Note: Descriptions are shown in the official language in which they were submitted.
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
1
High-Temperature Polymer/Inorganic Nanocomposites
BACKGROUND OF THE INVENTION
This application claims the benefit of U.S. Provisional
Application No. 60/048,921, filed June 6, 1997.
A nanocomposite is defined as an interacting mixture of
two phases, one of which is in the nanometer size range in at
least one dimension. Due to the nanoscale dimensions of the
reinforcement phase, nanocomposites display unique and
improved properties compared to that of micro- or macro-
composites. A wealth of unique properties and technological
opportunities are offered by these materials. Hence, over
the last few years, nanocomposite materials have become an
integral part of the synthesis of new materials for a wide
variety of applications including mechanical, optical,
magnetic and dielectric applications.
Polymer/inorganic nanocomposites have attracted much
attention as the properties of polymers are further enhanced
beyond what is achievable from more conventional particulate-
filled or micro-composites. Specifically, layered mica-type-
silicates have been widely used as inorganic reinforcements
for polymer matrices to create polymer nanocomposites with
nanoscale dispersion of the inorganic phase within the
polymer matrix. Layered silicate-polymer nanocomposites
having (i) polymer chains intercalated between the silicate
layers or (ii) individual silicate layers delaminated and
dispersed in a continuous polymer matrix, have been
fabricated.
Surface modification in the existing nanocomposites is
often achieved by using an organic surfactant such as alkyl
ammonium. The organic cation exchange at the oxide surface
provides favorable sites for interaction between the organic
and inorganic functionalities. That approach has been
exploited and has been shown to offer significant performance
advantages for a variety of commodity polymers. However, the
thermal instability of the surfactants used has hindered that
technology from realizing its full potential.
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
2
Significant new applications in automotive, aerospace,
electronic and food packaging industries will be realized by
fabricating nanocomposites having superior interfacial design
and enhanced thermal stability.
SOMMARY OF THE INVENTION
The invention provides new high-use temperature,
lightweight polymer/inorganic nanocomposite materials with
enhanced thermal stability and performance characteristics.
The invention provides two techniques that enhance the
thermal stability of the nanocomposite systems from their
current limits of 100 - 150 °C to over 250 °C. The two
unique approaches are based on innovative chemical design of
the organic-inorganic interface using (i) more thermally
stable surfactants/compatibility agents, and (ii) more
thermally stable synthetic organically-modified layered-
silicate reinforcements to create unique nanocomposites.
These approaches offer processibility through both solution
techniques, as well as solvent-free direct melt intercalation
technique.
The use of synthetic organically-modified layered
silicates (having built-in surfactants) eliminates the
poisonous alkyl ammonium surfactants which limit the
applications of nanocomposites as food packaging materials.
Recyclable and more cost-effective food packaging materials
are provided to replace unrecyclable multilayer plastic
packages.
The properties of these nanocomposites are optimized in
order to manufacture these materials in commercially
applicable forms, e.g. films, fibers and molded components.
The new technology provides hitherto unobtainable thermal
stability and performance characteristics, and has numerous
applications in automotive, aerospace, electronic and food
and beverage industries.
These and further and other objects and features of the
invention are apparent in the disclosure, which includes the
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
3
above and ongoing written specification, with the claims and
the drawings.
BRIEF DESCRIPTION OF THE DRAi8ING8
Figure 1 shows high temperature, high performance
organic/inorganic nanocomposites.
Figure 2 shows a chemical structure of the preferred
tetraphenyl phosphonium surfactant.
Figure 3 shows a schematic of polymer-layered silicate
nanocomposite structures.
