Note: Descriptions are shown in the official language in which they were submitted.
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
REACTIVE POLYMERIC VALVE,
DISPENSING DEVICES AND METHODS USING SAME
Technical Field of the Invention
The present invention is in the field of polymeric valves, and their use in
dispensing
systems and methods, particularly to polymeric valves useful in closed-loop
responsive drug
delivery devices.
Background of the Invention
One embodiment of the present invention relates to apparatus useful in
responsive
to devices capable of delivering drugs to a patient in a closed-loop system.
It may also be used
as a replacement for, or supplement to, body organism living systems for
medical or research
purposes.
Common therapeutic requirements that necessitate controlled release are: i) a
reduction in biological barriers to drug transport (e.g., the gastrointestinal
tract and liver first-
pass metabolism); ii) a reduction in the peaks and valleys of blood levels
leading to both
reduced drug-induced toxicity and an optimal pharmacological response; and
iii) the potential
for drug delivery at a specific region of the body (e.g., local or targeted
drug therapy).
Controlled release is typically achieved by incorporating (or 'encapsulating')
drugs in either
biodegradable or nondegradable polymers, which can precisely control the
release of the drug
2o to the body over specific times from a day to several years .
A major limitation in the usefulness of controlled release systems is that
these
implantable devices release drugs at a predetermined rate. Certain disease
states such as
diabetes, heart disease, hormonal disorders and cancer require drug
administration either at a
life-threatening moment or repeatedly at a certain, critical time of day.
Polymer drug delivery
systems require a detailed understanding of the diffusion of drugs out of the
tortuous pore
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
network in the polymer host matrix in order to manufacture predictable
products. The
development of highly detailed models for the release of drugs, including
effects such as
concentration-dependent diffusion and drug solubility, is hindered by the
complexity of the
pore network. Ordered micromachined pore networks in Si have been fabricated
to simplify
the modeling problem. Even the most reproducible polymer systems, however, are
not able to
respond to varying therapeutic needs in the patient.
Of the numerous types of responsive drug delivery systems investigated to
date, two
have reached the market: implantable infusion pumps and noninvasive
iontophoretic devices.
The implantable pumps have been investigated for more than ten years and are
currently used
to deliver insulin to patients with insulin-dependent diabetes. The most
significant
disadvantages to these systems are the requirement for major surgery and the
large implant
size.
Iontophoretic delivery systems are based on controlling the transport of ionic
drugs in
the presence of an externally applied electric field. The noninvasive
iontophoretic delivery
systems deliver drugs across the skin (i.e., transdermally). The disadvantages
of this approach
are that drug delivery is limited to small ionic drug molecules and that there
is potential
damage to the skin, as electricity is passed through the tissue. In addition,
metabolism of the
drug can occur before the drug reaches the systemic circulation.
Other approaches to responsive drug delivery appear to be less likely to
succeed.
Implants based on ultrasound can achieve only marginal increases in delivery
rate during
ultrasound over passive diffusion. Electromagnetically-mediated delivery
devices require a
large external electromagnet to induce changes in release rate from implants
containing
magnetic beads. Finally, several hydrogel-based, self regulated configurations
have been
proposed. Most of these systems are designed for insulin delivery and are
based on
2
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
immobilized glucose oxidase, which catalyzes the conversion of glucose to
gluconic acid.
The device responds to glucose by decreasing the pH in the microenvironment of
the
hydrogel, which can cause: (i) the hydrogel to swell by protonation of amine
residues of the
hydrogel, which decreases the resistance to diffusion of the insulin; and (ii)
an increase in the
solubility of insulin, which causes an increase in the driving force for
diffusion. While these
approaches have merit as currently practiced, they do not appear to be
designed with the rigor
of engineering principles, which will yield a configuration that could
actually be used
reliably. For example, after multiple on/off cycles of drug delivery, the
on/off drug delivery
ratio typically decreases. This suggests a short lifetime of such devices.
It is therefore an object of the invention to prepare smart implantable
devices with a
sufficiently long lifetime that are responsive to the patient's therapeutic
requirements and
deliver a certain amount of a drug in response to a biological stimulus.
Although described with respect to the field of drug delivery, it will be
appreciated
that similar advantages may obtain in other applications of the present
invention, including
~5 industrial, research and health care applications. Such advantages may
become apparent to
one of ordinary skill in the art, in light of the present disclosure or
through practice of the
invention.
Summary of the Invention
2o The present invention includes polymeric valves and valve devices. The
invention
also includes machines or instruments using those aspects of the invention.
The present
invention may also be used to upgrade, repair, or retrofit existing machines
or instruments
using methods and components known in the art. The present invention also
includes
methods and processes using the devices of the present invention, including
industrial
3
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
research and health care applications. The methods and processes of the
present invention
may be applied using procedures and protocols known and used in the arts to
which they
pertain.
The present invention may be used for artificial tissues and organs for
dispensing
biologically active compounds, such as antibiotics, hormones, drugs, (e.g.,
analgesics and
insulin) etc. The present invention may also find application in industrial
settings where
controllable dispensing of substances (e.g., lubricants, functional fluids,
oxidants or reducing
agents) is required.
Most General Valve
The present invention includes, in broadest terms, a valve governing an
opening in a
barrier material, comprising a reactive polymer adapted to reversibly increase
or decease in
size in response to a stimulus selected from the group of electrical,
chemical, electrochemical
or photochemical stimuli, so as to be capable of physically opening or closing
the opening in
response to a change in its electrical, chemical or electrochemical
environment. Accordingly,
the material used in accordance with the present invention may be barrier
materials selected
from any material capable of acting in accordance with the described function
in the
invention. including materials selected from the group consisting of
conducting polymers.
The conducting polymers may be selected from the group consisting of
polyanilines,
polypyrroles and polythiophenes, and mixtures thereof. The reactive polymer
may be
adapted to reversibly increase or decrease in size in response to an
electrical stimulus, and
additionally comprise an electrode in contact with the reactive polymer. The
reactive
polymer may be any polymer capable of reversible expansion and contraction in
response to a
4
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
stimuli, including those selected from the group consisting of hydrogels. The
hydrogel may
be selected from the group consisting of polyhydroxyethylmethacrylates.
In-Aperture Reactive Polymer
Also included in the present invention is, in broadest terms, a valve
governing an
opening in a barrier material having an interior surface, the valve comprising
a reactive
polymer disposed on the interior surface and adapted to reversibly increase or
decease in size
in response to a stimulus selected from the group of electrical, chemical,
electrochemical or
photochemical stimuli, so as to be capable of physically opening or closing
the opening in
response to a change in its electrical, chemical or electrochemical
environment. The barrier
1 o materials, and and reactive polymers may be as described above.
Reactive Polymer Mounted on Surface Opposing Aperture
The present invention also includes, in broadest terms, a valve governing an
opening
in a barrier material, the valve comprising: ( 1 ) a support member positioned
in opposition to
the opening; and (2) a reactive polymer disposed on the support member and
adapted to
1 > reversibly increase or decease in size in response to a stimulus selected
from the group of
electrical, chemical, electrochemical or photochemical stnnu 1l, so as to be
capable of
physically opening or closing the opening in response to a change in its
electrical. chemical,
electrochemical or photochemical environment. The barrier materials and
reactive polymers
may be as described above.
20 The valve may additionally comprise an impervious member disposed between
the
reactive polymer and the opening.
5
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Reactive Polymer Disposed in Crux of Angled Flap Piece Mounted on Surface
Opposing
Aperture
Also included in the present invention is, in broadest terms, a valve
governing an
opening in a barrier material, the valve comprising: ( 1 ) a support member
positioned in
opposition to the opening; (2) a closure member moveably attached to the
support member so
as to form an angle with the support member, and adapted to move between a
position
blocking the opening and a position away from the opening; and (3) a reactive
polymer
disposed in the angle and adapted to reversibly increase or decease in size in
response to a
stimulus selected from the group of electrical, chemical, electrochemical or
photochemical
stimuli. so as to be capable of moving said closure member between said
position blocking
the opening and the position away from the opening so as to open or close the
opening in
response to a change in the reactive polymer's electrical, chemical,
electrochemical or
photochemical environment.
The barrier material and reactive polymers may be as described above. The
reactive
polymer may be adapted to reversibly increase or decease in size in response
to an electrical
stimulus, and additionally comprise an electrode in contact with the reactive
polymer.
Tube Valve Device
The present invention also includes, in broadest terms, a valve governing an
opening
in a barrier material, the valve comprising: ( 1 ) a tubular container having
a longitudinal axis
2o aligned toward the opening; and (2) a reactive polymer disposed in the
tubular container and
adapted to reversibly increase or decease in size in response to a stimulus
selected from the
group of electrical, chemical. electrochemical or photochemical stimuli, such
that the reactive
polymer is capable of physically opening or closing the opening in response to
a change in its
6
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
electrical, chemical, electrochemical or photochemical environment. The
barrier materials
and reactive polymers may be as described above.
The valve may additionally comprise an impervious member disposed between the
reactive polymer and the opening. The reactive polymer may be adapted to
reversibly
increase or decease in size in response to an electrical stimulus, and may
additionally
comprise an electrode in contact with the reactive polymer.
Dumbbell-Shared Dispensing Device
Also included in the present invention is, in broadest terms, a device for
dispensing a
substance such as bioactive molecules and compounds, the device comprising: (
1 ) a first
to reservoir; and (2) a second reservoir; where the first and second reservoir
are connected by a
conduit having at least one opening and an interior surface, each opening
controlled by a
valve comprising a reactive polymer disposed on said interior surface and
adapted to
reversibly increase or decease in size in response to a stimulus selected from
the group of
electrical, chemical, electrochemical or photochemical stimuli, so as to be
capable of
physically opening or closing each opening in response to a change in its
electrical, chemical
or electrochemical environment.
The first and second reservoirs may be made of a barrier material as described
above.
such as those selected from the group consisting of conducting polymers as
described above.
The reactive polymer may be as described above.
2o The reactive polymer may be distributed substantially along the length of
the conduit.
The reactive polymer may be adapted to reversibly increase or decease in size
in response to
a chemical stimulus, and the conduit may have a plurality of apertures
distributed along the
length of the conduit so as to provide access to the reactive polymer from
outside the device.
Method Using Most General Valve
7
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
The present invention also includes, in broadest terms, a method of delivering
a
therapeutic agent, such as bioactive molecules and compunds to a tissue, the
method
comprising: (1) providing a reservoir of the therapeutic agent positioned so
as to provide the
therapeutic agent to the tissue, the reservoir comprising a barrier material
and having a valve
governing an opening in a barrier material comprising a reactive polymer
adapted to
reversibly increase or decease in size in response to a stimulus selected from
the group of
electrical, chemical, electrochemical or photochemical stimuli, so as to be
capable of
physically opening or closing the opening in response to the stimulus; and (2)
permitting the
stimulus to influence the reactive polymer so as to actuate the valve.
The stimulus may cause the reactive polymer to increase in size so as to cause
the
valve to open. The stimulus may also cause the reactive polymer to decrease in
size so as to
cause the valve to close. The therapeutic agent may treat a condition of the
tissue, the
stimulus may arise from the condition, and the stimulus may cause the valve to
open. The
therapeutic agent also may treat a condition of the tissue, the stimulus may
arise from the
treatment of the condition by the therapeutic agent, and the stimulus may
cause the valve to
close. In another embodiment the therapeutic agent may treats a condition of
the tissue, the
stimulus may arise from the condition, and the stimulus may cause the valve to
open, and
another stimulus may arise from the treatment of the condition by the
therapeutic agent, and
the other stimulus causes the valve to close.
?o Method Usin I~perture Reactive Polymer
Also included in the present invention is, in broadest terms, a method of
delivering a
therapeutic agent such as bioactive molecules and compounds to a tissue, the
method
comprising: (1) providing a reservoir of the therapeutic agent positioned so
as to provide the
therapeutic agent to the tissue, the reservoir comprising a barrier material
and having a valve
8
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
governing an opening in a barrier material having an interior surface, the
valve comprising a
reactive polymer disposed on the interior surface and adapted to reversibly
increase or
decease in size in response to a stimulus selected from the group of
electrical, chemical,
electrochemical or photochemical stimuli, so as to be capable of physically
opening or
closing the opening in response to the stimulus; and (2) permitting the
stimulus to influence
the reactive polymer so as to actuate the valve.
For example, the stimulus may cause the reactive polymer to increase in size
so as to
cause the valve to open; the stimulus may also cause the reactive polymer to
decrease in size
so as to cause the valve to close. Alternatively, the therapeutic agent also
may treat a
condition of the tissue, and the stimulus may arise from the condition, and
the stimulus may
cause the valve to open. In another embodiment the therapeutic agent may treat
a condition
of the tissue, the stimulus may arise from the treatment of the condition by
the therapeutic
agent, and the stimulus may cause the valve to close. The therapeutic agent
may alternatively
treat a condition of the tissue, the stimulus may arise from the condition,
the stimulus may
is cause the valve to open, and another stimulus may arise from the treatment
of the condition
by the therapeutic agent causing the valve to close.
