Note: Descriptions are shown in the official language in which they were submitted.
"F i ~- ~ i
CA 02388889 2002-06-05
r
SOYBEAN OIL HAVING HIGH OX1I~ATIVE STABILITY
F~~,I~p O~INiON
This invention relates to soybean oil and; in particular, to high oleic
soybean oil that-does not require hydrogenation or the: addition of
antioxidants to
achieve high oxidative stability:
gA~GROUI~TD OF THE:INYENTION
Soybean oil is currently thepredominantplant oil in the world. However,
soybean oii is relatively unstable w oxidation and therefore its use is
limited to
applications in which a high degree of oxidative stability is not required.
Soybean
oil contains high levels of polyunsafatty acids and is more pmne to
oxidation than oils with higher levels of tnonounsatutatal and satthated fatty
acids. The higher the degree of unsat<uation in an oil the more likely that
the oil
will go rancid (oxidize): Oxidation leads to the dent of off flavors and
odors in the oil as a result of the degr&dation process. Oils with high levels
of
polyunsatwated fatty acids are not often used in applications that require a
high
degree of oxidative stability; such as cooking for a long period of time at an
elevated temperat~u~e.
Several methods are available to increase the stability of soybean oil. One
commonly used method is catalytic hydrogenation, a process that reduces the
number of doublt bonds and raises the melting point of the fat°
Consequently,
hydrogenation also increases the saturated fatty acid content of oil: Another
approach to increase oxidative stabi.ity is dough the addition of
antioxidants.
Each of these approaches suffers from out or more drawbacks; for example,
2~ hydrogenation of oils has been linked to health, environmental and food
quality
concerns. One known consequence of hydrogenation of oils is the production of
traps fatty acid isomers which have been associated with deleterious Walth
effects
including increased risk of coronary heart disease: (Food Product Design,
November 1994). In the case of antioxidaats~ some are very expen~ve to acquire
and not all antioxidants withstand high temperatures. In addition, in many
cases a
food manufacturer does not want to use oils with added antioxidants if a label
with
unadulterated ingredients is desired. Thus, an oil which has a high oxidative
stability under high temperatures without raQuiring the addition of
antioxidants is
very desirable:
U.S. Patent No. 5;260,077; issued to Carridc ex al. an November 9,1993,
discloses a method of stabilizing riglyceride oils of high oleic acid content
by
addition of tocopherol, a natural aatioxidrmi. Tyre combination of the high
oleic
oiI and tocopherol results in a stable composition suitable for deep flying.
1
~:,: ki- : ~.
CA 02388889 2002-06-05
World Patent Publication W094/I I S I6, published on May 26, 1994,
discloses the isolation and characterization of nucleic acid fragments
encoding
fatty acid desaturase.enzymes; and their use to produce oil seeds with altered
tevtls of unsaturated fatty acids.
5 World Patent Publication W090/10380, published on September 20, 1990,
discloses a homogeneous assemblage of mature rapeseeds having an oleic acid
content of at least ?9 weight percent with respect to total fatty acids and an
erucic
acid content of not more than 2 weight percent. These seeds are alleged to
yield a
vegetable oil having high heat stability; this vegetable oil may be used as a
flying
10 oil:
European Patent Publication:EP 323,753, publislxtl on July 12, 1989,
discloses mature rapeseeds having, by weight; a total fatty acid content a
high
oleic acid content of at least 79% and riot more than 2% erucie acid. The oil
derived &om. these seeds is said to have increased heat stability.
15 "Clear Valley~ Canola Oil" Technical Bulletin No. SA 2069 (1995 Cargill
Foods) discloses a natural cauola salad oil with low levels of linolenic acid
and an
oxidative stability of at least 25 AOM hours.
"Van Den Bergh Design NH" Technical Bulletin No. FI93I8a discloses a
natural canola .oil with a high level of oleic acid and art oxidative
stability of at
ZO least 20 AOM hours:
"SVO Trisun 80" Technical Data Sheet discloses a natural sunflower oil
with 80% oleic acid and an oxidative stability of at least 35 AOM hours. "SVO
Trisun Extra" Technical Data Sheet discloses a natural sunflower oil with 85%
oleic acid and an oxidative stability of at least 60 AOM hours. "SVO
25 HS-Natural" Technical Data Sheet discloses a high oleic (80%) sunflower oil
with
added natural tocophemls (0.2%) arid an oxidative stability of at least 60-70
AOM
hours.
"Kraft Food Ingredients Soy~LL" Technical Data Shoet discloses a low
linolenic soybean oil with an oxidative stability of 23-25 hours:
30 Warner, K. et al. (( 1989) .IAOCS 66(4): 558-564) disclose the flavor and
oxidative stability of soybean, sunflower, and low erucic acid rapeseed oils.
The
oxidative stability of non-hydrogenated soybean oil, held at I00°C for
1, 2, or
3 days, is reported as 13.5, 15.0, 14.0 AOM hours, resp~tively. White, P. J.
and
Miller, L. A. ((1989) JAOCS 63(8): 1334-1338) disclose the oxidative
stabilities
35 of low linolenic acid, high stearic acid, and common soybean oils. Mounts,
T. L
et al. ((1989) JAOCS 65(4): 624-628) disclose the effect of alttred fatty
tecid
composition on soybean oil stability.
2
CA 02388889 2002-06-05
U.S. Patent No. 4,627,192 discloses a sunflower seed having an oleic acid
content of 80% or greater. U.S. Patent No. 4,743,402 discloses a high oleic
sunflower oil.
FR ?617675, published on 3anuary 13, 1989, discloses groundnut seeds with
an oleic acid content of 74-84% and linoleic acid content about 2-8%. The low
linoleic acid content is reported to ensure high storage stability.
World Patent Publication W091/1190b, published on August?2, 1991,
discloses safflower seeds having an oleic or linoleic acid content of at least
80%.
Oxidative stabilityis-also an important characteristic for industrial oil
10 applications. This problem is particularly acute for triglyceride oils
which tend to
deteriorate easily due to their high degree of unsaturation. The oxidation
proceeds
via a mechanism which is initiated by the formation of a free radical and-
occurs
rather easily in triglyceride oils d~ to the high content of active methylene
groups
adjacent to the double bonds. The overall effect is a high susceptibility of
the oil
15 to oxidation, which is further ~mplic~t~ by contact of the oil with metals,
such
as iron and copper, present in the equipment or material to be lubricated.
Metals
act as catalysts in the oxidation process and accelerate degradation of the
oil.
U.S. Patent No: 5,580,482, issued to Chasan ct al, on December 3, 1996,
discloses lubricant compositions stabilized against the effects of heat and
oxygen.
20 U.S: Patent No. 5, 413,725, issued to Lal et al. on May 9, 1995, discloses
pour point depressants for monounsaturated vegetable oils andfor high
monounsaturated vegetable oils/biodegradable base and fluid mixtures.
U.S. PatentNo. 5, 399,275, issued to Large et al. on March 21, 1995,
discloses environmentally friendly viscosity index improving compositions.
