Note: Descriptions are shown in the official language in which they were submitted.
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
PERMEABLE COMPOSITION, CONTROLLED RELEASE PRODUCT AND METHODS
FOR THE PRODUCTION THEREOF
FIELD OF INVENTION
The present invention relates to a permeable composition and a method for the
production thereof. The permeable composition may be comprised of a substrate
material.
Alternately, the permeable composition may be for use in coating a substrate
material. Further,
the present invention relates to a controlled release product comprised of the
permeable
composition and a method for the production thereof.
BACKGROUND OF INVENTION
Release control is desirable in numerous applications and in various fields.
For
instance, release control is often utilized or sought after in applications
relating to fertilizers,
pesticides and pharmaceuticals. In general terms, "release" is used to refer
to the exposure of an
originally contained agent, substrate or matter, referred to herein as the
"substrate material," to
the surrounding environment. The release of the substrate material is
facilitated by a releasing
medium (such as a solvent) and a releasing process (such as dissolution or
biodegradation).
Further, "control" refers to the ability to affect the release of the
substrate
material. The definition of control includes the manipulation of various
release variables,
including, but not limited to, the amount of substrate material released and
the release rate.
Extension of the release control concept to an appropriate application implies
that variable
release profiles can be attained through adjustment of the release control
technique. "Release
profile" refers to the correlation between the amount of substrate material
released and time.
In addition to permitting variability with respect to the release profile, it
is also
desirable that the release control technique be both reliable and cost
effective. Reliable release
control refers to a technique that is not unduly or significantly influenced
by environmental
conditions (such as temperature, abrasive handling, etc.), thereby inducing an
unpredictable
release of the substrate material.
-1-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Generally, the release control techniques conventionally utilized and employed
m
various applications have not been found to be fully satisfactory. For
instance, these release
control techniques are often not conducive to variable or adjustable release
control or such
variability is limited. Further, these techniques may not be reliable,
therefore limiting the ability
to attain a predictable release. Finally, these techniques may not be cost
effective, thereby
inhibiting their widespread usage.
As indicated, release control techniques are applicable to numerous
applications
and fields. Agriculture represents one such application in which release
control has become
increasingly important. More particularly, in recent decades, food producers
have instituted
more efficient farming techniques designed to better utilize agricultural
resources. As higher
demands are placed on agricultural production, growers have increasingly
focused upon
improving crop yields. Consequently, by way of example, fertilizers capable of
providing crops
with critical nutrients have become an integral tool in attempts to optimize
crop yields.
Basic fertilizers are comprised of rapidly degradable chemical compounds that
are released, almost immediately, as nutrient forms suitable for plant uptake.
This conversion is
generally performed by simple dissolution or natural soil degradation
processes. The unabated
nutrient release characteristic of these basic rapid release fertilizers tends
to have several
disadvantages. First, fertilizer costs are typically increased due to the
inefficient nutrient supply.
Generally, the initial rate of nutrient release from basic fertilizers is much
higher than the rate of
plant uptake. Consequently, a significant amount of fertilizer nutrients are
susceptible to losses
(i.e.: wasted) such as soil immobilization, leaching by rainfall, or
volatilization into the
atmosphere.
Second, basic fertilizers have difficulty achieving optimum plant nutrition.
In
order to compensate for a lack of release control and nutrient losses from
basic rapid release
fertilizers, growers tend to rely on high application rates or multiple
applications in an attempt to
meet crop nutritional requirements. Growers must also adjust fertilizer
application rates to
account for variable soil conditions or crop demand. Using such practices, it
is difficult to
ensure crops are neither deficiently nor excessively fertilized. Without
optimum plant nutrition,
crop yields cannot be maximized.
-2-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Third, the resulting need for multiple fertilizer applications tends to
increase labor
and equipment maintenance costs and operating time. Fourth, besides being
unavailable for
future stages of plant growth, lost nutrient chemicals can pose a potential
environmental hazard.
Once present in surface or subsurface drinking water supplies, leached plant
nutrients may
become contaminants. In the case of nitrogen based fertilizers, volatilization
contributes to the
amount of NO and NOX emissions in the atmosphere. Fifth, excess released
nutrients not
consumed by environmental losses may actually be toxic to plants, particularly
seedlings
sensitive to soil chemistry. Such plant damage is generally referred to as
crop "burning."
Therefore, there is a need in agriculture for controlled release fertilizer
products
and permeable fertilizer compositions capable of addressing the disadvantages
of basic fertilizers
which tend to have no release control. Specifically, there is a need for
fertilizer products and
compositions able to provide improved crop nutrition achieved through
variable, controlled
nutrient supplies capable of meeting disparate crop demands. Ideally, the
fertilizer product or
composition provides the correct amount of nutrients at the correct rate over
all or a portion of
the growing season. Further, there is a need to reduce fertilizer costs
associated with fertilizer
losses and multiple applications. Finally, there is a need to reduce
environmental damage
attributable to fertilizer nutrient losses and crop damage attributable to
excessive fertilizer
chemical concentrations in the soil.
Various attempts have been made to address the deficiencies of basic
fertilizers.
Specifically, the fertilizer industry has created numerous modified fertilizer
products and
compositions, which can be classified under the broad categories of
"stabilized fertilizers" and
"controlled release fertilizers." The term stabilized fertilizer is used to
refer to a fertilizer
amended with a chemical inhibitor designed to slow or suppress the natural
soil processes
responsible for converting the fertilizer into plant usable nutrients
("Controlled-Release and
Stabilized Fertilizers in Agriculture," Dr. Martin Trenkel, International
Fertilizer Industry
Association, December 1997, p.12).
Controlled release fertilizers are generally described as either uncoated or
slowly
degradable fertilizers or coated or encapsulated fertilizers. Generally
speaking, uncoated or
slowly degradable fertilizers tend to be chemically modified and rendered more
resistant to the
natural soil degradation mechanisms. For coated or encapsulated fertilizers, a
permeable or
porous coating composition is typically added to the surface of solid
fertilizer granules in order
-3-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
to slow water infiltration into the soluble nutrient core. Many of the
currently commercially
available controlled release fertilizers release nutrients in a gradual
fashion. That is, they
possess release profiles with a slower release rate than basic rapid release
fertilizers. However,
these fertilizers typically do not utilize any appreciable release control
technique. Therefore,
release profile variations are difficult or impossible to attain.
Fertilizers possessing controlled release properties without the use of a
governing
coating are typically classified into three general categories. First, "low or
limited solubility
fertilizers" include conventional soluble fertilizers that have been
chemically modified to
produce a new fertilizer compound of reduced solubility. Second, "matrix
fertilizers" are
comprised of granules including nutrient compounds dispersed throughout a
binder or carrier
material of typically low nutrient value. Although matrix fertilizers are
common, they tend to be
of relatively low commercial value. This is largely attributable to the fact
that, generally, a
substantial quantity of low nutrient value binder is required to form the
granule. Consequently,
the finished product tends to have a low nutrient quantity per unit weight of
fertilizer. Third,
"supergranules" are slow release fertilizers provided in the form of large
briquettes or sticks and
rely upon a low surface area/volume ratio to delay the complete dissolution of
the fertilizer.
Supergranules provide no appreciable control over nutrient release profiles
and are generally
used in insignificant quantities.
Urea-formaldehyde is the primary "low or limited solubility fertilizer" in use
today. The formation of urea-formaldehyde is achieved by reacting urea with
formaldehyde
under controlled conditions (temperature, time, pH, etc.) to form methylene
urea polymers of
various chain lengths. The initial nitrogen release from urea-formaldehyde
products is
associated with the dissolution of unreacted urea (usually less than 15% of
the fertilizer nitrogen
content). Before the remainder of the urea in the fertilizer can be released,
soil microbes must
first break down the polymers, thereby making additional urea available for
dissolution. The
longer the polymers, the longer the degradation time required to free the urea
from the polymer
chains. Therefore, some attenuation of the nitrogen release profile can be
achieved through
varying the degree of polymerization of the methylene ureas. Urea-formaldehyde
products are
available in granule and liquid forms.
However, there are several important disadvantages associated with the use of
urea-formaldehyde products. First, urea-formaldehyde products tend to be
approximately three
-4-
CA 02402212 2002-09-04
i~VO 01/66493 PCT/CA01/00301
to five times as expensive as urea. Second, urea-formaldehyde contains about
38% nitrogen.
However, some of the nitrogen contained in very long polymers may be released
after the
growing season, or not at all. Finally, formaldehyde is a toxic material.
Health concerns
associated with the handling of formaldehyde in production processes and the
usage of products
made from formaldehyde, have been raised.
Two additional low or limited solubility urea-based fertilizers are also
known.
The first is isobutylidene diurea (IBDU~ - 32% nitrogen). IBDU~ is formed via
a reaction with
urea and isobutyraldehyde, resulting in the formation of a single oligomer
(very short chain
polymer). The release rate of IBDU~ is largely influenced by its particle
size, where a smaller
granule corresponds to a faster release rate. The second is crotonylidene
diurea (CDU~ - 32.5%
nitrogen). CDU~ is a low solubility urea compound formed by a reaction of urea
and acetic
aldehyde. As with IBDU~, the nitrogen release rate of CDU~ is determined
largely by particle
size.
Commercially available "matrix fertilizers" typically employ the use of
degradable polymer matrices to carry nutrients such as nitrate, phosphate and
potassium
compounds. The matrix approach is seldom used for highly concentrated
fertilizers, such as
urea, because the Garner material may comprise as much as 40%, by weight of
the total fertilizer.
Generally, only low-grade fertilizers, such as NPK 10-10-10 (nitrogen - 10%,
phosphorous -
10%, potassium - 10%), are produced using the matrix approach.
Generally speaking, the various materials typically indicated to be suitable
for
fertilizer matrices are low solubility, insoluble or degradable substances,
such as elemental
sulphur, manure, apatite (calcium phosphate crystals), rock fines (and other
minerals) and
thermoplastic resins and cellulose. These low nutrient value matrix materials
can comprise 10 -
90%, by weight of the "fertilizer." However, the majority of these matrix
fertilizers are of
relatively low-value. In addition, the matrix fertilizers may provide slower
release than a low
solubility fertilizer but they do not typically have the capacity to maintain
a significant release of
nutrients over extended periods of time. The slow release properties of matrix
fertilizers result
from the fact that the matrix must be dissolved/degraded or water must migrate
through the
matrix to release the nutrients contained. As such, the matrix approach to
slow release fertilizers
provides limited control of the nutrient release rate.
-5-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
U.S. Patent No. 4,589,903 (Sato et. al.) describes a process involving
dissolving
synthetic wollastonite in concentrated sulphuric acid and blending the
solution with various
types of manure. The mixture is granulated and allowed to ferment. The low
quality pellets can
then be applied as fertilizers containing relatively low quantities of
nutrients and a large number
of beneficial microorganisms. The wollastonite and manure form the matrix of
the granules.
U.5. Patent No. 5,653,782 (Stern et. al.) describes a process by which
fertilizer
particles are preheated to a temperature in excess of the melting point of
sulphur, prior to being
mixed with solid sulphur prills. The superheated fertilizer melts the sulphur,
and as the mixture
is agitated in a pugmill, the fertilizer is "coated." Although the term
coating is used extensively
throughout the patent, it only refers to coating the particles prior to
agglomeration. The
resulting fertilizer is comprised of fertilizer particles contained in a
sulphur matrix.
Accordingly, this process is only suitable for coating those fertilizers
capable of withstanding
temperatures in excess of the melting point of sulphur (120°C) in a
range of 130 - 280°C. Many
fertilizers would melt or volatilize under such conditions. Urea, for example,
melts at 132°C.
Further, the matrix fertilizer may contain a fibrous medium able to absorb
water
into the granule core thereby dissolving and releasing nutrients (or a
herbicide) carried by the
fibres. The fibrous material may be an organic medium (cellulose). U.S. Patent
No. 5,471,786
(Clausen) describes the use of a fibrous medium containing a mineral. The
mineralized organic
material is lignite, consisting of peat (organic) and carbonaceous mineral
(coal). The
hydrophilic properties of the lignite make the product a suitable plant
growing medium. The
"planting blocks" are capable of retaining moisture even in conditions of dry
soil and low water
table.
Finally, absorptive cellulose fibers may be impregnated with plant nutrients
and
the resulting fibers subsequently bound in a matrix. Once placed in the soil,
the moisture and
nutrients stored in the fibers may be released. Some of these products may
possess degradable
coatings in order to prevent premature leaching, but they are not designed to
regulate the release
of nutrients. As such, these products may have some slow release properties
but without the
ability to significantly adjust the release profile. The "fertilizers"
produced also contain low
quantities of nutrients per unit weight, due to the presence of large
quantities of carrier fibers and
binders.
_6_
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
In contrast, coated or encapsulated fertilizers involve the application of a
coating
to a substrate material typically comprised of a solid, granular fertilizer.
In practice,
encapsulated fertilizers tend to be classified according to the composition of
the coating. The
most commonly used coating compositions are sulphur, synthetic polymers and a
combination of
sulphur and synthetic polymers.
Current commercially available sulphur coated fertilizer ("SCF") generally
consists of a water soluble or degradable fertilizer encapsulated by a sulphur
coating, a sealant
coating and typically a conditioner. Although the nutrient release from SCF
tends to be slower
than the release from basic fertilizers, the initial rate of nutrient release
is often still considered
to be too rapid. Therefore, it is desirable to obtain better control over the
release profile of SCF.
