Note: Descriptions are shown in the official language in which they were submitted.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
SHAPED PARTICLE AND COMPOSITION FOR BONE DEFICIENCY
AND METHOD OF MAKING THE PARTICLE
The present invention generally relates to a shaped particle as a
bone graft substitute and the use of such a substitute to repair, replace,
augment or improve a bone deficiency. The invention also relates to a
composition having such a particle in a suspension material to enhance
the utility of the particle as a bone graft substitute. Furthermore, a method
of making an improved hardened calcium sulfate material for a shaped
particle is provided.
Bone graft is used to fill spaces in bone tissue that are the result of
trauma, disease degeneration or other loss of tissue. Clinicians perform
bone graft procedures for a variety of reasons, often to fill a bone void
created by a loss of bone or compaction of cancellous bone. In many
instances the clinician also must rely on the bone graft material to provide
some mechanical support, as in the case of subchondral bone
replacement or compaction grafting around total joint replacement devices.
In these instances, clinicians pack the material into the defect to create a
stable platform to support the surrounding tissue and hardware.
There are several options available to the orthopaedic clinician for
bone graft material. Most commonly, the source of the graft material is
either the patient (autograft) or a donor (allograft). In autograft and, to a
lesser extent, in allograft there are biological factors such as proteins or
cells that are present that can assist in the fracture healing process.
Xenografts and bone graft substitutes are other options.
Autograft is taken from the patient's own body and is the most
commonly used graft material. The graft, which can come in the form of
chips or blocks, is harvested from an ectopic bone site within the body,
such as the iliac crest, and used in the deficient site. Autograft has the
potential draw back of increased pain and morbidity associated with a
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
2
second surgical procedure, in addition to having a limited supply of the
bone.
Allograft is another form of graft which comes from human bone
tissue donated to tissue banks, such as from a cadaver. Allograft is
available in a number of forms: granules or chips, blocks or struts, and
processed forms such as gels or putties. In addition to having a limited
supply, a serious drawback of allograft is the risk of disease transmission.
Xenografts are one such choice which come from non-human
bone-tissue donors and are often processed and mixed with other
components such as hydroxyapatite or other calcium salts. Again,
xenografts are not favored for human use because of concerns over
disease transmission and immunogenicity.
Given the disadvantages associated with autograft and allograft,
many have focused efforts on developing new synthetic bone substitute
materials that can fill the existing need.
Bone graft substitutes are materials other than human or non-human
bone tissue. The advantages of a synthetically derived substitute material
over human derived bone graft and naturally derived substitutes are: 1 )
more control over product consistency; 2) less risk for infection and
disease; 3) no morbidity or pain caused by harvesting of the patient's own
bone for graft; and 4) availability of the substitute in many different
volumes (that is, it is not limited by harvest site of the patient).
The biological and physical demands placed on a bone graft material
vary in response to the treatment indication. For instance, clinicians prefer
different physical forms of the materials (granules, blocks, dense, porous,
putty/paste, cement) depending on the difficulty filling a bone void
sufficiently with graft. Craniomaxillofacial defects typically pose relatively
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
3
low loadbearing requirements on the graft material. The size of the defect
may influence whether a conductive graft is sufficient or if an inductive
graft is required. In some instances, a graft's ability to withstand high load
and maintain structural support over a long period of time (such as in the
case of compaction grafting around a revision joint prosthesis) is more
important than the graft's ability to accelerate bone healing or bridge a gap
(such as in the case of grafting to achieve spine fusion). For this reason, it
is important to have more adaptable materials for bone graft over products
currently available in the art, which fall short of easily conforming to a
multitude of applications. Use of such a product would have the inherent
advantage of being less costly and more efficient for personnel in
orthopedics.
Two properties associated with currently available synthetic granules
have inherent disadvantages. First, it is difficult to get the granules from
the package into the defect. The granules are generally small, less than
10mm in any one dimension, and difficult to grasp individually. The
granules have no means to form an aggregate, so clinicians cannot handle
them in unison. Secondly, if the granules spill into an open surgical
wound, the granules stick to soft tissue, which makes it difficult to clear
them from the wound. Clinicians fear that if left in the wound, the granules
can cause further complications such as migration into the articulating
surfaces, potentially causing further damage.
Synthetic bone graft granules are commonly supplied in a simple
glass vial, and very little has been done to improve the handling
characteristics or ease the surgical procedure. There are a few
exceptions. Although a syringe-like device is available on the market to
assist in delivery of granules to the graft site, this does not address the
issue of preferential sticking of the granules to soft tissue in the wound.
Alternatively, demineralizing allograft products are commercially available
which come premixed in a gel or putty for improved handling.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
4
Other bone graft substitutes are known in the art. US Patent No.
5,676,700 is directed to interlocking structural elements for augmentation
or replacement of bone in which at least four posts of the element project
from a hub such that no more than two of the directions of any of the posts
lie in a common plane. The elements have posts with oval cross-sections
and in a preferred embodiment have an angle of 109.47 degrees between
each post.
US Patent No. 5,178,201 is directed to an implant method, as
opposed to a graft method, in which particles with from four to eight pins
which extend radially from a center have at least three pins which adhere
to a basic pattern. The body diameter of the particle is a maximum of 3
mm, and the specification does not teach tapering of the pins. US Patent
No. 5,458,970 teaches shaped particles comprising deformed fibers in
which the fiber is a zinc oxide whisker having a plurality of needle-like
portions being maximally 0.1 mm in length and extending from its nucleus
portion.
US Patent No. 5,258,028 is directed to an injectable micro-
implantation system utilizing textured micro particles maximally 3mm in
diameter and having a number of outwardly projecting pillar members.
WO 94/08912 teaches an aggregate having six arms in which the
arms are generally obelisk-shaped and have four sides each.
The method of making a product from a form of hydrated calcium
sulfate is known. Conversion of gypsum powders to plaster of Paris
powders (calcination) is well established, and the rehydration of the plaster
of Paris powder to convert to gypsum is also well known.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
US Patent No. 5,320,677 describes the formation of a composite
material of gypsum and a stronger component, such as wood fibers. The
technique then dehydrates the mix and rehydrates it. The method is a way
of mixing in and setting the wood fibers within calcium sulfate. A target
5 application for such a method is the preparation of wallboard.
German Patentschrift DE 3732281 C2 relates to the process of
compaction of gypsum, and the subsequent dehydration/rehydration at an
elevated temperature and pressure for the purpose of forming a
consolidation solid to create a more compact form of waste material for
easier disposal.
In the art of making shaped particles of calcium sulfate there is
lacking a process to form a small, detailed part with high density, strength
and resistance to dissolution in water. A major complication to this
processing is that calcium sulfate needs to be maintained below
temperatures of about 150°C-300°C and especially below
500°C to avoid
thermal decomposition to an insoluble anhydrous form which is difficult to
rehydrate. The low degradation temperature eliminates the possibilities of
a traditional, high-temperature sintering process to sinter the calcium
sulfate particles to one another, thereby strengthening and consolidating
the material. Sintering is herein defined as the bonding of powdered
particles by solid state diffusion.
Typical forming procedures for calcium sulfate are dry powder
pressing (as in pharmaceutical tableting) or casting of a plaster of Paris
slurry. The wall board industry uses various wet forming processes to
compact slurries of plaster of Paris into large sheets.
UK Patent 2 205 089 A is directed to a process for the production of
calcium sulphate alpha-hemihydrate. The calcium sulphate dihydrate is
molded, introduced to an autoclave, and in the presence of an adequate
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
6
amount of water in the pores, the crystal growth and crystal form of the
calcium sulphate alpha-hemihydrate is controlled by maintaining a
temperature between 110°C and 180°C and regulating the
atmospheric
pressure inside the autoclave.
It is an object of the present invention to provide a shaped particle for
use in treating a bone deficiency wherein said particle is shaped for use in
an array of particles interlocked with one another, comprising a center
portion and at least four tapered extremities projecting from said center
portion wherein said projections provide for interstitial spaces between
adjacent extremities, each extremity having a base attached at said center
portion, an opposite point, a length, and a circular transverse cross-
sectional configuration, wherein said interstitial spaces of one said particle
will accept at least one extremity of an adjacent said particle to facilitate
interlocking of adjacent particles in said array.
In specific embodiments of the present invention the particle has at
least three in a plane and the particle has six extremities. In other specific
embodiments the particle of is comprised of a material selected from the
group consisting of ceramic, bioactive glass, polymer, polymer/ceramic
composite, and polymer/glass composite. In a preferred embodiment the
particle is comprised of ceramic and more preferred is comprised of a
calcium salt such as calcium sulfate, calcium carbonate, calcium
phosphate and calcium tartarate, but most preferable is of calcium sulfate,
or gypsum.
In another embodiment of the present invention the particle is
comprised of a polymer such as polypropylene, polylactic acid, polyglycolic
acid and polycaprolactone.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
7
In a preferred embodiment the particle has a diameter of about 3-10
millimeters, more preferred is 4-8 millimeters, and most perferred is 6
millimeters.
It is another object of the present invention to provide an array which
contains multiple particles wherein said multiple particles are in a mixture
of particles comprised of different materials. In a specific embodiment the
different materials are selected from the group consisting of ceramic, such
as a calcium salt, bioactive glass, polymer, polymer/ceramic composite,
and polymer/glass composite.
In an additional object of the present invention there is a shaped
particle for the treatment of a bone deficiency wherein said treatment is
selected from the group consisting of augmentation of bone, repair of
bone, replacement of bone, improvement of bone, strengthening of bone
and healing of bone. In a specific embodiment the bone deficiency is
selected from the group consisting of a fracture, break, loss of bone, weak
bone, brittle bone, hole in bone, void in bone, disease of bone and
degeneration of bone.
In an additional embodiment the disease is selected from the group
consisting of osteoporosis, Paget's disease, fibrous dysplasia,
osteodystrophia, periodontal disease, osteopenia, osteopetrosis, primary
hyperparathyroidism, hypophosphatasia, fibrous dysplasia, osteogenesis
imperfecta, myeloma bone disease and bone malignancy.
