Note: Descriptions are shown in the official language in which they were submitted.
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
-1-
PYRROLO-ISOOUINOLINE AND TETRAHYDROPYRROLO
ISOQUINOLINE DERIVATIVES AND THEIR USE
AS MEDIATORS OF THE 5-HT7 RECEPTOR
Background to the Invention
The recently identified human 5-hydroxytryptamine-7 (5-HT7) receptor
subtype has been cloned, and the extensive distribution of its mRNA has been
reported. Highest levels of 5-HT7 receptor mRNA have been observed in the
hypothalamus, thalamus, brainstem and hippocampus, while lower levels have
been
found in the cerebral cortex, striatum, olfactory bulb and olfactory tubercle.
The
presence of 5-HT7 mRNA is not limited to the brain, it has also been found in
peripheral tissues, namely spleen, stomach, ileum, intestine. coronary artery,
descending colon, smooth muscle cells and heart.
Distribution and pharmacological studies have suggested that the 5-HT7
receptor may be involved in the vasodilation of blood vessels, and therefore
may play
a role in cardiovascular indications. 5-HT7 receptors also play a role in the
control of
circadian rhythms of spontaneous electrical activity in the suprachiasmatic
nucleus.
The high affinity of a number of antipsychotic agents for the 5-HT7 receptor
suggests
that this receptor may help mediate the therapeutic actions of these
compounds.
Octahydro-pyrrolo-isoquinoline derivatives, as antipsychotic neuroleptic
agents are disclosed in EP 10661 having the formula:
where R2 is hydrogen, alkyl, cycloalkyl, alkenyl, acyl, aryl or aralkyl.
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
-2-
Ancilated indole derivatives as antiphlogistics are disclosed in DE 77-
2740836 having the formula:
i
wherein:
R is phenyl or heterocycle,
R', RZ are alkyl, carboxyalkyl, phenyl, and
R3, R° is a 5 or 6 membered ring optionally containing 1-3, 5, O or N
atoms.
A series of novel 3-(2-aminoethyl)-pyrrolo[2,3-g]isoquinolin-5-one
derivatives are claimed that are effective pharmaceuticals for the treatment
of
anxiety, depression and related CNS disorders and other conditions such as
schizophrenia, sleep disorders, including instances of circadian rhythm, the
treatment
of alcohol and drug withdrawal and sexual dysfunction.
Summary of the Present Invention
This invention relates to novel 3-(2-aminoethyl)-pyrrolo[2,3-g]isoquinolin-5-
one derivatives, to processes for their preparation, to pharmaceutical
compositions
containing them and to their use in therapy. The compounds are believed to be
useful
for the treatment of CNS disorders such as anxiety, depression and related CNS
conditions and other conditions such as schizophrenia, sleep disorders,
including
instances of circadian rhythm, migraine headaches, the treatment of alcohol
and drug
withdrawal and sexual dysfunction by virtue of their ability to bind to the 5-
HT7
receptor subtype. The compounds of the present invention may also find utility
in the
treatment of cardiovascular and septic shock, hypotension, renal disorders,
diarrhea
and spastic colon.
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
-3-
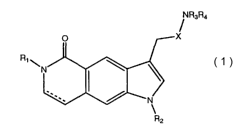
Compounds of the present invention are represented by the general formula
(1),
R~
Raga
O X
~N
N
R2
(1)
wherein:
Rl is hydrogen, alkyl of 1 to 8 carbon atoms, cycloalkyl of 3 to 8 carbon
atoms,
heterocycloalkyl of 3 to 8 members, alkylcycloalkyl of 4 to 9 carbon atoms,
alkylheterocycloalkyl of 4 to 9 members or (CH2)mAr or (CH2)mHet;
R2 is hydrogen, alkyl of 1 to 8 carbon atoms, (CH2)nAr or (CH2)nHet;
R3 is hydrogen, alkyl of 1 to 8 carbon atoms, alkylcycloalkyl of 4 to 9 carbon
atoms,
alkylheterocycloalkyl of 4 to 9 members, (CH2)nAr or (CH2)nHet;
R4 is hydrogen or alkyl of 1 to 8 carbon atoms;
R is hydrogen or alkyl of 1 to 4 carbon atoms;
X is [(CH=CH)R]n, (CHZ)n, [(C---C)R]n, CHR(CHZ)n, or CRZ(CHZ)n;
a dashed line represents an optional double bond;
m is an integer selected from 1 or 2; and
n is an integer selected from 0, 1 or 2;
or a pharmaceutically acceptable salt thereof.
