Note: Descriptions are shown in the official language in which they were submitted.
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
TITLE OF THE INVENTION
NICOTINYL ASPARTYL KETONES AS INHIBITORS OF CASPASE-3
BACKGROUND OF THE INVENTION
Apoptotic cell suicide is a fundamentally important biological process
that is required to maintain the integrity and homeostasis of multicellular
organisms.
Inappropriate apoptosis, however, underlies the etiology of many of the most
intractable of human diseases. In only the last few years, many of the
molecules that
participate in a conserved biochemical pathway that mediates the highly
ordered
process of apoptotic cell suicide have been identified. At the heart of this
pathway are
a family of cysteine proteases, the 'caspases', that are related to mammalian
interleukin-lI3 converting enzyme (ICE/caspase-1) and to CED-3, the product of
a
gene that is necessary for apoptotic suicide in the nematode C. elegans
(Nicholson et
al., 1997, Trends Biochem Sci 22:299-306). The role of these proteases in cell
suicide
is to disable critical homeostatic and repair processes as well as to cleave
key
structural components, resulting in the systematic and orderly disassembly of
the
dying cell.
The central importance of caspases in these processes has been
demonstrated with both macromolecular and peptide-based inhibitors (which
prevent
apoptosis from occurring in vitro and in vivo) as well as by genetic
approaches.
Inhibition of apoptosis via attenuation of caspase activity should therefore
be useful in
the treatment of human diseases where inappropriate apoptosis is prominent or
contributes to disease pathogenesis. Caspase inhibitors would thus be useful
for the
treatment of human diseases including, but not limited to, acute disorders
such as
cardiac and cerebral ischemia/ reperfusion injury (e.g. stroke), spinal cord
injury and
organ damage during transplantation, as well as chronic disorders such as
neurodegenerative diseases (e.g. Alzheimer's, polyglutamine-repeat disorders,
Down's, spinal muscular atrophy, multiple sclerosis), immunodeficiency (e.g.
HIV),
diabetes, alopecia and aging.
Ten caspases have so far been identified in human cells. Each is
synthesized as a catalytically dormant proenzyme containing an amino-terminal
prodomain followed by the large and small subunits of the heterodimeric active
enzyme. The subunits are excised from the proenzyme by cleavage at Asp-X
junctions (Nicholson et al., 1997, Trends Biochem Sci 22:299-306). The strict
requirement by caspases for Asp in the P1 position of substrates is consistent
with a
-1-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
mechanism whereby proenzyme maturation can be either autocatalytic or
performed
by other caspases. The three dimensional crystal structures of mature caspase-
1 and -
3 show that the large subunit contains the principle components of the
catalytic
machinery, including the active site Cys residue which is harbored within the
conserved pentapeptide motif, QACxG, l and residues that stabilize the
oxyanion of
the tetrahedral transition state (Wilson et al., 1994, Nature 370:270-75;
Walker et al.,
1994, Cell 78:342-52; Rotonda et al., 1996, Nat Struct Biol 3:619-25). Both
subunits
contribute residues which stabilize the P1 Asp of substrates while the small
subunit
appears to contain most of the determinants that dictate substrate specificity
and, in
particular, those which form the specificity-determining S4 subsite. One
distinctive
feature of these proteases is the absolute requirement for an aspartic acid
residue in
the substrate P1 position. The carboxylate side chain of the substrate P1 Asp
is
tethered by four residues in caspase-1 (Arg179, G1n238 from p20 and Arg341,
Ser347
from p10) that are absolutely conserved in all caspase family members.
Catalysis
involves a typical cysteine protease mechanism involving a catalytic dyad,
composed
of His237 and Cys285 (contained within an absolutely conserved QACxG
pentapeptide) and an 'oxyanion hole' involving G1y238 and Cys285. Inhibitors
bind,
however, in an unexpected non-transition state configuration (which raises
important
considerations for inhibitor design) with the oxyanion of the thiohemiacetal
being
stabilized by the active site His237.
Members of the caspase family can be divided into three functional
subgroups based on their substrate specificities which have been defined by a
positional-scanning combinatorial substrate approach. The principle effectors
of
apoptosis (group II caspases, which include caspases-2, -3 and -7 as well as
C. elegans
CED-3) have specificity for [P4]DExD[Pl], a motif found at the cleavage site
of most
proteins known to be cleaved during apoptosis. On the other hand, the
specificity of
group DI caspases (caspases-6, -8, -9 and -10, as well as CTL-derived granzyme
B) is
[P4](I,V,L)ExD[P1] which corresponds to the activation site at the function
between
the large and small subunits of other caspase proenzymes including group II
(effector)
family members. This and other evidence indicates that group III caspases
function as
upstream activators of group II caspases in a proteolytic cascade that
amplifies the
death signal. The role of group I caspases (caspases-1, -4 and -5) appears to
be to
mediate cytokine maturation and their role in apoptosis, if any, has not been
substantiated.
-2-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
A tetrapeptide corresponding to the substrate P4-P1 residues is
sufficient for specific recognition by caspases and as a consequence has
formed the
basis for inhibitor design. In addition to the requirement for a P1 Asp, the
P4 residue
in particular appears to be most important for substrate recognition and
specificity.
Caspase-1, for example, prefers a hydrophobic residue such as Tyr in P4 (which
corresponds to its YVHD cleavage site within proIL.-lf3) whereas caspase-3
(and other
group II enzymes) has a preference for an anionic Asp residue (which
corresponds to
the DXXD cleavage sites within most polypeptides that are cleaved by these
enzymes
during apoptosis). Peptide aldehydes, nitrites and ketones are potent
reversible
inhibitors of these proteases while compounds that form thiomethylketone
adducts
with the active site cysteine (e.g. peptide (acyloxy)methylketones) are potent
irreversible inhibitors. For example, the tetrapeptide aldehyde Ac-YVAD-CHO
(which was designed to mimic the YVHD caspase-1 recognition sequence within
proIL-1(3) is a potent inhibitor of caspase-1 (Ki < 1 nM) but a poor inhibitor
of
caspase-3 (Ki = 12 p,M) (Thornberry et al., 1992, Nature 356:768-74). In
contrast, the
Ac-DEVD-CHO tetrapeptide aldehyde (which was designed to mimic the caspase-3
recognition site) is a very potent inhibitor of caspase-3 (Ki < 1 nM) although
it is also
a weaker but reasonable inhibitor of caspase-1, presumably owing to
promiscuity in
the S4 subsite of this enzyme (Nicholson et al., 1995, Nature 376:37-43).
Several features plague these peptide-derived inhibitors as a platform
for drug design. In addition to their metabolic instability and membrane
impermeability, the slow-binding time-dependent inhibition of activity (e.g.
kon
caspase-l:Ac-YVAD-CHO = 3.8 x 105 M-ls-1; kon caspase-3:Ac-DEVD-CHO = 1.3
x 105 M-ls-1) precludes them from the rapid inhibition that may be necessary
to
abolish enzymatic activity in vivo. The present patent application describes
the
resolution of this issue with the discovery of several novel gamma-ketoacids
that
make highly suitable caspase inhibitors.
-3-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
SUMMARY OF THE INVENTION
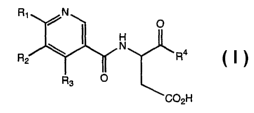
The present invention relates to compounds represented by formula I:
R~ /N
O
H
N
R2 ~ Ra
R3 O
C02H
as well as pharmaceutically acceptable salts, hydrates and esters thereof,
wherein:
R1 represents H, NH2, NHC1_6alkyl, NHC(O)C1_6alkyl,
NHC(O)OC1_6alkyl, or NHC(O)Aryl, said alkyl and the alkyl and aryl portions of
which are optionally substituted with 1-3 members selected from the group
consisting
of: C02H, C02C1_6alkyl, aryl, NH2, NHC1_3alkyl, NH-Aryl, N(C1_3alkyl)2 and
Hetcy
R2 is selected from the group consisting of:
(a) H, OH, halo, NH2, CN, C1_6alkyl, C2_6alkenyl, C2_6alkynyl,
C02H, Aryl and Hetcy;
(b) OC1_6alkyl and OC3_6alkenyl;
(c) -S(O)yCl_6alkyl, -S(O)yC3_6alkenyl, -S(O)yAryl and S(O)yHetcy,
wherein y is 0, 1 or 2;
(d) NHC 1 _6alkyl, NH-Aryl and NH-Hetcy;
(e) C(O)C1_6alkyl, C(O)C3_6alkenyl and C(O)Hetcy;
(f) C(O)NH2, C(O)NHC1_6alkyl, C(O)N(C1_6alkyl)2, C(O)NH-Aryl
and C(O)N(C 1 _6alkyl)-Aryl;
(g) NHC(O)C1_6alkyl, NHC(O)C3_6alkenyl, N(C1_6alkyl)C(O)C1_
(alkyl, N(C1_6alkyl)C(O)C3_6alkenyl and N(C1_6alkyl)C(O)Aryl;
(h) S(O)2NH2, S(O)2NHC1_6alkyl, S02NHHetcy, S(O)2NHC3_
galkenyl, S(O)2N(C1_6alkyl)2, S(O)2N(C1_galkyl)C3_galkenyl, S02NHAryI,
S02NH-Hetcy, S02N(C1_6alkyl)Aryl and S02N(C1_6alkyl)Hetcy;
(i) NHS02C1_6alkyl, NHS02C3_6alkenyl, N(C1_6alkyl)S02C1_
6alkyl and N(C1_6alkyl)S02C3_6alkenyl,
said C1_6alkyl, C2_6alkenyl, C3_6alkenyl and C2_(alkynyl groups and
portions in (a) through (i) above being optionally substituted with 1-6
members
-4-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
selected from the group consisting of: halo, OH, NH2~ CN, C02H, Hetcy, Aryl,
C02C1_6alkyl, OC1_6alkyl, O-Aryl, C02C3_4alkenyl, C(O)NH2, C(O)NHC1_3alkyl,
C(O)N(C1_3alkyl)2, C(O)NH-Aryl, C(O)N(C1_3alkyl)-Aryl, C(O)C1_3alkyl, C(O)C3_
4alkenyl, -S(O)yCl_3alkyl, -S(O)yC3_4alkenyl, S(O)y-(C1_3alkyl-aryl), wherein
y is
as previously defined; OC1_3alkyl-aryl, NH(C1_3alkyl-aryl),
N(C1_3alkyl)C(O)C1_
3alkyl, N(C1_3alkyl)C(O)C3_4alkenyl, N(C1_3alkyl)C(O)Aryl, N(C1_
3alkyl)C(O)Hetcy, S(O)2NH2, S(O)2NHC1_3alkyl, S(O)2NHC3_4alkenyl,
S(O)2NHAryl, S(O)2NHHetcy, S(O)2N(C1_3alkyl)2, S(O)2N(C1_3alkyl)C3_
4alkenyl, S(O)2N(C1_3alkyl)Aryl, S(O)2N(C1_3alkyl)Hetcy, NHS03H, NHS02C1_
3alkyl, NHS02C3_4alkenyl, NHS02Ary1, NHS02Hetcy, N(C1_3alkyl)S03H, N(C1_
3alkyl)S02C1_3alkyl, N(C1_3alkyl)S02C3_4alkenyl, N(C1_3alkyl)S02Ary1 and
N(C 1 _3alkyl)S02Hetcy;
R3 represents H, halo or C 1 _3alkyl, and
R4 is selected from the group consisting of: H, C1_6alkyl, C2_6alkenyl,
C2_6alkynyl and Hetcy, said C1_6alkyl, C2_6alkenyl and C2_6alkynyl groups
being
optionally substituted with 1-6 members selected from the group consisting of:
halo,
OH, NH2~ NHC1_l0alkyl, N(C1-l0alkyl)2, CN, C02H, Hetcy, Aryl, C02C1_6alkyl,
OC1_6alkyl, Oaryl, C02C1_3alkyl, C02C3_4alkenyl, C(O)NH2, C(O)NHC1_3alkyl,
C(O)N(C1_3alkyl)2, C(O)NH-Aryl, C(O)N(C1_3alkyl)-Aryl, C(O)C1_3alkyl, C(O)C3_
4alkenyl, -S(O)yCl_3alkyl, -S(O)yC3_4alkenyl, S(O)y-(C1_3alkyl-aryl), wherein
y is
as previously defined; OC1_3alkyl-aryl, NH(C1-3alkyl-aryl),
N(C1_3alkyl)C(O)C1_
3alkyl, N(C1_3alkyl)C(O)C3_4alkenyl, N(C1_3alkyl)C(O)Aryl, N(C1_
3alkyl)C(O)Hetcy, S(O)2NH2, S(O)2NHC1_3alkyl, S(O)2NHC3_4alkenyl,
S(O)2NHAryI, S(O)2NHHetcy, S(O)2N(C1_3alkyl)2, S(O)2N(C1_3alkyl)C3_
q.alkenyl, S(O)2N(C1_3alkyl)Aryl, S(O)2N(C1_3alkyl)Hetcy, NHS03H, NHS02C1_
3alkyl, NHS02C3-4alkenyl, NHS02Aryl, NHS02Hetcy, N(C1_3alkyl)S03H, N(C1_
3alkyl)S02C1_3alkyl, N(C1_3alkyl)S02C3_4alkenyl, N(C1_3alkyl)S02Aryl and
N(C 1 _3alkyl)S02Hetcy;
Aryl represents a 6-14 membered aromatic ring system and
Hetcy represents a 5-10 membered ring system, aromatic or non-
aromatic, containing at least one heteroatom and optionally containing up to 2
additional heteroatoms, said heteroatoms being selected from O, S(O)y with y
as
defined above and N,
-5-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
said Aryl and Hetcy groups and portions thereof being optionally
substituted with 1-6 members selected from the group consisting of: -(CH2)0-4-
C02H, -(CH2)0-3C02C1-3alkyl, halo, CN, NH2, phenyl, pyrrolidinyl, NHCH3, C1_
6alkyl, S02NH2 and S02CH3.
The invention also encompasses a pharmaceutical composition
comprising a compound of formula I in combination with a pharmaceutically
acceptable Garner.
