Note: Descriptions are shown in the official language in which they were submitted.
CA 02387146 2002-04-10
PCT/DE 00/03608 (WO 01/27254)
Gene transfer vectors for the therapy of autoimmune diseases
and diseases involving immunopathogenesis
The invention relates to a gene transfer vector which
comprises at least one nucleic acid molecule which comprises
a first nucleic acid sequence, encoding one or more
apoptosis-inducing ligand(s), a second nucleic acid sequence,
encoding one or more antigen(s), and, where appropriate, a
third nucleic acid sequence, encoding one or more
antiapoptosis molecule(s), and, where appropriate, a fourth
nucleic acid sequence, encoding one or more suicide
enzyme ( s ) .
The development of autoimmune diseases, such as multiple
sclerosis (MS) or diabetes (type 1), is due to an
uncontrolled activation of immune cells, which attack the
healthy cells in the body and destroy them. T lymphocytes,
which recognize fragments of endogenous proteins in
connection with endogenous MHC (major histocompatibility
complex) molecules, are crucially involved in the
pathogenesis of autoimmune diseases. T helper cells
(CD4+/CD8-) , which recognize a combination of peptide and MHC
Class II, have a key function in immunopathogenesis since
they stimulate the induction of autoreactive antibodies and
immune cells by secreting a variety of cytokines. Proteins
which are of cellular or viral origin, and which are
synthesized in cells, are degraded intracellularly in
proteasomes and presented on the cell surface, together with
MHC Class I molecules, to the T lymphocytes. These cells are
specifically recognized by cytolytic T cells (CD4-/CD8+) and
eliminated.
Normally, T cells which recognize endogenous proteins are
either eliminated (clonal deletion) or inactivated (anergy).
The consequence is tolerance to cells and structures which
are intrinsic to the body. In autoimmune diseases, this
tolerance is disturbed or inadequate. Autoimmune diseases are
CA 02387146 2002-04-10
- 2 -
possibly induced by viral or bacterial infections. It is
assumed that, because of similarities between pathogen-
specific and cell-type-specific proteins, uninfected cells
are also attacked by pathogen-specific antibodies or T cells.
In connection with chronically persisting viral infections,
inflammatory processes, some of which affect essential organs
such as the liver (hepatitis), also occur, even though the
immune response is primarily directed against pathogen-
specific structures. Such cases are said to involve immuno-
pathogenesis, since the damage and symptoms are primarily
caused by the body's own immune system and not by the
pathogen. A comparable situation is seen when transplanted
organs are rejected. In this case, a combination of the
donor's foreign MHC molecules and cellular proteins, with
which the molecules are complexed, is recognized by the
recipient's T cells as being foreign.
In accordance with present day knowledge, autoimmune diseases
and other diseases involving immunopathogenesis are treated
by a powerful immunosuppression and/or by administering
cytokines which have a regulatory effect, such as interferon
A large number of different preparations, provided by a
variety of manufacturers, are available for classical
chemotherapy using immunosuppressive agents, with these
preparations differing importantly in their areas of
application and their modes of action. A particularly serious
disadvantage of these substances is the occurrence of many
and various, and in some cases life-threatening, side-effects
(inter alia kidney damage, liver damage, inflammation of the
pancreas, anemia and fever), with these side-effects being
observed in a large number of patients. Such a nonspecific
inhibition of the immune system (e.g. with cyclosporin or
FK506) not only leads to a greatly attenuated immune defense,
and consequently to increased susceptibility to infectious
diseases, but also favors the development of tumors. For
example, the risk of being affected by Epstein Barr virus-
associated tumors is increased many times following organ
transplantations, where life-long immunosuppression is
required. The use of immunotherapeutic agents (e. g.
CA 02387146 2002-04-10
- 3 -
interferons) represents a marked improvement on classical
chemotherapy since it leads to fewer side-effects. However,
it is not possible to cure autoimmune diseases even with
these novel immunotherapeutic agents, since they do not act
specifically.
Antigen-presenting cells (APCs) play an important role both
in eliciting a T cell response and in inducing T cell
tolerance. The induction of an extensive activation and
multiplication of T cells is dependent on two signals which
the T cell has to receive. The first signal is conducted into
the T cell by the T cell receptor which recognizes an antigen
in connection with MHC on the surface of APCs. In the absence
of the second signal, i.e. what is termed the costimulatory
signal, the T cell becomes anergic. Anergy describes a state
in which the T cells are not reactive and do not multiply.
APCs are additionally able to influence the function of T
helper cells and to steer their properties either in the
direction of the Th(T helper cell)1 phenotype or in the
direction of the Th2 phenotype and thereby, for example,
promote the development of autoimmune diseases. The
combination of the different functions of the antigen-
presenting properties of the APCs determines the profile of
the T helper cell response. For this reason, APCs are able to
control whether an immune response proceeds immunogenically
or tolerogenically.
Fas is expressed on the surface of cells and mediates
apoptosis after it has interacted with the specific ligand
Fast or after it has bound an anti-Fas antibody which has an
agonistic effect. What is termed the "activation-induced cell
death" (AICD) of T cells, i.e. their programmed cell death
after the cells have been activated, is induced by an
autocrine feedback following the interaction of Fas and Fast
on one and the same activated T cell, an event which
underlines the importance of Fas-associated apoptosis for the
maintenance of T cell tolerance.
The Fas-mediated apoptosis of APCs plays a role in the
f
CA 02387146 2002-04-10
- 4 -
downregulation of immune responses. Activated T cells express
increased quantities of Fas ligand and in this way induce
apoptosis in the APCs. On the other hand, activated
macrophages also express Fas ligand and are able, for their
part, to induce apoptosis in the T cells. Thus, a large
quantity of Fas ligand on HIV-infected macrophages is, for
example, taken to be connected with the depletion of HIV-
specific CD4+ T cells.
The importance of Fas-dependent apoptosis for maintaining T
cell tolerance and avoiding autoimmune diseases has been
demonstrated, inter alia, by the fact that mutations which
affect the genes for Fas and the ligand of Fas, i.e. FasL,
respectively, lead to the development of autoimmune diseases
in 1pr/lpr and g1d/g1d mice, respectively. g1d/g1d and
lpr/1pr respectively designate mice which are homozygous for
inactivating mutations for Fast and Fas, respectively. Both
the clonal deletion of T cells in the periphery of the body,
and the maintenance of T cell tolerance to the body's own
antigens (autoantigens) and superantigens are disturbed in
lpr/1pr mice. Using a Fas gene which is artificially
introduced into, and then expressed in, the T cells to
correct the Fas-associated apoptosis defect in these cells
can prevent the development of autoimmune diseases in Ipr/lpr
mice.
Fas-mediated apoptosis also plays a crucial role in the
maintenance of immunoprivileged sites in the body. The
immunoprivileged state of the testes and the anterior chamber
of the eye require Fas ligand to be strongly expressed on the
corresponding parenchymal cells of these organs. In these
cases, it is assumed that the expression of Fas ligand on the
parenchyma) cells protects these tissues from destruction by
T cells by inducing apoptosis in the T cells. A viral
infection in the anterior chamber of the eye leads to
systemic T cell tolerance towards the virus. It is assumed
that APCs which present the Fas ligand together with
privileged antigens, which are derived from the privileged
cells, on their cell surfaces induce apoptosis of T cells in
CA 02387146 2002-04-10
- 5 -
the peripheral areas of the body and thereby bring about
systemic T cell tolerance.
In 1998, Zhang et a1. (Zhang et a1. (1998) Nat Biotechnol
16:1045-1049) demonstrated for the first time that Fas ligand
(FasL)-producing antigen-presenting cells (APCs) induced
antigen-specific T cell tolerance in a mouse model. It was
possible to produce T cell tolerance in mice using antigen-
presenting cells which, as a result of being infected with an
adenoviral vector, encoded adenoviral proteins in addition to
Fast. The T cell tolerance was specific for adenovirus
proteins and had no effect on an infection with mouse
cytomegalovirus. In a subsequent infection with an
adenovirus, mice which had been treated with the APCs
exhibited markedly prolonged persistence of the virus in the
liver, in conformity with a suppressed T cell response
against adenovirus-infected cells. Furthermore, the T cell
tolerance was dependent on the function of the Fas ligand
since it was not possible to induce any tolerance in Fas-
negative mice. A year later, the same research group
published experiments, which were likewise carried out in a
mouse model, on the induction of T cell tolerance toward
alloantigens (Zhang et a1. (1999) J Immunol 162: 1423-1430).
A further year later, the research group published
experiments which demonstrated that it was possible to use
Fast-expressing, antigen-presenting cells to prevent or treat
chronically inflammatory diseases as occur, for example,
following an infection of Fas-deficient mice with mouse
cytomegalovirus (Zhang et a1. (2000) J Clin Invest 105: 813-
821). In this experimental approach as well, the therapy was
based on inducing an antigen-specific, in this case mouse
cytomegalovirus-specific, T cell tolerance. For the above
described experiments, Zhang et a1. used adenoviral vectors,
which have the advantage of a high infection rate and ensure
satisfactory gene expression.
However, adenoviral vectors suffer from crucial disadvantages
precisely for use in humans; the foremost of these is the
expression of antigenic viral proteins, which has so far
CA 02387146 2002-04-10
- 6 -
thwarted gene therapy approaches using adenoviral vectors.
Since adenoviral vectors express regulatory and structural
proteins, the transduced cells are recognized by the immune
system, independently of the deliberately expressed antigens,
and eliminated. In order to avoid this reaction, the mice
described in the experiment had previously been made tolerant
to adenoviral proteins using appropriate Fast-positive APCs
which were expressing adenoviral proteins. A corresponding
approach to making the immune system tolerant toward a
potential viral pathogen is not suitable for humans.
The problem of the autocrine induction of apoptosis by
interaction of the artificially expressed Fast molecules with
the corresponding apoptosis receptors on the surface of the
APCs was ignored in the above-described experiments. In the
experiments of Zhang et al. (Zhang et a1. (1998) Nat
Biotechnol 16: 1045-1049; Zhang et a1. (1999) J Immunol 162:
1423-1430; Zhang et a1. (2000) J Clin Invest 105: 813-821),
Fas-deficient APCs were infected with vectors expressing the
Fas ligand. For an efficient therapeutic application, it is
imperative to protect the altered, antigen-presenting cells
from an autocrine stimulation of apoptosis and, at the same
time, to be able to avoid an uncontrollable immortalization
or even a transformation of the cells in the direction of a
tumor cell.
Furthermore, all the previous experiments have ignored the
problem of an unintentional stimulation of the immune system
resulting from expression of the antigen, for which T cell
tolerance is to be induced, without any simultaneous
expression of apoptosis-inducing ligands.
An object of the present invention is therefore the provision
of means for preventing or treating autoimmune diseases and
diseases involving immunopathogenesis.
The object is achieved by the subject matter defined in the
patent claims.
CA 02387146 2002-04-10
- 7 _
The invention is explained by the following figures.
Figure 1 shows, in diagrammatic form, the results of an
infection of mouse macrophages with an adenovirus which is
expressing Fast. Macrophages from CD95-deficient B6 mice were
purified and infected with adenoviruses which were expressing
either LacZ (AdLacZ, Ad/CV; Fast control) or Fast (AdFasL,
Ad/FL). (A) FACS was used to investigate the expression of
Fast on AdFasL-infected and uninfected macrophages. The
histogram shows the number of infected cells and the strength
of the Fast expression (Y axis, number of fluorescent cells;
X axis, strength of the fluorescence, determined using a
fluorescence-coupled anti-Fast antibody). (B) SlCr release
test for determining the ability of the infected, FasL-
expressing cells to induce apoptosis in target cells. The
macrophages which were infected with the two different
adenoviruses, and/or uninfected control macrophages, were
incubated with Slchromium- labeled, Fas+ target cells (A20
cells) and the lysis of the target cells was quantified by
the release of Slchromium into the culture supernatant.
Counts: number of Fast-expressing cells; FL2-H/PE: strength
of Fast expression; specific lysis (~): release of Slchromium
as related to a positive control in which maximum Slchromium
release was achieved by lyzing cells with SDS; E/T ratio:
ratio of effector cells (infected macrophages) to target
cells (A20 cells); M~-Ad/CV: macrophages infected with
AdLacZ (adenovirus which is expressing LacZ); MPG-Ad/FL:
macrophages infected with Ad-Fast (adenovirus which is
expressing FasL); M~-FL: macrophages transfected with a
Fast-expressing plasmid;
Figure 2 shows, in diagrammatic form, the result of
inhibiting the allogenic stimulation of T cells by FasL-
expressing antigen-presenting cells. Macrophages were
isolated from B6 1pr/Zpr mice and infected with adenoviruses
which were expressing either LacZ (AdLacZ) or Fast (AdFasL).
The infected macrophages were cocultured with T cells from
either (A) Fas-expressing B6 +/+ mice or (8) Fas-deficient B6
lpr/lpr mice and the stimulation of the T cells was measured
s.
CA 02387146 2002-04-10
_g_
by incorporating 3H-thymidine (mixed Lymphocyte reaction,
MLR). M~-CV: macrophages which are infected with AdLacZ
(adenovirus which is expressing LacZ); MQ~-FL: macrophages
which are infected with Ad-Fast (adenovirus which is
expressing FasL).
Figure 3 shows, in diagrammatic form, the result of
quantitatively analyzing the inflammation reaction in the
lung, the kidney and the liver. B6+~+ and B6 g1d/g1d mice were
infected intraperitoneally with mouse cytomegalovirus (1 x
105 pfu) and the degree of inflammation and of tissue damage
in the lung (upper panel), the kidney (middle panel) and the
liver (lower panel) was then assessed in accordance with a
relative scale of from 0 (no inflammation and/or damage) to 4
(strongest inflammation and/or damage). The thick lines in
the display represent the mean value ~ standard deviation of
the results from at least 5 mice at each investigation time.
Figure 4 shows, in diagrammatic form, the result of
decreasing the inflammations in the lung, the kidney and the
liver in mouse cytomegalovirus-infected mice which had been
treated, prior to the infection, with AdFasL-infected
antigen-presenting cells (APCs). B6 gId/g1d mice and B6
lpr/1pr mice were infected with mouse cytomegalovirus and, at
28 days after infection, treated with APCs which had either
been infected with Ad-CMVLacZ (Fast negative control), with
mouse cytomegalovirus (APC + MCMV), with AdFasL (Fast
positive control) or with mouse cytomegalovirus and AdFasL
(MCMV + AdFasL). The mice were treated four times with the
APCs at intervals of three days and examined four weeks after
the APC therapy had commenced. Lung, kidney and liver were
stained with hematoxylin and eosin and assessed by three
independent individuals. The thick lines in the display
represent the mean value ~ standard deviation of the
inflammation reaction in the different organs in the
variously treated mice. Lung gld and lung lpr: lung from B6
gId/g1d mice and B6 Ipr/1pr mice, respectively; liver gld and
liver lpr: liver from B6 g1d/g1d mice and B6 1pr/lpr mice,
respectively; kidney gld and kidney lpr: kidney from B6
CA 02387146 2002-04-10
_g-
gld/g1d mice and B6 1pr/1pr mice, respectively; APC-AdLacZ:
antigen-presenting cells infected with an adenovirus which is
expressing LacZ; APC+MCMV: antigen-presenting cells which are
infected with mouse cytomegalovirus; APC-AdFasL: antigen-
s presenting cells which are infected with an adenovirus which
is expressing Fast; APC-AdFasL+MCMV: antigen-presenting cells
which are infected with mouse cytomegalovirus and with an
adenovirus which is expressing Fast: * designates mean values
which differ significantly from the control group based on a
confidence interval of 95~ (P < 0.05).
Figure 5 shows, in diagrammatic form, the result of an
experiment for ascertaining the quantity of reactive T cells
which are specific for mouse cytomegalovirus from
cytomegalovirus (MCMV)-infected mice which, prior to the
infection, had been treated with AdFasL-infected antigen-
presenting cells (APCs). B6 1pr/1pr mice were infected with
mouse cytomegalovirus and treated with different APCs as
described in figure 4. Spleen cells were isolated from the
MCMV-infected mice 4 weeks after the APC therapy. The T cells
were stimulated in vitro with MCMV-infected APCs and the IL-
2-containing supernatant was isolated after 48 hours. APC-
AdLacZ: antigen-presenting cells which are infected with an
adenovirus which is expressing LacZ; APC-AdFasL: antigen-
presenting cells which are infected with an adenovirus which
is expressing Fast; APC-AdFasL+MCMV: antigen-presenting cells
which are infected with mouse cytomegalovirus and with an
adenovirus which is expressing Fast; * denotes mean values
which differ significantly from the control group based on a
confidence interval of 950 (P < 0.05).
Figure 6 shows, in diagrammatic form, the result of a
decreased production of autoantibodies in cytomegalovirus
(MCMV)-infected mice which, prior to infection, had been
treated with AdFasL-infected antigen-presenting cells (APC).
B6 g1d/g1d mice were infected with mouse cytomegalovirus and
treated with different APCs as described in figure 4. Sera
were isolated from the MCMV-infected mice 4 weeks after the
APC therapy. RF IgGl: rheumatoid factor; dsDNA IgGl:
CA 02387146 2002-04-10
-10-
autoantibodies directed against double-stranded DNA; APC-
AdLacZ: antigen-presenting cells which are infected with an
adenovirus which is expressing LacZ; APC-AdFasL: antigen-
presenting cells which are infected with an adenovirus which
is expressing Fast; APC-AdFasL+MCMV: antigen-presenting cells
which are infected with mouse cytomegalovirus and with an
adenovirus which is expressing Fast; * denotes mean values
which differ significantly from the control group based on a
confidence interval of 95~ (P c 0.05).
Figure 7 shows human macrophages which have been infected
with an adenovirus which is expressing LacZ. The virus
infected macrophages were identified using an X-Gal stain,
which detects (3-galactosidase (LacZ) in the infected cells by
means of its catalytic properties.
Figure 8 shows, in diagrammatic form, the results of an
experiment for demonstrating the modulating influence of
IL-10 .and tumor necrosis factor (TNF) on the function of
dendritic cells (DC) as antigen-presenting cells. Dendritic
cells were generated from peripheral blood mononuclear cells
in vitro by treating them with IL-4 and GM-CFS. The DCs were
treated either with TNF or IL-10 and subsequently incubated
with allogenic T cells; the stimulation and multiplication of
the T cells were then measured by incorporating 3H-labeled
thymidine. APC: antigen-presenting cells; DC (TNF): dendritic
cells which have been stimulated with tumor necrosis factor
(TNF); DC (IL-10): dendritic cells which have been stimulated
with interleukin-10 (IL-10); alto T cells: T cells from a
donor possessing an allogenic MHC pattern, i.e. an MHC
pattern which differs from that of the DCs. The X axis shows
the quantity of APCs which were used in the reaction. The Y
axis shows the radioactive disintegrations per minute (CPM)
as a measure of the incorporation of the radioactively
labeled nucleotide or as a measure of the stimulation of the
T cells.
Figure 9 shows, in diagrammatic form, the results of an
experiment for demonstrating an allogen-specific suppression
CA 02387146 2002-04-10
-11-
by tolerizing antigen-presenting cells (APCs). Dendritic
cells were generated from peripheral blood mononuclear cells
in vitro by treating them with IL-4 and GM-CFS. The DCs were
treated with either TNF or IL-10 and subsequently incubated
with allogenic T cells. Five days later, the T cells from
this reaction were incubated with antigen-presenting cells
from a third allogenic donor and the stimulation and
multiplication of the T cells were measured by the
incorporation of 3H-labeled thymidine. A, B and C denote
donors possessing different (allogenic) MHC patterns; MLR:
mixed lymphocyte reaction. The Y axis shows the radioactive
disintegrations per minute (CPM) as a measure of the
incorporation of the radioactively labeled nucleotide or as a
measure of the stimulation of the T cells. The compositions
of the first stimulation reaction (1st MLR) and the second
reaction (2nd MLR) are given under the individual bars.
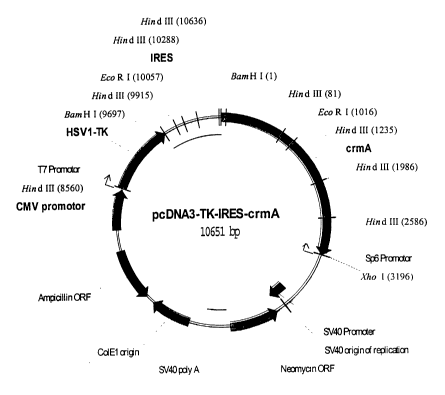
Figure 10 shows, in diagrammatic form, the construction of
the vectors according to the invention, i.e. (A) pcDNA3-TK-
IRES-crmA and (B) pcDNA3-Fast-IRES-PLP. Coding reading
frames, such as the nucleic acid sequence for the Fas ligand
(FasL), the proteolipid protein (PLP), the thymidine kinase
(TK) and crmA, and for resistance proteins such as neomycin
and ampicillin, are marked with light arrows. Eukaryotic
promoter elements which have regulatory activity, such as the
CMV promoter and the SV40 promoter, are depicted by dark
arrows, while prokaryotic promoters, such as the SP6 promoter
and the T7 promoter, are depicted by thin bent arrows.
