Note: Descriptions are shown in the official language in which they were submitted.
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
- 1 -
CORPOREAL ACCESS TUBE ASSEMBLY
RELATED APPLICATION
This application is a divisional application of a
parent Application 2Jo. 2,242,557 filed January 10, 1997.
FIELD OF THE INVENTIONS
These inventions relate generally to medical
catheters. They relate particularly to catheters used to
access either the stomach and/or intestine, or the
bladder, through a stoma or ostomy in the abdominal wall.
BACKGROUND OF THE INVENTIONS
The need to artificially introduce food into
the gastrointestinal tracts of individuals who can not
eat, ar will not eat, has been well known throughout and
even prior to this century. Before the mid-1970's,
feeding was done nasogastrically with red rubber or
polyvinylchloride feeding tubes. The use of enteral
feeding by means of nasogastric tubes exp;anded~
dramatically in the late 1970's with the :introduction of
tubes constructed of either silicone rubber or
polyurethane. Being constructed of stronger materials,
these tubes incorporated thinner walls, a:nd were
therefore smaller in outside diameter. These smaller
tubes were easier to insert and more comfortable for the
patient, and their introduction resulted :in a very rapid
growth of enteral nutrition via the nasog;astric route,
and increased interest in enteral nutrition in general.
CA 02389154 2002-07-04
WO 97125095 PCT/US97/00502
_. _ 2 _
By the 1980's problems with nasogastric feeding
were recognized by clinicians and the advantages of
direct gastrostomy access into the stomach through the
abdominal wa~.l had been described by Vaz,quez in U.S. Pat.
4,356,824, and by Moss in U.S. Pat. 4,543,085.
Refinements in securing gastrostomy tubes in the patient
were described by Parks in U.S. Pat. 4,666,433 and in
U.S. Pat. 4,685,901. .
The 1980's also saw the refinement of methods
for forming the gastrostomy stoma. Prior to the 1980's,
the stoma or gastrostomy was formed surgically by the
Stamm procedure, which required a surgical laporatoratomy
to insert the tube, usually a latex urologic Foley
retention catheter. A new method, called a "PEG", or
Percutaneous Endoscopic Gastrostomy, eliminated the need
for a surgical gastrostomy to place the gastrostomy tube
and dramatically expanded the interest i:n the use of
direct~gastrostomy tubes. The advantages of PEGs and the
PEG technique were described by Quinn et al in U.S. Pat.
4,795,430. The word "PEG" is used herein to identify
both the tube and the procedure.
Gastrostomy tubes can generally be organized
into three main groups, the third of which includes two
subgroups:
1. SPECIALTY TUBES placed at the i:.irne of gastric
surgery by the Stamm technique. The Moss and Vazquez
patent tubes are examples of this type.
2. PEG tubes which are used to form the initial
stoma or gastrostomy.
3. REPLACEMENT TUBES which are used to replace the
PEG tube after a period of time because the PEG has worn
out with use, or because a device which is more specific
to the patient's need is required. These tubes are
inserted into the original stoma created by either the
PEG or the Stamm technique.
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
_.. - 3 -
a. LOW PROFILE REPLACEMENT TUBES which are
preferred for active patients who wish to
conceal the tube's outer fitments during
periods when they are not receiving
feeding formula. The background for this
type of replacement tube is described by
Quinn et al in U.S. Pat. No. 5,125,897.
b. SIMPLE REPLACEMENT tubes which are less
complicated and less expensive are used
for patients who are not active and have
no need to hide their device. These
devices are direct modifications of the
original urologic Foley catheters used in
early gastrostomies. They are described
by Parks in U.S. Pat. Na. 4,666,433.
With some exceptions within individual designs,
gastrostomy tubes or tube assemblies of they
aforedescribed types each incorporate the following seven
features or components:
1. A tube to carry the enteral feeding formula
into the stomach and or the intestine.
2. An outflow port in the distal end of the tube.
The port or ports may be incorporated in the
end or the side wall of the tube. They may
also be incorporated in a separate, molded
bolus fastened to the distal end of the tube.
3. An administration set connector attached to the
proximal end of the tube, which is outside of
the patient.
4. A distal end retention device to hold the tube
in the stomach, e.g., an inflatable balloon or
a molded retention shape which can be deformed
with a stylet for insertion and removal.
5. An external bolster to secure the tube at the
point where it exits the skin. This bolster
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
_. - 4 -
maintains the proper distance :between the
external bolster and the internal retention
device, a distance corresponding to the
combined thickness of the individual patient's
skin, abdominal wall and stomach wall at the
site of the gastrostomy.
6. An anti-reflux valve to prevent leakage of
gastric acids from the patient when the
administration set is being changed or when
violent coughing causes excess:Lve back
pressure.
7. A measurement system to measure the patient's
abdominal wall thickness so that the tube
length between the retention device and the
external bolster can be adjusted to match this
thickness.
