Note: Descriptions are shown in the official language in which they were submitted.
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
METEIODS AND APPARATUS rOR DELIVERING MEDICAMENT TO TISSUE
MELD OF Ti-lE INVENTION
The present invention is directed t0 surgical methods and apparatus for
delivering
medicament to tissue. l~~lore particular(~~, the present invention is directed
to surgical methods
and apparatus for delivering medicament to tissue by first removing tissue to
form a hole or
channel in the tissue and then delivering medicament into the hole or channel
or into the tissue
surrounding the hole or channel. The methods and apparatus of the present
invention may be
applied in delivering growth factor to cardiac tissue dlll'lll~
transmyocardial revascularization.
BACKGROUND O1~ TIIE INVENTION
Cardiomyopathy ~C'Cll'c~lU Illealllllg "heart" and mt~ohcrlly meaning "muscle
disease")
refers to a group of disorders that directly damage the muscle of tire hearrt
walls, or
nryocamlimu. In these disorders, all chambers of the heart are affected. The
heart's function as
a pump is disnrpted, leading to an inadequate blood flow to Organs alld
tissues of the body.
Depending on the nature of the injury or abnormality in the heart muscle and
the resulting
structural changes in the heart chambers, one of three types of nonischemic
(that is, not caused
by heart attach) heart muscle disease may be present in a patient: clilcrt~cl
cmylu.wine,
hypertj~op~~ic, or re.sw~icto~.
Dilated congestive cardiomyopathy damages the fibers of the heart muscle,
weakening
the wails of the heart's chambers. The chambers thereby lose some of their
capacity to contract
forcefirlly and pump blood through the circulatory system. To COIIl1J2r1Sate
for the muscle
injury, the heart chambers enlarge or dilate which causes heart failure.
Hypertrophic
cardiomyopathy is characterized by a disorderly growth of heart muscle fibers
causing the heart
chambers to become thick walled and bulky. The thickening is generally most
strikin g in tire
walls of the left ventricle, the clamber of the heart which pumps blood
through the aorta to the
vital organs and tissues of the body. The distorted left ventricle contracts,
but the supply of
blood to the brain and other vital organs may be inadequate because blood is
trapped within the
heart during contractions. Restrictive cardiomyopathy causes abnormal cells,
proteins, or scar
tissue to infiltrate the muscle and structures of the heart, CalISlilg the
C11a111berS to become stiff
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
and bulky. The heart may initially contract normally, but the rigid chambers
restrict the return
of blood to the heart.
Massive or multiple heart attacks may also lead to severe heart damage as a
result of a
disruption of blood supply to heart muscle. The damage can result in
functional impairment and
structural abnormalities similar to those found in the other types of
cardiomyopathy. This type
of heart disease, resulting from coronary artery disease, is called
l.S'c~)~nllc ccrrcfinnryupcrllm~
(i.sclrerrric meaning "lacking o~:ygen").
Severe heart injury caused by a major heart attacks or multiple smaller heart
attacks may
result in heart enlargement and thinning of the chamber walls, abnormalities
which resemble
those observed in dilated cardiomyopathy. 1SC11e1111C ClrdlonlyOpathy
typically develops in
patients with severe coronary artery disease, often complicated by other
conditions such as
diabetes and hypertension.
Although heart failure symptoms in ischemic cardiom~~opathy are similar to
those found
in dilated cardiomyopathy, ischemic disease is more likely to be accompanied
by Sv111ptOmS Of
coronary artery disease, such as angina (which is chest pain resulting from
reduced oxygen
supply to the heart muscle). Diagnosis is typically based on a history of
heart attacks and
studies that demonstrate poor fU11Ct1011 111 llla~Or portions of the left
ventricle. The diagnosis can
be confirmed by coronary angiography, which reveals areas of narrowing and
blockage in the
coronary blood vessels.
Patients with ischemic cardiomyopathy are treated with medications that
relieve heart
failure symptoms and improve blood flow throu~,l~ the diseased coronary
arteries, such as
nitroglycerin, some types of calcium channel blockers, and angiotensin-
converting enzyme
(ACE) inhibitors. When symptoms of heart failure and coronary artery disease
cannot be
controlled with medications, coronary angioplasty or surgery may be
considered. Angioplasty
?5 and coronary artery bypass grafting may help increase blood flow to the
heart, which in turn
enhances heart muscle function.
When heart failure symptoms are advanced and Cannot be improved by drug
theraly~ or
surgery, patients may be referred for a heart transplant. Patients with
isciemic cardiomyopatlly
account for approximately one half of all heart transplant recipients. With a
limited supply of
donor hearts and complications resulting from heart transplant (such as organ
rejection),
surgeons have been exploring alternative procedures for treating severe
ischemic
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
cardiomyopathy. One such procedure is ~rcrn.wy~uwrmlinl r~mn.~~cnluri_cr~iun,
otherwise known
more simply as "TMR."
TMR procedures revascularize, that is, form new channels, in the heart muscle
or
myocardium. The newly formed channels penetrate through the entire heart wall,
which
S includes the epicardirrrn (the outer layer of the heart), the
errcluccrrclimn (the inner lining of the
heart), and the myocardium or muscular wall therebetween. As ischemic
cardiomyopathy more
often than not afflicts the left ventricle, the new channels are typically
formed in tire hearrt wall oi~
this chamber of the heart. Accordingly, oxygenated blood from the lungs
present in the left
ventricle awaiting to be pumped through the aorta is able to flow directly
into the newly formed
channels to nourish the heart muscle.
Pioneering methods for performing TMR involved the use of needles for
physically
puncturing holes in the heart wall. These methods resulted in only a temporary
delivery of
blood to the myocardium because the holes quichfy heated at the endocardiun~,
preventing
oxygenated blood from entering the myocardium. One of the more recent and
exciting methods
1S of performing TMR is through the use of lasers. It has been observed that
new holes or
channels formed in the heart wall by a laser tend to heal at the epicardium,
which prevents blood
loss, and promote blood perfusion into the ischemic region of the myocardium.
Lasers have proven to be a widely useful and applicable tool in modern medical
techniques, particularly in minimally invasive surgical procedures.
Technically speaking, a laser
(the word laser being an acronym for light amplification by .stimulated
c.~mission of radiation)
utilizes the natural oscillations of atoms or molecules between energy levels
for generating
coherent electromagnetic radiation. A laser is able to produce high-intensity
and hi~,lr-energy
light at a single frequency. The energy of laser light is measured in joules
(J), or watt-seconds
(W-s), and the power of a laser is measured in watts (W).
2S One of the conventional surgical apparatus for performing TMR consists of a
laser anti
an optical fiber. A surgeon places the end of the optical fiber against the
epicardium to ensure
that all the laser light is focused at the desired point, and then the laser
is fired. In order to form
the new channel completely through the heart v.vall and into the chamber, the
sur~~eon needs to
tactilely urge the optical fiber into and tiu-ou~~h the epicardium, the
myocardium, and tl~e
endocardium. Because of the nature of ischemic cardiomyopathy, the thickness
of the diseased
myocardium is irregular and greater than normal. Accordingly, the surgeon
needs to tactilely
urge the optical fiber through the heart wall at each location. This procedure
takes a certain
- J -
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
amount of time to accomplish safely and involves a certain amount of
guessworl: on the part of
the surgeon. This procedure is complicated by the beating of the heart.
Accordingly, the firing
of the laser needs to be synchronized with the beating of the heart. In
addition, irregularly
shaped holes may result if the surgeon does not urge the optical fiber into
the tissue at a
constant rate. For example, a cavity within the new hole may be formed if the
surgeon slowed
down or paused briefly at a particular location because more tissue at that
location would be
ablated by the increase in laser energy emitted over time. In addition, the
increase in emitted
laser energy may cause excessive trauma to the surrounding tissue at that
location.
In many surgical applications, it may be desired to drill as large a hole as
possible. For
example, in treating ischemic myocardium, holes with larger diameters have
larger inner surface
areas; accordingly, more blood is able to perfilse into the ischemic tissue.
The diffiiculty in
drilling relatively large holes (for example, about I 111111) with laser
ablation is that the area of the
lasing plenum increases exponentially with an increase in the diameter of the
hole (the la.s~iyT
plenrmr being defined as the "bottom" of the hole subject to emitted laser
energy). For example,
the ratio between the areas of the lasing plenum of a hole with a 0.~-mm
diameter and a hole
with a 1-mm diameter is four. Conventional practice has been to increase the
diameter of the
optical fiber and, accordingly, the diameter of the laser beam to form larger
holes. The power
of the laser may also be increased. However, increasing the diameter of the
laser beam results in
an increase in the amount of energy emitted and, accordingly, an increase in
the trauma of the
surrounding tissue. In addition, the power of the laser energy call only be
increased to a certain
point until the capacity of the optical fiber is exceeded.
Accordingly, in view of the foregoing, it is an object of~the present
invention to provide
methods and associated apparatus for delivering medicaments to tissue in a
consistent and
controlled manner.
It is another object of the present invention to provide surgical apparatus
for fornllng
either complete or partial 1101eS lil tISSlle alld then dellVerlllg
inedlCa111e11tS t0 the 1101eS and/or to
surrounding myocardium tissue.
It is a fill-ther object of the invention to provide surgical apparatus and
methods for
promoting angiogenesis and endothelial growth in tile I11~~OC11'dilllll.
It is yet another object of the present invention to provide methods and
associated
apparatus for delivering medicaments to the myocardium while performing
transmyocardial
revascularization.
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
sun~nnaa~~ or T~I:E >INVrNTioN
These and other objects are achieved by the surgical apparatus and associated
methods
of the present invention which provides a medicament delivery system which
forms holes or
channels in tissue by removing tissue and then delivers medicament to the hole
or channel or to
the tissue surrounding the hole or channel. Tissue is preferably removed with
laser ablation but
may be removed by other methods, for example, with high-frequency electrical
energy.
