Note: Descriptions are shown in the official language in which they were submitted.
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
CATALYTIC DISTILLATION REACTOR
CROSS-REFERENCE TO RELATED APPLICATIONS
The present application claims the benefit of 35 U.S.C. 111(b) provisional
application
Serial No. 60/66,025 filed November 17, 1999, and entitled Catalytic
Distillation Reactor for
Fischer-Tropsch Synthesis.
STATEMENT REGARDING FEDERALLY SPONSORED
RESEARCH OR DEVELOPMENT
Not applicable.
FIELD OF THE INVENTION
The present invention relates to a method and apparatus for converting
synthesis gas,
i.e., a mixture of carbon monoxide and hydrogen, to hydrocarbons, typically
referred to as the
Fischer-Tropsch reactions or the Fischer-Tropsch process. Particularly this
invention relates to
the use of a catalytic distillation reactor to achieve both reaction of the
syngas and separation of
the hydrocarbon product. Separation occurs through distillation and other mass
transfer
techniques. The invention also relates to the use of various catalyst
materials to promote and
control the Fischer-Tropsch reaction.
BACKGROUND OF THE INVENTION
Large quantities of methane, the main component of natural gas, are available
in many
areas of the world. Methane can be used as a starting material for the
production of other
hydrocarbons. The conversion of methane to hydrocarbons is typically carned
out in two steps.
In the first step methane is reformed with water or partially oxidized with
oxygen to produce
carbon monoxide and hydrogen (i.e., synthesis gas or syngas). In a second
step, the syngas is
converted to hydrocarbons.
This second step, the preparation of hydrocarbons from synthesis gas, is well
known in
the art and is usually referred to as Fischer-Tropsch synthesis, the Fischer-
Tropsch process, or
Fischer-Tropsch reaction(s). Catalysts for use in such synthesis usually
contain a catalytically
active metal from one of the Groups 8, 9, or 10 (in the New notation of the
periodic table of the
elements, which is followed throughout). In particular, iron, cobalt, nickel,
and ruthenium have
been abundantly used as the catalytically active metals. Cobalt and ruthenium
have been found
to be most suitable for catalyzing a process in which synthesis gas is
converted to primarily
hydrocarbons having five or more carbon atoms (i. e., where the CS+
selectivity of the catalyst
is high).
1
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
The Fischer-Tropsch reaction involves the catalytic hydrogenation of carbon
monoxide
to produce a variety of products ranging from methane to higher alkanes and
aliphatic alcohols.
The methanation reaction was first described in the early 1900's. The later
work by Fischer and
Tropsch dealing with higher hydrocarbon synthesis was described in the 1920's.
The process has been considered for the conversion of carbonaceous feedstock,
e.g.,
coal or natural gas, to higher value liquid fuel or petrochemicals. The first
major commercial
use of the Fischer-Tropsch process was in Germany during the 1930's. More than
10,000 B/D
(barrels per day) of products were manufactured with a cobalt based catalyst
in a fixed-bed
reactor. This work has been described by Fischer and Pichler in German Patent
No. 731,295
issued August 2, 1936.
Motivated by production of high-grade gasoline from natural gas, research on
the
possible use of the fluidized bed for Fischer-Tropsch synthesis was conducted
in the United
States in the mid-1940s. Based on laboratory results, Hydrocarbon Research,
Inc. constructed a
dense-phase fluidized bed reactor, the Hydrocol unit, at Carthage, Texas,
using powdered iron
as the catalyst. Due to disappointing levels of conversion, scale-up problems,
and rising natural
gas prices, operations at this plant were suspended in 1957. Research has
continued, however,
on developing Fischer-Tropsch reactors such as slurry-bubble columns, as
disclosed in U.S
Patent No. 5,348,982 issued September 20, 1994.
Commercial practice of the Fischer-Tropsch process has continued from 1954 to
the
present day in SASOL plants operated in South Africa. These plants use iron-
based catalysts,
and produce gasoline in relatively high-temperature fluid-bed reactors and wax
in relatively
low-temperature fixed-bed reactors.
Despite the research that has been done to date, the need exists for further
improvement
in commercial Fischer-Tropsch processes. For example, research is continuing
on the
development of more efficient Fischer-Tropsch catalyst systems and reaction
systems that
increase the selectivity for high-value hydrocarbons in the Fischer-Tropsch
product stream. In
particular, a number of studies describe the behavior of iron, cobalt or
ruthenium based
catalysts in various reactor types, together with the development of catalyst
compositions and
preparations.
There are significant differences in the molecular weight distributions of the
hydrocarbon products from different Fischer-Tropsch reaction systems. Product
distribution or
product selectivity depends heavily on the type and structure of the catalysts
and on the reactor
type and operating conditions. Accordingly, it is highly desirable to maximize
the selectivity of
the Fischer-Tropsch synthesis to the production of high-value liquid
hydrocarbons, such as
2
CA 02396581 2002-05-15
WO 01/36066 PCTNS00/31514
hydrocarbons with five or more carbon atoms per hydrocarbon chain. These
hydrocarbons,
which correspond to gasoline or diesel products, are expected to be in great
demand.
Traditional methods of Fischer-Tropsch synthesis produce a range of
hydrocarbons.
This range of hydrocarbons based on the carbon chain length of the hydrocarbon
is discussed in
U.S. Patent No. 4,619,910, which is incorporated herein by reference. This
well-known
distribution is known as the Anderson-Schulz-Flory distribution. In general,
the range of
hydrocarbons produced in Fischer-Tropsch processes may be characterized by the
Anderson-
Schulz-Flory distribution with a suitable value for the parameter alpha,
regardless of catalyst
type.
Because of the range of hydrocarbon products, typical systems that use the
Fischer-
Tropsch process provide a separation stage that follows the reaction stage.
The separation stage
is often one or more distillation columns. The distillation columns separate
the hydrocarbon
product into fractions according to boiling point. The lighter hydrocarbons,
having lower
boiling points, will vaporize and pass to the overhead region of a
distillation column, where
they can be removed as one product stream. The heavier hydrocarbons, having
higher boiling
points, will condense and fall to the lower region of the distillation column,
where they can be
removed as a separate product stream. In addition, any one or more of the
product streams
having intermediate compositions can be removed from the column at
intermediate points
between the top and the bottom and may then be sent to other columns for
further separation if
desired.
