Note: Descriptions are shown in the official language in which they were submitted.
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
hIPID-BI7~AYER ARRAYS AND METHODS OF MAKING AND USING SAME
This application was supported in part by a grant from
the NSF Biophysics Program, and by the MRSEC Program of the
NSF under Award DMR-9808677. Accordingly, the United States
Government has certain rights in this invention. This
invention was made with Government support under contracts
DMR-9808677, DCB-9807559 awarded by the National Science
1o Foundation. The Government has certain rights to this
invention
Field Of The Invention
The present invention relates to arrays of separated or
discreet lipid-bilayer regions on a substrate, and to methods
of making and using such arrays.
r_r_______
1) Bayerl, T.M. and Bloom, M., Biophys. J. 58:357-362,
( 1990 ) .
2) Johnson, S.J., et al., Biophys. J., 59:289-294,
(1991) .
3) Koenig, B.W., et al., Langmuir 12:1343-1350, (1996) .
4) Sackmann, E., Science 271:43-47, (1996).
5) Brian, A. and McConnell, H.M., Proc. Natl. Acad. Sci.
81:6159-6163, (1984).
6) Song, X.D. and Swanson, B.I., Langmuir 15:4710-4712,
(1999).
7) van Oudenaarden, A. and Boxer, S.G., Science
285:1046-1048, (1999).
8) Groves, J.T., et al., Proc. Natl. Acad. Sci. 95:935-
938, (1998).
9) Groves, J.T., et al., Proc. Natl. Acad. Sci.
94:13390-13395, (1997).
1
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
10) Groves, J.T. and Boxer, S.G., Biophys. J. 69:1972-
1975, (1995) .
11) Groves, J.T., et al., Science 275:651-653, (1997).
12 ) Groves, J. T . , et a1. , Langmuir 14 : 3347-3350, ( 1998 ) .
13) Cremer, P.S., et al., Langmuir 15:3893-3896, (1999).
14) Cremer, P.S. and Boxer, S.G., J. Phys. Chem. B
103:2554-2559, (1999).
15) Radler, J., et al., Langmuir 11:4539-4548, (1995).
16) Kumar, A., et al., Langmuir 10:1498-1511, (1994).
17 ) Bernard, A. , et a1. , Langmuir 14 : 2225-2229, ( 1998 ) .
18) James, C.D., et al., Langmuir 14:741-744, (1998).
19) Wilkinson, C.D.W., et al., J. Vac. Sci. Technol. B
16:3132-3136, (1998).
20) We used the same lipids as in reference 14;
different lipid compositions may expand to a different
extent.
21) Stelzle, M., et al., Biophys. J. 63:1346-1354,
(1992).
22) Chan, P.Y., et al., J. Cell Biol. 115:245 (1991).
23) Slender, et al., Biosensors and Bioelectronics
11:565-577 (1996).
24) Khuner, et al., Biophys J. 67:217-226 (1994).
Background Of The Invention
The ability to both functionalize and pattern surfaces
is of widespread interest. Lipid bilayers on solid supports
are especially challenging because they are two-dimensional
fluids. When bilayers are assembled on glass supports, they
are cushioned by a thin (5 - 20 A) layer of water, so that
3o both leaflets retain the fluidity that is an essential
feature of biological membranes. The result is that the
components are continually mixing and are free to diffuse
across the entire surface. Such supported membranes retain
many of the physical properties of natural cell membranes,
and they can interact with living cells if the necessary
2
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
components are present. Thus, the functionalization and
patterning of fluid supported lipid bilayers has applications
in the fabrication of biosensors to achieve separations, for
fundamental studies of membrane biophysics, and to construct
an interface between hard surfaces and living cells.
One of the present inventors has previously reported
methods for partitioning discreet regions of supported
bilayers on a planar substrate surface. In one earlier-
reported method, a supported bilayer expanse is partitioned
1o by scratching the surface of a previously assembled
continuous bilayer. The scratches function as barrier to
lateral diffusion by a combination of topographical and
tribological interactions. A second method involves
patterning the properties of the solid support using photo-
or electron beam lithography before the membrane is
assembled. Depending on the chemical composition of the
patterned material, lipids either do not assemble on the
patterned regions or they assemble but are immobile.
In either case, the effect of patterning is to confine
diffusive mixing to the corralled regions. Scratching is a
simple method to apply, but it is not well controlled or
entirely understood. Surface patterning requires the
application of a second material to the surface, and the
interaction of the lipids with the materials used is poorly
understood. In both cases, the glass support must be
chemically and/or physically altered.
Summary Of The Invention
The present invention includes, in one aspect, a method
of forming a pattern of separated lipid regions on a
substrate. The method includes the steps of forming over a
planar portion of a substrate, a lipid-bilayer expanse
sandwiched between a lower aqueous film and an upper aqueous
bulk phase. A blotter having an embossed pattern of surface
projections that (i) have contact surfaces capable of
3
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
supporting lipid-bilayer formation thereon, and (ii) form
separated regions bounded by such surfaces, is applied to the
bilayer expanse, serving to transfer regions of the lipid-
bilayer expanse on the substrate to the blotter's contact
surfaces. Removing the blotter leaves a pattern of separated
lipid regions corresponding to the separated regions formed
by the blotter projections.
