Note: Descriptions are shown in the official language in which they were submitted.
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
SYRINGE WITH RETRACTABLE NEEDLE ASSEMBLY
BACKGROUND OF THE INVENTION
The field of the present invention relates generally to apparatus and methods
for protection against an accidental sharps injury or stick from an
unprotected
needle.
For some time, the art has recognized the desirability of protecting personnel
from accidental sharps injuries or needle sticks. More recently, concerns have
been
expressed about the possibility of transmitting serious or potentially fatal
infection
as a result of such accidents. Most recently, legislation requiring the use of
safe
needle technology is pending in a number of States and before the Occupation
Safety and Health Administration. Although, the art has recognized the
desirability
of protecting against accidental sharps injuries or needle sticks, it is
believed that
practical protective devices are still not available.
U.S. Patent No. 5,209,739 discloses a hypodermic needle assembly and a
syringe, both having a retractable cannula. An elastomeric tube is connected
between the cannula and the passage to the fluid chamber. In each of the
embodiments, a separate mechanical device must be independently operated by
the
user to cause retraction of the cannula into a second compartment. Since the
fluid
must travel through the elastomeric tube to bypass the second compartment,
there is
a potential risk of injecting air directly into the patient if the elastomeric
tube
breaks.
European Patent No. 0 862 Al discloses a device in which a needle is
retracted into the syringe. In several of the embodiments, the device requires
the
user to independently operate a mechanical device to cause retraction of the
needle.
In the one embodiment which utilizes an elastic member, the elastic member is
not
preloaded and requires the user to depress the plunger to load the elastic
member
and therefter continue to apply pressure on the plunger to avoid premature
withdrawal of the plunger. As such, the device requires two hands for its
operation.
CA 02394351 2002-06-12
WO 01/41830 PCTIUSOO/33897
2
Various methods of providing a preloaded retraction assembly which permit
one hand operation are disclosed in co-owned PCT Application No.
PCT/US97/20646, International Publication No. WO 98/20923. While these
devices operate successfully, it has been found that the devices may have a
somewhat reduced shelf life since the retraction member remains in a
tensioned,
preloaded condition.
Other devices which allow the retraction member to be loaded by the user
have been introduced. However, these devices generally require a complicated
or
non-routine procedure to accomplish such. The further a device is from routine
operation, the generally less accepted it is by the medical community.
Additionally, some of these devices require a mechanical altering of the
device
which may be difficult to accomplish or may cause deformities which prevent
the
device from operating properly. See for example U.S. Patent Nos. 5,928,200 and
5,836,917.
Furthermore, many retractable systems employ a geometrically configured
retraction member which mates with a geometrically configured member of the
needle assembly. A common problem associated with such is the geometrically
configured retraction member is forward of the plunger sealing surface and
thereby
engages and seals the passageway through the needle assembly before all of the
fluid is expelled. As a result, pressure builds in the syringe body. As the
needle
assembly retracts, a fluid passage opens and the pressurized fluid is ejected
therefrom.
Another concern with prior art devices is the complicated and costly
manufacturing processes. With the tremendous number of syringes and other
needle devices used by the medical community, any substantial rise in cost of
the
products is undesirable and generally unacceptable.
Accordingly, there is a need for a syringe having an automatically retracted
used needle assembly that can be used in a conventional manner and does not
require elaborate manufacturing.
CA 02394351 2008-02-14
52256-1
3
SUMMARY OF THE INVENTION
The present invention is directed to a syringe and
a method for its manufacture. The syringe includes a hollow
body, a needle assembly positioned in the hollow body, and a
plunger assembly in the hollow body. The needle assembly is
retractable with an elastic member.
According to the present invention, there is
provided a syringe comprising: a hollow body having a
substantially open end and a substantially closed end that
defines an aperture; a needle assembly comprising a needle,
wherein the needle assembly is positioned in the hollow body
and passes through the aperture; and a plunger assembly in
the hollow body comprising: an elongated frame portion
having a first end and a sealing platform at a second end
and defining at least one longitudinally extending guide
track, wherein the sealing platform is sealingly engaged
with the hollow body, a retraction member releasably secured
to the sealing platform, wherein the retraction member is
attachable to the needle assembly; a catch member having a
guide configured to travel in the guide track; and an
elastic member extending between the retraction member and
the catch member, wherein the elastic member is put in
tension by moving the plunger assembly toward the
substantially closed end of the hollow body, and wherein
tension on the elastic member is released, thereby
retracting the needle into the hollow body, after the
retraction member attaches to the needle assembly.
In a first separate aspect of the present
invention, the syringe is actuated to put the elastic member
in tension as the plunger is inserted into the hollow body.
CA 02394351 2008-02-14
52256-1
3a
In a second separate aspect of the present invention, the retraction member
attaches to the needle assembly when the plunger is inserted substantially
into the
hollow body.
In a third separate aspect of the present invention, tension on the elastic
member is released, thereby retracting the needle into the hollow body, after
the
retraction member attaches to the needle assembly.
In a fourth separate aspect of the present invention, the retention assembly
holds the elastic member in tension prior to retraction as the plunger moves
toward
the needle assembly.
In a fifth separate aspect of the present invention, the mandrel seal moves
with the retraction member prior to retraction and releasably sealingly
engages the
retraction member and the plunger frame.
In a sixth separate aspect of the present invention, the needle seal
releasably
sealingly engages the needle assembly and one end of the hollow body.
In a seventh separate aspect of the present invention, the retaining fingers
releasably secure the needle assembly at one end of the hollow body.