Paraffins constitute a specific type of reaction product of the Fischer-
Tropsch synthesis
included within the hydrocarbons. Paraffins generally do not react further
under conditions
applicable to the Fischer-Tropsch synthesis. Water is also produced during
Fischer-Tropsch
synthesis. Recent research indicates that water can deactivate a Fischer-
Tropsch catalyst in
certain circumstances. Rothaemel, Hanssen, Blekkan, Schanke and Holmen, The
Effect of
Water on Cobalt Fischer-Tropsch Catalysts Studied by Steady-State Isotropic
Transient,
Kinetic Analysis, 38 Catalysis Today 79-84 (1997); Schanke, Hilmen, Bergene,
Kinnari, Rytter,
Adnanes and Holmen, Reoxidation and Deactivation of Supported Cobalt Fischer-
Tropsch
Catalysts, Energy & Fuels, Vol. 10 No.4 (July/August 1996) p. 867-872.
In addition, the catalytic Fischer-Tropsch synthesis, when practiced on a
commercial
scale, generates heat that must be removed from the reaction vessel. Fischer-
Tropsch synthesis
reactions are highly exothermic, and reaction vessels must be designed with
adequate heat
exchange capacity. Large scale reactors, which potentially offer the economic
advantages that
come with higher volumes, must presently include, at significant cost,
sufficient heat transfer
3
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
equipment within the reactor to remove the heat generated during the reaction.
The traditional
method for doing this, and a method that may be used in the present invention,
is to place heat
removal equipment inside the reaction vessel. A typical internal heat removal
arrangement
comprises a system of tubes within one or more reaction chambers. The tubes
contain a fluid
such as water, or any other acceptable fluid, which acts as the heat exchange
medium. In
operation, the heat generated within the reaction chamber passes through the
heat exchange
tubes and heats the fluid therein. The heat exchange fluid is then pumped
outside the reaction
vessel, where the heat is released, preferably through a heat exchanger. This
process can be
carried out continuously, with the heat exchange fluid circulating through the
reaction chamber.
A shortcoming of the internal heat exchange process is that the internal heat
exchange tubes
occupy reactor space. Internal heat removal equipment may therefore decrease
the reactor
volume that is available for Fischer-Tropsch synthesis, thus limiting the
capacities and
efficiencies for a given reactor.
Notwithstanding the foregoing patents and teachings, there remains a need for
a
continuous Fischer-Tropsch synthesis by which the production of certain
hydrocarbons can be
maximized and controlled.
The present invention overcomes the deficiencies of the prior art.
SUMMARY OF THE INVENTION
The present invention provides an apparatus and method for producing
hydrocarbons
according to the Fischer-Tropsch synthesis. Particularly, the invention
provides a catalytic
distillation reactor and its use for Fischer-Tropsch synthesis. In a preferred
catalytic distillation
reactor a single apparatus simultaneously achieves both the reaction of
hydrocarbons from
synthesis gas starting materials and the separation of the hydrocarbon product
into various
product streams.
A preferred embodiment includes a catalytic distillation reactor in which
synthesis gas
flows through one or more reaction chambers, which may include beds of
catalyst material,
such as one or more supported catalysts, including without limitation, cobalt,
ruthenium, iron
based catalysts, or other Fischer-Tropsch catalysts as are well known in the
art, at conversion-
producing conditions of temperature and pressure. The Fischer-Tropsch
reactions occur in the
reaction chambers. Heavier hydrocarbon products such as waxes fall to the
bottom of the
column reactor, where they can be removed, and progressively lighter gaseous
hydrocarbon
products flow to the upper regions of the column reactor. At one or more of
various points on
the column reactor, hydrocarbon products may be removed from the reactor.
Hydrocarbons
can be also condensed and refluxed into the reactor at any of one or more
various points.
4
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
An additional aspect of a preferred embodiment of the invention is that it
allows for
greater control of the Fischer-Tropsch product selectivity. As further
explained herein, in a
preferred embodiment, the conversion of synthesis gas feed to end hydrocarbon
products
occurs in a series of successive reaction chambers. The degree of conversion
may be optimized
by controlling the amount and type of catalyst material in each reaction
chamber, as well as the
reaction conditions in each reaction chamber, including the temperature,
pressure, and the
amount and concentration of reactants and products in the reaction chamber.
A further aspect of a preferred embodiment of the invention is that it allows
for
optimization of the hydrocarbon products produced. A typical Fischer-Tropsch
process
produces a range of hydrocarbon products including waxes, diesel, gasoline,
LPG (liquefied
petroleum gas) and gases such as methane, ethane, propane, and butane. A
preferred
embodiment of the present invention allows the more desirable product streams,
such as
kerosene and diesel, to be maximized, while the other product streams are
minimized.
Selectivity control is also enhanced since the heavy material will disengage
from the catalyst
and fall to the bottom. 'The bottom temperature will not boil the heaviest
hydrocarbons. The
light hydrocarbons are therefore in contact with the catalyst for a longer
time.
Another aspect of a preferred embodiment of the present invention is that it
allows for
the removal of water produced during Fischer-Tropsch synthesis from the
desired
hydrocarbon products. Water removal has the advantage of reducing the H20
partial pressure
in reactor sections, thus assisting with the Fischer-Tropsch synthesis. In
addition, water
removal increases the lifespan of a Fischer-Tropsch catalyst.
Still another aspect of a preferred embodiment of the present invention is
that it permits
the removal of paraffins produced during the Fischer-Tropsch synthesis.
Paraffins, which do
not generally react further under Fischer-Tropsch conditions, may be removed
at one or more
points of the catalytic distillation reactor. Removing paraffins has the
advantage of decreasing
the paraffins' partial pressure in various sections of the reactor, and
thereby assisting in the
Fischer-Tropsch synthesis.
A preferred embodiment of the present invention provides a still further
advantage of
providing a solution to the limitations of internal heat exchange equipment.
Hot fluids may be
pumped from one or more regions of the catalytic distillation reactor. These
heated fluids are
directed to one or more heat exchangers that are positioned outside of the
catalytic distillation
reactor. While passing through the heat exchanger, the fluids are cooled. Once
the fluids are
cooled as desired, they are returned to the catalytic distillation reactor
through return lines
where they can continue the process of reaction and separation. By providing
for a heat
5
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
exchange process outside the reaction vessel itself, the limitations
associated with internal heat
exchange means are avoided.
Thus, the present invention comprises a combination of features and advantages
that
enable it to overcome various problems of Fischer-Tropsch synthesis. The
various
characteristics described above, as well as other features, objects, and
advantages, will be
readily apparent to those skilled in the art upon reading the following
detailed description of the
preferred embodiments of the invention, and by referring to the accompanying
drawings.
Other objects and advantages of the invention will appear from the following
description. For a better understanding of this invention, reference is made
to the detailed
description thereof which follows, taken together with the subjoined claims.
