Note: Descriptions are shown in the official language in which they were submitted.
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
VACCINE FOR THE PREVENTION AND TREATMENT OF ALZHEIMER'S AND
AMYLOID RELATED DISEASES
RELATED APPLICATIONS
This application claims the benefit of priority under 35 U.S.C. 119(e) to
copending U.S.
Provisional Application No. 60/168,594, filed on November 29, 1999, and under
35 U.S.C. 120
of copending U.S. application No. , filed on November 28, 2000, the entire
contents
of both applications are incorporated herein by reference.
BACKGROUND OF THE INVENTION
The present invention relates to a new stereochemically based "non-self"
antigen
vaccine for the prevention and/or treatment of Alzheimer's and other amyloid
related diseases.
Amyloidosis refers to a pathological condition characterized by the presence
of
amyloid fibers. Amyloid is a generic term refernng to a group of diverse but
specific protein
deposits (intracellular and/or extracellular) which are seen in a number of
different diseases.
Though diverse in their occurrence, all amyloid deposits have common
morphologic properties,
stain with specific dyes (e.g., Congo red), and have a characteristic red-
green birefringent
appearance in polarized light after staining. They also share common
ultrastructural features
and common x-ray diffraction and infrared spectra.
Amyloid-related diseases can either be restricted to one organ or spread to
several
organs. The first instance is referred to as "localized amyloidosis" while the
second is referred
to as "systemic amyloidosis".
Some amyloidotic diseases can be idiopathic, but most of these diseases appear
as a
complication of a previously existing disorder. For example, primary
amyloidosis can appear
without any other pathology or can follow plasma cell dyscrasia or multiple
myeloma.
Secondary amyloidosis is usually seen associated with chronic infection (such
as tuberculosis)
or chronic inflammation (such as rheumatoid arthritis). A familial form of
secondary
amyloidosis is also seen in Familial Mediterranean Fever (FMF). This familial
type of
amyloidosis, as one of the other types of familial amyloidosis, is genetically
inherited and is
found in specific population groups. In these two types of amyloidosis,
deposits are found in
several organs and are thus considered systemic amyloid diseases. Another type
of systemic
amyloidosis is found in long-term hemodialysis patients. In each of these
cases, a different
amyloidogenic protein is involved in amyloid deposition.
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
"Localized amyloidoses" are those that tend to involve a single organ system.
Different amyloids are also characterized by the type of protein present in
the deposit. For
example, neurodegenerative diseases such as scrapie, bovine spongiform
encephalitis,
Creutzfeldt-Jakob disease and the like are characterized by the appearance and
accumulation of
a protease-resistant form of a prion protein (referred to as AScr or PrP-27)
in the central
nervous system. Similarly, Alzheimer's disease, another neurodegenerative
disorder, is
characterized by neuritic plaques and neurofibrillary tangles. In this case,
the plaque and blood
vessel amyloid is formed by the deposition of fibrillar A(3 amyloid protein.
Other diseases such
as adult-onset diabetes (Type II diabetes) are characterized by the localized
accumulation of
amyloid in the pancreas.
Once these amyloids have formed, there is no known, widely accepted therapy or
treatment which significantly dissolves the deposits in situ.
Each amyloidogenic protein has the ability to organize into ~3-sheets and to
form
insoluble fibrils which get deposited extracellularly or intracellularly. Each
amyloidogenic
protein, although different in amino acid sequence, has the same property of
forming fibrils and
binding to other elements such as proteoglycan, amyloid P and complement
component.
Moreover, each amyloidogenic protein has amino acid sequences which, although
different,
will show similarities such as regions with the ability to bind to the
glycosaminoglycan (GAG)
portion of proteoglycan (referred to as the GAG binding site) as well as other
regions which
will promote (3-sheet formation.
In specific cases, amyloidotic fibrils, once deposited, can become toxic to
the
surrounding cells. As per example, the A(3 fibrils organized as senile plaques
have been shown
to be associated with dead neuronal cells and microgliosis in patients with
Alzheimer's disease.
When tested in vitro, A/3 peptide was shown to be capable of triggering an
activation process of
microglia (brain macrophages), which would explain the presence of
microgliosis and brain
inflammation found in the brain of patients with Alzheimer's disease.
In another type of amyloidosis seen in patients with Type II diabetes, the
amyloidogenic protein IAPP has been shown to induce (3-islet cell toxicity in
vitro. Hence,
appearance of IAPP fibrils in the pancreas of Type II diabetic patients could
contribute to the
loss of the ~3 islet cells (Langerhans) and organ dysfunction.
People suffering from Alzheimer's disease develop a progressive dementia in
adulthood, accompanied by three main structural changes in the brain: diffuse
loss of neurons
in multiple parts of the brain; accumulation of intracellular protein deposits
termed
neurofibrillary tangles; and accumulation of extracellular protein deposits
termed amyloid or
senile plaques, surrounded by misshapen nerve terminals (dystrophic neurites).
