Note: Descriptions are shown in the official language in which they were submitted.
CA 02386648 2002-05-16
COMPOSITIONS AND METHODS FOR MANAGING BACTERIAL SKIN
GONDITIONS
10
FIELD OF THE INVENTION
The invention is in the field of compositions and methods for the prevention,
treatment and management of skin conditions involving bacterial contamination,
and
more particularly in the field of compositions and methods for the prevention,
treatment and management of acne using plant extracts.
BACKGROUND OF THE INVENTION
Bacterial contamination of the skin may contribute to various skin disorders,
including acne. Acne is a very common skin disorder involving the sebaceous
follicles
and is characterized by blackheads, whiteheads, papules, pustules, cysts, and
various
sized nodules and scars. The disorder affects skin areas where the sebaceous
glands
are most active and bacterial infection can occur in the sebaceous follicles.
A resident
anaerobic organism, Propionibacterium aches, proliferates in the environment
created
by the mixture of the excessive sebum and follicular cells and produces
chemotactic
factors and inflammatory mediators that may lead to inflammation (see Manual
of
Dermatologic Therapeutics. 5t" edition, 1995, Little, Brown and Company.
Boston,
pp.3-15).
A variety of acne treatment methods have been developed, including both
systemic and topical administration of antibiotics, and topical administration
of organic
peroxides, particularly benzoyl peroxide. However, many products used in the
treatment of acne are irritating and may cause inflammation of the skin. Many
of the
current products are only alcohol-soluble. Formulations that are alcohol-based
may be
irritating and are often very drying to the skin. Many of the acne treatments
are
effective only after extended periods of time and they can have a multitude of
side
effects. Side effects from current anti-acne treatments such as topical
clindamycin and
erythromycin or retinoids include local reactions such as burning, erythema
(redness),
peeling, dryness, and pruritus. Some therapies such as isotretinoin may have
extremely serious side effects including teratogenicity.
-1-
CA 02386648 2002-05-16
Several plants have been shown to exhibit antibiotic and antifungal activity
in in
vitro assays. For example, methanolic extracts of Epilobium angustifolium
aerial parts
and roots have been shown to demonstrate broad-spectrum antifungal effects as
well
as anti-bacterial effects against several bacteria, such as Escherichia coli,
Mycobacterium phlei, Pseudomonas aeruginosa, Staphylococcus aureus and
Staphylococcus epidermidis (see McCutcheon AR, 1996). Various plant tannins,
including some ellagitannins have been reported to have antimicrobial
properties (see
Vivas N, 2000; Kakiuchi N, 1986; and Yoshida T, 2000). However, the spectrum
of
antibacterial activity of tannins has been found to be unpredictable. For
example,
Oenothein B, an ellagitannin present in Epilobium angustifolium has been
reported to
exhibit antibacterial activity against Helicobacter pylori while being
inactive against
Staphylococcus aureus and Escherichia coli (see Yoshida T, 2000).
SUMMARY OF THE INVENTION
It has been discovered that Epilobium angustifolium (Canadian Willowherb)
extracts exhibit surprising antibacterial activity against Propionibacterium
aches.
Accordingly, in one aspect, the invention provides methods for the treatment
of a skin
condition involving Propionibacterium aches, in which a therapeutic or
prophylactic
dose of an extract of Epilobium angustifolium (Canadian Willowherb) is
administered.
In one aspect the invention provides a composition for treating a skin
condition
involving Propionibacterium aches, which comprises an extract of Epilobium
angustifolium (Canadian Willowherb) in a therapeutically or prophylactically
effective
amount sufficient to kill Propionibacterium aches, or prevent or inhibit the
growth
thereof. In some embodiments the compositions of the invention may include
formulations for topical use.
Other aspects of the invention include compositions and methods for the
prophylactic or therapeutic treatment of a skin condition involving
Propionibacterium
aches, comprising a prophylactically or therapeutically effective amount of an
oenothein extracted or prepared from Epilobium angustifolium (Canadian
Willowherb)
or from any other plant source. In an alternative embodiment, the oenothein
for use in
-2-
CA 02386648 2002-05-16
such compositions and methods may for example be oenothein A, a tautomer of
oenothein A or an isomer of oenothein A or any of their pharmacologically
acceptable
salts or oenothein B, a tautomer of oenothein B or an isomer of oenothein B
(such as,
for example oenothein D and F), or a derivative of oenothein B wherein a
hydroxy
group is for example replaced by a carboxylic acid group (such as, for
example,
oenothein G) (see Yoshida T., 1995) or any of their pharmacologically
acceptable
salts, or mixtures thereof.
In another aspect of the invention, new plant extracts found to contain an
oenothein may be used for treating a skin condition involving
Propionibacterium
acnes. For example, Epilobium spp. other than Epilobium angustifolium such as
Epilobium parviflorum may be used for making such extracts where they contain
oenothein A or oenothein B or tautomers or isomers or a derivative of
oenothein B
wherein a hydroxy group is for example replaced by a carboxylic acid group
(such as,
for example oenothein G) or any of their pharmacologically acceptable salts,
or
mixtures thereof. Various Onagreaceae plants such as, for example, Oneothera
laciniata or Oneothera erythrasepala, Lythracea plants such as, for example,
Woodfordia fructiosa or Cuphea hyssopifolia and Myrtaceae plants such as, for
example, Eucalyptus albs, Eucalyptus consideniana or Eucalyptus viminalis may
also
be used for making extracts containing oenothein A or oenothein B or tautomers
or
isomers or derivatives or oenotheins with similar chemical structures or
mixtures
thereof.