Method LJsin;~ Reactive Polymer Mounted on Surface Opposing Aperture
The present invention also includes, in broadest terms, a method of delivering
a
therapeutic agent to a tissue, the method comprising: ( 1 ) providing a
reservoir of the
2o therapeutic agent positioned so as to provide the therapeutic agent to the
tissue, the reservoir
comprising a barrier material and having a valve governing an opening in a
barrier material,
the valve comprising: (i) a support member positioned in opposition to the
opening; and (ii) a
reactive polymer disposed on the support member and adapted to reversibly
increase or
decease in size in response to a stimulus selected from the group of
electrical, chemical,
9
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
electrochemical or photochemical stimuli, so as to be capable of physically
opening or
closing the opening in response to the stimulus; and (2) permitting the
stimulus to influence
the reactive polymer so as to actuate the valve.
As an example the stimulus may cause the reactive polymer to increase in size
so as to
cause the valve to open, or the stimulus may cause the reactive polymer to
decrease in size so
as to cause the valve to close. In another embodiment, the therapeutic agent
may treat a
condition of the tissue, the stimulus may arise from the condition, and the
stimulus may cause
the valve to open. The therapeutic agent might also treat a condition of the
tissue, the
stimulus may then arise from the treatment of the condition by the therapeutic
agent, and the
stimulus may cause the valve to close. Alternatively, the therapeutic agent
may treat a
condition of the tissue, the stimulus may then arise from the condition, the
stimulus may
cause the valve to open, and then another stimulus may arise from the
treatment of the
condition by the therapeutic agent causing the valve to close.
Therapeutic Agent Dispensing System Comprising Most General Valve
Also included in the present invention is, in broadest terms, a system for
delivering a
therapeutic agent to a tissue, the system comprising: ( 1 ) at least one
reservoir of the
therapeutic agent positioned so as to provide the therapeutic agent to the
tissue, each
reservoir comprising a barrier material having at least one opening; and (2)
each opening
having a valve governing it. comprising a reactive polymer adapted to
reversibly increase or
?0 decease in size in response to a stimulus selected from the group of
electrical, chemical,
electrochemical or photochemical stimuli, so as to be capable of physically
opening or
closing the opening in response to the stimulus. The reservoir may comprise
silicon, or may
comprise a polymeric material. The system may be in the form of a sheet-like
material
comprising a plurality of reservoirs.
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Synthetic Reservoir Material Comprising Most General Valve
The present invention also includes, in broadest terms, a synthetic barrier
material
adapted to dispense at least one substance, the system comprising: ( 1 ) a
sheet-like material
comprising a plurality of reservoirs containing each substance, each reservoir
having an
opening; and (2) each opening having a valve governing it comprising a
reactive polymer
adapted to reversibly increase or decease in size in response to a stimulus
selected from the
group of electrical, chemical, electrochemical or photochemical stimuli, so as
to be capable of
physically opening or closing the opening in response to the stimulus. The
sheet-like material
may additionally comprise a plurality of reservoirs containing each substance,
each reservoir
having an opening with a valve governing it, the valve adapted to irreversibly
open the
opening in response to a stimulus selected from the group of electrical,
chemical,
electrochemical or photochemical stimuli.
Valve Incorporating Hinged Flap
Also included in the present invention is, in broadest terms, a valve
governing an
opening in a barrier material, the valve comprising: (1 ) a support member
positioned in
opposition to the opening including a moveable member adapted to move from a
position
away from the opening to a position covering the opening; and (?) a reactive
polymer
disposed on the support member so as to engage the moveable member and adapted
to
reversibly increase and decease in size in response to a stimulus selected
from the group of
3o electrical, chemical, electrochemical or photochemical stimuli. so as to be
capable of
reversibly physically moving the moveable member from a position away from the
opening
to a position covering the opening in response to a change in its electrical,
chemical or
electrochemical environment. The moveable member may be hinged onto the
support
member. The reactive polymer may be disposed between the moveable member and
the
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
support member, so as to be capable of moving the moveable member
substantially
perpendicular to the surface of the support member.
Valve Incorporating Slide Gate
The present invention also includes, in broadest terms, a valve governing an
opening
in a barrier material, the valve comprising: (1) a support member positioned
in opposition to
the opening including a moveable member adapted to slide from a position away
from the
opening to a position covering the opening; and (2) a reactive polymer
disposed on the
support member so as to engage the moveable member and adapted to reversibly
increase and
decease in size in response to a stimulus selected from the group of
electrical, chemical,
1 o electrochemical or photochemical stimuli, so as to be capable of sliding
the moveable
member between a position away from the opening and a position covering the
opening in
response to a change in its electrical, chemical or electrochemical
environment.
Im,_plant Device
Also included in the present invention is, in broadest terms, an implantable
device for
~ 5 dispensing a substance, the device comprising a capsule comprising a
reservoir adapted to
contain the substance, the reservoir having at least one opening controlled by
a valve, the
valve comprising a reactive polymer adapted to reversibly increase or decease
in size in
response to a stimulus selected from the group of electrical, chemical,
electrochemical or
photochemical stimuli, so as to be capable of physically opening or closing
the opening in
2o response to a change in its electrical, chemical or electrochemical
environment.
The implantable device may additionally comprise telemetry circuitry adapted
to send
a signal in response to the operation of the device. The implantable device
may additionally
comprise telemetry circuitry adapted to receive a signal in response to the
operation of the
device. The implantable device may additionally comprise a second reservoir
containing a
12
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
substance adapted to clear each aperture. The implantable device may also
comprise a
second reservoir containing an anticoagulant substance adapted to reduce
coagulation about
each aperture.
Telemetry System with Implantable Device
The present invention also includes, in broadest terms, a telemetry system for
monitoring the dispensing of a substance, the system comprising: (1) an
implantable device
for dispensing the substance, comprising a capsule that comprises a reservoir
adapted to
contain the substance, the reservoir having at least one opening controlled by
a valve, the
valve comprising a reactive polymer adapted to reversibly increase or decease
in size in
response to a stimulus selected from the group of electrical, chemical,
electrochemical or
photochemical stimuli, so as to be capable of physically opening or closing
the opening in
response to a change in its electrical, chemical or electrochemical
environment, and
comprising telemetry circuitry adapted to send a signal in response to the
operation of the
device; and (2) a remote device adapted to receive the signal from the
telemetry circuitry.
The remote device may be adapted to transmit a signal, and may additionally
comprise telemetry circuitry adapted to receive a signal from the remote
device. The
telemetry system may additionally comprise a second reservoir containing a
calibrant
substance adapted to calibrate the telemetry circuitry. The telemetry system
may also
comprise a second reservoir containing a calibrant substance adapted to
calibrate the
20 telemetry circuitry.
13
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Brief Description of the Drawings
Figure I is a side elevational sectional view of a device in accordance with
one
embodiment of the present invention.
Figure 2 is a side elevational sectional view of a device in accordance with
one
embodiment of the present invention.
Figure 3 is a side elevational sectional view of a device in accordance with
one
embodiment of the present invention.
Figure 4 is a perspective sectional view of a device in accordance with one
I o embodiment of the present invention.
Figure ~ is a side elevational sectional view of a device in accordance with
one
embodiment of the present invention.
Figure 6 is a side elevational sectional view of a device in accordance with
one
embodiment of the present invention.
IS
Detailed Description of the Preferred Embodiments)
In accordance with the foregoing summary, the following presents a detailed
description of the preferred embodiment of the invention that is currently
considered to be the
best mode.
2o The present invention includes several valve designs described herein.
The present invention also includes in vivo. telemetric responsive drug
delivery
systems such as a telemetric pill or a telemetric Norplant-like system. Both
the telemetric pill
and Norplant may be administered under local anesthesia without major surgery.
The
implants may have tiny orifices equipped with small irreversible metal valves
(TYPE 1) or
t4
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
reversible polymer valves (so-called "artificial muscle" valves) (TYPE II).
TYPE I based
systems may be used in cases where reversibility of drug delivery rate is not
a concern. They
may be simpler to make than polymeric valve based systems.
In a first embodiment of TYPE II polymeric valve devices, the valves may
simply
turn on and off drug release according to a square potential wave cycle, i.e.
they will be under
electrochemical control. A second-embodiment TYPE II implant may have a
separate
biosensor responding to the levels of an appropriate biological target
molecule (e.g. increase
or decrease in metabolic substrate, precursor, intermediate, product or by
product) and the
sensor may control the opening and closing of the polymeric valves via a small
battery. A
I o smart chip with any calculations and/or information storage and retrieval
and/or transmission
for therapeutics, sensor, and valve response characteristics may be
implemented in a third
embodiment. A miniaturized telemetry system may be employed for some
applications and
for testing various i~~ vivo drug delivery systems and components.
In a fourth embodiment, TYPE II drug delivery device, the biosensor may be
built
into the muscle material itself, and the opening and closing based on any
relevant
chemical/biological reaction, such as one that generates a metabolitic change,
such as a
change in pH. The latter may be accomplished, for instance, by an enzyme
reaction at the
muscle surface changing the local pH (e.g., glucose oaidase reacting with
glucose in the
patient's body) and thereby, swelling or shrinking the orifices of the drug
delivery reservoir
2o and automatically controlling the delivery rate of the dl'Llg (e.g..
insulin) to the patient.
Although, in principle, not needed in this case, the battery may still be used
as a safeguard
and as an additional means of control. Prototype drug delivery devices may be
tested on the
benchtop until separate biocompatibility studies warrant in vivo animal
testing. In vivo testing
IS
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
of initial sensor systems (e.g., potentiometric type Ca 2+, pH and COZ) may
start early in the
process.
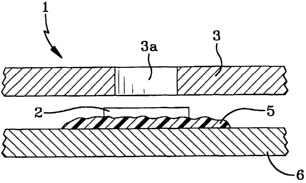
Figure 1 shows a valve 1 governing an opening in a barrier material 3. The
valve
utilizes a reactive polymer 5 attached to a barrier material 4 opposite the
opening, adapted to
reversibly increase or decrease in size in response to a stimulus such as
electrical, chemical,
electrochemical, or photochemical stimuli. When the polymer increases in size,
it expands
such that it effectively seals the opening, preventing material from passing
through. The
polymer may optionally have a plate 2 or other impervious member on its
surface. The plate
2 or other impervious member should be capable of sufficiently preventing
material from
1o passing through the opening when the polymer 5 expands and applies pressure
such that the
plate 2 or impervious member covers the opening. Additional intermediate
members such as
the plate 2 are preferred to effect closure as the hydrogel may be somewhat
porous. The
barrier material is preferably selected from conducting polymers such as
polyanilines,
polypyrroles and polythiophenes, or mixtures thereof. The reactive polymer is
preferably a
> > hydrogel such as polyhydroxyehtylmethacrylate.
Figure 2 shows another embodiment of a valve 6 governing an opening in a
barrier
material 8. The valve utilizes an intermediate support member 7 attached to a
barrier material
9 opposite the opening which is adapted to move from a position away from the
opening to a
position covering the opening. The valve also uses a reactive polymer 10
disposed on the
20 support member, adapted to reversibly increase or decrease in size in
response to a stimulus
such as electrical, chemical, electrochemical, or photochemical stimuli. When
the polymer
increases in size, it physically moves the movable support member 7 from a
position away
from the opening to a position covering the opening, effectively sealing the
opening and
preventing material from passing through.
16
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Figure 3 shows another embodiment of a valve 11 governing an opening in a
barrier
material 13. The valve comprises a tubular container 14 having a longitudinal
axis aligned
toward the opening, and a reactive polymer 15 disposed in the tubular
container 14, adapted
to reversibly increase or decrease in size in response to a stimulus such as
electrical,
chemical, electrochemical, or photochemical stimuli such that the polymer is
capable of
physically opening or closing the opening in response to a change in its
electrical, chemical,
electrochemical, or photochemical environment. The polymer may optionally have
a plate 12
or other impervious member on its surface. The plate 12 or impervious member
should be
capable of sufficiently preventing material from passing through the opening
when the
polymer 15 expands and applies pressure such that the plate 12 or impervious
member closes
the opening.
Figure 4 shows another embodiment of a valve 16 governing an opening 20 in a
barrier material 18 having an interior surface. The barrier material may have
an adjacent
upper 17 and lower 19 conductive material on its respective surfaces with
similar openings.
1 s The valve comprises a reactive polymer 21 disposed on the interior surface
of the opening 20,
adapted to reversibly increase or decrease in size in response to a stimulus
such as electrical,
chemical, electrochemical, or photochemical stimuli such that the polymer is
capable of
physically opening or closing the opening 20 in response to a change in its
electrical,
chemical, electrochemical, or photochemical environment.
Figure ~ shows a dumbell-shaped device 22 for dispensing a substance such as
molecules, the device being made of a barrier material 23. The device contains
a first
reservoir 24 and a second reservoir 2~, the two reservoirs connected by a
conduit having at
least one opening 27. Each opening 27 is controlled by a valve comprising a
reactive
polymer 26 adapted to reversibly increase or decrease in size in response to a
stimulus such
17
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
as electrical, chemical, electrochemical, or photochemical stimuli such that
the polymer is
capable of physically opening or closing the opening in response to a change
in its electrical,
chemical, electrochemical, or photochemical environment. The reactive polymer
26 may be
distributed substantially along the length of the conduit.