Figure 4 provides a table of the properties of nylon-6
and layered silicate-nylon nanocomposites.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring to Figure 1A, the current state-of-the-art is
schematically shown. Nanocomposites 1 have silicate layers 3
with alkyl ammonium surfactants 5 on the surface. The first
part of the invention, schematically shown in Figure 1B,
provides new organic/inorganic nanocomposite structures 11 by
substituting high temperature organic phosphonium cations 15
for the standard compatibilizing agent, viz., alkyl ammonium
cations. In the new invention, ion exchange occurs with the
more thermally stable organic phosphonium cations, e.g.
tetraphenyl phosphonium 15 (Figure 2). That modification
enhances the thermal stability of the nanocomposites from the
current level of about 100-150'C to approximately 175-200'C
or more without affecting other physical or mechanical
properties of the resulting nanocomposites.
The inventors have synthesized a new class of phenyl
phosphine-based arylene ether structural polymers that offer
excellent mechanical and thermo-oxidative properties. Those
polymers are somewhat similar in nature to phenyl
phosphonium, are qualified for space missions by NASA and are
being commercially produced.
As schematically shown in Figure 1C, the invention also
provides the innovative use of organically modified layered
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
4
aluminosilicates 23 (ORMLAS) that combine the layered
silicates and the organic compatibilizing agent 25 in a
single chemical compound 21, rendering the material thermally
stable, and highly organophilic. This dual function compound
is then miscible with a host of structural matrix resins such
as polyamides (nylon Tm 120'C), polyether imide (Ultem T9
215'C), polyimides (T9 >275'C) and poly arylene ethers (T9
>225-350'C). These approaches offer processibility through
both, solution techniques, as well as solvent-free direct
melt intercalation technique. Moreover, since the bonding of
the organic group to the inorganic Si atom is through the Si-
C bonds, the ORMLAS exhibits excellent thermal stability.
Fabricating ORMLAS layered silicates with high temperature
structural polymers offers an attractive combination of
properties such as high heat distortion temperature,
excellent impact resistance and excellent mechanical
properties.
The invention provides high use-temperature light-weight
polymer/inorganic nanocomposites which have outstanding
properties, compared to the state of the art layered silicate
nanocomposites that use alkyl ammonium as the surfactant. A
database of properties of control specimens is established
for nanocomposites made from mixtures of a number of
commodity polymers (e. g., Polystyrene, Nylon, modified
Polyetherimide, Polyethylene oxide) with montmorillonite
containing alkyl ammonium-surfactants (through cation-
exchange).
Superior polymer/layered silicate nanocomposites are
fabricated by using mixtures of polyetherimide (PEI) resins
with montmorillonite containing organic phosphonium
surfactants, e.g.. tetraphenyl phosphonium (TPP) (through
cation-exchange), via direct polymer melt intercalation
process.
Superior nanocomposites are fabricated by direct polymer
melt intercalating organically-modified layered
aluminosilicates (ORMLAS) with polyetherimide and
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
thermoplastic polyimide (PI) or polyarylene ether (PAE)
resins. The direct bonding of the organic surfactant group
to the structural Si atom through an Si-C bond provides a
"built-in" surfactant with enhanced thermal stability, and
also allows for easy tailoring of the organic-inorganic
interface through the organic groups.
Interfacial, thermal, mechanical and physical properties
of the fabricated nanocomposites are evaluated to optimize
the most promising nanocomposites.
These nanocomposites are designed by creating favorable
interactions at the polymer-layered silicate interfaces. That
is achieved by making the chemistry of the inorganic
reinforcement phase more compatible with the organic polymer
matrix, i. e., by making the layered silicate surfaces
organophilic. The normally hydrophilic silicate surfaces are
rendered organophilic after ion-exchange reactions of the
loosely-held cations in the interlayer spaces of the silicate
structure with organic cations.
Polymer-layered silicate nanocomposites have been
synthesized for a variety of commodity polymer systems.
Nanocomposites with properties much superior to that of the
corresponding unfilled and conventionally-filled polymers are
hence obtained. This unique combination of improved
properties, easy fabricability, and low-cost, offers
tremendous potential for commercial applications of these
materials.
