Note: Descriptions are shown in the official language in which they were submitted.
WO 01/39300 CA 02391501 2002-05-14 pCT/CA00/01309
IMPROVED CATHODE STRUCTURES FOR
DIRECT LIQUID FEED FUEL CELLS
Field Of The Invention
The present invention relates to cathode structures for
solid polymer fuel cells operating directly on a liquid
fuel stream in which the fuel is directly oxidized at the
anode. In particular, it relates to cathode structures
that provide improved performance in direct methanol fuel
cells.
Background Of The Invention
Electrochemical fuel cells convert reactants, namely
fuel and oxidants, to generate electric power and reaction
products. Electrochemical fuel cells generally employ an
electrolyte disposed between two electrodes, namely a
cathode and an anode. An electrocatalyst is needed to
induce the desired electrochemical reactions at the
electrodes. Solid polymer fuel cells operate in a range
from about 80EC to about 200EC and are particularly
preferred for portable and motive applications. Solid
polymer fuel cells employ a membrane electrode assembly
("MEA") which comprises a solid polymer electrolyte or
ion-exchange membrane disposed between the two electrode
layers. Flow field plates for directing the reactants
across one surface of each electrode are generally
disposed on each side of the MEA. The electrocatalyst used
may be a metal black, an alloy or a supported metal
catalyst, for example, platinum on carbon. The
electrocatalyst is typically incorporated at the
electrode/electrolyte interfaces.
A broad range of reactants have been contemplated for
use in solid polymer fuel cells and such reactants may be
delivered in gaseous or liquid streams. The oxidant stream
may, for example, be substantially pure oxygen but
preferably air, a dilute oxygen stream, is employed. The
fuel stream may be substantially pure hydrogen gas, a
WO 01/39300 CA 02391501 2002-05-14 pCT/CA00/01309
-2-
gaseous hydrogen-containing reformate stream derived from
a suitable feedstock, or a suitable gaseous or liquid
organic fuel mixture. A fuel cell operating on a liquid
fuel stream in which the fuel is reacted electrochemically
S at the anode (directly oxidized) is known as a direct
liquid feed fuel cell.
A direct methanol fuel cell (DMFC) is a type of fuel
cell in which methanol is directly oxidized at the anode.
Although it may be operated on aqueous methanol vapour, a
DMFC generally operates in a liquid feed mode on an
aqueous methanol fuel solution. There is often a problem
in DMFCs with substantial crossover of methanol fuel from
the anode to the cathode side through the membrane
electrolyte. The methanol that crosses over may react with
oxidant at the cathode and then cannot be recovered,
resulting in significant fuel inefficiency and
deterioration in fuel cell performance. To reduce
crossover, very dilute solutions of methanol (for example
about 5o methanol in water) are typically used as fuel
streams in liquid feed DMFCs. A considerable crossover of
water can also occur from the anode to cathode side in
addition to water that is produced at the cathode as a
result of the electrochemical reaction there. Because of
the extra water which may be present at the cathode in a
direct liquid feed fuel cell, a cathode construction
providing superior performance in a gas feed solid polymer
fuel cell will not necessarily give superior performance
in a DMFC even if both cell types are supplied with
similar oxidant streams.
Electrodes for solid polymer fuel cells generally
comprise a substrate (a porous electrically conductive
sheet material) and an electrocatalyst layer. The
electrocatalyst layer is located so as to be adjacent the
electrolyte when assembled into a MEA, and can be
deposited directly on the substrate or on the membrane
WO 01/39300 CA 02391501 2002-05-14 pCT/CA00/01309
-3-
electrolyte. Other materials, for example,
polytetrafluoroethylene (PTFE) and proton conducting
ionomer, are typically incorporated into the
electrocatalyst layer for purposes of controlling wetting
characteristics, providing an ionic pathway for protons to
the membrane electrolyte, acting as a binder, and the
like. The electrode substrate serves to distribute fluids
(for example, reactants and/or reaction products such as
water product at the cathode or carbon dioxide product at
a DMFC anode) from a flow field passage to an associated
electrocatalyst layer or vice versa. Electrode substrates
may be made, for example, of porous carbon cloth or paper.
As with the electrocatalyst layer, other materials, such
as PTFE and proton conducting ionomer, may be incorporated
into the substrate for similar purposes. For volumetric
energy density, the use of thinner electrodes and hence
MEAs is preferred.
A range of cathode constructions have been used in
gaseous oxidant feed solid polymer fuel cells. Prior art
cathodes comprising carbonaceous substrates typically
contain a significant amount of a hydrophobic additive
(for example, >10o by weight PTFE) and typically exceed
about 230 micrometers in thickness for purposes of
improved oxidant distribution and product water
management.
