Note: Descriptions are shown in the official language in which they were submitted.
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
Systems and Methods for Reducing Intraocular Pressure
BACKGROUND
Related Application:
The present application is related to and claims benefit of U.S. Provisional
patent
application 60/175,658, "Glaucoma Pressure Relief Valve and Drug Delivery
Device," filed
January 12, 2000, the contents of which are hereby incorporated by reference.
Field of the Invention:
The invention relates generally to systems and methods for reducing
intraocular pressure.
In one embodiment, the invention relates to implantable devices for drainage
of aqueous humor
to relieve high intraocular pressures characteristic of glaucoma.
Description of Related Art:
The eyeball is a substantially spherical structure whose shape and tone is
maintained by~
endogenous fluid materials that fill an external hollow collagenous globe. The
interior of the
eyeball is divided into two chambers, the anterior chamber arid the posterior
chamber.
Suspended between these chambers are the ocular lens and its supporting and
related tissues.
The posterior chamber is filled with a gelatinous material called vitreous
humor that is not
thought to contribute significantly to the pressure level within the eyeball,
termed intraocular
pressure (IOP). In contrast, the anterior chamber is filled with a watery
fluid called aqueous
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
humor that is constantly being produced and resorbed. This fluid exerts
pressure against the
overlying cornea and against all structures surrounding it. If the amount of
aqueous humor
produced is excessive, pressure within the anterior chamber and within the
eyeball will rise.
Normal IOP results from a healthy equilibrium between production and
resorption of aqueous
humor.
Aqueous humor is produced behind the base of the iris and flows into the
anterior
chamber. Resorption takes place through the trabecular meshwork system, from
whence the
fluid passes into scleral vessels to be taken up into the bloodstream. A
certain range of pressures
in the anterior chamber is considered normal, generally between 10 and 21 mm
Hg. The
pressure within the anterior chamber is determined by how rapidly aqueous
humor is produced
and how rapidly it is drained through the trabecular meshwork system.
Obstruction to the
drainage system may be a cause of elevated intraocular pressure. Persistence
of elevated IOP
produces the condition known as glaucoma, wherein an elevated IOP may damage
the optic
nerve and affect vision, leading eventually to blindness if not properly
treated.
A variety of treatments for glaucoma are available. Medical therapies endeavor
to reduce
IOP improving fluid outflow or reducing fluid production. Available medical
treatments may
include topical ophthalmic or systemic medications. Medical management may
fail, however,
because of poor patient compliance, high cost, or any one of a number of well-
recognized
complications and side effects. In the event that medical management is
unsuccessful, more
invasive treatments can be offered to the patient either to alter the normal
anatomy or to
introduce implantable drainage devices for relieving excesses of aqueous
humor. For example,
laser surgery may be recommended to alter the anatomy of the trabecular
meshwork and enhance
anterior chamber drainage; other laser-mediated ophthalmological procedures
are also available
for glaucoma treatment. Glaucomatous eyes that continue to have elevated
intraocular pressures
despite medical treatment and laser intervention may require a definitive
surgical procedure.
As one example, a conventional type of surgical intervention aims to create a
fistula or
other drainage channel out of the anterior chamber of the eye. The aqueous
humor is thereby
directed to flow into a surgically created subconjunctival or scleral pocket,
often called a "bleb,"
-2-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
from whence the fluid can be reabsorbed into the bloodstream. This operation
reduces
intraocular pressure by allowing excess fluid to flow out of the anterior
chamber. Several known
limitations accompany such procedures, however. First, normal wound healing
tends to interfere
with the patency of the fistula and with the dimension of the drainage pocket,
so that these
operations may have an unacceptable rate of failure. To increase the success
rate of this type of
surgery, physicians may recommend adjuvant treatment with agents that modulate
normal wound
healing. Such treatment increases the incidence of a second sort of problem
associated with
these procedures: excessive or overly rapid outflow of aqueous humor. It is
well known that
removal of too much aqueous humor too quickly can reduce intraocular pressure
precipitously to
dangerously low levels, a condition called hypotony, potentially causing a
number of sight
threatening complications. To prevent this problem, the surgical site must
heal sufficiently well
to produce controlled aqueous humor drainage. For this to occur, normal wound
healing is
essential. Those treatments that inhibit wound healing therefore increase the
risks associated
with excessive aqueous humor drainage. A third kind of problem accompanies
this type of
conventional drainage procedure: an increased risk of infection. Drainage of
aqueous humor
into a scleral or subconjunctival bleb poses a risk for infection by providing
a fluid milieu that
microorganisms can invade. Furthermore, if an infection becomes established in
the fluid-filled
pocket, the microorganisms can travel retrograde through the drainage channel
to enter the
anterior chamber of the eye and infect it as well, a much more serious
condition.
To address some of the problems associated with conventional surgery, a number
of
implantable devices have been proposed that endeavor to drain excessive fluid
from the anterior
chamber. The problems described above that affect soft tissue surgery also
affect implantation
surgery, however. Wound healing mechanisms are still called into play, even
though the surgery
includes the installation of an intraocular implant. Indeed, artificial
materials may overstimulate
local wound healing, leading to excessive scar tissue formation. Furthermore,
controlling the
outflow rate of aqueous humor remains essential, even if an artificial device
is involved in the
process. In addition, infection remains a risk. With a mechanical conduit
available to transmit
microorganisms from the outside to the interior of the eye, some mechanism is
desirable for
discouraging retrograde infection. Finally, the eye, like most tissues of the
body, has limited
tolerance for the long-standing presence of artificial materials. A locally
positioned implant may
-3-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
irritate the surrounding tissues. The eye, of course, is particularly
sensitive. A device to be
implanted on the surface of the eye may be perceived by the patient as a
chronic, persistent and
bothersome foreign body. Finally, since eye tissues are so delicate, implants
must be designed
and placed so that they do not damage vulnerable adjacent, subjacent or
overlying tissues. Even
if properly positioned initially, however, the implant can be displaced by
local tissue motion or
can be extruded by constrictive wound healing processes.