A main
-2-
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
constituent of these amyloid plaques is the amyloid-(3 peptide (A(3), a 40-42
amino-acid protein
that is produced through cleavage of the (3-amyloid precursor protein (APP).
Although
symptomatic treatments exist for Alzheimer's disease, this disease cannot be
prevented nor
cured at this time.
The use of a vaccine to treat Alzheimer's disease is possible in principle
(Schenk, D.
et al., (1999) Nature 400, 173-177). Schenk et al. show that, in a transgenic
mouse model of
brain amyloidosis (as seen in Alzheimer's disease), immunization with A(3
peptide inhibits the
formation of amyloid plaques and the associated dystrophic neurites. In that
study, a vaccine
using the human aggregated all-L peptide as immunogen prevented the formation
of (3-amyloid
plaque, astrogliosis and neuritic dystrophy in vaccinated transgenic mice.
However, it is apparent that there are a number of drawbacks to using an
endogenous protein as a vaccine (or a protein naturally present in the animal
being vaccinated).
Some of these drawbacks include:
~ Possible development of autoimmune disease due to the generation of
antibodies against "self" protein.
~ Difficulty in eliciting an immune response due to the failure of the host
immune
system to recognize "self" antigens.
~ Possible development of an acute inflammatory response.
SUMMARY OF THE INVENTION
The present invention relates to a stereochemically based "non-self" antigen
vaccine
for the prevention andlor treatment of Alzheimer's and other amyloid related
diseases. One aim
of the present invention is to provide a vaccine for the prevention and
treatment of Alzheimer's
and other amyloid related diseases, which overcomes the drawbacks associated
with using
naturally occurnng peptides, proteins or immunogens.
In an embodiment, a vaccine is provided which is produced using a "non-self"
peptide or protein synthesized from the unnatural D-configuration amino acids,
to avoid the
drawbacks of using "self" proteins. In accordance with the present invention,
the peptides need
not be aggregated to be operative or immunogenic as opposed to the prior art
vaccines.
In another embodiment, there is provided a method for preventing and/or
treating an
amyloid-related disease in a subject, which features administering to the
subject an antigenic
amount of an all-D peptide which elicits production of antibodies against the
all-D peptide, and
elicit an immune response by the subject, therefore preventing fibrillogenesis
and associated
cellular toxicity, wherein the antibodies interact with at least one region of
an amyloid protein,
-3-
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
e.g., (3 sheet region and GAG-binding site region, immunogenic fragments
thereof, protein
conjugates thereof, immunogenic derivative peptides thereof, immunogenic
peptides thereof,
and immunogenic peptidomimetics thereof. These vaccines may be used in the
prevention
and/or treatment of amyloid related diseases, and in the manufacture of
medicaments for
preventing and/or treating amyloid-related diseases.
In a further embodiment of the invention, a vaccine for preventing and/or
treating an
amyloid-related disease in a subject comprises an antibody which interacts
with amyloid
proteins to prevent fibrillogenesis, wherein the antibodies are raised against
an antigenic
amount of an all-D peptide interacting with at least one region of an amyloid
protein, e.g., (3
sheet region and GAG-binding site region, A(3 (I-42, all-D), immunogenic
fragments thereof,
protein conjugates thereof, immunogenic derivative peptides thereof,
immunogenic peptides
thereof, and immunogenic peptidomimetics thereof. These vaccines may be used
in the
prevention and/or treatment of amyloid related diseases, and in the
manufacture of
medicaments for preventing and/or treating amyloid-related diseases.
Still in a further embodiment, there is provided a method for preventing
and/or
treating an amyloid-related disease in a subject, which comprises
administering to the subject
an antigenic amount of an all-D peptide which interacts with at least one
region of an amyloid
protein, e.g., (3 sheet region and GAG-binding site region, A(3 (1-42),
immunogenic fragments
thereof, protein conjugates thereof, immunogenic derivative peptides thereof,
immunogenic
peptides thereof, and immunogenic peptidomimetics thereof, wherein the
compound elicits an
immune response by the subject and therefore prevents fibrillogenesis.
In a preferred embodiment of the present invention, the compound is a compound
of
Formula I:
R'-(P)-R" (I),
wherein
P is an all-D peptide interacting with at least one region of an amyloid
protein,
e.g., (3 sheet region and GAG-binding site region, A~3 (1-42, all-D),
immunogenic fragments thereof, immunogenic derivatives thereof, protein
conjugates thereof, immunogenic peptides thereof, and immunogenic
peptidomimetics thereof;
R' is an N-terminal substituent, e.g.:
~ hydrogen;
~ lower alkyl groups, e.g., acyclic or cyclic having 1 to 8 carbon atoms,
without or with functional groups, e.g., carboxylate, sulfonate and
phosphonate;
-4-
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
~ aromatic groups;
~ heterocyclic groups; and
~ acyl groups, e.g., alkylcarbonyl, arylcarbonyl, sulfonyl and phosphonyl
groups; and
R" is a C-terminal substituent, e.g., hydroxy, alkoxy, aryloxy, unsubstituted
or
substituted amino groups.