The invention also encompasses a method of treating cardiac and
cerebral ischemia/reperfusion injury (e.g. stroke), type I diabetes, immune
deficiency
syndrome (including AIDS), cerebral and spinal cord trauma injury, organ
damage
during transplantation, alopecia, aging, Parkinson's disease, Alzheimer's
disease,
Down's syndrome, spinal muscular atrophy, multiple sclerosis and
neurodegenerative
disorders, comprising administering to a mammalian patient in need of such
treatment
an effective amount of a compound of formula I.
DETAILED DESCRIPTION OF THE INVENTION
The invention is described using the following definitions unless
otherwise specified.
For purposes of this specification alkyl means linear, branched or
cyclic structures and combinations thereof, containing one to twenty carbon
atoms
unless otherwise specified. Examples of alkyl groups include methyl, ethyl,
propyl,
isopropyl, butyl, s- and t-butyl, pentyl, hexyl, heptyl, octyl, nonyl,
undecyl, dodecyl,
tridecyl, tetradecyl, pentadecyl, eicosyl, 3,7-diethyl-2,2-dimethyl- 4-
propylnonyl,
cyclopropyl, cyclopentyl, cycloheptyl, adamantyl, cyclododecylmethyl, 2-ethyl-
1-
bicyclo[4.4.0]decyl and the like.
Alkylcarbonyl signifies groups having the formula -C(O)-alkyl,
wherein alkyl is defined as above.
Alkylsulfonyl signifies groups having the formula -S(O)2-alkyl,
wherein alkyl is defined as above.
Fluoroalkyl means linear, branched,or cyclic alkyl groups and
combinations thereof, of one to ten carbon atoms, in which one or more
hydrogen but
no more than six is replaced by fluorine. Examples are -CF3, -CH2CH2F, and -
CH2CF3 and the like. Haloalkyl means linear, branched or cyclic alkyl groups
having
up to six halo groups attached.
-6-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Alkoxy means alkoxy groups of one to ten carbon atoms of a straight,
branched or cyclic configuration. Examples of alkoxy groups include methoxy,
ethoxy, propoxy, isopropoxy and the like.
Alkoxycarbonyl signifies groups having the formula -C(O)-alkoxy,
wherein alkoxy is defined as above.
Alkylthio means alkylthio groups of one to ten carbon atoms of a
straight, branched or cyclic configuration. Examples of alkylthio groups
include
methylthio, propylthio, isopropylthio, etc. By way of illustration, the
propylthio group
signifies -SCH2CH2CH3.
Aryl represents a 6-14 membered aromatic ring system, optionally
substituted with 1-6 members selected from the group consisting of: -(CH2)0-6-
C02H, -(CH2)0-6C02C1-6alkyl, OH, halo, CN, NH2, phenyl, naphthyl, pyrrolyl,
pyridyl, piperidyl, pyrrolidinyl, furanyl, thienyl, NHCH3, C1_6alkyl, NHS03H,
S02NH2, and S02CH3. Aryl is, for example, phenyl or naphthyl. When aryl is
present in a substituent on alkyl, alkenyl, alkynyl or Hetcy, it is optionally
substituted
as described above.
The groups -(CH2)0-6-C02H and -(CH2)0-6C02C1-6alkyl refer to
carboxylic acids and esters, alkanoic acids and alkyl esters thereof. Thus,
these
include C02H and C02C1_6alkyl.
Hetcy as used herein refers to a 5-14 membered ring system that is
aromatic, non-aromatic or partially aromatic, and that contains at least one
heteroatom. Up to 3 additional heteroatoms selected from O, S(O)y and N, with
y
representing 0, 1 or 2 are included. Hetcy is optionally substituted with 1-6
members
selected from the group consisting of: -(CH2)0-6-C02H, -(CH2)0-6C02C1-6alkyl,
OH, halo, CN, NH2, phenyl, naphthyl, pyrrolyl, pyridyl, piperidyl,
pyrrolidinyl,
furanyl, thienyl, NHCH3, C1_6alkyl, NHS03H, S02NH2, and S02CH3.
Heteroaryl is an aromatic subset of Hetcy, and thus includes, e.g., ,
pyridyl, furyl, thienyl, thiazolyl, isothiazolyl, imidazolyl, benzimidazolyl,
pyrazinyl,
pyrimidyl, quinolyl, isoquinolyl, benzofuryl, benzothienyl, pyrazolyl,
indolyl, purinyl,
isoxazolyl, oxazolyl and coumarinyl.
Halo includes F, Cl, Br and I.
For purposes of this specification, the following abbreviations have the
indicated meanings:
Alloc - allyloxycarbonyl
APCI - atmospheric pressure chemical ionization
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
BOC - t-butyloxycarbonyl
CBZ - carbobenzoxy
DCC - 1,3-dicyclohexylcarbodiimide
DIBAL - diisobutyl aluminum hydride
DIEA - N,N-diisopropylethylamine
DMAP - 4-(dimethylamino)pyridine
EDCI - 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride
EDTA - ethylenediaminetetraacetic acid,
tetrasodium salt
hydrate
ESI - electrospray ionization
FAB - fast atom bombardment
FMOC - 9-fluorenylmethoxycarbonyl
HMPA - hexamethylphosphoramide
HATU - O-(7-Azabenzotriazol-1-yl)N,N,N',N'-
tetramethyluronium hexafluorophosphate
HOBt - 1-hydroxybenzotriazole
HRMS - high resolution mass spectrometry
ICl - iodine monochloride
IBCF - isobutyl chloroformate
KHIVV>nS - potassium hexamethyldisilazane
LDA - lithium diisopropylamide
MCPBA - metachloroperbenzoic acid
Ms - methanesulfonyl = mesyl
Ms0 - methanesulfonate = mesylate
NBS - N-bromosuccinimide
NMM - 4-methylmorpholine
PCC - pyridinium chlorochromate
PDC - pyridinium dichromate
Ph - phenyl
PPTS - pyridinium p-toluene sulfonate
pTSA - p-toluene sulfonic acid
r.t. - room temperature
rac. - racemic
Tf0 - trifluoromethanesulfonate = triflate
_g_
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
TLC - thin layer chromatography
Alkyl group
abbreviations:
Me - methyl
Et - ethyl
n-Pr - normal propyl
i-Pr - isopropyl
n-Bu - normal butyl
i-Bu - isobutyl
s-Bu - secondary butyl
t-Bu - tertiary butyl
An aspect of the invention that is of interest relates to compounds of
formula I:
Ri /N
O
H
N
R2 ~ Ra
R3 O
~ C02H
as well as pharmaceutically acceptable salts, hydrates and esters thereof,
wherein:
R 1 represents H, NH2, NHC 1 _6alkyl, NHC(O)C 1 _6alkyl,
NHC(O)OC1_6alkyl, or NHC(O)Aryl, said alkyl and the alkyl and aryl portions of
which are optionally substituted with 1-3 members selected from the group
consisting
of: C02H, C02C1_6alkyl, aryl, NH2, NHC1_3alkyl, NH-Aryl, N(C1_3alkyl)2 and
Hetcy
R2 is selected from the group consisting of:
(a) H, OH, halo, NH2, CN, C1_6alkyl, C2_6alkenyl, C2-6alkynyl,
C02H, Aryl and Hetcy;
(b) OC1_6alkyl and OC3_6alkenyl;
(c) -S(O)yCl_6alkyl, -S(O)yC3_6alkenyl, -S(O)yAryl and S(O)yHetcy,
wherein y is 0, 1 or 2;
(d) NHC 1 _6alkyl, NH-Aryl and NH-Hetcy;
-9-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
(e) C(O)C1_6alkyl, C(O)C3_6alkenyl and C(O)Hetcy;
(f) C(O)NH2, C(O)NHC1_6alkyl, C(O)N(C1_6alkyl)2, C(O)NH-Aryl
and C(O)N(C1_galkyl)-Aryl;
(g) NHC(O)C1_6alkyl, NHC(O)C3_6alkenyl, N(C1_6alkyl)C(O)C1_
6alkyl, N(C1_6alkyl)C(O)C3_6alkenyl and N(C1_6alkyl)C(O)Aryl;
(h) S(O)2NH2, S(O)2NHC1_6alkyl, S02NHHetcy, S(O)2NHC3_
(alkenyl, S(O)2N(C1_galkyl)2, S(O)2N(C1_(alkyl)C3_6alkenyl, S02NHAryl,
S02NH-Hetcy, S02N(C1_6alkyl)Aryl and S02N(C1_6alkyl)Hetcy;
(i) NHS02C1_6alkyl, NHS02C3_6alkenyl, N(C1_6alkyl)S02C1_
6alkyl and N(C1_6alkyl)S02C3_6alkenyl,
said C1_6alkyl, C2_6alkenyl, C3_6alkenyl and C2_6alkynyl groups and
portions in (a) through (i) above being optionally substituted with 1-6
members
selected from the group consisting of: halo, OH, NH2~ CN, C02H, Hetcy, Aryl,
C02C1_6alkyl, OC1_6alkyl, O-Aryl, C02C3_4alkenyl,,C(O)NH2, C(O)NHC1_3alkyl,
C(O)N(C1_3alkyl)2, C(O)NH-Aryl, C(O)N(C1_3alkyl)-Aryl, C(O)C1_3alkyl, C(O)C3_
4alkenyl, -S(O)yCl_3alkyl, -S(O)yC3_4alkenyl, S(O)y-(C1_3alkyl-aryl), wherein
y is
as previously defined; OC 1 _3alkyl-aryl, NH(C 1 _3alkyl-aryl), N(C 1
_3alkyl)C(O)C 1 _
3alkyl, N(C1_3alkyl)C(O)C3_4alkenyl, N(C1_3alkyl)C(O)Aryl, N(C1_
3alkyl)C(O)Hetcy, S(O)2NH2, S(O)2NHC1_3alkyl, S(O)2NHC3_4alkenyl,
S(O)2NHAryl, S(O)2NHHetcy, S(O)2N(C1_3alkyl)2, S(O)2N(C1_3alkyl)C3_
4alkenyl, S(O)2N(C1_3alkyl)Aryl, S(O)2N(C1_3alkyl)Hetcy, NHS03H, NHS02C1_
3alkyl, NHS02C3_4alkenyl, NHS02Ary1, NHS02Hetcy, N(C1_3alkyl)S03H, N(C1_
3alkyl)S02C1_3alkyl, N(C1_3alkyl)S02C3_4alkenyl, N(C1_3alkyl)S02Ary1 and
N(C 1 _3alkyl)S02Hetcy;
R3 represents H, halo or C1_3alkyl, and
R4 is selected from the group consisting of: H, C1_6alkyl, C2_6alkenyl,
C2_6alkynyl and Hetcy, said C1_6alkyl, C2_6alkenyl and C2_6alkynyl groups
being
optionally substituted with 1-6 members selected from the group consisting of:
halo,
OH, NH2~ NHC 1 _ l palkyl ~ N(C 1 _ l0alkyl)2, CN, C02H, Hetcy, Aryl, C02C 1
_6alkyl,
OC1_6alkyl, Oaryl, C02C1_3alkyl, C02C3_4alkenyl, C(O)NH2, C(O)NHC1_3alkyl,
C(O)N(C1_3alkyl)2, C(O)NH-Aryl, C(O)N(C1_3alkyl)-Aryl, C(O)C1_3alkyl, C(O)C3_
4alkenyl, -S(O)yCl_3alkyl, -S(O)yC3_4alkenyl, S(O)y-(C1_3alkyl-aryl), wherein
y is
as previously defined; OC1_3alkyl-aryl, NH(C1_3alkyl-aryl),
N(C1_3alkyl)C(O)C1_
3alkyl, N(C1_3alkyl)C(O)C3_4alkenyl, N(C1_3alkyl)C(O)Aryl, N(C1_
-10-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
3alkyl)C(O)Hetcy, S(O)2NH2, S(O)2NHC1_3alkyl, S(O)2NHC3-4alkenyl,
S(O)2NHAryl, S(O)2NHHetcy, S(O)2N(C1_3alkyl)2, S(O)2N(C1_3alkyl)C3_
4alkenyl, S(O)2N(C1_3alkyl)Aryl, S(O)2N(C1_3alkyl)Hetcy, NHS03H, NHS02C1_
3alkyl, NHS02C3_4alkenyl, NHS02Ary1, NHS02Hetcy, N(C1_3alkyl)S03H, N(C1_
3alkyl)S02C1_3alkyl, N(C1_3alkyl)S02C3_4alkenyl, N(C1_3alkyl)S02Aryl and
N(C 1 _3alkyl)S02Hetcy;
Aryl represents a 6-14 membered aromatic ring system and
Hetcy represents a 5-10 membered ring system, aromatic or non-
aromatic, containing at least one heteroatom and optionally containing up to 2
additional heteroatoms, said heteroatoms being selected from O, S(O)y with y
as
defined above and N,
said Aryl and Hetcy groups and portions thereof being optionally
substituted with 1-6 members selected from the group consisting of: -(CH2)0-4-
C02H, -(CH2)0-3C02C1-3alkyl, halo, CN, NH2, phenyl, pyrrolidinyl, NHCH3, C1_
6alkyl, S02NH2 and S02CH3.
A particular aspect of the invention that is of interest relates to a subset
of compounds of formula I wherein:
R 1 represents H, NH2, NHC 1 _6alkyl, NHC(O)C 1 _6alkyl,
NHC(O)OC1_6alkyl or NHC(O)Aryl, said alkyl and the alkyl and aryl portions of
which are optionally substituted with 1-2 members selected from the group
consisting
of: C02H, C02C1_6alkyl, aryl, NH2, NHC1_3alkyl, NH-Aryl and N(C1_3alkyl)2.
Within this subset, all other variables are as originally defined.
