-
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
1/10
SEQUENCE LISTING
<110> STRYKER CORPORATION
<120> COMPOSITIONS AND THERAPEUTIC METHODS USING MORPHOGENIC
PROTEINS, HORMONES AND HORMONE RECEPTORS
<130> STK-4
<140>
<141>
<150> 60/156,261
<151> 1999-09-27
<160> 12
<170> PatentIn Ver. 2.1
<210> 1
<211> 1822
<212> DNA
<213> Homo Sapiens
<220>
<221> CDS
<222> (49)..(1341)
<400> 1
ggtgcgggcc cggagcccgg agcccgggta gcgcgtagag ccggcgcg atg cac gtg 57
Met His Val
1
cgc tca ctg cga get gcg gcg ccg cac agc ttc gtg gcg ctc tgg gca 105
Arg Ser Leu Arg Ala Ala Ala Pro His Ser Phe Val Ala Leu Trp Ala
10 15
ccc ctg ttc ctg ctg cgc tcc gcc ctg gcc gac ttc agc ctg gac aac 153
Pro Leu Phe Leu Leu Arg Ser Ala Leu Ala Asp Phe Ser Leu Asp Asn
20 25 30 35
gag gtg cac tcg agc ttc atc cac cgg cgc ctc cgc agc cag gag cgg 201
Glu Val His Ser Ser Phe Ile His Arg Arg Leu Arg Ser Gln Glu Arg
40 45 50
cgg gag atg cag cgc gag atc ctc tcc att ttg ggc ttg ccc cac cgc 249
Arg Glu Met Gln Arg Glu Ile Leu Ser Ile Leu Gly Leu Pro His Arg
55 60 65
ccg cgc ccg cac ctc cag ggc aag cac aac tcg gca ccc atg ttc atg 297
Pro Arg Pro His Leu Gln Gly Lys His Asn Ser Ala Pro Met Phe Met
70 75 80
ctg gac ctg tac aac gcc atg gcg gtg gag gag ggc ggc ggg ccc ggc 345
Leu Asp Leu Tyr Asn Ala Met Ala Val Glu Glu Gly Gly Gly Pro Gly
85 90 95
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
2/10
ggc cag ggc ttc tcc tac ccc tac aag gcc gtc ttc agt acc cag ggc 393
Gly Gln Gly Phe Ser Tyr Pro Tyr Lys Ala Val Phe Ser Thr Gln Gly
100 105 110 115
ccc cct ctg gcc agc ctg caa gat agc cat ttc ctc acc gac gcc gac 441
Pro Pro Leu Ala Ser Leu Gln Asp Ser His Phe Leu Thr Asp Ala Asp
120 125 130
atg gtc atg agc ttc gtc aac ctc gtg gaa cat gac aag gaa ttc ttc 489
Met Val Met Ser Phe Val Asn Leu Val Glu His Asp Lys Glu Phe Phe
135 140 145
cac cca cgc tac cac cat cga gag ttc cgg ttt gat ctt tcc aag atc 537
His Pro Arg Tyr His His Arg Glu Phe Arg Phe Asp Leu Ser Lys Ile
150 155 160
cca gaa ggg gaa get gtc acg gca gcc gaa ttc cgg atc tac aag gac 585
Pro Glu Gly Glu Ala Val Thr Ala Ala Glu Phe Arg Ile Tyr Lys Asp
165 170 175
tac atc cgg gaa cgc ttc gac aat gag acg ttc cgg atc agc gtt tat 633
Tyr Ile Arg Glu Arg Phe Asp Asn Glu Thr Phe Arg Ile Ser Val Tyr
180 185 190 195
cag gtg ctc cag gag cac ttg ggc agg gaa tcg gat ctc ttc ctg ctc 681
Gln Val Leu Gln Glu His Leu Gly Arg Glu Ser Asp Leu Phe Leu Leu
200 205 210
gac agc cgt acc ctc tgg gcc tcg gag gag ggc tgg ctg gtg ttt gac 729