The synthetic creation of polymer/inorganic
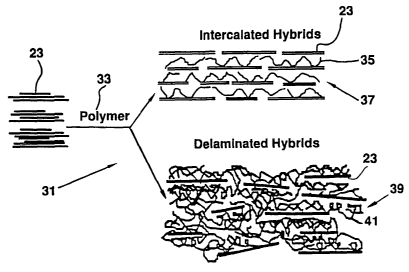
nanocomposites 31, schematically shown in Figure 3, exploits
the ability of the layered inorganic silicates 23 to
accommodate polymer 33 chains 35 between the layers 23
creating intercalated hybrids 37. Delaminated hybrids 39 are
created by dispersing individual layers 23 in a continuous
polymer matrix 41. Figure 3 shows a schematic representation
31 of the polymer/inorganic nanocomposite structures 37, 39
obtained using the layered silicates 23. Nanocomposites 37
have single polymer chains intercalated between the silicate
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
6
layers 23. In nanocomposites 39 the host silicate layers 23
are delaminated and dispersed in a continuous polymer matrix
41.
That synthetic design also exploits the ion-exchange
capacity of these layered-silicates 23 which allows for a
fine-tuning of their surface chemistry to create a favorable
organic-inorganic interface.
The advantages of the layered silicates in the synthesis
of those nanocomposites arise from their unique crystal
structure. Those layered silicate materials are fine-grained
and have crystal structures with a "platy" habit. Their
structure is common to the family of 2:1 layered- or
phyllosilicates, well-known examples of which are mica and
talc. The structure is composed of Si04 tetrahedra fused to
edge-shared octahedra of aluminum or magnesium hydroxides.
Layer stacking leads to regular Van der Waals gaps between
the layers, viz., interlayer or gallery. Isomorphic
substitution of cations is common (for example, A13+ or Fe3+
substituting for Si4+ in the tetrahedral network). That
leads to a net negative charge on the structure, which is
generally counter-balanced by cations residing in the
interlayer spacing. Those cations are more or less readily
exchanged and result in the cation-exchange capacity of the
materials.
Those interlayer cations are, for example, Na+ or K+ in
pristine layered silicates. For the synthesis of the
polymer/inorganic nanocomposites, an organophilic surface
chemistry is desired to create favorable interactions with
the organic polymer matrix. Therefore, those inorganic
cations are exchanged with various organic cations, e. g.,
alkyl ammonium cations. The hydrophilic silicate surface is
thus rendered organophilic. Those surfactants typically are
alkyl ammonium compounds e.g., dimethyl ditallow ammonium
bromide. One example is the use of dimethyl ditallow
ammonium bromide to cation-exchange with the Na+-
montmorillonite - a layered silicate. Such ion-exchanged
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
7
'organo'silicate clays are commercially available. Herein
lies the opportunity to tailor the interfacial chemistry of
those polymer/inorganic nanocomposites through creation of
the organic functional groups. The properties of the
nanocomposites can be optimized through interfacial surface
chemical design.
The synthesis of layered silicate nanocomposites has
involved intercalation of a suitable monomer followed by in
situ polymerization. Alternatively, polymer intercalation is
carried out from solution. Those techniques limit use in the
case of most technologically important polymers, since
suitable monomers and compatible polymer-silicate solvent
systems are not always available. The spectrum of
nanocomposite systems that can be synthesized is considerably
broadened by the advent of a more versatile and
environmentally-friendly synthetic approach, called direct
polymer melt intercalation. In that approach, the polymer
and the silicate are mixed, and the mixture is heated above
the softening point of the polymer. That technique allows
the synthesis of a much wider range of polymer/inorganic
nanocomposites. Polymers with varying degrees of polarity
and crystallinity are directly intercalated into organically-
modified layered silicates. Example of polymers use direct
polymer melt intercalation include polystyrene,
poly(dimethylsiloxane), poly(vinylidene fluoride), poly (E-
caprolactone), and (polyethylene oxide).
Delamination of the silicate layers can also be achieved
during nanocomposite synthesis through 'polymer melt
intercalation'. An example is the delamination of the
individual silicate layers achieved by suspending ditallow
ammonium-exchanged montmorillonite in PDMS
(Polydimethylsiloxane) at room temperature and sonicating for
2 minutes.