Another particular aspect of the invention that is of interest relates to a
subset of compounds of formula I wherein:
R2 is selected from the group consisting of:
(a) H, OH, halo, NH2, CN, C1_6alkyl, C2_6alkenyl, C2_6alkynyl,
C02H, Aryl and Hetcy;
(b) OC1_6alkyl and OC3_6alkenyl;
(c) -S(O)yCl_6alkyl, -S(O)yC3-6alkenyl, -S(O)yAryl and S(O)yHetcy,
wherein y is 0 or 2;
(d) NHC 1 _6alkyl;
(e) C(O)C1_6alkyl , C(O)C2_6alkenyl and C(O)Hetcy;
(f) C(O)NH2, C(O)NHC1_(alkyl, C(O)N(C1_6alkyl)2, C(O)NH-Aryl
and C(O)N(C1_6alkyl)-Aryl;
-11-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
(g) NHC(O)C1_6alkyl, NHC(O)C3_6alkenyl, N(C1_6alkyl)C(O)C1_
6alkyl, N(C1_6alkyl)C(O)C3_6alkenyl and N(C1_6alkyl)C(O)Aryl;
(h) S(O)2NH2, S(O)2~C1-6alkyl, S(O)2NHC3_6alkenyl,
S(O)2NHHetcy~ S(O)2N(C1_6alkyl)2 and S(O)2N(C1_6alkyl)C3_6alkenyl, and
(i) NHS02C1_6alkyl, NHS02C3_6alkenyl, N(C1_6alkyl)S02C1_
(alkyl and N(C1_6alkyl)S02C3_6alkenyl;
said C1_6alkyl, C2_6alkenyl, C3_6alkenyl and C2_6alkynyl groups and
the alkyl, alkenyl and alkynyl portions in (a) through (i) above being
optionally
substituted with 1-6 members selected from the group consisting of: halo, OH,
NH2,
CN, C02H, Hetcy, Aryl, C02C1_6alkyl, OC1_6alkyl, C02C1-3alkyl, C02C3_
4alkenyl, C(O)NH2, C(O)NHC1_3alkyl, C(O)N(C1_3alkyl)2, C(O)NH-Aryl,
C(O)N(C1_3alkyl)-Aryl, C(O)C1_3alkyl, C(O)C3_4alkenyl, -S(O)yCl_3alkyl, -
S(O)yC3_4alkenyl, S(O)y-(C1_3alkyl-aryl), wherein y is as previously defined;
OC1_
3alkyl-aryl, NH(C1_3alkyl-aryl), N(C1_3alkyl)C(O)C1_3alkyl,
N(C1_3alkyl)C(O)C3_
4alkenyl, N(C1_3alkyl)C(O)Aryl, N(C1_3alkyl)C(O)Hetcy, S(O)2NH2, S(O)2NHC1_
3alkyl, S(O)2NHC3_4alkenyl, S(O)2NHAryl, S(O)2NHHetcy, S(O)2N(C1_3alkyl)2,
S(O)2N(C1_3alkyl)C3_4alkenyl, S(O)2N(C1_3alkyl)Aryl, S(O)2N(C1_3alkyl)Hetcy,
NHS03H, NHS02C1_3alkyl, NHS02C3_4alkenyl, NHS02Ary1, NHS02Hetcy,
N(C1_3alkyl)S03H, N(C1_3alkyl)S02C1_3alkyl, N(C1_3alkyl)S02C3_4alkenyl,
N(C1_3alkyl)S02Aryl and N(C1_3alkyl)S02Hetcy;
Aryl represents a 6-14 membered aromatic ring system and
Hetcy represents a 5-14 membered ring system, aromatic, non-aromatic
or partially aromatic, containing at least one heteroatom and optionally
containing up
to 3 additional heteroatoms, said heteroatoms being selected from O, S(O)y
with y as
defined above and N,
said Aryl and Hetcy groups and portions thereof being optionally
substituted with 1-6 members selected from the group consisting of: -(CH2)0-4-
C02H, -(CH2)0-3C02C1-3alkyl, halo, CN, NH2, phenyl, pyrrolidinyl, NHCH3, C1_
(alkyl, S02NH2 and S02CH3. Within this subset, all other variables are as
originally
defined.
Another subset of compounds of the present invention that is of interest
relates to compounds of formula I wherein:
R3 represents H or C1_3alkyl. Within this subset, all other variables
are as originally defined.
-12-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Another subset of compounds of the present invention that is of interest
relates to compounds of formula I wherein:
R4 is selected from the group consisting of: H, C1_6alkyl, C2_6alkenyl,
C2_6alkynyl and Hetcy,
said C1_6alkyl, C2_6alkenyl and C2_6alkynyl groups being optionally
substituted with 1-6 members selected from the group consisting of: halo, OH,
NH2
CN, C02H, Hetcy, N(C1_10 alkyl)2, Aryl, C02C1_6alkyl, OC1_6alkyl, Oaryl,
C02C1_3alkyl, C02C3_4alkenyl, C(O)NH2, C(O)NHC1_3alkyl, C(O)N(C1_3alkyl)2,
C(O)NH-Aryl, C(O)N(C1_3alkyl)-Aryl, C(O)C1_3alkyl, C(O)C3_4alkenyl, -S(O)yCl_
3alkyl, -S(O)yC3_4alkenyl, S(O)y-(C1_3alkyl-aryl), wherein y is as previously
defined; OC1_3alkyl-aryl, NH(C1_3alkyl-aryl), N(C1_3alkyl)C(O)C1-3alkyl, N(C1_
3alkyl)C(O)C3_4alkenyl, N(C1_3alkyl)C(O)Aryl, N(C1_3alkyl)C(O)Hetcy,
S(O)2NH2, S(O)2NHC1_3alkyl, S(O)2NHC3_4alkenyl, S(O)2NHAryl,
S(O)2NHHetcy, S(O)2N(C1_3alkyl)2, S(O)2N(C1_3alkyl)C3_4alkenyl, S(O)2N(C1_
3alkyl)Aryl, S(O)2N(C1_3alkyl)Hetcy, NHS03H, NHS02C1_3alkyl, NHS02C3_
4alkenyl, NHS02Ary1, NHS02Hetcy, N(C1_3alkyl)S03H, N(C1_3alkyl)S02C1_
3alkyl, N(C1_3alkyl)S02C3-4alkenyl, N(C1_3alkyl)S02Ary1 and N(C1_
3alkyl)S02Hetcy;
Aryl represents a 6-14 membered aromatic ring system and
Hetcy represents a 5-10 membered ring system; aromatic or non-
aromatic, containing at least one heteroatom and optionally containing up to 2
additional heteroatoms, said heteroatoms being selected from O, S(O)y with y
as
defined above and N,
said Aryl and Hetcy groups and portions thereof being optionally
substituted with 1-3 members selected from the group consisting of: -(CH2)0-4-
C02H, -(CH2)0-3C02C1-3alkyl, halo, CN, NH2, phenyl, pyrrolidinyl, NHCH3, C1_
(alkyl, S02NH2 and S02CH3. Within this subset, all other variables are as
originally
defined.
A further subset of compounds within the present invention that is of
particular interest relates to compounds of formula I wherein:
R1 is selected from the group consisting of: H, NH2, NHC1_6alkyl,
NHC(O)C1_6alkyl, NHC(O)OC1_6alkyl and NHC(O)Aryl, said alkyl and the alkyl
and aryl portions of which are optionally substituted with 1-2 members
selected from
the group consisting of: C02H and C02C1_6alkyl, . Within this subset, all
other
variables are as originally defined.
-13-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Another subset of compounds of the present invention that is of
particular interest relates to a subset of compounds of formula I wherein:
R2 is selected from the group consisting of:
(a) H, OH, halo, NH2, C1_6alkyl, C2_6alkynyl, C02H, Aryl and
Hetcy;
(b) -S(O)yCl_6alkyl and S(O)yHetcy, wherein y is 0 or 2;
(c) C(O)Hetcy;
(d) C(O)NHC1_(alkyl and C(O)N(C1_6alkyl)2;
(e) NHC(O)C1_6alkyl;
(f) S(O)2NHC1_6alkyl, S(O)2NHHetcy and S(O)2N(C1_6alkyl)2,
and
(g) NHS02C 1_6 alkyl,
said C1_6alkyl and C2_6alkynyl groups and portions in (a) through (g)
above being optionally substituted with 1-2 members selected from the group
consisting of: CN, C02H, Aryl, O-Aryl, C02C 1 _6alkyl and OC 1 _6alkyl;
Aryl represents a 6-10 membered aromatic ring system and
Hetcy represents a 5-10 membered ring system, aromatic or non-
aromatic, containing at least one heteroatom and optionally containing up to 3
additional heteroatoms, said heteroatoms being selected from O, S and N,
said Aryl and Hetcy groups and portions thereof being optionally
substituted with 1-6 members selected from the group consisting of: -(CH2)0-6-
C02H and -(CH2)0-6C02C1-6alkyl. Within this subset, all other variables are as
originally defined.
Another subset of compounds of the present invention that is of
particular interest relates to compounds of formula I wherein R3 represents H.
Within
this subset, all other variables are as originally defined.
Another subset of compounds of the present invention that is of
particular interest relates to compounds of formula I wherein:
R4 is selected from the group consisting of: H and C1_4alkyl,
optionally substituted with a member selected from the group consisting of:
Hetcy,
N(C 1 _ l0alkyl)2, Aryl, O-Aryl, OC 1 _6alkyl, S(O)yC 1 _3alkyl, S (O)y-(C 1
_3alkyl-aryl),
wherein y is 0 or 2, OC1_3alkyl-aryl and NH(C1_3alkyl-aryl), wherein Aryl
represents
phenyl optionally substituted with 1-3 halo groups. Within this subset, all
other
variables are as originally defined.
-14-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Another subset of compounds of the present invention that is of
particular interest relates to compounds of formula I wherein:
R1 is selected from the group consisting of: H, NH2, NHC1_6alkyl,
NHC(O)C1_6alkyl, NHC(O)OC1_6alkyl and NHC(O)Aryl, said alkyl and the alkyl
and aryl portions of which are optionally substituted with 1-2 members
selected from
the group consisting of: C02H and C02C1_6alkyl;
R2 is selected from the group consisting of:
(a) H, OH, halo, NH2, C1_6alkyl, C2_6alkynyl, C02H, Aryl and
Hetcy;,
(b) -S(O)yCl_6alkyl and S(O)yHetcy, wherein y is 0 or 2;
(c) C(O)Hetcy;
(d) C(O)NHC 1 _6alkyl and C(O)N(C 1 _6alkyl)2;
(e) NHC(O)C 1 _6alkyl;
(f) S(O)2NHC1_6alkyl, S(O)2NHHetcy and S(O)2N(C1_6alkyl)2,
and
(g) NHS02C 1 _6 alkyl,
said C1_6alkyl and C2_6alkynyl groups and portions in (a) through (g)
above being optionally substituted with 1-2 members selected from the group
consisting of: CN, C02H, Aryl, O-Aryl, C02C1_6alkyl, OC1_6alkyl;
Aryl represents a 6-10 membered aromatic ring system and
Hetcy represents a 5-10 membered ring system, aromatic or non-
aromatic, containing at least one heteroatom and optionally containing up to 3
additional heteroatoms, said heteroatoms being selected from O, S and N,
said Aryl and Hetcy groups and portions thereof being optionally
substituted with 1-6 members selected from the group consisting of: -(CH2)0-6-
C02H and -(CH2)0-6C02C1-6alkyl;
R3 represents H, and
R4 is selected from the group consisting of: H and C1_4alkyl optionally
substituted with a member selected from the group consisting of: Hetcy, Aryl,
O-
Aryl, OC 1 _6alkyl, S(O)yC 1 _3alkyl, N(C 1 _ l0alkyl)2, S(O)y-(C 1 _3alkyl-
aryl), wherein
y is 0 or 2, OC1_3alkyl-aryl and NH(C1_3alkyl-aryl), wherein Aryl represents
phenyl
optionally substituted with 1-3 halo groups.
-15-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Representative examples of compounds of formula I are found in Table
I below.
TABLE I
Br
O
N / N
[J H
O
~C02H
OH
O
N / N
[J H
O
~C02H
NH2
O
N / N
3 H
0
~COZH
H
O~N ~ O
O N ~ I N~H
O ~C02H
O
I \ H II
N ~ N J'~
H
0
~COZH
-16-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
N / N
H
O
~C02H
H
HOZC~N I ~ H O
7 N~N
O '~ ~( H
O
~C02H
COzH
N \ O
B o N~N
H
O
~C02H
H
N ~ O
H02C~ I H
IOI N / N
H
9 0
COZH
C02H
0
N~N
'' ~ H
O
~C02H
-17-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
I/ N.~S wI
B'
11 O
~C02H
N O
H u
~ N~S
12 Br
w
o
~COzH
F
13 ~ I / N .~s
Ho
2
0
~COpH
F
14
I, N.~s ~I
0 0
~C02H
O F
HN I / N~S
15 O O
~COZH
CH3 I Nw O / F
~ N
~N~S \ I
16 ~
I / O O
COZH
-18-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
H
17 H3C02C~N N~ O
H ~
O I / N
H
O
~C02H
N~ O F
I H I
N~S
18
0
~C02H CI
N~ O CI /
I / N~O ~ I
19 Br
0
\COzH F
CF3C02' H +
N O F
~O I / N~S ~ ~ chiral
~cozH
O F
21
0
~coZH
N O F
22 \ I ,
a -
I / ° ~co2H
N S ~ ~ F
23 // o
~cozH
-19-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
H3C N~ O , F
N~S
24
0
~COZH
N
I N S~F
0 0
~cozH
26 H3COZC~N I / N~g ~ I F
O O
~COZH
2.7 i N O / F
\ I N I / N~g ~ I
O O
~COZH
N~
NC~N I / N . S \ I F
28
0 0
~cozH
N~ F
H .