Asp Ser Arg Thr Leu Trp Ala Ser Glu Glu Gly Trp Leu Val Phe Asp
215 220 225
atc aca gcc acc agc aac cac tgg gtg gtc aat ccg cgg cac aac ctg 777
Ile Thr Ala Thr Ser Asn His Trp Val Val Asn Pro Arg His Asn Leu
230 235 240
ggc ctg cag ctc tcg gtg gag acg ctg gat ggg cag agc atc aac ccc 825
Gly Leu Gln Leu Ser Val Glu Thr Leu Asp Gly Gln Ser Ile Asn Pro
245 250 255
aag ttg gcg ggc ctg att ggg cgg cac ggg ccc cag aac aag cag ccc 873
Lys Leu Ala Gly Leu Ile Gly Arg His Gly Pro Gln Asn Lys Gln Pro
260 265 270 275
ttc atg gtg get ttc ttc aag gcc acg gag gtc cac ttc cgc agc atc 921
Phe Met Val Ala Phe Phe Lys Ala Thr Glu Val His Phe Arg Ser Ile
280 285 290
cgg tcc acg ggg agc aaa cag cgc agc cag aac cgc tcc aag acg ccc 969
Arg Ser Thr Gly Ser Lys Gln Arg Ser Gln Asn Arg Ser Lys Thr Pro
295 300 305
aag aac cag gaa gcc ctg cgg atg gcc aac gtg gca gag aac agc agc 1017
Lys Asn Gln Glu Ala Leu Arg Met Ala Asn Val Ala Glu Asn Ser Ser
310 315 320
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
3/10
agc gac cag agg cag gcc tgt aag aag cac gag ctg tat gtc agc ttc 1065
Ser Asp Gln Arg Gln Ala Cys Lys Lys His Glu Leu Tyr Val Ser Phe
325 330 335
cga gac ctg ggc tgg cag gac tgg atc atc gcg cct gaa ggc tac gcc 1113
Arg Asp Leu Gly Trp Gln Asp Trp Ile Ile Ala Pro Glu Gly Tyr Ala
340 345 350 355
gcc tac tac tgt gag ggg gag tgt gcc ttc cct ctg aac tcc tac atg 1161
Ala Tyr Tyr Cys Glu Gly Glu Cys Ala Phe Pro Leu Asn Ser Tyr Met
360 365 370
aac gcc acc aac cac gcc atc gtg cag acg ctg gtc cac ttc atc aac 1209
Asn Ala Thr Asn His Ala Ile Val Gln Thr Leu Val His Phe Ile Asn
375 380 385
ccg gaa acg gtg ccc aag ccc tgc tgt gcg ccc acg cag ctc aat gcc 1257
Pro Glu Thr Val Pro Lys Pro Cys Cys Ala Pro Thr Gln Leu Asn Ala
390 395 400
atc tcc gtc ctc tac ttc gat gac agc tcc aac gtc atc ctg aag aaa 1305
Ile Ser Val Leu Tyr Phe Asp Asp Ser Ser Asn Val Ile Leu Lys Lys
405 410 415
tac aga aac atg gtg gtc cgg gcc tgt ggc tgc cac tagctcctcc 1351
Tyr Arg Asn Met Val Val Arg Ala Cys Gly Cys His
420 425 430
gagaattcag accctttggg gccaagtttt tctggatcct ccattgctcg ccttggccag 1411
gaaccagcag accaactgcc ttttgtgaga ccttcccctc cctatcccca actttaaagg 1471
tgtgagagta ttaggaaaca tgagcagcat atggcttttg atcagttttt cagtggcagc 1531
atccaatgaa caagatccta caagctgtgc aggcaaaacc tagcaggaaa aaaaaacaac 1591
gcataaagaa aaatggccgg gccaggtcat tggctgggaa gtctcagcca tgcacggact 1651
cgtttccaga ggtaattatg agcgcctacc agccaggcca cccagccgtg ggaggaaggg 1711
ggcgtggcaa ggggtgggca cattggtgtc tgtgcgaaag gaaaattgac ccggaagttc 1771