In another aspect, the blotter to which portions of the
lipid bilayer have been transferred is used to stamp a planar
1o substrate covered by an aqueous medium, thereby to deposit on
the substrate surface, a pattern of lipid bilayer regions
corresponding to the pattern of surface projections in the
blotter.
In both methods, the first substrates are formed of a
material suitable for lipid-bilayer formation over an aqueous
film, such as Si02, MgF2, CaF2, or mica. The blotter is formed
of a material capable of picking up a lipid-bilayer, such as
polydimethyl siloxane (PDMS), or surface-oxidized PDMS.
In a related aspect, the blotter to which portions of
2o the lipid bilayer have been transferred is used to stamp a
print medium, thereby to transfer the lipid-bilayer pattern
to the print medium. The medium may be further developed to
produce a colored pattern where the lipids were deposited.
Also disclosed is a surface-patterned device composed of
a substrate having a planar surface, and formed on this
surface, a pattern of lipid-bilayer regions sandwiched
between a lower aqueous film and an upper aqueous bulk phase.
The lipid regions are stably separated from one another by
self-limiting lateral diffusion, without physical barriers
3o between the regions on the substrate surface.
The substrate is formed of a suitable material for
supporting lipid-bilayer regions over an aqueous film, such
as SiOz, Mg F2, CaF2, or mica.
The lipid bilayer is formed of one or bilayer-forming
lipids, such as phosphatidylcholine,
4
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
phosphatidylethanolamine, phosphatidylserine, phosphatidic
acid, phosphatidylinositol, phosphatidylglycerol, and
sphingomyelin, and optionally, bilayer-compatible lipids such
as sterols and fatty acids. The device may contain 103 or
more discrete bilayer-compatible surface regions, and the
regions may be separated from one another by distances
between 1 um and about 10 um.
The device may be designed for use in detecting binding
events between one or more analytes and one or more selected
lipid-bilayer-anchored biomolecules. Exemplary biomoelcules
are transmembrane receptors or ion channels. Where the
biomolecules include polynucleotides, the regions are in the
form of an array of discreet regions, each carrying a
different polynucleotide at a different region.
Also forming part of the invention is a method for use
in detecting binding events between one or more analytes and
one or more selected lipid-bilayer-anchored biomolecules.
The method includes the steps of contacting a mixture
containing such analyte(s) with a surface detector array
2o device of the type just described and detecting binding of
the selected ligand to receptors which specifically bind it.
In still another aspect, the invention includes a
microfabrication process for producing a selected pattern on
the surface of a substrate. The method includes the steps of
forming, over a planar portion of such a substrate, a pattern
of lipid-bilayer regions sandwiched between a lower aqueous
film and an upper aqueous bulk phase. The pattern of lipid-
bilayer regions corresponds to the selected pattern, and the
lipid-bilayer regions are stably separated from one another
3o by self-limiting lateral diffusion, without physical barriers
between the regions on the substrate surface. Once the
pattern of lipid-bilayer regions is formed on the substrate,
the substrate is further processed to achieve the desired
selected pattern.
5
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
In another aspect, the invention includes a method of
forming an array of separated lipid regions on a substrate,
by the steps of forming lipid-bilayer surface regions on the
coplanar surfaces of a blotter of the type described above,
and the transferring the lipid regions to a substrate having
bilayer-compatible substrate regions separated by barriers.
The transferring is effective to partially fill the substrate
regions with lipid bilayers. Additional bilayer-forming
lipids are then added to the substrate regions, to fill each
substrate surface region with a lipid bilayer.
These and other objects and features of the invention
will become more fully apparent when the following detailed
description is read in conjunction with the accompanying
drawings.
Brief Description Of The Figures
Fig. 1 shows a portion of a patterned substrate device
formed in accordance with one aspect of the invention;
Figs. 2A through 2H are schematic illustration of two
2o methods used to create patterned bilayer and bilayer-free
regions on a substrate, in accordance with the invention,
where Fig. 2A illustrates patterning by blotting and Fig. 2B,
patterning by stamping;
Figs 3A through 3D are epifluorescence images of a
supported lipid bilayer formed by embossing, shown after
waiting for 30 minutes for self-limiting lateral expansion to
occur into the regions where lipid was removed (Fig. 3A), and
after an electric field (11V/cm and 1~A) was applied parallel
to the bilayer plane for 65 minutes creating a steady-state
gradient in the concentration of the negatively-charged
Texas-red labeled lipid component (Fig. 3B);
Figures 5A and 5B depict partial-fill and in-fill
perspective views.
