Note: Descriptions are shown in the official language in which they were submitted.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
1
BENZO[A]PHENAZIN-11-CARBOXAMIDE DERIVATIVES AND THEIR USE AS JOINT INHIBITORS
OF TOPOMERASE I AND II .
The present invention relates to substituted benzo[a]phenazine-11-carboxamides
and
derivatives thereof. These compounds are cytotoxic agents which have
demonstrated
topoisomerase I and topoisomerase II inhibition and have the ability to
circumvent multidrug
resistance mechanisms. They are therefore potential anticancer agents
The topoisomerases are important cellular targets for a number of successful
chemotherapeutic agents (Wang, Ann. Rev. Biochem, 65, 635-692, 1996) and are
essential
enzymes in the regulation of DNA topology which is required if cells axe to
divide and
proliferate (Wang, loc city- Digs that taxget topoisomerase II, for example
doxorubicin and
etoposide, have been widely used in cancer chemotherapy (Hande, Biophys. Acta
1400, 173-
184,1998) while those that specifically taxget topoisomerase I, principally
the camptothecin
analogues, have made an important impact more recently, an example being CPT-
11 for the
treatment of colon cancer (Dancey et ah Br. J. Cancer 74, 327-338, 1996). More
recently,
topoisomerases have been shown to be therapeutic targets for antifungal,
antibacterial and
antiviral drugs (Chen et ah Rev. Pharmacol. Toxicol, 34, 191-218, 1994).
In addition to those compounds that specifically target topoisomerase I or II,
several
joint inhibitors of topoisomerase I and II have been identified and may also
be beneficial in
the treatment of solid tumours. These compounds include intoplicine (Riou et
al ~ C~cer
Res. 53, 5987-5993, 1993), DACA/XR5000 (Finlay et al, Eur. J. Cancer 32A, 708-
714,
1996) and TAS-103 (Utsugi et ah J. Cancer Res, 88, 992-1002 1997) which are
all in clinical
evaluation. The advantage of joint inhibitors of topoisomerase I and II is
their ability to avoid
drug resistance and to target two key enzymes that affect the topology of DNA
which are
active at different points in the cell cycle.
It has now been found that a class of novel benzo[a]phenazine-11-carboxamides
are
inhibitors of topoisomerase I and topoisomerase II. Accordingly, the present
invention
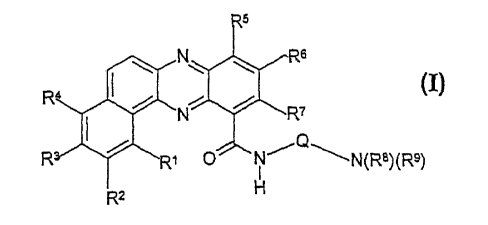
provides a compound which is a benzo[a]phenazine-11-carboxamide derivative of
formula (I)
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
2
6
R I
N ~ R7
~~N/~~N(R$)(R9)
I
R2 H
wherein each of Ri to R4, which are the same or different, is selected from
hydrogen, halogen,
hydroxyl, CI-C6 alkoxy which is unsubstituted or substituted, heteroaryloxy,
C1-C6 alkyl
which is unsubstituted or substituted, nitro, cyano, azido, amidoxime,
C02R1°, CON(R12)2,
OCON(Ri2)a, SRl°, SORII, SOZRIl, SOZN(Rl2)Z, N(R~2)2,
NRl°S02Ri1, N(SOZRII)2,
NRl°(CHZ)nCN, NRl°CORII, OCORII or CORIO;
each of RS to R7, which are the same or different, is selected from hydrogen,
halogen,
hydroxy, C1-C6 alkoxy, CI-C6 alkyl, SRl° and N(Rla)2;
Q is C1-C6 alkylene which is unsubstituted or substituted by (i) C1-C6 alkyl
which is
unsubstituted or substituted, (ii) hydroxy, provided that the hydroxy group is
not a to either of
the N atoms adjacent to Q in formula (I), (iii) COZRI°, or (iv)
CON(R12)Z;
R8 and R9, which are the same or different, are each hydrogen or C1-C6 alkyl,
or R$ and R9
together with the nitrogen atom to which they are attached form a saturated 5-
or 6-membered
N-containing heterocyclic ring which may include one additional heteroatom
selected from O,
N and S, or one of R8 and R9 is an alkylene chain optionally interrupted by O,
N or S, which
is attached to a carbon atom on the alkylene chain represented by Q to
complete a saturated 5-
or 6-membered N-containing heterocyclic ring as defined above;
Ri° is hydrogen, C1-C6 alkyl, C3-C1° cycloalkyl, benzyl or
phenyl;
RI1 is C1-C6 alkyl, C3-C1° cycloalkyl, benzyl or phenyl;
each R12, which are the same or different, is hydrogen, C1-C6 alkyl,
cycloalkyl, benzyl or
phenyl, or the two Riz groups form, together with the nitrogen atom to which
they are
attached, a 5- or 6-membered saturated N-containing heterocyclic ring which
may include 1
or 2 additional heteroatoms selected from O, N and S; and
nis l,2or3;
or a pharmaceutically acceptable salt thereof;
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
3
with the proviso that at least one of Ri to R4 is other than hydrogen.
In a preferred aspect of the invention the benzo[a]phenazine carboxamide-11-
derivative is of formula (Ia)
6
\ N \ R
R4 I (Ia)
/ _ ~ / 7
I N ' R13
3 / '
R RZ R1 O N CH~(CH2)p N(R$)(R9)
5 H
wherein Rl to R9 are as defined above;
p is 1 or 2; and
R13 is (i) hydrogen (ii) C1-C6 alkyl which is unsubstituted or substituted by
hydroxy, aryl or
N(R12)2 in which R12 is as defined above, (iii) C02R1°, (iv) CON(R12)2,
or (v) aryl.
When one of R8 and R9 in formula (I) is an alkylene chain which is attached to
a
carbon atom on Q, the compound of formula (I) has the following structure
(Ib):
Rs
N R6
\ ~ \
R4 I
/ N / R7
Y
R3 / Rl O~ N_yU_~ ~-Ri4
R2 H ~Z~
wherein R1 to R' are as defined above for formula (I);
R14 is hydrogen or Ci-C6 alkyl;
W is a direct bond or a Cl-CS alkylene chain; and
Y and Z form, together with the N and C atoms to which they are attached, a
saturated 5- or 6-
membered N-containing heterocyclic ring which may include one additional O, N
or S atom.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
4
A C1-C6 alkyl group rnay be linear or branched. A Ci-C6 alkyl group is
typically a C1-
Cd alkyl group, for example a methyl, ethyl, propyl, i-propyl, n-butyl, sec-
butyl or tert-butyl
group. A C1-C6 alkyl group is unsubstituted or substituted, typically by one
or more groups
selected from hydroxy-C1-C6 alkyl wherein the alkyl moiety is unsubstituted or
substituted as
specified herein for C1-C6 alkyl, C1-C6 alkoxy, phenyl, N(R12)2 wherein R12 is
as defined
above, and hydroxy. Examples of hydroxy-Cl-C6-alkyl include, for instance,
hydroxymethyl,
1-hydroxyethyl and 2-hydroxyethyl. C1-C6 alkylene is a CI-C6 alkyl group as
defined above
which is divalent.
An aryl group is typically an aromatic C6-C1° carbocyclic group, such
as phenyl or
naphthyl, which is unsubstituted or substituted by halogen, C1-C6 alkyl, OH,
CI-C6 alkoxy,
N02, N(R12)Z, COZRI°, CN or perhalo C1-C6 alkyl such as CF3.
A halogen is F, Cl, Br or I. Preferably it is F, Cl or Br.
A C1-Cb alkoxy group may be linear or branched. It is typically a C1-C4 alkoxy
group,
for example a methoxy, ethoxy, propoxy, i-propoxy, n-propoxy, n-butoxy, sec-
butoxy or tert-
butoxy group. A C1-C6 alkoxy group is unsubstituted or substituted, typically
by one or more
groups selected from N(R12)2, CON(R12)Z, hydroxy, C1-C6 alkoxy, C1-C6 alkyl,
C2-C6 alkenyl,
C2-C6 alkynyl, cyano, COZRI°, CORI°, a saturated 5- or 6-
membered N-containing
heterocyclic group or phenyl, the phenyl group being unsubstituted or
substituted by one or
more halogen atoms.
A C3-C1° cycloalkyl group may be cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl. Typically it is C3-C6 cycloalkyl. A C2-C6 alkenyl group contains
one or more
unsaturated bonds. It may be, for instance, vinyl, propenyl, butenyl or
pentenyl. A C2-C6
alkynyl group may be ethynyl, propynyl, butynyl or pentynyl. A saturated 5- or
6-membered
N-containing heterocyclic ring may be, for example, piperidine, piperazine,
morpholine or
pyrrolidine.
A heteroaryloxy group is a group -OHet in which Het is an unsaturated 5- or 6-
membered N-containing heterocyclic ring which may include one or more
additional O, N or
S atoms. Examples include furan, thiophene, pyrrole, indole, isoindole,
pyrazole, imidazole,
isoxazole, oxazole, thiazole, isothiazole, pyridine, quinoline, quinoxaline,
isoquinoline,
thienopyrazine, pyran, pyrimidine, pyridazine, pyra.zine, purine and triazine.
The aforesaid
heterocyclic ring may be unsubstituted or substituted by one or more
substituents, for instance
one or more substituents selected from OH, halogen, C1-C6 alkyl which is
unsubstituted or
substituted, for example by halogen (such as CF3), C j-C6 alkoxy, nitro and an
amino group
N(R12)2 as defined above.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
In a preferred aspect of the invention, Rl to R3 in formula (I), (Ia) or (Ib)
are each
hydrogen and R4 is other than hydrogen. Typically R4 is C1-C6 alkoxy, hydroxy,
C1-C6 alkyl,
hydroxy-C1-C6 alkyl, nitrite or halogen.
In a preferred series of compounds R4 in formula (I), (Ia) or (Ib) is Ci-C6
alkoxy or
'S hydroxy, R7 is hydroxy, and R1 to R3, RS and R6 are each hydrogen. Also
preferred are
compounds wherein R4 is C1-C6 alkoxy or hydroxy, R6 is C1-C6 alkoxy, halogen
or methylthio
and Rl to R3, RS and R7 are all hydrogen.
In formula (Ia) R13 is preferably C1-C6 alkyl, more preferably methyl.
In formulae (I) and (Ia) a preferred option for Q is a C2- or C3- alkylene
chain which is
substituted ~, to the adjacent amide nitrogen atom by C1-C6 alkyl which is
unsubstituted or
substituted as defined above. Preferably the substituent on Q is unsubstituted
C1-C6 alkyl or
hydroxy-C1-C6 alkyl such as hydroxymethyl or hydroxyethyl. Typically the CZ-
or C3-
alkylene chain is substituted ~ to the adjacent amide nitrogen atom by methyl,
ethyl,
isopropyl, hydroxymethyl, substituted hydroxymethyl or 1-hydroxyethyl.
Examples of preferred compounds of the invention are:
Compound Name Compound
Number
et oxy- enzo a p enazme- -car oxy is aci - 1
dimethylamino-ethyl)-amide
.~-riyaroxy-nenzo~alpnenazme-i 1-carnoxyiic
acia ~z-
dimethylamino-ethyl)-amide
4-Methoxy-benzo~aJphenazine-11-carboxylic 3-
acid (Z-
dimethylamino-ethyl)-amide
y roxy- enzo a p ena.zme- -car oxy is aci
dimethylamino-ethyl)-amide: hydrobromide salt
-Methoxy-benzo~aJphenazine-11-carboxylic acid
(2-
dimethylamino-ethyl)-amide
~-riyaroxy-nenzo~alpnenazme-i i-caxnoxyuc
acia ~~-
dimethylamino-ethyl)-amide
4-Nitro-benzo~aJphenazine-11-carboxylic acid 7 .
(2-
dimethylamino-ethyl)-amide
4-1W methylaminomethyl-3-hydroxy-benzo~aJphenazine-11-g
carboxylic acid (2-dimethylamino-ethyl)-amide
j-JW methylaminomethyl-4-hydroxy-benzo~aJphenazine-11-9 -
carboxylic acid (2-dimethylamino-ethyl)-amide
y-t~romo-4-metnoxy-nenzo~alpnenazme-1 ~-carboxylic10
acid (z-
dimethylamino-ethyl)-amide
4-Lyanomethoxy-benzo~aJphenazine-11-carboxylic11
acid (2-
dimethylamino-ethyl)-amide
4-t~enzyoxy-nenzo~alpnenazme-t 1-carboxylic 12
acid (~-
dimethylamino-ethyl)-amide
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
4-Prop- -yny oxy- enzo a p enazme- ~=car oxy 13
is aci
dimethylamino-ethyl)-amide
3,4-L~imethoxy- enzo a p Zeriazme- -car oxy 14
is aci
dimethylamino-ethyl)-amide
4-~thoxy-benzo [a p enazme- -car oxy is act 15
dimethylamino-ethyl)-amide
4-lsobutoxy-benzo[aJphenazine-I1=carboxy is 16
aci ( -
dimethylamino-ethyl)-amide
+-~~+-~moro-uenzytoxy)-benzo~a~phenazme-11-carboxylic1~
acid
(2-dimethylamino-ethyl)-amide
4-(z-Methoxy-ethoxy)-benzo[a]pheriazme- -car 1g
oxy is aci
dimethylamino-ethyl)-amide
X11-(~-lOmethylamino-ethylcarbamoyl)- enzo 19
a p enazm-
yloxy]-acetic acid ethyl ester
3-l3romo-4= y oxy- enzo a p enazme- -car oxy 20
1c aci
dimethylamino-ethyl)-amide
4-(~-riydroxy-ethoxy)-benzo[aJphenazine-I 21
-car oxy is aci
dimethylamino-ethyl)-amide
+-~ryrimiam-z-ytoxy)-benzo~a~phenazme-11-carboxylic22
acid (2= -
dimethylamino-ethyl)-amide
4-(z-Morpholm-4-yl-ethoxy)-benzo[aJphenazine-23
-car oxy is
acid (2-dimethylamino-ethyl)-amide
+-~j-~yano-propoxy)-nenzo~a~phenazme-11-carboxylic24
acid (2-
dimethylamino-ethyl)-amide
4-Methyl-benzo[aJphenazine-11=carboXy is aci 25
dimethylamino-ethyl)-amide
4-rmoro-nenzo~a~phenazme-11-carboxylic acid(2-26
dimethylamino-ethyl)-amide
4-(a-1)imethylamino-propoxy)-benzo a p enazme-2~
-car oxy is
acid (2-dimethylamino-ethyl)-amide
4-Methylsultanyl-benzo[a p enazme- -car oxy 2g
is aci
dimethylamino-ethyl)-amide
4-Larbamoylmethoxy-benzo[aJphenazine-~l-car 29
oxy is aci
dimethylamino-ethyl)-amide
4-Methoxy-benzo[aJphenazine-11-carboxy lc 30
aci -ammo-
hydroxy-propyl,)-amide
4-Methoxy-benzo[aJphenazine-11=carboxy is 31
aci
dimethylamino-propyl)-amide
4-tsromo-nenzo~a~phenazme-11-carboxylic acid 32
(~-
dimethylamino-ethyl)-amide
Acetic acid 11-(2-dimethy ammo-et y car amoy 33
-
benzo [a]phenazin-4-yl ester
~-~~-vxo-propoxy)-nenzo~a~phenazme-11-carboxylic34
acid (Z-
dimethylamino-ethyl)-amide
4-Methoxy-benzo[aJphenazine-1 -car oxy is 35
aci
dimethylamino-1-methyl-ethyl)-amide
4-~yano-nenzo~a~pnenazme-11-carboxylic acid 36
(2-
dimethylamino-ethyl)-amide
-
P;thyl-carbamic acid 3'7
II=(2- imet y ammo-et y car amoy -
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
benzo [~phenazyester
3- itro- enzo a p enazme- -car oxy is aci 3g
dimethylamino-ethyl)-amide
et anesu ony - enzo a p enazme- -car oxy is 39
aci
dimethylamino-ethyl)-amide
+-~moro-nenzo~a~pnenazme-11-carnoxylic acia 40
~z-
dimethylamino-ethyl)-amide
4-Azido-benzo~aJphenazine-11-carboxylic acid(2-41
dimethylamino-ethyl)-amide
4-~rrnno-eenzo~alpnenazme-11-carboxylic acia 42
~z-
dimethylamino-ethyl)-amide
[11-(2-Dimethylamino-ethylcarbarnoyI)=benzo 43
a p enazm-
yloxy]-acetic acid trifluoro-acetate salt
4-Acetylamino-benzo~aJphenazine-11-carboxylic44
acid (2=- -
dimethylamino-ethyl)-amide
imet y ammo-et y car amoy - enzo a p enazme- 45
carboxylic acid methyl ester
4-W s-(Methanesultonylamino)-benzo~aJphenazine-11-46
carboxylic acid (2-dimethylamino-ethyl)-amide
.~-~nmo-nenzo~a~pnenazme-1 i-carnoxylic acia 47
~L-
dimethylamino-ethyl)-amide
+-yv-riyaroxycarnamimiaoyl)-uenzo~a~pnenazme-11-carboxylc4g
acid (2-dimethylamino-ethyl)-amide
y roxymet y - enzo a p enazme- -car oxy is 49
aci
dimethylamino-ethyl)-amide
11-(~-l~imethylamino-ethylcarbamoyl)-benzo 50
[aJphenazine-4-
carboxylic acid, trifluoroacetate salt
4-Methylsultamoyl-benzo[aJphenazine-11-carboxylic- S1
acid (2-
dimethylamino-ethyl)-amide; trifluoro-acetate
:i-Methylsultamoyl-benzo[aJphenazine-11-carboxylic52
acid (2-
dimethylamino-ethyl)-amide; trifluoro-acetate
:i-Acetylammo-benzo[aJphenazine-11-carboxylic53
acid (2-
dimethylamino-ethyl)-amide.
4-mmetnylammo-benzo~a~pnenazme-11-carboxylic 54
acrd (~-
dimethylamino-ethyl)-amide
4-Methanesultonylamino-benzo~aJphenazine-11-carboXylic55
aci
(2-dimethylamino-ethyl)-amide
a-Methanesultonylamino-benzo~aJphenazine-11-carboxylic56
acW
(2-dimethylamino-ethyl)-amide
4=I7iinet y su arnoy - enzo a p enazme- -car 57
oxy is aci
dimethylamino-ethyl)-amide
a-LOmethylsulfamoyl-benzo~aJphenazine-11=carboxy5g
W cid ( -
dimethylamino-ethyl)-amide
4-~~yanometnyt-ammo)-benzo~a~phenazme-11-carboxylic59
acid
(2-dimethylamino-ethyl)-amide
4,1u-LOmethoxy-benzo~aJphenazine-11-carboxylic60
act
dimethylamino-ethyl)-amide
4-Methoxy-benzo[a]phenazlne- -car oxy is aci 61
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
imet y ammo--propy -aml a
enzo a p enazme=~dicar oxy 1c aci -ami a 6
dimethylamine-ethyl)-amide]; triflouroacetic
acid salt
1-(:hloro-4,1U-dimethoxy-benzo~aJphenazine-11-carboXy63
is aci
(2-dimethylamino-ethyl)-amide
.~-~umamoy-nenzo~a~pnenazme-1 i-carboxync 64
acia (~-
dimethylamino-ethyl)-amide
4-Methoxy-benzo~aJphenazine-11-carboxylic 65
acid (2=
dimethylamino-1,1-dimethyl-ethyl)-amide
2- itro- enzo a p enazme- -car oxy is aci 66
dimethylamino-ethyl)-amide
et oxy- -met y - enzo a p enazme- -cax oxy 6
is aci
dimethylamino-ethyl)-amide
i y roxy- enzo a p enazme- -car oxy is aci 6g
dimethylamino-ethyl)-amide
:i-J~imethylamino-2-~(4-methoxy-benzo[aJphenazme-69
carbonyl)-amino]-propionic acid methyl.ester.
Trifluoroacetic
acid salt
imet y ammo- -met oxy- enzo a p enazme- ~p
carbonyl)-amino]-propionic acid; hydrochloride
4-Methoxy-benzo~aJphenazine-11-carboxylic
aci
dimethylaminomethyl-propyl)-amide
4,1U-1W methoxy-benzo~aJphenazine-11-caxboxy
icy
dimethylamino-1-methyl-ethyl)-amide
oro- -met oxy- enzo a p enazme-, -car oxy ~3
is aci
dimethylamino-ethyl)-amide
et oxy- enzo a p enazme- -car oxy is aci . 74
dimethylarninomethyl-2-methyl-propyl)-amide
4-Methoxy-benzo~aJphenazine-11-carboxylic
acid (2-
dimethylamino-1-hydroxymethyl-ethyl)-amide
4-Methoxy-benzo~aJphenazine-11-carboxylic ~6
acid (1= -
dimethylaminomethyl-2-phenyl-ethyl)-amide
4-Methoxy-benzo~aJphenazine-11-carboxylic
acid (2-
dimethylamino-1-(S)-methyl-ethyl)-amide
4- a oxy- enzo a p enazme- -car oxy is aci ~g
dimethylamino-1-(R)-methyl-ethyl)-amide
4-Vitro-benzo~aJphenazine-11-carboxylic acid
dimethyla.mino-1-methyl-ethyl)-amide
itro- enzo a p enazme- -car oxy is aci gp
dimethylamino-1-methyl-ethyl)-amide
4-Methoxy-benzo~aJphenazine-11-carboxylic gl
acid (~-
dimethylamino-1-(S)-hydroxymethyl-ethyl)-amide
4-Methoxy-lU-methylamino-benzo[aJphenazine-~ g2
-car oxy is
acid (2-dimethylamino-ethyl)-amide
y roxy- -met oxy- enzo a p enazme- -car oxy g3
is aci
(2-dimethylamino-1 (R)-methyl-ethyl)-amide
4-Methoxy-benzo~aJphenazine=11-carboxylic g4
aci
dimethylaminomethyl-2-hydroxy-propyl)-amide
1u-Hydroxy-4-methoxy-benzo[a]phenazine-11-carboxyg5
is aci
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
9
(2-dimet y ammo-et y -ariu3e-
et oxy= enzo a p enazme- -car oxy ?c acs -pipersg6 -
m-
1-yl-ethyl)-amide
4-Methoxy-benzo~aJphenazine=1 T car oxy is g~
acs
dimethylamino-1-(2-hydroxyethyl)]-ethylamide
mo- -met oxy- enzo a p enazme- -car oxy is gg
acs
dimethylamino-ethyl)-amide
4-Methoxy-benzo~aJphenazine=Il=car oxy is g9
acs -morp o m-
4-yl-ethyl)-amide
4-Methoxy- enzo a p enazme- -car oxy is acs 90
-pyrro s m-
1-yl-ethyl)-amide
4-Methoxy-benzo~aJphenazine=1 I=cax oxy is 91
acs 1s-
hydroxy-ethyl)-amino]-ethyl}-amide
4-Methoxy-benzo~aJphenazine=il-car oxy is
acs
diethylamino-ethyl)-amide
4-Methoxy-y-methylsulfanyl-6emzo a p enazme- 93
-car oxy is
acid (2-dimethylamino-ethyl)-amide
4,~-1W methoxy-benzo~aJphenazine-1 I=carboxy 94
is acs
dimethylamino-ethyl)-amide
4,1 U-Uimethoxy-benzo~aJphenazine-11-car oxy 95
is acs
dimethylamino-1 (S)-hydroxymethyl-ethyl)-amide
4-Methoxy-benzo[aJphenazirie- -car oxy is 96
acs
methylamino-ethyl)-amide
tu-Hyaroxy-4-rnethoxy-benzo~aJphenazine-11-car9
oxy is acs
(2-dimethylamino-1 (S)-hydroxymethyl-ethyl)-amide
(K)-4-Methoxy-benzo~a]phenazine- -car oxy 9g
is acs
dimethylaminomethyl-2-methyl-propyl)-amide
4- et oxy- enzo a p enazme- -car oxy is acs 99
-met y -
pyrrolidin-3-(R)-yl)-amide
4-Methoxy- enzo a p enazme- -car oxy is acs 100
, - is -
dimethylamino-propyl)amide
Compounds of formula (I) may be prepared by a process which comprises:
(a) treating an activated derivative of a compound of formula (II):
6
N ~ R
R4 . (II)
N ~ R'
R3 / R1 C02H
5 R2
wherein R1 to R' are as defined above, with an amine of formula (III):
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
H2N/~\N(Rg)(R9) (III)
wherein Q, Rg and R9 are as defined above; or
(b) treating a compound of formula (IV):
5
R3 / Rl ~ v
Ra Rii
wherein Rl to R7 and Rl1 axe as defined above, with a compound of formula
(III) as defined
above, either in an organic solvent or neat and at an elevated temperature;
and
(c) if desired, converting one resulting benzo(a)phenazine-11-carboxamide
derivative of
10 formula (I) into another such derivative, and/or converting a
benzo[a]phenazine-11-
carboxamide derivative of formula (I) into a pharmaceutically acceptable salt
thereof.