In an embodiment, R' and R" are identical or different, wherein alkyl or aryl
group
of R' and R" are further substituted with functionalities such as halide
(e.g., F, Cl, Br, and I),
hydroxyl, alkoxyl, aryloxyl, hydroxycarbonyl, alkoxylcarbonyl,
aryloxycarbonyl, carbamyl,
unsubstituted or substituted amino, sulfo or alkyloxysulfonyl, phosphono or
alkoxyphosphonyl
groups.
When the compound has an acid functional group, it can be in the form of a
pharmaceutically acceptable salt or ester. When the compound has a basic
functional group, it
can be in the form of a pharmaceutically acceptable salt.
In a preferred embodiment of the present invention, the subject is a human
being.
In yet another embodiment of the present invention, the amyloid related
disease may
be Alzheimer's disease.
In another embodiment of the present invention, there is provided a method for
preventing and/or treating of an amyloid related disease in a subject,
comprising administering
to the subject an antigenic amount of a compound of Formula I:
R'-(P)-R" (I),
wherein
P is an all-D peptide interacting with at least one region of an amyloid
protein,
e.g., (3 sheet region and GAG-binding site region, A(3 (1-42, all-D),
immunogenic fragments thereof, immunogenic derivatives thereof, protein
conjugates thereof, immunogenic peptides thereof, and immunogenic
peptidomimetics thereof;
R' is an N-terminal substituent selected from the group consisting of:
~ hydrogen;
~ lower alkyl groups, e.g., acyclic or cyclic having 1 to 8 carbon atoms,
without or with functional groups, e.g., carboxylate, sulfonate and
phosphonate;
~ aromatic groups;
-5-
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
~ heterocyclic groups; and
~ acyl groups, e.g., alkylcarbonyl, arylcarbonyl, sulfonyl and phosphonyl
groups; and
R" is a C-terminal substituent, e.g., hydroxy, alkoxy, aryloxy, unsubstituted
or
substituted amino groups.
In accordance with this method, the compound elicits an immune response by the
subject, preventing fibrillogenesis.
In accordance with a preferred embodiment of the present invention, there is
provided a vaccine for preventing and/or treating an amyloid-related disease
in a subject,
comprising an antigenic amount of an all-D peptide which interacts with at
least one region of
an amyloid protein, e.g., (3 sheet region and GAG-binding site region, A(3 (1-
42, all-D) peptide,
immunogenic fragments thereof, protein conjugates thereof, immunogenic
derivative peptides
thereof, immunogenic peptides thereof, and immunogenic peptidomimetics
thereof, wherein
the compound elicits an immune response by the subject and prevents
fibrillogenesis.
BRIEF DESCRIPTION OF THE DRAWING
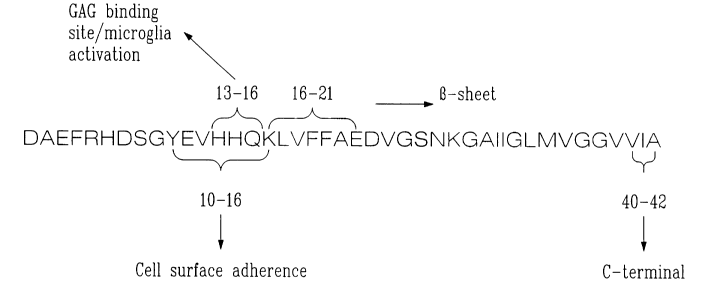
FIG. 1 illustrates the targeted sites for the antigenic fragments;
FIG. 2 illustrates the effect of 1 mg/ml of antibodies raised against D and L
forms of
A(3(16-21) on fibrillogenesis;
FIG. 3 illustrates the effect of 0.5 mg/ml of antibodies raised against D and
L forms
of A(3(16-21) on fibrillogenesis;
FIGS. 4A to 4C illustrate electron micrographs showing the effect of anti-D
KLVFFA peptide antibodies (FIG. 4B) and anti-L KLVFFA peptide antibodies(FIG.
4C) with
respect to a control (FIG. 4A) on fibrillogenesis;
FIGS. 5A to 5D illustrate the immunohistochemistry of anti-D KLVFFA on
aggregated A(3 peptide in brain sections of retrosplenial cortex (FIG. 5A) and
parietal cortex
(FIG. 5C) and the histochemistry (Thioflavin S assay) of anti-D KLVFFA on
aggregated A(3
peptide in the same brain sections of retrospinal cortex (FIG. 5B) and
parietal cortex (FIG. 5D);
FIGS. 6A to 6D illustrate the immunohistochemistry of anti-L KLVFFA antibodies
on aggregated A(3 peptide in brain sections of parietal cortex (FIG. 6A) and
entorhinal cortex
(FIG. 6C) and the histochemistry (Thioflavin S assay) of anti-L KLVFFA
antibodies on
aggregated A(3 peptide in the same brain sections of parietal cortex (FIG. 6B)
and entorhinal
cortex (FIG. 6D); and
-6-
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
FIG. 7 illustrates the response of rabbits to KLH-conjugated all-L and all-D
KLVFFA.
