Note: Descriptions are shown in the official language in which they were submitted.
CA 02390375 2002-05-07
WO 01/35071 PCT/US00/30815
CELL ANALYSIS AND SORTING APPARATUS
FOR MANIPULATION OF CELLS
FIELD OF THE INVENTION
This invention relates to cell analysis and sorting devices and methods for
manipulating cells using these devices. More particularly, the invention
relates to a cell
to analysis and sorting apparatus that can capture and hold single cells at
known locations
and then selectively release certain of these cells. A method of manipulating
the cells
using the cell analysis and sorting apparatus is also provided.
BACKGROUND OF THE INVENTION
Many recent technological advances have enhanced the study of cellular biology
and biomechanical engineering, most notably by improving methods and devices
for
carrying out cellular analysis. For example, in the past decade an explosion
in the
number of optical probes available for cell analysis has enabled an increase
in the amount
of information gleaned from microscopic and flow cytometric assays.
Microscopic
assays allow the researcher to monitor the time-response of a limited number
of cells
using optical probes. Flow cytometry, on the other hand, uses optical probes
for assays
on statistically significant quantities of cells for sorting into
subpopulations.
However, these mechanisms alone are insufficient for time-dependent analysis.
Microscopic assays can only track a few cells over time, and do not allow the
user to
track the location of individual cells. With flow cytometry, the user can only
observe
each cell once, and can only easily sort a cell population into three
subpopulations. Flow
cytometry techniques fail to provide for analysis of the same cell multiple
times, or for
arbitrary sorting of subpopulations. These kinds of bulk assay techniques
produce mean
statistics, but cannot provide the researcher with distribution statistics.
Advances in microsystems technology have also influenced many applications in
the fields of cell biology and biomedical engineering. Scaling down to the
micron level
allows the use of smaller sample sizes than those used in conventional
techniques.
SUBSTITUTE SHEET (RULE 26)
W~ O1/350~1 CA 02390375 2002-05-07 pCT/US00/30815
Additionally, the smaller size and ability to make large arrays of devices
enables multiple
processes to be run in parallel.
Integrated circuits have been fabricated on silicon chips since the 1950s, and
as
processing techniques improve, the size of transistors continues to shrink.
The ability to
produce large numbers of complex devices on a single chip sparked interest in
fabricating
mechanical structures on silicon as well. The range of applications for micro
electromechanical systems (MEMS) is enormous. Accelerometers, pressure
sensors, and
actuators are just a few of the many MEMS devices currently produced. Another
application of MEMS is in biology and medicine. Micromachined devices have
been
made for use in drug-delivery, DNA analysis, diagnostics, and detection of
cell
properties.
Manipulation of cells is another application of MEMS. For example, in the
early
1990's, Sato et al. described in his paper, which is hereby incorporated by
reference,
Individual and Mass Operation of Biological Cells using Micromechanical
Silicon
Devices, Sensors and Actuators, 1990, A21-A23:948-953, the use of pressure
differentials
to hold cells. Sato et al. microfabricated hydraulic capture chambers that
were used to
capture plant cells for use in cell fusion experiments. Pressure differentials
were applied
so that single cells were sucked down to plug an array of holes. Cells could
not be
individually released from the array, however, because the pressure
differential was
applied over the whole array, not to individual holes.
Bousse et al. in his paper, which is hereby incorporated by reference,
Micromachined Multichannel Systems for the Measurement of Cellular Metabolism,
Sensors and Actuators B, 1994, 20:145-150, described arrays of wells etched
into silicon
to passively capture cells by gravitational settling. Multiple cells were
allowed to settle
into each of an array of wells where they were held against flow due to the
hydrodynamics resulting from the geometry of the wells. Changes in the pH of
the
medium surrounding the cells were monitored by sensors in the bottom of the
wells, but
the wells lacked a cell-release mechanism, and multiple cells were trapped in
each well.
Another known method of cell capture is dielectrophoresis (DEP). DEP refers to
the
3o action of neutral particles in non-uniform electric fields. Neutral
polarizable particles
experience a force in non-uniform electric fields which propels them toward
the electric
field maxima or minima, depending on whether the particle is more or less
polarizable
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
than the medium it is in. By arranging the electrodes properly, an electric
field may be
produced to stably trap dielectric particles.
Microfabrication has been utilized to make electrode arrays for cell
manipulation
since the late 1980s. Researchers have successfully trapped many different
cell types,
including mammalian cells, yeast cells, plant cells, and polymeric particles.
Much work
involves manipulating cells by exploiting differences in the dielectric
properties of
varying cell types to evoke separations, such as separation of viable from non-
viable
yeast, and enrichment of CD34+ stem cells from bone marrow and peripheral
blood stem
cells. More relevant work on trapping cells in various two- and three-
dimensional
microfabricated electrode geometries has been shown by several groups.
However,
trapping arrays of cells with the intention of releasing selected
subpopulations of cells has
not yet been widely explored. Additionally, DEP can potentially induce large
temperature changes, causing not only convection effects but also profoundly
affecting
cell physiology.
These studies demonstrate that it is possible to trap individual and small
numbers
of cells in an array on a chip, but without the ability to subsequently
manipulate and
selectively release individual cells. This inability to select or sort based
on a biochemical
measurement poses a limitation to the kinds of scientific inquiring that may
be of interest.
The currently available mechanisms for carrying out cell analysis and sorting
are
2o thus limited in their applications. There is thus a need for an improved
method and
apparatus for sorting and releasing large quantities of cells that can easily
and efficiently
be used. In addition, there is a need for an analysis and sorting device that
allows the user
to look at each cell multiple times, and to track many cells over time.
Finally, there is a
need for a cell sorter that lets the user know the cell locations, and to be
able to hold and
selectively release the cells so that the user can arbitrarily sort based on
any aspect of the
cells' characteristic during time-responsive assays.
SUMMARY OF THE INVENTION
The present invention provides a cell sorting apparatus that is capable of
monitoring over time the behavior of each cell in a large population of cells.
The cell
analysis and sorting apparatus contains individually addressable cell
locations. Each
SUBSTITUTE SHEET (RULE 26)
W~ 01/35071 CA 02390375 2002-05-07 pCT/US00/30815 ,
location is capable of capturing and holding a single cell, and selectively
releasing that
cell from that particular location. In one aspect of the invention, the cells
are captured
and held in wells, and released using vapor bubbles as a means of cell
election. In
another aspect of the invention, the cells are captured, held and released
using electric
field traps.
According to one aspect of the present invention, the cell analysis and
sorting
apparatus has an array of geometric sites for capturing cells traveling along
a fluid flow.
The geometric sites are arranged in a defined pattern across a substrate such
that
individual sites are known and identifiable. Each geometric site is configured
and
to dimensioned to hold a single cell. Additionally, each site contains a
release mechanism
to selectively release the single cell from that site. Because each site is
able to hold only
one cell, and each site has a unique address, the apparatus allows the user to
know the
location of any particular cell that has been captured. Further, each site is
independently
controllable so that the user is able to arbitrarily capture cells at select
locations, and to
t 5 release cells at various locations across the array.
In one embodiment of the present invention, the geometric sites are configured
as
wells. As a fluid of cells is flown across the array of specifically sized
wells, cells will
fall into the wells and become trapped. Each well is sized and shaped to
capture only a
single cell, and is configured such that the cell will not escape into the
laminar flow of the
2o fluid above the well. The single cell can be held inside the well by
gravitational forces.
Each well can further be attached via a narrow channel to a chamber located
below the
well. Within the chamber is a heating element that is able to induce bubble
nucleation,
the mechanism for releasing the cell from the site. The bubble creates volume
expansion
inside the chamber which, when filled with fluid, will displace a jet of fluid
out of the
25 narrow channel and eject the cell out of the well. Fluid flow above the
well will sweep
the ejected cell away to be either collected or discarded.
In another embodiment of the present invention, the geometric sites are formed
from a three-dimensional electric field trap. Each trap comprises four
electrodes arranged
in a trapezoidal configuration, where each electrode represents a corner of
the trapezoid.
3o The electric fields of the electrodes create a potential energy well for
capturing a single
cell within the center of the trap. By removing the potential energy well of
the trap, the
4
SUBSTITUTE SHEET (RULE 26)
W~ 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
cell is ejected out of the site and into the fluid flow around the trap.
Ejected cells can
then be washed out and collected or discarded.