A variety of devices in the prior art purport to provide solutions for some or
all of these
problems. For example, certain prior art devices shunt aqueous humor to a
reservoir or drainage
area that is implanted in the sclera or subconjunctivally. As mentioned
earlier, however, these
devices face the problems of regulating aqueous outflow, resisting infection
and avoiding local
tissue irritation and trauma. The first problem, regulating aqueous outflow,
arises because the
drainage rate of this fluid depends substantially on the mechanical
characteristics of the implant
until there has been sufficient wound healing to restrict fluid outflow
biologically. Effective
balancing of biological and mechanical resistance to aqueous humor outflow
remains a problem
for implant-based drainage procedures. Prior art devices utilize a variety of
mechanisms to
restrict aqueous outflow. Each of these mechanisms, though, may become a
liability once
wound healing has been established. Restrictive elements within the implant,
when combined
with the restriction effected by wound healing, may inordinately reduce the
rate of aqueous
humor outflow, possibly to non-therapeutic levels. The second problem, the
possibility of
intraocular infection, arises because the presence of an implant provides a
conduit through which
bacteria can gain entry to the interior of the anterior chamber. Certain rior
art drainage devices
have introduced filters or valves or other conduit systems to impede the
retrograde transmission
of infection into the anterior chamber. These mechanisms have limitations,
however: even when
effective in resisting the transit of microorganisms, they have hydraulic
effects on fluid outflow
that may also impair effective drainage. Finally, the problem of local tissue
tolerance arises with
certain prior art devices because these foreign bodies may incite tissue
reactions culminating in
local inflammation or extrusion, and may further be perceptible or
uncomfortable for the patient:
these reactions to the presence of the implant may make its use clinically
unsuitable.
-4-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
Devices placed through the clear cornea to effect aqueous humor drainage are
intended to
avoid certain limitations accompanying scleral or subconjunctivally
implantation. Certain
devices, for example U. S. Patent No. 3,788,327 and U. S. Patent No.
5,807,302, and U. S. Patent
No. 5,743,868, provide for transcorneal conduits that drain anterior chamber
fluid onto the
surface of the cornea to mix with the tear film. The devices taught in the
abovementioned
patents contain certain features directed to the problems of outflow
regulation, microorganism
restriction, local tissue compatibility, and positional stability. These
problems, as previously
discussed, affect transcorneal devices as well. There remains a need,
therefore, for a
biocompatible anterior chamber drainage device that permits the well-
controlled outflow of
aqueous humor despite vagaries of wound healing. There remains a further need
for a drainage
device that can limit the ingress of microorganisms and thereby protect the
interior of the eye
from infection. In addition, there remains a need for an ophthalmological
drainage device that is
well tolerated and comfortable for the patient. Finally, the problem of
positional stability has not
been solved satisfactorily. A need exists in the art for a drainage device
that can be securely and
reliably positioned without fear of dislodging, migration, or extrusion.
In addition to the aforesaid needs for permanent or durable drainage of the
anterior
chamber in conditions such as glaucoma, there are additional needs for
temporary anterior
chamber drainage or decompression. For example, IOP elevation over short
intervals (1 hr - 2
wks) may exist following a number of ophthalmological procedures, including
cataract
extractions and repair of retinal detachment. Moreover, a physician may find
it advantageous to
use a shunt to temporarily control IOP in glaucoma before embarking upon other
surgical
procedures for the disorder that do not employ long-term shunting. A need
exists.for a device to
fulfill the need for short-term anterior chamber drainage in these and similax
situations.
A further need exists for providing a delivery system specifically adapted for
atraumatic
insertion of a transcorneal drainage device. Advantageously, such a delivery
system would be
able to hold the drainage device securely so that it could be positioned by
the surgeon. Such a
delivery system would further permit the ready release of the drainage device
when it is to be
inserted through the cornea. It is further desirable that the delivery system
be fabricated to avoid
introducing any additional damage to the delicate tissues of the corneal
epithelium and stroma.
-5-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
SUMMARY OF THE INVENTION
It is an object of the present invention to provide systems for reducing
intraocular
pressure. The systems of the present invention may include a shunt insertable
through the clear
'~ cornea of the eye into the anterior chamber to drain aqueous humor
therefrom. The shunt may
include a substantially cylindrical body with a channel through with that
permits drainage of
aqueous humor from the anterior chamber to the external surface of the clear
cornea; the shunt
may further include a head that rests against the outer surface of the clear
cornea, a foot that rests
against the inner surface of the cornea and an elongate filter retainable
within the channel of the
body that regulates the flow rate of aqueous humor therethrough and that
minimizes the ingress
of microorganisms. In one embodiment, aqueous humor is able to flow through an
aperture in
the foot to enter the channel in the body and pass therethrough, to exit
through a slit in the head,
flowing onto the surface of the cornea. In one embodiment, the head and the
foot are formed
integrally with the body. In another embodiment, the head, the foot, or the
body may be made
from a dehydratable polymer. In certain embodiments, the external surface of
the head or of the
foot may be configured to minimize cellular adhesion or adherence. In certain
embodiments, the
external surface of the body may be configured to encourage tissue adhesion or
adherence, or to
be attractive. The foot may be specifically shaped to facilitate introduction
of the shunt through
the cornea. In certain embodiments, the body is smaller in circumference than
the head or the
foot. The elongate filter may be retained within the channel of the body by
impaction or by any
other appropriate mechanism. The elongate filter may be positioned at the
proximal end of the
body or in any other position therein. .