In another aspect, the invention provides compositions and methods for the
prophylactic or therapeutic treatment of a skin condition involving
Propionibacterium
acnes, comprising a prophylactically or therapeutically effective amount of a
mixture of
oenothein and three tannins extracted from Epilobium angustifolium (Canadian
Willowherb) and characterized respectively by high performance liquid
chromatography column (HPLC). The retention times for the three tannins are in
the
ranges of about 11.42-11.49, 11.55-11.60 and 11.96-11.99 minutes upon gradient
elution in a 50% aqueous methanol solution on a SupelcosilT"" LC-18 column
with a
water/acetonitrile eluant having an initial composition of 98% water/2%
actetonitrile
and a composition of 65% water/35% acetonitrile at 10 minutes, 2% water/98%
-3-
CA 02386648 2002-05-16
10
acetronitrile at 20 minutes, and final composition of 98% water/2%
acetonitrile at 30-
50 minutes.
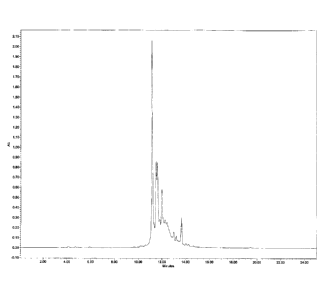
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a chromatogram of a tannin mixture exhibiting antibacterial
activity
against Propionibacterium acnes.
DETAILED DESCRIPTION OF THE INVENTION
In some aspects, the invention provides methods of treatment of skin
conditions
involving Propionibacterium acnes, in which a therapeutic or prophylactic dose
of an
extract of Epilobium angustifoliurn (Canadian Willowherb), or an oenothein, or
a tannin
mixture comprising the three tannins previously identified by HP~C and
optionally
oenothein is administered, such as by administration of a pharmacologically
acceptable formulation. Such formulations of the invention may comprise an
extract of
Epilobium angustifolium (Canadian Willowherb), or an oenothein or a
pharmacologically acceptable salt thereof, or a tannin mixture extracted from
Epilobium angustifolium (Canadian Willowherb) or from related Epilobium
species or
from any other plant source containing oenothein or such a tannin mixture and
a
pharmacologically acceptable excipient or carrier. In some embodiments, such
formulations may comprise a therapeutically or prophylactically effective
amount
sufficient to reduce Propionibacterium acnes or to prevent or inhibit the
growth of
Propionibacterium acnes. In particular embodiments, such formulations may
comprise
any one of the three aforementioned tannins by itself or in combination with a
pharmacologically acceptable excipient or carrier and/or an oenothein. In
other
embodiments, such formulations may comprise two of the three aforementioned
tannins by themselves or in combination with a pharmacologically acceptable
excipient or carrier and/or an oenothein.
As used herein "pharmacologically acceptable carrier" or "excipient" includes
any and all creams, gels, solvents, dispersion media, coatings, antibacterial
and
antifungal agents, isotonic and absorption delaying agents, and the like that
are
physiologically compatible and do not significantly adversely affect the
pharmaceutical
properties (e.g. toxicity and effectiveness) of the extract of Epilobium
angustifolium
-4-
CA 02386648 2002-05-16
(Canadian Willowherb), or the oenothein, or the tannin mixture extracted from
Epilobium angustifolium (Canadian Willowherb) or from related Epilobium
species or
from any other plant source containing oenothein or such a tannin mixture,
such as
are conventionally used in the casmetic and pharmaceutical arts. In one
embodiment,
the carrier is suitable for topical administration. In other embodiments, the
carrier is
suitable for internal administration. In some embodiments, oenotheins may
undergo
hydrolysis in acid or base, so that pharmacologically acceptable carriers or
excipients
may include pH buffers to maintain an acceptable pH for pharmaceutical
activity (see
Daniel et al., 1991, for a discussion of the effects of pH on ellagitannins).
A "therapeutically effective amount" refers to an amount effective, at dosages
and for periods of time necessary, to achieve the desired therapeutic result,
such as
reduction or elimination of acne. A therapeutically effective amount of an
extract of
Epilobium angustifolium (Canadian Willowherb), or of an oenothein, or of a
tannin
mixture extracted from Epilobium angustifolium (Canadian Willowherb) or from
related
Epilobium species or from any other plant source containing oenothein or such
a
tannin mixture may vary according to factors such as the acne state, age, sex,
and
weight of the individual, and the ability of the extract of Epilobium
angustifolium
(Canadian Willowherb), or of the oenothein, or of the tannin mixture extracted
from
Epilobium angustifolium (Canadian Willowherb) or from related Epilobium
species or
from any other plant source containing oenothein or such a tannin mixture to
elicit a
desired response in the individual. Dosage regimens may be adjusted to provide
the
optimum therapeutic response. A therapeutically effective amount is also one
in which
any toxic or detrimental effects of the extract of Epilobium angustifolium
(Canadian
Willowherb), or of the oenothein, or of the tannin mixture extracted from
Epilobium
angustifolium (Canadian Willowherb) or from related Epilobium species or from
any
other plant source containing oenothein or such a tannin mixture are
outweighed by
the therapeutically beneficial effects.