25 SUMMARY OlF ~,N
The present invention is directed-to a high oleic soybean oil having high
oxidative stability which comprises a C18:1 content of greater than 65% of the
fatty acid moieties in the oil, a combined C18:2 and C18:3 content of less
than
20% of the fatty acid moieties in the oil and an active oxygen method
induction
30 time of greater than SO hours wherein said oxidative stability is achieved
without
the addition of an antioxidant. The oil of this invention can be used as a
blending
source to make a blended oil product. It can be used in the preparation of
food.
BRIEF D,_ESCRI~TION OF Tl'IIE DR_A_WINGS
Figure Z depicts the peroxide values developed during the Active Oxygen
35 Method test for high oleic soybean oil compared with normal soybean oil.
Figure 2A presents the accumulation of polar materials in high oleic and
normal soybean oils during heating of the oils.
3
1 '~ "~ ~, I i
CA 02388889 2002-06-05
Figure 28 presents the accumulation of polymeric materials in high oleic
and normal soybean oils during heating of the oils.
Figure 3 compares the performance of high oleic soybean oil with normal
soybean oil in the Schaal Oven test:
5 Figure 4 compares the times required to make equivalent products as
indicated by iodine value (IV) from hydrogenation of high oleic and normal
soybean oils.
Figure 5 presents the Solid Fat Index measurements of a hydrogenated
high oleic soybean oiI compared with other fats.
10 DERAILED DESCRIPTION O~~~IENTION
The present invention relates to a high oleic soybean oil having high
oxidative stability which comprises a C 18:1 fatty acid content of greater
than 65%
by weight; combined levels of C 18:2 and C 18:3 fatty acid content of less
than
20% by weight, and an active oxygen method of greater than 50 hours wherein
15 said oxidative stability is achieved without the addition of as
antioxidant. A
soybean oil with "high oxidative stability" is a soybean oil that is less
susceptible
to oxidative degradation when compared to normal soybean oil.
A high oleic soybean seed is a soybean seed wherein oleic acid accounts for
greater than 65 percent of the fatty acid moieties in the oil and,
prefierably, greater
20 than 75 percent of the fatty acid moieties in the oil. Prefenrd high oleic
soybean
oil starting materials are disclosed in World Patent Publication W094/11516.
The soybeans used in the
present invention are described in Example I below.
The high oleic soybean oil of this invention has a C 18:1 content of 65 to
25 85% of the fatty acid moieties, a combined C 18:2 and C 18:3 content of
less tl~
20% of the fatty acid moieties. Preferably, the oil of the invention has a C
18:1
content of greater than auout 70% of the fatty acid moieties, a combined C
18:2
and C18:3 content of less than 15% of the fatty acid. More preferably, the oil
of
the invention has a C I 8: I content of greater than about 75% of the fatty
acid
30 moieties, a combined C18:2 and C18:3 content of less than 10% of the fatty
acid
moieties. Most preferably; the oil of the invention has a C 18:1 content of
freater
than about 80% of the fatty acid moieties, a combined C 18:2 and C 18:3
content of
less than 10% of the fatty acid.
A particularly advantageous feature of the present invention is that there is
35 no need for hydrogenation or other fractionation of the oil to achieve high
oxidative stability. Moreover,~it is not necessary to add antioxidants, such
as
natural antioxidants like tocophtrols, to the compositions of the invention in
order
to increase their stability.
4
i
:: ~..i..i~,'.. ii~. ~ I
CA 02388889 2002-06-05
A number of methods are well known to those skilled in' the art far
determining oxidative stability. The most commonly :used method is the Active
Oxygen Method (AOM). This is an accelerated oxidation test in which an oil is
aerated under a constant, elevated temperature (97.$°C) and degradation
is
monitored by measuring peroxide accumulation. The end point; or induction
time.
is determined by the number of hours required to reach a peroxide value of
104 meq/kg. Thus, the longer the induction time the more stable the oil.
Almost
all commercial oil samples specify a' AOM induction time as a component of the
technical data sheet.
The AOM induction time for the high oleic soybean oil of the invention is
greater than 50 hours. Preferably, the AOM induction is greater than 75 hours
and, most preferably, greater than 100 hours or even greater than 140 hours.
Another standard method now commonly usod'to evaluate the stability of
commercial cooking oils is the Oxidative Stability Index (OSI) which is
measuzed
automarically using a machine manufachn~ed by Ominion; Inc. of Rockland, MA,
USA.
The OSI machine works by bubbling air through oil heated to 1
I0°C. As
the oil oxidizes, volatile organic acids; primarily formic acid; is formed
which can
be collected in distilled water in a cell: The machine constaaatly.measunes
the
conductivity of the distilled water and the induction period is determined as
the
time it takes for this conductivity to begin a rapid rise. Although the data
derived
from the two methods do not always have a straight correlation; the OSI
induction
time values for mast oils are generally about half those of the AOM derived
values.
The OSI induction time value for the high oleic soybean oil of the invention
is greater than 25 hours. Preferably, the OSI induction time value is greater
than
50 hours and, most preferably, greater than 75 hours.
Vegetable oils are often used in high temperateve applications. Oil
oxidation is accelerated in the presence of heat. It is important that as oil
be able
to withstand these conditions for applications such as frying; baking,
roasting, etc.
In some cases, antioxidants may be added to improve stability but not ail
antioxidants withstand high temperatures. In addition, in many cases a food
manufacturer does not want to use oils with added antioxidants if a label with
unadulterated ingredients is desired. Therefore, an oil which is stable to
oxidation
under high temperatures, in the absence of any additives or otlxr processing
is
highly desirable. Overheating of oils oftc~w leads to thermal polymerization
of the
oil and oxidation products resulting in a gummy, varnish-Iike buildup on the
equipment used for heating and excessive foaming of the oil. As a result of
5
ii- ", J I
CA 02388889 2002-06-05
oxidation, a variety of degradation products are formed dependinb on the
conditions under which the oil is exposed. High temperature stability can be
evaluated by exposing the oils to high temperature and monitoring the
formation
of the undesirable degradation products. These include both volatile and
5 nonvolatile products and may be hydrocarbons; alcohols, aldehydes, ketones,
and
acids. The nonvolatile components can be further classified into,polar and
polymerized compounds. The polar and polymerized compounds present in a
degraded oil can be analyzed directly by reverse phase high performance liquid
chromatography as described in Lin, S.S. , 1991, Fats and oils oxidation.
14 Introduction to Fats and Oils Technology (Wan, P. J. ed.), pages 211-232,
Am. Oil
Chew. Sac.
The oil of this invention can 1x used in a variety of applications. In
general,
oxidative stability is related to flavor stability. The oil of this invention
can be
used in the preparation of foods. Exarnpl~ include, but are not limited to,
uses as
15 ingredients, as coatings, as salad oils, as spraying oils, as roasting
oils, and as
frying oils. Foods in which the oil rnay be used include, but are not limited
to,
crackers and snack foods, confectionery products, syrups and toppings, sauces
and
gravies, soups, batter and breading mixes, baking mixes and Boughs. Foods
which
incorporate .the oil of this invention may retain better flavor over longer
periods of
20 time due to the improved stability against oxidation imparted by this oil.
