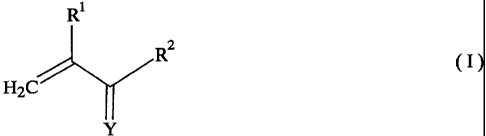Note: Descriptions are shown in the official language in which they were submitted.
CA 02392227 2008-07-28
1
THREE COMPONENT-CONTAINING ACRYLIC ADHESIVE COMPOSITION
TECHNICAL FIELD
The present invention relates to an adhesive composition for hard tissues
used in the medical and dental fields, showing strong adhesion to hard tissues
such as bone, nail and tooth, and being excellent in peripherical sealability.
More specifically, the present invention relates to a dental adhesive
composition
which is usually used for bonding to a tooth a resin material such as a dental
bonding material, a dental luting material, a dental composite resin or a
de_ntal
compomer, especially for the purpose of giving tooth excellent peripherical
sealability in odontotherapy.
BACKGROUND ART
In the restoration of teeth damaged by caries or the like, there have been
used a filling restorative material which is a so-called filling composite
resin or
filling compomer, a crown restorative material such as a dental metal alloy,
porcelain or a resin material, and the like. However, since these filling
restorative materials and crown restorative materials themselves do not
exhibit
adhesive properties, conventionally various dental adhesives have been used.
Among them, there has been preferably employed a so-called acidic etching-type
adhesive system in which a tooth surface is treated with a strong acidic
etching
agent such as phosphoric acid, and thereafter a bonding material is applied
thereto for adhesion.
ii
CA 02392227 2002-05-21
2
However, there are some defects in the above method of treating with the
acidic etching agent, such that washing with water for sufficiently removing
the
acid and drying again are necessitated after the treatment. Therefore, there
is a
defect that its procedures are complicated. According to the adhesive system
using an acidic etching agent, adhesive properties for enamel would be
improved.
However, it is difficult to give dentin high adhesive strength and excellent
peripherical sealability.
The term "peripherical sealability" as referred to herein means sealing
ability of an adhesive at the bonding portion (peripherical portion). The
peripherical sealability can be evaluated by immersing a sample in a solution
of a
colorant, for instance, an aqueous basic fuchsine solution. When the clearance
of
the bonding portion between a tooth and an adhesive is large, a large amount
of
the colorant penetrates into the clearance, so that the adherent is remarkably
colored at the peripherical portion. When the adherent is colored as described
above, it is thought that the clearance gives one of causations for secondary
caries by the invasion of bacteria or foreign substances giving a harmful
effect
on adhesive properties into the clearance.
As techniques using other adhesives, there have been proposed in
Japanese Patent Laid-Open Nos. Sho 62-223289 and Hei 3-240712 an adhesive
system in which a tooth is treated with a primer composition composed of an
acid or acidic monomer in place of the acidic etching agent, and a hydrophilic
monomer, and thereafter a bonding material is applied to the tooth without the
procedure of washing with water, i.e. a so-called adhesive system using a self-
etching primer. In this adhesive system, adhesive properties and peripherical
sealabilities have been improved to some extent especially for dentin.
CA 02392227 2002-05-21
3
However, in this adhesive system, the durability cannot be said to be
enough even though the self-etching primer is used. Accordingly, there often
occurs deterioration in peripherical sealability when the lowering of adhesive
strength is small in the durability test of adhesive strength. Therefore,
there are
often caused some problems in clinics such that leakage is caused between the
tooth and the restorative material after the passage of a given time period
from
restoration, and that caries again progresses from the peripherical portion,
thereby generating a so-called secondary caries. Therefore, in recent years,
there
have been desired further improvements in sealability at the peripherical
portion.
An object of the present invention is to provide an adhesive composition
for hard tissues, which exhibits excellent adhesive properties for hard
tissues,
especially tooth such as enamel, dentin or cement, and which is especially
excellent in peripherical sealability.
These and other objects of the present invention will be apparent from the
following description.
DISCLOSURE OF INVENTION
The present invention relates to an adhesive composition comprising:
(a) an acidic group-containing polymerizable monomer;
(b) a polymerizable monomer represented by the general formula (I):
Rl
RZ
(I)
H2C
Y
a
CA 02392227 2002-05-21
4
wherein Rl is hydrogen atom or methyl group; R2 is a halogen atom,
hydroxyl group, mercapto group, amino group or -O-R3-OH group,
wherein R3 is an alkylene group having 6 to 25 carbon atoms; and Y is
oxygen atom or sulfur atom; and
(c) a hydrophilic polymerizable monomer,
wherein the weight ratio of the polymerizable monomer (b) / acidic group-
containing polymerizable monomer (a) is 0.001 to 0.5.
BEST MODE FOR CARRYING OUT THE INVENTION
The acidic group-containing polymerizable monomer (a) is a component
for giving teeth excellent adhesive property.
It is preferable that the acidic group-containing polymerizable monomer
(a) has at least one polymerizable unsaturated group selected from the group
consisting of acryloyl group, methacryloyl group, vinyl group and vinylbenzyl
group. Among them, acryloyl group or methacryloyl group is preferable. In the
present specification, the term "(meth)acryl" collectively expresses both
methacryl and acryl.
The acidic group of the acidic group-containing polymerizable monomer
(a) includes at least one member selected from the group consisting of
phosphate
group, pyrophosphate group, thiophosphate group, carboxylate group and
sulfonate group.
Concrete examples of the acidic group-containing polymerizable
monomer (a) include a phosphate group-containing polymerizable monomer, a
pyrophosphate group-containing polymerizable monomer, a thiophosphate
group-containing polymerizable monomer, a carboxylate group-containing
sI
CA 02392227 2002-05-21
polymerizable monomer and a sulfonate group-containing polymerizable
monomer.
Concrete examples of the phosphate group-containing polymerizable
monomer include 6-(meth)acryloyloxyhexyl dihydrogen phosphate,
5 7-(meth)acryloyloxyheptyl dihydrogen phosphate, 8-(meth)acryloyloxyoctyl
dihydrogen phosphate, 9-(meth)acryloyloxynonyl dihydrogen phosphate,.
10-(meth)acryloyloxydecyl dihydrogen phosphate, 11-(meth)acryloyloxyundecyl
dihydrogen phosphate, 12-(meth)acryloyloxydodecyl dihydrogen phosphate,
16-(meth)acryloyloxyhexadecyl dihydrogen phosphate,
20-(meth)acryloyloxyeicosyl dihydrogen phosphate, di(meth)acryloyloxyhexyl
hydrogen phosphate, di(meth)acryloyloxyheptyl hydrogen phosphate,
di(meth)acryloyloxyoctyl hydrogen phosphate, di(meth)acryloyloxynonyl
hydrogen phosphate, di(meth)acryloyloxydecyl hydrogen phosphate,
6-(meth)acryloyloxyhexyl methylhydrogen phosphate, 6-(meth)acryloyloxyhexyl
ethylhydrogen phosphate, 8-(meth)acryloyloxyoctyl methylhydrogen phosphate,
10-(meth)acryloyloxydecyl methylhydrogen phosphate,
10-(meth)acryloyloxydecyl ethylhydrogen phosphate,
10-(meth)acryloyloxydecyl phenylhydrogen phosphate,
1,3-di(meth)acryloyloxypropyl-2-dihydrogen phosphate,
2-(meth)acryloyloxyethyl phenylhydrogen phosphate,
.2-(meth)acryloyloxyethyl 2'-bromoethylhydrogen phosphate,
(meth)acryloyloxyethyl phenyl phosphonate,
2-(meth)acryloyloxyethyl hexylhydrogen phosphate,
2-(meth)acryloyloxyethyl octylhydrogen phosphate,
2-(meth)acryloyloxyethyl decylhydrogen phosphate, and the like;
il
CA 02392227 2002-05-21
6
(5-methacryloxy)pentyl-3-phosphonopropionate, (6-methacryloxy)hexyl-
3-phosphonopropionate, (10-methacryloxy)decyl-3-phosphonopropionate,
(6-methacryloxy)hexyl-3-phosphonoacetate, (10-methacryloxy)decyl-
3-phosphonoacetate, and the like described in Japanese Patent Laid-Open No.
Hei 3-294286; 2-methacryloxyethyl (4-methoxyphenyl)hydrogen phosphate,
2-methacryloxypropyl (4-methoxyphenyl)hydrogen phosphate, and the like
described in Japanese Patent Laid-Open No. Sho 62-281885; phosphate group-
containing polymerizable monomers and their acid chlorides exemplified in
Japanese Patent Laid-Open Nos. Sho 52-113089, Sho 53-67740, Sho 53-69494,
Sho 53-144939, Sho 58-128393 and Sho 58-192891; and the like.
Concrete examples of the pyrophosphate group-containing polymerizable
monomer include di[2-(meth)acryloyloxyethyl] pyrophosphate,
di[4-(meth)acryloyloxybutyl] pyrophosphate, di[6-(meth)acryloyloxyhexyl]
pyrophosphate, di[8-(meth)acryloyloxyoctyl] pyrophosphate,
di[9-(meth)acryloyloxynonyl] pyrophosphate, di[10-(meth)acryloyloxydecyl]
pyrophosphate, di[10-(meth)acryloyloxyundecyl] pyrophosphate, and their acid
chlorides, and the like.
Concrete examples of the thiophosphate group-containing polymerizable
monomer include 6-(meth)acryloyloxyhexyl dihydrogen dithiophosphate,
8-(meth)acryloyloxyoctyl dihydrogen dithiophosphate,
9-(meth)acryloyloxynonyl dihydrogen dithiophosphate,
10-(meth)acryloyloxydecyl dihydrogen dithiophosphate, and their acid
chlorides,
and the like.
The carboxylate group-containing polymerizable monomer includes, for
instance, 4-(meth)acryloyloxyethoxycabonyl phthalic acid,
^
CA 02392227 2002-05-21
7
4-(meth)acryloyloxybutoxycarbonyl phthalic acid,
4-(meth)acryloyloxyhexyloxycarbonyl phthalic acid,
4-(meth)acryloyloxyoctyloxycarbonyl phthalic acid,
4-(meth)acryloyloxydecyloxycarbonyl phthalic acid, and their acid anhydrides;
5-(meth)acryloylaminopentyl carboxylic acid, 6-(meth)acryloyloxy-1,1-hexane
dicarboxylic acid, 8-(meth)acryloyloxy-1, 1 -octane dicarboxylic acid,
10-(meth)acryloyloxy-1,1-decane dicarboxylic acid, 11-(meth)acryloyloxy-1,1-
undecane dicarboxylic acid, 5-N-(meth)acryloylaminosalicylic acid and their
acid chlorides; and the like.
Concrete examples of the sulfonate group-containing polymerizable
monomer include 2-(meth)acrylamide-2-methylpropanesulfonic acid,
styrenesulfonic acid, 2-sulfoethyl (meth)acrylate, 6-sulfohexyl
(meth)acrylate,
p-(meth)acryloyloxybenzenesulfonic acid, and the like.
