Note: Descriptions are shown in the official language in which they were submitted.
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
TRICYCLIC DIHYDO~tPYRIMIDINE POTASSIUM CHANNEL OPENERS
TECHNICAL FIELD
Novel tricyclic dihydropyrimidine compounds and their derivatives can open
potassium channels and are useful for treating a variety of medical
conditions.
BACKGROUND OF INVENTION
Potassium channels play an important role in regulating cell membrane
excitability.
When the potassium channels open, changes in the electrical potential across
the cell
membrane occur and result in a more polarized state. A number of diseases or
conditions may
be treated with therapeutic agents that open potassium channels; for example,
see K. Lawson,
Pharmacol. Ther., v. 70, pp. 39-63 (1996); D.R. Gehlert et al., Prog. Neuro-
Psychopharmacol
& Biol. Psychiat., v. 18, pp. 1093-1102 (1994); M. Gopalakrishnan et al., Drug
Development
Research, v. 28, pp. 95-127 (1993); J.E. Freedman et al., The Neuroscientist,
v. 2, pp. 145-152
(1996); D. E. Nurse et al., Br. J. Urol., v. 68 pp. 27-31 (1991); B. B. Howe
et al., J.
Pharmacol. Exp. Ther., v. 274 pp. 884-890 (1995); D. Spanswick et al., Nature,
v. 390 pp.
521-25 (December 4, 1997); Dompeling Vasa. Supplementum (1992) 3434;
W09932495;
Grover, J Mol Cell Cardiol. (2000) 32, 677; and Buchheit, Pulmonary
Pharmacology &
Therapeutics (1999) 12, 103. Such diseases or conditions include asthma,
hypertension,
epilepsy, male sexual dysfunction, female sexual dysfunction, pain, bladder
overactivity,
stroke, diseases associated with decreased skeletal blood flow such as
Raynaud's phenomenon
and intermittent claudication, eating disorders, functional bowel disorders,
neurodegeneration,
benign prostatic hyperplasia (BPH), dysmenorrhea, premature labor, alopecia,
cardioprotection, coronary artery disease, angina and ischemia.
Bladder overactivity is a condition associated with the spontaneous,
uncontrolled
contractions of the bladder smooth muscle. Bladder overactivity thus is
associated with or
1
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
can cause diseases and/or conditions such as sensations of urgency, urinary
incontinence,
pollakiuria, bladder instability, nocturia, bladder hyerreflexia, and enuresis
(Resnick, The
Lancet (1995) 346, 94-99; Hampel, Urology (1997) 50 (Suppl 6A), 4-14; Bosch,
BJU
International (1999) 83 (Suppl 2), 7-9). Potassium channel openers (KCOs) act
as smooth
muscle relaxants. Because bladder overactivity and urinary incontinence can
result from the
spontaneous, uncontrolled contractions of the smooth muscle of the bladder,
the ability of
potassium channel openers to hyperpolarize bladder cells and relax bladder
smooth muscle
may provide a method to ameliorate or prevent bladder overactivity,
pollakiuria, bladder
instability, nocturia, bladder hyperreflexia, urinary incontinence, and
enuresis (Andersson,
Urology (1997) 50 (Suppl 6A), 74-84; Lawson, Pharmacol. Ther., (1996) 70, 39-
63; Nurse.,
Br. J. Urol., (1991) 68, 27-31; Howe, J. Pharmacol. Exp. Ther., (1995) 274,
884-890;
Gopalakrishnari, Drug Development Research, (1993) 28, 95-127).
The irritative symptoms of BPH (urgency, frequency, nocturia and urge
incontinence)
have been shown to be correlated to bladder instability (Pandita, The J. of
Urology (1999)
162, 943). Therefore the ability of potassium channel openers to hyperpolarize
bladder cells
and relax bladder smooth muscle may provide a method to ameliorate or prevent
the
symptoms of BPH. (Andersson; Prostate (1997) 30: 202-215).
The excitability of corpus cavernosum smooth muscle cells is important in the
male
erectile process. The relaxation of corporal smooth muscle cells allows
arterial blood to build
up under pressure in the erectile tissue of the penis leading to erection
(Andersson,
Pharmacological Reviews (1993) 45, 253). Potassium channels play a significant
role in
modulating human corporal smooth muscle tone, and thus, erectile capacity. By
patch clamp
technique, potassium channels have been characterized in human corporal smooth
muscle
cells (Lee, Int. J. Impot. Res. (1999) 11(4),179-188). Potassium channel
openers are smooth
muscle relaxants and have been shown to relax corpus cavernosal smooth muscle
and induce
erections (Andersson, Pharmacological Reviews (1993) 45, 253; Lawson,
Pharmacol. Ther.,
(1996) 70, 39-63, Vick, J. Urol. (2000) 163: 202). Potassium channel openers
therefore may
have utility in the treatment of male sexual dysfunctions such as male
erectile dysfunction,
impotence and premature ejaculation.
2
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The sexual response in women is classified into four stages: excitement,
plateau,
orgasm and resolution. Sexual arousal and excitement increase blood flow to
the genital area,
and lubrication of the vagina as a result of plasma transudation. Topical
application of KCOs
like minoxidil and nicorandil have been shown to increase clitoral blood flow
(J.J. Kim, J.W.
Yu, J.G. Lee, D.G. Moon, "Effects of topical K-ATP channel opener solution on
clitoral
blood flow", J. Urol. (2000) 163 (4): 240). KCOs may be effective for the
treatment of
female sexual dysfunction including clitoral erectile insufficiency,
vaginismus and vaginal
engorgement (I. Goldstein and J.R. Berman., "Vasculogenic female sexual
dysfunction:
vaginal engorgement and clitoral erectile insufficiency syndromes"., Int. J.
Impotence Res.
(1998) 10:584-S90), as KCOs can increase blood flow to female sexual organs.
Potassium channel openers may have utility as tocolytic agents to inhibit
uterine
contractions to delay or prevent premature parturition in individuals or to
slow or arrest
delivery for brief periods to undertake other therapeutic measures (Sanborn,
Semin. Perinatol.
(1995) 19, 31-40; Morrison, Am. J. Obstet. Gynecol. (1993) 169(5), 1277-85).
Potassium
channel openers also inhibit contractile responses of human uterus and
intrauterine
vasculature. This combined effect would suggest the potential use of KCOs for
dysmenhorrea
(Kostrzewska, Acta Obstet. Gynecol. Scand. (1996) 75(10), 886-91). Potassium
channel
openers relax uterine smooth muscle and intrauterine vasculature and therefore
may have
utility in the treatment of premature labor and dysmenorrhoea (Lawson,
Pharmacol. Ther.,
(1996) 70, 39=63).
Potassium channel openers relax gastrointestinal smooth tissues and therefore
may be
useful in the treatment of functional bowel disorders such as irritable bowel
syndrome
(Lawson, Pharmacol. Ther., (1996)70, 39-63).
Potassium channel openers relax airways smooth muscle and induce
bronchodilation.
Therefore potassium channel openers may be useful in the treatment of asthma
and airways
hyperreactivity (Lawson, Pharmacol. Ther., (1996) 70, 39-63; Buchheit,
Pulmonary
Pharmacology & Therapeutics (1999) 12, 103; Gopalakrishnan, Drug Development
Research,
(1993) 28, 95-127).
3
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Neuronal hyperpolarization can produce analgesic effects. The opening of
potassium
channels by potassium channel openers and resultant hyperpolarization in the
membrane of
target neurons is a key mechanism in the effect of opioids. The peripheral
antinociceptive
effect of morphine results from activation of ATP-sensitive potassium
channels, which causes
hyperpolarization of peripheral terminals of primary afferents, leading to a
decrease in action
potential generation (Rodrigues, Br J Pharmacol (2000) 129(1), 110-4). Opening
Of KATr
channels by potassium channel openers plays an important role in the
antinociception
mediated by alpha-2 adrenoceptors and mu opioid receptors. KCOs can potentiate
the
analgesic action of both morphine and dexmedetomidine via an activation of
KATP channels at
l0 the spinal cord level (Vergoni, Life Sci. (1992) 50(16), PL135-8; Asano,
Anesth. Analg.
(2000) 90(5), 1146-51).. Thus, potassium channel openers can hyperpolarize
neuronal cells
and have shown analgesic effects. Potassium channel openers therefore may be
useful as
analgesics in the treatment of various pain states including but not limited
to migraine and
dyspareunia (Lawson, Pharmacol. Ther., (1996) 70, 39-63; Gopalakrishnan, Drug
Development Research, (1993) 28, 95-127; Gehlert, Prog. Neuro-Psychopharmacol.
& Biol.
Psychiat., (1994) 18, 1093-1102).
Epilepsy results from the propagation of iionphysiologic electrical impulses.
Potassium channel openers hyperpolarize neuronal cells and lead to a decrease
in cellular
excitability and have demonstrated antiepileptic effects. Therefore potassium
channel openers
may be useful in the treatment of epilepsy (Lawson, Pharmacol. Ther., (1996)
70, 39-63;
Gopalakrishnan, Drug Development Research, (1993) 28, 95-127; Gehlert, Prog.
Neuro-
Psychopharmacol. & Biol. Psychiat., (1994) 18, 1093-1102).
Neuronal cell depolarization can lead to excitotoxicity and neuronal cell
death. When
this occurs as a result of acute ischemic conditions, it can lead to stroke.
Long-term
neurodegeneration can bring about conditions such as Alzheimer's and
Parkinson's diseases.
Potassium channel openers can hyperpolarize neuronal cells and lead to a
decrease in cellular
excitability. Activation of potassium channels has been shown to enhance
neuronal survival.
Therefore potassium channel openers may have utility as neuroprotectants in
the treatment of
neurodegenerative conditions and diseases such as cerebral ischemia, stroke,
Alzheimer's
4
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
disease and Parkinson's disease (Lawson, Pharmacol. Ther., (1996) 70, 39-63;
Gopalakrishnan, Drug Development Research, (1993) 28, 95-127; Gehlert, Prog.
Neuro-
Psychopharmacol & Biol. Psychiat., (1994) 18, 1093-1102; Freedman, The
Neuroscientist
(1996) 2, 145).
Potassium channel openers may have utility in the treatment of diseases or
conditions
associated with decreased skeletal muscle blood flow such as Raynaud's
syndrome and
intermittent claudication (Lawson, Pharmacol. Ther., (1996) 70, 39-63;
Gopalakrishnan, Drug
Development Research, (1993) 28, 95-127; Dompeling Vasa. Supplementum (1992)
3434;
and W09932495).
Potassium channel openers may be useful in the treatment of eating disorders
such as
obesity (Spanswick, Nature, (1997) 390, 521-25; Freedman, The Neuroscientist
(1996) 2,
145).
Potassium channel openers have been shown to promote hair growth therefore
potassium channel openers have utility in the treatment of hair loss and
baldness also known
as alopecia (Lawson, Pharmacol. Ther., (1996) 70, 39-63; Gopalakrishnan, Drug
Development Research, (1993) 28, 95-127).
Potassium channel openers possess cardioprotective effects against myocardial
injury
during ischemia and reperfusion. (Garlid, Circ. Res. (1997) 81(6), 1072-82).
Therefore,
potassium channel openers may be useful in the treatment of heart diseases
(Lawson,
Pharmacol. Ther., (1996) 70, 39-63; Grover, J. Mol. Cell Cardiol. (2000) 32,
677).
Potassium channel openers, by hyperpolarization of smooth muscle membranes,
can
exert vasodilation of the collateral circulation of the coronary vasculature
leading to increase
blood flow to ischemic areas and could be useful for the coronary artery
disease (Lawson,
Pharmacol. Ther., (1996) 70, 39-63, Gopalakrishnan, Drug Development Research,
(1993) 28,
95-127).
US 4918074, EP 183848 B1, EP 217142, EP 328700, JP 63060985, JP 63243029, JP
61227584, and Atwal, K.S., Bioorg. Med. Chem. Lett (1991) 1, 291-294 disclose
bicyclic 4,7-
dihydropyrazolo[1,5-a]pyrimidines.
5
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The compounds of the present invention are novel and hyperpolarize cell
membranes,
open potassium channels and relax smooth muscle cells.
SUMMARY OF THE INVENTION
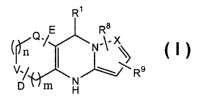
In its principle embodiment, the present invention discloses compounds of
formula (I):
R$
N' '
~~R9
D m
(I),
or a pharmaceutically acceptable salt, ester, amide, or prodrug thereof
wherein,
n is an integer of 0-1;
m is an integer of 1-2;
provided that when m is 2, n is 0;
R' is selected from aryl and heterocycle; .
Q is selected from C(O), S(O), and S(O)2;
V is selected from C(R6)(R~), O, S, and NR2, wherein R2 is selected from
hydrogen,
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkyl, alkynyl, aryl, arylalkoxy,
arylalkenyl,
arylalkyl, cyano, cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl,
heterocycle,
heterocyclealkyl, hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4
and R5 are
independently selected from hydrogen and lower alkyl;
R6 and R' are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, oxo, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined
above;
R8 and R9 are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
R~
Q /E
W
V.
N
6
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined above;
X is selected from N and CR3 wherein R3 is selected from hydrogen, alkenyl,
alkoxy,
alkoxyalkoxy, alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy,
arylalkenyl, arylalkyl,
carboxy, cyano, cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen,
heterocycle,
heterocyclealkyl, hydroxy, hydroxyalkyl, -NR4R5, and (NR4RS)alkyl wherein R4
and RS are as
defined above; and
D and E are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl; arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, oxo, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined
above.
DETAILED DESCRIPTION OF THE INVENTION
All patents, patent applications, and literature references cited in the
specification are
herein incorporated by reference in their entirety. In the case of
inconsistencies, the present
disclosure, including definitions, will prevail.
It is understood that the foregoing detailed description and accompanying
examples
are merely illustrative and are not to be taken as limitations upon the scope
of the invention.
Various changes and modifications to the disclosed embodiments will be
apparent to those
skilled in the art. Such changes and modifications, including without
limitation those relating
to the chemical structures, substituents, derivatives, intermediates,
syntheses, formulations
and/or methods of use of the invention, may be made without departing from the
spirit and
scope thereof.
In its principle embodiment, the present invention discloses compounds of
formula (I):
R8
N' \-
~ \ R9
m H
R'
Q /E
W
n
V.
N
7
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
(I),
or a pharmaceutically acceptable salt, ester, amide, or prodrug thereof
wherein,
n is an integer of 0-1;
m is an integer of 1-2;
provided that when m is 2, n is 0;
RI is selected from aryl and heterocycle;
Q is selected from C(O), S(O), and S(O)2;
V is selected from C(R6)(R~), O, S, and NR2, wherein R2 is selected from
hydrogen,
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkyl, alkynyl, aryl, arylalkoxy,
arylalkenyl,
arylalkyl, cyano, cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl,
heterocycle,
heterocyclealkyl, hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4
and RS are
independently selected from hydrogen and lower alkyl;
R6 and R' are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, oxo, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined
above;
R8 and R9 are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined above;
X is selected from N and CR3 wherein R3 is selected from hydrogen, alkenyl,
alkoxy,
alkoxyalkoxy, alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy,
arylalkenyl, arylalkyl,
carboxy, cyano, cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen,
heterocycle,
heterocyclealkyl, hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4
and RS are as
defined above; and
D and E are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
hydroxy, hydroxyalkyl, oxo, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined
above.
In another embodiment of the present invention, compounds have formula (I)
wherein,
R~ is aryl; X is CR3; R3 is hydrogen; and R8, R9, D, E, Q, V, m, and n are as
defined in
formula (I).
In another embodiment of the present invention, compounds have formula (I)
wherein,
R' is heterocycle; X is CR3; R3 is hydrogen; and Rg, R9, D, E, Q, V, m, and n
are as defined in
formula (I).
In a preferred embodiment, compounds of the present invention have formula
(II):
R'
Q N
Ra
V/ ~r
p N
H R
(II),
or a pharmaceutically acceptable salt, ester, amide, or prodrug thereof
wherein,
R~ is selected from aryl and heterocycle; .
Q is selected from C(O), S(O), and S(O)2;
V is selected from C(R6)(R~), O, S, and NR2, wherein Rz is selected from
hydrogen,
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkyl, alkynyl, aryl, arylalkoxy,
arylalkenyl,
arylalkyl, cyano, cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl,
heterocycle,
heterocyclealkyl, hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4
and RS are
independently selected from hydrogen and lower alkyl;
R6 and R' are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, oxo, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined
above;
R8 and R9 are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
9
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined above;
and
D and E are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, oxo, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined
above.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is heterocycle; Q is C(O); and Rg, R9, D, E, and V are as
defined in formula
(I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R1 is heterocycle; Q is S(O); and Rg, R9, D, E, and V are as
defined in formula
(I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, Rl is heterocycle; Q is S(O)Z; and Rg, R9, D, E, and V are as
defined in formula
(I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R1 is heterocycle; Q is C(O); V is S; and Rg, R9, D, and E are
as defined in
formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is heterocycle; Q is C(O); V is S; and Rg, R9, D, and E are
hydrogen.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is heterocycle; Q is C(O); V is CHZ; and Rg, R9, D, and E are
as defined in
formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is heterocycle; Q is C(O); V is CH2; E is alkyl; D is alkyl;
and R8 and R9 are
as defined in formula (I).
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is heterocycle; Q is C(O); V is CHZ; E is alkyl; D is alkyl;
and Rg and R9 are
hydrogen.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, Rl is heterocycle; Q is C(O); V is CH2; and R8, R9, D, and E are
hydrogen.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is heterocycle; Q is S(O)2; V is CH2; and R8, R9, D, and E
are as defined in
formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is heterocycle; Q is S(O)2; V is CH2; and Rg, R9, D, and E
are hydrogen.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, Rl is aryl; Q is C(O); and Rg, R9, D, E, and V are as defined in
formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R~ is aryl; Q is C(O); V is S; and R8, R9, D, and E are as
defined in formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is aryl; Q is C(O); V is S; and Rg, R9, D, and E are
hydrogen.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, Rl is aryl; Q is C(O); V is CHz; and Rg, R9, D, and E are as
defined in formula
(I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is aryl; Q is C(O); V is CH2; D is alkyl; E is alkyl; and Rg
and R9 are as
defined in formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is aryl; Q is C(O); V is CH2; D is alkyl; E is alkyl; and Rg
and R9 are
hydrogen.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is aryl; Q is C(O); V is CH2; R9 is aryl; and R8, D, and E
are as defined in
formula (I).
11
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is aryl; Q is C(O); V is CH2; R9 is aryl; and Rg, D, and E
are hydogen.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, RI is aryl; Q is C(O); V is CH2; R9 is heterocycle; and R8, D,
and E are as
defined in formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R~ is aryl; Q is C(O); V is CH2; R9 is heterocycle; and R8, D,
and E are hydogen.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, Rl is aryl; Q is C(O); V is CH2; R9 is halogen; and Rg, D, and E
are as defined in
formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R1 is aryl; Q is C(O); V is CH2; R9 is halogen; and Rg, D, and E
are hydogen.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is aryl; Q is C(O); V is CHZ; and Rg, R9, D, and E are
hydrogen.
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is aryl; Q is S(O); and Rg, R9, D, E, and V are as defined in
formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R' is aryl; Q is S(O)2; and Rg, R9, D, E, and V are as defined
in formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R~ is aryl; Q is S(O)2; V is CHZ; and R8, R9, D, and E are as
defined in formula
(I).
In another preferred embodiment of the present invention, compounds have
formula
(II) wherein, R~ is aryl; Q is S(O)2; V is CH2; and R8, R9, D, and E are
hydrogen.