29 H3C02C~N / N
O O
~COzH
N~ OII / F
Eco2c " ~
0 0
~co2H
-20-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
H
HOZC~N N~ O / F
31 '' ~o~ I / n"ys
i
0
~COZH
N
H3C02C~ N I
32
0 0
~COpH
N O / F
33 H3~~S ~ ~ N~s
0
~C02H
N O / F
34 H3C~S02I ~
I
O
~C02H
N~ O / F
35 ~~so21 ~ N~S ~
i
0
~C02H
N O / F
SOZ I / N~S
'NH
36 0
~C02H
-21-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
N~ O / F
3,~ NH.S02 / N~g
O
C02H
N O / F
~N, I / N
38 Sp2 ~S \
I
O
~C02H
CH3 N~ O / F
39 N~SO2 ~ /
0
~C02H
N~ O
H II
40 ~N.SOZ ~ / N~O
'''' ~v \
O
C02H
N O
H ~ II /
41 ~N.S02I / N~O
i ''~~
O ~C02H O \
N~ O F
42 ~N.S02 ~ / N J1 N \
\~O
\C02H F
-22-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
N~ O / F
43 ~'N N'SOZ I ~ N~S
I
O
~COzH
N~ O / F
44 \ ~ N,SO
0
COZH ~C02H
Br
O
45 N~N W
v ~O
~C02H
N~
O ~ N : S
46 t o -
~co2H
Nw H O
47 Br I ~ N
O
~C02H
CF3COZ ~ H +
N~ O / F
48 I ~ p ~ / N~g ~ ~ chirai
o
~COZH
-23-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
N~ O / F
49 H3COZC~N I / N~g
O 0
~COZH
N~ O / F
SO Ho2C~N I / N~g ~ I
101
~C02H
O I N~ H O / I F
S1 H3CpZC' v -NH / N~S
i
O
~COZH
N~ O / F
/ N~S
S2 H3cp2S
O
~COZH
N O / F
S3 w~soz I / N~s
i
0
~C02H
S4 N\ o
NC N,SO I / N~S
2
O
~C02H
-24-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
N O / F
H
55 CH3I ~ N~S
I
o
~C02H
N
H3CO2C~N_SO I / N~S
56
O
~COzH
N O
H
57 ~ N-S02 I / N J"
H
O
~C02H
N~ O
58 N, I / N N
sot ~ ~ _ ~/
0
~co2H
The compounds described herein, and in particular, in Table I, are
intended to include salts, enantiomers, esters and hydrates, in pure form and
as a
mixture thereof.
While chiral structures are shown below, by substituting into the
synthesis schemes an enantiomer other than the one shown, or by substituting
into the
schemes a mixture of enantiomers, a different isomer or a racemic mixture can
be
achieved. Thus, all such isomers and mixtures are included in the present
invention.
In another embodiment, the invention encompasses a method of
treating a caspase-3 mediated disease in a mammalian patient in need of such
treatment, comprising administering to said patient a compound of formula I in
an
amount effective to treat said caspase-3 mediated disease.
-25-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
In another embodiment, the invention encompasses a method of
treating cardiac and cerebral ischemia/reperfusion injury (e.g. stroke), type
I diabetes,
immune deficiency syndrome (including AIDS), cerebral and spinal cord trauma
injury, organ damage during transplantation, alopecia, aging, Parkinson's
disease,
Alzheimer's disease, Down's syndrome, spinal muscular atrophy, multiple
sclerosis
and neurodegenerative disorders, comprising administering to a mammalian
patient in
need of such treatment an effective amount of a compound of formula I.
In another embodiment, the invention encompasses a method of
treating acute disorders, including cardiac and cerebral ischemia/ reperfusion
injury
(e.g. stroke), spinal cord injury and organ damage during transplantation, in
a
mammalian patient in need of such treatment, comprising administering to said
patient
a compound of formula I in an amount effective to treat said acute disorder.
In another embodiment, the invention encompasses a method of
treating chronic disorders, including neurodegenerative diseases (e.g.
Alzheimer's,
polyglutamine-repeat disorders, Down's, spinal muscular atrophy, multiple
sclerosis),
immunodeficiency (e.g. HIV), diabetes, alopecia and aging, in a mammalian
patient in
need of such treatment, comprising administering to said patient a compound of
formula I in an amount effective to treat said chronic disorder.
In another embodiment, the invention encompasses a method of
treating a caspase-3 mediated disease in a mammalian patient in need of such
treatment, comprising administering to said patient a compound of formula I in
an
amount effective to treat said caspase-3 mediated disease.
In particular, these compounds are preferably useful to treat, prevent or
ameliorate in mammals and especially in humans, diseases including but not
limited
to:
cardiac and cerebral ischemia/reperfusion injury (e.g. stroke)
type I diabetes
immune deficiency syndrome (including AIDS)
cerebral and spinal cord trauma injury
organ damage during transplantation
alopecia
aging
Parkinson's disease
Alzheimer s disease
Down's syndrome
-26-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
spinal muscular atrophy
multiple sclerosis
neurodegenerative disorders.
The compound is administered to a mammalian patient in need of such
treatment or prevention an amount of a compound as described herein that is
effective
to treat or prevent the disease or condition.
The compounds described typically contain asymmetric centers and
may thus give rise to diastereomers and optical isomers. The present invention
includes all such possible diastereomers as well as their racemic and
resolved,
enantiomerically pure forms and pharmaceutically acceptable salts thereof.
Some of the compounds described herein contain olefinic double
bonds, and unless specified otherwise, are meant to include both E and Z
geometric
isomers.
The pharmaceutical compositions of the present invention comprise a
compound of formula I as an active ingredient or a pharmaceutically acceptable
salt
thereof in combination with a pharmaceutically acceptable carrier, and
optionally
other therapeutic ingredients. The term " pharmaceutically acceptable salts"
refers to
salts prepared from pharmaceutically acceptable bases including inorganic
bases and
organic bases. Representative salts derived from inorganic bases include
aluminum,
ammonium, calcium, copper, ferric, ferrous, lithium, magnesium, manganic
salts,
manganous, ammonium, potassium, sodium, zinc and the like. Particularly
preferred
are the calcium, magnesium, potassium, and sodium salts. Representative salts
derived from pharmaceutically acceptable organic bases include salts of
primary,
secondary and tertiary amines, substituted amines including naturally
occurring
substituted amines, cyclic amines, and basic ion exchange resins, such as
arginine,
betaine, caffeine, choline, N,N'-dibenzylethylenediamine, diethylamine, 2-
diethylaminoethanol, 2-dimethylaminoethanol, ethanolamine, ethylenediamine, N-
ethyl-morpholine, N-ethylpiperidine, glucamine, glucosamine, histidine,
hydrabamine,
isopropylamine, lysine, methylglucamine, morpholine, piperazine, piperidine,
polyamine resins, procaine, purines, theobromine, triethylamine,
trimethylamine,
tripropylamine, tromethamine and the like.
When the compound of the present invention is basic, salts may be
prepared from pharmaceutically acceptable non-toxic acids, including inorganic
and
organic acids. Examples of such acids include acetic, benzenesulfonic,
benzoic,
camphorsulfonic, citric, ethanesulfonic, fumaric, gluconic, glutamic,
hydrobromic,
-27-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
hydrochloric, isethionic, lactic, malefic, malic, mandelic, methanesulfonic,
mucic,
nitric, pamoic, pantothenic, phosphoric, succinic, sulfuric, tartaric, p-
toluenesulfonic
acid, and the like. Particularly preferred are citric, hydrobromic,
hydrochloric, malefic,
phosphoric, sulfuric and tartaric acids.
In the discussion of methods of treatment which follows, reference to
the compounds of formula I are meant to also include the pharmaceutically
acceptable
salts.
The ability of the compounds of formula I to inhibit caspase-3 make
them useful research tools in the field of apoptosis.
The magnitude of therapeutic dose of a compound of formula I will, of
course, vary with the nature of the severity of the condition to be treated
and with the
particular compound of formula I and its route of administration and vary upon
the
clinician's judgement. It will also vary according to the age, weight and
response of
the individual patient. An effective dosage amount of the active component can
thus
be determined by the clinician after a consideration of all the criteria and
using his/her
best judgement on the patient's behalf. A representative dose will range from
0.001
mpk/d to about 100 mpk/d.
An ophthalmic preparation for ocular administration comprising 0.001-
1% by weight solutions or suspensions of the compounds of formula I in an
acceptable
ophthalmic formulation may be used.
Any suitable route of administration may be employed for providing an
effective dosage of a compound of the present invention. For example, oral,
parenteral and topical may be employed. Dosage forms include tablets, troches,
dispersions, suspensions, solutions, capsules, creams, ointments, aerosols,
and the
like.
The compositions include compositions suitable for oral, parenteral
and ocular (ophthalmic). They may be conveniently presented in unit dosage
form
and prepared by any of the methods well-known in the art of pharmacy.
In practical use, the compounds of formula I can be combined as the
active ingredient in intimate admixture with a pharmaceutical carrier
according to
conventional pharmaceutical compounding techniques. The carrier may take a
wide
variety of forms depending on the form of preparation desired for
administration. In
preparing the compositions for oral dosage form, any of the usual
pharmaceutical
media may be employed, such as, for example, water, alcohols, oils, flavoring
agents,
preservatives, coloring agents and the like in the case of oral liquid
preparations, such
-28-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
as, for example, suspensions, elixirs and solutions; or carriers such as
starches, sugars,
microcrystalline cellulose, diluents, granulating agents, lubricants, binders,
disintegrating agents and the like in the case or oral solid preparations such
as, for
example, powders, capsules and tablets, with the solid oral preparations being
preferred over the liquid preparations. Because of their ease of
administration, tablets
and capsules represent the most advantageous oral dosage unit form in which
case
solid pharmaceutical carriers are obviously employed. If desired, tablets may
be
coated by standard aqueous or nonaqueous techniques.
Pharmaceutical compositions of the present invention suitable for oral
administration may be presented as discrete units such as capsules, cachets or
tablets
each containing a predetermined amount of the active ingredient, as a powder
or
granules or as a solution or a suspension in an aqueous liquid, a non-aqueous
liquid,
an oil-in-water emulsion or a water-in-oil emulsion. Such compositions may be
prepared by any of the methods of pharmacy but all methods include the step of
bringing into active ingredient with the carrier which constitutes one or more
necessary ingredients. In general, the compositions are prepared by uniformly
and
intimately admixing the active ingredient with liquid carriers or finely
divided solid
carriers or both, and then, if necessary, shaping the product into the desired
presentation. For example, a tablet may be prepared by compression or molding,
optionally with one or more accessory ingredients. Compressed tablets may be
prepared by compressing in a suitable machine, the active ingredient in a free-
flowing
form such as powder or granules, optionally mixed with a binder, lubricant,
inert
diluent, surface active or dispersing agent. Molded tablets may be made by
molding
in a suitable machine, a mixture of the powdered compound moistened with an
inert
liquid diluent. For example, each dosage unit may contain from about 0.01 mg
to
about 1.0 g of the active ingredient.
Method of Synthesis
Compounds of the present invention are conveniently prepared using
the procedures described generally below and more explicitly described in the
Example section thereafter.
Aspartyl aldehyde 1 is prepared as illustrated in Scheme 1. Reaction of
N-fluorenylmethyloxycarbonyl-L-aspartic acid ~3-tert-butyl ester (Fmoc-L-Asp
(OtBu)-OH) (2) (Novabiochem) with iso-butyl chloroformate (IBCF) followed by
-29-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
treating the reaction mixture with sodium borohydride gives alcohol 3. This is
oxidized under Swern conditions to give 1.
Scheme 1: Preparation of Aldehyde 1
O 1) IBCF, DIEA
FmocNH~OH ,h~~ -~goC - 0~C FmocNH' ~ OH
V '>
~C02-t-Bu 2) NaBH4, THF/MeOH ~CO~-t-Bu
2 3
O
FmocNH~
Swern ~/ ~ H
~ C02-t-Bu
1
This aldehyde may be loaded onto resin by the two-step procedure
shown in Scheme 2. Treatment of compound 4 (Webb et al, J. Am. Chem. Soc. 114,
3156 (1992)) with a commercial amino-Merrifield resin in the presence of EDCI
and
HOBT in dichloromethane followed by removal of the Boc group with
trifluoroacetic
acid (TFA) in dichloromethane afforded Resin A. Cleavage of the Fmoc group
using
piperidine in DMF provided Resin B. An appropriately substituted 3-
pyridylcarboxylic acid was then coupled with Resin B to provide Resin C using
a
peptide coupling reagent such as EDCI/HOBt or HATU/iPr2NEt. The desired
compound was then cleaved from the resin with 9:1 TFA:H20 with concomitant
cleavage of the t-butyl ester protecting group.
-30-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Scheme 2: Preparation of aldehyde caspase inhibitors
H H
,N N~ N~N~N~
H2N ~ 1 FmocNH~H IIO
~TFA O
EtOH. NH4CI _
~C02tBU
4
C02H
C02H
/ I ~NH EDCI, HOBT
,N N~ N~N N~
N
H N ~ O FmocNH~ O
~H H
piperidine
~C02tBu DMF \C02tBu
i _ ~ O i N O
\ ~ H \ ~ H
v
Resin B
R1 ~N
OH HATU/DIEA
R2
R3 O
Resin A
H H
Rt ~N N~N~N'
R2 \ ~ NH~H IOI 9:1 TFA/H20 R' N~ H O
R3 O ~ ~ R2 I / N~H
t-Bu-02C R3 O OH
Resin C
-31-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
The semicarbazide Resin D is prepared according to Scheme 3.
Treatment of compound 6 (Webb et al, J. Am. Chem. Soc. 114, 3156 (1992)) with
a
commercial amino-Merrifield resin in the presence of EDCI and HOBT in
dichloromethane followed by removal of the Boc group with trifluoroacetic acid
(TFA) in dichloromethane afforded Resin D.
Scheme 3: Preparation of semicarbazide Resin D
1. EDCI, HOBT
H ~ CHZCl2
Boc~ ~ N
2. TFA
O
NH2
6
COpH
H H
,N~N~
CF3CO2 +H3N
O
O
Resin D
Aspartyl ketone derivatives could be prepared as shown in Scheme 4.
An organometallic reagent such as an alkyl Grignard (RMgBr) can be added to
aldehyde 7 (prepared in direct analogy to aldehyde 1), and the resulting
alcohol
oxidized to the ketone with an oxidizing agent such as Dess-Martin
periodinane.
Ketone 9 can be loaded onto Resin D with catalytic HOAc in THF to provide
Resin E.
Cleavage of the Alloc group may be accomplished with Pd(PPh3)4 in the presence
of
pyrrolidine or tributyltin hydride, generating Resin F. This may be coupled
with
pyridine carboxylic acid derivatives as shown in Scheme 2 to generate
compounds of
the present invention.