Any of the acidic group-containing polymerizable monomers (a) can be
used alone or in combination of at least two kinds.
Among the acidic group-containing polymerizable monomers (a), the
phosphate group-containing polymerizable monomer, especially a phosphate
group-containing polymerizable monomer represented by the general formula
(Il)~
R4
O
11
O-R O -P -OR 6 (In
H2C I
O OH
wherein R4 is hydrogen atom or methyl group; R5 is an alkylene group having 6
to 25 carbon atoms; and R6 is hydrogen atom, an alkyl group or phenyl group,
m
CA 02392227 2002-05-21
8
can be suitably used, because there are exhibited excellent adhesive property,
adhesion durability, and peripherical sealability for enamel and dentin. It is
desired that the number of carbon atoms of the alkyl group represented by R6
is 1
to 20, preferably 1 to 10.
It is preferable that the content of the acidic group-containing
polymerizable monomer (a) in the adhesive composition of the present invention
is 1 to 70% by weight, preferably 5 to 60% by weight, more preferably 10 to
50% by weight, from the viewpoints of improvements in adhesive strength and
peripherical sealability for teeth.
The polymerizable monomer (b) is a component capable of improving
peripherical sealability. As the polymerizable monomer (b), there is used a
polymerizable monomer represented by the general formula (I):
R1
R2
~I)
H2C
Y
wherein R' is hydrogen atom or methyl group; R2 is a halogen atom, hydroxyl
group, mercapto group, amino group or -O-R3-OH group, wherein R3 is an
alkylene group having 6 to 25 carbon atoms; and Y is oxygen atom or sulfur
atom.
Representative examples of the polymerizable monomer represented by
the general formula (I) include a hydroxyl group-containing polymerizable
monomer represented by the general formula (III):
^
CA 02392227 2002-05-21
9
R1
O-R~ OH (IIn
H2C
0
wherein R' is hydrogen atom or methyl group; and R7 is an alkylene group
having 6 to 25 carbon atoms; and a polymerizable monomer represented by the
general formula (IV):
R1
R8
~
H2C
Y
wherein R' is hydrogen atom or methyl group; R8 is a halogen atom, hydroxyl
group, mercapto group or amino group; and Y is oxygen atom or sulfur atom.
These can be each used alone or in combination of at least two kinds.
Concrete examples of the hydroxyl group-containing polymerizable
monomer represented by the general formula (III) include 6-hydroxyhexyl
(meth)acrylate, 7-hydroxyheptyl (meth)acrylate, 8-hydroxyoctyl (meth)acrylate,
9-hydroxynonyl (meth)acrylate, 10-hydroxydecyl (meth)acrylate,
11-hydroxyundecyl (meth)acrylate, 12-hydroxydodecyl (meth)acrylate,
16-hydroxyhexadecyl (meth)acrylate, 20-hydroxyeicosyl (meth)acrylate, and the
like. These can be used alone or in combination of at least two kinds. Among
the hydroxyl group-containing polymerizable monomers represented by the
general formula (III), 8-hydroxyoctyl (meth)acrylate, 10-hydroxydecyl
(meth)acrylate, 12-hydroxydodecyl (meth)acrylate and 16-hydroxyhexadecyl
(meth)acrylate can be suitably used.
^
CA 02392227 2002-05-21
Concrete examples of the polymerizable monomer represented by the
general formula (IV) include (meth)acrylic acid, (meth)acrylic acid chloride,
(meth)acrylic acid bromide, 2-methylpropenethionic acid,
2-methylpropenedithionic acid, propenethionic acid, propenedithionic acid, and
5 the like. These can be used alone or in combination of at least two kinds.
Among the polymerizable monomers represented by the general formula (IV),
acrylic acid and methacrylic acid are preferable.
In the present invention, it is preferable that the hydroxyl group-
containing polymerizable monomer represented by the general formula (III) is
10 used together with the polymerizable monomer represented by the general
formula (IV), from the viewpoint of improvement in peripherical sealability.
In
this case, it is preferable that the weight ratio of the hydroxyl group-
containing
polymerizable monomer represented by the general formula (III) / the
polymerizable monomer represented by the general formula (IV) is 0.005 to 200,
preferably 0.05 to 20, from the viewpoint of improvement in peripherical
sealability.
In addition, it is preferable that the phosphate group-containing
polymerizable monomer represented by the general formula (II) is used as the
acidic group-containing polymerizable monomer (a), and that the hydroxyl
group-containing polymerizable monomer represented by the general formula
(III) is used as the polymerizable monomer (b), from the viewpoint of
improvement in the peripherical sealability. In this case, it is preferable
that the
group R5 in the phosphate group-containing polymerizable monomer represented
by the general formula (II) is identical to R' in the hydroxyl group-
containing
polymerizable monomer represented by the general formula (III), from the
CA 02392227 2002-05-21
11
viewpoint of further increasing the durability of peripherical sealability. In
addition, it is preferable that the number of carbon atoms of the alkylene
group
of R7 in the hydroxyl group-containing polymerizable monomer represented by
the general formula (III) is 8 to 25, from the viewpoints of further
increasing the
adhesion durability and the peripherical sealability.
In the present invention, the ratio of the acidic group-containing
polymerizable monomer (a) to the polymerizable monomer (b) is important. The
weight ratio of the polymerizable monomer (b) / the acidic group-containing
polymerizable monomer (a) is adjusted to 0.001 to 0.5, preferably 0.005 to
0.3,
more preferably 0.01 to 0.1, from the viewpoints of increasing the
peripherical
sealability and adhesive strength.
The hydrophilic polymerizable monomer (c) used in the present invention
refers to a compound having a solubility of at least 10%, more preferably at
least
30% in water of 25 C.
Concrete examples of the hydrophilic polymerizable monomer (c) include
2-hydroxyethyl (meth)acrylate, 2-hydroxypropyl (meth)acrylate,
3-hydroxypropyl (meth)acrylate, 1,3-dihydroxypropyl (meth)acrylate,
2,3-dihydroxypropyl (meth)acrylate, 2-hydroxypropyl-1,3-di(meth)acrylate,
3-hydroxypropyl-1,2-di(meth)acrylate, 2-trimethylammonium ethyl
(meth)acrylchloride, (meth)acrylamide, 2-hydroxyethyl (meth)acrylamide,
polyethylene glycol di(meth)acrylate, the number of oxyethylene groups of
which is at least 9, and the like. These can be used alone or in combination
of at
least two kinds. Among the hydrophilic polymerizable monomers (c), a
preference is given to hydrophilic polymerizable monomers of which alkylene
group has 1 to 3 carbon atoms, from the viewpoint of improvement in
mi
CA 02392227 2002-05-21
12
penetrability to the teeth.
It is desired that the content of the hydrophilic polymerizable monomer
(c) in the adhesive composition of the present invention is 5 to 95% by
weight,
preferably 10 to 90% by weight, more preferably 15 to 70% by weight, from the
viewpoints of improvements in adhesive property and peripherical sealability.
The adhesive composition of the present invention may contain water (d)
as occasion demands. When water (d) is contained in the adhesive composition,
adhesive property and peripherical sealability for teeth can be improved.
Water (d) may be any of those which do not substantially contain
impurities which give wrong influence to the exhibition of adhesive strength
between the teeth and the restorative material. Water (d) is preferably
distilled
water or ion-exchanged water.
It is desired that the content of water (d) in the adhesive composition of
the present invention is usually 0.1 to 80% by weight, preferably 0.5 to 70%
by
weight, more preferably 1 to 60% by weight, from the viewpoints of
improvements in adhesive property and peripherical sealability for teeth.
In the present invention, when the adhesive composition is very thinly
spread over the tooth surface with a dental air syringe or the like after the
adhesive composition is applied to the tooth surface, the adhesive composition
can be cured together with a dental bonding material, a dental luting material
and
a dental composite resin at the same time, so that a polymerization initiator
is not
necessarily required.
However, when the adhesive composition of the present invention is used
in combination with a dental luting material comprising a redox chemical
polymerization initiator, it is preferable that a reducing agent and/or an
oxidizing
CA 02392227 2002-05-21
13
agent is contained in the adhesive composition from the viewpoint of
improvement in adhesive property.
In addition, when the adhesive composition of the present invention is
coated in a thickness of at least 10 m, it is preferable that a
polymerization
initiator (e) is contained in the adhesive composition.
As the polymerization initiator (e), there can be employed a known
photopolymerization initiator and/or chemical polymerization initiator.
The photopolymerization initiator includes, for instance, a-diketones,
ketals, thioxanthones, acylphosphine oxides, coumarines, halomethyl group-
substituted-s-triazine derivatives, and the like.
Examples of the a-ketones include camphorquinone, benzyl,
2,3-pentadione, and the like.
Examples of the ketals include benzyl dimethyl ketal, benzyl diethyl ketal,
and the like. Examples of the thioxanthones include 2-chlorothioxanthone,
2,4-diethylthioxanthone, and the like.
Examples of the acylphosphine oxides include, for instance,
2,4,6-trimethylbenzoyldiphenylphosphine oxide,
2,6-dimethoxybenzoyldiphenylphosphine oxide,
2,6-dichlorobenzoyldiphenylphosphine oxide,
2,3,5,6-tetramethylbenzoyldiphenylphosphine oxide,
benzoyl di-(2,6-dimethylphenyl) phosphonate,
2,4,6-trimethylbenzoylethoxyphenylphosphine oxide, water-soluble
acylphosphine oxide compounds disclosed in Japanese Examined Patent
Publication No. Hei 3-57916, and the like.
Examples of the coumarines include compounds listed in Japanese Patent
^
CA 02392227 2002-05-21
14
Laid-Open No. Hei 10-245525, such as 3,3'-carbonylbis(7-
diethylamino)coumarine, 3-(4-methoxybenzoyl)coumarine, 3-thienoylcoumarine,
and the like.
Examples of the halomethyl group-substituted-s-triazine derivatives
include compounds listed in Japanese Patent Laid-Open No. Hei 10-245525,
such as 2,4,6-tris(trichloromethyl)-s-triazine, 2,4,6-tris(tribromomethyl)-s-
triazine and 2-methyl-4,6-bis(trichloromethyl)-s-triazine.
In addition, when the photopolymerization is carried out by ultraviolet ray
irradiation, a benzoyl alkyl ether, a benzoyl dimethyl ketal or the like is
preferable as a photopolymerization catalyst.
The photopolymerization initiator can be used alone or in combination of
at least two kinds.
The content of the photopolymerization initiator in the adhesive
composition of the present invention is not limited to specified ones. It is
desired
that the content is usually 0.01 to 10% by weight, preferably 0.1 to 5% by
weight.
