Note: Descriptions are shown in the official language in which they were submitted.
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
DETECTION OF STROKE EVENTS USING DIFFUSE OPTICAL TOMOGRAPHY
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority from U.S. Provisional Patent Application
Serial No.
60/178,526, filed January 24, 2000; U.S. Patent Application Serial No.
09/491,595, filed January
25, 2000, now pending; and U.S. Patent Application Serial No. 09/598,422,
filed June 19, 2000,
now pending.
FIELD OF THE INVENTION
The invention is in the field of diffuse optical tomography and stroke.
BACKGROUND OF THE INVENTION
Victims of stroke caused by an ischemic event in the brain can benefit from
treatment
with recombinant tissue plasminogen activator, a thrombolytic drug, within
three hours of the
ischemic event. On the other hand, if the stroke is caused by a bleed instead
of an ischemic
event, thrombolytic drugs are contraindicated. Thus, a quick and efficient
means of
distinguishing an ischemic event from a bleed in a stroke victim would aid a
health care provider
in managing the treatment of stroke victims. Furthermore, treatment protocols
need to be
tailored to individual patients. Therefore, continuous monitoring of cerebral
perfusion would
enable the health care provider to more effectively guide treatment in a
patient with a specific
pathology.
Diffuse optical tomography (DOT) refers to various non-invasive methods of
imaging
different tissues of a body or organ. Generally, DOT relies on the emission of
light from a light
source into the body, then detecting the light scattered from various tissues
of the body. For
example, since light scattered by hemoglobin in blood differs from light
scattered by other
-1-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
tissues, DOT has been applied to the imaging of blood within the body. In
addition, because the
absorption of light by deoxyhemoglobin is different from the absorption of
light by
oxyhemoglobin, DOT has been used to locate areas of high or low oxygenation in
the body by
determining decreases or increases in the intensity of scattered light.
However, the application of
DOT in various imaging scenarios in the clinic has been limited by the
inability to detect light
scattered by deep tissues. This can be due to either the inability of the
emitted light to reach deep
tissues, or the inability to detect and measure the weak intensity of light
scattered by the deep
tissues (i.e., no measurable contrast between scattered light and background).
SUMMARY OF THE INVENTION
The invention is based on the recognition that ischemic events deep in the
brain can be
detected using DOT to monitor collateral blood flow abnormalities in cortical
regions of the
brain arising from the deep ischemic events using contrast agents, such as
blood-borne tracer
dyes (also called tracers) and oxygen (e.g., oxyhemoglobin). By extrapolating
information from
the cortical regions, deep ischemic events as well as cortical ischemic events
in the brain can be
monitored even if the light used for DOT does not penetrate into the deep
portions of the brain,
or if the contrast in the light scattered from blood and solid tissues in the
deep portions of the
brain is not easily detected.
The invention is also based on the recognition that a brain bleed can be
imaged by DOT
using a blood-borne tracer dye or oxygen and detecting a localized region of a
lower
concentration of dye or oxygen or of no dye at all, as compared to an adjacent
region in the
brain. The imaging of a brain bleed is made possible by recognizing that blood
vessel
constriction and clotting at the site of the bleed will inhibit the dye or
oxygen from infiltrating
the region of the bleed while adjacent regions are unaffected. In addition,
the use of a tracer dye
or oxygen increases the contrast in light scattered by blood versus solid
tissue deep in the brain,
thereby allowing blood volume to be imaged even where the intensity of
scattered light is weak.
Accordingly, the invention features a method of detecting an ischemic event in
a brain in
a subj ect, using a first criterion, by ( 1 ) administering a dye bolus into
the bloodstream of the
subject; (2) directing light into the brain of the subject; (3) detecting
light emitted from the brain
-2-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
over time at a detection location, the dye being present in the brain for at
least a portion of the
detection time, and the light emitted from the brain in the presence of the
dye being different
from the light emitted from the brain in the absence of the dye, the magnitude
of the difference
corresponding to the concentration of the dye; (4) establishing a reference
time period
corresponding to the time a peak concentration of a dye bolus takes to reach
the detection
location in a normal brain; (5) determining a subject time period
corresponding to the time a
peak concentration of the dye bolus takes to reach the detection location in
the subject; and (6)
comparing the subject time period with the reference time period, where a
subject time period 1
or more seconds longer than the reference time period indicates an ischemic
event in the brain.
If the subject time period is 2 or more seconds longer than the reference time
period, then an
ischemic event in a deep portion of the brain is indicated.
In another aspect, the invention includes a method of detecting an ischemic
event in a
brain in a subj ect, using a second criterion, by ( 1 ) administering a dye
bolus into the bloodstream
of the subject; (2) directing light into the brain of the subject; (3)
detecting light emitted from the
brain over time at a detection location, the dye being present in the brain
for at least a portion of
the detection time, and the light emitted from the brain in the presence of
dye being different
from the light emitted from the brain in the absence of the dye, the magnitude
of the difference
corresponding to the concentration of the dye; (4) establishing a peak
reference concentration of
a dye bolus administered to a subject with a normal brain at the detection
location; (5)
determining a peak subject concentration of the dye bolus at the detection
location; and (6)
comparing the peak subject concentration with the peak reference
concentration, where a peak
subject concentration below the peak reference concentration indicates an
ischemic event in the
brain. If the peak subject concentration is below the peak reference
concentration but at least
50% of the peak reference concentration, then an ischemic event in a deep
portion of the brain is
indicated.
The invention also includes a method of detecting an ischemic event in a brain
in a
subject, using a third criterion, by (1) administering a dye bolus into the
bloodstream of the
subject; (2) directing light into the brain of the subject; (3) detecting
light emitted from the brain
over time at a detection location, the dye being present in the brain for at
least a portion of the
detection time, and the light emitted from the brain in the presence of dye
being different from
-3-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
the light emitted from the brain in the absence of the dye, the magnitude of
the difference
corresponding to the concentration of the dye; (4) establishing a reference
time period
corresponding to the time for the concentration of a dye bolus to vary from a
threshold (e.g., 10,
S, 2, or 1 % by volume) concentration to a peak concentration and back to the
threshold
concentration at the detection location in a normal brain; (5) determining a
subject time period
corresponding to the time for the concentration of the dye to vary from the
threshold
concentration to a peak concentration and back to the threshold concentration
at the detection
location; and (6) comparing the subject time period with the reference time
period, where a
subject time period longer than the reference time period indicates an
ischemic event in the brain.
If the subject time period is at least 2 seconds longer than the reference
time period, then an
ischemic event in a deep portion of the brain is indicated.
In still another aspect, the invention includes a method of detecting an
ischemic event in a
brain in a subject, using a fourth criterion, by (1) administering a dye bolus
into the bloodstream
of a subject; (2) directing light into the brain.of the subject; (3) detecting
light emitted from the
brain over time at a detection location, the dye being present in the brain
for at least a portion of
the detection time, and the light emitted from the brain in the presence of
the dye being different
from the light emitted from the brain in the absence of the dye, the magnitude
of the difference
corresponding to the concentration of the dye; (4) establishing a reference
map of cortical blood
flow in a normal brain; (5) obtaining a subject map of cortical blood flow in
the subject; and (6)
comparing the reference map with the subject map, where a continuous region of
decreased
blood flow in the subject map, compared to the reference map indicates an
ischemic event in the
brain. If the continuous region of decreased blood flow is at least 20 mm at
the longest diameter,
then an ischemic event in a deep portion of the brain is indicated. In
addition, this method can
further include (7) comparing the position of the region of decreased blood
flow with a map of
known brain vasculature; and (8) extrapolating the position of the ischemic
event in the brain of
the subject.
In addition, the invention includes a method of detecting an ischemic event in
a brain in a
subject using any combination (e.g., all) of the criteria specified above. The
methods described
above also need not specify all the steps; only the last comparing step is
required.
-4-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
The invention further includes a method of detecting a brain bleed in a
subject by ( 1 )
administering a dye into the bloodstream of the subject; (2) directing light
from a light source
into the brain of the subject; (3) detecting light emitted from the brain
while the dye is present in
a portion of the brain, the light emitted from the brain in the presence of
the dye being different
from the light emitted from the brain in the absence of the dye, the magnitude
of the difference
corresponding to the concentration of the dye, and the detecting step being
performed while the
dye is detectable in the blood circulation of the subject; and (4) determining
the concentration of
the dye in the portion of the brain, where a region of the portion with a
lower concentration of
the dye than an adjacent region of the portion indicates a brain bleed.
In another aspect, the invention includes a method of detecting a brain bleed
in a subject
by ( 1 ) administering a dye into the bloodstream of the subject; (2)
directing light from a light
source into the brain of the subject; (3) detecting light emitted from the
brain while the dye is
present in a portion of the brain, the light emitted from the brain in the
presence of the dye being
different from the light emitted from the brain in the absence of the dye, the
magnitude of the
difference corresponding to the concentration of the dye, and the detecting
step being performed
after the initial dye concentration in the blood circulation of the subject
has been reduced; and (4)
determining the concentration of the dye in the portion of the brain, where a
region of the portion
with a higher concentration of the dye than an adjacent region of the portion
indicates a brain
bleed.
In the methods of the invention, the dye can be indocyanine green or any other
suitable
dye described herein, the difference in light can be the amplitude of light,
and the subject can be
a mammal (e.g., a human). In addition, the methods can include directing light
into the brain
through the scalp of the subject from a plurality of light sources and
detecting light emitted from
the brain using a plurality of photodetectors (e.g., charged-coupled devices).
In the methods, light can be emitted and detected using a system including (I)
at least
two optical sources which during operation emit light into a sample at
spatially separated
locations; (2) at least two optical detectors positioned to receive light
emitted from the sample at
spatially separated locations in response to the light emitted from the
sources, wherein the signal
g(i j) produced by the jth detector in response to the optical radiation from
the ith source can be
expressed as g(i, j) = S'D~ f (i, j), where f(i j) depends only on the
properties of the head of the
-5-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
subject, f1 is a coupling coefficient for the ith source, and DJ is a coupling
coefficient for the jth
detector; and (3) an analyzer which during operation calculates the value of
the product SIDk for
at least one of the source-detector pairs based on the signals produced by the
detectors and
simulated values of f(ij) corresponding to a model of the optical properties
of the sample.
Further, the light can be directed by at least two optical sources and
detected by at least
two optical detectors, the sources coupling light into the brain at spatially
separated locations,
and the detectors positioned to receive light emitted from the sample at
spatially separated
locations and generating signals in response to the light from the sources. In
such a situation, the
method can further include (1) providing the signals generated by the
detectors, wherein the
signal g(i j) generated by the jth detector in response to the optical
radiation from the ith source
can be expressed as g(i, j) = S'D~ f (i, j~, where f(i j) depends only on the
properties of the
sample, ,$n is a coupling coefficient for the ith source, and DJ is a coupling
coefficient the jth
detector; and (2) calculating the value of the product SIDk for at least one
of the source-detector
pairs based on the signals generated by the detectors and simulated values of
f(i j) corresponding
to a model of the optical properties of the brain.