A "prophylactically effective amount" refers to an amount effective, at
dosages
and for periods of time necessary, to achieve the desired prophylactic result,
such as
preventing or inhibiting the growth of Propionibacterium acnes. A
prophylactically
-5-
CA 02386648 2002-05-16
effective amount can be determined as described above for the therapeutically
effective amount.
In particular embodiments, a preferred range for therapeutically or
prophylactically effective amounts of an extract of Epilobium angustifolium
(Canadian
Willowherb), or an oenothein, or a tannin mixture extracted from Epilobium
angustifolium (Canadian Willowherb) or from related Epilobium species or from
any
other plant source containing oenothein or such a tannin mixture may be
0.0003% to
99% by weight. These may be characterized by the percentage of dry matter
which
they contain. Dry matter is the solid residue remaining after removal of the
carrier or
solvent by drying, such as by drying a solution or suspension in an oven, by
lyophilization or evaporation under vacuum. Dry matter may be expressed in %
and
may also be referred to as plant solids concentration. Thus, a 100 gram or 100
mL
solution or suspension containing 5% dry matter by weight, yields 5 grams of
solids or
residue after drying. Alternative methods of drying may yield slightly
different values
for the percent by weight of dry matter, so that all such values recited
herein are
necessarily approximations. Dry matter values include suspended as well as
settled
solids. Any one of the three tannins previously identified by HPLC may be
effective by
itself in an amount of 0.0003% to 99%. In some embodiments the therapeutically
or
prophylactically effective amount may be 0.1 % to 50%. In other embodiments
the
therapeutically or prophylactically effective amount may be 0.1 % to 10%. One
method
of cosmetic, prophylactic or therapeutic treatment is to apply an extract of
Epilobium
angustifolium (Canadian Willowherb), or an oenothein, or a tannin mixture
extracted
from Epilobium angustifolium (Canadian Willowherb) or from related Epilobium
species or from other plant sources containing oenothein or such a tannin
mixture of
any component thereof topically to the acne area. Dosage values may vary with
the
severity of the condition to be alleviated. It is to be further understood
that for any
particular subject, specific dosage regimens may be adjusted over time
according to
the individual need and the judgement of the person administering or
supervising the
administration of the compositions, and that dosage ranges set forth herein
are
exemplary only and are not intended to limit the scope or practice of the
methods of
the invention.
_g_
CA 02386648 2002-05-16
In other embodiments the therapeutically or prophylactically effective amount
of
an extract of Epilobium angustifolium (Canadian Willowherb), or an oenothein,
or a
tannin mixture extracted from Epilobium angustifolium (Canadian Willowherb)
may be
combined or admixed with various therapeutically or prophylactically anti-acne
agents
conventionally used in the cosmetic and pharmaceutical arts.
In accordance with another aspect of the invention, therapeutic compositions
of
the present invention, comprising an extract of Epilobium angustifolium
(Canadian
Willowherb), or an oenothein, or a tannin mixture extracted from Epilobium
angustifolium (Canadian Willowherb) or from related Epilobium species or from
any
other plant source containing oenothein or such a tannin mixture, may be
provided in
containers having labels that provide instructions for use of the formulation
to: prevent
or treat acne. The labels may also disclose that the compositions comprise an
extract
of Epilobium angustifolium (Canadian Willowherb), or an oenothein, or a tannin
mixture extracted from Epilobium angustifolium (Canadian Willowherb) or from
related
Epilobium species or from any other plant source containing oenothein or such
a
tannin mixture.
A variety of methods may be used to purify oenotheins from natural sources for
use in the various aspects of the present invention. For example, U.S. Patent
No.
5,525,594 discloses methods of preparing oenothein B from plants. Similarly,
Ducrey
et a1.,1997 discloses oenothein A and oenothein B purification and
characterization
methods. Alternative purification methods may be used in accordance with the
present invention, provided that they may be used to produce a
pharmacologically
acceptable preparation of purified oenothein B suitable for use in the various
aspects
of the present invention.
Anti-Acne Procedures and Protocols
Overnight broth cultures of the P. acnes organisms were prepared. Cultures
were centrifuged, rinsed with saline and diluted to give 80% Transmittance at
wavelength of 530 nm and the suspension was used as the inoculum.
-7-
CA 02386648 2002-05-16
Test samples were inoculated with the organisms (zero time) and mixed well.
After the appropriate time, a sarnple was mixed with the broth blank and mixed
well.
Serial dilutions were performed, prepared and placed in duplicate using the
appropriate agar.
A control count was performed simultaneously on the cultures and plated in
duplicate.
