Note: Descriptions are shown in the official language in which they were submitted.
CA 02391819 2002-02-O1
WO 01/08610 PCT/IB00/01093
_1_
KIT FOR CHONDROCYTE CELL TRANSPLANTATION
BACKGROUND OF THE INVENTION
The invention relates to improvements in methods of chondrocyte cell
transplantation.
U.S. Patent No. 5,759,190, hereby incorporated by reference, describes one
method for transplantation for effective chondrocyte and/or cartilage
transplantation. U.S.
Provisional Patent Application No. 60/096,597, also hereby incorporated by
reference,
describes a second method for effective chondrocyte and/or cartilage
transplantatiop.
Brittberg et al., Treatment of Deep Cartilage Defects in the Knee with
Autologous
Chondrocyte Transplantation, New England Journal of Medicine, 331: 889-895
(October 6,
1994), also hereby incorporated by reference, describes a third method of
chondrocyte
transplantation. U.S. Patent Application No. 09/373,952, also hereby
incorporated by
reference, describes methods for effective chondrocyte cell and or cartilage
transplantation.
Heretofore, it was thought that successful chondrocyte cell and/or cartilage
cell transplantation required removal of damaged cartilage down to the
underlying bone.
BRIEF SUMMARY OF THE INVENTION
The present invention includes a system for implanting chondrocyte cells
andlor cartilage cells at a site of cartilage damage. The invention involves
first removing
damaged cartilage from a site of damaged cartilage, such that the depth of
removal of the
cartilage is sufficient to preserve a layer of protective covering, sometimes
referred to as a
subchondral layer, over the bone. One way of protecting the subchondral layer
is to remove
the damaged cartilage such that a thin layer of cartilage is left over the
subchondral layer.
The chondrocyte cells are then transplanted on top of this thin cartilage
layer. Leaving a
thin layer of cartilage over the subchondral layer limits or entirely prevents
bleeding from
the site of damaged cartilage.
In this way, chondrocyte cells and/or cartilage cells are implanted by various
methods without the use of a hemostatic barrier in the site of damage, as was
previously
thought necessary.
CON~IRMhTION COPY
W~ 01/08610 CA 02391819 2002-02-O1 pCT~B00/01093
-2-
In one embodiment, the present invention includes a method for the effective
treatment of articulating joint surface cartilage by the transplantation of
chondrocytes, to a
surface to be treated. The method includes the steps of placing chondrocytes
in a defect of
the articulating joint surface, and covering the surface to be treated with an
absorbable
covering cap. The present invention also includes a kit for chondrocyte
transplantation
including a covering cap and securing device.
BRIEF DESCRIPTION OF THE DRAWINGS
The present invention may be better understood by reference to the
description which follows taken together with the accompanying figures which
illustrate
particular embodiments the present invention wherein:
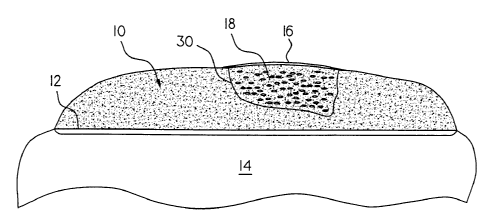
Fig. 1 is a drawing showing one embodiment of implantation of chondrocyte
cells and/or cartilage cells at a site of cartilage damage where the damaged
cartilage is
removed to a depth above the subchondral layer.
Fig. 2 is a drawing showing a second embodiment of implantation of
chondrocyte cells and/or cartilage cells at a site of cartilage damage where
the damaged
cartilage is removed to a depth above the subchondral layer.
Fig. 3 is a drawing showing a third embodiment of implantation of
chondrocyte cells and/or cartilage cells at a site of cartilage damage where
the damaged
cartilage is removed to a depth above the subchondral layer.
Fig. 4 is a drawing showing a covering cap or matrix used in the methods
according to the present invention.
DETAILED DESCRIPTION OF THE INVENTION
This invention concerns transplantation of chondrocyte cells and/or cartilage
cells into a site of cartilage damage without the use of a hemostatic barrier.
