Note: Descriptions are shown in the official language in which they were submitted.
CA 02401973 2009-05-25
METHOD FOR COLLECTING ANI) PRESERVING SEMEN
Field of the Invention
The present invention is directed generally to a niethod for collecting and
preserving semen. More particularly, the present invention is directed to a
method for
collecting semen into a wanned extender solution having a particulai- vohime.
Most
specifically, the present invention is directed to a method lor collecting
semen into a
collection vessel having a semen extender inaintained at a species appropriate
temperature and having a particular cliemical inakeup. The method for
collecting and
preserving semen in accordance witli the present invention results in greatly
improved
collected semen motility and longevity.
Background of the Invention
Evidence suggests that semen has been collected for artificial insemination
(Al)
since the 1300's. It is held that Arabian tribes stole senien from rival
tribes' stallions to
inseminate their own mares and that the semen from a poor stallion was also
used to
inseminate the rival tribes' mares. The first documented use of AI was in the
1780's by
the Italian physiologist, Spallanzani. I-tis insemination of a bitch, with
freshly collected
semen, resulted in the production of three puppies. At has continued to be
developed as a
tool in aninlal and human reproduction. Initially, a major problem with AI was
that the
collected semen had to be used the same day (and in cases such as with dogs,
almost
I
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
immediately) in order to achieve good results (i.e., pregnancy). In order for
Al to
develop to its full potential, a method had to be discovered to preserve semen
for use at a
later date.
The first successftil, and by far still the most common, way to preserve semen
for
later use is with semen extenders. Semen extenders are used to provide
nutrients for
speriu metabolism, to carry additives such as antibiotics and cryoprotectants
(for storage
at lower temperatures), and to provide multiple breedings from one semen
sample. In the
1930's, it was discovered that it was possible to use a buffered nutrient
medium to extend
the fertilizing life of semen for periods of up to three or four days.
Extended semen can
be maintained for a considerable length of time (times vary depending on the
species) if
the semen is chilled. Further, when a cryopreservative such as glycerol is
added,
extended semen can also be frozen and can remain viable (i.e., produce a
pregnancy) for
up to 20 years as shown in bovine seinen.
Semen extenders have traditionally been added post-collection. After the semen
is collected, the extender is normally added at a 1:1, 2:1, or 3:1 extender to
semen ratio,
depending on the initial motility, concentration, and species collected. In
practice, the
extender is added anywhere from a few minutes post-collection, up to
approximately
one-half to one hour post-collection.
Unprotected, freshly ejaculated semen loses motility and therefore fertilizing
capability rapidly, rendering it virtually useless in a matter of a few
minutes to a few
hours (time varies depending on species). In some species, such as the canine,
zero
percent motility can be reached in less than one hour. This loss of
fertilizing capability is
even a problem with extended semen. If the extended semen is chilled, the
reduction in
2
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
motility and other semen parameters can be slowed, but not eliminated. The
loss of
motility and fertilizing capability is especially a problem for frozen-thawed
semen. It is
generally accepted that fifty percent or more of the initial motility will be
lost during the
freezing and thawing process.
From its earliest stages as a spermatogonia to its final maturation, which,
from a
biochemical standpoint, does not normally occur until after it has entered the
female tract,
the spermatozoon needs a constant supply of nutrients to maintain its
metabolic activity
and to undergo the processes necessary for fertilization of the ova. As
spermatogonia
develop into sperinatozoa in the seminiferous tubules, their nutrient needs
are provided
by the Sertoli cells, also called nurse cells. These so-called "nurse cells"
secrete fluids
containing proteins necessaiy for the germ cells to grow and to mature into
the
spermatozoa. They also supply some of the energy requirements of the sperm
through
the production of lactate, which is converted to pyruvate by the spermatid
mitochondria.
As the spermatozoa are released into the lumen of the seminiferous tubules and
continue their journey tlirough the rete testes to the epididymis, they
continue to be
bathed in fluid rich in proteins, energy substrates such as glucose and
lactate, and a
variety of other substances. When the sperm cells reach the epididymis, they
are still
immotile. In the cauda epididymis, the sperm are concentrated and stored in a
highly
favorable environment. The epididymal cells secrete fluid that is low in pH
and has a
high potassium-to-sodium ratio. These conditions allow the sperm to be stored
and
matured and still remain viable for an extended period of time.
When the sperm are ready to be ejaculated, they are forced into the vas
deferens
and on into the urethra and then out of the body. Once the sperm reach the
urethra the
3
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
concentrated fluid in wh:ich they are contained is mixed with seminal fluid
secreted by the
accessory sex glands. The secretions of these glands contain buffers,
nutrients, and a
variety of other organic and inorganic substances. The buffers such as
phosphates and
carbonate buffers are essential for protection against pH shifts as the semen
is deposited
into the hostile enviromuent of the female tract. ln most species, the site of
semen
deposition, the vaginal vault, is extremely acidic. Furtller, as the sperm
continue to
metabolize, waste products, such as lactic acid, are produced which can lower
the pH
even more. The organic and inorganic ions such as sodium (Na2+), potassium
(K+), and
calcium (Ca2+) are necessary to initiate sperm motility and fertilizing
capability. Other
nutrients, such as fructose and sorbitol, are utilized by the sperm to meet
energy
requirements. Extenders were created in an attempt to hold the sperm in a
favorable
environment for cellular survival, while biochemically placing the cells in
suspended
animation (delaying their progression toward final maturation) until time for
their use.
Since Spallanzani's first documented use of Al in tlie 1780's, artificial
insemination has continued to develop its niche in reproduction. Because the
raw
(unprocessed) semen lost fertilizing capability rapidly after ejaculation,
Spallanzani
discovered that the semen had to be used soon after collection in order to
achieve good
results. It would be one hundred and fifty years before a method would be
developed to
extend the fertilizing life of spermatozoa after ejaculation.
With the development of semen extenders, semen could be preserved for use
many hours, or even days, post-ejaculation. First described in the 1930's, it
was
discovered that it was possible to use a buffered nutrient medium to extend
the fertilizing
life of semen for up to three or four days. Extenders are used in an attempt
to hold sperm
4
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
in optimal conditions until their use and also allow for multiple breedings
from one
semen sample.