Figure 6 shows an implantable device 28 for dispensing a substance. The device
28
comprises a capsule with a reservoir 35 adapted to contain the substance, the
reservoir having
at least one opening 32 controlled by a reversible valve of the present
invention. The device
28 may also comprise telemetry circuitry 20 adapted to send a signal in
response to the
operation of the device 28 as measured by a biosensor 31, which may be
contained in the
1 o device. An enclosed battery 29 may power the device. Substance passing
through the
openings) 32 in the drug reservoir 35 may then pass into the body through an
artificial
muscle membrane 33 comprising the outer wall of the capsule, through a
biocompatible
permeable outer membrane 34 and into the body. The device of the present
invention may
include or be used in conjunction with components of telemetric devices and
systems such as
t 5 sensors. data storage and retrieval circuitry and microprocessors,
transceivers, antennas. etc.
known Ill the art.
The many embodiments of the present invention make possible several
applications
for clinical, research, medical and industrial purposes. including examples
described below.
The lifetime of the implants may be determined principally by that of the in
viva
20 biosensor. Telemetry and controlled release may be used to study and extend
in vivo
biosensor lifetime. For example, one may use the present invention in
conjunction with
simple ion-selective electrodes (ISE's), gas permeable membranes, as well as
with enzyme-
and immuno- based biosensors. One may also study biocompatibility as another
example,
biocompatible polymers, and may use heparin and/or herudin anticoagulant drug
release
18
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
reservoirs, to keep the sensor surface functional for longer periods of time.
As another
example, simple screening tests may be performed in cell cultures
(fibroblasts), but more
complete informative tests may be performed in laboratory animal models. The
sensors
implanted in untethered animals may be studied telemetrically, and the sensor
results may be
compared with those of blood data from the laboratory.
The drug delivery systems may be tested in vitro. The artificial muscle
composition
and its modifications (e.g., incorporation of the sensor into the muscle) can
then be
optimized, and drug compatibility with the various BIO-MEMS components tested.
For example, melatonin/serotonin and insulin may be used as models for a
responsive
drug delivery system. Melatonin/serotonin regulates circadian rhythms and
insulin regulates
glucose levels. Additionally, a device for maintaining bone
fonnation/resorption can be
made using the present invention.
A drug/target molecule pair may be modeled for any given application. For
example,
models may be developed to establish the required mass of insulin or melatonin
incorporated
into a Norplant-like device of small volume (e.g., 136 mm'). For example rough
calculations
for the case of melatonin, based on the total body clearance (CL 100 mL/min
and
therapeutic concentration of melatonin (C~,,~~~100 pg/mL) yield a total of ~~
mg of melatonin
required to maintain Ctn~~ for a period of 1 year [Ex. mass of melatonin =
release rate x time
of release - CL x C~ne~ x time of release = 100 mL/min x 100 pg/mL x 1 y x (1
mg/109 pg) x
(60 min/h) x (24 h/day) x (365 day/y) = 5.3 mg]. An amount of 5 mg (assuming 1
g/cm3) will
only occupy 5% of the volume of the reservoir of the device (assuming 75% of
the device is
drug reservoir). In addition, if more reservoir volume is required, several of
these implants
may be administered (typically, up to six cylinders can be administered
simultaneously) as is
t9
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
routinely done for the Norplant system. Therefore, the volume of the device is
within
acceptable volume range for this example application.
For another example, the leakage rate of the selected drug molecules through
open
and closed metal (TYPE I) and polymer (TYPE II) valves may be calculated for
various
embodiments of the proposed valves. In this regard, the plunger approach
(e.g., Figures 1 and
5) and tube approach (e.g., Figure 3) may be more viable than the sphincter
approach
because, in the closed state, the drug has to move out laterally through a
thicker layer of
polymer (if no intermediate member is used). The model data may serve as input
for the
BIO-MEMS devices.
The expansion of the hydrogel triggered by an enzymatic reaction, upon sensing
a
specific analyte of interest, may result in the closing of the delivery of the
drug through a
pinhole. In anotherembodiment, an antibody, as well as an antigen-glucose
oxidase
conjugate, may be immobilized on a polymer by covalent immobilization. In the
absence of
antigen, glucose may not reach the active site of the antigen-glucose oxidase
conjugate
(prepared by conventional chemical conjugation methods) because of steric
hindrance. When
antigen enters the muscle, it may compete with the conjugate for the antigen
binding site on
the antibody. Thus. the conjugate may be released, which may allow for the
enzymatic
reaction to proceed, triggering the opening of the muscle.
Electropolymerization of PANI/hydrogel may be conducted from a mixture of a
2o hydrogel and a monomer (0.1 M aniline in 0.5 M HzSO:~) at - 0.2 V to + 0.8
V vs SCE by
using a potentiostat/galvanostat. The electrochemical deposition parameters
may be further
optimized, such as through the use of alternative hydrogel and redox polymer
systems.
Appropriate materials, such as carbon or gold may be used initially as a valve
seat for the
artificial muscle.
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Different hydrogels may be used in conjunction with the redox polymers. One
hydrogel is an acrylamide. It may be prepared by combining specific volumes of
a filtered 40
wt % acrylamide solution, a 2 wt% N,N-methylenebisacrylamide (MBA) solution
and 98% 2-
(dimethylamino) ethyl methacrylate (DMAEMA). The mixture may be deoxygenated
by
bubbling NZ through it for 15 minutes. A volume of 10-20 ~L of potassium
persulfate
solution may then be added to initiate the polymerization reaction. A second
type of
hydrogel may be hydroxyethyl methacrylate (HEMA) based. A HEMA based hydrogel
may
be P(HEMA-co-MMA) and may be prepared by combining a co-monomer feed of 75
mol%
HEMA and 25 mol% MMA, with 1 mol % ethylene glycol dimethacrylate (EGDMA) as
the
1o cross-linking agent and a trace amount of dimethoxy phenyl acetophenone
(DMPA) as the
photoinitiator. The polymerizations will be carried out at ambient conditions.
Three
different compositions of PHEMA-DMAEMA may be prepared and tested. The first
may
consist of 0.198 HEMA, 0.0495 DMAEMA and 0.752 HBO. The second may be composed
of 0.198 I-IEMA, 0.0494 DMAEMA, 0.00220 EGDMA, 0.450 HBO and 0.300 ethylene
1, glycol. The compositions above are all in volume fractions. The third PHEMA-
DMAEMA
composition may be 76 wt% HEMA, 10 wt% DMAEMA, 2 wt% EGDMA. 12 w-t% H~O and
a trace amount of DMPA.
For characterization of the first embodiment TYPE I and II devices in vimo,
the
kinetics of the release of target drugs may be determined. Individual
controlled release
2o implants may be placed in a beaker containing phosphate buffer saline (pI-
I=7.4) at 37°C.
Lead wires from the implants may be connected to an external potentiostat,
which may be
controlled by computer interface. The input current may be determined from
information
gained. At pre-selected times, the buffer may be removed and replaced with
fresh media.
21
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Analysis of drug concentrations may be performed by HPLC to determine the
cumulative
mass of drug released with time.
The second-embodiment implants TYPE I and II may have a separate sensor
responding to the levels of the proper biological target molecule and the
sensor may control
the opening of metal valves and opening and closing of polymer valves via a
small battery.
One may then incorporate the artificial muscle into a pill or Norplant-like
responsive
delivery system. The in vitro delivery of melatonin may be demonstrated while
sensing the
serotonin level. Similarly, insulin may be delivered while monitoring glucose.
Ca2+ sensing
and release of vitamin D may be demonstrated.
A similar configuration has been successfully prepared for iontophoretic
delivery
using silicone rubber. In this configuration, the drug from the reservoir may
be released to a
passive reservoir (i.e., space between artificial muscle and biocompatible
permeable
membranes) when the artificial muscle valve opens. From the passive reservoir.
the drug may
diffuse through the biocompatible membrane and reach the body to successfully
deliver
1, melatonin for extended periods (> 1 year).
One concern is structural and chemical modifications of the implant surfaces
(tested
in cell cultures (fibroblasts) and in animals (rats)). A controlled release of
heparin and
herudin may be used to keep sensor membranes and components functioning.
Simple
screening tests of drug reservoirs and biosensors may be performed in a cell
culture
20 (osteoblasts and fibroblasts work), and more complete tests may be carried
out in laboratory
animal models.
Biocompatibility evaluation may be performed for responsive drug delivery
systems
involving glucose/insulin in rats and sheep, for responsive drug delivery with
melatonin/serotonin in rats and non-human primates, or for bone
formation/resorption with
CA 02386151 2002-04-03
WO 01/26708 PCT/LJS00/28215
cell cultures. Biocompatibility implies both acceptance of implants,
measurable as absence
of immune response, as well as function for a sufficient period of time to
allow physiological
measurements. Biocompatibility of implants over varying durations of
implantation may be
established. This may be done by histological examination of tissue changes in
proximity to
implants (e.g., scarification), whole animal health monitoring, and
engineering evaluation of
the function of probes during (via telemetry) and after varying times in the
animal body.
Cell cultures of appropriate cells (fibroblasts, osteoblasts) may be used to
test the
biocompatibility and effectiveness of biosensors measuring pH and Ca. These
sensors may
be evaluated in cell culture to determine cell response. Cell attachment,
proliferation,
extracellular matrix synthesis, and survival may all be measured. With these
results the
biosensors may be optimized to provide the most biocompatible system.
Following the
assessment of biocompatibility, the effectiveness of the sensors may be
measured.
Measurements obtained with the sensors in culture may then be compared to
conventional
measures of pH and Ca2+ from the culture media and cells.
1 > A simple type of biosensor that may be employed in the responsive drug
delivery
system is a potentiometric sensor including pI-I, CO~, and Ca'+ sensors. Each
sensor/transmitter combination may be evaluated usin<~ the following protocol.
Following
calibration in known standards, telemetry may be implanted in animal models.
Telemetric
data may be compared with blood data taken at regular intervals.
2o Implantable telemetric biosensors of various types are known and the
present
invention may be used in conjunction with them. For example, potentiometric pH
sensors
based on an ion-selective membrane work may well for up to 8-9 days in blood,
and
considerably longer in subcutaneous tissue; sensors may remain functional
after 12 weeks.
Beyond that, anticoagulants may be used to help further extend the lifetime of
the sensors. An
?3
CA 02386151 2002-04-03
WO 01/26708 PCT/IJS00/28215
approach may be developed to extend biosensor in vivo lifetime by applying
technology from
the controlled release field, i.e., delivering anticoagulants around the
sensor membrane from a
polymer reservoir using the present invention. The purpose of the external
sleeve in this
embodiment is for the incorporation of a polymer reservoir for controlled
release of an
anticoagulant such as herudin or heparin.
From a biocompatibility point of view, optimal surface configurations reduce
(or even
prevent) protein deposition on the implant surface, and reduce tissue capsule
formation
around the implant to a minimum. Both of these goals may be achieved
separately or
possibly simultaneously. This embodiment may be designed with two different
aspects in
1o mind:, biocompatibility of the passive part of the implant i.e., the drug
reservoir, and
biocompatibility of the active parts i.e., the sensor and valves.
During the last two decades, cell culture studies undertaken by different
investigative
groups revealed that cells and bacteria respond favorably to certain surface
topographical
patterns. These topographic features include infinite grooves, ridges, and
finite pillars and
~ 5 wells smaller than the average tissue cell. Cells can attach, elongate,
migrate, and reproduce
along specific topographical features. Structures having an undercut and being
in the 1 to 3
pm size range promote good anchorage of a cell to a substrate. The local
hydrophilicity of
the surface may be modified (chemically and by using coatings and plasma
etching). Some
cells, such as fibroblasts, may grow in hydrophilic areas but apparently have
difficulty
2o adhering and spreading onto hydrophobic areas. Sharp edge definition may
thus be obtained
along edges of hydrophilic-hydrophobic interfaces on a lithographically-
patterned surface.
Besides topography of the implant, the chemistry of the implant may be
modified. Both SEM
and TEM of the exposed surfaces and in-situ AFM/STM may be used to evaluate
cell
24
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
adhesion. For valves and biosensors (active implant components) the same
approaches may
be investigated but in addition, the controlled release sleeve approach may be
evaluated.
The biocompatibility of the membrane surface of the enzyme sensor may be
enhanced
using the present invention in order to prolong the service life of the sensor
in vivo.
Biocompatible materials may be produced by either covalently attaching
anticoagulants
directly to the surface of the membrane or by employing polymeric coatings
that mimic the
surface of biological membranes (i.e., "stealth" material). One such material
is poly(MPC-
co-BMA), where MPC stands for methacryloyloxyethyl phosphoryl choline and BMA
for n-
butylmethacrylate. Because of its phosphoryl choline content, this polymer
mimics the
1o surface of phospholipid-based biological membranes. This polymer, which
behaves as a
hydrogel and shows very low cell and protein adsorption, also has excellent
blood
compatibility. Poly(MPC-co-BMA) may be employed as coating material to improve
the
biocompatibility of other polymers. In particular, it has been used as the
coating layer in a
glucose biosensor. It was demonstrated that the permeability of glucose was
not reduced in
the presence of the MPC-co-BMA polymer. On the contrary, the sensor showed
improved
sensitivity to glucose even in the presence of plasma proteins. This can be
explained by
taking into account the resistance of this polymer towards protein adhesion.
which
significantly decreases the fouling of the sensor surface. Preliminary studies
with membranes
prepared with plasticized Tecoflex and doped with the ionophore valinomycin
demonstrated
?o excellent response to potassium and thus, show promise for the use of
poly(MPC-co-BMA)
as a biocompatible coating layer for sensors.