In some preferred aspects of the present invention Rl is hydrogen, alkyl of 1
to 8 carbon atoms, alkylcycloalkyl of 4 to 9 carbon atoms,
alkylheterocycloalkyl of 4
to 9 members, (CH2)nAr or (CH2)nHet.
In other embodiments of the present invention R2 is hydrogen or alkyl of 1 to
8 carbon atoms.
X is preferably (CH2)n, and the optional double bond is preferably absent.
R3 and R4 are preferably hydrogen or alkyl.
Alkyl refers to straight or branched chain alkyl.
CA 02399545 2002-08-02
WO 01/57039 PCT/LJSO1/03430
-4-
Cycloalkyl refers to a saturated ring of 3 to 8 carbon atoms and preferably 5
to 6 carbon atoms such as cyclopentyl and cyclohexyl.
Heterocycloalkyl refers to a saturated ring of 3 to 8 carbon atoms having 1 or
2 heteroatoms selected from N, O and S. Preferably, heterocycloalkyl refers to
5 or 6
membered rings having at least one nitrogen heteroatom such as piperidinyl,
piperazinyl, pyrrolidinyl, imidazolidinyl, morpholinyl, thiomorpholinyl, and
pyrazolidinyl.
Ar is aryl and preferably is phenyl or naphthyl which may optionally be
substituted by one or more groups selected from flourine, chlorine, bromine,
iodine,
hydroxy, alkyl of 1 to 6 carbon atoms, trifluoromethyl, cyano and amino.
Het refers to heteroaryl and refers to a monocyclic 5 or 6 membered
heteroaryl group or a 9 or 10 membered bicyclic heteroaryl group. Preferred
heteroaryls have 1 or 2 heteroatoms selected from N, O and S, and, when 2
heteroatoms are present, it is preferred that at least one heteroatom is
nitrogen.
Exemplary monocyclic heteroaryl groups include pyridinyl, pyriminidinyl,
pyrazinyl,
furyl, thienyl, pyrrolyl, indolyl. Exemplary bicyclic heteraryl groups include
benzodioxanyl or quinolyl. Heteroaryl groups may optionally be substituted
with one
or more groups selected from flourine, chlorine, bromine, iodine, hydroxy,
alkyl of 1
to 6 carbon atoms, trifluoromethyl, cyano and amino.
The pharmaceutically acceptable salts are the acid addition salts which can be
formed from a compound of the above general formula and a pharmaceutically
acceptable acid such as phosphoric, sulfuric, hydrochloric, hydrobromic,
citric,
malefic, fumaric, acetic, lactic or methanesulfonic acid.
The compounds of this invention contain a chiral center, providing for various
steroisomeric forms such as racemic mixtures as well as optical isomers. The
individual optical isomers can be prepared directly or by asymmetric or
sterospecific
synthesis or by conventional separation of optical isomers from the racemic
mixture.
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
-5-
Detailed Description of the Invention
Compounds of the present invention may be prepared by those skilled in the
art of organic synthesis employing conventional methods which utilize readily
available reagents and starting materials. For example, reaction of an acidic
solution
(e.g. methanesulfonic acid) of 5-fluoroindanone with sodium azide affords the
required Schmidt rearrangement product. This can be alkylated with alkyl
halides,
alkylaryl halides or alkylheteroaryl halides under the influence of a base
such as
sodium hydride, and the product can be treated with hydrazine hydrate to
afford the
required aryl hydrazine. Reaction of the 1-aryl substituted hydrazine with a
substituted aldehyde derivative or protected derivative thereof (e.g. enol
ether or
dioxolan protecting group) under Fisher indole synthesis condition affords the
3-
substituted-pyrrolo[2,3-g]isoquinolin-5-one compound. 2-(3-chloropropyl)-1,3-
dioxolan is an example of suitably substituted and protected aldehyde
derivative, and
dilute sulfuric acid or dilute hydrochloric acid are suitable catalysts or co-
solvents for
the reaction. The basic amine may be alkylated directly for example by the
reaction
of an alkyl halide or alkylaryl halide under the influence of a base such as
sodium
hydride, or alternatively the basic amine may be directly acylated by the
action of
carboxylic acid halides, and the subsequent amide can be reduced by the action
of
lithium aluminum hydride. These step may be repeated to provide bis-alkylated
derivatives. The pyrrol nitrogen may be alkylated with alkyl halides or
alkylaryl
halides or alkylheteroaryl halides under the influence of a base such as
sodium
hydride. The optional double bond can be introduced by known and conventional
methods. For example reaction of a 3-substituted-1,6,7,8-tetrahydro-
pyrrolo[2,3-
g]isoquinolin-5-one with 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) affords
the
required 3-substituted-pyrrolo[2,3-g]isoquinolin-5-one compound of the present
invention.
