Note: Descriptions are shown in the official language in which they were submitted.
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
1
OSMOTIC BENEFICIAL AGENT DELIVERY SYSTEM
BACKGROUND OF THE INVENTION
Field of the Invention
The invention relates to osmotic beneficial agent delivery systems and
methods, and more particularly, the invention relates to an osmotic beneficial
agent
delivery system including an implant and a catheter for delivery of beneficial
agents
from the implant to a delivery site.
Brief Description of the Related Art
Osmotic delivery devices such as those described in U.S. Patent Nos.
4,111,202; 4,111,203; and 4,203,439 can deliver a beneficial agent at a
controlled
delivery rate over an extended period of time. Osmotic delivery systems are
very
reliable in delivering a beneficial agent over an extended period of time
called an
administration period. In general osmotic delivery systems operate by imbibing
fluid from an outside environment into the delivery system and releasing
corresponding amounts of a beneficial agent from the delivery system. The
osmotic
delivery systems are generally implanted in tissue which provides the fluid
2o environment from which fluid is drawn into the osmotic delivery system.
Osmotic delivery systems, commonly referred to as "osmotic pumps,"
generally include some type of capsule having one or more walls which
selectively
pass water into the interior of the capsule containing a water attracting
agent. The
absorption of water by the water attracting agent within the capsule reservoir
creates
an osmotic pressure within the capsule which causes a beneficial agent within
the
capsule to be delivered. The water attracting agent may be the beneficial
agent
being delivered to the patient, however, in most cases, a separate agent is
used
specifically for its ability to draw water into the capsule.
When a separate osmotic agent is used, the osmotic agent may be separated
so from the beneficial agent within the capsule by a movable dividing member,
such as
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
2
a membrane or a piston. The structure of the capsule is generally rigid such
that as
the osmotic agent takes in water from the environment and expands, the capsule
does not expand. As the osmotic agent expands, the agent causes the movable
dividing member to move, discharging the beneficial agent through an orifice
or exit
passage of the capsule. The beneficial agent is discharged through the exit
passage
at the same volumetric rate that water enters the osmotic agent through the
semipermeable walls of the capsule.
The rate at which the beneficial agent is discharged from the delivery device
is determined by many factors including the type of osmotic agent, the
permeability
~o of the semipermeable membrane, and the size and shape of the exit passage.
The
beneficial agent can be delivered at a controlled rate over periods of time
which may
be as long as a year.
One type of known osmotic delivery system includes a cylindrical capsule
having an osmotic tablet such as salt separated by a piston from a beneficial
agent
reservoir inside the capsule. A first open end of the capsule is provided with
a
membrane plug to provide a semipermeable wall. The membrane plug seals the
interior of the capsule from the exterior environment permitting only certain
liquid
molecules from the environment to permeate through the membrane plug into the
osmotic agent chamber of the capsule. An opposite end of the capsule includes
a
zo delivery orifice through which the beneficial agent is delivered at a
controlled
delivery rate.
It would be desirable to provide an osmotic delivery system including an
implant containing the beneficial agent which is implanted at one location and
a
catheter connected to the implant which delivers the beneficial agent to
another
25 location. It also would be desirable to provide such an osmotic delivery
system
which delivers the beneficial agent over an extended period. It would also be
desirable to provide and implantable system which allows delivery of a
beneficial
agent through a catheter to a treatment site, intravenously, or throughout a
treatment
region.
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
3
SUMMARY OF THE INVENTION
The present invention relates to an osmotic beneficial agent delivery system
including an implantable osmotic delivery device and a catheter for delivering
the
beneficial agent from the delivery device to a delivery location.
According to one aspect of the present invention, a beneficial agent delivery
system includes, an implant, an implantable docking station, and a catheter.
The
implant includes an implant housing having a proximal end and a distal end, an
osmotic agent contained within the housing, a beneficial agent reservoir
within the
~o housing, a fluid permeable membrane positioned in the proximal end of the
housing
which allows moisture to enter the housing and cause the osmotic agent within
the
housing to swell, and a fluid outlet at the distal end of the housing for
dispensing the
beneficial agent. The implantable docking station is configured to receive the
distal
end of the implant housing. The catheter is connected to the implantable
docking
~ s station with an inlet of the catheter arranged to receive the beneficial
agent
dispensed from the fluid outlet of the implant when the implant is received in
the
docking station.