In order for a semen extender to be effective, it must contain a number of
ingredients. It niust buffer the senien against shifts in pH due to the
continual metabolic
activity of the sperm during storage. It must also maintain an isotonic
environment. If
the extender is hypertonic, the sperm will shrivel and die. If the solution is
hypotonic, the
sperm will swell and burst. A number of ingredients liave been used to meet
these
requirements. The first successfiil buffer to be used in a semen extender was
the
phosphate buffer. Although the phosphate buffer worked, the sodium citrate
buffer soon
replaced it, because when mixed with egg yolk (a common nutrient in semen
extenders)
the mixture remained transparent. A variety of other buffers and various
combinations of
these buffers are now available. Some of these buffers are the tris buffer
solution, the tes
buffer, the test-yolk buffer (combined tes and tris buffers with egg yolk),
and tris-citrate
buffer. Regardless of which buffer is being used, all are added in the proper
concentrations to not only buffer the solution, but also to maintain the
isotonic nature of
the extender.
Semen extenders must contain adequate nutrients for sperm to metabolize during
storage. A variety of substances fill this need. Milk and egg yolk are common
protein
sources. A third protein source is irradiated bovine albumin.
Simple sugars are added to provide the sperm with energy. Fructose, glucose,
sucrose, sorbitol and pyruvate have all been used in semen extenders as
sources of
energy. Pyruvate, by its chemical nature, is the energy substrate most easily
utilized by
the sperm. However, it is not the most common energy source. The most common
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
energy substrate found in commercial semen extenders is fructose. Its ability
to easily be
converted to pyruvate within the sperm mitochondria and its cost effectiveness
make it an
ideal source of energy.
In addition to their roles as nutrient sources, proteins also help fulfill
another
requirement of the semen extender by serving as cryoprotectants. Unprotected,
the
membranes of the spermatozoa undergo configurational changes as the
temperature is
lowered (cold shock). However, the lecithin, lipoproteins, and phospholipids
from the
protein source provide protection from cold shock. As the semen is lowered
from body
tenlperature to 5 C, the temperature at wllich fresh chilled semen is held
during storage,
the sperm will undergo cold shock if not protected. When properly prepared,
chilling to
this temperature keeps the semen viable for a longer period of time than if
kept at
approximately rooni temperature (15 -20 C). Ptather, non-protein
cryoprotective agents
must be employed if the semen are to be frozen. This will be discussed in
detail below.
The final ingredient in inost semen extenders is an anti-microbial agent. The
anti-microbial agent is essential for reducing rnicrobial contamination and
preventing the
spread of diseases that can be transported in the semen. Through their control
of venereal
diseases, these agents have also been shown to improve conception rates. Some
common
antibacterial agents are penicillin, streptomycin, lincomycin, and gentamicin.
When an extender is to be used as the base media for freezing and long-term
storage of semen (cryopreservation), an additional cryoprotective agent must
be added to
protect spermatozoa from ice crystal fonnation. When semen is frozen without a
cryoprotectant, the ice crystals that form during the freezing process
puncture the cell
membrane and result in cell death. By using a cryoprotectant such as glycerol
or DMSO
6
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
(dimethylsulphoxidc), a large portion of the intracellular water is displaced
by the
cryoprotectant; theri.:fore, cellular damage due to ice crystal formation is
largely
prevented.
Semen exteriders liave continued to be used since their inception and a
variety of
extenders and cryol)rotectants are now available commercially. These products
fall into
three basic classes based on their protein source (egg yolk, milk, or
albumin). The egg
yolk based extende rs are used in many species, including cattle, sheep, dogs,
and humans.
Milk based extenders are used almost exclusively in the horse. The third class
of
extenders, serum albumin based extenders, are used in species such as dogs,
exotics and
humans.
Artificial insemination has been used for several centuries. However, it was
not
until the early 1900's, when semen extenders were developed, that semen could
be stored
and used at a time other than immediately after collection. The traditional
method of
extending semen was, and still is, to add the extender anywhere from a few
minutes up to
one hour post-collection. While this method does help preserve semen for use
at a later
time post-collection, data from this experiment suggests it is not the most
efficient
method.
Semen collection can be performed in a variety of ways depending on the
species.
Methods include the artificial vagina (AV), electro-ejaculation, digital
manipulation and
masturbation.
The first artificial vagina was developed at the University of Rome in 1914
for
use in the dog. Russian scientists developed other AV's for use in larger
species such as
the horse in 1933. Today's AV's, which vary in size and shape depending on
species,
7
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
consist of a tapered collection sleeve, in which the penis is placed. Attached
to the
tapered end is a collection container. Most AV's attempt to simulate natural
copulation
by providing suitable temperature, pressure and lubrication to induce
ejaculation.
The electro-ejaculator was developed in the 1940's. It consists of a probe
that is
placed into the rectum of the male to be collected. A low-voltage current (0
to 30 volts
with 0.5 to 1.0 amperage) is passed through the probe, stimulating
ejaculation. This
method is often used in bovine and ovine. The electro-ejaculator has also been
used in
humans with lower-body paralysis in order to obtain an ejaculate. While this
method of
collection is effective, it can produce lower quality semen samples when
compared to the
artificial vagina or digital manipulation. The electro-ejaculator should never
be used in
the equine due to possible tearing of the rectal tissue.
Digital manipulation is another method used for semen collection. This
technique
involves the physical manipulation of the penis by the collector to obtain an
ejaculate.
This method can be used alone with a collection container or combined with an
artificial
vagina. 1'his teclulique is commonly used in the porcine and the canine.
The most coinmon method for semen collection in the canine is with the use of
an
artificial vagina and digital manipulation. This technique involves the
collector utilizing
digital stimulation to encourage the dog to extend his penis and ejaculate
into the
artificial vagina. The index finger and thumb are placed in a u-shape behind
the bulbus
glandis, which helps to give the male the sensation of being "locked". It is
helpful with
some animals to allow the dog to step over the collector's arm, simulating the
natural tie.
Depending on the individual animal, variations in pressure, friction, and
movement may
be necessary to obtain an ejaculate.
8
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Regardless of which method is being used to obtain a semen sample, all must
use
some type of collection container. With the traditional collection methods,
the collection
containers (in the case of the canine, usually a plastic centrifuge tube) do
not have any
media placed in them prior to collection. After the semen is collected the
extender will
be added anywhere from a few minutes, up to one hour post-collection,
depending on the
protocol being used. While this method works and continues to be used today,
it may not
be the most efficient method.
Sperm are especially susceptible to changes in temperature. With natural
service,
semen is ejaculated into the warm moist environment of the female tract.
Iiowever, with
the traditional method of semen collection, semen is collected into a dry
container where
variations in temperature can be a real problem, especially if the collection
room is cold.