BRIEF DESCRIPTION OF THE DRAWINGS
For a detailed description of a preferred embodiment of the present invention,
reference
will now be made to the accompanying drawings, which form a part of the
specification, and
wherein:
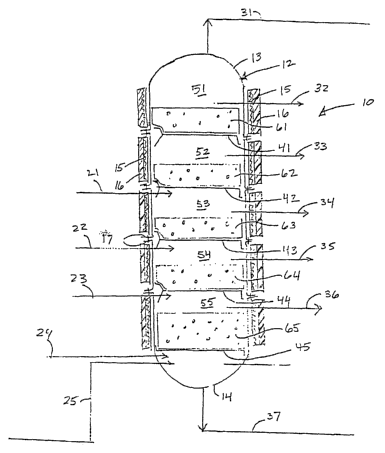
Figure 1 is a schematic view of an embodiment of a catalytic distillation
reactor
constructed in accordance with the present invention;
Figure 2 is a schematic view of an alternative embodiment of the present
reaction vessel
having different diameters at different vertical positions on the reaction
vessel;
Figure 3 is a schematic view of a second alternative embodiment of the present
reaction
vessel configured such that one reaction chamber contains no catalyst
material;
Figure 4 is a schematic view of a third alternative embodiment of the present
reaction
vessel having external heat exchange lines and heat exchangers;
Figure 5 is a schematic view of a fourth alternative embodiment of the present
reaction
vessel having external heat exchange lines, heat exchangers, water separation
stages, paraffin
separation stages and return lines;
Figure 6 is a view of a fifth embodiment of the present reaction vessel having
catalyst
beds which may be of varying thickness;
Figure 7 is a view of a plurality of the present reaction vessels running in
parallel and
surrounded by a common cooling medium;
Figure 8 is a view of a plurality of the present reaction vessels running in
parallel and
surrounded by individual cooling units.
DETAILED DESCRIPTION OF THE INVENTION
As described in detail below, a preferred embodiment of the present invention
includes
a reaction vessel that includes a catalyst for driving the reaction and an
apparatus and method
6
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
for continuously separating and recovering the reaction products. The vessel
also allows for the
continuous feed of various feedstocks into the vessel and for the continuous
removal of heat
from the vessel.
Vessel
Referring initially to Figure 1, a preferred embodiment of the present
catalytic
distillation reactor 10 includes a reaction vessel 12, which generally follows
the shape of any of
the various distillation columns and mass transfer reactors well known in the
art. According to
one preferred embodiment, the reactor is generally tubular or cylindrical. The
interior of
reaction vessel 12 is substantially in the form of a capped hollow tube.
During operation, the
reaction vessel 12 typically rests in an upright position. Reaction vessel 12
may also conform
to other shapes and configurations such as square, oval or rectilinear.
Reaction vessel 12 may
preferably be formed of multiple cylindrical sections. In this configuration,
each of the multiple
cylindrical sections includes a flange at each end so that the sections can be
bolted together to
form the overall reaction vessel 12 of Figure 1. Caps 13 and 14, disposed on
the upper and
lower end of the reaction vessel, respectively, act to seal the reaction
vessel 12 so that it can be
pressurized to conversion-promoting conditions. Reaction vessel 12 is
typically constructed of
any material capable of withstanding the temperatures and pressures
encountered in Fischer-
Tropsch synthesis. In one preferred embodiment, reaction vessel 12 is
constructed of carbon
steel.
In an alternative embodiment shown in Figure 2, the diameter of reaction
vessel 12
varies with vertical position. The reaction vessel shown in Figure 2 has three
horizontal
sections with different diameters. As is well known in the art, a distillation
column may be
designed to have an upper region having a larger diameter than a lower region
of the distillation
column. This is done to facilitate the expansion and flow of lighter gases in
the upper region of
the column. In Figure 2, three reaction zones 51, 52, 53 are shown, although
it will be
understood that more or fewer zones could be created, having different or
similar dimensions.
Because the reactors may be shapes other than cylindrical, as used herein, the
word "diameter"
will mean, without limitation, the traditional diameter of a circle as well as
any analogous
measurements for different shapes (e.g., the diagonal length of a square).
Positioned inside of reaction vessel 12 of Figure 1 are a plurality of trays
41, 42, 43, 44,
and 45, which define the lower boundaries of a plurality of reaction chambers
51, 52, 53, 54,
and 55, respectively. In a preferred embodiment, trays 41, 42, 43, 44, and 45
conform
substantially to the interior dimensions of said reaction vessel. It is also
preferred that each tray
lie in a substantially horizontal position within reaction vessel 12, although
it is contemplated
7
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
that the trays can be inclined. Trays 41, 42, 43, 44, and 45 can be
constructed of any material
suitable for use in a chemical reactor, including carbon steel. Trays 41, 42,
43, 44, and 45 are
typically fastened to the interior of the reaction vessel 12 by conventional
mechanical means,
such as, but not limited to, bolts, welds, screws, pins, hangers, and
interlocking fittings.
Although as shown in Figure 1 the positions of the individual trays 41, 42,
43, 44, and
45 correspond to the ends of the vessel segments, it will be understood that
trays 41, 42, 43, 44,
and 45 can be set at varying and adjustable vertical positions within reaction
vessel 12. The
reaction chambers 51-55 represent individual regions within the reaction
vessel 12 in which
simultaneous operations of reaction and physical separation take place. It is
not necessary that
the reaction chambers 51, 52, 53, 54, and 55 be equal in height. Similarly,
other embodiments
may have a different number of reaction chambers than that shown and the
reaction chambers
may each have different configurations as explained below.
Passageways through or around trays 51-55 may be provided by a series of
bubble caps,
downcomers, weirs, filters, sieves, sintered metal sieves, and/or other
standard items that are
typically used for mass transfer of gaseous and liquid materials in a
distillation column. Other
materials commonly used in distillation columns to assist in the distillation
process may be
used in reaction vessel 12 as a matter of engineering design choice and
optimization. Some
examples of such materials are baffles, plastic or metal saddles, and rings.
Furthermore, according to the present invention, each tray may have any one of
several
distinct configurations. For example, one or more trays may consist of a metal
tray and bubble
caps. Other trays may include a filter or sieve structure. Not every tray
needs to have the same
configuration and, in one preferred embodiment, each tray has a configuration
that has been
optimized for the particular reaction/separation combination to be performed
on that tray.
Positioned above trays 41-45 are catalytic materials 61, 62, 63, 64, and 65,
respectively.
'The catalytic materials preferably comprise all of the necessary components
of a Fischer
Tropsch catalyst or catalyst system. Thus, active catalyst components such as
catalytically
active metals for Fischer-Tropsch synthesis and their precursor and derivative
compounds, are
included within the definition of "catalytic material" as used herein. Support
materials such as
aluminas, silicas, and other catalyst support materials, as are well known in
the art, are likewise
included within the definition of "catalytic material" as used herein.