The system for delivering medicament to tissue in accordance with the present
invention may
be utilized to form a hole or channel in tissue, for example, cardiac tissue
(myocarcllum), and then to
deliver medicament, for example, a therapeutic went for the treatment of
cardiovascular disease,
a growth factor that promotes angiogenesis, a gene that encodes for said
growth factor, or any
other therapeutic agent or gene therapy went that promotes angiogenesis, to
the tissue by
partially or fully filling the hole or channel with the medicament, or by
injecting the tissue surrounding
the hole or channel with the medicament. This process may be repeated a
plurality of time to form
and fill a plurality of holes and CllallIleIS In a targeted area of tissue. In
contrast to conventional
systemic delivery approaches, the medicament-delivery system of the present
invention delivers
medicament in a controlled manner to specific targeted tissue.
The medicament-delivery system of the invention may form channels in targeted
tissue by
removing tissue with laser ablation. It has been found that tissue ablation
with laser energ~~ stimulates
a natural biological process of an~io~enesis in the bean. In addition,
administering IlledICilnlelltS SIICh
as growth factors that promote an~io~enesis Dave been fbund to promote
angiogenesis irl the heart.
Accordingly, a SyllergIStIC StlllllllatlOn al7d pl-o1170tIOn Of angio~,~enesis
in the heart is created by
augmenting the hearrt's natural angio~;enic response to laser ablation with
the delivery ofgrowth
factor to those areas of the myocardium which have been ablated. The coupling
of the heart's natural
response to the foCmatlon Of CllanIleIS Wlth the delivery of growth factor
into or adjacent to those
channels provides a benefit to patients not heretofore possible.
In a broad aspect of the present invention, a system for delivering
medicaments to tissue
includes an ablating and injecting device and a handpiece. ~'he ablating and
injecting device
includes an optical fiber and a delivery member formed together Into a unltaly
structure with
cladding. The optical fiber has an inlet for receiving laser energy from a
laser energy source and
an outlet for emitting laser energy. The delivery member has a lumen with an
inlet for receiving
medicament from a medicament source and an outlet for injecting medicament.
The handpiece
is adapted to receive the ablating and injecting device in a controlled and
movable relationship.
-5-
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
In use, a distal end of the handpiece is placed against the target tissue. The
ablating and
injecting device is advanced beyond the distal end of the handpiece and into
the tissue while
emitting laser energy from the optical fiber. The emitted laser energy ablates
the tissue as the
optical fiber advances. The ablating and injecting device is then retracted
from the tissue,
thereby resulting in a channel formed in the tissue. While the device
retracts, medicament is
injected from the delivery member into the channel, thereby providing a plug
within the channel.
The medicament may include growth factor alone or in combination with a
cellular matrix which
enhances angiogenesis in the tissue.
Other aspects, features, and advantages of the present invention will become
apparent to
those persons having ordinary skill in the alrt to which the present invention
pertains from the
following description takell 111 COn ~LI11Ct1011 \'VI2I7 the accompanying
drawings.
I3RiCl~ DCSCR11'TION O~ Tllr DRA~VIIVGS
FIG. 1 is a perspective view of an exemplary embodiment of a tissue drill of
the present
mventlon;
FIG. I A is a cross-sectional view of an exemplary optical fiber of the
invention taken
along line 1 A of FIG. 1;
FIG. 2A is a diagrammatic view of the exemplary tissue drill of the present
invention,
illustrating a handpiece receiving an optical fiber in a retracted position;
FIG. 2B is a diagrammatic view similar to that of F1G. 2A, illustrating the
optical fiber in
an advanced position;
FIG. 3A is a diagrammatic view of an exemplary handpiece of the tissue drill
of the
present invention, illustrating the 11a11dpleCe dISaSSelllbled;
FIG. 3B is a diagrammatic view similar to that of F1G. 3A, illustrating the
handpiece
assembled;
FIG. 4 is a schematic view of an exemplary optical fiber of the present
invention,
particularly illustrating an eccentric configuration of an outlet portion of
the optical fiber;
FIG. 5 is a schematic view of an end surface of the optical fiber illustrated
in FIG. 4;
FIG. 6 is a schematic view of another exemplary optical fiber of tile present
invention;
F1G. 7 is a schematic view of an end surface of the optical fiber illustrated
in F1G. 6;
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
FIG. 8 is a diagrammatic view of an exemplary end surface of an optical fiber
of the
present invention, particularly illustrating a relationship between emitted
laser enemy and
position of the end surface;
FIG. 9 is a schematic view of an exemplary source of laser energy of the
present
invention;
FIG. 10A is a schematic view of an exemplary tissue drill of the present
invention,
particularly illustrating a step of a preferred tissue-drilling procedure
implementing tl~e tissue
drill;
FIG. lOB is a view similar to that of FIG. 10A, illustrating a subsequent step
in the
tissue-drilling procedure;
FIG. l OC is a view similar to that of FIG. I OB, illustrating another
subsequent step in
the tissue-drilling procedure;
FIG. lOD is a view similar to that of FIG. l OC, illustrating yet another
subsequent step
in the tissue-drilling procedure;
FIG. 1 1 is a schematic view of tissue in which a hole has been drilled
according to an
exemplary method of the invention;
FIG. 12 is a schematic view of tissue in which a hole has been drilled
according to
another exemplary method of the invention;
FIG. 13 is a perspective view of an exemplary medicament delivery system
configured in
accordance with the present invention;
FIG. 14 is a schematic cross-sectional view of an exemplary ablating and
injecting device
for use in the medicament delivery system of the present invention;
FIG. 15 is a schematic view of an end surtoce oftl~e ablating and injecting
illustrated in
FIG. 14;
FIG. 16 is a schematic view of an exemplary source of laser energy and
medicament for
use in the medicament delivery system of the present invention;
FIG. 17A is a schematic view of an exemplary medicament delivery system of the
present invention, particularly illustrating a step of a preferred medicament-
delivery procedure
of the invention;
FIG. 17B is a view similar to that of FIG. 17A, illustrating a subsequent step
in tl~e
medicament-delivery procedure;
_7_
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
FIG. 18 is a perspective view of a tissue-removal and medicament-delivery
system in
accordance with the invention, particularly illustrating a coupling assembly
of the invention;
FIG. 19 is a cross-sectional view of an exemplary coupling assembly taken
along line
I9-19 of FIG. 18, with medicament injection and supply units shown
schematically;
S FIG. 20 is a diagrammatic view of an alternative embodiment of an exemplary
ablating
and injecting device for use in the medicament delivery system of the present
invention;
FIG. 21 is a diagrammatic view of another embodiment of an exemplary ablating
and
injecting device for use in the medicament delivery system of the present
invention;
FIG. 22 is a cross-sectional view of a tissue-removal and medicament-delivery
device of
IO the present invention, particularly configured to remove tissue with high-
frequency electrical
energy;
FIG. 23 is a cross-sectional view of an alternative embodiment of a tissue-
removal and
medicament-delivery device of the present invention;
FIG. 24 is a schematic view of a step of a tissue-removing procedure
incorporating the
1 S device of FIG. 22 or 23, particularly removing tissue with high-frequency
electrical energy
according to the invention;
FIG. 25 is a schematic view of a medicament-delivery step of the invention,
particularly
illustrating the delivery of medicament to tissue surrounding a hole or
channel formed in tissue;
FIG. 26 is a cross-sectional view of another elllbOdllllellt of an electrical-
energy tissue-
20 removal and medicament-delivery device in accordance with the invention;
F1G. 27 is a schematic view of another embodiment of a medicament-delivery
system of
the invention, particularly illustrating an ablating and ilecting device
received within a catheter
with rifling
FIG. 28 is a cross-sectional view of the medicament-delivery system of F1G.
27;
25 FIG. 29 is a developmental view of an exemplary catheter with rifling for
else In the
medicament-delivery system of FIG. 27;
FIG. 30 is a schematic view of an exemplary medicament delivery system of the
present
invention, illustrating needles around the perimeter of the head portion of
the handpiece;
FIG. 31 is a schematic view of the end surface of the head portion of FIG. 30;
30 FIG. 32 is a schematic view of the embodiment of FIG. 30, particularly
illustrating a step
of a preferred medicament-delivery procedure of the invention;
_g_
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
FIG. 33 is a schematic view of another exemplary embodiment of the head
portion of the
handpiece, illustrating nozzles around the perimeter of the head portion of
the handpiece;
FIG. 34 is an end view of yet another exemplary embodiment of the head portion
of the
handpiece, illustrating ports around the perimeter of the head portion of the
handpiece;
FIG. 35 is a perspective view of an alternative embodiment of a tissue-removal
and
medicament-delivery device of the present invention utilizing a single
delivery lumen;
FIG. 36 is a schematic cross-sectional view of the embodiment of FIG. 35;
FIG. 37 is a perspective view of yet another embodiment of a tissue-removal
and
medicament-delivery device of the present invention utilizing a single
delivery lumen;
F1G. 38 is a schematic cross-sectional view of yet another embodiment of of
the device
of the present invention utilizing at least one vacuum lumen and at least one
delivery lumen;
FIG. 39 a schematic cross-sectional view of a step of an alternate exemplary
embodiment of an electrical-energy tissue-removal and medicament-delivery
device of the
present invention; and
FIG. 40 is a schematic cross-sectional view of the embodiment of FIG. 39,
particularly
illustrating a step of a preferred medicament-delivery procedure of the
invention.
DE'I'AILCD DESCRII''1'ION O1~ l:Xl.Nll'LAR1' E11~1I30DIMCN'rS
Referring to the drawings in more detail, in FIG. 1 an exemplary embodiment of
a tissue
drill 50 of the present invention is illustrated in conjunction with a source
of laser energy 5~.
Exemplary tissue drill 50 forms holes or channels in tissue by laser ablation
in a consistent,
controllable, and programmable manner. The first portion of the following
description focuses
on the principles of tissue ablation and the forming of channels in tissue.