-32-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Scheme 4: Preparation of Ketone Derivatives
O OH O
AIIocNH~ AIIocNH~ AIIocNH~
V 'H RMgBr V 'R Dess-Martin
> >
~ COrt-Bu ~ COrt-Bu ~ COrt-Bu
8 9
Resin D
H H
N~N~N~
H2N~ IOI N/N N~
R
Pd(PPhg)4 AIIocNH~
~ COptBu 1-- R
pyrrolidine
O or ~C02tBu
Bu3SnH
O
Resin F Resin E
Alternatively, ketone derivatives containing a heteroattim in the 0-
position could be prepared from bromomethylketone 11. As shown in Scheme 5,
reaction of N-fluorenylmethyloxycarbonyl-L-aspartic acid D-tent-butyl ester
(Fmoc-L-
Asp (OtBu)-OH) (1) (Novabiochem) with iso-butyl chloroformate (IBCF) followed
by
treating the reaction mixture with an excess of diazomethane yields the
diazomethylketone intermediate 10. This intermediate is subjected in situ to a
1:1
mixture of AcOH and 45°lo aqueous hydrobromic acid (HBr) to give
compound 11 as
a white powder.
-33-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Scheme 5: Preparation of Bromomethylketone 11
II 1) IBCF, DIEA O
FmocNH~ THF, -78°C - 0°C FmocNH /N2
\0H
2) CH2N2, Et20
~C02-t-Bu ~C02-t-Bu
1 10
O
FmocNH gr
AcOH/45%HBr (1:1)
~C02-t-Bu
11
The general procedure for the solid phase synthesis of nicotinic acid
derivatives I incorporating a sulfide P1'sulfur side chain is illustrated in
Scheme 6.
Bromomethyl ketone 11 is mixed with Resin D in THF in the presence of AcOH
overnight to furnish Resin G. Nucleophilic displacement with an appropriate
thiol in
the presence of suitable bases give Resin H as shown. The Fmoc group on Resin
H is
cleaved with 20% (v) piperidine in DMF and the resultant Resin I reacted with
a
substituted nicotinic acid using O-(7-Azabenzotriazol-1-yl)N,N,N',N'-
tetramethyluronium hexafluorophosphate (HATU) as the activating agent and
diisopropylethylamine (DIEA) as the base, affording Resin J. The final product
I is
released from solid support by treating Resin J with trifluoroacetic acid
(TFA) in
water (9/l, v/v).
-34-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Scheme 6: Preparation of thiomethylketone derivatives
H H
NfiN~N~
FmocNH~B~ O
Z-(CH2)a SH / base
tt Rest ~ DMF
C02-t-BU
Resin G
R' N~
H O 9: t TFA/H20
RZ ~ / N~S-(CHz)a-Z
R3 O \ /OH
~O
Nicotinic acid derivatives bearing a carboxamide substituent in the 5-
postion were prepared as shown in Scheme 7. Nucleophilic displacement of the
bromine in Resin G with 4-fluorobenzylmercaptan in the presence of suitable
bases
followed by Fmoc cleavage with 20°l0 (v) piperidine in DMF gave Resin
K. This resin
was reacted with pyridinedicarboxylic acid using O-(7-Azabenzotriazol-1-
yl)N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU) as the activating
agent and diisopropylethylamine (DIEA) as the base, affording Resin L. This
resin
may be coupled with primary or secondary amines, or anilines using HATU and
DIEA to give the resin-bound amides (Resin M). The final product I is released
from
solid support by treating Resin M with trifluoroacetic acid (TFA) in water
(9/1, v/v).
-35-
R' ~N
Y 1 I 1) Pip/DMF
R2%~OH J 2) HATU/DIEA
TTR3 0O
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Scheme 7: Preparation of pyridinedlamide derivatives
F N~
HS I / HzN '
1)
iPr2NEt, DMF S(4-F-Bn)
t-Bu-02C~
2) Piperidine, DMF
Resin K
HO ~ I OH ~ HATU/DIEA
O O
H H
Nw H O RZNH, HATU, ~ I NON N'
R N I N~S-(CH2)a-Z DIEA HC~~NH O
z
O O \'0H 9:1 TFA/H20 O O C/ S(4-F-Bn)
t-Bu-02
la O WN
Nicotinic acid derivatives containing a sulfone in the 5-position were
prepared as shown in Scheme 8. Thus 5-bromonicotinic acid underwent Fischer
esterification followed by sodium thiomethoxide displacement of the bromine to
give
thioether 14. Oxidation of the sufide to the sulfoxide 15 could be
accomplished with
an oxidant such as MMPP. Pummerer rearrangement was achieved by warming with
TFAA, then concentrating and treating the residue with triethylamine in MeOH
to
give the thiol 16. This thiol may be alkylated with alkyl halides in DMF in
the
presence of an amine base. The resulting sulfide 17 can be oxidized to the
sulfone
with an oxidant such as MMPP, and the ester can be hydrolized under basic
conditions to give nicotinic acid derivative 18. This acid may be coupled to
Resin K
as previously described, and the resin cleaved with TFA/H20 to provide the
sulfone
derivatives which are examples of the present invention.
-36-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Scheme 8: Preparation of Sulfone Derivatives
Br \ COOH
Br \ COOMe MeS \ COOMe
MeOH I / MeSNa
N ----~ N
HZS04 DMF N
14
12 13
MMPP
RS \ COOMe HS \ COOMe O COOMe
E ~R-Br I ~ 1)TFAA
i
N DIEA, DMF N
2) Et3N, MeOH N
17 16
1 ) MMPP
2) LiOH
O~ ~O O COOH
R~S \ COOH 1) Resin K, O~ ,O
HATU, DIEA R~S N S \
i
N 2) TFA/H20 ~ ~ H O
18 N F
Nicotinic acid derivatives containing a sulfonamide in the 5-position
were prepared as shown in Scheme 9. The ester moiety of thiol 16 (or its
5 corresponding disulfide) may be hydrolized under basic conditions to give
acid 20
(or its corresponding disulfide). Oxidation to the sulfonyl chloride 21 may be
accomplished with chlorine gas in HOAc. Primary or secondary amines may be
reacted with 21 to give sulfonamides 22 . These acids may be coupled to Resin
K as
previously described, and the resin cleaved with TFA/H20 to provide the
sulfonamide
10 derivatives which are examples of the present invention.
-37-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Scheme 9: Preparation of Sulfonamide Derivatives
HS \ COOMe HS \ COOH C~ SO \ COOH
LiOH ~ C12
i
N HOAc N
19 20 21
R~NH
COOH
Q 1 ) Resin K, Q O
R2N~S \ N S \ H~ RzN~S/ \ COOH
H Q ~ / 2) T'FA/Hz0
N ~F N
22
Caspase inhibitors containing aryloxymethylketones and
acyloxymethyl ketones may be prepared as shown in Scheme 10. Resin G in DMF
may be treated with a phenol or substituted phenol in the presence of a base
such as
cesium carbonate to give Resin M. Alternatively, Resin G in DMF may be treated
with a carboxylic acid, preferably an aromatic acid, in the presence of a base
such as
potassium fluoride to give Resin M. Coupling of an appropriately substituted
nicotinic acid under standard conditions then provides Resin N, which may be
cleaved
with TFA/H20 to provide compounds of the present invention.
-38-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Scheme 10: Preparation of oxymethylketones
FmocN
1) ROH
base, DMF
2) Piperidine, DMF
R= aryl or acyl
R' H O 9:1 TFA/H20
R I i N~OR4
z
Rs O ~OH
IIO
Caspase inhibitors containing aryloxymethylketones and
acyloxymethyl ketones may be prepared as shown in Scheme 11. Resin O is
prepared
from Alloc-L-Asp (OtBu)-OH as described in Schemes 5 and 6. Treatment of Resin
O with a primary amine in DMF followed by Boc protection generates Resin P.
The
Alloc group may then be removed using a palladium catalyst and a hydride
source.
The resulting amine may be coupled with an appropriately substituted nicotinic
acid
under standard conditions to provide Resin Q. This may be cleaved with TFA/H20
to
provide compounds of the present invention.
-39-
H H
R~ ,N
R ~ I OH ~ HATU/DIEA
2
R3 U
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Scheme 11: Preparation of aminomethylketones
N~N~Nw N~N~Nw
AIIocNH~B O AIIocNH~ O
1) RNH2, DMF NRBoc
~C02-t-Bu -t-Bu-02C
N O 2) Boc20
Ii H ~i H
Resin O Resin P
R= aryl or acyl
1 ) Pd(PPh3)a
PhSiH3 R~ ,N
2) HATU/DIEA R2'~OH
R3 O
R~ ~/~1 H O 4. 9:1 TFA/H20 R~ N I N
R2~~~N~NHR . R2%
'R3 IOI OH R3 O
t-Bu-02
Resin D
The invention is further illustrated using the following non-limiting
examples.
-40-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 1
(3S)-3-[(5-BROMO-3-PYR>DYL)CARBONYL]AMINO-4-OXO-BUTANOIC ACID
O
N
Br - H
'C02H
Step 1:
t-Butyl (3S)-3-[(9H-9-fluorenylmethoxy)carbonyl]amino-4-hydroxy-butanoate (3)
H
FmocN
OOH
~C02-tBu
To a solution of N-Fmoc-L-aspartic acid ~i-tert-butyl ester (10.01 g,
24.3 mmol) in 100 mL of tetrahydrofuran (THF) at 0 °C was added N-
methylmorpholine (NMM, 2.9 mL, 26.7 mmol) followed by isobutyl chloroformate
(IBCF, 3.3 mL, 25.6 mmol). After stirnng for 45 minutes at 0 °C,. this
mixture was
cooled to -78 °C for 15 minutes. To the mixture was then added sodium
borohydride
(1.93 g, 51 mmol) followed by 20 mL MeOH. The mixture was stirred at -78
°C for
2h, the quenched with saturated aqueous ammonium chloride. The mixture was
extracted three times with ethyl acetate (250 mL). The combined organic layers
were
washed with brine, dried over magnesium sulfate, filtered and concentrated.
The
crude product was purified by flash chromatography. Eluting with hexanes/ethyl
acetate (1:1 to 1:9) afforded the desired product as a white powder (9.14 g).
1H NMR (400 MHz, acetone-d6): 8 7.85 (d, 2H), 7.69 (d, 2H), 7.41 (t, 2H), 7.32
(t,
2H), 7.02 (bd, 1H, NH), 4.70 (dd, 1H), 4.51-4.41 (m, 2H), 4.38-4.30 (m, 2H),
4.25 (t,
1H), 2.85 (dd, 1H), 2.70 (dd, 1H), 1.41 (s, 9H).
Step 2 t-Butyl (3S)-3-[(9H-9-fluorenylmethoxy)carbonyl]amino-4-oxo-butanoate
(1)
-41-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
O
FmocN
H
z
NCO 2-tBu
To a -78°C solution of oxalyl chloride (3.7 mL, 41.9 mmol) in 275
mL
CH2C12 was added DMSO (5.5 mL, 76.8 mmol) dropwise. The resulting solution
was stirred 20 min, then a solution of the alcohol from Step 1 (13.88 g, 34.9
mmol) in
75 mL CH2C12 was added via cannula. The mixture was stirred for 1h, then
diisopropylethylamine (18 mL, 105 mmol) was added . The mixture was stirred 1h
at
-78 °C, and -15 °C for 20 min, then poured into 1M HCI, and
extracted with CH2CI2.
The organic phase was washed with NaHC03 solution, brine and dried over MgS04.
Evaporation provided 13.97 g of the title compound.
Step 3: Semicarbazone
H i..,.,
N' N H
FmocN~ OH
H O
~C02-tBu
To a solution of crude aldehyde 2 (13.97 g, 35 mmol) in 100 mL EtOH
was added NaOAc (3.11 g, 38 mmol), followed by the Webb semicarbazide, TFA
salt
(11.36 g, 34.5 mmol) as a solution in 220 mL EtOH and 110 mL H20. The mixture
was stirred 15 h at RT, giving a ppt. The reaction mixture was partitioned
between
1M NaOH and EtOAc and extracted 3x with EtOAc. The combined organic layers
were washed with brine and dried over Na2S04. Purification by flash
chromatography (10% MeOH in CH2 C12 to 10°1o MeOH, 10°1o THF in
CH2Cl2)
provided 17.56 g of the title compound.
Step 4 Resin A
To a solution of HOBt (3.26 g, 24.1 mmol) in 250 mL CH2CI2 was
added a solution of semicarbazone (11.91 g, 20.1 mmol) in 100 mL CH2C12. Amino-
Merrifield resin (19.14 g, 13.4 mmol) was then added, and the suspension was
cooled
to 0 °C. EDCI (4.62 g, 24.12 mmol) was added in three portions, and the
mixture was
stirred 23 h at RT. The resin was then filtered and washed with aq. NH4C1
(6x), water
-42-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
(2x), aq. NaHC03 (3x), water (3x), 50% aq THF (3x), THF (3x), EtOAc (lx) and
CH2C12 (lx), then dried in vacuo to give 29.49 g of the title compound.
Step S Resin B
152 mg of resin was suspended in 2 mL DMF. Piperidine (0.4 mL) was
added, and the mixture was rotated for 25 min. The resin was filtered and
washed
with DMF (3 x), THF (3x) and CH2Cl2 (3x)
Step 6 (3S)-3-[(5-Bromo-3-pyridyl)carbamoyl)amino-4-oxo-butanoic acid
The resin from Step 5 was suspended in 1.6 mL DMF and EDCI (87
mg, 0.45 mmol), HOBt (70 mg, 0.45 mmol) and 5-bromonicotinic acid (77 mg, 0.38
mmol) were added. The mixture was rotated at RT for 3h, then filtered and
washed
with DMF, THF, CH2C12 and EtOAc (3x each). The resin was then treated with 9:1
TFA:H20 (2.5 mL) and rotated for 35 min. The resin was filtered and washed
with
9:1 TFA:H20 (2 x 2.5 mL), and the filtrates were concentrated. The residue was
dissolved in 1:1 HOAc:H20 and lyophilized to provide 19.3 mg of the title
compound.
1H NMR (D20, 400 MHz) 8 8.65 (m, 2H), 8.23 (br s, 1H), 5.00 (d,
1H), 4.27 (m, 1H), 2.51 (dd, 1H), 2.32 (dd, 1H).
