Note: Descriptions are shown in the official language in which they were submitted.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-1-
5-ALKYLPYRIDO[2,3-d]PYRIMIDINES TYROSINE KINASE INHIBITORS
FIELD OF THE INVENTION
This invention xelates to 5-alkylpyridopyrimidines as inhibitors of cyclin-
dependent kinases, particularly cyclin-dependent kinase 4. The compounds of
the
invention are useful for the treatment of inflammation, cell proliferative
diseases
such as cancer and restenosis, and neurodegenerative diseases such as
Alzheimer's disease.
SUMMARY OF THE RELATED ART
Cyclin-dependent kinases and related serine/threonine protein kinases are
cellular enzymes that perform essential functions in regulating cell division
and
proliferation. The cyclin-dependent kinase catalytic units, of which nine have
been
identif ed, are activated by regulatory units known as cyclines. The cyclin-
dependent kinases include (Cdlc) Cdkl, Cdlc2, Cdk4, CdkS, Cdk6, and Wee-
1 kinase. Increased activity or temporally abnormal activation of these
kinases
results in development of human tumors and other proliferative disorders such
as
restenosis. Compounds that inhibit Cdks, either by blocl~ing the interaction
between a cyclin and its lcinase partner, or by binding to and inactivating
the
kinase, cause inhibition of cell proliferation and thus are useful for
treating tumors
and other abnoi~nally proliferating cells.
Several compounds that hW ibit Cdks have demonstrated both preclinical
and clinical anti-tumor activity. For example, flavopiridol is a flavonoid
that is a
potent inhibitor of Cdk2 and Cdk4, and has been shown to inhibit several types
of
breast and lung cancer cells (Kaur et al., .l. Natl. Cancer Ir~st.,
1992;84:1736-1740;
Kaur et al., Int. J O~col., 1996;9:1143-1168). In addition, Olomoucine
[2-(hydroxyethylamine)-6-benzylamine-9-methylpurine] is a potent inhibitor of
Cdk2 and CdkS (Vesely et al., Eu~. J. Biochefn., 1994;224:771-786), and has
been
shown to inhibit proliferation of approximately 60 different human tumor cell
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
_2_
lines used by the National Cancer Institute (NCI) to screen for new cancer
therapies (Abraham et aL, Biol. Cell, 1995;83:105-120).
In addition to treating cancer, Cdk inhibitors have been shown to treat
cardiovascular disorders such as restenosis and atherosclerosis. Other
diseases in
which Cdk inhibitors are useful include those caused by a variety of
infectious
agents, including DNA and RNA viruses, and inflammatory disorders such as
rheumatoid arthritis.
An object of this invention is to provide a group of small molecular weight
organic compounds that are potent Cdk inhibitors, and as such are useful for
preventing and treating diseases caused by abnormally proliferating cells.
SUMMARY OF THE INVENTION
This invention provides 5-alkyl pyridopyrimidines that are useful for
treating inflammation, cell proliferative diseases such as cancer and
restenosis,
and neurodegenerative diseases such as Alzheimer's disease. The compounds of
the invention display unexpected improvements in pharmacokinetic properties
over prior art compounds, including unanticipated metabolic stability and low
clearance rates. In addition, the compounds of the invention are unexpectedly
selective inhibitors of Cdk4. The compounds of the invention are readily
synthesized, and can be administered to patients by a variety of methods.
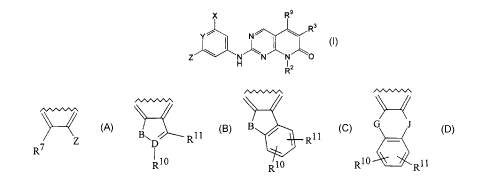
The compounds of the invention are those having the structure of
Formula I:
X R9
Y~ N~ ~ Rs
Z N N N O
~2
R
and pharmaceutically acceptable salts, esters, amides, and prodrugs thereof,
wherein:
R2 is (a) hydrogen;
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-3-
(b) lower alkyl optionally substituted with one, two, or three groups
independently selected from halogen, hydroxy, lower alkoxy,
amino, mono- or dialkylamino, carboxy, alkoxycarbonyl, thio
alkyl, nitrite, aryl, heteroaryl, or a carbocyclic group containing
from 3 to 7 members, up to two of which members are optionally
heteroatoms independently selected from oxygen, sulfur, and
nitrogen; or
(c) a carbocyclic group containing from 3 to 7 members, up to two of
which members are optionally heteroatoms independently selected
from oxygen, sulfur, and nitrogen, wherein the caxbocyclic group is
unsubstituted or substituted with one, two, or three groups
independently selected from halogen, hydroxy, lower alkyl, lower
alkoxy, amino, mono- or dialkylamino, aryl, and heteroaryl;
R3 is hydrogen, lower alkyl, lower alkoxy, halogen, trifluoromethyl, lower
alkynyl, lower alkenyl, nitrite, vitro, -COR4, -C02R4, -CONR4R5,
CONORS, -S02NR4R5, -S02R4, -S03R4, P(O)(OR4)(ORS), or
R4
-NR'1R5;
Y is N or CRS;
R9 is lower alkyl, haloallcyl, or aryl;
X and Z are independently hydrogen, halogen, lower alkyl, lower alkoxy,
trifluoromethyl, hydroxy, nitrite, vitro, -NR4R5, -N(O)R4R5,
-NR4RSR6W, -SR4, -C(O)R4, -C02R4, -CONR4R5, -S02NR4R5,
-S02R4, -S03R4, P(O)(OR4)(ORS), -T(CH2)mQR4,
-C(O)T(CH2)mQR4, or -NR4C(O)T(CH2)mQRS;
m is 1-6;
n is 0-6;
T is O, S, NR4, N(O)R4, NR4R5W, or CR4R5;
Q is O, S, NR4, N(O)R4, NR4RSW, C02, or a carbocyclic group containing from
3 to 7 members, up to four of which members are optionally heteroatoms
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-4-
independently selected from oxygen, sulfur, and nitrogen, wherein the
carbocyclic group is unsubstituted or substituted with one, two, or three
groups independently selected from halogen, hydroxy, hydroxyalkyl,
lower alkyl, lower alkoxy, alkoxycarbonyl, alkylcarbonyl,
alkylcarbonylamino, aminoalkyl, trifluoromethyl, N-hydroxyacetamide,
trifluoromethylalkyl, amino, or mono or dialkylamino;
R6 is lower alkyl, haloalkyl, or aryl;
R~ is NR4R5, N(O)R4R5, NR4RSR9X, OH, OR4, SR4, halo, COR4, (CH2)nR4,
C02R'~, CONR4R5, C(O)NR4S02R5, S(O)R4, S02R4, S02NR4R5,
S03R4, (CH2)nP(O)(OR4)2, NR4S02R5, aldehyde, nitrile, nitro, alkyl,
alkoxyalkyl, T(CH2)mQR4, C(O)T(CH2)mQR4, NR4C(O)T(CH2)mQRS,
or T(CH2)mC02R4;
W is an anion;
R4 and RS are independently hydrogen, lower alkyl, lower alkenyl, lower
alkynyl,
(CH2)nAr, arylalkyl, aryl, heteroaryl, heteroarylalkyl, cycloalkyl,
heterocycloalkyl, or heteroaryl, or R4 and RS together with the nitrogen to
which they are attached form a carbocyclic ring contaiung 3 to
8 members, up to four of which members are optionally carbonyl groups
or heteroatoms independently selected from oxygen, sulfur, S(O), S(O)2,
and nitrogen, wherein the carbocyclic group is unsubstituted or substituted
with one, two, three, or four groups independently selected from halogen,
hydroxy, hydroxyalkyl, lower alkyl, lower alkoxy, alkoxycarbonyl,
allcylcarbonyl, alkylcarbonylamino, aminoalkyl, aminoalkylcarbonyl,
trifluoromethyl, trifluoromethylalkyl, trifluoromethylallcylaminoalkyl,
amino, mono- or dialkylamino, N-hydroxyacetamido, aryl, heteroaryl,
carboxyallcyl, NR10S02R11, C(O)NRlORI 1, NRlOC(O)Rl l, C(O)OR10,
C(O)NRlOS02R11, (CH2)nS(O)nRlO, (CH2)n-heteroaryl,
O(CH2)n-heteroaryl, (CH2)nC(O)NR10R11, O(CH2)nC(O)OR10;
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-5-
and R4 additionally can be lower alkyl unsubstituted or substituted with one,
two,
or three groups independently selected from halogen, 5-oxo-4,5-dihydro-
1H 1,2,4-triazol-3-yl-sulfanyl, 5-oxo-4,5-dihydro-1H 1,2,4-triazol-3-yl-
sulfinyl, 5-oxo-4,5-dihydro-1H 1,2,4-triazol-3-yl-sulfonyl, or a
carbocyclic group containing from 3 to 7 members, up to four of which
members axe optionally heteroatoms independently selected from oxygen,
sulfur, and nitxogen, wherein the caxbocyclic group is unsubstituted or
substituted with one, two, or three groups independently selected from
halogen, hydroxy, hydroxyalkyl, lower alkyl, lower alkoxy,
alkoxycarbonyl, alkylcarbonyl, alkylcarbonylamino, aminoallcyl,
trifluoromethyl, N-hydroxyacetamide, trifluoromethylallcyl, amino, or
mono- or dialkylamino; and when Y is CRS, it is part of the part structure
r.~wn~
wherein R~ and Z are as defined above, or can be taken
Z
R
together with the carbons to which they are attached to form
~.~wv~s~ .~,~w~n~ a~.wwv~
B~D~ R11 G J
Rll or
R10
R1~ R10 R11
wherein:
G and J are independently CH2, NH, or O;
B is NH, S, CH2, or O;
D is C or N, provided that R10 is nothing when D is N; and
R10 and Rl 1 are independently hydxogen, halogen, lower alkyl, lower alkoxy,
or
alkylcarbonyl.
Preferred compounds have Formula I wherein Y is CRS. Of this group,
preferred compounds are those wherein R~ is NR4R5, and R4 and RS are taken
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-6-
together with the N to which they are attached to form a ring such as
piperazine,
piperidine, pyrrolidine, morpholine, each of which can be optionally
substituted.
The present invention also provides pharmaceutical compositions that
comprise a compound of Formula I together with a pharmaceutically acceptable
diluent, carrier, or excipient.
The present invention also provides methods for inhibiting cyclin-
dependent kinase and growth factor-mediated lcinase enzymes.
The present invention also provides a method of treating subjects suffering
from diseases caused by cellular proliferation. The method entails inhibiting
proliferation of tumorigenic cells of epithelial origin and vascular smooth
muscle
proliferation, and/or cellular migration by administering a therapeutically
effective
amount of a compound of Formula I to a subject in need oftreatment.
The invention also provides compounds useful in the diagnosis and
treatment of cancer, psoriasis, vascular smooth muscle cell proliferation
associated with atherosclerosis and postsurgical vascular stenosis and
restenosis in
mammals.
The present invention also provides a method of treating subjects suffering
from diseases caused by DNA tumor viruses such as herpes viruses.
DETAILED DESCRIPTION OF THE INVENTION
The novel compounds encompassed by the instant invention are those
described by the general Formula I set forth above, and the pharmaceutically
acceptable salts, esters, amides, and prodrugs thereof.
In addition to the compounds of Formula I, the invention encompasses, in
a preferred embodiment, compounds of Formula II:
X
3
Y~ ~ N
z \ N~ II
H
wherein X, Y, Z, and R3 are as defined above for Formula I.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
Preferred compounds of Formula II are those in which X and Z are
independently hydrogen, Cl, or F; Y is CRS; and R3 is hydrogen, CI, F, Br, or
CN.
In addition, the present invention also encompasses preferred compounds
of the Formula III:
R3
Z ~ . III
Especially preferred compounds of Formula III are those in which X and Z
are independently hydrogen, Cl, or F; Y is CRS; and R3 is hydrogen, Cl, F, Br,
or
CN.
In addition, the present invention also encompasses, as a further preferred
embodiment, compounds of the Formula IV:
X CH3
N / ~ R3
. IV
Z N N N O
H '
H C"CH
3 3
Preferred compounds of Formula IV axe those in which X and Z are
independently hydrogen, Cl, or F; Y is CRS; and R3 is hydrogen, Cl, F, Br, or
CN.
The most preferred invention compounds have the Formula V
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
_g_
R9
R3
N~
HN N N O
R2
X / Z
R~
wherein:
R2 is alkyl or cycloalkyl;
R3 is hydrogen or halo;
R9 is alkyl;
X and Z independently are hydrogen or halo;
R~ is NR4R5; and
v
R~' and RS are taken together with the nitrogen to which they are attached to
form
a 5- or 6-membered carbocyclic ring, optionally containing an oxygen,
nitrogen, or sulfur heteroatom, and optionally substituted with alkyl or
substituted allcyl groups.
Especially preferred compounds of Formula V are those wherein R~ is
N N N
N
or ~ , and such groups are optionally
O N
H
substituted by alkyl, acyl, amide, or the like.
Unless otherwise expressly stated, the following definitions are adhered to
throughout this disclosure.
By "alkyl," "lower alkyl," and "C 1-C 10 alkyl" in the present invention is
meant a straight or branched hydrocarbon radical having from 1 to 10 carbon
atoms and includes, for example, methyl, ethyl, n-propyl, isopropyl, n-butyl,
sec-butyl, isobutyl, tert-butyl, n-pentyl, iso-pentyl, n-hexyl, and the like.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-9-
By the term "halogen" in the present invention is meant fluorine, bromine,
chorine, and iodine.
"Alkenyl" means straight and branched hydrocarbon radicals having from
2 to 6 carbon atoms and one double bond and includes ethenyl, 3-buten-1-yl,
2-ethenylbutyl, 3-hexen-1-yl, and the like.
"Alkynyl" means straight and branched hydrocarbon radicals having from
2 to 6 carbon atoms and one triple bond and includes ethynyl, 3-butyn-1-yl,
propynyl, 2-butyn-1-yl, 3-pentyn-1-yl, and the like.
"Cycloalkyl" means a monocyclic or polycyclic hydrocarbyl group such as
cyclopropyl, cycloheptyl, cyclooctyl, cyclodecyl, cyclobutyl, adamantyl,
norpinanyl, decalinyl, norbornyl, cyclohexyl, and cyclopentyl. Such groups cam
be
substituted with groups such as hydroxy, keto, amino, alkyl, and dialkylamino,
and the like. Also included are rings in which 1 to 3 heteroatoms replace
carbons.
Such groups are termed "heterocyclyl," which means a cycloalkyl group also
bearing at least one heteroatom selected from O, S, or N, examples being
oxiranyl,
pyrrolidinyl, piperidyl, tetrahydropyran, and morpholine.
By "alkoxy," "lower alkoxy," and "C 1-C 10 alkoxy" is meant straight ox
branched chain alkoxy groups having 1-10 carbon atoms, such as, for example,
methoxy, ethoxy, propoxy, isopropoxy, n-butoxy, sec-butoxy, tert-butoxy,
pentoxy, 2-pentyloxy, isopentoxy, neopentoxy, hexoxy, 2-hexoxy, 3-hexoxy, and
3-methylpentoxy. In addition, alkoxy refers to polyethers such as -O-(CH2)2-O-
CH3, and the Iilce.
"Alkanoyl" groups are alkyl groups linked through a carbonyl, i.e.,
C1-CS-C(O)-. Such groups include fonnyl, acetyl, propionyl, butyryl, and
isobutyryl.
"Acyl" means an alkyl or aryl (Ar) group bonded through a carbonyl
group, i.e., R-C(O)-. For example, acyl includes a C1-C6 alkanoyl, including
substituted alkanoyl, wherein the alkyl portion can be substituted by NR4RSor
a
carboxylic or heterocyclic group. Typical acyl groups include acetyl, benzoyl,
and
3 0 the like.