These princilales of the
present invention are then readily applied to a system for delivering
medicaments to the tissue in
which the channels are formed, which will be discussed in more detail below.
Ablation is the process of fragmenting long molecules into short gaseous
molecules.
Much of the tissue in living organisms, including the human body, is made up
mostly of water
(e.g., about 75%) with organic material making up the remaining portion. The
molecules of
organic material consist of atoms of carbon, nitrogen, oxygen, and hydrogen
that are attached
together through covalent bonds. Ablation is the process of breaking these
covalent bonds.
Tissue drill 50 utilizes the ablation process to breal: molecules of tissue
apart, thereby forming
holes or channels in the tissue. The ablation process will be discuss in more
detail below.
-9-
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
Exemplary tissue drill 50 includes a handpiece 54 for I17a111pUlat1o11 by a
user and an
optical fiber 56, which is shown in FIG. 1 A, for t1'allSllllttlllg IaSer
ellel'gy 8'0111 laser energy
source 52. Optical fiber 56 has an outlet portion 58 for emitting laser
energy. Outlet 1)oaion 58
functions substantially as a drill bit. In operation, outlet portion 58 is
moved from a retracted
position (which is shown in the solid line) to an advanced position (which is
shown by the
phantom line) while emitting laser energy. Arrov.v A represents outlet portion
58 moving to the
advanced portion, and arrow L represents laser energy emitted from outlet
portion 58. Tissue is
ablated by laser energy as outlet portion 58 is advanced, thereby forming a
hole or a channel in
the tissue. Exemplary tissue drill 50 may also rotate outlet portion 58 while
moving to the
advanced position, which is represented by arrow R. After- reaching the
advanced position,
outlet portion 58 may be withdrawn to the retracted position, which is
represented by arrow Q.
The advancing and retracting of outlet portion 58 is preferably along a
central axis of optical
fiber 56. Any rotation of outlet portion 58 is preferably about the central
axis of optical fiber
56. The axial and rotational movement of outlet portion 58 will be discussed
in more detail
below.
Exemplary outlet pol-tion 58 of optical fiber 56 has an end surface 60 with an
outlet 62
from which laser energy is emitted. Outlet 62 is preferably offset from or
eccentric to the
central axis of outlet portion 58 so that as outlet portion 58 rotates, outlet
62 rotates about the
central axis. Accordingly, laser energy emitted from outlet 62 as outlet
portion 58 rotates is not
focused at a single point but is rather distributed about the central axis.
Alternatively speaking,
the eccentric relationship of outlet 62 with respect to the central axis of
outlet portion 58
preferably produces a gradient of laser energy as outlet portion 58 axially
advances, with the
highest level of laser energy at the central axis, wflich energy decreases
toward a peripheral
edge. The eccentricity of outlet portion 58 will also be discussed in more
detail below.
I-landpiece 54 may be implemented accorclin~ to a variety of
COIIfI~lIratI011S. For
example, handpiece 54 may be a flexible catheter utilized in endovascular
procedures and IlaVlll~
a plurality of lumens to facilitate visualization, tlusl~ing, and aspiration.
In this regard, outlet
portion 58 may advance beyond a distal end of the catheter to vascularize
tissue, such as on the
inside the left ventricle of the heart. Alternatively, handpiece 54 may be
formed as a trocar
sheath and positioned intercostally (i.e., between the ribs) for tissue
access. I-landpiece 54 may,
also be formed in a gooseneck-like configuration with a plurality of
al'tlClllated JOIIItS W111C11 Illa~'
be bent to assume and retalil a pa1-tlClllar Shape. ~'fOreOVel~, ha11dp12Ce
5~1 lllay be a COIICIIiIt \'Vlth
- 10-
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
flexible cable sheathing. Accordingly, in a general sense, handpiece S4
provides a "user
interface" for delivering outlet portion S8 to a target site, which may be
accomplished either by
direct physical manipulation by a surgeon or by programmed mechanical control.
An exemplary handpiece of the present invention is illustrated in FIGS. 2A and
2B.
Exemplary handpiece S4 may include a hotly portion 64 and a coupling portion
66. Exemplary
body portion 64 has a distal end 68. Exemplary coupling portion 66 is adapted
or configured to
receive optical fiber S6 in a controlled and axially movable relationship so
that outlet portion 58
may be advanced beyond distal end 68 of body portion 64. In addition, coupling
portion 66
may be adapted to receive optical fiber S6 in a rotatable relationship so that
at least outlet
portion 58 of optical fiber 56 may rotate. If handpiece 54 is configured as a
catheter or similar
flexible tubular member, the inner surface of tl~e tubular member serves as a
coupling portion by
receiving optical fiber S6 in a controlled, axially movable, and/or rotatable
relationship.
The retracted position of outlet portion S8 itS SIIO~VII I11 FIG. 2.A may be
defined as a
position in which end surface 60 is positioned substantially at or near distal
end 68 of body
portion 64. Accordingly, end surface 60 may project slightly beyond distal end
68 or,
alternatively, may be either proximal to or substantially aligned (or
coplanar) with distal end 68.
The advanced position of outlet portion S8 as shown in F1G. 2B may be defined
as a position in
which end surface 60 with outlet 62 projects a distance cf beyond distal end
68 of body portion
64. As will be discussed in more detail below, distance cl at which end
surface 60 projects
beyond distal end 68 is preferably predetermined, adjustable, and/or
prOgrillllillable.
With additional reference to FIGS. _~A and 3B, exemplary coupling portion 66
may
include a drive which is comprised of a tubular member 70 and a collar 72.
Tubular member 72
receives optical fiber S6 and may have a chuck 74 for retaining optical fiber
S6 thereto. Tubular
member 72 may also have annular threading 76 formed along a length thereof.
Collar 72 is
disposed within body portion 64 and has Complementary inner threading 78.
Exemplary tubular
member 72 is slidably and rotatably receivable within body portion 64 with
annular- threading 76
engaging with inner threading 78 of collar 72, aS Showll Ill FIG. 3B.
Accordingly, rotation of
tubular member 70 causes tubular member 70 to move axially. As optical fiber
56 is retained by
chuck 74, optical fiber 56 with outlet portion S8 moves axially with tubular
member 70. In an
alternative embodiment of handpiece 54 such as a catheter, rather thall
disposing couplinyl
portion 66 and a drive on iandpiece S4, these elements may be provided at a
proximal lOCatioll,
such as at laser apparatus S2. In this regard, catheter-configured handpiece
S4 retains optical
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
fiber 56 within a body portion which prevents buckling and which delivers
outlet portion 58 to a
target site but which is substantially free of coupling and drive apparatus.
Referencing FIG. 4, in addition to outlet portion 58, exemplary optical fiber
56 has all
elongate portion 80. A core 82 and a cladding 84 define optical fiber 56 and
extend along
elongate portion 80 and outlet portion 58. Core 82 has an inlet 86 for
receiving laser energy
and outlet 62 (see also FIG. 1 ) for emitting laser energy. Core 82 arid
cladding 84 may be made
of high-purity silica glass or sapphire, with core 82 having a higher index of
refraction than that
of cladding 84 so that modulated pulses of laser energy move along core 82
without penetrating
cladding 84. Although optical fiber 56 may be configured according to any
dimensions, for
many applications a length l~ of elongate portion 80 may range from about 0.5
meter (m) to
more than 2 m to provide a surgeon with sufficient maneuverability, and a
length I" of outlet
portion 58 may range up to about 50 millimeters (mm) so that holes of
different lengths may be
formed in tissue. For applications other than medical, optical fiber 56 may be
dimensioned
accordingly to accomplish the particular application.
1 S Core 82 of optical fiber 56 has an axis E along elongate portion 80 and an
aXls O at
outlet 62. With additional reference to FIG. 5, core 82 along outlet portion
58 angles away
from and is oblique to core 82 along elongate portion 80. At end surface 60,
axis O of core 82
at outlet 62 is offset from or eccentric to axis E of core 82 of elongate
portion 80 by a distance
8. Accordingly, laser energy emitted from outlet 62 is distributed about axis
E as optical fiber
56 rotates about axis of rotation E. Further, the distribution of laser energy
is across the entire
surface area of end surface 60 as optical fiber 56 make one complete
revolution. At end surface
60, outlet 62 may be configured so that axis O of core 82 is either oblique to
axis E or, as
shown, parallel to axis E.
An alternative exempalary embodiment of optical fiber 56 is illustrated in
FIGS. 6 and 7.
In addition to core 82 and cladding 84, exemplary optical fiber 56 may include
auxiliary cladding
88 disposed about outlet portion 58. Similar to tire embodiment shown in FIG.
4, to offset axis
O of outlet 62 from axis of rotation E by distance 8, core 82 of outlet
portion 58 is oblique to
core 82 of elongate portion 80. Auxiliary cladding 88 compensates for the
oblique relationship
of core 82 (and cladding 84) of outlet portion 58 with respect to core 82 (and
cladding 84) of
elongate portion 80. Auxiliary cladding 88 accordingly provides a preferred
cylinc(rical
configuration of outlet portion 58 so that outlet portion 58 rotates about
axis E as elongate
portion 80 rotates about axis E. Further, in addition to axis O at outlet 6~
being eccentric t~
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
axis E, axis O of core S2 may be oblique to axis E at outlet 62, rather than a
parallel relationship
as shown in FIG. 4.
As illustrated in FIGS. 6 and 7, end surface 60 (including outlet 62) is
substantially
perpendicular to axis E of exemplary optical fiber 56. To form the
perpendicular relationship,
core 82 and cladding 84 are ground or polished at an angle oblique to axis O,
thereby removing
portions of core 82 and cladding 84 shown by phantom line f. Accordingly,
exemplary end
surface 60 is substantially planar. Alternatively, end surface 60 may be
convex, concave, or
other configuration depending upon a particular implementation of outlet
portion 58.