In a specific embodiment the array of the present invention has
interlocking of adjacent particles which provides adequate porosity to allow
ingrowth from a host bone.
In a specific embodiment the porosity is between 40-80%. In a more
preferred embodiment the porisity is between 60 and 80%.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
8
In another object of the present invention there is an array of shaped
particles wherein said array comprises a plurality of shaped particles
comprising one or more shaped particles from the group consisting of a
first shaped particle comprising a center portion and at least four tapered
extremities projecting from said center portion wherein said projections
provide for interstitial spaces between adjacent extremities, each extremity
having a base attached at said center portion, an opposite point, a length,
and a circular transverse cross-sectional configuration, wherein said
interstitial spaces of one said particle will accept at least one extremity of
an adjacent said particle to facilitate interlocking of adjacent particles in
said array of shaped particles; a second shaped particle comprising a
center portion, at least two noncurved extremities, and at least three
curved extremities projecting from said center portion wherein said
projections provide for interstitial spaces between adjacent extremities,
each extremity having a base attached at said center portion, an opposite
point, a length, and a transverse cross-sectional configuration, wherein
said interstitial spaces of one said particle will accept at least one
extremity of an adjacent said particle to facilitate interlocking of adjacent
particles in said array; and a third shaped particle comprising a multi-ring
structure having at least four curved projections wherein said projections
provide for interstitial spaces between adjacent said projections, and
wherein said projections facilitate interlocking of adjacent particles in said
array.
In an additional object of the present invention is a shaped particle
for use in treating a bone deficiency wherein said particle is shaped for use
in an array of particles interlocked with one another, comprising a multi-
ring structure having at least four curved projections wherein said
projections provide for interstitial spaces between adjacent said
projections, and wherein said projections facilitate interlocking of adjacent
particles in said array. In specific embodiments the angles between the
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
9
curved projections are equal. In another embodiment the shaped particle
is composed of a polymer such as polypropylene, polylactic acid,
polyglycolic acid and polycaprolactone or a polymer/ceramic composite or
polymer/glass composite.
In another embodiment of the present invention is a a composition for
use in treating a bone deficiency comprising a suspension material; and a
shaped particle from the group consisting of a first shaped particle
comprising a center portion and at least four tapered extremities projecting
from said center portion wherein said projections provide for interstitial
spaces between adjacent extremities, each extremity having a base
attached at said center portion, an opposite point, a length, and a circular
transverse cross-sectional configuration, wherein said interstitial spaces of
one said particle will accept at least one extremity of an adjacent said
particle to facilitate interlocking of adjacent particles in said array of
shaped particles; a second shaped particle comprising a center portion, at
least two noncurved extremities, and at least three curved extremities
projecting from said center portion wherein said projections provide for
interstitial spaces between adjacent extremities, each extremity having a
base attached at said center portion, an opposite point, a length, and a
transverse cross-sectional configuration, wherein said interstitial spaces of
one said particle will accept at least one extremity of an adjacent said
particle to facilitate interlocking of adjacent particles in said array; and a
third shaped particle comprising a multi-ring structure having at least four
curved projections wherein said projections provide for interstitial spaces
between adjacent said projections, and wherein said projections facilitate
interlocking of adjacent particles in said array.
In specific embodiments the suspension material is selected from the
group consisting of starch, sugar, glycerin, blood, bone marrow, autrograft
material, allograft material, fibrin clot and fibrin matrix or the suspension
material is a binder capable of forming a gel such as collagen derivative,
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
cellulose derivative, methylcellulose, hydroxypropylcellulose,
hydroxypropylmethyl cellulose, carboxymethylcellulose, fibrin, and a
biological adhesive such as cryoprecipitate.
5 In another object of the present invention the suspension material
further comprises a biological agent, such as a growth factor, an antibiotic,
a strontium salt, a fluoride salt, a magnesium salt, a sodium salt, a bone
morphogenetic factor, a chemotherapeutic agent, a pain killer, a
bisphosphonate and a bone growth agent. In a specific embodiment the
10 growth factor is selected from the group consisting of platelet derived
growth factor (PDGF), transforming growth factor (3 (TGF- Vii), insulin-
related growth factor-I (IGF-I), insulin-related growth factor-II (IGF-II),
fibroblast growth factor (FGF), beta-2- microglobulin (BDGF II) and bone
morphogenetic protein (BMP). In a specific embodiment the antibiotic is
selected from the group consisting of tetracycline hydrochloride,
vancomycin, cephalosporins, and aminoglycocides such as tobramycin
and gentamycin.
In another specific embodiment the bone morphogenetic factor is
selected from the group consisting of proteins of demineralized bone,
demineralized bone matrix (DBM), bone protein (BP), bone morphogenetic
protein (BMP), osteonectin, osteocalcin and osteogenin. In an additional
specific embodiment the chemotherapeutic agent is selected from the
group consisting of cis-platinum, ifosfamide, methotrexate and doxorubicin
hydrochloride. In an additional specific embodiment the pain killer is
selected from the group consisting of lidocaine hydrochloride, bipivacaine
hydrochloride, and non-steroidal anti-inflammatory drugs such as ketorolac
tromethamine.
In another object of the present invention the composition further
includes a clotting factor composition. In a specific embodiment the
clotting factor composition comprises fibrinogen, thrombin and Factor XIII.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
11
In an additional object of the present invention there is a method to
treat a bone deficiency comprising the step of applying a shaped particle
to a bone deficiency wherein said shaped particle is selected from the
group consisting of a first shaped particle comprising a center portion and
at least four tapered extremities projecting from said center portion
wherein said projections provide for interstitial spaces between adjacent
extremities, each extremity having a base attached at said center portion,
an opposite point, a length, and a circular transverse cross-sectional
configuration, wherein said interstitial spaces of one said particle will
accept at least one extremity of an adjacent said particle to facilitate
interlocking of adjacent particles in said array of shaped particles; a
second shaped particle comprising a center portion, at least two
noncurved extremities, and at least three curved extremities projecting
from said center portion wherein said projections provide for interstitial
spaces between adjacent extremities, each extremity having a base
attached at said center portion, an opposite point, a length, and a
transverse cross-sectional configuration, wherein said interstitial spaces of
one said particle will accept at least one extremity of an adjacent said
particle to facilitate interlocking of adjacent particles in said array; and a
third shaped particle comprising a multi-ring structure having at least four
curved projections wherein said projections provide for interstitial spaces
between adjacent said projections, and wherein said projections facilitate
interlocking of adjacent particles in said array.
In another object of the present invention there is a method to treat a
bone deficiency comprising the steps of combining a shaped particle with
a suspension material wherein said particle is selected from the group
consisting of a first shaped particle comprising a center portion and at
least four tapered extremities projecting from said center portion wherein
said projections provide for interstitial spaces between adjacent
extremities, each extremity having a base attached at said center portion,
CA 02401421 2002-08-28
WO 01/66044 PCTNSO1/06043
12
an opposite point, a length, and a circular transverse cross-sectional
configuration, wherein said interstitial spaces of one said particle will
accept at least one extremity of an adjacent said particle to facilitate
interlocking of adjacent particles in said array of shaped particles a second
shaped particle comprising a center portion, at least two noncurved
extremities, and at least three curved extremities projecting from said
center portion wherein said projections provide for interstitial spaces
between adjacent extremities, each extremity having a base attached at
said center portion, an opposite point, a length, and a transverse cross-
sectional configuration, wherein said interstitial spaces of one said particle
will accept at least one extremity of an adjacent said particle to facilitate
interlocking of adjacent particles in said array; and a third shaped particle
comprising a multi-ring structure having at least four curved projections
wherein said projections provide for interstitial spaces between adjacent
said projections, and wherein said projections facilitate interlocking of
adjacent particles in said array; and applying said combination to a bone
deficiency.
In another object of the present invention there is a kit for the
treatment of a bone deficiency comprising a suspension material; and
multiple first shaped particles and multiple second shaped particles
wherein said first and second particles are shaped for use in an array of
particles interlocked with one another and wherein said particles are
selected from the group consisting of a first shaped particle comprising a
center portion and at least four tapered extremities projecting from said
center portion wherein said projections provide for interstitial spaces
between adjacent extremities, each extremity having a base attached at
said center portion, an opposite point, a length, and a circular transverse
cross-sectional configuration, wherein said interstitial spaces of one said
particle will accept at least one extremity of an adjacent said particle to
facilitate interlocking of adjacent particles in said array of shaped
particles;
a second shaped particle comprising a center portion, at least two
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
13
noncurved extremities, and at least three curved extremities projecting
from said center portion wherein said projections provide for interstitial
spaces between adjacent extremities, each extremity having a base
attached at said center portion, an opposite point, a length, and a
transverse cross-sectional configuration, wherein said interstitial spaces of
one said particle will accept at least one extremity of an adjacent said
particle to facilitate interlocking of adjacent particles in said array; and a
third shaped particle comprising a multi-ring structure having at least four
curved projections wherein said projections provide for interstitial spaces
between adjacent said projections, and wherein said projections facilitate
interlocking of adjacent particles in said array.
In a specific embodiment the kit further comprises a biological agent.
In another specific embodiment the kit further includes a clotting factor
composition, such as a composition comprising fibrinogen, thrombin and
Factor XIII. In another embodiment the kit further comprises a bowl
container for said multiple first and multiple second particles and a delivery
tool. In a specific embodiment the delivery tool is selected from the group
consisting of a spoon, a spatula, a scoop, a tweezer, forceps, a knife, a
hemostat, a syringe, a pipette, a cup and a ladle. In another specific
embodiment the bowl container is used for mixing said multiple first and
multiple second particles and a suspension material. In another specific
embodiment the bowl container is used for mixing said multiple first and
multiple second particles, said suspension material, and a biological agent.
In another embodiment there is a shaped particle for use in treating a
bone deficiency wherein said particle is shaped for use in an array of
particles interlocked with one another, comprising a center portion; at least
two noncurved extremities; and at least three curved extremities projecting
from said center portion wherein said projections provide for interstitial
spaces between adjacent extremities, each extremity having a base
attached at said center portion, an opposite point, a length, and a
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
14
transverse cross-sectional configuration, wherein said interstitial spaces of
one said particle will accept at least one extremity of an adjacent said
particle to facilitate interlocking of adjacent particles in said array.