6
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
Figs 4A and 4B are epifluorescence images of a supported
lipid bilayer formed by stamping, where the image was taken
immediately after photobleaching (Fig. 4A), and 46 minutes
after photobleaching (Fig. 4B) showing partial recovery of
the bleach as a result of lateral diffusion within the
printed line; and
Figs. 6A through 6C depict steps in the preparation of a
patterned substrate device constructed in accordance with
another embodiment of the invention.
Figs. 7A through 7C represent a top down view of the
bilayer arrays resulting from the practice of the present
invention, for example as depicted in figs. 6A-6C.
Detailed Description Of The Invention
I. Definitions
The terms below have the following meanings unless
indicated otherwise.
The term "aqueous" refers to a water-based liquid medium
that is not deleterious to lipids.
The term "aqueous thin film" refers to a film of aqueous
medium, typically 5-20 angstroms, and preferably about 10
angstroms, between a substrate surface and a lipid-bilayer
region;
The term "aqueous bulk phase" refers to the layer of
aqueous medium covering the lipid-bilayer regions on a
substrate and extending into the lateral spaces between
separated lipid-bilayer regions.
A surface "expanse" of lipid-bilayer refers to a
substantially uninterrupted planar expanse of lipid-bilayer
3o film on the surface of a substrate. The "expanse" is
partitioned into a plurality of separated lipid-bilayer
"regions" in accordance with the invention.
A "receptor" is a macromolecule capable of specifically
interacting with a ligand or analyte molecule. In cells,
receptors are typically associated with lipid bilayer
7
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
membranes, such as the extracellular, golgi or nuclear
membranes. Receptors for incorporation into expanses of
lipids in vitro (e.g., supported bilayers) may either be
purified from cells, recombinantly expressed, or, in the case
of small receptors, chemically synthesized.
A "ligand" or "analyte" is a molecule capable of
specifically binding to a receptor. Binding of the ligand to
the receptor is typically characterized by a high binding
affinity, i.e., Km>105, and can be detected either as a
1o change in the receptor's function (e.g., the opening of an
ion channel associated with or part of the receptor) or as a
change in the immediate environment of the receptor (e. g.,
detection of binding by surface plasmon resonance). Ligands
for incorporation into expanses of lipids in vitro (e. g.,
supported bilayers) may either be purified from cells,
recombinantly expressed, or, in the case of small ligands,
chemically synthesized.
Binding is "specific" if it results from a molecular
interaction between a binding site on a receptor and a
ligand, rather than from "non-specific" sticking of one
protein to another. In cases where the ligand binds the
receptor in a reversible manner, specificity of binding can
be confirmed by competing off labeled ligand with an excess
of unlabeled ligand according to known methods. Non-specific
interactions can be minimized by including an excess of a
protein (e.g., BSA) that does not have binding sites for
either the ligand or receptor.
II. Surface-Patterned Device
The present invention exploits the discovery herein that
lipid-bilayers can form stable, self-limiting monolayers on a
substrate surface, in effect, limiting the size of separated
lipid-bilayer regions by the lateral diffusion. Membrane-
forming lipids, when applied to a substrate surface, can
assemble into bilayers as a result of a balance of
8
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
hydrophobic interactions, interfacial surface tension and
repulsive intermolecular interactions; the interaction
between planar bilayers and oxide supports involves a balance
of van der Waals, electrostatic, hydration, and steric
forces. As a consequence of the balance among these forces,
supported lipid bilayers can expand only over a limited range
on the surface. Lateral expansion does depend on pH: at high
pH expansion is arrested, while at low pH it is initially
quite rapid but then slows down over the course of a few
minutes. The pH effect is likely related to the protonation
state of the substrate, e.g., glass surface and the resulting
water structure at the interface.
Expansion is ultimately self-limiting because the lipids
lose favorable interactions with each other. In one aspect,
the invention exploits this self-limiting lateral expansion
by gently removing bilayer material using a patterned polymer
stamp. Once material is removed, the remaining supported
membrane expands laterally, but the expansion halts leaving
an essentially free-standing, but bounded, stable, and fluid
2o region of bilayer material. Remarkably, it also proves
possible to transfer the material that is removed onto a
fresh surface, thereby stamping fluid bilayers in any desired
pattern.
Fig. 1 is a perspective view of a portion of a surface-
patterned device 20 constructed in accordance with the
invention. The device is fabricated from a substrate 22
whose surface 24 is formed of a material such as an oxidized
silicon or fused silica wafer. Exemplary dimensions of the
substrate are typically between about 0.5 cm to about 5 cm
3o per side and about 0.1 mm to about 1 cm in thickness.
Carried on the substrate surface are a plurality of
separated lipid-bilayer regions, such as regions 26, 28,
separated by channels, such as channel 30. The separated
regions may be completely separated from one another, e.g.,
completely surrounded by separating channels, or adjacent
9
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
regions may be joined by lipid-bilayer "bridges" that may
allow lipid diffusion between joined regions, but which are
stably formed by virtue of self-limiting diffusion of the
lipids making up the bridge.