With particular reference to FIG. 7, end surface 60 of exemplary optical fiber
56 has a
circumference CCS defined along an outer edge 90, and outlet 62 of core 82 has
a circumference
Co defined along outer edge 92. Circumference C~, and circumference C" are
coextensive along
an arc length a of outer edges 90 and 92. This relationship allows laser
energy to be emitted
from outlet 62 at outer edge 90 of end surtnce 60. As outlet portion 58
rotates, laser energy is
emitted along circumference C~, of rotating end surface 60. Arc length a may
rage ti-om a
single tangent point to several seconds, minutes, or degrees as desired.
Diameter d~ of outlet 62 is preferably greater than about one half of diameter
d~, of end
surface 60. Accordingly, outlet 62 has a surface area which is at least one
quarter of that of encf
surface 60. This relationship in surface area allows laser energy to be
emitted from a substantial
percentage of end surface 60. Further, laser energy is not emitted from the
entire end surface 60
simultaneously but rather over the time it takes outlet portion 58 to make one
revolution about
axis E. An exemplary commercial embodiment of optical tiber ~6 for use in
transmyocardial
revascularization entails a diameter d~, of end surface 60 (and outlet portion
58 of approximately
1 mm and a diameter d~ of outlet 62 of approximately 0.6 mm. Generally
speaking, the
dimensions of outlet portion 58 are determined by the type of procedure being
performed and
the desired size of the hole, with diameter d" of~outlet 62 being at least one
half of diameter d"
of end surface 60. For example, if a hole with a 1.5-mm diameter is desired,
then diameter d~, of
end surface 60 (and outlet portion 58) should be about 1.~ mm; diameter d" of
outlet 62 may
accordingly range from about 0.75 nun to slightly less than I .J mm, but is
preferably about O.S
mm. For many medical applications, it is contemplated that diameter d~, of end
surface 60 may
range from about 0.2 mm to more thal7 2.5 mm, with diameter d" of outlet 62
ranging from less
than about 0.1 mm to about 2 mm or more. For specific medical applications
such as
transmyocardial revascularization (which will be discussed below), diameter
d~, of end surface
- I _s -
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
60 may range from about 0.6 111111 to about 2 111111, \vlth Cllameter d" of
outlet 62 ranging from
about 0.3 mm to about I 111111.
With additional reference to FIG. 8, end surface 60 is schematically
illustrated during
rotation, with outlet 62 shown at progressive instances in time t,, t~, I3,
and t4 while rotating
about axis E. Because of the relationship between the surface areas of end
surface 60 and outlet
62, laser energy is continuously emitted from an area 94 of end surface 60. In
other words, area
94 represents an intersection of the positions of outlet 62 at every instance
of time while
rotating about axis E. Laser energy is accordingly emitted at intervals at
other areas of end
surface 60 depending upon the position of outlet 62 at a particular instance
in time.
The relationship between laser energy emitted from exemplary end surface 60
per
revolution of outlet 62 about axis E with respect to distance from axis E is
illustrated graphically
in FIG. 8. Emitted laser energy her revolution of outlet portion 58 decreases
from a constant
level at area 94 to a lower level at outer edge 90 of end surface 60. In the
graph, outer edge 90
is a distance from axis E substantially equal to radius r~, of end surface 60.
Depending upon a
particular configuration of exemplary end surface 60 and outlet 62, the
decrease in laser energy
or flux with respect to position may be a linear f1111Ct1011 aS ShOwil Or' a
nonlinear function. Also,
the relative level of energy per revolution at area 94 and at radius r~, is
illustrative only, as the
level of energy at the periphery of end surface 60 may valy according to the
particular surgical
procedure. For example, the energy flux at radius r~, may be at a relatively
low level when
compared to the constant level at area 94.
In accordance with this energy distribution her revolution of the present
invention, while
ablating tissue to form a hole, the transference of laser energy to peripheral
or surrounding
tissue is less than at a center of the hole being formed. This distribution of
laser energy may
limit trauma to tissue in which holes or channels are formed. l~lore
specifically, as outlet portion
58 moves through tissue while rotating and emitting laser energy, outer edge
90 of end surface
60 is adjacent to and contacts the surrounding tissue which defines the hole
being formed. As
the level of emitted laser energy at outer edge 90 is lower than that centered
about axis E
(which essentially defines the center of the hole being formed), damage to the
surrounding tissue
is reduced, resulting in less trauma to the tissue. It is believed that tissue
with a relatively low
level of trauma has a likelihood to experience angiogenesis, or the formation
of new blood
vessels in the tissue. This reduced-trauma feature of the present invention
will be discussed in
more detail below.
I4_
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
An exemplary process to form an eccentric outlet portion S8 as described above
involves
placing the distal end of optical fiber S6 within a Teflon'' tube at an angle,
with cladding 84
contacting the inner surface of the tube at one point. The tube may then be
filled with epoxy
which surrounds the distal end of optical fiber S6 except at the point at
which cladding 84
S contacts the tube. After the epoxy has cured and hardened, the tube is
removed, and the distal
surface of the epoxy and optical fiber S6 is polished to define end surface 60
at the point where
cladding 84 defines an annular edge of outlet portion S8. End surface 60 may
also be formed
with a lens to control the emission of the laser enemy in a particular manner.
An inner diameter
of the tube for forming outlet portion S8 essentially determines the diameter
of outlet portion S8
(i.e., diameter d~s of end surface 60). According to this process, optical
fibers S6 having outlet
portions S8 of different diameters may be formed, enabling surgeons to form
holes with a
variety of diameters. In addltloll, a plurality of outlet portions S8 each
having a different
diameter may be formed, each of which being able to be coupled to an optical
fiber, so that a set
of interchangeable "drill bits" is at a surgeons disposal during a particular
procedure. Optical
1S fiber S6 may be reusable or disposable, as may outlet portion S8 and
handpiece S4.
With further reference to FIGS. I allCi 3A, exemplary of handpiece S4 may
include a
head portion 96 connectable to a distal end of body portion 64 by a neck 98.
Distal end 68 of
body portion 64 is accordingly defined by a tissue end I 00 of head portion
96. Exemplary head
portion 96 may be conical so that tissue end 100 has a lamer diameter than
body portion 64.
Tissue end 100 provides a working surtnce or a tissue-engaging surface for
positioning
handpiece S4 over and against a surgical site in which a channel is to be
drilled into tissue.
Exemplary head portion 96 may also have an aperture 10? formed therein.
Aperture 102 may
function as a window for viewing a surgical site when tissue end 100 is placed
against tissue.
Aperture 102 may also function as a vent for exl~austin~ gases which may be
generated by laser
2S energy ablating tissue. AS Sh01v11 Ill FIG. I, exemplary neck 98 may be
angular to enhance the
positioning of head portion 96 against tissue. In this regard, neck 98 may be
configured as a
gooseneck with articulable joints for aSSllllllil~ and retaining a desired
shape. Exemplary head
portion 96 and neck 98 are preferably tubular, thereby providing an inner
continuum with body
portion 64 in which optical fiber S6 is receivable.
In particular procedures, it may be preferable to know where a hole has been
drilled in
tissue. However, the nature of the tissue or the size of the hole may render
it difficult for the
surgeon to determine where a hole has already been formed. Accordingly, the
newly formed
_ 1>_
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
hole drilled in tissue may be marked. In this regard, head portion 96 may
include apparatus for
marking where a hole has been drilled in tissue. For example, tissue end 100
may have an inking
device which dispenses biocompatible ink or dye on the tissue where a hole has
been formed.
The ink may be applied to the tissue through direct contact with tissue end
100 or, for example,
S by spraying. Exemplary handpiece 54 may have a reservoir for storing and
dispensing a colored
liquid or a particulate solid to the tissue. Fluorescent material may be used
to enhance
visualization. Other indicia may be applied to the tissue by handpiece 54 or
head portion 96 at
the target site; for example, alphanumeric indicia may indicate the parameters
of~the laser energy
emitted from outlet 62 to form a particular hole.
With further reference to FIGS. 2A to 3B, exemplary coupling portion 66 may
include a
spring 104 receivable against a seat 106 formed on a distal end of collar 72,
and a stop 108
disposed on a distal portion of tubular member 70. Spring 104 and stop 108
define a
mechanism for controlling a position of tubular member 70 within body portion
64, and may be
configured to facilitate the advancement and retraction of tubular member 70.
1S Exemplary source of laser energy S? is illustrated in FIG. 9. (_aser energy
source S2
includes a laser 1 10 for generating laser energy L. Lxemplary laser energy
source S2 may
include a drive assembly 1 12 for operatively associating with handpiece 54
and optical fiber 56,
and may also include a control unit 1 14 with a user interface 1 16. Exemplary
drive assembly
112 may include a coupler 1 18 for connecting with optical fiber S6, optics
120 for modifying
laser energy L as desired, and a clrive/motor 122. Exemplary coupler I 18 is
associated with
optics 120 for transferring laser ener';y L from laser I 10 to tl~e inlet of
optical fiber S6.
Exemplary coupler 1 18 is also associated with drive/motor 120 for rotating
optical fiber _56
As discussed above in reference to FIGS. 2.A and 2B, exemplary coupling
portion 66 of
handpiece S4 translates rotational movement of optical fiber 56 to axial
movement to advance
2S and to retract outlet portion S8. Exemplary drive assembly f 12 preferably
rotates optical fiber
S6. For example, coupler I 18 may secure and retain a proximal end of optical
fiber S6, with
motor/drive 122 rotating coupler 1 18 which also rotates optical fiber S6.
Drive assembly 1 12
may rotate optical fiber S6 in a first direction, for example, as shown by
arrow R, in F1G. 2A, to
cause optical fiber S6 to advance axially as shown by arrow A. When outlet
portion S8 reaches
the desired advanced position, drive assembly I 12 may then rotate optical
fiber S6 in an
opposite second direction, as shown by arrow R~ is F1G. 2B, to cause optical
fiber 56 to retract
axially as shown by arrow B. Exemplary drive assembly 1 l2 may oscillate
optical fiber S6 (that
16_
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
is, rotate optical fiber 56 clockwise and counterclockwise as shown by arrows
R, and R~) so
that outlet portion 58 reciprocates between the retracted position and the
advance position.