DETAILED DESCRIPTION OF THE INVENTION
For the purpose of the present disclosure, the following terms are defined
below.
The term "peptidomimetic" includes non-peptide compounds which mimic the
structural or the functional properties of a peptide.
The term "antigenic fragment thereof" includes fragments of peptides which are
capable of eliciting an immune response in a subject.
The term "amyloid related diseases" includes diseases associated with the
accumulation of amyloid which can either be restricted to one organ,
"localized amyloidosis",
or spread to several organs, "systemic amyloidosis". Secondary amyloidosis may
be associated
with chronic infection (such as tuberculosis) or chronic inflammation (such as
rheumatoid
arthritis), including a familial form of secondary amyloidosis which is also
seen in Familial
Mediterranean Fever (FMF) and another type of systemic amyloidosis found in
long-term
hemodialysis patients. Localized forms of amyloidosis include, without
limitation, diabetes
type II and any related disorders thereof, neurodegenerative diseases such as
scrapie, bovine
spongiform encephalitis, Creutzfeldt-Jakob disease, Alzheimer's disease,
Cerebral Amyloid
Angiopathy, and prion protein related disorders.
Except as otherwise expressly defined herein, the abbreviations used herein
for
designating the amino acids and the protective groups are based on
recommendations of the
ILJPAC-ILTB Commission on Biochemical Nomenclature (Biochemistry, 1972,
11:1726-1732).
The A(3(16-21) site is known to play an important role in initiating the
harmful
process of A(3 peptide amyloidogenesis. It is also known that when these
peptides are made
from D-amino acids, they retain their ability to interact with the natural all-
L-homologous
sequence, thereby preventing amyloidogenesis.
Other amyloid proteins which may be used in the present invention include,
without
limitation, IAPP, (32-microglubeline, amyloid A protein, and prion-related
proteins.
The vaccine of the present invention, prepared from all-D-A(3(16-21), D-A(3(10-
16),
D-A(3(1-40), D-A(3(1-42) or the C-terminal region of D-A(3(1-42), is believed
to elicit an
immune response in the host or in producing antibodies that recognize the
naturally occurring
target. As used herein, "all-D" includes peptides having >_ 75%, >_ 80%, >_
85%, >_ 90%, >_ 95%,
and 100% D-configuration amino acids. Also, the vaccine of the present
invention does not
present the drawbacks of using "self" proteins and does not need to be
aggregated to induce an
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
immune response. For example, the antibodies raised against the all-D-A(3(16-
21) peptide can
be expected to recognize the all-L-A(3(16-21) peptide sequence.
The elicited antibodies present in the host having received the vaccine of the
present
invention bind at the A(3(16-21) site or other sites such as the C-terminal
region of A(3 and have
the same or even greater ability to prevent amyloidogenesis as do the short
peptides themselves.
The vaccine of the present invention causes the generation of effective
antiamyloidogenic
antibodies in the vaccinated host.
A suggested immunization procedure is as follows:
a) prepare a vaccine from an all-D peptide having a sequence substantially the
same as that of a naturally occurnng (3 amyloid peptide, namely A~3 (all-L).
The
all-D peptide includes a full length A(3 (1-42, all-D), a peptide derived from
an
immunogenic fragment of A~3 (1-42, all-D), and a related peptidomimetic;
b) immunize a host with the vaccine to generate an antibody in the host with a
binding site capable of preventing fibrillogenesis.
Suitable pharmaceutically acceptable carriers include, without limitation, any
non-
immunogenic pharmaceutical adjuvants suitable for oral, parenteral,
intravascular (IV),
intraarterial (IA), intramuscular (IM), and subcutaneous (SC) administration
routes, such as
phosphate buffer saline (PBS).
The pharmaceutical carriers may contain a vehicle, which carnes antigens to
antigen-presenting cells. Examples of vehicles are liposomes, immune-
stimulating complexes,
microfluidized squalene-in-water emulsions, microspheres which may be composed
of
poly(lactic/glycolic) acid (PLGA). Particulates of defined dimensions (<5
micron) include,
without limitation, oil-in-water microemulsion (MF59) and polymeric
microparticules.