Promoters, activators, and
other materials that facilitate catalysis are also included within the
definition of "catalytic
material."
While catalytic materials 61-65 are shown occupying less than all of the
volume of their
respective chambers, the volume of the catalytic materials may be increased or
decreased. For
8
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
example, in some embodiments, the catalytic material fills each chamber. It is
further
contemplated that, in some configurations, the catalyst may be supported on a
packing material
or other support that is also capable of functioning as a distillation
packing, so as to enhance
separation. Alternatively, non-catalytic distillation packing or the like (not
shown), can be used
in conjunction with the catalytic materials) 61-65. In this case, the
distillation packing can be
used above one or more portions of catalytic material, or can be mixed with
the catalytic
material. Additionally and alternatively, the distillation packing, whether
catalytic or non-
catalytic, may be dump packed or structurally packed.
A plurality of feed lines 21, 22, 23, and 24 are preferably provided for
feeding the
desired gases into reaction vessel 12. Although four feed lines are shown, any
number of feed
lines, more or fewer than four, may be used. Preferably, each of the feed
lines 21-24 enters the
reaction vessel 12 into one of the reaction chambers 52-55, respectively, as
shown in Figure 1.
In other embodiments, feed lines 21, 22, 23, and 24 may be positioned
according to a variety of
configurations so as to achieve certain desired effects. For example, all feed
lines may enter the
reaction vessel in one reaction chamber. Compressors, heaters, and the like
(not shown) can be
provided on feed lines 21-24, so that the feed materials can be preheated and
pressurized if
desired. For example, it may be desired to preheat and pressurize the feed
materials such that
they enter the reactor at conditions compatible with those of the reaction
vessel 12 at their point
of entry.
According to a preferred embodiment, the reaction/separation products exit
reaction
vessel 12 through on or more of product lines 31, 32, 33, 34, 35, 36, and 37.
The compositions
of the various products passing through these product lines will vary
depending on operating
parameters, as described below.
Still referring to Figure 1, a plurality of heating coils 15 are preferably
positioned
around reaction vessel 12. Heating coils 15 may be selected from among the
heating coils
commonly used in the art for reactors and distillation columns. Insulation 16
is positioned
around heating coils 15 and is preferably placed around the exterior of
reaction vessel 12 and
coils 15 as shown in Figure 1. A separate heating coil 15 is preferably
disposed around each
individual reaction chamber S l, 52, 53, 54, and 55 and each coil 15 is
preferably individually
controlled so as to maintain each of the reaction chambers at a specific
desired temperature.
In the embodiment shown in Figure 1, a catalytic material 61-65 is present in
each of
the reaction chambers 51-55. In other embodiments of the present invention,
however, one or
more reaction chambers 51-55 may have no catalyst material present. By way of
illustration
and not limitation, in such an embodiment, any tray and its associated
reaction chamber that
9
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
does not contain catalytic material, would be configured to act essentially as
a stage of a
distillation column. Figure 3 shows a reaction vessel 70 configured so that
one reaction
chamber 72 does not contain a catalyst material. As shown in Figure 3, Fischer-
Tropsch
synthesis occurs in a lower reaction chamber 73 of the reaction vessel 70. The
conditions
present in lower reaction chamber 73 (the temperature, pressure, catalyst
material, etc.) are
chosen to optimize Fischer-Tropsch synthesis consistent with the relative
position of reaction
chamber 73 in the reaction vessel 70. Catalyst material 75 in reaction chamber
73 rests on tray
78 or is otherwise supported. Lighter hydrocarbons move upward from reaction
chamber 73.
These hydrocarbons may be moved upward through a series of pure distillation
stages that
contain no catalyst material, such as reaction chamber 72. Reaction chamber
72, defined by
trays 76 and 77, contains no catalyst material, and distillation in reaction
chamber 72 is
achieved through bubble caps 79 that are positioned on tray 77. Once the
lighter hydrocarbons
reach an upper region of reaction vessel 70, the hydrocarbons encounter a new
set of conditions
that promote Fischer-Tropsch synthesis in reaction chamber 71, which contains
catalyst
material 74. Reaction chamber 71 has conditions chosen to optimize Fischer-
Tropsch synthesis
in the relative position of upper reaction chamber 71. While the lighter
hydrocarbons migrate
to upper regions of the reaction vessel 70, heavier hydrocarbons move in the
opposite direction
to the lower areas of the reaction vessel 70. Thus, the individual reaction
chambers in the
present device can be uniquely tailored to promote Fischer-Tropsch synthesis
for the kinds of
hydrocarbons that predominate in each such reaction chamber.
Refernng now to Figure 6, a catalytic distillation reactor 10 is provided in
which layers
of catalyst material 61, 62, 63 of varying thickness are staged between
distillation/heat removal
chambers 51, 52. The thickness of the catalyst materials 61, 62, 63 may be
varied such as to
control the reaction and the temperature rise within the distillation/heat
removal chambers 51,
52. Any excess heat would be removed by the heat removal coils 15, which may
consist of
steam coils or any other acceptable heat removal system which is well known in
the art. The
heat removed from the chambers may then be disposed of by any acceptable means
(e.g., inter-
process heat exchange (not shown)).
Referring now to Figure 7, a catalytic distillation reactor segment is
provided having a
plurality of reaction chambers 51, 52, 53 running in parallel inside of an
outer shell 100.
Within the outer shell 100 and external to the reaction chambers 51, 52, 53 is
provided a
cooling medium which may be any acceptable cooling medium as is well known in
the art (e.g.,
steam). Preferably, the distillation reactor segment 200 is adapted to be
stacked on other
distillation reactor segments and would contain mechanisms for product removal
(such as those
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
shown in Figure 1, reference nos. 31-37), liquid redistribution (such as those
shown in Figure 3,
reference nos. 81 and 82), and gas/liquid feed streams (such as those shown in
Figure l,
reference nos. 21-24).
Refernng now to Figure 8, a plurality of catalytic distillation reactor
segments 200 are
run in parallel, each with separate external heat removal units 15 for
temperature control.
Preferably, the distillation reactor segments 200 are adapted to be stacked on
other distillation
reactor segments and would contain mechanisms for product removal (such as
those shown in
Figure 1, reference nos. 31-37), liquid redistribution (such as those shown in
Figure 3, reference
nos. 81 and 82), and gas/liquid feed streams (such as those shown in Figure 1,
reference nos.
21-24).
