Note: Descriptions are shown in the official language in which they were submitted.
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
DIRECT vLETHANOL CELL
WITH CIRCULATING ELECTROLYTE
BACKGROUND OF THE INVENTION
1. FIELD OF THE INVENTION
The present invention relates to fuel cell systems and, more particularly, to
fuel cell
systems having reduced reactant cross-over.
2. DESCRIPTION OF THE PRIOR ART
Due to the increasing demands for inexpensive, efficient and non-polluting
energy
sources, various alternatives have been pursued. One of such alternative
energy sources is
the electrochemical fuel cell. Such fuel cells convert a generally commonly
available fuel
and an oxidant to electricity leaving relatively safe by-products. A typical
fuel cell includes,
in addition to the fuel and oxidant, tvo generally planar electrodes (an anode
and a cathode),
and an electrolyte. Generally, the electrolyte is provided between the cathode
and the anode.
The electrodes are normally formed of a porous substrate that allows the fuel
and oxidant to
diffuse through and are usually covered on opposing surfaces with a catalyst
for the
respective reduction and oxidation (redox) reactions.
The redox reactions result in the production of protons and electrons at the
anode.
The electrodes are electrically connected, through an external load, so as to
provide a path for
the electrons generated by the redox reactions. To accommodate the flow of
protons from the
anode to the cathode, the cells are normally provided with an ion, or more
specifically, a
proton exchange membrane between the electrodes.,
In use, the fuel is passed through the porous anode substrate until it
contacts the
oxidation catalyst layer where it is oxidized. At the cathode, the oxidant
diffuses through the
porous cathode substrate and is reduced at the reduction catalyst layer: The
fuels and
oxidants for these cells are provided in a fluid state and consist of gases or
liquids. Examples
of fuels that can be used in fuel cells are hydrogen and lower alcohols such
as methanol. The
s0 oxidant is usually oxygen that can he supplied either as pure oxygen or as
air.
In the case of a hydro~~en fu:l cell. the fuel. hvdro~en, is providcd in a
~ascous state
and the following reactions take place:
SUBSTITUTE SHEET (RULE 26)
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
Anode: HZ -> 2H+ + 2e-
Cathode: %Z02 + 2H+ + 2e -~ HZO
As mentioned previously, the above oxidation reaction at the anode results in
the
production of protons and electrons. The electrons and conducted from the
anode to the
cathode by means of an electrical connection. The protons migrate from the
anode to the
cathode through the proton exchange membrane to react with the oxygen to form
water.
Fuel cells can be categorized as "indirect" or "direct". In the case of
indirect fuel
cells, the fuel, usually a lower alcohol, is first processed, or reformed,
before it is introduced
into the cell. With direct fuel cells, the fuel is not pre-processed, thereby
simplifying system.
In the case of a direct methanol fuel cell, the following reactions occur:
Anode: CH30H + HZO ---~ 6H+ + COZ + 6e-
Cathode: 1'/202 + 6H+ + 6e -~ 3H20
For the direct methanol fuel cell, the flow of protons and electrons are the
same as
that for the hydrogen fuel cell discussed above. The methanol fuel is provided
in a either a
liquid or vapour state. It is known that other types of fuels may be utilized
in such direct fuel
cells. Such fuels may include, by way of example, other simple alcohols, such
as ethanol,
dimethoxymethane, trimethoxymethane, and formic acid. Further, the oxidant may
be
provided in the form of an organic fluid having a high oxygen concentration or
hydrogen
peroxide solution, for example. Such direct methanol fuel cells are taught in
following US
Patents: 5,672,439; 5,874,182; and, 5,958,616.
The electrolyte used in fuel cells may be either liquid or solid. In the case
of a solid
electrolyte, the proton exchange membrane may also serve as a polymer
electrolyte
membrane (PEM), thereby providing two functions. As taught in US Patent
5,958,616, such
PEM's may comprise a hydrated sheet of a perfluorinated ion exchange membrane
such as a
polyperfluorosulfonic acid membrane, sold under the tradename NAFIONOO (E.I.
du Pont de
Nemours and Co.).