The oils of this invention can also be used as a blending source to make a
blendod oil product. By a blending source, it is meant that the oil of this
invention
can be mixed with other vegetable oils to improve the characteristics, such as
fatty
acid composition, flavor, and oxidative stability, of the other oils. The
amount of
25 oil of this invention which can be used will depend upon the desired
properties
sought to be achieved in the resulting final blended oil product. Examples of
blended oil products include, but are not limited to, margarines, shortenings,
fi_ying oils, salad oils, etc.
1n another aspect, the oils of this invention can be subjected to fiirrlur
30 processing such as hydrogenation, fractionation, interester'tftcation or
fat splitting
(hydrolysis).
In still another aspect, this invention concerns by-products made during the
production of the oils of this invention.
Methods for the extraction and processing of soybean seeds to produce
35 soybean oiI and meal are. well known throughout the soybean processing
industry.
In general, soybean oiI is produced using a series of steps which accomplish
the
extraction and puriFcation of an edible oil product from the oil bearing seed.
6
j j, , y ~
CA 02388889 2002-06-05
Soybean oils and soybean byproducts are produced using the generalized steps
shown in the. diagram below: .
Impurities Removed/
Byproducts Obtained
Soybean Seed
Defatted Soybean Flakes
Oil Extraction (Meal, Protein Products)
Degumming ~ Lecithin
Alkali or Physical Refining----1- Gums, Free Fatty Acids,
- Pigments
Water Washing ~- ~p
Bleaching - ---1- Color; Soap; Metal
(Hydrogenation)
(Winterization) Stearine .
Deodorization -- -1~ FPA, Tocopherols, Sterols,
Volatiles
Oil Products
Soybean seeds are cleaned, empered, dehulled, and flaked which increases
the efficiency of oil extraction. Oil extraction is usually accomplished by
solvent
(hexane) extraction but can also be achieved by a combination of physical
pressure andlor solvent extraction. The resulting oil is called crude oil. The
crude
oil may be degummed by hydrating phospholipids and other polar and neutral
lipid complexes which facilitate their separation from the nonhydradng;
triglyceride fraction (soybean oil). The resulting lecithin gums may be
further
7
CA 02388889 2002-06-05
processed to make commercially important lecithin products used ih a variety
of
food and industrial products as emulsification and release (antisticking)
agents.
Degummed oil may be further refined for the removal of impurities; primarily
free
fatty acids, pigments, and residual gums. Refining is accomplished by the
5 addition of caustic which reacts with free fatty acid to form soap and
hydrates
phosphatides and proteins in the crude oil. Water is used to wash out traces
of
soap formed during refining. The soapstock byproduct may be used directiy in
animal feeds or acidulated to recover the free fatty acids. Color is removed
through adsorption with a bleaching earth which removes most of the
chlorophyll
10 and carotenoid compounds. The refined oil can be hydrogenated resulting in
fats
with various melting properties and textures. Winterization (fractionation)
may be
used to remove stearine from the hydrogenated oil through crystallization
under
carefully controlled cooling conditions. Deodorization which is principally
steam
distillation under vacuum, is the last step and is designed to remove
compounds
15 which impart odor or flavor to the oil. Other valuable byproducts such as
tocopherols and sterols may be removed during the deodorization process.
Deodorizer distillate containing these byproducts may be sold for production
of
natural vitamin E and other high value pharmaceutical products. Refined,
bleached, (hydrogenated, fractionated) and deodorized oils and fats may be
20 packaged and sold directly or further processed into more specialized
products. A
more detailed reference to soybean seed processing, soybean oil production and
byproduct utilization can be found in Erickson, 1995, Practical Handbook of
Soybean Processing and Utilization, The American Oil Chemists' Society and
United Soybean Board.
25 Hydrogenation is a chemical reaction in which hydrogen is added to the
unsaturated fatty acid double bonds with the aid of a catalyst such as nickel.
High
oleic soybean oil contains unsaturated oleic, linoleic, and linolenicfatty
acids and
each of these can be hydrogenated. Hydrogenation has two primary effects.
First,
the oxidative stability of the oil is increased as a result of the reduction
of the
30 unsaturated fatty acid content. Second, the physical properties of the oil
are
changed because the fatty acid modifica'ons increase the melting point
resulting
in a semi-liquid or solid fat at room temperature.
There are many variables which affect the hydrogenation reaction which in
turn alter the composition of the final product. Operating conditions
including
35 pressure, temperature, catalyst type and concentration; agitation and
reactor design
are among the more important parameters which can be controlled Selective
hydrogenation conditions can be used to hydrogenate the more unsaturated fatty
acids in preference to the less unsaturated ones. Very light or brush
CA 02388889 2002-06-05
hydrogenation is often employed to increase>stability of liquid oils. Further
hydrogenation converts a liquid oil to a physically solid tat. The degree of
hydrogenation depends on the desired performance and melting characteristics
designed for the particular end product. Liquid shortenings, used in the
manufacture of baking products, solid fats and shortenings used for commercial
frying and roasting operations, and base stocks for margarine manufacture are
among the myriad of possible oil and fat products achieved through
hydrogenation. A more detailed description of hydrogenation and hydrogenated
products can be found in Patterson, H.B:W., 1994; Hydrogenation of Fats and
Oils: Theory and Practice. The American Oil Chemists' Society.
Interesterification refers to the exchange of the fatty acyl moiety between
an ester and an acid (acidolysis), an ester and an alcohol (alcoholysis) or an
ester
and ester (transesterification). Interesterification reactions are achieved
using
chemical or enzymatic processes. Random or ~irect~d transesterification
processes rearrange the fatty acids on the triglyceride molecule without
changing
the fatty acid composition. The modified triglyceride structure may result in
a fat
with altered physical properties. Directed interesterfication reactions using
lipases
are becoming of increasing interest for high value specialty products like
cocoa
butter substitutes. Products being commercially produced using
interesterification
reactions include but are not limited to shortenings, margarines, cocoa butter
substitutes and structured lipids containing medium chain fatty acids and
polyunsaturated fatty acids: Interesterificadon is further discussed in Hui,
Y.H.,
1996, Bailey's Industrial Oil and Fat Products, Volume 4; John Wiley & Sons.
Fatty acids and fatty acid methyl esters are two of the more important
oleochemicals derived from vegetables oils. Fatty acids are used for the
production of many products such as soaps, medium chain triglycerides, polyol
esters, alkanolamides, etc. Vegetable oils caa be hydrolyzed or piit into
their
corresponding fatty acids and glycerine. Fatty acids produced from various fat
splitting processes may be used crude or more oRen are purified into
~&~actions or
individual fatty acids by distillation and fractionation. Purified fariy acids
and
fractions thereof are converted into a wide variety of oleoehemicals, such as
diner
and trimer acids, diacids; alcohols, amines, amides, and esters. Fatty acid
methyl
esters are increasingly replacing fatty acids as starting materials for many
oleochemicals such as fatty alcohols, alkanolamides, a-sulfonated methyl
esters,
diesel oil components, etc. Glycerine is also obtained by the cleavage of
triglycerides using splitting or hydrolysis of vegetable oils. Further
references on
the commercial use of fatty acids and oleochemicals may be found in Erickson,
D.R. , 1995, Practical Handbook of Soybean Processing and Utilization, The
9
CA 02388889 2002-06-05
American Oil Chemists' Society, and United Soybean Board; Pryde, E.H., 1979,
Fatty Acids. The American 0i1 Chemists' Society: and Hui, Y:H:: 1996. Bailey's
Industrial Oil and Fat Products, Volume 4, John Wiley & Sons.