Summary Of The Invention
Preferred cathode constructions for liquid feed solid
polymer fuel cells operating on liquid aqueous fuel
streams differ from those of gaseous feed solid polymer
fuel cells. Unexpectedly, the use of less hydrophobic
additive in the cathode may be advantageous to fuel cell
performance. Further, thinner cathodes comprising a
porous carbonaceous support less than 230 micrometers
thick, and a carbon-based sublayer between the substrate
WO 01/39300 CA 02391501 2002-05-14
PCT/CA00/01309
-4-
and the electrocatalyst layer may be employed to provide
similar or better performance, and thus are advantageous
with regards to volumetric energy density of the fuel
cell.
An improved direct liquid feed solid polymer fuel
cell comprises a cathode, an anode, and a solid polymer
electrolyte. The cathode is supplied with a gaseous
oxidant stream and the anode is supplied with a liquid
fuel stream comprising fuel and water wherein the fuel is
directly oxidized at the anode. The cathode of the
improved fuel cell comprises a substrate and an
electrocatalyst layer. The substrate comprises a
carbonaceous support and a first hydrophobic additive. In
one embodiment the amount of the first hydrophobic
additive in the cathode substrate is less than loo by
weight. In another embodiment, the thickness of the
carbonaceous support in the cathode substrate is less than
230 micrometers and there is a carbon-based sublayer in
the cathode between the substrate and the electrocatalyst
layer. The embodiments can be combined.
The first hydrophobic additive may be PTFE. In
particular, the amount of the PTFE in the cathode
substrate may be about 6% by weight.
The thickness of the carbonaceous support in the
cathode substrate may be less than 230 micrometers and
greater than 75 micrometers. In particular, the thickness
of the carbonaceous support in the cathode substrate may
be about 150 micrometers. The loading of the carbon-based
sublayer may be less than about 0.7 mg/cm2. The thickness
of the carbon-based sublayer above the substrate may be
less than about 25 micrometers.
The carbon-based sublayer may comprise a second
hydrophobic additive. The second hydrophobic additive may
also be PTFE and may be present in an amount of from about
6o to 30o by weight of the sublayer. The preferred amount
WO 01/39300 CA 02391501 2002-05-14 pCT/CA00/01309
-5-
of the second hydrophobic additive may depend on the
manner in which the electrocatalyst layer is applied to
the cathode substrate. For instance, a larger amount (for
example, about 300) may be preferred if the
electrocatalyst layer is manually applied while a smaller
amount (for example, about 6%) may be preferred if the
electrocatalyst layer is applied by a spraying method.
The electrocatalyst layer in the cathode comprises
electrocatalyst and also may comprise a third hydrophobic
additive. The third hydrophobic additive may also be PTFE
and may be present in an amount of about 6o by weight of
the electrocatalyst layer.
Use of less than 10% by weight of the first
hydrophobic additive in the cathode substrate can result
in significant improvement in the performance of liquid
feed fuel cells in which the fuel stream comprises a
substantial amount of water (for example, typical DMFCs).
Use of a sublayer and a carbonaceous support less than 230
micrometers thick can provide a thinner cathode structure
with similar or better fuel cell performance.
Brief Description Of The Drawings
FIG. l is a schematic diagram of a direct methanol
solid polymer fuel cell.
FIG. 2a shows plots of output voltage as a function
of current density for the direct methc:I201 fuel cells
(~?T~IFCs; a.n th.e Examp:l_es whose cathodes ~:omp~-ise car_bcn
substrates with different amounts of PTFE.
FIG. 2b shows the output voltages at constant current
of the DMFCs shown in FIG. 2a, as a function of the
oxidant stoichiometry.
FI G. 3a shows p:J_ots of output vo_l.ta.ge a.s ~.. functi.cn
of current density for DMFCS in t:e Examples= vahose
cathodes compz-ise carbon su..bstrates o.r di.tfer_.in.g tJa.:i_cVn.esC
and; or T~rhi~ch also comprise a c~:wbon-based subl~:yer .
WO 01/39300 CA 02391501 2002-05-14 pCT/CA00/01309
-6-
FIG. 3b shows the output voltages at constant current
for the DMFCs shown in FIG. 3a, as a function of the
oxidant stoichiometry.
FIG. ~a shows plots of output voltage as ~. function
of current dens ity for DMFCs in ti=a Examples who se
cats=odes compz-ise carbon-based sublayers ;aith different
amounts of PTFE and whose electrocatalyst. layers were
applied in different ways.