In another preferred embodiment, compounds of the present invention have
formula (III):
R~
,Q N-N
I \ ~ Ra
R9
12
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
(III),
or a pharmaceutically acceptable salt, ester, amide, or prodrug thereof
wherein,
R~ is selected from aryl and heterocycle;
Q is selected from C(O), S(O), and S(O)2;
V is selected from C(R6)(R~), O, S, and NR2, wherein R2 is selected from
hydrogen,
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkyl, alkynyl, aryl, arylalkoxy,
arylalkenyl,
arylalkyl, cyano, cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl,
heterocycle,
heterocyclealkyl, hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4
and RS are
independently selected from hydrogen and lower alkyl;
R6 and R' are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, oxo, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined
above;
1 S Rg and R9 are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined above;
and
D and E are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, oxo, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined
above.
In another preferred embodiment of the present invention, compounds have
formula
(III) wherein, R' is heterocycle; Q is C(O); and Rg, R9, D, E, and V are as
defined in formula
(I).
13
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
In another preferred embodiment of the present invention, compounds have
formula
(III) wherein, R' is heterocycle; Q is S(O); and R8, R9, D, E, and V are as
defined in formula
(I).
In another preferred embodiment of the present invention, compounds have
formula
(III) wherein, R' is heterocycle; Q is S(O)Z; and Rg, R9, D, E, and V are as
defined in formula
(I).
In another preferred embodiment of the present invention, compounds have
formula
(III) wherein, R' is aryl; Q is C(O); and Rg, R9, D, E, and V are as defined
in formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(III) wherein, R' is aryl; Q is C(O); V is O; and R8, R9, D, and E are as
defined in formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(III) wherein, R1 is aryl; Q is C(O); V is O; and R8, R9, D, and E are
hydrogen.
In another preferred embodiment of the present invention, compounds have
formula
(III) wherein, Rl is aryl; Q is S(O); and Rg, R9, D, E, and V are as defined
in formula (I).
In another preferred embodiment of the present invention, compounds have
formula
(III) wherein, R~ is aryl; Q is S(O)2; and R8, R9, D, E, and V are as defined
in formula (I).
In another preferred embodiment, compounds of the present invention have
formula (IV):
R~
.Q ,N
V I ~Ra
E/v p N ~~\'~/ 9
H R
(IV),
or a pharmaceutically acceptable salt, ester, amide, or prodrug thereof
wherein,
Rl is selected from aryl and heterocycle;
Q is selected from C(O), S(O), and S(O)2;
V is selected from C(R6)(R~), O, S, and NR2, wherein R2 is selected from
hydrogen,
alkenyl, alkoxy, alkoxyalkoxy, alkoxyalkyl, alkyl, alkynyl, aryl, arylalkoxy,
arylalkenyl,
arylalkyl, cyano, cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl,
heterocycle,
14
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
heterocyclealkyl, hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4
and RS are
independently selected from hydrogen and lower alkyl;
R6 and R' are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, oxo, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined
above;
Rg and R9 are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, -NR4R5, and (NR4R5)alkyl wherein R4 and R5 are as
defined above;
and
D and E are independently selected from hydrogen, alkenyl, alkoxy,
alkoxyalkoxy,
alkoxyalkyl, alkyl, alkylthio, alkynyl, aryl, arylalkoxy, arylalkenyl,
arylalkyl, carboxy, cyano,
cycloalkyl, cycloalkylalkyl, haloalkoxy, haloalkyl, halogen, heterocycle,
heterocyclealkyl,
hydroxy, hydroxyalkyl, oxo, -NR4R5, and (NR4R5)alkyl wherein R4 and RS are as
defined
above.
In another preferred embodiment of the present invention, compounds have
formula
(IV) wherein, R' is heterocycle; Q is C(O); R8, R9, D, and E are as defined in
formula (I); and
V is as defined in formula (IV).
In another preferred embodiment of the present invention, compounds have
formula
(IV) wherein, R~ is heterocycle; Q is S(O); Rg, R9, D, and E are as defined in
formula (I); and
V is as defined in formula (IV).
In another preferred embodiment of the present invention, compounds have
formula
(IV) wherein, Rl is heterocycle; Q is S(O)Z; R8, R9, D, and E are as defined
in formula (I); and
V is as defined in formula (IV).
In another preferred embodiment of the present invention, compounds have
formula
(IV) wherein, R~ is aryl; Q is C(O); R8, R9, D, and E are as defined in
formula (I); and V is as
defined in formula (IV).
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
In another preferred embodiment of the present invention, compounds have
formula
(IV) wherein, R1 is aryl; Q is S(O); Rg, R9, D, and E are as defined in
formula (I); and V is as
defined in formula (IV).
In another preferred embodiment of the present invention, compounds have
formula
(IV) wherein, R1 is aryl; Q is S(O)2; R8, R9, D, and E are as defined in
formula (I); and V is as
defined in formula (IV).
Another embodiment of the present invention relates to pharmaceutical
compositions
comprising a therapeutically effective amount of a compound of formula (I-IV)
or a
pharmaceutically acceptable salt, ester, amide, or prodrug thereof in
combination with a
pharmaceutically acceptable carrier.
Another embodiment of the invention relates to a method of treating male
sexual
dysfunction including, but not limited to, male erectile dysfunction and
premature ejaculation,
comprising administering a therapeutically effective amount of a compound of
formula (I-IV)
or a pharmaceutically acceptable salt, ester, amide, or prodrug thereof.
Another embodiment of the invention relates to a method of treating female
sexual
dysfunction including, but not limited to, female anorgasmia, clitoral
erectile insufficiency,
vaginal engorgement, dyspareunia, and vaginismus comprising administering a
therapeutically effective amount of a compound of formula (I-IV) or a
pharmaceutically
acceptable salt, ester, amide, or prodrug thereof.
Yet another embodiment of the invention relates to a method of treating
asthma,
epilepsy, Raynaud's syndrome, intermittent claudication, migraine, pain,
bladder overactivity,
pollakiuria, bladder instability, nocturia, bladder hyperreflexia, eating
disorders, urinary
incontinence, enuresis, functional bowel disorders, neurodegeneration, benign
prostatic
hyperplasia (BPH), dysmenorrhea, premature labor, alopecia, cardioprotection,
and ischemia
comprising administering a therapeutically effective amount of a compound of
formula (I-IV)
or a pharmaceutically acceptable salt, ester, amide, or prodrug thereof.
16
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Definition of Terms
As used throughout this specification and the appended claims, the following
terms
have the following meanings.
The term "alkenyl," as used herein, refers to a straight or branched chain
hydrocarbon
containing from 2 to 10 carbons and containing at least one carbon-carbon
double bond
formed by the removal of two hydrogens. Representative examples of "alkenyl"
include, but
are not limited to, ethenyl, 2-propenyl, 2-methyl-2-propenyl, 3-butenyl, 4-
pentenyl, 5-
hexenyl, 2-heptenyl, 2-methyl-1-heptenyl, 3-decenyl and the like.
The term "alkenyloxy," as used herein, refers to an alkenyl group, as defined
herein,
appended to the parent molecular moiety through an oxy group, as defined
herein.
Representative examples of alkenyloxy include, but are not limited to, propen-
3-yloxy
(allyloxy), buten-4-yloxy, and the like.
The term "alkoxy," as used herein, refers to an alkyl group, as defined
herein,
appended to the parent molecular moiety through an oxy group, as defined
herein.
1 S Representative examples of alkoxy include, but are not limited to,
methoxy, ethoxy, propoxy,
2-propoxy, butoxy, tert-butoxy, pentyloxy, hexyloxy and the like.
The term "alkoxyalkoxy," as used herein, refers to an alkoxy group, as defined
herein,
appended to the parent molecular moiety through another alkoxy group, as
defined herein.
Representative examples of alkoxyalkoxy include, but are not limited to, tent-
butoxymethoxy,
2-ethoxyethoxy, 2-methoxyethoxy, methoxymethoxy, and the like.
The term "alkoxyalkyl," as used herein, refers to an alkoxy group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of alkoxyalkyl include, but are not limited to, tent-
butoxymethyl, 2-
ethoxyethyl, 2-methoxyethyl, methoxymethyl, and the like.
The term "alkoxycarbonyl," as used herein, refers to an alkoxy group, as
defined
herein, appended to the parent molecular moiety through a carbonyl group, as
defined herein.
Representative examples of alkoxycarbonyl include, but are not limited to,
methoxycarbonyl,
ethoxycarbonyl, tert-butoxycarbonyl, and the like.
17
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The term "alkyl," as used herein, refers to a straight or branched chain
hydrocarbon
containing from 1 to 10 carbon atoms. Representative examples of alkyl
include, but are not
limited to, methyl, ethyl, n-propyl, iso-propyl, n-butyl, sec-butyl, iso-
butyl, tert-butyl, n-
pentyl, isopentyl, neopentyl, n-hexyl, 3-methylhexyl, 2,2-dimethylpentyl, 2,3-
dimethylpentyl,
n-heptyl, n-octyl, n-nonyl, n-decyl, and the like.
The term "alkylcarbonyl," as used herein, refers to an alkyl group, as defined
herein,
appended to the parent molecular moiety through a carbonyl group, as defined
herein.
Representative examples of alkylcarbonyl include, but are not limited to,
acetyl, 1-oxopropyl,
2,2-dimethyl-1-oxopropyl, 1-oxobutyl, 1-oxopentyl, and the like.
The term "alkylcarbonyloxy," as used herein, refers to an alkylcarbonyl group,
as
defined herein, appended to the parent molecular moiety through an oxy group,
as defined
herein. Representative examples of alkylcarbonyloxy include, but are not
limited to,
acetyloxy, ethylcarbonyloxy, tert-butylcarbonyloxy, and the like.
The term "alkylsulfinyl," as used herein, refers to an alkyl group, as defined
herein,
appended to the parent molecular moiety through a sulfinyl group, as defined
herein.
Representative examples of alkylsulfinyl include,. but are not limited,
methylsulfinyl,
ethylsulfinyl, and the like.
The term "alkylsulfonyl," as used herein, refers to an alkyl group, as defined
herein,
appended to the parent molecular moiety through a sulfonyl group, as defined
herein.
Representative examples of alkylsulfonyl include, but are not limited,
methylsulfonyl,
ethylsulfonyl, and the like.
The term "alkylthio," as used herein, refers to an alkyl group, as defined
herein,
appended to the parent molecular moiety through a thio moiety, as defined
herein.
Representative examples of alkylthio include, but are not limited, methylthio,
ethylthio, tert-
butylthio, hexylthio, and the like.
The term "alkynyl," as used herein, refers to a straight or branched chain
hydrocarbon
group containing from 2 to 10 carbon atoms and containing at least one carbon-
carbon triple
bond. Representative examples of alkynyl include, but are not limited, to
acetylenyl, 1-
propynyl, 2-propynyl, 3-butynyl, 2-pentynyl, 1-butynyl and the like.
18
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The term "aryl," as used herein, refers to a monocyclic carbocyclic ring
system or a
bicyclic carbocyclic fused ring system having one or more aromatic rings.
Representative
examples of aryl include, azulenyl, indanyl, indenyl, naphthyl, phenyl,
tetrahydronaphthyl,
and the like.
S The aryl groups of this invention can be substituted with 1, 2, 3, 4, or 5
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkoxyalkoxy,
alkoxycarbonyl,
alkyl, alkylcarbonyl, alkylcarbonyloxy, alkylsulfinyl, alkylsulfonyl,
alkylthio, alkynyl, aryl,
aryloxy, azido, arylalkoxy, arylalkyl, aryloxy, carboxy, cyano, formyl,
halogen, haloalkyl,
haloalkox h drox , h drox alk 1 merca to nitro sulfam 1 sulfo sulfonate -
NRg°Rg~
Y~ Y Y Y Y Y~ P > > Y> > >
(wherein, Rg° and Rgl are independently selected from hydrogen, alkyl,
alkylcarbonyl, aryl,
arylalkyl and formyl), and -C(O)NR$ZRg3 (wherein, Rg2 and R83 are
independently selected
from hydrogen, alkyl, alkylcarbonyl, aryl, arylalkyl, and formyl).
The term "arylalkenyl," as used herein, refers to an aryl group, as defined
herein,
appended to the parent molecular moiety through an alkenyl group, as defined
herein.
Representative examples of arylalkenyl include, but are not limited to, 2-
phenylethenyl, 3-
phenylpropen-2-yl, 2-naphth-2-ylethenyl, and the like.
The term "arylalkoxy," as used herein, refers to an aryl group, as defined
herein,
appended to the parent molecular moiety through an alkoxy group, as defined
herein.
Representative examples of arylalkoxy include, but are not limited to, 2-
phenylethoxy, 3-
naphth-2-ylpropoxy, 5-phenylpentyloxy, and the like.
The term "arylalkoxycarbonyl," as used herein, refers to an arylalkoxy group,
as
defined herein, appended to the parent molecular moiety through a carbonyl
group, as defined
herein. Representative examples of arylalkoxy include, but are not limited to,
benzyloxycarbonyl, naphth-2-ylmethoxycarbonyl, and the like.
The term "arylalkyl," as used herein, refers to an aryl group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of arylalkyl include, but are not limited to, benzyl,
2-phenylethyl, 3-
phenylpropyl, 2-naphth-2-ylethyl, and the like.
19
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The term "aryloxy," as used herein, refers to an aryl group, as defined
herein,
appended to the parent molecular moiety through an oxy group, as defined
herein.
Representative examples of aryloxy include, but are not limited to, phenoxy,
naphthyloxy,
and the like.
The term "azido," as used herein, refers to an -N3 group.
The term "carbonyl," as used herein, refers to a -C(O)- group.
The term "carboxy," as used herein, refers to a -C02H group.
The term "carboxy protecting group," as used herein, refers to a carboxylic
acid
protecting ester group employed to block or protect the carboxylic acid
functionality while the
reactions involving other functional sites of the compound are carried out.
Carboxy-
protecting groups are disclosed in T.H. Greene and P.G.M. Wuts, Protective
Groups in
Organic Synthesis, 2nd edition, John Wiley & Sons, New York (1991), which is
hereby
incorporated herein by reference. In addition, a carboxy-protecting group can
be used as a
prodrug whereby the carboxy-protecting group can be readily cleaved in vivo,
for example by
I S enzymatic hydrolysis, to release the biologically active parent. T.
Higuchi and V. Stella
provide a thorough discussion of the prodrug concept in "Pro-drugs as Novel
Delivery
Systems", Vol 14 of the A.C.S. Symposium Series, American Chemical Society
(1975),
which is hereby incorporated herein by reference. Such carboxy-protecting
groups are well
known to those skilled in the art, having been extensively used in the
protection of carboxyl
groups in the penicillin and cephalosporin fields, as described in U.S. Pat.
No. 3,840,556 and
3,719,667, the disclosures of which are hereby incorporated herein by
reference. Examples of
esters useful as prodrugs for compounds containing carboxyl groups can be
found on pages
14-21 of "Bioreversible Carriers in Drug Design: Theory and Application",
edited by E.B.
Roche, Pergamon Press, New York (1987), which is hereby incorporated herein by
reference.
Representative carboxy-protecting groups are loweralkyl (e.g., methyl, ethyl
or tertiary butyl
and the like); benzyl (phenylmethyl) and substituted benzyl derivatives
thereof such
substituents are selected from alkoxy, alkyl, halogen, and nitro groups and
the like.
The term "cyano," as used herein, refers to a -CN group.
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The term "cycloalkyl," as used herein, refers to a saturated cyclic
hydrocarbon group
containing from 3 to 8 carbons. Representative examples of cycloalkyl include,
cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
The term "cycloalkylalkyl," as used herein, refers to cycloalkyl group, as
defined
herein, appended to the parent molecular moiety through an alkyl group, as
defined herein.
Representative examples of cycloalkylalkyl include, but are not limited to,
cyclopropylmethyl, 2-cyclobutylethyl, cyclopentylmethyl, cyclohexylmethyl and
4-cycloheptylbutyl, and the like.
The term "formyl," as used herein, refers to a -C(O)H group.
The term "halo" or "halogen," as used herein, refers to -Cl, -Br, -I or -F.
The term "haloalkyl," as used herein, refers to at least one halogen, as
defined herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of haloalkyl include, but are not limited to,
chloromethyl, 2-
fluoroethyl, trifluoromethyl, pentafluoroethyl, 2-chloro-3-fluoropentyl, and
the like.
The term "haloalkoxy," as used herein, refers to at least one halogen, as
defined
herein, appended to the parent molecular moiety through an alkoxy group, as
defined herein.
Representative examples of haloalkoxy include, but are not limited to, 2-
chloroethoxy,
difluoromethoxy, 1,2-difluoroethoxy, 2,2,2-trifluoroethoxy, trifluoromethoxy,
and the like.
The term "heterocycle," as used herein, refers to a monocyclic- or a bicyclic-
ring
system. Monocyclic ring systems are exemplified by any 5 or 6 membered ring
containing 1,
2, 3, or 4 heteroatoms independently selected from oxygen, nitrogen and
sulfur. The 5
membered ring has from 0-2 double bonds and the 6 membered ring has from 0-3
double
bonds. Representative examples of monocyclic ring systems include, but are not
limited to,
azetidine, azepine, aziridine, diazepine, 1,3-dioxolane, dioxane, dithiane,
furan, imidazole,
imidazoline, imidazolidine, isothiazole, isothiazoline, isothiazolidine,
isoxazole, isoxazoline,
isoxazolidine, morpholine, oxadiazole, oxadiazoline, oxadiazolidine, oxazole,
oxazoline,
oxazolidine, piperazine, piperidine, pyran, pyrazine, pyrazole, pyrazoline,
pyrazolidine,
pyridine, pyrimidine, pyridazine, pyrrole, pyrroline, pyrrolidine,
tetrahydrofuran,
tetrahydrothiophene, tetrazine, tetrazole, thiadiazole, thiadiazoline,
thiadiazolidine, thiazole,
21
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
thiazoline, thiazolidine, thiophene, thiomorpholine, thiomorpholine sulfone,
thiopyran,
triazine, triazole, trithiane, and the like. Bicyclic ring systems are
exemplified by any of the
above monocyclic ring systems fused to an aryl group as defined herein, a
cycloalkyl group as
defined herein, or another monocyclic ring system as defined herein.
Representative
examples of bicyclic ring systems include but are not limited to, for example,
benzimidazole,
benzothiazole, benzothiadiazole, benzothiophene, benzoxadiazole, benzoxazole,
benzofuran,
benzopyran, benzothiopyran, benzodioxine, 1,3-benzodioxole, cinnoline,
indazole, indole,
indoline, indolizine, naphthyridine, isobenzofuran, isobenzothiophene,
isoindole, isoindoline,
isoquinoline, phthalazine, pyranopyridine, quinoline, quinolizine,
quinoxaline, quinazoline,
tetrahydroisoquinoline, tetrahydroquinoline, thiopyranopyridine, and the like.
The heterocycle groups of this invention can be substituted with 1, 2,or 3
substituents
independently selected from alkenyl, alkenyloxy, alkoxy, alkoxyalkoxy,
alkoxycarbonyl,
alkyl, alkylcarbonyl, alkylcarbonyloxy, alkylsulfinyl, alkylsulfonyl,
alkylthio, alkynyl, aryl,
azido, arylalkoxy, arylalkoxycarbonyl, arylalkyl, aryloxy, carboxy, cyano,
formyl, halogen,
haloalkyl, haloalkoxy, hydroxy, hydroxyalkyl, mercapto, nitro, sulfamyl,
sulfo, sulfonate, -
NRg°R81 (wherein, R8° and R8~ are independently selected from
hydrogen, alkyl,
alkylcarbonyl, ,aryl, arylalkyl and formyl), and -C(O)NR8zR83 (wherein, Rg2
and R83 are
independently selected from hydrogen, alkyl, aryl, and arylalkyl).
The term "heterocyclealkyl," as used herein, refers to a heterocycle, as
defined herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of heterocyclealkyl include, but are not limited to,
pyrid-3-ylmethyl,
2-pyrimidin-2-ylpropyl, and the like.
The term "hydroxy," as used herein, refers to an -OH group.
The term "hydroxyalkyl," as used herein, refers to a hydroxy group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of hydroxyalkyl include, but are not limited to,
hydroxymethyl, 2-
hydroxyethyl, 3-hydroxypropyl, and the like.