When using a dry collection tube, the outside air temperature is quickly
transmitted to the
semen sample. One study on mouse spermatozoa showed that sperm collected at 0
to 4
C had significantly lower motility than that of sperm collected at 22 C.
Occasionally,
attempts to maintain the temperature of the collection tubes have been
performed.
Although attempting to niaintain the temperature of the collection tube is of
some help,
the semen is still being shocked. The semen is coming in contact immediately
witli the
collection container and any temperature variation can still be a problem.
Sperm are also susceptible to shifts in pH. While semen extenders do contain
buffers, the semen may not come in contact with these buffers until well after
collection.
The semen being collected into the dry collection tubes does not allow the
sperm to be
buffered immediately. Because sperm are continually metabolizing, the pII will
quickly
change in the absence of a buffer.
9
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Summary of The Invention
It is an object of the present invention to provide an improved method for
collecting and preserving semen.
Another object of the present invention is to provide a method for collecting
semen into a collection vessel having a particular extender solution volume.
A further object of the present invention is to provide a method for
collecting
semen into an extender solution maintained at a desired temperature.
Still ar.iother object of the present invention is to provide a method for
collecting
semen into an extender solution having a particular composition.
Modifying the way semen is collected and extended lessens the problems of
reduced motility and fertilizing capability seen in chilled extended semen.
Sperm are
easily susceptible to shock due to temperature and pH shifts. This shock to
the sperm can
result in decreased motility and other semen parameters and therefore
decreased semen
life. Protecting semen from temperature and pH shock upon collection would
extend the
functional life and :fertilizing capability of the spermatozoa. The method of
semen
collection and extension has been modified. A portion of'the extender is added
to the
collection vessel prior to semen collection, in an attempt to lessen or
prevent shock of the
spermatozoa.
The collection of semen samples into warmed extender media protects
spermatozoa from the initial cold and pH shock that can occur when traditional
collection
and extension methods are used. By collecting directly into the semen
extender, the
spermatozoa are immediately placed into a buffered environment, lessening the
chances
of pH shifts. Further, when the extender is warmed to the body temperature
appropriate
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
for the species being collected, the problem with temperature shock is also
lessened.
While extenders, have been added to the collection vessel prior to collection
in selected
species, for exaniple, for the treatment of sub-fertile aninials and in humans
for the
treatment of anti--sperm antibodies, this tecluiique has never been used with
animals
having normal feirtility nor as a routine methodology in humans.
Data obtained from experiments demonstrates that collection into warmed
extender media lessened the change of temperature and pH shock to the
spermatozoa
compared to that reported in previous studies, as shown by the improved semen
parameters. This rnodification to the collection/extension of semen in
accordance with
the present invention allows for improved preservation of spermatozoa over
time when
compared to traditional methods. Data analysis clearly shows that collecting
semen into
warmed extender rnedia improved the semen parameters evaluated. Specifically,
the
functional life spar- of the spermatozoa, measured as motility, was
significantly increased
in the treatment group as compared to the control (time to zero motility).
Some dogs were classified as "Tolerant" and "Tntolerant" depending on their
tolerance to traditional methods of semen collection. When comparing the
"Tolerant"
and "Intolerant" groups, both groups demonstrated improvement in motility in
the
treatment group. However, semen collected from those animals that were
"Intolerant" to
the traditional collection method appeared to demonstrate the most
improvement. By
collecting into the warmed extender, the temperature remained constant
preventing cold
shock to the spermatozoa. The spermatozoa also came in contact with the
buffers of the
extender immediately upon collection which lielped to prevent shifts in pH.
11
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
The treatment group maintained motility significantly longer than the control.
This held true for both the "Tolerant" and "Intolerant" groups, with the
"Intolerant"
samples demonstrating the greatest response to the methods of this disclosure.
This
improved motility over time led to the improvement seen in the time to last
full
insemination. Further, times to full acrosome reaction were delayed in the
treatment
group. By maintai ning a greater percent of motile (and therefore viable), non-
acrosorne
reacted sperm, the treatment group maintained a full insemination dose .for a
greater
length of time as compared to the control. By calculating the available sperm
pool (total
motile sperm per eiaculate), it was possible to observe that in animals with
good
concentration and volume had a greater number of inseminations upon collection
and
maintained at least one full insemination dose much longer due to the
treatment. In
animals that had lower concentrations and/or volumes, it was possible to get
an
insemination by using the treatment where no insemination would have been
available
using traditional methods.
By utilizing the collection method of the present invention in the
collection/extension procedure, it is possible to improve semen parameters in
both the
"Tolerant" and "Intolerant" animals, with the "Intolerant" animals appearing
to
demonstrate the most improvement. Semen from animals with a good volume and
concentration can be extended and maintained for a longer period of time as
compared to
traditional methods, allowing for improved ability in shipping fresh-extended
semen.
Animals that would not have an adequate semen sample for insemination, when
using
traditional methods, would now have to ability to be used for Al.
12
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Brief Description of The Drawinlzs
While the novel features of the method for collecting and preserving semen in
accordance with the present invention are set forth with particularity in the
appended
claims, a full and complete understanding of the invention may be obtained by
reference
to the detailed description of the preferred embodiment, as is set forth
subsequently, and
as illustrated in the accompanying drawings, in which:
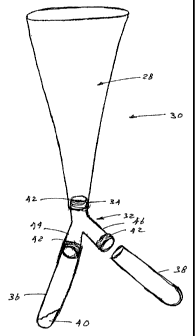
FIG. I is an exploded perspective view an artificial vagina utilizing a
collection vessel in
accordance with the present invention;
FIG. 2 is a perspective view showing a modified collection vessel for species
not
collected by the artificial vagina shown in Figure 1;
FIG. 3 is a graphical showing of the comparative motility of canine semen
obtained by
the methodology of the present invention compared with motility data of semen
obtained
by tradition methods;
FIG. 4 is a graphical showing of the survival time of canine spermatozoa from
five
different animals using semen treated by the methodology of the present
invention
compared with semen treated by tradition methods;
FIG. 5a is a graphical showing of the least-squares mean values of percent
motility of
porcine spermatozoa using semen treated by the methodology of the present
invention
compared with semen treated by tradition methods; and
FIG. 5b is a graphical showing of the least-squares mean values of percent
motility of
human spermatozoa using semen treated by the methodology of the present
invention
compared with semen treated by tradition methods.