"Amide" is an amino carbonyl group such as -CONR4R5.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-10-
The alkyl, alkenyl, alkoxy, and alkynyl groups described above are
optionally substituted, preferably by 1 to 3 groups selected from NR4R5,
phenyl,
substituted phenyl, thio C1-C6 alkyl, C1-C6 alkoxy, hydroxy, caxboxy,
C1-C6 alkoxycarbonyl, halo, nitrile, cycloalkyl, and a 5- or 6-membered
carbocyclic ring or heterocyclic ring having 1 or 2 heteroatoms selected from
nitrogen, substituted nitrogen, oxygen, and sulfur. "Substituted nitrogen"
means
nitrogen bearing C1-C6 alkyl or (CH2)nPh where n is 1, 2, or 3. Perhalo and
polyhalo substitution is also included.
Examples of substituted alkyl groups include 2-aminoethyl,
2-hydroxyethyl, pentachloroethyl, trifluoromethyl, 2-diethylaminoethyl,
2-dirnethylaminopropyl, ethoxycarbonylmethyl, 3-phenylbutyl,
methanylsulfanylmethyl, methoxymethyl, 3-hydroxypentyl, 2-carboxybutyl,
4-chlorobutyl, 3-cyclopropylpropyl, pentafluoroethyl, 3-morpholinopropyl,
piperazinylmethyl, and 2-(4-methylpiperazinyl)ethyl.
Examples of substituted alkynyl groups include 2-methoxyethynyl,
2-ethylsulfanylethynyl, 4-(1-piperazinyl)-3-(butynyl), 3-phenyl-5-hexynyl,
3-diethylamino-3-butynyl, 4-chloro-3-butynyl, 4-cyclobutyl-4-hexenyl, and the
like.
Typical substituted allcoxy groups include aminomethoxy,
trifluoromethoxy, 2-diethylaminoethoxy, 2-ethoxycarbonylethoxy,
3-hydroxypropoxy, 6-carboxhexyloxy, and the like.
Further, examples of substituted allcyl, alkenyl, and alkynyl groups include
dimethylaminomethyl, carboxymethyl, 4-dimethylamino-3-buten-1-yl,
5-ethylmethylamino-3-pentyn-1-yl, 4-morpholinobutyl,
4-tetrahydropyrinidylbutyl, 3-imidazolidin-1-ylpropyl, 4-tetrahydrothiazol-3-
yl-
butyl, phenylmethyl, 3-chlorophenylmethyl, and the like.
The term "anion" means a negatively charged counterion such as chloride,
bromide, trifluoroacetate, and triethylammonium.
By "heteroaryl" is meant one or more aromatic ring systems of 5-, 6-, or
7-membered rings containing at least one and up to four heteroatoms selected
from nitrogen, oxygen, or sulfur. Such heteroaryl groups include, for example,
thienyl, furanyl, thiazolyl, triazolyl, imidazolyl, (is)oxazolyl, oxadiazolyl,
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-11-
tetrazolyl, pyridyl, thiadiazolyl, oxadiazolyl, oxathiadiazolyl,
thiatriazolyl,
pyrimidinyl, (iso)quinolinyl, napthyridinyl, phthalimidyl, benzimidazolyl, and
benzoxazolyl. A preferred heteroaryl is pyridine.
By "aryl" is meant an aromatic carbocyclic group having a single ring
(e.g., phenyl), multiple rings (e.g., biphenyl), or multiple condensed rings
in
which at least one is aromatic, (e.g., 1,2,3,4-tetrahydronaphthyl, naphthyl,
anthryl,
or phenanthryl), which can be mono-, di-, or trisubstituted with, e.g.,
halogen,
lower alkyl, lower alkoxy, lower alkylthio, trifluoromethyl, lower acyloxy,
aryl,
heteroaiyl, and hydroxy. A preferred aryl is phenyl.
The term "cancer" includes, but is not limited to, the following cancers:
breast, ovary, cervix, prostate, testis, esophagus, glioblastoma,
neuroblastoma,
stomach, skin, keratoacanthoma, lung, epidennoid carcinoma, large cell
carcinoma, adenocarcinoma, bone, colon, adenocaxcinoma, adenoma, pancreas,
adenocarcinoma, thyroid, follicular carcinoma, undifferentiated carcinoma,
papillary carcinoma, seminoma, melanoma, sarcoma, bladder carcinoma, liver
carcinoma and biliary passages, kidney carcinoma, myeloid disorders, lymphoid
disorders, Hodgkin's, hairy cells, buccal cavity and pharynx (oral), lip,
tongue,
mouth, pharynx, small intestine, colon-rectum, large intestine, rectum, brain
and
central nervous system, and leukemia.
The term "pharmaceutically acceptable salts, esters, amides, and prodrugs"
as used herein refers to those carboxylate salts, amino acid addition salts,
esters,
amide's, and prodrugs of the compounds of the present invention which are,
within
the scope of sound medical judgment, suitable for use in contact with the
tissues
of patients without undue toxicity, irritation, allergic response, and the
like,
commensurate with a reasonable benefit/risk ratio, and effective for their
intended
use, as well as the zwitterionic forms, where possible, of the compounds of
the
invention. The term "salts" refers to the relatively nontoxic, inorganic and
organic
acid addition salts of compounds of the present invention. These salts can be
prepared in situ during the final isolation and purification of the compounds
or by
separately reacting the purified compound in its flee base form with a
suitable
organic or inorganic acid and isolating the salt thus formed. Representative
salts
include the hydrobromide, hydrochloride, sulfate, bisulfate, nitrate, acetate,
oxalate, valerate, oleate, palmitate, steaxate, laurate, borate, benzoate,
lactate,
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-12-
phosphate, tosylate, citrate, maleate, fumarate, succinate, tartrate,
naphthylate
rnesylate, glucoheptonate, lactobionate and laurylsulphonate salts, and the
like.
These may include canons based on the alkali and alkaline earth metals, such
as
sodium, lithium, potassium, calcium, magnesium and the like, as well as non-
toxic
ammonium, quaternary ammonium, and amine cations including, but not limited
to ammonium, tetramethylammonium, tetraethylammonium, methylamine,
dimethylamine, trimethylamine, triethylamine, ethylarnine, and the like. (See,
for
example, Berge S.M. et al., "Pharmaceutical Salts," J. Pharm. Sci., 1977;66:1-
19
which is incorporated herein by reference.)
Examples of pharmaceutically acceptable, non-toxic esters of the
compounds of this invention include C1-C6 alkyl esters wherein the alkyl group
is
a straight or branched chain. Acceptable esters also include CS-C7 cycloalkyl
esters as well as arylalkyl esters such as, but not limited to benzyl. C1-C4
alkyl
esters are preferred. Esters of the compounds of the present invention may be
prepared according to conventional methods.
Examples of pharmaceutically acceptable, non-toxic amides of the
compounds of this invention include amides derived from ammonia, primary
C1-C6 alkyl amines and secondary C1-C6 diallcyl amines wherein the alkyl
groups are straight or branched chain. In the case of secondary amines the
amine
may also be in the form of a 5- or 6-membered heterocycle containing one
nitrogen atom. Amides derived from ammonia, C1-C3 alkyl primary amines and
C 1-C2 dialkyl secondary amines are preferred. Amides of the compounds of the
invention may be prepared according to conventional methods.
The term "prodi-ug" refers to compounds that are rapidly transformed
in vivo to yield the 'parent compound of the above formulae, for example, by
hydrolysis in blood. A thorough discussion is provided in T. Higuclu and V
Stella,
"Pro-drugs as Novel Delivery Systems," vol. 14 of the A.C.S. Symposium Series,
and in Bioreversible Carriers in Drug_Desig-n, ed. Edward B. Roche, American
Pharmaceutical Association and Pergamon Press, 1987, both of which are hereby
incorporated by reference.
Representative compounds of the invention are shown below in Table 1.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-13-
TABLE 1
HN~ CH3 H3C CH3
~N ~ N~ \ F ~ N~ \
\ I ~ I ~ \ f
N N O H N ~ O
H3C CH3
1 4
O
H2N N
N O
ri3~ CH3
NH2
12 13
O O
N N
N N
H H
16 17
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-14
TABLE 1 (cont)
HN~~ c:H2 O
C1 ~ CI \ N
H
19 22
N CH~
O ~ I
Cl O
27
F
O
N N
N N
H H
34 35
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-15-
TABLE 1 (cont)
O
N N
N N
H H
36 37
Br
O
N N
N N
H H
38 40
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-16-
TABLE 1 (cont)
CH3
O
~ ~N
H C "'
3
O
41 45
N \ ~ Br CF3 CH3
~i ~ ~
HNI -N N- ' O
N 1
~N
O
47 54
c,~
c~~
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-17-
TABLE 1 (cont)
F
~2
O
CH2
F3C N F
N O
~2
74 86
O
N N
N N
H
OH
91 102
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-18-
TABLE 1 (cont)
H3C~
N CH2
N
O
110
N-N
O
N S%'~CH CH~
~N
118
H3C O
N
OOH
CHI
N
N
H
129
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-19-
TABLE 1 (cont)
H3C
O
139
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-20-
TABLE 1 (cont)
O
JH
192
202
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-21-
TABLE 1 (cont)
O
222
O/CH3
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-22
TABLE 1 (cont)
CH.,
228
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-23-
TABLE I (cont)
CH~
O
Cl
CH2 O OH 235
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-24
TABLE 1 (cont)
CH.,
NH2 L4b
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-25-
TABLE 1 (cont)
HN~ O
~N~C%-O
CH3
CH3 207
CH2CH3
CH3 \ ~CH3
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-26-
TABLE 1 (cont)
HN
N.
c~
O
O~CH3
CH30
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-27
TABLE 1 (cont)
-CH2CHCl2
~N~ ' O
~O
N"CH
3
CH3
The compounds of the present invention axe useful for treating cancer (for
example, leukemia and cancer of the lung, breast, prostate, and skin such as
melanoma) and other proliferative diseases including but not limited to
psoriasis,
HSV, HIV, restenosis, and atherosclerosis. To utilize a compound of the
present
invention to treat cancer, a patient having cancer is administered a
therapeutically
effective amount of a pharmaceutically acceptable composition comprising an
invention compound.
A further embodiment of this invention is a method of treating subjects
suffering from diseases caused by vascular smooth muscle cell proliferation.
Compounds within the scope of the present invention effectively inhibit
vascular
smooth muscle cell proliferation and migration. The method entails inhibiting
vascular smooth muscle proliferation, and/or migration by administering an
effective amount of a compound of Formula I to a subject in need of treatment.
.
The compounds of the present invention can be formulated and
administered in a wide variety of oral and parenteral dosage forms, including
transdermal and rectal administration. It will be recognized to those skilled
in the
art that the following dosage forms may comprise as the active component,
either
a compound of Formula I or a corresponding pharmaceutically acceptable salt,
ester, amide, prodrug, or solvate of a compound of Formula I.
A further embodiment of this invention is a pharmaceutical composition
comprising a compound of Formula I together with a pharmaceutically acceptable
carrier, diluent, or excipient therefor. For preparing pharmaceutical
compositions
with the compounds of the present invention, pharmaceutically acceptable
carxiexs
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
_2g_
can be either a solid or liquid. Solid form preparations include powders,
tablets,
pills, capsules, cachets, suppositories, and dispensable granules. A solid
caax~ier
can be one or more substances which may also act as diluents, flavoring
agents,
binders, preservatives, tablet disintegrating agents, or an encapsulating
material.
In powders, the carrier is a finely divided solid such as talc or starch which
is in a mixture with the finely divided active component. In tablets, the
active
component is mixed with the carrier having the necessary binding properties in
suitable proportions and compacted in the shape and size desired.
The compositions of this invention preferably contain from about 5% to
about 70% or more of the active compound. Suitable carriers include magnesium
carbonate, magnesium stearate, talc, sugar, lactose, pectin, dextrin, starch,
gelatin,
tragacanth, methylcellulose, sodium.carboxymethylcellulose, a low melting wax,
cocoa butter, and the like. A preferred form for oral use axe capsules, which
include the formulation of the active compound with encapsulating material as
a
carrier providing a capsule in which the active component with or without
other
carriers, is surrounded by a carrier, which is thus in association with it.
Similarly,
cachets and lozenges are included. Tablets, powders, capsules, pills, cachets,
and
lozenges can be used as solid dosage forms suitable for oral administration.
For preparing suppositories, a low melting wax, such as a mixture of fatty
acid glycerides or cocoa butter, is first melted and the active component is
dispersed homogeneously therein, as by stirring. The molten homogenous mixture
is then poured into convenient size molds, allowed to cool, and thereby to
solidify.
Liquid form preparations include solutions, suspensions, and emulsions
such as water or water/propylene glycol solutions. For parenteral injection,
liquid
preparations can be formulated in solution in aqueous polyethylene glycol
solution, isotonic saline, 5% aqueous glucose, and the like. Aqueous solutions
suitable for oral use can be prepared by dissolving the active component in
water
and adding suitable colorants, flavors, stabilizing and thickening agents as
desired.
Aqueous suspensions suitable for oral use can be made by dispersing the finely
divided active component in water and mixing with a viscous material, such as
natural or synthetic gums, resins, methylcellulose, sodium
carboxymethylcellulose, or other well-known suspending agents.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-29-
Also included are solid form preparations that are intended to be
converted, shortly before use, to liquid form preparations for oral
administration.
Such liquid forms include solutions, suspensions, and emulsions. These
preparations may contain, in addition to the active component, colorants,
flavors,
stabilizers, buffers, artificial and natural sweeteners, dispersants,
thickeners,
solubilizing agents, and the like. Waxes, polymers, microparticles, and the
like
can be utilized to prepare sustained-release dosage forms. Also, osmotic pumps
can be employed to deliver the active compound uniformly over a prolonged
period.
The pharmaceutical preparations of the invention are preferably in unit
dosage form. In such form, the preparation is subdivided into unit doses
containing appropriate quantities of the active component. The unit dosage
form
can be a packaged preparation, the package containing discrete quantities of
preparation, such as packeted tablets, capsules, and powders in vials or
ampules.
Also, the unit dosage form can be a capsule, tablet, cachet, or lozenge
itself, or it
can be the appropriate number of any of these in packaged form.
The therapeutically effective dose of a compound of Foxmula I will
generally be from about 1 mg/kg to about 100 mg/kg of body weight per day.
Typical adult doses will be about 50 mg to about 800 mg per day. The quantity
of
active component in a unit dose preparation may be varied or adjusted from
about
0.1 mg to about 500 mg, preferably about 0.5 mg to 100 mg according to the
particular application and the potency of the active component. The
composition
can, if desired, also contain other compatible therapeutic agents. A subject
in need
of treatment with a compound of Formula I is administered a dosage of about
1 mg to about 500 mg per day, either singly or in multiple doses over a 24-
hour
period.
The compounds of the present invention are capable of binding to and
inhibiting the activity of proteins having the ability to phosphorylate other
proteins, such as cdlcs, PDGFr, FGFr, c-Src, and EGFr-FL. Cdks form complexes
with cyclins, and these complexes phosphorylate lcey proteins allowing cells
to
proceed through the cell cycle (Meijer L., Progz°ess i~c Cell Cycle
Reseay~ch,
1995;1:351-363). The compounds of this invention inhibit this phosphorylation
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-30-
and therefore can be used as anti-proliferative agents for the treatment of
cancer
and/or restenosis and other proliferative diseases.
Because of their inhibitory activity against cdks and other kinases, the
compounds of the present invention are also useful research tools for studying
the
mechanism of action of those kinases, both in vitro and in vivo.
The examples presented below are intended to illustrate particular
embodiments of the invention, and are not intended to limit the scope of the
specification or the claims in any way.