In another embodiment there is a method for manufacturing a
shaped particle of calcium sulphate dihydrate comprising the steps of
making a shaped particle of calcium sulphate dihydrate; heating said
particle; and applying water to said particle.
In an additional embodiment there is a method for manufacturing a
shaped particle of calcium sulphate dihydrate comprising the steps
ofmaking a shaped particle of calcium sulphate dihydrate; heating in the
presence of pressure and moisture said particle of calcium sulphate
dihydrate to convert said particle to a-calcium sulphate hemihydrate
partially or in full; and applying water to said particle to convert said a -
calcium sulphate hemihydrate to said calcium sulphate dihydrate.
Other and further objects, features and advantages would be
apparent and eventually more readily understood by reading the following
specification and by reference to the company drawing forming a part
thereof, or any examples of the presently preferred embodiments of the
invention are given for the purpose of the disclosure.
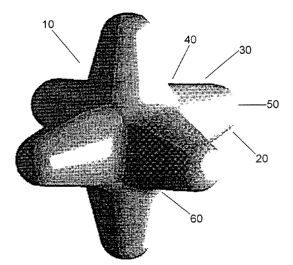
Figure 1 is a drawing of a preferred six-armed shaped particle of the
invention.
Figure 2 is a drawing of an array of interlocked six-armed shaped
particles of the invention.
Figure 3A through Figure 3D are drawings of a five-armed shaped
particle of the invention. Figure 3A is a top view of the particle. Figure 3B
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
is a view of the particle from an elevated side reference. Figure 3C is a
front view of the particle. Figure 3D is a right view of the particle.
Figure 4A through 4D are drawings of a six-armed shaped particle of
5 the invention having flat tips. Figure 4A is a top view of the particle.
Figure 4B is a view of the particle from an elevated side reference. Figure
4C is a front view of the particle. Figure 4D is a right view of the particle.
Figure 5A through 5D are drawings of a six-armed shaped particle of
10 the invention having rounded tips. Figure 5A is a top view of the particle.
Figure 5B is a view of the particle from an elevated side reference. Figure
5C is a front view of the particle. Figure 5D is a right view of the particle.
Figures 6A through 6D are drawings of a shaped particle of the
15 invention having an interlocked ring structure. Figure 6A is a top view of
the particle. Figure 6B is a view of the particle from an elevated side
reference. Figure 6C is a front view of the particle. Figure 6D is a right
view of the particle.
Figures 7A through 7D are drawings of different views of a six-armed
shaped particle of the invention having a propeller-like structure.
Figure 8A through Figure 8D are drawings of a six-armed shaped
particle of the invention. Figure 8A is a top view of the particle. Figure 8B
is a view of the particle from an elevated side reference. Figure 8C is a
front view of the particle. Figure 8D is a right view of the particle.
The term "bone deficiency" as used herein is defined as a bone
defect such as a break, fracture, void, diseased bone, loss of bone, brittle
bone or weak bone, injury, disease or degeneration. Such a defect may
be the result of disease, surgical intervention, deformity or trauma. The
degeneration may be as a result of progressive aging. Diseased bone
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
16
could be the result of bone diseases such as osteoporosis, Paget's
disease, fibrous dysplasia, osteodystrophia, periodontal disease,
osteopenia, osteopetrosis, primary hyperparathyroidism,
hypophosphatasia, fibrous dysplasia, osteogenesis imperfecta, myeloma
bone disease and bone malignancy. The bone deficiency may be due to a
disease or condition, such as a disease which indirectly adversely affects
bone. Furthermore, the bone malignancy being treated may be of a
primary bone malignancy or may be metastatic, originating from another
tissue or part of the body.
The term "ceramic" as used herein is defined as any non-metallic,
non-organic engineering material. An example of such a material is
hydroxylapatite, calcium sulphate, alumina or silica.
The term "gypsum" as used herein is defined as calcium sulfate in
the stable dehydrate state (CaS04CeH20) and includes the naturally
occurring mineral, the synthetically derived equivalents, and the dehydrate
material formed by the hydration of calcium sulfate hemihydrate
(CaS04Cf'/ZH20)(Plaster of Paris) or anhydrite calcium sulphate. The
gypsum may be obtained from commercially available sources.
The term "tapered" as used herein is defined as referring to an
extremity of a shaped particle wherein the width of one end of the
extremity is different in size from the width of another end of the extremity.
That is, the tapering of the extremity may be outward away from the center
of the particle or may be inward toward the center of the particle.
An object of the present invention is a shaped particle as part of
three-dimensional interlocking array of particles to be utilized in bone
graft.
A skilled artisan is aware that the particles may be utilized with inductive
graft in which the graft actively facilitates, either directly or indirectly,
bone
growth. In addition or alternatively, the particles may be utilized for a
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
17
conductive graft in which the graft is conducive to bone growth but does
not actively or directly facilitate it. In a specific embodiment conductive
graft utilizes shaped particles made from a ceramic, polymeric, glass
material, a polymeric/glass, or a polymeric/ceramic material. In another
specific embodiment the particles for conductive graft are augmented with
a biological agent. The material of the particle will be a biocompatible
ceramic or glass that may or may not eventually resorb or degrade within
the body as the bone heals and fills the bone void or improves the bone
deficiency. The particles will be of an appropriate size such that several
individual granules will be used to fill a small void while many can be used
to fill larger voids. The three-dimensional structure will allow the granules
to fill a volume and interlock with each other. In addition, the particles
will
be able to interlock with bone. The interlocking will enable the particles to
support some mechanical forces while maintaining stability and assist in
bone healing. The interlocking feature makes it possible for the particles
to resist some shear forces, unlike commercially available products. It will
also help to resist migration away from the implant site. The particles will
be able to fill odd bone defect shapes and sizes without necessarily
needing to carve a larger block to the approximate shape/size. The
interlocked particles also provide the ability for the entire implant to
behave
mechanically more like a single block as compared to current granular
products. The shapes would be such that a collection of these particles do
not aggregate into a solid, packed volume but instead leave an open,
interconnected porosity that is beneficial for bone healing. It is preferred
that the shape of the particles and/or the array of the shaped particles
allow the engineering or prediction of a specific porosity. For example, the
particles can be shaped to have such a design as to allow 40-80%
porosity upon agglomeration.
The purpose of having shaped particles is two-fold. First, the
capability to interlock provides resistance to shear forces and helps to
increase the stability when the graft is packed into a defect. Second,
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
18
porosity needs to be maintained when the shaped particles are
interlocked. It is known in the art that new bone growth can ingress into
pores ranging from 100-400 microns in size. The targeted total porosity
will range from 20% to 80%, which means that the array of interlocking
shaped particles of the invention will retain open spaces of 20-80% of a
specific volume of an array. It is important that a graft material provide
adequate porosity to allow ingrowth from the host bone. Alternatively, the
material must resorb or degrade away to allow for bone replacement. The
preferred embodiment is the combination of both of these properties.
The tapering of the extremities of the shaped particles improves
manufacturability, maximizes the open space between the extremities, and
provides greater mechanical stability in, for instance, the preferred shaped
particle of Figure 1 because the arms are thicker as you get closer to the
central body, which distributes loads over more mass of material.
The shaped particles of the present invention are illustrated in the
figures. Figure 1 shows a shaped particle (10) having an extremity (20),
and in a preferred embodiment the particle has six extremities. In a
preferred embodiment at least three of the extremities are in a common
plane. The extremities are tapered outwardly along the length (30) of the
extremity so that the base (40) of the extremity is wider than the tip (50) of
the extremity. In a preferred embodiment the tip (50) of the extremities are
rounded. The particle has an interstitial space (60) between the adjacent
extremities (20). In a preferred embodiment the radius of curvature of the
tip (50) of an extremity (20) is about 0.5mm and the radius of curvature of
the interstitial space (60) between adjacent extremities is about 0.5mm.
The preferred width of the entire particle is about 3-10 mm, and more
preferred 4-8mm, and most preferred is 6mm. The preferred width of a
base (40) of an extremity (20) is about 1.85mm, the preferred width of a tip
(50) of an extremity is about 1.19mm, and the preferred length (30) of an
extremity (20) is about 3mm. In a preferred embodiment the angles
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
19
between any of the adjacent extremities (20) are approximately equal. A
skilled artisan is aware that shaped particles may be used which are
greater in size than these measurements or smaller in size than these
measurements depending on the relevant application and bone deficiency.
It is preferred to keep the size of the particle small relative to the wound
site so that it will take many particles to fill the defect rather than one.
Figure 2 illustrates an array of shaped particles of the invention
wherein the extremities (20) of adjacent particles (10) are interlocked.
Figures 3A through 3D illustrate different views of a specific
embodiment wherein a five-armed shaped particle (100) is an object of the
invention. In a preferred embodiment of a five-armed shaped particle at
least three extremities lie in a plane. An extremity (110) is tapered
inwardly along its length (120) wherein the base (130) of the extremity
(110) is more narrow in width than the tip (141) of the extremity (110). An
interstitial space (150) is present between adjacent extremities. The tips
(141) of the extremities (110) are rounded in a specific embodiment.
Figures 3B through 3D illustrate that in a specific embodiment the tips (158
and 159) of two extremities (160 and 170, respectively) which are situated
about 180 degrees from one another are generally more conical in shape
than the tips (141 ) of the extremities (110). The extremities (160 and 170)
taper outwardly where the base (161 and 171, respectively) is wider than
the tips (158 and 159).
Figures 4A through 4D illustrate different views of a specific
embodiment wherein a six-armed shaped particle (300) is an object of the
invention. In a preferred embodiment at least three extremities lie in a
plane. An extremity (310) is tapered inwardly along its length (320)
wherein the base (330) of an extremity (310) is more narrow in width than
the tip (340) of the extremity (310). An interstitial space (350) is present
between adjacent extremities. The tips (340) have a generally flat surface.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
Figures 4B through 4D show the tips (360 and 361) of two extremities (370
and 380, respectively) are generally more conical in shape than the tips
(340) of the extremities (310) and are situated about 180 degrees from
one another in the particle (300).