Once the individual elements of the sensor are developed, a sensor may be
fabricated
for in vitro evaluation. Parameters to be investigated and optimized may
include detection
limit and detection range, response time, selectivity, and longevity. These
tests may all be
?5
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
conducted by immersing the sensor in phosphate-buffered solutions that are
spiked with
appropriate levels of the test analyte, such as clonidine.
Using non-silicon materials, one may produce inexpensive biosensors and
microvalve
technology in substrate materials with the desired electrical and protein
adsorption
characteristics. Non-silicon microstructures are proposed because silicon will
not be of much
use for this large volume application because of the large chip size required
and because
silicon is not biocompatible. Furthermore, by making the substrate with the
embedded smart
valves large and flexible, one may be able to accommodate much larger drug
reservoirs than
typically possible in silicon. Moreover, silicon cannot be made into a
flexible three
1o dimensional shape which might line a drug delivery reservoir (e.g., a
Norplant implant).
Micromachining tasks may also include the introduction of certain surface
topographies and
surface chemistries on passive parts of the implant. In early prototypes it is
desirable to
fabricate the structure shown in Figure 1 in a non-silicon flexible material.
For flexible
substrate materials one may investigate materials such as polyimide, Riston (a
dry
1, photoresist) and SU-8. Secondly, it may be desirable to develop a
reversible electrorelease
system in which micromachined reversible polymeric valves are used. 13y
designing
inexpensive MEMS, valves that can open and close many times the state of the
art may be
significantly advanced.
At least four methods may be used to fabricate artificial muscle valve seats.
Three of
2o these methods are meant as research vehicles only. The fourth methodology
is based on
flexible 3 photoresists. Of these four methods, only one is currently
preferred to lead to
manufacturable products.
The first research method is based on flex circuit. To hold the artit7cial
muscle it is
necessary to find a way to fabricate arrays of microholes or valve seats in a
l7exible substrate.
26
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Ideally, the artificial muscle polymerizes only inside the hole and not on the
outside substrate.
To confine polymerization to the inside of the holes the generic structure may
have a metal
layer sandwiched between two insulator layers. In the flex-circuit method, a
gold foil with a
thickness of 100 mm may be spin-coated with a first layer of non-
photosensitive polyimide
(e.g., 25 pm thick). The coated gold foil may be heated at 55°C for 2
hours to cure the
polyimide. In order to create a polyimide/metal/polyimide sandwich structure,
another layer
of polyimide may be spin coated onto the opposite face of the gold foil (e.g.,
25 Vim). The
structure should be heated at 55°C for 2 hours again to cure the second
layer of resist. Then
holes with a diameter ranging from 10 - 40 ~m may be drilled by laser
ablation. The
t o efficiency of the opening and closing of these through holes may be
studied as a function of
their diameter.
The second research valve seat fabrication method involves screen printing of
carbon
paste. First, a layer of non-photosensitive polyimide may be spin-coated onto
a sacrificial
substrate. The coated substrate may then be heated for 2 hours to cure the
resist.
Subsequently, carbon paste may be screen-printed onto this substrate and
heated at 400°C for
1 hour. After removing the sacrificial substrate, another layer of~ polyimide
may be spin-
coated on top of the carbon paste, and then heated again. Laser ablation of
micro-holes may
result in a structure similar to that of the first method but with carbon as
the conductor
sandwiched between the two thin layers of insulator.
2o PANI/hydrogel artificial muscle may be grown electrochemically onto
sidewalls of
the newly created holes. Passing an adequate amount of charge can control the
growrth of the
polymer, and in situ monitoring of the growth is possible by means of a
compound
microscope. The most suitable polymeric and metallic valves for a given
application may
27
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
then be determined. Both metal and polymer valve embodiments may be adapted
based on
modeling results.
Carbon forms a very good valve seat as the artificial muscle grows on it very
well. It
is therefore desirable to make carbon based valve seats. Carbon films may be
made by
pyrolyzing a patterned photoresist. To make this structure, the following
microfabrication
steps may be required. First a layer of 4 - 5 ~m of positive photoresist, such
as AZ-4330,
may be spin-coated onto a passivated silicon wafer. The passivation layer will
typically be a
1000 !~ layer of thermal Si02. After protecting the resist-coated front side
of the wafer with a
mechanical fixture the backside of the wafer may be patterned and etched in a
anisotropic
to KOH etch to create a cavity. The KOH etch may stop at the Si02 passivation
layer (SiOZ
does etch only very slowly in KOH) leaving a thin membrane of resist and SiOZ
suspended
over the cavity. The photoresist on the front side may now be patterned with
the desired hole
structures. As in the case of the flex circuit approach, one may explore a
range of hole
diameters (from 2 pm to SO pm lIl this method). Next, the patterned resist on
the front of the
~ 5 wafer may be pyrolized at 900° C for 1 hour in a gas mixture of 70%
nitrogen and 30%
hydrogen (forming gas). After the pyrolysis step, the thin layer of SiO~ may
be etched away
in a diluted HF solution and a first thin coat of photoresist deposited on the
front surface of
the valve seat. This thin layer of resist may subsequently be exposed from the
back, using the
holes in the pyrolyzed carbon as the in situ mask, and developed. The process
may then be
20 repeated for a thin resist layer on the back of the valve seat. The two
resist layers in front and
back of the carbon layer may isolate the top and bottom carbon surfaces and
enable
electropolymerization inside the carbon holes only.
28
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Riston and other non-silicon flexible substrates may be developed further to
make
valves in flexible substrates, as these flexible substrates may provide good
potential liners for
a drug reservoir.
Drug deposition methodology may be developed (e.g., drop delivery systems,
silkscreening, etc.). For example, drugs may be enclosed in the microchambers
with room
temperature processes. Closing off the drug release microchambers may be one
of the most
challenging micromachining tasks. To enclose the drugs in the microchambers
one may use
a dry sheet of photoresist such as Riston. Dry photoresist can be laminated in
sheet form at
room temperature and is quite adhering to many polymeric substrates. Leakage
may be
1 o studied by using dyes enclosed in the chambers and studying their leakage
in a centrifuge
setup.
In order to ascertain the morphology of the PANI/hydrogel combination, SEM
studies
may be carried out. The smoothness of the surface may be examined carefully
because it
may have a great effect on the degree or completeness of the closing and
opening of the
holes. The smoothness of the surface may be, in turn, determined by the
uniformity of PANI
growth.
The swelling and shrinking of the PANI/hydrogel system may be monitored in
.sim.
The swelling and shrinking processes of the PANI/hydrogel system in response
to both
chemical and electrochemical actuation may be studied in depth by monitoring
the
2o phenomenon with a compound microscope. The microscope may be connected to a
video
monitor, video camera, and a color video printer. Water immersion lenses may
be used for in
situ monitoring of the swelling and shrinking of the muscle structure.
The swelling and shrinking phenomenon of the polyaniline/hydrogel blend may be
studied quantitatively at a molecular level by means of an atomic force
microscope. The size
29
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
of the smallest valve seats in the flex circuit configuration may be 10
microns and in the C-
MEMS structure they may be as small as 2 microns. The artificial muscle, in
its swollen
state, may then bring that diameter further down to perhaps the angstrom
level. It is unknown
how small the holes eventually get in the swollen state of the hydrogel. The
mechanism of
swelling and shrinking may be investigated in more detail by these AFM
studies. Also the
morphology i.e., smoothness of the redox polymer/hydrogel surface may be
studied
extensively using this technique.
In situ resistance measurement of the PANI/hydrogel structure may also be
carried out
as a means of controlling the opening and closing of the valve. One may use a
bi-potentiostat
for this purpose. Two working electrodes in the form of closely spaced lines
may be
fabricated, and the PANI/hydrogel system may be deposited such that the gap
between the
lines is bridged. This may act like a microelectrochemical transistor with a
source and a
drain connected by the channel material. Perturbation of the channel material
by applying a
gate voltage may lead to a drain current proportional to the conductivity of
the channel
material. The presence of a hydrogel in a PANI/hydrogel blend is believed to
enhance the
influx of protons into the polyaniline matrix, hence resulting in a larger
change in
conductivity of PANI than with PANI alone.
Transmitters may be further miniaturized into pill-sized devices in accordance
with
known microcircuitry manufacturing techniques. In vitro and in vivo telemetric
pH data may
be collected and compared. This telemetric approach to studying
biocompatibility of
membrane materials may be a major help for in vivo sensor development in
general. The
"Pill-Transmitter" measurement device may have several applications for
integrating with
new and emerging technologies such as advanced biosensors, genetic sensors,
Micro-Electro-
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Mechanical-Systems (MEMS), and nano- and meso-scale sensors and devices. A
variety of
signal-conditioning modules have been designed to incorporate a large number
of sensors.
A telemetric chemical/biological sensor may include potentiometric sensors,
amperometric sensors, and bridge-type sensors. Recent efforts have been
focused on
measuring pressure, temperature, and pH in-utero to help doctors monitor a
fetus inside the
womb after corrective fetal surgery. The surgery may be performed in the
uterus through
tube-like endoscopic instruments (trocars). The pill-shaped biotelemeter may
be
subsequently inserted through such a trocar to monitor the health of the
mother and the fetus
in the months after surgery.
1 o This "pill-transmitter" has generated considerable interest in the medical
community
and potential applications range from responsive drug delivery, monitoring
gastrointestinal
pH and pressure, to measurements of glucose, lactate, blood gases, and other
physiological
and biological parameters in astronauts, soldiers, firefighters, and research
animals. Short-
term development efforts are targeted towards the following parameters: pI-I,
dissolved
1 > oxygen, heart rate, and ECG. Experience gained in this program may be
invaluable for work
on the responsive drug delivery pill.
One may subsequently verify in vivo performance of pH-biotelemetry transmitter
in
rats. One may then modify a biotelemeter to incorporate COz and OZ sensors,
and design O
biotelemetry circuit as a future platform for amperometric biosensors, e.g.
glucose.
2o It may be desirable to demonstrate telemetric electrochemical rupture of
metal
membranes by releasing a dye into a solution (e.g.. beaker experiments). A
biotelemetry
system for controlling the release of the dye may be developed and tested.
This prototype
system may serve as a feasibility model and may be the basis for the final
implantable
miniaturized system. A miniature receiver can be integrated into the final
drug delivery
31
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
device, as well as a controller/actuator circuit responsible for the drug
release. One may
miniaturize the receiver module of drug delivery system using chip-on-board
technologies.
One may demonstrate telemetric electrochemical rupture of metal membranes by
releasing a dye of known pH into a solution (beaker experiments) and use the
pH sensor
incorporated into the same drug delivery device to monitor the release. This
step may add a
sensing element to the drug delivery prototype system of the present
invention. The pH
telemeter developed and tested may be used initially to monitor the pH changes
and then
modified to become a part of the drug delivery system.
A telemetric modulation of dye release may be demonstrated with polymeric
valves
(beaker experiments), regulating the release by measuring the pl-I with a pH
sensor
incorporated into the same drug delivery device. This step involves the
refinement of the
control circuitry to modulate the drug release, i.e. to allow the release of
precise amounts of
the drug over a defined time period. The amount of the released drug may
depend on the
measured pH; the loop between sensor and actuator may be closed. The result is
a complete
~ 5 and functional sensor-actuator model of the responsive drug delivery
system.
A sensor signal conditioning module of a drug delivery system may be minimized
using chip-on-board technologies. A control module of drug delivery system may
also be
miniaturized. A miniaturized Norplant-sized drug delivery system may be built
using the
modules of the present invention, which may then be tested in vitro. This step
may involve
finalizing the packaging design and incorporating a drug reservoir (see Figure
6).
Telemetric modulation of drug release may be demonstrated with polymeric
valves in
a rat. One may regulate the release by measuring the concentration of a
biological trigger
molecule with a selective biosensor incorporated into the same drug delivery
device. This
step uses the system described above to verify its performance in vivo.
J7
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Methods and Materials
Intelligent Redox Polymers and Hydrogels The present invention includes
polymer valves
(also smart valves or artificial muscle to open and close small holes in the
drug reservoir).
Many types of intelligent redox polymers and hydrogels may be used in these
valves.
Intelligent polymers are polymers that respond to changes in the environment
in a predictable
and reproducible fashion. Two types of polymers that may be used in the
construction of the
proposed polymer valves are conducting polymers and hydrogels.
Polyanilines belong to the most widely used class of conducting polymers that
show a
1 o variety of interesting properties such as electrochromism, conductivity
switching, and the
ability to store charge and sensitivity to pH change of its microenvironment.
Polyaniline
(PANI) has also been used as an immobilization matrix for various enzymes and
receptors.