ctgtaataaa tgtcacaata aaacgaatga atgaaaaaaa aaaaaaaaaa a 1822
<210> 2
<211> 431
<212> PRT
<213> Homo Sapiens
<400> 2
Met His Val Arg Ser Leu Arg Ala Ala Ala Pro His Ser Phe Val Ala
1 5 10 15
Leu Trp Ala Pro Leu Phe Leu Leu Arg Ser Ala Leu Ala Asp Phe Ser
20 25 30
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
4/ 10
Leu Asp Asn Glu Val His Ser Ser Phe Ile His Arg Arg Leu Arg Ser
35 40 45
Gln Glu Arg Arg Glu Met Gln Arg Glu Ile Leu Ser Ile Leu Gly Leu
50 55 60
Pro His Arg Pro Arg Pro His Leu Gln Gly Lys His Asn Ser Ala Pro
65 70 75 80
Met Phe Met Leu Asp Leu Tyr Asn Ala Met Ala Val Glu Glu Gly Gly
85 90 95
Gly Pro Gly Gly Gln Gly Phe Ser Tyr Pro Tyr Lys Al.a Val Phe Ser
100 105 110
Thr Gln Gly Pro Pro Leu Ala Ser Leu Gln Asp Ser His Phe Leu Thr
115 120 125
Asp Ala Asp Met Val Met Ser Phe Val Asn Leu Val Glu His Asp Lys
130 135 140
Glu Phe Phe His Pro Arg Tyr His His Arg Glu Phe Arg Phe Asp Leu
145 150 155 160
Ser Lys Ile Pro Glu Gly Glu Ala Val Thr Ala Ala Glu Phe Arg Ile
165 170 175
Tyr Lys Asp Tyr Ile Arg Glu Arg Phe Asp Asn Glu Thr Phe Arg Ile
180 185 190
Ser Val Tyr Gln Val Leu Gln Glu His Leu Gly Arg Glu Ser Asp Leu
195 200 205
Phe Leu Leu Asp Ser Arg Thr Leu Trp Ala Ser Glu Glu Gly Trp Leu
210 215 220
Val Phe Asp Ile Thr Ala Thr Ser Asn His Trp Val Val Asn Pro Arg
225 230 235 240
His Asn Leu Gly Leu Gln Leu Ser Val Glu Thr Leu Asp Gly Gln Ser
245 250 255
Ile Asn Pro Lys Leu Ala Gly Leu Ile Gly Arg His Gly Pro Gln Asn
260 265 270
Lys Gln Pro Phe Met Val Ala Phe Phe Lys Ala Thr Glu Val His Phe
275 280 285
Arg Ser Ile Arg Ser Thr Gly Ser Lys Gln Arg Ser Gln Asn Arg Ser
290 295 300
Lys Thr Pro Lys Asn Gln Glu Ala Leu Arg Met Ala Asn Val Ala Glu
305 310 315 320
Asn Ser Ser Ser Asp Gln Arg Gln Ala Cys Lys Lys His Glu Leu Tyr
325 330 335
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
5/10
Val Ser Phe Arg Asp Leu Gly Trp Gln Asp Trp Ile Ile Ala Pro Glu
340 345 350
Gly Tyr Ala Ala Tyr Tyr Cys Glu Gly Glu Cys Ala Phe Pro Leu Asn
355 360 365
Ser Tyr Met Asn Ala Thr Asn His Ala Ile Val Gln Thr Leu Val His
370 375 380
Phe Ile Asn Pro Glu Thr Val Pro Lys Pro Cys Cys Ala Pro Thr Gln
385 390 395 400
Leu Asn Ala Ile Ser Val Leu Tyr Phe Asp Asp Ser Ser Asn Val Ile
405 410 415
Leu Lys Lys Tyr Arg Asn Met Val Val Arg Ala Cys Gly Cys His
420 425 430
<210> 3
<211> 102
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: OPX
<220>
<223> Each Xaa is independently selected from a
group of one or more specified amino acids as
defined in the specification.
