Note: Descriptions are shown in the official language in which they were submitted.
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
Cooling TherapieslDevice For Angioplasty with Restenosis.
10
CROSS-REFERENCE TO RELATED APPLICATIONS
This application is a continuation-in-part of U.S.
Patent Application No. 09/516,319, filed 3/1/00, entitled
"Method and Device for Performing Cooling-or Cryo-
Therapies for, e.g., Angioplasty with Reduced Restenosis
or Pulmonary Vein Cell Necrosis to Inhibit Atrial
Fibrillation" which is a continuation-in-part of U.S.
Patent Application Serial No. 09/052,545, filed 3/31/98,
entitled "Circulating Fluid Hypothermia Method and
Apparatus" and U.S. Patent Application Serial No.
09/215,038, filed 12/16/98, entitled "Inflatable Catheter
for Selective Organ Heating and Cooling and Method of
Using the Same"
CROSS-REFERENCE TO MICROFICHE APPENDIX
(none)
BACKGROUND OF THE INVENTION
Balloon angioplasty, or the technology of reshaping
of a blood vessel for the purpose of establishing vessel
patency using a balloon tipped catheter, has been known
since the late 1970's. The procedure involves the use of
a balloon catheter that is guided by means of a guidewire
through a guiding catheter to the target lesion or vessel
blockage. The balloon typically is equipped with one or
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
more marker bands that allow the interventionalist to
visualize the position of the balloon in reference to the
lesion with the aid of fluoroscopy. Once in place, i.e.,
centered with the lesion, the balloon is inflated with a
biocompatible fluid, and pressurized to the appropriate
pressure to allow the vessel to open.
Typical procedures are completed with balloon
inflation pressures between 8 and 12 atmospheres. A
percentage of lesions, typically heavily calcified
lesions, require much higher balloon inflation pressures,
e.g., upward of 20 atmospheres. At times, the balloon
inflation procedure is repeated several times before the
lesion or blockage will yield. The placement of stems
after angioplasty has become popular as it reduces the
rate of restenosis.
Restenosis refers to the renarrowing of the vascular
lumen following vascular intervention such as a balloon
angioplasty procedure or stmt insertion. Restenosis is
clinically defined as a greater than 50% loss of initial
lumen diameter. The mechanism or root causes of
restenosis are still not fully understood. The causes are
multifactorial, and are partly the result of the injury
caused by the balloon angioplasty procedure and stmt
placement. With the advent of stents, restenosis rates
have dropped from over 30% to 10-20%. Recently, the use
and effectiveness of low-dose radiation administered
intravascularly following angioplasty is being evaluated
as a method to alter the DNA or RNA of an affected
vessel's cells in the hope of reducing cell
proliferation.
Besides restenosis, another cardiological malady is
atrial fibrillation. Atrial fibrillation refers to very
rapid irregular contractions of the atria of the heart
resulting in a lack of synchronization between the
2
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
heartbeat and the pulse. The irregular contractions are
due to irregular electrical activity that originates in
the area of the pulmonary veins. A proposed device,
currently under development, for treating atrial
fibrillation is a balloon filled with saline that can be
ultrasonically agitated and heated. This device is
inserted in the femoral vein and snaked into the right
atrium. The device is then poked through the interatrial
septum and into the left atrium, where it is then angled
into the volume adjoining the suspect pulmonary vein with
the left atrium.
Research in atrial fibrillation indicates that
substantially complete circumferential necrosis is
required for a therapeutic benefit. The above technique
is disadvantageous in that circumferential portions of
the tissue, desired to be necrosed, are not in fact
affected. Other techniques, including RF ablation, are
similarly inefficient. Moreover, these techniques leave
the necrosed portions with jagged edges, i.e., there is
poor demarcation between the healthy and the necrosed
tissue. These edges can then cause electrical short
circuits, and associated electrical irregularities, due
to the high electric fields associated with jagged edges
of a conductive medium.
The above technique is also disadvantageous in that
heating is employed. Heating is associated with several
problems, including increased coagulum and thrombus
formation, leading to emboli. Heating also stimulates
stenosis of the vein. Finally, since tissues can only
safely be heated to temperatures of less than or about
75°C - 85°C due to charring and tissue rupture secondary
to steam formation. The thermal gradient thus induced is
fairly minimal, leading to a limited heat transfer.
Moreover, since heating causes tissues to become less
3
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
adherent to the adjacent heat transfer element, the
tissue contact with the heat transfer element is also
reduced, further decreasing the heat transfer.