All plates were incubated at 3711.0°C for the appropriate time and
resultant
colonies were then enumerated.
Example 1
This example shows the effectiveness of whole and clear extracts of Epilobium
angustifolium (Canadian Willowherb) in reducing Propionibacterium acnes after
one
hour of exposure.
Procedure for Making Canadian Willowherb Whole
Various methods may be used for making an extract from Epilobium
angustifolium (Canadian Willowherb). One method is as follows. In a beaker or
other
vessel, dried ground plant material was extracted with a variable amount of
solvent,
preferably an aqueous or aqueous ethanolic mixture such as aqueous methanol.
The
resultant primary extract may be heated, for example for a period of at least
20
minutes, and preferably 1 hour, then centrifuged and/or filtered to remove a
substantial portion of plant material. Alcoholic solvent may be removed and
the
resultant aqueous extract concentrated or diluted to the required dry matter
content
ranging from 0.5% to 99% by weight, preferably 5% to 20% by weight, most
preferably
5% by weight. This crude or primary extract is identified herein as Canadian
Willowherb Whole Extract. Guar gum or other gums may be added to the extract
to
obtain a homogeneous suspension. Preservatives such as antimicrobials and
antifungal agents or other agents such as acids or bases needed to adjust pH
may be
added to the extract.
_g_
CA 02386648 2002-05-16
Procedure for Making Canadian Willowherb Clear
Various methods may be employed for making Canadian Willowherb Clear.
One method is detailed as follows. The crude or primary Epilobium extract,
Canadian
Willowherb Whole Extract, may be treated with alcohol and decolorized with
charcoal
or other suitable decolorization agents to remove gums, chlorophyll and other
colored
components, and other unwanted constituents, and then filtered to obtain a
clarified
extract, known as Canadian Willowherb Clear Extract. The clarified extract may
be
diluted or concentrated to a desired dry matter content of plant material
ranging from
0.5% to 99% by weight, preferably 5% to 20% by weight, most preferably 5% by
weight. Preservatives such as antimicrobials and antifungal agents or other
agents
such as acids or bases needed to adjust pH may be added to the extract.
Results
Results are indicated in tabular form. Numbers indicate colony-forming units
(CFUs) per ml, and the reduction, or kill percentage.
This example shows the antibacterial effectiveness of a Canadian Willowherb
Whole Extract (CW5) and Canadian Willowherb Clear (CWSC) extracts containing
5%
of dry matter by weight and tested at a use level of 1 % against P. aches
evaluated by
performing time kill studies at 60 minutes.
_g_
CA 02386648 2002-05-16
Table 1: Time kill results of a 1 % use level of CW5 (whole extract, 5% dry
matter by
weight) and CWSC (clear extract, 5% dry matter by weight) extracts mixed in
water
after one-hour exposure time
Extract Use level Organisms ; Control 60 minutes
count
CW5 1.0% P. aches #11827 5.2 x 10' 5.8 x 10"
~% reduction = 88.8
CWSC 1.0% P. aches #11827 5.2 x 10 3.5 x 10
~% reduction = 99.3
Example 2
This example shows the antibacterial effectiveness of utilizing a 4% use level
of
an unpreserved Canadian Willowherb Whole Extract (5% dry matter content by
weight) against P. aches evaluated by performing further time kill studies at
2, 10 and
30 minutes. This corresponds to a total dry matter content of 0.2% by weight
in the
test solution.
Table 2: Time kill results of a 4% use level of CWSC (clear extract 5% dry
matter by
weight; unpreserved sample) extract mixed in water
Test Use Organisms Control2 10 30
Substance Level* __ count minutes minutes minutes
CWSC 4% P. aches 1.7 6.0 x 1.7 x 1.2 x
x 10 10 10 10
(unpreserved #11827
sam 1e % reduction 64.7 90.0 92.9
=
*Concentration of CWSC mixed with water by volume.
These results show a remarkable effectiveness of Canadian Willowherb Whole
Extract as an anti-P.acnes agent. The 4% use level corresponds to a total dry
matter
content of 0.2%, by weight, in the test solution. Most anti-acne treatments
require long
time exposures to be efficient and do not kill in vitro or in vivo in 2
minutes.
-10-
CA 02386648 2002-05-16
Example 3
This example shows the antibacterial effectiveness of different concentrations
of Epilobium angustifolium (Canadian Willowherb) clear extracts against P.
aches
evaluated by performing further time kill studies at 30, 60 and 120 minutes.