According to another aspect of the present invention, a beneficial agent
delivery system includes a substantially cylindrical implant containing a
beneficial
2o agent, an implantable tubular docking station arranged to removably receive
the
implant, and a catheter connected to the docking station and arranged to
receive the
beneficial agent from the implant and dispense the beneficial agent to a
treatment
site when the implant is received in the docking station.
According to a further aspect of the invention, a method for delivering
25 beneficial agents to a treatment site includes the steps of implanting a
docking
station and catheter combination in a body of a patient, and positioning the
catheter
and docking station such that a distal end of the catheter is located at a
treatment site
within the body; connecting an osmotic implant to the docking station with a
delivery orifice of the osmotic implant arranged to deliver a beneficial agent
from
so the osmotic implant to a lumen of the catheter; selectively passing aqueous
fluid into
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
4
the osmotic implant; and delivering the beneficial agent from the osmotic
implant
through the lumen of the catheter to the treatment site.
The present invention provides advantages of reliable delivery of a beneficial
agent to delivery site over an extended administration period in a safe and
convenient manner
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will now be described in greater detail with reference to the
preferred embodiments illustrated in the accompanying drawings, in which like
~o elements bear like reference numerals, and wherein:
FIG. 1 is a side view of a first embodiment of a beneficial agent delivery
system including an implant, a docking station, and a catheter;
FIG. 2 is a side view of the beneficial agent delivery system of FIG. 1 with
the implant positioned in the docking station;
FIG. 3 is a schematic side cross-sectional view of the beneficial agent
delivery system of FIG. 1 with the implant positioned in the docking station;
FIG. 4 is a side view of a second embodiment of a beneficial agent delivery
system including an implant, a docking station, and inner and outer catheters;
FIG. 5 is a side view of the beneficial agent delivery system of FIG. 4 with
2o the implant positioned in the docking station;
FIG. 6 is a side view of the beneficial agent delivery system of FIG. 1 with a
multi-port catheter; and
FIG. 7 is a side view of a third embodiment of a beneficial agent delivery
system including an implant, a docking station, and a catheter.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
For many medical and therapeutic treatments it would be desirable to
provide an implanted agent delivery catheter positioned to deliver a
beneficial agent
to a delivery site and a replaceable beneficial agent delivery device
removably
3o connectable to the catheter. The beneficial agent delivery system according
to the
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
present invention includes a catheter and docking station which are implanted
in a
patient with a distal end of the catheter positioned at the delivery site. The
catheter
and docking station are left in place while an implant containing the
beneficial agent
is removably connected to the catheter at the docking station and can be
replaced as
5 needed. The docking station provides a connection between the catheter and
the
implant and allows the implant to be replaced periodically while the catheter
and
docking station remain in place.
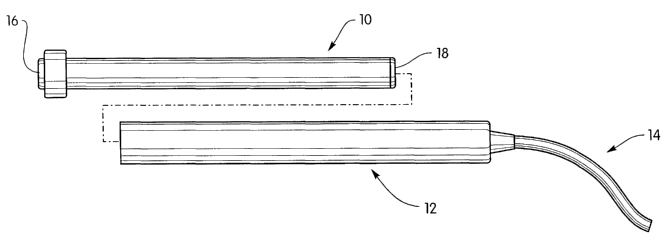
FIGS. 1 and 2 illustrate a beneficial agent delivery system including an
implant 10, a docking station 12, and a catheter 14. The implant 10 is any
known
1o implant for controlled delivery of a beneficial agent. Preferably, the
implant 10 is
an osmotic beneficial agent delivery device having a proximal end 16 with a
rate
controlling membrane and a distal end 18 with a beneficial agent delivery
orifice.
The distal end 18 of the implant 10 is inserted into the tubular docking
station 12
and allows the beneficial agent to be pumped from the implant into the
catheter 14
~5 for delivery to a delivery site. FIG. 2 illustrates the implant 10 inserted
in the
docking station.
FIG. 3 is a schematic representation of one embodiment of a beneficial agent
delivery system. The implant 10 includes a substantially cylindrical capsule
20, a
rate controlling membrane 22, and a distal end cap 24 with a beneficial agent
2o delivery orifice 26. The implant 10 may be of the type having an osmotic
agent
reservoir 28 and a beneficial agent reservoir 32 separated by a movable piston
30.
In this type of implant 10, the structure of the capsule 20 is generally rigid
such that
as the osmotic agent takes in water from the environment and expands, the
capsule
does not expand. As the osmotic agent expands, the osmotic agent causes the
25 movable piston 30 to move, discharging the beneficial agent through the
beneficial
agent delivery orifice 26. The beneficial agent is discharged through the
orifice 26
at the same volumetric rate that water enters the osmotic agent through the
semipermeable walls of the membrane 22.