The advantages and some of the most attractive
properties of these nanocomposites can be seen in the data in
Figure 4. Significant enhancements in properties of Nylon-6
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
8
are obtained as a result of forming a nanocomposite
containing 4% by weight layered silicate in the Nylon-6
matrix.
The data in Figure 4 show that the tensile strength and
modulus are enhanced without any loss in impact strength.
Loss of impact strength is usually witnessed with such
enhancements in the case of conventional filled polymers.
The significant increase in the heat distortion temperature
(i. e., 145'C up from 65'C) extends the use-temperature of
Nylon to under-the-hood structural components in automobiles
A reduction in water adsorption accompanies the
improvements in mechanical properties.
Considerable increases in heat resistance and thermal
stability are obtained with these nanocomposites.
Polydimethylsiloxane - layered silicate nanocomposites have a
delayed thermal decomposition compared to that of the pure
polymer - which decomposes into volatile cyclic oligomers.
The improved barrier properties of these nanocomposite
materials are demonstrated through measurements of relative
permeability of liquids and gases through the nanocomposites.
Dramatic reductions in permeability are obtained at low
inorganic contents compared to conventionally-filled polymers
with much higher filler contents. The large aspect ratio of
the silicate layers forces the solutes to follow more
tortuous paths in the polymer matrix around the silicate
layers. That results in much larger effective diffusion
distances, and hence lower permeabilities. The enhanced
thermal stability of the nanocomposites is also attributed to
the hindered out-diffusion of the volatile decomposition
products. Self-extinguishing characteristics in those
materials are related to the barrier properties rendered by
the silicate layers.
A key to obtaining superior properties at low inorganic
loadings is the homogeneous nanoscale dispersion of the
inorganic phase in the polymer, and the creation of favorable
interactions at the organic-inorganic interface. Favorable
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
9
interfacial chemistry leads to organic and inorganic phases
being dispersed at a nanometer level. The superior
properties of the new composites are obtained at low
inorganic loadings. The use of low inorganic contents leads
to significant advantages. High degrees of stiffness,
strength and barrier properties are obtained with far less
inorganic content than comparable glass- or mineral
reinforced polymers. Considerable weight savings are,
therefore, obtained.
Some commercial applications of these materials are, for
example, Nylon-layered nanocomposite automatic timing belt
cover. Other applications include airplane interiors, fuel
tanks, components in electrical and electronic parts, under-
the-hood automotive structural parts, brakes and tires.
Applications of nanocomposite barrier films may be used in
food packaging and in other applications are also
possibilities.
The fact that the nanocomposites yield significant
enhancements in properties at low inorganic loadings also
provides ease-of-manufacturing and several cost-benefits. It
allows for the use of simple manufacturing techniques (viz.,
extrusion, injection-molding and casting) which are normally
used for pure polymers. Therefore, the nanocomposites can be
manufactured at a much lower cost than the more conventional
fiber- or mineral-reinforced composites which require more
expensive fabrication procedures. That provides further
reasons for their commercial appeal.
Commodity polymers provide use-temperatures below 125'C.
Substructure applications for rockets and aircraft require
higher long term use-temperatures of about 175'C and 250'C.
The thermal stability of the current state-of-the-art
nanocomposite systems is often limited by the thermal
instability of the surfactants used to create favorable
interactions at the interface. Those surfactants typically
are alkyl ammonium compounds. One example is the use of
dimethyl ditallow ammonium bromide to ion-exchange with the
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
Na+-montmorillonite - a layered clay. The thermal stability
of the nanocomposite system is, therefore, limited by the
thermal stability of the alkyl ammonium compound.
Degradation of those surfactant molecules, and hence that of
the organic-inorganic interface, begins at temperatures
around 100 - 110'C.