Exemplary laser energy source 52 preferably controls when laser enemy L is
emitted
from outlet portion 58 of optical fiber 56. For example, control unit I 14 in
association with
laser 110 and drive assembly 1 12 may limit the emission of laser energy L to
only when outlet
portion 58 moves to the advance position. Laser energy L may then be
terminated during the
retraction of outlet portion 58. Alternatively, if drive assembly 1 12 is
reciprocating outlet
portion 58, laser energy L may be transmitted only during the advancing stroke
of outlet portion
58; the emission of laser enemy L may then be terminated at the end of the
advancing stroke.
The termination of laser energy L upon reaching the advanced position is
preferably automatic
and controlled by laser energy source 52. Alternatively, laser energy L may be
terminated by a
device such as a pressure sensor which determines when the distal end of
outlet portion 58
advanced completely through a section of tissue, e.g., the wall of the heart.
This control of laser
energy L is preferable during particular applications of tissue drill 50,
which will be discussed in
more detail below.
With further reference to F1G. I A, optical fiber » is preferably received
within a
housing 124. In addition to protecting optical fiber 56, exemplary housing 124
constrains any
torsional flexing or bending of optical fiber 56 which may result from the
rotation by drive
assembly 112. Exemplary optical fiber 56 may include a complementary coupler
126 for
connecting with coupler 1 18 of laser energy source 5?. Complementary coupler
126 preferably
provides a releasable association with coupler 1 18 so ti~at other optical
fibers in accordance with
the present invention may be connected to laser enemy source 5?. Exemplary
housing 124
preferably extends between coupler I 26 and chuck 74 of coupling portion 66 to
provide integral
protection of optical fiber 56 between laser energy source 52 and handpiece
54.
Exemplary laser energy source S2 may control a number of parameters of tissue
drill 50,
including distance d at which outlet portion 56 advances, a speed at which
outlet portion 56
advances, and a level at which laser enemy is emitted from outlet 62. Control
unit 1 14 in
association with user interface I 16 preferably controls, programs, monitors,
and/or adjusts each
of these parameters depending upon a particular tissue-drilling application.
For example, one
the many applications of tissue drill 50 is for drilling holes or channels
into or through heart
walls. This procedure is kn0\vll aS 11'Clil.1'lllJ'llL'CIl'CI~ICtI
re>>~cr.~~cmlcrni~u~iun or, n101'e Srlllply, as
_ 17_
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
TMR. FIGS. l0A through I OD schematically illustrate an exemplary T>t1R
procedure
implementing tissue drill 50 of the present invention.
A heart wall 130 is illustrated in FIG. 10.<1 and includes myocardium, or
heart muscle,
132 positioned between an outer serous layer or epicardium 134 and an inner
membrane or
S endocardium 136. It has been found to be medically beneficial to
revascularize the myocardium
of patients suffering from severe ischemic carcliomyopathy. The
revascularization of tloe
myocardium 132 involves forming new channels in the tissue. f3y implementing
exemplary
tissue drill 50 of the present invention, new channel may be formed in the
myocardium in a
controlled, consistent, and pCOgrammable Illallller.
Prior to a TMR procedure, the level at which laser I 10 is to generate laser
energy (_, and
the frequency at which laser energy L is to be pulsed may be determined. 111
addulon, distance cm
at which outlet portion 58 is to advance beyond distal end 68 and the speed at
which outlet
portion 58 is to rotate may be determined. These parameters may be stored In
COlltrol unit 1 14
and varied or programmed via user interface 1 16.
During the TI~IR procedure, access to the patient's chest cavity is provided,
preferably
by a minimally invasive procedure such as an intercostal incision using trocar
sheaths. Access to
the patient's heart is then provided, for example, by incising the
pericardium. With outlet
portion 58 in the retracted position, a surgeon may then maneuver head portion
96 of handpiece
54 into the chest cavity and position tissue surtace 100 against the
epicardium 134, as shown in
FIG. 10A. As discussed above, outlet portion SS may project slightly beyond
distal end 68 (that
is, tissue end 100) when in the retracted position to provide the surgeon with
a tactile feel of the
position of end surface 60 on the epicardium I 3=1.
V~~hen in the desired position on the epicardium 134, tissue drill 50 may be
activated.
This activation may be accomplished manually by an assistant via user
interface 1 16 or by the
surgeon with a foot or a hand trigger. Alternatively, activation of tissue
drill 50 may be
synchronized with the electrical activity of the heart through the use of an
electrocardiogram
(EKG) machine. Activation of tissue drill 50 causes laser energy source 52 to
generate and
transmit laser energy to optical fiber SG. Activation also causes optical
fiber 56 to rotate anti
advance outlet portion 58 through the epicardiunl I 34 and into the myocardium
132 of the heart
wall 130, as shown in FIG. 1013.
Outlet portion 58 continues to advance through the nlyOCardlUlTl 132 and
throu~~h the
endocardium 136. Vhhen end surface GO of outlet portion 58 has advanced
throu~,h the
18_
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
endocardium 136 and is positioned within the left ventricle of the patient's
heart aS shown 111
FIG. l OC, the emission of laser enemy is preferably terminated, and the
outlet portion 58 is
retracted. A new channel 138 through tl~e heart mall 1 30 results from this
procedure as shown
in FIG. 10D. Oxygenated blood from the lest ventricle may enter the new
channel I 38 through
the endocardium 136 and perfuse the tissue of the myocardium 132 surrounding
the new
channel 138. When handpiece 54 is configured as a catheter, outlet portion 58
advances
through the endocardium 136 and then into the nl)~OClrdll1111 f 32. Because
outlet portion 58
may be programmed to advance a predetermined distance, outlet portion 58 may
either continue
to advance completely through the epicardium 1 34 or begin to retract the
predetermined
distance within the myocardium 132, thereby forming a hole in the heart wall
130 rather than a
channel through the heart wall 130.
As mentioned above, reduced trauma to tl~e myocardium 1 34 surroundinv; the
new
channel 138 results from the eccentric relationship between outlet 62 and
rotational axis E. Tlris
reduced trauma may enable the surrounding tissue to regenerate vascular tissue
from the new
channel 138 and into the myocardium 1 34 or to experience angiogenesis. In
addition to the
eccentricity of outlet portion 58, the level oi~trauma inflicted on the
surrounding tissue is
mediated by the level of laser enemy emitted ti~om outlet 62, which will now
be discussed.
With reference to FIG. 9, the energy level at wlricl~ laser energy I. is
generated and
transmitted to optical fiber 56 may be varied, pro<~rammed, and controlled
according to each
tissue-drilling application. For example, tissue drill 50 nay be configured
for drilling holes in all
types of animal tissue and plant tissue, as well as other substances. The
parameters which define
the characteristics of laser ablation include frequency, energy her channel,
pulse width, and pulse
rate. As mentioned earlier, ablation is a process of~breal:in~ bonds between
atoms in molecules
by adding energy to the molecules. One preferred level oftlre laser energy L
for TMR
applications is to limit tl~e energy her pulse to less thall about 100
milliJoules per square
millimeter of area (mJ/mm~). l~lore preferably, an energy per pulse of about
30 I11J/IlllllZ has
been found to ablate cardiac tissue at a substantially reduced level of
trauma. Tlre energy per
pulse of laser 1 10 may be varied according to specific tissue-drilling
procedures.
With fiWher reference to FIGS. 3A and 3B, the drive may be configured to
control the rate
at which outlet portion 58 advances and retracts The rate of advancement is
controlled by the speed
at which optical fiber 56 rotates and the pltCll Of~tllf: COlllplelllelltaly
tlll-eildln~ of collar 72 and
tubular member 78. For smooth and continuous operation, it has been determined
that optical fiber
- 19-
CA 02392642 2002-05-27
WO 01/39682 PCT/LTS99/28570
56 and, accordingly, outlet portion 58 should rotate at a speed under about
5,000 revolutions per
minute (RPM). For TMR applications oftissue drill 50, a rotational speed
ranging from about 1,000
RPM to about 2,000 RPM is preferred. In this regard, a specific TMR
configuration of tissue drill 50
may be as follows. Optical fiber S6 may rotate at about 1,340 RfM. The pitch
of threading 76 and
78 may be configured so that outlet pol-tion 58 advances at a rate of about
15.5 millimeters per
second (mn~/s). With a rotational speed of 1,340 RPI\-1, it takes about 46
milliseconds (ms) for outlet
portion 58 to complete one rotation. For TMR applications, laser 1 I 0 may
emit pulses of laser
energy L of about ?0 nanoseconds (ns) in duration, with each pulse being
separated by about 4 ms.
The pulse rate may be about 10 pulses per revolution (or at about every
36° of rotation) or about 240
pulses per second.
Rather than advallClllg and retracting outlet portion SS at a constant rate as
described above,
tissue drill 50 may be configured such that outlet portion 58 moves at varying
rates of speed between
the retracted and advanced positions. The slower outlet portion 58 advances
(or retracts) while
emitting laser energy L, the more tissue that becomes ablated because the
tissue is subject to more
laser energy over time. Accordingly, a hole may be formed with a diameter
greater than diameter d~,
of end surface 60 (and outlet pol-tion 58) by aclvanclng outlet pol-tion 58 at
a speed which allows
laser energry L to ablate a greater amount of tissue. Alternatively, tile
power of laser energy L may
also be varied during the advancement of outlet portion S8 so that the tissue
is subjected to more or
less laser energy L. Generally speaking, a surgeon may program tissue drill 50
to ablate tissue at
varying levels of enerV~y per unit time to form holes of varying desired
diameters or configurations.