In any of the fuel cells mentioned above, it is important to maintain a
separation
between the anode and the cathode so as to prevent fuel from directly
contacting the cathode
and oxidizing thereon. For this reason, the proton exchange membrane must also
function as
a separator for the fuel and oxidant. However, the known membranes, although
functioning
well as proton exchangers and/or solid electrolytes, are not very efficient as
fuel separators
and a common problem in fuel cells is the incidence of fuel cross over, which
occurs when
-2-
SUBSTITUTE SHEET (RULE 26)
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
the fuel, prior to oxidation, diffuses through the membrane and contacts the
cathode. Apart
from the parasitic loss of fuel and oxidant from the system, such cross over
results in a short
circuit in the cell since the electrons resulting from the oxidation reaction
do not follow the
current path between the electrodes. Further, other disadvantages of fuel
cross over may
include structural changes on the cathode surface (i.e. sintering etc.) and
poisoning of the
reduction catalyst by fuel oxidation products.
One method of addressing this issue is to decrease the porosity of the
membrane
thereby preventing any fuel from crossing over. However, with this solution,
the flow of
proton will also be impeded, thereby resulting in decreased conductivity of
the cell and,
therefore, lower performance. As known in the art, fuel cell performance is
defined as the
voltage output from the cell at a given current density (or vice versa); thus,
the higher the
voltage at a given current density or the higher the current density at a
given voltage, the
better the performance.
The above mentioned US patents provide various solutions to the problem of
fuel
cross over in fuel cells. In each case, the solution provided lies in
improvements to the PEM.
For example, US Patents 5,672,439 and 5,874,182 teach novel PEM's having
essentially a
laminated structure wherein the PEM is provided with one or more layers of an
oxidation
catalyst for oxidizing any fuel that may diffuse through. US Patent 5,958,616
provides a
PEM having a plurality of voids for sequestering any fuel that may be passing
there-through.
However, such membranes are more expensive thereby adding to the cost of the
cell.
Another problem associated with PEM containing cells is that the membrane must
be
maintained in a hydrated state in order to function as a proton exchanger and
as an
electrolyte. This requires, therefore, a separate hydration system to ensure
that the membrane
does not dry out.
Thus, there exists a need for an improved fuel cell system that overcomes the
above
mentioned problem of fuel cross over as well as other deficiencies in the
known systems.
SUMMARY OF THE INVENTION
-3-
SUBSTITUTE SHEET (RULE 26)
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
BRIEF DESCRIPTION OF THE DRAWINGS
These and other features of the preferred embodiments of the invention will
become
more apparent in the following detailed description in which reference is made
to the
appended drawings wherein:
Figure 1 is an exploded side cross sectional views of a direct methanol fuel
cell
according to one embodiment of the invention.
Figures 2 to 6 are side cross sectional views of a direct methanol fuel cell
according to
other embodiments of the invention.
Figure 7 is a schematic illustration of a direct methanol fuel cell system
according to
one embodiment of the invention.
Figure 8 is a graph illustrating the Open Current Voltage (OCV) of a fuel cell
while in
operation.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
In general terms, the present invention provides a fuel cell wherein any un-
reacted
fuel is purged from the system so as to reduce or eliminate any fuel cross
over. As used
herein, the term "fuel cross over" is intended to mean the un-desired flow of
un-reacted fuel
from the anode to the cathode.
In a preferred embodiment, the invention provides a fuel cell having a
circulating
electrolyte that flows between the electrodes (the anode and the cathode) of
the cell and
which serves to remove any un-oxidized fuel that diffuses through the anode.
In this manner,
un-reacted fuel is removed from the fuel cell before it reaches the cathode,
thereby avoiding
fuel cross over.
In one embodiment, the fuel cell of the invention allows any un-reacted fuel
to be
recycled back to the cell.
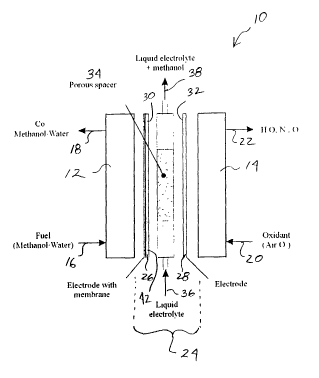
A direct methanol fuel cell according to one aspect of the invention is
illustrated in
Figure 1. As shown, the fuel cell 10 essentially consists of a planar
"sandwich" having, as its
outer surfaces, two end plates 12 and 14. The end plates may be formed as
commonly known
and may comprise materials such as polysulphon or other materials as will be
known to
persons skilled in the art. First end plate 12 is provided with a fuel inlet
16 and an outlet 18
for releasing un-reacted fuel and reaction products. Similarly, second end
plate 14 is
provided with an oxidant inlet 20 and an outlet 22 for un-reacted oxidant and
reaction
-4-
SUBSTITUTE SHEET (RULE 26)
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
products. The space between plates 12 and 14 essentially comprises the
reaction chamber 24
of the fuel cell.