An illustration of the preparation of compounds of the present invention is
I O shown in Schemes I and 2.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-3 i-
Scheme 1
CH3 CH3
CHO
N N\ I OH N\ I O
1. CH3MgBr
H3CS N NH
N NH oxid. ~ N NH
2.TPAP H3CS ~ H3CS
(Ef0)~P(O)CH~COZEt ~ NaH
CH3 CH3
Ni I \ Ni I \
oxidation
~ S N N O ~- H CS N N O
O 3
1. oxaziridine I 2. Boc-Pip-aniline
' CH3
CH3 N , I \
N ~ \ HN_ 'N N O
~\ I ~
HN. 'N N' \O TFA
N
N CNJ
H . TFA
CNJ I
I
Boc
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-32-
Scheme 2
N \ CHO N \
\S~i Mew NMO, TPAP \S~~ NH
1V ~ CH2C12 '
IOI ~3 R3
(Et0) ~~ C02Et NaH
NBS, DMF
\S
Q=H
'THF oxaziridine
Q=H,F
Q=Br
CHZCIZ
3 oxaziridine
CH2C12
~S
I I
0
t3
BocN~N NH2 TFA, CH2C12
DMSO
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-33-
Those having skill in the art will recognize that the starting materials may
be varied and additional steps employed to produce compounds encompassed by
the present invention, as demonstrated by the following examples.
As shown in Schemes 1 and 2, a 4-substituted amino-2-methansulfanyl-
pyrimidine-5-carboxaldehyde is reacted with an organometallic compound, such
as, for example, a Grignard reagent, to afford the corresponding secondary
alcohol. The alcohol is subsequently oxidized to the ketone. The ketone is
then
reacted with a trialkyl phosphonoacetate in the presence of base to produce
the
corresponding 8-substituted-5-alkyl-2-methylsulfanyl-8H-pyrido[2,3-d]pyrimidin-
7-one. The pyrido-pyrimidine then can be halogenated at the 6-position with a
common halogenating agent, such as, for example, N-bromosucccinimide (NBS).
The 2-methylsulfanyl derivative is oxidized to the corresponding
methylsulfoxide,
which is subsequently treated with a desired aniline to afford the 2-
phenylamino
invention compound.
When carrying out various reactions to prepare invention compounds, it
may be desirable to derivatize reactive groups such as amines, alcohols, and
acids,
with protecting groups that are readily removed when desired. Such protecting
groups simply avoid unwanted side reactions. Use of protecting groups is
common
in the art of organic chemistry, as described by Greeve and Wuts in Protective
Gf°oups ih Organic Synthesis, John Wiley and Sons, New York (2nd ed,
1991).
Typical hydroxy protecting groups include either forming gxoups such as
benzyl,
and acyl groups such as tert-butoxycarbonyl(Boc), formyl, and acetyl. Amino
protecting groups include benzyl, aryl such as acetyl, and trialkylsilyl
groups.
Carboxylic acid groups typically are protected by conversion to an ester that
can
be easily hydrolyzed, for example, trichloroethyl, tent-butyl, benzyl, and the
like.
Some of the invention compounds have one or more chiral centers, and
thus can exist as individual optical isomers and mixtuxes thereof. Compound
246
(Table 1), for example, can exist as an RS racemate, or as the individual R or
S
isomer. All individual isomers and mixtures thereof are included in this
invention.
Individual isomers are readily prepared by a chiral synthesis, or by
conventional
resolution techniques well-known to those skilled in the art.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-34-
The invention is illustrated further by the following detailed examples
which are not to be construed as limiting the invention in scope or spirit to
the
specific procedures described in them. The starting materials and various
intermediates utilized in the synthesis of invention compounds may be obtained
from commercial sources, prepared from commercially available organic
compounds, or prepared using well-known synthetic methods. The disclosures in
this application of all articles and references, including patents, are
incorporated
herein by reference.
EXAMPLE 1
8-CyclopefZtyl 6 fluoro-5-methyl 2-methylsulfauyl 8H pyrido~2,3-dJpyfimidi~Z-
7 ohe
CH3
N~ \ F
H3C\
S N N O
NaH (771 mg, 19.3 mmol) is suspended in dry THF (20 mL), and the
mixture is cooled to 0°C in an ice bath. Triethyl 2-fluoro-2-
phosphonoacetate
(3.9 mL, 19.3 mmol) is added dropwise with stirring, and the solution is
stirred at
room temperature for 30 minutes. A solution of 1-(4-cyclopentylamino-
2-methylsulfanyl-pyrimidin-5-yl)-ethanone in dry THF (40 mL) is added via a
cannula, and the reaction mixture is stirred at 24°C for 12 hours. The
reaction is
quenched by the addition of H20 (0.5 mL), and the THF is evaporated in vacuo.
The residue is partitioned between ethyl acetate and saturated aqueous sodium
chloride. The aqueous layer is extracted twice with fresh ethyl acetate, and
the
combined organic layers are dried over MgS04. After removal of the drying
agent
and evaporation of the solvent, the crude product is purified by
chromatography
on silica gel (eluting with 20%-30% ethyl acetate in hexanes) to give the
titled
compound as a colorless solid (0.61 g, 23%).
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-3 5-
EXAMPLE 2
8-Cycloperttyl 6 fZuoro-2-methanesulfirzyl 5-methyl 8H pyrido~2,3-
djpyr~imidi>z-
7 orre
N F
S 0
I I
O
8-Cyclopentyl-6-fluoro-5-methyl-2-methylsulfanyl-8H-
pyrido[2,3-d]pyrimidin-7-one (0.61 g, 2.08 mmol) from Example I and 3-phenyl-
1 p-nitrophenylsulfonyloxaziridine (0.65 g, 2.5 mmol) are dissolved in CH2C12
(20 mL) and stirred for I2 hours at 24°C. Following evaporation of the
solvent,
the crude product is purified by silica gel chromatography (eluting with
80%-100% ethyl acetate in hexanes) to provide the sulfoxide product as a white
solid (0.55 g, 86%).
EXAMPLE 3
4 ~4-(8-Cyclopenyl 6 fluoro-5-methyl 7 oxo-7,8-dihydro-
py~ido~2,3-dJpyrimidih-2 ylaynino) phenyl) pipe~azine-1-carboxylic acid te>~t
butyl ester~
CH3
N~ ~ \ F
H~N~N N' \O
N
N
O' 'O
I ,CH3
H3C~CH3
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-3 6-
8-Cyclopentyl-6-fluoro-2-methanesulfinyl-5-methyl-8H-
pyxido[2,3-d]pyrimidin-7-one (0.3 g, 0.97 mmol) from Example 2 and 4-(N Boc-
piperazin-1-yl)aniline (0.548 g, 1.94 mmol) are suspended in 1,4-dioxane (5
mL)
and heated to 80°C for 12 hours. Anhydrous DMSO (2.5 mL) is added, and
the
temperature is raised to 100°C. Heating is continued for 24 hours,
after which the
reaction mixture is cooled to 24°C and partitioned between ethyl
acetate and
saturated aqueous sodium bicarbonate. The organic layer is separated and
washed
with H20, and then with saturated aqueous sodium chloride. After drying over
anhydrous MgS04, the solvent is evaporated, and the residue is purified by
silica
gel chromatography to provide the titled compound as a yellow solid (0.23 g,
45%).
EXAMPLE 4
8-Cyclopehtyl 6 fluof o-S methyl 2-(4 pipes azih-1 yl plzenylamino)-8H
py~ido~2,3-dJpyrim.idin-7 one
H3
F
H~N~N~N~O
N
N
I
4-[4-(8-Cyclopentyl-6-fluoro-5-methyl-7-oxo-7, 8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-piperazine-1-carboxylic acid tert-
butyl ester (0.23 g, 0.44 mmol) from Example 3 is dissolved in a 1:1 mixture
of
trifluoroacetic acid (TFA)/CH2C12 (20 mL) and stirred at room temperature for
1 hour. Evaporation of the solvents, followed by the addition of anhydrous
diethyl
ether, gave an orange solid (compound 34) that is collected by filtration
(0.21 g,
74%). Mp 254-255°C.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-3 7-
C23H27N60F~1.93 TFA: Calcd C, 50.21; H, 4.54; N, 13.08. Found: C, 49.83; H,
4.45; N, 12.99.
EXAMPLE 5
6-BronZO-8-cyclopeutyl 5-methyl 2-metlzylsulfa~Zyl 8H pyrido~2,3-clJpyf~imidin-
7 ofZe
CH3
N~ \
H3C\
S ~N N p
8-Cyclopentyl-5-methyl-2-methylsulfanyl-8H-pyrido [2,3-d]pyrimidin-
7-one (1 g, 3.64 mmol) is dissolved in dry DMF (15 mL) and
N bromosuccinimide (0.97 g, 5.45 mmol) is added followed by benzoylperoxide
(0.13 g, 0.5 mmol). The resulting solution is stirred for 12 hours at
24°C. The
mixture is then partitioned between ethyl acetate and H20. The organic layer
is
washed with H20, and then with saturated aqueous sodium chloride solution and
dried over MgS04. Removal of the drying agent and evaporation of the solvent
gave the desired title product (0.86 g, 66%) which is used without further
purification.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-3 8
EXAMPLE 6
6-B~omo-8-cyclopentyl 5-methyl 2-(4 piperazin-1 yl phehylasnitzo)-8H
py~ido~2,3-dJpytintidin-7 one
CH3
N, ~ \ Br
H~N~N N' \O
N
N
I
H
6-Bromo-8-cyclopentyl-5-methyl-2-methylsulfanyl-8H-
pyrido[2,3-d]pyrimidin-7-one is oxidized as described in Example 2. The
sulfoxide is reacted with 4-(N-Boc-piperazin-1-yl)aniline as described in
Example 3. The N-Boc protecting group is removed by hydrolysis as described
above for 8-cyclopentyl-6-fluoro-5-methyl-2-methylsulfanyl-8H-
pyrido[2,3-d]pyrimidin-7-one to provide 6-Bromo-8-cyclopentyl-S-methyl-
2-(4-piperazin-1-yl-phenylamino)-8H-pyrido [2,3-d]pyr imidin-7-one
(compound 35). Mp >200°C (dec).
C23H~7N60Br 1.9 TFA: Calcd C, 45.90; H, 4.15; N, 11.97. Found: C, 45.53; H,
4.09; N, 11.76.
EXAMPLE 7
1-(4-Cyclopentyla~titzo-2-metltylsulfahyl pyrimidin-5 yl)-ethan-1-of
CH3
~OH
H3C~ ' 'N NH
S
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-39-
4-Cyclopentylamino-2-methanesulfanyl-pyrimidine-S-carboxaldehyde
(1.l g, 4.64 mmol) is dissolved in tetrahydrofuran (30 mL) under nitrogen and
then cooled with an ice bath. To this clear solution is slowly added methyl
magnesium bromide (4.4 mL, 13.2 mmol, 3 M in ether). The reaction is stirred
fox
1 hour with the ice bath still in place. The reaction is quenched with a small
amount of saturated aqueous ammonium chloride and then partitioned between
water and ethyl acetate. The layers are separated, and the aqueous layer is
extracted with ethyl acetate. The combined organic layers are washed with
brine
and then dried over magnesium sulfate. After filtration, the solvent is
removed
in vacuo to yield the titled compound as an oil (1.09 g, 90%). 1H NMR
(400 MHz, CDCl3): 8 1.42-1.59 (rn, SH), 1.60-1.76 (m, 4H), 2.04-2.06 (m, 2H),
2.49 (s, 3H), 4.38-4.43 (m, 1H), 4.69-4.74 (m, 1H), 6.28-6.30 (d, 1H); 7.57
(s,
1H).
EXAMPLE 8
1 S 1-(4 Cyclopefatylamiho-2-metlaylsulfafayl pys~imidin-S yl)-etlaa~ao~ae
CH3
N ~ w0
HsC.
N NH
1-(4-Cyclopentylamino-2-methylsulfanyl-pyrimidin-5-yI)-ethan-1-of
(1.09 g, 4.3 mmol) from Example 7 is dissolved in 100 mL of dichloromethane.
The solution is purged by bubbling nitrogen gas through it for 2 minutes. To
the
reaction solution axe added, in order: powdered molecular sieves (4 angstrom),
N methyl morpholine oxide (1.07 g, 8.6 g), and tetrapropylammonium
perruthenate (0.227 g, 0.645 mmol). The reaction mixture is stirred at
24°C for
2 hours, and small amounts of additional catalyst are periodically added. The
reaction mixture is then run through a silica column (1:1, ethyl
acetate:hexanes) to
2S yield the titled compound as a light yellow solid (0.74 g, 70%). 1H NMR
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-40-
(400 MHz, CDCl3): 8 1.51-1.78 (m, 6H), 2.02-2.08 (m, 2H), 2.49 (s, 3H), 2.53
(s,
3H), 4.47-4.53 (m, 1H), 8.53 (s, 1H), 9.21 (s, 1H); MS (M+1) 252.2.
EXAMPLE 9
8-Cyclope>ztyl 5-zzzetlzyl 2-methylszzlfanyl 8H pyrido~2,3-dJpyrimidih-7 ofze
H3
N, i w
H3C'S~N N p
A cooled (5°C) flask containing tetrahydrofuran (50 mL) is charged
with
sodium hydride (1.05 g, 26.3 mmol, 60% dispersion in mineral oil) under
nitrogen, and 1.0 g of triethyl phosphonoacetate is added. The cooling bath is
removed, and the mixture is stirred at 24°C until it becomes a
homogeneous
solution. The solution is diluted by the dropwise addition of a solution of
1-(4-cyclopentylamino-2-methylsulfanyl-pyrimidin-5-yl)-ethanone (3.0 g,
11.9 mmol) in tetrahydrofuran (25 mL). The reaction mixture is heated to
reflux
for 2 hours. The reaction mixture is cooled to 24°C, and diluted 50 mL
of water
and 50 mL of ethyl acetate. Following separation of the layers, the organic
layer is
dried over magnesium sulfate and concentrated in vacuo to near dryness. Hexane
is added, and the solid is stirred vigorously for 5 minutes before being
filtered to
yield the titled compound as a light pale orange solid (3.01 g, 92%). 1H NMR
(400 MHz, CDC13): 8 1.63-2.36 (m, 8H), 2.38 (s, 3H), 2.59 (s, 3H), 5.84-5.93
(m,
1H), 6.39 (s, 1H), 8.66 (s, 1H).
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-41-
EXAMPLE 10
8-Cyclopeutyl 2-metlaanesulfifZyl 5-methyl 8H pyr~ido~2,3-dJpyrimidiu-7 one
H3
N~ ~ \
H3C 'S ~ N N O
Ii
O
8-Cyclopentyl-5-methyl-2-methylsulfanyl-8H-pyrido [2,3-d]pyrimidin-
7-one (I.0 g, 3.63 mmol) from Example 9 is dissolved in dichloromethane
(15 mL), and (~)-ty°ahs-2-(phenylsulfonyl)-3-phenyloxaziridine is
added. The
reaction mixture is stirred at 24°C for 12 hours, and then the solution
is passed
through a silica column (2% MeOH in CH2Cl2) to yield the titled sulfoxide as a
white solid (0.67 g, 64%). 1H NMR (400 MHz, DMSO-d6): 8 1.53-2.19 (m, 8H),
2.45 (s, 3H), 2.87 (s, 3H), 5.75-5.84 (m, 1H), 6.64 (s, 1H), 9.19 (s, 1H).
EXAMPLE 11
4 ~~ (8-Cyclopehtyl S methyl 7 oxo-7,8-dilaydro pyrido,(2,3-dJpyrimidin-
2 ylamino) phenyl) pipe~azine-1-carboxylic acid te~~t butyl ester
BocN~ CH3
\/N / N i \
\ I ~ I .
N N N
H
O
8-Cyclopentyl-2-methanesulfinyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-
7-one (0.7 g, 2.4 mmol) from Example 10 and 4-(4'-N Boc-piperazinyl)-aniline
are dissolved in dimethylsulfoxide (8 mL) and heated to 90°C overnight.