Other common features of distillation columns may be incorporated into the
design of
the present reaction vessel. These include manholes or manways, which provide
access to the
interior and facilitate cleaning of the vessel, and inspection ports or
windows to permit visual
inspection of the interior of the reaction vessel while in use. It is also
common practice to
provide gangways or ladders on the exterior of the catalytic distillation
reactor to permit
physical access to all parts of the catalytic distillation reactor.
Catalysts
Catalytic materials 61-65 may be present in different amounts, concentrations,
forms
and configurations in each of the reaction chambers 51-55. T'he presence of
any mechanical
apparatus necessary to position the catalyst material within the column will
be understood and
will not be further recited herein. Such a mechanical apparatus may include,
by way of
illustrative example only, catalyst containers, holders, baskets, racks, or
nets. Similarly, any
suitable configuration may be employed for catalytic materials 61-65. For
example, fixed bed,
fluidized bed, slurry phase, slurry bubble column, or ebulliating bed systems,
among others,
may be used. Accordingly, the size and physical form of the catalyst materials
61-65 may vary
depending on the reaction chamber in which they are to be used.
The catalytic distillation reactor of the preferred embodiment is preferably
used with
catalysts active for Fischer-Tropsch synthesis. However, there is no
particular catalyst type that
must be used in the reaction vessel; indeed, reaction vessel 12 may be used
with any of the
Fischer-Tropsch catalysts now commonly used in Fischer-Tropsch synthesis
reactors, or with
other types of catalysts. In a similar vein, the preferred embodiment may
operate with any
physical form of the catalyst, or as it is sometimes called, the catalyst
system. In other words
the catalytic distillation reactor will function with packed bed, slurry bed,
or other types of
catalysts.
11
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
According to one preferred embodiment, the active catalyst components present
in the
catalyst materials include any metal known to promote Fischer-Tropsch
synthesis: By way of
illustration and not limitation, these active metals comprise Mn, Fe, Co, Ni,
Tc, Ru, Rh, Pd, Re,
Os, Lr, Pt, and combinations thereof, among others.
Active catalyst components used in the catalyst material of the preferred
embodiment
may be carried or supported on any suitable support, including but not limited
to supports
selected from the group including silica, titanic, titania/alumina, zirconia,
alumina, aluminum
fluoride, and fluorided alumina, silica, titanic, titania/alumina, and
combinations thereof. Other
supports, well known in the art, may also be used. Aluminum fluoride supports
are defined as
at least one aluminum fluoride (e.g., alpha-A1F3, beta-A1F3, delta-A1F3, eta-
A1F3,
gamma-A1F3, kappa-A1F3 and/or theta-A1F3). Preferred supports include silica,
alumina and
aluminum fluoride. Preferred aluminum fluoride supports are aluminum fluorides
that are
primarily alpha-A1F3 and/or beta-A1F3.
Other catalyst materials may be used. For example, U.S. Pat. Nos. 4,619,910;
4,670,472; and 4,681,867, hereby incorporated herein by reference, describe a
series of
catalysts for use in a slurry Fischer-Tropsch process in which synthesis gas
is selectively
converted to higher hydrocarbons of relatively narrow carbon number range.
Reactions of the
catalyst with air and water and calcination are specifically avoided in the
catalyst preparation
procedure. The catalysts are activated in a fixed-bed reactor by reaction with
CO + H2 prior to
slurrying in the oil phase in the absence of air.
Further, U.S. Pat. No. 4,477,595 discloses ruthenium on titanic as a
hydrocarbon
synthesis catalyst for the production of CS to C40 hydrocarbons, with a
majority of paraffins in
the CS to C20 range. U.S. Pat. No. 4,542,122 discloses a cobalt or cobalt-
thoria on titanic
having a preferred ratio of rutile to anatase as a hydrocarbon synthesis
catalyst. U.S. Pat. No.
4,088,671 discloses a cobalt-ruthenium catalyst where the support can be
titanic but preferably
is alumina for economic reasons. U.S. Pat. No. 4,413,064 discloses an alumina
supported
catalyst having cobalt, ruthenium and a Group 3 or Group 4 metal oxide, e.g.,
thoria. European
Patent No. 142,887 discloses a silica supported cobalt catalyst together with
zirconium,
titanium, ruthenium and/or chromium. The patents identified in this paragraph
are hereby
incorporated herein by reference.
Aluminas that have been treated with fluosilicic acid (H2SiF6) such as those
described
in European Patent Application No. EP 497,436, hereby incorporated herein by
reference, can
also be used as a support. The disclosed support comprises from about 0.5 to
about 10 weight
12
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
percent of fluorine, from 0.5 to about 5 weight percent of silica and from
about 85 to about 99
weight percent of alumina.
It has been found that higher selectivity and productivity catalyst materials
may be
produced when a promoter is used. The catalyst materials of the present
invention may
therefore be used with any of the following promoters: Sc, Y, La, Ti, Zr, Hf,
Rh, Pd, Os, Ir,
Pt, Re, Nb, Cu, Ag, Mn, B, P, and Ta for Co and/or Ru-containing catalysts,
and Na, K, Rb,
Cs, Mg, Ca, Sr, and Ba for Fe-containing catalysts. The amount of promoter
added to the
catalyst is typically sufficient to provide a weight ratio of elemental
promoter to elemental
catalyst metal of from about 0.00005:1 to about 0.5:1.
A preferred form of the desired catalyst material may be prepared by any of
the
methods known to those skilled in the art. By way of illustration and not
limitation, such
methods include impregnating the catalytically active compounds or precursors
onto a support;
extruding one or more catalytically active compounds or precursors together
with support
material to prepare catalyst extrudates, and/or precipitating the
catalytically active compounds
or precursors onto a support. Accordingly, the supported catalysts of the
present invention may
be used in the form of powders, particles, pellets, monoliths, honeycombs,
packed beds, foams,
and aerogels.
The most preferred method of preparation may vary among those skilled in the
art,
depending for example on the desired catalyst particle size. Those skilled in
the art are able to
select the most suitable method for a given set of requirements.
One method of preparing a supported metal catalyst, e.g., a supported cobalt,
cobalt/rhenium, or cobalt/rhenium/promoter catalyst is by incipient wetness
impregnation of
the support with an aqueous solution of a soluble metal salt such as nitrate,
acetate,
acetylacetonate or the like. Another method of preparing a supported metal
catalyst is by a
melt impregnation technique, which involves preparing the supported metal
catalyst from a
molten metal salt. One preferred method is to impregnate the support with a
molten metal
nitrate, e.g., Co(N03)2~6H20. Alternatively, the support can be impregnated
with a solution of
zero valent metal precursor. One preferred method is to impregnate the support
with a solution
of zero valent cobalt such as Co2(CO)g, Co4(CO)12 or the like in a suitable
organic solvent, e.g.,
toluene. Suitable rhenium compounds are the common water soluble ones, e.g.,
rhenium
heptoxide (Re20~) and ammonium perrhenate (NH4Re04).