The reaction chamber 24 includes a pair of generally porous electrodes
comprising an
anode 26 and a cathode 28 having opposing surfaces 30 and 32, respectively.
The electrodes
generally comprise sheets that are parallel to the plates 12 and 14. The
electrodes may be
made in any conventionally known manner and are formed of a porous material so
as to allow
the reactants to pass through. For example, electrodes for the present
invention may be
formed from a base of carbon cloth, or carbon fibre paper, having sprayed
thereon,
NAFIONO and/or E-TEK. Other electrode materials will be apparent to persons
skilled in
the art. For example, various porous carbon materials have been used to form
electrodes for
phosphoric acid fuel cells and such electrodes can be used, for example, in
direct methanol
fuel cells as well. Typically, the porous carbon electrodes are
polytetrafluroethylene (PTFE)
bonded and have carbon sheets or carbon fleece as a base structure. Corrosion
resistant
stainless steel foams can also be used as an the base structure.
Although not shown in Figure 1, the electrodes are electrically connected as
known in
the art to conduct the flow of electrons generated in the cell.
Each of the opposing surfaces 30 and 32 of the electrodes are provided with a
thin
catalyst layer (not shown) for catalyzing the oxidation and reduction
reactions of the cell.
The catalysts that are used in the invention may be any of those commonly
known such as
platinum (Pt), or a Pt and Ruthenium (Ru) combination. Various other catalysts
for the fuel
cell, such as carbon black, other noble metals etc., will be apparent to
persons skilled in the
art.
In the embodiment shown in Figure 1, the surface 30 of the anode 26 is
provided with
a proton exchange membrane 40. The membrane 40 preferably comprises a polymer
electrolyte membrane (PEM) as described above. In the preferred embodiment,
the polymer
electrolyte is acidic so as to act as an efficient hydrogen ion conductor and
also to neutralize
any COZ produced during the course of the reaction. In other embodiments, the
membrane
may be of any other commonly known material such as Gore-Tex~ etc.
A medium 34 is provided between the electrodes 26 and 28, through which an
electrolyte is flowed. In one embodiment, as shown in Figure 1, the medium 34
comprises a
porous spacer material positioned between the electrodes. The medium includes
an
electrolyte inlet 36 and an outlet 38 for the electrolyte and any reaction
components entrained
-5-
SUBSTITUTE SHEET (RULE 26)
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
therein. The electrolyte used in the preferred embodiment is an acidic
solution and more
preferably, comprises a solution of sulphuric acid.
In operation, the fuel is provided to the cell 10 via anode inlet 16 and,
after the
oxidation reaction, the resulting products and any un-reacted fuel is expelled
from the system
through outlet 18. Similarly, the oxidant for the reaction is introduced
through cathode inlet
20 and the products from the reduction reaction are expelled through cathode
outlet 22. The
fuel diffuses through the porous anode 26 and is oxidized at the catalyst
layer contained on
anode surface 30. A proton exchange membrane 42 provided on surface 30 aids in
conducting the protons towards the cathode. An electrical connection (not
shown) conducts
the electrons from the anode towards the cathode and through an external load.
However,
along with the protons generated by the oxidation reaction, a portion of any
un-reacted fuel,
and a portion of the reaction products may pass through the anode 26 and the
membrane 42
and enter the medium 34 containing a fluid electrolyte stream (not shown). The
electrolyte
enters the medium via inlet 36 and exits at outlet 38. In passing through the
medium 34, the
1 S electrolyte entrains any un-reacted fuel as well as any reaction products,
such as CO2. In this
manner, the electrolyte stream contained in medium 34 removes any potentially
damaging
products and reactants from the fuel cell system thereby maintaining the
performance of the
cell. However, being acidic, the fluid electrolyte does not impede the flow of
protons
between the anode and the cathode.