In yet another embodiment of the present invention, an integrated system is
proposed. The system can be a microfabrication-based dynamic array cytometer
(p,DAC)
having as one of its components the cell analysis and sorting apparatus
previously
described. To analyze a population of cells, the cells can be placed on a cell
array chip
containing a plurality of cell sites. The cells are held in place within the
plurality of cell
sites in a manner similar to that described above and analyzed, for example,
by
photometric assay. Using an optical system to detect fluorescence, the
response of the
1 o cells can be measured, with the intensity of the fluorescence reflecting
the intensity of the
cellular response. Once the experiment is complete, the cells exhibiting the
desired
response, or intensity, may be selectively released into a cell sorter to be
further studied
or otherwise selectively processed. Such an integrated system would allow
researchers to
also look at the cell's time response.
Further features and advantages of the present invention as well as the
structure
and operation of various embodiments of the present invention are described in
detail
below with reference to the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
This invention is pointed out with particularity in the appended claims. The
above
and further advantages of this invention may be better understood by referring
to the
following description when taken in conjunction with the accompanying
drawings, in
which:
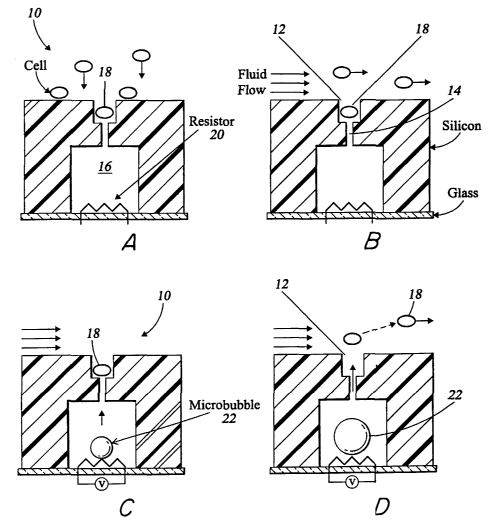
FIGS. 1 A, 1 B, 1 C, and 1 D show the mechanism by which one embodiment of the
present invention uses to capture, hold and release a single cell.
FIGS. 2A, 2B, and 2C show a process by which another embodiment of the
present invention uses to capture, hold and release a single cell.
FIGS. 3A and 3B show a top-down view of the cell sorting apparatus of FIG. 2.
FIG. 4 shows an exploded view of the cell sorting apparatus of FIG 2.
5
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
FIG. 5 shows an exploded view of yet another embodiment of the present
invention in which a cell sorting apparatus is integrated into a fluorescence-
detecting
system.
FIG. 6 is the thermodynamic pressure-volume diagram for water.
FIG. 7A shows a top view of a resistor of the present invention.
FIG. 7B shows a cross-section of the resistor of FIG. 7A.
FIG. 8 shows thermal resistances as seen by a heater of the present invention.
FIGS. 9A and 9B show flow lines for flow over rectangular cavities of
different
aspect ratios.
t 0 FIG. 10 shows a schematic of forces on a particle in a well.
FIG. 11 A shows a top view of a heater of the present invention.
FIG. 1 I B shows a cross-section of the heater of FIG. 1 I A.
FIG. 12A shows a side view of a cell well of the present invention.
FIG. 12B shows a top-down view of the cell well of FIG. 12A.
t5 FIGS. 13A, 13B, and I3C shows a top-down view of a silicon processing mask
set for use in the present invention.
FIG. 14 shows a top-down view of a glass processing mask.
FIG. 15 shows a diagram of a flow system for testing devices of the present
invention.
20 FIG. 16A shows a top-down view of a flow chamber of the present invention.
FIG. 16B shows a side view of the flow chamber of FIG. 16A.
FIG. 17 is a graph of pressure drop vs. flow rate for the flow chamber of
FIGS.
16A and 16B.
FIG. 18A shows a top-down view of the chamber base of flow chamber of FIG.
25 16A and 16B.
FIG. 18B shows a side view of the chamber base of FIG. 18A.
FIG. 18C shows a top-down view of the chamber lid of flow chamber of FIG.
16A and 16B.
FIG. 18D shows a side view of the chamber lid of FIG. 18C.
30 FIGS. 19A-19C show a process of fabricating a glass slide of the present
invention.
6
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/pS00/30815
FIGS. 20A-20H show a process of fabricating a silicon wafer of the present
invention.
FIGS. 21A-21D show a process of assembling the silicon wafer of FIGS. 20A-
20H onto the glass slide of FIGS. 19A-19C.
FIG. 22 is a graph of temperature v. resistance for platinum resistors of the
present
invention.
FIG. 23 shows a configuration for a resistor testing apparatus used in the
present
invention.
FIG. 24 is a graph of current v. voltage for the onset of boiling in platinum
line
IO resistors of the present invention.
FIG. 25 is a graph of current v. temperature for the platinum line resistors
of FIG.
24.
FIG. 26 is a graph of temperature v. resistance for a set of annealed platinum
line
resistors of the present invention.
t5 FIG. 27 is a graph of temperature v. resistance for a set of annealed
platinum line
resistors which were heated on a hot plate.
FIG. 28 is a graph of current v. voltage for a set of annealed platinum line
resistors of the present invention.
FIG. 29 is a graph of current v. temperature for the resistors of FIG. 28.
20 FIG. 30 is a graph of current v. voltage for the resistors of FIG. 28 under
repeated
boiling tests.
DETAILED DESCRIPTION OF THE INVENTION
25 FIGS. lA-1D illustrate an exemplary system of the present invention. A cell
site
10, shown in cross-section, contains a well 12 sized and shaped to hold a
single cell 18.
Connected to the bottom of the well 12 is a narrow channel 14 that opens into
a chamber
16 situated below the well. In this particular example, the well 12 and narrow
channel 14
are etched out of a silicon wafer. The silicon wafer is attached to a glass
slide on which
30 there is a platinum heater 20, and the alignment is such that the heater 20
is sealed inside
the chamber 16, which is filled with a fluid such as water.
7
SUBSTITUTE SHEET (RULE 26)
W~ ~1/35~~1 CA 02390375 2002-05-07 pCT/US00/30815
The well 12 function as a capture and hold mechanism. In operation, fluid
containing cells is flown over the top of the apparatus, and then the flow is
stopped. As
shown in FIG. 1A, the cells then settle and gravitational forces will allow
one cell 18 to
fall into and become trapped within the well 12. At this point the flow is
started again,
and the cell in the well is trapped while the cells not in well are flushed
away by
convection. FIG. 1 B shows how the well 12 is dimensioned and configured to
hold only
one cell 18 within the well I2 at a time. In addition, the well 12 is
configured such that
the cell 18 will not be swept out of the well due to laminar or fluid flow
above.
Experiments may be performed on the trapped cells, such as by adding a
reagant.
to When the experiments are concluded, the cells exhibiting the desired
characteristics may
be selectively released from the wells. In this example, when it is desired to
release cell
18 from the well 12, the operator can apply a voltage to the heating element
20 within the
chamber 16. The heating element 20 is then heated to a temperature above the
superlimit
of the fluid contained within the chamber 16 to initiate vapor bubble
nucleation at the
surface of the heating element 20, as seen in FIG. 1 C. In FIG. 1 D, a
microbubble 22 is
formed inside the chamber, creating a volume displacement. By adjusting the
voltage of
the heating element 20, the operator can control the size of the microbubble
22. When
the microbubble 22 is of sufficient size, the volume expansion in the chamber
will
displace a jet of fluid within the chamber 16 out of the narrow channel 14,
ejecting the
cell 18 out of the well 12. The released cell 18 can be swept into the fluid
flow outside
the well 12, to be later collected or discarded.
In another exemplary system of the present invention, the cell site 30
includes
electric field traps. Figures 2A-2C show, in cross-section, two cell sites on
a substrate
such as a microfabricated chip 36. Each site includes a plurality of
electrodes 32.
Preferably, each cell site 30 contains four electrodes, positioned in a
trapezoidal
configuration, as seen in Figures 3A and 3B. The cell site 30 is configured
and
positioned such that only one cell can be held within the site. The electrodes
32 create a
non-uniform electric field trap within which a single cell 34 can be held and
subsequently
released. FIG. 4 illustrates how the location and polarity of the electrodes
32 can create
3o an electric field trap for capturing the cell 34.
In use, cells in fluid medium flow over the cell sites 30, as shown in FIG.