5
Figures 5A through 5D illustrate different views of a specific
embodiment wherein a six-armed shaped particle (400) is an object of the
invention. In a preferred embodiment at least three extremities lie in a
plane. An extremity (410) is tapered inwardly along its length (420)
10 wherein the base (430) of an extremity (410) is more narrow in width than
the tip (440) of the extremity (410). An interstitial space (450) is present
between adjacent extremities. The tips (440) of the extremities (410) have
a generally rounded surface. Figures 5B through 5D show the tips (460
and 461 ) of two extremities (470 and 480, respectively) are generally more
15 conical in shape than the tips (440) and are situated 180 degrees from one
another in the particle (400).
It is preferred that the shaped particles represented in Figures 4 and
5 are made from a polymer, polymer/ceramic composite, or polymer/glass
20 composite. The tapering inwardly of the extremities (310 and 410) allows
these shaped particles to "snap-fit" into an adjacent particle.
Figures 6A through 6D illustrate different views of a specific
embodiment of the present invention wherein a shaped particle (500) is
similar to two interlocked rings positioned at about 90 degrees from one
another. Interstitial spaces (510) allow interlocking of the rings (520), or
curved projections, of an adjacent particle. The preferred composition
material of this structure is a polymer, a polymer/glass composite or a
polymer/ceramic composite. In a preferred embodiment the structure is
relatively compliant in comparison to a ceramic-based structure. A
preferred diameter of the entire particle (500) is about 6 mm, and a
preferred diameter of the ring (520) component of the structure is about
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
21
1 mm. The maximum number of rings would be such that the surface area
of the rings should not be more than 50% of the surface area of the
encompassed sphere - otherwise the parts would not interlock or nest with
each other. Using this as a starting point, then the diameter of the solid
structure of the ring (as an example at about 1 mm) becomes a factor. As
that diameter decreases the number of possible rings increases.
In the mathematical relationship between a radius of a "spherical"
particle, r, a thickness or diameter of rings, d, and a number of rings, n, a
surface area of a sphere is 4rrr2 and a surface area of the interlocking
rings is 2Trrdn. The objective is that the surface area of the rings is less
than or equal to 50% of the surface area of a sphere. The mathematical
relationship can be described as
2>Trdn <_ 0.50 (4>Trz), or
2rrrdn <_ 2rrrz, or
do <_r
Figures 7A through 7D illustrate a specific embodiment of the present
invention wherein a shaped particle (600) is similar to a propeller.
Interstitial spaces (610) allow interlocking of the extremities (620) of the
particle. The length (615) of an extremity (620) is curved generally as in a
propeller arm. The composition material of this structure is a ceramic,
polymer, bioglass, polymer/ceramic composite, or polymer/glass
composite. In a preferred embodiment the structure is relatively compliant
in comparison to a ceramic-based structure. A preferred diameter of the
entire particle (600) is about 6 mm, and a preferred diameter of the
extremities (620) component of the structure is about 1 mm. The
extremities (630 and 631), particularly as shown in Figure 7D, are
generally conical in shape, having a wider base (640 and 641,
respectively) tapering along the length (650 and 651, respectively) of the
extremity to a narrower tip (660 and 661, respectively). The extremities
(630 and 631) are positioned about 180 degrees relative to each other.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
22
Figures 8A through 8D illustrate different views of a specific
embodiment wherein a six-armed shaped particle (700) is an object of the
invention. In a preferred embodiment of a six-armed shaped particle at
least three extremities lie in a plane. An extremity (710) is tapered
inwardly along its length (720) wherein the base (730) of the extremity
(710) is more narrow in width than the tip (741 ) of the extremity (710). An
interstitial space (750) is present between adjacent extremities. The tips
(741) are rounded in a specific embodiment. Figures 8B through 8D
illustrate that in a specific embodiment the tips (702 and 704) of two
extremities (760 and 770, respectively) which are situated about 180
degrees from one another are generally more conical in shape than the
tips (741 ) of the extremities (710). The extremities (760 and 770) taper
outwardly where the base (761 and 771, respectively) is wider than the
tips (702 and 704, respectively).
A skilled artisan is aware that the surface to volume ratio of the
shaped particle of the present invention has influence upon several
factors, including the intended application of the bone graft, which dictates
the size of the particle needed and the dissolution rates, strength and
manufacturability.
Example 1 - Testing of Shaped Particles
The assessment of the shaped particles was based on two tests
designed to address interlocking of the particles and application to a
clinical-type case.
A) 'Slump' test-measure the ability of a pile of bone graft granules to
maintain its height before and after vibration.
B) Push-thru test-measure the resistance to push-thru of an
agglomeration of bone graft granules through a cylindrical defect in a
CA 02401421 2002-08-28
WO 01/66044 PCT/USOI/06043
23
porous foam block, which is a lab model used for human cancellous
bone.
The goal was to determine which of the designs provided the most
interlocking that was also an improvement over a design comparable to a
commercially available tablet-shaped product.
Eguipment:
A) 'Slump' test B) Push-Thru test
Tablets, 28 mL Tablets, 50mL
Shaped particle designs, 28mLShaped particle designs,
of each 50mL of each
100mL graduated cylinder (EXAX,Tinius-Olsen screw driven
No. mechanical
20025) test frame and
Scale (Mettler Toledo, AT261 # 2000 recorder
)
Vibrating, electronic pencil Porous foam block (General
(Ideal Plastics
Industries, Electric Marker) Manufacturing Company, FR3703)
Funnel (half angle 28) Polyethylene plunger and
stopper
Cuplike container (half angleImage pro Plus Software
12, base (Media
diameter 1.125") Cybernetics, V 3Ø1 )
Ring stand
Height gage (Mitutuyo, No.
192-112)
Base plate (1x6x6 inch cold-rolled
steel)
Watch with second hand
Three different shaped particles of the present invention (Six-armed
shaped particle, flared to bulb at end of arms of X-Y plane (Figure 8); Five-
armed shaped particle, flared to bulb at end of arms in X-Y place (Figure
3); Six-armed shaped particle, tapered straight to end of arms in all
directions (Figure 1 ); and one tablet-shaped geometry similar to
commercially available products. The shaped particle designs were
manufactured using clay formulation "50-dry". SLA molds were used to
form the design prototypes. The components were all made similarly,
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
24
though slightly different processing parameters were used with each to
insure proper drying and mold release, as follows:
1. Stereo lithographic models (SLA) were made of molds for each of the
three designs.
2. SLA molds were washed and dried.
3. Lubricant was applied to the surface of the SLA molds. Excess was
removed with a clean cloth and compressed air.
A. Two lubricants from Slide Products Inc. (Wheeling, IL) were
used: 42612N, 447126
B. PamO (International Home Foods, Parsippany, NJ) was used
as another lubricant
4. Clay formula 50-dry (81.6% gypsum, 1.1 % carboxymethyl cellulose,
4.1 % glycerin, 13% water) was rolled into sheets (about 1 mm thick),
big enough to cover the cavities in the molds.
~ Gypsum: FG-200, from BPB, Newarks, United Kingdom
~ carboxymethyl cellulose: 7HF, from Hercules, Wilmington, DE
~ Glycerine, USP: GX-195-1, from EM Science, Gibbstown, NJ
5. The mold halves were closed together and compacted using about
4000 Ibs. of force.
6. The molds were heated in a microwave oven to dry the water from
the parts.
A. Six-armed shaped particle, flared to bulb at the ends of arms in
X-Y plane heated for 4 min. at about 30% power.
B. Five-armed shaped particle X, flared to bulb at the ends of arms
in X-Y plane heated for 4:25 min. at about 30% power.
C. Six-armed shaped particle, straight, tapered arms, heated for
3:50 min. at about 30% power.
7. The molds were allowed to cool for approximately one minute.
8. The parts were removed from the mold and trimmed of any flashing
using an Exacto knife.
9. The parts were dried in a vacuum dessiccator for several hours prior
to further testing.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
Slump test
The slump test was conducted first since it was non-destructive.
5 Equal volumes (28mL) of each shaped particle design and the tablet
samples were measured using a 100mL graduated cylinder. These equal
volumes were weighed to determine the mass of material present.
The test begins by pouring the entire volume of individual shaped
10 particle designs into a starting container. Either a funnel (half angle
28°)
or a cuplike container (half angle 12° with a 1.125 inch flat base) was
used
to contain the shaped bone graft particles and provide a starting shape for
the pile. The container was then inverted and placed on a base through
which a vibration was applied for five seconds using an electronic,
15 vibrating pencil. The vibration was used to settle the shaped bone graft
particles into the container of choice and pre-pack them to that shape.
Following the vibration, the container was carefully removed. A height
gage was used to measure the initial height of the pile. Vibration was then
applied to the base plate, causing the pile to settle further. The height
20 gage was used again to measure this new height. The highest
particle/tablet was used as the height in all cases. This test was repeated
ten times for each design using each of the two containers (funnel and
cuplike container). From the data a difference in heights and the
percentage change in heights (relative to the initial height of the pile) were
25 calculated.
Table 1 shows the mass data collected for the three shaped particle
designs and the tablet geometry. The mass shown is for 28mL of
particles, as measured in a 100mL graduated cylinder. One data point
was collected for each design.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
26
Mass and mass per volume are important and related to the
dissolution time and the porosity of the agglomerated granules. If all
parameters were equal (material, density, surface-area-to-volume ratios,
etc.) it would be expected that the more mass per volume, the lower would
be the porosity of the agglomerate and the longer duration it would have
before dissolution. The dissolution rate would determine how much
material would disappear per unit of time and may also be influenced by
the surface-area-to-volume ratio and the material.
Table 1: Mass per 28 mL of particles
.yam to<~ ~ ~ ~P. ~~ : - - ~ Mass,.y er.::28.=ml: of
, %. ~ ~ iianutes~~:
P P
~ 9
~
z : . . .