The
reaction mixture is cooled to room temperature and partitioned between water
and
ethyl acetate. The organic layer is washed with sodium hydrogen carbonate,
brine,
and then dried over magnesium sulfate. Removal of the drying agent and
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-42-
concentration in vacuo gives the titled product as a yellow solid that is
recrystallized from water and acetonitrile (0.55 g, 45%). 1H NMR (400 MHz,
CDC13): 8 1.45 (s, 9H), 1.56-1.87 (m, 6H), 2.23-2.28 (m, 2H), 2.31 (s, 3H),
3.07 (m, 4H), 3.55 (m, 4H), 5.77-5.8I (m, IH), 6.19 (s, 1H), 6.90 (d, 2H),
7.42-7.44 (d, 2H), 7.47 (s, 1H), 8.55 (s, 1H); MS (M+1) 505.1. The Boc
protecting
group is removed by stirring in a l :l mixture of trifluoroacetic
acid/dichloromethane to give (Compound 1). Mp >215°C (dec).
EXAMPLE 12
8-Cyclope~ztyl S-ethyl 2-(4 piperazih-1 yl plZenylanZino)-8H pyrido,(2,3-
aJpy~imidih-7 ohe (Compound 193)
HN N N O
N
c~
N
H
1-(4-Cyclopentylamino-2-methylsulfanyl-pyrimidin-5-yl)-propan-1-o1.
4-Cyclopentylamino-2-methylsulfanyl-pyrimidine-5-carbaldehyde (4.07 g,
17.1 mmol) was dissolved in THF (60 mL) under nitrogen then cooled with an ice
bath. To this clear solution EtMgBr (13.4 mL, 40.3 mmol, Aldrich 3M in ether)
was slowly added. The reaction was stirred for 15 minutes with the ice bath
still in
place. The reaction was quenched with a small amount of sat. aq. NH4C1 then
partitioned between water and EtOAc. The layers were separated, the organic
layer dried over MgS04, and after filtration, the solvent was removed in vacuo
to
yield 1-(4-cyclopentylamino-2-methylsulfanyl-pyrimidin-5-yl)-propan-1-of as an
oil (4.55 g, 99%) which was used without further purification.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-43-
1-(4-Cyclopentyla~zino-2-methylsulfanyl py~ifnidin-5 yl) py°opan-1-ohe.
1-(4-Cyclopentylamino-2-methylsulfanyl-pyrimidin-5-yl)-propan-1-of (4.55 g,
17.1 mmol) was dissolved in toluene (80 mL) to which manganese (IV) oxide
(3.72 g, 42.8 mmol, Aldrich <5 micron, activated, ~85%) was subsequently
added.
The reaction was brought to reflux for 16 hours. The reaction was cooled to
zoom
temperature and filtered though a celite pad. The filtrate then was
concentrated in
vacuo to yield the product as a light yellow oil (3.79 g, 84%).
8-Cyclopentyl-5-ethyl-2-nzethylsulfanyl-8H py>~ido(2, 3-dJpy~imidin-7-ohe.
Under nitrogen, a cooled flask with THF (50 mL) was charged with NaH (1.23 g,
30.7 mmol, 60% dispersion in mineral oil) to which was added triethyl
phosphonoacetate (6.09 mL, 30.7 mmol). The cooling bath was removed, and the
reaction mixtuxe was stirred at ambient temperature until everything
dissolved. A
solution of the 1-(4-cyclopentylamino-2-methylsulfanyl-pyrimidin-5-yl)-propan-
1-one (3.0 g,.11.9 mmol) in THF (70 mL) was slowly added to the preformed
anion. Then the reaction mixture was brought to reflux for 60 hours. The
reaction
was cooled to room temperature and diluted with water and EtOAc. The layers
were separated, and the aqueous layer was extracted with EtOAc. The combined
organic extracts were washed with brine, dried over MgS04, filtered, and
concentrated in vacuo to give a waxy solid. The solid residue was triturated
with
hexanes to give a white solid after filtration (2.67 g, 66%).
8-Cyclopentyl-5-ethyl-2-methanesulfihyl-8H pyt°ido~2, 3-
dJpy°inaidih-
7-ohe. 8-Cyclopentyl-5-ethyl-2-methylsulfanyl-8H-pyrido [2,3-d]pyrimidin-7-one
(2.57 g, 8.88 mmol) was dissolved in CH2C12 (50 mL), and 2-benzene-sulfonyl-
3-phenyl-oxaziridine was added. The reaction mixture was stirred for 16 hours
at
room temperature. Then the solution was evaporated in vacuo to give an orange
oil. EtOAc was added and a white precipitate formed. This precipitate was
filtered
and washed with hexanes to yield a white solid (2.12 g, 78%).
4-~4-(8-Cyclopehtyl-S-ethyl-7-oxo-7, 8-dilzydro py~ido~2, 3-dJpy~imidin-
2 ylamino) phenyl) pipes°azine-1-carboxylic acid tef°t-butyl
ester. The sulfoxide,
8-cyclopentyl-5-ethyl-2-methanesulfmyl-8H-pyrido[2,3-d]pyrimidin-7-one (0.2 g,
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-44-
0.654 mmol) and 4-(4'-N Boc-piperazinyl)-aniline were dissolved in DMSO
(5 mL) and heated to 70°C for 16 hours. The reaction mixture was cooled
to room
temperature and partitioned between water and EtOAc. The organic layer was
washed with brine then dried over MgS04. After filtration and concentrate
in vacuo, orange solid was obtained which was purified by column
chromatography to yield the product as a yellow solid (0.160 g, 47%).
8-Cyclopentyl-5-ethyl-2-(4 pipey~azin-1 yl phe~ylamiv~o)-8H
py~ido~2, 3-dJpy~~i~2idin-7-one (235) ~4-[4-(8-Cyclopentyl-5-ethyl-7-oxo-
7, 8-dihydro-pyrido [2,3-dJpyrimidin-2-ylaxnino)-phenyl]-piperazine- I -
carboxylic
acid tert-butyl ester was dissolved in dichloromethane (2 mL), and
txifluoroacetic
acid (0.5 mL) was added. This mixture was stirred at room temperature for
1 S hours. The solvent was evaporated, then the solid was suspended in diethyl
ether and filtered to give a fluffy gxay solid (128 mg, 75%). 1H NMR (400 MHz,
DMSO-d6): 8 1.17 (m, 3H), 1.52-1.83 (m, 6H), 2.20 (m, 2H), 2.75 (m, 2H),
3.22 (m, 4H), 5.78 (m, 1H), 6.10 (s, 1H), 6.95 (d, 2H), 7.53 (d, 2H), 8.73 (s,
2H),
8.79 (s, 1H), 9.76 (s, 1H); CHN for C23H30N60 + 1.21 TFA.
EXAMPLE 13
2-~4 (4 Acetyl pipefazin-X yl) plaenylamihoJ 8-cyclope~ttyl S-methyl 8H
py~~ido~2,3-dJpy~~imidisz-7 one (Compound 198)
N'
HN~N N~O
N
c~
N
' 'O
I-[4-(4-Amino-phenyl)-pipexazin-1-yl]-ethane (0.075 g, 0.343 mrnol) and
8-cyclopentyl-5-methyl-2-methanesulfinyl-8H pyxido[2,3-d]pyrimidin-7-one
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-45-
(0.100 g, 0.343 mmol) ware dissolved in DMSO (5 mL) and heated to 70°C
for
I 6 hours. At this point, 20 mg more of the aniline was added, and heating was
continued for an additional 4 hours. The reaction was cooled to room
temperature
and partitioned between water and EtOAc. The organic layer was washed with
brine then dried over MgS04. Filtration and concentration in vacuo gave an
orange solid which was purified by column chromatography to yield a yellow
solid (0.049 g, 32%). Mp 261-263°C.
EXAMPLE 14
2-,(4-((3R,4S) Am.ifzometlayl t~~~uoromethyl py~rolidin-1 yl) plzenylaminoJ-
8-cyclopentyl 5-methyl 8H pyt~ido~2,3-dJpyfinZidin-7 ofZe (Compound 216)
HN N N O
N
FsC,, NH2
~(3R,4S)-1-(4 Aynino phenyl)-t~°ifluo~onzethyl py~~olidin-3 ylrnethylJ-
ca~bamic acid test-butyl ester. ((3S,4S)-4-Trifluoromethyl-pyrrolidin-3-
ylmethyl)-
carbamic acid tey t-butyl ester (1.0 g, 3.72 mmol), p-fluoro-nitrobenzene
(0.36 mL,
3.38 mmol) and diisopropyl ethyl amine (0.65 mL, 3.72 mmol) were dissolved in
acetonitrile (10 mL) and refluxed for 24 hours. The solvent was removed, and
the
mixture was triturated with hexanes and filtered to yield a crude yellow solid
(1.4 g). This product was dissolved in THF and treated with Raney Nickel under
a
hydrogen atmosphere until no further change in pressure was observable.
Following removal of the catalyst by filtration, the product aniline was
obtained
by evaporation of the solvent and used without further purification.
~(3R, 4S)-~4-(8-Cyclopentyl-S-methyl-7-oxo-7, 8-dihyd~o
pyf~ido~2,3-aJpyrimidin-2 ylamino) plZenylJ-trifluo~ornethyl pyrrolidin
3 ylmethyl,~-caf°bamic acid tee°t-butyl ester (222). [(3R,4S)-1-
(4-Amino-phenyl)-
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-46-
trifluoromethyl-pyrrolidin-3-ylmethyl]-carbamic acid tert-butyl ester and
8-cyclopentyl-5-methyl-2-methanesulfinyl-8H pyrido[2,3-d]pyrimidin-7-one wexe
coupled and deprotected as previously described for Example 11.
CHN for C25H29N601F3 + 1.6 TFA, mp >130°C (dec).
EXAMPLE 15
8-Cyclopentyl S-methyl 2-~4-,(2-(4H ~1,2,4Jt~iazol 3 ylsulfafzyl)-ethyl)
phefZylauzi~ZO~-8H pynido(2,3-dJpyrimidi~z-7 ohe (Compound 222)
N~ I w
HN~N~N
O
,N~S
N~-N
4-~2(4H ~l, 2, 4Jt~~iazol-3 ylsulfa~zyl)-ethyl) phenylamine. To a suspension
of hexane rinsed 60% sodium hydride (0.83 g), in dimethylformarnide (5 mL) at
0°C, was added a solution of 3-mercapto-1,2,4-triazole (2.0 g) in
dimethylformamide (10 mL) in portions. After 45 minutes 4-nitrophenethyl
bromide (4.1 g) was added, and the reaction mixture was stirred at room
temperature for 18 hours. 1 M Hydrochloric acid (70 mL) was added and the
aqueous phase was extracted with diethyl ether (3 x 100 mL), and the combined
organic extracts were concentrated to dryness. The resulting solid was
collected,
washed with diethyl ether (2 x 10 mL) and dried to yield the nitrobenzene
intermediate (3.17 g). M8: MH+, 251; MH- 248.9. A solution of this
intermediate
(1.0 g) was reduced using Raney Nickel (0.5 g) and hydrogen in THF (100 mL).
The sample was concentrated to dryness to yield the title compound (0.88 g),
MS:
MH+, 221; MH-, 219.
8-Cyclopentyl-5-methyl-2-~4-~2-(4H ~1,2,4Jtr°iazol-3 ylsulfanyl)-ethylJ-
phenylaminoJ-8H pyr-ido~2, 3-dJpyrimidiv~-7-one. A solution of 8-cyclopentyl-
5-methyl-2-methanesulfinyl-8H pyrido[2,3-d]pyrimidin-7-one (0.02 g), 4-[2(4H
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-47-
[1,2,4]triazol-3-ylsulfanyl)-ethyl]-phenylamine (0.0166 g) and trifluoroacetic
acid
(0.06 mL) in acetonitrile (2 mL) was heated at 80°C for 18 hours. The
reaction
mixture was cooled and the solvent removed in vacuo. 1 M Sodium hydroxide
(4 mL) was added and the aqueous phase was extracted with diethyl ether
(3 x 4 mL); sodium chloride (20 mg) was added after the first extraction. The
aqueous layer was acidified to pH = 1 and extracted with a mixture of ethyl
acetate/dichloromethane (9:I) (3 x 4 mL). The combined ethyl
acetate/dichloromethane extracts were concentrated to dryness and purified by
column chromatography using a gradient of ethyl acetate 60% to 100% in
hexanes. Concentration of the appropriate fractions yielded Compound 222
(0.014 g). 1H NMR (d6_DMSO): 8 1.58 (2H, m), 1.7 (2H, m), 1.88 (2H, m),
2.2 (2H, m), 2.38 (3H, s), 2.9 (2H, m), 3.4 (2H, m), 5.7 (IH, s), 5.8 (1H, m),
7.18(2H,d,J=9),7.6(2H,d,J=9),8.78(lH,s).
EXAMPLE 16
8-Cyclopeutyl 5-uZethyl 2-~4-(IH ~1,2,4j~riazol 3 ylsulfayzyl) phenylamihoJ 8H
pyr~ido~2,3-d~py~imidin-7 oyze (Compound 223)
N N N O
S N
~N
N =/
4-(IH ~l, 2, 4~t~iazol-3 ylsulfanyl) phevcylayniue. To a suspension of
hexanes rinsed 60% sodium hydride (1.16 g) in dimethylformamide (10 mL) at
0°C was added a solution of 3-mercapto-1,2,4-triazole (4.0 g) in
dimethylformamide (20 mL) dropwise. After 20 minutes, 1-fluoro-4-nitrobenzene
(5 g) in dimethylformamide (20 mL) was added, and the reaction mixture was
stirred at room temperature for 2 hours, then at 60°C for 18 hours. 1 M
Hydrochloric acid (100 mL) was added, and the solid was collected and dried. A
second crop of solid (2.1 g) was recovered by crystallization from the mother
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
_48_
liquors. To the combined solids was added dichloromethane (300 mL) and 1 M
sodium hydroxide (200 mL). The dichloromethane was further extracted with 1 M
sodium hydroxide (100 mL). The combined aqueous phases were extracted with
dichloromethane (2 x 300 mL) then acidified to pH =1. The solid formed was
S collected and dried to yield the nitrobenzene derivative (1.96 g). This
product was
reduced using Raney Nickel and hydrogen in tetrahydrofuran (100 mL).
Following removal of the catalyst, the sample was concentrated to dryness to
yield
the desired product (1.7 g), HS: MH+, 192.9; MH-, 190Ø
8-Cyclope3ztyl-5-methyl-2-~~-(1 H ~l, 2, 4Jt~ iazol-3 ylsulfanyl)-
phenylaminoJ-8H pyf°ido~2, 3-dJpyy°imidin-7-one. This compound
was prepared
from 4-(1H-[1,2,4]triazol-3-ylsulfanyl)-phenylamine and 8-cyclopentyl-S-methyl-
2-methanesulfmyl-8H pyrido[2,3-d]pyrimidin-7-one by the procedure of
Example 1S to give Compound 223. 1HNMR (D6-DMSO): 8 1.58 (2H, m),
1.75 (2H, m), 1.90 (2H, m), 2.2 (2H, m), 2.38 (3H, s), 5.82 (1H, m), 6.20 (1H,
s),
1S 7.4 (2H, d, J= 9), 7.73 (2H, d, J= 9), 8.85 (1H, bs), 8.82 (1H, s).
EXAMPLE 17
8-Cyclopentyl S-methyl 7 oxo-2-(4 pipe~azih-1 y1 phenylamiho)-7,8-dihydro-
py~ido,(2,3-dJpyf~imidine-6-carboxylic acid methyl estef~ (Compound 224)
O
N ~ ~ ~ o'
HN~N N O
N\
~Jlc
N
H
4-[4-(6-Bromo-8-cyclopentyl-S-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylarnino)-phenyl]-piperazine-1-carboxylic acid
tey~t-butyl ester (300 mg, O.S1S mmol), Pd(OAc)2 (23 mg, O.OS mmol),
1,2-bis(diphenylphosphino)-propane 64 mg, 0.1SS mmol), and triethylamine
(0.18 mL, 1.29 mmol) were combined in methanol and pressurized to S00 PSI in
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-49-
CO gas. The reaction mixture was heated to I00°C and stirred for 14
hours, then
allowed to cool to 24°C. Evaporation of the solvent, followed by
chromatography
on Si02 (45%-50% EtOAc in Hexanes) gave a yellow oil. This oil was dissolved
in CH2C12 (10 mL) and treated with 2 M HCl in diethyl ether (10 mL) at room
temperature. A white precipitate formed. After stirring for 3 hours at room
temperature, the solvent was evaporated. The residue was re-suspended in
anhydrous diethyl ether and filtered to give the titled compound as a yellow
solid
(34 mg), mp 195-205°C. IH NMR (dg-DMSO): 8 1.52 (br s, 2H), 1.71 (br s,
2H),
1.82 (br s, 2H), 2.I4 (br s, 2H), 2.30 (s, 3H), 3.18 (s, 4H), 3.27 (s, 4H),
3.76
IO (s, 3H), 5,8 (s, 1H), 6.96 (d, J = 8 Hz, 2H), 7.53 (d, J = 8 Hz, 2H), 8.85
(s, 1H),
9.04 (s, 1 H), 9.97 (s, 1 H).