Since an oxygen bolus can be used in stead of a dye bolus, the invention also
features a
method of detecting an ischemic event in a brain in a subject, using a first
criterion, by (1)
administering an oxygen bolus into the bloodstream of the subject; (2)
directing light into the
brain of the subject; (3) detecting light emitted from the brain over time at
a detection location,
the oxygen bolus being present in the brain for at least a portion of the
detection time, and the
light emitted from the brain in the presence of the oxygen bolus being
different from the light
emitted from the brain in the absence of the oxygen bolus, the magnitude of
the difference
corresponding to a difference in the concentration of total oxygen; (4)
establishing a reference
time period corresponding to a time a peak concentration of the oxygen bolus
takes to reach the
detection location in a normal brain; (5) determining a subject time period
corresponding to a
time a peak concentration of the oxygen bolus takes to reach the detection
location in the subject;
and (6) comparing the subject time period with the reference time period,
where a subject time
period 1 or more seconds longer than the reference time period indicates an
ischemic event in the
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
brain. If the subject time period is 2 or more seconds longer than the
reference time period, then
an ischemic event in a deep portion of the brain is indicated.
In another aspect, the invention includes a method of detecting an ischemic
event in a
brain in a subject, using a second criterion, by (1) administering an oxygen
bolus into the
bloodstream of the subject; (2) directing light into the brain of the subject;
(3) detecting light
emitted from the brain over time at a detection location, the oxygen bolus
being present in the
brain for at least a portion of the detection time, and the light emitted from
the brain in the
presence of the oxygen bolus being different from the light emitted from the
brain in the absence
of the oxygen bolus, the magnitude of the difference corresponding to a
difference in
concentration of total oxygen; (4) establishing a peak reference concentration
of the oxygen
bolus administered to a subject with a normal brain at the detection location;
(5) determining a
peak subject concentration of the oxygen bolus at the detection location; and
(6) comparing the
peak subject concentration with the peak reference concentration, where a peak
subject
concentration below the peak reference concentration indicates an ischemic
event in the brain. If
the peak subject concentration is below the peak reference concentration but
at least 50% of the
peak reference concentration, then an ischemic event in a deep portion of the
brain is indicated.
The invention also includes a method of detecting an ischemic event in a brain
in a
subject, using a third criterion, by (1) administering an oxygen bolus into
the bloodstream of the
subject; (2) directing light into the brain of the subject; (3) detecting
light emitted from the brain
over time at a detection location, the oxygen bolus being present in the brain
for at least a portion
of the detection time, and the light emitted from the brain in the presence of
oxygen bolus being
different from the light emitted from the brain in the absence of the oxygen
bolus, the magnitude
of the difference corresponding to the difference in concentration of total
oxygen; (4)
establishing a reference time period corresponding to the time for the
concentration of the
oxygen bolus to vary from a threshold concentration (e.g., about 85-95% oxygen
saturation) to a
peak concentration and back to the threshold concentration at the detection
location in a normal
brain; (5) determining a subject time period corresponding to the time for the
concentration of
the oxygen bolus to vary from the threshold concentration to a peak
concentration and back to
the threshold concentration at the detection location; and (6) comparing the
subject time period
with the reference time period, where a subject time period longer than the
reference time period
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
indicates an ischemic event in the brain. If the subject time period is at
least 2 seconds longer
than the reference time period, then an ischemic event in a deep portion of
the brain is indicated.
In still another aspect, the invention includes a method of detecting an
ischemic event in a
brain in a subject, using a fourth criterion, by (1) administering an oxygen
bolus into the
bloodstream of a subject; (2) directing light into the brain of the subject;
(3) detecting light
emitted from the brain over time at a detection location, the oxygen bolus
being present in the
brain for at least a portion of the detection time, and the light emitted from
the brain in the
presence of the oxygen bolus being different from the light emitted from the
brain in the absence
of the oxygen, the magnitude of the difference corresponding to the difference
in concentration
of total oxygen; (4) establishing a reference map of cortical blood flow in a
normal brain; (5)
obtaining a subject map of cortical blood flow in the subject; and (6)
comparing the reference
map with the subject map, where a continuous region of decreased blood flow in
the subject
map, compared to the reference map indicates an ischemic event in the brain.
If the continuous
region of decreased blood flow is at least 20 mm at the longest diameter, then
an ischemic event
in a deep portion of the brain is indicated. In addition, this method can
further include (7)
comparing the position of the region of decreased blood flow with a map of
known brain
vasculature; and (8) extrapolating the position of the ischemic event in the
brain of the subject.
In addition, the invention includes a method of detecting an ischemic event in
a brain in a
subject using any combination (e.g., all) of the criteria specified above. The
methods described
above also need not specify all the steps; only the last comparing step is
required.
The invention further includes a method of detecting a brain bleed in a
subject by (1)
administering oxygen into the bloodstream of the subject; (2) directing light
from a light source
into the brain of the subject; (3) detecting light emitted from the brain
while the oxygen is
present in a portion of the brain, the light emitted from the brain in the
presence of the oxygen
being different from the light emitted from the brain in the absence of the
oxygen, the magnitude
of the difference corresponding to the difference in concentration of total
oxygen, and the
detecting step being performed while the oxygen is detectable in the blood
circulation of the
subject; and (4) determining the concentration of the oxygen in the portion of
the brain, where a
region of the portion with a lower concentration of the oxygen than an
adjacent region of the
portion indicates a brain bleed.
_g_
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
In another aspect, the invention includes a method of detecting a brain bleed
in a subject
by (1) administering oxygen into the bloodstream of the subject; (2) directing
light from a light
source into the brain of the subject; (3) detecting light emitted from the
brain while the oxygen is
present in a portion of the brain, the light emitted from the brain in the
presence of the oxygen
being different from the light emitted from the brain in the absence of the
oxygen, the magnitude
of the difference corresponding to the difference in concentration of total
oxygen, and the
detecting step being performed after the initial oxygen concentration in the
blood circulation of
the subject has been reduced; and (4) determining the concentration of the
oxygen in the portion
of the brain, where a region of the portion with a higher concentration of the
oxygen than an
adjacent region of the portion indicates a brain bleed.
In the methods of the invention, the oxygen can be administered to the subject
by
temporary (e.g., for 1 minute) inhalation of oxygen-enriched atmosphere (e.g.,
50, 60, 70, 80, 90,
or 100% oxygen by volume), the difference in light can be the amplitude of
light, and the subject
can be a mammal (e.g., a human). In addition, the methods can include
directing light into the
brain through the scalp of the subject from a plurality of light sources and
detecting light emitted
from the brain using a plurality of photodetectors (e.g., charged-coupled
devices).
In the methods, light can be emitted and detected using a system including ( 1
) at least
two optical sources which during operation emit light into a sample at
spatially separated
locations; (2) at least two optical detectors positioned to receive light
emitted from the sample at
spatially separated locations in response to the light emitted from the
sources, wherein the signal
g(i j) produced by the jth detector in response to the optical radiation from
the ith source can be
expressed as g(i, j) = S' D~ f (i, j), where f(i j) depends only on the
properties of the head of the
subject, Sl is a coupling coefficient for the ith source, and DJ is a coupling
coefficient for the jth
detector; and (3) an analyzer which during operation calculates the value of
the product SIDk for
at least one of the source-detector pairs based on the signals produced by the
detectors and
simulated values of f(i j) corresponding to a model of the optical properties
of the sample.
Further, the light can be directed by at least two optical sources and
detected by at least
two optical detectors, the sources coupling light into the brain at spatially
separated locations,
and the detectors positioned to receive light emitted from the sample at
spatially separated
locations and generating signals in response to the light from the sources. In
such a situation, the
_9_
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
method can further include (1) providing the signals generated by the
detectors, wherein the
signal g(ij) generated by the jth detector in response to the optical
radiation from the ith source
can be expressed as g(i, j) = S'D~ f ~i, j~, where f(i,j) depends only on the
properties of the
sample, S'i is a coupling coefficient for the ith source, and DJ is a coupling
coefficient the jth
detector; and (2) calculating the value of the product SIDk for at least one
of the source-detector
pairs based on the signals generated by the detectors and simulated values of
f(i~ j) corresponding
to a model of the optical properties of the brain.
A "normal" brain or region of a brain as used herein is a brain or region
suitable to serve
as a control or reference tissue for testing of potentially affected tissue in
a subject brain. Thus, a
normal or reference brain or region can be a different brain than that of the
brain of a subject
under DOT examination, or the same brain as that of the subject. Where the
normal brain is the
same as the subject's, the control or reference tissue can be a matched,
symmetric region (e.g.,
right versus left hemisphere), or a region adjacent to the potentially
affected tissue. Due to
calibration considerations (discussed below), regions adjacent to the
potentially affected tissue
serve as better normal portions of the brain than symmetric regions, which in
turn are better
normal portions than regions of a different brain in a different individual.
A "deep" portion or region of a brain is greater than 1 cm below the inner
surface of the
skull.
"Oxygen" means any form of oxygen, including dissolved oxygen and oxygen
complexed
with hemoglobin (oxyhemoglobin). "Oxygen saturation" means the percentage of
total
hemoglobin that is in the form of oxyhemoglobin.
The methods of the invention provide for a quick, non-invasive means of
detecting and
distinguishing a bleed or ischemic event in the brain of a stroke patient or
suspected stroke
patient. The methods are particularly applicable for a patient exhibiting a
recent onset of one or
more symptoms of stroke. In such a patient, distinguishing a bleed from an
ischemic event in the
shortest amount of time is important for determining the optimal treatment for
the patient during
the critical first few hours after an ischemic stroke. In addition, the
methods can be applied
continuously to monitor the development of a stroke in a patient for fine
tuning of the treatment.
Unless otherwise defined, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
- 10-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
belongs. Although suitable methods and materials for the practice or testing
of the present
invention are described below, other methods and materials similar or
equivalent to those
described herein, which are well known in the art, can also be used. All
publications, patent
applications, patents, and other references mentioned herein are incorporated
by reference in
their entirety. In case of conflict, the present specification, including
definitions, will control. In
addition, the materials, methods, and examples are illustrative only and not
intended to be
limiting.
Other features and advantages of the invention will be apparent from the
following
detailed description, and from the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
Figs. 1-3 are schematic drawings of systems suitable for implementing the
methods of the
invention.
Fig. 4 is a schematic diagram of a source-detector pair for the system shown
in Fig. 3.
Fig. 5 is a flow chart summarizing the steps of one embodiment of the
calibration method
described herein.
Figs. 6A and 6B are schematic diagrams of blood vessels in a brain with a
cortical
ischemic event (Fig. 6A) and a brain with a deep ischemic event (Fig. 6B).
Fig 7A is a schematic drawing of a human head, showing the spatial
distribution of blood
flow abnormalities arising from a deep ischemic event.
Fig. 7B is a graph of time versus tracer concentration for the abnormality
seen in Fig. 7A.