The optical purity of resulting compounds that have an optically active
centre, for
instance the benzo[a]phenazine-11-carboxamide derivatives of formula (Ia) and
the salts
thereof, may be determined by the addition of an NMR shift reagent such as
2,2,2-trifluoro-
1 (9-anthryl) ethanol to NMR samples of the homochiral compounds.
The starting compounds of formula (II) and their esters (the compounds of
formula
(IV)) are novel and thus constitute a further aspect of the present invention.
In step (a) the carboxylic acid grouping in formula (II) may be activated as
the
corresponding acid chloride which may be obtained by treating the free
carboxylic acid of
formula (II) with thionyl chloride. Alternatively the carboxylic acid grouping
can be
activated by treatment with an appropriate amide-coupling reagent such as 1,1'-
carbonyldiimidazole.
The reaction between the activated derivative of the compound of formula ~(II)
and the
amine of formula (III) is typically conducted in an organic solvent. Suitable
solvents include
dimethylformamide and dichloromethane. The steps of activating the compound of
formula
(II) and treating the resulting activated derivative with the amine of formula
(III) may take
place without intermediate isolation of the activated derivative. In that case
the process
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
11
typically comprises combining the activating agent or coupling agent with the
compound of
formula (II) in an organic solvent and adding to the resulting reaction
mixture the amine of
formula (III).
A compound of formula (II) may be prepared by a process which comprises:
(a) treating a 1,2-naphthoquinone of formula (V):
R
R
wherein Rl to R4 are as defined above for formula (I), with a benzoic acid of
formula (VI):
s
H2N R6 (~
T ,,T ~ R7
C02H
or an ester or salt thereof, wherein R5, R6 and R' are as defined above for
formula (I), in an
organic solvent, optionally in the presence of an acid. The solvent may be,
for example,
ethanol or acetic acid. By using 1 to 5 equivalents of mineral acid in the
reaction mixture the
regioselectivity of the reaction may be controlled. The use of about 2
equivalents or more of
the acid, for instance from 1.5 to 5 equivalents, yields exclusively the
desired regioisomer
namely a benzo[a]phenazine-11-carboxylic acid of formula (II). The mineral
acid is
preferably hydrochloric acid, more preferably concentrated hydrochloric acid.
The salt of the
benzoic acid of formula (VI) is typically the acetate salt.
~ The 1,2-naphthoquinone of formula (V) may be prepared by treating the
corresponding
1-tetralone of formula (VII):
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
12
R
O
R
wherein Ri to R4 are as defined above for formula (I), with selenium dioxide
in accordance
with the procedure described in Tetrahedron Letters 1997, 4219-4220. The 1-
tetralones of
formula (VII) are known compounds or may be prepared from known compounds by
published methods, for instance as described in the reference examples which
follow, adapted
where necessary using conventional laboratory techniques to achieve the
desired definitions
of Rl to R~. Published methods include those described in J. Med. Chem 1997,
40, 3014-
3024; J. Org. Chem. 1984 , 4226; JACS. 1994, 116 pp. 4852-4857 and J. Med.
Chem. 1997
p.1049.
The benzoic acids of formula (VI) are known compounds or may be prepared from
known compounds using published methods, adapted where necessary using
conventional
laboratory techniques to achieve the desired definitions of RS to R'.
Published methods
include those described in J. Chem. Soc. Perkin Trans. I, 1984, p2019 and J.
Med. Chem
1987, p.843.
A compound of formula (II) may also be prepared by a process which comprises:
(a) treating a 2-halo-3-nitrobenzoic acid of formula (VIII):
02N
(VIII)
Hal
C02H
wherein Hal is Cl, Br, I or F, with a naphthylamine of formula (IX):
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
13
R~
\
i ~2
R3o~~ Ri
R2
wherein R1 to R4 are as defined above for formula (I); and
(b) submitting the resulting compound of formula (~):
~2N \
R4 \ I / (~
N
H
R3 / Ri C02H
R2
wherein R1 to R4 are as defined above, to reductive cyclisation.
Step (a) is typically conducted in an organic solvent. Suitable examples
include
butane-2,3-diol and ethylene glycol. Step (b) is generally carried out by
treatment of the
compound of formula (X) with NaBH4 in sodium methoxide, sodium ethoxide or
aqueous
NaOH. The process is described in J. Med. Chem. 1987, 30, 843-851.
A compound of formula (IV) may be prepared by esterification of a
corresponding
compound of formula (II) under standard reaction conditions, for instance by
treatment of the
free carboxylic acid compound of formula (II) with an alcohol of formula Rl1-
OH wherein
RI1 is as defined above.
Amines of formula (III) are known and commercially available compounds or may
be
produced from commercially available starting materials using conventional
techniques, for
instance as described in reference example 2 which follows.
A compound of formula (I) may be converted into another compound of formula
(I) by
conventional methods. For instance, a compound of formula (I) containing an
esterified
hydroxy group such as -OCOMe may be converted into a compound of formula (I)
containing a free hydroxy group by hydrolysis, for instance alkaline
hydrolysis. A compound
of formula (I) containing a free hydroxy group may be converted into a
compound of formula
(I) containing an esterified hydroxy group by esterification, for instance by
reaction with a
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
14
suitable carboxylic acid, acid halide or acid anhydride. A compound containing
a free
hydroxy group may also be converted to a compound containing a carbamic acid
ester
grouping, for instance by treatment with triethylamine and ethyl isocyanate in
an aprotic polar
solvent, for instance dimethylformamide.
A compound of formula (I) containing a vitro group may be converted into a
compound of formula (I) containing an amino group by reduction, for instance
by treatment
with indium and a saturated NHøCl solution in an organic solvent.
A compound containing a C1-C6 alkoxy group may be converted into a compound
containing a hydroxy group, for instance by treatment with boron tribromide in
a halogenated
hydrocarbon solvent, for instance dichloromethane, or with sodium thioethoxide
in dimethyl
formamide. A compound containing a hydroxy group may be converted into a
compound
containing an optionally substituted C1-C6 alkoxy group, for instance by
treatment with an
appropriate alkylating agent in the presence of a base. A compound containing
a carboxy
group may be converted to a compound containing a hydroxymethyl group by
reduction, for
instance by treatment with LiAlH4 in tetrahydrofuran.
A compound containing a halogen may be converted into a compound containing an
alkylsulfanyl or alkoxy group, for instance by treatment with a thioalkoxide
or alkoxide salt,
respectively, in an organic solvent. A compound containing a nitrite group may
be converted
into a compound containing an N-hydroxycarbamirnidoyl group, for instance by
treatment
with hydroxylamine (optionally in the form of a salt) in the presence of a
base such as
potassium carbonate.
A compound substituted by alkylaminomethyl at a benzene ring position may be
prepared under Marinich reaction conditions by treating a compound that is
substituted by
hydroxy ortho to the (unsubstituted) ring position in question with acetic
acid followed by
treatment with an alkylamine and a solution of formaldehyde in water. A
compound of
formula (I) may be acetylated, for instance on an amine group to form an
acetylamino
substituent, by treatment with acetyl chloride under suitable conditions.
Benzo[a]phenazine-11-carboxamide derivatives may be converted into
pharmaceutically acceptable salts, and salts may be converted into the free
compound, by
conventional methods. Pharmaceutically acceptable salts of the
benzo[a]phenzine-11-
carboxamide derivatives of formula (I) include salts of inorganic acids such
as hydrochloric
acid, hydrobromic acid and sulfuric acid, and salts of organic acids such as
acetic acid, oxalic
acid, malic acid, methanesulfonic acid, trifluoroacetic acid, benzoic acid,
citric acid and
tartaric acid. In the case of compounds of formula 1 wherein any one of RI-R4
and Rl° is
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
COON, the salts include both the above-mentioned salts and the salts of
sodium, potassium,
calcium and ammonium, which are prepared by treating the compound of formula 1
with or
the acid salts with the corresponding metal base or ammonia.
Mufti-drug resistance (MDR) is a phenomenon whereby cells which are typically
5 sensitive to chemotherapeutic agents develop resistance to those agents and
to a wide range of
unrelated drugs. MDR represents a major obstacle in the successful clinical
therapy of
cancer. Cancer cells which exhibit MDR can display a number of diverse
cellular alterations
including overexpression of P-glycoprotein (P-gp), overexpression of multidrug
resistance
associated protein (MRP), reduction in levels of topoisomerase II (termed
atypical drug
10 resistance) and qualitative changes in expression of topoisomerase I. MDR
is a very important
clinical problem with many tumors developing resistance to many
chemotherapeutic agents
including those that specifically target topoisomerase I and/or topoisomerase
II.
By simultaneously inhibiting topoisomerase I and II, compounds such as DACA
(Finlay ~t al, Eur. J. Cancer 32A, 708-714, 1996) have shown no loss of
activity when
15 resistance develops to camptothecin or amsacrine due to alteration of
either topoisomerase I
or II respectively. Qualitatively different cell cycle events have been
obtained with inhibitors
of topoisomerase I or II. (Kaufman, Biochim. Biophys. Acta 1400, 195-212,
1998). Joint
inhibitors of topoisomerases I and II appear to combine the properties of the
individual
specific inhibitors and act across the cell cycle (Iialdane et ah Cancer
Chemother. Pharmacol.
32: 463-470, 1993), resulting in a greater antitumour activity (Riou et ah
Cancer Res. 53,
5987-5993, 1993).
MDR due to the overexpression of membrane transporters such as P-glycoprotein
(Gottesman et ah ~u~ Rev. Biochem. 62, 385-427, 1993) and MRP (Loe et ah Eur.
J.
Cancer 32A, 945-957, 1996) is known to reduce the clinical efficacy of
chemotherapeutic
agents such as paclitaxel, etoposide and doxorubicin. Agents that avoid such
MDR .
mechanisms are predicted'to show therapeutic benefit in the treatment of
cancer.
Benzo[a]phenazine-11-carboxamide derivatives of formula I, their
pharmaceutically
acceptable salts and hydrates and solvates thereof (hereinafter referred to as
"the present
compounds") have been found in biological tests to have activity as inhibitors
of
topoisomerase I and II. In one aspect of the invention the present compounds
are joint
inhibitors of topoisomerase I and topoisomerase II.
The present compounds may therefore be used as inhibitors of topoisomerase I.
Alternatively the present compounds may be used as inhibitors of topoisomerase
II. In a
further embodiment they may be used as joint inhibitors of topoisomerase I and
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
16
topoisomerase II. They have been shown to kill human tumour cells and avoid
MDR
mechanisms. They therefore have potential in the treatment of cancer. Examples
of types of
cancer that the present compounds can be used to treat include leukaemias,
lymphomas,
sarcomas, carcinomas and adenocarcinomas. Specific examples include breast,
colon, brain,
lung, ovary, pancreatic, stomach and skin cancer.
A human or animal patient harbouring a tumour may be treated by a method
comprising the administration thereto of one of the present compounds. In
particular, a
method of treating human tumours, including those which express MDR, for
instance the
types of MDR referred to above, comprises administering a therapeutically
effective amount
of one of the present compounds to a patient harbouring a tumour. All types of
tumour may
thus be treated, both those which express MDR and those which do not. The
present
compound is administered in an amount effective to reduce or eliminate the
tumour. In one
aspect of the invention the present compound is administered orally. In
another aspect the
present compound is administered by a parenteral route, for instance
intravenously.
Owing to their activity as inhibitors of topoisomerase I and topoisomerase II
the
present compounds may also be used as antiviral, antibacterial or antifungal
agents.
The present compounds can be administered in a variety of dosage forms, for
example
orally such as in the form of tablets, capsules, sugar- or film-coated
tablets, liquid
solutions or suspensions or parenterally, for example intramuscularly,
intravenously or
subcutaneously. The present compounds may therefore be given by injection or
infusion.
The dosage depends on a variety of factors including
the age, weight and condition of the patient and the route of administration.
Typically,
however, the dosage adopted for each route of administration when a compound
of the
invention is administered alone to adult humans is 0.001 to 500 mg/kg, most
commonly in the
range of 0.01 to 100 mg/kg body weight. Such a dosage may be given, for
example, from 1 to
5 times daily by bolus infusion, infusion. over several hours and/or repeated
administration.
A benzo[a]phenazine-11-carboxamide derivative of formula (I) or a
pharmaceutically
acceptable salt thereof is formulated for use as a pharmaceutical or
veterinary composition
also comprising a pharmaceutically or veterirlarily acceptable carrier or
diluent. The
compositions are typically prepared following conventional methods and are
administered in a
pharmaceutically or veterinarily suitable form. An agent for use in the
treatment of tumours,
including those which express MDR, comprising one of the present compounds is
therefore
provided.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
17
The present compounds may be administered in any conventional form, for
instance as
follows:
A) Orally, for example, as tablets, coated tablets, dragees, troches,
lozenges, aqueous
or oily suspensions, liquid solutions, dispersible powders or granules,
emulsions, hard or soft
capsules, or syrups or elixirs. Compositions intended for oral use may be
prepared according
to any method known in the art for the manufacture of pharmaceutical
compositions and such
compositions may contain one or more agents selected from the group consisting
of
sweetening agents, flavouring agents, colouring agents and preserving agents
in order to
provide pharmaceutically elegant and palatable preparations.
Tablets contain the active ingredient in admixture with non-toxic
pharmaceutically
acceptable excipients which are suitable for the manufacture of tablets. These
eXCipients may
be for example, inert diluents, such as calcium carbonate, sodium carbonate,
lactose, dextrose,
saccharose, cellulose, corn starch, potato starch, calcium phosphate or sodium
phosphate;
granulating and disintegrating agents, for example, maize starch, alginic
acid, alginates or
sodium starch glycolate; binding agents, fox example starch, gelatin or
acacia; lubricating
agents, for example silica, magnesium or calcium stearate, stearic acid or
talc; effervescing
mixtures; dyestuffs, sweeteners, wetting agents such as lecithin, polysorbates
or lauryl
sulphate. The tablets may be uncoated or they may be coated by known
techniques to delay
disintegration and adsorption in the gastrointestinal tract and thereby
provide a sustained
action over a longer period. For example, a time delay material such as
glyceryl monostearate
or glyceryl distearate may be employed. Such preparations may be manufactured
in a known
manner, for example by means of mixing, 'granulating, tableting, sugar coating
or film coating
processes.
Formulations for oral use may also be presented as hard gelatin capsules
wherein the
active ingredient is mixed with an inert solid diluent, for example, calcium
carbonate, calcium
phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient
is present as
such, or mixed with water or an oil medium, for example, peanut oil, liquid
paraffin, or olive
oil.
Aqueous suspensions contain the active materials in admixture with excipients
suitable
for the manufacture of aqueous suspensions. Such excipients are suspending
agents, for
example, sodium carboxymethylcellulose, methylcellulose,
hydroxypropylmethylcellulose,
sodiiun alginate, polyvinylpyrrolidone gum tragacanth and gum acacia;
dispersing or wetting
agents may be naturally-occurring phosphatides, for example lecithin, or
condensation
products of an alkylene oxide with fatty acids, for example polyoxyethylene
stearate, or
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
18
condensation products of ethylene oxide with long chain aliphatic alcohols,
for example
heptadecaethyleneoxycetanol, or condensation products of ethylene oxide with
partial esters
derived from fatty acids and a hexitol such as polyoxyethylene sorbitol
monooleate, or
condensation products of ethylene oxide with partial esters derived from fatty
acids and
hexitol anhydrides for example polyoxyethylene sorbitan monooleate.
The said aqueous suspensions may also contain one or more preservatives, for
example, ethyl or n-propyl p-hydroxybenzoate, one or more colouring agents,
such as sucrose
or saccharin.
Oily suspension may be formulated by suspending the active ingredient in a
vegetable
oil, for example arachis oil, olive oil, sesame oil or coconut oil, or in a
mineral oil such as
liquid paraffin. The oily suspensions may contain a thickening agent, for
example beeswax,
hard paraffin or cetyl alcohol.
Sweetening agents, such as those set forth above, and flavouring agents may be
added
to provide a palatable oral preparation. These compositions may be preserved
by this addition
of an antioxidant such as ascorbic acid. Dispersible powders and granules
suitable for
preparation of an aqueous suspension by the addition of water provide the
active ingredient in
admixture with a dispersing or wetting agent, a suspending agent and one or
more
preservatives. Suitable dispersing or wetting agents and suspending agents are
exemplified by
those already mentioned above. Additional excipients, for example sweetening,
flavouring
and colouring agents, may also be present.
The pharmaceutical compositions of the invention may also be in the form of
oil-in-water emulsions. The oily phase may be a vegetable oil, for example
olive oil or
arachis oils, or a mineral oil, for example liquid paraffin or mixtures of
these. Suitable
emulsifying agents may be naturally-occurring gums, for example gum acacia or
gum
tragacanth, naturally occuring phosphatides, for example soy bean lecithin,
and esters or
partial esters derived from fatty acids and hexitol anhydrides, for example
sorbitan mono-
oleate, and condensation products of the said partial esters with ethylene
oxide, for example
polyoxyethylene sorbitan monooleate. The emulsion may also contain sweetening
and
flavouring agents. Syrups and elixirs may be formulated with sweetening
agents, for example
glycerol, sorbitol or sucrose. In particular a syrup for diabetic patients can
contain as carriers
only products, for example sorbitol, which do not metabolise to glucose or
which only
metabolise a very small amount to glucose.
Such formulations may also contain a demulcent, a preservative and flavouring
and
coloring agents;
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
19
B) Parenterally, either subcutaneously; or intravenously, or intramuscularly,
or
intrasternally, or by infusion techniques, in the form of sterile injectable
aqueous or
oleaginous suspensions. This suspension may be formulated according to the
known art using
those suitable dispersing of wetting agents and suspending agents which have
been mentioned
above. The sterile injectable preparation may also be a sterile injectable
solution or
suspension in a non-toxic paternally-acceptable diluent or solvent, for
example as a solution
in 1,3-butane diol. .
Among the acceptable vehicles and solvents that may be employed are water,
Ringer's
solution and isotonic sodium chloride solution. In addition, sterile, fixed
oils are
conventionally employed as a solvent or suspending medium. For this purpose
any bland
fixed oil may be employed including synthetic mono- or diglycerides. In
addition fatty acids
such as oleic acid find use in the preparation of injectables;
C) By inhalation, in the form of aerosols or solutions for nebulizers;
D) Rectally, in the form of suppositories prepared by mixing the drug with a
suitable
non-irritating excipient which is solid at ordinary temperature but liquid at
the rectal
temperature and will therefore melt in the rectum to release the drug. Such
materials are
cocoa butter and polyethylene glycols;
E) Topically, in the form of creams, ointments, j ellies, collyriums,
solutions or
suspensions.
Daily dosages can vary within wide limits and will be adjusted to the
individual
requirements in each particular case. In general, for administration to
adults, an appropriate
daily dosage is in the range of about 5 mg to about 500 mg, although the upper
limit may be
exceeded if expedient. The daily dosage 'can be administered as a single
dosage or in divided
dosages.
The invention will be further illustrated in the Examples which follow.
Reference Example 1: Preparation of Compounds of General Formula (II)
Reference Example 1A. 4-Methoxy-benzo[a]phenazine-11-carboxylic acid (IL1)
A mixture of 5-methoxy-[1,2]naphthoquinone (prepared by treatment of 5-
methoxytetralone with selenium dioxide, A. Bekaert et al Tetrahedron Letters
38,24, 4219-
4220, 1997)(1.98g) , 2,3-diamino-benzoic acid, diacetate salt ,
(J.Chem.Soc.Perkin.Trans I,
1984,p2019) (4.03g) and conc. hydrochloric acid(2.2mL) was heated to reflux in
ethanol(20mL) for 4 hours. The reaction mixture was cooled and the precipitate
collected by
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
filtration, and washed with ethanol and ether to yield the title compound as a
beige solid
(2.74g).
NMR: (DMSO) 4.05 (s,3H), 7.50(lH,d), 7.84-7.87(lH,m), 7.99(lH,d), 8.08-
8.10(lH,m),
8.41-8.49(3H,m), 8.63(lH,d).
5
Reference Example 1B. 4-Methyl-benzo[a]phenazine-11-carboxylic acid (IL2)
5-Methyl-1-tetralone was prepared from o-tolualdehyde according to the
literature
(J.Med Chem 1997,40,3014-3024). Treatment of 5-methyl-1-tetralone with
selenium dioxide
as described in Reference Example 1A yielded 5-methyl-[1,2]naphthoquinone.
This was
10 reacted with 2,3-diamino-benzoic acid, diacetate salt, as described in
Reference Example 1A
to yield the title compound.
NMR. d6-DMSO 9.02(lH,d), 8.51(lH,dd), 8.47-8.44(2H,m), 8.11-8.05(2H,m), 7.85-
7.78(2H,m), 2.79(3H,s).
Commencing with the appropriately substituted aldehydes, the following
compounds
15 of Formula (II) were prepared in an analogous manner:
4-Fluoro-benzo[a]phenazine-11-carboxylic acid (IL3) was prepared from 2-
fluorobenzaldehyde NMR, d6-DMSO, 8.97(lH,d), 8.51(lH,dd), 8.43(lH,dd),
8.38(lH,d),
8.11-8.06(2H,m), 7.95(lH,m), 7.80(lH,m);
3,4-Dimethoxy-benzo[a]phenazine-11-carboxylic acid (IL4) was prepared from 2,3-
20 dimethoxybenzaldehyde;
NMR, CDC13, 8.79(lH,d), 8.48(2H,d),8.37(lH,d), 8.05(lH,t),
7.95(lH,d),7.76(lH,d),
4.04(3H,s), 3.98(3H,s).
