Note: Descriptions are shown in the official language in which they were submitted.
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
TITLE OF THE INVENTION
CAPACITOR DEVELOPMENT
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention is directed to capacitors, and, more particularly,
super, or
ultracapacitors of the type utilizing bipolar electrode plates.
2. Background Art
Ultracapacitors, also known as super-capacitors, are well known in the art.
These
ultracapacitors store energy electrostatically by polarizing an electrolyte
solution.
Although the ultracapacitor is indeed an electrochemical device, there are no
chemical
1 ~ reactions involved in its energy storage mechanism. The historical
background and
operational characteristics and conventional structures can be found in
Ultracapacitors
For Portable Electronics, Xavier Andrieu, The Big Little Book of Capacitors,
Chapter 1 ~,
Pgs. 521-547.
An ultracapacitor is typically constructed from two or more bipolar electrode
plates separated from each other by electrolyte and a separator positioned
within the
electrolyte. The bipolar plates include a current collector comprised of an
electronically
conductive material, and, an electrode material associated with the current
collector. The
components of the capacitor are operatively secured and encased in a
surrounding
housing. The housing exposes electrical leads for connection with an item to
receive the
2~ electrical benefit of the capacitor.
In a conventional dielectric capacitor, energy is stored in the form of a
separated
electrical charge, wherein the greater the area for storing the charge, and
the closer the
separated charge, the greater the capacitance. In such a capacitor, the area
for storing the
charge is obtained from plates of a flat conductive material and the charged
plates are
separated with a dielectric material.
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
In an ultracapacitor, the area for storing a charge is conventionally obtained
from
a porous carbon material. The porous structure of the carbon allows the
chargeable area
to be much greater than the flat conductive plates of dielectric capacitors.
Furthermore,
an ultracapacitor's charge separation distance is typically determined by the
size of the
ions in the associated electrolyte, which are attracted to the charged
electrode. Such a
charge separation is substantially smaller than that which can be obtained
using
conventional dielectric materials. Accordingly, the combination of the
relatively large
surface area and the small charge separation results in superior capacitance
relative to
conventional capacitors.
2
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
SUMMARY OF THE INVENTION
The present invention is directed to a capacitor, and, more particularly, a
super
capacitor comprising at least one bipolar electrode plate each comprising a
current
collector and an electrode material associated with the current collector; an
electrolyte
comprising an organic aprotic solvent, a salt and means for stabilizing
electrochemical
activity between the solvent and at least one of the current collector and/or
the electrode
material; and, a separator positionable in the electrolyte so as to prevent
physical contact
between adjacently positioned electrode plates.
In a preferred embodiment of the invention, the electrolyte further includes
means
for increasing wettability of the electrode. The wettability increasing means
may
comprise a surfactant.
In such a preferred embodiment, the surfactant is selected from the group
comprising nonionic fluoro-alkanes.
In another preferred embodiment of the invention, the electrochemical activity
stabilizing means comprises a chemical component which can be reduced in place
of the
solvent. In such a preferred embodiment, the electrochemical activity
stabilizing means
is selected from the group comprising carbonates, such as vinylene carbonate,
spirolactones, anhydrides and oligomers.
In yet another preferred embodiment of the invention, the current collector is
selected from the group of materials having low electrical resistance,
substantial
impermeability to electrolyte penetration, chemical compatibility with at
least one of the
electrode material and the electrolyte, and, chemical stability.
In one such preferred embodiment, the current collector includes carbon
material,
while in another preferred embodiment the current collector comprises
aluminum.
In still another preferred embodiment of the invention, the capacitor further
includes a substantially inert frame structure. In such a preferred
embodiment, the frame
structure includes end plates having a current collector associated therewith.
Ultracapacitors fabricated in accordance with the above, and, more
particularly,
with carbonlgraphite fiber electroactive materials, a non-aqueous electrolyte,
and
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
polymer coated aluminum bipolar current collector plates have demonstrated
capacitance
of up to 0.95 F/cm', energy of up to 1 mAh/cm'. These capacitors have been
assembled
using commercially available components. Materials and components have been
selected
in relation with known and anticipated manufacturing constraints and in order
to enable
manufacturing scale-up of the capacitor.
