Note: Descriptions are shown in the official language in which they were submitted.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE I)E CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME DE _2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02387013 2008-05-16
25771-726
6-position substituted indolinones, the preparation thereof
and their use as pharmaceutical compositions
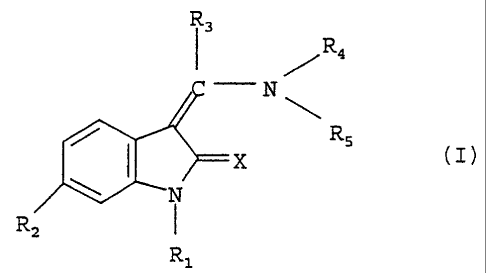
The present invention relates to new indolinones of general
formula
R3
R4
N
R5
)0!7>==
R2 I
R1
substituted in the 6 position, the tautomers, the
diastereomers, the enantiomers, the mixtures thereof and the
salts thereof, particularly the physiologically acceptable
salts thereof which have valuable properties.
The above compounds of general formula I wherein Rl denotes a
hydrogen atom or a prodrug group have valuable pharmacological
properties, in particular an inhibiting effect on various
kinases, especially receptor tyrosine kinases such as VEGFR2,
PDGFRa, PDGFRP, FGFR1, FGFR3, EGFR, HER2, IGF1R and HGFR, as
well as complexes of CDK's (Cyclin Dependent Kinases) such as
CDK1, CDK2, CDK3, CDK4, CDK5, CDK6, CDK7, CDK8 and CDK9 with
their specific cyclins (A, Bl, 22, C, Dl, D2, D3, E, F, G1,
G2, H, I and K) and to viral cyclin (cf. L. Mengtao in J.
Virology 71(3), 1984-1991 (1997)), and on the proliferation of
cultivated human cells, in particular endothelial cells, e.g.
in angiogenesis, but also on the proliferation of other cells,
in particular tumour cells.
CA 02387013 2007-12-05
25771-726
- 2 -
The other compounds of the above general formula I
wherein Rl does not denote a hydrogen atom or a prodrug group
are valuable intermediate products for preparing the
abovementioned compounds.
The present invention thus relates to the above
compounds of general formula I, whereby those compounds
wherein R1 denotes a hydrogen atom or a prodrug group have
valuable pharmacological properties, pharmaceutical
compositions containing the pharmacologically active
compounds, the use thereof and processes for preparing them.
According to one aspect of the present invention,
there is provided a compound of the formula I
R3
R4
N
Rs
X (I),
N
R2 I
R1
wherein: X denotes an oxygen or sulphur atom, R1 denotes a
hydrogen atom or a C1_4-alkoxycarbonyl or C2_4-alkanoyl group,
R2 denotes a carboxy group, a straight-chain or branched
C1_6-alkoxy-carbonyl group, a C4_7-cycloalkoxy-carbonyl or an
aryloxycarbonyl group; a straight-chain or branched
C1_6-alkoxy-carbonyl group, which is terminally substituted
in the alkyl moiety by a phenyl, heteroaryl, carboxy,
C1_3-alkoxy-carbonyl, aminocarbonyl, C,._3-alkylamino-carbonyl
or di-(C1_3-alkyl)-aminocarbonyl group; a straight-chain or
branched C2_6-alkoxy-carbonyl group, which is terminally
substituted in the alkyl moiety by a chlorine atom or a
CA 02387013 2007-12-05
25771-726
- 2a -
hydroxy, C1_3-alkoxy, amino, C1_3-alkylamino or
di-(C1_3-alkyl)-amino group; or an aminocarbonyl or
methylaminocarbonyl group or an ethylaminocarbonyl group
optionally substituted in the 2 position of the ethyl group
by a hydroxy or C1_3-alkoxy group; R3 denotes a hydrogen atom,
a C1_6-alkyl, C3_7-cycloalkyl, trifluoromethyl or heteroaryl
group, a phenyl or naphthyl group or a phenyl or naphthyl
group mono- or disubstituted by a fluorine, chlorine,
bromine or iodine atom, by a trifluoromethyl, C1_3-alkyl or
C1_3-alkoxy group, wherein, in the event of disubstitution,
the substituents are identical or different and wherein the
abovementioned unsubstituted as well as the mono- and
disubstituted phenyl and naphthyl groups are optionally
further substituted by a hydroxy, hydroxy-C1_3-alkyl or
C1_3-alkoxy-C1_3-alkyl group; by a cyano, carboxy, carboxy-
C1_3-alkyl, C1_3-alkoxycarbonyl, aminocarbonyl,
C1_3-alkylamino-carbonyl or di- (C1_3-alkyl) -aminocarbonyl
group; by a nitro group; by an amino, C1_3-alkylamino, di-
(C1_3-alkyl) -amino or amino-C1_3-alkyl group; by a
C1_3-alkylcarbonylamino, N- (C1_3-alkyl) -C1_3-alkyl-
carbonylamino, C1_3-alkylcarbonylamino-C1_3-alkyl,
N- (C7_3-alkyl) -C1_3-alkylcarbonylamino-Cl_3-alkyl, Cl_3-alkyl-
sulphonylamino, C1_3-alkylsulphonylamino-C1_3-alkyl,
N- (C1_3-alkyl) -C1_3-alkylsulphonylamino-C1_3-alkyl or
aryl-C1_3-alkylsulphonylamino group; by a cycloalkylamino,
cycloalkyleneimino, cycloalkyleneiminocarbonyl,
cycloalkyleneimino-C1_3-alkyl, cycloalkyleneiminocarbonyl-
C1_3-alkyl or cycloalkyleneiminosulphonyl-C1_3-alkyl group
having 4 to 7 ring members in each case, wherein in each
case the methylene group in position 4 of a 6- or 7-membered
cycloalkyleneimino group is optionally replaced by an oxygen
or sulphur atom, by a sulphinyl, sulphonyl, -NH or
-N(C1_3-alkyl) group; or by a heteroaryl or
heteroaryl-C1_3-alkyl group; R4 denotes a C3_-,-cycloalkyl
CA 02387013 2007-12-05
25771-726
- 2b -
group, wherein the methylene group in the 4 position of a 6-
or 7-membered cycloalkyl group is optionally substituted by
an amino, C1_3-alkylamino or di- (C1_3-alkyl) -amino group or is
optionally replaced by an -NH or -N(C1_3-alkyl) group; or a
phenyl group substituted by the group R6, which is optionally
further mono- or disubstituted by fluorine, chlorine,
bromine or iodine atoms or by C1_5-alkyl, trifluoromethyl,
hydroxy, C1_3-alkoxy, carboxy, C1_3-alkoxycarbonyl, amino,
acetylamino, C1_3-alkyl-sulphonylamino, aminocarbonyl,
Cl_3-alkyl-aminocarbonyl, di- (C1_3-alkyl) -aminocarbonyl,
aminosulphonyl, C1_3-alkyl-aminosulphonyl,
di-(C1_3-alkyl)-aminosulphonyl, nitro or cyano groups,
wherein the substituents are identical or different; R6
denotes a hydrogen, fluorine, chlorine, bromine or iodine
atom; a cyano, nitro, amino, C1_5-alkyl, C3_7-cycloalkyl,
trifluoromethyl, phenyl, tetrazolyl or heteroaryl group; a
group of formula
O
NH
CH
N O
H
wherein the hydrogen atoms bound to a nitrogen atom are
optionally in each case replaced independently of one
another by a C1_3-alkyl group; a C1_3-alkoxy group, a
C1_3-alkoxy-C1_3-alkoxy, phenyl-C1_3-alkoxy, amino-C2_3-alkoxy,
C1_3-alkylamino-C2_3-alkoxy, di- (C1_3-alkyl) -amino-C2_3-alkoxy,
phenyl-C1_3-alkylamino-C2_3-alkoxy, N- (C1_3-alkyl) -phenyl-C1_3-
alkylamino-C2_3-alkoxy, CS_7-cycloalkyleneimino-C2_3-alkoxy or
C1_3-alkylmercapto group; a carboxy, C1_4-alkoxycarbonyl,
aminocarbonyl, C1_3-alkylamino-carbonyl, N- (C1_5-alkyl) -C1_3-
alkylaminocarbonyl, phenyl-C1_3-alkylamino-carbonyl, N-(C1_3-
alkyl)-phenyl-C1_3-alkylamino-carbonyl, piperazinocarbonyl or
CA 02387013 2007-12-05
25771-726
- 2c -
N- (C1_3-alkyl) -piperazinocarbonyl group; a C1_3-
alkylaminocarbonyl or N- (C1_5-alkyl) -C1_3-alkylaminocarbonyl
group wherein an alkyl moiety is substituted by a carboxy or
C1_3-alkoxycarbonyl group or in the 2 or 3 position by a di-
(C1_3-alkyl) -amino, piperazino, N- (C1_3-alkyl) -piperazino or a
4- to 7-membered cycloalkyleneimino group; a C3_7-
cycloalkyl-carbonyl group; wherein the methylene group in
the 4 position of the 6- or 7-membered cycloalkyl moiety is
optionally substituted by an amino, C1_3-alkylamino or
di-(C1_3-alkyl)-amino group or replaced by an -NH or
-N(C1_3-alkyl) group; a 4- to 7-membered cycloalkyleneimino
group wherein a methylene group linked to the imino group is
optionally replaced by a carbonyl or sulphonyl group or the
cycloalkylene moiety is optionally fused to a phenyl ring or
one or two hydrogen atoms are optionally each replaced by a
C1_3-alkyl group and/or in each case the methylene group in
the 4 position of a 6- or 7-membered cycloalkyleneimino
group is optionally substituted by a carboxy,
C1_3-alkoxycarbonyl, aminocarbonyl, C1_3-alkylaminocarbonyl,
di- (C1_3-alkyl) -aminocarbonyl, phenyl-C1_3-alkylamino or N-
(C1_3-alkyl) -phenyl-Cl_3-alkylamino group or is optionally
replaced by an oxygen or sulphur atom or by a sulphinyl,
sulphonyl, -NH, -N(C1_3-alkyl) , -N(phenyl),
-N(Cl_3-alkyl-carbonyl) or -N(benzoyl) group; a Cl_4-alkyl
group substituted by the group R7, wherein R7 denotes a C3_7-
cycloalkyl group, wherein the methylene group in the 4
position of the 6- or 7-membered cycloalkyl group is
optionally substituted by an amino, C1_3-alkylamino or
di-(C1_3-alkyl)-amino group or is optionally replaced by
an -NH or -N(C1_3-alkyl) group or in a 5- to 7-membered
cycloalkyl group a-(CH2)z group is optionally replaced by a
-CO-NH group, a-(CH2)3 group is optionally replaced by a
-NH-CO-NH or -CO-NH-CO group or a-(CH2)4 group is optionally
replaced by a -NH-CO-NH-CO group, wherein in each case a
CA 02387013 2007-12-05
25771-726
- 2d -
hydrogen atom bound to a nitrogen atom is optionally
replaced by a C1_3-alkyl group; an aryl or heteroaryl group;
wherein, when the heteroaryl is a pyrrolyl, pyrazolyl or
imidazolyl group bound via a nitrogen atom, the heteroaryl
is optionally substituted by a C1_3-alkyl group, and wherein
the heteroaryl is a pyrrolidine, piperidino, morpholino,
thiomorpholino or piperazino group, the heteroaryl is
optionally substituted in the 4 position by a C1_3-alkyl,
phenyl-C1_3-alkyl, C1_3alkylcarbonyl or C1_4-alkoxycarbonyl
group or a hydrogen atom; a hydroxy or C1_3-alkoxy group; an
amino, C1_7-alkylamino, di- (C1_,-alkyl) -amino, phenylamino,
N-phenyl-C1_3-alkyl-amino, phenyl-C1_3-alkylamino,
N- (C1_3-alkyl) -phenyl-C1_3-alkylamino or di- (phenyl-
C1_3-alkyl) -amino group; an w-hydroxy-Cz_3-alkyl-amino,
N- (C1_3-alkyl) -co-hydroxy-C2_3-alkyl-amino, di- (co-hydroxy-C2_3-
alkyl) -amino, di- (m- (C1_3-alkoxy) -C2_3-alkyl) -amino or
N- (dioxo-lan-2-yl) -C1_3-alkyl-amino group; a C1_3-alkoxy-C1_3-
alkylamino, N- (C1_3-alkyl) -C1_3-alkoxy-C1_3-alkylamino or di-
(2-methoxy-ethyl)-amino group; a
C1_3-alkylcarbonylamino-C2_3-alkyl-amino or C1_3-
alkylcarbonylamino-C2_3-alkyl-N- (C1_3-alkyl) -amino group; a
C1_3-alkylsulphonylamino, N- (C1_3-alkyl) -C1_3-
alkylsulphonylamino, C1_3-alkylsulphonylamino-C2_3-alkyl-amino
or C1_3-alkylsulphonylamino-C2_3-alkyl-N- (C1_3-alkyl) -amino
group; a hydroxycarbonyl-C1_3-alkylamino or N- (C1_3-alkyl) -
hydroxycarbonyl-C1_3-alkyl-amino group; a guanidino group
wherein one or two hydrogen atoms are each optionally
replaced by a C1_3-alkyl group; a group of formula
-N(Re) -CO- (CH2)n-R9 (II),
wherein R8 denotes a hydrogen atom or a C1_3-alkyl group, n
denotes 0, 1, 2 or 3 and R9 denotes an amino, C,._4-alkylamino,
di- (C1_4-alkyl) -amino, phenylamino, N- (C1_4-alkyl) -
CA 02387013 2008-05-16
25771-726
2e -
phenylamino, benzylamino, N- (C1-4-alkyl) -benzylamino or C1_4-
alkoxy group, or a 4- to 7-membered cycloalkyleneimino
group, wherein in each case the methylene group in the 4
position of a 6- or 7-membered cycloalkyleneimino group is
optionally replaced by an oxygen or sulphur atom or by a
sulphinyl, sulphonyl, -NH, -N(C1_3-alkyl), -N(phenyl),
-N(Cl-3-alkyl-carbonyl) or -N(benzoyl) group, or, if n
denotes 1, 2 or 3, R9 is as defined herein or is a hydrogen
atom; a group of formula
-N(Rlo) - (CH2)m- (CO) o-Rll (III),
wherein Rlo denotes a hydrogen atom, a C1-3-alkyl group, a
C1_3-alkylcarbonyl, arylcarboriyl, phenyl-C1_3-alkyl-carbonyl,
C1_3-alkylsulphonyl, arylsulphonyl or phenyl-C1_3-
alkylsulphonyl group; m denotes 1, 2, 3 or 4; o denotes 1
or, if m denotes 2, 3 or 4, o denotes 0 or 1; and R11 denotes
an amino, C1._4-alkylamino, di-(C1-4-alkyl)-amino, phenylamino,
N- (C1-4-alkyl) -phenylamino, benzylamino, N- (C1-4-alkyl) -
benzylamino, C1-4-alkoxy or C1..3-alkoxy-Cl-3-alkoxy group, a
di- (C1_4-alkyl) -amino-C1_3-alkylamino group optionally
substituted in the 1 position by a C1_3-alkyl group or a 4-
to 7-membered cycloalkyleneimino group, wherein the
cycloalkylene moiety is optionally fused to a phenyl ring or
in each case the methylene group in the 4 position of the 6-
or 7-membered cycloalkyleneimino group is optionally
replaced by an oxygen or sulphur atom or by a sulphinyl,
sulphonyl, -NH, -N(C,.-3-alkyl), -N(phenyl), -N(C1-3-alkyl-
carbonyl) or -N(benzoyl) group; a C4-7-cycloalkylamino, C4-7-
cycloalkyl-C1_3-alkylamino or C4_7-cycloalkenylamino group
wherein position 1 of the ring is not involved in the double
bond and wherein the abovementioned groups are each
optionally further substituted at the amino-nitrogen atom by
a C5-7-cycloalkyl, C2_4-alkenyl or C1-4-alkyl group; or a 4- to
7-membered cycloalkyleneimino group, wherein the
CA 02387013 2008-05-16
25771-726
- 2f -
cycloalkylene moiety is optionally fused to a phenyl group
or to an oxazolo, imidazolo, thiazolo, pyridino, pyrazino or
pyrimidino group optionally substituted by a fluorine,
chlorine, bromine or iodine atom or by a nitro, C1_3-alkyl,
C1_3-alkoxy or amino group, and/or one or two hydrogen atoms
are each optionally replaced by a C1_3-alkyl, C5_7-cycloalkyl
or phenyl group; and/or the methylene group in the 3
position of a 5-membered cycloalkyleneimino group is
optionally substituted by a hydroxy, hydroxy-C1_3-alkyl, C1_3-
alkoxy or C1_3-alkoxy-C1_3-alkyl group; the methylene group in
the 3 or 4 position of a 6- or 7-membered cycloalkyleneimino
group is optionally in each case substituted by a hydroxy,
hydroxy-C1_3-alkyl, C1_3-alkoxy, C1_3-alkoxy-C1_3-alkyl,
carboxy, C1_.4-alkoxycarbonyl, aminocarbonyl, Cl_3-
alkylaminocarbonyl, di-(C1_3-alkyl)-aminocarbonyl, phenyl-
C1-3-alkylamino or N- (C1_3-alkyl) -phenyl-C1_3-alkyl-amino group
or is optionally replaced by an oxygen or sulphur atom or by
a sulphinyl, sulphonyl, -NH, -N(C1_3-alkyl-), -N(phenyl),
-N(phenyl-C1_3-alkyl-) , -N(C1_3-alkyl-carbonyl-) , -N(C1_4-
hydroxy-carbonyl-), -N(C1_4-alkoxy-carbonyl-), -N(benzoyl-)
or -N(phenyl-C1-3-alkyl-carbonyl-) group, wherein a methylene
group linked to an imino-nitrogen atom of the
cycloalkyleneimino group is optionally replaced by a
carbonyl or sulphonyl group or in a 5- to 7-membered
monocyclic cycloalkyleneimino group or a cycloalkyleneimino
group fused to a phenyl group, the two methylene groups
linked to the imino-nitrogen atom are each optionally
replaced by a carbonyl group, or R6 denotes a C1_4-alkyl group
which is substituted by a carboxy, Cl_3-alkoxycarbonyl,
aminocarbonyl, C1_3-alkylaminocarbonyl or di- (C1_3-alkyl) -
aminocarbonyl group or by a 4- to 7-membered
cycloalkyleneiminocarbonyl group, an N- (C1_3-alkyl) -C2_4-
alkanoylamino group which is additionally substituted in the
CA 02387013 2008-05-16
25771-726
- 2g -
alkyl moiety by a carboxy or C1_3-alkoxycarbonyl group, a
group of formula
-N(R12) -CO- (CH2)p-R13 (IV) ,
wherein R12 denotes a hydrogen atom, a C1_6-alkyl or C3_7-
cycloalkyl group or a C1_3-alkyl group terminally substituted
by a phenyl, heteroaryl, trifluoromethyl, hydroxy, C1_3-
alkoxy, aminocarbonyl, C1_4-alkylamino-carbonyl, di-(Cl_4-
alkyl)-amino-carbonyl, C1_3-alkyl-carbonyl, C1_3-alkyl-
sulphonylamino, N- (Cl_3-alkyl) -C1_3-alkyl-sulphonylamino, C1_3-
alkyl-aminosulphonyl or di-(C1_3-alkyl)-aminosulphonyl group
and p denotes 0, 1, 2 or 3 and R13 assumes the meanings of
the abovementioned group R7, or, if p denotes 1, 2 or 3,
R13 is as defined herein or R13 is a hydrogen atom; a group of
formula
-N (R14) - (CH2) q- (CO) r-R1s (V) ~
wherein R14 denotes a hydrogen atom, a C1_4-alkyl group or a
Cl_3-alkylcarbonyl, arylcarbonyl, phenyl-Cl_3-alkylcarbonyl,
heteroarylcarbonyl, heteroaryl-C1_3-alkylcarbonyl, C1_4-
alkylsulphonyl, arylsulphonyl, phenyl-C1_3-alkylsulphonyl,
heteroarylsulphonyl or heteroaryl-C1_3-alkyl-sulphonyl group,
q denotes 1, 2, 3 or 4, r denotes the number 1 or, if q is
2, 3 and 4, r is 0 or 1, and R15 assumes the meanings of the
abovementioned group R7; or a group of formula
-N (R16) -SOz-R17 (VI) ,
wherein R16 denotes a hydrogen atom or a C1_4-alkyl group
optionally terminally substituted by a cyano,
trifluoromethyl-carbonylamino or N-(C1_3-alkyl)-
trifluoromethyl-carbonyl-amino group and R17 denotes a C1_3-
alkyl group; an amino group substituted by a di-(C1_3-alkyl)-
amino-Cl_3-alkyl-carbonyl or di- (C1_3-alkyl) -amino-C1_3-alkyl-
CA 02387013 2007-12-05
25771-726
- 2h -
sulphonyl group and a di-(C1_3-alkyl)-aminocarbonyl-C1_3-alkyl
group, or an N- (C1_3-alkyl) -C1_5-alkylsulphonylamino or N-
(C1_3-alkyl)-phenylsulphonylamino group wherein the alkyl
moiety is additionally substituted by a cyano or carboxy
group; wherein all the single-bonded or fused phenyl groups
contained in the groups mentioned under R6 are optionally
mono- or disubstituted by fluorine, chlorine, bromine or
iodine atoms or by C1_5-alkyl, trifluoromethyl, hydroxy, C1_3-
alkoxy, carboxy, C1_3-alkoxycarbonyl, aminocarbonyl, C1_4-
alkylamino-carbonyl, di-(C1_4-alkyl)-amino-carbonyl,
aminosulphonyl, C1_3-alkyl-aminosulphonyl, di- (C1_3-alkyl) -
aminosulphonyl, C1_3-alkyl-sulphonylamino, nitro or cyano
groups, wherein the substituents are identical or different,
or two adjacent hydrogen atoms of the phenyl groups are
optionally replaced by a methylenedioxy group; and R5 denotes
a hydrogen atom or a C1_3-alkyl group, wherein by an aryl
group is meant a phenyl or naphthyl group optionally mono-
or disubstituted by a fluorine, chlorine, bromine or iodine
atom, by a cyano, trifluoromethyl, nitro, carboxy,
aminocarbonyl, C1_3-alkyl or C1_3-alkoxy group and by a
heteroaryl group is meant a monocyclic 5- or 6-membered
heteroaryl group optionally substituted by a C1_3-alkyl group
in the carbon skeleton, wherein the 6-membered heteroaryl
group contains one, two or three nitrogen atoms and the 5-
membered heteroaryl group contains an imino group optionally
substituted by a C1_3-alkyl or phenyl-C1_3-alkyl group, an
oxygen or sulphur atom or an imino group optionally
substituted by a C1_3-alkyl or phenyl-C1_3-alkyl group or an
oxygen or sulphur atom and additionally a nitrogen atom or
an imino group optionally substituted by a C1_3-alkyl or
phenyl-C1_3-alkyl group and two nitrogen atoms, and moreover
a phenyl ring may be fused to the abovementioned monocyclic
heterocyclic groups via two adjacent carbon atoms and the
bonding takes place via a nitrogen atom or via a carbon atom
CA 02387013 2007-12-05
25771-726
- 2i -
of the heterocyclic moiety or a fused phenyl ring; some or
all of the hydrogen atoms in the abovementioned alkyl and
alkoxy groups or in the alkyl moieties contained in the
above-defined groups of formula I are optionally replaced by
fluorine atoms; and wherein any carboxy group contained in
the abovementioned groups are optionally replaced by a
tert.butoxycarbonyl group; and wherein a hydrogen atom bound
to a nitrogen atom is optionally each replaced by hydroxyl,
benzoyl, pyridinoyl, formyl, acetyl, propionyl, butanoyl,
pentanoyl, hexanoyl, allyloxycarbonyl, methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl,
butoxycarbonyl, tert.butoxycarbonyl, pentoxycarbonyl,
hexyloxycarbonyl, octyloxycarbonyl, nonyloxycarbonyl,
decyloxycarbonyl, undecyloxycarbonyl, dodecyloxycarbonyl,
hexadecyloxycarbonyl, benzyloxycarbonyl,
phenylethoxycarbonyl, phenylpropoxycarbonyl, C1_3-
alkylsulphonyl-C2_4-alkoxycarbonyl, C1_3-alkoxy-C2_4-alkoxy-
C2_4-alkoxycarbonyl or an ReCO-O- (RfCRg) -0-CO group wherein Re
denotes a C1_8-alkyl, C5_7-cycloalkyl, phenyl or phenyl-C1_3-
alkyl group; Rf denotes a hydrogen atom, a C1_3-alkyl, C5_7-
cycloalkyl or phenyl group and R. denotes a hydrogen atom or
a C1_3-alkyl group; or wherein an amino nitrogen optionally
forms part of a phthalimido group, or a tautomer or
pharmaceutically acceptable salt thereof.
According to another aspect of the present
invention, there is provided a compound, tautomer or salt as
described herein, wherein: R1 and R3 are as described herein;
X denotes an oxygen atom; R2 denotes a carboxy group, a
straight-chain or branched C1_6-alkoxy-carbonyl group, a C5_-7-
cycloalkoxycarbonyl or a phenoxycarbonyl group; a straight-
chain or branched C1_3-alkoxy-carbonyl group, which is
terminally substituted in the alkyl moiety by a phenyl,
heteroaryl, carboxy, C1_3-alkoxycarbonyl, aminocarbonyl, C1_3-
CA 02387013 2007-12-05
25771-726
- 2j -
alkylaminocarbonyl or di-(C1_3-alkyl)-aminocarbonyl group; a
straight-chain or branched C2_3-alkoxy-carbonyl group, which
is terminally substituted in the alkyl moiety by a chlorine
atom or by a hydroxy, C1_3-alkoxy, amino, C1_3-alkylamino or
di-(C1_3-alkyl)-amino group; an aminocarbonyl or
methylaminocarbonyl group or an ethylaminocarbonyl group
optionally substituted in the 2 position of the ethyl group
by a hydroxy or C1_3-alkoxy group; R4 denotes a C3_7-cycloalkyl
group, wherein the methylene group in the 4 position of the
6- or 7-membered cycloalkyl group is optionally substituted
by an amino, C1_3-alkylamino or di- (C1_3-alkyl) -amino group or
is optionally replaced by an -NH or -N(C1_3-alkyl) group; or
a phenyl group substituted by the group R6 which is
optionally mono- or disubstituted by fluorine, chlorine or
bromine atoms or by C1_3-alkyl, trifluoromethyl, hydroxy,
C1_3-alkoxy, carboxy, C1_3-alkoxycarbonyl, amino, acetylamino,
aminocarbonyl, C1_3-alkyl-aminocarbonyl, di- (C1_3-alkyl) -
aminocarbonyl, nitro or cyano groups, wherein the
substituents are identical or different; R6 denotes a
hydrogen, fluorine, chlorine, bromine or iodine atom, or a
cyano, nitro, amino, C1_5-alkyl, C3_-,-cycloalkyl,
trifluoromethyl, phenyl, tetrazolyl or heteroaryl group; a
group of formula
O
CH NH
O
H
wherein a hydrogen atom bound to a nitrogen atom is
optionally replaced by a C1_3-alkyl group, a C1_3-alkoxy
group, an amino-C2_3-alkoxy, C1_3-alkylamino-C2_3-alkoxy, di-
(C1_3-alkyl) -amino-C2_3-alkoxy, phenyl-C1_3-alkylamino-C2_3-
alkoxy, N- (C1_3-alkyl) -phenyl-C1_3-alkylamino-C2_3-alkoxy,
CA 02387013 2007-12-05
25771-726
- 2k -
pyrrolidino-C2_3-alkoxy, piperidino-C2_3-alkoxy or C1_3-
alkylmercapto group; a carboxy, C1_4-alkoxycarbonyl,
aminocarbonyl, C1_3-alkylamino-carbonyl, phenyl-C1_3-
alkylamino-carbonyl or N-(C1_3-alkyl)-phenyl-C1_3-alkylamino-
carbonyl group; a C3_7-cycloalkyl-carbonyl group, wherein the
methylene group in the 4 position of the 6- or 7-membered
cycloalkyl moiety is optionally replaced by an -NH or
-N(C1_3-alkyl) group; a 4- to 7-membered cycloalkyleneimino
group, wherein a methylene group linked to the imino group
is optionally replaced by a carbonyl or sulphonyl group or
one or two hydrogen atoms are each optionally replaced by a
C1_3-alkyl group and/or in each case the methylene group in
the 4 position of a 6- or 7-membered cycloalkyleneimino
group is optionally substituted by a carboxy, C1_3-
alkoxycarbonyl, aminocarbonyl, C1_3-alkylaminocarbonyl, di-
(C1_3-alkyl) -aminocarbonyl, phenyl-C1_3-alkylamino or N- (C1_3-
alkyl)-phenyl-C1_3-alkylamino group or is optionally replaced
by an oxygen or sulphur atom or by a sulphinyl, sulphonyl,
-NH or -N(C1_3-alkyl) group; a C1_4-alkyl group terminally
substituted by the group R7, wherein R7 denotes a C5_7-
cycloalkyl group, wherein the methylene group in the 4
position of a 6- or 7-membered cycloalkyl group is
optionally replaced by an -NH or -N(C1_3-alkyl) group or in a
5- to 7-membered cycloalkyl group a - (CHz)Z group is
optionally replaced by a -CO-NH group, a-(CHZ)3 group is
optionally replaced by a -NH-CO-NH- or a-(CHz)4 group is
optionally replaced by a-NH-CO-NH-CO group, wherein in each
case a hydrogen atom bound to a nitrogen atom is optionally
replaced by a C1_3-alkyl group; a phenyl or heteroaryl group;
a hydroxy or C1_3-alkoxy group; an amino, C1_6-alkylamino,
di-(C1_6-alkyl)-amino, phenylamino, N-phenyl-C1_3-alkyl-amino,
phenyl-C1_3-alkylamino, N- (Cl_3-alkyl) -phenyl-C1_3-alkylamino
or di- (phenyl-C1_3-alkyl) -amino group; a
(o-hydroxy-C2_3-alkyl-amino, N- (C1_3-alkyl) -c)-hydroxy-
CA 02387013 2008-05-16
25771-726
- 21 -
C2_3-alkyl-amino, di- (co-hydroxy-C2_3-alkyl) -amino,
di- ((0- (C1_3-alkoxy) -C2_3-alkyl) -amino or
N-(dioxolan-2-yl)-C1_3-alkyl-amino group; a
C1_3-alkylcarbonylamino-C2_3-alkyl-amino or
C1_3-alkylcarbonylamino-C2_3-alkyl-N- (C1_3-alkyl) -amino group;
a C1_3-alkylsulphonylamino, N- (C1_3-alkyl) -
C1_3-alkylsulphonylamino, C1_3-alkylsulphonylamino-
-C2_3-alkyl-amino or C1_3-alkylsulphonylamino-C2_3-alkyl-
-N- (C1_3-alk.yl) -amino group; a hydroxycarbonyl-Cl_3-alkylamino
or N- (C1_3-alkyl) -hydroxycarbonyl-C1_3-alkyl-amino group; a
guanidino group wherein a hydrogen atom is optionally
replaced by a C1_3-alkyl group; a group of formula
-N(R8) -CO- (CHz)n-Ry (II) ,
wherein R8 denotes a hydrogen atom or a C1_3-alkyl group; n
denotes 0, 1, 2 or 3 and Ry denotes an amino, C1_3-alkylamino,
di-(Cl_3-alkyl)-amino, phenylamino, benzylamino or C1_4-alkoxy
group, or a 5- to 7-membered cycloalkyleneimino group,
wherein the methylene group in position 4 of the
cycloalkyleneimino group is optionally replaced by an oxygen
or sulphur atom or by an -NH, -N(C1_3-alkyl),-N(phenyl) ,
-N(C1_.3-alkyl-carbonyl) or -N(benzoyl) group, or, if n
denotes 1, 2 and 3, R9 is as defined herein or denotes a
hydrogen atom; a group of formula
-N(R10) - (CH2)m- (CO)o-Rll (III),
wherein Rlo denotes a hydrogen. atom or a C1_3-alkyl,
C1_3-alkylcarbonyl or C1_3-alkylsulphonyl group, m denotes 1,
2 or 3, o is 1 or, if m is 2 and 3, o is 0 or 1 and Rll
denotes an amino, C1_3-alkylamino, di- (C1_3-alkyl) -amino,
C1_4-alkoxy or C1_3-alkoxy-C1_3-alkoxy group or a 5- to 7-
membered cycloalkyleneimino group, wherein the methylene
group in position 4 of the cycloalkyleneimino group is
CA 02387013 2007-12-05
25771-726
- 2m -
optionally replaced by an oxygen or sulphur atom or by an
-NH, -N(C1_3-alkyl) , -N(phenyl), -N(C1_3-alkyl-carbonyl) or
-N(benzoyl) group; a C4_7-cycloalkylamino or
C4_7-cycloalkenylamino group wherein position 1 of the ring
is not involved in the double bond; a 4- to 7-membered
cycloalkyleneimino group, wherein the cycloalkylene moiety
is optionally fused to a phenyl group or one or two hydrogen
atoms is each optionally replaced by a C1_3-alkyl group
and/or the methylene group in position 3 of the
cycloalkyleneimino group is optionally substituted by a
hydroxy or C1_3-alkoxy group, in each case the methylene
group in the 4 position of a 6- or 7-membered
cycloalkyleneimino group is optionally substituted by a
hydroxy, hydroxy-C1_3-alkyl, C1_3-alkoxy, carboxy,
C1_3-alkoxycarbonyl, aminocarbonyl, C1_3-alkylaminocarbonyl,
di- (C1_3-alkyl) -aminocarbonyl, phenyl-C1_3-alkylamino or
N- (C1_3-alkyl) -phenyl-C1_3-alkylamino group or is optionally
replaced by an oxygen or sulphur atom or by a sulphinyl,
sulphonyl, -NH, -N(C1_3-alkyl), -N(phenyl),
-N(phenyl-C1_3-alkyl) , -N(C1_3-alkyl-carbonyl) , -N(C1_4-
alkoxy-carbonyl) , -N(benzoyl) or -N(phenyl-C1_3-alkyl-
carbonyl) group, wherein a methylene group linked to an
imino-nitrogen atom of the cycloalkyleneimino group is
optionally replaced by a carbonyl or sulphonyl group or in a
5- to 6-membered monocyclic cycloalkyleneimino group or a
cycloalkyleneimino group fused to a phenyl group, the two
methylene groups linked to the imino-nitrogen atom are each
optionally replaced by a carbonyl group; or R6 denotes a
C1_4-alkyl group which is terminally substituted by a
carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
C1_3-alkylaminocarbonyl or di-(C1_3-alkyl)-aminocarbonyl group
or by a 4- to 7-membered cycloalkyleneiminocarbonyl group;
CA 02387013 2007-12-05
25771-726
- 2n -
a group of formula
-N (R12) -CO- (CHZ) p-R13 ( IV)
wherein R12 denotes a hydrogen atom or a C1_3-alkyl,
C5_-,-cycloalkyl, phenyl-C1_3-alkyl or heteroaryl-C1_3-alkyl
group and p denotes 0, 1, 2 or 3 and R13 assumes the meanings
of the abovementioned group R7, or, if p denotes one of the
numbers 1, 2 and 3, R13 is as defined herein or denotes a
hydrogen atom; a group of formula
-N (R14) - (CHz) q- (CO) r-R15 (V)
wherein R14 denotes a hydrogen atom, a C1_4-alkyl group or a
C1_3-alkylcarbonyl, phenylcarbonyl, phenyl-C1_3-alkylcarbonyl,
heteroarylcarbonyl, heteroaryl-C1_3-alkylcarbonyl,
C1_4-alkylsulphonyl, phenylsulphonyl, phenyl-C1_3-
alkylsulphonyl, heteroarylsulphonyl or heteroaryl-C1_3-alkyl-
sulphonyl group, q denotes 1, 2, 3 or 4, r is 1 or, if q is
2, 3 or 4, r is 0 or 1 and R15 assumes the meanings of the
abovementioned group R7; a group of formula
-N(R16) -SO2-R17 (VI) ,
wherein R16 denotes a hydrogen atom or a C1_4-alkyl group
optionally terminally substituted by a cyano,
trifluoromethyl-carbonylamino or N-(C1_3-alkyl)-
trifluoromethyl-carbonyl-amino group and R17 denotes a
C1_3-alkyl group; or an amino group substituted by a
di- (C1_3-alkyl) -amino-C1_3-alkyl-carbonyl or di- (C1_3-alkyl) -
amino-C1_3-alkyl-sulphonyl group and a di- (C1_3-alkyl) -
aminocarbonyl-C1_3-alkyl group, wherein all the single-bonded
or fused phenyl groups contained in the groups mentioned
under R6 are optionally mono- or disubstituted by fluorine,
chlorine or bromine atoms or by C1_3-alkyl, trifluoromethyl,
hydroxy, C1_3-alkoxy, carboxy, C1_3-alkoxycarbonyl,
CA 02387013 2007-12-05
25771-726
- 2o -
aminocarbonyl, C1_3-alkyl-aminocarbonyl, aminosulphonyl,
Cl_3-alkyl-aminosulphonyl, nitro or cyano groups, wherein the
substituents are identical or different, or two adjacent
hydrogen atoms of the phenyl groups are optionally replaced
by a methylenedioxy group; and R5 denotes a hydrogen atom or
a C1_3-alkyl group; wherein, by a heteroaryl group as
mentioned above is meant a pyridinyl, pyrazinyl,
pyrimidinyl, pyridazinyl, pyrrolyl, furyl, thienyl,
oxazolyl, thiazolyl, pyrazolyl, imidazolyl or triazolyl
group optionally substituted in the carbon skeleton by a
C1_3-alkyl group wherein a hydrogen atom bound to a nitrogen
atom is optionally replaced by a C1_3-alkyl or
phenyl-C1_3-alkyl group and wherein the 5-membered heteroaryl
groups containing at least one imino group are bound via a
carbon or nitrogen atom; wherein a hydrogen atom bound to a
nitrogen atom in the abovementioned groups is optionally
replaced by hydroxyl, benzoyl, pyridinoyl, formyl, acetyl,
propionyl, butanoyl, pentanoyl, hexanoyl, allyloxycarbonyl,
methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
isopropoxycarbonyl, butoxycarbonyl, tert.butoxycarbonyl,
pentoxycarbonyl, hexyloxycarbonyl, octyloxycarbonyl,
nonyloxycarbonyl, decyloxycarbonyl, undecyloxycarbonyl,
dodecyloxycarbonyl, hexadecyloxycarbonyl, benzyloxycarbonyl,
phenylethoxycarbonyl, phenylpropoxycarbonyl,
C1_3-alkylsulphonyl-C2_4-alkoxycarbonyl, C1_3-alkoxy-C2_4-
alkoxy-C2_4-alkoxycarbonyl or an ReCO-O- (RfCRg) -0-CO group
wherein Re denotes a C1_$-alkyl, C5_-,-cycloalkyl, phenyl or
phenyl-C1_3-alkyl group, Rf denotes a hydrogen atom or a C1_3-
alkyl, C5_7-cycloalkyl or phenyl group and Rg denotes a
hydrogen atom or a C1_3-alkyl, or wherein an amino nitrogen
optionally forms part of a phthalimido group; and wherein
any carboxy group contained in the abovementioned groups is
optionally replaced by a tert.butoxycarbonyl group, and
wherein some or all of the hydrogen atoms in the
CA 02387013 2008-11-21
25771-726
- 2p -
abovementioned alkyl and alkoxy groups or in the alkyl
moieties contained in the above-defined groups of formula I
optionally replaced by fluorine atoms.
According to still another aspect of the present
invention, there is provided a compound, tautomer or salt as
described herein, wherein: X denotes an oxygen atom; R1
denotes a hydrogen atom; R2 denotes a carboxy group, a
straight-chain or branched C1_4-alkoxycarbonyl group or a
phenoxycarbonyl group; a straight-chain or branched
C1_3-alkoxy-carbonyl group, which is terminally substituted
in the alkyl moiety by a phenyl, carboxy,
C1_3-alkoxycarbonyl, aminocarbonyl, C1_3-alkylaminocarbonyl or
di-(C1_3-alkyl)-aminocarbonyl group; a straight-chain or
branched C2_3-alkoxy-carbonyl group which is terminally
substituted in the alkyl moiety by a hydroxy, C1_3-alkoxy,
amino, C1_3-alkylamino or di- (C1_3-alkyl) -amino group; or an
aminocarbonyl or methylaminocarbonyl group, or an
ethylaminocarbonyl group optionally substituted in the 2
position of the ethyl group by a hydroxy or C1_3-alkoxy group
or, if R4 denotes something other than an aminosulphonyl-
phenyl or N- (C1_5-alkyl) -C1_3-alkylaminocarbonyl-phenyl group,
the optional substituent further optionally denotes a
di- (C1_2-alkyl) -aminocarbonyl group; R3 denotes a C1_4-alkyl
group or a phenyl group which is optionally substituted by a
fluorine, chlorine or bromine atom or by a trifluoromethyl,
C1_3-alkyl, hydroxy or C1_3-alkoxy group; R4 denotes a C5_6-
cycloalkyl group, wherein the methylene group in position 4
of the C5-C6 cycloalkyl group when the C5-C6 cycloalkyl group
is a cyclohexyl group is optionally substituted by an amino,
C1_3-alkylamino or di- (C1_3-alkyl) -amino group or is
optionally replaced by an -NH or -N(C1_3-alkyl) group; a
phenyl group, a phenyl group disubstituted by C1_3-alkyl,
C1_3-alkoxy or nitro groups, wherein the substituents are
CA 02387013 2008-11-21
25771-726
- 2q -
identical or different; or a phenyl group substituted by the
group R6, which is optionally further substituted by a
fluorine, chlorine or bromine atom or by an amino or nitro
group, wherein R6 denotes a fluorine, chlorine or bromine
atom; a C1_3-alkyl, C1_3-alkoxy, nitro, amino or
C5_6-cycloalkyl group; a pyrrolyl, pyrazolyl, imidazolyl,
triazolyl or tetrazolyl group bound via a carbon atom,
wherein the abovementioned heteroaromatic groups in the
carbon skeleton are optionally substituted by a C1_3-alkyl
group or a hydrogen atom bound to a nitrogen atom is
optionally replaced by a C1_3-alkyl or phenyl-C,._3-alkyl
group; a group of formula
O
CH NH
--~'
H O
~
a carboxy, C1_4-alkoxycarbonyl, phenyl-C1_3-alkylamino-
carbonyl or C5_7-cycloalkyl-carbonyl group; a 5 or 6-membered
cycloalkyleneimino group, wherein the methylene group in
position 4 of the cycloalkyleneimino group is optionally
replaced by an oxygen or sulphur atom or by an -NH or
-N (C1_3-alkyl ) group; an unbranched C1_3-alkyl group
terminally substituted by the group R7, wherein R7 denotes a
C5_7-cycloalkyl group; wherein in a 5 or 6-membered
cycloalkyl group a - (CHz)Z group is optionally replaced by a
-CO-NH group, a-(CHz)3 group is optionally replaced by an
-NH-CO-NH- or a-(CH2)4 group is optionally replaced by an
-NH-CO-NH-CO group, wherein in each case a hydrogen atom
bound to a nitrogen atom is optionally replaced by a
C1_3-alkyl group; a phenyl or pyridinyl group or a pyrrolyl,
pyrazolyl, imidazolyl or triazolyl group bound via a carbon
or nitrogen atom, wherein the pyridinyl, pyrrolyl,
CA 02387013 2007-12-05
25771-726
- 2r -
pyrazolyl, imidazolyl and triazolyl groups in the carbon
skeleton are optionally substituted by a C1_3-alkyl group or
a hydrogen atom bound to a nitrogen atom is optionally
replaced by a C1_3-alkyl group; a hydroxy or C1_3-alkoxy
group; an amino, C1_6-alkylamino, di- (C1_6-alkyl) -amino,
phenylamino, N-phenyl-C1_3-alkylamino, phenyl-C1_3-alkylamino
or N- (C1_3-alkyl) -phenyl-C1_3-alkylamino group; a
m-hydroxy-C2_3-alkyl-amino, N- (C1_3-alkyl) -co-hydroxy-
C2_3-alkylamino, di- (c.o-hydroxy-C2_3-alkyl) -amino or
di- ((o- (C1_3-alkoxy) -C2_3-alkyl) -amino group; a
C1_3-alkylcarbonylamino-C2_3-alkyl-amino or
C1_3-alkylcarbonylamino-C2_3-alkyl-N- (C1_3-alkyl) -amino group;
a C1_3-alkylsulphonylamino, N- (C1_3-alkyl) -
C1_3-alkylsulphonylamino, C1_3-alkylsulphonylamino-
C2_3-alkylamino or C1_3-alkylsulphonylamino-C2_3-alkyl-
N-(C1_3-alkyl)-amino group; a hydroxycarbonyl-C1_3-alkylamino
or N- (C1_3-alkyl) -hydroxycarbonyl-C1_3-alkyl-amino group; a
guanidino group wherein a hydrogen atom is optionally
replaced by a C1_3-alkyl group; a group of formula
-N(R8) -CO- (CH2)n-R9 (II) ,
wherein R8 denotes a hydrogen atom or a C1_3-alkyl group, n
denotes 0, 1, 2 or 3 and R9 denotes an amino, C1_3-alkylamino,
di- (C1_3-alkyl) -amino or C1_4-alkoxy group, a 5- or 6-membered
cycloalkyleneimino group, wherein the methylene group in
position 4 of the cycloalkyleneimino group is optionally
replaced by an -NH, -N(Cl_3-alkyl) or -N(C1_3-alkyl-carbonyl)
group, or, if n denotes 1, 2 or 3, R9 is as defined herein or
R9 denotes a hydrogen atom; a group of formula
-N(Rlo) - (CH2)m- (CO)o-Rli (III),
wherein Rlo denotes a hydrogen atom or a C1_3-alkyl group, m
denotes 1, 2 or 3, o denotes 1 or, if m is 2 or 3, o denotes
CA 02387013 2008-11-21
25771-726
- 2s -
0 or 1 and Rll denotes an amino, C1_3-alkylamino, di- (C1_3-
alkyl)-amino, C1_4-alkoxy or methoxy-C1_3-alkoxy group or a 5-
or 6-membered cycloalkyleneimino group, wherein the
methylene group in position 4 of the cycloalkyleneimino
group is optionally replaced by an -NH, -N(C1_3-alkyl) or
-N(C1_3-alkyl-carbonyl) group; or an azetidino, pyrrolidino,
piperidino, 2,6-dimethyl-piperidino, 3,5-dimethyl-piperidino
or azepino group, wherein the methylene group in position 3
of the pyrrolidino group is optionally substituted by a
hydroxy group, the methylene group in position 4 of the
piperidino group is optionally substituted by a hydroxy,
hydroxy-C1_3-alkyl or C1_3-alkoxy group or is optionally
replaced by an oxygen or sulphur atom or by a sulphinyl,
sulphonyl, -NH, -N(C1_3-alkyl) , -N(C1_3-alkyl-carbonyl) ,
-N(benzoyl) or -N(phenyl-C1_3-alkyl-carbonyl) group, wherein
a methylene group linked to an imino-nitrogen atom of the
pyrrolidino, piperidino or azepino group is optionally
replaced by a carbonyl group; or R6 denotes a straight-chain
C1_3-alkyl group which is terminally substituted by a carboxy
or C1_3-alkoxy-carbonyl group; a group of formula
-N(R12) -CO- (CHz)p-R13 (IV) ,
wherein R12 denotes a hydrogen atom, a C1_3-alkyl or phenyl-
C1_3-alkyl group, p denotes 0, 1 or 2 and R13 denotes an
amino, C1_4-alkylamino, di- (C1_4-alkyl) -amino, benzylamino,
N- (C1_3-alkyl) -benzylamino, C1_3-alkoxy-C1_3-alkylamino,
N- (C1_3-alkyl) -C1_3-alkoxy-C1_3-alkylamino,
di-(2-methoxy-ethyl)-amino, di-(w-hydroxy-C2_3-alkyl)-amino
or aminocarbonyl-methyl-N-(methyl)-amino group, a pyrrolyl,
pyrazolyl or imidazolyl group bound via a nitrogen atom and
optionally substituted by a C1_3-alkyl group, or a
pyrrolidino, piperidino, morpholino, thiomorpholino or a
piperazino group optionally substituted in the 4 position by
CA 02387013 2008-11-21
25771-726
- 2t -
a C1_3-alkyl, phenyl-C1_3-alkyl, C1_3-alkylcarbonyl or
C1_4-alkoxycarbonyl group or, if p denotes 1 or 2, R13 is as
defined herein or denotes a hydrogen atom; a group of
formula
-N (R14) - (CH2) q- (CO) r-R15 (V)
wherein R14 denotes a hydrogen atom or a C1_4-alkyl,
C1_3-alkyl-carbonyl, phenylcarbonyl, phenyl-C1_3-
alkylcarbonyl, furyl-carbonyl, pyridinyl-carbonyl, furyl-
C1_3-alkylcarbonyl, pyridinyl-C1_3-alkylcarbonyl,
C1_4-alkylsulphonyl, phenylsulphonyl or phenyl-C1_3-
alkylsulphonyl group, q denotes 1, 2 or 3, r denotes 1 or,
if q is 2 or 3, r denotes 0 or 1 and R15 denotes an amino,
C1_4-alkylamino, di- (C1_4-alkyl) -amino, phenylamino,
N-(C1_4-alkyl)-phenylamino, benzylamino or
N-(C1_4-alkyl)-benzylamino group; or a group of formula
-N(R16) -S02-R17 (VI) ,
wherein R16 denotes a hydrogen atom or a C1_3-alkyl group
optionally terminally substituted by a cyano,
trifluoromethyl-carbonylamino or N-(C1_3-alkyl)-
trifluoromethyl-carbonyl-amino group and R17 denotes a
C1_3-alkyl group, and RS denotes a hydrogen atom, wherein a
hydrogen atom bound to a nitrogen atom in the abovementioned
groups is optionally replaced by an acetyl or
tert.butoxycarbonyl group, and the carboxy groups contained
in the abovementioned groups are optionally replaced with a
tert.butoxycarbonyl group.