EXAMPLE 2
(3S)-3-[(5-HYDROXY-3-PYR>DYL)CARBONYL]AMINO-4-OXO
BUTANOIC AC>D
1H NMR (D20, 400 MHz) 8 8.44 (m, 1H), 8.25 (m, 1H), 8.08 (m,
1H), 4.95 (d, 1H), 4.30 (m, 1H), 2.65 (dd, 1H), 2.48 (dd, 1H).
EXAMPLE 3
(3S)-3-[(5-AMINO-3-PYR117YL)CARBONYL]AMINO-4-OXO-BUTANOIC AC>D
1H NMR (D20, 400 MHz) S 8.22 (s, 1H), 7.98 (m, 1H), 7.95 (m, 1H),
4.95 (d, 1H), 4.28 (m, 1H), 2.65 (dd, 1H), 2.48 (dd, 1H).
-43-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 4
(3S)-3-[(6-(9H-9-(FLUORENYLMETHOXY)CARBONYLAMINO)-3
PYRIDYL)CARBONYL]AMINO-4-OXO-BUTANOIC ACID
1H NMR (d6-DMSO, 400 MHz) 8 8.86 (m, 1H), 8.77 (m, 1H), 8.17
(m, 1 H), 7.89 (m, 2H), 7.80 (m, 2H), 7.42 (m, 2H), 7.33 (m, 2H), 4.97 (d, 1
H), 4.4-4.2
(m, 4H), 3.0 (m, 1H), 2.7 (m, 1H).
EXAMPLE 5
(3S)-3-[(3-PYRIDYL)CARBONYL]AMINO-4-OXO-BUTANOIC ACID
1H NMR (D20, 400 MHz) 8 8.70 (br s, 1H), 8.50 (m, 1H), 8.02 (m, 1H), 7.40 (m,
1H), 4.96 (d, 1H), 4.27 (m, 1H), 2.50 (dd, 1H), 2.31 (dd, 1H).
EXAMPLE 6
(3S)-3-[(6-AMINO-3-PYRIDYL)CARBONYL]AMINO-4-OXO-BUTANOIC ACm
1H NMR (D20, 400 MHz) 8 8.15 (m, 1H), 8.00 (m, 1H), 6.88 (d, 1H), 4.98 (d,
1H),
4.30 (m, 1 H), 2.68 (dd, 1 H), 2.49 (dd, 1 H).
EXAMPLE 7
(3S)-3-[((6-((3-CARBOXYPROPANOYL)AMINO)-3-
PYRIDYL)CARBONYL)]AMINO-4-OXO-BUTANOIC ACID
Resin B was reacted with 6-aminonicotinic acid as described in
Example 1, Step 6. The resulting resin-bound aniline (104.8 mg) was suspended
in 2
mL of pyridine with 77 mg of succinic anhydride and heated to 94°C for
4 h. The
resin was washed with DMF (3 x), THF (3x) and CH2C12 (3x). The resulting resin
was treated with TFA as described in Example 1, Step 6 to provide the title
compound
as a mixture of cyclic acetals.
1H NMR (D20, 400 MHz) 8 8.7-8.1 (m, 2H), 7.35 (m, 1H), 4.95 (m,
0.6H), 4.80 (m, 0.4H), 4.28 (m,0.6H), 4.02 (m, 0.4H), 2.7-2.3 (m, 6H).
-44-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 8
2-(((5-((((1S)-2-CARBOXY-1-FORMYLETHYL)AMINO)CARBONYL)-2-
PYRLDYL)AMINO)CARBONYL)BENZOIC AC>D
1H NMR (D20/d6-acetone, 400 MHz) 8 8.55 (m, 1H), 8.15 (m, 1H),
7.95 (m, 1H), 7.83 (d, 1H), 7.56 (m, 1H), 7.50 (m, 1H), 7.44 (m, 1H), 5.00 (d,
1H),
4.32 (m, 1H), 2.68 (dd, 1H), 2.50 (dd, 1H).
EXAMPLE 9
(3S)-3-[((6-((4-CARBOXYBUTANOYL)AMINO)-3-
PYR>DYL)CARBONYL)]AMINO-4-OXO-BUTANOIC ACID
1H NMR (D20, 400 MHz) 8 8.50 (m, 1H), 8.26 (m, 1H), 7.45 (d, 1H),
4.95 (d, 1H), 4.28 (m, 1H), 2.65 (m, 2H), 2.43 (m, 2H), 2.27 (m, 2H), 1.75 (m,
2H).
EXAMPLE 10
4-(5-((((1S)-2-CARBOXY-1-FORMYLETHYL)AMINO)CARBONYL)-3-
PYRLDYL)BENZOIC ACID
Resin B was reacted with 5-bromonicotinic acid as described in
Example 1, Step 6. The resulting resin (0.5 mmol/g, 153 mg) was suspended in
DME
(2 mL). Aq. sodium carbonate (2M, 0.19 mL), 4-carboxyphenylboronic acid (64
mg,
0.38 mmol) and Pd(Ph3P)4 (9 mg, 0.008 mmol) were added, and the mixture was
heated at 90 °C for 16h, then cooled. The resin was filtered and washed
with aq
NH4Cl (3 x), H20 (3x), THF (3x) and CH2Cl2 (3x). The resulting resin was
treated
with TFA as described in Example 1, Step 6 to provide the title compound.
1H NMR (D20, 400 MHz) 8 8.82 (s, 1H), 8.79 (s, 1H), 8.30 (m, 1H),
7.82 (d, 2H), 7.64 (d, 2H), 4.98 (d, 1H), 4.27 (m, 1H), 2.49 (m, 2H), 2.32 (m,
2H).
EXAMPLE 11
(3S)-5-(BENZYLSULFANYL)-3-[(5-BROMO-3-PYRIDYL)CARBONYL]AMINO
4-OXOPENTANOIC ACID
Step 1: t-Butyl (3S)-5-bromo-3-[(9H-9-fluorenylmethoxy)carbonyl]amino-4-oxo-
pentanoate (11)
To a solution of N-Fmoc-L-aspartic acid (3-tert-butyl ester (21.0 g, 51.0
mmol) in 300 mL of tetrahydrofuran (THF) at -78 °C was added N-
methylmorliholine
-45-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
(NMM, 7.9 mL, 71.4 mmol) followed by isobutyl chloroformate (IBCF, 8.6 mL,
66.3
mmol). After stirring for 30 minutes at -78 °C, this mixture was warmed
to -15 °C
for 15 minutes. To the mixture was then added twice, in a 10 minutes interval,
a
solution of diazomethane in ether (1 M, 40 mL) with stirring. The mixture was
allowed to warm to 0°C and to it was added another 60 mL of the
diazomethane
solution. The solution was then warmed to room temperature and stirred for 10
minutes, recooled back to 0 °C and treated with a solution of HBr (48%
aqueous)/AcOH (1/1, v/v, 100 mL) for 5 minutes, diluted with ethyl acetate and
water.
The organic phase was separated, washed with water and brine, dried over
magnesium
sulfate, filtered and concentrated. The crude product was purified by flash
chromatography. Eluting with hexanes/ethyl acetate (3:1) afforded the desired
product as a white powder (20 g, 81 % yield).
1H NMR (400 MHz, acetone-d6): 8 7.85 (d, 2H), 7.69 (d, 2H), 7.41 (t,
2H), 7.32 (t, 2H), 7.02 (bd, 1H, NH), 4.70 (dd, 1H), 4.51-4.41 (m, 2H), 4.38-
4.30
(2xd, 2H), 4.25 (t, 1H), 2.85 (dd, 1H), 2.70 (dd, 1H), 1.41 (s, 9H).
Step 2: Preparation of Resin D
A suspension of amino-Mernfield resin (Novabiochem, 30 grams, 31.2
mmol), acid 6 (14.7 g, 46.8 mmol), EDCI (10.77 g, 56.12 mmol) and HOBT (8.6 g,
56.16 mmol) in DMF (240 mL) was shaken on a orbital shaker at 190 rpm
overnight.
The mixture was filtered and the residual resin washed sequentially with DMF,
methanol, dichloromethane and methanol and dried under vacuum. The resin then
was suspended in a solution of TFA/dichloromethane (1:2, 300 mL) and shaken
for 2h
on a orbital shaker. The suspension was filtered, washed with dichloromethane
(5x)
and methanol (5x) and then dried under vacuum overnight to yield Resin D (40.5
g,
0.81 mmol/g).
Step 3:
Loading of ketone 11 to Resin D
A suspension of ketone 11 (4.5 g, 9.22 mmol) and Resin D (8.8g, 7.13
mmol) in THF (70 mL) in the presence of AcOH (0.2 mL, 3.4 mmol) was shaken on
a
orbital shaker at 200 rpm overnight. The suspension was filtered and residual
resin
was washed sequentially with THF, dichloromethane, ethyl acetate and diethyl
ether.
Drying under high vacuum afforded Resin G (11.7 g).
-46-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Step 4:
Preparation of Resin I
O
O N ~~~'~
~NH
N
H2N
S
'COrt-Bu Resin I
To a suspension of Resin G (1.6 g) in DMF (6 mL) in a fritted reservoir
was added a solution of benzylmercaptan (5.5 mL, 1 M in DMF) and N,N-
diisopropylethylamine (DIEA) and the mixture was rotated on a disc (Glas-
CoITM)
for 3h and filtered. The resin was washed with DMF and then subjected to a
solution
of 20% piperidine in DMF for 20 minutes and then washed sequentially with DMF,
methanol, dichloromethane and methanol and dried under high vacuum to afford
Resin I.
Step 5:
(3S)-5-(Benzylsulfanyl)-3-[(5-bromo-3-pyridyl)carbonyl]amino-4-oxopentanoic
acid
Resin I (100 mg, 0.05 mmol) was suspended in 3 mL DMF and HATU
(96 mg, 0.25 mmol) and 5-bromonicotinic acid (51 mg, 0.25 mmol) were added.
DIEA (0.04 mL, 0.25 mmol) was then added and the mixture was rotated at RT for
3h, then filtered and washed with DMF, MeOH, THF, and CH2C12 (3x each). The
resin was then treated with 9:1 TFA:H20 (2.5 mL) and rotated for 35 min. The
resin
was filtered and washed with CH3CN and the filtrates were concentrated to
provide
16 mg of the title compound.
1H NMR ( 400 MHz, acetone-d6): 8 9.10 (br s, 1H, NH), 8.55 (br s,
1H), 8.45 (s, 2H), 7.35-7.20 (m, 5H), 5.25 (q, 1H), 3.75 (s, 2H), 3.50 (dd,
2H), 3.05
(dd, 1H), 2.88 (dd, 1H). MS: m/z 437.2, 439.2 (M+1).
-47-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 12
(3S)-5-(PHENYLSULFANYL)-3-[(5-BROMO-3-PYR>DYL)CARBONYL]AMINO-
4-OXOPENTANOIC ACID
Step 1:
Resin G (100 mg), DMF (3 ml) and thiophenol (26 p1) were mixed
together in a fritted syringe. It was shaken for 30 sec., then (iPr)2NEt (44
p,1) was
added. It was rotated for 2h. The resin was washed with DMF (4x), MeOH (4x),
THF (4x) and CH2C12 (4x). It was dried under a N2 flow for 10 min.
Step 2:
The resin thus obtained and 3.0 ml of a 20% piperidine/DMF v/v
solution were mixed together in a fritted syringe and it was rotated for 25
min. The
resin was washed with DMF (4x), THF (4x) and CH2C12 (4x). It was dried under
N2
flow for 10 min.
Step 3:
5-Bromonicotinic acid (51 mg) was coupled to the resin as described
previously and the coupled resin was treated with TFA/H20 to afford the title
compound ( 18 mg).
1H NMR ( 400 MHz, acetone-d6): 8 9.05 (br s, 1H, NH) 8.55 (br s,
1H), 8.40 (s, 2H), 7.35 (d, 2H), 7.25 (t, 2H), 7.16 (t, 1H), 5.20 (q, 1H),
4.20 (s, 1H),
3.05 (dd, 1H), 2.92 (dd, 1H). MS: m/z 423.0, 425.0 (M+1).
EXAMPLE 13
(3S)-5-(((3-(4-(FL,UOROBENZYL)SULFANYL)-1-CARBOXYMETHYL)-2
OXOPROPYL)AMINOCARBONYL)NICOTINIC AC)D
Step 1
A mixture of Resin K (1.52 g, 0.76 mmol), 3,5-pyridinedicarboxylic
acid (1.44g, 8.6 mmol) and HATU (1.10 g, 2.9 mmol) was suspended in 10 mL DMF
and shaken. Diethylisopropylamine (1.2 mL, 6.9 mmol) was then added and the
mixture rotated for 2.5 h. The resin was washed with DMF (3x), MeOH (3x), THF
(3x) and CH2Cl2 (3x) and dried to give Resin L.
-48-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Step 2
60 mg of Resin L was treated with 9:1 TFA:H20 and rotated for 15
min. The resin was, filtered and washed with acetonitrile, and the filtrates
were
concentrated to give 9 mg of the title compound.
1H NMR ( 400 MHz, acetone-d6): 8 9.28 (br s, 2H), 8.79 (m, 1H),
8.67 (d, 1H), 7.37 (m, 2H), 7.03 (m, 2H), 5.26 (dd, 1H), 3.73 (s, 2H), 3.58
(d, 1H),
3.45 (d, 1 H) 3 .10 (dd, 1 H), 2.90 (dd, 1 H).
EXAMPLE 14
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-
((DIETHYLAMINO)CARBONYL)-3-PYRIDYL)CARBONYL]AMINO-4
OXOPENTANOIC ACID
A mixture of Resin L (74 mg) and HATU (60 mg) was suspended in
DMF (2 mL). Excess diethylamine (~0.5 mL) was added and the mixture was
rotated
for 1.5h. The resin was washed with DMF (3x), MeOH (3x), THF (3x) and CH2C12
(3x). The resin was then treated with 9:1 TFA:H20 and rotated for 15 min. The
resin was filtered and washed with acetonitrile, and the filtrates were
concentrated to
give 13 mg of the title compound.
MS (APCI, neg. ion) m/z 474 (M-1, 100), 419 (45), 334 (15), 288 (43).
EXAMPLE 15
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-((BENZYLAMINO)CARBONYL)
3-PYR)DYL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
MS (APCI, neg. ion) m/z 508 (M-1, 100), 419 (45), 334 (15), 288 (43).