The docking station 12 according to the exemplary embodiment of FIG. 3
so includes a substantially cylindrical outer case 40. The docking station
case 40
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
6
according to this embodiment extends over at least '/Z of the length of the
implant
10, and preferably over substantially the entire length of the implant. The
catheter
14 is connected to the docking station 12 by a catheter hub 42 and strain
relief
portion 44. The docking station 12 may be fixed to the catheter 14 or to the
catheter
s hub 42 by an adhesive or other attachment mechanism. For example, for a
metal
catheter, the catheter 14 may be connected to the docking station 12 by
welding or
brazing.
The distal end cap 24 of the implant 10 includes an annular ridge 34 on the
distal end. This annular ridge 34 forms a fluid tight seal between the implant
10 and
~o the docking station 12. The seal can be improved by forming the annular
ridge 34
and/or the catheter hub of a resilient material. Alternative sealing
mechanisms may
also be provided, such as, an annular ridge on the catheter hub, or an annular
seal
between the exterior of the implant capsule 20 and the interior of the outer
case 40.
As shown in FIG. 3, a threaded connection 50 is provided between the
~ s implant capsule 20 and the docking station 12 to retain the implant
securely in the
docking station. The threaded connection 50 may be replaced with any other
known
connection system, such as a snap-fit connection, a mechanical locking
mechanism,
or the like.
A flange 54 is preferably provided on the implant 10 which can be grasped
2o during the implant exchange procedure. The system is preferably provided
with a
seal, such as the O-ring 52 which provides a seal between a distal end of the
docking station outer case 40 and the flange 54. The O-ring 52 prevents fluid
from
entering a space between the implant 10 and the outer case 40. This O-ring 52
also
can be used to prevent any beneficial agent from passing out of the system
between
25 the implant 10 and the docking station 12.
FIGS. 4 and 5 illustrate an alternative embodiment of the beneficial agent
delivery system including an implant 10, a docking station 12a, an inner
catheter
14a, and an outer catheter 14b. According to this embodiment, the docking
station
12a is in the form of a distal end cap received over the distal end of the
implant 10.
so The docking station 12a extends over only the distal end of the implant 10.
The
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
7
inner catheter 14a is connected to the distal end of the implant 10 and is
replaceable
with the implant. The outer catheter 14b is connected to the docking station
12a and
has an inner lumen which is sized to receive the inner catheter 14a. The outer
catheter 14b and the docking station 12a are implanted with a distal end of
the outer
catheter being placed accurately at the site of intended delivery of the
beneficial
agent.
The small inner catheter 14a has the advantage that the small lumen of this
catheter can be used to delivery the beneficial agent at a very slow flow
rate. The
inner catheter 14a preferably has a lumen diameter of 0.2 mm or less. This
small
~o lumen catheter accommodates an implant with a slow flow rate, such as 100
ml per
day or slower, and preferably 0.3 to 8 ml per day. The inner catheter 14a can
also
be replaced as a unit with the implant 10 and the outer catheter 14b can be
flushed
or used to draw blood when the implant is removed. Additionally, the inner
catheter 14a can be formed of materials which are impermeable to the
beneficial
~5 agent but does not necessarily have to be formed of a biocompatible
material
because it is shielded by the outer catheter 14b. Meanwhile, the outer
catheter 14b
can be formed of a biocompatible material, such as polyurethane, but does not
need
to be impermeable to the beneficial agent.
Preferably, there is as little space as possible between the inner and outer
2o catheters 14a, 14b to prevent fluid entrapment. The inner catheter 14a may
have a
length which is the same as or different from the length of the outer catheter
14b.
For example, the distal end of the inner catheter 14a may extend beyond the
distal
end of the outer catheter 14b and may have a plurality of holes for beneficial
agent
delivery over a treatment area.
25 FIG. 6 illustrates an alternative embodiment of a beneficial agent delivery
system for delivery of a beneficial agent throughout a targeted tissue site
rather than
at a single point. The system of FIG. 6 is substantially the same as the
system
shown in FIGS. 1 and 2 except that the distal end of the catheter 14c includes
a
plurality of holes 60. The holes 60 allow delivery of the beneficial agent
over an
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
8
area or region of the body. The arrangement, number, and size of the holes 60
will
vary depending on the delivery area and pattern desired.