Figure 2 illustrates another embodiment of the invention and shows the cell of
Figure
1 in an assembled state and wherein like numerals are used to identify like
elements. In the
cell 10a Figure 2, the fluid electrolyte is not flowed through a medium but
consists solely of
an electrolyte stream. However, such cell functions is the same manner as the
cell of Figure
1. Figure 2 also more clearly illustrates the electrical connection between
the electrodes 26
and 28. Specifically, the anode 26 is connected to an external load 44 by
means of a first
conductor 46. Similarly, the load 44 is connected to the cathode 28 by a
second conductor
48. Figure 2 also illustrates the use of a commonly known matrix 50 instead of
an electrolyte
membrane as in Figure 1.
Figure 3 illustrates yet another embodiment of the fuel cell of the invention,
wherein
elements common with Figure 1 are identified with like numerals. In the cell l
Ob of Figure
3, the anode 26 is provided with a PEM 42 as in Figure 1. However, in this
case, the cathode
28 is also provided with a coating 52 comprising a TeflonOO material. As
illustrated, the cell
-6-
SUBSTITUTE SHEET (RULE 26)
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
l Ob of Figure 3 includes a counter-current flow of oxidant with respect to
fuel. The acid
electrolyte follows the same direction as that of the fuel. The embodiment
shown in Figure 3
also illustrates the use of a screen mesh 53 as the fluid electrolyte medium
instead of the
porous spacer 34 of Figure 1.
S Figure 4 illustrates yet another embodiment of the fuel cell of the
invention. In this
case, the cell l Oc is of a similar structure as that of Figure 3. As in
Figure 1, the anode
surface 30 is provided with a PEM. However, in this case, the cathode surface
54 facing the
anode 26 is also provided with a PEM 56. As also illustrated in Figure 4, the
medium
through which the fluid electrolyte is passed comprises porous carbon material
58.
Figure 5 illustrates another embodiment of the invention wherein the cell l Od
is
generally of the same structure as that of Figure 4. However, in this case,
the plate 12 of the
anode side of the cell is not provided with outlet for the oxidation reaction
products. Instead,
such products, including and un-reacted fuel, is diverted to the fluid
electrolyte stream and
exits at a common outlet 60. Further the anode 62 of the cell of Figure 5
comprises a two-
phase electrode made of a porous carbon base and including fibre graphite and
a Pt/Ru
catalyst. As with Figure 4, the cathode is provided with a PEM 56.
Figure 6 illustrates yet another embodiment wherein the cell 10e comprises
generally
the cell of Figure S with some modifications. Firstly, the cell 10e is
provided with a fluid
electrolyte medium that comprises a dual channel conduit 64, which serves to
reduce fuel
cross over in two consecutive stages. Further, the anode 66 comprises another
two phase
structure comprising a gold plated screen with the desired catalyst.
Figure 7 illustrates a schematic representation of the process of the
invention. As can
be seen, fresh fuel, which, in the embodiment illustrated is methanol, is
provided to the
system 100 at inlet 102. Fresh oxidant, such as air, is provided to the system
at inlet 104.
The fuel is passed to a mixing tank 106, which will be discussed later,
through an inlet 108.
The outlet 110 of the mixing tank is fed to an inlet 112 of the cell 114. The
cell 114 includes
an outlet 116 for expelling the reaction products from the oxidation reaction.
Such products
are fed into a separator 118, which separates out any un-reacted fuel and
diverts same to the
mixing tank 106 where it is mixed with freshly supplied fuel. A vent 120
provided on the
separator 118 expels any reaction products (i.e. air, water, COZ) from the
system.
In the cell, which is of any of the designs mentioned above, the fuel is
oxidized to
produce a proton and electron stream. The proton stream is diverted to the
cathode where the
SUBSTITUTE SHEET (RULE 26)
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
reduction reaction takes place. The electrons generated in the oxidation
reaction are
conducted from the anode to the cathode through an external load 111 via
conductors 113 and
115. As discussed above, the invention provides the fuel cell with a
circulating electrolyte to
prevent any fuel cross over. As illustrated in Figure 7, the electrolyte is
provided from a
S storage tank 122 and is fed into the cell via inlet 124. The flowing
electrolyte collects any
un-reacted fuel and other reaction products and exits the cell through outlet
126. The
electrolyte stream is then fed to a separator 128, which separates the
electrolyte from the
reaction products and supplies re-generated electrolyte back to the storage
tank 122. The
separator also regenerates un-reacted fuel and returns same to the fresh fuel
inlet stream.