4
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
BRIEF DESCRIPTION OF THE DRAWINGS
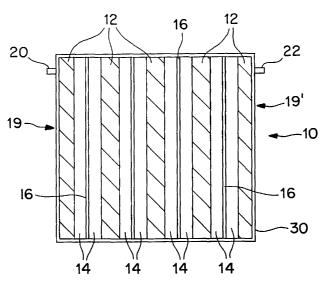
Fig. 1 of the drawings is a schematic cross-sectional view of the present
invention;
Fig. 2 of the drawings is a perspective view of a bipolar plate of the present
invention;
Fig. 3 of the drawings is a cross-sectional view of Fig. 2, taken along lines
3-3,
and showing the current collector material and the electrode material of the
present
invention;
Fig. 4 of the drawings is a graphical representation of the AC impedance of
the
present invention;
Fig. 5 of the drawings is a graphical representation of the normalized AC
impedance of the present invention;
Fig. 6 of the drawings is a graphical representation of the initial charge and
discharge voltage-time profile for the ultracapacitor of the present
invention;
Fig. 7 of the drawings is a graphical representation of the discharge voltage-
time
profile for the ultracapacitor of the present invention;
Fig. 8 of the drawings is a graphical representation of the discharge capacity
retention as a function of cycle number;
Fig. 9 of the drawings is a graphical representation of the initial power
performance, as illustrated by the voltage-time profile, for the capacitor of
the present
invention;
5
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
Fig. 10 of the drawings is a graphical representation of the normalized
voltage-
time profile during power testing of the capacitor of the present invention;
Fig. 11 of the drawings is a graphical representation of the Total Delivered
Energy as of function of time during discharge at different power rates;
Fig. 12 of the drawings is a graphical representation of comparative AC
impedance;
Fig. 13 of the drawings is a graphical representation of an initial charge and
discharge voltage-time profile;
Fig. 14 of the drawings is a graphical representation of comparative AC
impedance;
profile;
Fig. 1 ~ of the drawings is a graphical representation of comparative voltage-
time
Fig. 16 of the drawings is a graphical representation of comparative discharge
voltage-time profile;
Fig. 17 of the drawings is a graphical representation of initial power
performance;
Fig. 18 of the drawings is a graphical representation of normalized voltage-
time
profile during power testing;
Fig. 19 of the drawings is a graphical representation of the Total Delivered
Energy as function of time during discharge at different power rates;
6
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
Fig. 20 of the drawings is a graphical representation of the effect of the
electrolyte
additive on the charge behavior of an ultracapacitor;
Fig. 21 of the drawings is a graphical representation of the effect of the
maximum
charge voltage;
Fig. 22 of the drawings is a graphical representation of the effect of co-
solvent
based electrolyte on the total cell resistance;
Fig. 23 of the drawings is a graphical representation of the effect of co-
solvent
based electrolyte on the total cell resistance; and
Fig. 24 of the drawings is a graphical representation of initial charge-
discharge
voltage-time profile.
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
BEST MODE FOR PRACTICING THE INVENTION
While this invention is susceptible of embodiment in many different forms,
there
is shown in the drawings and will herein be described in detail, several
preferred
embodiments with the understanding that the present disclosure is to be
considered an
exemplification of the principles of the invention and is not intended to
limit the
invention to the embodiments so illustrated.
A schematic cross-sectional representation of ultracapacitor 10 is shown in
Fig. 1
as comprising bipolar electrode plates 12, electrolyte 14, separators 16,
housing 18,
housing end plates 19, 19' and exposed current collector leads 20, 22,
positioned through
corresponding end plates. As can be seen, the separators are positioned in
between
adjacently positioned bipolar electrode plates, and, within the electrolyte,
so as to prevent
inadvertent contact between the bipolar plates. Although an ultracapacitor
having five
bipolar electrode plates is shown in Fig. l, it will be readily understood to
those having
ordinary skill in the art that any number of desired plates are contemplated
by the present
invention.
A bipolar electrode plate 12, of the type shown in Fig. l, is shown in Fig. 2
as
comprising current collector 24 (Fig. 3) and electrode material 26 (see also
Fig. 3)
associated with the current collector. The combination of the current
collector 24 and
electrode material 26 are secured to and surrounded by frame structure 28.
Frame structure 28 is constructed from a substantially inert, non-
electronically
conducting material such as polyethylene and/or polypropylene. Alternatively,
glass
fiber filled polyethylene having a low temperature induced deformation and a
low
melting temperature may also be utilized. It is also contemplated that the
frame structure
be fabricated from other materials, such as low-density non-linear
polyethylene and/or
polypropylene. The frame structures can be manufactured through conventional
techniques such as die-cutting, injection molding and even extrusion. The
housing 18
(Fig. 1) can also be made out of the same material as the frame structure for
the bipolar
electrode plates. The end plates 19, 19' can further be fabricated from glass
reinforced
polyethylene sheets, and the associated end plate current collectors can be
fabricated
8
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
from the same material as the current collector itself, or for example,
expended copper
mesh or aluminum.