Fig. 8A is a schematic drawing of a human head, showing the spatial
distribution of
blood flow abnormalities arising from a cortical ischemic event.
Fig. 8B is a graph of time versus tracer concentration for the abnormality
seen in Fig. 8A.
Fig. 9A is a schematic representation of a human head, showing a brain bleed
(clear
region) surrounding a source bleed source (dot), while a dye bolus (hatched
region) is present in
the brain. The clear region represents a decreased concentration of dye due to
an inability of the
dye to infiltrate the bleed.
-11-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
Fig. 9B is a graph of position along line A versus tracer concentration for
the abnormality
seen in Fig. 9A.
Fig. 10 is a graph of time versus amplitude in a DOT experiment.
Fig. 11 is a schematic diagram of the optical arrangement of the sources and
detectors
used in Example 5 below.
Figs. 12A-12D are reconstructed DOT images of experimental data from Example 5
below.
Figs. 13A and 13B are graphs of peak image values derived from the images of
Figs.
12A-12D.
Fig 14 is an additional reconstructed image of the experimental data from
Example 5
below.
Fig. 15 is a graph of time versus amplitude obtained in the experiment of
Example 6.
Figs. 16A-16C are diagrams of a scalp cap for use in the methods of the
invention. Fig.
16A is a perspective view of the cap. Fig. 16B is a top view of the cap. Fig.
16C is a portion of
a cross-sectional view of the cap taken along line A-A in Fig. 16B, as
positioned on the scalp.
DETAILED DESCRIPTION
The invention relates to the use of diffuse optical tomography for detecting a
bleed or
ischemic event in a brain, or distinguishing one from the other. The various
light emitters and
detectors, dyes, oxygen, and algorithms used for carrying out DOT are
discussed below.
Introduction and General Considerations
The difficulty with using light to detect and monitor an ischemic stroke,
relative to a
brain bleed, arises from the significantly smaller abnormal blood volume
associated with
ischemia than the abnormal blood volume associated with a brain bleed.
Normally, perfused
brain tissue contains between 5-10% blood by volume. On the other hand, the
region of a bleed
contains about 100% blood. Further, as the bleed matures, the plasma diffuses
away, leaving a
pool of blood with a greater concentration of hemoglobin and thus higher
optical absorption.
-12-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
Therefore, a bleed will offer an intrinsic contrast exceeding a factor of 10-
20, and is detectable
by static DOT imaging of blood.
For an ischemic stroke, the blood volume may change by as little as a factor
of 2 because
ischemia is characterized by reduced, not increased, perfusion. In addition,
the oxygen
saturation will likely drop because of a higher oxygen extraction fraction
resulting from the
reduced perfusion. Depending on the wavelength of the measurement, the
contrast due to a
decrease in the ratio of oxyhemoglobin to deoxyhemoglobin can vary from -50%
at 750 nm to
+50% at 850 nm. The significantly smaller contrast of an ischemic stroke
relative to a bleed has
led many to believe that ischemic stroke cannot be detected, monitored, or
imaged using DOT.
The problem is overcome by recognizing that the reduced perfusion of a deep
ischemic
stroke has collateral effects extending to the cortex (see Example 1 below).
Using hemodynamic
magnetic resonance imaging (MRI), it was shown that the transit of blood in
the cortical region
adjacent to an ischemic stroke was delayed relative to brain tissue that is
not at risk for infarct.
The magnitude of the delay, as well as the spatial extent (size) of the blood
flow abnormality can
indicate the severity of an ischemic stroke. Changes in these parameters can
also provide
valuable feedback on the natural evolution of a stroke and the response of a
stroke to treatment.
When light of a certain wavelength (e.g., near infrared light) is shone on a
body surface, a
small percentage of the light is reflected by the surface while most of the
light enters the body.
Most of the light entering the body then travels small distances before its
direction is randomized
by multiple scattering events caused by materials within the body (including
tissues and contrast
agents). As the light migrates through tissues, there is a small probability
that a photon will be
absorbed, as compared to a scattering event. Since the scattering probability
is much greater
than the absorption probability in the transmission window between 700 nm and
900 nm, a small
but detectable amount of light is able to migrate through the scalp and skull
into the brain and
back out to the surface of the scalp. The volume sampled by the scattered
light depends on the
positioning of the light emitters) and collectors) relative to each other. If
the absorption
probability increases, e.g., in the presence of an absorbing dye or oxygen,
within a tissue, then
the amount of light detected will decrease.
In addition, a major problem in implementing optical methods for monitoring
brain
hemodynamics is distinguishing scalp signals from brain signals. This is true
with static
-13-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
measurements of blood volume (total hemoglobin concentration) and oxygen
saturation
(proportion of total hemoglobin that is oxyhemoglobin), as well as dynamic
bolus kinetic
measurements of blood flow. Three approaches can be used to help solve this
problem: (1)
small versus large separation -- compare optical measurements made with a 1 cm
optode
separation to that made with a separation greater than 2 cm; (2) left vs right
difference --
compare measurements made at symmetrical positions on the right and left
hemispheres of the
head; and (3) press the optodes firmly against the scalp.
( 1 ) The measurement made with the small optode separation will only see the
scalp and
skull while the measurement made with the larger separation will see the
scalp, skull, and brain
(see Example 6 below). Typically, blood is seen to arrive in the cortex a few
seconds before it
arrives in the scalp. This is confirmed by seeing a signal change with the
larger separation
measurement a few seconds before seeing a change with the smaller separation
measurement.
Thus, any delay in the arrival and/or transit of blood to the cortex can be
gauged by the
comparison of the larger separation measurement to the small separation
measurement.
(2) The hemodynamics on the left and right sides of the head are symmetric for
healthy
individuals. Any asymmetry is indicative of an abnormality.
(3) Pressing the optodes firmly against the head will blanch the scalp of
blood and thus
reduce the sensitivity to scalp signals.
Another problem arises from the blocking of optodes by hair. One solution for
this
problem is to provide a downward force on the optodes so that the optodes make
contact with the
skin surface. The optodes can then be moved from sided to side along the
surface of the scalp to
displace any hair blocking the optodes.
Another implementation strategy involves extracting small signal changes
resulting from
small concentrations of the vascular contrast agent (e.g., oxygen or dye).
While measurements
of light intensity or amplitude at the optode emitter wavelength are suitable
in DOT,
measurements at a wavelength different from the optode emitter wavelength are
also possible if
the dye is a fluorophore. Detection at the fluorophore's emitter wavelength
can be used to
measure relatively small concentrations of the dye within the blood. Thus, the
main advantage
of fluorescence detection is that smaller concentrations can be injected into
the subject for DOT
measurements, thereby permitting repeated injections every minute rather than
every 10 minutes,
- 14-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
as is typical with an absorptive contrast agent. The delay in re-
administration of an absorptive
dye such as indocyanine green (ICG) is necessary to allow the dye to be
metabolized by the liver.
Without clearance of the dye by the liver, the baseline level of dye in the
circulation would be
too high for detecting the next bolus injected into the patient. Of course,
nothing precludes
simultaneous monitoring at both an optode emitter wavelength and a fluorophore
emitter
wavelength. Such simultaneous monitoring can be used when a single contrast
agent having
both significant absorption at the optode emitter wavelength and fluorescence
wavelength is
injected into a patient as a bolus. Alternatively, simultaneous monitoring can
be useful if an
absorptive dye and a fluorescent dye are injected into the patient, either at
the same time or at
different times.
The problem with direct DOT imaging of deep tissue within the adult human
brain is that
very few photons actually reach the deep tissue and then escape the head to be
detected. Photon
counting detection methods provide the best signal-to-noise ratio when the
number of photons
detected per second is small (typically less than 1 x 10~ per second).
However, when using light
sources with an average power of 0.1 mW or smaller, the number of photons that
reach the
center of the brain and escape from the body is much less than the typical
background noise of
photons per second. This problem is best solved by using lasers with powers
greater than 1
W, but fear of tissue burning precludes the use of an emitter having that high
power. One
solution is to distribute the large optical power across multiple input sites,
thereby bringing the
total power to levels greater than 1 W without burning the tissue.
As an alternative, albeit no longer non-invasive method of illumination, a
catheter can be
threaded to the base of the brain. This catheter is then used as a source of
light that is detected at
the surface of the head.
Additional details regarding general considerations in DOT and other optical
systems can
be found in U.S. Patent Nos. 5,853,370; 5,353,799; 5,421,329; 5,282,467;
5,782,237; 5,553,614;
5,792,051; 5,902,235; 5,795,292; 5,697,367; 5,584,296; 5,482,034; 5,477,853;
5,465,714;
5,217,013; 5,140,989; 5,139,025; 4,817,623; 4,768,516; 4,725,147; 4,570,638;
and 5,779,631.
-15-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
Use of Dyes for Enhancing DOT Imaging of Blood
For blood flow measurements using DOT, a bolus of dye (also called tracer or
contrast
agent) can be injected into the blood stream of the patient The injection is
usually made
intravenously and usually through the arm, taking about 1 second to complete.
The bolus can be
1 to 10 ml (e.g., 5 ml) of dye. When a bolus of dye is introduced into the
blood stream, the bolus
travels relatively intact through the brain. As the bolus travels back through
the heart and to the
brain a second time, the bolus becomes dilute and spatially extended and
diffuse. After several
passes of the bolus through the heart (each cycle through the body taking
about 20-40 seconds),
it usually is no longer possible to recognize a spatially coherent contrast.
By monitoring the first
passage of the bolus through the brain, the amplitude of the detected light
decreases as the bolus,
which absorbs light, flows into the optically sampled volume of tissue. The
amplitude then
increases back to the baseline as the bolus flows out of the imaged region.
The duration of this
perturbation is a measure of blood flow, while the magnitude of the
perturbation can serve as a
measure of perfusion or blood volume. In addition, comparison of the onset and
offset of the
perturbation in different cortical regions of the brain can provide a relative
measure of the blood
transit time. All of these measures can serve to better characterize the state
and evolution of a
stroke by providing a spatial map or image of the abnormality in the brain
over time.
For additional details regarding the use of a dye bolus in DOT, see Patel et
al., Pediatric
Research 43:34-39, 1998. A device suitable for multiple, automated bolus
administrations is
described in U.S. Patent No. 5,722,956.
Useful dispersible chromophores include: drugs and dyes such as rifampin
(red),
~i-carotene (orange), tetracycline (yellow), indocyanine green (such as Cardio-
Greens), Evan's
blue, methylene blue; soluble inorganic salts such as copper sulfate (green or
blue), Cu(NH3)2+
(dark blue), Mn04 (purple), NiC 1 Z (green), Cr04 (yellow), Crz07z- (orange);
proteins such as
rhodopsin (purple and yellow forms) and green fluorescent protein (fluoresces
green under blue
light); and any of the Food and Drug Administration (FDA) approved dyes used
commonly in
foods, pharmaceutical preparations, medical devices, or cosmetics, such as the
well-characterized
non-toxic sodium salts FD&C Blue No. 1 (Brilliant Blue FCF), FD&C Green No. 3
(Fast Green
FCF), FD&C Red No. 3 (Erythrosine), FD&C Red No. 40 (ALLURAo Red AC), FD&C
Yellow
-16-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
No. 5 (Tartrazine), and FD&C Yellow No. 6 (Sunset Yellow FCF). Of these FD&C
dyes, Yellow
No. 5 is known to produce occasional allergic reactions.
