Note: Descriptions are shown in the official language in which they were submitted.
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
METHOD FOR TREATING CELLS RESISTANT TO ANTINEOPLASTIC AGENTS
Background of the Invention
The present invention relates to methods for treating cells resistant to
neoplastic agents.
Although there are now a number of cytotoxic agents that help to produce a
positive outcome in
cancer patient therapy, many cancer cells develop resistance or are resistant
to the neoplastic
agents currently of choice for chemotherapeutic treatment. The development of
drug resistance
substantially compromises the efficacy of cancer therapy.
Multidrug resistance cells are one example of cells that are resistant to
antineoplastic
to agents. In this case, the cells are resistant to more than one
antineoplastic agent. Multidrug
resistance is a well-defined phenomenon. Often cancer cells that become
resistant to one class
of anticancer drugs (i.e., Vinca alkaloids, anthracyclines, taxanes, including
paclitaxel,
epipodophyllotoxins, and the like) also demonstrate resistance to other
anticancer drugs.
Development of multidrug resistance creates a significant impediment in the
generation of
positive outcomes for many cancer patients. Multidrug resistant agents have a
number of
general features in common; they are generally lipophili, weakly basic
molecules of greater than
about 300 daltons or larger molecular weight. Multidrug resistant cells tend
to accumulate
anticancer drugs at a level lower than cells that are not multidrug resistant
(Beck, WT, Adv.
Enzym. Regul 1984, 22:207). Accumulation of drug at lower levels has been
shown in some
2o models to be associated with an increase in activity or in the amount of a
family of
transmembrane channel proteins.
An example of transmembrane channel proteins that are capable of decreasing
the
intercellular concentration of anti-cancer drugs are the integral membrane
proteins P-
glycoproteins (Pgp, Endicott JA and Ling, V. Annu. Rev. Biochem. 1989,
58:137). The proteins
appear to bind to antineoplastic agents and release the agents into the
extracellular milieu.
Expression of the MDRl cDNA, the DNA encoding Pgp, is sufficient to produce a
multidrug
resistance phenotype (Gros et al., Nature 1986, 323:728). These proteins are
present in rodents
and in man. Another protein associated with resistance to antineoplastic
agents is the
multidrug resistance-associated protein (MRP) (Grant CE et al., Cancer Res.
1994, 54:357).
3o MRP has been shown to confer multidrug resistance to doxorubicin,
vincristine, etoposide and
colchicine. For a review of other multidrug resistance associated proteins see
"Mechanisms of .
Drug Resistance" by Beck and Dalton in Cancer: Principles and Practice of
Oncology, p. 498-
512, eds DeVita et al., Lippincott-Raven, NY, 1997.
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
Elevated levels of Pgp have been observed in a variety of cancers including,
but not
limited to, Acute Myelogenous Leukemia, Non-Hodgkin's Lymphoma, multiple
myeloma as
well as in a variety of solid tumors including, but not limited to, cancers of
the adrenal, colon,
kidney, lung and breast (see Beck and Dalton, supra). Moreover, it is widely
recognized that
cancers having an origin in a variety of tissues and cells can develop
multidrug resistance.
Therefore, there is a need to identify and to use compounds that remain toxic
to otherwise
multidrug resistant cells.
In addition to multidrug resistance, there are other types of resistance to
antineoplastic
agents that have been observed. These include, for example, resistance to one
or more
to antineoplastic agents as a result of a mutated protein. One example of
resistance to
antineoplastic agents results from mutations in microtubules or in mutations
in tubulin dimers.
Cellular resistance to taxanes such as paclitaxel, can be multifactorial. For
example, cellular
resistance to the taxane family has been associated in some instances with a
mutation in the 13-
tubulin subunit. Again, as in the case of multidrug resistant cells, there is
a need for neoplastic
agents that remain toxic to taxane-resistant cells.
Summary of the Invention
The present invention relates to methods for treating cells with
discodermolide. In one
aspect, the invention relates to methods for treating cells with
discodermolide in vivo.
In another aspect of this invention, the invention relates to a method for
inhibiting the
2o growth of multidrug resistant cells comprising the step of contacting at
least one multidrug
resistant cell with a growth-inhibiting amount of discodermolide. In one
embodiment the
multidrug resistant cell is resistant to taxanes, for example paclitaxel. In
another embodiment
the multidrug resistant cells are growth inhibited ih vivo or in culture.
Preferably the cell is from
a mammal and more preferably from a human.
In another aspect of this invention, the invention relates to a method for
inhibiting the
growth of a cancer cell comprising the steps of: contacting at least one
cancer cell with a growth
inhibiting amount of discodermolide wherein the cancer cell is resistant to at
least one
antineoplastic agent. In a preferred embodiment the cancer cell is selected
from the group
consisting of a leukemia cell, a lymphoma cell and a solid tumor cell. In one
embodiment the
3o cancer cell is a multidrug resistant cell. In another embodiment the cell
comprises a mutation in
13-tubulin and in another embodiment, the cell over-produces glutathione.
Preferably the cell is
in a mammal.
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
The invention also relates to a method for promoting apoptosis in a multidrug
resistant
cell comprising the steps of :contacting a multidrug resistant cell with
discodermolide; and
inducing apoptosis in the cell. In a preferred embodiment the multidrug
resistant cell is resistant
to paclitaxel. The cell can be a cell in culture or in vivo. Preferably the
cell is from a mammal
and more preferably from a human.
The invention further relates to a method for inhibiting the growth of cancer
cells having
a f3-tubulin mutation comprising the steps of; contacting at least one cancer
cell with a growth
inhibiting amount of discodermolide wherein the cell comprises a mutation in
the protein f3-
tubulin; and inhibiting cell division in the cell. In one embodiment the cell
is resistant to
1o paclitaxel or to another antineoplastic agent. Preferably growth inhibition
occurs in vivo and
more preferably growth inhibition occurs in a mammal, preferably a human.
The invention also relates to a method for inhibiting growth of a tumor
resistant to at least one
antineoplastic agent comprising the step of: contacting a tumor with
discodermolide wherein the
tumor comprises cells resistant to at least one antineoplastic agent. In one
embodiment the cells
have a mutation in a 13-tubulin protein and in another embodiment the cells
overproduce
glutathione. In yet another embodiment, the cells are multidrug resistant. In
a preferred
embodiment, the at least one neoplastic agent is paclitaxel. In yet another
embodiment the cells
comprise raf 1 and wherein raf 1 is phosphorylated in the presence of
discodermolide.
Preferably the tumor is selected from the group of tumors consisting of lung,
prostate, colon,
breast, ovarian, kidney, brain, pancreatic esophageal, head and neck, gastric,
and liver tumors.
Brief Description of the Figures
Figure 1 is a table illustrating the antiproliferative activity of
discodermolide in various
cancer cell lines. Various cancer cell lines were incubated with increasing
concentrations of
discodermolide or paclitaxel and the ICso for cell proliferation is determined
by methylene blue
staining.
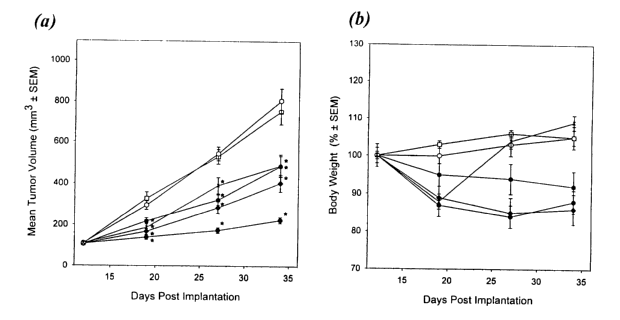
Figure 2(a) provides the mean tumor volumes and Figure 2(b) provides mean body
weights in a
study to assess paclitaxel-resistant (Pgp-1/MRP)-overexpressing human colon
tumor xenograft
(HCT 15 cells) sensitivity to discodermolide. --o-- refers to control solution
of 16.7% Crm.-
3o 8.3%EtOH/DSW, iv lx (d.14); --~-- refers to mice receiving discodermolide,
iv, 15 mg/leg, lx
(d.14);--~--refers to mice receiving discodermolide, iv, 7.5 mg/kg, lx (d.14);
--~ --refers to
mice receiving discodermolide, iv, 2.5 mg/kg, lx (d 14); --o-- refers to mice
receiving 12.5%
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
4
Crm-12.5%EtOH/DSW, iv, lx/day (d. 14-16); and --x-refers to mice receiving
paclitaxel, iv,
15 mg/kg, lx/day (d. 14-18).