In an eighth separate aspect of the present invention, the retaining arms
releasably secure the needle seal at one end of the hollow body.
In a ninth separate aspect of the present invention, it is contemplated that
combinations of the foregoing separate aspects may be incorporated into a
single
embodiment.
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
4
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is an expanded view of a syringe assembly in accordance with the
present invention.
Figure 2 is a cross sectional view of one embodiment of the syringe barrel.
Figure 3 is an exploded view of the closed end of the syringe barrel of
Figure 2.
Figure 3A is an exploded view of another embodiment of the closed end of
the syringe barrel.
Figure 4 is a side elevation of one embodiment of the needle assembly.
Figure 4A is a side elevation of another embodiment of the needle
assembly.
Figure 4B is an exploded view of the closed end of another embodiment of
the syringe barrel and another embodiment of the needle assembly.
Figure 5 is an isometric view of one embodiment of a plunger assembly.
Figure 6 is a cross sectional view of the plunger frame of the plunger
assembly of Figure 5.
Figure 6A is a cross sectional view of another embodiment of a plunger
assembly.
Figure 6B is an enlarged view of part of the plunger assembly of Figure 6A.
Figure 6C is one embodiment of a retraction assembly.
Figure 6D is one embodiment of a catch member.
Figure 7 is an exploded view of the sealing end of the plunger frame of
Figure 6.
Figure 7A is a cross sectional view of another embodiment of the sealing
end of a plunger frame.
Figure 8 is a cross sectional view of the plunger assembly of Figure 5.
Figure 9 is an exploded view of the first end of the plunger assembly of
Figure 8.
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
Figure 10 is a side elevation view of one embodiment of the mandrel of the
present invention.
Figure 10A is a side elevation view of another embodiment of the mandrel
and a cross sectional view of the mandrel seal of the present invention.
5 Figure 11 is a side elevation view of one embodiment of the catch member
of the present invention.
Figure 12 illustrates an embodiment of the syringe in an assembled but
unused condition.
Figure 13 illustrates the syringe of Figure 12 upon initial substantial
depression of the plunger assembly.
Figure 14 illustrates the syringe of Figure 12 after loading of the syringe.
Figure 15 illustrates the forward portion of the syringe of Figure 12 as it is
inserted in a patient.
Figure 16 illustrates the forward portion of the syringe of Figure 12 upon
substantial injection depression of the plunger assembly.
Figure 17 illustrates the forward portion of the syringe of Figure 12 after
the mandrel tip has entered the needle assembly cavity.
Figure 18 illustrates the forward portion of the syringe of Figure 12 upon
complete depression of the plunger assembly.
Figure 19 illustrates the syringe of Figure 12 after retraction of the needle.
Figure 20 shows the plunger assembly members formed from the first
injection mold shot in accordance with the preferred method of manufacture.
Figure 21 shows the plunger assembly as formed from the second injection
overmold shot in accordance with the preferred method of manufacture.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The preferred embodiments will be described with reference to drawing
figures where like numerals represent like elements throughout.
CA 02394351 2002-06-12
WO 01/41830 PCTIUSOO/33897
6
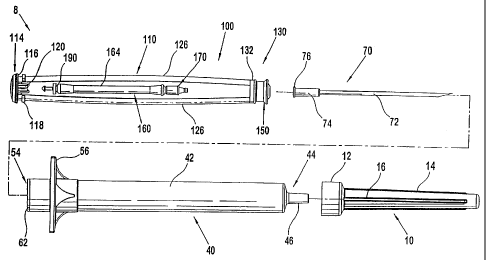
With reference to Figure 1, the syringe assembly 8 is comprised generally of
the cap member 10, the syringe barrel 40, the needle assembly 70, and the
plunger
assembly 100.
The cap member 10 includes an open, mating end 12 and a closed cone
section 14. The mating end 12 is preferably configured to slidingly engage the
syringe barrel 40. Alternatively, the mating end 12 may be provided with
threads
(not shown) which may engage corresponding threads (not shown) on the syringe
barrel 40. Other cap and corresponding barrel configurations are known and may
also be employed. The closed cone section 14 preferably includes a plurality
of
ribs 16 which assist gripping of the cap member 10.
Referring to Figures 2 and 3, one embodiment of the syringe barrel 40 is
comprised of a hollow body portion 42 which has a closed end 44 and an open
end
54. An external stabilized grip member 56 extends from the body 42 adjacent
to,
but forward of the open end 54. The grip member 56 may have various
configurations, the preferred elliptical configuration being shown. An
internal
annular shoulder 60 is defined in the hollow body 42 at approximately the same
position as the grip member 56. The open end 54 defines an open cavity 58 rear
of
the internal annular shoulder 60. An internal annular lip 62 may also be
provided
adjacent the open end 54.
The closed end 44 is defined by a truncated cone 46 which includes a
truncating plane having an aperture 48. Referring to Figure 3, in one
embodiment
of the syringe barrel 40, a retaining groove 50 is located on the interior of
the
syringe barrel 40 at a position adjacent to the closed end 44. The retaining
groove
50 retains the needle assembly 70 in position during use as will be described
in
more detail hereinafter. In one embodiment, the closed end 44 proximate the
truncated cone 46 has a generally convex taper 47 and at least one internal
ramp 52,
the functions of which will be described hereinafter.
Referring to Figure 3A, in another embodiment of the syringe barrel 40,
retaining fingers 51 are attached to the interior of the syringe barrel 40 at
a position
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
7
adjacent to the closed end 44. The retaining fingers 51 retain the needle
assembly
70 in position prior to retraction of the needle as will be described in more
detail
hereinafter.