In another aspect, this invention concerns the industrial use of the soybean
5 oil of this invention for industrial applications such as an industrial
lubricant for a
variety of end uses,: as a hydraulic fluid, etc. The industrial use of
vegetable oils
as a base fluid for lubricants has been known for many years. However,
interest
has intensified due to environmental concerns over the use of petroleum oils
in
environmentally sensitive areas. Vegetable oils are readily biodegradable,
have
10 low toxicity and have good lubricant characteristics. However, high pour
points
and rapid oxidation at high temperatures limit their use. Since the soybean
oil of
this invention is low in poiyunsaturates, as discussed herein; and has high
oxidative stability and high temperature stability; these characteristics also
make
the soybean oil of this invention desirable for industrial applications such
as as
15 industrial fluid, i:e., as industrial lubricant or as a hydraulic fluid,
ete. Additives
which can be used to make industrial lubricants and hydraulic fluids are
commercially available. Indeed, -some additives have been specially formulated
for use with high oleic vegetable oils:, Additives generally contain
antioxidants
and materials which retard foaming, wear, rust, etc.
20 One common method for measuring oxidative stability of industrial fluids
is the rotary bomb oxidation test (ASTM D-2272). The perfozznanee of the oil
of
this invention when compared to commercially available products using the
rotary
bomb oxidation test is set forth in the example below.
E LES
25 The present invention is further defined in the following Examples, in
which
all parts are by weight, percentages are by weight (to volume); and degrees
are
Celsius; unless otherwise ~tat~d. It should be understood that these Examples,
while indicating preferred embodiments of the invention, are given by way of
illustration only. From the above discussion and Chest Examples; one skilled
in
30 the art can ascertain the essential characteristics of this invention, and
without
departing from the spirit and scope thereof; can make various changes and
modifications of the invention to adapt it to various usages and conditions.
EXAMPLE I
Hi~g Oleic Soybeans
35 The production of a soybean oil high in oleic acid and low in
polyunsaturated fatty acids, without the need to perform chemical processing
of
the oil, requires the availability of soybeans which have a high content of
oleic
acid in the fatty acid fraction. High oleic soybeans were prepared by
recombinant
CA 02388889 2002-06-05
manipulation of the activity of oieoyl 12-desaturase. In soy.(Glycine max)
there
are two genes for this activity, one of which (GmFad 2-1 ) is expressed only
in the
developing seed (Heppard et al. (1996) Plant Physiol. 110:311-319): The
expression of this gene increases during the period of oit deposition,
starting
around 19 days after flowering, and its gene product is responsible for the
synthesis of the polyunsaturated fatty acids:found in soybean oil. GmFad 2-1
is
described in detail by Okuley, J. et aI. (1994) Plant-Cell 6:147-158 and in
W094/11516. It is available from the ATCC in the form of plasmid pSF2-169K
(ATCC accession number 69092): The other gene (GmFad 2-2) is expressed in
the seed, leafy root and stem of the soy plant at a constant level and is the
"housekeeping" 12-desaturase gene. The Fad 2-2 gene product is responsible for
the synthesis of polyunsaturated fatty acids for cell membranes.
GmFad 2-1 was placed under the control of a strong, seed-specific promoter
derived from xhe a'-subunit of the soybean (GTycine max) (3-congtycinin gene.
This promoter allows high level; seed specific expression of the trait gene.
It
spans the 606 by upstream of the start codon of the a' subunit of the Glycine
max
~-congylcinin storage protein. The (i-conglycinin promoter sequence represents
an allele of the published (3-conglycinin-gene (Doyle et al., (I986) J. Biol.
Chem.
261:9228-9238)-having differences at 27 n~leotide positions: It has been shown
to maintain seed specific expression patterns in transgenic plants (Barker et
al.,
(1988) Pros. Natl. Acad. Sci. 85:438-462 and Beachy et al., (1985) EMBO J.
4:3047-3053). The reading frame was terminated with a 3' fragment from the
phaseolin gene of green bean (Phaseolus vulgaris). This is a 1174 :bp stretch
of
sequences 3' of the Phaseolus vulgaris phaseolin gene stop codon (originated
from
clone described in Doyle et al., 1986).
The GmFad 2-1 open reading frame (ORF~ was in a sense orientation with
respect to the promoter so as to produce a gene silencing of the sense GmFad
2-1 cDNA and the endogenous GmFad 2-1 gene: This phenomenon, known as
"sense suppression" is an effective method for deliberately: tmninrg off genes
in
plants and is described in U.S: Patern No. 5,034,323.
For maintenance and replication of the plasmid in E. coti the GmFad 2-1
transcriptional unit described above was cloned into plasmid pGEM-9z(-)
(Promega Biotech, Madison WI, USA).
For identification of transforns~d soybean plants the ~-gtucuronidase gem
33 (GUS) from E: coli was used. The cassette used consisted of the three
modules;
the Cauliflower Mosaic Virus 35S promoter, the ~i-gbycuronidase gene (GUS)
from E. colt and a 0.77 kb DNA fragment containing the gene terminator from
the nopaline synthase (NOS) gene of the Ti plasmid of Agrobacterium
li
r ~ i,~ I: ; P~ I I
CA 02388889 2002-06-05
rumefaciens. The 35S promoter is a 1.4 kb promoter region from CaMV for
constitutive gene expression in most plant tissuesv(Odel1 et al. ( 1985)
Nature
303:810-812), the GUS gene a 1:85 kb fragment encoding the enzyme
(3-glucuronidase (Jefferson et al: (1986) PNAS USA 83:8447-8451)-and the NOS
S terminator a portion of the 3' end of the nopaline synthase coding region
(Fraley
et al., (1983) PNAS US 80:4803-4807). The GUS cassette was cloned into the
GmFad 2-I/pGEM-9z(-) construct and was designated pBS43:
Plasmid pBS43 was transformed into meristems of the elite soybean line
A2396, by the method of particle bombardment (Christou et al.; (1990) Trends
10 Biotechnol: 8:145-15I). Fertile planes were regenerated using methods well
known in the art.
From the initial population of transformed plants, a plant was selected
which was expressing GUS activity and which was also positive for the GmFad
2-1 gene (Event 260-OS) when evahrated by PCR. Small chips were taken from
15 a number of Rl seeds of plant 260-05 and screened for fatty acid
composition.
The chipped seed was then planted and germinated. Genomic DNA was
extracted from the leaves of the resulting plants and cut with the restriction
enzyme Bam HI. The blots :were probed with a-phaseolin°probe:
From the DNA hybridization pattern it was clear-that in the original
20 transformation event the GmFad 2-1 construct had become integrated at two
different loci in the soybean genome: At one locus (Locus A) tlx GmFad 2-1
construct was causing a silencing of the endogenous GmFad 2-1 gem, resulting
in a relative oleic acid content of about 85 % (compared with about 20 ~ in
elite
soybean varieties). At locus A there were two copies of pBS43. On the DNA
25 hybridization blot this was seen as two cosegregating bands. At the other
integration locus (Locus B) the GmFad 2-1 was over-expressing, thus decreasing
the oleic acid content to about 4 % .