The carriers of the present invention may also include chemical and genetic
adjuvants to augment immune responses or to increase the antigenicity of
antigenic
immunogens. These adjuvants exert their immunomodulatory properties through
several
mechanisms such as lymphoid cells recruitment, cytokine induction, and the
facilitation of
DNA entry into cells. Cytokine adjuvants include, without limitation,
granulocyte-macrophage
colony-stimulating factor, interleukin-12, GM-CSF, synthetic muramyl dipeptide
analog or
monophosphoryl lipid A. Other chemical adjuvants include, without limitation,
lactic acid
bacteria, Al(OH)3, muramyl dipeptides and saponins.
The peptide may be coupled to a carrier that will modulate the half-life of
the
circulating peptide. This will allow the control on the period of protection.
The peptide-carrier
may also be emulsified in an adjuvant and administrated by usual immunization
route.
_g_
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
The vaccine of the present invention will, for the most part, be administered
parenterally, such as intravascularly (IV), intraarterially (IA),
intramuscularly (IIVI),
subcutaneously (SC), or the like. In some instances, administration may be
oral, nasal, rectal,
transdermal or aerosol, where the nature of the vaccine allows for transfer to
the vascular
system. Usually a single injection will be employed although more than one
injection may be
used, if desired. The vaccine may be administered by any convenient means,
including syringe,
trocar, catheter, or the like. Preferably, the administration will be
intravascularly, where the
site of introduction is not critical to this invention, preferably at a site
where there is rapid
blood flow, e.g., intravenously, peripheral or central vein. Other routes may
find use where the
administration is coupled with slow release techniques or a protective matrix.
The use of the vaccine of the present invention in preventing and/or treating
Alzheimer's disease and other amyloid related diseases can be validated by
raising antibodies
against the corresponding all-D peptide and testing them to see if they can
effectively inhibit or
prevent the fibrillogenesis of the natural amyloid peptide (all-L).
The compounds used to prepare vaccines in accordance with the present
invention
have the common structure of Formula I:
R'-(P)-R" (I),
wherein
P is an all-D peptide interacting with at least one region of an amyloid
protein,
e.g., (3 sheet region and GAG-binding site region, A(3 (1-42, all-D),
immunogenic fragments thereof, immunogenic derivatives thereof, protein
conjugates thereof, immunogenic peptides thereof, and immunogenic
peptidomimetics thereof;
R' is an N-terminal substituent selected from the group consisting of:
~ hydrogen;
~ lower alkyl groups, e.g., acyclic or cyclic having 1 to 8 carbon atoms,
without or with functional groups, e.g., carboxylate, sulfonate and
phosphonate;
~ aromatic groups;
~ heterocyclic groups; and
~ acyl groups, e.g., alkylcarbonyl, arylcarbonyl, sulfonyl and phosphonyl
groups; and
R" is a C-terminal substituent, e.g., hydroxy, alkoxy, aryloxy, unsubstituted
or
substituted amino groups.
-9-
10-01-2002 CA0001413
CA 02388559 2002-05-15
JAN. 10.2002 10;52p.M S'~NABEI OGILV'f MTL 514 288 8389 N0. 0925 P. x/22
R' and R" may be identical or different; the alkyl or aryl group of R' end R"
may fiuther be
' substituted with organic funetionalities selected fi-om the group of halides
(F, Cl, Hr, and n, hydroxyl, ,
alkoxyl, aryloxyl, hydroxycerbonyl, alkoxylcarbonyl, aryloxycarbonyl,
carbarnyl, uneubstituted or
substituted amino, sulfo or sUcylwcysulfonyl, phosphvno or alkoxyphosphonyl,
and the like. .
Where a functional group is an acid, its pharmaceutically acceptable salt or
ester is in the
scope of this in~cntion. Where a functional group is a base, its
pharmaceutically acceptable salt is in
the scope of this invention.
In one embodiment, the preferred compounds are selected from the foil-length
peptide, A~
(1~2, all-D), and its lower homologues consisting of A~i (1-40, all-D), A~i (1-
35, ail-D), atld A~ (1-28, .
all-D). v
In another embodiment, the preferred compounds are selected from a goup of
short ,
peptides, e.g., A[3 (1-7, all-D), A~ (10-15, all-D), Ap (16-21, all-D), Ap (36-
42, all-D). The peptides .
can be shortened fiu~ther by removing one or more residues from either rnd or
both ends.
'fhc preferred compounds may also be all-D peptides derived from the peptides
above by
substitution of oae or more residues in the naturally occurring sequence. In
another embvdimem, the ,
preferred compounds are peptidomimetics of the above-said peptides.
tn a further embodiment, the preferred cornpoUnds may be coupled with a
carrier that wiU
modulate the biodistr~bution, immunogeaic property and the half life of the
coznpvunds.