Interposed between each lipid-bilayer region and the
underlying region of the substrate surface is an aqueous film
32 that is between about 5 A and 20 A (typically about 10 A)
in thickness. The degree of "bilayer-compatibility" of a
selected surface is a function of its intrinsic material
1o properties rather than its shape. The interactions between
lipid-bilayers and a substrate involve electrostatic and
hydration forces as well as attractive contributions from
long-range van der Waals forces. These forces act to
stabilize the single lipid bilayer, and to anchor the bilayer
region stably to the surface, to prevent the regions from
migrating laterally on the substrate surface. In a suitable
bilayer-compatible surface, an energetic minimum traps the
bilayer membrane between about 5 A and 20 A (typically about
10 A) away from the supporting surface, separated from the
2o supporting surface by an aqueous film of corresponding
thickness. Covering the lipid-bilayer and the channels
separating these regions is a bulk aqueous phase 34.
Bilayer-compatible surfaces are typically hydrophilic.
It will be appreciated that many materials suitable for use
in the microfabrication of a device according to the
invention will, when cleaned, present a bilayer-compatible
surface region. Exemplary materials having properties making
them suitable for bilayer-compatible surfaces include various
glasses, silicon oxides, including oxidized silicon (Si02),
MgF2, CaFz, mica, and various polymer films, such as thin
polyacrylamide or dextran films (see, e.g., 23; 24), both
incorporated herein by reference). Both types of polymer
films form a suitable bilayer-compatible surface that is
hydrated to provide a film of aqueous between the polymer
film and the supported bilayer membrane. Additional details
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
of suitable bilayer-compatible surfaces, and bilayer-barrier
regions are detailed in co-owned U.S. patent application SN
08,978,756, filed November 26, 1997, which is incorporated
herein.
The supported bilayer itself is a self-assembling, two-
dimensional fluid system, typically consisting of two opposed
leaflets of vesicle-forming lipid molecules. However, it can
be constructed as described below from any suitable membrane-
forming amphiphile. Most vesicle-forming lipids are long-
chain carboxylic acids, such as glycerides, having the
hydroxyl groups of the glycerol esterified with (i) fatty
acid chain(s), and (ii) a charged or polar moiety, such as a
phosphate-ester group. The vesicle-forming lipids are
preferably ones having two hydrocarbon chains, typically acyl
chains, and a polar head group. Long-chain carboxylic acids
with a phosphate group, or phospholipids, are particularly
well-suited for use with the present invention.
There are a variety of synthetic vesicle-forming lipids
and naturally-occurring vesicle-forming lipids, including the
2o phospholipids, such as phosphatidylcholine (PC),
phosphatidylethanolamine (PE), phosphatidylserine (PS),
phosphatidic acid, phosphatidylinositol (PI),
phosphatidylglycerol (PG), and sphingomyelin, where the two
hydrocarbon chains are typically between about 14-22 carbon
atoms in length, and have varying degrees of unsaturation.
The above-described lipids and phospholipids whose acyl
chains have varying degrees of saturation can be obtained
commercially or prepared according to published methods.
Other suitable lipids include glycolipids and sterols such as
3o cholesterol.
Preferred diacyl-chain lipids for use in the present
invention include diacyl glycerol, phosphatidyl ethanolamine
(PE) and phosphatidylglycerol (PG). These lipids are
preferred for use as the vesicle-forming lipid, the major
11
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
liposome component, and for use in the derivatized lipid
described below.
All of these phospholipids and others are available from
specialized suppliers of phospholipids (e. g., Avanti Polar
Lipids, Inc., Alabaster, Alabama) as well as from general
chemical suppliers, such as Sigma Chemical Co. (St. Louis,
MO ) .
The aqueous film and bulk aqueous phase may be any
suitable aqueous solution, such as a buffered saline solution
(e. g., PBS). The bulk solution can be readily changed
(taking care, of course, to keep the supported bilayer
submerged at all times) by, e.g., flow-through rinsing with a
solution having a different composition.
A number of different-dimension arrays of lipid regions
may be produced in accordance with the invention. They
include, as example, (i) a device containing a 1 cm2 array of
2500 identical 200 um square corrals or regions, (ii) a
device containing a 1 cm2 array of 10,000 identical 100 um
square regions, (iii) a device containing a 1 cm2 array of
about 37,000 identical 50 um square regions separated by 2 um
channels, and (iv) a device containing a 1 cm2 array of about
2.8 million 5 um square corrals or regions separated by 1 um-
wide channels.
Exemplary embodiments of the invention include devices
where the bilayer lipid expanses contain different
biomolecules, such as receptor protein molecules, ligand
protein molecules, or other protein molecules. Such devices
are particularly useful in biosensors, described more fully
in the applications section of the specification, and are
3o made as described below by fusing proteoliposomes to the
bilayer-compatible surface.
It is recognized that proteoliposome vesicles can be
fused to a glass surface to create a planar supported
membrane (5). This technique has been successfully applied
in a number of situations. In one example, the H2Kk protein
12
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
was reconstituted into egg phosphatidylcholine- cholesterol
vesicles by detergent dialysis, and the vesicles were used to
create a planar membrane on glass (5). The H2Kk-containing
membrane was capable of eliciting a specific cytotoxic
response when brought into contact with a cell.