..~ 7 ~~ ~ ~
~, . ~H. "s~
A) Six-armed shaped particle, 17.2175
flared to
bulb at end of arms of X-Y plane
B) Five-armed shaped particle, 20.2567
flared to
bulb at end of arms in X-Y place
C) Six-armed shaped particle, 21.2140
tapered
straight to end of arms in all
directions
D) Tablet geometry 31.3437
Table 2 shows the summarized results for the slump tests performed
on each of the different sample geometries using the funnel for a starting
form. Each sample was measured ten times. It was proposed that
maximizing the starting height and the height after vibration and
minimizing the change in height and percent change in height were the
ideal cases. The best value for the shaped particle designs tested for
each parameter is in bold. The tablets did not form a pile (tablets fell to
only one or two layers high) when the supporting container was removed,
qualitatively indicating poor interlocking relative to other samples.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
27
Table 2: Summarized
results for the
slump tests using
the funnel for
starting form.
Sample Starting Height Change Percent
height, after in change
in
H1 vibrationheight, height,
(inches) , H2 (inches) relative
to
(inches) starting
height
(inches)
A) Six-armed shaped 1.275 0.821 0.454 35.138
particle, flared 0.109 0.070 10.147 8.072
to bulb at
end of arms of X-Y
plane
(n=10)
B) Five-armed shaped1.223 0.806 0.418 33.350
particle, flared 0.161 0.069 0.146 8.675
to bulb at
end of arms in X-Y
plane
(n=10)
C) Six-armed shaped 1.114 0.829 0.285 24.734
particle, tapered 10.158 0.054 0.128 7.955
straight
to end of arms in
all
directions (n=10)
D) Tablet geometry, 0.662 0.578 0.084 12.342
(n=10) 10.055 0.032 10.056 6.981
Funnel
I 6-armlbulb arm:
H1 (inches) H2 (inches) D
T1 1.22 0.885 0.335
T2 1.56 0.738 0.822
T3 1.28 0.81 0.470
T4 1.18 0.76 0.420
T5 1.18 0.75 0.430
T6 1.3 0.790 0.51
T7 1.283 0.80 0.483
T8 1.121 0.926 0.195
T9 1.255 0.823 0.432
T10 1.285 0.929 0.356
CA 02401421 2002-08-28
WO 01/66044 PCTNSO1/06043
28
5- arm:
H1 (inches) H2 (inches)D
T1 1.344 0.093 0.441
T2 1.185 0.830 0.355
T3 1.180 0.75 0.430
T4 1.150 0.801 0.349
T5 1.760 0.89 0.470
T6 1.39 0.787 0.603
T7 1.103 0.656 0.447
T8 1.472 0.823 0.649
T9 0.959 0.812 0.147
T10 1.090 0.806 0.284
~ 6 armlstraight arm:
H1 H2 D
T1 1.132 0.890 0.242
T2 1.269 0.862 0.407
T3 1.219 0.801 0.418
T4 0.93 0.786 0.144
T5 0.967 0.849 0.118
T6 1.049 0.791 0.258
T7 1.050 0.789 0.261
T8 1.451 0.93 0.521
T9 1.020 0.829 0.191
T10 1.053 0.760 0.293
Tablet:
H1 H2 D
1 0.634 0.576 0.058
2 0.670 0.641 0.029
3 0.681 0.543 0.138
4 0.618 0.540 0.078
0.637 0.559 0.078
6 0.690 0.574 0.116
7 0.644 0.594 0.005
8 0.613 0.551 0.062
9 0.799 0.591 0.208
0.635 0.609 0.026
CA 02401421 2002-08-28
WO 01/66044 PCTNSO1/06043
29
Table 3 shows the summarized results for the slump tests performed
using the cuplike container for a starting form. As with the slump test
using the funnel for a starting container, maximizing the start height and
the height after vibration and minimizing the change in height and percent
change in height were the ideal cases. The best value for the shaped
particle designs tested in each column in bold.
Cunlike Container
Table 3: Summarized results for the slump tests using the cuplike
container for starting form.
Sample Starting Height Change Percent
height after in heightchange in
(inches) vibration(inches) height,
(inches) relative
to
sta rti
n g
height
(inches)
A) Six-armed shaped 0.970 0.860 0.111 11.184
particle, flared 10.056 0.027 0.051 4.696
to bulb at
end of arms of X-Y
plane
(n=10)
B) Five-armed shaped0.997 0.844 0.153 15.194
particle, flared 0.051 10.056 10.063 15.894
to bulb at
end of arms in X-Y
plane
(n=10)
C) Six-armed shaped 0.907 0.744 0.133 14.435
particle, tapered 10.062 10.052 10.067 16.854
straight
to end of arms in
all
directions (n=10)
D) Tablet geometry, 0.516 0.441 0.075 14.361
(n=10) 10.049 10.040 10.030 15.077
Actual test data are as follows.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
6 ArmIBulb
Arm
H1 H2 D
1 1.070 .870 0.20
2 0.975 .826 0.149
3 1.005 .880 0.125
4 0.891 .849 0.042
5 0.905 .821 0.084
6 0.951 .875 0.076
7 0.949 .886 0.063
8 0.940 .875 0.065
9 1.038 .890 0.148
10 0.979 .826 0.153
5-arm:
H1 H2 D
1 1.005 0.798 0.207
2 0.935 0.815 0.055
3 0.934 0.880 0.054
4 1.032 0.823 0.209
5 1.020 0.894 0.126
6 0.994 0.804 0.190
7 1.062 0.856 0.206
8 1.030 0.802 0.228
9 0.915 0.801 0.114
10 1.041 0.968 0.073
Tablet:
H1 H2 D
1 0.466 0.411 0.055
2 0.469 0.419 0.05
3 0.560 0.471 0.089
4 0.590 0.472 0.118
5 0.511 0.470 0.041
6 0.540 0.40 0.14
7 0.467 0.412 0.055
8 0.457 0.379 0.078
9 0.540 0.406 0.134
10 0.562 0.492 0.070
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
31
Data from the two slump tests were contradictory. From the test
using the funnel for support and shape of the initial pile, the six-armed
shaped particle with simple tapers was seen to be better than the other
designs. In the test using the cuplike container the six-armed shaped
particle with the arms in the X-Y plane flared to bulbs was seen as the
better design.
Push-thru test
The push-thru test was a mechanical test performed using a Tinius-
Olsen (Willow Grove, PA) screw-driven mechanical test frame. Once
tested using this procedure, the sample parts and the defects in the
porous blocks were considered to be damaged and not valid for additional
testing.
A polyethylene stopper was placed into the bottom of the pre-drilled,
0.750" hole (thru) in the porous foam block. Then, a volume
(approximately 8mL) of shaped particle is added to the hole and the top
plunger is inserted. The correct amount of shaped particles are added
when the plunger sits such that the fill mark just shows above the level of
the top of the porous foam block. The test block with plunger, stopper and
shaped particles are then transferred to the test frame. The part to be
tested is situated such that the stopper is over a solid block to temporarily
block the shaped particle and stopper from falling through. A pre-load of
ten pounds of force is then applied at a rate of 0.1 inches/minute. The
pre-load is then removed and the stopper is positioned over an opening
such that the plunger can press against the shaped particles and the
majority of resistance comes from frictional forces between the shaped
particle and the shaped particle and the walls. Additional resistance is
expected between the stopper/plunger and the walls, but this should be
small and consistent in all tests performed. Load is reapplied at a rate of
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
32
0.1 inches/minute until the resisting load drops to zero and the granules
are gone from the test block. Data is recorded using a load/displacement
graph. This test was repeated five times for each of the three shaped
particle designs and three times for the tablet geometry.
The data was analyzed using Image Pro Plus software (Media
Cybernetics) to determine the area under the curves. The assumption
was made that the load and displacement axes were both to the same
scale (displacement) which means that the value calculated for area under
the curve is not truly energy. The values of the area under the load-
displacement curve are useful for comparing one against the other and to
show relatively which design required more energy to force the granules
through the block.
Table 4 shows the summarized results for the push-thru testing on
each of the different geometries.
Table 4: Summarized results
for the push-thru tests.
Sample Area under Percentage
vs
load vs six-armed,
Displacement tapered
(in2)**
A) Six-armed shaped particle, 0.05710.015 0.655
flared to
bulb at end of arms of X-Y plane
(n=5)
B) Five-armed shaped particle, 0.058 10.009 0.667
flared to
bulb at end of arms in X-Y plane
(n=5)
C) Six-armed shaped particle, 0.087 0.019 1.000
tapered to
end of arms in all directions
(n=5)
D) Tablet geometry, "OsteoSet~-like"0.00310.003 0.034
shape (n=10)
**area under curve was measured using the Image Pro software package,
with both axes (load and displacement) calibrated as inches. This is not a
true energy measurement, but serves for comparative purposes.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
33
Maximizing the area under the load/displacement curve was ideal -
indicating the most energy required to overcome resistance of interlocking
and friction. The maximum value was found with the six-armed shaped
particle that was tapered on all arms and is listed in bold in the table.
Difference in the push-thru resistance between this design and each of the
other three designs was found to be statistically significant (student t-test,
two tail, unequal variance, p<0.05).
Observations during the testing showed that all three shaped particle
designs resisted push-thru similarly - the granules interlocked with
themselves and the walls of the foam block to resist the motion of the
plunger through nearly the entire thickness of the test block. The tablet
geometry did not offer much resistance, with only a short travel distance
required before all of the granules fell out of the bottom of the test block.
The tested granules can be listed in order of decreasing mass per
28mL volume: tablet geometry, six-armed shaped particle with tapered
arms, five-armed shaped particle, and six-armed shaped particle flared to
bulb at the end of arms in X-Y plane.
The conclusions of the slump testing and push-thru testing are as
follows:
Slump testing of the different designs was inconclusive. The test
using the funnel (28° half angle) showed the six-armed shaped particle
with tapered arms to be the best. The test using the cuplike container
(12°
half angle, 1.125" base) showed the six-armed shaped particle with X-Y
plane arms flared to bulbs to be the best. It was also seen that the tablets
behaved qualitatively worse compared to any of the shaped particle
designs, failing to interlock and retain much of the original pile height.