13
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Detailed Description of The Invention
While some current techniques of semen collection attempt to maintain the
temperature of the artificial vagina for the male's comfort, only a few
attempt to insulate
the collection container (porcine technique) and none attempt to maintain the
pI-I of the
specimen. Maintaining the spermatozoa at its preferred physiological
temperature and a
pH close to 7.4 will maintain the spermatozoa in optimum condition during
collection,
processing and in preparation for storage, and will improve post-storage semen
parameters.
The collection system of the present invention has been constructed to answer
the
physiological needs of the spermatozoa at the time of collection and lessen or
prevent
shock damage wliich would lead to decreased fertility to the spermatozoa. This
collection system could be used in any mammalian specie, including
domesticated and
non-domesticated animals, as well as in humans, and with any existing
extender/cryoprotectant.
In accordance with the present invention:
1) the samples of semen are collected into a measured volume of the protective
agents which protective agent or extender volume is provided in the range of
approximately 20% to 100% of the total expected semen volume that will be
placed in the specie-specific collection container,
2) the sample/media complex is maintained at physiological temperature, 32-38
C;
specific to each specie, and
3) only the sperm-rich fraction of the ejaculate are collected. A series of
specie
specific collection devises to allow for a true division of the ejaculate,
either into
14
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
fractions or a true-split ejaculate have been developed. These devises allow
easy
collection of the sperm-rich portion of the ejaculate while minimizing
contaminating by sperm-poor and gel fractions.
In preparation for extension/cryopreservation, the specie appropriate semen
collection
device is prepared. 'I'he extender/cryoprotectant is warmed to -3 to 0 C of
the expected
semen temperature upon ejaculation. Further, the collection vessel is warmed
to the same
temperature, A measured volume of the specie-appropriate
extender/cryoprotectant in the
volume range of 20-100% of the expected sperm-rich volume is added to the
collection
vessel. This media volume is set forth in Table 1 as follows:
TABLE 1. Specie-Specific Volumes of Extender/Cryoprotectant
Added to the Collection Vessel Prior to Collection for
Coinmon Domestic Mammal Species and Humans
Specie Media Volume % of Expected
(ML) Volume
Bovine 1-2 20
Canine 1 20-100
Equine 10-15 20-50
Ovine 1 l 00
Porcine 20-50 20-50
Human 1 20-100
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Where appropriate, the collection vessel is attached to the artificial vagina
and the
sample collected using the accepted teclinique for that specie. Once the
collection is
complete, the size of the ejaculate is determined (Total volume minus the
extender/cryoprotectant added prior to collection). Additional warmed media is
added to
dilute the semen sample to its final concentration (1:1 or 1:2 for
cryopreservation and 1:2
or 1:3 for extension; specie specific) , and processed using standard
iiidustry teclmiques.
A proprietary media based on the specific physiological needs of a motile
spermatozoa for each species has been developed. This will be discussed
shortly.
Collection techniques vary widely between species, therefore adding media to
the
collection vessel prior to collection presents unique problems which are
specie specific.
However, the collection vessels used to collect domestic mammal species and
human fall
into three general types as outlined in Table 2. The artificial vagina is
shown in Fig. 1
while the warmed collection vessel is depicted in Fig. 2.
'1 able 2. Collection Techniques
Technique Specie
Artificial Vagina Bovine, Equine, Canine
Warmed Collection Vessel Porcine
Dry, Room Temperature Collection Ovine, Bovine, Human
The porcine is collected by digital manipulation into a warmed collection
vessel
generally at 20, as depicted in Fig. 2, which has a mouth opening 22 covered
with gauze
to strain out the gel fraction of the ejaculate. While the vessel 20 is
generally warmed by
an outer warmed chamber 24, the vessel itself is dry before collection. The
appropriate
16
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
desired volume of extender media 26 is siniply added to the vessel prior to
placement of
the gauze. Unlike, the open, warmed, vessel 20 of the porcine, the artificial
vagina,
generally at 28 shown in Fig. 1, represents a closed system 30 for semen
collection.
Once the male's penis is placed into the system 30, all fluids ejaculated are
routed to the
collection vessel. While gel can be filtered similarly to the open container
by placing
gauze at the collection vessel opening, it is difficult to eliminate the sperm-
poor fractions
of the ejaculate. I-Iowever if a biocompatible y-tube 32 is placed at the
junction 34 of the
artificial vagiiia 30 and the collection vessel or vials 36 and 38, it is
possible to route the
sperm-rich portion in one direction and the sperm-poor fraction in the other.
The warnled
media 40 can then be placed in the collection vessel 36 used for the sperm-
rich fi-action.
The collection vessel shown in Figure 1 includes the artificial vagina 30
connected to a y-tube 32. Latex gaskets 42 are suitable for securing a tight
fit between
the artificial vagina 30 and the y-tube 32. Other kilown sealing agents may be
used. It is
preferred that the sealing agent bond the components together sufficiently to
allow the
assembled parts to remain integral during manipulation of the aninlal, but
preferably it
still is possible to disassemble the components after collection of a semen
sample.
Collection vessels, for example, vials 36 and 38 are connected to the ends
located near
the split ends 44 and 46 of the y-tube 32. Preferably the collection vials 36
and 38 fit
over the outer diameter of the ends 44 and 46 of the y-tube 32. Likewise, in
one
embodiment of this invention, the outer diameter of the smaller opening of the
artificial
vagina is slightly less than the inner diameter of the opening of the y-tube
32 connected
to the artificial vagina. Alternatively, the smaller operning of the
artificial vagina may fit
over the y-tube 32.
17
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Finally, in those species which have traditionally been collected into an
open, dry,
container that has been maintained at room temperature such as the container
20 of Fig.
2, it is necessary to change the shape of the container to accommodate the
inclusion of a
volume of extender media appropriate for each specie, such volume typically
being
between 1 and 10 mL. Because of the small collection volumes, it is also
necessary to
place insulation around the vessel to prevent cooling. However, the open end
22 of
container 20 must be large enough to accommodate the free end of the penis. A
collection device capable of both is shown in Figure 2.
It is not unknown for the collection process to take up to 45 minutes. The
insulation is chosen to minimize temperature variation of the collection
vessel and added
extender solution such that the temperature of the collection vessel 26 and
added extender
solution does not vary more than 2 or 3 C over a period of time up to 45
minutes. Of
course, it is understood that the extender solution and the collection vessel
are initially
within 2 or 3 C of an initial temperature appropriate for the species being
collected.