According to yet another aspect of the present
invention, there is provided a pharmaceutical composition
comprising a compound or salt as described herein and a
pharmaceutically acceptable carrier or diluent.
CA 02387013 2007-12-05
25771-726
- 2u -
According to a further aspect of the present
invention, there is provided a pharmaceutical composition as
described herein for treating excessive or anomalous cell
proliferation.
According to yet a further aspect of the present
invention, there is provided process for preparing a
pharmaceutical composition as described herein, wherein a
compound or salt as described herein is admixed with a
pharmaceutically acceptable carrier or diluent.
According to still a further aspect of the present
invention, there is provided use of a compound or salt as
described herein in manufacture of a medicament for
treatment of excessive or anomalous cell proliferation.
According to another aspect of the present
invention, there is provided use of a compound or salt as
described herein for treatment of excessive or anomalous
cell proliferation.
According to yet another aspect of the present
invention, there is provided a compound or salt as described
herein for treatment of excessive or anomalous cell
proliferation.
In the above general formula I
X denotes an oxygen or sulphur atom,
R1 denotes a hydrogen atom or a prodrug group such as a C1_4-
alkoxycarbonyl or C2_4-alkanoyl group,
R2 denotes a carboxy group, a straight-chain or branched C1_6-
alkoxy-carbonyl group, a C4_,-cycloalkoxy-carbonyl or an
aryloxycarbonyl group,
CA 02387013 2007-12-05
25771-726
- 2v -
a straight-chain or branched C1_6-alkoxy-carbonyl group,
which is terminally substituted in the alkyl moiety by a
phenyl, heteroaryl, carboxy, C1_3-alkoxy-carbonyl,
aminocarbonyl, C1_3-alkylamino-carbonyl or di- (C1_3-alkyl) -
aminocarbonyl group,
a straight-chain or branched C2_6-alkoxy-carbonyl group,
which is terminally substituted in the alkyl moiety by a
chlorine atom or a hydroxy, C1_3-alkoxy, amino, C1_3-
alkylamino or di-(C1_3-alkyl)-amino group,
CA 02387013 2002-03-19
- 3 -
an aminocarbonyl or methylaminocarbonyl group, an
ethylaminocarbonyl group optionally substituted in the 2
position of the ethyl group by a hydroxy or C1_3-alkoxy group
or, if R4 does not denote an aminosulphonyl-phenyl or
N-(C1_5-alkyl)-C1_3-alkylaminocarbonyl-phenyl group, it may also
denote a di- (Cl_2-alkyl) -aminocarbonyl group,
R3 denotes a hydrogen atom, a C1_6-alkyl, C3_,-cycloalkyl,
trifluoromethyl or heteroaryl group,
a phenyl or naphthyl group, a phenyl or naphthyl group mono-
or disubstituted by a fluorine, chlorine, bromine or iodine
atom, by a trifluoromethyl, Cl_3-alkyl or Cl_3-alkoxy group,
whilst in the event of disubstitution the substituents may be
identical or different and wherein the abovementioned
unsubstituted as well as the mono- and disubstituted phenyl
and naphthyl groups may additionally be substituted
by a hydroxy, hydroxy-Cl_3-alkyl or Cl_3-alkoxy-Cl_3-alkyl
group,
by a cyano, carboxy, carboxy-C1_3-alkyl,
C1_3-alkoxycarbonyl, aminocarbonyl, C1_3-alkylamino-carbonyl
or di-(C1_3-alkyl)-arninocarbonyl group,
by a nitro group,
by an amino, Cl_j-alkylamino, di- (C1_3-alkyl) -amino or
amino-Cl_3-alkyl group,
by a C1_3-alkylcarbonylamino, N- (Cl_3-alkyl) -Cl_3-alkyl-
carbonylamino, C1_3-alkylcarbonylamino-C1_3-alkyl,
N- (Cl_3-alkyl) -C1_3-alkylcarbonylamino-C1_3-alkyl, C1_3-alkyl-
sulphonylamino, C1_3-alkylsulphonylamino-C1_3-alkyl,
N- (Cl_3-alkyl) -Cl_3-alkylsulphonylamino-C,_3-alkyl or
aryl-C1_3-alkylsulphonylamino group,
CA 02387013 2002-03-19
- 4 -
by a cycloalkylamino, cycloalkyleneimino, cyclo-
alkyleneiminocarbonyl, cycloalkyleneimino-C1_3-alkyl,
cycloalkyleneiminocarbonyl-C1_3-alkyl or
cycloalkyleneiminosulphonyl-C1_3-alkyl group having 4 to 7
ring members in each case, whilst in each case the
methylene group in position 4 of a 6- or 7-membered
cycloalkyleneimino group may be replaced by an oxygen or
sulphur atom, by a sulphinyl, sulphonyl, -NH or
-N(C1_3-alkyl) group,
or by a heteroaryl or heteroaryl-C1_,-alkyl group,
R4 denotes a C3_,-cycloalkyl group,
whilst the methylene group in the 4 position of a 6- or 7-
membered cycloalkyl group may be substituted by an amino,
Cl_3-alkylamino or di- (Cl_3-alkyl) -amino group or replaced
by an -NH or -N(C1_3-alkyl) group,
or a phenyl group substituted by the group R6, which may
additionally be mono- or disubstituted by fluorine, chlorine,
bromine or iodine atoms, by C1_5-alkyl, trifluoromethyl,
hydroxy, C1_3-alkoxy, carboxy, C1_3-alkoxycarbonyl, amino,
acetylamino, C1_3-alkyl-sulphonylamino, aminocarbonyl,
Cl_3-alkyl-aminocarbonyl, di- (Cl_3-alkyl) -aminocarbonyl,
aminosulphonyl, C1_3-alkyl-aminosulphonyl,
di-(C1_3-alkyl)-aminosulphonyl, nitro or cyano groups, wherein
the substituents may be identical or different and wherein
R6 denotes a hydrogen, fluorine, chlorine, bromine or iodine
atom,
a cyano, nitro, amino, Cl_5-alkyl, C3_,-cycloalkyl,
trifluoromethyl, phenyl, tetrazolyl or heteroaryl group,
CA 02387013 2002-03-19
- 5 -
the group of formula
O
7NH
CH
N O
H
wherein the hydrogen atoms bound to a nitrogen atom may in
each case be replaced independently of one another by a
Cl_3-alkyl group,
a C1_3-alkoxy group, a C1_3-alkoxy-C1_3-alkoxy, phenyl-C1_3-alkoxy,
amino-C2_3-alkoxy, Cl_3-alkylamino-C2_3-alkoxy,
di- (Cl_3-alkyl) -amino-C2_3-alkoxy, phenyl-Cl_3-alkylamino-
CZ_3-alkoxy, N- (C1_3-alkyl) -phenyl-C1_3-alkylamino-C2_3-alkoxy,
CS_,-cycloalkyleneimino-C2_3-alkoxy or Cl_3-alkylmercapto group,
a carboxy, Cl_4-alkoxycarbonyl, aminocarbonyl, C1_3-alkyl-
amino-carbonyl, N- (Cl_5-alkyl) -Cl_3-alkylaminocarbonyl,
phenyl-C1_3-alkylamino-carbonyl,
N- (C1_3-alkyl) -phenyl-Cl_3-alkylamino-carbonyl,
piperazinocarbonyl or N-(C,_3-alkyl)-piperazinocarbonyl group,
a C1_3-alkylaminocarbonyl or N- (C1_5-alkyl) -
C1_3-alkylaminocarbonyl group wherein an alkyl moiety is
substituted by a carboxy or C1_3-alkoxycarbonyl group or in the
2 or 3 position by a di-(C1_3-alkyl)-amino, piperazino,
N- (Cl_3-alkyl) -piperazino or a 4- to 7-membered
cycloalkyleneimino group,
a C3_,-cycloalkyl-carbonyl group,
wherein the methylene group in the 4 position of the 6- or
7-membered cycloalkyl moiety may be substituted by an
amino, C1_3-alkylamino or di- (C1_3-alkyl) -amino group or
replaced by an -NH or -N(Cl_3-alkyl) group,
CA 02387013 2002-03-19
- 6 -
a 4- to 7-membered cycloalkyleneimino group wherein
a methylene group linked to the imino group may be
replaced by a carbonyl or sulphonyl group or
the cycloalkylene moiety may be fused to a phenyl ring or
one or two hydrogen atoms may each be replaced by a C1_3-
alkyl group and/or
in each case the methylene group in the 4 position of a 6-
or 7-membered cycloalkyleneimino group may be substituted
by a carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
C1_3-alkylaminocarbonyl, di-(C1_3-alkyl)-aminocarbonyl,
phenyl-Cl_3-alkylamino or N- (C1_3-alkyl) -
phenyl-C1_3-alkylamino group or
may be replaced by an oxygen or sulphur atom, by a
sulphinyl, sulphonyl, -NH, -N(C1_3-alkyl) , -N(phenyl) ,
-N(C1_3-alkyl-carbonyl) or -N(benzoyl) group,
a C1_4-alkyl group substituted by the group Rõ wherein
R7 denotes a C3_,-cycloalkyl group,
whilst the methylene group in the 4 position of a 6- or
7-membered cycloalkyl group may be substituted by an
amino, Cl_3-alkylamino or di- (Cl_3-alkyl) -amino group or
replaced by an -NH or -N(C1_3-alkyl) group or
in a 5- to 7-membered cycloalkyl group a-(CH2)2 group
may be replaced by a -CO-NH group, a-(CH2)3 group may
be replaced by a -NH-CO-NH or -CO-NH-CO group or a
-(CH2)4 group may be replaced by a -NH-CO-NH-CO group,
whilst in each case a hydrogen atom bound to a nitrogen
atom may be replaced by a C1_3-alkyl group,
CA 02387013 2002-03-19
- 7 -
an aryl or heteroaryl group,
a hydroxy or Cl_3-alkoxy group,
an amino, Cl_,-alkylamino, di- (Cl_,-alkyl) -amino,
phenylamino, N-phenyl-C1_3-alkyl-amino, phenyl-C1_3-alkyl-
amino, N- (C1_3-alkyl) -phenyl-C1_3-alkylamino or di-
(phenyl-Cl_3-alkyl) -amino group,
an w-hydroxy-C2_3-alkyl-amino, N- (Cl_3-alkyl) -w-hydroxy-
C2_3-alkyl-amino, di- (co-hydroxy-C2_3-alkyl) -amino,
di- ((o- (C1_3-alkoxy) -C2_3-alkyl) -amino or N- (dioxolan-
2-yl) -Cl_3-alkyl-amino group,
a Cl_3-alkylcarbonylamino-C2_3-alkyl-amino or
Cl_3-alkylcarbonylamino-C2_3-alkyl-N- (Cl_3-alkyl) -amino group,
a C1_3-alkylsulphonylamino, N- (Cl_3-alkyl) -C1_3-alkyl-
sulphonylamino, C1_3-alkylsulphonylamino-C2_3-alkyl-amino or
Cl_3-alkylsulphonylamino-CZ_3-alkyl-N- (Cl_3-alkyl) -amino
group,
a hydroxycarbonyl-Cl_3-alkylamino or N- (Cl_3-alkyl) -
hydroxycarbonyl-C1_3-alkyl-amino group,
a guanidino group wherein one or two hydrogen atoms may
each be replaced by a C1_3-alkyl group,
a group of formula
-N(R8)-CO-(CH2)n-Rg (II),
wherein
CA 02387013 2002-03-19
- 8 -
R. denotes a hydrogen atom or a C1_3-alkyl group,
n denates one of the numbers 0, 1, 2 or 3 and
R9 denotes an amino, Cl_4-alkylamino,
di- (Cl_4-alkyl) -amino, phenylamino,
N-(C1_4-alkyl)-phenylamino, benzylamino,
N- (Cl_4-alkyl) -benzylamino or Cl_4-alkoxy group, a 4- to
7-membered cycloalkyleneimino group, whilst in each
case the methylene group in the 4 position of a 6- or
7-membered cycloalkyleneimino group may be replaced by
an oxygen or sulphur atom, by a sulphinyl, sulphonyl,
-NH, -N(Cl_3-alkyl) , -N(phenyl), -N(C1_3-alkyl-carbonyl)
or -N(benzoyl) group, or, if n denotes one of the
numbers 1, 2 or 3, it may also denote a hydrogen atom,
a group of formula
-N(R10) - (CH2)m- (CO)o-R11 (III),
wherein
Rla denotes a hydrogen atom, a Cl_3-alkyl group, a
C1_3-alkylcarbonyl, arylcarbonyl, phenyl-C1_3-alkyl-
carbonyl, C1_3-alkylsulphonyl, arylsulphonyl or
phenyl-Cl_3-alkylsulphonyl group,
m denotes one of the numbers 1, 2, 3 or 4,
o denotes the number 1 or, if m denotes one of the
numbers 2, 3 or 4, o may also denote the number 0 and
Rll denotes an amino, Cl_,,-alkylamino,
di- (Cl_4-alkyl) -amino, phenylamino,
N-(C1_4-alkyl)-phenylamino, benzylamino,
N- (Cl_4-alkyl) -benzylamino, C1_4-alkoxy or
CA 02387013 2002-03-19
- 9 -
Cl_3-alkoxy-Cl_,-alkoxy group, a di- (Cl_4-alkyl) -amino-Cl_3-
alkylamino group optionally substituted in the 1
position by a C1_3-alkyl group or a 4- to 7-membered
cycloalkyleneimino group, wherein the cycloalkylene
moiety may be fused to a phenyl ring or in each case
the methylene group in the 4 position of a 6- or 7-
membered cycloalkyleneimino group may be replaced by an
oxygen or sulphur atom, by a sulphinyl, sulphonyl, -NH,
-N(Cl_3-alkyl) , -N(phenyl), -N(Cl_3-alkyl-carbonyl) or
-N(benzoyl) group,
a C4_,-cycloalkylamino, C4_,-cycloalkyl-C1_3-alkylamino or
C4_,-cycloalkenylamino group wherein position 1 of the ring
is not involved in the double bond and wherein the
abovementioned groups may each additionally be substituted
at the amino-nitrogen atom by a CS_,-cycloalkyl,
C2_4-alkenyl or Cl_4-alkyl group,
a 4- to 7-membered cycloalkyleneimino group, wherein
the cycloalkylene moiety may be fused to a phenyl group
or to an oxazolo, imidazolo, thiazolo, pyridino,
pyrazino or pyrimidino group optionally substituted by
a fluorine, chlorine, bromine or iodine atom, by a
nitro, Cl_3-alkyl, C1_3-alkoxy or amino group, and/or
one or two hydrogen atoms may each be replaced by a
Cl_3-alkyl, C5_,-cycloalkyl or phenyl group and/or
the methylene group in the 3 position of a 5-membered
cycloalkyleneimino group may be substituted by a
hydroxy, hydroxy-Cl_3-alkyl, Cl_3-alkoxy or
C,._3-alkoxy-C1_3-alkyl group,
the methylene group in the 3 or 4 position of a 6- or
7-membered cycloalkyleneimino group may in each case be
CA 02387013 2002-03-19
- 10 -
substituted by a hydroxy, hydroxy-C1_3-alkyl,
C1_3-alkoxy, Cl_3-alkoxy-Cl_3-alkyl, carboxy,
C1_4-alkoxycarbonyl, aminocarbonyl,
C1_3-alkylaminocarbonyl, di-(C1_3-alkyl)-aminocarbonyl,
phenyl-C1_3-alkylamino or N- (C1_3-alkyl) -phenyl-Cl_3-alkyl-
amino group or
may be replaced by an oxygen or sulphur atom, by a
sulphinyl, sulphonyl, -NH, -N(C1_3-alkyl-), -N(phenyl),
-N(phenyl-Cl_3-alkyl-) , -N(Cl_3-alkyl-carbonyl-) ,
-N(Cl_4-hydroxy-carbonyl-) , -N(Cl_4-alkoxy-carbonyl-) ,
-N(benzoyl-) or -N(phenyl-Cl_3-alkyl-carbonyl-) group,
wherein a methylene group linked to an imino-
nitrogen atom of the cycloalkyleneimino group may be
replaced by a carbonyl or sulphonyl group or in a 5-
to 7-membered monocyclic cycloalkyleneimino group or
a cycloalkyleneimino group fused to a phenyl group
the two methylene groups linked to the imino-
nitrogen atom may each be replaced by a carbonyl
group,
or R. denotes a Cl_4-alkyl group which is substituted by a
carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
C1_3-alkylaminocarbonyl or di- (Cl_3-alkyl) -aminocarbonyl group
or by a 4- to 7-membered cycloalkyleneiminocarbonyl group,
an N- (C1_3-alkyl) -CZ_4-alkanoylamino group which is additionally
substituted in the alkyl moiety by a carboxy or
C1_3-alkoxycarbonyl group,
a group of formula
-N(R12)-CO-(CH2)p-R13 (IV),
CA 02387013 2002-03-19
- 11 -
wherein
R12 denotes a hydrogen atom, a C1_6-alkyl or C3_,-cycloalkyl
group or a C1_3-alkyl group terminally substituted by a
phenyl, heteroaryl, trifluoromethyl, hydroxy, C1_3-alkoxy,
aminocarbonyl, C1_4-alkylamino-carbonyl, di-(C1_4-alkyl)-
amino-carbonyl, C1_3-alkyl-carbonyl, C1_3-alkyl-sulphonyl-
amino, N- (Cl_3-alkyl) -C1_3-alkyl-sulphonylamino,
C1_3-alkyl-aminosulphonyl or di-(C1_3-alkyl)-aminosulphonyl
group and
p denotes one of the numbers 0, 1, 2 or 3 and
R13 assumes the meanings of the abovementioned group R7,
or, if p denotes one of the numbers 1, 2 or 3, it may also
denote a hydrogen atom,
a group of formula
-N(R14)-(CH2)q-(CO)r-R15 (V),
wherein
R14 denotes a hydrogen atom, a C1_4-alkyl group, a
C1_3-alkylcarbonyl, arylcarbonyl, phenyl-C1_3-alkylcarbonyl,
heteroarylcarbonyl, heteroaryl-C1_3-alkylcarbonyl,
C1_4-alkylsulphonyl, arylsulphonyl,
phenyl-C1_3-alkylsulphonyl, heteroarylsulphonyl or
heteroaryl-C1_3-alkyl-sulphonyl group,
q denotes one of the numbers 1, 2, 3 or 4,
r denotes the number 1 or, if q is one of the numbers 2, 3
or 4, it may also denote the number 0 and
R15 assumes the meanings of the abovementioned group R7,
CA 02387013 2002-03-19
- 12 -
a group of formula
-N(R16)-SO2-Rl7 (VI),
wherein
R16 denotes a hydrogen atom or a C1_4-alkyl group optionally
terminally substituted by a cyano,
trifluoromethyl-carbonylamino or
N-(C1_3-alkyl)-trifluoromethyl-carbonyl-amino group and
R17 denotes a Cl_3-alkyl group,
an amino group substituted by a di-(C1_3-alkyl)-
amino-C1_3-alkyl-carbonyl or di- (C1_3-alkyl) -
amino-Cl_3-alkyl-sulphonyl group and a di- (Cl_3-alkyl) -
aminocarbonyl-C1_3-alkyl group,
or an N- (C1_3-alkyl) -Cl_5-alkylsulphonylamino or
N-(C1_3-alkyl)-phenylsulphonylamino group wherein the alkyl
moiety is additionally substituted by a cyano or carboxy
group,
wherein all the single-bonded or fused phenyl groups
contained in the groups mentioned under R. may be mono- or
disubstituted by fluorine, chlorine, bromine or iodine
atoms, by Cl_5-alkyl, trifluoromethyl, hydroxy, C1_3-alkoxy,
carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
C1_4-alkylamino-carbonyl, di-(C1_4-alkyl)-amino-carbonyl,
aminosulphonyl, C1_3-alkyl-aminosulphonyl,
di-(C1_3-alkyl)-aminosulphonyl, C1_3-alkyl-sulphonylamino,
nitro or cyano groups, wherein the substituents may be
identical or different, or two adjacent hydrogen atoms of
the phenyl groups may be replaced by a methylenedioxy
group,
CA 02387013 2002-03-19
- 13 -
and
R5 denotes a hydrogen atom or a C1_3-alkyl group,
wherein by an aryl group is meant a phenyl or naphthyl group
optionally mono- or disubstituted by a fluorine, chlorine,
bromine or iodine atom, by a cyano, trifluoromethyl, nitro,
carboxy, aminocarbonyl, Cl_3-alkyl or Cl_3-alkoxy group and
by a heteroaryl group is meant a monocyclic 5- or 6-membered
heteroaryl group optionally substituted by a C1_3-alkyl group
in the carbon skeleton, wherein
the 6-membered heteroaryl group contains one, two or three
nitrogen atoms and
the 5-membered heteroaryl group contains an imino group
optionally substituted by a C1_3-alkyl or phenyl-Cl_3-alkyl
group, an oxygen or sulphur atom or
an imino group optionally substituted by a C1_3-alkyl or
phenyl-C1_3-alkyl group or an oxygen or sulphur atom and
additionally a nitrogen atom or
an imino group optionally substituted by a C1_3-alkyl or
phenyl-C1_3-alkyl group and two nitrogen atoms,
and moreover a phenyl ring may be fused to the
abovementioned monocyclic heterocyclic groups via two
adjacent carbon atoms and the bonding takes place via a
nitrogen atom or via a carbon atom of the heterocyclic
moiety or a fused phenyl ring,
some or all of the hydrogen atoms in the abovementioned alkyl
and alkoxy groups or in the alkyl moieties contained in the
CA 02387013 2002-03-19
- 14 -
above-defined groups of formula I optionally being replaced by
fluorine atoms,
the saturated alkyl and alkoxy moieties with more than 2
carbon atoms which are present in the groups defined
hereinbefore also include the branched isomers thereof, such
as for example the isopropyl, tert.butyl, isobutyl group,
unless otherwise stated, and
additionally the hydrogen atom of any carboxy group present or
a hydrogen atom bound to a nitrogen atom, e.g. a hydrogen atom
of an amino, alkylamino or imino group or a saturated N-
heterocycle such as the piperidinyl group, may each be
replaced by a group which can be cleaved in vivo.
By a group which can be cleaved in vivo from an imino or amino
group is meant, for example, a hydroxy group, an acyl group
such as the benzoyl or pyridinoyl group or a C1_16-alkanoyl
group such as the formyl, acetyl, propionyl, butanoyl,
pentanoyl or hexanoyl group, an allyloxycarbonyl group, a
C1_16-alkoxycarbonyl group such as the methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, isopropoxycarbonyl,
butoxycarbonyl, tert.butoxycarbonyl, pentoxycarbonyl,
hexyloxycarbonyl, octyloxycarbonyl, nonyloxycarbonyl,
decyloxycarbonyl, undecyloxycarbonyl, dodecyloxycarbonyl or
hexadecyloxycarbonyl group, a phenyl-C1_6-alkoxycarbonyl group
such as the benzyloxycarbonyl, phenylethoxycarbonyl or
phenylpropoxycarbonyl group, a C1_3-alkylsulphonyl-
C2_4-alkoxycarbonyl, C1_3-alkoxy-C2_4-alkoxy-C2_4-alkoxycarbonyl or
ReCO-O- (RfCRg) -0-CO group wherein
R. denotes a C1_e-alkyl, CS_,-cycloalkyl, phenyl or phenyl-
C1_3-alkyl group,
CA 02387013 2002-03-19
- 15 -
Rf denotes a hydrogen atom, a Cl_3-alkyl, CS_,-cycloalkyl or
phenyl group and
R. denotes a hydrogen atom, a Cl_3-alkyl or ReCO-O- (RfCRg) -O
group wherein R. to R. are as hereinbefore defined,
wherein additionally the amino group may be a phthalimido
group, whilst the abovementioned ester groups may also be used
as a group which can be converted in vivo into a carboxy
group.
One sub-group of compounds of general formula I which deserves
special mention comprises those wherein
X, R. and R3 to RS are as hereinbefore defined and
R. denotes a straight-chain or branched C1_6-alkoxy-carbonyl
group, a C4_7-cycloalkoxycarbonyl or a aryloxycarbonyl group,
a straight-chain or branched C1_6-alkoxy-carbonyl group, which
is terminally substituted in the alkyl moiety by a phenyl,
heteroaryl, carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
Cl_3-alkylaminocarbonyl or di- (Cl_3-alkyl) -aminocarbonyl group,
a straight-chain or branched CZ_6-alkoxy-carbonyl group, which
is terminally substituted in the alkyl moiety by a chlorine
atom or a hydroxy, C1_3-alkoxy, amino, Cl_3-alkylamino or
di- (Cl_3-alkyl) -amino group,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
A second sub-group of compounds of general formula I which
deserves special mention comprises those wherein
CA 02387013 2002-03-19
- 16 -
X, R. and R3 to RS are as hereinbefore defined and
R2 denotes an aminocarbonyl or methylaminocarbonyl group, an
ethylaminocarbonyl group optionally substituted in the 2
position of the ethyl group by a hydroxy or C1_3-alkoxy group
or, if R4 does not denote an aminosulphonyl-phenyl or
N- (C1_5-alkyl) -Cl_3-alkylaminocarbonyl-phenyl group, R2 may also
denote a di-(C1_2-alkyl)-aminocarbonyl group,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
A third sub-group of compounds of general formula I which
deserves special mention comprises those wherein
X, R. to R3 and RS are as hereinbefore defined and
R4 denotes an R,- (Cl_,4-alkyl) -phenyl group, wherein
R, denotes an amino, C1_7-alkylamino, di- (Cl_7-alkyl) -amino,
phenylamino, N-phenyl-C1_3-alkyl-amino, phenyl-C1_3-alkyl-
amino, N- (Cl_3-alkyl) -phenyl-Cl_3-alkylamino or
di- (phenyl-Cl_3-alkyl) -amino group,
or a phenyl group substituted by the group of formula
-N(R12)-CO-(CH2)p-R13 (IV),
wherein R12, p and R13 are as hereinbefore defined,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
Preferred compounds of general formula I are those wherein
CA 02387013 2002-03-19
- 17 -
R1 and R3 are as hereinbefore defined and
X denotes an oxygen atom,
R2 denotes a carboxy group, a straight-chain or branched
C1_6-alkoxy-carbonyl group, a CS_,-cycloalkoxycarbonyl or a
phenoxycarbonyl group,
a straight-chain or branched C1_3-alkoxy-carbonyl group, which
is terminally substituted in the alkyl moiety by a phenyl,
heteroaryl, carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
Cl_3-alkylaminocarbonyl or di- (Cl_3-alkyl) -aminocarbonyl group,
a straight-chain or branched CZ_3-alkoxy-carbonyl group, which
is terminally substituted in the alkyl moiety by a chlorine
atom, by a hydroxy, C1_3-alkoxy, amino, C1_3-alkylamino or
di- (C1_3-alkyl) -amino group,
an aminocarbonyl or methylaminocarbonyl group, an
ethylaminocarbonyl group optionally substituted in the 2
position of the ethyl group by a hydroxy or C1_3-alkoxy group
or, if R4 does not denote an aminosulphonyl-phenyl or
N- (Cl_5-alkyl) -C1_3-alkylaminocarbonyl-phenyl group, it may also
denote a di- (C1_2-alkyl) -aminocarbonyl group,
R4 denotes a C3_7-cycloalkyl group,
whilst the methylene group in the 4 position of a 6- or 7-
membered cycloalkyl group may be substituted by an amino,
C1_3-alkylamino or di- (Cl_3-alkyl) -amino group or replaced
by an -NH or -N(Cl_3-alkyl) group,
or a phenyl group substituted by the group R6, which may
additionally be mono- or disubstituted by fluorine, chlorine
or bromine atoms, by C1_3-alkyl, trifluoromethyl, hydroxy,
CA 02387013 2002-03-19
- 18 -
C1_3-alkoxy, carboxy, C1_3-alkoxycarbonyl, amino, acetylamino,
aminocarbonyl, C1_3-alkyl-aminocarbonyl,
di-(C1_3-alkyl)-aminocarbonyl, nitro or cyano groups, wherein
the substituents may be identical or different and wherein
R. denotes a hydrogen, fluorine, chlorine, bromine or iodine
atom,
a cyano, nitro, amino, C,_5-alkyl, C3_7-cycloalkyl,
trifluoromethyl, phenyl, tetrazolyl or heteroaryl group,
the group of formula
O
CH NH
--t~~O
H
wherein a hydrogen atom bound to the'nitrogen atom may be
replaced by a C1_3-alkyl group,
a Cl_3-alkoxy group, an amino-C2_3-alkoxy, Cl_3-alkylamino-
C2_3-alkoxy, di- (Cl_3-alkyl) -amino-C2_3-alkoxy, phenyl-
C1_3-alkylamino-C2_3-alkoxy, N- (Cl_3-alkyl) -phenyl-
Cl_3-alkylamino-C2_3-alkoxy, pyrrolidino-C2_3-alkoxy,
piperidino-C2_3-alkoxy or C1_3-alkylmercapto group,
a carboxy, C1_4-alkoxycarbonyl, aminocarbonyl, Cl_3-alkyl-
amino-carbonyl, phenyl-C1_3-alkylamino-carbonyl or
N- (C1_3-alkyl) -phenyl-C1_3-alkylamino-carbonyl group,
a C3_7-cycloalkyl-carbonyl group,
wherein the methylene group in the 4 position of the 6- or
7-membered cycloalkyl moiety may be replaced by an -NH or
-N ( C1_3-alkyl ) group,
CA 02387013 2002-03-19
- 19 -
a 4- to 7-membered cycloalkyleneimino group, wherein
a methylene group linked to the imino group may be
replaced by a carbonyl or sulphonyl group or
one or two hydrogen atoms may each be replaced by a
C1_3-alkyl group and/or
in each case the methylene group in the 4 position of a 6-
or 7-membered cycloalkyleneimino group may be substituted
by a carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
C1_3-alkylaminocarbonyl, di-(C1_3-alkyl)-aminocarbonyl,
phenyl-Cl_3-alkylamino or N- (C1_3-alkyl) -
phenyl-C1_3-alkylamino group or
may be replaced by an oxygen or sulphur atom, by a
sulphinyl, sulphonyl, -NH or -N(Cl_3-alkyl) group,
a C,._4-alkyl group terminally substituted by the group Rõ
wherein
R7 denotes a C5_,-cycloalkyl group,
whilst the methylene group in the 4 position of a 6- or
7-membered cycloalkyl group may be replaced by an -NH
or -N(C1_3-alkyl) group or
in a 5- to 7-membered cycloalkyl group a-(CHZ)2 group
may be replaced by a -CO-NH group, a-(CH2)3 group may
be replaced by a -NH-CO-NH- or a-(CH2)4 group may be
replaced by a -NH-CO-NH-CO group, whilst in each case a
hydrogen atom bound to a nitrogen atom may be replaced
by a Cl_3-alkyl group,
a phenyl or heteroaryl group,
CA 02387013 2002-03-19
- 20 -
a hydroxy or C1_3-alkoxy group,
an amino, Cl_6-alkylamino, di- (Cl_6-alkyl) -amino,
phenylamino, N-phenyl-C1_3-alkyl-amino,
phenyl-Cl_,-alkylamino, N- (Cl_3-alkyl) -phenyl-Cl_3-alkylamino
or di- (phenyl-Cl_3-alkyl) -amino group,
a w-hydroxy-CZ_3-alkyl-amino, N- (Cl_3-alkyl) -W-hydroxy-
-C2_3-alkyl-amino, di- ((o-hydroxy-C2_3-alkyl) -amino,
di- (w- (Cl_3-alkoxy) -C2_3-alkyl) -amino or
N-(dioxolan-2-yl)-C1_3-alkyl-amino group,
a C1_3-alkylcarbonylamino-C2_3-alkyl-amino or
C1_3-alkylcarbonylamino-C2_3-alkyl-N- (Cl_3-alkyl) -amino group,
a Cl_3-alkylsulphonylamino, N- (Cl_3-alkyl) -
C1_3-alkylsulphonylamino, C1_3=alkylsulphonylamino-
-C2_3-alkyl-amino or C1_3-alkylsulphonylamino-Cz_3-alkyl-
-N- (Cl_3-alkyl) -amino group,
a hydroxycarbonyl-C1_3-alkylamino or
N- (C1_3-alkyl) -hydroxycarbonyl-Cl_3-alkyl-amino group
a guanidino group wherein a hydrogen atom may be replaced
by a Cl_3-alkyl group,
a group of formula
-N (Rg ) -CO- (CH2 ) n-Rg ( I I ) ,
wherein
R8 denotes a hydrogen atom or a C1_3-alkyl group,
n denotes one of the numbers 0, 1, 2 or 3 and
CA 02387013 2002-03-19
- 21 -
R9 denotes an amino, Cl_3-alkylamino, di- (C1_3-alkyl) -
amino, phenylamino, benzylamino or C1_4-alkoxy group, a
5- to 7-membered cycloalkyleneimino group, wherein the
methylene group in position 4 of the piperidino group
may be replaced by an oxygen or sulphur atom, by an
-NH, -N (Cl_3-alkyl) , -N (phenyl) , -N (Cl_3-alkyl-carbonyl)
or -N(benzoyl) group, or, if n denotes one of the
numbers 1, 2 or 3, it may also denote a hydrogen atom,
a group of formula
-N(R10)-(CH2)m-(CO)o-R11 (III),
wherein
Rlo denotes a hydrogen atom, a Cl_3-alkyl group, a
Cl_3-alkylcarbonyl or Cl_3-alkylsulphonyl group,
m denotes one of the numbers 1, 2 or 3,
o denotes the number 1 or, if m is one of the numbers 2
or 3, o may also denote the number 0 and
Rll denotes an amino, C1_3-alkylamino,
di- (C1_,-alkyl) -amino, C1_4-alkoxy or
C1_3-alkoxy-Cl_3-alkoxy group or a 5- to 7-membered
cycloalkyleneimino group, wherein the methylene group
in position 4 of the piperidino group may be replaced
by an oxygen or sulphur atom, by an -NH, -N(C1_3-alkyl),
-N(phenyl), -N(C1_3-alkyl-carbonyl) or -N(benzoyl)
group,
a C,4_7-cycloalkylamino or C4_,-cycloalkenylamino group
wherein position 1 of the ring is not involved in the
double bond,
CA 02387013 2002-03-19
- 22 -
a 4- to 7-membered cycloalkyleneimino group, wherein
the cycloalkylene moiety may be fused to a phenyl group
or
one or two hydrogen atoms may each be replaced by a
C1_3-alkyl group and/or
the methylene group in position 3 of the pyrrolidino
group may be substituted by a hydroxy or C1_3-alkoxy
group,
in each case the methylene group in the 4 position of a
6- or 7-membered cycloalkyleneimino group may be
substituted by a hydroxy, hydroxy-C1_3-alkyl,
C1_3-alkoxy, carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
Cl_3-alkylaminocarbonyl, di- (Cl_3-alkyl) -aminocarbonyl,
phenyl-Cl_3-alkylamino or N- (C,_3-alkyl) -phenyl-Cl_3-alkyl-
amino group or
may be replaced by an oxygen or sulphur atom, by a
sulphinyl, sulphonyl, -NH, -N(C1_3-alkyl), -N(phenyl),
-N(phenyl-C1_3-alkyl) , -N(Cl_3-alkyl-carbonyl) ,
-N(Cl_4-alkoxy-carbonyl) , -N(benzoyl) or
-N(phenyl-C1_3-alkyl-carbonyl) group,
wherein a methylene group linked to an imino-
nitrogen atom of the cycloalkyleneimino group may be
replaced by a carbonyl or sulphonyl group or in a 5-
to 6-membered monocyclic cycloalkyleneimino group or
a cycloalkyleneimino group fused to a phenyl group
the two methylene groups linked to the imino-
nitrogen atom may each be replaced by a carbonyl
group,
CA 02387013 2002-03-19
- 23 -
or R6 denotes a C1_4-alkyl group which is terminally
substituted by a carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
Cl_3-alkylaminocarbonyl or di- (Cl_3-alkyl) -aminocarbonyl group
or by a 4- to 7-membered cycloalkyleneiminocarbonyl group,
a group of formula
-N(R12)-CO-(CH2)p-R13 (IV),
wherein
R12 denotes a hydrogen atom, a C1_3-alkyl, CS_,-cycloalkyl,
phenyl-Cl_3-alkyl or heteroaryl-Cl_3-alkyl group and
p denotes one of the numbers 0, 1, 2 or 3 and
R13 assumes the meanings of the abovementioned group R,,
or, if p denotes one of the numbers 1, 2 or 3, it may also
denote a hydrogen atom,
a group of formula
-N(R14)-(CH2)q-(CO)r-R15 (V),
wherein
R14 denotes a hydrogen atom, a Cl_q-alkyl group, a
C1_3-alkylcarbonyl, phenylcarbonyl, phenyl-C1_3-alkyl-
carbonyl, heteroarylcarbonyl,
heteroaryl-C1_3-alkylcarbonyl, C1_4-alkylsulphonyl,
phenylsulphonyl, phenyl-C1_3-alkylsulphonyl-
heteroarylsulphonyl or heteroaryl-C1_3-alkyl-sulphonyl
group,
q denotes one of the numbers 1, 2, 3 or 4,
CA 02387013 2002-03-19
- 24 -
r denotes the number 1 or, if q is one of the numbers 2, 3
or 4, it may also denote the number 0 and
R15 assumes the meanings of the abovementioned group Rõ
a group of formula
-N(R16)-S02-R17 (VI),
wherein
R16 denotes a hydrogen atom or a C1_4-alkyl group optionally
terminally substituted by a cyano, trifluoromethyl-
carbonylamino or N- (C1_3-alkyl) -trifluoromethyl-
-carbonyl-amino group and
Rl, denotes a Cl_3-alkyl group,
an amino group substituted by a di- (Cl_3-alkyl) -
amino-C1_3-alkyl-carbonyl or di- (C1_3-alkyl) -
amino-Cl_3-alkyl-sulphonyl group and a di- (C1_3-alkyl) -
aminocarbonyl-C1_3-alkyl group,
wherein all the single-bonded or fused phenyl groups
contained in the groups mentioned under R6 may be mono-
or disubstituted by fluorine, chlorine or bromine
atoms, by C1_3-alkyl, trifluoromethyl, hydroxy,
C1_3-alkoxy, carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
C1_3-alkyl-aminocarbonyl, aminosulphonyl,
C1_3-alkyl-aminosulphonyl, nitro or cyano groups,
wherein the substituents may be identical or different,
or two adjacent hydrogen atoms of the phenyl groups may
be replaced by a methylenedioxy group, and
R. denotes a hydrogen atom or a C1_3-alkyl group,
CA 02387013 2002-03-19
- 25 -
whilst by a heteroaryl group as mentioned above is meant a
pyridinyl, pyrazinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
furyl, thienyl, oxazolyl, thiazolyl, pyrazolyl, imidazolyl or
triazolyl group optionally substituted in the carbon skeleton
by a C1_3-alkyl group wherein a hydrogen atom bound to a
nitrogen atom may be replaced by a C1_3-alkyl or
phenyl-C1_3-alkyl group and wherein the 5-membered heteroaryl
groups containing at least one imino group are bound via a
carbon or nitrogen atom,
a hydrogen atom bound to a nitrogen atom in the abovementioned
groups may be replaced by a group which can be cleaved in
vivo, particularly by an acetyl or tert.butoxycarbonyl group,
the carboxy groups contained in the abovementioned groups may
each be substituted by a group which can be cleaved in vivo
and may occur, for example, in the form of the
tert.butoxycarbonyl group,
some or all of the hydrogen atoms in the abovementioned alkyl
and alkoxy groups or in the alkyl moieties contained in the
above-defined groups of formula I optionally being replaced by
fluorine atoms and
the saturated alkyl and alkoxy moieties contained in the
abovementioned groups, which contain more than 2 carbon atoms,
may be straight-chain or branched, unless otherwise stated,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
One subgroup of preferred compounds of general formula I
deserving special mention comprises those wherein
X, R. and R3 to R. are as hereinbefore defined and
CA 02387013 2002-03-19
- 26 -
R2 denotes a straight-chain or branched Cl_6-alkoxy-carbonyl
group, a C5_7-cycloalkoxycarbonyl or a phenoxycarbonyl group,
a straight-chain or branched C1_3-alkoxy-carbonyl group, which
is terminally substituted in the alkyl moiety by a phenyl-
carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
C1_3-alkylaminocarbonyl or di-(C1_3-alkyl)-aminocarbonyl group,
a straight-chain or branched CZ_3-alkoxy-carbonyl group, which
is terminally substituted in the alkyl moiety by a hydroxy,
Cl_3-alkoxy, amino, Cl_3-alkylamino or di- (Cl_3-alkyl) -amino
group,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
A second sub-group of preferred compounds of general formula I
deserving special mention comprises those wherein
X, Rl and R3 to R. are as hereinbefore defined and
R2 denotes an aminocarbonyl or methylaminocarbonyl group, an
ethylaminocarbonyl group optionally substituted in the 2
position of the ethyl group by a hydroxy or C1_3-alkoxy group
or, if R4 does not denote an aminosulphonyl-phenyl or
N- (Cl_5-alkyl) -Cl_3-alkylaminocarbonyl-phenyl group, R2 may also
denote a di- (Cl_2-alkyl) -aminocarbonyl group,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
A third sub-group of preferred compounds of general formula I
deserving special mention comprises those wherein
X, Rl to R3 and RS are as hereinbefore defined and
CA 02387013 2002-03-19
- 27 -
R4 denotes an R,- (n-Cl_4-alkyl) -phenyl group, wherein
R7 denotes an amino, Cl_6-alkylamino, di- (C1_6-alkyl) -amino,
phenylamino, N-phenyl-C1_3-alkyl-amino, phenyl-C1_3-alkyl-
amino, N- (C1_3-alkyl) -phenyl-Cl_3-alkylamino or di- (phenyl-
C1_3-alkyl) -amino group,
or a phenyl group substituted by the group of formula
-N(R12)-CO-(CH2)p-R13 (IV),
wherein R12, p and R13 are as hereinbefore defined,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
Particularly preferred compounds of general formula I are
those wherein
X denotes an oxygen atom,
R. denotes a hydrogen atom,
R2 denotes a carboxy group, a straight-chain or branched
C1_4-alkoxycarbonyl group or a phenoxycarbonyl group,
a straight-chain or branched C1_3-alkoxy-carbonyl group, which
is terminally substituted in the alkyl moiety by a phenyl,
carboxy, C1_,-alkoxycarbonyl, aminocarbonyl,
Cl_3-alkylaminocarbonyl or di- (Cl_3-alkyl) -aminocarbonyl group,
a straight-chain or branched C2_3-alkoxy-carbonyl group which
is terminally substituted in the alkyl moiety by a hydroxy,
CA 02387013 2002-03-19
- 28 -
Cl_3-alkoxy, amino, Cl_3-alkylamino or di- (Cl_3-alkyl) -amino
group,
an aminocarbonyl or methylaminocarbonyl group, an
ethylaminocarbonyl group optionally substituted in the 2
position of the ethyl group by a hydroxy or C1_3-alkoxy group
or, if R4 does not denote an aminosulphonyl-phenyl or
N- (C1_5-alkyl) -Cl_3-alkylaminocarbonyl-phenyl group, it may also
denote a di- (Cl_2-alkyl) -aminocarbonyl group,
R3 denotes a C1_4-alkyl group or a phenyl group which may be