Chan, et a1. (22) demonstrated that a
glycosylphosphatidylinositol (GPI)-anchored membrane receptor
is laterally mobile in planar membranes formed from
proteoliposome fusion, and that this mobility enhances cell
adhesion to the membrane. Other applications employ a
combination of vesicle fusion, Langmuir-Blodgett methodology
and derivatized surfaces to prepare supported membranes.
In addition to incorporation of receptors or ion
channels into the bilayer membrane, the bilayer may be
derivatized with any of a number of groups or compounds to
create a surface having the desired properties. For example,
the liposomes may contain a ligand bound to the surface of
the lipid by attachment to surface lipid components.
Generally, such a ligand is coupled to the polar head group
of a vesicle-forming lipid. Exemplary methods of achieving
such coupling are described below.
It will be appreciated that the device is designed,
e.g., by microfabication, to include other elements needed
for carrying out the intended function of the device. For
example, in a device designed for use as a biosensor, the
substrate regions supporting the separated lipid-bilayer
regions may be electrodes, e.g., connected to the substrate
surface through a salt bridge, for measuring ion flow across
the membrane regions, for example, where the membrane regions
3o are constructed to contain ion channels. Alternatively, a
device for measuring transmembrane currents may include pH
sensitive dyes or the like that can be used to monitor ion
flow across the membrane. In still another embodiment , the
device is designed for measuring analyte binding to one or
more different biomolecules incorporated into the separated
13
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
bilayer regions. Here the device may be used in combination
with an optical scanning device for measuring binding of an
optically active analyte to one or more regions in the
device.
III. Construction of A Surface-Patterned Device
The invention contemplates three different methods for
forming surface-patterned arrays of lipid-bilayers regions on
a substrate.
to A. Blotting The first of these methods is referred to
as "blotting" and is illustrated in Fig. 2A. The method
involves first forming a continuous lipid-bilayer expanse on
a preferably planar substrate 42. Methods of forming the
lipid expanse are detailed elsewhere, including references 10
and 14, and as detailed in co-owned US patent application SN
08/978,756.
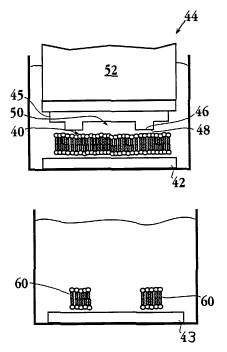
As shown in Figure 2B, there is then applied to the
expanse, a blotter 44 having an embossed pattern of surface
projections, such as projection 46 that (i) have contact
surfaces, such as surface 48, capable of supporting lipid-
bilayer formation thereon, and (ii) form separated regions,
such as region 50, bounded by such surfaces. The blotter is
preferably formed of a polymeric material, and a preferred
material is polydimethylsiloxane (PDMS), which may be surface
oxidized, e.g., by plasma treatment. The blotter also
contains a weight 52 for holding the blotter against the
substrate in a submerged condition.
According to an important aspect of the invention, this
blotting is effective to transfer lipid-bilayers regions in
3o contact with the contact surface48 to such surfaces, leaving
channels, such as channels 54 between now-separated lipid-
bilayer regions, such as regions 56, when the blotter is
removed. Removed lipid bilayer 60 may then be transferred to
a second glass surface 43, figure 2C, and deposited as shown
in figure 2D.
14
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
In another preferred embodiment is shown in figures 2E
through 2G, where bilayer material is pre-assembled from
vesicles 61 to ~~ink" oxidized PDMS surfaces 48 that are then
transferred to glass 42 by stamping to reliably transfer of
membrane patches 60 to glass 42.
As one exemplary method, vesicles were prepared from egg
phosphatidylcholine (egg PC) from Avanti Polar Lipids with 1
mol o N-(Texas Red sulfonyl)-1,2-dihexandeconoyl-sn-glycero-
3-phosphoethanolamine (Texas Red DHPE) from Molecular Probes.
The preparation of supported lipid bilayer from vesicles has
been outlined in detail elsewhere. The pH of the lipid
suspension was such that spreading is expected to occur. As
a result it is necessary to chose the amount of material
removed vs. amount of material left such that after expansion
a bilayer-free region remains. A Nikon E800 fluorescence
microscope equipped with a Photometrics Sensys CCD camera was
used to image the bilayers. Electrophoresis within the
membrane was performed using methods laid out previously, in
Millipore water with currents < 2~A which produces a
2o negligible amount of resistive heating.
Polydimethyl siloxane (PDMS) stamps were formed by
curing Sylgard 184 (Dow Corning) on silicon masters with
patterned photoresist. More preferably, Sylgard 182 may by
used because it does not harden below 65°C, suggesting that
the softness of the PDMS is important. The masters were
created using Shipley 3612 positive photoresist, 1~m thick,
on silicon wafers that were vapor primed with
hexamethyldisilazane. The patterning was achieved using
standard photolithographic techniques. The PDMS is a square
grid l~tm high; the width of the grid lines is 15 ~m and the
lines are 215 ~m apart. Using this stamp to emboss a lipid
bilayer should yield grid lines that are about 3 ~m wide and
227 ~tm apart .