Preliminary data from the canine, a specie resistant to current technology,
indicates that the inclusion of the protective agents prior to collection
maintains all semen
parameters (motility, viability, etc ... ) at higher levels than semen
prepared in the
tradition fashion. Canine semen were prepared for extension using the
commercially
available extender, Androhep (Minitube of America; Verona, WI). Split
ejaculates were
collected using the y-tube described above. In all cases, the ejaculate
fraction collected
into warmed extender maintained increased spermatozoa activity when compared
to the
fraction collected into a dry container. This is depicted in Fig. 3 with the
line 50
identifying the improved semen obtained in accordance with the present
invention.
18
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Further, as this technique can not be detrimental to semen quality; we have
used
the technique has been used, with permission of the dogs' owners but without
disclosing
the technique, in the breeding of six dogs. Semen were prepared for extension
as
described above or for cryopreservation using the cryoprotectant, Freezing
Medium-
TEST Yolk Buffer (Irvine Scientific; Santa Ana, CA). Breedings to date have
resulted in
the production oi,' five litters.
Extenders represent an osmotically balanced salt solution, containing both an
energy source (sugar), and a protein source [for both metabolites and as a
high
temperature (0-5 C) cryoprotectant]. Cryopreservatives add one of several
cryoprotective al;ents to this base media in order to protect the spermatozoa
at low
temperatures -30 to -196 C. Ringer's Lactate has many attributes of an
extender. It is an
osmotically balanced salt solution (as per its design as a fluid volume
replace solution
following dehydration, surgery or injury). It also has two sugar sources,
sodium lactate
and fructose. Further it has calcium, an essential element for sperm motility.
However, it
lacks the protein source of the classical extenders and earlier work suggests
high levels of
chloride may be detrimental to spermatozoa function. Short term semen quality
(culturing 1-24 hr) can be improved simply by substituting a variety of
calcium
compounds into the Ringer's lactate to replace the calciuni chloride. These
quality
improvements are shown in Figs 5a and 5b. Of the calcium compounds tested,
semen
function was maintained best in calcium phosphate, calcium carbonate, and
calcium
glucoilate. With the addition of a protein source, these modified Ringer's
Solutions
function as extenders, and with the addition of a cryoprotective agent they
also serve as
19
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
cryoprotective media and further improve the described collection process.
Fornnulation
of the modified compounds are listed in Table 3.
TABLE 3. Reformulation of IZinger's Lactate Solution for Use as a Semen
Extender
(mg/100 mL Water)
CaC12 Substitution with
Chemical CaCO3 Ca(H2PO4)2 Ca gluconate
aCl 600 600 600
Na lactate 310 310 310
CI 30 30 30
ructose 20 20 20
CaCO3 40 - -
Ca(H2PO4)2 - 82 -
Ca gl.uconate . - - 154
-------------------------- ----------------------------------------------------
----------------------------
Protein Source * 0.1 0.1 Ø1
*-protein sources will vary with specie and will be those currently used (egg
yolk, milk,
bovine albumin).
Unprotected, freshly ejaculated semen loses motility and therefore fertilizing
capability rapidly, rendering it virtually useless in a matter of a few
minutes to a few
hours (time varies depending on species). In some species, such as the canine,
zero
percent motility can be reached in less than one hour. This loss of
fertilizing capability is
even a problem with extended semen. If the extended semen is chilled, the
reduction in
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
motility and other semen parameters can. be slowed, but not eliminated. The
loss of
motility and fertilizing capability is especially a problem for frozen-thawed
semen. It is
generally accepted that fifty percent or more of the initial motility will be
lost during the
freezing and thawing process.
In theory, modifying the way semen is collected and extended may lessen the
problenis of reduced motility and fertilizing capability seen in chilled
extended semen.
These results should also apply to frozen-thawed semen. Sperm are easily
susceptible to
shock due to temperature and pH shifts. This shock to the sperm can result in
decreased
motility and otlier semen parameters and therefore decreased semen life. In
theory,
protecting semen from temperature and pH shock upon collection would extend
the
ftinctional life arid fertiliziiig capability of the spermatozoa. a portion of
the extender is
added to the collection vessel prior to semen collection, in an attempt to
lessen or prevent
shock of the spermatozoa.
In this invention, extension of the functional life span and fertilizing
capability of
semen has been attained through a modification of the traditional
collection/extension
method. Using the canine as a model, semen were collected into a measured
amount of
warmed extension media (-20% by volume of the expected volume of ejaculate = I
ml)
to determine if this method improved the life span and/or the fertilizing
capability of the
semen over the traditional method.
The canine was chosen for the model for a number of factors: (1) Canine sperm
are one of the more difficult cell types to niaintain for any length of time
outside the
body. Therefore, if semen parameters can be improved in the canine through the
modification of semen collection/extension, then theoretically the technique
should work
21
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
well in other species including humans, (2) Canine semen is similar in volume,
concentration, and sperm physiology to humans and works well as a model, (3)
Canines
are relatively eas.y to handle and collect, (4) And finally, there is a ready
supply of dogs
available in the community with known histories and therefore, there was no
need to
purchase and house test animals.
Prior to c.ollecting an animal, basic information such as name, age, breed,
AKC
registration number, owner's information, and date collected were recorded. In
order to
meet the requirennents set by the Animal Care and Use Committee, all
collections were
made under the supervision of a local veterinarian.
It was necessary to collect semen so that both the control and treatment
samples
were collected siniultaneously from the animal. This method of collection was
necessary
in order to eliminate the variation that can be seen between different
collections, or even
different collection fractions (traditional split collection), within the same
animal. No
traditional collection device allowed for this type of collection. Therefore,
it was
necessary to develop a collection device that could be used to fulfill this
need.
The modified artificial vagina with a biocompatible Y-tube as shown in Fig. I
and
as previously described allowed for a true split collection. This allowed one
ejaculate
from an animal to serve in both the control and treatment arms of the study.
Collection of the semen samples was done using digital manipulation and the
modified artificial vagina. Prior to collection the centrifuge tubes used to
collect the
semen were prepared. One tube was labeled control and no special procedures
were
taken. The other tube was labeled treatment and a measured aniount (lml) of
37C semen
22
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
extender was placed into the tube. This tube continued to be maintained at the
37'C
temperature.