Additional FDA approved dyes and colored drugs are described in the Code of
Federal
Regulations (CFR) for Food and Drugs (see Title 21 of CFR chapter 1, parts 1-
99). The table
below lists a number of suitable chromophores, their Chemical Abstract Service
(CAS)
Registration Numbers, colors, and absorption maxima.
Chromophore CAS Reg. No. Color Abs. Max.
(nm)
Yellow No. 1934-21-0 yellow 428
(3-carotene 7235-40-7 orange 466
rifampin 3292-46-1 red 475
Yellow No. 2783-94-0 yellow 480
6
tetracycline 60-54-8 yellow N/A
Red No. 40 25956-16-6 red 502
Red No. 3 16423-68-0 red 524
Blue No. 2 860-22-0 blue 610
Evan's Blue 314-13-6 blue 610
Green No. 2353-45-9 green 628
3
Blue No. 1 2650-18-2 blue 630
methylene 7220-79-3 blue 668/609
blue
indocyanine 3599-32-4 green 800 (mostly
green IR)
The dispersible chromophores listed above are generally (1) water-soluble at
physiological pH, although fat-soluble chromophores (such as (3-carotene) will
also work if they
are rapidly flushed from tissue, or (2) digestible or metabolizable through
enzymatic pathways
(such as methylene blue, which is rapidly metabolized by mitochondrial
reductases, and proteins
which are digested by proteases). In some cases, it may be possible to modify
a chromophore to
improve its dispersibility. A particular advantage of protein chromophores is
that they can be
conjugated to degradation inducing moieties, such as degradation signaling
polypeptides using
standard biochemical techniques. For example, green fluorescent protein can be
conjugated to
- 17-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
ubiquitin, which facilitates breakdown of the protein into small, invisible
peptides by the
eukaryotic ubiquitin proteolysis pathway.
Use of Oxygen for Enhancing DOT Imaging of Blood
Oxygen can provide the same benefits as described herein for dyes, with the
added
advantage that molecular oxygen can be introduced by non-invasive means, e.g.,
by inhalation.
Of course, a bolus of an oxygen-containing dye, such as oxyhemoglobin, can
also be injected
into the blood stream as detailed above.
A subject that is suspected of having a stroke event can be placed on a
standard inhaler or
respiratory device for controlling the concentration of oxygen in the inhaled
atmosphere.
Initially, the subject would breathe an ambient concentration of oxygen (about
20%) or even a
slightly enriched oxygen atmosphere. A concentration of oxygen lower than
ambient levels may
place the subject under some stress, though this is possible as well. At a
marked time point, the
oxygen concentration will be increased to a substantially higher concentration
(e.g., 50, 60, 70,
80, 90, or 100%) for a chosen time period (e.g., for 5 to 30 second, or one or
more minutes). The
period of time for oxygen inhalation will depend in part on the intended
measurement. If an
oxygen bolus is desired, then the period should be shorter than the cycle-time
of blood (i.e., the
time for a particular blood volume to return to the same position in the body,
which ranges from
20 to 40 seconds depending on numerous patient-specific factors), to produce a
detectable bolus
traveling through the blood stream. If only a generally increased
concentration of oxygen is
desired in the circulating blood, then the subject can be exposed to the
increased oxygen
atmosphere for longer than the cycle time, e.g., for 1 to 5 minutes.
For an oxygen bolus in the brain, the percentage oxygen saturation can be used
as a
measure of oxygen concentration. Oxygen saturation is typically expressed as
the proportion of
total hemoglobin that is oxyhemoglobin and is measured using standard methods
known in the
art. For a healthy individual, the baseline arterial oxygen saturation can
vary from 85-95%.
Thus, for an oxygen bolus to be detectable in a healthy individual initially
inhaling ambient air,
the concentration of oxygen in the oxygen-enriched air should be high enough
to induce a
measurable increase in arterial oxygen saturation (e.g., 97% or higher) above
baseline in the
brain. Of course, subjecting an individual to an initially oxygen-poor
environment (e.g., 10%
-18-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
oxygen atmosphere) can induce an artificially low base line (e.g., 50% oxygen
saturation) over
which a bolus can be detected. The particular baseline (or threshold)
saturation levels and the
peak saturation level for a bolus will vary from subject to subject according
to the individual
physiological parameters of each subject. The baseline is readily established
by monitoring the
oxygen saturation of a subject over time and before an oxygen bolus is
administered, e.g., by
inhalation of an oxygen-enriched environment.
Further details regarding the administration of an oxygen bolus can be found
in Edwards
et al., Lancet 2:770-771, 1988 and references cited therein.
Other than the non-invasive mode of administration, oxygen functions as any
other dye
when administered into the blood stream and provides the same advantages as
described herein.
Light Sources and Detectors of Scattered Light
There are numerous approaches for taking DOT measurements. In the simplest
approach,
a single optode delivers light to the scalp of an individual, and a single
optode detects the
scattered light. The optodes can be separated by 2.5 to 3.5 cm to maximize the
detection of light
that has traveled through the skull and into the cortex of the brain before
returning to the
detecting optode. For smaller optode separations, the detected light likely
travels only through
the skull. Larger optode separations result in smaller signal-to-noise ratios
and a larger volume
of tissue sampled, but have poor spatial resolution.
The 2.5 to 3.5 cm separation of emitters and detectors permits measurement of
flow/mean
transit time when a bolus moves through the position imaged by the pair of
optodes. To
diagnose and monitor the evolution of a stroke lesion, however, a spatial
array of measurements
are desirable. The array can provide measurements from region to region in the
cortex, as well
as the spatial extent (size) of any abnormality.
The wavelength of the light emitted from the optode should correspond to light
that is
detectably absorbed by the tissue or local blood to be imaged or that matches
the absorption
wavelength of a fluorescent or non-fluorescent dye. In the case of blood, a
bolus of dye can be
injected into the patient as described above and used to enhance the
absorption of light by the
blood in a time-dependent manner. For example, if a bolus of indocyanine green
is injected into
- 19-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
a patient for DOT, an appropriate wavelength for the light emitted by the
optode is 780-800 nm,
the peak absorption wavelength of indocyanine green.
The optodes (light sources and detectors) can be positioned on a patient's
head using any
means known in the art. For example, a stiff helmet made of Styrofoam and
having holes in
various positions around its hemisphere can be used to group, position, and
fix the optodes
against an individual's scalp.
Alternatively, a flexible and elastic cap made of rubber or nylon or the like,
similar to a
swimmer's cap, can be used to arrange the optodes. Referring to Figs. 16A-16C,
a device 400 is
formed from a flexible, elastic cap 410 containing holes 420 for placement of
a plurality of
optodes 430, only two of which are shown in Figs. 16A and 16B. The flexible
and elastic nature
of the cap helps ensure a fixed, tight fit over an individual's head. The two
optodes shown
include an optical fiber 432, which delivers light from a laser (not shown) to
scalp surface 402,
and a photodetector 434 for detecting scattered light. All optodes 430 are
flexibly attached to
cap 410 by rubber grommets 440. When cap 410 is tightly fitted over the head,
optodes 430 are
pressed against scalp surface 402, with pressure generated by displacement of
rubber grommets
440, such as displaced rubber grommet 440a (see Fig. 16C). To eliminate signal
artifacts from
hair shafts 460 interposed between scalp surface 402 and optodes 430, device
400 can be shifted
back and forth while cap 410 is placed over scalp surface 402. This shifting
movement causes,
for example, optode 432 to move hair fibers 460 away from the scalp area in
contact with optical
fiber 432 (see Fig. 16C). Other similar arrangements and devices (e.g., caps
or helmets) are
suitable for use in the methods of the invention.
In certain contexts, it is desirable to emit and detect light of two different
wavelengths. A
second wavelength is useful, for example, to determine the degree of
oxygenation (relative
amount of oxyhemoglobin) of the blood, while the first wavelength is used to
detect the
concentration of a dye introduced into the blood stream of a subject as
described herein.
Monitoring scattered light of the second wavelength can also help calibrate
the measurement of
dye concentration in blood by accounting for variations in the signals at the
first wavelength as a
result of changes in blood oxygenation.
-20-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
Circuitry for Detection of Scattered Light
A suitable circuitry for resolving small difference in light intensity
returning to one or
more optode from the brain is shown in Fig. 1. The circuit enables the use of
two wavelengths,
though only one wavelength is required for the methods of the invention. For
example, a single
wavelength of 780 nm would be appropriate when detecting a bolus of
indocyanine green
passing through the vasculature of the brain. The use of two wavelengths
enables more than one
dye to be used, each dye having a different absorption and/or fluorescent
wavelength. The two
dyes then offer collaborating signals that provide more reliable detection of
bleeds or ischemic
events. Further details regarding such benefits are discusses elsewhere
herein.
The phase encoding shown in the circuit of Fig. 1 can spatially distinguish
light emitted
into the head from sources at different locations (e.g., at 5 to 25 locations,
particularly at 18
locations) or at the same location but with different wavelengths. This
ability is important if the
optodes are to be powered continuously to fully resolve the passage of a
bolus. In other words, a
prototype DOT imager having only one light source can only energize one source
at a time,
which limits the data acquisition rate. A more efficient technique, using the
above circuit, is to
exploit the phase diversity afforded by coherent detection by modulating each
laser wavelength
at the same 2 kHz frequency but in phase quadrature with each other. Double-
balanced mixers
are insensitive to coherent signals which arrive exactly 90° out of
phase with the demodulation
clock. When a quadrature signal passes through the mixer, the DC level of the
resulting signal
averages out to zero, similar to uncorrelated noise. The mixer, fed by a
detector signal
containing both in-phase and quadrature components (generated by the two laser
sources), will
demodulate the in-phase signal only, and will completely ignore the quadrature
component. The
same holds true for the second mixer, fed with a "quadrature" demodulation
clock. Double-pole
post-detection filters are used to attenuate the strong second harmonic
component produced by
the quadrature source. This use of phase-encoding allows us to sample two
sources
simultaneously.