Push-thru testing showed that the six-armed shaped particle with
tapered arms offered the most resistance to push the granules all the way
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
34
through the porous foam test block. The other shaped particle designs
both required about 1/3 less energy to push the granules through the
same block. The tablets required only about 3% of the energy required to
push-thru the six-armed shaped particle with tapered arms. All of the
shaped particle designs were observed to resist push-thru until the plunger
was nearly all of the way through the test block. The tablet geometries fell
through after the plunger traveled only a short distance through the block.
Example 2 - Shaped Particle Characteristics
In a preferred embodiment a material for the ceramic component of a
bone grafting system of the present invention is calcium sulfate. Other
materials that could be used include: a calcium salt; hydroxylapatite, a
calcium phosphate; bioactive glass, a vitreous based glass (such as may
be used for maxio-cranio applications); calcium carbonate, a calcium
based mineral; various calcium phosphates, and calcium-rich minerals,
including tricalcium phosphate and orthophosphate; apatite/ wollastonite
glass ceramic, a calcium silicate often used in bone spacer applications;
resorbable polymers such as polysaccharides, polyglycolates, polylactic
acid (PLA), polyglycolic acid (PGA), polycaprolactone, polypropylene
fumarate (all of which can be blended or made to co-polymers to control
the desired properties of the product); and composites of resorbable
polymers and glass or ceramic fillers. Bioactive glass is a material whose
major components are CaO, Si02 and P205 and whose minor components
may be Na20, MgO, AI203, B203 and CaF2.
In a specific embodiment the shaped particle of the present invention
is colored to make it more visible. In another specific embodiment
differently shaped particles of the present invention are denoted with
different colors for better differentiation of the particles. In another
specific
embodiment the particles are coated or have contained within them an
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
agent such as green fluorescent protein or blue fluorescent protein to
make them fluorescent and therefore more visible.
The circular cross-section of the extremities, or arms, of the shaped
5 particle of the invention is beneficial for strength purposes, because an
equivalent response to loading will occur regardless of the application of
the load around the circumference. In contrast, an oval shape as is
utilized in commercially available products and in US Patent No. 5,676,700
has reduced resistance to loading when the loading is applied in the
10 direction of the axis of the shorter width of the oval compared to the axis
of
the longer width of the oval.
Example 3 - Suspension Material
15 It is an object of the present invention to utilize a suspension material
to suspend the shaped particles of the invention for easier application to a
bone deficiency.
A suspension material may be used as an additional component of a
20 system for a bone graft substitute to treat bone deficiency. The
suspension material may be a liquid, putty, dough or gel phase component
and may be mixed with the shaped particles described above at the time
of use or come as a pre-packaged system. The suspension material could
serve two potential functions: 1 ) to act as a binder to improve handling by
25 forming a putty-like material which is shapeable, and/or 2) to act as a
biological tool to assist in the healing through the addition of infection
control, bone growth, or other healing or biological agents. The
suspension material can provide standard suspension of particles within a
material or it may provide adhering of particles or connecting of particles in
30 a manner wherein the material is smaller in volume in an array than the
volume of the particles themselves.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
36
The suspension material can either be setting or non-setting in
response to time, temperature, presence of body fluid or other external
stimuli which might supply energy, such as ultraviolet radiation, magnetic
radiation, electromotive force (EMF), radiowaves, or ultrasound. In one
embodiment the suspension material will degrade once implanted. Ideally,
it would be derived from naturally occurring substances such as
carbohydrates, starches or glycerin. It should have a sufficient viscosity as
to help the granules adhere to each other to improve intraoperative
handling. Coating calcium salts of the preferred embodiment of the
shaped particles of the invention with this type of substance may also
decrease their affinity to stick to soft tissue, making it easier to remove
unwanted pieces from the application site. Fibrinogen/thrombin/Factor XIII
combinations may also provide a liquid or gel of appropriate viscosity to
use as a binder. The liquid may also be a synthetic material such as
calcium sulfate (plaster of Paris) that would set in situ. In another
embodiment, this binder could act as a carrier for a variety of agents
including but not limited to growth factors, bone morphogenic proteins,
fibrinogen/thrombin, antibiotics or some other therapeutic agent (see
Example 6).
In a specific embodiment the suspension material is blood, bone
marrow, autograft material, or allograft material. These materials are
preferentially derived from the patient with the bone deficiency being
treated. Alternatively, they are derived from a donor and preferable are
free from being the source of disease transmission.
In the invention, a suspension material is used which is compatible
with all synthetics (calcium phosphates, calcium sulfates, bioactive
glasses, and resorbable polymers). An example of a suspension material
is a mixing gel which can be mixed with the synthetic or natural products
(autograft or allograft) of choice by the clinician to produce a 'paste' for
application to a bone deficiency such as bone void filling. The suspension
CA 02401421 2002-08-28
WO 01/66044 PCT/US01/06043
37
material must have the appropriate viscosity and tackiness to agglomerate
the particles for easy application to the graft site. Once agglomerated, the
paste could be manipulated by hand or be transported by use of a tool
such as a scoop, spoon or syringe to the defect site.
The suspension material can also reduce the preferential sticking to
soft tissue. This adhesion to soft tissue may be caused by a number of
factors. Calcium phosphates are known for their affinity for many proteins,
as demonstrated by their use in chromatography columns for protein
isolation. Thus, their surface chemistry contributes to their preferential
sticking to soft tissues of the surgical site which is often covered in blood
and protein-containing body fluids. Secondly, many of these commercially
available products have rough surfaces that may mechanically adhere to
soft tissues such as coral-derived products which contain many
interconnected tubules that when fractured create a very rough surface. A
suspension material can minimize both effects. In the first case, the
suspension material alters the surface chemistry, thus reducing the
particles' affinity for proteins. In the second, the suspension material fills
in rough features, thereby reducing the particles' ability to mechanically
adhere to the tissue.
The suspension material of the present invention may be comprised
of biocompatible polymers, and in a specific embodiment the polymers are
bioresorbable. The polymers must be graftable into an animal without
causing unacceptable side effects. The polymers may be homopolymers
or copolymers and are preferably amorphous. A specific example is
polymers in which the units are derived from hydroxy carboxylic acids,
which are polyesters. Another example is poly(lactic acids) which may
originate from the polymerization of mixtures of L- and D-lactides in
proportions such that the poly(lactic acids) are amorphous. Another
example is copolymers consisting of units derived from lactic and glycolic
acids.
CA 02401421 2002-08-28
WO 01/66044 PCT/US01106043
38
A biocompatible polymer may or may not be degradable, depending
on the proposed use. Degradable polymers which are nontoxic and
implantable into organisms such as humans are preferable, and examples
include polyglycolic acid or polylactic acid. Other materials which may be
useful based on their biocompatibility and the ability to alter their
viscosity
and tackiness to prove useful in this invention include: polyvinylpyrolidone,
chitosin, glycerol, carboxymethylcellulose, methylcellulose, carrageenan,
hyaluronic acid, collagen-hydroxyapatite-hyaluronic acid composite,
alginate, dextrose, starches, cellulose gums or combinations of any of the
above listed items. A skilled artisan is aware that collagen or a derivative
of collagen is preferably treated prior to use in the invention so as not to
be immunoreactive, or alternatively a recombinant form of collagen may be
used.
A binder is a material that aids in the agglomeration of the particles
due to the tackiness of the binder both in a cohesive (with itself) and
adhesive (with the particles) nature. The final construct (binder plus
particles) still has flexibility and pliability so that it can fill a defect
completely. It is possible that plaster of Paris or a settable calcium
phosphate cement system may be used as a binder which will still
ultimately set to a firm construct. This would provide an improvement in
the immediate structural strength under a loading pattern that is
predominately compression. So, therefore, a binder may or may not
harden. In a preferred embodiment the binder hardens.
Examples of appropriate physiological materials which may be
included in the suspension material are saline, various starches,
hydrogels, polyvinylpyrrolidines, other polymeric materials,
polysaccharides, organic oils or fluids, all of which are well known and
utilized in the art. Materials that are biologically compatible, i.e., cause
minimal tissue reaction and are removed or metabolized without
cytotoxicity, are preferred. Biologically compatible saccharides such as
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
39
glucose or aqueous solutions of starch may be used. Certain fats may
also be used. In this connection, highly compatible materials include
esters of hyaluronic acids such as ethyl hyaluronate and
polyvinylpyrrolidone (PVP). PVP normally has the general empirical
formula [CHCH2)2N(CH 2)3C0]~ wherein n equal 25-500, a form otherwise
known as Plasdone O (trademark of GAF Corporation, New York, NY).
Another biocompatible material is a patient's own plasma. Blood may be
withdrawn from the patient, centrifuged to remove cells (or not) and mixed
with appropriate volume of particles and the mixture applied in the desired
locations.
In a preferred embodiment the suspension material is comprised of
the following: carboxymethylcellulose (maximum of 3 weight percent);
glycerol USP (maximum of 20 weight percent); and purified water USP
(maximum of 88.75 weight percent). The advantages to utilizing the
suspension material of the invention which are improvements over
currently available products derived from human tissue include: improved
handling; lower cost; no risk of disease; easier storage; longer shelf life;
ease of discarding any excess material; compatibility with all known
synthetics; and unlimited supply.
Example 4 - Polymeric Shaped Particle
In another object of the present invention the shaped particles of the
invention are of a polymeric phase. The material could be derived from a
wide variety of bioabsorbable, biocompatible polymers that will resorb or
degrade over time. These polymers could also be ceramic or glass filled
in order to boost the osteoconductivity of the polymer alone. The
polymers, or composites, also allow control of mechanical properties, such
as strength and stiffness, and control of degradation rates. The function of
this component is to offer compliance to a bone graft system comprised of
this material and the ceramic and suspension material phases described
above. In a preferred embodiment the polymeric shaped particles will
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
interlock with a ceramic-based particle, still maintaining a certain volume of
the combination that is open and has an interconnected porosity. The
polymeric granule also protects the ceramic components from brittle
fracture under compaction, acting as a buffer while the system is
5 compressed to fill a bone deficiency. In order to achieve these properties
it is envisioned that the polymeric shaped particles will be mostly plastic in
their behavior with a small portion of elastic response. This will insure that
the polymeric shaped particles will compress without too much rebound,
but that they will also serve as buffers between the ceramic granules. It is
10 also conceivable that the polymeric/composite granules may be used
without the ceramic granules in some indications where the ability to
compact the material is very important, such as in the compaction grafting
technique commonly used today in total joint revisions. No current
ceramic shaped particle system is suitable for compaction since they
15 would be pulverized by this technique.