The collection involved two individuals, one to collect the dog (collector)
and one
to manipulate the collection device (handler). The dog was encouraged to
extend his
penis and then the penis was placed into the lubricated AV. Using digital
manipulation,
the collector manipulated the dog until initial ejaculation. As the dog
ejaculated the
handler guided the Y-tube portion of the AV so as to collect relatively equal
amounts of
semen into each side of the collection device. Once the dog had finished
ejaculating the
AV was removed. The extension of the treatment sample was then completed
immediately using a 2:1 extender to semen ratio by volume. The control sample
was held
fifteen minutes post-collection before extending at the same ratio. This delay
was chosen
based on common veterinary practices.
Semen samples were evaluated at zero, one, six, twelve, eighteen, and twenty-
four
hours post-collection and at twenty-four hour intervals there after, until
zero percent
motility was reached. After the one-hour evaluation, semen was chilled to 5 C
and
stored. For the subsequent evaluations, a small amount (- .25m1) of semen was
removed
and warmed in a 37 C waterbath. Selected semen parameters were recorded at
each time
interval for both the treatment and control groups during the course of the
experiment.
Evaluations of a treatment ended when motility in that sample reached zero
percent. The
parameters evaluated included: volume, concentration, motility, forward
progression,
acrosome reaction, viability, and morphology. All standard semen parameters
were
evaluated on a Nikon Alphapot microscope equipped with phase optics (Nikon
Inc.; NY,
23
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
NY). Acrosome measurements were made on a Zeiss Standard microscope equipped
with fluorescence and cubes (Carl Zeiss Inc., NY, NY).
Volume
The initial total volume of semen was recorded upon ejaculation. It included
the
volume in both the control and the treatment (minus the volume of extender
added prior
to collection) arms of the study. The total volume of ejaculate was used to
calculate the
available sperm pool (discussed below) for both the control and treatment
groups.
Concentration
A slide designed to hold a measured volume of semen (3 microns; Microcell;
Conception Technologies, San Diego, CA) was used along with an eyepiece
micronieter
to determine the concentration. When viewed under the microscope at lOOX
magnification, the number of sperm in ten blocks of the micrometer (selected
at random)
is used to calculate the number of sperm per milliliter of an un-extended
sample.
Because the semen was extended at a 2:1 semen to extender ratio, it was
necessary to
multiply the observed count by three in order to calculate the actual
concentration. The
concentration was recorded in number of sperm per milliliter of ejaculate. The
concentration was averaged across time intervals for both the treatment and
control
samples. These averages were used to calculate an average concentration across
both the
treatment and control samples. This pooled average concentration was then used
to
calculate the available sperm pool (see below).
Motility
Because motility is one of the major criteria used in evaluating the
fertilizing
capability of spenn, the percent motile sperm was recorded. It is necessary
for sperm to
24
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
be motile in order to cross the zona pellucida and fertilize the oocyte. Once
motility is
lost, the sperm lose the ability to fertilize an ovum without assistance from
techniques
such as Intercytoplasmic Sperm Injection. Therefore, the logical end for the
evaluations
was when samples reached zero percent motility.
Using a Microcell slide and 100X power, a total of one hundred sperm were
counted. The number of moving sperm per one hundred gave the percent motility.
All
counts were made manually.
Forward Pro reg ssion
At each time point the forward progression of the motile sperm was evaluated
using a five-point scale as follows: 5+ sperm moving rapidly in a forward
direction
across microscope viewing field (in approximately one second or less), 4+
sperni nioving
steady but sloxver in a forward direction across the microscope field, 3+ in a
rapid side-
to-side motion with slow foi-ward progression, 2+ sperm moving in a side-to-
side
direction with no forward motion or in a circular or irregular pattern, 1+
sperin inoving
slightly side-to-side or in place with slight tail movement, and 0 no movement
detected.1b
Acrosome Reaction
In order for sperm to be able to fertilize the oocyte, they must first undergo
and
the acrosome reaction. This is a process where the acrosome cap of the sperm
head
dissolves and releases enzymes which allow the sperm to bind to the zona
pellucida of
the oocyte.
At each time point, the acrosome reaction was evaluated using a
Chlorotetracycline stain. When viewed under a fluorescent microscope, equipped
with a
520 m excitation filter and a 570 m barrier filter, the intact acrosome cap
appears a
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
fluorescent yellow. If the cap is visible, the sperm has not undergone the
acrosome
reaction. Both acrosome reacted and dead sperni (sperm which have lost the
selective
permeability of their membranes) loose their acrosomal cap there for appear
faded.
Because the acrosome reaction must not occur until the sperm are in the
proximity of the
oocyte, during storage it is desirable that the acrosome caps remain intact.
Therefore, the
percent of non-acrosome reacted sperm was recorded at each time point. These
numbers
were then used in the calculation of the available sperm pool.
Acrosomally Intact Sperm
Acrosome Reacted Spenn
Slides were prepared by mixing .5 microns of semen with 1 micron of
Chlorotetracycline stain. A coverslip was placed on the slide and the slide
was viewed
immediately using oil immersion under 1000X power. A total of one hundred
sperm
were counted and the percentage of non-acrosome reacted sperm was recorded.
Available Sperm Pool
26
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
The available sperm pool was calculated in order to determine the number of
motile, non-acrosome reacted sperm available for insemination and the number
of
insemination dosages at any given time point. lt was calculated by multiplying
the total
volume by the pooled average concentration, the percent motile sperm, and
percent of
non-acrosome reacted sperm (example: 2ml x 100million/ml x 50% motile x 50%
non-
acrosome reacted == 50 million available sperm). The available sperm pool was
then
divided by the nun:iber of sperm needed per insemination (number varies
depending on
species) in order to determine the number of inseminations.available. In the
canine, there
has been a great deal of controversy on the number of sperm needed per
insemination to
achieve a good pregnancy rate. Numbers range from 20 million to 200 million
motile
sperm per insemiriation. Based on communication with a local veterinarian (who
is a
canine reproductive specialist) and the fact that the number of non-acrosome
reacted
sperm (along with percent motile sperm) was included in calculating the
available sperm
pool, 60 million motile, non-acrosome reacted sperm was used as the
insemination
dosage needed to achieve a pregnancy in the canine. Survival time to last full
insemination (last time point with at least 60 million motile, non-acrosome
reacted
spermatozoa) was calculated and compared between the control and treatment
groups.
Viability
The viability or number of live cells was recorded at each time interval for
both
groups. Viability was accessed using Touladine Blue as a viability stain. Live
sperm do
not allow the stain to penetrate the cell membrane, while dead sperin (sperm
which have
lost the selective permeability of their membranes) accept the stain.