Figure 3(a) provides the mean tumor volumes and Figure 3(b) provides mean body
weights in a study to assess paclitaxel-resistant (Pgp-1)-overexpressing human
colon tumor
xenograft (MIP 101 cells) sensitivity to discodermolide. --a-- refers to
control solution of 16.7%
Crm.-8.3%EtOH/DSW, iv lx (d.14); --~-- refers to mice receiving
discodermolide, iv, 15
mg/kg, lx (d.14);--~--refers to mice receiving discodermolide, iv, 7.5 mg/kg,
lx (d.14); --O--
refers to mice receiving discodermolide, iv, 2.5 mg/kg, lx (d 14); --o--
refers to mice receiving
12.5% Crm-12.5%EtOH/DSW, iv, lx/day (d. 14-16); and-x--refers to mice
receiving
to paclitaxel, iv, 15 mg/kg, lx/day (d. 14-18).
Figure 4 assesses paclitaxel-resistant lA9PTX22 (13-tubulin mutation) cell
sensitivity to
discodermolide. --~-- refers to 1A9 cells receiving paclitaxel; --o-- refers
to lA9PTX22 cells
receiving paclitaxel; --~-- refers to 1A9 cells receiving discodermolide; and -
- Q -- refers to
lA9PTX22 cells receiving discodermolide.
Figure 5 illustrates results of experiments demonstrating that Paclitaxel-
resistant 1A9
PTX22 cells are sensitive to discodermolide in nude mice. Figure 5(a) refers
to treatments
starting 24 hours after animals were implanted subcutaneously (sc) with hollow
fibers (3
fibers/animal, one fiber/each cell line, six animals/compound). Paclitaxel was
administered IV,
once daily for 5 days at 15 mg/kg. Vehicle control was administered according
to the paclitaxel
2o schedule. Figure 5(b) refers to the identical regimen as 5(a) but here
discodermolide rather than
paclitaxel was administered iv, as a single 15 mg/kg injection. Vehicle
control was administered
according to the discodermolide schedule.
Description of the Preferred Embodiments
(+)-Discodermolide (hereinafter referred to as "discodermolide") is a
metabolite of the
marine sponge Discodermia dissolute (See Gunasekera, et al., J. Org. Chem.
55:4912, 1990.
Correction: J. Org. Chem. 56:1346, 1991).
/ 24
HO,,,,
15 . _
21
H 14 OH O' /NHZ
1I I~1
OH piscodermolide O
OH (+) 1
~'33~5N~
(593.80)
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
Despite their different chemical structure, discodermolide is believed to
function much
the same way as paclitaxel, the active substance in the drug TAXOL. Like
paclitaxel,
discodermolide acts to inhibit cold-induced depolymerization of purified
tubulin, and interferes
with microtubule dynamics in cells (ter Haar E, et al., Biochemistry 1996;
35:243-50).
Proliferating cells treated with the compound are axrested during mitosis, and
subsequently
undergo apoptosis (Balachandran R, et al., Anti-Cancer Drugs 1998; 9:67-76.
[Errata: Anti-
Cahcer Drugs 1998: 9:369-370]) In a number of studies, discodermolide as been
shown to be
more potent than paclitaxel in its ability to polymerize purified tubulin and
discodermolide binds
1o to tubulin competitively with paclitaxel. Paclitaxel has been shown to
disrupt microtubules in
tumor cells resulting in cell killing. Thus, investigators are working to
confirm the anti-
proliferative effects of discodermolide both in vitro and in vivo.
While investigators have demonstrated the superiority of discodermolide as
compared
with paclitaxel for killing cancer cells, the killing has been performed in
culture. . The present
15 invention provides data to demonstrate that discodermolide is effective for
inhibiting the growth
of cancer cells in vivo. In addition, the present invention demonstrates the
e~cacy of
discodermolide in vivo in cells that have demonstrated resistance to at least
one antineoplastic
agent. The studies described below demonstrate that discodermolide is useful
for treating cells
both in vivo and in culture and in treating cancer cells where the cancer
cells are resistant to at
20 least one antineoplastic agent because, for example, the cancer cells are
multidrug resistant; the
cancer cells over produce glutathione or because the cancer cells have a
mutation in one or more
proteins rendering the cells resistant to the antineoplastic agent..
Thus, the term "resistant to at least one neoplastic agent" is used herein to
refer to cells,
for example, that are multidrug resistant; cells that are resistant to
platinum or to other
25 alkylating agents because they tend to over produce glutathione and to
cells that have a mutation
in one or more cells that render the cells resistant to a particular
chemotherapeutic agent. For
example, it has been shown that cells resistant to taxanes include a mutation
in 13-tubulin protein
The present studies support the use of discodermolide in cases where one or
more antineoplastic
agents have failed to adequately inhibit growth of the cancer cells. The term
"resistance" is used
30 herein to refer to cells that are able to survive in the presence of at
least one neoplastic agent
where the normal cell counterpart (i.e., a growth regulated cell of the same
origin) would either
show signs of cell toxicity, cell death or cell quiescence (i.e., would not
divide).
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
The term "inhibit the growth off' as used in herein refers to the ability of a
particular
antineoplastic agent to limit or reduce the growth potential of a cell,
preferably a cancer cell.
Therefore, a particular antineoplastic agent can inhibit the growth of a cell
by reducing the rate
at which a particular cell divides, it can cause the cells to remain in a
quiescent (i.e., non-
dividing state) or it can induce cell cytotoxicity and/or cell death,
including apoptosis.
Examples of cancer cells from cancers resistant to at least one antineoplastic
agent that
can benefit from discodermolide therapy include leukemias and lymphomas, as
well as solid
tumors such as tumors of the colon, spleen, prostate, liver, lung and breast.
Figure 1 includes a
Table that illustrates that discodermolide is effective in inhibiting the
growth of a number of
1o different types of cancer cells.
In one aspect, this invention relates to the use of discodermolide to inhibit
the growth of
multidrug resistant cells. Multidrug resistance is a term known in the art
that refers to cells
which are resistant (i.e., the cells survive) to more than one antineoplastic
agent. The term
"antineoplastic agent" is used herein to refer to molecules that are able to
inhibit growth of a
15 cancer cell and are used in therapies to treat cancer in mammals. Cells can
be multidrug
resistant through a genetic mutation even though the cells have not been
exposed to one or more
antineoplastic agents. More commonly, multidrug resistance results from
exposure to one
antineoplastic agent which then selects for cells that are resistant to more
than one other
antineoplastic agent. Multidrug resistance is a major challenge in cancer
chemotherapy because
2o the resistance severely impairs the effectiveness of a number of clinically
important drugs.
Drugs that are known to induce multidrug resistance include, for example,
Actinomycin D,
anthracyclines such as daunorubicin, doxorubicin, etoposide, mitoxantrone,
taxanes, such as
paclitaxel, topoisomerase inhibitors such as etoposides, and Vinca alkaloids
such as vinblastine
and vincristine, vinorelbine and colchicine,. Multidrug resistance occurs both
in culture and in
25 vivo. In general, cell lines that display the multidrug resistance
phenotype are resistant to
natural products, but retain their sensitivity to alkylating agents and
antimetabolites.
A multidrug resistance gene family has been identified and appears to be part
of the ABC
(ATP-binding cassette) superfamily (reviewed by Bellamy in Ahhu. Rev. Pharm.
Toxicol. 1996,
36:161-83). The more common member of this family is the protein, Pgp which is
described
3o vide supra. A second protein associated with multidrug resistance is MRP.
MRP also confers
resistance to numerous natural products. Like Pgp, MRP can be elevated in
patients with acute
and chronic leukemia and solid tumors.