Just as gastrostomy tubes or tube assemblies
are used for enteral feeding, so suprapubic catheter
tubes or tube assemblies are used to administer drugs to,
or drain urine from, the bladder. Such tubes or tube
assemblies comprise the same seven features or components
referred to above in the context of gastronomy tubes or
tube assemblies. However, they access tree bladder
through a stoma formed in the abdominal wall above the
bladder or pubic area.
SUMMARY OF THE INVENTION
The present inventions seek to resolve problems
which clinical practice has shown to be inherent in the
aforedescribed seven features or componer,~ts of different
types of known gastrostomy and suprapubic: catheterization
tubes or tube assemblies. As described i.n the background
materials, there is now little key component design
commonality between PEGS, low profile replacement tubes
and simple replacement tubes, for example:. The
inventions disclosed herein embody improvements which can
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/OOS02
- 5 -
be incorporated into all of the tube types, thereby
providing a common component system for all gastrostomy
and superpubic catheterization tubes.
In doing so these inventions overcome the major
problems associated with existing tubes, which are:
Difficulty of insertion and removal.
Difficulty of obtaining accurate measurement of
required tube depth.
Lack of durability of internal retention devices.
Incompatibility of replacement gastrostomy tubes
with PEGS.
Valve failure.
Infection of the stoma.
Difficulty in cleaning of the stoma.
The need for many tube sizes.
Inability to secure the tube adequately with an
external bolster.
The need for special administration sets.
These problems manifest themselves in tub<_ components as
foflows
1. THE TUBE. Silicone and polyurethane are
the materials of choice for these tubes. Silicone is
softer and more compliant than polyurethane. Silicone
has a lower modulus of elasticity than urethane.
Softness is desirable-in medical catheters. However,
softness also increases the ability to kink and collapse,
which are undesirable characteristics. These problems
have heretofore been addressed by making tube walls
thicker in silicone tubes or by constructing the tubes
from the stronger, but less flexible, polyurethane. The
designer has had to make a choice between a smaller, but
less flexible, urethane tube and a larger, softer
silicone tube.
CA 02389154 2002-07-04
WO 97/25095 t'CT/US97/00502
__ . _
Flexibility, resistance to kinking and
resistance to collapsing are characteristics which are
particularly important in gastrostomy and suprapubic
catheterization tubes. Because these tubes exit the body
perpendicular to the skin, it is desirable to be able to ,
bend them as close to a right angle as possible so that
they can lie next to the skin. This problem is addressed
in Quinn et al U.S. Pat. No. 4,834,712. Tn addition,
some forms of gastrostomy tubes have extensions which
feed out of the stomach into the duodenum or jejunum.
These tube extensions must be able to negotiate from one
to five acute angle turns, depending whether they are
placed in the duodenum or further into the jejunum.
Tubes with a higher modulus, i.e., less flexible tubes,
can dig into the side walls of the intestine and resist
making the required tight turns as they move through the
intestine.
2. OUT FLOW PORT IN DISTAL END OF TUBE. The
problems with conventional nasogastric feeding tube
outlet ports as to insertion, flow and clogging are
described by Andersen et al. in U.S. Pat. No. 4,594,074.
These problems are also common to gastroatomy tubes.
3. ADMINISTRATTON SET CONNECTOR. Existing
low profile tubes have administration set connectors
which exit perpendicular to the patient's skin. This
configuration is described in Quinn et a7.. 5,125,897. To
prevent the administration set from kinki.ng and twisting,
special sets with right angle connectors must be used so
that the administration set tubing can li.e comfortably on
the patient's abdomen.
4. DISTAL END RETENTION DEVICE TO HOLD THE
TUBE IN THE STOMACF? or bladder. Tubes with inflatable
silicone retention balloons are easy to insert because
the uninflated balloons are formed completely flat '
against the tube wall. However, they are: unreliable
because the silicone balloon walls which are stretched
CA 02389154 2002-07-04
WO 97/25095 PCTlUS97100502
thin tend to break easily. In addition they must
incorporate a second inflation lumen in the tube to
access the balloon. This feature makes the tube larger
and also requires the incorporation of a balloon
inflation valve which adds cost and bulk to the product.
Pre-farmed, molded internal retention devices
must be deformed with a styles and are difficult to
insert and remove. They are generally reliable once they
are in place, however.
5. EXTERNAL BOLSTER. Existing technology for
bolsters is well known, as the aforementioned prior art
illustrates. Some bolsters secure the tube, but do not
bend it at a right angle to position. it out of the way,
next to the skin. Others secure and bend it at a right
angle, but axe rigid parts which can be uncomfortable for
the patient. None of these rigid bolsters achieve full,
right-angle bending of the tube due to the stiffness of
the tubing.
6. ANTI-REFLUX VALVE. Existing valves
include flapper valves which clog and malfunction.
Furthermore, it is often necessary to open the valve to
decompress the stomach or bladder, so location of the
valve is also important. Some gastrostomy valves are
positioned so that special decompression sets are
required to activate them if feeding is not taking place.