The release mechanism for SCF is typically water infiltration through pores
and
cracks in the sulphur coating. There tend to be two sources of the
imperfections encountered in
sulphur coatings. First, properties inherent in molten sulphur introduce
defects within the
sulphur coating. The fertilizer coating process basically involves spraying a
granular substrate
material with an atomized spray of coating material. As the fine coating
droplets strike the
substrate particles, they spread-out and freeze over the granule surface. A
description of a
typical coating process is provided in U. S. Patent No. 3,991,225 (Blouin).
The relatively high
surface tension and viscosity of molten sulphur may result in less than ideal
granule wetting and
coverage, thereby inducing a portion of the coating imperfections.
Second, the formation of additional coating imperfections is attributable to
the
allotropic nature of sulphur crystals. At various points during the freezing
of molten sulphur and
the aging of solid sulphur, a variety of atomic structures may be present.
These sulphur
structures include polymeric, amorphous, monoclinic crystalline and
orthorhombic crystalline
sulphur. As differential, physical variations in the structure of sulphur
occur, imperfections
(voids and fissures) of various sizes are formed between the sulphur crystals.
Additional cracks
and voids are formed as the sulphur crystals are subjected to thermal changes,
resulting in
differential expansion and contraction between the crystals. Although the
amount and formation
rate of the defects within sulphur can be influenced by the thermal history of
the material, the
formation of crystalline sulphur and therefore imperfections, tends to be
inevitable.
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Increasing the sulphur coating thickness does not provide effective control of
nutrient release as imperfections form regardless of coating thickness. In the
case of
commercially produced SCF, an increase in coating weight does have the effect
of decreasing
the overall nutrient release. However, the reduced release is simply a result
of more fertilizer
granules receiving a heavier, layered coating which does not allow any
nutrient release within
the growing season (termed "lock-off'). Products containing a significant
number of "locked-
off' granules are inefficient as more fertilizer must be applied to achieve
the total desired
nutrient quantity.
Therefore, in the case of conventional SCF, control over the coating process
may
minimize the number of major coating defects, but there is no effective method
of accurately
controlling the formation of crystal imperfections. Due to a lack of
imperfection control, the
permeability of the sulphur coat cannot be significantly varied. Consequently,
sufficient
attenuation of the nutrient release profile is not possible with conventional
sulphur coating
technologies.
In an attempt to reduce the initial rate of nutrient release, a sealant may be
added
to the surface of the sulphur coating. The sealant fills the coating
imperfections that would
otherwise transmit water into the fertilizer granule core relatively quickly.
The sealants selected
are typically hydrophobic waxes, oils, polyethylene resins or combinations
thereof. These
temporary sealants are subject to being degraded by soil microbes prior to
water penetration
through the sulphur coat and into the fertilizer core. Thus, a microbiocide is
often applied to the
sealant in order to prevent premature degradation of the sealant. As such,
sealants act to only
delay water contact with the sulphur coating. In addition, sealants often only
partially survive
typical fertilizer handling operations, resulting in a discontinuous
encapsulation of the sulphur
coating.
In addition, in order to obtain a relatively free-flowing product that may be
easily
handled, conditioners may also be added to SCF. Conditioners are typically
minerals such as
finely divided clay or diatomaceous earth, which counteract the "stickiness"
of the sealant.
SCF may also be undesirable due to the fact sulphur is a brittle material.
Even
well formed coatings are prone to cracking and chipping during fertilizer
handling operations.
In the event the sulphur coating remains intact after handling, the micro-
pores and fissures
_g_
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
within the coating are generally enlarged, resulting in further degradation of
any release control
properties.
The insufficient ability to control the release of the substrate material from
SCF
S has resulted in release profiles which are not ideal or even desirable for
many applications. This
deficiency is exacerbated by the poor coating durability exhibited by
conventional SCF. Thus,
in summary, SCF lacks desirable performance attributes. First, significant
control over a
generally undesirable nutrient or substrate release profile is typically not
attainable using
conventional sulphur coatings. Second, typical fertilizer handling operations
damage relatively
fragile sulphur coatings of SCF, resulting in a release profile that tends to
be undesirable,
unreliable and invariable.
In the case of SCF, recent technological developments have focussed upon
improving the sulphur coating durability and/or the coating process. For
example, U. S. Patent
No. 4,636,242 (Timmins) describes the modification of elemental sulphur using
a dialkyl
polysulphide plasticizer. Timmins indicates that the admixture is capable of
reducing the
viscosity of molten sulphur (resulting in better granule coverage) and
plasticizing the solidified
coating (resulting in a more flexible coating). These developments may
somewhat reduce the
rapid, initial nutrient release associated with conventional SCF and improve
the handling
characteristics of the coated fertilizer as compared with SCF. However, no
significant release
control technique appears evident.
Synthetic polymer coated fertilizer ("PCF") is typically comprised of solid
fertilizer particles as the substrate material surrounded by a polymer coating
(i.e.: polyethylene,
polyurethane, polyolefin, alkyd resin, etc.). The are several advantages of
PCF as compared to
SCF. First, PCF typically possesses a less rapid, initial rate of release and
sustained nutrient
supply longer into the growing season. Second, polymer coatings are typically
more durable
than sulphur coatings and therefore, less susceptible to damage during
handling. Third, due to
the lighter coating material, PCF usually possesses a higher nutrient content,
by total weight of
fertilizer. In the case of commercially available SCF, the sulphur coating may
comprise up to
30% of the total fertilizer weight. By comparison, PCF seldom contains more
than 15% coating
material, by weight of fertilizer.
-9-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
However, there are some disadvantages associated with PCF. There may be
environmental concerns. Polymer coatings may breakdown very slowly (or not at
all), resulting
in a plastic residue in the soil system. Further, due to increased process and
material costs, PCF
is generally significantly more expensive than other controlled release
fertilizers, including SCF.
S
Water infiltration through the porous or permeable polymer coat provides the
release mechanism for PCF. Depending upon the technology, the porosity or
permeability of the
polymer coating may be fixed or variable. In the case of fixed porosity or
permeability coatings,
no significant control over the nutrient release profile is attainable. A
degree of nutrient release
attenuation can be achieved with variable permeability polymer coatings.
However, due to
complex manufacturing processes and expensive materials, the high cost of
these products often
prohibits their usage in agriculture. The largest market for PCF tends to be
horticulture and
"high-end" lawn fertilizers.
Commercially available synthetic polymer and sulphur coated fertilizers
("PSCF") typically include approximately 15% sulphur coating and less than 2%
polymer
coating. Sulphur is the primary fertilizer coating used in conjunction with
the secondary
polymer coating which is designed to act as an improved sealant. Polymer
sealants are typically
more durable than traditional sealants and they do not require the addition of
a conditioner to the
coated particles.
PSCF is an attempt to combine the lower initial rate of release and durability
of
polymer coatings with the low-cost of a sulphur coating. The release profile
of most PSCF is
still predominantly governed by the primary sulphur coating. The polymer
topcoat is generally
provided to limit degradation of the sulphur coating during handling. More
expensive PSCF
may incorporate a polymer coating capable of providing a degree of release
control (i.e.: a
variable permeability membrane).
Further, in the field of construction materials (such as sulphur concrete and
the
like), the addition of filler materials, including mineral fillers and fibers,
to elemental sulphur
has been used to create materials with highly desirable "permanent"
durability. For example, U.
S. Patent No. 4,484,950 (Hinkebein) discloses an invention in which mixtures
of molten sulphur
and crystalline phosphate fibers are cast into various structures. The focus
of Hinkebein is to
- 10-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
provide a strong, durable material suitable for such long-term applications as
tanks, pipes and
pavement.
U. S. Patent No. 4,026,719 (Simic) describes a material comprised of sulphur,
sulphur plasticizer (such as dicyclopentadiene) and a reinforcing filler such
as mica, talc (platy
silicates) or glass fibers. The composition is described as useful for durable
coatings for floors
and slabs. Simic also refers to the potential use of the composition for
"water impoundment"
applications (such as lining irngation ditches), thereby implying an
impermeable (or very low
permeability) material is produced.
In the above mentioned references and others similar in nature, fibrous
materials
may be used to mechanically reinforce the properties of sulphur compositions
in an extreme
fashion (i.e.: ultimate strengthening and durability, minimizing or
eliminating permeability,
etc.). Therefore, it is feasible that filler reinforcement could improve the
durability of controlled
release products or compositions. However, direct application of the
reinforcing techniques
described would likely result in an impermeable (or unacceptably low
permeability) composition
or controlled release product, thereby "locking off' the substrate material.
"Stabilization" is used herein to refer to methods designed to reduce the
formation of defects (voids and fissures) at the material crystal level, as
described above. Such
defects may be formed as a result of differential crystal movement caused by
allotropic crystal
conversion and/or thermally induced expansion and contraction of the crystals.
Stabilization
techniques for materials such as sulphur may be classified as chemical
stabilization or physical
stabilization.
Chemically stabilized sulphur has been used in various construction materials,
such as sulphur concrete. According to A. H. Vroom, "Sulphur Polymer Concrete
and its
Applications," VII International Congress on Polymers in Concrete, September
22 - 25, 1992,
Moscow, pp. 606 - 619, a polymeric sulphur concentrate (SRX) is added to
molten elemental
sulphur. Upon freezing, the SRX polymer is indicated to promote formation of
micro sulphur
crystals, as opposed to macro sulphur crystals. Apparently, as the modified
sulphur experiences
crystal conversion and/or thermal changes, less differential movements are
experienced by the
fine crystals, thereby reducing defect formation.
-11-
CA 02402212 2002-09-04
WO 01/66493 PCT/CAOI/00301
Dicyclopentadiene, styrene and limonene are examples of polymeric polysulphide
plasticizers that, when added to molten sulphur, tend to substantially reduce
the amount of
crystalline sulphur formed upon freezing (i.e.: more amorphous and polymerized
sulphur is
present in the cooled material) (B. R. Currell et. al., "New Uses of Sulphur,"
Advances in
Chemistry Series 140, 1975, pp. 1 - 17). However, these chemical admixtures
generally do not
provide permanent stabilizing as sulphur crystals are eventually formed over
time.
Polymeric polysulphides have also been used in various sulphur based
construction materials such as road markings and masonry coatings. However, in
the case of
sulphur coated fertilizers, such plasticizing techniques are generally not
compatible with the
fertilizer coating process. During fertilizer coating, molten mixtures are
sprayed onto the
fertilizer granule substrate material. Once added to molten sulphur, polymeric
polysulphides
tend to increase the viscosity and crystallization time of the molten mixture,
as described in U. S.
Patent No. 4,129,453 (Simic). Therefore, during spraying, the modified
polymeric sulphur tends
to exhibit very poor granule wetting and may even agglomerate fertilizer
granules, as the
modified sulphur requires more time to freeze (R. Jerome Timmins, "Modified
Sulphur Coated
Urea," 198th ACS National Meeting, Miami Beach, Florida, September 10 - 15,
1989, Paper 23,
p. 3).
Alternately, fine particulate filler materials have been used to physically
stabilize
sulphur compositions, primarily in construction material applications. Once
dispersed
throughout molten sulphur, the particulate inclusions serve as centers for
crystallization during
freezing, thereby promoting the growth of "uniform, dense, fine-crystal
structures" as described
in Yu. I. Orlowsky and B. P. Ivashkevich, "Peculiarities of Technology of
Production of Sulphur
Polymer Concrete...," VII International Congress on Polymers in Concrete,
September 22 - 25,
1992, Moscow, p. 664. The stabilized crystal structure apparently experiences
less and smaller
defects during differential crystal movement induced by sulphur crystal
conversion and thermal
expansion and contraction. Therefore, dispersed particulate filler materials
in sulphur may
reduce the uncontrollable release mechanism currently utilized in coating
applications such as
SCF (i.e.: voids and fissures).
Release mechanisms for known or conventional controlled release products and
compositions may be generally classified into two categories. The first
category is solvent
-12-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
infiltration through a conductme coating for the substrate material. The
second category is
solvent infiltration through a conductive matrix including the substrate
material.
Regarding the first category of release mechanisms, a soluble substrate
material
may be encapsulated with a coating possessing pores introduced at the time of
manufacturing
(for example, SCF or PCF as described in "Controlled-Release and Stabilized
Fertilizers in
Agriculture," Dr. Martin Trenkel, International Fertilizer Industry
Association, December 1997,
pp. 23 - 26). Upon contact with the coating, the appropriate solvent can enter
the core of the
substrate material via the pores and dissolve the substrate material, thereby
releasing it to the
surrounding environment.
By employing this first release mechanism, control over the release rate may
only
be achieved by varying the porosity or permeability of the coating. However,
many existing
coating technologies lack the ability to accurately or significantly vary the
coating porosity or
permeability. Although polymer coatings with a degree of permeability control
exist, the high
cost of such coatings often prohibit their widespread application. For
example, fertilizers coated
with variable permeability polymers are seldom used in mass agriculture
applications due to the
high cost. The high cost of variable permeability PCF is typically a result of
relatively
expensive coating materials and relatively complex coating processes.
Regarding the second category of release mechanism, fibrous media may be
impregnated with soluble substrate materials. For instance, the absorbent
fibers may be
agglomerated with the substrate, forming a fibrous "matrix." Appropriate
solvents may then
migrate throughout the fibers, releasing the soluble substrate material.
U. S. Patent No. 5,019,564 (Lowe et al) discloses an invention whereby plant
fibers are used to absorb organic pesticides prior to being loosely
agglomerated into relatively
non-friable "granules." Upon exposure to water, the pesticides absorbed within
the fibers are
released from the "granules" more slowly than pesticides introduced directly
to the agricultural
environment.