In other embodiments, the systems of the present invention may include an
implant that
can be placed across the cornea to drain the anterior chamber of the eye. The
implant may
include a head, a foot, a tubular conduit between the foot and the head that
has an interior
channel in fluid communication with the anterior chamber, and a filter that
can be impacted
within the anterior chamber to regulate outflow of aqueous humor and to
restrict incursion or
minimize ingress of microorganisms or obstruct their passage.
-6-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
In yet other embodiments, the systems of the present invention may include a
transcorneal shunt and may further include a delivery device for implanting
the shunt in this
transcorneal position. In certain embodiments, the transcorneal shunt to be
implanted with the
delivery device may have a head, a foot, a substantially cylindrical body
between the head and
the foot having a channel therethrough, and a filter positioned within the
channel to regulate the
flow rate of aqueous humor through the channel and further to restrict the
ingress of
microorganisms. In certain embodiments, the delivery device may include a tip
dimensionally
adapted for holding the shunt and for positioning the shunt for insertion
through the external
surface of the cornea, and may further include a plunger slidable from a
proximal position to a
distal position wherein sliding the plunger dislodges the shunt and urges it
through the external
surface of the cornea into a transcorneal position.
It is a further object of the present invention to provide methods for
decreasing anterior
chamber fluid pressure, thereby to treat glaucoma and other disorders
characterized by elevated
anterior chamber pressure. These methods may include the steps of providing a
transcorneal
shunt, providing a delivery device for positioning the shunt in the
transcorneal position, incising
a pilot hole through the exterior surface of the cornea to permit the
insertion of the shunt
therethrough, and employing the delivery device to insert the shunt into the
transcorneal position.
In one practice of the invention, the shunt that is provided may have a
substantially cylindrical
body, the head, a foot and a filter. It is yet another object of the present
invention to provides
methods for temporary drainage of anterior chamber fluid, thereby to decrease
intraocular
pressure. Temporary drainage is understood to take place over a short term,
for example, from
one hour to several weeks, using a device that may be removable at the
conclusion of the
temporary drainage period or that may be biodegradable, to be resorbed at the
end of that
temporary period. Such a device may be useful for implantation following those
procedures that
might be followed by increases in IOP, or may be useful as a temporary
correction for disorders
characterized by increased IOP.
The shunt according to the present invention is intended to solve certain of
the
abovementioned problems that have persisted within the ophthalmological arts
for treatment of
elevated IOP. First, the shunt, its delivery device and the methods for their
use are adapted for
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
positioning of a drainage system across the clear cornea, thereby avoiding the
difficulties that
accompany subconjunctival or subscleral drainage. Second, the outflow of
aqueous humor is
consistently regulated by a filtration system without implicating mechanisms
of wound healing,
so that a predictable outflow rate can be calculated to avoid the dangers of
hypotony on one hand
and inadequate drainage on the other. Third, the filter provides a tortuous
path to inhibit
bacterial ingress; in addition, the slit opening in the head is shaped and
sized to resist bacterial
invasion; furthermore, the head itself is fabricated from a material that
resists cellular adhesion,
including the adhesion of microorganisms. Fourth, the device is made of
materials well tolerated
by the cornea. The head and the foot resist cellular adhesion and discourage
scarring over the
device, while the body is made of materials that encourage cellular adhesion,
thereby to affix the
device securely in the transcorneal position. These and other objects,
features and advantages of
the present invention will become more evident from the following discussion
and drawings,
wherein like numbers represent like components.
BRIEF DESCRIPTION OF THE DRAWINGS
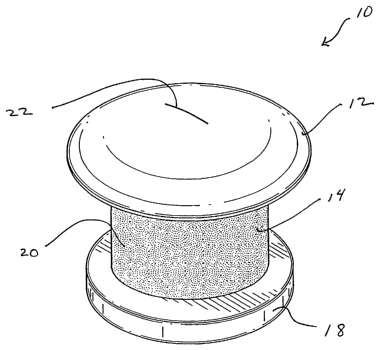
Fig. 1 is a perspective drawing of an embodiment of the present invention.
Fig. 2 is an exploded view of an embodiment of the present invention showing
an insertion path
of the filter.
Fig. 3 is a cross-section view of an embodiment of the present invention.
Fig. 4 is an anatomic cross section showing a shunt in position according to
the present
invention.
Fig. 5 is a schematic diagram of an embodiment of the present invention.
Fig. 6 A-D show perspective and cross-sectional views of a delivery device
according to the
present invention.
_g_
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
Fig. 7 A-B show a perspective and a cross-sectional view of an alternative
embodiment of a
delivery device according to the present invention.
DETAILED DESCRIPTION
With reference to Fig. l, a perspective view of a shunt 10 according to the
present
invention may be seen. In a representative embodiment, the shunt 10 may be
approximately one
millimeter long with an outer diameter of approximately 0.5 mm. While the
shunt 10 illustrated
in this and the following figures is shown as a cylindrical structure, it is
understood that other
shapes of tubular conduits may be suitable as well. For example, the shunt 10
may assume a
more oval shape or a more lenticular shape. Fig. 1 shows the shunt 10 from its
top or external
aspect. The shunt 10 dimensionally adapted for transcorneal positioning. The
head 12 will be
located on the external or epithelial surface of the cornea when the shunt 10
is in position. As
shown in this figure, the head 12 may be dome-shaped to provide a continuous
transition surface
from the device to the cornea. This shape may also be well tolerated by the
patient's eyelid.
