Note: Descriptions are shown in the official language in which they were submitted.
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
CATHETER WITH STYLET LUMEN
FIELD OF THE INVENTION
This invention relates generally to catheters for use in delivery of drug, and
in particular to a
catheter suitable for drug delivery and comprising a stylet lumen.
BACKGROUND OF THE INVENTION
Delivery of drug to a specific treatment site represents a substantial
challenge in the design of
drug delivery systems. Site-specific drug delivery can be particularly
challenging when the drug is to
be delivered long-term (e.g., several hours to several days, weeks, or
months). One approach to
accomplish site-specific drug delivery involves the use of a catheter, which
can be positioned at a
treatment site to facilitate localized delivery of drug from a drug reservoir
that may be some distance
from the treatment site. Long-term drug delivery requires that such catheters
be biocompatible, drug
non-reactive, impermeable, and flexible (e.g., not sharp or easily breakable
while implanted in the
body).
The problem is further complicated where it is desirable to consistently
deliver drug in
relatively small amounts at very low volume rates, and thus requires a small
drug delivery lumen.
Catheters having a small inner lumen for drug delivery are often extremely
difficult to handle due to,
for example, their fragility and their small outer diameters. Adapting a
catheter having a larger outer
diameter to have a smaller inner lumen can provide a catheter that is
relatively easy to handle, but too
stiff or too thick for implantation through tortuous bends in the implantation
pathway that leads to the
treatment site.
Various approaches have been taken to placing a catheter in a desired location
in the body in
non-drug delivery contexts. Two commonly used types of dilatation catheters
used in balloon
angioplasty are referred to as "over-the-wire" catheters, and "non-over-the-
wire" catheters. A non-
over-the-wire catheter acts as its own guide wire, and thus there is no need
for a separate guide wire
lumen. An over-the-wire catheter is one in which a separate guide wire lumen
is provided in the
catheter. Placement of this type of catheter in the body is a two-step
process. A guide wire is used to
establish a path to, for example, a stenosis in a blood vessel. The catheter
is then advanced over the
guide wire until the distal end of the catheter reaches its destination.
During advancement of the
catheter over the guidewire, frictional forces build, which increase with
increasing length of the guide
wire lumen, thus hampering placement of the catheter. The so-called "monorail"
catheter was
developed as an approach to reduce these fiictional forces and thus to
facilitate implantation of the
catheter. See, for example, U.S. Patent Nos. 4,762.129; and 5,350,395. In
monorail-type catheters, a
separate guidewire lumen is provided, which is substantially shorter than the
overall length of the
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
catheter. A guidewire is introduced into a vessel of a subject, then the
catheter is inserted over the
guidewire, using the guidewire lumen. Because the guidewire lumen is shorter
than the overall length
of the catheter, the frictional forces as the catheter is moved over the
guidewire are reduced compared
with catheters in which the guidewire lumen run the length of the catheter.
Since the guide wire lumen
in these monorail catheters is open at both ends, there is still a requirement
for a two-step process for
catheter placement.
The aforementioned catheters were typically used for balloon angioplasty, and
as such had
relatively large outer diameters. The relatively large outer diameter of these
catheters renders them
unsuitable for implantation and drug delivery to sites in the body such as the
spinal cord.
Furthermore, a catheter design which requires a two-step process for
implantation is also unsuitable
for delivery of drugs, in part because introducing a guidewire into such
locations carries with it the
inherent risk of damaging tissue at the implantation site.
"Microbore" catheters have been developed for delivery of drugs to various
sites in the body
(see, e.g., U.S. Patent No. x,820,610). Such catheters typically have a
delivery lumen inner diameter
of about 0.015 inch, and are thus adapted for delivery of volumes of
formulation in the range of
milliliters per day. However, there are many treatment situations which it is
desirable to deliver a
given drug to a relatively inaccessible site in the body over an extended
period of time, for example,
several hours, days, or weeks. In many of these situations, the drug is needed
in only very low
quantities and/or at a low volume rate over an extended time period. In these
instances, the features of
consistency, accuracy, and reliability of very low volume rate delivery which
are indispensable for
particular types of treatment are simply not met with existing microbore
catheters. Furthermore,
because of the small outer diameter, as well as the flexibility of the
catheters, both desirable features
for delivery to relatively inaccessible sites in the body, the catheters are
difficult to implant, as they
lack the required stiffness.
In general, catheters for use in drug delivery must meet the opposing demands
of having
sufficient stiffness so as to allow a user to implant the catheter to
relatively inaccessible sites in the
body, yet possessing sufficient flexibility to allow guidance through tortuous
passageways, at the same
time being capable of delivering microliter and submicroliter quantities of a
drug formulation per day.
There is thus a need in the field for a drug delivery catheter that is
biocompatible, flexible, and suitable
for delivery of drug at low volume rates, and readily implantable. The present
invention addresses
these problems, and provides related advantages as well.
SUMMARY OF THE INVENTION
The present invention features a catheter suitable for drug delivery. The
catheter comprises a
catheter body comprising a proximal and a distal end, and defining a drug
delivery lumen and a stylet
-2-
CA 02389028 2002-05-13
WO 01/41858 PCT/LTS00/33476
lumen. The stylet lumen comprises a distal end configured to permit a stylet
to abut the stylet lumen
distal end, and a proximal aperture which is distal to the proximal end of the
catheter, providing entry
of the stylet into the side of the catheter. The stylet lumen is adapted for
slidably receiving a stylet
which can be used to guide the catheter to the intended site in the body of a
subject and thus to
facilitate implantation of the catheter.
In some embodiments, the stylet lumen and the delivery lumen are juxtaposed.
1n some of
these embodiments, the catheter body comprises two integral, juxtaposed
elongate members which
define the delivery lumen and the stylet lumen. In other of these embodiments,
the catheter body
comprises a single elongate member through which both delivery and stylet
lumen extend. In other
embodiments, the stylet lumen and the delivery lumen are coaxial.
In some embodiments, the stylet lumen distal end is at least partially closed
or is completely
closed. In other embodiments, the stylet lumen distal end has one or more
openings, which openings
are sized such that the stylet cannot pass through. Thus, the stylet is pushed
against the stylet lumen
distal end to position the catheter body within the subject. In those
embodiments in which the stylet
lumen distal end comprises one or more openings, fluid from an anatomical site
in the body of the
subject can be sampled through the stylet lumen.
In other aspects, the invention features a drug delivery system comprising a
drug delivery
device and a catheter of the invention and a method for delivery of drug using
the catheter of the
invention.
A primary object of the invention is to provide a catheter that can be readily
handled,
implanted, and cut to length, and that can be readily adapted for use in
accurate, consistent, and
reliable delivery of drug at a particularly low volume rate, e.g., microliter
or submicroliter quantities
of a liquid or semisolid drug formulation per day.
Another object of the invention is to provide a catheter that is pre-attached
to a drug delivery
device, thus eliminating the need for a physician or other health case worker
to effect the connection
between the catheter and the delivery device. The catheter-delivery device
assembly may be provided
with a stylet positioned in the stylet lumen. The entire assembly may be
provided as a sterile unit.
Another object of the invention is to provide a catheter that can be used with
a variety of drug
delivery systems to accomplish site-specific drug delivery.