Cleavage sites for selected restriction endonucleases, such
as BamHI, EcoRI, XhoI and HindIII, are identified with the
name of the nuclease. Regulatory nucleic acid sequences, such
as the SV40 virus polyadenylation sequence (SV40polyA) and
the IRES, i.e. the internal ribosome binding site, are marked
by thin bars.
The expressions "vector" or "gene transfer vector" which are
used here denote naturally occurring or artificially created
organisms and constructs for the uptake, replication,
expression or transfer of nucleic acids in cells. Viruses,
CA 02387146 2002-04-10
-12-
such as retroviruses, adenoviruses, adeno-associated viruses,
poxviruses, alphaviruses or herpesviruses are examples of
vectors. Bacteria, such as listerias, shigellas or
salmonellas, are also examples of vectors. Liposomes or naked
DNA, such as bacterial plasmids, virus-derived plasmids,
phagemids, cosmids, bacteriophages or artificially prepared
nucleic acids, such as artificial chromosomes, are further
examples of viruses.
The expression "apoptosis receptor" which is used here
denotes polypeptides which are located in the cytoplasmic
membrane of cells and which initiate apoptosis in the cell
following interaction with, and activation by, a specific
ligand. Examples of apoptosis receptors are polypeptides
which belong to the subfamily of tumor necrosis factor
receptors which are characterized by cytoplasmic death
domains, for example CD95/Fas/Apol, TRAIL-R1, TRAIL-R2 and
Apo3.
The expressions "ligand" or "apoptosis-inducing ligand" or
"apoptosis ligand" which are used here denote a membrane-
located polypeptide which can interact with apoptosis
receptors. The binding of the ligands to the apoptosis
receptors activates the receptors and induces apoptosis in
the cells which are carrying the receptors. Examples of
apoptosis ligands are CD95L/FasL/ApolL, TRAIL and Apo3L.
The expression "antiapoptosis molecules" which is used here
denotes polypeptides which inhibit apoptosis in the cell.
These polypeptides may be of cellular or viral origin.
Antiapoptosis molecules furthermore denote nucleic acid
molecules, including nucleic acids which are complementary to
nucleic acids, which encode apoptosis-inducing polypeptides.
The expression "antigen" which is used here denotes
polypeptides which comprise either a complete protein or
parts of a protein which include single or several T cell
epitopes and, after proteolytic processing by the cell, are
presented by MHC molecules and bound by T cell receptors.
CA 02387146 2002-04-10
-13-
The expression "suicide enzyme" which is used here denotes
polypeptides which convert substances, which are only
slightly toxic or are not toxic, into toxic substances or
alter them in such a way that they can be used or converted
by enzymes in the cell.
The expression "IRES" which is used here denotes viral
nucleic acid sequences which enable binding of functionally
active ribosomes to take place, independently of the cellular
regulatory sequences, such as the 5'-Cap structure. IRES
sequences are characterized by a strong secondary structure.
IRES sequences have been described, for example in picorna-
viruses.
An object of the present invention is to enable a selective,
antigen-specific immunotherapy to be achieved in cases of
autoimmune diseases and diseases involving
immunopathogenesis. Individual T cell clones possessing
defined specificity for cellular or pathogen-specific
proteins are to be eliminated and immulological tolerance
toward an antigen thereby generated or restored.
The invention relates to a gene transfer vector which
comprises at least one nucleic acid molecule which comprises
a first nucleic acid sequence, which encodes one or more
apoptosis-inducing ligand(s), a second nucleic acid sequence,
which encodes one or more antigen(s), and, where appropriate,
a third nucleic acid sequence, which encodes one or more
antiapoptosis molecule(s), and, where appropriate a fourth
nucleic acid sequence, which encodes one or more suicide
enzyme(s). Preference is given to a gene transfer vector
which comprises a nucleic acid molecule which comprises the
first three, or all four, or the first two and the fourth,
nucleic acid sequences. Preference is furthermore given to a
gene transfer vector which comprises two nucleic acid
molecules, with the first and second nucleic acid sequences
being present on a first nucleic acid molecule and the third
and fourth. nucleic acid sequences being present on a second
CA 02387146 2002-04-10
-14-
nucleic acid molecule. Particular preference is given to a
gene transfer vector, with the first and second nucleic acid
sequences being functionally linked to each other such that
the expression of the second nucleic acid sequence is
dependent on the expression of the first nucleic acid
sequence and/or the third and fourth nucleic acid sequences
being functionally linked to each other such that the
expression of the fourth nucleic acid sequence is dependent
on the expression of the third nucleic acid sequence.
The gene transfer vectors according to the invention can be
used for treating autoimmune diseases and other diseases
which are due to immunopathogenesis. Immunopathogenesis
denotes damage to cells, tissues or organs which is caused by
cellular or humoral immune mechanisms, i.e. by lymphocytes or
antibodies or complement-mediated mechanisms. The vectors
according to the invention can be used to recombinantly alter
cells of the body ex vivo. By means of these gene therapy
vectors, the cells which are to be modified obtain a number
of new properties which make them suitable for treating
autoimmune diseases and other diseases which are due to
immunopathogenesis. Within the meaning of this invention,
suitable denotes that the modified cells are able to attract
the immune cells which are involved in the pathogenesis, to
recognize these cells specifically and to destroy them by
inducing apoptosis.
The vectors according to the invention can be based on a
large number of vector systems which are nowadays available
and which are able to carry a number of different genes or
functional regions and express the corresponding gene
products in eukaryotic cells. In the case of vectors which
are based on viral systems, nucleic acid sequences are
packaged into these vectors using packaging cell lines or
other in vitro systems. The vectors can then either penetrate
into the cells actively or be taken up by these cells.
Nonviral vectors are introduced into the target cells by way
of a variety of transfer processes which are based on
physical and biological mechanisms. An important property of
CA 02387146 2002-04-10
-15-
the vectors according to the invention is that no viral
proteins, or other proteins which are connected with the
vector system, which might interfere with the function of the
cells modified by the vector systems are synthesized in the
modified cells. Within the meaning of the invention, an
expression of viral or other proteins would be harmful if the
modified cells, which produce these proteins, are recognized
and destroyed by immune cells. Within the meaning of this
invention, this recognition would be harmful if it thereby
impairs the natural function of the immune system in
recognizing and destroying viral or bacterial pathogens,
degenerate cells or other cells or pathogens which are
normally recognized by the immune system. It is also harmful
if it thereby restricts the efficiency of the cells in
destroying the immunopathogenic immune cells.
The cells which have been modified by means of the vectors
according to the invention, which vectors comprise the
combination according to the invention of nucleic acid
sequences, express antigens which are recognized by
immunopathogenic immune cells. These immunopathogenic cells
play a particular role for the pathogenesis of a defined
disease since they specifically recognize (endogenous)
antigens and coordinate an immune reaction against these
antigens and the antigen-expressing cells. The antigens which
are introduced into the cells together with the vectors
according to the invention can be specific for particular
diseases or specific for the affected organ or tissue or the
affected cell type. The vectors according to the invention
additionally encode apoptosis-inducing ligands. These
apoptosis-inducing ligands induce natural cell death in the
immunopathogenic immune cells which recognize the antigens.
The vectors according to the invention may encode one or more
different apoptosis-inducing ligands. The modified cells only
recognize and destroy those immune cells which recognize the
artificially synthesized antigenic epitope and therefore
physically come into contact with the modified cells.
The nucleic acid sequences which are responsible for inducing
CA 02387146 2002-04-10
-16-
the apoptosis (apoptosis-inducing ligands) and the nucleic
acid sequences which encode the antigenic polypeptides
(antigens) can be functionally coupled or linked to each
other at the transcriptional level such that it is not
possible for the antigens to be expressed without the
apoptosis-inducing ligands being expressed. This greatly
increases the safety of the vectors according to the
invention since this thereby prevents it from being possible
for the disease-causing immune cells to recognize the altered
cells, and for the immune cells to be thereby stimulated,
without apoptosis being simultaneously induced in these
latter cells.
The nucleic acid sequences in the vectors according to the
invention can additionally carry genes or functional regions
which prevent the cells which have been altered by the vector
according to the invention from themselves initiating
apoptosis, by way of autocrine mechanisms, and in this way
destroying themselves (antiapoptosis molecules). This greatly
increases the efficiency of the vectors and of the altered
cells. The genes or functional regions either encode
regulators of the activity of apoptosis-inducing factors or
prevent them being expressed.
Where appropriate, the vectors according to the invention can
additionally comprise nucleic acid sequences (suicide genes)
which encode polypeptides which make it possible, if desired,
to eliminate the recombinantly modified cells after they have
been reinfused into the body. A functional coupling which is
comparable to the functional coupling of the nucleic acid
sequences which encode the antigen and the apoptosis-inducing
ligands can be present in the case of the nucleic acid
sequences which encode the antiapoptosis molecules and the
suicide enzymes. This coupling ensures that cells which can
no longer be eliminated from the body on account of the
antiapoptosis molecules can be removed by the function of the
suicide enzymes.
The nucleic acid sequences for the antiapoptosis molecules
CA 02387146 2002-04-10
-17-
and the suicide enzymes can be located on the same nucleic
acid molecule on which the nucleic acid sequences for the
apoptosis-inducing ligands and the antigens are located, or
they can be located on a different nucleic acid molecule.
The invention furthermore relates to a gene transfer vector
as a therapeutic agent. The invention furthermore relates to
the use of the gene transfer vectors for producing a
therapeutic composition for preventing or treating autoimmune
diseases, e.g. rheumatoid arthritis, systemic lupus
erythematodes, Sjogren's syndrome, polymyositis,
dermatomyositis, polymyalgia rheumatica, temporal arteritis,
spondylarthropathies, such as Bechterew's disease, Crohn's
disease, ulcerative colitis, celiac disease, autoimmune
hepatitis, type I diabetes mellitus, adrenal insufficiency,
thyroiditis, psoriasis, dermatitis, herpetiformis, pemphigus
vulgaris, alopecia, multiple sclerosis and myasthenia gravis,
or for preventing or treating chronically inflammatory
processes which are due to immunopathogenesis, for example
chronic inflammations following viral or bacteria l
infections, such as chronic hepatitis in the case of
hepatitis B virus or hepatitis C virus infections, or
encephalitis following infection with the measles virus, and
for preventing or treating transplant rejections.
The invention furthermore relates to the use of gene transfer
vectors for the ex vivo modification of eukaryotic cells, in
particular animal or mammalian cells, in particular human
cells.
Retroviral vectors
Preference is given to the gene transfer vectors being
retroviral vectors and, in particular, vectors which are
based on lentiviruses. These constitute a suitable platform
for developing efficient vectors for transferring nucleic
acids into cells. The insertion of a desired foreign gene
into a suitable vector, and the packaging into retroviral
particles, can be carried out using methods which have
CA 02387146 2002-04-10
-18-
already been described in detail, and are state of the art.
The recombinant viruses which are produced are subsequently
isolated and incubated in vivo or ex vivo with the desired
target cells. A large number of different retroviral systems
have thus far being described, with these systems being
suitable for transferring the combinations of nucleic acids
according to the invention. Preference is therefore given to
using retroviral, and, in particular, lentiviral, gene
transfer vectors for transferring the combinations of nucleic
acid sequences according to the invention into eukaryotic
cells.
Retroviral gene transfer vectors according to the invention
can be based on a variety of retroviruses such as type B, C
or D retroviruses and also spumaviruses and lentiviruses.
Examples of representatives of suitable retrovirus families
are those which are described on pages 2-7 in "RNA Tumor
Viruses" and also a large number of xenotrophic retroviruses,
such as NZB-X1, NZB-X2 and NZB9-1, and polytrophic
retroviruses, such as MCF and MCF-MLV. These retroviruses can
be obtained from stocks or collections, such as the American
Type Culture Collection ("ATCC", Manassas, Va.), or can be
isolated from biological material using current and published
molecular biological techniques.
Retroviruses which are particularly suitable for preparing
retroviral gene transfer vectors comprise representatives
from the group of avian leukemia viruses, bovine leukemia
viruses, mouse leukemia viruses, mink cell focus-inducing
viruses, mouse sarcoma viruses, gibbon leukemia viruses, cat
leukemia viruses, reticuloendothelial viruses and Rous
sarcoma viruses. Mouse leukemia viruses such as the
representatives 4070A and 1504A, Abelson (ATCC No. VR-999),
Friend (ATCC No. VR-245), Graffi, Gross (ATCC No. 590),
Kirsten, Harvey sarcoma virus and Rauscher (ATCC No. VR-998),
and also the Moloney mouse leukemia virus (ATCC No. VR-190),
are particularly suitable. The Rous sarcoma virus, including
Bratislava, Bryan high titer (e. g. ATCC Nos. VR-334, VR-657,
CA 02387146 2002-04-10
-19-
VR-726, VR-659 and VR-728), Bryan Standard, Carr-Zilber,
Engelbreth-Holm, Harris, Prague (e.g. ATCC Nos. VR-772 and
45033) and Schmidt-Ruppin (e.g. ATCC Nos. VR-724, VR-725 and
VR-354), are also particularly suitable.
In the case of special applications, which are described in
the invention, components of the nucleic acid molecules in
the retroviral gene transfer vectors can also be derived from
other retroviruses than those which are listed. For example,
retroviral vector long terminal repeat (LTR) regions can be
derived from the mouse sarcoma virus while the tRNA binding
site can be derived from the Rous sarcoma virus, the
packaging signal from the mouse leukemia virus and the origin
for the second strand DNA synthesis from the avian leukemia
virus.
It is furthermore possible to use nucleic acid molecules for
retroviral vectors which contain a 5' LTR, a tRNA binding
site, a packaging signal, one or more heterologous sequences,
an origin of second strand DNA synthesis, and a 3' LTR, with
the nucleic acid molecule not containing any sequences
encoding Gag/Pol or Env. LTRs contain three elements, i.e.
the U5, R and U3 regions. These elements contain a large
number of signals which are of importance for the biological
activity of retroviruses, for example promoters and enhancer
elements which are located in the U3 region. LTRs within a
provirus can be characterized unambiguously with the aid of
the characteristic sequence duplications at the ends of the
genome. The 5' LTRs which are preferably used in the present
invention contain a 5' promoter element and a minimal LTR
sequence which enables the vector nucleic acid to be reverse-
transcribed and integrated into the genome of the target
cell. The 3' LTR region contains a polyadenylation signal and
LTR sequences which are required for the reverse
transcription and integration of the vector nucleic acid into
the genome of the target cell.
The tRNA binding site and the origin of the second strand DNA
synthesis are required for biological activity, and the
CA 02387146 2002-04-10
-20-
identification of these components is state of the art. For
example, retroviral tRNAs bind, by means of Watson-Crick base
pairing, to a tRNA binding site and are packaged into the
virus particles together with the retroviral genome. The tRNA
is then used by the reverse transcriptase as a primer for the
DNA synthesis. The tRNA binding sequence is located
immediately downstream of the 5' LTR and can be readily
identified by its location. In the same way, the origin of
the second strand DNA synthesis is of great importance for
retroviral second strand DNA synthesis. This region, which is
termed a polyuridine tract, is located directly upstream of
the 3' LTR.
In addition to the 5' and 3' LTRs, the tRNA binding sequence
and the origin of second strand DNA synthesis, the nucleic
acid sequences in retroviral gene transfer vectors can
contain a packaging signal and, in addition to this, one or
more heterologous sequences which are described in detail
below.
For example, use is made of retroviral gene transfer vectors
which do not possess nucleic acid sequences encoding Gag/Pol
or Env. For example, retroviral gene transfer vectors which
do not possess any sequences encoding Gag/Pol or Env can be
produced by preparing vector constructs which possess an
extended packaging signal. The term "extended packaging
signal" defines a nucleotide sequence which exceeds the
minimal sequence which is required for specifically packaging
nucleic acids. Use of the extended packaging sequence makes
it possible to prepare virus stocks which have a higher
titer, with this being due to an increased quantity of RNA
being packaged. For example, the minimal packaging signal of
the Moloney mouse leukemia virus (Mo-MLV) is encoded by a
sequence which begins at the end of the 5' LTR and contains
the Pst I cleavage site. The extended packaging signal of Mo-
MLV contains sequences beyond nucleotide 567, including the
start of the Gag/Pol gene (nucleotide 621), and ends beyond
nucleotide 1560. Therefore, a retroviral gene transfer vector
which does not possess any extended packaging signal can be
CA 02387146 2002-04-10
-21-
prepared from Mo-MLV by deleting the sequence extending
beyond nucleotide 567.
It is furthermore possible to use nucleic acid sequences, for
retroviral gene transfer vectors, in which the packaging
signal partially or entirely overlaps the retroviral Gag/Pol
sequence but has nevertheless been completely deleted or
truncated upstream of the start codon of the Gag/Pol gene. It
is furthermore possible to use nucleic acid sequences, for
retroviral gene transfer vectors, which contain a packaging
signal which is extended in the 5' region upstream of the
start of the Gag/Pol gene. If these retroviral vectors are
used, preference should be given to using packaging cell
lines, for producing the recombinant virus particles, in
which the 5' terminal end of the Gag/Pol gene in a Gag/Pol
expression cassette is modified such that it exhibits a codon
usage in the Gag gene which is modified and which differs
from the wild-type HIV-1 Gag sequence.
It is furthermore possible to use nucleic acid sequences, for
retroviral gene transfer vectors, which possess a 5' LTR, a
tRNA binding site, a packaging signal, an origin of second
strand DNA synthesis and a 3' LTR region, with the nucleic
acid sequence not possessing any retroviral nucleic acid
sequence upstream of the 5' LTR. These vectors do not possess
any Env-encoding sequence upstream of the 5' LTR. It is
furthermore possible to use nucleic acid sequences, for
retroviral gene transfer vectors, which contain a 5' LTR, a
tRNA binding sequence, a packaging signal, an origin of
second strand DNA synthesis and a 3' LTR but which do not
contain any retroviral packaging signal sequence downstream
of the 3' LTR. The term "packaging signal sequence", which is
used here, defines a sequence which is required for packaging
an RNA genome.
Suitable packaging cell lines for establishing the
abovementioned retroviral gene transfer vectors are already
available and have been used many times for preparing cell
lines (also termed vector cell lines) for producing
recombinant vector particles.
CA 02387146 2002-04-10
-22-
Among the retroviral vectors, lentiviral vectors are
particularly suitable for transferring the combinations,
according to the invention, of nucleic acid sequences since
they are able to insert nucleic acid sequences into a large
number of resting and postmitotic cells, such as neuronal
cells, liver cells, muscle cells and hematopoietic stem
cells, and to cause the sequences to be expressed. The
lentiviral vector particles can be produced by the triple
infection of mammalian cells with (i) a Gag/Pol expression
vector, (ii) a transfer construct which contains the
packaging signal, the foreign nucleic acid sequences) and
the flanking LTRs, and (iii) an expression vector for a coat
protein. In this connection, it is possible to use coat
proteins from various amphotrophic or xenotrophic
retroviruses and also other viruses, such as the coat
proteins of the Moloney mouse leukemia virus (Mo-MLV) and the
MLV isolate 4070A, and also the vesicular stomatitis virus
(VSV) G glycoprotein or the rabies G glycoprotein. Lentiviral
vectors which have been prepared in this way are able to
stably transfect a large number of different cells.
Lentiviral vectors which contain the central polyuridine
tract and the terminator sequence of the HIV Pol gene exhibit
an increased transduction efficiency, with this increased
efficiency being due to an improved nuclear translocation of
the vector.
In order to improve the safety of lentiviral gene therapy
vectors, it is possible to prepare different functional
packaging constructions containing deletions in the accessory
HIV-1 and/or SIV-1 genes Vif, Vpr, Vpu and Nef. It is
furthermore possible to develop functional lentiviral vectors
which no longer require the viral transactivator protein Tat.
Furthermore, in order to minimize the appearance of
replication-competent recombinants, it is possible to develop
self-inactivating vector systems in which, for example, an
extensive segment of the U3 region in the 5' and/or 3' LTR,
including the TATA box and the sites for binding the
transcription factors SP1 and NF-KB, is deleted within the
vector. These modifications remove a large part of the viral
CA 02387146 2002-04-10
-23-
transcription elements. Furthermore, deleting the U3 region
prevents a possible interference between the promoter located
in the LTR and internal promoters and drastically reduces the
danger of activating neighboring cellular genes at the site
of integration of the lentiviral vector. Synthetically
prepared, codon-adapted HIV and/or SIV Gag/Pol and Env genes
are used, for example, to circumvent the Rev dependence of
the lentiviral Gag/Pol and Env genes. Alternatively, it is
possible to use constitutive transport elements (CTE) from
other viruses, such as, for example, the Mason-Pfitzer monkey
virus CTE or the monkey retrovirus type 1 (SRV-1) CTE, and
also the hepatitis B virus post-transcriptional regulatory
element (PRE) and the Rous sarcoma virus post-transcriptional
direct repeat (DR) element, in order to enable the HIV/SIV
Gag, Gag/Pol and Env gene transcripts to be exported in a
Rev-independent manner. These methods enable the transactive
Rev protein to be excluded from the lentiviral vectors,
thereby contributing to an increase in the safety of this
vector type. A compilation of the lentiviral vector systems
which are currently in use is given, for example, in the
review by Buchschacher and Wong Staal (Buchschacher et al.