Fig. 1 shows a
first embodiment where damaged cartilage 10 (damaged either through traumatic
injury or
otherwise defective) is removed to a depth above a subchondral layer 12
covering a bone
14. The thickness of the remaining cartilage layer over the subchondral layer
will vary on
the site of damage, but is thick enough to prevent or limit the amount of
bleeding at the site
of damage.
WO 01/08610 CA 02391819 2002-02-O1 pCT~B00/01093
-3-
In one embodiment, the present invention includes a cartilage repair
implantation method. The implantation method includes harvesting cartilage
cells from a
non-weight bearing surface of a patient, culturing the chondrocyte cells in a
suitable growth
media, securing a covering cap 16 over the cartilage defect area leaving one
edge of
covering cap 16 unsecured, injecting the cultured chondrocytes in growth media
under
covering cap 16, and securing the open edge of covering cap 16 to the edge of
the cartilage
defect.
In one embodiment, covering cap 16 preferably is a cell free cap 16 and is
used as a patch to cover the damaged area and under which cultured chondrocyte
cells such
as autologous or homologous chondrocyte cells are transplanted. Covering cap
16 is
sutured or otherwise held in place over the area of defect. Covering cap 16 is
formed, for
example, of a collagen membrane such as Chondro-Cell~ (a commercially
available Type
II collagen matrix pad, Ed. Geistlich Sohne, Switzerland) or Chondro-Gide ~ (a
commercially available Type I collagen matrix pad, Ed. Geistlich Sohne,
Switzerland), or
any other suitable membrane that will be absorbed or resorbed by the body, as
discussed
below. The cultured chondrocyte cells in a suitable transplant media 18 are
injected under
covering cap 16. Transplant media 18, for example, includes DMEM/F12 media (up
to 250
ml), autologous serum (25 ml to a final concentration of 10%) , L-ascorbic
acid (7.5 ml at
concentration of 300 micromoles per liter), Fungizone ~ (2 ml at concentration
of 2.2
micromoles per liter), and gentomycin sulfate (1.25 ml at concentration of 70
micromoles
per liter). The cultured chondrocyte cells were previously grown in a culture
media, for
example, including DMEM/F12 media (up to 500 ml), L-ascorbic acid (15 ml at
concentration of 300 micromoles per liter) , Fungizone ~ (4.0 ml at
concentration of 2.2
micromoles per liter), gentomycin sulfate (2.5 ml at concentration of 70
micromoles per
liter), and fetal calf, porcine, kangaroo, or other blood serum (100 ml to
final concentration
of 20%). Preferably, the cultured chondrocyte cells are moved from the growth
media into
the transplant media approximately 72 hours before transplantation. By cell-
free is meant
that covering cap 16 contains no living or dead cells.
In one embodiment, the present invention is as follows. For an autologous
implant, a cartilage biopsy is harvested by arthroscopic technique from a non-
weight
bearing area in a joint of a patient and transported to a laboratory in a
growth media
containing 20% fetal calf serum. The cartilage biopsy is then treated with an
enzyme such
WD ~1/0g61~ CA 02391819 2002-02-O1 pCT/IB~~/01093
-4-
as trypsin ethylenediaminetetraacetic acid (EDTA), a proteolytic enzyme and
binding agent,
to isolate and extract cartilage chondrocyte cells. The extracted chondrocyte
cells are then
cultured in the growth media from an initial cell count of about 50,000 cells
to a final count
of about 20 million chondrocyte cells or more.
Three (3) days before reimplantation, the growth media is exchanged for a
transplant media which contains 10% autologous serum (that is, serum extracted
from the
patient's blood as described below). Then, the cultured chondrocyte cells in
the transplant
media are injected under partially secured covering cap 16.
It is understood that the area of cartilage defect 30 can be treated directly,
enlarged slightly, or sculpted by surgical procedure prior to injection of
cultured
chondrocyte cells (as described in U.S. Patent No. 5,989,269, the entire
disclosure and
teachings of which are hereby incorporated by reference), to accommodate and
promote
cartilage cell growth. The culturing procedure as well as the growth and
transplant media
are described by way of example, in detail below, starting first with a
description of a
laboratory procedure used to process the harvested cartilage biopsy and to
culture the
chondrocyte cells according to the present invention.