SUMMARY OF THE INVENTION
The present invention provides an enhanced method
and device to inhibit or reduce the rate of restenosis
following angioplasty or stmt placement. The invention
is similar to placing an ice pack on a sore or
overstrained muscle for a period of time to minimize or
inhibit the bio-chemical events responsible for an
associated inflammatory response. An embodiment of the
invention generally involves placing a balloon-tipped
catheter in the area treated or opened through balloon
angioplasty immediately following angioplasty. A so-
called "cryoplasty" balloon, which can have a dual
balloon structure, may be delivered through a guiding
catheter and over a guidewire already in place from a
balloon angioplasty. The dual balloon structure has
benefits described below and also allows for a more
robust design, providing significant safety advantages to
the patient because two balloons must be broken if
cooling fluid is to deleteriously infuse into the
patient.
The dual balloon may be centered in the recently
opened vessel with the aid of radio opaque marker bands,
indicating the "working length" of the balloon. In
choosing a working length, it is important to note that
typical lesions may have a size on the order of 2-3 cm.
A biocompatible heat transfer fluid, which may contain
contrast media, may be infused through the space between
the dual balloons. While this fluid does not circulate
in this embodiment, once it is chilled or even frozen by
thermal contact with a cooling fluid, it will stay
4
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
sufficiently cold for therapeutic purposes.
Subsequently, a biocompatible cooling fluid with a
temperature between about, e.g., - 40°C and -60°C, may be
injected into the interior of the inner balloon, and
circulated through a supply lumen and a return lumen.
The fluid exits the supply lumen through a skive in the
lumen, and returns to the refrigeration unit via another
skive and the return lumen.
The biocompatible cooling fluid chills the
biocompatible heat transfer fluid between the dual
balloons to a therapeutic temperature between about,
e.g., 0°C and -50°C. The chilled heat transfer fluid
between the dual balloons transfers thermal energy
through the balloon wall and into the adjacent intimal
vascular tissue for the appropriate therapeutic length of
time. Upon completion of the therapy, the circulation of
the biocompatible cooling fluid is stopped, and the heat
transfer fluid between the dual balloons withdrawn
through the annular space. Both balloons may be collapsed
by means of causing a soft vacuum in the lumens. Once
collapsed, the cryoplasty catheter may be withdrawn from
the treated site and patient through the guiding
catheter.
In more detail, in one aspect, the invention is
directed to a device to treat tissue, including an outer
tube, an an inner tube disposed at least partially within
the outer tube, and a dual balloon including an inner
balloon and an outer balloon, the inner balloon coupled
to the inner tube at a proximal and at a distal end, the
outer balloon coupled to the inner tube at a distal end
and to the outer tube at a proximal end. A first
interior volume is defined between the outer balloon and
the inner balloon in fluid communication with an inlet in
the volume between the outer tube and the inner tube.
'S
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
Variations of the invention may include one or more
of the following. The inner tube may further define a
guidewire lumen, a supply lumen, and return lumen. The
supply lumen may define a hole or skive such that a fluid
flowing in the supply lumen may be caused to flow into a
volume defined by the inner balloon, and the return lumen
may define a hole or skive such that a fluid flowing in a
volume defined by the inner balloon may be caused to flow
into the return lumen. The guidewire lumen may extend
from a proximal end of the inner tube to a distal end of
the inner tube. The device may further comprise at least
two radially extending tabs disposed around a
circumference of the inner tube to substantially center
the inner tube within the dual balloon. The device may
further comprise at least one marker band disposed on the
inner tube to locate a working region of the device at a
desired location. The device may further comprise a
source of chilled fluid having a supply tube and a return
tube, the supply tube coupled in fluid communication to
the supply lumen and the return tube coupled in fluid
communication to the return lumen. A source of fluid may
also be included, the source of fluid coupled in fluid
communication to a volume between the inner balloon and
the outer balloon. The fluid may be a perfluorocarbon
such as Galden fluid. The fluid may also include
contrast media.
In another aspect, the invention is directed to a
method of reducing restenosis after angioplasty in a
blood vessel. The method includes inserting a catheter
into a blood vessel, the catheter having a balloon. The
balloon is then inflated with a perfluorocarbon such that
an exterior surface of the balloon is in contact with at
least a partial inner perimeter of the blood vessel, the
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
perfluorocarbon having a temperature in the range of
about -10°C to -50°C.
Variations of the method may include one or more of
the following. The method may include disposing the
catheter at a desired location using at least one radio
opaque marker band. The method may include flowing the
perfluorocarbon into the balloon using a supply lumen and
exhausting the perfluorocarbon from the balloon using a
return lumen. The balloon may be a dual balloon, and the
method may further include providing a heat transfer
fluid in the volume between the dual balloons. The heat
transfer fluid may include a contrast media fluid. The
method may include disposing the catheter such that at
least a portion of the balloon is in a coronary artery or
in a carotid artery.
In yet another aspect, the invention is directed to
a method of reducing atrial fibrillation. The method
includes inserting a catheter at least partially into the
heart, the catheter having a balloon, a portion of the
balloon located in the left atrium and a portion of the
balloon located in a pulmonary vein. The balloon is then
inflated with a perfluorocarbon such that an exterior
surface of the balloon is in contact with at least a
partial circumference of the portion of the pulmonary
vein adjacent the left atrium, the perfluorocarbon having
a temperature in the range of about -10°C to -50°C.