While this shape seems particularly advantageous, other shapes of the head may
be designed to
provide the same advantages. For example, a minimally protruding flat head 12
with rounded
edges may be equally well tolerated. Other appropriate designs may be
determined using no
more than routine experimentation. The undersurface (not shown) of the head 12
may be flat or
curved suitably to match the shape of the corneal surface whereupon the device
is to be
positioned. The head 12, the body 14, and the foot 18 may all be formed
integrally as a unit, or
the head 12 or the foot 18 may be formed integrally with the body. In another
embodiment, each
component may be disassemblable from the others.
Copolymers of hydroxyethyl methacrylate (HEMA) may be used in the fabrication
of
components of the shunt. In one embodiment, the head 12 is formed from a
smooth material to
inhibit tissue and bacterial adherence and is highly hydrated and wettable
with tears. The head
12 may have a surface ingredient comprising a HEMA polymer such as HEMA plus
methacrylic
acid that is well known in the art for inhibiting cell adhesion. As an
example, poly 2-
-9-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
hydroxyethyl methacrylate (PHEMA) may be used for the shunt casing. In one
embodiment, the
base material for the tissue integration layer coating that attracts cells may
include HEMA and
cyclohexylmethacrylate. Covalently crosslinked hydrogels used in contact
lenses and having
equillibrium water content at least 15% by weight (and more preferably at
least
20% by weight), may be included in the composition of the casing, in
particulax copolymers of
esters of acrylic and methacrylic acid with di- and polyhydroxy compounds.
Examples of
suitable polyhydroxy compounds include ethylenglycol, diethylenglycol,
triethylenglycol, 1,2-
propandiol, glycerol, glycerolmonoacetate, glucose and the like. Such esters
may be further
copolymerized with vinylpyrrolidone, acrylic and methacrylic acid, acrylamide,
N-substituted
acrylamide, and many other similar compositions, as will be apparent to
practitioners in the art.
A number of specific compositions of such hydrogels are known in the art, many
of which would
be suitable and readily identifiable to skilled artisans using no more than
routine
experimentation. Typical crosslinkers are diacrylates and dimethacrylates of
the above diols and
polyols. In certain embodiments, the surface of the body 14 may include a
tissue integration
layer comprising a crosslinked polymer, for example a composition comprising
HEMA and a
alkylmethacrylate, particularly cyclohexylmethacrylate and particularly in
such a composition
where the said alkylmethacrylate is used in a higher concentration than HEMA.
The tissue
integration layer may be smooth, patterned or porous. In an exemplary
embodiment, a shunt
consistent with the present invention would be characterized by certain
physical characteristics,
including reversible hydration, shape memory, localized surface regions with
hydrophilic or
hydrophobic properties, localized surfaces with different hydration properties
and localized
surfaces having different cellular adhesion properties.
Bacterial invasion is further resisted by the slit 22 traversing the head 12.
The slit 22
permits the outflow of aqueous humor that has passed through the shunt to flow
onto the clear
cornea, thereby to enter the tear film. While the slit 22 depicted in this
figure is a single elongate
aperture, it is understood that other slit configurations may advantageously
provide for aqueous
humor outflow and restriction of bacterial incursion. For example, a pattern
of multiple small
slits may be designed. Or, for example, a slit or series of slits may less
elongated and more
rounded than this figure depicts. Other slit arrangements may be readily
envisioned by
practitioners of ordinary skill.
-10-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
The foot 18 may be made from materials similar to the head 12. This figure
shows a top.
or outer surface of the foot 18 adapted for contact with the inner or
endothelial surface of the
cornea. As shown here, the foot 18 may be flat, or it may be curved to fit the
shape of the
corneal surface it contacts. Furthermore, the foot 18 may be tapered or
frustoconical to facilitate
its insertion through the cornea. In the depicted embodiment, the foot 18 is
wider than the body
14. The inner surface (not shown) of the foot 18 bears an aperture through
which aqueous humor
enters the shunt 10. These and other features of the foot 18 will be shown in
other figures.
With further reference to Fig. l, the body 14 of the shunt 10 is positioned
between and is
connected to the head 12 and the foot 18. The body 18 may be made from a solid
HEMA
polymer and coated with a hydrogel, such as a copolymer of HEMA and
cyclohexylmethacrylate, that serves to promote cell adhesion. The coating 20
of the body 18 is
receptive to tissue attachment, so that the body 18 may be securely anchored
in position. This
feature enables the shunt 10 to resist in situ motion and displacement.
Furthermore, this feature
serves to prevent bacterial ingrowth along the transcorneal channel within
which the shunt 10 is
positioned. To further promote tissue ingrowth and cell attachment, the
coating 20 of the body
18 may be treated with surface alterations such as texturing, roughening or
introduction of
patterned irregularities. Combining HEMA polymers that promote cell adhesion
on the body 14
with HEMA polymers that resist cell adhesion on the head 12 and the foot 18
permits the shunt
both to become firmly attached to the cornea where the body 14 passes
therethrough, and also
to resist the attachment of bacteria to the head 12 with potential subsequent
invasion.
It is understood in the art that devices made of HEMA are well tolerated by
the eye. In
addition, a device made from dehydrated polymer, such as HEMA, may be
dehydrated to be
reduced to a smaller size for implantation through a small incision. This
feature may facilitate
insertion of the shunt through a pilot hole or similar small access route with
minimal tissue
disruption. After a dehydrated shunt 10 according to the present invention is
properly
positioned, it may imbibe water from the surrounding tissues and swell to its
predetermined size.