Apart from above mentioned advantages, further advantages of the present
invention
include: improved cell heat dissipation; hydration of the PEM; removal of
unwanted reaction
products (e.g. C02). Further with the invention, any lost catalyst may also be
recovered.
Examples
The following examples are used to illustrate the present invention and should
not be
considered to limit same in any way.
1. Manufacture of PEM:
For our investigations we used NAFION plus E-TEK electrodes (Single sided ELAT
electrode 4 mg/cm2 Pt/Ru). Form the literature you get a very good idea of how
to make own
electrodes and how to prepare them properly. The base material often is a
carbon cloth
(35mm)[10] with Vulcan XC72 (30%PTFE, 20-30 pm) on both sides. As catalyst (30-
40%PTFE) 20%Pt on Vulcan XC 72 diluted with XC72 is used. At the end NAFION
solution
is sprayed on the surface (M=1100kg/kmol, -~-SOA, 0-2,7 mg/cm2 dry weight)
which should
diffuse for 10 min and dry for app. 2h at 80°C.
One major point concerns the preparation of NAFION. Before NAFION can be used
several
steps of preparation have been done namely boiling in
~ 3% H202
~ deionised water
~ 0.5 M H2S04
~ deionised water
_g_
SUBSTITUTE SHEET (RULE 26)
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
for over one hour each [10]. Afterwards the NAFION membranes have to be pre-
dried (45
min. on a 60°C heated vacuum table. Then the catalyst layer has to be
hot pressed onto the
membrane at 125°C and lOSatm for 120s [9] (140°C for 3 minutes
[1]). The assembly has to
be sandwiched between two uncatalysed carbon-cloth gas-diffusion backings (E-
TEK). The
parameters for pressing are
~ temperature of app.140°C
~ pressure of app.1000kg/cm2
~ for 3min
2. Electrodes
The used electrodes have been ordered by E-TEK. The EFCG electrode on TGPH-
120 Toray Carbon Paper has a loading of 4mg/cm2 Pt/Ru. The ordered area is
23*23cm.
3. Test Results
The first built system ran with hydrogen and oxygen using a 0.5 M H2S04
electrolyte
between the anode and cathode. We did not heat the system up, so the
temperature was app.
20°C. Graph 1 shows the recorded voltage/current-density curve. Because
of our limited test
equipment we were only able to go up to 2.5 A which corresponds to 550 mA/cm2.
This
system works fine and provides S50 mA/cm2 @ 0.35 V.
0,9 -
0 , g ._ __-
07
0,6
0,5
0,4
0,3
02
01
0,0 200,0 400,0 600,0
[mA/cm2]
-9-
SUBSTITUTE SHEET (RULE 26)
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
Graph 1: Voltage/Current density curve with hydrogen and oxygen at 20°C
without pressure
and 0.5 M H2S04 as electrolyte.
The next step was to record U-I curves with the same system setup but air
instead of
pure oxygen. Graph 2 shows the graph and we reached only 300mA/cmz @ 0.1 V.
The
assumption is that a system running with air instead of pure oxygen has to be
made
pressurized.
__. .._._ _. -.
0,7
0,6
0,5
0,4 '
0,3
0,2
0,1
0
0,0 100,0 200,0 300,0 400,0
[mA/cm2]
Graph 2: Voltage/Current density curve with hydrogen and air at 20°C
and pressure less
In order to have best conditions for first tests with methanol pure oxygen was
used
again. The system setup stays the same, but a new feeding system for methanol
as fuel and
for the circulating electrolyte has been introduced.
The first experiments did not lead to any promising results because there was
a
leakage problem at the anode side. The first used material (kind of neoprene)
was to porous.
So a special sealing gel (from the automotive sector) which is resistant again
water alcohol
solutions and high temperatures has been used. The good thing is that it
remains plastic and
therefore the cell can be opened again without any efforts. In order to avoid
contact problems
the O-ring sealing at the anode and cathode have been removed and this special
sealing gel
- 10-
SUBSTITUTE SHEET (RULE 26)
CA 02391398 2002-05-22
WO 01/39307 PCT/CA00/01376
has been used. This arrangement makes also sure that there is enough contact
between the
electrode and the carbon contact plate.