Variations of the method may include one or more of
the following. The balloon may have a working region
having a length of between about 5 mm and 10 mm. The
method may further include inserting a wire having a
needle point from the femoral vein into the right atrium
and forming a hole using the needle point in the
interatrial septum between the right atrium and the left
atrium. A guide catheter may then be inserted into the
7
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
right atrium. A guide wire may further be inserted
through the guide catheter into the right atrium and
further into a pulmonary vein. The catheter may then be
disposed over the guidewire into a volume defined by the
joint of the right atrium and the pulmonary vein.
Advantages of the invention may include one or more
of the following. The invention inhibits or reduces the
rate of restenosis following a balloon angioplasty or any
other type of vascular intervention. At least the
following portions of the vascular anatomy can benefit
from such a procedure: the abdominal aorta (following a
stmt or graft placement), the coronary arteries
(following PTCA or rotational artherectomy), the carotid
arteries (following an angioplasty or stmt placement),
as well as the larger peripheral arteries.
When the invention is used to treat atrial
fibrillation, the following advantages inure. The cooled
tissue is adherent to the heat transfer element,
increasing the heat transfer effected. Since very cold
temperatures may be employed, the temperature gradient
can be quite large, increasing the heat transfer rate.
In both embodiments, heat transfer does not occur
primarily or at all by vaporization of a liquid, thus
eliminating a potential cause of bubbles in the body.
Nor does cooling occur primarily or at all by a pressure
change across a restriction or orifice, this simplifying
the structure of the device. Thrombus formation and
charring, associated with prior techniques, are minimized
or eliminated.
Additional advantages will be apparent from the
description that follows, including the drawings and
claims.
~8
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1A shows a side schematic view of a catheter
according to a first embodiment of the invention.
FIG. 1B shows a cross-sectional view of the catheter
of FIG. 1A, as indicated by lines 1B-1B in FIG. 1A.
FIG. 1C shows an alternate cross-sectional view of
the catheter of FIG. 1A, as indicated by lines 1B-1B in
FIG. 1A.
FIG. 2A shows a side schematic view of a catheter
according to a second embodiment of the invention.
FIG. 2B shows a cross-sectional view of the catheter
of FIG. 2A, as indicated by lines 2B-2B in FIG. 2A.
FIG. 3 shows a schematic view of a catheter in use
according to a third embodiment of the invention.
FIG. 4 shows a cross-sectional view of the catheter
of FIG. 3 .
FIG. 5 shows an alternative cross-sectional view of
the catheter of FIG. 3.
FIG. 6 shows an alternative cross-sectional view of
the catheter of FIG. 3.
FIG. 7 shows a schematic view of the warm balloon of
the catheter of FIG. 3.
DETAILED DESCRIPTION
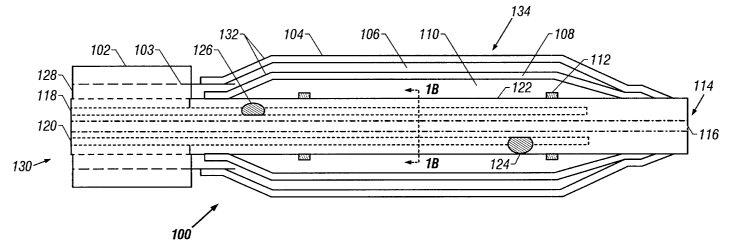
Referring to FIG. 1A, a catheter 100 is shown
according to a first embodiment of the invention. The
catheter 100 has a proximal end 130 and a distal end 114.
Of course, this figure is not necessarily to scale and in
general use the proximal end 130 is far upstream of the
features shown in FIG. 1A.
The catheter 100 may be used within a guide catheter
102, and generally includes an outer tube 103, a dual
9
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
balloon 134, and an inner tube 122. These parts will be
discussed in turn.
The guide catheter 102 provides a tool to dispose
the catheter 100 adjacent the desired location for, e.g.,
angioplasty or reduction of atrial fibrillation. Typical
guide catheter diameters may be about 6 french to 9
french, and the same may be made of polyether blockamide,
polyamides, polyurethanes, and other similar materials.
The distal end of the guide catheter is generally
adjacent the proximal end of the dual balloon 134, and
further is generally adjacent the distal end of the outer
tube 103.
The ability to place the guide catheter is a
significant factor in the size of the device. For
example, to perform angioplasty in the carotid arteries,
which have an inner diameter of about 4 to 6 mm, a
suitably sized guide catheter must be used. This
restricts the size of the catheter 100 that may be
disposed within the guide catheter. A typical diameter
of the catheter 100 may then be about 7 french or less or
about 65 to 91 mils. In a second embodiment described
below, a catheter for use in the coronary arteries is
described. Of course, which catheter is used in which
artery is a matter to be determined by the physician,
taking into account such factors as the size of the
individual patient's affected arteries, etc.