(2000) Blood 95: 2499-2504).
In addition to retroviral and lentiviral vectors, it is also
possible to use a large number of other viral and nonviral
gene transfer vectors which can likewise be employed for
transferring the nucleic acid sequence combinations according
to the invention. Since these vector systems are to be
employed for generating therapeutically utilizable,
recombinantly modified cell lines, the viral vector systems
are modified, for safety reasons, such that they are no
longer able to replicate lytically. Preference is given to
the gene transfer vectors being vectors which are based on
adenoviruses, adenoassociated viruses, poxviruses,
alphaviruses or herpesviruses.
Adeaoviral vectors
The preparation of recombinant adenoviral vectors (Ad
CA 02387146 2002-04-10
-24-
vectors), including the E1/E3, E1/E4 and gutless vectors, is
state of the art and can be carried out in accordance with
published protocols. For example, (1) the desired nucleotide
sequence can be inserted into a pBHGl1 plasmid in order to
produce recombinant E1/E3-deleted Ad vectors following the
transfection of 293 cells and subsequent intracellular
recombination; (2) the desired nucleotide sequence can first
of all be integrated into the E1 region of one of a large
number of E1-deleted Ad vectors, and cotransfected with a
ClaI-digested H5d11014 vector, and the recombinant, El/E4-
deleted Ad vectors can be isolated following the transfection
of 293 E4 cells and subsequent intracellular homologous
recombination, and (3) the desired nucleotide sequence can
first of all be inserted, together with an appropriate
quantity of a stuffer sequence, e.g. which is derived, for
example, from bacteriophage lambda DNA, into the ArAd plasmid
in order, subsequently, to ensure efficient packaging of the
recombinant gutless adenovirus vector genomes following
transfection into 293 cells and infection with an HS.CBALP
helper virus. Equilibrium sedimentation in a cesium chloride
gradient can be used, for example, to free the recombinant
gutless adenovirus vector particles from contaminating helper
viruses due to the vector particles having a lower density
than the helper virus.
Adenoassociated viruses
It is furthermore possible to use a variety of adeno-
associated virus (AAV) vector systems, which have already
been developed, for the gene transfer. A detailed description
of the construction of AAV vectors has been published and is
state of the art.
In recombinant AAV vectors, all the coding sequences are
usually replaced with the desired heterologous nucleic acid
sequences. The recombinant AAVs are prepared by
cotransfecting an AAV vector, which carries the desired gene,
and a helper AAV plasmid, which possesses all the essential
AAV genes, into adenovirus-infected cells, which provide all
CA 02387146 2002-04-10
-2 5-
the helper functions which are required for AAV replication
and the production of vector particles. However,
disadvantages of this vector system for use in gene therapy
are the low titers of recombinant vectors and possible
contaminations of the vectors with wild-type AAV and
infectious helper viruses. Furthermore, the size of the
foreign sequences to be integrated into AAV vectors is
limited to 5 kb.
Poxviruses
Alternative viral vector systems for transferring nucleic
acids which encode a desired foreign gene are based on
representatives of the poxvirus family, including the
vaccinia viruses and avian poxviruses. Vector systems of this
nature are prepared as will now be described using the
example of recombinant vaccinia viruses. The DNA encoding the
desired gene is first of all integrated into a suitable
vector such that it is located in the vicinity of a vaccinia
promoter and a flanking vaccinia DNA sequence such as the
sequence encoding thymidine kinase (TK). This vector is then
transfected into cells, with these latter simultaneously
being infected with vaccinia viruses. By means of an
homologous recombination, the insert containing the foreign
gene is then recombined into the viral genome. The resulting
TK-positive recombinants can be established by culturing the
viruses on cells in the presence of 5-bromodeoxyuridine and
subsequently isolating plaques.
Alternatively, it is possible to use other avian poxviruses
for the gene transfer, such as fowlpox and canarypox viruses.
Recombinant avian poxviruses which are expressing immunogens
derived from organisms which are pathogenic to humans can
induce a protective immune response after having been
administered to mammals. The use of avian poxviruses is
particularly advantageous for an application in humans and
other mammals since representatives of the avian pox genus
only replicate productively in receptive avian species and
not in mammalian cells. Methods for preparing recombinant
CA 02387146 2002-04-10
-26-
avian poxviruses are state of the art and are based on
genetic recombination mechanisms which are comparable with
those which have previously been described for producing
recombinant vaccinia viruses.
Alphaviruses
Vectors derived from representatives of the alphavirus genus,
such as Sindbis and Semliki forest viruses, can also be used
for transferring the nucleotide sequences of selected genes.
The preparation and use of vectors based on Sindbis virus are
state of the art and have been published many times.
Bacteria
Preference is furthermore given to the gene transfer vectors
being bacteria, in particular Listeria monocytogenes (Ompl,
DactA, ~plcB), Shigella flexneri (~aroA, OvirG) and
Salmonella typhimurium. A very promising DNA delivery system
for recruiting and activating antigen-specific cells makes
use of bacterial suicide vectors which are based, for
example, on attenuated Listeria monocytogenes (~mpl, DaCtA,
~plcB), Shigella flexneri (DarOA, wire) and Salmonella
typhimurium isolates. The preparation and use of such
bacterial gene transfer systems have been published in detail
and are state of the art. Thus, "suicide" strains of L.
monocytogenes are able, for example, to infect professional
antigen-presenting cells selectively. After the bacteria have
been taken up into the cytoplasm of the target cell, they are
destroyed by means of a Listeria-specific phage lysin,
resulting in the release of the plasmid DNA transported by
the bacteria, with this DNA subsequently penetrating into the
cell nucleus. Important advantages of this bacterial system
lie in the oral administration of the bacteria and the
selective introduction of plasmids into APCs, which assume a
central role in inducing a cellular immune response. The
suicide strains of the invasive bacteria Shigella flexneri
and Salmonella typhimurium have been attenuated by deleting
genes which are essential for producing metabolites involved
in cell wall synthesis. After infecting mammalian cells,
f
CA 02387146 2002-04-10
-27-
these bacterial strains lyse due to the lack of these
metabolites.
PZasmid DNA
Preference is furthermore given to the gene transfer vectors
being plasmids. Naked plasmid DNA is suitable as a vector for
the nucleic acid sequence combinations according to the
invention. These expression vectors contain, by way of
example, the following essential elements: one or more strong
constitutive and/or inducible promoters, a transcription
terminator, such as that of bovine growth hormone, an
antibiotic resistance or another marker for selecting the
transformed organism, the first, second and/or third and/or
fourth nucleic acid sequence according to the invention, and
an origin of replication which enables the plasmid to be
produced in a suitable host organism. A second generation of
linear DNA plasmids, i.e. what are termed the MIDGE
transfection vectors, are particularly suitable for
efficiently transferring the nucleic acid sequences according
to the invention. MIDGE vectors are composed of the two
strands of a DNA polymer which contains an arbitrary number
of the desired coding sequences and the promoter and
terminator sequences which are required for expressing
foreign genes, with the strands being linked, at both ends,
with loops of single-stranded deoxyribonucleotides such that
a covalently closed molecule is formed. Apart from the
foreign genes which are desired for the medical application,
together with the regulatory units which are required for an
efficient expression, MIDGES do not contain any further
coding sequences as are required, for example, for amplifying
and selecting customary DNA transfer vectors. Thus, these
vectors do not, for example, possess any Ori sequences, which
contain potential integration sites, or any sequences
encoding antibiotics as selection markers, which latter
sequences are under the control of promoters which frequently
cannot be completely shut down in mammalian cells.
The efficiency of this system for transferring genes and for
CA 02387146 2002-04-10
-28-
achieving an improved expression of foreign genes in
mammalian and human cells can be increased, in particular, by
attaching a heterologous class of peptides, i.e. what are
termed nuclear localization signals (NLS, nuclear
localization sequences). For example, use of the NLS derived
from the SV-T antigen can improve the importation into the
nucleus of MIDGE-like constructs and increase the expression
of foreign genes. Furthermore, specific targeting can be
achieved by coupling the MIDGES to tissue-specific ligands.
Furthermore, in analogy with naked DNA, the MIDGES can be
coupled to a large number of nonviral gene transfer systems
in order to ensure more efficient uptake into the target
cell.
Furthermore, it is possible to use gene transfer vectors
which contain components of eukaryotic DNA transposons.
Transposons are naturally occurring genetic elements which
are able to move from one position to the next within a
chromosome. For example, representatives of the Tc1/mariner
family, such as the sleeping beauty transposon, can be used
for preparing suitable vectors for employment in mammalian
cells. An advantage of these vectors is that it is possible
to integrate multiple regulatory sequences into the vectors.
These vectors integrate into the host cell genome and enable
the desired foreign sequences to be expressed continuously
over a long period.
Systems for transferring nucleic acids into eukaryotic cells
In addition, a large number of methods have been described
for transferring genes into mammalian cells on the basis of
nonviral systems. The nonviral vectors are frequently
employed in combination with particle-mediated gene transfer
or with viral vector systems.
In brief, the nucleic acid sequence combination according to
the invention can be integrated into a conventional gene
transfer vector, or a combination of several conventional
gene transfer vectors, which possesses) suitable control
CA 02387146 2002-04-10
-29-
elements for enabling the desired foreign genes) to be
expressed efficiently with high yields. The vectors according
to the invention can then be coupled to synthetic gene
transfer molecules, for example polymeric DNA-binding
rations, such as polylysine, protamine and albumin, or bound
to ligands which mediate specific cell targeting, such as
asialoorosomucoid, insulin, galactose, lactose or
transferrin.
Furthermore, the efficiency of the uptake of naked DNA can be
increased by using biologically degradable latex beads. Due
to the endocytosis which is mediated by the latex beads, DNA-
loaded latex beads are taken up into the target cells with an
increased efficiency. The efficiency of this method can be
increased by an increase in the hydrophobicity, and an
improved disaggregation which accompanies this, of the beads
in the endosome, resulting in the DNA being more efficiently
released in the cytoplasm.
Furthermore, various liposome compositions and also
immunostimulatory reconstituted influenza virosomes (IRIV,
immunopotentiating reconstituted influenza virosomes) are
also suitable for use as vehicles for transferring the
vectors according to the invention into mammalian and human
cells (US Pat. No. 5,879,685). Furthermore, foreign nucleic
acid sequences can be integrated into a vector containing
suitable control sequences, bound to synthetic gene transfer
molecules, such as polymeric DNA-binding rations (e. g.
polylysine, protamine and albumin), and coupled to cell
targeting ligands, such as asialoorosornucoid, insulin,
galactose, lactose or transferrin. Another administration
system is based on the packaging of sequences, which contain
the desired genes under the control of different tissue-
specific and/or constitutive promoters, into liposomes. In
addition, the transfer of the previously described nucleic
acids can be increased by combination with a photopolymerized
hydrogel material. Another customary method for transferring
nucleic acids is their administration using a portable
particle gun, and also the use of ionizing radiation for
CA 02387146 2002-04-10
-30-
activating the gene transfer.
Other methods for optimizing the efficiency of viral vector
transduction comprise varying the multiplicity of infection
(M.O.I), depleting ions, such as phosphate ions, adding
polycationic substances, such as protamine sulfate, varying
the contact time, the temperature and the pH, and
centrifuging cells and virus or vector stocks together with
each other.
The above-described gene transfer systems can be used for
genetically manipulating isolated human or mammalian cells,
in particular antigen-presenting cells (monocytes,
macrophages, dendritic cells and B cells). If retroviral gene
transfer vectors are used, the cells can be converted by
stimulation into the S phase in order to make it possible to
infect these cells . Cells which are in the first quarter to
half of the S phase have been found to be particularly
susceptible to being transfected with retroviruses.
Polypeptides possessing antigenic epitopes
The vectors according to the invention are characterized by
the fact that they comprise nucleic acid sequences which
encode proteins, or parts of proteins or polypeptides, which
are recognized by immune cells (antigens). Within the meaning
of the invention, immune cells are lymphocytes which possess
regulatory or cytolytic properties, such as CD8+/CD4- T cells
or CD8-/DC4+ T cells or CD8-/CD4-/CD56+ killer cells (NK
cells). Within the meaning of this invention, proteins or
polypeptides are proteins derived from human or animal cells.
These proteins or polypeptides are located either on the
surface of the cells which are attacked, e.g. glycoproteins
on cell membranes as a result of their function, or are
located in the interior of the cells, e.g. regulatory
proteins. These proteins or polypeptides are processed in the
cell and presented to the immune cells of the body in the
context of Class 1 or Class 2 MHC molecules. The proteins or
polypeptides within the meaning of this invention are
CA 02387146 2002-04-10
-31-
recognized by the endogenous immune cells and lead to a
stimulation of these cells, i.e. to a multiplication of the
cells, which multiplication can be measured by the release of
messenger substances (IFN-y, IL2, TNF, inter alia) or by the
cytolytic activity of the immune cells.
The vectors according to the invention are characterized by
the fact that they comprise nucleic acid sequences which
encode polypeptides which possess one or more linear or
structural epitopes. These epitopes can be recognized by
immunopathogenic T cells after they have been presented by
way of MHC molecules. These peptide regions and epitopes can
also be encoded and expressed in the context of other,
immunogenic or nonimmunogenic, polypeptides, for example as
fusion proteins or in the form of exchangeable cassettes in
proteins, with the cassettes encoding regions or epitopes or
combinations of epitopes of the proteins. Within the meaning
of this invention, epitopes of the proteins are those regions
of tyke proteins, or those amino acid sequences, which are
recognized by immune cells such as T cells or NK cells. These
epitopes can be recognized both by immune cells derived from
healthy individuals and by immune cells derived from
individuals suffering from autoimmune diseases.
The vectors according to the invention can be used, for
example, for treating autoimmune diseases, chronically
inflammatory processes which are due to immunopathogenesis,
and tissue and organ rejection reactions. For example,
autoreactive T cells, which recognize endogenous proteins and
structures, are known to be involved in many autoimmune
diseases. The vectors according to the invention can comprise
nucleic acid sequences which encode and express these
endogenous proteins and structures. A11 diseases where T
cells are involved in the pathogenesis and where the proteins
and structures attacked by the T cells have been identified
are suitable for treatment.
Rheumatologic diseases are one group of autoimmune diseases .
In rheumatoid arthritis, for example, the joints are attacked
CA 02387146 2002-04-10
-32-
and clinical complications are joint destruction, kidney
damage and amyloidosis. Systemic lupus erythematodes affects
and damages various organs and tissues, such as the central
nervous system and the kidneys. The Sjogren's syndrome
affects exocrine glands such as salivary glands. Polymyositis
and dermatomyositis are autoimmune diseases of the
musculature and the skin and lead to myasthenia and
paralysis. Polymyalgia rheumatica and temporal arteritis are
inflammatory diseases of the blood vessels and cause
myasthenia and loss of sight. Spondylarthropathies, such as
Bechterew's disease, once again affect the joints and lead to
rigidity.
A number of gastrointestinal diseases constitute a further
group of autoimmune diseases. Crohn's disease affects the
entire intestinal tract and leads to bleeding, stenoses and
fistulae, and not infrequently results in the development of
tumor diseases. Ulcerative colitis is an inflammatory disease
of the large intestine and leads to perforation and bleeding.
Celiac disease affects both the small intestine and the large
intestine and results in weight loss. Autoimmune hepatitis is
an inflammatory disease of the liver with liver cirrhosis and
liver transplantation as a consequence.
Endocrinological diseases can likewise be attributed to
autoimmune reactions. Type I diabetes mellitus is an
inflammatory disease of the pancreas and leads to diabetes
and damage to the blood vessels, for example with impairment
of the kidneys, of the peripheral nervous system and the
eyes, and may subsequently require kidney and pancreas
transplants. Adrenal insufficiency and thyroiditis are also
autoimmune diseases involving T cell pathogenesis.
Furthermore, a number of skin diseases are classed as
belonging to the autoimmune disease group. Some examples are
psoriasis, dermatitis herpetiformis and pemphigus vulgaris:
in the case of these diseases, infections can induce
complications. Alopecia leads to hair loss. Finally, there
are also neurologic diseases which have to be attributed to
autoimmune reactions. For example, multiple sclerosis affects
CA 02387146 2002-04-10
-33-
the central and peripheral nervous system while myasthenia
gravis affects the musculature. Paralyses can arise as
complications in association with both these neurologic
autoimmune diseases.
The vectors according to the invention can also be used for
treating chronically inflammatory processes which are due to
immunopathogenesis, for example chronic inflammations
following viral or bacterial infections, such as chronic
hepatitis in the case of hepatitis B virus infections or
hepatitis C virus infections, or encephalitis following
infection with the measles virus.
In addition, the vectors according to the invention can be
used for treating tissue and organ rejection reactions. As a
rule, the immune response against foreign structures on the
surfaces of the cells of the organ to be transplanted, which
structures are essentially formed by the MHC (major
histocompatibility complex) molecules which are present on
all cells, excludes the transplantation of tissues and organs
when the donor and recipient are not compatible with each
other or when the immune response of the donor is not
suppressed. In the sense of organ transplants, compatible
means that the different alleles for Class I and Class II MHC
in the donor and the recipient match to such a substantial
extent that no inflammatory reaction occurs.
Normally, T cells recognize, by means of their T cell
receptors, fragments of nonendogenous proteins associated
with endogenous MHC. T cells which recognize endogenous
fragments of proteins together with endogenous MHC are either
inactivated by a variety of pathways or are not activated. In
the case of an immune response against an organ which is to
be transplanted, the T cells of the recipient recognize the
combination of antigen and foreign MHC even if the same
antigen is not recognized in combination with endogenous MHC.
The vectors according to the invention, which are employed,
in connection with a transplantation, for the purpose of
CA 02387146 2002-04-10
-34-
inducing tolerance toward the organ which is to be
transplanted or which has already been transplanted, encode
and express proteins and structures of the organ types which
are to be transplanted or which have already been
transplanted. The vectors which are employed, for example, in
connection with a pancreas transplantation, encode
characteristic proteins derived from the pancreas. Vectors
which are employed in association with kidney or liver
transplantations encode characteristic proteins derived from
liver or kidney cells. The encoded proteins also include
those proteins which are not organ-specific, such as
endothelial cells, but which are present in the transplanted
organ and constitute a target of the inflammatory reaction in
the case of organ rejection.
In that which follows, the choice of possible antigenic
proteins which can be encoded and expressed by the vectors
according to the invention is described in more detail, by
way of example, for three autoimmune diseases, i.e. multiple
sclerosis, myasthenia gravis, rheumatoid arthritis, and Type
I diabetes mellitus.
Multiple sclerosis
Multiple sclerosis (MS), which is a disease of the human
central nervous system, is characterized by perivascular
inflammations and by demyelination. The collections of
activated T cells in the early MS lesions, and in the
surrounding, still unaffected regions, of the white medullary
body, underline the role of cell-mediated immunity (T cells)
in the development of multiple sclerosis. The investigations
carried out on a generally accepted animal model, i.e.
experimental autoimmune encephalomyelitis (EAE), which can be
induced by immunizing with myelin components, have
demonstrated that EAE can be transferred from one animal to
another by activated, myelin-specific T cells. As a rule,
immune cells directed against different components of the
myelin, components of astrocytes and proteins which are not
derived from cells of the central nervous system can be
CA 02387146 2002-04-10
-35-
detected in a patient suffering from multiple sclerosis. In
addition, different immune cells recognize various regions of
a component (epitopes), with an increase in the number of
recognized epitopes correlating with aggravation of the
disease.
Myelin basic protein (MBP) is a constituent of myelin which
can be separated off and relatively easily purified. About
300 of the myelin in the central nervous system (CNS)
consists of MPB. MBP was the first protein constituent of
myelin which was demonstrated to have inflammation-inducing
properties. By way of example, the vectors according to the
invention encode and express the following epitopes, singly
or in combination: AA 1-20, AA 7-26, AA 16-38, AA 38-55,
AA 50-68, AA 61-82, AA 71-89, AA 83-102, AA 94-117, AA 108-
131, AA 124-141, AA 131-145, AA 139-153, AA 148-162, AA 153-
170, AA 80-102, AA 81-99, AA 82-100, AA 83-99, AA 85-99, AA
86-99 and AA 159-169.
With a content of more than 50$, myelin.proteolipid protein
(PLP) is the largest constituent of myelin in the central
nervous system. PLP is a membrane protein having strongly
hydrophobic properties. It is mainly CD4+, PLP-specific T
cells which have been identified by proliferation experiments
in the blood of individuals suffering from multiple
sclerosis. By way of example, the vectors according to the
invention encode and express the following epitopes,
individually or in combination: AA 40-60, AA 89-106, AA 103
120, AA 125-143, AA 139-154, AA 1-275, AA 95-117, AA 139-151
and AA 185-206.