-11-
CA 02386648 2002-05-16
Table 3: Time kill result of Canadian Willowherb Clear Extract (CWSC) extract
tested
in water at use levels of 1-5%
Test Use Organisms Control 30 60 minutes120
Substance Level* count minutes minutes
CWSC 1 % P.acnes #118271.2 x 1.1 x 1.1 x 10 1.1 x
10 10 10
reduction = 8.3 8.3 8.3
CWSC 2% P. acnes 1.2 x 9.2 x 6.8 x 10 4.5 x
10 10 10
#11827 23.3 43.3 62.5
reduction =
CWSC 2.5% P.acnes #'118271.7 x 5.9 x 3.1 x 10 5.0 x
10 10 10
reduction = 65.3 81.8 70.1
CWSC 3% P"acnes #118271.5 x 5.1 x 0 0
10~ 10
reduction = 99.7 100 100
CWSC 4% P.acnes #11827 6.2 x 1.5 x 10 5.5 x
( 1.5 x 10 10 10
reduction = 99.6 99.0 99.9
~
CWSC 5% P.acnes #11827 8 x 1~ 2 x 10 1.6 x
~ 2 10~~ 10
reduction = 93.3 99.8 99.9
j
'Concentration of CWSC mixed with water by volume.
Epilobium angustifolium (Canadian Willowherb) extracts may for example be
diluted for use at use levels of approximately 1 %, 2%, 2.5%, 3%, 4%, and 5%
(by
volume) corresponding respectively to an approximate total dry matter content,
by
weight, of 0.05%, 0.10%, 0.125'%, 0.15%, 0.20% and 0.25%. CWSC clear aqueous
extracts are also effective against Propionibacterium acnes, with surprisingly
strong
activity for extracts having at least 0.15% dry matter or solids by weight in
the clear
aqueous extract. Similarly, preparations having at least an equivalent
concentration of
oenothein B may be advantageous, such as concentrations of oenothein B of at
least
about 0.00075% (or in alternative embodiments, of at least about 0.00050%,
0.00055%, 0.00060%, 0.00065%, 0.00080%, 0.00085%, 0.00090%, 0.00095%,
0.001 %, or any range of values between such concentrations). In some
embodiments, it may for example be desirable to use such extracts having an
activity
that yields a reduction of P. acnes in such tests of at least 90%, 95%, 96%,
97%, 98%,
-12-
CA 02386648 2002-05-16
99% or 99.9%. In some embodiments extracts having a P. acnes reduction
activity of
100% may be used.
Example 4
Concentrated extracts cantaining higher amounts of oenothein B were also
tested. These extracts contained approximately 50% oenothein B (technical
grade)
and 90-98% oenothein B (pure grade), by weight.
Procedure for PrJ~aringi Technical Grade Oenothein B~50+%~urity)
A variety of methods may be employed to prepare technical grade of oenothein
B, comprising at least 50% oenathein B. Column chromatography may be employed
as well as the following method. Canadian Willowherb Clear aqueous extract,
containing 0.5 to 99% dry matter by weight, preferably 5 to 20% dry matter, by
weight,
can be concentrated by evaporation under pressure or evaporated by other
method to
contain about 20% dry matter, by weight. Alternatively, Canadian Willowherb
Whole
aqueous extract (crude or primary extract) can be concentrated by evaporation
under
pressure, or evaporated by other method, to contain about 20% dry matter, by
weight.
Addition of base, such as 4.0 M sodium hydroxide solution, with stirring to
ensure
thorough mixing, will produce a k>right yellow to dark precipitate. The
resultant solution
can be filtered to dryness under vacuum through various filters such as a #4
Whatman
filter (Whatman International L.td.). The retained yellow to brown solid is
then
suspended in a minimal amount of water and treated with 2.0 N hydrochloric
acid,
drop wise, until the solution is acidic (about pH 4). Solution can be left to
dry at room
temperature, or by alternate method, and then ground to obtain the dried
technical
grade oenothein B, comprising at least 50% oenothein B, by weight.
Alternatively, the technical grade oenothein B may be prepared by subjecting
the crude (Canadian Willowherb Whole Extract) or clear (Canadian Willowherb
Clear
Extract) to column chromatography as outlined in the procedure for preparing
oenothein B pure grade (90-98% pure oenothein B).
The purity of the technical grade oenothein B may be determined by high
performance liquid chromatography using the following method. The dried ground
-13-
CA 02386648 2002-05-16
material (20mg) was dissolved in 1 ml 50% aqueous methanol and injected onto a
SupelcosiITM LC-18 (Supelco) column (the injection volume may be in the range
of
1.5 to 5 microlitres). Elution was carried out by a solvent gradient at a flow
rate of 1
mL/min under the following conditions (volume percentages):
~ 0-10 min 98%water with 0.1 % v/v trifluoroacetic acid (TFA);
~ 10-20 min 65% water with 0.1 % v/v TFA and 35% acetonitrile with 0.1 % v/v
TFA;
~ 20-30 min 2% water with 0.1 % v/v TFA and 98% acetonitrile with 0.1 % v/v
TFA;
~ 30-50 min 98% water with 0.1 % v/v TFA and 2% acetonitrile with 0.1 % v/v
TFA.
Detection was carried out at wavelengths ranging from 210 nm to 400 nm. At
270 nm oenothein B has a retention time of about 11.01-11.15 minutes under
these
conditions.
Procedure for Preparing Pure Grade Oenothein B j90-98% purity)
In a beaker or other vessel, dried ground plant material was extracted with
aqueous methanol or any other suitable solvent, under heat for at least 20
minutes.
The solution was filtered and they residue was squeezed to near dry while the
mixture
was still warm. The methanolic extract solution was evaporated under vacuum.