The impregnated support is dried and reduced with hydrogen or a hydrogen
containing gas. The hydrogen reduction step may not be necessary if the
catalyst is prepared
13
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
with zero valent cobalt. In another preferred method, the impregnated support
is dried,
oxidized with air or oxygen and reduced in the presence of hydrogen.
Typically, at least a portion of the metals) of the catalytic metal component
of the
catalyst materials of the present invention is present in a reduced state, i.
e., in the metallic state.
Therefore, it is normally advantageous to activate the catalyst prior to use
by a reduction
treatment, in the presence of hydrogen at an elevated temperature. Typically,
the catalyst is
treated with hydrogen at a temperature in the range of from about 75°C
to about 500°C, for
about 0.5 to about 24 hours at a pressure of about 1 to about 75 atm. Pure
hydrogen may be
used in the reduction treatment, as may a mixture of hydrogen and an inert gas
such as nitrogen,
or a mixture of hydrogen and other gases as are well known in the art, such as
carbon monoxide
and carbon dioxide. Reduction with pure hydrogen and reduction with a mixture
of hydrogen
and carbon monoxide are preferred. The amount of hydrogen may range from about
1 % to
about 100% by volume.
As stated above, the catalyst material, its physical form, and the
concentration of its
contents can be optimized in each reaction chamber so as to result in a
desired reaction scheme.
Indeed, the catalyst material should be selected for each reaction chamber so
as to optimize the
reactions occurring in said reaction chamber.
The recycling or refluxing of materials is common in distillation columns and
is also
part of a preferred embodiment. One or more recycle lines or reflux lines may
take materials
from any reaction chamber and return the materials to the reaction vessel 12
at another point.
Preferably, as shown in Figure 3, a recycle stream 81 will take product from
the top reaction
chamber and deposit the product at a lower point of the reaction vessel 12.
Once returned to a
relatively lower position of the reaction vessel 12, the recycled light
hydrocarbons that were
present in the top reaction chamber 51 may undergo additional Fischer-Tropsch
reaction. Also
as shown in Figure 3, reflux line 82 may remove product from a lower reaction
chamber and
deposit the product in a higher reaction chamber. As will be understood, the
recycle and reflux
lines 81, 82 may be configured in a number of ways (not shown). A recycle line
81 or reflux
line 82 may merge with one or more feed lines 21-24 as one way of returning
products to the
reaction vessel 12. In another embodiment, a reflux or recycle line may
directly reenter the
reaction vessel 12 as shown in Figure 3. Further, the recycle lines 81 may
diverge from one or
more product lines 31-37, as shown, as a way of returning fluids found in the
product lines to
the reaction vessel 12. While in a recycle line 81, fluids may undergo
heating, cooling,
pressurization, or depressurization as needed to place the products in a
physical condition
appropriate for return to the reaction vessel 12.
14
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
Operation
In operation, reactants and other processing materials, if any, preferably
enter reaction
vessel 12 through feed lines 21, 22, 23, and 24. The reactants typically used
to form
hydrocarbons according to the Fischer-Tropsch process comprise hydrogen, H2,
and carbon
monoxide, CO. Preferably, H2 and CO are combined and injected into the
reaction vessel
together as syngas through each of the feed lines 21, 22, 23, and 24.
Alternatively, the reactants
HZ and CO may be individually injected into reaction vessel 12 through one or
more of the feed
lines 21-24. According to one preferred embodiment, one or more H2/CO
feedstock mixtures
enter reaction vessel 12 at multiple points through feed lines 21, 22, 23, and
24. The H2/CO
molar ratio may vary for each of feed lines 21, 22, 23, and 24. The molar
ratio of hydrogen to
carbon monoxide may also be varied between the streams entering reaction
chambers 51-55, so
as to control the hydrocarbon product distribution. Similarly, other
conditions related to feed
lines 21, 22, 23, and 24 such as flow rate, temperature, and pressure may vary
for each
particular feed line.
Nitrogen, which is not a raw material for the Fischer-Tropsch synthesis, is
typically
used as a purge gas when starting up or shutting down reaction vessel 12
before and after a
Fischer-Tropsch synthesis run. Nitrogen, which is an inert element and will
not react with the
reactants or products typically found during Fischer-Tropsch synthesis, is
pumped into the
reaction vessel 12. The nitrogen purges vessel 12 by displacing any materials
that are in the
reaction vessel 12. Nitrogen may be fed into reaction vessel 12 through feed
lines 21, 22, 23,
and 24, or through any combination of these feed lines. Preferably nitrogen is
admitted to
reaction vessel 12 through a dedicated nitrogen line 25 as shown in Figure 1.
The concentrations of feed materials and their injection points, the reaction
temperatures and pressures, and the catalyst types and amount of catalyst used
in various
reaction chambers 51-55 in reaction vessel 12 may all be varied in accordance
with the present
invention to control the product distribution, conversion, and selectivity.
Generally speaking,
the product lines disposed in the bottom or lower end of reaction vessel 12
will remove heavier
(larger chain hydrocarbons) reaction products. Waxes, for example, will
typically exit through
bottom product line 37. Progressively lighter hydrocarbons will pass to
progressively upper
reaction chambers of the reactor vessel 12, where they may be drawn off in one
of the upper
product lines.
According to one embodiment of the invention, the components of the present
column
are configured such that the following petroleum products are produced from
the reaction
vessel. Product line 36, next in order above bottom line 37, draws primarily
diesel fuel from
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
the reaction vessel 12. Product line 35 draws primarily kerosene and product
line 34 draws
primarily jet fuel. Product line 33 draws primarily gasoline and product line
32 draws
primarily LPG. Coming off top line 31 will be gaseous materials, comprising
methane, ethane,
propane and butane. It should be understood that other embodiments of the
present invention
may contain a number of product lines different from that just described.
H2/CO mixtures suitable as a feedstock for conversion to hydrocarbons
according to the
process of the preferred embodiment can be obtained from light hydrocarbons
such as methane
by means of steam reforming, partial oxidation, or other processes well known
in the art.