Myelin oligodendrocyte protein (MOG) is a relatively small
constituent (0.01-0.05$) of myelin. MOG has a molecular
weight of 26-28 kDa and is composed of an immunoglobulin-like
variable domain and two hydrophobic (potential) transmembrane
domains. Investigations carried out on animal models, in
which MOG induces both a T cell-mediated inflammatory
reaction and demyelinating antibodies, and investigations
carried out in association with multiple sclerosis, have
CA 02387146 2002-04-10
-36-
identified the glycoprotein as being an important antigen in
demyelinating autoimmune diseases of the central nervous
system. By way of example, the vectors according to the
invention encode and express the following epitopes of MOG,
individually or in combination: AA 1-22, AA 35-55, AA 36-45,
AA 34-56, AA 43-57, AA 64-96, AA 92-106, AA 134-148, AA 1-26,
AA 14-39, AA 27-50, AA 38-60, AA 50-74, AA 63-87, AA 76-100,
AA 89-113, AA 101-125, AA 162-178, AA 168-182 and AA 14-36.
The myelin-associated basic protein on oligodendrocytes
(myelin-associated oligodendrocytic basic protein, MOBP) is
one of the main constituents of myelin. Patients suffering
from multiple sclerosis which proceeds in phases exhibit a
cellular immune reaction against MOBP. By way of example, the
vectors according to the invention encode and express the
following epitopes, individually or in combination: AA 1-19,
AA 11-29, AA 21-39, AA 31-49, AA 37-60, AA 41-59, AA 51-69,
AA 83-99, AA 1-60 and AA 27-50.
The oligodendrocyte-specific protein (OSP) represents about
7% of total myelin. OSP is a transmembrane protein having a
length of 207 amino acids. A comparison of the tertiary
structure of OSP showed homology with peripheral myelin
protein 22 in the CNS. It has been possible to detect
antibodies against OSP in the spinal fluid of individuals who
have contracted multiple sclerosis which proceeds in phases.
T cells having a specificity for OSP have been demonstrated
to be present in humans. By way of example, the vectors
according to the invention encode and express the following
epitopes, individually or in combination: AA 52-71, AA 72-91,
AA 82-101, AA 102-121, AA 131-151, AA 142-161, AA 182-201 and
AA 192-207.
Myelin-associated glycoprotein (MAG) is a constituent of
myelin in the central and peripheral nervous system. MAG is a
membrane protein having a molecular weight of 100 kDa and is
composed of five extracellular immunoglobulin-like domains, a
single transmembrane domain and a cytoplasmic domain. Two
isoforms (L and S forms), which are formed by alternative
CA 02387146 2002-04-10
-37-
splicing, have been described, with these forms being
detected at different times during myelin formation. MAG is
located in the periaxonal membranes of the myelin-forming
Schwann cells and oligodendrocytes and is thought to be
connected with glia-axon interactions. By way of example, the
vectors according to the invention encode and express the
following epitopes, individually or in combination: AA 20-34,
AA 124-137, AA 354-377 and AA 570-582.
The proteins which will now be described constitute other
targets for pathogenic immune cells. Glycoprotein P0, as a
constituent of the peripheral nervous system and the Schwann
cells. PO has a molecular weight of 30 kDa and constitutes
more than 50~ of the mass of the compact myelin in the
peripheral nervous system. Peripheral myelin protein 22 (PMP-
22/PAS-II) has a molecular weight of 22 kDa. PMP-22 is not
specific for Schwann cells but is also expressed in other
tissues such as the lung, the stomach and the heart.
p170k/SAG (Schwann cell membrane glycoprotein) is a
glycoprotein which has a molecular weight of 170 kDa. SAG is
produced by myelinating and nonmyelinating Schwann cells.
Oligodendrocyte myelin glycoprotein (OMgp) is a glycoprotein
having homology with MPB which is expressed exclusively in
the central nervous system. Schwann cell myelin protein (SMP)
is another glycoprotein which is formed by Schwann cells and
was discovered in chickens. The glycoprotein exhibits 44~
homology with MAG. Other polypeptides which have been
identified as targets for autoimmunoreactive cells are
transaldolase, 5100, alpha B crystallin, 2',3'-cyclic
nucleotide 3'-phosphodiesterase (CNP) and GFAP. By way of
example, the vectors according to the invention furthermore
encode these proteins, or fragments thereof, in particular
regions, or combinations of regions, which carry T cell
epitopes.
Myasthenia gravis
Myasthenia gravis, an autoimmune disease which leads to
progressive muscle weakness, is caused by autoantibodies
CA 02387146 2002-04-10
-38-
directed against the acetylcholine receptor on muscle cells.
Production of the autoantibodies is dependent on specific T
helper cells. 10~ of patients with myasthenia gravis suffer
from epithelial tumors of the thymus. The tumor cells
synthesize mRNAs which encode the a and E subunits of the
acetylcholine receptor and may possibly, in this way,
mistakenly sensitize T cells in the thymus, or circulating T
cells, against epitopes of the acetylcholine receptor.
However, the mechanism of tolerance induction is also
possibly disturbed in the maturing thymocytes. Regions in the
subunits of the acetylcholine receptor which constitute
epitopes for B and T cells have been described on many
occasions. By way of example, the vectors according to the
invention encode and express subunits of the acetylcholine
receptor, or fragments of these subunits, in particular the
following regions, individually or in combination: AA a1-437,
AA a3-181, AA a37-114, AA a37-181, AA a62-90, AA a73-90, AA
a75-90, AA a75-115, AA a130-178, AA a138-167, AA a144-156, AA
a149-158, AA a149-163, AA a146-160 and AA x201-219.
Rheumatoid arthritis
Rheumatoid arthritis was one of the first systemic diseases
to be attributed to autoimmune mechanisms. Essentially two
aspects of rheumatoid arthritis suggest that a fundamental
disturbance of the immune system is the cause of the disease.
The first of these is the frequently very massive
infiltration of lymphocytes, including CD4+ T cells, in the
inflamed hypertrophic synovial tissue, and the second is the
production of large quantities of rheumatoid factor by B
cells and plasma cells in the synovium. Rheumatoid factors
are antibodies which are directed against structure-imparting
regions of the heavy chain of IgG. The specific tissue damage
in the joints and in extraarticular structures is attributed
to the inflammatory panni and to accretions of granular
cells, which are termed rheumatoid nodes. The most important
argument in support of T cells participating in the
development of rheumatoid arthritis is the strong association
of the disease with particular MHC Class II haplotypes and
CA 02387146 2002-04-10
-3 9-
' the observation that, in the mouse model, the disease can be
adaptively transferred by isolated T cells. A very wide
variety of antigens have been described as being possible
targets of the autoreactive immune cells, including
structures in the connective tissue, such as collagen,
proteoglycans and antigens on chondrocytes, heat shock
proteins and exogenous viral or bacterial antigens. By way of
example, the vectors according to the invention encode these
proteins or fragments thereof, in particular regions or
combinations of regions, which carry T cell epitopes, for
example Type II collagen (CII).
Type 1 diabetes mellitus
Type I diabetes mellitus is an autoimmune disease with
multifactorial causes which include genetic predisposition.
The destruction of the insulin-producing (3 cells is caused by
T lymphocytes. Both CD4+ and CD8+ T cells are involved in the
pathogenesis and both subtypes are required equally for the
development of the inflammatory reaction. Experiments with
NOD mice, which constitute an animal model for diabetes, have
identified CD8+ T cells as being the functional effector
cells. It was possible to transmit the disease by
transferring CD8+ T cells from prediabetic NOD mice to SCID
NOD mice, which, while exhibiting the genetic predisposition,
do not possess any immune cells.
A number of possible autoantigens have already been
identified in diabetes. Antibodies directed against various
autoantigens have been detected in prediabetic patients and
in patients in whom diabetes has recently been diagnosed. In
addition, CD4+ and CD8+ T cells have been detected whose
specificity differs to some degree. Within the context of the
invention which is described here, the gene therapy vectors
carry functional regions or genes which encode and express
antigenic proteins, protein fragments, or epitopes and
combinations of epitopes, which constitute targets for CD4+
and/or CD8+ T cells. Within the meaning of this invention,
antigenic proteins are those proteins which are recognized,
in conjunction with MHC Class I or MHC Class II, on syngenic
CA 02387146 2002-04-10
-40-
cells by T cells from individuals suffering from diabetes.
One target of the autoimmune mechanisms in Type I diabetes is
the tyrosine phosphatase IA-2. Autoantibodies against the
protein, which autoantibodies are almost always directed
against the intracellular domain of the protein, can be
detected in patients suffering from diabetes. In individuals
with a genetic predisposition to diabetes, autoantibodies
against IA-2 are a clear marker of a rapid aggravation of the
disease. As an antigenic target for T cells in diabetes, the
vectors can encode and express the entire IA-2 protein, or
protein fragments thereof. By way of example, the vectors
according to the invention encode and express the following
regions, individually or in combination, which regions have
been identified as being epitope-carrying regions in
conjunction with MHC-DR4: AA 654-674, AA 709-732, AA 753-771,
AA 797-817, AA 854-872 and AA 955-975.
Among the possible autoantigens in Type I diabetes, insulin
and its precursor, i.e. proinsulin, are the only proteins
which are exclusively synthesized in the beta cells.
Autoantibodies directed against proinsulin can be detected in
prediabetic and diabetic patients. Diabetes can be produced
in NOD mice by transferring proinsulin-specific T cells, an
observation which demonstrates the importance of proinsulin
as a target of the cellular immune response. As an antigenic
target for T cells in diabetes, the vectors can encode and
express the entire alpha chain, the entire beta chain, the
linking peptide, the entire proinsulin protein or protein
fragments, individually or in combination. By way of example,
the vectors according to the invention encode and express the
following regions, which have been identified as being
epitope-carrying regions: AA 1-15 (p1-15) and AA 35-50 (p35-
50), from the region of the linking peptide between the alpha
and beta chains; AA 6-80 (a6-80) from the region of the alpha
chain; AA 9-23 and AA 10-25 (b10-25), from the region of the
beta chain.
Glutamic acid decarboxylase 65 (GAD65) is one of the target
CA 02387146 2002-04-10
-41-
structures for autoimmune reactions in patients suffering
from Type I diabetes. Mononuclear cells in the blood of
patients react with cell division to the protein, and,
furthermore, more than 70~ of patients possess antibodies
directed against GAD65. A combination of antibodies directed
against GAD65 and IA-2 in individuals who, because of their
MHC type, exhibit a genetic predisposition for diabetes is a
strong indication that a Type I diabetes is developing. As an
antigenic target for T cells in diabetes, the vectors can
encode and express the entire GAD65 protein or protein
fragments thereof. By way of example, the vectors according
to the invention encode and express the following regions,
individually or in combination, which regions have been
identified as being epitope-carrying regions in conjunction
with MHC-DQ8: AA 1-60, AA 51-120, AA 101-115, AA 111-180, AA
171-240, AA 206-220, AA 231-300, AA 291-360, AA 351-420,
AA 411-480, AA 431-445, AA 461-475, AA 471-530, AA 521-585,
AA 536-550, AA 121-140, AA 201-220, AA 231-250 and AA 471-
490. GAD65 is also a target of CD8+ T cells. Vectors can
therefore also encode and express the region AA 15-23. Other
possible regions are AA 505-519 and AA 521-535.
The heat shock protein Hsp60 constitutes another target for
activated immune cells in Type I diabetes. The vectors encode
and express, for example, the AA 437-460 region (peptide
277?, which has been identified as being a T cell epitope in
NOD mice. A number of epitopes or regions in the Hsp60
protein which contain T cell epitopes have been identified in
individuals suffering from diabetes and in healthy control
individuals. By way of example, the vectors according to the
invention encode the entire Hsp60 protein or fragments
thereof, in particular the following regions, or combinations
of regions, which carry T cell epitopes: AA 1-20, AA 16-35.,
AA 31-50, AA 46-65, AA 61-80, AA 106-125, AA 121-140, AA 136-
155, AA 151-170, AA 166-185, AA 195-214, AA 240-259,
AA 255-275, AA 271-290, AA 286-305, AA 301-320, AA 346-365,
AA 421-440, AA 436-455, AA 466-485 and AA 511-530.
Other targets of the autoimmune reaction in Type I diabetes
are constituted by proteins, which have not yet been
CA 02387146 2002-04-10
-42-
' characterized, in the cell membrane of the beta cells. The
islet cell protein ICA69 and a protein with a molecular
weight of 38 kDa in the secretory granules (Roep et a1.
(1991) .Lancet 337: 1439-1441) have been identified as being
further antigens. By way of example, the vectors according to
the invention encode these proteins, or fragments thereof, in
particular regions, or combinations of regions, which carry T
cell epitopes.
Expression of apoptosis-inducing ligands
The vectors according to the invention are characterized by
the fact that they comprise nucleic acid sequences which
encode one or more apoptosis-inducing ligands. Apoptosis is a
process which is controlled at the level of the genes and
which is involved in the regulation of homeostasis, the
development of tissues and organs and the removal of cells of
the immune system which are no longer required. Apoptosis is
also involved in the elimination of cells which have
undergone changes due to damage to their chromosomes or due
to an infection with viral pathogens. Normal cells are
protected from apoptosis by what are termed "survival
signals". However, proapoptotic signals, which are elicited
by damage or infection, initiate a sequence of events which
end with the death of the cells . Cell death by apoptosis is
characterized by a thickening of the chromatin, a
fragmentation of the chromosomal DNA, a type of blistering of
the membrane, a shrinking of the cell and, finally,
decomposition of the dead cell in a membrane-enclosed vesicle
(apoptotic bodies).
Essentially two signal pathways leading to the elicitation of
apoptosis exist in the cell. The two pathways react to
different inducers. The pathway which is relevant to the
present invention leads, by way of the stimulation of
apoptosis receptors on the surface of the cells, such as
CD95/Fas/Apol, TRAIL or Apo3, and mediation by adapter
molecules, such as FADD and TRADD, to the activation of
caspase 8 (FLICE, initiator ca spa se) , which has a regulatory
CA 02387146 2002-04-10
-43-
action, and subsequent caspases, such as caspase 3 (effector
caspase), which induce the apoptosis. By contrast, other
signals, such as DNA damage, the lack of growth factors,
defective cell adherence or activated oncogenes, lead to the
induction of a signal cascade in which factors from
mitochondria, such as cytochrome C and APAF1, and factors of
the Bcl family, are involved. This cascade leads to the
activation of caspase 9 (initiator caspase) and,
subsequently, to the activation of caspase 3.
The apoptosis receptors belong to a subfamily of what are
termed "death receptors", which form a part of the tumor
necrosis factor (TNF) receptor superfamily. The members of
this family are characterized by from two to four copies of
cysteine-rich extracellular domains. The apoptosis receptors
in turn possess intracellular "death domains" (DD) which are
indispensable for the further transmission of the apoptosis
signal. By means of adapter proteins, the apoptosis signal is
communicated to the caspases, which finally initiate
apoptosis by way of a number of steps.
The apoptosis-inducing ligands are membrane-located or
soluble proteins, for example CD95L/FasL/ApolL, Apo2L/TRAIL
or Apo3L, which interact with specific receptor molecules,
such as CD95/Fas/Apol, TRAIL or Apo3, on other cells, or on
the same cell, and thereby induce apoptosis in the receptor-
carrying cells. The apoptosis-inducing ligands belong, for
example, to the tumor necrosis factor (TNF) superfamily.
Members of the TNF family are characterized by structural and
biochemical properties. The members are Type II transmembrane
proteins, with the exception of Lyrnphotoxin (LT) a, and (3,
which can also be converted by proteolytic cleavage into
soluble ligands. The ligands occur as trimers having three
identical subunits which, by interaction with their specific
apoptosis receptors, cause these receptors to become
trimerized.
Caspases are a group of cysteine proteases . 14 caspases have
so far been identified in mammals (including humans).
CA 02387146 2002-04-10
-44-
Caspases recognize motifs consisting of 4 amino acids, which
they cut on the carboxyl side of the amino acid aspartate.
Caspases are synthesized as zymogens, i.e. precursor proteins
with a very low activity, with these zymogens being activated
by proteolytic cleavage: The activated enzymes are hetero-
tetramers, comprising in each case two identical cleaved
subunits. A number of caspases are activated by
autoproteolytic cleavage while others are activated by
further caspases. What are termed the initiator caspases
(caspase 8 and caspase 9) start the avalanche of self-
augmenting caspase activity by activating what are termed
effector caspases (caspase 3) by means of proteolytic
cleavage.
Adapter proteins constitute a link between the effectors
(caspases) and the regulators (Bcl-2 family receptors) of
apoptosis. The adapters interact physically, by means of
homotypic interactions, with the three groups of factors by
way of what are termed death domains (DD), death effector
domains (DED) and caspase recruitment domains (CARD).
The vectors according to the invention comprise nucleic acid
sequences which encode apoptosis-inducing ligands. For
example, the vectors encode the ligand CD95L, which binds to
the receptor molecule CD95/Fas/Apo-1. CD95 is a glycosylated
membrane protein (Type I) having a molecular weight of about
45-52 kDa (335 amino acids). Several soluble forms of the
protein also arise as a result of differential splicing of
the messenger RNA. Expression of the CD95 molecule is
stimulated by interferon-y (IFN-y), by TNF and by the
activation of T cells. Under natural conditions, CD95-
mediated apoptosis is induced by CD95 binding with the
natural ligand CD95L (Fast, Apo-1-L). CD95L is a TNF-related
membrane protein (Type II) which likewise occurs in soluble
form, as a result of proteolytic cleavage, and can bind to
CD95. The interaction of CD95L with CD95 leads to a Ca2+-
independent (i.e. different from the induction due to
perforin/granzyme) apoptosis of the CD95-carrying cells.
CA 02387146 2002-04-10
-45-
In addition, the vectors according to the invention can
comprise nucleic acid sequences which encode the protein
TRAIL (APO-2L), which binds to the specific receptor
molecules TRAIL-R1 (DR4), TRAIL-R2 (KILLER, DR5, TRICK2),
TRAIL-R3 (LIT, DcR1) and TRAIL-R4 (TRUNDD, DcR2). TRAIL has
been demonstrated to be naturally present on a number of
cells, such as Type II interferon-stimulated monocytes,
cytomegalovirus-infected fibroblasts, and Type I interferon-
stimulated and antigen-stimulated T cells or NK cells. TRAIL
induces apoptosis in a number of transformed tumor cell lines
and activated T cells. It has been possible to detect the
mRNA which encodes the TRAIL-R2 receptor in a very wide
variety of tissues, such as spleen, thymus and lymphocytes in
the peripheral blood. Cells and tissues which express mRNA
for TRAIL-R2 are susceptible to TRAIL-mediated apoptosis.
In addition, the vectors according to the invention can
comprise nucleic acid sequences which encode the APO-3 ligand
(Apo3L/TWEAK). Apo3L is a Type II transmembrane protein
having a length of 149 amino acids. The extracellular region
of ApoL3 exhibits a high degree of homology with TNF. Apo3L
mRNA has been detected in a very wide variety of lymphoid and
nonlymphoid tissues. ApoL3 binds to the receptor molecule
Apo3 (DR3, WSL-1, TRAMP, LARD) and induces apoptosis in the
receptor-carrying cells. The receptor for Apo3L is
principally expressed on the cell surface of lymphocytes for
example on unstimulated resting lymphocytes in the peripheral
blood (PBL), phytohemagglutinin (PHA)-treated PBL, CD4+ T
cells, CD8+ T cells and B cells. The induction of apoptosis
by way of Apo3L is mediated by FADD/MORT1. ApoL3-mediated
apoptosis is blocked by the viral caspase inhibitors CrmA,
obtained from cowpox virus, and by the baculovirus p35
protein.
Inhibition of apoptosis in the recombinantly modified cells
The vectors according to the invention are characterized by
the fact that they comprise, where appropriate, nucleic acid
sequences which encode one or more antiapoptosis molecules.
CA 02387146 2002-04-10
-46-
An interaction of CD95, TRAIL, TRAMP, TNF or lymphotoxin with
their specific receptors can take place not only between
different cells but also on one and the same cell and thereby
lead, in an autocrine manner, to the induction of apoptosis.
An example of this is the apoptosis of activated immune
cells, i.e. what is termed activation-induced cell death,
which leads to the removal of immune cells which are no
longer required. T cells which have been stimulated, by way
of their T cell receptor, to multiply express CD95 on their
cell surface, with this CD95 interacting with CD95L on the
membrane.
In order to prevent such an autocrine induction, or a
paracrine induction, by other cells, for example in cell
culture when the starting cells are being modified, in the
cells which have been modified with the vectors according to
the invention, the vectors can comprise nucleic acid
sequences which encode intracellular inhibitors of apoptosis
(antiapoptosis molecules). These inhibitors interact either
with the different receptor molecules in the cell membrane,
with the adapter molecules which transmit the death signal
between the receptors and the caspases, or with the caspases
themselves. These inhibitors are derived either from the
cells themselves, and are involved in the regulation of
apoptosis, or they are viral proteins which prevent apoptosis
occurring in virus-infected cells until sufficient viral
progeny have been produced and released.