When the photopolymerization initiator is used as the polymerization
initiator (e), the photopolymerization initiator may be used alone. However,
in
order to further accelerate photo-thermosetting properties, it is preferable
that the
photopolymerization initiator is used together with a reducing agent.
The reducing agent includes tertiary amines, aldehydes, thiol group-
containing compounds, and the like.
Examples of the tertiary amines include 2-dimethylaminoethyl
methacrylate, N,N-bis[(meth)acryloyloxyethyl]-N-methylamine, ethyl
4-dimethylaminobenzoate, butyl 4-dimethylaminobenzoate, butoxyethyl
4-dimethylaminobenzoate, N-methyldiethanolamine,
CA 02392227 2002-05-21
4-dimethylaminobenzophenone, and the like.
Examples of the aldehydes include dimethylaminobenzaldehyde,
terephthalaldehyde, and the like.
Examples of the thiol group-containing compounds include
5 2-mercaptobenzoxazole, decanethiol, 3-mercaptopropyltrimethoxysilane,
thiobenzoic acid, and the like.
These reducing agents can be used alone or in combination of at least two
kinds.
The content of the reducing agent in the adhesive composition of the
10 present invention is not limited to specified ones. It is desired that the
content is
usually 0.01 to 10% by weight, preferably 0.05 to 7% by weight, more
preferably
0.1 to 5% by weight.
In addition, as the chemical polymerization initiator, for instance, there
can be suitably used a redox polymerization initiator comprising an oxidizing
15 agent and a reducing agent.
When the redox polymerization initiator is used, there is a necessity to
divide the adhesive composition into at least two wrapped portions. However,
when the adhesive composition of the present invention is used together with
the
other material, for instance, a restorative material such as a dental bonding
material, a composite resin, a compomer, a resin for denture base, a resin
cement
or a resin-reinforceable glass ionomer cement, it is possible to include only
one
of the oxidizing agent and the reducing agent in the adhesive composition, as
long as at least one of the oxidizing agent and the reducing agent is
contained in
the restorative material.
The oxidizing agent includes, for instance, organic peroxides such as
CA 02392227 2002-05-21
16
diacyl peroxides, peroxy esters, dialkyl peroxides, peroxy ketals, ketone
peroxides and hydroperoxides.
Concrete examples of the diacyl peroxides include benzoyl peroxide,
2,4-dichlorobenzoyl peroxide, m-toluoyl peroxide, and the like.
Concrete examples of the peroxy esters include t-butylperoxy benzoate,
bis-t-butylperoxy isophthalate, 2,5-dimethyl-2,5-bis(benzoylperoxy)hexane,
t-butylperoxy-2-ethylhexanoate, t-butylperoxy isopropyl carbonate, and the
like.
Concrete examples of the dialkyl peroxides include dicumyl peroxide,
di-t-butyl peroxide, lauroyl peroxide, and the like.
Concrete examples of the peroxy ketals include 1,1-bis(t-butylperoxy)
3,3,5-trimethylcyclohexane, 1,1-bis(t-butylperoxy)cyclohexane,
1,1-bis(t-hexylperoxy)cyclohexane, and the like.
Concrete examples of the ketone peroxides include methyl ethyl ketone
peroxide, cyclohexanone peroxide, methyl acetoacetate peroxide, and the like.
Concrete examples of the hydroperoxides include t-butyl hydroperoxide,
cumene hydroperoxide, p-diisopropylbenzene peroxide, and the like.
As the reducing agent, aromatic tertiary amines, aliphatic tertiary amines,
sulfinic acids and their salts can be cited as preferred ones.
Concrete examples of the aromatic tertiary amines include
N,N-dimethylaniline, N,N-dimethyl-p-toluidine, N,N-dimethyl-m-toluidine,
N,N-diethyl-p-toluidine, N,N-dimethyl-3,5-dimethylaniline,
N,N-dimethyl-3,4-dimethylaniline, N,N-dimethyl-4-ethylaniline,
N,N-dimethyl-4-i-propylaniline, N,N-dimethyl-4-t-butylaniline,
N,N-dimethyl-3,5-di-t-butylaniline, N,N-bis(2-hydroxyethyl)-
3,5-dimethylaniline, N,N-di(2-hydroxyethyl)-p-toluidine,
~
CA 02392227 2002-05-21
17
N,N-bis(2-hydroxyethyl)-3,4-dimethylaniline, N,N-bis(2-hydroxyethyl)-
4-ethylaniline, N,N-bis(2-hydroxyethyl)-4-i-propylaniline,
N,N-bis(2-hydroxyethyl)-4-t-butylaniline, N,N-bis(2-hydroxyethyl)-
3,5-di-i-propylaniline, N,N-bis(2-hydroxyethyl)-3,5-di-t-butylaniline,
n-butoxyethyl 4-dimethylaminobenzoate, (2-methacryloyloxy)ethyl
4-dimethylaminobenzoate, and the like.
Concrete examples of the aliphatic tertiary amines include trimethylamine,
triethylamine, N-methyldiethanolamine, N-ethyldiethanolamine,
N-n-butyldiethanolamine, N-lauryldiethanolamine, triethanolamine,
(2-dimethylamino)ethyl methacrylate, N-methyldiethanolamine dimethacrylate,
N-ethyldiethanolamine dimethacrylate, triethanolamine monomethacrylate,
triethanolamine dimethacrylate, triethanolamine trimethacrylate, and the like.
Concrete examples of the sulfinic acids or their salts include
benzenesulfinic acid, sodium benzenesulfinate, potassium benzenesulfinate,
calcium benzenesulfinate, lithium benzenesulfinate, toluenesulfinic acid,
sodium
toluenesulfinate, potassium toluenesulfinate, calcium toluenesulfinate,
lithium
toluenesulfinate, 2,4,6-trimethylbenzenesulfinic acid, sodium
2,4,6-trimethylbenzenesulfinate, potassium 2,4,6-trimethylbenzenesulfinate,
calcium 2,4,6-trimethylbenzenesulfinate, lithium 2,4,6-
trimethylbenzenesulfinate,
2,4,6-triethylbenzenesulfinic acid, sodium 2,4,6-triethylbenzenesulfinate,
potassium 2,4,6-triethylbenzenesulfinate, calcium 2,4,6-
triethylbenzenesulfinate,
2,4,6-i-propylbenzenesulfinic acid, sodium 2,4,6-triisopropylbenzenesulfinate,
potassium 2,4,6-triisopropylbenzenesulfinate, calcium
2,4,6-triisopropylbenzenesulfinate, and the like.
These oxidizing agents and reducing agents can be used alone or in
m
CA 02392227 2002-05-21
18
combination of at least two kinds.
The content of the chemical polymerization initiator in the adhesive
composition of the present invention is not limited to specified ones. It is
desired
that the content is usually 0.01 to 10% by weight, preferably 0.05 to 7% by
weight, more preferably 0.1 to 5% by weight.
For the purpose of improvements in thermosetting property and
mechanical strength, there may be contained in the adhesive composition of the
present invention a hydrophobic polymerizable monomer other than the acidic
group-containing polymerizable monomer (a), the polymerizable monomer (b)
and the hydrophilic polymerizable monomer (c) (hereinafter simply referred to
as
"hydrophobic polymerizable monomer").
The hydrophobic polymerizable monomer includes, for instance, esters of
carboxylic acids such as a-cyanoacrylic acid, (meth)acrylic acid, a-
halogenated
acrylic acids, crotonic acid, cinnamic acid, sorbic acid, maleic acid and
itaconic
acid; (meth)acrylamide and derivatives of (meth)acrylamide; vinyl esters;
vinyl
ethers; mono-N-vinyl derivatives, styrene derivatives, and the like. Among
them,
(meth)acrylic acid esters are preferably used.
Concrete examples of the hydrophobic polymerizable monomer include
the following monomers. The term "monofunctional monomer" as referred to
herein means a monomer having one olefinic double bond.
(A) Monofunctional Monomer
Methyl (meth)acrylate, ethyl (meth)acrylate, i-propyl (meth)acrylate,
i-butyl (meth)acrylate, benzyl (meth)acrylate, lauryl (meth)acrylate,
2,3-dibromopropyl (meth)acrylate, 3-methacryloyloxypropyl trimethoxysilane,
11-methacryloyloxyundecyl trimethoxysilane, and the like.
CA 02392227 2002-05-21
19
(B) Bifunctional Monomer
Ethylene glycol di(meth)acrylate, triethylene glycol di(meth)acrylate,
propylene glycol di(meth)acrylate, neopentyl glycol di(meth)acrylate,
1,6-hexanediol di(meth)acrylate, 1,10-decanediol di(meth)acrylate, bisphenol A
diglycidyl (meth)acrylate, 2,2-bis[4-(meth)acryloyloxyethoxyphenyl]propane,
2,2-bis[4-(meth)acryloyloxypolyethoxyphenyl]propane,
2,2-bis[4-[3-(meth)acryloyloxy-2-hydroxypropoxy]phenyl]propane,
1,2-bis [3-(meth)acryloyloxy-2-hydroxypropoxy] ethane, pentaerythritol
di(meth)acrylate,1,2-bis(3-methacryloyloxy-2-hydroxypropoxy)ethane,
[2,2,4-trimethylhexamethylenebis(2-carbamoyloxyethyl)] dimethacrylate, and
the like.
(C) Monomers Having Tri- or More Functional Group
Trimethylolpropane tri(meth)acrylate, trimethylolethane tri(meth)acrylate,
tetramethylolmethane tri(meth)acrylate, pentaerythritol tetra(meth)acrylate,
N,N'-(2,2,4-trimethylhexamethylene)bis[2-(aminocarboxy)propane-l,3-diol]
tetramethacrylate, 1,7-diacryloyloxy-2,2,6,6,-tetraacryloyloxymethyl-4-
oxyheptane, and the like.
The above-mentioned hydrophobic polymerizable monomers can be used
alone or in combination of at least two kinds:
It is desired that the content of the hydrophobic polymerizable monomer
in the adhesive composition of the present invention is usually at most 60% by
weight, preferably at most 40% by weight, especially from the viewpoint of
maintaining adhesive strength against enamel.
In addition, in the present invention, in order to supplement the solubility
of the acidic group-containing polymerizable monomer (a), the polymerizable
i
CA 02392227 2002-05-21
monomer (b) and the polymerization initiator in the adhesive composition, a
volatile organic solvent can be contained in the adhesive composition.
As the volatile organic solvent, it is desired to use a volatile organic
solvent usually having a boiling point of at most 150 C, preferably at most
5 100 C at atmospheric pressure.
Preferred examples of the volatile organic solvent include alcohols such
as ethanol, methanol, 1-propananol and isopropananol; ketones such as acetone
and methyl ethyl ketone; ester compounds such as ethyl acetate, methyl acetate
and ethyl propionate; ethers such as 1,2-dimethoxyethane, 1,2-diethoxyethane
10 and tetrahydrofuran; hydrocarbon compounds such as heptane, hexane and
toluene; halogenated hydrocarbon compounds such as chloroform and
dichloromethane; and the like. These volatile organic solvents may be used
alone or in combination of at least two kinds. Among them, a water-soluble
volatile solvent such as ethanol or acetone is preferable.