The term "lower alkyl," as used herein, is a subset of alkyl as defined herein
and refers
to a straight or branched chain hydrocarbon group containing from 1 to 4
carbon atoms.
22
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Representative examples of lower alkyl include, but are not limited to,
methyl, ethyl, n-
propyl, iso-propyl, n-butyl, iso-butyl, tert-butyl, and the like.
The term "mercapto," as used herein, refers to a -SH group.
The term "(NR4R5)alkyl," as used herein, refers to a -NR4R5 group, as defined
herein,
appended to the parent molecular moiety through an alkyl group, as defined
herein.
Representative examples of (NR4R5)alkyl include, but are not limited to,
aminomethyl,
dimethylaminomethyl, 2-(amino)ethyl, 2-(dimethylamino)ethyl, and the like.
The term "nitro," as used herein, refers to a -NOZ group.
The term "oxo," as used herein, refers to a =O moiety.
The term "oxy," as used herein, refers to a -O- moiety.
The term "sulfamyl," as used herein, refers to a -SOZNR94R95 group, wherein,
R94 and
R95 are independently selected from hydrogen, alkyl, aryl, and arylalkyl, as
defined herein.
The term "sulfinyl," as used herein, refers to a -S(O)- group.
The term "sulfo," as used herein, refers to a -S03H group.
1 S The term "sulfonate," as used herein, refers to a -S(O)20R96 group,
wherein, R96 is
selected from alkyl, aryl, and arylalkyl, as defined herein.
The term "sulfonyl," as used herein, refers to a -S02- group.
The term "thio," as used herein, refers to a -S- moiety.
Preferred compounds of formula (I) include, but are not limited to:
9-(3-bromo-4-fluorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-
one;
9-(3-bromo-4-fluorophenyl)-5,9-dihydro-4H-pyrazolo [ 1,5-a]thiopyrano[3,4-
d]pyrimidin-8(7H)-one;
9-( 1-naphthyl)-5,6,7,9-tetrahydropyrazolo [5,1-b]quinazolin-8(4H)-one;
9-(2-naphthyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-one;
9-(3,4-dibromophenyl)-5,6,7,9-tetrahydropyrazolo[S,1-b]quinazolin-8(4H)-one;
9-(3,4-dichlorophenyl)-5,6,7,9-tetrahydropyrazolo [5,1-b]quinazolin-8(4H)-one;
9-(3-bromophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-one;
9-(3-chlorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-one;
23
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
9-[4-chloro-3-(trifluoromethyl)phenyl]-5,6,7,9-tetrahydropyrazolo [5,1-
b]quinazolin-
8(4H)-one;
9-[4-fluoro-3-(trifluoromethyl)phenyl]-5,6,7,9-tetrahydropyrazolo [5,1-
b]quinazolin-
8(4H)-one;
9-[3-(trifluoromethoxy)phenyl]-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-
one;
9-(3-cyanophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-one;
9-(3-methylphenyl)-5,6,7,9-tetrahydropyrazolo [5,1-b]quinazolin-8(4H)-one;
8-(3-bromo-4-fluorophenyl)-5,8-dihydro-4H,7H-faro[3,4-d]pyrazolo[1,5-
a]pyrimidin-
7-one;
(-) 9-(3-bromo-4-fluorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-
one;
(+) 9-(3-bromo-4-fluorophenyl)-5,6,7,9-tetrahydropyrazolo[S,l-b]quinazolin-
8(4H)-
one;
9-(3-bromo-4-fluorophenyl)-5,6,7,9-tetrahydro-4H-pyrazolo[1,5-a]thiopyrano[3,2-
d]pyrimidine 8,8-dioxide,
9-(3-chloro-4-hydroxyphenyl)-5,6,7,9-tetrahydropyrazolo [5,1-b]quinazolin-
8(4H)-
one;
3-bromo-9-(3-bromo-4-fluorophenyl)-5,6,7,9-tetrahydropyrazolo[S,1-b]quinazolin-
8(4H)-one;
9-(3-chloro-4-fluorophenyl)-5,6,7,9-tetrahydropyrazolo [5,1-b]quinazolin-8(4H)-
one;
9-(3,4-difluorophenyl)-5,6,7,9-tetrahydropyrazolo [5,1-b]quinazolin-8(4H)-one;
9-(4-fluorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-one;
9-[4-(trifluoromethyl)phenyl]-5,6,7,9-tetrahydropyrazolo[S,1-b]quinazolin-
8(4H)-one;
9-(4-cyanophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-one;
9-(4-chloro-3-nitrophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-
one;
9-(4-chloro-3-fluorophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
24
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
9-(3-bromo-4-fluorophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazo1o[5,1-
b]quinazolin-8(4H)-one;
9-[4-fluoro-3-(trifluoromethyl)phenyl]-7,7-dimethyl-5,6,7,9-
tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
9-(3,4-dichlorophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-
8 (4H)-one;
9-(4-chloro-3-nitrophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-
8(4H)-one;
9-(3,4-dibromophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-one;
9-[3-fluoro-4-(trifluoromethyl)phenyl]-7,7-dimethyl-5,6,7,9-
tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
9-(3-nitrophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-
one;
9-(3-cyanophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-
one;
7,7-dimethyl-9-(5-nitro-3-thienyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-
one;
9-(5-bromo-2-hydroxyphenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
9-(5-chloro-2-hydroxyphenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
9-(2-hydroxy-5-nitrophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
9-(3,5-dibromo-2-hydroxyphenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
9-(3-bromo-5-chloro-2-hydroxyphenyl)-7,7-dimethyl-5,6,7,9-
tetrahydropyrazolo[S,1-
b] quinazo lin-8 (4H)-one;
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
9-(3,5-dichloro-2-hydroxyphenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
9-(3,4,5-trifluorophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo [5,1-
b]quinazolin-
8(4H)-one;
9-(3,4-dichlorophenyl)-3-(3-fluorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
9-(3,4-dichlorophenyl)-3-(3-chlorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
9-(3,4-dichlorophenyl)-3-(4-carboxyphenyl)-5,6,7,9-tetrahydropyrazolo[S,1-
b]quinazolin-8(4H)-one;
9-(3,4-dichlorophenyl)-3-(2-thienyl)-5,6,7,9-tetrahydropyrazolo [5,1-
b]quinazolin-
8(4H)-one;
9-(3,4-dichlorophenyl)-3-[2-(trifluoromethyl)phenyl]-5,6,7,9-
tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one;
3-bromo-9-(3,4-dichlorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-
one;
(+) 3-bromo-9-(3,4-dichlorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-one;
(-) 3-bromo-9-(3,4-dichlorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-one;
(+) 9-(3,4-dichlorophenyl)-3-(2-thienyl)-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-
8(4H)-one;
(-) 9-(3,4-dichlorophenyl)-3-(2-thienyl)-5,6,7,9-tetrahydropyrazolo[5,1-
b]quinazolin-
8(4H)-one;
9-(3,4-dichlorophenyl)-3-(2-furyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-
one and pharmaceutically acceptable salts, esters, amides, or prodrugs
thereof.
Preparation of Compounds of The Invention
26
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The compounds and processes of the present invention will be better understood
in
connection with the following synthetic schemes and methods which illustrate a
means by
which the compounds of the invention can be prepared.
The compounds of this invention can be prepared by a variety of synthetic
routes.
Representative procedures are shown in Schemes 1-20.
Scheme 1
R~
E
\Q~E 1 HN,X) p\ _~ I N.X>
+ ~~,,~Ra N~9J~Rs
m O CHO H2N R9 m H R
(1) (2) (3) (4)
Fused pyrimidines of general formula (4), wherein R', X, Q, R8, R9, D and E
are as
defined in formula (I) and m is an integer 1-2, can be prepared according to
the method of
Scheme 1. A carbonyl component of general formula (1) can be treated with an
aldehyde of
general formula (2) and an amino heterocycle of general formula (3) in a
solvent such as
ethanol, acetonitrile or dimethylformamide with heating to provide fused
pyrimidines of
general formula (4).
Scheme 2
R'
~~Q~E R' ~i v a ~~Q~ ~ N,X~
V~O + CHO + H2N R9~ R V N~9~~Ra
H R
(5) (2) (3) (6)
Fused pyrimidines of general formula (6), wherein R', X, Q, V, R8, R9, D, and
E are as
defined in formula (I), can be prepared according to the method of Scheme 2. A
carbonyl
component of general formula (5) can be treated with an aldehyde of general
formula (2) and
an amino heterocycle of general formula (3) in a solvent such as ethanol;
acetonitrile or
dimethylformamide with heating to provide fused pyrimidines of general formula
(6).
Carbonyl components of general formula (5) may be prepared using the
procedures described
27
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
in (Dodd, J.H., Journal of Heterocyclic Chemistry 27 (1990) 1453; Terasawa,
T., Journal of
Organic Chemistry 42 (1977) 1163).
Scheme 3
R~
V-O/E R~ HN'X~ V O/~ N'IX
~ + ~i ~Re ~ ~i,,w
+ CHO H2N R9J ~ N R9 Rs
D D H
(7) (2) (3) (8)
Fused pyrimidines of general formula (8), wherein R', X, Q, V, R8, R9, D, and
E are as
defined in formula (I), can be prepared according to the method of Scheme 3. A
carbonyl
component of general formula (7) can be treated with an aldehyde of general
formula (2) and
an amino heterocycle of general formula (3) in a solvent such as ethanol,
acetonitrile or
dimethylformamide with heating to provide fused pyrimidines of general formula
(8).
Carbonyl components of general formula (7) may be prepared as described in
(Nakagawa, S.,
Heterocycles 13 (1979) 477; D'Angelo, J., Tetrahedron Letters 32 (1991) 3063).
Scheme 4
R'
V.Q ~ HN'X Q N~X
%~ + R + ~ /,rte a V ~ , y
CHO HzN R9 R N ~s~'~ Ra
E D O E D H R
(9) (2) (3) (1 0)
Fused pyrimidines of general formula (10), wherein R1, X, Q, V, R8, R9, D, and
E are
as defined in formula (I), can be prepared according to the method of Scheme
4. A carbonyl
component of general formula (9) can be treated with an aldehyde of general
formula (2) and
an amino heterocycle of general formula (3) in a solvent such as ethanol,
acetonitrile or
dimethylformamide with heating to provide fused pyrimidines of general formula
( 10).
28
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Scheme 5
O O R~
RO R~ HN' X, RO N' X
+ ~/,,~Rs _
p O + CHO H2N Rs p N~9J~Rs
R
R (2) (3) R H
(11 ) (12)
R'
O
N~X
(12) O~N~s~r~Rs
D H R
(13)
Fused pyrimidines of general formula (13), wherein Rl, X, Rg, R9, and D are as
defined in formula (I), can be prepared according to the method of Scheme 5. A
dicarbonyl
component of general formula (11), wherein R' is selected from Cl and OAc and
R is selected
from lower alkyl, cyanoalkyl, and carboxy protecting group, can be treated
with an aldehyde
of general formula (2) and an amino heterocycle of general formula (3) in a
solvent such as
ethanol, acetonitrile or dimethylformamide with heating to provide fused
pyrimidines of
general formula (12). In the case where R' -is OAc, cleavage of the acetyl
group may be
required to induce cyclization to provide fused pyrimidines of general formula
(13). In the
case where R' is Cl, cyclization can proceed directly without the isolation of
(12) to provide
fused pyrimidines of general formula (13).
Many of the starting aryl and heteroaryl aldehydes necessary to carry out the
methods
described in the preceeding and following Schemes may be purchased from
commercial
sources or may be synthesized by known procedures found in the chemical
literature.
Appropriate literature references for the preparation of aryl and heteroaryl
aldehydes may be
found in the following section or in the Examples. For starting materials not
previously
described in the literature the following Schemes are intended to illustrate
their preparation
through a general method.
The preparation of aldehydes used to synthesize many preferred compounds of
the
invention may be found in the following literature references: Pearson, Org.
Synth. Coll. Vol
29
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
V (1973), 117; Nwaukwa, Tetrahedron Lett. (1982), 23, 3131; Badder, J. Indian
Chem. Soc.
(1976), 53, 1053; Khanna, J. Med. Chem. (1997), 40, 1634; Rinkes, Recl. Trav.
Chim. Pays-
Bas (1945), 64, 205; van der Lee, Recl. Trav. Chim. Pays-Bas (1926), 45, 687;
Widman,
Chem. Ber. (1882), 15, 167; Hodgson, J. Chem. Soc. (1927), 2425; Clark, J.
Fluorine Chem.
(1990), 50, 411; Hodgson, J. Chem. Soc. (1929), 1635; Duff, J. Chem. Soc.
(1951), 1512;
Crawford, J. Chem. Soc. (1956), 2155; Tanouchi, J. Med. Chem. (1981), 24,
1149;
Bergmann, J. Am. Chem. Soc. (1959), 81, 5641; Other: Eistert, Chem. Ber.
(1964), 97,
1470; Sekikawa, Bull. Chem. Soc. Jpn. (1959), 32, 551.
Scheme 6
Rio Rio
R~2
H O H O
(20) (21 )
Rio Rio
\ R~2
/ /
RO OR RO OR
(22) (23)
Meta, para-disubstituted aldehydes of general formula (21), wherein R'°
is selected
from alkyl, haloalkyl, halo, haloalkoxy, alkoxy, alkylthio, -NRg2Rg3, and -
C(O)NRg2Rg3
wherein R82 and Rg3 are independently selected from hydrogen, alkyl,
alkylcarbonyl, aryl,
arylalkyl, and formyl and R'2 is selected from nitro, halo, and alkylcarbonyl,
can be prepared
according to the method described in Scheme 6. A para substituted aldehyde of
general
formula (20) or the corresponding acetal protected aldehyde of general formula
(22), wherein
R is selected from alkyl or together with the oxygen atoms to which they are
attached form a
5 or 6 membered ring wherein 1,3-dioxolanes are preferred, may by subjected to
conditions of
an electrophilic aromatic substitution reaction to provide aldehydes of
general formula (21 ) or
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
protected aldehydes of general formula (23). Preferred protecting groups for
compounds of
general formula (22) and (23) include dimethyl or diethyl acetals or the 1,3-
dioxolanes:
These protecting groups can be introduced at the beginning and removed at the
end to provide
substituted aldehydes of general formula (21 ) using methods well known to
those skilled in
the art of organic chemistry.
Scheme 7
Rio Rio Rio
--. I ~ ~ I ~ R~z
HO I ~ HO / ~ HO
(25) CHO CHO
(26) (27)
Aldehydes of general formula (27), wherein R~° is selected from alkyl,
haloalkyl, halo,
haloalkoxy, alkoxy, alkylthio, -NR82R83, and -C(O)NRgZRg3 wherein R82 and Rg3
are
independently selected from hydrogen, alkyl, alkylcarbonyl, aryl, arylalkyl,
and formyl and
R~Z is selected from nitro, halo, and alkylcarbonyl, can be prepared by the
method described
in Scheme 7. A meta substituted phenol (25) is converted to the para
substituted
salicylaldehyde (26) by reaction with a base such as sodium hydroxide and a
reagent such as
trichloromethane or tribromomethane, known as the Reimer-Tiemann reaction. An
alternate
set of reaction conditions involves reaction with magnesium methoxide and
paraformaldehyde
(Aldred, J. Chem. Soc. Perkin Trans. 1 (1994), 1823). The aldehyde (26) may be
subjected to
conditions of an electrophilic aromatic substitution reaction to provide meta,
para
disubstituted salicylaldehydes of general formula (27).
31
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Scheme 8
Rio Rio
\ R~z I \ R~2
HO ~ HO
CHO
(28) (27)
An alternative method of preparing meta, para disubstituted salicylaldehydes
of
general formula (27), wherein R'° is selected from alkyl, haloalkyl,
halo, haloalkoxy, alkoxy,
alkylthio, -NRg2Rg3, and -C(O)NRg2Rg3, wherein Rg2 and Rg3 are independently
selected from
hydrogen, alkyl, alkylcarbonyl, aryl, arylalkyl, and formyl and R~Z is
selected from nitro, halo,
and alkylcarbonyl, can be used as described in Scheme 8. A meta, para
disubstituted phenol
of general formula (28) can be reacted with a base such as sodium hydroxide
and a reagent
such as trichloromethane or tribromomethane, known as the Reimer-Tiemann
reaction, to
provide disubstituted salicylaldehydes of general formula (27). An alternate
set of reaction
conditions involves reaction with magnesium methoxide and paraformaldehyde
(Aldred, J.
Chem. Soc. Perkin Trans. 1 (1994), 1823).
Scheme 9
Br Rio Rio
R~z ~z R~z
R
~ i
RO OR RO OR H O
1 S (29) (23) (21 )
An alternative method of preparing benzaldehydes of general formula (21),
wherein
R'2 is selected from alkyl, haloalkyl, chlorine, fluorine, haloalkoxy, alkoxy,
alkylthio, nitro,
alkylcarbonyl, arylcarbonyl, -NR8zRg3, and -C(O)NRg2R83 wherein R82 and R83
are
independently selected from hydrogen, alkyl, alkylcarbonyl, aryl, arylalkyl,
and formyl, and
R'° is selected from alkyl, hydroxyalkyl, alkylthio, alkylcarbonyl, and
formyl, is described in
Scheme 9. Protected benzaldehydes of general formula (29), wherein R is
selected from alkyl
or together with the oxygen atoms to which they are attached form a 5 or 6
membered ring
32
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
wherein 1,3-dioxolanes are preferred, can be converted to the 3,4-
disubstituted benzaldehyde
of general formula (23) via conversion of the bromide to an intermediate
lithio or magnesio
derivative, followed by reaction with an appropriate electrophile such as an
aldehyde,
dialkyldisulfide, a Weinreb amide, dimethylformamide, an alkyl halide or other
electrophile
followed by deprotection of the acetal to provide benzaldehydes of general
formula (21 ).
Scheme 10
R~° Rio Rio
~ Br \ R~2 I \ R~2
~, ~ i
RO OR RO OR H O
(31 ) (23) (21 )
An alternative method of preparing benzaldehydes of general formula (21 ),
wherein
Rl° is selected from alkyl, haloalkyl, chlorine, fluorine, haloalkoxy,
alkoxy, alkylthio, -
NRg2Rg3, and =C(O)NRg2R83 wherein Rg2 and R83 are independently selected from
hydrogen,
alkyl, alkylcarbonyl, aryl, arylalkyl, and formyl, R12 is selected from alkyl,
hydroxyalkyl,
alkylthio, alkylcarbonyl, arylcarbonyl, and formyl, can be used as described
in Scheme 10.
Protected benzaldehydes of general formula (31), wherein R is selected from
alkyl or together
with the oxygen atoms to which they are attached form a 5 or 6 membered ring
wherein 1,3-
dioxolanes are preferred can be processed as described in Scheme 9 to provide
benzaldehydes
of general formula (21 ).
33
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Scheme 11
R'° Rio
~ O~R~s
O H O H
(32) (33)
Benzaldehydes of general formula (33), wherein R1° is selected from
hydrogen, alkyl,
alkylsulfonyl, aryl, heteroaryl, cyano, haloalkyl, halo, haloalkoxy, nitro,
alkoxy, alkylthio, -
S NR82Rg3, and -C(O)NRg2Rg3 wherein Rg2 and Rg3 are independently selected
from hydrogen,
alkyl, alkylcarbonyl, aryl, arylalkyl, and formyl, and R'3 is selected from
hydrogen, alkyl,
arylalkyl, and haloalkyl wherein preferred haloalkyl groups are selected from
difluoromethyl,
2,2,2-trifluoroethyl and bromodifluoromethyl, can be prepared as described in
Scheme 11. 3-
Hydroxybenzaldehyde of general formula (32) can be treated with suitable
alkylating reagents
such as benzylbromide, iodomethane, 2-iodo-1,1,1-trifluoroethane,
chlorodifluoromethane, or
dibromodifluoromethane in the presence of base such as potassium carbonate,
potassium tert-
butoxide or sodium tent-butoxide, to provide benzaldehydes of general formula
(33). The
synthesis of useful 3-hydroxybenzaldehydes of general formula (32) may be
found in the
following literature references: J. Chem. Soc. (1923), 2820; J. Med Chem.