2A. By
adjusting the electric field of each electrode 32, a potential energy well can
be created
8
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
within each cell site 30. The potential energy well is of sufficient strength
to capture a
single cell 34 traveling along the fluid flow and to hold the cell 34 within
the center of the
trap, as seen in FIG. 2B. When the operator selects to release a cell 34, he
can adjust the
electric fields of the electrodes 32 forming the trap. FIG. 2C shows how this
in turn
s removes the potential energy well, releasing the cell 34 back into the fluid
flow. The cell
34 can then be collected or discarded.
The electrodes forming the electric field trap are preferably thin-film poles
formed
of gold. This creates a three-dimensional electric field trap that is
effective in holding a
cell against the laminar flow of the fluid surrounding the electrodes.
Further, while only
Io one or two cell sites are illustrated, it is understood that the drawings
are merely
exemplary of the kind of site that can be included in the cell sorting
apparatus of the
present invention. The cell sorting apparatus can contain anywhere from a
single cell site
to an infinite number of cell sites, for sorting mass quantities of cells.
Moreover, while
the embodiments herein are described as holding cells, it is understood that
what is meant
15 by cells includes biological cells, cellular fragments, particles,
biological molecules, ions,
and other biological entities.
Because the cell sorting apparatus of the present invention allows the
operator to
know the location of each cell in the array of cell sites, the operator is
able to manipulate
the cells and arbitrarily sort the cells based on their characteristic under
time-responsive
20 assays. One such method contemplates using scanning techniques to observe
dynamic
responses from cells. As shown in F1G. 5, an integrated cellular analysis
system 100 is
proposed in which cells are tested using light-emitting assays to determine
the cell's
response to stimuli over time. The integrated system can be a microfabrication-
based
dynamic array cytometer (p.DAC). The tested cells are placed on a cell array
chip 110
?5 similar to the cell sorting apparatus above, to be held in place within the
plurality of cell
sites, such as those described above. Using an optical system 120 to detect
fluorescence,
the response of the cells can be measured, with the intensity of the
fluorescence reflecting
the intensity of the cellular response. Once the experiment is complete, the
cells
exhibiting the desired response, or intensity, may be selectively released, to
be collected
3o or later discarded. Such an integrated system would allow researchers to
look at the cell's
time response.
9
SUBSTITUTE SHEET (RULE 26)
W~ 01/3$071 CA 02390375 2002-05-07 pCT/US00/30815
Any light-emitting assay in which the cell's response may vary in time is
suited
for study using this proposed system. It is ideally suited for finding
phenotype
inhomogeneities in a nominally homogeneous cell population. Such a system
could be
used to investigate time-based cellular responses for which practical assays
do not
currently exist. Instead of looking at the presence/absence or intensity of a
cell's
response to stimulus, the researcher can look at its time response.
Furthermore, the
researcher can gain information about a statistically significant number of
cells without
the potential of masking important differences as might occur in a bulk
experiment.
Specific applications may include the study of molecular interactions such as
receptor-
ligand binding or protein-protein interactions. Signal transduction pathways,
such as
those involving intracellular calcium, can also be investigated.
An advantage of the proposed integrated system is that the full time-response
of
all the cells can be accumulated and then sorting can be performed. This is
contrasted
with flow cytometry, where each cell is only analyzed at one time-point and
sorting must
l5 happen concurrently with acquisition. Geneticists can look at gene
expression, such as
with immediate-early genes, either in response to environmental stimuli or for
cell-cycle
analysis. Another large application area is drug discovery using reporter-gene
based
assays. The integrated system can also be used to investigate fundamental
biological
issues dealing with the kinetics of drug interactions with cells, sorting and
analyzing cells
2o that display interesting pharmacodynamic responses. Another application is
looking at
heterogeneity in gene expression to investigate stochastic processes in cell
regulation.
Finally, once temporal responses to certain stimuli are determined, the
integrated system
can be used in a clinical setting to diagnose disease and monitor treatment by
looking for
abnormal time responses in patients' cells.
25 One objective of the present invention is to provide a cell analysis and
sorting
apparatus which uses hydraulic forces to capture individual cells into
addressable
locations, and can utilize microbubble actuation to release these individual
cells from
their locations. In developing this apparatus, it was necessary to model and
understand
many physical phenomena, not the least important of which includes the theory
behind
30 bubble nucleation on micro-heaters. Further, it was necessary to design a
device with the
proper dimensions so that single particles, or cells, could be held in wells
against a flow.
Biological cells were not used in these experiments, as polystyrene
microspheres of the
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
same dimensions were thought to be more robust for testing purposes. The
fabrication
process had to be designed in order to build chips with the desired
attributes, and various
problems which arose needed to be resolved. Finally, it was necessary to
understand the
heating of the resistors so that sufficiently high temperatures could be
reached.
Under the theory of bubble nucleation, pool boiling takes place when a heater
surface is submerged in a pool of liquid. As the heater surface temperature
increases and
exceeds the saturation temperature of the liquid by an adequate amount, vapor
bubbles
nucleate on the heater. The layer of fluid directly next to the heater is
superheated, and
bubbles grow rapidly in this region until they become sufficiently large and
depart
1o upwards by a buoyancy force. While rising the bubbles either collapse or
continue
growing depending on the temperature of the bulk fluid.
There are two modes of bubble nucleation: homogeneous and heterogeneous.
Homogeneous nucleation occurs in a pure liquid, whereas heterogeneous
nucleation
occurs on a heated surface.
15 In a pure liquid containing no foreign objects, bubbles are nucleated by
high-
energy molecular groups. According to kinetic theory, pure liquids have local
fluctuations in density, or vapor clusters. These are groups of highly
energized molecules
which have energies significantly higher than the average energy of molecules
in the
liquid. These molecules are called activated molecules and their excess energy
is called
2o the energy of activation. The nucleation process occurs by a stepwise
collision process
that is reversible, whereby molecules may increase or decrease their energy.
When a
cluster of activated molecules reaches a critical size, then bubble nucleation
can occur.
In order to determine at what temperature water will begin to boil in the
homogeneous nucleation regime, it was useful to know the thermodynamic
superheat
25 limit of water. FIG. 6 is the thermodynamic pressure-volume diagram for
water, which
shows a region of stable liquid to the far left, stable vapor to the far
right, metastable
regions, and an unstable region in the center of the dashed curve. The dashed
line is
called the spinodal, and to the left of the critical point represents the
upper limit to the
existence of a superheated liquid. Along this line, Equation ( 1-1 ) holds
true, and within
30 the spinodal, Equation ( 1-2 ) applies.
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pcT/US00/30815
O -0 c><_><)
T
Cy ~ 0
T
The van der Waals and Berthelot equations of state were used to calculate the
superheat limit of water.
a
CI'+ T"vz ~w-b~= RT
Where v is the specific volume, R is the gas constant, and a and b are
constants. n=0 for
the van der Waals equation, n=1 for the Berthelot equation, and n=0.5 for the
modified
Berthelot equation. a and b were computed using Equation ( 1-3 ), given the
fact that at
the critical point, Equations ( 1-4 ) and ( 1-5 ) are true.
to
apl
=o
~, T,,
a=p
avz -° (i-5)
T.,
Using the above equations, the thermodynamic superheat limit of water was
computed. The results are shown below in Table 1.
Equation of State T/T~~ (T~r 647K) Superheat Limit (C)
Van der Waals 0.844 273
Modified Berthelot 0.893 305
Berthelot 0.919 322
Table 1. Thermodynamic superheat limit of water ca~cmatea mtn s equanons or
sate.
These values represent the temperature above which homogeneous nucleation
must begin.
12
SUBSTITUTE SHEET (RULE 26)
WO 01/3$071 CA 02390375 2002-05-07 pCT/US00/30815
A kinetic limit of superheat may also be computed using the kinetic theory of
the
activated molecular clusters. The kinetic limit of superheat for water is
about 300°C.
When liquid is heated in the presence of a solid surface, heterogeneous
nucleation
usually occurs. In this regime, bubbles typically nucleate in cavities
(surface defects) on
the heated surface. The degree of superheat necessary to nucleate a bubble in
a cavity is
inversely dependent on the cavity radius, as shown in Equation ( 1-6 ).
2~Tro,
T" -Tsar - h~,P~~a ( 1-6)
Where T,v is the surface temperature, T,.u, is the saturation temperature (
100°C for water),
6 is the surface tension, h;~ is the latent heat of vaporization, P~ is the
vapor density, and
r~ is the cavity radius. For example, the surface temperature necessary to
nucleate
bubbles in water with a surface that has a 1 ~tm cavity radius is about
133°C. For a 0.1 ~tm
cavity radius the temperature to nucleate a bubble is about 432°C, well
above the highest
thermodynamic water superheat limit of 322°C.