Figure 4 depicts one emodiment of the needle assembly 70. In this
embodiment, the needle assembly 70 is comprised of a needle 72 which is
centrally
positioned in the conical projection 74. The conical projection 74 generally
complements the interior of the truncated cone 46 of the syringe barrel 40.
Immediately adjacent to the projection 74 is a sealing ring 76. The projection
74
and the sealing ring 76 preferably are formed as a unitary molding, but may be
formed as separate components. The interior passage 78 of the needle assembly
70
communicates with the hollow needle 72 and the geometrically configured cavity
80 extending into the rear surface of the needle assembly 70. The cavity 80
preferably has a cylinder portion 80a and a hemispheric portion 80b which
complement the geometrically configured tip 176 of the plunger mandrel 170
(shown in Figure 10). The needle assembly 70 is positioned within the syringe
barrel 40 such that the needle 72 extends through the aperture 48 and the
sealing
ring 76 is positioned in and retained by the retaining groove 50. Preferably,
the
sealing ring 76 sealingly engages the truncated cone 46 of the syringe barrel
40 and
the conical projection 74.
Figure 4A depicts another embodiment of the needle assembly 70. In this
embodiment, the needle assembly 70 has a needle seal lip 75 that engages and
retains a needle seal 77. The needle seal lip 75 may comprise an annular
collar or
other surface that retains the needle seal 77. When the needle assembly 70 is
inserted into the truncated cone 46 of the syringe barrel 40, the needle seal
77 is
positioned between and sealingly engages the needle seal lip 75 and the closed
end
44 of the syringe barrel 40. Alternately, the needle seal 77 may sealingly
engage
the conical projection 74 and the closed end 44 of the syringe barrel 40. To
facilitate sealing engagement of the needle seal 77 with the closed end 44 of
the
syringe barrel 40, the closed end 44 may have a shelf 43 (shown in Figure 3A)
that
CA 02394351 2002-06-12
WO 01/41830 PCTIUSOO/33897
8
engages the needle seal 77. The needle seal 77 is preferably annular, and may
comprise an 0-ring.
Other embodiments of the syringe barrel 40 and needle assembly are shown
in Figure 4B. In these embodiments, a needle seal 53 (shown in cross-section)
is
inserted through the aperture 48 at the closed end 44 of the syringe barrel
40. The
needle seal 53 is positioned between and sealingly engages the conical
projection
74 and the truncated cone 46. In this embodiment, a smaller end 55 of the
truncated
cone 46 opens up to receive the needle seal 53 and then closes to retain the
seal 53
in sealing engagement. To accomplish this opening and closing, the smaller end
55
of the truncated cone 46 is comprised of a plurality of retaining arms 57. A
first
end 61 of each retaining arm 57 is attached to a larger end 59 of the
truncated cone
46. The first ends 61 are arranged about the circumference of the larger end
59.
Each of the retaining arms 57 has a second end 63 that may move between an
open
position and a closed position when the retaining arms 57 are bent. An area
defined
by the second ends 63 of the retaining arms 57 while in the closed position is
smaller than an area defined by the second ends 63 of the retaining arms 57
while in
the open position. The area defined by the arms 57 in the closed position is
smaller
than a cross-sectional area of the needle seal 53 so that the needle seal 53
will not
pass out through the aperture. When the needle seal retainer is in the closed
position, the needle seal 53 sealingly engages the truncated cone 46 and the
conical
projection 74, and when the needle seal retainer is in the open position, the
needle
seal 53 is preferably released from sealing engagement with the truncated cone
46
and the conical projection 74. Retaining fingers 51 are attached to the
interior of
the syringe barrel 40 at a position adjacent to the closed end 44. The
retaining
fingers 51 help retain the needle assembly 70 in position prior to retraction
of the
needle as will be described in more detail hereinafter.
In all of the embodiments described herein, the needle assembly 70 is
retained in a position that is adjacent to the closed end 44 of the barrel 40
by a
needle assembly retainer prior to retraction. In the embodiments shown in
Figures
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
9
3 and 4, the needle assembly retainer comprises the sealing ring 76 that is
positioned in and retained by the retaining groove 50. In the embodiment shown
in
Figures 3A and 4B, the needle assembly retainer comprises the retaining
fingers
51.
The retaining fingers 51 are preferably disposed about the circumference of
a larger end 59 of the truncated cone 46. A first end 65 of each of the
retaining
fmgers 51 is connected with the syringe barrel 40 at a location adjacent to
the
closed end 44.The retaining fingers 51 preferably comprise L-shaped members
with
a retaining lip 67 near a second end 69. The retaining fingers 51 are bendable
between a closed position and an open position. While in the closed position,
the
second ends 69 of the fingers 51 define an area that is smaller than the area
defined
by the second ends 69 when they are in the open position. The smaller area
defined
by the fingers 51 in the closed position is smaller than a cross-sectional
area of the
needle assembly 70, such that the needle assembly does not pass through the
needle
assembly retainer when the fingers 51 are in the closed position.
Referring to Figures 1 and 5-11, the plunger assembly 100 includes a
plunger frame 110, a retraction assembly 160, a thumb pad 104 and a sealing
member 150. The plunger frame 110 includes a first end 114 and a sealing end
130
with a pair of opposed connecting rods 126 extending therebetween. The opposed
connecting rods 126 define opposed retraction assembly guide tracks 128.