Reference Example 1C. 4-Bromo-benzo[a]phenazine-11-carboxylic acid (IL5)
2-Bromobenzyl bromide was converted into 5-bromotetralone according to the
literature (J. Org. Chem. 1984, p4226). Treatment of 5-bromotetralone with
selenium dioxide
as described in Referene Example 1A yielded 5-bromo-[1,2]naphthoquinone, which
was
coupled with 2,3-diamino-benzoic acid, diacetate salt , as described in
Reference Example 1A
to yield the title compound.
NMR d6-DMSO, 9.24(lH,d), 8.48(2H,m), 8.33(lH,m), 8.26(lH,m), 8.20(lH,d),
8.08(lH,t),
7.87(lH,t)
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
21
Reference Example 1D. 4-Cyano-benzo[a]phenazine-11-carboxylic acid (IL6)
To a solution of 5-bromotetralone (see Reference Example 1C)(lS.Og) in N,N-
dimethylformamide (20mL) was added copper (I) cyanide(6.39g) and the reaction
mixture
heated to reflux for 20 hours. The reaction mixture was then cooled to
80°C ~d a solution of
FeC13.6H20 (24g) in water (38mL) was added. After stirring for a further 45
minutes the
reaction mixture was cooled, diluted.with water, extracted into toluene,
washed with water,
dried (MgSOø) and the solvent removed iy~ vacuo to yield 5-cyano-1-tetralone
as a yellow
solid.
Treatment of 5-cyano-1-tetralone with selenium dioxide as described in
Reference
Example 1A yielded the corresponding 5-cyano-[1,2]naphthoquinone, which was
coupled
with 2,3-diamino-benzoic acid, diacetate salt , as described to yield the
title compound.
NMR (d6-DMSO), 8.10-8.18(2H,m), 8.33(lH,d), 8.43(2H,m), 8.48-8.54(2H,m),
9.48(lH,d).
MS DCI/NH3 m/z 300 (MH+)
Reference Example 1E. 4-Chloro-benzo[a]phenazine-11-carboxylic acid (IL7)
S-Amino-1-tetralone was prepared from a-tetralone according to the literature
(J. Am.
Chem. Soc. 1994, p4852). A mixture of 5-amino-1-tetralone (80mg) and
concentrated
hydrochloric acid (1mL) was cooled to 0°C, A solution of sodium nitrite
(35mg) in water
(O.SmL) was added dropwise to the stirring solution. The cold diazonium
solution was then
poured rapidly onto a stirring solution of copper (I) chloride (62mg) in
concentrated
hydrochloric acid (1mL). The reaction mixture was allowed to warm to ambient
temperature
and then stirred for 1.5 hours. The mixture was then extracted with ethyl
acetate, washed with
water, dried (MgS04) and the solvent removed i~ vacuo to yield 5-chloro-1-
tetralone as a
brown solid (87mg).
Treatment of 5-chloro-tetralone with selenium dioxide as described in
Reference
Example 1A yielded 5-chloro-[1,2]naphthoquinone which was coupled with 2,3-
diamino-
benzoic acid, diacetate salt, as described in Reference Example 1A to yield
the title
compound.
Reference Example 1F. 4-Methanesulphonyl-benzo[a]phenazine-11-carboxylic acid
(IL8)
5-Methylsulphanyl-1-tetralone was prepared from 5-hydroxy-1-tetralone
according to
the literature (J. Med. Chem. 1997, p1049). A mixture of S-methylsulphanyl-1-
tetralone
(221mg) and 3-chloroperbenzoic acid (595mg) was stirred at room temperature
for 2 hours.
The reaction mixture was then extracted with dichloromethane, washed with
water, dried
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
22
(Na2S04) and the solvent removed iy~ vacuo to yield 5-methanesulphonyl-1-
tetralone as an
off white solid (210mg)
Treatment of 5- methanesulphonyl-1-tetralone with selenium dioxide as
described in
Reference Example 1A yielded 5-methanesulfonyl-[1,2]naphthoquinone which was
coupled
with 2,3-diamino-benzoic acid, diacetate salt, as described in Reference
Example 1A to yield
the title compound.
NMR, d6-DMSO, 9.61(lH,d), 9.03(lH,d), 8.56(2H,m), 8.43(lH,d), 8.32(lH,m),
8.16(2H,m),
3.51(3H,s). MS, DCI/NH3 m/z 353 (MH+),
Reference Example 1G. 4-Azido-benzo[a]phenazine-11-carboxylic acid (IL9)
5-Amino-1-tetralone was prepared from a-tetralone according to the literature
(J. Am.
Chem. Soc. 1994, p4852). To a cold solution of 5-amino-1-tetralone (415mg) in
water (2mL)
and concentrated hydrochloric acid (SmL) was added a solution of sodium
ilitrite (186mg) in.
water (l.2mL), maintaining low temperature. After 40 minutes a solution of
sodium azide
(184mg) in water (l.2mL) was added dropwise. The reaction mixture was allowed
to warm to
room temperature. After one further hour the reaction was quenched with water,
extracted into
ether, washed with sodium bicarbonate solution, dried (MgS04) and the solvent
removed iu
vacuo to yield crude material which was purified using flash chromatography to
yield 5-
azido-1-tetralone (77mg).
Treatment of 5-azido-1-tetralone with selenium dioxide as described in
Reference Example
1A yielded 5-azido-[1,2]naphthoquinone which was coupled with 2,3-diamino-
benzoic acid,
diacetate salt , as described in Reference Example 1A to yield the title
compound.
Reference Example 1H. Benzo[a]phenazine-4,11-dicarboxylic acid 4-methyl ester
(II.10)
Methyl 5-oxo-5,6,7,8-tetrahydro-1-naphthoate was prepared according to the
literature
(J. Org. Chem. 1976, p2918) from 2-methyl-2,6,7,8-tetrahydro-chromen-5-one
(Tetrahedron
Letters, 1975, p3407). Treatment ofmethyl S-oxo-5,6,7,8-tetrahydro-1-
naphthoate with
selenium dioxide as described in Reference Example 1A yielded the
corresponding 1,2-
naphthoquinone, 5,6-dioxo-5,6-dihydro-naphthalene-1-carboxylic acid methyl
ester, which
was coupled with 2,3-diamino-benzoic acid, diacetate salt , as described in
Reference
Example 1A to yield the title compound.
NMR, d6-DMSO, 9.47(lH,d), 9.02(lH,d), 8.53(lH,d), 8.44(2H,rn), 8.20(lH,d),
8.10(2H,m),
4.03(3H,s).
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
23
Reference Example 1I. 4-Ethoxy-benzo[a]phenazine-11-carboxylic acid (IL11)
A mixture of 5-hydroxy-1-tetralone (2.00g) and sodium hydroxide (493mg) was
warmed in ethanol (40mL) to 50°C. Ethyl iodide (3.94mL) was then added
and the reaction
mixture was heated at reflux for 16 hours. The reaction mixture was diluted
with 2N
hydrochloric acid, extracted into ethyl acetate, dried and the solvent removed
i~ ~acuo to
yield 5-ethoxy-1-tetralone as a white solid (2.06g)
Treatment of 5-ethoxy-1-tetralone with selenium dioxide as described in
Reference
Example 1A yielded 5-ethoxy-[1,2]naphthoquinone which was coupled with 2,3-
diamino-
benzoic acid, diacetate salt , as described to yield the title compound.
NMR: d6-DMSO. 1.52(3H,q), 4.33(2H,t), 7.50(lH,d), 7.85-7.90(lH,m), 8.00(lH,d),
8.08-
8.11(lH,m), 8.45-8.55(3H,m), 8.68(lH,d).
Reference Example 1J. 4-Methylsulfanyl-benzo[a]phenazine-11-carboxylic acid
(IL 12)
To a stirred solution of 4-fluoro-benzo[a]phenazine-11-carboxylic acid
(IL3,see
Reference Example 1B)( 58mg) in dry DMSO(3mL) was added sodium
thiomethoxide(SSmg)
and the reaction mixture heated at 100°C for 1.5 hours and 130°C
for 1 hour.The reaction
mixture was then cooled to room temperature, quenched with acetic acid,
extracted with ethyl
acetate, washed with water, dried and the solvent removed iy~ ~,acuo to Yield
the title
compound as a red solid(37mg).
NMR, d6-DMSO, 9.00(lH,d), 8.52(2H,m), 8.45(lH,d), 8.11(2H,m), 7.91(2H,m),
2.70(3H,s).
MS, DCI/NH3, m/z=321(MH+, 100%)
Reference Example 1K. 4-Benzyloxy-benzo[a]phenazine-11-carboxylic acid (IL13)
To a solution of 4-methoxy-benzo[a]phenazine-11-carboxylic acid (IL1, see
Reference
Example lA)(441mg) in dichloromethane(30mL) cooled to 0°C was added a
1.0M solution of
boron tribromide in dichloromethane(7.25mL). The reaction mixture was allowed
to warm to
room temperature arid then stirred for 16 hours. The mixture was then poured
onto ice/water
yielding 4-hydroxy-benzo[a]phenazine-11-carboxylic acid as a red/brown solid
which was
collected by filtration and air dried (230mg).
NMR: d6-DMSO. 7.35(lH,d), 7.75(lH,m), 7.92(lH,d), 8.08(lH,m), 8.47-8.55(4H,m),
10.72(lH,broad).
A mixture of 4-hydroxy-benzo[a]phenazine-11-carboxylic acid (80mg), sodium
hydroxide(34mg)
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
24
and benzyl bromide(100~,L) in ethanol(2mL) was heated to reflux for 4 hours.
The reaction
mixture was then cooled, diluted with ethyl acetate, washed with dilute acid,
dried(MgS04)
and the solvent removed i~ vacuo to yield the crude title compound as a brown
solid(SOmg).
NMR: d6-DMSO. Includes 5.35(2H,s)
The following compounds were prepared in an analogous manner from 4-hydroxy-
ben~zo[a]phenazine-11-carboxylic acid using the appropriate alkylating
reagent;
4-Prop-2-ynyloxy-benzo[a]phenazine-11-carboxylic acid (IL14) was prepared
using propargyl
bromide;
4-Isobutoxy-benzo[a]phenazine-11-carboxylic acid (IL15) was prepared using
isobutyl
bromide;
4-(4-Chloro-benzyloxy)-benzo[a]phenazine-11-carboxylic acid (IL16) was
prepared using p-
chlorobenzyl bromide;
4-(2-Methoxy-ethoxy)-benzo[a]phenazine-11-carboxylic acid (IL17) was prepared
using 2-
bromoethyl methyl ether;
4-Ethoxycarbonylmethoxy-benzo[a]phenazine-11-carboxylic acid (IL18) was
prepared using
ethyl bromoacetate. Sodium ethoxide in dry ethanol was used for this reaction;
4-[2-(tc~~-Butyl-dimethyl-silanyloxy)-ethoxy]-benzo[a]phenazine-11-carboxylic
acid (IL19)
was prepared using tey.~-butyl-(2-iodo-ethoxy)-dimethyl-silane. The te~.~-
Butyl-(2-iodo-.
ethoxy)-dimethyl-silane was prepared using standard procedures from 2-iodo-
ethanol.
Reference Example 1L. Benzo[a]phenazine-11-carboxylic acid (IL20)
A mixture of 1,2-naphthoquinone(commercially available, 2.0g) and 2,3-diamino-
benzoic acid, diacetate salt , (3.79g) was heated to reflux in acetic
acid(30mL) for 2 hours.
The reaction mixture was cooled and the solvent removed i~ vacuo to yield a
gum. This was
purified using flash chromatography to yield a 2:1 mixture of the desired
title compound to
the undesired benzo[a]phenazine-8-carboxylic acid (840mg). The two isomers
were separated
after further modification (see Reference Example 3B below)
NMR d6-DMSO,includes 9.07-9.09(lH,m), 9.21-9.22(lH,m). 2:1 ratio
Reference Example 1M. 4-Methylsulfamoyl-benzo[a]phenazine-11-carboxylic acid
(IL21)and 3-methylsulfamoyl-benzo[a]phenazine-11-carboxylic acid (IL22)
Benzo[a]phenazine-11-carboxylic acid methyl ester (IV.20, see Reference
Example
3B, 220mg) was heated under nitrogen to 180°C in chlorosulphonic
acid(2mL) for 6 hours.
The reaction was then cooled, poured onto ice/water and the pale yellow solid
collected by
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
filtration to give approximately a 1:1 mixture of 4-chlorosulfonyl-
benzo[a]phenazine-11-
carboxylic acid and 3-chlorosulfonyl-benzo[a]phenazine-11-carboxylic acid
(182mg). NMR
d6-DMSO, includes 9.41(lH,s), 9.30(lH,d).
The mixture of 4-chlorosulfonyl-benzo[a]phenazine-11-carboxylic acid and 3-
5 chlorosulfonyl-benzo[a]phenazine-11-carboxylic acid (182mg) was dissolved in
dichloromethane(SrriL). To this was added a 40% solution of methylamine in
water (SmL)
and the reaction mixture was stirred vigorously for 4 hours. The reaction
mixture was then
poured onto dichloromethane, acidified (2N HCl), extracted into
dichloromethane, dried
(MgS04) and the solvent removed ih vacuo to give a ~l:l mixture of the title
compounds
10 (160mg). The two isomers were separated after further chemical
modification.
NMR (CDCl3 +d4MeOH) includes 9.38(lH,s), 9.22(lH,d), 2.61(3H,s), 2.55(3H,s).
4-Dimethylsulfamoyl-benzo[a]phenazine-11-carboxylic acid (IL23)and 3-
dimethylsulfamoyl-benzo[a]phenazine-11-carboxylic acid (IL24) were prepared in
an
analogous manner by reaction of dimethylamine with the mixture of 4-
chlorosulfonyl-
15 benzo[a]phenazine-11-carboxylic acid and 3-chlorosulfonyl-benzo[a]phenazine-
11-
carboxylic acid. The two isomers were separated after further chemical
modification. NMR
(d6-DMSO) of mixture includes 9.06(lH,d), 9.42(lH,s), 2.93(6H,s)
4-Sulfamoyl-benzo[a]phenazine-11-carboxylic acid (IL25) and 3-sulfamoyl-
benzo[a]phenazine-11-carboxylic acid (IL26) were prepared in an analogous
manner by
20 reaction of ammonium hydroxide with the mixture of 4-chlorosulfonyl-
benzo[a]phenazine-
11-carboxylic acid and 3-chlorosulfonyl-benzo[a]phenazine-11-carboxylic acid.
The two
isomers were separated after further chemical modification. NMR (d6-DMSO) of
mixture
includes, 9.39(lH,d), and 9.60(lH,s)
25 Reference Example 1N. 4-Nitro-benzo[a]phenazine-11-carboxylic acid (IL27)
and 3-nitro-
benzo[a]phenazine-11-carboxylic acid (IL28)
Concentrated sulphuric acid (SmL) and concentrated nitric acid (SmL) were
mixed
together at 0°C. To this mixture was added benzo[a]phenazine-11-
carboxylic acid (IL20)
(100mg) and the reaction mixture allowed to warm slowly to room temperature.
After 24
hours the reaction mixture was poured onto water yielding a yellow
precipitate. This was
collected by filtration to yield a 4:1 mixture of 4-vitro-benzo[a]phenazine-11-
carboxylic acid
(IL27) and 3-vitro-benzo[a]phenazine-11-carboxylic acid (IL28). The two
isomers were
separated after further chemical modification.
NMR, d6-DMSO, includes 9.82(lH,d), 9.46(lH,d), 1:4 ratio
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
26
MS MH@320
Reference Example 10. 4-Amino-benzo[a]phenazine-11-carboxylic acid (IL29) and
3-
amino-benzo[a]phenazine-11-carboxylic acid (IL30)
To the 4:1 mixture of 4-nitro-benzo[a]phenazine-11-carboxylic acid (IL27) and
3-
nitro-benzo[a]phenazine-11-carboxylic acid (IL28)(52mg) in ethanol (SmL) from
Reference
Example 1N was added ammonium chloride solution (3mL) and indium (cat.) The
reaction
mixture was heated to reflux, then cooled and filtered through a bed of
celite. The filtrate was
diluted with water, extracted into dichloromethane, dried (MgS04) and the
solvent removed ih
vacuo to yield the title compounds as a mixture (48mg). Separated after
further modification.
NMR, d6-DMSO, 6.95(lH,d), 7.55(lH,t), 7.75(2H,m), 8.05(2H,m), 8.20(lH,d),
8.65(lH,d)
Reference Example 1P. 3-Bromo-4-hydroxy-benzo[a]phenazine-11-carboxylic acid
(IL31)
A mixture of 4-hydroxy-benzo[a]phenazine-11-carboxylic acid (see Reference
Example 1K, 102mg) and bromine(0.04mL) was stirred in chloroform (3mL) at room
temperature for 20 hours. The solvent was removed i~ vacuo and the residue was
purified
using flash chromatography (10% methanol in dichloromethane) to yield the
title compound
(24mg) as a yellow-brown solid.
MS DCI/NH3 , MH+ 369/371 (1:1)
NMR (CDC13), 7.94(IH,d), 8.1-8.2(2H,m), 8.52-8.62(2H,m), 9.1(lH,d).
Reference Example 1Q. 2-Nitro-benzo[a]phenazine-11-carboxylic acid (IL32)
7-Nitro-1-tetralone was prepared according to the literature (J. Am. Chem.
Soc, 1994,
116, pp4852-4857). Treatment of 7-nitro-1-tetralone with selenium dioxide as
described in
Reference Example 1A yielded the corresponding 7-nitro-[1,2]naphthoquinone,
which was
coupled with 2,3-diamino-benzoic acid, diacetate salt , as described in
Reference Example 1A
to yield the title compound.
Reference Example 1R. 2-Methoxy-benzo[a]phenazine-11-carboxylic acid (IL33)
8-Amino-naphthalen-2-of was prepared from 8-amino-2-naphthalenesulphonic acid
(commercially available) according to the literature (J. Org. Chem. 1949,
p351). To a
solution of 8-amino-naphthalen-2-of (B.OOg) in dry N,N-dimethylformamide
(80mL) was
added sodium hydride (60% dispersion in mineral oil, 3.2g) carefully. After
stirring for 4
hours the reaction mixture was cooled in an ice bath and methyl iodide
(3.13mL) was added
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
27
dropwise. The reaction mixture was then stirred at room temperature for 3
days. Water
(lOmL) was then added and the volatiles were removed in vacuo~ The residue was
dissolved
in chloroform, washed with water, dried (MgS04) and the solvent removed in
vacuo to yield a
dark oil. This was purified using flash chromatography (chloroform) to yield 7-
methoxy-
naphthalen-1-ylamine as a dark brown liquid (2.92g).
7-Methoxy-naphthalen-1-ylamine was treated with 2-bromo-3-vitro-benzoic acid
according to the analogous procedure described by G.W.Rewcastle et al (J. Med.
Chem. 1987,
p843) to yield 2-(7-methoxy-naphthalen-1-ylamino)-3-vitro-benzoic acid.
Reductive
cyclisation using sodium borohydride (J. Med Chem. 1987, p843) yielded the
desired title
compound.
NMR, d6-DMSO, 14.44(lH,broad,s), 8.55(lH,d), 8.48(lH;dd), 8.42(lH,dd),
8.23(lH,d),
8.10-8.04(2H,m), 7.86(lH,d), 7.56(lH,dd), 4.02(3H,s).
MH+ @305
3-Methoxy-benzo[a]phenazine-11-carboxylic acid (IL34) was prepared in
analogous
manner starting with 5-amino-naphthalene-2-sulfonic acid (commercially
available).
NMR, d6-DMSO, 14.60(lH,broad,s), 8.94(lH,d), 8.51(2H,d), 8.23(lH,d), 8.10-
7.96(2H,m),
7.67(lH,d), 7.55(lH,dd), 3.99(3H,s).
MH+ ~a 305
Reference Example 1S. 9-Bromo-4-methoxy-benzo[a]phenazine-11-carboxylic acid
(IL35)
A mixture of 4-methoxy-benzo[a]phenazine-11-carboxylic acid (ILl)(100mg) and
bromine(5 drops) in chloroform(7mL) was stirred at room temperature for 3
days. The
reaction mixture was reduced in vacuo and the desired product was isolated
using flash
chromatography (25mg).
MS MH+ @383/385 (1:l)
NMR ,CDCl3, 14.47(lH,br,s), 8.86(lH,d), 8.33(lH,d), 7.95(lH,t), 7.78(lH,d),
6.97(lH,d),
6.12(lH,d), 5.75(lH,d), 3.94(3H,s)
Reference Example 1T. 4,10-Dimethoxy-benzo[a]phenazine-11-carboxylic acid
(IL36)
2-Amino-6-methoxy-3-vitro-benzoic acid methyl ester was prepared using a
procedure
analogous to that described in the literature (Kim et al, J.Med. Chem. 1993,
p2335).
Hydrolysis of the methyl ester was achieved using potassium hydroxide in
refluxing ethanol
for 2 hours to yield 2-amino-6-methoxy-3-vitro-benzoic acid. Hydrogenation of
the vitro
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
2~
group was performed in acetic acid/water over palladium on carbon catalyst on
the Parr
apparatus at SOpsi H2 to yield 2,3-diamino-6-methoxy-benzoic acid. This was
reacted with 5-
methoxy-[1,2]naphthoquinone as described in Reference Example 1A to yield the
desired title
compound.
NMR, d6-DMSO, 4.05(3H,s), 4.10(3H,s), 7.45(lH,d), 7.78-7.82(lH,m), 7.92(lH,d),
8.03(lH,d),8.36-8.40(2H,m), 8.78(lH,d)
Reference Example 1U. 4-Methoxy-8-methyl-benzo[a]phenazine-11-carboxylic acid
(IL37)
2,3-Diamino-4-methylbenzoic acid was prepared from 4-methyl anthranilic acid
according to the method described by Rewcastle et al (J.Med Chem. 1987, p843).
This was
reacted with 5-methoxy-[1,2]naphthoquinone as described in Reference Example
1A to yield
the desired title compound.
NMR, d6-DMSO, 2.97(3H,s), 4.06(3H,s), 7.50(lH,d), 7.85-7.90(lH,m),
7.97(lH,d),8.02(lH,d), 8.45-8.50(2H,m), 8.57(lH,d).
Reference Example 1V. 9-Chloro-4-methoxy-benzo[a]phenazine-11-carboxylic acid
(IL38)
5-Chloro-3-nitroanthranilic acid was prepared according to the procedure
described by
Flippin et al (Biorg. Med. Chem. Lefts 1996, p477). Hydrogenation of this
material in ethyl
acetate using palladium on carbon at SOpsi H2 for 2 hours yielded 2,3-diamino-
5-chloro-
benzoic acid. This was reacted with 5-methoxy-[1,2]naphthoquinone as described
in
Reference Example 1A to yield the desired title compound.
NMR, d6-DMSO, 4.05(3H,s), 7.48,(lH,d), 7.82-7.86(lH,m), 7.92(lH,d),
8.30(lH,d),
8.48(lH,d),.8.54(lH,d), 8.68(lH,d), 14.1(lH,broad).