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
R4
R
HN
/ F / F / F
-6-
Ri
wN I \
HN H2
R3R4 Ra
R1\
R2
Compounds of the present invention bind with very high affinity to the 5-HT7
receptor and consequently, they are useful for mediating the 5-HT7 receptor in
mammals. Compounds of the present invention are useful in the treatment of
central
nervous system disorders associated with dysfunction of the 5-HT7 receptor
such as
anxiety, depression and related conditions (e.g. GAD) and other conditions
such as
schizophrenia, sleep disorders, including instances of circadian rhythm,
migraine
headaches, the treatment of alcohol and drug withdrawal and sexual dysfunction
by
virtue of their ability to bind to the 5-HT7 receptor subtype. Similarly, the
compounds of the present invention may also find utility in the treatment of
cardiovascular and septic shock, hypotension, renal disorders, diarrhea and
spastic
colon.
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
-
5-HT7 Receptor Binding Assay
High affinity for the serotonin SHT7 receptor was established by testing the
claimed compound's ability to displace [3H] LSD binding in CHO cells stably
transfected with the human SHT7 receptor. Human cloned receptor membranes of
the
serotonin-7 subtype, expressed in a Chinese Hamster Ovary (CHO) cell line are
purchased from BioSignal Drug Discovery Technology, Montreal, Canada. The
frozen ampoules of receptor membranes are reconstituted in 50.0 mM Tris.HCl
buffer, at pH 7.4 to give 15-20qg of tissue protein per 100.0 q1 of
suspension. The
diluted membranes are kept cold on ice and immediately used in subsequent
binding
experiments.
Binding experiments are performed in a 96 well microtiter plate format, in a
total volume of 200 ~.~1. To each well is added: 80.0 ~1 of incubation buffer
made in
50 mM Tris.HCl buffer, pH 7.4 and containing 10.0 mM MgCl2 and 0.5 mM EDTA;
E.~l of [3H] LSD (S.A., 86.0 Ci/ mmol, Amersham Life Science), 5.0 - 6.0 nM.
The
15 dissociation constant, KD of [ 3H]LSD at the human serotonin 5-HT7 receptor
is 2.9
nM, as determined in saturation studies with increasing concentrations of [
3H]LSD.
The reaction is initiated by the final addition of 100.0 p1 of tissue
suspension. Non-
specific binding is measured in the presence of 10.0 E.~M methiothepin, added
in 20.0
~1 volume.
20 Test compounds when needed are added in 20.0 q1 volume. The reaction
proceeds in the dark for 120 minutes at room temperature, at which time, the
bound
ligand receptor complex is filtered off on a 96 well unifilter with a Packard
~ Filter-
mate 196 Harvester. The filtermate is air dried and the radioactivity is
measured in a
Packard TopCount~ equipped with six photomultiplier detectors, after drying
and
addition of 40.0 Ed Microscint ~-20 scintillant to each shallow well. The
unifilter
plate is heat sealed and counted in a Packard TopCount ~ with a tritium
efficiency of
31.0%.
Specific binding is defined as the total radioactivity bound less the amount
bound in the presence of 10.0 NM unlabeled methiothepin. Binding in the
presence of
varying concentrations of test drugs is expressed as percent of specific
binding in the
absence of drug. These results are then plotted as log % bound vs log
concentration
of test drug. Nonlinear regression analysis of data points with a computer
assisted
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
_g_
program Prism~ yields both the IC SO and the K; values of test compounds with
95%o
confidence limits. Alternatively, linear regression line of decline of data
points is
plotted, from which the IC So value can be read off and the K ; value
determined by
solving the following equation:
Ki = ICS/( 1 + L/KD)
where L is the concentration of the radioactive ligand used and K D is the
dissociation
constant of the ligand for the receptor, both expressed in nM.