Fourth generation segregant lines (R4 plants); generated from the original
transformant, were allowod to grow to maturity. R4 seeds; which contained
30 only the silencing Locus A (e.g., G94-1) did not contain any detectable
GmFad
2-1 mRNA (when measured by Northern blotting) in samples recovered 20 days
after flowering. GmFad 2-2 mRNA, although reduced somewhat compared with
controls, was not suppressed. Thus the GmFad 2-1 sense construct had the
desired effect of preventing the expression of the GmFad 2-1 gene and thus
35 increasing the oleic acid content of the seed. All plants homozygous for
the
GmFad 2-1 silencing locus had an identical Southern blot profile over a
rntmber
of generations. This indicates that the insert was stable and at the same
position
in the genome over at least four generations. A summary of the oleic acid
12
~. '~ i':, ; y I
CA 02388889 2002-06-05
contents found in the different generations of recombinant soybeanplants and
seeds is presented in Table 1.
TABLE ~
Plant ID Generation Planted' Seed Analyzed' Bulk Oleic Acid (~)
6253 R0:1 R1:2 84.1
6276 R0:1 R1:2 84.2 °1
G29b R0:1 R 1:2 84.19b
6313 R0:1 Rl :2 83.8 %
6328 R0:1 RI :2 84.09&
6168-18? RI :2 R2:3 84.4 9'°
G 168-171 R1:2 R2:3 85.2 ~
6168-59-4 R2:3 R3:4 84.06
6168-72-1 R2:3 R3:4 84.1 °X
GI68-72-2 R2:3 R3:4 84:5 %
G 168-72-3 R2:3 R3 :4 84.3
6168-72-4 R2:3 R3:4 83.3 °6
'R0:1 indicates the seed and the plant grown from seed after selfm,g of the
first
generation transformant. R1:2 indicates the seed and the plant grown from seed
after selfing of the second generation transformant. R2:3 indicates the seed
and
the plant grown from seed after selfing of the third ge~ration transformant.
R3:4 indicates the seed and the plant grown from seed after selfing of the
fourth
generation transformant.
Io
High Oleic ~gvbean O~on ~,r~d ess'pg
High oleic and normal soybean oils each were produced on the laboratory
bench or at a commercial pilot plant using industry tandard methods as
described
below. Commercial samples of other high stability oils and shortenings used
for
comparison were obtained from the manufacturers and stored frozen under
nitrogen. These included samples of soybean flying oil, clear liquid
shortening,
heavy duty shortening, low linolenic soybean oil, and high oleic canola oil.
High
w oleic corn oil was produced using industry standard conditions similar to
those
described above.
Part A: LarQe~pilot,~h scale processing of oil
Harvested soybeans (97.5 kg) were tempered by spraying the seed with
water to raise the moisture to 8.7%. The water and seed wen blended for
approximately 10 minutes and allowed to equilibrate for approximately 21
hours.
Tempered seed was cracked using a Ferrell-Ross Cracking Roll set at 3.5 on the
cracking roll scale. Hulls were separated on a Kice Multi-aspirator Model 6F6
13
i
"j: ii~ - ~~ ~ I
CA 02388889 2002-06-05
using a dil~erential air pressure of0.8-1.2 inches of water: The aspirated
soybean
meats were cooked in a two tray Simon-Rosedowns cooker heated to
approximately 40°C for 10-30 minutes. Warmed soybean meats were dropped
into the second tray and heated to 60-75°C for 1 S-25 minutes. The
cooked
soybean meats were flaked to a thickness of approximately 0.4 mm in a E. R.
and
F. Turner Flaking Roil. The resulting soybean flakes were extracted with
hexane
in a Crown Iron Works Loop Extractor (Type II) using a total residence time of
60 minutes and a solvent-to-solids ratio of approximately 1.5:1 (wt:wt). The
solvent temperature was 50-60°C. The miscella (hexane/oil mixture) was
desolventized using a Tetra-Laval Scraped Surface Heat Exchanger followed by
complete desolventization in the lab using a rotary evaporator. The crude oil
was
collected and held under nitrogen until further processing.
Crude oil was water degummed in the following manner. The oil was
heated to 60-70°C and a volume of 90°C water equivalent to 2% of
the oil volume
was added and mixed for 15 minutes at 75-80°C; solids were then
separated by
centrifugation. Degummed oil was refined by heating to 70-80°C. A
volume of .
an 85% phosphoric acid solution equivalent to 0.1 % of the degummed oil volume
was added and the solution mixed for 30 minutes: Enough NaOH was added to
reach 16 Be to neutralize free fatty acids; an additional 0.08% w/w excess of
2.0 NaOH was added and the solution mixed for 30 minutes while heating to
80-85°C: Solids were separated by centrifugation. The oil was water-
washed by
heating to 75-80°C and adding 95°C water to 10% (v/v); mixed for
10 minutes at
80-90°C, and centrifuged. Water-washed oil was bleached by loading 1200
g of
oil into a 2L Pan reactor and adding a bleaching clay (Clarion 470 SA;
American
Colloid Co.) to 0:5% (w/w) undervacuum and heating to 110°C for 30
minutes
before cooling to 65°C. The oil was removed and 30 g "filter aid" was
added and
the oil filtered. These steps were repeated until all the oil was bleached.
The oil
was deodorized by loading 2200 g into 5 L glass deodorizer under vacuum and
heated to 100°C. Steam was added at 3%(w/w~h and the oil was brought to
240°C with continuous sparging for 1 h at temperature. The oiI was then
cooled
to 70°C with sparging and the oil removed from the deodorizer. Thirty
ppm citric
acid was added to mimic industry standards during deodorization. Deodorized
oil
- was stored frozen under nitrogen.
Part ~: Small llg~oratoryy scale processing of oil
Harvested soybeans were heated in the microwave to 180°F, cooled
to mom
temperature and cracked using a Roskamp TRC 6S0-6 Crack and Roll. Soybean
hulls were removed using a Kice Aspirator and the remaining meats were heated
to 180°F and flaked in a Roskamp TRC 9I2 Flake and Roll: Crude oil was
14
;j. ~.I I
CA 02388889 2002-06-05
extracted in a glass, water jacketed extraction vessel heated to 60°C
far
~S minutes using a solvent fo solids ratio of approximately 4:1. The
hexane/oil
miscelIa was collected and the extraction repeated. The miscella was
desolventized using a rotary evaporator Ieavin~ crude oil.
A volume of an 85% phosphoric acid solution equal to 0.1 % (v/v) of the
crude oil was added and the solution heated to 6S-70°C for 10 minutes
while
stirring. Wann (60°C) NaOH (8% aqueous solution) was added dropwise to
the
oil to neutralize the free fatty acids and the H3P04 with an additional 0.2%
wt/wt
excess: The solution was stirred for five minutes and the solids separated by
centrifugation. The oil was water washed by adding hot water to 20% (v/v) as
the
sample was heated to 90°C with rapid agitation. The oil and water were
allowed
to cool at room temperature for 10 minutes and then separated by
centrifugation.