Therefore, when
27
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
viewed under the microscope at lOOX, the live sperm appeared clear, with a
bluish puiple
halo, while the dead sperm appeared completely bluish purple.
Slides were prepared by mixing .5 microns of semen with .5 microns of
Touladine
Blue stain and smearing the mixture across the slide. The slides were then
dried on a
slide warmer plate at 56 C. A total of one hundred sperm were counted and the
number
of live sperm gave the percent viable.
Morphology
Morphology was recorded at the initial time point, approximately one-half way
through the evaluations, and when zero percent motility was reaclied, for both
the
treatment and control samples by three investigators, to verify that
morphology did not
change due to treatment. Morphology was not used in calculating the available
sperm
pool as the number of normal sperm fell within the acceptable range of sixty
to eighty
percent or greater.
The morphology slides were prepared by smearing .5 microns of semen across a
slide. The slides were then dried on a slide warmer plate at 56 C. Dried
slides were then
stained using an Eosin-l-lematoxylin stain using the following steps: (1)
slides were
dipped ten times in Methanol, (2) slides were then transferred and dipped into
Eosin ten
times, (3) slides were transferred and dipped ten times in the Hematoxylin,
(4) slides
were then rinsed by dipping ten times into de-ionized water, and finally, (5)
slides were
dried on a slide warmer plate at 56 C. A total of one hundred sperm were
counted and
the number of head, mid-piece, and tail defects were noted at each evaluation
along with
the percentage of normal sperm. This was done to confirni that there was no
change in
morphology over time.
28
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
l;xperiment Design
The study was designed as a split-plot with repeated measures having a
specific
end point. Becau:;e a portion of the same ejaculate from each dog was
represented in
both the treatment and control arms of the study, the design was a true split
plot. 'l'he
same ejaculate wus evaluated over time, resulting in 'the repeated-measures
factor.
However, individual animals had a significantly different number of repeated
measures
due to the fact that when samples reached zero percent motility the data
collection ended.
Because of the specific ending point being zero percent motility, the zero was
an actual
number and was included in the data analysis. An alpha level of .05 was set
for
consideration of whether a statistical difference was detected.
Sample Numbers
A total of ten animals were used in the study. The criteria for participation
were
that the dogs had sufficient volume so the ejaculate could be represented in
both the
treatment and control arms of the study and that there was motile sperm upon
ejaculation.
Having ten dogs represented in each group along with the repeated measures
factor
allowed for more than ample numbers of observations to show a statistical
difference.
Data Analysis
All data were analyzed using the Statistical Program for the Social Sciences
(SPSS) version 8.0 on a Gateway Solo laptop computer. Comparisons between
treatment
and control groups for motility, acrosome reaction, viability, and morphology
were
analyzed using the General Linear Model (GLM). Motility at any specific time
point,
time to zero motility, and time to last full insemination were compared
between the
29
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
treatment and control groups using paired t-tests. Chi-square analysis was
used for
comparison of the forward progression data.
Preliminary data analysis revealed there was no a dog-treatment interaction.
However, the dogs could be classified into two groups based on based on the
their
tolerance to traditional methods. Dogs whose control samples did not have at
least
twenty percent motility at the zero hour evaluation and/or did not maintain at
least twenty
percent motility at the one hour evaluation were labeled "Intolerant" to
traditional
methods. All other dogs were considered to be "Tolerant" to traditional
methods. Of the
ten dogs represented in this study five were considered "Tolerant" and five
were
considered "Intolerant." General observations were made based on this
tolerance or
intolerance designation.
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Motility
As expected, there was a difference in motility due to time (P<.001). Also
there
was an overall difference between the treatment and control groups for
motility (P<.001),
The difference between treatment by time versus control by time was
significant
(P<.001). In addition, speimatozoa in the treatment group had significantly
higher
motility compared to the control at all time points past the initial
evaluation up to 192
hours, where there were too few animals for an accurate analysis. The
following graph
shows the difference between the treatment and control groups when all animals
were
included in the analysis.
100
90 ; - - ` =
80 P<.001
e; 60 l- - - Treatment
Control
40 i = - - - --
30 -
20 \ - - -
10 - -~ - - -
0 - r-t r - r,- r- r-r- =-.
0 18 72 144 216 288 360 432 504 576
Hours
Treatment by Time versus Control by Time-All Animals
31
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
There was also a difference between the "Tolerant" and "Intolerant" animals
(P<.001).
The following graphs show the difference between the treatment by time versus
control
by time for both tlle "Tolerant" and "Intolerant" animals (.
100
90 ~ . _
P<.001 -
70 -- - Treatment
60~-- - - ~
50 Control 40
I r - 30
~.-=.
~ ...
--- --- - - -
20 =; - - -=
10 =',- --- - -
0 , ,
0 12 48 120 192 264 336 408 480 552
Hours
Treatment by Time versus Control by Time-"Tolerant" Animals
100 .._..,.
- - - - - - - -
80 - - ~ P<.001
~ -.
70 ~ ; -- - _
60 - - -Treatment
50 - - - - -- - - Control
40 ~- -- - - - -
30 `ti - --- - - - - -- -
~~ --- -- -
10 a ~_-~~ -- - - 0
~-r z-- ~ -
0 6 18 48 96 144 192 240 288
Hours
Treatment by Time versus Control by Time-"Intolerant" Animals
32
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Tune to Zero Percent Motility
The analysis of time to zero percent motility showed. a difference due to the
treatment (P ---.001). The average time to zero percent motility for the
control group was
129.6 hours (std. error +/- 30.75) with a range of 12 to 288 hours. The
average time to
zero percent rnotility for the treatment group was 276 hours (std. error +/-
49.74) with a
range of 48 to 600 hours. When all dogs were included in the analysis,
spermatozoa
survival time iiicreased in the treatment group an average of 378.31% over the
control.
300, - P<.001
,~.