It is another object of the invention to provide a catheter that is suitable
for delivery of drug to
a distal treatment site within a subject, particularly sites that are highly
sensitive or fragile (e.g., the
spinal cord).
An advantage of the catheter of the present invention lies in the fact that a
proximal section of
the catheter comprises a delivery lumen (and not a stylet lumen), which
proximal section extends
proximally beyond the aperture which receives the stylet. This proximal
section has a single lumen
-3-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
and therefore can be cut to length and attached to a delivery device. This
feature eliminates the
possibility that the delivery device will be inadvertently attached to the
wrong lumen (i.e., the stylet
lumen), an error which might otherwise occur if the stylet and delivery lumen
proximal ends were co-
terminal.
Another advantage of the catheter of the present invention is that, since the
stylet lumen is
separate from the drug delivery lumen, the stylet lumen can be larger than the
drug delivery lumen.
Thus, the stylet lumen can accommodate a stylet which is of sufficient size so
as to provide the
stiffness necessary to guide the catheter to the site of treatment.
A further advantage of the catheter of the present invention is conferred by
openings) in the
stylet lumen distal end. This opening(s), when present, allows one to sample
fluid from an anatomical
site in the body of the subject. A fluid sample can provide diagnostic
information, and can establish
the location or placement of the distal end of the catheter.
Another important advantage of the invention is that the catheter can
facilitate delivery of
extremely small volumes of drug (e.g., submicroliter volumes) and at low
volume delivery rate, yet is
easily handled, e.g., by a clinician during implantation. This advantage ofthe
catheter is provided by
the combined characteristics of the delivery lumen, which provides the drug
delivery conduit, and the
stylet lumen, which provides for ease of guiding of the catheter to the
intended site of delivery of
formulation. Furthermore, by facilitating delivery of small volumes of drug to
a specific treatment
site, the catheter reduces the economic costs as well as the risks associated
with systemic dosing.
Another important advantage is that the side exit of the stylet allows for pre-
attachment of the
catheter to a delivery device. A single- or mufti-lumen catheter would not be
pre-attached, as such an
arrangement would not allow facile removal of the stylet, which is needed for
implantation, but which
must subsequently be removed.
The small outer diameter of the catheter body confers the advantage of
allowing access
through small bore needles during implantation or to sites in the body
accessible only by fine
passageways.
These and other objects, advantages and features of the present invention will
become
apparent to those skilled in the art upon reading this disclosure in
combination with drawings wherein
like numerals refer to like components throughout.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic drawing showing the general features of an exemplary
catheter of the
invention.
-4-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
Figure 2 is a cut-away view of the portion of the catheter body comprising the
stylet lumen
proximal aperture, and shows the arrangement of the stylet lumen and delivery
lumen. Figure 3 is
a cut-away view of the distal end of the catheter body, and shows stylet
lumen, delivery lumen,
delivery lumen distal aperture, and stylet lumen closed distal end.
Figure 4 is a cross-sectional view of the portion of the catheter body
comprising the stylet
lumen proximal aperture, and shows the arrangement of stylet lumen proximal
aperture, and delivery
lumen.
Figure 5 is a cross-sectional view of the distal section of the catheter body,
and shows stylet
lumen, and delivery lumen.
Figure 6 shows the catheter body distal end, with delivery lumen distal
aperture.
Figures 7A, 7B, 8A and 8B illustrate alternative exemplary embodiments of the
stylet lumen
proximal aperture. Figures 7A and 8A are cut away views; Figures 7B and 8B are
perspective views.
Figures 9-13 illustrate an alternative exemplary embodiment ofthe catheter
having integral,
juxtaposed arrangement of stylet and delivery lumen.
Figures 14-17 illustrate an alternative exemplary embodiments of the catheter
having coaxial
arrangement of stylet and delivery lumen.
Figures 18-20 depict exemplary embodiments in which the stylet lumen distal
end comprises
one or more openings. Figure 18 is a cut-away view; Figures 19 and 20 are end
views.
Figure 21 is a cut-away view of a delivery system of the invention comprising
a catheter of the
invention, and an attachment element, and attached for use with a drug
delivery device.
Figure 22 illustrates an embodiment of the invention in which the catheter
body is attached to
a drug delivery device. In this example, a stylet is positioned within the
stylet lumen.
Figures 23A and 23B illustrate an embodiment of the invention in which the
catheter body is
attached to the drug delivery device, and a connector further secures the
catheter body to the delivery
device. Figure 23A is a perspective view; Figure 23B is a cut-away view.
DETAILED DESCRIPTION OF THE INVENTION
Before the present catheter, method of drug delivery, and specific devices and
formulations
used in connection with such are described, it is to be understood that this
invention is not limited to
the particular embodiments described, as such methods, devices, and
formulations may, of course,
vary. It is also to be understood that the terminology used herein is for the
purpose of describing
particular embodiments only, and is not intended to limit the scope of the
present invention which will
be limited only by the appended claims.
-5-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
It must be noted that as used herein and in the appended claims, the singular
forms "a," "an,"
and "the" include plural referents unless the context clearly dictates
otherwise. Thus, for example,
reference to "a catheter" includes one or more catheters, reference to "a
formulation" includes mixtures
of different formulations, and reference to "the method of delivery" includes
reference to equivalent
steps and methods known to those skilled in the art, and so forth.
Unless defined otherwise. all technical and scientific terms used herein have
the same meaning
as commonly understood by one of ordinary skill in the art to which this
invention belongs. Although
any methods and materials similar or equivalent to those described herein can
be used in the practice or
testing of the present invention, the preferred methods and materials are now
described. All
publications mentioned herein are incorporated herein by reference to disclose
and describe the specific
methods and/or materials in connection with which the publications are cited.
The publications discussed herein are provided solely for their disclosure
prior to the filing
date of the present application. Nothing herein is to be construed as an
admission that the present
invention is not entitled to antedate such publication by virtue of prior
invention. Further, the dates of
publication provided may be different from the actual publication dates which
may need to be
independently confirmed.
Definitions
"Drug delivery device" as used herein is meant to encompass any device that
comprises a drug
reservoir and that facilitates movement of drug from the drug reservoir to a
site external to the drug
delivery device. "Drug delivery device" thus encompasses controlled drug
release devices, as well as
devices that release drug in an unpatterned (e.g., substantially unregulated)
manner. Controlled drug
release devices are particularly preferred for use with the catheter of the
present invention.
"Controlled release" as used herein (e.g., in the context of "controlled drug
release") is meant
to encompass release of substance (e.g., a drug) at a selected or otherwise
controllable rate, interval,
and/or amount. "Controlled release" thus encompasses, but is not necessarily
limited to, substantially
continuous delivery, patterned delivery (e.g., intermittent delivery over a
period of time that is
interrupted by regular or irregular time intervals), and delivery of a bolus
of a selected substance (e.g.,
as a pre-determined, discrete amount of a substance over a relatively short
period of time (e.g., a few
seconds or minutes).
The term "controlled drug release device" is meant to encompass any device
that provides for
controlled release of a drug or other desired substance, and that can be
adapted for use with a catheter
ofthe invention, e.g., a drug delivery device that provides for controlled
release of drug through a
catheter of the invention, and at a rate that is suitable to
-6-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
accomplish delivery of a therapeutically effective amount of drug to a
treatment site according to the
methods of the invention.