U. S. Patent No. 5,762,678 (Hiles) describes the development of a soil
enhancing
complex in which the soft cores of cellulose fibers are digested, resulting in
hollow, "micro-
capillaries" composed of the cellulose wall material. The processed "micro-
capillaries" may
-13-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
then absorb water and plant nutrients within the cellulose tubes and walls.
The laden "micro-
fibers" are subsequently agglomerated into pellets and coated with a moisture
retaining hydrogel.
A gelatinous polymer coating is then applied for the purpose of retaining the
integrity of the
pellet. The contained nutrients may then be gradually released into the soil
environment.
U. S. Patent No. 5,364,627 (Sony) discloses a technology wherein the
releasable
agent is dispersed throughout the cross sections of polymer fibers. This
dispersion is
accomplished by mixing the agent with the molten polymer, prior to spinning
the mixture into
fibers. The release of the agent is accomplished via solvent migration through
contiguously
arranged agent particles contained within the fiber matrix. Should the
releasable agent not be
arranged contiguously within the fiber, mechanical action (i.e.: chewing) may
be required to
expose the releasable agent to solvent contact.
In order to achieve release, the "sponge" or "wick drain" matrix approaches
described in the above patents, and several others, rely on solvent
transmission through channels
or openings contained within the fibrous media. While such techniques are
conducive to gradual
release, and perhaps controlled release, they are generally not suitable for
applications such as
high nutrient content fertilizers. One of the factors determining the value of
fertilizer products is
the nutrient content, by weight of fertilizer. When used in slow release
fertilizer applications,
the matrix approaches previously described appear to result in a low value
fertilizer product due
to dependence on a large quantity of non-nutrient, carrier fibers and binders.
Finally, the dispersion of fillers within the permeable composition or
controlled
release product is also relevant. In this regard, U. S. Patent No. 4,129,453
(Simic) describes a
construction material comprised of plasticized sulphur, reinforcing asbestos
fibers and dispersing
agents, such as talc or mica which aid in achieving dispersal of the asbestos.
The dispersing
agent is necessary to avoid "lumpiness" of the molten material mixture. Such
dispersing agents
are not applied directly on the filler. Rather they added to the plasticized
sulphur prior to filler
mixing.
SUMMARY OF INVENTION
The present invention is directed generally at permeable compositions and at
methods for producing such permeable compositions. The present invention is
also directed at
-14-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
controlled release products which include the permeable compositions of the
present invention
and at methods for producing such controlled release products.
The permeable compositions of the present invention include a matrix material
and a particulate filler material which is dispersed throughout the matrix
material. The
permeability of the permeable compositions is derived at least in part from
interfacial
passageways between the matrix material and the external surfaces of the
particles of filler
material. These interfacial passageways are located at the interfaces between
the matrix material
and the external surfaces of the particles of filler material. Additional
permeability of the
permeable compositions may be derived from the matrix material if the matrix
material is itself
permeable or degradable or if the matrix material contains imperfections.
The controlled release products of the present invention include a substrate
material which is coated with at least one layer of the permeable composition
of the present
1 S invention.
The invention is based upon the discovery that the permeability of the
permeable
compositions can be controlled by controlling the interfaces and the
interfacial passageways.
The ability to control the permeability of the permeable compositions makes
the
compositions of the present invention attractive for use in numerous
applications. either to
control permeability per se or to control the release of substrate materials
which are either coated
with the permeable composition or are included as a component of the permeable
composition.
For example, in agricultural applications the permeable compositions of the
present invention may be useful for coating or incorporating substrate
materials such as seeds,
fertilizers, pesticides and herbicides. Similarly, in pharmaceutical
applications the permeable
compositions of the present invention may be useful for coating or
incorporating substrate
materials such as vitamins and medicines.
Preferably the permeability of the compositions is controlled in the present
invention by applying a degradable surface treatment material to the external
surfaces of the
particles of filler material before the filler material is dispersed
throughout the matrix material.
When the surface treated particles of filler material are dispersed throughout
the matrix material
-15-
CA 02402212 2002-09-04
WO 01/66493 PCTlCA01/00301
the interfacial passageways are defined by the surface treatment material
which is present on the
external surfaces of the particles of filler material and the surface
treatment material provides
degradable interfaces between the matrix material and the external surfaces of
the particles of
filler material.
By selecting the relative proportions and the physical and chemical
characteristics
of the matrix material, the filler material and the surface treatment
material, the permeability of
the permeable compositions can be controlled. By controlling the thickness and
the integrity of
the coating layer of a permeable composition that is applied to a substrate
material, the.
permeability of a controlled release product can be further controlled.
The term "substrate material" as used herein refers to any material, whether
organic, inorganic, natural or synthetic which is intended to be delivered or
released or exposed
to an environment. The substrate material may, for example but without
limiting the generality
of the foregoing, be comprised of seeds, fertilizers, pesticides, herbicides,
fungicides, medicines,
vitamins, or foods.
The term "matrix material" as used herein refers to any material, whether
organic,
morgamc, natural or synthetic which is capable of providing a matrix for the
filler material in the
permeable composition. The matrix material may or may not include one or more
substrate
materials. Depending upon the intended application of the invention, the
matrix material may be
permeable or impermeable, and may be physically and chemically stable or may
be degradable.
The term "particulate filler material" as used herein refers to any
particulate
material, whether organic, inorganic, natural or synthetic. Filler material
may be of any particle
shape or particle size. There is no lower or upper limit to the particle size
of the filler material as
long as the particle size of the filler material is compatible with the
intended application of the
invention. The particles of filler material may also have any shape (i.e.,
aspect ratio and surface
area per unit volume) as long as the particle shape of the filler material is
compatible with the
intended application of the invention. Although a fibrous (i.e., high aspect
ratio) filler material
may be preferred for some applications of the invention, low aspect ratio
filler materials may
also be used in the invention and may be preferred for some applications.
-16-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
The term "degradable surface treatment material" as used herein refers to any
material, whether organic, inorganic, natural or synthetic, which may be
effectively applied to
the external surfaces of particles of filler material, which will provide
degradable interfaces
between the matrix material and the external surfaces of the particles of
filler material, and
which will subsequently degrade either entirely or in part through processes
such as dissolution,
thermal decomposition, biological degradation or chemical decomposition. The
surface
treatment material may be in solid or liquid form.
The term "interfacial passageways" as used herein refers to gaps, spaces or
pores
which are formed at the interfaces between the matrix material and the
external surfaces of the
particles of filler material. These interfacial passageways provide pathways
for the migration of
substances throughout the permeable composition. An interfacial passageway may
be defined
by a single particle of filler material or may be defined by a plurality of
particles of filler
material which are contiguous or are interconnected by gaps, spaces or pores.
Two or more
interfacial passageways may also be interconnected to form a network of
interfacial passageways
in the matrix.
The term "degradable interfaces" as used herein refers to interfaces that are
formed when surface treated particles of filler material are dispersed
throughout the matrix
material, (i.e., interfaces between the matrix material and the surface
treatment material and
interfaces between the surface treatment material and the external surfaces of
the particles of
filler material). In the permeable compositions of the present invention, the
interfacial
passageways are initially either fully or partially filled with degradable
surface treatment
material in order to provide the degradable interfaces. These degradable
interfaces are fully or
partially degradable upon degradation of the surface treatment material to
facilitate some
migration of substances through the interfacial passageways. Prior to
degradation, the
degradable interfaces may facilitate the transfer of forces between the matrix
material and the
filler material, thus enabling the filler material to perform a mechanical
reinforcement function
in the permeable composition.
The term "permeable composition" as used herein refers to a composition which
is comprised of a matrix material, a particulate filler material, and at least
some interfacial
passageways between the matrix material and the external surfaces of the
particles of filler
material. The matrix material may or may not include a substrate material.
-17-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
The term "controlled release product" as used herein refers to a product which
is
comprised of a substrate material and a coating on the substrate material
which includes at least
one layer of a permeable composition. The term "release" as used herein refers
to the exposure
of the substrate material to a surrounding environment. The term "control"
refers to the ability
to affect the release of the substrate material from the controlled release
product.
The term "stable" as used herein refers to a material which does not tend to
be
prone to physical or chemical degradation through processes such as
dissolution, thermal
decomposition, biological degradation or chemical decomposition.
In a first preferred aspect, the invention is a permeable composition
comprising:
(a) an amount of a matrix material;
(b) an amount of a particulate filler material dispersed throughout the matrix
material, wherein each of the particles of filler material is comprised of an
external surface; and
(c) interfacial passageways between the matrix material and the external
surfaces of
the particles of filler material.
In a second preferred aspect, the invention is a controlled release product
comprising:
(a) a substrate material;
(b) a permeable composition coating the substrate material in a coating layer,
the
permeable composition comprising:
(i) an amount of a matrix material;
-18-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
(ii) an amount of a particulate filler material dispersed throughout the
matrix
material, wherein each of the particles of filler material is comprised of an
external surface; and
(iii) interfacial passageways between the matrix material and the external
surfaces of the particles of filler material.
In a third preferred aspect, the invention is a method for producing a
permeable
composition, the method comprising the steps of:
(a) providing an amount of a particulate filler material, wherein each of the
particles
of filler material is comprised of an external surface;
(b) applying an amount of a degradable surface treatment material to the
external
surfaces of the particles of filler material to form surface treated particles
of filler
material; and
(c) dispersing the surface treated particles of filler material throughout an
amount of
a matrix material to form the permeable composition such that interfacial
passageways between the matrix material and the external surfaces of the
particles of filler material are defined by the surface treatment material and
such
that degradable interfaces between the matrix material and the external
surfaces
of the particles of filler material are provided by the surface treatment
material.
In a fourth preferred aspect, the invention is a method for producing a
controlled
release product, the method comprising the steps of:
(a) providing an amount of a particulate filler material, wherein each of the
particles
of filler material is comprised of an external surface;
(b) applying an amount of a degradable surface treatment material to the
external
surfaces of the particles of filler material to form surface treated particles
of filler
material; and
-19-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
(c) dispersing the surface treated particles of filler material throughout an
amount of
a matrix material to form a permeable composition such that interfacial
passageways between the matrix material and the external surfaces of the
particles of filler material are defined by the surface treatment material and
such
that degradable interfaces between the matrix material and the external
surfaces
of the particles of filler material are provided by the surface treatment
material;
(d) providing a substrate material; and
(e) applying the permeable composition to the substrate material in a coating
layer to
form the controlled release product.
The substrate material may be incorporated into the permeable composition as a
component of the matrix material or the substrate material may be coated with
the permeable
composition. A permeable composition comprising a substrate material may also
be coated with
a permeable composition which does not comprise a substrate material.
The substrate material may be selected from a wide range of materials
depending
upon the application of the invention. In the preferred embodiment, however,
the substrate
material is comprised of a fertilizer. The fertilizer may be incorporated into
the permeable
composition to form a "matrix type fertilizer" or particles of the fertilizer
may be coated with the
permeable composition to form a "coated type fertilizer". A matrix type
fertilizer may also be
coated with a permeable composition to form a combination fertilizer if
desired. Preferably the
fertilizer is a urea fertilizer.
The primary functions of the matrix material are to provide a matrix for the
filler
material and to provide support for the interfacial passageways. The matrix
material may also
increase the durability of a permeable composition or controlled release
product or serve as a
carrier for a substrate material which has been incorporated into a permeable
composition or
controlled release product.
The matrix material may be comprised of any organic or inorganic material
which
is suitable for the intended application of the permeable composition or
controlled release
-20-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
product. Depending upon the intended application, the matrix material may be
permeable or
impermeable and may either be chemically and physically stable or be
degradable.
In the preferred embodiment the substrate material is comprised of a
fertilizer. In
one preferred embodiment of permeable composition, the matrix material is
preferably
comprised of sulphur. In a second preferred embodiment of permeable
composition, the matrix
material is preferably comprised of fertilizer as a substrate material and
sulphur as a carrier
material. The sulphur is most preferably elemental sulphur.
In the preferred embodiment, sulphur is preferred because it is inexpensive
relative to other possible matrix materials. Elemental sulphur is particularly
preferred because it
is inexpensive relative to chemically stabilized sulphur materials. Although
elemental sulphur
tends to be chemically unstable and prone to deterioration due to abrasion and
other physical
stresses, these tendencies are minimized by the presence of the filler
material in the matrix,
which serves in the preferred embodiment to mechanically reinforce and
physically stabilize the
elemental sulphur matrix material.
The primary function of the filler material is to act with the surface
treatment
material and the matrix material to form the interfacial passageways. The
filler material may
also provide mechanical reinforcement for the matrix material or physical
stabilization of the
matrix material.
The filler material may be comprised of any particulate organic or inorganic
material which is compatible with the matrix material and which may be surface
treated with the
surface treatment material. Preferably the filler material is relatively
stable and relatively
impermeable.
In the preferred embodiment where the substrate material is comprised of a
fertilizer, the filler material is preferably comprised of wollastonite. Most
preferably, the filler
material in the preferred embodiment is comprised of wollastonite fibers.
The primary function of the surface treatment material is to define the
interfacial
passageways when the filler material is dispersed throughout the matrix
material by forming
-21 -
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
degradable interfaces between the matrix material and the external surfaces of
the particles of
filler material.
A possible secondary function of the surface treatment material may be to
facilitate at least a temporary bond between the matrix material and the
filler material which may
enable the filler material to perform a mechanical reinforcement function in
the permeable
composition. This bond will tend to deteriorate upon degradation of the
degradable interfaces.