Preferably the hydrogen is provided by free hydrogen, although some Fischer-
Tropsch catalysts
have sufficient water gas shift activity to convert some water to hydrogen for
use in the
Fischer-Tropsch process. It is preferred that the molar ratio of hydrogen to
carbon monoxide in
the feed be greater than 0.5:1 and preferably from about 0.67:1 to 2.5:1. More
preferably, the
feed gas stream contains hydrogen and carbon monoxide in a molar ratio of
about 2:1. The
feed gas may also contain carbon dioxide. The feed gas stream should contain a
low
concentration of compounds or elements that have a deleterious effect on the
catalyst, such as
poisons. For example, the feed gas may need to be pre-treated to ensure that
it contains low
concentrations of sulfur or nitrogen compounds such as hydrogen sulfide,
ammonia and
carbonyl sulfides.
The Fischer-Tropsch process is typically run in a continuous mode. In this
mode, the
gas hourly space velocity through a reaction chamber 51-55 typically may range
from about
100 volumes/hour/volume catalyst (v/hr/v) to about 10,000 v/hr/v and
preferably from about
300 v/hr/v to about 2,000 v/hr/v. The temperature in each reaction chamber 51-
55 is typically
in the range from about 160°C to about 300°C. Preferably, each
reaction chamber 51-55 is
operated at conversion promoting conditions at temperatures from about
190°C to about 260°C.
The reaction chamber pressure is typically in the range of about 80 psig (653
kPa) to about
1000 psig (6994 kPa), preferably, from 80 psig (653 kPa) to about 600 psig
(4237 kPa), more
preferably, from about 140 psig (1066 kPa) to about 400 psig (2858 kPa), and
still most
preferably at about 150 psig.
As feed lines 21-24 deposit syngas materials into a given reaction chamber 51-
55,
simultaneous operations of reaction and separation take place. In the presence
of catalyst
material, the syngas reactants form hydrocarbons. In each reaction chamber 51-
55, the
materials present are also subjected to the physical affects caused by the
temperature in the
reaction chamber. With respect to the hydrocarbons, if the temperature at a
given point in the
column is above a particular hydrocarbon's boiling point, the molecules of
that hydrocarbon
16
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
will vaporize and become gaseous. Other heavier hydrocarbons will remain as
liquids.
Gravitational forces will thus act to physically separate the liquids and
gases such that the gases
will rise to the top of each reaction chamber 51-55 and liquids will remain at
the bottom. Thus,
in each reaction chamber 51-55, the temperature may be selected so as to
control the amount of
product that vaporizes or remains liquid.
In operation, liquids formed in one reaction chamber 51-55 will migrate in a
downward
direction, toward the next lower reaction chamber. Gases formed in one
reaction chamber 51-
55 will conversely migrate in an upward direction toward the next upper
reaction chamber.
Once a molecule has migrated from one reaction chamber 51-55 to another
reaction chamber
51-55, this molecule will thereupon be subject to further reaction and
physical separation
according to the configuration present in the new reaction chamber. By a
succession of such
operations, the catalytic distillation reactor achieves its simultaneous
objectives of reaction and
separation.
In a reaction chamber configured so as to contain a fixed bed catalyst
material, the
reaction step occurs in and around the fixed bed in a manner similar to that
found in fixed bed
Fischer-Tropsch reactors. Fixed bed Fischer-Tropsch catalyst materials
typically consist of a
monolithic or f support material in which are present the active catalyst
components along with
the necessary activators and promoters. The support material provides the
structure of the
catalyst material. In this configuration, the catalyst material does not move.
The support
material will have interstices and voids through which the reactants and
products may migrate
into and out of the catalyst material. As stated above, the catalyst bed may
be structured so that
it does not occupy the entire volume of the reaction zone.
In a reaction chamber configured to contain a fluidized bed of catalyst
material, the
reaction step takes place throughout the area containing the fluidized bed and
in a manner
similar to that found in fluidized bed Fischer-Tropsch reactors. A fluidized
bed for Fischer-
Tropsch synthesis typically consists of solid/gas phases. The catalyst
material is present as a
solid. The solid catalyst material consists of loosely separated particles
that are of a size and
mass chosen so that they may be entrained by the gases passing upward through
the reaction
chamber. In operation, the particles comprising the catalyst material are
turbulently mixed by
the entraining gases.
In a reaction chamber configured to contain a solid/liquid slurry catalyst
material, the
reaction will occur in a manner similar to that found in Fischer-Tropsch
reactors containing a
solid/liquid slurry. A solid/liquid slurry for Fischer-Tropsch synthesis
typically consists of
solid-liquid phases. The catalyst material is again present as a solid. The
solid catalyst material
17
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
consists of separate particles that are of a size and mass chosen so that they
may be slurried by
the liquids passing through the reaction chamber. A typical slurry catalyst
for Fischer-Tropsch
synthesis is described in U.S. Patent No. 5,348,982, hereby incorporated
herein by reference.
Referring now to Figure 4, a preferred embodiment of the present invention
includes
heat exchangers 91A, 91B that are external to the column. In this embodiment,
heat removal
may be achieved by first drawing fluids from reaction vessel 12 through a
series of heat
exchange lines 92A and 92B. Heat exchange lines 92A and 92B lead from various
reaction
chambers 51-55 in reaction vessel 12 to one or more heat exchangers 91A and
91B. The heat
exchangers are positioned externally from the catalytic distillation reaction
vessel 12. Heat
exchangers 91A and 91B may be selected from any of a wide variety of heat
exchangers
commercially available. While in one preferred embodiment, heat exchange lines
92A and 92B
are attached to the reaction vessel 12 so as to draw fluids from two of the
reaction chambers 51-
55 of Figure 1, other heat exchange line arrangements may be designed. For
example, in
another embodiment, the number of heat exchange lines may be varied and the
heat exchange
lines positioned differently. Also by means of illustration and not
limitation, heat exchange
lines may draw fluids from each reaction chamber 51-55. The heat exchange
lines 92A and
92B may draw either liquids or gases from the reaction chambers 51-55. Return
lines 93A and
93B, leading from heat exchangers 91A and 91B, direct cooled fluids back into
reaction vessel
12. In one preferred embodiment, a return line is linked to each of reaction
chambers 51-55,
although other embodiments are possible without departing from the scope of
the present
embodiment. The fluids, that are returned to the reaction vessel 12 in this
embodiment, may as
shown in Figure 4 but need not be, returned to the same reaction chamber 51-55
from which
they were drawn. The fluids present in the reaction chamber therefore
constitute the heat
exchange medium in an external heat exchange process. Accordingly, heat
exchange
equipment internal to the reaction vessel 12 is eliminated or minimized. The
removal of heat
by external heat exchangers in accordance with the present embodiment thus
also allows
control of the temperatures in specific reaction chambers 51-55 by removing
fluids from a
specific reaction chamber 51-55 and returning the cooled fluids to the same
reaction chamber.
It is therefore possible to control the temperature in individual reaction
chambers 51-55 by
providing heat exchange equipment for that reaction chamber.