The outer tube 103 houses the catheter 100 while the
latter traverses the length of the guide catheter 102.
The outer tube 103 may have a diameter of about 4 french
to 7 french, and the same may be made of polyether
blockamide, poly-butylene terephtalate, polyurethane,
polyamide, polyacetal polysulfone, polyethylene, ethylene
tetrafluoroethylene, and other similar materials.
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
The distal end of the outer tube 103 adjoins the
proximal end of the dual balloon 134. The outer tube 103
provides a convenient location for mounting a proximal
end of an outer balloon 104 within the dual balloon 134,
and further may provide an inlet 128 for providing a
fluid such as a liquid to a first interior volume 106
between the dual balloons. In some cases, an inlet 128
per se may not be necessary: the fluid, which may also be
a sub-atmospheric level of gas or air, may be provided
during manufacture in the first interior volume 106. In
this case, the proximal and distal ends of the first
interior volume may be sealed during manufacture. The
inlet 128 may be at least partially defined by the
annular volume between the interior of the outer tube 103
and the exterior of the inner tube 122.
The dual balloon 134 includes an outer balloon 104
and an inner balloon 108. Between the two is the first
interior volume 106. The outer balloon 104 may be
inflated by inflating the interior volume 106. The inner
balloon 108 has a second interior volume 110 associated
with the same. The inner balloon 108 may be inflated by
inflating the second interior volume 110.
To avoid the occurrence of bubbles in the
bloodstream, both the inner balloon 108 and the outer
balloon 104 may be inflated using biocompatible liquids,
such as Galden° fluid, perfluorocarbon-based liquids, or
various contrast agents. There is no need that the fluid
inflating one of the interior volumes be the same fluid
as that inflating the other. Additional details on these
fluids are described below.
In the case of the first interior volume 106, this
fluid may be, e.g., stationary or static: in other words,
it need not be circulated. In the case of the second
interior volume 110, this fluid would in general be
11
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
circulated by an external chiller (not shown). The
chiller may be, e.g., a gear pump, peristaltic pump, etc.
It may be preferable to use a gear pump over a
peristaltic pump as the attainable pressure of the former
is generally greater than that of the latter. Moreover,
gear pumps have the advantageous property of being
linear, i.e., their output varies in direction proportion
with their revolutions per minute. Two types of gear
pumps which may be employed include radial spur gear
pumps and helical tooth gear pumps. Of these, the
helical tooth gear pump may be more preferable as the
same has been associated with higher pressures and a more
constant output. The ability to achieve high pressures
may be important as the cooling fluid is required to pass
through a fairly narrow, e.g., five to seven french,
catheter at a certain rate. For the same reason, the
viscosity of the fluid, at the low temperatures, should
be appropriately low. In this way, e.g., the flow may be
increased. For example, an appropriate type of fluid may
be Galden~ fluid, and in particular Galden~ fluid item
number "HT-55", available from Ausimont Inc. of
Thorofare, NJ. At -55°C, this fluid has a viscosity of
2.1 centiStokes. At -70°C, this fluid has a viscosity of
3.8 centiStokes. It is believed that fluids with such
viscosities at these temperatures would be appropriate
for use .
The so-called "cones" of the balloons 108 and 104,
indicated generally by reference numeral 132, may be made
somewhat thicker than the remainder of the balloon
sections. In this way, the heat transfer efficiency in
these sections is significantly less than over the
remainder of the balloon sections, this "remainder"
effectively defining a "working region" of the balloon.
In this way, the cooling or "cryoplasty" may be
12
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
efficiently localized to the affected area rather than
spread over the length of the balloon.
The inner tube 122 is disposed within the interior
of the dual balloon 134 and within the interior of the
guide catheter 102. The inner tube 122 includes a supply
lumen 120, a return lumen 118, and a guidewire lumen 116.
The guidewire lumen 116 may have sizes of, e.g., 17 or 21
mils inner diameter, in order to accommodate current
standard sized guidewires, such as those having an outer
diameter of 14 mils. This structure may be preferable,
as the pressure drop encountered may be substantially
less. In use, the supply lumen 120 may be used to supply
a circulating liquid to the second interior volume 110.
The return lumen 118 may be used to exhaust the
circulating liquid from the second interior volume to the
external chiller. As may be seen from FIG. 1A, both
lumens 118 and 120 may terminate prior to the distal end
114 of the catheter 100. The lumen arrangement may be
seen more clearly in Fig. 1B. Fig. 1C shows an alternate
such arrangement, and one that may provide an even better
design for minimal pressure drop. In this design, lumens
118' and 120' are asymmetric about guidewire lumen 116'
A set of radio opaque marker bands 112 may be
disposed on the inner tube 122 at locations substantially
adjacent the cones 132 to define a central portion of the
"working region" of the balloons 104 and 108. This
working region is where the "cryoplasty" procedures
described below may substantially occur.