Reference Compounds tested using the above procedure:
K. value and 95 % confidence interval
Compound 5-HT7 binding
Ki (nM)
Clozapine 6.3 (2.6 -11.0) nM
Loxapine 43.0 (26.0 - 68.0) nM
The compounds of the present invention showed activity in the above test, for
example:
Compound 5-HT7 binding
Ki (nM)
Compound 1 14
The following non-limiting specific examples are included to illustrate the
synthetic procedures used for preparing compounds of formula 1. In these
examples,
all chemicals and intermediates are either commercially available or can be
prepared
by standard procedures found in the literature or are known to those skilled
in the art
of organic synthesis. Several preferred embodiments are described to
illustrate the
invention. However, it should be understood that the invention is not intended
to be
limited to the specific embodiments.
Intermediate 1
6-Fluoro-3,4-dihydro-2H-isoquinolin-1-one
Sodium azide (7.8 g, 120 mmol) was added portionwise to a stirred solution of
5-fluoro indanone (9 g, 60 mmol) in methanesulfonic acid (35 mL) and
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
-9-
dichloromethane (35 mL), while the temperature was maintained between 22
°-29°C.
Once addition was completed. the mixture was stirred at room temperature for
16
hours. The mixture was cooled to 0 °C and neutralized by the addition
of SN-NaOH
solution and the organic layer separated. The aqueous layer was washed with
dichloromethane (3 x 50 mL) and the combined organics washed with water (50
mL),
brine (50 mL) and dried over anhydrous sodium sulfate. Filtration and
concentration
gave a light oil (7.8 g) which was purified by flash silica gel chromatography
(eluted
with diethyl ether) to afford the titled compound as a white solid.
m.p. 107-108°C
M+ 165
Elemental Analysis for: C9H~FN0
Calculated: C, 65.45; H, 4.88; N, 8.48
Found: C, 65.01; H, 5.10; N, 8.33
Intermediate 2
6-Hydrazino-3,4-dihydro-2H-isoquinolin-1-one
A solution of 6-fluoro-3,4-dihydro-2H-isoquinolin-1-one (3.6 g, 0.022 mole)
and
hydrazine (17.3 mL, 0.55 mole) was stirred at reflux in dioxane (150 mL) under
nitrogen for 48 hours. The reaction mixture was concentrated under vacuo,
water
added (150 mL), and the product isolated by filtration. The product was
thoroughly
washed with diethyl ether to give the titled compound quantitatively as a
white solid.
m.p. 158-160°C
MS(EI) m/e 177 (M+)
Elemental Analysis for: C9H11N30 0.75H20
Calculated: C, 56.68; H, 6.61; N, 22.03
Found: C, 56.69; H, 6.11; N, 22.23
Compound 1
3-(2-Amino-ethyl)-1 6 7,8-tetral~dro-pyrrolof2,3-glisoduinolin-5-one
A solution of 6-hydrazino-3,4-dihydro-2H-isoquinolin-1-one (0.5 g, 2.3 mmole)
and
2-(3-chloropropyl)-1,3-dioxolan (0.31 mL, 2.3 mmole) was stirred at reflux in
degassed EtOH/H.,O (5/1, 90 mL) solution under nitrogen for 24 hours. The
reaction
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
- 10-
mixture was partitioned between saturated aqueous NaHCO 3 and isopropyl
acetate.
The combined organic layers were dried over anhydrous sodium sulfate and
concentrated for chromatography on a silica gel column using CHZCh/MeOH/NH40H
(9.0/1.0/0.1) solution to give an oil. The oil containing two isomers was
separated by
Primesphere C-5 reversible preparative HPLC (90%CH 3CN/HZO + 0.1 %TFA) and
the desired product was freeze-dried under vacuo to give the titled compound
as a
yellow colored solid.