The system of FIG. 6 is particularly useful for medical applications in which
it is desirable to deliver a beneficial agent over an area or region of the
body. For
example, in bone repair it may be more efficacious to deliver a drug to the
entire
tissue area between two fracture points. The region between the bone at a
fracture
point would be saturated with the drug to encourage bone reformation
throughout
the region. According to one preferred embodiment of the invention, the
catheter
14c or at least the distal tip of the catheter is formed of a bioerodible
material, such
~o as PLGA, so that removal of the catheter is not required.
FIG. 7 illustrates an alternative embodiment of system for connecting a
catheter 114 to an implant 110 with a docking station 112. According to this
embodiment, the implant 110 is provided with a connector 118 in the form of a
flow
guide which is attached to the distal end of the implant. The connector 118
may be
~ 5 attached to the implant 110 by any known mechanical connecting method,
such as
adhesive, crimping, threading, welding, and the like. At the proximal end of
the
catheter 114, an alignment tube 120 is threaded into or otherwise attached to
the
catheter. An outer casing 122 is attached to the alignment tube 120 and
secured to
the proximal end of the catheter 114. The outer casing 122 is connected to the
2o catheter 114 by any known mechanical connecting method, such as adhesive,
crimping, threading, welding, and the like. The alignment tube 120 and the
outer
casing 122 form the docking station 112 for removably attaching the implant
110 to
the catheter 114.
The implant 110 according to the embodiment of FIG. 7 is replaceably
25 connected to the docking station 112 by inserting the alignment tube 120
into the
connector 118. The implant 110 is preferably secured in the docking station by
a
locking mechanism (not shown) which may be a threaded nut, a clamp, or other
mechanical fastener.
In operation of all of the embodiments of the present invention, the docking
3o station 12 and catheter 14 are implanted and may be left in place for an
extended
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
9
period of time, preferably many years. The implant 10 will be implanted, and
removed and replaced periodically. The time period for replacement with vary
depending on the payload and delivery rate of the implant 10. The removal and
replacement of the implant 10 from the docking station 12 may be done by hand
or
with the assistance of specially designed tools. Although the implant 10,
docking
station 12, and catheter 14 are all preferably implanted within the body of a
patient,
the system may also be used with the docking station and implant positioned
outside
of the body.
One example of an application for the present invention is for the
~o intravenous delivery of factor VIII for treatment of hemophilia A. The
catheter is
implanted into a patient with a distal end of the catheter positioned in a
blood vessel.
The implant containing the factor VIII is connected to the catheter by the
docking
station for intravenous delivery. The implant is replaced on a regular basis
as long
as treatment is continued. The treatment can also be changed by changing the
llllplant.
Other uses of the delivery system of the present invention include
intrapericadial, intrathecal, inner ear, vascular, and other treatments. For
example,
anti-athersclerotic agents can be delivered for treatment of coronary artery
disease,
anti-thrombotic agents can be delivered to reduce restenosis, opiates
(fentanyl or
2o sufentanil) can be delivered for treatment of chronic malignant pain,
baclofen can be
delivered for treatment of spasticity (spinal, cerebral palsy), chemotherapy
agents
can be delivered to the inner ear and other organs, gentamicin can be
delivered to
the inner ear to treat tinnitus, and anti-thrombotic agents (such as heparin)
can be
delivered to vascular grafts to promote graft patency.
Examples of semipermeable materials for the membrane plug 22 include, but
are not limited to, polyurethane, polyetherblockamide (PEBAX, commercially
available from ELF ATOCHEM, Inc.), injection-moldable thermoplastic polymers
with some hydrophilicity such as ethylene vinyl alcohol (EVA), and hydrophilic
acrylate polymers, such as hydroxyethyl methacrylate (HEMA). In general, the
ao membrane plug 30 is made from semipermeable materials having a water uptake
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
ranging from 1 % to 80%, and preferably less than 50% . The composition of the
semipermeable membrane plug 22 is permeable to the passage of external liquids
such as water and biological liquids, and it is substantially impermeable to
the
passage of beneficial agents, osmopolymers, osmagents, and the like.
s Other materials for the membrane plug 22 are hytrel polyester elastomers
(DuPont), cellulose esters, cellulose ethers and cellulose ester-ethers, water
flux
enhanced ethylene-vinyl acetate copolymers, semipermeable membranes made by
blending a rigid polymer with water-soluble low molecular weight compounds,
and
other semipermeable materials well known in the art. The above cellulosic
~o polymers have a degree of substitution, D.S., on the anhydroglucose unit,
from
greater than 0 up to 3 inclusive. "Degree of substitution" or "D.S." means the
average number of hydroxyl groups originally present on the anhydroglucose
unit
comprising the cellulose polymer that are replaced by a substituting group.