As noted above, the proximal portion of the outer
balloon 104 is mounted on the outer tube 103 at its
distal end. The distal end of the outer balloon 104 is
secured to the distal end of the catheter 100 and along
the inner tube 122. In contrast, both the proximal and
distal ends of the inner balloon 108 may be secured to
13
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
the inner tube 122 to create a sealed second interior
volume 110.
At least two skives 124 and 126 may be defined by
the inner tube 122 and employed to allow the working
fluid to exit into the second interior volume 110 and to
exhaust the same from the second interior volume 10. As
shown in the figure, the skive l24 is in fluid
communication with the lumen 120 and the skive 126 is in
fluid communication with the lumen 118. Here, "fluid
l0 communication" refers to a relationship between two
vessels where a fluid pressure may cause a net amount of
fluid to flow from one vessel to the other.
The skives may be formed by known techniques. A
suitable size for the skives may be from about 50 mils to
125 mils.
A plurality of tabs 119 may be employed to roughly
or substantially center the inner tube 122 within the
catheter 100. These tabs may have the shape shown, the
shape of rectangular or triangular solids, or other such
shapes so long as the flow of working fluid is not unduly
impeded. In this specification, the phrase "the flow of
working fluid is not unduly impeded" is essentially
equated to the phrase "substantially center". The tabs
119 may be made of polyether blockamide, poly-butylene
terephtalate, polyurethane, polyamide, polyacetal
polysulfone, polyethylene, ethylene tetrafluoroethylene,
and other similar materials, and may have general
dimensions of from about 3 mils to 10 mils in height, and
by about 10 mils to 20 mils in width.
In a method of use, the guide catheter 102 may be
inserted into an affected artery or vein such that the
distal tip of the guide catheter is just proximal to an
affected area such as a calcified area or lesion. Of
14
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
course, it is noted that typical lesions do not occur in
the venous system, but only in the arterial.
This step provides a coarse estimate of proper
positioning, and may include the use of fluoroscopy. The
guide catheter may be placed using a guide wire (not
shown). Both the guide catheter and guide wire may
already be in place as it may be presumed a balloon
angioplasty or stmt placement has previously been
performed.
The catheter 100 may then be inserted over the guide
wire via the lumen 116 and through the guide catheter
102. In general, both a guide wire and a guide catheter
are not strictly necessary - one or the other may often
suffice. During insertion, the dual balloon 134 may be
uninflated to maintain a minimum profile. In fact, a
slight vacuum may be drawn to further decrease the size
of the dual balloon 134 so long as the structural
integrity of the dual balloon 134 is not thereby
compromised.
When the catheter 100 is distal of the distal tip of
the guide catheter 102, a fine positioning step may occur
by way of the radio opaque marker bands 112. Using
fluoroscopy, the location of the radio opaque marker
bands 112 can be identified in relation to the location
of the lesion. In particular, the catheter may be
advantageously placed at the location of the lesion and
further such that the lesion is between the two marker
bands. In this way, the working region of the balloon
134 will substantially overlap the affected area, i.e.,
the area of the lesion.
Once placed, a biocompatible heat transfer fluid,
which may also contain contrast media, may be infused
into the first interior volume 106 through the inlet 128.
While the use of contrast media is not required, its use
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
may allow early detection of a break in the balloon 104
because the contrast media may be seen via fluoroscopy to
flow throughout the patient's vasculature. Subsequently
a biocompatible cooling fluid may be circulated through
the supply lumen 120 and the return lumen 118. Before or
during the procedure, the temperature of the
biocompatible cooling fluid may be lowered to a
therapeutic temperature, e.g., between -40°C and -60°C,
although the exact temperature required depends on the
nature of the affected area. The fluid exits the supply
lumen 120 through the skive 124 and returns to the
chiller through the skive 126 and via the return lumen
118. It is understood that the respective skive
functions may also be reversed without departing from the
scope of the invention.
The biocompatible cooling fluid in the second
interior volume 110 chills the biocompatible heat
transfer fluid within the first interior volume 106 to a
therapeutic temperature of, e.g., between about -25°C and
-50°C. The chilled heat transfer fluid transfers thermal
energy through the wall of the balloon 104 and into the
adjacent intimal vascular tissue for an appropriate
therapeutic length of time. This time may be, e.g.,
about % to 4 minutes.
Upon completion of the therapy, the circulation of
the biocompatible cooling fluid may cease. The heat
transfer fluid within the first interior volume 106 may
be withdrawn though the inlet 128. The balloons 104 and
108 may be collapsed by pulling a soft vacuum through any
or all of the lumens 124, 126, and 128. Following
collapse, the catheter 100 may be withdrawn from the
treatment site and from the patient through the guide
catheter 102.