<400> 3
Cys Xaa Xaa His Glu Leu Tyr Val Ser Phe Xaa Asp Leu Gly Trp Xaa
1 5 10 15
Asp Trp Xaa Ile Ala Pro Xaa Gly Tyr Xaa Ala Tyr Tyr Cys Glu Gly
20 25 30
Glu Cys Xaa Phe Pro Leu Xaa Ser Xaa Met Asn Ala Thr Asn His Ala
35 40 45
Ile Xaa Gln Xaa Leu Val His Xaa Xaa Xaa Pro Xaa Xaa Val Pro Lys
50 55 60
Xaa Cys Cys Ala Pro Thr Xaa Leu Xaa Ala Xaa Ser Val Leu Tyr Xaa
65 70 75 80
Asp Xaa Ser Xaa Asn Val Ile Leu Xaa Lys Xaa Arg Asn Met Val Val
85 90 95
Xaa Ala Cys Gly Cys His
100
<210> 4
<211> 97
<212> PRT
CA 02385887 2002-03-18
WO 01/23563 PCT/LTS00/26528
6/10
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Generic-Seq-7
<220>
<223> Each Xaa is independently selected from a
group of one or more specified amino acids as
defined in the specification.
<400> 4
Leu Xaa Xaa Xaa Phe Xaa Xaa Xaa Gly Trp Xaa Xaa Xaa Xaa Xaa Xaa
1 5 10 15
Pro Xaa Xaa Xaa Xaa Ala Xaa Tyr Cys Xaa Gly Xaa Cys Xaa Xaa Pro
20 25 30
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Asn His Ala Xaa Xaa Xaa Xaa Xaa
35 40 45
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Cys Cys Xaa Pro
50 55 60
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Leu Xaa Xaa Xaa Xaa Xaa Xaa Xaa
65 70 75 80
Val Xaa Leu Xaa Xaa Xaa Xaa Xaa Met Xaa Val Xaa Xaa Cys Xaa Cys
85 90 95
Xaa
<210> 5
<211> 102
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Generic-Seq-8
<220>
<223> Each Xaa is independently selected from a
group of one or more specified amino acids as
defined in the specification.
<400> 5
Cys Xaa Xaa Xaa Xaa Leu Xaa Xaa Xaa Phe Xaa Xaa Xaa Gly Trp Xaa
1 5 10 15
Xaa Xaa Xaa Xaa Xaa Pro Xaa Xaa Xaa Xaa Ala Xaa Tyr Cys Xaa Gly
20 25 30
Xaa Cys Xaa Xaa Pro Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Asn His Ala
35 40 45
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
50 55 60
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
7/10
Xaa Cys Cys Xaa Pro Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Leu Xaa Xaa
65 70 75 80
Xaa Xaa Xaa Xaa Xaa Val Xaa Leu Xaa Xaa Xaa Xaa Xaa Met Xaa Val
85 90 95
Xaa Xaa Cys Xaa Cys Xaa
100
<210> 6
<211> 97
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Generic-Seq-9
<220>
<223> Each Xaa is independently selected from a
group of one or more specified amino acids as
defined in the specification.
<400> 6
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
1 5 10 15
Pro Xaa Xaa Xaa Xaa Xaa Xaa Xaa Cys Xaa Gly Xaa Cys Xaa Xaa Xaa
20 25 30
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
35 40 45
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Cys Xaa Pro
50 55 60
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Leu Xaa Xaa Xaa Xaa Xaa Xaa Xaa
65 70 75 80
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Cys Xaa Cys
85 90 95
Xaa
<210> 7
<211> 102
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Generic-Seq-10
<220>
<223> Each Xaa is independently selected from a
group of one or more specified amino acids as
defined in the specification.