Current collector 24 is fabricated from materials having low electrical
resistance,
impermeability to electrolyte penetration and permeation, chemical
compatibility, and
electrochemical stability. A preferred material is polyethylene coated
aluminum foil,
commercially available under the trade name COER-Xal from a company called
Rexam
Graphics of South Hadley, Massachusetts. It has been observed that the use of
such
material provides several advantageous features. Specifically:
- aluminum foil provides absolute separation of electrolyte in the bipolar
electrode construction;
- the PE coating onto the aluminum foil provides for additional mechanical and
chemical stability to the aluminum foil;
- the PE coating onto the aluminum foil provides a "hot-melt" adhesive
characteristic to the current collector, thus enhancing the adhesion of the
carbon electrode
onto the current collector; and
- the COER-Xal provides good electrical conductivity and is available in
fairly
thin configurations (75 um Al, 25 um PE on each side).
As an alternative to the above, it is also contemplated that other materials,
such as
a polyethylene-carbon paper (25 um) and a polyethylene-carbon composite
material can
also be used.
With respect to electrode material 26, such material can be fabricated from
conventional types of electrode materials presently available for the
fabrication of super,
ultracapacitors. One example of acceptable electrode material, and generally
the most
frequently described, is based on the utilization of a high surface area
activated carbon
powder. The surface area of the carbon is generally greater than 1,500 mZ/g.
These
carbons generally have a total pore volume of 1 ml/g, and an average pore size
of 20 A.
in order to fabricate an electrode material using these carbons, the selected
carbon
powder must be mixed with an appropriate "binder" and than applied onto a
current
collector to which the carbon powder must adhere.
9
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
A second acceptable type of carbon electrode material is based on activated
carbon fibers, generally available in the form of woven and or non-woven
felts, fabrics,
foams, or webs. Carbon fibers having similar surface area than the ones
measured for the
activated carbons are available commercially. The use of carbon fiber webs
simplifies
the electrode assembly process by eliminating the need of compounding the
activated
carbon powder with a binder and by eliminating the need to apply, generally
via a solvent
coating process, the compounded carbon powder onto a current collector. It is
believed
that the main difference between the carbon powders and the carbon fibers
reside in the
trade off between the energy storage capacity of the carbon electrode and its
ease of
processing.
Electrolyte 14 as shown in Fig. 1 is comprised of an orUanic aprotic solvent,
a
salt, a stabilizer and a surfactant. The solvent can comprise conventionally
known
materials, such as the ones described by Dr. Ue In J. Electrochem. Soc. 141, 1
1 ( 1994)
2989-2996. For example only, propylene carbonate ("PC") can be used. Although
PC
l 5 has proven to be a good solvent, it has a high viscosity (n = 2.5 cp) and
consequently has
a less than optimum conductivity. Accordingly, solvent mixtures have also been
considered and tested. Acetonitrile (n=0.3 cp) and y-buryrolacrone (n=l .7 cp)
have been
initially selected as a co-solvent to PC.
The salt used in the electrolyte fornmlation comprises tetra ethylene ammonium
tetra fluoroborate. However, other salts, such as fluorinated alkyl ammoniums,
among
several other conventional salts, are likewise contemplated for use.
Inasmuch as the electrochemical stability of organic aprotic electrolytes in
contact
with carbon powder/fiber anodes is of concern to the capacitor industry, the
present
invention addresses such a concern by utilizing an additive/chemical component
that can
preferentially be reduced in place of the solvent. For example, in a preferred
embodiment, the additive may comprise a carbonate (vinylene carbonate), a
spriolactone.
an anhydride and/or an oligomer.
In order to increase the wettability of the carbon electrode by the
electrolyte,
surfactants are added to the electrolyte solution. These surfactants are
generally selected
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
to be nonionic fluoro-alkanes (SaiNippon Ink F-142d, F177 and 3M FC-171 and FC
170C are examples).
In support of the advantageous benefits from the ultracapacitors constructed
in
accordance with the above, numerous analyses were performed and graphically
depicted
in Figs. 4 - 24, wherein the additives (stabilizers and surfactants) were used
in
combination with conventional electrode materials (i.e. TSW2, TSW3 carbon
fiber
electrode material, commercially available from a company called Toyobo in
Japan, and,
Satin based electrodes from a company called Calgon, located in the United
Kingdom).
Further information regarding the analyses can be found in corresponding U.S.