Reference Example 2 : Preparation of Compounds of General Formula (III
Reference Example 2A. 4-Aza-DL-leucine methyl ester. hydrochloride (IIL1)
Methanol (150mL) was saturated with anhydrous hydrogen chloride gas. To this
was
added 4-aza-DL-leucine (4.86g)(commercially available) and the reaction
mixture was stirred
overnight at room temperature. The solvent was removed i~ yacuo to yield the
title compound
(quantitative yield)
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
29
Reference Example 2B. N1,N1-Dimethyl-butane-1,2-diaxnine (IIL2)
Methyl N-(tee.~-butoxycarbonyl)-2-aminobutyrate was prepared as described in
the
literature( J.Med. Chem. 1989,p1886). Treatment of this compound with
diisobutyl.
aluminium hydride in toluene at -78° C for 1.5 hours yielded the
corresponding aldehyde
(prep see H.W. Scheeren et al, J. Org. Chem. 1990, p3998).
A mixture of the aldehyde (1.85g), dimethylaxnine hydrochloride (1.61 g),
sodium
acetate (1.21 g) and sodium cyanoborohydride (0.83g) in methanol was stirred
at room
temperature for 24 hours. The pH was adjusted to 6-7 using acetic acid and
monitored during
the reaction. The reaction mixture was then concentrated i~ vacuo~ ~d ~e
residue dissolved
I O in ethyl acetate and washed with water. The organic layer was dried
(MgS04) and the solvent
removed ih vacuo to yield a colourless oil, which was purified using flash
chromatography to
yield the desired dimethylamine derivative as a pale oil (0.56g). To this
compound (260mg)
was added a 4.0M solution of HCl in dioxane(2mL) carefully and the reaction
mixture stirred
for 90 minutes. The reaction mixture was then concentrated i~ vacuo to yield
the desired title
compound as an off white solid (quantitative yield).
Reference Example 2C. 3~,~-Trimethyl-butane-1, 2-diamine. Hydrochloride salt
(IIL3)
N-(tert-butoxycaxbonyl)-DL-valine methyl ester was prepared from DL-valine
using
standard preparative techniques. This was converted into the desired title
compound using an
analogous procedure to that described in Reference Example 2B.
Enantiomerically pure (R)-3~,~-Trimethyl-butane-1, 2-diamine. Hydrochloride
salt (IIL3.a)
was prepared by using D-valine as the starting material.
Reference Example 2D. ~,~-Dimethyl-3-phenyl-propane-1,2-diamine.
Hydrochloride salt (IIL4)
N-(tent-butoxycarbonyl)-DL-phenylalanine methyl ester was prepared from DL-
phenylalanine using standard preparative techniques. This was converted into
the desired title
compound using an analogous procedure to that described in Reference Example
2B.
30.
Reference Example 2E. (S)-NI,NI-Dimethyl-propane-1,2-diamine. Hydrochloride
salt (IILS)
2-(S)-[N-(tey.~-Butoxycarbonyl)amino]propanal was prepared from L-alanine
according
to the procedure described in the literature(Chakravarty et al, J. Med. Chem
1989, p1886). A
mixture of the aldehyde(2.62g), dimethylamine hydrochloride(2.47g), sodium
acetate(1.99g)
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
and sodium cyanoborohydride(1.43g) in methanol (45mL) was stirred at room
temperature for
18 hours. The reaction mixture was dissolved in ethyl acetate, washed with
water, dried
(MgS04), and the solvent removed ih vacuo to yield a viscous oil. This was
dissolved in
dichloromethane, extracted with citric acid, basified with sodium hydroxide,
and re-extracted
5 with ethyl acetate. The organic layer was reduced i~ vacuo to yield the
dimethylamino
derivative as a white solid (586mg).
To this compound (366mg) was added a 4.0M solution of hydrochloric acid in
dioxane (S.SmL) at room temperature. After stirring for 30mins the volatiles
were removed iy~
vacuo to yield the desired title compound as a viscous oil (313mg)
Reference Example 2F. (R)-~,~-Dimethyl-propane-1,2-diamine. Hydrochloride salt
(IIL6)
2-(R)-[N-(tent'Butoxycarbonyl)amino]propanal was prepared from D-alanine Me-
ester
hydrochloride according to the procedure described in the
literature(Chalcravarty et al, J. Med.
Chem 1989, p1886).
A mixture of the aldehyde(16.21g), dimethylamine hydrochloride(15.28g), sodium
acetate(11.53g) and sodium cyanoborohydride(8.24g) in methanol (250mL) was
stirred at
room temperature for 18 hours maintaining pH at 6-7 with AcOH. The reaction
mia~ture was
dissolved in ethyl acetate, washed with, water, dried (MgS04), and the solvent
removed iy~
vacuo to yield a viscous oil which was purified using flash chromatography to
yield the
dimethylamino derivative as a white solid(10.81g).
To this compound (3.17g) was added a 4.0M solution of hydrochloric acid in
dioxane
(20mL) at room temperature. After stirring for 1 hour the volatiles were
removed i~ vacuo to
yield the desired title compound as a viscous oil (2.79g)
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
31
Reference Example 2G. (S)-2-Amino-3-dimethylamino-propan-1-ol. Hydrochloride
salt (IIL7)
N-L(test-Butoxy)carbonyl]-O-(tee.~-butyldimethylsilyl)-R-serine methyl ester
was
prepared according to the literature (H.W. Scheeren et al, J. Org. Chem. 1990,
p3998) from
D-serine methyl ester hydrochloride . Treatment of this compound with
diisobutyl aluminium
hydride in toluene at -70°C for 2 hours yielded the correponding
aldehyde (H.W. Scheeren et
al, J. Org. Chem. 1990, p3998).
A mixture of the crude aldehyde(4.43g) ,dimethylamine.hydrochloride (2.26g),
sodium cyanoborohydride (1.31g) and sodium acetate (1.83g) was stirred in
methanol (SSmL)
for 24 hours at room temperature. Aqueous work-up yielded the dimethylamine
derivative.
This was dissolved in dioxane and to this was added a 4.0 M solution of
hydrochloric acid in
dioxane and the mixture stirred for 20 minutes. Concentration of the mixture
i~ vacuo Yielded
the crude desired title compound as a white solid.
Reference Example 2H. 3(S)-Amino-4-dimethylamino-butan-2(S)-ol. Hydrochloride
salt
(III.8)
Hydrogen chloride gas was bubbled through a solution of D-threonine (20g) in
methanol (SOmL).. The resulting solution was stirred for 5 hours at room
temperature and
then reduced i~ vacuo to yield D-threonine methyl ester hydrochloride as a
white solid
(quantitative yield). Treatment with di-tert-butyl Bicarbonate in acetonitrile
with
triethylamine yielded N-(tent-butoxycarbonyl)-D-threonine methyl ester.
Treatment of this
compound with tert-butyldirnethylsilyl chloride in dichloromethane with
imidazole yielded
the corresponding TBDMS protected alcohol.
Treatment of this compound with diisobutyl aluminium hydride in toluene at -
78° C
for 4 hours yielded the corresponding aldehyde (prep see H.W. Scheeren et al,
J. Org. Chem.
1990, p3998). Reductive amination was carried out as described in Reference
Example 2G to
yield the corresponding dimethylamino derivative. Deprotection using 4.0M HCl
in dioxane
as described in Reference Example 2G yielded the title compound as a golden
oil.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
32
Reference Example 2I. 3-Amino-4-dimethylamino-butan-1-ol. Hydrochloride salt
(IIL9)
DL-Homoserine was treated with hydrogen chloride gas in methanol to yield the
corresponding lactone. Treatment with di-tert-butyl Bicarbonate in
acetonitrile with
triethylamine yielded the N-tey.t-butoxycarbonyl protected derivative.
S Treatment of this compound with diisobutyl aluminium hydride in toluene at -
78° C for 4
hours yielded the corresponding lactol (prep see H.W. Scheeren et al, J. Org.
Chem. 1990,
p3998).
Reductive amination was carried out as described in Reference Example 2G to
yield
the corresponding dimethylamino derivative, 3-[N-(te~t-Butoxycarbonyl)amino]-4-
dimethylamino-butan-1-ol. Deprotection using 4.0M HCl in dioxane as described
in
Reference Example 2G yielded the desired title compound.
Reference Example 2J. 1-Methyl-3-(R)-aminopyrrolidine. Hydrochloride salt
(III.10)
A solution of 3R-(-)-1-benzyl-3-aminopyrrolidine(commercially available,
847mg) in
tert-butanol (lOmL) and 1.0N sodium hydroxide solution (4.8mL) was treated
dropwise with
a solution of di-tey.t-butyl Bicarbonate (l.Obg) in tert-butanol (SmL). After
1.5 hours tert-
butanol was removed 1n vacuo ~d ~e residue dissolved in ethyl acetate, washed
with water,
dried (MgSO~,) and the solvent removed zn vacuo to yield the desired 3N-te~.t-
butoxycarbonyl
protected derivative as a colourless gum (1.26g).
A solution of the 3N-tert-butoxycarbonyl protected derivative (800mg) in
tetrahydrofuran was
stirred over palladium hydroxide catalyst under an.atmosphere of hydrogen for
24 hours. The
reaction mixture was then filtered through celite and the solvent removed in
vacuo to Yield the
desired 3N-test-butoxycarbonyl-3-(R)-aminopyrrolidine (quantitative yield).
To a solution of N-tent-butoxycarbonyl-3-(R)-aminopyrrolidine (437mg) in
methanol
(IOmL) was added 37% formaldehyde solution in H20 (0.52mL) and sodium
borohydride(271mg). The reaction mixture was stirred for 24 hours at room
temperature and
then reduced in vacuo. The residue was dissolved in chloroform, washed with
brine and
NaHC03 solution, dried (MgSO~) and the solvent removed iyt vacuo to yield the
desired 3N-
test-butoxycarbonyl-3-(R)-amino-1~=methylpyrrolidine as a gum (390mg).
Deprotection with
4.0M HCl solution in dioxane as described above yielded the desired title
compound.
Reference Example 2K. Ni,NI,NZ,NZ-Tetramethyl-propane-I,2,3-triamine
trihydrochloride (IIL11)
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
33
To ice cooled thionyl chloride ( 35 mL), 1,3-bis(dimethylamino)-propan-2-of
(4.91 g,
33.58 mmol, commercially available) was added dropwise over 45 mires with
stirring. After
the addition was complete the mixture was stirred for a further 4 h. Excess
thionyl chloride
was removed under reduced pressure to give 8.9 g of a cream solid of the
product as the
hydrochloride salt. The free base was obtained by treating a suspension of the
hydrochloride
salt in toluene (18 mL) with sodium hydroxide (2.4 eq) in water (14 mL) for 30
min. The
organic layer was removed, dried over MgS04, filtered and the solvent removed
i~ vacuo to
yield 2-chloro-N,N,NI,Ni-tetramethylpropane-1,3-diamine as a yellow liquid.
2-Chloro-N,N,Nl,N1-tetramethylpropane-1,3-diamine (1.6 g, 9.71 mmol) as a
solution
in toluene (14 mL) was treated with potassium phthalimide (1.98 g, 10 .68
mmol). The stirred
mixture was heated at reflux for 18 h under an inert atmosphere, cooled to
ambient
temperature and the solvent removed under reduced pressure to yield a beige
solid,
recrystallisation from diethyl ether afforded a fawn solid (1.85 g). This
solid (0.953, 3.46
mmol) as a solution in ethanol (10 rnL) was treated with hydrazine hydrate
(0.22 mL, 6.93
mmol) and the mixture stirred at ambient temperature for 18 h. The suspension
was removed
by filtration and the filtrate acidified with 2 mL of 2 M hydrochloric acid
and the solvent
removed iy~ vacuo to give the product as a cream solid (795 mg). M.pt 126.5 -
128 °C, MH+
432
Reference Example 3 : Preparation of Compounds of General Formula (IV)
Reference Example 3A. 4-Methoxy-benzo[a]phenazine-11-carboxylic acid methyl
ester
(IV.l)
Acetyl chloride (4.6mL) was added dropwise to a suspension of 4-methoxy-
benzo[a]phenazine-11-carboxylic acid (ILl,see Reference Example 1A, 4.9g) in
methanol
(SOmL). The mixture was heated to reflux for 4 hours. The volatiles were then
removed iu
vacuo to yield the title compound as a dark solid (quantitative yield).
NMR, d6-DMSO, 8.76(lH,d), 8.41(2H,d), 8.26(lH,d), 8.00(lH,t), 7.90(lH,d),
7.78(lH,t),
7.43(lH,d), 4.08(3H,s), 4.03(3H,s).
Reference Example 3B. Benzo[a]phenazine-11-carboxylic acid methyl ester (IV.2)
The mixture of benzo[a]phenazine-11-carboxylic acid (IL20) and
benzo[a.]phenazine-
8-carboxylic acid (885mg), prepared as described in Reference Example 1L
above, was
heated to reflux in a mixture of methanol(40mL) and acetyl chloride(920~,L)
for 90 minutes.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
34
The reaction mixture was then cooled slowly to yield the title compound as a
single isomer
which was collected by filtration (377mg).
NMR, d6-DMSO, 9.53-9.55(lH,m), 9.06(lH,d), 8.57(lH,d), 8.47(lH,d), 8.35(lH,d),
8.10-
8.11(lH,m), 8.09-8.02(lH,m), 7.95-8.01(2H,m), 4.21(3H,s)
Reference Example 3C. 4-Dimethylamino-benzo[a]phenazine-11-carboxylic acid
methyl
ester (IV.3) and 3-dimethylamino-benzo[a]phenazine-11-carboxylic acid methyl
ester (IV.4)
To the 4:1 mixture of 4-amino-benzo[a]phenazine-11-carboxylic acid (IL29) and
3-
amino-benzo[a]phenazine-11-carboxylic acid (IL30)(48mg) in N,N-
dimethylformamide(lOmL) from Reference Example 1N was added methyl
iodide(O.SmL)and
diisopropylethylamine (2.OmL). The reaction mixture was heated to 100°C
for 4 hours. The
mixture was cooled, diluted with ethyl acetate, washed with water, dried
(MgS04) and the
solvent removed z~ vacuo to yield the title compounds as a red solid (29mg).
Purified after
further chemical modification.
Reference Example 3D. 10-Fluoro-4-methoxy-benzo[a]phenazine-11-carboxylic acid
methyl ester (IV.S)
Methyl 2-amino-6-fluoro-3-nitrobenzoate was prepared according to the
literature (J.
Med. Chem 1993, p2335). Hydrogenation over palladium on carbon in methanol
yielded 2,3-
diamino-6-fluoro-benzoic acid methyl ester. This compound was reacted with 5-
methoxy-
[1,2]naphthoquinone in cold ethanol acid with concentrated HCl to yield the
desired title
compound.
NMR, CDC13, ~ 4.08(3H,s), 4.20(3H,s), 7.20(lH,d), 7.65-7.75(2H,m), 7.92(lH,d),
8.35(lH,dd), 8.53(lH,d), 8.91(lH,d)
MS m/e 337 (MH+, 100%)
Reference Example 3E. 4-Methoxy-10-methylamino-benzo[a]phenazine-11-
carboxylic.
acid methyl ester (IV.6)
A mixture of 10-fluoro-4-methoxy-benzo[a]phenazine-11-carboxylic acid methyl
ester
(IV.S) (100mg) and 2.0M solution of methylamine in tetrahydrofuran was stirred
at room
temperature for 18 hours. The solvent was then removed i~ vacuo to yield the
crude desired
title compound as an orange solid.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
Reference Example 3F. 10-Amino-4-methoxy-benzo[a]phenazine-11-carboxylic acid
methyl ester (IV.7)
A mixture of 10-fluoro-4-methoxy-benzo[a]phenazine-11-carboxylic acid methyl
ester
(IV.S) (466rng) and sodium azide (900mg) in N,N-dimethylformamide(lOmL) was
heated at
5 90°C for 18 hours. The reaction mixture was then cooled, diluted with
water, and then sodium
hydroxide solution was added resulting in a brown precipitate. This was
collected by filtration
and washed with water and ether to yield the desired title compound as a
yellow solid
(242mg).
NMR, CDCl3, 4.08(3H,s), 4.20(3H,s), 7.25(lH,d), 7.70-7.72(2H,m), 7.90(lH,d),
8.36(lH,d),
10 8.52(lH,d), 8.85(lH,d).
MS DCI/NH3 m/z 334 (MH+, 100%)
Example 1: Preparation of Compounds of General Formula (I)
15 Example 1A. 4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-
amide
A mixture of 4-methoxy-benzo[a]phenazine-11-carboxylic acid (IL1) (129mg) and
1,1'-carbonyldiimidazole(138mg) was stirred in dry N,N-dimethylformamide (8mL)
at room
temperature for 4 hours. To this mixture was added N,N-dimethylethylenediamine
20 (commercially available)(O.SmL) and the reaction mixture was stirred at
room temperature for
a further 30 minutes. The volatiles were then removed i~ vacuo. The residue
was dissolved in
dichloromethane, washed with water, dried (MgSO~) and the solvent removed rye
vacuo to
provide crude product. This was purified using flash chromatography. (5%
methanol in
dichloromethane) to yield the title compound as a bright yellow solid (120mg).
25 The following compounds of formula (I) were prepared in an analogous manner
using
the appropriate starting acid of formula (II) and amine of formula (III).
3-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide
was
prepared from 3-methoxy-benzo[a]phenazine-11-carboxylic acid (IL34) and N,N-
dimethylethylenediamine;
30 2-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide was
prepared from 2-methoxy-benzo[a]phenazine-11-carboxylic acid (IL33) and N,N-
dimethylethylenediamine;
4-Nitro-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide was
prepared
from the mixture of 4-nitro-benzo[a]phenazine-11-carboxylic acid (IL27) and 3-
nitro-
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
36
benzo[a]phenazine-11-carboxylic acid (IL28), and N,N-dimethylethylenediamine
and then
purified using flash chromatography;
4-Benzyloxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide
was
prepared from 4-benzyloxy-benzo[a]phenazine-11-carboxylic acid (II.13) and N,N-
dimethylethylenediamine;
4-Prop-2-ynyloxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide was
prepared from 4-prop-2-ynyloxy-benzo[a]phenazine-11-carboxylic acid (IL14) and
N,N-
dimethylethylenediamine;
3,4-Dimethoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide was
prepared from 3,4-dimethoxy-benzo[a]phenazine-11-carboxylic acid (IL4) and N,N
dimethylethylenediamine;
4-Ethoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide
was'
prepared from 4-ethoxy-benzo[a]phenazine-11-carboxylic acid (IL11) and N,N-
dimethylethylenediamine;
4-Isobutoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide
was
prepared from 4-isobutoxy-benzo[a]phenazine-11-carboxylic acid (II.15) and N,N-
dimethylethylenediamine;
4-(4-Chloro-benzyloxy)-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)
amide was prepared from 4-(4-chloro-benzyloxy)-benzo[a]phenazine-11-carboxylic
acid
(IL 16) and N,N-dimethylethylenediamine;
4-(2-Methoxy-ethoxy)-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide
was prepared from 4-(2-methoxy-ethoxy)-benzo[a]phenazine-11-carboxylic acid
(IL17) and
N,N-dimethylethylenediamine;
[11-(2-Dimethylamino-ethylcarbamoyl)-benzo[a]phenazin~4-yloxy]-acetic acid
ethyl ester
was prepared from 4-ethoxycarbonylmethoxy-benzo[a]phenazine-11-carboxylic acid
(IL18)
and N,N-dimethylethylenediamine;
3-Brorno-4-hydroxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide
was prepared from 3-bromo-4-hydroxy-benzo[a]phenazine-11-carboxylic acid
(IL31) and
N,N-dimethylethylenediamine;
4-[2-(tert-Butyl-dimethyl-silanyloxy)-ethoxy]-benzo[a]phenazine-11-carboxylic
acid (2-
dimethylamino-ethyl)-amide was prepared from 4-[2-(tent-butyl-dimethyl-
silanyloxy)-
ethoxy]-benzo[a]phenazine-11-carboxylic acid (II.19) and N,N-
dimethylethylenediamine;
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
37
4-Methyl-benzo[a]phenazine-11-carboxylic acid(2-dimethylamino-ethyl)-amide was
prepared
from 4-methyl-benzo[a]phenazine-11-carboxylic acid (IL2) and N,N-
dimethylethylenediamine; .
4-Fluoro-benzo[a]phenazine-11-carboxylic acid(2-dimethylamino-ethyl)-amide was
prepared
from- 4-fluoro-benzo[a]phenazine-11-carboxylic acid (IL3) and N,N-
dimethylethylenediamine;
4-Methylsulfanyl-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-.ethyl)-
amide was
prepared from 4-methylsulfanyl-benzo[a]phenazine-11-carboxylic acid (IL12) and
N,N-
dimethylethylenediamine;
4-Bromo-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide was
prepared
from ~ 4-bromo-benzo[a]phenazine-11-carboxylic acid (IL5) and N,N-
dimethylethylenediamine;
4-Cyano-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide was
prepared
from 4-cyano-benzo[a]phenazine-11-carboxylic acid (IL6) and N,N-
dimethylethylenediamine;
3-Nitro-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide was
prepared
from the mixture of 4-vitro-benzo[a]phenazine-11-carboxylic acid (IL27) and 3-
nitro-
benzo[a]phenazine-11-carboxylic acid (IL28), and N,N-dimethylethylenediamine,
and then
purified using flash chromatography;
4-Methanesulfonyl-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide
was prepared from 4-methanesulphonyl-benzo[a]phenazine-11-carboxylic acid
(IL8) and
N,N-dimethylethylenediamine;
4-Chloro-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide
was prepared
from 4-chloro-benzo[a]phenazine=11-carboxylic acid (IL7) and N,N-
dimethylethylenediamine;
4-Azido-benzo[a]phenazine-11-carboxylic acid(2-dimethylamino-ethyl)-amide was
prepared
from ' 4-azido-benzo[a]phenazine-11-carboxylic acid (IL9) and N,N-
dimethylethylenediamine; l l -(2-Dimethylamino-ethylcarbamoyl)-benzo
[a]phenazine-4-
carboxylic acid methyl ester was prepared from benzo[a]phenazine-4,11-
dicarboxylic acid 4-
methyl ester (II.10) and N,N-dimethylethylenediamine;
4-Methylsulfamoyl-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide;
trifluoro-acetate was prepared from the mixture of 4-methylsulfamoyl-
benzo[a]phenazine-11-
carboxylic acid (IL21)and 3-methylsulfamoyl-benzo[a]phenazine-11-carboxylic
acid(IL22),
and N,N-dimethylethylenediamine. The two isomers were separated using
preparative HPLC;
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
38
3-Methylsulfamoyl-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide;
trifluoro-acetate was prepared from the mixture of 4-methylsulfamoyl-
benzo[a]phenazine-11-
carboxylic acid (IL21) and 3-methylsulfamoyl-benzo[a]phenazine-11-carboxylic
acid (IL22),
and N,N-dimethylethylenediamine. The two isomers were separated using
preparative HPLC;
4-Dimethylsulfamoyl-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide
was prepared from the mixture of 4-dimethylsulfamoyl-benzo[a]phenazine-11-
carboxylic acid
(IL23)and 3-dimethylsulfamoyl-benzo[a]phenazine-11-carboxylic acid (IL24), and
N,N-
dimethylethylenediamine. The two isomers were separated using flash
chromatography;
3-Dimethylsulfamoyl-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide
was prepared from the mixture of 4-dimethylsulfamoyl-benzo[a]phenazine-11-
carboxylic acid
(IL23)and 3-dimethylsulfamoyl-benzo[a]phenazine-11-carboxylic acid (IL24), and
N,N
dimethylethylenediamine. The two isomers were separated using flash
chromatography;
3-Sulfamoyl-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide
was
prepared from the mixture of 4-sulfamoyl-benzo[a]phenazine-11-carboxylic acid
(IL25) and
3-sulfamoyl-benzo[a]phenazine-11-carboxylic acid (IL26), and N,N
dimethylethylenediamine. 3-Sulfamoyl-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-ethyl)-amide was purified using flash chromatography;
2-Nitro-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide was
prepared
from 2-vitro-benzo[a]phenazine-11-carboxylic acid (IL32) and N,N-
dimethylethylenediamine;
9-Bromo-4-methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide
was prepared from 9-bromo-4-methoxy-benzo[a]phenazine-11-carboxylic acid
(IL35) and
N,N-dimethylethylenediamine;
4-Nitro-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-1-methyl-ethyl)-
amide was
prepared from the mixture of 4-vitro-benzo[a]phenazine-11-carboxylic acid
(IL27) and 3-
vitro-benzo[a]phenazine-11-carboxylic acid (IL28), and 1-dimethylamino-2-
propylamine, and
then purified using flash chromatography;
3-Nitro-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-1-methyl-ethyl)-
amide was
prepared from the mixture of 4-vitro-benzo[a]phenazine-11-carboxylic acid
(IL27) and 3
vitro-benzo[a]phenazine-11-carboxylic acid (IL28), and 1-dimethylamino-2-
propylamine, and
then purified using flash chromatography;
Example 1B. 4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-1-
methyl-ethyl)-amide .