The energy per unit time may be adjusted by varying either the speed at which
outlet polrtion 58
advances (which varies the time the tissue is subject to laser energy) or the
level of laser energy, or
both.
In order to form the substantially cylindrical hole 138 shown 111 FIG. l OD,
tissue drill 50
?5 advanced outlet portion 58 at a substantially constant speed, and laser
energy source 52 emitted laser
energy at a substantially constant level. However, if a conical-shaped hole
140 as S110wn Ill F1G. I 1
is desired, with the apes: of the hole 140 positioned at the epicardium I 34
and the base of the hole
140 positioned at the endocardium 136, then tissue drill 50 may be configured
to advance outlet
portion 58 at a decreasing rate (i.e., moving at a slower and slower speed)
ve~hile advancing through
the heart wall 130 from the epicardium 134 to the endocardium I 36.
Accordingly, a greater amount
of tissue is ablated as outlet pol-tion 58 advances at a slower speed The
resulting hole 140 has a
diameter substantially equal to diameter d" ofoutlet portion S8 at the
epicardium 1 34 and a diameter
-20-
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
larger than diameter d~s at the endocarciium 136. By fornung the hole 140 with
a relatively lame
diameter at the endocardium 136 improves the patency of the hole and,
therefore, the perfusion of
the blood into the myocardium 132. In addition, by forming a hole with as
small a diameter as
possible at the epicardium 134 minimizes bleeding and trauma.
With reference to FIG. 12, another noncylindrically shaped hole 142 is shown.
Rather than
forming hole 142 by advancing outlet portion 58 fi-om the epicardium 134 to
the endocardium 136 as
shown in FIG. I I, hole 142 is formed endovascularly, with outlet portion 58
advancing from the
endocardium 136 and into the myocardium 132 a predetermined distance c/. As
mentioned above, to
form holes endovascularly, handpiece 54 may be conti~,ureci as a catheter,
with access to the left
ventricle of the heart provided through, for example, a femoral arrtery and
the aolrta. To form hole
142 with a diameter greater than diameter d~, of end surfirce 60 at tire
endocardium 136, tissue drill
50 is configured to advance outlet portion >8 relatively slowly at or near the
epicardium I 36 and then
to increase the speed. This results in more tissue being ablated at or near
the endocardium 136 tllall
at the ~~170tt0111~~ of hole 142 wit111I1 tile illyOCa1'dllllll I32. LaSel-
energy may also be emitted whole
1 S outlet portion 58 retracts to ablate more tissue toward the endocardium
136. The speed of
advancement may be varied by varying either the revolutions per second at
which outlet portion 58
rotates or the pitch of threading 76 and/or 7S, or both. As mentioned above,
rather than varying the
speed at which outlet polrtion 58 advances, the level of emitted laser energy
I_ may be varied. In this
regard, to form hole 142, tissue drill 50 may be configured to emit laser
energy L at a relatively Nigh
level when outlet polrtion SS begins to advance, and then to decrease the
level as outlet portion S8
advances distance c/.
Alternatively, rather than adJustrng the speed or the enemy level, outlet
portion 58 may
reciprocate a multiple of times either at increasing depths or at decreasing
depths. For example,
referencing FIG. 12, if the desired depth of tire hole to be formed is
distance cl (that is, the distance
end surface 60 advances beyond the distal end ofhandpiece 54), then tissue
drill 50 may be
configured to advance outlet portion 58 a distance cl on a first stroke and
then to advance outlet
portion 58 a distance which incrementally decreases for each subsequent stroke
for a predetermined
number of strokes. Accordingly, even though the speed at which outlet portion
58 advances and the
level at which laser energy L is emitted, hole 142 Illay be formed with a
relatively large dlallleter at
the endocardium 136 and tapered toward the epicardium l34 because tissue
toward the endocardium
134 is subject to repeated laser enemy with the multiple strokes of outlet
polrtion 58. Therefore, a
greater portion of this tissue is ablated because of the increased level of
energy received per unit time.
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
Alternatively, rather than decreasing the distance of the stroke, the distance
of each multiple stroke
may be incrementally increased to form hole 140 of F1G. 1 1. In addition, if
it is desired to form a
hole with a relatively large-diameter inner chamber, then tissue drill 50 lay
pause outlet portion 58 at
a predetermined distance for a predetermined amount oftime to concentrate
laser energy at one
location to ablate a relatively large portion of tissue at that location.
Delivery of Medicament to Tissue
An exemplary system for delivering medicament to tissue which is configured in
accordance
with the present invention is illustrated in FIG. 13. Exemplary medicament
delivery system is
referenced with numeral l 50 and may be utilized to form a hole or channel in
tissue, for example,
cardiac tissue (nryocardium), and then to deliver medicament, for example, a
therapeutic agent for
the treatment of cardiovascular disease, or a growth factor, to the tissue by
partially or fillly filling
the hole or channel with tile medicament, by injectin~~ n~eciicament into the
tissue surrounding the
hole or channel, or by administering medicament to a region which includes the
hole or channel anti
the surrounding tissue. This process may be repeated to form and fill a
plurality of holes and
channels in a targeted area of tissue. In contrast to conventional systemic
delivery approaches, the
system I 50 of the present invention delivers medicament in a controlled
manner to specific targeted
tissue. The terms lmle and clmmue~l used herein indicate any space formed in
tissue or through a
section of tissue, which space may be substantially re~mllar i1 shape, such as
circular, elliptical,
curvilinear, or rectilinear, or SIIbStalltially irregular IIl shape.
The exemplary embodiment of delivery system 150 illustrated in F1G. 13 forms
the holes or
channels by removing tissue with laser ablation. As mentioned above, it has
been found that tissue
ablation with laser energy StlrlllllateS a llatlll'al b1010~ICaI prOCeSS Of
illl~lO~eIIeSIS I11 the heart. In
addition, angiogenic-enhancing growth factors have been found to promote
angiogenesis in the hear
Accordingly, a synergistic stimulation and promotion of angiogenesis in the
heart is created by
augmenting the bean's natural angiogenic response to laser ablation with tl~e
delivery ofgroWh
factor to those areas of the myocardium which soave been ablated. The coupling
of the heal-t's natural
response to the f01-lllatl0rl Of CllallilelS \'Vlth the delivery of angiogenic
growth factor into or adjacent
to those channels provides a benefit to patients not possible prior to the
present invention.
~9edicament delivery system 150 may include many Of the 51111e elelllelltS aS
eXelllplaly tissue
drill 50 discussed above. Elements of medicament delivery system I 50 which
are substantially
analogous to elements of tissue drill 50 use like reference numerals with the
addition of a prime (').
For example, IlledICalllelll delivery system 150 includes a handpiece S=I'
V~lllCll Illay be S1117Sta11t11IIy
77 _
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
the same as handpiece 54 of tissue drill 50. 'fliis referencin g convention
will be used in the
description hereunder, and the earlier description of such analogous elements
will not be repeated in
connection with medicament delivery system I 50.
In cardiac applications of the system 150 of tl~e invention, the
administration of medicament
such as endothelial growth factor to cardiac tissue such as myocardium
promotes cardiovascular
angiogenesis. Growth factors are proteins that stimulate or enhance cell
growth. Growtlrfactor
proteins may be packaged in carrier molecules to specifically enhance
angiogenesis. For example, the
naked DNA of the growth-factor pl'otelll may be COlllbllled with a cellular
matrix. Examples of
cellular matrixes include fibrin, plasma, and ally other stnlcture that
enhances the biocompatibility of
the growth factor in the tissue, the angiogenic activity of the growth factor,
and/or the sustained
release of the growth factor into the tissue. There are many commercially
available growth factors
that promote angiogenesis, such as vascular endothelial growth factor (VEGF),
basic fibroblast
growth factor (bFGF), transforming ~;rowtl~ factor-beta (TGF-(3), and platelet-
derived growth factor
(PDGF). The term nmc~iccrrncm~ used herein may include growth factor alone,
growth factor in
combination with a cellular matrix, or growth tractor In COn1b111i1tI0n wlth
any other component that is
known to assist in the delivery of the growth factor. In addition,
IlledICalllent could include any other
substance that stimulates angiogenic activity in the heart.
To deliver medicament to myocardium, exemplary medicament delivery system 150
includes
a tissue-removal device for forming boles or channels in tissue, such as a
source of laser energy and
medicament 152 and an ablatin'; and injecting device 154. As discussed in more
detail below,
ablating and injecting device 154 includes an optical fiber which receives
laser energy tl'Olll SOUI-Ce
152 for ablating tissue to form a channel, and a delivery member which
receives medicament from
source 152 for injection into the channel. In cardiac applications, system I
50 is able to deliver
growth factor directly into the ischemic myocardium of a patient to promote
the grova~th of
endothelial cells.
With additional reference to FIGS. 1=! and I 5, exemplary ablating and
injecting device ) S4
may include an optical fiber 156 and a delivery member I 58 molded together
into a unitary structure
with cladding 160. As described above, exemplary optical fiber 156 may include
a core S2' and a
cladding 84', with core 82' having all lrllet 86' for receiving laser energy
and outlet 62' for
emitting laser energy, as indicated by arrow L in F1G. 13. Exemplary delivery
member 1 S8 may
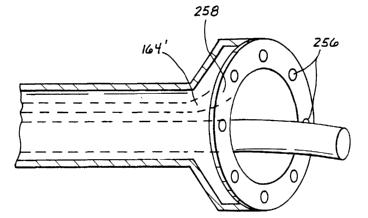
include a wall 162 in v.vhich is defined a lumen I 64 with an inlet 166 for
receiving medicament
and an outlet 168 for providing medicament, which is indicated by arrow Nl in
F1G. 13.
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
Ablating and injecting device 154 has an end surface 170 and an outlet portion
172. Ocltlets 62'
and 168 may be substantially coplanar with end surface 170.