7. TUBE MEASUREMENT AND STZING. PEGs and
simple gastrostomy tubes, for example, are=_ sized to the
patient after they are inserted. The position of the
external bolster is approximated by simultaneously
tugging on the internal retention member and then pushing
the external member down on the skin. This is an
imprecise method. Low profile replacement tubes have
separate stoma depth measuring tools which are pre-
inserted into the stoma. The clinician then selects a
tube which corresponds to this measurement. from a large
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
_ g _
selection o.f tube lengths. These tubes can not be
adjusted for a change in patient size.
The present inventions include a reinforced
silicone tube which has the same modulus as an
unreinforced silicone tube and walls which can not be
collapsed or kinked. The invention has walls which are
approximately the same thickness as a comparable urethane
tube. The new tube is therefore superior to both
silicone and urethane.
The present inventions include a one piece
anti-reflux slit valve located in the set which
automatically opens flow in either direction when the
luer of a regular set connector is inserted. It cannot
be damaged by a stylet.
The present irwentions include a pre-formed,
pre-inflated silicone balloon with unique. deformation
characteristics for both insertion and removal. Because
it is pre--formed, the balloon walls are approximately
0.012 to 0.030 inches thick during use, versus~the .004
inch thick walls of inflated balloons. It also has .
unique retention qualities in relation to much larger
retention devices.
The present inventions include a simple,
silicone bolster which both secures the catheter and
bends it at a right angle to the patient's skin. The
bolster presents minimum bulk and maximum access to the
stoma for air_ circulation and cleaning. It is secure but
can be easily adjusted as the patient's condition
changes.
The present inventions include a system which
eliminates the need for pre-measurearent devices and
greatly reduces the number of necessary sizes for low
profile replacement tubes. For the first time, it also
provides a means for precisely measuring the bolster
position. This pre-measurement and precise bolster
positioning are accomplished by a unique tube marking
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
- 9 -
system in combination with the bolster.
In addition to the aforedescribed, these
improvements and others are also embodied in a PEG tube
and insertion asserr~bly invention. This invention
incorporates most of the components of the low profile
replacement tube. After insertion, the PEG tube assembly
which remains embodies all of the features of the
replacement tube.
The PEG tube assembly is inserted by the same
method as a conventional PEG. Therefore no stylet is
required. In addition, because the internal retention
balloon is inserted in its inflated state:, the feeding
set connector has no inflation/deflation valve or lumen.
After insertion into the :~tomac:h, the tube is
cut off at a predetermined point indicated by a wide
black marker~band encircling the PEG tube:. An
inflation/deflation lumen in the wire reinforced PEG tube
is occluded by a plug extending approximately 5
centimeters below the black marker band. This plug
retains the air in the retention balloon when the tube is
subsequently cut at the marker band.
After the tube is cut at the marker band, the
external bolster of the invention and retention ring are
slipped over the tube. The feeding set connector is then
threaded into the tube. Tube depth is adjusted and the
external bolster is anchored. The device: is now ready to
function as a low profile gastrostomy tub>e, just like the
replacement tube.
To remove the tube, it is cut c>ff at a marker
line below the marker band. This line i~c normally
positioned 10 to 15 centimeters from the retention
balloon. The marker line is below the ai.r
inflation/deflation line plug, so the air line is opened
when the tube is cut. The open air :Line allows air to
escape from the balloon during removal, thereby allowing
the balloon to deform as it is being pulled out of the
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
-- _ - 10 -
stoma.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing and other objects of these
inventions are illustrated more or less diagrammatically
in the drawings, in which:
FIGURE 1 is an illustration of a replacement
tube assembly embodying features of the inventions, with
the tube assembly in place accessing a p,atient's stomach;
FIGURE 2 is an enlarged side elevational view
of the replacement tube assembly illustrated in FIGURE 1;
FIGURE 3 is a longitudinal sectional view
through the bolus end of the replacement tube assembly
illustrated in FIGURES 1_ and 2;
FIGURE 4 is a top plain view of the bolus end
illustrated in FIGURE 3;
FIGURE S is a longitudinal sectional view,
similar to FIGURE 3, showing mare of the replacement tube
assembly embodying features of the inventions;
FIGURE 6 is a side view, similar to FIGURE 2
but partially in section illustrating a near-completely
assembled replacement tube assembly embodying features of.