The induction of apoptosis can be inhibited at the various
stages of the cascade comprising receptors, adapters and
caspases. In particular viruses have invented a large number
of strategies for protecting themselves against apoptosis as
an immune mechanism. The present invention uses these viral
and cellular mechanisms in order to protect the cells, which
have been altered by the vectors, from autocrine induction of
apoptosis.
The vectors according to the invention can, for example,
comprise nucleic acid sequences which encode adenoviral
CA 02387146 2002-04-10
-47-
proteins from the E3 region of the virus. These proteins
inhibit membrane-bound biochemical processes which are
induced when the TNFR is activated. The protein E3-14.7K
inhibits the release of arachidonic acid by phospholipase A2
(PLAZ) as a consequence of stimulation by way of the TNF
receptor. The adenoviral proteins E3-14.5K and E3-10.4K form
the complex RID, which prevents translocation of PLAZ from
the cytoplasm to the cell membrane following stimulation of
the TNFR. In addition, RID causes a rapid internalization,
and lysosomal degradation, of membrane-bound CD95.
In addition, the vectors according to the invention can
comprise nucleic acid sequences which encode proteins which
exhibit a high degree of homology with the DED domains. These
proteins are termed FLIPS, while the viral proteins are
termed vFLIPs. The signal cascade between receptors and FADD,
on the one hand, and caspase 8 (FLICE), on the other hand, is
blocked, and apoptosis thereby prevented, by means of a
homotypic interaction of the FLIPs/vFLIPs with the adapter
protein FADD by way of the DED domains.
In addition, the vectors according to the invention can
comprise nucleic acid sequences which encode the proteins
which will now be described. The proteins MC159 (Bertin et
aI. (1997) Proc Nat1 Acad Sci U S A 94: 1172-1176) and MC160
from the Molluscum contagiosum virus bind to FADD and thereby
prevent caspase 8 and FADD from being recruited. The proteins
BORFE2 (E1.1) from herpesvirus BHV-4 and E8 from the equine
herpesvirus EHV-2 (Bertin et a1. (1997) Proc Nat1 Acad Sci U
S A 94: 1172-1176) bind inactive procaspase 8 and in this way
prevent an interaction with FADD and consequently activation.
The proteins K13 from HHV-8 and ORF71 from herpesvirus
saimiri also possess DE.D domains and function in the same
way. Furthermore, viral proteins can be encoded which bind,
and thereby inactivate, signal factors (FADD, TRADD, TRAF) in
the apoptosis cascade. The adenovirus protein E1B-19K, which
exhibits homology with the cellular protein Bcl-2, interacts
with FADD and thereby blocks signal transfer from TNFR and
CD95. In addition, E1B-19K is furthermore able to replace
CA 02387146 2002-04-10
-48-
Bcl-2 functionally and to prevent activation by way of the
mitochondria (via caspase 9). The Epstein Barr virus LMP-1
protein interacts with various TRAF molecules (TNFR-
associated factors) and thereby blocks signal transfer from
TNFR. In addition, LMP-1 also binds TRADD. However, the
apoptosis signal cascade is not induced, presumably due to
the binding site for TRADD being modified. LMP-1 additionally
induces expression of the antiapoptotic proteins A20, Bcl-2
and Mcl-1. The vectors according to the invention furthermore
express, for example, the SV40 LT protein, which mediates
resistance to Fas-induced apoptosis by way of a protein
kinase C-mediated pathway. For example, the vectors according
to the invention encode the polyoma proteins ST and MT, both
of which mediate resistance to CD95-mediated and/or TNFR-
mediated apoptosis. The ST protein achieves this by binding
and inhibiting the protein PP2A. The MT protein directly
activates signal pathways which promote survival of the cell.
These pathways include the PI3 kinase, which subsequently
phosphorylates, and thereby inactivates, the protein Bad,
which has a proapoptotic action.
The vectors according to the invention can likewise encode
caspase inhibitors in order to prevent autocrine induction of
apoptosis in the modified cells. The p35 protein from the
baculoviruses Autographica californica nuclear polyhedrosis
virus (AcNPV) and Bombyx mori NHV (BmNPV) is synthesized in
the early phase of virus replication and prevents apoptosis
by a number of very different stimuli. p35 blocks the
induction of apoptosis by ligands of TNF and CD95. p35 is
cleaved by a number of caspases (caspases 1, 3, 6, 7, 8 and
10). However, the cleavage products are not released but
remain bound and form, together with the caspases, a stable
inhibitory complex. p35 can, for example, be encoded by the
vectors according to the invention. The vectors according to
the invention can also encode viral proteins which exhibit
homology with cellular serpins. Serpins are chymotrypsin-like
serine proteases and inhibit a number of different caspases.
CrmA from the cowpox virus inhibits CD95L-induced and TNFR-
induced apoptosis by blocking caspases 1 and 8. The SPI-1 and
CA 02387146 2002-04-10
-4 9-
SPI-2 proteins from the harepox virus, and the protein B13R
from the vaccinia virus, exhibit a high degree of homology
with CrmA and likewise inhibit apoptosis due to CD95L and
TNF. The Serp-1 and Serp-2 proteins of the myxoma virus and
the SPI-4 protein of the harepox virus possess corresponding
antiapoptotic properties. Furthermore, it is possible to
encode proteins which are homologous with cellular cIAPs,
such as vIAPs, or cellular proteins, such as FLAME-1 or
I-FLICE.
Cydia pompnella granulosis virus (CpGP), Orgyiapse
audotsugata polyhedro$is virus (OpMNPV) and AcNPV encode
viral IAPs (vIAPs) which act in the signal cascade above p35.
While vIAPs bind and inhibit inactive procaspases and caspase
8, they are unable to inhibit already activated caspases, as
p35 can. While cIAPs interact with TRAF molecules, they can
also inhibit apoptosis due to non-TNFR-associated processes.
A conserved RING Finger motif and at least one so-called BIR
motif (baculovirus IAP repeat) are required for antiapoptotic
activity. FLAME-1 is a cellular protein which inhibits
apoptosis due to CD95/TNF receptor (Srinivasula et a.I. (1997)
J Bio1 Chem 272: 18542-18545). FLAME-1 exhibits a high degree
of homology to caspase 10 and caspase 8 (FLICE). Two adjacent
regions are located in the amino terminal region and exhibit
homology with the DED domains of FADD which make homotypic
interactions with other DED proteins possible. A third
adjacent region exhibits homology with the functional caspase
domain of caspases 8 and 10. While FLAME-1 interacts directly
with FADD, caspase 8 and caspase 9, it does not possess any
caspase activity. FLAME-1 therefore acts as a dominant
negative repressor of apoptosis by means of CD95/TNF
receptor. The inhibitory effect ensues as a result of the
functionally inactive FLAME-1 protein blocking the receptor
complex composed of CD95/TNR receptor/FADD. I-FLICE is
another cellular inhibitor (Hu et al. (1997) J Bio1 Chem 272:
17255-17257) which can be encoded and expressed by the
vectors according to the invention in order to prevent
apoptosis in the recombinantly modified cells. I-FLICE
exhibits structural homologies with FLICE/caspase 8 and
CA 02387146 2002-04-10
-50-
caspase 10. In the amino terminal region, there are two
adjacent domains having homology with FADD DED domains. In
the carboxy terminal region, a domain exists which exhibits
homology with the caspase domain. However, I-FLICE does not
exhibit any caspase activity and acts as a dominant negative
inhibitor of apoptosis by means of CD95/TNF receptors. In
contrast to FLAME-1, I-FLICE only binds to FLICE/caspase 8
and caspase 10 and not to FADD. I-FLAME is therefore not
recruited by the binding of CD95L/TNF to the receptor complex
consisting of CD95/TNR receptor/FADD. The inhibitory effect
is brought about by complexing, and thereby inactivating,
caspases 8 and 10.
An inhibition of apoptosis in the cells modified by the
vectors according to the invention can also be produced by
inhibiting, e.g. by means of an antisense approach,
expression of the proteins (apoptosis receptors, adapters and
caspases) which are involved in the induction.
The antisense RNAs which are encoded and synthesized by the
vectors according to the invention may, on the one hand, be
directed exclusively against a protein in the apoptosis
signal chain and inhibit apoptosis by way of a particular
pathway, for example by way of membrane-associated receptors
,or by way of a mitochondria-mediated pathway. On the other
hand, the antisense RNA may include various regions which are
specific for different targets in the signal cascade for
inducing apoptosis. These different regions in the antisense
RNA are specific for receptor proteins, adapter proteins
and/or caspases. The vectors according to the invention
either synthesize a single antisense RNA or a combination of
different antisense RNAs which are specifically directed, for
example, against individual apoptosis receptors, caspases or
adapter molecules. Alternatively, the vectors express a
single antisense RNA which contains several regions which are
in each case specific for individual apoptosis receptors,
caspases or adapter molecules and, in combination, prevent
the expression of several proteins which are involved in
apoptosis.
s
CA 02387146 2002-04-10
-51-
In connection with this invention, the vectors carry, for
example, functional regions which synthesize the antisense
RNA for apoptosis receptors in eukaryotic cells. Blocking the
expression of receptor molecules which start signal cascades
which have a proapoptotic action blocks autocrine stimulation
of apoptosis in the gene therapy vector-modified cell at the
earliest possible stage.
The vectors according to the invention can, for example,
encode CD95-specific antisense RNAs and block expression of
CD95/Fas. Blocking CD95 prevents induction of apoptosis by
way of CD95L/FasL. For example, the gene therapy vectors
encode and express TNFR-specific antisense RNAs which block
expression of TNFR and protect the cell from TNF-mediated
apoptosis. Alternatively, the vectors encode and express, for
example, TRAIL-R1-specific and/or TRAIL-R2-specific antisense
RNAs which prevent the expression of the TRAIL-R1 and/or
TRAIL-R2 receptors, respectively. This makes the cells
resistant to TRAIL-mediated apoptosis. Alternatively, the
gene therapy vectors encode and express TRAMP receptor-
specific antisense RNAs, which prevent expression of TRAMP
receptors in the altered cells. This makes the cells
resistance to TRAMP-mediated apoptosis.
The vectors according to the invention can furthermore
comprise nucleic acid sequences which encode, for example,
antisense RNA for adapter proteins in eukaryotic cells. Since
these adapter molecules function one level below the
apoptosis receptors and one adapter molecule is used by
several apoptosis receptors, more than only one apoptosis
signal pathway can be blocked by blocking the expression of a
single adapter protein. Resistance to different apoptosis-
inducing ligands can be achieved by blocking a single adapter
molecule. The vectors according to the invention synthesize
either a single antisense RNA or a combination of different
antisense RNAs which are specifically directed against
individual adapter proteins. Alternatively, the vectors
express an antisense RNA which contains individual regions
CA 02387146 2002-04-10
-52-
which are in each case specific for an adapter protein and,
in combination, prevent the expression of several adapter
proteins.
In connection with this invention, the gene therapy vectors
can, for example, encode FADD-specific antisense RNAs which
prevent expression of FADD. Inhibiting FADD expression blocks
signal transfer by the apoptosis receptors CD95, TNFR, TRAIL-
R1, TRAIL-R2 and TRAMP. The cells are thereby resistant to an
induction of apoptosis by way of CD95L, TNF, TRAIL and CD3.
Furthermore, the vectors can, for example, encode TRADD-
specific antisense RNAs which prevent expression of TRADD.
TRADD is specifically involved in the induction of apoptosis
by means of TNF/TNFR. Inhibiting the expression of TRADD
thereby specifically blocks the induction of apoptosis by
means of TNF/TNFR. In addition, the vectors can encode APAFl-
specific antisense RNAs. APAF1 is an adapter protein which
plays a central role in the induction of apoptosis by way of
the mitochondria-associated pathway. APAF1 is released from
the mitochondria, together with cytochrome C, and associates,
in the cytoplasm of the cell, with dATP to form a trimeric
complex which activates caspase 9. Inhibition of the
synthesis of APAF1 leads to a blockade of apoptosis by way of
the mitochondria-associated pathway.
In connection with this invention, the gene therapy vectors
can furthermore encode antisense RNA against caspases, e.g.
caspase 1, 3, 8 or 9, in eukaryotic cells. The vectors
according to the invention synthesize either a single
antisense RNA or a combination consisting of different
antisense RNAs which are specific directed against individual
caspases. Alternatively, the vectors express an antisense RNA
which contains individual regions which are in each case
specific for a caspase and, in combination, inhibit the
expression of several caspases.
Suicide enzymes
The vectors according to the invention are characterized by
a,
CA 02387146 2002-04-10
-53-
the fact that they comprise, where appropriate, nucleic acid
sequences which encode suicide enzymes by which the
recombinantly modified cells can, at any time, for example in
vivo, be eliminated. For example, the suicide genes encode
and express enzymes which convert biological substrates
(prodrugs), which are supplied to the body from the exterior,
into toxic substances and/or modify the substances in such a
way that these substances are used as substrates by the
enzymes of the cell. Alternatively, suicide enzymes encode
substances which are themselves toxic, but the expression of
these genes is strictly controlled in the recombinantly
modified cell. In connection with this invention, the genes
encoding substances which are themselves toxic are strongly
suppressed in the cell and are not synthesized. By adding
biological or chemical substances, the genes which encode the
toxic proteins are activated and the cells will then die. The
vectors which are described in connection with this invention
preferably encode suicide genes which convert prodrugs, which
are not toxic or are only slightly toxic, into toxic
substances.
The prodrugs which are used must exhibit a markedly lower
toxicity than the activated substances and must constitute
good substrates for the activating enzymes. In addition,
these substances must be sufficiently chemically stable under
physiological conditions and possess good pharmacological and
pharmacokinetic properties. Depending on the type, some
prodrugs are taken up into the cells and converted
intracellularly into the toxic substance. Other prodrugs are
activated extracellularly. Accordingly, the prodrugs or the
activated toxic substances must be readily taken up by the
cells.
For example, the vectors according to the invention encode
and express herpes simplex virus (HSV) thymidine kinase (TK).
HSV TK phosphorylates acyclovir to acyclovir diphosphate,
which is further phosphorylated by cellular kinases. Because
of its substrate specificity, the eukaryotic cell thymidine
kinase cannot phosphorylate acyclovir, for which reason
CA 02387146 2002-04-10
-54-
uninfected cells or HSV TK-negative cells are resistant to
guanosine analogs. The HSV-infected and/or thymidine kinase-
positive cells, which have, for example, been modified with
the therapy vectors of this invention, can be selectively
eliminated by systemically administering acyclovir or
gancyclovir.
Furthermore, the vectors according to the invention can
encode varicella zoster virus (VZV) thymidine kinase. The
action of VZV TK is comparable with that of HSV TK, with the
difference that VZV TK uses 6-methoxypurine
arabinonucleoside.
In addition, the vectors which are mentioned in this
invention can encode enzymes which activate the following
prodrug/enzyme systems: carboxylesterase (CA) activates
irinotecan; cytosine deaminase (CD) activates 5-
fluorocytosine (5-FC); carboxypeptidase G2 (CPG2) activates
2-chloroethyl-2-mesyloxyethylaminobenzoyl-L-glutamic acid
(CMDA) and CJS278H and also the self-activating prodrugs
doxorubicin and daunorubicin; cytochrome P450 (Cyt 450)
activates cyclophosphamide (CP), ifosfamide (IF), ipomeanol
and 2-aminoanthracene (2-AA); deoxycytidine kinase (dCK)
activates cytosine arabinose (ara-C); nitroreductase (NR)
activates CB1954 (5-aziridinyl-2,4-dinitrobenzamide); purine
nucleoside phosphorylase (PNP) activates 6-methylpurine-2'-
deoxyribonucleoside (6-MePdR); thymidine phosphorylase (TP)
activates 5'-deoxy-5-fluorouridine (5'-DFUR); xanthine
guanine phosphoribosyl transferase (XGPRT)activates
6-thioxanthine (6-TX) and 6-thioguanine (6-TG). In addition,
the vectors according to the invention can encode bacterial
uracil phosphoribosyl transferase, which activates 5-
fluorouracil, or encode a fusion protein consisting of
cytosine deaminase (FCY1) and Saccharomyces cerevisiae uracil
phosphoribosyl transferase (FUR1), which activates
fluorocytosine (5-FC).
CA 02387146 2002-04-10
-55-
Vectors according to the invention for gene therapy
The invention relates to a gene transfer vector which
comprises at least one nucleic acid molecule which comprises
a first nucleic acid sequence, encoding one or more
apoptosis-inducing ligand(s), a second nucleic acid sequence,
encoding one or more antigen(s), and, where appropriate, a
third nucleic acid sequence, encoding one or more
antiapoptosis molecule(s), and, where appropriate, a fourth
nucleic acid sequence, encoding one or more suicide
enzyme ( s ) .
Particular preference is given to a gene transfer vector in
which the first and second nucleic acid sequences are linked
to each other functionally such that expression of the second
nucleic acid sequence is dependent on expression of the first
nucleic acid sequence, i.e. expression of the antigens is
physically coupled to expression of the apoptosis-inducing
ligands and is dependent on the latter. Particular preference
is furthermore given to a gene transfer vector in which the
third and fourth nucleic acid sequences are functionally
linked to each other such that expression of the third
nucleic acid sequence is dependent on expression of the
fourth nucleic acid sequence, i.e. expression of the
antiapoptosis molecules is always coupled to expression of
the suicide enzymes and is dependent on this expression.
For treating diseases such as autoimmune diseases, or
diseases which are due to an immunopathogenesis or diseases
which are based on the rejection of transplanted tissues or
organs, antigen-presenting cells (APCs) can be treated with
one of the vectors according to the invention. APCs can be
treated, for example, with a gene transfer vector which
comprises nucleic acid sequences which encode antigens,
apoptosis-inducing ligands, antiapoptosis molecules and
suicide enzymes. Furthermore, the APCs can be treated with
any type of combination of vectors, for example with several
of the vectors according to the invention, which vectors
encode different antigens. The APCs can, for example, also be
CA 02387146 2002-04-10
-56-
treated with a combination of vectors according to the
invention which encode antigens and vectors according to the
invention which encode antiapoptosis molecules. Combinations
of vectors are of value when, for example, the individual
nucleic acid regions in the vectors are so large that they,
for example, exert a negative influence on the preparation or
use of the vector or when APCs are to be treated with vectors
which encode different antigens, apoptosis ligands,
antiapoptosis molecules or suicide enzymes.
Control elements for expressing gene information
The gene transfer vectors according to the invention comprise
nucleic acids which, apart from the first to fourth above-
described nucleic acid sequences, can contain additional
sequences and functional regions, e.g. for controlling and
regulating the expression of genes in mammalian cells, for
example.
These sequences and functional regions can be promoters
and/or promoter elements, preferably viral promoter sequences
for expressing gene sequences in mammalian cells. Some
examples of viral promoter sequences are the early SV40
promoters, the mouse mammary tumor virus (MMTV) LTR promoter,
the Type I human immunodeficiency virus (HIV-1) LTR promoter,
the adenovirus major late promoter (Ad MLP) and the herpes
simplex virus (HSV) promoter. Furthermore, promoters of
nonviral genes, for example promoters of murine 3-
phosphoglycerate kinase, of human ubiquitin C and of the
murine metallotheionein gene, are also suitable for
efficiently expressing gene sequences in mammals. In this
connection, expression can be effected using a constitutive
promoter or a regulatable (inducible) promoter. Thus, a
glucocorticoid-inducible promoter can, by way of example, be
used in certain cell types, such as hormone-stimulatable
cells.
The expression rates can normally be increased by combining
the abovementioned promoter elements with what are termed
enhancer elements. In this connection, viral enhancer
CA 02387146 2002-04-10
-57-
elements are frequently particularly efficient since they
normally have a broader host spectrum than do enhancers
derived from mammalian cells. Very efficient representatives
of viral enhancers include the SV40 early gene enhancer and
the promoter/enhancer combinations from the Rous sarcoma
virus LTR and human cytomegalovirus. Furthermore, it is also
possible to use regulatable enhancer elements which are only
active, for example, in the presence of inducers, such as
hormones or metal ions.
These sequences and functional regions can furthermore be
leader sequences and/or processing sequences, e.g. a protease
cleavage site, preferably the adenoviral three-part leader
sequence and a large number of leader sequences of mammalian
proteins such as the leader of the erythropoietin gene and
the tPA leader, in order to mediate efficient secretion of
foreign proteins from mammalian cells.
In addition, these sequences and functional regions can be
transcription termination sequences and polyadenylation
sequences. Some very efficient poly A signals for use in
mammalian expression vectors are derived, for example, from
bovine growth hormone, from mouse (3-globin, from the early
SV40 transcription unit and from the herpes simplex thymidine
kinase gene. Prokaryotic transcription terminators have been
described in detail and incorporating them has a large number
of positive effects on gene expression. In eukaryotes, a
consensus sequence having the nucleotide sequence ATC AAA
(A7T) TAG GAA GA has been identified in the termination
region of 9 genes.