Accordingly, for surfaces with cavity sizes well below 1 pm, it is likely that
homogeneous nucleation will occur since the liquid will reach the superheat
limit before a
bubble nucleates in a cavity. Micromachined surfaces tend to have very smooth
surfaces.
For instance, the platinum resistors are only 3-6pm wide, and 0.1 pm thick, so
it is
unlikely that cavities will exist on the surface which are large enough for
heterogeneous
nucleation to occur. The largest likely nucleation cavity would be the
thickness of the
resistor, which is 0.1 ~tm, and results in a boiling temperature for
heterogeneous
nucleation above the thermodynamic superheat limit as shown above. Thus, it
was
assumed that homogeneous nucleation was the most likely method of bubble
nucleation
to occur for the resistors of this invention.
However, when platinum films are annealed, thermal grooving and agglomeration
can take place at the grain boundaries. A groove will develop on the surface
of a hot
polycrystalline material where a grain boundary meets the surface. As the
surface gets
hotter, the grooves deepen, initiating holes, and the platinum begins the
process of balling
up in order to reduce surface area. This process is called agglomeration. The
agglomeration rate is insignificant at anneal temperatures below 700°C.
However, for a
13
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
600°C anneal of platinum for 1 hour, the onset of agglomeration can
cause small voids in
the platinum with radii of up to about 0.5 Vim. In this case, heterogeneous
nucleation
would be possible at a temperature of about 166°C.
Next, it was desirable to predict the electrical current necessary to achieve
a
certain temperature of the resistor. The schematic and boundary conditions for
this
resistor model are shown in FIG. 7A and 7B. For the cross-sectional slice
through the
resistor (7B), the water above the heater was 450pm thick, corresponding to
the height of
the silicon chamber containing the water. It was assumed that the ambient
temperature
was maintained at the top of the water in the well since above this there was
silicon with
l0 water at the ambient temperature flowing over the top of it. The bottom of
the glass slide
was also assumed to be at the ambient temperature since it was contacting a
surface at the
ambient temperature. The resistor was about 10,000 times thinner than the
glass slide and
had ohmic heating, or power generation equal to IZR for the entire volume of
the resistor.
First, the characteristic time for the heat to conduct through the two
bounding
surfaces was calculated using Equation ( 1-7 ).
L2
z ~ a ( 1_7
Where L is the characteristic length for conduction and a is the thermal
diffusivity of the
material.
Using this relation, it was found that the characteristic time for conduction
through lmm of glass was about 2.3 seconds. Similarly, the characteristic time
for
conduction through 450~m of water was 1.38 seconds. Accordingly, for this
system the
time to reach steady state would be about four times greater than the highest
characteristic
time, about 9 seconds. As established above, homogeneous bubble nucleation was
likely
to occur, which is a molecular process and thus may be assumed to be
approximately
instantaneous. The time for a bubble to nucleate was therefore far shorter
than the 9
seconds necessary for the system to reach steady state, so steady state
conditions are
unlikely to be achieved before the bubble nucleates.
14
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT~S00/30815
It was then necessary to determine the dominant modes of heat transfer from
the
resistor to its surroundings. The purpose of this model was to predict the
temperature of
the heater for a given current, before the onset of boiling. For this model,
heat transfer
due to radiation was neglected.
A lumped model approach was taken for this analysis. This approximation was
checked by computing the Biot number for the resistor.
Bi = ht = 7x10-y « 1
kP,
( 1-8 )
Where t is the platinum resistor thickness (0.1 pm) and kn, is the thermal
conductivity of
0 platinum (71.5 W/mK). It was assumed in this model a heat transfer
coefficient of
h=SW/mzK as a high bound for natural convection. The Biot number measures the
ratio
of internal conduction resistance to external convection resistance. Since the
Biot
number was much less than unity, the lumped body approximation was used and an
assumption was made that the entire resistor was at a uniform temperature.
FIG. 8 shows the thermal resistances between the resistor and the ambient
temperature. For the purpose of this order of magnitude estimate of the heat
transfer
mechanisms, steady state conditions were used in determining thermal
resistances. First,
the thermal resistance due to convection through the water was computed. For
this case it
was assumed there was natural convection since the water above the heater was
stagnant,
and boiling was not occurnng. The thermal resistance due to convection was
calculated
below.
1 _ I _ , K
R'~""e"'°° = hA - hwL 6.67x10 W ( 1-9 )
Where w is the resistor width (3pm) and L is the resistor length (1000p.m).
Next the thermal resistance due to conduction through the platinum resistor,
glass
slide, and water were computed. The resistance due to conduction was given by:
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
_ L
Rconduction ~ ( 1-1~ )
Where L is the length through which heat conducts, and A is the cross-
sectional area. For
the platinum, the length through which heat conducts was very long ( l2mm) and
the
cross-sectional area was very small, resulting in a high thermal resistance:
RPr°tin°,n = Lr~' = 5.4x10 K ( t-1 t )
k,,ttw W
Where t is the platinum film thickness (0.1 pm), Lpt is the length through
which heat
conducts ( l2mm), w is the width of the resistor (3~m), and kPt is the
conductivity of
platinum (71.SW/mK). Similarly, the thermal resistances of the glass and water
were
t 0 computed.
L
kgLw 4.1x105 K
W ( i-12 )
R",°,e, = L'° = 2.2x105 K 1-13 )
k",Lw W (
Where L~ is the length of glass through which heat conducts ( lmm), k~ is the
conductivity
of glass (0.81 W/mK), L is the length of the resistor ( 1000pm), w is the
width of the
resistor (3~m), L,s, is the length of water through which heat conducts
(450~m), and k~" is
the conductivity of water (0.67W/mK).
From this it was shown that Rs,ass arid RWa,er were the dominant thermal
resistances
for the system. Thus, heat transfer due to convection in the water and
conduction through
the platinum were negligible.
2o An estimate the temperature of the resistor as a function of time for a
given
current using semi-infinite body theory was then made. For small times (t<lms)
it was
assumed that both the water and glass are semi-infinite bodies with initial
temperature Ta.
At t=0, a constant heat flux (due to the resistor) is applied at the water-
glass interface
(x=0). The one-dimensional temperature profile was computed using the infinite
16
SUBSTITUTE SHEET (RULE 26)
W~ Ol/350~1 CA 02390375 2002-05-07 pCT/US00/30815
composite solid solution. The region x>0 is water, x=0 is the resistor, and
x<0 is the
glass. A one-dimensional model was used for short times since the length of
the resistor
(L=1000pm) was much less than the width of the resistor (L=6um). The
temperature was
assumed to be constant along the resistor, and lateral conduction was
neglected for small
times. This model will break down when the lateral conduction becomes
significant, and
when the assumption of semi-infinite bodies becomes invalid. The boundary
conditions
for this problem are given below.
T =Tz,x=O,t>0
( 1-14 )
i i
qiai - qzaz x = 0,t > 0 ( 1-15 )
K, K,
q~ + q3 = ~l ( 1-t6 )
to Where K is the thermal conductivity (0.61 W/mK for water and 0.88 W/mK for
glass), q
is the heat flux, and the subscript '1' denotes water, and '2' denotes glass.
The solution for the temperature profiles in water and air for a constant heat
flux q
(W/m') applied at x=0 is given by Equations ( 1-17 ) and ( 1-18 ).
T - T = 2q a,azt ler c x
i ,~ ( )
K, az + Kz a, f 2 a,t 1-17
T -T = 2q a~azt ie~fc x
z ~ ( i-18 )
K, az + h'z a, 2 azt
~5
Where a is the thermal diffusivity ( 1.47x 10-'m/s'' for water and 4.4x 10-
'm/sz for glass)
and To is the initial temperature of the body.
The solution was also used to check the semi-infinite body assumption. For
times
equal to or less than l ms, and a reasonable heat flux such as 2.5x 107 W/m2,
the heat
2o penetration depths into the glass and water were less than 100pm. The total
thickness of
the water was 450~m and of the glass was lmm, so the semi-infinite body
assumption
17
SUBSTITUTE SHEET (RULE 26)
W~ 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
held true. The one-dimensional model was sufficient for determining the
temperature of
the resistor at small times.
Using the theory described above, it was possible to predict the power
necessary to
form a bubble. Since homogeneous bubble nucleation was assumed, the bubbles
would
form at approximately the superheat limit of water. The value of 305°C
given by the
modified Berthelot equation (Table 1 ) was used. Next, the infinite composite
solid
solution was used to calculate the temperature of the heater for a given time,
say 1 ms.