(1986), 29, 1982;
Monatsh. Chem. (1963), 94, 1262; Justus Liebigs Ann. Chem. (1897), 294, 381;
J. Chem.
Soc. Perkin Traps. 1 (1990), 315; Tetrahedron Lett. (1990), 5495; J. Chem.
Soc. Perkin
Traps. 1 ( 1981 ), 2677.
34
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Scheme 12
OH
O
R~2 R~z
~,
O~H O H
(34) (35)
Benzaldehydes of general formula (35), wherein R12 is selected from hydrogen,
alkyl,
alkylsulfonyl, aryl, heteroaryl, cyano, haloalkyl, halo, haloalkoxy, nitro,
alkoxy, alkylthio, -
NRgzRx3, and -C(O)NR8zR83 wherein R82 and Rg3 are independently selected from
hydrogen,
alkyl, alkylcarbonyl, aryl, arylalkyl, and formyl, and R13 is selected from
hydrogen, alkyl,
arylalkyl, and haloalkyl wherein preferred haloalkyl groups are selected from
difluoromethyl,
2,2,2-trifluoroethyl, and bromodifluoromethyl, can be prepared as described in
Scheme 12. 4-
Hydroxybenzaldehydes of general formula (34) can be treated with suitable
alkylating
reagents such as benzylbromide, iodomethane, 2-iodo-1,1,1-trifluoroethane,
chlorodifluoromethane, or dibromodifluoromethane, in the presence of base such
as
potassium carbonate, potassium tert-butoxide or sodium tert-butoxide to
provide
benzaldehydes of general formula (35). The synthesis of useful 4-
hydroxybenzaldehydes of
general formula (34) may be found in the following literature references:
Angyal, J. Chem.
Soc. (1950), 2141; Ginsburg, J. Am. Chem. Soc. (1951), 73, 702; Claisen,
Justus Liebigs
Ann. Chem. (1913), 401, 107; Nagao, Tetrahedron Lett. (1980), 21, 4931;
Ferguson, J. Am.
Chem. Soc. (1950), 72, 4324; Barnes, J. Chem. Soc. (1950), 2824; Villagomez-
Ibarra,
Tetrahedron (1995), 51, 9285; Komiyama, J. Am. Chem. Soc. (1983), 105, 2018;
DE 87255;
Hodgson, J. Chem. Soc. (1929), 469; Hodgson, J. Chem. Soc. (1929), 1641.
35
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Scheme 13
R'° Rio
NH2 R'2
O H O H
(36) (21 )
An alternate method for introduction of substituents at the 3-position of
benzaldehydes
of general formula (21), wherein R'° is selected from hydrogen, alkyl,
alkylsulfonyl, aryl,
heteroaryl, cyano, haloalkyl, halo, haloalkoxy, vitro, alkoxy, alkylthio, and -
C(O)NRgZR83,
wherein Rg2 and R83 are independently selected from hydrogen, alkyl,
alkylcarbonyl, aryl,
arylalkyl, and formyl can be used as described in Scheme 13. This method, also
known as the
Sandmeyer reaction, involves converting 3-amino benzaldehydes of general
formula (36) to
an intermediate diazonium salt with sodium nitrite. The diazonium salts can be
treated with a
bromine or iodine source to provide the bromide or iodide. The Sandmeyer
reaction and
conditions for effecting the transformation are well known to those skilled in
the art of
organic chemistry. The types of R'2 substituents that may be introduced in
this fashion
include cyano, hydroxy, or halo. In order to successfully carry out this
transformation it may
in certain circumstances be advantageous to perform the Sandmeyer reaction on
a protected
. aldehyde. The resulting iodide or bromide can be treated with unsaturated
halides, boronic
acids or tin reagents in the presence of a palladium catalyst such as
tetrakis(triphenylphosphine)palladium (0) to provide benzaldehydes of general
formula (21 ).
The diazonium salts may also be treated directly with unsaturated halides,
boronic acids or tin
reagents in the presence of a palladium catalyst such as
tetrakis(triphenylphosphine)palladium
(0) to provide benzaldehydes of general formula (21 ).
36
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Scheme 14
NH2 Rio
R12
~/
O~H O H
(37) (21 )
An alternate method for introduction of substituents at the 4-position of
benzaldehydes
of general formula (21), wherein R'2 is selected from hydrogen, alkyl,
alkylsulfonyl, aryl,
heteroaryl, cyano, haloalkyl, halo, haloalkoxy, vitro, alkoxy, alkylthio, and -
C(O)NR82R83,
wherein R82 and R83 are independently selected from hydrogen, alkyl,
alkylcarbonyl, aryl,
arylalkyl, and formyl, can be used as described in Scheme 14. This method,
also known as
the Sandmeyer reaction, involves converting 4-amino benzaldehydes of general
formula (37)
to an intermediate diazonium salt with sodium nitrite and then treating the
diazonium salts in
a similar manner as that described in Scheme 13. The types of R'°
substituents that may be
introduced in this fashion include cyano, hydroxy, or halo. The Sandmeyer
reaction and
conditions for effecting the transformation are well known to those skilled in
the art of
organic chemistry. In order to successfully carryout this transformation it
may in certain
circumstances be advantageous to perform the Sandmeyer reaction on a protected
aldehyde.
Scheme 15
NHZ (CI) Br
OCF3 ~ OCF3
1 ) Ac20
2) BuLi, DMF
Br 3) H2S04
4) Sandmeyer O H
4-Bromo-3-(trifluoromethoxy)benzaldehyde or 4-chloro-3-
(trifluoromethoxy)benzaldehyde can be prepared as described in Scheme 15. The
commercially available 4-bromo-2-(trifluoromethoxy)aniline can be protected on
the amino
group with a suitable N-protecting group well known to those skilled in the
art of organic
chemistry such as acetyl or tert-butoxycarbonyl. The bromine can then be
converted to the
37
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
lithio or magnesio derivative and reacted directly with dimethylformamide to
provide the 4-
aminoprotected-3-(trifluoromethoxy)benzaldehyde derivative. Removal of the N-
protecting
group followed by conversion of the amine to a bromide or chloride via the
Sandmeyer
method of Scheme 14 provides 4-bromo-3-(trifluoromethoxy)benzaldehyde or 4-
chloro-3-
(trifluoromethoxy)benzaldehyde.
Scheme 16
CF3 CF3 CF3
y ~ NOZ ~ N02
-~ ~ / --~ I /
O OH O OH
OH
CF3 CF3 CF3
NH2 ~ I ~ Y I j Y
CHO
OH OH
(38) (39)
4-Trifluoromethylbenzaldehydes of general formula (39), wherein Y is selected
from
cyano, nitro, and halo may be prepared according to the method of Scheme 16. 4-
Trifluoromethylbenzoic acid is first nitrated, using suitable conditions well
known in the
literature such as nitric acid with sulfuric acid, and the carboxylic acid
group reduced with
borane to provide 3-nitro-4-trifluoromethylbenzyl alcohol. From this benzyl
alcohol may be
obtained the 3-nitro-4-trifluoromethylbenzaldehyde by oxidation with typical
reagents such as
manganese dioxide. The nitro benzylic alcohol can be reduced to the aniline
using any of a
number of different conditions for effecting this transformation among which a
preferred
method is hydrogenation over a palladium catalyst. The aniline can be
converted to either a
halo or cyano substituent using the Sandmeyer reaction described in Scheme 13.
Benzyl
alcohols of general formula (38) can be oxidized using conditions well known
to those skilled
38
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
in the art such as manganese dioxide or swern conditions to provide
benzaldehydes of general
formula (39).
For certain aromatic ring substitutions of R1 for compounds of the present
invention it
is preferable to effect transformations of the aromatic ring substitutions
after the aldehyde has
been incorporated into the core structure of the present invention. As such,
compounds of the
present invention may be further transformed to other distinct compounds of
the present
invention. These transformations involve Stille, Suzuki and Heck coupling
reactions all of
which are well known to those skilled in the art of organic chemistry. Shown
below are some
representative methods of such transformations of compounds of the present
invention to
other compounds of the present invention.
Scheme 17
R~° Rio
R~2
R» ~ R»
1. Di-t-butyldicarbonate
E R8 DMAP, MeCN E R8
N,~ X Q / N _~ X
n
~ / 2. Pd(PPh3)a, R~2SnR3 (~ _
N~ R9 DMF, 110 ° C V~ N~\R9
D m H D m H
(41 ) (42)
Dihydropyridines of general formula (42), wherein R8, R9, D, E, Q, V, X, m,
and n are
as defined in formula (I), RI° is selected from hydrogen, alkyl,
alkylcarbonyl, alkylsulfonyl,
aryl, heteroaryl, cyano, haloalkyl, chlorine, fluorine, haloalkoxy, nitro,
alkoxy, and alkylthio,
and -C(O)NR82Rg3 wherein R8z and R83 are independently selected from hydrogen,
alkyl,
alkylcarbonyl, aryl, arylalkyl, and formyl, R11 is selected from hydrogen,
hydroxy, alkoxy,
haloalkoxy, and arylalkoxy, R12 is selected from alkyl, vinyl, aryl,
heteroaryl, cyano and the
like, can be prepared as described in Scheme 17. Compounds of general formula
(41),
wherein Y is selected from bromine, iodine, and triflate, are protected with a
tert-
butoxycarbonyl (Boc) group using standard procedures. The aromatic bromide,
iodide, or
39
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
triflate can be treated with a suitable tin, boronic acid, or unsaturated
halide reagent in the
presence of a palladium catalyst with heating in a solvent such as
dimethylformamide to
effect a coupling reaction that provides dihydropyridines of general formula
(42). The
conditions for this transformation also effect the removal of the Boc
protecting group.
Scheme 18
Rt2
Rio Rio
R~i ~ R~~
1. Di-t-butyldicarbonate
E R8 DMAP, MeCN
Q / N'\ X Q /E R X
c ~ cf ~ ~N,~ ~'
~ / 2. Pd(PPh3)a, R~2SnR3 l1
N~ R DMF, 110 ° C V/ N~ R9
D m H D _~m H
(43)
(44)
Dihydropyridines of general formula (44), wherein R8, R9, D, E, Q, V, X, m,
and n are
as defined in formula (I), R~2 is selected from hydrogen, alkyl,
alkylcarbonyl, alkylsulfonyl,
aryl, heteroaryl, cyano, haloalkyl, chlorine, fluorine, haloalkoxy, nitro,
alkoxy, alkylthio, and -
C(O)NRg2R83 wherein Rg2 and Rg3 are independently selected from hydrogen,
alkyl,
alkylcarbonyl, aryl, arylalkyl, and formyl, R1' is selected from hydrogen,
hydroxy, alkoxy,
haloalkoxy, and arylalkoxy, R~° is selected from alkyl, vinyl, aryl,
heteroaryl, cyano and the
like, can be prepared as described in Scheme 18. Dihydropyridines of general
formula (43),
wherein Y is selected from bromine, iodine, and triflate, can be protected
with a tert-
butoxycarbonyl (Boc) group using standard procedures. The aromatic bromide,
iodide, or
triflate can be reacted with a suitable tin, boronic acid, or unsaturated
halide reagent in the
presence of a palladium catalyst with heating in a solvent such as
dimethylformamide to
effect a coupling reaction that provides dihydropyridines of general formula
(44). The
conditions for this transformation also effect the removal of the Boc
protecting group.
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Scheme 19
~o ~~o
Y
R~1~ 1. Di-t-butyldicarbonate R"
DMAP, MeCN
CF3
N ~ X 2. Pd(PPh3)a ~ Q /E N
~~ \~ R'Zn CF3 ~~ \
V~ N~~Rs ~/~ ~~Rs
D m H D m
(41 ) (47)
Dihydropyridines of general formula (47), wherein Rg, R9, D, E, Q, V, X, m,
and n are
as defined in formula (I), R'° is selected from hydrogen, alkyl,
alkylcarbonyl, alkylsulfonyl,
aryl, heteroaryl, cyano, haloalkyl, chlorine, fluorine, haloalkoxy, nitro,
alkoxy, alkylthio, and -
C(O)NRg2R83 wherein R82 and R83 are independently selected from hydrogen,
alkyl,
alkylcarbonyl, aryl, arylalkyl, and formyl, and R1' is selected from hydrogen,
hydroxy,
alkoxy, haloalkoxy, and arylalkoxy, can be prepared as described in Scheme 19.
Dihydropyridines of general formula (41), wherein Y is selected from bromine,
iodine, and
triflate can be protected with a tert-butoxycarbonyl (Boc) group using
standard procedures.
The aromatic bromide, iodide, or triflate can be treated with a suitable
halozinc reagent in the
presence of a palladium catalyst with heating in a solvent such as
dimethylformamide to
effect a coupling reaction that provides dihydropyridines of general formula
(47). The
conditions for this transformation also effect the removal of the Boc
protecting group. The
types of meta substituents that may be introduced in this fashion include
trihalopropenyl and
more specifically the trifluoropropenyl group.
41
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Scheme 20
CF3
~R~o
Rio
i , R»i
R~~
1. Di-t-butyldicarbonate
DMAP, MeCN
a s
Q. 2. Pd(PPh3)4 Q ~E N ' X
w
n /E N \ \ , ~ \
~' ~ R Zn CF3 V ~ ~ Rs
U~ ~~R9 N
~N D m H
D m
(43) (48)
Dihydropyridines of general formula (48), Rg, R9, D, E, Q, V, X, m, and n are
as
defined in formula (I), Rl° is selected from hydrogen, alkyl,
alkylcarbonyl, alkylsulfonyl, aryl,
heteroaryl, cyano, haloalkyl, chlorine, fluorine, haloalkoxy, nitro, alkoxy,
alkylthio, -
C(O)NR82R83 wherein Rg2 and R83 are independently selected from hydrogen,
alkyl,
alkylcarbonyl, aryl, arylalkyl, and formyl, R" is selected from hydrogen,
hydroxy, alkoxy,
haloalkoxy, and arylalkoxy, can be prepared as described in Scheme 20.
Dihydropyridines of
general formula (43), wherein Y is selected from bromine, iodine, and triflate
can be protected
with a tert-butoxycarbonyl (Boc) group using standard procedures. The aromatic
bromide,
iodide, or triflate can be treated with a suitable halozinc reagent in the
presence of a palladium
catalyst with heating in a solvent such as dimethylformamide to effect a
coupling reaction that
provides dihydropyridines of general formula (48). The conditions for this
transformation
also effect the removal of the Boc protecting group. The types of para
substituents that may
be introduced in this fashion include trihalopropenyl and more specifically
the
trifluoropropenyl group.
42
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Scheme 21
R' R'
( ~O~ I N'X R$ NBS ( ~Q~ I N'X Rs
V~ m N ~ V~ m N
H ~ H Br
(50) (51 )
Pd(0), (HO)2BR9 R'
or Q, E
Pd(0), (R)sSnR9 (~n ~ ~ N~X R$
or V~ m N
Pd(0), XR9 ~ H R9
(5~) . (52)
R=alkyl or phenyl
X=I or Br
Fused pyrimidines of general formula (52), wherein Rl, X, Q, V, R8, D, E, m
and n are
as defined in formula (I) and R9 is selected from alkenyl, alkynyl, aryl and
heterocycle, can be
prepared according to the method of Scheme 21. Fused pyrimidines of general
formula (50)
may be treated with N-bromosuccinimide (NBS) in a solvent such as methylene
chloride to
provide bromides of general formula (S 1 ). Bromides of general formula (51 )
may be treated
with a palladium (0) catalyst such as tetrakis(triphenylphosphine)palladium
(0), an
organoborane reagent and a base such as cesium fluoride or potassium carbonate
under
Suzuki conditions which are known to those of skill in the art (Syn. Comm. 11,
1981, 513;
JOC 49, 1984, 5237; Tet. Lett. 26, 1985, 5997; Tet. Lett. 28, 1987, 5093; and
Tet. Lett. 28,
1987, 5097) to provide fused pyrimidines of general formula (52). Bromides of
general
formula (51) may also be treated with a palladium (0) catalyst such as
tetrakis(triphenylphosphine)palladium (0) and a tin reagent under Stille
conditions which are
known to those of skill in the art (JACS 101, 1979, 4992) to provide fused
pyrimidines of
general formula (52). Bromides of general formula (51 ) may also be treated
with a palladium
(0) catalyst, an aryl halide (Br or I) or a heterocyclic halide (Br or I) and
a base such as
triethylamine under biaryl coupling conditions or Heck conditions which are
known to those
of skill in the art to provide fused pyrimidines of general formula (52).
43
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The compounds and processes of the present invention will be better understood
by
reference to the following examples, which are intended as an illustration of
and not a
limitation upon the scope of the invention. Further, all citations herein are
incorporated by
reference.
Example 1
9-~4-bromo-3-fluorophenyl)-5,6,7,9-tetrahydropyrazolo [5,1-b]quinazolin-8(4H)-
one
A solution of 1,3-cyclohexanedione (0.56 g, 5 mmol), 3-bromo-4-
fluorobenzaldehyde
(1.01 g, 5 mmol), and 3-aminopyrazole (0.41 g, 5 mmol) in ethanol (5 mL) was
heated at
reflux for 24 hours. After the reaction mixture was allowed to cool to ambient
temperature,
the volatiles were evaporated at reduced pressure and the resulting residue
was
chromatographed on silica gel, eluting with 5% ethanol/methylene chloride to
provide 0.9 g
(49 %) of the title compound.
1H NMR (DMSO-d6) 8 1.94 (m, 2H), 2.25 (m, 2H), 2.63 (m, 2H), 5.72 (d, 1H),
6.19 (s, 1H),
7.1 (m, 1 H), 7.23 (t, 1 H), 7.31 (d, 1 H), 7.4 (dd, 1 H), 10.55 (s, 1 H); MS
(ESI-) m/z: 362 (M-
H)-; Analysis Calculated for C16Hi3FBrN30: C, 53.06; H, 3.62; N, 11.60. Found:
C, 52.92; H,
4.02; N, 11.48.
Example 2
9-1,3-bromo-4-fluoro~hen~)-5,9-dihydro-4H-~ rah zolo[1,5-a]thiopyrano[3,4-
d]pyrimidin-
8 7H -one
A solution of 3,5-thiopyrandione (0.13 g, 1 mmol) prepared as described in
(Fehnel,
E.A., J. Amer. Chem. Soc., (1955), 77, 4241-4244), 3-bromo-4-
fluorobenzaldehyde (0.203 g,
1 mmol), and 3-aminopyrazole (0.082 g, 1 mmol) in ethanol (2 mL) were heated
at reflux for
24 hours. After the reaction mixture was allowed to cool to ambient
temperature, the volatiles
were evaporated at reduced pressure and the resulting residue was
chromatographed on silica
gel, eluting with 5% ethanol/methylene chloride to provide 0.045 g (12 %) of
the title
compound.
mp 160-163 °C;
44
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
1 H NMR (DMSO-db) 8 3.15 (d, 1 H), 3 .5 (d, 1 H), 3 .6 (d, 1 H),3.9 (d, 1 H),
5. 8 (d, 1 H), 6.26 (s,
1 H), 7.13 (m, 1 H), 7.29 (t, 1 H), 7.38 (d, 1 H), 7.42 (dd, 1 H), 10.86 (s, 1
H); MS (ESI-) m/z:
380 (M-H)-; Analysis Calculated for CASH"BrFN30S~0.25C2H60: C, 47.52; H, 3.22;
N,
10.73. Found: C, 47.57; H, 2.89; N, 10.29.
Example 3
9-( 1-naphthyl)-5,6,7,9-tetrahydropyrazolo [5,1-b]q_uinazolin-8(4H)-one
A solution of 1,3-cyclohexanedione (0.11 g, 1 mmol), 1-naphthaldehyde (0.16 g,
1
mmol), and 3-aminopyrazole (0.11 g, 1.27 mmol) in ethanol (10 mL) were heated
at 80 °C in
a sealed 20 mL vial for 3 days. After the reaction mixture was allowed to cool
to ambient
temperature, the solvent was evaporated at reduced pressure and the resulting
residue was
chromatographed on silica gel, eluting with 5% ethanol/methylene chloride to
provide 0.14 g
(44%) of the title compound.