In one embodiment, growth media (herein, "the growth media") used to treat
the cartilage biopsy during the culturing process and to grow the cartilage
chondrocyte cells
is prepared by mixing together 2.5 ml gentomycin sulfate (concentration 70
micromole/liter), 4.0 ml amphotericin (concentration 2.2 micromole/liter;
tradename
Fungizone~ , an antifungal available from Squibb), 1 S ml 1-ascorbic acid (300
micromole/liter), 100 ml fetal calf serum (final concentration 20%), and the
remainder
DMEM/F12 media to produce about 400 ml of growth media. (The same growth media
is
also used to transport the cartilage biopsy from the hospital to the
laboratory in which the
chondrocyte cells are extracted and multiplied.)
Blood obtained from the patient is centrifuged at approximately 3,000 rpm to
separate the blood serum from other blood constituents. The separated blood
serum is saved
and used at a later stage of the culturing process and transplant procedure.
Cartilage biopsy previously harvested from a patient for autologous
transplantation is shipped in the growth media described above to the
laboratory where it
will be cultured. The growth media is decanted to separate out the cartilage
biopsy, and
w0 O1/08C10 CA 02391819 2002-02-O1 pCT/IB00/01093
-5-
discarded upon arrival at the laboratory. The cartilage biopsy is then washed
in plain
DMEM/F12 at least three times to remove the film of fetal calf serum on the
cartilage
biopsy.
The cartilage biopsy is then washed in a composition which includes the
growth media described above, to which 28 ml trypsin EDTA (concentration
0.055) has
been added. In this composition it is incubated for five to ten minutes at
37°C, and 5%
C02. After incubation, the cartilage biopsy is washed two to three times in
the growth
media, to cleanse the biopsy of any of the trypsin enzyme. The cartilage is
then weighed.
Typically, the minimum amount of cartilage required to grow cartilage
chondrocyte cells is
about 80-100 mg. A somewhat larger amount, such as 200 to 300 mg, is
preferred. After
weighing, the cartilage is placed in a mixture of 2 ml collagenase
(concentration 5,000
enzymatic units; a digestive enzyme) in approximately 50 ml plain DMEM/F12
media, and
minced to allow the enzyme to partially digest the cartilage. After mincing,
the minced
cartilage is transferred into a bottle using a funnel, and approximately 50 ml
of the
collagenase and plain DMEM/F12 mixture is added to the bottle. The minced
cartilage is
then incubated for 17 to 21 hours at 37°C, and S% COZ.
In one embodiment, the incubated minced cartilage is then strained using 40
pm mesh, centrifuged (at 1054 rpm, or 200 times gravity) for 10 minutes, and
washed twice
with growth media. The chondrocyte cells are then counted to determine their
viability,
following which the chondrocyte cells are incubated in the growth media for at
least two
weeks at 37°C, and 5% COz, during which time the growth media is
changed, preferably,
three or four times.
Preferably, at least three days before re-implantation in the patient, the
chondrocyte cells are removed by trypsinization and centrifugation from the
growth media,
and transferred to a transplant media containing 1.25 ml gentomycin sulfate
(concentration
70 micromole/liter), 2.0 ml amphotericin (concentration 2.2 micromole/liter;
tradename
Fungizone~, an antifungal available from Squibb), 7.5 ml 1-ascorbic acid (300
micromole/liter), 25 ml autologous blood serum (final concentration 10%), and
the
remainder DMEM/F12 media to produce about 300 ml of transplant media.
Before or during the chondrocyte transplantation procedure, covering cap 16
is cut to a size suitable to fit over the damaged cartilage area. Covering cap
16 is secured by
WO 01/08610 CA 02391819 2002-o2-O1 pCT/IB00/01093
_6_
adhesive or mechanical retention devices or means, or a combination of both
adhesive or
mechanical retention devices or means, to the cartilage defect area without
impairing the
further in situ differentiation of the chondrocytes and the regeneration of
the natural
cartilage matrix material. For example, covering cap 16 is sutured, adhered
with adhesive,
and/or secured with retention pins to the area of cartilage defect 30.