The oil was dehydrated using very rapid agitation'under vacuum at 85-
95°C for
30 minutes or until all moisture (bubbles, condensation) had been removed. The
vacuum was then broken with nitrogen. The oil was bleached by adding 2%
(wt/wt) Activated Bleaching Earth (AOCS #Z1077) and the solution mixed under
vacuum for 30 minutes at 85-95°C before cooling to 80°C. The
vacuum was
broken with nitrogen and 1 % (wt/wt) of diatomaceous earth was added and the
mixtwe filtered through a prepared bed of diatomaceous earth.
Citric acid was added to approximately 50 ppm, and the oil was deodorized
at 240°C with steam (4 mL water per 100 g oil) in a glass deodorizer
for
approximately 1 hour. The oil was cooled to 80°C with spargit~g, and it
was
further cooled to 40°C under nitrogen. The refined, bleached, and
deodorized
oil was stored frozen under a nitrogen atmosphere.
EXAMPLE 3
Comp~~itional Analv~~
Uils produced in Example 2 were analyzed far composition as described
below. Compositional data for the oils is given in Table 2.
Fatty a~i com sition: Fatty acid composition was determined essentially
by the methods described in AOCS Ce lc-89. Fatty acid methyl esters were
prepared as follows. Ten pL oil was mixed with 1 mL hexane and 0.25 mL of a
3 % sodium methoxide solution for 30 minutes. Acetic acid (0.1 mL of a 10%
solution) was added, the sample was mixed and the layers separated by
centrifugation. The resulting fatty acid methyl esters extracted in the hexane
layer were resolved by gas chromotography (GC). Hewlett Packatd 5890GC
(Wilmington, DE) equipped with a SP2340 column (60 m, 0.25 mm ID,
0.20 micron film thickness) (Supelco, BelIefonie, PA). Column temperature
was 150°C at injection and the temperature programed from 150°C
to 200°C at
CA 02388889 2002-06-05
2°Clmin over 40 minutes. Injector and: detector temperatures were
215°C and
230°C, respectively.
Pgroxide value. free ~atty.~cids: and color: Peroxide values were determined
by titration essentially by AOCS method Cd 8-53 and the data expressed as
5 milliequivalents peroxide/kg oil. Free fatty acid values were determined by
AOCS method Ca Sa-40 and the data expressed as % free fatty acids (as oleic
acid). Color was measured using a Lovibond Tintometer and a S-114" tube
according to AOCS method Ccl3b-45.
Tocooheroj content: Tocopheml content was determined by normal phase
HPLC using a Rainin Instrument Dynamax HPLC and data acquisition system
equipped with a Milton Roy spectromonitor LJV detecwr. HPLC conditions were:
Waters pPorasil 3.9 x 300 mm silica column (unbonded; irregular shaped), a
solvent system of hexane! isopropyl alcohol (98.5!1.5) with a flow rate of
1.5 mL/minTotal run time for each sample was 7.0 minutes. Samples for HPLC
15 analysis were prepared by adding 900 ~I, of hexanelisopropyl alcohol
{98.5/1.5)
to 100 ~L of refined, bleached and deodorized oil. 40 ~L was injected into the
HPLC. Absorbance was monitored at 295 nm. Data are expressed as mg
tocopheroU 104 g of oil.
These data show the compositional analysis of the soybean oil of the
20 invention and the vegetable oils with which it was compared. The
performance of
these oils was then further evaluated in the examples as discussed below.
16
i~
CA 02388889 2002-06-05
Cs O 00 M 00 N O,
r 0~0 N ~ O
rr wr
f' E
p
t~
id 'H Vl
~r_,. 1 1 1 1 1 1
Z Z
V~'lN~c~~1~t~11ht~'1d
~", O O O Q O O O O O
a
o c e~-. 0 0 0 0 0
O .. vp ~ N N M N tV
O
ualogb'',oF
w o 0 0 0 o co 0 0 0
a,
~og.~-~oo ogc'~,
~, o o c o o c o 0 0
_o
a ~ N ~n O ~n op N O O O
f-1 ~ N I'~ C O N V1 ~C O a
U
c
N
G ~O QI O~ N ~"1 ~ C !~ 00
U '" ~ o' ~' ~', '~ ~ ~, '~,
V ~ ~" m
1 1 1 1 1 1
;v U '-
..
a
- ~ ~_ ,yo o; N oo vo ao .. ~ .n
G~ON~~N!'~
w
O N
...1 M M ~ M 1n !~
V e~i it et N Vi N
eW0 O O, vD ~ ~ V!
'~' ~G O M O~ O ~''1 ~ ~ O
U ... ... ~'
a m
a '&
ee ~o
.5 ~ m
E.. ~ a $ '~ ~ ~. z? ~'
a a ~ v .5~ w-
','u~'ugue
.!3
'~ $
17
i ~.I-It ~ a ~ I
CA 02388889 2002-06-05
EXAMPL~_4
Oxidative Stability .
The high oleic and normal soybean oils produced in Example 2 were
evaluated for oxidative stability by AOM and OSI. All other commercial oils
used for comparison were evaluated by OSI only. OSI determinations were made
at 110°C using the Oxidative Stability Instrument (Omnion, Inc,
Rockland, MA)
using official ROCS methods (AOCS Method Cd 12b-92). Samples were run in
duplicate and the data presented are the average values for each sample. AOM
determinations were made using official AOCS methods (Cd 12-S7). The tests
were run at 97.8°C and the induction times given represent the number
of hours
required to reach 100 milliequivalents peroxide per kg of oil tested.
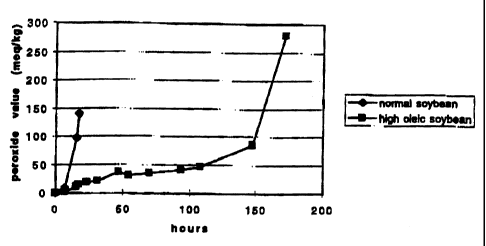
Figure 1 shows a graph comparing the AOM results of high oleic and normal
soybean oil. The average AOM induction time for high oleic soybean oil was
145 hours compared with 1 S hours for normal soybean oil. The raw data used to
make the graph is given in Table 3. Values obtained from the OSI
determinations
are given in Table 4. High oleic soybean oil had an OSI induction time of
80.7 hours compared with 6.9 hours for normal soybean oil.
TABLE 3
AUM values for normal
and high oleic soybean
oils
Time (hours) Normal Soybean Oil High Oleic Soybean
Oil
0 0 0
7 9.77 1.93
15 97.06 11.68
17 141.04 15.2
23 18.76
31 20.83
46.5 36.48
54.5 31.1
70 34.97
94 41.75
108 46.85
147.5 86.84
171.5 279.54
18
~~ i I i
CA 02388889 2002-06-05
4~
OSI induction times for various vegetable oils
O~I jnduction Time(hours)
high oleic soybean 80.7 +I - 3.2
normal soybean 6.9 +/ - 0.4
high oleic sunflower 18.2 +/ - 0.1
high oleic corn 15.4 +/ - 0.2
low Iinolenic soybean 6.5 +/ - 0.3
high oleic canola 20.9 +I - 2.3
soybean frying oil 21.2 + I - i .0
clear liquid shortening31:8 +I - 0.9
heavy duty shortening 84.8 +/ - 1.2
' The compositions of these oils are given in Table 2
E
High Temperature S~abilit~Q~H~gh (,)leic Soybean Oil
High temperature stability of refinad~ bleached; and deodorized-high oleic
soybean oil produced in Example 2 was compared to the stability for normal
soybean oil and other commercial oil samples. Stability was determined by
heating the oils to frying temperature and monitoring the formation of polar
and
polymer degradation products by HPLC. High oleic soybean oil developed polar
and polymer materials to a lesser extent than normal soybean oil as shown in
Figure 2A and 2B.