FIG. 4b shows the output voltages at constant current
for the DMFCs shown in FIG. 4a, as a function of the
oxygen stoichiometry.
Detailed Description Of Preferred Embodiments
The improved cathode structures described herein are
suitable for use in fuel cells in which the supplied
liquid fuel stream comprises substantially more water than
typical gas feed fuel streams (that is, more than
humidified hydrogen or methanol/water vapor fuel streams).
For instance, the improved cathode structures are suitable
for use in liquid feed fuel cells such as liquid feed
direct methanol fuel cells (DMFCs). The liquid fuel
stream in a DMFC comprises at least the same number of
moles of water as methanol (since the anode reaction
requires one mole of water for each mole of methanol). In
fact, typically dilute solutions of methanol in water are
employed in order to reduce crossover of methanol from the
anode to cathode.
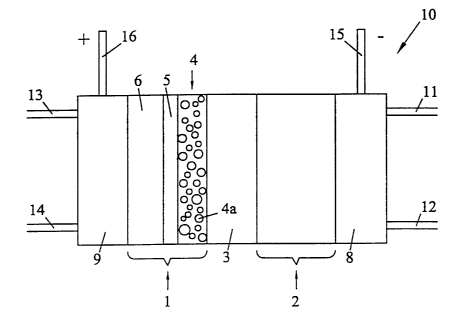
FIG. 1 shows a schematic diagram of a liquid feed
DMFC comprising an improved cathode structure. For
purposes of illustration, a preferred series stack of fuel
cells is represented merely by a single liquid feed fuel
cell 10 in FIG. 1. Fuel cell 10 contains a membrane
electrode assembly (MEA) comprising a porous cathode 1 and
porous anode 2 that are bonded to a solid polymer membrane
electrolyte 3. Liquid fuel flow field 8 and oxidant flow
WO 01/39300 CA 02391501 2002-05-14 pCT/CA00/01309
field 9 are pressed against anode 2 and cathode 6
respectively on the faces opposite the membrane
electrolyte 3. Fuel cell 10 has a liquid fuel stream
inlet 11, a liquid fuel stream outlet 12, an oxidant inlet
13, and an oxidant outlet 14. Electrical power is
obtained from the fuel cell via negative and positive
terminals 15 and 16 respectively.
As shown, cathode 1 comprises an electrocatalyst
layer 4, a carbonaceous substrate 6, and a carbon-based
sublayer 5 between electrocatalyst layer 4 and substrate
6. Each of electrocatalyst layer 4, substrate 6, and
sublayer 5 comprises an amount of a suitable hydrophobic
additive (for example, PTFE). Electrocatalyst layer 4
also comprises proton conducting ionomer dispersed
throughout (for example, NAFIONT"') . The hydrophobic
additive serves to modify the wetting characteristics of
each component and may also serve as a binder. The
ionomer provides ionic pathways from electrocatalyst
particles 4a in layer 4 to membrane electrolyte 3.
Cathode 1 further comprises several features leading
to improved fuel cell performance in liquid feed fuel
cells. Substrate 6 preferably comprises less than 10% by
weight PTFE additive and is therefore more hydrophilic
than substrates with more PTFE. The combination of the
carbonaceous support in substrate 6 (preferably less than
about 230 micrometers thick) and the carbon-based sublayer
(preferably less than about 0.7 mg/cm2 and 25 micrometers
thick) provides a shorter path for water removal than
thicker substrates. While these features might be
expected to adversely affect the distribution of oxidant
to the cathode electrocatalyst, it has been found that a
net performance improvement can be realized nonetheless.
(Like electrocatalyst layer 4, the carbon-based sublayer 5
is very thin relative to substrate 6 and is thus generally
CA 02391501 2002-05-14
WO 01/39300 PCT/CA00/01309
_g_
not so significant compared to the thickness of the
substrate.)
Without being bound by any particular theory, the
observed performance improvements are believed to relate
to the substantial diffusion of water across the membrane
electrolyte in such liquid feed fuel cells, which
significantly alters the water management situation at the
cathode. For instance, in certain DMFC embodiments, water
crossover from the anode may account for up to 900 of the
water at the cathode. In such cases, it seems to be
relatively more important to get the water out of the
cathode electrocatalyst layer than it is to get
distributed oxidant in. It is generally easier to move
water away from the electrocatalyst layer through a
thinner substrate, although the trade-off may be poorer
distribution of the oxidant to the electrocatalyst layer.
Further, it is easier to move water through a substrate
that is more hydrophilic (for example, has less
hydrophobic additive) although again this tends to make it
harder to distribute gaseous oxidant to the cathode
electrocatalyst.