Varying degrees of dehydration are possible, depending on the particular
hydrogel formulation.
Even if dehydration only yields a small decrease in size, this may facilitate
implantation.
-11-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
Furthermore, implanting the dehydrated device in its transcorneal position and
allowing it to
imbibe water and hence enlarge will secure its tight fit in the intended
position.
Fig. 2 presents a perspective view of the shunt 10 as seen from the bottom or
interior
aspect. In the depicted embodiment, when the shunt 10 is positioned
anatomically, the foot 18
lies on the inner aspect or endothelium of the cornea and projects into the
anterior chamber. In
this figure, the body 14 and the head 12 may be also seen. The shunt 10 is
provided with a
channel 24 the passes through the foot 18 and the body 14 to approach the
undersigned of the
head. As illustrated in the previous figure, a slit (not shown) on the head 12
permits the egress of
aqueous humor that has flowed through the channel 24. A filter 28 regulates
the flow of aqueous
humor from the anterior chamber to the external aspect of the eye and provides
a tortuous path
through the channel 24 to impede the passage of bacteria. In one embodiment,
the filter 28 may
be made of titanium. Other materials such as ceramics and polymers may also be
suitable for the
filter 28. In certain embodiments, the filter 28 is impactable within the
channel 24 of the body
14. The filter 28 may be intended to form a permanent element of the shunt I0.
Alternatively,
the filter 28 may be removable and replaceable in those embodiments where
access to the
channel 24 is provided without disrupting the transcorneal position of the
shunt 10. For
example, a removable head I2 may permit access to the filter 28 so that it can
be removed and
replaced. As another example, the head I2 may be provided with an access port
(not shown)
located so that access to the filter 28 would be available without disrupting
the position of the
head 12. That access port and its attachment to the head 12 could, in certain
embodiments, be
integrated with the slit system described previously. Other arrangements may
be readily
envisioned by practitioners in these arts. The filter may be housed within a
rigid housing. This
housing may be inserted and removed from the shunt body 14 after the tissue
integration layer
has affixed the body 14 in position, without disrupting the affixation of the
casing in the eye.
As shown in Fig. 2, the filter 28 may be fabricated as a cylinder to be
inserted within the
channel 24 by a press fit. In the illustrated embodiment, the channel 24 has
smooth walls 30.
The filter 28, with representative dimensions of approximately 0.02 by 0.02
in., abuts the wall of
the channel 24 to be securely fixed therein. The depicted filter 28 contains a
network of pores
with pore size approximately 0.5 microns. The size of the pores is
dimensionally adapted for
-12-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
controlling fluid flow rate at approximately two microliters per minute. This
flow rate, obtained
by fabricating the size of the pores and the length of the flow path to
provide appropriate
resistance to flow, is sufficient to reduce the excess intraocular pressure
associated with
glaucoma while preventing ocular hypotony. While the previously described
arrangement of
pore size and flow path length appears particularly advantageous for the
systems of the present
invention, it is understood that other arrangements of pore size and flow path
length may also be
suitable. It is further understood that hydraulic characteristics of metals,
ceramics or polymers
may vary and that specifications for filters made from these substances may
vary also while still
falling within the scope of the invention, with the intent of any filter being
to provide consistent,
predictable and pathophysiologically desirable rates of aqueous humor outflow
while interfering
with retrograde passage of microorganisms.
Fig. 3 shows a shunt 10 according to the present invention in cross-section.
This figure
illustrates a fluid path for aqueous humor from the anterior chamber through
the channel 24
passing through the body I4 to drain out through the slit 22 in the head 12.
This figure shows the
head 12, the body 14 and the foot 18 all fabricated integrally as a unit. This
figure also shows a
single linear slit 22 penetrating the head 12. The depicted slit 22 extends
axially through the
head 12. Other slit arrangements may be envisioned as well. An irregular slit
path, for example,
may be provided. Multiple slits or a combination of slits and other shaped
perforations may also
be provided. In this figure, a coating 20 with an irregular surface has been
applied to the outer
aspect of the body 14. A filter 28 is shown disposed securely within the
channel 24. As
illustrated in this figure, the filter 28 occupies the mid portion of the
channel 24. Other positions
of the filter 28 may also be suitable. For example, the filter 28 may be
positioned more
proximally or more distally then is illustrated here.
Fig. 4 shows an anatomic cross section with the shunt 10 in its anatomic
position
traversing the cornea 104. As previously described, surfaces of the depicted
embodiment may
be made from different materials with different properties, in particular,
with a surface resistant
to cell adhesion or protein deposition and with a surface attractive to cell
adhesion, as described
above. The head 12 of the device is seen resting on the corneal surface 118.
The shunt 10 is
provided with a passage therethrough that permits fluid within the anterior
chamber 108 to flow
-13-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
across the clear cornea 104 to the outside surface of the eye. Fluid entering
the interior passage
of the shunt 10 will then exit the device and flow onto the outer corneal
surface 118, from
whence it commingles with the tear film. This figure shows the head 12 of the
shunt 10 in
contact with the outer corneal surface 118. This figure further shows the foot
18 in contact with
the inner corneal surface 122, although such contact is not necessary for
satisfactory positioning.