16
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
To inhibit restenosis, the following therapeutic
guidelines may be suggested:
Minimum Average Maximum
Temperature -20C -55C -110C
of heat
transfer
fluid
Temperature 0C to -10C -20C to -50C to
achieved at -30C -100C
intimal wall
Depth of lOths of mm 1 mm 3 mm
penetration
of
intema/media
Length of 30 seconds 1-2 min 4-5 min
time fluid is
circulating
Substantially the same catheter may be used to treat
atrial fibrillation. In this method, the catheter is
inflated as above once it is in location. The location
chosen for treatment of atrial fibrillation is such that
the working region spans a portion of the left atrium and
a portion of the affected pulmonary vein. Thus, in this
embodiment, the working region of the catheter may have a
length of about 5 mm to 30 mm. The affected pulmonary
vein, of the four possible pulmonary veins, which enter
the left atrium, may be determined by electrophysiology
studies.
To maneuver the catheter into this location, a
catheter with a needle point may first be inserted at the
femoral vein and routed up to the right atrium. The
needle of the catheter may then be poked through the
17
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
interatrial septum and into the left atrium. The
catheter may then be removed if desired and a guide
catheter disposed in the same location. A guide wire may
be used through the guide catheter and may be maneuvered
at least partially into the pulmonary vein. Finally, a
catheter such as the catheter 100 may be placed in the
volume defining the intersection of the pulmonary vein
and the left atrium.
A method of use similar to that disclosed above is
then employed to cool at least a portion of, and
preferably all of, the circumferential tissue. The
coldness of the balloon assists in the adherence of the
circumferential tissue to the balloon, this feature
serving to increase the overall heat transfer rate.
The catheter 100 above may be particularly useful
for procedures in the carotid arteries by virtue of its
size. For use in the coronary arteries, which are
typically much smaller than the carotid artery, an even
,smaller catheter may be desired. For example, one with an
outer diameter less than 5 french may be desired.
Referring to FIG. 2A, a catheter 200 is shown
according to a second embodiment of the invention. This
embodiment may be particularly useful for use in the
coronary arteries because the dimensions of the catheter
200 may be considerably smaller than the dimensions of
the catheter 100. However, in several ways the catheter
200 is similar to the above-described catheter 100. In
particular, the catheter 200 has a proximal end 230 and a
distal end 214 and may be used within a guide catheter
202. The catheter 200 includes an outer tube 203, a dual
balloon 234, and an inner tube 222.
The ability to place the guide catheter is a
significant factor in the size of the device. For
example, to perform angioplasty in the coronary arteries,
18
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
which have an inner diameter of about 1 ~ to 4 ;~ mm, a
suitably sized guide catheter may be used. This then
restricts the size of the catheter 200 which may be
disposed within the guide catheter. A typical diameter
of the catheter 200 may then be about 3 french or less or
about 35-39 mils. The same may be placed in the femoral
artery in order to be able to track to the coronary
arteries in a known manner.
Analogous to these features in the catheter 100, the
outer tube 203 houses the catheter 200 and may have an
outside diameter of about 5 french to 7 french, and the
same may be made of similar materials. The distal end of
the outer tube 203 adjoins the proximal end of the dual
balloon 234. The outer tube 203 provides a mounting
location for an outer balloon 204, and further provides
an inlet 228 for providing a fluid such as a liquid to a
first interior volume 206 between the dual balloons. As
noted in connection with catheter 100, an inlet 228 per
se may not be necessary: the fluid, which may also be a
sub-atmospheric level of air, may be provided in the
first interior volume 206. Also as above, the proximal
and distal ends of the volume may be sealed during
manufacture. The inlet 228 may be at least partially
defined by the annular volume between the interior of the
outer tube 203 and the exterior of the inner tube 222.
The dual balloon 234 includes an outer balloon 204
and an inner balloon 208. These balloons are basically
similar to balloons 104 and 108 described above, but may
be made even smaller for use in the smaller coronary
arteries.
The same types of fluids may be used as in the
catheter 100.
The inner tube 222 is disposed within the interior
of the dual balloon 234 and within the interior of the
19
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
guide catheter 202. The inner tube 222 includes a supply
lumen 220 and a return lumen 218.
A set of radio opaque marker bands 212 may be
disposed on the inner tube 222 for the same reasons
disclosed above in connection. with the marker bands 112.
As noted above, the proximal portion of the outer
balloon 204 is mounted on the outer tube 203 at its
distal end. The distal end of the outer balloon 204 is
secured to the distal end of the catheter 200 and along
the inner tube 222. In contrast, both the proximal and
distal ends of the inner balloon 208 may be secured to
the inner tube 222 to create a sealed second interior
volume 210.
At least two skives 224 and 226 may be defined by
the inner tube 222 and employed to allow the working
fluid to exit into the second interior volume 210 and to
exhaust the same from the second interior volume 210.