the inventions;
FIGURE 7 is an end view of the set-connector
cap in the replacement tube assembly of FIGURES 1-6;
FIGURE 8 is an enlarged, side view of a set-
connector for the replacement tube assemk>ly of FIGURES 1-
6, partially in section;
FIGURE 9 is a side elevational view of a
replacement tube assembly embodying features of the
inventions, illustrated in its unassembled form prior to
insertion through a stoma formed in a patient's stomach;
FIGURE 10 is a front elevational view of the
bolster component for the replacement tube assembly of
FIGURE 9 taken along line 10-10 of FIGURE 9, with the
tube component removed;
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/OOSU2
_ 11 _
FIGURE 11 is a bottom plan view of the bolster
component of FIGURE 10, taken along line 11-11 of FIGURE
9 with the tube component removed;
FIGURE 12 is a longitudinal, section taken
through the replacement tube assembly of FIGURE 9 showing
a stylet partially inserted and the tube assembly about
to be inserted through a stoma;
FIGURE 13 is a view similar to FIGURE 12,
showing the stylet driven completely into the tube
assembly to distend the retention balloon component
immediately prior to insertion;
FIGURE 14 is a view similar to FIGURE 13, but
showing the balloon component configuration as the
balloon passes through the stoma;
FIGURE 15 is a side elevational view of a
replacement tube assembly in a set of three lengths, the
assembly being the shortest of the three and having~a
gauging system embodying features of the inventions
imprinted along its length;
FIGURE 16 is a side elevational view of the
intermediate length replacement tube assembly in the set
of three, also having a gauging system embodying features
of the inventions imprinted along its length;
FIGURE 17 is a side elevat:ional view of the
longest replacement tube assembly in the set of three,
also having the gauging system imprinted <along its
length; .
FIGURE 18 is a side elevational view of a
replacement tube assembly in place in a patient's stoma
(in section) with the bolster positioned using the
gauging system of the inventions;
FIGURE 19 is a view, similar to FIGURE 17,
showing a replacement tube assembly carrying a gauging
system which is a variation of that shown in FIGURES 15-
38;
CA 02389154 2002-07-04
WO 97/2505 PCT/US97I00502
12
FIGURE 20 is a view of the replacement tube
assembly seen in FIGURE 19, showing the opposite side of
the tube segment~and the gauging system;
FIGURE 21 is a longitudinal sectional view
through the bolus end of a replacement tube assembly
embodying features of another form of the inventions, a
form in which the balloon is accessed by an inflation and
deflation lumen;
FIGURE 22 is a sectional view taken along line
22-22 of FIGURE 21;
FIGURE 23 is a sectional view through the set
connector for the form of replacement tube assembly shown
in FIGURE 21;
FIGURE 24 is a view similar to FIGURE 1
illustrating the inventions embodied in a suprapubic
catheter tube assembly;
FIGURE 2~ is a view similar to~ FIGURE 1
illustrating the inventions embodied in a PEG tube
assembly;
FIGURE 26 is a side e,levational view of a PEG
and insertion tube assembly embodying features of the
present inventions;
FIGURE 27 is an enlarged side elevational view
of a portion of the PEG tube assembly after. it is severed
from the assembly of FIGURE 25;
FIGURE 28 is a side elevational view of a
feeding set adaptor readyy far mating with the PEG tube
assembly of FIGURE 27; and
FIGURE 29 is a side elevationa:l view of the
assembled PEG tube after a feeding set adaptor has been
mated.
DESCRIPTION OF THE PREFERRED EMBODIMENT
Referring now to the drawings, and particularly
to FIGURE 1, the inventions disclosed to are embodied
here in a replacement gastrostomy tube assembly shown
SUBSTITUTE SHEET (RU~E.26~
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
- 13 -
generally at 10. 'the tube assembly 10 is shown in place,
extending through a stoma S in a patient, from a feeding
formula supply tube 11 outside the patient's abdominal
wall A to inside the patient's stomach S'T_. The stoma S
may be formed in a conventional manner by one of several
well-known procedures hereinbefore referred to.
The tube assembly 10 is a replacement tube
assembly in the sense that has hereinbefore been
described. The tube assembly 10 is designed to be easily
connected to, and disconnected from, a conventional
feeding formula supply tube 11 in a manne r hereinafter
discussed.
The inventions are illustrated here in a
gastrostomy tube assembly. However, as will hereinafter
be discussed, the inventions may find equally
advantageous application in other tube assemblies, such
as PEG and jejunostomy tubes, for example, or other
corporeal access environments like suprapubic catheter
assemblies.
Referring now to FIGURE 2, the replacement
gastrostomy tube assembly 10 is seen to comprise a short
segment 15 of tube formed of silicone rubber and
embodying features of the invention. The, gastrostomy
tube segment 15, which is constructed in a manner
hereinafter discussed in detail, has a bolus 16 connected
in fluid communication with the tube segment at the
latter's discharge end 17, and a set connector 18
connected in fluid communication with the tube segment at
the latter's inlet end 19. The bolus 16 and the set
connector 18 are also formed of silicone rubber.
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
-' . - 14 -
Adjacent the bolus tip 16, and encircling the
tube segment 15 near the discharge end 17, is a tire-
shaped balloon 20 which also embodies features of the ,
invention and will hereinafter be discussed in detail.
Suffice it to say at this point that the balloon 20 is
filled with a fluid medium such as air or water. Air is
preferred and, in the present illustration, is employed.
Approximately intermediate the ends 17 and 19
of the tube segment 15 is a right-angle :bolster 21
through which the tube segment passes. The bolster 21
construction and arrangement on the tube segment 15,
which comprises additional features of t:he invention,
grips the tube segment at a selected distance from the
balloon 20, and forces the segment slightly past a right
angle configuration so that the set connector 18 lies
immediately adjacent to the patient's abdomen when in
place. The construction and operation o:~ the bolster 21
will also hereinafter be discussed in detail.