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
The cells which are resistant to at least one antineoplastic agent are
preferably contacted
with discodermolide ih vivo, preferably in a mammal. Although the cells can
also be contacted
with discodermolide in culture. A preferred mammal in this invention is a
human; however,
veterinary applications are additionally included within the scope of this
invention.
Multidrug resistance can be monitored in culture or in vivo. Methods for
monitoring and
assessing multidrug resistance are well known and for that reason will not be
described in detail
here. In culture, cells which are able to grow or to survive in the presence
of more than one
antineoplastic agent as compared with matched, growth controlled cultures of
cells including
those obtained from normal, differentiated tissue are said to be multidrug
resistant.
to It is also possible to assess multidrug resistance in vivo and to assess
multidrug resistance
over time for or during a particular treatment regime. For example,
immunocytochemical assays
are known in the art that assess levels of Pgp protein or other proteins
belonging to the multidrug
resistance family of proteins. In these assays it is possible to compare
levels of the proteins in
cancer cells as compared with normal, growth controlled cells. RNA assays or
immunoblots
15 have been described in the literature to monitor multidrug resistance as
have iu situ hybridization
studies and flow cytometric assays.
To demonstrate the growth inhibiting, and preferably cytotoxic effect of
discodermolide
for multidrug resistant cells in vivo, two different human tumor xenografts
(HCT-15 and MIP
101) are separately implanted subcutaneously in athymic nude mice (see Example
2 below).
20 NVP XAA296-NX results in statistically significant (p < 0.01) and
reproducible inhibition of
tumor growth in both tumor models. The HCT-15 model is completely refractory
to paclitaxel
treatment, while the MIP 1 O1 model is resistant to paclitaxel when
administered at 15 mg/kg,
once daily for the first five days.
Discodermolide administered as a single injection produces dose-dependent,
statistically
25 significant (p < 0.01) inhibition of tumor growth for all tested doses in
both xenograft models.
Toxicity, as measured by body weight loss, appears to be tumor-dependent since
an independent
experiment demonstrates that naive (non-tumor bearing) athymic nude mice dosed
with single
injections of discodermolide lost no more than 4% of body weight one week
after dosing and
fully recovered to the control levels in 3 weeks after dosing. These
experiments demonstrate the
3o antitumor efficacy of discodermolide in two tumor models that were
resistant to paclitaxel. In
both cases discodermolide was able to induce apoptosis. In further experiments
it was
demonstrated that discodermolide promoted phosphorylation of Raf 1, Bcl-2 and
Bcl-xL
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
In another aspect of this invention, the invention relates to methods for
inhibiting the
growth of cancer cells having mutation in a cellular protein that renders the
cells resistant to at
Least one antineoplastic agent. An example of this mechanism of resistance are
cells having a !3-
tubulin mutation that renders the cells resistant to taxanes, such as
paclitaxel. In this aspect of
the invention, the invention involves contacting at least one cell with a
growth-inhibiting amount
of discodermolide. Again, methods for determining whether or not cancer cells
are growth
inhibited are well known in the art. These studies include cell quantitation
using cell counting
techniques monitored over time, flow cytommetry, and the like. In vivo, cell
growth inhibition
can be monitored in blood borne tumors by assessing tumor load over time.
Similarly, the size
to of the tumor ih situ can be monitored as can the progression or lack
thereof of metastases. All of
these methods are well known to those of ordinary skill in the art of oncology
drug testing.
Recently published results of clinical studies suggest that mutations in (3-
tubulin are
associated with resistance of solid tumors to paclitaxel (Monzo M, et al. J.
Clih. Oncol. 1999,
17,(6):1786-1793). More than one mutation in the l3-tubulin proteins have been
described.
15 These include a mutation in Alanine364 and in several leucines within the
13-tubulin amino acid
sequence. This invention demonstrates that cells that are refractory to
paclitaxel and have a 13-
tubulin mutation are sensitive to discodermolide therapy. Example 3 details
experiments
assessing discodermolide sensitivity using a paclitaxel-resistant ovarian
carcinoma cell line,
lA9PTX22 and its parental cell line, 1A9, which is sensitive to paclitaxel. In
these studies cells
2o remain sensitive to discodermolide irrespective of the presence of a 13-
tubulin mutation.
There are several mutations in at least one 13-tubulin protein that have been
described as
conferring resistance to cancer cells for at least one antineoplastic agent.
The amino acid
sequences for the two isotypes of native human 13-tubulin are provided below
as SEQ ID NO:1
and SEQ ID NO:2:
25 Table I
Amino Acid sequence of 13-tubulin* (SEQ ID NO:1)
mreivhiqag qcgnqigakf wevisdehgi dptgtyhgds dlqldrisvy yneatggkyv
61 prailvdlep gtmdsvrsgp fgqifrpdnf vfgqsgagnn wakghytega elvdsvldw
121 rkeaescdcl qgfqlthslg ggtgsgmgtl liskireeyp drimntfsw pspkvsdtw
30 181 epynatlsvh qlventdety cidnealydi cfrtlrlttp tygdlnhlvs gtmecvttcl
241 rfpgqlnadl rklavnmvpf prlhffinpgf apltsrgsqq yraltvpdlt qqvfdalcnmrn
30I aacdprhgry ltvaavfrgr msmkevdeqm lnvqnknssy fvewipnnvk tavcdipprg
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
361 lkmavtfign staiqelfkr iseqftamfr rkaflhwytg egmdemefte aesnznndlvs
421 eyqqyqdata eeeedfgeea eeea
*swissprot: locus TBBS HCJMAN, accession P04350
Amino Acid sequence of 13-tubulin~ (SEQ ID N0:2)*
mreivhlqag qcgnqigakf wevisdehgi dptgtyhgds dlqlerinvy yneatggnyv
61 pravlvdlep gtmdsvrsgp fgqifrpdnf vfgqsgagnn wakghytega elvdavldw
121 rkeaescdcl qgfqlthslg ggtgsgmgtl liskmreefp drimntfsw pspkvsdtw
181 epynatlsvh qlventdety cidnealydi cfrtlklttp tygdlnhlvs atmsgvttcl
241 rfpgqlnadl rklavnmvpf prlhffinpaf apltsrgsqq yrgltvpelt qqmfdakrnmn
l0 301 aacdprhgry ltvaavfrgr msmkevdeqm lsvqsknssy fvewipnnvk tavcdipprg
361 lkmavtfign staiqelfkr iseqftamfr rkaflhwytg egmdemefte aesnmndlvs
421 eyqqyqdata eqgefeeeae eeva
In yet another aspect of this invention, the invention relates to the use of
Discodermolide
15 to treat cells resistant to platinating agents such as cisplatin and its
analogues. In experiments
using methods identical to those described in Example 1, below, the ovarian
cell lines 2008 and
C 13 are tested for sensitivity to discodermolide and to paclitaxel. C 13 is
resistant to paclitaxel
and demonstrates an overproduction of glutathione. In experiments comparing
paclitaxel and
discodermolide, 2008 cells have ICsos of 0.06 and 0.8 for discodermolide and
paclitaxel
2o respectively while the cisplatin resistant cells C13 have ICsos of 0.03 and
12. These results
demonstrate the utility of discodermolide as a treatment for cancer cells
resistant to cisplatin or
that overproduce glutathione.
The term "contacting" is used in this invention to refer to any suitable
delivery method
fox bringing discodermolide in contact with the cancer cells that are
resistant to at least one
25 antineoplastic agent. For culture applications, merely adding solutions of
discodermolide in a
pharmaceutically acceptable buffer of cell culture medium is sufficient. For
ih vivo applications,
discodermolide can be delivered to the cancer cells resistant to at least one
antineoplastic agent
using any suitable method known to those of ordinary skill in the art of drug
delivery.
Intravenous delivery and peritoneal delivery is preferred and those skilled in
the art of drug
3o delivery are familiar with the apparati designed for drug delivery via this
route of administration.
Similarly, the pharmaceutically acceptable formulations comprising
pharmacologically
active discodermolide alone, or in combination with one or more
pharmaceutically acceptable
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
carriers, preferably suitable for parenteral application will be readily
discernible to those of
ordinary skill in the art. Such formulations may include suitable excipients.