In a representative positioning, the shunt 10 of the present invention may be
placed in the
superior aspect of the clear cornea, overlain by the upper lid during neutral
gaze. Embodiments
of the shunt 10 according to the present invention may be constructed to span
the corneal stroma
between the tear film on the outer corneal surface 118 and the anterior
chamber 108. In certain
embodiments, a shunt 10 may include at least the following components: (a) a
body 14 made
from a hydrogel and having an outer surface in direct contact with stromal
tissue; (b) a head 12
protruding from the cornea and having an external surface in contact with the
tear film and in at
least intermittent contact with the inner aspect of the eyelid (not shown);
(c) a foot 18 protruding
into the anterior chamber 108. In the described embodiment, at least the
external surface of the
body 14 and the head 12 have different properties with respect to cell
adhesion and water
wettability. In a particularly preferred embodiment, the external surface of
the head 12 is non-
adherent for cells and is well wettable with tears and is highly hydrated,
whereas the external
surface of the body 14 is less hydrated and highly adherent for cells. Fig. 4
also schematically
shows other anatomic structures. The lens 100 is shown dividing the anterior
chamber 108 from
the posterior chamber 102. Lateral to the lens 100 are the ciliary processes
114 of the ciliary
body 112, which structures are responsible for the production of aqueous
humor. Anterior to the
lens 100 is the iris 120.
Fig. 5 illustrates schematically an embodiment of the shunt 10 according to
the present
invention. In the depicted embodiment, the body 14 is traversed by a channel
24 approximately
0.017 in. to 0.018 in. in diameter. In the depicted embodiment, the channel
24: is approximately
0.048 in. in length. A filter 28 is shown within the channel 24. The filter 28
has a vertical height
of approximately 0.020 inches. It is advantageous that the filter be
configured to retain
microorganisms such as bacteria, viruses, fungi and spores thereof. The foot
18 is shown to have
a tapered edge 16 to facilitate inserting the shunt 10 across the cornea. The
tapered edge 16
depicted in this figure slants at a 45 degree angle over a distance of
approximately 0.008 inches.
-14-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
The foot 18 may have an overall vertical height of approximately 0.013 inches.
Other sizes and
shapes of the foot 18 may be envisioned that facilitate insertion of the shunt
I O across the cornea
while allowing the foot 18 to remain properly located within the anterior
chamber. For example,
the foot 18 may be provided with a folding or pleating arrangement which
minimizes its size
with dehydration and expands to a larger size with rehydration. In other
embodiments, the foot
18 may have a frustoconical shape or an inverted frustoconical shape that can
be folded to
facilitate its insertion. In certain embodiments, the foot 18 is larger than
the body 14, as is shown
in this figure. While the filter 28 shown in this figure is positioned in
distal end of the channel
24, other positions for the filter 28 are consistent with the present
invention. For example, the
filter 28 may be positioned more approximately in the channel 24, or it may
occupy a made
positioned in the channel, or it may be fabricated with pore size and fluid
pathway length
sufficient to allow the filter 28 to occupy substantially all of the channel
24.
In certain embodiments, a shunt 10 according to the present invention may be
formed
from a shape memory polymer that can be converted into a deformed shape
suitable for insertion
through a small incision, to return to its preselected shape in response to
hydration or in response
to body temperature. For example, a shunt 10 in the state of partial
dehydration with a softening
temperature TS that is higher than room temperature and preferably near body
temperature may
be initially inserted into the transcorneal position through an access
incision (e.g., a slit, an
excision, a puncture or any other access incision familiar to skilled
artisans), and may then, upon
rehydration and temperature increase, expand to assume its preselected size
and shape.
Methods for manufacturing a shunt according to the present invention may
include .
fabrication in a disposable mold or by machining with the tissue integration
layer being applied
as a curable composition. For example, the corneal implant or shunt can be
cast from a mixture
of HEMA, methacrylic acid, dimethacrylate crosslinker, and a free radical
initiator in a single
part silicone mold with a cavity formed by imprinting with a die shaped in a
preselected shape.
Alternatively, the corneal implant or shunt can be machined and then a tissue
integration layer
can be applied to an outer surface of the shunt. The tissue integration layer
being a curable
composition comprising a copolymer of HEMA with alkylmethacrylate, monomer
HEMA, a
dimethacrylate crosslinker, a free radical initiator and a volatile solvent.
Other methods for
-15-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
manufacturing a corneal implant or shunt according to these systems and
methods should be
readily identifiable by practitioners of ordinary skill in the relevant arts.
Systems and methods of the present invention may advantageously employ a
delivery
device adapted for holding a shunt or other drainage device, positioning the
shunt or drainage
device in a preselected position adjacent to the cornea and inserting the
shunt or drainage device
across the corneal surface to occupy a transcorneal position. In certain
embodiments, the
delivery device may include an insertion tip adapted for releasably holding
the shunt and for
positioning the shunt for insertion through the external surface of the
cornea, and may further
include an inserter slidable from a proximal to a distal position wherein
sliding the inserter from
the proximal to the distal position dislodges the shunt from the insertion tip
and urges it through
the external surface of the cornea into the transcorneal position.
Advantageously, a pilot hole or
other small access wound may be created in the corneal surface or may be
extended into or
through the corneal stroma before inserting the shunt or drainage device to
decrease resistance
when the delivery system is used to deliver the device into its preselected
transcorneal position.
The delivery device according to the present invention may, in certain
embodiments, be adapted
for indicating to the operator that the shunt has been properly positioned.
Fig. 6A shows a delivery device 200 suitable for inserting a shunt according
to the
present invention into a transcorneal position. The delivery device 200
depicted in this figure
has an ergonomic design with a proximal elongate shaft 206, a grip area 210,
an inserter that
includes a slidable tip piece 212, and an insertion tip 214. The shaft 206 and
the grip area 210
are formed from a body housing 202, preferably made from a lightweight plastic
material. The
forward portion of the delivery device 200 includes a hollow distal housing
226 within which the
slidable tip piece 212 may be moved anteriorly and posteriorly. The grip area
210 features a
proximal protuberance 204 and a distal protuberance 208 between which the
delivery device 200
is grasped with a pencil grip, allowing the shaft 206 to rest on the
operator's first dorsal web
space. The pencil grip is particularly suitable for guiding the insertion tip
214 with precision,
although other types of gripping are available for the device 200 at the
operator's discretion. At
the distal end of the insertion tip 214 is an insertion aperture 218 into
which a shunt (not shown)
may be placed.