Referring now to FIGURES 3 and 4, the bolus 16
and its connection to the discharge end 17 of the tube
segment 15 is shown in substantial detail. The bolus 16
may be of the design and construction il:Lustrated and
described in the Quinn U.S. Patent No. 5,451,216,
assigned to the same assignee as the present application
and invention. The bolus 16 comprises a body 30 having a
tube 15 receiving section 31, a central passage section
32, and a nose section 33.
The tube segment 15, at its discharge end 17,
is glued inside the receiving section 31 of the bolus 16
with a silicone based adhesive. A passage 35 extending
axially through the passage section 32 of the bolus 16 is
then in continuous fluid communicat_LOn with the tube 15. '
A radially extending discharge port 36 is
formed through the bolus from the passage 35. It is '
through this port that enteral feeding discharge takes
place.
CA 02389154 2002-07-04
WO 97/25095 NCT/US97/00502
- 15 -
The nose section 33 of the bolus 16 has an
axial, stylet-receiving pocket 39 formed therein. In
this sense the bolus 16 is different than that disclosed
in the aforementioned co-pending application. The pocket
39 is designed to receive the tip of a stylet (not shown
in this FIGURE) in a manner hereinafter discussed in
detail, both as to the way the stylet is employed and its
purpose.
Referring now to FIGURES 5 and 5A, the portion
of the tube segment 15 which joins the bolus 16 is shown
in enlarged, longitudinal and transverse sections. The
tube segment 3.5 comprises a silicane body 41 containing a
stainless steel wire coil spring 42. The coil spring 42
extends from the receiving end (not shown) of the tube
segment l5 to a point 43 immediately adjacent, but not
within, the balloon 20. Accordingly, the: balloon 20
surrounds a tube body portion 45 which is unsupported by
the spring 42.
The tail spring 42 is inserted into an extruded
silicone tube. Liquid. silicone is introduced into the
tube so that it flows the length of the tube, coating and
covering the wire and adhering it to the inside of the
tube. The liquid silicone sets to unitize the original
tube, the coil spring 42 and the coating into a generally
cylindrical wall having an inner surface 46 and an outer
surface 47.
The balloon 20 is tire-shaped, as has been
pointed out. It is formed of conventional silicone film
which is 0.030 of an inch thick in this embodiment. Using
the language of vehicle tire construction, it comprises a
casing 51 having an outside diameter of 0.600 inches.
The casing 51 has, at its inside diameter which
corresponds to the outside diameter of the tube body 41,
a pair of beads 52 and 53. The beads 52 and 53 are glued
to the outer surface of the tube body 41 'with a silicone
adhesive in a conventional manner.
CA 02389154 2002-07-04
WO 97/25095 PCT/US97100502
- 16 -
The balloon 20 is preformed in the shape
illustrated. As such, air is trapped in the space 55
when assembled. The beads 52 and 53 are bonded to the
tube body 41 to assemble the tube <~nd balloon.
The side-walls 56 and 57 of the tire-shaped '
balloon casing 51 are preferably spaced from each other
by 0.200 inches from outer surface to outer surface. The
side-wall 56 extends perpendicular to the axis of the
tube 15 for a distance of 0.100, i.e., it defines a
substantially flat outer surface 58 extending outwardly
from the bead 52 for 0.100 inches. Connecting the side
walls 56 and 57 is the tread wall 59 of the casing 51.
It defines a semi-circle in cross-section. The radius of
the semi-circle is 0.100 inches.
The aforedescribed balloon 20 configuration
provides important advantages. Its flat retention
surface 58 is 500 of its diameter externally of the beads
52 and 53. In this shape it is very resistant to
distortion when functioning in its tube assembly 10
retaining capacity. It also presents a wide, stable,
flat retaining surface 58.
Referring now to FIGURES 6-8, the set connector
18 at the inlet end 19 of the tube 15 is shown in
enlarged (FIGURE 6) and then further enlarged (FIGURES 7
and 8) form. The connector 18 comprises a generally
cylindrical fitting 61 also molded of silicone rubber.
The fitting 61 has a unitarily formed body 62 and cap 63,
with the cap flexibly attached to one end of the body by
an easily bendable arm 64.
The fitting body 62 also has an axial passage
65 formed through i.t. Seated in thc.~ passage 65, ,
approximately intermediate its ends, is a conventional
slit valve insert 66. The valve insert 66 is also molded
of silicone and includes a slit 6'7 which is forced open
into a generally round shape by the feed3.ng supply tube
connector tip (not shown) when the tip is inserted for
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
_ 17 _
feeding purposes. When the tip is z:emoved, and the valve
66 is subjected to pressure from below, t:he valve slit 67
closes.