The term "low volume rate drug delivery" as used herein generally refers to
delivery of a liquid
or semisolid drug at a volume rate of from about 0.01 ~l/day to about 200
pl/day, usually about
0.04 pl/day to about 20 ~I/day, more usually about 0.1 ~l/day to about 8.0
~1/day.
The term "treatment site" as used herein is meant to refer to a desired site
for delivery of drug
from a drug delivery device of the invention, and/or a site from which fluid
sampling is desired, e.g.,
for diagnosis and/or prognosis. "Treatment site" is thus meant to include,
although is not necessarily
limited to, a subcutaneous, percutaneous, intravenous, intrathecal,
intramuscular, intra-arterial,
intravascular, intraperitoneal, intraspinal, epidural, intracranial,
peritumoral, or intratumoral (i.e.,
within a cancerous growth) site within a subject, as well as
sites within or near a selected organ or tissue (e.g., central nervous system
(e.g., spinal fluid, brain,
etc.), peripheral nervous system, kidney, liver, pancreas, heart (e.g.,
intrapericardial), lung, eye, ear
(e.g., inner ear), lymph nodes, breast, prostate, ovaries, testicles, thyroid,
spleen, etc. ), digestive
system (e.g., stomach, gastrointestinal tract, etc.), skeletal muscle, bone,
urinary bladder, gall bladder,
adrenal gland, adipose tissue, parathyroid gland, uterus, fallopian tube,
skin, into a vessel associated
with the circulatory system (e.g., artery, arteriole, blood vessel, vein,
capillary bed, lymph vessel,
particularly arteries that feed a selected organ or tissue)), a tumorous
growth (e.g., cancerous tumor
(e.g., solid tumor), cyst, etc.), at a site associated with a microbial
infection (e.g., bacterial, viral,
parasitic or fungal infection), or to an autologous or synthetic graft (e.g.,
a vascular graft).
The term "access site" or "implantation site" is used to refer to a site on or
in a subject at
which a catheter of the invention is introduced for implantation and
positioning within the subject's
body, e.g., for delivery of drug to a desired treatment site. For example,
where a catheter is implanted
in a subject for delivery of drug to the spinal cord, the access site or
implantation site can be a
subcutaneous site at which a proximal end of the catheter is substantially
retained, and the treatment
site is a position within or adjacent the spinal cord (treatment site) at
which a distal end of the catheter
is positioned for delivery of drug.
"Drug delivery system" as used herein is meant to refer to a combination of a
catheter of the
invention and a drug delivery device suitable for use in delivery of a drug to
a treatment site,
preferably a controlled drug release device.
The term "subject" is meant any subject, generally a mammal (e.g., human,
canine, feline,
equine, bovine, etc.), to which drug delivery is desired.
The term "impermeable" means that the material is sufficiently impermeable to
environmental
fluids as well as ingredients contained within the dispensing device such that
the migration of such
materials into or out of the device through the impermeable device is so low
as to have substantially no
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
adverse impact on the activity or function of the drug retained within the
device during the delivery
period.
The terms "drug formulation", "formulation" and "drug", used interchangeably
herein, are
meant to encompass any substance suitable for delivery to a treatment site of
a subject, which
substances can include pharmaceutically active drugs, as well as biocompatible
substances that do not
exhibit a pharmaceutical activity in and of themselves, but that provide for a
desired effect at a
treatment site, e.g., to flush or irrigate a treatment site (e.g., saline).
The term "proximal end" (or "first end") is used herein in connection with
components and/or
structures which are closer to a clinician or other individual who is using
the catheter and/or devices of
the invention in a medical treatment setting. Conversely, the term "dista.l
end" (or "second end") is
used herein in connection with components and/or structures which are closer
to the treatment site or
sampling site within the body of the subject being treated.
Overview of the Invention
The catheter of the present invention comprises a catheter body which defines
a delivery lumen
which generally extends the length of the catheter body, from at or near the
catheter body proximal end
to at or near the catheter body distal end. The catheter of this invention
further defines a stylet lumen.
The delivery lumen and the stylet lumen are generally separated from one
another by an impermeable
material, e.g., the stylet lumen and delivery lumen are not in fluid
communication. While the delivery
lumen extends from at or near a proximal end to at or near a distal end of the
catheter body, the stylet
lumen has a proximal aperture distal to the catheter body proximal end. The
relative position of the
stylet proximal aperture provides a side entry for inserting a stylet into the
catheter. The invention is
also advantageous in that the catheter body comprises a proximal section or
region that defines a
delivery lumen, but not a stylet lumen. This feature of the stylet lumen
provides an important
advantage by allowing the proximal extension to be cut to any desired length
without interfering with
the stylet lumen.
Another salient feature of the stylet lumen defined by the catheter body is
its distal end, which
is configured to permit a stylet to abut the stylet lumen distal end, i.e.,
the stylet cannot pass through
the stylet lumen distal end. This feature confers a further advantage in that
the stylet lumen is adapted
to slidably receive a stylet, which is used to push the catheter to a site of
treatment in the body. The
stylet provides the stiffness necessary to guide the catheter to the site of
treatment without the need for
introduction of a guidewire prior to insertion of the catheter. Thus, the risk
of tissue damage by the
stylet is effectively reduced or eliminated. The distal end of the stylet
lumen is sufficiently close to the
catheter body end so that control over the direction of the catheter body
distal end is maintained. The
_g_
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
stylet lumen distal end may be closed. or may have one or more openings. When
the stylet lumen
distal end comprises one or more openings, the openings) allow sampling of
fluid from an anatomical
site in the body of the subject. A fluid sample can be further analyzed for
diagnostic purposes, and
can also provide information as to the location of the distal end of the
catheter within the body. As an
example, when the catheter is to be used for delivery to an intrathecal site,
a fluid sample which
comprises cerebrospinal fluid is an indication that intrathecal implantation
has been achieved.
In one embodiment of the catheter of the present invention, the stylet lumen
has a larger inner
diameter than the delivery lumen, e.g., the delivery lumen diameter is on the
order of hundredths to
thousandths of an inch. In this embodiment, fluid sampling through the stylet
lumen would be more
practical than through the delivery lumen.
The catheter body is made of a highly flexible, biocompatible material. After
placement of the
catheter in the body, the stylet is generally removed. thus effectively
removing the stiffness conferred
by the stylet, so that the feature of catheter body flexibility is restored.
The flexibility feature is
important in a catheter which is maintained in the body over extended periods
of time, since damage to
the tissues surrounding the implanted catheter upon movement by the subject
into which the catheter is
implanted is reduced or eliminated.
While the catheter of the invention is primarily intended for use in drug
delivery, it may also
be used in the course of other medical procedures, including, e.g., to
facilitate sampling of fluid (e.g.,
spinal fluid) from the treatment site, etc.; delivery of energy, such as in an
ablation treatment; in
imaging methods, e.g., delivery of ultrasound energy; or a microendoscopic or
angioscopic-type
device.
The catheter body, stylet lumen, delivery lumen, and various exemplary
embodiments of the
catheter will now be described in more detail.
CATHETER BODY MATERIALS AND GENERAL CHARACTERISTICS
The catheter body is generally a flexible elongate structure comprising a
proximal end, a distal
end, and an outer surface. The catheter body can be any suitable shape
including, but not limited to,
tubular, elliptical, cylindrical, etc., and may be either smooth on the
catheter outer surface, or may
comprise ridges (e.g., longitudinal, axial, or circumferential) or other
surface variations as will be
desirable for the specific applications for which the catheter is used.