As shown in Figure 6, in one embodiment of the plunger frame, the first end
114 of the plunger frame 110 includes a terminating plate 116 extending
between
and bridging the opposed connecting rods 126. An annular thumb pad retaining
ring 117 extends about the terminating plate 116. Additionally, a guide member
118 may extend outward from each connecting rod 126 proximate the terminating
plate 116. A retention assembly 120 extends inward from the terminating plate
116
between the opposed connecting rods 126. This embodiment of the retention
assembly 120 includes a pair of opposed L-shaped members 122, each L-shaped
member having a beveled catch 124 extending therefrom. Other retention
CA 02394351 2002-06-12
WO 01/41830 PCT/USOO/33897
assemblies which permit inward passage and then retention of a geometrically
configured tip are within the scope of the invention.
As shown in Figures 6A and 6B, another embodiment of the plunger frame
has a retention assembly that comprises a plural.ity of retention teeth 103
arranged
5 along a surface of the connecting rods 126 of the plunger assembly,
substantially
parallel to a longitudinal axis of the elongated frame portion of the plunger
assembly.
Each component of the plunger frame 110 is preferably manufactured from
polypropylene or glass filled polypropylene. Other materials, including
various
10 plastics, may also be used. As described in more detail hereinafter, the
plunger
frame 110, in addition to components of the retraction assembly 160, is
preferably
formed as a first shot of a multiple shot injection molding procedure.
As shown in Figure 7, in one embodiment of the plunger assembly, the
sealing end 130 includes a sealing platform 132 extending between the
connecting
rods 126 and including an apertured cylinder 134 terminating in an apertured
pressure cone 138. The apertures are preferably concentric such that a
continuous
hollow integral shaft 140 passes through the sealing end 130 from the sealing
platform 132 to the pressure cone 138. The hollow shaft 140 is preferably
tapered
such that the diameter is greater within the sealing platform 132 than within
the
pressure cone 138. Additionally, an internal annular ring 142 extends into the
hollow shaft 140 proximate the pressure cone 138. An external annular
retaining
ring 136 is positioned about the juncture of the cylinder 134 and pressure
cone 138.
The function of the taper and the internal and external rings 136 and 142 will
be
described in more detail hereinafter.
As shown in Figure 7A, in another embodiment of the plunger assembly, the
hollow shaft 140 has a first end 141 and a second end 143, wherein the first
end 141
defines a cross-sectional area that is smaller than a cross-sectional area
defined by
the second end 143. The transition between the smaller and larger cross-
sectional
areas is depicted to be abrupt, but may alternately be gradual.
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
11
Referring to Figure 8, the plunger sealing member 150 is positioned about
the cylinder 134 and the external retaining ring 136 and is maintained in
position by
the external ring 136. The sealing member 150 includes annular seals 152 and
154
at each end with a narrower portion 156 positioned therebetween. When the
plunger assembly 100 is positioned in the syringe barrel 40, annular seal 152
sealingly engages the inside surface of the hollow body portion 42 with an
area of
open space about the narrower portion 156. Annular seal 154 may also sealingly
engage the hollow body 142, but may also include a passage to prevent creating
a
vacuum in the narrower portion 156. The sealing member 150 is preferably
manufactured from an elastomer. A material found to be suitable is KratonTM
manufactured by Shell Oil. A preferred material is KratonTM G2706 manufactured
by Shell Oil. As will be described in greater detail hereinafter, the sealing
member
150 is preferably overmolded directly in position, but may be manufactured
separately and subsequently positioned about the cylinder 134 and retaining
ring
136.
The first end 114 of plunger frame 110 can be utilized with the terminating
plate 116 and no thumb pad 104. However, it is preferable to provide a thumb
pad
104 about the terminating plate 116 and retained by the retaining ring 117 as
shown
in Figure 9. The thumb pad 104 is also preferably manufactured from an
elastomer, preferably KratonTM. As with the sealing member 150, it is
preferable
that the thumb pad 104 be overmolded directly in position, but it too may be
manufactured separately and subsequently positioned and secured about the
terminating plate 116.
The preferred retraction assembly 160 will be described with reference to
Figures 5, 6C, 6D, 8, 10, 10A and 11. The retraction assembly 160 includes a
mandrel 170, a catch member 190 and an elastic member 164.
Referring to Figure 10, one embodiment of the mandrel 170 includes a
generally cylindrical body 172 with a tapered portion 174 extending from one
end
and a shaft portion 180 extending from the other. The tapered portion 174
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
12
terminates in a geometrically configured tip 176. A mandrel annular retaining
ring
178 extends about the cylindrical body 172 proximate the juncture with the
tapered
portion 174. The mandrel 170 is releasably secured to the plunger frame by a
retention means, which includes the retaining ring 178. The mandrel retaining
ring
178 preferably is part of the mandrel 170 and does not separate from the
mandrel
170. The shaft portion 180 includes a plurality of barbs 182 or the like
extending
therefrom for retaining the elastic member 164.
In another embodiment of the mandrel 170, as shown in Figure 10A, the
cylindrical body 172 has a groove 173 and a mandrel seal 171 (a cross-section
of
which is depicted) is positioned about the groove 173 between the mandrel 170
and
the hollow shaft 140. The mandrel seal 171 releasably and sealingly engages
the
mandrel 170 and the hollow shaft 140. The mandrel seal 171 is depicted as an 0-
ring, but may be of any configuration that releasably and sealingly engages
the
hollow shaft 140 and the mandrel 170.