In one embodiment, using a 1 ml syringe and a 16 gauge needle, the cultured
chondrocyte cells in transplant media (about 0.6 ml containing about l Ox 106
chondrocyte
cells) was drawn up into the barrel of the syringe. A 23 gauge short needle
was switched
for the 16 gauge needle and the cultured chondrocyte cells were inj ected
under the secured
covering cap 16 into the area of cartilage defect 30. The unsecured opening of
covering
cap 16 was then secured (for example, with adhesive) prior to removal of the
needle and
then the needle was carefully withdrawn. No leakage of cells occurred.
Suitable adhesive includes a biocompatible glue, such as organic fibrin glue
(e.g., Tisseel~, fibrin based adhesive, Baxter, Austria or a fibrin glue
prepared in the
surgical theater using autologous blood samples).
Fig. 2 shows a second embodiment where damaged cartilage 10 is removed
to a depth above a subchondral layer 12 covering a bone 14. Chondrocyte cells
are
previously grown on a matrix 15 formed, for example, of a collagen membrane
such as
Chondro-Cell~ (a commercially available Type II collagen matrix pad, Ed.
Geistlich
Sohne, Switzerland) or Chondro-Gide~ (a commercially available Type I collagen
matrix
pad, Ed. Geistlich Sohne, Switzerland), or any other suitable membrane that
will be
absorbed or resorbed by the body to form a chondrocyte cell-loaded matrix 20.
The
chondrocyte cell-loaded matrix 20 is then glued, for example, using a
biocompatible glue 22
such as Tisseal~ (a commercially available fibrin based adhesive, Baxter,
Austria) into the
area of damaged cartilage. The cultured chondrocyte cells were previously
grown on the
matrix 15 in a culture media, for example, including DMEM/F12 media (up to 500
ml), L-
ascorbic acid (15 ml at concentration of 300 micromoles per liter) , Fungizone
~ (4.0 ml at
concentration of 2.2 micromoles per liter), gentomycin sulfate (2.5 ml at
concentration of
70 micromoles per liter), and fetal calf, porcine, kangaroo, or other blood
serum (100 ml to
final concentration of 20%). At some point after the chondrocyte cells are
grown on the
matrix but before transplantation, the growth media is exchanged for a
transplant media
WO 01/08610 CA 02391819 2002-o2-O1 PCT/IB00/01093
7-
including DMEM/F12 media (up to 250 ml), autologous serum (25 ml to a final
concentration of 10%) , L-ascorbic acid (7.5 ml at concentration of 300
micromoles per
liter), Fungizone ~ (2 ml at concentration of 2.2 micromoles per liter), and
gentomycin
sulfate (1.25 ml at concentration of 70 micromoles per liter). The exchange of
transplant
media for growth media takes place approximately 72 hours before
transplantation.
Fig. 3 shows a third embodiment which is identical to the embodiment in
Fig. 2 but uses pins 24 to hold the chondrocyte cell-loaded matrix in place
rather than fibrin
glue. Pins 24 are a commercially available lactide co-polymer pin, sold under
the name
OrthoPinTM and available from Ed. Geistlich Sohne, Switzerland.
Preferably, covering cap 16 or matrix 15 is a material which will support
chondrocyte cell growth and which, over time will be absorbed or resorbed in a
body of a
patient receiving the implant. The transplantation procedure may be by
arthroscopic,
minimally invasive or open surgery technique. The method of the invention also
contemplates the use of suitable allogenic and xenogenic chondrocyte cells for
the repair of
a cartilage defect.
A suitable covering cap 16 or matrix 15 is a solid or gel-like, scaffold
characterized by being able to hold a stable form for a period of time to
enable it to be
secured over or in the cartilage defect and to promote growth of chondrocytes
cells in the
cartilage defect.