15 The content of the volatile organic solvent in the adhesive composition of
the present invention is not limited to specified ones. It is desired that the
content is usually at most 60% by weight, preferably at most 30% by weight.
When the volatile organic solvent is contained in the adhesive
composition, in order not to impair its adhesive property, it is preferable
that the
20 volatile solvent is evaporated as much as possible with a dental air
syringe or the
like after the adhesive composition is applied to a tooth.
In addition, a polymerization inhibitor, a colorant, a fluorescent, an
ultraviolet absorbent and the like may be properly added to the adhesive
composition of the present invention as occasion demands.
Furthermore, for the purpose of imparting antibacterial property to the
m
CA 02392227 2002-05-21
21
adhesive composition of the present invention, there may be contained in the
adhesive composition of the present invention a cationic antibacterial
compound
such as cetyl pyridium chloride, chlorhexidine hydrochloride, a benzalkonium
chloride, (meth)acryloyloxydodecyl pyridium bromide,
(meth)acryloyloxyhexadecyl pyridium chloride or (meth)acryloyloxydecyl
ammonium chloride.
In addition, for the purpose of imparting acid resistance to the adhesive
composition of the present invention, there may be contained in the adhesive
composition of the present invention a known fluorine compound which releases
a fluorine ion, such as sodium fluoride, lithium fluoride or cetylamine
hydrofluoride.
Furthermore, a filler can be contained in the adhesive composition of the
present invention within an amount which would not impair the fluidity of the
adhesive composition. The filler includes an inorganic filler, an organic
filler or
their composites.
The inorganic filler includes, for instance, minerals of which basic
material is silica, such as silica, kaolin, clay and mica; ceramics of which
basic
material is silica, comprising A1203, B203, Ti02, Zr02, BaO, La203, Sr02, CaO
P205 and the like; glass such as lanthanum glass, barium glass, strontium
glass,
sodium glass, lithium borosilicate glass, zinc glass, fluoroaluminum
borosilicate
glass, borosilicate glass and bio glass; crystal silica, hydroxylapatite,
alumina,
titanium oxide, yttrium oxide, zirconia, calcium phosphate, barium sulfate,
aluminum hydroxide, and the like.
The organic filler includes, for instance, organic resins such as polymethyl
methacrylate, polyfunctional methacrylate polymer, polyamide, polystyrene,
m
CA 02392227 2002-05-21
22
polyvinyl chloride, chloroprene rubber, nitrile rubber and styrene-butadiene
rubber. In addition, there can be also used an inorganic/organic composite
filler
in which the inorganic filler is dispersed in these organic resins, or in
which the
inorganic filler is coated with the above-mentioned organic resin. These
fillers
can be used alone or in combination of at least two kinds.
For the purpose of controlling the fluidity of the adhesive composition,
these fillers may be used after previously subjecting to a surface treatment
with a
known agent for surface treatment, such as a silane coupling agent as occasion
demands.
The agent for surface treatment includes, for instance,
vinyltrimethoxysilane, vinyltriethoxysilane, vinyltrichlorosilane,
vinyltri((3-methoxyethoxy)silane, y-methacryloyloxypropyltrimethoxysilane,
y-glycidoxypropyltrimethoxysilane, y-mercaptopropyltrimethoxysilane,
y-aminopropyltriethoxysilane, and the like.
The content of the filler in the adhesive composition of the present
invention is not limited to specified ones. It is desired that the content is
usually
at most 30% by weight, preferably at most 10% by weight.
In addition, it is preferable that the viscosity of the adhesive composition
is controlled to at most 1 Pa-s at 30 C by dispersing a filler having an
average
particle diameter of 0.001 to 30 m in the adhesive composition, from the
viewpoints of penetrability, coatability, thermosetting property for teeth.
When
the adhesive composition of the present invention is used as an adhesive, it
is
preferable to control the viscosity to 0.1 to 1 Pa-s at 30 C.
The adhesive composition of the present invention can be used in various
forms. For instance, the adhesive composition of the present invention can be
~
CA 02392227 2002-05-21
23
used as an agent for pretreatment in order to bond to teeth a material to be
bonded, such as a dental bonding material, a resin cement, a glass ionomer
cement, a zinc phosphate cement, a polycarboxylate cement, a silicate cement
or
a zinc oxide eugenol cement, or to enhance adhesive properties (form 1). In
addition, the adhesive composition also can be used as an adhesive for bonding
a
dental composite resin or a compomer for filling with teeth (form 2).
Furthermore, the adhesive composition can be used as a fissure sealant
material
applied to pit and fissure, or a coating material for root and a proximal
teeth
portion (form 3), or can be used as a dentinal canal sealant material for the
purpose of suppressing hyperesthesia (form 4).
When the adhesive composition is used in the form 1, it is preferable that
the content of the acidic group-containing polymerizable monomer (a) in the
adhesive composition is 5 to 40% by weight, that the content of the
polymerizable monomer (b) is 0.005 to 20% by weight, and that the content of
the hydrophilic polymerizable monomer (c) is 10 to 90% by weight, and it is
more preferable that water (d) is contained in a content of 5 to 60% by
weight.
In this case, the weight ratio of (b)/(a), that is, the polymerizable monomer
(b)/the acidic group-containing polymerizable monomer (a) is adjusted to 0.001
to 0.5.
In addition, when the adhesive composition is used in the forms 2 to 4, it
is preferable that the content of the acidic group-containing polymerizable
monomer (a) in the adhesive composition is 10 to 50% by weight, that the
content of the polymerizable monomer (b) is 0.01 to 25% by weight, and that
the
content of the hydrophilic polymerizable monomer (c) is 5 to 50% by weight,
and it is more preferable that water (d) is contained in a content of 0.1 to
10% by
m
CA 02392227 2002-05-21
24
weight. In this case, the weight ratio of the polymerizable monomer (b)/the
acidic group-containing polymerizable monomer (a) is adjusted to 0.001 to 0.5.
In addition, it is preferable to include in the adhesive composition a
hydrophobic
polymerizable monomer other than the acidic group-containing polymerizable
monomer (a), the polymerizable monomer (b) and the hydrophilic polymerizable
monomer (c) in a content of 5 to 40% by weight, from the viewpoints of more
improving adhesive property and peripherical sealability. In addition, for the
purpose of improvements in coatability and mechanical strength, a filler may
be
contained in this adhesive composition within a range of at most 30% by
weight.
The,adhesive composition of the present invention can be used for not
only hard tissues such as teeth but also crown restorative materials such as
metals, porcelains, ceramics and composite resin cured products. Further, the
adhesive composition of the present invention may be used in combination with
a commercially available dental metal primer, a primer for bonding porcelain,
an
acidic etching agent or a tooth face cleaning agent such as a hypochlorite.
Among them, it is especially preferable that the adhesive composition of the
present invention is used in combination with the acidic etching agent,
because
more excellent peripherical sealability can be imparted to enamel.
Next, the present invention will be described more specifically on the
basis of the following examples, but the present invention is by no means
limited
thereto.
The abbreviated names and symbols are as follows:
[Acidic Group-Containing Hydrophobic Polymerizable Monomers]
m
CA 02392227 2002-05-21
MDP: 10-Methacryloyloxydecyl dihydrogen phosphate
MOP: 8-Methacryloyloxyoctyl dihydrogen phosphate
ADDP: 12-Acryloyloxydodecyl dihydrogen phosphate
MHEP: 16-Methacryloyloxyhexadecyl ethylhydrogen phosphate
5 MEPP: Methacryloyloxy ethylphenyl phosphonate
MDPP: 10-Methacryloyloxydecyl phosphonic acid
4-MHPT: 4-Methacryloyloxyhexyl oxycarbonyl phthalic acid
[Hydroxyl Group-Containing Polymerizable Monomers]
10 4HM: 4-Hydroxybutyl methacrylate
6HM: 6-Hydroxyhexyl methacrylate
8HM: 8-Hydroxyoctyl methacrylate
1OHM: 10-Hydroxydecyl methacrylate
12HA: 12-Hydroxydodecyl methacrylate
15 16HA: 16-Hydroxyhexadecyl methacrylate
[Hydrophilic Polymerizable Monomers]
HEMA: 2-Hydroxyethyl methacrylate
9G: Polyethylene glycol dimethacrylate (number of moles of
20 oxyethylene group added being 9)
14G: Polyethylene glycol dimethacrylate (number of moles of
oxyethylene group added being 14)
[Polymerization Initiator and Reducing Agent]
25 CQ: Camphorquinone
- --- --------
CA 02392227 2002-05-21
26
TMDPO: 2,4,6-Trimethylbenzoyldiphenylphosphine oxide
DEPT: N,N-Di(2-hydroxyethyl)-p-toluidine
TPBSS: Sodium 2,4,6-triisopropylbenzenesulfinate
DMAB: N,N-Dimethylaminobenzophenone
[Polymerization Inhibitor]
BHT: t-Butylhydroxytoluene
[Other Polymerizable Monomers]
Bis-GMA: Bisphenol A diglycidyl methacrylate
UDMA: [2,2,4-Trimethylhexamethylenebis(2-carbamoyloxyethyl)]
dimethacrylate
Example 1
An adhesive composition composed of 20 parts by weight of MDP,
0.02 parts by weight of methacrylic acid, 40 parts by weight of HEMA and
40 parts by weight of distilled water was prepared. The adhesive strength and
peripherical sealability were determined in accordance with the following
testing
method for adhesive strength and testing method for peripherical sealability.
The
results are shown in Table 1.
[Testing Method for Adhesive Strength]
Bovine front teeth were smoothly wet-grinded with #1000 silicon carbide
paper (manufactured by Nippon Kenshi K.K.) to expose their enamel surfaces or
dentin surfaces, and thereafter water on the surfaces was blown away with a
CA 02392227 2008-07-28
27
dental air syringe. An adhesive tape having a thickness of about 150 m with a
hole opening having a diameter of 3 mm was pasted to the exposed enamel
surfaces or dentin surfaces, and the adhesive composition obtained in Example
1
was applied to the hole with a brush. The adhesive composition was allowed to
stand for 30 seconds, and thereafter dried with an air syringe to a degree
such
that the adhesive composition had no fluidity. A photopolymerizable dental
bonding material "Clearfil Megabond" (manufactured by Kuraray Co., Ltd.,
trade mark) was applied onto the dried adhesive composition with a brush so
that
its thickness became about 100 m. The resulting coat was photo-irradiated
with
a dental photoirradiator "LIGHTEL II" (manufactured by Gunma Ushio Denki
K.K., trade mark) for 10 seconds to cure the coat. Further, a commercially
available photopolymerizable dental composite resin "Clearfil AP-X"
(manufactured by Kuraray Co., Ltd., trade mark) was mounted on the cured coat,
and covered with a film made of EVAL (manufactured by Kuraray Co., Ltd.,
registered trademark). Thereafter, the slide glass was pressed over the film,
and
photo-irradiated for 40 seconds with the above-mentioned photoirradiator to
cure
the composite resin.