The mandrel seal 171 initially is in sealing engagement with the mandrel 170
and the first end 141 of the hollow shaft 140 and inhibits the passage of
fluid
therebetween prior to retraction of the needle. The sealing engagement creates
holding forces between the hollow shaft 140 and the mandrel seal 171, as well
as
between the mandrel seal 171 and the mandrel 170. The holding forces inhibit
movement of the seal 171 relative to the mandrel 170 and relative to the
hollow
shaft 140, and thus inhibit retraction of the needle. Accordingly, the
retention
means that releasably secures the mandrel 170 to the plunger frame includes
the
mandrel seal 171.
In the smaller-diameter area, the holding force between the mandrel seal 171
and the mandrel 170 is greater than the holding force between the mandrel seal
171
and the hollow shaft 140, such that the mandrel seal 171 does not move
relative to
the mandrel 170, but instead moves relative to the hollow shaft 140 when the
mandrel 170 is moved relative to the hollow shaft 140.
CA 02394351 2002-06-12
WO 01/41830 PCT/USOO/33897
13
The desired distribution of holding forces may provided by various means.
Preferably, the mandrel 170 has an annular groove 173 about the circumference
of
the mandrel 170 and located at a seal position on the mandrel 170. The annular
groove 173 is configured so that the mandrel seal 171 abuts or fits into the
groove
173 and is held in place on the mandrel 170 at the seal position so long as
the
mandrel seal 171 is located in the smaller-area portion of the hollow shaft
140.
Once the mandrel seal 171 moves from the smaller area to the larger area of
the
hollow shaft 140, the mandrel seal 171 may either remain in the groove 173 or
expand away from the groove 173, as described in greater detail below.
It is noted that configurations other than a groove may also be employed to
retain the mandrel seal fixed with respect to the mandrel. For example, the
mandrel
seal could be positioned between two annular collars on the mandrel.
Additionally,
any other non-smooth or irregular surface may be employed to mechanically
inhibit
movement of the seal relative to the mandrel. Alternately, an adhesive could
be
used to attach the mandrel seal to the mandrel. The mandrel seal could also be
formed as an integral part of the mandrel.
Referring to Figure 8, in one embodiment of the retraction assembly 160, an
annular stop 184 extends about the cylindrical body 172 of the mandrel 170
adjacent the end of the hollow shaft. The stop 184 is preferably elastomeric
and
therefore is preferably formed in conjunction with the elastic member 164.
Referring to Figures 5 and 11, one embodiment of the catch member 190
includes an elongated plate 192 which is sized such that each end of the
elongated
plate 192 extends into and travels within a respective retraction assembly
guide
track 128 of the plunger frame 110. Extending from one side of the elongated
plate
192 is a shaft 194 with barbs 196 or the like extending therefrom for
retaining the
elastic member 164. In the embodiment shown in Figure 11, a second shaft 198
extends from the opposite side of the elongated plate 192 and terminates in a
geometrically configured catch tip 200. The tip 200 is configured to mate with
and
be retained by the retention assembly 120 of the plunger frame 110.
CA 02394351 2002-06-12
WO 01/41830 PCTIUSOO/33897
14
As shown in Figures 6C and 6D, another embodiment of the catch member
105 has a guide 107 configured to travel in the guide track 128 between the
connecting rods of the plunger frame 110. The catch member 105 has a catch
tooth
109 situated on either side of the catch member 105. Each catch tooth 109
engages
with one of the retention teeth 103 (the retention teeth 103 are part of the
retention
assembly shown in Figures 6A and 6B) at a time. After one of the retention
teeth
103 receives one of the catch teeth 109, the catch tooth 109 is inhibited from
moving away from the first end 114 of the elongated frame portion.
Accordingly,
the catch member 105 is retained by the retention teeth 103 of the retention
assembly and the elastic member 164 is thereby held in tension on one end by
the
sealing platform and on the other end by the catch member 105.
The mandrel 170 and the catch member 190 105 are preferably
manufactured from the same material as the plunger frame 110. As such, these
components can also be formed during the first shot of the multiple shot
injection
molding procedure used to form the plunger frame.110.
The elastic member 164 extends between the mandrel 170 and the catch
member 190 105. The elastic member 164 is manufactured from a resilient
material, which is preferably an elastomer, but which can be other materials,
for
example a stainless steel spring or the like. The elastic member 164 is
preferably
manufactured from KratonTM. In the preferred method of manufacture described
below, the elastic member 164 is formed between the mandrel 170 and the catch
member 190 105 with a second injection, overmolding shot. Do to the elastic
member 164 being directly overmolded over the barbed shafts 180 and 194 and
the
innate bonding property of the preferred material, there is generally not a
need for
additional securing means, for example adhesive, to maintain the elastic
member
164 secured to the mandrel 170 and catch member 190 105. It is contemplated
that
the elastic member 164, irrespective of the material from which it is
manufactured,
may also be manufactured separately and secured to the mandrel 170 and catch
member 190 105. Since the sealing member 150, thumb pad 104 and stop 184 are
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
all also preferably manufactured from the same material as the elastic member
164,
they are also preferably formed during the second injection, overmolding shot.
Having described the components of the preferred syringe 8, its assembly
and use will now be described with reference to Figures 12-19. The needle
5 assembly 70 is positioned in the syringe barrel 40 with the needle 72
extending
through the aperture 48.
In the embodiment shown in Figures 3 and 4, the needle assembly 70 is
inserted until the sealing ring 76 seats in and is retained by the syringe
body
retaining groove 50.
10 In the embodiment shown in Figures 3A and 4A, the needle assembly 70
with the needle seal 77 is inserted into the truncated cone 46 of the syringe
barrel
40 and is retained by the retaining fingers 51. When the needle assembly 70 is
being inserted into the truncated cone 46, it contacts the retaining fingers
51.