substituted by a fluorine, chlorine or bromine atom, by a
trifluoromethyl, C1_3-alkyl, hydroxy or C1_3-alkoxy group,
R4 denotes a C5_6-cycloalkyl group,
wherein the methylene group in position 4 of the
cyclohexyl group may be substituted by an amino,
Cl_3-alkylamino or di- (Cl_3-alkyl) -amino group or replaced
by an -NH or -N(C1_3-alkyl) group,
a phenyl group, a phenyl group disubstituted by C1_3-alkyl,
C1_3-alkoxy or nitro groups, wherein the substituents may be
identical or different, or
a phenyl group substituted by the group R6+ which may
additionally be substituted by a fluorine, chlorine or bromine
atom or by an amino or nitro group, wherein
R6 denotes a fluorine, chlorine or bromine atom,
a C1_3-alkyl, Cl_3-alkoxy, nitro, amino or C5_6-cycloalkyl group,
a pyrrolyl, pyrazolyl, imidazolyl, triazolyl or tetrazolyl
group bound via a carbon atom, wherein the abovementioned
heteroaromatic groups in the carbon skeleton may be
CA 02387013 2002-03-19
- 29 -
substituted by a C1_3-alkyl group or a hydrogen atom bound to a
nitrogen atom may be replaced by a C1_3-alkyl or
phenyl-C1_3-alkyl group,
the group of formula
O
NH
CH
N O
H
a carboxy, C1_4-alkoxycarbonyl, phenyl-C1_3-alkylamino-carbonyl
or C5_7-cycloalkyl-carbonyl group,
a 5 or 6-membered cycloalkyleneimino group, wherein
the methylene group in position 4 of the piperidino group
may be replaced by an oxygen or sulphur atom, by an -NH or
-N(Cl_3-alkyl) group,
an unbranched C1_3-alkyl group terminally substituted by the
group Rõ wherein
R7 denotes a CS_7-cycloalkyl group,
wherein in a 5 or 6-membered cycloalkyl group a-(CHz)2
group may be replaced by a -CO-NH group, a -(CH2)3 group
may be replaced by an -NH-CO-NH- or a-(CH2)4 group may
be replaced by an -NH-CO-NH-CO group, whilst in each
case a hydrogen atom bound to a nitrogen atom may be
replaced by a C1_3-alkyl group,
a phenyl or pyridinyl group or a pyrrolyl, pyrazolyl,
imidazolyl or triazolyl group bound via a carbon or
nitrogen atom, wherein the abovementioned heteroaromatic
groups in the carbon skeleton may be substituted by a
, CA 02387013 2002-03-19
- 30 -
C1_3-alkyl group or a hydrogen atom bound to a nitrogen
atom may be replaced by a C1_3-alkyl group,
a hydroxy or Cl_3-alkoxy group,
an amino, Cl_6-alkylamino, di- (Cl_6-alkyl) -amino,
phenylamino, N-phenyl-C1_3-alkylamino,
phenyl-C1_3-alkylamino or N- (C1_3-alkyl) -phenyl-
Cl_3-alkylamino group,
a w-hydroxy-CZ_3-alkyl-amino,
N- (Cl_3-alkyl) -w-hydroxy-CZ_3-alkylamino,
di- (w-hydroxy-C2_3-alkyl) -amino or
di- (co- (C1_3-alkoxy) -CZ_3-alkyl) -amino group,
a Cl_,-alkylcarbonylamino-CZ_3-alkyl-amino or
Cl_3-alkylcarbonylamino-C2_3-alkyl-N- (Cl_3-alkyl) -amino group,
a C1_3-alkylsulphonylamino, N- (C,_3-alkyl) -
C1_3-alkylsulphonylamino, C1_3-alkylsulphonylamino-
-C2_3-alkylamino or Cl_3-alkylsulphonylamino-
-C2_3-alkyl-N- (Cl_3-alkyl) -amino group,
a hydroxycarbonyl-C1_3-alkylamino or
N- (Cl_3-alkyl) -hydroxycarbonyl-Cl_3-alkyl-amino group,
a guanidino group wherein a hydrogen atom may be replaced
by a C1_3-alkyl group,
a group of formula
-N(R8)-CO-(CH2)n-Rg (II),
wherein
CA 02387013 2002-03-19
- 31 -
R. denotes a hydrogen atom or a Cl_3-alkyl group,
n denotes one of the numbers 0, 1, 2 or 3 and
R9 denotes an amino, Cl_3-alkylamino,
di- (C1_3-alkyl) -amino or Cl_4-alkoxy group, a 5- or 6-
membered cycloalkyleneimino group, wherein the
methylene group in position 4 of the piperidino group
may be replaced by an -NH, -N(Cl_3-alkyl) or
-N(C1_3-alkyl-carbonyl) group, or, if n denotes one of
the numbers 1, 2 or 3, R9 may also denote a hydrogen
atom,
a group of formula
-N(R10) - (CH2) m- (CO)o-R11 (III),
wherein
Rlo denotes a hydrogen atom or a Cl_3-alkyl group,
m denotes one of the numbers 1, 2 or 3,
o denotes the number 1 or, if m is one of the numbers 2
or 3, o may also denote the number 0 and
Rll denotes an amino, C1_3-alkylamino,
di- (C1_3-alkyl) -amino, C1_4-alkoxy or methoxy-C1_3-alkoxy
group or a 5- or 6-membered cycloalkyleneimino group,
wherein the methylene group in position 4 of the
piperidino group may be replaced by an -NH,
-N(Cl_3-alkyl) or -N(C1_3-alkyl-carbonyl) group,
an azetidino, pyrrolidino, piperidino,
2,6-dimethyl-piperidino, 3,5-dimethyl-piperidino or
azepino group, wherein
CA 02387013 2002-03-19
- 32 -
the methylene group in position 3 of the pyrrolidino
group may be substituted by a hydroxy group,
the methylene group in position 4 of the piperidino
group may be substituted by a hydroxy,
hydroxy-Cl_3-alkyl or Cl_3-alkoxy group or
may be replaced by an oxygen or sulphur atom, by a
sulphinyl, sulphonyl, -NH, -N(C1_3-alkyl) , -N(C1_3-alkyl-
carbonyl), -N(benzoyl) or -N(phenyl-C1_3-alkyl-carbonyl)
group,
wherein a methylene group linked to an imino-
nitrogen atom of the pyrrolidino, piperidino or
piperazino group may be replaced by a carbonyl
group,
or R6 denotes a straight-chain C1_3-alkyl group which is
terminally substituted by a carboxy or C1_3-alkoxy-carbonyl
group,
a group of formula
-N(R12)-CO-(CH2)p-R13 (IV),
wherein
R12 denotes a hydrogen atom, a C1_3-alkyl or phenyl-Cl_3-
alkyl group,
p denotes one of the numbers 0, 1 or 2 and
R13 denotes an amino, Cl_4-alkylamino, di- (Cl_4-alkyl) -amino,
benzylamino, N-(C1_3-alkyl)-benzylamino,
C1_3-alkoxy-Cl_3-alkylamino, N- (C1_3-alkyl) -
CA 02387013 2002-03-19
- 33 -
C1_3-alkoxy-C1_3-alkylamino, di- (2-methoxy-ethyl) -amino,
di-(w-hydroxy-CZ_3-alkyl)-amino or aminocarbonyl-methyl-N-
(methyl)-amino group,
a pyrrolyl, pyrazolyl or imidazolyl group bound via a
nitrogen atom and optionally substituted by a C1_3-alkyl
group,
a pyrrolidino, piperidino, morpholino, thiomorpholino or a
piperazino group optionally substituted in the 4 position
by a Cl_3-alkyl, phenyl-Cl_3-alkyl, Cl_3-alkylcarbonyl or
C1_4-alkoxycarbonyl group or, if n denotes the number 1 or
2, it may also denote a hydrogen atom,
a group of formula
-N(R14) - (CH2) q- (CO) r-R15 (V) ,
wherein
R14 denotes a hydrogen atom, a Cl_4-alkyl, C1_3-alkyl-
carbonyl, phenylcarbonyl, phenyl-C1_3-alkylcarbonyl, furyl-
carbonyl, pyridinyl-carbonyl, furyl-C1_3-alkylcarbonyl,
pyridinyl-C1_3-alkylcarbonyl, C1_4-alkylsulphonyl,
phenylsulphonyl or phenyl-C1_3-alkylsulphonyl group,
q denotes one of the numbers 1, 2 or 3,
r denotes the number 1 or, if q is one of the numbers 2 or
3, it may also denote the number 0 and
Rls denotes an amino, Cl_4-alkylamino, di- (C1_4-alkyl) -amino,
phenylamino, N-(C1_4-alkyl)-phenylamino, benzylamino or
N- (Cl_9-alkyl) -benzylamino group,
CA 02387013 2002-03-19
- 34 -
or a group of formula
-N(R16)-SO2-Rl7 (VI),
wherein
R16 denotes a hydrogen atom or a C1_3-alkyl group optionally
terminally substituted by a cyano,
trifluoromethyl-carbonylamino or
N-(C1_3-alkyl)-trifluoromethyl-carbonyl-amino group and
R17 denotes a Cl_3-alkyl group,
wherein all the single-bonded or fused phenyl groups
contained in the groups mentioned under R6 may be
substituted by a fluorine, chlorine or bromine atom, by
a methyl, trifluoromethyl, methoxy, nitro or cyano
group and
R5 denotes a hydrogen atom,
wherein a hydrogen atom bound.to a nitrogen atom in the
abovementioned groups may be replaced by an acetyl or
tert.butoxycarbonyl group,
the carboxy groups contained in the abovementioned groups may
also be present in the form of the tert.butoxycarbonyl
precursor group and
the saturated alkyl and alkoxy moieties contained in the
abovementioned groups, which contain more than 2 carbon atoms,
may be straight-chain or branched, unless otherwise stated,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
CA 02387013 2002-03-19
- 35 -
One subgroup of particularly preferred compounds of general
formula I deserving special mention comprises those wherein
X, Rl, R3 and R5 are as hereinbefore defined,
R2 denotes a straight-chain or branched C1_q,-alkoxycarbonyl
group or a phenoxycarbonyl group,
a straight-chain or branched C1_3-alkoxycarbonyl group, which
is terminally substituted in the alkyl moiety by a phenyl-
carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
Cl_3-alkylaminocarbonyl or di- (Cl_3-alkyl) -aminocarbonyl group,
or
a straight-chain or branched CZ_3-alkoxy-carbonyl group, which
is terminally substituted in the alkyl moiety by a hydroxy,
Cl_3-alkoxy, amino, Cl_3-alkylamino or di- (Cl_3-alkyl) -amino
group, and
R4 denotes an R,- (n-Cl_3-alkyl) -phenyl group, wherein
R. denotes an amino, C1_6-alkylamino, di- (Cl_4-alkyl) -amino,
cw-hydroxy-C2_3-alkyl-amino,
N- (Cl_3-alkyl) -ou-hydroxy-C2_3-alkyl-amino,
di- (w-hydroxy-C2_3-alkyl) -amino or
di- ((0- (Cl_3-alkoxy) -C2_3-alkyl) -amino group,
or a phenyl group substituted by the group of formula
-N(R12)-CO-(CH2)p-R13 (IV),
wherein R12, p and R13 are as hereinbefore defined,
CA 02387013 2002-03-19
- 36 -
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
A second subgroup of particularly preferred compounds of
general formula I deserving special mention comprises those
wherein
X, Rl, R3 and RS are as hereinbefore defined,
R2 denotes an aminocarbonyl or methylaminocarbonyl group, an
ethylaminocarbonyl group optionally substituted in the 2
position of the ethyl group by a hydroxy or C1_3-alkoxy group
or, if R4 does not denote an aminosulphonyl-phenyl or
N- (Cl_5-alkyl) -C1_3-alkylaminocarbonyl-phenyl group, R2 may also
denote a di-(C1_2-alkyl)-aminocarbonyl group and
R4 denotes a R,- (n-Cl_3-alkyl) -phenyl group, wherein
R7 denotes an amino, C1_6-alkylamino, di- (Cl_4-alkyl) -amino,
cw-hydroxy-C2_3-alkyl-amino, N- (Cl_3-alkyl) -w-hydroxy-
-C2_3-alkyl-amino, di- (w-hydroxy-CZ_3-alkyl) -amino or
di- (co- (Cl_3-alkoxy) -C2_3-alkyl) -amino group,
or a phenyl group substituted by the group of formula
-N(R12)-CO-(CH2)p-R13 (IV),
wherein R12, p and R13 are as hereinbefore defined,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
Most particularly preferred compounds of general formula I are
those wherein
CA 02387013 2002-03-19
- 37 -
X denotes an oxygen atom,
R. and R. each denote a hydrogen atom,
R. denotes a methoxycarbonyl, ethoxycarbonyl or aminocarbonyl
group,
R3 denotes a phenyl group and
R4 denotes a phenyl group monosubstituted by the group R6,
wherein
R6 denotes an N-methyl-imidazol-2-yl group,
an unbranched C1_3-alkyl group which is terminally substituted
by a Cl_4-alkylamino, di- (Cl_4-alkyl) -amino, piperidino or
2,6-dimethyl-piperidino group,
a group of formula
-N(R12)-CO-(CH2)p-R13 (IV),
wherein
R12 denotes a Cl_3-alkyl group,
p denotes one of the numbers 1 or 2 and
R13 denotes a di- (Cl_3-alkyl) -amino group,
or a group of formula
-N(R14) - (CH2) q- (CO) r-R15 (V) ,
wherein
CA 02387013 2002-03-19
- 38 -
R14 denotes a Cl_3-alkyl-carbonyl or Cl_3-alkylsulphonyl
group,
q denotes one of the numbers 1, 2 or 3,
r denotes the number 1 or, if q is one of the numbers 2 or
3, r may also denote the number 0 and
Rls denotes a di- (Cl_3-alkyl) -amino group,
wherein the saturated alkyl moieties contained in the
abovementioned groups which contain more than 2 carbon atoms
may be straight-chain or branched, unless otherwise stated,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
A subgroup of most particularly preferred compounds of general
formula I deserving special mention comprises those wherein
X, Rl, R3 and R. are as hereinbefore defined,
RZ denotes a methoxycarbonyl or ethoxycarbonyl group and
R4 denotes a di- (Cl_3-alkyl) -amino-Cl_3-alkylphenyl group or
a phenyl group substituted by the group of formula
-N(R12)-CO-(CH2)p-R13 (IV),
wherein R12, p and R13 are as hereinbefore defined,
the tautomers, the diastereomers, the enantiomers, the
mixtures thereof and the salts thereof.
CA 02387013 2002-03-19
- 39 -
The following are mentioned as examples of particularly
preferred compounds:
(a) 3-Z- [1- (4- (piperidin-l-yl-methyl) -anilino) -1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone,
(b) 3-Z-[(1-(4-(piperidin-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-carbamoyl-2-indolinone,
(c)' 3-Z- [1- (4- (piperidin-l-yl-methyl) -anilino) -1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone,
(d) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone,
(e) 3-Z- [1- (4- ( (2, 6-dimethyl-piperidin-l-yl) -methyl) -anilino) -
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone,
(f) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-acetyl-amino) -ani-
lino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone,
(g) 3-Z-[1-(4-(N-(3-dimethylamino-propyl)-N-acetyl-amino)-ani-
lino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone,
(h) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone,
(i) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone,
(j) 3-Z-[1-(4-(N-acetyl-N-dimethylaminocarbonylmethyl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone,
(k) 3-Z-[1-(4-ethylaminomethyl-anilino)-i-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone,
CA 02387013 2002-03-19
- 40 -
(1) 3-Z- [l- (4- (1-methyl-imidazol-2-yl) -anilino) -1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone,
(m) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-arnino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone,
(n) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone,
(o) 3-Z-[1-(4-(N-(3-dimethylamino-propyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone,
(p) 3-Z-[1-(4-(N-dimethylaminocarbonylmethyl-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone,
(q) 3-Z-[1-(4-(N-((2-dimethylamino-ethyl)-carbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone,
(r) 3-Z-[l-(4-(N-(2-dimethylamino-ethyl)-N-acetyl-amino)-ani-
lino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone and
(s) 3-Z-[1-(4-methylaminomethyl-anilino)-i-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
the tautomers, the mixtures and the salts thereof.
Another subgroup of compounds of general formula I comprises
those wherein
X denotes an oxygen or sulphur atom,
CA 02387013 2002-03-19
- 41 -
R1 denotes a hydrogen atom or a prodrug group such as a
Cl_4-alkoxycarbonyl or C2_4-alkanoyl group,
R. denotes a carboxy group, a straight-chain or branched
C1_6-alkoxycarbonyl group, a C5_7-cycloalkoxycarbonyl or phenyl-
C1_3-alkoxycarbonyl group, an aminocarbonyl or
Cl_2-alkylaminocarbonyl group or, if R. does not denote an
aminosulphonyl-phenyl or N-(C1_5-alkyl)-C1_3-alkylaminocarbonyl-
phenyl group, a di-(C1_2-alkyl)-aminocarbonyl group,
R3 denotes a hydrogen atom, a C1_6-alkyl, C3_7-cycloalkyl,
trifluoromethyl or heteroaryl group,
a phenyl or naphthyl group, a phenyl or naphthyl group mono-
or disubstituted by a fluorine, chlorine, bromine or iodine
atom, by a trifluoromethyl, C1_3-alkyl or C1_3-alkoxy group,
whilst in the event of disubstitution the substituents may be
identical or different and wherein the abovementioned
unsubstituted as well as the mono- and disubstituted phenyl
and naphthyl groups may additionally be substituted
by a hydroxy, hydroxy-Cl_3-alkyl or Cl_3-alkoxy-Cl_3-alkyl
group,
by a cyano, carboxy, carboxy-C1_3-alkyl,
C1_3-alkoxycarbonyl, aminocarbonyl, C1_3-alkylamino-carbonyl
or di- (Cl_3-alkyl) -aminocarbonyl group,
by a nitro group,
by an amino, Cl_3-alkylamino, di- (C1_3-alkyl) -amino or
amino-Cl_3-alkyl group,
by a C1_3-alkylcarbonylamino, N- (C1_3-alkyl) -C1_3-alkyl-
carbonylamino, C1_3-alkylcarbonylamino-C1_3-alkyl,
N- (C1_3-alkyl) -C1_3-alkylcarbonylamino-C1_3-alkyl, Cl_3-alkyl-
CA 02387013 2002-03-19
- 42 -
sulphonylamino, C1_3-alkylsulphonylamino-C1_3-alkyl,
N- (C1_3-alkyl) -Cl_3-alkylsulphonylamino-C1_3-alkyl or
aryl-C1_3-alkylsulphonylamino group,
by a cycloalkylamino, cycloalkyleneimino, cyclo-
alkyleneiminocarbonyl, cycloalkyleneimino-C1_3-alkyl,
cycloalkyleneiminocarbonyl-C1_3-alkyl or
cycloalkyleneiminosulphonyl-C1_3-alkyl group having 4 to 7
ring members in each case, whilst in each case the
methylene group in position 4 of a 6- or 7-membered
cycloalkyleneimino group may be replaced by an oxygen or
sulphur atom, by a sulphinyl, sulphonyl, -NH or
-N(Cl_3-alkyl) group,
or by a heteroaryl or heteroaryl-C1_3-alkyl group,
R4 denotes a C3_7-cycloalkyl group,
whilst the methylene group in the 4 position of a 6- or 7-
membered cycloalkyl group may be substituted by an amino,
Cl_3-alkylamino or di- (Cl_3-alkyl) -amino group or replaced
by an -NH or -N(C1_3-alkyl) group,
or a phenyl group substituted by the group R6, which may
additionally be substituted by a fluorine, chlorine, bromine
or iodine atom, by a Cl_5-alkyl, trifluoromethyl, Cl_3-alkoxy,
carboxy, C1_3-alkoxycarbonyl, aminosulphonyl, nitro or cyano
group, wherein
R6 denotes a hydrogen, fluorine, chlorine, bromine or iodine
atom,
a cyano, nitro, C1_5-alkyl, C3_7-cycloalkyl, trifluoromethyl,
phenyl, tetrazolyl or heteroaryl group,
CA 02387013 2002-03-19
- 43 -
a C1_3-alkoxy group optionally sunstituted by 1 to 3 fluorine
atoms, a Cl_3-alkoxy-C1_3-alkoxy, phenyl-Cl_3-alkoxy,
amino-C2_3-alkoxy, Cl_3-alkylamino-Cz_3-alkoxy,
di- (C1_3-alkyl) -amino-Cz_3-alkoxy, phenyl-Cl_3-alkylamino-
C2_3-alkoxy, N- (C1_3-alkyl) -phenyl-Cl_3-alkylamino-C2_3-alkoxy,
C5_7-cycloalkyleneimino-C2_3-alkoxy or Cl_3-alkylmercapto group,
a carboxy, C1_4-alkoxycarbonyl, aminocarbonyl, C1_3-alkyl-
amino-carbonyl, N- (Cl_5-alkyl) -Cl_3-alkylaminocarbonyl,
phenyl-C1_3-alkylamino-carbonyl,
N- (C1_3-alkyl) -phenyl-Cl_3-alkylamino-carbonyl,
piperazinocarbonyl or N-(C1_3-alkyl)-piperazinocarbonyl group,
a C1_3-alkylaminocarbonyl or N- (Cl_5-alkyl) -
C1_3-alkylaminocarbonyl group wherein an alkyl moiety is
substituted by a carboxy or C1_3-alkoxycarbonyl group or is
substituted in the 2 or 3 position by a di-(C1_3-alkyl)-amino,
piperazino, N-(C1_3-alkyl)-piperazino or a 4- to 7-membered
cycloalkyleneimino group,
a 4- to 7-membered cycloalkyleneimino group, wherein
a methylene group linked to the imino group may be
replaced by a carbonyl or sulphonyl group or
the cycloalkylene moiety may be fused to a phenyl ring or
one or two hydrogen atoms may each be replaced by a
C1_3-alkyl group and/or
in each case the methylene group in the 4 position of a 6-
or 7-membered cycloalkyleneimino group may be substituted
by a carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
C1_3-alkylaminocarbonyl, di- (C1_3-alkyl) -aminocarbonyl,
phenyl-C1_3-alkylamino or N- (Cl_3-alkyl) -
phenyl-C1_3-alkylamino group or
CA 02387013 2002-03-19
- 44 -
may be replaced by an oxygen or sulphur atom, by a
sulphinyl, sulphonyl, -NH, -N(C1_3-alkyl) , -N(phenyl) ,
-N (C1_3-alkyl-carbonyl) or -N(benzoyl) group,
a C1_4-alkyl group which may be substituted
by a hydroxy or Cl_3-alkoxy group,
by an amino, Cl_,-alkylamino, di- (Cl_,-alkyl) -amino,
di-N- (Cl_3-alkyl) -amino-Cz_3-alkylamino,
tri-N, N, N' - (Cl_3-alkyl) -amino-C2_3-alkylamino, phenylamino,
N-phenyl-C1_3-alkyl-amino, phenyl-C1_3-alkylamino,
N- (Cl_3-alkyl) -phenyl-C1_3-alkylamino or di-
(phenyl-Cl_3-alkyl) -amino group,
by a C1_3-alkylcarbonylamino, N- (C1_3-alkyl) -
C1_3-alkylcarbonylamino, C1_3-alkoxycarbonyl-C1_3-alkylamino
or N- (C1_3-alkyl) -Cl_3-alkoxycarbonyl-Cl_3-alkylamino group,
by a C4_,-cycloalkylamino, C4_,-cycloalkyl-C1_3-alkylamino or
C4_,-cycloalkenylamino group wherein position 1 of the ring
is not involved in the double bond and wherein the
abovementioned groups may each additionally be substituted
at the amino-nitrogen atom by a C1_3-alkyl group wherein
some or all of the hydrogen atoms are replaced by fluorine
atoms, by a CS_,-cycloalkyl, CZ_,-alkenyl or C1_4-alkyl group,
by a 4- to 7-membered cycloalkyleneimino group, wherein
a methylene group linked to the imino group may be
replaced by a carbonyl or sulphonyl group or
the cycloalkylene moiety may be fused to a phenyl group
or to an oxazolo, imidazolo, thiazolo, pyridino,
pyrazino or pyrimidino group optionally substituted by
CA 02387013 2002-03-19
- 45 -
a fluorine, chlorine, bromine or iodine atom, by a
nitro, C1_3-alkyl, Cl_3-alkoxy or amino group or
one or two hydrogen atoms may each be replaced by a
Cl_3-alkyl, CS_7-cycloalkyl or phenyl group and/or
in each case the methylene group in the 4 position of a
6- or 7-membered cycloalkyleneimino group may be
substituted by a hydroxy, carboxy, C1_4-alkoxycarbonyl,
aminocarbonyl, C1_3-alkylaminocarbonyl,
di- (Cl_3-alkyl) -aminocarbonyl, phenyl-C1_3-alkylamino or
N- (Cl_3-alkyl) -phenyl-Cl_3-alkylamino group or
may be replaced by an oxygen or sulphur atom, by a
sulphinyl, sulphonyl, -NH, -N ( C1_3 -alkyl ) , -N (phenyl ) ,
-N(C1_3-alkyl-carbonyl) or -N(benzoyl) group,
by a carboxy, C1_3-alkoxycarbonyl, aminocarbonyl,
C1_3-alkylaminocarbonyl or di- (C1_3-alkyl) -aminocarbonyl
group or
by a 4- to 7-membered cycloalkyleneiminocarbonyl group,
an amino, pyrrolidino, piperidino, morpholino, benzoylamino or
N- (C,_3-alkyl) -benzoylamino group,
an N- (C1_3-alkyl) -C2_4-alkanoylamino group which is additionally
substituted in the alkyl moiety by a carboxy or
C1_3-alkoxycarbonyl group,
a group of formula
-N(Rg) -CO- (CH2)n-Rg (II),
wherein
CA 02387013 2002-03-19
- 46 -
Re denotes a hydrogen atom or a C1_3-alkyl group,
n denotes one of the numbers 0, 1, 2 or 3 and
R9 denotes an amino, C1_4-alkylamino, phenylamino,
N-(C1_4-alkyl)-phenylamino, benzylamino,
N- (C1_4-alkyl) -benzylamino or di- (C1_4-alkyl) -amino group, a
4- to 7-membered cycloalkyleneimino group, whilst in each
case the methylene group in the 4 position of a 6- or 7-
membered cycloalkyleneimino group may be replaced by an
oxygen or sulphur atom, by a sulphinyl, sulphonyl, -NH,
-N (C1_3-alkyl) , -N (phenyl) , -N (C1_3-alkyl-carbonyl) or
-N(benzoyl) group, or, if n denotes one of the numbers 1,
2 or 3, it may also denote a hydrogen atom,
a group of formula
-N(R10) - (CH2)m- (CO)o-R11 (III),
wherein
Rlo denotes a hydrogen atom, a C1_3-alkyl group, a
C,._3-alkylcarbonyl, arylcarbonyl, phenyl-C1_3-alkylcarbonyl,
C1_3-alkylsulphonyl, arylsulphonyl or
phenyl-C1_3-alkylsulphonyl group,
m denotes one of the numbers 1, 2, 3 or 4,
o denotes one of the numbers 0 or 1 and
R11 denotes an amino, C1_4-alkylamino, phenylamino,
N-(C1_4-alkyl)-phenylamino, benzylamino,
N- (Cl_,-alkyl) -benzylamino or di- (Cl_4-alkyl) -amino group, a
4- to 7-membered cycloalkyleneimino group, wherein the
cycloalkylene moiety may be fused to a phenyl ring or in
each case the methylene group in the 4 position of a 6- or
CA 02387013 2002-03-19
- 47 -
7-membered cycloalkyleneimino group may be replaced by an
oxygen or sulphur atom, by a sulphinyl, sulphonyl, -NH,
-N(Cl_3-alkyl) , -N(phenyl), -N(C1_3-alkyl-carbonyl) or
-N(benzoyl) group, a Cl_j-alkoxy group or a
di- (Cl_4-alkyl) -amino-C1_3-alkylamino group optionally
substituted in the 1 position by a C1_3-alkyl group,
or an N- (C1_3-alkyl) -C1_5-alkylsulphonylamino or
N-(C1_3-alkyl)-phenylsulphonylamino group wherein the alkyl
moiety is additionally substituted by a cyano or carboxy
group,
wherein all the single-bonded or fused phenyl groups
contained in the groups mentioned under R. may be mono- or
disubstituted by fluorine, chlorine, bromine or iodine
atoms, by Cl_5-alkyl, trifluoromethyl, Cl_3-alkoxy, carboxy,
C1_3-alkoxycarbonyl, aminosulphonyl, nitro or cyano groups,
wherein the substituents may be identical or different, or
two adjacent hydrogen atoms of the phenyl groups may be
replaced by a methylenedioxy group,
and
R5 denotes a hydrogen atom or a C1_3-alkyl group,
wherein by an aryl group is meant a phenyl or naphthyl group
optionally mono- or disubstituted by a fluorine, chlorine,
bromine or iodine atom, by a trifluoromethyl, C1_3-alkyl or
C1_3-alkoxy group and
by a heteroaryl group is meant a monocyclic 5- or 6-membered
heteroaryl group optionally substituted by a C1_3-alkyl group,
wherein the 6-membered heteroaryl group contains one, two or
three nitrogen atoms and the 5-membered heteroaryl group
contains an imino group optionally substituted by a C1_3-alkyl
group, an oxygen or sulphur atom or an imino group optionally
CA 02387013 2002-03-19
- 48 -
substituted by a C1_3-alkyl group and an oxygen or sulphur atom
or one or two nitrogen atoms, and moreover a phenyl ring may
be fused to the abovementioned monocyclic heterocyclic groups
via two adjacent carbon atoms,
the saturated alkyl and alkoxy moieties present in the groups
defined above which contain more than 2 carbon atoms also
include the branched isomers thereof such as, for example, the
isopropyl, tert.butyl or isobutyl group, unless otherwise
stated, and
additionally any carboxy, amino or imino group present may be
substituted by a group which can be cleaved in vivo,
the isomers and the salts thereof.
According to the invention the new compounds are obtained, for
example, by the following methods known in principle from the
literature:
a. reacting a compound of general formula
R3
Z1
/ I
X (VII) ,
~ N
Rz' I
R18
wherein
X and R3 are as hereinbefore defined,
R2' has the meanings given for R2 hereinbefore,
R1B denotes a hydrogen atom or a protecting group for the
nitrogen atom of the lactam group, wherein one of the groups
CA 02387013 2002-03-19
- 49 -
RZ' and R18 may also denote a bond to a solid phase optionally
formed via a spacer and the other one of the groups R2' and R18
has the abovementioned meanings, and Z1 denotes a halogen
atom, a hydroxy, alkoxy or aryl-alkoxy group, e.g. a chlorine
or bromine atom, a methoxy, ethoxy or benzyloxy group,
with an amine of general formula
RS
H - N/ (VIII) ,
\ R4
wherein
R4 and R5 are as hereinbefore defined,
and if necessary subsequently cleaving any protecting group
used for the nitrogen atom of the lactam group or cleaving
from a solid phase.
The protecting group for the nitrogen atom of the lactam group
may be, for example, an acetyl, benzoyl, ethoxycarbonyl,
tert.butyloxycarbonyl or benzyloxycarbonyl group and
the solid phase may be a resin such as a
4-(2',4'-dimethoxyphenylaminomethyl)-phenoxy resin, the bond
preferably being formed via the amino group, or a p-
benzyloxybenzyl alcohol resin, wherein the bond is
conveniently formed via an intermediate member such as a 2,5-
dimethoxy-4-hydroxy-benzyl derivative.
The reaction is conveniently carried out in a solvent such as
dimethylformamide, toluene, acetonitrile, tetrahydrofuran,
dimethylsulphoxide, methylene chloride or mixtures thereof,
optionally in the presence of an inert base such as
triethylamine, N-ethyl-diisopropylamine or sodium hydrogen
carbonate at temperatures between 20 and 175 C, whilst any
CA 02387013 2002-03-19
- 50 -
protecting group used can be cleaved at the same time by
transamidation.
if Z1 in a compound of general formula VII denotes a halogen
atom, the reaction is preferably carried out in the presence
of an inert base at temperatures of between 20 and 120 C.
If Z. in a compound of general formula VII denotes a hydroxy,
alkoxy or arylalkoxy group, the reaction is preferably carried
out at temperatures between 20 and 200 C.
If a protecting group used subsequently has to be cleaved,
this is conveniently done either hydrolytically in an aqueous
or alcoholic solvent, e.g. in methanol/water, ethanol/water,
isopropanol/water, tetrahydrofuran/water, dioxan/water,
dimethylformamide/water, methanol or ethanol in the presence
of an alkali metal base such as lithium hydroxide, sodium
hydroxide or potassium hydroxide at temperatures between 0 and
100 C, preferably at temperatures between 10 and 50 C,
or advantageously by transamidation with an organic base such
as ammonia, butylamine, dimethylamine or piperidine in a
solvent such as methanol, ethanol, dimethylformamide and the
mixtures thereof or in an excess of the amine used, at
temperatures between 0 and 100 C, preferably at temperatures
between 10 and 50 C.
Cleaving from any solid phase used is preferably carried out
using trifluoroacetic acid and water at temperatures between 0
and 35 C, preferably at ambient temperature.
b. In order to prepare a compound of general formula I wherein
R2 has the meanings given hereinbefore, with the exception of
the carboxy group:
reacting a compound of general formula
CA 02387013 2002-03-19
- 51 -
R3
R4
N
Rs
(IX) ,
HOOC N
I
Rl
wherein
Rl and R3 to RS are as hereinbefore defined, or the reactive
derivatives thereof, with a compound of general formula
H - R19 (X) ,
wherein
R19 denotes a Cl_6-alkanol, a C4_7-cycloalkanol or an aromatic
alcohol,
a C1_6-alkanol which is terminally substituted in the alkyl
moiety by a phenyl, heteroaryl, carboxy, C1_3-alkoxy-carbonyl,
aminocarbonyl, C1_3-alkylamino-carbonyl or
di- (C1_3-alkyl) -aminocarbonyl group,
a C2_6-alkanol which is terminally substituted in the alkyl
moiety by a chlorine atom or a hydroxy, C1_3-alkoxy, amino,
C1_3-alkylamino or di- (C1_3-alkyl) -amino group,
an amino or methylamino group, an ethylamino group optionally
substituted in the 2 position of the ethyl group by a hydroxy
or C1_3-alkoxy group or a di- (C1_2-alkyl) -amino group.
The esterification or amidation is preferably carried out in a
solvent such as methylene chloride, diethylether,
tetrahydrofuran, toluene, dioxan, acetonitrile,
CA 02387013 2002-03-19
- 52 -
dimethylsulphoxide or dimethylformamide, optionally in the
presence of an inorganic or a tertiary organic base,
preferably at temperatures between 20 C and the boiling
temperature of the solvent used. The reaction with a
corresponding acid is preferably carried out in the presence
of a dehydrating agent, e.g. in the presence of isobutyl
chloroformate, tetraethyl orthocarbonate, trimethyl
orthoacetate, 2,2-dimethoxypropane, tetramethoxysilane,
thionylchloride, trimethylchiorosilane, phosphorus
trichloride, phosphorus pentoxide,
N,N'-dicyclohexylcarbodiimide, N,N'-dicyclohexyl-
carbodiimide/N-hydroxysuccinimide, N,N'-dicyclohexyl-
carbodiimide/1-hydroxy-benzotriazole, 2-(1H-benzotriazol-l-
yl)-1,1,3,3-tetramethyluronium-tetrafluoroborate, 2-(1H-
benzotriazol-l-yl)-1,1,3,3-tetramethyluronium-
tetrafluoroborate/1-hydroxy-benzotriazole,
N,N'-carbonyldiimidazole or triphenylphosphine/carbon
tetrachloride, and optionally with the addition of a base such
as pyridine, 4-dimethylaminopyridine, N-methyl-morpholine or
triethylamine, conveniently at temperatures between 0 and
150 C, preferably at temperatures between 0 and 100 C, and the
acylation with a corresponding reactive compound such as an
anhydride, ester, imidazolide or halide thereof, is optionally
carried out in the presence of a tertiary organic base such as
triethylamine, N-ethyl-diisopropylamine or N-methyl-morpholine
at temperatures between 0 and 150 C, preferably at
temperatures between 50 and 100 C.
c. In order to prepare a compound of general formula I,
wherein R4 denotes a C1_,,-alkyl group substituted by the group
Rõ wherein
R7 denotes an amino, Cl_7-alkylamino, di- (Cl_7-alkyl) -amino,
phenylamino, N-phenyl-C1_3-alkyl-amino, phenyl-C1_3-alkyl-
amino, N- (Cl_3-alkyl) -phenyl-C1_3-alkylamino or
di- (phenyl-C1_3-alkyl) -amino group,
CA 02387013 2002-03-19
- 53 -
aco-hydroxy - C2 _ 3- a l kyl - ami no ,
N- (Cl_3-alkyl ) -w-hydroxy-C2_3-alkyl-amino,
di- (co-hydroxy-C2_3-alkyl) -amino,
di- (co- (Cl_3-alkoxy) -C2_3-alkyl) -amino or
N-(dioxolan-2-yl)-C1_3-alkyl-amino group,
a Cl_3-alkylcarbonylamino-C2_3-alkyl-amino or
Cl_3-alkylcarbonylamino-Cz_3-alkyl-N- (C1_3-alkyl) -amino group,
a C1_3-alkylsulphonylamino, N- (Cl_3-alkyl) -
C1_3-alkylsulphonylamino,
Cl_3-alkylsulphonylamino-C2_3-alkyl-amino or
C1_3-alkylsulphonylamino-C2_3-alkyl-N- (Cl_3-alkyl) -amino
group,
a group of formula
-N(R10) - (CH2)m- (CO)o-R11 (III),
wherein
Rlo denotes a hydrogen atom, a Cl_3-alkyl group, a
C1_3-alkylcarbonyl, arylcarbonyl, phenyl-C1_3-alkyl-
carbonyl, C1_3-alkylsulphonyl, arylsulphonyl or phenyl-
C1_3-alkylsulphonyl group,
m denotes one of the numbers 1, 2, 3 or 4,
o denotes the number 1 and
Rll denotes an amino, Cl_4-alkylamino,
di- (Cl_4-alkyl) -amino, phenylamino,
N-(C1_4-alkyl)-phenylamino, benzylamino,
N- (Cl_4-alkyl) -benzylamino, C1_4-alkoxy or
. CA 02387013 2002-03-19
- 54 -
C1_3-alkoxy-Cl_3-alkoxy group, a di- (C1_4-alkyl) -amino-Cl_3-
alkylamino group optionally substituted in the 1
position by a C1_3-alkyl group, or a 4- to 7-membered
cycloalkyleneimino group, wherein the cycloalkylene
moiety may be fused to a phenyl ring or in each case
the methylene group in the 4 position of a 6- or 7-
membered cycloalkyleneimino group may be replaced by an
oxygen or sulphur atom, by a sulphinyl, sulphonyl, -NH,
-N(C1_3-alkyl) , -N(phenyl) , -N(Cl_3-alkyl-carbonyl) or
-N(benzoyl) group,
a C4_,-cycloalkylamino, C4_7 -cycloalkyl-Cl_3-alkylamino or
C4_7-cycloalkenylamino group wherein position 1 of the ring
is not involved in the double bond and wherein the
abovementioned groups may each additionally be substituted
at the amino-nitrogen atom by a CS_7-cycloalkyl,
C2_4-alkenyl or Cl_,,-alkyl group,
or a 4- to 7-membered cycloalkyleneimino group, wherein
the cycloalkylene moiety may be fused to a phenyl group
or to an oxazolo, imidazolo, thiazolo, pyridino,
pyrazino or pyrimidino group optionally substituted by
a fluorine, chlorine, bromine or iodine atom, by a
nitro, Cl_3-alkyl, C1_3-alkoxy or amino group, and/or
one or two hydrogen atoms may each be replaced by a
C1_3-alkyl, CS_,-cycloalkyl or phenyl group and/or
the methylene group in the 3 position of a 5-membered
cycloalkyleneimino group may be substituted by a
hydroxy, hydroxy-Cl_3-alkyl, Cl_3-alkoxy or
Cl_3-alkoxy-C1_3-alkyl group,
in each case the methylene group in the 3 or 4 position
of a 6- or 7-membered cycloalkyleneimino group may be
CA 02387013 2002-03-19
- 55 -
substituted by a hydroxy, hydroxy-C1_3-alkyl,
Cl_3-alkoxy, C1_3-alkoxy-Cl_3-alkyl, Cl_4-alkoxycarbonyl,
aminocarbonyl, C1_3-alkylaminocarbonyl, di- (C1_3-alkyl) -
aminocarbonyl, phenyl-C1_3-alkylamino or N- (Cl_3-alkyl) -
phenyl-Cl_3-alkylamino group or
may be replaced by an oxygen or sulphur atom, by a
sulphinyl, sulphonyl, -NH, -N(C1_3-alkyl-), -N(phenyl),
-N(phenyl-C1_3-alkyl-) , -N(Cl_3-alkyl-carbonyl-) ,
-N(Cl_4-alkoxy-carbonyl-) , -N(benzoyl-) or
-N(phenyl-C1_3-alkyl-carbonyl-) group,
wherein a methylene group linked to an imino-
nitrogen atom of the cycloalkyleneimino group may be
replaced by a carbonyl or sulphonyl group or in a 5-
to 7-membered monocyclic cycloalkyleneimino group or
a cycloalkyleneimino group fused to a phenyl group
the two methylene groups linked to the imino-
nitrogen atom may each be replaced by a carbonyl
group:
reacting a compound of general formula
R3 Rs
I -
A Za
-N \ /
X (XI),
N
R
z'
Ria
wherein
R3, R5 and X are as hereinbefore defined,
Rz' has the meanings given for R2 hereinbefore,
R18 denotes a hydrogen atom or a protecting group for the
nitrogen atom of the lactam group, wherein one of the groups
CA 02387013 2002-03-19
- 56 -
R2' and R18 may also denote a bond to a solid phase optionally
formed via a spacer and the other one of the groups RZ' and R18
has the abovementioned meanings, A denotes a C1_4-alkyl group
and Z. denotes a leaving group, for example an alkyl or
arylsulphonyloxy group such as the methylsulphonyloxy,
ethylsulphonyloxy, p-toluenesulphonyloxy or
trifluoromethanesulphonyloxy group, with an amine of general
formula
H-R7, (XII ) ,
wherein
R7, has the meanings given for R7 hereinbefore, and
subsequently, if necessary, cleaving any protecting group used
for the nitrogen atom of the lactam group, or cleaving from a
solid phase.