2501 QTreallTut
m Cortrot
200
SY; Tk
Murs 750 1
100 r~
50-
Ti-ealnert Corrtrol
Treatments
Time to Zero Percent Motility-All Animals
33
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Further, the difference in the survival time of spermatozoa between the
"Tolerant"
and "Intolerartt" animals was significant (P<.001). Spermatozoa in the
treatnient group
from the "1'olerant" dogs showed a 203.29% improvement in survival time over
the
control, while spermatozoa in the treatment group from the "Intolerant" dogs
showed a
553.33% improvement in survival time over the control.
v< n32
400 J
350 E~B ETJ T
300 ~' 250
Hours 200 10
-
. !~
~.-
: Treatment Control
Treatment
Time to Zero Percent Motility-"Tolerant" Animals
.... _._
- P<.016
400 ~ -
350 O Treatment
300 Control 250
Hours 200
150
100
0
Treatment Control
Treatment
Time to Zero Percent Motility-"Intolerant" Animals
34
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Time to Last Full Insemination
The analysis of time to last full insemination showed a difference due to
treatment
(P<.03). The average iime to last full insemination for the control group was
6.7 hours
(std. error +/- 2.72). 'The average time to last full inseniination for the
treatment group
was 49.9 (std. error +/- 18.75). The treatment group averaged 1306.67% of the
control.
Nine of the ten animals fell within. a 95% confidence interval (CI) and
maintained an
average of 640.74% of the control (Figure 5.7). A comparison of "Tolerant" and
"Intolerant" groups could not be performed due to the wide variation in volume
and
concentration seen in individual animals.
50--..
- - ` El1'reairnent r '
35-
N Control
30 -- i
Hours 25
20-
;.~ 10-
}}Y.~,yJ
Treatment Control
Treatnient
Time to Last Full Insemination-Animals Within the 95% CI
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Forward Progression
The analysis of forward progression showed a difference between the treatment
and control (P<.001). There was also a difference between treatment by time
and control
by time at time points lhour through 288 hours (P<.001). The treatment group
maintained a higher level of forward progression over time than the control
group.
_..
4
3 5 - - - --- P<.001
- - - -
3- -
(D Treathmrrt
2 5 ~ - - - - { Controi
. ;;
Fo-w ind 2 - - - -
Progression
~ - - - -- - --
l.5 ,, - ' - - -. - - -, - -
-
U5
U T 1 ~
0 1 6 12 18 24 48 72 96 120 144 168 192 216 240 264 288 312
Homs
Forward Progression-Treatment by Time versus Control by Time
36
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Acrosome Reaction
The analysis of the percentage of the non-acrosome reacted, motile sperm
showed
a difference between the treatment and control groups (P<.001). There was also
a
difference between the treatment by time and the control by time (P<.017). The
following chart shows the maintenance of a higlier percentage of non-acrosome
reacted
sperm in the treatment group over time as compared to the control group over
time.
120
100 - - - :
O. . .. . ~ , .. ; .. . . . ...___-..~s.__~._,. -_.- -
$0
O
v Treatment
Control
E~ 60 -4 - -
~ t
U s
z 40 --`- - -
. ~
20 - -' ~ - -
0
~10 DO 00 \D cr N O 00 ~D ch N O 00 ~ V O~ ~ O~ d' W M 00 M 00 N ~
=----~ N N M M d ~ ~ ~
Hours
Non-acrosome Reacted-Treatment by Time versus Control by Time
37
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Viability
The analysis of viability showed an overall difference between the treatment
and
control groups (P<.001). The following graph demonstrates the treatments
increased
viability over time as compared to the control group.
120 --~ --...........100 '`- - - - - - -
P<.001
-'7'r23tITE1lt
=~ ,
Corrtrol
..,
ce 6p
>
= .\
. .. .. . ~ . . ~ ~ ~ . . . .. ~ i
. . . ~ . . ~ .. - ~ ~ ... ' ..~...s
~ ~ ~ . . . _ ---- --- :--"
20 ~ - - - -
Q . . .,.: .,_. -_. ~ ,
C 00 00 "D ~t N 1 O 00 l0 '~ N d' N O
<~7 M M d' Ln
11Uun
Viability over Time-Treatment versus Control
38
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
Morphology
Morpliology for all dogs fell within the acceptable range of greater than
sixty
percent. There was no difference in morphology between the treatment and
control
groups (P>.062), as expected. However, there was a difference in morphology
over time
(P<.001). T'his difference was only in tail abnormalities and was expected
with the loss
of motility and changes associated with cell death. There was also a
difference between
the three investigators (P<.002). However, each investigator found no
clifference
between the treatment and control groups.
39
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
In accordance with the present invention, collection into warmed extender
media
lessens the cold and pH shock to the spermatozoa reported in previous studies,
as shown
by the improved semen parameters. This novel modification to the
collection/extension
of semen allows for improved preservation of spermatozoa over time when
compared to
traditional methods. From the data analysis, it is clear that collecting semen
into warmed
extender media improved the semen parameters evaluated. Specifically, the
fiinctional
life span of the spermatozoa, measured as motility, was significantly
increased in the
treatment group as compared to the control. When comparing animals grouped as
"Tolerant" and "Intolerant," both groups demonstrated improvement in motility
in the
treatment group. IIowever, semen collected from those animals that were
"Intolerant" to
the traditional collection method appeared to demonstrate the most
improvement. By
collecting into the warmed extender, the temperature remained constant
preventing cold
shock to the spermatozoa. The spermatozoa also came in contact with the
buffers of the
extender immediately upon collection which helped to prevent shifts in pH.
The treatment group maintained motility significantly longer than the control
(time to zero motility). This held true for both. the "Tolerant" and
"Intolerant" grotips,
with the "Intolerant" samples demonstrating the greatest response to
treatment. This
improved motility over time led to the improvement seen in the time to last
full
insemination. Further, times to full acrosome reaction were delayed in the
treatment
group. By maintaining a greater percent of motile (and therefore viable), non-
acrosome
reacted sperm, the treatment group maintained a full insemination dose for a
greater
length of time as compared to the control. By calculating the available sperm
pool (total
motile sperm per ejaculate), it was possible to observe that in animals with
good
CA 02401973 2002-09-03
WO 01/64936 PCT/US01/06467
concentration and voluime had a greater number of inseminations upon
collection and
maintained at least one full insemination dose much longer due to the
treatment. In
animals that had lower concentrations and/or volumes, it was possible to get
an
insemination by using the treatment where no insemination would have been
available
using traditional methoids.
By utilizing this modification in the collection/extension method, it is
possible to
improve semen pararneters in both the "Tolerant" and "Intolerant" animals,
with the
"Intolerant" animals appearing to demonstrate the most improvement. Semen from
animals with a good. volume and concentration can be extended and maintained
for a
longer period of time as compared to traditional methods, allowing for
improved ability
in shipping fresh-extended semen. Animals that would not have an adequate
semen
sample for insemination, when using traditional methods, would now have to
ability to be
used for AI.
WHAT IS CLAIMED IS:
41