A plurality of tabs 219 may be employed to roughly
or substantially center the inner tube 222 within the
catheter 200 as in catheter 100. These tabs may have the
same general geometry and design as tabs 119. Of course,
they may also be appropriately smaller to accommodate the
smaller dimensions of this coronary artery design.
The tabs 119 and 219 are particularly important in
the catheters 100 and 200, as contact by the inner tube
of the outer tube may also be associated with an
undesired conductive heat transfer prior to the working
fluid reaching the working region, thereby deleteriously
increasing the temperature of the working fluid at the
working region.
The method of use of the catheter 200 is generally
the same as for the catheter 100. Known techniques may
be employed to place the catheter 200 into an affected
coronary artery. For the catheter 200, an external
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
guidewire may be used with appropriate attachments to the
catheter.
Referring to Fig. 3, an alternative embodiment of a
catheter 300 which may be employed in PV ablation is
detailed. In this figure, a dual balloon system 301 is
shown; however, the balloons are not one within the other
as in Fig. 1. In this embodiment, a warm balloon 302 is
distal of a cold balloon 304. Warm balloon 302 may be
used to anchor the system 301 against movements, which
may be particularly useful within a beating heart. Cold
balloon 304 may then be employed to cryo-ablate a
circumferential lesion at the point where a pulmonary
vein 306 enters the left atrium 308.
Within the cold balloon 304, a working fluid may be
introduced via an outlet port 308 and may be retrieved
via an inlet port 310. Ports 308 and 310 may be skived
in known fashion into the catheter shaft lumens whose
design is exemplified below.
As noted above, the warm balloon 302 serves to
anchor the system 301 in the pulmonary vein and left
atrium. The warm balloon 302 also serves to stop blood,
which is traveling in the direction indicated by arrow
312, from freezing upon contact with the cold balloon
304. In this way, the warm balloon 302 acts as an
insulator to cold balloon 304.
As the warm balloon 302 does not require connective
heat transfer via a circulating working fluid, it may be
served by only one skived port, or by two ports, such as
an inlet port 314 and an outlet port 316, as shown in
Fig. 3. In some embodiments, a separate lumen or lumens
may be used to fill the warm balloon. In an alternative
embodiment, a valve mechanism may be used to fill the
warm balloon using fluid from the cold balloon. In the
case where only one port is used to fill the warm
21
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
balloon, draining the same requires a slight vacuum or
negative pressure to be placed on the lumen servicing the
inlet/outlet port. A benefit to the two lumen design is
that the warm balloon may be inflated and deflated in a
more expeditious manner.
Typical pressures within the warm balloon may be
about 1-2 atm (10-30 psi), and thus maintains a fairly
low pressure. An appropriate fluid will be biocompatible,
and may be Galden fluid, DSW, and so on. Typical
pressures within the cold balloon may be about 5-7 atm,
for example about 6 atm (e.g., at about 100 psi), and
thus maintains a higher pressure. An appropriate fluid
may be Galden fluid, e.g., HT-55, DSW, and so on. The
volume of fluid required to fill the cold balloon may
vary, but may be about 4-8 cc. The cold balloon may be
about 2 to 2 ~ cm long, and have a diameter of 1 to 2
cm.
In some embodiments, the warm balloon may be glued
or otherwise attached to the cold balloon. In the case
where only one port is used to fill the warm balloon,
draining both balloons may simply entail closing either
the return lumen or the supply lumen, and drawing a
vacuum on the other. In this way, both the cold and warm
balloons may be evacuated. Tn any case, a standard
medical "indeflator" may be used to pressurize and de-
pressurize the various lumens and balloons.
Fig. 4 shows an embodiment of the arrangement of
lumens within the catheter. In particular, supply and
return lumens for the cold balloon 304 are shown by
lumens 318 and 320, respectively. Supply and return
lumens for the warm balloon 302 are shown by lumens 322
and 324, respectively, although as noted only one may be
used as required by the dictates of the user. A
guidewire lumen 326 is also shown. An alternative
',22
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
arrangement is shown in Fig. 5, where the corresponding
lumens are shown by primes.
In the above lumen designs, the exterior blood is
exposed to the cold supply flow. Referring to Fig. 6, an
alternative lumen design is shown in which the cold fluid
supply lumen 328 is exposed to only the cold fluid return
lumen 330. An insulation space 332 may also be employed.
In this way, the heat flux from the exterior flow is
minimized and the cold fluid may reach the cold balloon
at a lower temperature. One drawback to such a system is
that the operational pressure may be higher.
Referring back to Fig. 4, the overall catheter outer
diameter may be about .130", e.g. about 10 french,
including an insulation sleeve and guide discussed below.
The catheter shaft 303 itself may be about .110" and may
be made of, e.g., polyethylene (PE), and preferably a
combination of a low density PE and a high density PE.