1H NMR (DMSO-d6) ~ 1.95 (m, 2H), 2.21 (m, 2H), 2.72 (m,2H), 5.62 (s, 1 H),
7.00 (d, 1 H),
7.15-7.95 (m, 7H), 8.61 (d, 1H), 10.45 (s, 1H); MS (APCI+) m/z: 316 (M+H)+;
Analysis
Calculated for C2oHI~N30: C, 76.17; H, 5.43; N, 13.32. Found: C, 75.99; H,
5.48; N, 13.27.
Example 4
9 ~2-naphthyl)-5,6,7,9-tetrahydropyrazolo [5,1-b],quinazolin-8(4H)-one
2-Naphthaldehyde (0.16 g, 1 mmol) was treated according to the procedure
described
in Example 3 to provide 0.16 g (51%) of the title compound.
1H NMR(DMSO-d6) 8 1.91 (m, 2H), 2.25(m, 2H), 2.68(m,2H), 5.74(d, 1H), 6.37(s,
1H), 7.20-
7.90(m, 8H), 10.50(s, 1H); MS(APCI+) m/z: 316(M+H)+; Analysis Calculated for
CZOH,~N30: C, 76.17; H, 5.43; N, 13.32. Found: C,75.97; H, 5.50; N, 13.35.
Example 5
9-(3,4-dibromophenyl)-5,6,7,9-tetrahydropyrazolor5,1-b],quinazolin-8(4H)-one
A solution of 1,3-cyclohexanedione (0.11 g, 1 mmol), 3,4-dibromobenzaldehyde
(0.26
g, 1 mmol) and 3-aminopyrazole (0.11 g, 1.27 mmol) in ethanol ( 10 mL) was
heated at 80 °C
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
in a sealed 20 mL vial for 3 days. After the reaction mixture was allowed to
cool to ambient
temperature, the resulting solid was isolated by filtration and
recrystallization from acetone to
provide 0.23 g (56%) of the title compound.
'H NMR(DMSO-d6) 1.91 (m, 2H), 2.25 (m, 2H), 2.64 (m,2H), 5.78(d, 1H), 6.19(s,
1H), 6.95-
7.65 (m, 4H), 10.59 (s, 1H); MS(APCI+) m/z: 423(M+H)+; Analysis Calculated for
C16H13Br2N3~: C, 45.42; H, 3.10; N, 9.93; Br, 37.77. Found: C, 45.17; H, 3.22;
N, 9.88; Br,
37.59.
Example 6
9-(3,4-dichlorophenyl)-5,6,7,9-tetrahydropyrazolo[51-b]quinazolin-8(4H)-one
3,4-Dichlorobenzaldehyde (0.18 g, 1 mmol) was treated according to the
procedure
described in Example 3 to provide 0.18 g (55%) of the title compound.
~H NMR(DMSO-db) 8 1.95 (m, 2H), 2.25 (m, 2H), 2.64 (m, 2H), 5.78 (d, 1 H),
6.20 (s, 1 H),
7.00-7.58 (m, 4H), 10.50 (s, 1H); MS(APCI+) m/z: 334(M+H)+; Analysis
Calculated for
C~6H,3C1zN30: C, 57.50; H, 3.92; N, 12.57; Cl, 21.22. Found: C, 57.29; H,
4.06; N, 12.53;
Cl, 21.45.
Example 7
9-(3-bromophenyl)-5 6 7 9-tetrahydropyrazolof5 1-b]quinazolin-8~4H)-one
3-Bromobenzaldehyde (0.19 g, 1 mmol) was treated according to the procedure
described in Example 3 to provide 0.21 g (60%) of the title compound.
1H NMR(DMSO-d6) 8 1.93 (m, 2H), 2.25 (m, 2H), 2.65 (m, 2H), 5.78 (d, 1 H),
6.20 (s, 1 H),
7.05-7.40 (m, 5H), 10.55 (s, 1H); MS(APCI+) m/z: 344(M+H)+; Analysis
Calculated for
C16H,4BrN30: C, 55.83; H, 4.10; N, 12.21; Br, 23.21. Found: C, 55.95; H, 4.30;
N, 12.14; Br,
23.30.
Example 8
9-(3-chlorophenyl)-5,6,7,9-tetrahydropyrazolo(5 1-b]'quinazolin-8(4H)-one
46
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
3-Chlorobenzaldehyde (0.14 g, 1 mmol) was treated according to the procedure
described in Example 3 to provide 0.17 g (49%) of the title compound.
1H NMR(DMSO-d6) 8 1.95 (m, 2H), 2.25 (m, 2H), 2.65 (m, 2H), 5.75 (d, 1H), 6.20
(s, 1H),
7.02-7.38 (m, 5H), 10.55 (s, 1H); MS(APCI+) m/z: 300(M+H)+; Analysis
Calculated for
C,6Hi4C1N30: C, 64.11; H, 4.71; N, 14.02; Cl, 11.83. Found: C, 63.81; H, 4.82;
N, 14.30; Cl,
11.96.
Example 9
9-[4-chloro-3-(trifluoromethyl)phenyl]-5,6,7,9-tetrahydropyrazolo [5,1-
blguinazolin-8(4H)-
one
4-Chloro-3-trifluoromethylbenzaldehyde (0.21 g, 1 mmol) was treated according
to
the procedure described in Example 3 to provide 0.17 g (45%) of the title
compound.
1H NMR(DMSO-d6) 8 1.95 (m, 2H), 2.25 (m, 2H), 2.65 (m, 2H), 5.78 (d, 1H), 6.30
(s, 1H),
7.30-7.61 (m, 4H), 10.59 (s, 1H); MS(APCI+) m/z: 368 (M+H)+; Analysis
Calculated for
Cl~H,3C1F3N30: C, 55.52; H, 3.56; N, 11.43; Cl, 9.64; F, 15.50. Found: C,
55.50; H, 3.67; N,
11.59; Cl. 9.68; F, 15.15.
Example 10
9-[4-fluoro-3-(trifluoromethyl)phenyl]-5,6,7,9-tetrahydropyrazolo f 5,1-
b]quinazolin-8(4H)-
one
4-Fluoro-3-trifluoromethylbenzaldehyde (0.19 g, 1 mmol) was treated according
to the
procedure described in Example 3 to provide 0.16 g (46%) of the title
compound.
'H NMR(DMSO-d6) 8 1.95 (m, 2H), 2.25 (m, 2H), 2.65 (m, 2H), 5.79 (d, 1H), 6.29
(s, 1H),
7.35-7.48 (m, 4H), 10.60 (s, 1H); MS(APCI+) m/z: 352(M+H)+; Analysis
Calculated for
C1~H13F4N30: C, 58.12; H, 3.73; N, 11.96; F, 21.63. Found: C, 54.49; H, 3.90;
N, 11.07; F,
22.79.
Example 11
9-[3-(trifluoromethoxy)phenyl]-5,6,7,9-tetrahydropyrazolo[5,1-blguinazolin-
8(4H)-one
47
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
3-Trifluoromethoxybenzaldehyde (0.19 g, 1 mmol) was treated according to the
procedure described in Example 3 to provide 0.17 g (50%) of the title
compound.
1H NMR(DMSO-d6) b 1.95 (m, 2H), 2.25 (m, 2H), 2.65 (m, 2H), 5.78 (d, 1 H),
6.25 (s, 1 H),
7.05-7.40 (m, 5H), 10.05 (s, 1H); MS(APCI+) m/z: 350(M+H)+; Analysis
Calculated for
C,~H~4F3N302: C, 58.45; H, 4.04; N, 12.03; F, 16.32. Found: C, 58.43; H, 3.93;
N, 11.90; F,
15.92.
Example 12
9-(3-cyanophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-bl4uinazolin-8(4H)-one
3-Cyanobenzaldehyde (0.13 g, 1 mmol) was treated according to the procedure
described in Example 3 to provide 0.16 g (55%) of the title compound.
~H NMR(DMSO-db) 8 1.95 (m, 2H), 2.25 (m, 2H), 2.65 (m, 2H), 5.78 (d, 1 H),
6.25 (s, 1 H),
7.30-7.66 (m, 5H), 10.60 (s, 1H); MS(APCI+) m/z: 291 (M+H)+; Analysis
Calculated for
C»H14N40: C, 70.33; H, 4.86; N, 19.30. Found: C, 70.31; H, 4.95; N, 19.36.
Example 13
9-(3-methylphenyl)-5,6,7,9-tetrahydropyrazolo[S,1-b]guinazolin-8(4H)-one
3-Methylbenzaldehyde (0.12 g, 1 mmol) was treated according to the procedure
described in Example 3 to provide 0.17 g (60%) of the title compound.
1H NMR (DMSO-d6) 1.90 (m, 2H), 2.21 (s, 3H), 2.24 (m, 2H), 2.61 (m, 2H), 5.70
(d, 1H),
6.19 (s, 1 H), 6.85-7.30 (m, 5H), 10.40 (s, 1 H); MS (APCI+) m/z: 280 (M+H)+;
Analysis
Calculated for C17H,~N30: C, 73.10; H, 6.13; N, 15.04. Found: C, 72.92; H,
6.17; N, 15.35.
Example 14
8-(3-bromo-4-fluorophenyl)-5,8-dihydro-4H,7H-faro[3.4-d]pyrazolof 1 5-
a]pyrimidin-7-one
A solution of methyl 4-chloroacetate (0.108 g, 1 mmol), 3-bromo-4-
fluorobenzaldehyde (0.203 g, 1 mmol) and 3-aminopyrazole (0.082 g, 1 mmol) in
ethanol (2
mL) was heated at reflux for 24 hours. After the reaction mixture was allowed
to cool to
ambient temperature, the volatiles were evaporated at reduced pressure and the
resulting
48
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
residue was chromatographed on silica gel, eluting with 5% ethanol/methylene
chloride to
provide 0.045 g (12 %) of the title compound.
H NMR (DMSO-d6) ~ 5.02 (q, 2H), 5.85 (d, 1 H), 6.3 (s, 1 H), 7.25 (m, 1 H),
7.31 (t, 1 H), 7.39
(d, 1 H), 7.52 (dd, 1 H), 11.08 (s, 1 H); MS (ESI+) m/z: 352 (M+H)+; Analysis
Calculated for
S C,4H9BrFN3O2: C, 48.02; H, 2.59; N, 12.00. Found: C, 48.40; H, 2.87; N,
11.65.
Example 1 S
(-) 9-(3-bromo-4-fluorophen~)-5,6,7,9-tetrahydropyrazolo[5,1-b]guinazolin-
8(4H)-one
The product from Example 1 (0.6 g) was chromatographed on a Chiracel OD 4.6 x
250 Prep Model column, eluting with 10% ethanol/hexane to provide 0.259 g of
the title
compound (retention time 12.0 min).
[a]23D -35.85° (DMSO); ~H NMR (DMSO-d6) 8 1.93 (m, 2H), 2.25 (m, 2H),
2.62 (m, 2H),
5.72 (d, 1 H), 6.19 (s, 1 H), 7.1 (m, 1 H), 7.22 (t, 1 H), 7.31 (d, 1 H), 7.4
(dd, 1 H), 10.55 (s, 1 H);
MS (ESI+) m/z: 362 (M+H)+; Analysis Calculated for C,6H~3N3BrF0: C, 53.06; H,
3.62; N,
11.60. Found: C, 52.83; H, 3.77; N, 11.28.
Example 16
~~3-bromo-4-fluorophenyl)-5,6,7,9-tetrahydro~yrazolo~5,1-b]guinazolin-8(4H)-
one
The product from Example 1 (0.6 g) was chromatographed on a Chiracel OD 4.6 x
250 Prep Model column, eluting with 10% ethanol/hexane to provide 0.252 g of
the title
compound (retention time 14.639 min).
[a]23D +35.88° (DMSO); ~H NMR (DMSO-d6) 8 1.92 (m, 2H), 2.25 (m, 2H),
2.55 (m, 2H),
5.72 (d, 1 H), 6.19 (s, 1 H), 7.1 (m, 1 H), 7.23 (t, 1 H), 7.32 (d, 1 H), 7.4
(dd, 1 H), 10.5 5 (s, 1 H);
MS (ESI+) m/z: 362 (M+H)+; Analysis Calculated for C,6H,3N3BrF0: C, 53.06; H,
3.62; N,
11.60. Found: C, 52.81; H, 3.72; N, 11.54.
Example 17
9-(3-bromo-4-fluorophenyl)-5,6,7,9-tetrahydro-4H-R ray zoloL,S-
a]thiopyranoj3,2-
d]pyrimidine 8,8-dioxide
49
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
A solution of tetrahydrothiopyran-3-one-l,l-dioxide (0.74 g, 5 mmol), 3-bromo-
4-
fluorobenzaldehyde (1.01 g, 5 mmol), and 3-aminopyrazole (0.41 g, 5 mmol) in
ethanol (5
mL) was heated at reflux for 24 hours. After the reaction mixture was allowed
to cool to
ambient temperature, the solid that precipitated was filtered off, washed with
ethanol, and
dried to provide the title compound.
H NMR (DMSO-d6) 8 2.23 (m, 2H), 2.63 (m, 2H), 3.26 (m, 1 H), 3.42 (m, 1 H),
5.63 (d, 1 H),
6.32 (s, 1 H), 7.22 (m, 1 H), 7.3 (d, 1 H), 7.31 (t, 1 H), 7.48 (dd, 1 H),
10.17 (s, 1 H); MS (ESI+)
m/z: 400 (M+H)+; Analysis Calculated for C,SH13N3BrFO2S: C, 45.11; H, 3.26; N,
10.53.
Found: C, 45.13; H, 3.50; N, 10.40.
Examples 18-53 were prepared according to General Procedure A
General Procedure A
1,3-Cyclohexanedione or 4,4-dimethyl-1,3-cyclohexanedione (0.2-0.5 mmol), an
aldehyde (0.2-0.5 mmol) and 3-aminopyrazole (0.2-0.5 mmol) in absolute ethanol
(2 mL)
were combined in a 1:1:1 molar ratio and heated at 80 °C for 3 days.
The mixture was
allowed to cool to ambient temperature and the solvent was removed by
evaporation at
reduced pressure. The crude products were purified by either flash column
chromatography
(5% MeOH in methylene chloride as eluent), recrystallization from ethanol or
preparative
TLC (5% MeOH in methylene chloride as solvent).
Example 18
9-(3-chloro-4-hydroxyphenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-
one
1,3-Cyclohexanedione, 3-chloro-4-hydroxybenzaldehyde and 3-aminopyrazole were
processed as described in General Procedure A to provide the title compound.
~HNMR(DMSO-d6) 1.95 (m, 2H), 2.25 (m, 2H), 2.65 (m,2H), 5.70 (s, 1H), 6.07 (s,
1H), 6.80-
6.90 (m, 2H), 7.00 (s, 1 H), 7.24 (s, 1 H); MS(APCI+) m/z: 316 (M+H)+.
Example 19
3-bromo-9-(3-bromo-4-fluorophenyl)-5,6,7,9-tetrahydropyrazolof5,1-b~quinazolin-
8(4H)-one
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The product from Example 1 and N-bromosuccinimide were processed as described
in
Example 48 to provide the title compound.
~HNMR(DMSO-d6) 1.94 (m, 2H), 2.25 (m, 2H), 2.70 (m,2H), 6.19 (s, 1H), 7.12 (m,
1H), 7.25
(m, 1H), 7.44 (s, 1H), 7.46 (m, 1H); MS(APCI+) m/z: 439 (M+H)+.
Example 20
9-(3-chloro-4-fluorophenyl)-5,6,7,9-tetrahydropyrazolo [5 1-bLguinazolin-8(4H)-
one
1,3-Cyclohexanedione, 3-chloro-4-fluorobenzaldehyde and 3-aminopyrazole were
processed as described in General Procedure A to provide the title compound.
'H NMR (DMSO-d6) 8 10.54 (s, 1H), 7.24-7.33 (m, 3H), 7.04-7.10 (m, 1H), 6.19
(s, 1H),
5.73 (d, 1H), 2.55-2.71 (m, 2H), 2.18-2.33 (m, 2H), 1.82-2.01 (m, 2H); MS
(APCI+) m/z: 318
(M+H)+.
Example 21
9-(3,4-difluorophenyl)-5,6,7,9-tetrahydropyrazolo[5 1-blquinazolin-8(4H -one
1,3-Cyclohexanedione, 3,4-difluorobenzaldehyde and 3-aminopyrazole were
processed as described in General Procedure A to provide the title compound.
'H NMR (DMSO-d6) b 10.53 (s, 1 H), 7.25-7.33 (m, 2H), 7.08-7.17 (m, 1 H), 6.19
(s, 1 H),
5.72 (d, 1H), 2.55-2.71 (m, 2H), 2.18-2.2.34 (m, 2H), 1.82-2.01 (m, 2H); MS
(APCI+) m/z:
302 (M+H)+.
Example 22
9-(4-fluorophenyl)-5,6,7,9-tetrahydropyrazolof 5 1-b~quinazolin-8(4H)-one
1,3-Cyclohexanedione, 4-fluorobenzaldehyde and 3-aminopyrazole were processed
as
described in General Procedure A to provide the title compound.
1H NMR (DMSO-d6) b 10.46 (s, 1H), 7.39 (d, 1H), 7.11-7.16 (m, 2H), 7.01-7.08
(m, 2H),
6.19 (s, 1 H), 5.70 (d, 1 H), 2.60-2.69 (m, 2H), 2.18-2.34 (m, 2H), 1.82-2.01
(m, 2H); MS
(APCI+) m/z: 284 (M+H)+.
51
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Example 23
9-l4-(trifluoromethyl)phenyl]'-5 6 7 9-tetrahydro~,yrazolo[5 1-b]guinazolin-
8(4H)-one
1,3-Cyclohexanedione, 4-trifluoromethylbenzaldehyde and 3-aminopyrazole were
processed as described in General Procedure A to provide the title compound.
'H NMR (DMSO-d6) 8 10.56 (s, I H), 7.61 (d, 2H), 7.32 (d, 2H), 6.26 (s, 1 H),
5.73 (d, 1 H),
2.58-2.69 (m, 2H), 2.19-2.32 (m, 2H), 1.82-2.02 (m, 2H); MS (APCI+) m/z: 334
(M+H)+.
Example 24
9-(4-cyanophenyl)-5,6,7.9-tetrahydropyrazolo f 5 1-b]guinazolin-8(4H)-one
1,3-Cyclohexanedione, 4-cyanobenzaldehyde and 3-aminopyrazole were processed
as
described in General Procedure A to provide the title compound.
~H NMR (DMSO-d6) 8 10.60 (s, 1 H), 7.71 (d, 2H), 7.28 (d, 2H), 6.25 (S, 1 H),
5.74 (D, 1 H),
2.55-2.69 (M, 2H), 2.18-2.30 (M, 2H), 1.80-2.00 (M, 2H); MS (APCI+) m/z: 291
(M+H)+.
Example 25
9-(4-chloro-3-nitrophenyl)-5 6 7 9-tetrahydropyrazolo[5 1-b]guinazolin-8(4H)-
one
1,3-Cyclohexanedione, 4-chloro-3-nitrobenzaldehyde and 3-aminopyrazole were
processed as described in General Procedure A to provide the title compound.
1H NMR (DMSO-d6) 8 1.95 (m, 2H), 2.25 (m, 2H), 2.62 (m, 2H), 5.79 (d, 1H),
6.25 (s, 1H),
7.20 (m, 2H), 7.6 (m, 1 H), 7.8 (m, 1 H), 10.6 (s, 1 H).