The reaction is conveniently carried out in a solvent such as
methylene chloride, tetrahydrofuran, 1,4-dioxan, toluene,
acetonitrile, dimethylsulphoxide, dimethylformamide, dimethyl-
acetamide, N-methylpyrrolidone or the mixtures thereof,
optionally with the addition of water as a co-solvent and/or
with the addition of an inert auxiliary base, e.g. sodium
hydrogen carbonate, pyridine, 2,4,6-trimethylpyridine,
quinoline, triethylamine, N-ethyldiisopropylamine, N-ethyl-
dicyclohexylamine, 1,4-diazabicyclo[2,2,2]octane or 1,8-
diazabicyclo[5,4,0]undec-7-ene, at temperatures between -50 C
and +100 C, preferably between -10 C and +50 C, while any
protecting group used may be cleaved at the same time by
transamidation.
If any protecting group used for the nitrogen atom of the
lactam group has to be removed or if the compound has to be
CA 02387013 2002-03-19
- 57 -
cleaved from a solid phase this is carried out as described
under method (a) above.
If according to the invention a compound of general formula I
is obtained which contains an alkoxycarbonyl group, this may
be converted by hydrolysis into a corresponding carboxy
compound, or
if a compound of general formula I is obtained which contains
an amino or alkylamino group, this may be converted by
reductive alkylation into a corresponding alkylamino or
dialkylamino compound, or
if a compound of general formula I is obtained which contains
an amino or alkylamino group, this may be converted by
acylation or sulphonation into a corresponding acyl or
sulphonyl compound, or
if a compound of general formula I is obtained which contains
a carboxy group, this may be converted by esterification or
amidation into a corresponding ester or aminocarbonyl
compound, or
if a compound of general formula I is obtained which contains
a cycloalkyleneimino group wherein a methylene group is
replaced by a sulphur atom, this may be converted by oxidation
into a corresponding sulphinyl or sulphonyl compound, or
if a compound of general formula I is obtained which contains
a nitro group, this may be converted by reduction into a
corresponding amino compound, or
if a compound of general formula I is obtained wherein R4
denotes a phenyl group substituted by an amino, alkylamino,
aminoalkyl or N-alkyl-amino group, this may subsequently be
converted, by reaction with a corresponding cyanate, iso-
CA 02387013 2002-03-19
- 58 -
cyanate or carbamoyl halide, into a corresponding urea
compound of general formula I, or
if a compound of general formula I is obtained wherein R4
denotes a phenyl group substituted by an amino, alkylamino,
aminoalkyl or N-alkyl-amino group, this may subsequently be
converted, by reaction with a corresponding compound which
transfers the amidino group or by reaction with a
corresponding nitrile, into a corresponding guanidino compound
of general formula I.
The subsequent hydrolysis is preferably carried out in an
aqueous solvent, e.g. in water, methanol/water, ethanol/water,
isopropanol/water, tetrahydrofuran/water or dioxan/water, in
the presence of an acid such as trifluoroacetic acid,
hydrochloric acid or sulphuric acid or in the presence of an
alkali metal base such as lithium hydroxide, sodium hydroxide
or potassium hydroxide at temperatures between 0 and 100 C,
preferably at temperatures between 10 and 50 C.
The subsequent reductive alkylation is preferably carried out
in a suitable solvent such as methanol, methanol/water,
methanol/water/ammonia, ethanol, ether, tetrahydrofuran,
dioxan or dimethylformamide, optionally with the addition of
an acid such as hydrochloric acid in the presence of
catalytically activated hydrogen, e.g. hydrogen in the
presence of Raney nickel, platinum or palladium/charcoal, or
in the presence of a metal hydride such as sodium borohydride,
lithium borohydride, sodium cyanoborohydride or lithium
aluminium hydride at temperatures between 0 and 100 C,
preferably at temperatures between 20 and 80 C.
The subsequent acylation or sulphonylation is preferably
carried out with the corresponding free acid or a
corresponding reactive compound such as the anhydride, ester,
imidazolide or halide thereof, preferably in a solvent such as
CA 02387013 2002-03-19
- 59 -
methylene chloride, diethylether, tetrahydrofuran, toluene,
dioxan, acetonitrile, dimethylsulphoxide or dimethylforrnamide,
optionally in the presence of an inorganic or tertiary organic
base at temperatures between -20 and 200 C, preferably at
temperatures between 20 C and boiling temperature of the
solvent used. The reaction with the free acid may optionally
be carried out in the presence of an acid-activating agent or
a dehydrating agent, e.g. in the presence of isobutyl
chloroformate, tetraethyl orthocarbonate, trimethyl
orthoacetate, 2,2-dimethoxypropane, tetramethoxysilane,
thionyl chloride, trimethylchlorosilane, phosphorus
trichloride, phosphorus pentoxide, N,N'-dicyclohexyl-
carbodiimide,
N,N'-dicyclohexylcarbodiimide/N-hydroxysuccinimide,
N,N'-dicyclohexylcarbodiimide/1-hydroxy-benzotriazole,
2-(1H-benzotriazol-l-yl)-1,1,3,3-tetramethyluronium-
tetrafluoroborate, 2-(1H-benzotriazol-i-yl)-1,1,3,3-
tetramethyluronium-tetrafluoroborate/1-hydroxy-benzotriazole,
N,N'-carbonyldiimidazole or triphenylphosphine/carbon
tetrachloride, and optionally with the addition of a base such
as pyridine, 4-dimethylamino-pyridine, N-methyl-morpholine or
triethylamine, conveniently at temperatures between 0 and
150 C, preferably at temperatures between 0 and 100 C. The
reaction with a corresponding reactive compound may optionally
be carried out in the presence of a tertiary organic base such
as triethylamine, N-ethyl-diisopropylamine, N-methyl-
morpholine or pyridine or by using an anhydride in the
presence of the corresponding acid at temperatures between 0
and 150 C, preferably at temperatures between 50 and 100 C.
The subsequent esterification or amidation is conveniently
carried out by reacting a corresponding reactive carboxylic
acid derivative with a corresponding alcohol or amine as
described hereinbefore.
CA 02387013 2002-03-19
- 60 -
The subsequent oxidation of the sulphur atom is preferably
carried out in a solvent or mixture of solvents, e.g. in
water, water/pyridine, acetone, methylene chloride, acetic
acid, acetic acid/acetic anhydride, dilute sulphuric acid or
trifluoroacetic acid, usefully at temperatures of between -80
and 100 C depending on the oxidising agent used.
In order to prepare a corresponding sulphinyl compound of
general formula I the oxidation is expediently carried out
with one equivalent of the oxidising agent used, e.g. with
hydrogen peroxide in glacial acetic acid, trifluoroacetic acid
or formic acid at 0 to 20 C or in acetone at 0 to 60 C, with a
peracid such as performic acid in glacial acetic acid or
trifluoroacetic acid at 0 to 50 C or with m-chloroperbenzoic
acid in methylene chloride, chloroform or dioxan at -20 to
80 C, with sodium metaperiodate in aqueous methanol or ethanol
at -15 to 25 C, with bromine in glacial acetic acid or aqueous
acetic acid optionally in the presence of a weak base such as
sodium acetate, with N-bromosuccinimide in ethanol, with
tert.butyl hypochlorite in methanol at -80 to -30 C, with
iodobenzodichloride in aqueous pyridine at 0 to 50 C, with
nitric acid in glacial acetic acid at 0 to 20 C, with chromic
acid in glacial acetic acid or in acetone at 0 to 20 C and
with sulphuryl chloride in methylene chloride at -70 C, the
resulting thioether-chlorine complex is expediently hydrolysed
with aqueous ethanol.
In order to prepare a sulphonyl compound of general formula I
the oxidation is expediently carried out starting from a
corresponding sulphinyl compound with one or more equivalents
of the oxidising agent used or starting from a corresponding
mercapto compound, expediently with two or more equivalents of
the oxidising agent used, e.g. with hydrogen peroxide in
glacial acetic acid/acetic anhydride, trifluoroacetic acid or
in formic acid at 20 to 100 C or in acetone at 0 to 60 C, with
a peracid such as performic acid or m-chloroperbenzoic acid in
CA 02387013 2002-03-19
- 61 -
glacial acetic acid, trifluoroacetic acid, methylene chloride
or chloroform at temperatures between 0 and 60 C, with nitric
acid in glacial acetic acid at 0 to 20 C, with chromic acid,
sodium periodate or potassium permanganate in acetic acid,
water/sulphuric acid or in acetone at 0 to 20 C.
The subsequent reduction of a nitro group is preferably
carried out by hydrogenolysis, e.g. with hydrogen in the
presence of a catalyst such as palladium/charcoal or Raney
nickel in a solvent such as methanol, ethanol, ethyl acetate,
dimethylformamide, dimethylformamide/acetone or glacial acetic
acid, optionally with the addition of an acid such as
hydrochloric acid or glacial acetic acid at temperatures of
between 0 and 50 C, but preferably at ambient temperature, and
at a hydrogen pressure of 1 to 7 bar, but preferably 3 to 5
bar.
The subsequent preparation of a corresponding urea compound of
general formula I is conveniently carried out with an
inorganic cyanate or a corresponding isocyanate or
carbamoylchloride, preferably in a solvent such as
dimethylformamide and optionally in the presence of a tertiary
organic base such as triethylamine at temperatures between 0
and 50 C, preferably at ambient.
The subsequent preparation of a corresponding guanidino
compound of general formula I is conveniently carried out by
reacting with a compound which transfers the amidino group
such as 3,5-dimethylpyrazole-l-carboxylic acid amidine,
preferably in a solvent such as dimethylformamide and
optionally in the presence of a tertiary organic base such as
triethylamine at temperatures of between 0 and 50 C,
preferably at ambient temperature.
CA 02387013 2002-03-19
- 62 -
In the reactions described hereinbefore, any reactive groups
present such as carboxy, hydroxy, amino, alkylamino or imino
groups may be protected during the reaction by conventional
protecting groups which are cleaved again after the reaction.
For example, a protecting group for a carboxyl group may be a
trimethylsilyl, methyl, ethyl, tert.butyl, benzyl or
tetrahydropyranyl group and
protecting groups for a hydroxy, amino, alkylamino or imino
group may be an acetyl, trifluoroacetyl, benzoyl,
ethoxycarbonyl, tert.butoxycarbonyl, benzyloxycarbonyl,
benzyl, methoxybenzyl or 2,4-dimethoxybenzyl group and
additionally, for the amino group, a phthalyl group.
Any protecting group used is optionally subsequently cleaved
for example by hydrolysis in an aqueous solvent, e.g. in
water, isopropanol/water, tetrahydrofuran/water or
dioxan/water, in the presence of a acid such as
trifluoroacetic acid, hydrochloric acid or sulphuric acid or
in the presence of an alkali metal base such as lithium
hydroxide, sodium hydroxide or potassium hydroxide, at
temperatures between 0 and 100 C, preferably at temperatures
between 10 and 50 C.
However, a benzyl, methoxybenzyl or benzyloxycarbonyl group is
cleaved, for example, hydrogenolytically, e.g. with hydrogen
in the presence of a catalyst such as palladium/charcoal in a
solvent such as methanol, ethanol, ethyl acetate,
dimethylformamide, dimethylformamide/acetone or glacial acetic
acid, optionally with the addition of an acid such as
hydrochloric acid or glacial acetic acid at temperatures
between 0 and 50 C, but preferably at ambient temperature, and
at a hydrogen pressure of 1 to 7 bar, but preferably 3 to 5
bar.
CA 02387013 2002-03-19
- 63 -
A methoxybenzyl group may also be cleaved in the presence of
an oxidising agent such as cerium(IV)ammonium nitrate in a
solvent such as methylene chloride, acetonitrile or
acetonitrile/water at temperatures of between 0 and 50 C, but
preferably at ambient temperature.
A 2,4-dimethoxybenzyl group, however, is preferably cleaved in
trifluoroacetic acid in the presence of anisole.
A tert.butyl or tert.butyloxycarbonyl group is preferably
cleaved by treating with an acid such as trifluoroacetic acid
or hydrochloric acid, optionally using a solvent such as
methylene chloride, dioxan, ethyl acetate or ether.
A phthalyl group is preferably cleaved in the presence of
hydrazine or a primary amine such as methylamine, ethylamine
or n-butylamine in a solvent such as methanol, ethanol,
isopropanol, toluene/water or dioxan at temperatures between
20 and 50 C.
Moreover, chiral compounds of general formula I obtained may
be resolved into their enantiomers and/or diastereomers.
Thus, for example, the compounds of general formula I obtained
which occur as racemates may be separated by methods known per
se (cf. Allinger N. L. and Eliel E. L. in "Topics in
Stereochemistry", Vol. 6,,,,Wiley Interscience, 1971) into their
optical antipodes and compounds of general formula I with at
least 2 asymmetric carbon atoms may be resolved into their
diastereomers on the basis of their physical-chemical
differences using methods known per se, e.g. by chromatography
and/or fractional crystallisation, and, if these compounds are
obtained in racemic form, they may subsequently be resolved
into the enantiomers as mentioned above.
CA 02387013 2002-03-19
- 64 -
The enantiomers are preferably separated by column separation
on chiral phases or by recrystallisation from an optically
active solvent or by reacting with an optically active
substance which forms salts or derivatives such as e.g. esters
or amides with the racemic compound, particularly acids and
the activated derivatives or alcohols thereof, and separating
the mixture of diastereomeric salts or derivatives thus
obtained, e.g. on the basis of their differences in
solubility, whilst the free antipodes may be released from the
pure diastereomeric salts or derivatives by the action of
suitable agents. Optically active acids in common use are e.g.
the D- and L-forms of tartaric acid or dibenzoyltartaric acid,
di-o-tolyltartaric acid, malic acid, mandelic acid,
camphorsulphonic acid, glutamic acid, N-acetylglutamic acid,
aspartic acid, N-acetylaspartic acid or quinic acid. An
optically active alcohol may be for example (+)- or
(-)-menthol and an optically active acyl group in amides, for
example, may be a(+)- or (-)-menthyloxycarbonyl group.
Furthermore, the compounds of formula I obtained may be
converted into the salts thereof, particularly for
pharmaceutical use into the physiologically acceptable salts
with inorganic or organic acids. Acids which may be used for
this purpose include for example hydrochloric acid,
hydrobromic acid, sulphuric acid, phosphoric acid, fumaric
acid, succinic acid, lactic acid, citric acid, tartaric acid,
maleic acid or methanesulphonic acid.
Moreover, if the new compounds of formula I thus obtained
contain a carboxy group, they may subsequently, if desired, be
converted into the salts thereof with inorganic or organic
bases, particularly for pharmaceutical use into the
physiologically acceptable salts thereof. Suitable bases for
this purpose include for example sodium hydroxide, potassium
hydroxide, cyclohexylamine, ethanolamine, diethanolamine and
triethanolamine.
CA 02387013 2002-03-19
- 65 -
The compounds of general formulae VII to XII used as starting
materials are known from the literature in some cases or may
be obtained by methods known from the literature or may be
obtained by the methods described hereinbefore and in the
Examples. For example, the compounds of general formula VI
are described in German Patent Application 198 24 922.5.
Moreover, the compounds of general formula XI may be obtained
from the compounds of general formula I wherein R4 denotes a
C1_4-alkyl-phenyl group substituted in the alkyl moiety by a
hydroxy group, for example, by reacting with alkyl- or
arylsulphonyl chlorides.
As already mentioned, the new compounds of general formula I
wherein R1 denotes a hydrogen atom or a prodrug group have
valuable pharmacological properties, particularly inhibitory
effects on various kinases, especially on receptor-tyrosine
kinases such as VEGFR2, PDGFRa, PDGFR(3, FGFR1, FGFR3, EGFR,
HER2, IGF1R and HGFR, as well as on complexes of CDK's (Cyclin
Dependent Kinases) such as CDK1, CDK2, CDK3, CDK4, CDK5, CDK6,
CDK7, CDK8 and CDK9 with their specific cyclins (A, Bl, B2, C,
Dl, D2, D3, E, F, G1, G2, H, I and K) and on viral cyclin, on
the proliferation of cultivated human cells, particularly
endothelial cells, e.g. in angiogenesis, but also on the
proliferation of other cells, particularly tumour cells.
The biological properties of the new compounds were tested by
the following standard procedure, as follows:
Human umbilical endothelial cells (HUVEC) were cultivated in
IMDM (Gibco BRL), supplemented with 10 % foetal calf serum
(FBS) (Sigma), 50 M of 9-mercaptoethanol (Fluka), standard
antibiotics, 15 g/ml of endothelial cell growth factor (ECGS,
Collaborative Biomedical Products) and 100 g/ml of heparin
CA 02387013 2002-03-19
- 66 -
(Sigma) on gelatine-coated culture dishes (0.2 % gelatine,
Sigma) at 37 C, under 5 % CO2 in a water-saturated atmosphere.
In order to investigate the inhibitory activity of the
compounds according to the invention the cells were starved
for 16 hours, i.e. kept in culture medium without growth
factors (ECGS + heparin). The cells were detached from the
culture dishes using trypsin/EDTA and washed once in serum-
containing medium. Then they were seeded out in amounts of 2.5
x 103 cells per well.
The proliferation of the cells was stimulated with 5 ng/ml of
VEGF165 (vascular endothelial growth factor; H. Weich, GBF
Braunschweig) and 10 g/ml of heparin. As a control, 6 wells
in each dish were not stimulated.
The compounds according to the invention were dissolved in
100% dimethylsulphoxide and added to the cultures in various
dilutions in triplicate, the maximum dimethyl sulphoxide
concentration being 0.3 %.
The cells were incubated for 76 hours at 37 C, then for a
further 16 hours 3H-thymidine (0.1 Ci/well, Amersham) was
added in order to determine the DNA synthesis. Then the
radioactively labelled cells were immobilised on filter mats
and the radioactivity incorporated was measured in a i3-
counter. In order to determine the inhibitory activity of the
compounds according to the invention the mean value of the
non-stimulated cells was subtracted from the mean value of the
factor-stimulated cells (in the presence or absence of the
compounds according to the invention).
The relative cell proliferation was calculated as a percentage
of the control (HUVEC without inhibitor) and the concentration
CA 02387013 2002-03-19
- 67 -
of active substance which inhibits the proliferation of the
cells by 50 s (IC50) was determined.
The test results of the following compounds (a) to (s) of
general formula I are given by way of example:
(a) 3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone,
(b) 3-Z-[(1-(4-(piperidin-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-carbamoyl-2-indolinone,
(c) 3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone,
(d) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone,
(e) 3-Z-[1-(4-((2,6-dimethyl-piperidin-l-yl)-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone,
(f) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-acetyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone,
(g) 3-Z- [1- (4- (N- (3-dimethylamino-propyl) -N-acetyl-amino) -
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone,
(h) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone,
(i) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone,
(j) 3-Z-[1-(4-(N-acetyl-N-dimethylaminocarbonylmethyl-amino)-
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone,
CA 02387013 2002-03-19
- 68 -
(k) 3-Z-[1-(4-ethylaminomethyl-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone,
(1) 3-Z- [1- (4- (1-methyl-imidazol-2-yl) -anilino) -1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone,
(m) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-arnino)-
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone,
(n) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone,
(o) 3-Z- [1- (4- (N- (3-dimethylamino-propyl) -N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone,
(p) 3-Z-[1-(4-(N-dimethylaminocarbonylmethyl-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone,
(q) 3-Z-[1-(4-(N-((2-dimethylamino-ethyl)-carbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone,
(r) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-acetyl-amino) -
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
and
(s) 3-Z-[1-(4-methylaminomethyl-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone.
CA 02387013 2002-03-19
- 69 -
The following Table contains the results found:
Compound IC50 [ m]
(a) 0.04
(b) 0.35
(c) 0.01
(d) 0.02
(e) 0.05
(f) 0.01
(g) 0.003
(h) 0.01
(i) 0.03
(j) 0.02
(k) 0.03
(1) 0.1
(m) 0.02
(n) 0.02
(o) 0.01
(p) 0.02
(q) 0.02
(r) 0.01
(s) 0.04
In view of their inhibitory effect on the proliferation of
cells, particularly endothelial cells and tumour cells, the
compounds of general formula I are suitable for treating
diseases in which the proliferation of cells, particularly
endothelial cells, plays a part.
Thus, for example, the proliferation of endothelial cells and
the concomitant neovascularisation constitute a crucial stage
in tumour progression (Folkman J. et al., Nature 3-aa, 58-61,
(1989); Hanahan D. and Folkman J., Cell $fi, 353-365, (1996)).
Furthermore, the proliferation of endothelial cells is also
CA 02387013 2002-03-19
- 70 -
important in haemangiomas, in metastasisation, rheumatoid
arthritis, psoriasis and ocular neovascularisation (Folkman
J., Nature Med. 1, 27-31, (1995)). The therapeutic usefulness
of inhibitors of endothelial cell proliferation was
demonstrated in the animal model for example by O'Reilly et
al. and Parangi et al. (O'Reilly M.S. et al., Cell $$, 277-
285, (1997); Parangi S. et al., Proc Natl Acad Sci USA .43.,
2002-2007, (1996)).
The compounds of general formula I, their tautomers, their
stereoisomers or the physiologically acceptable salts thereof
are thus suitable, for example, for treating tumours (e.g.
plate epithelial carcinoma, astrocytoma, Kaposi's sarcoma,
glioblastoma, lung cancer, bladder cancer, carcinoma of the
neck, melanoma, ovarian cancer, prostate cancer, breast
cancer, small-cell lung cancer, glioma, colorectal carcinoma,
urogenital cancer and gastrointestinal carcinoma as well as
haematological cancers, such as multiple myeloma), psoriasis,
arthritis (e.g. rheumatoid arthritis), haemangioma,
angiofibroma, eye diseases (e.g. diabetic retinopathy),
neovascular glaucoma, kidney diseases (e.g.
glomerulonephritis), diabetic nephropathy, malignant
nephrosclerosis, thrombic microangiopathic syndrome,
transplant rejections and glomerulopathy, fibrotic diseases
(e.g. cirrhosis of the liver), mesangial cell proliferative
diseases, arteriosclerosis and damage to the nerve tissue and
also for inhibiting the reocclusion of blood vessels after
treatment with a balloon catheter, in vascular prosthetics or
after the insertion of mechanical devices for keeping blood
vessels open (e.g. stents), or other diseases in which cell
proliferation or angiogenesis are involved.
By reason of their biological properties the compounds
according to the invention may be used on their own or in
conjunction with other pharmacologically active compounds, for
example in tumour therapy, in monotherapy or in conjunction
CA 02387013 2002-03-19
- 71 -
with other anti-tumour therapeutic agents, for example in
combination with topoisomerase inhibitors (e.g. etoposide),
mitosis inhibitors (e.g. vinblastin, taxol), compounds which
interact with nucleic acids (e.g. cis-platin,
cyclophosphamide, adriamycin), hormone antagonists (e.g.
tamoxifen), inhibitors of metabolic processes (e.g. 5-FU
etc.), cytokines (e.g. interferons), kinase inhibitors,
antibodies, or in conjunction with radiotherapy, etc. These
combinations may be administered either simultaneously or
sequentially.
For pharmaceutical use the compounds according to the
invention are generally used for warm-blooded vertebrates,
particularly humans, in doses of 0.01-100 mg/kg of body
weight, preferably 0.1-20 mg/kg. For administration they are
formulated with one or more conventional inert carriers and/or
diluents, e.g. with corn starch, lactose, glucose,
microcrystalline cellulose, magnesium stearate,
polyvinylpyrrolidone, citric acid, tartaric acid, water,
water/ethanol, water/glycerol, water/sorbitol,
water/polyethylene glycol, propylene glycol, stearyl alcohol,
carboxymethylcellulose or fatty substances such as hard fat or
suitable mixtures thereof in conventional galenic preparations
such as plain or coated tablets, capsules, powders, injectable
solutions, ampoules, suspensions, solutions, sprays or
suppositories.
CA 02387013 2002-03-19
- 72 -
The Examples which follow are intended to illustrate the
invention:
Abbreviations used:
FMOC = 9-fluorenylmethoxycarbonyl
HOBt = 1-hydroxy-lH-benzotriazole
TBTU = O-benzotriazol-l-yl-N,N,N',N'-tetramethyluronium-
tetrafluoroborate
DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene
Preparation of the starting compounds:
Gnl i d = hage Example T
2.0 g of Rink resin (MBHA resin, made by Messrs Novabiochem)
are left to swell in 30 ml of dimethylformamide. Then 40 ml of
30% piperidine in dimethylformamide are added and the mixture
is shaken for 7 minutes to cleave the FMOC protecting group.
The resin is then washed repeatedly with dimethylformamide.
Then 0.4 g of 2-indolinone-6-carboxylic acid (prepared
analogously to Langenbeck et al., Justus Liebigs Ann. Chem.
499, 201-208 (1932)), 297 mg HOBt, 706 mg TBTU and 0.9 ml of
N-ethyl-diisopropylamine in 30 ml of dimethylformamide are
added and the mixture is shaken for 1 hour. Then the solution
is suction filtered and the resin is washed five times with 30
ml of dimethylformamide and three times with 30 ml of
methylene chloride. To dry it, nitrogen is blown through the
resin.
Yield: 1.9 g of charged resin
CA 02387013 2002-03-19
- 73 -
Snl i = haaP xam]a1 e TT
1.9 g of the resin obtained in Example I are stirred with 6 ml
of acetic anhydride and 6 ml of triethyl orthobenzoate for 3
hours at 110 C. Then the mixture is left to cool and the resin
is washed with dimethylformamide and subsequently with
methylene chloride.
Yield: 1.9 g of moist resin
The following charged resins are prepared analogously to
Example II:
(1) resin charged with 3-Z-(1-ethoxy-methylene)-6-carbamoyl-2-
indolinone
Prepared by reacting the resin obtained according to Example I
with triethyl orthoformate
(2) resin charged with 3-Z-(1-methoxy-l-methyl-methylene)-6-
carbamoyl-2-indolinone
Prepared by reacting the resin obtained according to Example I
with trimethyl orthoacetate
(3) resin charged with 3-Z-(1-methoxy-i-ethyl-methylene)-6-
carbamoyl-2-indolinone
Prepared by reacting the resin obtained according to Example I
with trimethyl orthopropionate
(4) resin charged with 3-Z-(1-methoxy-l-propyl-methylene)-6-
carbamoyl-2-indolinone
Prepared by reacting the product of Example I and trimethyl
orthobutyrate
CA 02387013 2002-03-19
- 74 -
F_xam= l e T T T
N- (4-ni _roz henyl) -N-methyl -m. _hanes 3lphonamide
3.0 g of N-methyl-4-nitroaniline are dissolved in 20 ml of
pyridine and 2.4 g of inethanesulphonic acid chloride added
dropwise at room temperature. The mixture is stirred for 12
hours at room temperature. After this time the mixture is
poured onto water, the precipitate formed is filtered off and
dried at 50 C in vacuo.
Yield: 4.0 g (87 t of theory),
Rf value: 0.5 (silica gel, ethyl acetate/toluene = 7:3)
Melting point: 107-108 C
E.xamz l e TV
N-( -di m hylamino-ethyl )-N-methy sulphonyl-4-ni roa i l i n_
38.9 g of N-methylsulphonyl-4-nitroaniline are dissolved in
2.0 1 of acetone, 51.9 g of 1-chloro-2-dimethylamino-ethane,
77.4 g of potassium carbonate and 5.0 g of sodium iodide are
added and the mixture is stirred for a total of 4 days at
50 C, while after 12 hours a further 25.9 g of 1-chloro-2-
dimethylamino-ethane, 49.8 g of potassium carbonate and 5.0 g
of sodium iodide in 500 ml of acetone are added and after 36
hours another 26.0 g of 1-chloro-2-dimethylamino-ethane, 50.0
g of potassium carbonate and 5.0 g of sodium iodide in 100 ml
of acetone are added. After this time the mixture is filtered
and the filtrate evaporated down. The residue is stirred with
ether, suction filtered and dried at 40 C.
Yield: 25.3 g (49 0 of theory),
Rf value: 0.5 (silica gel, methylene chloride/methanol/ammonia
= 9:1:0.1)
C11H17N30aS
ESI mass spectrum: m/z = 288 [M+H+]
CA 02387013 2002-03-19
- 75 -
The following compounds are prepared analogously to Example
IV:
(1) 4-[N-(3-dimethylamino-propyl)-N-methylsulphonyl-amino]-
nitrobenzene
(2) N-carboxymethyl-N-methylsulphonyl-4-nitroaniline
(3) N-cyanomethyl-N-methylsulphonyl-p-phenylenediamine
(4) 4- [N- (2- (N-benzyl-N-methyl-amino) -ethyl) -N-
methylsulphonyl-amino]-nitrobenzene
(5) 4-[N-(3-phthalimido-2-yl-propyl)-N-methylsulphonyl-amino]-
nitrobenzene
(6) 4 - [N- ( 3 - (N-benzyl -N-methyl -amino ) -propyl ) -N-
methylsulphonyl-amino]-nitrobenzene
F'.xami 1 eV
N-(dimethylaminocarbonyl-methyl)-N-methylsulphonyl-4-
nit-rnanilin
7.0 g of N-carboxymethyl-N-methylsulphonyl-4-nitroaniline, 2.5
g of dimethylamine hydrochloride, 8.1 g of TBTU and 3.9 g of
HOBT are dissolved in 125 ml of dimethylformamide and at 0 C
17.6 ml of N-ethyl-diisopropylamine are added. The mixture is
stirred for 4 hours at room temperature, diluted with 1 1 of
water and the precipitate formed is suction filtered. After
washing with water, ethanol and ether the residue is dried at
70 C in vacuo.
Yield: 5.3 g (69 % of theory),
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
CiiHisNa0sS
ESI mass spectrum: m/z = 300 [M-H-]
CA 02387013 2002-03-19
- 76 -
The following compounds are prepared analogously to Example V:
(1) 4-[(N-dimethylaminocarbonylmethyl)-amino]-nitrobenzene
prepared from 4-(N-carboxymethyl-amino)-nitrobenzene and
dimethylamine hydrochloride
(2) 4-(N-methylaminocarbonylmethyl-N-methylsulphonyl-amino)-
nitrobenzene
Prepared from N-carboxymethyl-N-methylsulphonyl-4-nitroaniline
and methylamine hydrochloride
(3) 4-[(N-(methylcarbamoyl-methyl)-N-methyl-amino)-methyl]-
nitrobenzene
Prepared from 4-[(N-carboxymethyl-N-methyl-amino)-methyl]-
nitrobenzene and methylamine hydrochloride
(4) 4-[(N-(dimethylcarbamoyl-methyl)-N-methyl-amino)-methyl]-
nitrobenzene
Prepared from 4-[(N-carboxymethyl-N-methyl-amino)-methyl]-
nitrobenzene and dimethylamine hydrochloride
Rxam= le V T
4- [N- (- im thyl amino-et-hyl) -N-ac-Pty1 - mi nol -ni rob n._ne
3.6 g of 4-(2-dimethylamino-ethylamino)-nitrobenzene
(according to Gabbay et al., J. Am. Chem. Soc. 91, 5136
(1969)) are dissolved in 50 ml of methylene chloride and 5.0
ml of triethylamine are added. 1.3 ml of acetyl chloride are
slowly added dropwise to this mixture at room'temperature and
the mixture is stirred for 2 hours at room temperature. After
this time another 5.0 ml of triethylamine and 1.3 ml of
acetylchloride are added and the mixture is refluxed for
another 2 hours. The solvent is removed, the residue is taken
up in ethyl acetate and the organic phase is extracted twice
CA 02387013 2002-03-19
- 77 -
with water. After drying over MgSO4 the solvent is removed and
the residue dried in vacuo.
Yield: 2.0 g (45 's of theory),
Rf value: 0.55 (silica gel, methylene
chloride/methanol/ammonia = 9:1:0.1)
Ci2Hi7N303
ESI mass spectrum: m/z = 252 [M+H`]
The following compounds are prepared analogously to Example
VI:
(1) 4- [N- ( 3 -dimethylamino-propyl ) -N-acetyl -amino] -nitrobenzene
Prepared from 4-(3-dimethylamino-propylamino)-nitrobenzene
(according to Gabbay et al., J. Am. Chem. Soc. 21õ 5136 (1969)
and acetyl chloride
(2) 4-[N-(2-dimethylamino-ethyl)-N-propionyl-amino]-
nitrobenzene
Prepared from 4-(2-dimethylamino-ethylamino)-nitrobenzene and
propionyl chloride
(3) 4-[N-acetyl-N-(dimethylaminocarbonylmethyl)-amino]-
nitrobenzene
Prepared from 4-[N-(dimethylaminocarbonylmethyl)-amino]-
nitrobenzene and acetyl chloride
(4) 4-[N-(2-dimethylamino-ethyl)-N-butyryl-amino]-nitrobenzene
Prepared from 4-(2-dimethylamirno-ethylamino)-nitrobenzene and
butyryl chloride
(5) 4-[N-(2-dimethylamino-ethyl)-N-isobutyryl-amino]-
nitrobenzene
Prepared from 4-(2-dimethylamino-ethylamino)-nitrobenzene and
isobutyryl chloride
CA 02387013 2002-03-19
- 78 -
(6) 4-[N-(2-dimethylamino-ethyl)-N-benzoyl-amino]-nitrobenzene
Prepared from 4-(2-dimethylamino-ethylamino)-nitrobenzene and
benzoyl chloride
(7) 4-[N-(2-dimethylamino-ethyl)-N-acetyl-amino]-1,3-
dinitrobenzene
Prepared from 4-(2-dimethylamino-ethyl-amino)-1,3-
dinitrobenzene and acetyl chloride
(8) 4- [N- (2-dimethylamino-ethyl) -N- (furan-2-carbonyl) -amino] -
nitrobenzene
Prepared from 4-(2-dimethylamino-ethylamino)-nitrobenzene and
furan-2-carbonyl chloride
(9) 4-[N-(2-dimethylamino-ethyl)-N-(2-methoxy-benzoyl)-amino]-
nitrobenzene
Prepared from 4-(2-dimethylamino-ethylamino)-nitrobenzene and
2-methoxy-benzoyl chloride
(10) 4-[N-(2-dimethylamino-ethyl)-N-(pyridine-3-carbonyl)-
amino]-nitrobenzene
Prepared from 4-(2-dimethylamino-ethylamino)-nitrobenzene and
nicotinic acid chloride
(11) 4- [N- (2-dimethylamino-ethyl) -N- (phenyl-acetyl) -amino] -
nitrobenzene
Prepared from 4-(2-dimethylamino-ethylamino)-nitrobenzene and
phenylacetyl-chloride
(12) 4-[N-(2-dimethylamino-ethyl)-N-acetyl-amino]-3-bromo-
nitrobenzene
Prepared from 4-[N-(2-dimethylamino-ethyl)-amino]-3-bromo-
nitrobenzene and acetyl chloride
(13) N-acryloyl-N-methyl-4-nitro-aniline
CA 02387013 2002-03-19
- 79 -
Prepared from 4-methylamino-nitrobenzene and acrylic acid
chloride
(14) N-acryloyl-N-isopropyl-4-nitro-aniline
Prepared from 4-isopropylamino-nitrobenzene and acrylic acid
chloride
(15) N-acryloyl-N-benzyl-4-nitro-aniline
Prepared from 4-benzylamino-nitrobenzene and acrylic acid
chloride
(16) N-bromoacetyl-N-methyl-4-nitro-aniline
Prepared from 4-methylamino-nitrobenzene and bromoacetyl
chloride
(17) N-bromoacetyl-N-isopropyl-4-nitro-aniline
Prepared from 4-isopropylamino-nitrobenzene and bromoacetyl
chloride
(18) N-bromoacetyl-N-benzyl-4-nitro-aniline
Prepared from 4-benzylamino-nitrobenzene and bromoacetyl
chloride
Examp1e VTT
r7- (i m thylaminomet-hyl rarhonyl) -N-met-.hyl -4-ni _ro-ani 1 i ne
1.8 g of dimethylamine hydrochloride and 5.5 g of potassium
carbonate are placed in 80 ml of acetone and 4.2 g of N-
bromoacetyl-N-methyl-4-nitroaniline are added in three batches
at room temperature. The mixture is stirred for 12 hours at
room temperature. After this time the mixture is filtered and
the filtrate is evaporated down. The residue is dissolved in
ethyl acetate, washed twice with water, dried over sodium
sulphate and finally concentrated by rotary evaporation.
Yield: 2.8 g (79 % of theory),
Rf value: 0.5 (silica gel, ethyl acetate/methanol = 7:3)
CA 02387013 2002-03-19
- 80 -
Melting point: 121-122 C
The following compounds are prepared analogously to Example
VII:
(1) N-(piperidin-1-yl-methylcarbonyl)-N-methyl-4-nitroaniline
(2) N-(morpholin-4-yl-methylcarbonyl)-N-methyl-4-nitroaniline
(3) N-[(4-benzyl-piperazin-1-yl)-methylcarbonyl]-N-methyl-4-
nitroaniline
.(4) N-(pyrrolidin-1-yl-methylcarbonyl)-N-methyl-4-nitroaniline
(5) N-[(N-aminocarbonylmethyl-N-methyl-amino)-methylcarbonyl]-
N-methyl-4-nitroaniline
(6) N-[(N-benzyl-N-methyl-amino)-methylcarbonyl]-N-methyl-4-
nitroaniline
(7) N-[di-(2-methoxyethyl)-amino-methylcarbonyl]-N-methyl-4-
nitroaniline
(8) N-(dimethylaminomethylcarbonyl)-N-isopropyl-4-nitro-
aniline
(9) N-(piperidin-1-yl-methylcarbonyl)-N-isopropyl-4-nitro-
aniline
(10) N-[(4-tert.butoxycarbonyl-piperazin-1-yl)-
methylcarbonyl]-N-isopropyl-4-nitro-aniline
(11) N-[(N-benzyl-N-methyl-amino)-methylcarbonyl]-N-benzyl-4-
nitro-aniline
(12) N-(dimethylaminomethylcarbonyl)-N-benzyl-4-nitro-aniline
CA 02387013 2002-03-19
- 81 -
(13) N-(piperidin-1-yl-methylcarbonyl)-N-benzyl-4-nitro-
aniline
(14) N-[di-(2-hydroxyethyl)-amino-methylcarbonyl]-N-methyl-4-
nitroaniline
(15) N-[(N-(2-methoxyethyl)-N-methyl-amino)-methylcarbonyl]-N-
methyl-4-nitroaniline
(16) N-[(N-(2-dimethylamino-ethyl)-N-methyl-amino)-
methylcarbonyl]-N-methyl-4-nitroaniline
(17) N-[(4-methyl-piperazin-1-yl)-methylcarbonyl]-N-methyl-4-
nitroaniline
(18) N-[(imidazol-l-yl)-methylcarbonyl]-N-methyl-4-
nitroaniline
(19) N-[(phthalimido-2-yl)-methylcarbonyl]-N-methyl-4-
nitroaniline
Fxamsla VITT
AT- r ( -di m hy] aminn- . hyl) -rar.banyl 1-N-benzyl -4-ni ro- n i l i n.
0.5 g of dimethylamine hydrochloride, 1.1 ml of triethylamine
and 1.2 g of N-acryloyl-N-benzyl-4-nitro-aniline are dissolved
in 50 ml of methanol and stirred for 24 hours at room
temperature. After this time the mixture is evaporated down.
The residue is purified over an aluminium oxide column
(activity 2-3) with methylene chloride/ethanol 50:1 as eluant.
Yield: 1.4 g (98 % of theory),
Rf value: 0.8 (aluminium oxide, methylene chloride/ethanol =
20:1)
Melting point: 73 C
CA 02387013 2002-03-19
- 82 -
The following compounds are prepared analogously to Example
VIII:
(1) N-[(2-dimethylamino-ethyl)-carbonyl]-N-isopropyl-4-nitro-
aniline
Prepared from N-acryloyl-N-isopropyl-4-nitro-aniline and
dimethylamine hydrochloride
(2) N-[(2-dimethylamino-ethyl)-carbonyl]-N-methyl-4-nitro-
aniline
Prepared from N-acryloyl-N-methyl-4-nitro-aniline and
dimethylamine hydrochloride
(3) N-[(2-(4-tert.butoxycarbonyl-piperazin-1-yl)-ethyl)-
carbonyl]-N-methyl-4-nitro-aniline
Prepared from N-acryloyl-N-methyl-4-nitro-aniline and
N-tert.butoxycarbonyl-piperazine
(4) N-[(2-(piperidin-l-yl)-ethyl)-carbonyl]-N-methyl-4-nitro-
aniline
Prepared from N-acryloyl-N-methyl-4-nitro-aniline and
piperidine
(5) N-[(2-(N-benzyl-N-methyl-amino)-ethyl)-carbonyl]-N-methyl-
4-nitro-aniline
Prepared from N-acryloyl-N-methyl-4-nitro-aniline and
N-benzyl-N-methyl-amine
Fxam=le TX
4(4 methyl ~i_p razine ~-yl )-n; rc~benzene
31.5 g of 4-chloro-i-nitrobenzene and 44.4 ml of 1-
methylpiperazine are combined and stirred for 18 hours at
90 C. Then the solution is poured onto ice water and the
precipitate formed is suction filtered, washed with water and
CA 02387013 2002-03-19
- 83 -
recrystallised from ethanol/water 1:1. The residue is dried in
vacuo at 75 C.
Yield: 44.0 g (99 % of theory),
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
Melting point: 108-112 C
The following compounds are prepared analogously to Example
IX:
(1) N-(2-dimethylaminoethyl)-N-methyl-4-nitroaniline
Prepared from 1-fluoro-4-nitrobenzene and 1-dimethylamino-2-
methylamino-ethane
(2) N-(3-dimethylaminopropyl)-N-methyl-4-nitroaniline
Prepared from 1-fluoro-4-nitrobenzene and 1-dimethylamino-3-
methylamino-propane
(3) 4-(N-carboxymethyl-amino)-nitrobenzene
Prepared from 1-fluoro-4-nitrobenzene and glycine
(4) N-cyclohexyl-p-phenylenediamine
Prepared from 1-fluoro-4-nitrobenzene and cyclohexylamine
(5) 6-[N-(2-dimethylamino-ethyl)-N-methylsulphonyl-amino]-3-
phthalimido-2-yl-nitrobenzene
Prepared from 2-nitro-4-phthalimido-2-yl-fluorobenzene,
N-(2-dimethylamino-ethyl)-methanesulphonamide and sodium
hydride as base
(6) 6-[N-(2-dimethylamino-ethyl)-N-methylsulphonyl-amino]-1,3-
dinitrobenzene
Prepared from 2,4-dinitro-chlorobenzene, N-(2-dimethylamino-
ethyl)-methanesulphonamide and sodium hydride as base
CA 02387013 2002-03-19
- 84 -
(7) 4-[N-(2-dimethylamino-ethyl)-N-methylsulphonyl-amino]-3-
chloro-nitrobenzene
Prepared from 2-fluoro-5-nitro-chlorobenzene, N-(2-dimethyl-
amino-ethyl)-methanesulphonamide and sodium hydride as base
(8) 4-(2-dimethylamino-ethyl-amino)-1,3-dinitrobenzene
Prepared from 1-chloro-2,4-dinitro-benzene and N,N-dimethyl-
ethylenediamine
(9) 4- [N- (2-dimethylamino-ethyl) -N- (ethylsulphonyl) -amino] -
nitrobenzene
Prepared from 1-fluoro-4-nitro-benzene, N-(2-dimethylamino-
ethyl)-ethanesulphonamide and sodium hydride as base
(10) 4-[N-(2-dimethylamino-ethyl)-N-(propylsulphonyl)-amino]-
nitrobenzene
Prepared from 1-fluoro-4-nitro-benzene, N-(2-dimethylamino-
ethyl)-propanesulphonamide and sodium hydride as base
(11) 4-[N-(2-dimethylamino-ethyl)-N-(butylsulphonyl)-amino]-
nitrobenzene
Prepared from 1-fluoro-4-nitro-benzene, N-(2-dimethylamino-
ethyl)-butanesulphonamide and sodium hydride as base
(12) 4- [N- (2-dimethylamino-ethyl) -N- (benzylsulphonyl) -amino] -
nitrobenzene
Prepared from 1-fluoro-4-nitro-benzene, N-(2-dimethylamino-
ethyl)-C-phenylmethanesulphonamide and sodium hydride as base
(13) 4- [N- (2-dimethylamino-ethyl) -N- (phenylsulphonyl) -arnino] -
nitrobenzene
Prepared from 1-fluoro-4-nitro-benzene, N-(2-dimethylamino-
ethyl)-benzenesulphonamide and sodium hydride as base
(14) 4-[N-(2-dimethylamino-ethyl)-N-(isopropylsulphonyl)-
amino]-nitrobenzene
CA 02387013 2002-03-19
- 85 -
Prepared from 1-fluoro-4-nitro-benzene, N-(2-dimethylamino-
ethyl)-isopropylsulphonamide and sodium hydride as base
(15) 4-[N-(2-dimethylamino-ethyl)-amino]-3-bromo-nitrobenzene
Prepared from 2-bromo-l-fluoro-4-nitro-benzene and N,N-
dimethyl-ethylenediamine
(16) 4-isopropylamino-nitrobenzene
Prepared from 1-fluoro-4-nitrobenzene and isopropylamine
(17) 4-benzylamino-nitrobenzene
Prepared from 1-fluoro-4-nitrobenzene and benzylamine
Fxami l_e X
4-(imidazol-4-yl)-ni_robenzene
9.5 g of 2-phenylimidazole are carefully dissolved in 50 ml of
concentrated sulphuric acid and 5.8 g of ammonium nitrate are
added to this solution at 0 C. After a further 60 minutes
stirring at 0 C the mixture is poured onto ice water, made
basic with ammonia water and the precipitate formed is suction
filtered and recrystallised from ethanol.