The following DMFC examples have been included to
illustrate different embodiments and aspects of the
present cathode structures, but these should not be
construed as limiting in any way. For instance, the
improved cathode structures described may also be used in
liquid feed fuel cells operating on liquid fuel mixtures
comprising a substantial amount of water and another fuel
such as, for example, ethanol, dimethyl ether.
Examples
Experimental DMFCs were assembled using various
modified cathode structures and were then subjected to
fuel cell performance tests. Modifications to the cathode
WO 01/39300 CA 02391501 2002-05-14 pCT/CA00/01309
-9-
included: varying the amount of PTFE in the cathode
substrate, varying the thickness of the cathode substrate,
incorporating a carbon-based sublayer in the cathode,
varying the amount of PTFE in the sublayer, and varying
the method of application of the electrocatalyst layer.
In a first series of fuel cells, denoted A to I, the
membrane electrolyte employed in each cell was NAFIONTM
117. The active area of the electrodes was about 49 cm2.
The anodes comprised electrocatalyst layers comprising
unsupported platinum/ruthenium (at about 4 mg/cm2 loading)
and NAFIONTM ionomer (at about 0.4 m g/cm2loading). The
anode electrocatalyst layers were manually applied on 229
micrometer thick TGP grade (product from Toray) carbon
fibre paper substrates in slurry form and were then
impregnated afterwards with the ionomer. The cathodes
comprised electrocatalyst layers consisting of unsupported
platinum (also at about 4 mg/cm2loading) and 6o by weight
PTFE. The cathode electrocatalyst layers were also
manually applied in slurry form onto TGP grade (product
from Toray) carbon fibre paper substrates. Different
thicknesses of carbon fibre paper were used as cathode
substrates, however, and the substrates were also
impregnated with varying amounts of PTFE additive.
Further, some cathodes also employed a carbon-based
sublayer between the carbon fibre paper substrate and the
electrocatalyst layer. The details of the cathode
structures employed for each cell of this first series are
summarized in Table 1 below.
WO 01/39300 CA 02391501 2002-05-14 pCT/CA00/01309
-10-
Table 1
Cell % wt. Nominal Sublayer
of PTFE in thickness of incorporated
substrate substrate (in at cathode?
micrometers) I
A 3.70 152 Yes
B 6.40 152 Yes
C 12.50 152 Yes
D 19.70 152 Yes
E 11.7% 229 No
F 11.8% 229 Yes
G 12.30 152 No
H 13.2% 76 Yes
I 13.20 152* Yes
* This 152 micrometer substrate was prepared by stacking
two pieces of 76 micrometer PTFE-impregnated carbon fibre
paper together.
PTFE was introduced into the carbon fibre paper
substrates by impregnation with an appropriate aqueous
PTFE suspension followed by drying. Where applicable,
approximately 0.46 mg/cmz loading of a sublayer containing
carbon black and 26o wt. PTFE was applied by screen
printing a carbon-based slurry onto the appropriate
substrate and then drying the substrate. (Sublayer
loadings less than about 0.7 mg/cm2 have been found to be
advantageous. Greater amounts may be detrimental.) The
slurry consisted of polyethylene glycol (liquid),
polycarbonate (liquid), Shawinigan carbon black (Chevron
Chemical C50 grade), and PTFE (60% by weight PTFE in a
dilute water suspension) in a weight ratio of about
WO 01/39300 CA 02391501 2002-05-14 pCT/CA00/01309
-11-
33.3/20.1/2.28/0.81. The applied sublayer penetrated the
pores of the carbon fibre paper typically to a depth of
about 35 micrometers (as observed under a scanning
electron microscope). Some sublayer material remained
above the surface of the carbon fibre paper, typically
with a thickness of about 10 micrometers.
The performance characteristics determined for these
experimental DMFCs included output voltage versus current
density (at constant fuel flow rate and constant oxidant
stoichiometry except, in the case of the latter, at the
lowest current densities where there may be substantial
competition for the available oxygen at the cathode for
methanol oxidation from methanol crossover) and output
voltage versus oxidant stoichiometry (at constant current
density). Stoichiometry is defined as the ratio of
reactant supplied to the fuel cell to reactant consumed in
the electrochemical reactions in the fuel cell. This
testing was done at about 97EC. Compressed air was used as
the oxidant stream and 0.45M aqueous methanol was used as
the liquid fuel stream, both at 3 bar absolute pressure.