The surface treatment material may also aid in dispersing the filler material
throughout the matrix material. Alternatively, if desired or necessary, a
second surface treatment
agent may be utilized for the purpose of aiding in the dispersal of the filler
material.
The surface treatment material may be comprised of any substance which is
compatible with the intended application of the invention and which is capable
of providing the
degradable interfaces due to dissolution, thermal decomposition, biological
degradation,
chemical decomposition or some other process. Preferably the surface treatment
material will
also provide a bond between the matrix material and the filler material which
will deteriorate
upon degradation of the degradable interfaces.
In the preferred embodiment where the substrate material is comprised of a
fertilizer, where the matrix material is comprised of sulphur, and where the
filler material is
comprised of wollastonite, the surface treatment material is preferably
comprised of a
naphthalene sulphonate formaldehyde copolymer.
The durability, permeability and other properties of the permeable composition
will depend upon the following controllable design factors:
1. the choice of matrix material, filler material, and surface treatment
material;
2. the chemical and physical characteristics of the matrix material;
3. the chemical and physical characteristics of the filler material (including
particle
shape and particle size);
4. the chemical and physical characteristics of the surface treatment
material; and
5. the relative proportions of matrix material, filler material and surface
treatment
material in the permeable composition.
-22-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
The durability, permeability and other properties of the controlled release
product
will depend upon further controllable design factors relating to the thickness
and the integrity of
the coating of permeable composition which is applied to the substrate
material.
These design factors make it possible to tailor a permeable composition and
controlled release product to provide a specific desired performance. This
desired performance
may relate to the permeability of a permeable composition or to the release
profile for a substrate
material which is included in a permeable composition or in a controlled
release product.
The matrix material is selected to be compatible with the filler material, the
surface treatment material and with the intended application of the invention.
A relatively
durable and stable matrix material may provide a more extended release profile
for a substrate
material. A degradable matrix material may provide a shortened release profile
for a substrate
material. Elemental sulphur, the preferred matrix material in the preferred
embodiment for
fertilizer applications, tends to disintegrate under physical stress or in the
presence of water or
humidity, thus releasing the fertilizer into the surrounding environment more
quickly than if the
matrix material were more physically stable. The matrix material is preferably
compatible with
the environment in which the permeable composition is to be used and is
preferably cost
effective.
The filler material is selected to be compatible with the matrix material, the
surface treatment material and with the intended application of the invention.
A relatively stable
filler material will likely be able to perform a physical reinforcement
function for the matrix
over the entire expected service life of the permeable composition. A
degradable filler material
may not provide good physical reinforcement performance, but may provide a
shortened release
profile for a substrate material as the filler material degrades. The filler
material is preferably
compatible with the environment in which the permeable composition is to be
used and is
preferably cost effective.
The surface treatment material is selected to be compatible with the matrix
material, the filler material and with the intended application of the
invention. The surface
treatment material is preferably compatible with the environment in which the
permeable
composition is to be used and is preferably cost effective. Preferably the
surface treatment
- 23 -
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
material enhances or at least does not interfere with the dispersal of the
filler material throughout
the matrix material.
The particle size and shape of the filler material is selected to be
compatible with
the intended application of the invention and with the functions to be
performed by the filler
material. High aspect ratio filler materials will tend to provide more
physical reinforcement for
the matrix material than low aspect ratio filler materials. On the other hand,
high aspect ratio
filler materials will have a higher surface area per unit volume than low
aspect ratio filler
materials and may therefore be more difficult to disperse in the matrix
material.
The following general trends can be suggested for design factors pertaining to
permeable compositions and controlled release products:
1. the amount of mechanical reinforcement (or physical stabilization) provided
to a
matrix by a filler material will increase as the amount of filler material
increases;
2. the amount of mechanical reinforcement provided to a matrix by a filler
material
will generally increase as the aspect ratio of the filler material increases;
3. the amount of mechanical reinforcement provided to a matrix by a filler
material
will generally increase as the stability of the filler material increases;
4. the dispersability of a filler material throughout a matrix material may
tend to
decrease as the aspect ratio of the filler material increases (due to an
increase in
surface area per unit volume of filler material);
S. the permeability of a permeable composition may tend to decrease and the
release
profile for a controlled release product may tend to extend as the amount of
mechanical reinforcement or physical stabilization of a matrix material
increases
(due to a potential increase in durability, stability or integrity of the
permeable
composition);
6. the permeability of a permeable composition may tend to increase and the
release
profile for a controlled release product may tend to shorten as the number of
interfacial passageways in the permeable composition increases (due to an
increase in the number of potential flow pathways in the permeable
composition);
7. the permeability of a permeable composition may tend to increase and the
release
profile for a controlled release product may tend to shorten as the amount of
-24-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
surface treatment material applied to a filler material increases (due to a
potential
increase in the size of the interfacial passageways);
8. the permeability of a permeable composition may tend to decrease and the
release
profile for a controlled release product may tend to extend as the stability
of a
S matrix material increases (due to a potential increase in the durability,
stability or
integrity of the permeable composition);
9. the permeability of a permeable composition may tend to decrease and the
release
profile for a controlled release product may tend to extend as the stability
of a
surface treatment material increases (due to a potential increase in the
amount of
time required to degrade the degradable interfaces);
10. the release profile for a controlled release product may tend to extend as
the
thickness of a coating layer increases (due to increased length of the
interfacial
passageways); and
11. the release profile for a controlled release product may tend to extend as
the
integrity of a coating layer increases (due to reduced permeability of the
permeable composition).
This list of trends is not exhaustive. It must also be cautioned that some of
the
trends may be subject to interdependence of design factors. For example,
although increasing
the amount of filler material in a matrix may reduce permeability by
increasing the durability,
stability and integrity of a permeable composition, an increase in amount of
filler material also
offers the potential for increased permeability due to an increased number of
interfacial
passageways. The net effect of an increase in the amount of filler material
may therefore be
dependent upon properties such as the initial stability and durability of the
matrix material.
With this caution in mind, the design factors can be manipulated in the
application of the invention to provide an overall net effect which will
assist in the design of a
permeable composition or controlled release product having desired properties.
In addition, each of the design factors may be varied within a particular
permeable composition or controlled release product to refine further the
properties of the
composition or product. As one example, a single permeable composition may
include more
than one type of filler material or particles of filler material having
varying shapes and sizes. As
- 2~ -
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
a second example, a single permeable composition may contain particles of
filler material having
varying types or amounts of surface treatment material applied to them.
SUMMARY OF DRAWINGS
S
Embodiments of the invention will now be described with reference to the
accompanying drawings, in which:
Figure 1 provides a graph of durability test results for Example 1 performed
for
various surface coatings;
Figure 2 provides a graph of the release control test results for Example 1
for the
Wollastonite sugar treatment series performed for surface treated
wollastonite;
Figure 3 provides a graph of the release control test results for Example 1
for the
Wollastonite sulphonate treatment series performed for surface treated
wollastonite;
Figure 4 provides a graph of the release control test results for Example 1
for the
muscovite mica aluminum sulphate treatment series performed for surface
treated mica;
Figure 5 provides a graph of the results of a controlled release assessment
study
for Example 2;
Figure 6 provides a graph of the results of a mechanical durability assessment
study for Example 2; and
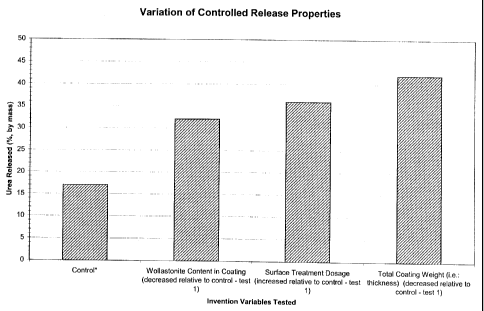
Figure 7 provides a graph of the results of a variation of controlled release
properties for Example 3.
DETAILED DESCRIPTION
The present invention relates to a variety of applications and fields
requiring or
desiring a mechanism for achieving a relatively reliable and relatively
controlled release of a
substrate material either contained within a permeable composition or having a
coating applied
-26-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
thereto. Via attenuation of the release mechanism, the present invention may
provide variable
rates of release. Specifically, the release control technique of the present
invention is believed to
be amenable to producing permeable compositions and controlled release
products possessing a
variety of release profiles designed to meet specific product requirements.
For example. with
respect to agricultural applications, the controlled release fertilizer
products and permeable
fertilizer compositions of the present invention may allow the tailoring of
nutrient (substrate
material) release profiles to disparate crop nutritional requirements, soil
conditions and/or
growing season length.
Further, the present invention may provide improved durability to the
permeable
compositions and controlled release products to reduce potential physical
degradation under
applied stresses (such as, abrasive handling operations, thermal stresses,
etc.). Thus, such
damage, which would normally unduly influence the release profile, may be
mitigated. In
addition, as a result of improved durability, the amount of the permeable
composition or the
1 S release control product required to withstand applied stresses may be
reduced. Thus, the
resulting composition or product may possess higher concentrations of valuable
substrate
material and lower concentrations of lesser valued materials.
As a result of the above, the present invention may provide a relatively cost
effective manner of introducing release profile variability to those
applications currently lacking
such an attribute (such as sulphur coated fertilizers). The present invention
may also provide a
less expensive release control alternative to those products currently
employing costlv_ release
control techniques (such as variable permeability polymer membrane coatings).
As described further below, the preferred embodiment of the within invention
is
particularly applicable to agricultural applications, such as fertilizers and
pesticides. However,
the permeable compositions, release control products and methods described
herein are further
applicable to other applications and fields such as pharmaceuticals, medicines
and
nutraceuticals.
With respect to agricultural applications, and in particular fertilizers, the
present
invention may provide growers with several advantages. Utilizing variable
release profiles,
growers can conceivably select compositions and products designed to meet
their specific crop
and soil demands. Improved substrate material (fertilizer or nutrient)
delivery may be attainable
-27-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
with reduced numbers of applications. Further, the initial rate of substrate
material release may
be reduced, thereby minimizing soil toxicity and crop "burning" associated
with uncontrolled
release fertilizers and some controlled release fertilizers (such as
conventional SCF). By
reducing the initial rate of substrate material release, fewer losses may be
incurred. A reduction
in the required number of applications throughout the growing season may
represent a
substantial savings on labour and equipment operating and maintenance costs.
As well, fewer
losses may result in decreased potential for water contamination or harmful
volatilization
emissions.
The present invention is directed at a relatively durable, cost effective
method of
obtaining controlled release for those applications requiring such an
attribute. In particular, the
within invention is directed at a permeable composition comprising an amount
of a matrix
material, an amount of a particulate filler material dispersed throughout the
matrix material,
wherein each of the particles of filler material is comprised of an external
surface, and interfacial
passageways between the matrix material and the external surfaces of the
filler material.
In a first aspect of the invention, the matrix material of the permeable
composition is comprised of a substrate material such that the filler material
is dispersed
throughout the substrate material. In a second aspect of the invention, the
permeable
composition is used in coating a substrate material. In a third aspect of the
invention, the
invention is directed at a controlled release product comprised of a substrate
material and a
permeable composition coating the substrate material in a coating layer.
Finally, the present
invention is further directed at a method for producing the permeable
composition and a method
for producing the controlled release product.
In all aspects of the invention, a degradable surface treatment material is
preferably applied to the external surfaces of the particles of filler
material such that the
interfacial passageways are defined by the surface treatment material and such
that the surface
treatment material provides degradable interfaces between the matrix material
and the external
surfaces of the particles of filler material.
Generally, the release control mechanism of the present invention is related
to the
selection and interplay of a number of factors: the filler material properties
(including particle
size, aspect ratio and surface area); filler material quantity or amount;
surface treatment material
-28-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
properties; surface treatment material quantity or amount; and the thickness
of the permeable
composition. Further, the properties of the filler material and the quantity
of filler material also
affect the potential mechanical reinforcing and physical stabilizing abilities
of the within
invention. Finally, a number of other factors are also considered in the
selection of the filler
material and surface treatment material of the invention: the dispersion
capabilities of the filler
material; the compatibility of the filler material and surface treatment
material with the method
of production of the permeable composition and controlled release product; the
environmental
acceptance of the filler material and surface treatment material; and the
costs of the filler
material and surface treatment material. All of these factors and
considerations are discussed in
detail below.
The substrate material of the present invention may be any substrate, agent or
matter desired or required to be released to the surrounding environment in a
relatively
controlled manner. For instance, the substrate material may be, but is not
limited to, one or more
of the following: seeds; fertilizers (such as urea, ammonium phosphate, etc.);
herbicides;
fungicides; pesticides; pharmaceuticals; vitamins; veterinary medicines; and
foods. In the
preferred embodiment of the invention, the substrate material is comprised of
a fertilizer. Any
fertilizer suitable for the particular application may be used. However, in
the preferred
embodiment, the fertilizer is comprised of Urea ((NHZ)zC0). Urea has the
highest content of
nitrogen commonly commercially available for granular fertilizers (46%, by
weight).
The filler material of the present invention may be any particulate material
compatible with the particular application of the invention. Preferably the
filler material
possesses an aspect ratio in which the size of the filler particle is greater
in one dimension than
in another dimension. In other words, the filler material preferably has an
aspect ratio greater
than 1. Examples of fillers possessing an aspect ratio greater than one
include fillers which are
comprised of plate structures, fillers which are comprised of fiber structures
and combinations
thereof. Preferably, the filler material is comprised of a fiber structure.