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
Figure 3A depicts an epifluorescence image of a
supported lipid bilayer after it was embossed for ten minutes
in ultrapure water. The image was taken approximately 30
minutes after embossing, at which point the bilayer has
essentially finished expanding into the region where the
membrane was removed. A dark grid pattern is clearly visible
in the image where the lipids have been removed, and the
pattern was shown to be stable under water for at least one
week. The expansion of the bilayer is responsible for the
1o ragged edges of the pattern, as the expansion front of a
bilayer proceeds in finger-like projections on glass
surfaces. The fluorescence intensity in the grid (dark)
areas is very close to background levels, indicating that
lipid material has been nearly completely removed from the
grid area, that is, both leaflets of the bilayer appear to be
removed. The grid lines are approximately 19 ~tm wide rather
than the expected 3 Vim. However, as one of the properties of
PDMS is that it is deformable, it would not be surprising if
the area of the PDMS in contact with the bilayer during
2o embossing was larger than expected.
To determine if this pattern functions as a barrier to
lateral diffusion we applied an electric field parallel to
the plane of the bilayer. Electrophoresis causes the
negatively charged Texas Red-labeled lipids to be drawn
towards the positive electrode, forming a gradient at steady
state as they build up against the boundary of a patterned
region. Figure 3B depicts an image of the same area as
Figure 3A after an electric field of 11 V/cm and 1 (A was
applied for 65 minutes. A concentration gradient of bright
3o fluorescence is seen on the right side of each partitioned
region demonstrating that the lipids are mobile within the
square corrals but are confined. Upon removing the field,
the lipids relax back to uniformity; the electric field
direction can be reversed and the gradient forms along the
16
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
opposite side. Thus, it is seen that the bilayer-free
regions created by removing lipids with the PDMS stamp serve
as stable barriers to lateral diffusion. Fluorescence
recovery after photobleaching (FRAP) experiments were also
performed (data not shown), and these demonstrated recovery
within the patterned regions but not across boundaries.
Figure 3E depicts a pattern made by depositing small
bilayer regions onto surfaces, separated by relatively larger
barrier regions. Figure 3F depicts bilayer regions
surrounded by similarly sized barrier regions. Figures 3G
through 3I depict photographs of fluorescent material
migrating under the effect of an applied electric field
across the pan-handle shaped bilayer region. This
demonstrates that the shape of a bilayer region may be used
in conjunction with external forces to concentrate, dilute,
mix, or transfer materials.
An interesting situation arises at the outside borders
of the embossed region. A stamp half the size of the
bilayer-covered region was used, so that only half the
2o bilayer area was patterned. Because the patterned areas at
the borders were adjacent to a larger reservoir of material
then those in the interior, as the bilayer expands the
barriers at the edges should be and are erased. This result
means that all that is necessary to create barriers to
diffusion is to remove enough material so that after the
subsequent expansion the bilayer regions are left
unconnected. Additionally, it is possible to erase all of
the barriers to diffusion by incubating with vesicles, i.e.
adding material back in. After incubation the lipids are
once again free to diffuse across the entire surface.
B. Stamping In this method, which is illustrated in
Figs. 2A through 2D, lipid-bilayer regions are transferred
from a first substrate covered with a planar lipid expanse,
as above, to the contact surfaces of a blotter 44, to produce
a pattern of bilayer regions, such as regions 60, on the
17
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
coplanar blotting surfaces of the blotter, as shown. The
step of forming the bilayers on the blotter surfaces may be
similar to the blotting method described above, where the
blotter is applied to a planar lipid expanse on a substrate,
or may be by direct formation of lipid bilayer regions on the
blotter surfaces. In this second method, vesicles are placed
in contact with the blotter surfaces under water, e.g., for
at least an hour, until a layer of lipid bilayer forms on the
exposed surfaces. Excess vesicles are then removed, e.g., by
1o shaking them off the blotter.
In the final step of the method, shown in Fig. 2D, the
blotter is stamped on second substrate 43, to transfer the
lipid-bilayer regions on the blotter surfaces to the
substrate.