The inlet and outlet ports or inlet/outlet port of
the warm balloon may be skived from the lumens 322 and
324. Referring to Fig. 7, the warm balloon 302 itself
may be made of a sleeve 332 of silicone tubing of, e.g.,
35 durometer on the "D" scale, and held in place by two
pieces of PET heat shrink tubing 334. Alternative
methods of securing the warm balloon during inflation may
include metal bands or an adhesive.
Referring back to Fig. 3, marker bands 336 may be
employed within either or both of the cold balloon and
warm balloon to assist the physician is disposing the
same in an appropriate location. The marker bands
typically denote the working areas of the balloons, and
may be made of Pt, Iridium, Au, etc.
In the ablation procedure, the working cold fluid
may exit the circulation system or chiller at, e.g.,
about -85°C. The circulation system or chiller may be,
.23
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
e.g., a two-stage heat exchanger. The fluid may then
enter the catheter at about -70°C to about -75°C, and may
strike the balloon at about -55°C to about -65°C. The
overall procedure may take less than a minute to
circumferentially ablate the desired tissue up to several
minutes. Of course, these numbers are only exemplary and
the same depend on the design of the system and fluids
used.
Mapping electrodes 338 may be employed at the distal
end of the warm balloon. These mapping electrodes may
each have a wire attached, the wires extending down,
e.g., the supply and return lumens for the warm fluid or
the cold fluid. The mapping electrodes 338 may be used
to detect stray electrical fields to determine where
ablation may be needed and/or whether the ablation
procedure was successful. The mapping electrodes may
typically be about 2-3 mm apart from each other.
Construction of the warm balloon typically involves
adhering the same to the shaft 303 and skiving the inlet
and outlet ports. In some instances, it may be desired
to place a silicone sleeve 340 on the proximal and/or
distal ends of the warm and/or cold balloons. The
silicone sleeve 340 may then serve to further insulate
the non-working sections of the balloons from blood that
would otherwise potentially freeze during a procedure.
The silicone sleeve would typically be attached only at a
portion of its length, such as that indicated by circle
342, so that the same may slide along the balloon as the
balloon is inflated. In addition to insulation effects,
the silicone sleeve also serves to assist in collapsing
the balloon during deflation.
The entire catheter shaft 303 may be surrounded by
an insulation catheter sleeve 344 (see Fig. 4). 'Sleeve
344 may have a thickness of, e.g., 0.01 inches, and may
24
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
be made of a foamed extrusion, e.g., that with voids of
air disposed within. The voids further assist the
insulating effect since their heat transfer is extremely
low. A void to polymer ratio of, e.g., 20% to 30o may be
particularly appropriate. Such foamed extrusions are
available from, e.g., Applied Medical Resources in Laguna
Niguel, CA, or Extrusioneering, Inc., in Temecula, CA.
To prevent damage to tissue other than where the
ablation is to occur, such as at the insertion site near
the femoral vein and around the puncture point through
the atrial septum, an insulation sleeve may be used as
noted above.
The invention has been described above with respect
to particular embodiments. It will be clear to one of
skill in the art that numerous variations may be made
from the above embodiments with departing from the spirit
and scope of the invention. For example, the invention
may be combined with stmt therapies or other such
procedures. The dual balloon disclosed may be used after
angioplasty or may be an angioplasty balloon itself.
Furthermore, while the invention has occasionally been
termed herein a "cryoplasty catheter", such a term is for
identification purposes only and should not be viewed as
limiting of the invention. Fluids that may be used as
heat transfer fluids include perfluorocarbon-based
liquids, i.e., halogenated hydrocarbons with an ether
bond, such as FC 72. Other materials that may be used
include CFCs, Freon~, or chemicals that when placed
together cause an endothermic reaction. Preferably, low
viscosity materials are used as these result generally in
a lessened pressure drop. The balloons may be made,
e.g., of Pebax, PET/PEN, PE, PA 11/12, PU, or other such
materials. Either or both of the dual balloons may be
doped to improve their thermal conductivities. The shaft
CA 02400753 2002-08-30
WO 01/64145 PCT/USO1/06648
of inner tube 122 may be made of Pebax, PBT, PI/PEI, PU,
PA 11/12, SI, or other such materials. The precise
shapes and dimensions of the inner and outer lumens,
while indicated in, e.g., Figs. 1B, 1C, and 2B, may vary.
The lumen design shown in Figs. 1B-1C may be employed in
the catheter of Fig. 2A and vice-versa. Embodiments of
the invention may be employed in the field of cold
mapping, where a circle of tissue is cooled to see if the
affected part has been reached. If the affected tissue
is that which is being cooled, a more vigorous cooling
may be instituted. Other variations will be clear to one
of skill in the art, thus the invention is limited only
by the claims appended hereto.
WHAT IS CLAIMED
26