Alternately, the extract could be dried down by lyophilization after removal
of solvent
by evaporation, or concentrated by other means. The concentrated extract
solution,
about 40 grams, was subjected to column chromatography using varying
concentrations of aqueous-acetone, in a Diaion (HP-20) column (Mitsubishi
Chemical
Corporation). This provided a water eluate, a 30% acetone eluate, and a 60%
acetone
eluate. The eluates were dried down. The water eluate, about 10 grams, and the
30%
acetone eluate, about 16 grams, both contain oenothein B. The combined water
and
30% acetone eluate was subjected again to a Diaion column, eluting with water,
and
then 10% aqueous acetone. The resultant eluate was dried down, yielding about
4
grams of material. A 2 gram sample subjected to a MCI GEL CHP20P 37-75 micron
column (Mitsubishi Chemical Corporation) was eluted with 20% aqueous methanol
to
yield fractions A to G. Fractions A to D, combined, gave about 1.16 gram
oenothein B,
-14-
CA 02386648 2002-05-16
at a purity of about 88%. The 1.16 gram was further subjected to column
chromatography in a LiChroprep RP-18 25-40 micrometer (Merck) utilizing
water: methanol (9:1) as eluent, and yielding about 0.74 grams of oenothein B,
of a
purity about 92-94%. Further subjections to column chromatography in another
MCI
GEL CHP20P 37-75 micron column eluting with 20% aqueous methanol yields
fractions ranging in purity from 92-98%.
The purity of the pure grade oenothein B may be determined by high
performance liquid chromatography using the following method. The dried ground
material (20mg) was dissolved in 1 mL 50% aqueous methanol and injected onto a
SupelcosiITM LC-18 (Supelco) Column (the injection volume may be in the range
of
1.5 to 5 microlitres). Elution was carried out by a solvent gradient at a flow
rate of 1
mL/min under the following conditions (volume percentages):
~ 0-10 min 98%water with 0.1 % v/v trifluoroacetic acid (TFA);
~ 10-20 min 65% water with 0.1 % v/v TFA and 35% acetonitrile with 0.1 % v/v
TFA;
~ 20-30 min 2% water with 0.1 % v/v TFA and 98% acetonitrile with 0.1 % v/v
TFA;
~ 30-50 min 98% water with 0.1 % v/v TFA and 2% acetonitrile with 0.1 % v/v
TFA.
Detection was carried out at wavelengths ranging from 210 nm to 400 nm. At
270 nm oenothein B has a retention time of about 11.01-11.15 minutes under
these
conditions.
-15-
CA 02386648 2002-05-16
Results
Table 4: Kill Test Results for Oenothein B Technical Grade (a solid which
contains
approximately 50% oenothein B by weight), dissolved in water for testing
Final EquivalentOrganisms Control 30 60 120
ConcentrationUse Level count minutes minutes minutes
of Oenotheinof CWSC*
B by Weight
0.0018% 3.6% P. acnes #118271.5 x 10 9.2 x 4.7 x 0
10" 10"
reduction = 93.9 96.9 100
0.007% 14% P. ac:nes #118278.9 x 10 9.2 x 8.7 x 7.8 x
10 10 10
i % reduction 99.0 99.0 91.2
~ =
0.014% 28% P. acnes #118278.9 x 10 6.1 x 7.2 x 4.8 x
I 10 10 10
reduction = 99.3 99.2 94.6
0.028% 56% P. acnes #118279.75 x 8.0 x 7.7 x 8.2 x
10 10 10 10
reduction = 99.2 67.2 99.9
* This is the approximate concentration of CWSC mixed with water to obtain an
oenothein B concentration equivalent to the Final Concentration tested as
prepared
from oenothein B Technical Grade.
-16-
CA 02386648 2002-05-16
Table 5: Kill Test Results for Oenothein B Pure Grade (a solid which contains
approximately 90% to 98% oenothein B by weight), dissolved in water for
testing
ConcentrationEquivalentOrganisms Control30 60 120
of OenotheinUse Level count minutes minutes minutes
B, by Weightof CWSC*
0.0003% 1.2% P. acnes #11827 1.7 6.6 x 5.5 x 7.5 x
x 10 10 10
reduction = 106 61.2 67.6 55.9
0.0006% 2.4% P. acnes #11827 1.5 7.6 x 4.3 x 0
x 10 10
io reduction = 106 94.9 97.1 100
0.0035% 14% P. acnes #11827 8.9 9.0 x 8.3 x 7.0 x
x 10 10 10
io reduction = 106 99.0 99.1 92.1
0.007% 28% P. acnes #11827 8.9 6.8 x 6.9 x 7.3 x
x 10 10 10
reduction = 106 99.2 99.2 91.8
0.014% 56% P. acnes #11827 9.75 1.5 x 7.7 x 8.2 x
x 10' 10 10
io reduction = 106 98.5 I 92.1 99.1
* This is the approximate concentration of CWSC mixed with water to obtain an
oenothein B concentration equivalent to the Final Concentration tested as
prepared
from oenothein B Pure Grade.
