Note: Descriptions are shown in the official language in which they were submitted.
WO 00/66499 PCT/DE00/01389
Method and Device
for the Purification of Sewage Water by using a Modified Membrane Filtration
The invention concerns a method for the purification of sewage water which
combines the
principles of membrane filtration and electrolysis and, therefore, allows a
high efficiency in
purification with nearly no backwash operation required.
Known are plants that use the activated sludge process, working in continuous
or batch op-
eration mode. The disadvantages of those approaches are their big requirements
as regards
floor space, spatial dimension and/or time needed for sedimentation processes.
The elimi-
nation of phosphorus is effected mainly by activated sludge extraction. Plants
working in
batch operation mode where the dry substance content is in excess of 7kg/rn3
with a high
level of aerobic stabilization of activated sludge still have a relatively
high P load being 5 mg
- 15 mg per liter. In industrial-scale treatment plants, P elimination to less
than 2mg/Itr is
not achieved unless flocculation / precipitation processes are employed.
Micro-filtration plants, in form of plate-type or tube-type filters, are very
efficient in BOD /
COD elimination but even here the N03- and P04~ contents in the effluent are
rather high.
Since an activated-sludge-and-water mixture is directly loaded onto the
membranes they
calcify, mineralize and become clogged soon so that they can be cleaned only
by an in-
creased backwash operation, which is very energy-consuming and limits the
plant's per-
formance parameters.
Electrolysis cells in sewage water purification applications have the
advantage of achieving
high COD degradation rates together with a good nitrogen and phosphorus
elimination pro-
vided they are equipped with sacrificial anodes that allow the formation of
salts. The disad-
vantage here, however, is the high volume of sludge which does flocculate well
due to its
high iron contents but requires a special finish of tank base as this kind of
sludge shows
nearly no tendency to slide off from the tank base. Furthermore, certain micro
organisms
are admitted to the effluent, and the number of germs here is significantly
greater than in
micro-filtration applications.
Therefore, it is the object of the invention to have sewage water purified in
such a way that
high purification rates are achieved in both the biological load and the
mineral matter as
well as a low number of germs as regards residual micro organisms to produce a
high-
quality effluent water after purification. The water's quality shall be so
that it can be used
without problems as service water in domestic installations. The treatment
costs shall be
not significantly higher than those accrued in larger communal plants. The
units shall be of
a compact design that can be used in both indoor and outdoor installations as
well as retro-
fit kids to existing treatment plants.
CA 02386959 2002-04-05
WO 00/66499 PCT/DE00/01389
_2_
According to the invention the problem shall be solved by suggesting a method
and a device
for executing the latter, in which completely new features are involved, and
where a high
rate in biological degradation is achieved together with a good COD, N and P
elimination at
a low number of germs as well as low-level operating costs.
The activated sludge method is used in most cases as a
nitrification/denitrification process
in the further clarification of sewage water. During the denitrification stage
the activated
sludge tends to settle but is kept suspended by agitators or slight aeration
in order to have
active surfaces of sludge particles available. The settling behavior during
the denitrification
stage is very good (settling rate: 1 meter in 30 minutes as a rule, similar to
the SBR
method).
The denitrification stage is used to stop the circulation for a short time,
i.e. about 15 - 20
minutes, and draw off the clarified water as is comes into existence after
such stop. Alter-
natively, this may also be achieved through a baffle plate thickener, for
example. The aim is
to maintain a high activated sludge proportion in the activated sludge tank /
denitrification
tank, which makes it possible that - due to the high dry substance contents (>
5kg/kgd) -
load fluctuations can be buffered safely. The biological purification,
however, is not fully
utilized to capacity but will be interrupted when the oxygen consumption
begins to drop
(depletion). This allows an energy-wise optimum operation.
The clarified water phase carrying a low portion of activated sludge enters
the device ac-
cording to invention, which comprises a combination of electrolysis cell and
membrane fil-
tration. To form the membrane and to serve as a support to said membrane an
electrically
conductive material is employed which then becomes the cathode. Especially
suited for that
purpose are noble metals, metal grids, wire gazes or coated membranes using
carbon fi-
bers, stainless steel or carbon fiber structures with or without coating as a
base material.