A stainless steel rod was bonded to the cured surface with a commercially
available dental resin cement "Panavia 21" (manufactured by Kuraray Co., Ltd.,
trade mark) to give a test piece. After 30 minutes, the test piece was
immersed
in water of 37 C for 24 hours, and thereafter a thermal cycle comprising -
immersing the test piece in cold water of 4 C for one minute and in hot water
of
60 C for one minute was carried out 2000 times. Thereafter, its adhesive
strength was determined. A universal tester (manufactured by Instron) was used
for the determination of the adhesive strength, and the tensile adhesive
strength
CA 02392227 2008-07-28
28
was determined under the conditions of a cross head speed of 2 mm/minute. The
determination value for each adhesive strength was expressed as an average
value of the determination values of 8 test pieces.
[Testing Method for Peripherical Sealability]
A cavity having a diameter of about 4 mm and a depth of about 3 mm was
formed by using a dental air turbine so that the cervical line of the molar
portion
of a human evulsed tooth was positioned at the center. The adhesive
composition obtained in Example 1 was applied to the internal surface of the
cavity, and the adhesive composition was allowed to stand for 30 seconds, and
thereafter dried with an air syringe to a degree such that the adhesive
composition had no fluidity. A photopolymerizable dental bonding material
"Clearfil Megabond" (manufactured by Kuraray Co., Ltd., trade mark), was
applied to the internal surface of the cavity so that its thickness became
about
100 m. The resulting coat was photo-irradiated for 10 seconds with a dental
photoirradiator "LIGHTEL II" (manufactured by Gurima Ushio Denki K.K.,
trade mark) to cure the coat. Further, a commercially available
photopolymerizable dental composite resin "Clearfil AP-X" (manufactured by
Kuraray Co., Ltd., trade mark) was filled in the cavity, and photo-irradiated
for
40 seconds with the above-mentioned photoirradiator to cure the composite
resin.
Subsequently, in order to prevent the penetration of the colorant from the tip
of
tooth root and the scissure at crown and the like, "Clearfil Megabond"
(manufactured by Kuraray Co., Ltd., trade mark) was applied to the portions
other than the cavity restorative portion and its surroundings, and photo-
irradiated for 30 seconds with the above-mentioned photoirradiator to cure the
^
CA 02392227 2002-05-21
29
resin.
The resulting test piece was immersed in water at 37 C for 24 hours, and
thereafter a thermal cycle comprising immersing in cold water of 4 C for one
minute and in hot water of 60 C for one minute was carried out 2000 times.
Thereafter, the test piece was immersed in a 0.2% aqueous basic fuchsine
solution at 37 C for 24 hours, and thereafter the test piece was taken out
from the
solution and washed with water. The test piece was dried with a dental air
syringe, and its restored portion was divided into three portions lengthwise
using
a low-speed diamond cutter to give three slices per tooth. Fifteen slices were
prepared from five molar teeth from human teeth in total.
The evaluation of the penetration of the colorant was made by observing
both the cavity margin of a tooth top side (enamel side) and the cavity margin
of
a gingival side (dentin side) by naked eyes with a light microscope
(magnification: 25 times), obtaining the following scores, and averaging the
scores of 15 slices.
Score 0: No penetration of a colorant to wall and bottom of the cavity is
recognized.
Score 1: No penetration of a colorant to the bottom of the cavity is
recognized, but as to the wall of the cavity, penetration of the
colorant to at most 1/2 of the wall of the cavity is recognized.
Score 2: No penetration of a colorant to the bottom of the cavity is
recognized, but as to the wall of the cavity, penetration of the
colorant to at least 1/2 of the wall of the cavity is recognized.
Score 3: Penetration of a colorant to wall and bottom of the cavity is
CA 02392227 2002-05-21
recognized.
Examples 2 to 7 and Comparative Examples 1 to 3
Adhesive compositions in which the amount of methacrylic acid was
5 changed were prepared as shown in Table 1. The adhesive strength test and
peripherical sealability test were carried out in the same manner as in
Example 1
using these adhesive compositions. The results are also shown in Table 1.
CA 02392227 2002-05-21
31
r. o 0 0~, "? od r.' ~~c u
bp
U
N 'o M 00
0 N ,-~ O O
d n
cO O
rA
..~~ N '~ ~t d ~ N C p C
N N
r-4 N N
M O ~ ~ ~ O N N
N N Q ~t d ~ 01 CN
e-1 r-1 O O
M ~o h l~
C5 c~ O~ p C
.,,
a~ .
3 a ~
a, ^
~ :b
~. g va
õ
1-4
O
~ U ~ =V bCA UC/) :U
u y,a V~ ~. V1
O a4-4
~~+
.~ i~ =n ,~ U U ~ .~
.C O
~ A~~ W A'G E-~ C7
d a
CA 02392227 2002-05-21
32
~ M N dp- dp ~ tV vi Cfi ~ `--+
W t/~ N
V1 M
> cV OO OO O Q~F ~ ~ Cfi
cd
O cn p .--~
U~ N O ~ ~ p 0~ c~ cv
~.+
O
.,,
a)
.a ^
o ~i ,~ õ E" =~ G
cl -cm
o a
.. a~
pm o o~ cn ti
4-4 4-4 04
cn
(U
> co
~ a=
^i
CA 02392227 2002-05-21
33
As is clear from the results shown in Table 1, when the adhesive
compositions of the present invention in which the weight ratio of methacrylic
acid/MDP is controlled to the range of 0.001 to 0.5 (Examples 1 to 7) are
used, it
can be seen that excellent adhesive strength and excellent peripherical
sealability
are exhibited.
On the other hand, when the adhesive composition in which methacrylic
acid is not contained (Comparative Example 1) is used, it can be seen that
excellent adhesive strength is exhibited, but peripherical sealability is
drastically
deteriorated.
In addition, when the adhesive composition in which the weight ratio of
methacrylic acid/MDP is controlled to 1.0 or 2.0 (Comparative Examples 2 and
3) is used, it can seen that adhesive strength is clearly lowered, and that
peripherical sealability is deteriorated.
Examples 8 to 14 and Comparative Examples 4 to 6
Adhesive compositions composed of MDP, 1OHM, HEMA, distilled
water and the like were prepared as shown in Table 2. The adhesive strength
test
and peripherical sealability test were carried out in the same manner as in
Example 1 using these adhesive compositions. The results are also shown in
Table 2.
^i
CA 02392227 2002-05-21
34
T.-I O p O ~ ~? 00 oo ~O
p
~
rM-+ pN ~ ~ dQ' M O 00 N rt ~
~ N N dp dO p N O CO
N
~
M
ca ~-+ N p 'd ~ pp" N O C
W O OO N O p p ~ ~ d^ et
00 06 06 C C
M 00
~ pC 01
O O
N '"'~ d= V~
00
00 O C
.~ a
... v~
wo
g o a
0 0
~ co
o tn
~r'' =~ ~ ~~ >
=~h c~7
~C =-~ Q a,
= CA 02392227 2002-05-21
ry
^~ ~ N xn,,, M
k,., o o c~ Q o o ~t
V
~..~ r. i CV N
..+
=..,
.a
O p 'll VJ
0
,~ C~ ,.^p ~.~+ H ,=~"~ =A
b `~ :c~ =~ ' '" A
o
o~~
4-4
p
.~a, ~ ,~ ~ =q~
~
A
~ d w
^
CA 02392227 2002-05-21
36
As is clear from the results shown in Table 2, when the adhesive
compositions in which the weight ratio of 1OHM/MDP is controlled to the range
of 0.001 to 0.5 (Examples 8 to 14) are used, it can be seen that 'excellent
adhesive
strength and excellent peripherical sealability are exhibited.
On the other hand, when the adhesive composition in which 1OHM is not
contained (Comparative Example 4) is used, it can be seen that excellent
adhesive
strength is exhibited, but peripherical sealability is drastically
deteriorated.
In addition, when the adhesive composition in which the weight ratio of
10HM/MDP is controlled to 1.0 or 2.0 (Comparative Examples 5 and 6) is used,
it can be seen that adhesive strength is clearly lowered, and that
peripherical
sealability is deteriorated.
Examples 15 to 18
Adhesive compositions composed of MEPP, methacrylic acid, HEMA,
distilled water, CQ, DEPT, TPBSS and DMAB were prepared as shown in Table
3. The adhesive strength test and peripherical sealability test were carried
out in
the same manner as in Example 1 using these adhesive compositions. The
results are also shown in Table 3.
Comparative Examples 7 to 10
Adhesive compositions in which methacrylic acid was removed from the
adhesive compositions used in Examples 15 to 18 were prepared as shown in
Table 3. The adhesive strength test and peripherical sealability test were
carried
out in the same manner as in Example 1 using these adhesive compositions. The
results are also shown in Table 3.
^i
CA 02392227 2002-05-21
37
b
M 00 00
00 N N cn cn 01 0~
O O
AO
U
I~ O M v1 tn M M M
M p M M p ~ N N N O O
.-,
tn n i!1 tn M ~O M M M
~ c`, N C
dN' dN' C C O N N O C
V1 W) N M rF M
~ t--: C O
M
N
O
=.~ +
16
~r n
A=+ y ~,,,~ U
o
.~ ~
~ a
O ~ N =U >O y c~ b N
U f C~ 0 N [/~ .'C
p^ V r3 4-4 ~Q C/) a
4.4
Ur U p
*ZZ
ACY A~ ~oi W A g C7
a
^
CA 02392227 2002-05-21
38
tn N G~
O~ O~ O C) Q v) N M
a0 00
W M O M M ~ v1 N ~ N O ~ 1-i
~
00 cq Cq CO 'n N cl O C`1 O ~ ~
V
CD 0
M
~ O ~ ~ O in N ~ O 0~0 o~0 N N
O
=~
'd ^
cn
3 14 ; En
a. $ o
o
o . ~ o ~
V c~tl v A~ 0 u~%) O
~ p U '3- w C/)
V~ O 0
EO
>
N A,q ~d ,r
p ..r QI ~ ~y r.'H+ ~ ~j ~ ...~ =+ N ~-. = Eb
=~ v~ ~, 0 W Fq ~CC ~ -b $ a
A U A A A~ E-~ C7
CA 02392227 2002-05-21
39
As is clear from the results shown in Table 3, when the adhesive
compositions in which methacrylic acid is blended (Examples 15 to 18) are
used,
it can be seen that excellent adhesive strength and excellent peripherical
sealability are exhibited.