m.p. 82-86°C
MS(ESI) m/e 230 (M+H)+
Elemental Anal siy s for: C13H15N30.C2HF3O2. H20
Calculated: C, 49.86; H, 4.60; N, 11.24
Found: C, 49.98; H, 4.65; N, 11.39
Compound 2
3-(2-Amino-ethyl)-6-benzyl-1,6,7,8-tetrahydro-pyrrolof2,3-~lisoauinolin-5-one
A solution of 6-fluoro-3,4-dihydro-2H-isoquinolin-1-one (1.5 g, 9.1 mmole) and
cesium carbonate (3.3 g, 10 mmole) in acetonitrile (100 mL) was stirred at
room
temperature under nitrogen. To this was added benzyl bromide (1.2 mL, 10
mmole)
and the mixture refluxed for 15 hours. The reaction mixture was concentrated
for
chromatography on a silica gel column using EtOAc/hexane (9/1) to give the
expected product, 2-N-benzyl-6-fluoro-3,4-dihydro-2H-isoquinolin-1-one, as an
oil
(2.2 g, 95%).
A solution of 2-N-benzyl-6-fluoro-3,4-dihydro-2H-isoquinolin-1-one (2.2g, 8.6
mmole) and hydrazine (6.7 mL, 0.215 mole) was stirred at reflux in dioxane
(150
mL) under nitrogen for 48 hours. The reaction mixture was concentrated for
chromatography on a silica gel column by using EtOAc/MeOH (9/1) to give the
expected product, 2-N-benzyl-6-hydrazino-3,4-dihydro-2H-isoquinolin-1-one,
quantitatively as an oil.
A solution of 2-N-benzyl-6-hydrazino-3,4-dihydro-2H-isoquinolin-1-one (1.l g,
4.1
mmole), 2-(3-chloropropyl)-1,3-dioxolan (0.6 mL, 4.1 mmole) and 1.0 M ethereal
HCI (4.1 mL) was stirred at reflux in degassed EtOH/H~O (5/1, 200 mL) solution
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
- 11 -
under nitrogen for 24 hours. The reaction mixture was partitioned between
saturated
aqueous NaHC03 and isopropyl acetate. The combined organic layers were dried
over anhydrous sodium sulfate and concentrated for a multiple chromatography
on a
silica gel column using CH ZCh/MeOH/NH,OH (9.0/1.0/0.1) solution to give a
free
base of the desired product as an off-white colored solid (0.35g, 1.1 mmole,
27%).
The product in methanol was treated with 1.0 M ethereal HCl (1.1 mL, 1.1
mmole)
and recrystallized from CHZCh/MeOH (1/1) to afford the titled compound as an
off-
white colored solid.
m.p. 262-263°C
MS(ESI) m/e 320 (M+H)+
Elemental Analysis for: C20H21N30.HC1.H20
Calculated: C, 64.25; H, 6.47; N, 11.24
Found: C, 64.31; H, 6.09; N, 11.09
Compound 3
3-(2-Amino-ethyl)-6-benz~nvrrolof 2,3-~lisoquinolin-5-one
A solution of 3-(2-Amino-ethyl)-6-benzyl-1,6,7,8-tetrahydro-pyrrolo[2,3-g]iso-
quinolin-5-one (1 mmole) and 2,3-dichloro-5,6-dicyanobenzoquinone (2 mmole) in
chlorobenzene (15 mL) at reflux under nitrogen for 24 hours provides the
titled
compound as a yellow colored solid.
MS(ESI) m/e 228 (M+H)+
Elemental Analysis for: C13H13N30
Calculated: C, 68.70; H, 5.70; N, 18.49
Found: C, 68.51; H, 5.59; N, 18.29
Compound 4
3-(2-N-Benzvlamino-ethyl)-1,6,7,8-tetrahvdro-nyrrolof2,3-~lisoauinolin-5-one
A solution of 3-(2-amino-ethyl)-1,6,7,8-tetrahydro-pyrrolo[2,3-g]isoquinolin-5-
one
(0.229 g, 1 mmole, compound 1 above) and triethylamine (1 mmole) in CH ZCl,
(15
mL) was treated with benzoyl chloride (1 equivalent) at 0°C and the
mixture stirred at
room temperature for 16 hours. Water (25 mL) was added, the organics separated
and washed with water (15 mL), brine (15 mL) and dried (MgS04). Filtration and
CA 02399545 2002-08-02
WO 01/57039 PCT/IJSO1/03430
-12-
concentration in vacuo gave the required amide as a yellow colored oil (0.26
g, 78%
yield). A THF solution (15 mL) of the amide was cooled to 0 °C under a
N2
atmosphere and treated with the dropwise addition of lithium aluminum hydride
(1M
THF, 1mL). The mixture was refluxed for 30 minutes, cooled to 0°C and
the excess
hydride reagent treated with saturated NH 4C1 solution. The mixture was
filtered,
concentrated in vacuo, dissolved in ethyl acetate ( 1 S mL), washed with water
(2 x 20
mL), brine (15 mL) and dried over anhydrous sodium sulfate. Filtration and
concentration in vacuo afforded the titled amine as a yellow oil.