However, as indicated, a
combination of different filler materials may also be used. In the discussion
that follows,
"fibrous filler" shall describe any filler material possessing an aspect ratio
greater than one (such
as fibers and plates) while "non-fibrous filler" shall describe any filler
material not possessing an
aspect ratio greater than one.
-29-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
It is believed that the filler material provides mechanical reinforcement to
the
permeable composition or controlled release product of the within invention.
The mechanical
filler material reinforcement may improve the strength and durability of the
composition or
product. As a result, the composition or product may be better able to
withstand externally
applied stresses, such as abrasive handling, thermal shock, etc.
Further, it is believed that the filler material may physically stabilize the
permeable composition or controlled release product. In particular, it has
been found that
fibrous filler materials may substantially reduce the incidence and/or size of
material defects
resulting from inevitable material alterations, such as allotropic crystal
conversion and/or
thermally induced expansion and contraction of material crystals. Particularly
in the case where
the matrix material is comprised of sulphur (as discussed below), the physical
stabilizing action
exerted by filler material inclusions inhibits or minimizes the uncontrollable
release mechanism
(i.e.: cracks and defects) currently employed in applications such as sulphur
coated fertilizers.
Finally, the filler material forms or facilitates the formation of the release
control
mechanism of the present invention. More particularly, the interfaces between
the longitudinal
surface area of the fibrous filler material and the matrix material provides
passageways and/or
sites for passageway formation. Once formed, these interfacial passageways
serve as conduits
for solvent transmission into the core of the composition or product. Where
the substrate
material is capable of dissolution or degradation, the interfacial passageways
also serve as
channels for the transmission of the substrate material.
As indicated, any organic, inorganic, natural or synthetic filler material may
be
used. For instance, the filler material may be comprised of one or a
combination of the
following (listed in no particular order): (1) inorganic (mineral) fillers
such as wollastonite
(calcium metasilicate), calcium metaphosphate fibers, asbestos, mica, talc,
kaopolite, glass
fibers, ceramic fibers (i.e.: alumina-silica fibers), vitreous fibers (i.e.:
blast furnace slag fibers),
basalt fibers or a combination thereof; (2) organic fillers such as plastic
fibers (i.e.:
polypropylene, polyethylene, polyvinyl alcohol, etc.), cotton, hemp or
cellulose. In the preferred
embodiment, the filler material is comprised of wollastonite. Wollastonite is
a fibrous mineral
comprised of acicular, calcium metasilicate (CaSi03) crystals.
-30-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
The particular filler matenal is selected to be suitable for or compatible
with the
particular application of the invention. Factors affecting the selection of
the filler material
include, but are not limited to, the desired stability of the filler material,
its environmental
acceptability and cost effectiveness. Preferably, the filler material is
thermally, chemically and
S physically stable upon contact with the matrix material and the method of
production (i.e.:
preferably, the filler does not substantially melt, volatilize, decompose,
dissolve, disintegrate,
etc., during its production or useful service life of the composition or
product). The filler
material is also preferably environmentally compatible with the intended
application. Finally,
the filler material cost is preferably compatible with the economics of the
intended application.
It has been found that the following properties of the filler material may
affect the
release control mechanism, mechanical reinforcing abilities and stabilizing
abilities of the
present invention: (1) filler particle size (length x diameter/thickness); (2)
filler particle aspect
ratio (ratio of length to diameter/thickness); and (3) filler particle surface
area. Any filler
particle size, filler particle aspect ratio and filler particle surface area
capable of producing a
permeable composition having the desired properties may be utilized.
In the preferred embodiment the filler material is comprised of wollastonite.
Table 1 provides a representative listing of commercially available
wollastonite products which
may be suitable for use as a filler material in the preferred embodiment of
the invention. Table
lA includes wollastonite products which have actually been tested and have
been found to be
suitable for use as a filler material. Table 1B includes additional
wollastonite products which are
expected, because of their physical properties, also to be suitable for use as
a filler material in
the preferred embodiment of the invention.
The wollastonite products listed in Table 1 are all produced and sold by Nyco
Minerals, Inc. or by its licensees or related companies.
Table 1 is not intended to provide an exhaustive list of suitable wollastonite
products. Other wollastonite products produced and sold by Nyco Minerals, Inc.
or by other
producers of wollastonite may also be suitable for use in the invention.
-31 -
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Table 1A
Tested Wollastonite
Filler
Materials
Example NYCO Product Names
8 NYAD'R' M 100
9 NYAD" M 200
NYAD'R' M 325
11 NYAD" M 400
12 NYAD~ M 1250
Table 1B
Potential
Wollastonite
Filler
Materials
Example NYCO~ Product Names
1 NYAD G'R'
2 NYGLOS'R' 20
3 NYGLOS'R' M15
4 NYGLOS'R' 12
NYGLOS'R' 8
6 NYGLOS" 5
NYGLOS" 4
13 NYAD G'R' Special
14 ULTRAFIBE'R' 55
NYCOR'R' R
16 RRIMGLOS'M I
12 RRIMGLOS'-M II
18 NYAD'R' 200
19 NYAD'R' 325
NYAD'R' 400
21 NYAD'R' 475
22 NYAD'R' 1250
23 NYGLOS'R' M3
-32-
CA 02402212 2006-O1-06
24 NYGLOS M20
25 NYGLOS~' M50
26 NYAD~' M475
A filler material of any particle size, aspect ratio and surface area may be
useful
in the invention. More particularly, however, the range of preferable particle
sizes, aspect ratios
and surface areas may be classified according to a general preferred range, a
more preferable
range and a most preferable range as set out in Table 2. Although the ranges
of Table 2 may be
generally applicable to any filler material dispersed throughout any matrix
material, the ranges
have been found to be particularly applicable in the preferred embodiment in
which the filler
material is comprised of wollastonite and the matrix material is comprised of
sulphur. Thus, for
example, the wollastonite may have a particle width of between about 3 microns
and about 40
microns, a particle length of between about 10 microns and about 600 microns
and a particle
surface area of between about 1 m2/cm~ and about 15 m2/cm3.
Table 2
Ranges of Filler
Particle Size,
Aspect Ratio
and Surface
Area
Parameter Min/Max General More Most Preferable
Preferred Preferable Range
Range Range
Minimum < 3 microns 3 microns 3 microns
Average* Particle
Diameter/ThicknessMaximum >40 microns 25 microns 15 microns
Range (microns)
Minimum <10 microns 10 microns 10 microns
Average* Particle
Length Range Maximum >600 microns100 microns SO microns
(microns)
Minimum <2:1 2:1 3:1
* P
i
l
Average M~imum >20:1 13:1 10:1
art
c
e
Aspect
Ratio Range
-33-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Minimum < 1.5 4.5 6.0
m''/cm3 of m'/cm~ of m~/cm' of
specific specific specific filler
filler filler
Average* Particle volume volume volume
Surface Area Maximum >15.0 15.0 15.0
Range
mz/cm3 of m''/cm3 m'/cm3 of
of
specific specific specific filler
filler filler
volume volume volume
*The average dimensions of particles of filler material may be determined by
any
suitable method as may be practiced in the art.
In an exemplary method for determining particle thickness, length and aspect
ratio, average particle dimensions are determined by computerized analysis of
images generated
by an optical microscope equipped with a camera and an automated stage. A
small amount of a
sample to be analyzed is placed in a transparent container using an
appropriate liquid for
dispersal. A camera is then used to generate a digital signal which is
subsequently processed by
image analysis software. The software defines the border of each image by
setting a grayscale
threshold value. The grayscale image is converted into a binary image.
Corresponding screen
pixels are then used to represent an "area" for each projected two-dimensional
image of particles
within the original field of view. Analysis tools then eliminate particles
that are determined to
be "crossed" or "touching". The remaining particles are then measured to
determine maximum
1~ diameter (length) and minimum diameter (width). At least 1,000 and
preferably 5,000 or more
particles are typically analyzed for each sample. The results are then
weighted by area and the
mean average length, mean average diameter and mean average aspect ratio are
determined.
Suitable image analysis software suited is commercially available. Suitable
image analysis
systems are also commercially available.
An exemplary method for determining specific surface area utilizes a
commercially available automated instrument (Micromeritics~ ASAP 2000). In
this method, the
physical adsorption of an inert gas is conducted at a pressure which is within
the range of
linearity for derivation of specific surface area using the Brunauer, Emmet
and Teller (BET)
standard model for gas adsorption on particulate solids.
-34-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
In general, the particle size of the filler material is preferably less than
the
thickness of the permeable composition. Larger filler sizes are potentially
suitable for relatively
large thicknesses of the permeable composition, while smaller filler sizes are
generally more
suited to relatively small thicknesses of the permeable composition. It may be
desirable to
reduce the weight (i.e.: thickness) of the permeable composition in order to
maximize the value
of the composition or product, which tends to be derived from the amount (by
weight) of the
substrate material (such as fertilizer) contained inside the composition or
product.
It has further been found that the quantity of filler material present in the
composition or product affects its release control mechanism, mechanical
reinforcing abilities
and stabilizing abilities. Any filler quantity capable of producing a
permeable composition
having the desired properties may be utilized. However, the range of
preferable filler quantity
may also be classified according to a general range, a preferable range and a
most preferable
range as set out in Table 3. Although the ranges of Table 3 may be generally
applicable to any
filler material dispersed throughout any matrix material, the ranges have been
found to be
particularly applicable in the preferred embodiment in which the filler
material is comprised of
wollastonite and the matrix material is comprised of sulphur.
Table 3
Suitable Ranges of Filler
Quantity
Minimum QuantityMaximum Quantity
General Mass of Filler Required2% 50%
(by
mass of matrix material )
General Volume of Filler 1.4% 34.5%
Required (by
volume of matrix material)
Preferable Mass of Filler 5% 30%
Required (by
mass of matrix material)
Preferable Volume of Filler 3.5% 20.7%
Required
(by volume of matrix material)
Most Preferable Mass of Filler10% 20%
Required (by mass of matrix
material)
-35-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Most Preferable Volume of Filler 6.9% 13.8%
Required (by volume of matrix
material)
However, the permeable composition may be comprised of more voluminous
filler material having a lower specific gravity ~~ithin a greater thickness of
the composition or
less voluminous filler material having a greater specific gravity within a
smaller thickness of the
composition. Therefore, the preferable ranges of filler quantities may be
broadened.
Accordingly, in the preferred embodiment, the general range of filler quantity
is approximately
0.5% to 200% filler, by volume of matrix material. The preferable range of
filler quantity is 1
to 120% filler, by volume of matrix material. The most preferable range of
filler quantity is
approximately 2% to 80% filler, by volume of matrix material.
As indicated, a degradable surface treatment material is preferably applied to
the
external surfaces of the particles of filler material. It is believed that the
surface treatment of the
particles of filler material facilitates the release control mechanism of the
present invention and
may facilitate compatibility of specific filler materials with specific matrix
materials (i.e.:
provides or improves filler dispersion). As well, in order for the filler
material to be
mechanically reinforcing, the surface treatment material also preferably
provides for a transfer of
any externally applied loads from the matrix material to the particles of
filler material across the
degradable interface, at least until such time that the interface in fact
degrades.
Specifically, it is believed that the surface treatment material applied to
the
external surfaces of the particles of filler material initiates or enhances
the formation of the
interfacial passageways between the matrix material and the external surfaces
of the particles of
filler material. Thus, surface treatment refers to the application of a
surface treatment material to
the surface area of the particulate filler material. The surface treatment
material is preferably
soluble or degradable in the presence of a solvent or degradation process
specific to the surface
treatment.
More particularly, the surface treatment material provides degradable
interfaces
between the matrix material and the external surfaces of the particles of
filler material. Thus,
upon contact with an appropriate solvent or degradation agent, the surface
treatment material is
-36-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
dissolved or degraded, thereby forming or enhancing the interfacial passageway
between the
longitudinal surface of the particles of filler material and the matrix
material. The dissolution or
degradation of the surface treatment material provides interfacial passageways
where none
would otherwise exist and/or augments those interfacial passageways that may
already exist.
Therefore, the surface treatment material is believed to provide the primary
element of control over the release rate of the substrate material.
Accordingly, it is further
believed that control over the release profile may be achieved by selection
and variation of one
or more of the factors described below.
First, the surface treatment material or materials may be selected to control
the
rate of formation or enhancement of the interfacial passageways. By selecting
surface treatment
materials of varied solubility or degradation properties, it is believed that
adjustment of the
surface treatment dissolution or degradation rate may be attained. Second, the
thickness of the
permeable composition may be selected to control the rate of formation or
enhancement of the
interfacial passageways. By adjusting the thickness, the length of the
particles of filler material
is varied proportionally. Consequently, the rate at which complete interfacial
passageways are
formed or enhanced along the surface of the filler particles may be
controlled.
Third, the amount or dosage of the surface treatment material applied to the
external surfaces of the particles of filler material may be selected to
control the size of the
interfacial passageways. Upon dissolution or degradation of the surface
treatment material, a
correlation between the amount of surface treatment material applied and the
properties (i.e.,
size, effectiveness etc.) of the interfacial passageway may be observed.
Fourth, the volume or
amount of the surface treated filler material used may be selected to control
the amount of
interfacial passageways. By adjusting the amount of the surface treated filler
material contained
within the permeable composition, it is believed that the number of
interfacial passageways
induced or enhanced in the composition may be controlled. Finally, the volume
of the total filler
material may be selected to control the amount of interfacial passageways. In
applications
where greater durability and slower release are required, the extent of the
surface treatment
material applied to the particles of filler material may be adjusted. In such
cases, the total filler
material content may be increased to enhance durability and the amount of
surface treated filler
material may be reduced in order to achieve the desired release profile.