Further insertion of the needle assembly 70 moves the retaining fingers 51
into the
15 open position so that the needle assembly 70 may pass through the opening
defined
by the retaining fingers 51.
When the needle assembly 70 is inserted sufficiently into the truncated cone
46, the retaining fingers 51 return to the closed position. The retaining
fingers 51
are formed from an elastic material so that they return to the closed position
if they
are displaced from the closed position and no external forces are acting on
the
retaining fingers 51. If a force is applied tending to push the needle
assembly 70
out of the truncated cone 46 toward the open end of the barre140 when the
retaining
fmgers 51 are in the closed position, the needle assembly 70 will contact at
least
one of the retaining lips 67, and at least one of the retaining fingers 51
will resist
that force. The orientation of the surface of the lips 67 in a substantially
perpendicular relationship to the direction in which such a force would be
applied
decreases the component of the force that would act to spread the fingers 51
into
the open position.
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
16
In the embodiment shown in Figure 4B, the needle seal 53 is inserted into
the truncated cone 46 through the aperture 48 rather than through the open end
of
the syringe barrel 40. To insert the needle seal 53 into the truncated cone 46
through the aperture 48, the retaining arms 57 are moved into the open
position and
held in the open position so that the needle seal 53 may pass through the
opening
defined by the arms 57. Once the needle seal 53 is sufficiently inserted into
the
truncated cone 46, the arms 57 are released and move toward the closed
position.
The arms 57 are formed of an elastic material so that the arms 57 return to
the
closed position if they are displaced from the closed position and no external
forces
are acting on the arms 57. Once the needle seal 53 is inserted into the
truncated
cone 46 and the arms 57 return to the closed position, the needle seal 53 is
retained
in the truncated cone 46 by the arms 57.
The needle assembly 70 may be inserted through the aperture 48 or through
the open end of the barrel 40. If the needle assembly 70 is inserted through
the
open end of the barrel 40, it is done in the same manner as described above in
connection with the embodiments shown in Figures 3A and 4A. Accordingly, the
needle assembly 70 contacts the retaining fingers 51 and pushes them into the
open
position. Once the needle assembly 70 is sufficiently inserted, the retaining
fingers
51 move into the closed position and retain the conical projection 74 of the
needle
assembly 70. If the needle assembly 70 is inserted through the aperture 48, it
may
be inserted in the same manner as, and at the same time as, the seal 53. If
inserted
through the aperture 48, the retaining fingers 51 need not move to the open
position
to accept the needle assembly 70.
In the embodiments shown in Figures 3, 3A, 4, and 4A, the cap member 10
may be mated with the closed end 44 of the syringe barrel 40 either before or
after
insertion of the needle assembly 70. In the embodiments shown in Figure 4B,
the
cap member 10 may be mated with the closed end 44 of the syringe barrel 40
after
insertion of the needle seal 53.
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
17
The plunger assembly 100 is assembled by assembling the plunger frame
100, which already has the thumb pad 104 and sealing member 150 positioned
thereon, and the retraction assembly 160. As explained above, the elastic
member
164 is preferably molded directly to the mandrel 170 and catch member 190 105,
to
form the retraction assembly 160. If not formed integrally, the elastic member
164
is secured to the mandrel and catch member barbed shafts 180 and 194.
With the retraction assembly 160 complete, the mandrel 170 is inserted
through the hollow shaft 140 passing through the plunger frame sealing end
130. In
the embodiment shown in Figures 7 and 10, the mandrel 170 is inserted until
the
mandrel retaining ring 178 is secured by the sealing end internal annular ring
142.
The mandrel retaining ring 178 forms a fluid tight seal with the plunger
sealing end
130 proximate the pressure cone 138, thereby sealing the hollow shaft 140. The
resilient stop 184 abuts against the rear surface of the sealing platform 132,
thereby
forming a fluid tight seal about that end of the hollow shaft 140. As the
plunger
assembly is withdrawn from the hollow body 42, a vacuum is created therein.
The
seal provided by the resilient stop 184 helps prevent air or other materials
from be
pulled past the mandrel 170 into the syringe body 42 by the internal vacuum
force.
In the embodiment shown in Figures 7A and 10A, the mandrel 170 is
inserted to place the mandrel seal 171 within the first end 141 of the hollow
shaft
140. The mandrel seal 171 may abut a pressure cone 138 or a lip (not shown) on
the hollow shaft 140 that inhibits excessive insertion of the mandrel 170.
With the mandrel 170 in place, the plunger assembly 100 is ready to be
inserted into the syringe barrel 40 through the open end 54. The annular seals
152
and 154 sealingly engage the inside of the syringe barrel 40 as the plunger
assembly
100 is inserted. The plunger assembly 100 is inserted approximately half-way
into
the syringe barrel 40 until the catch member 190 105 abuts the shoulder 60, as
shown in Figure 12. The syringe 8 is ready for packaging and delivery. It
should
be noted that at this time the elastic member 164 is not tensioned. This helps
increase the shelf life of the syringe 8 since the elastic member 164 is not
under
CA 02394351 2002-06-12
WO 01/41830 PCTIUSOO/33897
18
constant tension. If shelf life is not a concern, the catch member 190 105 can
be
secured to the retention assembly 120 prior to packaging, whereby the syringe
8
would have a preloaded elastic member.
After removing the syringe assembly 8 from the packaging, the operator can
hold the syringe in a typical one hand manner, i.e. with two fingers abutting
the grip
member 56 and the thumb on the thumb pad 104. The operator presses on the
thumb pad 104 to depress the plunger assembly 100 into the syringe barrel 40
with
a substantially complete depression to expel air from the syringe hollow body
42.