In a preferred embodiment the shaped particle of polymer has as the
ends of its extremities a bubble shape which may provide a "snap-fit" for
adjacent interlocking polymeric shaped particles, such as the particles
20 illustrated in Figures 4 and 5.
Example 5 - A Bone Graft System
Together, the three components of the invention which provide a
25 bone graft substitute system, including a ceramic shaped particle, a
suspension material, and a polymeric shaped particle, will offer the
clinician several options when approaching a grafting procedure. The
most basic option would be to use the ceramic granules alone when the
defect is contained and does not have to provide a lot of mechanical or
30 structural support. When the suspension material is added the clinician
will be able to work with the granules outside of the bone deficiency site to
shape the aggregate. The suspension material may also offer the
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
41
possibility to introduce infection control or active agents to promote bone
healing and growth. The addition of the polymeric shaped particles to the
ceramic shaped particles offers the clinician the ability to compress the
graft into a deficient site. This would be beneficial when more structural
support and stability was required of the implant and might also be more
suited to larger volume defects. The system may also include allograft
material, such as chips, blocks, putties and gels) or in addition or
alternatively may include autograft material.
In a specific embodiment the system will include multiple shaped
particles wherein the particles are of different shapes. The different
shapes which may be included are illustrated in the figures herein or may
have variations of these shapes. In addition or alternatively these multiple
particles may be comprised of different materials.
As seen from the currently available products, the typical approach to
address the breadth of properties required from bone graft materials is to
provide multiple bone graft materials with the intention to apply each to a
specific class of indications. If the clinician requires a mixture of
properties
or attributes, the clinician must mix the currently available products from
different manufacturers to obtain a desirable set of attributes or move on to
another product already designed with the right set of attributes. Thus, in
the present invention, a system of products that may be used either
independently or mixed with any of the other constituents in the system is
provided. A list of the constituents envisioned include: a bioceramic
component with osteoconductive properties that is available as a shaped
particle; suspension material that aids primarily in the delivery of the
shaped particles; a compliant shaped particle with improved mechanical
properties that mimics the compliance of allograft cancellous bone; a fibrin
matrix (see Example 7) that can act as a carrier as with the suspension
material but can provide some enhancement to bone healing, as well as
act as a carrier for the following items; antibiotics, cancer therapy,
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
42
osteoporosis therapies, or therapies for other bone mineralization
disorders that can affect the overall efficacy of a bone graft material
depending on the complications associated with the graft procedure;
growth factors, bone morphogentic proteins, or protein fragments that can
further enhance bone healing and/or have a specific high affinity for the
fibrin matrix (these factors may utilize wide variety of pathways to meet the
end results such as influencing the development of mesenchymal stem
cells, growth and reproduction of osteoblast/osteoclast/osteocytes,
chemotoxic agents that encourage mitogenesis and re-population by the
osteoblasts/osteoclasts/osteocytes, angiogenic agents, etc.); cells which
may also be delivered using a fibrin matrix which are beneficial to bone
healing such as osteoblasts, osteoclasts, and/or osteocytes; allograft bone
and bone products; and other biological agents.
In a preferred embodiment these components are compatible with
autograft. It is generally known that clinicians prefer to use autograft over
existing synthetics since it is the tissue which is trying to be emulated.
Clinicians will mix in autograft and/or blood to fill in the missing aspects
or
properties (primarily to capture the bioactive aspects) of the currently
available products in an object of the present invention.
The present bone graft system invention offers several improvements
over current bone graft substitutes: all components may be
resorbable/degradable in-vivo (current products offered include both
resorbable/degradable and permanent structure); interlocking structure
increases mechanical strength and stability of the granular structure
(particularly under shear forces) relative to the current designs of random
and regular, non-interlocking structures; interlocking structure that also
maintains open, interconnected porosity which allows the individual
shaped particles (especially ceramic) to be dense and therefore less likely
to chip and break than current porous (ceramic) structures which are
friable and weak; dense shaped particles will not adhere to soft tissues as
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
43
will the currently available porous ceramic structures; offering product as a
shaped particle allows the clinician to fill a large range of defect sizes,
whereas current products offer granule and block forms; a multi-
component system allows the clinician to tailor the bone graft to the needs
of the patient without having to utilize many different product offerings
(current products do not offer this flexible, systematic approach); the
addition of antibiotics to the system allows the clinician to graft at an
earlier
stage in cases where infection is a concern; and the addition of biological
factors which may hasten the bone healing process to or onto a
component of the system of the invention can provide superior mechanical
support which will offer an advantage over the current delivery system (a
collagen sponge) for such molecules.
The integral advantage of a system of the invention is that it
eliminates the need to develop a specific product for each specific
indication. The clinician can now mix/match the components of the system
as needed to provide the desirable mixture of attributes, thus having the
ability to tailor or design a bone graft product for each patient to suit his
or
her unique needs and specific complications. This results in a lower cost
to the patient who will be charged only for the products used.
Flexibility in pharmaceutical choice to match infectious agents is also
an advantage of the present invention. In the case of antibiotics, the
clinician can choose the appropriate antibiotic based on the culture results
from the wound. In the case of some currently available products, the
clinician has only one choice for an antibiotic (tobramycin).
There is also provided greater ease of storage and lower distribution
costs as compared to products which directly incorporate bioactive
proteins, cells, or pharmaceuticals. These 'active' ingredients have
specific storage conditions and limited shelf lives. If the products are
pre-mixed, the manufacturer runs the risk of having to dispose of the entire
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
44
product at expiration rather than the 'active' ingredient with the shorter
shelf life. This also eliminates issues caused by the potential for
interactions between the 'active' ingredients and the device during long
storage times.
Furthermore, if the bone graft already contains the pharmaceutical or
bioactive protein or cells, then the product may be limited in its use to
treat
larger defects for fear of over dosing. Similar issues are encountered in
treating small defects where the dose may be too small to have a
beneficial outcome. Giving the clinician the ability to set the dose allows
that the proper dose will be used in all cases.
Example 6 - Addition of Biological Agents to the System
In a preferred embodiment of the present invention a biological agent
is included in the suspension material. Examples include antibiotics,
growth factors, fibrin (see Example 7), bone morphogenetic factors, bone
growth agents, chemotherapeutics, pain killers, bisphosphonates,
strontium salt, fluoride salt, magnesium salt, and sodium salt.
In contrast to administering high doses of antibiotic orally to an
organism, the present invention allows antibiotics to be included within the
suspension material of the composition for a local administration. This
reduces the amount of antibiotic required for treatment of or prophalaxis
for an infection. Administration of the antibiotic by the suspension material
in a composition would also allow less diffusing of the antibiotic,
particularly if the antibiotic is contained within a fibrin matrix (see
Example
7). Alternatively, the particles of the present invention may be coated with
the antibiotic and/or contained within the particle or the suspension
material. Examples of antibiotics are tetracycline hydrochloride,
vancomycin, cephalosporins, and aminoglycocides such as tobrarnycin
and gentamicin.
CA 02401421 2002-08-28
WO 01/66044 PCT/US01/06043
Growth factors may be included in the suspension material for a local
application to encourage bone growth. Examples of growth factors which
may be included are platelet derived growth factor (PDGF), transforming
5 growth factor ~3 (TGF- ~3), insulin-related growth factor-I (IGF-I), insulin-
related growth factor-II (IGF-II), fibroblast growth factor (FGF), beta-2-
microglobulin (BDGF II) and bone morphogenetic protein (BMP). The
particles of the present invention may be coated with a growth factor
and/or contained within the particle or the suspension material.
Bone morphogenetic factors may include growth factors whose
activity is specific to osseous tissue including proteins of demineralized
bone, or DBM (demineralized bone matrix), and in particular the proteins
called BP (bone protein) or BMP (bone morphogenetic protein), which
actually contains a plurality of constituents such as osteonectin,
osteocalcin and osteogenin. The factors may coat the shaped particles of
the present invention and/or may be contained within the particles or the
suspension material.
Bone growth agents may be included within the suspension material
of the composition of the invention in a specific embodiment. For instance,
nucleic acid sequences which encode an amino acid sequence, or an
amino acid sequence itself may be included in the suspension material of
the present invention wherein the amino acid sequence facilitates bone
growth or bone healing. As an example, leptin is known to inhibit bone
formation (Ducy et al., 2000). Any nucleic acid or amino acid sequence
which negatively impacts leptin, a leptin ortholog, or a leptin receptor may
be included in the composition. As a specific example, antisense leptin
nucleic acid may be transferred within the composition of the invention to
the site of a bone deficiency to inhibit leptin amino acid formation, thereby
avoiding any inhibitory effects leptin may have on bone regeneration or
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
46
growth. Another example is a leptin antagonist or leptin receptor
antagonist.
The nucleic acid sequence may be delivered within a nucleic acid
vector wherein the vector is contained within a delivery vehicle. An
example of such a delivery vehicle is a liposome, a lipid or a cell. In a
specific embodiment the nucleic acid is transferred by carrier-assisted
lipofection (Subramanian et al., 1999) to facilitate delivery. In this method,
a cationic peptide is attached to an M9 amino acid sequence and the
cation binds the negatively charged nucleic acid. Then, M9 binds to a
nuclear transport protein, such as transportin, and the entire DNA/protein
complex can cross a membrane of a cell.
An amino acid sequence may be delivered within a delivery vehicle.
An example of such a delivery vehicle is a liposome. Delivery of an amino
acid sequence may utilize a protein transduction domain, an example
being the HIV virus TAT protein (Schwarze et al., 1999).
In a preferred embodiment the biological agent of the present
invention has high affinity for a fibrin matrix (see Example 7).
In a specific embodiment, the particle of the present invention may
contain within it or on it a biological agent which would either elute from
the particle as it degrades or through diffusion.