In addition, these sequences and functional regions can be
translation control elements. Thus, an optimal Kozak sequence
(CC(A/G)CcaugG) promotes the initiation of the translation of
eukaryotic mRNAs. In this connection, particular significance
for optimal translation initiation must be attached to the
purines A or G in position -3 and the G directly upstream of
the start codon.
CA 02387146 2002-04-10
-58-
In addition, the efficiency with which cDNA gene sequences,
which do not contain any introns, are expressed can in some
cases be significantly increased 10-20 fold by fusing an
intron in the 5' region of the ORF. In this connection, a
synthetic intron SIS, which was been produced by fusing an
adenovirus splice donor to an immunoglobulin gene splice
acceptor, or an SV40 19S late mRNA intron, by way of example
for a large number of different introns, possesses particular
efficacy in a variety of cells.
In addition, translation initiation at the correct start
codon can be severely impaired by the presence of additional
AUG codons in the 5'-untranslated region. Such an inhibition
can be minimized by the presence of a translation termination
codon which is in frame with the upstream AUG. Furthermore,
translation is frequently impaired by the tendency of defined
sequences in the 5'-untranslated region (UTR) to form
secondary structures. Furthermore, destabilizing motifs
within foreign gene sequences can have a negative effect on
expression rates. Representative sequences of this nature
are, by way of example, AU-rich sequences in the 3' UTR
region of many unstable mammalian mRNAs. UUAUUUAUU and
UUAUUUA(U/A)(U(/A) are very efficient destabilizing sequence
motifs. These sequence motifs should be removed or
inactivated in order to increase expression of the desired
foreign genes.
In addition, the efficiency with which the desired nucleic
acid sequence is expressed can be increased by selecting and
using suitable, host-specific codons (codon usage).
An expression vector normally contains a combination of a
promoter, a polyadenylation signal and a transcription
termination sequence. Furthermore, enhancers, introns having
functional splice donor and acceptor sites, and also leader
sequences, can, if required, be incorporated in a modular
manner into the constructs. Expression constructs are
frequently contained in a replicon, for example in extra-
chromosomal elements (e.g. plasmids) which are able to
CA 02387146 2002-04-10
-59-
survive stably in a host, such as a mammalian cell. Mammalian
replication systems contain those which are derived from
animal viruses and require transactive factors for
replication: For example, plasmids which contain the
replication systems of papova viruses, such as SV40 or
polyoma viruses, replicate in extremely high copy number in
the presence of the appurtenant viral T antigen. Other
examples of mammalian replicons include those which are
derived from the bovine papilloma virus and from the Epstein
Barr virus. Furthermore, a replicon can contain two
replication systems which ensure survival in the host, e.g. a
replication system for gene expression in mammals and a
system for amplifying the vector in bacteria. The plasmid
pMT2 is an example of such a mammalian/bacterial shuttle
vector.
Regulating the expression of the apoptosis-inducing ligands
The nucleic acid sequences which encode apoptosis-inducing
ligands can be regulated such that the expression of the
ligands can be switched on and switched off. Expression of
the apoptosis-inducing ligands can be completely switched off
in transduced cells. This is of particular importance for
preparing stable, vector-producing cell lines or for
generating the bait cells. Since most cells carry apoptosis
receptors constitutively on their surfaces, including also
the packaging cell lines which can be used for preparing
viral gene transfer vectors and the antigen-presenting cells
which are to be transduced, transduction of these cells with
the gene for an apoptosis-inducing ligand would lead to
paracrine and autocrine induction of apoptosis. Switching off
the expression of the ligand in the packaging lines and/or in
the transduced cells in culture prevents nonspecific
interaction between these cells and makes it possible to
culture them.
Expression of the ligands is preferably switched off using
the methods which will now be described. 1. By means of using
the RevTet system supplied by ClonTech, USA. 2. Another
CA 02387146 2002-04-10
-6~-
possibility, which is based on the principle of double
infection with an expression vector and a regulatory vector,
is based on using bacterial regulatory systems in eukaryotes.
The gene for the apoptosis-inducing ligand is under the
control of the strong prokaryotic T7 promoter. Since T7
polymerase is not present either in the packaging line or in
the cells which are to be transduced subsequently, the ligand
is not expressed. It is only when a second regulatory vector
is used to cotransduce the gene for T7 polymerase that the
ligand is then expressed in the doubly transduced cells.
3. Another suitable system is the Cre-loxP system from
bacteriophage P1.
Coexpressing antigens and apoptosis-inducing ligands or
antiapoptosis molecules and suicide enzymes
Preference is given to the vectors according to the invention
only expressing the antigens, or constituents of antigens,
which are recognized by immune cells in conjunction with the
ligands which have an apoptotic effect. This coexpression is
achieved by coupling the nucleic acid sequences on a joint
transcript. This prevents the transduced cells from
stimulating specific T cells in vivo, by presenting
constituents of the antigen, without destroying the T cells.
An internal ribosomal entry site (IRES) can be used to
express two or more genes under the transcriptional control
of a constitutive or regulatable promoter. For example, the
IRESs from picorna viruses, from hepatitis C virus or from
BiP (immunoglobulin heavy chain binding protein), or
retroviral IRES sequences, are used.
Another possibility for expressing two or more genes under
the transcriptional control of a strong promoter consists in
using sequences which require a shift in the ribosomal
reading frame during translation, as a result of which a stop
codon is overlooked. Normally, these frame-shift signals at
the RNA level require the ribosomes to be displaced, at a
specific site, into the -1 reading frame (in the 5'
CA 02387146 2002-04-10
-61-
orientation) and to continue the translation in the new
reading frame. Such -1 frame-shift signals have been
described in a large number of different virus families, such
as retroviruses, coronaviruses, astroviruses, totiviruses,
podoviruses, siphoviruses, luteoviruses and dianthoviruses.
By way of example, the frame-shift sequence in the Rous
sarcoma virus (RSV) consists of 2 essential components: a
homopolymeric slippery sequence, consisting of the sequence
(AAAUUUA) and an RNA secondary structure which is located a
few nucleotides downstream. The following slippery consensus
sequence has been constructed by comparing the slippery
sequences in different viruses: it consists of a sequence
which comprises 7 nucleotides and which contains 2
homopolymeric triplets (X-XXY-YYZ) (Brierley (1995) J Gen
Viro1 76 (Pt 8): 1885-1892).
In addition to vector-specific nucleic acid sequences, which,
for example, enable the vectors to replicate in bacteria or
eukaryotic cells, or regulatory nucleic acid sequences, which
enable the coding regions to be expressed, or nucleic acid
sequences which enable the vector to be packaged or the
nucleic acid to be packaged into a vector, vectors according
to the invention also comprise, for example, nucleic acids,
as well, which encode one or more suicide enzymes and one or
more antiapoptosis molecules. The expression of the
antiapoptosis molecules is always coupled to the expression
of the suicide enzymes and is dependent on this latter
expression.
Target cells for treatment with therapeutically active
nucleic acids
Antigen-presenting cells (APCs)
For the therapy of autoimmune diseases or diseases involving
immunopathogenesis, syngenic antigen-presenting cells from
the individuals who are to be treated are used for preparing
the bait cells. The MHC pattern of the antigen-presenting
cells and the reactive cells is consequently identical and it
is only those T cells which react autoaggressively, or
CA 02387146 2002-04-10
-62-
recognize the foreign antigens in conjunction with endogenous
MHC, which are attracted and eliminated. When transplant
rejections are being treated or prevented, antigen-presenting
cells are purified from the organ donor. These cells are
allogenic (different MHC pattern) in relation to the
recipient. In this case it is necessary to recognize and
eliminate the T cells which recognize cellular antigens
together with foreign MHC from the donor.
Lymphocytes, accessory cells and effector cells constitute
the most prominent representatives of the acquired immune
system. Lymphocytes are able to recognize foreign antigens
specifically and to stimulate a specific humoral and cell-
mediated immune response. Different subpopulations of
lymphocytes are known to differ in the nature of their
antigen recognition and their specific effector functions.
B lymphocytes are the producers of antibodies. They recognize
extracellular antigens and and antigens which are presented
on the surfaces of cells and differentiate into antibody-
secreting plasma cells following contact with an antigen.
T lymphocytes, which are the mediators of the cell-mediated
immune response, can be subdivided into several subtypes of
which the CD4+ T helper cells and CD8+ cytotoxic T cells are
the most important. Helper and cytotoxic T cells exhibit a
restricted specificity for antigens. They only recognize
peptide antigens which are presented on the surface of an
endogenous cell together with MHC class II or MHC class I,
respectively. Following the specific recognition of a
specific MHC class II/peptide complex, T helper cells secrete
cytokines which stimulate T cells and other immune cells,
such as B cells and macrophages, to proliferate and
differentiate. Cytotoxic T cells (CTL) lyse cells which are
presenting peptides from nonendogenous proteins together with
MHC class I proteins on their surfaces. On the other hand,
what are termed the suppressor T cells are a subtype of T
helper cells which produce cytokines which suppress
particular immune functions. A third class of lymphocytes,
i.e. the natural killer (NK) cells, are a component of the
innate immune response for combating viruses and
CA 02387146 2002-04-10
-63-
intracellular pathogens.
Antigen-presenting cells (APCs) are of very great importance
for regulating the immune system. These cells take up foreign
antigens, process them into small peptides and present them,
together with MHC proteins, on their surfaces. Two classes of
APCs are distinguished. Professional APCs present the
generated peptides on MHC class I and class II proteins and,
in addition, express costimulatory proteins such as B7.1 and
B7.2. The most important representatives of these APCs are
dendritic cells and macrophages, and also B cells. These
cells stimulate T helper cells and cytotoxic T cells which,
by means of their T cell receptor, recognize peptides which
are complexed with MHC class II and MHC class I,
respectively. On the other hand, nonprofessional APCs, which
present MHC/peptide complexes but do not present any
costimulatory proteins, are only recognized by T cells which
have already been activated. The professional APCs are the
main target cells into which the vectors according to the
invention are to be preferentially inserted ex vivo.
The purification of different populations of blood
lymphocytes has been described in a large number of
publications and is state of the art. Mononuclear cells can
be purified, for example, from the peripheral blood by means
of Ficoll-Hypaque density gradient centrifugation.
Furthermore, other methods based on the antibody-mediated
recognition of immune cells can be used for positively and
negatively selecting cell populations. By way of example,
such methods are immunomagnetic selection, "panning" on
immobilized monoclonal antibodies, antibodies/complement-
mediated cell lysis and the cell sorting of fluorescence-
labeled cells. CD3 is a suitable surface marker for selecting
T cells. Specific T helper cells are selected with the aid of
the CD4 marker and cytotoxic T cells are selected with the
aid of the CD8 marker. Other T cell subpopulations can be
(pre)selected with the aid of the markers CD30, CD45RA (naive
T cells) and CD45R0 (activated T cells and memory T cells).
Activated T cells can be separated with the aid of the CD69
CA 02387146 2002-04-10
-64-
marker protein. Monocytes can be purified using specific
antibodies (AB) directed against the surface markers CD14 and
CDllb, while natural killer cells can be purified using anti-
CD16 ABs and B cells can be purified using markers CD19 and
CD22. APCs can be purified using specific antibodies directed
against HLA-DR.
Suitable methods for separating T cells from mononuclear cell
populations are based, for example, on rosetting T cells
using sheep red blood cells (SRBC). In addition this method
makes it possible to isolate nonrosetting cell populations
(B lymphocytes, monocytes and macrophages). In this
connection, preference is given to using SRBCs which have
been treated with neuraminidase or 2-aminoethylisothiouronium
bromide (AET), since these treated SRBCs exhibit an increased
binding of T cells. The different T cell populations can be
positively identified or separated using the previously
described methods based on using antibodies to recognize
specific surface markers.
B cells can, by way of example, be very efficiently purified,
in accordance with the already described methods, using CD19-
specific antibodies. The methods of cell sorting by means of
FACS and the use of immunomagnetic beads are particularly
suitable. By way of example, monocytes can be isolated by
adherence to L-leucine methyl ester matrices, for example,
gradient sedimentation through colloidal silica particles and
flow cytometry. However, the former methods activate
monocytes. Another very suitable method for purifying large
quantities of nonactivated monocytes is counterflow
centrifugation using an elutriator (counterflow centrifugal
elutriation, CCE).
By way of example, natural killer cells can be isolated from
Ficoll-Hypaque gradients-purified lymphocyte populations by
means of negative selection using anti-CD3, anti-CDS, anti-
CD19, anti-CD14 and anti-erythrocyte antibodies.
Immune cells can also be generated from other cell
CA 02387146 2002-04-10
~ -65-
~ populations by means of cytokine stimulation. For example,
white blood cells and differentiating precursor cells and
stem cells can be stimulated by a large number of growth
factors. In particular, the cytokines IL-3, IL-4, IL-5, IL-6,
IL-9, CM-CSF, M-CSF and G-CSF, which are produced by
activated T helper cells and activated macrophages, stimulate
myeloid stem cells, which then differentiate into pluripotent
stem cells, granulocyte-monocyte precursor cells, eosino-
philic precursor cells, basophilic precursor cells,
megakaryocytes and erythroid precursor cells. The
differentiation is modulated by growth factors such as
GM-CSF, IL-3, IL-6, IL-11 and erythropoietin (EPO).
Pluripotent stem cells then differentiate into lmyphoid stem
cells, bone marrow stroma cells, precursor T cells, precursor
B cells, thymocytes, T helper cells, cytotoxic T cells and B
cells. This differentiation is modulated by growth factors
such as IL-3, IL-4, IL-6, IL-7, GM-CSF, M-CSF, G-CSF, IL-2
and IL-5. Granulocyte-monocyte precursors differentiate into
monocytes, macrophages and neutrophils. This differentiation
is modulated by the growth factors GM-CSF, M-CSF and IL-8.
Eosinophilic precursors differentiate into eosinophils. This
process is modulated by GM-CSF and IL-5. The differentiation
of basophilic precursors into mast cells and basophils is
stimulated by GM-CSF, IL-4 and IL-9. Following stimulation
with GM-CSF, EPO and IL-6, megakaryocytes produce blood
platelets. Stimulated by EPO, erythroid precursor cells
differentiate into red blood cells. The purity of the
isolated cell populations is monitored by FRCS analysis using
specific antibodies.
The proportion of the dendritic cells in the blood is less
than 0.2%. For this reason, it is not necessary to directly
purify this cell population. However, by way of example,
cytokines can be used to generate relatively large quantities
of dendritic cells from CD14-positive blood rnonocytes. The
cytokines TNF-a, GM-CSF and IL4 are used, in suitable
concentrations, for differentiating dendritic cells from
monocytes. This method has been published on a number of
occasions and is state of the art.
CA 02387146 2002-04-10
-66-
It is possible to take up all the mononuclear cells in cell
culture medium and to stimulate them nonspecifically. For
example, the B cells are stimulated to proliferate using
crosslinking antibodies directed against the B cell receptor
or using lectins (pokeweed mitogen) or IL-4. For example, T
lymphocytes can be stimulated preferentially by incubating
with a CD3-binding agent, such as the monoclonal antibody
OCT-3. A CD3-binding agent is a ligand which binds CD3
molecules on the surfaces of cells. The ligand can be an
antibody, such as OCT-3, which is able to crosslink two or
more CD3 molecules. This crosslinking induce s the
proliferation and activation of CD3+ cells, such as
T lymphocytes. Furthermore, the activation of T lymphocytes
by CD3-binding agents can be enhanced by varying particular
parameters, such as the concentration of the binding agent,
the incubation time, the cell number, the incubation
temperature, the binding affinity of the agent, the avidity
and the efficiency of the cell activation. Furthermore, T
cells in peripheral blood monocytes can be stimulated with
phytohemaglutinin (PHA). By way of example, other lymphocyte
stimulators are tetanus toxoid, concanavalin A (Con A),
ionomycin and PMA.
Furthermore, nucleic acids which contain unmethylated CpG
motif sequences together with the sequence motif Pur-Pur-CG-
Pyr-Pyr are particularly suitable for activating the
mammalian B cells, monocytes, macrophages and dendritic
cells. In this connection, CpG-containing bacterial or insect
DNA, and also CpG-containing synthetic oligodeoxynucleotides
(ODNs) having a minimal length of 8 nucleotides, can be
suitable for stimulating the abovementioned cell populations.
An enhanced effect can be achieved by using chemically
modified,. and thereby stabilized, oligonucleotides, such as
phosphorothioate-linked ODNs, by way of example. Mammalian
immune cells, such as B cells, monocytes, macrophages and
dendritic cells, are activated directly following contact
with CpG-containing nucleic acids, a fact which is expressed
in the enhanced surface expression of MHC class II molecules
CA 02387146 2002-04-10
-67-
and costimulatory molecules and in the increased
transcription of defined cytokine mRNAs and the secretion of
proinflammatory cytokines such as TNF-a, IFN-y, IL12 and IL6.
In particular, modulation of the immunological properties of
CpG-containing ODNs can be achieved by specifically selecting
or changing the sequences flanking the CpG motifs.
On the one hand, proliferation of the cells which are
subsequently to be transduced is necessary in order, for
example, to enable the retroviral vectors to be integrated
into the cellular genome. On the other hand, the
multiplication of the cells following transduction with a
retroviral vector leads to an increase in the probability
that these cells will be recognized by appropriate T cells.
For the therapy of autoimmune diseases or of diseases
involving immunopathogenesis, syngenic antigen-presenting
cells from the individuals who are to be treated are used for
preparing the bait cells. The MHC pattern of the antigen-
presenting cells and the reacting cells is consequently
identical and only those T cells which react
autoaggressively, or which recognize the foreign antigens in
conjunction with endogenous MHC, are attracted and
eliminated. When transplant rejections are being treated or
prevented, antigen-presenting cells are purified from the
peripheral blood of the organ donor. These cells are
allogenic (different MHC pattern) in relation to the
recipient. In this case, it is necessary to recognize and
eliminate the T cells which recognize cellular antigens
together with foreign MHC from the donor.
Cul turiag APCs
The mammalian or human cells can be cultured in a suitable
nutrient medium to which at least one defined growth factor
has been added. A large number of growth factors, which
promote the growth of different cell types, have been
described. Typical representatives of such growth factors are
cytokine mitogens, such as IL-2, IL-10, IL-12 and IL-15,
CA 02387146 2002-04-10
-68-
which, for example, promote the growth and activation of
lymphocytes. Other cell types are in particular [lacuna] by a
different class of growth factors, such as hormones,
including the human pregnancy hormone chorionic gonadotrophin
(hCG) and human growth hormone. The selection of suitable
growth factors for defined cell populations has been
described in detail and is state of the art.
The following implementation examples serve to explain the
invention and are not to be construed as being limiting.
Data from animal experiments
Using adenoviral gene transfer to transfect murine antigen-
presenting cel.Is (APCs) with the Fas ligand gene
A peritoneal lavage was carried out in Fas (CD95)-deficient
C57BL(B6) mice and the peritoneal macrophages were isolated.
5 x 106 macrophages per well were cultured for a period of 24
hours in complete DMEM medium in a 6-well plate and
subsequently transfected with an adenoviral vector (AdFasL),
which led to expression of the Fas ligand (Fast, CD95L) gene,
or with a control vector (AdLac2, expression of the (3-
galactosidase). After 48 hours, the transfected macrophages
were tested for the expression and functionality of Fast. In
the Facs analysis, it was found that almost 90°s of the
AdFasL-transfected macrophages (AdFasL-APCs) were expressing
the Fast gene on the cell surface whereas it was not possible
to observe any relevant Fast expression on the macrophages
which had been transfected with AdLacZ (AdLacZ-APCs)
(fig. 1A). In order to investigate whether the Fast
expression was also functional, AdFasL-transfected
macrophages were employed in a cytotoxicity test using Fas-
expressing A20 target cells. A concentration-dependent
cytolysis of the target cells was then observed in the
cocultures with AdFaL-APCs, whereas it was not possible to
detect any lysis, or only possible to detect slight
spontaneous lysis, in the cocultures of AdLacZ-APCs and A20
target cells (fig. 1B).
Inhibition of the allogenic proliferation of T cells by Fas
CA 02387146 2002-04-10
-69-
ligand-expressing APCs
In order to establish whether Fast expression on the AdFasL-
APCs can modulate the interaction with T cells and suppress
an allogen-specific T cell response, AdFasL-APCs and AdLacZ-
APCs were generated from B6-1pr/1pr mice (H-2Db) and
cocultured, in a mixed lymphocyte reaction (MLR), with T
cells from Fas-deficient MRL-1pr/1pr mice (H-2Dk) or Fas-
expressing MRL-+/+ control mice (H-2Dk). Allogenic T cell
proliferation was determined by the incorporation of [3H]-
thymidine. It was found that the allogenic proliferation of
the H-2Dk T cells was significantly reduced in the cocultures
with H-2Db Fast-APCs as compared with the cocultures of H-2Dk
T cells with H-2Db LacZ-APCs (fig. 2A). By using the Fas-
deficient T cells from MRL-lpr/lpr mice, it was furthermore
possible to demonstrate that this suppression effect was due
to the interaction of Fas with Fas ligand and consequently
required the functional expression of both the molecules
(fig. 2B) .