This is similar to standard syringe operation. As the plunger assembly 100 is
depressed, the catch member 190 105 is retained by the shoulder 60 such that
the
catch member 190 105 cannot travel forward. However, the plunger frame 110
continues its forward travel. Since the catch member 190 105 is retained but
the
plunger frame 110 and secured mandrel 170 continue forward, the elastic member
164 begins to stretch and tension.
As travel continues forward, the catch element 200 109 of the catch member
190 105 is received by the retention assembly 120. In the embodiment shown in
Figure 11, the catch element is a geometrically configured catch tip 200. In
the
embodiment shown in Figures 6C and 6D, the catch element is comprised of catch
teeth 109 and the retention assembly is comprised of retention teeth 103. As
shown
in Figure 13 for the embodiment with a catch tip 200, the catch element 200
109 is
secured by the retention assembly 120 of the plunger frame 110. The elastic
member 164 is thereby secured in a loaded condition between the secured
mandrel
170 and the secured catch member 190 105. As the catch element 200 109 and
retention assembly 120 mate, an audible "click" may occur to provide a signal
of
proper mating to the operator.
Additionally, the syringe barrel annular lip 62 adjacent the first end 114 of
the plunger frame will discourage complete depression of the plunger assembly
100
as the catch element 200 109 is received by the retention assembly 120.
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
19
With the elastic member 164 loaded, the syringe 8 can be loaded in a typical
fashion by removing the cap 10, inserting the needle 42 into a desired vial or
the
like, and withdrawing the plunger assembly 100 to draw up a desired dose as
shown
in Figure 14. Since the elastic member 164 is tensioned between two components
secured to the plunger frame 110, withdrawal of the plunger assembly 100 will
not
trigger the elastic member 164. Instead, the plunger assembly 100 will operate
as a
standard syringe plunger.
Once any air has been purged from the syringe barrel 40 in a known manner,
the device 8 is ready for injection of the needle 72 into the patient. As
stated above,
the elastic member 164 is tensioned between two fixed components, and
therefore,
is not acting to move the plunger assembly 100 in either direction. As such,
the
user does not have to maintain constant pressure on the plunger assembly, but
is
free to hold the syringe 8 in the traditional dart like fashion between their
thumb
and forefinger of one hand, and use the other hand to pinch the patient's skin
at the
point of insertion for subcutaneous injection, spread the skin for
intramuscular
injection, and stabilize the skin for IV injection. These methods of injection
are the
generally preferred methods in the medical field.
As the needle 72 is inserted, a rearward force, indicated by the arrow A in
Figure 15, is applied against the needle assembly 70. To resist this force,
the
needle assembly sealing ring 76 is secured within the retaining groove 50 in
the
embodiment shown in Figures 3 and 4. Additionally, since the syringe barrel
tapered surface 47 about the truncated cone 46 is convex, the rearward force
causes
the syringe barrel surface to urge inward, as indicated by arrows B, thereby
creating
a tighter retention force about the needle assembly 70. Once the user has
inserted
the needle 72 into the patient, the user injects the substance into the
patient by
depressing the thumb pad 104. In the embodiments shown in Figures 3A, 4A, and
4B, the force applied against the needle assembly is resisted by the retaining
fingers
51.
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
As shown in Figure 16, upon substantial depression of the plunger assembly
100, the mandrel tip 176 begins to enter the needle assembly cavity 80. At
approximately the same time, the first annular seal 152 meets and is deflected
by
the ramps 52 adjacent the closed end 44 of the syringe barrel 40, thereby
breaking
5 the fluid tight seal. Any fluid trapped between the plunger sealing member
150 and
the needle assembly 70 is permitted to pass the deflected annular seal 152
into the
open space around narrower portion 156. The second annular seal 154 may remain
in sealing engagement with the syringe barrel 40 to prevent any unwanted
inward
or outward flow past the sealing member 150. However, the annular seal 154 may
10 include a small passage to let trapped air about the narrower portion 156
escape.
The mandrel tip 176 passes through the needle assembly cavity cylindrical
portion 80a into the geometrically configured cavity hemispherical portion 80b
whereby the mandrel 170 is secured to the needle assembly 70 as shown in
Figure
17.
15 At approximately the same time the mandrel 170 and needle assembly 70
attach, the retaining fingers 51 are moved into the open position by the
plunger
assembly 100 so that the needle assembly 70 may pass through the opening
defined
by the fingers 51. A holding force exerted by the fingers 51 on the needle
assembly
70 is lower when the fingers 51 are in the open position than when the fingers
51
20 are in the closed position. The tension in the elastic member is sufficient
to
overcome any remaining holding force exerted by the fingers 51 on the needle
assembly 70.
Once the mandrel 170 is secured to the needle assembly 70 as shown in
Figure 17, the mandrel tip 176 has moved as far into the needle assembly 70 as
possible, yet the plunger frame 110 has not completed its full stroke. As
such,
continued force on the thumb pad 104 will continue to move the plunger frame
110
forward. Since the mandrel 170 position is fixed and the plunger frame 110 is
being forced forward, the mandrel is pushed backward.
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
21
In the embodiment shown in Figures 7 and 10, the retaining ring 178 will
be forced inward past the plunger sealing end retaining ring 136, thereby
releasing
the mandrel 170 as shown in Figure 18. That is, the mandrel retaining ring 178
moves behind the retaining ring 136 as shown in phantom.