Representative materials include, but are not limited to, one selected from
the group
~s consisting of cellulose acylate, cellulose diacylate, cellulose triacylate,
cellulose
acetate, cellulose diacetate, cellulose triacetate, mono-, di-, and
tricellulose
alkanylates, mono-, di-, and tricellulose aroylates, and the like. Exemplary
cellulosic polymers include cellulose acetate having a D.S. up to 1 and an
acetyl
content up to 21 % ; cellulose acetate having a D.S. of 1 to 2 and an acetyl
content of
21 % to 35 % ; cellulose acetate having a D.S. of 2 to 3 and an acetyl content
of 35 %
to 44.8 % , and the like. More specific cellulosic polymers include cellulose
propionate having a D. S. of 1.8 and a propionyl content of 39.2 % to 45 % and
a
hydroxyl content of 2.8 % to 5.4 % ; cellulose acetate butyrate having a D.S.
of 1.8
and an acetyl content of 13 % to 15 % and a butyryl content of 34 % to 39 % ;
cellulose acetate butyrate having an acetyl content of 2 % to 29 % , a butyryl
content
of 17 % to 53 % and a hydroxyl content of 0.5 % to 4.7 % ; cellulose acetate
butyrate
having a D.S. of 1.8, and acetyl content of 4% average weight percent and a
butyryl
content of 51 % ; cellulose triacylates having a D.S. of 2.9 to 3 such as
cellulose
trivalerate, cellulose trilaurate, cellulose tripalmitate, cellulose
trisuccinate, and
3o cellulose trioctanoate; cellulose diacylates having a D.S. of 2.2 to 2.6
such as
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
11
cellulose disuccinate, cellulose dipalmitate, cellulose dioctanoate, cellulose
dipentate; coesters of cellulose such as cellulose acetate butyrate and
cellulose,
cellulose acetate propionate, and the like.
Materials which may be used for the capsule 20 and the outer case 40
should be sufficiently strong to ensure that the capsule and outer case will
not leak,
crack, break, or distort under stresses to which they would be subjected
during
implanting or under stresses due to the pressures generated during operation.
The
capsule 20 and outer case 40 may be formed of chemically inert and
biocompatible,
natural or synthetic materials which are known in the art. The material of the
~o capsule 20 and outer case 40 is preferably a non-bioerodible material which
remains
in the patient after use, such as titanium. However, the material of the
capsule may
alternatively be of bioerodible material which bioerodes in the environment
after
dispensing of the beneficial agent. Generally, preferred materials for the
capsule
are those acceptable for human implants.
In general, typical materials of construction suitable for the capsule 20 and
outer case 40 according to the present invention include non-reactive polymers
or
biocompatible metals or alloys. The polymers include acrylonitrile polymers
such
as acrylonitrile-butadiene-styrene terpolymer, and the like; halogenated
polymers
such as polytetraflouroethylene, polychlorotrifluoroethylene, copolymer
2o tetrafluoroethylene and hexafluoropropylene; polyimide; polysulfone;
polycarbonate; polyethylene; polypropylene; polyvinylchloride-acrylic
copolymer;
polycarbonate-acrylonitrile-butadiene-styrene; polystyrene; and the like.
Metallic
materials useful for the capsule include stainless steel, titanium, platinum,
tantalum,
gold, and their alloys, as well as gold-plated ferrous alloys, platinum-plated
ferrous
alloys, cobalt-chromium alloys and titanium nitride coated stainless steel.
In general, materials suitable for use in the piston 30 are elastomeric
materials including the non-reactive polymers listed above, as well as
elastomers in
general, such as polyurethanes and polyamides, chlorinated rubbers, styrene-
butadiene rubbers, and chloroprene rubbers.
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
12
The osmotic tablet is an osmotic agent which is a fluid-attracting agent used
to drive the flow of the beneficial agent. The osmotic agent may be an
osmagent, an
osmopolymer, or a mixture of the two. Species which fall within the category
of
osmagent, i.e., the non-volatile species which are soluble in water and create
the
osmotic gradient driving the osmotic inflow of water, vary widely. Examples
are
well known in the art and include magnesium sulfate, magnesium chloride,
potassium sulfate, sodium chloride, sodium sulfate, lithium sulfate, sodium
phosphate, potassium phosphate, d-mannitol, sorbitol, inositol, urea,
magnesium
succinate, tartaric acid, raffmose, and various monosaccharides,
oligosaccharides
~o and polysaccharides such as sucrose, glucose, lactose, fructose, and
dextran, as well
as mixtures of any of these various species.