Yield: 8.0 g (64 s of theory),
Rf value: 0.6 (silica gel, ethyl acetate/ethanol = 10:1)
C9H7N302
Mass spectrum: m/z = 189 [M+]
The following compounds are prepared analogously to Example X:
(1) 4-(imidazol-2-yl)-nitrobenzene
Prepared from 4-(imidazol-2-yl)-benzene
(2) 4-(5-methyl-imidazol-4-yl)-nitrobenzene
Prepared from 4-methyl-5-phenyl-imidazole (J. Heterocycl.
Chem. 1983, 20, 1277-1281)
CA 02387013 2002-03-19
- 86 -
Fxa n? f- X T
4- (2- (imi(jazol-4-y1 ) -ethylene) -ni _roh _n . _n .
1.5 g of 4-nitrobenzaldehyde and 7.45 g of (N-trityl-imidazol-
4-yl-methyl)-triphenylphosphonium chloride are dissolved in 75
ml of tetrahydrofuran and to this solution 3.0 ml of DBU are
added dropwise at room temperature. After a further 120
minutes stirring at room temperature the mixture is poured
onto water and the precipitate formed is suction filtered. The
product is taken up in 25 ml of iN hydrochloric acid and
refluxed for 4 hours. After this time it is neutralised with
ammoniacal water, extracted with ethyl acetate and the organic
phase is washed with water, dried over sodium sulphate and
evaporated down. The residue is purified over a silica gel
column with methylene chloride/methanol 10:1 as eluant.
Yield: 1.0 g of (47 s of theory),
Rf value: 0.6 (silica gel, ethyl acetate/ethanol = 10:1)
Melting point: 185-188 C
F=xatn= 1 e XT T
4- (lai = eridin-1 -yl-methyl) -ni troh _n .ene
40.0 g of 4-nitrobenzyl bromide are dissolved in 500 ml of
methylene chloride, 51.5 ml of triethylamine are added and
18.3 ml of piperidine are carefully added dropwise. After the
end of the exothermic reaction the mixture is refluxed for
another 30 minutes. After cooling it is washed with water and
the organic phase is dried over sodium sulphate. Finally, the
organic phase is evaporated down.
Yield: 36.3 g of (89 % of theory),
Rf value: 0.6 (silica gel, methylene chloride/methanol = 9:1)
Ci2Hi6Nz0a
Mass spectrum: m/z = 221 [M+)
CA 02387013 2002-03-19
- 87 -
The following compounds are prepared analogously to Example
XII:
(1) 4-[(2,6-dimethyl-piperidin-l-yl)-methyl]-nitrobenzene
(2) 3-(N,N-dimethyl-aminomethyl)-nitrobenzene
(3) 4-(N,N-dimethyl-aminomethyl)-nitrobenzene
(4) 4-(2-dimethylamino-ethyl)-nitrobenzene
(5) 4-(2-diethylamino-ethyl)-nitrobenzene
(6) 4-(diethylamino-methyl)-nitrobenzene
(7) 4-(N-benzyl-N-methyl-aminomethyl)-nitrobenzene
(8) 4-(N-ethyl-N-methyl-aminomethyl)-nitrobenzene
(9) 4-[N-(n-hexyl)-N-methyl-aminomethyl]-nitrobenzene
(10) 4-(thiomorpholin-4-yl-methyl)-nitrobenzene
(11) 4-[(4-methyl-piperazine-1-yl)-methyl]-nitrobenzene
(12) 4-(imidazol-1-yl-methyl)-nitrobenzene
(13) 4-[2-(4-hydroxy-piperidin-1-yl)-ethyl-amino]-nitrobenzene
(14) 4-[(3-hydroxy-pyrrolidin-l-yl)-methyl]-nitrobenzene
(15) 4-(1,2,4-triazol-l-yl-methyl)-nitrobenzene
(16) 4-(1,2,3-triazol-2-yl-methyl)-nitrobenzene
(17) 4-(1,2,3-triazol-l-yl-methyl)-nitrobenzene
CA 02387013 2002-03-19
- 88 -
(18) 4-[(N-ethoxycarbonylmethyl-N-methyl-amino)-methyl]-
nitrobenzene
(19) 4-[(N-aminocarbonylmethyl-N-methyl-amino)-methyl]-
nitrobenzene
(20) 4-(azetidin-1-yl-methyl)-nitrobenzene
(21) 4-[(di-(2-methoxy-ethyl)-amino)-methyl]-nitrobenzene
(22) 4-[N-(N-tert.butoxycarbonyl-3-amino-propyl)-N-methyl-
aminomethyl]-nitrobenzene
(23) 4-[(N-propyl-N-methyl-amino)-methyl]-nitrobenzene
(24) 4-[(N-(2-dimethylamino-ethyl)-N-methyl-amino)-methyl]-
nitrobenzene
(25) 4- [ (N- (3-dimethylamino-propyl) -N-methyl-amino) -methyl] -
nitrobenzene
(26) 4-[(N-(2-methoxy-ethyl)-N-methyl-amino)-methyl]-
nitrobenzene
(27) 4-[(N-(2-hydroxy-ethyl)-N-methyl-amino)-methyl]-
nitrobenzene
(28) 4-[(N-(dioxolan-2-yl-methyl)-N-methyl-amino)-methyl]-
nitrobenzene
(29) 4-(3-oxo-piperazine-1-yl-methyl)-nitrobenzene
CA 02387013 2002-03-19
- 89 -
F.xa le XTTI
4- f (N-carboxymethyl-N-methyl -amino) -methyll -ni trob .nzene
7.33 g of 4-[(N-ethoxycarbonylmethyl-N-methyl-amino)-methyl]-
nitrobenzene are dissolved in 140 ml of ethanol, 34.0 ml of iN
sodium hydroxide solution are added and the mixture is stirred
for half an hour at room temperature. After this time the
mixture is neutralised with 34 ml of 1N hydrochloric acid, the
solvent removed, the residue taken up in methylene chloride
and extracted with water. The aqueous phase is evaporated
down and the residue is recrystallised from methylene
chloride.
Yield: 5.43 g (84 % of theory),
Rf value: 0.4 (silica gel, methylene chloride/methanol = 2:1)
CioHiaN20a
Mass spectrum: m/z = 223 [M+]
Fxamp1 e XTV
4- ( T-1~ e _lhyl -aminomethyl) -ni _robenzene
6.0 g of 4-nitrobenzyl bromide are dissolved in 25 ml of
ethanol, combined with 25 ml of 10% ethanolic ethylamine
solution and refluxed for 2 hours. Then the solution is
concentrated by rotary evaporation, the residue is taken up
with methylene chloride and washed with dilute sodium
hydroxide solution. Finally the organic phase is evaporated
down.
Yield: 2.3 g (46 % of theory),
Rf value: 0.2 (silica gel, methylene chloride/methanol = 9:1)
C9H12N202
ESI mass spectrum: m/z = 179 [M-H-]
The following compounds are prepared analogously to Example
XIV:
(1) 4-[N-(4-chlorobenzyl)-aminomethyl]-nitrobenzene
CA 02387013 2002-03-19
90 -
(2) 4-(N-cyclohexyl-aminomethyl)-nitrobenzene
(3) 4-(N-isopropyl-aminomethyl)-nitrobenzene
(4) 4-(N-propyl-aminomethyl)-nitrobenzene
(5) 4-(N-methyl-aminomethyl)-nitrobenzene
(6) 4-(N-butyl-aminomethyl)-nitrobenzene
(7) 4-(N-methoxycarbonylmethyl-aminomethyl)-nitrobenzene
(8) 4-(N-benzyl-aminomethyl)-nitrobenzene
(9) 4-(aminomethyl)-nitrobenzene
(10) 4-(pyrrolidin-1-yl-methyl)-nitrobenzene
(11) 4-(morpholin-4-yl-methyl)-nitrobenzene
(12) 4-(hexamethyleneiminomethyl)-nitrobenzene
(13) 4-(4-hydroxy-piperidin-1-yl-methyl)-nitrobenzene
(14) 4-(4-methoxy-piperidin-1-yl-methyl)-nitrobenzene
(15) 4-(4-methyl-piperidin-1-yl-methyl)-nitrobenzene
(16) 4-(4-ethyl-piperidin-1-yl-methyl)-nitrobenzene
(17) 4-(4-isopropyl-piperidin-1-yl-methyl)-nitrobenzene
(18) 4-(4-phenyl-piperidin-1-yl-methyl)-nitrobenzene
(19) 4-(4-benzyl-piperidin-1-yl-methyl)-nitrobenzene
CA 02387013 2002-03-19
- 91 -
(20) 4-(4-ethoxycarbonyl-piperidin-1-yl-methyl)-nitrobenzene
(21) 4-(N,N-dipropyl-aminomethyl)-nitrobenzene
(22) 4-(4-tert.butoxycarbonyl-piperazin-1-yl-methyl)-
nitrobenzene
(23) 4-(2-morpholin-4-yl-ethyl)-nitrobenzene
(24) 4-(2-pyrrolidin-1-yl-ethyl)-nitrobenzene
(25) 4-(2-piperidin-1-yl-ethyl)-nitrobenzene
(26) 4-(N-ethyl-N-benzyl-aminomethyl)-nitrobenzene
(27) 4-(N-propyl-N-benzyl-aminomethyl)-nitrobenzene
(28) 4-[N-methyl-N-(4-chlorobenzyl)-aminomethyl]-nitrobenzene
(29) 4-[N-methyl-N-(4-bromobenzyl)-aminomethyl]-nitrobenzene
(30) 4- [N-methyl -N- ( 4 - f luorobenzyl ) -aminomethyl ] -nitrobenzene
(31) 4-[N-methyl-N-(4-methylbenzyl)-aminomethyl]-nitrobenzene
(32) 4-[N-methyl-N-(3-chlorobenzyl)-aminomethyl]-nitrobenzene
(33) 4- [N-methyl -N- ( 3 , 4 -dimethoxybenzyl ) -aminomethyl ] -
nitrobenzene
(34) 4-[N-methyl-N-(4-methoxybenzyl)-aminomethyl]-nitrobenzene
(35) 4-(N-2,2,2-trifluoroethyl-N-benzyl-aminomethyl)-
nitrobenzene
CA 02387013 2002-03-19
- 92 -
(36) 4-[N-2,2,2-trifluoroethyl-N-(4-chlorobenzyl)-
aminomethyl]-nitrobenzene
(37) 4-(thiomorpholin-4-yl-methyl)-nitrobenzene
(38) 4-(azetidion-1-yl-methyl)-nitrobenzene
(39) 4-(3,4-dihydropyrrolidin-1-yl-methyl)-nitrobenzene
(40) 4-(3,4-dihydropiperidin-1-yl-methyl)-nitrobenzene
(41) 4-(2-methoxycarbonyl-pyrrolidin-l-yl-methyl)-nitrobenzene
(42) 4-(3,5-dimethyl-piperidin-1-yl-methyl)-nitrobenzene
(43) 4-(4-phenyl-piperazin-1-yl-methyl)-nitrobenzene
(44) 4-(4-phenyl-4-hydroxy-piperidin-1-yl-methyl)-nitrobenzene
(45) 4-[N-(3,4,5-trimethoxybenzyl-N-methyl-aminomethyl)-
nitrobenzene
(46) 4-[N-(3,4-dimethoxybenzyl)-N-ethyl-aminomethyl]-
nitrobenzene
(47) 4- [N- ( 2 , 6 -dichlorobenzyl ) -N-methyl ) -aminomethyl ] -
nitrobenzene
(48) 4- [N- (4-trifluoromethylbenzyl) -N-methyl) -aminomethyl] -
nitrobenzene
(49) 4-(N-benzyl-N-isopropyl-aminomethyl)-nitrobenzene
(50) 4-(N-benzyl-N-tert.butyl-aminomethyl)-nitrobenzene
(51) 4-(N,N-diisopropyl-aminomethyl)-nitrobenzene
CA 02387013 2002-03-19
- 93 -
(52) 4-(N,N-diisobutyl-aminomethyl)-nitrobenzene
(53) 4-(2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-methyl)-
nitrobenzene
(54) 4-(2,3-dihydro-isoindol-2-yl-methyl)-nitrobenzene
(55) 4-(6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-nitrobenzene
(56) 4-(1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
nitrobenzene
(57) 4-[N-(2-hydroxyethyl)-N-benzyl-aminomethyl]-nitrobenzene
(58) 4-[N-(1-ethyl-pentyl)-N-(pyridin-2-yl-methyl)-
aminomethyl]-nitrobenzene
(59) 4-(piperin-1-yl-methyl)-1,3-dinitrobenzene
(60) 4-(N-phenethyl-N-methyl-aminomethyl)-nitrobenzene
(61) 4-[N-(3,4-dihydroxy-phenethyl)-N-methyl-aminomethyl]-
nitrobenzene
(62) 4-[N-(3,4,5-trimethoxy-phenethyl)-N-methyl-aminomethyl]-
nitrobenzene
(63) 4-[N-(3,4-dimethoxy-phenethyl)-N-methyl-aminomethyl]-
nitrobenzene
(64) 4-[N-(3,4-dimethoxy-benzyl)-N-methyl-aminomethyl]-
nitrobenzene
(65) 4-[N-(4-chloro-benzyl)-N-methyl-aminomethyl]-nitrobenzene
CA 02387013 2002-03-19
- 94 -
(66) 4-[N-(4-bromo-benzyl)-N-methyl-aminomethyl]-nitrobenzene
(67) 4-[N-(4-fluoro-benzyl)-N-methyl-aminomethyl]-nitrobenzene
(68) 4-[N-(4-methyl-benzyl)-N-methyl-aminomethyl]-nitrobenzene
(69) 4-[N-(4-nitro-phenethyl)-N-methyl-aminomethyl]-
nitrobenzene
(70) 4-(N-phenethyl-N-benzyl-aminomethyl)-nitrobenzene
(71) 4-(N-phenethyl-N-cyclohexyl-aminomethyl)-nitrobenzene
(72) 4-[N-(2-(pyridin-2-yl)-ethyl)-N-methyl-aminomethyl]-
nitrobenzene
(73) 4-[N-(2-(pyridin-4-yl)-ethyl)-N-methyl-aminomethyl]-
nitrobenzene
(74) 4-[N-(pyridin-4-yl-methyl)-N-methyl-aminomethyl]-
nitrobenzene
(75) 4-(N,N-dibenzyl-aminomethyl)-nitrobenzene
(76) 4- [N- (4-nitro-phenethyl) -N-propyl-aminomethyl] -
nitrobenzene
(77) 4-(N-benzyl-N-(3-cyano-propyl)-aminomethyl)-nitrobenzene
(78) 4-(N-benzyl-N-allyl-aminomethyl)-nitrobenzene
(79) 4- [N-benzyl-N- (2, 2, 2-trifluoroethyl) -aminomethyl] -
nitrobenzene
CA 02387013 2002-03-19
- 95 -
(80) 4-LN-(2-benzo(1,3)dioxol-5-yl-methyl)-N-methyl-
aminomethyl]-nitrobenzene
(81) 4-(7-chloro-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-nitrobenzene
(82) 4-(7,8-dichloro-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-nitrobenzene
(83) 4-(7-methoxy-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-nitrobenzene
(84) 4-(7-methyl-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-nitrobenzene
(85) 4-(7,8-dimethoxy-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-nitrobenzene
(86) 4-(6,7-dichloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-nitrobenzene
(87) 4-(6,7-dimethyl-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-nitrobenzene
(88) 4-(6-chloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
nitrobenzene
(89) 4-(7-chloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
nitrobenzene
(90) 4-(6-methoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
nitrobenzene
(91) 4-(7-methoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
nitrobenzene
CA 02387013 2002-03-19
- 96 -
(92) 4-[(2,3,4,5-tetrahydro-azepino(4,5-b)pyrazin-3-yl)-
methyl]-nitrobenzene
(93) 4-[(7-amino-2,3,4,5-tetrahydro-azepino(4,5-b)pyrazin-3-
yl)-methyl]-nitrobenzene
(94) 4-[(2-amino-5,6,7,8-tetrahydro-azepino(4,5-d)thiazol-6-
yl)-methylJ-nitrobenzene
(95) 4-[(5,6,7,8-tetrahydro-azepino(4,5-d)thiazol-6-yl)-
methyll-nitrobenzene
F'xamx 1 p XV
4- (1,i-di oxo-thi omor= holin-4-yl-methyl )-ni _rob .n ._ne
6.0 g of 4-(thiomorpholin-4-yl-methyl)-nitrobenzene are
dissolved in 100 ml of methylene chloride and 10.3 g of meta-
chloroperbenzoic acid are slowly added. After a further 3
hours stirring at room temperature the precipitate obtained is
filtered off.
Yield: 6.2 g (91 % of theory)
Rf value: 0.5 (silica gel, methylene chloride/methanol = 1:1)
C11H14N204S
Mass spectrum: m/z = 270 [M+]
The following compound is prepared analogously to Example XV:
(1) 4-(1-oxo-thiomorpholin-4-yl-methyl)-nitrobenzene
F.xampl P XVI
4 [N ('I-ami no-z _ropyl ) -N-methy1 qulnhon,yl -ami no1 -nitrobenzene
9.5 g of 4-[N-(3-phthalimido-2-yl-propyl)-N-methylsulphonyl-
amino]-nitrobenzene are dissolved in 200 ml of ethanol, 11.5
ml of hydrazine hydrate are added and the mixture is stirred
for 1.5 hours at 50 C. After cooling the residue is largely
CA 02387013 2002-03-19
- 97 -
evaporated down, water is added and the solution is extracted
with methylene chloride. The organic phase is dried,
evaporated down and purified over a silica gel column with
methylene chloride/methanol/ammonia 9:1:0.1.
Yield: 2.5 g (39 % of theory)
Rf value: 0.2 (silica gel, methylene chloride/methanol = 9:1)
CioHisN304S
ESI mass spectrum: m/z = 272 [M-H-]
The following compound is prepared analogously to Example XVI:
(1) 6-[N-(2-dimethylamino-ethyl)-N-methylsulphonyl-amino]-3-
amino-nitrobenzene
Prepared from 6-[N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino]-3-phthalimido-2-yl-nitrobenzene
RxamnlP XVTT
4-(1 -mPthyl-imidazol-2-yl)-ni_trnhP_n!7.enP
7.5 g of 4-(imidazol-2-yl)-nitrobenzene are dissolved in 50 ml
of dimethylsulphoxide and at 0 C 5.0 g of potassium
tert.butoxide are added. After one hour of stirring at room
temperature 2.6 ml of methyl iodide are added dropwise and the
mixture is stirred for one hour at room temperature. After
this time the residue is poured onto ice water and the
precipitate formed is suction filtered, washed with water and
dried.
Yield: 6.1 g (76 % of theory)
Rf value: 0.6 (silica gel, methylene chloride/methanol = 10:1)
Melting point: 186-187 C
CA 02387013 2002-03-19
- 98 -
The following compounds are prepared analogously to Example
XVII:
(1) 4-(1-ethyl-imidazol-2-yl)-nitrobenzene
Prepared from 4-(imidazol-2-yl)-nitrobenzene and ethyl iodide
(2) 4-(i-benzyl-imidazol-2-yl)-nitrobenzene
Prepared from 4-(imidazol-2-yl)-nitrobenzene and benzyl
bromide
Fxams 1 _ XVTTT
4- [ (N- (2- (2-methoxy-ethoxy) -ethyl) -N-methyl-amino) -methyl] -
nitrob nzene
5.0 g of 4-methylaminomethyl-nitrobenzene are dissolved in 30
ml of dimethylformamide and 4.6 g of 2-(2-methoxy-ethoxy)-
ethyl chloride are added. After six hours' stirring at 100 C
the solvent is removed and the residue is taken up in ethyl
acetate. The organic phase is washed with water and dried
over sodium sulphate. After the elimination of the solvent the
residue is purified over an aluminium oxide column (activity
2-3) with toluene/ethyl acetate 5:1 as eluant.
Yield: 2.3 g (29 % of theory)
Rf value: 0.5 (aluminium oxide, toluene/ethyl acetate = 5:1)
Ci3H2oNz0a
ESI mass spectrum: m/z = 267 [M-H"]
Fxa=1 p XLX
4- (N-~thyl-N- r butoxycarhonyl -aminomethyl) - i.rob .n . _n _
2.2 g of 4-(ethylaminomethyl)-nitrobenzene are dissolved in 50
ml of ethyl acetate and stirred with 2.6 g of di-tert-butyl.
dicarbonate (tert.butoxycarbonyl-anhydride) for 30 minutes at
room temperature. Then the solution is washed with water and
evaporated down.
Yield: 3.4 g of theory
CA 02387013 2002-03-19
- 99 -
Rf value: 0.3 (silica gel, methylene chloride/methanol =
50:1)
Melting point: 85 C
The following compounds are prepared analogously to Example
XIX:
(1) 4-[N-(4-chlorophenyl-methyl)-N-tert.butoxycarbonyl-
aminomethyl]-nitrobenzene
(2) 4-(N-tert.butoxycarbonyl-aminomethyl)-nitrobenzene
(3) 4-(N-cyclohexyl-N-tert.butoxycarbonyl-aminomethyl)-
nitrobenzene
(4) 4-(N-isopropyl-N-tert.butoxycarbonyl-aminomethyl)-
nitrobenzene
(5) 4-(N-methyl-N-tert.butoxycarbonyl-aminomethyl)-
nitrobenzene
(6) 4-(N-propyl-N-tert.butoxycarbonyl-aminomethyl)-
nitrobenzene
(7) 4-(N-butyl-N-tert.butoxycarbonyl-aminomethyl)-nitrobenzene
(8) 4-(N-methoxycarbonylmethyl-N-tert.butoxycarbonyl-
aminomethyl)-nitrobenzene
(9) 4-(N-benzyl-N-tert.butoxycarbonyl-aminomethyl)-
nitrobenzene
CA 02387013 2002-03-19
- 100 -
(10) 4-[N-(3-trifluoroacetylamino-propyl)-N-rnethylsulphonyl-
amino]-nitrobenzene
Prepared from 4-[N-(3-amino-propyl)-N-methylsulphonyl-amino]-
nitrobenzene and trifluoroacetic acid anhydride
(11) 4-[(4-tert.butoxycarbonyl-piperazin-l-yl)-methyl]-
nitrobenzene
F.xam= l e XX
4- (jain _ridin-l-yl -mPtt.hyl ) -ani l i ne
37.0 g of 4-(piperidin-l-yl-methyl)-nitrobenzene are dissolved
in 300 ml of methanol, 8.0 g of Raney nickel are added and the
mixture is hydrogenated for 85 minutes with 3 bars of hydrogen
at room temperature. The catalyst is filtered off and the
filtrate is evaporated down.
Yield: 24.0 g (75 % of theory),
Rf value: 0.4 (silica gel, methylene chloride/methanol = 9:1)
Ci2Hi8Nz
ESI mass spectrum: m/z = 191 [M+H+]
The following compounds are prepared analogously to Example
VIII:
(1) 4-[(2,6-dimethyl-piperidin-1-yl)-methyl]-aniline
(2) N-(2-dimethylamino-ethyl)-N-methylsulphonyl-p-
phenylenediamine
(3) 3-(dimethylaminomethyl)-aniline
(4) 4-(dimethylaminomethyl)-aniline
(5) 4-(2-dimethylamino-ethyl)-aniline
(6) 4-[N-(2-dimethylamino-ethyl)-N-acetyl-amino]-aniline
CA 02387013 2002-03-19
- 101 -
(7) 4-[N-(3-dimethylamino-propyl)-N-acetyl-amino]-aniline
(8) 4- [N- (2-dimethylamino-ethyl) -N-benzoyl-amino] -aniline
(9) 4-[N-(2-dimethylamino-ethyl)-N-propionyl-amino]-aniline
(10) 4-[N-(2-dimethylamino-ethyl)-N-butyryl-amino]-aniline
(11) 4-[N-(2-dimethylamino-ethyl)-N-isobutyryl-amino]-aniline
(12) 4-(N-tert.butoxycarbonyl-aminomethyl)-aniline
(13) 4-(N-ethyl-N-tert.butoxycarbonyl-aminomethyl)-aniline
(14) 4-[N-(4-chlorophenyl-methyl)-N-tert.butoxycarbonyl-
aminomethyl]-aniline
(15) 4-(N-cyclohexyl-N-tert.butoxycarbonyl-aminomethyl)-
aniline
(16) 4-(N-isopropyl-N-tert.butoxycarbonyl-aminomethyl)-aniline
(17) 4-(N-propyl-N-tert.butoxycarbonyl-aminomethyl)-aniline
(18) 4-(N-methyl-N-tert.butoxycarbonyl-aminomethyl)-aniline
(19) 4-(N-butyl-N-tert.butoxycarbonyl-aminomethyl)-aniline
(20) 4-(N-methoxycarbonyl-methyl-N-tert.butoxycarbonyl-
aminomethyl)-aniline
(21) 4-(N-benzyl-N-tert.butoxycarbonyl-aminomethyl)-aniline
(22) 4-(pyrrolidin-1-yl-methyl)-aniline
CA 02387013 2002-03-19
- 102 -
(23) 4-(morpholin-4-yl-methyl)-aniline
(24) 4-(hexamethyleneiminomethyl)-aniline
(25) 4-(4-hydroxy-piperidin-l-yl-methyl)-aniline
(26) 4-(4-methoxy-piperidin-1-yl-methyl)-aniline
(27) 4-(4-methyl-piperidin-l-yl-methyl)-aniline
(28) 4-(4-ethyl-piperidin-1-yl-methyl)-aniline
(29) 4-(4-isopropyl-piperidin-1-yl-methyl)-aniline
(30) 4-(4-phenyl-piperidin-1-yl-methyl)-aniline
(31) 4-(4-benzyl-piperidin-1-yl-methyl)-aniline
(32) 4-(4-ethoxycarbonyl-piperidin-1-yl-methyl)-aniline
(33) 4-(N,N-dipropyl-aminomethyl)-aniline
(34) 4-(4-tert.butoxycarbonyl-piperazin-1-yl-methyl)-aniline
(35) 4-(2-morpholin-4-yl-ethyl)-aniline
(36) 4-(2-pyrrolidin-1-yl-ethyl)-aniline
(37) 4-(2-piperidin-1-yl-ethyl)-aniline
(38) 4-(N-propyl-N-benzyl-aminomethyl)-aniline
(39) 4-[N-(n-hexyl)-N-methyl-aminomethyl]-aniline
(40) 4-[N-methyl-N-(4-chlorobenzyl)-aminomethyl]-aniline
CA 02387013 2002-03-19
- 103 -
(41) 4- [N-methyl -N- ( 4 -bromobenzyl ) - aminomethyl ] -aniline
(42) 4-[N-methyl-N-(4-methylbenzyl)-aminomethyl]-aniline
(43) 4- [N-methyl-N- (4-fluorobenzyl) -aminomethyl] -aniline
(44) 4-[N-methyl-N-(3-chlorobenzyl)-aminomethyl]-aniline
(45) 4-[N-methyl-N-(3,4-dimethoxybenzyl)-aminomethyl]-aniline
(46) 4-[N-methyl-N-(4-methoxybenzyl)-aminomethyl]-aniline
(47) 4-(N-2,2,2-trifluoroethyl-N-benzyl-aminomethyl)-aniline
(48) 4- [N-2, 2, 2-trifluoroethyl-N- (4-chlorobenzyl) -
aminomethyl]-aniline
(49) 4-(thiomorpholin-4-yl-methyl)-aniline
(50) 4-(1-oxo-thiomorpholin-4-yl-methyl)-aniline
(51) 4-(1,1-dioxo-thiomorpholin-4-yl-methyl)-aniline
(52) 4-(azetidion-1-yl-methyl)-aniline
(53) 4-(3,4-dihydropyrrolidin-l-yl-methyl)-aniline
(54) 4-(3,4-dihydropiperidin-l-yl-methyl)-aniline
(55) 4-(2-methoxycarbonyl-pyrrolidin-l-yl-methyl)-aniline
(56) 4-(3,5-dimethyl-piperidin-l-yl-methyl)-aniline
(57) 4-(4-phenyl-piperazin-1-yl-methyl)-aniline
(58) 4-(4-phenyl-4-hydroxy-piperidin-1-yl-methyl)-aniline
CA 02387013 2002-03-19
- 104 -
(59) 4-[N-(3,4,5-trimethoxy-benzyl)-N-methyl-aminomethyl]-
aniline
(60) 4-[N-(3,4-dimethoxy-benzyl)-N-ethyl-aminomethyl]-aniline
(61) 4-(N-benzyl-N-ethyl-aminomethyl)-aniline
(62) 4-[N-(2,6-dichlorobenzyl)-N-methyl-aminomethyl]-aniline
(63) 4-[N-(4-trifluoromethylbenzyl)-N-methyl-aminomethyl]-
aniline
(64) 4-(N-benzyl-N-isopropyl-aminomethyl)-aniline
(65) 4-(N-benzyl-N-tert.butyl-aminomethyl)-aniline
(66) 4-(diethylamino-methyl)-aniline
(67) 4-(2-diethylamino-ethyl)-aniline
(68) 4-(N,N-diisopropyl-aminomethyl)-aniline
(69) 4-(N,N-diisobutyl-aminomethyl)-aniline
(70) 4-(2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-methyl)-aniline
(71) 4-(2,3-dihydro-isoindol-2-yl-methyl)-aniline
(72) 4-(6,7-dimethoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-aniline
(73) 4-(1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-aniline
(74) 4-[N-(2-hydroxy-ethyl)-N-benzyl-aminomethyl]-aniline
CA 02387013 2002-03-19
- 105 -
(75) 4-[N-(1-ethyl-pentyl)-N-(pyridin-2-yl-methyl)-
aminomethyl]-aniline
(76) 4-(piperidin-1-yl-methyl)-3-nitro-aniline
(77) 4-(piperidin-1-yl-methyl)-3-amino-aniline
(78) 4-(N-benzyl-N-methyl-aminomethyl)-aniline
(79) 4-(N-ethyl-N-methyl-aminomethyl)-aniline
(80) 4-(N-phenethyl-N-methyl-aminomethyl)-aniline
(81) 4-[N-(3,4-dihydroxy-phenethyl)-N-methyl-aminomethyl]-
aniline
(82) 4-[N-(3,4,5-trimethoxy-phenethyl)-N-methyl-aminomethyl]-
aniline
(83) 4-[N-(3,4-dimethoxy-phenethyl)-N-methyl-aminomethyl]-
aniline
(84) 4-[N-(3,4-dimethoxy-benzyl)-N-methyl-aminomethyl]-aniline
(85) 4-[N-(4-chloro-benzyl)-N-methyl-aminomethyl]-aniline
(86) 4-[N-(4-bromo-benzyl)-N-methyl-aminomethyl]-aniline
(87) 4- [N- (4-fluoro-benzyl) -N-methyl-aminomethyl] -aniline
(88) 4-[N-(4-methyl-benzyl)-N-methyl-aminomethyl]-aniline
(89) 4-[N-(4-nitro-phenethyl)-N-methyl-aminomethyl]-aniline
(90) 4-(N-phenethyl-N-benzyl-aminomethyl)-aniline
CA 02387013 2002-03-19
- 106 -
(91) 4-(N-phenethyl-N-cyclohexyl-aminomethyl)-aniline
(92) 4- [N- (2- (pyridin-2-yl) -ethyl) -N-methyl-aminomethyl] -
aniline
(93) 4- [N- (2- (pyridin-4-yl) -ethyl) -N-methyl-aminomethyl] -
aniline
(94) 4-[N-(pyridin-4-yl-methyl)-N-methyl-aminomethyl]-aniline
(95) 4-(N,N-dibenzylaminomethyl)-aniline
(96) 4-[N-(4-nitro-benzyl)-N-propyl-aminomethyl]-aniline
(97) 4-[N-benzyl-N-(3-cyano-propyl)-aminomethyl]-aniline
(98) 4-(N-benzyl-N-allyl-aminomethyl)-aniline
(99) 4-[N-benzyl-N-(2,2,2-trifluoroethyl)-aminomethyl]-aniline
(100) 4-[(benzo(1,3)dioxol-5-yl-methyl)-methyl-aminomethyl]-
aniline
(101) 4-(7-chloro-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-aniline
(102) 4-(7,8-dichloro-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-aniline
(103) 4-(7-methoxy-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-aniline
(104) 4-(7-methyl-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-aniline
CA 02387013 2002-03-19
- 107 -
(105) 4-(7,8-dimethoxy-2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-aniline
(106) 4-(6,7-dichloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-aniline
(107) 4-(6,7-dimethyl-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-aniline
(108) 4-(6-chloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
aniline
(109) 4-(7-chloro-1,2,3,4-tetrahydro-isoquinolin-2-yl-methyl)-
aniline
(110) 4-(6-methoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-aniline
(111) 4-(7-methoxy-1,2,3,4-tetrahydro-isoquinolin-2-yl-
methyl)-aniline
(112) 4-(2,3,4,5-tetrahydro-azepino(4,5-b)pyrazin-3-yl-
methyl)-aniline
(113) 4-(7-amino-2,3,4,5-tetrahydro-azepino(4,5-b)pyrazin-3-
yl-methyl)-aniline
(114) 4-(2-amino-5,6,7,8-tetrahydro-azepino(4,5-d)thiazol-6-
yl-methyl)-aniline
(115) 4-(5,6,7,8-tetrahydro-azepino(4,5-d)thiazol-6-yl-
methyl)-aniline
(116) 4-(4-methyl-piperazin-1-yl)-aniline
(117) 4-[N-(2-dimethylamino-ethyl)-N-methyl-amino]-aniline
CA 02387013 2002-03-19
- 108 -
(118) 4-[N-(3-dimethylamino-propyl)-N-methyl-amino]-anil ine
(119) N-(3-dimethylamino-propyl)-N-methylsulphonyl-p-
phenylenediamine
(120) 4-[(N-dimethylaminocarbonylmethyl-N-methylsulphonyl)-
amino] -aniline
(121) N-(4-aminophenyl)-N-methyl-methanesulphonamide
(122) 4-(imidazol-4-yl)-aniline
(123) 4-(tetrazol-5-yl)-aniline
(124) 4-[N-(2-dimethylamino-ethyl)-N-propionyl-amino]-aniline
(125) N-(dimethylaminomethylcarbonyl)-N-methyl-p-
phenylenediamine
(126) N-[(2-dimethylamino-ethyl)-carbonyl]-N-methyl-p-
phenylenediamine
(127) 4-(N-acetyl-N-dimethylaminocarbonylmethyl)-amino)-
aniline
(128) N-methylaminocarbonylmethyl-N-methylsulphonyl-p-
phenylenediamine
(129) N-aminocarbonylmethyl-N-methylsulphonyl-p-
phenylenediamine
(130) 4-(imidazolidin-2,4-dion-5-ylidene-methyl)-aniline
(131) 4-(imidazolidin-2,4-dion-5-yl-methyl)-aniline
CA 02387013 2002-03-19
- 109 -
(132) 4-(2-oxo-pyrrolidin-l-yl-methyl)-aniline
(133) N-cyanomethyl-N-methylsulphonyl-p-phenylenediamine
(134) 4-[2-(imidazol-4-yl)-ethyl]-aniline
(135) 4-[(4-methyl-piperazin-1-yl)-methyl]-aniline
(136) 4-[N-(2-(N-benzyl-N-methyl-amino)-ethyl)-N-
methylsulphonyl-amino]-aniline
(137) 4 - [N- ( 3 - (N-benzyl -N-methyl -amino) -propyl ) -N-
methylsulphonyl-amino]-aniline
(138) N-cyclohexyl-p-phenylenediamine
(139) 4-(pyridin-4-yl-methyl)-aniline
(140) 4-(imidazol-l-yl-methyl)-aniline
(141) 4-benzyl-aniline
(142) N-(3-trifluoroacetylamino-propyl)-N-methylsulphonyl-p-
phenylenediamine
(143) tert.butyl 4-amino-phenylacetate
(144) 4-(imidazol-2-yl)-aniline
(145) 4-(1-methyl-imidazol-2-yl)-aniline
(146) 4-(1-ethyl-imidazol-2-yl)-aniline
(147) 4-(1-benzyl-imidazol-2-yl)-aniline
CA 02387013 2002-03-19
- 110 -
(148) 4-[N-(2-dimethylamino-ethyl)-N-methylsulphonyl-amino]-3-
amino-aniline
(149) 4-[N-(2-dimethylamino-ethyl)-N-methylsulphonyl-amino]-3-
chloro-aniline
(150) 4-[N-(2-dimethylamino-ethyl)-N-acetyl-amino]-3-amino-
aniline
(151) 4-[N-(2-dimethylamino-ethyl)-N-acetyl-amino]-3-bromo-
aniline
(152) 4-[2-(4-hydroxy-piperidin-1-yl)-ethyl-amino]-aniline
(153) N-(2-dimethylamino-ethyl)-N-ethylsulphonyl-p-
phenylenediamine
(154) N-(2-dimethylamino-ethyl)-N-propylsulphonyl-p-
phenylenediamine
(155) N-(2-dimethylamino-ethyl)-N-isopropylsulphonyl-p-
phenylenediamine
(156) N-(2-dimethylamino-ethyl)-N-butylsulphonyl-p-
phenylenediamine
(157) N-(2-dimethylamino-ethyl)-N-benzylsulphonyl-p-
phenylenediamine
(158) N-(2-dimethylamino-ethyl)-N-phenylsulphonyl-p-
phenylenediamine
(159) 4-((3-hydroxy-pyrrolidin-1-yl)-methyl)-aniline
(160) 4- [N- (2-dimethylamino-ethyl) -N- (furan-2-carbonyl) -
amino] -aniline
CA 02387013 2002-03-19
- 111 -
(161) 4-[N-(2-dimethylamino-ethyl)-N-(2-methoxy-benzoyl)-
amino] -aniline
(162) 4-[N-(2-dimethylamino-ethyl)-N-(pyridine-3-carbonyl)-
amino] -aniline
(163) 4-[N-(2-dimethylamino-ethyl)-N-(phenyl-acetyl)-amino]-
aniline
(164) N-(piperidin-1-yl-methylcarbonyl)-N-methyl-p-
phenylenediamine
(165) N-(morpholin-4-yl-methylcarbonyl)-N-methyl-p-
phenylenediamine
(166) N-[(4-benzyl-piperazin-l-yl)-methylcarbonyl]-N-methyl-p-
phenylenediamine
(167) N-(pyrrolidin-l-yl-methylcarbonyl)-N-methyl-p-
phenylenediamine
(168) 4-(5-methyl-imidazol-4-yl)-aniline
(169) N-[(2-dimethylamino-ethyl)-carbonyl]-N-isopropyl-p-
phenylenediamine
(170) N-[(2-dimethylamino-ethyl)-carbonyl]-N-benzyl-p-
phenylenediamine
(171) N-(N-aminocarbonylmethyl-N-methyl-amino)-
methylcarbonyl)-N-methyl-p-phenylenediamine
(172) N-[(N-benzyl-N-methyl-amino)-methylcarbonyl]-N-methyl-p-
phenylenediamine
CA 02387013 2002-03-19
- 112 -
(173) N-[di-(2-methoxyethyl)-amino-methylcarbonyl]-N-methyl-p-
phenylenediamine
(174) N-[(2-(4-tert.butoxycarbonyl-piperazin-1-yl)-ethyl)-
carbonyl]-N-methyl-p-phenylenediamine
(175) N- [ (2- (piperidin-1-yl) -ethyl) -carbonyl] -N-methyl-p-
phenylenediamine
(176) N-[(2-(N-benzyl-N-methyl-amino)-ethyl)-carbonyl]-N-
methyl-p-phenylenediamine
(177) N-(dimethylaminomethylcarbonyl)-N-isopropyl-p-
phenylenediamine
(178) N-(piperidin-l-yl-methylcarbonyl)-N-isopropyl-p-
phenylenediamine
(179) N-[(4-tert.butoxycarbonyl-piperazin-1-yl)-
methylcarbonyl]-N-isopropyl-p-phenylenediamine
(180) N-[(N-benzyl-N-methyl-amino)-methylcarbonyl]-N-benzyl-p-
phenylenediamine
(181) N-(dimethylaminomethylcarbonyl)-N-benzyl-p-
phenylenediamine
(182) N-(piperidin-1-yl-methylcarbonyl)-N-benzyl-p-
phenylenediamine
(183) 4-(1,2,4-triazol-l-yl-methyl)-aniline
(184) 4-(1,2,3-triazol-2-yl-methyl)-aniline
(185) 4-(1,2,3-triazol-i-yl-methyl)-aniline
CA 02387013 2002-03-19
- 113 -
(186) 4-[(N-ethoxycarbonylmethyl-N-methyl-amino)-methyl]-
aniline
(187) 4-[(N-aminocarbonylmethyl-N-methyl-amino)-methyl]-
aniline
(188) 4-(azetidin-l-yl-methyl)-aniline
(189) 4-[(di-(2-methoxy-ethyl)-amino)-methyl]-aniline
(190) 4-[(N-(2-(2-methoxy-ethoxy)-ethyl)-N-methyl-amino)-
methyl]-aniline
(191) 4-[N-(N-tert.butoxycarbonyl-3-amino-propyl)-N-methyl-
aminomethyl]-aniline
(192) 4-[(N-(methylcarbamoyl-methyl)-N-methyl-amino)-methyl]-
aniline
(193) 4-[(N-(dimethylcarbamoyl-methyl)-N-methyl-amino)-
methyl]-aniline
(194) 4-[(N-propyl-N-methyl-amino)-methyl]-aniline
(195) 4-[(N-(2-dimethylamino-ethyl)-N-methyl-amino)-methyl]-
aniline
(196) 4-[(N-(3-dimethylamino-propyl)-N-methyl-amino)-methyl]-
aniline
(197) 4-[(N-(2-methoxy-ethyl)-N-methyl-amino)-methyl]-aniline
(198) 4-[(N-(2-hydroxy-ethyl)-N-methyl-amino)-methyl]-aniline
(199) 4-[(N-(dioxolan-2-yl-methyl)-N-methyl-amino)-methyl]-
aniline
CA 02387013 2002-03-19
- 114 -
(200) 4-(3-oxo-piperazin-1-yl-methyl)-aniline
(201) N-[di-(2-hydroxyethyl)-amino-methylcarbonyl]-N-methyl-p-
phenylenediamine
(202) N-[(N-(2-methoxyethyl)-N-methyl-amino)-methylcarbonyl]-
N-methyl-p-phenylenediamine
(203) N-[(N-(2-dimethylamino-ethyl)-N-methyl-amino)-
methylcarbonyl]-N-methyl-p-phenylenediamine
(204) N-[(4-methyl-piperazin-l-yl)-methylcarbonyl]-N-methyl-p-
phenylenediamine
(205) N-[(imidazol-l-yl)-methylcarbonyl]-N-methyl-p-
phenylenediamine
(206) N-[(phthalimido-2-yl)-methylcarbonyl]-N-methyl-p-
phenylenediamine
FrxAms 1 e XXI
4- (4-hy roxymethyl-Kjip ri di n-1 -yl -mP hyl -ami no) -ani 1 i np-
1.1 g of 4-(4-ethoxycarbonyl-piperidin-1-yl-methyl-amino)-
aniline are suspended in 15 ml of tetrahydrofuran. 175 mg of
lithium borohydride are added at room temperature, stirred for
24 h, another 175 mg of lithium borohydride are added and
after a further 7.5 hours 15 ml of water are added and the
mixture is stirred for 10 minutes. It is extracted three times
with 15 ml of ethyl acetate. The combined organic phases are
washed with water and saturated saline solution, dried over
sodium sulphate and concentrated by rotary evaporation. The
residue is purified over a silica gel column with methylene
chloride/methanol/ammonia 4:1:0.01 as eluant.