EXAMPLE 16
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-((N-PHENYL-N
METHYLAMINO)CARBONYL)-3-PYRIDYL)CARB AMOYL] -4
OXOPENTANOIC ACID
MS (APCI, neg. ion) m/z 508 (M-l, 70), 419 (100), 279 (30).
-49-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 17
(3S)-3-[((6-((3-(CARBOXYMETHYL)PROPANOYL)AMINO)-3
PYRll~YL)CARBONYL)]AMINO-4-OXO-BUTANOIC ACID
1H NMR ( 400MHz, acetone-d6): 810.35 (br s, 1H), 8.88 (s, 1H), 8.40-
8.23 (m, 3H), 4.55 (t, 1H), 3.62 (s, 3H), 3.10 (dd, 1H), 2.91-2.83 (m, 2H),
2.75-2.65
(m, 2H), 2.60 (dd, 1H). MS: m/z 352.4 (M+1).
EXAMPLE 18
(3S)-5-[(2'CHLORO-6'-FLUOROBENZYL)SULFANYL]-3-[(5-BROMO-3-
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
1H NMR (400 MHz, acetone-d6): 8 9.05 (br s, 1H), 8.85 (br s, 1H),
8.55 (br s, 1H, NH), 8.40 (br s, 1H), 7.35-7.25 (m, 2H), 7.12-7.06 (m, 1H),
5.18 (q,
1H), 3.90 (s, 2H), 3.75 (s, 2H), 3.05 (dd, 1H), 2.90 (dd, 1H). MS: m/z 489.6
(M+1).
EXAMPLE 19
(3S)-5-[(2' CHLORO-6'-FLUOROBENZYLOXY]-3-[(5-BROMO-3
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC AC>D
1H NMR ( 400 MHz, acetone-d6): b 9.13 (br s, 1H), 8.97 (br s, 1H),
8.55 (br s, 1 H), 8.45 (br s, 1 H), 7.48-7.37 (m, 1 H), 7.31 (t, 1 H), 7.15
(q, 1 H), 5.10 (q,
1H), 4.82-4.72 (m, 2H)4.58-4.45 (m, 2H), 2.98-2.80 (m, 2H). ). MS: m/z 473.2,
475.1,
477.0 (M+1).
EXAMPLE 20
(3S)-5-((4-FLUOROBENZYL)SULFANYL)-3-[(5-(ISOPROPOXYMETHYL)-3-
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC AC>D
Step 1 3-Bromo-5-hydroxymethylpyridine
To a suspension of 5-bromonicotinic acid (8.5 g, 42 mmol) in 120 mL
EtOH was added conc. sulfuric acid (2 mL). The mixture was heated to reflux
for
15h, then cooled and concentrated. The residue was partitioned between ether
and
saturated NaHC03. The organic phase was washed with brine, dried over MgS04
and evaporated to give 9.4 g of a colorless oil. This material was dissolved
in 150 mL
of EtOH and treated with NaBH4 (10.3 g, 272 mmol). The mixture was stirred for
2
days at room temperature, then was quenched with 30 mL water and was
concentrated. The residue was diluted with water and was extracted 4x with
CH2C12.
The combined organic phases were washed with brine, filtered through cotton
and
-50-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
evaporated to give 7 g of an oil. Purification by flash chromatography (ether)
gave 3.2
g of the title compound.
Step 2 3-Bromo-5-(isopropoxymethyl)pyridine
To a 0 °C solution of 3-bromo-5-hydroxymethylpyridine (211 mg,
1.22
mmol) in CH2C12 (10 mL) was added Et3N (0.4 mL) and MsCI (0.13 mL, 1.7 mmol).
The solution was stirred for 45 min, then isopropanol (1 mL) was added, and
the
solution was stirred overnight. Concentration followed by flash chromatography
(50% ether/hexanes) provided 130 mg of 3-bromo-5-chloromethylpyridine. This
material was dissolved in isopropanol and treated with sodium hydride and
tetramethylammonium iodide. The reaction mixture was warmed to 50 °C
overnight,
then concentrated and partitioned between EtOAc and water. The organic phase
was
washed with brine and dried over MgS04 to give 130 mg of the title compound.
Step 3 5-(Isopropoxymethyl)nicotinic acid
A mixture of 3-bromo-5-(isopropoxymethyl)pyridine (130 mg, 0.56
mmol), PdCl2(Ph3P)2 (9 mg, 0.013 mmol), PPh3 (26 mg, 0.10 mmol) and Bu3N (125
mg, 0.67 mmol) were combined in a 2 mL vial with a stirbar. Degassed water
(0.03
mL, 1.6 mmol) was added and the vial was placed in a stainless steel bomb. The
bomb was charged with CO (200 psi), and heated to 120 °C for 20 h (ref:
J. Org.
Chem. 46, 4614, 1981). The bomb was cooled and depressurized, and the reaction
mixture was partitioned between ethyl acetate and aq. ammonium chloride. The
organic phase was washed with brine and dried over MgS04 to give 75 mg of the
title
compound contaminated with Ph3P.
Step 4 3S)-5-((4-Fluorobenzyl)sulfanyl)-3-[(5-(isopropoxymethyl)-3-
pyridyl)carbonyl]amino-4-oxopentanoic acid
Unpurified 5-(isopropoxymethyl)nicotinic acid (75 mg) was added to
Resin K as described previously, then cleaved with TFA:H20 to provide 38 mg of
the
title compound.
1H NMR ( 400 MHz, acetone-d6): 8 9.17 (br s, 1H), 8.94 (br s, 1H),
8.6 (m, 2H), 7.37 (m, 2H), 7.05 (m, 2H), 5.26 (q, 1H), 4.78 (s, 2H), 3.80 (m,
1H), 3.72
(s, 2H), 3.52 (dd, 2H), 3.07 (dd, 1H), 2.92 (dd, 1H).
-51-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 21
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5
((DIISOPROPYLAMINO)CARBONYL)-3-PYR117YL)CARBONYL]AMINO-4
OXOPENTANOIC ACID
MS (APCI, neg. ion) m/z 502 (M-1, 100), 362 (20), 316 (16).
EXAMPLE 22
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-PHENYL-3-
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
1H NMR ( 400 MHz, acetone-d6): 8 9.10 (m, 2H), 8.67-8.56 (m, 2H),
7.78 (d, 2H), 7.58-7.48 (m, 3H), 7.42-7.35 (m, 2H), 7.05 (t, 2H), 5.30 (m,
1H), 3.75
(s, 2H), 3.53 (dd, 2H), 3.10 (dd, 1H), 2.90 (dd, 1H). MS: m/z 453.3 (M+1).
EXAMPLE 23
(3S)-5-(4-FLUOROBENZYLSULFANYL)-4-OXO-3-[((5-(PHENYL-1-ETHYNYL)-
3-PYR117YL)CARBONYL]AMINO)PENTANOIC ACID
1H NMR ( 400 MHz, acetone-d6): 8 9.08 (br s, 1H), 8.92 (br s, 1H),
8.58 (d, 1H), 8.40 (s, 1H), 7.65-7.60 (m, 2H), 7.50-7.43 (m, 3H), 7.40-7.33
(m, 2H),
7.02 (t, 2H), 5.28 (q, 1H), 3.72 (s, 2H), 3.52 (dd, 2H), 3.10 (dd, 1H), 2.90
(dd, 1H).
MS: m/z 477.2 (M+1).
EXAMPLE 24
((3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(6-METHYL-3-
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
1H NMR ( 400 MHz, acetone-d6): 8 9.25 (br s, 1H), 8.88-8.75 (m,
2H), 7.98 (d, 1H), 7.40-7.32 (m, 2H), 7.05 (t, 2H), 5.28 (q, 1H), 3.71 (s,
2H), 3.50 (dd,
2H), 3.08 (dd, 1H), 2.90 (dd, 1H), 2.82 (s, 3H). MS: m/z 391.3 (M+1).
EXAMPLE 25
(3S)-5-(4-FLUOROBENZYLSULFANYL)-4-OXO-3-[(5-
(PIPERIDINOCARB ONYL)-3-PYRIDYL)CARB ONYLJ AMINOPENTANOIC
ACID
1H NMR ( 300 MHz, acetone-d6): 88.7 (m, 2H), 8.6 (d, 1H),8.25 (m,
1H), 7.40-7.32 (m, 2H), 7.1-7.0 (m, 2H), 5.25 (m, 1H), 3.71 (s, 2H), 3.50 (dd,
2H),
3.35 (m, 2H), 3.1 (m, 3H), 2.90 (dd, 1H), 1.9-1.5 (m, 6H).
-52-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 26
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-
((((METHYLCARBOXY)PROPYL)AMINOCARBONYL)-3-
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC ACLD
1H NMR ( 400 MHz, acetone-d6): 8 9.27 (br s, 1H), 9.20 (br s, 1H),
8.69 (br s, 1H), 8.64 (m, 1H), 7.40-7.32 (m, 2H), 7.05 (t, 2H), 5.28 (m, 1H),
3.72 (s,
2H), 3.59 (s, 3H), 3.50 (dd, 2H), 3.47 (m, 2H), 3.08 (dd, 1H), 2.90 (dd, 1H),
2.43 (t,
2H), 1.90 (m, 2H). MS m/z 520.3 (M+1).
EXAMPLE 27
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-((ETHYL(4
PYRIDYLMETHYL)AMINO)CARBONYL)-3-PYRIDYL)CARBONYL]AMINO-4
OXOPENTANOIC ACID
MS (APCI, neg. ion) m/z 537 (M-1, 100), 397 (40), 351 (45).
EXAMPLE 28
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-((BUTYL(2
CYANOETHYL)AMINO)CARB ONYL)-3-PYRIDYL)CARB ONYL] AMINO-4
OXOPENTANOIC ACID
MS (APCI, neg. ion) m/z 527 (M-1, 100), 474 (30), 387 (20), 288 (35).
EXAMPLE 29
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5
(((METHYLCARBOXY)BUTYL)AMINOCARBONYL)-3
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
1H NMR ( 400MHz, acetone-d6): 8 9.15 (br s, 1H), 8.65 (s, 1H), 8.58
(m, 1H), 8.15 (br s, 1H, NH), 7.40-7.33 (m, 2H), 7.05 (t, 2H), 5.28 (q, 1H),
3.75 (s,
2H), 3.58 (s, 3H), 3.50 (dd ABX, 2H), 3.50-3.43 (m, 2H), 3.05 (dd, 1H), 2.92
(dd,
1H), 2.35 (t, 2H), 1.67 (m, 4H). MS m/z 534.3 (M+1).
-53-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 30
(3 S )-5-(4-FLUOROBENZYLSULFANYL)-3-[ (5
(((METHYLCARBOXY)ETHYL)AMINOCARBONYL)-3
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
1H NMR ( 400 MHz, acetone-d6): b 9.28 (br s, 1H, NH), 9.18 (br s,
1H), 8.68 (s, 1H), 8.62 (m, 1H), 8.27 (br s, 1H, NH), 7.39-7.34 (m, 2H), 7.05
(t, 2H),
5.25 (q, 1H), 4.09 (q, 2H), 3.73 (s, 2H), 3.68 (q, 2H), 3.50 (dd, ABX, 2H),
3.07 (dd,
1H), 2.90 (dd, 1H), 2.65 (t, 2H), 1.18 (t, 3H). MS: m/z 520.4 (M+1).
EXAMPLE 31
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[((6-((3
CARBOXYPROPANOYL)AM1N0)-3-PYRIDYL)CARBONYL)]AMINO-4-OXO
PENTANOIC ACID
1H NMR (400 MHz, acetone-d6): 8 9.05 (s, 1H), 8.50 (m, 1H, NH),
8.38 (d, 1H), 7.45 (d, 1H), 7.39-7.35 (m, 2H), 7.05 (t, 2H), 5.25 (m, 1H),
3.72 (s, 2H),
3.50 (dd, ABX, 2H), 3.08 (dd, 1H), 2.90 (s, 4H), 2.85 (dd, 1H). ~ MS: m/z
492.3
(M+1).
EXAMPLE 32
((3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-
(((METHYLCARBOXY)PENTYL)AMINOCARBONYL)-3-
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
1H NMR (300 MHz, acetone-d6): 8 9.21 (s, 1H), 8.78 (s, 1H), 8.68 (d,
1H), 8.24 (br s, 1H, NH), 7.42-7.35 (m, 2H), 7.05 (t, 2H), 5.28 (q, 1H), 3.72
(s, 2H),
3.60 (s, 3H), 3.50 (dd ABX, 2H), 3.45 (t, 2H), 3.08 (dd, 1H), 2.91 (dd, 1H),
2.30 (t,
2H), 1.69-1.58 (m, 4H), 1.49-1.38 (m, 2H). MS: m/z 548.3 (M+1).
-54-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 33
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-(PROPYLTHIO)-3
PYR117YL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
Step 1
Methyl5-bromonicotinate
To a solution of 5-bromonicotinic acid (10 g, 49.5 mmol) in MeOH
(200 mL) was added 2 mL conc H2S04. The mixture was heated to reflux
overnight,
then cooled and concentrated. The residue was diluted with aq NaHC03 and
extracted three times with ethyl acetate. The combined organic layers were
washed
with brine, dried over MgS04 and concentrated to give 8.75 g of the title
compound.
Step 2
Methyl 5-(thiomethyl)nicotinate
To a solution of methyl 5-bromonicotinate (5.0 g, 23.1 mmol) in DMF
(100 mL) was added NaSMe (1.78 g, 25.5 mmol). The mixture was heated to 80
°C
and stirred overnight. The mixture was cooled, diluted with aq NaHC03 and
extracted three times with ethyl acetate. The combined organic layers were
washed
with brine, dried over MgS04 and concentrated to give 2.0 g of the title
compound.
Step 3
Methyl 5(sulfinylmethyl)nicotinate
To a solution of methyl 5-(thiomethyl)nicotinate (2.93 g, 16.0 mmol) in
1:1 CH2C12/MeOH (160 mL) was added MMPP (80°l0, 4.94 g, 8.0 mmol)
portionwise. The mixture was diluted with water, then concentrated. Aq. NaHC03
was added, and the mixture was extracted three times with CH2C12. The organic
extracts were washed with brine, filtered through cotton and evaporated to
give the
title compound which was used in the next step without purification.