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
39
A mixture of 4-methoxy-benzo[a]phenazine-11-carboxylic acid methyl ester
(IV.1)
(350mg) and 1-dimethylamino-2-propylamine(2mL)(commercially available) was
heated to
110°C under N2 for 4 hours. The reaction mixture was then cooled and
the excess amine was
removed ih vacuo. The residue was then purified using flash chromatography
(silica, ethyl
acetate and then 25% methanol in ethyl acetate) to yield the title compound as
a yellow solid
( 164mg)
The following compounds of formula (I) were prepared in an analogous manner
using
the appropriate starting ester of formula (IV) and the appropriate amine of
Formula (III):
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (3-dimethylamino-propyl)-amide
was
prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid methyl ester
(IV.1) and 3-
(dimethylamino) propylamine(commercially available);
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (3-amino-2-hydroxy-propyl)-
amide was
prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid methyl ester
(IV.l) and 1,3-
diamino-2-hydroxypropane (commercially available);
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-propyl)-amide
was
prepared from
4-methoxy-benzo[a]phenazine-11-carboxylic acid methyl ester (IV.1) and N2,N2-
dimethyl-
propane-1,2-diamine (commercially available);
4-Dimethylamino-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide was
prepared from the mixture of 4-dimethylamino-benzo[a]phenazine-11-carboxylic
acid methyl
ester (IV.3) and 3-dimethylamino-benzo[a]phenazine-11-carboxylic acid methyl
ester (IV.4),
and N,N-dimethylethylenediamine, followed by flash chromatography purification
to remove
the minor isomer;
4-Methoxy-10-methylamino-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-
amide was prepared from 4-methoxy-10-methyla~nino-benzo[a]phenazine-11-
carboxylic acid
methyl ester (IV.6) and N,N-dimethylethylenediamine;
10-Amino-4-methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide
was prepared from 10-amino-4-methoxy-benzo[a]phenazine-11-carboxylic acid
methyl ester
(IV.7 and N,N-dimethylethylenediamine; and
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-methylamino-ethyl)-amide was
prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid methyl ester
(IV.1) and N-
methylethylenediamine;
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
Example 1C. 4-Methaxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
1,1-
dimethyl-ethyl)-amide
A mixture of 4-methoxy-benzo[a]phenazine-11-carboxylic acid (IL1) and thionyl
chloride was heated to reflux for 6 minutes. Thionyl chloride was then removed
iu vacuo~ The
5 residue was dissolved in dry dichloromethane at 0°C and 1-
dimethylamino-2-methyl-2-
aminopropane (commercially available) was added. After stirring for 2 hours
the reaction
mixture was dissolved in dichloromethane, washed with sodium bicarbonate
solution, dried
(MgS04) and the solvent removed i~ vacuo to provide crude product. This was
purified using
flash chromatography to yield the title compound.
10 The following compounds of formula (I) were prepared in an analogous manner
using
the appropriate starting acid of formula (II) and the appropriate amine of
Formula (III)
3-Dimethylamino-2-[(4-methoxy-benzo[a]phenazine-11-carbonyl)-amino]-propionic
acid
methyl ester was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid
(II.1) and
4-aza-DL-leucine methyl ester. hydrochloride (IIL 1) in the presence of
pyridine. This was
15 purified using preparative HPLC (isocratic 60% waterl40% MeCN) to yield the
trifluoroacetate salt of the desired compound;
4,10-Dimethoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide was
prepared from 4,10-dimethoxy-benzo[a]phenazine-11-carboxylic acid(IL36) and
N,N-
dimethylethylenediamine;
20 Lengthened reaction times (over 1 hour) with thionyl chloride results in
chlorination of the
phenazine nucleus. Hence 1-Chloro-4,10-dimethoxy-benzo[a]phenazine-11-
carboxylic acid
(2-dimethylamino-ethyl)-amide was prepared from 4,10-dimethoxy-
benzo[a]phenazine-11-
carboxylic acid(IL36) and N,N-dimethylethylenediamine;
4-Methoxy-8-methyl-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide
25 was prepared from 4-methoxy-8-methyl-benzo[a]phenazine-11-carboxylic acid
(IL37) and
N,N-dimethylethylenediamine;
9-Chloro-4-methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide
was prepared from 9-chloro-4-methoxy-benzo[a]phenazine-11-carboxylic acid
(IL38) and
N,N-dimethylethylenediamine;
30 4,10-Dimethoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-1-
methyl-ethyl)-
amide was prepared from 4,10-dimethoxy-benzo[a]phenazine-11-carboxylic acid
(IL36) and
1-dimethylamino-2-propylamine(commercially available);
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
41
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (1-dimethylaminomethyl-propyl)-
amide
was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (IL 1) and
N1,N1-
dimethyl-butane-1,2-diamine.hydrochloride (IIL2) in the presence of
triethylamine;
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (1-dimethylaminomethyl-2-methyl-
propyl)-amide was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid
(IL1)
and 3Nl,Nl-trimethyl-butane-1,2-diamine.Hydrochloride salt (IIL3) in the
presence of
triethylamine;
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (1-dimethylaminomethyl-2-phenyl-
ethyl)
amide was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (IL1)
and N1,NI
dimethyl-3-phenyl-propane-1,2-diamine.Hydrochloride salt (IIL4) in the
presence of
triethylamine;
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-1-(S)-methyl-
ethyl)-
amide was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (IL1)
and (S)-
N1,NI-dimethyl-propane-1,2-diamine.Hydrochloride salt (IILS) in the presence
of
txiethylamine;
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-1(R)-methyl-
ethyl)-
amide was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (ILl)
and (R)-
NI,NI-dimethyl-propane-1,2-diamine.Hydrochloride salt (IIL6) in the presence
of
triethylamine;
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-1(S)-
hydroxymethyl-
ethyl)-amide was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid
(IL1) and
(S)-2-Amino-3-dimethylawino-propan-1-ol.Hydrochloride salt (IIL7) in the
presence of
triethylamine;
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (1(S)-dimethylaminomethyl-2(S)-
hydroxy-
propyl)-amide was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid
(IL1) and
3(S)-Amino-4-dimethylamino-butan-2(S)-ol. Hydrochloride salt (IILB) in the
presence of
triethylamine;
4-Methoxy-benzo[a]phenazine-11-carboxylic acid [1-dimethylamino-1-(2-
hydroxyethyl)]-
ethylamide was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid
(II.1) and 3-
Amino-4-dimethylamino-butan-1-ol.Hydrochloride salt (IIL9) in the presence of
triethylamine;
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-piperidin-1-yl-ethyl)-amide
was
prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (IL1) and 1-(2-
aminoethyl)piperidine (commercially available);
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
42
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-morpholin-4-yl-ethyl)-amide
was
prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (II.1) and 4-(2-
aminoethyl)morpholine (commercially available);
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-pyrrolidin-1-yl-ethyl)-amide
was
prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (ILl) and 1
(aminoethyl)pyrrolidine (commercially available);
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-diethylamino-ethyl)-amide
was
prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (II.1) and N,N-
diethylethylenediamine (commercially available);
4-Methoxy-benzo[a]phenazine-11-carboxylic acid ~2-[bis-(2-hydroxy-ethyl)-
amino]-ethyl~-
amide was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (ILl)
and N,N-
bis(2-hydroxyethyl)ethylenediamine (commercially available);
4,10-Dimethoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-1(S)
hydroxymethyl-ethyl)-amide was prepared from 4,10-dimethoxy-benzo[a]phenazine-
11
carboxylic acid (IL36) and (S)-2-amino-3-dimethylamino-propan-1-
ol.Hydrochloride salt
(IIL7) in the presence of aqueous sodium carbonate;
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (1-methyl-pyrrolidin-3-(R)-yl)-
amide was
prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (IL1) and 1-
methyl-3-(R)-
aminopyrrolidine. Hydrochloride salt (IIL 10) in the presence of
triethylamine;
(R)-4-Methoxy-benzo[a]phenazine-11-carboxylic acid(1-dimethylaminomethyl-2-
methyl-
propyl)-amide was prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid
(ILl) and
(R)-3N1,N1-trimethyl-butane-1, 2-diamine. Hydrochloride salt (IIL3.a) in the
presence of
triethylamine;
trihydrochloride(III.l 1) in the presence of triethylamine;
4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2,3-bis)-dimethylamino-
propyl)amide was
prepared from 4-methoxy-benzo[a]phenazine-11-carboxylic acid (ILl)
andNl,NI,NZ,N2-
tetramethyl-propane-1,2,3-triamine.
Example 2: Interconversion of compounds of Formula (I)
Compounds of Formula (I) prepared as described in Example 1 were converted
into
other compounds of Formula (I) as described below.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
43
Example 2i. 4-Hydroxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-
amide: hydrobromide salt
To a solution of 4-methoxy-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-
ethyl)-amide (L1) (727mg) in dry dichloromethane (lSmL) cooled to -5°C
was added a 1.0M
solution of boron tribromide in dichloromethane (13.6mL). After stirring for 4
hours the
reaction mixture was poured onto ice/water yielding a precipitate which was
collected by
filtration. This was triturated from a hot methanol/ethyl acetate mixture to
yield the title
compound as a beige solid (SOSmg).
Example 2ii. 3-Hydroxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-
amide
To a solution of 3-methoxy-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-
ethyl)-amide (170mg) in dry N,N-dimethylformamide(3mL) was added sodium
thioethoxide(380mg). The reaction mixture was then heated to reflux under
argon for 3 hours.
The reaction mixture was cooled, acidified (dilute HCl) and volatiles removed
in vacuo. The
residue was purified using column chromatography (20% methanol in
dichloromethane) to
yield the title compound as a red solid (142mg).
2-Hydroxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide
was
prepared in an analogous manner from 2-methoxy-benzo[a]phenazine-11-carboxylic
acid (2-
dimethylamino-ethyl)-amide.
Example 2iii. 4-Cyanomethoxy-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-
ethyl)-amide
To a suspension of 4-hydroxy-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-ethyl)-amide: hydrobromide salt(230mg) in dry N,N-
dimethylformamide(3mL) was added potassium tent-butoxide (175mg) and then
bromoacetonitrile(47~,L). The reaction mixture was heated to 100°C for
1 hour. The reaction
mixture was then cooled, diluted with ethyl acetate, washed with sodium
carbonate solution
and brine, dried (MgS04) and the solvent removed ih vacuo to provide crude
product. This
was purified using flash chromatography (silica, 25% MeOH in ethyl acetate) to
yield the title
compound as a yellow solid (74mg).
The following compounds of formula (I) were prepared in an analogous manner
using
4-hydroxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide:hydrobromide salt and the appropriate alkylating reagent;
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
44
4-(Pyrimidin-2-yloxy)-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide
was prepared using 2-bromopyridine;
4-(2-Morpholin-4-yl-ethoxy)-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-ethyl)-
amide was prepared using N-(2-chloroethyl)morpholine hydrochloride;
4-(3-Cyano-propoxy)-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide
was prepared using 4-bromobutyronitrile;
4-(3-Dimethylamino-propoxy)-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-
ethyl)-amide was prepared using 3-dimethylaminopropyl chloride hydrochloride;
4-Carbamoylmethoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide
was prepared using 2-bromoacetamide;
4-(2-Oxo-propoxy)-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide
was prepared using chloroacetone; and
[11-(2-Dimethylamino-ethylcarbamoyl)-benzo[a]phenazin-4-yloxy]-acetic acid
te~.~-butyl
ester was prepared using tertbutyl bromoacetate.
Example 2iv. Ethyl-carbamic acid 11-(2-dimethylamino-ethylcaxbamoyl)-
benzo[a]phenazin-
4-yl ester.
A mixture of 4-hydroxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino
ethyl)-amide: hydrobromide salt(540mg), triethylamine (0.51mL) and ethyl
isocyanate
(0.29mL) was stirred in dry N,N-dimethylformamide(3mL). The product slowly
precipitated
from the reaction mixture and was collected by filtration and washed with
ether to yield the
title compound as a yellow solid (210mg).
Example 2v. Acetic acid 11-(2-dimethylamino-ethylcarbamoyl)-benzo[a]phenazin-4-
yl
ester
A mixture of 4-hydroxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino
ethyl)-amide: hydrobromide salt(45mg), triethylamine (7lp,L) and acetyl
chloride(20p,L) in
dichloromethane(l.4mL) was stirred at room temperature for 2 hours: All
volatiles were
removed in vacuo and the residue was purified using column chromatography (I0%
methanol
in dichloromethane) to yield the title compound as a yellow solid (27mg).
Example 2vi. [11-(2-Dimethylamino-ethylcarbamoyl)-benzo[a]phenazin-4-yloxy]-
acetic
acid trifluoroacetate salt
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
To a solution of [11-(2-dimethylamino-ethylcarbamoyl)-benzo[a]phenazin-4-
yloxy]-
acetic acid test-butyl ester (l8mg) in dry dichloromethane (1mL) was added
trifluoroacetic
acid (1mL). After stirring for 4 hours the solvent was removed in vacuo to
yield crude
product. This was triturated with ether to yield the title compound as a
yellow solid (1 Omg).
5
Example 2vii. Benzo[a]phenazine-4,I1-dicarboxylic acid 4-amide 11-[(2-
dimethylamine-
etlryl)-amide]; triflouroacetic acid salt
11-(2-Dimethylamino-ethylcarbamoyl)-benzo[a]phenazine-4-carboxylic acid methyl
ester (200mg) was sonicated in methanol(20mL) to give a fine suspension. To
this was added
10 sodium cyanide (22mg). The mixture was then sparged with anhydrous ammonia
for 15 rains.
The reaction mixture was stirred at room temperature for 10 days and on each
day the mixture
was sparged with ammonia. After 10 days the volatiles were removed in vacuo
and the
residue was purified using flash chromatography to yield crude product. This
was further
purified using preparative HPLC (isocratic; 80:20 H20/acetonitrile) to yield
the title
15 compound as a yellow solid (lOmg).
Example 2viii. 11-(2-Dimethylamino-ethylcarbamoyl)-benzo[a]phenazine-4-
carboxylic
acid, trifluoroacetate salt
11-(2-Dimethylamino-ethylcarbamoyl)-benzo[a]phenazine-4-carboxylic acid methyl
20 ester (200mg) was sonicated in a mixture of methanol (4mL) and ammonium
hydroxide(20mL). The suspension was then heated to 50°C for 92 hours.
All volatiles were
then removed in vacuo to yield crude product, which was purified using
preparative HPLC to
yield the title compound (24mg)
25 Example 2ix. 4-Hydroxymethyl-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-
ethyl)-amide
To a solution of 11-(2-dimethylamino-ethylcarbamoyl)-benzo[a]phenazine-4-
carboxylic acid methyl ester (317mg) in tetrahydrofuran (l8mL) and 2-propanol
(lOmL)at
0°C was added lithium borohydride (2.0M solution in tetrahydrofuran,
1.97xnL,) The reaction
30 mixture was stirred at room temperature overnight, and then quenched with
ammonium
chloride solution. The reaction mixture was extracted with ethyl acetate,
dried (MgS04) and
concentrated ivc vacuo. The residue was purified using flash chromatography
(10% MeOH in
dichloromethane) to yield the title compound as a yellow solid (98mg).
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
46
Example 2x. 4-(N-Hydroxycarbamimid~I)-benzo[a]phenazine-11-carboxylic acid (2-
dimethylarnino-ethyl)-amide
A mixture of 4-cyano-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-
amide (20mg) , potassium carbonate(37mg) and hydroxylamine hydrochloride(l9mg)
was
heated to reflux in ethanol (SrnL) for 18 hours. The reaction mixture was
filtered, and the
filtrate was collected and the solvent removed in vacuo to yield the title
compound (20mg)
Example 2xi. 4-Dimethylaminomethyl-3-hydroxy-benzo[a]phenazine-11-carboxylic
acid
(2-dimethylamino-ethyl)-amide
3-Hydroxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide
(26mg) was sonicated in acetic acid (2mL) to give a fine suspension. To this
was added a 40%
solution of dimethylamine in water (3mL) and a 37% solution of formaldehyde in
water
(3mL). The reaction mixture was left stirring for 2 days. The volatiles were
then removed iu
vacuo to yield the title compound (29mg).
3-Dimethylaminomethyl-4-hydroxy-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-ethyl)-amide was prepared in an analogous manner from 4-hydroxy-
benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide.
Example 2xii. 4-(2-Hydroxy-ethoxy)-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-ethyl)-amide
To a solution of 4-[2-(test-butyl-dimethyl-silanyloxy)-ethoxy]-
benzo[a]phenazine-11-
carboxylic acid (2-dimethylamino-ethyl)-amide (125iiig) in
tetrahydrofuran(SmL) was added
a 1.0M solution of tetrabutyl ammonium fluoride (l.2mL). After stirring for
1.5 hours the
reaction mixture was diluted with ethyl acetate, washed with water, dried
(MgS04) and the
solvent removed in vacuo to yield crude product which was purified using flash
chromatography to yield the title compound as an orange solid (24mg).
Example 2xiii. 4-Amino-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl
amide
A mixture of 4-vitro-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-
amide(176mg), indium(154mg) and saturated ammonium chloride solution (SmL) in
ethanol(20mL) was heated to reflux for 3 hours. The reaction mixture was
cooled, quenched
with water and then filtered through celite. The filtrate was concentrated ih
vacuo, and the
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
47
residue was treated with sodium bicarbonate solution, extracted into
chloroform, dried
(MgSOø) and the solvent removed iu vacuo to Yield the title compound as a red
solid (163mg)
3-Amino-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-amide was
prepared
in an analogous manner from 3-nitro-benzo[a]phenazine-11-carboxylic acid (2
dimethylamino-ethyl)-amide.
Example 2xiv. 4-Acetylamino-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-
ethyl)-amide
To a solution of 4-amino-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino
ethyl)-axnide(20mg) in tetrahydrofuran(5mL) was added pyridine(O.lmL) and
acetyl
chloride(20~L). After stirring for 1 hour the reaction mixture was extracted
into ethyl acetate,
washed with sodium bicarbonate solution, dried (MgS04) and the solvent removed
i~ vacuo.
The residue was triturated with ether to yield the title compound as a yellow
solid (1 Omg)
3-Acetylamino-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-ethyl)-
amide
was prepared in an analogous manner from 3-amino-benzo[a]phenazine-11-
carboxylic acid
(2-dimethyla.mino-ethyl)-amide and acetyl chloride;
4-Methanesulfonylamino-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-
amide was prepared in an analogous manner from 4-amino-benzo[a]phenazine-11-
carboxylic
acid (2-dimethylamino-ethyl)-amide and methanesulphonyl chloride;
3-Methanesulfonylamino-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-
amide was prepared in an analogous manner from 3-amino-benzo[a]phenazine-11-
carboxylic
acid (2-dimethylamino-ethyl)-amide and methanesulphonyl chloride;
4-Bis-(Methanesulfonylamino)-benzo[a]phenazine-11-carboxylic acid (2-
dirnethylamino-
ethyl)-amide was prepared in a similar manner from 4-amino-benzo[a]phenazine-
11-
carboxylic acid (2-dimethylamino-ethyl)-amide using excess methanesulphonyl
chloride, and
triethylamine as base.
Example 2xv. 4-(Cyanomethyl-amino)-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-ethyl)-amide -
To a solution of 4-amino-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
ethyl)-amide(69mg) in methanol (lOmL) was added formaldehyde (37% solution,
l.OmL),
potassium cyanide(102mg)and 2N HCl (l.OmL). The reaction mixture was heated to
50°C for
3 hours. The reaction mixture was then cooled, diluted with water and sodium
bicarbonate
solution, extracted into dichloromethane, dried (MgS04) and the solvent
removed iy~ vacuo to
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
48
yield crude product. This was purified using flash chromatography (10%
methanol in
dichloromethane) to yield the title compound as a violet coloured solid (l3mg)
Example 2xvi. 3-Dimethylamino-2-[(4-methoxy-benzo[a]phenazine-11-carbonyl)-
amino]-propionic acid; hydrochloride
A mixture of 3-dimethylamino-2-[(4-methoxy-benzo[a]phenazine-11-carbonyl)-
amino]-propionic acid methyl ester(150mg) and 1M HCl (SOmL) was heated to
reflux for 1
hour. After cooling, all volatiles were removed iy~ vacuo to yield the title
compound as a red
solid (quantitative yield) .
Example 2xvii. 4,10-Dihydroxy-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-
ethyl)-amide
To a cold solution of 4,10-dimethoxy-benzo[a]phenazine-11-carboxylic acid (2
dimethylamino-ethyl)-amide (96mg) in dichloromethane(2mL)was added a 1.0M
solution of
boron tribromide in dichloromethane (2.14mL, 9 equivalents). The reaction
mixture was
stirred for 16 hours and then ice was added with sodium carbonate and sodium
chloride. The
organics were extracted into dichloromethane, dried (MgS04), and the solvent
removed Iy~
vacuo to .yield an orange compound which was recrystallised from
dichloromethane/methanol/hexane to yield the title compound (6mg).
Example 2xviii. 10-Hydroxy-4-methoxy-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-ethyl)-amide
To a cold solution of 4,10-dimethoxy-benzo[a]phenazine-11-carboxylic acid (2
dimethylamino-ethyl)-amide (300mg) in dichloromethane (25mL) was added a 1.0M
solution
of boron tribromide in dichloromethane (1.63mL, 2.2 equivalents). The reaction
mixture~was
stirred for 6 hours and then ice was added with sodium carbonate and sodium
chloride. The
organics were extracted into dichloromethane, dried (MgSO4), and the solvent
removed iu
vacuo to yield a yellow solid which was purified using flash chromatography to
yield the title
compound (6lmg)
10-Hydroxy-4-methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
1 (R)-methyl-ethyl)-amide was prepared in an analogous manner from 4,10-
dimethoxy-
benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-1(R)-methyl-ethyl)-
amide; and
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
49
10-Hydroxy-4-methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
1(S)-
hydroxymethyl-ethyl)-amide was prepared in an analogous manner from 4,10-
dimethoxy-
benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-1(S)-hydroxymethyl-
ethyl)-amide.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
Example 2xix. 4-Methoxy-9-methylsulfanyl-benzo[a]phenazine-11-carboxylic acid
(2-
dimethylamino-ethyl)-amide
A mixture of 9-chloro-4-methoxy-benzo[a]phenazine-11-carboxylic acid (2-
5 dimethylamino-ethyl)-amide(85mg) and sodium thiomethoxide (43mg) in N,N-
dimethylformamide (1mL) was heated to 120°C for 6 hours and 60°C
for 16 hours. The
reaction mixture was then cooled, diluted with ethyl acetate, washed with
water, dried
(MgS04) and the solvent removed iy~ vacuo to yield a yellow solid which was
purified using
flash chromatography to yield the desired title compound (37mg).