Yield: 200 mg (27 % of theory)
CA 02387013 2002-03-19
- 115 -
Rf value: 0.4 (silica gel, methylene chloride/methanol/ammonia
4:1:0.01)
Melting point: 157 C
F.xampl p XXT T
mPt-hyl 4-m -hoxycarbonylmethyl--l-n i ro-b n .oa
54.3 g of methyl 3-nitro-benzoate and 29.0 g of methyl
chloroacetate are dissolved in 100 ml of dimethylformamide and
this solution is added dropwise at -10 C to a solution of 78.5
g of potassium-tert. butoxide in 500 ml of dimethylformamide.
The mixture is stirred for another 10 minutes at room
temperature and after this time the solution is poured onto
350 ml of concentrated hydrochloric acid in 2 1 of ice water.
The solution is stirred for 0.5 hours, the precipitate
obtained is suction filtered and washed with water. The
product is recrystallised from 150 ml of methanol and dried at
40 C in vacuo.
Yield: 48.3 g of (51 %- of theory), contains about 20 0 of
methyl 6-methoxycarbonylmethyl-3-nitro-benzoate
Rf value: 0.7 (silica gel, petroleum ether/ethyl acetate =
1:1)
Melting point: 65-73 C
The following compound is prepared analogously to Example
XXII:
(1) ethyl 4-methoxycarbonylmethyl-3-nitro-benzoate
Prepared from ethyl 4-thoxycarbonylmethyl-3-nitro-benzoate
RxamIl1 e XXTTT
mPi- yl 2-i ndolinone-5-carboxy a_P
48.3 g of methyl 4-methoxycarbonylmethyl-3-nitro-benzoate are
dissolved in 800 ml of concentrated acetic acid, 5.0 g of
CA 02387013 2002-03-19
- 116 -
palladium on carbon (10%) are added and the solution is
hydrogenated for 2.5 hours at room temperature and 50 psi. The
catalyst is filtered off and the filtrate is evaporated down.
The residue is taken up in 150 ml of tert.-butylmethyl ether,
filtered again and dried in vacuo at 100 C.
Yield: 28.6 g (98 % of theory),
Rf value: 0.4 (silica gel, methylene chloride/methanol = 10:1)
Melting point: 208-211 C
The following compound is prepared analogously to Example
XXIII:
(1) ethyl 2-indolinone-6-carboxylate
Prepared from ethyl 4-methoxycarbonylmethyl-3-nitro-benzoate
Fxa 1P XXTV
1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-ethoxycarbonyl-
2-indalinone
15.0 g of ethyl 2-indolinone-6-carboxylate, 49.6 ml of
triethyl orthobenzoate and 150 ml of acetic anhydride are
stirred for 4 hours at 110 C. After this time the solvent is
removed, the residue is recrystallised from petroleum ether
and dried in vacuo at 50 C.
Yield: 16.9 g (61 % of theory),
Rf value: 0.5 (silica gel, petroleum ether/methylene
chloride/ethyl acetate = 5:4:1)
Melting point: 98-100 C
C22H2iNOs
The following compounds are prepared analogously to Example
XXIV:
(1) 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-methoxycarbonyl-
2-indolinone
CA 02387013 2002-03-19
- 117 -
Prepared from methyl 2-indolinone-6-carboxylate, triethyl
orthobenzoate and acetic anhydride
(2) 1-acetyl-3-(1-ethoxy-l-ethyl-methylene)-6-ethoxycarbonyl-
2-indolinone
Prepared from ethyl 2-indolinone-6-carboxylate, triethyl
orthopropionate and acetic anhydride
CA 02387013 2002-03-19
- 118 -
Preparation of the final compounds:
R.xam l= A l
3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-i-phenyl-
mPtlyl PnPI -6- ar amoyl-2-i dol i non -trifl ioroa .._a _P
300 mg of resin obtained according to Example II are suspended
in 3 ml of dimethylformamide and shaken with 0.2 g of 4-
(piperidin-l-yl-methyl)-aniline for 22 hours at 70 C. Then it
is filtered off and the resin is washed several times with
methylene chloride, methanol and dimethylformamide. Then 1 ml
of methanolic ammonia is added for 2 hours in order to
eliminate the acetyl group. Then after further washing 4 ml of
10% trifluoroacetic acid in methylene chloride are added
during another 60 minutes, the resin is separated off and the
solution is evaporated down.
Yield: 69 mg
Rf value: 0.1 (silica gel, methylene chloride/methanol = 9:1)
C28H28N402
Mass spectrum: m/z = 452 (m+)
The following compounds are prepared analogously to Example 1:
(1) 3-Z-(l-Anilino-l-phenyl-methylene)-6-carbamoyl-2-
indolinone
Prepared from the resin obtained according to Example II and
aniline
C22H17N302
Mass spectrum: m/z = 355 (m+)
(2) 3-Z-[1-(4-dimethylaminomethyl-anilino)-l-phenyl-
methyleneJ-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-dimethylaminomethyl-aniline
C25H24N402
Mass spectrum: m/z = 412 (m`)
CA 02387013 2002-03-19
- 119 -
(3) 3-Z- [1- (4- (2-diethylamino-ethyl) -anilino) -i-phenyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-(2-diethylamino-ethyl)-aniline
C28H30N4O
2
Mass spectrum: m/z = 454 (m+)
(4) 3-Z- [1- (4- (morpholin-4-yl-methyl) -anilino) -i-phenyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-(morpholin-4-yl-methyl)-aniline
R. value: 0.50 (silica gel, methylene chloride/methanol = 4:1)
C27H26N403
Mass spectrum: m/z = 454 (m')
(5) 3-Z-[1-(4-(l-oxo-thiomorpholin-4-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-(1-oxo-thiomorpholin-4-yl-methyl)-aniline
Rf value: 0.30 (silica gel, methylene chloride/methanol = 9:1)
C27H26N4O3S
Mass spectrum: m/z = 486 (m+)
(6) 3-Z-[1-(4-(1,1-dioxo-thiomorpholin-4-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-(1,1-dioxo-thiomorpholin-4-yl-methyl)-aniline
Rf value: 0.30 (silica gel, methylene chloride/methanol = 9:1)
C27H26N4O4S
Mass spectrum: m/z = 502 (m+)
(7) 3-Z- [1- (4- (benzylaminomethyl) -anilino) -1-phenyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
CA 02387013 2002-03-19
- 120 -
Prepared from the resin obtained according to Example II and
4-[N-(phenyl-methyl)-N-tert.butoxycarbonyl-aminomethyl]-
aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol = 4:1)
C30H26Na0z
Mass spectrum: m/z = 474 (m')
(8) 3-Z-[1-(4-(amino-methyl)-anilino)-1-phenyl-methylene]-
6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-(N-tert.butoxycarbonyl-aminomethyl)-aniline
R. value: 0.10 (silica gel, methylene chloride/methanol = 4:1)
C23H20N402
Mass spectrum: m/z = 384 (m+)
(9) 3-Z- [1- (4- (2, 6-dimethylpiperidin-1-yl-methyl) -anilino) -
1-phenyl-methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-(2,6-dimethylpiperidin-l-yl-methyl)-aniline
Rf value: 0.45 (silica gel, methylene chloride/methanol = 4:1)
Cs0H32Na0z
Mass spectrum: m/z = 480 (m+)
(10) 3-Z-[1-(4-(pyrrolidin-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-(pyrrolidin-1-yl-methyl)-aniline
Rf value: 0.15 (silica gel, methylene chloride/methanol = 4:1)
C27H26N402
Mass spectrum: m/z = 438 (m+)
(11) 3-Z-[1-(3-(dimethylaminomethyl)-anilino)-1-phenyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin.obtained according to Example II and
3-dimethylaminomethyl-aniline
Rf value: 0.23 (silica gel, methylene chloride/methanol = 4:1)
CA 02387013 2002-03-19
- 121 -
C25H24N402
Mass spectrum: m/z = 412 (m+)
(12) 3-Z-[1-(3-(N-methyl-N-ethyl-aminomethyl)-anilino)-
1-phenyl-methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
3-(N-methyl-N-ethyl-aminomethyl)-aniline
Rf value: 0.23 (silica gel, methylene chloride/methanol = 4:1)
C26H26N4O2
Mass spectrum: m/z = 426 (m+)
(13) 3-Z-[1-(3-(methylaminomethyl)-anilino)-i-phenyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-(N-tert.butoxycarbonyl-N-methyl-aminomethyl)-aniline
Rf value: 0.06 (silica gel, methylene chloride/methanol = 4:1)
C24H22N402
Mass spectrum: m/z = 399 (m+H+)
(14) 3-Z-[1-(3-hydroxymethyl-anilino)-1-phenyl-methylene]-
6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example II and
3-amino-benzyl alcohol
Rf value: 0.7 (silica gel, methylene chloride/methanol = 4:1)
C23H19N3O3
Mass spectrum: m/z = 385 (m+)
(15) 3-Z-[1-(4-(methoxycarbonylmethyl-aminomethyl)-anilino)-
1-phenyl-methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-(N-methoxycarbonylmethyl-N-tert.butoxycarbonyl-aminomethyl)-
aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
C26H24N404
Mass spectrum: m/z = 457 (m+H+)
CA 02387013 2002-03-19
- 122 -
(16) 3-Z-[1-(4-(N-methylsulphonyl-N-(dimethylaminocarbonyl-
methyl)-amino)-anilino)-1-phenyl-methylene]-6-carbamoyl-
2-indolinone
Prepared from the resin obtained according to Example II and
4-(N-methylsulphonyl-N-(dimethylaminocarbonylmethyl)-amino)-
aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
C27H27N505S
Mass spectrum: m/z = 533 (m+)
(17) 3-Z- [l- (4- (N-acetyl-aminomethyl) -anilino) -1-phenyl-
methylene]-6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example II and
4-(N-acetyl-aminomethyl)-aniline
Rf value: 0.70 (silica gel, methylene chloride/methanol = 4:1)
C25H22N403
Mass spectrum: m/z = 426 (m')
(18) 3-Z-[1-(3,4-dimethoxy-anilino)-i-phenyl-methylene]-
6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example II and
3,4-dimethoxy-aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
C24H21N304
Mass spectrum: m/z = 415 (m+)
(19) 3-Z-[1-(4-(morpholin-4-yl)-anilino)-1-phenyl-methylene]-
6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-morpholin-4-yl-aniline
Rf value: 0.20 (silica gel, methylene chloride/methanol = 9:1)
C26H24N403
Mass spectrum: m/z = 440 (m')
(20) 3-Z-[1-(4-acetylamino-anilino)-1-phenyl-methylene]-
6-carbamoyl-2-indolinone
CA 02387013 2002-03-19
- 123 -
Prepared from the resin obtained according to Example II and
4-acetylamino-aniline
Rf value: 0.25 (silica gel, methylene chloride/methanol = 9:1)
C24H2ON403
Mass spectrum: m/z = 412 (m+)
(21) 3-Z- [1- (4-amino-anilino) -1-phenyl-methylene] -6-carbamoyl-
2-indolinone
Prepared from the resin obtained according to Example II and
4-amino-aniline
Rf value: 0.40 (silica gel, methylene chloride/methanol = 9:1)
C22HisN402
Mass spectrum: m/z = 370 (m+)
(22) 3-Z-[1-(4-N-methyl-N-acetyl-amino-anilino)-i-phenyl-
methylene]-6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example II and
4-(N-methyl-N-acetyl-amino)-aniline
C25H22N403
Mass spectrum: m/z = 426 (m+)
(23) 3-Z-[1-(4-ethoxycarbonyl-anilino)-i-phenyl-methylene]-
6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example II and
ethyl 4-amino-benzoate
C25H21N304
Mass spectrum: m/z = 427 (m+)
(24) 3-Z-[1-(4-carboxy-anilino)-i-phenyl-methylene]-
6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example II and
4-amino-benzoic acid
R. value: 0.11 (silica gel, methylene chloride/methanol = 9:1)
C23H17N3Q4
Mass spectrum: m/z = 398 (m-H+)
CA 02387013 2002-03-19
- 124 -
(25) 3-Z-[1-(4-benzylcarbamoyl-anilino)-1-phenyl-methylene]-
6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example II and
4-amino-benzoic acid-benzylamide
Rf value: 0.21 (silica gel, methylene chloride/methanol = 9:1)
C30H24N403
Mass spectrum: m/z = 488 (m+)
(26) 3-Z-[1-(cyclohexyl-amino)-i-phenyl-methylene]-6-
carbamoyl-2-indolinone
Prepared from the resin obtained according to Example II and
cyclohexylamine
Rf value: 0.60 (silica gel, methylene chloride/methanol = 9:1)
C22H23N302
Mass spectrum: m/z = 361 (m+)
(27) 3-Z-[1-(4-amino-cyclohexyl-amino)-i-phenyl-methylene]-
6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-amino-cyclohexylamine
C22H24N402
Mass spectrum: m/z = 376 (m+)
(28) 3-Z-[1-(N-methyl-piperidine-4-yl-amino)-1-phenyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
4-amino-i-methyl-piperidine
Rf value: 0.15 (silica gel, methylene chloride/methanol = 4:1)
C22H24N402
Mass spectrum: m/z = 376 (m+)
(29) 3-Z- [1- (4- (piperidin-1-yl-methyl) -anilino) -1-methyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example 11(2)
and 4-(piperidin-1-yl-methyl)-aniline
Rf value: 0.30 (silica gel, methylene chloride/methanol = 4:1)
CA 02387013 2002-03-19
- 125 -
C23H26N4Q2
Mass spectrum: m/z = 390 (m+)
(30) 3-Z-[1-(3-dimethylaminomethyl-anilino)-i-methyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example 11(2)
and 3-dimethylaminomethyl-aniline
Rf value: 0.51 (silica gel, methylene chloride/methanol = 4:1)
C20H22N4O2
Mass spectrum: m/z = 351 (m+H+)
(31) 3-Z- [1- (4- (N-methyl-N-benzyl-aminomethyl) -anilino) -
1-methyl-methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example 11(2)
and 4-(N-methyl-N-benzyl-aminomethyl)-aniline
Rf value: 0.73 (silica gel, methylene chloride/methanol = 4:1)
C26H26N402
Mass spectrum: m/z = 426 (m+)
(32) 3-Z-[1-(4-(N-methylsulphonyl-N-(2-dimethylamino-ethyl)-
amino)-anilino)-1-methyl-methylene]-6-carbamoyl-2-indolinone-
trifluoroacetate
Prepared from the resin obtained according to Example 11(2)
and 4-(N-methylsulphonyl-N-(2-dimethylamino-ethyl)-amino)-
aniline
C22H27N504S
Mass spectrum: m/z = 458 (m+H)
(33) 3-Z-[1-(4-chloro-anilino)-1-methyl-methylene]-6-
carbamoyl-2-indolinone
Prepared from the resin obtained according to Example 11(2)
and 4-chloro-aniline
Rf value: 0.10 (silica gel, methylene chloride/methanol = 9:1)
C17H14C1N3O2
Mass spectrum: m/z = 327/329 (m+)
CA 02387013 2002-03-19
- 126 -
(34) 3-Z-[1-(3-chloro-anilino)-1-methyl-methylene]-6-
carbamoyl-2-indolinone
Prepared from the resin obtained according to Example 11(2)
and 3-chloro-aniline
Rf value: 0.11 (silica gel, methylene chloride/methanol = 9:1)
C17H14ClN3O2
Mass spectrum: m/z = 327/329 (m`)
(35) 3-Z-[1-(4-methoxycarbonyl-anilino)-1-methyl-methylene]-
6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example 11(2)
and methyl 4-amino-benzoate
Rf value: 0.11 (silica gel, methylene chloride/methanol = 9:1)
C19H17N304
Mass spectrum: m/z = 351 (m+)
(36) 3-Z- [1- (4-carboxy-anilino) -1-methyl-methylene] -6-
carbamoyl-2-indolinone
Prepared from the resin obtained according to Example 11(2)
and 4-amino-benzoic acid
C18H15N3O4
Mass spectrum: m/z = 336 (m-H+)
(37) 3-Z-[1-(4-methyl-3-nitro-anilino)-1-methyl-methylene]-
6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example 11(2)
and 4-methyl-3-nitro-aniline
Rf value: 0.82 (silica gel, methylene chloride/methanol = 4:1)
C18H16N4O4
Mass spectrum: m/z = 352 (m+)
(38) 3-Z- [1- (4- (piperidin-1-yl-methyl) -anilino) -1-propyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example 11(4)
and 4-(piperidin-1-yl-methyl)-aniline
R. value: 0.37 (silica gel, methylene chloride/methanol = 4:1)
CA 02387013 2002-03-19
- 127 -
C25H30N402
Mass spectrum: m/z = 418 (m')
(39) 3-Z-[1-(3-dimethylaminomethyl-anilino)-1-propyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II(4)
and 3-dimethylaminomethyl-aniline
Rf value: 0.42 (silica gel, methylene chloride/methanol = 4:1)
C22H26N402
Mass spectrum: m/z = 378 (m+)
(40) 3-Z-[1-(4-(N-methyl-N-benzyl-aminomethyl)-anilino)-1-pro-
pyl-methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example 11(4)
and 4-(N-methyl-N-benzyl-aminomethyl)-aniline
Rf value: 0.81 (silica gel, methylene chloride/methanol = 4:1)
C28H30N4Q2
Mass spectrum: m/z = 454 (m+)
(41) 3-Z-[1-(4-(N-methylsulphonyl-N-(2-dimethylamino-ethyl)-
amino)-anilino)-1-propyl-methylene]-6-carbamoyl-2-indolinone-
trifluoroacetate
Prepared from the resin obtained.according to Example 11(4)
and 4-(N-methylsulphonyl-N-(2-dimethylamino-ethyl)-amino)-
aniline
Rf value: 0.59 (silica gel, methylene chloride/methanol = 4:1)
C24H31N504''S
Mass spectrum: m/z = 486 (m+H+)
(42) 3-Z-[1-(4-chloro-anilino)-1-propyl-methylene]-6-
carbamoyl-2-indolinone
Prepared from the resin obtained according to Example 11(4)
and 4-chloro-aniline
Rf value: 0.17 (silica gel, methylene chloride/methanol = 9:1)
C19H18C1N302
Mass spectrum: m/z = 355/357 (m+)
CA 02387013 2002-03-19
- 128 -
(43) 3-Z-[1-(3-chloro-anilino)-1-propyl-methylene]-6-
carbamoyl-2-indolinone
Prepared from the resin obtained according to Example II(4)
and 3-chloro-aniline
R. value: 0.12 (silica gel, methylene chloride/methanol = 9:1)
C19H18C1N3O2
Mass spectrum: m/z = 355/357 (m+)
(44) 3-Z-[1-(4-methoxycarbonyl-anilino)-1-propyl-methylene]-
6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example 11(4)
and methyl 4-amino-benzoate
R. value: 0.8 (silica gel, methylene chloride/methanol = 4:1)
CaiHz iN309
Mass spectrum: m/z = 379 (m+)
(45) 3-Z-[1-(4-carboxy-anilino)-1-propyl-methylene]-
6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example 11(4)
and 4-amino-benzoic acid
Cz0Hi9N30a
Mass spectrum: m/z = 364 (m-H+)
(46) 3-Z-[1-(4-methyl-3-nitro-anilino)-i-propyl-methylene]-
6-carbamoyl-2-indolinone
Prepared from the resin obtained according to Example 11(4)
and 4-methyl-3-nitro-aniline
R. value: 0.86 (silica gel, methylene chloride/methanol = 4:1)
C20H20N404
Mass spectrum: m/z = 380 (m+)
CA 02387013 2002-03-19
- 129 -
Rxamile 2
3-Z-[1-(3-(piperidin-l-yl-methyl)-anilino)-1-phenyl-
mei-hy1PnP1-6-carbamoyl-2-indolinone-triflLOroacetate
2.0 g of resin obtained according to Example II are reacted
analogously to Example 1 with 2.0 g of 3-aminobenzyl alcohol
in 20 ml of dimethylformamide for 22 hours at 70 C. Then the
solvent is suction filtered and the resin is washed several
times with dimethylformamide and methylene chloride. Then 200
mg of the moist charged resin are suspended in 2 ml of
methylene chloride and left to stand with 0.2 ml of
methanesulphonic acid chloride and 0.1 ml of triethylamine for
2 hours at room temperature. Then the resin is washed several
times with methylene chloride, suspended in 2 ml of inethylene
chloride and combined with 0.2 ml of piperidine. After 1 hour
the resin is washed with methylene chloride and
dimethylformamide and then treated with trifluoroacetic acid
analogously to Example 1.
Yield: 15 mg
Rf value: 0.30 (silica gel, methylene chloride/methanol = 4:1)
C2eHzaNa02
Mass spectrum: m/z = 452 (m+)
The following compounds are prepared analogously to Example 2:
(1) 3-Z-[1-(3-(diethylaminomethyl)-anilino)-i-phenyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
diethylamine
Rf value: 0.80 (silica gel, methylene chloride/methanol = 4:1)
C27H28N402
Mass spectrum: m/z = 440 (m+)
(2) 3-Z-[1-(3-(benzylaminomethyl)-anilino)-i-phenyl-
methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
CA 02387013 2002-03-19
- 130 -
Prepared from the resin obtained according to Example II and
benzylamine
Rf value: 0.80 (silica gel, methylene chloride/methanol = 4:1)
C3 0H26N402
Mass spectrum: m/z = 474 (m+)
(3) 3-Z-[1-(3-(N-methyl-N-benzyl-aminomethyl)-anilino)-
1-phenyl-methylene]-6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
N-methyl-benzylamine
Rf value: 0.80 (silica gel, methylene chloride/methanol = 4:1)
C3iH28N402
Mass spectrum: m/z = 488 (m+)
(4) 3-Z- [1- (3- (butylaminomethyl) -anilino) -1-phenyl-methylene] -
6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
butylamine
Rf value: 0.40 (silica gel, methylene chloride/methanol = 4:1)
C27H28N4O2
Mass spectrum: m/z = 440 (m+)
(5) 3-Z-[1-(3-(aminomethyl)-anilino)-i-phenyl-methylene]-
6-carbamoyl-2-indolinone-trifluoroacetate
Prepared from the resin obtained according to Example II and
ammonia
C23H2ON402
Mass spectrum: m/z = 385 (m+H+)
(6) 3-Z- [1- (3- (N- (3-dimethylaminopropyl) -N-methyl-amino-
methyl)-anilino)-1-phenyl-methylene]-6-carbamoyl-2-indolinone-
trifluoroacetate
Prepared from the resin obtained according to Example II and
1-dimethylamino-3-methylaminopropane
Rf value: 0.67 (silica gel, methylene chloride/methanol = 4:1)
C29H33N502
CA 02387013 2002-03-19
- 131 -
Mass spectrum: m/z = 484 (m+H+)
(7) 3-Z-[1-(3-(N-(2-dimethylaminoethyl)-N-methyl-aminomethyl)-
anilino)-i-phenyl-methylene]-6-carbamoyl-2-indolinone-
trifluoroacetate
Prepared from the resin obtained according to Example II and
1-dimethylamino-2-methylaminoethane
Rf value: 0.40 (silica gel, methylene chloride/methanol = 4:1)
C28H31N502
Mass spectrum: m/z = 470 (m+H+)
Fx m 1~-g-"~
3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-1-phenyl-
methylPne] -6-ethoxycarhonyl- .-;n ol;nonP
1.5 g of 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 1.1 g of 4-(piperidin-l-yl-
methyl)-aniline are dissolved in 15 ml of dimethylformamide
and stirred for 45 minutes at 100 C. After cooling 5.0 ml of
piperidine are added and the mixture is stirred for another 3
hours at room temperature. The solvent is removed and the
residue purified over an aluminium oxide column (activity:
2-3) with methylene chloride/ethanol (100:3) as eluant.
Yield: 1.1 g(58% of theory),
Rf value: 0.5 (aluminium oxide, methylene chloride/ethanol =
100:3)
C30H31N303
Mass spectrum: m/z = 481 [M+]
The following compounds are prepared analogously to Example 3:
(1) 3-Z-[1-(4-bromo-anilino)-i-phenyl-methylene]-6-ethoxy-
carbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-bromoaniline
Rf value: 0.4 (silica gel, toluene/ethyl acetate = 5:1)
CA 02387013 2002-03-19
- 132 -
C24H19BrN2O3
Mass spectrum: m/z = 462/464 [M+]
(2) 3-Z-[1-(3-(dimethylaminomethyl)-anilino)-1-phenyl-
methylenel-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 3-(dimethylaminomethyl)-
aniline
Rf value: 0.5 (aluminium oxide, methylene chloride/ethanol =
30:1)
C27H27N303
ESI mass spectrum: m/z = 442 [M+H']
(3) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(dimethylaminomethyl)-
aniline
Rf value: 0.7 (aluminium oxide, ethyl acetate/ethanol = 20:1)
C27H27N303
ESI mass spectrum: m/z = 442 [M+H+]
(4) 3-Z- [1- (4- [ (2, 6-dimethyl-piperidin-l-yl) -methyl] -anilino) -
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4- [(2,6-dimethyl-piperidin-l-
yl) -methyl] -aniline
Rf value: 0.6 (silica gel, methylene chloride/ethanol = 5:1)
C32H35N303
Mass spectrum: m/z = 509 [M+]
(5) 3-Z-[1-(4-(2-dimethylamino-ethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from i-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(2-dimethylamino-ethyl)-
aniline
CA 02387013 2002-03-19
- 133 -
Rf value: 0.2 (silica gel, methylene chloride/ethanol = 5:1)
C28H29N3O3
Mass spectrum: m/z = 455 [M+]
(6) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-acetyl-amino) -
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-acetyl-amino)-aniline
R. value: 0.4 (aluminium oxide, methylene chloride/ethanol =
20:1)
C30H32N4Q4
Mass spectrum: m/z = 512 [M+]
(7) 3-Z-[l-(4-tert.butyloxycarbonyl-anilino)-i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-tert.butyloxycarbonyl-
aniline
Rf value: 0.4 (aluminium oxide, methylene chloride/ethanol =
40:1)
C29H28N205
Mass spectrum: m/z = 484 [M+]
(8) 3-Z- [1- (4- (N- (3-dimethylamino-propyl) -N-acetyl-amino) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(N-(3-dimethylamino-propyl)-
N-acetyl-amino)-aniline
Rf value: 0.2 (aluminium oxide, methylene chloride/ethanol =
40:1)
C31H34N404
Mass spectrum: m/z = 526 [M+]
CA 02387013 2002-03-19
- 134 -
(9) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and N-(2-dimethylamino-ethyl)-N-
methylsulphonyl-p-phenylenediamine
Rf value: 0.3 (aluminium oxide, methylene chloride/ethanol =
40:1)
C29H32N4O5S
Mass spectrum: m/z = 548 [M+]
(10) 3-Z- [1- (4- (4-methyl-piperazin-1-yl) -anilino) -i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(4-methyl-piperazin-1-yl)-
aniline
Rf value: 0.3 (aluminium oxide, ethyl acetate)
C29H3DN403
ESI mass spectrum: m/z = 483 [M+H`]
(11) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methyl-amino)-
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-methyl-amino)-aniline
Rf value: 0.5 (aluminium oxide, methylene chloride/ethanol =
20:1)
C29H32N403
ESI mass spectrum: m/z = 485 [M+H+]
(12) 3-Z-[1-(4-(N-(3-dimethylamino-propyl)-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(N-(3-dimethylamino-propyl)-
N-methyl-amino)-aniline
Rf value: 0.5 (aluminium oxide, ethyl acetate)
CA 02387013 2002-03-19
- 135 -
C30H34N403
ESI mass spectrum: m/z = 499 [M+H+]
(13) 3-Z-[1-(4-(N-methyl-acetylamino)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-amino-N-methyl-acetanilide
Rf value: 0.3 (silica gel, methylene chloride/ethanol = 15:1)
C27H25N304
Mass spectrum: m/z = 455 [M+]
(14) 3-Z-[l-(4-(N-methyl-methylsulphonylamino)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and N-(4-aminophenyl)-N-methyl-
methanesulphonamide
R. value: 0.8 (aluminium oxide, ethyl acetate)
C26H2SN3O5S
Mass spectrum: m/z = 491 [M+]
(15) 3-Z-[1-(4-(N-(3-dimethllamino-propyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and N-(3-dimethylamino-propyl)-N-
methylsulphonyl-p-phenylenediamine
Rf value: 0.6 (silica gel, methylene chloride/ethanol/ammonia
= 5:2:0.01)
C30H34N405''S
ESI mass spectrum: m/z = 563 [M+H+]
(16) 3-Z-[1-(4-(N-dimethylaminocarbonylmethyl-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 136 -
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(N-
dimethylaminocarbonylmethyl-N-methylsulphonyl)-amino)-aniline
Rf value: 0.6 (silica gel, methylene chloride/ethanol = 10:1)
C29H30N406''S ESI mass spectrum: m/z = 561 [M-H-]
(17) 3-Z-[1-(4-(imidazol-4-yl)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(imidazol-4-yl)-aniline
R. value: 0.5 (silica gel, methylene chloride/ethanol/ammonia
= 10:1:0.01)
C27H22N403
Mass spectrum: m/z = 450 [M+]
(18) 3-Z- [1- (4- (tetrazol-5-yl) -anilino) -1-phenyl-methylene] -6-
ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(tetrazol-5-yl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/ethanol = 5:1)
C2sH2oN603
ESI mass spectrum: m/z = 451 [M-H-]
(19) 3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(N-benzyl-N-methyl-
aminomethyl)-aniline
Rf value: 0.4 (silica gel, methylene chloride/ethanol = 10:1)
C33H31N303
ESI mass spectrum: m/z = 516 [M-H-]
(20) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-propionyl-amino)-
aniline)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 137 -
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-[N-(2-dimethylamino-ethyl)-
N-propionyl-amino]-aniline
Rf value: 0.2 (silica gel, methylene chloride/ethanol = 5:1)
C31H34N404
ESI mass spectrum: m/z = 525 [M-H-]
(21) 3-Z- [1- (4- (pyrrolidin-l-yl-methyl) -anilino) -1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(pyrrolidin-1-yl-methyl)-
aniline
Rf value: 0.1 (silica gel, methylene chloride/ethanol = 5:1)
C29H29N303
ESI mass spectrum: m/z = 466 [M-H-]
(22) 3-Z-[1-(4-(N-methyl-N-phenethyl-aminomethyl)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(N-phenethyl-N-methyl-
aminomethyl)-aniline
Rf value: 0.4 (silica gel, methylene chloride/ethanol = 10:1)
C34H33N3O3
ESI mass spectrum: m/z = 530 [M-H-]
(23) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and N-dimethylaminomethylcarbonyl-
N-methyl-p-phenylenediamine
Rf value: 0.1 (silica gel, methylene chloride/ethanol = 10:1)
C29H30N404
ESI mass spectrum: m/z = 497 [M-H-]
CA 02387013 2002-03-19
- 138 -
(24) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-ethylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and N-(2-dimethylamino-ethyl)-N-
ethylsulphonyl-p-phenylenediamine
Rf value: 0.6 (silica gel, methylene chloride/ethanol = 5:1)
C30H34N4O5''S
ESI mass spectrum: m/z = 561 [M-H-]
(25) 3-Z-[1-(4-(N-tert.butoxycarbonyl-N-ethyl-aminomethyl)-
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and 4-(N-tert.butoxycarbonyl-N-
ethyl-aminomethyl)-aniline
R. value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C32H35N3O5
ESI mass spectrum: m/z = 540 [M-H-]
(26) 3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-1-ethyl-
methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-ethyl-methylene)-6-
ethoxycarbonyl-2-indolinone and 4-(piperidin-1-yl-methyl)-
aniline
Rf value: 0.9 (silica gel, methylene chloride/ethanol = 5:1)
C26H31N303
ESI mass spectrum: m/z = 432 [M-H-]
(27) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-i-ethyl-methylene]-6-ethoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-ethyl-methylene)-6-
ethoxycarbonyl-2-indolinone and N-(2-dimethylamino-ethyl)-N-
methylsulphonyl-p-phenylenediamine
Rf value: 0.3 (silica gel, methylene chloride/ethanol = 5:1)
C25H32N405S
CA 02387013 2002-03-19
- 139 -
ESI mass spectrum: m/z = 499 [M-H-]
(28) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(dimethylaminomethyl)-
aniline
Rf value: 0.6 (silica gel, methylene chloride/methanol = 5:1)
C26H25N303
ESI mass spectrum: m/z = 428 [M+H+]
(29) 3-Z-[1-(4-[(2,6-dimethyl-piperidin-1-yl)-methyl]-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4- [(2,6-dimethyl-piperidin-l-
yl) -methyl] -aniline
Rf value: 0.5 (RP 8, methanol/five percent saline solution =
4:1)
C31H33N303
ESI mass spectrum: m/z = 496 [M+H+]
(30) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(2-dimethylamino-ethyl)-N-
methylsulphonyl-p-phenylenediamine
Rf value: 0.6 (silica gel, methylene chloride/methanol = 5:1)
C28H30N4O5''S
ESI mass spectrum: m/z = 533 [M-H-]
(31) 3-Z-[1-(4-(N-(3-dimethylamino-propyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
CA 02387013 2002-03-19
- 140 -
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(3-dimethylamino-propyl)-N-
methylsulphonyl-p-phenylenediamine
Rf value: 0.5 (aluminium oxide, methylene chloride/methanol =
30:1)
C29H32N405S
ESI mass spectrum: m/z = 547 [M-H-]
(32) 3-Z-[1-(4-(N-dimethylaminocarbonylmethyl-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-dimethylaminocarbonyl-
methyl-N-methylsulphonyl)-amino)-aniline
Rf value: 0.5 (aluminium oxide, methylene chloride/methanol =
20:1)
C28H28N4O6S
ESI mass spectrum: m/z = 547 [M-H-]
(33) 3-Z-[1-(4-(N-acetyl-N-dimethylaminocarbonylmethyl-amino)-
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-acetyl-N-
dimethylaminocarbonylmethyl)-amino)-aniline
Rf value: 0.6 (silica gel, methylene chloride/methanol = 10:1)
C29H28N405
ESI mass spectrum: m/z = 511 [M-H-]
(34) 3-Z-[1-(4-(N-dimethylaminocarbonylmethyl-amino)-anilino)-
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4- (N-dimethylaminocarbonyl-
methyl) -amino) -aniline
Rf value: 0.6 (aluminium oxide, methylene chloride/methanol =
30:1)
C27H26N404
CA 02387013 2002-03-19
- 141 -
ESI mass spectrum: m/z = 469 [M-H-]
(35) 3-Z- [1- (4- (N- (3-dimethylamino-propyl) -N-acetyl-amino) -
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(3-dimethylamino-propyl)-N-
acetyl-p-phenylenediamine
Rf value: 0.5 (aluminium oxide, methylene chloride/methanol =
20:1)
C30H32N404
ESI mass spectrum: m/z = 511 [M-H-]
(36) 3-Z-[1-(4-(N-methylaminocarbonylmethyl-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-methylaminocarbonylmethyl-
N-methylsulphonyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C27H26N406S
ESI mass spectrum: m/z = 533 [M-H-]
(37) 3-Z-[1-(4-((imidazolidin-2,4-dion-5-ylidene)-methyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((imidazolidin-2,4-dion-5-
ylidene)-methyl)-aniline
Rf value: 0.4 (silica gel, methylene chloride/methanol = 10:1)
C27H20N405
ESI mass spectrum: m/z = 479 [M-H-]
(38) 3-Z-[1-(4-(N-((2-dimethylamino-ethyl)-carbonyl)-N-methyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
CA 02387013 2002-03-19
- 142 -
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-((2-dimethylamino-ethyl)-
carbonyl)-N-methyl-p-phenylenediamine
Rf value: 0.5 (aluminium oxide, methylene chloride/methanol =
20:1)
C29H30N4Q4
ESI mass spectrum: m/z = 497 [M-H-]
(39) 3-Z-[1-(4-(N-tert.butoxycarbonyl-aminomethyl)-anilino)-1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-tert.butoxycarbonyl-
aminomethyl)-aniline
Rf value: 0.3 (aluminium oxide, methylene chloride/methanol =
20:1)
C29H29N305
ESI mass spectrum: m/z = 498 [M-H-]
(40) 3-Z-[l-(4-(2-oxo-pyrrolidin-1-yl-methyl)-anilino)-1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(2-oxo-pyrrolidin-1-yl-
methyl)-aniline
Rf value: 0.3 (silica gel, methylene chloride/methanol = 20:1)
C2aH2sN304
ESI mass spectrum: m/z = 466 [M-H-]
(41) 3-Z-[1-(4-(N-aminocarbonylmethyl-N-methylsulphonyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-aminocarbonylmethyl-N-
methylsulphonyl-p-phenylenediamine
Rf value: 0.7 (silica gel, methylene chloride/methanol = 5:1)
Ca6H24N40eS
ESI mass spectrum: m/z = 519 [M-H-]
CA 02387013 2002-03-19
- 143 -
(42) 3-Z- [1- (4- (thiomorpholin-4-yl-methyl) -anilino) -1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(thiomorpholin-4-yl-
methyl)-aniline
Rf value: 0.4 (silica gel, methylene chloride/methanol = 15:1)
C28H27N303S
ESI mass spectrum: m/z = 484 [M-H-]
(43) 3-Z-[1-(4-(1,1-dioxo-thiomorpholin-4-yl-methyl)-anilino)-
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(1,1-dioxo-thiomorpholin-4-
yl-methyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C28H27N305S
ESI mass spectrum: m/z = 516 [M-H-]
(44) 3-Z-[1-(4-(N-cyanomethyl-N-methylsulphonyl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-cyanomethyl-N-methyl-
sulphonyl-p-phenylenediamine
Rf value: 0.6 (silica gel, methylene chloride/methanol = 10:1)
C26H22N405S
ESI mass spectrum: m/z = 501 [M-H-]
(45) 3-Z-[1-(4-(N-tert.butoxycarbonyl-ethylaminomethyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-ethyl-N-
tert.butoxycarbonyl-aminomethyl)-aniline
Rf value: 0.6 (silica gel, methylene chloride/methanol = 10:1)
C31H33N305
ESI mass spectrum: m/z = 526 [M-H-]
CA 02387013 2002-03-19
- 144 -
(46) 3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-anilino)-1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-benzyl-N-methyl-
aminomethyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C3aHz9N303
ESI mass spectrum: m/z = 502 [M-H-1
(47) 3-Z-[1-(4-(1-oxo-thiomorpholin-4-yl-methyl)-anilino)-1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(1-oxo-thiomorpholin-4-yl-
methyl)-aniline
Rf value: 0.7 (silica gel, methylene chloride/methanol = 10:1)
C28H27N304S
ESI mass spectrum: m/z = 500 [M-H-1
(48) 3-Z-[1-(4-(2-(imidazol-4-yl)-ethyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(2-(imidazol-4-yl)-ethyl)-
aniline
Rf value: 0.4 (silica gel, methylene chloride/methanol = 5:1)
CzaHaeNa03
ESI mass spectrum: m/z = 463 [M-H-1
(49) 3-Z-[1-(4-(morpholin-4-yl-methyl)-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(morpholin-4-yl-methyl)-
aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C28H27N304
ESI mass spectrum: m/z = 468 [M-H-1
CA 02387013 2002-03-19
- 145 -
(50) 3-Z- [1- (4- ( (4-methyl-piperazin-1-yl) -methyl) -anilino) -i-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((4-methyl-piperazin-1-yl)-
methyl)-aniline
Rf value: 0.4 (silica gel, methylene chloride/methanol 5:1)
C29H30N403
ESI mass spectrum: m/z = 481 [M-H-]
(51) 3-Z-[1-(4-((2-(N-benzyl-N-methyl-amino)-ethyl)-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-(N-benzyl-N-methyl-
amino)-ethyl)-N-methylsulphonyl-amino)-aniline
Rf value: 0.7 (silica gel, methylene chloride/methanol = 10:1)
C34H34N405S
ESI =mass spectrum: m/z = 609 [M-H-]
(52) 3-Z-[1-(4-cyclohexylamino-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-cyclohexyl-p-
phenylenediamine
Rf value: 0.8 (silica gel, methylene chloride/methanol = 10:1)
C29H28N203
ESI mass spectrum: m/z = 451 [M-H-]
(53) 3-Z-[1-(4-(pyridin-4-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(pyridin-4-yl-methyl)-
aniline
Rf value: 0.6 (silica gel, methylene chloride/methanol/ammonia
= 5:1:0.01)
CA 02387013 2002-03-19
- 146 -
C29H23N303
ESI mass spectrum: m/z = 460 [M-H-]
(54) 3-Z-[1-(4-(imidazol-l-yl-methyl)-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(imidazol-l-yl-methyl)-
aniline
R. value: 0.4 (silica gel, methylene chloride/methanol/ammonia
= 10:1:0.01)
C27H22N403
ESI mass spectrum: m/z = 449 [M-H-]
(55) 3-Z-[l-(4-(imidazol-l-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(imidazol-1-yl-methyl)-
aniline
Rf value: 0.4 (silica gel, methylene chloride/methanol/ammonia
= 10:1:0.01)
C27H22N403
ESI mass spectrum: m/z = 449 [M-H-]
(56) 3-Z-[1-(N-methyl-piperidine-4-yl-amino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-amino-l-methyl-piperidine
Rf value: 0.3 (silica gel, methylene chloride/methanol = 5:1)
C23H25N303
ESI mass spectrum: m/z = 390 [M-H-]
(57) 3-Z-[1-(4-(imidazol-4-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(imidazol-4-yl-methyl)-
aniline
CA 02387013 2002-03-19
- 147 -
Rf value: 0.2 (silica gel, methylene chloride/methanol = 5:1)
C27H22N4O3
ESI mass spectrum: m/z = 449 [M-H-]
(58) 3-Z- [1- (4- ( (4-hydroxy-piperidin-1-yl) -methyl) -anilino) -1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4- ((4-hydroxy-piperidin-l-
yl) -methyl) -aniline
R. value: 0.1 (silica gel, methylene chloride/methanol = 10:1)
C29H29N303
ESI mass spectrum: m/z = 482 [M-H-]
(59) 3-Z-[1-(4-((4-methoxy-piperidin-1-yl)-methyl)-anilino)-1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((4-methoxy-piperidin-l-
yl)-methyl)-aniline
Rf value: 0.4 (silica gel, methylene chloride/methanol = 10:1)
C30H3iN304
ESI mass spectrum: m/z = 496 [M-H-]
(60) 3-Z-[1-(4-benzyl-anilino)-1-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-benzyl-aniline
Rf value: 0.6 (silica gel, methylene chloride/methanol = 10:1)
C30H24N203
Melting point: 224 C
(61) 3-Z- [1- (4- (N- (3-trifluoroacetylamino-propyl) -N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(3-trifluoroacetylamino-
propyl)-N-methylsulphonyl-p-phenylenediamine
CA 02387013 2002-03-19
- 148 -
Rf value: 0.5 (aluminium oxide, methylene chloride/methanol =
20:1)
C29H27F3N406S
ESI mass spectrum: m/z = 615 [M-H-]
(62) 3-Z-[1-(4-tert.butoxycarbonylmethyl-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
ethoxycarbonyl-2-indolinone and tert.butyl 4-
aminophenylacetate
Rf value: 0.5 (aluminium oxide, ethyl acetate)
C30H30N205
ESI mass spectrum: m/z = 497 [M-H-]
(63) 3-Z-[1-(4-tert.butoxycarbonyl-anilino)-i-ethyl-
methylene]-6-ethoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-ethylmethylen)-6-
ethoxycarbonyl-2-indolinone and 4-tert.butoxycarbonyl-aniline
Rf value: 0.4 (aluminium oxide, methylene chloride/ethanol =
20:1)
C25H28N205
ESI mass spectrum: m/z = 435 [M-H-]
(64) 3-Z-[1-(4-(4-tert.butoxycarbonyl-piperazin-1-yl-methyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(4-tert.butoxycarbonyl-
piperazin-1-yl-methyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C33H36N405
ESI mass spectrum: m/z = 567 [M-H-]
(65) 3-Z-[1-(4-(1-methyl-imidazol-2-yl)-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 149 -
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(1-methyl-imidazol-2-yl)-
aniline
Rf value: 0.6 (silica gel, methylene chloride/methanol = 5:1)
C27H22N403
ESI mass spectrum: m/z = 449 [M-H-]
(66) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-3-nitro-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-
2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 6-(N-(2-dimethylamino-ethyl)-
N-methylsulphonyl-amino)-3-amino-nitrobenzene
Rf value: 0.6 (silica gel, methylene chloride/methanol = 5:1)
C28H29N507S
ESI mass spectrum: m/z = 578 [M-H-]
(67) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-3-amino-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-
2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-methylsulphonyl-amino)-3-amino-aniline
Rf value: 0.5 (aluminium oxide, methylene chloride/methanol =
20:1)
C28H3iN505S
ESI mass spectrum: m/z = 548 [M-H-]
(68) 3-Z-[1-(4-((3-(N-benzyl-N-methyl-amino)-propyl)-N-methyl-
sulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(3-(N-benzyl-N-methyl-
amino)-propyl)-N-methylsulphonyl-amino)-aniline
Rf value: 0.6 (silica gel, methylene chloride/methanol = 10:1)
C35H36N405S
CA 02387013 2002-03-19
- 150 -
ESI mass spectrum: m/z = 623 [M-H-]
(69) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-3-chloro-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-methylsulphonyl-amino)-3-chloro-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C28H29C1N4O5S
ESI mass spectrum: m/z = 567/569 [M-H-]
(70) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-amino)-
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-
dimethylaminomethylcarbonyl-N-methyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C28H28N404
ESI mass spectrum: m/z = 483 [M-H-]
(71) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-acetyl-amino) -
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-acetyl-amino)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C29H30N404
ESI mass spectrum: m/z = 497 [M-H-]
(72) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-propionyl-amino)-
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-propionyl-amino)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
CA 02387013 2002-03-19
- 151 -
C30H32N404
ESI mass spectrum: m/z = 511 [M-H-]
(73) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-butyryl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-butyryl-amino)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C31H34N4O4
ESI mass spectrum: m/z = 525 [M-H-]
(74) 3-Z-[l-(4-(N-(2-dimethylamino-ethyl)-N-isobutyryl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-isobutyryl-amino)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C31H34N404
ESI mass spectrum: m/z = 525 [M-H-]
(75) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-benzoyl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-benzoyl-amino)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C34H32N404
ESI mass spectrum: m/z = 559 [M-H-]
(76) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-acetyl-amino)-3-
amino-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-acetyl-amino)-3-amino-aniline
CA 02387013 2002-03-19
- 152 -
Rf value: 0.5 (aluminium oxide, methylene chloride/methanol =
20:1)
C29H31N5O4
ESI mass spectrum: m/z = 512 [M-H-]
(77) 3-Z-[1-(4-(4-hydroxymethyl-piperidin-1-yl-methyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(4-hydroxymethyl-piperidin-
1-yl-methyl-amino)-aniline
Rf value: 0.3 (silica gel, methylene chloride/methanol = 5:1)
C30H31N304
ESI mass spectrum: m/z = 496 [M-H-]