-16-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
Fig. 6B shows a cross-section of the distal part of a delivery device 200
according to the
present invention with the slidable tip piece 212 advanced anteriorly. The
slidable tip piece 212
slides coaxially along a fixed plunger 220. Fig. 6B shows the slidable tip
piece 212 in a forward
position relative to the fixed position of the plunger 220 within the distal
housing 226. In this
position, a chamber is formed between the distal end 230 of the plunger and
the insertion
aperture 218 within the insertion top 214 that is dimensionally adapted for
releasably holding the
shunt 10. In this figure, shunt 10 may be seen positioned within the insertion
tip 2I4 of the
slidable tip piece 212, just inside the insertion aperture 218. In this
figure, the insertion tip 214
at the distal end of the tip piece 212 is shown in contact with the surface of
the cornea 228. So
positioned, the anterior face of the shunt 10 is seated approximately flush
with the distal insertion
tip 214, with the posterior face of the shunt 10 abutting against the distal
end 230 of the plunger
220. In this position, furthermore, a posterior chamber 222 is formed
posterior to the back end
228 of the slidable tip piece 212 and anterior to the fixed backstop 224. This
posterior chamber
222 provides a space into which the slidable tip piece 212 can be pushed by a
posteriorly
directed force. Such a posteriorly directed force may be produced for the
slidable tip piece 212
when the operator advances the delivery device unit 200 forward with its
distal insertion tip 214
in contact with the surface 228 of the cornea. The surface 228 of the cornea
resists the forward
motion of the distal insertion tip 214 and forces the slidable tip piece 212
backwards. The
position of the plunger 220, by contrast, is fixed within the delivery device
200. Therefore, as
the slidable tip piece 212 is forced relatively backward, the plunger 220 is
propelled relatively
forward by the continuing advancement of the delivery device 200 in the
operator's hand. The
plunger 220 and the shunt 10 in contact with the distal end 230 of the plunger
220 continue to .
move forward so that the shunt is urged past the surface 228 of the cornea
into its transcorneal
position. Passage of the shunt 10 through the surface 228 of the cornea may be
facilitated by
providing a small insertion site or pilot hole into which the foot of the
shunt (not shown) may
enter. The axial length of the sliding chamber 222 may be approximately the
same as the length
of the shunt 10. This design mitigates against pushing the shunt 10 too far
into the eye.
The extent of rearward displacement of the slidable tip piece 212 may be seen
in Fig. 6C.
In this figure, the insertion tip Z I4 is visible distal to the distal housing
226, the slidable tip piece
-17-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
212 having been pushed proximally into the distal housing 226. This figure
also shows the distal
end 230 of the plunger visible through the insertion aperture 218 of the
distal insertion tip 214,
indicating that the distal end 230 of the plunger may be approximately flush
with the distal end
of the insertion tip 214 when the slidable tip piece 212 has been pushed fully
backward.
Fig. 6D shows in cross-section the positions of the delivery device structures
when the
shunt 10 has been pushed through the corneal surface to occupy its
transcorneal position acxoss
the corneal stroma 232. The slidable tip piece 212 is in its full rearward
position, with its back
end 228 abutting the backstop 224 of the plunger. The plunger 220 itself is
not moveable within
the distal housing 226. Instead, forward advancement of the delivery device
200 has pushed the
slidable tip piece 212 backward relative to the plunger 220. The shunt 10,
remaining in contact
with the distal end 230 of the-plunger, is urged thereby through the corneal
surface 228,
advantageously through a pilot hole or incision or insertion site, to occupy
its transcorneal
position. Further forward directed pressure on the delivery device 200 meets
with resistance as
the distal insertion tip 214 of the no-longer-displaceable slidable tip piece
212 presses against the
corneal surface 228. Encountering this resistance, the operator knows to apply
no further
pressure.
Other mechanisms may be envisioned to inform the operator that the shunt 10
has been
correctly positioned. For example, the posterior chamber 222 may be equipped
with notches or
tabs (not shown) that mate with correlative structures on the slidable tip
piece 212 when the
slidable tip piece 212 has been fully displaced rearwardly. The engagement of
these mated
structures with each other may produce an audible or tactilely perceptible
click, informing the
operator that full rearward displacement of the slidable tip piece 212 and
hence full forward
positioning of the shunt 10 has taken place. The engagement of the mated
structures may be
permanent, so that the slidable tip piece cannot be returned to its forward
position, or the
engagement may be releasable by a latch, a button or similar mechanism. Other
equivalent
structures for signaling the operator about the position of the shunt may be
readily envisioned by
practitioners in these arts. In certain embodiments, the entire slidable tip
piece 212 or the
insertion tip 214 may be made from transparent materials, while the plunger
may be made from
opaque or brightly colored materials. This arrangement may permit the operator
easily to
-18-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
perceive the relative positions of these structures with respect to each
other. Alternatively, all
the distal structures may be made from transparent materials so that the
operator can easily
visualize the corneal surface through the transparent areas of the delivery
device 200.