Example 2xx. 4,9-Dimethoxy-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-
ethyl)-amide
A mixture of 9-chloro-4-methoxy-benzo[a]phenazine-11-carboxylic acid (2-
dimethylamino-ethyl)-amide(85mg) and a 25% solution of sodium methoxide in
methanol(4mL) was heated to reflux for 6 hours. The reaction mixture was then
cooled,
diluted with ethyl acetate, washed with water, dried (MgS04) and the solvent
removed za
vacuo to Yield a yellow solid which was purified using flash chromatography to
yield the
desired title compound (42mg).
Example 2.xxi. 4-Methoxy-benzo[a]phenazine-11-carboxylic acid (2-dimethylamino-
1-
hydroxyniethyl-ethyl)-amide
A mixture of 3-dimethylamino-2-[(4-methoxy-benzo[a]phenazine-11-carbonyl)-
amino]-propionic acid methyl ester(335mg) and lithium borohydride(72mg)in
tetrahydrofuran
(lOmL) and isopropanol (lOmL) was stirred at room temperature for 18 hours.
Another 5
equivalents of lithium borohydride were added and the mixture stirred for
another 18 hours.
The reaction was quenched with ammonium chloride solution and extracted with
ethyl
acetate, dried (MgS04) and the solvent removed iu vacuo to yield a brown gum.
Purification
using flash chromatography and trituration with ether yielded the desired
title compound as
an orange powder.
Example 3: Biological testing of compounds of formula (I)
The cytotoxicity of compounds of formula (I) was measured using the H69
parental
(H69/P) human small cell lung carcinoma cell line and the drug resistant human
small cell
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
51
lung carcinoma cell line H69/LX4 which overexpresses P-glycoprotein (Pgp). The
cytotoxicity, as measured by the ICSO (concentration required to give 50% cell
kill) in the
H69/LX4 cell line divided by the cytotoxicity in the H69/P cell line gives an
indication of the
degree to which a compound is affected by Pgp-dependent MDR and is termed the
resistance
factor (Rf) of the compound.
H69/P and H69/LX4 cells were pipetted into 96-well tissue culture plates and
then
allowed to incubate at 37°C for 4 h. A range of concentrations from
0.01 nM to S~M of
compounds of formula (I) or the standards TAS-103, Doxorubicin and Topotecan
were then
added. The plates were incubated for 5-6 days before adding AlamarBlue to each
well and
returning the plates to the incubator for 5-8 h to allow colour development.
The cell numbers
in the plates at the end of this period were directly proportional to the
absorbance measured at
a wavelength of 570 nm (reference wavelength 600 nm).
The compounds of formula (I) were active in the range 5 nM to 5 ~,M. Specific
results
for selected compounds are listed in table 1.
Table 1.
Compound H69/P ICso(nM)H69/LX4 ICSO(nM)Rf
TAS-103 21 22 1.1
Doxorubicin 27.3 3700 135
Topotecan 15.9 61.5 3.9
4 35 48 1.4
3 3 5 49 1.4
11 28 24 0.9
94 19 25 1.3
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
52
6g 19 25 1.3
75 23 28 1.2
g1 20 25 1.2
7g 24 31 1.3
9g 21 20 1
g4 19 19 1
The cytotoxicity of the compounds described herein was also measured using the
COR-L23 parental (COR-L23/P) human non-small cell lung carcinoma cell line and
also the
drug resistant human non-small cell lung carcinoma cell line COR-L231R which
overexpresses multidrug resistance associated protein (MRP). The cytotoxicity,
as measured
by the ICSO (concentration required to give 50% cell kill) in the L23/R cell
line divided by the
cytotoxicity in the L23/P cell line gives an indication of the degree to which
a compound may
be affected by MRP-dependant MDR and is termed the resistance factor (Rf) of
the
compound.
L23/P and L23/R. cells were pipetted into 96-well tissue culture plates and
then
allowed to incubate at 37°C for 4 h. A range of concentrations from
0.01nM to S~,M of
compounds of formula (I) or the standards TAS-103, Doxorubicin and Topotecan
were then
added. The plates were incubated for 5-6 days before proliferation was
assessed using the
sulphurhodamine B (SRB) assay as described by Skehan et al, J Natl Cancer Inst
1990, 82,
pp1107-1112.
Compounds were active in the range 1nM to S~,M. Specific examples axe listed
in Table 2.
Table 2.
Compound L23/P ICSO(nM)L23/R ICso(nM) Rf
TAS-103 16.3 22 1.3
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
53
Doxorubicin 20.3 326.8 16.1
Topotecan 13.6 20.8 1.5
4 14.7 16.8 1.1
3 14.4 19.9 1.4
11 6.1 17.7 2.9
94 5.7 3.8 0.7
68 13.1 44.4 3.4
75 13.0 17.2 1.3
f1 4 12.4 3.1
78 7.6 8.9 1.2
98 9.6 8.8 0.9
The cytotoxicity of the compounds described herein was also measured using the
Jurkat human leukaemia cell line (JL~) and also the amsacrine-resistant Jurkat
human
leukaemia cell line (JL~ and the doxorubicin-resistant Jurkat human leukaemia
cell line
(JLD). The cytotoxicity, as measured by the ICSO (concentration required to
give 50% cell kill)
in the JLA or JLD cell line divided by the cytotoxicity in the JL~ cell line
gives an indication of
the degree to which a compound may be affected by atypical drug resistance and
is termed the
resistance factor (Rf) of the compound. The method used has been described
previously
(Finlay et al, Eur J. Cancer 32A, 708-714, 1996). Compounds were active in the
range 1nM to
S~,M. Specific examples are listed in Table 3.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
54
Table 3.
Compound JL~ ICso(nlVnJLA ICso(nlV~RyJLA~JI~C)JLD ICso(nM)Rf(.nnlJLc)
TAS-103 5.4 302 55.9 384 71.1
Doxorubicin7.0 25.9 3.7 109 15.6
4 19.0 26.6 1.4 22.8 1.2
3 27.0 21.6 0.8 24.3 0.9
2 37 96 2.6 107 2.9
35 28 19 0.7 25 0.9
78 21 14 0.7 17 0.8
81 8.7 9.2 1.1 9.3 1.1
84 4.4 9.8 2.2 7.2 1.6
87 16 16 1.0 17 1.0
94 16 1.8 14 1.6
9.2
98 8.6 18 2.1 14 1.6
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
SS
Compounds were also studied for their ability to stabilize cleavable complexes
in the
presence of either topoisomerase I or II essentially as described previously
(Finlay et al, Eur J.
Cancer 32A, 708-714, 1996). Presence of cleavable complexes was indicated by
an increase
in the number and intensity of bands observed after electrophoresis and
autoradiography. The
results are expressed as the effective concentration range where an increase
in cleavable
complexes was observed relative to controls in the absence of drug. A number
of compounds
described herein were tested using these protocols and compounds showed
poisoning of
topoisomerases I and II in the range 0.01 - 20 ~,M. Specific examples are
listed in Table 4.
Table 4
Effective concentration
range (p.Nl)
Compound Topoisomerase Topoisomerase
I II
TAS-103 0.3-10.0 0.3-10.0
3 0.1-3.0 0.1-3.0
4 0.1-3.0 0.1-3.0
35 0.03-1.0 0.03-1.0
78 0.03-1.0 0.03-1.0
84 0.03-1.0 0.03-1.0
Example 4: Pharmaceutical composition
Tablets, each weighing 0.15 g and containing 25 mg of a compound of the
invention
can be manufactured as follows:
Composition for 10,000 tablets
Compound of the invention (250g)
lactose (800 g)
corn starch (415 g)
talc powder (30 g)
magnesium stearate (5 g)
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
56
The compound of the invention, lactose and half of the corn starch axe mixed.
The
mixture is then forced through a sieve 0.5 mm mesh size. Corn starch (10 g) is
suspended in
warm water (90 ml). The resulting paste is used to granulate the powder. The
granulate is
dried and broken up into small fragments on a sieve of 1.4 mm mesh size. The
remaining
quantity of starch, talc and magnesium stearate is added, carefully mixed and
processed into
tablets.
Example 5: Characterisation of compounds of formula (I)
The compounds prepared in Example 3 were characterised by proton N.M.R.
I O spectroscopy and mass spectrometry. AlI proton NMR was performed at 400
MHz.
Characterisation by mass spectrometry was performed using desorption chemical
ionisation or
electrospray ionisation. The results are set out in the following Table.
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
57
,~ M
Wit'
.-. N yr
~O 00 .-r x r1
vi ~ d: ~--1 N "~ r~i ~
0o M r~ N '-' ~ ~ . WI
.--i N N ~ ~ ~ +~ t~
N x O x, ~ o0
N s~ N ~
"' ~ rte, ~, ~ '-' x x ''~., ° ,~~., t~ v~
N °° t-~' ~ ,'2a, ~ N ~ N ~ ~ ~ ~ O N
tn ~i ,~ ~O x N 'r,Ti' ~; N l~ ~o N
cV ~n of r~ N a d- II N ~o ~ "d ~ d- °
rn Wit;
Vi ~ ,~ N ~ ~ ~-N °' ~ h ~ a~ r, ~ ~' ~. co
~ox~~,~d~'-,
'-'~ t~ 'J N ~ ~D II [~ s-v ~ DD N N v~
N d' ~ "x,
N co N ~ ~ 3 ~ pp ~ xp 0~1, ,.~
M ~ x V'1
x .~ r~.i x ~ ~ r-~i ,--i ~ 00
N M ~ r' t-~i N ~ N ~rj ~i M
~ ~: M ~ N II ~ ~ r~ 'd
'-' ~ ; ~ ~ ~ ~ M ~ d- , pxp ~~~., due- ~p x V'7 ~ ~ .-fl cn
O x W ~ ~ d; ~ 00 d. r'' O ~ \O ~ 00
~ O ~ op r-, " ~ oo ~ ~ v~1 N ~ N
I~ N O ~ ~ ~ ,~~, N d
dx' d"
.-, N ~ p, "d N ~ N r~i ~ x ~ ~'' ~ ~ ,zj N N
Cfl II "C3 .-i ~ O N M \O 'J N ,.~,
x i~ m ~ ~ ''~'~ d' r-, dv ,~ oho '. ~n d0-, ~ ~ ~ ,~ o v '~i
N cn ~-: '~ n r.., N ~ Two co cn
a~ ~. a1 . y ,.-yo
o°,o h vi o0 r-'~ x , ~I, 01 din- N ~,.' ~ ~ ~ ~ O ~ , r~ ~r O
Nx_~~ '-'~~~ *~oo ~ x ~ N N~~~-~N moo
"~dN~ t~i° 't1~,0.-~~N_~ ~ ~ N ~~ ~NO°1,
N o0 rWt ~ ~ ~ ~ ~ ~ ~ '-'
'd: M ~, ~ rv V1 O ~ ~ O1
M r~-~ II ~ l~ N M a ,x I~ r'' ,-a O ~ ~ r~
r: ~ N ~ O N h x, ~ N r.-~ ~ ~ .-~, V7 x ~ x ~i
r-~ ~ ~ ~ ,-, x r~i N
N x N r.~~ 0o s.i ,~ r-' N ~ ~ N r-
O N ono x ~ ~p N N ~ O ~ ~D ~ x O~ N N
00 ~ O ~ ~p '"~, ~ ~ o0
cn ~ ~n ~-, N °° d; ~'' x O~ oo "~'~' ,
x '"' ~ ~ ~j rW1 ~ N O ~ '~ a ~ ~ ~ O ~ ;
r--, x ,-, a1 pp V1 "~C3 ~ ~ ~ ~ N ~ ~ Q1 . C!~ °
N ~i M ~ r~_i l~
"~d~ O ~ ~ O ~ ~ ~ ~ O r.~ ~ O ~ ,~~~ U ~ 00 r~ U ~ W '_'' "C~ ~ l
CJ~ ~ ~ C/~ II ~ Vl ~ h ~ '° C/~ ~ l~ C/W.r ~ ~ n U
O d- ~ oo N ~n M N ~O d- ,~ ''' ~ ~ ~ ~ x '~ ~ d- ~p
Wit; d; ~ oo ~ ° '~J oo vo ~ a~ o ~ O oo "~.'L,' ~ ~ U
~ oo '~ ~ b m ~ ~ 'b ~ N ~ ~ ~--~ ~1 0o t~ '° ~-1 °° '~
o, ~-° '~" -d ~ ~ '-' 'o
v~ .-~ 00
+m.~ ~.,.~.~ M ° ~ o0
M cd cd a1 c~i
m d' ..~-.
+ ~ + U x
a
N
c'~ _cd ~ O~- ~.O O O~ O~ O~ O~ O~
N O N z ~ N O
O
N ~'
N x , N x N x x 'd dx-
U U U N U U U U U
U
i
N M et ~l7 ~O I~ 00 C1
0
z
U
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
58
i +' N
N
( ~ a ~ N ~ O
, x O ~O x d : M
l~ ~ ~ V1 0 ~ N N
0 N ~ ~i '
'
N I I ~ .
d - ~ , ,~ x ~ ~ x r
N N ~ tn
x ~ ~ ~ ~ ~ 01
~O
N O ~ ~ ~ II ~
N x M ,..;n II ~' II ~ ''--~N
t x c N ~' ~i ~ "L~ x,
N o cn
W o "T'
r
~
'
O ~ Do W c5' ~ , "'
...r ' l ~ "~ ,- d
r-, ~ r
O ' C3 u~ '
'
00 x [~ ~ M ~ " ~ M r-~ N ~ op d
N ~ x -c3 N ~ ~,i ~ ~ ~
~ p N -~ ~ c '? ' . o
''~ "~ i
~ _ ~ 23 ~ ~ ~ N N ~ o ~ J N
N 'd p ~C, d, t~
~ Q o ~ oo op
l~ .
'
T3 ~ '~ n x ' ~~ ~ n oo ~, ' ~.y0 r' ~ -~'
~' c II ' ' n O
c ~
n ~ x , ~ ~ N ~ ~ M '. ~ ~ a, -: ~
' ~ t II : N ~ M
N ~ ~ ~ ~t O ~ d ~ v~ ~ M O
. ' w d; 1.
,.~,
00 ~," D1 ~ x . ~ 01
~: ,~ ~
N ~ ' ~ ~ ~ x ~ x x -~ II ,~, ~ '~ M
" '~~ "-~
-.~ o, o
N ~ o
' ' ~ ~ ~ O ~ O ~ y N ~' ~ ~ ~ o
ll ~ ~
'
+ d 1 r O M
~ 00 ~, ~ x ~ .,..; r~ , ~G ~ m N
~ (~ O .r d ,~
OO ~ ~ .O ri
cn ~ r~i ~ ~ p ~' N t' ~ _ w
N ~ ; ~ dV"
~ ~ w,r
N
Op ~ ~ O o r r M ~
p ~ ~ ~ ~ ~ 00 a ~ l~ d
~
~ M ~ p ~ ~ ~ ~' ~ ~
~ V
0
1 0 N s-s
N X00 1 ~~ ~ ~' ~' p ~ N ~ ~~~ d.
c- ' N
01 ~
V0'' N ~p N I~ ,-~ N N F"'~ op o0 r~ ~ ~ M
~ ~ ~ N x r~ N ~
3 d ~ ~ O ~ x
~ ~ x
r~ .-s r~ ' 1 N ~ N O i~
~ T d' c ~ o
~ ~ N " r1 O o O
N '
m N ~ ~ ~ O ~ ~ ~ ~ ~ ~ N ~ ~ r
~'Ci "."' '' t
~ l~ l~
~'
r II F,.,~~ ~ r r.~, ~"'~ r~
x' ''~ ~ II r- a ~, , , r, a-, r
"-' ~ N 'fl c~j ,
O '
' TS
N "d
_ ~ ,.... 'c3 ~-r~ ~ _' '~ N
~ ''xN''~ ~0 +; .,~ c> o~
~ ~'11 ~ ~~ ~~
~ i
~~
_ ~ O , t. p o x ~ oo O
t , , _ m
O ~ N ~
p
+. ~ M ~ ~ N ~ T3 ' N ~ ~ N
l~ ~ o cn ~ ~ V1
o d. ,~ . .-~
~ ~,~ O
x
~ N N ~ N t' ~ N ~ ~ x ~ ~ ~3 ~ ~p ~ ~
~ O oo
a1
n ~ a x N ~ ~ N
~ ~ N N
j
~~ l~ .-;~ ~ r1 ~ N C~ ~ x, va
N v~ N N N O "'~'~ ~ r~_ i-; r~-~
N O _. ., r~'
x ~
x ~Ndx. ~d ~d',~,CV 'r~' MMyO
..~N ~0ldN- ~,' N
~r' M O , N ~ O1
j '-' ~ ~
j
i ~ ''
~ ~ '
"
x' ,b N T3 ~. O O'' ~,'~,-, p~ O
N T3 'd ~ d
,-d 't'J T3 N ~
~ a
' ~
~
~
~r~' "r~,'r~, '"r~rx'x,' x "~x M~~ -~r ~
~ N ~ ~~ ~, ~ l~ r~r
~ ~ ~ N ~ ~ ~ u~~
r-' ~ t~ ~ N ~ U n U ~ ~.1 0 0 U ~-; ~l N U
~ ,-~ U a ~ n ,~ ~ N ..-.
<.r,
~ v'n-,. ~-1 ~ oo ~ cn ,--~ ~ N -: ~ ~ ~
~ 0~ ~ o~ ~ o t~ U
m o ~ 0 U ~d ~.
o "~' o0
t 0o
U t~ oo U ~t U t 0 ~-
oo ~ . ,
a; c
~d ~
_ _ ~
o 0
-~ :~ .
a ~
0 O OO O~p ~
~n r~,0 r~ r~~~ ~ O r
+ + NN
~ ~~ ~~+ x
xM ~U ~~ ~U ~~ ~~ ~ ~~ W~mcNn ~ld~-~
p ~
. ~~ d .