Preferred delivery
formulations are provided in the examples below. These formulations include
combinations of
discodermolide with cremaphor, propylene glycol, propylene glycol with DSW,
ethanol DSW or
with saline.
In culture, effective doses used are typically those at the ICso concentration
(see Figure
1). In vivo acute toxic doses are determined in clinical trial by treating at
fractions of the ICSo
and assess toxicity. Effective doses of discodermolide for the mouse are about
(+/- 5 mg/kg) 15
mg/lcg given as one treatment every three weeks; for the rat; about (+l- 0.5)
3 mg/kg
to administered as one treatment every three weeks; and for marmoset; about
(+/- 0.5) 1 mg/kg
given as one treatment every three weeks. Preferred dosages and dosing regimes
for man will of
course be perfected following clinical trials using methods well known to
those of ordinary skill
in the art of clinical trials and will be optimized for particular types of
cancer; however expected
dosages are preferably from about 10 mg/kg to about 300 mg/kg in humans and
more preferably
from about 50 mg/kg to about 150 mg/kg of discodermolide.
While particular embodiments of the invention will be described in detail, it
will be
apparent to those of ordinary skill in the art that these embodiments axe
exemplary rather than
limiting.
2o Example 1
In vitro growth inhibition of multidrug resistant cells
Preparation of compound solutions
A stock solution of discodermolide (natural product) at 10 mg/ml in 95 % v/v
ethanol is
prepared and stored at -20 °C. Aliquots are diluted directly either in
cell culture media (for in
vitro assays) or in phosphate buffered saline (PBS; for all in vivo
experiments).
Cells and cell culture conditions
The following cell lines are obtained from the American Type Culture
Collection
(ATCC, Rockville, MD, USA): human colon carcinomas HCT-15 (CCL 225) and HCT-
116
(CCL 247), human lung adenocarcinoma A549 (CCL 185), human large cell
carcinoma NCI-
3o H460 (HTB 177), estrogen-independent breast carcinoma MDA-MB-231 (HTB 177),
prostate
cancer cell line Du 145 (HTB 81). The human KB-31 (drug-sensitive) and KB-8511
(multidrug-
resistant, Pgp170 overexpressing) epidermoid carcinoma cells axe obtained from
Dr. R. M.
Baker, Roswell Park Memorial Institute (Buffalo, NY, USA) and have been
previously
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
11
described (Akiyam S, et al. Somatic Cell Molec Genetics 1985;11:117-126 and
Fojo A, et al..
Cancer Res. 1997;45:3002-3007). The human metastatic prostate carcinoma PC-3M
is obtained
from Dr. I. J. Fidler (MD Anderson Cancer Center, Houston, TX, USA). The
estrogen-
dependent human breast carcinoma cell line MCF-7/ADR (multidrug resistant) is
a subline of
the MCF-7 cell line (ATCC HTB 22) and is obtained from Dr. D. Fabbro (Novartis
Pharma AG,
Basel, Switzerland) and has been previously described (Blobe GC, et al. J.
Biol. Chem. 1993;
268:658-664).
Antiproliferative assay
For the antiproliferative assays, cells are seeded at 1.5 x 103/well into 96-
well microtiter
1o plates and incubated overnight. Compounds are added in serial dilutions on
day 1. The plates are
than incubated for additional 5 days. This allowed the control cultures to
undergo at least 3 cell
divisions. After incubation the cells are fixed with 3.3 % v/v glutaraldehyde,
washed with water
and stained with 0.05% w/v methylene blue. After washing, the dye is eluted
with 3 % v/v HCl
and the optical density measured at 665 nm with a SpectraMax 340 (Bucherer,
Basel,
Switzerland). ICSO values are determined by a computerized system (SoftPro,
Bucherer, Basel,
Switzerland) using the formula (OD test - OD start) / (OD control - OD start)
x 100. ICSO is
defined as the drug concentration which leads to 50% of cells per well
compared to control
cultures (100%) at the end of the incubation period.
Material
2o Natural discodermolide (sample 1) is obtained from Harbor Branch
Oceanographic
Institution (Ft. Pierce, FL, USA). Synthetic discodermolide is prepared using
any number of
methods described in the art including, for example, the methods of Smith AB,
PCT Publication
Number WO 00/04865, the contents of which is incorporated by reference herein.
Paclitaxel is
obtained from Calbiochem (La Jolla, CA, USA). Cell culture materials are from
Integra
BioSciences (Wallisellen, Switzerland). For HPLC, solvents are HPLC Gradient
grade from
Merck (Darmstadt, Germany). Liquid media, fetal bovine serum (FBS) and media
additives are
from Gibco/BRL (Basel, Switzerland).
Results
Antiproliferative activity
3o The antiproliferative profile of discodermolide is determined against a
panel of human
tumor lines. As shown in Table 1, the compound showed potent antiproliferative
activity in
vitro with the ICso values in the low nanomolar range (~ 2 - 24 nM) for drug-
susceptible cell
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
12
lines. Paclitaxel, is a potent cytotoxic agent but is much less active than
discodermolide against
HCT-15 colon cells (~ 120 vs. ~ 8 nM ICSO respectively). In the Pgp170-
overexpressing,
multidrug resistant I~B-851 l and MCF-7/ADR lines, the loss of activity of
paclitaxel was
several fold higher than that shown by discodermolide.
Discussion
Discodermolide is more potent than paclitaxel against MCF-7/ADR cells, which
is
multidrug resistant due to overexpression of Pgp170, protein kinase-C, and
glutathione S-
transferase.
l0 Example 2
In vivo growth inhibition of multidrug resistant cells
Cell lines and tissue culture
All cell lines that are used in animal studies are free of Mycoplasma
contamination
(Rapid Detection System by Gen-Probe, Inc., San Diego, CA) and viral
contamination (MAP
15 testing by MA BioServices, Inc., Rockville, MD). The HCT-15 human colon
tumor cell line is
purchased from the American Type Culture Collection, Rockville, MD, Accession
Number
ATCC CCL 225. The MIP 101 human colon tumor cell line is obtained from Dr. R.
Kramer
(Bristol Meyers Squibb) and was previously described (Miles RM, et aI. Cancer
Invest.
1987;5(6):545-52). These cells are Pgp-1 (human Pgp) overexpressors, making
the cells
2o resistant to paclitaxel. All cell lines are propagated and expanded in RPMI
1640 medium
containing 10% heat-inactivated FBS (Life Technologies, Grand Island, N~. Cell
expansions
for implantation are performed in T225 tissue culture flasks. Cells are
harvested at 70-90%
confluency, washed once with HBSS containing 10% FBS, and are suspended in
plain HBSS.
Animals and tumor implantations
25 Outbred athymic (nulnu) female mice ("Hsd:Athymic Nude-nu" from Harlan
Sprague
Dawley, Indianapolis, IN) are anesthetized with Metofane (Mallinckrodt
Veterinary, Inc.,
Mundelein, IL,). A cell suspension (100 ~.L) containing 1x106 cells is then
injected sc into the
right axillary (lateral) region of each animal. Tumors are allowed to grow
until a volume of
approximately 100 mm3 was achieved. At this point, mice bearing tumors are
sorted into groups
30 of eight for the study. The sorting process produced groups balanced with
respect to mean and
range of tumor size.