EXAMPLE 18
The following compounds are prepared essentially according to the
procedures described in Examples 1 to 17 and as illustrated in Schemes I and
2:
(a) 8-Cyclopentyl-5-methyl-2-(4-piperazin-1-yl-phenylaznino)-8H-
pyrido[2,3-d]pyrimidin-7-one trifluoroacetic acid salt (Compound 1), mp
>215°G
(dec);
(b) 8-(1-Methylethyl)-5-methyl-2-(4-piperazin-1-yl-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 2), mp >235°C (dec);
(c) 8-Cyclopentyl-5-methyl-2-(4-fluoro-3-methylphenylamino)-8H-
pyrido[2,3-d]pyrirnidin-7-one (Compound 3);
(d) 8-(1-Methylethyl)-5-methyl-2-(4-fluoro-3-methylphenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 4);
(e) 8-Cyclohexyl-S-methyl-2-(4-fluoro-3-methylphenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 5);
(f) 8-Cyclohexyl-5-methyl-2-[4-(4-propanoylpiperazin-
1-yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 6);
(g) 8-Cyclopentyl-5-methyl-2-[4-(4-propanoylpiperazin-
1-yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetic acid salt
(Compound 7), mp 235-237°C;
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-50-
(h) 8-(1-Methylethyl)-5-methyl-2-[4-(4-propanoylpiperazin-
1-yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 8);
(i)- 8-Cyclohexyl-5-methyl-2-(4-piperazin-1-yl-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 9);
(j) 8-Cyclopentyl-5-methyl-2-(4-pyridylphenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 10);
(k) 8-(1-Methylethyl)-5-methyl-2-(4-pyridylphenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 11);
(1) 8-Cyclopentyl-5-methyl-2-[4-(3-aminopyrrolidinyl)phenylamino]-8H-
pyrido[2,3-d]pyrimidin-7-one trifluoroacetic acid salt (Compound 12),
mp >195°C (dec);
(m) 8-(1-Methylethyl)-5-methyl-2-[4-(3-aminopynolidinyl)phenylamino]-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 13), mp >227-229°C;
(n) N-(1- f 4-[(8-cyclopentyl-5-methyl-7-oxo(8-hydropyridino[2,3-d]-
pyrimidin-2-yl))amino]phenyl]pyrrolidin-3-yl)-3,3-dimethylbutanamide
(Compound 14);
(o) N-(1-~4-[(5-methyl-8-(1-methylethyl)-7-oxo(8-hydropyridino[2,3-d]-
pyrimidin-2-yl))amino]phenyl~pyrrolidin-3-yl)-3,3-dimethylbutanamide
(Compound 15);
(p) 8-Cyclopentyl-5-methyl-2-(3-chloro-4-piperazin-1-yl-phenylamino)-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 16), mp 234-237°C;
(c~ 8-Cyclohexyl-5-methyl-2-(3-chloro-4-piperazin-1-yl-phenylamino)-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 17);
(r) 8-(1-Methylethyl)-5-methyl-2-(3-chloro-4-piperazin-1-yl-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 18);
(s) 8-Cyclopentyl-6-fluoro-5-methyl-2-(3-chloro-4-piperazin-1-yl-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 19);
(t) 8-Cyclohexyl-6-fluoro-5-methyl-2-(3-chloro-4-piperazin-1-yl-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 21);
(u) 8-(1-Methylethyl)-6-fluoro-5-methyl-2-(3-chloro-4-piperazin-1-yl-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 20);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-51-
(v) 8-Cyclopentyl-5-methyl-2-(3-chloro-4-morpholin-4-yl-phenylamino)-
8H-pyrido[2,3-d]pyrirnidin-7-one (Compound 22);
(w) 8-(1-Methylethyl)-5-methyl-2-(3-chloro-4-morpholin-4-yl-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 23);
(x) 8-Cyclohexyl-5-methyl-2-(3-chloro-4-moipholin-4-yl-phenylamino)-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 24);
(y) 2-( f 3-chloro-4-[4-(3-morpholin-4-ylpropyl)piperidyl]phenyl}amino)-
8-cyclopentyl-5-methyl-8-hydropyridino[2,3-d]pyrimidin-7-one (Compound 25);
(z) 2-( f 3-chloro-4-[4-(3-moxpholin-4-ylpropyl)piperidyl]phenyl}amino)-
8-(1-methylethyl)-5-methyl-8-hydropyridino[2,3-d]pyrimidin-7-one
(Compound 26);
(aa) 2-(~3-chloro-4-[4-(3-morpholin-4-ylpropyl)piperidyl]phenyl}amino)-
8-cyclohexyl-5-methyl-8-hydropyridino[2,3-d]pyrimidin-7-one (Compound 27);
(bb) 2-(~3-chloro-4-[4-(3-piperazinylpropyl)piperidyl]phenyl}amino)-
8-cyclopentyl-6-fluoro-5-methyl-8-hydropyridino[2,3-d]pyrimidin-7-one
(Compound 28);
(cc) 2-( f 3-chloro-4-[4-(3-piperazinylpropyl)piperidyl]phenyl}amino)-
8-( 1-methylethyl)-6-fluoro-5 -methyl-8-hydropyridino [2,3 -d]pyrimidin-7-one
(Compound 29);
(dd) 2-( f 3-chloro-4-[4-(3-piperazinylpropyl)piperidyl]phenyl}amino)-
8-cyclohexyl-6-fluoro-5-methyl-8-hydropyridino [2,3-d]pyrimidin-7-one
(Compound 30), mp >80°C (dec);
(ee) 2-(~3-chloro-4-[4-(3-piperazinylpropyl)piperidyl]phenyl}amino)-
8-cyclopentyl-5-methyl-8-hydropyridino[2,3-d]pyrimidin-7-one (Compound 31);
(f~ 2-({3-chloro-4-[4-(3-piperazinylpropyl)piperidyl]phenyl}amino)-
8-( 1-methylethyl)-5-methyl-8-hydropyridino [2,3-d]pyrimidin-7-one
(Compound 32);
(gg) 2-( f 3-chloro-4-[4-(3-piperazinylpropyl)piperidyl]phenyl}amino)-
8-cyclohexyl-5-methyl-8-hydropyridino[2,3-d]pyrimidin-7-one (Compound 33);
(gg2) 8-Cyclopentyl-2-[4-(piperazin-1-yl)-phenylamino]-6-fluoro-5-
methyl-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetate (Compound 34),
mp 254-255°C;
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-52-
(gg3) 8-Cyclopentyl-2-[4-(piperazin-1-yl)-phenylamino]-b-bromo-5-
methyl-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetate (Compound 35),
mp >200°C;
(hh) 8-Cyclopentyl-2-[4-(3,5-dimethyl-piperazin-1-yl)-phenylamino]-
b-fluoro-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one hydrochloride
(Compound 36), mp >220°C;
(ii) 8-Cyclopentyl-2-(3-fluoro-4-piperazin-1-yl-phenylamino)-5-methyl-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 37);
(jj) 6-Bromo-8-cyclopentyl-2-[4-(3,5-dimethyl-piperazin-1-yl)-
phenylamino]-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one hydrochloride
(Compound 38), mp >230°C;
(klc) 8-Cyclopentyl-6-fluoro-2-(3-fluoro-4-piperazin-1-yl-phenylamino)-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 39);
(11) 6-Bromo-8-cyclopentyl-2-(3-fluoro-4-piperazin-1-yl-phenylamino)-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 40);
(nun) 8-Cyclopentyl-2-[4-(3,5-dimethyl-piperazin-1-yl)-phenylamino]-
S-methyl-8H-pyrido[2,3-d]pyrimidin-7-one hydrochloride (Compound 41);
(nn) 2-(3-Chloro-4-piperazin-1-yl-phenylamino)-8-cyclopentyl-5-methyl-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 42);
(oo) 2-(3-Chloro-4-piperazin-1-yl-phenylamino)-8-cyclopentyl-6-fluoro-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 43);
(pp) b-Bromo-2-(3-chloro-4-piperazin-1-yl-phenylamino)-8-cyclopentyl-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 44);
(qq) 8-Cyclopentyl-5-methyl-2-(4-morpholin-4-yl-phenylamino)-8H-
pyrido[2,3-d]pyrirnidin-7-one trifluoroacetate (Compound 45), mp 227-
229°C;
(rr) 8-Cyclopentyl-6-fluoro-5-methyl-2-(4-morpholin-4-yl-phenylamino)-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 46);
(ss) 6-Bromo-8-cyclopentyl-5-methyl-2-(4-morpholin-4-yl-phenylamino)-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 47);
(tt) 8-Cyclopentyl-2-(3-fluoro-4-morpholin-4-yl-phenylamino)-5-methyl-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 48);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-53-
(uu) 8-Cyclopentyl-6-fluoro-2-(3-fluoro-4-morpholin-4-yl-phenylamino)-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 49);
(w) 6-Bromo-8-cyclopentyl-2-(3-fluoro-4-morpholin-4-yl-phenylamino)-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 50);
(ww) 2-(3-Chloro-4-morpholin-4-yl-phenylamino)-8-cyclopentyl-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 51);
(xx) 2-(3-Chloro-4-morpholin-4-yl-phenylamino)-8-cyclopentyl-6-fluoro-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 52);
(yy) 6-Bromo-2-(3-chloro-4-morpholin-4-yl-phenylamino)-8-cyclopentyl-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 53);
(zz) 8-Cyclopentyl-5-methyl-2-{4-[4-(2,2,2-trifluoro-ethyl)-piperazin-
1-yl]-phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 54),
mp 198-199°C;
(aaa) 8-Cyclopentyl-6-fluoro-5-methyl-2-~4-[4-(2,2,2-trifluoro-ethyl)-
piperazin-1-yl]-phenylamino~-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 55);
(bbb) 6-Bromo-8-cyclopentyl-5-methyl-2-{4-[4-(2,2,2-trifluoro-ethyl)-
piperazin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 56);
(ccc) 8-Cyclopentyl-5-methyl-2-~4-[4-(3-piperazin-1-yl-propyl)-
piperidin-1-yl]-phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetate
(Compound 57), mp >80°C (dec);
(ddd) 8-Cyclopentyl-6-fluoro-5-methyl-2-~4-[4-(3-piperazin-1-yl-propyl)-
piperidin-1-yl]-phenylamino~-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 58),
mp >230°C;
(eee) 6-Bromo-8-cyclopentyl-5-methyl-2-~4-[4-(3-piperazin-1-yl-propyl)-
piperidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 59);
(fff) 8-Cyclopentyl-5-methyl-2- f 4-[4-(3-morpholin-4-yl-propyl)-
piperidin-1-yl]-phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 60);
(ggg) 8-Cyclopentyl-6-fluoro-5-methyl-2-~4-[4-(3-morpholin-4-yl-
propyl)-piperidin-1-yl]-phenylamino ] -8H-pyrido [2, 3 -d]pyrimidin-7-one
(Compound 61);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-S4-
(hhh) 6-Bromo-8-cyclopentyl-S-methyl-2-{4-[4-(3-morpholin-4-yl-
propyl)-piperidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 62);
(iii) 6-Bromo-8-cyclopentyl-S-methyl-2-~4-[4-(3-morpholin-4-yl-propyl)-
S piperidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 63);
(jjj) 2-(4- f 4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl}-
phenylamino)-8-cyclopentyl-S-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 64);
(kkl~) 2-(4-{4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl}-
phenylamino)-8-cyclopentyl-6-fluoro-S-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 65);
(111) 2-(4-~4-[3-(3-Amino-pyrrolidin-1-yl)-propyl]-piperidin-1-yl}-
phenylamino)-6-bromo-8-cyclopentyl-S-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 66);
1S (mmm) 8-Cyclopentyl-2-(3-fluoro-4-[4-(3-piperazin-1-yl-pxopyl)-
piperidin-1-yl]-phenylamino}-S-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 67);
(nnn) 8-Cyclopentyl-6-fluoro-2-~3-fluoro-4-[4-(3-piperazin-1-yl-propyl)-
piperidin-1-yl]-phenylamino } -S-methyl-8H-pyrido [2, 3-d]pyrimidin-7-one
(Compound 68);
(ooo) 6-Bxomo-8-cyclopentyl-2- f 3-fluoro-4-[4-(3-piperazin-1-yl-propyl)-
piperidin-1-yl]-phenylamino}-S-methyl-8H-pyxido[2,3-d]pyrimidin-7-one
(Compound 69);
(ppp) 8-Cyclopentyl-2-{3-fluoro-4-[4-(3-morpholin-4-yl-propyl)-
2S piperidin-1-yl]-phenylamino}-S-methyl-8H-pyrido[2,3-d]pyximidin-7-one
(Compound 70);
(qqq) 8-Cyclopentyl-6-fluoro-2- f 3-fluoro-4-[4-(3-morpholin-4-yl-
propyl)-piperidin-1-yl]-phenylamino } -S-methyl-8H-pyrido [2, 3-d]pyrimidin-7-
one
(Compound 71);
(rrr) 6-Bromo-8-cyclopentyl-2-~3-fluoro-4-[4-(3-morpholin-4-yl-propyl)-
piperidin-I -yl]-phenylamino}-S-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 72);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-55-
(sss) 2-[4-(3-Amino-pyrrolidin-1-yl)-phenylamino]-8-cyclopentyl-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 73), mp >215°C
(dec);
(ttt) 2-[4-(3-Amino-pyrrolidin-1-yl)-phenylamino]-8-cyclopentyl-
6-fluoro-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 74);
(uuu) 2-[4-(3-Amino-pyrxolidin-1-yl)-phenylamino]-6-bromo-
8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 75);
(vw) 2-[4-(3-Amino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-
8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 76);
(www) 2-[4-(3-Amino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-
8-cyclopentyl-6-fluoro-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 77);
(xxx) 2-[4-(3-Amino-pyrrolidin-1-yl)-3-fluoro-phenylamino]-6-bromo-
8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 78);
(yyy) 8-Cyclopentyl-5-methyl-2-{4-[3-(2,2,2-trifluoro-ethylamino)-
pyrrolidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetate
(Compound 79), mp >160°C (dec);
(zzz) 8-Cyclopentyl-6-fluoro-5-methyl-2- f 4-[3-(2,2,2-trifluoro-
ethylamino)-pyrrolidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 80);
(aaaa) 6-Bromo-8-cyclopentyl-5-methyl-2- f 4-[3-(2,2,2-trifluoro-
ethylamino)-pyrrolidin-1-yl]-phenylamino } -8H-pyrido [2, 3 -d]pyrimidin-7-one
(Compound 8I);
(bbbb) 2-[4-(3-Amino-pyrrolidin-1-yl)-3-chloro-phenylamino]-
8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetate
(Compound 82), mp >215°G (dec);
(cccc) 2-[4-(3-Amino-pyrrolidin-1-yl)-3-chloro-phenylamino]-
8-cyclopentyl-6-fluoro-5-methyl-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 83), mp 221 °C;
(dddd) 2-[4-(3-Arnino-pyrrolidin-1-yl)-3-chloro-phenylamino]-6-bromo-
8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 84);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-56-
(eeee) 2-[4-(3-Aminomethyl-4-trifluoromethyl-pyrrolidin-1-yl)-