On the other hand, when the adhesive compositions in which methacrylic
acid is not contained (Comparative Examples 7 to 10) are used, it can be seen
that excellent adhesive strength is exhibited, but peripherical sealability is
drastically deteriorated.
Examples 19 to 23
Adhesive compositions composed of MDP, 1OHM, HEMA, distilled
water, CQ, DEPT, TPBSS and DMAB were prepared as shown in Table 4. The
adhesive strength test and peripherical sealability test were carried out in
the
same manner as in Example 1 using these adhesive compositions. The results
are also shown in Table 4.
Comparative Examples 11 to 15
Adhesive compositions in which 1OHM was removed from the adhesive
compositions used in Examples 19 to 23 were prepared as shown in Table 4.
The adhesive strength test and peripherical sealability test were carried out
in the
same manner as in Example 1 using these adhesive compositions. The results
are also shown in Table 4.
^i
CA 02392227 2002-05-21
.-4 v, ,--l
c~ ~ o 0 0 o O o 0 00 00 o O
..,
cz
N p M tn tn V1 O v1 n M M
O M M O M O C C N N O C
rA
O M p 0 VS O v1 tq M ~7' ~
M ~j l- O M C 06 06
O O
O V~ -~ ~ O N V~ M tI ~ M C*)
N *'i Cj dN' ~'N' O M O O O N N O
~ -n O u1 ~ 6 CO
O M O O Cj
E-+
...,
.O
cn
O +~ .tf ~ V]
O p
[ 4-
O y~"j ~ '~ = ~ `=s
r4
q ^ ^ y 3 a 9 o p4 v~ o v~ 4-4
Va~ ~~ ~.-, va Q a~i a .~ E-' ~
~'aW ~
V A~ A o~W A~F~ C7
'~ ~ ~ a
a
CA 02392227 2002-05-21
41
-r1 O v1 v 1 N 0 M
.~-+ OM OM OM M O O 00 00 r-i r+
~~ O O M M v1 O -n v~ ~ ~
.-+ v v1
C M O O N 14
~ O ~ V1 M N M
~ rcf) ..q ~ O Q O O M O O O 00 00 r-+ ~--i
t~
rQ V1 v~ 00 ~G
v r"q r" i O ~ N~ O M O O O N N `-i
.V-.4-~ CD O In dtn p tvi p p O o6 a0 r4 ~--i
^p O O ~ fn
F. .r
O bD A
O aa a~ ~ C
0 C%]
4-4
O O
> > A x rn c7 w ~ A S
c7
~ U A~ A~~ W A r. E2
, Q <C a
^i
CA 02392227 2002-05-21
42
As is clear from the results shown in Table 4, when the adhesive
compositions in which 1OHM is blended (Examples 19 to 23) are used, it can be
seen that excellent adhesive strength and excellent peripherical sealability
are
exhibited.
On the other hand, when the adhesive compositions in which 1OHM is not
contained (Comparative Examples 11 to 15) are used, it can be seen that
excellent adhesive strength is exhibited, but peripherical sealability is
drastically
deteriorated.
Examples 24 to 31
Adhesive compositions prepared by adding methacrylic acid, acrylic acid,
methacrylic acid chloride or 2-methylpropenedithionic acid to a composition
composed of MDPP or 4-MHPT, HEMA, distilled water, DEPT, TPBSS and the
like were prepared as shown in Table 5. The adhesive strength test and
peripherical sealability test were carried out in the same manner as in
Example 1
using these adhesive compositions. The results are also shown in Table 5.
Comparative Examples 16 and 17
Adhesive compositions in which methacrylic acid was removed from an
adhesive composition used in Example 24 and an adhesive composition used in
Example 28 were prepared as shown in Table 5. The adhesive strength test and
peripherical sealability test were carried out in the same manner as in
Example 1
using these adhesive compositions. The results are also shown in Table 5.
a
CA 02392227 2002-05-21
43
W) W) tn 00 \~c \c
M O~ O O O O~ dN tn N ~tr; G O N
C~
-I'1 V~ 4/1 tn ~t ~D ~D
M O O r' O N ~ N~ C C
O ~T d= O ~
M M
O~ O O O ~~~ N O ~~ O C
V~ i/~ v1 V1 ~. M M
N O ~ o O O O N c~ tn N O tri wi C C
O O O O r; (~l CV ~ N O =-~ v1 v~
O t O ON ON O O
V) v1 V'1 ~ N1-t
N kn O O O O O cl N= N N Q O O O O
kn
4:
E-+ in tn i/'1 in 00 N N
N O p O O ~', ~ N O O O O 6
V1 v1 v'1 v~ t*~ N N N
N ~ O ~ O O O cV cV vi N~ O O O O
G d~ O N N
.~
v] ~i = .~, R~ ~, p 'C,,,ii f~ ~
En
t~~p o U b ~=~" p a
p~+A t7;d U ~ 0= . N .,'~.~ ~
o~g 04 ~ OQa=..
a b
V~~ ~ ~ b o~ o o ~
a~ .ti cd
~ F+ ~' ~ ~i ~ % ~ ~ ~ ~ =~ ~ =~ ~
w Q .ti o
00 ~W A=~H CA
7
ad~ ~ a
CA 02392227 2002-05-21
44
O In tn
O O O O tn N O N N
~ h i/'~ 00
0 O 0 O c-4~ try N O ~ ~
,r e)
-O
W
~ ..~. LD 'G; L~ ==~' ~~''' '~ y
ou r. u.~ O O O^
cu O
A ~ :b '1~ N ' -, EO O ~ .~
0 O El
O c~ U A '-= O'~ }' E~ :~ ~, a~i
N
0(~ ~ V U V 0 O A ~El
Q' It, 0 0 O
o o o v
w
~ "
CA~~~
o~ W A'~ E t7
a
mI
CA 02392227 2002-05-21
As is clear from the results shown in Table 5, when the adhesive
compositions in which methacrylic acid, acrylic acid, methacrylic acid
chloride
or 2-methylpropenedithionic acid is blended (Examples 24 to 31) are used, it
can
be seen that excellent adhesive strength and excellent peripherical
sealability are
5 exhibited.
On the other hand, when the adhesive compositions in which methacrylic
acid and the like are not contained (Comparative Examples 16 and 17) are used,
it can be seen that excellent adhesive strength is exhibited, but peripherical
sealability is drastically deteriorated.
Examples 32 to 40
Adhesive compositions composed of MDP,10HM, HEMA, distilled
water, methacrylic acid or acrylic acid, DEPT and TPBSS were prepared as
shown in Table 6. The adhesive strength test and peripherical sealability test
were carried out in the same manner as in Example 1 using these adhesive
compositions. The results are also shown in Table 6.
CA 02392227 2002-05-21
46
O O N tn N It
O -+ O O ~ O C
p p V1 O N d '-4 M
M N O ~ M oo ~ M r O
00 O O O u? O 0 M 0~ M M
M N tn M M r-+ p CO N O O
N r, M O N N
cM NO OW) OM M r--i CO N N CO C
N V1 M M
~ M NO p tn M O M r+ C C N N O O
G~l
-/) O O O N tn O ~ Q
M N p I/'1 M C M r"i O C N N C
O N Q Q: in N
~ M N O O O O cM -; O `-' C N N 6 C
cC
N V1 O N Q N N
M ~ O ~ ~ M ~--i vp O cO
N O O O ( V? O p r- O
M N O tn M M --i ~ N O
^ rs. o
o
o
=+-^ ~ N a~ .~ ' ~ ~' A
O d 10 v ~ p =~ ~ b a~)
:d 3~ a~q c w~ o
aa v~ a v~
:a 4-4
4-4
V 51 =+:1. `n
cn IiI '.~
=Fw
^i
CA 02392227 2002-05-21
47
As is clear from the results shown in Table 6, when the adhesive
compositions of the present invention in which the weight ratio of methacrylic
acid/MDP or the weight ratio of acrylic acid/MDP is controlled to the range of
0.001 to 0.5 (Examples 32 to 40) are used, it can be seen that very excellent
peripherical sealability is exhibited.
Examples 41 to 50
Adhesive compositions composed of a phosphate group-containing
polymerizable monomer having a different alkylene chain length, a hydroxyl
group-containing polymerizable monomer having a different alkylene chain
length, HEMA and distilled water were prepared as shown in Table 7. The
adhesive strength test and peripherical sealability test were carried out in
the
same manner as in Example 1 using these adhesive compositions. The results
are also shown in Table 7.
^i
= CA 02392227 2002-05-21
48
~ 000~ OOOOM nj nj ~: ~ ~''?
O d ~ O ~-=~ ~-+ 66
~ 0 0 0~ O O M O O In ~
Q ' ~~
e-+ N
O ~N d' O ~ H 6 O
00 O O~ O O O O O M W)
et `~ O dN' dN O O 0~0 N 0~0 ~ ~ ~
CO O
~ 00~0 OOOMO W) in M N ,b. Nt
~C-i
N V~ N ,-i M ~O v~
ED q 0~00 000C) 0 nj clj O N~ O O
~t ~F O N N
WNt 00 00~00 nj nj O 00 N M M
d d O N N O O
ONOO OMQOO nj O
r O Nt d CO N N O O
Irl 000 00000 C.j ~j C v?
et O ti H O O
N In O O O O M O O O O M~ M M
.--~ dN' dN' O ,-~-~ ,~-~ C G
~/7 V1 N I~ D
~0 0 0
p0000 nj
O O
Cd
=~ C~
O o
b4 ~ "'" N
~=~~ ~'~ ~'
~=aa~i aGo
a ~,
~ ~~
o Cg o õb p o o~'
0 0~ ~~= 1 O 0
(5-00 C7.0 o,~2=~ 0CQ ~ cn~ o
a v~
>,=~ ~ 3 .c ~ o 4-4
(.j R w a '" o
0aR,
p~
oo oo ~: ~G A o 'ao.? a .a o 'a'b
~aa a a~ o
a, xw ~a;c7~ w A a E-~ t7
^
CA 02392227 2002-05-21
49
Comparative Examples 18 to 21
Adhesive compositions composed of a phosphate group-containing
polymerizable monomer having a different alkylene chain length, 4HM, HEMA
and distilled water were prepared as shown in Table 8. The adhesive strength
test and peripherical sealability test were carried out in the same manner as
in
Example 1 using these adhesive compositions. The results are also shown in
Table 8.