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
13
Drugs and formulations
Discodermolide is isolated from the sponge Discodermia dissoluta using the
methods of
(Gunasekera SP, et al. supra). Multiple batches of compound of similar purity
(all > 95% pure,
as determined by mass spectrometry, and nuclear magnetic resonance analysis)
are used
throughout the various studies. Solid discodermolide is dissolved in pure
ethanol to create a stock
solution which is diluted just before dosing with Cremophor EL (Crm) and DSW
to a final
concentration of 16.7% Cremophor EL, 8.3% ethanol and 75% DSW. The compound is
administered intravenously (iv). In the first study discodermolide is tested
against both tumors at
five different dosing schedules: (i.) 30 mg/kg dosed as two 15 mg/kg
injections on day 1 of the
to experiment, (ii.) 30 mg/kg dosed as three 10 mg/kg injections on days 1, 2,
and 3, (iii.) 15 mg/kg
dosed as a single injection on day l, (iv.) 20 mg/kg dosed as a 15 mg/kg
injection on day 1,
followed by a 5 mg/kg injection on day 11, and (v.) 10 mg/kg dosed as a 7.5
mg/kg injection on
day 1 followed by a 2.5 mg/kg injection on day 11. In the second study
discodermolide is dosed
as one injection on the first day of the experiment, at 2.5 mg/kg, S mg/kg,
7.5 mg/kg, 10 mg/kg,
15 12.5 mg/kg, or 15 mg/kg. In addition the 7.5 mg/kg injection on day 1
followed by a 2.5 mg/kg
injection on day 11 is repeated from the first study. The actual doses,
regimens and routes of
administration used for the specific models are discussed in each section
separately. Positive control
animals receive clinical formulations of paclitaxel (TAXOL) diluted 4-fold
with DSW and
administered iv once daily for five consecutive days. Vehicle control for
paclitaxel is
20 administered according to paclitaxel's schedule. In the first study vehicle
controls for
discodermolide (16.7% Cremophor EL, 8.3% ethanol and 75% DSW) are administered
as three
daily injections on days 1, 2, and 3, or as two injections on days 1, and 1 I.
In the second study
vehicle control for discodermolide is administered as a single injection on
day 1.
Tumors are measured, and individual animal body weights are recorded once
weekly.
25 Standard experiments are conducted for 3 full weeks from the initial
dosing.
To assess toxicity of discodermolide on non-tumor bearing animals, four groups
of 8
naive nude mice are dosed with the compound, iv, once (Smg/kg, 7.5 mg/kg, 10
mg/kg, or 15
mg/kg). The control group is dosed with the vehicle alone (16.7% Cremophor EL,
8.3% ethanol
and 75% DSW). Body weights are recorded once weekly.
3o Calculations of results
Antitumor activity is expressed as % T/C (comparing 0 tumor volumes for
treatment
group to vehicle control group). Regressions are calculated using the formula:
(1-T/To) x 100%,
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
14
where T is the tumor volume for the treatment group at the end of the
experiment, and To is the
tumor volume at the beginning of the experiment.
Statistical significance of the results is uniformly evaluated using a one-
tailed Student's
t-test following analysis of our representative experiments.
Results
A. Subcutaneous HCT-15 colon tumor model
Results are determined for the first experiment in the HCT-1S colon tumors
with
discodermolide administered at: (i.) 30 mg/kg dosed as two 1S mg/kg injections
on day 1 of the
experiment, (ii.) 30 mg/kg dosed as three 10 mg/kg injections on days 1, 2,
and 3, (iii.) 15 mg/kg
to dosed as a single injection on day l, (iv.) 20 mg/lcg dosed as a 15 mg/kg
injection on day 1,
followed by a 5 mg/kg injection on day 1 l, and (v.) 10 mg/lcg dosed as a 7.5
mg/kg injection on
day 1 followed by a 2.S mg/lcg injection on day 1 l.. All animals dosed with
30 mg/kg of
discodermolide administered as three 10 mg/kg injections on days 1, 2, and 3,
are sacrificed in
the beginning of the third week of the experiment due to excessive body weight
loss. Four
15 animals from the group dosed 20 mg/kg administered as a 15 mg/kg injection
on day 1, followed
by a 5 mg/lcg injection on day 11, die in the second week of the experiment.
The remaining four
animals from this group are sacrificed due to the excessive body weight loss.
Discodermolide
administered as a single 15 mg/kg injection on day 1 produces 43% T/C with
18.9% body
weight loss. Two 1S mg/kg injections on day 1 (30 mg/kg total dose) results in
22% TIC, and
20 24.6% body weight loss. Administration of the compound at 10 mg/kg, as a
7.S mg/kg injection
on day 1 followed by a 2.5 mg/kg injection on day 11, gives 31% T/C associated
with 17.3%
body weight loss. All antitumor efficacy results are statistically significant
(p < 0.01). Paclitaxel,
administered at 15 mg/kg, daily, for the first 5 days, is inactive in this
experiment (95% T/C). In
the second study discodermolide is dosed as one injection on the first day of
the experiment, at
25 2.5 mg/kg, S mg/lcg, 7.5 mg/kg, 10 mg/kg, 12.5 mg/kg, or 15 mg/kg. In
addition the 7.5 mg/kg
injection on day 1 followed by a 2.5 mglkg injection on day 11 is repeated
from the first study.
Discodermolide dosed at 10 mg/kg, administered as a 7.5 mg/kg injection on day
1 followed by
a 2.5 mg/kg injection on day 11, gives 29% T/C with 11.4% body weight loss.
Single injections
of 2.5 mg/kg, S mg/kg, 7.5 mg/kg, 10 mg/kg, 12.5 mg/lcg, or 15 mg/kg
discodermolide on day 1
30 of the experiment produces 62% T/C, 44% T/C, 43% T/C, 40% T/C, 28% T/C, and
27% T/C,
respectively. Corresponding body weight changes are 3.5% (gain), -1.9%, -7.9%,
-8.S%, -12.9%,
and -11.0%. All antitumor efficacy results are statistically significant (p <
0.01). In the second
study paclitaxel is inactive (94% T/C). Repeat of the dose response in the
third study, produces
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
results similar to those obtained in the second experiment. Single injections
of 2.5 mg/kg, 5
mg/kg, 7.5 mg/kg, 10 mg/kg, 12.5 mg/kg, or 15 mg/kg discodermolide on day 1 of
the
experiment produces 54% T/C, 43% T/C, 23% T/C, 27% T/C, 25% T/C, and 19% T/C,
respectively. Corresponding body weight changes are 1.7% (gain), -8.9%, -
23.3%, -17.6%, -
20.1%, and -13.4%. All antitumor efficacy results are statistically
significant (p < 0.01). In the
third study paclitaxel is inactive (81% T/C). Results for the HCT15 xenograft
model and
corresponding mean body weights are provided in Figure 2.
B. Subcutaneous MIP 101 colon tumor model.
Results for the first experiment in the MIP 101 colon tumors with
discodermolide is
to determined for data points gathered from mice administered: (i.) 30 mg/kg
dosed as two 15
mg/kg injections on day 1 of the experiment, (ii.) 30 mg/kg dosed as three 10
mg/kg injections
on days 1, 2, and 3, (iii.) 15 mg/kg dosed as a single injection on day 1,
(iv.) 20 mg/kg dosed as
a 15 mg/kg injection on day 1, followed by a 5 mg/kg injection on day 11, and
(v.) 10 mg/kg
dosed as a 7.5 mg/kg injection on day 1 followed by a 2.5 mg/kg injection on
day 11. All
15 animals dosed with 30 mg/kg of discodermolide administered as three 10
mg/kg injections on
days 1, 2, and 3, die on day 5 of the experiment. All animals from the group
dosed with 20
mg/kg administered as a 15 mg/kg injection on day 1, followed by a 5 mg/kg
injection on day 11
are sacrificed in the beginning of the third week of the experiment due to the
excessive body
weight loss. Discodermolide is administered as a single 15 mg/kg injection on
day 1 produced
36% T/C (treated vs. control) with 18.9% body weight loss. Two 15 mg/lcg
injections on day 1
(30 mg/kg total dose) result in 24% T/C, and 23.4% body weight loss.
Administration of the
compound at 10 mg/kg, as a 7.5 mg/kg injection on day 1 followed by a 2.5
mg/kg injection on
day 11, gives 38% T/C associated with 21.3% body weight loss. All antitumor
efficacy results
are statistically significant (p < 0.01 ). Paclitaxel, administered at 15
mg/kg, daily, for the first 5
days, is inactive in this experiment (82% T/C). In the second study
discodermolide is dosed as
one injection on the first day of the experiment, at 2.5 mg/kg, 5 mglkg, 7.5
mg/kg, 10 mg/lcg,
12.5 mg/kg, or 15 mg/kg. In addition the 7.5 mg/kg injection on day 1 followed
by a 2.5 mg/lcg
injection on day 11 is repeated from the first study. Results are presented
graphically in Figure 2.