phenylamino]-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 85);
(fff~ 2-[4-(3-Aminomethyl-4-trifluoromethyl-pyrrolidin-1-yl)-
phenylarnino]-8-cyclopentyl-6-fluoro-5-methyl-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 86);
(gggg) 2-[4-(3-Aminomethyl-4-trifluoromethyl-pyrrolidin-1-yl)-
phenylsmino]-6-bromo-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 87);
(hhhh) 2-[4-(3-Trifloroethylaminomethyl-pyrrolidin-1-yl)-phenylamino]-
8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 88);
(iiii) 2-[4-(3-Trifloroethylaminomethyl-pyrrolidin-1-yl)-phenylamino]-
8-cyclopentyl-6-fluoro-5-methyl-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 89);
(jjjj) 2-[4-(3-Trifloroethylasninomethyl-pyrrolidin-1-yl)-phenylamino]-
6-bromo-8-cyclopentyl-5-methyl-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 90);
(kkkk) 8-Cyclopentyl-2-[4-(3,3-dimethyl-piperazin-1-yl)-phenylamino]-
5-methyl-8H-pyrido[2,3-d]pyrirnidin-7-one hydrochloride (Compound 91),
mp >150°C (dec);
(1111) 8-Cyclopentyl-2-[4-(3,3-dimethyl-piperazin-1-yl)-phenylamino]-
6-fluoro-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 92);
(mmmm) 6-Bromo-8-cyclopentyl-2-[4-(3,3-dimethyl-piperazin-1-yl)-
phenylamino]-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one hydrochloride
(Compound 93), mp >200°C (dec);
(nnnn) 8-Cyclopentyl-5-methyl-2-[4-(3,3,4-trimethyl-piperazin-1-yl)-
phenylarnino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 94);
(oooo) 8-Cyclopentyl-6-fluoro-5-methyl-2-[4-(3,3,4-trimethyl-piperazin-
I-yl)-phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 95);
(pppp) 6-Bromo-8-cyclopentyl-5-methyl-2-[4-(3,3,4-trimethyl-piperazin-
1-yl)-phenylsrnino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 96);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-57-
(qqqq) 2-[4-(4-Acetyl-piperazin-1-yl)-phenylamino]-8-cyclopentyl-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 97);
(rrrr) 2-[4-(4-Acetyl-piperazin-1-yl)-phenylamino]-8-cyclopentyl-
6-fluoro-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 98),
mp 267-269°C;
(ssss) 2-[4-(4-Acetyl-piperazin-1-yl)-phenylamino]-6-bromo-
8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 99);
(tttt) 8-Cyclopentyl-2-{4-[4-(2-hydroxy-ethyl)-3,5-dimethyl-piperazin-
1-yl]-phenylamino}-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 100), mp 156-159°C;
(uuuu) 8-Cyclopentyl-6-fluoro-2-{4-[4-(2-hydroxy-ethyl)-3,5-dimethyl-
piperazin-1-yl]-phenylamino}-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound I01);
(vwv) 6-Bromo-8-cyclopentyl-2-{4-[4-(2-hydroxy-ethyl)-3,5-dimethyl-
piperazin-1-yl]-phenylamino}-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 102);
(wwww) 8-Cyclopentyl-5-methyl-2-(4-perhydro-1,4-diazepin-1-yl-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one hydrochloride (Compound 103),
mp 172°C (dec);
(xxxx) 8-Cyclopentyl-6-fluoro-5-methyl-2-(4-perhydro-1,4-diazepin-1-yl-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one hydrochloride (Compound 104),
mp 192°C (dec);
(yyyy) 6-Bromo-8-cyclopentyl-5-methyl-2-(4-perhydro-1,4-diazepin-1-yI-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 105);
(zzzz) 8-Cyclopentyl-5-methyl-2-[4-(4-methyl-piperazin-1-yl)-
phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 106),
mp 211-213°C;
(aaaaa) 8-Cyclopentyl-6-fluoro-5-methyl-2-[4-(4-methyl-piperazin-1-yl)-
phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 107);
(bbbbb) 6-Bromo-8-cyclopentyl-5-methyl-2-[4-(4-methyl-piperazin-1-yl)-
phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 108);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-58-
(ccccc) 8-Cyclopentyl-5-methyl-2-[4-(4-methyl-perhydro-1,4-diazepin-
1-yl)-phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 109),
mp >185°C (dec);
(ddddd) 8-Cyclopentyl-6-fluoro-5-methyl-2-[4-(4-methyl-perhydro-
1,4-diazepin-1-yl)-phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 110);
(eeeee) 6-Bromo-8-cyclopentyl-5-methyl-2-[4-(4-methyl-perhydro-
1,4-diazepin-1-yl)-phenylamino]-8H-pyrido [2, 3 -d]pyrimidin-7-one
(Compound 111);
(fffff) ~4-[4-(8-Cyclopentyl-5-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-piperazin-1-yl~-acetic acid
(Compound 112);
(ggggg) f 4-[4-(8-Cyclopentyl-6-fluoro-5-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-piperazin-1-yl}-acetic acid
(Compound 113);
(hhhhh) f 4-[4-(6-Brorno-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-piperazin-1-yl~-acetic acid
(Compound 114);
(iiiii) 8-Cyclopentyl-5-methyl-2-(4-~4-[3-(1H-tetrazol-5-yl)-propyl]-
piperidin-1-yl)-phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 115);
(jjjjj) 8-Cyclopentyl-6-fluoro-5-methyl-2-(4- f 4-[3-(1H-tetrazol-5-yl)-
propyl]-piperidin-1-yl}-phenylamino)-8H-pyridoj2,3-d]pyrimidin-7-one
(Compound 116);
(kkl~l~k) 6-Bromo-8-cyclopentyl-5-methyl-2-(4- f 4-[3-(1H-tetrazol-5-yl)-
propyl]-piperidin-1-yl~-phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 117);
(11111) 8-Cyclopentyl-5-methyl-2-(4- f 4-[3-(5-oxo-4,5-dihydro-1H-
1,2,4-triazol-3-ylsulfanyl)-propyl]-piperidin-1-yI}-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 1I8);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-59-
(mmnlmln) 8-Cyclopentyl-6-fluoro-5-methyl-2-(4-~4-[3-(5-oxo-
4, 5 -dihydro-1 H-1,2,4-triazol-3-ylsulfanyl)-propyl]-piperidin-1-yl } -
phenylamino)-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 119);
(nnnnn) 6-Bromo-8-cyclopentyl-5-methyl-2-(4-{4-[3-(5-oxo-4,5-dihydro-
1 H-1,2,4-triazol-3 -ylsulfanyl)-propyl] -piperidin-1-yl } -phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 120);
(ooooo) 8-Cyclopentyl-5-methyl-2-(4-{4-[3-(5-oxo-4,5-dihydro-1H-
1,2,4-triazole-3 -sulfinyl)-propyl]-piperidin-1-yl } -phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 12I);
(ppppp) 8-Cyclopentyl-6-fluoro-5-methyl-2-(4- f 4-[3-(5-oxo-4,5-dihydro-
1 H-1,2,4-triazole-3 -sulfinyl)-propyl]-piperidin-1-yl } -phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound I22);
(qqqqq) 6-Bromo-8-cyclopentyl-5-methyl-2-(4-~4-[3-(5-oxo-4,5-dihydro-
1 H-1,2,4-triazole-3 -sulfinyl)-propyl]-piperidin-1-yl } -phenylamino)-8 H-
pyrido[2,3-d]pyrimidin-7-one (Compound 123);
(rrrrr) 8-Cyclopentyl-5-methyl-2-{4- f 4-[3-(5-oxo-4,5-dihydro-1H-
1,2,4-triazole-3-sulfonyl)-propyl]-piperidin-1-yl}-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 124);
(sssss) 8-Cyclopentyl-6-fluoro-5-methyl-2-(4-~4-[3-(5-oxo-4,5-dihydro-
1H-1,2,4-triazole-3-sulfonyl)-propyl]-piperidin-1-yl}-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 125);
(ttttt) 6-Bromo-8-cyclopentyl-5-methyl-2-(4- f 4-[3-(5-oxo-4,5-dihydro-
1 H-1,2,4-triazole-3 -sulfonyl)-propyl]-piperidin-1-yl } -phenylamino)-8H-
pyrido[2,3-d]pyriinidin-7-one (Compound 126);
(uuuuu) N-(2- f 1-[4-(8-Cyclopentyl-5-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-piperidin-4-yl}-ethyl)-N-hydroxy-
acetamide (Compound 127);
(vvwv) N-(2-{1-[4-(8-Cyclopentyl-6-fluoro-5-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-piperidin-4-yI} -ethyl)-N-hydroxy-
acetamide (Compound 128);
(www~%vw) N-(2-{I-[4-(6-Bromo-8-cyclopentyl-5-methyl-7-oxo-
7, 8-dihydro-pyrido [2, 3-d]pyrimidin-2-ylamino)-phenyl]-piperidin-4-yl } -
ethyl)-
N-hydroxy-acetamide {Compound 129);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-60-
(xxxxx) N-(3-{1-[4-(8-Cyclopentyl-5-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-piperidin-4-yl}-propyl)-N-hydroxy-
acetamide (Compound 130);
(yyyyy) N-(3-{1-[4-(8-Cyclopentyl-6-fluoro-5-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-piperidin-4-yl}-propyl)-N-hydroxy-
acetamide (Compound 131);
(zzzzz) N-(3-{1-[4-(6-Bromo-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-piperidin-4-yl}-propyl)-N-hydroxy-
acetarnide (Compound 132);
(aaaaaa) 2-(Benzofuran-5-ylamino)-8-cyclopentyl-5-methyl-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 133);
(bbbbbb) 8-Cyclopentyl-2-(1H-indol-5-ylamino)-5-methyl-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 134);
(cccccc) 2-(Benzo[b]thiophen-5-ylamino)-8-cyclopentyl-5-methyl-8H-
IS pyrido[2,3-d]pyrimidin-7-one (Compound 135);
(dddddd) 8-Cyclopentyl-2-(2,3-dimethyl-1H-indol-5-ylamino)-5-methyl-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 136);
(eeeeee) 2-(9H-Garbazol-3-ylamino)-8-cyclopentyl-5-methyl-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 137);
(ffffffj 8-Cyclopentyl-2-(1H-indazol-5-ylamino)-5-methyl-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 138);
(gggggg) 2-(2-Acetyl-benzofuran-5-ylamino)-8-cyclopentyl-5-methyl-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 139);
(hhhhhh) 8-Cyclopentyl-5-methyl-2-(4-morpholin-4-yl-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 140), mp 227-229°C;
(iiiiii) 8-Cyclopentyl-2-[4-(3,5-dimethyl-piperazin-I-yl)-phenylamino]-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 141);
(jjjjjj) 2-(3-Chloro-4-piperazin-I-yl-phenylamino)-8-cyclopentyl-
5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetate (Compound 142), mp
234-237°C;
(kkkl~kk) 8-Cyclopentyl-5-methyl-2-(4-piperidin-1-yl-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 143);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-61-
(111111) 8-Cyclopentyl-5-methyl-2-[4-(4-methyl-piperazin-1-yl)-
phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 144);
(mmmmmm) N-{1-[4-(8-Cyclopentyl-5-methyl-7-oxo-7,8-dihydro-
pyrido [2,3-d]pyrimidin-2-ylamino)-phenyl]-piperidin-4-yl}-acetamide
(Compound 145);
(rmnnrm) 8-Cyclopentyl-5-methyl-2-(4-piperazin-1-yl-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one trifluoroacetate (Compound 146), mp 237-
240°C;
(oooooo) 8-Cyclopentyl-6-fluoro-5-methyl-2-(4-piperazin-1-yl-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 147),
mp 254-255°C;
(pppppp) 6-Iodo-8-cyclopentyl-5-methyl-2-(4-piperazin-1-yl-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 148);
(qqqqqq) 2-[3-Chloro-4-(3-amino-pyrrolidin-1-yl)-phenylamino]-8-
cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetate
(Compound 149), mp >215°C (dec);
(rrrrrr) 8-Cyclopentyl-5-methyl-2-[4-(4-(2,2,2-trifluoroethyl)-piperazin-1-
yl)-phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 150),
mp 198-199°C;
(ssssss) 8-Cyclopentyl-2-(4-fluoro-phenylamino)-5-methyl-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 151), mp 217-220°C;
(tttttt) 8-Cyclopentyl-5-methyl-2-phenylamino-8H-pyrido[2,3-
d]pyrimidin-7-one (Compound 152), mp 180-183°C;
(uuuuuu) 8-Cyclopentyl-2-(3,4-dichlorophenylamino)-5-methyl-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 153), mp 225-230°C;
(www) 8-Isopropyl-5-methyl-2-(4-piperazin-1-yl-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one trifluoroacetate (Compound 154), mp >235°C
(dec);
(wwvwvww) 8-Isopropyl-5-methyl-2-[4-(4-propionyl-piperazin-1-yl)-
phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 15S);
(xxxxxx) 8-Cyclohexyl-5-methyl-2-(4-piperazin-1-yl-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 156);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-62-
(yyyyyy) 2- f 4-[4-(3-Morpholin-4-yl-propyl)-piperidin-1-yl]-
phenylamino } -8-cyclohexyl-6-fluoro-5-methyl-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 157), mp 206-209°C;
(zzzzzz) 8-Cyclopentyl-5-methyl-2-[4-(2H-1,2,4-triazol-3-
ylsulfanylmethyl)-phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 158);
(aaaaaaa) 8-Cyclopentyl-5-methyl-2-[4-(2H-1,2,4-triazole-3-
sulfinylmethyl)-phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 159);
(bbbbbbb) 8-Cyclopentyl-5-methyl-2-[4-(2H-1,2,4-triazole-3-
sulfonylmethyl)-phenylamino]-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 160);
(ccccccc) 8-Cyclopentyl-5-methyl-2-[4-(5-oxo-4,5-dihydro-1,2,4-
oxadiazol-3-ylmethyl)-phenylamino]-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 161);
(ddddddd) 8-Cyclopentyl-5-methyl-2-{4-[2-(2H-1,2,4-triazol-3-
ylsulfanyl)-ethyl-phenylamino }-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 162);
(eeeeeee) 8-Cyclopentyl-5-methyl-2- f 4-[2-(2H-1,2,4-triazole-3-sulfinyl)-
ethyl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 163);
(fffffff) 8-Cyclopentyl-5-methyl-2- f 4-[2-(2H-1,2,4-triazole-3-sulfonyl)-
ethyl]-phenylamino}-8H-pyrido[2,3-d]pyrirnidin-7-one (Compound 164);
(ggggggg) 8-Cyclopentyl-5-methyl-2- f 4-[2-(5-oxo-4,5-dihydro-1,2,4-
oxadiazol-3-yl)-ethyl]-phenylamino } -8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 165);
(hhhhhhh) 8-Cyclopentyl-5-methyl-2-[4-(3H-1,2,3-triazol-4-
ylsulfanylmethyl)-phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 166);
(iiiiiii) 8-Cyclopentyl-5-methyl-2- f 4-[2-(3H-1,2,3-triazol-4-ylsulfanyl)-