Rearranging equation ( I-17 ) to solve for the heat flux, or power per unit
area at position
x=0, it was derived:
to
p (K, a, + Kz a, kT - T, )
Lw 2 a,a=t
( 1-19 )
For an initial temperature of 20°C, and the other properties given
above, the heat
flux necessary to heat the resistor to 305°C in lms was computed from(
1-19 ) to be
1.32x10' W/m''. For typical resistor dimensions of w=6pm and L=I500~m, the
~5 necessary power was about 120mW.
The micromachined wells must be of the proper dimensions to ensure that
particles which settle into them remain held in the wells once a flow above
them is
initiated. The theory of slow viscous flow over cavities has been well
characterized and
the streamlines for various geometries have been calculated and experimentally
verified.
20 FIG. 9 shows the flow pattern for laminar flow over a rectangular cavity
for two
different width to height aspect ratios. From these flow patterns it was seen
that there
was a separating flow line which penetrates slightly into the cavity. Below
this line there
were one or two vortices, depending on the aspect ratio of the cavities. A
particle below
the separating flow line would not be swept out of the cavity by a slow flow
in the
25 laminar range, though the vortex may agitate the particle.
An order of magnitude calculation was performed in order to compare the
relative
sizes of the gravity force pulling a particle down, compared to the viscous
shear force
pulling a particle out of the well. A diagram of a particle in a well with
flow over the top
is shown in FIG. 10.
18
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
The force of gravity acting on the particle was dependent on the difference in
density between the particle and the water, 0p. The density of water is
approximately
1000kg/m3, and the density of the polystyrene beads used in the experiments
was given
by the manufacturer as 1060kg/m3. The density of cells ranges from 1050-
1100kg/m3.
Accordingly, the force of gravity, Fs was computed as shown:
F~ =~p3~ra3g (1-20)
Where a is the particle radius (5x 1 O-bm), and g is the gravitational
constant.
The viscous shear force acting on the particle was computed by assuming the
top
of the particle was at the top of the well, and that the flow prof 1e was
parabolic. The
shear stress at the wall was:
_ _du
r~~' p d ( 1-21 )
Y ~=o
Where ~C is the viscosity of water ( 1 x 10-3kg/ms) and u(y) is the velocity
profile as a
function ofy, the distance from the wall.
Assuming a parabolic velocity profile in the flow chamber, the flow profile
was
calculated for a known chamber height and volume flow rate.
u~Y) = by Y~h -Y) ( 1-22 )
__ Q
V wh ( 1-23 )
u(Y) = w~ Y~~z -Y) ( 1-24 )
_du _ 6Q
d wh Z ( 1-25 )
Y y=o
Where V is the average flow velocity, w is the chamber width, and h is the
chamber
height.
19
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
The viscous shear force on the cell was estimated as the wall shear stress
multiplied by the area being effected, approximately ~a'.
F,, = r",era z = P 6Q era''
wh ' ( 1-26 )
Where a is the cell radius. Finally the ratio of gravity to viscous force was
computed.
4 3
_ ~P 3 ~a g _ 20pagivh '' ( 1-27 )
F,. ,u 6Q ~a z 9f~Q
w
Using the flow chamber dimensions in FIG. 16, and a range of reasonable flow
rates, this ratio was computed.
to
1 F
Q=1 '~' CV =2.1~m~~ ~ =292 (1-Z8)
min s F,,
F
Q=10'~ CV =21'~m~-a ~ =29 (1-29)
min s Fv
1 F
Q=100 '~ CV =210-'n~--~ ~' =3 (1-30)
min s F,.
It was necessary that the ratio of forces be greater than one so that the
gravity
force was stronger than the viscous force. These numbers were used to aid in
determining a range of acceptable operating flow rates.
Another relevant piece of information was the time it took for the particles
to
settle. At low Reynolds number, an isolated rigid spherical particle will
settle with its
Stokes velocity.
0 2a z (PS - P)g
U -
(1-31)
SUBSTITUTE SHEET (RULE 26)
CA 02390375 2002-05-07
WO 01/35071 PCT/US00/30815
Where a is the sphere radius (S~m for a polystyrene bead), ps is the density
of the bead
(about 1060kg/m~), p is the density of water ( 1000kg/m3), and ,u is the
viscosity of water.
Using these values a Stokes velocity was calculated as:
U° = SxlO-6 m = 5 Pm
s s
(1-32)
Using this velocity to check the associated Reynolds number it was found that
Re= pU'~a -3x10-' « 1
p (1-33)
Thus, the assumption of low Reynolds number was valid. The Reynolds number
is the ratio of inertial effects to viscous forces. For this case, only the
highly viscous
to regime applied and inertial effects were negligible.
Another value which was checked was the Peclet number. This is the ratio of
sedimentation to diffusion. For the particles to settle, the Peclet number
must be
sufficiently high, otherwise the particles will diffuse throughout the liquid.
Pe =
( 1-34 )
D" __ kT = 4x10m4 n?~
6~rua s ( 1-35 )
Pe = 6.x10- » 1 ( 1-36 )
Where D" is the Brownian diffusivity, and k is the Boltzmann's constant
(1.381x10-~6 erg/cm). Thus the Peclet number was sufficiently high for
settling to
dominate over diffusion.
The value calculated above for the Stokes velocity is that for an isolated
particle;
however, in the case at hand there were many beads settling at once. This was
taken into
2o account in the calculation of the hindered velocity. A function of the
particle volume
fraction is multiplied by the Stokes velocity to result in the hindered
velocity of particles
in the suspension.
21
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
( 1-37 )
U = ~°.~(~)
f(~)_(1-~)5.~ =0.95 (
-6 rrt ,urrt ( 1-39 )
U = 4.75x10 = 4.75-
S
Where ~ is the particle volume fraction (about 0.01 for this case).
Accordingly, the time
necessary for all the particles to settle to the bottom of the flow chamber
was calculated
using the hindered velocity and the chamber height, the maximum distance to be
traveled.
5
t, = h =166s = 2.76 min
U ( 1-40 )
Where h is the chamber height (790um). This settling time was used as a
guideline in
experiments.
A more reasonable assumption for calculating the settling time was that the
o distance the particles fell is an average of half the chamber height. For
this case a settling
time of about 83 seconds was obtained.
For the given pressure increase associated with the bubble formation in the
large
sealed well, the flow rate out of the channel in the top of the well was
calculated. Since
the Reynolds number was in the creeping flow regime (Re< 1 ), inertial effects
neglected,
I S and the initial, instantaneous flow out of the channel was computed using
the steady state
equation for flow through a circular aperture at low Reynolds number.
Q - OPC 3
3~ ( t-4t )
Where O is the volume flow rate, dP is the pressure drop, c is the aperture
radius (~2.5 or
2o 4pm), and ,u is the water viscosity.
Since the pressure change due to the bubble formation was not easily
calculable,
the volume flow rate out of the chamber was estimated in a different way.
Because water
is incompressible, it was assumed in the model that the bubble formation as a
volume
injection into the chamber resulted in the same volume being ejected from the
chamber
22
SUBSTITUTE SHEET (RULE 26)
WD 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
over the characteristic bubble formation time. For instance, if it took lms to
form a
l0~tm diameter bubble, then the resulting volume flow rate out of the chamber
was
calculated as follows.
V = 4 Jrr3 = 5.24x10-m,n3
( 1-42 )
Q = v = 5.24x1Om' m3
t s ( 1-43 )
Using the volume flow rate the average velocity of fluid out of the channel
was
calculated, and it is seen that the Reynolds number of the flow was indeed
low.
V = Q = 27 nzm
arc z s (
Re = pV ~ = 0.067 < 1
( 1-45 )
Where c is the channel radius (2.S~m). The force of the fluid jet on the
particle was
calculated using the Stokes drag force:
Fo = 6~r~aV = 2.5x10-'' N
( t-46 )
Where a is the radius of the spherical particle (S~m for polystyrene beads).
Comparing
this to the gravitational force ( 1-20 ) pulling the particle down, it was
found that the force
of the jet on the particle was much greater than the force of gravity.
Fb = Op 4 ~ra3g = 3.1x10m3 N « Fo
( 1-47 )
Fo V
F °' a' ( 1-48 )
23
SUBSTITUTE SHEET (RULE 26)
W~ 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
Where dp is the difference in densities between the water and the polystyrene
beads (60
kg/m3). It was seen that as the particle radius increased, the effect of
gravity increased.