In the embodiments shown in Figures 7A and 10A, as the needle user
continues depressing the plunger, the plunger frame continues moving forward
relative to the mandrel 170, so that the mandrel 170 is moved from a first
position
in the smaller-area first end 141 of the hollow shaft 140 to a second position
in the
larger-area second end 143 of the hollow shaft 140.
As the mandrel 170 moves from the first position toward the second
position, the holding force between the hollow shaft 140 and the mandrel seal
171
is overcome and the mandrel seal 171 slides against the hollow shaft 140. The
mandrel seal 171 moves with the mandrel 170 because the holding force between
the mandrel seal 171 and the mandrel 170 is greater than the holding force
between
the mandrel seal 171 and the hollow shaft 140.
Once the mandrel seal 171 moves into the larger area of the second end 143
of the hollow shaft 140, the mandrel seal 171 either expands away from the
mandrel 170 or remains engaged with the mandrel 170. The seal 171 would expand
if it were compressed around the mandrel 170 by the hollow shaft 140 during
the
assembly of the syringe. If the seal 171 expands, the area defined by an
opening in
the seal 171 (the inner seal area) preferably becomes large enough as the seal
171
moves from the first position to the second position, such that the mandrel
170 may
retract the needle through the inner seal area.
In any case, the mandrel seal 171 no longer secures the mandrel 170 to the
hollow shaft 140 after the mandrel seal 171 moves from the first position to
the
second position within the hollow shaft 140.
Since the mandrel 170 is under the load of the elastic member 164 but no
longer secured to the hollow shaft 140, the load of the elastic member 164
automatically retracts the mandrel 170 into the plunger frame 110 between the
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
22
connecting rods 126. Through the connection of mandrel tip 176 and the
geometrically configured needle assembly cavity 80, the needle assembly 70 is
also
retracted into the plunger frame 110, as shown in Figure 19.
In one embodiment, the tapered pressure cone 138 on the sealing end of the
plunger frame 110 contacts the convex, tapered portion 47 of cone 46 and
causes it
to spread slightly. This reduces the retaining force of cone 46 on the needle
assembly 70 to assist retraction of the needle assembly 70. Furthermore, since
the
tapered portion 47 is convex, the forward fluid and plunger force, as
indicated by
arrow C in Figure 18, urge the tapered portion 47 outward, as indicated by
arrows
D, further easing the retaining force and thereby reducing the requisite
retraction
force. This flexing preferably occurs simultaneously or slightly after the
mandrel
176 enters the hemispherical portion 80a of the needle assembly cavity 80.
Referring again to Figure 19, as the plunger assembly 100 completes its
stroke, the thumb pad 104 enters the open cavity 58 at the end of the syringe
barrel
40. In the preferred embodiment, the thumb pad 104 is manufactured from a
resilient material which sealingly engages the syringe barrel 40 wall, thereby
closing the open end 54 and preventing any inadvertent fluid flow out of the
syringe
barrel 40. Additionally, since the thumb pad 104 enters and is recessed in the
open
cavity 58, it makes it difficult for anyone to inadvertently or intentionally
remove
the plunger assembly 100 and expose the used needle 72. The thumb pad 104
preferably has a semi-domed configuration which enhances its inaccessibility.
Additionally, the thumb pad 104 is preferably inserted past the inner annular
lip 62
and retained thereby, further enhancing inaccessibility.
The method of manufacture of one embodiment of the syringe will now be
described with reference to Figures 20 and 21. A first shot injection mold
procedure is utilized to form the plunger frame 110 components (the first end
114,
the sealing end 130 and the connecting rods 126 extending therebetween), the
mandrel 170, and the catch member 190 105 in a single shot of the desired
material,
in the preferred embodiment, polypropylene. The resultant component is shown
in
CA 02394351 2002-06-12
WO 01/41830 PCT/US00/33897
23
Figure 20. The mandrel 170 and the catch member 190 105 are maintained in
position relative to one another and the plunger frame 110 by runners 210
extending from the components 170 and 190 to the connecting rods 126. The
formed plunger frame 110, mandrel 170 and catch member 190 105 are then
positioned in a second mold cavity. Using a second injection, overmold shot of
the
desired material, in the preferred embodiment, KratonTM, the elastic member
164,
stop 184, sealing member 150 and thumb pad 104 are formed directly over the
corresponding parts of the plunger frame 110, mandrel 170 and catch member 190
105 as shown in Figure 21. After the plunger assembly 100 is removed from the
second mold, the runners 210 are trimmed off the mandrel 170 and connecting
rods
126, thereby freeing the mandrel 170. The mandrel 170 is then pushed through
the
plunger sealing end hollow shaft 140 until it is retained in position by the
interaction of the mandrel retaining ring 178 and the plunger sealing end
retaining
ring 136. After the mandrel 170 is secured, the runners 210 can be trimmed
between the catch member 190 105 and the connecting rods 126. While it is
possible to trim all of the runners 210 at the same time, it is preferable to
maintain
the runners 210 supporting the catch member 190 105 to avoid excessive
movement
of the retraction assembly 160 during insertion of the mandrel 170. Once the
mandrel 170 is inserted and the runners 210 are trimmed, the plunger assembly
100
is ready for use in accordance with the above.
This method of overmolding a resilient, elastomeric material about a frame
assembly is also contemplated for use in forming various other medical and non-
medical articles. With respect to medical articles, retractable blood
collection
devices, automated lancets, syringes with tensioned or tensionable elastomeric
inner or outer sheaths, and butterfly devices are among the articles
considered.
While the present invention has been described in terms of the preferred
embodiments, other variations which are within the scope of the invention as
defined in the claims will be apparent to those skilled in the art.
* * *