The inlet end 19 of the tube 15 is seated in,
and glued with a silicone adhesive to, a cylindrical end
section 69 of the passage 65 in the fitting body 62. The
cap 63, at the other end of the body 62, includes a plug
71 which is received in the passage 65 when it is
desirable to disconnect the replacement tube assembly 10
from the feeding tube 11. An annular locking shoulder 72
is formed on the plug 71 and is adapted t.o snap fit into
a corresponding annular locking depression encircling the
passage 65.
Referring again to FIGURE 2, anal also to
FIGURES 9-11, the construction and function of the
bolster 21 will now be described. The bolster 21
comprises a molded silicone rubber body 81 and a molded
silicone rubber O-ring 82. The bolster body 81 is formed
in a split configuration so as to have two legs, 83 and
84, joined at corresponding one ends by a bridge 85. The
legs 83 and 84 may be spread to the position shown in
FIGURE 9 so that the tube segment 15 is essentially
straight. In this position, the O-ring 82 is positioned
off the bolster, freely encircling the tube.
When the legs 83 and 84 are brought together,
as seen in FIGURE 2, the tube segment 15 is bent slightly
past a right angle configuration, i.e., the angle is
slightly less than 90° whereby the tube segment outside
the bolster i.s actually inclined slightly toward the
abdominal wall. In this position of the legs 83 and 84,
the O-ring 82 is snapped into place in an annular
depression 86 to maintain the bolster 21 and the tube
segment 15 is this position.
FIGURES 10 and 11 show the bolster in side and
end views, a~> taken from FTGURE 9. As seen in FIGURES 9-
11, the leg 83 is formed with a substantially semi-
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
_. . _ 1g
cylindrical trough 87 extending axially along one side of
it. The trough 87 curves outwardly to terminate at one
end at the bridge 85. At its outer end, the trough 87 .
becomes a cylindrical passage section 88 as it passes
through an annular collar 89 which form; what amounts to
a foot on the leg 83. The other leg 84 is also formed
with a substantially semi-cylindrical trough 91 extending
axially along one side of it. The trough 91 also curves
outwardly to terminate at one end at the bridge 85.
Immediately adjacent this curve, a cylindrical passage
section 92 is formed through the leg 84, perpendicular to
the trough 91.
The bolster 21 is fabricated by molding it in a
body 81 without legs. The legs 83 and 84 are formed by
~~ cutting the body 81 on the L-shaped path best seen in
FIGURE 2. It will thus be seen that the normal state of
the body is with the legs 83 and 84 lying flush against
each other. In this relationship the two troughs 87 and
91, and the two passage sections 88 and 92, collectively
form a generally L-shaped passage 95 extending entirely
through the bolster, with the passage section 88 inclined
slightly past a right angle. The leg 84 and, thus, the
body 81 has a flat bottom surface 95.
The tube segment 15 is threaded through the
passage section 88 in the leg 83 and the passage section
92 in the leg 84 while the legs are spread into the
attitude seen in FIGURES 9-11. When it is desirable to
bend the tube segment 15 into slightly greater than a
right-angle, in a manner hereinafter discussed, the legs
83 and 84 are simply brought together and the O-ring 82
snapped in place. Because the tube segment 15 has a coil
spring 42 built into it, it does not kink and become
blocked inside the bolster 21.
Referring now to FTGURES 12-14 and FIGURE 1, a
replacement gastronomy tube assembly 10 is shown being
prepared for insertion (FIGURES 12 and 13?_ into the
CA 02389154 2002-07-04
WO 97/25095 PCT/ITS97/00502
- 19 -
patient's stomach ST through a prefrarmed stoma S. FIGURE
1 shows it inserted and secured. FIGURE 1~ shows it
being inserted (the: patient is not strown here) . The
stoma S has previously been formed with a PEG. When the
PEG is removed, as it normally is after a short period of
use, a replacement assembly is inserted.
Referring initially to FIGURE 12, a rigid metal
stylet 96 of known construction is inserted, tip 97
first, through the set connector 18 into the tube segment
15. The stylet 96 is inserted using its handle 98 until
its tip 97 reaches and seats in the pocket 39 of the
bolus 16. Further insertion of the styles then stretches
the balloon 20, as seen in FIGURE 13.
According to the invention, the stylet 96 is
forced into the tube segment 15 until it has stretched
the balloon out into the configuration shown in FIGURE
13. At this point, the volume of the balloon 20 is
actually greater that it is in its relaxed form (FIGURE
12) so that a partial vacuum forms within the balloon,
causing it to collapse inward to some extent.
With the balloon 20 in a greatly reduced
diameter form, the bolus 16 is inserted through the stoma
S, followed by the balloon and the lower portion of the
tube segment 15. As the balloon 20 passes through the
stoma S it flattens out rearwardly into the configuration
shown in FIGURE 14, thus facilitating passage through the
stoma. Once the balloon has clearly entered the stomach,
the styles 96 is pulled out. The balloon 20 expands to
its normal size and shape as the tube segment 15 under
the balloon becomes shorter and thicker again. The tube
assembly 10 is then drawn outwardly until the flat
surface 58 on the outer side 56 of the balloon 20 rests
against the stomach wall lining. With the styles 96
completely removed, the plug 71 is inserted into the bore
65 through the set connector for sanitary reasons.