Figure 4A shows epifluorescence images of the material
that is stamped using the first inking procedure and a grid
pattern that is identical to that shown in Figure 3A. The
bright pattern of fluorescence corresponds to the region that
was removed in Figure 3A, giving further evidence that the
2o embossing method actually removes bilayer material. In order
to test whether the transferred lipids are themselves
assembled into a fluid bilayer, a region of the fluorescence
just below the center (Figure 4A) was photobleached. Figure
4B is the same region 46 minutes later, and it is evident
that much of the fluorescence in the bleached region has
recovered. Close inspection indicates that recovery is not
complete; we suspect that the removal and deposition
processes as currently practiced leave some regions that are
not fully covered, though sufficiently connected to permit
long-range diffusion. The fluorescence level of the
transferred lipids is approximately 60o that of the
fluorescence level of the surface from which the lipids were
removed; of the 400 lost approximately loo was lost in the
removal and 30o was lost in the stamping. We have done
little to optimize this system, and variations in the
18
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
methods, the stamp material or the stamp topography may prove
fruitful. Similar results were obtained with the second
method. We note in passing that there is an inversion of the
membrane leaflet that is in contact with the receiving
surface in these two methods. In the first method, the side
of the supported membrane originally in contact with the
glass remains in contact after transfer and printing. In the
second method, where the assembly is initially on the PDMS
surface, the transfer should place the side that is in
1o contact with the bulk solution when on PDMS in closest
contact with the receiving glass surface. This may prove
useful for inverting the orientation of self-assembled
systems.
Patterns formed by stamping could also be erased by
incubating with vesicles as was the case for patterns formed
by embossing. The addition of more material to a surface
that is clean except in the patterned region, is another
demonstration that the patterns are maintained by the self-
limiting lateral expansion of supported lipid bilayers.
2o C. Lipid-Region Augmentation A third method of forming
lipid regions is illustrated in Figs. 5A, 5B, and 5C. In
this method, discreet lipid regions are formed on the contact
surfaces of a blotter, as above. These lipid regions are
then transferred by stamping to corresponding regions of a
substrate, such as substrate 70 in Figs. 5A, 5B, and 5C.
Preferably, the substrate is one like that described in co-
owned US patent application SN 08/978,756, having lipid-
bilayer compatible surface regions, or lipid regions, such as
regions 72, 74, separated by lipid-bilayer barriers, such as
3o barrier 76. Contact surfaces 46a and 46b on blotter 46
preferably have a one-to-one correspondence with lipid
regions72 and 74 on the substrate, and can be placed in
registry therewith, such that each blotter contact surface
deposits its lipid bilayer on a corresponding lipid region of
the substrate.
19
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
As illustrated in Fig. 5A, the lipid transfer stamping
is effective to partially fill each substrate lipid,
producing islands of lipid bilayers within each region.
According to one aspect of this method, the amount of lipid
transferred to each of the substrate regions in the blotter-
transfer step is selectively varied, by selectively varying
the surface area of each of contact surface of the blotter.
Thus, in Figs. 5A, it might be assumed that the four areas
shown have increasing amount of lipid, in progressing in
to right-to-left and back-to-front directions. Figs. 7A shows a
top down view of such distribution of different amounts of a
first lipid material into different, spaced-apart locations.
Fig. 6A depicts the "inked" stamped method disclosed above,
resulting in Fig 6B patches 60 spatially distributed on
surface 42 separated by barrier regions. In-fill is depicted
in figure 6C by the addition of lipid vesicles 61 which
contact patches 60 and/or surface 42 and fill in the bilayer
region up to the point of the bilayer barriers. Referring
back to Figures 7A through 7C, Fig. 7A is a top down view of
the step depicted in figure 6B. Figures 7B represents a top
down view of Fig. 6C, illuminating only lipid component
attributable to patches 60. Figure 7C depicts the
illumination of the in-fill lipid vesicle material. Thus,
taken together, Figures 7A through 7C demonstrate how the
instant invention provides methods and devices for creating
arrays of independent, supported lipid bilayer regions,
supported by barrier regions, wherein each bilayer region
comprises a different lipid composition or ratio or lipids.
Following the transfer step, the substrate is exposed to
lipid vesicles effective to form lipid-bilayer regions that
"fill in" or complete the lipid coverage in each of the
substrate's lipid regions as shown in Fig. 5B. Figs. 5e and
5f, under different illumination conditions, show the amounts
of the first and second bilayer forming materials in each
location. This can be done, for example, by exposing the
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
partially filled substrate to lipid vesicles, similar to the
producing of planar expanses on the substrate as discussed
above. With this filling in, each lipid region on the
substrate, such as regions 72, 74, is covered with a lipid
bilayer region having a defined ratio of lipid material
deposited by blotter transfer to lipid material subsea_uently
added.
The method is useful, for example, in producing arrays
with different selected amount of two or more lipids, and/or
different ratios of lipid/non-lipid biomolecules, e.g.,
lipid/receptor protein. In the latter case, for example, a
blotter having different-size contact surface areas might be
prepared with a lipid-bilayer containing a fixed ratio of
lipid/protein. When this material is transferred to the
substrate, different selected amount of lipid and protein are
transferred to the different-sized blotter surface regions,
and thus different selected amounts of protein are
transferred to the individual substrate lipid regions.
Following lipid addition to the substrate, each region will
2o have a different selected lipid/protein ratio.
Other methods of forming lipid-bilayer regions with
selected ratios of components are also contemplated, in
accordance with the invention. For example, a grid a small-
sized lipid regions can be blotted to remove different-
selected size interior regions. By filling in these interior
regions with lipid vesicles or by stamping from a blotter,
desired ratios of components can be deposited in each array
region.