I measured the OCV which lied between 0.7 and 0.8. The recording of U-I curves
failures because the cell voltage broke down under load.
S The next step was to keep this system but to increase the temperature to
50,60, 90 °C.
The results became better, always making first tests with a little fan. The
problem is that it
still was not possible to record curves because the voltage falls very rapidly
under load (even
when measuring resistance-free). Because of the boiling point at 64°C
of methanol I stayed at
a temperature of 60°C.
The change of the molarity of the electrolyte was the next step. So mixtures
of 0.5, 1,
5 and 10 M H2S04 have been tried out. The improvement was very little so the
conclusion
was that this influence is negligible.
Mixtures of 1, 2, S and 10 M MeOH I even put in pure methanol but I did not
get
improvements.
Because we are circulating the electrolyte it is possible to run with higher
methanol
concentrations.
The next step was to build a vapor feed system. We thought that the problem
could be
that no methanol comes to the fine pores when putting a load on the cell
because those
electrodes are gas diffusion electrodes. The temperature in the test rig was
>90°C. We also
did not get results because the OCV only reached app. 0.35 V and the cell did
not even
manage to power the fan.
All those experiments have been made without pressure and so the next step
will be to
build a system where the pressure can be changed.
Although the invention has been described with reference to certain specific
embodiments, various modifications thereof will be apparent to those skilled
in the art
without departing from the spirit and scope of the invention as outlined in
the claims
appended hereto.
-11-
SUBSTITUTE SHEET (RULE 26)
08-03-2002 CA 02391398 2002-05-22
CA0001376
As described above, the present invention provides a fuel cell system for the
electrochemical production of electricity from liquid and gaseous fuels on the
anodic side and
oxygen and air on the cathodic side, whereby the electrode reactions are
happening in catalyst
regions (interfaces) contained in porous electrodes and the reaction products
are continuously
removed in circulating gas streams which also provide new gas supply and in a
circulating
electrolyte which serves also as a heat managing liquid stream, thereby
characterized, that the
speed of electrolyte circulation determines the build-up of the fuel or
reactant cross-over
gradient in the cell and the removed methanol is reclaimed in a distillation
loop.
In the fuel cell system of the invention, separators or matrix may be attached
to the
electrodes to reduce the methanol outflow (at the anode) or minimize the
reaction of the
methanol on the air-cathode. Further, one of the separators (on the anode) can
be of the PE-
Membrane type. The matrix or separator barriers may be chosen from microporous
materials
like asbestos.
In the fuel cell system of the invention, the circulating electrolyte is a
good
conductive salt solution selected from the group of battery electrolytes with
a pH of neutral to
low acidic values. Examples of such electrolytes include KSCN or NH4SCN,
acidified
K2S04, or selected strong organic acids (Superacids).
With fuel cell systems according to one embodiment of the invention, the
temperature
of the cell is high enough to allow a methanol distillation recovery loop
(over 70 deg.C.).
Further, the fuel feed can be as an aqueous solution of methanol or as
methanol
vapour. The fuel feed can be such that the concentration of the methanol (% in
water or
methanol gas vapor pressure) can be increased to give a higher anode voltage
simultaneous
with the adjustment of the methanol barriers and the speed of electrolyte
circulation which
reduce the crossover which will then tend to increase.
With fuel cell systems according to one embodiment, the electrodes can be
porous all-carbon
electrodes (the baked carbon type) in tubular or plate shape, carrying the
proper catalysts for
the anode and cathode reactions. Further, the electrodes can be of the type
used for PAFC
systems, sprayed or layered PTFE bonded porous carbon layers on a woven carbon
(graphite)
sheet or carbon fleece or carbon fiber carrier. The electrodes can be
stainless steel screen
supported plate (foil) structures layered with mixtures of activated carbon
and suitable
catalyst and fillers which are pore-formers (e.g. bicarbonates) or repellent
binders (e.g. PTFE
or PE.). In one embodiment, a CARBON/ PTFE/NAFION mix is used to produce the
anodes
of the DMFC, whereby the carrier is stainless steel wool.
12
AMENDED SHEET