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
_.. - 20 -
Turning now to the tube measurement system
which embodies features of the inventions described and
illustrated herein, attention is invited to FIGURES 15-17
which a first form of the system is shown. There, three
different replacement gastronomy tube assemblies are seen
at 10A, lOB and 10C. The tube assemblies 10A, lOB and
lOC are identical in construction and operation to the
assembly 10 hereinbefore described. As will be seen,
however, the segments 15A, 158 and 15C of gastronomy tube
are progressively longer (by approximately 1.25
centimeters).
In this form of the system, three different
replacement assembly lengths are provided to cover the
normal variations in stoma depth encountered in patients,
a range of from approximately 0.75 to 5.00 centimeters.
The tube 15A has a measuring gauge 110 embodying features
of the invention imprinted on its side and covering the
shorter-range of stoma depths of 0.75 to 2.5 centimeters.
The tube 15 has a measuring gauge 111 innprinted on its
side and covering the mid-range of stoma depths of 2.0 to
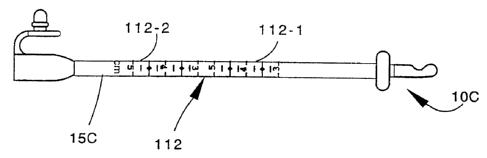
4.0 centimeters. The tube 15C has a measuring gauge 112
imprinted on its side and covering the upper-range of
stoma depths of 3,0 to 5.0 centimeters.
As will be seen, the measuring gauge 110
comprises two identical sets of gauge markings 110-1 and
110-2 imprinted on its side. The marking set 110-2 is
positioned so that corresponding centimeter gradations in
the 110-1 set (1.0 centimeter, for example) are spaced
precisely the length of the aforedescribed bolster
passage 95 from each other, for reasons hereinafter
explained. As will. also be seen, tlm centimeter markings
in the 110-1 set are read right-side-up from the bolus 16
end of the assembly while the centimeter markings in the
110-2 set are read right-side-up from the set connector
18 end, i.e., up-side-down from the bolu~~ end.
CA 02389154 2002-07-04
WO 97/25095 fCT/US97/00502
- 21 -
The measuring gauges 111 and 1.12 each have two,
identical sets of gauge markings also; markings 111-1 and
111-2 in the case of gauge 111 and 1.12-1 and 112-2 in the
case of gauge 112. The sets in both gauges are, like the
gauge 110, spaced from each other a distance
corresponding to the length of the passage 95 through the
bolster 21.
Referring now to FIGURE 18, with the tube
assembly 111 (for example) in position so that the
balloon 20 rests against the stomach lining, the
physician reads the 2.5 centimeter gauge marking on the
tube segment at the abdomen surface. While this is being
done the bolster 21 is in its open and displaced position
shown in FIGURES 12-14. Bolster legs 83 and 84 are then
brought together around the silicone tube 15 and held
manually. Tube segment 1S is then pulled through the
bolster 21 until the paired 2.5 centimeter gauge marking
is precisely aligned with the end surface of the bolster
from which it has emerged. The tube segment 25 and
bolster 21 position is then as seen in FIGURE 18. Then
the bolster legs 83 and 8~ are secured by an O-ring 82.
With this done, the physician knows that the flat surface
95 of the bolster 21 is resting flush against the
patient's abdomen, but the balloon is not putting
inordinate pressure on the stomach lining -- in other
words, an ideal fit has been achieved.
Referring now to FIGURES i9 and 20, a second
form of measurement system embodying features of the
invention is shown. Fiere, one length of tube 15X is used
for the full range of stoma depths. The tube 15X is the
same length as the previously described tube 15C. Unlike
the tube 15C, however, it has a full-range measuring
gauge 113 on one side of it, with the indicia in black,
and a full range measuring gauge 114 of the other side of
it, with the indicia in red.
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/OU502
- 22 -
The black indicia gauge 113 measures the tube
length from the balloon 20. The red indicia gauge 114,
on the other side of the tube 15X, has corresponding
indicia (3.0 centimeters and 3.0 centimeters, for
example) spaced from each other by the J.ength of the
bolster passage 95. This second form of gauging system
is used in a manner identical to that hereinbefore
described in relation to the form.
Referring now to FIGURES 21-23~ another
embodiment of the gastronomy replacement tube assembly is
illustrated generally at 210. The replacement tube
assembly 210 is similar to the tube assembly 10
hereinbefore; described except that it utilizes a tube
segment 215 which has an inflation/deflation lumen 214 in
it and a radial aperture 213 connecting that lumen with
the inside of the balloon 220.
Tixe inflation/deflation lumen 214 communicates,
at its opposite end, with the set connector 218. A lumen
access port 264 is formed in the body 262 of the
connector 218 and a silicone rubber slit valve plug 268
seals the outer end of that port.