The following are exemplary compounds for use in the manufacture of a
medicament for preventing err treating Alzheimer's disease and other arnyloid
related diseases:
SEQ )D NO: 1 A(3 (1~t2, all D)
DAF~RHDSGYEVHHQKLVFFAEDvGSNKGA>zGLMVGGwlA
5EQ m NO: 2 A~ (1~0, all-D)
DAEFIiHDSGYEVHHQKLVFFAF.DVGSNKGAIIGLMVGGw
SEQ 1D NO: 3 A~ (1-35, all.D)
DAEFR1~5GYEVHHQKLV'FFAEDVGSNKGAIIGLM
SEQ 1Z7 NO: 4 A~ (1-28, a11-D) DA>sFRHDSGYEVHHQKLVFFAEDVGSNK
SEQ m NO: 5 A[3 (1-7, all-D) DAEFRHD
SEQ >D NO: 6 A~ (10-16, all-D) YEVHHQK
SEQ )D NO: 7 Ap (16 21, all-D) KLVFFA
SEQ 1D NO: 8 A~i (362, all-D) VGGWIA ,
SEQ 1D NO: 9 Lys-Ile-V~-phe-Phe-Ala (all-D)
5EQ 1D NO: 10 Lys-Lys-Leo-Val-Phe-Yhe.Ala (ail-D)
-10-
AMENDED SHEET
r_r.t __:;~lnml~nnnrJ 1F~~f) ~mDt.~lr.:h~ii~ r'.UU!
10-01-2002 CA0001413
CA 02388559 2002-05-15
1AN. 10. 2002 I0:53p.M S'~P.BEY C~GILVY MTL 5I4 288 8389 NQ, 0725 P. 8/22 '
SEQ ID NO: 11 Lys-Phe-Val-Phe-Phe-Ala (all-D) '
SEQ 1T.7 NO: I2 Ala Phe-Phe-vat-Lcu-Lys (all-D)
SEQ ID NO: 13 Lys-Leu-Val-Phe (all-D)
SEQ m Nv: 14 Lys-AIa-Val-Phe-phe-Ala (all D)
SEQ D7 N0:15 Lys-Leu-VaI-Phe-Phc (ah-D)
SEQ >D NO: 16 Lys-Vai-Va1-Phe-Phc-Ala (all-D)
SEQ 1D NO: 17 Lys-De-Val Phe-Phe-Ala-NHz (all D) ,
SEQ m NO: 18 Lya-Leu-Val-Phe-Phc-Ala-NHz (all-D)
SEQ ID NO: 19 Lys-Phe-Val-Phe Phe-Ala-NH= (all-D)
SEQ >D NO: 2o AIa-Phe-Phe-Val-Leu-Lys-NHi (all-D) ;
5EQ 1D NO: 21 Lys-Leu-Val-Phe-NIA (a11-D) ;
SEQ 1D NO: 22 Lys-Ala-Val-Phe-Phe-Ala-NHZ (all-D)
SEQ >D NO. 23 Lys-Leu-Va1-phe.phe-NH= (all-D)
5EQ >D NO: 24 Lya-Val-Val-Phe-Phe-A1a-NH2 (alI-D)
SEQ ID NO: 25 Lys-Leu-Val-Phe-Phe-Ala-Glt1 (all-D) '
SEQ 1D NO: 26 Lys-Leu-Val-Phe Phe-Ala-Gln-NHz (all-D)
SEQ B? NO: 27 His-His-Gln-Lys-Leu.Yal-Phe-Phe-Ala-Gln (sll-D)
SEQ ID NO: 28 Asp-Asp-Asp (ell-D)
SEQ ID NO: 29 Lys-Val-Asp-Asp-Gln-Asp (aI1 D)
SEQ >D NO: 30 His His-Gtn-Lys (all-D) ,
SEQ D7 N0: 31 Phe-Phe-NH-CHiCIizSOzH (all-D)
SEQ 1D NO: 32 Phe-P'he-NH-CHzCHzCH:SO3H (alI-D) _ . ,
SEQ >D NO: 33 Phe-Phe-NH-CH=CH_CH~CH=S03H (a11-D)
SEQ 1D NO: 34 Phe-Tyr-NH-CHzCH=SO,H (all-D) ~ ,
SEQ 1D NO: 35 Phe-Tyr NH-CHzCHzCHzSO~i (all-D)
5EQ B7 NO: 36 Phe-Tyr NH-CHzCH:Cfi2CHzS03H (all-D)
S>;Q m NO: 37 HOsSCHzCH= Phe-Phe (all-D)
SEQ )D NO: 38 HO,SCHZCHzCH~-Phe-Phe (all-D)
SEQ )D NO: 39 H03SCHZCHiCtIZCH~-Phe-phe (a11-D)
SEQ II3 NO: 40 HO~SCHZCHz-Phe-'Iyr (all-D)
SEQ >D NO: 41 H03SC13zCHZCHz-Phe-Tyr (all-D)
5EQ >D NO: 42 H03SCIizGHzCHzCHz-Phe-Tyr (ah-D)
SEQ ID NO: 43 HO~SCHZCHz-Leu-Val-Phe-Phe-Ala (all-D)
-I1-
AMENDED SHEET
r _ r _ _ _ . ~ . ~ n m ~ mnn~ 1 ~ ~ ~n h-mPt . ml' . : b7~~ r . i_4Y
10-01-2002 CA0001413
CA 02388559 2002-05-15 _ _
JAN, 10. 2002 I0:53AM SWABEY OGILVY MTL 51~ 288 8389 N0. 0925 P. 9/2"c
SEQ LD NO: 44 H03SCIiZCH~CHI Leu-Val.Phe-Phe-Ala (all D)
. SEQ ~ NO: 45 HO~SCHZC:HiCH~CHZ Leu-Val-Phe-Phe-Ala (aIl D)
SEQ 117 NO: 46 Leu-Val-Phe-Phe-Ala NIA-GHiCHzSOjH (a11-D) ,
SEQ TD NO: 47 Leu-Va1-Phe-Phe-Ala-NH-CHzCH~CHzS0~3 (all-D) ,
SEQ 1D NO: 48 Leu-Val Ylu Phe-Ala-NH-CIiiCHiCIi2CHaS03FI (all-D).