The circuit 20 shown in Fig. 1 is such a quadrature encoded system, as
described
immediately above, that places the signals from the two source wavelengths
into mutually
orthogonal phases at one carrier frequency, centered at about 2 kHz. The
electrical signals
representing the intensity of each source wavelength are extracted from the
single detector
-21-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
channel using synchronous or phase-sensitive detection. The 7555
microprocessor 22 serves as
the master clock, which is divided down and split into I and Q (in-phase and
quadrature,
respectively) outputs 24 and 26. The I and Q outputs 24 and 26 are then used
to directly
modulate the bias current through the two-laser diode sources 28 and 30,
respectively. Since the
received signals will experience a slight but significant propagation delay
through the detector,
the demodulator clocks are delayed by the exact same amount using adjustable R-
C phase
shifters 24a and 26a. The pots are adjusted to maximize the signal level of
the main component
while nulling the quadrature component to zero. The output of the double-
balanced mixers 34
and 36 are then separated by the two-stage lowpass filters 38 and 40 to remove
any AC
components, and buffered through output buffers 42 and 44, yielding the two
synchronously
rectified DC signals, "I" output (46) and "Q" output (48).
Another approach for energizing and distinguishing multiple sources
simultaneously is to
encode different emitting optodes with different modulation frequencies. A
schematic of such a
system is shown in Fig. 2. The frequency encoded system 50 operates in a
manner similar to the
circuit 20 shown in Fig. 1, except that each source wavelength 52 and 62 is
modulated and
demodulated at its own unique frequency. This is essentially the equivalent of
two separate
single-channel subsystems SOa and SOb operating independently. Each master
clock operates at
a slightly different frequency. The frequencies must differ by at least three
times the baseband
(post-detection) bandwidth and should not be within the same distance of any
of the frequency
harmonics to avoid interchannel cross-talk.
Subsystems SOa and 50b are nearly identical and contain components that
operate as
described above. Subsystem SOa synchronizes a 780 nm laser source 52 with a
photo detector 55
using a 2 kHz masterclock 53, in order to improve the signal-to-noise ratio of
the detected signal.
The signal from photodetector 55 passes through a double-balanced mixer 54, to
demodulate the
2 kHz carrier signal to a DC level, before separation by a lowpass filter 56,
yielding output 58.
Similarly, subsystem SOb synchronizes a 830 nm laser source 62 with a photo
detector 65 using a
2.2 kHz masterclock 63. The signal from photodetector 65 passes through a
double-balanced
mixer 64 before separation by a lowpass filter 66, yielding output 68.
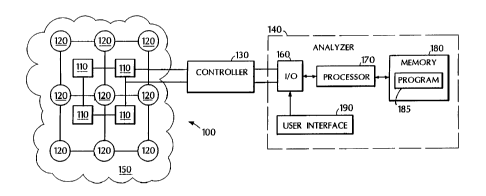
A schematic diagram of a DOT array system is shown in Fig. 3. The system 100
includes
an array of spatially separated light sources 110 and spatially separated
detectors 120. During
-22-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
use, the array of sources and detectors is positioned over a sample 150 to be
imaged, e.g., a
patient's head to image blood within the brain. A controller 130 connected to
light sources 110
sequentially triggers them to couple light into sample 150, which is a highly
scattering media
(e.g., blood and brain tissue) that causes the light to become diffuse within
the sample. For each
sequentially triggered source, each detector 120 measures the light that
reaches it through sample
150. Controller 130 is also connected to detectors 120 and selectively
channels the signals from
the detectors. An analyzer 140 is connected to controller 130 and analyzes the
signals measured
by detectors 120.
The signal g(i j) measured by the jth detector in response to light coupled
into the sample
by the ith source can be expressed as:
g(i, j) = S'D~ f (i, j) (1)
where f(ij) is the transmittance of the sample from the ith source to the jth
detector, and ,S'i and
Dj are coupling coefficients for the ith source to the jth detector,
respectively. The transmittance
f(i j) depends only on the optical properties, e.g., the spatially varying
absorption and scattering
coefficients, and can be numerically calculated by a forward calculation if
the optical properties
are known. Conversely, if the transmittance f(i j) can be calculated from the
measured signals
g(ij), an inverse calculation can be performed on f(ij) to yield the optical
properties of the
sample (e.g., a brain) and reveal, e.g., the presence of an object (e.g.,
blood flowing through a
blood vessel) hidden in the sample (e.g., a brain). The source coupling
coefficient S'i includes all
of the factors associated with coupling light generated from the ith source
into the sample. The
detector coupling coefficient DJ includes all of the factors associated with
coupling light out of
the sample to generate an output signal at the jth detector.
For example, in one embodiment shown in Fig. 4, each source 120, e.g., the ith
source,
includes a diode laser 200 for producing the optical radiation, an optical
fiber 210, and a lens 220
for coupling the optical radiation into fiber 210, which includes an end 215
adjacent sample 150
for directing the optical radiation into the sample. Each detector 130, e.g.,
the jth detector,
includes an optical fiber 250 having an end 255 adjacent sample 150 for
receiving the optical
-23-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
radiation emitted from sample 150, a photodetector 260 for measuring the
intensity of the optical
radiation received by fiber 250, and an amplifier 270 for amplifying the
output of photodetector
260 to give the measured signal g(ij). In this embodiment, the source coupling
coefficient S' is
the product of the fluence P~ produced by diode laser 200, the coupling
coefficient CLr of the
lens 220 to the fiber 210, the transmission coefficient T~F of fiber 210, and
the coupling
coefficient CFT at the interface of fiber 210 and sample 150. Similarly, the
detector coupling
coefficient DJ is the product of the coupling coefficient CTF at the interface
of fiber 250 and
sample 150, the transmission coefficient TLF of fiber 260, and the coupling
coefficient of the
sample fluence produced by the diode laser PL, the coupling coefficient CFp
between fiber 260
and photodetector 260, the efficiency yPE of photodetector 260, and the gain A
of amplifier 270.
In other embodiments, the light source can include a laser other than a diode
laser, e.g.,
an ultrafast laser, or instead it can include an incoherent source. Also, the
sources can include a
common light source that selectively couples light into one of multiple fibers
that deliver the
light to spatially separated locations on the sample. Alternatively, the
sources need not include
optical fibers at all. For example, the lasers themselves can be positioned
adjacent the sample or
can include beam delivery optics to direct the light to the sample through
free space. However,
this method does not remove the blood from the scalp as described above.
Furthermore, the light
sources can provide light at multiple wavelengths by including, e.g., multiple
diode lasers.
Calibration Issues
Calibration of signals generated from the methods of the invention can be
performed
using any means in the art, as well as using the new calibration methods
described below. In
general, the relationship between absorption and concentration of a natural
tracer, such as
oxyhemoglobin, is described by the Beer-Lambert law and modified versions
thereof. The
calculation of cerebral blood flow from the concentration of tracer is based
on the Fick principle,
which states that the rate of accumulation (Q) of tracer in the organ of
interest is equal to the
difference between the rate of arrival and the rate of departure of the
tracer. Assuming that the
time that the time taken to inject the tracer bolus is by comparison
relatively short enough to
ignore, a measurement of the amount accumulated in an organ can be made at a
specific time t (if
inducing the tracer at time 0), provided that t is less than the minimum
transit time through the
-24-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
organ, i.e., no tracer would be leaving the organ. Then the accumulation of
tracer (Q) can be
expressed as:
Q(t) = to F ' Ca ~t (A)~
where C~ is the arterial concentration of tracer. When flow F is constant, the
Eq. (A) can be
derived as:
F = Q(t) ~ Jo C"dl (B).
For example, blood flow measurements can be made by near-infrared spectroscopy
(NIRS) using oxyhemoglobin as tracer in transmittance mode as described in
Edwards et al., J.
Appl. Physiol. 75:1885-1889, 1993. The experiment described in the reference
immediately
above was performed in the forearms of six healthy young adults. For
comparison, blood flow
was also measured by venous occlusion plethysmography, and the relation
between flow
calculated by NIRS (y) and plethysmography (x) was y = 0.93x + 0.30.
Calculation of the
signals generated by the methods of the invention can be similarly performed.
Cerebral blood
flow can also be measured using the calculations employed in Skov et al.,
Pediatr. Res. 30:570-
573, 1991; Bucher et al., Pediatr. Res. 33:56-60, 1993; Fallon et al., Ann.
Thorac. Surg. 56:1473-
1477, 1993; and Elwell et al., J. Appl. Physiol. 77:2753-2760, 1994. In
addition, tracer dyes can
be used as a more flexible alternative to oxyhemoglobin in NIRS. Measurements
using the dye
ICG can produce a relatively high signal-to-noise (S/N) ratio than when using
oxyhemoglobin as
tracer. See, e.g., Patel et al., supra.
However, many of the past studies do not show that optical methods utilizing a
tracer can
be used to quantify cerebral blood flow (CBF), despite their calibration
methods. At best, they
show that a flow index can be measured that is proportional to the real flow,
but that the
proportionality constant varies from study to study. This indicates that the
proportionality
constant depends on the experimental design. Eq. (A) shows that flow F is
equal to the
concentration of a tracer measured at a given time Q(t) divided by the
integrated arterial
concentration C~(t) feeding into the organ of interest. Thus, an accurate
quantitative measure of
-25-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
F requires a quantitative measure of Q(t) and C~(t). This requires absolute
quantification of the
absorption coefficient from the optical measurements, since the concentration
and absorption
coefficient are linearly related through the extinction coefficient of the
tracer. If the tracer were
uniformly distributed throughout the tissue sampled by the diffusing light,
then this would be a
simple task. However, in the case of analyzing the CBF, the tracer has
different concentrations
in the scalp, skull, and brain. Quantification of tracer concentration in this
case can be done by
imaging. To make the situation worse, the tracer is not uniformly distributed
in each tissue type
as it is localized to blood vessels which comprise ~5% of the tissue. Thus,
assuming no
saturation of the optical signal by the tracer, one needs to know the volume
fraction of the blood
vessels in order to quantify the tracer concentration. However, this is not
likely to be sufficient
since saturation easily occurs with ICG as the tracer, since the dye increases
the absorption
coefficient of the blood vessels such that the sensitivity of detection of the
blood vessels drops
relative to the surrounding tissue matrix. All of these factors introduce
systematic errors
rendering an absolute measurement of CBF difficult to achieve. Further, a
relative measurement
of CBF is not likely to be accurate since these factors change from patient to
patient, and can
also change within a patient from measurement to measurement.
Our solution, therefore, is to self calibrate the measured flow index in
abnormal tissue
(stroke) against normal tissue. The ratio of these flow indices will nearly
equal the ratio of the
true flow in each region since the systematic errors will nearly cancel in the
ratio. This new
method is described below.
Other than the circuitry considerations discussed above, the signals still
must be
calibrated correctly. To analyze the measured values g(i j) discussed above, a
calibration is
required to convert the measured values for g(i j) into the transmittance
values f(i j). According
to Equation (1), this requires a calibration for the value of SiDJ for every
source-detector pair. As
will be described below, analyzer 140 determines self consistent values for
,S'~DJ based on the set
of measured values g(ij) and the results of a numerical calculation for f(i j)
corresponding to an
approximate model of the sample. Once the calibration is determined, the same
set of measured
values g(ij) can be used to calculate f(i j) according to Equation ( 1 ), from
which the analyzer can
perform the inverse calculation. Because the same set of measured values are
used for the
-26-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
calibration and the inverse calculation, the optical properties determined by
the analyzer do not
include systematic errors caused by fluctuations in the source and detector
coupling coefficients.