- ~
N N N tY1 N N ~. cn
O
z
N
N N ~ ~ O
x' x, rte, d'
N N N N N N N N U
N U U U U U U U
U
O r-i ~'1 ~ ~-1 ~ ~ r~ e-I
r
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
59
-: zs o ~: d= d=
-: o, ~ x ~ N o0
c,~ '~' ~ ~ '-° x ,.:, ~ .-: II oo ~J o0
a--s ~ ~ t~ N .c~ ' ~ ''' h a,
°~°~ o
II a1 _. ~-, _ ~ ~ °° ~;
d. ~ ~ ~ M ~' CO N N ~ o ~t ,.~,' N y, ~ r_-~i ~ x_,
II ~ ~ N _N "C ~ r~i "~ ~ ~ oo ~ x t~ N
s~ ~t ~ h
~-.~ TJ ~' ~ ~ W o0 ~ ~ ~ I~I ~' l~ 4'1 ~ p ~ OO x ~ .,..~ ~ W.r
II
x ,."L~, ~ °° ~ a II p II cn .,.~ ..~~ ~ ~ ~ ~ "~,' e-v N o0
,~.,~ op ~
N ~ II N ~.. h ,~ ~ co -~ ,~ ~ ~ °~ .-: ,-. ~ r-:
~-rj ~-. ~ 00 ~ .~ cn ~ ~ N x ~ O M
01 l~ .~ ~ I~ ~ ~ ~ ~ ~ '~', ~ ~ O1 ~ ~ ~ ~ r~ r"' N
~ II ~j ~ r~ x ~ ~, O ~-r~ r' ~, ~ ~, ~ ..-~ M
_ O '-' W_ r l~ ~ ~ ~ N 01 O ~ ~ V7
is ~ "~ ~p ~, ~ ~ O ono N ~ ~ ~' M N ~ ~ °° ~ cn ~ o0
d. ~ ~ II o0 pp '.~. ,s~,i' N T3 N (~ d1 ~ O ~.~f (~ N "d ~
~ CO pp ~ .-i t~ ~ II ~ ~ 'L~ ~ s1 "a
'~~' ~ N a' ~ N ~, ~ N ~ x N ~ ~ ~d y 'L3
r,~ a
00 w ~ M .~-' II ~n ~ ~ M "'CS 01 i.; N a '-' x V7 N
N ~ _ ,--~ r.~''., "x, 'T' .-~ ~ ~ x oho cn
a >-v o0 00 r, N ~ ~O ~ ~ ~ O1
N 01 x M ~ M ~ yr M pip x ~ V1 ~ N pp ~ ~ l,~ ~--' e"~ ~ i 1
x oo '~ _ _ t~ t~ M ~ II ~ cn ~ as O
O ~ N ~ ° x II ~ N N oo '' ~ N +.e N N pp ' ~I' t~ p ~O oo ~ N
N co i-W O ~ h N Wit: x N ~ l~ 'r3 '~ ~ N "-~ ~ o~o
II ~ ~ II ~? "yn N ~ ~ ~ ~ N ~ ~ "~ 'cJ ~.-
h ~ ~ ~ p a
~j~ ~~~ ~~ ~_ 01 ~h~'dd'MOO ~ ~ ~~M~~-s
~ d' N N oo ~ pMp l~ x ''w~ ~ "~ ~ ~' ,~I, ~ x O d- ~ ~!1 N V 'r.~,
N l~ x N ~ 'd N O ~ N N ~ ~ wr
N
"' N ~ ~.a ~ ~ '"C3 M ~ 000 O ~ rx'n ~ ~ ~ x, ~ 0p0 r:
''~'~ ~ N l~ a1 ~j ~ N N 01
d; N ~ ~~ ,~, 'n ~ ,x" ~_i ~ x ~ N N ~ ~ ~. p
x ts,~xN x°~ i ,"...,0'~~pxmoo M~"~ isi~ p~'~~
N ~ ~ a l~ ~ M ~ N 'p ~ l~ pp ~ pip ~ . N ~ ,~,~ ~ ~d ~,~~ ~ ,-d
+~~ ~~M~h,~C~. ~ppj~h~~~.~t'cn ~ ~ M~u COWN.rIOx
N ~ ~ ~ -N N ~' ~ i~ N N ~-' ~ N ~ N O N ~n ~O ~ V ~ ~ ~ r~
~ i~ ~ . . N ~ 'd 'd ~ ~V., ~ h
d' ~ ~ . ~ ~-Nr~~ ~ M ~ . ., ~ .--i x ~ ~ . ~ O ~ ~ ., x M M M M N M
" M w cn ~ N cn ~p op N M M cn ~ r. .--. ' 00
d' ~ O .-, ~ O ~ U 'e N wr ~~ ~ ~ .-~ V 00 ~ .-~ ~ N "~~ U ~ ri 00
N eon ~ ~ ~ oo N ~ p t~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ n x o ~ n ~ ~ n ~
d~ oo ~ U ~ II ~ ~ oo II U ~ .,..; N U t~ oo U cn ~ ° U ~f ~ ,~ ~ W--;
o ~ ~ ~ ~ ~
0 0 0
o ~. ~~t~-.~ o oxd.o xoo° ~, +
cn ~ d- + M M m M
+~ ~ ~zMx o x~~' ~~ N x x
N 01 -I- N ~'~" O U N r,., -I- N U N ~'' U P1 x U N x 01 M
O O
O ~ O O, O O
z z z z N w
x ~° x x x x x x
V1 x M ~1 Imn
U U U U U U U U
Cv O r-I N M <t ~ 'O
N N N N N N N
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
N ~
N rx O ~ ,..~~ 'C3
N a>
001 ~ O t~ N ~ x t~ "Ci ~ ~-a
II II m N c~i N ~ ~ ~ II ~ .-:
+-~ ~"' ~ x x N ~'' vp d- ~ x ~p ~ ~ O
V7 x '~ O op -r-~ ~ ~ ~ N ~ ~Lj
Na1~ x ~ N ~°I' ~x jj
h ~h
N ~ N x ~ ~ ~ ~ ~ N ~ O '~ v0 '~ ~' d' oho
~r t~ r.~ 01 t~ "~' x ~ ,.~., ~ x ~ 00
~O N
Oi i~ %~ N 'C r~ 0 ~ ' N due" ~ ~ ~ ~"~ M r~ 'd ~ N
t~ a1 ~ ~ ~ r-"Li ~ ~' N ~ o0 ~ ~' oo ~ N r
II II ,~ x x N
~~~ V7~ v~7~ '~~~.~ cn~' N ~-'~ x0-0 01~'-' ~D
r., .M O N
~ N N0~1~ N°O ,~xx0 N~01 ~0~1.r;~ ~~~ N
N ~ -~ ,~ oo ,'2', ~O oo ~'d r-,
n .-: 'n l~ l~ ~ N °' M ,~ ~ p °~° II '~~' ~ '~ '.'~ +~ o
a; W o ~ ~ a, ~ .~ ~:
vo ~ II II %=. d' x ~~, ~ ~-: x o ~ oo ~ ,-~ Ti
cy ,..~ ~ N ~ o N ~ N +-" ~ "~ ~ ~ ~ ~ II N x ~ wd
-i i x 01 \O ~ ''~ ,~., N
c~n x ~ ~ ~ ,.~, ~ U h ~ ~ N v0 x ~ x M ~. ,-N~ "~ ~ N 01
., N x r~i ~ ~ ~ N ~ "~d ~ ~ due' ~ VN1 N ~ N ~ 00 ~ ~ ~ s~ V~1
II ''~'' II II x ~ ~ ~' II oo ,'I', N %-sr,~ ~-; ,-~ ~'-' ~ N ~n
d- ~ Uys h ~ ,~ h M s-~ x N "~ N h O N W yr +~ V7 ~' N
N oo N 'J +~ ,-cj oo Q..' '-' '"1''' ~ ''-' ~ O op '~' ~ p~ ~ 'r,'L,' 01 v0
N~ °~-a~ x-~~ °~o.~ x~ ooh-:a,~~ll o;~_~,-;
N rte; x N pip x ~'~ ~ N ~ .N~. ~ ~ <j ~ h i1 N ..~ ~ I~ ~-~'
"~' V? ~ ~ ~ x y; ~ ~; TJ oo "~ N °° II '.d 'r? ,~ N ~ ~ N II t
o 'i' O cn ~''? "~~' ~ x ~ O N ,~ ~ ~., II .-s x '-' cn
. ~p x ~ U _pp u~ ~ ~ N ~-, ~~'Z3 x "'~'~~h ~ uTJ
cn ,I~, d; ~ _~ M N ~ 01 '~ tn ~ ~ N N r~ ~r pip' -~ a
N -E-a pp N N ~ d' ~'~' ~' oo ~ r' ~ '~'' ~' x c~~'n ~ r., N 'M,, t~ 'x N
~~ II Mx~~lvox ~ ~~N~~OO~ ~r II N N°°x~,~, x
~' N
~ 'd ~ r-, N O ~ ~ I~ ri7 ,.-i ~ ~' ,-, ..~ ~ ~ 00 01 '~ 41 s1 O V~1 N l\ O \O
'~ O '~ ~.~, ~ ~ ~,; V7 ~ s~ II ~ "~ ,--. ~ h ~ _ ~~,~ .~ ~p v~ 00 t~ ~ ~ 01
II
-,-W d' x ~ ~' ~ O r~i ~ '"' p ~ x x ~ "'.' '~ N '~ N tx ~' .~-v ~
N wr x 00 F.-~ N W ~ "d wr N '~ ~ x r~ x x i-y0 .~.i ~ ~ N N
r~ "d 'r ° N ~2, ,-, ,-Wi +-~ N r, d~ ~ O l0 ~i x'
N ~t"~d~II N 0~01~ Ur~OMr-' ~'W..wp~~"~I~ ~~oo~O M
~ 'WO r-, r., ~ N cn _ ~ cn 't-~ N a
m oo ~ ~ t~ II a ~t oo ~ oy
-.~ x p~ l~ ~ V7 N ~"'~ ~ ,-~ II N r,, ~ II ~ ~O M x N v ~ ~. ~ 01
'd ~ ~d ~ "d a~ oo ~ ~ '"~ ',2,' h O oo N O~ N v-, h
N,r ~ ° N ~ ~ ~b buN ~ ~~Z ~b~°~~O°'"~ 'a,-a "-'
..-r M N ..-, 's ,~ ~ ~ ,.~,' .-~ N ~ ,~ -~S''- M ,~ ~ ,-, ~-rj ~ ,~ ,.-r ,-,
,-, ,-,
i '.r01 u~~r U ~~~ c~ C/~ ~~~ pp O wr~s
U o ,.-~ oo U N U N N ~O y~ U o o co ~ N ~n U ~ ~ ~ U N cn U
(~ o, o o ~ ~r x ~ cn t~ oo a~ ~1 o a\ oo ~,' ~ o <r; a, ~ of N ~ ~ Wit; ~ ,-
mo (~
U M oo ~ U oo a U N ~ oo cn U ~f t~ oo O ~ ~t o0 os U 1l ~i '°c~ h oo U
t~ oo U
o O o ,--i o
i-~ o ~ r~ ~ ~ ~o
cn o M \ ~ M -F~ cn o M '~ ~ ~ M o M
N ~- °O r~ ~ l~ ° Z x N v, ~ r,
U N -~ +oU No_oo U Nx Cj°~ _+Mx Nx cr~~j NxU
~1 ~ ~ W ~ M ~ ~ d' o ~ ~ ~ ~ M U d' ~ ~ ~ ~ C~ ~ ~ ~1
d'
z . z z z z z ~ z z z
x x x x x ~° x x x
x
U U U U U U U U U
00 C1 O ~ N M '~!' V7
N N M M M M M M
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
61
,,-; :-:
i _ ~ N ,~N, N ~ M
~i cn ~ O ~ x x~,
'_-n . ~ ~ co ~_O ~ ~ N ~ +~ O~
'.-yn m II , ~I, oo ~n ~ '~_.'fa'' '~--(i'' . '~' 'E--~ ,-"'C.~' N
p ~ x ~ ,~ O oc7 ''"'~'', '~ .~ ~ -~ ~' oo v~1~
II r., N t~ ~ ~ h r.~.W.'' ~' v-''[~, N l~ "
~1 x N N d. "~ ~ ~ ,.~ V? ~ 'J ~ ,~ ~ ~ .a N ~ N
N ~ ~~ ~~. ooo~ x ~"~ ,--. ,~ o0
II ~ ~'''~~ ~%N ~ N~ DMZ ~O
01 ~, +..~ + ~ '-, ~ .~ t-, N N ~ ~ d- I I O ~ ~ cn O
"~d" 'r~rx~ ~'~N'' ~ ~N~ ~~x~ t~'d ~,"Nry~ M41
U ~, ,--. ~O'.
0o c-n a, x ~ o x Ct- ~ cYi v-i m ~~,~ ~ ~ x o O -.-. o '''x
-a l~
f"' ~~~ N ~ ~ ~ N h ~ ~ ~'N ~ O
~N'' ~ ~ a\ ~ ~ +-~ ~C3 ~ ~ ~ ~ ~ cd x~, ~ ~ O~
O N ~ ~ ~ x x N ~ rte' ~ ~ N ~O r~-~ C<' pp
~t "rT~ r,'Z,''" ~ x x oo ~ ono ~ O M ~ ~' r~ N
pp oo ~ .~~,~ ~ N ~ O ~ ~ Oy 01 d- 01 ~~ ~ '~' ~ ~ M ~ ~ N
o ~ ~-: ,-~: v~ x ~n x 'x oo ~ M ~ oo ~ m x ~ ~ ~ x o ~ o°
'r' N x x t~. ,.~ °1 U r'' II N ~. ~ ~. ~-: U "~' ~ oo ~ U "Z' ,~ oo
[v ~ ~ ~ N ~ 00 ~ ~ ~ pp N N N N 00 ~ 01 i ~
N ate; ~ n ~ ~ N ~ ~ ~ 'a N ° °° ~x.' oo ~ ~ 'o ~ ~
~ x ~ ° x ~ a;
l~ d- ',~, ~,' N ~ r~~" x co ~0 01 h N o0 ~ oo .~ M l~
.'~'~" l~ N ~ ,~ dM'.' o ~ .~ w~ o0 h ~ ~ "t~ ~ O ~; M N ~ p ~ ,.Cj ~Ih TJ
00 ~ d- N ~ ,.-N~ ~--i x p~ +' ''d ~_ p1 ,.fl r~ ,~, o,.~ ~' ~i
''~'r, ~~," N N x I~I :~ c~i ~ ~x ~ II r~n N o ,.~'C,' "'~~' '-'' N x' x, o0
+~ s-s N ' "-i ,~ 'C~ a ~ ~'' ~ '-i ~ cn ~ .-i N ~ r~i l~
N ~ "CJ ~ M O . ,~'., O '~ ,_~, O ~ c<1 ~ ~ ~ ~ r~ M ~ p N O
'r' oo "u ~ x a ~ o x ~ ~r ~: ~ ~ ~ ~ ~ c.,~, ~ x o 00 ,~ ~ ~ oo '.' 00
~O N N N dy-. ~ ~ U .-~ O ~ ,x" ~ cV op a1 ~ U ~.~,' ~ M
1 ~i N ~ x ~ Z vi N r~i ~ p N N ~ N ~fl ~ ~ ~ ~ ~ p '~ v~ O
II
~_n ,,~ N ~ ~ N ~ oo x d~ ~ x '~ ~..~ 01 ~ x O ~i Wn
~d=T~ N a~p~pO~~'d~~~ v"~~ Q''''~"i~,"~~~p~V1~~TS
~ ~ ~ ~ o ~ ~ 'P' r'' o x ~ ~
_ ~ 'J ill" N oo M
o .~~~ ~ ~ ~ ~ ~ N '.tea " N ~ ~ .~ ~ ono N +; ~ x ~ N ~,; ~ c j
.-~ O x O ~ ..~j ~ ~ O a N .,~ 'd ,xi" . ~ I~ M p~ 0 l0
~ l~
N C/1 ..fl r_.'~ x v7 01 ~ ~ r, m ~ x o0
U N ~ ~ 'r' ~ M x x 'r '-' o0 '"~ ,--~ ~' ~ N
oo ~...o U U ~ U a
r~ N r~ O ~ ~ ~.~ ~ ~ u~
00~rvo~O ' M~~~U~x~~:~~uo,c~Y,,o~~~~x~ o'NO.U d
l~ 00 .-, ~ o0 'd ~ o0 ~~ ~ CO T3 W N.s U C~ 0o ,~ ~ .~- t\ 'd t~ 0o V f~ ~ h
0
O
~ o
t~ M ~ . ~
+-' x + t~~ r.~,cno~,~~ -~ ~~ ,.~.,01
+\+ Z~ Z+ ~~+_+M+ x Z+ ~
~ oo ~ U n U x H N ~ U ~ ~ ~ ~ U x U N ~
'.. r-, ~ d' ~ ~ U ~ a ~ W.~ a M ~ -~-. ~
m d-
z o ~ ~ z z z M z z
d1 ~ ~ N ~ ~ O ~ N m ~ c~1 N
""~~' '~~' "'"L' O m cxn
U U U ~ U U U U U
U U
M M M M r1 '~' er d' d' d'
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
62
~ r-~~ ~ _ _ N N
U~ zx o °°o ~x ~ ,~N,''' U
~ "O .~ ~ ~ ~n t' ~ ~ U ~ a"
N N II
rp ~ ~ ~ . ~' pNp ~ rv ~
N ~ ~ oo w'-~.r y ~ +~ Oi ~ ~ Q, 'F.Zi ~ oo ~.r
O M ~ ~ N O M O N ~ 00
01 CO ~ a x _ N ,-i ~ ~ N ~r N ~r '~' ~ 'd M x t~
M Wn l~ tn ~ ~ ~.., p ~ O i
e~ 'r~' ~' ~ '~',~ ONO II N O h pp ~ pp "a d' "~,~' m ~ O
O
M ~ ~ ,..1 ~ h Cp ~ ~ _N x -CS 00
N '.'' z3. din- U O ;.fl N v~1 (, 'Ci _
TJ
U'~,~'~ ~N ~h 'ON ~x oo ~ co.~ U~ k
N ~., U _~O.~j~ _ ~ ~~ ~~ Nr-,~~
boo ~~z~~°~~ ~'~ x o ~Z
o .~; o
O _ ~ V1 00 pp ~ _ M ~ ~ ~--~ ~ 01 pp 'd
M ~ x m ~ ~n N M O ~ oo ~ t ~ N x N
cn 't
y' N r~i O ,.fl N ~ ~ ,-, ~ V7 N
~~ O V1%'~ ~~ ~'~ ~~ M~ N'~00 <t
p 'N +'~ ,.N~,~-'~~~~~~ ~ s-s 00'~ lax' t~~ x~0oo
U ~i U
~ v~~ ~ 'r~ x oo ~ ~ Ov N r~-, N ~ N ~' N ~ x O x
n M ~ ~ ~ N a1 ~ ~n
N ~ N ~ ~ ~. ~ 01 ~ ~ ~s ~ ~O
O pp x ~' d. ~ N N 'C3 ~ ~, O 'd .
r~ "C~ t~11 00 "'~~ i~ M ~.' ~ ~ ~ 'G ~ .~ (~ ~ 00
~"' s~ x ~ s~ 'd ~ ~.i O N r~ ~i
-~, N x ~ N x + ~ ~ N ~," ~ ~,~ + ~ ~ +.~ <j- r.~," ~ y
00 ~ ~ 'W ..~ t-~ cCi r' ~ M t~ ~i O N ~ ~ ~'n O w.r
II M +~ 'C3 M ~n N O 'n 'J ~ cn '-' ~ ~ ~ CO ,..~ a x x ''~ ~' o\
0o M ~ a~ N II oo N in ~ oo M O z U Os
2'3 U '~' ~ U x oo ~ oo °~ ~ "-' "' ~ cn
oo .~j oo Q ~ .~-; N ,-:
~n s~ N ,~ I~ ~ cn M Tf U ~
N V7 OVO N ~ ~.i s~ N ~ ~ i~ W t ~ ~ ~ ~ vi ~ ~i ~
01 O ~ l~ ~ N r~~
N ~'' a ~ ,-, a tx two
'° v0 .-; v0 ~ '~ 'x~° N x ,~,~ d- ,-: ~ x p ~ ~ x ~ ~
'~° .-: ,.'~~ 00
v~ ~ ~ .~ cn N O i-~ ~ a1
M ~, N ~. O p O pp ~, O d' ~ 00
~ d"'r-~- o o~po O N ~ M ~ ~a cn ~ N r-~~ ~ N ~ ~
QN.~j~t~ ~N~t- ~ 3 ~ v "~d M~''~'m~~~O
M x rn '-' x cn ~ ~ cu ~ '~.-!i, x' '-n 'x, '--G' .-' O y-1 ''~' a~
~Cj ~ ~ U _ ~_ ,~, 00 .~ ,-, ~, ~ 00 ._, M _~ a M _~ ~ U 00 U
v a l~ 00 ~r t~ "d l~ 0o U a ,-D v..s o0 U N o0 U N o0 ~ '° t~ '-, ~ l~
~x x x~~
U + U x ~, N U N o0 o U N o U ~ U "~ N
U ~ ~ ~ f~ ~ ~ ° ~ ~ ~ ° ~ C~ ~
'r' O O p d- "' - ~ O . O M
O ~ l0 d' M M M V1 ~ O
N N N N ~ ~ ~-~- ~ dw- ~ M N cZn
x cry x ~ x x O x O
M N N N ~' d' d- N N N
U U U U U U U U U U
\p I~ 00 C1 O r~ N M 'et'
~t 'd' ~f' et W V7 u7 rn V7 V7
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
63
M
-N N ~ .-, 'd ~ M
~~ N a' ~? ~ x '~' N
ai N ~ U ~-; e~ x ~ x co '~ ~o ~~ ~ ,-
o~~ x~°~,y x~ ~~ ~~ o~:
~ rp -~-~ tn ~ oo +-~ ~ TS N ~ N \O N ~ N o0
01 N '"' '~'~ ,.~' ,-r O ~ ~ O r~i N ~ ~ O\ lp ~ x ~ ~n 'r~
'-' N ~ ~ ~ ~ N ~ 'x ,~, d: ~ ~ ~' '-' v~1 ~ O~ x' r~
~O~O p~r~ ~~ x"C7 0~0~'C3x
yt o ~ N N .~.j a> ~ ~ t~ .~ ,--~ ,.~ ~.,.~ ~ ~ m ~p o0
N oo ..-. t'~ x ~ ~ rx ~ ~ oo ° ~ M x II ~ d: v~
N ri ,.-1 ~' ~ U 1-4 Q N ~.,' ~-I ~ 00 '~' ~ .~''~,~ ..fl 00 O
~ 'd Q1 N N ~..,-~ ~ '"' ~ M ~ ~ d' N ~ _ M ,-,
M F.~' ~ W ~ N o0 r~ N ~ ~ ° ~ .-s O ~_ _r'' rx N \p
w W.r 4'7 pp v..y ~ ~ M ~ x ~I ~ ~, ~ ~° 00
.' ~ ° 00 01 00 ~ ~ _N oo N O N O h ~i N M O~ '~ r~
,~, ~ l0 M II ~ M O ~ ..fl ~ c0 ~ ~ +.; "C~ _M x ~ O ~ N "L~
~,' 00 ~ ~ '~ M ~ ~ ~i ~ h
'd M ~ O x x O Q1 ~ 00 00
~N r~'r~,"x~ N~~ ~~ Nr'' dv If ~ '~ ~r~
-s ~ '-~ ~ ~ ,-~
N,-,"d ~o~o~o~orsM r~'i ~O ~O N~x~ NoNo ~~i N~rx~o°o
r, V1 '~ ~ Op
n ~ ~ ~ N oo ~ ~ ~ U N ,~I, x ~ N ~ ~ x a ~ M
~ N f.~, ~ +.~ ~ ~ ~~ ~ 00 V7 ~_.i p ~ ~ N d- 01
"~c~~y'J N N~i~'..~ x~ x~ N oo~ ~G~ ooh a1 N~o N
N ~ ~ "~ x ~ ~ ,~ II c~ ~ ' M x ~ d-
00 ,~, I~ l0 cn N x " O ~- ~ h W~ 'd
m d1 ~ ~ ° ~ "~ 00 x M +-~ V7 ~ ~ 00
,.-. d- ~ ~ II ~ ~,j ~ N ,~, N ~ II x ~ ~ M '~ j~ '~ "~~' ,-,
c~n ~ 00 ~ ~' 'd II ~ '-' ~ ~ ~' v ~ ~ ~ ~ ~ h y.r
v~ ,~.~" ,~ ~ N "d
M .,,; yn ~ ~ l~ ~ ~ N ~ ~ ~ ~ ~ d1
WM..r N x "'.' x '~ ~ O x ~ ~ ~ V7
M W ~ ~ 'r ~ 'l~ ~ U x ~ ~ ~ 00 M lp O I~ M ~, O1
~ M N ~ ~ ~ ,-, ~ ~ ~r ~ i ~ ~ ry
N I~ O ~ ~ _'"~~ O N ~r rs N ~. ~ ~ O v~~ ~_ ~ ~. O1 'r-~ ~ +~-~
m r~ ~ ~ ~ ~ ~ N M ~~ vi ~ N ~ 00 ~ ~ ~ rx ~ ~ y"~ I~ , N ~ ,.~~, ",p
.~N N~~ ~~ ~~,~oox x~~ .r~~ ~ ~,~~~.~ x N N ~ N ~ ~'
~ ~D ~ ~ N ~i ~O ~s x
n ~ ~ ~ v, ~ ~ M ~ x ~ o ~r a x n °~ ~
N ~; ~ '~' ~ U ~ ~ ~ mo cYi t~ ~ U '° co os ~ °° N
d; ~ p ~' x o d; ,-. ~ ~ II t~ M II ~ ~ ~-1 N II II '~~- jj
O ~ d~ ~ N h '~ M ~' ~ N 'd ~ U '._' O N '~ ~ U "'~ ~'' ~, Q ~ O
''"L'~ l~ r.., -r-~ "t7 N ~ r~n ~ °~p ~ T3 ~ (\ "d x ~. r~n b "C N ~
C/~ x "'
M 0 ,~ t~ . x ~ . ., r-, ~ ~ '~ ~p x r~' ~ M ~ ~ O ~.>~ cn N x ~ ~ M r~ ~ n
.~ ~'C3M.-M,~~~.M-~oo~U"~ ~v..r'-' ~'~~,~o~~~~a~~wU
~: U ~ ~n U ~Wo ~1 M ~ o 0o U co . ~n '-, o~ U ~n . 00
t~id't~N ~010~Od;UOx ~,~N ~r~.-i\Or~~ON,-,~Y(~M~41
a ~ Wo U I~ o; U o0 0, ... t~ ~ d- ~t oo U i ,~ "w.~s i h d- N l~ oo U oo ~ I~
~
-. .-. vo o ,--. .-. Wit- o
N M ~ M ° M O~ M due' o ~ dN,, d'
,~, ,n o ~ o0 00 ~ ~ ~ ~ O
+ ~+ zx ~°~z+ °~~ ~~~sH++o°~
Q o o '~ ~- 0 0
z z z, z z z w U z
,N~, N N NQ NO N~ cT
x x x x_
N m x N N N ~ M x N N
U U U U U U U U U U U
\o l~ 00 C1 O r1 N M et In ~D
~f7 1n N V'7 ~D ~D ~D ~O ~D \G ~C
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
64
N
N M ~d: Zj ~ N W.r~
a
d' 01 ~ ~ s-s ~ ~ ~ ~-~" ,~N, x l~ ~ d'
y N 'd ~O M r.~ O v0 vi ~ N o0
00 ~~., x ,~ ~ 00 ~ N O N x O\ rv
M ~ c~ ~ N ~, "~-'" ~ op ~ "~,'~'' ~ ~ N ..~ N ~ pp 'd
M m
~r ~ .~ ~ 'd ~ p r' j ~ ~ II ~:
~o, ~° ~x ~°oo~ ~N°' 'd~
m ~, ~, ~-:,,I~, ~ ~: x ~ V dy ~ ,.~s t\ yo x o0
0 0 ~ ~' ~ N ~ x °~
. O M ~ ~ ~ -d ._~ ,~ v, t~ ~:
M cO ~ t~ i x III N oo ~d ~ ~p x N N ~ 00
h d0-. N ~ ~ r~i 01 ~'' co x' N \O d. l\ x' s~ 00
N N '~ ~'" '~ a1 ,-~ '~ II " II oo .-: ~ d.
r.~i r~ ~ 00 N ~O x ,-, V7 ~' ,_, N ~--a ~ ~ ~ r., -N
m dx. O ,~2', ~ ~ ~i ,--~ oo ~ N ''"' O x "~ N x ~ "d
O a~ ~ ~ N ~ ,--~ ~ II ~. ~ '-' a; M .~ x M ~ cd m ~
O dx' 'd r.~N ~ ~ ~' ,x CV ~ ~ .