For typical cells, the radius ranges from S~m (red blood cells) to 20~m (most
other cells)
to 100pm (embryos and eggs). This device will most likely be used for cells on
the order
of 5-IO~m in radius so the above calculation was representative of the
expected
applications.
DESIGN OF THE COMPONENTS
io A. RESISTIVE HEATERS
In order to heat the water to a sufficiently high temperature for microbubble
formation, resistive heaters were used. The heaters were made of thin-f Im
platinum on
standard glass slides. In designing the heaters it was necessary first to
determine a range
of resistances and currents to attain the desired power output. The design
constraint for
t5 this step was the need to keep the current density below the
electromigration limit of
platinum, while retaining an adequate degree of ohmic heating. The
electromigration
limit is the maximum current density which platinum can endure before the
atoms begin
to migrate leaving the resistor inoperable.
The electromigration limit of platinum was reported to be J=9x 106 A/cm''. It
was
2o necessary to design the resistors to operate at a current density below
this limit.
The resistance of a line heater is calculated as follows.
pL
R=
tw ( ~ -49 )
Where R is the resistance (S2), L is the length of the resistor (m), t is the
film thickness
25 (m), w is the width of the resistor (m), and p is resistivity of platinum
(S2m).
The power output of a resistor is a function of the current and resistance, as
shown
below.
p=I2R (i-50)
24
SUBSTITUTE SHEET (RULE 26)
CA 02390375 2002-05-07
WO 01/35071 PCT/US00/30815
J = I < 9x106 A
wt cmZ ( 1-51 )
Where I is the current (A) and J is the current density.
Accordingly, as the currents were limited by the electromigration limit, the
resistances needed to be sufficiently high to achieve the desired power
output. The power
output necessary to form a bubble was estimated by using the numbers from Lin
et al.'s
paper, 'Microbubblc Powered Actuator', herein incorporated by reference, where
microbubbles were formed on a polysilicon line heater. Their resistor was on
top of a
thin dielectric layer, which was on a silicon wafer. It was reasonable to
assume that the
heat dissipation of this configuration might well be greater than the heat
dissipation of the
l0 platinum line resistor fabricated on a glass slide. Also, a liquid with a
higher boiling
necessary to nucleate bubbles under these conditions was approximately 65mW.
Slide ResistorLen Width Resistance Electromi rationMax Power
Name th um Ohms Limit mA mW
um
Slidel 1 3000 3 1000 22 467
2 2500 3 833 22 389
3 500 3 167 22 78
4 1000 3 333 22 156
5 1000 4 250 29 207
6 2000 3 667 22 311
7 1500 3 500 22 233
8 1000 5 200 36 259
Slide2 1 3000 3.6 483 22 226
2 2500 3,6 400 22 187
3 500 3 167 22 78
4 1000 3.6 150 22 70
5 1000 6 167 43 311
6 2000 3.6 317 22 14F
7 1500 3.6 233 22 109
8 1000 3,5 180 22 84
Slide3 1 3000 6 625 43 1166
2 2500 6 521 43 972
3 500 3 208 22 97
4 1000 3 417 22 194
5 1000 6 208 43 389
6 2000 6 417 43 778
7 1500 6 313 43 583__
8 1000 6 208 43 389
Table 2. Resistor dimensions, resistances, and electromigration limits.
IS Using this as a guideline, the resistances were chosen to range from 16752-
100052,
yielding maximum powers before electromigration of 70-1166mW. These powers
were
chosen to be up to an order of magnitude greater than necessary to avoid
reaching the
electromigration limit in the operation of the resistors.
SUBSTITUTE SHEET (RULE 26)
CA 02390375 2002-05-07
WO 01/35071 PCT/US00/30815
The resistivity of platinum actually varies with temperature and film
deposition
conditions, but for these calculations it was taken to be 1 x 10-~S2m. This is
the value for
bulk platinum, however the resistivity of thin film platinum can vary widely.
Heater
widths range from 3 -6pm and lengths range from 500 -3000~m. Some heaters were
designed to have a narrow region, 100~m long in the center, which would be
hotter than
the rest of the resistor. FIG. 1 1A is a top view of a heater configuration,
while FIG. 11B
shows a cross-sectional view of the heater and its dimensions. A table of
resistor
dimensions, maximum currents, and maximum power outputs is also shown in Table
2.
The lines connecting the contact pads to the heaters were designed to have a
far
to lower resistance than the heaters. This was done to ensure that the lines
did not heat up,
and that they remained approximately at the ambient temperature. The connector
line
widths wcre chosen to be 1500~m with lengths of l2mm. The total resistance of
each
line was about 7.752.
~ 5 B. WELLS
Square wells were micromachined into silicon in order to hold cells. It was
necessary to choose a range of dimensions for these wells to allow for tests
with different
particle sizes and flow rates. The final goal was to have the ability to trap
one particle in
each of an array of wells.
Chip Number Well Dimension Hole Dimension
um um
1 16 5
b 16 8
2 10 5
2b 10 8
3 20 5
3b 20 8
4 30 5
4b 30 8
5 40 5
5b 40 8
6 50 5
6b 50 8
Table 3. Well Dimensions
Side lengths of the wells were chosen to range from 10~m, corresponding to the
smallest test bead size, up to SO~m. Well sizes ranging from 10-SO~m were
chosen.
26
SUBSTITUTE SHEET (RULE 26)
W~ 01/35071 CA 02390375 2002-05-07 pCT/pS00/30815
Narrow channel widths of S~m and 8~m were chosen since both these sizes are
smaller
than the minimum test particle size of 10~m and it is necessary that particles
not be able
to settle down into the narrow channel. The table of well dimensions is shown
in Table 3.
A diagram of the well geometry is shown in FIG. 12A, which shows a side view,
while
s FIG. 12B shows a top-down view.
Photomasks for use in the device fabrication were created using standard mask
layout software. The mask set for the silicon processing are shown in FIGS.
13A-13C
and the glass mask set is shown in FIG. 14A and 14B.
Three masks were designed for the silicon portion of the device processing.
One
to mask was created for the cell wells (FIG. 13A), one for the narrow channels
within the
wells (FIG. 13B), and one for the large wells (FIG. 13C) etched from the
backside of the
wafer to enclose the heaters. Two masks were made for the fabrication of the
platinum
heaters on the glass slides. One mask (FIG. 14) was designed to pattern the
metal.
In order to test the finished devices, a fluidic system as illustrated in FIG.
15 was
l5 designed and assembled. A syringe pump 150 was used as the flow source for
the bulk
fluid, and flow rates ranging from 1 to 100 ~L/min were specified. Beads,
cells, or cell
stimuli were injected through the sample injection valve 152. A pressure
sensor 154 was
located before the flow chamber 156 so that the pressure drop across the
chamber could
be monitored. All fluid was outlet into a waste beaker 158 which could be
reused if
2o desired.
A schematic of the flow chamber 156 is shown in FIGS. 16A and 16B. The flow
chamber was machined from plexiglass so that it was clear and a microscope was
used to
observe cell behavior from above the chamber. HPLC (high-performance liquid
chromatography) fittings were used with tube dimensions of 1/16 inch outer
diameter and
25 0.020 inch inner diameter. The gasket between the slide and the top cover
were made
from PDMS (poly dimethyl siloxane), a flexible polymer. A seal was formed by
screwing the top plate down onto the bottom plate. Aluminum molds were
machined in
order to create PDMS gaskets of the proper dimensions. Gaskets were compressed
until a
hard stop was reached. The stop was provided by the spacers, made of metal
shim stock,
3o in order to accurately specify the channel height. The aspect ratio of the
channel's width
to height was greater than 10, allowing the assumption of a parabolic velocity
profile-
plane Poiseuille flow.
27
SUBSTITUTE SHEET (RULE 26)
CA 02390375 2002-05-07
WO 01/35071 PCT/US00/30815
The height of the flow chamber was 790~m (determined by thickness of metal
spacer). Flow rates ranged from 1 to 100 pL/min and corresponded to Reynolds
numbers
of 0.001-0.1. In this creeping flow regime, the entrance length for fully
developed flow
was calculated to be negligible. These calculations are shown below.
min
m
min = 1 .77
-
Ac S ~ 1-52 )
_ Amax~m
vmaxr 1 77
-
Ac S ~ 1-53 )
min
h
Re v = 0.0011
min -
~ 1-54 )
h V K
n,:~
Rc - = 0.1 1
n,~ ,
. ( t _55 )
v
h Re
,
ma
x
X~ = 2.6,um
~
30 ~ 1-56 )
Where Vm;~ is the minimum average velocity, Qm;~ is the minimum volume flow
rate
( 1 pL/min), A~ is the cross-sectional area of the channel (h=790pm, w=12mm),
Vm;~ is the
maximum average velocity, Amax is the maximum volume flow rate ( 100pL/min),
Re is
t o the Reynolds number, v is the kinematic viscosity of water ( 1 x 10-
6m2/s), and X~ is the
entrance length for fully-developed flow.