Discodermolide dosed at 10 mg/kg, administered as a 7.5 mg/kg injection on day
1 followed by
3o a 2.5 mg/kg injection on day 11, gives 35% T/C with 11.4% body weight loss.
One animal in
that group dies from apparent drug toxicity. Single injections of 2.5 mg/kg, 5
mg/kg, 7.5 mg/kg,
10 mg/kg, I2.5 mg/kg, or 15 mg/kg discodermolide on day 1 of the experiment
produces 59%
T/C, 57% T/C, 46% T/C, 37% TIC, 22% T/C, and 18% T/C, respectively.
Corresponding body
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
16
weight losses are 7.9%, 10.2%, 13.2%, 18.7%, 1 S.5%, and 11.7%. All antitumor
efficacy results
are statistically significant (p < 0.01 ). In the second study paclitaxel
showed statistically
significant inhibition of tumor growth (54% T/C, p < 0.01). Results are
summarized in Figure 3.
C. Toxicity of discodermolide in non-tumor bearing animals.
Naive animals are dosed with 5, 7.5, 10, or 15 mg/kg of discodermolide
administered as a single
injection of the experiment. One week after dosing animals demonstrate the
following body
weight changes: +8.7%, +1.2%, -1.2%, and-4.1%, respectively. Two weeks after
dosing
corresponding body weight changes are +9.4%, +3.2%, +1.2%, and -0.4%. Three
weeks after
dosing all animals gained weight as follows: +9.4%, +7.7%, +5.6%, and +5.9%.
Animals dosed
to with the vehicle alone demonstrate the following body weight changes in
each week of the
experiment: +5.5%, +4.7%, and +5.9%. After a single iv administration the
compound caused
only minimal, and transient, body weight loss in these animals, and only at l
Omg/kg, and 15
mg/kg doses.
D. Discussion
15 In both colon models, HCT-15, and MIP 101, discodermolide, administered as
single
injection, demonstrated a dose-dependent, statistically significant inhibition
of tumor growth at
all doses between 2.5 mg/kg and 15 mg/kg. The HCT-15 model is totally
refractory to treatment
with paclitaxel, while the MIP 101 model shows no response in the first study
(82% T/C),
although in the second study paclitaxel produces a modest, but statistically
significant inhibition
20 of tumor growth (54% T/C, P < 0.01).
For both tumor models only two dosing schedules from the first study are
repeated in the
second experiment; the repeated schedules demonstrate good reproducibility
between the two
experiments. In the HCT-15 model a single 15 mg/kg injection of discodermolide
produces 43%
T/C in the first study, and 27% T/C in the second study. A total dose of 10
mg/kg administered
25 as two injections, a 7.5 mg/kg on day 1 followed by a 2.5 mg/kg on day 11
resulted in 31% T/C
in the first study, and 29% T/C in the retest. In the MIP 101 model a single I
S mglkg inj action of
the compound gave 36% T/C in the first experiment, and 18% T/C in the retest.
Discodermolide
at a total dose of 10 mg/kg administered as two injections, a 7.5 mg/kg on day
1 followed by a
2.5 mg/kg on day 11 produces 38% T/C in the first study, and 35% T/C in the
retest.
3o In the HCT-15 model the dose response study is repeated in the third
experiment.
Antitumor efficacy of the discodermolide demonstrates good reproducibility for
all doses
between 2.5 mg/lcg, and 15 mg/kg (62% T/C and 54% T/C for 2.5 mg/kg, 44% T/C
and 43%
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
17
T/C for 5 mg/kg, 43% TlC and 23% T/C for 7.5 mg/kg, 40% T/C and 27% T/C for 10
mg/kg,
28% T/C and 25% T/C for 12.5 mg/kg, 27% T/C and 19% T/C for 15 mglkg). In the
repeat of
the dose response study body weight losses are higher, reaching 23%, compared
to 13% in the
original study.
In conclusion, discodermolide shows dose-dependent antitumor efficacy in two
known
multidrug resistant tumor lines grown as xenografts in nude mice.
Example 3
Ih vitro and Ih vivo antitumor effect of discodermolide to paclitaxel
resistant cells having 13-
tubulin mutation.
to Cell lines and tissue culture
All cell lines are free of Mycoplasma contamination (Rapid Detection System by
Gen-
Probe, Inc., San Diego, CA). The LS 174T human colon tumor cell line is
purchased from the
American Type Culture Collection, Rockville, MD. The 1A9 and the lA9PTX22
ovarian tumor
cell lines are obtained from Dr. T. Fojo, Medicine Branch, Division of
Clinical Sciences,
15 National Cancer Institute, National Institutes of Health, Bethesda, MD
20892. The 1A9 is a
clone of the ovarian carcinoma cell line, A2780 (Eva A, et al. Nature 1982,
295:116-119.). The
lA9PTX22 subline is isolated as an individual clone from the 1A9 cell line in
a single step
selection by exposure to 5 ng/mL paclitaxel in the presence of 5 ~,g/mL
verapamil. The
lA9PTX22 cell line is found to be 24-fold more resistant to paclitaxel than
the parental 1A9
20 (Giannakakou P, et al., J. Biol. Chem. 1997, 272(4):17118-17125).
Resistance to paclitaxel is
maintained following 2 years of culturing in a drug-free media, and was
attributed to the A1a364
-> Thr mutation in (3-tubulin that is found in the lA9PTX22 cell line. All
cell lines are
propagated and expanded in RPMI 1640 medium containing 10% heat-inactivated
FBS (Life
Technologies, Grand Island, N~ in a tissue culture incubator (37 °C,
controlled, humidifed
25 atmosphere containing 5% COa). Cell expansions are performed in T75 tissue
culture flasks
(COSTAR, Corning, N~. For hollow fiber preparations, cells are harvested at 70-
90% confluency
using 0.25% Trypsin-EDTA (Life Technologies, Grand Island, N~
In vitro cytotoxicity assay
1A9 and lA9PTX22 cells are plated in 96-well plates at 5x104 cells/well,
placed in a
3o tissue culture incubator and allowed to attach overnight. The next morning,
the number of
viable cells in the "time 0" plate (3 wells for each cell line) is determined
using an MTT assay
(Alley MC, et al., Cauce~ Res. 1988, 48:589-601).
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
18
At the same time drugs are added in serial, I O-fold dilutions, to the
experimental plates.
Corresponding vehicles are added to control plates. Experimental and control
plates are then
incubated in the tissue culture incubator for 72 hours. After the incubation
the number of viable
cells is determined in each plate using the MTT assay. ICSOS (defined as a
concentration of a
given compound causing 50% inhibition of cell growth) are determined by
comparing cell
growth in the drug-treated plate (T-To) to the cell growth in the
corresponding control plate (C-
To). For each experiment results are calculated using average numbers from two
sets of plates.
Preparation of hollow fibers
PVDF hollow fibers (Spectrum, Gardena, CA) are soaked in 70% EtOH for 72 hours
to before use. After this step, all handling of fibers are done under a
biological laminar flow hood
using aseptic procedures. Individual fibers are flushed with 3mL of the ice-
cold tissue culture
media using a syringe equipped with a 20-gauge needle. Next, each fiber is
filled with an
appropriate cell suspension (1x106 cells/mL for the 1A9 and lA9PTX22 cells,
and 0.5x106
cells/mL for the LS 174T cells), and both ends of the fiber are sealed with a
hot flat needle
15 holder. The entire length of the fiber is then sealed into 1.5 cm
microcapsules (further called
"hollow fibers"), each containing approximately I5 uL of the appropriate cell
suspension. After
separation, individual hollow fibers are placed in 6 well plates (6 fibers in
5 mL media per well),
and are incubated overnight at 37 °C in the tissue culture incubator.