In the description that follows, it is assumed that the sources provide
continuous wave
(CW) optical radiation and the detectors measure the intensity of the optical
radiation, in which
case g(i j), f(ij), S'i, and DJ are all real-valued. However, the calibration
techniques described
herein can also be applied to other diffuse optical measurement techniques in
which the sources
do not provide CW radiation. For example, in some techniques, the amplitude of
the optical
radiation provided by the source is modulated to create photon density waves
in the sample, and
the detectors are configured to measure the amplitude and phase of the photon
density waves
after propagation through the sample. In this case the values for g(i j), f(i
j), S'i, and DJ can be
complex. For a general reference on DOT with modulated optical radiation see,
e.g., O'Leary et
al., Phys. Rev. Lett. 69: 2658, 1992. Furthermore, in other techniques, each
source provides a
temporally coherent light pulse, e.g., a picosecond pulse, and the detectors
are time-gated to
measure the temporal delay of the diffuse light pulse in addition to its
intensity. For a general
reference on such time-domain DOT techniques, see, e.g., Patterson et al.,
Appl. Opt. 28: 2331,
1989; and Arridge, Inverse Problems 15:841-893, 1999.
Calibration Method. Assuming NS sources and Nd detectors and following
Equation (1)
above, the coupling coefficient of the ith source and jth detector can be
expressed as:
Sr - 1 ~ g(1~.1) (2)~
Nd .i=i .f (t> >) ~ D.i
(_> >) (3)~
NS ~=i.f(1>>OS'
SrD; = g(1>>) (4).
f(l~.i)
Equation (2) is the average of all measurements made with source i and
Equation (3) is the
average of all measurements made with detector j. Substituting Equations (3)
and (4) into
-27-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
Equation (2) we obtain a relationship between the source coupling coefficient
Sl and the detector
coupling coefficient Dk, corresponding to the ith source and the kth detector,
respectively:
N ik
S i = Ns ~ gO, .%) -_ As
N~ .i-1 NS g(ti>J) .f~tZ~k) k pk
.f(t~.l)' ~ ' '
ir_l.f (tt~ .l )
and likewise between DJ and Sl, corresponding to the source and detector
coupling coefficients
for the jth detector and the Ith source, respectively:
Dj - Nn ~ g(l>>) -_ Aii (6).
NS '-' .f(i>j) ~N g(1>») . I(i>») . Si si
.~I(t>>.I) g(1>>.l)
Thus,
i j
S D = L(Z~j~k~l) (7)~
SiDk
where
L(i, j,k,l) = AskA~i (8).
Note that L(i j,k,~ is a function only of the number of sources and detectors,
NS and Nd, the
measurements, g(i j), and the optical properties of the medium as reflected
through f(i j). In other
words, L(i j,k,~ is not dependent on the coupling coefficients.
Combining Equations (2), (3), and (7), the coupling coefficient product SIDk
must
minimize the following expression to be consistent with each other and with
the measurements
g(iJ)~
-28-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
N,. N~ L(i, j k, l) 1
j ~ k .f(i~j)-g(i~j)~
;_' ;-' S D
Assuming that f(i j) can be calculated from an approximate model, the value
for SIDk can be
calculated by minimizing Equation (9) with respect to SIDk. The minimization
can be repeated
for every source-detector pair, or alternatively, once the value for SIDk is
determined for the l-k
source-detector pair, the other coupling coefficient products can be
determined from Equation
(7). Although the calibration is non-linear, with no unique solution for the
individual coupling
coefficients Sl and DJ, an exact solution exists for the coupling coefficient
product of every
source-detector pair, which is all that is needed for the calibration. The
minimization of
Equation (9) can be performed using standard techniques known in the art, see,
e.g., Press et al.
in Numerical Recipes in C: The Art of Scientific Computing (Cambridge U.
Press, New York,
1988).
To perform the minimization in Equation (9), analyzer 140 makes a numerical
forward
calculation for f(i j) based on an approximate model of the sample. An initial
model can be that
the sample is homogenous with a constant absorption coefficient pa and a
constant reduced
scattering coefficient ps . Once the calibration is performed, an inverse
calculation can provide
perturbative corrections to the model to show spatial variations in the
optical properties of the
sample. Numerical techniques for the forward and inverse calculations are
known in the art and
will be briefly described in the next section. However, for the simple case of
an infinite
homogeneous sample, the model forward calculation simplifies to the following
expression:
i
.f(i~j)= 3ps exp -~3l~swa)Zlr~ (10)~
4~Ir
where (r~ I is the distance between the i'" source and the j'" detector on the
sample surface.
If the background optical properties are not known, then an iterative
procedure can be
used to find ~Q, ps , and the coupling coefficients. The procedure involves
estimating p,a and
-29-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
p,s to calculate f(i j), and then minimizing F(S~Dk) in Equation (9) to fmd
the coupling
coefficient ,SdDk. The procedure is repeated with different values of pa and
ps to minimize
F(~a, ps ,ftDk) with respect to all three parameters. For spectroscopic
applications where
constant values of pa and ps are expected over the spatial extent of the
measurement, the
technique provides an accurate determination of the absolute values of pa and
ps .
Once all of the coupling coefficients S'D' are determined based on the initial
model
calculation, experimental values for f(i,j) are calculated from Equation (1)
based on the
determined coupling coefficients and the measurements g(i j). The analyzer
then performs an
inverse calculation on the experimental values for f(i j) to determine
perturbations to the
homogeneous model for the sample. If necessary, the calibration can be
repeated for an
improved model of the sample based on the results of the inverse calculation.
In turn, the inverse
calculation can be repeated for experimental values f(i j) calculated from
Equation (1) using the
revised calibration. This iterative process can be repeated until the results
for the spatially
varying optical properties of the sample begin to converge.
The steps performed by the analyzer to carry out the calibration and analysis
of
measurements g(i j) for imaging applications is summarized by the flow chart
in Fig. 5:
In step 300, the analyzer receives measured signals g(i j) from the detectors.
In step 310, an initial model for the sample is input into the analyzer, for
example, the
initial model may treat the sample as being homogeneous. In this case, values
for pa and p,s
are estimated if they are otherwise unknown.
In step 320, the analyzer calculates values for f(i j) based on the sample
model and a
forward calculation. For example, if the sample is modeled to be homogeneous
and infinite,
Equation (10) can be used.
In step 330, the coupling coefficient SdDk is determined by minimizing F(SlDk)
with
respect to SIDk in Equation (9), the other parameters in Equation (9) being
specified by the
measurements for g(i j) and the values for f(i j) from the model forward
calculation in step 320.
In step 340, steps 310-330 are repeated as necessary with additional estimates
for p a and
p s , until values are found for ,S~Dk, p Q , and p s that optimally minimize
F(S~Dk).
-30-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
In step 350, the values for all of the remaining coupling coefficients S'IY
are determined
by either: 1 ) calculating SD' using Equation (7) and the value for .S~Dk
determined in step 340;
or 2) replacing the argument SrDk for F in Equation (9) with the coupling
coefficients SD'
corresponding to each of the remaining source-detector and determining the
value S'D' that
minimizes F.
In step 360, the calibration coefficients S'D' determined in steps 340 and
350, and the
measurements g(i j) from step 300 are used to calculate experimental values
for,f(ij) based on
Equation ( 1 ).
In step 370, an inverse calculation is performed on the variation of the
experimental
values for f(ij) from the expected values for a homogeneous medium calculated
in step 360 to
determine spatial.variations in the optical properties of the sample, e.g., an
object hidden with a
highly scattering sample.
In step 380, if necessary, the model for the sample estimated in step 310 is
revised based
on the results from step 370, then steps 320, 330, and 350 are repeated, one
time, to recalculate
the calibration coefficients S'D' based on the revised sample model.
In step 390, if necessary, steps 360-370 are repeated, one time, using the
recalculated
calibration coefficients from step 380 to improve the determination of the
spatial variations in the
optical properties of the sample. Steps 380-390 can be iteratively repeated as
necessary until the
spatially varying optical properties determined in step 390 converge to within
a desired accuracy.
For spectroscopic applications in which the optical properties of the sample
are expected
to be homogeneous, the method can be terminated at step 340 because the
calibration technique
provides direct determination of the absolute values of pa , and ps .
Also, in other embodiments, the summations in the Equations above need not be
over
every source and detector in the experimental apparatus. For example, source-
detector
measurements between any two sources and any two detectors are sufficient to
determine all of
the source-detector coupling coefficient products corresponding to that set of
sources and
detectors. This example would be equivalent to setting NS 2 and N~=2 in the
above Equations.
Once some of the coupling coefficient products are determined based on a
subset of all possible
source-detector measurements, the remaining coupling coefficient products can
be determined
with relatively fewer additional measurements.
-31-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
Forward and Inverse Calculations. Techniques for the forward and inverse
calculations
of light propagation within the sample are known in the art. See, e.g.,
Arridge, supra. An
exemplary formulation of the calculation is described below.
The absorption coefficient pa and diffusion coefficients D, where D = u~~3(p~,
+ ps )~, are
expanded into a spatially independent background term pa° and Do,
respectively, and a
perturbative spatially dependent terms 8pa(r) and 8D(r), respectively. These
terms are then
incorporated into the diffusion equation, whose formal solution can be
expressed as an integral
equation by use of the appropriate Green function corresponding to the
sample's boundary
conditions.
In the present case, the light energy density is expanded in a perturbative
series, i.e., U(r)
= Uo(r) + U,(r) + ..., and solved to first order. The first-order perturbative
solution to the
heterogeneous equation, in the limit in which Ui far less than Uo, is given
by:
Uo(rs,rd) =Mexp(ikolrs -rdI)/(4~Dolrs -rd~) (11)
Uurs~rd~= -Swa(r)uDolUo(rs~r)G(r~rr~)-f'SD(r)~Uo(rs~r)WG(r~r~) d3r (12)
y o
where M is the amplitude of the source located at rs, G(r,rd) is the Green
function solution of the
homogeneous equation at detector position r~, a is the speed of light in the
medium, and ko = [(-
upa°+ic~)/Do] 1~2 is the photon density wave number, where c~ is the
source modulation angular
frequency. If an infinite medium is assumed, the Green function is
G(r, rd ) = exp(iko Ir - rd I) /(4~Ir - r~ ) . The integral in Equation ( 12)
is over the entire sample
volume. In the embodiment described above, the source is a CW source without
any modulation.
Thus, co goes to zero and ko is purely imaginary, so that both Uo(r) and U~(r)
are real-valued.
The first term in the integral of Equation (12) corresponds to absorbing
inhomogeneities,
whereas the second term corresponds to scattering inhomogeneities. Where it is
known that one
-32-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
or other type of inhomogeneity dominates, the non-dominant term can be dropped
from Equation
(12).