MS(ESI) m/e 320 (M+H)+
Elemental Analysis for: C20H21N30
Calculated: C, 75.21; H, 6.63; N, 13.16
Found: C, 75.03; H, 6.59; N, 13.29
Pharmaceutical Composition
Applicable solid carriers can include one or more substances which may also
act as flavoring agents, lubricants, solubilizers, suspending agents, fillers,
glidants,
compression aids, binders or tablet-disintegrating agents or an encapsulating
material.
In powders, the carrier is a finely divided solid which is in admixture with
the finely
divided active ingredient. In tablets, the active ingredient is mixed with a
Garner
having the necessary compression properties in suitable proportions and
compacted in
the shape and size desired. The powders and tablets preferably contain up to
9990 of
the active ingredient. Suitable solid carriers include, for example, calcium
phosphate,
magnesium stearate, talc, sugars, lactose, dextrin, starch, gelatin,
cellulose, methyl
cellulose, sodium carboxymethyl cellulose, polyvinylpyrrolidine, low melting
waxes
and ion exchange resins.
Liquid carriers may be used in preparing solutions, suspensions, emulsions,
syrups and elixirs. The active ingredient of this invention can be dissolved
or
suspended in a pharmaceutically acceptable liquid carrier such as water, an
organic
solvent, a mixture of both or pharmaceutically acceptable oils or fat. The
liquid
Garner can contain other suitable pharmaceutical additives such as
solubilizers,
emulsifiers, buffers, preservatives, sweeteners, flavoring agents, suspending
agents,
thickening agents, colors, viscosity regulators, stabilizers or osmo-
regulators.
Suitable examples of liquid carriers for oral and parenteral administration
include
CA 02399545 2002-08-02
WO 01/57039 PCT/USO1/03430
-13-
water (particularly containing additives as above e.g. cellulose derivatives,
preferably
sodium carboxymethyl cellulose solution), alcohols (including monohydric
alcohols
and polyhydric alcohols e.g. glycols) and their derivatives, and oils (e.g.
fractionated
coconut oil and arachis oil). For parenteral administration the carrier can
also be an
oily ester such as ethyl oleate and isopropyl myristate. Sterile liquid
carriers are used
in sterile liquid form compositions for parenteral administration.
Liquid pharmaceutical compositions which are sterile solutions or suspensions
can be utilized by, for example, intramuscular, intraperitoneal or
subcutaneous
injection. Sterile solutions can also be administered intravenously. Oral
administration may be either liquid or solid composition form.
Preferably the pharmaceutical composition is in unit dosage form, e.g. as
tablets or capsules. In such form, the composition is sub-divided in unit dose
containing appropriate quantities of the active ingredient; the unit dosage
forms can
be packaged compositions, for example packeted powders, vials, ampoules,
prefilled
syringes or sachets containing liquids. The unit dosage form can be, for
example, a
capsule or tablet itself, or it can be the appropriate number of any such
compositions
in package form.
The therapeutically effective dosage to be used in the treatment of a specific
disease must be subjectively determined by the attending physician. The novel
method of the invention for treating conditions related to or are affected by
the 5-
HT7 receptor comprise administering to warm-blooded animals, including humans,
an effective amount of at least one compound of Formula ( 1 ) and its non-
toxic,
pharmaceutically acceptable addition salts. The compounds may be administered
orally, rectally, parenterally or topically to the skin and mucosa. The usual
daily dose
is depending on the specific compound, method of treatment and condition
treated.
The usual daily dose is 0.01 - 1000 mg/Kg for oral application, preferably 0.5
- 500
mg/Kg, and 0.1 - 100 mg/Kg for parenteral application, preferably 0.5 - 50
mg/Kg.