Other electron-emitting substances are also suitable.
To form the anode, sacrificial materials such as ferrous material, aluminum,
etc., or a com-
bination of the same can be used.
Also, graphite electrodes are possible. Arranging the cathode and the anodes
in an appro-
priate way will create a gap through which the sewage water can flow,
whereupon the ion-
ized constituents of the sewage water such as S042-, N03-, NOz-, CI-, P042-,
bacteria and vi-
ruses, etc., wander to the anode where the electron exchange processes take
place, and
finally convert into a gaseous state or sludge without adhesive power to drop
down from the
anode.
Fe3+ dissolved away from the anode will accelerate said settling behavior, but
graphite or
stainless steel anodes will do as well in case no higher anodic effect is
wanted.
CA 02386959 2002-04-05
WO 00/66499 PCT/DE00/01389
-3-
By way of an appropriate practical example below the invention shall be
explained in more
detail, with:-
Figure 1 - depicting the front view of the device for further purification
of the sewage water after biological treatment;
Figure 2 - depicting the side view of the device as shown in figure 1;
Figure 3 - depicting the bird's view of the device as shown in figure 1.
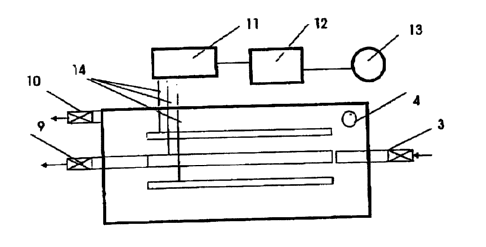
Through a controllable inlet (3) that can be shut off the sewage water coming
from an up-
stream clarification stage flows into the tank (1). The inlet is arranged in
such a way that
inside the same a high flow rate is maintained at the anodes (5) as well as at
the cathode,
or the membrane (6), respectively. The tank (1) is equipped with a gastight
cover (2). Also,
there is a vent pipe (4) provided. Both the anodes (5) and the cathodic
membrane (5) are
arranged on a spacer frame (7). The slanting base (8) is inclined in two
directions so that
the sludge extractor (10) is situated at the low point of this surface.
The direct current as needed for the electrolysis is supplied from the mains
connection (13)
using a regulating transformer (12) and a rectifier (11). The spacer frame's
(7) design al-
lows the gap length between anode (5) and cathodic membrane (6) to be adjusted
so that it
can be geared to the sewage water's electrolytic behavior. The same applies to
the strength
of current, voltage and duration. For regulating purposes a commonly known
control unit
may be employed.
Process control depends on water quality and hardness. The voltage to be fed
to the elec-
trodes shall be set to such a value that a slight positive OH- ion pressure
develops at the
cathode, or inside the same, so that a negative field is built up in front of
the membrane,
which in turn makes that metal that ions are converted into hydroxides
preferably before
they contact the membrane, or even enter into the membrane cell. There is a
correlation
between voltage / current flow, pore size and flow rate, or negative pressure
inside the
membrane.
With domestic sewage water, for example, a voltage of 2V - 4V, a pore size of
lNm - 2Nm,
and a positive pressure of 20mm water column is sufficient to achieve effluent
values being
< 100mg/Itr COD, < 5mg/Itr NH4-N, < 2mg/Itr P, and number of germs < 10.
By increasing the pore size to 1Nm - 2Nm in diameter the active pore area
increases from
0.03Nmz - 0.2Nmz with known micro-filtration to 0.78Nm2 - l.5Nm2.
As a result, the negative pressure at the membrane is lessening, i.e. 20cm
water column is
sufficient to guarantee a free outlet, as well as backwash pressure values
being O.lbar
0.2bar. Throughputs as high as 100 Itr/mz - 200 Itr/m2 can be achieved.
CA 02386959 2002-04-05
WO 00/66499 PCT/DE00/01389
-4-
The device to be configured to execute the method includes the below described
elements
which are interconnected by their functional tasks.