Dimensions
In general, the dimensions of the catheter (e. g. , overall length, outer
diameter, inner diameter,
wall thiclrness, etc. ) can be varied as required or desired, and will vary
according to a variety of
-9-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
factors (e.g., the treatment site for delivery, the drug delivery device used
in connection with the
catheter, etc.). For example, the inner diameter of the drug delivery lumen of
catheter can be equal to,
or can be greater or less than, the diameter of an orifice from which drug
flows from a drug reservoir
of a drug delivery device that is to be used with the catheter. In one
embodiment, the delivery lumen of
the catheter can comprise an inner diameter that is equal to or greater than
the diameter of the orifice
of a drug release device to be used with the catheter, and then taper over its
length to a relatively
smaller inner diameter, e.g., to provide a drug delivery outlet at the distal
end. In general, the inner
and outer diameters of at least the proximal end of the catheter is preferably
of a size sufficient to
provide a leak-resistant or leak-proof drug flow path from a drug reservoir of
the drug delivery device
through the catheter drug delivery conduit.
In general, the catheter may have an overall length in the range from about
0.4 inch to about
80 inches, e.g., from about 2 inches to about 60 inches, e.g., from about 4
inches to about 40 inches,
e.g., from about 6 inches to about 30 inches.
In general, the catheter of the invention can be described as comprising a
proximal section and
a distal section. In its distal section, the catheter body defines a delivery
lumen and a stylet lumen. In
its proximal section, the catheter body does not define a stylet lumen, but
defines a delivery lumen
which is continuous with the delivery lumen defined by the catheter body in
its distal section. Thus,
the proximal section can be cut to length without disturbing the stylet lumen.
The catheter body proximal section (the section of the catheter having a
delivery lumen but no
stylet lumen) can be of any desired length. For example, the length of the
distance between the stylet
proximal aperture and the proximal end of the catheter body can be from about
0.40 inches to about 4
inches or more.
The catheter body outer diameter can be substantially the same throughout its
length, or can
be varied (e.g., tapered, greater over the distal section than the proximal
section, etc.). In one
exemplary embodiment, the outer diameter of the catheter body changes abruptly
at the stylet aperture
such that the section of the catheter body which defines the delivery lumen
and not the stylet lumen
(i.e., the proximal section) has a smaller outer diameter than the section of
the catheter body which
defines both a delivery lumen and a stylet lumen. Alternatively, the proximal
and distal ends can have
substantially the same outer diameter.
In exemplary, non-limiting embodiments, the outer diameter of the distal
section of the
catheter body (the section comprising both delivery lumen and a stylet lumen)
is generally from about
0.01" (0.01 inch) to about 0.100", e.g., from about 0.015" to about 0.080",
e.g., from about 0.020" to
about 0.070", or from about 0.030" to about 0.060". In one embodiment, the
catheter body distal
section outer diameter is from about 0.040" to about 0.050". The outer
diameter of the proximal
section of the catheter body is usually from about 0.01 inch to about 0.100
inch.
-10-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
Delivery lumen inner diameter
The portion of the drug delivery conduit defined by the delivery lumen is of
an inner diameter
compatible with the desired delivery characteristics for the drug. For
example, in some embodiments,
the delivery lumen inner diameter is compatible for delivery at a relatively
low volume rate, e.g., as
low as about 0.01/pl/day. As with the catheter outer diameter, the delivery
lumen inner diameter can
be substantially the same throughout the entire length of the catheter, or can
vary along the catheter's
length. For example, the drug delivery conduit catheter can be tapered or
narrowed at any point along
the catheter body, e.g., tapered at a distal end, or can be widened at any
point along the catheter body,
e.g., widened over a distal portion of the catheter.
In exemplary, non-limiting embodiments, the inner diameter of the catheter
body defining the
delivery lumen can be from about 0.0002" to about 0.025 ", from about 0.0005"
to about 0.015 ", from
about 0.001" to about 0.0125", or from about 0.002" to about 0.010".
In one preferred embodiment, the lumen comprises a nickel titanium alloy. The
inner diameter
of the delivery lumen is about 0.003" to 0.006", and the outer diameter of the
distal section of the
catheter is about 0.005" to about 0.012".
Stylet lumen
Generally, the stylet lumen is adapted to slidably receive the stylet, which
is then removed
once the catheter has been placed at the desired site in the body. Where the
stylet is removed from the
catheter, it may be preferable to enhance the slideability of the surfaces of
the stylet, stylet lumen inner
wall, e.g., by Teflon or parylene coating of the stylet, etc.
In general, the inner diameter of the stylet lumen can be of a desirable size,
e.g., from about
0.0070 inch to about 0.030 inch, e.g., from about 0.0075 inch to about 0.025
inch, or from about
0.010 inch to about 0.020 inch.
The distance between the stylet distal end and the distal end of the catheter
body can vary,
depending on the implantation site and treatment site of catheter placement
contemplated, on the
application, and on the relative stiffness of the distal section of the
catheter body desired during
implantation. In general, this distance is less than about 8 inches, e.g.,
less than about 4 inches, e.g.,
less than about 2 inches, and generally between 0.004 inch and 4 inches, e.g.,
between about 0.04 inch
and about 0.8 inch.
The stylet lumen is configured such that the stylet abuts the stylet lumen
distal end, i.e., the
stylet cannot pass through the stylet lumen distal end. In some embodiments,
the stylet lumen distal
end is closed.
-11-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
In other embodiments, the stylet lumen distal end has one or more openings, as
long as the
stylet cannot pass through the stylet lumen distal end. Stylet lumen distal
end openings) are referred
to herein as "stylet lumen distal aperture(s)" or "sampling aperture(s)." In
these embodiments, the
openings) is dimensioned such that the stylet cannot pass through. A sampling
aperture may have a
diameter from about 5% to about 95%, e.g., from about 10% to about 80%, from
about 25% to about
75 %, the diameter of the stylet. Thus, for example, if the stylet has a
diameter of about 0.006 ", an
opening in the stylet lumen distal end may be about 0.005" or less. However,
the openings) is large
enough to permit fluid sampling, e.g. to allow any fluid present at a given
anatomical site to pass
through upon application of negative pressure, at or near the stylet lumen
proximal aperture. In some
of these embodiments, sampling apertures are provided by a porous area, such
as a mesh, a filter, or a
porous membrane.
The proximal end of the stylet lumen comprises an opening, the "stylet lumen
proximal
aperture", which is adapted to receive a stylet. Once the catheter body has
been implanted, it is
generally desirable to remove the stylet. To prevent infection after
implantation, the stylet lumen may
be filled with a sterile material, may be filled with a material containing an
anti-microbial agent, and/or
can be sealed off. The stylet lumen proximal aperture can be configured such
that, upon removal of
the stylet, the stylet lumen is sealed off from the environment (e.g., the
atmosphere or the body). The
stylet lumen aperture may comprise a self sealing element, e.g., a diaphragm,
an iris-type closure, or a
septum.
The stylet lumen proximal aperture can also be configured to receive a locking
device or a
connector, e.g., to secure a drug reservoir onto the catheter body. Any of a
number of known
connectors are suitable for this purpose.