Implantation of hollow fibers
2o Outbred athymic (nulnu) female mice ("Chrls:Athymic Nude-nu", Charles River
Laboratories, Wilmington, MA) are anesthetized with ip injections of
Ketamine/Xylazine (150
mg/kg, and 12 mg/kg body weight, respectively). For the subcutaneous
implantation an 11-gauge
trocar containing one or two hollow fibers is inserted into an incision made
with scissors at the
nape of the neck of an animal, and fibers are released by retracting the
trocar while depressing
25 the plunger. This procedure is repeated until all three hollow fibers are
implanted. One wound
clip is used to close the skin incision After the surgery each animal receives
a single,
subcutaneous injection of 0.4 mg/kg butorphenol to relieve any potential pain.
Animals recover
from the anesthesia on a heating pad, before returning to their cages.
In vivo Hollow Fiber Assay
3o One day after the implantation (3 hollow fibers/animal, each hollow fiber
containing one
cell line: LS 174T, 1A9, and lA9PTX22) animals are randomly sorted into five
groups of six
mice/group. The first group is sacrificed; hollow fibers are retrieved, and
processed according to
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
19
a published procedure (Hollingshead MG, et al. Life Sciences 1995, 57(2):131-
141) , to
determine the number of viable cells in each fiber (To.)
The remaining groups are treated as follows:
Group 1: Discodermolide, 15 mg/kg, iv, once.
Group 2: Vehicle for discodermolide (16.7% Crem. EL, 8.3% Ethanol, 75% DSW),
iv, once.
Group 3: Paclitaxel, 15 mg/lcg, iv, daily for 5 days.
Group 4: Vehicle for paclitaxel (12.5% Cremophor EL, 12.5% Ethanol, 75% DSW),
iv, daily for
5 days.
On day 6 alI animals are sacrificed, and hollow fibers are retrieved, and
processed according to
1o Hollingshead, MG, (supra) to determine the number of viable cells in each
fiber (T- for fibers
from animals treated with experimental compounds, C - for fibers from animals
treated with
corresponding vehicles). Antitumor activity is expressed as % Mean t1T / Mean
t1C [comparing
cell growth for treatment group to vehicle control group, where % Mean OT /
Mean ~C = (Mean
T - Mean To / Mean C - Mean To) x 100%]. Regressions are calculated using the
formula: (1-
Mean T / Mean To) x 100%. Statistical significance of the results is uniformly
evaluated using a
two-tailed Student's t-test.
Experimental compounds and formulations
Discodermolide is isolated from the sponge Discodermia dissoluta using the
method of
Gunasekera SP (supra). Multiple batches of compound of similar purity (all >
95% pure, as
determined by mass spectrometry, and nuclear magnetic resonance analysis) are
used throughout
the various studies. For the in vitro cytotoxicity assays all compounds are
dissolved in DMSO and
are added to the plates with cells to obtain desired concentrations. The
amount of DMSO in cell
cultures did not exceed 0.1% v/v. Paclitaxel is purchased from Sigma/Aldrich,
(St. Louis, MO).
For the in vivo hollow fiber assay, solid discodermolide is dissolved in pure
ethanol to create a
stock solution which is diluted just before dosing with Cremophor EL and DSW
to a final
concentration of 16.7% Cremophor EL, 8.3% ethanol and 75% DSW. The compound is
administered to mice as a single, 15 mg/kg iv injection. Positive control
animals receive clinical
formulations of paclitaxel (TAXOL) diluted 4-fold with DSW (12.5% Cremophor
EL, 12.5%
ethanol and 75% DSW final concentrations) and administered iv, at 15 mg/kg,
once daily for five
3o consecutive days. Vehicle controls are administered according to the
corresponding drug
schedules.
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
Results
In vitro cytotoxicity
Results of the first experiment are summarized in Figure 5, however, all
experiments
were performed in duplicate. In the 1A9 cell line the ICsos for paclitaxel,
and discodermolide
are 0.8 ng/ml, and 6 ng/ml, respectively. In the paclitaxel-resistant lA9PTX22
cell line, the
corresponding ICsos are: IS ng/ml, and 3 ng/ml.. In the 1A9 cell line ICsos
for, paclitaxel, and
discodermolide are 0.4 ng/ml, and 3 ng/ml, respectively. In the paclitaxel-
resistant lA9PTX22
cell line the corresponding ICsos are 9 ng/ml, and 3 ng/ml.
Ih vivo hollow fiber assay
1o Results of the antitumor activity of paclitaxel and discodermolide against
three human
solid tumor cell lines subcutaneously implanted into nude mice in hollow
fibers are summarized
in Figure 4. Paclitaxel, dosed iv, at 15 mg/kg, once daily, for 5 days
produced TlC of 3%, -8%,
and 79%, in LS 174T, 1A9, and lA9PTX22 cell lines, respectively.
Discodermolide,
administered as a single, 15 mg/kg, iv injection, gave 7%, 8%, and -13% T/C in
the respective
15 cell lines. Paclitaxel, dosed iv, at 15 mg/kg, once daily, for 5 days
produced T/C of 8%, 2%, and
90%, in LS 174T, 1A9, and lA9PTX22 cell lines, respectively. Discodermolide is
administered
as a single, 15 mg/kg, iv injection, gave 13%, I4%, and 13% T/C in the
respective cell lines. In
both experiments animals dosed with paclitaxel lost 5% of their body weights,
and animals
dosed with discodermolide lost 10% of their body weights.
20 Discussion
The lA9PTX22 cell line was derived from a 1A9 clone of an ovarian carcinoma
cell line A2780
(Eva A, et al. Nature 1982, 295:116-119) by exposure to paclitaxel
(Giannakakou P, et al. J.
Biol. Chem. 1997, 272(4):17118-17125). The lA9PTX22 cell line shows 24-fold
resistance to
paclitaxel iu vitro, compared to the parental 1A9. This level of resistance is
maintained after the
cell line is cultured for 2 years in the absence of paclitaxel. It was shown
that the lA9PTX22 cell
line contains an Ala3s4 -> Thr mutation in J3-tubulin, and that paclitaxel
does not induce
polymerization of the mutated tubulin prepared from the lA9PTX22 cells
(Giannakakou P.,
supra). Taken together these data suggest that mutations in (3- tubulin are
likely responsible for
the resistance to paclitaxel.
Here we examine ih vitro, and in vivo sensitivity of the 1A9 and lA9PTX22 cell
lines to
discodermolide, a natural product, that, like paclitaxel, exerts its
cytotoxicity by stabilizing
tubulin polymers. In two, separate in vitro cell growth inhibition
experiments, both, 1A9 and
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
21
lA9PTX22 cell lines showed similar sensitivity to discodermolide (for 1A9
ICsos were 6 ng/mL,
and 3 ng/mL, and for lA9PTX22 ICsos were 3ng/mL for both experiments). In
contrast, the
lA9PTX22 cell line is 20-fold more resistant to paclitaxel then the parental
1A9 cells (for IA9
cells ICsos were 0.8 ng/mL, and 0.4 ng/mL, and for lA9PTX22 cells ICSOS are 15
ng/mL, and 9
ng/mL). Doxorubicin, used as a mechanistically unrelated cytotoxic control,
produced the ICsos
of 3 ng/mL for 1A9 cells, and 3-5 ng/mI for lA9PTX22 cells, showing that the
latter is only
slightly (2 fold) less sensitive to this compound than the parental 1A9 cell
line. In order to
determine how the in vitro sensitivity of the paclitaxel-resistant lA9PTX22
cell line to
discodermolide translates into the in vivo response, the compound is tested in
the hollow fiber
assay. A third cell line, colon carcinoma LS 174T is used in this experiment
as an additional
positive control. Both discodermolide and paclitaxel are dosed iv, at optimal
concentrations and
dosing schedules as described in Example 2. Discodermolide is administered
once, at 15
mg/kg, and paclitaxel is administered once daily for 5 days at 15 mg/kg. The
lA9PTX22 cell
line is sensitive to treatment with discodermolide (13% regression in the
first study, and 13%
T/C in the retest, both p < 0.01), but is completely refractory to paclitaxel
(79% T/C in the first
study, and 90% T/C in the retest, both p > 0.05). The LS 174T cell line is
equally sensitive to
both compounds. These results suggest that discodermolide can provide an
effective therapy
against tumors resistant to paclitaxel due to mutations in tubulin.