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
8/10
<400> 7
Cys Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
1 5 10 15
Xaa Xaa Xaa Xaa Xaa Pro Xaa Xaa Xaa Xaa Xaa Xaa Xaa Cys Xaa Gly
20 25 30
Xaa Cys Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
35 40 45
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
50 55 60
Xaa Xaa Cys Xaa Pro Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Leu Xaa Xaa
65 70 75 80
Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa
85 90 95
Xaa Xaa Cys Xaa Cys Xaa
100
<210> 8
<211> 5
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic
Peptide
<220>
<223> Each Xaa is independently selected from a
group of one or more specified amino acids as
defined in the specification.
<400> 8
Cys Xaa Xaa Xaa Xaa
1 5
<210> 9
<211> 5
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic
Peptide
<220>
<223> Each Xaa is independently selected from a
group of one or more specified amino acids as
defined in the specification.
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
9/10
<400> 9
Cys Xaa Xaa Xaa Xaa
1 5
<210> 10
<211> 102
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Generic amino
acid sequence
<220>
<223> Each Xaa may be any amino acid residue
<400> 10
Cys Xaa Xaa Xaa Xaa Leu Xaa Val Xaa Phe Xaa Asp Xaa Gly Trp Xaa
1 5 10 15
Xaa Trp Xaa Xaa Xaa Pro Xaa Gly Xaa Xaa Ala Xaa Tyr Cys Xaa Gly
20 25 30
Xaa Cys Xaa Xaa Pro Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Asn His Ala
35 40 45
Xaa Xaa Gln Xaa Xaa Val Xaa Xaa Xaa Asn Xaa Xaa Xaa Xaa Pro Xaa
50 55 60
Xaa Cys Cys Xaa Pro Xaa Xaa Xaa Xaa Xaa Xaa Xaa Xaa Leu Xaa Xaa
65 70 75 80
Xaa Xaa Xaa Xaa Xaa Val Xaa Leu Xaa Xaa Tyr Xaa Xaa Met Xaa Val
85 90 95
Xaa Xaa Cys Xaa Cys Xaa
100
<210> 11
<211> 96
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic
amino acid (COPS)
<400> 11
Leu Tyr Val Asp Phe Ser Asp Val Gly Trp Asp Asp Trp Ile Val Ala
1 5 10 15
Pro Pro Gly Tyr Gln Ala Phe Tyr Cys His Gly Glu Cys Pro Phe Pro
20 25 30
Leu Ala Asp His Phe Asn Ser Thr Asn His Ala Val Val Gln Thr Leu
35 40 45
CA 02385887 2002-03-18
WO 01/23563 PCT/US00/26528
10/10
Val Asn Ser Val Asn Ser Lys Ile Pro Lys Ala Cys Cys Val Pro Thr
50 55 60
Glu Leu Ser Ala Ile Ser Met Leu Tyr Leu Asp Glu Asn Glu Lys Val
65 70 75 80
Val Leu Lys Tyr Asn Gln Glu Met Val Val Glu Gly Cys Gly Cys Arg
85 90 95
<210> 12
<211> 96
<212> PRT
<213> Artificial Sequence
<220>
<223> Description of Artificial Sequence: Synthetic
amino acid (COP7)
<400> 12
Leu Tyr Val Asp Phe Ser Asp Val Gly Trp Asn Asp Trp Ile Val Ala
1 5 10 15
Pro Pro Gly Tyr His Ala Phe Tyr Cys His Gly Glu Cys Pro Phe Pro
20 25 30
Leu Ala Asp His Leu Asn Ser Thr Asn His Ala Val Val Gln Thr Leu
35 40 45
Val Asn Ser Val Asn Ser Lys Ile Pro Lys Ala Cys Cys Val Pro Thr
50 55 60
Glu Leu Ser Ala Ile Ser Met Leu Tyr Leu Asp Glu Asn Glu Lys Val
65 70 75 80
Val Leu Lys Tyr Asn Gln Glu Met Val Val Glu Gly Cys Gly Cys Arg
85 90 95