Enzymes like glucose oxidase, urease, and lipase, etc., have been successfully
immobilized
into the PANI matrix by physical entrapment or electropolymerization from
monomer
solutions. Some promising applications of conducting polymer based actuators
are in
micromachining, actuator microflaps for aircraft wings, microscopic valves and
pumps for
parts of a "complete analytical laboratory on a chip", and in artificial
muscles for robotic
prosthetic devices. The operation of these actuators relies on mechanical
movement in
response to change in pH, charge, temperature, etc. Similar actuators have
been described for
polypyrrole.
Polymers most efficient in transforming molecular energy into mechanical
energy
may be hydrogels. A hydrogel is a water-swollen network (more or less cross-
linked) of
hydrophilic homo- or copolymers. Various conditions that may be used to
control the
hydrogel actuators are temperature change, light, radiation, electric field,
and change of pH of
33
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
the liquid. Electric fields have been used to bend an ionic polymer gel. A
"gel fish" has been
made to swim in response to the changing electric field on the hydrogel, and a
"Gel hand"
(gel fingers) has been constructed that will grab an egg softly without
breaking it. Hydrogels
have also been used extensively in controlled delivery systems in
pharmaceuticals and
medicine.
Microfabricated Responsive Drug Delivery Systems The BIO-MEMS approach to
closed-
loop responsive drug therapy has its genesis in the electrorelease of drugs.
An electrorelease
system has been described based on microporous membranes. In this system, the
molecules
to be released may be physically entrapped in pores of a porous membrane which
may be
covered in areas by a non-porous barrier layer material (e.g., a conductor
such as gold). A
second electrode, physically separated from the non-porous barrier layer, may
act as a
counter electrode. Release may be initiated by applying a small voltage
between the barrier
layer and the counter electrode and electrochemically dissolving or disrupting
the barrier
layer. The electrorelease rate would then be controlled by the number of pores
that are
electrochemically opened. The rate may also be varied by separating the
microporous
membrane into individual electrorelease zones. This system was further
improved in the
present invention by using BIO-MEMS devices based on metal and polymer.
In the present invention valves may be either thin metal films or blends of
redox
polymers and hydrogels.
l3iocompatibility of Passive and Active Components. Biocompatibility can be
described as
the sum of interactions between an implant and the surrounding tissue after
implantation.
Generally, there is a tissue reaction in response to injury and to the
presence of the implant.
34
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
The tissue response represents a healing attempt resulting in protein
depositions on the
implant and subsequent formation of a dense fibro-granulous tissue capsule
surrounding the
implant and isolating it from the body's internal tissue environment. The
degree of tissue
response is modified by the chemical and physical properties of the implant
itself and by the
modifications of the implant due to the tissue environment (corrosion,
degradation, swelling,
and embrittlement). Both the protein deposits on the implant and the tissue
capsule
surrounding it affect the properties and function of the implant itself. For
drug delivery and
sensor systems there are special potential contributors to tissue responses
that deal with the
designed function of the implant. Such special aspects relating to tissue
compatibility
1 o include: (a) the leaching of drugs and other leacheables from the implant
into the
implant/tissue interface, (b) the implant surface porosity (as dictated by
release control
features) which has been identified as a major modifier of tissue responses,
and (c) the
specific surface chemical and physico-chemical properties such as charge,
energy, pI-I, and
electrical charge changes, which all may be specifically designed to
facilitate and improve
t 5 sensing or delivery mechanisms but have their own distinct effect on
tissue responses.
Protein depositions on the implant and tissue capsules surrounding the
implant, on the other
hand impede proper sensing of tissue parameters and controlled delivery of
drugs into the
target tissues.
All of these interactions may play minimal roles for devices implanted for
less than
2o one week. But they may impede tissue acceptability and proper device
function significantly
when implanted for months to years. It may therefore be essential that every
design step be
discussed and tested with respect to its biocompatibility.
In the delivery implant of the present invention there is a passive'
component, i.e. the
drug reservoir, and two active' components, i.e. the biosensor and metal or
polymer valve.
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
For making passive components more biocompatible, biocompatible surface
chemistry/coatings may be used, and surface topography experimentally
modified. One goal
may be to minimize tissue response to the implant and abolish progressive
chronic
inflammatory response. The chronic inflammatory response can be controlled by
holding the
surface topography within a one micrometer range. Such topography may be
produced on
devices such as catheters by ion beam etching, micromachining, and casting
methods.
Histological responses to such engineered surfaces show elimination of
macrophages at the
interface and formation of a fibroblast layer which is adherent to the surface
and one to two
cell layers thick.
The biosensors and the polymer valves in the implant may involve immobilized
enzymes, antibodies, gas permeable membranes, ion selective membranes,
hydrogels, and
redox polymers, etc. Controlled release strategies may be used to deliver
anticoagulants such
as heparin and herudin over the sensor and valve surfaces to keep the sensor
membranes and
valves active. Telemetry may be used to monitor the continued functioning of
the in vivo
sensors and valves by comparing telemetric animal data with blood measurement
data Iron
the laboratory.
Chemical Sensors and Biosensors~. The simplest chemical sensors for responsive
drug
delivery may be electrochemical pH, Ca2+, and COZ sensors. These are all
potentiometric
devices (i.e., they measure a voltage) and their operation, in principle at
least, should pose
less problems for extending their lifetime in an irr vivo environment than
amperometric
devices (i.e., they measure a current) because the latter are area dependent.
More problematic
for in vivo operation are biosensors.
36
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
A biosensor is a device that combines a biologically active element with an
appropriate transducer. When designing such a sensor, three important
components need to
be considered: the nature of the biologically active element (e.g., enzyme,
antibody, binding
protein, etc.) which is responsible for the selectivity of the device, the
transducer which
provides the measurable signal or response, and the immobilization procedure
which defines
the efficiency of the interaction between the biologically active element and
the transducer.
Beyond the potentiometric probes listed above, enzyme biosensors may be used
to
regulate the delivery of drugs from the microfabricated drug reservoirs. Given
the
requirement to operate in vivo, the appropriate biosensors may need to meet
the following
conditions: (a) avoid use of toxic reagents, (b) be relatively small in size,
and (c) be reliable
over extended periods of time. The sensors used may monitor biochemical
indicators related
to a patient's health condition and activate a valve to release an appropriate
amount of drug.
If these enzyme sensors are used in vivo then no components should be capable
of
leaching out for long periods of time. Accordingly, immobilization schemes for
enzymes are
important. Several immobilization approaches have been employed in the
development of
enzyme biosensors. The immobilization of enzymes on surfaces may be
accomplished both
by physical and chemical methods. Physical methods of immobilization include
the
absorption of protein to surfaces by various weak interactions such as
electrostatic,
hydrophobic/hydrophilic, and van der Waals forces. Though the method is simple
and cost-
2o effective, it suffers from leaching of the protein from the immobilization
support. Physical
adsorption generally leads to dramatic changes in the protein
microenvironment. Polymer
entrapment involves the incorporation of an enzyme into a matrix (organic
polymer or'
hydrogel) that forces the enzyme to remain close to the electrode surface. One
way to
accomplish this is by entrapping the enzymes within electropolymerized films,
such as
37
CA 02386151 2002-04-03
WO 01/26708 PCT/IJS00/28215
polypyrrole, polyaniline, poly(o-phenylenediamine), and polyindole. In
addition, the enzyme
can be trapped on the surface of the sensor using semipermeable (dialysis)
membranes.
These semipermeable membranes have pores that are small enough to restrict
leaching of the
enzyme, but large enough to allow substrates to reach the enzyme. Attachment
of enzymes
by chemical means involves the formation of strong covalent or coordination
bonds between
the protein and the immobilization support. Covalent attachment typically
involves some
type of cross-linking agent, such as glutaraldehyde, or employs condensation
reactions where
a functionalized surface reacts with functional groups on the enzyme.
Sensors based on immobilized enzymes have been widely used in ire vivo
monitoring
1o (e.g., glucose), food monitoring (e.g., freshness), diagnostics (e.g.,
immunoassays), process
monitoring (e.g.. fermentation), and environmental monitoring (e.g.,
wastewater).
Immobilization can be defined as the attachment of target molecules to a
support resulting in
reduced or complete loss of mobility. Proteins have been immobilized on hollow
fiber
modules, packed beds, suspended particles, and in hydrogels. Immobilized
enzymes have
1 i become popular reagents in a variety of fields because they combine a high
specificity toward
a particular compound (or family of compounds) with the convenience and cost
effectiveness
of a reusable immobilized catalyst.
For the responsive insulin/glucose device of the present invention, a glucose
sensor
may be used. Glucose sensors have been studied more intensively than almost
any other
?o implantable sensor, therefore implantable glucose sensor work is being used
as a point of
reference. The concept of continuously monitoring glucose has been popular
since 1962. At
that time, a design of a sensor was published for use during cardiovascular-
surgery. During
the following decade, efforts were directed toward developing and testing
implantable
systems and enthusiasm for a device that could mimic the glucose/insulin
control system
38
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
rose. Besides the obvious advantage of serving as an artificial endocrine
pancreas. such a
system could be coupled with telemetry hardware and thereby give the patient
advance
warning of hypoglycemia.
Sensor thrust may be started with in vivo measurements on the previously
developed
potentiometric probes for pH, Ca2+, and COZ and their biocompatibility
improved. Enzymes
may then be affixed to the pH or COZ sensor and, upon reacting with their
substrates, a
resulting pH or CO~ change may be monitored. In all cases, the chemical sensor
responses
may be investigated to determine whether they are proportional to one of the
selected target
molecules. In most cases, only a change in pH or CO~ is needed rather than an
absolute value,
1 o making the challenge of long-time in vivo sensing somewhat easier. A
target for the in vivo
sensor performance may be reliable in vivo operation for at least one month.
Recently the
current problems with glucose sensors have been summarized in two general
areas: (i) the
reliability and stability of glucose monitoring methods, and (ii) algorithms
for subcutaneous
insulin infusion. The pH sensor of the present invention may be used as the
basis for the
i~~ vivo glucose sensor.
There may be no potentiometric serotonin/melatonin sensor available (in vivo
or in-
vitro) and no in vivn immunosensors are currently known. As in the case of the
glucose
sensor, sensing schemes may be developed for melatonin and serotonin based on
enzymes
that cause a change in pH which may be detected by the underlying pH sensor.
An in vivo
2o immunosensor may also be developed in accordance with the present
invention. The
immunosensor principle that may be used is an electrochemical one because of
size and
power consumption constraints. The combination of electrochemical detection
with an
immunological reaction may provide an analytical technique with excellent
selectivity and
detection limits. Electrochemical immunoassays based on labels that are
electroactive or
39
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
catalyze the production of an electroactive reaction product have been
developed and applied
to the determination of compounds of clinical and environmental importance.
Detection
limits as low as the zeptomole range (a few thousand molecules) have been
reached for the
determination of large molecules such as proteins, using microcapillary
immunoreactors with
electrochemical detection.
An immunosensor combines the elements of the immunoassay technique into a
single
device that responds directly to the concentration of the target analyte. The
analyte
determines the choice of antibody to provide selectivity for the sensor.
The present invention provides for the development of an electrochemical
immunosensor. The sensor concept may be generally applicable to the detection
of any
analyte for which a suitable antibody, binding protein, molecularly imprinted
polymer, or
other selective binding material exists and a suitable label can be attached
to the analyte
without substantially interfering with binding to the antibody. An
immunosensor based on a
recognition element such as an antibody has advantages relative to other
possible sensor
~ 5 types for the target application of an implantable sensor that would
control release of a
therapeutic drug. Antibodies have exceptional selectivity for a wide range of
molecules.
Antibodies have large binding constants with target molecules (Kf typically
106 to 1 Ol 1 ),
which translates to very low detection limits. lmmunosensors lend themselves
to the use of a
generic label for sensing many different species. Thus, a general sensor may
be developed
that is applicable to the sensing of a wide range of molecules with the target
analyte being
determined by the antibody.
Two of the main disadvantages of antibody-based sensors are not as important
in the
intended application of an implantable sensor. First, the fragility of the
biorecognition
element (e.g., an antibody) is of a lesser concern because the sensor may be
implanted in
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
mammals. This environment is friendly for biomolecules with respect to
parameters such as
temperature, pH, ionic strength, and exposure to toxic molecules that can
denature
biomolecules if not controlled. The antibody may be sequestered behind a
biocompatible
membrane where it may not be subject to destruction by the host's immune
defense system.
This problem is even further reduced where humanized monoclonal antibodies are
used, i.e.
antibodies in which the only non-human parts are the CDRs.
The second disadvantage is that antibodies bind rapidly with target molecules,
but
release them slowly as reflected by the large binding constant. This is a
problem for
applications that require rapid reversibility for the sensor. However, in many
applications
to rapid reversibility is not required (i.e., response times of up to an hour
are acceptable in many
applications). Whenever a faster response is required, antibodies with faster
off rates may be
used.
Micro Electro Mechanical Systems'. For over two decades silicon and integrated
circuit (IC)
fabrication techniques have been promoted as the optimum choice of material
and fabrication
methodology, respectively, for miniaturized chemical and mechanical sensors.