Based on Equations (11) and (12), the forward calculation corresponds to:
.f~l .7~= U°~rs')'rr~ti))+Ul(rs'~,rdi)) (13).
' M
In other embodiments, it is also possible to expand the perturbation series
beyond the first term.
For the image reconstruction, i.e., the inverse calculation, the integral in
Equation (12) is
digitized into a sum over voxels (i.e., volume elements), and equated to a
series of the values for
Ul (r5 '~, rd~~ ), which is extracted from the measurements g(i j) and the
calibration. This yields a
set of coupled linear equations that relates to the values of 8p,a and 8D in
each voxel within the
sample, which correspond to spatial variations in ~a and ~5. Many numerical
methods are
available for solving this system of equations including, for example,
Simultaneous Iterative
Reconstruction Technique (SIRT) and single value decomposition (SVD). For a
reference on
SIRT, see, e.g., Kak et al., in Principles of Computerized Tomographic
Imaging, (IEEE, New
York, 1988), Chap. 6, p. 211. For a reference on SVD, see, e.g., Press et al.,
supra, at Chap. 2, p.
52.
Software Implementation. The calibration method can be implemented in hardware
or
software, or a combination of both. The method can be implemented in computer
programs
using standard programming techniques following the method and figures
described herein.
Program code is applied to input data to perform the functions described
herein and generate
output information. The output information is applied to one or more output
devices such as a
display monitor.
Each program is preferably implemented in a high level procedural or object
oriented
programming language to communicate with a computer system. However, the
programs can be
implemented in assembly or machine language, if desired. In any case, the
language can be a
compiled or interpreted language.
Each such computer program is preferably stored on a storage medium or device
(e.g.,
ROM or magnetic diskette) readable by a general or special purpose
programmable computer,
-33-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
for configuring and operating the computer when the storage media or device is
read by the
computer to perform the procedures described herein. The computer program can
also reside in
cache or main memory during program execution. The calibration method can also
be
implemented as a computer-readable storage medium, configured with a computer
program,
where the storage medium so configured causes a computer to operate in a
specific and
predefined manner to perform the functions described herein.
For example, referring again to Fig. 3, analyzer 140 includes a processor 170,
and
input/output control card 160, a user interface 190 such as a keyboard and
monitor, and a
memory 180. The memory stores a program 185 specifying the steps of the
calibration method.
When executed, the program causes the processor to carry out the steps of the
calibration
method.
Additional details regarding the calibration method immediately above are
described in
U.S. Provisional Application Serial No. 60/154,423, filed September 17, 1999.
Once the signals have been calibrated and sorted, indicating changes, if any,
in the
scattered light at each individual location, the separate data points at each
position can be
assembled into an image, which is then conveyed via a visual display. The
displayed image can
indicate an actual or modeled outline of a tissue or organ, with a region of
abnormality indicated
by a different color or shade of gray. Alternatively, the spatial abnormality
can be indicated by a
rough outline (e.g., a box) of the affected region, with a caption of text
stating the nature of the
abnormality (e.g., increased blood volume or delayed blood flow). Other
techniques for
displaying images containing target structures such as abnormalities are also
suitable for use in
the invention.
Distinguishing a Cortical Ischemic Event from a Deep Ischemic Event in the
Brain
It is possible to distinguish sub-cortical from cortical lesions through their
hemodynamic
spatio-temporal signatures (see Example 2 below). For instance, a sub-cortical
lesion is likely to
induce a broad spatial and temporal hemodynamic variation within the cortex.
To illustrate the
phenomenon, one must first note that major arteries feeding the brain enter at
the organ's center.
Blood then flows towards the cortex via branching arterioles (Figs. 6A and
6B). In general,
however, each portion of the cortex is fed by more than one set of arterioles
and associated
-34-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
capillaries, an architecture that many believe evolved to minimize tissue
damage when an
ischemic event occurs deep in the brain.
Thus, an ischemic event deep in the brain will not completely shut off the
flow of blood
to a cortical region. Instead, referring to Fig. 6A, an ischemic event 510 in
a deep portion 550
leads to a diffuse, affected region 520 of decreased and delayed blood flow to
cortical portion
530 of a brain 500 due to a longer vessel path taken by the blood in
arterioles 540. In addition,
collateral blood flow will mix tracer from unaffected brain tissue with blood
that contains little
tracer because of the ischemia, resulting in a less pronounced dilution of the
tracer than seen
immediately downstream of the ischemic event. This is analogous to cars
detouring around a
major accident on a highway by exiting the highway, taking back roads
(collateral arterioles) and
thereby affecting cars headed to other destinations (other regions of the
cortex), then re-entering
the highway to reach their destination. The detoured cars arrive at their
faraway destination (the
cortex of the brain) later than would have been expected and cause cars
traveling elsewhere to be
delayed as well.
In contrast, referring to Fig. 6B, a cortical lesion 560 in brain 500 causes a
more
localized, affected region 570 in cortical portion 530 that exhibits a delay
in the transit of a
contrast agent (such as a dye or an oxygen bolus) as well as a greater
reduction in the quantity of
the tracer that moves through local region 570. Referring to the car analogy
above, if a major
accident on the highway occurred just before the destination, an observer
would (1) see few cars
making it to the destination because there are no exits to leave the highway;
and (2) little effect
on other cars using other highways because the detoured cars, if they can exit
the highway at all,
only need to travel a short distance to their destination and would not affect
other cars traveling
to other destinations much at all.
The result is that more tracer will arrive in cortical region 530 with deep
lesion 510
relative to cortical lesion 560. In addition, with deep lesion 510, the peak
tracer concentration
will be delayed more with respect to the normal tracer curve as compared to
the delay caused by
cortical lesion 560. This greater delay occurs because the tracer must travel
a greater distance,
via collateral arterioles, from the lesion to cortical region 530 in brain
500.
In summary, as compared to a normal tracer curve, a cortical lesion will cause
the tracer
peak to be delayed by a small amount and have a significantly reduced peak
concentration, while
-35-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
a sub-cortical or deep lesion will cause a longer delay in the peak arrival of
the tracer and a
smaller reduction in the peak concentration. Thus, thresholds on both the
temporal and
concentration characteristics will enable discernment of deep and cortical
lesions. This threshold
approach is also supported by the spatial extent (size) of the abnormal blood
flow in the cortex.
A small spatial extent will be consistent with a cortical lesion, while a
large spatial extent will
suggest a deep lesion. Thresholds can be set as follows: if (1) the peak delay
relative to the
normal curve is greater than about 2 seconds, (2) the peak amplitude is
reduced by less than
about 50% of the normal peak amplitude, (3) the spatial extent of the blood
flow anomaly is
greater than about 20 mm in diameter, and/or (4) the minimal period of time
required for passage
of a dye bolus at a detection point is about 2 or more seconds longer than a
normal reference
period of time, then a deep ischemic event is indicated.
Of course, the detection of any ischemic event, whether deep or cortical, is
based on
thresholds broader than the ones described immediately above. An ischemic
event is detected if
(1) the peak delay relative to the normal curve is greater than about 1
second, (2) the peak
amplitude is reduced as compared to the normal peak amplitude, (3) a blood
flow anomaly is
detected, and/or (4) the minimal period of time required for passage of a dye
bolus at a detection
point is longer than a normal reference period of time.
If the size of the region of abnormal blood flow is measured and mapped to a
particular
region of the brain, this information can be used to determined which blood
vessel and where
along its path the blockage has occurred. This is possible because the
architecture of brain
vasculature is well known and has been exhaustively catalogued in the human
population. See,
e.g., Kretschmann et al., "Arteries of the Brain and their Vascular
Territories," In: Cranial
Neuroimaging and Clinical Neuroanatomy, Thieme Medical Publishers, Inc., New
York, pp 191-
199, 1992. In other words, given the known blood vessel path, direction of
blood flow, and
interconnections among arteries and arterioles, one can deduce the location of
the blockage
responsible for the cortical blood flow abnormality detected by DOT. For
example, referring
back to Figs. 6A and 6B, the locations of lesions 510 and 560 can be deduced
from the position
and size of affected regions 520 and 570, respectively.
The invention will be further described in the following examples, which do
not limit the
scope of the invention defined in the claims.
-36-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
EXAMPLES
Example 1: Distinguishing Deep Ischemic Events from Cortical Ischemic Events
One can distinguish a deep ischemic event from a cortical ischemic event by
the
differences in the hemodynamic spatio-temporal signatures generated by DOT.
For a patient
suffering from a stroke, a health care provider first withdraws a 1 ml volume
of blood from the
patient and mixes the blood with 1 ml of a solution containing 0.1 mg/kg body
weight of
indocyanine green dye (Pulsion-green, Pulsion, Munich, Germany) dissolved in
water. After a
period of about 1 S seconds to allow binding of the dye to plasma proteins,
the 2 ml mixture is
injected as a bolus into the patient through a venous catheter in the arm.
The patient's head is first fitted with a helmet containing optodes emitting
red light at 780
nm and optode detectors spaced throughout the scalp surface covered by the
helmet. Light is
emitted by the a laser and passes through the optodes and into the brain
through the scalp and
skull of the patient. Any light that is reflected and/or scattered by the
brain and passes out
through the scalp and skull is detected. The amplitude of detected light at a
point above the scalp
is inversely correlated with the concentration of dye in the cortical region
of the brain below that
point.
If the patient is experiencing an ischemic event in a deep portion of the
brain, a diffuse
blood flow abnormality will be evident in a cortical region of the brain. As
shown in Figs. 7A
and 7B, as the dye bolus passes the cortical region of the brain adjacent to a
deep ischemic event,
the bolus is both diffuse and delayed in comparison to a reference dye bolus
trace. The reference
dye bolus trace can be an averaged or estimated normal trace, an actual trace
of the patient's
brain under normal (i.e., without an ischemic event) conditions, or a trace of
a normal matched
portion of the brain (e.g., an unaffected hemisphere of the brain). As
indicated in the graph of
Fig. 7B, the peak concentration of dye in the affected cortical region is
about 55% of the peak
normal peak concentration, showing the reduction in the amount of dye moving
through the
cortical region that would be expected by a deep ischemic event.
The deep ischemic event also leads to an increase of about 3 seconds in the
time it takes
the peak concentration to be detected after the bolus is injected or passes a
reference point as
compared to a normal peak concentration (t,), showing that the bolus of dye
moving through the
-37-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
cortical region of the brain has been delayed by a deep ischemic event.
Finally, the size of the
blood flow abnormality (30 mm at the longest diameter) is indicated by the
longer period of time
required for the bolus to completely traverse the affected cortical region
(t3), as compared to the
time required for the bolus to traverse the unaffected region (t2). In this
instance, t3 is about 3
seconds longer than t2. In other words, the minimal amount of time required
for the dye
concentration to advance from zero to the peak, then back down to zero is far
longer than under
normal conditions. This result is indicative of a disruption of the flow of
the dye bolus by the
deep ischemic event, of which only the collateral effects of the disruption in
the cortex is being
detected by DOT. See general discussion above under "Distinguishing a Cortical
Ischemic
Event from a Deep Ischemic Event in the Brain."