Sample Preparation: Oil samples (5 mL) were heated in glass tubes in an
aluminum block on a hot plate controlled with a PMC Dataplate 520 temperature
controller and timer. Oils were heated to 190°C for 10 hours each day
and
allowed to cool before reheating. Fifty ~L samples for HPLC analysis were
collected after I 0, 20, 30, 40, 50, and 60 hours of heating. Samples were
stored at
-20°C until they Could be assayed.
Reverse Phas~Higl~Pgrformau~~ Liquid C~I~rg,~~otoaranhv: Methods used
for reverse phase HPLC analysis of heated oils are similar to those of Lin
(1991).
Prior to HPLC analysis, 50 p,L samples were brought to room temperature and
950 ~L of an isopropyl alcohol /hexane (20:80, v/v) solution was added and the
samples vortexed. The HPLC system consisted of a Rainin Instrument Dynamax
HPLC and data acquisition system, two Rabbit-HP solvent delivery pumps,
Spectra Physics SP888018875 Autosampler, and Milton Roy spectmmonitor W
detector set at 254 nm. The column was a Beckman Ultrasphere 4:6 x 25 cm.
Gradient conditions changed from 40:60 to 90:10 isopropanol:methanol over
37 minutes. Resulting chromatographs were integrated and the areas underneath
19
i is . ;~
CA 02388889 2002-06-05
the peaks corresponding to palar and polymeric materials identified. Data was
expressed as total polar and total polymer peak area units.
EXAMPLE 6
Schaalwen Test
5 The oxidative stability of the instant high oleic soybean oil was also
determined in an accelerated aging test known as a Schaal Oven Test. Oils used
in this test were those produced in Example 2.
Oil samples {15 mL) were placed in a 30 mL beaker with a watch glass
cover and kept in a forced draft oven at 63°C. Oxidative degradation
was
10 measured as titrable peroxide equivalents according to AOCS Method Cd 8-53.
Samples for analysis were collected at various times during the heating of the
oils. Figure 3 presents the results from this test.
Tlle Ef~~Q,f~~or .~bset~ of Toc~~' ea rol
15 on O~C~tivl: ~ ba ilit5!
Part A: Comparison of Normal an~H~ Oleic Soyn Oils
Tocopherols are naturally occurring antioxidants and are present at
different levels in different oilseeds. The tocopheml content of he extracted
oils may also vary depending on the conditions used to manufacture the oil.
20 Specifically, more or less tocopherol can remain in the oil depending on
the time
and temperature conditions used during deodorization. The. tocopherol contents
for high oleic and normal soybean oiI were measured to determine if varying
levels could be affecting stability of tl~ two oils. These data are included
in
Table 2. High oleic and normal soybean.oil did not differ in either total
25 tocopherol content or in the ratio of individual tocopherols. While typical
for
the deodorizing conditions used here, these values are somewhat higher than
values obtained for commercially produced oils which are ge~rally around
100 mg total tocopheroll100 g oil, depending;on the supplier. High oleic
sunflower oil, high oleic corn oil and high oleic canola oil all had somewhat
30 lower tocopherol contents than the soybean oils; these values ranged from
46 mg (sunflower) to 73 mg (corn) per 100 g oil:
High oleic soybean oil and the high oleic sunflower oil of Table 5 have
similar fatty acid compositions with regard to oxidation potential. The
difference between the OSI induction dune for high oleic soybtan (80.7) atxi
35 high oleic sunflower (34.3 hours), suggests that factors other than fatty
acid
composition alone are affecting the oxidation rate. Tocopherols are known to
exert a strong antioxidant affect which is dependent upon concentration. The
soybean and sunflower oils differed in both the total tocopherol content and
individual tocopherols present. To determine whether tocopherols were
'i ~. I i
CA 02388889 2002-06-05
responsible for the difference in OSI, we added back individual tocopherols to
achieve both the relative ratios and total amount of tocopherol (Table 4)
present
in the high oleic soybean oil. As shown in Table 5, the OSI of high oleic
sunflower was increased to that of high oleic soybean by matching both the
individual and total tocopherols present in high oleic soybean oil:
TABLE 5
The effect on OSI induction time of adding tocopherols
to high oleic oils to match the content present in high oleic soybean oil
High Oleic High Oleic
Soybean Oil Sunflower Oil
Fatty acid Composition
C16:0' 6.4 3.0
C180 3.3 4.3
C18:1 85.6 - 87.0
C18:2 1.6 4.1
C18:3 2.2 0.0
PV 0.02 0.01
FFA 0.3 0.01
total tocopherol (beginning) 160.4 46.7
wt % (beginning)
alpha 5.1 95.1
gamma 71.5 4.9
delta 23.4 0.0
OSI (beginning) 80.7 34.3
total tocopherol (ending) 193.0
~ % (1~g~8)
alpha 25.1
g~ 58:0
delta 17.0
OSI (ending) 74.6
The effect of tocopherols was also examined by producinghigh oleic
soybean
oils with lower tocopherol content (equal to concentrationsfound in high
oleic
sunflower) and measuring stability by AOM. The
oils were produced by
varying both the time and temperature during deodorization
of refined, bleached
high oleic soybean oil. Temperatures used were 240C
and Z65C.
Deodorization times ranged from 0 to 360 hours. Tableshows the
6
deodorization conditions, the resulting oil compositions
and AOM stability.
21
i i
~,.~ , y ~ i
CA 02388889 2002-06-05
TAB
Deodorization of High Oleic Soybean Oil
Conditions 1 2 3 4 5 6 7
temperature, °C. 240 240 240 240 240 265 265
time at 0 30 60 120 240 240 3b0
temperature,
min.
tocopherols, 192.3 t77.9 177.5 161.4 138:5 32.5 28.1
total, mgt 100 g
AOM (hours) 122.0 111.3 111.0 107.5 105.8 94.8 94.5
While tocopherol content was significantly reduced in the high oleic
soybean oil, it can be seen from Tabie 6 that its oxidative stability, as
measured
by AOM induction time, was not reduced by more than 25 ~ in the sample with
the lowest tocopherol content. An induction time of 94 hours is significantly
longer than the comparable OSI values for any of the other oils listed in
Table 4.
n~mrt,~ a
Hydrogenation gf Normai~,~ High Olric Soybean Oils
This example illustrates the advantages of hydrogenating the high oleic
soybean oil of this invention.
High oleic and normal soybean oils were hydrogenated using methods
standard in the industry as described below.