This was
largely based on the success of silicon and IC fabrication in the electronics
industry. The
advantages of small, planar, and batch fabricated sensors over serially
manufactured large
sensors in terms of size and cost reduction were obvious and the integration
of electronics
with the sensing function as well as the possibility of redundancy and
multifunctional arrays
were seen as additional desirable features. In the case of mechanical devices
such as
temperature sensors, pressure sensors, accelerometers, and gyros, etc. these
predicted
advantages have largely been proven correct and a small (currently about 1 %
of the IC
Industry) but growing MEMS industry resulted.
41
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
In cases where silicon is the only substrate and does not play any role in the
sensing
mechanism itself, as is the case with the current chemical/biological sensor
application, there
may be advantages in using silicon but these are often not as significant as
in the case of
mechanical sensors.
An overwhelming determining factor for substrate choice may be the final
package of
the device. A chemical sensor on an insulating substrate is almost always
easier to package
than a chemical sensor on a piece of silicon with conductive edges in need of
insulation.
Indeed, the saw cuts of an individual die may leave unpassivated silicon
sides, which are
exposed to the electrolyte. Sensor packaging is so important in sensors, and
especially in
chemical sensors, that, as a rule, sensor design should start by addressing
the issue of
packaging before that of the sensor itself. In this context, an easier to
package substrate has a
significant advantage. The latter is the most important reason why recent
chemical sensor
development in industry has retrenched from a move towards integration on
silicon in the
1970's and early 1980's, to a hybrid thick film on ceramic approach in the
late 1980's; and
~5 nineties. In academic circles in the US, chemical sensor integration with
electronics
continued until the late eighties; in Europe and Japan such efforts are still
ongoing.
There are other reasons why silicon and thin film technology may not be
optimum for
chemical sensor manufacturing. With hydrogels and membranes constituting most
chemical
sensors, optimum thicknesses are in the range of 20 to 100 Vim. Thus, thick
film processes are
20 more suited to the chemical sensor construction. Moreover, most chemical
sensor materials
are incompatible with IC processing. The very point of using silicon (i.e.,
its standardness) is
forfeited in a chemical sensor environment. Another area in which B10-MEMS
differs
considerably from mechanical MEMS applications is in the amount of integration
of
electronics on the chip and in how to make array elements. In IC technology,
integration is a
42
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
necessity, while in BIO-MEMS a modular approach is often preferred. Too much
integration
is a problem of central importance to the manufacturing yield of chemical and
biological
sensors.
Since it may be crucial to subject the chip to as few processing steps as
possible,
producing the electronics on a separate die from the bioprobe(s), and placing
it on-board only
if the application absolutely makes it essential is clearly a better approach.
Besides yield,
which is linked to materials incompatibility, another major problem associated
with
integrating electronics in chemical or biological sensors is leakage of liquid
leading to
shunting of the high impedance electronics.
t0 This points toward the need for a non-Silicon approach in building
disposable
inexpensive chemical and biological sensors and structures of the type of the
present
invention. The structure may be fabricated along with similar drug delivery
devices in a non-
silicon flexible material. The required electronics may be fabricated on a
separate chip well
isolated from the wet environment of the drug delivery reservoir. Rigid and
brittle silicon
t 5 may not be of use for this large volume application because the silicon
chip size that is
required may be much too large and silicon is not biocompatible. Moreover,
silicon cannot
be made into a flexible three-dimensional shape to line a drug delivery
reservoir (e.g.. a
Norplant implant). Furthermore, by making the substrate with the embedded
smart valves
large and flexible, it will accommodate much larger drug reservoirs than
typically possible in
20 silicon. For flexible substrate materials, materials such as AZ-4000,
polymide, Riston (a dry
photoresist), and SU=8 may be investigated.
l3ioteleniety. Meaningful measurements of physiological, electrochemical, and
biological
parameters in animals and humans may use wireless data transmission techniques
to reduce
43
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
the impact of the measurement process on the research subject. Hardwired
systems, although
technically applicable with the present inventions, limit the mobility of the
animal or human
and often yield false physiological measurements due to the stress factor
involved. A modern
biotelemetric measurement system for chronic, untethered monitoring should be
small and
either implantable in the body or attachable to the skin. A long lifetime of
the system may
also be desired, and typically ranges from a few weeks to several months. Long
operational
lifetimes may be especially critical for applications involving implants.
Wireless transmission of signals in today's biomedical research is primarily
based on
RF techniques (radio frequencies). The frequency of the RF signal needs to be
chosen such
1o that its absorption by biological tissue is small and the available
bandwidth is sufficient for
the desired application. Various modulation schemes are used, e.g., FM
(frequency
modulation), AM (amplitude modulation), PCM (pulse code modulation), or PIM
(pulse
interval modulation). The latter is especially well suited for low-power low-
bandwidth
biotelemetry applications.
t 5 An essential part of a biotelemetry system is its package, or
encapsulation material.
The electronics of the biotelemeter must be protected from moisture and ions,
and the
encapsulation material must be biocompatible if the system is to be implanted.
The
biocompatibility requirements are less stringent if the system is attached to
the outside of the
body. Similar considerations apply when comparing subcutaneously and
intravenously
2o implanted sensors or biotelemetry devices.
The use of biotelemetry in clinical applications is becoming more widespread.
Typical methods of transmitting biotelemetric RF-based. Within this family of
devices, the
most variation lies within the choice of transmission formatting. These
typically include
direct FM blocking (squegging), oscillation and frequency, and/or time
division multiplexing.
44
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
There are several requirements for radio frequency and optical technologies. A
coil
may be needed in RF transmission for generating the output signal and the
coupling
coefficient may have to be adequate for transmitting the data to the exterior.
In the case of
optical technologies, transmission may be limited to those cases in which a
sufficient optical
path is available between transmitter and receiver.
The ionic and volume conduction properties of body fluids may be used to
transmit
data from an implanted telemetry device. Current thus injected will produce,
relative to
different body locations, various potential differences which in turn can be
registered by
surface electrodes. This approach of using body fluids as the transmission
medium sidesteps
1 o the problems related to the radio frequency and optical technologies, and
conceptually, the
technology's underlying premise is sound and simple. Because there is no need
for radio
coils, the size of the telemetric device may be limited only by the
constraints of its power
supply. Furthermore, the technology is not limited to a particular body region
-- indeed, its
only requirement is an ionic fluid medium for data transmission. This
environment is typical
of the human body. Drawbacks with this approach, however, include the problem
that using
tissue as the transmission medium necessarily limits the usable modulation
techniques.
Furthermore, ionic fluids could introduce reliability problems due to motion
artifacts
(osmotic pressure, circulatory effects, muscle pressure, and skin movement,
etc.) that have
not been studied in detail. For these reasons the RF based systems are
favored.
Dru~llTarget Pairs. Insulin/glucose and melatonin/serotonin are described
herein as primary
examples, but any drug/target system may be used. As another system, a bone
formation/resorption pair may also be used.
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
The emerging understanding of the circadian biological clock in humans has
resulted
in circadian-timed therapeutic intervention, which is rapidly finding
applications in the
treatment of jet lag, sleep disorders, some sorts of depressions, peptic
ulcers, cardiac
ischemia, hypertension, asthma, cancer, and shift-workers' performance. It has
been observed
that optimal circadian drug timing results in improved drug efficacy and/or
lower toxicity. In
addition, by optimally timing therapy it may be possible to reduce the drug's
dosage. A
specific example includes the treatment of cancer patients where circadian
timing of surgery,
anticancer drugs, radiation therapy, and biological agents have shown to
improve toxicity
profiles, enhance tumor control, and ultimately, patient survival. This is
also true in the case
1o of AIDS treatment and in diabetes, since the levels of glucose have been
linked to the
circadian clock. In light of the above, it is evident that a better
understanding of the circadian
rhythms at the cellular and molecular levels is needed for the design of
therapies that are most
favorably, reproducibly, and economically adjusted to each individual
patient's needs.
Serotonin and Melcrtoni~z There are a number of biomolecules that are involved
in the
regulation of circadian rhythms, and those include melatonin and serotonin.
Serotonin is a
Central Nervous System neurotransmitter, which is a precursor of melatonin. It
is perhaps
the most implicated neurotransmitter substance in the etiology of various
disorders of the
central nervous system, which include depression, anxiety, aggression,
obsessive-compulsive
2o disorder, schizophrenia, obesity and eating disorders, panic, hypertension,
migraine, stroke,
nausea, autism, and Alzheimer's disease. Like serotonin, melatonin is part of
the regulation
of a number of physiological functions and rhythms, such as sexual behavior,
hormone
secretion, brain function, and the sleep-wakefulness cycle. Melatonin is a
pineal-gland
hormone that regulates the sleep-wake cycle, seasonal reproduction, and
locomotor activity.
46
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Melatonin is known to modulate a variety of cellular and subcellular processes
in the retina of
vertebrates, such as dopamine release, CAMP accumulation, and interactions
with
photoreceptors. In that respect, the development of implantable micromedical
devices capable
of detection of melatonin and serotonin and their responsive delivery,
depending on the local
levels of these biomolecules, should allow for the control and regulation of
circadian
rhythms. Moreover, glucose levels are affected by circadian rhythms; thus, a
glucose
responsive delivery system in conjunction with the serotonin/melatonin systems
of the
present invention will not only be useful in the treatment of diabetes, but
also should provide
insight into the circadian regulation of this disease.
Discussion
From the foregoing description, different drugs can be released at different
times and
the release rate may be sent by opening more "holes". The drug release valves
of the prior art
only work once. The small current, applied between a counter electrode and the
metal valve
electrode, causes either local hydrolysis of water at the metal valve
electrode surface and
subsequent bursting of the metal valve electrode or direct dissolution of the
metal electrode.
In both cases the drugs stored underneath the metal are released.
As an improvement, the present invention includes reversible polymeric valves
in
silicon. Also included are irreversible metal and reversible polymeric valves
in non-silicon
flexible substrates. which may then be incorporated into a telemetric,
responsive dru~~
delivery system.
The "artificial muscle" described refers to inter alia a chemo-electro-
mechanical
actuator, such as those consisting of a blend of a hydrogel such as poly(2-
hydroxyethyl
methacrylate) (PHEMA), poly (2-hydroxyethyl methacrylate-co-methyl
methacrylate)
47
CA 02386151 2002-04-03
WO 01/26708 PCT/LTS00/28215
(PHEMA-co-MMA), polyacrylamide (PA), and an electronically conducting redox
polymer
like polyaniline (PANI), polypyrrole (PPy) or their derivatives. The redox
polymers, which
form the "electronic backbone" of the muscle, are sensitive to pH, applied
potential, and
chemical potential in their microenvironment. Hydrogels, which form the "ionic
body" of the
muscle, provide a cross-linked network of hydrophilic homopolymers or
copolymers and
exhibit dramatic effects on swelling and shrinking upon changing pH, solvent,
temperature,
electric field, or ambient light conditions. By making a blend, some of the
desirable
properties of a hydrogel, i.e., very large swelling (e.g., 300% of the
original size) and
shrinking, are retained while the redox polymer, which by itself does not
swell or shrink that
1o much (e.g., 20%), makes the swelling/shrinking process much faster and
controllable with a
small electrochemical bias. The enhanced rate of swelling and shrinking is
expected because
of the distribution of protons in the hydrogel, which increases the proton
access around the
redox polymer electronic backbone. Moreover, the incorporation of a redox
polymer makes
it feasible to deposit the artificial muscle material locally and selectively
within a chosen
1 > microstructure.
By designing inexpensive reversible polymer valves that can open and close
many
times, the state of the art in responsive drug delivery can be significantly
advanced.
Preliminary data was obtained using sphincter-type valves using poly(2-
hydroxyethyl
methacrylate) (PHEMA) and polyaniline (PANI) as the hydrogel and the redox
polymer
20 components respectively of the actuator material. The method of
incorporation of
electropolymerized polyaniline in the hydrogel was optimized in order to
obtain a redox
polymer/hydrogel blend with a smooth morphology and the largest percentage of
swelling
and shrinking. Two different approaches were used to deposit various
PANI/PHEMA
combinations on a TEM gold grid, then characterizing the blend by scanning
electron
48
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
microscopy (SEM). The TEM gold grid was used to simulate an array of holes. In
a first
approach, PANI was deposited on a TEM gold grid by electropolymerization from
a 0.5 M
HZS04 solution containing 0.1 M aniline by cycling the electrode potential
between -0.2 V
and +0.8 V vs. SCE. In this approach, the gold grid was first dip-coated with
the hydrogel
and exposed to a UV source (270 nm) for 90 seconds. In a second approach, a
mixture of the
hydrogel and the monomer solution was electropolymerized, and the
PANI/hydrogel blend
was exposed to the UV source for the same duration as in the first approach.
From Approach
1 (Electropolymerization of PANI on PHEMA) it is evident that PANI was not
deposited
uniformly throughout the hydrogel although the hydrogel gave a smooth rounded
hole.