Therapeutic use of Fas ligand-expressing antigen-presenting
cells in a mouse model of virus-induced autoimmune disease
The infection of Fas ligand-deficient C57/BL6(B6) g1dlgld
mice with murine cytomegalovirus (MCMV) induces a chronic
autoimmune inflammatory reaction. In order to test whether an
antigen-specific suppression of the T cells by AdFasL-APCs
can also be achieved in vivo, AdFasL-APCs and AdLacZ-APCs
were tested therapeutically in a mouse model of chronic
autoimmune disease. In the mouse model employed, a chronic
inflammatory reaction in various organs was induced by the
intraperitoneal infection of Fas-deficient B6-lpr/lpr mice or
Fast-deficient B6-g1d/g1d mice with 1 x 106 PFU of the mouse
cytomegalovirus (MCMV), whereas it was not possible to detect
any relevant organ changes in B6-+/+ mice after the virus-
infected cells has been eliminated. The reasons for the
chronic inflammation in B6-Ipr/1pr and B6-g1d/gld mice was
not a delay in the elimination of virus-infected cells but,
instead, a persistent activation of T cells, which were
principally responsible for the inflammatory infiltrates in
CA 02387146 2002-04-10
-70-
the organs; for this reason, this mouse model was
outstandingly suitable for testing the effectiveness of the
novel therapy concept. Furthermore, the chronic inflammation
exhibited a marked autoimmune component since it was possible
to detect an increase in autoantibodies. Fig. 3 shows the
histological severity, over time, of the inflammatory
reaction in the lung, kidney and liver in MCMV-infected
B6-+/+ and Fast-deficient B6-gld/gld mice.
Significant improvement in the MCMV-induced chronic
inflammatory reaction achieved by treating with Fas Zigand-
expressing APCs
28 days after the infection with MCMV, B6-gZd/g1d and
B6-Zpr/Zpr mice were treated with AdLacZ-APCs or AdFasL-APCs
(1 x 106 APCs i.p. every 3rd day for a period of 12 days). In
addition, some of these APCs were pulsed with MCMV in vitro
prior to administration. 4 weeks after the beginning of the
treatment, the organs were removed and the severity of the
inflammatory reaction was determined (fig. 4) . A significant
improvement in the inflammation was found in the case of the
B6-g1d/gld mice treated with AdFasL-APCs, with this
improvement being augmented still further by the APCs being
previously pulsed with MCMV. In addition, it was possible to
demonstrate that the observed therapeutic effect was Fas-
mediated since it was not possible to detect any improvement
in Fas-deficient B6-1pr/lpr mice.
Significant reduction in MCMV-specific T cells in the spleen
following treatment with Fas ligand-expressing APCs
At the same time, the spleen was also removed from the
B6-gZdlgld mice and the spleen cells were cocultured with
MCMV-pulsed APCs, derived from B6 mice, over a period of
48 hours in a mixed lymphocyte reaction (MLR). A significant
reduction in IL-2 secretion was seen in the case of the
spleen cells obtained from the animals which had been treated
with AdFasL-APCs (fig. 5). This consequently demonstrated
that the AdFasL-APCs had suppressed the T cells, in an
antigen-specific manner, in vivo.
CA 02387146 2002-04-10
-71-
Decreased production of autoantibodies in MCMV-infected
B6-g1d/gld mice as a result of having been treated with Fas
ligand-expressing APCs
Since MCMV-infected B6-g1d/g1d mice develop high con-
centrations of autoantibodies, an experiment was carried out
to investigate whether the autoantibody production can be
influenced by administrating AdFasL-APCs. For this, the
levels of rheumatoid factor IgG1 and anti-double stranded DNA
IgGl autoantibodies were determined in the serum of mice
which had been treated either with AdLacZ-APCs, AdFasL-APCs
or MCMV-pulsed AdFasL-APCs. A significant reduction in
autoantibody production was found in the animals which were
treated with AdFasL-APCs, with it being possible to augment
the suppression even further by previously pulsing the
AdFasL-APCs with MCMV (fig. 6).
Example using primary human cells
Effective transfection of primary human macrophages with an
adenoviral vector
Primary monocytes, which had been isolated by leukapheresis,
were differentiated in vitro, over a period of 7 days, into
macrophages or, by adding IL-4 and GM-CSF to the culture
medium, into dendritic cells (DCs). Subsequently, the
macrophages and DCs were transfected with AdLacZ at varying
concentrations. It was found that, in contrast to other cells
(e. g. fibroblasts or murine macrophages), both macrophages
and DCs are significantly more difficult to transfect.
However, when a high MOI (500) was used, it was possible,
after 72 hours, to demonstrate successful transfection in
approx. 30% of the macrophages by means of detecting the (3-
galactosidase by means of an x-Gal staining (fig. 7). It was
possible to increase the transfection rate markedly by using
a variety of cytokines and also lipofectamine.
Inhibiting the allogenic proliferation of T cells with
tolerogenic dendritic cells
A tolerogenic DC phenotype was also investigated
CA 02387146 2002-04-10
-72-
independently of the adenoviral transfection. Other research
groups have shown that a suppressive DC phenotype is
generated by adding IL-10 to the DC culture on day 5, whereas
the addition of TNF at the same point in time leads to a
strongly activating DC phenotype, an observation which has
been attributed, inter alia, to the differential expression
of costimulating molecules. An investigation was therefore
carried out to determine whether these two DC populations
differ in their ability to induce an allogenic T cell
stimulation in the MLR (fig. 8). It was found that IL-10-
matured DCs induce a smaller proliferation of allogenic
T cells than do TNF-treated DCs. It was possible to augment
this effect still further by adding an anti-Fas antibody
(clone CH-11).
Demonstrating an allogen-specific suppression brought about
by tolerizing APCs
In order to investigate whether the observed suppression of
the T cell proliferation is allogen-specific, the T cells
were isolated 5 days after beginning the MLR and then
employed in a 2nd MLR against APCs derived from a third donor
(third party) (fig. 9). In this connection, it was also
possible to demonstrate that the IL-10-matured DCs brought
about allogen-specific suppression of the T cells since the
reaction of the T cells, which had initially been cocultured
with IL-10-matured DCs, on the APCs obtained from the third
donor proceeded in an unimpaired manner.
Construction of vectors according to the invention
The vectors pcDNA3-TK-IRES-crmA (fig. 10A) and pcDNA3-FasL-
IRES-PLP (fig. 10B) are taken as examples of vectors
according to the invention.
pcDNA3-Fast-IRES-PLP (fig. 10A) comprises nucleic acid
sequences which encode, for example, the apoptosis-inducing
ligand Fast and, by way of example, the antigen proteolipid
protein (PLP). The two regions are linked by an IRES sequence
such that the antigen is translated from the same mRNA as is
CA 02387146 2002-04-10
-73-
the apoptosis-inducing ligand. The vector is based on the
cloning vector pcDNA3. The nucleic acid regions which encode
Fast and PLP were transcribed from RNA into cDNA by means of
the polymerase chain reaction (PCR). Methods for isolating
the RNA from cells, and the use of PCR for transcribing
specific RNA molecules into cDNA, are state of the art. The
oligonucleotides which were used as primers for the PCR
comprise, at their 5' ends, cleavage sites for endonucleases
which were subsequently used for cloning the cDNAs into the
vector pcDNA3. The region which encodes Fast was cloned into
the vector pcDNA3, as the first region, by way of the
cleavage sites for HindIII and BamHI. Subsequently, the PLP-
encoding fragment was inserted by way of the cleavage sites
for BamHI and EcoRI. Finally, the nucleic acid fragment which
encompasses the IRES was cloned in by way of the recognition
sequence for BamHI, between Fast and PLP. The techniques for
isolating nucleic acids, for cleaving nucleic acids and for
purifying nucleic acid cleavage products, and also the
ligation of individual nucleic acid fragments, and the
replication of the artificially generated nucleic acids in
bacteria, are state of the art.
pcDNA3-TK-IRES-crmA (fig. 10B) comprises nucleic acid
sequences which encode a suicide enzyme, such as thymidine
kinase, and an antiapoptosis molecule, such as crmA. The
expression of crmA is coupled, by an IRES sequence, to the
expression of TK such that crmA can only be expressed
together with the TK. The vector is based on the clone vector
pcDNA3, and the cloning strategy, and the preparation of the
vector according to the invention, are comparable with those
for pcDNA3-Fast-IRES-PLP. The region which encodes the
thymidine kinase was cloned into the vector pcDNA3, as the
first region, by way of the cleavage sites for HindIII and
BamHI. Subsequently, the crmA-encoding fragment was inserted
by way of the cleavage sites for BamHI and XhoI. Finally, the
nucleic acid fragment comprising the IRES was cloned in, by
way of the recognition sequence for BamHI, between Fast and
PLP. The nucleic acid sequences of the two vectors pcDNA3-
FasL-IRES-crmA and pcDNA3-TK-IRES-PLP are listed as SEQ ID
Image
CA 02387146 2002-04-10
-75-
SEQUENCE LISTING
<110> Schwarzmann, Fritz
<120> Gene transfer vectors for the therapy of autoimmune diseases and
diseases involving immunopathogenesis
<130> SCW-002 PCT
<140> xx
<141> 2000-10-12
<150> DE 199 48 983.1
<151> 1999-10-12
<160> 2
<170> PatentIn Ver. 2.1
2 0 <210> 1
<211> 10651
<212> DNA
<213> Artificial Sequence
<220>
<223> Plasmid
<400> 1
gatccatgaa tatttctaaa aaacttaaaa gttatacatt gataggtgga ttagctgtat 60
3 0 ttggagctct tggttctgca agctttggct ttaagcaatc agataagagt aacgataaca 120
cgcaattagt taatcaagca agaacgctag atgctaattc tgttagactt gcaggtcttg 180
gacaaaatgg ttcgttgttc aatacagttc ttagagatgt tgatgataac tttataacag 240
cagctaatgg aacaattatc aaattagata gttttactaa accattatat ggtttagatc 300
taagtgatga ttttgctgga tacaaagtaa aacaaatagt ttcagattac acaactagca 360
gaaatagatt tgatcaaaga caaacaagag catattatgc tctgttggtt aatgatgaag 420
ctaacgttca tttaaaaaga attaatacta actcaaatag aattggtaat agaaacaaca 480
attctaagtt tgtaattggt ggtgttgata atccagctca cgtaattaga tttactgatg 540
atgggactaa atttaatttt acaaagcaaa ctcaaggtga aattgttaat gacttcattt 600
tagatgcgcc aatcttacct aaagatttac acccagattg atataactta tacattcaaa 660
gaaagatctt accaaatgac gtaaacactg cagttgttcc ttgaccagta ggtagagtta 720
gtggaacaaa tgctgatgat gggatgtttg attttgggaa tggtcaaata actaatacag 780
CA 02387146 2002-04-10
-76-
atcctattgctcaaactaaaaccactactgataatcaaaatccttcaacttttaattcag840
gagcaatgcctggtgcaaacaatagatacgattctcaattgaatgtcaagcatagaatta900
aaacatctttccaattagatgaaaaatttgtttatccagaatgaactggttctgaagaga960
ataaaaatattacaagattagctactggaagtttgccaagcaacgaaagatattgaattc1020
ttgacataccagggactccacaagttactttaaaagaagattcagttaacgtattttcaa1080
gactatacttaaactcagttaattctttatcattcattggtgatagtatttatatttttg1140
gtacttctgaattaccatcattatgatactattcattcccaactagattatctgatctaa1200
ccgctttgaatcaagttaaaacagatgatattgaagcttcaagcactgataacggtacaa1260
caacaaacggaacaacgacaacaactgatacatctagtggttcaacaggtgctggaacag1320
gaaatactactaacacttctcaaacagtttctaatcctactttaaatacttatcgtagtt1380
ttggaattgatagtaaaccaacttctgcaaacaaaatagatgaaactaattgagcagatc1440
ctaatgttattgaagcaagaatatatgctgaatacagattaggtattcaaaatgaaattc1500
caataactaatgcaggaaactttatccgaaacacaattggtggtgttggttttacttcaa1560
caggttcaagagtagttttaagagcttcttataacggtgatcaacgtccaactggaaact1620
tccaacctttcttatacgtatttggttatttaggataccaacaaactagaacaggaactt1680
tctgatacggaacatataaactattaaacaacagcccttacgacgtattagatgctgcaa1740
gagtaggtactgaaaccaatcaatttagaagaacttcattaacataccctgttatgggtg1800
gatayctaactgaagaaggtgctagaagtttctctaatactccatatataagagcacaag1860
gtgacacaccagaaagccgaagcatcttccaatctggctaytctgataatacttatgagt1920
2 acattcaatcagttttaggatttgatggaattagaaataacttaaatgttggggttaaag1980
0
catcaagcttcttaaactcaaatagaccaaatccaaacggtctagaaatgattgctgcaa2040
caacatacttaagatcacaaattggattagctagaacatctggattaccaaaccaacaac2100
cattcggaacaactcaccaagttatttcagtatcacctggtgatcagttctcatcaatta2160
agaatattagaacaatcttccctggtaaccagttatgatacttcttattcacaaatgaaa2220
2 ataataaatctagtgtttatacattaagattagctgactcaagtaaccctgatgcgtcaa2280
5
gctcattcagtccaacaagtttaattgacgttaatgaaattggtgtaatcttacctttat2340
tagacaattcattctatacagtaaatgctgctggtaatgttgcattgttctcatcaaacc2400
ctggttctcctggatcatatactgctgtaaatacatttaatcagaacttatctgatattg2460
cttttgaaggttctggtgctaaatatacatctgatttctgaggaacaatccaattcaaac2520
30 ccgatgagtacttaattcaaaatgggttcactagtcaagtggctagaaacttcgttacaa2580
accaaagcttcttaaacagtttagttgacttcactcctgctaatgctggtactaactacc2640
gtgtagtggttgatcctgatggtaatttaacaaaccaaaacctacctctaaaagttcaga2700
tccaatacttagatggtaagtattatgatgctaaattaaagaacaataatttagtaacat2760
tctcttataacaactttgctgctttaccttcatgagtagtgcctacagcaattggtagta2820
35 cattaggtattcttgcaattatgatcatcttaggattagctatcggtattcctttaagag2880
ctcaaagaaaattacaagacaaagggttcaaaacaacattcaaaaaagttgataccttga2940
ctgctgctgttggttcagtttacaagaagattattacccaaactgctaacgttaagaaaa3000
aacctgctgctttaggtgctggtaaatctggtgataagaaacctgctgctgctgctaaac3060
ctgctgctccagctaaaccatctgcaccaaaagctagctcaccagctaaaccaactgcgc3120
40 ctaaatctggtgcgcctacaaaaccaactgctcctaagccagctgctccaaaaccaaccg3180
ctcccaaagaataactcgagcatgcatctagagggccctattctatagtgtcacctaaat3240
CA 02387146 2002-04-10
_77_
gctagagctcgctgatcagcctcgactgtgccttctagttgccagccatctgttgtttgc3300
ccctcccccgtgccttccttgaccctggaaggtgccactcccactgtcctttcctaataa3360
aatgaggaaattgcatcgcattgtctgagtaggtgtcattctattctggggggtggggtg3420
gggcaggacagcaagggggaggattgggaagacaatagcaggcatgctggggatgcggtg3480
ggctctatggcttctgaggcggaaagaaccagctggggctctagggggtatccccacgcg3540
ccctgtagcggcgcattaagcgcggcgggtgtggtggttacgcgcagcgtgaccgctaca3600
cttgccagcgccctagcgcccgctcctttcgctttcttcccttcctttctcgccacgttc3660
gccggctttccccgtcaagctctaaatcggggcatccctttagggttccgatttagtgct3720
ttacggcacctcgaccccaaaaaacttgattagggtgatggttcacgtagtgggccatcg3780
ccctgatagacggtttttcgccctttgacgttggagtccacgttctttaatagtggactc3840
ttgttccaaactggaacaacactcaaccctatctcggtctattcttttgatttataaggg3900
attttggggatttcggcctattggttaaaaaatgagctgatttaacaaaaatttaacgcg3960
aattaattctgtggaatgtgtgtcagttagggtgtggaaagtccccaggctccccaggca4020
ggcagaagtatgcaaagcatgcatctcaattagtcagcaaccaggtgtggaaagtcccca4080
ggctccccagcaggcagaagtatgcaaagcatgcatctcaattagtcagcaaccatagtc4140
ccgcccctaactccgcccatcccgcccctaactccgcccagttccgcccattctccgccc4200
catggctgactaattttttttatttatgcagaggccgaggccgcctctgcctctgagcta4260
ttccagaagtagtgaggaggcttttttggaggcctaggcttttgcaaaaagctcccggga4320
gcttgtatatccattttcggatctgatcaagagacaggatgaggatcgtttcgcatgatt4380
2 gaacaagatggattgcacgcaggttctccggccgcttgggtggagaggctattcggctat4440
0
gactgggcacaacagacaatcggctgctctgatgccgccgtgttccggctgtcagcgcag4500
gggcgcccggttctttttgtcaagaccgacctgtccggtgccctgaatgaactgcaggac4560
gaggcagcgcggctatcgtggctggccacgacgggcgttccttgcgcagctgtgctcgac4620
gttgtcactgaagcgggaagggactggctgctattgggcgaagtgccggggcaggatctc4680
ctgtcatctcaccttgctcctgccgagaaagtatccatcatggctgatgcaatgcggcgg4740
ctgcatacgcttgatccggctacctgcccattcgaccaccaagcgaaacatcgcatcgag4800
cgagcacgtactcggatggaagccggtcttgtcgatcaggatgatctggacgaagagcat4860
caggggctcgcgccagccgaactgttcgccaggctcaaggcgcgcatgcccgacggcgag4920
gatctcgtcgtgacccatggcgatgcctgcttgccgaatatcatggtggaaaatggccgc4980
ttttctggattcatcgactgtggccggctgggtgtggcggaccgctatcaggacatagcg5040