Catheter bodv materials
The catheter body comprises a biocompatible material, more preferably an
implantable grade
biocompatible material. The material of the catheter body that defines the
drug delivery conduit is
preferably substantially drug-impermeable and comprises a materials) that does
not react in an
unintended manner with the active agent formulation. The catheter body can be
made of a single
material, or can comprise two or more materials layered upon one another.
Exemplary materials include, but are not necessarily limited to, biocompatible
polymers,
elastomers, metals, metal alloys, glasses, laminates of hydrophilic polymers
and hydrophobic
polymers, multilaminates or polymer, metals, and/or glasses; and the like.
Exemplary biocompatible polymeric materials include, but are not necessarily
limited to,
homopolymers and copolymers of vinyl acetate (e.g., ethylene vinyl acetate
copolymer); homopolymers
and copolymers of acrylates (e.g., poly(methyl) methacrylate (PMMA),
polyethylmethacrylate,
-12-
CA 02389028 2002-05-13
WO 01/41858 PCT/LTS00/33476
ethylene glycol dimethacrylate, ethylene dimethacrylate and hydroxymethyl
methacrylate);
polyurethanes; polyethylenes; polyvinylchlorides; polycarbonates; polyamides;
polysulfones;
polyesters; polyimides; halogenated polymers (e.g., polytetrafluoroethylene
(PTFE), polyvinyl
fluoride, polychlorotrifluoroethylene, copolymers tetrafiuoroethylene and
hexafluoropropylene; PFA,
and the like); polyolefins (e.g., high density polyethylene (HDPE), low
density polyethylene (LDPE),
linear low density polyethylene (LLDPE), polypropylenes, and the like);
polystyrenes; nylons;
urethanes; homopolymers and copolymers of acrylonitrile (e.g., acrylonitrile-
butadiene-styrene
polymer, styrene acrylonitrile, polycarbonate-acrylonitrile-butadiene-styrene;
and the like);
polyvinylpyrrolidone; 2-pyrrolidone; polyacrylonitrile butadiene; cellulose
acetate; polyethylene
terephtholate; polymethylpentene; polyisobutylene; polymethylstyrene;
polyvinylidine chloride and
homopolymers and copolymers of polyvinylidine chloride (e.g.,
polyvinylchloride-acrylic copolymers);
PEBAXTM; HYTRELTM; and other similar compounds known to those skilled in the
art. Further
exemplary polymers are described in Plastics Materials 6'h ed., May 1995, J.A.
Brydson, Butterworth-
Heinemann, publishers.
Suitable, biocompatible elastomers include, but are not necessarily limited
to, biocompatible
elastomers such as medical grade synthetic (e.g., silicone) rubbers; polyvinyl
chloride elastomers;
polyolefins; homopolymeric and copolymeric elastomers; urethane-based
elastomers; natural rubbers;
and fluorinated polymers (e.g., PTFE), and the like.
Metallic materials suitable for the catheter body comprise stainless steel,
titanium, platinum,
tantalum, gold and their alloys; gold-plated ferrous alloys; platinum-plated
titanium, stainless steel,
tantalum, gold and their alloys as well as other ferrous alloys; cobalt-
chromium alloys; titanium
nitride-coated stainless steel, titanium, platinum, tantalum, gold, and their
alloys; TEFLONTM; nickel
titanium; and superelastic nickel titanium.
In one embodiment, the catheter body comprises nickel titanium, particularly
superelastic
nickel titanium (NITINOLTM). In another embodiment, the catheter body
comprises a silicone or a
polyurethane elastomer.
The catheter body can comprise additional materials or agents. For example,
the catheter can
comprise a coating on an internal wall of the delivery lumen to facilitate
transport of drug through the
delivery lumen, or to impart other desirable characteristics to the catheter.
The catheter lumen can
also comprise coatings that reduce the risk of infection, e.g., a silver
coating, a coating or treatment
with an antimicrobial agent(s), etc. The outer wall of the catheter body can
comprise a coating or be
treated to facilitate implantation of the catheter within the subject, to
reduce the risk of infection,
and/or to impart other desirable characteristics to the catheter.
-13-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
Stylet
The catheter of the present invention is positioned in the body by use of a
stylet which abuts
the distal end of the stylet lumen, thus effectively allowing one to push the
catheter through the body.
The stylet facilitates pushing of the catheter to an implantation site. The
characteristics) conferred on
the catheter by the stylet can be varied by, for example, varying the
materials from which the stylet is
made, varying the structure of the stylet (e.g., a coil-like structure, a rod-
like structure, other structure
that provides compression strength), and varying the thickness of the stylet.
The stylet can be provided in a variety of configurations (e.g., geometrical
shapes, e.g.,
substantially straight or in a pre-set shape to give all or a portion of the
catheter a desired
configuration to facilitate access to a specific region) and a variety of
dimensions (e.g., length,
diameter, etc. ) as suitable to provide the desired compression strength.
The stylet diameter can be substantially the same throughout its length, or
may vary, e.g., the
stylet diameter can be greater over those portions of the catheter where
increased relative stiffness is
desired and less over those portions of the catheter where increased relative
flexibility is desired. The
dimensions and/or configuration of the stylet can be varied with the
dimensions of the stylet lumen. In
some embodiments it may be desirable to use a stylet having a combination of
structural
configurations.
Materials for the stylet can be selected according to the desired design,
e.g., to provide for
compression strength. Exemplary materials for use in the stylet include, but
are not necessarily limited
to, metals (e.g., stainless steel wire, parylene-coated or Teflon-coated
stainless steel), metal alloys),
polymers (e.g., particularly polymers of relatively high flex modulus, e.g.,
carbon fiber,) and the like.
An example of a compression stylet is a wire-like element, preferably teflon-
coated stainless steel,
having a diameter within the range of from about 0.006" to about 0.030".
The invention will now be described in further detail and with reference to
the drawings. The
embodiments described below or in the figures are only exemplary and are not
meant to be limiting in
any way.
EXEMPLARY SPECIFIC EMBODIMENTS OF THE CATHETER OF THE INVENTION
Referring generally to one non-limiting embodiment of the catheter of the
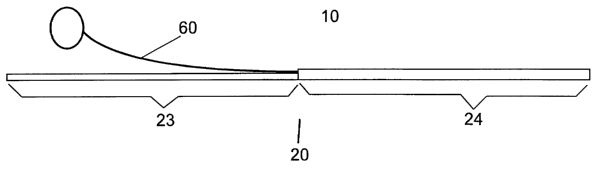
invention illustrated
in Figure 1, the catheter 10 of the invention comprises a catheter body 20
which comprises a proximal
section 23 and a distal section 24. The distal section defines both delivery
and stylet lumen. The
proximal section defines a delivery lumen, which is continuous with the
delivery lumen of the distal
section. A stylet 60 enters the stylet lumen at the stylet lumen proximal
aperture.