Approach 2 (Electropolymerization from a solution of HEMA dispersed in the
aniline
monomer solution) shows that the PANI was deposited uniformly but a smooth,
rounded hole
was not obtained. By using a combination of Approach 1 and 2 :
electropolymerization of
the monomer/HEMA mixture onto a TEM gold grid first coated with HEMA solution,
it was
attempted to obtain an artificial muscle with a smooth hole morphology and
uniform PANI
~5 distribution. Smooth artificial muscle morphology with uniform PANI
distribution was
indeed obtained using this combination deposition approach. The latter
hydrogel/redox
polymer deposition method was the one used to observe the swelling and
shrinking of the
muscle material.
The swelling and shrinking processes of the PANI/hydrogel system in response
to
2o electrochemical actuation were studied in depth by monitoring the
phenomenon with a
microscope connected to a video monitor, CCD camera, video recorder, and color
video
printer. The microscope has a 20X objective and 40X water immersion lens. The
lens was
immersed in 0.5 M HZS04 during the monitoring process and a potential between -
0.2 V and
+0.8 V was applied to the electrode at a scan rate of 50 mV/s for a duration
of 1 ~ cycles.
49
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
Real-time images of the artificial muscle were captured by the CCD camera,
which was
connected to the microscope. These real-time images were viewed on the video
monitor and
recorded onto videocassettes. The in situ monitoring of the artificial muscle
blend of PANI
and PHEMA-PVP showed a significant change in the size of the opening when this
blend
was cycled in the potential range of -0.2V and +0.8V (SCE) in 0.5 M HZSO~. The
change in
the longest length between the largest opening and the smallest opening is
approximately
150%. For comparison, real-time swelling and shrinking of PANI were also
examined.
Under identical experimental conditions, no significant change in the size of
the opening was
observed for PANI alone.
1o It is well known that the properties of an implanted material can affect
cell and tissue
response. Therefore, the materials chosen for use in this implantable system
potentially are
first evaluated for their biocompatibility unless otherwise known to be so.
Initially, well-
characterized materials may be assessed in cell cultures to determine cell
response. For
example, silicone rubber, a prime candidate for use in the outer casing of the
COZ gas sensor
1 i because of its well-documented biocompatibility, may be evaluated in a
Iibroblast cell
culture. Results from these studies may be used to determine the
appropriateness of this
material and/or what modifications are necessary. Once an acceptable material
has been
identified in cell culture. it may be tested 11? hlvU. All the materials which
may be used in the
implantable drug delivery system may be subjected to these series of tests
independently, in
20 order to optimize the overall biocompatibility of the entire system.
For instance, it may also be desirable that bone cell-biomaterial interactions
in vitro
be appreciated, and methods may be developed and used to control these
interactions. Rat
bone marrow, and recently marrow from humans, is routinely cultured under
conditions that
result in the formation of bone-forming osteoblasts and bone-resorbing
osteoclasts. These
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
cultures may be used to assess the effects of biomaterials in various forms,
e.g., bulk, ionic,
and particulate, on the formation and functions of bone cells. For example,
sublethal
concentrations of metal ions which can be released from orthopedic and dental
implants were
found to inhibit the normal differentiation of bone cells. Knowing that
biomaterials can have
adverse effects on bone cells, basic research is being conducted to understand
the effects of
osteotropic biomolecules, e.g., BMP-2 and vitamin D analogs, on bone cell
responses. In
concurrent studies, these and other osteotropic biomolecules are then being
used to modify
the surfaces of orthopedic and dental biomaterials for the purpose of inducing
desired bone
cell responses. For example, a biodegradable material loaded with BMP-2
drastically
improved the formation of osteoblasts in pluripotent cell cultures. In the
present invention,
the expertise described above may be used to develop devices that sense
markers of bone
catabolism and deliver pharmacologic agents to counter these effects.
Evaluation of the sensor and delivery technologies in an animal model may be
essential prior to evaluation in human studies. Primates offer the distinct
advantage of close
phylogenetic relationship with humans. The rhesus monkey is widely accepted as
a primate
model for many aspects of human physiology. Its circadian timing organization
closely
resembles that of humans in many respects. Rhesus are day-active and exhibit
consolidated
sleep. Thus, they are uniquely suited to evaluating therapeutic strategies of
circadian rhythm
manipulation. Several long-term studies of circadian rhythms in unrestrained
rhesus have
2o been performed, which includes studying the effects of light and gravity on
the rhesus
circadian timing system. It is important to note that this is the same system
that may be used
for initial testing of the responsive drug melatonin/serotonin delivery
system. Telemetric
devices have been used in unrestrained rhesus for periods of over one year to
study light
masking, properties of endogenous body temperature, and activity rhythms. The
primate test
51
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
system developed for these studies enables the recording of physiological and
behavioral data
from eight monkeys simultaneously. The rhesus module provides a controlled
environment
with visual isolation and individual control of module light timing and
intensity, as well as
monitoring of ambient temperatures. In addition to telemetry of heart rate and
body
temperature, activity may be monitored through motion sensors. Food and water
intake may
be monitored continuously. Urinary excretion of reproductive hormones may be
measured in
female rhesus. Performance rhythms were examined using a microcomputer-based
test
system, which supplies food as a reward, and provides environmental
enrichment. A
telemetry implant has been developed for long-term measurement of brain
temperature. The
capability also exists to restrain rhesus monkeys for catheterization and
acute sampling.
Thus, repeated measurement of blood gases and blood hormone levels should be
another
capability of a lab utilizing the present invention.
While there is a substantial amount of knowledge on biocompatibility of
passive
implant materials, much less has been done to elucidate fouling mechanisms of
sensor
~5 membranes. Since sensor membranes are often electrically active (e.g., in
an ion selective
membrane) or need to be gas permeable (e.g., a CO~ or O~ sensor), the
biocompatibility
issues are significantly different from those in the case of an "inert"'
implant. Because these
membranes are part of a biosensor one may, by implanting and monitoring the
sensor
performance, conduct further research on fouling mechanisms of the membranes.
It was determined that a pH sensor based on an ion selective poly (vinyl
chloride)
membrane (PVC) retains its electrochemical properties following chronic
exposure to a
physiologic environment. Miniaturized, H~+- selective electrodes were
implanted
subcutaneously in a rat for up to 21 days. Electrode stability, sensitivity,
selectivity. response
time, and internal resistance were measured following implantation and
compared to pre-
52
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
implant values. Sensitivity and selectivity were decreased only slightly with
time. Electrode
stability and response times show small changes over the implant duration.
With the
exception of drift, ion-selective PVC membranes retain much of their capacity
for accurate
electrochemical measurements following as long as 21 days of direct and
continuous
exposure to rat subcutaneous tissue.
A combination of a typical reference electrode and pH sensor may be mounted in
a
dual lumen catheter; one lumen for the reference and one for the pH sensor. It
may be
desirable to extend these measurements to in vivo telemetric data collection
in rats beyond 21
days (results indicate that the sensors remain viable as long as 12 weeks
after implantation).
It may be desirable to maximize the pH sensor's life-time by slowly releasing
heparin or
herudin over the sensor surface. The anticoagulants may be released from a
controlled
release polymer reservoir. In other words, not only may the proposed drug
delivery device
release drugs to fight disease, it also may release chemicals to keep the
sensor surface
functional. In many instances one can set up the detection scheme such that
one only needs
t 5 to measure a pH change so that drift is less of an issue.
Voltage measuring potentiometric type sensors for in vivo measurements may be
solely implemented, as they are less sensitive to changes in electrode area by
fouling than
current measurement-based amperometric devices. A potentiometric pH sensor is
perhaps
the least challenging in vivo biosensor. A more challenging bio-sensor for in
vivo
?0 applications is a potentiometric gas sensor incorporating a gas permeable
membrane. The
sensor developed for this purpose under the present invention is a CO~ sensor.
The CO~
sensor is based on an Ir/IrOx pH electrode and a Ag/AgCI reference electrode.
The two
electrodes are put into silicone tubing. Both ends of the tubing are sealed
with silicone
adhesive. The tubing is then filled with an electrolyte solution. The whole
sensor body is
53
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
then covered with a plastic tubing for physical protection. The COZ sensor was
calibrated in
a NaHC03 solution. The sensitivity was about 60mV/pC02. The response time was
less than
minutes.
Principles of enzymology and enzyme biosensors may be used in accordance with
the
5 present invention. This includes the use of molecular biology techniques to
overexpress
enzymes in bacteria and yeast, protein isolation through the use of
bioseparation tools,
enzyme immobilization, site directed and surface functionalization. These
principles may be
used to produce enzyme biosensors with either electrochemical or optical
detection. Glucose
oxidase, urease, and lipase all have been immobilized into a PANI matrix by
1 o electropolymerization from monomer solutions and by physical entrapment
procedures.
These immobilized enzyme systems have been used to make microsensors and
sensor arrays
for glucose, urea, and triglycerides. Since the artificial muscle of the
present invention is also
based on PANI and similar redox polymers, enzymes may be incorporated directly
into the
artificial muscle.
Potentiometric enzyme electrodes for urea may be prepared by the covalent
attachment of urease to a polypyrrole film. In particular, a polypyrrole film
may be f rst
deposited on the surface of a glassy-carbon electrode and then functionalized
to incorporate
nitro groups that were electrochemically reduced to amine groups. The enzyme
urease was
attached to the film's amino groups through its carboxyl functionalities
(i.e., C-terminus.
2o aspartic and glutamic acid residues) by a carbodiimide reaction. The pH
response of the
polypyrrole film after incorporation of the enzyme was evaluated
potentiometrically.
resulting in a slope of -54 ~ 1 mV/pH unit. Urease catalyzes the hydrolysis of
urea, which
causes a change in pH that can be detected by the polypyrrole film. The main
advantage of
the proposed system is that the enzyme is directly attached to the transducer,
allowing for
54
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
more rugged and easily miniaturized sensors. Furthermore, the covalent
immobilization of
the enzyme to the surface (rather than passive adsorption) reduces the
possibility of losing
enzyme into the bulk test solutions through leaching.
It may also be desirable to produce sensors with enhanced biocompatibility.
For
example, heparin was covalently attached on the surface of derivatized
cellulose triacetate
membranes, which were subsequently impregnated with the potassium-selective
ionophore
valinomycin. The resulting ion-selective electrodes responded to potassium and
had
selectivity coefficients on the same order of magnitude as those of
conventional poly (vinyl
chloride)-based electrodes. It was found that the heparin layer does not
significantly alter the
response characteristics of the electrodes. The biological activity of the
immobilized heparin
was measured in terms of its inactivation of blood coagulation factor Xa. It
was found that
the covalently anchored heparin was able to inactivate factor Xa. Therefore,
by covalently
attaching heparin on the surface of ion-selective electrodes, sensors with
improved blood
compatibility characteristics were prepared.
Segmented polyurethanes (SPUs) have been used to fabricate implantable devices
and
prostheses due to their thromboresistance and excellent mechanical properties.
SPUs have
also been used in the fabrication of solid-state electrochemical sensors
because they
demonstrate good adhesion properties. A silicone-modified SPU. BioSpan-S, has
a surface
which is blood compatible and more stable in vivo towards hydrolysis of the
polyether
2o segment compared to unmodified SPU. With the goal of developing
biocompatible ion-
selective electrodes. membranes containing valinomycin, and other additives
such as the
lipophilic salt potassium tetrakis[3.5-bis-(trifluoromethyl)phenyl~borate. and
plasticizers such
as DOS and DOP were prepared with BioSpan-S. The sensors demonstrated
excellent
response characteristics for potassium ions. Membranes containing the sodium
ionophore and
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
the ammonium ionophore were also prepared. In both cases, the response was
Nernstian and
the selectivity pattern was comparable to other matrixes such as polyvinyl
chloride). The
thrombogenicity of the membranes was tested by scanning electron microscopy
after being in
contact with blood plasma. In comparison with other polymers such as PVC and
non-
modified polyurethane, the silicone-modified membranes showed significantly
less platelet
adhesion, even when containing the ionophore and the plasticizer. These
results prove the
suitability of the SPU polymer for its use in biocompatible sensors.
Yet another embodiment of the present invention is an in vivo immunosensor.
The
immunosensor may be based on the principle of a selective antibody recognition
element
1o with a displaceable labeled analyte that is detectable electrochemically.
When labeled
analyte is bound to the antibody, the label may not be detectable
electrochemically because of
separation from the electrode surface. Target analyte may displace the label
from the
antibody, rendering it diffusable to an electrode for electrochemical
detection. Thus, increase
in analyte concentration may cause an increase in electrochemical signal.
Physically, the
immunosensor may consist of four main elements: (i) an outer semipermeable,
biocompatiblc
membrane, (ii) a sensing layer that contains the antibody recognition element
and the labeled
analyte, (iii) a thin separation layer (termed the diffusion layer) through
which released
labeled analyte can diffuse to the electrode, and (iv) an electrode system for
electrochemical
detection.
2o The outer membrane may serve as a protection layer that provides
biocompatibility
with the host, prevents large proteins from entering the sensing layer and
reaching the
underlying electrode where fouling by adsorption could occur, allows small
analyte
molecules to pass, and is impenetrable by the labeled analyte. The sensing
layer may consist
56
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
of a host matrix such as a polymer network where the antibody and analyte
labeled with an
electroactive compound (referred to now as labeled analyte) are immobilized.