In the determination of output voltage versus current
density, the fluid flow rates were such that, at current
densities of 300 mA/cm2, the oxidant and fuel
stoichiometries were 2 and 3 respectively. In the
determination of output voltage versus oxidant
stoichiometry, the same fuel stoichiometry was used and
voltage was determined as a function of oxidant
stoichiometry at a constant 200 mA/cm2 current density.
FIG. 2a shows the output voltage versus current
density plots for DMFCs A, B, C, and D. These fuel cells
have similar cathodes except for the amount of PTFE in the
carbon substrate. Fuel cell D, with the highest PTFE
content in the cathode substrate, performed significantly
worse than the other cells. FIG. 2b shows the output
voltage versus oxidant stoichiometry plots for the same
CA 02391501 2002-05-14
WO 01/39300 PCT/CA00/01309
-12-
cells. Here, the ability to maintain a high output
voltage at lower oxidant stoichiometry is indicative of
better performance. Again, fuel cell D, with the highest
PTFE content in the substrate, performed significantly
worse than the other cells. Fuel cell C, with 6.4o PTFE
in the substrate, shows the best performance in both
figures. (Note that some hysteresis is observed in the
plots in FIG. 2b and in later FIGS. 3b and 4b. The
oxidant stoichiometry is first decreased stepwise until
the output voltage drops significantly. The oxidant
stoichiometry is then increased stepwise until the output
voltage recovers. In general, the output voltage is
higher during the increase in stoichiometry than it is
during the decrease in stoichiometry. This hysteresis may
result from the cathode getting wetter as the
stoichiometry decreases, with the possible formation of
new water pathways from water deposits in the cathode.
The presence of new water pathways may then improve water
removal once the oxidant stoichiometry is increased again.
Furthermore, the cathode potential changes when the
oxidant stoichiometry is varied in this way. A change in
the cathode potential can result in the removal of
strongly bound adsorbates from the cathode
electrocatalyst, and hence a refreshing of the cathode
electrocatalyst.)
FIGS. 3a and 3b show the output voltage versus
current density plots and the output voltage versus
oxidant stoichiometry plots for DMFCs C and E to I
inclusive. These fuel cells have roughly the same amount
of PTFE in the cathode substrate but differ in cathode
substrate thickness and/or presence of a sublayer at the
cathode. Cells C and I show similar or better performance
to that of cell E (which is a conventional cathode)
although the overall thickness of the cathodes in cells C
and I is about 67 micrometers thinner than that of cell E.
CA 02391501 2002-05-14
WO 01/39300 PCT/CA00/01309
-13-
FIGS. 3a and 3b also show a marked improvement in
performance with the use of a sublayer in cells with a 152
micrometer thick cathode substrate (comparing cells C and
I to cell G which has no sublayer).
In a second series of fuel cells, denoted J, K, and
L, the membrane electrolyte employed in each cell was
NAFIONT"' 115. The electrodes were similar to those of
cells A to I except that each cathode had a 0.6 mg/cm2
carbon-based sublayer having different amounts of PTFE and
each had NAFIONT"' ionomer in the electrocatalyst layer (at
about 0.6 mg/cm2 loading). In the cathode of cell J, the
sublayer was applied using a spray technique which results
in somewhat more sublayer material remaining above the
surface of the substrate. The thickness of the sprayed
sublayer above the carbon fibre paper surface as observed
under a scanning electron microscope was about 22
micrometers thick on average (actual range from about 15
to 25 micrometers thick). Further, each cathode substrate
was 229 micrometers thick and contained about 6o by wt.
PTFE. Finally, in some cathodes, the electrocatalyst
layer was applied using a spray technique. Table 2 below
summarizes the differences between these cells.
Table 2
Cell o wt. Method of
of PTFE in application of
sublayer electrocatalyst
layer
J 6o Spray
K 30o Spray
300 Manual
CA 02391501 2002-05-14
WO 01/39300 PCT/CA00/01309
-14-
The performance characteristics were determined as
above except that testing here was done at 110EC and 0.4M
aqueous methanol was used as the fuel stream. Also, in
this series, the performance characteristics were
determined at constant fuel stoichiometry.
FIGS. 4a and 4b show the output voltage versus
current density plots and the output voltage versus
oxidant stoichiometry plots for DMFCs J, K, and L. The
performance of cell K is substantially worse than the
other two, indicating that the preferred amount of PTFE in
the sublayer may depend on the manner in which the
electrocatalyst layer is applied to the cathode substrate.
While particular elements, embodiments and
applications of the present invention have been shown and
described, it will be understood, of course, that the
invention is not limited thereto since modifications may
be made by those skilled in the art without departing from
the spirit and scope of the present disclosure,
particularly in light of the foregoing teachings.