Referencing F1G. 16, exemplary laser energy and medicament source 152 may
include a
laser 110', a drive motor 112', a control unit 1 14', a user interface 116',
and a coupler 1 18' as
S described above. Source 152 may also include a medicament supply 174 for
providing
medicament 1V1 and an injection unit 176 connected to supply 174, both of
which are connected
to control unit 1 14'. A coupler 178 connects lines from laser optics 120' and
injection unit 176
into the unitary ablating and injecting device I 54.
Analogous to optical fiber S6 described above, exemplary ablating and
injecting device
154 is rotatable about an axis of rotation E and translatable betmeen an
advanced position and a
retracted position. With additional reference to FIGS. 17A and 17B, system 150
may form a
channel 138 in myocardium 132, either partially tllrou<~h the myocardium or
completely through
the myocardium. In accordance with the present invention, as distal portion
l72 of ablating and
injecting device 154 retracts from tile advanced position to the retracted
position, as shown by
arrow B, control unit 1 14' activates injection unit 176 to inject medicament
M from supply 174
into delivery member 158 and through outlet l68 into channel 138, as shown in
FIG. 17A.
When device 154 is in the retracted position and handpiece 54' is moved away,
a discrete
amount 179 of medicament is left within channel 138, as shown in FIG. 17(3.
The discrete
amount 179 may partially or fully till channel 135. Tioe procedure may be
repeated a plurality of
times at different locations in the myocardium 132, thereby seeding the
myocardium with
medicament such as angiogenesis-promoting ~~rowtl~ factor. Exemplary injection
unit 176 may
inject medicament through tile use of hydraulics, pneumatics, aerosol, or
other means.
In addition, injection unit 176 may be con(i~ured as an injection jet nozzle
which utilizes
high pressure to create a fluid COIlllllil Of 111ed1C8111e11t for injection
into tissue. A jet injector may
also be used to form a hole in or through tissue with high-pressure fluid
(wluch may contain
medicament), either by tearing (or expanding) the tissue or by removing the
tissue, or a
combination of both. The jet injector may be configured to deliver medicament
to tile tissue
while f01-nlitl~ the hole or channel therein.
Regarding the coupling of ablating and injecting device 154 to laser energy
and
medicament source 152, reference is made to FIGS. 18 and 19 in which an
exemplary
embodiment of a coupling assembly 180 is illustrated. Exemplary coupling
assembly 180
includes a housing 182 which is adapted to receive a reel 184 in a rotatable
and sealed
_2:~_
CA 02392642 2002-05-27
WO 01/39682 PCT/LTS99/28570
relationship. Reel 184 includes a passage I 86 formed axially therethrougl in
which ablating and
injecting device 1 S4 is securely received. Reel 184 also includes an annular
channel 188 and a
through hole 190 extending between passage 186 and channel I 88. Delivery
member I S8
extends from device 154 into through hole 190 to be in communication with
channel 188. A
feeding tube 192 extends between a port 194 of housing I 82 and the medicament
injection and
supply units 176 and 174.
A plurality of o-rings 196 may be used to seal reel 184 within housing 182,
device I S4
within passage 186, and delivery member 1 _58 within through hole 190. Rings
196 may be low-
friction Teflon'x seals. Specialized couplings, such as a Touly-Borst valve
coupling, may be
used to connect device 1 S4 to reel l 84. I-lousing 182 may include structure
such as stops to
limit the axial translation of reel 184. Altlough exa<~~,erated in tl~e
drawings, tolerances
between reel 184 and housing 182 may be on the order of less than about O.OOS
inch. In
addition, housing 182 may be of a two-piece desi~~n with nvo halves hinged
together to allow
easy access to the inside ofthe housing.
Coupling assembly 180 allows ablating and injecting device I S4 to rotate
about
rotational axis E under power from drive unit I 12' while receiving laser
energy and medicament.
For example, device 1 S4 may be driven about 40 revolutions in one direction
(yielding the advanced
position), and then driven about 40 revolutions in the other direction
(yielding the retracted position).
Because of the secure coupling with device 1 S4, reel I 84 is driven by device
1 S4 to rotate about axis
E, that is device 1 S4 may act as a drive shat. ~~~l~en it is desired to
deliver medicament to tissue,
injection unit 176 injects medicament tlu-ou~h tube I 92 (which is indicated
by arrow M) and into a
space 198 defined within channel 1 S8 and beUveen reel I 84 and Dousing I 82.
Medicament is
accordingly urged and/or injected into the lumen I 64 of delivery member I S8.
It~ledicalnent may be
continuously injected into delivery lumen 1 S8 while reel 184 rotates. As
described above, the
injection of medicament into delivery lumen 158 may be limited to when device
154 is retracting.
With general reference to FIG. 13, rather than coupling delivery member 1 S8
to medicament
supply 174 at source system 152, exemplary Dandpiece _S4' may include an
assembly for injecting
medicament into delivery member 1 S8 (not shown). For example, a pressure
capsule, such as a CO,
capsule, may inject medicament into the inlet 166 o('lumen 164 and out of the
outlet 168. Delivery
member 158 may be coiled within handpiece S4' when in the retracted
positioned, and may then
uncoil while being driven to the advanced position. In the embodiment Wlth all
111)eCtloll aSSelllbly at
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
handpiece 54', delivery member 1 S8 may have a relatively shoe overall lennh
as the handpiece is
positioned at or near the tissue targeted to receive medicament.
Alternative configurations of the ablating and injecting device of the present
invention
are shown in FIGS. 20 and 21. Referencing FIG. 20, exemplary ablating and
injecting device 154'
S includes an optical fiber 156' and a delivery member 158' molded together
into a unitary structure
with cladding 160'. Exemplary delivery member 1 SS' may be crescent shaped in
cross-section, as
shown. As discussed above, the diameter d" of the outlet of optical fiber 156'
is preferably at
least one half of the diameter d~, of the end surface 170' of device l S4'.
For example, diameter
do may be about 0.6 mm and diameter d" may be abollt 1.0 mm. Accordingly, as
device 154'
rotates about axis E, laser energy emitted from optical fiber 156' ablates
tissue along the entire
radial sweep of axis E, thereby forming a channel of about 1.0 mm in diameter,
which is
described above (see FIG. 8).
Referencing FIG. 21, exemplary ablotiy anti injecting device I S4" includes a
pair of optical
fibers 156cr and 156h and a delivery member 1 SS" molded to~,ether into a
unitary structure with
cladding 160". Exemplary delivery member I SS" may be rectilinear shaped in
cross-section, as
shown. Tlle outlet of each optical fiber l S6 preferably has a diameter d" of
approximately one
quarter of the diameter d~, of the end surface 170" of device 154'. Therefore,
collectively the
diameters d~ of the optical fibers 156~r and I 56h comprise about one half of
the diameter d~, of
the end surface 170". For example, dian7eter d" may be about 0.3 nun and
diameter cl~, of end
surface 170" 177x)' be about 1.0 111171. Alterllallvely, any number of~ fibers
may be used in
multiple-fiber device ( 54", such as four 0. I 5-mm diameter fibers.
Optical fiber becomes more flexible when its diameter is reduced. 1t follows
that the pair
Of Optical fibers 1 56C1 al7d 1567 Of FIG. 2 I each W~Itll a Cliallletei' Of
about 0.~ 171117 are 1770re
flexible than the single optical fiber 156' of FIG. 20 with a diameter of
about 0.6 mm. As such,
device 154" is more flexible and is able to follow a more tortuous path than
device 154'.
Accordingly, device 154' shown in FIG. 20 is useiill in direct-visualization
procedures in
conjunction with a handiiece as described above, such as in illtra-operative
or traps-tlloraclC
procedures, which do not require the optical fiber to bend through tortuous
paths. Device I 54"
shown in F1G. 21 is usefill in indirect-visualization procedures in
conjunction with a catheter
and a scope, such as traps-septal and endovascular procedures. For example,
device 154" may
be inserted into a femoral artery, through tl7e aOrtIC 11-C17, and into the
left ventricle to ablate
tissue from the endocardium to the epicardium.
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
Rather than fOrrllln~ ChaIlIlelS Ill tissue with laser ablation as described
above, tissue may
be removed to form channels in accordance with the present invention for
medicament delivery
by other methods, for example, by high-frequency electrical energy or radio-
frequency (RF)
energy. Referencing FIG. 22, an exemplary embodiment of a tissue-removal and
medicament-
delivery device 200 which uses electrical energy to remove tissue in
accordance with the present
invention is illustrated. Device 200 includes an electrode 202 disposed on a
distal tip of the
device, an insulator 204 proximal to the electrode ?0?, anti a body 20G. A
delivery lumen 208 is
formed axially through electrode 202, insulator ?0-I, and body ?06, and I~as
an outlet 210 in a
distal end of device 200.
An alternative embodiment of an electrical-energy tissue-removal and
medicament-
delivery device 200' of the invention is illustrated in F1G. 2 3. Rather than
having an axial
delivery lumen, device 200' includes at least one delivery lumen 210 formed
longitudinally
through at least the body 20G'. As shown in FIG. 2 3, two delivery lumens ?
l2cr and 21?h are
formed through the body 206' and extend into the insulator 204'. Each lumen ?
12 has an outlet
214 formed on a side 216 of device 200'. In the exemplary embodiment, outlets
214n and 214h
may be substantially diametrically opposed within the device. Alternatively,
each lumen ? 12
may have a plurality of outlets which form an array of ports on the side 216
of device 200' .
With additional reference to FIG. 2=4, in use, tl~e tissue-removal and
medicament-delivery
devices 200 and 200' generate high-frequency electrical enemy, which in turn
generates an
ionized plasma corona ? 16 and converts tissue 1 32 to gas to create a channel
or hole in the
tissue. A ground plate 21 S ma~~ be provided such that tissue 132 is
positioned between the
ground plate and electrode 202. '1'I~e ground plate 218 may be used to control
conduction paths
(shown by the dashed arrows) formed by tl~e positively charged electrode 202,
v.vhich controls
the formation of channels in tissue. After holes or channels are formed in
tissue, medicament
such as growth factor that promotes angiugenesis may be delivered to the
tissue within the hole
or channel itself via the delivery lumen as described above. Alternatively,
with reference to FIG.