-14-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
Figure 2 shows a cut-away view of the portion of the catheter body 20
comprising the stylet
lumen aperture 73. In this cut-away view, the stylet lumen proximal aperture
73 is adapted to slidably
receive a stylet, which enters the stylet lumen 70. The delivery lumen 50 is
shown juxtaposed to the
stylet lumen 70. Figure 3 is a cut-away view of the distal end of the catheter
body 20, and shows the
delivery lumen 50, the delivery lumen outlet 54 defined at a distal end 22 of
the catheter body, the
stylet lumen 70, and the stylet lumen distal end 72. Figure 4 is a cross-
sectional view of the catheter
body at a point just proximal of the stylet lumen proximal aperture 73 (i.e.,
at the junction of the
proximal and distal sections of the catheter), and shows the arrangement of
the delivery lumen 50
relative to the stylet lumen proximal aperture 73. Figure 5 is a cross-
sectional view of the distal
portion 24 of the catheter body 20, and shows the arrangement of the delivery
lumen 50 relative to the
stylet lumen 70. Figure 6 depicts the distal end 22 of the catheter body 20,
and shows the delivery
lumen distal aperture 54. In the exemplary embodiment shown in Figure 6, the
stylet lumen distal end
is closed.
Outer diameter of catheter body at the function between the proximal and
distal sections
While the outer diameter of the catheter body can change abruptly at the
stylet lumen aperture,
as described above, the outer diameter could also be substantially uniform
over, for example, the
junction between the proximal and distal sections. An exemplary variation is
shown in Figures 7A
(cut-away view) and 7B (perspective view), which illustrate catheter body 20
having an outer diameter
which is substantially uniform between the catheter body proximal section 23
and the catheter body
distal section 24. The stylet lumen 70 curves toward the catheter body outer
surface, such that the
stylet lumen aperture 73 is an opening in the sidewall of the catheter body,
as shown in Figure 7B.
Alternatively, as shown in Figures 8A (cut-away view) and 8B (perspective
view), a guidance groove
120, which serves to guide the stylet into the stylet lumen 70, is provided in
the outer surface of the
catheter body Z0.
In these embodiments, the easy cut-to-length feature can be retained, provided
the proximal
portion of the catheter body is cut at a site proximal to the stylet aperture,
or, alternatively, the
guidance groove.
First and second elongate members integral and juxtaposed
In one exemplary variation of the catheter body of the invention, as shown in
Figures 9-13, the
catheter body 20 comprises a first elongate member 30 defining the delivery
lumen 50. The catheter
body further comprises a second elongate member 40 which is juxtaposed to and
integral with the first
elongate member and which defines the stylet lumen 70. The first elongate
member is substantially
-15-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
longer than the second elongate member. The first and second elongate members
may be made of the
same or of different materials. Figure 10 shows a cut-away view of this
exemplary variation and
shows the arrangement of the delivery lumen 50 relative to the stylet lumen
70. Figure 11 is a cross-
sectional view of the portion of the catheter body comprising the stylet
aperture 73 and delivery lumen
50. Figure 12 is a cross-sectional view of the distal portion of the catheter
body of this variation,
comprising both delivery lumen 50 and stylet lumen 70.
In a fiuther variation, the second elongate member 40 can comprise a proximal
extension 45
which is not integral with the first elongate member 30, as shown in Figure
13.
Coaxial stylet and delivery lumen
The stylet lumen and the delivery lumen can be coaxial, i.e., the catheter
body can comprise an
elongate first member and an outer member which are coaxial and which define
the delivery and stylet
lumen, respectively. In these embodiments, the catheter body comprises a first
elongate member 30
defining a delivery lumen 50, and a second elongate member 40 defining,
together with the first
elongate member, a stylet lumen 70. Various aspects of this variation is shown
in Figures 14-16.
Figure 14 shows the proximal section 23 and the distal section 24 of the
catheter body 20. Figure 15
is a cut-away view illustrating how the stylet lumen 70 is defined by the
first 30 and second 40
elongate members of the catheter body 20, and the delivery lumen 50 is defined
by the first elongate
member 30 of the catheter body. The first elongate member is substantially
longer than the outer
member, and defines a delivery lumen extending from the catheter body proximal
end 21 to the
catheter body distal end 22. The second elongate member defines a stylet lumen
70 opening into a
stylet lumen proximal aperture 73 which is distal to the catheter body
proximal end, and wherein the
second elongate member defines a stylet lumen terminating at a distal end 72.
As shown in Figure 16,
the first elongate member 30 is substantially completely enclosed within the
second elongate member
40 along the length of the second elongate member. In this embodiment, the
first elongate member 30
and second elongate member 40 are fused to one another for a short distance
near the stylet lumen
proximal aperture 73. As shown in Figure 17, the stylet lumen 70 is defined by
the outer wall 36 of
the first elongate member 30 and the inner wall 44 of the second elongate
member 40 of the catheter
body 20, and the delivery lumen 50 is defined by the inner wall 37 of the
first elongate member 30 of
the catheter body. The first 30 and second 40 elongate members can be made
from the same materials
or from different materials.
The second elongate member 40 and first elongate members) 30 can be associated
in a variety
of ways to provide the catheter 10 of the invention. For example, the inner
and outer members may be
joined by connecting walls (not shown) at or near the stylet lumen proximal
aperture 73 and/or at a
-16-
CA 02389028 2002-05-13
WO 01/41858 PCT/L1S00/33476
distal end 22 of the catheter body 20. Alternatively or in addition, the first
and second elongate
members can be joined together at their ends by crimping, heat fusion,
ultrasonic welding,
radiofrequency welding, heat bonding, solvent bonding, soldering, etc. The
first and second elongate
members can be joined by a mechanical element, such as a press fit or locking
element. Alternatively
or in addition, the first and second elongate members can be connected by an
adhesive material, which
may be placed within a space between the first and second elongate members,
minimally at the extreme
proximal and distal ends of the first elongate member so as to hold the first
elongate member in place
within the second elongate member. Suitable adhesives include, but are not
necessarily limited to,
epoxy resins, ethylene vinyl acetate-based adhesives, polyurethane,
cyanoacrylates, W curable
adhesives, RTV silicone adhesives, as well as other silicone-based adhesives,
solvent bonding adhesive
substances, polyisobutylene (PIB)-based adhesives, and the like. Additional
suitable adhesives are
well known in the art, see, e.g., Handbook of Adhesive Technolo~y A. Pizzi and
K.L. Mittal, Jan.
1994, Marcel Dekker, publisher.
Stylet lumen distal end apertures)
Figures 18-20 depict exemplary embodiments in which the stylet lumen distal
end comprises
one or more openings. Figure 18 is a cut-away view of the distal end 22 of the
catheter body, showing
the delivery lumen distal aperture 54, and the stylet lumen 70 with stylet 60
positioned in stylet lumen
and abutting stylet lumen distal end 72. The stylet lumen distal end 72 is
defined in part by an inner
surface of the catheter body and provides a surface against which force can be
exerted via a stylet to
push the catheter into place. Figure 19 is an end view of an exemplary
embodiment in which stylet
lumen distal end has one opening 74. Figure 20 is an end view of an exemplary
embodiment in which
stylet lumen distal end has a plurality of openings 74. Openings 74 are
dimensioned to be smaller than
diameter of stylet.
ATTACHMENT ELEMENTS
The catheter of the invention can be modified to be permanently fixed to a
drug delivery device
(e.g., the catheter can be an extension of a drug delivery device component
(e.g., outer sheath of a drug
delivery device) or can be attached by welding, adhesive bonding, etc.).