(78) 3-Z-[l-(4-(2-(4-hydroxy-piperidin-1-yl)-ethyl)-anilino)-
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(2-(4-hydroxy-piperidin-l-
yl)-ethyl-amino)-aniline
Rf value: 0.3 (silica gel, methylene chloride/methanol = 5:1)
C30H31N304
ESI mass spectrum: m/z = 496 [M-H-]
(79) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-propylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(2-dimethylamino-ethyl)-N-
propylsulphonyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C30H34N405S
ESI mass spectrum: m/z = 561 [M-H-]
(80) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-butylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
CA 02387013 2002-03-19
- 153 -
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(2-dimethylamino-ethyl)-N-
butylsulphonyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C31H36N4O5'S
ESI mass spectrum: m/z = 575 [M-H-]
(81) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-phenylsulphonyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(2-dimethylamino-ethyl)-N-
phenylsulphonyl-p-phenylenediamine
R. value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C33H32N405''S
ESI mass spectrum: m/z = 595 [M-H-]
(82) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-benzylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(2-dimethylamino-ethyl)-N-
benzylsulphonyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C34H34N405S
ESI mass spectrum: m/z = 609 [M-H-]
(83) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-ethylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(2-dimethylamino-ethyl)-N-
ethylsulphonyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C29H32N405'S
ESI mass spectrum: m/z = 547 [M-H-]
CA 02387013 2002-03-19
- 154 -
(84) 3-Z-[1-(4-((imidazolidin-2,4-dion-5-yl)-methyl)-anilino)-
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4- ((imidazolidin-2,4-dion-5-
yl ) -methyl ) -aniline
Rf value: 0.6 (silica gel, methylene chloride/methanol = 5:1)
C27H22N405
ESI mass spectrum: m/z = 481 [M-H-]
(85) 3-Z-[1-(4-((3-hydroxy-pyrrolidin-1-yl)-methyl)-anilino)-
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((3-hydroxy-pyrrolidin-l-
yl)-methyl)-aniline
Rf value: 0.1 (silica gel, methylene chloride/methanol = 10:1)
C28H27N304
ESI mass spectrum: m/z = 468 [M-H-]
(86) 3-Z-[1-(4-(cyclohexylyl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(cyclohexyl-methyl)-aniline
(Eur. J. Med. Chem. Chim. Ther. 1992, 27, 537-544)
Rf value: 0.6 (silica gel, methylene chloride/methanol = 10:1)
C30H3aN203
ESI mass spectrum: m/z = 465 [M-H-]
(87) 3-Z-[1-(4-(cyclohexyl-carbonyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(cyclohexyl-carbonyl)-
aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C30H28N204
ESI mass spectrum: m/z = 479 [M-H-]
CA 02387013 2002-03-19
- 155 -
(88) 3-Z-[1-(4-diethylaminomethyl-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(diethylamino-methyl)-
aniline
Rf value: 0.4 (silica gel, methylene chloride/methanol = 10:1)
C28H29N303
ESI mass spectrum: m/z = 454 [M-H-]
(89) 3-Z- [1- (4- (N- (n-hexyl) -N-methyl-aminomethyl) -anilino) -1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(n-hexyl)-N-methyl-
aminomethyl)-aniline
Rf value: 0.6 (silica gel, methylene chloride/methanol = 10:1)
C31H35N303
ESI mass spectrum: m/z = 496 [M-H-]
(90) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N- (furan-2-
carbonyl)-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-(furan-2-carbonyl)-amino)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C32H30N405
ESI mass spectrum: m/z = 549 [M-H-]
(91) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N- (2-methoxy-
benzoyl)-amino)-anilino)-1-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-(2-methoxy-benzoyl)-amino)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
CA 02387013 2002-03-19
- 156 -
C35H34N405
ESI mass spectrum: m/z = 589 [M-H"]
(92) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N- (pyridine-3-
carbonyl)-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-(pyridine-3-carbonyl)-amino)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C33H31N504
ESI mass spectrum: m/z = 560 [M-H-]
(93) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N- (phenyl-acetyl) -
amino)-anilino)-1-phenyl-methylenel-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-(phenyl-acetyl)-amino)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C35H34N404
ESI mass spectrum: m/z = 573 [M-H-]
(94) 3-Z- [1- (4- (N-ethyl-N-methyl-aminomethyl) -anilino) -1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-ethyl-N-methyl-
aminomethyl)-aniline
Rf value: 0.3 (silica gel, methylene chloride/methanol = 10:1)
C27H27N303
ESI mass spectrum: m/z = 440 [M-H-]
(95) 3-Z-[1-(4-(imidazol-2-yl)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(imidazol-2-yl)-aniline
CA 02387013 2002-03-19
- 157 -
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C26H2ON403
ESI mass spectrum: m/z = 435 [M-H-1
(96) 3-Z-[1-(4-(i-ethyl-imidazol-2-yl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(1-ethyl-imidazol-2-yl)-
aniline
Rf value: 0.4 (silica gel, methylene chloride/methanol = 10:1)
C28H24N403
ESI mass spectrum: m/z = 463 [M-H-1
(97) 3-Z-[1-(4-(1-benzyl-imidazol-2-yl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(1-benzyl-imidazol-2-yl)-
aniline
Rf value: 0.3 (silica gel, methylene chloride/methanol = 20:1)
C33H26N4Q3
ESI mass spectrum: m/z = 525 [M-H-1
(98) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-
isopropylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(2-dimethylamino-ethyl)-N-
isopropylsulphonyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C30H34N4O5S
ESI mass spectrum: m/z = 561 [M-H-1
(99) 3-Z-[1-(4-(N-(piperidin-1-yl-methylcarbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
CA 02387013 2002-03-19
- 158 -
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(piperidin-1-yl-
methylcarbonyl)-N-methyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C31H32N4O4
ESI mass spectrum: m/z = 523 [M-H-]
(100) 3-Z-[1-(4-(N-(morpholin-4-yl-methylcarbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylenel-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(morpholin-4-yl-
methylcarbonyl)-N-methyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C30H30N405
ESI mass spectrum: m/z = 525 [M-H-]
(101) 3-Z-[1-(4-(N-((4-benzyl-piperazin-1-yl)-methylcarbonyl)-
N-methyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-((4-benzyl-piperazin-1-yl)-
methylcarbonyl)-N-methyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C37H37Ns04
ESI mass spectrum: m/z = 614 [M-H-]
(102) 3-Z-[l-(4-(N-(pyrrolidin-1-yl-methylcarbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylenel-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(pyrrolidin-l-yl-
methylcarbonyl)-N-methyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C30H30N404
ESI mass spectrum: m/z = 509 [M-H-]
CA 02387013 2002-03-19
- 159 -
(103) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-acetyl-amino) -3-
bromo-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(2-dimethylamino-ethyl)-
N-acetyl-amino)-3-bromo-aniline
Rf value: 0.6 (silica gel, methylene chloride/methanol = 5:1)
C29H29BrN4O4
ESI mass spectrum: m/z = 575/577 [M-H-]
(104) 3-Z-[1-(4-(5-methyl-imidazol-4-yl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(5-methyl-imidazol-4-yl)-
aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol/ammonia
= 10:1:0.01)
C27H22N403
ESI mass spectrum: m/z = 449 [M-H-]
(105) 3-Z- [1- (4- (N- ( (2-dimethylamino-ethyl) -carbonyl) -N-
isopropyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-((2-dimethylamino-ethyl)-
carbonyl)-N-isopropyl-p-phenylenediamine
Rf value: 0.1 (silica gel, methylene chloride/methanol = 10:1)
C31H34N404
ESI mass spectrum: m/z = 525 [M-H"]
(106) 3-Z- [1- (4- (N- ( (2-dimethylamino-ethyl) -carbonyl) -N-
benzyl-amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-
2-indolinone
CA 02387013 2002-03-19
- 160 -
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-((2-dimethylamino-ethyl)-
carbonyl)-N-benzyl-p-phenylenediamine
Rf value: 0.1 (silica gel, methylene chloride/methanol = 10:1)
C31H34N404
ESI mass spectrum: m/z = 525 [M-H-]
(107) 3-Z-[1-(4-(N-butyl-N-tert.butoxycarbonyl-aminomethyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-butyl-N-
tert.butoxycarbonyl-aminomethyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C33H37N305
ESI mass spectrum: m/z = 554 [M-H-]
(108) 3-Z- [1- (4- (N- ( (N-aminocarbonylmethyl-N-methyl-amino) -
methylcarbonyl)-N-methyl-amino)-anilino)-i-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(N-aminocarbonylmethyl-N-
methyl-amino)-methylcarbonyl)-N-methyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C29H29N50s
ESI mass spectrum: m/z = 526 [M-H-]
(109) 3-Z-[1-(4-(N-((N-benzyl-N-methyl-amino)-methylcarbonyl)-
N-methyl-amino)-anilino)-i-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-((N-benzyl-N-methyl-amino)-
methylcarbonyl)-N-methyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C34H32N4O4
ESI mass spectrum: m/z = 559 [M-H-]
CA 02387013 2002-03-19
- 161 -
(110) 3-Z-[1-(4-(N-(di-(2-methoxyethyl)-amino-methylcarbonyl)-
N-methyl-amino)-anilino)-i-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(di-(2-methoxyethyl)-amino-
methylcarbonyl)-N-methyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C32H36N406
ESI mass spectrum: m/z = 571 [M-H-]
(111) 3-Z- [1- (4- (N- ( (2- (4-tert.butoxycarbonyl-piperazin-1-yl) -
ethyl)-carbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-((2-(4-tert.butoxycarbonyl-
piperazin-1-yl)-ethyl)-carbonyl)-N-methyl-p-phenylenediamine
Rf value: 0.8 (silica gel, methylene chloride/methanol = 5:1)
C36H41N5O6
ESI mass spectrum: m/z = 638 [M-H-]
(112) 3-Z- [1- (4- (N- ( (2- (piperidin-l-yl) -ethyl) -carbonyl) -N-
methyl-amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-
2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-((2-(piperidin-1-yl)-
ethyl)-carbonyl)-N-methyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 5:1)
C32H34N404
ESI mass spectrum: m/z = 537 [M-H-]
(113) 3-Z- [1- (4- (N- ( (2- (N-benzyl-N-methyl-amino) -ethyl) -
carbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-((2-(N-benzyl-N-methyl-
amino)-ethyl)-carbonyl)-N-methyl-p-phenylenediamine
= CA 02387013 2002-03-19
- 162 -
Rf value: 0.4 (silica gel, methylene chloride/methanol = 10:1)
C35H34N404
ESI mass spectrum: m/z = 573 [M-H-]
(114) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-isopropyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-
(dimethylaminomethylcarbonyl)-N-isopropyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C30H32N4O4
ESI mass spectrum: m/z = 511 [M-H-]
(115) 3-Z- [1- (4- (N- (piperidin-1-yl-methylcarbonyl) -N-
isopropyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(piperidin-1-yl-
methylcarbonyl)-N-isopropyl-p-phenylenediamine
R. value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C33H36N404
ESI mass spectrum: m/z = 551 [M-H-]
(116) 3-Z-[l-(4-(N-((4-tert.butoxycarbonyl-piperazin-l-yl)-
methylcarbonyl)-N-isopropyl-amino)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-((4-tert.butoxycarbonyl-
piperazin-1-yl)-methylcarbonyl)-N-isopropyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C37H43N506
ESI mass spectrum: m/z = 652 [M-H-]
CA 02387013 2002-03-19
- 163 -
(117) 3-Z- [1- (4- (N- ( (N-benzyl-N-methyl-amino) -methylcarbonyl) -
N-benzyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-((N-benzyl-N-methyl-amino)-
methylcarbonyl)-N-benzyl-p-phenylenediamine
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C40H36N404
ESI mass spectrum: m/z = 635 [M-H-]
(118) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-benzyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and N-(dimethylaminomethyl-
carbonyl)-N-benzyl-p-phenylenediamine
R. value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C34H32N404
ESI mass spectrum: m/z = 559 [M-H-]
(119) 3-Z-[1-(4-(N-(piperidin-1-yl-methylcarbonyl)-N-benzyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(5-methyl-imidazol-4-yl)-
aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C37H36N404
ESI mass spectrum: m/z = 559 [M-H-]
(120) 3-Z-[1-(4-(1,2,4-triazol-2-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(i-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(1,2,4-triazol-1-yl-
methyl)-aniline
R. value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
CA 02387013 2002-03-19
- 164 -
C26H21N503
ESI mass spectrum: m/z = 450 [M-H-]
(121) 3-Z-[1-(4-(1,2,3-triazol-2-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(1,2,3-triazol-2-yl-
methyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 20:1)
C26H21N503
ESI mass spectrum: m/z = 450 [M-H-]
(122) 3-Z-[1-(4-(1,2,3-triazol-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(1,2,3-triazol-1-yl-
methyl)-aniline
Rf value: 0.4 (silica gel, methylene chloride/methanol = 9:1)
C26H21N503
ESI mass spectrum: m/z = 450 [M-H-]
(123) 3-Z-[1-(4-((N-aminocarbonylmethyl-N-methyl-amino)-
methyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((N-aminocarbonylmethyl-N-
methyl-amino)-methyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C27H26N404
ESI mass spectrum: m/z = 469 [M-H-]
(124) 3-Z- [1- (4- ( (di- (2-methoxy-ethyl) -amino) -methyl) -
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((di-(2-methoxy-ethyl)-
amino)-methyl)-aniline
CA 02387013 2002-03-19
- 165 -
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C30H33N305
ESI mass spectrum: m/z = 514 [M-H-]
(125) 3-Z-[1-(4-(pyrrolidin-l-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(pyrrolidin-1-yl-methyl)-
aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C28H27N303
ESI mass spectrum: m/z = 452 [M-H-]
(126) 3-Z- [1- (4- ( (di- (2-hydroxy-ethyl) -amino) -methyl) -
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4- ((di-(2-hydroxy-ethyl)-
amino) -methyl) -aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C28H29N305
ESI mass spectrum: m/z = 486 [M-H-]
(127) 3-Z-[1-(4-((N-ethoxycarbonylmethyl-N-methyl-amino)-
methyl)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((N-ethoxycarbonylmethyl-N-
methyl-amino)-methyl)-aniline
Rf value: 0.5 (aluminium oxide, methylene chloride/ethanol =
40:1)
C29H29N305
ESI mass spectrum: m/z = 498 [M-H-]
(128) 3-Z-[1-(4-(azetidin-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 166 -
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(azetidin-1-yl-methyl)-
aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol/ammonia
= 9:1:0.5)
C27H25N303
ESI mass spectrum: m/z = 438 [M-H"]
(129) 3-Z-[1-(4-(N-propyl-N-tert.butoxycarbonyl-aminomethyl)-
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-propyl-N-
tert.butoxycarbonyl-aminomethyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C32H35N305
ESI mass spectrum: m/z = 540 [M-H-]
(130) 3-Z- [l- (4 ( (N- (2- (2-methoxy-ethoxy) -ethyl) -N-methyl-
amino)-methyl)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-
2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((N-(2-(2-methoxy-ethoxy)-
ethyl)-N-methyl-amino)-methyl)-aniline
R. value: 0.4 (silica gel, methylene chloride/methanol = 9:1)
C30H33N305
ESI mass spectrum: m/z = 514 [M-H"]
(131) 3-Z-[1-(4-((N-(tert.butoxycarbonyl-3-amino-propyl)-N-
methyl-amino)-methyl)-anilino)-i-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(N-(N-tert.butoxycarbonyl-
3-amino-propyl)-N-methyl-aminomethyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C33H38N4O5
ESI mass spectrum: m/z = 571 [M+H+]
CA 02387013 2002-03-19
- 167 -
(132) 3-Z-[1-(4-((N-(methylcarbamoyl-methyl)-N-methyl-amino)-
methyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((N-(methylcarbamoyl-
methyl)-N-methyl-amino)-methyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C28H28N4O4
ESI mass spectrum: m/z = 483 [M-H-]
(133) 3-Z-[1-(4-((N-(dimethylcarbamoyl-methyl)-N-methyl-
amino)-methyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-
2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((N-(dimethylcarbamoyl-
methyl)-N-methyl-amino)-methyl)-aniline
Rf value: 0.3 (silica gel, methylene chloride/methanol = 10:1)
C29H30N4O4
ESI mass spectrum: m/z = 497 [M-H-]
(134) 3-Z-[1-(4-methyl-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-methyl-aniline
Rf value: 0.4 (silica gel, methylene chloride/methanol = 9:1)
C24H20N203
ESI mass spectrum: m/z = 383 [M-H-]
(135) 3-Z-[1-(4-((N-propyl-N-methyl-amino)-methyl)-anilino)-1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((N-propyl-N-methyl-amino)-
methyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C28H29N3O3
CA 02387013 2002-03-19
- 168 -
ESI mass spectrum: m/z = 454 [M-H-]
(136) 3-Z- [1- (4- ( (N- (2-hydroxy-ethyl) -N-methyl-amino) -methyl) -
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((N-(2-hydroxy-ethyl)-N-
methyl-amino)-methyl)-aniline
Rf value: 0.5 (aluminium oxide, methylene chloride/ethanol =
40:1)
C27H27N304
ESI mass spectrum: m/z = 456 [M-H-]
(137) 3-Z-[1-(4-((N-(2-dimethylamino-ethyl)-N-methyl-amino)-
methyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((N-(2-dimethylamino-
ethyl)-N-methyl-amino)-methyl)-aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C29H32N403
ESI mass spectrum: m/z = 483 [M-H-]
(138) 3-Z-[1-(4-((N-(3-dimethylamino-propyl)-N-methyl-arnino)-
methyl)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 1-acetyl-3-(l-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-((N-(3-dimethylamino-
propyl)-N-methyl-amino)-methyl)-aniline aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C30H3aNa03
ESI mass spectrum: m/z = 497 [M-H-]
(139) 3-Z-[1-(4-(3-oxo-piperazin-1-yl-methyl)-anilino)-1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 169 -
Prepared from 1-acetyl-3-(1-ethoxy-l-phenylmethylene)-6-
methoxycarbonyl-2-indolinone and 4-(3-oxo-piperazin-1-yl-
methyl)-aniline aniline
Rf value: 0.46 (silica gel, methylene chloride/methanol = 9:1)
C28H26N404
ESI mass spectrum: m/z = 481 [M-H-]
Exam l
3-Z-[1-(4-carboxy-anilino)-i-phenyl-methylene]-6-ethoxy-
canconyl - 2 - i ndol inone
485 mg of 3-Z-[1-(4-tert.butoxycarbonyl-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone are dissolved in 15
ml of methylene chloride and 6.0 ml of trifluoroacetic acid
are added. The mixture is stirred for 2 hours at room
temperature. Then the solvent is removed and the residue
recrystallised from ether.
Yield: 375 mg (87 %~ of theory),
Rf value: 0.3 (silica gel, methylene chloride/methanol = 10:1)
C2sH2oN20s
Mass spectrum: m/z = 428 [M+]
The following compounds are prepared analogously to Example 4:
(1) 3-Z-[1-(4-aminomethyl-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-(N-tert.butoxycarbonyl-aminomethyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Rf value: 0.5 (silica gel, methylene chloride/methanol/ammonia
= 5:1:0.01)
C24H21N3O3
ESI mass spectrum: m/z = 398 [M-H-]
(2) 3-Z-[1-(4-ethylaminomethyl-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 170 -
Prepared from 3-Z-[1-(4-(N-tert.butoxycarbonyl-ethylamino-
methyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Rf value: 0.4 (silica gel, methylene chloride/methanol/ammonia
= 10:1:0.01)
C26H2sN303
ESI mass spectrum: m/z = 426 [M-H-]
(3) 3-Z-[1-(4-carboxymethyl-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-tert.butoxycarbonylmethyl-anilino)-i-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Rf value: 0.1 (aluminium oxide, methylene
chloride/ethanol/ammonia = 5:1:0.01)
C26H22N205
ESI mass spectrum: m/z = 441 [M-H-]
(4) 3-Z-[1-(4-carboxy-anilino)-1-ethyl-methylene]-6-ethoxy-
carbonyl-2-indolinone
Prepared from 3-Z-[1-(4-tert.butoxycarbonyl-anilino)-i-ethyl-
methylene]-6-ethoxycarbonyl-2-indolinone
Rf value: 0.1 (aluminium oxide, methylene chloride/ethanol =
20:1)
C2iH2oN20s
ESI mass spectrum: m/z = 379 [M-H-]
(5) 3-Z- [1- (4- (piperazin-1-yl-methyl) -anilino) -1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-(4-tert.butoxycarbonyl-piperazin-1-yl-
methyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Rf value: 0.1 (silica gel, methylene chloride/methanol/ammonia
= 10:1:0.01)
C28H28N403
ESI mass spectrum: m/z = 469 [M+H+]
CA 02387013 2002-03-19
- 171 -
(6) 3-Z-[1-(4-butylaminomethyl-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
Prepared from 3-Z-[l-(4-(N-butyl-N-tert.butoxycarbonyl-
aminomethyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C28H29N303
ESI mass spectrum: m/z = 454 [M-H-]
(7) 3-Z-[1-(4-ethylaminomethyl-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-(N-tert.butoxycarbonyl-N-ethyl-
aminomethyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
Rf value: 0.3 (silica gel, methylene chloride/methanol/ammonia
= 10:1:0.01)
C27H2'7N303
ESI mass spectrum: m/z = 442 [M+H']
(8) 3-Z-[1-(4-ethylaminomethyl-anilino)-1-phenyl-methylene]-6-
carbamoyl-2-indolinone
Prepared from 3-Z-[1-(4-(N-tert.butoxycarbonyl-ethylamino-
methyl)-anilino)-i-phenyl-methylene]-6-carbamoyl-2-indolinone
Rf value: 0.2 (silica gel, methylene chloride/methanol/ammonia
= 5:1:0.01)
C25H24N402
ESI mass spectrum: m/z = 411 [M-H-]
(9) 3-Z-[1-(4-(N-(piperazin-1-yl-methylcarbonyl)-N-isopropyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 3-Z-[1-(4-(N-((4-tert.butoxycarbonyl-piperazin-
1-yl)-methylcarbonyl)-N-isopropyl-amino)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 172 -
Rf value: 0.35 (silica gel, methylene chloride/methanol = 9:1)
C32H35N504
ESI mass spectrum: m/z = 552 [M-H-]
(10) 3-Z- [1- (4- (N- ( (2- (piperazin-l-yl) -ethyl) -carbonyl) -N-
methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-
2-indolinone
Prepared from 3-Z-[1-(4-(N-((2-(4-tert.butoxycarbonyl-
piperazin-1-yl)-ethyl)-carbonyl)-N-methyl-amino)-anilino)-i-
phenyl-methyleneJ-6-methoxycarbonyl-2-indolinone
Rf value: 0.4 (silica gel, methylene chloride/methanol/ammonia
= 5:1:0.01)
C31H33N504
ESI mass spectrum: m/z = 540 [M+H+]
(11) 3-Z-[1-(4-(N-propyl-aminomethyl)-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-(N-propyl-N-tert.butoxycarbonyl-
aminomethyl)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Rf value: 0.35 (silica gel, methylene chloride/methanol = 9:1)
C27H27N303
ESI mass spectrum: m/z = 440 [M-H-]
(12) 3-Z-[1-(4-((N-(3-amino-propyl)-N-methyl-amino)-methyl)-
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-((N-(tert.butoxycarbonyl-3-amino-
propyl)-N-methyl-amino)-methyl)-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
Rf value: 0.35 (silica gel, methylene chloride/methanol = 9:1)
C2eH3 oN403
ESI mass spectrum: m/z = 471 [M+H+]
CA 02387013 2002-03-19
- 173 -
Example 5
3-Z-[1-(4-methylaminomethyl-anilino)-1-phenyl-methylene]-6-
P-hoxycarbonyyl-2-indolinone
100 mg of 3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone are
dissolved in 20 ml of ethanol, 0.2 ml of 1N hydrochloric acid
are added and the mixture is hydrogenated for 70 minutes at
room temperature and 50 psi hydrogen pressure. The reaction
solution is filtered and the filtrate concentrated by rotary
evaporation. The residue is dried in vacuo at 100 C.
Yield: 50 mg (53 % of theory),
Rf value: 0.3 (silica gel, methylene chloride/ethanol/ammonia
= 5:1:0.01)
C26H25N3O3
ESI mass spectrum: m/z = 426 [M-H-]
The following compounds are prepared analogously to Example 5:
(1) 3-Z-[1-(4-methylaminomethyl-anilino)-i-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Rf value: 0.2 (silica gel, methylene chloride/methanol/ammonia
= 10:1:0.01)
C25H23N303
ESI mass spectrum: m/z = 412 [M-H"]
(2) 3-Z- [1- (4- (N- (2-methylamino-ethyl) -N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 3-Z-[1-(4-((2-(N-benzyl-N-methyl-amino)-ethyl)-
N-methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 174 -
Rf value: 0.3 (silica gel, methylene chloride/methanol/ammonia
= 10:1:0.01)
C27H28N405S
ESI mass spectrum: m/z = 519 [M-H-1
(3) 3-Z- [1- (4- (N- (2-amino-ethyl) -N-methylsulphonyl-amino) -
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-(N-cyanomethyl-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Rf value: 0.5 (silica gel, methylene chloride/methanol/ammonia
= 5:1:0.01)
C2eHz6N405S
ESI mass spectrum: m/z = 505 [M-H-1
(4) 3-Z- [1- (4- (N- (3-methylamino-propyl) -N-methylsulphonyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 3-Z-[1-(4-(N-(3-(N-benzyl-N-methyl-amino)-
propyl)-N-methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
Rf value: 0.3 (silica gel, methylene chloride/methanol/ammonia
= 5:1:0.01)
C28H30N405S
ESI mass spectrum: m/z = 533 [M-H-1
(5) 3-Z- [1- (4- (N- (piperazin-1-yl-methylcarbonyl) -N-methyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 3-Z-[1-(4-(N-((4-benzyl-piperazin-1-yl)-
methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
Rf value: 0.5 (silica gel, methylene chloride/methanol = 9:1)
C30H31N5O4
ESI mass spectrum: m/z = 524 [M-H-1
CA 02387013 2002-03-19
- 175 -
(6) 3-Z- [1- (4- (N- (methylamino-methylcarbonyl) -N-methyl-amino) -
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 3-Z-[l-(4-(N-((N-benzyl-N-methyl-amino)-
methylcarbbnyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
Rf value: 0.3 (silica gel, methylene chloride/methanol = 9:1)
C27H26N4O4
ESI mass spectrum: m/z = 469 [M-H-]
(7) 3-Z- [1- (4- (N- ( (2-methylamino-ethyl) -carbonyl) -N-methyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Prepared from 3-Z-[1-(4-(N-((2-(N-benzyl-N-methyl-amino)-
ethyl)-carbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
Rf value: 0.3 (silica gel, methylene chloride/methanol/ammonia
= 5:1:0.01)
C28H28N404
ESI mass spectrum: m/z = 483 [M-H-]
Exams l e 6
3-Z-[1-(4-ureidomethyl-anilino)-1-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinon_
300 mg of 3-Z-[1-(4-aminomethyl-anilino)-i-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone are dissolved in 15 ml of
methanol and 200 ml of triethylamine are added. Then 400 mg of
potassium cyanate in 5 ml of water are added. After 2 days of
stirring at room temperature the reaction solution is
concentrated by rotary evaporation, the residue taken up in
methylene chloride and washed once with water and once with
saturated sodium chloride solution. The organic phase is
dried over sodium sulphate and concentrated by rotary
evaporation. The residue is dried in vacuo at 100 C.
Yield: 100 mg of (21 % of theory),
CA 02387013 2002-03-19
- 176 -
Rf value: 0.7 (silica gel, methylene chloride/methanol = 5:1)
C25H22N404
ESI mass spectrum: m/z = 441 [M-H-]
Fxamz l_ e 7
3-Z-[1-(4-guanidinomethyl-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
300 mg of 3-Z-[1-(4-aminomethyl-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone are dissolved in 5 ml of
dimethylformamide and 300 ml of triethylamine are added. Then
700 mg of 3,5-dimethylpyrazol-i-carboxylic acid amidine in 5
ml of dimethylformamide are added. After one day of stirring
at room temperature the reaction solution is concentrated by
rotary evaporation. The residue is dried at 100 C in vacuo.
Yield: 200 mg (87 % of theory),
Rf value: 0.1 (Reversed phase RP 8, methanol/five percent
saline solution = 6:4)
C25H23N503
Mass spectrum: m/z = 441 [M+]
F=xaID ~~P 8
3-Z-[1-(4-acetylaminomethyl-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl - 2 - indol inone
100 mg of 3-Z-[1-(4-aminomethyl-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone are dissolved in 5 ml of
glacial acetic acid, 0.1 ml of acetic anhydride is added and
the mixture is stirred for 10 minutes at room temperature.
After this time the reaction solution is poured onto saturated
soda solution and extracted four times with methylene
chloride. The combined organic phases are washed with
saturated saline solution, dried over sodium sulphate and
concentrated by rotary evaporation. The residue is dried at
100 C in vacuo.
CA 02387013 2002-03-19
- 177 -
Yield: 20 mg (23 % of theory),
Rf value: 0.4 (silica gel, methylene chloride/methanol = 10:1)
C26H23N304
ESI mass spectrum: m/z = 440 [M-H-]
The following compounds are prepared analogously to Example 8:
(1) 3-Z- [1- (4- (N-methylsulphonyl-aminomethyl) -anilino) -1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-aminomethyl-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone and methanesulphonyl
chloride/triethylamine
Rf value: 0.7 (silica gel, methylene chloride/methanol = 5:1)
C25H23N305S
ESI mass spectrum: m/z = 476 [M-H-]
(2) 3-Z-[1-(4-(4-benzoyl-piperazin-1-yl-methyl)-anilino)-i-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-(piperazin-i-yl-methyl)-anilino)-i-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone and benzoyl
chloride
Rf value: 0.7 (silica gel, methylene chloride/methanol = 10:1)
C35H32N404
ESI mass spectrum: m/z = 571 [M-H-]
(3) 3-Z- [1- (4- ( (N- (3-acetylamino-propyl) -N-methyl-amino) -
methyl)-anilino)-1-phenyl-methylenel-6-methoxycarbonyl-2-
indolinone
Prepared from 3-Z-[1-(4-((N-(3-amino-propyl)-N-methyl-amino)-
methyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
Rf value: 0.3 (silica gel, methylene chloride/methanol = 9:1)
C30H32N404
ESI mass spectrum: m/z = 511 [M-H-]
CA 02387013 2002-03-19
- 178 -
F, xam= 1 P 9
3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-1-phenyl-
mPr ylene]-h-carboxy-2-indolinone
0.8 g of 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone are dissolved in 30
ml of ethanol, 8.3 ml of iN sodium hydroxide solution are
added and the mixture is stirred for 1 hour at 80 C. After
cooling, it is neutralised with 8.3 ml of iN hydrochloric
acid. The precipitate formed is suction filtered, washed with
water, ethanol and ether and dried in vacuo at 100 C.
Yield: 0.7 g of (89 % of theory),
Rf value: 0.2 (silica gel, methylene chloride/methanol = 5:2)
C2BH27N303
Mass spectrum: m/z = 453 [M+]
The following compounds are prepared analogously to Example 9:
(1) 3-Z-[1-(4-bromo-anilino)-i-phenyl-methylene]-6-carboxy-
2-indolinone
Prepared from 3-Z-[1-(4-bromo-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
Rf value: 0.4 (silica gel, toluene/ethyl acetate = 5:1)
C22HisBrN203
ESI mass spectrum: m/z = 435/437 [M+H+]
(2) 3-Z- [1- (3- (dimethylaminomethyl) -anilino) -i-phenyl-
methylene]-6-carboxy-2-indolinone
Prepared from 3-Z-[1-(3-(dimethylaminomethyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Rf value: 0.7 (Reversed phase RP 8, methanol/five percent
saline solution = 4:1)
C25H23N303
ESI mass spectrum: m/z = 414 [M+H+]
CA 02387013 2002-03-19
- 179 -
(3) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-i-phenyl-
methylene]-6-carboxy-2-indolinone
Prepared from 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
Rf value: 0.7 (Reversed phase RP 8, methanol/five percent
saline solution = 4:1)
C25H23N303
ESI mass spectrum: m/z = 412 [M-H-]
(4) 3-Z- [1- (4- [ (2,6-dimethyl-piperidin-1-yl) -methyl] -anilino) -
1-phenyl-methylene]-6-carboxy-2-indolinone
Prepared from 3-Z-[1-(4-[(2,6-dimethyl-piperidin-1-yl)-
methyl]-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
Rf value: 0.6 (Reversed phase RP 8, methanol/five percent
saline solution = 4:1)
C30H31N303
ESI mass spectrum: m/z = 482 [M+H+]
(5) 3-Z-[1-(4-(1-methyl-imidazol-2-yl)-anilino)-i-phenyl-
methylene]-6-carboxy-2-indolinone
Prepared from 3-Z-[1-(4-(l-methyl-imidazol-2-yl)-anilino)-1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Rf value: 0.6 (Reversed phase RP 8, methanol/five percent
saline solution = 4:1)
C26H20N403
ESI mass spectrum: m/z = 435 [M-H-]
(6) 3-Z-[1-(4-(N-acetyl-N-dimethylaminocarbonylmethyl-amino)-
anilino)-i-phenyl-methylene]-6-carboxy-2-indolinone
Prepared from 3-Z-[1-(4-(N-acetyl-N-
dimethylaminocarbonylmethyl-amino)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Rf value: 0.3 (silica gel, methylene chloride/methanol = 10:1)
C28H26N405
ESI mass spectrum: m/z = 497 [M-H"]
CA 02387013 2002-03-19
- 180 -
(7) 3-Z-[1-(4-ethylaminomethyl-anilino)-1-phenyl-methylene]-6-
carboxy-2-indolinone
Prepared from 3-Z-[1-(4-ethylaminomethyl-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
Rf value: 0.6 (Reversed phase RP 8, methanol/five percent
saline solution = 4:1)
C25H23N303
ESI mass spectrum: m/z = 412 [M-H"]
(8) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-carboxy-2-indolinone
Prepared from 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-
methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-
2-indolinone
Rf value: 0.6 (Reversed phase RP 8, methanol/five percent
saline solution = 4:1)
C27H26N404
ESI mass spectrum: m/z = 469 [M-H-]
(9) 3-Z-[1-(4-(N-tert.butoxycarbonyl-ethylaminomethyl)-
anilino)-1-phenyl-methylene]-6-carboxy-2-indolinone
Prepared from 3-Z-[1-(4-(N-tert.butoxycarbonyl-ethylamino-
methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
Rf value: 0.4 (silica gel, methylene chloride/methanol = 10:1)
C30H31N305
ESI mass spectrum: m/z = 512 [M-H-]
(10) 3-Z-[1-(4-((N-carboxymethyl-N-methyl-amino)-methyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-((N-ethoxycarbonylmethyl-N-methyl-
amino)-methyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl
-2-indolinone
CA 02387013 2002-03-19
- 181 -
Rf value: 0.4 (silica gel, methylene chloride/methanol = 6:1)
C27H25N305
ESI mass spectrum: m/z = 470 [M-H-]
Examsnl e 10
3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-1-phenyl-
methylene] -6-methoxycarbonyl -2-indol innnf-
0.9 g of 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-carboxy-2-indolinone are suspended in 35 ml of
dimethylformamide and 0.4 g of carbonyldiimidazole are added.
The mixture is stirred for 14 hours at 80 C. After this time
20 ml of methanol are added and the mixture is stirred for
another 3 hours at 50 C. The solvent is removed and the
residue is purified over a silica gel column with methylene
chloride/methanol (3:1) as eluant.
Yield: 0.5 g of (490 of theory),
Rf value: 0.5 (aluminium oxide, methylene chloride/methanol =
30:1)
C29H29N303
ESI mass spectrum: m/z = 468 [M+H+]
The following compounds are prepared analogously to Example
10:
(1) 3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-1-phenyl-
methylene]-6-benzyloxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and benzyl alcohol
Rf value: 0.6 (aluminium oxide, methylene chloride/methanol =
30:1)
C35H33N303
Mass spectrum: m/z = 543 [M+]
(2) 3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-1-phenyl-
methylene]-6-isopropyloxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 182 -
Prepared from 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and isopropanol
Rf value: 0.4 (aluminium oxide, methylene chloride/isopropanol
= 30:1)
C31H33N3O3
Mass spectrum: m/z = 495 [M']
(3) 3-Z- [1- (4- (piperidin-1-yl-methyl) -anilino) -1-phenyl-
methylene]-6-propyloxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and n-propanol
Rf value: 0.7 (silica gel, methylene chloride/methanol = 5:1)
C31H33N303
Mass spectrum: m/z = 495 [M+]
(4) 3-Z- [1- (4- (piperidin-l-yl-methyl) -anilino) -1-phenyl-
methylene]-6-butyloxycarbonyl-2-indolinone
Prepared from 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and n-butanol
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C32H35N303
Mass spectrum: m/z = 509 [M+]
(5) 3-Z-[1-(4-bromo-anilino)-1-phenyl-methylene]-6-carbamoyl-
2-indolinone
Prepared from 3-Z-[1-(4-bromo-anilino)-1-phenyl-methylene]-
6-carboxy-2-indolinone and ammonia
Rf value: 0.5 (silica gel, methylene chloride/methanol = 10:1)
C22H16BrN2O3
Mass spectrum: m/z = 432/434 [M-H-]
(6) 3-Z- [1- (4- (piperidin-1-yl-methyl) -anilino) -1-phenyl-
methylene]-6-ethylcarbamoyl-2-indolinone
Prepared from 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and ethylamine gas
Rf value: 0.6 (silica gel, methylene chloride/methanol = 5:1)
CA 02387013 2002-03-19
- 183 -
C30H32N402
Mass spectrum: m/z = 480 [M+]
(7) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-1-phenyl-
methylene]-6-[(2-methoxy-ethoxy)-carbonyl]-2-indolinone
Prepared from 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and methylglycol
Rf value: 0.8 (silica gel, methylene chloride/methanol = 4:1)
C25H23N303
ESI mass spectrum: m/z = 470 [M-H-]
(8) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-i-phenyl-
methylene]-6-[(2-dimethylamino-ethoxy)-carbonyl]-2-indolinone
Prepared from 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and
2-dimethylaminoethanol aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 5:2)
C29H32N403
ESI mass spectrum: m/z = 483 [M-H-]
(9) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-1-phenyl-
methylene]-6-[(2-N-tert.butoxycarbonyl-amino-ethoxy)-
carbonyl]-2-indolinone
Prepared from 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and 2-N-
tert.butoxycarbonyl-amino-ethanol aniline
Rf value: 0.8 (silica gel, methylene chloride/methanol = 5:2)
C32H36N405
ESI mass spectrum: m/z = 412 [M-H-]
(10) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-1-phenyl-
methylene]-6-[(2,2,2-trifluoroethoxy)-carbonyl]-2-indolinone
CA 02387013 2002-03-19
- 184 -
Prepared from 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and 2,2,2-
trifluoroethanol aniline
Rf value: 0.5 (silica gel, methylene chloride/methanol = 5:1)
C27H24F'3N303
ESI mass spectrum: m/z = 494 [M-H-]
F~mp1P ~~
3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-1-phenyl-
methylenp] - 6 - .arbamoyl - . - indol ; non .
0.9 g of 3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-i-phenyl-
methylene]-6-carboxy-2-indolinone, 0.8 g of TBTU and 0.4 g of
HOBT are suspended in 25 ml of dimethylformamide and 1.0 ml of
triethylamine are added. The mixture is stirred for 15
minutes at room temperature. After this time ammonia gas is
introduced at 10-15 C over a period of 15 minutes and the
mixture is stirred for 1.5 hours at room temperature. The
precipitate formed is suction filtered, washed with water,
ethanol and ether and dried at 100 C in vacuo.
Yield: 0.6 g (64 % of theory),
Rf value: 0.4 (Reversed phase RP 8, methanol/five percent
saline solution = 6:4)
C28H28N402
ESI mass spectrum: m/z = 453 [M+H+]
The following compounds are prepared analogously to Example
11:
(1) 3-Z- [1- (4- (piperidin-l-yl-methyl) -anilino) -1-phenyl-
methylene]-6-dimethylcarbamoyl-2-indolinone
Prepared from 3-Z- [1- (4- (piperidin-l-yl-methyl) -anilino) -
1-phenyl-methylene]-6-carboxy-2-indolinone and dimethylamine
hydrochloride/diisopropylethylamine
CA 02387013 2002-03-19
- 185 -
Rf value: 0.5 (silica gel, methylene chloride/methanol = 5:1)
C30H32N402
ESI mass spectrum: m/z = 481 [M+H+]
(2) 3-Z- [1- (4- (piperidin-l-yl-methyl) -anilino) -1-phenyl-
methylene]-6-(N-ethyl-N-methyl-carbamoyl)-2-indolinone
Prepared from 3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and N-ethyl-
N-methyl-amine
Rf value: 0.5 (aluminium oxide, methylene chloride/ethanol =
20:1)
C31H34N402
ESI mass spectrum: m/z = 495 [M+H+]
(3) 3-Z-[l-(4-(piperidin-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methylcarbamoyl-2-indolinone
Prepared from 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and methylamine
hydrochloride/diisopropylethylamine
Rf value: 0.3 (aluminium oxide, methylene chloride/ethanol =
20:1)
C29H30N402
ESI mass spectrum: m/z = 467 [M+H+]
(4) 3-Z-[1-(3-(dimethylaminomethyl)-anilino)-1-phenyl-
methylene]-6-methylcarbamoyl-2-indolinone
Prepared from 3-Z-[1-(3-(dimethylaminomethyl)-anilino)-
i-phenyl-methylene]-6-carboxyl-2-indolinone and methylamine
hydrochloride/triethylamine
Rf value: 0.3 (silica gel, methylene chloride/ethanol = 2:1)
C26H26N402
Mass spectrum: m/z = 426 [M+]
(5) 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-(2-hydroxyethyl-carbamoyl)-2-indolinone
CA 02387013 2002-03-19
- 186 -
Prepared from 3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and
ethanolamine/diisopropylethylamine
Rf value: 0.5 (aluminium oxide, methylene chloride/methanol =
20:1)
C30H32N403
ESI mass spectrum: m/z = 495 [M-H-]
(6) 3-Z-[1-(4-(piperidin-l-yl-methyl)-anilino)-1-phenyl-
methylene]-6-diethylcarbamoyl-2-indolinone
Prepared from 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-carboxy-2-indolinone and diethylamine
hydrochloride/diisopropylethylamine
Rf value: 0.8 (aluminium oxide, methylene chloride/methanol =
10:1)
C32H36N402
ESI mass spectrum: m/z = 509 [M+H+]
(7) 3-Z-[1-(4-(N-tert.butoxycarbonyl-ethylaminomethyl)-
anilino)-1-phenyl-methylene]-6-carbamoyl-2-indolinone
Prepared from 3-Z-[1-(4-(N-tert.butoxycarbonyl-
ethylaminomethyl)-anilino)-1-phenyl-methylene]-6-carboxy-2-
indolinone
Rf value: 0.3 (silica gel, toluene/ethyl acetate/ethanol =
4:2:1)
C30H32N404
ESI mass spectrum: m/z = 511 [M-H-]
(8) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-carbamoyl-2-indolinone
Prepared from 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-
methyl-amino)-anilino)-1-phenyl-methylene]-6-carboxy-2-
indolinone
Rf value: 0.5 (silica gel, methylene chloride/methanol/ammonia
= 5:1:0.01)
CA 02387013 2002-03-19
- 187 -
C27H27N5O3
ESI mass spectrum: m/z = 468 [M-H-]
F'xam 1 P- 12
3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
x r.itriG acid
3.25 g of citric acid monohydrate are placed in 50 ml of
methanol and 5.0 g of 3-Z-[1-(4-(N-dimethylaminomethyl-
carbonyl-N-methyl-amino)-anilino)-i-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone are added at room temperature.