-37-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
As indicated, the applied surface treatment material may also serve as a
dispersing agent for the filler material. In certain applications, untreated
filler material may not
be readily dispersible in specific matrix materials. Inability to achieve
filler dispersal may be a
function of various interference mechanisms (i.e.: statically charged filler
particles, poor matrix
material wetting properties, etc.) In such cases, the surface treatment
material and the dosage or
amount are selected to not only initiate or enhance interfacial passageway
formation, but to also
render the filler material dispersible in the matrix material.
In certain applications where filler surface treatment is not specifically
required
for dispersal, the surface treatment of the filler material may result in
substantially reducing the
mixing effort required for dispersal. From a practical or production process
standpoint, it may
be desirable to select surface treatment materials and amounts or dosages
which not only initiate
or enhance interfacial passageway formation, but also improve dispersal of the
filler material
particles within the matrix material.
The surface treatment material may be comprised of any solid, liquid, organic,
inorganic, natural or synthetic surface treatment agent capable of providing
the desired
degradable interfaces between the matrix material and the external surfaces of
the particles of
filler material. For example, the surface treatment material may be comprised
of one or more of
the following (listed in no particular order): aluminum sulphate; sodium
lauryl sulphate; alkyl
sulphates; substituted phenol ethoxy phosphate esters; hydrated lime; tridecyl
alcohol
ethoxylate; octylphenol ethoxylate; sorbitol monooleate ethoxylate; canola
oil; sodium
silicate; calcium chloride; sugar; potassium chloride; ammonium sulphate;
naphthalene;
butylnaphthalene; naphthalene sulphonate; calcium lignosulphonate; naphthalene
sulphonate
formaldehyde condensates; sodium alkyl benzene sulphonates; styrene butadiene;
dairy
products; polyoxyalkylene glycol ether; polypropylene glycol monobutyl ether;
lecithin;
polyvinyl alcohol; detergent and combinations thereof.
Preferably, the surface treatment material is water soluble so that the
degradable
interfaces degrade in the presence of water. In the preferred embodiment, the
surface treatment
material is comprised of a naphthalene sulphonate formaldehyde copolymer.
As indicated, the surface treatment material provides degradable interfaces,
which
interfaces may degrade through various actions such as dissolution, thermal
decomposition,
-38-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
biological degradarion, chemical decomposition, etc. Where the substrate
material is also
selected to degrade, the mode of surface treatment material degradation
selected is typically the
same as that required to degrade the substrate material. For example, in the
case of substrate
materials which are degradable through dissolution by a particular solvent,
the surface treatment
material selected is typically also soluble in the same solvent.
In addition to degradability, the surface treatment material is also
preferably
selected to provide one or more of the following further desirable properties
to the composition
or product of the present invention. First, the surface treatment material is
preferably thermally,
chemically and physically stable upon contact with the matrix material and the
production
method (i.e.: the surface treated filler material must not substantially melt,
volatilize,
decompose, dissolve, disintegrate, etc., during the production method).
Second, in cases where
the filler material could not otherwise be dispersed in a matrix material
during production (due
to electrostatic repulsion, surface tension effects, etc), the surface
treatment material preferably
renders the filler material compatible with the matrix material to provide
filler dispersal. In
cases where the filler material is difficult to disperse in the matrix
material, the surface treatment
material may be used to improve filler dispersal. Third, the surface treatment
material is
preferably environmentally compatible with the intended application. Fourth,
the surface
treatment material cost is preferably compatible with the economics of the
intended application.
Finally, it is also generally desirable that the type of surface treatment
material be
compatible (i.e.: bondable) with the filler material and the matrix material
for the purpose of
maintaining the mechanical reinforcing properties of the filler material
particles. Incompatible
surface treatment materials may induce "slippage" along the filler
material/matrix material
interface, thereby reducing the filler material's mechanical reinforcing
capability.
The quantity or dosage of the surface treatment material applied to the
particles of
filler material has been found to affect both the release control mechanism
and dispersion of
filler material in the present invention. Any amount, quantity or dosage of
surface treatment
material capable of producing a permeable composition having the desired
properties may be
utilized. However, the range of preferable amounts of surface treatment
material may be
classified according to a general range, a preferable range and a most
preferable range as set out
in Table 4. Although the ranges of Table 4 may be generally applicable, the
ranges have been
found to be particularly applicable in the preferred embodiment in which the
filler material is
-39-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
comprised of wollastonite and the matrix material is comprised of sulphur.
Further, the ranges
have been further found to be particularly applicable in the preferred
embodiment in which the
surface treatment material is comprised of naphthalene sulphonate formaldehyde
copolymer.
Table 4
Ranges of Amounts or Dosages of
Surface Treatment Material
Minimum Maximum
Quantity Quantity
General Surface Treatment Material0.1% 20%
Mass
Dosage Required (by mass of Filler
Material)
General Surface Treatment Material
Volume
Dosage Required (surface treatment0.0002 cm3/m2 0.04 cm3/mz
material
volume/filler material surface
area)
Preferable Surface Treatment Material0.5% 10%
Mass
Dosage Required (by mass of filler
material)
Preferable Surface Treatment Material
Volume Dosage Required (surface 0.001 cm3/m2 0.02 cm3/m2
treatment
material volume/filler material
surface area)
Most Preferable Fiber Surface 1% 5%
Treatment
Mass Dosage Required (by mass
of filler
material)
Most Preferable Surface Treatment
Material
Volume Dosage Required (surface 0.002 cm3/m2 0.01 cm3/m2
treatment
material volume/filler material
surface area)
However, the permeable composition may be comprised of more voluminous
surface treatment material having a lower specific gravity within a lower
surface area filler
material or less voluminous surface treatment material having a higher
specific gravity within a
higher surface area filler material. Therefore, the preferable ranges of
surface treatment material
quantities may be broadened. Accordingly, in the preferred embodiment, the
general range of
surface treatment material quantity is approximately 0.00005 cm3 surface
treatment material/m2
of surface area of filler material to 0.6 cm3 surface treatment material/m2 of
surface area of filler
material. The preferable range of surface treatment material quantity is
0.0003 cm3 surface
treatment material/m2 of surface area of filler material to 0.3 cm3 surface
treatment material/m2
-40
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
of surface area of filler material. 1'he most preferable range of surface
treatment material
quantity is approximately 0.0005 cm3 surface treatment material/m2 of surface
area of filler
material to 0.1 cm3 surface treatment material/m2 of surface area of filler
material.
S As indicated above, the permeable composition of the present invention is
comprised of an amount of a matrix material. In one aspect of the invention,
the matrix material
is comprised of a substrate material and a Garner material. In a further
aspect of the invention,
the permeable composition including the matrix material is used in coating a
substrate material.
Whether the matrix material is comprised of the substrate material or used for
coating the
substrate material, the matrix material acts as a Garner for the filler
material. Specifically, the
filler material is dispersed throughout the matrix material. Further, the
matrix material facilitates
the control release mechanism of the present invention. By increasing or
decreasing the
thickness or dimensions of the permeable composition comprised of the matrix
material, the
"length" of the interfacial passageways formed around individual particles of
filler material or
contiguously arranged particles of filler material is also increased or
decreased. Therefore, the
time required to degrade the surface treatment material may be increased or
decreased as the
thickness or dimensions of the permeable composition are varied.
As indicated, the matrix material is comprised of a carrier material in one
aspect
of the invention. Further, in all aspects of the invention, the matrix
material acts as a carrier.
Thus, the matrix material, and the carrier material, may be comprised of any
organic, inorganic,
natural or synthetic material or a combination thereof capable of acting as a
carrier for the filler
material and capable of facilitating the formation or enhancing the formation
of the interfacial
passageways. For example, suitable or preferred matrix materials and carrier
materials are
comprised of one or a combination of (listed in no particular order):
polyurethane,
polypropylene, polyethylene, latex, sulphur and resins. In the preferred
embodiment, the matrix
material is comprised of sulphur. Accordingly, where the matrix material is
comprised of a
carrier material, the carrier material is comprised of sulphur.
In the preferred embodiment the sulphur is preferably elemental sulphur, which
is
less expensive than chemically modified sulphur. Elemental sulphur also
potentially offers more
flexibility in designing the permeable composition, since the relative
instability of elemental
sulphur in comparison with chemically modified sulphur may be advantageous for
avoiding the
effects of "lock-off '. In the short term, the inclusion of the filler
material throughout the
-41 -
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
elemental sulphur matrix material will serve in the preferred embodiment to
mechanically
reinforce and physically stabilize the elemental sulphur, thus counteracting
some of the potential
disadvantages of using a relatively unstable matrix material.
The particular matrix material and earner material selected will be dependent
upon the desired properties of the permeable composition and/or controlled
release product and
the intended application thereof. Matrix material properties, including
carrier material
properties, may be governed by factors such as matrix material strength,
permeability, cost
and/or environmental/health acceptability. For example, sulphur is often used
for fertilizer
applications due to its relatively low cost and acceptability for eventual
long-term degradation in
the environment.
The thickness of the permeable composition, including the thickness of any
coating layer of the permeable composition, has been found to affect the
release control
mechanism of the present invention. Any thickness of permeable composition,
including any
thickness of a coating layer thereof, capable of producing an end product
having the desired
properties may be utilized. However, the range of preferable thicknesses of
permeable
composition may be classified according to a general range and a preferable
range as set out in
Table 5. Although the ranges of Table 5 may be generally applicable, the
ranges have been
found to be particularly applicable in the preferred embodiment in which the
filler material is
comprised of wollastonite, the matrix material is comprised of sulphur and the
surface treatment
material is comprised of naphthalene sulphonate formaldehyde copolymer.
Further, the following ranges of thicknesses are particularly applicable where
the
permeable composition is applied to the substrate material in a coating layer.
Specifically, the
ranges set out in Table 5 are approximate and have been calculated from the
applied sulphur
coating weights, assuming a spherical coating substrate material with a
diameter of 2.2 mm.
Such a substrate material size is representative of many, but not all, prilled
or granulated
fertilizers.
- 42 -
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Table 5
Ranges of Thickness of the Permeable
Composition
Minimum Maximum
Value Value
General Weight of Permeable Composition
Applied to Substrate Material 1 S% 30%
(%, by weight
of coated product or substrate
material)
General, Approximate Thickness 45 microns 90 microns
Applied to
Substrate Material (microns)
Preferable Weight of Permeable
Composition
Applied to Substrate Material 15% 25%
(%, by weight
of coated product or substrate
material)
Preferable Approximate Thickness 45 microns 75 microns
Applied to
Substrate Material (microns)
In general, it is desirable to minimize the weight or thickness of the
permeable
composition or coating layer for economic reasons. Matrix materials represent
an additional
material expense in production. Also, typically the value of the end product
is directly related to
the amount of valuable substrate material in the product, not the amount of
matrix or carrier
material (such as sulphur).
The invention may be utilized in other products, particularly coated products
such
as those using polymer coatings (including polymer coated fertilizers or
pharmaceuticals) or
very small granular substrate materials. In these applications, the coatings
are generally applied
much more thinly than shown above in Table 5. Therefore, the preferable ranges
of thickness of
a coating layer of the permeable composition may be broadened. Accordingly, in
the preferred
embodiment, the general range of thickness of the coating layer is
approximately 2 to 100
microns and the preferable range of thickness of the coating layer is 20 to 75
microns.
Where the matrix material is comprised of the substrate material (rather than
applying the matrix material as a coating on the substrate material), the
dimensions of the
permeable composition preferably range from prill/granules of approximately 1
mm (or less) to
- 43 -
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
mm. However, the invention may also be used in much larger products such
fertilizer macro-
granules, tablets or briquettes. Such products may range in size from 25 mm
(or less) granules
or tablets to 1 SO mm (or more) briquettes.
In the method aspect of the invention, the invention is directed at a method
for
producing the permeable composition and a method for producing the controlled
release product.
In either case, the application or product is first selected for use with the
present invention. For
example, as discussed above, suitable applications or products include those
that require a cost-
effective method of achieving controlled release properties (such as
controlled release
fertilizers).
Both of the methods include the steps for the production of the permeable
composition. Specifically, the method is comprised of providing an amount of a
particulate filler
material, wherein each of the particles of filler material is comprised of an
external surface, and
applying an amount of a degradable surface treatment material to the external
surfaces of the
particles of filler material to form surface treated particles of filler
material. The properties and
parameters of the filler material and the surface treatment material are
preferably as described
above. Therefore, the filler material and the surface treatment material are
selected according to
their required or desired properties discussed previously. Further, the
selected surface treatment
material is applied to the surface area of the selected filler material at the
required or desired
amount or dosage as discussed previously, using any conventional or known
mechanism or
process suitable for applying surface treatment materials to particle
surfaces.
Further, the method for producing the permeable composition is further
comprised of dispersing the surface treated particles of filler material
throughout an amount of a
matrix material to form the permeable composition such that interfacial
passageways between
the matrix material and the external surfaces of the particles of filler
material are defined by the
surface treatment material and such that degradable interfaces between the
matrix material and
the externals surfaces of the filler material are provided by the surface
treatment material. The
properties and parameters of the matrix material are preferably as described
above.