Species which fall within the category of osmopolymer are hydrophilic
polymers that swell upon contact with water, and these vary widely as well.
Osmopolymers may be of plant or animal origin, or synthetic, and examples of
~s osmopolymers are well known in the art. Examples include: poly(hydroxy-
alkyl
methacrylates) with molecular weight of 30,000 to 5,000,000,
poly(vinylpyrrolidone) with molecular weight of 10,000 to 360,000, anionic and
cationic hydrogels, polyelectrolyte complexes, polyvinyl alcohol) having low
acetate residual, optionally cross-linked with glyoxal, formaldehyde or
2o glutaraldehyde and having a degree of polymerization of 200 to 30,000, a
mixture
of methyl cellulose, cross-linked agar and carboxymethylcellulose, a mixture
of
hydroxypropyl methylcellulose and sodium carboxymethylcellulose, polymers of N-
vinyllactams, polyoxyethylene-polyoxypropylene gels, polyoxybutylene-
polyethylene block copolymer gels, carob gum, polyacrylic gels, polyester
gels,
25 polyurea gels, polyether gels, polyamide gels, polypeptide gels, polyamino
acid
gels, polycellulosic gels, carbopol acidic carboxy polymers having molecular
weights of 250,000 to 4,000,000, Cyanamer polyacrylamides, cross-linked indene-
maleic anhydride polymers, Good-Rite polyacrylic acids having molecular
weights
of 80,000 to 200,000, Polyox polyethylene oxide polymers having molecular
CA 02395362 2002-06-25
WO 01/47595 PCT/US00/34315
13
weights of 100,000 to 5,000,000, starch graft copolymers, and Aqua-Keeps
acrylate
polymer polysaccharides.
Although the present invention has been described with respect to an osmotic
system having as osmotic agent and a beneficial agent, it should be understood
that
the osmotic agent may be incorporated into the beneficial agent.
In one embodiment of the invention, the beneficial agents contained in the
beneficial agent reservoir 32 are flowable compositions such as liquids,
suspension,
slurries, pastes, or powders and are poured into the capsule 20 prior to
insertion of
the membrane plug 22. Alternatively, such flowable compositions may be
injected
io with a needle through a delivery port or membrane plug, which allows for
filling
without air bubbles. Still further alternatives may include any of the wide
variety of
techniques known in the art for forming capsules used in the pharmaceutical
industry.
Animals to whom drugs may be administered using systems of this invention
include humans and other animals. The invention is of particular interest for
application to humans and household, sport, and farm animals, particularly
mammals.
The present invention applies to the administration of beneficial agents in
general, which include any physiologically or pharmacologically active
substance.
2o The beneficial agent may be any of the agents which are known to be
delivered to
the body of a human or an animal such as drug agents, medicaments, vitamins,
nutrients, or the like.
The beneficial agent can be present in this invention in a wide variety of
chemical and physical forms, such as solids, liquids and slurries. On the
molecular
25 level, the various forms may include uncharged molecules, molecular
complexes,
and pharmaceutically acceptable acid addition and base addition salts such as
hydrochlorides, hydrobromides, sulfate, laurylate, oleate, and salicylate. For
acidic
compounds, salts of metals, amines or organic cations may be used. Derivatives
such as esters, ethers and amides can also be used. An active agent can be
used
3o alone or mixed with other active agents.
CA 02395362 2002-06-25
WO 01/47595 PCT/LJS00/34315
14
According to other embodiments of the present invention, the implant 10
may take different forms. For example, the piston 30 may be replaced with a
member such as a diaphragm, partition, pad, flat sheet, spheroid, or rigid
metal
alloy, and may be made of any number of inert materials. Furthermore, the
osmotic
device may function without the piston 30, having simply an interface between
the
osmotic agent/fluid additive and the beneficial agent or having the osmotic
agent
incorporated in the beneficial agent.
~ o While the invention has been described in detail with reference to the
preferred embodiments thereof, it will be apparent to one skilled in the art
that
various changes and modifications can be made and equivalents employed,
without
departing from the present invention.