These results show the surprising effectiveness of oenotheins, particularly
oenothein B, as an anti- P. acnes agent in the treatment of conditions
involving P.
acnes such as acne and other skin conditions. Accordingly, in some embodiments
it
may be advantageous to use purified oenothein B to prepare medicaments for the
treatment of skin conditions involving P. acnes. In some embodiments such
purified
oenothein B preparations may contain 0.1 % to 10% oenothein B by weight. In
other
embodiments such purified oenothein B preparations may contain 50% or more
oenothein B by weight (such as 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90% or 100%
oenothein B).
-17-
CA 02386648 2002-05-16
Example 5
A mixture of oenothein B and three tannins isolated from extracts of Epilobium
angustifolium (Canadian Willowherb) was also shown to exhibit antibacterial
activity
against Propionibacterium acnes.
Procedure for Making Tannin Mixture
In a beaker or other vessel, 293 g of dried ground plant material was
extracted
with aqueous methanol or any other suitable solvent, under heat for at least
20
minutes. The solution was filtered and the residue was squeezed to near dry
while the
mixture was still warm. The methanolic extract solution was evaporated under
vacuum. Alternately, the extract could be dried down by lyophilization after
removal of
solvent by evaporation. The concentrated extract solution, about 40 grams, was
subjected to column chromatography using varying concentrations of aqueous-
acetone, in a Diaion (HP-20) Column (Mitsubishi Chemical Corporation). This
provided
a water eluate, a 30% acetone eluate, and a 60% acetone eluate. Finally,
washing the
column with methanol provided a methanol eluate, which contains mainly color
pigments. The eluates were dried down. The water eluate yielded 10 grams of
solids,
the 30% acetone eluate yielded '16 grams of material and the 60% acetone
eluate
yielded 7 grams of material. The dried eluates (20mg) were dissolved in 1m1
50%
aqueous methanol and injected onto a SupelcosiITM LC-18 (Supelco) column (the
injection volume may be in the range of 1.5 to 5 microlitres). Elution was
carried out
by a solvent gradient at a flow rate of 1 mUmin under the following conditions
(volume
percentages):
~ 0-10 min 98%water with 0.1 °/~ v/v trifluoroacetic acid (TFA);
~ 10-20 min 65% water with 0.1 % v/v TFA and 35% acetonitrile with 0.1 % v/v
TFA;
~ 20-30 min 2% water with 0.1 % v/v TFA and 98% acetonitrile with 0.1 % v/v
TFA;
~ 30-50 min 98% water with 0.1 % v/v TFA and 2% acetonitrile with 0.1 % v/v
TFA.
Detection was carried out at wavelengths ranging from 210 nm to 400 nm.
Figure 1 is a chromatogram at 270 nm utilizing an injection volume of 1.5
microlitres.
This gradient is the same one used for the determination of Oenothein B.
-18-
CA 02386648 2002-05-16
The four components referred to in this four tannin mixture are the ones
outlined in Table 5. As noted below, these four components may comprise about
64%
of the total sample. The identification of one of these tannins as oenothein B
has
been confirmed.
The eluates contained at least four tannins; oenothein B is one of the major
tannins in the eluates. The 30% acetone eluate contains oenothein B plus three
other
tannins, and was dried down to obtain a quantity of 16 grams (which represents
the
dried solids from the eluate, comprising oenothein B plus three other tannins -
about
64% of the total - as well as other solid material from the extract - about
36% of the
total weight).
The 60% acetone eluate contains three tannins that are more polar than
oenothein B, and was dried down to obtain a quantity of 7 grams.
The tannins may be obtained in variable amounts. For example, one mixture
(the dried solids from the 30% acetone eluate) was found to contain the
tannins,
identified by HPLC in the following composition:
Table 6: Tannin Mixture Chromatographic Characterisation
annin Retention Percent Identification
Time
(Minutes) Composition
1 11.150 28.56 Oenothein
B
2 11.493 10.86
3 11. 598 12.26
4 11.989 12.31
Total 63.99
-19-
CA 02386648 2002-05-16
Rcaci il+e
Table 7: Kill Test Result for Tannin Mixture Isolated from Extracts of
Epilobium
angustifolium (Canadian Willowherb)
ConcentrationEquivalent Organisms Control 60
of CW Tannin Use bevel count minutes
of
Mixture* CWSC**
0.0003% 0.54% P. acnes # 118271.1 x 1.1 x 10'
10
(unpreserved) % reduction = 99.9
* Weight percent of isolated solids dissolved in water.
** This is the approximate concentration of CWSC mixed with water needed to
obtain
an equivalent concentration of 'tannins 1 through 4 as in the Concentration of
CW
Tannin Mixture.