In case of the membrane being in clean state, which is the sewage water to be
purified can
flow unhindered through the cathodic membrane's (6) pores, the power required
for the
electrolysis is fed via the electrode terminal points (14) to the anodes (5)
and the cathodic
membrane (6). The clarified water is discharged through the clarified water
outlet (9) in
unpressurized state, or under slight sub-atmospheric pressure. The water level
is as shown
by position mark min. (16). In the event the level of sewage water before
purification rises,
for example, to an elevation as shown by position mark max. (15) - maybe due
to a slight
drop in the cathodic membrane's (6) active pore area - a backwashing of the
cathodic
membrane (6) by using a dedicated backwash pump (not shown) is carried out
through the
outlet (9), with the inlet (3) being shut off at the same time. Dilute acid
may be added to
the backwash water in order to prevent the cathodic membrane (6) from being
clogged with
hydroxides. At short interval of time a pole reversal of power supply is
effected, whereupon
the particles are blasted off from both the anodes (5) and the cathodic
membrane (6), and
then swirled away by the sewage water flow. Then, the power supply via the
electrode ter-
minal points (14) is switched off. The sludge settles onto the inclined base
(8) and is - as
already described - removed through the sludge extractor (10).
In unpressurized operation mode, i.e. with a free outlet and a differential
pressure being
maintained at 20cm water column, the throughput per unit area is some
1751tr/m2h -
2001tr/m2h.
For example, a floor space of 0.6mz would be sufficient for an 8-person
household, produc-
ing 1,200 liter of sewage water per day, with a nitrification /
denitrification time of 16h/8h,
taking into account a peak load of Qdlo x 1201tr/h.
It is mainly hydrogen and ammonium ions that wander to the cathode, where they
convert
into gaseous state.
The particular advantage offered by the combination of method and device is
based on the
fact that the anodic effect does not transport the activated residual sludge
to the cathode
(i.e. the membrane), which - due to its repellent behavior - does not capture
salt forming
constituents such as S04-- or P04--. The whole surface of the membrane becomes
negatively
charged by the OH' ions that form at the cathode membrane (6). Metal ions
attracted by
the cathode are caught in a thin-layer zone right before the membrane where
they are con-
verted into metal hydroxides and deposited so that they are not able to reach
the mem-
brane's interior. By regulating the current conduction, both excessive and
insufficient for-
mation of OH- ions can be avoided as otherwise metal hydroxides would
precipitate inside
the membrane.
This process is easy to control as after a certain time of direct contact the
conductivity will
CA 02386959 2002-04-05
WO 00/66499 PCT/DE00/01389
-5-
decrease, as the current conduction will do. Both current conduction and
sewage water flow
are parameters to be used to create conditions easy to control.
Redox measurements in the space between anode and cathode, or in the membrane
efflu-
ent water, can exactly demonstrate the situation as described. In addition,
the cathodic
membrane (6) will receive new, active features to allow the physicochemical
treatment of
the sewage water and at the same time offer protection against clogging, or
adsorption of
salts and settling of bacterial cultures onto the membrane.
Hence, the membrane is not simply a mechanical sludge separating element but
an active
member in the physicochemical treatment of the sewage water.
Low power consumption, being some lkWh per 1 cubic meter of sewage water, will
allow
energy savings to a great extent which is due to the fact that the biological
OZ influx can be
Berated. To tackle the problem of membrane calcifying dilute acid may be added
to the
backwash water, which will cause adsorbed hydroxides to be removed, and at the
same
time the pH value to be brought back to normal as the excessive formation of
OH~ ions by
overvoltage will shift it to basic. In case of high-calcareous sewage water, a
commonly
known electrolysis cell or other appropriate aids or facilities may be
installed upstream.
CA 02386959 2002-04-05
WO 00/66499 PCT/DE00/01389
-6
Key to the Figures
1 - Tank
2 - Gastight Cover
3 - Controllable Inlet
4 - Vent Pipe
- Anodes
6 - Cathodic Membrane
7 - Spacer Frame
8 - Inclined Base
9 - Clarified Water
Outlet
10- Sludge Extractor
11- Rectifier
12 - Regulating Transformer
13 - Mains Connection
14 - Electrode Terminal Points
- Position Mark (max.)
16 - Position Mark (min.)
CA 02386959 2002-04-05