Another embodiment of the invention includes one or more water separation
stages.
The water separation stage may follow one of several designs. In a preferred
embodiment, the
water separation stage may be a settling tank wherein water and hydrocarbons
settle and
separate. Refernng to Figure 5, water separation is achieved by pumping
materials into water
18
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
separation tanks 94A and 94B. When the fluids are condensed to liquid form,
the water will
physically separate from the liquid hydrocarbons. Once the water has
separated, it can be
pumped off; the remaining hydrocarbons can then be directed to an appropriate
location. The
hydrocarbons may either be fed back to the reaction vessel or to a product
tank. Water
separation may also occur in a flash separation drum. In a preferred
embodiment, the water
separation occurs in conjunction with the heat removal operation. Refernng
again to Figure 5,
fluids drawn from reaction vessel 12 are first passed through heat exchangers
91 A or 91 B.
Upon cooling, hot fluids will condense, or partially condense, to liquid form.
The fluids next
pass to water separation tanks 94A and 94B. It is there that water physically
separates from
other liquids and can be removed.
In another embodiment (not shown), water separation may also be achieved in
conjunction with fluid recycle and reflex. In this embodiment, fluids pumped
through the
recycle and reflex lines are again passed into a water separation tank. Once
the liquids have
separated in the water separation tank, the water layer may be pumped off.
When recycling
fluids from the top of the reaction vessel, the fluids may first pass through
a heat exchanger or
condenser to cool the fluids. The fluids may then pass into a water separation
tank. When
refluxing fluids from the bottom of the reaction vessel the fluids may also
pass through water
separation tanks that will separate out water. Refluxed fluids can themselves
be cooled or
reheated.
Other embodiments of the invention may also include one or more paraffin
separation
stages. Refernng to Figure 5, paraffin separation is achieved by pumping
materials into a
paraffin separator 95. The paraffin separator itself may follow a membrane
separation process,
a chemical separation process, or be a mufti-stage distillation column. The
paraffin separator
should be designed so as to separate paraffms from olefins. The paraffins,
which are no longer
reactive in the Fischer-Tropsch synthesis, may then be removed to product
storage. The olefins
may be returned to the reaction vessel for further Fischer-Tropsch reaction.
Paraffin separation
may also take place during recycle and reflex operations. In such an
embodiment fluids
pumped through the recycle and reflex lines will pass through a water
separation stage and then
a paraffin separation stage. In this manner, reactive olefins can be separated
from the non-
reactive paraffins. The olefins may be returned to the reaction vessel in the
recycle and reflex
return lines.
A variety of standard control equipment and measurement devices will assist in
the
operation of the catalytic distillation reactor. Thermocouples or other
temperature measuring
devices may be positioned within the reaction vessel 12. Preferably, a
plurality of temperature
19
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
measuring devices may be present at different positions in each reaction
chamber such as
reaction chambers 51-55 of Figure 1. In this manner the temperature in each
particular reaction
chamber 51-55 may be measured and/or monitored. Hot spots, cool spots,
temperature spikes
and excessive temperature gradients typically should be avoided. Thus, by
careful temperature
measurement, the proper temperature differential may be maintained between
adjacent reaction
chambers 51-55 in order to promote the optimum mass transfer between the
reaction chambers.
Flow regulators, not shown, typically control the passage of hydrocarbons
through feed
lines 21-24, product lines 31-37, recycle and reflux lines 81, 82 and heat
exchanger lines 92A,
92B, 93A and 93B. Flow regulator equipment may include valves, which may be
either
manual or automatic. In addition, fluid flows may be measured with standard
measuring
devices such as manometers and flow meters.
Examples
Example 1: Pelleted Catalyst
Catalyst pellets 3mm in diameter containing 20 wt% Cobalt with 0.5% Rhenium on
gamma
alumina were dumped into the reactor. Catalyst produced by standard incipient
wetness
techniques. The catalyst was reduced in the reactor at 350C with 50:50 mixture
of H2/N2 for
16 hours. The overall space velocity during the runs were 2 NL/hr/g-cat. There
were 4 catalyst
sections each containing 10 grams of catalyst. The temperature was
225°C at a pressure of 150
psig. The overhead product above the top catalyst section was condensed at
20°C. The entire
condensed hydrocarbon stream was used as reflux after water removal via
decanting. Overhead
liquid product was recycled to the top of the reactor. Heavier liquid products
were removed
from the bottom of the reactor. Syn gas with a 2:1 ratio of H2/CO was fed at
the bottom of the
reactor.
Run No. Run 2 Run 1
CO Conversion 100% 100%
CS+ (g CS+/hr/kg-cat) 250 310
Methane (wt% HC product) 4% 4%
C02 from CO 1 % 1
The carbon number distribution does not follow the standard Anderson-Schulz-
Flory
distribution common to Fischer-Tropsch. The figure below shows that there is
potential for
significant chain limiting ability.
Example 2' Pelleted Catalyst in a Structured Wire Mesh Packing Material
Catalyst pellets of 3 mm in diameter were rolled into a structured wire mesh
packing material.
The pellets were evenly distributed through out the packing. The catalyst
pellets were identical
CA 02396581 2002-05-15
WO 01/36066 PCT/US00/31514
to those used in Example 1. 20 grams catalyst was loaded into each of the four
reactor sections.
The catalyst was reduced identically as Example 1. Temperature of the reactor
was 225°C. The
space velocity was overall 2 NL/hr/g-catalyst. The syn gas feed was fed to two
separate
sections of catalyst at the lowest catalyst section and the second section
from the top. The
overhead product above the top catalyst section was condensed at 20°C.
The entire condensed
hydrocarbon stream was used as reflux after water removal via decanting. The
overhead liquid
product was refluxed to the top of the reactor and the next to the bottom
catalyst section evenly.
Syn gas feed was 2:1 ratio at both feed locations.
Run No. Run 1 Run 2
CO Conversion 85% 65%
CS+ (g CS+/hr/kg-cat) 200 80
Methane (wt% HC product) 13% 30%
C02 from CO 9% 12%
Pressure (psig) 270 200
Without further elaboration, it is believed that one skilled in the art can,
using the
description herein, utilize the present invention to its fullest extent. While
a preferred
embodiment of the invention has been shown and described, modifications
thereof can be made
by one skilled in the art without departing from the spirit or teaching of
this invention. The
embodiments described herein are exemplary only and are not limiting. Many
variations and
modifications of the system and apparatus are possible and are within the
scope of the
invention. Accordingly, the scope of the protection is not limited to the
embodiments described
herein, but is only limited by the claims that follow, the scope of which
shall include all
equivalents of the subject matter of the claims.
21