Table 8
Comparative Examples
18 19 20 21
Adhesive Composition (parts by weight)
Phosphate Group-Containing
Polymerizable Monomer (a)
MOP 15 0 0 0
MDP 0 15 0 0
ADDP 0 0 15 0
MHEP 0 0 0 15
4HM 0.3 0.3 0.3 0.3
HEMA (c) 42.5 42.5 42.5 42.5
Distilled Water (d) 42.5 42.5 42.5 42.5
4HM/Phosphate Group-Containing 0.02 0.02 0.02 0.02
Polymerizable Monomer (a)
(weight ratio)
Adhesive Stren2th
Enamel of Bovine Tooth (MPa) 17.1 21.4 19.0 17.4
Dentin of Bovine Tooth (MPa) 17.8 21.3 18.9 17.8
Peripherical Sealability
Tooth Top Side (Enamel Side) 1.5 1.3 1.4 1.7
Gingival Side (Dentin Side) 1.3 1.5 1.6 2.0
CA 02392227 2008-07-28
As is clear from the results shown in Table 7, when the adhesive
compositions in which the phosphate group-containing polymerizable monomer
and the hydroxyl group-containing polymerizable monomer having an alkylene
chain length of at least 6 are blended (Examples 41 to 50) are used, it can be
seen
5 that excellent adhesive strength and excellent peripherical sealability are
exhibited. Especially, in the case of the combination of the phosphate group-
containing polymerizable monomer and the hydroxyl group-containing
polymerizable monomer in which the number of alkylene chain length is the
same (Examples 42, 25, 47 and 50), further excellent peripherical sealability
is
10 exhibited.
On the other hand, as is clear from the results shown in Table 8, when the
adhesive compositions in which 4HM is contained as the hydroxyl group-
containing polymerizable monomer (Comparative Examples 18 to 21) are used,
it can be seen that excellent adhesive strength is exhibited, but peripherical
15 sealability is drastically deteriorated.
Example 51 and Comparative Example 22
Some tests were carried out in accordance with the testing method for
adhesive strength and the testing method for peripherical sealability of
Example
20 1 using the adhesive composition of Example 3 or Comparative Example 1.
However, as the photopolymerizable bonding material, a bonding material
composed of 61 parts by weight of Bis-GMA, 31 parts by weight of HEMA,
5 parts by weight of colloidal silica [AerosilTM 380, manufactured by Nippon
Aerosil K.K.], 2 parts by weight of CQ, and 1 part by weight of DMAB was
25 prepared and used in place of "Clearfil Megabond" (manufactured by Kuraray
CA 02392227 2008-07-28
51
Co., Ltd., trade mark).
As a result, since the adhesive composition of Example 3 had an adhesive
strength of 17.5 MPa against bovine enamel and an adhesive strength of
15.8 MPa against bovine dentine, adhesive strength is excellent. In addition,
since the adhesive composition of Example 3 had a score of 0.5 for the cavity
margin of a tooth top side (enamel side) and a score of 0.4 for the cavity
margin
of a gingival side (dentin side), peripherical sealability is excellent.
On the other hand, since the adhesive composition of Comparative
Example 1 had an adhesive strength of 17.1 MPa against bovine enamel and an
adhesive strength of 15.2 MPa against bovine dentine, the adhesive strength is
excellent. However, since the score for the cavity margin of a tooth top side
(enamel side) was 2.1 and the score for the cavity margin of a gingival side
(dentin side) was 2.4, peripherical sealability is clearly deteriorated.
Example 52
An adhesive composition composed of 35 parts by weight of MDP,
2 parts by weight of inethacrylic acid, 40 parts by weight of HEMA, 5 parts by
weight of Bis-GMA, 10 parts by weight of 14G, 5 parts by weight of distilled
water, 2.5 parts by weight of TMDPO, 0.5 parts by weight of DMAB and
0.05 parts by weight of BHT was prepared. Adhesive strength was determined
in accordance with the testing method for adhesive strength described below
using this adhesive composition. As a result, since the adhesive strength
against
bovine enamel was 17.5 MPa and the adhesive strength against bovine dentine
was 14.6 MPa, adhesive strength is excellent.
In addition, peripherical sealability was determined in accordance with the
CA 02392227 2008-07-28
52
testing method for periphet'ical sealability described below. As a result,
since the
score for the cavity margin of a tooth top side (enamel side) was 0.3 and the
score for the cavity margin of a gingival side (dentin side) was 0.3,
peripherical
sealability is excellent.
[Testing Method for Adhesive Strength]
Bovine front teeth were smoothly wet-grinded with #1000 silicon carbide
paper (manufactured by Nippon Kenshi K.K.) to expose their enamel surfaces or
dentin surfaces, and thereafter water on the surfaces was blown away with a
dental air syringe. An adhesive tape having a thickness of about 150 m with a
hole opening having a diameter of 3 mm was pasted to the exposed enamel
surfaces or dentin surfaces, and the adhesive composition obtained in Example
52 was applied in a thickness of about 100 m to the hole with a brush. After
30 seconds, the resulting coat was photo-irradiated with a dental
photoirradiator
"LIGHTEL II" (manufactured by Gunma Ushio Denki K.K., trade mark) i for
30 seconds to cure the coat. Further, a commercially available
photopolymerizable dental composite resin "Clearfil AP-X" (manufactured by
Kuraray Co., Ltd., trade mark)i was mounted on the cured coat, and covered
with
a film made of EVAL (manufactured by Kuraray Co., Ltd., registered trade
mark).
Thereafter, the slide glass was pressed over the film, and photo-irradiated
for
40 seconds with the above-mentioned photoirradiator to cure the composite
resin.
A stainless steel rod was bonded to the cured surface with a commercially
available dental resin cement "Panavia 21" (manufactured by Kuraray Co., Ltd.,
trade mark) to give a test piece. After 30 minutes, the test piece was
immersed
in water of 37 C for 24 hours, and thereafter a thermal cycle comprising
CA 02392227 2008-07-28
53
immersing the test piece in cold water of 4 C for one minute and in hot water
of
60 C for one minute was carried out 2000 times. Thereafter, its adhesive
strength was determined. A universal tester (manufactured by Instron) was used
for the determination of the adhesive strength, and the tensile adhesive
strength
was determined under the conditions of a cross head speed of 2 mm/minute. The
determination value for each adhesive strength was expressed as an average
value of the determination values of 8 test pieces.
[Testing Method for Periplierical Sealability]
A cavity having a diameter of about 4 mm and a depth of about 3 mm was
formed by using a dental air turbine so that the cervical line of the molar
portion
of a human evulsed tooth was positioned at the center. The adhesive
composition obtained in Example 52 was applied to the internal surface of the
cavity so that its thickness became about 100 m. After 30 seconds, the
resulting coat was photo-irradiated for 30 seconds with a dental
photoirradiator
"LIGHTEL II" (manufactured by Gunma Ushio Denki K.K., trade mark) to cure
the coat. Further, a commercially available photopolymerizable dental
composite resin "Clearfil AP-X" (manufactured by Kuraray Co., Ltd., trade
mark) was filled in the cavity, and photo-irradiated for 40 seconds with the
above-mentioned photoirradiator to cure the composite resin. Subsequently, in
order to prevent the penetration of the colorant from the tip of tooth root
and the
scissure at crown and the like, "Clearfil Megabond" (manufactured by Kuraray
Co., Ltd., trade mark), was applied to the portions other than the cavity
restorative portion and its surroundings, and photo-irradiated for 30 seconds
with
the above-mentioned photoirradiator to cure the resin.
^
CA 02392227 2002-05-21
54
The resulting test piece was immersed in water at 37 C for 24 hours, and
thereafter a thermal cycle comprising immersing in cold water of 4 C for one
minute and in hot water of 60 C for one minute was carried out 2000 times.
Next, the test piece was immersed in a 0.2% aqueous basic fuchsine solution at
37 C for 24 hours, and thereafter the test piece was taken out from the
solution
and washed with water. The test piece was dried with a dental air syringe, and
its restored portion was divided into three portions lengthwise using a low-
speed
diamond cutter to give three slices per tooth. Fifteen slices were prepared
from
five molar teeth from human teeth in total. The evaluation of the penetration
of
the colorant was made in accordance with the evaluation method of Example 1.
Comparative Example 23
An adhesive composition in which methacrylic acid was removed from
the adhesive composition used in Example 52 was prepared. Adhesive strength
was determined in accordance with the testing method for adhesive strength of
Example 52 using this adhesive composition. As a result, since the adhesive
strength against bovine enamel was 17.2 MPa and the adhesive strength against
bovine dentine was 14.3 MPa, adhesive strength is excellent. On the other
hand,
peripherical sealability was determined in accordance with the testing method
for
peripherical sealability of Example 52. However, since the score for the
cavity
margin of a tooth top side (enamel side) was 1.5 and the score for the cavity
margin of a gingival side (dentin side) was 2.0, peripherical sealability is
drastically deteriorated as compared to Example 52.
Exam lp e 53
^
CA 02392227 2002-05-21
An adhesive composition composed of 34 parts by weight of MDP,
2 parts by weight of 1OHM, 40 parts by weight of HEMA, 5 parts by weight of
Bis-GMA, 5 parts by weight of 9G, 5 parts by weight of UDMA, 5 parts by
weight of distilled water, 3 parts by weight of TMDPO, 0.5 parts by weight of
5 CQ, 0.5 parts by weight of DMAB and 0.05 parts by weight of BHT was
prepared. Adhesive strength was determined in accordance with the testing
method for adhesive strength of Example 52 using this adhesive composition.
As a result, since the adhesive strength against bovine enamel was 16.5 MPa
and
the adhesive strength against bovine dentine was 14.1 MPa, adhesive strength
is
10 excellent. In addition, peripherical sealability was determined in
accordance
with the testing method for peripherical sealability of Example 52. As a
result,
since the score for the cavity margin of a tooth top side (enamel side) was
0.3
and the score for the cavity margin of a gingival side (dentin side) was 0.3,
peripherical sealability is excellent.
Comparative Example 24
An adhesive composition in which 1OHM was removed from the adhesive
composition used in Example 53 was prepared. Adhesive strength was
determined in accordance with the testing method for adhesive strength of
Example 52 using this adhesive composition. As a result, since the adhesive
strength against bovine enamel was 16.6 MPa and the adhesive strength against
bovine dentine was 13.9 MPa, adhesive strength is excellent. On the other
hand,
peripherical sealability was determined in accordance with the testing method
for
peripherical sealability of Example 52. However, since the score for the
cavity
margin of a tooth top side (enamel side) was 1.5 and the score for the cavity
CA 02392227 2002-05-21
56
margin of a gingival side (dentin side) was 2.0, peripherical sealability is
clearly
deteriorated as compared to Example 53.
Example 54
Adhesive strength was determined in accordance with the testing method
for adhesive strength described below using the adhesive composition of
Example 52. As a result, since the adhesive strength against bovine enamel was
21.5 MPa and the adhesive strength against bovine dentine was 14.1 MPa,
adhesive strength is excellent. In addition, peripherical sealability was
determined in accordance with the testing method for peripherical sealability
described below. As a result, since the score for the cavity margin of a tooth
top
side (enamel side) was 0.1 and the score for the cavity margin of a gingival
side
(dentin side) was 0.6, peripherical sealability is excellent.