Fig. 7A illustrates yet another embodiment of a delivery device 200 according
to the
present invention. The outer shape of this embodiment may be similar to the
outer shape of the
delivery device 200 depicted in figures 6 A-D, with, for example, a body
housing 202 that
extends rearwards to form a shaft (not shown) and a grip area 210
ergonomically formed with a
proximal protuberance 204 and a distal protuberance 208. In the depicted
embodiment, an
insertion aperture 218 is provided at the distalmost part of the insertion tip
214 into which the
shunt (not shown) may be releasably inserted. In the depicted embodiment,
however, the fixed
tip piece 244 and the insertion tip 214 are fixed relative to the delivery
device 200. A trigger 240
is provided in proximity to the grip area 210. The trigger 240 is located
slidably within a cutout
notch 242 through the distal housing 226. The trigger notch 242 permits the
forward
displacement of the trigger 240 relative to the distal housing 226. As shown
in this figure, the
trigger is in proximity to the grip area 210, although any other convenient
location for the trigger
mechanism 240 may be selected. The trigger 240 may have a roughened,
corrugated or irregular
surface so that it is more maneuverable by an operator.
Fig. 7B shows a longitudinal cross-section of the delivery device 200 taken at
line A-A'
of Fig. 7A. While the body housing 202 is shown here as hollow, the body
housing 202
proximal to the trigger shaft 250 may be solid or configured in any convenient
manner. The
distal housing 226, however, is sufficiently hollow to permit axial motion of
a slidable plunger
248 therethrough. In the depicted embodiment, the distal housing 226 also
bears a cutout trigger
notch 242 into which the trigger shaft 250 may be advanced. As shown in this
figure,
advancement of the trigger shaft 250 forwardly also urges the slidable plunger
248 forward
relative to the position of the distal housing 226. This figure shows a
chamber 216 present
within the insertion tip 214 of the fixed tip piece 224. This chamber 216 is
dimensionally
adapted for releasably retaining a shunt (not shown) according to the present
invention. When
the delivery device 200 depicted in this figure is used to insert and position
a shunt, the operator
may advance the trigger 240 to the forwardmost position of the trigger notch
242, thereby
-19-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
advancing the trigger shaft 250 and its affixed slidable plunger 248 so that
the slidable plunger
248 advances into the chamber 216 and displaces the shunt (not shown)
therefrom. The insertion
tip 214 of the delivery device 200 is adapted for contacting the outer surface
of the cornea during
shunt delivery. The operator holds the delivery device 200 securely, with its
insertion tip 214 in
contact with the corneal surface in a preselected position, and the operator
then simultaneously
advances the trigger 240 forward to insert the shunt through the cornea in the
designated area. As
has been mentioned previously, a variety of materials may be used for the
fabrication of the
delivery device 200. In particular, the distal elements of the delivery device
may be made of
transparent materials. The slidable plunger 248 may also be made of
transparent materials, so as
to facilitate visualization of the shunt. Alternatively, the insertion tip 214
and/or the fixed tip
piece 244 may be made of transparent materials, while the slidable plunger 248
is made of an
opaque material that may be brightly colored so that its relative position can
be readily
visualized.
By referring to the above described drawings, one may appreciate certain
methods for
decreasing anterior chamber fluid pressure according to the present invention.
In one practice of
the invention, a shunt is provided to drain aqueous humor, and a delivery
device is provided
suitable .for inserting the shunt. The shunt may be adapted for draining
aqueous humor at a
preselected rate and further for resisting the incursion of microorganisms.
After adequate
anesthesia has been provided, a site is selected for insertion of the drainage
shunt. A pilot hole
may be created that extends across the external surface of the cornea, and
that may extend
through the corneal stroma and further extend into the anterior chamber. The
dimensions of the
pilot hole are to be determined by the individual operator, based on surgical
judgment and the
individual patient's anatomy. A needle, a trocar, a scalpel, or any of the
multitude of instruments
familiar to ophthalmologic practitioners may be used to form the pilot hole or
similar insertion
site. The shunt may be inserted by the operator into the delivery device, or
the shunt may be pre-
inserted in the delivery device during its manufacture. While certain
exemplary dimensions for
shunt sizes have been disclosed herein, it is understood that a range of shunt
sizes may be
available to fit the variations in individual anatomy. It is further
understood that delivery devices
of various sizes may be provided to engage the different sized shunts, or that
a single sized
delivery device may be suitable for implanting shunts of all different sizes.
With the shunt
-20-
CA 02397166 2002-07-09
WO 01/50943 PCT/USO1/00350
secured in the insertion tip of the delivery device, the operator advances
fine delivery device
toward the external surface of the cornea. When the delivery device reaches
the preselected
position on the cornea, the shunt is urged into its transcorneal position
using the mechanisms of
the delivery device for advancing and displacing the shunt. When the shunt has
been properly
positioned to extend through the cornea, it will be able to drain aqueous
humor onto the corneal
suxface. Proper positioning of the shunt may be evidenced by the presence of a
visible droplet of
aqueous humor on the head of the implanted device.
It should be understood that such a device may be useful for implantation
following those
procedures that might be followed by increases in IOP or may be useful as a
temporary
correction for disorders characterized by increased IOP. In the case of a
temporary correction
following retina surgery, cataract extractions or other invasive ophthalmic
surgeries, the device
will be implanted for two hours up to one month, or until IOP has stabilized.
In contrast,
permanent or otherwise long term implants with the device of the current
invention would be
used in the case of treating glaucoma in diabetic patients.
It is understood that the specification provided above, with its drawings and
descriptions,
is only exemplary of the present invention and certain illustrative
embodiments. It is further
understood that changes and modifications may be made to the various
components and
structures of the stmt and its delivery systems and methods without departing
from the scope of
the present invention. Rather, the present invention is understood to be
defined by the following
claims.
-21-