By inserting the round, blunt needle tip T of
an inflation/deflation needle, with a side hole, through
the slit valve plug 268Y the balloon 220 can either be
filled with air or evacuated of air. This permits the
assembly 210 to be introduced into, or removed from, a
stoma even more easily.
CA 02389154 2002-07-04
WO 97/25095 PCT/US97/00502
- 23 -
Referring now to FIGURE 24, the inventions
disclosed are embodied here in a suprapubic catheter tube
assembly shown generally at 310. The assembly 310 is
substantially identical in construction .and operation to
the replacement gastrostomy tube assembly 10 hereinbefore
discussed, except that it is introduced into the bladder
B through the urinary urethra. It then accesses the
abdominal wall through a stoma above the bladder in the
pubic area. Accordingly, it is not described
independently in greater detail.
Referring now to FIGURE 25, a PEG tube assembly
embodying features of the inventions is shown generally
at 410. The PEG tube assembly 410 is, in many respects,
identical to the replacement tube assembly 10
hereinbefore discussed. To the extent that it is, the
PEG tube assembly 410 features are not described in great
detail. To the extent that it differs, the following
discussion will be sufficient to an understanding of its
construction and operation. _
Turning t.o FIGURE 26, the PEG tube assembly 410
or, more precisely, the bulk of it, begins life as a
component of a PEG insertion unit 411. ':Che PEG insertion
unit 411 comprises a conventional plastic lead-in tube
412 with a solid plastic fitting 413 seated in, and glued
to, one end. A placement wire 414 is anchored in the
tube 412 and protrudes in a loop 416 from the other end.
The PEG tube assembly (portion) 410 seen in
FIGURE 26 comprises a tube segment 415 identical to that
shown at 215 in FIGURES 21-23, with the E:xceptian that it
has several additional features. As seen in FIGURE 27,
the tube segment 415 has a deflation lumen 416 extending
along its length, with a radial access port 417
communicating with the retention balloon 420. The lumen .
416 at the end of the tube 415 is plugged at 421 (there
is no bolus attached).
CA 02389154 2002-07-04
WO 97125095 PCT/US97/00502
- 24 -
The tube 415 is, in this illustration, about 25
centimeters long. At a point 10-15 centimeters from the
capped end 421 a thin black marker line 425 is imprinted
encircling the tube 415. Between the marker line 425 and
the end 426 of the tube 415 in which the fitting 413 is
glued, a wide black marker band 430 is imprinted
encircling the tube.
Starting from the end 426 of the tube 415, the
deflation lumen 416 is plugged at 432 with silicone
rubber (after the lumen is initially formed) to a point
below the band 430 but above the line 425. Below the
plug 432, the lumen 416 is open to the balloon 420. It
will thus be seen that the marker band 430 encircles
portion of the tube 415 which does not contain an open
lumen 416.
The PEG tube insertion unit 411 is pulled into
the patient's stomach, in a conventional manner, by the
placement ~~~ire 414. A snare wire (not shown) in a
cannula (not shown) which has been inserted into the
patient's stomach by piercing the abdomen and stomach
walls is used to snare the placement wire loop 416 (which
has been led in to the stomach through the esophagus) and
pull it out through the access stoma formed by the
piercing. The wire 414, lead in tube 412, and tube 415
continue to be pulled until the retention balloon 420
seats against the stomach wall..
At this point the tube 415 is severed at the
marker band 430. A feeding set adaptor 435 (see FIGURE
28) is then threaded into the open end of the tube 415 at
the marker band 430. Before this is done, however, a
bolster 441 and retention ring 482 identical to those
previously discussed are placed over the tube 415, as
seen in FIGURE 29.
As will be seen in FIGURE 28, the adaptor 435
is similar to the adaptor 18 seen in FTGURE 6 except that
it is threaded into the tube 41S instead of being glued
CA 02389154 2002-07-04
WO 97/25095 fCT/US97/U0502
- 25 -
onto it. This is facilitated by an externally threaded
metal fitting 436 which is glued into the body 437 of the
adaptor 435. The threads 438 on the fitting turn into,
and are gripped by, the tube 415.
The tube 415 is now ready to be anchored to the
patient's abdomen using the bolster 441 and procedure
identical to that previously described. To this end the
tube carries gauging indicia (not shown) which are also
identical t~ those previously described.
After the PEG tube has been u:~ed for feeding
purposes in a known manner for a period, it is removed
and replaced with an aforedescribed replacement tube
assembly 10. To do this the bolster 421 is opened. Then
the tube 415 is cut at the black line 425. This opens
the lumen 416. The retention balloon c~~n now deflate and
deform as the~PEG tube assembly (or ~~lhat remains of it
after the feeding set adaptor end i:a severed) 410 is
pulled out through the stoma.
While preferred embodiments of the invention
have been described, it should be understood that the
invention is not so limited and modifications may be made
without departing from the invention. T'he scope of the
invention is defined by the appended claims, and all
devices that come within the meaning of the claims,
either literally or by equivalence, are intended to be
embraced therein.