These results show the surprising effectiveness of a tannin mixture of the
invention in the treatment of conditions involving P. acnes, such as acne and
other
skin conditions. Accordingly, in some embodiments it may be advantageous to
use
oenothein B in combination with other tannins, particularly tannins obtainable
from
Epilobium angustifolium (Canadian Willowherb), to prepare medicaments for the
treatment of skin conditions, such as conditions involving P. acnes. In some
embodiments such preparations may contain mixtures of tannins in the following
amounts (in weight percent): (1) Oenothein B any value or any range within the
values
of from 0.1 to 1.5% (for example from a minimum value of any one of 0.1 %,
0.2%,
0.3%, 0.4%, 0.5%, 0.6%, 0.7%, 0.8%, 0.9% or 1.0% to a higher maximum value of
0.5%, 0.6%, 0.7%, 0.8%, 0.9%, 1.0%, 1.1 %, 1.2%, 1.3%, 1.4% or 1.5%); (2)
Tannin 2
any value or any range within the values of from 0.01-1.0% (for example from a
minimum value of any one of 0.01 %, 0.02%, 0.03%, 0.04%, 0.05%, 0.06%, 0.07%,
0.08%, 0.09%, 0.10%, 0.11, 0.12, 0.13, 0.14, 0.15, 0.16, 0.17, 0.18, 0.19,
0.20 to a
higher maximum value of 0.80%, 0.81 %, 0.82%, 0.83%, 0.84%, 0.85%, 0.86%,
0.87%, 0.88%, 0.89%, 0.90%, 0.91 %, 0.92%, 0.93%, 0.94%, 0.95%, 0.96%, 0.97%,
0.98%, 0.99% or 1.00%); (3) Tannin 3 any value or any range within the values
of
from 0.01-1.0% (for example from a minimum value of any one of 0.01%, 0.02%,
0.03%, 0.04%, 0.05%, 0.06%, 0.07%, 0.08%, 0.09%, 0.10%, 0.11, 0.12, 0.13,
0.14,
0.15, 0.16, 0.17, 0.18, 0.19, 0.20 to a higher maximum value of 0.80%, 0.81 %,
0.82%,
-20-
CA 02386648 2002-05-16
0.83%, 0.84%, 0.85%, 0.86%, 0.87%, 0.88%, 0.89%, 0.90%, 0.91 %, 0.92%, 0.93%,
0.94%, 0.95%, 0.96%, 0.97%, 0.98%, 0.99% or 1.00%); (4) Tannin 4 any value or
any
range within the values of from 0.01-1.0% (for example from a minimum value of
any
one of 0.01 %, 0.02%, 0.03%, 0.04%, 0.05%, 0.06%, 0.07%, 0.08%, 0.09%, 0.10%,
0.11, 0.12, 0.13, 0.14, 0.15, 0.15, 0.17, 0.18, 0.19, 0.20 to a higher maximum
value of
0.80%, 0.81 %, 0.82%, 0.83%, 0.84%, 0.85%, 0.86%, 0.87%, 0.88%, 0.89%, 0.90%,
0.91 %, 0.92%, 0.93%, 0.94%, 0.95%, 0.96%, 0.97%, 0.98%, 0.99% or 1.00%).
Conclusion
Although various embodiments of the invention are disclosed herein, many
adaptations and modifications may be made within the scope of the invention in
accordance with the common general knowledge of those skilled in this art.
Such
modifications include the substitution of known equivalents for any aspect of
the
invention in order to achieve the same result in substantially the same way.
Numeric
ranges are inclusive of the numbers defining the range. In the claims, the
word
"comprising" is used as an open-ended term, substantially equivalent to the
phrase
"including, but not limited to".
References:
The following documents are incorporated herein by reference:
McCutcheon AR. Ethnopharmacology of Western North American Plants with
Special Focus on the Genus Artemisia. Ph.D. thesis, University of British
Columbia,
May, 1996.
Vivas N, Augustin M, Lonvaud-Funel A. Influence of Oak Wood and Grape
Tannins on the Lactic Acid Bacterium OEnococcus oeni (Leuconostoc oenos,
8413).
Journal of the Science of Food and Agriculture, 2000, 80(11 ):1675-1678.
Kakiuchi N, Hattori M, Nishizawa M, Yamagishi T, Okuda T, Namba T. Studies
on Dental Caries Prevention by Traditional Medicines: VIII. Inhibitory Effect
of Various
Tannins on Glucan Synthesis by Glucosyl Transferase from Streptococcus Mutans.
Chemical and Pharmaceutical Bulletin (Tokyo), 1986, 34(2):720-725.
-21
CA 02386648 2002-05-16
Yoshida T, Hatano T, Ito H. Chemistry and Function of Vegetable Polyphenols
with High Molecular Weights. Biofactors, 2000, 13(1-4):121-125.
Yoshida T., Chou T., Shingu T., Okuda T., Oenotheins D, F, and G
Hydrolysable Tannin Dimers from Oenothera laciniatia. Phytochemistry, 1995,
40(2):555-561.
Daniel EM, Ratnayake S, Kinstle T, Stoner GD. The effects of pH and rat
intestinal contents on the liberation of ellagic acid from purified and crude
ellagitannins. , 1991, J. Natural Products 54(4):946-952.
Ducrey B., Marston A., Gohring S., Hartmann R., Hostettmann K., Inhibition of
5a-Reductase and Aromatase by the Ellagitannins Oenothein A and Oenothein B
from
Epilobium Species. Planta Medica, 1997, 63:111-114.
-22-