The biological agent may be a pain killer. Examples of such a pain
killer are lidocaine hydrochloride, bipivacaine hydrochloride, and non-
steroidal anti-inflammatory drugs such as ketorolac tromethamine.
Other biological agents which may be included in the suspension
material or contained on or in the particles of the present invention are
chemotherapeutics such as cis-platinum, ifosfamide, methotrexate and
CA 02401421 2002-08-28
WO 01/66044 PCTNSO1/06043
47
doxorubicin hydrochloride. A skilled artisan is aware which
chemotherapeutics would be suitable for a bone malignancy.
Another biological agent which may be included in the suspension
material or contained on or in the particles of the present invention is a
bisphosphonate. Examples of bisphosphonates are alendronate,
clodronate, etidronate, ibandronate,
(3-amino-1-hydroxypropylidene)-1,1-bisphosphonate (APD),
dichloromethylene bisphosphonate, aminobisphosphonatezolendronate
and pamidronate.
The biological agent may be either in purified form, partially purified
form, commercially available or in a preferred embodiment are
recombinant in form. It is preferred to have the agent free of impurities or
contaminants.
Example 7 - Addition of Fibrinogen to the Composition
It is advantageous to include into the composition of shaped particles
and suspension material any factor or agent which attracts, enhances, or
augments bone growth. In a specific embodiment the composition further
includes fibrinogen which, upon cleaving by thrombin, gives fibrin. In a
more preferred embodiment Factor XIII is also included to crosslink fibrin,
giving it more structural integrity.
Fibrin is known in the art to cause angiogenesis (growth of blood
vessels) and in an embodiment of the present invention acts as an
instigator of bone growth. It is preferred to mimic signals which are
normally present upon, for instance, breaking of bone to encourage
regrowth. It is known that fibrin tends to bind growth factors which
facilitate this regrowth.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
48
In an object of the present invention the inclusion of fibrin into the
composition is twofold: 1) to encourage bone growth; and 2) to act as a
delivery vehicle.
The fibrin matrix is produced by reacting three clotting factors -
fibrinogen, thrombin, and Factor XIII. These proteins may be
manufactured using recombinant techniques to avoid issues associated
with pooled-blood products and autologous products. Currently, the
proteins are supplied in a frozen state ready for mixing upon thawing.
However, lypholization process development allows that the final product
will either be refrigerated or stored at room temperature and reconstituted
immediately prior to use. In a preferred embodiment the clotting factors
are recombinant in form.
Only fibrinogen and thrombin are required to produce a fibrin matrix
in its simplest form. However, the addition of Factor XIII provides the
ability to strengthen the matrix by means of cross linking the fibrin fibrils.
Specific mixtures of the three proteins may be provided to generate the
appropriate reaction time, degradation rate, and elution rate for the
biological agents.
Modifications can be made by altering the fibrin component. One
expected modification would be to use hyaluronic acid or a collagen gel
instead of or in addition to a fibrin component. Other variations may be
inclusion of additional clotting factors in the fibrin matrix. Additional
examples of clotting factors are known in the art and may be used, but in a
specific embodiment they are clotting factors relevant to a bone disorder.
The clotting factors may be purified, partially purified, commercially
available, or in recombinant form. In a specific embodiment thrombin
alone is used with the patient's own blood or bone marrow aspirate to
produce a fibrin matrix.
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
49
In a specific embodiment a biological agent as described above is
contained within the fibrin matrix.
Example 8 - Method of Making a Calcium Sulfate-based Shaped
Particle
In another object of the present invention, an improved method for
making a calcium sulfate-based shaped particle, is provided. Calcium
sulfate materials are typically not very strong when formed using
conventional forming techniques. Plaster of Paris (CaS04~'/zH20; calcium
sulfate hemihydrate) can be mixed with water and set through the
following reaction to form gypsum (CaS04~2H20; calcium sulfate
dihydrate):
CaS04~'/ZH20 + 1'/2H20 ~ CaS04~2H20
However, in order to have a pourable slurry, an excess of water is
required which increases porosity leading to a weaker material. In
addition, the high surface area to volume ratio of the porous component
can lead to increased dissolution rates of the material in an aqueous
environment.
Another option is to utilize a gypsum material and form it into a shape
through compaction of slurry casing. Since the gypsum is already fully
hydrated the material will not set through a reaction as above. If water is
used in the processing it is simply dried off, again leading to porosity in
the
final form.
This process invention allows the material to be formed using
techniques that can provide the desired component geometry and
reasonable density in the dried component. A secondary process of heat
treatment and hydration is then used to tailor the final material properties,
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
namely for the purpose of increasing the strength and decreasing the
dissolution rate. It should be possible to control these properties with the
control of the forming process and the subsequent
dehydration/rehydration. In specific embodiments the heating steps are
5 performed at a pressure greater than ambient pressure, such as in an
autoclave at 120-150 degrees Celsius or 25-50 PSI. A skilled artisan is
aware that the calcium sulfate composition of the invention may be of the
a or ~3 form depending on the heat and pressure parameters utilized, and
either form may be used or generated in the present invention.
In the process a heat treatment and hydration process is applied to
gypsum after it is formed into a shape (through pressing, casting, injection
or other means known in the art). Similarly, the process could be done on
a shaped component of plaster of Paris that was formed by some
non-water based process (i.e.: die compaction). The intention of this
secondary processing is to control the strength and dissolution of the
gypsum for use in a bone grafting application. By proper control of the
secondary processing it is possible to tailor the material properties of the
component.
Steps in process:
1 ) convert shaped gypsum to shaped plaster of Paris using heat
(approximately 150°C)
CaS04~2H20 ~ CaS04~'/ZH20 + 1'/2H20
2) convert shaped plaster of Paris back to gypsum, encouraging
recrystallization, to improve strength and dissolution properties
CaS04~2H20 + excess of H20 -~ CaS04~2H20 + excess of H20
3) dry components of excess water
Alternative embodiments for products with which this secondary
processing could be useful are any application where a stronger gypsum
CA 02401421 2002-08-28
WO 01/66044 PCT/USO1/06043
51
with more resistance to dissolution by water could be used such as
controlled release applications, consumer products, and mold making).
It is an object of the present invention to combine the known
substeps of conversion of gypsum powders to plaster of Paris powders
(calcination) and rehydration of the plaster of Paris powder to get the
material back to gypsum to generate a stronger gypsum.
In this invention, any calcium sulfate may be used which is capable
of hydration reaction. This includes gypsum formed in the exhaust gas
desulfurization process, gypsum formed as a by-product by neutralization
of waste sulfuric acid, gypsum formed as a by-product in the phosphoric
acid reproduction process, and calcined gypsum (especially gypsum
hemihydrate formed by refining such gypsum product by a known
recrystallization method and calcining the refined gypsum). In a preferred
embodiment the gypsum is commercially available.
A skilled artisan is aware that the application of water to the particle
in the rehydration step helps to control material properties, including
strength, dissolution rates and density.
Example 9 - Forming a Shaped Particle
The process involves the following general steps:
1. A clay-gypsum powder is mixed with processing aids (such as
binders and lubricants) and water to wet and make the clay plastic.
2. A forming operation such as pressing, rolling, extrusion or injection
shapes the clay to the desired form.
3. The clay is set in the mold or is in contact with the mold to make a
shape with enough green strength to be handled. Setting immediately
following the forming should also be good for maintaining the particle
geometry and tolerance.
CA 02401421 2002-08-28
WO 01/66044 PCT/US01/06043
52
4. The pieces can then be transported to the next processing step or
to packaging.
The following is a preferred specific embodiment of the process:
1. Make clay-Gypsum powder about 75 to 85 w/c (weight percent);
carboxymethyl cellulose or other binder material about 0 to 5 w/c; water
about 10 to 25 w/c.
2. Press clay-using a split mold, press the gypsum clay under an
applied load (approximately 3000 Ibs-force).
3. Set clay in mold-Apply heat to the mold with the gypsum clay.
Apply the heat such that the temperature of the parts achieves
approximately 100~C in about 5 minutes. Setting occurs through
dehydration.
4. Remove pieces from mold.
It is generally preferred that the ceramic material for the shaped
particle of the invention should not be too hard, sticky or dry.
There are many materials that may be suitable for use as binders,
including carboxymethyl cellulose, hydroxypropylmethyl cellulose, or
polyacrylate.
The shaping methods of the present invention can include pressing
in a split mold, injection molding, rolling and extrusion.
The 'setting' action for the clay can be by simple dehydration or could
be some more complex reaction that is mitigated by the combination of
binders, water and gypsum and controlled by some external stimuli such
as heat, radiation or chemical addition.
All patents and publications mentioned in the specification are
indicative of the level of those skilled in the art to which the invention
CA 02401421 2002-08-28
WO 01/66044 PCT/USOI/06043
53
pertains. All patents and publications are herein incorporated by reference
to the same extent as if each individual publication was specifically and
individually indicated to be incorporated by reference.
Ducy, P., Amling, M., Takeda, S., Priemel, M., Schilling, A.F., Beil,
F.T., Shen, J., Vinson, C., Rueger, J.M., and Karsenty, G. 2000. Leptin
inhibits bone formation through a hypothalamic relay: a central control of
bone mass. Cell 100:197-207.
Schwarze, S.R., Ho, A., Vocero-Akbani, A. and S.F. Dowdy, 1999. In
vivo protein transduction: delivery of a biologically active protein into the
mouse. Science 285: 1569-1572.
Subramanian, A., Ranganathan, P. and S.L. Diamond, 1999. Nuclear
targeting peptide scaffolds for lipofection of nondividing mammalian cells.
Nature Biotechnology 17: 873-877.
One skilled in the art readily appreciates that the present invention is
well adapted to carry out the objectives and obtain the ends and
advantages mentioned as well as those inherent therein. Particles,
compositions, treatments, methods, kits, procedures and techniques
described herein are presently representative of the preferred
embodiments and are intended to be exemplary and are not intended as
limitations of the scope. Changes therein and other uses will occur to
those skilled in the art which are encompassed within the spirit of the
invention or defined by the scope of the pending claims.