Example 26
9-(4-chloro-3-fluorophenyl)-7 7-dimethyl-5 6 7 9-tetrahydroQyrazolo[5 1-
b]quinazolin-8(4H)-
one
4,4-Dimethyl-1,3-cyclohexanedione, 4-chloro-3-fluorobenzaldehyde and 3-
aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
52
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
H NMR (DMSO-d6) S 10.45 (s, 1 H), 7.45 (t, 1 H), 7.31 (d, 1 H), 7.11 (dd, 1
H), 9.95 (dd, 1 H),
6.15 (s, 1H), 5.71 (d, 1H), 2.61-2.70 (m, 2H), 1.80 (t, 2H), 1.02 (s, 3H),
0.94 (s, 3H); MS
(APCI+) m/z: 346 (M+H)+.
Example 27
9-(3-bromo-4-fluorophenyl)-7,7-dimethyl-5 6 7 9-tetrahydropyrazolol5 1-
b]guinazolin-8(4H)-
one
4,4-Dimethyl-1,3-cyclohexanedione, 3-bromo-4-fluorobenzaldehyde and 3-
aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
1 H NMR (DMSO-d6) 8 10.48 (s, 1 H), 7.40 (dd, 1 H), 7.30 (d, 1 H), 7.24 (t, 1
H), 7.10-7.16 (m,
1H), 6.14 (s, 1H), 5.70 (d, 1H), 2.62-2.71 (m, 2H), 1.80 (t, 2H), 1.02 (s,
3H), 0.94 (s, 3H); MS
(APCI+) m/z: 391 (M+H)+.
Example 28
9-f4-fluoro-3-(trifluoromethyl)phenyl]-7 7-dimethyl-5 6 7 9-
tetrahydropyrazolo[5 1
b]quinazolin-8(4H)-one
4,4-Dimethyl-1,3-cyclohexanedione, 4-fluoro-3-trifluoromethylbenzaldehyde and
3-
aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
1H NMR (DMSO-d6) b 10.53 (s, 1H), 7.35-7.52 (m, 3H), 7.32 (d, 1H), 6.24 (s,
1H), 5.72 (d,
1 H), 2.63-2.71 (m, 2H), 1.81 (t, 2H), 1.02 (s, 1 H), 0.93 (s, 1 H).
Example 29
9-(3,4-dichlorophenyl)-7,7-dimethyl-5 6 7 9-tetrahydrop razolo[5 1-
b]guinazolin-8(4H, -one
4,4-Dimethyl-1,3-cyclohexanedione, 3,4-dichlorobenzaldehyde and 3-
aminopyrazole
were processed as described in General Procedure A to provide the title
compound.
53
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
'H NMR (DMSO-d6) 8 10.49 (s, 1H), 7.50 (d, 1H), 7.31-7.35 (m, 2H), 7.07 (dd,
1H), 6.14 (s,
1 H), 5.71 (d, 1 H), 2.62-2.75 (m, 2H), 1.80 (t, 2H), 1.02 (s, 3H), 0.94 (s,
3H); MS (APCI+)
m/z: 363 (M+H)+.
Example 30
9-(4-chloro-3-nitrophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-
blquinazolin-8(4H)-
one
4,4-Dimethyl-1,3-cyclohexanedione, 4-chloro-3-nitrobenzaldehyde and 3-
aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
'H NMR (DMSO-d6) 8 10.55 (s, 1H), 7.81 (d, 1H), 7.65 (d, 1H), 7.45 (dd, 1H),
7.33 (d, 1H),
6.24 (s, 1H), 5.73 (d, 1H), 2.61-2.73 (m, 2H), 1.82 (t, 2H), 1.02 (s, 3H),
0.94 (s, 3H); MS
(APCI+) m/z: 373 (M+H)+.
Example 31
9-(3,4-dibromophenyl)-7,7-dimethyl-5,6,7,9-tetrahydrop ray_ zolo[5,1-
b~quinazolin-8(4H)-one
4,4-Dimethyl-1,3-cyclohexanedione, 3,4-dibromobenzaldehyde and 3-aminopyrazole
were processed as described in General Procedure A to provide the title
compound.
' H NMR (DMSO-d6) 8 10.51 (s, 1 H), 7.62 (d, 1 H), 7.47 (d, 1 H), 7.31 (d, 1
H), 7.02 (dd, 1 H),
6.12 (s, 1H), 5.71 (d, 1H), 2.59-2.74 (m, 2H), 1.80 (t, 2H), 1.02 (s, 3H),
0.94 (s, 3H); MS
(APCI+) m/z: 452 (M+H)+.
Example 32
9-[3-fluoro-4-(trifluoromethyl)phenyl]-7,7-dimethyl-5,6,7,9-
tetrahydropyrazolo[5,1-
b]quinazolin-8~4H)-one
4,4-Dimethyl-1,3-cyclohexanedione, 3-fluoro-4-trifluoromethylbenzaldehyde and
3-
aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
54
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
H NMR (DMSO-d6) 8 10.54 (s, 1 H), 7.66 (t, 1 H), 7.33 (d, 1 H), 7.22 (d, 1 H),
7.13 (d, 1 H),
6.23 (s, 1H), 5.74 (d, 1H), 2.62=2.72 (m, 2H), 1.81 (t, 2H), 1.03 (s, 3H),
0.94 (s, 3H); MS
(APCI+) m/z: 380 (M+H)+.
Example 33
7,7-dimethyl-9-(3-nitrophenyl)-5.6,7,9-tetrahydropyrazolo[5,1-b]guinazolin-
8(4H)-one
4,4-Dimethyl-1,3-cyclohexanedione, 3-nitrobenzaldehyde and 3-aminopyrazole
were
processed as described in General Procedure A to provide the title compound.
~H NMR (DMSO-db) 8 10.44 (s, 1H), 8.03-8.08 (m, 1H), 7.91-7.94 (m, 1H), 7.57-
7.59 (m,
2H), 7.33 (d, 1H), 6.30 (s, 1H), 5.74 (d, 1H), 2.62-2.72 (m, 2H), 1.75-1.84
(m, 2H), 1.03 (s,
3H), 0.93 (s, 3H); MS (APCI+) m/z: 339 (M+H)+.
Example 34
9-(3-cyanophenyl)-7,7-dimethyl-5,6,7,9-tetrahydro~yrazoloj5,1-b]guinazolin-
8(4H)-one
4,4-Dimethyl-1,3-cyclohexanedione, 3-cyanobenzaldehyde and 3-aminopyrazole
were
processed as described in General Procedure A to provide the title compound.
~H NMR (DMSO-d6) 810.47 (s, 1H), 7.64-7.69 (m, 1H), 7.54-7.57 (m, 1H), 7.44-
7.48 (m,
2H), 7.31 (d, 1H), 6.20 (s, 1H), 5.72 (d, 1H), 2.59-2.76 (m, 2H), 1.81 (t,
2H), 1.02 (s, 3H),
0.92 (s, 3H); MS (APCI+) m/z 319 (M+H)+.
Example 35
7 7-dimethyl-9-(5-nitro-3-thienyl)-5,6,7,9-tetrahydropyrazolo[S,1-b]quinazolin-
8(4H)-one
4,4-Dimethyl-1,3-cyclohexanedione, 4-formyl-2-nitrothiophene and 3-
aminopyrazole
were processed as described in General Procedure A to provide the title
compound.
~H NMR (DMSO-d6) 8 10.55 (s, 1 H), 7.68 (d, 1 H), 7.62 (d, 1 H), 6.24 (s, 1
H), 5.72 (d, 1 H),
2.62-2.70 (m, 2H), 1.79-1.87 (m, 2H), 1.04 (s, 3H), 1.01 (s, 3H); MS (APCI+)
m/z: 345
(M+H)+.
Example 36
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
9-(5-bromo-2-hydroxyphen~)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo(5,1-
b]guinazolin-
8 4H -one
4,4-Dimethyl-1,3-cyclohexanedione, 5-bromo-2-hydroxybenzaldehyde and 3-
aminopyrazole were processed as described in General Procedure A to provide
the title
S compot~nd~. -
H NMR (DMSO-d6) 8 10.44 (s, 1 H), 9.86 (s, 1 H), 7.26 (d, 1 H), 7.13 (dd, 1
H), 6.93 (d, 1 H),
6.66 (d, 1H), 6.30 (s, 1H), 5.66 (d, 1H), 2.60-2.69 (m, 2H), 1.78-1.85 (m,
2H), 1.02 (s, 3H),
0.95 (s, 3H); MS (APCI) m/z: 389 (M+H)+.
Example 37
9-(5-chloro-2-hydroxyphen~)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo [5,1-
blquinazolin-
8 4H -one
4,4-Dimethyl-1,3-cyclohexanedione, 5-chloro-2-hydroxybenzaldehyde and 3-
aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
H NMR (DMSO-d6) 8 10.45 (s, 1 H), 9.84 (s, 1 H), 7.26 (d, 1 H), 7.02 (dd, 1
H), 6.79 (d, 1 H),
6.71 (d, 1 H), 6.31 (s, 1 H), 5.66 (d, 1 H), 2.60-2.70 (m, 2H), 1.76-1.84 (m,
2H), 1.02 (s, 3H),
0.95 (s, 3H); MS (APCI+) m/z: 344 (M+H)+.
~ Example 38
~2-hydroxy-5-nitrophenyl)-7,7-dimethyl-5,6,7,9-tetrahydropyrazolo[5,1-
blguinazolin-
8 4H -one
4,4-Dimethyl-1,3-cyclohexanedione, 2-hydroxy-5-nitrobenzaldehyde and 3-
aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
1H NMR (DMSO-d6) 8 11.13 (s, 1 H), 10.46 (s, 1 H), 7.95 (dd, 1 H), 7.87 (d, 1
H), 7.25 (d, 1 H),
6.86 (d, 1H), 6.38 (s, 1H), 5.66 (d, 1H), 2.64-2.68 (m, 2H), 1.77-1.84 (m,
2H), 1.02 (s, 3H),
0.91 (s, 3H); MS (APCI+) m/z: 355 (M+H)+.
56
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Example 39
9-(3,5-dibromo-2-hydroxyphenyl)-7,7-dimethyl-5,6.7,9-tetrahydrop r~[5,1-
b]quinazolin-
8 4H -one
4,4-Dimethyl-1,3-cyclohexanedione, 3,5-dibromo-2-hydroxybenzaldehyde and 3-
aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
~ H NMR (DMSO-db) 8 10.79 (s, 1 H), 7.59 (d, 1 H), 7.42 (d, 1 H), 6.65 (d, 1
H), 6.42 (s, 1 H),
5.81 (d, 1H), 2.60-2.76 (m, 2H), 1.82 (t, 2H), 1.06 (s, 3H), 1.02 (s, 3H); MS
(APCI+) m/z:
468 (M+H)+.
Example 40
9-(3-bromo-5-chloro-2-hydroxyphenyl)-7,7-dimethyl-5,6,7,9-
tetrahydropyrazolo[5,1-
b~QUinazolin-8(4H)-one
4,4-Dimethyl-1,3-cyclohexanedione, 3-bromo-5-chloro-2-hydroxybenzaldehyde and
3-aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
1H NMR (DMSO-d6) 8 1.02 (s, 3H), 1.06 (s, 3H), 1.84 (t, 2H,), 2.62-2.75 (m,
2H),), 5.79 (d,
1 H), 6.43 (s, 1 H), 6.54 (d, 1 H), 7.40 (d, 1 H), 7.48 (d, 1 H), 10.78 (s, 1
H); MS (APCI+) m/z:
423 (M+H)+.
Example 41
~3,5-dichloro-2-hydrox~phen~l~l-7.7-dimethyl-5,6,7.9-tetrahydroR ra~zolo~5,l-
b]!quinazolin-
8 4H -one
4,4-Dimethyl-1,3-cyclohexanedione, 3,5-dichloro-2-hydroxybenzaldehyde and 3-
aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
~H NMR (DMSO-d6) b 7.34-7.38 (m, 1H), 6.58 (d, 1H), 6.43 (s, 1H), 5.77 (d,
1H), 2.62-2.81
(m, 2H), 1.83 (t, 2H), 1.05 (s, 3H), 1.00 (s, 3H); MS (APCI+) m/z: 379 (M+H)+.
57
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Example 42
7,7-dimethyl-9-(3,4.5-trifluorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-
blguinazolin-8(4H~
one
4,4-Dimethyl-1,3-cyclohexanedione, 3,4,5-trifluorobenzaldehyde and 3-
aminopyrazole were processed as described in General Procedure A to provide
the title
compound.
1H NMR (DMSO-d6) 8 10.51 (s, 1H), 7.33 (d, 1H), 7.00-7.07 (m, 2H), 6.15 (s,
1H), 5.71 (d,
1H), 2.58-2.77 (m, 2H), 1.82 (t, 2H), 1.02 (s, 3H), 0.96 (s, 3H); MS (APCI+)
m/z: 347
(M+H)+.
Example 43
9-(3,4-dichlorophenyl)-3-(3-fluorophen~)-5,6,7,9-tetrahydropyrazolo[S,1-
b]guinazolin-
8 4H -one
The product from Example 48 (0.041 g, 0.1 mmol) in dimethoxyethane/methanol
(1.5
mL) was treated with 3-fluorophenylboronic acid (0.13 mmol), cesium fluoride
(0.2 mmol)
and tetrakis(triphenylphosphine)palladium(0) ( 0.008 g, 0.006 mmol). The
mixture was
heated at 100 °C for 48 hours and then allowed to cool to ambient
temperature. The reaction
mixture was filtered through Celite and the filtrate concentrated under
reduced pressure. The
residue was purified by HPLC chromatography.
1H NMR (DMSO-d6) ~ 1.95 (m, 2H), 2.28 (m, 2H), 2.75 (m, 2H), 6.2 (s, 1H), 7.10
(m, 2H),
7.3 (m, 1 H), 7.4 (m, 3 H), 7.65 (s, 1 H), 10.5 (s, 1 H).
Example 44
3-(3-chlorophen~)-9 ~3,4-dichlorophenyl)-5,6,7,9-tetrahydropyrazolo[5.1-
blquinazolin-
8 4H -one
The product from Example 48 and 3-chlorophenylboronic acid were processed as
described in Example 43 to provide the title compound.
1HNMR(DMSO-d6) 8 1.95 (m, 2H), 2.25 (m, 2H), 2.74 (m, 2H), 6.20 (s, 1H), 7.10
(m, 1H),
7.30 (m, 1H), 7.45-7.55 (m, 4H), 7.65 (s, 1H); MS(APCI+) m/z: 444 (M+H)+.
58
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Example 45
3 ~4-carboxyphen~)-9-(3,4-dichlorophenyl~,-5,6,7,9-tetrahydropyrazolo[5,1-
b~guinazolin-
8 4H -one
The product from Example 48 and 4-carboxyphenylboronic acid were processed as
described in Example 43 to provide the title compound.
'H NMR(DMSO-d6) 8 1.95 (m, 2H), 2.25 (m, 2H), 2.74 (m, 2H), 6.22 (s, 1H), 7.12
(m, 1H),
7.44 (s, 1 H), 7.54 (m, 1 H), 7.62 (d, J=8, 2H), 7.77 (m, 1 H), 7.98 (d, J=8,
2H); MS (APCI+)
m/z: 454 (M+H)+.
Example 46
9-(3,4-dichlorophenyl -L3-(2-thien~)-5,6,7.9-tetrahydropyrazolol5,1-
b]quinazolin-8(4H)-one
The product from Example 48 and 2-tributylstannylthiophene were processed as
described in Example 51 to provide the title compound as light yellow solid.
~H NMR
(DMSO-d6) b 1.95 (m, 2H), 2.27 (m, 2H), 2.75 (m, 2H), 6.22 (s, 1 H), 7.11 (d,
1 H), 7.14 (dd,
1 H), 7.22 (d, 1 H), 7.45 (s, 1 H), 7.48 (d, 1 H), 7.52 (s, 1 H), 7.53 (d, 1
H), 10.30 (s, 1 H); MS
(ESI+) m/z 416 (M + H+); MS (ESI-) m/z 414 (M-H)- Anal. Calcd for
C20H15C12N30S: C,
57.70; H, 3.63; N, 10.09. Found: C, 57.53; H, 3.46; N, 9.76.
Example 47
9-y3,4-dichlorophenyl)-3-[2-(trifluoromethyl)phenyl]-5,6,7,9-
tetrahydropyrazolo[5,1-
b]quinazolin-8(4H -one
The product from Example 48 and 2-(trifluoromethyl)phenylboronic acid were
processed as described in Example 43 to provide the title compound.
~H NMR (DMSO-d6) b 1.95 (m, 2H), 2.25 (m, 2H), 2.74 (m, 2H), 6.28 (s, 1H),
7.10 (m, 1H),
7.30 (m, 1H), 7.40-7.95 (m, SH), 10.22 (s, 1H); MS(APCI+) m/z: 478 (M+H)+.
Example 48
3-bromo-9-(3,4-dichlorophenYl)-5,6,7,9-tetrahydropyrazolo[5,1-b]guinazolin-
8(4H -one
59
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The product from Example 6 (0.52 g, 1.56 mmol) in dichloromethane was treated
with
N-bromosuccinimide (0.28 mg, 1.56 mmol) and allowed to stir at ambient
temperature
overnight. The mixture was filtered and the filter cake washed with CHzCl2 to
provide the
title compound (0.54 g) as a solid.
1H NMR (DMSO-d6) 8 1.94 (m, 2H), 2.24 (m, 2H), 2.77 (m, 2H), 6.20 (s, 1H),
7.06 (d, J=8,
1 H), 7.40 (s, 1 H), 7.44 (s, 1 H), 7.55 (d, J=8, 1 H); MS(APCI+) m/z 411
(M+H)+.
Example 49
3-bromo-9-(3,4-dichlorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H)-one
The product from Example 48 was subjected to chiral column chromatography
(Whelko column, 2.11 cm x 25 cm, eluting with 80:20 hexane:(CH30H:CHZCIz 2:1))
to
provide two enantiomers. The faster moving enantiomer, retention time 25
minutes, was
isolated as an off white solid.
'H NMR (DMSO-db) 8 1.94 (m, 2H), 2.26 (m, 2H), 2.72 (m, 2H), 6.18 (s, 1H),
7.07 (d, 1H),
7.42 (s, 1 H), 7.48 (s, 1 H), 7.52 (d, 1 H), 10.57 (s, 1 H); MS (ESI+) m/z:
412 (M+H)+; MS
(ESI-) m/z: 410 (M-H)'; Anal. Calcd for Cl6HizBrC12N30: C, 46.52; H, 2.93; N,
10.17.
Found: C, 46.49; H, 3.11; N, 9.92.
Example 50
3-bromo-9-(3,4-dichlorophenyl)-5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-
8(4H1-one
The product from Example 48 was subjected to chiral column chromatography
(Whelko column, 2.11 cm x 25 cm, eluting with 80:20 hexane:(CH30H:CHZCl2 2:1))
to
provide two enantiomers. The slower moving enantiomer, retention time 31.2
minutes, was
isolated as an off white solid.
1H NMR (DMSO-db) 8 1.94 (m, 2H), 2.26 (m, 2H), 2.72 (m, 2H), 6.18 (s, 1H),
7.07 (d, 1H),
7.42 (s, 1 H), 7.48 (s, 1 H), 7.52 (d, 1 H), 10.57 (s, 1 H); MS (ESI+) m/z:
412 (M+H)+; MS
(ESI-) m/z: 410 (M-H)'; Anal. Calcd for C,6H12BrC12N30: C, 46.52; H, 2.93; N,
10.17.
Found: C, 46.78; H, 2.92; N, 10.02.
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Example 51
9-(3,4-dichlorophenyl)-3-(2-thienyl)-5 6 7 9-tetrahydrowrazolo~5 1-
b],quinazolin-8(4H)-one
The product from Example 49 (0.2 g, 0.5 mmol) in DMF (4 mL) was treated with
tetrakis(triphenylphosphine)palladium(0) (0.11 g) and 2-
tributylstannylthiophene (0.41 g, 1.1
mmol). The reaction mixture was heated at 110 °C for 20 hours. After
cooling to ambient
temperature, the mixture was filtered through Celite and the filtrate diluted
with ethylacetate.