Covering cap 16 or matrix 15 is stable for a period of time sufficient to
allow
full cartilage repair and then be absorbed or resorbed by the body over time,
for example,
within two to three months from implantation without leaving any significant
traces and
without forming toxic degradation products. The terms "absorbed" and
"resorbed" are
meant to include processes by which covering cap 16 or matrix 15 is broken
down by
natural biological processes, and the broken down covering cap 16 or matrix 15
and
degradation products thereof are taken up and disposed of, for example, in
cells, across
tissues or by way of diffusion or osmosis, through such systems as the
lymphatics or blood
vessels. Accordingly, covering cap 16 or matrix 15 preferably is a
physiologically
absorbable or resorbable, non-antigenic membrane-like material.
As shown in Fig. 4 covering cap 16 or matrix 15 preferably is in a sheet like
form having one relatively smooth side 26 and one relatively rough or porous
side 28.
CA 02391819 2002-02-O1
WO 01/08610 PCT/IB00/01093
_g_
Smooth side 26 is relatively more dense than rough or porous side 28. Rough
side 28, for
example, is fibrous and typically faces cartilage defect 30 and promotes
chondrocyte cell
ingrowth, while the smooth side 26 typically faces away from cartilage defect
30 and
impedes tissue ingrowth.
In one embodiment, covering cap 16 or matrix 15 is formed of polypeptides
or proteins. Preferably, the polypeptides or proteins are obtained from
natural sources, e.g.,
from mammals. Artificial materials, however, having physical and chemical
properties
comparable to polypeptides or proteins from natural sources, may also be used
to form
covering cap 16 or matrix 15. In another embodiment, covering cap 16 or matrix
15 is
formed from hyaluronic acid or derivatives thereof.
It is also preferred that covering cap 16 or matrix 15 is reversibly
deformable
without mechanical destruction as it is handled by the user so it can be
manipulated and
then returns to its original shape. This deformation is completely reversible
once covering
cap 16 or matrix 15 is introduced into the joint or is placed on the surface
to be treated, for
example, in an arthroscopic procedure.
The material forming covering cap 16 or matrix 15 may be uncrosslinked or
partially or fully crosslinked. A preferred material from which covering cap
16 or matrix 15
is formed is collagen such as obtained from equine, porcine, fetal calf,
kangaroo, bovine,
ovine, and chicken. As set forth above, suitable materials from which covering
cap 16 or
matrix 15 is formed include Chondro-Cell~ (a commercially available type II
collagen
matrix pad, Ed. Geistlich Sohne, Switzerland), and Chondro-Gide~ (a
commercially
available type I collagen matrix pad, Ed. Geistlich Sohne, Switzerland), as
discussed above.
A covering cap 16 or matrix 15 formed of collagen Type I is somewhat stiffer
than one
formed from collagen Type II, although Type II collagen matrixes may also be
used as
covering cap 16 or matrix 15 in the present invention.
It has been found under electron microscopy that the chondrocytes cultured
on the dense or smooth side 26 of covering cap 16 or matrix 15 did not grow
into the
structure of covering cap 16 or matrix 15, whereas chondrocyte cells cultured
on rough or
porous side 28 of covering cap 16 did grow into the porous or rough side 28 of
covering cap
16 or matrix 15, and furthermore showed the presence of proteoglycans and no
signs of
fibroblast structures. This result indicates that when covering cap 16 or
matrix 15 is secured
WO 01/08610 CA 02391819 2002-02-O1 pCT/IB00/01093
-9-
over or in the area cartilage defect 30, the rough or porous side should face
toward the area
of damaged cartilage. This will enable the chondrocyte cells to penetrate the
rough or
porous side of covering cap 16 or matrix 1 S and produce a smooth cartilage
surface in line
with the intact surface of the damaged cartilage.
The present invention encompasses still other methods of cell transplantation
so long as such methods avoid the prior necessity of using a hemostatic
burner.
The subjoined claims therefore are intended to be construed to cover not only
those embodiments of this invention disclosed above but also to cover all such
embodiments, variants and equivalents of the invention as may be made by those
skilled in
the art to which the invention pertains, which embodiments, variants and
equivalents are
within the true spirit and scope of this invention.