M ~~ TS ~ 01 ai "~ ~ ~ ~, '~ ~ "d ~p II
O N r~_ r~ ~ ~ O ~' O1 N ~ ~ O1 '~ I~ ~ N x ~ dyn r-~' 'd
~ ,.O ~ N wr ,..0
0 ~ O '° M ~ ~ ,~, O ~ ~ 00 ~ ,-i ''~~ ,.1 .,~ II '-' xi ~ II l~ rx
~ \O ON0 0 ~ x ~ \O ~ .-i ~ ~ ~ ~ 0 ~' ~ ~ rx ~'
00 '~ II ~ ~ "~ j o0 00 ~ N l~ N ~ ~ Wo °° ~ ~ ''' 'r x
r~ "L3 .~~~,P ~ ~ 000 M ~ ~ ~ ~ N ~ ,~ N ~ a _ N 001 ~ r~ 0~1 N
01 ~ N ~ M Or~O ~ ~ M 'C3 d: ~ .-~~ ..a I' ~ O~O M \O ~ 00 Q1
xa,_N~ ~ ~x_~
~ II M Wit; a ;.° d. ~ ,~ ~ o ~ x ~ N wo ~ II _
N Nl~~ ~01..fl~~oOr~ O~~rj +-e~~"~0~1~ ~O~ ~N.~'~j~'dtn
~ I~ N ~ x 01 ~ ~ ~ N d- ~ N ,x
M lp ~ V1 ~ ~ '~ O ri ~ ~ ~ ,_, O ~ V1 (~ h ~ 0 ..~ ~ M
~ r~i l' ~ ~ ~ ~ ~ ~ pp ~ 'd V ~ ~ N s..; ~-~j
pp ~ ~ O V7 W n ~' ~'' II x', ~ p M ,-, r'' ' '-~ ~: ~r.~i ~n II ~n O
., 01 N oy I I ~ o O r° ~ ~p .~ ,,.~ ,,~ I I x ~ M ~ ~ ''. ' a~ a1 ~-,
~ ~t .~ oo ,~ ~n ~ o '~' +' d''~., ",~', °° ~' r., ~' U ,-, N ~
oo '~ 00 0
rte, N O s"' r'' ~ O ~ . . 'd ' ~ °~ +" x '~ "~d
'-' ~ x N N ~ N N M w'~.r M d ~ M ~ Z l~ N x (~ N '~-~G _.-a
N ~ ~ Wr x ~' d1 00 .-1 ~ ~ O ".' ~ M ~ ~.r ~ ~ M a 01 ~i ~'
,~ oo ~ o N ,~ U x ~ oo ..~ ~. p ~ o ..-, ~n oo v, U ~
M ~ Ov ~ N x ~ ~ ~ x ~ ~ .d- ~ ~ x ~ ~ ~ ~ x ~ ~ N l~ N ~ ~n
.~: o r ~, ,-O .-. h ~' O ~"~ N i-.., ~ ~n ~ oo ~ U ~' N "'-' U ~ "-~ '''
r-, ~ r~n 'C3 ~ .~ TJ ~~-, ~ ~ p O +-~ ~' N N d' II 'd ~ ,-W T3 ~ ~ 'T3 N o0
x~ocr;xx Nx~x ~~x~U'xxx~~,~ Nxxhx N~~~
~r ~' U ~ ~ ~ ~ l ~ . ~ N ~ ~, M ~ ~ V ~ '~ ~,.j ~ a ~ ~ ~ ~ ~ 01
O V1 M O o0 I t~ O ~ d' O~ O ~O ~n t~ V~ o0 N t~ N
d: N ~ .-i V7 ~ M l~ I~ ~ ~ d1 O1 ~ r--~ M ~ ~ <t' ~ ~ ~ ~ O \O 01 ~ d' I~ 41
~,O
co wr U ~' o0 d' N t~ oo O "Cj l~ M "d oo V'i ~ d- i h d' ~ d- t~ oo d- N l~
oo w.s 'G
M
o
M o M ~ dM- ~' M -I-~ M ~ o -I-
o ~ i~ ~' ~ o ~
\ -I- ~ o ~ +
U N x U N x o N U a~ o~ o U
~ M a
0 0 o z o , o z o
dZ- o dZ- U d" ~p ~ U N oZo
N O N , N N O N
N N N N N N N N
U U U U U U U U
r ~ r ~ r
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
'o ~ _ . o '~ II
H
~ M QQ a"' 01 ~ ~ h _
r1
v~ p r-~i N o0 ,~i d' ,.~i ~-WO
dW-,
''~' '".' p .-~ N °~ ~ III ~ ~o ,..'L,~ ~ x, ~ '"'' M
CO ~ ~' pp +; ~' ~-, (~ h (~ h '--i ~ .--i ~ ~ ~ t~1
~.o ~ O ~', M ~-. 'l~ x ~ 's "d w ~~j x N ~ p1
0o N N ~ d~'. r~' °~ N '~'' "'a _.~ ~ O "d O a ~ O
I I
N x ~ ~ a1 ~ ~ "~Cf ~ N ,~'~, O ~~ ~O ~ r-~ ,~ ~ d'
N ,-i d. ~1, t-, ~ r1 ~ II O ~ ~O ~ x ~ x ~ ~ ~ N %'~ ~ 'd
M f"' ~ N N I I ~ 'r
h 00 ,..p I~ l~ ~, 'C3 ~ O ' ~ pNp "~C~ O ~'' "-' i~ x 01~ r~
'd V1 ~ H ~ N ~f1 ~' M
~' bhp x N +-~ ~ ~_' ~ ~ ~ ~ x ~_ x _ r'~ a1 ~ N
~_i ~ ~ ~ x ,.~ ~ ~ "~ N x ~n O ~ ~n ~ co ~ ~ ~ dp'.'
00 ~ N N ~ a1 0o O ~i o0
wi V ~ ~' ~ N x ~ ~ N h N x M ~"_' ~ ~ d. N
a i
"d ~ ~"~ N ~ x ~ ~ ~ f"' r-~f ~ ~ 'd O ~ O .-~" r~ ~' l~
00 I\ ~' .-.~ a t' M ~ O 'd 't3 "C3 l~ l~ a °~ M
tx ~ _ ~ ~p ~ ~ N ~ ,~i ~ ~ x o0 ~ x ~p x d. 00
o, ~ ~, N o, ~ ~p ~ ; ~ r~ x ~--~ x r: x ~-: '., °° a I I
~ ~ M ~ ~
r-~ ,.~ ~' ~ ~ ~ N l~ oo ~ ~ V7 ~ ~n I~
N t~ N ~O "d M ''~ N t~ 00 00 ~ . ~n ~O ~ oo d-
~ ~ "d 00 ,r~~" "'~ ~ rv ~ M x ~ N ~ N N
,_, ~ f-, N ,-m-, ~,' ~ N ~ N " ~ x ~ 't~ '"''
0o c~ r~ .-
~e ~_~ a d. ~ 's ~ ~ N _ .9 "C~
'~ x ~ ~ ~ ,~ ~ 'r ~ ~ V? o ~ ~ ~
M Wo wo ,.-. o, ~ ,...,
'~ "',hi zj °° ,~,~ o~ O ~ '~ ~ I ~ 'Wo ~n M 'd M N ~ M o0
"CS yr 'd o0 a ~ t~ ~ N N '~~' ~' ~ x N rte., N x ~ N v'i N
-a ~ M ~ '"' ~ n I I ~ :-: s=.
'~ ° H II oo '~ N ''d ~ '--. ~ ~n yt
~ r' ''' ~ N rx oo x ~, N ~ ~ o0 N x ~ o0 '..~ N r~ o0 ,ti °,~ O O
00 ~ 01 '~ ~,' y,r 'd ~ I~ ~ ~ xt ws 'd
Ui _
'~''~,-~~~~~N~~O Nxx~~x ~ °~'-'x~°o.~ x°° II
°~.-.
r-, r.~ ~ II cn '~"' .--i r~ Z ~ '~ n ~ II ~' Ct
w'd ~~~~~ ~Uj N ~O r-, ~ ~~~ ~ p ~ M H h'~ N r~
'x, x ~ O ~M'' ~' ~ t~ N M ~-i ~ d= ~ 'W'' ~ N M p ~ m l~ ~ '-' ~ 'd
'~ V '~ 'Y' d: U N ~ N ~ U ~n ~ oo '~.~'~ U ~: o a ~ '~' ~l oo x U oo ~ x II
M 'o II ~ ~1 ~ .-. U oo ~ U ~ ~ :-:
0 0 °. N ~ U ~: ~' ~ ~ ~ II d- ~ ~ U ~ ~ M ~1 n c~ d 'n U ,~ x ,~ ,~
oN,,
rx ~ N\O'C3 tn'CJU,~'~~ ~"'aO N ~al~~Wo ~~~ '~txt~t~"t~
"~r~-~t~ Nr~t~.~t~i x~ Nx~_ x N~Ms-;"~i
cn N M ~ ~ ~ r~ ~ r~ H '--~ _cn
Q1 'r w.s 's ~ ~ ~ N O ~ ..~-~ '~ a y.s 'd l~ ~ ,.~, ~"° U
trj O ~ N 01 O ' M O N r~, O O V7 d1 ~ \O
~x~'-~~n~o,.~MO~°o~.-~~;voo~a,~~ooo,~°oox °oo Noa,~
~r~U"C3d'~'C3I~d"N~t'~d'oo,-i~Nh~d'l~~d'oo,~ i d'~t~iooNU
~
tn
'1- -I- ~ ~ ° ~ '~' o 'a-.y' o N 'a-.i' d~
z ~ x ~ ~~ z~- z~
-I' _F' ~ M -I- M ~ ~ .'~. ~ + yr c _F'
~1 ~ ~ ~ ~ ° °
M N N N M M M N
O U U ' 0 U U O O
~~z, z z ~ z
x x x x x x x x
M a1 M M N N M M
N N N N N N N N
U U U U U U U U
~f7 ~O I~ 00 01 O r1 N
l~ r t~ I~ l~ 00 GO o0
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
66
~ N ~ II ~ ~ ~ ~ x,~ r-,
N 'O x N
p ; ' ~ M ~ ~ 'fl
1 Z' TS
~n '~ ~ (
N
,. ~, x _~ ,x
, s~ i~ r~, ~.,..~ ~
- ~ ~ ~ "d
''" ~--~ . ~
-'
r-,
h 11 -d ~ ~ O ,- d. ~"' x N
~ " ~ x ~
r, , , ~ C , r~ 'r
,. ,~ y.~, N r , .,~
~ N .I~ ~ ~ M 0o ,-~
.~ O
~ r _
M ~"' W
"'' ~ 0
x V
_ pp - a o T, O ~ a
~ W 00
d
.
t
1
I~ "C3 0 01 M ~ [w _
~ ~ N p
r~ ~ 01 o0
~O "~d ~ r ~ ~.,
rx ~ s-s ~ ~' ~ b '~
~W-' O
N
"y
r
'.'~ ~ ~ ,. x ~ ~ ~ ~
~i ~ O ~
~ ~ ~
r ~ x x ,~, r~.~
~ op r 7 ~ r ~ M N ~ M
o ,-~ ,~ ,~ ~ 01 Z3
0 "x t~ W _ N a3 ~.
yr d. ~ ~D
N ,-m n O~ op N ~ ~ ~'n , ~ ~O ~ ~ N N V ~O x
~ , ~ p1
M
~
-a
cn ~ ~ op N ~O ~ O oo W-, '~ ~ N h ~
N ~ ~ O
x ,-~
oo
N p N
~' II "~ 00 .~ ~ I\
: N
s~ x ~- y d ~ ~ ~ "CS k N
x' ~ ~ ~i ~
'-' d1 'd ~ ~ 00 r~ V1 " ' ~' n
II 00 N N x "~ r~
~ x '
x
II
x o x
M
t\ 'p oo x ~ ~p l~ oo N ~O t~ O
~ ~ 0 d
~ '
r'' ~ r~, '~ II ~ II M _ ~ t~ , O
-i t~ ~ ~ oo jj 01
x, N
~~ + ~ ~ ~ m N ~' '~.'
o ~ o d
a
t~ ~ oo . ,- x ~ -d
N II N
~
~
~ xr~
N ~ x ~' N x TJ 1~ l~ M ~ ux
~0
~ ~ r.~C, "~"r 0 N N ~ N ~ ~ AIL ~ ~
"CJ x ~ N o0
x
M ~ x ~ ~ dN. o0 ~
c~n r~ d: '
~D
1
D1 N N N pp ~ ~ ~, ~ 's
01 M
0 M N
~ i~ ~ x ~ t~ r~
-~ ~ I~ '~ -r N ~ C ~ ~ l~ ~ ~ pxp r..
~ ~ "d t~ ~ '
N ~
" '~
a
~ ~ . M ~~ ~ O~ ~
~p ~x 'n ~ x .
~ "~ r
~
w
~p
,xI ~ x, NW N ~~M ~ d
x W ~ II ..
~ ~i n
a dv r"' ~ ~ t~ in r"' r l0
~ ~ ''~ ~ ~ v co
i N j ~' M
O N M ~ II v? ,-: O ~~,-t ~ M
p N tn ~n ~ ~ ~ ~ ~i
. ~ ~
N
00
~ h M 00 ~ ~ ~ W ~ ~
~ t '~ ~ ,-~
v~
r h +~
~ _ ~
N~ mx~x ~ U''~' N x V7~"~d U"dO~ ~d" M M
N ~ M N~
x
II U ~ ~ '' ~ ~ "~' O . ~' ~i ~' ~- ~'.'
',~,' O ri O -, ~ ~ .-. , U
N ~ r U U d: '''
~O 01 ~. ~ ~ d;
l~ i U
01 ~ ~
,_, -i N ~, ~ ,- U "~~'' ~ N o0
. U M II t ~ c'? ~ h x ~-1
~ II d' n co II II O N
II ,~ ~ ~ ~ ~.
~ o
.-;
--:
:~ y U ,.. " N ,~ U U
, _
U
r.~ N x ~ r~ ~ ~' "C3 N r-~i M ~ ~ x ~ ~ N
x ~ x x x cn r~i
~ r~
M M ,--~ ~ N +~ ~ ~ N ~ oo C ~
'~ 'd N .-, ~ N II cn d-
,-, N N
o x x .~ a ..mn U M h
~. ' ~
N N ~ N M O ~ '
~ ~ 0 ~i N
n
N Q V p' tn x ~ o r ~ O O
-i O~ .-i r N O T r.~ ~
r 1 d: ~ O O
a ~ ~r -~ d' d- .-. d- d- ~ ~ o; U .-, d- d-
...~ ~ ~--. ~ .~ ~ ~ t~ ,-. ~r ,-, .-. N
oo ~ oo
~
p M ~ O~
~'
M ~ M M x d: -j- M M M
x ~ ~ O ~ N x
~ x~, z x
~
~ ~ o ~ ~ ~ ~~
o ~ + x
U x o N ; o U ~ x o, ~ U U
o ~ U ~
l~ ~
M M M N M N M N
d- dw t d- dw n ~ d-
~ N ~ Z Z Z
d c d d
" n - -
M d- N ~n d- N d- d'
N N N N N N N N
U U U U U U U U
M '~t ~ ~D l~ 00 C1 O
00 00 00 00 00 00 OO G1
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
67
y~~~ , N ,
.-a x ~n
pip ~ ~ pp 00 ~ M ~ 00
!l _~ ~ '
~
_ ~ ~ 1l
~ ~ ~
~
N ' r~ TS N " sy
x cat ,--~ "~J '~ ~ cn
cd '~
,
,
~ N ~ ~ ~ N Ov ~ ~ ~ ~ t~ vi
~ o .i~ o
- o ~ ~ h ~ ~ 00 x ~ o I
, N ~ ~ ~
M ~ 1
t~ ~; 00 . ~"dv ~~ 01'_''-C) 01 O
ue"
p d d: ~ ~ 01 M ~ ~ l0 Op
~ 00 00
~ o x ~ N
l~ x ~ ~ ~ x O ~ ~ N ~,' ~ ~
~
d' O ~ r~ ~ o0 v O"
M .--r ~, -.~-~x ~ rv o0 ri ~ ~ ~ t~ N ~ ~
N N
, l!7
x ~ ~
n n
M ~ O t' ~ N O ,'2,' ~ ~ ~ N ~ '"'' d-
~ N . h
~. pMp O ~ N ~ ~ jj ~ o
~' ~ jj o
O ~; Ov ->~ ~ ~ "C~ h ~ N "C3 r~
~ ~
r_ . t
N , G "~' ~ ''~ ~ O ~ '~' o0
~ ~ '~'' r~
~
~ ' d: ' ~ x ,
~ ,
r N ~ N r D1 ~ l~ -~
' ~ N oo d\ yi
N ~ ~ c~ M ~ ~
O
~ r~ t~ ~ ~ ~ ~' ~n
~ ~ co o0 l~ o0
~ 'd ~
d: ~ ~ ~.,~ N O~ O t
M M x ~ ~ l~ ~ x N N ~~ N
N N ~ N N
~ ~ M ~ O ~ ~ x ~ N
~ x
~ lW ~O ~ N x
.r d' ,~ 00
M ~ M ~ ~ ~ 41 ~ 00 lp
~
~ "T' '~' '~' 'C3 'd "C7 . ~
~ ~a ~
r ,. l~ N N c,i ~ ~ ~t
., _ ~ ~ ~ x x ~ ~i ~i ~ ~
W x
N " ~ ~'', 0 l r N ~., N
~ M ~ 1 ' O O ~ ~-' x ~ ~ ~ '
~ ~ 00 t~ I~ O O
~' W
N O ~n x [ ..., II M ~ ~ II
. x --~ y N ~ oo ,..
j '.r v~ O O ~n ~
~ ~ ~ ~ M
M 00 M ~ ~ ~ V1 I~ 00 II II ~ N d: ~
~ ~
~ oo ~ Q1 . ~ l~ ~ ~' b O l~ oo ~ ,~ "
V'1 ~ ~d oo oo O ' O
N -~'
oo x o M N N x ~ N N F,
l N N
~C
~ ~ ,,. ~ O M
N ~ ~ , M
n x M x
h ~
in ,-~ o o O v 0 O ~ N ~O ~n x ~ N ~--
t~ ~ o 1 '-' U t~ ~ N Oi d ~ N
~ ' ~ O o ~ ~ ~ 0 of ~ -. ,~ ~ ~ U
U o U ' M ~ U ''-' '
o ~
a
N~,. ~ o ~N ~' o . n . ~N
,~.~ ~M ~ ~N II N~ II , ~-1M
: : II ~N~ II II ~ ~
~
~ U ~: ~ , U h ~ U " ' '"'' U U
-, . .- ~ ~ ~ ~ 'd s~
~ ~ ~ ~d s
~d ~
-a ~
~M+-' xr~r~ir~~r~ix r~~r~ ~r~~ir~ ~ Nr~-ir~~iN
d- t~ -~ v~ ~ ~ M ~ ~: ~ M yo
os O -, -, ~ xr~~r~ir~ ~ ~
- ~ ., V
-.
-~ k
~
, . . U , as
O , , r N O ~ N , d' V1 O
~ ~i t~ 0 N ~' ~ N
V 0 ~n ~ ~ 01
N
N W C yn 1 (~ d' O p
0o O~ 1 N O d' N ~ ~n ~O 01 O
01 ,x I1 lmn 1 v1 r O d:
O a1 O M .-a 01
00 00 N ~
.-y~ p d' O ~i' U C~l d' N d- ~O d- N d'
oo r7-a d' N I~ oo N l~ d' co oo O l~ N
l~ oo oo 01
N N
M M M M ~ + ~ ~ M M 0 M
~ ~
d',~~,, d'~ d'+ .+ _h ~\ ~d'~\ ~~ d'
a~ U a~ U a~ \ ~ ~n U a~ o U a~ U
~ ~ ~ o N
o
l~ ~ W ~ W ap- ~ ~ ~ ~ ~ ~ G~
~ ~ ~
N
O O O O O O
z
N
N N M N N N N
U U U U U U U
.-~ N M ~t ~ ~o t~
CA 02392873 2002-05-28
WO 01/46157 PCT/GB00/04609
68
N
_ ~ ~
r-~ 01 ~ N
WO N O
,-- ~ r~ M ~
'-' ~ " O
i-:
r-, d' i ~ 01
N ~
_ _
l ~ M ,~n I~ x,~ [~ ~, ~
d. ~, 00
QOO ~ ,-~~~ ~'C~~
~~'M
l~ ~, "L3 ~ t~ ~O
N x
a>
~' N ~
"
~ a ~i ~ ~0 00
-, ~O a ~ ~.
~n ~ ~ N ~
N ~ N
00
00 000~~ ~~ N ~
~' a
N x ~ ~O ~ rx
o0
~ ~1 ~
'r O, r-, +-, V7 ~ h N
x
II cn x,~ N "'~~' N
~ .=f
~s _
N., ~ '.~ ~ x d' pp
N
~ 'd t~ ~ O ' 'C3
01 t~ O ~ o x '~
M x _ d; o -~
N ~ d1 x
~o ~ ~ ~ N co a>
v0 ~ N ~ M O
x w.s
~ r N
N "V? Nd'
oN ~
r~r~ r ~-~ d
d -' N M ~ "
~ ~ f- M
_~ -. x O 00
: ~ ~ O d N
'd v ;
'd
l~ ~ l\
~ O
T3
~ ~ l~0 ~ ~
~
s
a1
~i oo
m
'-' 'n ~opNN Nun NCO
~ ~
0 "
' ~
00 0 ~ O o_o N ~j l~
~
d' N ~ 'C3 N M oo
N Q.., ~ ~ ~ -
'~ ~ ' ~ m
~ ~'
oo ~ ~-: '
N r~
x a a ~ ~ ~ M
~
., II U x .,x ~ U ~:o
~ en ~o ~ .
_ N ~ d' 0 ~ ~ ~ ~ 00
cd '-'
~ d ~
~i V 00 ~ w
~ M .~ oo ~j
~ '"'"s
y--.
~ a O O in U. N ~ 00~i~
No~o~ O~~r~i i N
~ ~000~1
~
~ ri ~~ ~~ , ~m ~
.-~ U N M t~ ~
O r-.
~ ~
0
~ ~ 00 M
~1'
'~I' ~I' 'I_
M
-i-
d'
N N N
O O O
Z N
o
o
V'1 M ~1
N N N
U U U
0
0