The dispersal step may be performed in any manner and using any mechanism
suitable for dispersing the surface treated particles throughout the matrix
material. For instance,
any compatible mixing process or mixing apparatus may be used. Preferably, the
surface treated
-44-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
particles are substantially uniformly dispersed or mixed throughout the matrix
material by the
dispersing step. In the preferred embodiment, the surface treated particles of
filler material and
the matrix material in fluid form (for example molten, emulsified, etc.) are
mixed together using
known or conventional means, mechanisms or processes. Further, the surface
treated particles of
filler material may undergo a pre-dispersal process or step where required to
facilitate the
subsequent dispersal of the particles in the matrix material. For instance,
the surface treated
particles of filler material may be pre-heated prior to the dispersing step in
order to facilitate
mixing the filler material with a thermoplastic or thermosetting matrix
material.
In one aspect of the method for producing the permeable composition where the
matrix material is comprised of the substrate material, the dispersing step is
further comprised of
dispersing the surface treated particles of filler material throughout the
substrate material. The
properties and parameters of the substrate material are preferably as
described above. Further,
the dispersal step may be performed in any manner and using any mechanism
suitable for
dispersing the surface treated particles of filler material throughout the
matrix material including
the substrate material. For instance, any compatible mixing process or mixing
apparatus may be
used. Preferably, the surface treated particles are substantially uniformly
dispersed or mixed
throughout the substrate material by the dispersing step. Further, the
substrate material may
undergo a pre-dispersal process or step where required to facilitate the
subsequent dispersal of
the particles of filler material in the substrate material. For instance, the
substrate material may
be pre-heated prior to the dispersing step in order to facilitate dispersal of
the substrate material
within the thermoplastic or thermosetting matrix material.
Finally, in this aspect of the method for producing the permeable composition,
the method is preferably further comprised of the step of forming the
permeable composition
into discrete particles. The permeable composition may be formed into discrete
particles having
any desired dimensions or configuration using any known or conventional
processes or
mechanisms suitable or compatible for use with the particular permeable
composition. For
instance, the discrete particles of permeable composition may be formed by
such techniques as
prilling, granulating or extrusion.
In a further aspect of the method for producing the permeable composition
where
the matrix material is not comprised of the substrate material, the method may
be further
comprised of the step of applying the permeable composition to a substrate
material in a coating
-45-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
layer. With respect to the method for producing a controlled release product,
the method is
comprised of the steps of providing the amount of the particulate filler
material, applying the
amount of the degradable surface treatment material to the external surfaces
of the particles of
filler material and dispersing the surface treated particles of filler
material throughout the
amount of the matrix material to form the permeable composition, all as
described above.
However, the method for producing the controlled release product is further
comprised of the
steps of providing a substrate material and applying the permeable composition
to the substrate
material in a coating layer to form the controlled release product. In either
method, the
properties and parameters of the substrate material and the coating layer are
preferably as
described above.
The permeable composition may be applied to the substrate material in a
coating
layer using any known or conventional processes or mechanisms suitable or
compatible for use
with the particular permeable composition and capable of achieving the desired
coating layer.
For instance, the coating layer may be applied using conventional techniques
such as atomized
coating applications.
Further, where applying a coating layer, the substrate material may undergo a
pre-
application process or step where required to facilitate the subsequent
application of the
permeable composition to the substrate material. For instance, the substrate
material may be
pre-treated with an application of a primer material selected to promote or
facilitate the later
adhesion of the coating layer of permeable composition to the substrate
material.
Finally, with respect both the method for producing the permeable composition
and the method for producing the controlled release product, the methods may
further include
additional processing steps, where desired or required, to achieve or produce
the desired end
product. For instance, the composition or the product may be subjected to
cooling or curing
steps or additional materials may be applied as further coatings.
A detailed description is provided below of the preferred embodiment of the
invention in the production of sulphur coated fertilizers (SCF). SCF was
selected as an example
of the application of the invention given that conventional SCF tends to be a
high-value, high
volume product, which typically lacks the ability to achieve reliable,
significant release control
in a cost-effective fashion. Traditionally, release control in SCF is achieved
via the application
-46-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
of costly polymer coatings applied over the primary sulphur coating or the use
of expensive,
chemical sulphur modifiers (sulphur plasticizers or stabilizers), which often
posses only limited
performance. Additionally, conventional SCF tends not to be mechanically
durable, and thus
has unreliable release properties.
S
In the preferred embodiment, the filler material is comprised of wollastonite
(calcium metasilicate) for several reasons. First, the relatively high
particle strength and high
particle aspect ratios provide excellent reinforcement of a sulphur matrix.
Significant variability
in wollastonite particle size, aspect ratio and surface area also provides
capacity for relatively
significant release control and formation of interfacial passageways. Second,
wollastonite has
relatively high thermal stability, which makes it suitable for use in sulphur
matrices, which are
applied in molten form at temperatures in the approximately 130 to
160°C temperature range.
Third, wollastonite material cost is compatible with the economics of the
production. Finally,
wollastonite is an inert mineral, which is acceptable for use in agricultural
applications.
Further, in the preferred embodiment, the surface treatment material for the
wollastonite is comprised of a naphthalene sulphonate formaldehyde copolymer
(nsf
copolymer), in aqueous solution. Nsf copolymer was selected as the surface
treatment material
for several reasons. First, nsf copolymer is hydrophilic and water soluble,
and is, therefore,
degradable (soluble) in the presence of the fertilizer releasing agent
(water). Nsf copolymer also
effectively improves wollastonite particle dispersion in molten sulphur.
Second, nsf copolymer
is substantially stable at the temperatures used to apply molten sulphur
coatings to fertilizers.
Third, nsf copolymer material cost is compatible with the economics of SCF
production.
Finally, the use of nsf copolymer is accepted in agricultural applications.
In the preferred embodiment, the nsf copolymer is applied to the external
surfaces
of the particles of wollastonite filler material using the following process
regime. First, the
wollastonite is pre-heated in order to facilitate a nsf copolymer coating over
substantially the
entire surface area of the wollastonite particles. The wollastonite is
preferably pre-heated to a
temperature range of about 60 to 90 °C (most preferably about 70 - 80
°C). Second, the surface
treatment material , or nsf copolymer, is also pre-heated. The required amount
of nsf copolymer,
in aqueous solution and in the dosages discussed previously, is preferably
heated to a
temperature range of about 50 - 70 °C (most preferably about 60 to 70
°C). Water may be added
-47-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
to the nsf copolymer solution in order to dilute the surface treatment
material, thereby
facilitating complete coverage of the wollastonite particles.
Third, in a suitable surface treatment mixer/agitator, the pre-heated
wollastonite is
sprayed with the heated surface treatment material solution and subjected to
high-shear mixing.
Suitable surface treatment agitators (such as pugmills, ribbon mixers, etc)
are utilized.
Agitation/mixing action must be sufficient to disperse the surface treatment
material or nsf
copolymer over the surface area of the wollastonite particles, however the
agitation action must
not be so intense as to damage or break the wollastonite particles. In the
preferred embodiment,
this was accomplished using a 1 kg capacity, table-top, rotating paddle-mixer
with an agitation
speed ranging from about 60 to 150 rpm (preferably about 90 to 120 rpm). The
mixture of
wollastonite and nsf copolymer is agitated for approximately 15 to 30 minutes.
The mixture
temperature is maintained in the temperature range of about 50 to 70 °C
during the surface
treatment process.
Fourth, the surface treated wollastonite is removed from the agitator/mixer
and
allowed to air-dry under ambient conditions for approximately 24 hours. The
wollastonite is
thinly spread-out and periodically agitated by hand, during drying. Finally,
as the surface
treatment solution dries, the wollastonite particles may become agglomerated.
In order to break-
up any particle clusters, the dried surface treated wollastonite particles are
agitated with a paddle
mixer at approximately 60 rpm for approximately 10 minutes.
Once the permeable composition is prepared as described above, the preferred
embodiment of the controlled release product is prepared as follows. First,
the substrate material
or fertilizer granules are prepared by pre-heating them to a temperature range
of about 70 - 90 °C
(preferably approximately 80 °C). Further, if necessary, fertilizer
priming may be performed. In
order to improve or facilitate bonding between the permeable composition
(sulphur/surface
treated particles of wollastonite) and the substrate material (fertilizer
granules), the fertilizer
granules may be "primed" (i.e.: coated) with a relatively thin layer of
elemental sulphur. The
molten elemental sulphur possesses a lower viscosity than the molten
sulphur/surface treated
wollastonite particle mixture and, therefore, tends to adhere better to the
fertilizer substrate. The
elemental sulphur primer may be applied in low quantities of about 2 to 10%,
by weight of the
final, coated fertilizer product or controlled release product (preferably 4
to 7%, by weight of the
final fertilizer coated product). The coating regime for elemental sulphur
"priming" is the same
-48
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
as that described below for the application of the coating layer of the
permeable composition to
the substrate material.
Second, the surface treated particles of wollastonite filler material are pre-
heated
in order to facilitate mixing of the surface treated wollastonite particles
with molten sulphur
matrix material. The surface treated wollastonite particles are pre-heated to
a temperature in a
range of about 130 to 145 °C.
Third, the pre-heated, surface treated wollastonite is added to the molten
sulphur
at the required quantity, ass discussed previously. The wollastonite particles
and sulphur may be
mixed together using any conventional high-shear mixing means (such as
pneumatic agitators).
Mixing is preferably performed until the wollastonite is substantially fully
dispersed and a
substantially homogeneous mixture is achieved.
1 S Fourth, a coating layer of the permeable composition (sulphur/surface
treated
wollastonite particles) is applied to the substrate material. The application
of the sulphur/surface
treated wollastonite particle coating to the fertilizer substrate is
consistent with conventional or
known techniques for applying elemental sulphur coatings to fertilizers.
However, adjustments
to the conventional sulphur coating regime are typically required due to the
presence of the
wollastonite particles in the coating mixture. The following coating
application process
description is applicable to the application of sulphur/surface treated
wollastonite particle
coatings to fertilizer granules.
Using any conventional granule fluidizing means (such as a rotating drum), the
fertilizer granules are fluidized (i.e.: a moving bed, sheet, etc of
fertilizer granules is induced).
The degree of granule fluidization must be sufficient to adequately expose the
discrete fertilizer
granules to an applied coating mixture spray. The coating mixture is then
delivered to the
fertilizer granules. In particular, the molten sulphur/surface treated
wollastonite mixture is
pumped via conventional means to a series of conventional, fluid atomizing
nozzles. The
nozzles are equipped with sufficient orifice size and mechanical clean-out
capabilities (i.e.:
orifice needles) to prevent or inhibit plugging of the nozzles by the
wollastonite particles.
Via the atomizing nozzles, the molten sulphur/surface treated wollastonite
coating
mixture fluid stream is contacted with atomizing air at a sufficient flow rate
and pressure to
-49-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
atomize (produce discrete droplets) the coating mixture fluid stream. The air
must also be
heated to prevent freezing of the molten coating mixture fluid stream during
atomizing. As the
molten, atomized coating mixture droplets are sprayed from the atomizing
nozzles, they strike
the fluidized granule bed and spread over the surface of the discrete
fertilizer granules before
freezing. As a system of atomized coating mixture droplets coalesce and freeze
on the granule
surface, a uniform sulphur/surface treated wollastonite coating is formed.
The following detailed coating application regime, as set out in Table 6, is
applicable to bench-scale production of the preferred embodiment of the
controlled release
product of the within invention having a sulphur/surface treated wollastonite
coating.
Table 6
Bench Scale Process
Regime for the Application
of Sulphur/Surface
Treated Wollastonite
Coatings to Fertilizer
Granules
General Regime ParametersSpecific Regime Regime Variable
Variables Value
Fertilizer Amount 5 kg
Fertilizer Granule Fluidizing Drum Length3 ft.
Fluidizing Fluidizing Drum Diameter2 ft.
Fluidizing Drum Rotation20 to 50 rpm
Speed
Coating Mixture Flowrate2 to 6 gph
Coating Mixture Flow10 to 30 psi
Coating Mixture DeliveryPressure
Coating Mixture 140 to 160 C
Temperature
Atomizing Air Flowrate1 to 5 cfin
Coating Mixture AtomizingAtomizing Air Flow 20 to 70 psi
Pressure
Atomizing Air Flow 140 to 170 C
Temperature
Once the permeable composition coating layer is applied, the controlled
release
product may be cooled using conventional air cooling means. Further, although
not required,
-50
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
additional coatings may be applied over the permeable composition coating
layer, such as
polymeric sealants. If desired, such additional coatings may be applied for
the purpose of
additional release control, product coloration, etc.
Test Results - Example One
Various tests have been conducted on the preferred embodiment of the
controlled
release product of the present invention, including assessments of durability,
controlled release
characteristics and dispersal. Test results related to these technological
elements are presented,
following a description of the test specimen preparation.
In order to facilitate analysis, the specimens in Example 1 were prepared as
"macro-granules" having a granule size larger than typical fertilizers.
Test Specimen Preparation Procedure
The test specimens are prepared as follows:
1. Urea specimens 13 mm in diameter are cast from molten urea, cooled and
weighed. The surfaces of the specimens are inspected to ensure nn s;~";f,~a"t
defects (i.e.: holes, dents, etc.) are present.
2. Coating additives are heated to approximately 140 ° C, prior to
mixing with
sulphur at 130 - 140 ° C. The mix temperature is adjusted to 135 - 140
° C, if
necessary.
3. The cooled urea specimens are dipped in the coating mixture.
4. The coated specimens are cooled, weighed and the % coating weight is
calculated:
Coating Weight = mass of coating x 100
mass of coating + mass of urea
5. Test coats are performed prior to specimen coating to ensure the amount of
coating additive results in an average % coating weight between 20 - 22%. The
applied coating weight is determined by the viscosity of the coating mix.
-51-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Holding the sulphur temperature constant, the amount of coating additive is
adjusted to ensure the coating weight is consistent for all test groups.