Step 4
Methyl5-mercaptonicotinate
To a solution of unpurified sulfoxide in CH2C12 (110 mL) was added
TFAA (50 mL). The solution was heated to reflux for 4h then cooled and
concentrated. The residue was dissolved in 1:1 MeOH/Et3N (60 mL) and
evaporated.
This was repeated two more times, then the residue was dissolved in CH2Cl2 and
-55-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
washed with aq NH4C1, and brine, filtered through cotton and concentrated to
give the
title compound as a mixture with the corresponding disulfide.
Step 5
5-Mercaptonicotinic acid
To a solution of methyl 5-mercaptonicotinate (1.0 g, 5.9 mmol) in
MeOH (30 mL) and water (20 mL) was added LiOH~H20 (744 mg, 17.7 mmol). The
mixture was stirred at room temperature for 3h, then concentrated. The residue
was
dissolved in water, acidified to pH 3 with 1M HCI, and extracted three times
with
ethyl acetate. The combined organic layers were dried over MgS04 and
concentrated
to give the title compound as a mixture with the corresponding disulfide.
Step 6
5-(Propylthio)nicotinic acid
To a solution of 5-mercaptonicotinic acid (90 mg, 0.58 mmol) in DMF
was added DIEA (0.35 mL, 2.1 mmol) followed by 1-bromopropane (0.09 mL, 0.93
mmol). The mixture was stirred at room temperature for 24h, then partitioned
between NaHC03 and EtOAc. The organic phase was evaporated to give 33 mg of
propyl 5-(propylthio)nicotinate. The aqueous phase was acidified to pH 3 and
extracted three times with EtOAc. The organic layers were washed with brine,
dried
over MgS04 and concentrated to give 35 mg of the title compound.
S tep 7
(3S)-5-(4-Fluorobenzylsulfanyl)-3-[(5-(propylthio)-3-pyridyl)carbamoyl]amino-4-
oxopentanoic acid
Resin I (88 mg) was treated with of 5-(propylthio)nicotinic acid (35
mg) as described in Example 12 to provide 20 mg of the title compound.
MS m/z 451 (M+1).
-56-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 34
(3S)-5-(4-FLUOROBENZYLSULFANYL)-4-OXO-3-([(5-(PROPYLSULFONYL)-3
PYR)T7YL)CARBONYL]AMINO)PENTANOIC ACID
Step 1
Propyl 5-(propylsulfonyl)nicotinate
To a solution of propyl 5-(propylthio)nicotinate (33 mg, 0.14 mmol) in
1:1 CH2 Cl2:MeOH (1.2 mL) was added MMPP (80°10,110 mg, 0.22 mmol), and
the
mixture was stirred overnight, then partitioned between EtOAc and NaHC03 . The
organic phase was washed with brine, dried over MgS04 and evaporated to give
21
mg of the title compound.
Step 2
5-(Propylsulfonyl)nicotinic acid
To a solution of propyl 5-(propylsulfonyl)nicotinate (21 mg, 0.08
mmol) in 1:1 MeOH:H20 (1 mL) was added LiOH~H20 (34 mg, 0.8 mmol). The
mixture was stirred for 1h, then acidified to pH 3 with 1M HCI. The mixture
was
extracted three times with ethyl acetate, washed with brine and dried over
MgS04 to
give 13 mg of the title compound.
Step 3
(3S)-5-(4-Fluorobenzylsulfanyl)-3-[(5-(propylsulfonyl)-3-
pyridyl)carbamoyl]amino-4-
oxopentanoic acid
Resin I (60 mg) was treated with 5-(propylsulfonyl)nicotinic acid (13
mg) as described in Example 12 to provide 15 mg of the title compound.
MS m/z 483 (M+1).
-57-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 35
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-((DIETHYLAMINO)SULFONYL)
3-PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
MS m/z 512 (M+1).
EXAMPLE 36
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5
((ISOPROPYLSULFONYL)AMINO)-3-PYR117YL)CARBONYL]AMINO-4
OXOPENTANOIC ACID
MS m/z 498 (M+1).
EXAMPLE 37
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5
((CYCLOPROPYLAMINO)SULFONYL)-3-PYRIDYL)CARBONYL]AMINO-4
OXOPENTANOIC ACID
MS m/z 496 (M+1).
EXAMPLE 38
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-((PYRROLIDINO)SULFONYL)-3-
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
Step 1
5-(Chlorosulfonyl)nicotinic acid
To a solution of 5-mercaptonicotinic acid (130 mg) in AcOH (10 mL)
was added a slow stream of chlorine gas for 5 min. The solution was then
evaporated
using toluene as an azeotrope to give 161 mg of the title compound.
Step 2
5-((Pyrrolidino)sulfonyl)nicotinic acid
To a suspension of 5-(chlorosulfonyl)nicotinic acid (50 mg, 0.22
mmol) in CH2C12 (1.5 mL) was added pyrrolidine (10 drops), giving a solution.
After
2.5h, the reaction was partitioned between CH2C12 and dilute NaHC03. The
aqueous
phase was acidified to pH3 and extracted three times with CH2C12. The combined
organic phases were filtered through cotton and evaporated to give 42 mg of
the title
compound.
-58-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Step 3
(3S)-5-(4-Fluorobenzylsulfanyl)-3-[(5-((pyrrolidino)sulfonyl)-3-
pyridyl)carbonyl]amino-4-oxopentanoic acid
Resin I (80 mg) was treated with 5-((pyrrolidino)sulfonyl)nicotinic
acid (42 mg) as described in Example 12 to provide 20 mg of the title
compound.
MS m/z 510 (M+1).
EXAMPLE 39
(3S)-5-(4-FLUOROBENZYLSULFANYL)-3-[(5-(((N-CYCLOPROPYL-N-
METHYL)AMINO)SULFONYL)-3-PYRIDYL)CARBONYL]AMINO-4-
OXOPENTANOIC ACID
MS m/z 510 (M+1).
EXAMPLE 40
(3S)-5-PHENOXY-3-[(5-((CYCLOPROPYLAMINO)SULFONYL)-3-
PYRIDYL)CARBONYL]AMINO-4-OXOPENTANOIC ACID
To a suspension of Resin G (200 mg, 0.086 mmol) in DMF (6 mL) was
added phenol (41 mg, 0.43 mmol) and cesium carbonate (140 mg, 0.43 mmol). The
suspension was rotated for 2.5 h, then washed with DMF (3x), water (3x), DMF
(3x),
THF (3x), MeOH (3x), and CH2C12 (3x). The resulting resin was suspended in 20%
piperidine/DMF, rotated for 10 min, then washed with DMF (3x), THF (3x), MeOH
(3x), and CH2Cl2 (3x) to give Resin M.
Resin M (200 mg, 0.086 mmol), 5-((cyclopropylamino)sulfonyl)
nicotinic acid (46 mg, 0.189 mmol), and HATU (72 mg, 0.189 mmol) were
suspended
in DMF (4 mL) then treated with DIEA (0.033 mL, 0.2 mmol). The suspension was
rotated 2.5 h, then washed with DMF (3x), THF (3x), MeOH (3x), and CH2C12
(3x).
The resulting resin was rotated with 9:1 TFA:H20 for 10 min, then filtered and
washed with acetonitrile. The filtrate was concentrated to give the title
compound.
1H NMR (500 MHz , acetone-d6): 9.32 (m, 1H), 9.03 (m, 1H), 8.74
(m, 1H, NH), 8.62 (m, 1H), 8.45 (m, 1H, NH), 7.32-7.22 (m, 2H), 7.01-6.88 (m,
3H), 5.21-5.06 (m, 2H), 4.30 (q, 1H), 3.16-2.89 3.16 (m, 2H), 2.31 (m, 1H),
0.59
(m, 2H), 0.52 (m, 2H). MS m/z 448.3 (M+1).
-59-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
EXAMPLE 41
(3S)-3-[(5-((CYCLOPROPYLAMINO)-SULFONYL)-3
PYR>DYL)CARBONYL]AMINO-5-(( 1-NAPHTHYLCARBONYL)OXY)-4
OXOPENTANOIC ACID
To a suspension of Resin G (200 mg, 0.086 mmol) in DMF (6 mL) was
added 1-naphthoic acid (74 mg, 0.43 mmol) and potassium fluoride (25 mg, 0.43
mmol). The suspension was rotated for 2.5 h, then washed with DMF (3x), water
(3x), DMF (3x), THF (3x), MeOH (3x), and CH2C12 (3x). The resulting resin was
converted to the title compound in the same manner as described in Example 52.
1H NMR (500 MHz , acetone-d6): 9.38 (m, 1H), 9.15 (m, 1H), 8.95-
8.8 (m, 2H, 2 NH), 8.29 (m, 1H), 8.17 (m, 1H), 7.99 (m, 1H), 7.65-7.51 (m,
3H),
7.15 (m, 1H), 5.49-5.35 (q, 2H), 5.30-5.22 (q, 1H), 3.20-2.97 (m, 2H), 2.32
(m, 1H),
0.58 (m, 2H), 0.51 (m, 2H). MS m/z 526.5 (M+1).
EXAMPLE 42
(3S)-5-(2,6-DIFLUOROBENZYLAMINO)-3-[(5
((CYCLOPROPYLAMINO)SULFONYL)-3-PYR>DYL)CARBONYL]AMINO-4
OXOPENTANOIC ACID
To a suspension of Resin O (500 mg, 0.225 mmol) in DMF (15 mL)
was added 2,6-difluorobenzylamine (0.11 mL, 1.12 mmol). The suspension was
rotated for 1.5 h, then washed with DMF (3x), THF (3x), MeOH (3x), and CH2C12
(3x). The resin was resuspended in CH2C12 and treated with di-t-
butyldicarbonate
(0.15 mL, 0.67 mmol) and DIEA (0.12 mL, 0.67 mmol). The mixture was rotated
for
2 h, then washed with DMF (3x), THF (3x), MeOH (3x), and CH2C12 (3x). The
resulting Resin P was suspended in CH2C12 (15 mL) and treated with Pd(PPh3)4
(25
mg, 0.023 mmol) and phenylsilane (0.66 mL, 5.4 mmol). The mixture was rotated
for
min, then washed with DMF (3x), THF (3x), MeOH (3x), and CH2C12 (3x). The
resulting resin was converted to the title compound in the same manner as
described
in Example 52.
30 MS m/z 497.3 (M+1).
-60-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
Assays for Determining Biological Activity
(a) Measurement of caspase activity by cleavage of a fluorogenic substrate
(b) A fluorogenic derivative of the tetrapeptide recognized by caspase-3 and
corresponding to the P1 to P4 amino acids of the PARP cleavage site, Ac-DEVD-
AMC (AMC, amino-4-methylcoumarin) was prepared as follows: i) synthesis of N
Ac-Asp(OBn)-Glu(OBn)-Val-C02H, ii) coupling with Asp(OBn)-7-amino-4
methylcoumarin, iii) removal of benzyl groups.
COON
0 O O
'N N Y 'N N Y 'N ~ O
H I H I H
O O
~ COOH
COOH
Standard reaction mixtures (300 p,L final volume), contained Ac-
DEVD-AMC and purified or crude caspase-3 enzyme in 50 mM Hepes/KOH (pH
7.0), 10% (v/v) glycerol, 0.1 % (w/v) CHAPS, 2 mM EDTA, 5 mM dithiothreitol,
and
were incubated at 25°C. Reactions were monitored continuously in a
spectrofluorometer at an excitation wavelength of 380 nm and an emission
wavelength of 460 nm.
(c) Cell Death Detection ELISA (Whole Cell Assay)
Photometric immunoassay. for the qualitative and quantitative in vitro
determination of cytoplasmic histone-associated-DNA-fragments (mono- and
oligonucleosomes) after induced cell death. This assay was performed using the
commercially available kit from Boehringer Mannheim, cat. No. 1 920 685.
(d) In Vivo Myocardial Ischemia and Reperfusion Injury in Rats
Male Sprague-Dawley rats (300-400g) were fasted overnight, and then
anesthetized with intraperitoneal administration of sodium pentobarbital (65
mg/kg).
To monitor heart rate and aortic pressure the left carotid artery was isolated
and a
cannula placed in the vessel. The aortic cannula was interfaced with a
pressure
transducer which was connected to a physiologic recorder. The left jugular
vein was
isolated and cannulated for administration of a caspase inhibitor compound or
vehicle
-61-
CA 02386411 2002-04-04
WO 01/27085 PCT/CA00/01196
(2 % dimethylsulfoxide in 0.9% NaCI). A left thoracotomy was performed in the
region overlying the heart and the pericardium opened, exposing the heart. The
origin
of the left coronary artery was visualized and a 4.0 suture passed under the
artery
approximately 2 - 3 mm from its origin. The ends of the suture were passed
through
a short length of 2 mm id tubing and coronary artery occlusion effected by
placing
tension on the suture such that the tube compressed the artery. After initial
placement
of the suture/occluder, the thoracotomy was closed with a small clamp and
opened
only to effect occlusion and reperfusion of the artery. A Lead II
electrocardiograph
(ECG) signal was obtained by placing subdermal platinum leads and continuously
monitored. After a baseline period of 20-30 minutes the left coronary artery
was
occluded for 45 minutes. The period of reperfusion was 3 hours. The caspase
inhibitor or vehicle was administered as a first bolus 5 minutes before the
onset of
ischemia and a second bolus was administered again at the onset of
reperfusion.
Additionally, an infusion was initiated immediately after the first bolus
dose. Control
animals received the vehicle alone in equal volumes to the caspase inhibitor
treated
animals. At the end of reperfusion the animals were euthanized and infarct
size
determined using a dual staining technique (1.5% w/v triphenyltetrazolium
chloride to
demarcate infarct tissue and 0.25% w/v Evan's blue to demarcate the area at
risk of
infarct. The heart was subsequently cut transversely into 4 slices of equal
thickness,
and infarct size and area at risk quantified using planimetry.
Using the above procedure, it is demonstrated that administration of a
caspase inhibitor reduces infarct size in the rat subjected to 45 minutes of
regional
ischemia and 3 hours of reperfusion.
-62-