Finally, the detection electrode may consist of a thin film of deposited
conductive
material such as gold or platinum. For that, an interdigitated array (IDA) may
be used as the
detection electrode, which consists of a pair of electrodes in which each
electrode is made of
parallel strips of metal that are separated by insulating material. One major
advantage of the
IDA is the redox cycling of the detected molecule that occurs when the
potentials applied to
the two electrodes cause reduction to occur at the cathode and oxidation at
the anode,
enabling lower detection limits due to the enhanced current for each label
molecule. It is
1 o anticipated that planar sensors on the order of I or 2 mm on edge can be
fabricated for
implantation. As a first example of immunosensor, a system for clonidine was
developed, a
drug used in the treatment of alcoholism.
In making disposable micro medical devices, one may often compete with
continuous
processes rather than serial processes, and silicon batch technology is often
not suited to
produce sufficiently inexpensive disposables. As an example, refer to the mass
production of
an amperometric glucose sensor (cost target ten cents per sensor). The
competing processes
to make glucose sensors involve such proven technology as doctor's blade on a
continuous
moving web. This dilemma may be solved by converging IC and thick film
processes from
silicon wafers to large photosensitive sheets or even continuous processes
with the resist
material in a web format.
When there are no electronics on the chip and one only desires to fabricate an
array of
chemical sensors, a modular approach may be preferred. When combining BIO-MEMS
chambers holding different drugs each new drug added to the substrate may
interfere with the
chemical activity of the previously deposited drug and can cripple the yield
of the finished
57
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
array. A critical consequence of the integrated array approach is also that
the largely non-
standard materials and their modes of deposition need to be reinvented for
each new element
added to the array. To increase the manufacturing yield dramatically, one may
benefit by
fabricating a different wafer (sheet or web) with only one type of drug and,
after cutting out
the individual sensors, combine them into an array with pick and place
techniques. This
modular approach enables the independent development of different drug and
sensor
chemistries and obviates all compatibility issues. In a factory environment
this eases the
fabrication of different drug delivery panels on demand. This approach entails
a drug/sensor
array somewhat larger than an integrated silicon approach, but for most
applications this is
quite adequate. In a modular approach, sensors may be built separately on
individual wafers,
sheets, or rolls and then put in a drug delivery system with pick and place
techniques. Since
one can produce sensors on large sheets or even in a continuous mode,
manufacturing costs
may be reduced much beyond wafer batch technologies
As non-silicon flexible substrates, polyamides (combinations of photo-
sensitive and
non-photosensitive polyamides), AZ-4000, dry photoresists and SU-8 were
investigated. For
disposable electrochemical valves and drug reservoirs there may be versions
made on
Polyimide and Pyralux. The current preferred material embodiment of the
individual drug
delivery chamber is Pyralux, a negative dry resist. The manufacturing
procedure of an
individual chamber with an Ag valve is already demonstrated. Since the Pyralux
comes in
rolls it may eventually be possible to make these type of drug delivery
systems on a
continuous base but for now one can use 5 by 5 inch sheets which may fit both
on lithography
equipment and a silkscreen machine.
A critical element in tile configuration, integration, and application of any
chronically
implanted, ingested, or indwelling sensor, drug delivery, or other medical
intervention
58
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
element is the associated measurement subsystem. This subsystem may include
signal
conditioning, data acquisition, communications, and other necessary elements.
Sensors
integrated with these 'measurement platforms" include those that measure
biopotentials,
biophysical, and most recently, biochemical and biological parameters.
Chemical and
biological sensors are generally either potentiometric or amperometric, and
can be monitored
using either electrochemical or optical techniques. For an ambulatory patient
or subject,
these systems must allow chronic, untethered, continuous operation over
periods ranging
from hours, to days, to weeks or even months depending on the application and
specific
method of interface with the body.
The chemical/biological sensor of the present invention may improve previously
developed potentiometric pH, Ca2+, and CO~ sensors and provide enzyme and
immunosensors capable of surviving in vivo for extended periods of time.
Besides these
sensors, other in vivo biosensors for the selected target molecules may be
produced.
including an in vivo immunosensor.
Potentiometric glucose sensors may be constructed. The rapid, selective, and
sensitive response of natural sensory systems that employ enzymes may be used
in the
development of biosensors. Glucose sensors may generally follow one of two
approaches.
The first approach involves placing sensors into blood vessels such as the
versa cava or the
carotid artery. The second involves using a microdialysis probe or more
commonly, an
amperometric enzymatic-based transducer. The risks of thrombosis and
hematogenous
spread of infection are believed to militate against the long-term use of
intravascular sensors
although not impossible. While the exact relationship between blood and
subcutaneous
glucose concentrations is still being investigated, recent work suggests that
mass transfer
59
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
modeling methods may significantly improve the estimates of blood glucose
levels that are
based on subcutaneous data.
Furthermore, there are significant advantages associated with subcutaneous
sensors:
clinical safety, ease of insertion and removal, ease of coupling these sensors
to a telemetry
system, and cost. There is substantial evidence that subcutaneous placement of
a glucose
sensor will work and will lead to a much longer life of the sensor than when
contacting blood
directly. These expectations are confirmed by subcutaneous pH measurements.
Potentiometric glucose sensors may be built in which the reaction of glucose
oxidase with
glucose acidifies the local environment and this acidification may be measured
with the pH
t o probe described earlier.
The enzyme that may be used as the sensing element in the production of the
biosensor for serotonin may be monoamine oxidase. This enzyme is commercially
available
from a variety of sources and its enzymatic activity, as well as its stability
and other
important biochemical properties, have been well characterized. For the
detection of
melatonin, one may employ melatonin deacetylase. This enzyme may be isolated
from
Xenopus retinal tissue after homogenation and cell lysis. The gene for this
enzyme has not
been cloned and one task may be to clone and overexpress melatonin deacetylase
in E. coli
and/or yeast to provide an alternative source of enzyme for our studies.
Briefly, the gene of
interest may be isolated from a cDNA library by using the polymerase chain
reaction and
appropriately designed primers. The primers may contain unique restriction
sites, so that
after amplification of the gene of interest, efficient cloning into a high
expression vector may
be ensured.
The recombinant plasmid, containing the gene of interest may be introduced
into
competent E. coli or yeast cells by transformation. The transformed cells may
be screened
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
for the presence of the gene in the correct orientation with respect to the
promoter region of
the plasmid by restriction endonuclease digestion, followed by agarose gel
electrophoresis.
Plasmids having the insert in the correct orientation may be isolated, and the
sequence of the
cloned gene may be determined by DNA sequencing. The protein encoded by the
gene may
be expressed and isolated by using biochromatography (ion exchange and gel
filtration
chromatography). Alternatively, a poly-histidine tail may be attached to
melatonin
deacetylase by a gene-fusion approach, which may allow facile purification by
using affinity
chromatography. The purity of the isolated enzyme may be verified by gel
electrophoresis.
Serotonin and melatonin sensors may then be produced. Serotonin (5-
i o hydroxytryptamine), a biochemical precursor of melatonin, is a
neurotransmitter in the
Central Nervous System. The principal route of metabolism of serotonin
involves
monoamine oxidase (MAO) forming 5-hydroxyindole acetic acid. Melatonin
deacetylase is
an enzyme that degrades melatonin to 5-methoxytryptamine with the release of
acetic acid. It
is found in several tissues, and it may be the dominant breakdown pathway of
melatonin in
the retina as a mechanism of ocular melatonin clearance. Both these enzymes
may catalyze
reactions that result in a change in pH in their microenvironment. Thus. they
may be coupled
with a pH detection system to yield biosensors for serotonin and melatonin.
respectively.
At least two different strategies may be employed to design these biosensors.
The
enzymes may be covalently attached to a conducting polymer (e.g.,
polypyrrole), or the
enzymes may be entrapped behind a semipermeable membrane at the tip of the pH
transducer. The first approach may allow for incorporation of the enzyme in
close proximity
to or within the artificial muscle, which may lead to a direct coupling of
enzyme action (pH
change) and muscle contraction/expansion. The second approach has the
advantage that
detection and actuation may be independent of each other and regulated by the
control
61
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
circuitry. This may allow for circadian rhythms to be closely monitored, and
for the delivery
dosage to be adjusted. A variety of semipermeable membranes may be evaluated
for enzyme
entrapment. Two important parameters to be evaluated may be the thickness and
porosity of
the membranes, which control the diffusion rate of the substrate and,
ultimately, the working
range and response time of the sensor.
An in vivo immunosensor may be based on the principle of a selective antibody
recognition element with a displaceable labeled analyte that is detectable
electrochemically.
When labeled analyte is bound to the antibody, the label may not be detectable
electrochemically because of the distance separating it from the electrode
surface. Target
t 0 analyte may displace the label from the antibody, rendering it diffusable
to an electrode for
electrochemical detection. Thus, increase in analyte concentration may cause
an increase in
electrochemical signal. A major goal may be identification of the proper
label. Experience
points to p-aminophenol (PAP) as an electroactive molecule with reversible
electrochemistry
that is suitable for detection with an IDA. Consequently, PAP might be used
and suitability
~ 5 determined, although necessary substitutions in the ring for coupling may
be problematic for
cycling. Good alternative candidates may be substituted ferrocenes. These have
been
thoroughly investigated as mediators for glucose sensors.
One may then begin trapping of the labeled molecule. An important goal in
immunosensor development is developing a restraining system to trap the
labeled molecule
2o within the sensing layer. This may be accomplished either by binding the
labeled analyte to
the hydrogel matrix with a long tether such as polyethylene glycol), or by
attaching the label
to a dendrimer molecule that is too large to permeate the outer membrane and,
thus, escape
the sensing layer. The latter has been exploited in the development of
immunoassays. In this
case, the dendrimer may be attached to the analyte as a linker and have
multiple electroactive
62
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
labels attached to the dendrimer. This approach has the advantage of multiple
labels being
displaced by each captured analyte molecule, which results in increased
sensitivity. Other
dendrimer systems (e.g., poly(amidoamine) [PAMAM] and peptidic dendrimers),
their
optimum size with respect to diffusion, electrochemical sensitivity, and
interferences with the
analyte binding event may be investigated. The properties of the labeled
compounds may be
investigated by using cyclic voltammetry with conventional electrodes such as
Au or Pt discs.
The diffusion layer may be a thin layer that serves to prevent detection of
bound
labeled analyte by the electrode. This layer may consist of the same type of
polymer as the
sensing layer, but without antibody. It should be as thin as possible to
minimize diffusional
t o distances to the electrode surface.
The applicability of the IDA and redox cycling to electrochemical immunoassay
has
been demonstrated. These IDAs, which consist of an array of Pt fingers one
micrometer wide
and spaced by one micrometer with a microdeposited Ag/AgCI reference electrode
and a Pt
auxiliary electrode, may be used for studies of recycling the labeled
dendrimers.
Clonidine may be used as the test analyte for developing the prototype sensor.
This is
an antihypertensive drug used for the treatment of alcoholism. The therapeutic
blood level is
0.2 - 2 ng/mL (low nM range). The availability of anti-clonidine antibodies
has allowed for
the development of immunoassays for clonidine. In that respect, one may attach
the
dendrimer label by using conventional chemical attachment methods (e.g..
carbodimide-
mediated conjugation) to minimize interference with the antibody binding site
at the other
end of the molecule. The average association constant of labeled clonidone may
be
determined with standard tests such as a competitive ELISA with the unlabeled
ligand.
The response of the serotonin and melatonin biosensors may be characterized
with
respect to response time, selectivity, sensitivity, and reproducibility using
concentrations of
63
CA 02386151 2002-04-03
WO 01/26708 PCT/US00/28215
these two compounds that are physiologically relevant. For example, it is
known that during
the day the levels of melatonin in healthy individuals range from 10-20 pg/mL
(-~- 0.1 nM),
whereas nocturnal levels are at the high end 200 pg/mL (~lnM). The range of
serotonin
levels is 50-200 ng/mL. The optimized biosensors may be integrated within the
responsive
delivery system. Changes in serotonin/melatonin levels may trigger the
sensitive artificial
muscle to release the right amount of melatonin. This system can thus be used
to develop an
automatic feedback melatonin delivery system. It should be noted that a
melatonin sensor by
itself might be sufficient to provide information regarding the controlled
delivery of
melatonin to the astronaut. By incorporating a serotonin biosensor in addition
to the
1 o melatonin biosensor, and a utilizing broader insight of the circadian
rhythm physiology, a
patient's response to melatonin treatment may be obtained.
The preferred embodiments herein disclosed are not intended to be exhaustive
or to
unnecessarily limit the scope of the invention. The preferred embodiments were
chosen and
described in order to explain the principles of the present invention so that
others skilled in
the art may practice the invention. Having shown and described preferred
embodiments of
the present invention, it will be within the ability of one of ordinary skill
in the art to make
alterations or modifications to the present invention, such as through the
substitution of
equivalent materials or structural arrangements, or through the use of
equivalent process
steps, so as to be able to practice the present invention without departing
from its spirit as
2o reflected in the appended claims, the text and teaching of which are hereby
incorporated by
reference herein. It is the intention, therefore, to limit the invention only
as indicated by the
scope of the claims and equivalents thereof.
64