Electrical connections to the contact pads were made using a probe station.
Contact pads were positioned outside of the PDMS gasket and were thus kept
outside of
the fluid flow.
In order to ensure the proper flow characteristics of the flow chamber, dye
was
injected into the flow and the resulting profile was observed. The results
were used to
discover problems such as blockages in the flow chamber and correct them. When
a
uniform flow was established, lOpm diameter beads were injected into the flow
and
observed under a microscope.
2o The pressure drop across the flow chamber was monitored using a pressure
transducer. The majority of the pressure drop was caused by the connector
tubing, but by
comparing the pressure reading to the theoretical value, the presence of
bubbles and other
blockages to the flow may be detected.
28
SUBSTITUTE SHEET (RULE 26)
WO ~1/3$~~1 CA 02390375 2002-05-07 pCT/US00/30815
The pressure versus flow rate plot for the flow chamber is shown in FIG. 17.
The
theoretical value is plotted with the experimental measurements. When these
two values
do not match, a blockage in the chamber or tubing is probable.
The pressure drop through the tubing was calculated using the following
equation.
DP - _ 8~a~ ~
pry. ( 1-57 )
Where ,u is the viscosity of water ( 1 x 10-~kg/ms), r is the tube radius
(0.254mm), and dx is
the tube length (m). The pressure drop through the chamber was calculated to
be
negligible in comparison. The flow chamber schematic with dimensions is shown
in
o FIGS.18A-18D.
FABRICATION OF THE COMPONENTS
The platinum heaters were fabricated on standard lx3in glass slides using a
lift-
l5 off process. The process flow is shown in FIGS. 19A-19C. In the first step
illustrated as
FIG. 19A, photoresist was spun onto the glass slide, exposed using mask 4, and
developed. Next. 100 of titanium and 1000 of platinum were evaporated onto the
slide, as seen in FIG. 19B. The titanium served as an adhesion layer between
the glass
and the platinum. In the following step, the slide was submerged in acetone to
dissolve
20 the photoresist and lift away the metal which was deposited on top of the
photoresist, as
depicted in FIG. 19C. Only the platinum resistors were left on the glass
slide. Some
slides were then annealed in a tube furnace at 600°C for 1 hour. While
not used in this
example, it is contemplated that photoresist may be applied manually to the
slide to attach
the silicon chip to the slide.
25 The silicon chip process flow is shown in FIGS. 20A-20H. Double Side
Polished
(DSP) four inch diameter silicon wafers were used. In the first step shown as
FIG. 20A,
1 um of thermal oxide was grown on the wafer. Next the oxide was patterned
using mask
1, FIG. 20B. Resist was spun on top of the oxide and patterned using mask 2.
The
resulting configuration was called a nested mask, shown as FIG. 20C.
29
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
First the photoresist mask was used to etch the narrow 5 pm trenches, then the
oxide mask was used to etch the cell wells, as shown in FIGS. 20D and 20E.
Next the
wafer was turned over and photoresist was deposited and patterned on the back
side using
mask 3 (FIG. 20F). A deep silicon etch was then performed to etch through the
wafer
and intersect the narrow trenches etched previously (FIG. 20G) to obtain a
finished wafer
(FIG. 20H).
A complete device consisted of a silicon chip attached to a glass slide by
photoresist, as shown in FIGS. 21 C and D. The resist provided a water-tight
seal so that
volume expansion in the bubble wells resulted in a burst of fluid being pushed
through
~o the narrow channel and ejecting a cell.
To facilitate the assembly process, alignment marks were fabricated on the
glass
slide and matching holes were etched in the silicon chip. The alignment
tolerances were
sufficiently large (about 2mm) that the chip could be aligned to the slide by
hand using
just the naked eye, while still positioning the bubble wells over the platinum
heaters.
l5 Photoresist was painted onto the silicon chip around the bubble wells using
a
toothpick. Drops of water were deposited into each well using a pipette, then
the glass
slide was visually aligned from above and stuck down onto the chip. The drops
of water
served to fill the bubble wells and get pushed through the narrow channel to
fill it with
water. The device was now ready to be tested in the flow chamber.
20 Next, the resistance of the platinum resistors were studied. The film
thickness was
first measured using a profilometer. The platinum thickness measurements
ranged from
about 800-900A, so the average value of 850A was used in the subsequent
calculations.
The resistance along metal lines wide enough not to be strongly affected by
variation of a
few microns was measured using a multimeter. The lines used for this
measurement were
25 measured in an optical microscope to be about 1510pm wide. The length of
the lines was
about 8mm. Knowing the width, thickness, and length of these lines, as well as
the
measured resistance, the resistivity of the thin film platinum at room
temperature was
determined. The measured resistance was 1552, and the computed resistivity was
calculated below.
p = ~R = 2.41x10-' S2m ~ 1-58 )
SUBSTITUTE SHEET (RULE 26)
CA 02390375 2002-05-07
WO 01/35071 PCT/US00/30815
Where t is the film thickness (8500, w is the line width (1513p.m), R is the
measured
resistance ( 1552), and L is the length of the line (8mm). This resistivity
was more than
twice the value for bulk platinum ( 1 x 10-' SZm), but was a reasonable value
for thin film
platinum. This is because bulk platinum is a crystalline material, whereas
thin film
platinum is polycrystalline and the grain boundaries significantly increase
resistance.
Next, the resistance of the resistors was measured with a multimeter. Using
the value
of resistivity from above, the line width of each resistor was determined. The
line widths
were also measured using an optical microscope to an accuracy of about ~1 ~tm.
The
l0 results of this measurement for two different resistor slides are shown in
Table 4.
ResistorL um R Ohms Com uted Line Measured Desi
# Width um um n um
Slide 1 3000 1020 8.34 8 3
1
2 2500 845 8.39 8 3
3 500 185 7.66 8 3
4 1000 347 8.17 8 3
5 1000 272 10.42 10 4
6 2000 672 8.44 9 3
7 1500 504 8.44 8 3
8 1000 260 10.90 10 5
Slide 1 3000 850 10.01 10 6
3
2 2500 728 9.74 10 6
3 500 247 5.74 6 3
4 1000 479 5.92 6 3
5 1000 316 8.97 9 6
6 2000 620 9.15 9 6
7 1500 450 9.45 10 6
8 1000 270 10.50 10 6
Table 4. Resistance measurements and calculated, measured, and designed line
widths.
From this it was determined that the measured and calculated line widths were
within
the range of error for the measurements, confirming the resistivity
calculation. The
resulting plot of normalized resistance versus temperature is shown in FIG.
22. The
resistance was normalized using the resistance at room temperature. This curve
was used
later to predict the temperature of a resistor, knowing the resistance at room
temperature
and measuring the resistance during operation.
Using the cross-sectional area of the resistors, the maximum current before
electromigration was calculated. It was known that maximum current density
before
electromigration is 9x 106 A/cm''. Using this the maximum current for each
resistor was
calculated. The results of this are shown in Table 5.
31
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT~S00/30815
Resistor Com uted Line Width Maximum Current
# um mA
Slide 1 8.3 71.3
1
2 8.4 71.7
3 7.7 65.5
4 8.2 69.9
5 10.4 89.1
6 8.4 72.1
7 8.4 72.1
8 10.9 93.2
Slide 1 -. 10.0 -. -85.6-..
3
2 9.7 83.2
3 5.7 49.1
4 5.9 50.6
5 9.0 76.7
6 9.1 78.2
7 9.5 80.8
8 10.5 89.8 -
Table S. Computed electromigration limits for resistors.
These results were used as guidelines during testing of microbubble devices to
avoid burning out the resistors.
The main objective for the resistors was that they be able to reach high
enough
temperatures to boil water. The resistors were tested on a probe station using
an
HP4145b to vary the voltage and measure the resulting current through the
resistor. A
to PDMS gasket was placed on top of the slide and filled with water. The
gasket contained
the water and kept it from touching the electrical contacts and probes. FIG.
23 is a
schematic of this configuration.