Alternatively, the catheter of
the invention can comprise an attachment element 85 for attaching the catheter
to a drug delivery
device (see, e.g., Figure 21). The attachment element 85 facilitates and
maintains a connection
between a drug delivery device and a catheter of the invention, and thus
maintains a drug flow pathway
from a drug reservoir of a delivery device to a treatment site, during use in
drug delivery to a treatment
site. Such an attachment element may provide for permanent or reversible
attachment of the drug
-17-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
delivery device to the catheter, and preferably provides for a leak-proof seal
between the catheter and
the drug delivery device.
Any of a variety of attachment elements are compatible for use in the drug
delivery system of
the invention. The attachment element can be provided as a portion of or
component associated with
the catheter proximal end, the drug delivery device distal end, or a
combination of both. The
attachment element can be fashioned from or attached to a proximal section of
the catheter body,
which proximal portion defines a proximal portion of the delivery lumen. For
example, the attachment
element can be a press fit lock fashioned from or attached to a portion of
such the catheter proximal
extension. In another example, the attachment element is a combination of a
threaded connector
elements, luer lock elements, bayonet connectors, etc. Alternatively, the
attachment element can be
provided by a catheter receiving element positioned at the distal end of the
drug delivery device, e.g.,
the proximal end of the catheter can permanently or removably inserted into
the body of the drug
delivery device. In this latter embodiment, the proximal end of the catheter
may not require any
additional elements to accomplish attachment to the drug delivery device.
FURTHER VARIATIONS
The catheter may comprise a single drug outlet at or near the distal end for
delivery of drug at
or near a treatment site, or may comprise a plurality of such drug outlets
(e.g., in the form of side holes
along a portion of the distal end of the catheter that communicate with a drug
delivery conduit defined
by the inner diameter of the inner member, the outer member, or both). The
catheter may comprise a
single drug delivery conduit (e.g., defined by a single inner member), or may
comprise a plurality of
drug delivery conduits (e.g., defined by one or more inner members positioned
within a single outer
member).
The catheter of the invention can be further modified by providing a
radiopaque marker at one
or more locations along its length, or by impregnating or coating all or a
portion of the catheter body
with appropriate radiopaque dyes or other radiopaque materials. Suitable
radiopaque maskers can
comprise metal rings (e.g., platinum, palladium, gold, etc.) The provision of
radiopaque maskers is
well known in the art.
In other variations contemplated by the invention, the distal end of the
catheter is shaped so as
to allow for smooth passage through such tortuous bends. For example, the
distal end of the catheter
can be provided as a rounded tip that allows for the catheter to move smoothly
around such bends
(e.g., where a square-ended catheter tip might catch on the sidewalk of a
vessel or duct, thus
frustrating implantation or placing the subject at risk of injury). In other
variations, the distal end of
the catheter optionally ends in a one-way valve such as a duck bill valve to
prevent retrograde flow
into the catheter delivery lumen, with external pressure at that distal end.
Alternatively or in addition,
-18-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
the distal end may comprise a porous plug that serves as a filter element
preventing particulate matter
(including bacteria) from exiting from the catheter and into the treatment
site.
The catheter can also be provided as a multi-lumen catheter, e.g., a catheter
comprising a
plurality of delivery lumen and/or a plurality of stylet lumen. The catheter
can also comprise a distal
extension, which is positioned adjacent the distal end of the catheter body.
For example, the distal
extension can be floppy (i.e., flexible, e.g., has a low flexural modulus
relative to the flexural modulus
of the body of the catheter which defines both the stylet lumen and delivery
lumen). The flexibility of
the floppy distal extension facilitates the catheter's negotiation of curves
and tortuous bends (e.g., in
vessels, ducts, or arteries) during implantation, and further reduces the risk
of damage to the
surrounding tissue. The catheter distal extension can be of any desired
length, generally from about
0.4 inch to about 8 inches, usually from about 0.8 inch to about 4 inches.
DRUG DELIVERY DEVICES SUTTABLE FOR USE WTTH THE CATHETER OF THE INVENTION
The catheter of the invention can be provided in connection with a drug
delivery device to
provide a drug delivery system. In this embodiment, it may be desirable to
include a component that
facilitates attachment of the catheter to the drug delivery device and/or
stabilize such attachment, e.g.,
substantially diminish movement of the catheter in a direction perpendicular
to the longitudinal axis of
the drug delivery device (e.g., to provide strain relief), so as to reduce
risk of breakage of the catheter
at the attachment site.
As exemplified in Figure 21, the drug delivery device 80, having a proximal
end 82 and a
distal end 83, which distal end defines a drug delivery orifice 84. The drug
delivery orifice provides a
drug flow pathway from a drug reservoir (not shown) within the drug delivery
device, and may be
provided as a distinct opening or as a series of openings, e.g., as in the
context of a rate-limiting
membrane, which membrane defines a plurality of openings through which drug
may flow from the
drug reservoir. The distal end 83 of the drug delivery device is attached to
catheter body proximal
section 23 comprising delivery lumen 50 to provide a flow pathway from a drug
delivery device
reservoir in the drug delivery device 80 through the drug delivery device
orifice 84 and into the drug
delivery lumen 50 of the catheter body 20 of catheter 10. The catheter body
proximal section 23 thus
communicates with the drug delivery device in a manner that facilitates
movement of drug from the
drug
delivery device 80, through the catheter body 20, and out the drug delivery
outlet 54 of the catheter
body 20.
As exemplified in Figure 22, a catheter of the present invention can be
provided to a user pre-
attached to a delivery device. The catheter body 20 is shown attached to a
delivery device 80 at the
-19-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
proximal end 21 of the catheter body, with a stylet 60 positioned in the
stylet lumen. The entire
assembly or component thereof, e.g., catheter, stylet, and delivery device,
can be provided as a sterile
unit, in a sterile package (not shown).
A delivery system of the invention may further comprise a mechanical connector
130, which
facilitates attachment of the catheter to the drug delivery device. At least a
portion of the mechanical
connector 130 is adapted for attachment to (e.g., insertion into), or stable
positioning into, stylet lumen
proximal aperture 73. Another portion of the connector is adapted for
connecting (detachably or
fixedly) to delivery device 80. The mechanical connector serves to facilitate
and/or stabilize the
connection between the catheter and the delivery device, and may further serve
to seal the stylet lumen.
As exemplified in Figures 23A (perspective view) and 23B (cut-away view),
mechanical
connector 130 has a distal end 132 which can be detachably attached to (e.g.,
inserted into) proximal
sylet lumen aperture 73 once catheter body 20 is in position in the body of
the subject, and after stylet
is withdrawn. In this exemplary embodiment, connector 130 has a proximal end
131 which is attached
to delivery device 80, thereby locking catheter body onto delivery device.
Mechanical connector 130
can be of any type, e.g., it may have a plurality of barbs on its surface
which engage stylet lumen inner
surface; it may be configured such that it expands once inserted into stylet
lumen, thereby locking into
place; it may have threads that screw into stylet lumen which may be
conversely threaded to accept
connector. Insertion of a connector can also serve as a mechanical barrier to
limit or prevent
introduction of unwanted materials, e.g., microorganisms, into the stylet
lumen. A connector can also
serve to limit possible leakage of fluid from the body of the subject and out
through the stylet lumen
proximal aperture.