The invention provides more thermally stable
surfactants, which optimize the dispersion of the inorganic
phase, and also enhance the compatibility at the organic-
inorganic interface through the creation of favorable
interactions. The invention therefore provides enhanced
use-temperatures of the nanocomposites. One part elevates
the use-temperature of the nanocomposite system by using more
thermally stable surfactants than the currently used alkyl
ammonium compounds.
The first part provides tetra-phenyl phosphonium
compounds (with thermal stability in the range of 190' -
200'C) to carry out a cation-exchange with the layered
silicate reinforcement. Tetraphenyl phosphonium is a
reactive salt with a net positive charge, as shown in Figure
2. The salt readily ion exchanges with the cations on the
surface of the inorganic phase attaching itself from the
oxide surface and thus rendering the surface organophilic.
That surface modified system lends itself to direct melt
intercalation. Because tetraphenyl phosphonium is a high
temperature organic moiety with thermal stability in excess
of 200'C, the first part of the invention provides
nanocomposites which will satisfy the need for long-term use-
temperatures of 175-200'C.
The second part of the invention extends the use-
temperature of the nanocomposites to over 250'C. It is based
on the innovative use of organically-modified layered
alumino-silicates (ORMLAS) that combine the layered silicate
and the organic surfactant/compatibility agent in a single
chemical compound. The organic surfactant groups are bonded
to the structural Si atom through thermally stable Si-C
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
11
bonds. The thermal stability of the overall system is
therefore greatly enhanced. Therefore, those materials
provide unique inorganic layered silicate reinforcements
having markedly more thermally-stable surfactants "built-in"
to the chemical structure. Those materials are miscible in a
host of structural matrix resins, such as polyamides (nylon
Tm 120'C) and polyether imide (Ultem T9 215'C), polyimides
(T9 >275'C) and poly arylene ether (PAE Tm >325-350'C).
Those ORMLAS materials are synthesized using the sol-gel
process where the organic groups are incorporated into the
molecular structure through the use of organically modified
silicon alkoxides, i. e., precursors containing Si-CxHx
bonds. The organic functionality is therefore directly
bonded to the structural Si atom by the Si-C bond. The
organically modified aluminosilicate will be synthesized
using mixtures of organically-modified silicon alkoxides and
solutions of aluminum chloride. The standard approach
combines an alcohol solution of aluminum chloride with an
alcohol solution of organically functionalized alkoxysilane.
The organically functionalized trialkoxysilane, e. g.,
alkyltriethoxysilane, provides alkyl organic groups connected
directly through Si-C bonds. The mixture is then condensed
(crosslinked) to form a gel at appropriate pH conditions by
the addition of NaOH. The gel is aged, filtered, washed with
distilled water, and then dried in vacuum. That procedure
yields a stable layered organophilic compound. The resulting
material is either precipitated as a powder, dried and ground
to appropriate particle size, or cast into various shapes and
forms. In that case, the ORMLAS is precipitated as a powder
or ground and classified it into appropriate size particles
for incorporation with the polymer matrix for direct melt
intercalation. A unique feature of those ORMLAS compounds is
that they are especially engineered to delaminate in the
presence of a variety of polymer resins - thus promoting the
dispersion of the inorganic layers in the polymer matrix.
That versatile and innovative new feature yield
CA 02401052 2002-09-20
WO 01/70492 PCT/US00/07708
12
nanocomposites which will satisfy needs for a range of use-
temperatures extending to long-term use-temperatures over
250'C.
In addition to the enhanced thermal stability, the use
of synthetic organically-modified layered silicates (ORMLAS)
having built-in surfactants eliminates the poisonous alkyl
ammonium surfactants which limit the applications of
nanocomposites as food packaging materials. The elimination
of the ion-exchange also lowers the processing costs.
Therefore, that technique yields recyclable and more cost-
effective food packaging materials with superior gas-barrier
properties to replace unrecyclable multilayer plastic food
packaging materials.
While the invention has been described with reference to
specific embodiments, modifications and variations of the
invention may be constructed without departing from the scope
of the invention, which is defined in the following claims.