Example 4
Discodermolide induces Raf 1 phosphorylation
Cell culture conditions:
A549, a human non-small cell lung carcinoma and MDA-MB-435, a human
breast carcinoma, used in this study are obtained from the American Type
Culture Collection
(ATCC, Rockville, MD, USA). MDA-MB-435 cells are maintained in MEM containing
10%
FBS, 1% sodium pyruvate, 1% MEM non-essential amino acids, and 15 mM HEPES
(pH=7.4).
A549 cells are maintained in RPMI 1640 containing 10% FBS. 1A9, a single-cell
clone of the
human ovarian carcinoma cell line A2780 and PTX22, the paclitaxel-resistant
subline, used in
this study are obtained from M. Wartman (Novartis Pharmaceuticals). 1A9 cells
and PTX22
cells are maintained in RPMI 1640 supplemented with 10% FBS. PTX22 maintenance
media
also contained l5ng/mL paclitaxel and S~g/mL verapamil. Drug is removed from
the media for
5-7 days before use in an experiment. All maintenance media contained 100
units/mL penicillin
and 100 ~,g/mL streptomycin.
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
22
Antiproliferative assays:
Cell lines are trypsinized and counted using a Coulter counter. Cells were
plated in 96
well plates (190 ~L/well) at the following densities: 1,000 cells/well for
A549 and 3,000
cells/well for MDA-MB-435. The number of cells plated results in cell
densities of 75-90%
confluence by the time of harvest. Plates are seeded on day 0. On day 1 test
compounds are
added to triplicate wells in a final volume of 10 ~L media. Initial cell
density for each cell line
is measured on day 1 by adding 10 ~,L MTS mixture (see below), incubating for
4 h and
recording absorbance at 490 nm (A490). Two or three days after test compound
addition, 10
~L/well of MTS mixture is added to the test plates and A49o was read 4 h
later. A49o values for
to wells containing cells are corrected for media absorbance, then normalized
to initial density
readings to determine percent net growth. Percent net growth is calculated as
(A49o + drug -
Aa9o initial)/(A49o - drug - A49o initial). Graphs of percent net growth as a
function of compound
concentration are used to calculate concentrations resulting in 50% growth
inhibition (ICso).
MTS mixture is prepared fresh on day of addition to cell plates at a ratio of
10 ~,L of a 0.92
mg/mL solution of phenazine methosulfate (PMS) to 190 ~L of a 2 mg/mL solution
of MTS (3-
(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H
tetrazolium,
inner salt). PMS and MTS solutions are prepared in buffered saline containing
0.2 g/L KCI, 8.0
g/L NaCI, 0.2 g/L KH2P04, 1.15 g/L NaaHP04 , 133 mg/L CaCl2 ~ 2 H20, 100 mg/L
MgCla ~ 6
H20, (pH = 7.35) and stored as foil-wrapped aliquots at -20 °C. Test
compounds are prepared as
2o stock solutions in DMSO. Test compound dilutions are made in 2% DMSO/cell
maintenance
media and diluted into assay plates to give 0.1% DMSO final in all wells.
Western Blots:
Cells are plated at a density of 1.5 x 106 cells per 100 mm plate. The next
day cells are
treated with vehicle control (0.1% DMSO) or test compound for 24 h. Cells are
harvested by
washing monolayers twice with PBS, then lysing with 300 u1 of lysis buffer[20
mM Tris (pH
8.0), 2 nM EDTA, 100 mM NaCI, 0.5% NP40, 0.0125% DOC, 2.5% glycerol, 1 mM
vanadate,
25 mM sodium fluoride and protease inhibitor cocktail (1:500 dilution,
Sigma)]. Lysates were
spun at 12,000 x g and the supernatant transferred to a new tube. Protein
concentration of the
lysates is determined using BCA protein assay Reagent (Pierce). Samples (75
~.g) are resolved
by SDS-PAGE on a 7.5% or 14% tris-glycine gel for Raf 1 and Bcl-xL,
respectively, and
transferred to nitrocellulose. Immunodetection is performed as described by
Amersham Vistra
Fluorescence Western blotting Kit directions with the following modifications:
the membrane is
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
23
blocked with 5% milk (Carnation Non-Fat Dry Milk) in buffer containing 20 mM
Tris~HCI
(pH=7.4), 100 mM NaCI, and 0.2% Tween 20 (TBST) overnight and is subsequently
incubated
for 2 h with primary antibody (Santa Cruz C-12 for Raf 1 and Santa Cruz H-62
for Bcl-xL),
followed by fluorescein-linked anti-rabbit Ig in 5% milk and anti-fluorescein
alkaline
s phosphatase conjugate in TBST at 1:500 and 1:2000 dilutions, respectively. A
Storm 860
(Molecular Dynamics) is used to detect fluorescent product according to the
manufacturer's
instructions.
Results
1o The effects on cell proliferation following a 72 h exposure to
discodermolide was
measured by MTS assays. The ICSO values are determined in two independent
experiments to be
45 and 60 nM for A549 cells, 3 and 15 nM for MDA-MB-435 cells, 10 and 39 nM
for 1A9 cells
and 23 and 37 nM for PTX-22 cells. Cells are treated with 50, 100 and 180 nM
discodermolide
and MDA-MB-435 cells are treated with 2, 20, 50 and 90 nM discodermolide to
determine the
15 effects of discodermolide treatment on Raf 1 and Bcl-xL phosphorylation.
The paclitaxel
concentration used as a positive control in the study was the same as used in
Example 2.
Both Raf 1 and Bcl-xL are phosphorylated following 24 hour treatment of A549
and
MDA-MB-435 cells with paclitaxel or discodermolide. Phosphorylation of Raf 1
is observed by
2o the appearance of additional bands migrating more slowly and
phosphorylation of Bcl-xL; is
observed by the broadening of a single band. Raf 1 phosphorylation is
concentration-dependent,
since in A549 and MDA-MB-435 cells the doublet is only observed at the higher
concentrations
tested. These results are consistent with previous studies in which the
effects of paclitaxel on
Raf 1 phosphorylation are also shown to be concentration dependent (Tortes K
and Horwitz SB.
25 Cancer Res. 1998:58:3620-3626). Interestingly, Raf 1 phosphorylation is not
required for cell
death since low concentrations of paclitaxel led to apoptosis without Raf 1
phosphorylation
perhaps through p21 and or p53 mediated apoptotic pathways. The minimum
paclitaxel
concentration required for Raf 1 phosphorylation coincides with the induction
of the G2/M
block suggesting that Raf 1 activation may be a component of the signal
cascade activated
3o during the mitotic checkpoint. Since the discodermolide concentration
required to induce Raf 1
phosphorylation was greater than the ICSO value, it is likely that similar
concentration specific
discodermolide activities also exist. For example, treatment of A549 and MDA-
MB-435 cells at
the ICso values is likely to induce cell death but not Raf 1 phosphorylation.
CA 02401104 2002-09-16
WO 01/74355 PCT/US00/08904
24
In order to determine whether discodermolide is potentially potent on tumor
cells that are
resistant to paclitaxel, we examined the effects of discodermolide on 1A9
ovarian carcinoma
cells and a paclitaxel resistant subline, PTX-22. Paclitaxel resistance is not
due to reduced
paclitaxel accumulation but is associated with failure of tubulin
polymerization in cells, cellular
extracts or purified tubulin . This subline is reported to be 20-30-fold less
sensitive to paclitaxel
but does retain sensitivity to Vinca alkaloids. The paclitaxel 72 hour IC50
value shifts from 6 nM
on 1A9 cells to 80 nM on PTX-22 cells (13-fold less sensitive). Interestingly,
discodermolide
shows no cross-resistance as measured by ICSO, which are 25 and 30 nM on 1A9
and PTX-22
to cells, respectively. Raf 1 is phosphorylated in 1A9 parental cells treated
with both paclitaxel and
discodermolide and in the PTX-22 cells treated with discodermolide., Raf 1
phosphorylation is
not observed in the PTX-22 cells following paclitaxel treatment. These studies
suggest that
discodermolide may be useful in treating tumors that are paclitaxel-resistant.