6. Those specimens possessing a % coating weight outside a range of 19 - 25%
are
rej ected.
Durability Testing
The durability testing was performed as follows:
1. Five coated specimens are weighed.
2. The specimens are added to the durability tester, which consists of a
shaker
capable of holding the samples and a ceramic impact charge. The mass ratio of
impact charge to specimens is 42:1.
3. The specimens are shaken for 10 minutes, thereby subjecting the coatings to
violent collisions with the ceramic impact charge.
4. The urea specimens and any coating remaining on the specimens are weighed.
The specimens are inspected to ensure no significant loss of urea substrate
has
occurred and the % coating loss is calculated:
° initial mass of specimens - mass of specimens after test X 100
/o Coating Loss =
initial mass of coating on specimens
In addition to a control test of specimens coated with pure sulphur,
durability
testing for Example 1 was conducted on specimens coated with sulphur and non-
fibrous fillers,
and specimens coated with sulphur and fibrous fillers, as set out in Table 7.
Table
7
Durability
Testing
Program
Test Coating Coating Inclusion Content
Inclusion In Coating (%, by weight
of sulphur)
None
1 (control sulphur coat)Not Applicable
-52-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Fine Silica Sand
2 (Non-Fiber) 15%
Ground Expanded Shale
3 (Non-Fiber) 15%
Powdered Carbon
4 (Non-Fiber) 15%
Wollastonite
(Fiber) 15%
Muscovite Mica
6 (Fiber or Plate) 12%*
Cellulose (w/kaolin)
7 (Fiber) 2.5%*
In some cases less than 15%, by weight of sulphur, coating additive resulted
in
the maximum permissible average coating weight of 22%. Such tests are denoted
with an
asterisk. All test specimen sets possessed similar average coating weights in
the 20 to 22%
5 range.
Figure 1 provides a graph of the durability test results for Example 1. As is
evident from the durability test results, fibrous filler material inclusions
improve the physical
durability of sulphur based coatings. Of the fibrous filler materials tested
to date, wollastonite
inclusions were found to induce the most substantial improvement in sulphur
based coating
durability. Therefore, it is observed that fibrous filler materials provide
reinforcement of sulphur
coatings.
Further, as is evident from the durability test results, the addition of
fibrous filler
materials to sulphur based coatings results in greater coating durability than
is possessed by
those coatings containing non-fibrous filler materials. Therefore, it is
observed that fibrous filler
materials possess greater reinforcing properties than non-fibrous filler
materials (i.e.: materials
without a particulate aspect ratio).
Release Control Testing
The release control testing was performed as follows:
-53-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
1. Five coated specimens of known urea content are each placed in 200 ml of
water
at l5-20°C.
2. After 4 hours of submersion each urea specimen is removed from water and
assessed for mass loss. The assessment involves determining loss of the urea
substrate (via dissolution) by pressing on the coating. Any specimens that
rupture
are dried and weighed. The total % urea released is calculated:
° initial specimen mass - ruptured specimen mass
/o urea released= x 100
initial urea mass
3. Ruptured specimens are removed from the testing program.
4. Remaining specimens are placed in water again and the amount of urea
release is
assessed every 15 hours thereafter.
In agriculture applications, the releasing solvent is generally water.
Therefore,
for the purposes of controlled release testing, hydrophilic substances have
been selected as
appropriate surface treatment materials. Based on positive coating durability
results, controlled
release testing has been conducted primarily on wollastonite. However, a test
series for
muscovite mica (KA13Si301o(OH)2) was also conducted in order to verify the
release control
technique. Mica is comprised of thin, alumino-silicate plates. The release
control testing
program for Example 1 is set out in Table 8.
Table
8
Release
Control
Testing
Program
Test Filler Surface Surface TreatmentFiller Dosage in
Coating
# Material Treatment Dosage (%, by (%, by weight
weight
Tested Material of filler) of sulphur)
None Not Not Not
8 (control ApplicableApplicable Applicable
sulphur
coat)
-54-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Not
9 WollastoniteNone Applicable 15%
WollastoniteSugar 2% 15%
11 WollastoniteSugar 5% 15%
12 WollastoniteSugar 10% 15%
13 WollastoniteSulphonate2% 15%
14 WollastoniteSulphonate5% 15%
Muscovite Not
Mica None Applicable 12%*
Muscovite Aluminum
16 Mica Sulphate 5% 12%*
Muscovite Aluminum
17 Mica Sulphate 10% 12%*
* Maximum filler content before 22% coating weight was exceeded.
5 Relating to Example 1:
Figure 2 provides a graph of the release control test results for the
wollastonite
sugar treatment series;
-55-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
2. Figure 3 provides a graph of the release control test results for the
wollastomte
sulphonate treatment series; and
3. Figure 4 provides a graph of the release control test results for the
muscovite mica
aluminum sulphate treatment series.
Although Figure 2, Figure 3 and Figure 4 are based upon somewhat limited data,
a number of possible trends can be ascertained from the data.
First, as indicated by the Figures, specimens coated with sulphur and
untreated
wollastonite or sulphur and untreated muscovite mica released urea much more
slowly than
specimens coated with pure sulphur. Therefore, it is observed that fibrous
filler inclusions
within sulphur are capable of physically stabilizing sulphur. By substantially
reducing the
incidence and/or size of coating defects at the crystal level, the
uncontrollable release
mechanism employed by conventional sulphur coatings (i.e.: the defects) is
observed to be
substantially reduced. The formation of defects at the crystal level is
potentially minimized via
further adjustment of the filler material content in the coating or permeable
composition.
Second, as indicated by the Figures, specimens coated with sulphur and
untreated
wollastonite released urea at a different rate than specimens coated with
sulphur and untreated
muscovite mica. Therefore, it is observed that a degree of control over
sulphur crystal
imperfection formation can be achieved via filler selection.
Third, as indicated by the Figures, specimens coated with a matrix of sulphur
and
surface treated wollastonite released urea at a faster rate than specimens
coated with a matrix of
sulphur and untreated wollastonite. Therefore, it is observed that appropriate
surface treatment
of fibrous filler materials initiates interfacial passageway formation and/or
enhances existing
interfacial passageways between the longitudinal surface area of the filler
particles and matrix
material.
Fourth, as indicated by the Figures, specimens coated with sulphur and fibrous
fillers with higher surface treatment dosages released urea at a faster rate
than specimens coated
with sulphur and fibrous fillers with lower surface treatment dosages.
Therefore, it is observed
that adjustment of the surface treatment dosage provides a facet of control
over the release
profile.
-56-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Fifth, as indicated in Figure 4, specimens coated with a matrix of sulphur and
surface treated muscovite mica released urea at a faster rate than specimens
coated with a matrix
of sulphur and untreated muscovite mica. Figure 4 further suggests the
potential significance of
choice of filler material, since muscovite mica arguably exhibits tendencies
toward "lock off
when incorporated into a permeable composition in a similar manner to
wollastonite. This in
turn suggests that different filler materials may require different designs
for the permeable
composition in order to achieve the same desired properties.
Filler Dispersion Testing
The filler dispersion testing for Example 1 was performed as follows:
1. During durability and release control testing specimen preparation, the
dispersal
properties of the filler material in molten sulphur were observed and
qualitatively
assessed.
2. Filler dispersion was qualitatively rated as poor, fair, good or very good.
Filler dispersal assessments were conducted on the fibrous fillers tested for
release control, as set out above. The filler dispersion test results for
Example 1 are set out in
Table 9.
Table
9
Qualitative
Assessment
of
Filler
material
Dispersal
in
Sulphur
Test Filler Surface Surface TreatmentDispersal
# Material Treatment Dosage (%, by Rating
weight
Tested Material of filler material)
Not
18 WollastoniteNone Applicable Poor
19 WollastoniteSugar 2% Poor
-57-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
20 WollastoniteSugar 5% Fair
21 WollastoniteSugar 10% Fair
22 WollastoniteSulphonate2% Good
23 WollastoniteSulphonate5% Very Good
Muscovite Not
24 Mica None Applicable Good
Muscovite Aluminum
25 Mica Sulphate 5% Very Good
Muscovite Aluminum
26 Mica Sulphate 10% Very Good
First, as is evident from the dispersal assessment, untreated muscovite mica
possessed better dispersal properties in sulphur than untreated wollastonite.
Therefore, it is
observed that various fibrous fillers possess differing dispersal properties
in molten sulphur.
Second, as is evident from the dispersal assessment, various surface
treatments
(of similar dosages) imparted differing dispersal properties to wollastonite.
Therefore, it is
observed that surface treatment material selection can be used to improve the
dispersal of treated
fibrous fillers in molten sulphur.
Finally, as is evident from the dispersal assessment, surface treated filler
dispersal
properties in sulphur varied with surface treatment dosage. Therefore, it is
observed the surface
treatment dosage can be used to improve the dispersal of treated fillers in
molten sulphur.
-58-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301 -
Test Results - Example Two
Urea is high-value, nitrogen fertilizer possessing the highest content of
nitrogen
commonly commercially available in a solid, granular form (46% nitrogen, by
weight).
Utilizing the aforementioned invention elements and invention process regime,
the sulphur
coated urea (SCU) product set out in Table 10 was produced on a bench-scale.
The SCU product of Example 2 was prepared having a granule size comparable
with a typical fertilizer to facilitate comparison with typical fertilizers.
Table 10
Example Composition of SCU Product
Produced
Composition Parameter Composition Parameter Value
Fertilizer Substrate Utilized Urea
Quantity of Sulphur Primer Utilized5%, by total weight of coated
product
Quantity of Sulphur/Surface 15%, by total weight of coated
Treated product
Wollastonite Coating Applied
Quantity of Surface Treated 15%, by weight of sulphur.
Wollastonite
in Coating
Wollastonite Particles NYAD~ M 1250
Wollastonite Surface Treatment nfs copolymer
Material
Wollastonite Surface Treatment 3%, by weight of Wollastonite
Material
Dosage
The SCU product with the above composition was subjected to assessments of its
controlled release properties and its mechanical durability. With regards to
controlled release
testing, the SCU product was tested relative to a commercially available
polymer coated urea
product (PCU) (control). PCU products in general are among the best performing
and highest
value controlled release fertilizer (CRF) products available on the market.
The PCU product
-59
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
tested possessed a "200 day release claim." Such claims are typically
established using field
trials designed to measure the CRF's sustained releasing action under field
conditions. For the
purposes of this evaluation however, an accelerated laboratory testing
procedure was used to
assess the release profile of the SCU product, relative to the 200 day release
claim PCU product.
The controlled release testing results for Example 2 are provided in Figure 5.
The
SCU product exhibited a nitrogen release profile close to that of a
substantially more expensive,
commercially available PCU product with a 200 day release claim. Therefore,
the ability of the
invention to produce a product with controlled release properties is verified.
With regards to mechanical durability, the SCU product's mechanical durability
was assessed relative to two commercially available SCU products (controls).
The SCU control
products also possessed additional product coatings over the primary sulphur
coating, in the
form of polymeric (wax) coatings.
The mechanical durability of the SCU products was assessed using a laboratory
procedure, which subjects the SCU to abrasive handling procedures, as follows:
1. 100 g of fertilizer sample is dropped down an 11-ft PVC tube, into a metal
container. The coated granules are subjected to violent collisions with the
walls
of the container and each other.
2. The sample drop is repeated 9 more times, for a total sample drop of 10
times.
3. 15 g of the mechanically stressed sample is then submersed in 15 °C
water for 24
hours.
4. The sample is then removed from the water, dried and weighed. The mass of
urea dissolved is used to calculate the % urea released, after abrasion.
The mechanical durability test results for Example 2 are provided in Figure 6
and
Table 11 below.
Table 11
Mechanical Durability Test Results
Description % urea release before % urea release after
abrasion (%, by mass) abrasion (%, by mass)
-6U-
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
Commercial SCU No. 58 91
1
(with polymer wax
overcoat)
Commercial SCU No. 27 57
2
(with polymer wax
overcoat)
SCU (utilizing the 17 34
within
invention)
The SCU product produced with the invention exhibited superior mechanical
durability, as compared to two commercially available SCU products possessing
additional
coating materials. Therefore, the ability of the invention to produce a
mechanically durable (i.e.:
reliable) product with controlled release properties is verified.
Test Results - Example Three
Utilizing the sulphur coated urea (SCU) product described in Table 10 above as
a
"control," a number of factors potentially affecting the controlled release
properties of the SCU
product were varied as set out in Table 12 below. For each product described
in Table 12, the
primer coating weight was fixed at approximately 5%, by total weight of the
product. Only the
weight of the coating of the permeable composition was reduced where indicated
in Test #4.
The results of varying the factors on the controlled release properties of the
SCU
product of Example 3 are also set out in Table 12 and Figure 7.
Table
12
Assessment
of
Release
Control
Factors
Test Description or WollastoniteSurface Coating Urea
#
Variable Tested Content Treatment Weight Released
(%, (%,
by weight Material by total after
of 1 day
sulphur) Dosage (%, weight static
of
by weight product) dissolutio
of
wollastonite) n (%,
by
mass)
-61 -
CA 02402212 2002-09-04
WO 01/66493 PCT/CA01/00301
I
1 Control 15 3 20 17
Wollastonite Content
2 in Coating S 3 20 32
(decreased relative
to
control - test
1 )
Surface Treatment
3 Material Dosage 1 S 5 20 36
(increased relative
to
control - test
1)
Total Coating
Weight
4 (i.e. thickness) 1 S 3 16 42
(decreased relative
to
control - test
1 )
-62-