Provisional Patent Application Serial No. 60/165,865, filed November 16, 1999,
from
which the present application depends, the entirety of which is incorporated
herein by
reference. In particular, and with respect to the graphs:
Fig. 4 of the drawings is a graphical representation of the AC impedance of
the
present invention based on TSW2 carbon fiber electrodes, as measured.
Fig. 5 of the drawings is a graphical representation of the normalized AC
impedance of the present invention based on TSW2, illustrating the effect of
adhesion
between the current collector and the electroactive material. Better adhesion
provides for
lower internal resistance.
Fig. 6 of the drawings is a graphical representation of the initial charge and
discharge voltage-time profile for the ultracapacitor assembled using the TSW2
based
electrodes. Although, some differences in charge time are observed, discharge
time is
almost constant for all systems. This behavior is further illustrated in
Figure 7.
Fig. 7 of the drawings is a graphical representation of the discharge voltage-
time
profile for the ultracapacitor assembled using the TSW2 based electrodes.
Fig. 8 of the drawings is a graphical representation of the discharge capacity
retention as a function of cycle number as illustrated for the initial 5
cycles for the
capacitor assembled using the TSW2 based electrodes.
Fig. 9 of the drawings is a graphical representation of the initial power
performance, as illustrated by the voltage-time profile, for the capacitor
assembled using
the YSW2 based electrodes.
11
CA 02389727 2002-04-30
WO 01/37295 PCTNS00/31588
Fig. 10 of the drawings is a graphical representation of the normalized
voltage-
time profile during power testing of the capacitor assembled using the TSW2
based
electrodes. These results indicate a good capacity retention for these
electrodes. This is
further illustrated in Figure 11.
Fig. 11 of the drawings is a graphical representation of the Total Delivered
Energy as of function of time during discharge at different power rates.
Fig. 12 of the drawings is a graphical representation of the comparative AC
impedance between the TSW2 and TSW3 electrode material considered by the
manufacturer as "equivalents."
Fig. 13 of the drawings is a graphical representation of the initial charge
and
discharge voltage-time profile for the TSW2 and TSW3 electrode material.
Fig. 14 of the drawings is a graphical representation of the comparative AC
impedance between the TSW2 and TSW3 electrode material and the Satin electrode
material.
1 ~ Fig. 15 of the drawings is a graphical representation of the comparative
voltage-
time profile between capacitor assembled using the TSW2 carbon paper and the
Satin
carbon fabric.
Fig. 16 of the drawings is a graphical representation of the comparative
discharge
voltage-time profile between capacitor assembled using the TSW2 carbon paper
and the
Satin carbon fabric.
Fig. 17 of the drawings is a graphical representation of the initial power
performance, as illustrated by the voltage-time profile, for the
ultracapacitor assembled
using the Satin carbon fabric.
Fig. 18 of the drawings is a graphical representation of the normalized
voltage-
time profile during power testing of the ultracapacitor assembled using the
Satin based
electrodes. These results indicate a good capacity retention for these
electrodes. This is
further illustrated in Figure 19.
Fig. 19 of the drawings is a graphical representation of the Total Delivered
Energy as function of time during discharge at different power rates.
12
CA 02389727 2002-04-30
WO 01/37295 PCT/US00/31588
Fig. 20 of the drawings is a graphical representation of the effect of the
electrolyte
additive on the charge behavior of an ultracapacitor. Capacitor #1 10103 has
no
electrolyte additive and shows long self decomposition behavior during the
initial charge,
while capacitor #102902 shows a normal charge behavior.
Fig. 21 of the drawings is a graphical representation of the effect of the
maximum
charge voltage.
Fig. 22 of the drawings is a graphical representation of the effect of co-
solvent
based electrolyte on the total cell resistance, for the ultracapacitor
assembled using
TSW3 electrode material.
Fig. 23 of the drawin~'s is a graphical representation of the effect of co-
solvent
based electrolyte on the total cell resistance, for the ultracapacitor
assembled using Satin
fabric. Tests performed using a 1:1 mixture of propylene carbonate and
acetronitrile
indicate that although the electrolyte conductivity is increased, as expected,
the
electrochemical stability of the electrolyte is reduced. This may be due in
part to water
contamination and to the presence of other residual impurities.
Fig. 24 of the drawings is a graphical representation of the initial charge-
discharge voltage-time profile for capacitor assembled using the Satin fabric
and co-
solvent based electrolyte (#101501).
The foregoing description and drawings merely explain and illustrate the
invention and the invention is not limited thereto except insofar as the
appended claims
are so limited as those skilled in the art who have the disclosure before them
will be able
to make modifications and variations therein without departing from the scope
of the
invention.
13