The solution formed is evaporated down, the residue is washed
with ether and recrystallised from ethyl acetate.
Yield: 6.3 g (90 % of theory),
Rf value: 0.6 (silica gel, methylene chloride/methanol/ammonia
= 5:1:0.01)
Melting point: 198 C
C2eH2sNa05 x C6HeO7
ESI mass spectrum: m/z = 483 [M-H-]
Elemental analysis: calc.: C 60.34 H 5.37 N 8.28
found: 59.98 5.25 8.13
The following compound is prepared analogously to Example 12:
(1) 3-Z- [1- (4- (dimethylaminomethyl) -anilino) -1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone x methanesulphonic
acid
Prepared from 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-i-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone and
methanesulphonic acid
Rf value: 0.6 (silica gel, methylene chloride/methanol/ammonia
= 5:1:0.01)
Melting point: 275 C
C26H25N3O3 X CH4O3S
ESI mass spectrum: m/z = 426 [M-H-]
CA 02387013 2002-03-19
- 188 -
Elemental analysis: calc.: C 61.92 H 5.59 N 8.03 S 6.12
found: 61.43 5.87 7.85 5.39
The following compounds may be prepared analogously to the
foregoing Examples:
(1) 3-Z-(1-anilino-l-phenyl-methylene)-6-ethoxycarbonyl-
2-indolinone
(2) 3-Z-[1-(4-nitro-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(3) 3-Z- [1- (4-fluoro-anilino) -1-phenyl-methylene] -6-ethoxy-
carbonyl-2-indolinone
(4) 3-Z-[1-(4-chloro-anilino)-1-phenyl-methylene]-6-ethoxy-
carbonyl-2-indolinone
(5) 3-Z- [1- (4-iodo-anilino) -1-phenyl-methylene] -6-
ethoxycarbonyl-2-indolinone
(6) 3-Z-[1-(4-cyano-anilino)-1-phenyl-methylene]-6-ethoxy-
carbonyl-2-indolinone
(7) 3-Z-[1-(4-methoxy-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(8) 3-Z-[1-(4-ethoxy-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(9) 3-Z-[1-(4-trifluoromethyl-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(10) 3-Z-[1-(4-methyl-anilino)-i-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 189 -
(11) 3-Z-[1-(4-methylmercapto-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(12) 3-Z-[1-(4-aminomethyl-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(13) 3-Z-[1-(4-(isopropylaminomethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(14) 3-Z- [1- (4- (anilinomethyl) -anilino) -1-phenyl-methylene] -
6-ethoxycarbonyl-2-indolinone
(15) 3-Z-[1-(4-(propylaminomethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(16) 3-Z-[1-(4-(butylaminomethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(17) 3-Z-[1-(4-(isobutylaminomethyl)-anilino)-i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(18) 3-Z-[1-(4-(cyclohexylaminomethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(19) 3-Z- [1- (4- (benzylaminomethyl) -anilino) -1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(20) 3-Z- [1- (4- ( (N-ethyl-N-methyl-amino) -methyl) -anilino) -
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(21) 3-Z- [1- (4- ( (N-methyl-N-propyl-amino) -methyl) -anilino) -
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(22) 3-Z-[1-(4-((N-isopropyl-N-methyl-amino)-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 190 -
(23) 3-Z-[1-(4-((N-ethyl-N-propyl-amino)-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(24) 3-Z-[1-(4-((N-ethyl-N-isopropyl-amino)-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(25) 3-Z-[1-(4-(dipropylaminomethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(26) 3-Z-[1-(4-(diisopropylaminomethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(27) 3-Z-[1-(4-((N-benzyl-N-ethyl-amino)-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(28) 3-Z-[1-(4-(dibenzylaminomethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(29) 3-Z-[1-(4-(3,6-dihydro-2H-pyridin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(30) 3-Z-[1-(4-(3,5-dimethyl-piperidin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(31) 3-Z-[1-(4-(azepan-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(32) 3-Z-[1-(4-(piperazin-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(33) 3-Z-[1-(4-(morpholin-4-yl-methyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(34) 3-Z-[1-(4-(thiomorpholin-4-yl-methyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 191 -
(35) 3-Z-[1-(4-(1-oxo-thiomorpholin-4-yl-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(36) 3-Z- [1- (4- (1, 1-dioxo-thiomorpholin-4-yl-methyl) -anilino) -
i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(37) 3-Z-[1-(4-(acetylamino-methyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(38) 3-Z- [1- (4- (2-amino-ethyl) -anilino) -1-phenyl-methylene] -
6-ethoxycarbonyl-2-indolinone
(39) 3-Z- [1- (4- (2-methylamino-ethyl) -anilino) -i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(40) 3-Z- [1- (4- (2-ethylamino-ethyl) -anilino) -i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(41) 3-Z- [1- (4- (2-diethylamino-ethyl) -anilino) -1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(42) 3-Z- [1- (4- (2-piperidin-1-yl-ethyl) -anilino) -i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(43) 3-Z-[1-(4-(2-acetylamino-ethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(44) 3-Z- [1- (4- (3-amino-propyl) -anilino) -1-phenyl-methylene] -
6-ethoxycarbonyl-2-indolinone
(45) 3-Z-[1-(4-(3-dimethylamino-propyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(46) 3-Z-[1-(4-(N-aminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 192 -
(47) 3-Z-[1-(4-(N-methylaminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(48) 3-Z-[1-(4-(N-ethylaminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(49) 3-Z-[1-(4-(N-diethylaminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(50) 3-Z-[1-(4-(N-(piperidin-1-yl-methylcarbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(51) 3-Z-[1-(4-(N-(morpholin-4-yl-methylcarbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(52) 3-Z-[1-(4-(N-(piperazin-1-yl-methylcarbonyl)-N-methyl-
amino]-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(53) 3-Z-[1-(4-(N-(2-amino-ethylcarbonyl)-N-methyl-amino)-
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(54) 3-Z-[1-(4-(N-(2-methylamino-ethylcarbonyl)-N-methyl-
amino)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(55) 3-Z-[1-(4-(N-(2-diethylamino-ethylcarbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(56) 3-Z- [1- (4- (N-acetyl-N- (2-aminoethyl) -amino) -anilino) -
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 193 -
(57) 3-Z-[1-(4-(N-acetyl-N-(2-methylamino-ethyl)-amino)-
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(58) 3-Z-[1-(4-(N-acetyl-N-(2-methylamino-propyl)-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(59) 3-Z- [1- (4- (N-acetyl-N- (2-piperidin-1-yl-ethyl) -amino) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(60) 3-Z-[1-(4-(N-acetyl-N-(aminocarbonylmethyl)-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(61) 3-Z- [1- (4- (N-acetyl-N- (dimethylaminocarbonylmethyl) -
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(62) 3-Z-[1-(4-(N-acetyl-N-(piperidin-1-yl-carbonylmethyl)-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(63) 3-Z-[1-(4-(N-methyl-N-(aminocarbonyl)-amino)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(64) 3-Z-[1-(4-(N-methyl-N-(methylaminocarbonyl)-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(65) 3-Z-[1-(4-(N-methyl-N-(dimethylaminocarbonyl)-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(66) 3-Z-[1-(4-(N-methyl-N-(piperidin-l-yl-carbonyl)-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(67) 3-Z-[1-(4-(N-(2-aminoethyl)-N-methylsulphonyl-amino)-
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 194 -
(68) 3-Z-[1-(4-(N-(2-methylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(69) 3-Z-[1-(4-(N-(2-ethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(70) 3-Z- [1- (4- (N- (2-diethylamino-ethyl) -N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(71) 3-Z-[1-(4-(N-(2-pyrrolidin-1-yl-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(72) 3-Z-[1-(4-(N-(2-piperidin-1-yl-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl=methylene]-6-ethoxycarbonyl-2-
indolinone
(73) 3-Z-[1-(4-(N-(2-piperazin-1-yl-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(74) 3-Z-[1-(4-(N-(2-(morpholin-4-yl)-ethyl)-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(75) 3-Z-[1-(4-(N-(aminocarbonylmethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(76) 3-Z-[1-(4-(N-(methylaminocarbonylmethyl)-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
195 -
(77) 3-Z-[1-(4-(N-(ethylaminocarbonylmethyl)-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(78) 3-Z- [1- (4- (N- (N- (2-dimethylamino-ethyl) -N-methyl-amino) -
carbonylmethyl)-N-methylsulphonyl-amino)-anilino)-i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(79) 3-Z-[1-(4-(N-(diethylaminocarbonylmethyl)-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(80) 3-Z-[1-(4-(N-(pyrrolidin-1-yl-carbonylmethyl)-N-methyl-
sulphonyl-amino)-anilino)-i-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(81) 3-Z-[1-(4-(N-(piperidin-1-yl-carbonylmethyl)-N-
methylsulphonyl-amino)-anilino)-i-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(82) 3-Z-[1-(4-(N-(piperazin-1-yl-carbonylmethyl)-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(83) 3-Z-[1-(4-(N-((morpholin-4-yl)-carbonylmethyl)-N-methyl-
sulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(84) 3-Z-[1-(4-(2-dimethylamino-ethoxy)-anilino)-i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(85) 3-Z-[1-(4-(3-dimethylamino-propoxy)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(86) 3-Z-[1-(4-(aminocarbonylmethyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 196 -
(87) 3-Z- [1- (4- (2-aminocarbonyl-ethyl) -anilino) -1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(88) 3-Z- [1- (4- (pyridin-2-yl) -anilino) -1-phenyl-methylene] -
6-ethoxycarbonyl-2-indolinone
(89) 3-Z-[1-(4-(pyridine-3-yl)-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(90) 3-Z-[1-(4-(pyridin-4-yl)-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(91) 3-Z-[1-(4-(N-acetyl-N-methyl-amino)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(92) 3-Z-[1-(4-(N-ethylcarbonyl-N-(dimethylaminocarbonyl-
methyl)-amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(93) 3-Z-[1-(carbamoylmethyl-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(94) 3-Z-[1-(4-dimethylcarbamoylmethyl-anilino)-i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(95) 3-Z-[1-(4-(piperidin-1-yl-methyl)-anilino)-methylene]-
6-ethoxycarbonyl-2-indolinone
(96) 3-Z- [1- (4- (piperidin-l-yl-methyl) -anilino) -propylidene] -
6-ethoxycarbonyl-2-indolinone
(97) 3-Z- [1- (4- (piperidin-l-yl-methyl) -anilino) -butylidene] -
6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 197 -
(98) 3-Z-[1-(4-(N-(3-dimethylamino-propyl)-N-acetyl-amino)-
anilino)-methylene]-6-ethoxycarbonyl-2-indolinone
(99) 3-Z-[1-(4-(N-(3-dimethylamino-propyl)-N-acetyl-amino)-
anilino)-ethylidene]-6-ethoxycarbonyl-2-indolinone
(100) 3-Z- [1- (4- (N- (3-dimethylamino-propyl) -N-acetyl-amino) -
anilino)-propylidene]-6-ethoxycarbonyl-2-indolinone
(101) 3-Z- [1- (4- (N- (3-dimethylamino-propyl) -N-acetyl-amino) -
anilino)-butylidene]-6-ethoxycarbonyl-2-indolinone
(102) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-methylene]-6-ethoxycarbonyl-2-indolinone
(103) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-propylidene]-6-ethoxycarbonyl-2-indolinone
(104) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-butylidene]-6-ethoxycarbonyl-2-indolinone
(105) 3-Z-[1-(4-tetrazol-5-yl-anilino)-methylene]-6-
ethoxycarbonyl-2-indolinone
(106) 3-Z- [1- (4-tetrazol-5-yl-anilino) -ethylidene] -6-ethoxy-
carbonyl-2-indolinone
(107) 3-Z-[1-(4-tetrazol-5-yl-anilino)-propylidene]-6-ethoxy-
carbonyl-2-indolinone
(108) 3-Z-[1-(4-tetrazol-5-yl-anilino)-butylidene]-6-
ethoxycarbonyl-2-indolinone
(109) 3-Z-[1-(4-carboxy-anilino)-methylene]-6-ethoxycarbonyl-
2-indolinone
CA 02387013 2002-03-19
- 198 -
(110) 3-Z- [1- (4-carboxy-anilino) -propylidene] -6-
ethoxycarbonyl-2-indolinone
(111) 3-Z-[1-(4-carboxy-anilino)-butylidene]-6-ethoxycarbonyl-
2-indolinone
(112) 3-Z- [1- (4- (N- (3-dimethylamino-propionyl) -N-
dimethylaminocarbonylmethyl-amino)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(113) 3-Z- [1- (4- (N- (4-dimethylamino-butyryl) -N-
dimethylaminocarbonylmethyl-amino)-anilino)-i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(114) 3-Z-[1-(4-(N-dimethylaminocarbonylmethyl-N-(2-
dimethylamino-ethylsulphonyl)-amino)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(115) 3-Z- [1- (4- (N-dimethylaminocarbonylmethyl-N- (3-
dimethylamino-propylsulphonyl)-amino)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(116) 3-Z-[1-(4-((2-hydroxy-ethyl)-amino-methyl)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(117) 3-Z-[1-(4-((2-methoxy-ethyl)-amino-methyl)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(118) 3-Z- [1- (4- ( (2-dimethylamino-ethyl) -amino-methyl) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(119) 3-Z- [1- (4- ( (3-dimethylamino-propyl) -amino-methyl) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 199 -
(120) 3-Z-[1-(4-((N-tert.butoxycarbonyl-2-amino-ethyl)-amino-
methyl)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(121) 3-Z-[1-(4-((N-tert.butoxycarbonyl-3-amino-propyl)-amino-
methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(122) 3-Z- [1- (4- ( (2-amino-ethyl) -amino-methyl) -anilino) -1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(123) 3-Z-[l-(4-((3-amino-propyl)-amino-methyl)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(124) 3-Z-[l-(4-((2-acetylamino-ethyl)-amino-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(125) 3-Z- [1- (4- ( (3-acetylamino-propyl) -amino-methyl) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(126) 3-Z-[1-(4-((2-methylsulphonylamino-ethyl)-amino-methyl)-
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(127) 3-Z-[1-(4-((3-methylsulphonylamino-propyl)-amino-
methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(128) 3-Z-[l-(4-(N-(N-tert.butoxycarbonyl-2-amino-ethyl)-N-
methyl-amino-methyl)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(129) 3-Z- [1- (4- (N- (2-amino-ethyl) -N-methyl-amino-methyl) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 200 -
(130) 3-Z- [1- (4- (N- (2-acetylamino-ethyl) -N-methyl-amino-
methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(131) 3-Z-[1-(4-(N-(2-methylsulphonylamino-ethyl)-N-methyl-
amino-methyl)-anilino)-1-phenyl-methylene]-6-ethoxyc.arbonyl-2-
indolinone
(132) 3-Z-[1-(4-(carboxymethyl-amino-methyl)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(133) 3-Z-[1-(4-(ethoxycarbonylmethyl-amino-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(134) 3-Z-[1-(4-(carbamoylmethyl-amino-methyl)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(135) 3-Z-[1-(4-(dimethylcarbamoyl-methyl-amino-methyl)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(136) 3-Z-[1-(4-(methylcarbamoyl-methyl-amino-methyl)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(137) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-amino-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(138) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-nitro-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(139) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-acetylamino-anilino)-i-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
, ..
- 201 -
(140) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-methylsulphonylamino-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(141) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-cyano-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(142) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-hydroxy-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(143) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-methoxy-anilino)-i-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(144) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-ethoxycarbonyl-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(145) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-carboxy-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(146) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-carbamoyl-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(147) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-chloro-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(148) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-fluoro-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
CA 02387013 2002-03-19
- 202 -
(149) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-bromo-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(150) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-methyl-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(151) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3-trifluoromethyl-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(152) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3,5-dibromo-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(153) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-3,5-dichloro-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(154) 3-Z- [1- (4- (dimethylaminomethyl) -3-amino-anilino) -1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(155) 3-Z-[1-(4-(dimethylaminomethyl)-3-nitro-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(156) 3-Z-[1-(4-(dimethylaminomethyl)-3-acetylamino-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(157) 3-Z-[1-(4-(dimethylaminomethyl)-3-
(methylsulphonylamino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(158) 3-Z-[1-(4-(dimethylaminomethyl)-3-cyano-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 203 -
(159) 3-Z-[1-(4-(dimethylaminomethyl)-3-hydroxy-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(160) 3-Z-[1-(4-(dimethylaminomethyl)-3-methoxy-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(161) 3-Z- [1- (4- (dimethylaminomethyl) -3- (ethoxycarbonyl) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(162) 3-Z-[1-(4-(dimethylaminomethyl)-3-carboxy-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(163) 3-Z-[1-(4-(dimethylaminomethyl)-3-carbamoyl-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(164) 3-Z- [1- (4- (dimethylaminomethyl) -3-chloro-anilino) -1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(165) 3-Z-[1-(4-(dimethylaminomethyl)-3-fluoro-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(166) 3-Z-[1-(4-(dimethylaminomethyl)-3-bromo-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(167) 3-Z- [1- (4- (dimethylaminomethyl) -3-methyl-anilino) -i-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(168) 3-Z- [1- (4- (dimethylaminomethyl) -3-trifluoromethyl-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(169) 3-Z-[1-(4-(dimethylaminomethyl)-3,5-dibromo-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(170) 3-Z-[1-(4-(dimethylaminomethyl)-3,5-dichloro-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 204 -
(171) 3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-
N-methyl-amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(172) 3-Z-[1-(4-(N-(imidazo-i-yl-methylcarbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(173) 3-Z-[1-(4-(N-(phthalimido-2-yl-methylcarbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(174) 3-Z-[1-(4-(N-aminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(175) 3-Z-[1-(4-(N-acetylaminomethylcarbonyl-N-methyl-arnino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(176) 3-Z-[1-(4-(N-methylsulphonylaminomethylcarbonyl-N-
methyl-amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-
indolinone
(177) 3-Z- [1- (4- (N- ( (N- (2-methoxyethyl) -N-methyl-amino) -
methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(178) 3-Z- [1- (4- (N- ( (N- (2-dimethylaminoethyl) -N-methyl-amino) -
methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(179) 3-Z- [1- (4- (N- ( (di- (2-hydroxyethyl) -amino) -
methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(180) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-anilino)-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 205 -
(181) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-anilino)-ethylidene]-6-ethoxycarbonyl-2-indolinone
(182) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-anilino)-propylidene]-6-ethoxycarbonyl-2-indolinone
(183) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-
amino)-anilino)-butylidene]-6-ethoxycarbonyl-2-indolinone
(184) 3-Z-[1-(4-(dimethylaminomethyl)-anilino)-methylene]-6-
ethoxycarbonyl-2-indolinone
(185) 3-Z- [1- (4- (dimethylaminomethyl) -anilino) -ethylidene] -6-
ethoxycarbonyl-2-indolinone
(186) 3-Z- [1- (4- (dimethylaminomethyl) -anilino) -propylidene] -6-
ethoxycarbonyl-2-indolinone
(187) 3-Z- [1- (4- (dimethylaminomethyl) -anilino) -butylidene] -6-
ethoxycarbonyl-2-indolinone
(188) 3-Z-[1-(4-(N-dimethylaminocarbonylmethyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(189) 3-Z-[1-(4-(N-(3-dimethylamino-propyl)-N-acetyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(190) 3-Z-[1-(4-((imidazolidin-2,4-dion-5-ylidene)-methyl)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(191) 3-Z- [1- (4- (N- ( (2-dimethylamino-ethyl) -carbonyl) -N-
methyl-amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
CA 02387013 2002-03-19
- 206 -
(192) 3-Z-[1-(4-(N-tert.butoxycarbonyl-aminomethyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(193) 3-Z-[1-(4-(2-oxo-pyrrolidin-1-yl-methyl)-anilino)-i-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(194) 3-Z-[1-(4-(N-aminocarbonylmethyl-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(195) 3-Z-[1-(4-(N-cyanomethyl-N-methylsulphonyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(196) 3-Z- [1- (4- (2- (imidazol-4-yl) -ethyl) -anilino) -1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(197) 3-Z- [1- (4- ( (2- (N-benzyl-N-methyl-amino) -ethyl) -N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(198) 3-Z-[1-(4-cyclohexylamino-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(199) 3-Z- [1- (4- (imidazol-i-yl-methyl) -anilino) -1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(200) 3-Z-[1-(4-(imidazol-i-yl-methyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(201) 3-Z-[1-(N-methyl-piperidine-4-yl-amino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(202) 3-Z-[1-(4-(imidazol-4-yl-methyl)-anilino)-i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 207 -
(203) 3-Z-[1-(4-((4-hydroxy-piperidin-1-yl)-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(204) 3-Z-[1-(4-((4-methoxy-piperidin-1-yl)-methyl)-anilino)-
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(205) 3-Z- [1- (4-benzyl-anilino) -1-phenyl-methylenel-6-
ethoxycarbonyl-2-indolinone
(206) 3-Z- [1- (4- (N- (3-trifluoroacetylamino-propyl) -N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(207) 3-Z-[1-(4-(4-tert.butoxycarbonyl-piperazin-l-yl-methyl)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(208) 3-Z- [1- (4- (1-methyl-imidazol-2-yl) -anilino) -1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(209) 3-Z-[1-(4-(1-methyl-imidazol-2-yl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(210) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-3-amino-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(211) 3-Z- [1- (4- ( (3- (N-benzyl-N-methyl-amino) -propyl) -N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(212) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-acetyl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(213) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-butyryl-amino)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 208 -
(214) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-isobutyryl-
amino)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(215) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-benzoyl-amino) -
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(216) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-acetyl-amino) -3-
amino-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(217) 3-Z- [1- (4- (4-hydroxymethyl-piperidin-1-yl-methyl) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(218) 3-Z- [1- (4- (2- (4-hydroxy-piperidin-1-yl) -ethyl) -anilino) -
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(219) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-propylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(220) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-butylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(221) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-phenylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(222) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-benzylsulphonyl-
amino)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(223) 3-Z-[1-(4-((imidazolidin-2,4-dion-5-yl)-methyl)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 209 -
(224) 3-Z- [1- (4- ( (3-hydroxy-pyrrolidin-1-yl) -methyl) -anilino) -
1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(225) 3-Z- [1- (4- (cyclohexylyl-methyl) -anilino) -1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(226) 3-Z- [1- (4- (cyclohexyl-carbonyl) -anilino) -i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(227) 3-Z-[1-(4-diethylaminomethyl-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(228) 3-Z- [1- (4- (N- (n-hexyl) -N-methyl-aminomethyl) -anilino) -1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(229) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N- (furan-2-
carbonyl)-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(230) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N- (2-methoxy-
benzoyl)-amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(231) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N- (pyridine-3-
carbonyl)-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(232) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N- (phenyl-acetyl) -
amino)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(233) 3-Z- [1- (4- (imidazol-2-yl) -anilino) -1-phenyl-methylene] -
6-ethoxycarbonyl-2-indolinone
(234) 3-Z-[1-(4-(1-ethyl-imidazol-2-yl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 210 -
(235) 3-Z-[1-(4-(1-benzyl-imidazol-2-yl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(236) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-
isopropylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(237) 3-Z-[1-(4-(N-((4-benzyl-piperazin-1-yl)-methylcarbonyl)-
N-methyl-amino)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(238) 3-Z-[1-(4-(N-(pyrrolidin-1-yl-methylcarbonyl)-N-methyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(239) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-acetyl-amino) -3-
bromo-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(240) 3-Z-[1-(4-(5-methyl-imidazol-4-yl)-anilino)-i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(241) 3-Z- [1- (4- (N- ( (2-dimethylamino-ethyl) -carbonyl) -N-
isopropyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(242) 3-Z- [1- (4- (N- ( (2-dimethylamino-ethyl) -carbonyl) -N-
benzyl-amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(243) 3-Z-[1-(4-(N-butyl-N-tert.butoxycarbonyl-aminomethyl)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 211 -
(244) 3-Z-[1-(4-(N-((N-aminocarbonylmethyl-N-methyl-amino)-
methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(245) 3-Z-[1-(4-(N-((N-benzyl-N-methyl-amino)-methylcarbonyl)-
N-methyl-amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(246) 3-Z- [1- (4- (N- (di- (2-methoxyethyl) -amino-methylcarbonyl) -
N-methyl-amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(247) 3-Z- [1- (4- (N- ( (2- (4-tert.butoxycarbonyl-piperazin-l-yl) -
ethyl)-carbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(248) 3-Z- [1- (4- (N- ( (2- (piperidin-l-yl) -ethyl) -carbonyl) -N-
methyl-amino)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(249) 3-Z- [1- (4- (N- ( (2- (N-benzyl-N-methyl-amino) -ethyl) -
carbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(250) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-isopropyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(251) 3-Z- [1- (4- (N- (piperidin-1-yl-methylcarbonyl) -N-
isopropyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(252) 3-Z-[1-(4-(N-((4-tert.butoxycarbonyl-piperazin-l-yl)-
methylcarbonyl)-N-isopropyl-amino)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 212 -
(253) 3-Z-[1-(4-(N-((N-benzyl-N-methyl-amino)-methylcarbonyl)-
N-benzyl-amino)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(254) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-benzyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(255) 3-Z-[1-(4-(N-(piperidin-1-yl-methylcarbonyl)-N-benzyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(256) 3-Z- [1- (4- (1, 2, 4-triazol-2-yl-methyl) -anilino) -i-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(257) 3-Z-[1-(4-(1,2,3-triazol-2-yl-methyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(258) 3-Z-[1-(4-(1,2,3-triazol-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(259) 3-Z-[1-(4-((N-aminocarbonylmethyl-N-methyl-amino)-
methyl)-anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(260) 3-Z- [1- (4- ( (di- (2-methoxy-ethyl) -amino) -methyl) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(261) 3-Z- [1- (4- ( (di- (2-hydroxy-ethyl) -amino) -methyl) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(262) 3-Z-[1-(4-((N-ethoxycarbonylmethyl-N-methyl-amino)-
methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
CA 02387013 2002-03-19
- 213 -
(263) 3-Z- [1- (4- (azetidin-1-yl-methyl) -anilino) -1-phenyl-
methylene]-6-ethoxycarbonyl-2-indolinone
(264) 3-Z-[1-(4-(N-propyl-N-tert.butoxycarbonyl-aminomethyl)-
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(265) 3-Z- [l- (4- ( (N- (2- (2-methoxy-ethoxy) -ethyl) -N-methyl-
amino)-methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(266) 3-Z-[1-(4-((N-(tert.butoxycarbonyl-3-amino-propyl)-N-
methyl-amino)-methyl)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(267) 3-Z-[1-(4-((N-(methylcarbamoyl-methyl)-N-methyl-amino)-
methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(268) 3-Z-[1-(4-((N-(dimethylcarbamoyl-methyl)-N-methyl-
amino)-methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(269) 3-Z-[1-(4-((N-propyl-N-methyl-amino)-methyl)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(270) 3-Z- [1- (4- ( (N- (2-dimethylamino-ethyl) -N-methyl-amino) -
methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(271) 3-Z-[1-(4-((N-(3-dimethylamino-propyl)-N-methyl-amino)-
methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(272) 3-Z- [1- (4- ( (N- (2-methoxy-ethyl) -N-methyl-amino) -methyl) -
anilino)-i-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 214 -
(273) 3-Z- [l- (4- ( (N- (2-hydroxy-ethyl) -N-methyl-amino) -methyl) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(274) 3-Z-[1-(4-((N-(dioxolan-2-yl-methyl)-N-methyl-amino)-
methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(275) 3-Z-[1-(4-(3-oxo-piperazin-l-yl-methyl)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(276) 3-Z-[1-(4-(N-(piperazin-1-yl-methylcarbonyl)-N-
isopropyl-amino)-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(277) 3-Z- [1- (4- (N- ( (2- (piperazin-1-yl) -ethyl) -carbonyl) -N-
methyl-amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(278) 3-Z- [1- (4- ( (N- (3-amino-propyl) -N-methyl-amino) -methyl) -
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(279) 3-Z- [1- (4- (N- (3-methylamino-propyl) -N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(280) 3-Z-[l-(4-Ureidomethyl-anilino)-1-phenyl-methylene]-6-
ethoxycarbonyl-2-indolinone
(281) 3-Z-[1-(4-guanidinomethyl-anilino)-1-phenyl-methylene]-
6-ethoxycarbonyl-2-indolinone
(282) 3-Z-[1-(4-(N-methylsulphonyl-aminomethyl)-anilino)-1-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(283) 3-Z-[1-(4-(4-benzoyl-piperazin-1-yl-methyl)-anilino)-i-
phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 215 -
(284) 3-Z- [1- (4- ( (N- (3-acetylamino-propyl) -N-methyl-amino) -
methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(285) 3-Z-[1-(4-((N-(3-methylsulphonylamino-propyl)-N-methyl-
amino)-methyl)-anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-
2-indolinone
(286) 3-Z-[1-(4-((N-carboxymethyl-N-methyl-amino)-methyl)-
anilino)-1-phenyl-methylene]-6-ethoxycarbonyl-2-indolinone
(287)3-Z-(1-anilino-l-phenyl-methylene)-6-methoxycarbonyl-
2-indolinone
(288) 3-Z-[1-(4-nitro-anilino)-1-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinone
(289) 3-Z-[1-(4-fluoro-anilino)-1-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinone
(290) 3-Z-[1-(4-chloro-anilino)-1-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinone
(291) 3-Z-[1-(4-bromo-anilino)-1-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinone
(292) 3-Z- [1- (4-iodo-anilino) -1-phenyl-methylene] -6-
methoxycarbonyl-2-indolinone
(293) 3-Z-[1-(4-cyano-anilino)-i-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinone
(294) 3-Z-[1-(4-carboxy-anilino)-i-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 216 -
(295) 3-Z-[1-(4-methoxy-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(296) 3-Z-[1-(4-ethoxy-anilino)-1-phenyl-methylene]-6-methoxy-
carbonyl-2-indolinone
(297) 3-Z- [1- (4-trifluoromethyl-anilino) -i-phenyl-methylene] -
6-methoxycarbonyl-2-indolinone
(298) 3-Z-[1-(4-methylmercapto-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
(299) 3-Z- [1- (4- (isopropylaminomethyl) -anilino) -i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(300) 3-Z- [1- (4- (anilinomethyl) -anilino) -1-phenyl-methylene] -
6-methoxycarbonyl-2-indolinone
(301) 3-Z-[1-(4-(isobutylaminomethyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(302) 3-Z-[1-(4-(cyclohexylaminomethyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(303) 3-Z-[1-(4-(benzylaminomethyl)-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(304) 3-Z-[1-(4-((N-methyl-N-propyl-amino)-methyl)-anilino)-
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(305) 3-Z-[1-(4-((N-isopropyl-N-methyl-amino)-methyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(306) 3-Z-[1-(4-((N-ethyl-N-propyl-amino)-methyl)-anilino)-
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 217 -
(307) 3-Z- [1- (4- ( (N-ethyl-N-isopropyl-amino) -methyl) -anilino) -
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(308) 3-Z-[1-(4-(dipropylaminomethyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(309) 3-Z-[1-(4-(diisopropylaminomethyl)-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(310) 3-Z- [1- (4- ( (N-benzyl-N-ethyl-amino) -methyl) -anilino) -
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(311) 3-Z- [1- (4- (dibenzylaminomethyl) -anilino) -1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(312) 3-Z-[1-(4-(3,6-dihydro-2H-pyridin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(313) 3-Z-[1-(4-(3,5-dimethyl-piperidin-1-yl-methyl)-anilino)-
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(314) 3-Z-[1-(4-(azepan-1-yl-methyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(315) 3-Z- [1- (4- (2-amino-ethyl) -anilino) -i-phenyl-methylene] -
6-methoxycarbonyl-2-indolinone
(316) 3-Z-[1-(4-(2-methylamino-ethyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(317) 3-Z-[1-(4-(2-ethylamino-ethyl)-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(318) 3-Z-[1-(4-(2-dirnethylamino-ethyl)-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 218 -
(319) 3-Z-[1-(4-(2-diethylamino-ethyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(320) 3-Z- [1- (4- (2-piperidin-1-yl-ethyl) -anilino) -1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(321) 3-Z- [1- (4- (2-acetylamino-ethyl) -anilino) -1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(322) 3-Z- [1- (4- (3-amino-propyl) -anilino) -1-phenyl-methylene] -
6-methoxycarbonyl-2-indolinone
(323) 3-Z-[1-(4-(3-dimethylamino-propyl)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(324) 3-Z-[1-(4-(N-aminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(325) 3-Z-[1-(4-(N-ethylaminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(326) 3-Z-[1-(4-(N-diethylaminomethylcarbonyl-N-methyl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(327) 3-Z-[1-(4-(N-dipropylaminomethylcarbonyl-N-methyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
(328) 3-Z-[1-(4-(N-((N-ethyl-N-methyl-amino)-methylcarbonyl)-
N-methyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(329) 3-Z-[1-(4-(N-((N-ethyl-N-propyl-amino)-methylcarbonyl)-
N-methyl-amirio) -anilino) -1-phenyl-methylene] -6-
methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 219 -
(330) 3-Z-[1-(4-(N-((N-methyl-N-propyl-amino)-methylcarbonyl)-
N-methyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(331) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-ethyl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(332) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-propyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
(333) 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-butyl-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(334) 3-Z- [1- (4.- (N- (2-amino-ethylcarbonyl) -N-methyl-amino) -
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(335) 3-Z-[1-(4-(N-(2-diethylamino-ethylcarbonyl)-N-methyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
(336) 3-Z- [1- (4- (N-acetyl-N- (2-aminoethyl) -amino) -anilino) -
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(337) 3-Z- [1- (4- (N-acetyl-N- (2-methylamino-ethyl) -amino) -
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(338) 3-Z- [1- (4- (N-acetyl-N- (3-methylamino-propyl) -amino) -
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(339) 3-Z-[l-(4-(N-acetyl-N-(2-piperidin-1-yl-ethyl)-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(340) 3-Z- [1- (4- (N-acetyl-N- (aminocarbonylmethyl) -amino) -
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 220 -
(341) 3-Z- [1- (4- (N-acetyl-N- (piperidin-1-yl-carbonylmethyl) -
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
(342) 3-Z- [1- (4- (N-methyl-N- (aminocarbonyl) -amino) -anilino) -
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(343) 3-Z- [1- (4- (N-methyl-N- (methylaminocarbonyl) -amino) -
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(344) 3-Z-[1-(4-(N-methyl-N-(dimethylaminocarbonyl)-amino)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(345) 3-Z-[1-(4-(N-methyl-N-(piperidin-1-yl-carbonyl)-amino)-
anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(346) 3-Z-[1-(4-(N-(2-ethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
(347) 3-Z-[1-(4-(N-(2-diethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
l
(348) 3-Z- [1- (4- (N- (2-pyrrolidin-1-yl-ethyl) -N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(349) 3-Z- [1- (4- (N- (2-piperidin-1-yl-ethyl) -N-methylsulphonyl-
amino)-anilino)-i-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
(350) 3-Z-[1-(4-(N-(2-piperazin-1-yl-ethyl)-N-methylsulphonyl-
amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
CA 02387013 2002-03-19
- 221 -
(351) 3-Z- [1- (4- (N- (2- (4-morpholin-1-yl) -ethyl) -N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(352) 3-Z-[1-(4-(N-(ethylaminocarbonylmethyl)-N-
methylsulphonyl-amino)-anilino)-i-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(353) 3-Z-[1-(4-(N-(diethylaminocarbonylmethyl)-N-
methylsulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(354) 3-Z-[1-(4-(N-(pyrrolidin-1-yl-carbonylmethyl)-N-methyl-
sulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(355) 3-Z-[1-(4-(N-(piperidin-1-yl-carbonylmethyl)-N-methyl-
sulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(356) 3-Z-[1-(4-(N-(piperazin-1-yl-carbonylmethyl)-N-methyl-
sulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(357) 3-Z-[1-(4-(N-((morpholin-4-yl)-carbonylmethyl)-N-methyl-
sulphonyl-amino)-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(358) 3-Z-[1-(4-(2-dimethylamino-ethoxy)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(359) 3-Z-[1-(4-(3-dimethylamino-propoxy)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(360) 3-Z-[1-(4-(aminocarbonylmethyl)-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 222 -
(361) 3-Z-[1-(4-(2-aminocarbonyl-ethyl)-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(362) 3-Z- [1- (4- (pyridin-2-yl) -anilino) -1-phenyl-methylene] -
6-methoxycarbonyl-2-indolinone
(363) 3-Z-[1-(4-(pyridine-3-yl)-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
(364) 3-Z- [1- (4 ( (N-phenethyl-N-methyl-amino) -methyl) -anilino) -
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(365) 3-Z-[1-(4-(N-acetyl-N-methyl-amino)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(366) 3-Z-[1-(4-(N-ethylcarbonyl-N-(dimethylaminocarbonyl-
methyl)-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-
2-indolinone
(367) 3-Z-[1-(4-(N-methyl-N-methylsulphonyl-amino)-anilino)-
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(368) 3-Z-[1-(4-carboxymethyl-anilino)-1-phenyl-methylene]-6-
methoxycarbonyl-2-indolinone
(369) 3-Z-[1-(4-carbamoylmethyl-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
(370) 3-Z-[1-(4-dimethylcarbamoylmethyl-anilino)-i-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(371) 3-Z-[1-(4-tetrazol-5-yl-anilino)-1-phenyl-methylene]-
6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 223 -
(372) 3-Z- [1- (4- (piperidin-1-yl-methyl) -anilino) -methylene] -
6-methoxycarbonyl-2-indolinone
(373) 3-Z- [1- (4- (piperidin-1-yl-methyl) -anilino) -ethylidene] -
6-methoxycarbonyl-2-indolinone
(374) 3-Z- [1- (4- (piperidin-1-yl-methyl) -anilino) -propylidene] -
6-methoxycarbonyl-2-indolinone
(375) 3-Z- [1- (4- (piperidin-1-yl-methyl) -anilino) -butylidene] -
6-methoxycarbonyl-2-indolinone
(376) 3-Z- [1- (4- (N- (3-dimethylamino-propyl) -N-acetyl-amino) -
anilino)-methylene]-6-methoxycarbonyl-2-indolinone
(377) 3-Z- [1- (4- (N- (3-dimethylamino-propyl) -N-acetyl-amino) -
anilino)-ethylidene]-6-methoxycarbonyl-2-indolinone
(378) 3-Z- [1- (4- (N- (3-dimethylamino-propyl) -N-acetyl-amino) -
anilino)-propylidene]-6-methoxycarbonyl-2-indolinone
(379) 3-Z-[1-(4-(N-(3-dimethylamino-propyl)-N-acetyl-amino)-
anilino)-butylidene]-6-methoxycarbonyl-2-indolinone
(380) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-methylene]-6-methoxycarbonyl-2-indolinone
(381) 3-Z- [1- (4- (N- (2-dimethylamino-ethyl) -N-methylsulphonyl-
amino)-anilino)-ethylidene]-6-methoxycarbonyl-2-indolinone
(382) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-propylidene]-6-methoxycarbonyl-2-indolinone
(383) 3-Z-[1-(4-(N-(2-dimethylamino-ethyl)-N-methylsulphonyl-
amino)-anilino)-butylidene]-6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 224 -
(384) 3-Z-[1-(4-tetrazol-5-yl-anilino)-methylene]-6-methoxy-
carbonyl-2-indolinone
(385) 3-Z-[1-(4-tetrazol-5-yl-anilino)-ethylidene]-6-methoxy-
carbonyl-2-indolinone
(386) 3-Z-[1-(4-tetrazol-5-yl-anilino)-propylidene]-6-methoxy-
carbonyl-2-indolinone
(387) 3-Z-[1-(4-tetrazol-5-yl-anilino)-butylidene]-6-methoxy-
carbonyl-2-indolinone
(388) 3-Z-[1-(4-carboxy-anilino)-methylene]-6-methoxycarbonyl-
2-indolinone
(389) 3-Z- [1- (4-carboxy-anilino) -ethylidene] -6-
methoxycarbonyl-2-indolinone
(390) 3-Z-[1-(4-carboxy-anilino)-propylidene]-6-methoxy-
carbonyl-2-indolinone
(391) 3-Z- [1- (4-carboxy-anilino) -butylidene] -6-
methoxycarbonyl-2-indolinone
(392) 3-Z-[1-(4-(N-benzyl-N-methyl-aminomethyl)-anilino)-1-
methyl-methylene]-6-methoxycarbonyl-2-indolinone
(393) 3-Z-[1-(4-(2,3,4,5-tetrahydro-benzo(d)azepin-3-yl-
methyl)-anilino)-1-methyl-methylene]-6-methoxycarbonyl-2-
indolinone
(394) 3-Z-[1-(4-((benzo(1,3)dioxol-5-yl-methyl)-methyl-amino-
methyl)-anilino)-1-methyl-methylene]-6-methoxycarbonyl-2-
indolinone
CA 02387013 2002-03-19
- 225 -
(395) 3-Z-[1-(4-(N-phenethyl-N-methyl-aminomethyl)-anilino)-1-
methyl-methylene]-6-methoxycarbonyl-2-indolinone
,
(396) 3-Z- [1- (4- (N- (3,4-dimethoxy-benzyl) -N-methyl-a-mino-
methyl)-anilino)-1-methyl-methylene]-6-methoxycarbonyl-2-
indolinone
(397) 3-Z- [1- (4- (N- (4-Chloro-benzyl) -N-methyl-amino-methyl) -
anilino)-i-methyl-methylene]-6-methoxycarbonyl-2-indolinone
(398) 3-Z-[1-(4-(N-(4-methylbenzyl)-N-methyl-amino-methyl)-
anilino)-1-methyl-methylene]-6-methoxycarbonyl-2-indolinone
(399) 3-Z- [1- (4- (N- (4-fluoro-benzyl) -N-methyl-amino-methyl) -
anilino)-1-methyl-methylene]-6-methoxycarbonyl-2-indolinone
(400) 3-Z- [1- (4- (N- (4-bromo-benzyl) -N-methyl-amino-methyl) -
anilino)-1-methyl-methylene]-6-methoxycarbonyl-2-indolinone
(401) 3-Z- [1- (4- (N- (3-dimethylamino-propionyl) -N-
dimethylaminocarbonylmethyl-amino)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(402) 3-Z- [1- (4- (N- (4-dimethylamino-butyryl) -N-
dimethylaminocarbonylmethyl-amino)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(403) 3-Z-[1-(4-(N-dimethylaminocarbonylmethyl-N-(2-
dimethylamino-ethylsulphonyl)-amino)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
(404) 3-Z-[1-(4-(N-dimethylaminocarbonylmethyl-N-(3-
dimethylamino-propylsulphonyl)-amino)-anilino)-1-phenyl-
methylene]-6-methoxycarbonyl-2-indolinone
CA 02387013 2002-03-19
- 226 -
(405) 3-Z- [l- (4- ( (2-hydroxy-ethyl) -amino-methyl) -anilino) -1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(406) 3-Z-[1-(4-((2-methoxy-ethyl)-amino-methyl)-anilino)-1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(407) 3-Z-[1-(4-((2-dimethylamino-ethyl)-amino-methyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(408) 3-Z-[1-(4-((3-dimethylamino-propyl)-amino-methyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(409) 3-Z-[1-(4-((N-tert.butoxycarbonyl-2-amino-ethyl)-amino-
methyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
(410) 3-Z-[1-(4-((N-tert.butoxycarbonyl-3-amino-propyl)-amino-
methyl)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-
indolinone
(411) 3-Z- [1- (4- ( (2-amino-ethyl) -amino-methyl) -anilino) -1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(412) 3-Z-[l-(4-((3-amino-propyl)-amino-methyl)-anilino)-1-
phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(413) 3-Z- [1- (4- ( (2-acetylamino-ethyl) -amino-methyl) -anilino) -
1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(414) 3-Z-[1-(4-((3-acetylamino-propyl)-amino-methyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
(415) 3-Z-[1-(4-((2-methylsulphonylamino-ethyl)-amino-methyl)-
anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.