25, medicament may be delivered into the walls of the hole or channel 138 and
into the tissue
132 surrounding the hole or channel 138 via tl~e delivery lumens ? 12, as
indicated by arrows 1~~1.
Referencing FIG. 26, another exemplary embodiment of an electrical-energy
tissue-
removal and medicament-delivery device 200" of tile invention is illustrated.
Device 200"
includes a cathode 220 disposed on a distal tip of tl~e device, an InSrllatOr
204" proximal to the
electrode 202, an anode 222 proximal to the insulator, and a body 206". A
delivery lumen 208
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
is formed axially through the device 200". Electrical-conducting leads (not
shown) connect the
cathode and anode 220 and 222 to a power source. When activated, conduction
paths (shown
in dashed lines) between the cathode 220 and the anode 220 define the ionized
plasma cornea
216' which converts tissue to gas to form channels.
S Another exemplary embodiment of a medicament-delivery system 230 of the
present
invention is illustrated in FIGS. 27, 28, and 29. System 230 includes an
ablating and injecting
device 232 received within a catheter 234. Exemplary ablating and injecting
device 232 includes
a pair of optical fibers 236a and 236h and a pair of delivery members 238a and
238h. Cladding
240 configures the fibers 236 and 238 into a unitary and cylindrical device.
Optical fibers 236
and delivery members 238 may be configured and function in accordance with the
description
provided above. Exemplary device 232 also includes rifling tracks 242 formed
on an annular lip
244 thereof, preferably at a distal end of the device, and exemplary catheter
234 111C1lIdeS rifllllg
246 formed on an inner surface thereof for slidingly engaging with the rifling
tracks 242 of the
ablating and injecting device 232. Accordingly, as ablating and injecting
device 232 rotates, the
rifling 246 translates the device 232 axially within catheter 234, between the
advanced and
retracted positions as described above.
Exemplary medicament-delivery system 230 is particularly useful in
endovascular
procedures which may entail guiding the ablating and injecting device 232 and
catheter 234
through tortuous paths to its final destination. Accordingly, it is preferable
to maximize as
much as possible the flexibility of the ablating and injecting device 232. As
such, it is preferable
for the diameters of the optical fibers 236 to be as SIllaIl as possible while
still capable of
carrying sufficient laser energy to ablate tissue. In a preferred embodiment,
the diameter of each
optical fiber 236 may be about 0.25 nun. The overall diameter of the device
232 nay then be
about 0.5 mm.
In an alternative embodiment of the ablating and injecting device of tile
present
invention, the medicament may be delivered directly to the tissue surrounding
the channel
instead of delivering the medicament into tile channel or into the tissue by
way of tile channel.
This may be advantageous where it is desirable to avoid systemic
administration of the
medicament, which could occur through washout of medicament when it is
delivered directly
into the channel. Various configurations of this alternate embodiment are
shown in FIGS. 30-
40.
_2S_
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
Referencing FIG. 30, alternate head portion 100' Ilas one or more needles 2S0
on tire
outer rim of its tissue-engaging surtace for penetrating the tissue around the
perimeter of the
tissue channel opening. This provides access directly to the tissue
surrounding the channel. ~I'I~e
delivery device is provided with one or more medicament lumens 164' which are
in fluid
communication with needles 250. Needles 2S0 pierce the tissue around the
perimeter of the
channel opening and deliver medicament by way of lumen or lumens 164'. The
medicament
diffuses through the tissue without having to enter into the channel, thus
avoiding 111ed1Calllen2
washout and the possibility of systemic delivery of tile medicament. F1G. 31
illustrates the end
surface of head portion 100' having an array of needles 2S0 around its
perimeter. FIG. 32
illustrates medicament 2S2 diffusing into the tissue surrounding channel 138.
Other
embodiments of alternate head portion 100' are illustrated in FIGS. 33 and 34.
FIG. 33
illustrates head portion 100' having a IloZZle Or ilOZZIeS 2J=1 around its
perimeter. Nozzles 2S4
are adapted to atomize tile medicament when head portion 100' is placed up
against the tissue
surrounding the channel opening. As in the previous embodiment using needles
250, the
1S medicament is diffused directly into the tissue and not into the channel
where it can be washed
out into the patient's system. F1G. 3=4 illustrates yet another embodiment
wherein head portion
100' has a port or ports 2S6 on the head portion perimeter for diffusing
medicament directly
into the tissue surrounding the channel opening.
FIGS. 3S and 36 illustrate that medicament can be provided to head portion
100'
through a single lumen 164" which is in tluid CO IllIl111111cat1011 wltll all
811rllllilr lllallltold 2S8
Wh1C17 c01T11111Ir71CateS throu~.~h the perimeter ofhead portion 100' to ports
256. F1G. 37
illustrates an alternate embodiment wherein single lumen 16=I" Iras an annular
geometry. Those
skilled in the art will appreciate that this sin<,le lumen embodiment
incorporating manifold 2S8
can also be utilized with nozzles 2S4 or needles 250. Similarly, it will be
appreciated by those
skilled in the ao that other means for diflusin~, medicament directly into the
tissue surrounding
the channel opening can be utilized for like effect.
FIG. 38 illustrates yet another exemplary embodiment wherein at least one
delivery
lumen 264 is in fluid communication with delivery outlet 266 and at least one
vacuum lumen
268 is in communication with a vacuum source (not shown) and terminates in
vacuum outlet
270. By providing a vaCllllill to head portion 100' through lumen 268 to
outlet 270 the clinician
can insure that medicament Catl be delivered directly to the tissue through
lumen 264 and outlet
266. It will be appreciated by those skilled in the art that this embodiment
could include a
_2c~_
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
plurality of vaclllllll 1r1111eI1S alld a plurality of delivery llllllellS t0
nrax11111Ze the effectiveness of
the invention.
Referencing F1G. 39, an alternate excnrplary embodiment of an electrical-enemy
tissue-
removal and medicament-delivery device 200" is illustrated wherein the
medicament can be
directly delivered into the tissue walls and dit~used into the tISSlle
Slll'rolltldlilg the channel. As
in the embodiment of FIG. 23, two delivery lumens 212a' and 212b' are
provided, each having
its respective outlet 214x' and 214b'. In tire exemplal>> embodiment, outlets
214a' and 214b'
may be substantially diametrically opposed mitlrin the device. In this
embodiment, vacuum
lumens 212c' and 212d' are provided longitudinally through the body of device
200" and in
communication with outlets 214c' and 214d', respectively. Outlets 214c' and
214d' may also be
diametrically opposed to each other. Vacuum lumens 212c' and 212d' are in
communication
with a vacuum source (not shown) which provides a vacuum through lrlnrens
212c' and 212d' to
outlets 214c' and 214d' to drav.v the tissue channel wall up against outlets 2
I 4c' and 214d'. Due
to their proximity, sufficient vacuum can be provided to also draw the tissue
wall up against
I S outlets 214a' and 214b'. Medicament 252 can then be provided through
delivery lumens 212x'
and 212b' to outlets 214x' and 214b' and directly into the tissue wall of the
channel as illustrated
in FIG. 40. In the embodiment shown, outlets 21=la' alld 21=4b' are distal to
outlets 214c' and
214d'. However, it is also possible to configure the outlets so that 214c' and
214d' outlets are
distal to 214a' and 214b' outlets and to confl<,ure the delivery and vacuum
lumens accordingly.
As is the case with the embodiment of FIGS. 30-s~, IIrIS elllbodlillellt also
pel'ilrltS IlledICalllellt
to be diffused into tire tissue surrounding tire channel without having
systemic washout of tire
medicament.
In addition to removin '; tissue to form boles or channels by laser ablation
or by high-
frequency electrical enemy, holes or channels may be formed in tissue
mechanically with hot tips or
biopsy needles, ultrasonically, or hydraulically with high-pressure water. The
medicament call be
growth factor, which may take many forms. For example, ,rowtlr factor may be
delivered as a
protein solution. Alternatively, ~;rov.~~th factor may be combined with a
fibrin, collagen, or plasma to
form a cellular matrix gel. Growrth factor may also be mixed into a semi-solid
using a biocompatible
matrix. Further, growth factor may be delivered to tissue in an atomized form
under pressure. Tlre
medicament can also be a gene drat encodes for said <~rovvtlr factor, or any
other therapeutic
ajent or gene therapy agent that promotes an~;iolenesis or any therapeutic
agent for the
treatment of cardiovascular disease. Whatever the form may be, the
angio~enesis-promoting
_;0_
CA 02392642 2002-05-27
WO 01/39682 PCT/US99/28570
growth-factor solution is administered throu~fh the delivery lulnen(s) to
enhance and accelerate the
angiogenic process. The gro\vth factor solution may be driven into the channel
and/or tlSSlle LISIIIs,
for example, a pnet1111at1C SyStenl, a IlleC11a111Ca1 S~'SlClll (t;.~;., a
syringe-type system witll a plunger), a
hydraulic system (e.g., using fluids or gas), or a gravitational system.
Those skilled in the art will understand that the embodiments of the present
invention
described above exemplify the present invention and do not limit the scope of
the invention to these
specifically illustrated and described embodiments. The scope of the invention
is determined by the
terms of the appended claims and their legal equivalents, rather than by the
described examples. In
addition, the exemplary embodiments provide a follildall011 f1~0r11 \\'111C11
llllnlerollS alterllatlveS alld
I~ 1170d1fiCat10T1S May be n lade, which alternatives alld IllOC11f1C1t1011S
al'e aISO \\'itlllll tile SCOpe Oftlle
present invention as defined in the appended claims
- J I -