The diluted filtrate was washed with brine solution, dried over MgS04 and
concentrated under
reduced pressure. The residue was chromatographed on silica gel eluting with
hexane:ethylacetate (1:1) to yield 0.14 g of the title compound as a light
yellow solid.
1H NMR (DMSO-d6) 8 1.95 (m, 2H), 2.27 (m, 2H), 2.75 (m, 2H), 6.22 (s, 1H),
7.11 (d, 1H),
7.14 (dd, 1 H), 7.22 (d, 1 H), 7.45 (s, 1 H), 7.48 (d, 1 H), 7.52 (s, 1 H),
7.53 (d, 1 H), 10.30 (s,
1 H); MS (ESI+) m/z: 416 (M+H)+; MS (ESI-) m/z 414 (M-H)-; Anal. Calcd for
C2oHi5C12N3OS: C, 57.70; H, 3.63; N, 10.09. Found: C, 57.80; H, 3.70; N, 9.86.
Example 52
9-(3,4-dichlorophenyl)-3-(2-thienyl)-5 6 7 9-tetrah~~ rv azolo[5 1-
b]guinazolin-8(4H)-one
The product from Example 50 was processed as described in Example 51 to
provide
the title compound as a light yellow solid.
~H NMR (DMSO-d6) 8 1.95 (m, 2H), 2.27 (m, 2H), 2.75 (m, 2H), 6.22 (s, 1H),
7.11 (d, 1H), .
7.14 (dd, 1 H), 7.22 (d, 1 H), 7.45 (s, 1 H), 7.48 (d, 1 H), 7.52 (s, 1 H),
7.53 (d, 1 H), 10.30 (s,
1 H); MS (ESI+) m/z: 416 (M+H)+; MS (ESI-) m/z: 414 (M-H)-; Anal. Calcd for
CzoH,5C12N30S: C, 57.70; H, 3.63; N, 10.09. Found: C, 57.78; H, 3.60; N, 9.85.
Example 53
9-(3,4-dichlorophenyl)-3-(2-furyl)-5 6 7 9-tetrahydropyrazolol5 1-bctuinazolin-
8(4H)-one
The product from Example 6, tetrakis(triphenylphosphine)palladium(0) (0.11 g)
and 2-
tributylstannylfuran were processed as described in Example 51 to provide the
title
compound.
61
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
IH NMR (DMSO-d6) 8 1.95 (m, 2H), 2.28 (m, 2H), 2.78 (m, 2H), 6.22 (s, 1H),
6.57 (dd, 1H),
6.64 (d, 1 H), 7.09 (d, 1 H), 7.42 (s, 1 H), 7.53 (d, 1 H), 7.64 (s, 1 H),
7.65 (d, 1 H), 9.97 (s, 1 H);
MS (ESI+) m/z 400 (M+H)+; MS (ESI-) m/z 398 (M-H)-; Anal. Calcd for
C2oH,5C12N302: C,
60.02; H, 3.78; N, 10.50. Found: C, 60.06; H, 3.76; N, 10.33.
Determination of Potassium Channel Opening Activity
Membrane Hyperpolarization Assays
Compounds were evaluated for potassium channel opening activity using primary
cultured guinea-pig urinary bladder (GPB) cells.
For the preparation of urinary bladder smooth muscle cells, urinary bladders
were
removed from male guinea-pigs (Hartley, Charles River, Wilmington, MA)
weighing 300-400
g and placed in ice-cold Ca2+-free Krebs solution (Composition, mM: KCI, 2.7;
KHzP04, 1.5;
NaCI, 75; Na2HP04, 9.6; NaZHP04~7H20, 8; MgS04, 2; glucose, 5; HEPES, 10; pH
7.4).
Cells were isolated by enzymatic dissociation as previously described with
minor
modifications in (Klockner, U. and Isenberg, G., Pflugers Arch. 1985, 405, 329-
339), hereby
incorporated by reference. The bladder was cut into small sections and
incubated in 5 mL of
the Kreb's solution containing 1 mg/mL collagenase (Sigma, St. Louis, MO) and
0.2 mg/mL
pronase (Calbiochem, La Jolla, CA) with continuous stirring in a cell
incubator for 30
minutes. The mixture was then centrifuged at 1300 x g for 5 minutes, and the
pellet
resuspended in Dulbecco's PBS (GIBCO, Gaithersburg, MD) and recentrifuged to
remove
residual enzyme. The cell pellet was resuspended in 5 mL growth media
(composition:
Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum,
100
units/mL penicillin, 100 units/mL streptomycin and 0.25 mg/mL amphotericin B)
and further
dissociated by pipetting the suspension through a flame-polished Pasteur
pipette and passing
it through a polypropylene mesh membrane (Spectrum, Houston, TX). The cell
density was
adjusted to 100,000 cells/mL by resuspension in growth media. Cells were
plated in clear-
bottomed black 96-well plates (Packard) for membrane potential studies at a
density of 20,000
cells/well and maintained in a cell incubator with 90% air:10% COZ until
confluent. Cells
62
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
were confirmed to be of smooth muscle type by cytoskeletal staining using a
monoclonal
mouse anti human-a-smooth muscle actin (Biomeda, Foster City, CA).
Functional activity at potassium channels was measured by evaluating changes
in
membrane potential using the bis-oxonol dye DiBAC(4)3 (Molecular Probes) in a
96-well
cell-based kinetic assay system, Fluorescent Imaging Plate Reader (FLIPR)
(K.S. Schroeder
et al., J. Biomed. Screen., v. 1 pp. 75-81 (1996)), hereby incorporated by
reference.
DiBAC(4)3 is an anionic potentiometric probe which partitions between cells
and extracellular
solution in a membrane potential-dependent manner. With increasing membrane
potential
(for example, K+ depolarization), the probe further partitions into the cell;
this is measured as
an increase in fluorescence due to dye interaction with intracellular lipids
and proteins.
Conversely, decreasing membrane potential (hyperpolarization by potassium
channel openers)
evokes a decrease in fluorescence.
Confluent guinea-pig urinary bladder cells cultured in black clear-bottomed 96-
well
plates were rinsed twice with 200 mL assay buffer (composition, mM: HEPES, 20;
NaCI,
120; KCI, 2; CaCl2, 2; MgCl2, l; glucose, 5; pH 7.4 at 25 °C)
containing 5 p,M DiBAC(4)3
and incubated with 180 mL of the buffer in a cell incubator for 30 minutes at
37 °C to ensure
dye distribution across the membrane. After recording the baseline
fluorescence for 5
minutes, the reference or test compounds, prepared at 10 times the
concentration in the assay
buffer, were added directly to the wells. Changes in fluorescence were
monitored for an
additional 25 minutes. Hyperpolarization responses were corrected for any
background noise
and were normalized to the response observed with 10 pM of the reference
compound P 1075,
N"-cyano-N-(tert-pentyl)-N'-(3-pyridinyl)guanidine, which was assigned as
100%. P 1075 is a
potent opener of smooth muscle KATP channels (Quast et al., Mol. Pharmacol.,
v. 43 pp. 474-
481 (1993)) and was prepared using the procedures described in (Manley, J.
Med. Chem.
(1992) 35, 2327-2340), hereby incorporated by reference.
Routinely, five concentrations of P1075 or test compounds (log or half log
dilutions)
were evaluated and the maximal steady-state hyperpolarization values
(expressed as
relative to P1075) plotted as a function of concentration. The ECSO
(concentration that elicits
50% of the maximal response for the test sample) values were calculated by non-
linear
63
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
regression analysis using a four parameter sigmoidal equation. The maximal
response of each
compound (expressed as % relative to P1075) is reported. Stock solutions of
compounds
were prepared in 100% DMSO and further dilutions were carried out in the assay
buffer and
added to a 96-well plate. The maximal steady-state hyperpolarization values
(expressed as
relative to P 1075) and the ECSO values for representative compounds of the
present invention
are shown in Table 1.
Table 1
Membrane Hyperpolarization (MHP) in Guinea-Pig Bladder (GPB) Cells
Maximal
Response
xample # (% P1075) C50(I~M)
1 87 0.031
2 100 0.040
3 34 24
4 96 0.429
98 0.122
6 95 0.130
7 98 0.550
8 92 1.12
9 94 0.187
100 0.290
11 90 1.71
12 85 2.86
13 50 10
14 89 0.258
103 0.051
16 105 0.020
17 89 0.910
64
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
18 74 5.5
19 81 0.39
20 83 0.26
21 75 2.3
22 59 7.8
25 66 0.31
26 75 0.34
27 99 0.036
28 96 0.28
29 100 0.045
30 113 0.37
31 96 0.001
32 92 0.16
33 58 7.3
34 38 16
36 103 0.34
38 69 4.6
39 110 1.5
40 107 1.4
41 103 1.4
42 105 0.23
43 95 3.2
44 87 2.5
45 106 0.27
46 118 0.038
48 113 0.033
In vitro Functional models
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Compounds of the present invention were evaluated for functional potassium
channel
opening activity using tissue strips obtained from Landrace pig bladders.
Landrace pig bladders were obtained from female Landrace pigs of 9-30 kg.
Landrace
pigs were euthanized with an intraperitoneal injection of pentobarbital
solution, Somlethal~ ,
J.A: Webster Inc., Sterling MA. The entire bladder was removed and immediately
placed into
Krebs Ringer bicarbonate solution (composition, mM: NaCI, 120; NaHC03, 20;
dextrose, 11;
KCI, 4.7; CaCl2, 2.5; MgS04, 1.5; KH2PO4, 1.2; K2EDTA, 0.01, equilibrated with
5%
COZ/95% Oz pH 7.4 at 37 °C). Propranolol (0.004 mM) was included in all
of the assays to
block ~i-adrenoceptors. The trigonal and dome portions were discarded. Strips
3-5 mm wide
and 20 mm long were prepared from the remaining tissue cut in a circular
fashion. The
mucosal layer was removed. One end was fixed to a stationary glass rod and the
other to a
Grass FT03 transducer at a basal preload of 1.0 gram. Two parallel platinum
electrodes were
included in the stationary glass rod to provide field stimulation of 0.05 Hz,
0.5 milli-seconds
at 20 volts. This low frequency stimulation produced a stable twitch response
of 100-500
centigrams. Tissues were allowed to equilibrate for at least 60 minutes and
primed with 80
mM KCI. A control concentration response curve (cumulative) was generated for
each tissue
using the potassium channel opener P 1075 as the control agonist. P 1075
completely
eliminated the stimulated twitch in a dose dependent fashion over a
concentration range of 10-
9 to 10-5 M dissolved in DMSO using 1/2 log increments. After a 60 minute
rinsing period, a
concentration response curve (cumulative) was generated for the test agonist
in the same
fashion as that used for the control agonist P 1075. The maximal efficacy of
each compounds
(expressed as % relative to P 1075) is reported. The amount of agent necessary
to cause 50%
of the agent's maximal response (EDSO) was calculated using "ALLFIT" (DeLean
et al., Am.
J. Physiol., 235, E97 (1980)), hereby incorporated by reference, and agonist
potencies were
expressed as pD2 (the negative logarithm). Agonist potencies were also
expressed as an index
relative to P 1075. The index was calculated by dividing the EDSO for P 1075
by the EDSO for
the test agonist in a given tissue. Each tissue was used for only one test
agonist, and the
indices obtained from each tissue were averaged to provide an average index of
potency.
These data are shown in Table 2.
66
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Table 2
Functional Potassium Channel Opening Activity in Isolated Bladder Strips
Landrace
Pig Bladder
Efficacy
Example# (%P1075) pDz Index
1 96 6.47 0.26
15 100 6.67 0.21
16 95 6.34 0.28
As shown by the data in Tables 1 and 2, the compounds of this invention reduce
stimulated contractions of the bladder and therefore may have utility in the
treatment of
diseases prevented by or ameliorated with potassium channel openers.
Compounds of the present invention may exist as stereoisomers wherein,
asymmetric
or chiral centers are present. These stereoisomers are "R" or "S" depending on
the
configuration of substituents around the chiral carbon atom. The terms "R" and
"S" used
herein are configurations as defined in IUPAC 1974 Recommendations for Section
E,
Fundamental Stereochemistry, Pure Appl. Chem., 1976, 45: 13-30. In particular,
the
stereochemistry at the point of attachment of R', as shown in formula (I)-
(IV), may
independently be either (R) or (S), unless specifically noted otherwise. The
present invention
contemplates various stereoisomers and mixtures thereof and are specifically
included within
the scope of this invention. Stereoisomers include enantiomers and
diastereomers, and
mixtures of enantiomers or diastereomers. Individual stereoisomers of
compounds of the
present invention may be prepared synthetically from commercially available
starting
materials which contain asymmetric or chiral centers or by preparation of
racemic mixtures
followed by resolution well-known to those of ordinary skill in the art. These
methods of
resolution are exemplified by (1) attachment of a mixture of enantiomers to a
chiral auxiliary,
separation of the resulting mixture of diastereomers by recrystallization or
chromatography
and liberation of the optically pure product from the auxiliary or (2) direct
separation of the
mixture of optical enantiomers on chiral chromatographic columns.
67
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The term "pharmaceutically acceptable carrier," as used herein, means a non-
toxic,
inert solid, semi-solid or liquid filler, diluent, encapsulating material or
formulation auxiliary
of any type. Some examples of materials which can serve as pharmaceutically
acceptable
carriers are sugars such as lactose, glucose and sucrose; starches such as
corn starch and
potato starch; cellulose and its derivatives such as sodium carboxymethyl
cellulose, ethyl
cellulose and cellulose acetate; powdered tragacanth; malt; gelatin; talc;
excipients such as
cocoa butter and suppository waxes; oils such as peanut oil, cottonseed oil,
safflower oil,
sesame oil, olive oil, corn oil and soybean oil; glycols; such a propylene
glycol; esters such as
ethyl oleate and ethyl laurate; agar; buffering agents such as magnesium
hydroxide and
aluminum hydroxide; alginic acid; pyrogen-free water; isotonic saline;
Ringer's solution; ethyl
alcohol, and phosphate buffer solutions, as well as other non-toxic compatible
lubricants such
as sodium lauryl sulfate and magnesium stearate, as well as coloring agents,
releasing agents,
coating agents, sweetening, flavoring and perfuming agents, preservatives and
antioxidants
can also be present in the composition, according to the judgment of the
formulator.
The present invention provides pharmaceutical compositions which comprise
compounds of the present invention formulated together with one or more non-
toxic
pharmaceutically acceptable carriers. The pharmaceutical compositions can be
formulated for
oral administration in solid or liquid form, for parenteral injection or for
rectal administration.
Further included within the scope of the present invention are pharmaceutical
compositions comprising one or more of the compounds of formula (I)-(IV)
prepared and
formulated in combination with one or more non-toxic pharmaceutically
acceptable
compositions. The pharmaceutical compositions can be formulated for oral
administration in
solid or liquid form, for parenteral injection or for rectal administration.
The pharmaceutical compositions of this invention can be administered to
humans and
other mammals orally, rectally, parenterally , intracisternally,
intravaginally, intraperitoneally,
topically (as by powders, ointments or drops), bucally or as an oral or nasal
spray. The term
"parenterally," as used herein, refers to modes of administration which
include intravenous,
intramuscular, intraperitoneal, intrasternal, subcutaneous, intraarticular
injection and infusion.
68
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Pharmaceutical compositions of this invention for parenteral injection
comprise
pharmaceutically acceptable sterile aqueous or nonaqueous solutions,
dispersions,
suspensions or emulsions and sterile powders for reconstitution into sterile
injectable
solutions or dispersions. Examples of suitable aqueous and nonaqueous
carriers, diluents,
solvents or vehicles include water, ethanol, polyols (propylene glycol,
polyethylene glycol,
glycerol, and the like), suitable mixtures thereof, vegetable oils (such as
olive oil) and
injectable organic esters such as ethyl oleate. Proper fluidity may be
maintained, for example,
by the use of a coating such as lecithin, by the maintenance of the required
particle size in the
case of dispersions, and by the use of surfactants.
These compositions may also contain adjuvants such as preservative agents,
wetting
agents, emulsifying agents, and dispersing agents. Prevention of the action of
microorganisms may be ensured by various antibacterial and antifungal agents,
for example,
parabens, chlorobutanol, phenol, sorbic acid, and the like. It may also be
desirable to include
isotonic agents, for example, sugars, sodium chloride and the like. Prolonged
absorption of
the injectable pharmaceutical form may be brought about by the use of agents
delaying
absorption, for example, aluminum monostearate and gelatin.
In some cases, in order to prolong the effect of a drug, it is often desirable
to slow the
absorption of the drug from subcutaneous or intramuscular injection. This may
be
accomplished by the use of a liquid suspension of crystalline or amorphous
material with poor
water solubility. The rate of absorption of the drug then depends upon its
rate of dissolution
which, in turn, may depend upon crystal size and crystalline form.
Alternatively, delayed
absorption of a parenterally administered drug form is accomplished by
dissolving or
suspending the drug in an oil vehicle.
Suspensions, in addition to the active compounds, may contain suspending
agents, as,
for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and
sorbitan esters,
microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-agar,
tragacanth, and
mixtures thereof.
If desired, and for more effective distribution, the compounds of the present
invention
can be incorporated into slow-release or targeted-delivery systems such as
polymer matrices,
69
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
liposomes, and microspheres. They may be sterilized, for example, by
filtration through a
bacteria-retaining filter or by incorporation of sterilizing agents in the
form of sterile solid
compositions, which may be dissolved in sterile water or some other sterile
injectable medium
immediately before use.
The active compounds can also be in micro-encapsulated form, if appropriate,
with
one or more excipients as noted above. The solid dosage forms of tablets,
dragees, capsules,
pills, and granules can be prepared with coatings and shells such as enteric
coatings, release
controlling coatings and other coatings well known in the pharmaceutical
formulating art. In
such solid dosage forms the active compound can be admixed with at least one
inert diluent
such as sucrose, lactose, or starch. Such dosage forms may also comprise, as
is normal
practice, additional substances other than inert diluents, e.g., tableting
lubricants and other
tableting aids such a magnesium stearate and microcrystalline cellulose. In
the case of
capsules, tablets and pills, the dosage forms may also comprise buffering
agents. They may
optionally contain opacifying agents and can also be of such composition that
they release the
active ingredients) only, or preferentially, in a certain part of the
intestinal tract in a delayed
manner. Examples of embedding compositions which can be used include polymeric
substances and waxes.
Injectable depot forms are made by forming microencapsulated matrices of the
drug in
biodegradable polymers such as polylactide-polyglycolide. Depending upon the
ratio of drug
to polymer and the nature of the particular polymer employed, the rate of drug
release can be
controlled. Examples of other biodegradable polymers include poly(orthoesters)
and
poly(anhydrides) Depot injectable formulations are also prepared by entrapping
the drug in
liposomes or microemulsions which are compatible with body tissues.
The injectable formulations can be sterilized, for example, by filtration
through a
bacterial-retaining filter or by incorporating sterilizing agents in the form
of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile injectable
medium just prior to use.
Injectable preparations, for example, sterile injectable aqueous or oleaginous
suspensions may be formulated according to the known art using suitable
dispersing or
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
wetting agents and suspending agents. The sterile injectable preparation may
also be a sterile
injectable solution, suspension or emulsion in a nontoxic, parenterally
acceptable diluent or
solvent such as a solution in 1,3-butanediol. Among the acceptable vehicles
and solvents that
may be employed are water, Ringer's solution, U.S.P. and isotonic sodium
chloride solution.