Upon ramping the voltage across resistors from zero to about 20-30 V, there
was
violent bubbling originating not from the hot part of the resistor, but from
the edges of the
wide connector lines. It was evident that the bubbles were gas bubbles and not
water
vapor bubbles because the bubbles did not condense when the heater was turned
off.
Further experimentation revealed that electrolysis of the water was occurring
and the
water was being broken down into hydrogen and oxygen. After flushing the
slides,
gaskets, and glassware for several minutes with deionized water, and testing
again, the
problem of electrolysis was eliminated.
When the problem of electrolysis was eliminated, the resistors were once again
tested in water. When the resistor reached a sufficient temperature, boiling
occurred
along the length of the heater. After the power was turned off, small air
bubbles
32
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
remained on the resistor due to the dissolved gas coming out of solution, as
described
previously. In subsequent tests, the air bubbles served as nucleation sites
for boiling, the
inception of boiling occurred at a much lower temperature. When boiling begins
and
bubbles form on the resistor, the heat dissipation into the water increases
drastically. This
is a favorable phenomenon for the operation of the device because the onset of
boiling is
represented as a sharp increase in current on the I-V curve. This is because
when the heat
dissipation increases, the temperature decreases, resulting in a lower
resistance and thus a
higher current through the resistor. An I-V curve for the onset of boiling on
a line resistor
is shown in FIG. 24.
In this I-V curve it is shown that for the first run when no bubbles were
present on
the line, there is a sharp jump in current at the onset of boiling. For the
second run,
residual bubbles were left on the heater and served as nucleation sites for
boiling resulting
in a smooth I-V curve with boiling beginning at a lower temperature. The two
curves are
very close after the boiling begins for run 1.
In later tests, when no dissolved gas came out of solution, the jump in the I-
V curve
occurred during each heating cycle for the resistors, since there were no
residual air
bubbles left when the power was turned off.
Using the calibration given in FIG. 22 for the temperature-resistance
relationship of
the resistor, the temperature of the resistor for each current was plotted to
find the boiling
temperature. The current vs. temperature plot corresponding to the I-V curve
shown
above is in FIG. 25. On this plot water is shown to boil at approximately
308°C, at which
point the temperature drops rapidly due to the increased convective heat
transfer
associated with boiling.
The boiling points for the 5 resistors tested ranged from 250°C to
308°C. The
lowest calculated value for the superheat limit of water was found to be
273°C, so these
measured boiling points suggest that the bubble nucleation occurs either in
the
homogeneous regime, or by a weak heterogeneous mechanism.
After a considerable amount of testing of the resistors characterized above, a
drift in
the boiling temperature became apparent. In order to determine the reason for
this, the
resistors were recalibrated as described in the previous section. The
temperature versus
normalized resistance curve is shown in FIG. 26. The dramatic change in
temperature-
resistance characterization led to the testing of a second generation of
resistors. It is
33
SUBSTITUTE SHEET (RULE 26)
WO 01/35071 CA 02390375 2002-05-07 pCT/US00/30815
thought that these changed characteristics are caused over time by the heating
of the
resistors. The operation of the resistors effectively caused them to anneal
themselves.
Annealing changed the geography of the platinum grain boundaries and thus
changed the
resistivity of the resistors.
In order to avoid this effect in future testing, new resistor slides were
annealed at
600°C for 1 hour as the last step in their process. This temperature is
higher than
operating temperatures are likely to reach, but not so high that major
agglomeration will
result. Once the anneal was ccmplete, the new resistors were characterized as
described
above for the first generation resistors.
First, the resistivity of the platinum at room temperature was found to be
2.056x 10~'S2m, less than the unannealed resistors that were 2.41 x 10~'S2m.
Next the
resistances were measured using a multimetcr, and the line widths were
computed as
before, as shown in Table 6.
Resistor L um R Ohms Com uted Line Desi n um
# Width um
Slide 1 3000 553 13.12 6
3
2 2500 481 12.57 6
3 500 146 8.28 3
4 1000 281 8.61 3
5 1000 205 11.80 6
6 2000 409 11.83 6
7 1500 310 11.70 6
I 8 1000 186 13.00 6
S
Table 6. Measured resistances and computed line widths of second generation
resistors.
The temperature-resistance characteristic or the resistors was then measured
on a
hotplate as described above, and is shown in FIG. 27.
At this point, the bubble formation characteristics of the resistors were
tested as
described previously with boiled, deionized water. Voltages were ramped up by
O.SV
steps with delay times of 1 ms using the HP4145b, as before. None of these
tests resulted
in residual gas bubbles since the delay time was short, and the maximum
voltage used
was just above the bubble nucleation voltage, determined by testing. All
resulting vapor
bubbles condensed back into the liquid phase within one minute of stop of
current flow.
A resulting I-V curve is shown in Figure 28, and the corresponding temperature
curve is shown in Figure 29. From the curve we can see that the onset of
boiling
occurred at about 200°C, a much lower temperature than for the first
generation resistors,
34
SUBSTITUTE SHEET (RULE 26)
W~ 01/3$071 CA 02390375 2002-05-07 pCT/US00/30815
and well below the superheat limit of water. For the 8 second generation
resistors tested,
boiling points ranged from 128°C-200°C, with the majority of the
temperatures above
180°C. This suggests that the boiling is in the heterogeneous
nucleation regime as
discussed earlier. The cavity radii corresponding to these boiling inception
temperatures
are calculated from Equation ( 1-59 )
_ 26TSO,
h~~ Pv ~T". - T,~~ ~ ( 1-59 )
The results of this calculation are shown in Table7.
Resistor Boilin Tem eratureCavit Radius
# C um
1 200.7 0.33
2 198.3 0.34
3 170.4 0.47
4 183.2 0.40
5 128 1.19
6 188 0.38
7 189 0.37
8 169 0.48
Table 7. E3ubble nucleation cavity radii corresponding to measured boiling
temperatures.
From this we can see that bubbles were nucleated in radii ranging from 0.3-
l.2pm. As discussed previously, these cavities were most likely formed during
the
i5 600°C anneal, during which the grooves at the grain boundaries
widened creating
cavities.
The second generation resistors were also tested for the repeatability of
their boiling
temperatures. I-V curves were measured as in the previous section, and then
remeasured
for the same conditions several times. Between measurements, time was given
for the
vapor bubbles to dissipate so that the characteristic jump in the I-V curve at
boiling could
be observed with each measurement. The boiling point was found to be very
repeatable,
and an example of the results is shown in Figure 30. This result demonstrated
the
potential of a control system based on a jump in the I-V curve at the onset of
boiling,
since the boiling point remained fixed.
Another interesting result from this testing is that for a particular
resistor, the
bubbles tended to nucleate in the same locations on the resistor each time.
This
SUBSTITUTE SHEET (RULE 26)
W~ 01/35071 CA 02390375 2002-05-07 PCT/US00/30815
strengthens the hypothesis that the bubbles are nucleating in the
heterogeneous regime, in
cavities created by thermal grooving caused by the annealing.
RESULTS
s
The cell chip was attached to the glass resistor slide as described earlier,
and then
tested in two ways. First tests were done with stagnant fluid on the device.
Then the
device was put into the flow chamber for testing. The results of these tests
are described
below.
to For these tests, several drops of bulk solution were placed on top of the
cell chip,
and contained by the PDMS gasket. A drop of the polystyrene bead solution was
then
added to the bulk fluid and allowed to settle. The bulk solution was a 0.05%
solution of
Triton x-100 surfactant in deionized water. The bead solution was about 1%
beads
diluted in the same bulk solution. Some of the beads settled into wells, as
shown in
~s Figure 42. When voltage across the resistor was ramped up by the HP4145b,
an I-V
curve with a jump similar to that in FIG. 24 was produced, demonstrating that
boiling had
occurred. Consequently, the bubble formation under the well caused a volume
expansion
which rapidly ejected the beads from the well. First the beads are in the
well, and then
they are rapidly expelled. This sequence was also captured on videotape, and
the process
2o was repeated multiple times with the same success.
Preliminary dynamic testing was performed in the flow chamber. Beads were
ejected in a similar way to the static test, and carried away in the flow. The
preliminary
tests suggested that the beads are held in the wells against a reasonable flow
rate, and are
ejected into the flow when a microbubble forms.
25 While the invention has been particularly shown and described above with
reference to several preferred embodiments and variations thereon, it is to be
understood
that additional variations could be made in the invention by those skilled in
the art while
still remaining within the spirit and scope of the invention, and that the
invention is
intended to include any such variations, being limited only by the scope of
the appended
3o claims.
36
SUBSTITUTE SHEET (RULE 26)