The compounds listed above may be modified by removing or inserting oae or
more amino
acid residues, or by substituting one or more amino acid residues with other
amino acid or nan~amino
acid fragments.
The following are e~lary compounds derived from compound 18
(all D
KLVFFA-NIA;; SEQ : 18) by substituting one or two amino acid residues)
ID NO with other ammo
acids.
5EQ ID NO: 49 Lys-Leu-Vai-Trp-Phc-Ale-NFIz(all-D) ,
SEQ 117 NO: 50 Lys-Leu-Val-Phe-Trp-Ala- N& (al)-D)
SEQ m NO: S I Lys-Leu-Val-Trp-?rp-Aia- NHj (all-D)
SEQ 1D NO: 5Z Lys-Leu-Yal-Tyr-Phe-Ala- NHz (all D)
SEQ ID NO: 53 Lys-Leu-Val-Phe-Tyr-AIa- NH;, (all-D)
SEQ m NO: 54 Lys-Leu.Va1-Tyr-?yr-AIa- NHz (all-D)
SEQ D7 NO: 55 Lys-Leu-Val-Thf-Phe-Ala- NfIi (all-D) .
SEQ ID NO: 56 Lys-Leu-Val-Phe-Thi-Ala- NHz (all-D) .
SEQ m NO: 57 Lys-Lea-Yal-Thi-Thi-Ala- NI~i (a11-D) .
SEQ >D NO: 58 Lys-Leu-Val-Cha-Phe-Ala- NHz (all-D)
SEQ ID~NO: 59 ~ ' Lys-Lcu=Va1-Phe-Cha-Ata- NHz (all-D)
SEQ >D NO: 60 Lys-Leu-Val-Cha-Cha-Ala- NHz (all-D)
SEQ >D NO: 61 Lys Leu-VaI-pgly-Phc-Ala-Nfiz (all-D)
SEQ 117 NO: 6Z Lys-Leu-Val-Phe-Pgly flla-NHS (all-D) ,
SEQ D7 NO: 63~ Lys-Leu Yel pgly-pgly-Ala- NH;, (all-D).
For the above compounds,
the terms Thi,
Cha and Pgly are
intended to mean
thienylalanine,
cyclohexylalaaine
and phenylglycine,
respectively.
Rabbits were immunized with all-D or all-L KLVFFA. Results of the antibody
titers ;
obtained are shows in FIG. 7. As seen in FIG. 7, the vaccine of the present
invention causes production
of antibodies.
The present invention encompasses various types of immune responses triggered
using
the vaccine of the present invention, e.g., amyloid therapies using the
vaccine approach.
-12-
AMENDED SHEET
r_~~ _~:+~inm»~7nn~ 1RWI1 Cm~~n .m ..~~,.~~, ~ .~_ww
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
In accordance with the present invention, there is also provided a vaccine
which
triggers a preferential TH-2 response or a TH-1 response, according to the
type of
immunization used. By inducing a TH-2 response, anti-inflammatory cytokine
production such
as IL,-4, Il-10 and TGF-(3, as well as the production of IgG 1 and IgG 2b
antibody classes, are
favored. Such type of response would be preferred, as a major inflammatory
response in the
brain of the patients with AD would be avoided. On the other hand, with a
preferred TH-1
response, a pro-inflammatory response with a production of inflammatory
cytokines such as IL-
1, Il-6, TNF and IFN gamma would be favored. This type of response would more
likely
trigger activation of the macrophage population. These macrophages would then
phagocytose
any particulate deposits (such as plaques) via a complement-activated process
as well as via
antibody-mediated process. This approach would be beneficial to clear already
organized
senile plaques and prevent the formation of new fibrillary deposits.