In contrast, if the patient is experiencing an ischemic event in the cortex of
the brain,
several distinguishing characteristics of the abnormal trace of dye
concentration can be seen in
Figs. 8A and 8B. First, as shown in Fig. 8B, the peak dye concentration is
reduced by a greater
amount (80%) from normal than is seen for a deep ischemic event (e.g., 45% in
Fig. 7B) because
the source of the dye flow blockage, rather than its downstream effects, is
directly detected by
DOT. Second, the increase in the time required for the peak concentration to
travel from a
reference point, e.g., bolus injection, to the detection site, relative to
normal (t,) is only about 1
second because the detected region is closer to the source of the ischemic
event. Third, the
minimal amount of time needed for the dye concentration to vary from zero to a
peak, and back
down to zero (t3) is only about 1 second longer than that for the normal trace
(t2). These
characteristics are analogous to the "ripple effect" seen on the water's
surface formed from a
pebble thrown into a pond. As observations are made closer to the ischemic
event, the effect is
both briefer and more intense than when the observation is made farther away
from the event.
Example 2: Detecting a Brain Bleed in a Human Stroke Patient
A brain bleed can also be detected by DOT following the procedure described in
Example 1, except that a measurement is made at a single time point after the
dye is distributed
uniformly throughout the circulatory system and the brain, instead of
measuring the movement
of a dye bolus through the brain over time. Figs. 9A and 9B show the head of
an individual
experiencing a brain bleed and the expected position-dependent trace of the
dye concentration,
-38-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
respectively. The dye is shown by the hatched region, and the pooling of blood
at the bleed is
shown by the clear region within the hatched region, with the source of the
bleed indicated as a
dot. As traced in the graph of Fig. 9B, the dye concentration along line A
passing through the
source of the bleed differs from a normal trace by the presence of a region of
a lower
concentration of dye. This phenomenon is caused by the clotting and blood
vessel constriction
associated with the bleed, which inhibits the dye from infiltrating the bleed
region. However, the
clotting and blood vessel constriction would not be apparent immediately after
the bleed begins,
since some time is required for these physiological and biochemical events to
occur. Regions in
the brain adjacent to the bleed area are not affected.
Alternatively, a brain bleed can be detected by allowing sufficient time for
the dye to
infiltrate the bleed. Once the dye enters the bleed, the dye is trapped in the
bleed by the clotting
and blood vessel constriction mentioned above. Meanwhile, the dye in the rest
of the circulation
is being removed from the body, e.g., by the liver, or degrades. If a
measurement of dye
concentration is made at this later time point, the presence of a bleed can be
seen by an inverse of
the phenomenon shown in Figs. 9A and 9B; namely, that a higher dye
concentration is seen in
the bleed region, while the rest of the brain exhibits a lower or zero dye
concentration. While at
this later time (about 10 minutes following bolus injection) the dye
concentration in the bleed
may be greater than the surrounding tissue, it will always be smaller than the
peak concentration
observed in the surrounding tissue at earlier times (about 1 minute following
injection).
Example 3: Modeling Blood Flow
Tubes were impregnated in a solution containing IntralipidTM (Flock et al.,
Lasers in
Surgery and Medicine 12:510-519, 1992; and Van Staveren et al., Applied Optics
30:4507-4514,
1991) and India ink. IntralipidTM, an aqueous suspension of lipid droplets, is
a common, fat-rich
supplement given intravenously to patients in a hospital. The concentration of
IntralipidTM in the
solution was adjusted to give the solution light scattering properties similar
to that in a human
brain, the concentration of India ink was adjusted to give the solution light
absorption properties
similar to that in a human brain. A dilute India ink solution having
absorption properties similar
to blood was passed through the tubes. The liquid was circulated through the
tubes with a
pulsatile pump to give a temporal flow distribution similar to that found in
arteries. Optodes
-39-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
were then placed on the surface of the slab to deliver light (780 nm) and
collect the diffusely
remitted light. The separation between optodes was adjusted to 1, 2, 3, and 4
cm for different
experiments. A bolus of concentrated India ink (dye) was then injected into
the tubes upstream
of the position of the optodes. As the dye flowed past the optodes, the light
detected decreased
in intensity due to the increased absorption of light by the dye. After the
dye agent passed the
imaging area, the optical signal returned to baseline. The experiment was then
repeated at a
different flow speed.'
The amplitude of detected light at the two flow speeds and with a optode
separation of 4
cm is graphed in Fig. 10. The "Fast Flow" (shown as the first inverted peak at
about 5 seconds)
is about 4 times as fast as the "Slow" speed (shown as the second inverted
peak at about 17
seconds). "Fast Flow" data points are graphed as solid square symbols, while
the "Slow" flow
symbols are graphed as solid circles. The graph shows that distinct variations
in amplitude could
be detected as the bolus passed through the region imaged, indicating that
cerebral blood flow
could be monitored using DOT.
Example 4: Detection of a Brain Bleed in a Piglet Model using a Calibration
Method
A piglet model was used in this study. The experimental set-up was a hybrid
system of
diffuse optical tomography and X-ray CT. Measurements were made on the bench
of the X-ray
scanner.
A one-week old piglet weighing 3 kg was sedated, intubated, and ventilated
during the
experiment. The femoral artery was catheterized for continuous blood pressure
monitoring, fluid
infusions, and blood extractions. Some of the blood taken from the femoral
artery was delivered
through two small needles inserted 2 cm through scalp, skull, and brain tissue
to produce an
artificial brain bleed. The separation of insertion points for the two needles
was about 2 cm. The
combined thickness of the scalp and skull was about 1 cm, so the blood was
injected into the
brain at a depth of about 1 cm. The injection speed was controlled by a step
motor at a rate of
42 p,l/min. A total of 625 p,1 of blood was injected in I S minutes for each
bleed . Images were
reconstructed every 15 seconds. Bleed A was created first, then bleed B. The
optical probe was
placed on top of the piglet's head.
-40-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
Referring to Fig. 1 l, the probe has 16 detectors (large circles) and 9
sources (pairs of
small circles) at each of 780 nm and 830 nm. The positions of bleeds A and B
relative to the
probe are also shown in Fig. 11. Before injecting bleed A, a baseline was
measured and used to
find the coupling coefficient of each source-detector channel and also the
background optical
properties. The calculated absorption and effective scattering coefficients
were 0.0672 cm ~ and
8.44 cm I, respectively, at 780 nm, and 0.0666 cm's and 7.61 cm ~,
respectively, at 830 nm.
These values correspond to an oxygen saturation (S02) of 58% and a total
hemoglobin content
(HbT) of 78 pl/mol. After bleed A was generated, another baseline was measured
before
injecting bleed B. X-ray CT images were taken after the optical measurements
to identify the
positions of the bleeds.
2D images were reconstructed based on the DOT measurements using simultaneous
iterative reconstruction technique (SIRT) and assuming that the piglet head is
semi-infinite.
Figs. 12A-12D show the time-course of the reconstructed images of bleed A at
both
wavelengths, with and without using the calibration method described in detail
above to correct
the DOT measurements. Figs. 12A-12D show that the application of the
calibration method
greatly reduces the presence of artifacts in the bottom and right sides of the
images. The
calibration method also improves the image amplitude.
Figs. 13A and 13B show a time-course plot of the peak image value of bleed A
at the two
wavelengths. Without calibration (Fig 13A), the plot is noisy with no clear
build up of the bleed.
After the calibration (Fig. 13B), however, the development of the bleed is
clearly visible.
Fig. 14 shows time-course images of both bleeds A and B as the volume of bleed
B was
increased. The base line and coupling coefficient were the same as those used
in reconstructing
images of bleed A. The respective intensities of the A and B bleeds differ
because their depths
differ.
These results indicate that the use of a dye bolus in conjunction with the
calibration
method described herein further facilitate the methods of detecting deep
ischemic events or
bleeds in the brain.
-41-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
Example 5: Monitoring Cerebral Blood Flow in an Adult Human
A 100 mW, 808 nm laser diode coupled to a 1 mm diameter fused silica fiber was
used to
deliver light to an adult human head. Four diode detectors were coupled to the
scalp via 3 mm
diameter plastic fibers. The collecting fibers were positioned 1, 2, 3, and 4
cm from the emitting
fiber. These four separations were chosen to discriminate between scalp
signals and skull
signals. Measurements made at 1 and 2 cm separations were sensitive to changes
in the optical
properties of only the skull and scalp. On the other hand, measurements made
at 3 and 4 cm
separations were sensitive to changes in the optical properties of the brain,
skull, and scalp. The
remitted intensity at the four positions was sampled at 4 Hz. Baseline data
was collected for 10
seconds, followed by the bolus injection. The bolus consisted of a 2 ml saline
solution of 40 mg
of indocyanine green injected into a vein in the arm of the volunteer. This
injection was
immediately followed by a 10 ml saline flush.
The data is summarized in the graph of Fig. 15, showing the attenuation of the
optical
signal caused by passing of the bolus of indocyanine green through the brain
of the adult human
volunteer. The results indicate that, at 20 seconds following the injection of
the dye bolus, the
signal at 3 and 4 cm separations dropped significantly, while the drop in the
signals at 1 and 2 cm
separations was delayed by about 2 seconds. The delay at 1 and 2 cm
separations indicated that
the signal decay at the 3 and 4 cm separation for the initial 2 seconds was
entirely due to the
arrival of indocyanine green in the brain. The small increases in each signal
at about 34 seconds
resulted from the re-circulation of the indocyanine green bolus.
It appeared that the concentration of indocyanine green actually drops by more
than a
factor of ten during re-circulation. At first thought, this result should have
led to a much greater
increase in the optical signal, and, in fact, the signal might have been
expected to return to
baseline. However, the dye concentration is likely large enough to have
saturated the optical
attenuation. This is further evidenced by uniform distribution of the signal
during the second
passage of the bolus, which has a concentration about 10 times smaller than
the bolus of the first
passage.
These results show that detecting a dye bolus in an adult brain is possible.
The blood
flow can then be calculated by applying Fick's principle as described in Patel
et al., supra;
-42-
CA 02397145 2002-07-23
WO 01/52719 PCT/USO1/02226
Edwards et al., Lancet 2:770-771, 1988; Edwards et al., J. App. Physiol.
75:1884-1889, 1993; or
Kuebler et al., J. Cerebral Blood Flow Met. 18:445-456, 1988.
Other Embodiments
It is to be understood that while the invention has been described in
conjunction with the
detailed description thereof, the foregoing description is intended to
illustrate and not limit the
scope of the invention, which is defined by the scope of the claims. Other
aspects, advantages,
and modifications are within the scope of the following claims.
For example, the methods of the invention can be accomplished with any
combination of
one or more fluorescent and/or non-fluorescent dyes injected (e.g., as a
bolus) into the blood
stream of an individual.
What is claimed is:
- 43 -