In addition, sterile, fixed oils are conventionally employed as a solvent or
suspending
medium. For this purpose any bland fixed oil can be employed including
synthetic mono- or
diglycerides. In addition, fatty acids such as oleic acid are used in the
preparation of
inj ectables.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders,
and granules. In such solid dosage forms, the active compound is mixed with at
least one
inert, pharmaceutically acceptable excipient or carrier such as sodium citrate
or dicalcium
phosphate and/or a) fillers or extenders such as starches, lactose, sucrose,
glucose, mannitol,
and silicic acid; b) binders such as carboxymethylcellulose, alginates,
gelatin,
polyvinylpyrrolidinone, sucrose, and acacia; c) humectants such as glycerol;
d) disintegrating
agents such as agar-agar, calcium carbonate, potato or tapioca starch, alginic
acid, certain
silicates, and sodium carbonate; e) solution retarding agents such as
paraffin; f) absorption
accelerators such as quaternary ammonium compounds; g) wetting agents such as
cetyl
alcohol and glycerol monostearate;) absorbents such as kaolin and bentonite
clay; and i)
lubricants such as talc, calcium stearate, magnesium stearate, solid
polyethylene glycols,
sodium lauryl sulfate, and mixtures thereof. In the case of capsules, tablets
and pills, the
dosage form may also comprise buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and hard-
filled gelatin capsules using such excipients as lactose or milk sugar as well
as high molecular
weight polyethylene glycols and the like.
The solid dosage forms of tablets, dragees, capsules, pills, and granules can
be
prepared with coatings and shells such as enteric coatings and other coatings
well known in
the pharmaceutical formulating art. They may optionally contain opacifying
agents and can
also be of a composition that they release the active ingredients) only, or
preferentially, in a
71
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
certain part of the intestinal tract in a delayed manner. Examples of
embedding compositions
which can be used include polymeric substances and waxes.
Compositions for rectal or vaginal administration are preferably suppositories
which
can be prepared by mixing the compounds of this invention with suitable non-
irritating
excipients or carriers such as cocoa butter, polyethylene glycol or a
suppository wax which
are solid at ambient temperature but liquid at body temperature and therefore
melt in the
rectum or vaginal cavity and release the active compound.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, microemulsions, solutions, suspensions, syrups and elixirs. In
addition to the
active compounds, the liquid dosage forms may contain inert diluents commonly
used in the
art such as, for example, water or other solvents, solubilizing agents and
emulsifiers such as
ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl
alcohol, benzyl
benzoate, propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (in
particular,
cottonseed, groundnut, corn, germ, olive, castor, and sesame oils), glycerol,
tetrahydrofurfuryl
alcohol, polyethylene glycols and fatty acid esters of sorbitan, and mixtures
thereof.
Besides inert diluents, the oral compositions can also include adjuvants such
as
wetting agents, emulsifying and suspending agents, sweetening, flavoring, and
perfuming
agents.
Dosage forms for topical or transdermal administration of a compound of this
invention include ointments, pastes, creams, lotions, gels, powders,
solutions, sprays,
inhalants or patches. The active component is admixed under sterile conditions
with a
pharmaceutically acceptable carrier and any needed preservatives or buffers as
may be
required. Ophthalmic formulation, ear drops, eye ointments, powders and
solutions are also
contemplated as being within the scope of this invention.
The ointments, pastes, creams and gels may contain, in addition to an active
compound of this invention, excipients such as animal and vegetable fats,
oils, waxes,
paraffins, starch, tragacanth, cellulose derivatives, polyethylene glycols,
silicones, bentonites,
silicic acid, talc and zinc oxide, or mixtures thereof.
72
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Powders and sprays can contain, in addition to the compounds of this
invention,
excipients such as lactose, talc, silicic acid, aluminum hydroxide, calcium
silicates and
polyamide powder, or mixtures of these substances. Sprays can additionally
contain
customary propellants such as chlorofluorohydrocarbons.
Compounds of the present invention may also be administered in the form of
liposomes. As is known in the art, liposomes are generally derived from
phospholipids or
other lipid substances. Liposomes are formed by mono- or multi-lamellar
hydrated liquid
crystals that are dispersed in an aqueous medium. Any non-toxic,
physiologically acceptable
and metabolizable lipid capable of forming liposomes may be used. The present
compositions in liposome form may contain, in addition to the compounds of the
present
invention, stabilizers, preservatives, excipients, and the like. The preferred
lipids are the
natural and synthetic phospholipids and phosphatidylcholines (lecithins) used
separately or
together.
Methods to form liposomes are known in the art. See, for example, Prescott,
Ed.,
Methods in Cell Biology, Volume XIV, Academic Press, New York, N. Y., (1976),
p 33 et
seq.
The terms "pharmaceutically acceptable salts, esters and amides," as used
herein, refer
to carboxylate salts, amino acid addition salts, zwitterions, esters and
amides of compounds of
formula (I)-(IV) which are, within the scope of sound medical judgement,
suitable for use in
contact with the tissues of humans and lower animals without undue toxicity,
irritation,
allergic response, and the like, are commensurate with a reasonable
benefit/risk ratio, and are
effective for their intended use.
The compounds of the present invention can be used in the form of
pharmaceutically
acceptable salts derived from inorganic or organic acids. By "pharmaceutically
acceptable
salt" is meant those salts which are, within the scope of sound medical
judgement, suitable for
use in contact with the tissues of humans and lower animals without undue
toxicity, irritation,
allergic response and the like and are commensurate with a reasonable
benefit/risk ratio.
Pharmaceutically acceptable salts are well-known in the art. For example, S.
M. Berge et al.
describe pharmaceutically acceptable salts in detail in J. Pharmaceutical
Sciences, 1977, 66: 1
73
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
et seq. The salts can be prepared in situ during the final isolation and
purification of the
compounds of the invention or separately by reacting a free base function with
a suitable
organic acid. Representative acid addition salts include, but are not limited
to acetate,
adipate, alginate, citrate, aspartate, benzoate, benzenesulfonate, bisulfate,
butyrate,
camphorate, camphorsufonate, digluconate, glycerophosphate, hemisulfate,
heptanoate,
hexanoate, fumarate, hydrochloride, hydrobromide, hydroiodide, 2-
hydroxyethansulfonate
(isethionate), lactate, maleate, methanesulfonate, nicotinate, 2-
naphthalenesulfonate, oxalate,
pamoate, pectinate, persulfate, 3-phenylpropionate, picrate, pivalate,
propionate, succinate,
tartrate, thiocyanate, phosphate, glutamate, bicarbonate, p-toluenesulfonate
and undecanoate.
Also, the basic nitrogen-containing groups can be quaternized with such agents
as lower alkyl
halides such as methyl, ethyl, propyl, and butyl chlorides, bromides and
iodides; dialkyl
sulfates like dimethyl, diethyl, dibutyl and diamyl sulfates; long chain
halides such as decyl,
lauryl, myristyl and stearyl chlorides, bromides and iodides; arylalkyl
halides like benzyl and
phenethyl bromides and others. Water or oil-soluble or dispersible products
are thereby
obtained. Examples of acids which can be employed to form pharmaceutically
acceptable
acid addition salts include such inorganic acids as hydrochloric acid,
hydrobromic acid,
sulphuric acid and phosphoric acid and such organic acids as oxalic acid,
malefic acid,
succinic acid and citric acid.
Basic addition salts can be prepared in situ during the final isolation and
purification
of compounds of this invention by reacting a carboxylic acid-containing moiety
with a
suitable base such as the hydroxide, carbonate or bicarbonate of a
pharmaceutically
acceptable metal cation or with ammonia or an organic primary, secondary or
tertiary amine.
Pharmaceutically acceptable salts include, but are not limited to, cations
based on alkali
metals or alkaline earth metals such as lithium, sodium, potassium, calcium,
magnesium and
aluminum salts and the like and nontoxic quaternary ammonia and amine cations
including
ammonium, tetramethylammonium, tetraethylanunonium, methylamine,
dimethylamine,
trimethylamine, triethylamine, diethylamine, ethylamine and the like. Other
representative
organic amines useful for the formation of base addition salts include
ethylenediamine,
74
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
ethanolamine, diethanolamine, piperidine, piperazine and the like. Preferred
salts of the
compounds of the invention include phosphate, tris and acetate.
The term "pharmaceutically acceptable ester," as used herein, refers to esters
of
compounds of the present invention which hydrolyze in vivo and include those
that break
down readily in the human body to leave the parent compound or a salt thereof.
Examples of
pharmaceutically acceptable, non-toxic esters of the present invention include
Ci-to-C6 alkyl
esters and CS-to-C~ cycloalkyl esters, although C~-to-C4 alkyl esters are
preferred. Esters of
the compounds of formula (I)-(IV) may be prepared according to conventional
methods. For
example, 9-(3,4-dichlorophenyl)-3-(4-carboxyphenyl)-5,6,7,9-
tetrahydropyrazolo[5,1-
b]quinazolin-8(4H)-one can be treated with an acid, such as HCI, in an
alcoholic solvent, such
as methanol, to provide the ester 9-(3,4-dichlorophenyl)-3-(4-
methoxycarbonylphenyl)-
5,6,7,9-tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-one.
The term "pharmaceutically acceptable amide," as used herein, refers to non-
toxic
amides of the present invention derived from ammonia, primary C,-to-C6 alkyl
amines and
secondary C1-to-C6 dialkyl amines. In the case of secondary amines, the amine
may also be
in the form of a 5- or 6-membered heterocycle containing one nitrogen atom.
Amides derived
from ammonia, C,-to-C3 alkyl primary amides and C,-to-C2 dialkyl secondary
amides are
preferred. Amides of the compounds of formula (I)-(IV) may be prepared
according to
conventional methods. For example, 9-(3,4-dichlorophenyl)-3-(4-carboxyphenyl)-
5,6,7,9-
tetrahydropyrazolo[5,1-b]quinazolin-8(4H)-one can be treated with a
chloroformate, such as
isobutylchloroformate, in an organic solvent, such as tetrahydrofuran or
methylene chloride at
a temperature of about 0 °C to ambient temperature, to provide an
intermediate anhydride
which can then be treated with an amine, such as dimethylamine, to provide 9-
(3,4-
dichlorophenyl)-3-(4-dimethylaminocarbonylphenyl)-5,6,7,9-tetrahydropyrazolo
[5,1-
b]quinazolin-8(4H)-one. It is further intended that amides of the present
invention include
amino acid and peptide derivatives of the compounds of formula (I), as well.
The term "pharmaceutically acceptable prodrug" or "prodrug,"as used herein,
represents those prodrugs of the compounds of the present invention which are,
within the
scope of sound medical judgement, suitable for use in contact with the tissues
of humans and
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
lower animals without undue toxicity, irritation, allergic response, and the
like, commensurate
with a reasonable benefit/risk ratio, and effective for their intended use.
Prodrugs of the
present invention may be rapidly transformed in vivo to the parent compound of
the above
formula, for example, by hydrolysis in blood. A thorough discussion is
provided in T.
Higuchi and V. Stella, Pro-drugs as Novel Delivery Systems, V. 14 of the
A.C.S. Symposium
Series, and in Edward B. Roche, ed., Bioreversible Carriers in Drug Design,
American
Pharmaceutical Association and Pergamon Press (1987), hereby incorporated by
reference.
Dosage forms for topical administration of a compound of this invention
include
powders, sprays, ointments and inhalants. The active compound is mixed under
sterile .
conditions with a pharmaceutically acceptable carrier and any needed
preservatives, buffers or
propellants which can be required. Opthalmic formulations, eye ointments,
powders and
solutions are also contemplated as being within the scope of this invention.
Actual dosage levels of active ingredients in the pharmaceutical compositions
of this
invention can be varied so as to obtain an amount of the active compounds)
which is
effective to achieve the desired therapeutic response for a particular
patient, compositions and
mode of administration. The selected dosage level will depend upon the
activity of the
particular compound, the route of administration, the severity of the
condition being treated
and the condition and prior medical history of the patient being treated.
However, it is within
the skill of the art to start doses of the compound at levels lower than
required for to achieve
the desired therapeutic effect and to gradually increase the dosage until the
desired effect is
achieved.
The present invention contemplates pharmaceutically active compounds either
chemically synthesized or formed by in vivo biotransformation to compounds of
formula (I-
(IV).
The compounds of the invention, including but not limited to those specified
in the
examples, possess potassium channel opening activity in mammals (especially
humans). As
potassium channel openers, the compounds of the present invention may be
useful for the
treatment and prevention of diseases such as asthma, epilepsy, male sexual
dysfunction,
female sexual dysfunction, pain, bladder overactivity, stroke, diseases
associated with
76
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
decreased skeletal blood flow such as Raynaud's phenomenon and intermittent
claudication,
eating disorders, functional bowel disorders, neurodegeneration, benign
prostatic hyperplasia
(BPH), dysmenorrhea, premature labor, alopecia, cardioprotection, coronary
artery disease,
angina and ischemia.
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat bladder overactivity, sensations of
incontinence
urgency, urinary incontinence, pollakiuria, bladder instability, nocturia,
bladder hyerreflexia,
and enuresis may be demonstrated by (Resnick, The Lancet (1995) 346, 94-99;
Hampel,
Urology (1997) 50 (Suppl 6A), 4-14; Bosch, BJU International (1999) 83 (Suppl
2), 7-9;
Andersson, Urology (1997) 50 (Suppl 6A), 74-84; Lawson, Pharmacol. Ther.,
(1996) 70, 39-
63; Nurse., Br. J. Urol., (1991) 68, 27-31; Howe, J. Pharmacol. Exp. Ther.,
(1995) 274, 884-
890; Gopalakrishnan, Drug Development Research, (1993) 28, 95-127).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat male sexual dysfunction such as male
erectile
dysfunction, impotence and premature ejaculation may be demonstrated by
(Andersson,
Pharmacological Reviews (1993) 45, 253; Lee, Int. J. Impot. Res. (1999)
11(4),179-188;
Andersson, Pharmacological Reviews (1993) 45,253; Lawson, Pharmacol. Ther.,
(1996) 70,
39-63, Vick, J. Urol. (2000) 163: 202).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat female sexual dysfunction such as
clitoral erectile
insufficiency, vaginismus and vaginal engorgement may be demonstrated by (J.J.
Kim, J.W.
Yu, J.G. Lee, D.G. Moon, "Effects of topical K-ATP channel opener solution on
clitoral
blood flow", J. Urol. (2000) 163 (4): 240; I. Goldstein and J.R. Berman.,
"Vasculogenic
female sexual dysfunction: vaginal engorgement and clitoral erectile
insufficiency
syndromes"., Int. J. Impotence Res. (1998) 10:584-S90).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat benign prostatic hyperplasia (BPH)
may be
demonstrated by (Pandita, The J. of Urology (1999) 162, 943; Andersson;
Prostate (1997) 30:
202-215).
77
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat premature labor and dysmenorrhoea
may be
demonstrated by (Sanborn, Semin. Perinatol. (1995) 19, 31-40; Morrison, Am. J.
Obstet.
Gynecol. (1993) 169(5), 1277-85; Kostrzewska, Acta Obstet. Gynecol. Scand.
(1996) 75(10),
886-91; Lawson, Pharmacol. Ther., (1996) 70, 39-63).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat functional bowel disorders such as
irritable bowel
syndrome may be demonstrated by (Lawson, Pharmacol. Ther., (1996) 70, 39-63).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat asthma and airways hyperreactivity
may be
demonstrated by (Lawson, Pharmacol. Ther., (1996) 70, 39-63; Buchheit,
Pulmonary
Pharmacology & Therapeutics (1999) I2, I03; Gopalakrishnan, Drug Development
Research,
( 1993) 28, 95-127).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat various pain states including but
not limited to
migraine and dyspareunia may be demonstrated by (Rodrigues, Br. J. Pharmacol.
(2000)
129(1), 110-4; Vergoni, Life Sci. (1992) 50(16), PL135-8; Asano, Anesth.
Analg. (2000)
90(5), 1146-51; Lawson, Pharmacol. Ther., (1996) 70, 39-63; Gopalakrishnan,
Drug
Development Research, (1993) 28, 95-127; Gehlert, Prog. Neuro-Psychopharmacol.
& Biol.
Psychiat., (1994) 18, 1093-I 102).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat epilepsy may be demonstrated by
(Lawson,
Pharmacol. Ther., (1996) 70, 39-63; Gopalakrishnan, Drug Development Research,
(1993) 28,
95-127; Gehlert, Prog. Neuro-Psychopharmacol & Biol. Psychiat., (1994) 18,
1093-1102).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat neurodegenerative conditions and
diseases such as
cerebral ischemia, stroke, Alzheimer's disease and Parkinson's disease may be
demonstrated
by (Lawson, Pharmacol. Ther., (1996) 70, 39-63; Gopalakrishnan, Drug
Development
78
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
Research, (1993) 28, 95-127; Gehlert, Prog. Neuro-Psychopharmacol. & Biol.
Psychiat.,
(1994) 18, 1093-1102; Freedman, The Neuroscientist (1996) 2, 145).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat diseases or conditions associated
with decreased
skeletal muscle blood flow such as Raynaud's syndrome and intermittent
claudication may be
demonstrated by (Lawson, Pharmacol. Ther., (1996) 70, 39-63; Gopalakrishnan,
Drug
Development Research, (1993) 28, 95-127; Dompeling Vasa. Supplementum (1992)
3434;
W09932495).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat eating disorders such as obesity may
be demonstrated
by (Spanswick, Nature, (1997) 390, 521-25; Freedman, The Neuroscientist (1996)
2, 145).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat alopecia may be demonstrated by
(Lawson,
Pharmacol. Ther., (1996) 70, 39-63; Gopalakrishnan, Drug Development Research,
(1993) 28,
95-127).
The ability of the compounds of the.present invention, including but not
limited to
those specified in the examples, to treat myocardial injury during ischemia
and reperfusion
may be demonstrated by (Garlid, Circ Res (1997) 81(6), 1072-82; Lawson,
Pharmacol. Ther.,
(1996) 70, 39-63; Grover, J. Mol. Cell Cardiol. (2000) 32, 677).
The ability of the compounds of the present invention, including but not
limited to
those specified in the examples, to treat coronary artery disease may be
demonstrated by
(Lawson, Pharmacol. Ther., (1996) 70, 39-63, Gopalakrishnan, Drug Development
Research,
(1993) 28, 95-127).
Aqueous liquid compositions of the present invention are particularly useful
for the
treatment and prevention of asthma, epilepsy, hypertension, Raynaud's
syndrome, male sexual
dysfunction, female sexual dysfunction, migraine, pain, eating disorders,
urinary
incontinence, functional bowel disorders, neurodegeneration and stroke.
When used in the above or other treatments, a therapeutically effective amount
of one
of the compounds of the present invention can be employed in pure form or,
where such
79
CA 02391291 2002-05-10
WO 01/36422 PCT/US00/32333
forms exist, in pharmaceutically acceptable salt, ester, amide or prodrug
form. Alternatively,
the compound can be administered as a pharmaceutical composition containing
the
compound of interest in combination with one or more pharmaceutically
acceptable
excipients. The phrase "therapeutically effective amount" of the compound of
the invention
means a sufficient amount of the compound to treat disorders, at a reasonable
benefit/risk
ratio applicable to any medical treatment. It will be understood, however,
that the total daily
usage of the compounds and compositions of the present invention will be
decided by the
attending physician within the scope of sound medical judgement. The specific
therapeutically effective dose level for any particular patient will depend
upon a variety of
factors including the disorder being treated and the severity of the disorder;
activity of the
specific compound employed; the specific composition employed; the age, body
weight,
general health, sex and diet of the patient; the time of administration, route
of administration,
and rate of excretion of the specific compound employed; the duration of the
treatment; drugs
used in combination or coincidental with the specific compound employed; and
like factors
well known in the medical arts. For example, it is well within the skill of
the art to start doses
of the compound at levels lower than required to achieve the desired
therapeutic effect and to
gradually increase the dosage until the desired effect is achieved.
The total daily dose of the compounds of this invention administered to a
human or
lower animal may range from about 0.003 to about 10 mg/kg/day. For purposes of
oral
administration, more preferable doses can be in the range of from about 0.01
to about 5
mg/kg/day. If desired, the effective daily dose can be divided into multiple
doses for purposes
of administration; consequently, single dose compositions may contain such
amounts or
submultiples thereof to make up the daily dose.