ethyl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 167);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-63-
(jjjjjjj) 8-Cyclopentyl-5-methyl-2- f 4-[4-(5-oxo-4,5-dihydro-1,2,4-
oxadiazol-3-yl)-piperidin-I-yl]-phenylamino }-8H-pyrido [2,3-d]pyrirnidin-7-
one
(Compound 168);
(kkklckkk) 8-Cyclopentyl-5-methyl-2-{4-[4-(2H-1;2,4-triazol-3-
S ylsulfanyl)-piperidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrirnidin-7-one
(Compound 169);
(I1111I1) 8-Cyclopentyl-5-methyl-2- f 4-[4-(2H-1,2,4-triazole-3-sulfinyl)-
piperidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 170);
(mmmmn~n) 8-Cyclopentyl-5-methyl-2- f 4-[4-(2H-1,2,4-triazole-3-
sulfonyl)-piperidin-1-yl]-phenylamino}-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 171), mp 235-237°C;
(nnmmnn) 8-Cyclopentyl-5-methyl-2- f 4-[4-(2H-tetrazol-5-yl)-piperidin-
I-yI]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 172);
(ooooooo) 1-[4-(8-Cyclopentyl-5-methyl-7-oxo-7,8-dihydro-pyrido[2,3-
d]pyrimidin-2-ylamino)-phenyl]-piperidine-4-carboxylic acid (1H-tetrazol-5-yl)-
amide (Compound 173);
(ppppppp) 8-Cyclopentyl-5-methyl-2-~4-[4-(3H-1,2,3-triazol-4-
ylsulfanyl)-piperidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 173);
(qqqqqqq) 3-[4-(8-Cyclopentyl-5-methyl- 7-oxo-7,8-dihydro-pyrido[2,3-
d]pyrimidin-2-ylamino)-phenyl]-N-( 1 H-tetrazol-5-yl)-propionamide
(Compound 174);
(rrrrrrr) 2-[4-(8-Cyclopentyl-5-methyl-7-oxo-7,8-dihydro-pyrido[2,3-
d]pyrimidin-2-ylamino)-phenoxy]-N-(IH-tetrazol-5-yl)-acetamide
(Compound 175);
(sssssss) 8-Cyclopentyl-5-methyl-2-[4-(5-oxo-4,S-dihydro-1,2,4-
oxadiazol-3-ylmethoxy)-phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 176), mp >195°C (dec);
(ttttfitt) 8-Cyclopentyl-5-methyl-2-(4-~4-[2-(2H-1,2,4-triazole-3-sulfmyl)-
ethyl]-piperidin-1-yl } -phenylamino)-8H-pyrido [2, 3 -d]pyrimidin-7-one
(Compound 177);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-64-
(uuuuuuu) 8-Cyclopentyl-5-methyl-2-(4-{4-[2-(2H-1,2,4-triazole-3-
sulfonyl)-ethyl]-piperidin-1-yl}-phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 178);
(wwwv) 8-Cyclopentyl-5-methyl-2-(4-{4-[2-(3H-1,2,3-triazol-4-
ylsulfanyl)-ethyl]-piperidin-1-yl}-phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-
one
(Compound 179);
(~n~w~wwww) 8-Cyclopentyl-5-methyl-2-(4- f 4-[2-(2H-1,2,4-triazol-3-
ylsulfanyl)-ethyl]-piperidin-1-yl}-phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-
one
(Compound 180), mp 234-237°C;
(xxxxxxx) 8-Cyclopentyl-5-methyl-2-(4-{4-[2-(5-oxo-4,5-dihydro-1,2,4-
oxadiazol-3-yl)-ethyl]-piperidin-1-yl } -phenylamino)-8H-pyrido [2, 3 -
d]pyrimidin-
7-one (Compound 181);
(yyyyyyy) 8-Cyclopentyl-5-methyl-2-{4-[4-(2-oxo-2,3-dihydro-1,2,3,5-
oxathiadiazol-4-yl)-piperidin-1-yl]-phenylamino }-8H-pyrido [2,3-d]pyrimidin-7-
one (Compound 182);
(zzzzzzz) 8-Cyclopentyl-2-~4-[4-(2,2-dioxo-2,3-dihydro-1,2,3,5-
oxathiadiazol-4-yl)-piperidin-1-yl]-phenylamino}-5-methyl-8H-pyrido[2,3-
d]pyrimidin-7-one (Compound 183);
(aaaaaaaa) 8-Cyclopentyl-5-methyl-2-~4-[4-(1-oxo-2,5-dihydro-1H-
1,2,3,5-thiatriazol-4-yl)-piperidin-1-yI]-phenylamino}-8H-pyrido[2,3-
d]pyrimidin-7-one (Compound 184);
(bbbbbbbb) 8-Cyclopentyl-2-{4-[4-(l,l-dioxo-2,5-dihydro-1H-1,2,3,5-
thiatriazol-4-yl)-piperidin-1-yl]-phenylamino } -5-methyl-8H-pyrido [2, 3 -
d]pyrimidin-7-one (Compound I85);
(cccccccc) N- f I-[4-(8-Cyclopentyl-5-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-piperidine-4-carbonyl}-
methanesulfonamide (Compound 186);
(dddddddd) 8-Cyclopentyl-5-methyl-2- f 4-[3-(2H-1,2,4-triazol-3-
ylsulfanyl)-pyrrolidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one
{Compound 187);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-65-
(eeeeeeee) 8-Cyclopentyl-5-methyl-2-{4-[3-(2H-I,2,4-triazole-3-
sulfmyl)-pyrrolidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 188);
(ffffffff) 8-Cyclopentyl-5-methyl-2-{4-[3-(2H-1,2,4-triazole-3-sulfonyl)-
pyrrolidin-1-yl]-phenylarnino}-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 189);
(gggggggg) 8-Cyclopentyl-5-methyl-2-{4-[3-(3H-1,2,3-triazol-4-
ylsulfanyl)-pyrrolidin-1-yl]-phenylamino }-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 190);
(hhhhhhhh) 8-Cyclopentyl-5-methyl-2-{4-[3-(5-oxo-4,5-dihydro-1,2,4-
oxadiazol-3-yl)-pyrrolidin-1-yl]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 191);
(iiiiiiii) 8-Cyclopentyl-5-methyl-2-{4-[4-(3-hydroxypropyl)piperidin-1-
yI]-phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetate
(Compound 192), mp 180-184°C;
(jjjjjjjj) 8-Cyclopentyl-5-propyl-2-(4-piperazin-1-yl-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one trifluoroacetate (Compound 193);
(kkkkht~lckk) 8-Cyclopentyl-5-ethyl-2-(4-piperazin-1-yl-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 194);
(11111111) 8-(1-Methylethyl)-5-ethyl-2-(4-piperazin-1-yl-phenylamino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 195), mp >235°C (dec);
(mmmmmmmm) 8-(1-Methylethyl)-5-ethyl-2-(4-piperazin-1-yl-
phenylamino)-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetate
(Compound 196), mp >235°C (dec);
(nnnnnnnn) 8-Cyclopentyl-5-methyl-2-[4-(3-hydroxypyrrolidin-1-
yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 197),
mp 225-226°C;
(oooooooo) 8-Cyclopentyl-5-ethyl-2-[4-(4-acetylpiperazin-1-
yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 198);
(pppppppp) 8-Cyclopentyl-5-methyl-6-fluoro-2-[4-(4-acetylpiperidin-1-
yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 199),
mp 267-269°C;
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-66-
(qqqqqqqq) 8-Cyclopropyl-5-methyl-2-[4-(4-acetamidopiperidin-1-
yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 200), mp
221°C
(dec);
(qqqqqqqr) 8-Cyclopropyl-5-methyl-6-fluoro-2-[4-(4-acetamidopiperidin-
1-yl) phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 201),
mp >250°C;
(rrrrrrrr) 8-Cyclopentyl-5-methyl-2-[4-(homopiperazin-1-
yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one hydrochloride
(Compound 202), mp 172°C (dec);
(ssssssss) 8-Cyclopentyl-5-methyl-6-fluoro-2-[4-(homopiperazin-I-
yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one hydrochloride
(Compound 203), mp 192°C (foam);
(tttttttt) 8-Cyclopentyl-5-methyl-6-fluoro-2-[4-(3,3-dimethyl-4-
acetylpiperazin-1-yl)phenylamino]-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 204), mp 200-204°C;
(uuuuuuuu) 8-Cyclopentyl-5-methyl-2-[4-(3,3-dimethyl-4-
acetylpiperazin-1-yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 205), mp 192-196°C;
(vwwvvv) 8-Cyclopentyl-5-methyl-6-fluoro-2-[4-(4-methylpiperazin-1-
yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one trifluoroacetate
(Compound 206);
(vvwwvvwwwW) 8-Cyclopentyl-5-methyl-2-[4-(N-
methylacetamido)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 207), mp 185-187°C;
(xxxxxxxx) 8-Cyclopentyl-5-methyl-2- f 4-[2-(2-
hydroxyethoxy)ethylamino]phenylamino~-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 208), mp 122-126°C;
(yyyyyyyy) 8-Cyclopentyl-5-methyl-2-[4-(3-oxopiperazin-1-
yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 209), mp
>235°C
(dec);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-67-
(zzzzzzzz) 8-Cyclopentyl-5-methyl-2-[4-(2-
methoxyethoxy)phenylamino]-8H-pyrido [2,3-d]pyrimidin-7-one
(Compound 210), mp 156-157°C;
(aaaaaaaaa) 8-Cyclopentyl-5-methyl-2-(9H carbazol-3-yl amino)-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 211);
(bbbbbbbbb) 8-Cyclopentyl-5-methyl-2-(1H-indazol-5-yl)amino-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 212);
(ccccccccc) 8-Cyclopentyl-5-methyl-2-(2-acetylbenzofuran-5-yl)amino-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 213);
(ddddddddd) 8-Cyclopentyl-5-methyl-2-[(4-piperidin-1-yl)phenylamino]-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 214);
(eeeeeeeee) 8-Cyclopentyl-5-methyl-2-(2,3-dimethylindol-S-yl)amino-
8H-pyrido[2,3-d]pyrimidin-7-one (Compound 215);
(fffffffff) 8-Cyclopentyl-5-isopropyl-2-[4-(3,5-methyl-4R-
aminomethylpyrrolidin-1-yl)phenylamino]-8H-pyrido[2,3-d]pyrimidin-7-one
(Compound 216);
(ggggggggg) 8-Cyclopentyl-5-methyl-2-~4-[4-(2-hydroxyethyl)piperazin-
1-yl)]phenylamino}-8H-pyrido[2,3-d]pyrimidin-7-one (Compound 217),
mp 171-173°C;
(hhhhhhhhh) 8-Cyclopentyl-5-methyl-2-{4-[4-(3-
morpholinopropyl)piperidin-1-yl]phenylamino ~ -8H-pyrido [2,3-d]pyrimidin-7-
one
trifuoroacetic acid salt (Compound 218), mp 178-181 °C;
(iiiiiiiii) 8-Cyclopentyl-5-methyl-2-(benzofuran-5-yl)amino-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 219);
(jjjjjjjjj) 8-Cyclopentyl-5-methyl-2-(indol-5-yl)amino-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 220); and
(I~l~lckl~l~kl~lc) 8-Cyclopentyl-5-methyl-2-(thionaphthen-5-yl)amino-8H-
pyrido[2,3-d]pyrimidin-7-one (Compound 221);.
8-Cyclopentyl-6-iodo-5-methyl-2-(4-piperazin-1-yl-phenylamino)-8H
pyrido[2,3-d]pyrimidin-7-one (Compound 225), mp 185-198°C (dec);
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-68-
8-Cyclopentyl-2-~4-[1-(3,S-dimethyl-piperazin-1-yl)-methanoyl]-
phenylamino}-S-methyl-8H pyrido[2,3-d]pyrimidin-7-one (Compound 226),
mp 181 (foam);
8-Cyclopentyl-2-[4-(3,S-dimethyl-piperazin-1-yl)-phenylamino]-S-
S trifluoromethyl-8H pyrido[2,3-d]pyrimidin-7-one (Compound 227),
mp 200(foam);
6-Bromo-8-cyclopentyl-2-[4-(3,3-dimethyl-piperazin-1-yl)-phenylamino]-
S-methyl-8H pyrido[2,3-d]pyrimidin-7-one (Compound 228), mp >200 (dec);
8-Cyclopentyl-2-[4-(3,S-dimethyl-piperazin-I -yl)-phenylamino]-6-iodo-S-
methyl-8H pyrido[2,3-d]pyrimidin-7-one (Compound 229), mp 22S-226°C
(dec);
6-Chloro-8-cyclopentyl-2-[4-(3,S-dimethyl-piperazin-1-yl)-phenylamino]-
S-methyl-8H pyrido[2,3-d]pyrimidin-7-one (Compound 230), mp
>2S0°C;
8-Cyclopentyl-S-methyl-2-[4-( 1 H-[ 1,2,4]triazol-3-ylsulfanyl)-
phenylamino]-8H pyrido[2,3-d]pyrimidin-7-one (Compound 231);
1 S 4-[4-(8-Cyclopentyl-S-methyl-7-oxo-7,8-dihydro-pyrido[2,3-d]pyrimidin-
2-ylamino)-phenyl]-piperazine-I-carbaldehyde (Compound 232), mp 244-
247°C;
8-Cyclopentyl-2-(4-piperazin-1-yl-phenylamino)-S-trifluoromethyl-8H
pyrido[2,3-d]pyrimidin-7-one (Compound 233), mp >27S°C (dec);
8-( 1-Ethyl-propyl)-S-methyl-2-(4-piperazin-1-yl-phenylamino)-8H
pyrido[2,3-d]pyrimidin-7-one (Compound 234), mp >180°C (dec);
[4-(8-Cyclopentyl-S-methyl-7-oxo-7,8-dihydro-pyrido[2,3-d]pyrimidin-2-
ylarnino)-benzyl]-phosphonic acid (Compound 235), mp >2S0°C;
6-Chloro-8-cyclopentyl-S-methyl-2-(4-piperazin-1-yl-phenylamino)-8H
pyxido[2,3-d]pyrimidin-7-one (Compound 236), mp 188°C (dec);
2S 2-[4-(3,S-Dimethyl-piperazin-1-yl)-phenylamino]-8-(1-ethyl-propy1)-S-
methyl-8H pyrido[2,3-d]pyrimidin-7-one (Compound 237), mp >18S°C (dec);
8-Cyclopentyl-2-[4-(2-hydroxy-ethylamino)-phenylamino]-S-methyl-8H
pyrido[2,3-d]pyrimidin-7-one (Compound 238), mp 197-200°C;
3-[4-(8-Cyclopentyl-S-methyl-7-oxo-7,8-dihydro-pyrido[2,3-d]pyrimidin-
2-ylamino)-phenyl]-N,N diethyl-propionamide (Compound 239), mp I38-
I40°C;
8-Cyclopentyl-6-fluoro-2-[4-(2-hydroxy-ethyl)-phenylamino]-S-methyl-
8H pyrido[2,3-d]pyrimidin-7-one (Compound 240), mp 241-244°C;
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-69-
8-Cyclopentyl-2-[4-(2-hydroxy-ethyl)-phenylamino]-5-methyl-8H
pyrido[2,3-d]pyrimidin-7-one (Compound 241), mp 191-194°C;
4-(8-Cyclopentyl-5-methyl-7-oxo-7, 8-dihydro-pyrido [2,3-cl]pyrimidin-2-
ylamino)-benzoic acid (Compound 242);
8-Cyclopentyl-2-[4-(3,3-dimethyl-piperazin-1-yl)-phenylamino]-5-methyl-
8H pyrido[2,3-d]pyrimidin-7-one (Compound 243), mp >150°C (dec);
[4-(8-Cyclopentyl-5-methyl-7-oxo-7, 8-dihydro-pyrido [2,3 -d]pyrimidin-2-
ylamino)-benzyl]-phosphonic acid diethyl ester (Compound 244),
mp >250°C (foam);
8-Cyclopentyl-6-fluoro-2-[4-(2-methoxy-ethylamino)-phenylamino]-5-
methyl-8H pyrido[2,3-d]pyrimidin-7-one (Compound 245), mp 147-149°C;
(S)-2-Amino-3-[4-(8-cyclopentyl-6-fluoro-5-methyl-7-oxo-7,8-dihydro-
pyrido[2,3-d]pyrimidin-2-ylamino)-phenyl]-propionic acid (Compound 246),
mp 238°C (dec);
8-Cyclopentyl-2-[4-(2-methoxy-ethoxy)-phenylamino]-5-methyl-8H
pyrido[2,3-d]pyrimidin-7-one (Compound 247), mp 156-157°C;
8-Cyclopentyl-2-(4-isopropylamino-phenylamino)-5-methyl-8H
pyrido[2,3-d]pyrimidin-7-one (Compound 248), mp 216-220°C;
8-Cyclopentyl-2-(4-hydroxy-3, 5-dimethyl-phenylamino)-5-methyl-8H
pyrido[2,3-d]pyrimidin-7-one (Compound 249), mp 252-254°C;
8-Cyclopentyl-6-fluoro-2-(4-hydroxy-3,5-dimethyl-phenylamino)-5-
methyl-8H pyrido[2,3-d]pyrimidin-7-one (Compound 250), mp 241-248°C;
8-Cyclopentyl-6-fluoro-2-(4-hydroxy-3,5-dimethyl-phenylamino)-5-
methyl-8H pyrido[2,3-d)pyrimidin-7-one (Compound 251), mp 240°C (dec).