ttggctacccgtgatattgctgaagagcttggcggcgaatgggctgaccgcttcctcgtg5100
ctttacggtatcgccgctcccgattcgcagcgcatcgccttctatcgccttcttgacgag5160
ttcttctgagcgggactctggggttcgaaatgaccgaccaagcgacgcccaacctgccat5220
cacgagatttcgattccaccgccgccttctatgaaaggttgggcttcggaatcgttttcc5280
gggacgccggctggatgatcctccagcgcggggatctcatgctggagttcttcgcccacc5340
ccaacttgtttattgcagcttataatggttacaaataaagcaatagcatcacaaatttca5400
caaataaagcatttttttcactgcattctagttgtggtttgtccaaactcatcaatgtat5460
cttatcatgtctgtataccgtcgacctctagctagagcttggcgtaatcatggtcatagc5520
tgtttcctgtgtgaaattgttatccgctcacaattccacacaacatacgagccggaagca5580
taaagtgtaaagcctggggtgcctaatgagtgagctaactcacattaattgcgttgcgct5640
cactgcccgctttccagtcgggaaacctgtcgtgccagctgcattaatgaatcggccaac5700
CA 02387146 2002-04-10
_78_
gcgcggggagaggcggtttgcgtattgggcgctcttccgcttcctcgctcactgactcgc5760
tgcgctcggtcgttcggctgcggcgagcggtatcagctcactcaaaggcggtaatacggt5820
tatccacagaatcaggggataacgcaggaaagaacatgtgagcaaaaggccagcaaaagg5880
ccaggaaccgtaaaaaggccgcgttgctggcgtttttccataggctccgcccccctgacg5940
agcatcacaaaaatcgacgctcaagtcagaggtggcgaaacccgacaggactataaagat6000
accaggcgtttccccctggaagctccctcgtgcgctctcctgttccgaccctgccgctta6060
ccggatacctgtccgcctttctcccttcgggaagcgtggcgctttctcaatgctcacgct6120
gtaggtatctcagttcggtgtaggtcgttcgctccaagctgggctgtgtgcacgaacccc6180
ccgttcagcccgaccgctgcgccttatccggtaactatcgtcttgagtccaacccggtaa6240
gacacgacttatcgccactggcagcagccactggtaacaggattagcagagcgaggtatg6300
taggcggtgctacagagttcttgaagtggtggcctaactacggctacactagaaggacag6360
tatttggtatctgcgctctgctgaagccagttaccttcggaaaaagagttggtagctctt6420
gatccggcaaacaaaccaccgctggtagcggtggtttttttgtttgcaagcagcagatta6480
cgcgcagaaaaaaaggatctcaagaagatcctttgatcttttctacggggtctgacgctc6540
agtggaacgaaaactcacgttaagggattttggtcatgagattatcaaaaaggatcttca6600
cctagatccttttaaattaaaaatgaagttttaaatcaatctaaagtatatatgagtaaa6660
cttggtctgacagttaccaatgcttaatcagtgaggcacctatctcagcgatctgtctat6720
ttcgttcatccatagttgcctgactccccgtcgtgtagataactacgatacgggagggct6780
taccatctggccccagtgctgcaatgataccgcgagacccacgctcaccggctccagatt6840
2 tatcagcaataaaccagccagccggaagggccgagcgcagaagtggtcctgcaactttat6900
0
ccgcctccatccagtctattaattgttgccgggaagctagagtaagtagttcgccagtta6960
atagtttgcgcaacgttgttgccattgctacaggcatcgtggtgtcacgctcgtcgtttg7020
gtatggcttcattcagctccggttcccaacgatcaaggcgagttacatgatcccccatgt7080
tgtgcaaaaaagcggttagctccttcggtcctccgatcgttgtcagaagtaagttggccg7140
2 cagtgttatcactcatggttatggcagcactgcataattctcttactgtcatgccatccg7200
5
taagatgcttttctgtgactggtgagtactcaaccaagtcattctgagaatagtgtatgc7260
ggcgaccgagttgctcttgcccggcgtcaatacgggataataccgcgccacatagcagaa7320
ctttaaaagtgctcatcattggaaaacgttcttcggggcgaaaactctcaaggatcttac7380
cgctgttgagatccagttcgatgtaacccactcgtgcacccaactgatcttcagcatctt7440
30 ttactttcaccagcgtttctgggtgagcaaaaacaggaaggcaaaatgccgcaaaaaagg7500
gaataagggcgacacggaaatgttgaatactcatactcttcctttttcaatattattgaa7560
gcatttatcagggttattgtctcatgagcggatacatatttgaatgtatttagaaaaata7620
aacaaataggggttccgcgcacatttccccgaaaagtgccacctgacgtcgacggatcgg7680
gagatctcccgatcccctatggtcgactctcagtacaatctgctctgatgccgcatagtt7740
35 aagccagtatctgctccctgcttgtgtgttggaggtcgctgagtagtgcgcgagcaaaat7800
ttaagctacaacaaggcaaggcttgaccgacaattgcatgaagaatctgcttagggttag7860
gcgttttgcgctgcttcgcgatgtacgggccagatatacgcgttgacattgattattgac7920
tagttattaatagtaatcaattacggggtcattagttcatagcccatatatggagttccg7980
cgttacataacttacggtaaatggcccgcctggctgaccgcccaacgacccccgcccatt8040
40 gacgtcaataatgacgtatgttcccatagtaacgccaatagggactttccattgacgtca8100
atgggtggactatttacggtaaactgcccacttggcagtacatcaagtgtatcatatgcc8160
CA 02387146 2002-04-10
_79_
aagtacgccccctattgacgtcaatgacggtaaatggcccgcctggcattatgcccagta8220
catgaccttatgggactttcctacttggcagtacatctacgtattagtcatcgctattac8280
catggtgatgcggttttggcagtacatcaatgggcgtggatagcggtttgactcacgggg8340
atttccaagtctccaccccattgacgtcaatgggagtttgttttggcaccaaaatcaacg8400
ggactttccaaaatgtcgtaacaactccgccccattgacgcaaatgggcggtaggcgtgt8460
acggtgggaggtctatataagcagagctctctggctaactagagaacccactgcttactg8520
gcttatcgaaattaatacgactcactatagggagacccaagcttatggcttcgtacccct8580
gccatcaacacgcgtctgcgttcgaccaggctgcgcgttctcgcggccatagcaaccgac8640
gtacggcgttgcgccctcgccggcagcaagaagccacggaagtccgcccggagcagaaaa8700
tgcccacgctactgcgggtttatatagacggtccccacgggatggggaaaaccaccacca8760
cgcaactgctggtggccctgggttcgcgcgacgatatcgtctacgtacccgagccgatga8820
cttactggcgggtgctgggggcttccgagacaatcgcgaacatctacaccacacaacacc8880
gcctcgaccagggtgagatatcggccggggacgcggcggtggtaatgacaagcgcccaga8944
taacaatgggcatgccttatgccgtgaccgacgccgttctggctcctcatatcggggggg9000
aggctgggagctcacatgccccgcccccggccctcaccctcatcttcgaccgccatccca9060
tcgccgccctcctgtgctacccggccgcgcgataccttatgggcagcatgaccccccagg9120
ccgtgctggcgttcgtggccctcatcccgccgaccttgcccggcacaaacatcgtgttgg9180
gggcccttccggaggacagacacatcgaccgcctggccaaacgccagcgccccggcgagc9240
ggcttgacctggctatgctggccgcgattcgccgcgtttacgggctgcttgccaatacgg9300
tgcggtatctgcagggcggcgggtcgtggcgggaggattggggacagctttcggggacgg9360
ccgtgccgccccagggtgccgagccccagagcaacgcgggcccacgaccccatatcgggg9420
acacgttatttaccctgtttcgggcccccgagttgctggcccccaacggcgacctgtata9480
acgtgtttgcctgggccttggacgtcttggccaaacgcctccgtcccatgcacgtcttta9540
tcctggattacgaccaatcgcccgccggctgccgggacgccctgctgcaacttacctccg9600
2 ggatggtccagacccacgtcaccaccccaggctccataccgacgatctgcgacctggcgc9660
5
gcacgtttgcccgggagatgggggaggctaactgaggatccactagtaacggccgccagt9720
gtgctggaattaattcgctgtctgcgagggccagctgttggggtgagtactccctctcaa9780
aagcgggcatgacttctgcgctaagattgtcagtttccaaaaacgaggaggatttgatat9840
tcacctggcccgcggtgatgcctttgagggtggccgcgtccatctggtcagaaaagacaa9900
tctttttgttgtcaagcttgaggtgtggcaggcttgagatctggccatacacttgagtga9960
caatgacatccactttgcctttctctccacaggtgtccactcccaggtccaactgcaggt10020
cgatcgagcatgcatctagggcggccgcactagaggaattcgcccctctccctccccccc10080
~ccctaacgttactggccgaagccgcttggaataaggccggtgtgtgtttgtctatatgtg10140
attttccaccatattgccgtcttttggcaatgtgagggcccggaaacctggccctgtctt10200
cttgacgagcattcctaggggtctttcccctctcgccaaaggaatgcaaggtctgttgaa10260
tgtcgtgaaggaagcagttcctctggaagcttcttgaagacaaacaacgtctgtagcgac10320
cctttgcaggcagcggaaccccccacctggcgacaggtgcctctgcggccaaaagccacg10380
tgtataagatacacctgcaaaggcggcacaaccccagtgccacgttgtgagttggatagt10440
tgtggaaagagtcaaatggctctcctcaagcgtagtcaacaaggggctgaaggatgccca10500
gaaggtaccccattgtatgggaatctgatctggggcctcggtgcacatgctttacatgtg10560
tttagtcgaggttaaaaaagctctaggccccccgaaccacggggacgtggttttcctttg10620
CA 02387146 2002-04-10
-80-
aaaaacacga tgataagctt gccacaaccc g 10651
<210> 2
<211> 8116
<212> DNA
<213> Artificial Sequence
<220>
<223> Plasmid
<400> 2
gatccttccagctgaacaaagtcagccacaaagcagactagccagccggctacaattgga60
gtcagagtcccaaagacatgggcttgttagagtgctgtgcaagatgtctggtaggggccc120
cctttgcttccctggtggccactggattgtgtttctttggggtggcactgttctgtggct180
gtggacatgaagccctcactggcacagaaaagctaattgagacctatttctccaaaaact240
accaagactatgagtatctcatcaatgtgatccatgccttccagtatgtcatctatggaa300
ctgcctctttcttcttcctttatggggccctcctgctggctgagggcttctacaccaccg360
gcgcagtcaggcagatctttggcgactacaagaccaccatctgcggcaagggcctgagcg420
2 caacggtaacagggggccagaaggggaggggttccagaggccaacatcaagctcattctt480
0
tggagcgggtgtgtcattgtttgggaaaatggctaggacatcccgacaagtttgtgggca540
tcacctatgccctgaccgttgtgtggctcctggtgtttgcctgctctgctgtgcccgtgt600
acatttacttcaacacctggaccacctgcgactctattgccttccccagcaagacctctg660
ccagtataggcagtctctgtgctgacgccagaatgtatggtgttctcccatggattgctt720
2 tccctggcaaggtttgtggctccaaccttctgtccatctgcaaaacagctgagttccaaa780
5
tgaccttccacctgtttattgctgcatttgtgggggctgcagctacactggtttccctgc840
tcaccttcatgattgctgccacttacaactttgccgtccttaaactcatgggccgaggca900
ccaagttctgagaattctgcagatatccatcacactggcggccgctcgagcatgcatcta960
gagggccctattctatagtgtcacctaaatgctagagctcgctgatcagcctcgactgtg1020
30 ccttctagttgccagccatctgttgtttgcccctcccccgtgccttccttgaccctggaa1080
ggtgccactcccactgtcctttcctaataaaatgaggaaattgcatcgcattgtctgagt1140
aggtgtcattctattctggggggtggggtggggcaggacagcaagggggaggattgggaa1200
gacaatagcaggcatgctggggatgcggtgggctctatggcttctgaggcggaaagaacc1260
agctggggctctagggggtatccccacgcgccctgtagcggcgcattaagcgcggcgggt1320
3 gtggtggttacgcgcagcgtgaccgctacacttgccagcgccctagcgcccgctcctttc1380
5
gctttcttcccttcctttctcgccacgttcgccggctttccccgtcaagctctaaatcgg1440
ggcatccctttagggttccgatttagtgctttacggcacctcgaccccaaaaaacttgat1500
tagggtgatggttcacgtagtgggccatcgccctgatagacggtttttcgccctttgacg1560
ttggagtccacgttctttaatagtggactcttgttccaaactggaacaacactcaaccct1620
4 atctcggtctattcttttgatttataagggattttggggatttcggcctattggttaaaa1680
0
aatgagctgatttaacaaaaatttaacgcgaattaattctgtggaatgtgtgtcagttag1740
CA 02387146 2002-04-10
-81-
ggtgtggaaagtccccaggctccccaggcaggcagaagtatgcaaagcatgcatctcaat1800
tagtcagcaaccaggtgtggaaagtccccaggctccccagcaggcagaagtatgcaaagc1860
atgcatctcaattagtcagcaaccatagtcccgcccctaactccgcccatcccgccccta1920
actccgcccagttccgcccattctccgccccatggctgactaattttttttatttatgca1980
gaggccgaggccgcctctgcctctgagctattccagaagtagtgaggaggcttttttgga2040
ggcctaggcttttgcaaaaagctcccgggagcttgtatatccattttcggatctgatcaa2100
gagacaggatgaggatcgtttcgcatgattgaacaagatggattgcacgcaggttctccg2160
gccgcttgggtggagaggctattcggctatgactgggcacaacagacaatcggctgctct2220
gatgccgccgtgttccggctgtcagcgcaggggcgcccggttctttttgtcaagaccgac2280
ctgtccggtgccctgaatgaactgcaggacgaggcagcgcggctatcgtggctggccacg2340
acgggcgttccttgcgcagctgtgctcgacgttgtcactgaagcgggaagggactggctg2400
ctattgggcgaagtgccggggcaggatctcctgtcatctcaccttgctcctgccgagaaa2460
gtatccatcatggctgatgcaatgcggcggctgcatacgcttgatccggctacctgccca2520
ttcgaccaccaagcgaaacatcgcatcgagcgagcacgtactcggatggaagccggtctt2580
gtcgatcaggatgatctggacgaagagcatcaggggctcgcgccagccgaactgttcgcc2640
aggctcaaggcgcgcatgcccgacggcgaggatctcgtcgtgacccatggcgatgcctgc2700
ttgccgaatatcatggtggaaaatggccgcttttctggattcatcgactgtggccggctg2760
ggtgtggcggaccgctatcaggacatagcgttggctacccgtgatattgctgaagagctt2820
ggcggcgaatgggctgaccgcttcctcgtgctttacggtatcgccgctcccgattcgcag2880
2 cgcatcgccttctatcgccttcttgacgagttcttctgagcgggactctggggttcgaaa2940
0
.
tgaccgaccaagcgacgcccaacctgccatcacgagatttcgattccaccgccgccttct3000
atgaaaggttgggcttcggaatcgttttccgggacgccggctggatgatcctccagcgcg3060
gggatctcatgctggagttcttcgcccaccccaacttgtttattgcagcttataatggtt3120
acaaataaagcaatagcatcacaaatttcacaaataaagcatttttttcactgcattcta3180
2 gttgtggtttgtccaaactcatcaatgtatcttatcatgtctgtataccgtcgacctcta3240
5
gctagagcttggcgtaatcatggtcatagctgtttcctgtgtgaaattgttatccgctca3300
caattccacacaacatacgagccggaagcataaagtgtaaagcctggggtgcctaatgag3360
tgagctaactcacattaattgcgttgcgctcactgcccgctttccagtcgggaaacctgt3420
cgtgccagctgcattaatgaatcggccaacgcgcggggagaggcggtttgcgtattgggc3480
30 gctcttccgcttcctcgctcactgactcgctgcgctcggtcgttcggctgcggcgagcgg3540
tatcagctcactcaaaggcggtaatacggttatccacagaatcaggggataacgcaggaa3600
agaacatgtgagcaaaaggccagcaaaaggccaggaaccgtaaaaaggccgcgttgctgg3660
cgtttttccataggctccgcccccctgacgagcatcacaaaaatcgacgctcaagtcaga3720
ggtggcgaaacccgacaggactataaagataccaggcgtttccccctggaagctccctcg3780
35 tgcgctctcctgttccgaccctgccgcttaccggatacctgtccgcctttctcccttcgg3840
gaagcgtggcgctttctcaatgctcacgctgtaggtatctcagttcggtgtaggtcgttc3900
gctccaagctgggctgtgtgcacgaaccccccgttcagcccgaccgctgcgccttatccg3960
gtaactatcgtcttgagtccaacccggtaagacacgacttatcgccactggcagcagcca4020
ctggtaacaggattagcagagcgaggtatgtaggcggtgctacagagttcttgaagtggt4080
40 ggcctaactacggctacactagaaggacagtatttggtatctgcgctctgctgaagccag4140
ttaccttcggaaaaagagttggtagctcttgatccggcaaacaaaccaccgctggtagcg4200
CA 02387146 2002-04-10
-82-
gtggtttttttgtttgcaagcagcagattacgcgcagaaaaaaaggatctcaagaagatc4260
ctttgatcttttctacggggtctgacgctcagtggaacgaaaactcacgttaagggattt4320
tggtcatgagattatcaaaaaggatcttcacctagatccttttaaattaaaaatgaagtt4380
ttaaatcaatctaaagtatatatgagtaaacttggtctgacagttaccaatgcttaatca4440
gtgaggcacctatctcagcgatctgtctatttcgttcatccatagttgcctgactccccg4500
tcgtgtagataactacgatacgggagggcttaccatctggccccagtgctgcaatgatac4560
cgcgagacccacgctcaccggctccagatttatcagcaataaaccagccagccggaaggg4620
ccgagcgcagaagtggtcctgcaactttatccgcctccatccagtctattaattgttgcc4680
gggaagctagagtaagtagttcgccagttaatagtttgcgcaacgttgttgccattgcta4740
caggcatcgtggtgtcacgctcgtcgtttggtatggcttcattcagctccggttcccaac4800
gatcaaggcgagttacatgatcccccatgttgtgcaaaaaagcggttagctccttcggtc4860
ctccgatcgttgtcagaagtaagttggccgcagtgttatcactcatggttatggcagcac4920
tgcataattctcttactgtcatgccatccgtaagatgcttttctgtgactggtgagtact4980
caaccaagtcattctgagaatagtgtatgcggcgaccgagttgctcttgcccggcgtcaa5040
tacgggataataccgcgccacatagcagaactttaaaagtgctcatcattggaaaacgtt5100
cttcggggcgaaaactctcaaggatcttaccgctgttgagatccagttcgatgtaaccca5160
ctcgtgcacccaactgatcttcagcatcttttactttcaccagcgtttctgggtgagcaa5220
aaacaggaaggcaaaatgccgcaaaaaagggaataagggcgacacggaaatgttgaatac5280
tcatactcttcctttttcaatattattgaagcatttatcagggttattgtctcatgagcg5340
2 gatacatatttgaatgtatttagaaaaataaacaaataggggttccgcgcacatttcccc5400
0
gaaaagtgccacctgacgtcgacggatcgggagatctcccgatcccctatggtcgactct5460
cagtacaatctgctctgatgccgcatagttaagccagtatctgctccctgcttgtgtgtt5520
ggaggtcgctgagtagtgcgcgagcaaaatttaagctacaacaaggcaaggcttgaccga5580
caattgcatgaagaatctgcttagggttaggcgttttgcgctgcttcgcgatgtacgggc5640
2 cagatatacgcgttgacattgattattgactagttattaatagtaatcaattacggggtc5700
5
attagttcatagcccatatatggagttccgcgttacataacttacggtaaatggcccgcc5760
tggctgaccgcccaacgacccccgcccattgacgtcaataatgacgtatgttcccatagt5820
aacgccaatagggactttccattgacgtcaatgggtggactatttacggtaaactgccca5880
cttggcagtacatcaagtgtatcatatgccaagtacgccccctattgacgtcaatgacgg5940
30 taaatggcccgcctggcattatgcccagtacatgaccttatgggactttcctacttggca6000
gtacatctacgtattagtcatcgctattaccatggtgatgcggttttggcagtacatcaa6060
tgggcgtggatagcggtttgactcacggggatttccaagtctccaccccattgacgtcaa6120
tgggagtttgttttggcaccaaaatcaacgggactttccaaaatgtcgtaacaactccgc6180
cccattgacgcaaatgggcggtaggcgtgtacggtgggaggtctatataagcagagctct6290
35 ctggctaactagagaacccactgcttactggcttatcgaaattaatacgactcactatag6300
ggagacccaagcttatgcagcagcccttcaattacccatatccccagatctactgggtgg6360
acagcagtgccagctctccctgggcccctccaggcacagttcttccctgtccaacctctg6420
tgcccagaaggcctggtcaaaggaggccaccaccaccaccgccaccgccaccactaccac6480
ctccgccgccgccgccaccactgcctccactaccgctgccacccctgaagaagagaggga6540
4 accacagcacaggcctgtgtctccttgtgatgtttttcatggttctggttgccttggtag6600
0
gattgggcctggggatgtttcagctcttccacctacagaaggagctggcagaactccgag6660
CA 02387146 2002-04-10
-83-
agtctaccagccagatgcacacagcatcatctttggagaagcaaataggccaccccagtc6720
caccccctgaaaaaaaggagctgaggaaagtggcccatttaacaggcaagtccaactcaa6780
ggtccatgcctctggaatgggaagacacctatggaattgtcctgctttctggagtgaagt6840
ataagaagggtggccttgtgatcaatgaaactgggctgtactttgtatattccaaagtat6900
acttccggggtcaatcttgcaacaacctgcccctgagccacaaggtctacatgaggaact6960
ctaagtatccccaggatctggtgatgatggaggggaagatgatgagctactgcactactg7020
ggcagatgtgggcccgcagcagctacctgggggcagtgttcaatcttaccagtgctgatc7080
atttatatgtcaacgtatctgagctctctctggtcaattttgaggaatctcagacgtttt7140
tcggcttatataagctctaaggatccactagtaacggccgccagtgtgctggaattaatt7200
cgctgtctgcgagggccagctgttggggtgagtactccctctcaaaagcgggcatgactt7260
ctgcgctaagattgtcagtttccaaaaacgaggaggatttgatattcacctggcccgcgg7320
tgatgcctttgagggtggccgcgtccatctggtcagaaaagacaatctttttgttgtcaa7380
gcttgaggtgtggcaggcttgagatctggccatacacttgagtgacaatgacatccactt7940
tgcctttctctccacaggtgtccactcccaggtccaactgcaggtcgatcgagcatgcat7500
ctagggcggccgcactagaggaattcgcccctctccctcccccccccctaacgttactgg7560
ccgaagccgcttggaataaggccggtgtgtgtttgtctatatgtgattttccaccatatt7620
gccgtcttttggcaatgtgagggcccggaaacctggccctgtcttcttgacgagcattcc7680
taggggtctttcccctctcgccaaaggaatgcaaggtctgttgaatgtcgtgaaggaagc7740
agttcctctggaagcttcttgaagacaaacaacgtctgtagcgaccctttgcaggcagcg7800
2 gaaccccccacctggcgacaggtgcctctgcggccaaaagccacgtgtataagatacacc7860
0
tgcaaaggcggcacaaccccagtgccacgttgtgagttggatagttgtggaaagagtcaa7920
atggctctcctcaagcgtagtcaacaaggggctgaaggatgcccagaaggtaccccattg7980
tatgggaatctgatctggggcctcggtgcacatgctttacatgtgtttagtcgaggttaa8040
aaaagctctaggccccccgaaccacggggacgtggttttcctttgaaaaacacgatgata8100
2 agcttgccacaacccg 8116
5