Hydrogenation reactions were carried out using 0.04 ~ nickel catalyst
(Nysosel 325, Engelhard Corp), 75 ml oil, 104°C, under 90 psi hydrogen,
stirred at ?50 rpm in a reactor made by Autoclave Engineer, Inc., (EZE - Seal
Reactor). Changes in the fatty acid composition of the oil during the reaction
was monitored by refractive izidex. Oil samples were collected with iodine
values (IVs) ranging from about 95 to 45. Oils were filtered through celite
and
deodorized using conditions described above. Hydrogenated oil samples were
evaluated for free fatty acids, peroxides, fatty acid composition, and OSI
induction time using the method described above. Solid fat index of each oil
.- sample was determined using ROCS Method Cd 10-57. The solid fat index is a
measure of the solids content at a given temperature, and is an important test
in
characterizing the physical properties of a fat.
22
G II
CA 02388889 2002-06-05
a
"~h ~..~.N
r en ~ N o; oo N o 0o m
a
_u v ~o 0 0 0 0 ~ M ~~, c~ M
0
0o vi o,~ v°' . a So
U ~ M ~ ~ O~ ~ ~ ~' 1p
r
U
r.
o .D o, o. -~ ~r o~ M r. e~~~ o~
N M N ~ N
rr
N
U
0
00 ~O ~ .-~ .~, 00 ~r N N ~!
h
v H
M N t~ ~O O~ N N,
et b ~ ~ N M M ~G ~
1p ~ !r1 V'7 ..w M
'-i ~ l~ 00
O
LL r, t~1 V1 M N Y1 ~ N
h GO N ~ ~ r' ~ .fir t~~f V1
GL
fi, a N ao l~ ., v0 h eh O~
°.r ,~ 0D N vC G0 ~ "~", h
O
ti N ... M M O~ '~
o Gr
.. v ~ ~ M Yh1 ~ ~~ ~~" N
> "'~a~a~o~on D~
... ~"~' t~ v~ ..
> po .~ ov
.5!
E ~ o ~5 0 'We
H rip ~~ ~ ,erb
~, :~ s .~"~ $ g '
23
i i, i ~ i
CA 02388889 2002-06-05
Using high oleic soybean oil as the base oil for hydrogenation reactions has
several advantages over using normal soybean oil. Significantly less time is
required to reach any given product, as represented by IV, from high oleic
soybean oil when compared with normal soybean oil (Figure 4). The resulting
hydrogenated products also have several advantages including lower trans fatty
acids and substantially longer OSI induction times. Figure 5 presents the
solid
fat index values for one of the hydrogenated high oleic soybean oil products
compared with hydrogenated normal soybean oil products and a variety of
commercial fats.
EXAMPLE 9
Stabil~y of ~~gh oleic soybean oil as a ~~;t~on o~ composition
Oil from high oleic soybean seed grown during subsequent growing
seasons was harvested and the oil extracted and processed by the conditions
set
forth in Example 2. Compositional data was obtained using the methods
IS described in Example 3. High oleic soybean oils from these productions
varied
slightly in fatty acid composition, tocopherol content, and AOM/OSI induction
times as shown in Table 8.
_T~m'~~oFi$
Coglposition and stabilities of soybeap oils
IA, 95 PR, 95 IA, 96 (Control)
Fatty Acid Qomposition
C16:0 G.3 7.1 6.4 10.1
CI8:0 3.7 3.7 3.1 3.5
C 18:1 84.0 81.7 82. G 17.9
C18:2 1.6 2.0 2.3 58.6
C18:3 2.4 3.6 3.7 8.2
Quality
pV 0.0 0.0 0.06
FFA 4.01 0.01 0.01
color 1.SY 0.3R ~ 0.9Y 1.8Y 0.2R
O.1R
AOM, hr 141 107 82 14
OSI, hr 52-80 56-63 43 6
tocop6erols (mg1100g) 137 170 87.9
gamins 101.0 115.0 54.9
delta 25.5 43.5 29.6
alpha 10.1 11.0 3.4
24
;,, air
CA 02388889 2002-06-05
EXAMP~rE 10
Hi h oleic soybegn oil as a blending source
art ~1: Ble_n~,iflg with normal soybean oil
High oleic soybean oil produced in Example 2 was blended-with normal
5 soybean oil to varying percentages and the oxidative stability of the
blended oils
was evaluated by OSI. Table 9 shows the effect the increase in oxidative
stability of normal soybean oil by blending with high oleic soybean oil.
The effect on oxidative stability of normal soybean oil by blending with high
oleic soybean oil.
Fatty
Acid
Compositiaat
9b normal soybean '
oil
in bled OSI f~hrs~C 16:0 C 18:0C 18:1 C I8:2C 18:3
0 76.4 6.3 3.7 84.6 1.7 2.3
2.s 63.9 6.6 3.6 82.9 3 2.5
46.3 6.7 3.4 80.9 4:6 2.6
38.4 6.9 3.7 78.4 6.9 2.8
Is 30.5 7 3.7 75:8 9.1 3.1
25.7 7.4 3.8 72.9 11.5 3.3
100 6.0 10:1 3.5 17:9 58.6 8.2
10 Part B: Blending with low linoignje g,,r~,~,Qy n oil
High oleic soybean oiI produced in Example 2 was blended with a low
Iinolenic acid soybean oil and the resulting blend evaluated for oxidative
stability
by OSI. Table 10 shows the composition and oxidative stability of the blend.
TABLE 10
The effect on oxidative scabilry of low linolenic soybean oil by blending with
high oleic soybean oi!
% low linolenic OSI Inducti~
soybean oil C 16:0C 18:0 C 18:1C 18:2 C I Time (hours)
in blend 8:3
0 6.4 3.7 84.4 1.0 2.2 72.7
5p 7.3 4.0 61.2 23.2 2.6 18.6
100 8.3 4.3 37.7 44.4 3.4 10.9
is
EXAMPLE I1
~valuatioa of high oleic soybean ~] use in i u~,trial fluid
end hvli~~aulic fluid avpl'ca
The performance of the oil of this invention use in industrial fluid and
20 hydraulic fluid applications was compared to commercially available
industrial
zs
.y j.i . g:
CA 02388889 2002-06-05
oil products using the rotary bomb oxidation test (RBOT) (ASTM D-2272).
This test is used to evaluate the oxidation characteristics of turbine,
hydraulic,
transformer and gear oils. The test apparatus consists of a pressurized bomb
axially rotating at an angle of 30° from the horizontal in a bath at
150°C.
Fifty grams of test oil with or without commercial additive and 5 g of water
are
charged .to the bomb containing a copper catalyst coil. The bomb is initially
pressurized with oxygen to 90 psi at room temperature. The 150°C bath .
temperature causes this pressure to increase to approximately 200 psi. As
oxidation occurs, the pressure drops, and the failure point is taken as a 2S
psi
drop from the maximum pressure attained at i50°C. The results are
reported as
the number of minutes to the 25 psi loss, as shown in Table I1 below.
T~,BLE 11
Performance of oiis is Rotary Bomb Oxidation test
RBOT Minutes
high oleic soybean oil, no additive 22
high oleic soybean oil + 4.5% Lubriml 7653 250
control soybean oil, no additive I8
control soybean oil + 4.5% Lubrizol 7653 49
Mobil BAL 224H (canole-based) hydraulic fluid 29
Mobile DTI: 13M (petroleum-based) 216
Pep Boys ATF (petroleum-based) 135
These data show the superior performance of the oil of the present invention
in
industrial fluid and hydraulic fluid applications which require high oxidative
stability.
26