Both approaches (i.e. TH-1 and TH-2) are of value. The antigen used could be
the
peptides which contain regions responsible for cellular adherence, i.e.,
region 10-16, regions
responsible for the GAG binding site, i.e., 13-16, regions responsible for the
(3 sheet 16-21 or
regions for 40-42. These peptides could be presented in such a way that either
a preferential
TH-1 or TH-2 response is obtained, depending on the type of adjuvant used, or
depending on
the route of administration of the vaccine. For example, a mucosal
immunization via nasal
administration is possible, since it is known that such a route of
administration would favor a
TH-2 response.
The present invention will be more readily understood by refernng to the
following
examples, which are given to illustrate the invention rather than to limit its
scope.
EXAMPLE I
An in vitro validation procedure to test the effectiveness of all-D peptide
vaccines
derived from fibrillogenic proteins was performed in rabbits or mice to
demonstrate that
antibodies can be raised against A(3 16-21 (all-D) (see FIG. 7). The
antibodies produced were
tested to prove that they effectively prevent the fibrillogenesis of natural
A(3(1-40, all-L) in
vitro. Standard assays for fibrillogenesis were used to evaluate activity,
such as those based on
Thioflavine T, circular dichroism and solubility.
This approach could also be used to establish which areas of the A(3 peptide
are
most effective when used in the form of all-D peptides to prepare
antifibrillogenic vaccines.
One way this could be performed is as follows:
a) rabbits or mice are immunized with a series of overlapping all-D peptides
generated from the A(3(1-42) sequence, e.g., A(3(1-6), A(3(2-8), A(3(4-10),
etc.
-13-
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
b) antisera are prepared from the immunized rabbits or mice.
c) these antisera are tested to see which parts of the A(3 sequence produce
antisera
which most effectively prevents fibrillogenesis in the standard assays for
fibrillogenesis mentioned above.
EXAMPLE II
Effect of Antibodies Against D- and L-A~3(16-21) Peptide Vaccine on
Fibrillogenesis
A validation procedure to test anti-fibrillogenic activity of antibodies
raised against
D- and L- A(3(16-21) peptide was performed.
Rabbits were immunized with D- or L-A(3 (16-21) peptide. Antibodies raised
were
tested for their antifibrillogenic activities by ThT assay and by electron
microscopy (EM).
Antibodies raised against the D- and L- forms of KLVFFA were capable of
blocking
the fibrillogenesis process as seen either by the Thioflavin T assay (ThT)
(FIGS. 2 and 3) and
by EM (FIGs. 4A to 4C). In the ThT assay, fibril formation is monitored by the
increase in
fluorescence with time. As seen in the Figures, the antibodies were capable of
inhibiting such
an increase in fluorescence, proving that these antibodies were inhibiting
fibrillogenesis.
As can be seen in these figures (FIGS. 2 to 4), antibodies raised against the
D-
peptide have a better anti-fibrillogenic activity than anti-L antibodies.
These results were also confirmed by EM (FIGs. 4A to 4C) where both anti-D and
anti-L KLVFFA peptide blocked the fibril formation when compared to control
(FIG. 4A).
Moreover, again the anti-D peptide has a greater anti-fibrillogenic activity
(FIG. 4B) than the
anti-L peptide (FIG. 4C). This goes along with the ThT assay where the
decrease in
fluorescence was greater with the anti-D peptide antibody than with the anti-L
peptide
antibody.
EXAMPLE III
Antibody Binding Assay
Brain sections were stained with antibodies raised against KLVFFA peptide (D
and
L forms). As seen in FIGS SA to SD and 6A to 6D, the antibodies were not
capable of binding
to aggregated (ThioS positive) A~i. It can be seen from both sets of figures,
which were stained
for both plaques (ThioS) and anti-peptides that the antibodies are recognizing
A(3 at the surface
of the cells but are not capable of binding to plaques. These results show
that the anti-
KLVFFA peptide antibody is recognizing the non-fibrillary A~3 but does not
bind to aggregated
A~3. There was no difference between the anti-D and anti-L peptide antibodies
in this assay.
-14-
CA 02388559 2002-05-15
WO 01/39796 PCT/CA00/01413
These results clearly prove that the antibody recognizes only the non-
aggregated
form and blocks the fibrillogenesis. By having such activity, the vaccine of
the present
invention 1) prevents A(3 from organizing itself into a fibril and 2) prevents
an inflammatory
response being triggered by such an antibody binding to an insoluble form,
since the antibody is
not able to bind to aggregated A(3.
While the invention has been described in connection with specific embodiments
thereof, it will be understood that it is capable of further modifications and
this application is
intended to cover any variations, uses, or adaptations of the invention
following, in general, the
principles of the invention and including such departures from the present
disclosure as come
within known or customary practice within the art to which the invention
pertains and as may
be applied to the essential features hereinbefore set forth, and as follows in
the scope of the
appended claims.
-15-