The invention has a variety of uses in microfabrication,
3o and printing, as well as in diagnostic and drug-screening
applications, as can be appreciated by those skilled in the
art.
For example, in microfabrication, a pattern of lipid
regions formed in accordance with the invention could be used
to affix a non-lipid molecule, e.g., a protein, e.g., that
21
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
has a lipid-compatible moiety. After securing the
biomolecule to the surface, e.g., with a fixative, the
original lipid pattern could be removed. Likewise, in
printing, a pattern of lipid bilayer regions formed on a
stamp can be transferred to a selected print medium, e.g.,
paper, then subsequently used to capture a, lipid-compatible
dye. After the dye is captured and affixed to the medium,
the lipids may be removed, e.g., by a wash step.
to Experimental
Small unilamellar vesicles were prepared from egg
phosphatidylcholine (egg PC, Avanti Polar Lipids) with 1 mol%
Texas Redo 1,2-dihexadecanoyl-sn-glycero- 3-
phosphoethanolamine, triethylammonium salt (Texas Red~ DHPE,
Molecular Probes) or 3 mol% Marina Blue~ 1,2-dihexadecanoyl-
sn-glycero- 3-phosphoethanolamine (Marina BlueO DHPE,
Molecular Probes). The appropriate amounts of each kind of
lipids were mixed together in chloroform, dried under N2, and
put under vacuum for at least 40 minutes. The lipids were
then reconstituted with Millipore (18MS2) water and were
passed 19 times through an Avanti extruder containing a
membrane with 50nm pores. The extruded vesicles were stored
at 4°C and were used within 3 days. It is preferable to use
50nM extruded vesicles prepared less than three days prior to
use, and more preferably, prepared just prior to use.
A Nikon E800 fluorescence microscope equipped with a
Photometrics Sensys CCD camera was used to image the
bilayers. To image the Texas Red fluorophores a Texas Red
filter (Chroma Technology Corp.) set was used; Marina Blue
3o fluorophore were imaged using a Cascade Blue filter set
(Chroma Technology Corp.). The Cascade Blue filter set
matches the spectrum of Marina Blue reasonably well.
Electrophoresis within the membrane was performed, using
methods laid out previously, in Millipore water with currents
22
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
< 2,uA which produces a negligible amount of resistive
heating. Diffusion coefficients of the fluorescent probes
were determined by Fourier analysis of the time evolution of
a fluorescence profile using a custom fitting program.
Polydimethyl siloxane (PDMS) stamps were formed by
curing Sylgard 182 (Dow Corning) on silicon masters with
patterned photoresist at 70°C for 80 minutes. The masters
were created using Shipley 3612 negative photoresist, lfun or
1.65,can thick, on silicon wafers, which were vapor primed with
hexamethyldisilazane. The patterning was achieved using
standard photolithographic techniques. After developing, the
wafers were vapor primed again with hexamethyldisilazane to
assist in the subsequent removal of the PDMS. Flat PDMS
stamps were formed by curing on silicon wafers that were
vapor primed with hexamethyldisilazane. Surface oxidation of
PDMS was carried out using a plasma cleaner (Harrick
Scientific) under high power for 15 to 60 seconds while a
small amount of air was leaked into the chamber. Glass
slides were prepared by washing in ICNx7 detergent (ICN,
2o Costa Mesa, CA) followed by exhaustive rinsing in distilled
water and then baking in a kiln at 400°C for at least 4
hours. It is preferable to use such prepared glass within
three days of cleaning.
Microcontact printing of the lipid bilayers was carried
out as follows. Within 30 minutes of oxidation the PDMS was
brought into contact with a solution of lipid vesicles
(either in water or mixed with 5mM Tris, 50mM NaCl, at pH 8
buffer) for 1 min, then the excess vesicle were washed away
with large amounts of water. More preferably, a 10 mM Tris,
100 mM NaCl, pH 8.0 can be used instead of 18 meg-ohm
ultrapure water. Keeping the PDMS in water at all times the
"inked" PDMS was then brought into contact, using a light
weight (5.2g), with a glass surface for preferably 10-15
seconds in a solution of 5mM Tris, 50mM NaCl, at pH 8Ø
23
CA 02388173 2002-03-14
WO 01/26800 PCT/US00/27774
Permanent grid patterns, which serve as barriers for
membrane partitioning, were produced on glass by microcontact
printing bovine serum albumin (BSA) as described in detail
elsewhere.9 In brief, a 201 solution of 80~.g/ml of Alexa488
labeled BSA (Molecular Probes) in a l7mM, pH 8.0 phosphate
buffer was placed on an oxidized PDMS stamp, allowed to sit
for lOmin, the excess was shaken off, and then the stamp was
dried under N2. The stamp was brought into contact with a
glass slide using a light weight (14g) for 30 seconds. The
1o slide was rinsed vigorously in deionized water to remove the
excess BSA and dried under N2.
While the invention has been described with reference to
specific methods and embodiments, it will be appreciated that
various modifications may be made without departing from the
invention.
24