[Testing Method for Adhesive Strength]
Bovine front teeth were smoothly wet-grinded with #1000 silicon carbide
paper (manufactured by Nippon Kenshi K.K.) to expose their enamel surfaces or
dentin surfaces, and thereafter water on the surfaces was blown away with a
dental air syringe. An adhesive tape having a thickness of about 150 m with a
hole opening having a diameter of 3 mm was pasted to the exposed enamel
surfaces or dentin surfaces. A 40% aqueous phosphoric acid solution was
applied to the hole portion, and washed with water after 15 seconds passed.
Water was gently wiped off with absorbent cotton so that the tooth etched by
phosphoric acid would not be dried. The adhesive composition obtained in
Example 52 was immediately applied to the hole with a brush so that its
CA 02392227 2008-07-28
57
thickness became about 100 m. After 30 seconds, the resulting coat was photo-
irradiated with a dental photoirradiator "LIGHTEL II" (manufactured by Gunma
Ushio Denki K.K., trade mark) for 30 seconds to cure the coat. Further, a
commercially available photopolymerizable dental composite resin "Clearfil AP-
X" (manufactured by Kuraray Co., Ltd., trade mark) was mounted on the cured
coat, and covered with a film made of EVAL (manufactured by Kuraray Co.,
Ltd., registered trademark). Thereafter, the slide glass was pressed over the
film,
and photo-irradiated for 40 seconds with the above-mentioned photoirradiator
to
cure the composite resin.
A stainless steel rod was bonded to the cured surface with a commercially
available dental resin cement "Panavia 21" (manufactured by Kuraray Co., Ltd.,
trade mark) to give a test piece. After 30 minutes, the test piece was
immersed
in water of 37 C for 24 hours, and thereafter a thermal cycle comprising
immersing the test piece in cold water of 4 C for one minute and in hot water
of
60 C for one minute was carried out 2000 times. Thereafter, its adhesive
strength was determined. A universal tester (manufactured by Instron) was used
for the determination of the adhesive strength, and the tensile adhesive
strength
was determined under the conditions of a cross head speed of 2 mm/minute. The
determination value for each adhesive strength was expressed as an average
value of the determination values of 8 test pieces.
[Testing Method for Peripherical Sealability]
A cavity having a diameter of about 4 mm and a depth of about 3 mm was
formed by using a dental air turbine so that the cervical line of the molar
portion
of a human evulsed tooth was positioned at the center. A 40% aqueous
CA 02392227 2008-07-28
58
phosphoric acid solution was applied to the internal surface of the cavity,
and
washed with water after 15 seconds. Water was gently wiped off with absorbent
cotton so that the tooth etclied by phosphoric acid would not be dried. The
adhesive composition obtained in Example 52 was immediately applied to the
internal surface of the cavity so that its thickness became about 100 m.
After
30 seconds passed, the resulting coat was photo-irradiated for 30 seconds with
a
dental photoirradiator "LIGHTEL II" (manufactured by Gunma Ushio Denki
K.K., trade mark) to cure the coat. Further, a commercially available
photopolymerizable dental composite resin "Clearfil AP-X" (manufactured by
Kuraray Co., Ltd., trade mark) was filled in the cavity, and photo-irradiated
for
40 seconds with the above-mentioned photoirradiator to cure the composite
resin.
Subsequently, in order to prevent the penetration of the colorant from the tip
of
tooth root and the scissure at crown and the like, "Clearfil Megabond"
(manufactured by Kuraray Co., Ltd., trade mark) was applied to the portions
other than the cavity restorative portion and its surroundings, and photo-
irradiated for 30 seconds with the above-mentioned photoirradiator to cure the
resin.
The resulting test piece was immersed in water at 37 C for 24 hours, and
thereafter a thermal cycle comprising immersing in cold water of 4 C for one
minute and in hot water of 60 C for one minute was carried out 2000 times.
Thereafter, the test piece was immersed in a 0.2% aqueous basic fuchsine
solution at 37 C for 24 hours, and thereafter the test piece was taken out
from the
solution and washed with water. The test piece was dried with a dental air
syringe, and its restored portion was divided into three portions lengthwise
using
a low-speed diamond cutter to give three slices per tooth. Fifteen slices were
^
CA 02392227 2002-05-21
59
prepared from five molar teeth from human teeth in total. The evaluation of
the
penetration of the colorant was made in accordance with the evaluation method
of Example 1.
Comparative Example 25
Adhesive strength was determined in accordance with the testing method
for adhesive strength of Example 54 using the adhesive composition of
Comparative Example 23. As a result, since the adhesive strength against
bovine
enamel was 21.2 MPa and the adhesive strength against bovine dentine was
14.1 MPa, adhesive strength is excellent. On the other hand, peripherical
sealability was determined in accordance with the testing method for
peripherical
sealability of Example 54. However, since the score for the cavity margin of a
tooth top side (enamel side) was 0.8 and the score for the cavity margin of a
gingival side (dentin side) was 2.4, peripherical sealability is clearly
deteriorated
as compared to Example 54.
Example 55
Adhesive strength was determined in accordance with the testing method
for adhesive strength described below using the adhesive composition of
Example 24. As a result, since the adhesive strength against bovine enamel was
18.6 MPa and the adhesive strength against bovine dentine was 9.6 MPa,
adhesive strength is excellent. In addition, peripherical sealability was
determined in accordance with the testing method for peripherical sealability
described below. As a result, since the score for the cavity margin of a tooth
top
side (enamel side) was 0.2 and the score for the cavity margin of a gingival
side
CA 02392227 2008-07-28
(dentin side) was 0.4, perip;terical sealability is also excellent.
[Testing Method for Adhesive Strength]
Bovine front teeth were smoothly wet-grinded with #1000 silicon carbide
5 paper (manufactured by Nippon Kenshi K.K.) to expose their enamel surfaces
or
dentin surfaces, and thereafter water on the surfaces was blown away with a
dental air syringe. An adhesive tape having a thickness of about 150 m with a
hole opening having a diameter of 4 mm was pasted to the exposed enamel
surfaces or dentin surfaces, and the adhesive composition obtained in Example
10 24 was applied to the hole with a brush. The adhesive composition was
allowed
to stand for 30 seconds, and thereafter dried with an air syringe to a degree
such
that the adhesive composition had no fluidity.
A stainless steel rod was bonded to the applied surface of this adhesive
composition with a commercially available dental resin cement "Panavia 21"
15 (manufactured by Kuraray Co., Ltd., trade mark) to give a test piece. After
30 minutes, the test piece was immersed in water of 37 C for 24 hours, and
thereafter a thermal cycle comprising immersing the test piece in cold water
of
4 C for one minute and in hot water of 60 C for one minute was carried out
2000 times. Thereafter, its adhesive strength was determined. A universal
tester
20 (manufactured by Instron) was used for the determination of the adhesive
strength, and the tensile adliesive strength was determined under the
conditions
of a cross head speed of 2 mm/minute. The determination value for each
adhesive strength was expressed as an average value of the determination
values
of 8 test pieces.
CA 02392227 2008-07-28
61
[Testing Method for Periphcrical Sealability]
A cavity having a diameter of about 4 mm and a depth of about 3 mm was
formed by using a dental air turbine so that the cervical line of the molar
portion
of a human evulsed tooth was positioned at the center. An impression was
prepared by using a commercially available dental rubbery elastic impression
material "Exafine" (manufactured by K.K. G. C., trade mark), and thereafter a
cured product of a dental crown material "Estenia" (manufactured by Kuraray
Co., Ltd., trade mark) was prepared. The surface of the cured product was
coated with a 40% aqueous phosphoric acid solution for 5 seconds, washed with
water, and dried. Further, a dental porcelain bonding material "Clearfil
Porcelain
Bond" (manufactured by Kuraray Co., Ltd., trade mark) was applied thereto, and
dried with a dental air syringe.
The adhesive composition obtained in Example 24 was applied to the
internal surface of the cavity, and the adhesive composition was allowed to
stand
for 30 seconds, and thereafter dried with the air syringe to a degree such
that the
adhesive composition had iio fluidity. The cured product of Estenia
(manufactured by Kuraray Co., Ltd., trade mark) was glued with a dental resin
cement "Panavia 21" (manufactured by Kuraray Co., Ltd., trade mark), and
thereafter an excess paste was removed. After 5 minutes passed from gluing,
"Oxyguard II" (manufactured by Kuraray Co., Ltd., trade mark) was applied to
the peripherical portion to cure the surface of the resin cement, and after
10 minutes passed, Oxyguard II was removed with a water gun. Subsequently,
in order to prevent the penetration of the colorant from the tip of tooth root
and
the scissure at crown and the like, "Clearfil Megabond" (manufactured by
Kuraray Co., Ltd., trade mark) was applied to the portions other than the
cavity
^
CA 02392227 2002-05-21
62
restorative portion and its surroundings, and photo-irradiated for 30 seconds
with
the above-mentioned photoirradiator to cure the resin.
The resulting test piece was immersed in water at 37 C for 24 hours, and
thereafter a thermal cycle comprising immersing in cold water of 4 C for one
minute and in hot water of 60 C for one minute was carried out 2000 times.
Thereafter, the test piece was immersed in a 0.2% aqueous basic fuchsine
solution at 37 C for 24 hours, and thereafter the test piece was taken out
from the
solution and washed with water. The test piece was dried with a dental air
syringe, and its restored portion was divided into three portions lengthwise
using
a low-speed diamond cutter to give three slices per tooth. Fifteen slices were
prepared from five molar teeth from human teeth in total. The evaluation of
the
penetration of the colorant was made in accordance with the evaluation method
of Example 1.
Comparative Example 26
Adhesive strength was determined in accordance with the testing method
for adhesive strength of Example 55 using the adhesive composition of
Comparative Example 16. As a result, since the adhesive strength against
bovine
enamel was 18.3 MPa and the adhesive strength against bovine dentine was
9.3 MPa, adhesive strength is excellent. On the other hand, peripherical
sealability was determined in accordance with the testing method for
peripherical
sealability of Example 55. However, since the score for the cavity margin of a
tooth top side (enamel side) was 1.7 and the score for the cavity margin of a
gingival side (dentin side) was 1.9, peripherical sealability is deteriorated
as
compared to Example 55.
w
CA 02392227 2002-05-21
63
INDUSTRIAL APPLICABILITY
Since the adhesive composition of the present invention strongly bonds to
a tooth and shows excellent peripherical sealability, it can be suitably used
for
bonding a hard tissue with a resin material in the medical field and the
dental
field.
In addition, the adhesive composition of the present invention can
improve peripherical sealability especially for a tooth, so that the
penetration of
the bacterial caries into the bonding portion can be suppressed, whereby
regeneration of caries can be prevented. Therefore, the adhesive composition
is
highly valuable in the contribution to dental therapy.