EXAMPLE 19
Biological Assays
Several of the invention compounds have been evaluated in standard
assays routinely used to measure inhibition of cyclin-dependent kinase enzymes
and other serine/threonine protein kinases. The assays were carried out as
follows:
Cdlcl and Cdk2 enzyme assays for IC50 determinations and kinetic
evaluation are performed as follows. 96-well filter plates (Millipore
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
_70_
MADVN6550) are used. The total volume is 0.1 mL 20 mM TRIS (pH 7.4),
50 mM NaCI, 1 mM dithiothreitol, 10 mM MgCl2, 12 mM ATP containing
0.25 ~,Ci [32P]ATP, 20 ng enzyme (Cdk2/cyclin E, Cdk2/cyclin A, or
Cdkl/cyclin B), 1 ~g retinoblastoma protein, and appropriate dilutions of the
particular invention compound. All components except the ATP are added to the
wells, and the plate is placed on a plate mixer for 2 minutes. The reaction is
initiated by addition of [32P]ATP, and the plate is incubated at 25°C
fox
minutes. The reaction is terminated by addition of 0.1 mL 20% TCA. The plate
is kept at 4°C for at least 1 hour to allow the substrate to
precipitate. The wells are
10 then washed five times with 0.2 mL 10% TCA, and 32P incorporation is
determined with a beta plate counter (Wallac Inc., Gaithersburg, MD).
The Cdk4 enzyme assay for IC50 determination and kinetic evaluation is
performed as follows. 96-well filter plates (Millipore MADVN6550) are used.
The total volume is 0.1 mL containing a final concentration of 20 mM TRIS
15 (tris[hydroxymethyl]aminomethane) (pH 7.4), 50 mM NaCI, 1 mM
dithiothreitol,
10 mM MgCl2, 25 ~M ATP containing 0.25 ~Ci [32P]ATP, 20 ng Cdk4, 1 ~g
retinoblastoma protein, and appropriate dilutions of a compound of the present
invention. All components except the ATP axe added to the wells, and the plate
is
placed on a plate mixer for 2 minutes. The reaction is started by adding
[32P]ATP,
and the plate is incubated at 25°C for 15 minutes. The reaction is
terminated by
addition of 0~1 mL 20% trichloroacetic acid (TCA). The plate is kept at
4°C for at
Ieast 1 hour to allow the substrate to precipitate. The wells are then washed
five
times with 0.2 mL 10% TCA, and 32P incorporation is determined with a beta
plate counter (Wallac Inc., Gaithersburg, MD).
Fox PDGF receptor (PDGFr) and FGF receptor (FGFr) tyrosine kinase
assays, full-length cDNAs for the mouse PDGF-~ and human FGFl(flg) receptor
tyrosine kinases are obtained from J. Escobedo and prepared as described
previously (Escobedo et al., J. Biol. Chem., 1988;262:1482-1487). PCR primers
are designed to amplify a fragment of DNA that codes for the intracellular
tyrosine lcinase domain. The fragment is inserted into a baculovirus vector,
cotransfected with AcMNPV DNA, and the recombinant virus is isolated. SF9
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-71-
insect cells are infected with the virus to overexpress the protein, and. the
cell
lysate is used for the assay.
PDGFr and FGFr enzyme assays are performed in 96-well plates
(100 ~L/incubation/well), and conditions are optimized to measure the
S incorporation of 32P from [y32P]ATP into a glutamate-tyrosine co-polymer
substrate. Briefly, ~to each well is added X2.5 ~L incubation buffer
containing
2S rnM Hepes (pH 7.0), 1S0 mM NaCl, 0.1% Triton X-100, 0.2 mM PMSF,
0.2 mM Na3V04, 10 mM MnCl2, and 7S0 ~g/mL Poly (4:1) glutamate-tyrosine
followed by 2.S ~,L inhibitor and S ~.L enzyme lysate (7.S ~g/~.~L FGFr or
6.0 ~.g/~.L PDGFr) to initiate the reaction. Following a 10-minute incubation
at
2S°C, 10 mL [y32P]ATP (0.4 ~,Ci plus SO ~,M ATP) is added to each well,
and
samples are incubated for an additional 10 minutes at 2S°C. The
reaction is
terminated by the addition of 100 ~.L 30% trichloroacetic acid (TCA)
containing
mM sodium pyrophosphate and precipitation of material onto glass fiber mats
1S (Wallac). Filters are washed three times with 1S% TCA containing 100 mM
sodi~,un pyrophosphate, and the radioactivity retained on the filters is
counted in a
Wallac 1250 Betaplate reader. Nonspecific activity is defined as radioactivity
retained on the filters following incubation of samples with buffer alone (no
enzyme). Specific enzymatic activity (enzyme plus buffer) is defined as total
20 activity minus nonspecific activity. The concentration of a compound that
inhibited specific activity by SO% (ICSO) is determined based on the
inhibition
curve.
The c-Src protein kinase assay is carried out as follows. c-Src kinase is
purified from baculovirus infected insect cell lysates using an antipeptide
2S monoclonal antibody directed against the N-terminal amino acids amino acids
2-17) of c-Src. The antibody, covalently linked to 0.65 ym latex beads, is
added to
a suspension of insect cell lysis buffer comprised of 1 SO mM NaCl, SO mM Tris
(pH 7.S), 1 mM DTT, 1% NP-40, 2 mM EGTA, 1 mM sodium vanadate, 1 mM
PMSF, 1 ~.glmL each of leupeptin, pepstatin, and aprotinin. Insect cell lysate
containing c-Src protein is incubated with these beads for 3 to 4 hours at
4°C with
rotation. At the end of the lysate incubation, the beads are rinsed three
times in
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
_72_
lysis buffer, resuspended in lysis buffer containing 10% glycerol, and frozen.
These latex beads are thawed, rinsed three times in assay buffer (40 mM Tris
(pH 7.5), 5 mM ~,g C12) and suspended in the same buffer. Tn a Millipore 96-
well
plate with a 0.65 ~.m polyvinylidine membrane bottom are added the reaction '
components: 10 ~,L c-Src beads, 10 ~,L 2.5 mg/mL poly GluTyr substrate, 5 ~uM
ATP containing 0.2 ~,Ci [32P]ATP, 5 ~,L DMSO containing inhibitors or as a
solvent control, and buffer to make the final volume 125 ~L. The reaction is
started at room temperature by addition of ATP and quenched 10 minutes later
by
the addition of 125 qL 30% TCA, 0.1 M sodium pyrophosphate for 5 minutes on
ice. The plate is then filtered and the wells washed with two 250-mL aliquots
of
15% TCA, 0.1 M pyrophosphate. The filters are then punched, counted in a
liquid
scintillation counter, and the data examined for inhibitory activity in
comparison
to a known inhibitor such as erbstatin. The method has been described by
Thompson et al., J. Med. Chem., 1994;37:598-609.
The results of the foregoing assays for several representative invention
compounds axe presented in Table 1. The metabolic stability of representative
Compounds was evaluated in human liver microsomes (HLM) and is given in
Table 1 as the time in minutes (T Half) required for one-half of the parent
compound to disappear after being added to a HLM homogluate.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-73-
w
xx ~~ z zz z z z
~ ° ~~c~~~~~o ~~-~~ ~~~~~oo
~.zzzzzz,~ zMz~ zzzz n n ~ n z
w~ ICI°zz~z °zM ~ z ~ ~° ~zzzzzz ~ z
a- Wn d- co ,~.-. d- ,--~ ~ ,-, 00 0 ~n ~n d-
O O ~ \O N ~ ~n \O l~ ~ ~ d' M O 01 W
U ~7 ,~, O N ~ O O ~ .--i ~ O 41 O O O O O N d' ~ ~
~.,U ~.oozoozooooo ooooocici n ~o
U N
M
~1 U'~ tn tn V7 ~ t!7 t~ tn In O ~ 01 V') tn
~', r, U U ~. n n n n n n n n n n n n n n n M n n z n
R-,
a1
00 M 00 ~
O l~ l~ M I~ l~ d: O~ Q1
U ~n n n n n ~' ~ n n n n n ~ n o n ~ '~ n n n
U
N
''-' p ~n ,--~ l~ I~ ~ l~ l~ l~
U ~~.. n n n n n ~ n n n n n n n n '~ n n n n ~ n
U
.-.
U ~ ~t~ ~1~01 U N ~ N01M ~ V) 01OM001d'
m ,-O ..O M ,-O M N ~ O "~3 ~t3 '~c3 ~ ~' ~Cf ~ O 01 N 0o M O 00
O ~~N ~N N "a M u'ra~-~ N 'rN O ~ N ~ N N ~
O i i i ~ ~ ~ i i ~ ~ i ~ i
v'W n ~n ~n d' l~ O N ~n O ~n ~O ~ ~ d- N o0 l~ O ~n ~D O
~ M M 01 M N p0 n 'w-~ ~ ~ ~ ~ ~ ~ n 01 ~ 00 N O 00
n n N ~ N N n ~ % ~ ~-1 N % N ~ N '-' N N .--~ y
+~
O
~-~ N l~ N ~ in t~ oo M 01 N O ~O a1 ~ o0 O .-~ N M l~ N
O ,--.~ ~ d- ~n ~n l~ l~ oo O O O d- d' ~ ~n ~n ~n in 01
r~ r~ r-, r-1 r-I r-I w-1 r1 r1 r1 r--I
~z z
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-74-
H
U ° N N
U ~, ~ o
~O ~ M
U ( ~.I dm.. ~ °~ n o0
m M .-m~ ~n N ~ N O O ~n ~n
O ~n ~O O d' oo I~ ~n oo .-~ l~ d- oo ~n M ~O ~n O t~ N
~O o0 N ~ ~ N ~ O O M M O d' d' O d: M O ~ O
r~",~ ~- O CV O O O O O O O O O O O O O O r-i O O ~ O
~M°, ~ o
~N~v ~n n n n n n n n n n n n n n n n n°'''n'~'
~U
d- o ~ dN- ~n M
O ~ ~ O1 M ~ 01 d: 00 M
U ~- /\ /~ /\ ~-' ~' /~ ~ ~ ~ n n n n n n ~ N O N n ri
H
U
N
O ~ ~n ,-~ 00
U ~ n ~ n n n n n n N ~ n n n n n ~ n ~ n n n
U
U
U V ~O M 01 U U d' ~O l~ ~D V O V
M .-~
v~ ..~ N WO N N O 01 00 N ~ l~
N CO N
''
"
O N ,--a ,-~ .-r ~ ,-.,
~ N N N ~ ,--., O ~
L3 ,_, 0o O
d tn ~
n
~
~ N ~ ~
~n t -~ pp
, ~n N O , ~
l '"' N
O N
~
'~ n N
~
M N o0 N M l~ O
O l~
O
O O~
N N N N ~ N ,-a ,--~ ..-~ ~ N
~ ,-.,
,-~
O
M vo t~ oo O~ O ,-~ N M ~ ~W o ~ oo ~o ~ oo N ~W o ~ 00
a1 a1 01 01 01 O O O O O O O O O ~ ~ ~--~ N N N N N
~ N N N N N N N N N N N N N N N N NIII
O
z
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-75-
w
H
~U
U '-'
0
L7 'n
wH
V7 O ~ ~ M ~
O ~ ~O ~ N N n ~n t~
O .--i O N d; M O N
~-. O O O O O O .--~ n O
M W N
c~., ~ ° l~ ~ d; t~
M U U ~.° n ~ n M n n n
H
~_~n
O ~ M lp d: l!7
U ~°'°'-'n n n°'n'°
H
J
a,
0
~ ~'~n~'nnnnnn
?r H
U O d' 01 U CO
'~
~p N '~N ~ ~ '~N ,d;
~
N d' 00 t~ .--y~ ,.fl
pp ~O O
b~A Nd-~p~O1 dy.,~d-d',~
r-, ,--i ,-~ '
~ N
N ~'~
N c~
~,
N
O
~d cd
01 N ~ 00 .-y
OD O
N N N N N N N
N N
U
Z
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
-76-
Vaxious properties of preferred 5-methylpyridopyrimidin-7-ones such as
8-cyclopentyl-5-methyl-2-[(4-piperazinylphenyl)-amino]-8-hydxopyridino [2,3-
d]pyrimidin-7-one (Compound 1), including IC50, stability, and clearance rate,
are displayed in Table 2.
Table 2
Compoutzd5-Me IC50 T%z in Clearance
(~M)
Cdkl/ Cdlc2/Cdk2/ Cdk4 c-Src PDGFrHLM (mL/min/lcg)
FGFr
cyclincycliiicyclin (min)
B A E
1 Yes >5 >5 >5 0.0071.077 83 25.5 (n
= 2)
57 Yes >5 >5 >5 0.151 6.08
73 Yes >1.7 >1.7 >5 0.061>5 80
79 Yes >5 >5 >5 0.975NA 34 8
~
152 Yes >1.7 4.91 >5 2.15NA >50 4
From the xesults displayed in Table 2, it is clear that Compound 1 and
other invention compounds specifically inhibit Cdk4, and has relatively little
effect on Cdkl and Cdlc2. Furthezmoxe, Compound 1 is relatively more stable,
and
cleared at a slower rate, compared to prior art compounds. These results
indicate
that the methyl group in the S-position confexs unique properties onto the
pyridopyrimidine and is a preferred embodiment.
Fo~r~ulatiofi Exauiples
As noted above, the invention compounds will typically be formulated
with common excipients, diluents, and carriers to provide compositions that
are
well-suited for convenient administration to mammals. The following examples
illustrate typical compositions that are provided in a further embodiment of
this
invention.
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
EXAMPLE 20
Tablet Formulation
Ingredient Amount
Compound 12 50 mg
Lactose 80 mg
Cornstarch (for mix) 10 mg
Cornstarch (for paste)8 mg
Magnesium Steaxate 2 mg
( 1 %)
150 mg
Compound 12 is mixed with the lactose and cornstarch (for mix) and
blended to uniformity to a powder. The cornstarch (fox paste) is suspended in
6 mL of water and heated with stirring to form a paste. The paste is added to
the
mixed powder, and the mixture is granulated. The wet granules are passed
through
aNo. 8 hard screen and dried at 50°C. The mixture is lubricated with 1%
magnesium stearate and compressed into a tablet. The tablets are adminstered
to a
patient at the rate of 1 to 4 each day for prevention and treatment of
atherosclerosis.
EXAMPLE 21
Pa~entet~al Solutiosz
In a solution of 700 mL of propylene glycol and 200 mL of water for
injection is added 20.0 g of Compound 38. The mixture is stirred and the pH is
adjusted to 5.5 with hydrochloric acid. The volume is adjusted to 1000 mL with
water for injection. The solution is sterilized, filled into 5.0 mL ampoules,
each
containing 2.0 mL (40 mg of Compound 38), and sealed under nitrogen. The
solution is administered by injection to a patient suffering from cancer and
in need
of treatment.
The invention and the manner and process of making and using it, are now
described in such full, clear, concise, and exact terms as to enable any
person
skilled in the art to which it pertains, to make and use the same. It is to be
understood that the foregoing describes preferred embodiments of the present
CA 02401368 2002-08-27
WO 01/70741 PCT/USO1/02657
_78,
invention and that modifications rnay be made therein without departing from
the
spirit or scope of the present invention as set forth in the claims. To
particularly
point out and distinctly claim the subject matter regarded as invention, the
following claims conclude this specification.