Drug delivery devices suitable for use in conjunction with the catheters of
the invention may
be based on any of a variety of drug delivery systems. For example, the drug
delivery device can be
based upon a diffusion-based delivery system, e.g., erosion-based delivery
systems (e.g., polymer-
impregnated with drug placed within a drug-impermeable reservoir in
communication with the drug
delivery conduit of the catheter of the invention), electrodiffusion systems,
and the like. In other
embodiments, the controlled drug release device is based upon a convective
drug delivery system, e.g.,
systems based upon electroosmosis, vapor pressure pumps, electrolytic pumps,
effervescent pumps,
piezoelectric pumps, osmotic pumps, etc.
In one embodiment, the drug delivery device comprises an osmotic pump, such as
an osmotic pump
similar to that described in U.S. Pat. No. 5,728,396. In one embodiment, the
osmotic pump is a
DUROSTM osmotic pump.
-20-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
Although controlled release of drug is described above as being primarily
attributed to
characteristics of the drug delivery device, other aspects, features, or
embodiments of the invention can
facilitate controlled release of drug, and are within the scope of and
contemplated by the present
invention. For example, characteristics of the catheter (e. g. , dimensions of
the drug delivery conduit,
particularly the inner diameter of the delivery lumen) can facilitate or
further facilitate controlled
release of drug from a drug reservoir to the treatment site. In another
example, the catheter can be
loaded with polymer that provides for controlled diffusion of drug from a drug
reservoir.
Any of a wide variety of drugs can be delivered using the drug delivery system
of the
invention. Drugs suitable for delivery are preferably provided as flowable
formulations, and are
generally provided as liquids or semisolids. The drugs may be anhydrous or
aqueous solutions,
suspensions or complexes, and may be formulated with pharmaceutically
acceptable vehicles or
carriers, as well as additional inert or active ingredients. The drugs of
formulations suitable for
delivery using the invention may be in various forms, such as uncharged
molecules, components of
molecular complexes or pharmacologically acceptable salts. Also, simple
derivatives of the agents
(such as prodrugs, ethers, esters, amides, etc.) that are easily hydrolyzed by
body pH, enzymes, etc.,
can be employed. Preferably the agents are formulated so as to remain stable
for long periods of
storage on the shelf or under refrigeration, as well as for long periods
stored in an implanted drug
delivery system of the invention.
Of particular interest is the treatment of diseases or conditions that require
long-term therapy,
e.g., chronic and/or persistent diseases or conditions for which therapy
involves treatment over a
period of several days (e.g., about 3 days to 10 days), to several weeks
(e.g., about 3 or 4 weeks to 6
weeks), to several months or years, up to including the remaining lifetime of
the subject. Subjects who
are not presently suffering from a disease or condition, but who are
susceptible to such may also
benefit from prophylactic therapies using the devices and methods of the
invention.
USE OF THE CATHETER OF THE INVENTION
The catheter ofthe invention can be used in a wide variety of subjects. For
example, the
catheter can be implanted with an associated drug delivery device at any
convenient site within the
subject's body and oriented for delivery to any desired treatment site. In one
embodiment, the catheter
and the associated drug delivery device are partially or completely implanted,
with at least portion of
the drug delivery device retained at an accessible, external or subcutaneous
site within the subject's
body (e.g., under the skin of the arm, shoulder, neck, back, or leg) or within
a body cavity (e.g., within
the mouth). The site of implantation can be at a site close (e.g., within a
few centimeters, e.g., within
about 0.8 inch), or at a site relatively distant (e. g. , more than about 12
inches, generally greater than
about 20 inches to about 40 inchess) from the treatment site, and thus the
ultimate site of drug
-21-
CA 02389028 2002-05-13
WO 01/41858 PCT/US00/33476
delivery. A single catheter and/or drug delivery device, or two or more
catheters and/or drug delivery
devices can be implanted in a subject during the course of a therapeutic
program.
In one embodiment, the catheter is primed with drug prior to implantation,
e.g., the drug
delivery conduit is substantially pre-filled with drug. Priming of the
catheter reduces delivery start-up
time, i. e., time related to movement of the drug from the drug delivery
device to the distal end of the
catheter. This feature is particularly advantageous where the drug delivery
device releases drug at or
below a low or very low volume rate (e.g., 0.4 p.l/day) or less. The drug used
to prime the catheter
may be the same drug that is delivered from the drug delivery device, or may
be a different drug or
different formulation of the drug, e.g., the catheter itself may provide for a
component of the
therapeutic regimen.
The catheter can be designed for temporary use, or to remain implanted in the
subject for an
extended period. e.g., from several days, to several weeks or months, and can
be designed to be
substantially permanently implanted in the subject (e.g., for the subject's
remaining lifespan). The
drug delivery devices can be removably attached to the catheter, or may be
permanently affixed.
Where the drug delivery device is removably attached, the drug delivery device
can be removed
following a desired drug administration period, and. where desirable replaced
with a similar or
different drug delivery device.
The devices ofthe present invention (e.g., catheter, drug delivery system
comprising a drug
delivery device and catheter) are preferably rendered sterile prior to use.
This may be
accomplished by separately sterilizing each component, e.g., by gamma
radiation, steam sterilization
or sterile filtration, etc., then aseptically assembling the final system.
Alternatively, the devices may be
assembled, then terminally sterilized using any appropriate method.
To implant the catheter, the stylet is inserted into the catheter, and used to
position the catheter
within the subject's body during implantation. Once the catheter is positioned
where desired, the stylet
is withdrawn. The empty lumen can then be filled with a material (e.g.,
liquid, solid, or semi-solid),
which material preferably comprises an antimicrobial agent (e.g.,
bacteriostatic or bactericidal agent),
and/or can be sealed off, i.e., the stylet lumen proximal aperture plugged
with a solid material,
crimped, or heat-sealed. Alternatively, the stylet can be substantially
permanently positioned within
the catheter stylet lumen.
Insertion of the catheter is generally accomplished in a manner similar to
insertion of any of a
variety of catheters, e.g., under aseptic conditions with at least some local
or general anesthesia
administered to the subject. Where the catheter comprises radiopaque material,
insertion of the
catheter can be monitored by X-ray or other means of visualization of the
insertion process.
After implanting, the proximal section of the catheter can be cut to length
and the drug
delivery device attached. The drug delivery device and any remaining portion
of the catheter
-22-
CA 02389028 2002-05-13
WO 01/41858 PCT/L1S00/33476
completely implanted in the subject. The drug delivery device, and generally
at least a portion of the
catheter, are retained at an access site as described above.
Where desired, the drug delivery device and/or catheter can be anchored within
the subject by
any suitable conventional means.' For example, sutures can be used to secure a
proximal end ofthe
drug delivery device and/or catheter at or near an implantation site.
Following implantation, the
catheter defines a drug delivery conduit that provides for transport of drug
from a proximal catheter
end to a distal catheter end, where the catheter proximal end is preferably
maintained at the initial
access site or implantation site and the catheter distal end is positioned so
a drug delivery outlet of the
catheter is positioned at, within, or adjacent the desired treatment site. The
invention as shown and
described is considered to be the one of the most practical and preferred
embodiments. It is
recognized, however, that the departures may be made therefrom which are
within the scope of the
invention and that obvious modifications will occur to one skilled in the art
upon reading this
disclosure.
-23-